Abstract
Objective
Maternal thyroid autoimmunity and thyroid function in early pregnancy may impact fetal neurodevelopment. We aimed to investigate how thyroid autoimmunity and thyroid function in early pregnancy were associated with language acquisition in offspring at 12–36 months of age.
Methods
This study was embedded in the prospective Odense child cohort. Mother–child dyads were excluded in case of maternal intake of thyroid medication during pregnancy. The parents completed MacArthur–Bates Communicative Development Inventories (MB-CDI) every third month to assess their offspring’s productive vocabulary. All completed reports for each child were included in the analyses. Logistic growth curve models evaluated associations between MB-CDI scores and levels of maternal thyroid peroxidase antibodies (TPOAb), free thyroxine (FT4), and thyrotropin, respectively, measured in early pregnancy (median gestational week 12). All models were stratified by offspring sex and adjusted for maternal age, education, pre-pregnancy body mass index, parity, breastfeeding, and offspring age.
Results
The study included 735 mother–child dyads. Children born to mothers with TPOAb ≥11 kIU/L, opposed to TPOAb <11 kIU/L, had a lower probability of producing words at age 18–36 months for girls (OR = 0.78, P < 0.001) and 33–36 months for boys (OR = 0.83, P < 0.001). The probability of producing words was higher in girls at 30–36 months of age with low-normal maternal FT4 vs high-normal FT4 (OR = 0.60, P < 0.001), and a similar trend was seen in boys. Results were ambiguous for thyrotropin.
Conclusion
In women without known thyroid disease, TPOAb positivity in early pregnancy was negatively associated with productive vocabulary acquisition in girls and boys. This association was not mediated by a decreased thyroid function, as low-normal maternal FT4, unexpectedly, indicated better vocabulary acquisition. Our results support that maternal thyroid autoimmunity per se may affect fetal neurodevelopment.
Keywords: thyroid, thyroid autoimmunity, language acquisition, MacArthur–Bates communicative development inventories, Odense child cohort
Introduction
Fetal neurodevelopment is vulnerable to alterations in the prenatal environment, and various exposures may have lifelong consequences (1). Early language acquisition is an indicator of fetal neurodevelopment (2) and is of particular interest as it predicts later intelligence quotient (IQ) (3) and academic achievements (4, 5, 6). Healthy children speak their first words around 12 months of age (7, 8). By the second year, the expressive vocabulary accelerates, averaging 258 words at 24 months in Danish children (9). Significant individual differences exist in the timing and pace of early language acquisition, with faster vocabulary growth for females (7, 8, 10) and children of highly educated parents (7). Also, genetic (11), endocrine (1, 12), and environmental factors (13) are likely to be part of complex interactions affecting early language acquisition.
The prevalence of thyroid autoimmunity assessed by thyroid peroxidase antibodies (TPOAb) among pregnant women, with or without thyroid dysfunction, is estimated to be 5–14% (14). Whether maternal thyroid autoimmunity per se affects the fetus is much debated (15), and studies in this field are conflicting (16, 17, 18, 19). Maternal TPOAb positivity in early pregnancy has been associated with poorer intelligence scores on the Bayley Scale of Infant Development in the offspring at 25–30 months (16). In two mother–child cohorts from the Netherlands (Generation R) and the United Kingdom (ALSPAC), respectively, an association was found between maternal TPOAb positivity and lower non-verbal IQ in children at age 5–8 years in the Generation R cohort (20) but not in the ALSPAC cohort (20). No association existed between maternal TPOAb positivity and language performance, measured with the Language Development Survey, in children at 30 months (18).
During normal pregnancy, the maternal thyroid gland increases its hormone production, as the fetus relies on maternal thyroid hormone supply in the first half of pregnancy (21). The high level of human chorionic gonadotropin (hCG) in early pregnancy stimulates the maternal thyroid due to its structural and functional similarities with thyrotropin (TSH) (21). As a result, maternal serum TSH decreases, and serum thyroxine increases during the first trimester (22). Current guidelines recommend TSH assessment to estimate maternal thyroid function during pregnancy (22), although free thyroxine (FT4) may be a more appropriate measure of the thyroid status in the first trimester due to the hCG-induced reduction in maternal TSH levels (23).
TPOAb positivity is a risk factor for developing maternal hypothyroidism during pregnancy, as the increased demands for thyroid hormones may not be met in case the reserve capacity of the thyroid function is compromised. A sufficient maternal thyroid hormone supply in early pregnancy is crucial for normal fetal neurodevelopment (23). Accordingly, maternal hypothyroidism has been associated with poorer language performance (24) and lower child IQ (25). Whereas overt hypothyroidism in pregnancy clearly has negative implications for both the pregnancy and the fetus, evidence is conflicting in the case of minor thyroid dysfunction (16, 26). Most observational studies have shown that mild maternal hypothyroidism has a negative impact on various aspects of fetal neurodevelopment, while the few randomized studies performed in this area had a neutral outcome (26, 27). Studies investigating the association between maternal thyroid function within the normal range and language acquisition in the offspring are scarce.
In the present study, we add further knowledge to this area, focusing on language acquisition in children born to women without known thyroid disorders. Based on a large and well-characterized Danish cohort of pregnant women, we investigated whether coinciding maternal thyroid autoimmunity and subtle variations in thyroid function in early pregnancy are associated with the vocabulary development in the offspring up to the age of 36 months, and whether such an association differs between offspring sexes.
Methods
Population
This study was part of the Odense child cohort (OCC), a single-center prospective study of pregnant women and their offspring in Odense municipality, Denmark. The women were recruited consecutively in the period 2010–2012 (28). Of 6707 eligible pregnant women, 4017 were invited to the OCC, of whom 2874 were included. Comparisons between included pregnant women and non-participants are described elsewhere (28).
Maternal thyroid autoantibodies and hormones
The mothers had a fasting blood sample drawn at median gestational week 12 (range: 8–20 weeks). Blood samples, stored at Odense Patient data Explorative Network (OPEN) at −80°C, were analyzed for TPOAb (ACN code: 10066), FT4 (ACN code: 10160), and TSH (ACN code: 10172) using electrochemiluminescence immunoassay on the E801 module at the Roche Cobas 8000 platform (Roche Diagnostics GmbH). The intra-assay coefficients of variation for TPOAb were 15.0% and 12.5% at concentrations of 17.5 and 24.0 kIU/L, respectively; for FT4, coefficients of variation were 3.6% and 3.8% at concentrations of 16.2 and 35.1 pmol/L, respectively; for TSH, coefficients of variation were 7.2% and 4.6% at concentrations of 0.08 and 11.0 mIU/L, respectively (29).
Child productive vocabulary
We used the validated Danish adaptation (8) of the MacArthur–Bates Communicative Development Inventories (MB-CDIs) to assess early language development (30). The MB-CDI consists of checklists assessing the parents’ self-reported knowledge of their children's language skills, with specific questions about receptive and productive vocabulary, gestures, and grammar. In this study, we only included data on productive vocabulary, as this has proven more reliable than receptive vocabulary (31). The MB-CDI consists of two instruments: (1) the MB-CDI: Words and Gestures(MB-CDI: WG), which is normed for toddlers and includes 410 commonly used words from 20 semantic categories; and (2) the MB-CDI: Words and Sentences(MB-CDI: WS), which is normed for older children up to 3 years of age and consists of 725 words from 22 semantic categories. Electronic versions of these two instruments were sent out to the parents every third month when the offspring was 12–18 months (MB-CDI: WG) and 21−36 months (MB-CDI: WS) for a maximum of nine possible measurements per child.
Covariates
Possible covariates of the effect of maternal thyroid autoantibodies and thyroid hormones on language acquisition were detected through previous literature. Offspring vocabulary size is positively associated with increasing offspring age (32) and maternal education level (33) but negatively associated with multiparity (34). Previous studies have shown positive associations between breastfeeding and cognition (35). Breastfeeding might be influenced by maternal age, smoking, BMI, and education level (36). Further, thyroid hormone levels (37) and education (38) are associated with BMI. Thyroid hormone levels are also associated with age (22) and smoking (39). Thus, several possible covariates were included in this study (Supplementary Figure 1, see section on supplementary materials given at the end of this article).
Maternal age, pre-pregnancy BMI (<18.5; 18.5–25; >25), parity (nulliparous, primiparous, or multiparous), highest achieved education (low, intermediate, or high), breastfeeding (weeks of exclusive breastfeeding) (40), and smoking status during pregnancy (yes or no) were obtained from questionnaires collected during pregnancy and post partum or from hospital records using social security numbers. Education was categorized as low (‘high school or less’), intermediate (‘high school + 1–4 years’), and high (‘high school + more than four years’). Offspring sex and birth characteristics were derived from obstetric/pediatric hospital records. Information about the mother’s country of birth was retrieved from a register in Odense Municipality. Presence of thyroid disease was determined through a questionnaire distributed during gestational week 28, asking about the use of over-the-counter and prescription medication, including the name of the drug, duration, dosage, and use in gestational week 4–9, 10–14, 15–19, 20–24, and 25–29 (41). Mothers were excluded if they received thyroid medication at any time during pregnancy.
Statistical analysis
Baseline characteristics are reported as mean ± standard deviation (s.d.), median (25th; 75th percentile), or numbers (percentage). Data normality was inspected via histograms and quantile-quantile (QQ) plots. Maternal and offspring characteristics were compared between subgroups using the Wilcoxon rank sum test for non-normally distributed continuous data, independent t-test for normally distributed continuous data, and χ 2-test for categorical data.
Data from all completed MB-CDI reports for each child was included in the analyses. Productive vocabulary scores were operationalized as binary values for each word in the MB-CDI (1 = produced; 0 = not produced) to account for by-item variance. The optimal functional form of the compiled trajectories was identified by logistic mixed-effects unconditional means models (i.e. intercept-only models including all relevant covariates) at step 1, followed by unconditional growth models (i.e. by including offspring age as a predictor) at step 2. The effect of maternal thyroid autoimmunity (TPOAb) and thyroid function (FT4 and TSH) on MB-CDI scores were then evaluated at step 3 in separate conditional growth models, with each variable added as a predictor (Model 1-6). Model 1 was divided according to TPOAb <11.0 kIU/L vs ≥11.0 kIU/L, based on the validated cut-off level for TPOAb positivity at Odense University Hospital (42). Model 2 used TPOAb as a continuous variable. Model 3 arbitrarily divided FT4 in <20th percentile vs >80th percentile. Model 4 used FT4 as a continuous variable. Model 5 arbitrarily divided TSH in ≤2.5 mIU/L vs >2.5 mIU/L. A similar analysis was conducted using 3.5 mIU/L as the threshold (Supplementary Figure 2 and Supplementary Table 1); however, the statistical power was limited due to few cases with TSH >3.5 mIU/L. Model 6 used TSH as a continuous variable.
Random intercepts by participants were added to all models to account for repeated measures. Model comparison by means of (Bayesian) Akaike Information Criterion and log-likelihood tests was then used to compare the added fit value at step 3 compared to steps 1 and 2. Estimates from conditional growth models are reported as standard odds ratios (ORs) with standard error (SE) and 95% confidence intervals (CIs). Significant effects were interpreted by calculating average predicted probabilities for each conditional growth model, using representative values for the continuous predictors and average values for categorical predictors. The predicted probabilities were then plotted with 95% CI for each model. The average predicted probabilities with SE and 95% CI are also compiled in tables. As TSH was not normally distributed, a logarithmic transformation was applied before inclusion in the models. Analyses were run separately for girls and boys since previous studies have shown a gender difference in early productive vocabulary scores on the MB-CDI (8, 10). All regression models were performed as complete case analyses, assuming missingness at random and with acceptable power even after missing case deletion. To evaluate this approach, all models were repeated with imputation.
In sensitivity analyses, all models were adjusted for maternal ethnicity, smoking, and diabetes mellitus (including gestational diabetes mellitus), as these factors may interfere with the association between maternal thyroid function and offspring language acquisition (29). In an additional sensitivity analysis, TPOAb-positive women (i.e. TPOAb ≥11.0 kIU/L) were excluded to determine whether the results were significantly different in offspring from TPOAb-negative mothers. Lastly, a sensitivity analysis was done including mothers with missing information on the use of thyroid medication during pregnancy.
All analyses were run in R version 4.3.0 (43) using the lme4 package version 1.1-33 (44) for model fitting and the sjPlot package version 2.8.14 (45) for estimating predicted probabilities. Model convergence was aided by means of Newton–Levenberg–Marquardt optimization. Statistical tests were two-sided, and the level of statistical significance was P < 0.05.
Results
Characteristics of participants
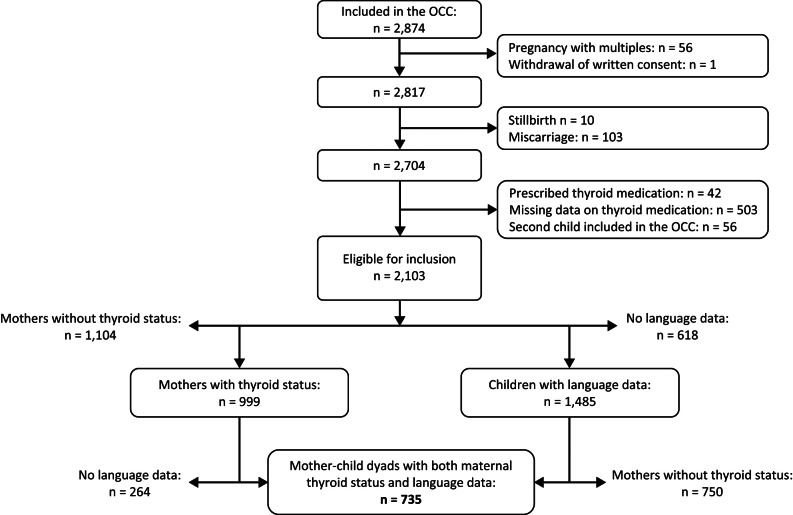
A flowchart of included participants is shown in Fig. 1. We excluded women with stillbirth (n = 10), miscarriage (n = 103), pregnancy with multiples (n = 56), women taking prescribed thyroid medication (n = 42), and women with missing information about thyroid medication (n = 503). Of the remaining women, 56 were pregnant more than once during the inclusion period, and only the first pregnancy was included. Of the eligible mother–child dyads, 735 (352 girls and 383 boys) were included in the analysis, all having a maternal first-trimester thyroid status and at least one MB-CDI report (Fig. 1).
Figure 1.
Flowchart of participants. OCC, Odense Child Cohort.
Table 1 shows the baseline characteristics of the included mother–child dyads. Mothers carrying girls were comparable to those carrying boys in age, parity, smoking, education level, ethnicity, and first-trimester TPOAb and TSH levels. Mothers carrying girls had significantly lower pre-pregnancy BMI and higher FT4 than mothers carrying boys. Boys had significantly higher body weight and length at birth than girls, while gestational age at delivery was similar for both sexes. The period of exclusive breastfeeding was similar between girls and boys.
Table 1.
Baseline characteristics of mother–child dyads. Data are presented as mean ± s.d.or median (25th; 75th percentile) for continuous variables or as n (%) for categorical variables. Values in bold indicate statistical significance.
| All (n = 735) | Girls (n = 352) | Boys (n = 383) | P* | |
|---|---|---|---|---|
| Maternal characteristics | ||||
| Maternal age (years) | 30.3 ± 4.5 | 30.2 ± 4.3 | 30.4 ± 4.7 | 0.57 |
| Pre-pregnancy BMI (kg/m2) | 23.4 (21.3;26.6) | 22.8 (21.1;26.0) | 23.8 (21.5;27.1) | 0.003 |
| Parity | 0.31 | |||
| Nulliparous | 436 (59.3) | 202 (57.4) | 234 (61.1) | |
| Primi- and multi-parous | 299 (40.7) | 150 (42.6) | 149 (38.9) | |
| Smoking | 0.69 | |||
| Yes | 25 (3.4) | 11 (3.1) | 14 (3.7) | |
| No | 710 (96.6) | 341 (96.9) | 369 (96.3) | |
| Education | 0.29 | |||
| Low | 207 (28.2) | 91 (25.8) | 116 (30.3) | |
| Intermediate | 373 (50.8) | 179 (50.9) | 194 (50.6) | |
| High | 147 (20.0) | 77 (21.9) | 70 (18.3) | |
| Missing | 8 (1.0) | 5 (1.4) | 3 (0.8) | |
| Ethnicity | 0.93 | |||
| European | 708 (96.3) | 341 (96.9) | 367 (95.8) | |
| Non-European | 17 (2.3) | 8 (2.3) | 9 (2.4) | |
| Missing information | 10 (1.4) | 3 (0.8) | 7 (1.8) | |
| Thyroid status | ||||
| TPOAb ≥11.0 (kIU/L) | 52.6 (24.0;149.5) | 42.6 (23.1;137.0) | 83.5 (24.8;159.0) | 0.18 |
| n (%) | 88 (12.0) | 46 (13.1) | 42 (11.0) | |
| TPOAb <11.0 (kIU/L) | 647 (88.0) | 306 (47.3) | 341 (52.7) | 1.00 |
| FT4 (pmol/L) | 14.12 ± 1.91 | 14.27 ± 2.02 | 13.99 ± 1.79 | 0.03 |
| TSH (mIU/L) | 1.43 (0.92;2.06) | 1.41 (0.88;1.92) | 1.46 (0.93;2.16) | 0.29 |
| Offspring characteristics | ||||
| Gestational age (days) | 282 (276;288) | 282 (275;287) | 281 (274;287) | 0.51 |
| Birth weight (g) | 3567 ± 473 | 3501 ± 457 | 3628 ± 481 | <0.001 |
| Missing birth weight, n (%) | 1 (0.14) | 0 (0) | 1 (0.26) | |
| Birth length (cm) | 52.1 ± 2.1 | 51.7 ± 2.0 | 52.4 ± 2.0 | <0.001 |
| Missing birth length, n (%) | 7 (0.95) | 3 (0.85) | 4 (1.04) | |
| Exclusive breastfeeding (weeks) | 33 (16;48) | 33 (17;50) | 34 (15;46) | 0.30 |
| Missing data on breastfeeding, n (%) | 152 (20.7) | 70 (19.9) | 82 (20.4) |
*Difference between sexes, tested with independent t-test or Wilcoxon rank sum test for continuous variables and χ2 tests for categorical variables.
FT4, free thyroxine; BMI, body mass index; TPOAb, antibodies against thyroid peroxidase; TSH, thyrotropin.
Included mother–child dyads did not differ significantly from the non-included dyads according to maternal age, pre-pregnancy BMI, education, thyroid function tests, and offspring sex (Supplementary Table 2). There were significantly more mothers of European origin, non-smokers, and nulliparous in the included group compared to the non-included group. Included offspring had significantly higher body weight, length, and gestational age at birth than non-included offspring. Included offspring were also breastfed longer than those excluded.
Productive vocabulary scores
The median age of the offspring at the first completed MB-CDI: WG report was 12 months (range: 12–18, interquartile range; IQR = 0) for both girls and boys. The median age of the offspring at the first completed MB-CDI: WS report was 21 months for both girls (range: 21–36, IQR = 3) and boys (range: 21–33, IQR = 3). The median number of completed MB-CDI reports was five (total range: 1–9, IQR = 4) for both girls and boys. Thirty-seven girls and 48 boys had only one completed MB-CDI report.
Girls produced significantly more words at 12–33 months than boys. The mean number of produced words equalized between sexes at 36 months because of the ceiling effects (Supplementary Table 3); however, the numbers of completed MB-CDI reports at 36 months were only 51 for girls and 41 for boys.
Maternal TPOAb and productive vocabulary
In model 1 (TPOAb <11 vs ≥11 kIU/L), the OR of the interaction effect of TPOAb ≥11 kIU/L and offspring age on productive vocabulary was 0.78 (P < 0.001) for girls and 0.83 (P < 0.001) for boys (Table 2). In model 2 (TPOAb as a continuous variable), the OR of the interaction effect of TPOAb and offspring age on productive vocabulary was 1.02 (P = 0.38) for girls and 0.84 (P < 0.001) for boys (Table 2).
Table 2.
Association between maternal TPOAb at gestational week median 12 (range: 8–20 weeks) and productive vocabulary from the MacArthur–Bates Communicative Development Inventories (MB-CDI) reports in the offspring aged 12–36 months (models 1 and 2). The association between maternal TPOAb and the probability of producing words from the MB-CDI are estimated by conditional growth models, including effects of time (offspring age) and main effects of covariates. The outputs of the conditional growth models are reported as odds ratios (OR), standard error (SE), and 95% CI. Numbers represent complete cases included in the analyses. P-values <0.05 are significant and marked in bold.
| Girls (n = 277) | Boys (n = 298) | |||||||
|---|---|---|---|---|---|---|---|---|
| OR | SE | 95% CI | P | OR | SE | 95% CI | P | |
| TPOAb (<11.0 vs ≥11.0 kIU/L) | ||||||||
| Intercept | 1.00 | 0.00 | 1.00; 1.00 | <0.001 | 1.00 | 0.00 | 1.00; 1.00 | <0.001 |
| Offspring age | 10.27 | 6.41×10-3 | 10.41; 10.40 | <0.001 | 9.79 | 6.16 × 10−3 | 9.68; 9.91 | <0.001 |
| TPOAb (≥11.0) | 0.99 | 0.09 | 0.83; 1.18 | 0.92 | 1.06 | 0.09 | 0.89; 1.27 | 0.49 |
| Maternal age | 0.89 | 0.10 | 0.73; 1.07 | 0.22 | 0.76 | 0.10 | 0.63; 0.92 | 0.005 |
| Breastfeeding | 1.19 | 0.08 | 1.02; 1.39 | 0.03 | 1.24 | 0.08 | 1.05; 1.46 | 0.01 |
| Maternal education | ||||||||
| Linear | 1.21 | 0.09 | 1.01; 1.44 | 0.03 | 1.01 | 0.09 | 0.84; 1.21 | 0.92 |
| Quadratic | 1.11 | 0.09 | 0.94; 1.31 | 0.23 | 0.87 | 0.09 | 0.73; 1.02 | 0.09 |
| Primi- and multi-parous | 0.78 | 0.10 | 0.64; 0.94 | 0.009 | 0.96 | 0.09 | 0.80; 1.16 | 0.68 |
| Pre-pregnancy BMI | ||||||||
| <18.5 | 0.88 | 0.10 | 0.72; 1.08 | 0.22 | 1.10 | 0.09 | 0.92; 1.31 | 0.29 |
| >25.0 | 1.04 | 0.08 | 0.88; 1.23 | 0.62 | 0.96 | 0.09 | 0.81; 1.14 | 0.67 |
| Offspring age × TPOAb (≥11.0) | 0.78 | 0.02 | 0.75; 0.81 | <0.001 | 0.83 | 0.02 | 0.80; 0.86 | <0.001 |
| TPOAb (Linear) | ||||||||
| Intercept | 1.00 | 0.00 | 1.00; 1.00 | <0.001 | 1.00 | 0.00 | 1.00; 1.00 | <0.001 |
| Offspring age | 09.97 | 6.33×10-3 | 9.84; 10.09 | <0.001 | 9.84 | 6.43 × 10−3 | 9.72; 9.96 | <0.001 |
| TPOAb (linear) | 0.81 | 0.10 | 0.67; 0.98 | 0.03 | 1.12 | 0.07 | 0.97; 1.29 | 0.61 |
| Maternal age | 0.88 | 0.10 | 0.73; 1.07 | 0.21 | 0.77 | 0.10 | 0.64; 0.93 | 0.005 |
| Breastfeeding | 1.19 | 0.08 | 1.01; 1.39 | 0.033 | 1.23 | 0.08 | 1.04; 1.44 | 0.010 |
| Maternal education | ||||||||
| Linear | 1.19 | 0.09 | 1.00; 1.42 | 0.05 | 1.02 | 0.09 | 0.85; 1.22 | 0.97 |
| Quadratic | 1.11 | 0.09 | 0.93; 1.31 | 0.24 | 0.87 | 0.09 | 0.73; 1.03 | 0.09 |
| Primi- and multi-parous | 0.78 | 0.10 | 0.64; 0.94 | 0.011 | 0.95 | 0.09 | 0.79; 1.14 | 0.67 |
| Pre-pregnancy BMI | 0.88 | 0.10 | 0.72; 1.07 | 0.19 | 1.11 | 0.09 | 0.93; 1.32 | 0.30 |
| <18.5 | ||||||||
| >25.0 | 1.04 | 0.09 | 0.88; 1.23 | 0.66 | 0.96 | 0.09 | 0.81; 1.14 | 0.67 |
| Offspring age × TPOAb | 1.02 | 0.02 | 0.98; 1.06 | 0.38 | 0.84 | 0.02 | 0.81; 0.87 | <0.001 |
BMI, body mass index; TPOAb, thyroid peroxidase antibodies.
Comparing TPOAb-positive vs TPOAb-negative mothers, plotted predicted probabilities by offspring age (Fig. 2A and B) show that the productive vocabulary was lower with maternal TPOAb ≥11 kIU/L in girls at age 12–36 months and in boys at 15–36 months. Group-by-group contrasts (Supplementary Table 4) show that the effect was significant for girls in the age range of 18–36 months, while for boys only at 33–36 months. Comparing TPOAb-negative vs TPOAb-positive mothers, girls produced a mean of 498 vs 417 words at 30 months, respectively, while the corresponding figures for boys were 423 vs 409, respectively (Supplementary Table 5).
Figure 2.
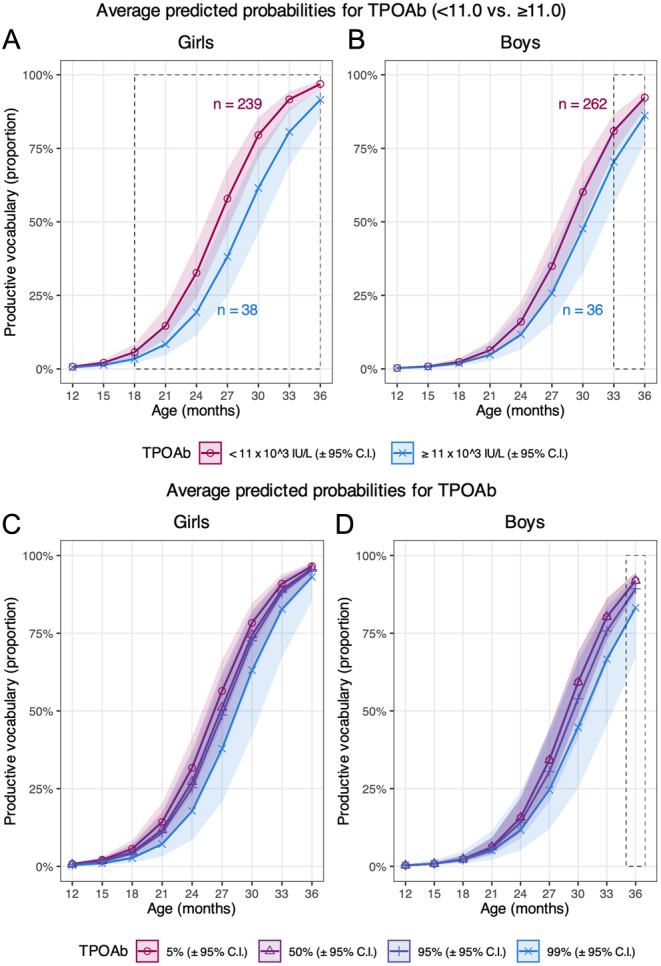
Graphs A and B illustrate the average predicted probability of productive vocabulary score according to maternal thyroid peroxidase antibody (TPOAb) status plotted by offspring age for girls and boys, respectively (model 1). Graphs C and D illustrate the predicted probabilities of productive vocabulary at the 5th, 50th, 95th, and 99th percentile of TPOAb by offspring age for girls and boys, respectively (model 2). The lines represent the predicted mean trajectories with corresponding 95% confidence intervals. The dotted lines show at which age groups the effect was significant. Numbers in A and B represent complete cases included in the analyses according to maternal TPOAb level.
Using TPOAb as a continuous variable (model 2), the plotted predicted probabilities (Fig. 2C) show a lower productive vocabulary with higher TPOAb levels in girls in the entire age range, although the effect of TPOAb did not reach statistical significance (P = 0.38). In boys, the productive vocabulary scores were almost equal across TPOAb levels at 12–21 months (Supplementary Table 6). The predicted probability of producing words was lower with higher TPOAb levels from 24 months to 36 months, although the difference was only significant at age 36 months when comparing the 5th (girls: 10.6 kIU/L, boys: 10.6 kIU/L) and 99th(girls: 242 kIU/L, boys: 241 kIU/L) percentile (Fig. 2B, Supplementary Table 6).
Maternal FT4 and productive vocabulary
In model 3 (FT4 <20% vs >80% percentile), the OR of the interaction effect of FT4 >80% and offspring age on productive vocabulary was 0.77 (P < 0.001) for girls and 0.90 (P < 0.001) for boys (Table 3). In model 4 (FT4 as a continuous variable), the OR of the interaction effect of FT4 with offspring age on productive vocabulary was 0.60 (P < 0.001) for girls and 1.13 (P = 0.002) for boys (Table 3).
Table 3.
Association between maternal FT4 (gestational week median 12, range: 8–20 weeks) and productive vocabulary from the MacArthur–Bates Communicative Development Inventories (MB-CDI) in the offspring aged 12–36 months (models 3 and 4). The association between FT4 and the probability of producing words from the MB-CDI are estimated by conditional growth models, including effects of time (offspring age) and main effects of covariates. The outputs of the conditional growth models are reported as odds ratios (OR), standard error (SE), and 95% CI. Numbers represent complete cases included in the analyses. P-values <0.05 are significant and marked in bold.
| Girls | Boys | |||||||
|---|---|---|---|---|---|---|---|---|
| OR | SE | 95% CI | P | OR | SE | 95% CI | P | |
| FT4 (<20% vs >80% percentile) | ||||||||
| Girls, n = 97 | ||||||||
| Boys, n =111 | ||||||||
| Intercept | 1.00 | 0.00 | 1.00; 1.00 | <0.001 | 1.00 | 0.00 | 1.00; 1.00 | <0.001 |
| Offspring age | 10.88 | 0.02 | 10.56; 11.21 | <0.001 | 10.69 | 0.01 | 10.44; 10.96 | <0.001 |
| FT4 (>80%) | 1.26 | 0.15 | 0.95; 1.69 | 0.11 | 0.97 | 0.14 | 0.74; 1.28 | 0.83 |
| Maternal age | 0.88 | 0.17 | 0.64; 1.22 | 0.46 | 0.88 | 0.15 | 0.65; 1.19 | 0.40 |
| Breastfeeding | 1.14 | 0.13 | 0.88; 1.47 | 0.33 | 1.24 | 1.14 | 0.95; 1.62 | 0.12 |
| Maternal education | ||||||||
| Linear | 1.37 | 0.15 | 1.02; 1.84 | 0.04 | 0.93 | 0.16 | 0.68; 1.26 | 0.63 |
| Quadratic | 1.18 | 0.14 | 0.89; 1.55 | 0.25 | 0.81 | 0.14 | 0.61; 1.07 | 0.14 |
| Primi- and multi-parous | 0.92 | 0.17 | 0.66; 1.28 | 0.61 | 0.85 | 0.16 | 0.62; 1.17 | 0.32 |
| Pre-pregnancy BMI | ||||||||
| <18.5 | 0.75 | 0.14 | 0.57; 0.99 | 0.045 | 1.08 | 0.15 | 0.80; 1.45 | 0.62 |
| >25 | 1.16 | 0.14 | 0.88; 1.52 | 0.29 | 0.97 | 0.14 | 0.73; 1.27 | 0.80 |
| Offspring age × FT4 (>80%) | 0.77 | 0.03 | 0.72; 0.82 | <0.001 | 0.90 | 0.03 | 0.84; 0.95 | <0.001 |
| FT4 (linear) | ||||||||
| Girls, n = 277 | ||||||||
| Boys, n = 298 | ||||||||
| Intercept | 1.00 | 0.00 | 1.00; 1.00 | <0.001 | 1.00 | 0.00 | 1.00; 1.00 | <0.001 |
| Offspring age | 15.90 | 0.04 | 14.65; 17.26 | <0.001 | 8.59 | 0.04 | 7.99; 9.24 | <0.001 |
| FT4 (linear) | 1.13 | 0.09 | 0.95; 1.34 | 0.18 | 0.89 | 0.09 | 0.74; 1.07 | 0.21 |
| Maternal age | 0.89 | 0.10 | 0.73; 1.08 | 0.22 | 0.77 | 0.10 | 0.64; 0.93 | 0.01 |
| Breastfeeding | 1.19 | 0.08 | 1.02; 1.40 | 0.03 | 1.22 | 0.08 | 1.04; 1.44 | 0.02 |
| Maternal education () | ||||||||
| Linear | 1.17 | 0.09 | 0.98; 1.40 | 0.08 | 1.02 | 0.09 | 0.85; 1.22 | 0.87 |
| Quadratic | 1.12 | 0.09 | 0.95; 1.33 | 0.19 | 0.86 | 0.09 | 0.73; 1.02 | 0.09 |
| Primi- and multi-parous | 0.77 | 0.10 | 0.63; 0.93 | 0.01 | 0.95 | 0.09 | 0.79; 1.14 | 0.58 |
| Pre-pregnancy BMI | 0.86 | 0.10 | 0.70; 1.05 | 0.14 | 1.10 | 0.09 | 0.92; 1.31 | 0.30 |
| <18.5 | ||||||||
| >25 | 1.03 | 0.09 | 0.87; 1.21 | 0.77 | 0.96 | 0.09 | 0.81; 1.14 | 0.63 |
| Offspring age × FT4 (linear) | 0.60 | 0.05 | 0.55; 0.65 | <0.001 | 1.13 | 0.04 | 1.05; 1.23 | 0.002 |
BMI, body mass index; FT4, free thyroxine.
Plotted predicted probabilities (Fig. 3A) show that the MB-CDI scores tended to be larger for the 80th percentile of FT4 compared to the 20th percentile in girls at age 12–18 months, while the opposite association applied at age 21–36 months; however, these effects were not statistically significant (Fig. 3A, Supplementary Table 7). For boys, the productive vocabulary tended to be larger for the 20th percentile of FT4 compared to the 80th percentile in all age groups, but the effect was, as in girls, insignificant (Fig. 3B, Supplementary Table 7). When comparing mothers with FT4 levels <20% vs >80%, the girls produced a mean of 486 vs 471 words at 30 months, respectively, while the corresponding figures for boys were 428 vs 393, respectively (Supplementary Table 5).
Figure 3.
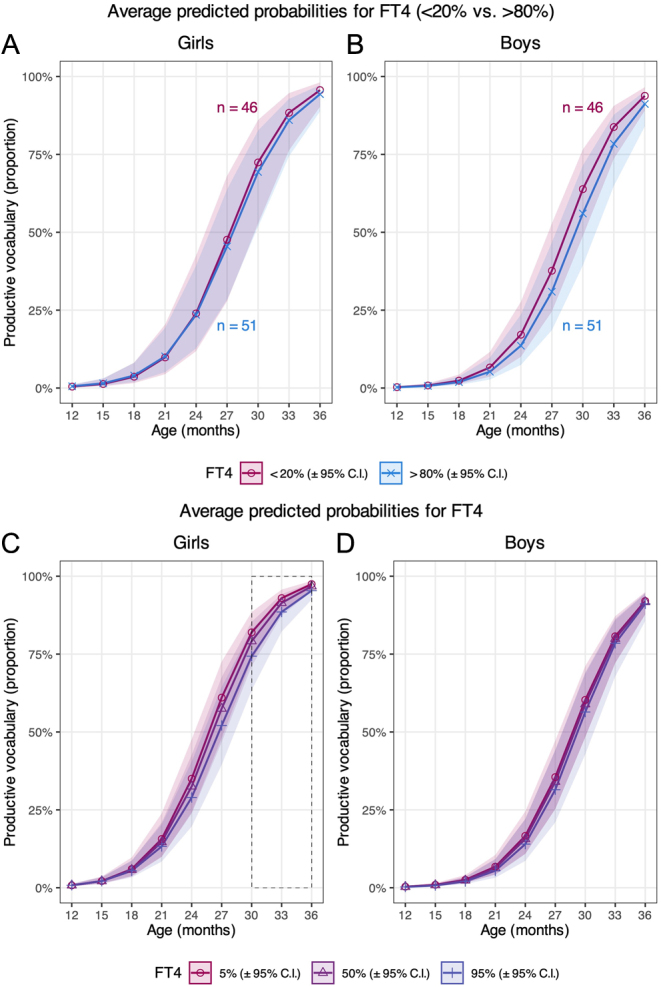
Graphs A and B illustrate the predicted probabilities of productive vocabulary according to maternal free thyroxine (FT4) levels in early pregnancy plotted by offspring age, of girls and boys, respectively (model 3). Graphs C and D illustrate the predicted probabilities of productive vocabulary at the 5th, 50th, and 95th percentile of maternal FT4 in early pregnancy plotted by offspring age, of girls and boys, respectively (model 4). The lines represent the predicted mean trajectories of girls and boys, respectively, with corresponding 95% CIs. Numbers in A and B represent complete cases included in the analyses of maternal FT4 <20th and >80th percentile.
Using FT4 as a continuous variable, plotted predicted probabilities show that the productive vocabulary at 18–36 months increased in both girls and boys with lower FT4 levels (Fig. 3C and D). At age 12–15 months, the probability of producing words was independent of the maternal FT4 level. For girls at age 30–36 months, the predicted probabilities of MB-CDI scores were significantly lower with maternal FT4 at the 95th percentile (girls: 17.6 pmol/L, boys: 17.7 pmol/L) compared to those with maternal FT4 at the 5th percentile (girls: 11.5 pmol/L, boys: 11.6 pmol/L). A similar effect was not observed for boys (Supplementary Table 8).
Maternal TSH and productive vocabulary
In model 5, the OR of the interaction effect of TSH >2.5 mIU/L (vs TSH ≤2.5 mIU/L) with increasing offspring age on productive vocabulary was 1.06 (P < 0.001) for girls and 1.29 (P < 0.001) for boys (Table 4). In model 6 (TSH as a continuous variable), the OR of the interaction effect of TSH with offspring age on productive vocabulary was 1.20 (P < 0.001) for girls and 1.39 (P < 0.001) for boys (Table 4).
Table 4.
Association between early pregnancy maternal thyrotropin (TSH) and productive vocabulary in the offspring aged 12–36 months (models 5 and 6). The association between maternal TSH and the productive vocabulary is estimated by conditional growth models, including effects of time (offspring age) and main effects of covariates. The outputs of the conditional growth models are reported as odds ratios (OR), standard error (SE), and 95% confidence intervals (95% CI). Numbers represent complete cases included in the analyses. P-values <0.05 are significant and marked in bold.
| Girls (n = 277) | Boys (n = 298) | |||||||
|---|---|---|---|---|---|---|---|---|
| OR | SE | 95% CI | P | OR | SE | 95% CI | P | |
| TSH (≤2.5 vs >2.5 mIU/L) | ||||||||
| Intercept | 1.00 | 0.00 | 1.00; 1.00 | <0.001 | 1.00 | 0.00 | 1.00; 1.00 | <0.001 |
| Offspring age | 9.93 | 6.26 × 10−3 | 9.80; 10.05 | <0.001 | 9.35 | 6.13 × 10−3 | 9.24; 9.46 | <0.001 |
| TSH (>2.5 mIU/L) | 1.00 | 0.08 | 0.85; 1.17 | 0.97 | 0.74 | 0.09 | 0.62; 0.88 | <0.001 |
| Maternal age | 0.89 | 0.10 | 0.73; 1.08 | 0.22 | 0.77 | 0.10 | 0.64; 0.93 | 0.006 |
| Breastfeeding | 1.19 | 0.08 | 1.02; 1.40 | 0.03 | 1.20 | 0.08 | 1.02; 1.42 | 0.026 |
| Maternal education | ||||||||
| Linear | 1.18 | 0.09 | 0.99; 1.41 | 0.06 | 1.02 | 0.09 | 0.85; 1.22 | 0.86 |
| Quadratic | 1.11 | 0.09 | 0.94; 1.32 | 0.22 | 0.87 | 0.09 | 0.73; 1.03 | 0.10 |
| Primi- and multi-parous | 0.77 | 0.10 | 0.64; 0.93 | 0.008 | 0.94 | 0.09 | 0.78; 1.13 | 0.53 |
| Pre-pregnancy BMI | ||||||||
| <18.5 | 0.87 | 0.10 | 0.71; 1.06 | 0.17 | 1.10 | 0.09 | 0.92; 1.31 | 0.32 |
| >25 | 1.03 | 0.09 | 0.87; 1.22 | 0.70 | 0.96 | 0.09 | 0.81; 1.14 | 0.64 |
| Offspring age × TSH (>2.5 mIU/L) | 1.06 | 0.02 | 1.03; 1.10 | <0.001 | 1.29 | 0.02 | 1.24; 1.34 | <0.001 |
| TSH (linear) | ||||||||
| Intercept | 1.00 | 0.00 | 1.00; 1.00 | <0.001 | 1.00 | 0.00 | 1.00; 1.00 | <0.001 |
| Offspring age | 9.31 | 0.00 | 9.13; 9.50 | <0.001 | 8.49 | 0.00 | 8.33; 8.65 | <0.001 |
| TSH (linear) | 0.87 | 0.08 | 0.74; 1.03 | 0.102 | 0.65 | 0.09 | 0.54; 0.78 | <0.001 |
| Maternal age | 0.88 | 0.10 | 0.73; 1.07 | 0.207 | 0.76 | 0.10 | 0.63; 0.92 | 0.005 |
| Breastfeeding | 1.19 | 0.08 | 1.01; 1.40 | 0.036 | 1.20 | 0.08 | 1.02; 1.41 | 0.029 |
| Maternal education | ||||||||
| Linear | 1.18 | 0.09 | 0.99; 1.41 | 0.067 | 1.02 | 0.09 | 0.85; 1.23 | 0.825 |
| Quadratic | 1.11 | 0.09 | 0.94; 1.32 | 0.220 | 0.86 | 0.09 | 0.73; 1.02 | 0.090 |
| Primi- and multi-parous | 0.77 | 0.10 | 0.64; 0.94 | 0.009 | 0.93 | 0.10 | 0.77; 1.12 | 0.442 |
| Pre-pregnancy BMI | ||||||||
| <18.5 | 0.87 | 0.10 | 0.71; 1.06 | 0.164 | 1.09 | 0.09 | 0.91; 1.31 | 0.328 |
| >25 | 1.03 | 0.09 | 0.87; 1.22 | 0.726 | 0.96 | 0.09 | 0.81; 1.14 | 0.617 |
| Offspring age × TSH | 1.20 | 0.02 | 1.15; 1.25 | <0.001 | 1.39 | 0.02 | 1.33; 1.45 | <0.001 |
BMI, body mass index; TSH, thyrotropin.
Visual inspection of the plotted predicted probabilities shows an ambiguous influence of maternal TSH on productive vocabulary. A larger productive vocabulary was indicated for girls with maternal TSH >2.5 mIU/L than those with maternal TSH ≤2.5 mIU/L (Fig. 4A and B). For boys until the age of 27 months, the predicted probabilities suggest that the productive vocabulary was larger with maternal TSH ≤2.5 mIU/L. However, at 27–30 months, the curvilinear line followed the same trend as for girls, showing better productive vocabulary with maternal TSH >2.5 mIU/L (Fig. 4A and B). Considering the individual age subgroups, the predicted probabilities did not depend on the TSH level to any major extent (Supplementary Table 9).
Figure 4.
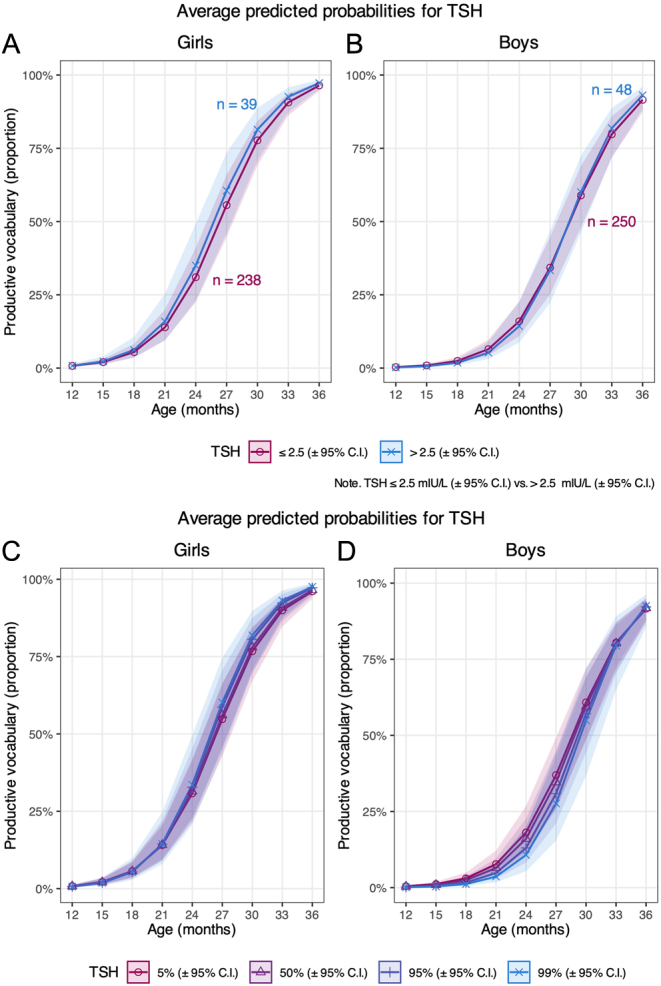
Graphs (A) and (B) show the predicted probabilities of productive vocabulary according to maternal thyrotropin (TSH) ≤2.5 or >2.5 mIU/L and plotted by offspring age of girls and boys, respectively (model 5). Graphs (C) and (D) show the predicted probabilities of productive vocabulary at the 5th, 50thth, 95th, and 99th percentile of maternal TSH and plotted by offspring age of girls and boys, respectively (model 6). The lines represent the predicted mean trajectories with corresponding 95% CIs. Numbers in (A) and (B) represent complete cases included in the analyses of maternal TSH ≤2.5 or >2.5 mIU/L, respectively.
Using TSH as a continuous variable, plotted predicted probabilities showed almost identical productive vocabularies across the 5th, 50th, 95th, and 99th percentiles of maternal TSH in both girls and boys at all age subgroups (Fig. 4C and D, Supplementary Table 10).
Sensitivity analysis
By excluding 76 maternal smokers and 30 mothers with diabetes mellitus and/or gestational diabetes mellitus, respectively, the association between maternal FT4 levels and productive vocabulary (model 4) for boys became non-significant (P = 0.159 and P = 0.35, respectively; data not shown). All other patterns of significance did not change. By excluding 88 mother–child dyads with maternal TPOAb positivity (TPOAb ≥11 kIU/L), the association between TSH levels and productive vocabulary (model 5) for boys became non-significant (P = 0.439; data not shown), while all other results did not change significantly. In addition, the results were not affected significantly by excluding 73 mother–child dyads with non-European mothers. Lastly, we included mother–child dyads with missing information about thyroid medication during pregnancy. This yielded an additional eight mother–child dyads with both thyroid function measures and MB-CDI data. Including these individuals in the analyses, the results were unchanged for models 1–5, while in model 6, the association between TSH (continuous) and productive vocabulary became non-significant for boys (P = 0.472; data not shown). Imputation analyses confirmed the significant and independent influence of maternal TPOAb positivity on productive language acquisition (data not shown).
Covariates and offspring productive vocabulary
All conditional growth models showed a strong positive association between productive vocabulary scores and offspring age (P < 0.001 for all models). Tables 2, 3, and 4 show coefficient estimates of other covariates included in the six models. These estimates reflect the influence of each covariate on the mean productive vocabulary probability scores across all offspring ages when the other covariates are kept constant. Breastfeeding and maternal education influenced productive vocabulary positively, while maternal age, pre-pregnancy BMI <18.5, and parity had a negative influence (Tables 2, 3, and 4).
Discussion
In the present study, we demonstrated significantly lower productive vocabulary scores in girls at age 18–36 months and boys at age 33–36 months when the mothers were TPOAb-positive compared to those of TPOAb-negative mothers in early pregnancy. Likewise, vocabulary scores were lower in boys at 36 months of age when maternal TPOAb was at the 99th percentile compared to the 5th percentile during early pregnancy. Importantly, the effect of TPOAb was not mediated by coexisting maternal thyroid dysfunction. In fact, the probability of producing words over time was greater with low-normal maternal FT4, although this effect was only statistically significant for girls at 30–36 months in one of the models. As for maternal TSH, a tendency was found between higher levels and higher vocabulary scores in the children; however, the results regarding the influence of TSH were generally more ambiguous, possibly due to the fact that plasma TSH shows large fluctuations in the first trimester and large variations among individuals. Taken together, our results suggest that maternal thyroid autoimmunity per se might have a negative impact on early language acquisition.
In a previous study by Pop et al. (46), 5-year-old children of TPOAb-positive mothers had lower verbal scores on the McCarthy Scale compared to children of TPOAb-negative mothers, all with normal thyroid function. Further, a study by Li et al. (16) found lower intelligence scores, including evaluation of language, in children at 25–30 months if the mothers had TPOAb levels >50 IU/mL, compared to TPOAb-negative mothers. Yet other studies were unable to demonstrate any association between maternal thyroid autoimmunity and offspring language skills (17, 18, 19, 47). The conflicting results may rely on differences in the methods used, offspring age, and the timing of maternal thyroid function assessment.
In our study, we used a standardized and norm-referenced tool for measuring early language acquisition, i.e. the MB-CDI parent reports. Only one study, by Henrichs et al. (48), has previously employed this instrument for investigating the association between thyroid function in euthyroid treatment-naive women and productive language in children. The authors found an association between maternal FT4 below the 5th percentile (i.e. <10.96 pmol/L) and a delay in expressive language (vocabulary scores <15th percentile) (48). However, unlike the present study, the study by Henrichs et al. did not include any measures of thyroid autoimmunity, and it only included one MB-CDI report at 18 months, which was based on the short form comprising 112 out of the original 600 words (48).
The possible impact of maternal thyroid autoimmunity on fetal neurodevelopment has important implications, yet some issues remain unanswered. In current guidelines (22), the focus is to disclose thyroid dysfunction in pregnancy by screening, followed by adequate treatment. Based on the current and previous studies (16, 46), it could be argued that screening of pregnant women should include measurement of TPOAb, disregarding the thyroid functional status. For such an approach to be justified, evidence must be provided that treatment of thyroid autoimmunity, if possible, is beneficial for the course of pregnancy and/or the fetus. Indeed, in a randomized, placebo-controlled study, Negro et al. showed that TPOAb levels decreased significantly more in euthyroid TPOAb-positive pregnant women when supplemented with selenium 200 µg/day, as compared to untreated women (49). Importantly, fewer women progressed to hypothyroidism in the selenium-supplemented group (49). However, no neurocognitive assessment of the offspring was done in that study, and no between-group differences were found regarding infant weight, length, cranial perimeter, or APGAR score, or in the risk of maternal hypertension, preeclampsia, placental abruption, or premature delivery (49). Whether selenium supplementation has any role in TPOAb-positive pregnant women is intriguing. The results by Negro et al. (49) need to be confirmed, including a thorough neurocognitive evaluation of the offspring.
It is well-known that TPOAb-positive euthyroid women carry a higher risk of both single and recurrent pregnancy loss (50). It has been suggested that this is caused by an underlying mild thyroid hormone deficiency. Although the context of our study was different, our results to some extent oppose such an assumption, as the effect of TPOAb was not mediated by a low FT4. The effect of LT4 treatment in TPOAb-positive euthyroid women was investigated in the recent T4LIFE trial (51), in which 187 women with recurrent pregnancy loss were randomized to receive either LT4 or placebo prior to pregnancy. No difference was found between the two groups regarding live birth rate, pregnancy loss, and preterm birth (51). These results are in line with similar neutral outcomes in previous trials (52, 53). However, it remains to be elucidated whether such an approach in TPOAb-positive euthyroid women is beneficial for the neurocognitive development of the fetus.
It is unclear if thyroid autoimmunity per se affects pregnancy and the fetus. In euthyroid women (on LT4 or treatment-naïve) with thyroid autoimmunity and recurrent miscarriages or unexplained infertility, increased levels of non-organ specific antibodies and immune components have been demonstrated (54). It is likely that thyroid autoimmunity merely reflects a more widespread immune dysregulation, including increased numbers of T cells and elevated levels of inflammatory cytokines. These immunological aberrations may directly or indirectly impact the placenta, the fetal–placental circulation, or the fetal development, as supported by a recent study (55).
Our study has several strengths. We investigated a cohort of mother–child dyads with repeated measurements over time and extensive information about maternal and offspring characteristics. We used the standardized and norm-referenced MB-CDI parent report for measuring early language development at several time points up to the age of 36 months. The high number of MB-CDIs per child provided a solid estimation of the inter- and intraindividual variability and patterns of change over time.
Some limitations exist. First, due to the increased thyroxine-binding globulin level and decreased albumin level in pregnancy, the reliability of the solid-phase extraction LC-MS method is higher for measuring FT4 than the electrochemiluminescence immunoassay as used in our study (22). Liquid chromatography-tandem mass spectrometry is not commonly assessable and is much more expensive and is therefore rarely used (22). Second, blood sampling of the mothers took place from 8th to 20th gestational week. Ideally, this was done in all participants before the 14th week, as fetal brain development relies on the maternal thyroid function only until this point (56). However, it is likely that the time of blood sampling is much less critical in relation to maternal autoimmunity. Third, even though we included several covariates, some residual confounding could persist, including the influence of maternal IQ, parental comorbidities, and/or parental speech-hearing deficiency. Fourth, information regarding thyroid medication, education, breastfeeding, smoking habits, and pre-pregnancy BMI was collected through self-reported questionnaires, which may have introduced response bias. Fifth, selection bias cannot be ruled out since not all invited mothers accepted inclusion in our study (28). Finally, we do not have information on whether the MB-CDI reports were filled in by the mothers or the fathers, which theoretically may have introduced recall bias in case some TPOAb-positive mothers suffered from cognitive deficits (i.e. ‘brain fog’) (57).
In conclusion, our study finds a lower probability of producing words in both girls and boys of TPOAb-positive mothers (TPOAb ≥11.0 kIU/L) compared to TPOAb-negative mothers (<11.0 kIU/L). Importantly, this was unrelated to coexisting thyroid dysfunction in terms of low-normal levels of FT4. Our study supports that maternal thyroid autoimmunity has a negative influence on early fetal neurodevelopment. The mechanisms behind such an interaction are unknown. Also, it remains to be demonstrated how the harmful impact of thyroid autoimmunity on the fetus can be diminished or even avoided.
Supplementary Materials
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the study reported.
Funding
The first author is supported by a PhD scholarship funded by the Danish Medicines Agency, the Region of Southern Denmark (grant number: 20/14658), and a grant from ‘Overlægerådets Forskningsfond’ at Odense University Hospital (grant number: A4641). The Odense child cohort study is supported by Odense University Hospital, Municipality of Odense. This study was also supported by the Danish Mental Health Fund (Psykiatriens Forskningsfond, grant number: 180811221). None of the funding sources had any involvement in the study design, collection/analysis/interpretation of data, or writing the report.
Ethical approval
The study was approved by the Regional Scientific Ethical Review Committee (Project ID S-20090130) and the Danish Data Protection Agency (j.no. 18/15692). All parents received written and oral information about the OCC project before providing written consent for participation.
Author contribution statement
MSA, SJB, DG, DB, and NB contributed to the study conception and design. FT and KRR were responsible for data analysis. AFD contributed to data management. KRR, FT, MSA, and SJB interpreted the data. KRR wrote the first draft of the manuscript. All authors commented on previous versions of the manuscript. All authors read and approved the final version of the manuscript.
Acknowledgements
The authors would like to thank the families in Odense Child Cohort for their participation and commitment to the study and the technicians at Hans Christian Andersen’s Children’s Hospital for carefully examining the children.
References
- 1.Räikkönen K Pesonen AK Roseboom TJ & Eriksson JG. Early determinants of mental health. Best Practice and Research 201226599–611. ( 10.1016/j.beem.2012.03.001) [DOI] [PubMed] [Google Scholar]
- 2.Liao S-F Liu J-C Hsu C-L Chang M-Y Chang T-M & Cheng H. Cognitive development in children with language impairment, and correlation between language and intelligence development in kindergarten children with developmental delay. Journal of Child Neurology 20153042–47. ( 10.1177/0883073814535486) [DOI] [PubMed] [Google Scholar]
- 3.Flensborg-Madsen T & Mortensen EL. Language development and intelligence in midlife. British Journal of Developmental Psychology 201937269–283. ( 10.1111/bjdp.12271) [DOI] [PubMed] [Google Scholar]
- 4.Elbro C Dalby M & Maarbjerg S. Language-learning impairments: A 30-year follow-up of language-impaired children with and without psychiatric, neurological and cognitive difficulties. International Journal of Language and Communication Disorders 201146437–448. ( 10.1111/j.1460-6984.2011.00004.x) [DOI] [PubMed] [Google Scholar]
- 5.Bleses D Makransky G Dale PS Højen A & Ari BA. Early productive vocabulary predicts academic achievement 10 years later. Applied Psycholinguistics 2016371461–1476. ( 10.1017/S0142716416000060) [DOI] [Google Scholar]
- 6.Dale PS Paul A Rosholm M & Bleses D. Prediction from early childhood vocabulary to academic achievement at the end of compulsory schooling in Denmark. International Journal of Behavioral Development 202347123–134. ( 10.1177/01650254221116878) [DOI] [Google Scholar]
- 7.Frank MC Braginsky M Yurovsky D & Marchman VA. Variability and Consistency in Early Language Learning: the Wordbank Project: Cambridge: MA: MIT Press; 2021. [Google Scholar]
- 8.Bleses D Vach W Slott M Wehberg S Thomsen P Madsen TO & Basbøll H. The Danish communicative developmental inventories: validity and main developmental trends. Journal of Child Language 200835651–669. ( 10.1017/S0305000907008574) [DOI] [PubMed] [Google Scholar]
- 9.Bleses D Vach W Slott M Wehberg S Thomsen P Madsen TO & Basbøll H. Early vocabulary development in Danish and other languages: a CDI-based comparison. Journal of Child Language 200835619–650. ( 10.1017/S0305000908008714) [DOI] [PubMed] [Google Scholar]
- 10.Wallentin M & Trecca F. Cross-cultural sex/gender differences in produced word content before the age of 3 years. Psychological Science 202334411–423. ( 10.1177/09567976221146537) [DOI] [PubMed] [Google Scholar]
- 11.Hayiou-Thomas ME Dale PS & Plomin R. The etiology of variation in language skills changes with development: A longitudinal twin study of language from 2 to 12 years. Developmental Science 201215233–249. ( 10.1111/j.1467-7687.2011.01119.x) [DOI] [PubMed] [Google Scholar]
- 12.Mumm H Dreyer AF Bleses D Glintborg D Jensen TK Boye H Trecca F & Andersen MS. Maternal cortisol levels in third trimester and early language development: A study of 1093 mother-child pairs from the Odense Child Cohort. Journal of Neuroendocrinology 202335e13314. ( 10.1111/jne.13314) [DOI] [PubMed] [Google Scholar]
- 13.Olesen TS, Bleses D, Andersen HR, Grandjean P, Frederiksen H, Trecca F, Bilenberg N, Kyhl HB, Dalsager L, Jensen IK, et al.Prenatal phthalate exposure and language development in toddlers from the Odense Child Cohort. Neurotoxicology and Teratology 20186534–41. ( 10.1016/j.ntt.2017.11.004) [DOI] [PubMed] [Google Scholar]
- 14.Bliddal S, Derakhshan A, Xiao Y, Chen LM, Männistö T, Ashoor G, Tao F, Brown SJ, Vafeiadi M, Itoh S, et al.Association of thyroid peroxidase antibodies and thyroglobulin antibodies with thyroid function in pregnancy: an individual participant data meta-analysis. Thyroid 202232828–840. ( 10.1089/thy.2022.0083) [DOI] [PubMed] [Google Scholar]
- 15.De Leo S & Pearce EN. Autoimmune thyroid disease during pregnancy. Lancet 20186575–586. ( 10.1016/S2213-8587(1730402-3) [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Shan Z, Teng W, Yu X, Li Y, Fan C, Teng X, Guo R, Wang H, Li J, et al.Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25–30 months. Clinical Endocrinology 201072825–829. ( 10.1111/j.1365-2265.2009.03743.x) [DOI] [PubMed] [Google Scholar]
- 17.Kampouri M Margetaki K Koutra K Kyriklaki A Karakosta P Anousaki D Chalkiadaki G Vafeiadi M Kogevinas M & Chatzi L. Maternal mild thyroid dysfunction and offspring cognitive and motor development from infancy to childhood: the Rhea mother-child cohort study in Crete, Greece. Journal of Epidemiology and Community Health 20217529–35. ( 10.1136/jech-2019-213309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghassabian A, Bongers-Schokking JJ, De Rijke YB, Van Mil N, Jaddoe VWV, De Muinck Keizer-Schrama SMPF, Hooijkaas H, Hofman A, Visser W, Roman GC, et al.Maternal thyroid autoimmunity during pregnancy and the risk of attention deficit/hyperactivity problems in children: the Generation R Study. Thyroid 201222178–186. ( 10.1089/thy.2011.0318) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams FLR Watson J Ogston SA Visser TJ Hume R & Willatts P. Maternal and umbilical cord levels of T4, FT4, TSH, TPOAb, and TgAb in term infants and neurodevelopmental outcome at 5.5 years. Journal of Clinical Endocrinology and Metabolism 201398829–838. ( 10.1210/jc.2012-3572) [DOI] [PubMed] [Google Scholar]
- 20.Derakhshan A Korevaar TIM Taylor PN Levie D Guxens M Jaddoe VWV Nelson SM Tiemeier H & Peeters RP. The association of maternal thyroid autoimmunity during pregnancy with child IQ. Journal of Clinical Endocrinology and Metabolism 20181033729–3736. ( 10.1210/jc.2018-00743) [DOI] [PubMed] [Google Scholar]
- 21.Stagnaro-Green A & Pearce E. Thyroid disorders in pregnancy. Nature Reviews 20128650–658. ( 10.1038/nrendo.2012.171) [DOI] [PubMed] [Google Scholar]
- 22.Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, Grobman WA, Laurberg P, Lazarus JH, Mandel SJ, et al.2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 201727315–389. ( 10.1089/thy.2016.0457) [DOI] [PubMed] [Google Scholar]
- 23.de Escobar GM Ares S Berbel P Obregón MJ & del Rey FE. The changing role of maternal thyroid hormone in fetal brain development. Seminars in Perinatology 200832380–386. ( 10.1053/j.semperi.2008.09.002) [DOI] [PubMed] [Google Scholar]
- 24.Chen Y Luo ZC Zhang T Fan P Ma R Zhang J & Ouyang F. Maternal thyroid dysfunction and neuropsychological development in children. Journal of Clinical Endocrinology and Metabolism 2023108339–350. ( 10.1210/clinem/dgac577) [DOI] [PubMed] [Google Scholar]
- 25.Andersen SL Andersen S Liew Z Vestergaard P & Olsen J. Maternal thyroid function in early pregnancy and neuropsychological performance of the child at 5 years of age. Journal of Clinical Endocrinology and Metabolism 2018103660–670. ( 10.1210/jc.2017-02171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casey BM, Thom EA, Peaceman AM, Varner MW, Sorokin Y, Hirtz DG, Reddy UM, Wapner RJ, Thorp JM, Saade G, et al.Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. New England Journal of Medicine 2017376815–825. ( 10.1056/NEJMoa1606205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reading R. Antenatal thyroid screening and childhood cognitive function. Child: Care, Health and Development 201238605–605. ( 10.1111/j.1365-2214.2012.01404.x) [DOI] [Google Scholar]
- 28.Kyhl HB Jensen TK Barington T Buhl S Norberg LA Jørgensen JS Jensen DFG Christesen HT Lamont RF & Husby S. The Odense Child Cohort: aims, design, and cohort profile. Paediatric and Perinatal Epidemiology 201529250–258. ( 10.1111/ppe.12183) [DOI] [PubMed] [Google Scholar]
- 29.Andersen MS Jensen TK Dreyer AF Madsen JB Christesen HT Brandslund I Bilenberg N & Glintborg D. Free thyroxine in early pregnancy is an independent negative predictor of 3rd trimester HbA1c. Odense child cohort. Clinical Endocrinology 202195508–519. ( 10.1111/cen.14492) [DOI] [PubMed] [Google Scholar]
- 30.Fenson L Marchman VA Thal DJ Dale PS Reznick JS & Bates E. The MacArthur-Bates Communicative Development Inventories - User’s Guide and Technical Manual: Baltimore: Brookes Publishing Company; 2006. [Google Scholar]
- 31.Law J & Roy P. Parental report of infant language skills: a review of the development and application of the communicative development inventories. Child and Adolescent Mental Health 200813198–206. ( 10.1111/j.1475-3588.2008.00503.x) [DOI] [PubMed] [Google Scholar]
- 32.Barbu S Nardy A Chevrot JP Guellaï B Glas L Juhel J & Lemasson A. Sex differences in language across early childhood: family socioeconomic status does not impact boys and girls equally. Frontiers in Psychology 187461–10. ( 10.3389/fpsyg.2015.01874) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fink G McCoy DC & Yousafzai A. Contextual and socioeconomic variation in early motor and language development. Archives of Disease in Childhood 2020105421–427. ( 10.1136/archdischild-2019-317849) [DOI] [PubMed] [Google Scholar]
- 34.Gurgand L Lamarque L Havron N Bernard JY Ramus F & Peyre H. The influence of sibship composition on language development at 2 years of age in the ELFE birth cohort study. Developmental Science 202326e13356. ( 10.1111/desc.13356) [DOI] [PubMed] [Google Scholar]
- 35.Anderson JW Johnstone BM & Remley DT. Breast-feeding and cognitive development: A meta-analysis. American Journal of Clinical Nutrition 199970525–535. ( 10.1093/ajcn/70.4.525) [DOI] [PubMed] [Google Scholar]
- 36.Reynolds R, Kingsland M, Daly J, Licata M, Tully B, Doherty E, Farragher E, Desmet C, Lecathelinais C, McKie J, et al.Breastfeeding practices and associations with pregnancy, maternal and infant characteristics in Australia: a cross-sectional study. International Breastfeeding Journal 2023188. ( 10.1186/s13006-023-00545-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biondi B. Thyroid and obesity: an intriguing relationship. Journal of Clinical Endocrinology and Metabolism 2010953614–3617. ( 10.1210/jc.2010-1245) [DOI] [PubMed] [Google Scholar]
- 38.Sart G Bayar Y & Danilina M. Impact of educational attainment and economic globalization on obesity in adult females and males: empirical evidence from BRICS economies. Frontiers in Public Health 2023111102359. ( 10.3389/fpubh.2023.1102359) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Åsvold BO Bjøro T Nilsen TIL & Vatten LJ. Tobacco smoking and thyroid function: a population-based study. Archives of Internal Medicine 20071671428–1432. ( 10.1001/archinte.167.13.1428) [DOI] [PubMed] [Google Scholar]
- 40.Bruun S Wedderkopp N Mølgaard C Kyhl HB Zachariassen G & Husby S. Using text messaging to obtain weekly data on infant feeding in a Danish birth cohort resulted in high participation rates. Acta Paediatrica 2016105648–654. ( 10.1111/apa.13382) [DOI] [PubMed] [Google Scholar]
- 41.Jensen RC Glintborg D Timmermann CAG Nielsen F Boye H Madsen JB Bilenberg N Grandjean P Jensen TK & Andersen MS. Higher free thyroxine associated with PFAS exposure in first trimester. The Odense child cohort. Environmental Research 2022212113492. ( 10.1016/j.envres.2022.113492) [DOI] [PubMed] [Google Scholar]
- 42.Jensen EA Petersen PH Blaabjerg O Hansen PS Brix TH & Hegedüs L. Establishment of reference distributions and decision values for thyroid antibodies against thyroid peroxidase (TPOAb), thyroglobulin (TgAb) and the thyrotropin receptor (TRAb) Clinical Chemistry and Laboratory Medicine 200644991–998. ( 10.1515/CCLM.2006.166) [DOI] [PubMed] [Google Scholar]
- 43.R Core Team R: A Language and Environment for Statistical Computing: Vienna Austria: R Foundation for Statistical computing; 2023. [Google Scholar]
- 44.Bates D Mächler M Bolker BM & Walker SC. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 201567. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 45.Lüdecke D. sjPlot: data visualization for statistics in social science. R package version 2.8.14 2023. Available at: https://CRANR-project.org/package=sjPlot [Google Scholar]
- 46.Pop VJ de Vries E van Baar AL Waelkens JJ de Rooy HA Horsten M Donkers MM Komproe IH van Son MM & Vader HL. Maternal thyroid peroxidase antibodies during pregnancy: A marker of impaired child development? Journal of Clinical Endocrinology and Metabolism 1995803561–3566. ( 10.1210/jcem.80.12.8530599) [DOI] [PubMed] [Google Scholar]
- 47.Nelson SM Haig C McConnachie A Sattar N Ring SM Smith GD Lawlor DA & Lindsay RS. Maternal thyroid function and child educational attainment: prospective cohort study. BMJ 2018360k452. ( 10.1136/bmj.k452) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Henrichs J, Bongers-Schokking JJ, Schenk JJ, Ghassabian A, Schmidt HG, Visser TJ, Hooijkaas H, De Muinck Keizer-Schrama SMPF, Hofman A, Jaddoe VVW, et al.Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: the Generation R Study. Journal of Clinical Endocrinology and Metabolism 2010954227–4234. ( 10.1210/jc.2010-0415) [DOI] [PubMed] [Google Scholar]
- 49.Negro R Greco G Mangieri T Pezzarossa A Dazzi D & Hassan H. The influence of selenium supplementation on postpartum thyroid status in pregnant women with thyroid peroxidase autoantibodies. Journal of Clinical Endocrinology and Metabolism 2007921263–1268. ( 10.1210/jc.2006-1821) [DOI] [PubMed] [Google Scholar]
- 50.Thangaratinam S Tan A Knox E Kilby MD Franklyn J & Coomarasamy A. Association between thyroid autoantibodies and miscarriage and preterm birth: meta-analysis of evidence. BMJ 2011342d2616. ( 10.1136/bmj.d2616) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Dijk MM, Vissenberg R, Fliers E, van der Post JAM, van der Hoorn MP, de Weerd S, Kuchenbecker WK, Hoek A, Sikkema JM, Verhoeve HR, et al.Levothyroxine in euthyroid thyroid peroxidase antibody positive women with recurrent pregnancy loss (T4LIFE trial): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. Diabetes and Endocrinology 202210322–329. ( 10.1016/S2213-8587(2200045-6) [DOI] [PubMed] [Google Scholar]
- 52.Dhillon-Smith RK, Middleton LJ, Sunner KK, Cheed V, Baker K, Farrell-Carver S, Bender-Atik R, Agrawal R, Bhatia K, Edi-Osagie E, et al.Levothyroxine in women with thyroid peroxidase antibodies before conception. New England Journal of Medicine 20193801316–1325. ( 10.1056/NEJMoa1812537) [DOI] [PubMed] [Google Scholar]
- 53.Wang H, Gao H, Chi H, Zeng L, Xiao W, Wang Y, Li R, Liu P, Wang C, Tian Q, et al.Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing in vitro fertilization and embryo transfer a randomized clinical trial. JAMA 20173182190–2198. ( 10.1001/jama.2017.18249) [DOI] [PubMed] [Google Scholar]
- 54.Kim NY Cho HJ Kim HY Yang KM Ahn HK Thornton S Park JC Beaman K Gilman-Sachs A & Kwak-Kim J. Thyroid autoimmunity and its association with cellular and humoral immunity in women with reproductive failures. American Journal of Reproductive Immunology 20116578–87. ( 10.1111/j.1600-0897.2010.00911.x) [DOI] [PubMed] [Google Scholar]
- 55.Ru X Yang M Teng Y Han Y Hu Y Wang J Tao F & Huang K. Association of maternal thyroid peroxidase antibody during pregnancy with placental morphology and inflammatory and oxidative stress responses. Frontiers in Endocrinology 2023141–13. ( 10.3389/fendo.2023.1182049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jansen TA Korevaar TIM Mulder TA White T Muetzel RL Peeters RP & Tiemeier H. Maternal thyroid function during pregnancy and child brain morphology: A time window-specific analysis of a prospective cohort. Lancet. Diabetes and Endocrinology 20197629–637. ( 10.1016/S2213-8587(1930153-6) [DOI] [PubMed] [Google Scholar]
- 57.Ettleson MD Raine A Batistuzzo A Batista SP McAninch E Teixeira MCTV Jonklaas J Laiteerapong N Ribeiro MO & Bianco AC. Brain fog in hypothyroidism: understanding the patient’s perspective. Endocrine Practice 202228257–264. ( 10.1016/j.eprac.2021.12.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a