ABSTRACT
Objective:
The development of coronavirus disease 2019 (COVID-19) vaccines was a crucial preventative measure toward controlling the pandemic. Several side effects have been reported. This study investigated the long-term side effects reported by the Saudi population. post-COVID-19 vaccination.
Methods:
The cross-sectional study involved Saudi participants of both genders, aged ≥16 years, and had received at least one dose of any of the available vaccines in Saudi Arabia. They were asked to fill out an online questionnaire divided into three sections: Demographics, medical history, and side effects that appeared post-COVID-19 vaccines.
Results:
The findings indicated that the undesirable effects were reported by 82% of the participants. These side effects involve three categories: The most common, additional or reported, and persistent side effects. The most common side effects were pain at the site of injection (88.16%), bone pain/joint pain (68.7%), and fatigue (68.46%). Menstrual disorders (n = 46), hair loss (n = 34), and memory problems (n = 19) were reported by participants as additional side effects. Among all side effects, fatigue, joint pain, hair loss, and menstrual disorders were the most persistent side effects. Moreover, 190 participants reported that they were diagnosed with diseases soon after receiving the COVID-19 vaccine including COVID-19, thyroid gland disorder, and irritable bowel disease. The quality of life of some of the participants was affected by post-COVID-19 vaccines, as 25.28% had anxiety, 21.22% had depression, and 33.16% had discomfort.
Conclusion:
These findings may contribute to understanding the effect of COVID-19 vaccines on the Saudi population’s health and public opinion about these vaccines.
Keywords: Coronavirus, covid, covid-19, pandemic, vaccine
Introduction
Infectious diseases pose a formidable threat to global health and security, making them the leading cause of mortality worldwide. This presents a substantial challenge for the global community in terms of public health and overall well-being.[1] Coronavirus disease 2019 (COVID-19) is an infectious disease caused by the severe acute respiratory syndrome coronavirus 2. The COVID-19 outbreak has led to major health, social, and economic burdens.[2,3] Globally, health-care systems were not prepared for such a crisis; therefore, fast and effective solutions were needed urgently. Self-quarantine was obligatory worldwide, subsequently, Saudi Arabia implemented several preventative measures including partial and complete lockdown and travel restrictions.[4,5] Repurposing of the already available drugs such as hydroxychloroquine,[6] remdesivir,[7] and favipiravir[8] has also been investigated. However, these drugs demonstrated ineffective therapeutic efficacies and safety profiles on severely affected COVID-19 patients. The development of COVID-19 vaccines to control the pandemic has been a worldwide priority.[9] In 2020, Pfizer/BioNTech (BNT162b2) was the first COVID-19 vaccine that obtained authorization from the Food and Drug Administration (FDA) and granted World Health Organization (WHO) approval for emergency use.[10] The approval of the Oxford/AstraZeneca vaccine was then released in January 2021 by the European Commission and obtained authorization for emergency use by the WHO in February 2021. Other vaccines that also obtained the emergency use listing by the WHO include Moderna (mRNA 1273) and Johnson and Johnson’s Janssen Ad26.COV 2.S.
In Saudi Arabia, four vaccines were approved, including Pfizer/BioNTech, Oxford/AstraZeneca, Moderna, and Janssen (Johnson and Jhonson).[11] A two-dose regimen program of Pfizer/BioNTech or Oxford/AstraZeneca vaccines was first made available for high-risk candidates such as first-line health-care workers and citizens with chronic diseases. Shortly after, a two-dose regimen program of the available vaccines was mandatory for both Saudi citizens and residents, which was then followed by the booster-dose phase.[12]
Although these vaccines are designed to prevent lethal complications related to COVID-19, a range of mild-to-severe side effects have been reported globally. Mild side effects include localized pain at the site of injection, tenderness, swelling, itching, tenderness, and swelling of armpit glands. Other mild systematic side effects include headache, fatigue, fever, chills, night sweats, muscle pain, joint pain, decreased appetite, nausea, and vomiting.[13-17] Moreover, on October 12, 2022, updates issued by the Centers for Disease Control and Prevention reported several severe side effects post-COVID-19 vaccine administration. These include anaphylaxis, myocarditis and pericarditis, Guillain–Barré syndrome, thrombosis with thrombocytopenia syndrome, and death.[18]
According to the Saudi Ministry of Statistics, 67,979,420 vaccine doses were administered to the Saudi Arabian population.[19] There have been several studies examining short-term side effects of COVID-19, but data on long-term side effects of these vaccines within different regions of Saudi Arabia are limited. Furthermore, effective interventions will be developed with the understanding of COVID-19 vaccination side effects and public opinion of COVID-19 vaccinations. Herein, we aim to explore the most common long-term side effects acquired post-COVID-19 vaccination among the Saudi population and their effects on the overall quality of life.
Materials and Methods
Study design
This was a cross-sectional survey-based study completed between the 12th and 22nd of October 2022, to evaluate the effect of the COVID-19 vaccine on the health of the Saudi Arabian population, using a validated self-reporting questionnaire created on Google Forms, which was dispersed randomly through all social media applications. The study protocol was approved by the King Abdulaziz University Hospital Ethics Committee (reference no. 30-18, 5, 2022). All procedures were conducted in compliance with the institution’s ethical guidelines.
Participants were directed to a link with a brief introduction to the aim, purpose of the study, and informed consent, which included statements about voluntary participation. In addition, our questionnaire guaranteed participants anonymity, and that our results were to be reported as grouped information and findings. All participants signed an online consent form. The questionnaire also gave participants the choice to withdraw before submitting their answers and at any stage.
The design of the questionnaire was divided into four sections, shown in Appendix A, and was as follows: Section one was about participant’s demographics, including gender, nationality, age, marital status, educational level, and employment status. The second section questions were about the participant’s medical history: They were asked to specify any health problems, chronic diseases, comorbidities, smoking and exercising habits, number of COVID-19 vaccine doses, date of delivery of the final dose, and type/brand name of the vaccine/s, and the participant was asked to list the accompanying medications before and after receiving the vaccine. The list of medications includes and is not limited to paracetamol, non-steroidal anti-inflammatory drugs, or supplements such as Vitamin D, zinc, and multivitamins. In the third section, participants were asked about post-vaccination symptoms and diseases; when they appeared, how long the symptoms lasted, and whether they had been hospitalized due to these symptoms. If their lymph nodes have been removed surgically, and if they were diagnosed with any disease after receiving the COVID-19 vaccine, or if they have been treated with cupping. The last section covered the quality of life of the participant post-vaccination. The questions examined in this section include, but are not limited to: continuous monitoring of the participant’s vital signs, whether the participant’s overall well-being has been affected, whether the participant is willing to receive another annual seasonal vaccine dose, whether the participant believes the COVID-19 vaccination is safe and whether a family member of the participant contracted an infection or died after receiving the vaccination. In terms of persistent side effects, participants were asked whether side effects persisted longer than 2 months after the last dose received.
Inclusion criteria
Inclusion criteria included all adults, both genders, and aged 16 years and above that are nationals of the Kingdom of Saudi Arabia.
Exclusion criteria
Participants who were not Saudi nationals, those who were under 16 years of age, and who did not receive at least one COVID-19 vaccination shot.
Sample size
One thousand six hundred and sixty-two responses were received from different age groups, regions, and nationalities. The study sample included participants who received at least one vaccination dose at the Saudi Ministry of Health COVID-19 vaccination clinics with one of the three pharmaceutical brands which were Pfizer, AstraZeneca, and/or Moderna. The data sheet and results were exported to a Microsoft Excel spreadsheet which was cleaned up, and accordingly, our final sample of participants that fit the exclusion/inclusion criteria dropped to 1503.
Statistical analysis
Microsoft Excel was used for statistical analysis to obtain descriptive analysis for the data collected, frequencies, and percentages to compare between variables. Graphpad prism was used to generate figures.
Results
Demographics and general characteristics
A total of 1662 participants filled in the questionnaire by October 12, 2022. However, one hundred and forty-eight participants were excluded, because they did not meet the inclusion criteria of this study. Around one-third of the participants (30.1%) were male (n = 453), whereas 69.9% (n = 1050) were female and both received at least one dose of the vaccines available in Saudi Arabia. The age of the participants ranged from 16 to >65 years old and they are from 12 different Saudi Arabia provinces. The majority of the participants were from Makkah (n = 914), Riyadh (n = 302), and Eastern (n = 118) provinces [Table 1].
Table 1.
Demographics and general characteristics of the participants
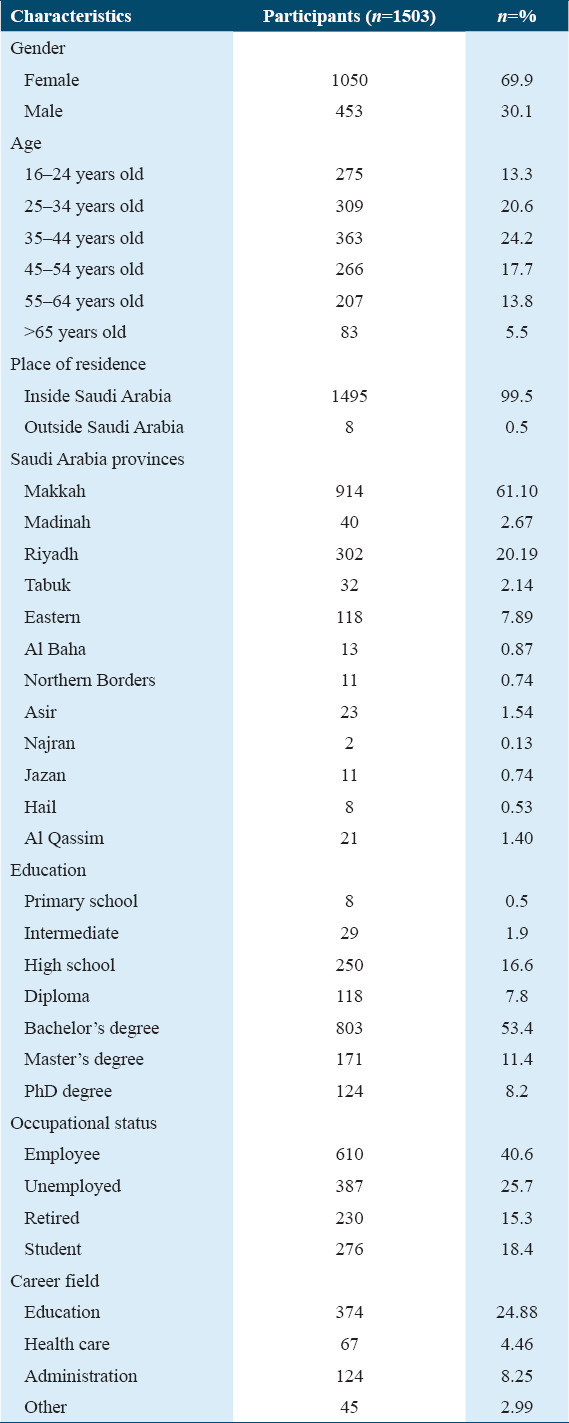
The health status of the participants was determined according to information they provided on faintness, smoking habits, and mental and physical health [Table 2]. The vast majority of the participants (n = 1090) reported that they performed physical exercise. In addition, 80.8% of the participants were non-smokers and 80.8% did not suffer from any common health issues. However, 40.16% of the participants were affected by chronic diseases, including obesity (11.43%), diabetes (9.05%), hypertension (10.57%), high cholesterol (9.64%), thyroid gland disorder (8.39%), asthma (5.48%), heart diseases (2.25%), arthritis (2.11%), rheumatoid arthritis (1.85%), and other (20.61%). The minority of the participants have also reported that they have been suffering from several other health issues such as psychological issues (6.52%), behavioral disorders (0.79%), and physical disability (11%).
Table 2.
General health status of the participants
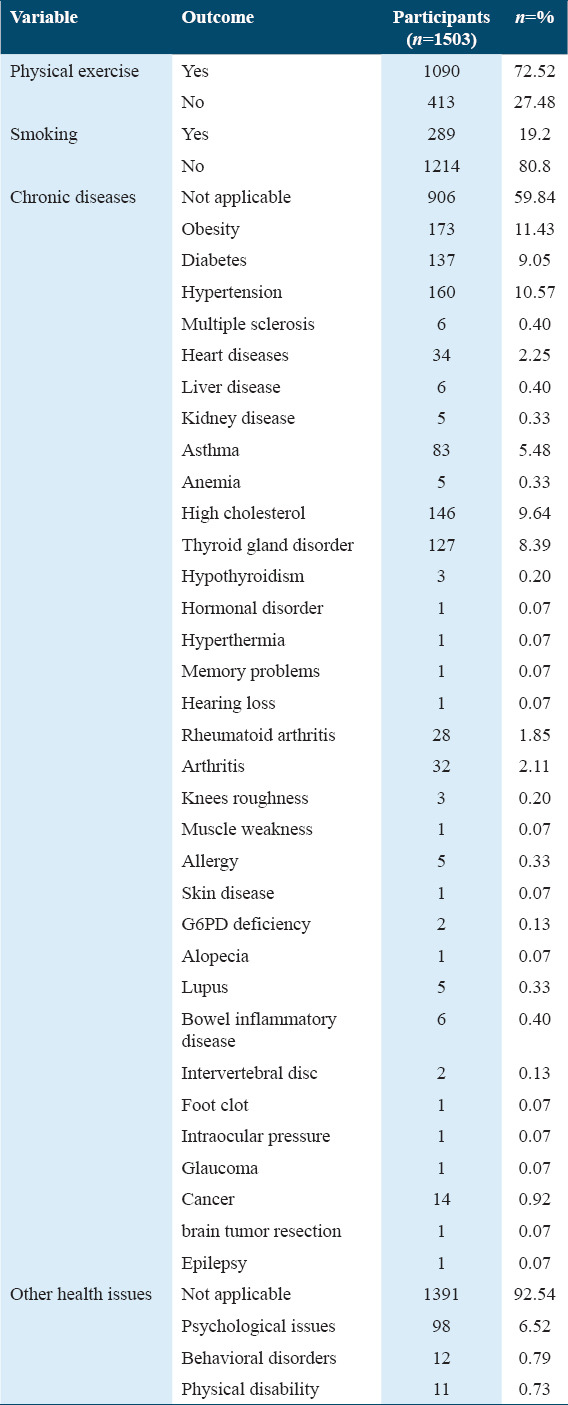
The participants were asked about the type of COVID-19 vaccine, the number of vaccine doses they have received, and how many times they have been infected with the virus [Table 3]. Most of the participants received only the Pfizer vaccine (68.7%), whereas 17.2% were vaccinated with Pfizer and AstraZeneca vaccines and 6.6% were vaccinated with AstraZeneca, Pfizer, and Moderna vaccines. Furthermore, over than three-quarters (n = 1157) of the participants received three doses of the COVID-19 vaccine with the last dose being administered within 9–12 months. Moreover, around half of the participants (n = 700) reported that they had not been diagnosed with COVID-19. In contrast, 53% of the participants reported that they had been infected with the virus at least once (n = 804), 37% of which claimed that they were infected after receiving the vaccine.
Table 3.
COVID-19 vaccination and infection status
Medications and supplements during vaccination
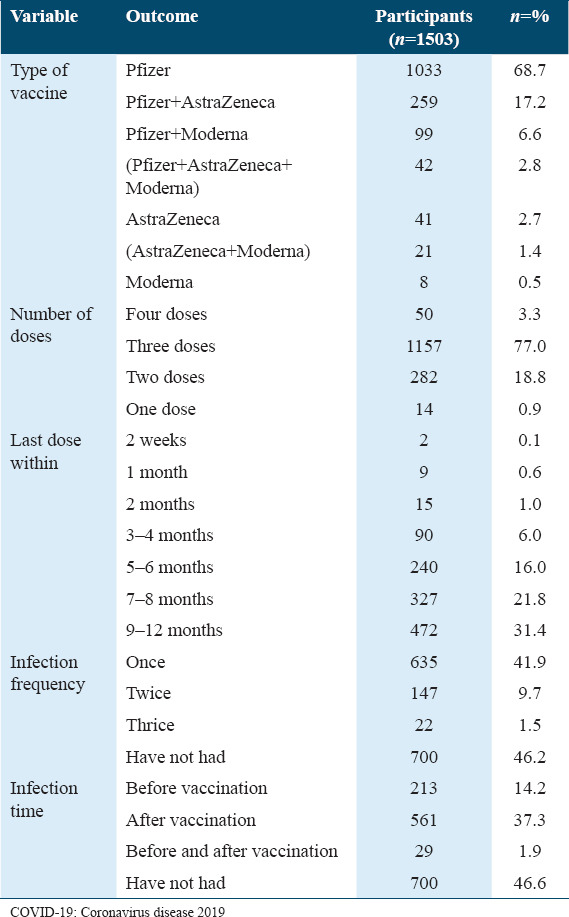
The participants have been asked about the medications and supplements that they may have been taken after receiving the COVID-19 vaccine [Table 4]. Most of the participants reported that they have not had any medication (61.74%) or supplements (40.59%) post-COVID-19 vaccination (n = 928) and (n = 610), respectively. In addition, 29.61% of the participants specified that they had had paracetamol (n = 445) and the rest of the participants reported that they had been taken a variety of medications [Table 4]. Vitamin D, C, and multivitamins were the most used supplements post-COVID-19 vaccine: 36.39%, 32%, and 23.75%, respectively. Some of the participants have also reported that they have had other supplements such as zinc (n = 302) and iron (n = 205).
Table 4.
Medications and supplements used during COVID-19 vaccination
Post-vaccine side effects
The participants were asked whether they suffered from any side effects after receiving the vaccine to document the common and specific side effects among the Saudi population. The results indicated that out of all participants (n = 1503), 1233 (82.04%) reported having side effects after the COVID-19 vaccine. The majority of them (n = 845) noted that these side effects started soon after getting vaccinated and before 24 h had passed [Figure 1].
Figure 1.
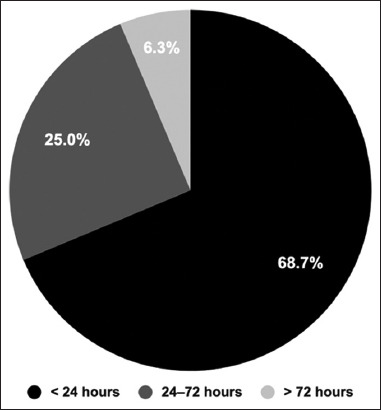
The onset of Coronavirus disease 2019 vaccination side effects. The participants were asked when the side effects started after receiving the vaccine. The pie chart is divided based on the onset of the side effects; black <24 h, dark grey 24–72 h, and >72 h
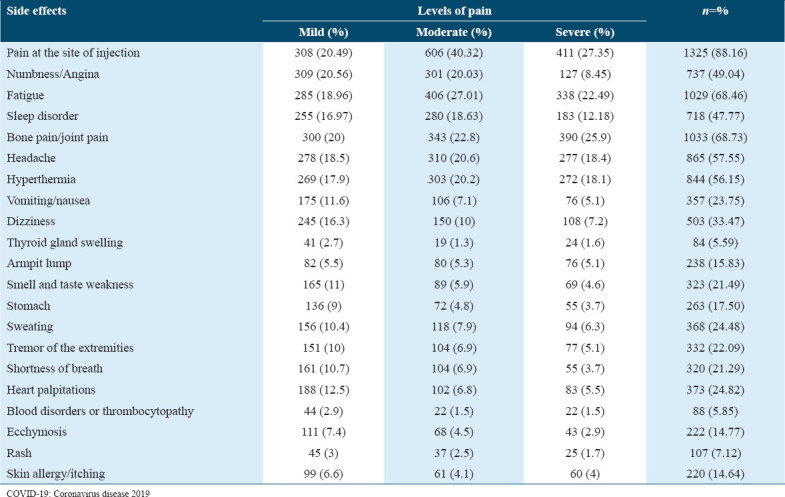
The participants identified the wide range of post-vaccination side effects. [Table 5] shows these side effects and their severity levels. The most common side effects were pain at the site of injection (88.16%), bone pain/joint pain (68.7%), fatigue (68.46%), headache (57.6%), hyperthermia (56.2%), numbness/angina (49.04%), sleep disorder (47.77%), and dizziness (33.5%).
Table 5.
The side effects and their severity after COVID-19 vaccination
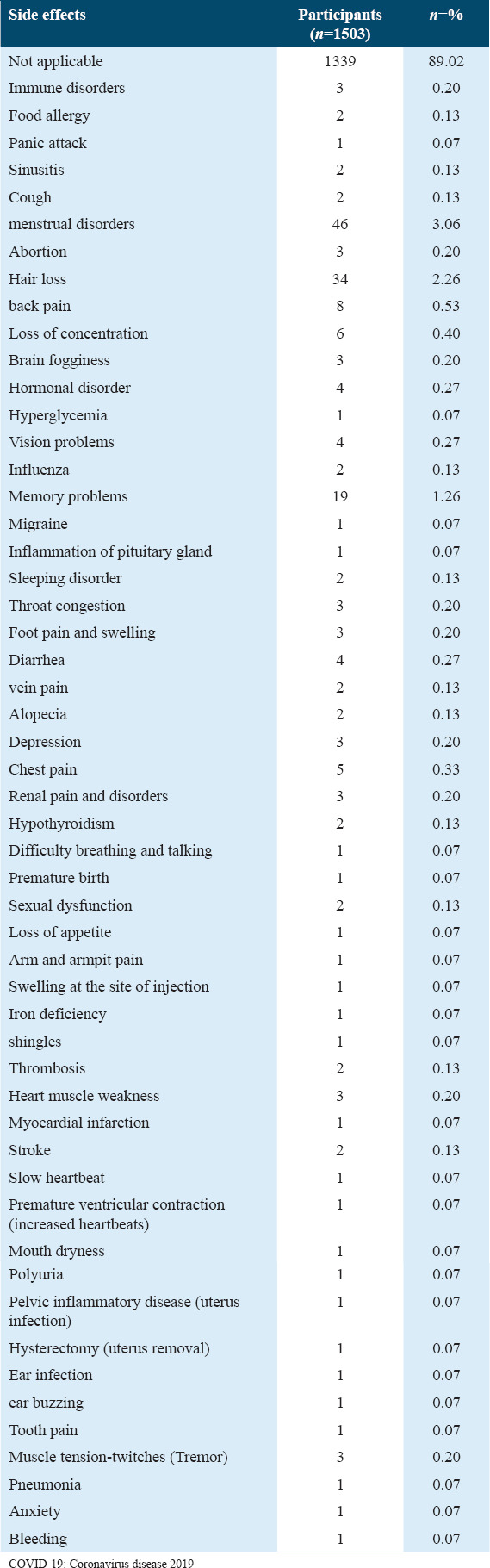
Eleven percent of the participants (n = 165) reported they were suffering from additional side effects [Figure 2]; these were not mentioned in [Table 5]. Among these side effects, menstrual disorders, hair loss, and memory problems were mentioned by many individuals: n = 46, n = 34 and n = 19, respectively [Table 6].
Figure 2.
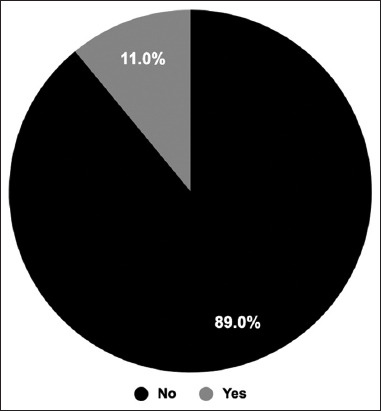
Additional side effects. The participants were asked whether they had any additional side effects. The pie chart shows the percentage of the participants who said yes, they had or no they had not
Table 6.
Additional side effects of COVID-19 vaccination reported by participants
Persistent side effects
The participants were asked if they had any persistent side effects post-COVID-19 vaccination to identify long-term side effects. The results showed that 312 out of 1503 participants reported that they had persistent side effects [Figure 3]. These include fatigue (n = 52), joint pain (n = 44), hair loss (n = 42), and menstrual disorders (n = 35). In addition, less frequent persistent side effects such as headache (n = 28), muscle pain (n = 23), irritated skin (n = 19), shortness of breath (n = 19), pain at the injection site (n = 19), memory problems (n = 17), heart weakness (n = 17), and heart palpitation (n = 17) had been reported [Table 7].
Figure 3.
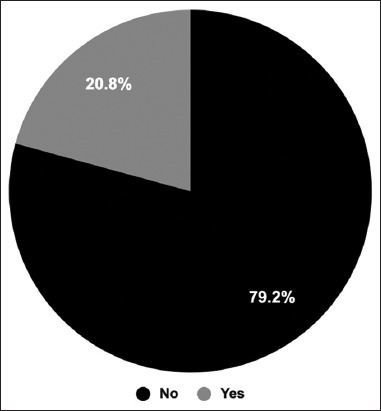
Persistent side effects post-Coronavirus disease 2019 vaccination. The pie chart shows the percentages of participants who had persistent side effects compared to the percentage of participants who did not have persistent side effects
Table 7.
Persistent side effects post-COVID-19 vaccinations
Post-COVID-19 vaccination diseases
By the time they answered our study’s questionnaire, the majority of the participants had already gotten three doses. Therefore, the participants have been asked about diseases that may have appeared after vaccination. Around 12.64% of participants (n = 190) reported that they were diagnosed with a disease after receiving the vaccine [Figure 4].
Figure 4.
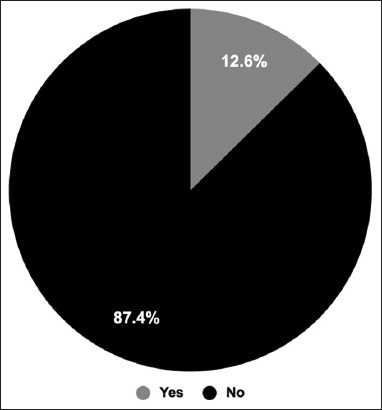
Coronavirus disease 2019 post-(COVID-19) diseases. The participants were asked whether they had been diagnosed with any disease after receiving the COVID-19 vaccine. The pie chart shows the percentage of participants who developed a disease after receiving COVID-19 vaccine compared to the participants who did not develop any diseases
Most of the participants who have been diagnosed with diseases post-COVID-19 reported that the disease appeared after the second dose (n = 82) and the third dose (n = 72) [Figure 5]. Moreover, the largest percentage (n = 160) of them were diagnosed with a disease after the vaccination with the Pfizer type [Figure 5].
Figure 5.
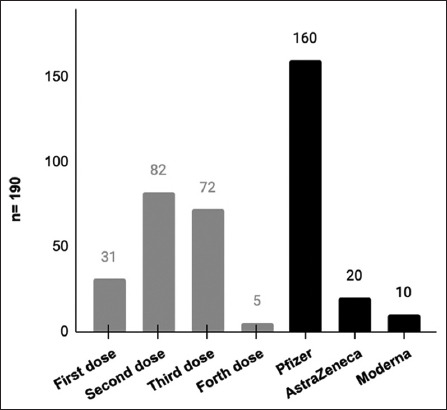
The disease appeared after any dose and which type of vaccine. Participants were asked to determine after which dose, they were diagnosed with the disease, as well as after any type of vaccine. The bar chart shows the comparison between the number of participants, who were diagnosed with diseases (n = 190) after each dose and the type of vaccine
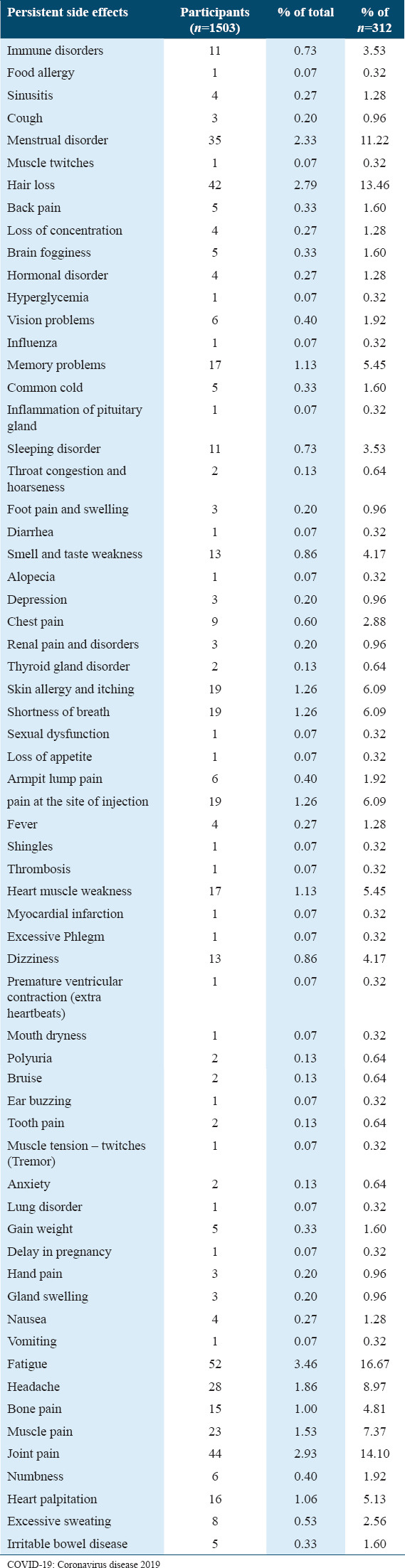
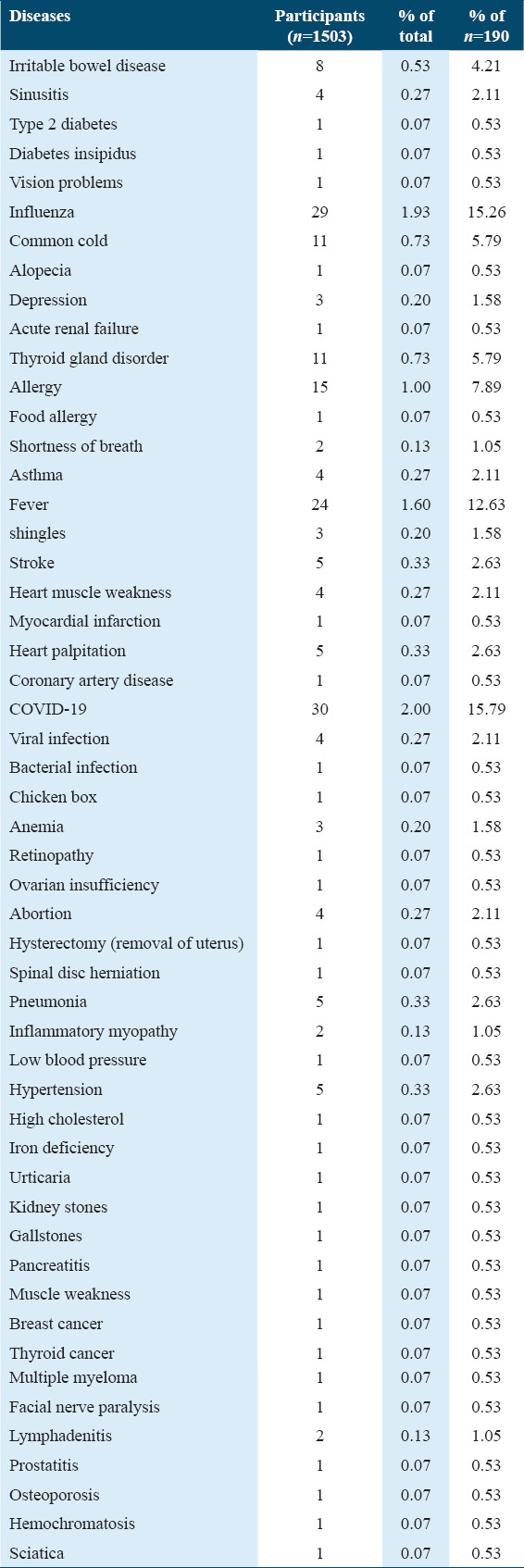
Based on their participants’ (n = 190) reports, Table 8 presents the list of diseases noted post-COVID-19 vaccination. The most common diseases documented were COVID-19 (n = 30), influenza (n = 29), fever (n = 24), allergy (n = 15), thyroid gland disorder (n = 11), common cold (n = 11), and irritable bowel disease (n = 8).
Table 8.
The diseases that appeared after receiving the COVID-19 vaccine
Quality of life and COVID-19 vaccine
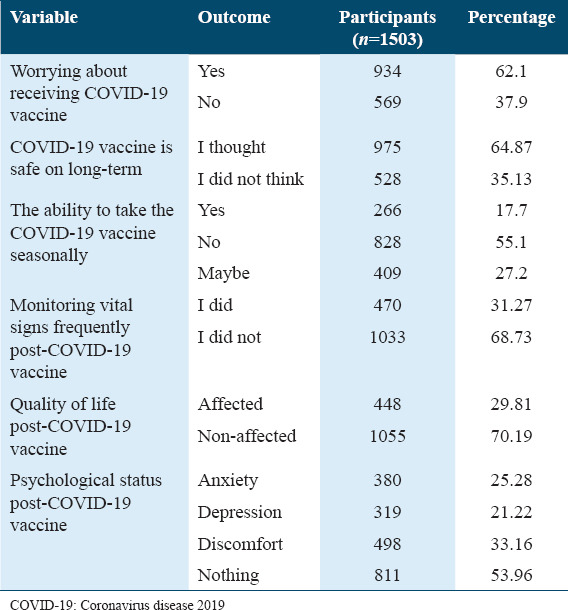
In this part, we assess whether the quality of life of individuals has been affected after receiving COVID-19 vaccines [Table 9]. The results show that the majority of the participants (62.1%) were worried about receiving COVID-19 vaccine. On the other hand, only 35.13% of individuals (n = 528) thought that COVID-19 vaccines were unsafe in the long term, whereas 64.87% of the participants (n = 975) felt safe. Moreover, nearly 55% disagreed to take the seasonal COVID-19 vaccine, whereas 17.7% and 27.2% of the participants agreed and selected “agree maybe,” respectively. Thirty-one percent of participants kept monitoring their vital signs frequently. Likewise, 30% of participants considered that their quality of life was affected after the vaccine. Therefore, regarding their participants’ psychological status, some of them felt anxiety, depression, and discomfort (25.28%, 21.22%, and 33.16%, respectively).
Table 9.
The effect of the COVID-19 vaccine on the quality of life
Seventy-four percent (n = 1112) of the study participants in our study were satisfied with their health status after taking the vaccine, whereas the other participants (26%) were unsatisfied [Figure 6].
Figure 6.
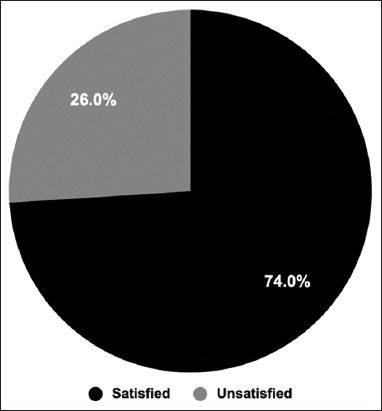
Participants’ satisfaction with their health status. The participants were asked whether they were satisfied with their health status post-Coronavirus disease 2019 vaccination. The pie chart shows the percentage of satisfied participants compared to unsatisfied ones
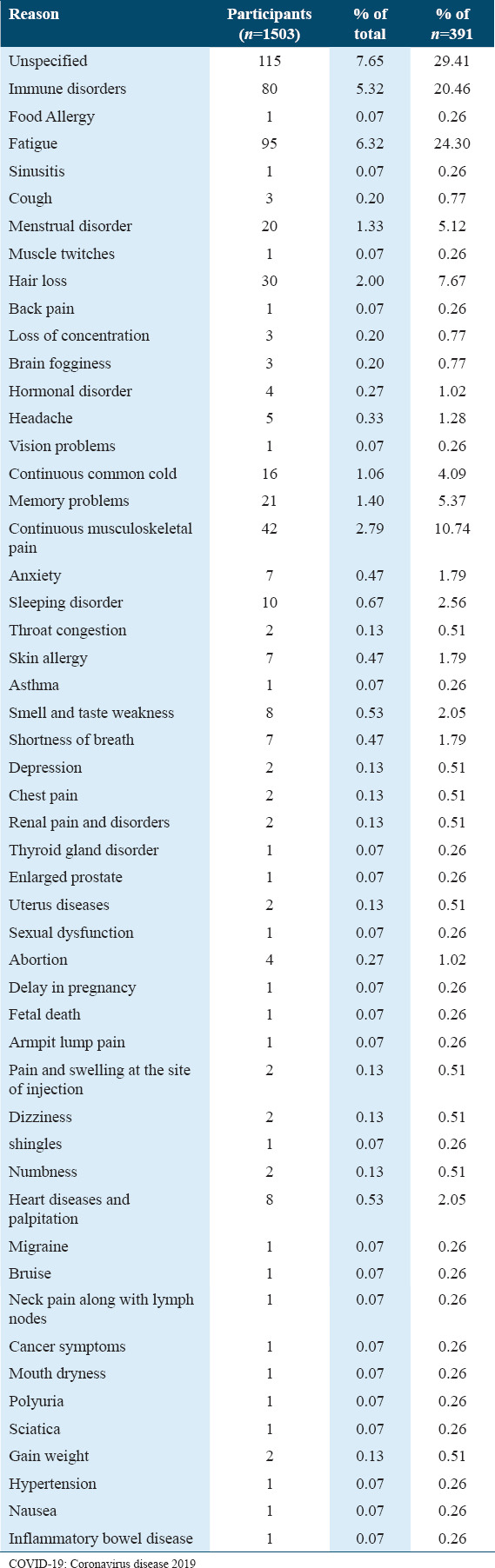
Unsatisfied participants (n = 391) felt dissatisfied for a number of reasons, the most mentioned were fatigue (n = 95), immune disorders (n = 80), musculoskeletal pain (n = 42), hair loss (n = 30), memory problems (n = 21), menstrual disorder (n = 20), continuous common cold (n = 16), and sleeping disorder (n = 10) [Table 10].
Table 10.
The reasons behind the unsatisfaction of the participants with their health status post-COVID-19 vaccine
The participants were asked if they had undergone cupping treatment after receiving the COVID-19 vaccine. The results showed that 91.8% of the participants had not undergone cupping treatment after the COVID-19 vaccine [Figure 7]. Participants reported a reduction in the side effects after treating with cupping (65.8%).
Figure 7.
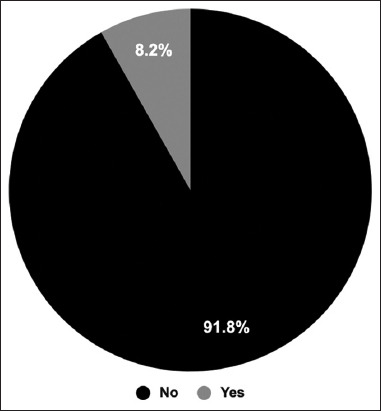
Cupping therapy (Hijama) among participants after receiving Coronavirus disease 2019 vaccines. The participants were asked if they were treated with cupping after vaccination. The pie chart shows the percentage of participants who had undergone cupping
Discussion
The globe is in the midst of perpetuating the COVID-19 pandemic affecting all nations. Experts are speculating that COVID-19 is going to keep oscillating worldwide infinitely.[20] Furthermore, with the emerging new variants, of which a few are considered “variants of concerns” according to WHO,[21] the only solution to prevent future surges in COVID-19 cases is to maintain a threshold of herd immunity through vaccination campaigns. However, people are becoming hesitant to be immunized due to the unpleasant side effects that follow. Therefore, it is crucial for vaccine manufacturers to continuously develop the available vaccinations by appraising the reported side effects in published research studies. Moreover, it is pivotal to address people’s concerns by creating a self-reporting system that is directly connected to an empathetic health-care system. As there is no self-reporting platform in Saudi Arabia, the only way to get people to report side effects is by means of questionnaires. In this study, people are asked to report experiencing short-term side effects as well as long-term side effects post-COVID-19 vaccination.
Generally, side effects can be categorized based on the impacted site into two groups: Local reactions and systemic reactions. In this study, the majority of local side effects reported involve minor injection site’s reactions including injection site pain (1.3%) and swelling (0.3%). In addition, minor systematic symptoms described include fatigue (3.5%), fever (1.3%), headache (1.9%), muscle pain (1.5%), diarrhea (0.1%), vomiting (0.1%), and flu-like symptoms (0.19%). Our findings are in accordance with the recent study conducted in Italy.[22] The majority of the side effects disappeared after 1–7 days (65.49%) whereas others reported recovery from side effects at longer duration from 1 month to 3–6 months (4.45%) and (2.26%), respectively. The previously mentioned side effects significantly impacted young ages between 18 and 44 (13.4%) compared to the <45 age groups (8.5%). Nevertheless, the aforementioned side effects were found to be the expected side effects announced by the FDA and also in alignment with age groups expected to be more susceptible.[23]
Some of the self-reported long-term side effects are found to be frequently reported by female participants including menstrual irregularities (3.33%). The disturbance in menstrual included missed cycles, irregular cycles, and decreased or increased in menses. This is the first Saudi study to report such post-COVID-19 vaccine disorder among Saudi females. This report is in agreement with some research studies that examined disturbances in the menstrual cycle or in menses among females after COVID-19 vaccines.[24,25] Similarly, to the later publications, most of the acquired menstrual disorders appeared after the booster dose (74.4%) and the second dose (23.5%). However, unlike aforesaid research studies, Saudi female participants did not retain normal menstrual patterns to the date of this study. Unfortunately, gender bias is common in many drug trials, COVID-19 vaccine manufacturers did not break this cycle and thus did not include side effects that occur due to gender differences during test trials.[26] To bridge this gap, further clinical investigations are required to better understand the causative prognostic factor behind this physiological disorder among Saudi females. Supporting our results are the findings of Govindapala et al., who found different responses in females toward COVID-19 vaccination.[27] Another prevalent side effect reported in this study among Saudi females was telogen effluvium (4%) reported post-booster vaccine (76.47%) and second vaccine (17.64%). This manifestation includes hair shedding, excessive hair loss, and hair thinning. Although, to this date, there is no research study that examined hair loss among females post-COVID-19 vaccination. However, an abundance of data from research studies has reported telogen effluvium among patients post-COVID-19 infection.[28] The latter research study proposed that the hair fall manifestation could be triggered due to psychological stress. However, patients with immune-compromised conditions such as Alpaca Areata reported hair shedding immediately after the COVID-19 vaccine. This could indicate an underlying immune-mediated response that could explain such conditions after COVID-19 immunization.[29]
Other highly reported post-vaccination side effects that also persisted to the date of this study involve fatigue (6.25%), arthralgia (2.79%), headache (1.66%), memory problems (1.19%), shortness of breath (1.196%), and myalgia (1.26%). The priorly mentioned side effects were found to be the common denominator of the reported concerns in many other research studies.[30,31] It seems that COVID-19 vaccines evoke an immune response that could be attributed to all these side effects. Subsequently, many studies embarked on investigations to detect possible immunological factors that got antagonized by these vaccines. In light of that, one study suggested that COVID-19 vaccines commence activation of the initial stages of the immune response causing a subsequent surge in cytokines production more specifically interferons type 1. This type of interferon has a ubiquitous range of actions throughout the whole body including the respiratory system.[32] Notably, many post-COVID-19 vaccine side effects are linked to cytokines production namely fatigue, fever, and shortness of breath. Strikingly, it was found that prolonged exposure to this type of cytokine as a therapy induces chronic fatigue, loss of concentration, and depression,[33] which could indicate that COVID-19 vaccines might impose a similar harmful immune response.
Other side effects that reported are concerning the cardiovascular system include heart palpitations (1.1%), myocardial infarction (0.1%), cardiomyopathy (0.1%), thrombosis (0.1%), chest pain (0.6%), and premature ventricular contraction (0.1%). Many research studies delved into confirming cardiovascular dysfunction cases that arise from post-COVID-19 vaccines. Certainly, a direct link between COVID-19 immunizations and cardiovascular dysfunctions was proven clinically. Collectively, research studies suggested that COVID-19 vaccinations induce inflammatory responses that explain the pathological cardiovascular manifestations.[34-37]
Other rare and major side effects that also self-reported by participants after vaccination were premature birth (0.1%), Bell’s palsy (0.1%), shingles (0.1%), tooth pain (0.2%), swollen eyelids (0.1%), ear buzzing (0.1%), mouth dryness (0.1%), and vision problems (0.4%). Some participants reported non-communicable side diseases post-COVID-19 vaccine including breast cancer (0.1%), thyroid cancer (0.1%), blood cancer (0.1%), and acute kidney failure (0.1%). Although, the above-stated side effects are scarcely reported, nevertheless, clinicians must run vigilant examinations for patients who acquire new conditions post vaccines.
Cupping therapy is a common practice in traditional medicine, involving local suction of the skin by special cups. This therapy is believed to enhance blood flow and prompt swift healing of chronic pains in the neck and the lower back, according to the National Center for Complementary and Integrative Health.[38] Many studies have demonstrated promising preventive and therapeutic benefits of cupping therapy. These studies investigated the therapeutic performance of the cupping procedure on pathological conditions such as pain and high fever due to infection of the upper respiratory tract,[39] pulmonary dysfunctions in asthmatic children,[40] type 2 diabetes mellitus,[41] autoimmune diseases such as rheumatoid arthritis, hypertension,[42] myocardial infarction, cardiac arrhythmias, and chronic fatigue syndrome.[43] However, sparse studies have assessed the effects of cupping therapy in this new pandemic COVID-19.[44] Cupping therapy may ameliorate the most common symptoms and signs of COVID-19 infection. This study showed a reduction in the side effects among people who were treated with cupping (65.8%).
In response to clinical infections caused by viruses from the influenza family, respiratory syncytial viruses, and rhinoviruses, the active metabolite of Vitamin D, 1,25-dihydroxy Vitamin D (1,25 [OH] 2D), activates innate antiviral effector systems and controls inflammation. Vitamin D can be taken as a dietary supplement and is primarily produced by the skin as a result of exposure to sunshine.[45]
Previous research that examined the effects of Vitamin D supplements on the efficiency of the COVID-19 vaccine and immunity, as well as the impact of Vitamin D on alleviating the symptoms of infection with COVID-19, has revealed several encouraging findings.[46] Similarly to that, it was found to reduce the mortality risk among COVID-19 patients,[47] On the contrary, according to Jolliffe et al., Vitamin D has no effect on boosting immunity and does not improve the efficiency of the COVID-19 vaccine.[48]
Overall, many adverse side effects were frequently reported by COVID-19 vaccine recipients and a few side effects were gender-dependent on the physiological differences reported by female participants mostly after the booster immunization.
The questionnaire created in this study pushed the boundaries by formulating difficult questions that were not tackled by all the previously reported Saudi publications. Participants are encouraged to disclose all experienced side effects. In addition, this study highlighted side effects that appeared among Saudi females that were not examined by other counterpart Saudi researchers. Nonetheless, the main limitation of this self-reporting study is recall bias by participants. Another limitation is that participants could have experienced a prior infection with the COVID-19 virus but did not prove it by COVID-19 test or got a false-negative. This is crucial as the reported adverse effects could be due to post-COVID-19 infection. In addition, this study eliminated survey entries from non-Saudis. In addition, Online surveys have their limitations and since a particular stratum of society takes part in online surveys, the findings may not be true representative of findings.
Further research is needed to evaluate the vaccine’s effectiveness and potential side effects in specific populations, such as immunocompromised individuals, pregnant women, children, and older adults. These studies help tailor vaccine recommendations and identify any specific safety concerns. Future research will focus on developing strategies to manage and mitigate vaccine side effects effectively. This includes refining vaccination protocols, optimizing vaccine delivery methods, and developing interventions to address common and rare adverse events. Future studies may also investigate the relationship between vitamin supplementation and the magnitude and effectiveness of the immune response and reduction of undesirable effects following vaccination. It is important to note that the COVID-19 vaccine development and rollout have been rapid, and ongoing research aims to provide a comprehensive understanding of potential adverse events and optimize vaccination strategies to ensure public health and safety.
Ethical Approval and Consent to Participate
The study protocol was approved by the King Abdulaziz University Hospital Ethics Committee (reference no. 30-18, 5, 2022). All participants signed an online consent form indicating their informed consent.
Availability of Data and Material
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Competing Interests
The authors certify that there is no conflict of interest to declare.
Funding Statement
NA (no funding received for this study).
Authors’ Contributions
SAA conceived the idea and designed the study. All authors participated in designing the questionnaire. EZR collected the data and analyzed the results. AHG, NHH ASG, AO, and KK performed the literature search and wrote the manuscript. AO translated the questionnaire and participants’ answers. AHG and NHH created the graphical abstract. SAA supervised the project and reviewed the original manuscript. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
We greatly thank the study participants for their participation.
References
- 1.Tabish SA. Recent trends in emerging infectious diseases. Int J Health Sci (Qassim) 2009;3:V–VIII. [PMC free article] [PubMed] [Google Scholar]
- 2.Estimated COVID-19 Burden. 2022. [[Last accessed on 2022 Nov 20]]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/burden.html .
- 3.Alabdulmonem W, Shariq A, Rasheed Z. COVID-19:A global public health disaster. Int J Health Sci (Qassim) 2020;14:7–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Algaissi AA, Alharbi NK, Hassanain M, Hashem AM. Preparedness and response to COVID-19 in Saudi Arabia:Building on MERS experience. J Infect Public Health. 2020;13:834–8. doi: 10.1016/j.jiph.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahmud I, Al-Mohaimeed A. COVID-19:Utilizing local experience to suggest optimal global strategies to prevent and control the pandemic. Int J Health Sci (Qassim) 2020;14:1–3. [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Cremades M, Solans BP, Hughes E, Ernest JP, Wallender E, Aweeka F, et al. Optimizing hydroxychloroquine dosing for patients with COVID-19:An integrative modeling approach for effective drug repurposing. Clin Pharmacol Ther. 2020;108:253–63. doi: 10.1002/cpt.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19-preliminary report. N Engl J Med. 2020;383:1813–36. doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 8.Cai Q, Yang M, Liu D, Chen J, Shu D, Xia J, et al. Experimental treatment with favipiravir for COVID-19:An open-label control study. Engineering (Beijing) 2020;6:1192–8. doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaur SP, Gupta V. COVID-19 vaccine:A comprehensive status report. Virus Res. 2020;288:198114. doi: 10.1016/j.virusres.2020.198114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO Issues its First Emergency Use Validation for a COVID-19 Vaccine and Emphasizes Need for Equitable Global Access. 2020. [[Last accessed on 2022 Oct 25]]. Available from: https://www.who.int/news/item/31-12-2020-who-issues-its-first-emergency-use-validation-for-a-covid-19-vaccine-and-emphasizes-need-for-equitable-global-access .
- 11.Vaccines Approved for Use in Saudi Arabia. 2022. [[Last accessed on 2022 Oct 19]]. Available from: https://covid19.trackvaccines.org/country/saudi-arabia .
- 12.Interim Guidelines for the Use of SARS-CoV-2 Vaccine. 2021. [[Last accessed on 2022 Oct 27]]. Available from: https://covid19.cdc.gov.sa/professionals-health-workers/interim-guidelines-for-the-use-of-sars-cov-2-vaccine .
- 13.Abu-Halaweh S, Alqassieh R, Suleiman A, Al-Sabbagh MQ, AbuHalaweh M, AlKhader D, et al. Qualitative assessment of early adverse effects of pfizer-biontech and sinopharm COVID-19 vaccines by telephone interviews. Vaccines (Basel) 2021;9:950. doi: 10.3390/vaccines9090950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Shitany NA, Harakeh S, Badr-Eldin SM, Bagher AM, Eid B, Almukadi H, et al. Minor to moderate side effects of pfizer-biontech covid-19 vaccine among Saudi residents:A retrospective cross-sectional study. Int J Gen Med. 2021;14:1389–401. doi: 10.2147/IJGM.S310497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaur RJ, Dutta S, Bhardwaj P, Charan J, Dhingra S, Mitra P, et al. Adverse events reported from COVID-19 vaccine trials:A systematic review. Indian J Clin Biochem. 2021;36:427–39. doi: 10.1007/s12291-021-00968-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klugar M, Riad A, Mekhemar M, Conrad J, Buchbender M, Howaldt HP, et al. Side effects of mRNA-based and viral vector-based COVID-19 vaccines among German healthcare workers. Biology (Basel) 2021;10:752. doi: 10.3390/biology10080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menni C, Klaser K, May A, Polidori L, Capdevila J, Louca P, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID symptom study app in the UK:A prospective observational study. Lancet Infect Dis. 2021;21:939–49. doi: 10.1016/S1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selected Adverse Events Reported after COVID-19 Vaccination. 2022. [[Last accessed on 2022 Oct 14]]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html .
- 19.Ministry Statistics. 2022. [[Last accessed on 2022 Nov 11]]. Available from: https://www.moh.gov.sa/en/pages/default.aspx .
- 20.Phillips N. The coronavirus is here to stay-here's what that means. Nature. 2021;590:382–4. doi: 10.1038/d41586-021-00396-2. [DOI] [PubMed] [Google Scholar]
- 21.WHO Tracking SARS-CoV-2 Variants. 2022. [[Last accessed on 2022 Dec 10]]. Available from: https://www.who.int/activities/tracking-sars-cov-2-variants .
- 22.Beccia F, Regazzi L, Marziali E, Beccia V, Pascucci D, Mores N, et al. BNT162b2 COVID-19 vaccine safety among healthcare workers of a tertiary hospital in Italy. Vaccines (Basel) 2023;11:477. doi: 10.3390/vaccines11020477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.(CDC) C for DC and P. Reactions and Adverse Events of the Pfizer-BioNTech COVID-19 Vaccine. 2022. [[Last acessed on 2022 Dec 10]]. Available from: https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html .
- 24.Edelman A, Boniface ER, Benhar E, Han L, Matteson KA, Favaro C, et al. Association between menstrual cycle length and coronavirus disease 2019 (COVID-19) vaccination:A U. S. Cohort. Obstet Gynecol. 2022;139:481–9. doi: 10.1097/AOG.0000000000004695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Male V. Menstrual changes after covid-19 vaccination. Br Med J. 2021;374:n2211. doi: 10.1136/bmj.n2211. [DOI] [PubMed] [Google Scholar]
- 26.Vijayasingham L, Heidari S, Munro J, Omer S, MacDonald N. Resolving sex and gender bias in COVID-19 vaccines R and D and beyond. Hum Vaccin Immunother. 2022;18:2035142. doi: 10.1080/21645515.2022.2035142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Govindapala D, Dhanaratna D, Senarath U, Lamabadusuriya D, Senaratne T, Wijenayake W, et al. Reactogenicity and persistence of IgG antibodies against SARS-CoV-2 among recipients of ChAdOx1 nCoV-19 vaccine:A single center experience from Sri Lanka. Int J Health Sci (Qassim) 2023;17:36–43. [PMC free article] [PubMed] [Google Scholar]
- 28.Hussain N, Agarwala P, Iqbal K, Omar HM, Jangid G, Patel V, et al. A systematic review of acute telogen effluvium, a harrowing post-COVID-19 manifestation. J Med Virol. 2022;94:1391–401. doi: 10.1002/jmv.27534. [DOI] [PubMed] [Google Scholar]
- 29.Gallo G, Mastorino L, Tonella L, Ribero S, Quaglino P. Alopecia areata after COVID-19 vaccination. Clin Exp Vaccine Res. 2022;11:129–32. doi: 10.7774/cevr.2022.11.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nassar RI, Alnatour D, Thiab S, Nassar A, El-Hajji F, Basheti IA. Short-term side effects of COVID-19 vaccines:A cross-sectional study in Jordan. Hum Vaccin Immunother. 2022;18:2082792. doi: 10.1080/21645515.2022.2082792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guezguez F, Romdhani M, Boutaleb-Joutei A, Chamari K, Ben Saad H. Management of long-COVID-19 patients with sleep disorders:Practical advice to general practitioners. Libyan J Med. 2023;18:2182704. doi: 10.1080/19932820.2023.2182704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sprent J, King C. COVID-19 vaccine side effects:The positives about feeling bad. Sci Immunol. 2021;6:eabj9256. doi: 10.1126/sciimmunol.abj9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borsini A, Pariante CM, Zunszain PA, Hepgul N, Russell A, Zajkowska Z, et al. The role of circulatory systemic environment in predicting interferon-alpha-induced depression:The neurogenic process as a potential mechanism. Brain Behav Immun. 2019;81:220–7. doi: 10.1016/j.bbi.2019.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Das BB, Kohli U, Ramachandran P, Nguyen HH, Greil G, Hussain T, et al. Myopericarditis after messenger RNA coronavirus disease 2019 vaccination in adolescents 12 to 18 years of age. J Pediatr. 2021;238:26–32. doi: 10.1016/j.jpeds.2021.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diaz GA, Parsons GT, Gering SK, Meier AR, Hutchinson IV, Robicsek A. Myocarditis and pericarditis after vaccination for COVID-19. JAMA. 2021;326:1210–2. doi: 10.1001/jama.2021.13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montgomery J, Ryan M, Engler R, Hoffman D, McClenathan B, Collins L, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. J Am Med Assoc Cardiol. 2021;6:1202–6. doi: 10.1001/jamacardio.2021.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simone A, Herald J, Chen A, Gulati N, Shen AY, Lewin B, et al. Acute myocarditis following COVID-19 mRNA vaccination in adults aged 18 years or older. J Am Med Assoc Intern Med. 2021;181:1668–70. doi: 10.1001/jamainternmed.2021.5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.(NCCIF) NC for C and IH Cupping. 2022. [[Last accessed on 2022 Dec 20]]. Available from: https://www.nccih.nih.gov/health/cupping .
- 39.Liu Y. Cupping therapy for 103 cases of high fever due to infection of the upper respiratory tract. J Tradit Chin Med. 2002;22:124–5. [PubMed] [Google Scholar]
- 40.Hong J, Fu M, Wang X, Gao Z. Effects of cupping therapy on the pulmonary functions in asthmatic children. J Tradit Chin Med. 2006;26:7. [PubMed] [Google Scholar]
- 41.Baghdadi H, Abdel-Aziz N, Ahmed NS, Mahmoud HS, Barghash A, Nasrat A, et al. Ameliorating role exerted by al-hijamah in autoimmune diseases:Effect on serum autoantibodies and inflammatory mediators. Int J Health Sci (Qassim) 2015;9:207–32. [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmed SM, Madbouly NH, Maklad SS, Abu-Shady EA. Immunomodulatory effects of blood letting cupping therapy in patients with rheumatoid arthritis. Egypt J Immunol. 2005;12:39–51. [PubMed] [Google Scholar]
- 43.Arslan M, Yeşilçam N, Aydin D, Yüksel R, Dane S. Wet cupping therapy restores sympathovagal imbalances in cardiac rhythm. J Altern Complement Med. 2014;20:318–21. doi: 10.1089/acm.2013.0291. [DOI] [PubMed] [Google Scholar]
- 44.Cheng SI. Medical acupuncture as a treatment for novel COVID-19-Related respiratory distress:Personal experience from a frontline anesthesiologist. Med Acupunct. 2021;33:83–5. doi: 10.1089/acu.2020.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martineau AR, Cantorna MT. Vitamin D for COVID-19:Where are we now? Nat Rev Immunol. 2022;22:529–30. doi: 10.1038/s41577-022-00765-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Inserra F, Tajer C, Antonietti L, Mariani J, Ferder L, Manucha W. Vitamin D supplementation:An alternative to enhance the effectiveness of vaccines against SARS-CoV-2? Vaccine. 2021;39:4930–1. doi: 10.1016/j.vaccine.2021.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Annweiler C, Hanotte B, Grandin de l'Eprevier C, Sabatier JM, Lafaie L, Célarier T. Vitamin D and survival in COVID-19 patients:A quasi-experimental study. J Steroid Biochem Mol Biol. 2020;204:105771. doi: 10.1016/j.jsbmb.2020.105771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jolliffe DA, Vivaldi G, Chambers ES, Cai W, Li W, Faustini SE, et al. Vitamin D supplementation does not influence SARS-CoV-2 vaccine efficacy or immunogenicity:Sub-studies nested within the CORONAVIT randomised controlled trial. Nutrients. 2022;14:3821. doi: 10.3390/nu14183821. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.


