Abstract
The actin-based cytoskeleton is considered a fundamental driving force for cell differentiation and development. Destrin (Dstn), a member of the actin-depolymerizing factor family, regulates actin dynamics by treadmilling actin filaments and increasing globular actin pools. However, the specific developmental roles of dstn have yet to be fully elucidated. Here, we investigated the physiological functions of dstn during early embryonic development using Xenopus laevis as an experimental model organism. dstn is expressed in anterior neural tissue and neural plate during Xenopus embryogenesis. Depleting dstn promoted morphants with short body axes and small heads. Moreover, dstn inhibition extended the neural plate region, impairing cell migration and distribution during neurulation. In addition to the neural plate, dstn knockdown perturbed neural crest cell migration. Our data suggest new insights for understanding the roles of actin dynamics in embryonic neural development, simultaneously presenting a new challenge for studying the complex networks governing cell migration involving actin dynamics.
Keywords: Destrin, F-actin, Neural crest, Neurulation, Xenopus laevis
INTRODUCTION
Actin filaments directly or indirectly participate in various cellular processes: Maintaining cell shape and polarity, migration, division, organelle transport, vesicle trafficking, axonal growth, muscle contraction, and phagocytosis (Svitkina, 2018 Jan 2, Winder and Ayscough, 2005). Moreover, participation largely depends on the assembly, disassembly, and treadmilling of actin filaments, termed “actin dynamics” (Bernstein and Bamburg, 2010). The family of actin-depolymerizing factors (ADFs) is primarily known as a regulator of actin dynamics, as they facilitate actin filament severing and depolymerization (Bamburg, 1999, Bamburg and O'Neil, 2002). The ADFs family members are evolutionarily conserved and are found in all eukaryotes (Maciver and Hussey, 2002). In addition, most mammals express 3 isoforms of ADF proteins, that is, destrin (DSTN, also known as ADF), cofilin-1 (CFL1), and cofilin-2 (CFL2). DSTN and CFL1 are most abundant in nonmuscle tissues, whereas CFL2 is predominantly expressed in muscles (Bamburg, 1999).
These dynamizing actin proteins play diverse roles in health and disease. Actin rod formation activity of ADFs engages in the occurrence of several neurodegenerative diseases in the early stages (Bernstein et al., 2006). Furthermore, it has been suggested that DSTN and CFL1 are involved in cytokinesis and gametogenesis, contributing to cancers and infertility in humans (Bamburg and O'Neil, 2002). CFL1 knockout mice are embryonic lethal at E11.5 to 12.5 and present defects in neural tube closure and neuronal cell migration, whereas DSTN knockout mice remain alive but exhibit abnormal cornea thickening, leading to postnatal blindness (Bellenchi et al., 2007, Ikeda et al., 2003, Tahtamouni et al., 2013). CFL1 appears essential for neuronal development and differentiation, but the DSTN requirement has yet to be studied. Thus, it is necessary to understand the developmental roles of DSTN to establish the exquisiteness of the ADF as an actin dynamics regulator in physiological processes.
Therefore, we investigated the developmental roles of dstn in embryogenesis, particularly during neurulation and neural crest development, using Xenopus laevis. We used the morpholino oligonucleotide (MO)-mediated knockdown of dstn in Xenopus-developing embryos. Our results indicated that dstn mediates the extension of the neural plate region by modulating actin dynamics, and dstn is involved in neural crest cell migration during Xenopus embryogenesis without affecting neural crest gene expression.
MATERIALS AND METHODS
Xenopus Maintenance and Embryo Manipulation
Adult Xenopus laevis were obtained from the Korean Xenopus Resource Center for Research and maintained in plastic aquarium tanks circulating dechlorinated water at 18 °C (recommended by the Institutional Review Board of Kyungpook National University, Korea). Ovulation was induced in Xenopus females by injecting 1,000 International units of human chorionic gonadotrophin, and eggs were fertilized in vitro and manipulated for further experiments, as described previously (Kim et al., 2018).
Experiments were strictly conducted according to the Animal Care and Use Committee guidelines, consistent with international laws and policies (National Institute of Health) Guide for the Care and Use of Laboratory Animals, publication no. 85-23, 1985). The Institutional Review Board of Kyungpook National University in Korea approved the experimental use of amphibians (2021-0017). All research group members were trained to care for and use experimental organisms appropriately.
Plasmid Construction, MO Design, and Microinjections
For messenger ribonucleic acid (mRNA) injection or in situ hybridization using RNA probes, dstn was designed based on National Center for Biotechnology Information and Xenbase sequences. For mRNA synthesis, plasmids were constructed using a pCS107 vector with restriction enzymes BamH1 and Xho1. The RNA probe sequence containing 200 to 300 base pairs of full-length genes was labeled with digoxigenin (DIG) and inserted into a T-easy vector (pGEM, Promega). Next, the pCS107 vectors and T-easy were linearized by Apa1, a restriction enzyme. Then, the capped mRNAs were synthesized using the SP6 mMessage mMachine kit (Invitrogen). Finally, DIG-labeling RNA probes were generated using the T7 mMessage mMachine kit (Invitrogen). The dstn MO is 25 nucleotides long and has the following base composition: 5′-TCCGAACACCTGATGCCATTGTTGA-3′. The control MO has the following sequence: 5′-CCTCTTACCTCAGTTACAATTTATA-3′. The MO was obtained from Gene Tools. For the rescue experiments, mutant constructs (dstn*) not recognized by dstn MO were subcloned into the pCS107 vector using the abovementioned method. Finally, MOs and mRNAs were injected into the fertilized Xenopus embryos.
Whole-Mount In Situ Hybridization
Xenopus embryos were fixed in MEMFA (4% paraformaldehyde, 0.1 M (3-(N-morpholino) propanesulfonic acid) [pH 7.4], 1 mM MgSO4, and 2 mM Ethylene glycol tetraacetic acid) at 4 °C overnight. The embryos were dehydrated before storage in 100% methanol at −20 °C, and whole-mount in situ hybridization (WISH) was performed as described previously (Kim et al., 2018). Probes were detected using an alkaline phosphatase-labeled anti-DIG antibody (1:1000, Roche) and Nitroblue tetrazolium dichloride/5-bromo-4-chloro-3-indolyl-phosphate staining solution (Roche).
Real-Time Quantitative Polymerase Chain Reaction
Total RNA was extracted from the Xenopus embryos, and complementary deoxyribonucleic acid was synthesized using the first strand complementary deoxyribonucleic acid synthesis kit (Takara). Furthermore, real-time quantitative polymerase chain reaction was performed with TB Green Premix Ex Taq (Takara) and specific primers (Table 1) using CFX Connect Real-Time PCR System (Bio-Rad).
Table 1.
The primer sequences for real-time PCR
| Gene | Forward primer | Reverse primer |
|---|---|---|
| odc | 5'-GCTTCTGGAGCGGGCAAAGGA-3' | 5'-CAACATGGAAACTCACACC-3′ |
| dstn | 5′- CGGGGGAAGCAACAAAACAG-3′ | 5′- CCCATTGCTTTTGGACACCTG-3′ |
Alcian Blue Staining
Xenopus embryos (NF. St. 49) were fixed in MEMFA and rehydrated in 50% Ethanol (EtOH). Then, the embryos were incubated in 0.04% Alcian blue (Sigma) staining solution (10 mM MgCl2 and 80 % EtOH) for 3 days. Next, the stained embryos were bleached using a bleaching solution (3% H2O2, 5% formamide, 0.5× saline sodium citrate) and washed in 0.1× trypsin to dissolve soft tissue. Finally, embryos were transferred into buffered EtOH (0.25× saline sodium citrate, 75% EtOH) for storage.
Immunofluorescence and Confocal Microscopy
Xenopus embryos were fixed with MEMFA, and Alexa Fluor 488 phalloidin (1:1000, Invitrogen) was used to visualize F-actin. Image processing and analysis were performed using an Olympus FV1200 confocal microscope.
Western Blotting
Embryonic lysates were prepared from embryos using lysis buffer (137 mM NaCl, 20 mM Tris-HCl, 20 mM NP-40, 10% glycerol) with 1 mM phenylmethylsulfonyl fluoride, 5 mM sodium vanadate, and 1 mM protease inhibitor cocktail (Roche). The proteins were loaded and separated via 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. The membrane was blocked using a blocking solution (5% skim milk) at room temperature for 1 h. The proteins were detected with monoclonal anti-Flag antibody (1:1,000, Abcam) and monoclonal anti-beta actin (1:1,000, Santa Cruz). The secondary antibodies used were anti-mouse Immunoglobin G Horseradish peroxidase-linked antibodies (1:2,000, Santa Cruz).
Quantification and Statistics
Confocal and optical microscope imaging data were analyzed using ImageJ software (National Institutes of Health) and Zen software (Zeiss), respectively. Statistical analyses were performed using GraphPad (Dotmatics) Prism 7. The results are presented as the mean ± standard deviation from at least 3 independent experiments (exact numbers are shown in graphs; n). The data were analyzed using Student's t-test, and the significance level was considered as *P < .05, **P < .01, ***P < .001, ****P < .0001.
RESULTS
dstn Is Expressed in Anterior Neural Tissue During Xenopus Embryogenesis
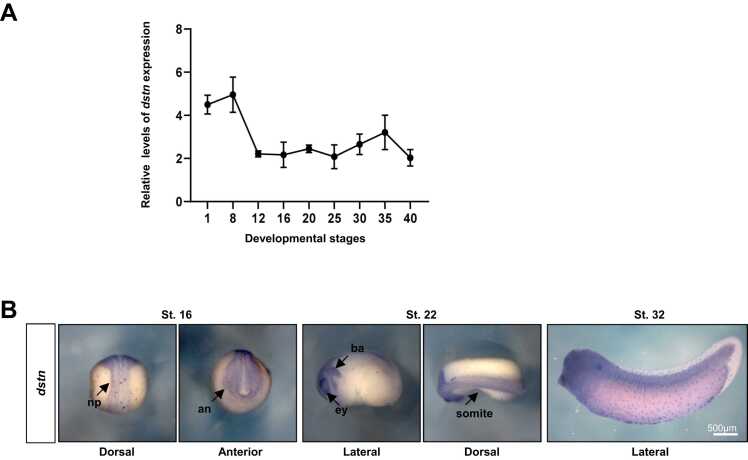
To better understand the developmental roles of dstn, we used real-time quantitative polymerase chain reaction to examine dstn expression at 9 landmark stages of Xenopus embryogenesis spanning blastula (Nieuwkoop and Farber stage 8; NF. St. 8), gastrula (NF. St. 12), neurula (NF. St. 16), early tailbud (NF. St. 22 and 26), and the late tailbud stage of development (NF. St. 32, 36, and 40) (Fig. 1A). Temporal expression data revealed that dstn was a maternal gene and expressed throughout each landmark stage of embryonic development (Fig. 1A). The tissue-specific expression of dstn analyzed by WISH was exhibited in neural tissues being localized in the anterior neural tissue, neural plate, eyes, branchial arch, and somites during the early tailbud development stages (NF. St. 16 and 22) (Fig. 1B). Furthermore, their expression, representing a dotted pattern, was detected in the Xenopus epithelium during the late tailbud development stage (NF. St. 32). The spatiotemporal expression pattern suggests that dstn has potential roles in neural tissue induction and neural crest development.
Fig. 1.
dstn is expressed in anterior neural tissues and the epidermis during Xenopus embryogenesis. (A) Temporal expression of dstn analyzed by real-time qPCR at 9 landmark stages of embryonic development. (B) Spatial expression patterns of dstn were examined by whole-mount in situ hybridization (WISH). np, neural plate; an, anterior neural; ey, eye; ba, branchial arch; qPCR, quantitative polymerase chain reaction.
dstn Morphants Showed Phenotypic Abnormalities in the Malformed Body Axis and Small-Sized Head
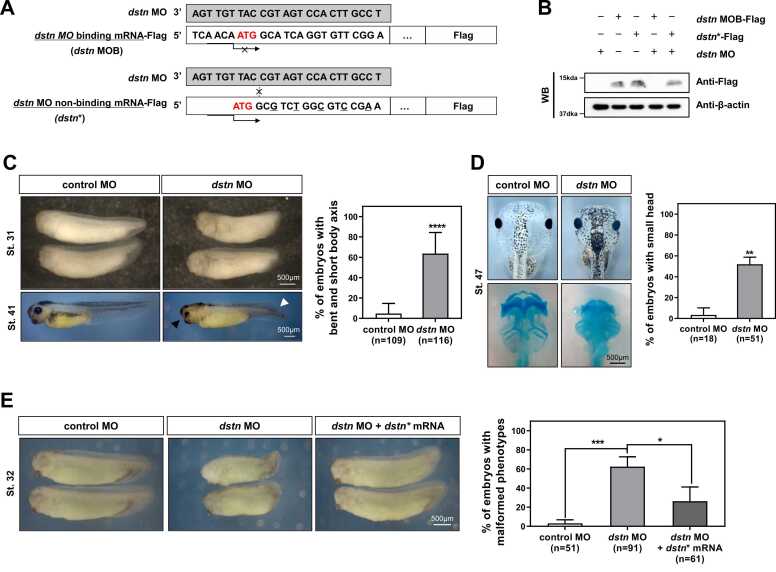
To delineate the roles of dstn during Xenopus embryogenesis, we performed a loss of function study by microinjecting MOs targeting the dstn gene. Additionally, we designed an MO binding construct (dstn MOB) and rescue construct (dstn*) that cannot bind with MO (Fig. 2A). The efficacy of dstn MO was analyzed using Western blotting. These results showed that dstn MOs effectively inhibited the translation of Flag-tagged dstn MOB mRNA constructs (Fig. 2B). However, this inhibition did not affect the Flag-tagged MO non-binding mRNA construct of dstn (dstn*) in which MOB sites were mutated to prevent specific MOB (Fig. 2B). The control MO (40 ng) or dstn specific MO (40 ng) was injected into the 2-cell staged Xenopus embryos. The dstn morphants exhibited developmental abnormalities at late tailbud stages (NF. St. 31 and 41) compared with control MO-injected embryos. Most dstn knockdown embryos had small-sized heads, delayed eye development, bent tails, and shortened body axes (Fig. 2C). The delayed eye development in the knockdown embryos was recovered during the later stages of development. However, the small-sized heads persisted in the morphant embryos (NF. St. 47) (Fig. 2D). Thus, we performed Alcian blue staining to observe the effects of dstn on cartilage formation. Our results showed that the overall cartilage size was reduced in dstn morphants, but there were no structural anomalies observed in dstn knockdown embryos (Fig. 2D). Further, to verify that the developmental abnormalities were specifically induced by dstn depletion, rescue experiments were performed by microinjecting dstn* mRNA with dstn MO. These results showed that the embryos effectively recovered the developmental anomalies observed in dstn morphants (Fig. 2E). Collectively, our data suggest dstn is crucial for Xenopus embryogenesis.
Fig. 2.
dstn knockdown embryos show developmental malformations: short body axis and small-sized heads. (A) A graphical representation indicating dstn morpholino oligonucleotide (MO), dstn MO binding mRNA (dstn MOB)-Flag, dstn MO non-binding mRNA (dstn*)-Flag construct that codes for destrin but possess a mutated 5′-UTR, which dstn MO cannot target. Co-delivery of the dstn MO and rescue mRNA produced the same phenotypes as wild-type dstn mRNA. (B) Western blotting confirmed the efficacy of dstn MO. Anti-β-actin was used as a loading control. (C) dstn MO (40 ng) was injected into the Xenopus embryos at the 2-cell stage, and the embryos were then fixed at developmental stages 31 and 41. White arrows represent the defects in tail development, and black arrows represent head and eye anomalies compared with control embryos. The statistical analysis showed that more than 60% of dstn morphants exhibited short and bent axes compared to control embryos. (D) Analysis of cartilage formation in dstn MO-injected embryos was conducted by fixing the embryos at stage 47, followed by staining with Alcian blue. The graphical representation indicated that more than 50% of dstn morphants showed small-sized heads compared to the control embryos. (E) Rescue experiments were performed by microinjecting the dstn* mRNA with dstn MO at the 2-cell stage of embryos. The developing embryos fixed at stage 32 showed that the phenotypic malformations of the small-sized head and bent axis were effectively recovered by co-injecting the dstn* and dstn MO. A graphical representation indicated that microinjection of dstn* mRNA with dstn MO considerably rescued phenotypic malformations as observed in dstn morphants. The significance levels are shown as *P < .05, **P < .01, ***P < .001, ****P < .0001, ns, not significant.
Knockdown of dstn Impaired the Neural Plate Extension and Inhibited Cell Migration During Xenopus Embryogenesis
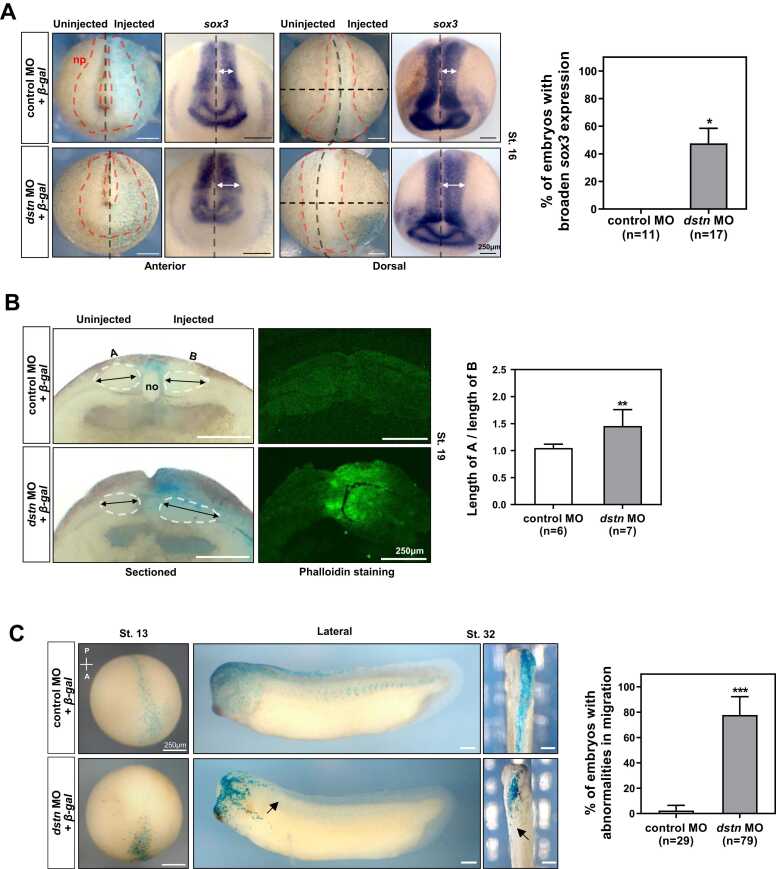
Considering the tissue-specific expression of dstn on the neural plate (Fig. 1B), we investigated the roles of dstn in neurulation during Xenopus development. To demonstrate the effect of dstn during neurulation, dstn MO was unilaterally microinjected into 1 blastomere of 2-cell staged Xenopus embryos, and the expression of neural plate marker sox3 was observed. As shown in the neural plate marker, the sox3 expression pattern resulted in a 20% wider neural plate on the MO-injected side of embryos compared with the MO-injected side of the control embryos (Fig. 3A). Furthermore, we cross-sectioned the embryos after dstn knockdown to observe the internal anatomy of neurula stage embryos (NF. St. 19). The gross internal structure showed a clear difference in distribution and position of mesodermal cells on the injected side (B) of the embryos compared with the uninjected side (A), although the notochord was formed normally in the control and morphant embryos (Fig. 3B). The polymerization and depolymerization of actin filaments are important in neurulation (Schoenwolf et al., 1988, Ybot-Gonzalez and Copp, 1999). Since dstn plays a crucial role in regulating actin dynamics (Bamburg, 1999), we examined the effect of dstn inhibition on actin filaments during neurulation using fluorescent phalloidin that can effectively bind to actin filaments (Baldwin et al., 2022, Yamagishi and Abe, 2015). Confocal microscopy revealed the accumulation of actin filaments on the dstn MO-injected side of developing embryos (Fig. 3B) compared with evenly distributed filaments on the uninjected side and in control embryos.
Fig. 3.
dstn mediates neural plate extension and perturbs cell migration. (A) dstn MO was coinjected with beta-galactosidase (β-gal) mRNA into 1 blastomere of 2-cell staged embryos, and the embryos were then fixed at the late neurula stage (NF. St.16). β-gal staining indicated the injected side of the embryos. The length of the white-headed arrows exhibits the neural plate expansion in the dstn MO-injected side of the embryos compared to the shorter white-headed arrows in the control MO-injected side, as shown in the anterior and dorsal views. A graphical representation showed that approximately 50% of the dstn MO-injected embryos exhibited wider neural plates than the control embryos (scale bar: 250 µm). (B) Cross-sectional analysis of neurula stage embryos (NF. St.19) showed a difference in mesoderm distribution and position between the MO-injected side (B) and the uninjected side (A). Further, phalloidin immunostaining exhibited an accumulation of actin filaments (increased intensity) on the injected side of the embryos compared with the uninjected side. A graph representing the mean intensity of phalloidin staining showed a significant change in phalloidin intensity in dstn morphants compared to the control. Black arrows represent variation in the distribution and position of mesodermal cells (scale bar: 250 µm), no (notochord). (C) The dstn MO and β-gal mRNA microinjections indicated that cell migration was highly limited. Black arrows represent the reduced and restricted cell migration in dorsal (only dorsal view for early-stage embryos) and lateral views of developing embryos. Cell migration was restricted only to the head regions in the dstn-depleted embryos compared to cell migration from the head to the tail axis in control embryos. Statistical analysis of dstn morphants and control embryos revealed significant cell migration restriction in dstn-depleted embryos compared to control embryos (scale bar: 250 µm). *P < .05, **P < .01, ***P < .001.
Neurulation is a dynamic process that requires harmonized changes in cell shape and movements (Wallingford, 2005); furthermore, it depends on convergent extension and apical constriction (Copp et al., 2003; Wallingford, 2005). To elucidate whether dstn-suppression-induced neural plate extension depends on cell movements, we injected dstn MO with beta-galactosidase (β-gal) mRNA into the D.1.2 blastomere of developing embryos. The D.1.2 blastomere continues to divide and migrate throughout the spinal cord from head to tail and eventually differentiates according to the fate of each cell (Bauer et al., 1994, Moody, 1989). As shown in Figure 3C, β-gal staining at early (NF. St. 13) and later (NF. St. 32) stages of embryogenesis indicated cell movements. The dorsal view of embryos in the gastrula stage (NF. St. 13) indicated restricted cell movements after dstn depletion compared with control embryos (Fig. 3C). Moreover, unlike the expression of β-gal as observed in control embryos (from head to tail), the expression of β-gal was limited in head regions of dstn morphants (NF. St. 32), suggesting the involvement of dstn in cell migration (Fig. 3C). Altogether, our findings showed that the extended neural plate region observed in the dstn MO-injected side of the embryos exhibited an accumulation of actin filaments compared to the evenly distributed filaments in the uninjected side of the embryos and control MO-injected embryos. Owing to the accumulation of actin filaments, we speculated that the extension of the neural plate might involve actin filaments and interfere with restricted cell migration in dstn morphants.
dstn is Required for Neural Crest Migration During Xenopus Embryogenesis
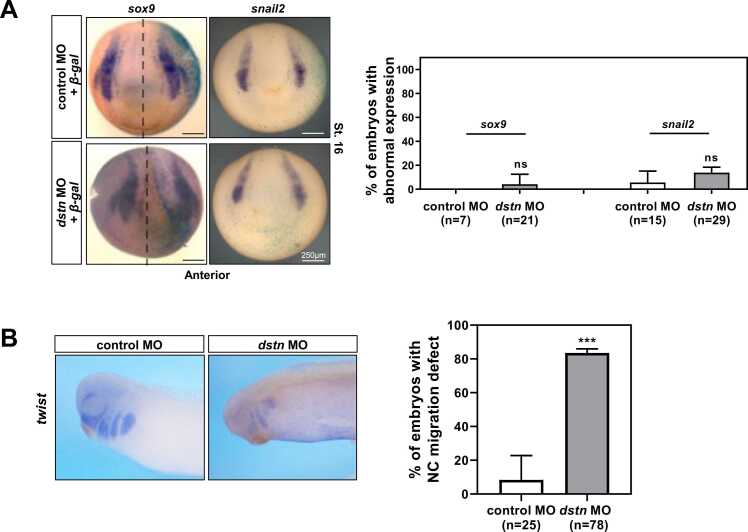
Given the conflicting results between mouse studies showing that CFL1 is important for neurogenesis but DSTN can have different functions (Gurniak et al., 2005) and the study showing that DSTN can affect Neural crest migration in chicken models (Vermillion et al., 2014), the roles of DSTN during neural crest development should be further studied. We wondered whether dstn could regulate the expression of neural crest genes, which are crucial for early development. Neural crest cells are vertebrate stem cells that migrate and differentiate to generate various cell types throughout early development (Almeida et al., 2010). Hence, we performed WISH using neural crest markers, snail2 and sox9. Following dstn depletion, snail2 and sox9 did not appear to be significantly affected, indicating that dstn does not affect neural crest specification (Fig. 4A). We discovered that dstn plays a role in cell migration during neurulation by affecting actin filament accumulation (Fig. 3) and these results raise the possibility that dstn may also be involved in neural crest migration. To examine the effect of dstn on neural crest migration, we performed WISH using embryos at stage 27 and the neural crest marker gene twist, which plays a role in the dissociation of neural crest cells by repressing E-cadherins in delaminating cells. Knockdown of twist led to upregulation of E-cadherins and inhibition of cell dispersion (Simões-Costa and Bronner, 2015). Our WISH twist data showed that dstn knockdown led to the downregulation of twist and inhibited neural crest migration. Therefore, our data indicate that dstn is not involved in neural crest specification but in migration.
Fig. 4.
dstn knockdown effects on neural crest specifiers during Xenopus embryogenesis. (A) Embryos were microinjected with dstn MO and β-gal mRNA and analyzed for neural crest-specific genes, sox9, and snail2. The uninjected side of the embryos served as an internal control. Statistical analysis showed no significant change in the expression of neural crest specifiers on the dstn MO-injected side of the embryos compared to the uninjected and control MO-injected sides (scale bar: 250 µm). (B) WISH analysis of twist in stage 27 embryos injected with control MO or dstn MO. Statistical analysis indicated that 80% of dstn morphants had defective neural crest migration compared to control MO-injected embryos (scale bar: 500 µm). nc, neural crest. ***P<.001, ns, not significant.
DISCUSSION
Numerous independent studies have demonstrated cell-type-specific roles for DSTN and CFL1 in embryonic development (Gurniak et al., 2005, Hotulainen et al., 2005, Zhang et al., 2016). DSTN and CFL1 mutant mice showed the significance of CFL1 in neural crest migration and morphogenesis of neural tubes, whereas roles for DSTN during embryonic development were unappreciated (Gurniak et al., 2005). In previous studies, CFL1 was considered indispensable for cell migration, and DSTN-depleted chick cells revealed minor roles during neural crest migration (Vermillion et al., 2014). In contrast, DSTN and CFL1 exhibited overlapping roles in mammalian nonmuscle cells regulating actin dynamics (Hotulainen et al., 2005).
To understand the specific roles and detailed mechanisms of DSTN compared to CFL1 in embryonic development, we investigated the localization and expression pattern of dstn during Xenopus laevis embryogenesis. The maternal expression of dstn and its localization in anterior neural tissues, including neural plate, brain, and eyes (Fig. 1), implies it performs significant roles during Xenopus development. The loss of function study revealed that dstn deficiency induced developmental abnormalities, such as small-sized heads, delayed eye development, bent tails, and shortened body axes (Fig. 2). However, the delayed eye development was recovered during the later stages of embryonic development, although the smaller-sized head persisted throughout the last stage embryos (Fig. 2). The most plausible explanation for the delayed eye development at stage 41 and presentation of normal eyes at stage 47 is the regrowth ability of Xenopus eyes in embryos and tadpoles (Kha et al., 2018). dstn might affect the eye size at early development stages; meanwhile, dstn might not be involved in maintaining eye growth and size at later developmental stages. The smaller eyes observed during the early stages regained their normal size due to the regrowth ability of Xenopus eyes. However, regarding head size, dstn appeared to possess long-term effects that could not be recovered.
Additionally, our data present dstn roles during neurulation. The neural plate area labeled with sox3 was expanded on the dstn MO-injected side of the embryos (Fig. 3A). Further, sox3 overexpression is responsible for neural plate expansion by increasing cell proliferation (Archer et al., 2011). Conversely, dstn knockdown resulted in restricted migration of cells, meaning there might be a possibility that cell accumulation led to sox3 overexpression, which ultimately resulted in the expansion of the neural plate—transverse histological sections of neural tissues at NF. St. 19 showed that the loss of dstn expanded the neural plate through an accumulation of F-actin. However, it did not affect neural tube formation (Fig. 3B). Likewise, the spatial expression of neural crest specifiers (sox9 and snail2) was not affected by dstn depletion (Fig. 4A). Contrastingly, the expression of twist (neural crest specifier) was downregulated in dstn morphants (Fig. 4B). The contradictory expression of sox9, snail2, and twist in dstn morphants is possibly due to the roles of sox9 and snail2 in neural crest specifications since they act upstream of twist. In comparison, twist is a neural crest marker that plays a role in neural crest cell migration. Further, twist is involved in dissociating neural crest cells by repressing E-cadherins in delaminating cells. Thus, twist knockdown led to E-cadherins upregulation and cell dispersion inhibition (Simões-Costa and Bronner, 2015). Therefore, our results showed that dstn is required for neural crest cell migration but does not interfere with neural crest specification and differentiation.
Cell migration is a critical process in living organisms, and actin dynamics play a crucial role in regulating cell migration (Schaks et al., 2019). Thus, we conducted a migration assay using β-gal and WISH analyses of the neural crest marker, twist, to determine the effect of dstn inhibition on cell migration during neurulation and neural crest development (Figs. 3C and 4B). These results demonstrate that dstn, a key ADF, modulates cell migration during embryogenesis in neural and neural crest development. Our data expand upon the previous results in mouse and chick cells, revealing the vital and independent roles of dstn during cell migration.
Additional pioneering studies have also shown the involvement of DSTN and CFL1 in neurodegenerative disorders, such as Alzheimer’s, by promoting the formation of hyperphosphorylated Tau and amyloid beta (Bamburg et al., 2010, Kang and Woo, 2019, Rush et al., 2018). Our data provided convincing evidence that small-sized heads and expanded neural plates in dstn morphants indicate the involvement of ADFs with neurodegenerative disorders.
In conclusion, by regulating cell migration, dstn plays a significant role during Xenopus embryogenesis, particularly in neurulation and neural crest development. Thus, our study suggests new insights for understanding the roles of actin dynamics in embryonic development.
Author Contributions
Youni Kim, Hyun Kyung Lee, and Kyeong-Yeon Park performed the experiments; Youni Kim, Tayaba Ismail, Hongchan Lee, and Hyun-Shik Lee performed data analysis and wrote the manuscript. Hyun Kyung Lee, Tayaba Ismail, HongYeol Ryu, and Dong-Hyung Cho reviewed and edited the manuscript. Taeg Kyu Kwon, Tae Joo Park, Taejoon Kwon, and Hyun-Shik Lee designed experiments, interpreted the results. Hong Yeol Ryu, Dong-Hyung Cho, and Hyun-Shik Lee critically analysed the manuscript and funding acquisition is provided by Hyun-Shik Lee. All the authors read and approved the final manuscript.
Declaration of Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Korea Environment & Technology Institute (KEITI) through the Core Technology Development Project for Environmental Diseases Prevention and Management, which was funded by the Korea Ministry of Environment (MOE) (grant number 2022003310001) and the National Research Foundation of Korea and the Ministry of Science & ICT (grant number 2021R1A2C1010408).
ORCID
Youni Kim: https://orcid.org/0000-0002-3052-6754
Hyun-Kyung Lee: https://orcid.org/0000-0003-4989-698X
Kyeong-Yeon Park: https://orcid.org/0009-0009-2509-9047
Tayaba Ismail: https://orcid.org/0000-0001-9970-4793
Hongchan Lee: https://orcid.org/0000-0001-9009-209X
Hong Yeoul Ryu: https://orcid.org/0000-0002-3367-9887
Dong-Hyung Cho: https://orcid.org/0000-0002-8859-0310
Taeg Kyu Kwon: https://orcid.org/0000-0003-1204-2059
Tae Joo Park: https://orcid.org/0000-0003-3176-177X
Taejoon Kwon: https://orcid.org/0000-0002-9794-6112
Hyun-Shik Lee: https://orcid.org/0000-0002-3837-9867
References
- Almeida A.D., Wise H.M., Hindley C.J., Slevin M.K., Hartley R.S., Philpott A. The F-box protein Cdc4/Fbxw7 is a novel regulator of neural crest development in Xenopus laevis. Neural Dev. 2010;5:1–20. doi: 10.1186/1749-8104-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer T.C., Jin J., Casey E.S. Interaction of Sox1, Sox2, Sox3 and Oct4 during primary neurogenesis. Dev. Biol. 2011;350:429–440. doi: 10.1016/j.ydbio.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin A.T., Popov I.K., Wallingford J.B., Chang C. Assays for apical constriction using the Xenopus model. Methods Mol. Biol. 2022;2438:415–437. doi: 10.1007/978-1-0716-2035-9_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamburg J.R. Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu. Rev. Cell Dev. Biol. 1999;15:185–230. doi: 10.1146/annurev.cellbio.15.1.185. [DOI] [PubMed] [Google Scholar]
- Bamburg J.R., Bernstein B.W., Davis R.C., Flynn K.C., Goldsbury C., Jensen J.R., Maloney M.T., Marsden I.T., Minamide L.S., Pak C.W., et al. ADF/Cofilin-actin rods in neurodegenerative diseases. Curr. Alzheimer Res. 2010;7:241–250. doi: 10.2174/156720510791050902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamburg J.R., O'Neil P.W. ADF/cofilin and actin dynamics in disease. Trends Cell Biol. 2002;12:598–605. doi: 10.1016/s0962-8924(02)02404-2. [DOI] [PubMed] [Google Scholar]
- Bauer D.V., Huang S., Moody S.A. The cleavage stage origin of Spemann's Organizer: analysis of the movements of blastomere clones before and during gastrulation in Xenopus. Development. 1994;120:1179–1189. doi: 10.1242/dev.120.5.1179. [DOI] [PubMed] [Google Scholar]
- Bellenchi G.C., Gurniak C.B., Perlas E., Middei S., Ammassari-Teule M., Witke W. N-cofilin is associated with neuronal migration disorders and cell cycle control in the cerebral cortex. Genes Dev. 2007;21:2347–2357. doi: 10.1101/gad.434307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein B.W., Bamburg J.R. ADF/cofilin: a functional node in cell biology. Trends Cell Biol. 2010;20:187–195. doi: 10.1016/j.tcb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein B.W., Chen H., Boyle J.A., Bamburg J.R. Formation of actin-ADF/cofilin rods transiently retards decline of mitochondrial potential and ATP in stressed neurons. Am. J. Physiol.-Cell Physiol. 2006;291:C828–C839. doi: 10.1152/ajpcell.00066.2006. [DOI] [PubMed] [Google Scholar]
- Copp A.J., Greene N.D., Murdoch J.N. The genetic basis of mammalian neurulation. Nat. Rev. Genet. 2003;4:784–793. doi: 10.1038/nrg1181. [DOI] [PubMed] [Google Scholar]
- Gurniak C.B., Perlas E., Witke W. The actin depolymerizing factor n-cofilin is essential for neural tube morphogenesis and neural crest cell migration. Dev. Biol. 2005;278:231–241. doi: 10.1016/j.ydbio.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Hotulainen P., Paunola E., Vartiainen M.K., Lappalainen P. Actin-depolymerizing factor and cofilin-1 play overlapping roles in promoting rapid F-actin depolymerization in mammalian nonmuscle cells. Mol. Biol. Cell. 2005;16:649–664. doi: 10.1091/mbc.E04-07-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S., Cunningham L.A., Boggess D., Hobson C.D., Sundberg J.P., Naggert J.K., Smith R.S., Nishina P.M. Aberrant actin cytoskeleton leads to accelerated proliferation of corneal epithelial cells in mice deficient for destrin (actin depolymerizing factor) Hum. Mol. Genet. 2003;12:1029–1036. doi: 10.1093/hmg/ddg112. [DOI] [PubMed] [Google Scholar]
- Kang D.E., Woo J.A. Cofilin, a master node regulating cytoskeletal pathogenesis in Alzheimer's disease. J. Alzheimers Dis. 2019;72:S131–S144. doi: 10.3233/JAD-190585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kha C.X., Son P.H., Lauper J., Tseng K.A.-S. A model for investigating developmental eye repair in Xenopus laevis. Exp. Eye Res. 2018;169:38–47. doi: 10.1016/j.exer.2018.01.007. [DOI] [PubMed] [Google Scholar]
- Kim Y., Jeong Y., Kwon K., Ismail T., Lee H.-K., Kim C., Park J.-W., Kwon O.-S., Kang B.-S., Lee D.-S., et al. Physiological effects of KDM5C on neural crest migration and eye formation during vertebrate development. Epigenet. Chromatin. 2018;11:72. doi: 10.1186/s13072-018-0241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciver S.K., Hussey P.J. The ADF/cofilin family: actin-remodeling proteins. Genome Biol. 2002;3:1–12. doi: 10.1186/gb-2002-3-5-reviews3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody S.A. Quantitative lineage analysis of the origin of frog primary motor and sensory neurons from cleavage stage blastomeres. J. Neurosci. 1989;9:2919–2930. doi: 10.1523/JNEUROSCI.09-08-02919.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush T., Martinez-Hernandez J., Dollmeyer M., Frandemiche M.L., Borel E., Boisseau S., Jacquier-Sarlin M., Buisson A. Synaptotoxicity in Alzheimer's disease involved a dysregulation of actin cytoskeleton dynamics through cofilin 1 phosphorylation. J. Neurosci. 2018;38:10349–10361. doi: 10.1523/JNEUROSCI.1409-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaks M., Giannone G., Rottner K. Actin dynamics in cell migration. Essays Biochem. 2019;63:483–495. doi: 10.1042/EBC20190015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenwolf G.C., Folsom D., Moe A. A reexamination of the role of microfilaments in neurulation in the chick embryo. Anat. Record. 1988;220:87–102. doi: 10.1002/ar.1092200111. [DOI] [PubMed] [Google Scholar]
- Simões-Costa M., Bronner M.E. Establishing neural crest identity: a gene regulatory recipe. Development. 2015;142:242–257. doi: 10.1242/dev.105445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina T. The actin cytoskeleton and actin-based motility. Cold Spring Harb. Perspect. Biol. 2018 Jan 2;10(1):a018267. doi: 10.1101/cshperspect.a018267. PMID: 29295889; PMCID: PMC5749151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahtamouni L.H., Shaw A.E., Hasan M.H., Yasin S.R., Bamburg J.R. Non-overlapping activities of ADF and cofilin-1 during the migration of metastatic breast tumor cells. BMC Cell Biol. 2013;14:45. doi: 10.1186/1471-2121-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermillion K.L., Lidberg K.A., Gammill L.S. Expression of actin-binding proteins and requirement for actin-depolymerizing factor in chick neural crest cells. Dev. Dyn. 2014;243:730–738. doi: 10.1002/dvdy.24105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford J.B. Neural tube closure and neural tube defects: studies in animal models reveal known knowns and known unknowns. Am J Med Genet C Semin Med Genet. 2005 May 15;135C(1):59–68. doi: 10.1002/ajmg.c.30054. PMID: 15806594. [DOI] [PubMed] [Google Scholar]
- Winder S.J., Ayscough K.R. Actin-binding proteins. J. Cell Sci. 2005;118:651–654. doi: 10.1242/jcs.01670. [DOI] [PubMed] [Google Scholar]
- Yamagishi Y., Abe H. Reorganization of actin filaments by ADF/cofilin is involved in formation of microtubule structures during Xenopus oocyte maturation. Mol. Biol. Cell. 2015;26:4387–4400. doi: 10.1091/mbc.E15-01-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ybot-Gonzalez P., Copp A.J. Bending of the neural plate during mouse spinal neurulation is independent of actin microfilaments. Dev. Dyn.: Off. Publ. Am. Assoc. Anat. 1999;215:273–283. doi: 10.1002/(SICI)1097-0177(199907)215:3<273::AID-AJA9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Zhang C., Zhang W., Lu Y., Yan X., Yan X., Zhu X., Liu W., Yang Y., Zhou T. NudC regulates actin dynamics and ciliogenesis by stabilizing cofilin 1. Cell Res. 2016;26:239–253. doi: 10.1038/cr.2015.152. [DOI] [PMC free article] [PubMed] [Google Scholar]