Abstract
Summary
We report a case of a 59-year-old woman with Cushing’s disease who developed hyperthyroidism following treatment of hypercortisolaemia. The patient with a history of recurrent hospitalisations caused by multi-sited soft tissue abscesses was admitted with sepsis. Both her medical history and physical examination suggested Cushing’s syndrome. The initial hormonal diagnostic process, conducted after sepsis treatment, brought forth conflicting results. However, hormonal assessment repeated 3 months later indicated pituitary hypercortisolaemia, which was confirmed through bilateral inferior petrosal sinus sampling and was successfully treated with transsphenoidal pituitary surgery. Three months after the surgery, the patient was readmitted to our epartment with symptoms of hyperthyroidism, which was confirmed by laboratory tests. Thyroid scintiscans indicated Graves’ disease. However, the absence of anti-thyroid stimulating hormone antibodies suggested other etiologies of hyperthyroidism. Eventually, the patient underwent radioiodine therapy. Currently, her condition is improving and she has had no recurrence of abscesses, severe infections, or hyperthyroidism. In conclusion, while clinical manifestation of hypercortisolaemia might be non-specific, its treatment may trigger the development of autoimmune diseases.
Learning points
The presence of recurrent severe infections should prompt physicians to consider the possibility of hypercortisolaemia.
Chronic hypercortisolism is debilitating and can lead to significant disability.
Dexamethasone suppression testing in patients with active or recent severe inflammatory or infectious illnesses may produce misleading or confusing results.
Clinicians should be aware of the potential development of autoimmune diseases following successful treatment of hypercortisolaemia.
Patient Demographics: Adult, Female, White, Poland
Clinical Overview: Pituitary, Pituitary, Thyroid, Cushing's disease, Hyperthyroidism
Publication Details: Unique/unexpected symptoms or presentations of a disease, May, 2024
Background
Clinical manifestations of hypercortisolaemia might be variable and non-specific, leading to a potential delay in diagnosis. While one of its symptoms is susceptibility to infections, the treatment of hypercortisolaemia results in the cessation of immunosuppression, which can subsequently contribute to the development of autoimmune diseases (1). Here we describe a case of a 59-year-old woman with a history of soft tissue and muscle abscesses recurring over several years who was diagnosed with advanced, long-term Cushing’s disease. Following neurosurgical treatment of hypercortisolaemia, the patient developed symptoms of hyperthyroidism due to autoimmune thyroid disease 3 months later.
Case presentation
A 59-year-old woman with a history of type 2 diabetes mellitus, hypertension, osteoporosis, and recurrent hospitalisations due to multiple soft tissue and muscle abscesses over several years was admitted to the Department of Internal Medicine and Metabolic Diseases of University Clinical Hospital in Bialystokt with symptoms of sepsis.
According to the medical records, the patient had been hospitalised multiple times over the course of at least 10 years due to recurrent abscesses in various locations, including oral cavity, trunk, buttock, and calf. Based on these records, the patient had undergone at least four surgical drainage procedures for abscesses in different areas. One month before admission to our hospital, the patient had been hospitalised in the Department of Surgery due to left buttock abscess with necrosis of ischial tuberosity. The CT scan had revealed abscesses of iliopsoas muscle and both buttocks, as well as an enlarged and irregularly outlined left adrenal gland measuring 59 × 25 mm. On admission to our department, the patient was in a poor condition. Physical examination revealed cachexia, thin and atrophic skin with numerous small scars from previous cutaneous abrasions, excessive chin hair, poor dental condition with severe hypodontia, a moon-shaped face, facial plethora, central fat redistribution, proximal muscle wasting, oedema in arms and legs, and a deep cavity in the left buttock abscess. Additionally, the patient reported a history of easy bruising and amenorrhoea for several years. Laboratory tests showed elevated inflammatory markers, anaemia, and low plasma levels of TSH, fT3, and fT4 (TSH: 0.19 μIU/mL, normal range (NR): 0.35–4.94 μIU/mL; fT3: 1.99 pg/mL, NR: 1.71–3.71 pg/mL; fT4: 0.98 pg/mL, NR: 0.7–1.48 pg/mL). Blood and abscess cavity swab cultures were positive for methicillin-susceptible Staphylococcus aureus.
Investigation
Abdominal and pelvic CT, repeated at our department to obtain an adrenal washout protocol, revealed abscesses in the right iliopsoas and both gluteal muscles as well as two left adrenal gland lesions, measuring 18 × 22 mm and 34 × 22 mm, with densities of −14 HU and −22 HU, respectively (Fig. 1). Considering the combination of phenotypic features, the patient’s medical history, and the results of imaging tests, we suspected Cushing’s syndrome. Despite the patient’s severe inflammatory state, which could have affected laboratory assessments, we decided to proceed with the basic diagnostic tests for Cushing’s syndrome, considering that, in case of hypercortisolism, the use of steroidogenesis inhibitors could significantly improve the patient’s condition. Hormonal tests revealed increased midnight cortisol (529.73 nmol/L; upper limit of normal: 49.66 nmol/L) and 24-h urinary free cortisol (UFC; 777.93 nmol/24 h; upper limit of normal: 485.52 nmol/24 h), indicating hypercortisolaemia. The patient underwent antibiotic therapy, red blood cell transfusion, abscess drainage, and negative-pressure wound therapy for the abscess cavity. The administered treatment led to a significant improvement in the patient’s condition, which allowed us to forgo the planned steroidogenesis inhibitor therapy. Two weeks after the resolution of inflammation, we reevaluated the patient for hypercortisolaemia and observed elevated midnight cortisol (259.35 nmol/L) and UFC (753.10 nmol/24 h). The adrenocorticotrophic hormone (ACTH) level was 21.94 ng/L, suggesting ACTH-dependent Cushing’s syndrome. Pituitary magnetic resonance imaging (MRI) showed a right-sided 6 mm lesion, described as Rathke’s cleft cyst or, less likely, an atypical microadenoma (Fig. 2). Furthermore, we performed low-dose dexamethasone (DXM) suppression test, which confirmed hypercortisolaemia. To confirm the pituitary aetiology of hypercortisolaemia, we performed high-dose dexamethasone (DXM) suppression test. Despite its limited accuracy (2), it was the only test for differential diagnosis of ACTH-dependent Cushing’s syndrome available in our department at that time. The absence of cortisol suppression in high-dose DXM test did not indicate pituitary Cushing’s syndrome (Table 1). However, due to the relatively short period since the resolution of the inflammatory state, we decided to repeat the diagnostic process at a later time point.
Figure 1.
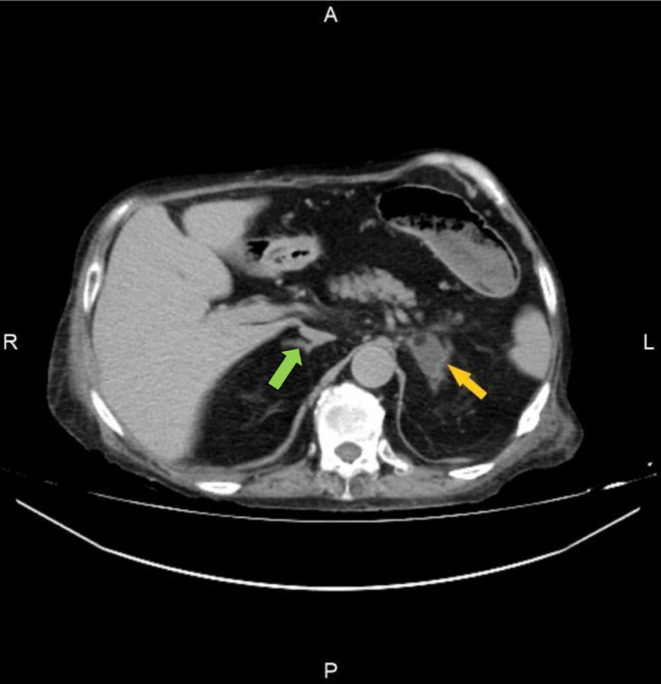
Left adrenal gland hyperplasia (yellow arrow) compared to the normal right adrenal gland (green arrow) in the abdomen CT.
Figure 2.

The lesion of pituitary gland in pituitary MRI.
Table 1.
The results of dexamethasone (DXM) suppression test 2 weeks after the resolution of the inflammatory state.
| At 0 min* | DXM test | ||
|---|---|---|---|
| Low dose | High dose | ||
| Serum cortisol, nmol/L | 297.97 | 438.68 | 342.12 |
| 24-h UFC, nmol/24 h | 753.10 | 1721.38 | 608.28 |
*Before DXM administration.
UFC, urinary free cortisol.
The patient was readmitted to our department after 3 months. Her condition had improved significantly and there were no clinical or laboratory signs of inflammation. However, leukocytosis with neutrophilia and low TSH, fT3, and fT4 plasma concentrations were still observed. During the diagnostic process, we confirmed elevated midnight cortisol (320.04 nmol/L) and UFC in the upper limit of normal (420.97 nmol/24 h). The ACTH concentration was 27.98 ng/L. Subsequently, we observed no blood or urinary cortisol suppression in low-dose DXM test again, while high-dose DXM test showed proper suppression of cortisol levels (Table 2). We then performed a corticotropin-releasing hormone (CRH) test, which revealed an over two-fold increase in ACTH and an almost two-fold increase in cortisol levels after stimulation. This observation, along with cortisol suppression in the high-dose DXM test, indicated pituitary ACTH-dependent Cushing’s syndrome (Table 3).
Table 2.
The results of dexamethasone (DXM) suppression test 3 months after the resolution of the inflammatory state.
| At 0 min* | DXM test | ||
|---|---|---|---|
| Low dose | High dose | ||
| Serum cortisol, nmol/L | 372.47 | 206.93 | 132.43 |
| 24-h UFC, nmol/24 h | 420.97 | 178.76 | 57.93 |
*Before DXM administration.
UFC, urinary free cortisol.
Table 3.
The results of CRH stimulation test.
| 0 min | 15 min | 30 min | 45 min | 60 min | 90 min | |
|---|---|---|---|---|---|---|
| Serum cortisol, nmol/L | 460.75 | – | 786.31 | 849.77 | 852.53 | 717.34 |
| Plasma ACTH, ng/L | 27.98 | 62.19 | 62.39 | 64.89 | 61.34 | – |
CRH, corticotropin-releasing hormone; ACTH, adrenocorticotropic hormone.
To establish an unequivocal diagnosis, we referred the patient to the Department of Neurosurgery for bilateral inferior petrosal sinus sampling (BIPSS). However, before the referral, we conducted thyroid scintiscan, which revealed an even Technetium-99m uptake equal to 1.29%. Additional hormonal tests showed decreased levels of follicle-stimulating hormone (FSH) and luteinising hormone (LH), while oestradiol and prolactin (PRL) were within the normal range. Based on the hormonal and imaging evaluation in conjunction with the patient’s clinical features, we concluded that the low concentrations of TSH, FSH, and LH were a result of hypercortisolaemia.
Subsequently, the patient was referred to the Department of Neurosurgery, where BIPSS was performed, confirming the pituitary origin of hypercortisolism.
Treatment
The patient underwent a successful transsphenoidal pituitary surgery, following which the cortisol level was measured at 74.49 nmol/L. Histopathological examination of the excised lesion confirmed a pituitary adenoma positive for ACTH staining with Ki67 proliferation index <1%. Subsequently, the patient received hydrocortisone substitution therapy at 30 mg per day. Comprehensive hormonal assessment, conducted 2 weeks after the surgery, revealed low ACTH concentration (3.47 ng/L) and morning cortisol (92.98 nmol/L). The TSH level returned to the normal range, and gonadotropin concentrations were appropriate for the menopausal state.
Outcome and follow-up
Three months later, the patient was readmitted to our department with symptoms of fever, severe weakness, weight loss, and heart palpitations. In addition to the abnormalities observed previously, physical examination revealed arrhythmia, identified in ECG as atrial fibrillation. Laboratory tests revealed a significantly decreased concentration of TSH (<0.008 μIU/mL), elevated fT3 (5.54 pg/mL) and fT4 (>5.00 pg/mL) levels, and increased concentrations of anti-TPO and anti-TG antithyroid autoantibodies. Anti-thyroid stimulating hormone receptor (anti-TSHR) antibodies (TRAbs) and thyroid-stimulating immunoglobulin (TSI) remained within the normal ranges. Ultrasonography of the thyroid gland revealed an enlarged, slightly hypoechogenic, hypervascular goitre with several small nodules. Fine needle aspiration biopsy of the lesions confirmed their benign nature. During the hospitalisation, the patient was administered high doses of methimazole, which resulted in a decrease in thyroid hormone concentrations, restoration of cardiac sinus rhythm, and improvement in the patient’s condition. To further investigate the potential causes of hyperthyroidism, the patient was readmitted to our department several weeks later, after having achieved euthyroid state. We performed thyroid I-131 scintigraphy, which revealed homogeneous, significantly increased tracer uptake (86.8% after 24 h). Ophthalmological examination did not reveal any signs of ophthalmopathy. Based on imaging tests’ results, Graves’ disease was suspected. However, due to the absence of TRAbs, we repeated nuclear imaging of the thyroid before making a final decision regarding the appropriate treatment. Over the course of several months, the patient has undergone two more I-131 scintiscans, both showing comparable thyroid images with an even iodine uptake of 80% and 75% after 24 h, respectively (Fig. 3). The anti-TSHR antibodies titre remained negative.
Figure 3.
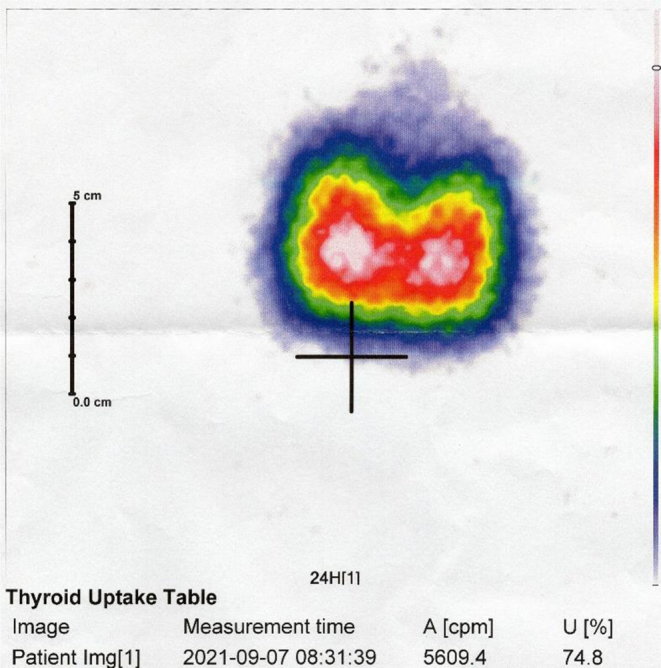
The homogeneous iodine uptake during the 3rd thyroid scintigraphy.
Throughout the diagnostic process, the patient required gradually reduced doses of antithyroid drugs. However, every attempt to withdraw pharmacological treatment resulted in recurrent hyperthyroidism. Therefore, after 18 months of antithyroid drug administration, the patient underwent radioiodine therapy, achieving and maintaining euthyroid state. Currently, the patient requires only hydrocortisone substitution in the previously established dose. Her condition is improving, with no reoccurrence of abscesses, any other severe infections, or hyperthyroidism (Figs 4 and 5).
Figure 4.
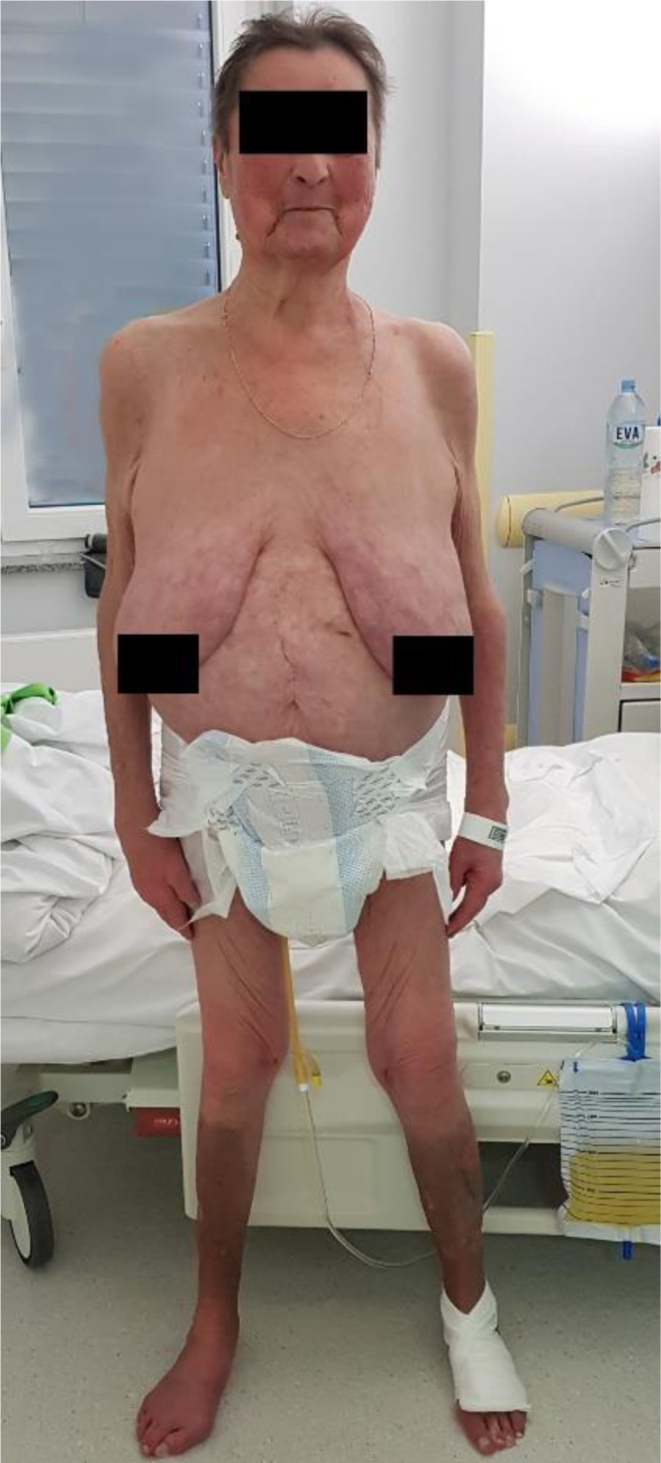
The appearance of the patient before the neurosurgical treatment of hypercortisolaemia.
Figure 5.
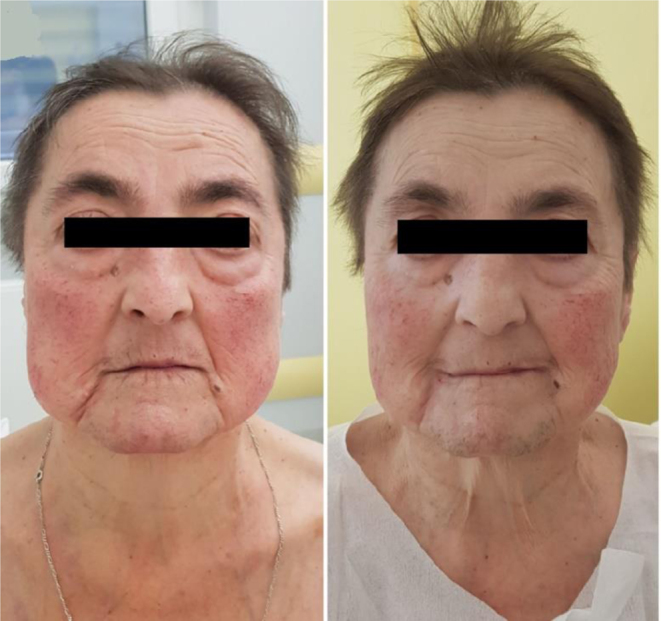
The appearance of the patient before and 5 months after the neurosurgical treatment of hypercortisolaemia.
Discussion
To the best of our knowledge, recurrent, multi-sited abscesses have never been reported as a major consequence of long-lasting Cushing’s syndrome. Unfortunately, during frequent hospitalisations at various surgery departments, the patient’s symptoms had never been associated with hypercortisolism; therefore, further investigations into immune system impairment had never been pursued.
In hypercortisolaemic patients, restoring normal cortisol levels may improve the control of chronic infections. In cases of severe, life-threatening conditions due to hypercortisolism, urgent medical treatment aimed at lowering cortisol concentration is recommended (3). Concerned that the patient would not survive without administration of steroidogenesis inhibitors, we performed basic hormonal tests, which initially indicated hypercortisolaemia. Fortunately, the patient’s condition improved during the hormonal diagnostics, eliminating the need for steroidogenesis inhibitor administration. The initial hormonal differential diagnosis has brought conflicting results regarding the putative aetiology of hypercortisolaemia. We were aware that the patient’s acute condition could have influenced the hormonal results (4); therefore, we conducted a detailed diagnostic process 3 months after the resolution of inflammation, which, unlike previous results, indicated pituitary ACTH-dependent Cushing’s syndrome. We eventually confirmed hypercortisolaemia, although the unclear results of hormonal and imaging tests presented challenges in determining the exact source of cortisol hypersecretion. Although the post-inflammatory hormonal tests indicated pituitary hypercortisolism, the results of the initial diagnostic process, combined with the observation of macronodular unilateral adrenal hyperplasia on the abdominal CT, raised doubts about the source of hypercortisolism. As in patients with ACTH-dependent Cushing’s syndrome the adrenal glands may typically appear normal or show bilateral hyperplasia due to chronic ACTH hyperstimulation, the coincidental unilateral adrenal macronodules associated with Cushing’s disease have been documented in only a small number of cases (5). These inconsistencies prompted us to conduct a careful investigation to determine the source of hypercortisolism and avoid misdirected surgery.
Additionally, given the patient’s history of recurrent abscesses over at least 10 years, we suspected the patient had been experiencing chronic, long-lasting hypercortisolism at a mild level or cyclic hypercortisolism. However, since the diagnostic criteria for cyclic Cushing’s syndrome require at least three episodes of hypercortisolism interspersed with periods of normocortisolaemia (6), the lack of past cortisol measurements in our patient prevented verification of this hypothesis.
Three months after the surgical treatment of hypercortisolaemia, the patient developed hyperthyroidism. The absence of anti-TSHR antibodies and the presence of thyroid nodules led us to consider Graves’ disease, Hashitoxicosis, or disseminated thyroid autonomy. Guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists (7, 8) state that a negative TRAb titre does not exclude the diagnosis of Graves’ disease but indicates the need for assessing radioiodine uptake during thyroid scintigraphy. In case the scintiscan reveals homogeneous, high tracer uptake, as it was in the case of our patient, Graves’ disease can be diagnosed. Additionally, thyroid hypervascularisation on ultrasonography indicates higher probability of Graves’ disease compared to Hashitoxicosis (7, 9). Another important factor supporting the theory of an autoimmune background of hyperthyroidism is the presence of positive anti-TPO and anti-TG antibodies, as well as the fact that in patients with remission of hypercortisolism restoration of immune function can promote the onset of autoimmune disorders, of which thyroid autoimmunity is the most prevalent (1). Considering all these factors, we favoured the hypothesis of an autoimmune background of the patient’s hyperthyroidism in the form of Graves’ disease, particularly since TRAb-negative cases of Graves’ disease are observed in as many as 18% of cases (10).
The presented case highlights the importance of considering the possibility of Cushing’s syndrome in patients with recurrent severe infections. Furthermore, clinicians should be aware of the potential onset of autoimmune diseases after successful treatment of hypercortisolaemia.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this case report.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Patient consent
This study was exempt from ethical approval procedures being a case report study that describes the patient treated in our Department. The study was in line with the ethical standards of Declaration of Helsinki or comparable ethical standards. Written informed consent for publication of her clinical details and clinical images was obtained from the patient.
Author contribution statement
ES was involved in the diagnostic and therapeutic process and writing the article. AŁ, MK and GZieliński were involved in the diagnostic and therapeutic process. IK and MK-K contributed to the diagnostic and therapeutic process, writing of the article and final approval of the version to be submitted.
References
- 1.Colao A Pivonello R Faggiano A Filippella M Ferone D Somma Di C Cerbone G Marzullo P Fenzi G & Lombardi G. Increased prevalence of thyroid autoimmunity in patients successfully treated for Cushing’s disease. Clinical Endocrinology 20005313–19. ( 10.1046/j.1365-2265.2000.01018.x) [DOI] [PubMed] [Google Scholar]
- 2.Fleseriu M, Auchus R, Bancos I, Ben-Shlomo A, Bertherat J, Biermasz NR, Boguszewski CL, Bronstein MD, Buchfelder M, Carmichael JD, et al.Consensus on diagnosis and management of Cushing’s disease: a guideline update. Lancet. Diabetes and Endocrinology 20219847–875. ( 10.1016/S2213-8587(2100235-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuevas-Ramos D Lim DST & Fleseriu M. Update on medical treatment for Cushing’s disease. Clinical Diabetes and Endocrinology 2016216. ( 10.1186/s40842-016-0033-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newell-Price J Trainer P Besser M & Grossman A. The diagnosis and differential diagnosis of Cushing’s syndrome and pseudo-Cushing’s states. Endocrine Reviews 199819647–672. ( 10.1210/edrv.19.5.0346) [DOI] [PubMed] [Google Scholar]
- 5.Albiger NM Occhi G Sanguin F Iacobone M Casarrubea G Ferasin S Mantero F & Scaroni C. Adrenal nodules in patients with Cushing’s disease: prevalence, clinical significance and follow-up. Journal of Endocrinological Investigation 201134e204–e209. ( 10.3275/7349) [DOI] [PubMed] [Google Scholar]
- 6.Meinardi JR Wolffenbuttel BHR & Dullaart RPF. Cyclic Cushing’s syndrome: a clinical challenge. European Journal of Endocrinology 2007157245–254. ( 10.1530/EJE-07-0262) [DOI] [PubMed] [Google Scholar]
- 7.Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, Rivkees SA, Samuels M, Sosa JA, Stan MN, et al.2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid 2016261343–1421. ( 10.1089/thy.2016.0229) [DOI] [PubMed] [Google Scholar]
- 8.Bahn RS, Burch HB, Cooper DS, Garber JR, Greenlee MC, Klein I, Laurberg P, McDougall IR, Montori VM, Rivkees SA, et al.ATA / AACE Guidelines hyperthyroidism and other causes of thyrotoxicosis. Endocrine Practice 201117456–520. ( 10.4158/ep.17.3.456) [DOI] [PubMed] [Google Scholar]
- 9.Singh I & Hershman JM. Pathogenesis of hyperthyroidism. Comprehensive Physiology 2016767–79. ( 10.1002/cphy.c160001) [DOI] [PubMed] [Google Scholar]
- 10.Zuhur SS Bilen O Aggul H Topcu B Celikkol A & Elbuken G. The association of TSH-receptor antibody with the clinical and laboratory parameters in patients with newly diagnosed Graves’ hyperthyroidism: experience from a tertiary referral center including a large number of patients with TSH-receptor antibody-negative patients with Graves’ hyperthyroidism. Endokrynologia Polska 20217214–21. ( 10.5603/EP.a2020.0062) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a