Key Points
-
•
VWF O-glycans critically influence VWF biosynthesis and trafficking into WPBs in human ECs.
-
•
O-glycan inhibition leads to VWF A1 domain activation and formation of significantly smaller WPBs.
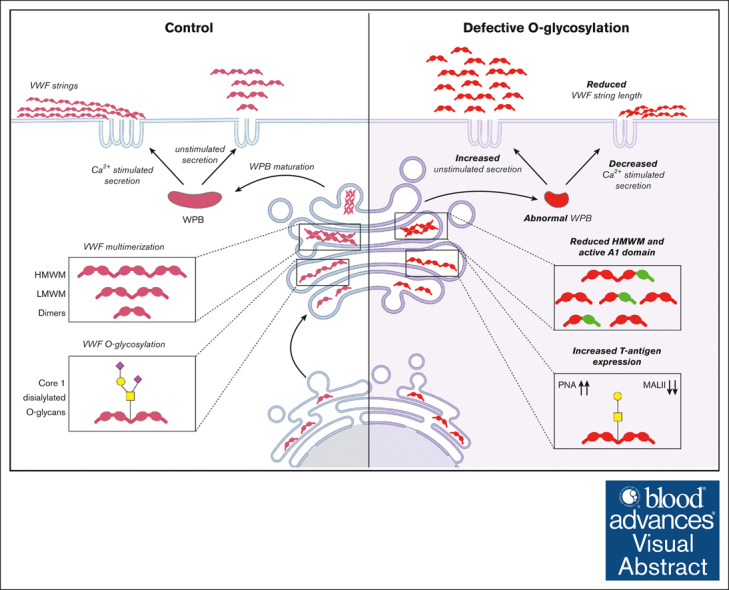
Visual Abstract
Abstract
von Willebrand factor (VWF) undergoes complex posttranslational modification within endothelial cells (ECs) before secretion. This includes significant N- and O-linked glycosylation. Previous studies have demonstrated that changes in N-linked glycan structures significantly influence VWF biosynthesis. In contrast, although abnormalities in VWF O-linked glycans (OLGs) have been associated with enhanced VWF clearance, their effect on VWF biosynthesis remains poorly explored. Herein, we report a novel role for OLG determinants in regulating VWF biosynthesis and trafficking within ECs. We demonstrate that alterations in OLGs (notably reduced terminal sialylation) lead to activation of the A1 domain of VWF within EC. In the presence of altered OLG, VWF multimerization is reduced and Weibel-Palade body (WPB) formation significantly impaired. Consistently, the amount of VWF secreted from WPB after EC activation was significantly reduced in the context of O-glycosylation inhibition. Finally, altered OLG on VWF not only reduced the amount of VWF secreted after EC activation but also affected its hemostatic efficacy. Notably, VWF secreted after WPB exocytosis consisted predominantly of low molecular weight multimers, and the length of tethered VWF string formation on the surface of activated ECs was significantly reduced. In conclusion, our data therefore support the hypothesis that alterations in O-glycosylation pathways directly affect VWF trafficking within human EC. These findings are interesting given that previous studies have reported altered OLG on plasma VWF (notably increased T-antigen expression) in patients with von Willebrand disease.
Introduction
von Willebrand factor (VWF) is a large multimeric plasma sialo-glycoprotein that plays critical roles in normal hemostasis.1 Under normal conditions, in vivo expression of VWF is limited to endothelial cells (ECs) and megakaryocytes.2,3 Before secretion, VWF undergoes complex posttranslational modification that includes significant glycosylation.3,4 N-linked glycosylation begins in the endoplasmic reticulum, through the addition of high-mannose oligosaccharide chains onto 12 sites in the VWF monomer.5 After transport to the Golgi apparatus, these initial N-linked glycan (NLG) structures undergo further modification, leading to the formation of complex branching carbohydrate chains.6 O-linked glycosylation of the VWF dimer also takes place in the Golgi, leading to the formation of 10 O-linked glycan (OLG) chains. O-glycosylation is initiated by the addition of GalNAc residues onto specific serine or threonine residues, leading to the formation of the precursor Tn antigen (GalNAcα1-Ser/Thr).7,8 This initial O-glycan is then extended by core 1 β3-galactosyltransferase 1 (C1GALT1), which catalyzes addition of galactose residues to convert Tn into T antigens (Gal1-3βGalNAcα1-Ser/Thr; Figure 1A).9 Sialylated core 1 T antigens constitute the predominant OLG on human VWF.10,11 Importantly, 8 of the OLG chains on VWF are present in 2 clusters located either side of the A1 domain (cluster 1 includes T1248, T1255, T1256, and S1263; and cluster 2 includes T1468, T1477, S1486, and T1487).12 Although most N- and O-glycans of VWF are capped by sialic acid,13 covalently linked ABO(H) blood group carbohydrate determinants are also present as terminal sugar residues on a proportion of both the N-linked (13%) and O-linked (1%) chains.14
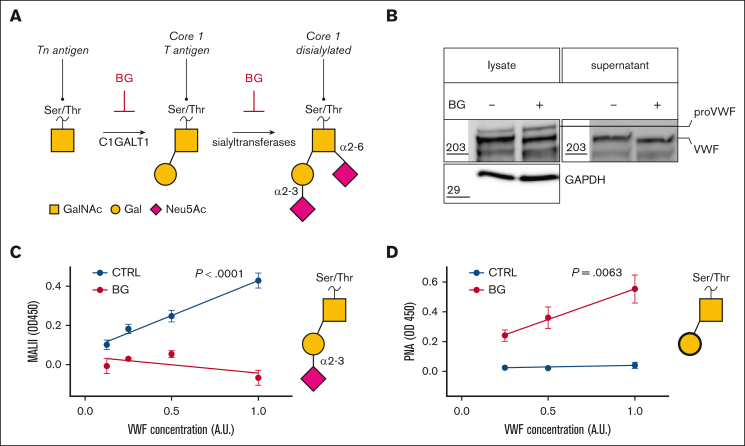
Figure 1.
BG treatment of HUVECs alters VWF O-linked glycosylation. (A) Inhibitory effect of BG on O-glycan biosynthetic processing; (B) VWF immunoblot in HUVEC cell lysates and conditioned media after BG treatment (GAPDH loading control; representative blot of n = 3). (C) M amurensis lectin II (MAL-II) binding to VWF secreted from BG-treated vs untreated HUVEC controls cells (CTRL) (mean and standard error of the mean [SEM]; n = 4; simple linear regression; P < .0001). (D) PNA lectin binding to VWF secreted from BG-treated vs untreated HUVEC CTRLs (mean and SEM; n = 4; simple linear regression; P = .0063). A.U., XXX; proVWF, XXX.
Previous studies have demonstrated that the NLG and OLG structures on VWF (notably terminal sialylation and ABO[H] blood group determinants) play important roles in regulating susceptibility to ADAMTS13 proteolysis15, 16, 17, 18 and in vivo clearance.19, 20, 21, 22, 23, 24 In addition, specific NLG structures on VWF have also been shown to influence VWF biosynthesis within ECs.25,26 For example, EC treatment with tunicamycin inhibited precursor NLG sugar chain attachment to VWF, which in turn led to impaired dimerization.25 Consequently, VWF secretion was ablated and intracellular VWF levels were significantly reduced.25 Similarly, EC incubation with castanospermine inhibited glucosidase activity in the ER and thus NLG chain development on VWF.26 Unlike tunicamycin, castanospermine treatment did not prevent dimerization but nonetheless significantly attenuated VWF secretion.26
There are conflicting data regarding whether O-glycan carbohydrates influence VWF biosynthesis.27, 28, 29 Using site-directed mutagenesis, Nowak et al reported no significant difference in secreted recombinant VWF (rVWF) levels for a range of O-glycan variants expressed in human embryonic kidney 293 (HEK293T) cells compared with wild type (VWF-WT).27 Importantly, however, previous studies have shown that rVWF expression in HEK293T cells does not lead to Weibel-Palade body (WPB) formation.30 Conversely, Badirou et al found that expression of murine rVWF missing all OLG was significantly reduced in COS-7 cells compared with VWF-WT.28 In keeping with the concept that OLG may affect VWF biosynthesis, a recent GWAS study implicated OLG processing genes in determining VWF biosynthesis.31 Furthermore, previous studies also demonstrated that OLG structures influence mucin biosynthesis and in particular intracellular trafficking.32 In this study, we therefore investigated the hypothesis that OLG may specifically affect VWF trafficking in ECs capable of producing WPBs.
Materials and methods
Cell culture
Human umbilical vein endothelial cells (HUVECs) were cultured in EC growth medium (PromoCell, Heidelberg, Germany) supplemented with 18% heat-inactivated fetal bovine serum (FBS) and 1% penicillin and streptomycin. All experiments were performed using HUVECs between passages 3 and 5. HEK293 cells were cultured in Dulbecco modified Eagle medium (Merck, Darmstadt, Germany) supplemented with 10% FBS and 1% penicillin and streptomycin. HEK293 cells were used before passage 20.
OLG inhibition with BG and ITZ
HUVECs were plated on gelatin-coated plates and allowed to reach confluency. Confluent monolayers were treated with 2 mM benzyl-N-acetyl-galactosaminide (GalNAc-O-benzyl; BG;Merck) or vehicle (dimethyl sulfoxide) for 72 hours. After 72 hours, HUVECs were either fixed, or the media was changed to allow for the collection of steady-state secreted VWF into the supernatant. To determine intracellular VWF levels, HUVECs were lysed in PBS containing 1% Triton in the presence of proteases inhibitors on ice. Similar to BG treatment, HUVEC monolayers were treated with 2 μM itraconazole (ITZ; Merck) for 72 hours. VWF:Ag and VWF collagen binding (VWF:CB) activity levels in cell supernatants and lysates were determined using ELISA, as previously described.33 VWF multimers were analyzed by agarose gel electrophoresis as before.34 Secreted angiopoietin-2 (Angpt-2) levels in HUVEC supernatant were determined by commercial ELISA (Bio-Techne, R&D systems) following the manufacturer’s instructions.35
Western blot analysis
BG-treated, ITZ-treated, or control cell lysate and supernatant samples were diluted with SDS sample buffer and DTT before boiling at 95°C for 10 minutes. Boiled samples were loaded in Bis-Tris 4% to 12% gels (Invitrogen, Thermo Fisher Scientific, Waltham, MA) and separated for 40 minutes. Proteins were transferred onto a nitrocellulose membrane and blocked with 5% BSA-PBS-Tween 0.1% for 1 hour. Membranes were then probed with primary anti-VWF (1:1000; DAKO Agilent Technologies, Santa Clara, CA), anti-GAPDH (1:2500; Abcam, Cambridge, United Kingdom), anti-C1GALT1 (1:1000; Santa Cruz Biotechnology, Dallas, TX), or anti α-tubulin (1:2500; Abcam) antibodies overnight at 4°C and subsequently incubated with HRP–conjugated secondary antibodies. Blots were visualized with enhanced chemiluminescence substrates in an Amersham Imager 680.
Lectin analysis of VWF OLG determinants
VWF glycans were assessed using specific lectin ELISAs as previously described.23 In brief, Polysorb flat bottom plates were coated using deglycosylated polyclonal anti-VWF (1:250; Dako, Agilent Technologies). Nonspecific binding was blocked with Protein-Free Blocking Buffer (Thermo Fisher Scientific, Abingdon, United Kingdom). Conditioned media from control or BG-treated HUVEC were diluted in PBS and loaded onto the plate and incubated for 2 hours at 37°C. Biotinylated lectins Peanut agglutinin (PNA; 1 μg/mL) or Maackia amurensis lectin II (2.5 μg/mL; both Vector Laboratories) were diluted in PBS-T and incubated for 1 hour at 37°C. Lectin binding was detected with high sensitivity streptavidin–horseradish peroxidase (Pierce, Thermo Fisher Scientific) and subsequent incubation with substrate 3,3’,5,5’-Tetramethylbenzidine (R&D Systems). The reaction was subsequently stopped with 1M H2SO4. Absorbance was read at 450 nm using a SpectraMax Reader. All ELISAs were repeated 3 times, and dilutions were measured per duplicate.
Immunofluorescence microscopy
Paraformaldehyde-fixed glass coverslips of control and BG-treated cells were permeabilized with 0.1% Triton X-100 (in PBS containing 0.05% sodium azide [NaN3] and 0.01% BSA) for 15 minutes and blocked with 3% BSA in PBS for 45 minutes. Coverslips were then incubated in a humidifying chamber with primary antibodies for 1 hour at room temperature. Subsequently, the coverslips were incubated in a humidifying chamber with fluorescently labeled secondary antibodies (1:1000; Invitrogen, Thermo Fisher Scientific) for 1 hour at room temperature in the dark. Coverslips were mounted onto glass slides, and images were acquired using a Leica Stellaris 8 STED 3D/Falcon/confocal system (Leica microsystems, Ashbourne, Ireland) with Leica HC PL APO CS2 100×/1.40 oil immersion objective. WPB morphology (>8000 per treatment) in ECs were assessed with an automated script in ImageJ/Fiji, in which the WPB length was measured by Feret diameter (the longest distance between any 2 points in the selection boundary), and circularity was assessed by the following formula: . Nanobodies directed against the activated VWF-A1 domain and the VWF-A3 domain were kindly provided by P. J. Lenting (Paris, France). Nanobodies were conjugated to Alexa-488 (nano-active A1) and Alexa-647 (nano-A3) and used as previously described.36
Transient expression of VWF OLG variants in HEK293 cells
The expression vector pcDNA-VWF encoding recombinant full-length human VWF has previously been described.27 Additional rVWF variants were prepared by site-directed alanine mutagenesis of individual serine and threonine residues. These included VWF-ΔC1 in which the N-terminal cluster 1 OLGs were removed (containing T1248A, T1255A, T1256A, and S1263A) and VWF-ΔC2 in which the C-terminal cluster 2 OLGs (containing T1468A, T1477A, S1486A, and T1487A) flanking the VWF-A1 domain were removed. Finally, a VWF-ΔC1+ΔC1 variant missing both OLG clusters (containing T1248A, T1255A, T1256A, S1263A, T1468A, T1477A, S1486A, T1487A) was also generated, as previously described in detail.27 These variants were kindly provided by T. A. McKinnon (Imperial College London, London, United Kingdom). All rVWF variants were transiently expressed in HEK293 cells. In brief, HEK293 were grown to confluence and then transfected using TurboFect (Thermo Fisher). 24 hours later, the media was changed back to DMEM containing 10% FBS, and cells were allowed to express the rVWF variants for a further 48 hours before pseudo-WPB formation was assessed.
Histamine-induced VWF release and string formation under shear
BG-treated or control HUVEC were washed and starved for 15 minutes in release medium (M199 containing 0.2% BSA) prior to stimulation assay. After washing, cells were incubated in release medium supplemented with histamine (100 μM final concentration; Merck) for 30 minutes. VWF:Ag and VWF:CB activities in the supernatant were then assessed by ELISA, as detailed above.
To assess VWF string formation, BG-treated and control HUVEC were plated on gelatin-coated 6 channel μ-slide (ibidi). After 24 hours, the HUVEC were activated under flow (1.5 mL/min) with histamine (100 μM). Tethered VWF strings on the HUVEC cell surface were visualized by flowing over washed platelets in brightfield (Zeiss Axiovert 200) at 37°C. Whole blood samples were collected from healthy consenting volunteers in accordance with RCSI research ethics (REC1391 and REC1504) and the Declaration of Helsinki. Washed platelets were obtained from whole blood of healthy blood donors, as previously described.37 Alternatively, tethered VWF strings were also visualized using fluorescently labeled anti-VWF antibodies (Zenon labeling kit; Thermo Fisher) in the green channel using a Leica Stellaris 8 STED 3D/Falcon/confocal system (Leica microsystems) and Okolab incubation system with temperature (37°C) and CO2 (5%) control.
Data presentation and statistical analysis
All images were analyzed using ImageJ/Fiji. GraphPad Prism 10.0 was used to plot and analyze data. Data are presented as mean and standard deviation unless stated otherwise. Data that are normally distributed are tested for significance by a Student t test or 1-way analysis of variance for ≥3 conditions. Data that are not normally distributed are tested for significance by a Mann-Whitney t test (or Kruskal-Wallis for ≥3 conditions). A P value <.05 was considered significant.
Results
BG treatment of HUVECs alters VWF O-linked glycosylation
BG has been shown to inhibit key steps in OLG development (Figure 1A).38,39 To investigate whether OLG influences VWF biosynthesis in human ECs, HUVECs were treated with BG. Consistent with reduced O-linked glycosylation, a reduction in molecular weight was observed for VWF in cell lysates and for VWF secreted into the supernatant from BG-treated HUVEC compared with untreated controls (Figure 1B). Since previous studies demonstrated that BG treatment resulted in reduced sialylation of mucin OLG,38 the effects of BG on VWF OLG chains were assessed using lectin ELISAs. In keeping with the mucin studies, treatment of HUVEC with BG resulted in significantly (P < .001) reduced M amurensis lectin II binding to secreted VWF, consistent with a reduction in terminal α2-3 sialylation (Figure 1C). Furthermore, BG treatment was also associated with a significant (P = .0063) increase in binding of PNA lectin (which binds to nonsialylated core 1 glycans) to secreted VWF compared with untreated controls (Figure 1D). Together, these data demonstrate that BG treatment of ECs directly affects VWF O-glycosylation, resulting in a partial reduction in terminal α2-3 linked sialylation and a consequent increase in T-antigen expression.
O-linked sialylation influences VWF secretion and alters WPB morphology
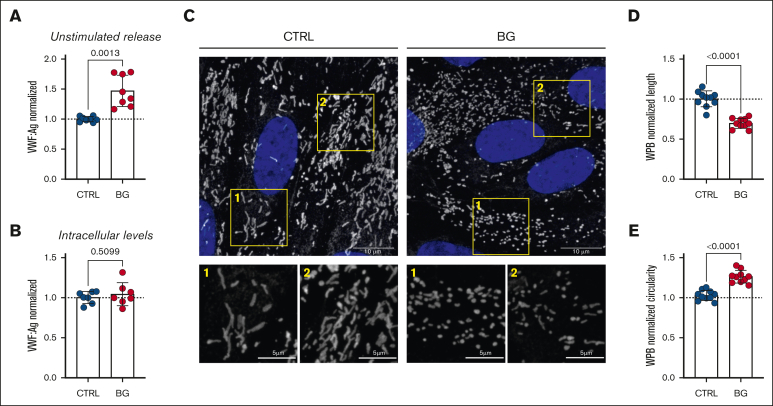
Previous studies investigating a putative role for OLGs in regulating VWF biosynthesis were performed in cells that did not form WPB storage organelles.27,28 Consequently, we next investigated whether alterations in O-linked sialylation influenced VWF trafficking in WPB-containing human ECs. Importantly, incubation with BG was associated with a significant increase (P = .0013) in unstimulated VWF secretion from HUVECs (Figure 2A). In contrast, no effect on steady-state intracellular VWF concentration in HUVEC lysates was observed (Figure 2B). To further investigate the mechanisms through which OLGs influence VWF synthesis and trafficking within human ECs, immunofluorescence microscopy was used to assess WPB morphology. In contrast to normal HUVECs, WPB morphology was markedly altered in HUVECs incubated with BG compared with controls (Figure 2C). In particular, inhibition of O-linked sialylation resulted in the formation of WPB that were significantly shorter (P < .0001; Figure 2D) and rounder (P < .0001; Figure 2E) than the elongated cigar-shaped WPBs seen in control HUVECs. Collectively, these results indicate that alterations in O-linked glycosylation significantly affect WPB formation and VWF secretion in ECs.
Figure 2.
O-linked sialylation influences VWF secretion and alters WPB morphology. (A) Unstimulated VWF:Ag secretion levels from HUVECs incubated with or without BG (2 mM for 72 hours; n = 8 from 4 independent experiments; Welch t test; P = .0013). (B) VWF:Ag levels in unstimulated HUVEC cell lysates after treatment with or without BG (n = 8, from 4 independent experiments; t test; P = .5099). (C) Immunofluorescent images of HUVEC treated with BG (2 mM for 72 hours) compared with untreated CTRLs (VWF in gray; DAPI [4′,6-diamidino-2-phenylindole] in blue; representative images of 3 independent experiments); scale bars are set at 10 μm for overview images and at 5 μm for the zoomed regions; (D) Automated assessment of WPB length; results are depicted as normalized to CTRL (n = 10 images, from 3 independent experiments; t test; P < .0001). (E) Automated assessment of WPB circularity (data are shown as normalized to CTRL) in CTRL and BG-treated cells (n = 10 images, from 3 independent experiments; t test; P < .0001).
C1GALT1 influences VWF OLG determinants and WPB morphology
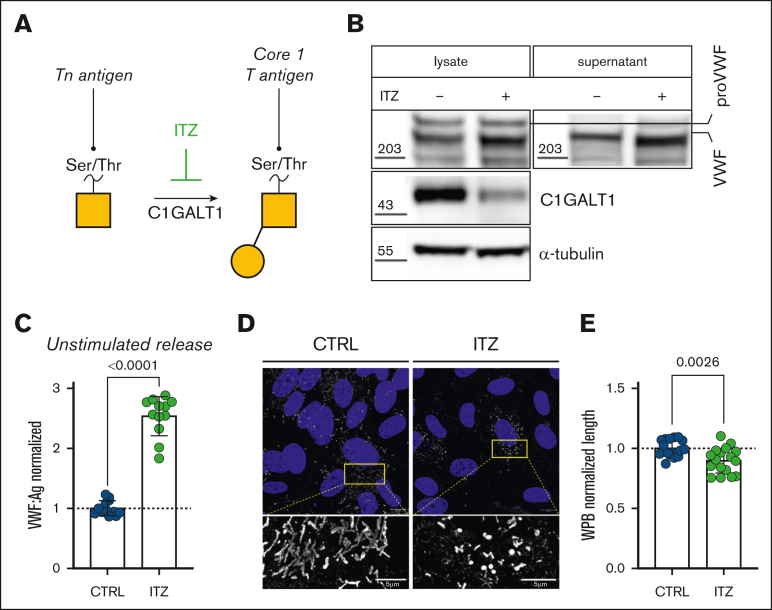
C1GALT1 plays a key role in T-antigen synthesis by catalyzing the transfer of galactose residues onto precursor Tn antigens (Figure 3A).40,41 ITZ has been shown to inhibit T-antigen formation in cancer cell lines by targeting C1GALT1 for proteasomal degradation.40,41 To further evaluate the effects of OLGs in regulating VWF biosynthesis, HUVECs were next treated with ITZ. Consistent with the notion that ITZ reduces C1GALT1 in other cell types,41 we observed markedly reduced C1GALT1 expression in ITZ-treated HUVECs compared with untreated controls (Figure 3B). Similar to BG, a reduction in molecular weight was also observed for (1) VWF in cell lysates and (2) VWF secreted into the supernatant from ITZ-treated HUVECs compared with untreated controls (Figure 3C). In agreement with our BG observations, ITZ treatment was associated with a significant increase (P < .0001) in unstimulated basal VWF secretion from HUVECs (Figure 3D). Moreover, immunofluorescence microscopy confirmed that WPB morphology was markedly altered in HUVEC incubated with ITZ compared with controls (Figure 3E), with WPB significantly reduced in length (P < .0026) compared with WPB in untreated control HUVECs (Figure 3F). Interestingly, ITZ treatment had a more marked effect on unstimulated VWF secretion but a less significant effect on WPB formation compared with BG treatment.
Figure 3.
C1GALT1 influences VWF OLG determinants and WPB morphology. (A) Inhibitory effect of ITZ on O-glycan biosynthetic processing. (B) C1GALT1 immunoblot in HUVEC cell lysates after ITZ treatment (α-tubulin loading control; representative blot, n = 3). VWF immunoblot in HUVEC cell lysates and conditioned media after ITZ treatment (GAPDH loading control; representative blot, n = 3). (C) Unstimulated VWF:Ag secretion levels from HUVECs incubated with or without ITZ (2 μM for 48-72 hours; n = 12, from 4 independent experiments; Mann-Whitney test; P < .0001). (D) Immunofluorescent images of HUVECs treated with ITZ (2 μM for 48-72 hours) compared with untreated CTRLs (VWF in gray; DAPI in blue; representative images of 3 independent experiments). Scale bars are set at 10 μm for overview images and at 5 μm for the zoomed regions. (E) Automated assessment of WPB length (data are shown as normalized to CTRL) in CTRL and ITZ-treated cells (n = 10 images, from 3 independent experiments; t test; P = .0026).
Altogether, these observations further support the hypothesis that OLG truncations significantly influence WPB length and VWF secretion.
Effects of VWF OLG truncation on Golgi morphology and WPB cargo storage
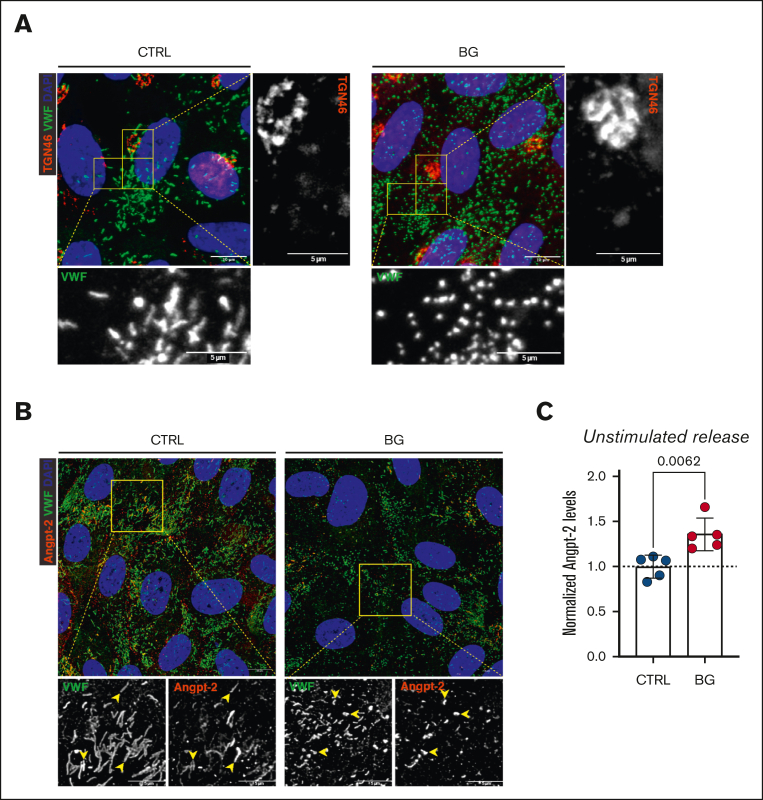
Previous studies have shown that the length of WPB is determined before they bud off from the trans-Golgi network.42,43 In addition, Ferraro et al demonstrated that normal Golgi ribbon architecture was needed for the packing of VWF quanta into nascent WPBs.42 Consequently, unlinking of the Golgi into ministacks led to the formation of short WPBs.42, 43, 44 To investigate whether OLG truncation induced unlinking of the Golgi, we next performed immunofluorescent costaining of trans-Golgi network and VWF in control and BG-treated cells. No significant alteration in Golgi morphology was observed in control and BG-treated cells (Figure 4A). In BG-treated cells that were producing short WPBs, the Golgi appeared continuous, indicating that the BG treatment does not affect the Golgi homeostasis. Angpt-2 is another EC glycoprotein stored within WPB and secreted after EC activation.45,46 Despite the small and stubby WPB formed in BG-treated HUVECs, we observed that Angpt-2, which does not have any known OLG sites, was still recruited into WPB in these cells (Figure 4B). Importantly, however, consistent with enhanced unstimulated VWF secretion levels, a significant (P = .0062) increase in basal Angpt-2 secretion was also seen after HUVEC incubation with BG (Figure 4C). These data suggest that alterations in VWF OLGs leading to alterations in WPB morphology may have additional biological effects by affecting secretion of other WPB cargo components.
Figure 4.
Effects of VWF OLG truncation on Golgi morphology and WPB cargo storage. (A) Immunofluorescent images of VWF (green), TGN46 (red), and DAPI (blue) of BG-treated HUVECs (2 mM for 72 hours) compared with untreated CTRLs (representative images of 2 independent experiments). (B) Immunofluorescence images of VWF (green), Angpt-2 (red), and DAPI (blue) of BG-treated HUVECs compared with untreated CTRL cells (yellow arrowheads indicate WPBs positive for Angpt-2; representative images of n = 5). (C) Angpt-2 levels secreted into conditioned media from BG-treated HUVECs compared with untreated CTRLs (n = 5; t test; P = .0062).
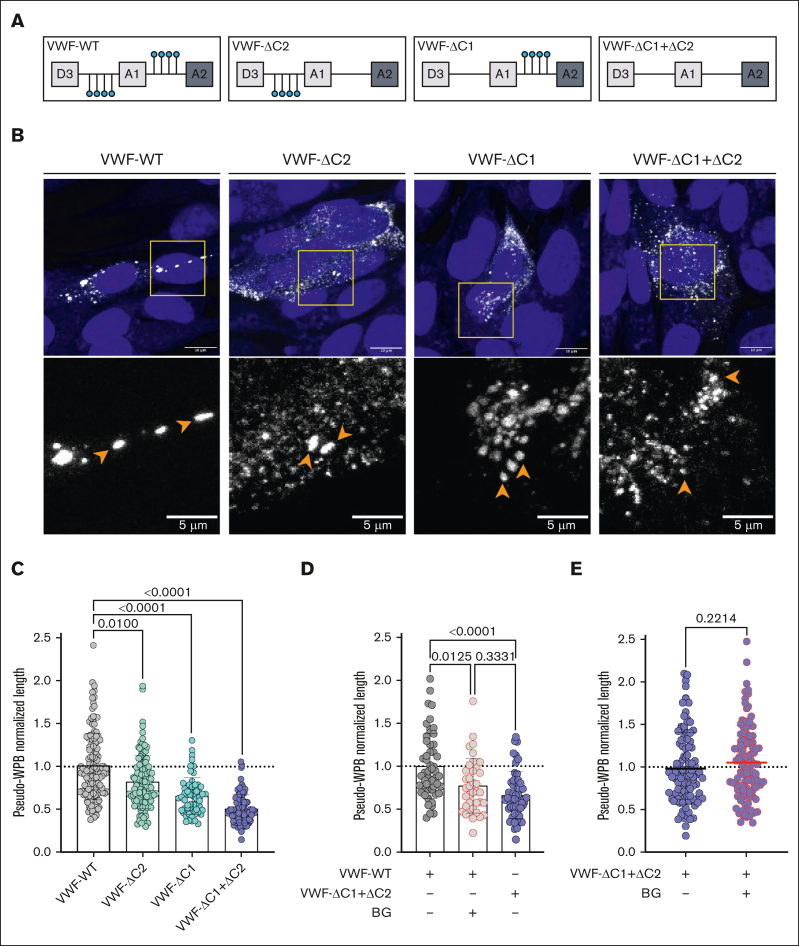
O-glycan clusters in VWF affect pseudo-WPB morphology
To confirm that the effects of BG and ITZ were modulated by changes in VWF O-glycan structures, a series of rVWF OLG mutants were examined. These included rVWF in which the N-terminal (VWF-ΔC1) and C-terminal (VWF-ΔC2) OLG clusters at either side of the VWF A1 domain were removed using alanine mutagenesis of serine and threonine residues, as previously described (Figure 5A).27 In addition, a rVWF variant lacking both OLG clusters (VWFΔC1+ΔC2)27 was also studied. Nowak et al previously reported that expression and secretion of these variants was not significantly different to VWF-WT.27 In contrast to previous studies,27,28 HEK293 cells were used for these expression studies as these have been shown to produce pseudo-WPB after rVWF expression.30 Consistent with our OLG inhibitor data, expression of VWF-ΔC1, VWF-ΔC2, and VWF-ΔC1+ΔC2 in HEK293 cells all resulted in the formation of short and stubby WPBs (Figure 5B-C). Interestingly, the magnitude of this effect on WPB morphology was most marked for VWF-ΔC1+ΔC2, in which both OLG clusters either side of the A1 domain were absent (Figure 5B-C). In addition, loss of the N-terminal OLG cluster (VWF-ΔC1) had a significantly greater effect on pseudo-WPB morphology than the removal of the C-terminal OLG cluster (VWF-ΔC2; P = .014; Figure 5C).
Figure 5.
O-glycan clusters in VWF affect pseudo-WPB morphology. (A) Schematic representation illustrating OLG clustered around the VWF-A1 domain and the recombinant OLG mutants generated. (B) Immunofluorescent images of HEK293 cells expressing VWF-WT, VWF-ΔC2, VWF-ΔC1, and VWF-ΔC1+ΔC2. Orange arrowheads in zoomed images point to pseudo-WPBs (representative images of n = 3). Scale bars are set at 10 μm for overview images and at 5 μm for the zoomed regions. (C) Pseudo-WPB length in VWF-WT, VWF-ΔC2, VWF-ΔC1, and VWF-ΔC1+ΔC2 expressing HEK293 cells (n = 3; Kruskal-Wallis test; P = .01; P < .0001; and P < .0001). (D) Pseudo-WPB length in VWF-WT, VWF-WT treated with BG, and VWF-ΔC1+ΔC2 expressing HEK293 cells normalized to VWF-WT (n = 2 independent experiments; Kruskal-Wallis test with multiple comparisons; P = .0125; P < .0001; P = .3331). (E) Pseudo-WPB length in VWF-ΔC1+ΔC2 and VWF-ΔC1+ΔC2 treated with BG expressing HEK293 cells normalized to VWF-ΔC1+ΔC2 (n = 3 independent experiments; Mann-Whitney test; P = .0125; P < .0001; P = .3331).
In keeping with the HUVEC data, BG treatment of HEK293 transfected with VWF-WT resulted in pseudo-WPB that were significantly (P = .0125) reduced in length compared with untreated controls (Figure 5D). Furthermore, this BG-induced reduction in pseudo-WPB size was similar to VWF-ΔC1+ΔC2 expressed in HEK293 cells. Finally, BG treatment of HEK293 cells producing VWF-ΔC1+ΔC2 had no additional effect in reducing WPB size (Figure 5E). Together, our findings demonstrate, to our knowledge, for the first time that OLG determinants on VWF play a critical role in regulating WPB size.
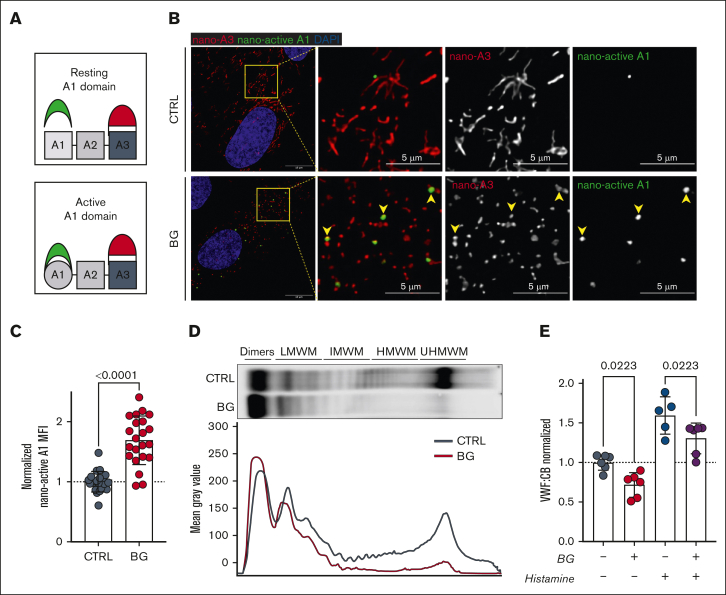
OLG inhibition leads to VWF-A1 domain activation and reduced HMWMs
Loss of O-linked sialylation has recently been reported to result in A1 domain activation mediated via destabilization of the flanking autoinhibitory module (AIM).47 Consequently, we next studied the effect of BG-induced OLG inhibition on A1 domain activation using a specific nanobody toward activated A1 (Figure 6A).48 Interestingly, intracellular A1 domain activation was significantly (P < .0001) increased in BG-treated cells compared with controls (Figure 6B-C). Recent studies have demonstrated that shorter WPB are associated with reductions in high molecular weight multimers (HMWMs) and attenuated hemostatic capacity of secreted VWF to capture platelets on the surface of activated ECs.42,43 Consistently, we observed reduced HMWMs in conditioned media from BG-treated HUVECs compared with untreated controls (Figure 6D). Moreover, VWF:CB was also significantly reduced (P = .0223) for VWF secreted from BG-treated cells under steady-state conditions or in response to histamine activation (Figure 6E).
Figure 6.
OLG inhibition leads to VWF-A1 domain activation and reduced HMWMs. (A) Figure illustrates use of nanobodies to detect activated VWF-A1 domain (nano-active A1 in green) vs normal VWF-A3 domain (nano-A3 in red). (B) Immunofluorescence images of nanobody interactions with BG-treated HUVECs compared with untreated CTRLs (VWF-A3 in red; active-A1 in green and DAPI in blue; representative images of n = 3). Yellow arrowheads point to WPBs positive for both inactive and active VWF. Scale bars are set at 10 μm for overview images and at 5 μm for the zoomed regions; (C) Mean fluorescence intensity (MFI) for nano-active A1 binding in BG-treated HUVECs vs untreated controls (n = 22, from 4 independent experiments; Welch t test; P < .0001). (D) VWF multimer blot and densitometry of conditioned media from BG-treated HUVECs compared with untreated CTRLs (representative images of 2 independent experiments). (E) VWF:CB for VWF secreted from HUVECs incubated with or without BG (1) under steady-state conditions and (2) after histamine stimulation (n = 6 from 3 independent experiments; 1-way analysis of variance [ANOVA] with multiple comparisons; CTRL vs BG, P = .0223; CTRL his vs BG his, P = .0223). his, histamine; IHWM, XXX; LHWM, XXX; UHWM, XXX.
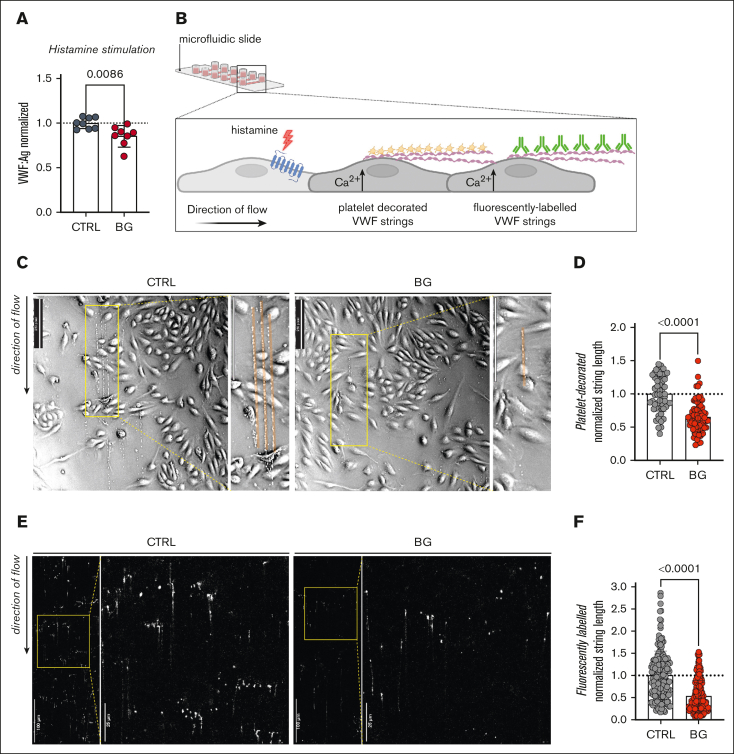
In keeping with the reduction in WPB size, BG treatment of HUVECs was also associated with a significant reduction (P = .0002) in total VWF:Ag secreted after histamine stimulation. (Figure 7A). To further elucidate the effects of OLG inhibition in human ECs on VWF biology, we next investigated the formation of tethered VWF strings and platelet-decorated VWF strings on the surface of histamine-activated HUVECs under shear conditions (Figure 7B). Consistent with the reduction in WPB length, platelet-decorated VWF strings on the surface activated HUVECs were significantly (P < .0001) shorter after BG treatment (Figure 7C-D). Furthermore, we observed that fluorescently labeled VWF strings from BG-treated HUVECs after histamine stimulation were also significantly reduced in length (Figure 7E-F). Collectively, these findings confirm that OLG truncation significantly influences VWF activation, multimerization, and the ability of VWF to capture platelets on the surface of activated ECs.
Figure 7.
Truncation of OLG causes reduces VWF strings on activated ECs. (A) VWF:Ag secretion after histamine (100 μM) stimulation from HUVECs incubated with or without BG (n = 8, from 4 independent experiments; t test; P = .0086). (B) Histamine activation of HUVECs under shear results in the production of tethered VWF strings on the EC surface, which can be detected using platelets or fluorescent anti-VWF antibodies. (C) Platelet-decorated VWF string visualized in brightfield after histamine stimulation of BG-treated HUVECs compared with untreated CTRLs (representative images of 2 independent experiments; direction of the flow from top to bottom; flow rate, 1.5 mL/min [2 dyn/cm2]; scale bars, 250 pixels). (D) Platelet-decorated VWF string length for HUVECs treated with BG compared with untreated CTRLs (Mann-Whitney test; P < .0001). (E) Fluorescently labeled VWF strings after histamine stimulation of BG-treated HUVEC compared with untreated CTRLs (representative images of 2 independent experiments; direction of the flow from top to bottom; flow rate, 1.5 mL/min [2 dyn/cm2]). (F) Fluorescently labeled VWF string length for HUVECs treated with BG compared with untreated CTRLs (Mann-Whitney test; P < .0001).
Discussion
O-glycan determinants have been implicated in regulating several key aspects of VWF biology.12,13 For example, previous studies performed after tail vein injection in VWF−/− mice and rats consistently reported that loss of OLG was associated with a significant reduction in VWF plasma half-life.20,28 Subsequent studies have confirmed that sialylation on OLG plays a critical role in protecting VWF against rapid clearance mediated via the macrophage galactose lectin.49,50 Conversely, previous studies investigating whether OLG influences VWF biosynthesis reached differing conclusions.27, 28, 29 Nowak et al performed alanine mutagenesis to remove OLG sites in human VWF individually or in combination and then studied rVWF expression in HEK293T cells.27 In this model, no significant OLG effects on rVWF synthesis or secretion were observed. Conversely, Badirou et al investigated the significance of OLGs by performing mutagenesis to remove OLG sites in murine rVWF and then assessed expression by either hydrodynamic expression in VWF−/− mice28,51 or after transient transfection of Cos-7 cells.28 In contrast to Nowak et al, they reported that loss of OLGs was associated with a significant reduction in rVWF expression.27 These differences likely relate in large part to the fact that the studies involved human and murine rVWF variants expressed in different cell lines (HEK293T cells, murine primary hepatocytes, and Cos-7 cells).
In this study, we adopted a different strategy to investigate a putative role for OLG in regulating VWF biosynthesis using O-glycosylation inhibitors in human ECs (HUVEC). This approach is similar to the original studies that showed that NLG sugars influence VWF trafficking and secretion.26,52 Our HUVEC findings demonstrate that OLG truncation on VWF leads to a significant reduction in WPB length, an increase in VWF steady-state secretion, and a reduction in VWF exocytosis after EC activation. Consistent with these HUVEC data, expression of rVWF variants lacking OLG clusters flanking the VWF-A1 domain also resulted in the formation of significantly shortened pseudo-WPB in HEK293 cells compared with those seen after VWF-WT expression. Together, these data suggest that the OLG structures, particularly the cluster located on the N-terminal side of the VWF-A1 domain, directly influence WPB formation. Recent studies have demonstrated that WPB length can be similarly reduced by a variety of agents (including statins and nocodazole) that lead to unlinking of the Golgi ribbon into ministack.42, 43, 44 However, we observed no significant change in Golgi in HUVECs after OLG truncation. Consequently, it seems likely that OLG truncation may instead affect the availability of VWF quanta for packing into nascent WPB.
The mechanism(s) through which OLG truncation influences VWF secretion and WPB morphology remain to be fully elucidated. However, previous studies have shown that the N- and C-terminal flanking regions of the A1 domain cooperatively form an AIM that reduces accessibility to the GPIbα-binding site located within the VWF-A1 domain.53, 54, 55 Notably, OLG structures are present in both the N-AIM and C-AIM regions and are largely conserved in mammals.47 Interestingly, Voos et al recently reported that desialylation of OLG chains located within the AIM regions leads to destabilization of the autoinhibitory effect and VWF-A1 domain activation.47 In keeping with the hypothesis that OLGs influence AIM, we further demonstrate that BG-induced loss of O-linked sialylation also results in VWF-A1 activation in human ECs. Thus, in contrast to the previous mutagenesis studies, which involved loss of complete OLG chains,27,28 our findings highlight that relatively modest changes in OLG structures, such as reductions in terminal α2-3 sialylation, have the potential to significantly influence multiple aspects of VWF biosynthesis and secretion.
Previous OLG mutagenesis studies did not observe any effect on VWF multimerization for rVWF variants compared with that of the VWF-WT expressed in the same cells.27, 28, 29 However, those VWF expression studies were performed in murine hepatocytes and HEK293T cells in which the expression of HMWM was limited. Conversely, after BG treatment of HUVECs, we observed a significant decrease in HMWMs and a consistent reduction in VWF:CB activity. These findings are interesting given that very recent electron cryo-microscopy studies have reported a novel direct role for the VWF-A1 domain in directing VWF tubule formation and concatamerization.56,57 In addition, we observed that OLG truncation was associated with a significant reduction in the length of tethered platelet-decorated VWF strings on the surface of activated ECs under flow. This finding is consistent with previous reports showing that unlinking of the Golgi ribbon with simvastatin or nocodazole treatment not only led to a reduction in WPB size but also to the formation of significantly shorter VWF strings.42, 43, 44 Together, these findings raise the intriguing possibility that therapeutic modulation of WPB organelle size might offer a novel approach to antithrombotic therapy.44
From a clinical perspective, it is important to highlight that reduced VWF biosynthesis and secretion have been shown to constitute the most common pathogenic mechanism in patients with type 1 VWD (plasma VWF:Ag levels <30 IU/dL) and Low VWF (plasma VWF levels 30-50 IU/dL), respectively.58, 59, 60, 61 Furthermore, van Schooten et al demonstrated that binding of the lectin PNA to plasma VWF was significantly enhanced in a significant subgroup of patients with type 1 VWD compared with healthy controls.62 These lectin data suggest that O-linked T-antigen expression on plasma VWF is significantly increased in these patients. Our data show that increased T-antigen expression on VWF can influence multiple aspects of EC biosynthesis and secretion. Importantly, the RIIIS/J mouse provides a naturally occurring proof-of-principle that glycosylation abnormalities targeted specifically to ECs can directly cause VWD without necessarily leading to other multisystem pleiotropic effects.63 This inbred strain of mice has significantly reduced plasma VWF levels compared with other murine strains and consequently displays a bleeding phenotype. Previous studies have demonstrated that reduced plasma VWF levels in RIIIS/J mice are due to aberrant expression of an N-acetylglactosaminyltransferase in ECs, which results in altered VWF glycosylation and consequently pathological enhanced VWF clearance.63 Critically however, RIIIS/J mice with this EC–specific glycosylation abnormality do not have multisystem abnormalities such as those typically observed in patients with congenital disorders of glycosylation.64,65 Further studies will be required to elucidate the biological mechanism(s) underpinning the increase in O-linked T-antigen expression in patients with type 1 VWD. These studies will be complicated by the fact that there is significant interindividual heterogeneity in VWF glycosylation in both normal people and in patients with VWD.23 Furthermore, alterations in VWF sialylation may occur during EC biosynthesis or after digestion by circulating neuraminidases present in the plasma.23,66 Nevertheless, we hypothesize that alterations in O-linked glycosylation or sialylation machinery within ECs could contribute to the pathogenesis of VWD, particularly in families in whom the disease is not linked to the VWF locus on chromosome 12.
Conflict-of-interest disclosure: J.S.O. has served on the speaker’s bureau for Baxter, Bayer, Novo Nordisk, Sobi, Boehringer Ingelheim, Leo Pharma, Takeda, and Octapharma; served on the advisory boards of Baxter, Sobi, Bayer, Octapharma CSL Behring, Daiichi Sankyo, Boehringer Ingelheim, Takeda, and Pfizer; and received research grant funding awards from 3M, Baxter, Bayer, Pfizer, Shire, Takeda, and Novo Nordisk. The remaining authors declare no competing financial interests.
Acknowledgments
J.S.O. is supported by funds from the National Institutes of Health for the Zimmerman Program (HL081588) and a Science Foundation Ireland Principal Frontiers for the Future award (20/FFP-A/8952). The authors acknowledge RCSI’s Super-Resolution Image Consortium facility funded by Science Foundation Ireland (18/RI/5723).
Authorship
Contribution: E.K. and J.S.O. conceived the study and contributed to data collection and interpretation; D.D., P.E.B., M.G., and S.E assisted with data collection; I.S. assisted with image analysis; E.K., D.D., P.E.B., M.G., S.E., R.B., and J.S.O. contributed to the literature review, final draft writing, and critical revision and approved the final version of the manuscript; and all the authors have participated sufficiently in this work, taken public responsibility for the content, and made substantial contributions to this research.
Footnotes
Presented in the abstract form as oral presentations at the 30th International Society on Thrombosis and Haemostasis Congress, London, United Kingdom, July 2022 and the 31st International Society on Thrombosis and Haemostasis Congress, Montreal, Canada, June 2023.
Data are available for sharing on request from the corresponding author, James S. O’Donnell (jamesodonnell@rcsi.ie).
References
- 1.Leebeek FW, Eikenboom JC. von Willebrand's disease. N Engl J Med. 2016;375(21):2067–2080. doi: 10.1056/NEJMra1601561. [DOI] [PubMed] [Google Scholar]
- 2.Wagner DD. Cell biology of von Willebrand factor. Annu Rev Cell Biol. 1990;6:217–246. doi: 10.1146/annurev.cb.06.110190.001245. [DOI] [PubMed] [Google Scholar]
- 3.Lenting PJ, Christophe OD, Denis CV. von Willebrand factor biosynthesis, secretion, and clearance: connecting the far ends. Blood. 2015;125(13):2019–2028. doi: 10.1182/blood-2014-06-528406. [DOI] [PubMed] [Google Scholar]
- 4.Preston RJ, Rawley O, Gleeson EM, O'Donnell JS. Elucidating the role of carbohydrate determinants in regulating hemostasis: insights and opportunities. Blood. 2013;121(19):3801–3810. doi: 10.1182/blood-2012-10-415000. [DOI] [PubMed] [Google Scholar]
- 5.Titani K, Kumar S, Takio K, et al. Amino acid sequence of human von Willebrand factor. Biochemistry. 1986;25(11):3171–3184. doi: 10.1021/bi00359a015. [DOI] [PubMed] [Google Scholar]
- 6.Canis K, McKinnon TA, Nowak A, et al. Mapping the N-glycome of human von Willebrand factor. Biochem J. 2012;447(2):217–228. doi: 10.1042/BJ20120810. [DOI] [PubMed] [Google Scholar]
- 7.Brockhausen I, Schutzbach J, Kuhns W. Glycoproteins and their relationship to human disease. Acta Anat. 1998;161(1-4):36–78. doi: 10.1159/000046450. [DOI] [PubMed] [Google Scholar]
- 8.Ju T, Otto VI, Cummings RD. The Tn antigen-structural simplicity and biological complexity. Angew Chem Int Ed Engl. 2011;50(8):1770–1791. doi: 10.1002/anie.201002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanisch FG. O-glycosylation of the mucin type. Biol Chem. 2001;382(2):143–149. doi: 10.1515/BC.2001.022. [DOI] [PubMed] [Google Scholar]
- 10.Canis K, McKinnon TA, Nowak A, et al. The plasma von Willebrand factor O-glycome comprises a surprising variety of structures including ABH antigens and disialosyl motifs. J Thromb Haemost. 2010;8(1):137–145. doi: 10.1111/j.1538-7836.2009.03665.x. [DOI] [PubMed] [Google Scholar]
- 11.Solecka BA, Weise C, Laffan MA, Kannicht C. Site-specific analysis of von Willebrand factor O-glycosylation. J Thromb Haemost. 2016;14(4):733–746. doi: 10.1111/jth.13260. [DOI] [PubMed] [Google Scholar]
- 12.Ward S, O'Sullivan JM, O'Donnell JS. The biological significance of von Willebrand factor O-linked glycosylation. Semin Thromb Hemost. 2021;47(7):855–861. doi: 10.1055/s-0041-1726373. [DOI] [PubMed] [Google Scholar]
- 13.Ward S, O'Sullivan JM, O'Donnell JS. von Willebrand factor sialylation-a critical regulator of biological function. J Thromb Haemost. 2019;17(7):1018–1029. doi: 10.1111/jth.14471. [DOI] [PubMed] [Google Scholar]
- 14.Ward SE, O'Sullivan JM, O'Donnell JS. The relationship between ABO blood group, von Willebrand factor, and primary hemostasis. Blood. 2020;136(25):2864–2874. doi: 10.1182/blood.2020005843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowen DJ. An influence of ABO blood group on the rate of proteolysis of von Willebrand factor by ADAMTS13. J Thromb Haemost. 2003;1(1):33–40. doi: 10.1046/j.1538-7836.2003.00007.x. [DOI] [PubMed] [Google Scholar]
- 16.McGrath RT, van den Biggelaar M, Byrne B, et al. Altered glycosylation of platelet-derived von Willebrand factor confers resistance to ADAMTS13 proteolysis. Blood. 2013;122(25):4107–4110. doi: 10.1182/blood-2013-04-496851. [DOI] [PubMed] [Google Scholar]
- 17.McGrath RT, McKinnon TA, Byrne B, et al. Expression of terminal alpha2-6-linked sialic acid on von Willebrand factor specifically enhances proteolysis by ADAMTS13. Blood. 2010;115(13):2666–2673. doi: 10.1182/blood-2009-09-241547. [DOI] [PubMed] [Google Scholar]
- 18.McKinnon TA, Chion AC, Millington AJ, Lane DA, Laffan MA. N-linked glycosylation of VWF modulates its interaction with ADAMTS13. Blood. 2008;111(6):3042–3049. doi: 10.1182/blood-2007-06-095042. [DOI] [PubMed] [Google Scholar]
- 19.Sodetz JM, Pizzo SV, McKee PA. Relationship of sialic acid to function and in vivo survival of human factor VIII/von Willebrand factor protein. J Biol Chem. 1977;252(15):5538–5546. [PubMed] [Google Scholar]
- 20.Stoddart JH, Jr., Andersen J, Lynch DC. Clearance of normal and type 2A von Willebrand factor in the rat. Blood. 1996;88(5):1692–1699. [PubMed] [Google Scholar]
- 21.Grewal PK, Uchiyama S, Ditto D, et al. The Ashwell receptor mitigates the lethal coagulopathy of sepsis. Nat Med. 2008;14(6):648–655. doi: 10.1038/nm1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Sullivan JM, Aguila S, McRae E, et al. N-linked glycan truncation causes enhanced clearance of plasma-derived von Willebrand factor. J Thromb Haemost. 2016;14(12):2446–2457. doi: 10.1111/jth.13537. [DOI] [PubMed] [Google Scholar]
- 23.Aguila S, Lavin M, Dalton N, et al. Increased galactose expression and enhanced clearance in patients with low von Willebrand factor. Blood. 2019;133(14):1585–1596. doi: 10.1182/blood-2018-09-874636. [DOI] [PubMed] [Google Scholar]
- 24.Chion A, O'Sullivan JM, Drakeford C, et al. N-linked glycans within the A2 domain of von Willebrand factor modulate macrophage-mediated clearance. Blood. 2016;128(15):1959–1968. doi: 10.1182/blood-2016-04-709436. [DOI] [PubMed] [Google Scholar]
- 25.Wagner DD, Mayadas T, Marder VJ. Initial glycosylation and acidic pH in the Golgi apparatus are required for multimerization of von Willebrand factor. J Cell Biol. 1986;102(4):1320–1324. doi: 10.1083/jcb.102.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKinnon TA, Goode EC, Birdsey GM, et al. Specific N-linked glycosylation sites modulate synthesis and secretion of von Willebrand factor. Blood. 2010;116(4):640–648. doi: 10.1182/blood-2010-02-267450. [DOI] [PubMed] [Google Scholar]
- 27.Nowak AA, Canis K, Riddell A, Laffan MA, McKinnon TA. O-linked glycosylation of von Willebrand factor modulates the interaction with platelet receptor glycoprotein Ib under static and shear stress conditions. Blood. 2012;120(1):214–222. doi: 10.1182/blood-2012-02-410050. [DOI] [PubMed] [Google Scholar]
- 28.Badirou I, Kurdi M, Legendre P, et al. In vivo analysis of the role of O-glycosylations of von Willebrand factor. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0037508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carew JA, Quinn SM, Stoddart JH, Lynch DC. O-linked carbohydrate of recombinant von Willebrand factor influences ristocetin-induced binding to platelet glycoprotein 1b. J Clin Invest. 1992;90(6):2258–2267. doi: 10.1172/JCI116112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michaux G, Hewlett LJ, Messenger SL, et al. Analysis of intracellular storage and regulated secretion of 3 von Willebrand disease-causing variants of von Willebrand factor. Blood. 2003;102(7):2452–2458. doi: 10.1182/blood-2003-02-0599. [DOI] [PubMed] [Google Scholar]
- 31.Sabater-Lleal M, Huffman JE, de Vries PS, et al. Genome-wide association transethnic meta-analyses identifies novel associations regulating coagulation factor VIII and von Willebrand factor plasma levels. Circulation. 2019;139(5):620–635. doi: 10.1161/CIRCULATIONAHA.118.034532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gouyer V, Leteurtre E, Delmotte P, et al. Differential effect of GalNAcalpha-O-bn on intracellular trafficking in enterocytic HT-29 and Caco-2 cells: correlation with the glycosyltransferase expression pattern. J Cell Sci. 2001;114(pt 8):1455–1471. doi: 10.1242/jcs.114.8.1455. [DOI] [PubMed] [Google Scholar]
- 33.O'Donnell J, Boulton FE, Manning RA, Laffan MA. Amount of H antigen expressed on circulating von Willebrand factor is modified by ABO blood group genotype and is a major determinant of plasma von Willebrand factor antigen levels. Arterioscler Thromb Vasc Biol. 2002;22(2):335–341. doi: 10.1161/hq0202.103997. [DOI] [PubMed] [Google Scholar]
- 34.Swinkels M, Atiq F, Burgisser PE, et al. Quantitative 3D microscopy highlights altered von Willebrand factor alpha-granule storage in patients with von Willebrand disease with distinct pathogenic mechanisms. Res Pract Thromb Haemost. 2021;5(6) doi: 10.1002/rth2.12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fogarty H, Ward SE, Townsend L, et al. Sustained VWF-ADAMTS-13 axis imbalance and endotheliopathy in long COVID syndrome is related to immune dysfunction. J Thromb Haemost. 2022;20(10):2429–2438. doi: 10.1111/jth.15830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wohner N, Sebastian S, Muczynski V, et al. Osteoprotegerin modulates platelet adhesion to von Willebrand factor during release from endothelial cells. J Thromb Haemost. 2022;20(3):755–766. doi: 10.1111/jth.15598. [DOI] [PubMed] [Google Scholar]
- 37.Kenny M, Stamboroski S, Taher R, Bruggemann D, Schoen I. Nanofiber topographies enhance platelet-fibrinogen scaffold interactions. Adv Healthc Mater. 2022;11(14) doi: 10.1002/adhm.202200249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delannoy P, Kim I, Emery N, et al. Benzyl-N-acetyl-alpha-D-galactosaminide inhibits the sialylation and the secretion of mucins by a mucin secreting HT-29 cell subpopulation. Glycoconj J. 1996;13(5):717–726. doi: 10.1007/BF00702335. [DOI] [PubMed] [Google Scholar]
- 39.Byrd JC, Dahiya R, Huang J, Kim YS. Inhibition of mucin synthesis by benzyl-alpha-GalNAc in KATO III gastric cancer and Caco-2 colon cancer cells. Eur J Cancer. 1995;31A(9):1498–1505. doi: 10.1016/0959-8049(95)00248-h. [DOI] [PubMed] [Google Scholar]
- 40.Lin MC, Chien PH, Wu HY, et al. C1GALT1 predicts poor prognosis and is a potential therapeutic target in head and neck cancer. Oncogene. 2018;37(43):5780–5793. doi: 10.1038/s41388-018-0375-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun X, Zhan M, Sun X, Liu W, Meng X. C1GALT1 in health and disease. Oncol Lett. 2021;22(2):589. doi: 10.3892/ol.2021.12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferraro F, Kriston-Vizi J, Metcalf DJ, et al. A two-tier Golgi-based control of organelle size underpins the functional plasticity of endothelial cells. Dev Cell. 2014;29(3):292–304. doi: 10.1016/j.devcel.2014.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferraro F, Mafalda Lopes da S, Grimes W, et al. Weibel-Palade body size modulates the adhesive activity of its von Willebrand factor cargo in cultured endothelial cells. Sci Rep. 2016;6 doi: 10.1038/srep32473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferraro F, Patella F, Costa JR, Ketteler R, Kriston-Vizi J, Cutler DF. Modulation of endothelial organelle size as an antithrombotic strategy. J Thromb Haemost. 2020;18(12):3296–3308. doi: 10.1111/jth.15084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karampini E, Fogarty H, Elliott S, et al. Endothelial cell activation, Weibel-Palade body secretion, and enhanced angiogenesis in severe COVID-19. Res Pract Thromb Haemost. 2023;7(2) doi: 10.1016/j.rpth.2023.100085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fogarty H, Ahmad A, Atiq F, et al. VWF-ADAMTS13 axis dysfunction in children with sickle cell disease treated with hydroxycarbamide vs blood transfusion. Blood Adv. 2023;7(22):6974–6989. doi: 10.1182/bloodadvances.2023010824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Voos KM, Cao W, Arce NA, et al. Desialylation of O-glycans activates von Willebrand factor by destabilizing its autoinhibitory module. J Thromb Haemost. 2022;20(1):196–207. doi: 10.1111/jth.15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hulstein JJ, de Groot PG, Silence K, Veyradier A, Fijnheer R, Lenting PJ. A novel nanobody that detects the gain-of-function phenotype of von Willebrand factor in ADAMTS13 deficiency and von Willebrand disease type 2B. Blood. 2005;106(9):3035–3042. doi: 10.1182/blood-2005-03-1153. [DOI] [PubMed] [Google Scholar]
- 49.Ward SE, O'Sullivan JM, Drakeford C, et al. A novel role for the macrophage galactose-type lectin receptor in mediating von Willebrand factor clearance. Blood. 2018;131(8):911–916. doi: 10.1182/blood-2017-06-787853. [DOI] [PubMed] [Google Scholar]
- 50.Ward SE, O’Sullivan JM, Moran AB, et al. Sialylation on O-linked glycans protects von Willebrand factor from macrophage galactose lectin-mediated clearance. Haematologica. 2022;107(3):668–679. doi: 10.3324/haematol.2020.274720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pruss CM, Golder M, Bryant A, et al. Pathologic mechanisms of type 1 VWD mutations R1205H and Y1584C through in vitro and in vivo mouse models. Blood. 2011;117(16):4358–4366. doi: 10.1182/blood-2010-08-303727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wagner DD, Marder VJ. Biosynthesis of von Willebrand protein by human endothelial cells: processing steps and their intracellular localization. J Cell Biol. 1984;99(6):2123–2130. doi: 10.1083/jcb.99.6.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deng W, Voos KM, Colucci JK, et al. Delimiting the autoinhibitory module of von Willebrand factor. J Thromb Haemost. 2018;16(10):2097–2105. doi: 10.1111/jth.14251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deng W, Wang Y, Druzak SA, et al. A discontinuous autoinhibitory module masks the A1 domain of von Willebrand factor. J Thromb Haemost. 2017;15(9):1867–1877. doi: 10.1111/jth.13775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arce NA, Cao W, Brown AK, et al. Activation of von Willebrand factor via mechanical unfolding of its discontinuous autoinhibitory module. Nat Commun. 2021;12(1):2360. doi: 10.1038/s41467-021-22634-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anderson JR, Li J, Springer TA, Brown A. Structures of VWF tubules before and after concatemerization reveal a mechanism of disulfide bond exchange. Blood. 2022;140(12):1419–1430. doi: 10.1182/blood.2022016467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Javitt G, Yeshaya N, Khmelnitsky L, Fass D. Assembly of von Willebrand factor tubules with in vivo helical parameters requires A1 domain insertion. Blood. 2022;140(26):2835–2843. doi: 10.1182/blood.2022017153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Flood VH, Christopherson PA, Gill JC, et al. Clinical and laboratory variability in a cohort of patients diagnosed with type 1 VWD in the United States. Blood. 2016;127(20):2481–2488. doi: 10.1182/blood-2015-10-673681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lavin M, Aguila S, Schneppenheim S, et al. Novel insights into the clinical phenotype and pathophysiology underlying low VWF levels. Blood. 2017;130(21):2344–2353. doi: 10.1182/blood-2017-05-786699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Atiq F, Blok R, van Kwawegen C, et al. Type 1 VWD classification revisited: novel insights from combined analysis of the LoVIC and WiN studies. Blood. 2024;143(14):1414–1424. doi: 10.1182/blood.2023022457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O'Donnell JS. Low VWF: insights into pathogenesis, diagnosis, and clinical management. Blood Adv. 2020;4(13):3191–3199. doi: 10.1182/bloodadvances.2020002038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Schooten CJ, Denis CV, Lisman T, et al. Variations in glycosylation of von Willebrand factor with O-linked sialylated T antigen are associated with its plasma levels. Blood. 2007;109(6):2430–2437. doi: 10.1182/blood-2006-06-032706. [DOI] [PubMed] [Google Scholar]
- 63.Mohlke KL, Purkayastha AA, Westrick RJ, et al. Mvwf, a dominant modifier of murine von Willebrand factor, results from altered lineage-specific expression of a glycosyltransferase. Cell. 1999;96(1):111–120. doi: 10.1016/s0092-8674(00)80964-2. [DOI] [PubMed] [Google Scholar]
- 64.Francisco R, Brasil S, Poejo J, et al. Congenital disorders of glycosylation (CDG): state of the art in 2022. Orphanet J Rare Dis. 2023;18(1):329. doi: 10.1186/s13023-023-02879-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lefeber DJ, Freeze HH, Steet R, Kinoshita T. In: Essentials of Glycobiology. Varki A, Cummings RD, Esko JD, et al., editors. Cold Spring Harbor Laboratory Press; 2022. Congenital disorders of glycosylation; pp. 599–614. [Google Scholar]
- 66.Yang WH, Aziz PV, Heithoff DM, Mahan MJ, Smith JW, Marth JD. An intrinsic mechanism of secreted protein aging and turnover. Proc Natl Acad Sci U S A. 2015;112(44):13657–13662. doi: 10.1073/pnas.1515464112. [DOI] [PMC free article] [PubMed] [Google Scholar]