Abstract
Interstitial pneumonia (IP) is a severe organ manifestation of cytomegalovirus (CMV) disease in the immunocompromised host, in particular in recipients of bone marrow transplantation (BMT). Diagnostic criteria for the definition of CMV-IP include clinical evidence of pneumonia together with CMV detected in bronchoalveolar lavage or lung biopsy. We have used the model of syngeneic BMT and simultaneous infection of BALB/c mice with murine CMV for studying the pathogenesis of CMV-IP by controlled longitudinal analysis. A disseminated cytopathic infection of the lungs with fatal outcome was observed only when reconstituting CD8 T cells were depleted. Neither CD8 nor CD4 T cells mediated an immunopathogenesis of acute CMV-IP. By contrast, after efficient hematolymphopoietic reconstitution, viral replication in the lungs was moderate and focal. The histopathological picture was dominated by preferential infiltration of CD8 T cells confining viral replication to inflammatory foci. Notably, after clearance of acute infection, CD62Llo and CD62Lhi subsets of CD44+ memory CD8 T cells were found to persist in lung tissue. One can thus operationally distinguish an early CMV-positive IP (phase 1) and a late CMV-negative IP (phase 2). According to the definition, phase 2 histopathology would not be diagnosed as a CMV-IP and could instead be misinterpreted as a CMV-induced immunopathology. We document here that phase 1 as well as phase 2 pulmonary CD8 T cells are capable of exerting effector functions and are effectual in protecting against productive infection. We propose that antiviral “stand-by” memory-effector T cells persist in the lungs to prevent virus recurrence from latency.
Even though cytomegaloviruses (CMV) typically cause disease with multiple organ involvement, the lungs are a particularly conspicuous organ site of CMV pathogenesis, latency, and reactivation. Specifically, CMV-associated interstitial pneumonia/pneumonitis (CMV-IP) is the most fatal among the clinical manifestations of human CMV (hCMV) disease in the immunocompromised host, such as recipients of bone marrow transplantation (BMT) and solid organ transplants, and recipients of heart-lung transplants in particular (for reviews, see references 10, 17, 18, 43, 45, and 56). While the incidence of hCMV infection is similar after autologous and allogeneic BMT, CMV-IP is more common after allogeneic BMT, specifically in association with graft-versus-host (GvH) disease (26, 27, 50, 55). However, in the less frequent cases of CMV-IP observed after autologous BMT, the disease was just as severe and the fatality rate was outstandingly high (11, 25, 41).
Experimental CMV-IP occurring during lethal infection of immunodepleted BALB/c mice with murine CMV (mCMV) confirmed the role of the lungs as a prominent site of CMV pathogenesis in the immunodeficient host and identified stromal and parenchymal lung cells, such as interstitial fibrocytes, alveolar epithelial cells, and endothelial cells, as target cells of productive mCMV infection in the absence of leukocytic infiltrates (40). Selective exogenous reconstitution of the infected recipients with presensitized antiviral T cells by means of cell transfer demonstrated antiviral control (38, 40) and, notably, also prevention of lung histopathology (39), mediated by CD8 T cells but not by CD4 T cells. The validity of this mCMV model was proven by clinical application of T-cell transfer as a specific preemptive cytoimmunotherapy of hCMV disease after BMT (44), and an originally suspected immunopathology caused by the transferred CD8 T cells was not observed in patients (for a review, see reference 43). Extension of the mCMV infection model by a concurrent experimental BMT, either a syngeneic BMT (19, 32) or a BMT performed across a single major histocompatibility complex (MHC) class I antigen difference (1, 33), documented a preferential recruitment of endogenously reconstituted CD8 T cells into the infected lungs. Specifically, CD8 T cells were recruited much more efficiently than CD4 T cells, and recruitment to infected lungs by far exceeded the recruitment to uninfected lungs (19). A curative antiviral rather than an immunopathological function of CD8 T cells in pulmonary infiltrates was concluded from four facts: (i) T-cell infiltration correlated with a decline in virus replication (1, 19), (ii) depletion of CD8 T cells during reconstitution abolished the antiviral control with fatal outcome (32), (iii) CD8 T cells isolated from pulmonary infiltrates showed an ex vivo MHC-restricted cytolytic activity against infected but not against uninfected target cells (19), and (iv) adoptive transfer of pulmonary infiltrate-derived CD8 T cells controlled virus replication in various organs of indicator recipients, the lungs included (1). While a correlation between reconstitution of CD8 T cells after BMT and control of infection was also observed in patients (42), the mCMV model has provided proof of the protective principle.
A special role of the lungs in the biology of CMV is also inferred from studies on mCMV latency, reactivation, and recurrence. Thus, in the quite diverse models of neonatal infection (3, 37) and infection of BMT recipients (22–24, 49), the lungs consistently proved to be a prominent site of viral latency, as indicated by a high copy number of latent viral genomes and an associated high risk of transcriptional reactivation and recurrent infection upon secondary immunoablative treatment. By controlling virus replication during acute infection, CD8 T cells limited the load of latent viral genomes in the lungs and, accordingly, reduced the risk of recurrent infection of the lungs (49).
While all this cumulative evidence argued for a protective role of the immune response to mCMV as well as hCMV, Grundy et al. highly influenced the field by proposing an immunopathogenesis of CMV-IP based on their interpretation of clinical data as well as on data from specifically designed murine models (13–15). In essence, the authors highlighted the clinical data on the statistical association between CMV-IP and GvH disease, and this line of argument was supported by murine infection models in which a GvH reaction which was experimentally enforced by administration of fairly high numbers of mature allogeneic T cells caused lung pathology that was preventable by immunosuppression. A second argument was the absence of CMV-IP in athymic nude mice. A third argument was the usually benign course of lung infection by hCMV during earlier stages of AIDS, which the authors ascribed to a lack of CD4 T-cell-driven immunopathology. Finally, Grundy et al. cited cases in which antiviral therapy had apparently failed to resolve established pulmonary symptoms. Since then, the literature on clinical CMV-IP has been filled with case reports that interpret single clinical observations in favor or disfavor of Grundy's immunopathogenesis hypothesis. Rebuttal came primarily from clinicians who outlined in detail that the immunopathological characteristics ascribed to mCMV pneumonia do not apply to most cases of hCMV pneumonia (4, 29). Specifically, Morris (29) concluded that the murine model is not valid.
However, the pathogenesis and histopathology of murine CMV-IP have so far not been thoroughly studied by longitudinal analysis in a setting of BMT that is more closely related to the clinical situation. Based on the finding that hCMV can also result in severe CMV-IP after autologous BMT (11, 25, 41), the present study is focused on syngeneic BMT and on the role of CD8 T cells in mCMV-associated pathogenesis during acute and latent infection of the lungs.
MATERIALS AND METHODS
BMT, infection, and in vivo T-cell subset depletion.
Animal experiments were approved by the Ethics Commission, permission no. 177-07/931-17, according to German federal law. Donors and recipients of a syngeneic experimental BMT were 8-week-old, female BALB/c (haplotype H-2d) mice. For hematoablative conditioning, recipients were total-body γ irradiated with a single dose of 6 Gy delivered by a 137Cs γ-ray source. About 6 h after irradiation, reconstitution was initiated by intravenous (i.v.) infusion of 5 × 106 tibial and femoral donor bone marrow cells, isolated and depleted of mature CD8 T cells as described previously (1). About 2 h after BMT, recipients were infected subcutaneously in the left hind footpad with 105 PFU of purified, cell culture-propagated mCMV, strain Smith ATCC VR-194/1981 (24). Under the specific conditions chosen for BMT, a large number of recipients were expected to survive the infection (19, 49). At days 7 and 14 during reconstitution, regenerating CD4 and CD8 T-cell subsets were depleted in vivo by i.v. infusion of 1 mg of monoclonal antibodies (MAb) YTS 191.1 and YTS 169.4 (6), respectively (kindly provided by the group of S. Jonjic, University of Rijeka, Rijeka, Croatia). The efficacy of the depletions was controlled by cytofluorometric analysis as documented previously (32).
Quantitation of virus infection and T-cell infiltration in tissues.
The in vivo infection was quantitated either by the yield of infectious virus, measured as centrifugally enforced PFU contained in organ homogenates, or by the number of infected cells present in lung tissue sections and detected either by in situ hybridization (ISH) of viral DNA or by immunohistochemical staining (IHC) of viral protein. T-cell infiltration was quantitated by IHC.
(i) Determination of virus titers in organs.
Infectious virus was quantitated in organ homogenates by a plaque assay performed on nearly confluent second-passage mouse fetal fibroblast monolayers under conditions of centrifugal enhancement of infectivity as described previously (19, 40). The virus titers represent the number of infectious units per organ and are expressed as PFU* to indicate the enhancement. It is worth noting that 1 PFU* corresponds to ca. 25 viral genomes, whereas a noncentrifugal PFU represents ca. 500 viral genomes (24).
(ii) ISH of viral DNA in intranuclear inclusion bodies.
Detection of viral DNA was performed as described previously (32). In essence, in order to get an optimal sensitivity of detection, a mixture of digoxigenin (digoxigenin-11-dUTP)-tagged plasmids containing HindIII fragments A, I, and K, altogether representing 51.2 kbp of the mCMV Smith genome, was used as the specific probe. Labeling was performed with alkaline phosphatase-conjugated antidigoxigenin antibody and new fuchsin as the substrate, yielding a brilliant red. ISH detects infected cells in the late phase of viral replication, when viral DNA accumulates for packaging and nucleocapsid assembly in an intranuclear inclusion body.
(iii) Two-color IHC.
Infected lung cells and lung-infiltrating T cells were simultaneously visualized in 2-μm paraffin-embedded sections of lung tissue by two-color IHC as described in detail previously (19). In essence, infected lung cells were visualized by detection of intranuclear viral IE1 protein with MAb CROMA 101 (kindly provided by S. Jonjic, University of Rijeka) using the alkaline phosphatase-anti-alkaline phosphatase (APAAP) method with new fuchsin as the substrate (red staining). T cells were visualized by staining of membrane CD3ɛ, using the avidin-biotin-peroxidase complex (ABC) method with diaminobenzidine tetrahydrochloride as the substrate, followed by enhancement with nickel sulfate hexahydrate (black staining). Viral IE1 protein is present in the nucleus of the infected cell throughout the viral replication cycle. Therefore, IE1-specific IHC detects more infected cells than does ISH. In our experience, the ratio between IHC-positive and ISH-positive cells is ca. 2:1 during florid infection of permissive tissues.
(iv) Quantitation of histological data.
To avoid artificial compression of lung tissue, it was crucial to distend alveolar spaces by instillation of the fixative (phosphate-buffered saline [PBS] [pH 7.4] containing 4% [vol/vol] formalin) into the trachea. The numbers of infected tissue cells (red) and infiltrating T cells (black) were counted for representative areas of tissue sections (in the case of the lungs, usually 100-mm2 areas) compiled from sections representing the organ morphology and taken in distances from each other (>10 μm) chosen to exclude redundant counting of the same cells. Numbers of infected tissue cells were related to the number of lung cells counted in uninfected lung tissue after hematoxylin staining of nuclei. It should be mentioned that infiltrating leukocytes were found not to be infected, with the exception of a very few alveolar macrophages. Therefore, the reported percentage of infected cells in the lungs refers to the stromal-parenchymal compartment. An estimate of the total number of infected cells per lung was made by relating section volumes (2 μm by 100 mm2) to the average total volume of a lung embedded in paraffin (0.135 cm3). The extrapolation to the total organ volume results in an overestimation because sections may cut the same nucleus more than once depending on the ratio between nucleus (assumed to be a sphere) diameter, D, and thickness of the tissue section, d. Extrapolated numbers N* were corrected by using the empirical formula N = N* × d/D. Since nuclei, in particular those of endothelial cells, are ellipsoids rather than spheres, and since the size and form of nuclei differ between cell types in the lungs, the estimate should be seen as a rough approximation to give an impression of the order of magnitude.
Isolation and immunomagnetic enrichment of pulmonary CD8 T cells.
Mononuclear leukocytes were isolated from lung tissue as described previously (19) by collagenase-DNase digestion of lung parenchyma followed by Ficoll density gradient centrifugation. Analyses were performed with cells pooled from at least 10 mice per group. It is important to emphasize that intravascular leukocytes had been largely removed by perfusion, but the presence of intracapillary leukocytes that stick to endothelial cells can never be excluded. Cells were either used directly for cytofluorometric analyses of the phenotypes present in the pulmonary infiltrate population (see below) or were subjected to positive immunomagnetic sorting (MiniMacs separation unit; Miltenyi Biotec Systems, Bergisch-Gladbach, Germany) for the purification of CD8 T cells (anti-CD8a MicroBeads, catalog no. 494-01; Miltenyi Biotec Systems) as described in more detail previously (1). The purity of the population was found to be ca. 96% as determined by cytofluorometric reanalysis with phycoerythrin (PE)-conjugated MAb anti-CD8a (clone 53-6.7, rat immunoglobulin G2a [IgG2a]; catalog no. 01045A; PharMingen, San Diego, Calif.).
Assay of CD3ɛ-redirected cytolytic activity.
The CD3ɛ assay measures the total cytolytic potential of an effector cell population by antigen-independent polyclonal signaling via the T-cell receptor (TCR)-CD3 complex. Target cells were 51Cr-labeled Fc receptor-expressing P815 mastocytoma cells armed with Fc receptor-bound anti-CD3ɛ MAb. Immunomagnetically purified pulmonary CD8 T cells were tested as effector cells. A standard 4-h 51Cr release assay was performed with graded numbers of effector cells and with 103 target cells per 0.2-ml microwell. Data represent the mean percentage of specific lysis from three replicate cultures. It should be recalled from previous work that P815 cells are not lysed by mCMV-activated pulmonary infiltrate T cells in the absence of anti-CD3ɛ MAb and that no cytolytic activity was triggered by anti-CD3ɛ MAb in unprimed T cells isolated from the lungs after BMT with no infection (19).
Three-color cytofluorometric analyses.
Cytofluorometric analyses were performed with a FACSort (Becton Dickinson, San Jose, Calif.) by using CellQuest software (Becton Dickinson) for data processing. Overlaps in the emission spectra of fluorescent dyes were compensated for throughout, and thresholds were set in the forward-versus-side scatter (FSC-vs-SSC) plot to exclude particles the size of erythrocytes or smaller and to exclude dead cells during data acquisition. For calculations, a “lymphocyte gate” was set in the FSC-vs-SSC plot so as to largely exclude macrophages and residual granulocytes from the analysis but include all living T cells. Staining for the expression of TCR α/β (see below) was used to define the lymphocyte gate. This is of particular importance, since the scatter characteristics of activated pulmonary infiltrate T cells differ somewhat from those of resting T cells in lymphoid organs. Basic technical aspects, such as the preparation of cells for cytofluorometric analysis, the composition of buffers, and the blocking of nonspecific binding sites, have been described previously (1). Throughout, fluorescence channel 1 (FL-1) represents the fluorochrome fluorescein (fluorescein isothiocyanate; FITC), FL-2 represents the fluorochrome R-PE, and FL-3 represents either the tandem fluorochrome PE-Cy5, also known as Cy-Chrome, or the tandem fluorochrome PE-Texas Red, also known as RED613 or duochrome.
(i) Determination of CD8/CD4 subset ratios among α/β T cells.
Pulmonary infiltrate cells retrieved from the Ficoll interphase were labeled with FITC-conjugated MAb anti-CD8 (clone 53-6.7, rat IgG2a; catalog no. 01044A; Becton Dickinson), PE-conjugated MAb anti-TCR α/β (clone H57-597, hamster IgG; catalog no. 01305A; PharMingen), and PE-Cy5-conjugated MAb anti-CD4 (clone H129.19, rat IgG2a; catalog no. 09008A; PharMingen). The analysis was restricted to α/β T cells by setting an electronic gate on signals with positive FL-2.
(ii) CD44 and CD62L activation phenotyping of CD8 T cells.
Pulmonary infiltrate cells retrieved from the Ficoll interphase were labeled with FITC-conjugated MAb anti-CD44 (clone IM7, rat IgG2b; catalog no. 01224D; PharMingen) and PE-conjugated MAb anti-CD8a (see above). CD62L was labeled indirectly with biotinylated MAb anti-CD62L (clone MEL-14, rat IgG2a; catalog no. 01262D; PharMingen) and streptavidin-RED613 (catalog no. 19541-010; Life Technologies). The analysis was restricted to CD8 T cells by setting an electronic gate on signals with positive FL-2.
(iii) Detection of intracellular IFN-γ.
The functional capacity of pulmonary CD8 T cells to produce gamma interferon (IFN-γ) (35) was tested by antigen-independent polyclonal signaling via the TCR-CD3 complex. Pulmonary infiltrate cells retrieved from the Ficoll interphase were seeded in replicate 0.2-ml cultures in 96-well round-bottomed microwell plates at a concentration of 106 cells per culture. The composition of the culture medium, which included 10 μg of brefeldin A per ml, was described previously (20). For polyclonal signaling, cells were stimulated with 0.4 μg (per culture, i.e., per 106 cells) of MAb anti-CD3ɛ (clone 145-2C11, hamster IgG; Dianova catalog no. 1530-14; Southern Biotechnology Associates Inc., Birmingham, Ala.). It should be noted that a log2 titration of anti-CD3ɛ from 0.2 to 1.6 μg gave identical results (data not shown). The cells were harvested after 5 h of incubation and processed for cytofluorometric analysis as described previously (20). Surface staining was performed with FITC-conjugated anti-CD62L (clone MEL-14, rat IgG2a; catalog no. 01264D; PharMingen) and PE-Cy5-conjugated MAb anti-CD8a (clone 53-6.7, rat IgG2a; catalog no. 01048A; PharMingen). After washing, cell fixation was performed for 20 min at ca. 20°C by addition of 100 μl of PBS containing 2% (vol/vol) paraformaldehyde. Cells were washed to remove the fixative and permeabilized as described (20). For intracellular IFN-γ staining, an aliquot of 2 × 106 cells was labeled with 0.05 μg of PE-conjugated MAb anti mouse IFN-γ (clone XMG1.2, rat IgG1; catalog no. 18115A; PharMingen). For isotype control, another aliquot was incubated with 0.05 μg of PE-conjugated rat IgG1 (clone R3-34, isotype control rat IgG1; catalog no. 20615A; PharMingen).
Analysis of in vivo antiviral activity of pulmonary CD8 T cells by adoptive transfer.
Recipients of adoptive cell transfer were 8-week-old, female BALB/c mice that were immunosuppressed by γ irradiation with a dose of 6.5 Gy and infected in the left hind footpad with 105 PFU of purified mCMV. Under these conditions, all mice die of multiple-organ CMV disease (40) between days 10 and 18 after infection unless they receive protective T cells (39). Graded numbers of immunomagnetically purified pulmonary CD8 T cells (see above) were transferred i.v. 6 h after γ irradiation and 2 h before infection, and antiviral function was assessed for lungs and spleen of the recipients on day 12 after infection by virus plaque assay.
RESULTS
Control of pulmonary mCMV infection after BMT depends on CD8 T-cell reconstitution.
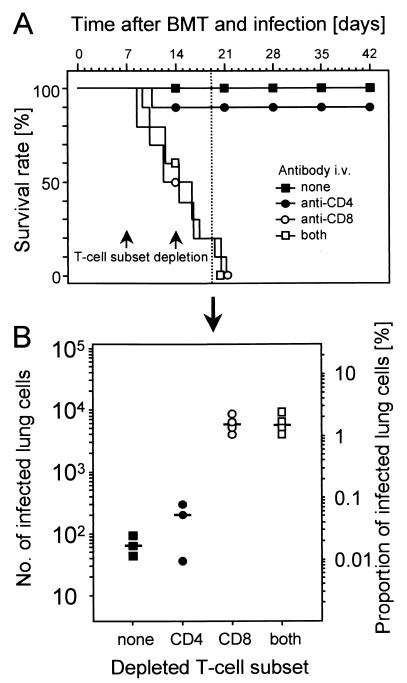
Syngeneic BMT was performed with concomitant experimental mCMV infection. Based on previous work in this model (19, 32, 49), conditions of BMT and infection were chosen to permit a high survival rate of the recipients due to successful and timely hematopoietic reconstitution. In the particular experiment shown in Fig. 1, all recipients survived long term (Fig. 1A, solid squares). The protective principle within the reconstituting myeloid and lymphoid cell lineages was revealed by specific in vivo depletion of T-cell subsets during the process of reconstitution after BMT. The efficacy of the depletions was controlled by cytofluorometric analysis of pulmonary infiltrate cells. In accordance with a previous report (32), depletion reduced the respective T-cell subsets to ≤0.5% of the T cells (not shown here). Selective depletion of CD4-positive cells, which includes CD4 T cells and subsets of bone marrow myeloid cells, had no significant effect on the survival rate (Fig. 1A, solid circles). By contrast, selective depletion of CD8 T cells inevitably resulted in death from the disease within 3 weeks (Fig. 1A, open circles). Simultaneous depletion of both T-cell subsets did not accelerate the lethal course (Fig. 1A, open squares). It must be emphasized that depletion of CD8 T cells is not associated with lethal disease after BMT in the absence of infection (not shown). In accordance with previous work (32; for a review, see reference 21), this experiment has thus unequivocally identified CD8 T cells as the protective principle preventing CMV-associated mortality after BMT. CD4 T cells were not required for protection, nor could they functionally substitute for the eliminated CD8 T cells in this particular experimental setting. The result is also decisive regarding the controversial hypotheses of “viral pathogenesis” versus “immunopathogenesis” of CMV disease. Recipients survived when CD8 T cells or both CD8 and CD4 T cells were present, and they died when CD8 T cells or both subsets were absent. Thus, clearly, the observed lethal outcome of mCMV infection was not the result of an immunopathology.
FIG. 1.
Role of T-cell subsets in the control of CMV disease during hematopoietic reconstitution. (A) Effect of selective T-cell subset depletion on survival rate. BMT and mCMV infection were performed on day 0, and T-cell subsets were depleted by two consecutive (on days 7 and 14) i.v. infusions of MAbs directed against CD4 or CD8. Shown are Kaplan-Meyer plots for 10 recipients per group. Solid and open symbols indicate the presence and absence of CD8 T cells, respectively. (B) Effect of selective T-cell subset depletion on mCMV replication in the lungs. With additional recipients in each of the four experimental groups, the number of infected lung tissue cells was determined by quantitative viral DNA ISH on day 19, that is, at a stage when CMV disease was prefinal in CD8-depleted recipients. Symbols correspond to those in panel A and represent data from individual recipients. The median value is marked by a horizontal bar. The left-hand scale shows the absolute numbers of ISH-positive lung cells present in representative 100-mm2 areas compiled from lung tissue sections. The right-hand scale relates these numbers to the number of nucleated stromal and parenchymal cells detected by hematoxylin staining. The total number of lung cells is ca. 6 × 107.
Since CMV disease is characterized by multiple-organ involvement (18, 32), one could argue that CD8 T cells prevent a lethal course of disease by controlling virus replication at vital tissue sites, including liver, adrenal glands, and the bone marrow stroma (9, 32), but contribute to pathogenesis specifically in the lungs. As a first approach to understanding the role of CD8 T cells in the lungs, we quantitated the infection of lung tissue in relation to the presence of T-cell subsets by counting the number of productively infected lung cells. Productive infection was visualized by ISH staining of viral DNA accumulated in intranuclear inclusion bodies during viral DNA packaging and nucleocapsid assembly in the late phase of the viral replication cycle. The analysis was performed at day 19 after BMT and infection, that is, at a prefinal stage of CMV disease (Fig. 1B). The extent of infection of the lungs precisely reflected the overall course of disease. In the two experimental groups with reconstituting CD8 T cells (Fig. 1B, solid symbols), the proportion of infected lung cells was between 0.01 and 0.1%, whereas in both groups that were depleted of CD8 T cells (open symbols), between 1 and 3% of the stromal and parenchymal lung cells, in absolute terms ca. 0.6 × 106 to 1.8 × 106 cells, were found to be in the late phase of infection. In conclusion, reconstitution of CD8 T cells after BMT is essential to prevent massive infection of the lungs.
Viral histopathology in lungs devoid of T-cell infiltrates.
The histopathological consequences of viral replication and T-cell control in the lungs were studied by two-color IHC, simultaneously visualizing infected cells by red staining of intranuclear viral IE1 protein and infiltrating T cells by black membrane staining of CD3ɛ (Fig. 2).
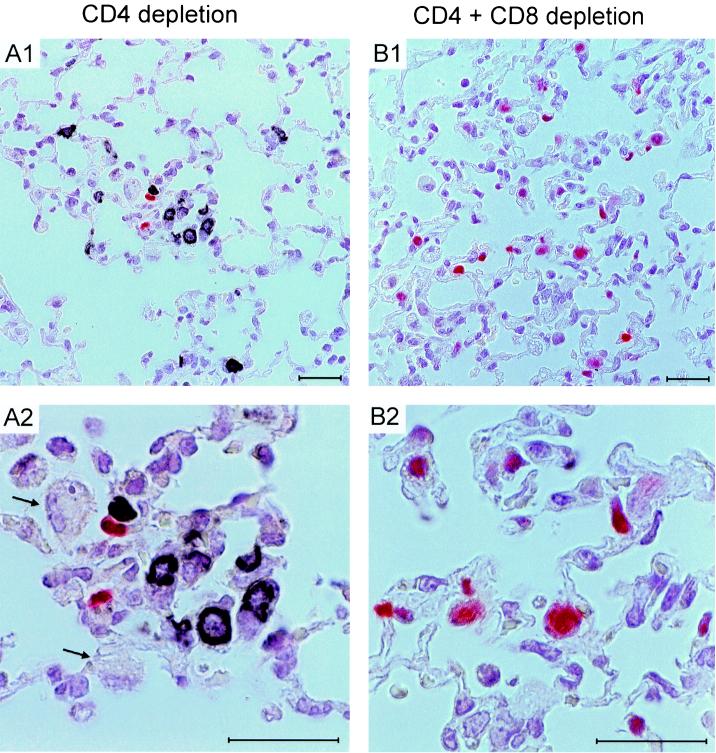
FIG. 2.
Viral histopathology in the lungs after T-cell subset depletion. A two-color IHC analysis of lung tissue was performed on day 19 after BMT and mCMV infection (corresponding to Fig. 1) for recipients depleted either selectively of CD4 T cells (A1, overview; A2, details) or depleted of both T-cell subsets (B1, overview; B2, details). Infected cells are visualized by red staining of intranuclear viral IE1 protein, and infiltrating T cells are visualized by black staining of membrane CD3ɛ. Counterstaining was performed with hematoxylin. The arrows in panel A2 point to uninfected alveolar macrophages recruited to the inflammatory focus. Bars, 25 μm.
In the experimental group that was selectively depleted of CD4 T cells, infected cells were rare in the lungs and were actually not seen in every tissue section. Notably, infiltrating blastoid CD8 T cells were not distributed randomly in lung tissue but formed inflammatory foci specifically at the sites at which infected lung cells were located (Fig. 2, panel A1 for overview and A2 for details). Alveolar macrophages were also recruited into these foci (Fig. 2A2). As a side aspect, one should note that the alveolar macrophages shown in Fig. 2A2 did not express detectable IE1 protein. From this part of the experiment, we draw the following conclusions: (i) extravasation and recruitment of CD8 T cells to the site of infection do not require help by CD4 T cells, (ii) infiltrating CD8 T cells focus their action towards infected cells, and (iii) in reverse conclusion, CD8 T cells do not damage uninfected parts of tissue, at least not by cell-to-cell delivery of effector functions.
A strikingly different picture was seen when both T-cell subsets were depleted during reconstitution (Fig. 2, panel B1 for overview and B2 for details). Numerous infected cells, distributed randomly throughout the lung parenchyma, were now detected in every tissue section. By cell counting in representative 100-mm2 areas of lung tissue, the proportion of IE1-expressing cells was found to range between ca. 2 and 6%. Complete absence of CD3ɛ staining gives visible proof of the efficacy of T-cell depletion. Alveolar macrophages were frequent but were not found colocalized to infected cells (Fig. 2B1). Altogether, focal infection, as it was seen in the presence of CD8 T cells, was here replaced by disseminated infection. A comparison of the overall tissue architecture (Fig. 2, compare panels A and B) shows a more pronounced widening of alveolar septa and a higher degree of alveolar collapse in the absence of T-cell infiltrates! From this part of the experiment, we draw the following conclusions. (i) Infection of the lungs bursts in the absence of CD8 T cells. In the reverse conclusion, the CD8 T cells shown in Fig. 2A were antivirally active and responsible for limiting the spread of infection in the lungs. (ii) Histopathological characteristics of disseminated interstitial pneumonia develop in absence of T cells, that is, neither CD8 T cells nor CD4 T cells cause the histopathological alterations seen in the acute phase of CMV infection.
In conclusion, the lung pathology found after BMT and acute CMV infection is not a CMV-associated T-cell-mediated immunopathology but represents an authentic viral pneumonia.
Persistence of T-cell infiltrates after clearance of productive infection.
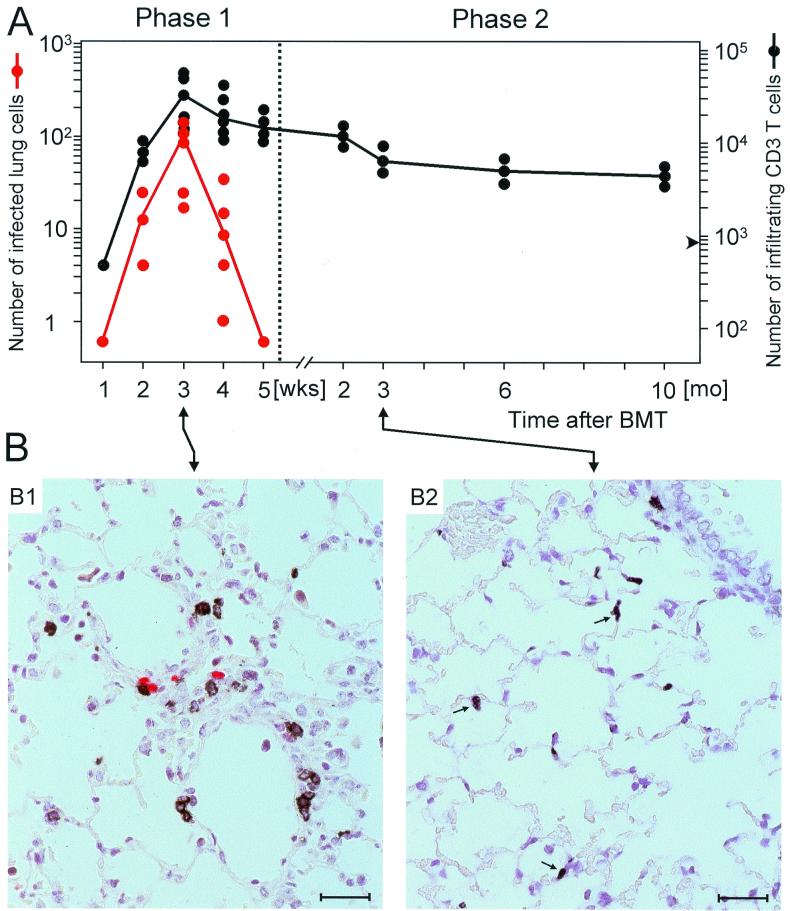
Efficient reconstitution of antiviral CD8 T cells after BMT leads to control of CMV infection and prevents a lethal course of disease, but there might be sequelae accounting for late CMV-associated morbidity in survivors of acute CMV disease (5). We therefore performed a longitudinal study of pulmonary infection and T-cell infiltration by two-color IHC, correlating the number of T cells in the lungs with the number of infected cells for an observation period of 10 months after BMT and primary infection, with no experimental depletion of T-cell subsets performed (Fig. 3). In accordance with previous data on the yield of infectious virus (19), the number of infected lung cells reached its peak after 3 weeks and declined sharply thereafter. By 5 weeks, productive infection was resolved. It should be recalled from previous work that the viral genome is not cleared from the lungs but is maintained in a state of latency (22, 24). Infiltration of T cells paralleled the infection during the first 3 weeks, but the infiltrates persisted long term, with only a slow and moderate decline in cell numbers during the observation period (Fig. 3A). By extrapolation, one can predict that infiltrates persist for the life span of the recipients. On the basis of this kinetics, we can now operationally define two phases of CMV-associated histopathology. Phase 1 is defined by the simultaneous presence of infected lung cells and T-cell infiltrates, whereas phase 2 is defined by the presence of T-cell infiltrates in the absence of productive infection. Histopathology representative of these two defined phases is documented in Fig. 3B for the third week (Fig. 3B1, phase 1) and for the third month (Fig. 3B2, phase 2). Figure 3B1 shows a perivascular inflammatory focus with colocalization of infected cells (red) and infiltrating blastoid T cells (black). By contrast, Fig. 3B2 documents a scattered distribution of small, apparently resting T cells (black) in lung parenchyma in the absence of infected cells. The tissue architecture in this late phase is not severely impaired, which is consistent with long-term survival of the recipients and which implies that neither CD8 nor CD4 T cells cause visible tissue damage. In fact, the presence of interstitial T cells is the only significant histopathological sign. In summary, phase 1 histopathology can be characterized as a CMV-positive IP with inflammatory foci, whereas phase 2 histopathology can be characterized as a diffuse and very moderate CMV-negative IP with marked T-cell presence in the interstitium and little involvement of alveolar spaces. From a pathologist's point of view, and with no further information being provided, phase 2 histopathology in clinically asymptomatic BMT recipients would not easily be diagnosed as being associated with CMV. Rather, the presence of T-cell infiltrates in the absence of an infectious agent may tempt one to diagnose an “idiopathic” pneumonia or an immunopathological condition of unknown etiology. That it is in fact an “aged” CMV pneumonia is only revealed by longitudinal analysis of the events.
FIG. 3.
Longitudinal analysis of pulmonary infection and T-cell infiltration after BMT. Histopathology in the lungs was studied by quantitative two-color IHC, with infected cells being visualized by red staining of intranuclear viral IE1 protein (red dots) and infiltrating T cells by black staining of membrane CD3ɛ (black dots). (A) Time course of infection and T-cell infiltration. Each symbol represents data for individual recipients. The lines connect median values. Negative counts are depicted only on the first occasion in the kinetics and remained negative throughout. The left-hand scale and the right-hand scale show the numbers of infected lung cells and of lung-infiltrating T cells, respectively, for representative 100-mm2 areas compiled from several lung tissue sections. In a control group receiving BMT but not infection, the tissue architecture was not pathologically altered (19) (not shown), and in phase 2, the average number of T cells per 100 mm2 was 880 (arrowhead), in comparison to ca. 200 in lungs of normal mice. (B) Examples of lung histology representative of phase 1 (B1, CMV-positive IP) and of phase 2 (B2, CMV-negative IP). (B1) Perivascular inflammatory focus seen 3 weeks after BMT and infection in tissue that connects two neighboring vessels. T cells located within the inflammatory focus are activated lymphoblasts, as shown by halo-like membrane staining (see also Fig. 2A2). (B2) Random distribution of residual interstitial T cells after clearance of productive infection, as observed after 3 months. Note that most of the T cells (some of which are marked by an arrow) are considerably smaller than those in phase 1 and apparently represent resting cells. Counterstaining was performed with hematoxylin. Bars, 25 μm.
Activation phenotypes of phase 1 and 2 pulmonary CD8 T cells.
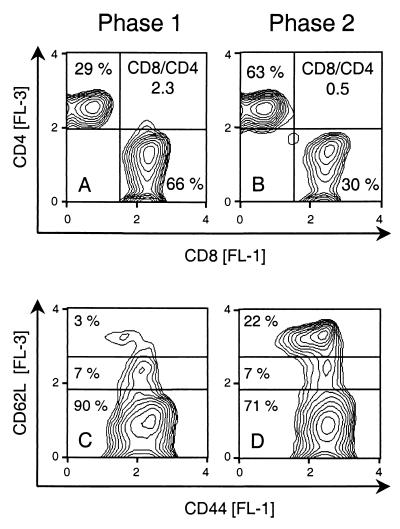
While the preceding experiments have provided conclusive evidence for a protective role of infiltrating CD8 T cells during phase 1, a contribution to late histopathology still has to be considered. As a first approach to understanding the role of CD8 T cells during phase 2, we isolated lymphocytes from phase 1 and 2 pulmonary infiltrates for a comparative cytofluorometric analysis of their phenotypes (Fig. 4). In accordance with our previous work (19), the T-cell population in the phase 1 infiltrates was largely dominated by CD8 T cells. It is instructive to recall from the previous work that the CD8/CD4 T-cell ratio in lung infiltrates is ca. 0.2 after BMT with no mCMV infection, as it is in normal lungs of immunocompetent BALB/c mice (19), whereas this ratio was 2.3 in phase 1 infiltrates of the experiment documented in Fig. 4A. In phase 2, the absolute number of T cells in lung tissue had declined by a factor of ca. 10 (recall Fig. 3A). In addition, the CD8/CD4 ratio had declined to 0.5 (Fig. 4B). Thus, the absolute number of CD8 T cells had actually declined significantly between phase 1 and phase 2, by a factor of ca. 20. The CD44 cell surface glycoprotein (also called Pgp-1 and Ly-24) is required for extravasation of activated T cells into an inflammatory site (8). Accordingly, CD8 T cells isolated from pulmonary infiltrates all expressed CD44, regardless of whether they were isolated during phase 1 or phase 2 (Fig. 4C and D). Like CD44, the CD62L member of the selectin family (also called l-selectin, Ly-22, and MEL-14 antigen) contributes to the recruitment of leukocytes into areas of inflammation by mediating their initial tethering and rolling on endothelial surfaces. However, upon activation of lymphocytes, CD62L is rapidly shed from the cell surface as a result of proteolytic cleavage (12, 54; for a review, see reference 53). Activated cells are therefore supposed to display the phenotype CD44hi CD62Llo, and naive T cells and quiescent memory T cells are supposed to display the phenotypes CD44lo CD62Lhi and CD44hi CD62Lhi, respectively (28; for a review, see reference 2). In phase 1 pulmonary infiltrates, 90% of the CD8 T cells showed the phenotype of activated cells (Fig. 4C). This finding is in agreement with the antiviral effector function performed by CD8 T cells during acute infection of the lungs. Note that the few CD62Lhi CD8 T cells seen in phase 1 were CD44lo. These cells likely represent naive CD8 T cells, probably residual intracapillary leukocytes. In phase 2 pulmonary infiltrates, the activated population was still prominent (ca. 71%), but a significant population of CD44hi CD62Lhi memory T cells (ca. 22%) had emerged in the infiltrates (Fig. 4D). For completeness, it should be mentioned that a small population (ca. 7%) of CD62Ldim CD8 T cells was present in both phases. We do not presently know any role for these cells. In conclusion, the cellular composition of phase 1 and phase 2 pulmonary infiltrate T-cell populations differs in that the proportion of CD8 T cells is reduced in phase 2 while the number of CD8 T cells with memory phenotype is increased. However, the proportion of CD8 T cells with the activation phenotype CD44hi CD62Llo is still quite considerable in phase 2.
FIG. 4.
Phenotypes of phase 1 and phase 2 pulmonary infiltrate T cells. Pulmonary infiltrate cells were isolated at 4 weeks (phase 1) and at 10 weeks (early in phase 2) after BMT and mCMV infection. (A and B) Three-color cytofluorometric analysis was performed for the marker combinations FITC (FL-1)-CD8, PE (FL-2)-TCRα/β, and PE-Cy5 (FL-3)-CD4. A gate was set on lymphocytes, and the analysis was restricted to ca. 20,000 α/β T cells by a second gate set on positive FL-2. (C and D) Three-color cytofluorometric analysis was performed for the marker combinations FITC (FL-1)-CD44, PE (FL-2)-CD8, and RED613 (FL-3)-CD62L. A gate was set on lymphocytes, and the analysis was restricted to ca. 10,000 CD8 T cells by a second gate set on positive FL-2. FL-3 (ordinate) versus FL-1 (abscissa) log fluorescence intensities are shown for gated cells as contour plots in a 70% log-density mode (threshold, 2%; smoothing factor, 5). Percentages of relevant T-cell subsets and the ratios of CD8 to CD4 T cells (CD8/CD4) are indicated.
Functional properties of phase 1 and 2 CD8 T cells.
The finding that a considerable number of phase 2 pulmonary infiltrate CD8 T cells still displayed the phenotype of activated T cells suggested a functional activity. Since the proportion of CD8 T cells specific for any single antigenic peptide of mCMV is proposed to be very low, as it was concluded previously for the immunodominant IE1 peptide (19), the antigen-independent method of eliciting effector function by polyclonal signaling via the CD3-TCR complex was chosen to measure the functional competence of whole CD8 populations. Specifically, the cytolytic potential of immunomagnetically purified phase 1 and 2 pulmonary infiltrate CD8 T cells was tested by CD3ɛ-redirected lysis. In accordance with previous work (19), phase 1 CD8 T cells were cytolytically active (Fig. 5). Notably, even though with somewhat lower cytolytic potential, purified phase 2 CD8 T cells were also functional in this assay (Fig. 5). Accordingly, analysis of perforin gene expression by reverse transcriptase-PCR revealed a similar amount of perforin transcripts for phase 1 and 2 pulmonary infiltrate CD8 T cells in this particular experiment (not shown). It should be mentioned, however, that cytolytic activity during phase 2 is variable and that detection of low activities in the infiltrates requires enrichment for CD8 T cells. From previous work (19) it should be recalled that cytolytic activity is undetectable throughout the kinetics in pulmonary infiltrates after BMT performed in the absence of infection. In conclusion, the experiment has demonstrated that phase 2 CD8 T cells, at least some of them, possess the capacity to exert cytolytic effector function upon engaging their CD3-TCR complex. This quality is dependent upon prior sensitization during a preceding infection of the lungs.
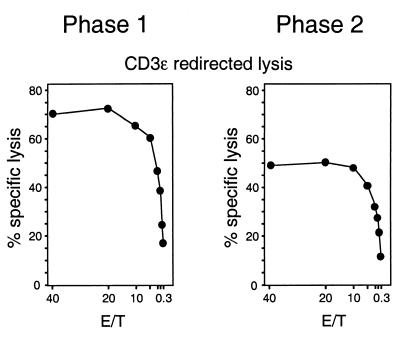
FIG. 5.
Cytolytic activity of pulmonary infiltrate CD8 T cells after polyclonal CD3ɛ signaling. Immunomagnetically purified CD8 T cells (phase 1, 4 weeks; phase 2, 16 weeks) were tested for cytolytic activity by the assay of CD3ɛ-redirected lysis with effector-to-target cell ratios (E/T) as indicated. Note that T cells isolated from lungs after BMT with no infection did not exert detectable cytolytic activity at an E/T ratio of 40 (data not shown here; see reference 19).
The expression of IFN-γ is another relevant effector quality of CD8 T cells. The capacity to synthesize IFN-γ upon polyclonal signaling via the CD3-TCR complex was tested for phase 1 and 2 pulmonary infiltrate cells by stimulation with MAb anti-CD3ɛ (Fig. 6A). For comparison, pulmonary T cells isolated at the same time post-BMT from the lungs of uninfected BMT recipients were analyzed accordingly (Fig. 6B). Three-color cytofluorometric analysis was used to combine cell phenotyping for expression of CD8 and CD62L with measurement of accumulated intracellular IFN-γ.
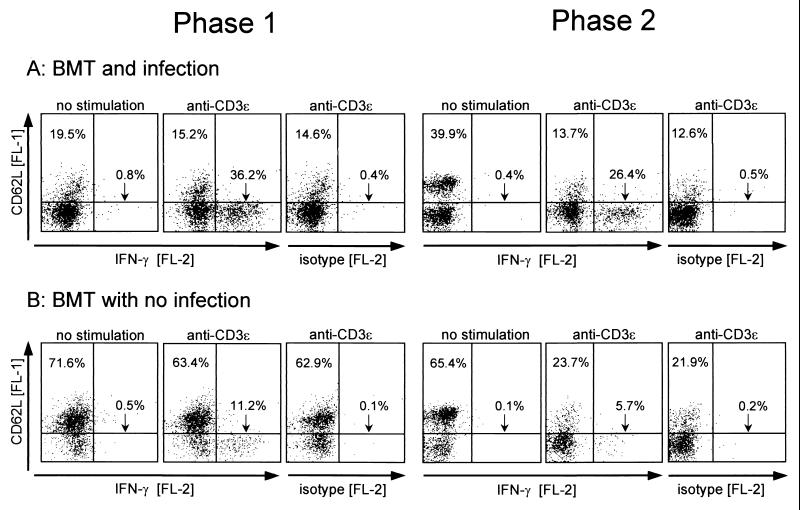
FIG. 6.
Production of IFN-γ after polyclonal CD3ɛ signaling. (A) BMT and mCMV infection. (B) BMT with no infection. Pulmonary infiltrate cells (phase 1, 4 weeks; phase 2, 16 weeks) were stimulated for 5 h with MAb anti-CD3ɛ in the presence of brefeldin A. Control groups were treated accordingly except for polyclonal CD3ɛ stimulation. Three-color cytofluorometric analysis was performed for the marker combination FITC (FL-1)-CD62L, PE (FL-2)-IFN-γ, and PE-Cy5 (FL-3)-CD8. A gate was set on lymphocytes, and the analysis was restricted to ca. 25,000 CD8 T cells by a second gate set on positive FL-3. FL-1 (ordinate) versus FL-2 (abscissa) log fluorescence intensities are shown for gated cells as dot plots, with 10,000 dots displayed. Percentages are indicated for CD62Lhi CD8 T cells (upper left quadrants) and for CD62Llo CD8 T cells with intracellular accumulation of IFN-γ (lower right quadrants). The isotype controls (PE-conjugated rat IgG1) are shown for cells stimulated with anti-CD3ɛ.
In phase 1 after infection (Fig. 6A, left panel), ca. 36% of the gated CD8 T cells were capable of expressing IFN-γ, and these cells displayed the CD62Llo phenotype. The few CD62Lhi cells (ca. 20%) were mostly refractory to stimulation by anti-CD3ɛ. In accordance with their CD44lo phenotype (recall Fig. 4), this finding is indicative of naive cells. It should be noted that IFN-γ expression discriminates between two qualitatively different subsets of CD8+ CD62Llo T cells. We concluded this from the finding that intensification of the triggering by higher doses of MAb anti-CD3ɛ did not increase the number of responding cells (not shown). After BMT with no infection (Fig. 6B, left panel), many more CD8 T cells in phase 1 (ca. 72%) displayed the CD62Lhi phenotype of resting cells and accordingly, most of these cells (ca. 63% of the CD8 T cells) were also refractory to stimulation by anti-CD3ɛ. CD8 T cells producing IFN-γ were less frequent than after infection (ca. 11% versus ca. 36%), but were also of the CD62Llo phenotype.
In phase 2 after infection (Fig. 6A, right panel), the percentage of CD62Lhi CD8 T cells was elevated (ca. 40% versus ca. 20% in phase 1) and the percentage of CD62Llo IFN-γ producers was still substantial, ca. 26%. Remarkably, unlike in phase 1, many of the CD62Lhi cells were now susceptible to stimulation by anti-CD3ɛ, as is indicated by the shift to a CD62Llo phenotype. This feature is consistent with the known loss of CD62L upon activation of quiescent memory CD8 T cells to memory effector cells (28). After BMT with no infection (Fig. 6B, right panel), many cells among the prevalent CD62Lhi population also showed a memory-type downregulation of CD62L in response to stimulation with anti-CD3ɛ, but only a few CD62Llo cells (ca. 6% of the CD8 T cells) were able to respond with production of IFN-γ.
In conclusion, infection alters the pulmonary CD8 T-cell population quantitatively and qualitatively. It increases the absolute and relative number of CD8 T cells that can respond to a CD3-TCR-mediated stimulus with the production of IFN-γ.
Phase 1 and phase 2 pulmonary infiltrate CD8 T cells protect against productive infection.
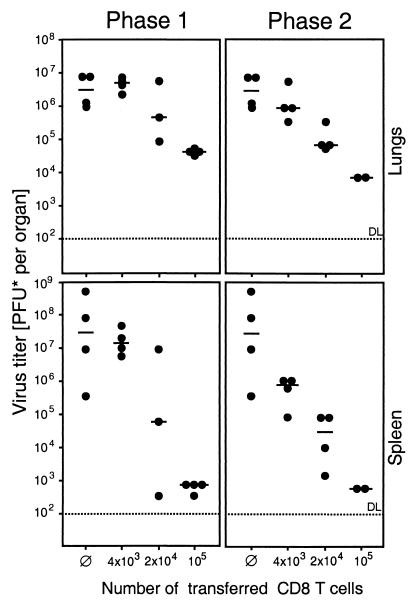
The finding that “stand-by” effector cells persist in the lungs for many months after clearance of the productive infection raised the question of their functional role, immunopathology or antiviral surveillance. Evidence in favor of antiviral surveillance was provided by adoptive transfer of immunomagnetically purified phase 1 and 2 pulmonary infiltrate CD8 T cells into immunocompromised and lethally infected indicator recipients. Pulmonary CD8 T cells derived from both phases controlled the acute infection in a dose-dependent manner not only at the site of origination, namely in the lungs, but also in the spleen (Fig. 7) and in other organs of the indicator recipients (not shown). Notably, the efficacy per cell was even somewhat higher in phase 2.
FIG. 7.
Comparison of the in vivo antiviral function of phase 1 and phase 2 CD8 T cells. Graded numbers of immunomagnetically purified pulmonary infiltrate CD8 T cells (phase 1, 4 weeks; phase 2, 12 weeks) were transferred by i.v. infusion into immunocompromised and infected indicator recipients. Infectious virus in lungs and spleen of the recipients was measured on day 12 after infection and cell transfer by a virus plaque assay. PFU*, PFU determined under conditions of centrifugal enhancement of infectivity. Dots represent individual transfer recipients. The median values are marked by a horizontal bar. The dotted line indicates the detection limit (DL) of the assay. Ø, positive control of virus replication in the absence of cell transfer.
In conclusion, phase 2 pulmonary infiltrate CD8 T cells can exert an antiviral effector function in vivo.
DISCUSSION
Since the time when Grundy et al. (14) postulated an immunopathogenesis triggered by CMV infection, the pathogenesis of CMV-IP has been a matter of ongoing debate. While a number of pros and cons have meanwhile been collected from clinical data and murine models (for reviews, see references 4 and 29), a systematic study of lung histopathology after CMV infection in the specific context of BMT was clearly missing. Murine models of GvH disease and mCMV infection have been used to advocate the immunopathogenesis hypothesis (14, 15, 46), although these models were by design unrelated to the particular conditions imposed by the kinetics of hematopoietic and lymphopoietic reconstitution after BMT. A model of allogeneic BMT in the rat confirmed the postulated correlation between GvH immunogenetics of the transplantation and CMV-IP but did not confirm immunological symptoms of a GvH disease (48) as it was proposed by the immunopathogenesis hypothesis. Likewise, we have evidence for murine CMV-IP caused in the absence of GvH disease by extensive virus replication resulting from a failure in antiviral CD8 T-cell control after BMT performed across an MHC class I difference (31, 33). In the present report, a murine model of syngeneic BMT was used to study the time course of CMV-associated histopathology of the lungs under conditions of endogenous reconstitution of an antiviral immune response. We have operationally defined two phases in the kinetics that will be discussed consecutively: phase 1 is characterized by pulmonary infiltrates and acute infection of the lungs, while phase 2 is characterized by the persistence of interstitial T cells during viral latency after clearance of productive infection.
Phase 1 scenario: CD8 T cells prevent disseminated viral pneumonia.
The acute phase of pulmonary mCMV infection after syngeneic BMT was characterized by a vigorous and preferential recruitment of reconstituted CD8 T cells that confined the infection by the formation of inflammatory foci. This process was independent of CD4 T cells. The infection of the lungs was moderate, with only ca. 0.04% (ca. 25,000 cells) of the lung stromal and parenchymal cells expressing viral IE1 protein at the peak of infection. At the same time, the number of CD8 T cells in the infiltrates was ca. 5 million, that is, ca. 200 CD8 T cells were present to control 1 infected cell. The actual ratio visible in the inflammatory foci was much lower, which is explained by the fact that infection was already controlled in many foci. The focal character of the inflammation as well as the correlation between CD8 T-cell infiltration and decline of the infection implied an antiviral function of the CD8 T cells. Activation of the CD8 T cells was indicated by blastoid morphology in the immunohistology, by a CD62Llo phenotype, and by a significant proportion of cells that produced IFN-γ upon CD3-TCR triggering.
Proof of the protective principle was provided by two complementary approaches: (i) CD8 T cells isolated from phase 1 infiltrates controlled the infection in cell transfer recipients and (ii) selective elimination of reconstituting CD8 T cells led to an enhancement of lung infection by a factor of 100, that is, ca. 4% of the lung cells, or, in absolute terms, ca. 2.5 million lung cells, were then infected. A disseminated IP with widening of alveolar septa and partial collapse of alveoli was seen also after combined depletion of CD4 and CD8 T cells, that is, in the absence of any T-cell infiltration. It is hence obvious that phase 1 CMV-IP does not represent a T-cell-driven immunopathology but is caused primarily by cytolytic infection, with a likely contribution of cytokines and chemokines secreted by infected cells as well as by alveolar macrophages and, possibly, by natural killer cells. Clinical observations in BMT recipients have implicated TH2-type cytokines and lack of T-cell-mediated cytotoxicity in the pathogenesis of CMV-IP (47). In a clinical setting, it is difficult to evaluate the contributions of these two parameters to the pathogenesis. We would propose that the failure in the CD8 T-cell control was decisive for the development of CMV-IP in these patients and that the TH2-dominated cytokine profile is an epiphenomenon associated with the CD8 T-cell deficiency. This interpretation is also in accordance with clinical cases of early CMV-IP occurring prior to engraftment and in the absence of GvH disease following T-cell depleted allogeneic BMT (7, 16, 30). In previous models of CMV-IP, reviewed and interpreted by J. Grundy (13), an apparent lack of correlation between virus titers and histopathology associated with T-cell infiltration was used as an argument in favor of the immunopathogenesis hypothesis. Clearly, in our model as well, the lung histopathology is visually dominated by the infiltrating cells, particularly at later stages of phase 1 when the number of infected cells had already declined due to the effect of antiviral CD8 T cells. It is also evident that antiviral treatment at that stage would not resolve the histopathological characteristics. Nonetheless, these infiltrates are protective in that they prevent extensive viral destruction of the tissue, a conclusion that is in accordance with the progressive CMV-IP observed in athymic nu/nu mice (46). The antiviral function of CD8 T cells also explains the benign course of pulmonary hCMV infection during earlier stages of AIDS (for a review, see reference 45). While J. Grundy proposed a lack of CD4-driven immunopathology as a result of CD4 T-cell depletion in the patients, we infer from our data obtained by selective depletion of the CD4 subset (recall Fig. 1 and 2) that residual CD8 T cells are likely to suffice for controlling the infection except in later stages of disease, when the numbers of CD8 T cells have also declined.
In conclusion, timely reconstitution of CD8 T cells is critical for the termination of acute infection and thus for the prevention of disseminated viral pneumonia.
Phase 2 scenario: persistence of pulmonary T cells after clearance of acute infection.
A striking feature is the persistence of functionally competent pulmonary T cells after clearance of productive mCMV infection, which is reminiscent of persistent lymphocytosis observed in survivors of clinical CMV-IP (5). This CMV-negative IP can be viewed as an “aged” CMV-IP in survivors in whom the antiviral response has largely prevented viral histopathology. In fact, the presence of high numbers of interstitial T cells per se indicated a pathological condition, whereas the alveolar architecture was apparently intact. With no further information, an association with CMV is impossible to recognize. The T cells in phase 2 differed from those in the inflammatory foci of phase 1 by the following features: (i) they were no longer organized in foci but were randomly distributed in the lung parenchyma, (ii) they were no longer lymphoblasts but were resting cells by morphological criteria, (iii) the proportion of CD8 T cells had declined, even though it was still elevated compared with that in normal lungs or time-matched lungs after BMT with no infection (19), and (iv) many of the CD8 T cells represented memory cells, as was indicated by their CD62Lhi phenotype in conjunction with responsiveness to triggering via their CD3-TCR complex. The latter property discriminates memory cells from naive cells, which are also CD62Lhi but are refractory to triggering by MAb anti-CD3ɛ. Notably, however, a high proportion of phase 2 CD8 T cells had maintained or possibly reacquired the CD62Llo phenotype, and a significant proportion of the CD8 T cells were able to respond by producing IFN-γ.
A remarkable set of experiments that are related to these findings have previously been reported by Tanaka et al. (51, 52). After clearance of acute mCMV infection in the lungs, a fulminant and rapidly fatal pneumonia was elicited by in vivo application of MAb anti-CD3ɛ. Since virus recurrence did not occur in the short period until death, only 24 to 48 h after injection of the MAb, it was obvious that the pneumonia was not caused by cytolytic virus replication. On the other hand, the pneumonia was CMV associated in that the effect was not observed with naive mice. The cause of pneumonia then was a “cytokine storm,” which included IFN-γ and tumor necrosis factor alpha (51), which in turn induced nitric oxide formation via expression of inducible nitric oxide synthetase (52). In addition, we propose also direct damage to lung tissue by elicited cytolytic effector function, such as perforin release, from memory-effector CD8 T cells. From the facts that virus replication was clearly not involved in this acute disease and that the pneumonia was prevented by immunosuppression, the authors believed they had provided direct evidence for an immunopathogenesis of CMV-IP. In order to explain the lack of disease in naive mice, they proposed a role for CMV infection in modulating the responsiveness of pulmonary T cells to stimulation via the CD3ɛ molecule of the CD3-TCR complex.
We have here documented that the proposed modulation of T-cell responsiveness does indeed occur as a result of CMV infection. What has actually happened in the experiments performed by Tanaka and colleagues? In essence, they have observed the in vivo consequences of polyclonal T-cell stimulation via the CD3-TCR complex, that is, the in vivo correlate of the in vitro CD3ɛ-redirected lysis assay and the CD3ɛ-mediated induction of IFN-γ synthesis. A quantitative comparison between phase 2 pulmonary infiltrates of infected and uninfected BMT recipients makes it obvious why pneumonia is induced preferentially in the infected group: the extrapolated number of CD8 T cells at 3 months after BMT and infection was ca. 0.5 million per lung, of which 26.4% (recall Fig. 6) were able to respond with IFN-γ synthesis, that is, ca. 105 CD8 T cells had the potential to respond to in vivo polyclonal stimulation. For BMT with no infection, the extrapolated number of CD8 T cells was ca. 40,000 per lung, of which only 5.4% were able to respond with IFN-γ synthesis, that is, only ca. 2000 CD8 T cells had the potential to respond to in vivo polyclonal stimulation. In summary, mCMV infection in our model of syngeneic BMT has amplified the number of functionally competent CD8 T cells residing in the lungs by a factor of 50! In addition, cytokine production by CD4 T cells will also be stimulated. That a simultaneous polyclonal in vivo triggering of the effector functions of so many T cells by MAb anti-CD3ɛ will result in an acute cytokine syndrome and associated pneumonia is evident. Was this now the proof of an immunopathogenesis of late CMV-IP?
Stand-by effector cells: guardians of CMV latency?
The experiments by Tanaka et al. (51, 52) as well as the data presented here have identified a powerful CMV-amplified response potential of the pulmonary CD8 T-cell population, and this indeed entails a risk of immunopathogenesis. However, disease will develop only if the effector functions are called up by polyclonal stimulation. At the moment, we see no disease correlate for such polyclonal in vivo triggering via the CD3-TCR complex, but there may be risk factors that could elicit immunopathogenesis mediated by the tissue-resident T cells. After allogeneic BMT, recruitment of antiviral CD8 T cells into the lungs by CMV-associated chemokines might indeed also attract GvH-reactive donor T cells, as was implied by the immunopathogenesis hypothesis (14). However, there is a major problem with this idea: while the statistical correlation between clinical GvH disease and human CMV-IP is undoubted, owing to the Minnesota Bone Marrow Transplantation Program study published in 1986 (27), the authors of this study have specifically emphasized that GvH disease preceded symptomatic hCMV infection by more than a month. One may therefore rather propose that GvH disease causes lung damage, which predisposes the lungs to fulminant CMV infection resulting in CMV-IP.
What else can be the physiological role of the persisting pulmonary T cells? An evident hint for answering this question was given by the antiviral efficacy of phase 2 CD8 T cells in adoptive transfer recipients. We propose that these cells represent tissue-resident “stand-by” effector cells ready for eliminating latently infected lung cells before virus recurrence, as soon as reactivation of viral gene expression has led to the presentation of antigenic peptides. There is clear evidence in support of an immunosurveillance function of T cells in maintaining CMV latency in general and specifically also in the lungs. We have shown previously in the murine model that the lungs carry a particularly high load of latent mCMV genome that entails a high statistical risk of intermittent transcriptional reactivation (3, 37, 49). However, infectious virus remained undetectable (24) unless an immunoablative treatment was performed (22–24, 49). These experiments have also demonstrated that recurrent virus can indeed originate from lung tissue and does not have to be imported from a distant site (23). Notably, as shown by Polic et al. (34), selective depletion of lymphocyte subsets revealed a redundant and hierarchical control of mCMV latency, with CD8 T cells being the principal subset.
In a way, the stand-by effector cells are reminiscent of any ordinary memory T cell, with the notable exception that they stay long term at the extralymphoid tissue site and that a significant subpopulation display the CD62Llo phenotype of acutely sensitized memory-effector cells, as opposed to the CD62Lhi phenotype of quiescent memory T cells in lymphoid tissues. Taken together, both features are likely to reflect a frequent engagement of CD8 T cells in antiviral responses that keeps them busy and causes them to reside long term in the lungs. Analysis of viral transcription in latently infected lungs has in fact revealed a focal and random transcription of the immediate-early gene ie1, with a frequency of ca. 10 transcriptional events per lung at any moment in time (22, 23). If this transcription leads to presentation of antigenic peptides, specifically of the immunodominant ie1-encoded nonapeptide YPHFMPTNL (for a review, see reference 36), only a few CD8 T cells out of the large pool of stand-by memory-effector cells would be needed to terminate primordial reactivation. Such a limited and local delivery of effector functions would not be associated with any notable tissue damage. We propose that transient but iterative presentation of antigenic peptides based on the observed random transcriptional activity (22) accounts for frequent pulses of memory T-cell stimulation. Such a mechanism explains the persistence of pulmonary infiltrate CD8 T cells in latently infected lungs.
Conclusion.
The murine model presented here has given experimental evidence against an immunopathogenesis of primary CMV-IP. In the acute phase, protective antiviral CD8 T cells confine viral replication to inflammatory foci and eventually terminate productive infection of the lungs. Disseminated CMV-IP is caused by extensive cytolytic infection in the absence of CD8 T cells and with no pathogenetic involvement of CD4 T cells. After resolution of productive infection, stand-by memory-effector cells remain in the lungs as guardians of viral latency. Their elimination by secondary immunoablative events (23, 34) can result in virus recurrence and late CMV disease. In addition, the persistence of tissue-resident memory-effector cells in the lungs entails a risk of immunopathogenesis after unspecific polyclonal triggering of the T-cell effector functions under disease conditions that still need to be defined.
ACKNOWLEDGMENTS
We thank Ronda Cardin (at the time of this study at St. Jude Children's Research Hospital, Memphis, Tenn.) for her detailed protocol of intracellular IFN-γ staining after polyclonal stimulation. We appreciated the expert technical assistance of Doris Thomas and Doris Dreis. Claudia Trummer helped by searching literature.
Support was provided by the Deutsche Forschungsgemeinschaft, Sonderforschungsbereich 490, individual project B1, “Immune control of latent cytomegalovirus infection,” and Sonderforschungsbereich 432, individual project A10, “Influence of cytomegalovirus infection on the risk of leukaemia relapse after bone marrow transplantation.”
REFERENCES
- 1.Alterio de Goss M, Holtappels R, Steffens H-P, Podlech J, Angele P, Dreher L, Thomas D, Reddehase M J. Control of cytomegalovirus in bone marrow transplantation chimeras lacking the prevailing antigen-presenting molecule in recipient tissues rests primarily on recipient-derived CD8 T cells. J Virol. 1998;72:7733–7744. doi: 10.1128/jvi.72.10.7733-7744.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashton-Rickardt P G, Opferman J T. Memory T lymphocytes. Cell Mol Life Sci. 1999;56:69–77. doi: 10.1007/s000180050007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balthesen M, Messerle M, Reddehase M J. Lungs are a major organ site of cytomegalovirus latency and recurrence. J Virol. 1993;67:5360–5366. doi: 10.1128/jvi.67.9.5360-5366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barry, S. M., M. A. Johnson, and G. Janossy. Cytopathology or immunopathology? The puzzle of cytomegalovirus pneumonitis revisited. Bone Marrow Transplant., in press. [DOI] [PMC free article] [PubMed]
- 5.Chien S M, Chan C K, Kasupski G, Chamberlain D, Fyles G, Messner H. Long-term sequelae after recovery from cytomegalovirus pneumonia in allogeneic bone marrow transplant recipients. Chest. 1992;101:1000–1004. doi: 10.1378/chest.101.4.1000. [DOI] [PubMed] [Google Scholar]
- 6.Cobbold S P, Jayasuriya A, Nash A, Prospero T D, Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature (London) 1984;312:548–550. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- 7.Couriel D, Canosa J, Engler H, Collins A, Dunbar C, Barrett A J. Early reactivation of cytomegalovirus and high risk of interstitial pneumonitis following T-depleted BMT for adults with hematological malignancies. Bone Marrow Transplant. 1996;18:347–353. [PubMed] [Google Scholar]
- 8.DeGrendele H C, Estess P, Siegelman M H. Requirement for CD44 in activated T cell extravasation into an inflammatory site. Science. 1997;278:672–675. doi: 10.1126/science.278.5338.672. [DOI] [PubMed] [Google Scholar]
- 9.Dobonici M, Podlech J, Steffens H-P, Maiberger S, Reddehase M J. Evidence against a key role for transforming growth factor-β1 in cytomegalovirus-induced bone marrow aplasia. J Gen Virol. 1998;79:867–876. doi: 10.1099/0022-1317-79-4-867. [DOI] [PubMed] [Google Scholar]
- 10.Einsele H, Hebart H, Bokemeyer C, Kanz L, Jahn G, Müller C A. Cytomegalovirus infection following hematopoietic stem cell transplantation. Monogr Virol. 1998;21:106–118. [Google Scholar]
- 11.Enright H, Haake R, Weisdorf D, Ramsay N, McGlave P, Kersey J, Thomas W, McKenzie D, Miller W. Cytomegalovirus pneumonia after bone marrow transplantation: risk factors and response to therapy. Transplantation. 1993;55:1339–1346. doi: 10.1097/00007890-199306000-00024. [DOI] [PubMed] [Google Scholar]
- 12.Gallatin W M, Weissman I L, Butcher E C. A cell surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983;304:30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- 13.Grundy J E. Virologic and pathogenetic aspects of cytomegalovirus infection. Rev Infect Dis. 1990;12(Suppl. 7):S711–S719. doi: 10.1093/clinids/12.supplement_7.s711. [DOI] [PubMed] [Google Scholar]
- 14.Grundy J E, Shanley J D, Griffiths P D. Is cytomegalovirus interstitial pneumonitis in transplant recipients an immunopathological condition? Lancet. 1987;ii:996–999. doi: 10.1016/s0140-6736(87)92560-8. [DOI] [PubMed] [Google Scholar]
- 15.Grundy J E, Shanley J D, Shearer G M. Augmentation of graft-versus-host reaction by cytomegalovirus infection resulting in interstitial pneumonitis. Transplantation. 1985;39:548–553. doi: 10.1097/00007890-198505000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Hertenstein B, Hampl W, Bunjes D, Wiesneth M, Duncker C, Koszinowski U H, Heimpel H, Arnold R, Mertens T. In vivo/ex vivo T cell depletion for GvHD prophylaxis influences onset and course of active cytomegalovirus infection and disease after BMT. Bone Marrow Transplant. 1995;15:387–393. [PubMed] [Google Scholar]
- 17.Ho M. Epidemiology of cytomegalovirus infection. Rev Infect Dis. 1990;12:701–710. doi: 10.1093/clinids/12.supplement_7.s701. [DOI] [PubMed] [Google Scholar]
- 18.Ho M. Cytomegaloviruses. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. New York, N.Y: Churchill Livingstone; 1995. pp. 1351–1364. [Google Scholar]
- 19.Holtappels R, Podlech J, Geginat G, Steffens H-P, Thomas D, Reddehase M J. Control of murine cytomegalovirus in the lungs: relative but not absolute immunodominance of the immediate-early 1 nonapeptide during the antiviral cytolytic T-lymphocyte response in pulmonary infiltrates. J Virol. 1998;72:7201–7212. doi: 10.1128/jvi.72.9.7201-7212.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holtappels R, Thomas D, Podlech J, Geginat G, Steffens H-P, Reddehase M J. The putative natural killer decoy early gene m04 (gp34) of murine cytomegalovirus encodes an antigenic peptide recognized by protective antiviral CD8 T cells. J Virol. 2000;74:1871–1884. doi: 10.1128/jvi.74.4.1871-1884.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koszinowski U H, Reddehase M J, Jonjic S. The role of T-lymphocyte subsets in the control of cytomegalovirus infection. In: Thomas D B, editor. Viruses and the cellular immune response. New York, N.Y: Marcel Dekker, Inc.; 1993. pp. 429–445. [Google Scholar]
- 22.Kurz S K, Rapp M, Steffens H-P, Grzimek N K A, Schmalz S, Reddehase M J. Focal transcriptional activity of murine cytomegalovirus during latency in the lungs. J Virol. 1999;73:482–494. doi: 10.1128/jvi.73.1.482-494.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurz S K, Reddehase M J. Patchwork pattern of transcriptional reactivation in the lungs indicates sequential checkpoints in the transition from murine cytomegalovirus latency to recurrence. J Virol. 1999;73:8612–8622. doi: 10.1128/jvi.73.10.8612-8622.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurz S K, Steffens H-P, Mayer A, Harris J R, Reddehase M J. Latency versus persistence or intermittent recurrences: evidence for a latent state of murine cytomegalovirus in the lungs. J Virol. 1997;71:2980–2987. doi: 10.1128/jvi.71.4.2980-2987.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ljungman P. Cytomegalovirus interstitial pneumonia in autologous bone marrow transplant recipients. Bone Marrow Transplant. 1994;13:209–212. [PubMed] [Google Scholar]
- 26.Meyers J D, Flournoy N, Thomas E D. Risk factors for cytomegalovirus infection after human marrow transplantation. J Infect Dis. 1986;153:478–488. doi: 10.1093/infdis/153.3.478. [DOI] [PubMed] [Google Scholar]
- 27.Miller W, Flynn P, McCullough J, Balfour H H, Jr, Goldman A, Haake R, McGlave P, Ramsay N, Kersey J. Cytomegalovirus infection after bone marrow transplantation: an association with acute graft-v-host disease. Blood. 1986;67:1162–1167. [PubMed] [Google Scholar]
- 28.Mobley J L, Rigby S M, Dailey M O. Regulation of adhesion molecule expression by CD8 T cells in vivo. II. Expression of L-selectin (CD62L) by memory cytolytic T cells responding to minor histocompatibility antigens. J Immunol. 1994;153:5443–5452. [PubMed] [Google Scholar]
- 29.Morris D J. Cytomegalovirus pneumonia—a consequence of immunosuppression and pre-existing lung damage rather than immunopathology? Respir Med. 1993;87:345–349. doi: 10.1016/0954-6111(93)90047-4. [DOI] [PubMed] [Google Scholar]
- 30.Nagler A, Elishoov H, Kapelushnik Y, Breuer R, Or R, Engelhard D. Cytomegalovirus pneumonia prior to engraftment following T-cell depleted bone marrow transplantation. Med Oncol. 1994;11:127–132. doi: 10.1007/BF02999860. [DOI] [PubMed] [Google Scholar]
- 31.Oettel O. Histopathology of murine cytomegalovirus infection after MHC class-I disparate bone marrow transplantation. M.D. thesis. Mainz, Germany: Johannes Gutenberg University; 2000. [Google Scholar]
- 32.Podlech J, Holtappels R, Wirtz N, Steffens H-P, Reddehase M J. Reconstitution of CD8 T cells is essential for the prevention of multiple-organ cytomegalovirus histopathology after bone marrow transplantation. J Gen Virol. 1998;79:2099–2104. doi: 10.1099/0022-1317-79-9-2099. [DOI] [PubMed] [Google Scholar]
- 33.Podlech J, Steffens H-P, Holtappels R, Mayer A, Alterio de Goss M, Oettel O, Wirtz N, Maiberger S, Reddehase M J. Cytomegalovirus pathogenesis after experimental bone marrow transplantation. Monogr Virol. 1998;21:119–128. [Google Scholar]
- 34.Polic B, Hengel H, Krmpotic A, Trgovcich J, Pavic I, Lucin P, Jonjic S, Koszinowski U H. Hierarchical and redundant lymphocyte subset control precludes cytomegalovirus replication during latent infection. J Exp Med. 1998;188:1047–1054. doi: 10.1084/jem.188.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prussin C, Metcalfe D D. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J Immunol Methods. 1995;188:117–128. doi: 10.1016/0022-1759(95)00209-x. [DOI] [PubMed] [Google Scholar]
- 36.Reddehase, M. J. The immunogenicity of human and murine cytomegaloviruses. Curr. Opin. Immunol., in press. [DOI] [PubMed]
- 37.Reddehase M J, Balthesen M, Rapp M, Jonjic S, Pavic I, Koszinowski U H. The conditions of primary infection define the load of latent viral genome in organs and the risk of recurrent cytomegalovirus disease. J Exp Med. 1994;179:185–193. doi: 10.1084/jem.179.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reddehase M J, Mutter W, Koszinowski U H. In vivo application of recombinant interleukin 2 in the immunotherapy of established cytomegalovirus infection. J Exp Med. 1987;165:650–656. doi: 10.1084/jem.165.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reddehase M J, Mutter W, Münch K, Bühring H-J, Koszinowski U H. CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J Virol. 1987;61:3102–3108. doi: 10.1128/jvi.61.10.3102-3108.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reddehase M J, Weiland F, Münch K, Jonjic S, Lüske A, Koszinowski U H. Interstitial murine cytomegalovirus pneumonia after irradiation: characterization of cells that limit viral replication during established infection of the lungs. J Virol. 1985;55:264–273. doi: 10.1128/jvi.55.2.264-273.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reusser P, Fisher L D, Buckner C D, Thomas E D, Meyers J D. Cytomegalovirus infection after autologous bone marrow transplantation: occurrence of cytomegalovirus disease and effect on engraftment. Blood. 1990;75:1888–1894. [PubMed] [Google Scholar]
- 42.Reusser P, Riddell S R, Meyers J D, Greenberg P D. Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood. 1991;78:1373–1380. [PubMed] [Google Scholar]
- 43.Riddell S R. Pathogenesis of cytomegalovirus pneumonia in immunocompromised hosts. Semin Respir Infect. 1995;10:199–208. [PubMed] [Google Scholar]
- 44.Riddell S R, Watanabe K S, Goodrich J M, Li C R, Agha M E, Greenberg P D. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257:238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 45.Salomon N, Perlman D C. Cytomegalovirus pneumonia. Semin Respir Infect. 1999;14:353–358. [PubMed] [Google Scholar]
- 46.Shanley J D, Thrall R S, Forman S J. Murine cytomegalovirus replication in the lungs of athymic BALB/c nude mice. J Infect Dis. 1997;175:309–315. doi: 10.1093/infdis/175.2.309. [DOI] [PubMed] [Google Scholar]
- 47.Sparrelid E, Emanuel D, Fehninger T, Andersson U, Andersson J. Interstitial pneumonitis in bone marrow transplant recipients is associated with local production of TH2-type cytokines and lack of T cell-mediated cytotoxicity. Transplantation. 1997;63:1782–1789. doi: 10.1097/00007890-199706270-00013. [DOI] [PubMed] [Google Scholar]
- 48.Stals F S, Steinhoff G, Wagenaar S S, van Breda Vriesman J P, Haverich A, Dormans P, Moeller F, Bruggeman C A. Cytomegalovirus induces interstitial lung disease in allogeneic bone marrow transplant recipient rats independent of acute graft-versus-host response. Lab Investig. 1996;74:343–352. [PubMed] [Google Scholar]
- 49.Steffens H-P, Kurz S, Holtappels R, Reddehase M J. Preemptive CD8 T-cell immunotherapy of acute cytomegalovirus infection prevents lethal disease, limits the burden of latent viral genomes, and reduces the risk of virus recurrence. J Virol. 1998;72:1797–1804. doi: 10.1128/jvi.72.3.1797-1804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sullivan K M, Meyers J D, Flournoy N, Storb R, Thomas E D. Early and late interstitial pneumonia following human bone marrow transplantation. Int J Cell Cloning. 1986;4(Suppl. 1):107–121. doi: 10.1002/stem.5530040712. [DOI] [PubMed] [Google Scholar]
- 51.Tanaka K, Koga Y, Lu Y-Y, Zhang X-Y, Wang Y, Kimura G, Nomoto K. Murine cytomegalovirus-associated pneumonitis in the lungs free of the virus. J Clin Investig. 1994;94:1019–1025. doi: 10.1172/JCI117415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanaka K, Nakazawa H, Okada K, Umezawa K, Fukuyama N, Koga Y. Nitric oxide mediates murine cytomegalovirus-associated pneumonitis in lungs that are free of the virus. J Clin Investig. 1997;100:1822–1830. doi: 10.1172/JCI119710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tedder T F, Steeber D A, Chen A, Engel P. The selectins: vascular adhesion molecules. FASEB J. 1995;9:866–873. [PubMed] [Google Scholar]
- 54.Tedder T F, Steeber D A, Pizcueta P. L-Selectin-deficient mice have impaired leukocyte recruitment into inflammatory sites. J Exp Med. 1995;181:2259–2264. doi: 10.1084/jem.181.6.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wingard J R, Chen D Y, Burns W H, Fuller D J, Braine H G, Yeager A M, Kaiser H, Burke P J, Graham M L, Santos G W. Cytomegalovirus infection after autologous bone marrow transplantation with comparison to infection after allogeneic bone marrow transplantation. Blood. 1988;71:1432–1437. [PubMed] [Google Scholar]
- 56.Zaia J A. Epidemiology and pathogenesis of cytomegalovirus disease. Semin Hematol. 1990;27(Suppl. 1):5–10. [PubMed] [Google Scholar]