This cohort study identifies the factors associated with likelihood of microsatellite instability and KRAS biomarker testing among patients with metastatic colorectal cancer.
Key Points
Question
What are the social and demographic factors associated with the receipt of biomarker testing in patients with metastatic colorectal cancer?
Findings
In this retrospective cohort study of 41 061 patients, factors such as older age, treatment at community facilities, lower educational level in area of residence, and treatment at South Central regional facilities were associated with a reduced likelihood of microsatellite instability and KRAS testing.
Meaning
Findings of this study highlight the sociodemographic-based disparities in biomarker testing, which can inform the development of strategies that promote equity in cancer care and improve outcomes for underserved populations.
Abstract
Importance
Among patients with metastatic colorectal cancer (mCRC), data are limited on disparate biomarker testing and its association with clinical outcomes on a national scale.
Objective
To evaluate the socioeconomic and demographic inequities in microsatellite instability (MSI) and KRAS biomarker testing among patients with mCRC and to explore the association of testing with overall survival (OS).
Design, Setting, and Participants
This cohort study, conducted between November 2022 and March 2024, included patients who were diagnosed with mCRC between January 1, 2010, and December 31, 2017. The study obtained data from the National Cancer Database, a hospital-based cancer registry in the US. Patients with mCRC and available information on biomarker testing were included. Patients were classified based on whether they completed or did not complete MSI or KRAS tests.
Exposure
Demographic and socioeconomic factors, such as age, race, ethnicity, educational level in area of residence, median household income, insurance type, area of residence, facility type, and facility location were evaluated.
Main Outcomes and Measures
The main outcomes were MSI and KRAS testing between the date of diagnosis and the date of first-course therapy. Univariable and multivariable logistic regressions were used to identify the relevant factors in MSI and KRAS testing. The OS outcomes were also evaluated.
Results
Among the 41 061 patients included (22 362 males [54.5%]; mean [SD] age, 62.3 [10.1] years; 17.3% identified as Black individuals, 78.0% as White individuals, 4.7% as individuals of other race, with 6.5% Hispanic or 93.5% non-Hispanic ethnicity), 28.8% underwent KRAS testing and 43.7% received MSI testing. A significant proportion of patients had Medicare insurance (43.6%), received treatment at a comprehensive community cancer program (40.5%), and lived in an area with lower educational level (51.3%). Factors associated with a lower likelihood of MSI testing included age of 70 to 79 years (relative risk [RR], 0.70; 95% CI, 0.66-0.74; P < .001), treatment at a community cancer program (RR, 0.74; 95% CI, 0.70-0.79; P < .001), rural residency (RR, 0.80; 95% CI, 0.69-0.92; P < .001), lower educational level in area of residence (RR, 0.84; 95% CI, 0.79-0.89; P < .001), and treatment at East South Central facilities (RR, 0.67; 95% CI, 0.61-0.73; P < .001). Similar patterns were observed for KRAS testing. Survival analysis showed modest OS improvement in patients with MSI testing (hazard ratio, 0.93; 95% CI, 0.91-0.96; P < .001). The median (IQR) follow-up time for the survival analysis was 13.96 (3.71-29.34) months.
Conclusions and Relevance
This cohort study of patients with mCRC found that older age, community-setting treatment, lower educational level in area of residence, and treatment at East South Central facilities were associated with a reduced likelihood of MSI and KRAS testing. Highlighting the sociodemographic-based disparities in biomarker testing can inform the development of strategies that promote equity in cancer care and improve outcomes for underserved populations.
Introduction
As the third most prevalent and second leading cause of cancer-related deaths in the US, colorectal cancer (CRC) poses a formidable challenge to the global health care community.1 Initial diagnosis reveals metastatic disease in approximately 20% of patients with CRC,1 and the outcome is poor, with a 5-year survival rate of 15.6%.2
Nevertheless, precision oncology has emerged as a transformative approach to managing metastatic CRC (mCRC), a factor in substantially improved outcomes.3,4 Genetic biomarkers, such as microsatellite instability (MSI) and somatic alteration in the BRAF and RAS oncogenes have been the mainstay of precision medicine. In particular, KRAS-positive alteration is reported to be present in 35% to 45% of CRC cases.5 Numerous clinical trials have shown that anti–epidermal growth factor receptor monoclonal antibodies combined with chemotherapy improved survival outcomes for patients with wild-type KRAS CRC tumors.6 Additionally, the MSI pathway encompasses approximately 15% to 20% of sporadic CRC and the majority of hereditary CRC.7 Immune checkpoint inhibitors, such as pembrolizumab and nivolumab, have demonstrated remarkable responses in patients with MSI-high CRC, offering a promising first-line treatment for mCRC.8,9
Due to the prevalence and importance of the mentioned genetic alterations, guidelines recommend comprehensive biomarker testing for all patients with mCRC.10 Unfortunately, the health care field is plagued by a lack of access to several disciplines of the care process. Multiple studies have identified socioeconomic and demographic disparities associated with inequitable survival outcomes for patients with CRC.11,12 Numerous factors support these disparities, including genetic and behavioral characteristics,13,14 limited access to guideline-recommended screening,15,16 and discrepancies in treatment access and quality.17,18,19 Furthermore, reports have been emerging of disparate genetic testing access and counseling for patients with CRC.20,21 However, few studies have assessed the national discrepancies in access to genomic testing in CRC.
Therefore, the era of precision medicine could play a role in the exacerbation of preexisting disparities and widening of the gap in outcomes across specific populations. Consequently, in this study, we sought to evaluate the socioeconomic and demographic inequities in MSI and KRAS biomarker testing among patients with mCRC and to explore the association of testing with overall survival (OS).
Methods
Data Source
Data were obtained from the National Cancer Database (NCDB), a hospital-based cancer registry that includes demographic, clinical, and pathological data on more than 70% of patients with cancer diagnosed in the US.22 In accordance with the Common Rule, this cohort study was exempt from ethics review and informed consent requirement because it used deidentified patient data. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Study Design
Patients diagnosed with CRC between January 1, 2010, and December 31, 2017, were identified. Only patients with clinical stage IV were included, as biomarker testing was initially recommended for patients with metastatic disease. Given that the NCDB only reports MSI and KRAS biomarker tests in CRC, patients with available documentation on the completion (or noncompletion) of either test were included. Patients diagnosed before January 1, 2010, were excluded since KRAS testing was not prevalent before that period. In 2018, the NCDB changed the MSI and KRAS test variables and, since then, no longer reports patients who did not undergo these tests. Patients aged 80 years or older were excluded since the decision to perform biomarker testing may have been altered by the choice of not administering systemic therapy. Accordingly, patients were classified based on the completion (or noncompletion) of either MSI or KRAS test.
Demographic, Socioeconomic, and Clinical Variables
The MSI and KRAS testing variables were recorded as site-specific factor 7 and site-specific factor 9, respectively. The NCDB reports that the tests were performed at any time between the date of diagnosis and first-course treatment. Nevertheless, the exact timing of testing was not reported. Sociodemographic variables were included, such as age, sex, race, ethnicity, insurance type, median household income, facility type, facility location (eTable 1 in Supplement 1), distance to facility, geographic location (US Census division), and educational level in area of residence. Race and ethnicity variables recorded in the NCDB were self-identified social constructs reported in the patients’ medical records. Educational level was assessed at the zip code level based on the percentage of adult residents in the area without a high school diploma. Median household income was also estimated by matching the zip code of the patient’s residence with files derived from the American Community Survey data. Clinical factors included Charlson Comorbidity Index, administration of chemotherapy and immunotherapy, surgery of the primary and nonprimary sites, and lung or liver metastases. A definition of socioeconomic and demographic factors is provided in eAppendix in Supplement 1.
Statistical Analysis
The χ2 test assessed the socioeconomic and clinical categorical characteristic differences among the cohorts. Univariable and multivariable logistic regressions were conducted to determine the relevant independent factors associated with MSI and KRAS testing. Relative risk (RR) ratios and 95% CIs were calculated using Poisson models with robust covariance. A patient was considered to be positive for the event or outcome if the patient received biomarker testing. Univariable and multivariable Cox proportional hazards regression models were used to identify significant survival factors. The inclusion of clinical variables, such as guideline-recommended treatment options in the multivariable model, enabled the objective assessment of the association between testing and OS. Kaplan-Meier curves were produced based on test completion. The survival follow-up time was calculated from the date of diagnosis to the date of last contact or all-cause death. Due to missing date of testing, the date of diagnosis was used as the start of the follow-up period for the survival analysis. Treatment interventions were initiated within a short period after diagnosis and testing. The relatively short period between diagnosis, testing, and treatment, as defined by the NCDB, partially accounted for immortal time bias. Patients with missing follow-up data were not included in the survival analysis.
Two-sided P < .05 was considered to be statistically significant. The study was conducted between November 2022 and March 2024. SAS, version 9.4 (SAS Institute Inc), and R, version 4.2.3 (R Project for Statistical Computing), were used to analyze the data.
Results
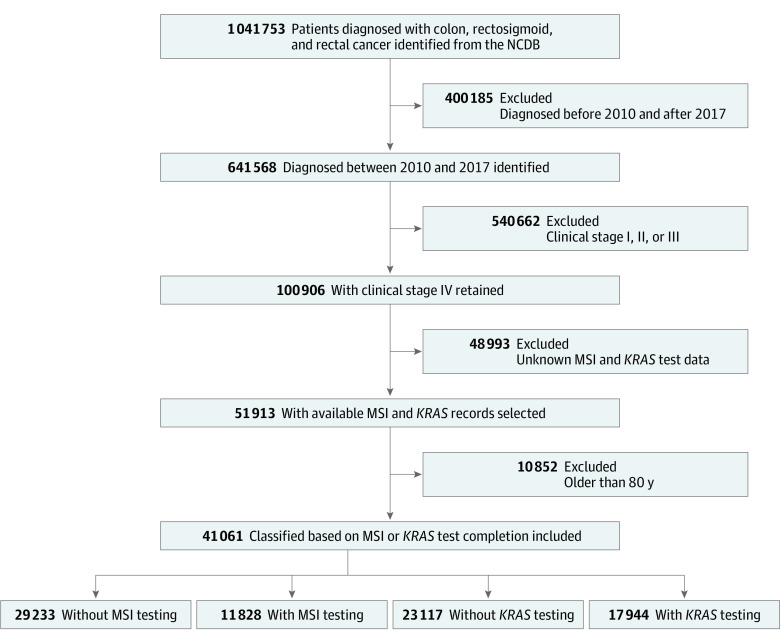
A total of 41 061 patients (22 362 males [54.5%], 18 699 females [45.5%]; mean [SD] age, 62.3 [10.1] years) diagnosed with mCRC between 2010 and 2017 were included (Figure). Of these patients, only 28.8% underwent KRAS testing and 43.7% underwent MSI testing. The population consisted of individuals who identified as Black (17.3%), White (78.0%), or other (4.7%) race with Hispanic (6.5%) or non-Hispanic (93.5%) ethnicity and who lived in a metropolitan setting (85.0%). Additionally, a substantial proportion of patients were covered by Medicare (43.6%), treated at a comprehensive community cancer program (40.5%) and South Atlantic facilities (21.3%), and resided in an area with lower educational level (51.3%). The clinical and sociodemographic characteristics of patients are shown in Table 1.
Figure. Study Flow Diagram.
MSI indicates microsatellite instability; NCDB, National Cancer Database.
Table 1. Baseline Sociodemographic and Clinical Characteristics Between Patients With and Without Biomarker Testing .
| Characteristic | MSI testing, No. (%) | P value | KRAS testing, No. (%) | P value | ||
|---|---|---|---|---|---|---|
| Without (n = 29 233) | With (n = 11 828) | Without (n = 23 117) | With (n = 17 944) | |||
| Age group, y | ||||||
| 18-49 | 2981 (57.0) | 2249 (43.0) | <.001 | 2296 (43.9) | 2934 (56.1) | <.001 |
| 50-59 | 7326 (67.5) | 3529 (32.5) | 5566 (51.3) | 5289 (48.7) | ||
| 60-69 | 9800 (73.6) | 3517 (26.4) | 7582 (56.9) | 5735 (43.1) | ||
| 70-79 | 9126 (78.3) | 2533 (21.7) | 7673 (65.8) | 3986 (34.2) | ||
| Racea | ||||||
| Black | 5297 (74.7) | 1793 (25.3) | <.001 | 4156 (58.6) | 2934 (41.4) | <.001 |
| White | 22 648 (70.7) | 9406 (29.3) | 17 902 (55.8) | 14 152 (44.2) | ||
| Othera | 1288 (67.2) | 629 (32.8) | 1059 (55.2) | 858 (44.8) | ||
| Ethnicityb | ||||||
| Hispanicb | 1882 (70.6) | 784 (29.4) | .48 | 1509 (56.6) | 1157 (43.4) | .75 |
| Non-Hispanica | 27 351 (71.2) | 11 044 (28.8) | 21 608 (56.3) | 16 787 (43.7) | ||
| Sex | ||||||
| Male | 16 057 (71.8) | 6305 (28.2) | .003 | 12 533 (56.0) | 9829 (44.0) | .26 |
| Female | 13 176 (70.5) | 5523 (29.5) | 10 584 (56.6) | 8115 (43.4) | ||
| Insurance type | ||||||
| Private | 10 346 (64.6) | 5680 (35.4) | <.001 | 7810 (48.7) | 8216 (51.3) | <.001 |
| No insurance | 1848 (76.6) | 566 (23.4) | 1450 (60.1) | 964 (39.9) | ||
| Medicaid | 3001 (70.3) | 1269 (29.7) | 2416 (56.6) | 1854 (43.4) | ||
| Medicare or otherc | 13 675 (76.5) | 4210 (23.5) | 11 155 (62.4) | 6730 (37.6) | ||
| Unknown | 363 (1.2) | 103 (0.9) | 286 (61.4) | 180 (38.6) | ||
| Educational level in area of residence, %d | ||||||
| ≥17.6 | 7457 (75.3) | 2452 (24.7) | <.001 | 5961 (60.2) | 3948 (39.8) | <.001 |
| 10.9-17.5 | 8243 (73.8) | 2922 (26.2) | 6564 (58.8) | 4601 (41.2) | ||
| 6.3-10.8 | 7682 (69.3) | 3396 (30.7) | 6027 (54.4) | 5051 (45.6) | ||
| <6.3 | 5582 (65.5) | 2944 (34.5) | 4364 (51.2) | 4162 (48.8) | ||
| Unknown | 269 (70.2) | 114 (29.8) | 201 (52.5) | 182 (47.5) | ||
| Facility type with CP | ||||||
| Community | 2885 (78.4) | 796 (21.6) | <.001 | 2236 (60.7) | 1445 (39.3) | <.001 |
| Comprehensive | 12 344 (74.1) | 4304 (25.9) | 9601 (57.7) | 7047 (42.3) | ||
| Academic | 8710 (67.8) | 4134 (32.2) | 6895 (53.7) | 5949 (46.3) | ||
| Integrated network | 5294 (67.1) | 2594 (32.9) | 4385 (55.6) | 3503 (44.4) | ||
| Median household income, US$ | ||||||
| <40 227 | 6689 (76.0) | 2118 (24.0) | <.001 | 5364 (60.9) | 3443 (39.1) | <.001 |
| 40 227-50 353 | 6784 (73.3) | 2466 (26.7) | 5367 (58.0) | 3883 (42.0) | ||
| 50 354-63 332 | 6602 (70.6) | 2753 (29.4) | 5184 (55.4) | 4171 (44.6) | ||
| ≥63 333 | 8864 (67.0) | 4369 (33.0) | 6982 (52.8) | 6251 (47.2) | ||
| Area of residence | ||||||
| Metropolitan | 24 681 (70.7) | 10 237 (29.3) | <.001 | 19 535 (55.9) | 15 383 (44.1) | .003 |
| Urban | 4051 (73.5) | 1461 (26.5) | 3217 (58.4) | 2295 (41.6) | ||
| Rural | 501 (79.4) | 130 (20.6) | 365 (57.8) | 266 (42.2) | ||
| Facility locatione | ||||||
| New England | 1235 (63.5) | 710 (36.5) | <.001 | 1004 (51.6) | 941 (48.4) | <.001 |
| Middle Atlantic | 4964 (71.1) | 2018 (28.9) | 3878 (55.5) | 3104 (44.5) | ||
| South Atlantic | 6356 (72.6) | 2403 (27.4) | 5007 (57.2) | 3752 (42.8) | ||
| East North Central | 5259 (72.5) | 1992 (27.5) | 4198 (57.9) | 3053 (42.1) | ||
| East South Central | 1877 (75.1) | 622 (24.9) | 1567 (62.7) | 932 (37.3) | ||
| West North Central | 2206 (71.6) | 877 (28.4) | 1591 (51.6) | 1492 (48.4) | ||
| West South Central | 2760 (75.2) | 912 (24.8) | 2198 (59.9) | 1474 (40.1) | ||
| Mountain | 1268 (66.4) | 642 (33.6) | 1012 (53.0) | 898 (47.0) | ||
| Pacific | 3308 (66.7) | 1652 (33.3) | 2662 (53.7) | 2298 (46.3) | ||
| Travel distance, miles | ||||||
| ≤5 | 9877 (73.9) | 3486 (29.5) | <.001 | 7821 (58.5) | 5542 (41.5) | <.001 |
| 5.1-10 | 6655 (71.0) | 2712 (29.0) | 5220 (55.7) | 4147 (44.3) | ||
| 10.1-25 | 7024 (69.9) | 3019 (30.1) | 5503 (54.8) | 4540 (45.2) | ||
| >25 | 5677 (68.5) | 2611 (31.5) | 4573 (55.2) | 3715 (44.8) | ||
| Year of diagnosis | ||||||
| 2010 | 4022 (85.8) | 665 (14.2) | <.001 | 3145 (67.1) | 1542 (32.9) | <.001 |
| 2011 | 3831 (81.6) | 865 (18.4) | 2886 (61.5) | 1810 (38.5) | ||
| 2012 | 4214 (79.3) | 1101 (20.7) | 3085 (58.0) | 2230 (42.0) | ||
| 2013 | 4285 (76.1) | 1348 (23.9) | 3109 (55.2) | 2524 (44.8) | ||
| 2014 | 3891 (73.0) | 1441 (27.0) | 2887 (54.1) | 2445 (45.9) | ||
| 2015 | 3466 (66.7) | 1733 (33.3) | 2776 (53.4) | 2423 (46.6) | ||
| 2016 | 3029 (58.7) | 2131 (41.3) | 2633 (51.0) | 2527 (49.0) | ||
| 2017 | 2495 (49.5) | 2544 (50.5) | 2596 (51.5) | 2443 (48.5) | ||
| CCI | ||||||
| 0 | 21 081 (70.4) | 8867 (29.6) | <.001 | 16 459 (55.0) | 13 489 (45.0) | <.001 |
| 1 | 5608 (72.8) | 2100 (27.2) | 4429 (57.5) | 3279 (42.5) | ||
| ≥2 | 2544 (74.7) | 861 (25.3) | 2229 (65.5) | 1176 (34.5) | ||
| Grade | ||||||
| Well-differentiated | 1206 (66.8) | 600 (33.2) | <.001 | 967 (53.5) | 839 (46.5) | <.001 |
| Moderately differentiated | 11 607 (63.9) | 6545 (36.1) | 9159 (50.5) | 8993 (49.5) | ||
| Poorly differentiated | 5577 (65.2) | 2981 (34.8) | 4572 (53.4) | 3986 (46.6) | ||
| Unknown | 10 843 (86.4) | 1702 (13.6) | 8419 (67.1) | 4126 (32.9) | ||
| Immunotherapy | ||||||
| Not administered | 25 301 (75.3) | 8279 (24.7) | <.001 | 20 489 (61.0) | 13 091 (39.0) | <.001 |
| Administered | 3854 (52.3) | 3512 (47.7) | 2565 (34.8) | 4801 (65.2) | ||
| Unknown | 78 (67.8) | 37 (32.2) | 63 (54.8) | 52 (45.2) | ||
| Chemotherapy | ||||||
| Not administered | 9449 (84.3) | 1762 (15.7) | <.001 | 9104 (81.2) | 2107 (18.8) | <.001 |
| Administered | 18 868 (65.9) | 9772 (34.1) | 13 195 (46.1) | 15 445 (53.9) | ||
| Unknown | 916 (75.7) | 294 (24.3) | 818 (67.6) | 392 (32.4) | ||
| Liver metastasis at presentation | ||||||
| Absent | 6351 (71.6) | 2524 (28.4) | <.001 | 5393 (60.8) | 3482 (39.2) | <.001 |
| Present | 22 643 (71.0) | 9258 (29.0) | 17 529 (54.9) | 14 372 (45.1) | ||
| Unknown | 570 (79.1) | 151 (20.9) | 467 (65.8) | 254 (35.2) | ||
| Lung metastasis at presentation | ||||||
| Absent | 21 130 (69.5) | 9266 (30.5) | <.001 | 16 938 (55.7) | 13 458 (44.3) | <.001 |
| Present | 7533 (75.8) | 2411 (24.2) | 5712 (57.4) | 4232 (42.6) | ||
| Unknown | 570 (79.1) | 151 (20.9) | 467 (65.8) | 254 (35.2) | ||
| Surgery of primary site | ||||||
| No | 18 107 (85.0) | 3190 (15.0) | <.001 | 13 564 (63.7) | 7733 (36.3) | <.001 |
| Yes | 11 075 (56.2) | 8625 (43.8) | 9508 (48.3) | 10 192 (51.7) | ||
| Unknown | 51 (79.7) | 13 (20.3) | 45 (70.3) | 19 (29.7) | ||
| Surgery of nonprimary site | ||||||
| No | 25 093 (74.8) | 8474 (25.2) | <.001 | 19 658 (58.6) | 13 909 (41.4) | <.001 |
| Yes | 4113 (55.2) | 3342 (44.8) | 3441 (46.2) | 4014 (53.8) | ||
| Unknown | 27 (69.2) | 12 (30.8) | 18 (46.2) | 21 (3.8) | ||
Abbreviations: CCI, Charlson Comorbidity Index (range: 0-25, with the highest score indicating a higher number of comorbidities); CP, cancer program; MSI, microsatellite instability.
Race data obtained from the National Cancer Database (NCDB) were self-identified in the patients’ medical records. Other races and non-Hispanic ethnicities include American Indian, Aleutian, or Eskimo; Asian Indian; Asian Indian or Pakistani; Chamorran; Chinese; Filipino; Fiji Islander; Guamanian; Hawaiian; Hmong; Japanese; Kampuchean; Korean; Laotian; Melanesian; Micronesian; New Guinean; Other; Other Asian; Pacific Islander; Polynesian; Samoan; Tahitian; Thai; Tongan; Unknown origin; and Vietnamese.
Ethnicity data obtained from the NCDB were self-identified in the patients’ medical records. Hispanic ethnicity includes Mexican, Puerto Rican, Cuban, South or Central American (except Brazil), Dominican Republic, or Spanish origin.
Other insurance type includes other governmental insurance.
Educational level was assessed at the zip code level based on the percentage of adult residents without a high school diploma.
US Census division.
Relative risk estimates for the event of MSI testing revealed factors associated with a lower likelihood of MSI testing (Table 2). Older patients had lower odds of MSI testing, with the lowest odds in the age group 70 to 79 years (RR, 0.70; 95% CI, 0.66-0.74; P < .001), followed by the 60-to-69-year (RR, 0.75; 95% CI, 0.72-0.78; P < .001) and 50-to-59-year (RR, 0.84; 95% CI, 0.81-0.87; P < .001) groups, compared with the age group of 18 to 49 years. Other significant factors included living in an area where the percentage of adults with no high school diploma was 17.6% or greater compared with an area with less than 6.3% (RR, 0.84; 95% CI, 0.79-0.89; P < .001), treatment at a community vs an academic cancer program (RR, 0.74; 95% CI, 0.70-0.79; P < .001), rural vs metropolitan residency (RR, 0.80; 95% CI, 0.69-0.92; P < .001), East South Central vs New England facilities (RR, 0.67; 95% CI, 0.61-0.73; P < .001). The association of factors with MSI testing in the univariable analysis is reported in eTable 2 in Supplement 1.
Table 2. Multivariable Logistic Regression Results With Relative Risk Estimates for Microsatellite Instability Testing.
| Characteristics | MSI testing | ||
|---|---|---|---|
| OR (95% CI) | P value | RR (95% CI) | |
| Age group, y | |||
| 18-49 | 1 [Reference] | NA | NA |
| 50-59 | 0.68 (0.63-0.73) | <.001 | 0.84 (0.81-0.87) |
| 60-69 | 0.55 (0.50-0.59) | <.001 | 0.75 (0.72-0.78) |
| 70-79 | 0.50 (0.45-0.55) | <.001 | 0.70 (0.66-0.74) |
| Racea | |||
| White | 1 [Reference] | NA | NA |
| Black | 0.97 (0.90-1.04) | .35 | 0.98 (0.94-1.02) |
| Othera | 0.98 (0.87-1.10) | .73 | 1.00 (0.94-1.06) |
| Ethnicityb | |||
| Non-Hispanica | 1 [Reference] | NA | NA |
| Hispanicb | 0.97 (0.87-1.07) | .51 | 0.98 (0.93-1.04) |
| Sex | |||
| Male | 1 [Reference] | NA | NA |
| Female | 1.06 (1.01-1.12) | .02 | 1.04 (1.01-1.06) |
| Insurance type | |||
| Private | 1 [Reference] | NA | NA |
| No insurance | 0.88 (0.78-0.98) | .03 | 0.93 (0.86-1.00) |
| Medicaid | 0.89 (0.82-0.97) | .008 | 0.94 (0.90-0.99) |
| Medicare or otherc | 0.89 (0.83-0.95) | <.001 | 0.94 (0.90-0.97) |
| Unknown | 0.67 (0.53-0.87) | .002 | 0.80 (0.68-0.93) |
| Educational level in area of residence, %d | |||
| <6.3 | 1 [Reference] | NA | NA |
| 6.3-10.8 | 0.89 (0.83-0.96) | .003 | 0.94 (0.91-0.98) |
| 10.9-17.5 | 0.76 (0.69-0.82) | <.001 | 0.86 (0.82-0.90) |
| ≥17.6 | 0.72 (0.65-0.79) | <.001 | 0.84 (0.79-0.89) |
| Unknown | 1.35 (0.52-3.52) | .54 | 1.20 (0.66-2.18) |
| Facility type with CP | |||
| Academic | 1 [Reference] | NA | NA |
| Community | 0.59 (0.53-0.65) | <.001 | 0.74 (0.70-0.79) |
| Comprehensive | 0.67 (0.63-0.71) | <.001 | 0.81 (0.78-0.84) |
| Integrated network | 1.02 (0.95-1.10) | .007 | 1.01 (0.98-1.05) |
| Area of residence | |||
| Metropolitan | 1 [Reference] | NA | NA |
| Urban | 0.97 (0.88-1.05) | .42 | 0.98 (0.93-1.03) |
| Rural | 0.67 (0.54-0.84) | <.001 | 0.80 (0.69-0.92) |
| Facility locatione | |||
| New England | 1 [Reference] | NA | NA |
| Middle Atlantic | 0.65 (0.58-0.74) | <.001 | 0.81 (0.76-0.86) |
| South Atlantic | 0.55 (0.48-0.62) | <.001 | 0.73 (0.69-0.78) |
| East North Central | 0.55 (0.49-0.62) | <.001 | 0.74 (0.69-0.79) |
| East South Central | 0.46 (0.39-0.53) | <.001 | 0.67 (0.61-0.73) |
| West North Central | 0.53 (0.46-0.61) | <.001 | 0.72 (0.67-0.77) |
| West South Central | 0.50 (0.44-0.58) | <.001 | 0.70 (0.65-0.75) |
| Mountain | 0.77 (0.66-0.90) | .001 | 0.88 (0.82-0.95) |
| Pacific | 0.82 (0.72-0.93) | .002 | 0.90 (0.84-0.95) |
| Travel distance, miles | |||
| ≤5 | 1 [Reference] | NA | NA |
| 5.1-10 | 1.07 (1.00-1.15) | .04 | 1.04 (1.00-1.08) |
| 10.1-25 | 1.06 (0.99-1.14) | .09 | 1.02 (0.99-1.06) |
| >25 | 1.20 (1.10-1.30) | <.001 | 1.09 (1.05-1.14) |
| Year of diagnosis | |||
| 2010 | 1 [Reference] | NA | NA |
| 2011 | 1.47 (1.31-1.65) | <.001 | 1.32 (1.21-1.44) |
| 2012 | 1.80 (1.61-2.01) | <.001 | 1.51 (1.39-1.64) |
| 2013 | 2.04 (1.82-2.28) | <.001 | 1.67 (1.54-1.81) |
| 2014 | 2.59 (2.31-2.89) | <.001 | 1.94 (1.79-2.10) |
| 2015 | 3.84 (3.44-4.30) | <.001 | 2.41 (2.24-2.61) |
| 2016 | 5.78 (5.17-6.46) | <.001 | 2.94 (2.73-3.17) |
| 2017 | 9.95 (8.89-11.14) | <.001 | 3.71 (3.45-4.00) |
| Median household income, US$ | |||
| ≥63 333 | 1 [Reference] | NA | NA |
| 50 354-63 332 | 1.02 (0.95-1.10) | .64 | 1.01 (0.97-1.05) |
| 40 227-50 353 | 0.96 (0.88-1.04) | .28 | 0.97 (0.93-1.02) |
| <40 277 | 0.96 (0.87-1.06) | .44 | 0.97 (0.92-1.03) |
| Unknown | 0.62 (0.25-1.58) | .32 | 0.77 (0.43-1.37) |
| CCI | |||
| 0 | 1 [Reference] | NA | NA |
| 1 | 1.09 (1.02-1.16) | .01 | 1.04 (1.01-1.08) |
| ≥2 | 1.11 (1.01-1.22) | .03 | 1.05 (1.00-1.11) |
| Immunotherapy | |||
| Not administered | 1 [Reference] | NA | NA |
| Administered | 1.51 (1.42-1.62) | <.001 | 1.19 (1.15-1.23) |
| Unknown | 1.41 (0.89-2.23) | .15 | 1.19 (0.92-1.53) |
| Chemotherapy | |||
| Not administered | 1 [Reference] | NA | NA |
| Administered | 1.76 (1.65-1.88) | <.001 | 1.48 (1.42-1.55) |
| Unknown | 1.28 (1.09-1.50) | .002 | 1.23 (1.11-1.36) |
| Surgery primary site | |||
| No | 1 [Reference] | NA | NA |
| Yes | 5.34 (5.05-5.65) | <.001 | 2.80 (2.70-2.90) |
| Unknown | 1.20 (0.63-2.30) | .59 | 1.18 (0.75-1.85) |
| Surgery nonprimary site | |||
| No | 1 [Reference] | NA | NA |
| Yes | 1.23 (1.15-1.31) | <.001 | 1.08 (1.05-1.12) |
| Unknown | 0.58 (0.28-1.22) | .15 | 0.78 (0.49-1.22) |
Abbreviations: CCI, Charlson Comorbidity Index (range: 0-25, with the highest score indicating a higher number of comorbidities); CP, cancer program; MSI, microsatellite instability; NA, not applicable; OR, odds ratio; RR, relative risk.
Race data obtained from the National Cancer Database (NCDB) were self-identified in the patients’ medical records. Other races and non-Hispanic ethnicities include American Indian, Aleutian, or Eskimo; Asian Indian; Asian Indian or Pakistani; Chamorran; Chinese; Filipino; Fiji Islander; Guamanian; Hawaiian; Hmong; Japanese; Kampuchean; Korean; Laotian; Melanesian; Micronesian; New Guinean; Other; Other Asian; Pacific Islander; Polynesian; Samoan; Tahitian; Thai; Tongan; Unknown origin; and Vietnamese.
Ethnicity data obtained from the NCDB were self-identified in the patients’ medical records. Hispanic ethnicity includes Mexican, Puerto Rican, Cuban, South or Central American (except Brazil), Dominican Republic, or Spanish origin.
Other insurance type includes other governmental medical services, such as TRICARE, military, and Veterans Affairs.
Educational level was assessed at the zip code level based on the percentage of adult residents without a high school diploma.
US Census division.
For the event of KRAS testing, older patients (aged 70-79 years) had the lowest likelihood for testing compared with the group aged 18 to 49 years (RR, 0.81; 95% CI, 0.78-0.84; P < .001). Other factors associated with a lower likelihood for KRAS testing (Table 3) included living in an area where the percentage of adults with no high school diploma was 17.6% or greater compared with an area with less than 6.3% (RR, 0.92; 95% CI, 0.88-0.96; P < .001), Medicaid compared with private insurance (RR, 0.94; 95% CI, 0.91-0.98; P < .001), treatment at a community compared with an academic cancer program (RR, 0.92; 95% CI, 0.88-0.96; P < .001), and East South Central facilities compared with New England facilities (RR, 0.78; 95% CI, 0.73-0.83; P < .001). The association of factors with KRAS testing in the univariable analysis is reported in eTable 3 in Supplement 1.
Table 3. Multivariable Logistic Regression Results With Relative Risk Estimates for KRAS Testing.
| Characteristics | KRAS testing | ||
|---|---|---|---|
| OR (95% CI) | P value | RR (95% CI) | |
| Age group, y | |||
| 18-49 | 1 [Reference] | NA | NA |
| 50-59 | 0.84 (0.78-0.90) | <.001 | 0.93 (0.90-0.96) |
| 60-69 | 0.77 (0.71-0.83) | <.001 | 0.90 (0.87-0.92) |
| 70-79 | 0.64 (0.59-0.70) | <.001 | 0.81 (0.78-0.84) |
| Sex | |||
| Male | 1 [Reference] | NA | NA |
| Female | 0.97 (0.93-1.02) | .21 | 0.99 (0.97-1.01) |
| Racea | |||
| White | 1 [Reference] | NA | NA |
| Black | 1.05 (0.99-1.12) | .11 | 1.02 (0.99-1.06) |
| Othera | 0.93 (0.84-1.03) | .14 | 0.97 (0.92-1.01) |
| Ethnicityb | |||
| Non-Hispanica | 1 [Reference] | NA | NA |
| Hispanicb | 1 (0.92-1.10) | <.001 | 1.00 (0.96-1.05) |
| Educational level in area of residence, %c | |||
| <6.3 | 1 [Reference] | NA | NA |
| 6.3-10.8 | 0.95 (0.89-1.01) | .10 | 0.98 (0.95-1.01) |
| 10.9-17.5 | 0.85 (0.79-0.92) | <.001 | 0.93 (0.90-0.96) |
| ≥17.6 | 0.84 (0.77-0.92) | <.001 | 0.92 (0.88-0.96) |
| Unknown | 1.12 (0.51-2.48) | .78 | 1.06 (0.72-1.54) |
| Insurance type | |||
| Private | 1 [Reference] | NA | NA |
| No insurance | 0.91 (0.83-1.00) | .06 | 0.96 (0.91-1.01) |
| Medicaid | 0.88 (0.81-0.94) | <.001 | 0.94 (0.91-0.98) |
| Medicare or otherd | 0.93 (0.87-0.98) | .01 | 0.97 (0.94-0.99) |
| Unknown | 0.83 (0.68-1.02) | .07 | 0.91 (0.82-1.02) |
| Facility type with CP | |||
| Academic | 1 [Reference] | NA | NA |
| Community | 0.85 (0.78-0.92) | <.001 | 0.92 (0.88-0.96) |
| Comprehensive | 0.89 (0.85-0.94) | <.001 | 0.95 (0.92-0.97) |
| Integrated network | 0.97 (0.91-1.04) | .36 | 0.99 (0.96-1.02) |
| Area of residence | |||
| Metropolitan | 1 [Reference] | NA | NA |
| Urban | 1.02 (0.95-1.10) | .62 | 1.01 (0.97-1.05) |
| Rural | 1.02 (0.85-1.22) | .87 | 1.01 (0.92-1.11) |
| Facility locatione | |||
| New England | 1 [Reference] | NA | NA |
| Middle Atlantic | 0.87 (0.78-0.97) | .01 | 0.94 (0.89-0.99) |
| South Atlantic | 0.79 (0.71-0.88) | <.001 | 0.90 (0.85-0.94) |
| East North Central | 0.74 (0.66-0.82) | <.001 | 0.87 (0.83-0.91) |
| East South Central | 0.60 (0.52-0.68) | <.001 | 0.78 (0.73-0.83) |
| West North Central | 0.92 (0.81-1.04) | .19 | 0.96 (0.91-1.02) |
| West South Central | 0.78 (0.69-0.88) | <.001 | 0.89 (0.84-0.94) |
| Mountain | 0.99 (0.87-1.15) | .97 | 1.00 (0.94-1.07) |
| Pacific | 0.98 (0.88-1.10) | .75 | 0.99 (0.94-1.05) |
| Travel distance, miles | |||
| ≤5 | 1 [Reference] | NA | NA |
| 5.1-10 | 1.03 (0.97-1.09) | .28 | 1.01 (0.99-1.04) |
| 10.1-25 | 0.99 (0.94-1.05) | .84 | 1.00 (0.97-1.02) |
| >25 | 0.99 (0.93-1.07) | .93 | 1.00 (0.96-1.03) |
| Year of diagnosis | |||
| 2010 | 1 [Reference] | NA | NA |
| 2011 | 1.30 (1.19-1.42) | <.001 | 1.16 (1.10-1.22) |
| 2012 | 1.52 (1.40-1.66) | <.001 | 1.26 (1.20-1.33) |
| 2013 | 1.54 (1.41-1.68) | <.001 | 1.27 (1.21-1.34) |
| 2014 | 1.62 (1.49-1.77) | <.001 | 1.31 (1.25-1.37) |
| 2015 | 1.69 (1.54-1.84) | <.001 | 1.33 (1.27-1.40) |
| 2016 | 1.78 (1.63-1.95) | <.001 | 1.36 (1.30-1.43) |
| 2017 | 1.79 (1.64-1.96) | <.001 | 1.37 (1.30-1.44) |
| Median household income, US$ | |||
| ≥63 333 | 1 [Reference] | NA | NA |
| 50 354-63 332 | 1.03 (0.96-1.10) | .41 | 1.01 (0.98-1.04) |
| 40 227-50 353 | 0.99 (0.92-1.06) | .74 | 0.99 (0.96-1.03) |
| <40 277 | 0.97 (0.89-1.05) | .41 | 0.98 (0.94-1.02) |
| Unknown | 0.97 (0.45-2.07) | .93 | 0.98 (0.68-1.42) |
| CCI | |||
| 0 | 1 [Reference] | NA | NA |
| 1 | 1.08 (1.02-1.14) | .008 | 1.04 (1.01-1.07) |
| ≥2 | 0.92 (0.84-0.99) | .03 | 0.95 (0.91-0.99) |
| Immunotherapy | |||
| Not administered | 1 [Reference] | NA | NA |
| Administered | 1.68 (1.59-1.79) | <.001 | 1.22 (1.19-1.25) |
| Unknown | 1.32 (0.89-1.96) | .17 | 1.14 (0.95-1.37) |
| Chemotherapy | |||
| Not administered | 1 [Reference] | NA | NA |
| Administered | 3.77 (3.56-3.99) | <.001 | 2.43 (2.34-2.54) |
| Unknown | 1.82 (1.59-2.08) | <.001 | 1.60 (1.46-1.75) |
| Surgery primary site | |||
| No | 1 [Reference] | NA | NA |
| Yes | 1.61 (1.54-1.68) | <.001 | 1.26 (1.24-1.29) |
| Unknown | 0.72 (0.41-1.26) | .25 | 0.82 (0.57-1.18) |
| Surgery nonprimary site | |||
| No | 1 [Reference] | NA | NA |
| Yes | 1.14 (1.07-1.20) | <.001 | 1.05 (1.02-1.08) |
| Unknown | 1.18 (0.61-2.31) | .62 | 1.09 (0.84-1.41) |
Abbreviations: CCI, Charlson Comorbidity Index (range: 0-25, with the highest score indicating a higher number of comorbidities); CP, cancer program; NA, not applicable; OR, odds ratio; RR, relative risk.
Race data obtained from the National Cancer Database (NCDB) were self-identified in the patients’ medical records. Other races and non-Hispanic ethnicities include American Indian, Aleutian, or Eskimo; Asian Indian; Asian Indian or Pakistani; Chamorran; Chinese; Filipino; Fiji Islander; Guamanian; Hawaiian; Hmong; Japanese; Kampuchean; Korean; Laotian; Melanesian; Micronesian; New Guinean; Other; Other Asian; Pacific Islander; Polynesian; Samoan; Tahitian; Thai; Tongan; Unknown origin; and Vietnamese.
Ethnicity data obtained from the NCDB were self-identified in the patients’ medical records. Hispanic ethnicity includes Mexican, Puerto Rican, Cuban, South or Central American (except Brazil), Dominican Republic, or Spanish origin.
Educational level was assessed at the zip code level based on the percentage of adult residents without a high school diploma.
Other insurance type includes other governmental insurance.
US Census division.
Unadjusted survival analysis (eFigures 1 and 2 in Supplement 1) revealed that improved median OS for patients was associatedwith MSI testing vs without testing (23.89 [95% CI, 23.30-24.48] months vs 12.29 [95% CI, 12.01-12.57] months) and with KRAS testing vs without testing (20.40 [95% CI, 20.01-20.79] months vs 10.68 [95% CI, 10.34-11.02] months). The median (IQR) follow-up period for the survival analysis was 13.96 (3.71-29.34) months. In the multivariable Cox proportional hazards regression model, MSI testing (hazard ratio [HR], 0.93; 95% CI, 0.91-0.96; P < .001) and KRAS testing (HR, 0.97; 95% CI, 0.95-1.00; P = .03) were associated with a modest improvement in OS after adjusting for confounders compared with no MSI and KRAS testing. Patterns of increased MSI (14.2% to 50.5%) and KRAS (32.9% to 48.5%) testing are reported between 2010 and 2017, with a 9.2% increase in MSI testing compared with the 0.5% decrease in KRAS testing during the last year of the study (eFigure 3 in Supplement 1). Table 4 highlights the factors associated with OS in this patient cohort.
Table 4. Univariable and Multivariable Cox Proportional Hazards Regression Models for Overall Survival.
| Characteristics | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| MSI testing | ||||
| No | 1 [Reference] | NA | 1 [Reference] | NA |
| Yes | 0.62 (0.60-0.63) | <.001 | 0.93 (0.91-0.96) | <.001 |
| KRAS testing | ||||
| No | 1 [Reference] | NA | 1 [Reference] | NA |
| Yes | 0.71 (0.69-0.72) | <.001 | 0.97 (0.95-1.00) | .03 |
| Age, y | ||||
| 18-49 | 1 [Reference] | NA | 1 [Reference] | NA |
| 50-59 | 1.16 (1.12-1.20) | <.001 | 1.06 (1.02-1.10) | .007 |
| 60-69 | 1.43 (1.38-1.49) | <.001 | 1.16 (1.12-1.21) | <.001 |
| 70-79 | 1.90 (1.83-1.97) | <.001 | 1.30 (1.24-1.35) | <.001 |
| Sex | ||||
| Male | 1 [Reference] | NA | 1 [Reference] | NA |
| Female | 0.96 (0.94-0.98) | <.001 | 0.98 (0.96-1.00) | .07 |
| Ethnicitya | ||||
| Non-Hispanic | 1 [Reference] | NA | 1 [Reference] | NA |
| Hispanic | 0.83 (0.79-0.87) | <.001 | 0.84 (0.79-0.88) | <.001 |
| CCI | ||||
| 0 | 1 [Reference] | NA | 1 [Reference] | NA |
| 1 | 1.18 (1.15-1.21) | <.001 | 1.08 (1.05-1.11) | <.001 |
| ≥2 | 1.60 (1.54-1.66) | <.001 | 1.28 (1.24-1.34) | <.001 |
| Grade | ||||
| Well-differentiated | 1 [Reference] | NA | 1 [Reference] | NA |
| Moderately differentiated | 1.03 (0.97-1.08) | .40 | 1.12 (1.06-1.18) | <.001 |
| Poorly differentiated | 1.59 (1.50-1.69) | <.001 | 1.80 (1.70-1.91) | <.001 |
| Insurance type | ||||
| Private | 1 [Reference] | NA | 1 [Reference] | NA |
| No insurance | 1.38 (1.31-1.45) | <.001 | 1.20 (1.14-1.26) | <.001 |
| Medicaid | 1.26 (1.21-1.31) | <.001 | 1.14 (1.09-1.18) | <.001 |
| Medicare or otherb | 1.56 (1.52-1.60) | <.001 | 1.13 (1.10-1.16) | <.001 |
| Unknown | 1.32 (1.19-1.47) | <.001 | 1.08 (0.98-1.20) | .14 |
| Educational level in area of residence, %c | ||||
| ≥17.6 | 1 [Reference] | NA | 1 [Reference] | NA |
| 10.9-17.5 | 1.03 (1.00-1.06) | .11 | 0.99 (0.96-1.03) | .62 |
| 6.3-10.8 | 0.97 (0.94-1.00) | .02 | 0.98 (0.94-1.02) | .29 |
| Unknown | 1.02 (0.91-1.15) | .69 | 1.06 (0.69-1.62) | .78 |
| Facility type with CP | ||||
| Academic | 1 [Reference] | NA | 1 [Reference] | NA |
| Community | 1.24 (1.19-1.29) | <.001 | 1.07 (1.02-1.12) | .003 |
| Comprehensive | 1.20 (1.17-1.23) | <.001 | 1.13 (1.10-1.17) | <.001 |
| Integrated network | 1.16 (1.13-1.20) | <.001 | 1.13 (1.09-1.16) | <.001 |
| Median household income, US$ | ||||
| ≥63 333 | 1 [Reference] | NA | 1 [Reference] | NA |
| 50 354-63 332 | 1.09 (1.06-1.12) | <.001 | 1.05 (1.01-1.08) | .007 |
| 40 227-50 353 | 1.16 (1.12-1.19) | <.001 | 1.08 (1.04-1.12) | <.001 |
| <40 277 | 1.20 (1.17-1.24) | <.001 | 1.10 (1.05-1.15) | <.001 |
| Unknown | 1.13 (1.01-1.26) | .04 | 0.97 (0.65-1.46) | .89 |
| Year of diagnosis | ||||
| 2010 | 1 [Reference] | NA | 1 [Reference] | NA |
| 2011 | 0.96 (0.92-1.00) | .07 | 0.97 (0.93-1.01) | .18 |
| 2012 | 0.93 (0.89-0.97) | <.001 | 0.92 (0.88-0.96) | <.001 |
| 2013 | 0.95 (0.91-0.99) | .01 | 0.96 (0.92-1.00) | .07 |
| 2014 | 0.96 (0.92-1.00) | .05 | 0.98 (0.94-1.02) | .31 |
| 2015 | 0.91 (0.87-0.95) | <.001 | 0.93 (0.89-0.97) | .002 |
| 2016 | 0.86 (0.82-0.90) | <.001 | 0.91 (0.87-0.95) | <.001 |
| 2017 | 0.81 (0.77-0.85) | <.001 | 0.83 (0.79-0.87) | <.001 |
| Immunotherapy | ||||
| Not administered | 1 [Reference] | NA | 1 [Reference] | NA |
| Administered | 0.65 (0.63-0.67) | <.001 | 0.85 (0.82-0.87) | <.001 |
| Unknown | 0.85 (0.68-1.05) | .13 | 0.97 (0.77-1.21) | .76 |
| Chemotherapy | ||||
| Not administered | 1 [Reference] | NA | 1 [Reference] | NA |
| Administered | 0.31 (0.30-0.32) | <.001 | 0.37 (0.36-0.38) | <.001 |
| Unknown | 0.41 (0.38-0.44) | <.001 | 0.49 (0.45-0.52) | <.001 |
| Lung metastasis at presentation | ||||
| Absent | 1 [Reference] | NA | 1 [ Reference] | NA |
| Present | 1.33 (1.30-1.37) | <.001 | 1.16 (1.13-1.19) | <.001 |
| Unknown | 1.51 (1.39-1.64) | <.001 | 1.07 (0.98-1.17) | .14 |
| Liver metastasis at presentation | ||||
| Absent | 1 [Reference] | NA | 1 [Reference] | NA |
| Present | 1.10 (1.07-1.13) | <.001 | 1.27 (1.23-1.30) | <.001 |
| Unknown | 1.52 (1.33-1.73) | <.001 | 1.09 (0.95-1.26) | .21 |
| Surgery primary site | ||||
| No | 1 [Reference] | NA | 1 [Reference] | NA |
| Yes | 0.46 (0.45-0.47) | <.001 | 0.53 (0.52-0.55) | <.001 |
| Unknown | 0.54 (0.39-0.74) | <.001 | 0.58 (0.42-0.82) | .002 |
| Surgery nonprimary site | ||||
| No | 1 [Reference] | NA | 1 [Reference] | NA |
| Yes | 0.56 (0.54-0.58) | <.001 | 0.81 (0.79-0.84) | <.001 |
| Unknown | 0.56 (0.38-0.83) | .003 | 0.78 (0.53-1.15) | .21 |
| Area of residence | ||||
| Metropolitan | 1 [Reference] | NA | 1 [Reference] | NA |
| Urban | 1.11 (1.08-1.15) | <.001 | 1.04 (1.00-1.08) | .07 |
| Rural | 1.16 (1.06-1.26) | .001 | 1.11 (1.02-1.22) | .02 |
| Facility locationd | ||||
| New England | 1 [Reference] | NA | 1 [Reference] | NA |
| Middle Atlantic | 0.91 (0.86-0.96) | .001 | 0.85 (0.80-0.90) | <.001 |
| South Atlantic | 1.03 (0.98-1.09) | .28 | 1.02 (0.96-1.08) | .56 |
| East North Central | 1.08 (1.02-1.14) | .01 | 1.08 (1.02-1.14) | .008 |
| East South Central | 1.07 (1.00-1.15) | .04 | 1.03 (0.97-1.11) | .35 |
| West North Central | 1.03 (0.97-1.10) | .35 | 1.06 (1.00-1.13) | .07 |
| West South Central | 0.96 (0.90-1.02) | .21 | 0.84 (0.79-0.89) | <.001 |
| Mountain | 1.01 (0.94-1.09) | .73 | 0.94 (0.87-1.00) | .07 |
| Pacific | 0.98 (0.93-1.04) | .50 | 0.98 (0.92-1.04) | .45 |
| Travel distance, miles | ||||
| ≤5 | 1 [Reference] | NA | 1 [Reference] | NA |
| 5.1-10 | 0.94 (0.92-0.97) | <.001 | 0.98 (0.95-1.01) | .22 |
| 10.1-25 | 0.90 (0.87-0.93) | <.001 | 0.99 (0.97-1.03) | .75 |
| >25 | 0.90 (0.87-0.93) | <.001 | 0.99 (0.96-1.03) | .71 |
Abbreviations: CCI, Charlson Comorbidity Index (range: 0-25, with the highest score indicating a higher number of comorbidities); CP, cancer program; HR, hazard ratio; MSI, microsatellite instability; NA, not applicable.
Ethnicity data obtained from the NCDB were self-identified in the patients’ medical records. Hispanic ethnicity includes Mexican, Puerto Rican, Cuban, South or Central American (except Brazil), Dominican Republic, or Spanish origin.
Other insurance type includes other governmental insurance.
Educational level was assessed at the zip code level based on the percentage of adult residents without a high school diploma.
US Census division.
Discussion
This nationwide analysis found associations of socioeconomic and demographic factors with decreased MSI and KRAS testing. Sociodemographic factors, including community-setting treatment, treatment at East South Central facilities, lower educational levels in area of residence, and older age, were associated with a reduced likelihood of MSI and KRAS testing. Rural residence was associated with lower odds of MSI testing only. Slightly improved OS outcomes were reported for patients who underwent MSI tests.
Patients treated at community cancer programs and comprehensive community cancer programs had lower odds of receiving biomarker testing than those at academic programs. Academic facilities may be more likely to order biomarker testing due to their research focus, access to specialized resources, and involvement in clinical trials. A recent survey revealed that oncologists at academic centers were more likely to order biomarker testing at the time of initial biopsy than oncologists in community cancer programs (76% vs 52%) and to involve the patient’s family in biomarker testing discussions (85% vs 59%; P = .009).23 Furthermore, in a survey by the Association of Community Cancer Centers, over half of the respondents indicated their programs had no standard protocol for biomarker testing, whereas 14% were unsure if any protocols existed in their institutions.24 The same survey revealed that patients at these facilities required psychosocial support, genetic counseling, and financial assistance.24 Given that most US patients with cancer are treated in community settings, addressing methods to increase compliance with biomarker testing in community cancer programs is crucial.25
Moreover, patients residing in areas with lower educational level demonstrated lower MSI and KRAS testing rates, possibly due to a limited understanding of biomarker testing’s utility. While we could not assess linguistic abilities due to insufficient information in the NCDB and, although Hispanic ethnicity was not associated with a lack of testing, we contend that patients’ understanding of medical terminology and the purpose of testing were below physicians’ expectations. This disparity is exemplified in an analysis reporting that almost half of the patients could not describe specific terms related to biomarker testing immediately after an explanation by their oncologists.26 False perceptions toward biomarker testing can be attributed to lower health literacy levels and limited efforts by clinicians to explain comprehensively the importance of testing, both of which can be deterrents for patients with lower educational levels.
Patients residing in rural areas were less likely to receive biomarker testing. These patients could face logistic challenges in accessing specialized tertiary centers or diagnostic facilities that offer genetic testing. This finding aligns with the geographic association between US Census divisions and the outcome, where East South Central facilities had the lowest likelihood of MSI and KRAS testing, followed by West South Central compared with New England facilities. The widespread rural landscape in these regions may explain the association. According to data from the US Census Bureau, states such as Mississippi, Arkansas, Alabama, and Kentucky are among the top states with rural populations.27 It is also important to analyze rurality in the context of the vicinity of urban or metropolitan areas given that these states also have the lowest urban populations and are not in proximity to major urban cities. Additionally, national data recorded the lowest levels of bachelor’s degree attainment in South Central states, such as Kentucky, Louisiana, Arkansas, and Mississippi.28 These demographic factors may explain the lower rates of MSI and KRAS testing in these regions given the association of rurality and educational level with the primary outcome. We believe that this finding poses a serious dilemma because the Centers for Disease Control and Prevention reported that Mississippi and Kentucky in East South Central, along with Louisiana and Arkansas in West South Central, exhibited notably higher incident CRC cases than other states.29 Consequently, the same states had among the highest rates of CRC deaths over the years studied and up to the present date.29 Although granular state-specific data would assist in identifying the risk factors for the suboptimal outcomes, the lower likelihood for patients to undergo testing, compounded by the high incidence in these regions, may be associated with these worse outcomes.
Patients with late-onset disease (≥50 years) were less likely to undergo biomarker testing. This finding can be explained by the initial recommendation of MSI testing for younger patients with a hereditary component, such as Lynch syndrome. Prior to the US Food and Drug Administration approval of pembrolizumab for MSI-high tumors, MSI testing was predominantly performed to identify patients likely to have Lynch syndrome and to assess outcome.30,31 Alternatively, the gradual decrease in likelihood with the incremental increase in age groups for both tests may implicate the possible lack of compliance by physicians and/or patients. Patients with late-onset disease may not have been fully aware of the importance of MSI and KRAS testing due to the relative novelty of the field during the present study. The findings align with historical evidence of the increased acceptance of genetic testing in younger patients.32 It is plausible that concerns regarding the burdensome treatment after testing and its implications for quality of life may have deterred older patients, considering their high comorbidity score and limited life expectancy. On the other hand, physicians’ perceptions may have dissuaded them from testing older patients due to concerns about toxic effects and/or perceived limited effectiveness.
The results of the present study did not show an association with insurance, which may be interpreted in different ways. First, health coverage alone may not explain disparities in testing, and other factors can be considered. Second, access to private insurance and government insurance (Medicaid and Medicare) differs by state. Third, while some states had legislation mandating coverage for biomarker testing during the study period, others did not implement such legislation until 2021, suggesting that insurance did not play a crucial role in accessing biomarker testing during the study.33
While this study did not find any association between race and ethnicity and biomarker testing, evidence of racial and ethnic disparities is documented in the literature.34 The lack of significance after adjusting for covariates suggests that racial and ethnic disparities may be mediated, at least partially, through socioeconomic, demographic, and clinical variables. Moreover, race and ethnicity can serve as a precursor for a cascade of socioeconomic disparities. The multifaceted association among these variables confirms the complexity of drawing conclusions from analyses of social determinants of health.
Clinical Implications
Survival benefits were associated with MSI and KRAS testing, as demonstrated by the Kaplan-Meier analysis and univariable Cox proportional hazards regression model. Nevertheless, the benefit was diluted when therapeutic interventions (and other factors) were accounted for in the multivariable analysis. The difference between the univariable and multivariable associations was the nature of the intervention itself given that testing guides therapeutic choices. These findings highlight the prominence of confounding clinical variables on survival outcomes and the importance of timely treatment follow-up after testing, given the abundant literature supporting the advantages of tailored therapeutics.8,35,36,37,38 Additionally, the significant difference between the unadjusted and adjusted models suggests that testing acts as a surrogate of overall cancer care, reflecting broader systemic disparities in treatment access and quality. Patients without biomarker testing can have a perpetual vulnerability of lack of access to other treatment options. In short, disparities in MSI and KRAS testing can compromise optimal mCRC treatment.
Temporal Patterns of Biomarker Testing
Despite the apparent underuse of biomarkers, our study demonstrated a progressive increase in testing. The percentage of patients who were tested significantly increased from 32.9% to 48.5% for KRAS and from 14.2% to 50.5% for MSI. Although KRAS testing was integrated into national guidelines earlier than MSI testing, MSI testing became more prevalent than KRAS testing during the last year of the study, with a 9.2% increase in MSI testing and 0.5% decrease in KRAS testing between 2016 and 2017. The treatment landscape for mCRC has been rapidly expanding to include various MSI-tailored immunotherapies associated with substantially improved outcomes, more so than KRAS-guided treatments. Nevertheless, while these findings may be affected by historical and contemporary societal structural policies, the recent improvement in testing implies that familiarization with this novel field could help incorporate testing that is concordant with national recommendations.
Limitations
The study’s retrospective nature did not account for nonobservable confounding variables. Moreover, data were extrapolated from the NCDB, which collects predesignated variables from hospitals, limiting the ability to report the specific timing of testing and the type of test used. Due to the unavailability of the exact time of testing, we considered the date of diagnosis as the start of the follow-up period for the survival analysis. Meanwhile, it is important to highlight the geographic context of educational level and median household incomes in the analysis. Additionally, the NCDB does not report patients who did not undergo the test after 2017, restricting the inclusion of patients with recent years of diagnosis. We also acknowledge that biomarker testing is a surrogate of overall care and that the heterogeneity of institutions within the NCDB is a factor in variations in quality of care. Nevertheless, using the NCDB, which encompasses the largest number of facilities and spans US geography, to investigate topics of disparities provides valuable insight into the implementation of clinical practice.
Conclusions
In this cohort study of patients with mCRC, older age, lower educational level in area of residence, community-setting treatment, and treatment at East South Central facilities were associated with a lower likelihood of MSI and KRAS testing. By highlighting the sociodemographic-based disparities in biomarker testing using national registries, we can develop strategies for promoting equity in cancer care and improving outcomes for underserved populations. Further research is encouraged to assess biomarker testing at the state level rather than the regional level, with particular emphasis on sociodemographically vulnerable populations.
eAppendix. Definition of Socioeconomic and Demographic Factors
eFigure 1. Kaplan Meier Overall Survival Curves Based on MSI Testing
eFigure 2. Kaplan Meier Overall Survival Curves Based on KRAS Testing
eFigure 3. Pattern of Biomarker Testing per Year of Diagnosis
eTable 1. US Census Division of the Regions by States
eTable 2. Univariable Association of Factors With MSI Testing
eTable 3. Univariable Association of Factors With KRAS Testing
eReferences
Data Sharing Statement
References
- 1.Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233-254. doi: 10.3322/caac.21772 [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute . Cancer stat facts: colorectal cancer. Accessed October 19, 2023. https://seer.cancer.gov/statfacts/html/colorect.html
- 3.Hong DS, Fakih MG, Strickler JH, et al. KRASG12C inhibition with sotorasib in advanced solid tumors. N Engl J Med. 2020;383(13):1207-1217. doi: 10.1056/NEJMoa1917239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Nicolantonio F, Vitiello PP, Marsoni S, et al. Precision oncology in metastatic colorectal cancer - from biology to medicine. Nat Rev Clin Oncol. 2021;18(8):506-525. doi: 10.1038/s41571-021-00495-z [DOI] [PubMed] [Google Scholar]
- 5.Han CB, Li F, Ma JT, Zou HW. Concordant KRAS mutations in primary and metastatic colorectal cancer tissue specimens: a meta-analysis and systematic review. Cancer Invest. 2012;30(10):741-747. doi: 10.3109/07357907.2012.732159 [DOI] [PubMed] [Google Scholar]
- 6.Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28(31):4697-4705. doi: 10.1200/JCO.2009.27.4860 [DOI] [PubMed] [Google Scholar]
- 7.Moreira L, Balaguer F, Lindor N, et al. ; EPICOLON Consortium . Identification of Lynch syndrome among patients with colorectal cancer. JAMA. 2012;308(15):1555-1565. doi: 10.1001/jama.2012.13088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.André T, Shiu KK, Kim TW, et al. ; KEYNOTE-177 Investigators . Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med. 2020;383(23):2207-2218. doi: 10.1056/NEJMoa2017699 [DOI] [PubMed] [Google Scholar]
- 9.Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18(9):1182-1191. doi: 10.1016/S1470-2045(17)30422-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benson AB, Venook AP, Al-Hawary MM, et al. Colon cancer, version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19(3):329-359. doi: 10.6004/jnccn.2021.0012 [DOI] [PubMed] [Google Scholar]
- 11.Warren Andersen S, Blot WJ, Lipworth L, Steinwandel M, Murff HJ, Zheng W. Association of race and socioeconomic status with colorectal cancer screening, colorectal cancer risk, and mortality in southern US adults. JAMA Netw Open. 2019;2(12):e1917995. doi: 10.1001/jamanetworkopen.2019.17995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramkumar N, Colla CH, Wang Q, O’Malley AJ, Wong SL, Brooks GA. Association of rurality, race and ethnicity, and socioeconomic status with the surgical management of colon cancer and postoperative outcomes among Medicare beneficiaries. JAMA Netw Open. 2022;5(8):e2229247. doi: 10.1001/jamanetworkopen.2022.29247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Augustus GJ, Ellis NA. Colorectal cancer disparity in African Americans: risk factors and carcinogenic mechanisms. Am J Pathol. 2018;188(2):291-303. doi: 10.1016/j.ajpath.2017.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fulton JE. Physical inactivity is more common among racial and ethnic minorities in most states. Accessed October 19, 2023. https://blogs.cdc.gov/healthequity/2020/04/01/physical-inactivity/
- 15.Gawron AJ, Yadlapati R. Disparities in endoscopy use for colorectal cancer screening in the United States. Dig Dis Sci. 2014;59(3):530-537. doi: 10.1007/s10620-013-2937-x [DOI] [PubMed] [Google Scholar]
- 16.May FP, Yang L, Corona E, Glenn BA, Bastani R. Disparities in colorectal cancer screening in the United States before and after implementation of the Affordable Care Act. Clin Gastroenterol Hepatol. 2020;18(8):1796-1804.e2. doi: 10.1016/j.cgh.2019.09.008 [DOI] [PubMed] [Google Scholar]
- 17.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544-573. doi: 10.1002/cncr.24760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tramontano AC, Chen Y, Watson TR, Eckel A, Hur C, Kong CY. Racial/ethnic disparities in colorectal cancer treatment utilization and phase-specific costs, 2000-2014. PLoS One. 2020;15(4):e0231599. doi: 10.1371/journal.pone.0231599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakkila BF, Kerekes D, Nunez-Smith M, et al. Evaluation of racial disparities in quality of care for patients with gastrointestinal tract cancer treated with surgery. JAMA Netw Open. 2022;5(4):e225664. doi: 10.1001/jamanetworkopen.2022.5664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dharwadkar P, Greenan G, Stoffel EM, et al. Racial and ethnic disparities in germline genetic testing of patients with young-onset colorectal cancer. Clin Gastroenterol Hepatol. 2022;20(2):353-361.e3. doi: 10.1016/j.cgh.2020.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dillon J, Ademuyiwa FO, Barrett M, et al. Disparities in genetic testing for heritable solid-tumor malignancies. Surg Oncol Clin N Am. 2022;31(1):109-126. doi: 10.1016/j.soc.2021.08.004 [DOI] [PubMed] [Google Scholar]
- 22.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683-690. doi: 10.1245/s10434-007-9747-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boehmer L, Roy UKB, Schrag J, et al. Identifying barriers to equitable biomarker testing in underserved patients with NSCLC: a mixed-methods study to inform quality improvement opportunities. J Clin Oncol. 2021;39(28 suppl):123. doi: 10.1200/JCO.2020.39.28_suppl.123 [DOI] [Google Scholar]
- 24.Association of Community Cancer Centers . Assessing the status of biomarker testing in metastatic colorectal cancer and the challenges faced by community cancer care teams. February 2021. Accessed March 20, 2024. https://www.accc-cancer.org/docs/projects/colorectal-cancer-biomarker/mcrc_survey-summary_final-(1).pdf?sfvrsn=20c6687_0
- 25.Petrelli NJ. A community cancer center program: getting to the next level. J Am Coll Surg. 2010;210(3):261-270. doi: 10.1016/j.jamcollsurg.2009.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blee SM, Shah RP, Pinheiro APM, et al. Physician communication and patient understanding of molecular testing terminology. Oncologist. 2021;26(11):934-940. doi: 10.1002/onco.13930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.US Census Bureau. Census Bureau data . Accessed March 2024. https://data.census.gov/
- 28.World Population Review. Educational attainment by state. Accessed October 19, 2023. https://worldpopulationreview.com/state-rankings/educational-attainment-by-state
- 29.US Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute . US cancer statistics data visualizations tool. Accessed March 2024. https://www.cdc.gov/cancer/dataviz
- 30.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96(4):261-268. doi: 10.1093/jnci/djh034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.US Food and Drug Administration . FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. Accessed May 12, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-first-tissuesite-agnostic-indication
- 32.Aro AR, Hakonen A, Hietala M, et al. Acceptance of genetic testing in a general population: age, education and gender differences. Patient Educ Couns. 1997;32(1-2):41-49. doi: 10.1016/S0738-3991(97)00061-X [DOI] [PubMed] [Google Scholar]
- 33.Sadigh G, Goeckner HG, Kazerooni EA, et al. State legislative trends related to biomarker testing. Cancer. 2022;128(15):2865-2870. doi: 10.1002/cncr.34271 [DOI] [PubMed] [Google Scholar]
- 34.Bruno DS, Hess LM, Li X, Su EW, Patel M. Disparities in biomarker testing and clinical trial enrollment among patients with lung, breast, or colorectal cancers in the United States. JCO Precis Oncol. 2022;6:e2100427. doi: 10.1200/PO.21.00427 [DOI] [PubMed] [Google Scholar]
- 35.Lenz HJ, Van Cutsem E, Luisa Limon M, et al. First-line nivolumab plus low-dose ipilimumab for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: the phase II CheckMate 142 study. J Clin Oncol. 2022;40(2):161-170. doi: 10.1200/JCO.21.01015 [DOI] [PubMed] [Google Scholar]
- 36.Zocche DM, Ramirez C, Fontao FM, Costa LD, Redal MA. Global impact of KRAS mutation patterns in FOLFOX treated metastatic colorectal cancer. Front Genet. 2015;6:116. doi: 10.3389/fgene.2015.00116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loupakis F, Ruzzo A, Cremolini C, et al. KRAS codon 61, 146 and BRAF mutations predict resistance to cetuximab plus irinotecan in KRAS codon 12 and 13 wild-type metastatic colorectal cancer. Br J Cancer. 2009;101(4):715-721. doi: 10.1038/sj.bjc.6605177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408-1417. doi: 10.1056/NEJMoa0805019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Definition of Socioeconomic and Demographic Factors
eFigure 1. Kaplan Meier Overall Survival Curves Based on MSI Testing
eFigure 2. Kaplan Meier Overall Survival Curves Based on KRAS Testing
eFigure 3. Pattern of Biomarker Testing per Year of Diagnosis
eTable 1. US Census Division of the Regions by States
eTable 2. Univariable Association of Factors With MSI Testing
eTable 3. Univariable Association of Factors With KRAS Testing
eReferences
Data Sharing Statement