Abstract
Background
Chronic plaque psoriasis is the most common type of psoriasis, and it is characterised by redness, thickness, and scaling. First‐line management of chronic plaque psoriasis is with topical treatments, including vitamin D analogues, topical corticosteroids, tar‐based preparations, dithranol, salicylic acid, and topical retinoids.
Objectives
To compare the effectiveness, tolerability, and safety of topical treatments for chronic plaque psoriasis, relative to placebo, and to similarly compare vitamin D analogues (used alone or in combination) with other topical treatments.
Search methods
We updated our searches of the following databases to February 2011: the Cochrane Skin Group Specialised Register, CENTRAL in The Cochrane Library (2011, Issue 2), MEDLINE (from 1948), EMBASE (from 1980), Science Citation Index (from 2008), Conference Proceedings Citation Index ‐ Science (from 2008), BIOSIS (from 1993), Dissertation Abstracts via DialogClassic (all publication years), and Inside Conferences (all publication years).
We identified ongoing and unpublished studies from the UK Clinical Research Network Study Portfolio and the metaRegister of Controlled Trials. We checked the bibliographies of published studies and reviews for further references to relevant trials, and we contacted trialists and companies for information about newly published studies.
A separate search for adverse effects was undertaken in February 2011 using MEDLINE and EMBASE (from 2005).
Final update searches for both RCTs and adverse effects were undertaken in August 2012. Although it has not been possible to incorporate RCTs and adverse effects studies identified through these final searches within this review, we will incorporate these into the next update.
Selection criteria
Randomised trials comparing active topical treatments against placebo or against vitamin D analogues (used alone or in combination) in people with chronic plaque psoriasis.
Data collection and analysis
One author extracted study data and assessed study quality. A second author checked these data. We routinely contacted trialists and companies for missing data. We also extracted data on withdrawals and on local and systemic adverse events. We defined long‐term trials as those with a duration of at least 24 weeks.
Main results
This update added 48 trials and provided evidence on 7 new active treatments. In total, the review included 177 randomised controlled trials, with 34,808 participants, including 26 trials of scalp psoriasis and 6 trials of inverse psoriasis, facial psoriasis, or both. The number of included studies counted by Review Manager (RevMan) is higher than these figures (190) because we entered each study reporting a placebo and an active comparison into the 'Characteristics of included studies' table as 2 studies.
When used on the body, most vitamin D analogues were significantly more effective than placebo, with the standardised mean difference (SMD) ranging from ‐0.67 (95% CI ‐1.04 to ‐0.30; 1 study, 119 participants) for twice‐daily becocalcidiol to SMD ‐1.66 (95% CI ‐2.66 to ‐0.67; 1 study, 11 participants) for once‐daily paricalcitol. On a 6‐point global improvement scale, these effects translate into 0.8 and 1.9 points, respectively. Most corticosteroids also performed better than placebo; potent corticosteroids (SMD ‐0.89; 95% CI ‐1.06 to ‐0.72; I² statistic = 65.1%; 14 studies, 2011 participants) had smaller benefits than very potent corticosteroids (SMD ‐1.56; 95% CI ‐1.87 to ‐1.26); I² statistic = 81.7%; 10 studies, 1264 participants). On a 6‐point improvement scale, these benefits equate to 1.0 and 1.8 points, respectively. Dithranol, combined treatment with vitamin D/corticosteroid, and tazarotene all performed significantly better than placebo.
Head‐to‐head comparisons of vitamin D for psoriasis of the body against potent or very potent corticosteroids had mixed findings. For both body and scalp psoriasis, combined treatment with vitamin D and corticosteroid performed significantly better than vitamin D alone or corticosteroid alone. Vitamin D generally performed better than coal tar, but findings relative to dithranol were mixed. When applied to psoriasis of the scalp, vitamin D was significantly less effective than both potent corticosteroids and very potent corticosteroids. Indirect evidence from placebo‐controlled trials supported these findings.
For both body and scalp psoriasis, potent corticosteroids were less likely than vitamin D to cause local adverse events, such as burning or irritation. Combined treatment with vitamin D/corticosteroid on either the body or the scalp was tolerated as well as potent corticosteroids, and significantly better than vitamin D alone. Only 25 trials assessed clinical cutaneous dermal atrophy; few cases were detected, but trials reported insufficient information to determine whether assessment methods were robust. Clinical measurements of dermal atrophy are insensitive and detect only the most severe cases. No comparison of topical agents found a significant difference in systemic adverse effects.
Authors' conclusions
Corticosteroids perform at least as well as vitamin D analogues, and they are associated with a lower incidence of local adverse events. However, for people with chronic plaque psoriasis receiving long‐term treatment with corticosteroids, there remains a lack of evidence about the risk of skin dermal atrophy. Further research is required to inform long‐term maintenance treatment and provide appropriate safety data.
Keywords: Humans; Administration, Topical; Adrenal Cortex Hormones; Adrenal Cortex Hormones/adverse effects; Adrenal Cortex Hormones/therapeutic use; Bone Density Conservation Agents; Bone Density Conservation Agents/adverse effects; Bone Density Conservation Agents/therapeutic use; Chronic Disease; Facial Dermatoses; Facial Dermatoses/drug therapy; Psoriasis; Psoriasis/drug therapy; Randomized Controlled Trials as Topic; Scalp Dermatoses; Scalp Dermatoses/drug therapy; Vitamin D; Vitamin D/adverse effects; Vitamin D/analogs & derivatives; Vitamin D/therapeutic use
Plain language summary
Skin treatments for chronic plaque psoriasis
Chronic plaque psoriasis is the most common type of psoriasis. Although any part of the body may be affected, the most commonly affected sites are the elbows, knees, and scalp. 'Topical' treatments (i.e. treatments applied to the skin) are usually tried first. These include vitamin D products, topical corticosteroids, tar‐based preparations, dithranol, salicylic acid, and vitamin A products. As chronic plaque psoriasis is a long‐term condition, it is important to find out which treatments work best and what adverse effects they have. This review describes average benefits of different treatments, while recognising that individuals will vary in their experience of each treatment.
The evidence was based on 177 studies, which, in total, included 34,808 people. Studies were typically about 7 weeks' long, but this ranged from 1 week to 52 weeks. Vitamin D products were found to work better than placebo (the base cream or ointment). Potent topical corticosteroids (strong, e.g. betamethasone dipropionate) and very potent (very strong, e.g. clobetasol propionate) topical corticosteroids were also effective.
Some studies compared vitamin D products directly with potent or very potent corticosteroids. These products had similar effects when applied to the body, but corticosteroids worked better than vitamin D for scalp psoriasis. Treatment that combined vitamin D with a corticosteroid was more effective than vitamin D alone and more effective than the topical corticosteroid alone. Vitamin D products generally performed better than coal tar, but studies found conflicting results when comparing vitamin D with dithranol.
Whether applied to the body or to the scalp, potent corticosteroids were less likely than vitamin D to cause 'local adverse events', such as skin irritation or burning, and people were therefore more likely to stop using vitamin D products. When studies examined whether topical treatments had effects within the body ('systemic adverse events'), we found no difference between placebo and any other treatment. However, this may be because many trials did not properly assess systemic adverse events, rather than because there really was no difference.
More long‐term studies would help doctors and people with psoriasis decide on the best way to treat this chronic condition.
Background
Description of the condition
Psoriasis is a chronic inflammatory skin disease with a prevalence ranging from between 1% and 2% in the UK and northern European populations (Hellgren 1967; Krueger 1984) to 0.1% to 0.3% in the Far East (Simons 1949) and China (Yip 1984). Psoriasis comprises multiple phenotypes and may be localised (e.g. to the skin‐fold areas (inverse psoriasis), the palms, or the soles) or widespread. Types of widespread psoriasis include guttate, generalised pustular, and erythrodermic (Griffiths 2007). Chronic plaque psoriasis may be localised or widespread and accounts for 90% of psoriasis cases (Griffiths 2007); it is characterised by red patches of thickened skin (plaques) covered in silver scales (Figure 1). Any area of the body may be affected, but the main areas are the knees, elbows, lower back, and scalp. There is a wide spectrum of disease severity from a single plaque to involvement of more than 90% of the skin surface. Psoriasis may be classified as 'mild', 'moderate', or 'severe', although these categories are difficult to define precisely (Krueger 2000). Psoriatic arthritis accompanies the cutaneous (skin) manifestations of psoriasis in 5% to 30% of cases (Barisic‐Drusko 1994; Krueger 1984; Salvarani 1995; Zanolli 1992). Recent improvements in the classification criteria may reduce the wide variation in reported prevalence of psoriatic arthritis (Taylor 2006). Psoriasis occurs in 5% of people with Crohn's disease (Lee 1990).
1.
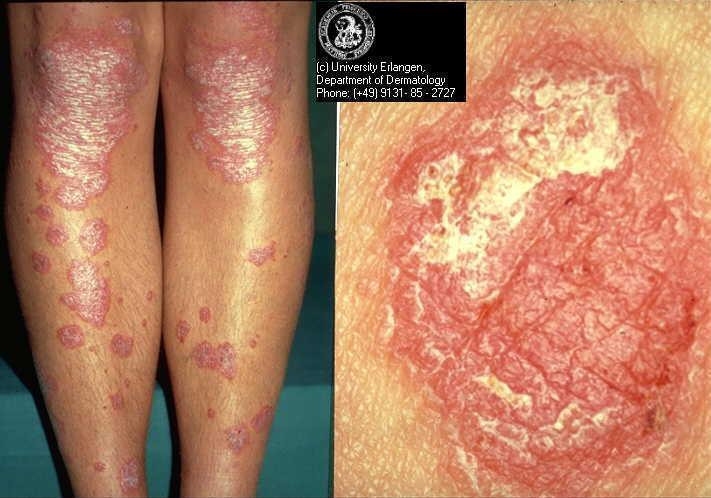
Chronic plaque psoriasis Source: Dermis Dermatology Atlas Online (used with permission)
Causes
The way that psoriasis develops is complicated and appears to be influenced by many factors, including genetic changes, local trauma, infections, certain drugs (such as beta‐blockers, lithium, chloroquine, and non‐steroidal anti‐inflammatory drugs (NSAIDs)), the duration of antipsoriatic treatments, endocrine factors, sunlight, alcohol, smoking, and stress (Tagami 1997). The skin lesions of psoriasis are shown in Figure 2, and they are characterised by cells multiplying too quickly (epidermal hyperproliferation), cells not maturing normally (abnormal keratinocyte differentiation), and the presence of cells that cause inflammation (a lymphocyte inflammatory infiltrate) (Barker 1991; Griffiths 2003; Stern 1997). Psoriasis is now recognised as an immune‐mediated disorder, with tumour necrosis factor alpha (TNFα), dendritic cells, and T‐cells all contributing to its pathogenesis (Griffiths 2007a). Several genes interact with environmental factors to induce the development of psoriasis, and different combinations of changes in several genes and environmental factors can produce the same clinical picture of psoriasis (Bhalerao 1998; Brandrup 1978; Farber 1974; Lomholt 1963; Willan 1808). A locus (plural = loci) is the specific location of a gene on a chromosome, and its position is defined using the letters 'p' (for a chromosome's short arm) and 'q' (for a long arm). At least nine chromosomal psoriasis susceptibility loci were originally identified (Griffiths 2007a). The strongest association and linkage is to a locus within the major histocompatibility complex, the area affecting immune response (Genetic Analysis of Psoriasis Consortium 2010; Henseler 1992; Russell 1972; Svejgaard 1974; Tazi‐Ahnini 1999a; Tazi‐Ahnini 1999b; Trembath 1997). Other linkage studies have reported linkage to 4q and 17q (Matthews 1996; Tomfohrde 1994) and 16q and 20q (Nair 1997; Trembath 1997). Proinflammatory CD4‐positive T helper cells produce interferon gamma (produced by Th1) or interleukin (IL)‐17 (produced by Th17). These cells interact with dendritic cells, macrophages, mast cells, and neutrophils, causing inflammation (Ghoreschi 2007). A meta‐analysis of 3 genome‐wide association studies (GWAS) has identified 15 new susceptibility loci (Tsoi 2012) for psoriasis. This brings the total number of loci associated with psoriasis to 36. Several of these loci are involved in the regulation of the skin's innate immune response. They provide confirmation of the role of several existing biologic therapies as well as new targets for drug development.
2.
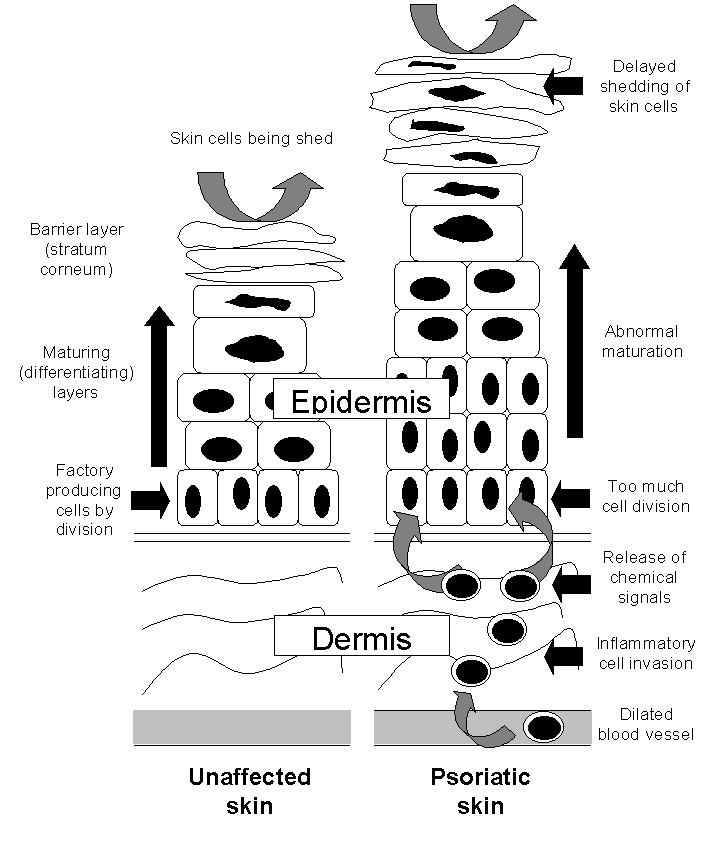
The epidermis in the skin of people with and without psoriasis
Impact
Until identified as a single disease by von Hebra in 1841, psoriasis was thought to be a variant of leprosy and regarded as contagious (de Jong 1997). The misconception may persist: In a survey of people with psoriasis in 1997, almost three‐quarters of respondents reported that others thought their condition was contagious, and a similar proportion feared swimming and taking part in sporting activities (Watts 1998). Psoriasis can lead to social isolation (van de Kerkhof 1997a), stigmatisation (Gupta 1998; van de Kerkhof 1997a), and fear of other people's reactions, adversely affecting the quality of daily life (Finlay 1994; Finlay 1995a; Finlay 1995b; Finlay 2001; McKenna 2003; Ortonne 2000; Richards 2003; Stern 1995). Psychological distress induced by psoriasis may also impair the response to treatment (Fortune 2003).
Description of the intervention
Treatment of psoriasis should always be appropriate to its severity and importance to that individual: It should never be more unpleasant, intolerable, or dangerous than the disease itself (Camp 1992). Topical treatments include vitamin D analogues, topical corticosteroids, tar‐based preparations, dithranol, salicylic acid, and topical retinoids (Baadsgaard 1995; Corbett 1976; Fredriksson 1980; Goeckerman 1931; Ingram 1953; Kragballe 1988; Kragballe 1989; Langner 1996; Staberg 1989; Unna 1916; Van de Kerkhof 1996a), but there is no evidence‐based 'treatment ladder' by which to sequence treatments (Van de Kerkhof 2008). Emollients are generally used in a supportive role as an addition to topical treatments, to normalise hyperproliferation, differentiation, and to exert anti‐inflammatory effects (Fluhr 2008). The two classes of topical treatment for psoriasis that are most commonly prescribed in developed countries are vitamin D analogues and topical corticosteroids, because they are considered more cosmetically acceptable than tar and dithranol preparations (Baadsgaard 1995; Kragballe 1988; Van de Kerkhof 1996a).
Topical corticosteroids (specifically glucocorticoids) are available in four potencies: mild, moderate, potent, and very potent, which are assessed using the vasoconstrictor assay (BMA 2012). The benefit of topical steroids is that in cream formulations, they are easy to apply, cosmetically acceptable, do not stain the skin, and rarely cause irritation. There are several adverse effects of corticosteroids, including cutaneous atrophy, rebound after discontinuation of treatment, and decreasing response to the drug (tachyphylaxis) (du Vivier 1975; Lee 1998; Kao 2003). Glucocorticoids (GC) exert their effects either via interaction with cell membranes (non‐genomic effects) or downstream with the genome and via interaction with intracellular fluid in GC receptors and downstream with the genome (genomic effects). The genomic effects are of two types: "transrepression (inhibition of synthesis of regulatory proteins) and transactivation (induction of the synthesis of regulatory proteins)" (Bos 2008). Transactivation appears to mediate certain adverse reactions, such as cutaneous atrophy. Immunomodulation seems to be the result of GC‐mediated transrepression, that is, silencing of proinflammatory genes, such as TNFα. Non‐steroidal GC receptor ligands (selective GC receptor agonists) have recently been identified and may reduce the side‐effects of GC without loss of immunosuppressive effects (Bos 2008).
The naturally occurring active metabolite of vitamin D, calcitriol (1a,25‐dihydroxyvitamin D3) (Langner 1996), and two synthetic vitamin D analogues, calcipotriol (Kragballe 1988; Kragballe 1989; Staberg 1989) and tacalcitol (1a,24‐dihydroxyvitamin D3) (Baadsgaard 1995; Van de Kerkhof 1996a), are effective when applied topically in psoriasis (Mason 2002a). These agents bind to vitamin D receptors (VDR), which in turn bind to vitamin D‐responsive elements (VDRE) in multiple genes. 'Switching on' (transactivation of) these genes inhibits the multiplication of cells and stimulates their differentiation (Figure 2). VDRs also suppress the inflammatory component of psoriasis by inhibiting the production of proinflammatory cytokines (small proteins that affect cell‐cell interaction), such as interleukin‐1 (IL‐1). Vitamin D analogues all have the potential to induce abnormally high levels of calcium in the blood serum (hypercalcaemia) and urine (hypercalciuria). Although calcipotriol ointment causes no elevation of total serum calcium when used at the recommended dose of 100 g per week (Mortensen 1993), there are significant elevations in both serum and urinary calcium when the dose is increased to 300 g per week (Bourke 1993a; Bourke 1994). Topical vitamin D analogues are cosmetically acceptable; they are not known to cause skin atrophy; and they are not usually associated with rebound when therapy is discontinued. However, at least 25% of people are reported to have little or no response to topical vitamin D analogues (Holick 1996; Mee 1998).
Urea or salicylic acid may be used to reduce thickness and scaling of the skin; combination with other products can improve their absorption. However, these can also irritate the skin. Topical immunosuppressants, such as methotrexate, and topical macrolactams, such as tacrolimus, are relatively new treatments, and their effectiveness, tolerability, and longer‐term effects are less clear than with the more established products. This review also considers combination products involving any of the above treatments.
The Cochrane Library has three published Cochrane reviews of interventions for psoriasis. Owen 2000 assessed the impact of antistreptococcal interventions for guttate and chronic plaque psoriasis. The review found that "although both antibiotics and tonsillectomy have frequently been advocated for patients with recurrent guttate psoriasis or chronic plaque psoriasis, there is to date no good evidence that either intervention is beneficial." Chalmers 2000 reviewed all treatments, excluding antistreptococcal interventions, for guttate psoriasis. The review identified only one relevant trial and no evidence of the effectiveness of any topical interventions. Chalmers 2006 assessed interventions, including topical treatments, for chronic palmoplantar pustulosis (a disease that is closely related to psoriasis and used to be considered a variant of psoriasis). Chalmers 2006 found that topical steroids under hydrocolloid occlusion were effective in inducing remission. In addition, The Cochrane Library has four published Cochrane review protocols, which cover interventions for nail psoriasis (Velema 2009), interventions for scalp psoriasis (Jales 2012), phototherapy (Chen 2011), and the biological agent ustekinumab (Roberts 2011).
Why it is important to do this review
Chronic plaque psoriasis is a condition for which there is no known cure, and currently, available treatments may only temporarily clear the skin (Bonifati 1998; Griffiths 2004). Clinical practice varies between and within different countries. By focusing on topical treatments for psoriasis, either as monotherapy or in combination, this review assesses the relative effectiveness, tolerability, and safety of these treatments and so helps to determine how best to induce remission and delay recurrence in people receiving topical treatment. Table 1 provides a list of acronyms used in the review.
1. List of acronyms.
| Acronym | Full name |
| BC | baseline comparability demonstrated (clinical/demographic) |
| BD | twice daily |
| BMD | betamethasone dipropionate |
| BMV | betamethasone valerate |
| BSA | Body Surface Area |
| Btw‐patient | Between‐patient |
| CI | confidence interval |
| dys | days |
| EQ‐5D | EuroQOL |
| FU | follow up (includes treatment period) |
| I² | heterogeneity statistic |
| IAGI | Investigator Assessment of Global Improvement (change score) |
| IGA | Investigator Global Assessment (static score) |
| IQR | interquartile range |
| ISGA | Investigator's Static Global Assessment Score |
| LAE | local adverse effects |
| LCD | liquor carbonis distillate |
| LF | loss to follow up (per cent of participants randomised, not contributing to primary outcome measure) |
| MEMS | Medication Event Monitoring System |
| mPASI | modified Psoriasis Area Severity Index |
| NA | not available/not applicable |
| NR | not reported |
| OD | once daily |
| OM | once in the morning |
| ON | once at night |
| ODS | overall disease severity |
| PAGI | Patient Assessment of Global Improvement (change score) |
| PASI | Psoriasis Area Severity Index |
| PDI | Psoriasis Disability Index |
| PGA | Patient Global Assessment (static score) |
| PMAQ‐3w | Medication Adherence Questionnaire, version 3W |
| pt | point |
| QOL | quality of life |
| RD | risk difference |
| SD | standard deviation |
| SMD | standardised mean difference |
| TCP | two‐compound product |
| TD | three times daily |
| TLPSS | Total Local Psoriasis Severity Score |
| TSS | Total Severity Score/total sum score |
| UV | ultra violet |
| VDRE | Vitamin D‐Responsive Element |
| wks | weeks |
| yrs | years |
Structure of the Review
The structure of the review is provided to facilitate navigation:
Objectives
Methods
Results
Description of the studies
Risk of bias in the included studies
-
Effects of the interventions
-
(1) Primary outcome measures
(a) Investigator's Assessment of Overall Global Improvement (IAGI)/Investigator's Global Assessment of Disease Severity (IGA)
(b) Total Severity Scores (TSS)
(c) Psoriasis Area and Severity Index (PASI)
(d) Patient Assessment of overall Global Improvement (PAGI)/Patient Global Assessment of Disease Severity (PGA)
(e) Combined end point (IAGI/TSS/PASI/PAGI)
-
(2) Secondary outcome measures
(a) Withdrawal rates (total rate; withdrawal because of adverse events; withdrawal because of treatment failure)
-
(b) Adverse events (local and systemic)
(i) Findings from the main review
(ii) Findings from the separate search for additional studies of adverse events
(c) Quality of life measures
(d) Economic outcomes (not updated in 2011)
(e) Concordance or adherence with treatment (not updated in 2011)
-
Discussion
Authors' conclusions
Under 'Primary outcome measures', we report findings for each of the 19 analyses (including sensitivity analyses). We also do this under 'Secondary outcome measures' for subsections (a) and (b). We did not update the sections on Economic outcomes (2d) and Concordance (2e) in 2011 because of resource constraints.
Objectives
To compare the effectiveness, tolerability, and safety of topical treatments for chronic plaque psoriasis, relative to placebo, and to similarly compare vitamin D analogues (used alone or in combination) with other topical treatments.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials in the review. Trials could be either placebo‐controlled or head‐to‐head with a vitamin D preparation (head‐to‐head trials compare two active treatments with each other). The types of study design eligible for inclusion were as follows: parallel‐group (between‐patient), cross‐over, and within‐patient designs. For within‐patient studies, where study participants serve as their own control, we included only those studies that clearly adopted a left‐right design, and we excluded studies where multiple plaques were treated with more than two products. If no useful effectiveness, withdrawal, or adverse events data were available, either from the published paper or from sponsors or trialists, we excluded the study.
In addition to findings on adverse events from the main review, we undertook separate searches for additional safety and tolerability studies. The searches for longer‐term adverse events included studies of any design that included humans (i.e. not only animals; either humans only or humans and animals). However, studies with fewer than 10 participants (including case reports) were not eligible for inclusion. We did not restrict the search for concordance/adherence studies by study design (i.e. non‐randomised studies were eligible for inclusion).
Types of participants
People of any age with chronic plaque psoriasis affecting the body, limbs, scalp, or a combination of the aforementioned. We did not limit participant type by area of involvement, disease severity, or skin area treated.
Types of interventions
Topical treatments, including the following:
vitamin D preparations, e.g. calcipotriol;
corticosteroids, e.g. betamethasone valerate;
coal tar;
dithranol, also known as anthralin;
salicylic acid;
urea;
topical retinoids;
topical immunosuppressants, e.g. methotrexate;
topical macrolactams, e.g. ascomycin derivatives, such as tacrolimus; and
combination products, e.g. corticosteroids with coal tar or corticosteroids with vitamin D.
We compared topical treatments with vehicle (placebo). We also compared vitamin D analogues with other topical treatments. We selected vitamin D analogues for this comparison because they are first‐line treatments in many developed countries (van de Kerkhof 1998). We based the potency of topical corticosteroids on classifications from a previous review (Mason 2002b).
The review included any topical treatment for psoriasis, except for products for which (a) no licence was obtained and (b) research into the product was discontinued. The reason for this exclusion criterion is that these products are unlikely to be of interest to people making decisions about health care, such as policy‐makers, people with psoriasis, or clinicians. Although they may be of interest to researchers, lessons from the research into 'failed' molecules are likely to have been reflected in the development of subsequent products.
Trials of systemic or ultraviolet (UV) (phototherapy) treatments with adjunctive topical treatment were not eligible for inclusion in the review.
Types of outcome measures
Table 2 provides an overview of the effectiveness outcome measures included in the review. We provide details of how we used the primary outcomes to derive a 'combined end point' in the section 'Measures of treatment effect'.
2. Overview of outcome measures on effectiveness.
| Outcome | Acronym | Construct | Scale, minimum | Scale, maximum | Notes |
| * Investigator's Assessment of Overall Global Improvement | IAGI | Improvement from baseline variably defined. Common taxonomy ranges from worse to cleared | 4‐pt | 7‐pt | Calculated means and standard deviations by assigning zero to 'worse' (or equivalent). Higher scores indicate greater improvement |
| Investigator's Global Assessment of Disease Severity | IGA | Static equivalent of the IAGI | 4‐pt | 7‐pt | Calculated means and standard deviations by assigning zero to 'clear' (or equivalent). Higher scores indicate more severe disease |
| Total Severity Score | TSS | Redness (erythema), thickness (infiltration) and scaling (sometimes also itching (pruritis)) of target plaque(s). Scored separately then summed | 0 to 3 | 0 to 24 | Also known as the Local Psoriasis Severity Index or the Total Sum Score. Higher scores indicate more severe disease |
| Psoriasis Area and Severity Index | PASI | Redness, thickness, and scaliness of the lesions (each graded on a 0 to 4 scale), weighted by the area of involvement (0 to 6) and summed | 0 to 68 (without head) | 0 to 72 (including head) | Higher scores indicate more severe disease |
| * Patient's Assessment of Overall Global Improvement | PAGI | Assessed as IAGI | 4‐pt | 7‐pt | Less often reported than IAGI. Majority of included trials use 5‐pt scale |
| Patient's Global Assessment of Disease Severity | PGA | Assessed as IGA | 4‐pt | 5‐pt | Rarely reported (5/177 studies) |
| * IAGI/PAGI data are entered as a negative values; thus, a reduction denotes a positive improvement for the active treatment consistent with TSS and PASI measures. | |||||
Primary outcomes
Investigator's Assessment of Overall Global Improvement (IAGI)/Investigator's Global Assessment of Disease Severity (IGA).
Total Severity Scores (TSS).
Psoriasis Area and Severity Index (PASI).
Patient Assessment of overall Global Improvement (PAGI)/Patient Global Assessment of Disease Severity (PGA).
Secondary outcomes
Withdrawal rates (total rate; withdrawal due to adverse events; withdrawal due to treatment failure).
Adverse events (local and systemic).
Quality of life measures.
Economic outcomes.
Concordance or adherence with treatment.
Search methods for identification of studies
We aimed to identify all relevant randomised controlled trials (RCTs) regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
Search strategies used for the previous version of the review (Mason 2009; see also Acknowledgements) were revised where appropriate and rerun. We did not restrict the searches by body area affected. The information specialists updated the search strategies to reflect changes in the interfaces and MeSH (Medical Subject) headings, as well as to incorporate terms for newly licensed products.
In February 2011, the following databases were searched for effectiveness RCTs of psoriasis treatments:
the Cochrane Skin Group Specialised Skin Register (searched 8 February 2011) using the search strategy in Appendix 1;
the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (2011, Issue 2) using the search strategy in Appendix 2;
MEDLINE via OVID (from 1948) using the strategy in Appendix 3;
EMBASE via OVID (from 1980) using the strategy in Appendix 4;
Science Citation Index (SCI) via the Institute for Scientific Information (ISI) Web of Knowledge interface (now known as Thomson Reuters) (from 2008) using the strategy in Appendix 5;
Conference Proceedings Citation Index ‐ Science (CPCI‐S) via the ISI web of Knowledge interface (from 2008) using the strategy in Appendix 5;
BIOSIS via the DialogClassic interface (from 1993) using the strategy in Appendix 6;
Dissertation Abstracts via DialogClassic interface (from inception) using the strategy in Appendix 7;
Inside Conferences via DialogClassic interface (from inception) using the strategy in Appendix 7;
System for Information on Grey Literature in Europe (SIGLE) via WebSPIRS interface (search not updated) using the strategy in Appendix 8;
National Research Register (NRR) (CD‐ROM interface, issue 2004/4) using the strategy in Appendix 2; and
the UK Clinical Research Network Study Portfolio (http://public.ukcrn.org.uk/search/) using the strategy in Appendix 2.
To comply with Cochrane policy (stipulating that reviews must be published within 12 months of the electronic searches being run), further searches for this update were run on 23, 24, and 29 August 2012. Although it was not possible to incorporate RCTs identified through this search within this review, we listed relevant references in the 'Characteristics of studies awaiting classification' tables. They will be incorporated into the next update of the review.
Searching other resources
References from published studies and reviews
We checked these for further references to relevant trials.
Unpublished literature
We routinely contacted trialists and companies for newly published studies and missing data.
The metaRegister of Controlled Trials (http://www.controlled‐trials.com/mrct/) was searched in August 2012 for ongoing and unpublished trials.
Adverse effects
On 2 February 2011, the following databases were searched for studies of adverse events of specific psoriasis treatments:
MEDLINE via OVID (see Appendix 9); and
EMBASE via OVID (see Appendix 10).
We limited searches to English‐language papers published in the years between 2005 to 2011. In MEDLINE, the search was designed to omit records with the following publication types: 'note', 'comment', and 'editorial'.
We also considered relevant adverse effects studies identified during the screening for effectiveness trials.
These searches were updated in August 2012, identifying 537 new references. We will incorporate these studies into the next update of this review.
Concordance/adherence
We did not undertake searches for concordance/adherence in the 2011 review update because of resource constraints.
Language restrictions
There were no language restrictions when searching for effectiveness RCTs or concordance/adherence studies. We restricted searches for studies of adverse events to those published in English.
Data collection and analysis
Selection of studies
Two authors (AM and JM) screened titles and (where available) abstracts identified from the searches, and another author (MC) acted as an arbiter when necessary. In our protocol, we stated our intention that we would exclude studies meeting only some of the inclusion criteria stated above. However, this was infeasible, because we would have needed to cite large numbers of studies (over 1000). Therefore, we listed as excluded only those studies that we deemed potentially eligible for inclusion and for which we retrieved full papers, but which subsequently failed to meet the inclusion criteria.
For the separate search for studies exploring adverse events, we deemed studies as eligible if they addressed safety or tolerability issues, focused on drugs included in the main review, and were longer‐term in follow‐up (> 12 weeks). Short‐term studies (with follow‐up < 12 weeks) were eligible for inclusion only if they were designed specifically to consider adverse effects, tolerability, or safety. Studies that included fewer than 10 participants (including case reports) were not eligible for inclusion.
For the separate search for studies of concordance/adherence with treatment, studies were eligible if they addressed adherence with topical treatment in people with any type of psoriasis. This section was not updated because of resource constraints.
Data extraction and management
Applying methods from our original review (Mason 2002a), we summarised the major attributes of trials, including treatment forms, doses and duration, inclusion and exclusion criteria, level of blinding, within‐patient or between‐patient (parallel‐group) design, method of generation of the randomisation sequence, concealment of allocation, numbers of participants randomised, baseline comparability, loss to follow up, primary and secondary outcomes, withdrawals, and adverse events. One reviewer (AM) extracted the data, and another reviewer (HH) checked these data.
We extracted data from trials on four primary outcomes:
IAGI (Investigator's Assessment of Global Improvement) or the IGA (Investigator's Global Assessment of Disease Severity).
TSS (Total Severity Score).
PASI (Psoriasis Area and Severity Index).
PAGI (Patient Assessment of Global Improvement) or the PGA (Patient Global Assessment of Disease Severity).
Where available, we also extracted data on quality of life, economic outcomes, and concordance/adherence.
In addition, we extracted data on withdrawal due to any reason, such as adverse events or treatment failure, as well as adverse events due to local and systemic effects.
For each outcome measure under a comparison, we included the same treatment options regardless of data availability (see Data and analyses). We did this for three reasons. Firstly, an inclusive approach makes clear that there is an absence of data, not that data have been omitted. Secondly, if data subsequently become available when the review is updated in future, the correct structure is in place for data entry. Thirdly, this approach ensures treatments are always ordered identically regardless of outcome.
Assessment of risk of bias in included studies
Assessment of methodological quality
The quality assessment included an evaluation of each included study, based on the following components, which are considered to be associated with biased estimates of treatment effect (Juni 2001): (a) the method of generation of the randomisation sequence; (b) the method of allocation concealment ‐ we considered this 'adequate' if the assignment could not be foreseen; (c) who was blinded/not blinded (participants, clinicians, outcome assessors); and (d) how many participants were lost to follow up.
In addition, the quality assessment included the following: (e) baseline assessment of the participants for age, sex, duration, and severity of psoriasis; and (f) baseline comparability of intervention and control groups. We recorded the information in the 'Characteristics of included studies' section.
Measures of treatment effect
Summarising primary outcomes with standardised mean differences
We extracted data on four primary outcome measures:
IAGI (Investigator's Assessment of Global Improvement) or the IGA (Investigator's Global Assessment of Disease Severity)
TSS (Total Severity Score)
PASI (Psoriasis Area and Severity Index)
PAGI (Patient Assessment of Global Improvement) or the PGA (Patient Global Assessment of Disease Severity)
Trials often reported more than one measure, but none of the trials reported all measures. We therefore devised a 'combined end point', which allowed more data to contribute to an overall analysis and facilitated treatment comparisons. We labelled this 'super' outcome as outcome (e) throughout the review.
We constructed the combined end point by taking IAGI (or IGA) data when available, and failing this, TSS, PASI, or PAGI (PGA) data in that order of availability. For PASI and TSS, some included trials reported change scores and others reported end point scores. In view of the mix of end point/change scores and of the variation in scale, we analysed findings using a standardised mean difference statistic (SMD) in a random‐effects model. Table 2 summarises the characteristics of the outcome measures.
We also expressed SMDs in physical units adjusting by the appropriate pooled standard deviation estimate (Table 3).
3. Summary of imputed standard deviation values.
| Type of study/score |
Placebo IAGI (change)/IGA (end point) |
Placebo TSS |
Placebo PASI |
Placebo PAGI (change)/PGA (end point) |
H2H IAGI (change)/IGA (end point) |
H2H TSS |
H2H PASI |
H2H PAGI (change)/PGA (end point) |
| Between‐patient (end point) | 0.93 | 1.33 | 3.76 | 1.13 | 1.01 | 1.65 | 3.61 | 1.12 |
| Within‐patient (end point) | 1.08 | 1.49 | 7.17 | NA | NA | 1.50 | 2.58 | NA |
| Between‐patient (change) | 1.17 | 1.52 | 5.75 | 1.31 | 1.10 | 1.73 | 7.85 | 1.20 |
| Within‐patient (change) | 1.02 | 1.58 | NA | 1.53 | 0.96 | 1.94 | NA | 0.83 |
| Within‐patient (% change) | NA | 0.18 | NA | NA | NA | NA | NA | NA |
| Between‐patient (% change) | NA | NA | 0.37 | NA | NA | 0.13 | 0.33 | NA |
| Scalp between‐patient (end point) | 1.08 | 1.74 | NA | 1.06 | 1.06 | 1.94 | NA | 1.18 |
| Scalp within‐patient (end point) | 1.33 | NA | NA | NA | NA | NA | NA | NA |
| Scalp between‐patient (change) | 1.20 | NA | NA | 1.28 | 1.30 | 1.75 | NA | 1.20 |
| Scalp between‐patient (% change) | NA | NA | NA | NA | NA | 0.25 | NA | NA |
| NA: not available; H2H: head‐to‐head; IGA [PGA]: Investigator [Patient] Global Assessment of Disease Severity; IAGI [PAGI]: Investigator (patient) Assessment of Global Improvement; TSS: Total Severity Score; PASI: Psoriasis Area and Severity Index | ||||||||
Secondary outcomes
We summarised data on adverse events, quality of life measures, economic outcomes, and concordance as narratives. We summarised withdrawal data using the risk difference (RD) metric and pooled using a random‐effects model. We felt this was more appropriate than a fixed‐effect model since definitions of withdrawal and adverse events vary between trials.
Unit of analysis issues
Within‐patient studies are statistically analogous to cross‐over studies, and results should be adjusted by the correlation coefficient (Section 16.4.6, Cochrane Handbook for Systematic Reviews of Interventions; Higgins 2011). No study included in the review reported this statistic, and we did not have access to patient‐level data, so could not estimate it directly.
On the subject of cross‐over studies, the Cochrane Handbook for Systematic Reviews of Interventions states (Section 16.4.5; Higgins 2011) the following: "A common situation is that means and standard deviations (or standard errors) are available only for measurements on E [experimental group] and C [control group] separately. A simple approach to incorporating cross‐over trials in a meta‐analysis is thus to take all measurements from intervention E periods and all measurements from intervention C periods and analyse these as if the trial were a parallel‐group trial of E versus C. This approach gives rise to a unit‐of‐analysis error (see Chapter 9, Section 9.3) and should be avoided unless it can be demonstrated that the results approximate those from a paired analysis, as described in Section 16.4.4. The reason for this is that confidence intervals are likely to be too wide, and the trial will receive too little weight, with the possible consequence of disguising clinically important heterogeneity. Nevertheless, this incorrect analysis is conservative, in that studies are under‐weighted rather than over‐weighted. While some argue against the inclusion of cross‐over trials in this way, the unit‐of‐analysis error might be regarded as less serious than some other types of unit‐of‐analysis error."
Consequently, we included within‐patient studies as though they were parallel‐group studies, accepting that they are under‐weighted. To explore whether it was appropriate to combine these trials, we undertook two sensitivity analyses. First, we considered how effect size varied for within‐ and between‐patient studies. If the magnitude of effect varied consistently between the two study designs, this strongly suggested a non‐zero correlation coefficient and an appropriateness to separate the trials. Second, we used sensitivity analysis to explore the impact on pooled findings of varying the correlation coefficient (rho) for within‐patient studies. This analysis used the generic inverse variance measure, with SMDs and their standard errors estimated from the formulae in the Cochrane Handbook for Systematic Reviews of Interventions (Section 16.4.6.4) (Higgins 2011). These estimated SMDs differ slightly from those that RevMan estimates for continuous outcomes, even when the correlation coefficient is zero (which is the assumption implicit in the latter model).
The analyses found no evidence that the magnitude of effect varied consistently. Within‐patient trials did not consistently demonstrate smaller or larger effects than between‐patient trials. Varying the value of rho had no significant effect on the findings: As rho increased, the effect size increased and the confidence intervals (usually) widened, but the magnitudes of changes were small and non‐significant at the 5% level.
In the interests of statistical purity, these trials could (a) be reported separately or (b) be removed altogether. The drawback of option (a) is that it makes an already complex review even more complex and less accessible; the disadvantage of option (b) is that it removes data that might be of interest to clinicians and people with psoriasis. On balance, we preferred to report relevant randomised data wherever possible to help inform pragmatic decision‐making.
Dealing with missing data
We routinely contacted trialists and companies for missing data.
Where studies did not report estimates of variance, we derived them from confidence intervals (CIs) or from P values where possible. Where we could not obtain estimates of variance, we imputed them deterministically by pooling the standard deviations of treatment cohorts fully reported in trials and adjusted for scale size.
We made separate imputations for each outcome measure (see Table 3):
for within‐patient studies;
for between‐patient (parallel‐group) studies;
for end point scores;
for change scores; and
for scalp trials.
Within‐patient designs are statistically analogous to cross‐over studies, and the precision of their findings within a meta‐analysis needs adjustment for within‐patient correlation. We attempted to explore this by sensitivity analysis.
Data synthesis
We analysed findings using a standardised mean difference statistic (SMD) in a random‐effects model. However, this model cannot perfectly address all the sources of design complexity that arise when summarising findings across studies. Three of the main sources of complexity are listed below. Other sources of complexity include variation in trial duration, disease severity, participant demographics, treatment application method, dosing frequency, drug potency and vehicle.
Study design (within‐ versus between‐patient)
Trials were either between‐patient or within‐patient designs. The former randomise participants into separate (parallel) groups; the latter randomise treatments to the left or right side of the same participant. Within‐ and between‐patient trials have different variance structures. Moreover, the two responses (left and right) of within‐patient studies may be correlated (See Unit of analysis issues for further details).
Absence of a simple one‐to‐one correspondence between papers, trials, and comparisons
There were instances of single papers reporting either multiple trials or multiple analyses within a single trial. Therefore, simple counts of numbers of participants and numbers of studies contributing data to the analysis were misleading, and we made adjustments accordingly (Table 4). Thus, these numbers may not match the numbers estimated in RevMan, which does not account for these factors.
4. Overview of analyses: evidence of effectiveness outcomes.
| Comparison No. | Comparison Label | No. studies (NB: a study may contribute to more than one comparison) | Per cent studies with between‐patient design | No. participants |
| 01 | Vitamin D analogues vs. placebo | 30 | 60% | 4986 |
| 02 | Corticosteroid (potent) vs. placebo | 13 | 85% | 2216 |
| 03 | Corticosteroid (very potent) vs. placebo | 10 | 70% | 1264 |
| 04 | Dithranol vs. placebo | 3 | 0% | 47 |
| 05 | Vitamin D combination products vs. placebo | 5 | 100% | 2058 |
| 06 | Other treatment vs. placebo | 26 | 46% | 1450 |
| 07 | Vitamin D analogues vs. corticosteroid (potent) | 14 | 64% | 3542 |
| 08 | Vitamin D analogues vs. corticosteroid (very potent) | 2 | 100% | 82 |
| 09 | Vitamin D combined with corticosteroid vs. corticosteroid | 5 | 100% | 2113 |
| 10 | Vitamin D alone or in combination vs. dithranol | 8 | 88% | 1284 |
| 11 | Vitamin D alone or in combination vs. other vitamin D analogue | 4 | 75% | 513 |
| 12 | Vitamin D alone or in combination vs. vitamin D + corticosteroid | 17 | 94% | 5856 |
| 13 | Vitamin D alone or in combination vs. other treatments: complex regimens | 9 | 89% | 2936 |
| 14 | Vitamin D alone or in combination vs. other treatment: long‐term studies (> 24 wks) | 1 | 100% | 297 |
| 15 | Vitamin D analogues vs. other treatment | 19 | 68% | 2364 |
| 16 | Flexural/facial psoriasis: placebo‐controlled trials | 2 | 100% | 122 |
| 17 | Flexural/facial psoriasis: vitamin D alone or in combination vs. other treatment | 4 | 75% | 588 |
| 18 | Scalp psoriasis: placebo‐controlled trials | 14 | 93% | 3011 |
| 19 | Scalp psoriasis: vitamin D alone or in combination vs. other treatments | 12 | 100% | 5413 |
Body area targeted for treatment
Whereas the majority of trials investigated chronic plaque psoriasis on the body, some trials focused on scalp psoriasis; some reported findings for both scalp and body psoriasis; and some were of inverse (flexural) or facial psoriasis. One trial of body and scalp psoriasis reported overall outcomes (IAGI/PAGI), a scalp‐only outcome (TSS), and a body‐only outcome (modified PASI) (Van de Kerkhof 2002a). Ortonne 2010 reported findings separately for treatment of the body and treatment of the face. We previously used sensitivity analysis to investigate the scalp psoriasis trials (Mason 2009), but in this update we analysed trials of inverse psoriasis (comparisons 16 and 17) and scalp trials (comparisons 18 and 19) separately from the trials of body psoriasis (see Sensitivity analysis).
Subgroup analysis and investigation of heterogeneity
We examined findings by agent class (as our primary analysis) and individual topical agent (within‐class analysis).
When comparing trials both within and across therapeutic classes, the summary estimates may demonstrate substantial heterogeneity. Ideally, we would seek to identify the reasons for individual differences, but publications rarely report sufficient detail to make a robust investigation feasible. Reasons might include differences in trial design, length of follow‐up, disease severity, participant selection, adherence, adequacy of concealment of allocation, adequacy of blinding, and source of funding (Mason 2002a).
The Cochrane Handbook for Systematic Reviews of Interventions explicitly endorses the combination of 'apples and oranges' "if they are used to contribute to a wider question about fruit" (Section 9.5.1; Higgins 2011). Our purpose was to identify whether classes of topical treatments work and are safe. To this end, there is a fundamental difference between heterogeneity that makes it uncertain whether individual people with psoriasis will derive any benefit from a treatment and heterogeneity that makes the size of a positive benefit imprecise. Clinicians and those with psoriasis will still value information about a treatment that is beneficial even though its magnitude is poorly understood. However, we clearly stated the presence of heterogeneity where it occurred and used the Cochrane Handbook for Systematic Reviews of Interventions as a guide to interpretation (Section 8.5.2; Higgins 2011).
Sensitivity analysis
We used a meta‐analysis with a random‐effects estimation both for measures of effect and for pooling of risk differences for adverse events. We quantified heterogeneity using the I² statistic. If we identified outliers, we undertook a sensitivity analysis to investigate the implications of their exclusion on the pooled summary statistics. In addition, we undertook sensitivity analyses to investigate the impact of within‐patient versus between‐patient trials, and to explore the impact on pooled findings of varying the correlation coefficient (see Unit of analysis issues). In some comparisons, there were no, or relatively few, studies that included both within‐patient and between‐patient designs, few participants contributing data, or both. We used the following criteria to help decide whether we should have included an analysis in the sensitivity analysis:
frequently‐used products in clinical practice; and
for within‐/between‐patient sensitivity analysis: whether it included both within‐patient and between‐patient designs
Where at least two within‐patient trials were included in a pooled comparison, we explored the potential influence of the correlation coefficient.
Based on these criteria, we selected six comparisons (analyses 1, 2, 3, 4, 7, and 18) for sensitivity analysis. To ensure sufficient data were available, we analysed the combined end points. These analyses cover vitamin D analogues, dithranol, and corticosteroids, which are amongst the most frequently used products in clinical practice.
Other
We involved a consumer throughout the review process to help ensure the readability of the final review.
Results
Description of studies
Results of the search
For this update, the RCT searches identified 3749 records:
MEDLINE: 1312
EMBASE: 2008
SCI: 253
BIOSIS: 44
Dissertation Abstracts: 1
Inside Conferences: 0
CENTRAL: 70
UK Clinical Research Network: 20
Skin Group Specialised Register: 41
The total number of new records assessed after deduplication against each other and previously identified records was 2637.
We added records from the searches in February 2011 to those identified from searches run in 2008 (see Mason 2009). The total number of records screened for this review over time is now 5414.
From the 2011 searches, we retrieved 148 papers and screened these for eligibility. (Some papers were multiple reports of the same trial).
In 40 trials (some of which were consequently excluded), some or all outcome data were missing. We contacted trialists or sponsors to request missing data, receiving data for 25 of these trials. We excluded trials that reported no useable outcome data. We did not contact trialists or sponsors for missing adverse events or withdrawal data, although some sponsors provided this spontaneously.
We included 48 new randomised controlled trials in the updated review. Compared with the previous version of this review (Mason 2009), studies were larger (mean number of participants: 284 versus 164), had a longer treatment duration (10 weeks versus 6 weeks) and follow up (11 weeks versus 8 weeks), and were more likely to be parallel‐group in design (88% versus 63%). The new studies were also more likely to include an active control group (60% versus 43%) and patient‐reported outcomes (44% versus 24%), and there were relatively more scalp trials (21% versus 12%). The 48 trials provided evidence on 7 new active treatments.
Included studies
The updated review included 177 studies, with 34,808 participants.
The number of included studies counted by RevMan is 190, because we entered each study reporting a placebo and an active comparison into the 'Characteristics of included studies' table as two studies.
Of the included studies, 106 of these were placebo‐controlled; 84 compared treatments head‐to‐head, with 15 trials reporting both placebo‐controlled and head‐to‐head comparisons. The 15 trials reporting both head‐to‐head and placebo comparisons contributed only once to the analysis of study characteristics, unless the trial involved entirely distinct participants in its placebo‐controlled and active‐controlled analyses. For example, the trial by Guenther 2002 compared treatments against each other (Guenther 2002 (H)) and against placebo (Guenther 2002 (P)). This study contributed only once to the analysis of study characteristics (number of participants, proportion of males, etc). However, two trials reported placebo and head‐to‐head analyses involving entirely separate participants (Barker 1999 (H) and Barker 1999 (P); Grattan 1997 (H) and Grattan 1997 (P)). Therefore, the total number of studies contributing data to the analysis of study characteristics and quality assessment was 177 (106 placebo + 84 head‐to‐head ‐15 double‐counted trials (with placebo and active comparators) and 2 trials that each report 2 separate studies (Barker 1999 (H) and Barker 1999 (P); Grattan 1997 (H) and Grattan 1997 (P)).
There were 26 trials of scalp psoriasis (Barrett 2005; Buckley 2008; Cook‐Bolden 2010; Duweb 2000; Elie 1983; Ellis 1988; Franz 1999; Franz 2000; Green 1994; Jarratt 2004; Jemec 2008 (H) and Jemec 2008 (P); Kiss 1996; Klaber 1994; Klaber 2000b; Köse 1997; Kragballe 2009; Lepaw 1978; Luger 2008; Olsen 1991; Pauporte 2004; Poulin 2010; Reygagne 2005; Shuttleworth 1998; Tyring 2010; Van de Kerkhof 2002a; Van de Kerkhof 2009). Six trials investigated inverse psoriasis, facial psoriasis, or both (Gribetz 2004; Kreuter 2006 (H) and Kreuter 2006 (P); Lebwohl 2004; Liao 2007; Ortonne 2003; Ortonne 2010). One trial evaluated psoriasis in children (Oranje 1997). Most trials were conducted in ambulatory care settings, but four trials were of hospitalised participants (Grattan 1997 (H) and Grattan 1997 (P); Kragballe 1991a; Monastirli 2000; Van der Vleuten 1995).
One hundred and twenty‐three trials adopted a between‐patient (parallel‐group) design; 53 were within‐patient studies; and one trial used both designs (Henneicke‐v. Z. 1993). The trial by Levine (Levine 2010 (H) and Levine 2010 (P)) was a within‐patient trial that randomised participants to two of seven treatment options. Therefore, the pair‐wise comparisons we analysed (e.g. calcipotriol versus placebo) included a mixture of within‐ and between‐patient designs: Some participants received calcipotriol on one side and placebo on the other; other participants received calcipotriol on one side and another active treatment on the other side.
The 177 studies included 34,808 participants. Of these studies, 133 provided data on the age of participants. The mean age of all participants for which studies provided data was 47.2 years (range = 2 to 97 years) (N = 28,921). Data on the gender of participants (N = 28,941) were available from 140 studies. Overall, participants were more likely to be male; the mean proportion of males was 56.7% (range = 30% to 100%).
Almost half the studies (77/177 = 44%) did not clearly report the overall baseline severity of study participants (e.g. participants with mild to moderate disease) (Figure 3). One hundred studies explicitly reported baseline severity or reported sufficient information on global severity scores, such as the mean and variation in baseline PASI or the percentage of body surface area (BSA) affected, to allow us to infer global severity using guidance on the interpretation of severity scores (Finlay 2005; Krueger 2000). In the 100 trials where severity was assessable, we classified participant severity as mild (5 studies), mild to moderate (36 studies), mild to severe (6) or very severe (2 studies), moderate (12 studies), moderate to severe (27 studies), moderately severe (2 studies), moderately severe to very severe (2 studies), and severe (8 studies).
3.
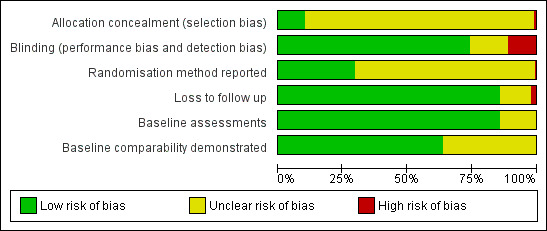
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Seventy‐seven studies provided insufficient information to allow an assessment of clinical severity to be made; we could not make assessments of the clinical characteristics of participants in studies reporting only the mean PASI (with no information about variation) or reporting only localised (e.g. TSS) scores. One example of a study that included participants with a wide range of severity scores is the trial by Cunliffe 1992, where the mean baseline PASI was 9.0 (suggesting moderately severe disease, according to Finlay 2005), but where individual participant scores ranged from 0.6 to 41.2. Another example is the study by Olsen 1996 (1), where participant BSA involvement averaged 12%, but ranged from 1% to 80%. It is unclear how participant severity was distributed within these ranges (i.e. whether these extremes were 'outliers' or whether a sizeable proportion of participants were clustered at the extreme ends of the distribution).
Even where trialists classified participant severity, it was not always clear that this was consistent with published guidance, which itself does not always provide consistent messages. For example, Finlay 2005 states that a PASI score > 10, a BSA involvement > 10%, or Dermatology Life Quality Index (DLQI) score > 10 constitutes severe disease. However, Krueger 2000 argues that BSA is unreliable as an indicator of severity, which is better proxied by quality of life assessments. However, the included studies rarely assessed quality of life. Given this lack of clarity and the absence of adequate severity data in around half (44%) of the included studies, we could not use sensitivity analysis to investigate the impact of baseline participant severity, nor could we reliably use severity to investigate inter‐study heterogeneity.
All 177 studies provided data on treatment duration (mean: 7 weeks; range = 1 to 52) and follow‐up duration (mean: 9 weeks; range = 2 to 52), where 'follow‐up duration' was defined as including the treatment period. Commonly used outcomes assessed by the studies included the following:
individual signs (erythema, scaling, induration) (105/177 studies = 59%);
Total Severity Score, Total Sign Score, or equivalent (83 studies = 47%);
PASI (65 studies = 37%);
IAGI/IGA (113 studies = 64%); and
PAGI/PGA (52 studies = 29%).
Outcome measures employed by small numbers (< 5) of trials included the following;
Local Psoriasis Severity Index (scale not reported);
Jacoby assessment score (0 to 7 score transformed to % clinical improvement); and
investigator assessment of skin staining.
Trials seldom assessed quality of life (9 trials = 5%).
Participant‐reported outcomes included the following:
overall participant assessment (relative efficacy, speed of response, irritation, staining, ease of application);
participant global assessment of acceptability of treatment, participant assessment of likely adherence; and
participant assessment of cosmetic acceptability.
We grouped placebo‐controlled trials by type of treatment (e.g. vitamin D products) and grouped head‐to‐head trials in a similar way (e.g. vitamin D versus potent corticosteroid). We included 19 comparisons in the review. Since many trials did not specify participants' disease severity, it was not possible to use severity to inform pooling decisions. The primary analysis explored the results of pooling within these 19 comparison groups using a random‐effects model. In addition, we undertook sensitivity analyses for five comparisons using the 'combined end point'. These analyses used pooled data to explore within‐ and between‐patient trial variation.
In 90% (159/177) of the studies included in the review, participants applied their own treatments. Nurses applied treatments in 1 trial (Geilen 2000); participants' parents delivered some care in a trial of childhood psoriasis (Oranje 1997); and the delivery method was unclear in 16 studies.
Excluded studies
We excluded 43 studies, of which we had newly added 16 studies in this update of the review (see 'Characteristics of excluded studies' tables). The most common reasons for exclusion from the update were that the study did not report adequate data and requests for missing data from trialists or sponsors were unsuccessful (N = 5), or that the study did not provide a comparison of interest (N = 6). Two studies were not randomised (Kaur 2004; Vena 2005); one study assessed multiple plaques (Buder 2010); and two evaluated unlicensed products that were not subsequently marketed (Agrawal 2010; Rhemus 2006). We also excluded trials of nail psoriasis that we had previously included (Mason 2009), as the topic is now covered by a separate Cochrane review (de Vries 2013).
Studies awaiting classification
Update searches were run on 23, 24, and 29 August 2012. For each database searched, we have shown below the numbers of records identified. The total number of new records assessed (after deduplication against each other and previously identified records) was 1865. Relevant studies from these searches (10 references) are listed in the Studies awaiting classification section, but we did not include them in the main review.
MEDLINE: 1203
EMBASE: 1140
SCI: 129
BIOSIS: 37
Dissertation Abstracts: 1
Inside Conferences: 0
CENTRAL: 26
UK Clinical Research Network: 0
Skin Group Specialised Register: 67
Ongoing studies
The metaRegister of Controlled Trials was searched for ongoing and unpublished trials during the final searches for this review in August 2012 http://www.controlled‐trials.com/mrct/ using the following phrases:
"psor* AND topical NOT completed", which retrieved 127 hits;
"psor* AND calcipot% NOT completed", which retrieved 7 hits;
"psor* AND vitamin D NOT completed", which retrieved 21 hits;
"(psor* AND topical AND corticost%) NOT completed", which retrieved 63 hits; and
"psor* AND tar NOT completed", which retrieved 8 hits.
In total, we identified 10 potentially relevant trials. We provide details in the 'Characteristics of ongoing studies' tables.
Risk of bias in included studies
We extracted and tabulated data on six quality indicators. Summary findings are presented narratively, with characteristics for all studies presented in the 'Characteristics of included studies' tables. Figure 3 is a graphical representation of the overview of the risk of bias. All included trials were randomised, but only 47/177 (27%) clearly reported the method used to randomise participants. Concealment of treatment allocation was explicitly adequate in 15 trials, but most trials (151/177 = 85%) blinded participants to treatment allocation. Most (164/77 = 93%) trials reported loss to follow up data, and 142 trials (80%) demonstrated that groups were comparable at baseline.
Allocation
Of the 177 studies assessed for quality, 15 (8.5%) explicitly achieved adequate concealment of treatment allocation (low risk of bias). Concealment was unclear in the majority of studies (160 studies = 90.4%), so the risk of bias was also unclear. Concealment was inadequate in 2 studies (1.1%) (high risk of bias) (Figure 3).
Blinding
Most (131/177 = 74%) studies were double‐blind, with 20 studies adopting a single‐blind (investigator‐only) approach. Eighteen studies were 'open' (no blinding), and in the remaining 8 studies, the blinding approach adopted was unclear (Figure 3). Twenty trials explicitly stated that the outcome assessor was blinded to treatment allocation. However, the outcome assessor will also have been blinded in double‐blind trials where the investigator also assessed outcomes.
Incomplete outcome data
We defined 'loss to follow up' as the number of enrolled participants who failed to contribute data for the analysis.
Of the 177 studies assessed for quality, 13 (7%) provided no data on loss to follow up. For the remaining 164 studies, the mean percentage loss to follow up was 6.1% (range = 0% to 31.5%). Fifty‐one studies reported that there was no loss to follow up. Four studies lost more than 25% of their participants to follow‐up, and we classified them as having high risk of bias for this dimension (Henneicke‐v. Z. 1993; Lin 2007; Maier 2004; Weinstein 2003) (see Figure 3).
Where studies did not report estimates of variance, we derived them from confidence intervals (CIs) or from P values where possible. Where we could not obtain estimates of variance, we imputed them (see Table 3). In total, we imputed estimates of variance for at least 1 outcome measure in 45 studies (7 of which were scalp trials); details are in the notes section of the 'Characteristics of included studies' tables.
Other potential sources of bias
Method of generation of the randomisation sequence
Only randomised controlled trials were eligible for inclusion in the review. However, 130 studies (73%) did not clearly report the randomisation method used. Fourteen studies reported a block randomisation design, and 27 studies used computerised methods (5 studies used both). Four reported that sequential allocation had been used (3 of these studies were published in the 1970s); 1 study used the toss of a coin; and another study used a sealed envelope method. It could be argued that we should have excluded trials with sequential allocation from the review, but this might discriminate against studies with better reporting methods in favour of those not stating the randomisation method.
Baseline assessment of the participants for age, gender, and clinical characteristics
We coded studies as follows: y (baseline assessments for age, gender, and clinical characteristics), p (at least one type of assessment), and NR (not reported or unclear). Most studies (121/177 = 68.4%) provided baseline assessments of age, gender, and clinical characteristics. Forty‐three studies (24.3%) provided a partial assessment, and 13 studies (7.3%) reported no relevant data.
Baseline comparability of intervention and control groups
We coded studies as follows: y (comparability demonstrated, low risk of bias), p (comparability partially demonstrated, risk of bias unclear), and NR (comparability not demonstrated or unclear, risk of bias unclear or high, depending on whether groups were clearly non‐comparable). Studies could demonstrate comparability by reporting data for each group, by reporting the outcome of statistical tests (e.g. P values), or both. One hundred and sixteen studies (65.5%) demonstrated that the groups were comparable at baseline; 27 studies (15.3%) demonstrated partial comparability; and 34 studies (19.2%) did not clearly demonstrate comparability between the groups. No study found that groups were non‐comparable (high risk of bias).
Data extraction method for the review
To minimise errors and reduce potential biases being introduced by review authors, the recommended approach is that data extraction should be undertaken independently by at least two people, preferably from complementary disciplines (Section 7.6.2, Higgins 2011). However, in this review, one reviewer (AM) extracted the data, and another reviewer (HH) checked these data.
Effects of interventions
Primary outcome measures
The review analyses 19 comparisons. Of these, 8 are topical treatment versus placebo analyses, and 11 are head‐to‐head analyses of a topical treatment against a vitamin D analogue (i.e. 1 commonly used class of treatments). Some analyses are a 'catch all' category; for example, analysis 6 includes 'Other treatment versus placebo', which covers 26 treatments for body psoriasis for which there is less research evidence (both in terms of numbers of studies and numbers of participants contributing data). Similarly, analysis 15 incorporates 12 head‐to‐head comparisons of vitamin D analogues for body psoriasis that are not easily classified under the other head‐to‐head comparisons. Scalp trials (comparisons 18 and 19) and trials of inverse psoriasis (comparisons 16 and 17) are analysed separately from the trials of body psoriasis.
Table 4 summarises the 19 analyses. Table 2 gives details of the outcome measures considered. The number of participants and number of studies are adjusted manually from those reported in the Tables and Figures to allow for within‐patient studies, studies contributing more than once to a single analysis, and studies contributing to multiple analyses. Therefore, numbers of participants and studies reported sometimes differ from the numbers estimated by RevMan.
For each of the 19 analyses, we analysed data on 5 effectiveness outcome measures, where available. The fifth measure is a 'combined end point' that uses data from the four primary outcome measures.
(a) Investigator's Assessment of Overall Global Improvement (IAGI)/Investigator's Global Assessment of Disease Severity (IGA)
Analysis 1: Vitamin D analogues versus placebo
This comparison included eight vitamin D analogues for body psoriasis (see Analysis 1.1 and Table 5). Twenty trials with 3771 participants reported IAGI data on 7 of these treatments. Thirteen trials were between‐patient design, and 7 were within‐patient studies. Treatment duration ranged from 4 weeks to 12 weeks. The pooled SMD across all treatments was ‐0.95 (95% CI ‐1.17 to ‐0.74; I² statistic = 89.0%), but there was considerable variation between treatments, so we removed pooling across subgroups. Six treatments were significantly more effective than placebo, with the effect size ranging from ‐0.67 (becocalcidiol twice daily) to ‐1.66 (paricalcitol once daily). There was considerable between‐study variation in the IAGI SMD for calcitriol. The pooled effect was ‐1.03 (95% CI ‐1.71 to ‐0.36), but this ranged from ‐0.26 (95% CI ‐0.99 to 0.47) for Langner 2001 (P) to ‐3.11 (95% CI ‐3.57 to ‐2.66) for Perez 1996. The magnitude of the IAGI SMD for the Perez study was the highest across all comparisons and treatments. For the 'combined end point' of this analysis, we explored the impact of removing this trial from the pooled findings using sensitivity analysis. The presence of considerable heterogeneity within this subgroup means that the estimated average benefit should be interpreted with caution (Higgins 2011).
1.1. Analysis.
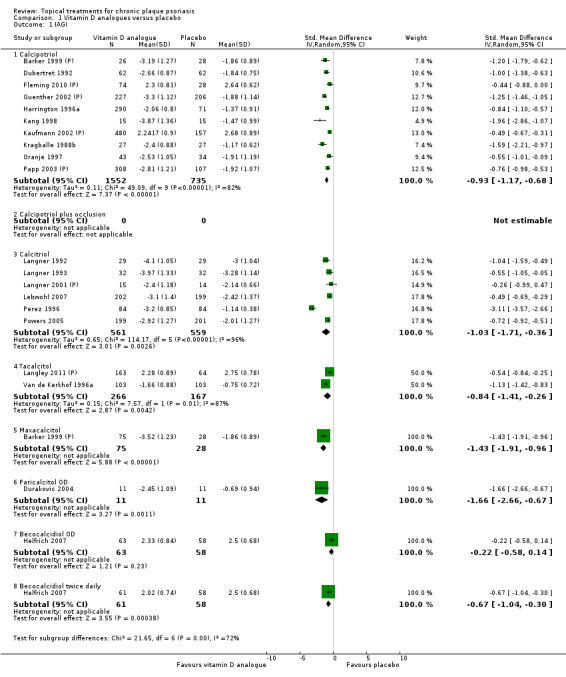
Comparison 1 Vitamin D analogues versus placebo, Outcome 1 IAGI.
5. Analysis 01: Trial characteristics and outcomes: vitamin D vs. placebo.
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Calcipotriol OD/BD | Effect size [CI] | (SMD ‐0.93; 95% CI ‐1.17 to ‐0.68) | (SMD ‐1.15; 95% CI ‐1.41 to ‐0.89) | (SMD ‐0.65; 95% CI ‐0.75 to ‐0.55) | (SMD ‐0.64; 95% CI ‐0.97 to ‐0.30) | (SMD ‐0.96; 95% CI ‐1.15 to ‐0.77) |
| 02 Calcipotriol plus occlusion | Effect size [CI] | NA | (SMD ‐0.15; 95% CI ‐0.44 to 0.14) | ‐ | ‐ | (SMD ‐0.15; 95% CI ‐0.44 to 0.14) |
| 03 Calcitriol OD/BD | Effect size [CI] | (SMD ‐1.03; 95% CI ‐1.71 to ‐0.36) | (SMD ‐1.22; 95% CI ‐2.38 to ‐0.07) | ‐ | (SMD ‐0.59; 95% CI ‐0.76 to ‐0.41) | (SMD ‐0.92; 95% CI ‐1.54 to ‐0.29) |
| 04 Tacalcitol OD | Effect size [CI] | (SMD ‐0.84; 95% CI ‐1.41 to ‐0.26) | (SMD ‐0.66; 95% CI ‐0.95 to ‐0.36) | (SMD ‐0.27; 95% CI ‐0.56 to 0.03) | (SMD ‐0.24; 95% CI ‐0.53 to 0.05) | (SMD ‐0.73; 95% CI ‐1.09 to ‐0.37) |
| 05 Maxacalcitol OD | Effect size [CI] | (SMD ‐1.43; 95% CI ‐1.91 to ‐0.96) | (SMD ‐1.61; 95% CI ‐2.10 to ‐1.12) | ‐ | ‐ | (SMD ‐1.43; 95% CI ‐1.91 to ‐0.96) |
| 06 Paricalcitol OD | Effect size [CI] | (SMD ‐1.66; 95% CI ‐2.66 to ‐0.67) | (SMD ‐2.15; 95% CI ‐3.24 to ‐1.06) | ‐ | ‐ | (SMD ‐1.66; 95% CI ‐2.66 to ‐0.67) |
| 07 Becocalcidiol OD | Effect size [CI] | (SMD ‐0.22; 95% CI ‐0.58 to 0.14) | (SMD ‐0.02; 95% CI ‐0.37 to 0.34) | ‐ | ‐ | (SMD ‐0.22; 95% CI ‐0.58 to 0.14) |
| 08 Becocalcidiol BD | Effect size [CI] | (SMD ‐0.67; 95% CI ‐1.04 to ‐0.30) | (SMD ‐0.46; 95% CI ‐0.83 to ‐0.10) | ‐ | ‐ | (SMD ‐0.67; 95% CI ‐1.04 to ‐0.30) |
| All treatments | Effect size [CI]; I² statistic | (SMD ‐0.95; 95% CI ‐1.17 to ‐0.74): I² statistic: 89.0% |
(SMD ‐1.04; 95% CI ‐1.33 to ‐0.74) I² statistic: 93.0% | (SMD ‐0.58; 95% CI ‐0.71 to ‐0.45): I² statistic: 42.3% |
(SMD ‐0.54; 95% CI ‐0.72 to ‐0.36): I² statistic: 55.5% |
(SMD ‐0.90; 95% CI ‐1.07 to ‐0.72); I² statistic: 87.5% |
| ‐ | No. participants | 3771 | 2647 | 2357 | 1467 | 4986 |
| ‐ | Between‐patient design | 13 | 9 | 8 | 5 | 18 |
| ‐ | Within‐patient design | 7 | 10 | 1 | 0 | 12 |
| ‐ | Treatment duration | 4 wks to 12 wks | 4 wks to 12 wks | 3 wks to 8 wks | 8 wks to 8 wks | 3 wks to 12 wks |
| Sensitivity analyses | Within‐patient trials | ‐ | ‐ | ‐ | ‐ | (SMD ‐1.11; 95% CI ‐1.58 to ‐0.64) |
| ‐ | Between‐patient trials | ‐ | ‐ | ‐ | ‐ | (SMD ‐0.80; 95% CI ‐0.96 to ‐0.63) |
| ‐ | Calcitriol, Perez 1996 removed | ‐ | ‐ | ‐ | (SMD ‐0.60; 95% CI ‐0.78 to ‐0.41) | |
| ‐ | Calcipotriol BD | ‐ | ‐ | ‐ | ‐ | (SMD ‐1.02; 95% CI ‐1.23 to ‐0.82) |
| ‐ | Calcipotriol OD | ‐ | ‐ | ‐ | ‐ | (SMD ‐0.76; 95% CI ‐1.13 to ‐0.40) |
| ‐ | correlation coefficient (rho) = 0 All trials |
‐ | ‐ | ‐ | ‐ | (SMD ‐0.85; 95% CI ‐1.00 to ‐0.71); I² statistic: 87.8% |
| ‐ | rho = 0 Btw‐patient trials rho = 0.25 Within‐patient trials |
‐ | ‐ | ‐ | ‐ | (SMD ‐0.87; 95% CI ‐1.01 to ‐0.72); I² statistic: 88.8% |
| ‐ | rho = 0 Btw‐patient trials rho = 0.50 Within‐patient trials |
‐ | ‐ | ‐ | ‐ | (SMD ‐0.88; 95% CI ‐1.03 to ‐0.73); I² statistic = 90.3% |
| ‐ | rho = 0 Btw‐patient trials rho = 0.75 Within‐patient trials |
‐ | ‐ | ‐ | ‐ | (SMD ‐0.91; 95% CI ‐1.07 to ‐0.75); I² statistic: 93.2% |
For acronyms, see Table 1.
Analysis 2: Corticosteroid (potent) versus placebo
This comparison included 10 potent corticosteroids for body psoriasis (see Analysis 2.1 and Table 6), although no effectiveness data were available for budesonide. Nine studies with 1867 participants reported IAGI data on 6 of these 10 treatments. Eight trials were between‐patient design, and one was a within‐patient study (Stein 2001). Treatment duration ranged from 3 to 12 weeks. The SMD across all 6 treatments for IAGI was ‐1.00 (95% CI ‐1.18 to ‐0.82; I² statistic = 57.6%). All six treatments performed statistically significantly better than placebo.
2.1. Analysis.
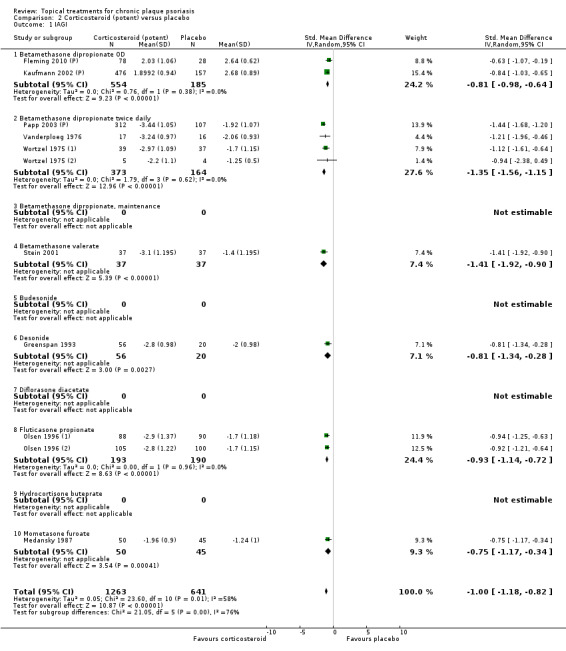
Comparison 2 Corticosteroid (potent) versus placebo, Outcome 1 IAGI.
6. Analysis 02: Trial characteristics and outcomes: potent steroids vs. placebo.
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Betamethasone dipropionate OD | Effect size [CI] | (SMD ‐0.81; 95% CI ‐0.98 to ‐0.64) | (SMD ‐0.74; 95% CI ‐1.16 to ‐0.32) | (SMD ‐0.79; 95% CI ‐1.44 to ‐0.14) | ‐ | (SMD ‐0.80; 95% CI ‐0.96 to ‐0.64) |
| 02 Betamethasone dipropionate BD | Effect size [CI] | (SMD ‐1.35; 95% CI ‐1.56 to ‐1.15) | (SMD ‐0.77; 95% CI ‐1.48 to ‐0.06) | (SMD ‐1.21; 95% CI ‐1.44 to ‐0.97) | ‐ | (SMD ‐1.35; 95% CI ‐1.56 to ‐1.15) |
| 03 Betamethasone dipropionate, maintenance | Effect size [CI] | (SMD ‐0.95; 95% CI ‐1.62 to ‐0.27) | ‐ | ‐ | (SMD ‐0.95; 95% CI ‐1.62 to ‐0.27) | |
| 04 Betamethasone valerate | Effect size [CI] | (SMD ‐1.41; 95% CI ‐1.92 to ‐0.90) | (SMD ‐1.09; 95% CI ‐2.00 to ‐0.18) | ‐ | ‐ | (SMD ‐1.33; 95% CI ‐1.78 to ‐0.89) |
| 05 Budesonide | Effect size [CI] | ‐ | ‐ | ‐ | ‐ | ‐ |
| 06 Desonide | Effect size [CI] | (SMD ‐0.81; 95% CI ‐1.34 to ‐0.28) | (SMD ‐1.16; 95% CI ‐1.70 to ‐0.61) | ‐ | ‐ | (SMD ‐0.81; 95% CI ‐1.34 to ‐0.28) |
| 07 Diflorasone diacetate | Effect size [CI] | ‐ | (SMD ‐0.32; 95% CI ‐0.73 to 0.09) | ‐ | ‐ | (SMD ‐0.32; 95% CI ‐0.73 to 0.09) |
| 08 Fluticasone propionate | Effect size [CI] | (SMD ‐0.93; 95% CI ‐1.14 to ‐0.72) | ‐ | ‐ | ‐ | (SMD ‐0.93; 95% CI ‐1.14 to ‐0.72) |
| 09 Hydrocortisone buteprate | Effect size [CI] | ‐ | (SMD ‐0.46; 95% CI ‐0.77 to ‐0.15) | ‐ | ‐ | (SMD ‐0.46; 95% CI ‐0.77 to ‐0.15) |
| 10 Mometasone furoate | Effect size [CI] | (SMD ‐0.75; 95% CI ‐1.17 to ‐0.34) | (SMD ‐1.12; 95% CI ‐1.55 to ‐0.68) | ‐ | ‐ | (SMD ‐0.75; 95% CI ‐1.17 to ‐0.34) |
| All treatments | Effect size [CI]; I² statistic | (SMD ‐1.00; 95% CI ‐1.18 to ‐0.82); I² statistic: 57.6% | (SMD ‐0.77; 95% CI ‐1.01 to ‐0.52); I² statistic: 46.7% | (SMD ‐0.97; 95% CI ‐1.31 to ‐0.62); I² statistic: 79.6% | ‐ | (SMD ‐0.89; 95% CI ‐1.06 to ‐0.72); I² statistic: 65.1% |
| ‐ | No. participants | 1867 | 553 | 1158 | 0 | 2216 |
| ‐ | Between‐patient design | 8 | 6 | 3 | 0 | 11 |
| ‐ | Within‐patient design | 1 | 1 | 0 | 0 | 2 |
| ‐ | Treatment duration | 3 wks to 12 wks | 2 wks to 12 wks | 4 wks to 8 wks | ‐ | 2 wks to 12 wks |
| Sensitivity analyses | Within‐patient trials | ‐ | ‐ | ‐ | ‐ | (SMD ‐1.33; 95% CI ‐1.78 to ‐0.89) |
| ‐ | Between‐patient trials | ‐ | ‐ | ‐ | ‐ | (SMD ‐0.85; 95% CI ‐1.03 to ‐0.67) |
| ‐ | correlation coefficient (rho) = 0 All trials |
‐ | ‐ | ‐ | ‐ | (SMD ‐0.89; 95% CI ‐1.06 to ‐0.72) I² statistic: 77.7% |
| ‐ | rho = 0 Btw‐patient trials rho = 0.25 Within‐patient trials |
‐ | ‐ | ‐ | ‐ | (SMD ‐0.89; 95% CI ‐1.06 to ‐0.72) I² statistic: 78.0% |
| ‐ | rho = 0 Btw‐patient trials rho = 0.50 Within‐patient trials |
‐ | ‐ | ‐ | ‐ | (SMD ‐0.90; 95% CI ‐1.07 to ‐0.73) I² statistic: 78.6% |
| ‐ | rho = 0 Btw‐patient trials rho = 0.75 Within‐patient trials |
‐ | ‐ | ‐ | ‐ | (SMD ‐0.91; 95% CI ‐1.08 to ‐0.74) I² statistic: 80.2% |
For acronyms, see Table 1. Both within‐patient trials compared betamethasone valerate with placebo.
Analysis 3: Corticosteroid (very potent) versus placebo
This comparison included three very potent corticosteroids for treatment of psoriasis of the body (see Analysis 3.1 and Table 7). Five studies with 515 participants reported IAGI data on 2 of the 3 treatments. There were four between‐patient trials and 1 within‐patient study (Beutner 2006). Treatment duration ranged from two to four weeks. The IAGI SMD across both treatments was ‐1.87 (95% CI ‐2.38 to ‐1.36; I² statistic = 78.7%). Both clobetasol propionate and halobetasol performed statistically significantly better than placebo. In Analysis 18.1, we present placebo‐controlled scalp trials of very potent corticosteroids.
3.1. Analysis.
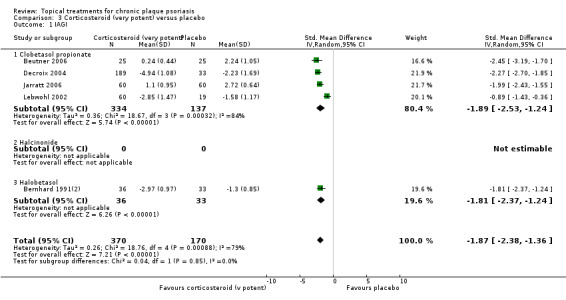
Comparison 3 Corticosteroid (very potent) versus placebo, Outcome 1 IAGI.
7. Analysis 03: Trial characteristics and outcomes: v. potent steroids vs. placebo.
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Clobetasol propionate | Effect size [CI] | (SMD ‐1.89; 95% CI ‐2.53 to ‐1.24) | (SMD ‐1.35; 95% CI ‐1.80 to ‐0.89) | ‐ | (SMD ‐1.01; 95% CI ‐1.55 to ‐0.47) | (SMD ‐1.65; 95% CI ‐2.10 to ‐1.20) |
| 02 Halcinonide | Effect size [CI] | ‐ | ‐ | ‐ | ‐ | ‐ |
| 03 Halobetasol | Effect size [CI] | (SMD ‐1.81; 95% CI ‐2.37 to ‐1.24) | ‐ | ‐ | (SMD ‐1.25; 95% CI ‐1.46 to ‐1.04) | (SMD ‐1.36; 95% CI ‐1.65 to ‐1.07) |
| All treatments | Effect size [CI], N, I² | (SMD ‐1.87; 95% CI ‐2.38 to ‐1.36); I² statistic: 78.7% | (SMD ‐1.35; 95% CI ‐1.80 to ‐0.89); I² statistic: 75.3% | ‐ | (SMD ‐1.22; 95% CI ‐1.42 to ‐1.02); I² statistic: 0% | (SMD ‐1.56; 95% CI ‐1.87 to ‐1.26); I² statistic: 81.7% |
| ‐ | No. participants | 515 | 545 | 0 | 283 | 1264 |
| ‐ | Between‐patient design | 4 | 3 | 0 | 1 | 7 |
| ‐ | Within‐patient design | 1 | 0 | 0 | 2 | 3 |
| ‐ | Treatment duration | 2 wks to 4 wks | 2 wks to 4 wks | 2 wks to 2 wks | 2 wks to 4 wks | |
| Sensitivity analyses | Within‐patient trials | ‐ | ‐ | ‐ | ‐ | (SMD ‐1.52; 95% CI ‐2.02 to ‐1.02) |
| ‐ | Between‐patient trials | ‐ | ‐ | ‐ | ‐ | (SMD ‐1.58; 95% CI ‐1.99 to ‐1.17) |
| ‐ | correlation coefficient (rho) = 0 All trials |
‐ | ‐ | ‐ | ‐ | (SMD ‐1.52; 95% CI ‐1.80 to ‐1.24) I² statistic: 81.6% |
| ‐ | rho = 0 Btw‐patient trials rho = 0.25 Within‐patient trials |
‐ | ‐ | ‐ | ‐ | (SMD ‐1.52; 95% CI ‐1.80 to ‐1.25) I² statistic: 82.2% |
| ‐ | rho = 0 Btw‐patient trials rho = 0.50 Within‐patient trials |
‐ | ‐ | ‐ | ‐ | (SMD ‐1.53; 95% CI ‐1.80 to ‐1.26) I² statistic: 83.3% |
| ‐ | rho = 0 Btw‐patient trials rho = 0.75 Within‐patient trials |
‐ | ‐ | ‐ | ‐ | (SMD ‐1.55; 95% CI ‐1.80 to ‐1.29) I² statistic: 85.9% |
For acronyms, see Table 1.
18.1. Analysis.
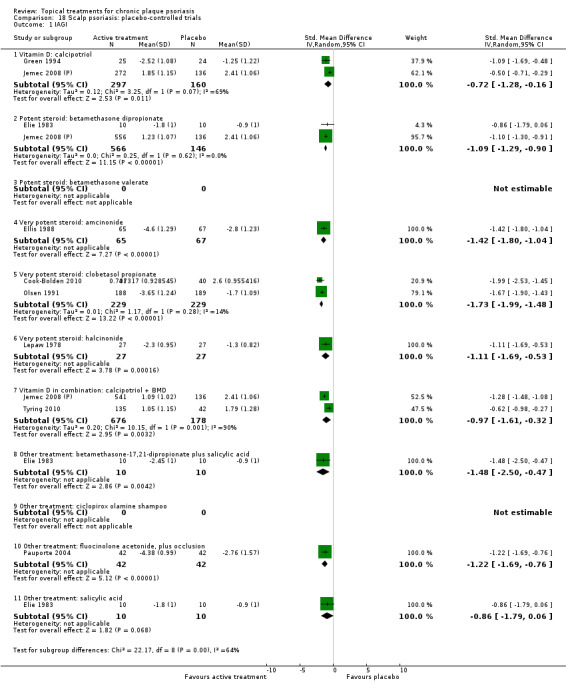
Comparison 18 Scalp psoriasis: placebo‐controlled trials, Outcome 1 IAGI.
Analysis 4: Dithranol versus placebo
Our review did not identify any study comparing dithranol against placebo and which reported IAGI data.
Analysis 5: Vitamin D combination products versus placebo
This comparison included treatment with combined calcipotriol and betamethasone dipropionate used either once or twice daily on the body (see Analysis 5.1 and Table 8). Five parallel‐group studies with 2058 participants contributed data on both dosing options. Treatment duration ranged from four to eight weeks. The IAGI SMD across treatments was ‐1.44 (95% CI ‐1.76 to ‐1.12; I² statistic = 89.4%), with twice‐daily combination treatment (SMD ‐1.90; 95% CI ‐2.09 to ‐1.71) achieving a significantly larger effect than once‐daily treatment (SMD ‐1.21; 95% CI ‐1.50 to ‐0.91). However, the difference between once‐ and twice‐daily dosing was not statistically significant when benefit was assessed using the PASI (see Analysis 5.3). In Analysis 18.1, we report placebo‐controlled trials of combination vitamin D/steroid treatments for scalp psoriasis.
5.1. Analysis.
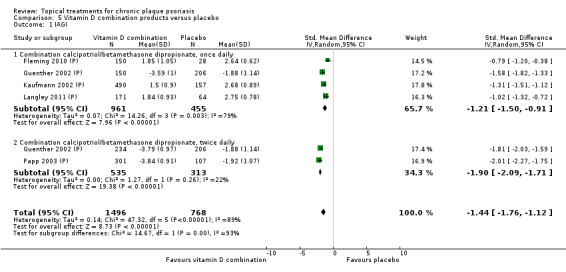
Comparison 5 Vitamin D combination products versus placebo, Outcome 1 IAGI.
8. Analysis 05: Trial characteristics and outcomes: vitamin D combination vs. placebo.
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Combination calcipotriol/betamethasone dipropionate, OD | Effect size [CI] | (SMD ‐1.21; 95% CI ‐1.50 to ‐0.91) | ‐ | (SMD ‐1.14; 95% CI ‐1.57 to ‐0.70) | (SMD ‐0.69; 95% CI ‐0.98 to ‐0.40) | (SMD ‐1.21; 95% CI ‐1.50 to ‐0.91) |
| 02 Combination calcipotriol/betamethasone dipropionate, BD | Effect size [CI] | (SMD ‐1.90; 95% CI ‐2.09 to ‐1.71) | ‐ | (SMD ‐1.41; 95% CI ‐1.86 to ‐0.97) | ‐ | (SMD ‐1.90; 95% CI ‐2.09 to ‐1.71) |
| All treatments | Effect size [CI], N, I² statistic | (SMD ‐1.44; 95% CI ‐1.76 to ‐1.12); I² statistic: 89.4% | ‐ | (SMD ‐1.24; 95% CI ‐1.53 to ‐0.95); I² statistic: 87.6% | (SMD ‐0.69; 95% CI ‐0.98 to ‐0.40); I² statistic: NA | (SMD ‐1.44; 95% CI ‐1.76 to ‐1.12); I² statistic: 89.4% |
| ‐ | No. participants | 2058 | 0 | 2056 | 235 | 2058 |
| ‐ | Between‐patient design | 5 | 0 | 5 | 1 | 5 |
| ‐ | Within‐patient design | 0 | 0 | 0 | 0 | 0 |
| ‐ | Treatment duration | 4 wks to 8 wks | ‐ | 4 wks to 8 wks | 8 wks | 4 wks to 8 wks |
For acronyms, see Table 1.
5.3. Analysis.
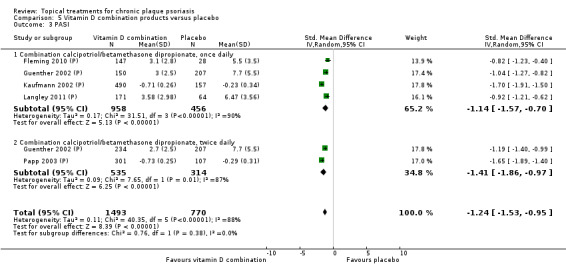
Comparison 5 Vitamin D combination products versus placebo, Outcome 3 PASI.
Analysis 6: Other treatment versus placebo
This comparison comprised all other treatments for psoriasis of the body not included in the first five analyses; therefore, we removed pooling. None of the studies assessed the same treatment, which means that findings should be interpreted with caution.
In total, we included 26 treatments in this analysis (see Analysis 6.1 and Table 9). Eight studies with 364 participants reported IAGI data on 8 of these 26 treatments. Four trials were between‐patient design, and four were within‐patient studies. Treatment duration ranged from 3 to 12 weeks.
6.1. Analysis.
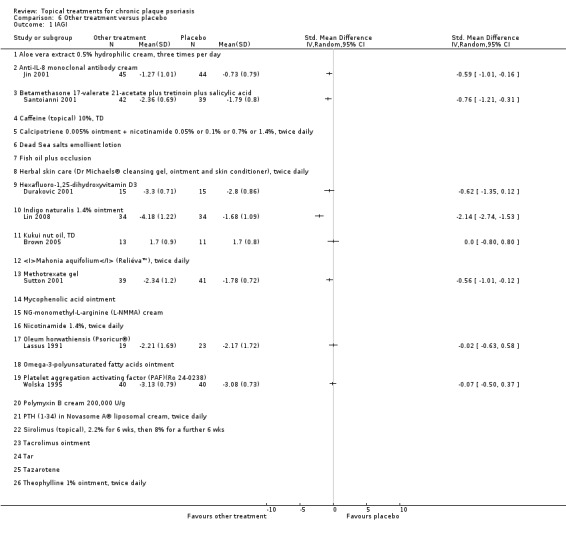
Comparison 6 Other treatment versus placebo, Outcome 1 IAGI.
9. Analysis 06: Trial characteristics and outcomes: other treatments vs. placebo.
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Aloe vera extract | Effect size [CI] | ‐ | ‐ | (SMD ‐1.58; 95% CI ‐2.16 to ‐0.99) | ‐ | (SMD ‐1.58; 95% CI ‐2.16 to ‐0.99) |
| 02 Anti‐IL‐8 monoclonal antibody cream | Effect size [CI] | (SMD ‐0.59; 95% CI ‐1.01 to ‐0.16) | (SMD ‐0.70; 95% CI ‐1.13 to ‐0.27) | ‐ | ‐ | (SMD ‐0.59; 95% CI ‐1.01 to ‐0.16) |
| 03 Betamethasone 17‐valerate 21‐acetate plus tretinoine plus salicylic acid | Effect size [CI] | (SMD ‐0.76; 95% CI ‐1.21 to ‐0.31) | ‐ | (SMD ‐0.54; 95% CI ‐0.99 to ‐0.10) | (SMD ‐0.80; 95% CI ‐1.26 to ‐0.35) | (SMD ‐0.76; 95% CI ‐1.21 to ‐0.31) |
| 04 Caffeine (topical) 10%, TD | Effect size [CI] | ‐ | ‐ | (SMD ‐0.39; 95% CI ‐0.84 to 0.06) | ‐ | (SMD ‐0.39; 95% CI ‐0.84 to 0.06) |
| 05 Calcipotriene 0.005% ointment + nicotinamide 0.05% or 0.1% or 0.7% or 1.4%, BD | Effect size [CI] | ‐ | (SMD ‐0.48; 95% CI ‐0.81 to ‐0.15) | ‐ | ‐ | (SMD ‐0.48; 95% CI ‐0.81 to ‐0.15) |
| 06 Dead sea salts emollient lotion | Effect size [CI] | ‐ | ‐ | (SMD 0.57; 95% CI ‐0.36 to 1.51) | ‐ | (SMD 0.57; 95% CI ‐0.36 to 1.51) |
| 07 Fish oil plus occlusion | Effect size [CI] | ‐ | (SMD ‐1.05; 95% CI ‐1.64 to ‐0.46) | ‐ | ‐ | (SMD ‐1.05; 95% CI ‐1.64 to ‐0.46) |
| 08 Herbal skin care (Dr Michaels® cleansing gel, ointment and skin conditioner), BD | Effect size [CI] | (SMD ‐2.96; 95% CI ‐4.19 to ‐1.74) | ‐ | (SMD ‐2.96; 95% CI ‐4.19 to ‐1.74) | ||
| 09 Hexafluoro‐1,25‐dihydroxyvitamin D3 | Effect size [CI] | (SMD ‐0.62; 95% CI ‐1.35 to 0.12) | (SMD ‐1.13; 95% CI ‐1.91 to ‐0.35) | ‐ | ‐ | (SMD ‐0.62; 95% CI ‐1.35 to 0.12) |
| 10 Indigo naturalis 1.4% ointment | Effect size [CI] | (SMD ‐2.14; 95% CI ‐2.74 to ‐1.53) | (SMD ‐1.64; 95% CI ‐2.13 to ‐1.15) | ‐ | ‐ | (SMD ‐2.09; 95% CI ‐2.62 to ‐1.56) |
| 11 Kukui nut oil, TD | Effect size [CI] | (SMD 0.00; 95% CI ‐0.80 to 0.80) | (SMD 0.33; 95% CI ‐0.48 to 1.14) | (SMD ‐0.03; 95% CI ‐0.84 to 0.77) | (SMD 0.00; 95% CI ‐0.80 to 0.80) | (SMD 0.00; 95% CI ‐0.80 to 0.80) |
| 12 Mahonia aquifolium (Reliéva™), BD | Effect size [CI] | ‐ | ‐ | ‐ | ‐ | (SMD ‐0.77; 95% CI ‐1.06 to ‐0.48) |
| 13 Methotrexate gel | Effect size [CI] | (SMD ‐0.56; 95% CI ‐1.01 to ‐0.12) | (SMD ‐0.48; 95% CI ‐0.92 to ‐0.04) | (SMD ‐1.58; 95% CI ‐2.16 to ‐0.99) | ‐ | (SMD ‐1.05; 95% CI ‐2.04 to ‐0.06) |
| 14 Mycophenolic acid ointment | Effect size [CI] | ‐ | (SMD ‐1.44; 95% CI ‐2.67 to ‐0.22) | ‐ | ‐ | (SMD ‐1.44; 95% CI ‐2.67 to ‐0.22) |
| 15 NG‐monomethyl‐L‐arginine (L‐NMMA) cream | Effect size [CI] | ‐ | (SMD 0.08; 95% CI ‐0.60 to 0.75) | ‐ | ‐ | (SMD 0.08; 95% CI ‐0.60 to 0.75) |
| 16 Nicotinamide 1.4%, BD | Effect size [CI] | ‐ | (SMD ‐0.20; 95% CI ‐0.60 to 0.20) | ‐ | ‐ | (SMD ‐0.20; 95% CI ‐0.60 to 0.20) |
| 17 Oleum horwathiensis | Effect size [CI] | (SMD ‐0.02; 95% CI ‐0.63 to 0.58) | (SMD ‐0.77; 95% CI ‐1.40 to ‐0.14) | ‐ | ‐ | (SMD ‐0.02; 95% CI ‐0.63 to 0.58) |
| 18 Omega‐3‐polyunsaturated fatty acids ointment | Effect size [CI] | ‐ | ‐ | ‐ | ‐ | ‐ |
| 19 Platelet aggregation activating factor (PAF) (Ro 24‐0238) | Effect size [CI] | (SMD ‐0.07; 95% CI ‐0.50 to 0.37) | ‐ | ‐ | (SMD ‐0.07; 95% CI ‐0.50 to 0.37) | |
| 20 Polymyxin B cream, 200,000 U/g | Effect size [CI] | ‐ | (SMD 0.13; 95% CI ‐0.59 to 0.85) | ‐ | ‐ | (SMD 0.13; 95% CI ‐0.59 to 0.85) |
| 21 PTH (1‐34) in Novasome A® liposomal cream, BD | Effect size [CI] | ‐ | (SMD ‐2.31; 95% CI ‐3.26 to ‐1.36) | ‐ | ‐ | (SMD ‐2.31; 95% CI ‐3.26 to ‐1.36) |
| 22 Sirolimus (topical), 2.2% for 6 wks, then 8% for a further 6 wks | Effect size [CI] | ‐ | (SMD ‐0.39; 95% CI ‐0.98 to 0.21) | ‐ | ‐ | (SMD ‐0.39; 95% CI ‐0.98 to 0.21) |
| 23 Tacrolimus ointment | Effect size [CI] | ‐ | (SMD 0.06; 95% CI ‐0.52 to 0.63) | ‐ | ‐ | (SMD 0.06; 95% CI ‐0.52 to 0.63) |
| 24 Tar | Effect size [CI] | ‐ | (SMD ‐0.45; 95% CI ‐1.11 to 0.22) | ‐ | ‐ | (SMD ‐0.45; 95% CI ‐1.11 to 0.22) |
| 25 Tazarotene | Effect size [CI] | ‐ | (SMD ‐0.86; 95% CI ‐1.11 to ‐0.62) | ‐ | ‐ | (SMD ‐0.86; 95% CI ‐1.11 to ‐0.62) |
| 26 Theophylline 1% ointment, BD | Effect size [CI] | ‐ | ‐ | (SMD ‐2.87; 95% CI ‐4.13 to ‐1.62) | ‐ | (SMD ‐2.87; 95% CI ‐4.13 to ‐1.62) |
| All treatments | (not pooled) | ‐ | ‐ | ‐ | ‐ | ‐ |
| ‐ | No. participants | 364 | 907 | 529 | 105 | 1450 |
| ‐ | Between‐patient design | 4 | 5 | 8 | 2 | 12 |
| ‐ | Within‐patient design | 4 | 12 | 1 | 0 | 14 |
| ‐ | Treatment duration | 3 wks to 12 wks | 3 wks to 12 wks | 2 wks to 12 wks | 3 wks to 12 wks | 2 wks to 12 wks |
For acronyms, see Table 1.
Four treatments performed statistically significantly better than placebo: anti‐IL‐8 monoclonal antibody cream; betamethasone 17‐valerate 21‐acetate plus tretinoin plus salicylic acid, indigo naturalise 1.4% ointment, and methotrexate gel. The effect size for the IAGI ranged from ‐0.56 (Sutton 2001; methotrexate gel) to ‐2.14 (Lin 2008; indigo naturalise 1.4% ointment).
In four treatments, the difference relative to placebo was not statistically significant: hexafluoro‐1,25‐dihydroxyvitamin D3, kukui nut oil, oleum horwathiensis, and platelet aggregation activating factor (PAF). No treatment was statistically significantly less effective than placebo.
Analysis 7: Vitamin D analogues versus corticosteroid (potent)
This comparison included eight vitamin D analogue‐potent corticosteroid contrasts for body psoriasis (see Table 10). Eight studies with 2655 participants reported IAGI data for 6 of the 8 intervention‐comparator contrasts (see Analysis 7.1). Seven trials were between‐patient design, and one was a within‐patient study (Medansky 1996). Treatment duration ranged from three to eight weeks. Overall, there was no statistically significant difference between vitamin D analogues and potent corticosteroids: The SMD across all 6 treatments for IAGI was 0.17 (95% CI ‐0.04 to 0.37; I² statistic = 83.4%). In light of the high level of heterogeneity and inconsistency across treatments (Higgins 2011). We removed pooling. Vitamin D analogues performed statistically significantly better than one potent corticosteroid. This finding came from a single between‐patient study in which 99 participants contributed data (Bruce 1994). The SMD for calcipotriol against fluocinonide 0.05% ointment was ‐0.58 (95% CI ‐0.99 to ‐0.18; I² statistic = NA). Calcipotriol was statistically significantly less effective than both diflorasone diacetate 0.05% ointment (SMD 0.27; 95% CI 0.02 to 0.52) and betamethasone dipropionate (SMD 0.43; 95% CI 0.28 to 0.58; I² statistic = 50.3%). We found no statistically significant difference between calcipotriol and betamethasone valerate, calcitriol and betamethasone dipropionate, or calcitriol and betamethasone valerate.
10. Analysis 07: Trial characteristics and outcomes: vitamin D vs. potent steroids.
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Calcipotriol vs. betamethasone dipropionate | Effect size [CI] | (SMD 0.43; 95% CI 0.28 to 0.58) | ‐ | (SMD 0.36; 95% CI 0.22 to 0.51) | ‐ | (SMD 0.43; 95% CI 0.28 to 0.58) |
| 02 Calcipotriol vs. betamethasone valerate | Effect size [CI] | (SMD ‐0.02; 95% CI ‐0.21 to 0.17) | (SMD ‐0.26; 95% CI ‐0.41 to ‐0.11) | (SMD ‐0.12; 95% CI ‐0.22 to ‐0.02) | (SMD ‐0.26; 95% CI ‐0.38 to ‐0.14) | (SMD ‐0.12; 95% CI ‐0.26 to 0.02) |
| 03 Calcipotriol vs. desoxymetasone | Effect size [CI] | ‐ | ‐ | (SMD 0.15; 95% CI ‐0.73 to 1.02) | ‐ | (SMD 0.15; 95% CI ‐0.73 to 1.02) |
| 04 Calcipotriol vs. diflorasone diacetate | Effect size [CI] | (SMD 0.27; 95% CI 0.02 to 0.52) | (SMD 0.40; 95% CI 0.15 to 0.65) | ‐ | ‐ | (SMD 0.27; 95% CI 0.02 to 0.52) |
| 05 Calcipotriol vs. fluocinonide | Effect size [CI] | (SMD ‐0.58; 95% CI ‐0.99 to ‐0.18) | (SMD ‐0.50; 95% CI ‐0.92 to ‐0.07) | ‐ | ‐ | (SMD ‐0.58; 95% CI ‐0.99 to ‐0.18) |
| 06 Calcitriol vs. betamethasone dipropionate | Effect size [CI] | (SMD 0.21; 95% CI ‐0.04 to 0.45) | (SMD 0.27; 95% CI 0.02 to 0.51) | (SMD 0.39; 95% CI 0.14 to 0.63) | ‐ | (SMD 0.21; 95% CI ‐0.04 to 0.45) |
| 07 Calcitriol vs. betamethasone valerate | Effect size [CI] | (SMD ‐0.19; 95% CI ‐0.91 to 0.53) | ‐ | ‐ | ‐ | (SMD ‐0.19; 95% CI ‐0.91 to 0.53) |
| 08 Tacalcitol vs. betamethasone valerate | Effect size [CI] | ‐ | (SMD 0.41; 95% CI 0.09 to 0.74) | ‐ | ‐ | (SMD 0.41; 95% CI 0.09 to 0.74) |
| All treatments | Effect size [CI]; I² statistic | (SMD 0.17; 95% CI ‐0.04 to 0.37); I² statistic: 83.4% | (SMD 0.11; 95% CI ‐0.22 to 0.44); I² statistic: 86.7% | (SMD 0.12; 95% CI ‐0.07 to 0.32); I² statistic: 86.2% | (SMD ‐0.26; 95% CI ‐0.38 to ‐0.14); I² statistic: 0% | (SMD 0.11; 95% CI ‐0.07 to 0.30); I² statistic: 85.6% |
| ‐ | No. participants | 2655 | 891 | 3185 | 738 | 3542 |
| ‐ | Between‐patient design | 7 | 2 | 7 | 1 | 9 |
| ‐ | Within‐patient design | 1 | 4 | 2 | 1 | 5 |
| ‐ | Treatment duration | 3 wks to 8 wks | 3 wks to 6 wks | 4 wks to 8 wks | 6 wks | 3 wks to 8 wks |
| Sensitivity analyses | Within‐patient trials | ‐ | ‐ | ‐ | ‐ | (SMD 0.17; 95% CI ‐0.20 to 0.54) |
| ‐ | Between‐patient trials | ‐ | ‐ | ‐ | ‐ | (SMD 0.10; 95% CI ‐0.11 to 0.31) |
| ‐ | correlation coefficient (rho) = 0 All trials |
‐ | ‐ | ‐ | ‐ | (SMD 0.10; 95% CI ‐0.08 to 0.28); I² statistic: 90.5% |
| ‐ | rho = 0 Btw‐patient trials rho = 0.25 Within‐patient trials |
‐ | ‐ | ‐ | ‐ | (SMD 0.10; 95% CI ‐0.07 to 0.28) I² statistic: 91.3% |
| ‐ | rho = 0 Btw‐patient trials rho = 0.50 Within‐patient trials |
‐ | ‐ | ‐ | ‐ | (SMD 0.11; 95% CI ‐0.07 to 0.29) I² statistic: 92.4% |
| ‐ | rho = 0 Btw‐patient trials rho = 0.75 Within‐patient trials |
‐ | ‐ | ‐ | ‐ | (SMD 0.12; 95% CI ‐0.06 to 0.30) I² statistic: 94.3% |
For acronyms, see Table 1.
7.1. Analysis.
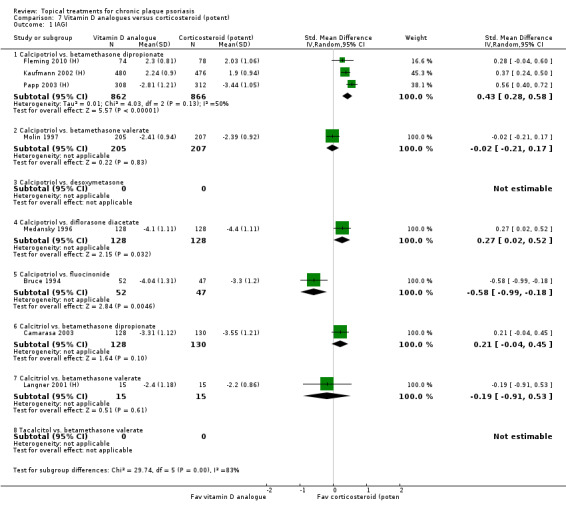
Comparison 7 Vitamin D analogues versus corticosteroid (potent), Outcome 1 IAGI.
Analysis 8: Vitamin D analogues versus corticosteroid (very potent)
This comparison included one vitamin D analogue, calcipotriol ointment, versus a very potent corticosteroid contrast, clobetasol propionate foam, for psoriasis of the body (see Table 11 and Analysis 8.1). One study with 42 participants reported IAGI data. Koo 2006 was a between‐patient study with a treatment duration of two weeks, which found no significant difference between the treatments (SMD 0.19; 95% CI ‐0.42 to 0.80).
11. Analysis 08: Trial characteristics and outcomes: vitamin D vs. v. potent steroids.
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Calcipotriol vs. clobetasol propionate | Effect size [CI] | (SMD 0.19; 95% CI ‐0.42 to 0.80) | ‐ | (SMD ‐0.32; 95% CI ‐0.95 to 0.30) | (SMD 0.42; 95% CI ‐0.20 to 1.03) | (SMD ‐0.06; 95% CI ‐0.57 to 0.44); I² statistic: 25.7% |
| All treatments | No. participants | 42 | 0 | 40 | 42 | 82 |
| ‐ | Between‐patient design | 1 | 0 | 1 | 1 | 2 |
| ‐ | Within‐patient design | 0 | 0 | 0 | 0 | 0 |
| ‐ | Treatment duration | 2 wks | ‐ | 6 wks | 2 wks | 2 wks to 6 wks |
8.1. Analysis.

Comparison 8 Vitamin D analogues versus corticosteroid (very potent), Outcome 1 IAGI.
Analysis 9: Vitamin D combined with corticosteroid versus corticosteroid
This comparison considered vitamin D analogues‐steroid combination against potent or very potent corticosteroids for body psoriasis (see Analysis 9.1 and Table 12). The comparison included three contrasts: calcipotriol plus betamethasone dipropionate versus betamethasone dipropionate, calcipotriol plus betamethasone dipropionate versus clobetasol propionate, and calcipotriol plus clobetasol propionate versus clobetasol propionate.
9.1. Analysis.
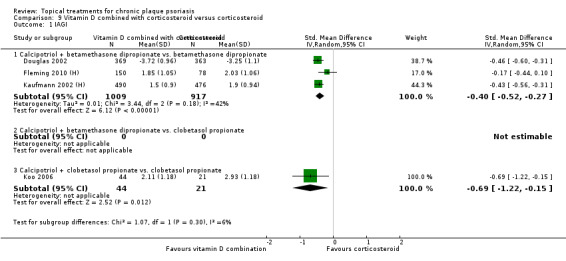
Comparison 9 Vitamin D combined with corticosteroid versus corticosteroid, Outcome 1 IAGI.
12. Analysis 09: Trial characteristics and outcomes: vitamin D + steroid vs. steroid.
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Calcipotriol + betamethasone dipropionate vs. betamethasone dipropionate | Effect size [CI] | (SMD ‐0.40; 95% CI ‐0.52 to ‐0.27) | ‐ | (SMD ‐0.44; 95% CI ‐0.55 to ‐0.33) | ‐ | (SMD ‐0.40; 95% CI ‐0.52 to ‐0.27) |
| 02 Calcipotriol + betamethasone dipropionate vs. clobetasol propionate | Effect size [CI] | ‐ | (SMD 0.45; 95% CI 0.09 to 0.81) | ‐ | ‐ | (SMD 0.45; 95% CI 0.09 to 0.81) |
| 03 Calcipotriol + clobetasol propionate vs. clobetasol propionate | Effect size [CI] | (SMD ‐0.69; 95% CI ‐1.22 to ‐0.15) | ‐ | ‐ | (SMD ‐0.28; 95% CI ‐0.80 to 0.24) | (SMD ‐0.69; 95% CI ‐1.22 to ‐0.15) |
| ‐ | Effect size [CI], I² | (SMD ‐0.41; 95% CI ‐0.53 to ‐0.29); I² statistic: 32.0% | (SMD 0.45; 95% CI 0.09 to 0.81): I² statistic: NA | (SMD ‐0.44; 95% CI ‐0.55 to ‐0.33) I² statistic: 22.4% | (SMD ‐0.28; 95% CI ‐0.80 to 0.24) I² statistic: NA | (SMD ‐0.26; 95% CI ‐0.52 to ‐0.00); I² statistic: 84.4% |
| ‐ | No. participants | 1991 | 122 | 1876 | 65 | 2113 |
| ‐ | Between‐patient design | 4 | 1 | 3 | 1 | 5 |
| ‐ | Within‐patient design | 0 | 0 | 0 | 0 | 0 |
| ‐ | Treatment duration | 2 wks to 8 wks | 4 wks | 4 wks to 8 wks | 2 wks | 2 wks to 8 wks |
Four between‐patient trials reported IAGI data for 1991 participants on 2 of the 3 intervention‐comparator contrasts. Treatment duration ranged between two and eight weeks. As these treatment comparisons were very different, we only pooled subtotals. In all but one trial (Fleming 2010 (H)), combination treatment was significantly more effective than corticosteroid alone. Three of the four trials compared calcipotriol/betamethasone dipropionate against betamethasone dipropionate (SMD ‐0.40; 95% CI ‐0.52 to ‐0.27; I² statistic = 41.8%), and one trial compared combined treatment with calcipotriol and clobetasol against clobetasol alone (SMD ‐0.69; 95% CI ‐1.22 to ‐0.15).
Analysis 10: Vitamin D alone or in combination versus dithranol
This comparison considered vitamin D analogues against dithranol (Analysis 10.1 and Table 13). We identified three intervention‐comparator contrasts: calcipotriol versus dithranol, calcitriol versus dithranol, and tacalcitol versus dithranol. Five between‐patient trials reported IAGI data for 1108 participants on 2 of these 3 intervention‐comparator contrasts. Treatment duration ranged from 8 weeks to 12 weeks. There was some variation in the dithranol regimens employed by trials and in the baseline severity of trial participants. These factors may help explain the high level of heterogeneity found in the pooled results.
10.1. Analysis.
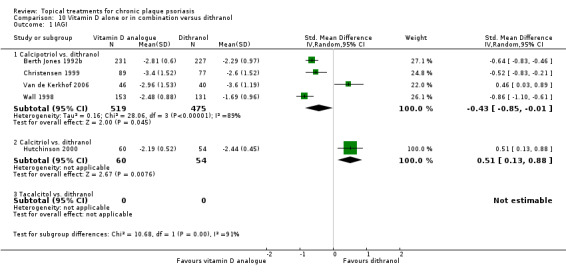
Comparison 10 Vitamin D alone or in combination versus dithranol, Outcome 1 IAGI.
13. Analysis 10: Trial characteristics and outcomes: vitamin D vs. dithranol.
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Calcipotriol vs. dithranol | Effect size [CI] | (SMD ‐0.43; 95% CI ‐0.85 to ‐0.01) | (SMD ‐0.54; 95% CI ‐1.16 to 0.08) | (SMD 0.73; 95% CI ‐0.55 to 2.00) | (SMD ‐0.05; 95% CI ‐0.90 to 0.80) | (SMD 0.07; 95% CI ‐0.57 to 0.71) |
| 02 Calcitriol vs. dithranol | Effect size [CI] | (SMD 0.51; 95% CI 0.13 to 0.88) | (SMD 0.13; 95% CI ‐0.24 to 0.50) | (SMD ‐0.21; 95% CI ‐0.58 to 0.16) | ‐ | (SMD 0.51; 95% CI 0.13 to 0.88) |
| 03 Tacalcitol vs. dithranol | Effect size [CI] | ‐ | (SMD ‐0.18; 95% CI ‐0.60 to 0.25) | (SMD ‐0.07; 95% CI ‐0.50 to 0.36) | ‐ | (SMD ‐0.18; 95% CI ‐0.60 to 0.25) |
| All treatments | Effect size [CI], I² statistic | (SMD ‐0.24; 95% CI ‐0.72 to 0.25); I² statistic:93.0% | (SMD ‐0.27; 95% CI ‐0.73 to 0.20); I² statistic: 80.6% | (SMD 0.36; 95% CI ‐0.33 to 1.04); I² statistic: 94.5% | (SMD ‐0.05; 95% CI ‐0.90 to 0.80); I² statistic: 92.5% | (SMD 0.09; 95% CI ‐0.44 to 0.63); I² statistic: 94.9% |
| ‐ | No. participants | 1108 | 386 | 796 | 544 | 1284 |
| ‐ | Between‐patient design | 5 | 3 | 5 | 2 | 7 |
| ‐ | Within‐patient design | 0 | 1 | 0 | 0 | 1 |
| ‐ | Treatment duration | 8 wks to 12 wks | 4 wks to 8 wks | 8 wks to 12 wks | 8 wks to 12 wks | 4 wks to 12 wks |
The SMD for the IAGI was ‐0.24 (95% CI ‐0.72 to 0.25; I² statistic = 93.0%). The presence of considerable heterogeneity means that the estimated average benefit should be treated with caution and pooling was therefore removed (Higgins 2011). Data from four trials contributed to the SMD for the calcipotriol versus dithranol: ‐0.43 (95% CI ‐0.85 to ‐0.01; I² statistic = 89.3%), indicating that calcipotriol was statistically significantly more effective than dithranol. Three of these four trials found a significant difference in favour of calcipotriol (Berth Jones 1992b; Christensen 1999; Wall 1998), but the trial by Van de Kerkhof 2006 found outpatient treatment with short contact dithranol to be significantly more effective than calcipotriol alone.
Data from one trial contributed to the SMD for the calcitriol versus dithranol: 0.51 (95% CI 0.13 to 0.88; I² statistic = NA), indicating that dithranol was statistically significantly more effective than calcitriol.
Analysis 11: Vitamin D alone or in combination versus other vitamin D analogue
Our review identified three intervention‐comparator contrasts in this comparison: calcipotriol versus calcitriol, calcipotriol versus tacalcitol, and calcipotriol versus maxacalcitol for body psoriasis (see Analysis 11.1 and Table 14). Three trials involving 498 participants contributed IAGI data for all 3 intervention‐comparator contrasts (one trial for each contrast). Two trials were between‐patient, and one was within‐patient in design (Barker 1999 (H)). Treatment duration ranged from 8 to 12 weeks. The SMD for the IAGI was ‐0.06 (95% CI ‐0.51 to 0.38; I² statistic = 82.2%). The presence of substantial heterogeneity reflects differences in the findings from the two intervention‐comparator contrasts underlying this statistic. We found a statistically significant difference in favour of calcipotriol in the analysis against tacalcitol (SMD ‐0.47; 95% CI ‐0.73 to ‐0.21), but there was no significant difference relative to calcitriol (SMD 0.00; 95% CI ‐0.25 to 0.25) or between calcipotriol and maxacalcitol (SMD 0.43; 95% CI ‐0.12 to 0.98). In light of the inconsistency of results across treatments, we only pooled subtotals (Higgins 2011).
11.1. Analysis.
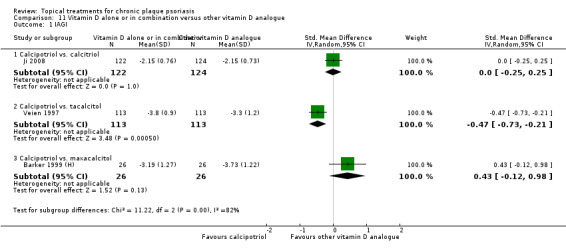
Comparison 11 Vitamin D alone or in combination versus other vitamin D analogue, Outcome 1 IAGI.
14. Analysis 11: Trial characteristics and outcomes: vitamin D vs. vitamin D.
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Calcipotriol vs. calcitriol | Effect size [CI] | (SMD 0.00; 95% CI ‐0.25 to 0.25) | (SMD ‐0.32; 95% CI ‐0.57 to ‐0.07) | (SMD ‐1.11; 95% CI ‐2.22 to 0.01) | (SMD 0.04; 95% CI ‐0.21 to 0.29) | (SMD ‐0.41; 95% CI ‐1.46 to 0.64) |
| 02 Calcipotriol vs. tacalcitol | Effect size [CI] | (SMD ‐0.47; 95% CI ‐0.73 to ‐0.21) | (SMD ‐0.45; 95% CI ‐0.68 to ‐0.22) | ‐ | ‐ | (SMD ‐0.47; 95% CI ‐0.73 to ‐0.21) |
| 03 Calcipotriol vs. maxacalcitol | Effect size [CI] | (SMD 0.43; 95% CI ‐0.12 to 0.98) | (SMD 0.13; 95% CI ‐0.41 to 0.68) | ‐ | ‐ | (SMD 0.43; 95% CI ‐0.12 to 0.98) |
| All treatments | Effect size [CI], I² | (SMD ‐0.06; 95% CI ‐0.51 to 0.38); I² statistic: 82.2% | (SMD ‐0.31; 95% CI ‐0.55 to ‐0.06); I² statistic: 46.9% | (SMD ‐1.11; 95% CI ‐2.22 to 0.01); I² statistic: NA | (SMD 0.04; 95% CI ‐0.21 to 0.29); I² statistic: NA | (SMD ‐0.17; 95% CI ‐0.62 to 0.27); I² statistic: 78.5% |
| ‐ | No. participants | 498 | 563 | 15 | 250 | 513 |
| ‐ | Between‐patient design | 2 | 2 | 1 | 1 | 3 |
| ‐ | Within‐patient design | 1 | 1 | 0 | 0 | 1 |
| ‐ | Treatment duration | 8 wks to 12 wks | 8 wks to 12 wks | 8 wks | 12 wks | 8 wks to 12 wks |
| Sensitivity analyses | TSS data from Ji 2008 used in combined end point: 01 Calcipotriol vs. calcitriol |
‐ | ‐ | ‐ | (SMD ‐0.52; 95% CI ‐1.19 to 0.15; I² statistic: 44.9%) | |
| ‐ | TSS data from Ji 2008 used in combined end point: all treatments |
‐ | ‐ | ‐ | (SMD ‐0.28; 95% CI ‐0.66 to 0.10; I² statistic: 70.6%) |
Analysis 12: Vitamin D alone or in combination versus vitamin D + corticosteroid
Our review identified 12 intervention‐comparator contrasts for body psoriasis, involving 3 vitamin D analogues, 2 combination products, and 7 different corticosteroids (see Analysis 12.1 and Table 15 ). Eleven parallel‐group trials involving 4791 participants contributed IAGI data for 9 of these 12 intervention‐comparator contrasts. Treatment duration ranged from two to eight weeks. There were no IAGI data for three contrasts: calcipotriol versus calcipotriol plus diflucortolone valerate, calcipotriol versus calcipotriol plus fluocinonide acetonide, and calcitriol versus calcitriol plus diflucortolone valerate.
12.1. Analysis.
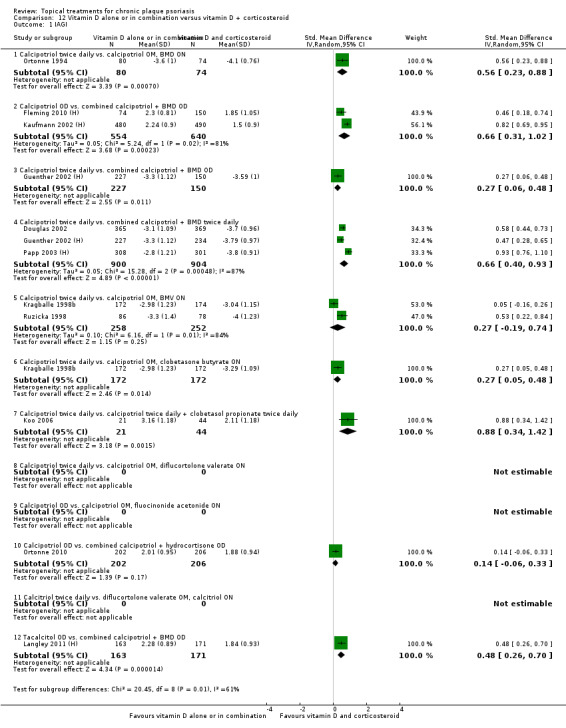
Comparison 12 Vitamin D alone or in combination versus vitamin D + corticosteroid, Outcome 1 IAGI.
15. Analysis 12: Trial characteristics and outcomes: vitamin D vs. vitamin D + steroid.
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Calcipotriol BD vs. calcipotriol OM, BMD ON | Effect size [CI] | (SMD 0.56; 95% CI 0.23 to 0.88) | ‐ | (SMD 0.46; 95% CI 0.10 to 0.82) | ‐ | (SMD 0.56; 95% CI 0.23 to 0.88) |
| 02 Calcipotriol OD vs. combined calcipotriol + BMD OD | Effect size [CI] | (SMD 0.66; 95% CI 0.31 to 1.02) | ‐ | (SMD 0.67; 95% CI 0.23 to 1.11) | ‐ | (SMD 0.66; 95% CI 0.31 to 1.02) |
| 03 Calcipotriol BD vs. combined calcipotriol + BMD OD | Effect size [CI] | (SMD 0.27; 95% CI 0.06 to 0.48) | (SMD 0.25; 95% CI 0.03 to 0.48) | (SMD 0.52; 95% CI 0.38 to 0.67) | ‐ | (SMD 0.43; 95% CI 0.20 to 0.66) |
| 04 Calcipotriol BD vs. combined calcipotriol + BMD BD | Effect size [CI] | (SMD 0.66; 95% CI 0.40 to 0.93) | ‐ | (SMD 0.64; 95% CI 0.46 to 0.83) | ‐ | (SMD 0.66; 95% CI 0.40 to 0.93) |
| 05 Calcipotriol BD vs. calcipotriol OM, BMV ON | Effect size [CI] | (SMD 0.27; 95% CI ‐0.19 to 0.74) | ‐ | (SMD 0.43; 95% CI ‐0.07 to 0.93) | ‐ | (SMD 0.27; 95% CI ‐0.19 to 0.74) |
| 06 Calcipotriol BD vs. calcipotriol OM, clobetasone butyrate ON | Effect size [CI] | (SMD 0.27; 95% CI 0.05 to 0.48) | ‐ | (SMD 0.17; 95% CI ‐0.04 to 0.38) | ‐ | (SMD 0.27; 95% CI 0.05 to 0.48) |
| 07 Calcipotriol BD vs. calcipotriol BD + clobetasol propionate BD | Effect size [CI] | (SMD 0.88; 95% CI 0.34 to 1.42) | ‐ | ‐ | (SMD 0.70; 95% CI 0.16 to 1.23) | (SMD 0.88; 95% CI 0.34 to 1.42) |
| 08 Calcipotriol BD vs. calcipotriol OM, diflucortolone valerate ON | Effect size [CI] | ‐ | ‐ | (SMD 0.08; 95% CI ‐0.29 to 0.44) | ‐ | (SMD 0.08; 95% CI ‐0.29 to 0.44) |
| 09 Calcipotriol OD vs. calcipotriol OM, fluocinonide acetonide ON | Effect size [CI] | ‐ | ‐ | (SMD 0.53; 95% CI ‐0.11 to 1.18) | ‐ | (SMD 0.53; 95% CI ‐0.11 to 1.18) |
| 10 Calcipotriol OD vs. combined calcipotriol + hydrocortisone OD | Effect size [CI] | (SMD 0.14; 95% CI ‐0.06 to 0.33) | ‐ | (SMD 0.08; 95% CI ‐0.11 to 0.28) | ‐ | (SMD 0.14; 95% CI ‐0.06 to 0.33) |
| 11 calcitriol BD vs. diflucortolone valerate OM, calcitriol ON | Effect size [CI] | ‐ | ‐ | (SMD 0.24; 95% CI ‐0.09 to 0.57) | ‐ | (SMD 0.24; 95% CI ‐0.09 to 0.57) |
| 12 Tacalcitol OD vs. combined calcipotriol + BMD OD | Effect size [CI] | (SMD 0.48; 95% CI 0.26 to 0.70) | ‐ | (SMD 0.47; 95% CI 0.25 to 0.69) | (SMD 0.46; 95% CI 0.24 to 0.68) | (SMD 0.48; 95% CI 0.26 to 0.70) |
| All treatments | Effect size [CI], I² statistic | (SMD 0.48; 95% CI 0.32 to 0.65), I² statistic: 86.9% | (SMD 0.25; 95% CI 0.03 to 0.48) | (SMD 0.47; 95% CI 0.34 to 0.59), I² statistic: 82.3% | (SMD 0.49; 95% CI 0.29 to 0.69), I² statistic: 0% | (SMD 0.46; 95% CI 0.33 to 0.59), I² statistic: 83.3% |
| ‐ | No. participants | 4791 | 301 | 5703 | 399 | 5856 |
| ‐ | Between‐patient design | 11 | 1 | 15 | 2 | 16 |
| ‐ | Within‐patient design | 0 | 0 | 1 | 0 | 1 |
| ‐ | Treatment duration | 2 wks to 8 wks | 4 wks | 2 wks to 12 wks | 2 wks to 8 wks | 2 wks to 12 wks |
For acronyms, see Table 1.
Overall, vitamin D alone appeared to be less effective than vitamin D plus corticosteroid: The SMD for the IAGI was 0.48 (95% CI 0.32 to 0.65; I² statistic = 86.9%). This finding applied to all but two of the intervention‐comparator contrasts: There was no statistically significant difference between twice‐daily calcipotriol and a regimen of calcipotriol (morning) plus betamethasone valerate (night time) (SMD 0.27; 95% CI ‐0.19 to 0.74) (Kragballe 1998b), or between once‐daily calcipotriol and once‐daily treatment with a combined product containing calcipotriol and hydrocortisone (SMD 0.14; 95% CI ‐0.06 to 0.33) (Ortonne 2010). The study by Ortonne 2010 also reported findings separately for the face (see Analysis 17.1).
17.1. Analysis.
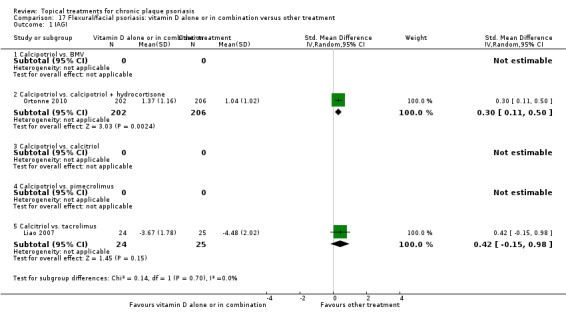
Comparison 17 Flexural/facial psoriasis: vitamin D alone or in combination versus other treatment, Outcome 1 IAGI.
In light of the observed heterogeneity between the intervention‐comparator contrasts, we only pooled subtotals (Higgins 2011).
Analysis 13: Vitamin D alone or in combination versus other treatments: complex regimens
This comparison summarises findings on complex regimens for body psoriasis, defined here as treatment sequences that do not consist of a simple head‐to‐head comparison between two active treatments (see Analysis 13.1 and Table 16). We identified 12 intervention‐comparator contrasts, and IAGI data were available for 10 of these. Data from 2755 participants contributed to the IAGI analysis, based on findings from seven trials. Six of the trials were between‐patient (parallel‐group) in design, and one was a within‐patient study (Austad 1998). Trial duration varied between 6 and 12 weeks. As the interventions and comparators were highly variable and because two trials each contributed three pair‐wise contrasts, we did not pool the data.
13.1. Analysis.
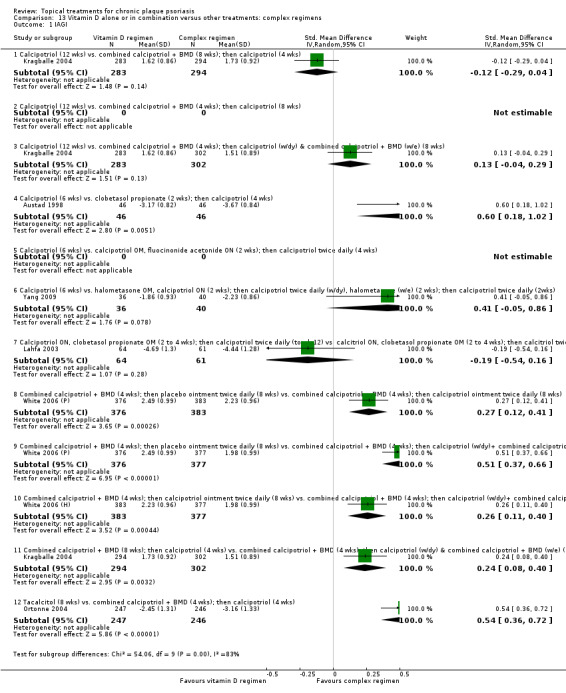
Comparison 13 Vitamin D alone or in combination versus other treatments: complex regimens, Outcome 1 IAGI.
16. Analysis 13: Trial characteristics and outcomes: vitamin D vs. other treatments: complex regimens.
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) | Effect size [CI] | (SMD ‐0.12; 95% CI ‐0.29 to 0.04) | ‐ | (SMD ‐0.04; 95% CI ‐0.19 to 0.11) | (SMD ‐0.14; 95% CI ‐0.30 to 0.02) | (SMD ‐0.12; 95% CI ‐0.29 to 0.04) |
| 02 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (8 wks) | Effect size [CI] | ‐ | ‐ | (SMD 0.29; 95% CI ‐0.04 to 0.62) | ‐ | (SMD 0.29; 95% CI ‐0.04 to 0.62) |
| 03 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | Effect size [CI] | (SMD 0.13; 95% CI ‐0.04 to 0.29) | ‐ | (SMD 0.10; 95% CI ‐0.05 to 0.25) | (SMD 0.10; 95% CI ‐0.06 to 0.26) | (SMD 0.13; 95% CI ‐0.04 to 0.29) |
| 04 Calcipotriol (6 wks) vs. clobetasol propionate (2 wks); then calcipotriol (4 wks) | Effect size [CI] | (SMD 0.60; 95% CI 0.18 to 1.02) | (SMD 0.63; 95% CI 0.21 to 1.05) | ‐ | ‐ | (SMD 0.60; 95% CI 0.18 to 1.02) |
| 05 Calcipotriol (6 wks) vs. calcipotriol OM, fluocinonide acetonide ON (2 wks); then calcipotriol BD (4 wks) | Effect size [CI] | ‐ | ‐ | (SMD 0.66; 95% CI 0.01 to 1.32) | ‐ | (SMD 0.66; 95% CI 0.01 to 1.32) |
| 06 Calcipotriol (6 wks) vs. halometasone OM, calcipotriol ON (2 wks); then calcipotriol BD (w/dy), halometasone (w/e) (2 wks); then calcipotriol BD (2wks) | Effect size [CI] | (SMD 0.41; 95% CI ‐0.05 to 0.86) | ‐ | (SMD 1.13; 95% CI 0.64 to 1.62) | ‐ | (SMD 0.41; 95% CI ‐0.05 to 0.86) |
| 07 Calcipotriol ON, clobetasol propionate OM (2 to 4 wks); then calcipotriol BD (to wk12) vs. calcitriol ON, clobetasol propionate OM (2 to 4 wks); then calcitriol BD (to wk12) | Effect size [CI] | (SMD ‐0.19; 95% CI ‐0.54 to 0.16) | ‐ | (SMD ‐0.27; 95% CI ‐0.62 to 0.09) | ‐ | (SMD ‐0.19; 95% CI ‐0.54 to 0.16) |
| 08 Combined calcipotriol + BMD (4 wks); then placebo ointment BD (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol ointment BD (8 wks) | Effect size [CI] | (SMD 0.27; 95% CI 0.12 to 0.41) | ‐ | (SMD 0.25; 95% CI 0.10 to 0.39) | (SMD 0.28; 95% CI 0.13 to 0.42) | (SMD 0.27; 95% CI 0.12 to 0.41) |
| 09 Combined calcipotriol + BMD (4 wks); then placebo ointment BD (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy)+ combined calcipotriol + BMD (w/e) (8 wks) | Effect size [CI] | (SMD 0.51; 95% CI 0.37 to 0.66) | ‐ | (SMD 0.59; 95% CI 0.45 to 0.74) | (SMD 0.71; 95% CI 0.56 to 0.85) | (SMD 0.51; 95% CI 0.37 to 0.66) |
| 10 combined calcipotriol + BMD (4 wks); then calcipotriol ointment BD (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) + combined calcipotriol + BMD (w/e) (8 wks) | Effect size [CI] | (SMD 0.26; 95% CI 0.11 to 0.40) | ‐ | (SMD 0.30; 95% CI 0.16 to 0.45) | (SMD 0.44; 95% CI 0.29 to 0.58) | (SMD 0.26; 95% CI 0.11 to 0.40) |
| 11 Combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | Effect size [CI] | (SMD 0.24; 95% CI 0.08 to 0.40) | ‐ | (SMD 0.15; 95% CI ‐0.01 to 0.30) | (SMD 0.23; 95% CI 0.07 to 0.39) | (SMD 0.24; 95% CI 0.08 to 0.40) |
| 12 Tacalcitol (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | Effect size [CI] | (SMD 0.54; 95% CI 0.36 to 0.72) | ‐ | (SMD 0.49; 95% CI 0.31 to 0.67) | (SMD 0.54; 95% CI 0.36 to 0.72) | (SMD 0.54; 95% CI 0.36 to 0.72) |
| All treatments | (not pooled) | ‐ | ‐ | ‐ | ‐ | ‐ |
| ‐ | No. participants | 2755 | 46 | 2991 | 2508 | 2936 |
| ‐ | Between‐patient design | 6 | 0 | 8 | 4 | 8 |
| ‐ | Within‐patient design | 1 | 1 | 0 | 0 | 1 |
| ‐ | Treatment duration | 6 wks to 12 wks | 6 wks | 2 wks to 12 wks | 8 wks to 12 wks | 2 wks to 12 wks |
For acronyms, see Table 1.
Six intervention‐comparator contrasts for which IAGI data were available found a significant difference between regimens. A two‐week regimen with clobetasol propionate followed by four weeks' treatment with calcipotriol was more effective than six weeks of monotherapy with calcipotriol (SMD 0.60; 95% CI 0.18 to 1.02). All treatments were applied twice‐daily in this within‐patient study (Austad 1998).
The remaining five more effective regimens all involved once‐daily treatment with a combined product containing calcipotriol and betamethasone dipropionate, and the same study (White 2006 (P)) reported three contrasts. This study involved an initial 'treatment' phase consisting of 4 weeks' treatment with the combined product, followed by an 8‐week maintenance phase. In one maintenance phase, participants received placebo ointment for 8 weeks. This was significantly less effective than maintenance with once‐daily calcipotriol (SMD 0.27; 95% CI 0.12 to 0.41), and it was also less effective than maintenance with once‐daily calcipotriol on weekdays and the combined product at the weekend (SMD 0.51; 95% CI 0.37 to 0.66). When these two comparator regimens were compared directly (White 2006 (H)), the alternating regimen using weekday/weekend treatments during the maintenance phase was significantly more effective than once‐daily calcipotriol for maintenance (SMD 0.26; 95% CI 0.11 to 0.40). Kragballe 2004 compared 3 12‐week treatment regimens, 2 of which were 'complex'. When we compared these complex regimens, eight weeks' once‐daily combination treatment with calcipotriol and betamethasone dipropionate, followed by four weeks of once‐daily calcipotriol was significantly less effective than a regimen consisting of four weeks' once‐daily combination treatment, followed by eight weeks of once‐daily calcipotriol on weekdays and the combined product at weekends (SMD 0.24; 95% CI 0.08 to 0.40). A separate trial compared 8 weeks with tacalcitol to combination ointment for 4 weeks, followed by calcipotriol for 4 weeks (Ortonne 2004). Participants applied all treatments once daily (at night time). Monotherapy with tacalcitol was significantly less effective than the complex regimen (SMD 0.54; 95% CI 0.36 to 0.72).
In 4 of the 10 intervention‐comparator contrasts for which IAGI data were available, there was no significant difference in effect. The study by Kragballe 2004 (see paragraph above) found no difference between twice‐daily calcipotriol and the two comparator complex regimens. One regimen consisted of eight weeks' once‐daily treatment with a combined product containing calcipotriol and betamethasone dipropionate, followed by four weeks of once‐daily calcipotriol. Relative to calcipotriol monotherapy, the SMD for the IAGI was ‐0.12 (95% CI ‐0.29 to 0.04). When compared with a regimen consisting of four weeks' once‐daily treatment with a combined product containing calcipotriol and betamethasone dipropionate, followed by eight weeks of once‐daily calcipotriol on weekdays and the combined product at weekends, twice‐daily calcipotriol appeared to be slightly less effective, although the difference was not statistically significant (SMD 0.13; 95% CI ‐0.04 to 0.29).
Yang 2009 compared a six‐week course of twice‐daily calcipotriol with a complex routine using halometasone and calcipotriol. For two weeks participants applied halometasone in the morning and calcipotriol at night; over the next two weeks, halometasone was applied twice daily at weekends and calcipotriol twice daily during the week. For the final two weeks, treatment reverted to twice‐daily calcipotriol. Although there was a trend in favour of the complex regimen (SMD 0.41; 95% CI ‐0.05 to 0.86), the difference was not statistically significant.
Lahfa 2003 compared two different vitamin D products combined with a very potent corticosteroid. In the initial phase, participants applied dual therapy (clobetasol propionate in the mornings and the vitamin D analogue (calcipotriol or calcitriol) at night) until achieving clearance or marked improvement or until 4 weeks had elapsed. Participants then switched to a monotherapy maintenance phase with the vitamin D product for the remainder of the 12‐week study period. The IAGI results showed the 2 treatment regimens to be equivalent: ‐0.19 (95% CI ‐0.54 to 0.16).
Analysis 14: Vitamin D alone or in combination versus other treatment: long‐term studies (> 24 weeks)
This comparison included active‐controlled studies of psoriasis of the body that were at least 24 weeks in duration (see Analysis 14.1 and Table 17). One longer‐term placebo‐controlled trial of the body (Katz 1991a) and two long‐term scalp trials (Luger 2008; Poulin 2010) did not meet the inclusion criteria for this comparison, and they are evaluated elsewhere (Analysis 2, Analysis 19, and Analysis 18, respectively).
14.1. Analysis.
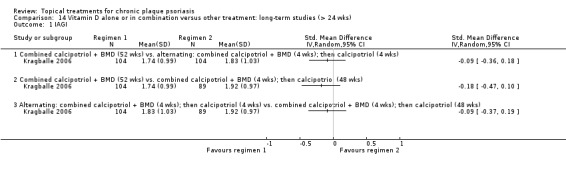
Comparison 14 Vitamin D alone or in combination versus other treatment: long‐term studies (> 24 wks), Outcome 1 IAGI.
17. Analysis 14: Trial characteristics and outcomes: vitamin D vs. other treatment: long‐term studies (> 24 wks).
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Combined calcipotriol + BMD (52 wks) vs. alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | Effect size [CI] | (SMD ‐0.09; 95% CI ‐0.36 to 0.18) | ‐ | ‐ | ‐ | (SMD ‐0.09; 95% CI ‐0.36 to 0.18) |
| 02 Combined calcipotriol+BMD (52 wks) vs. combined calcipotriol+ BMD (4 wks); then calcipotriol (48 wks) | Effect size [CI] | (SMD ‐0.18; 95% CI ‐0.47 to 0.10) | ‐ | ‐ | ‐ | (SMD ‐0.18; 95% CI ‐0.47 to 0.10) |
| 03 Alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | Effect size [CI] | (SMD ‐0.09; 95% CI ‐0.37 to 0.19) | ‐ | ‐ | ‐ | (SMD ‐0.09; 95% CI ‐0.37 to 0.19) |
| All treatments | (no pooling) | ‐ | ‐ | ‐ | ‐ | ‐ |
| ‐ | No. participants | 297 | 0 | 0 | 0 | 297 |
| ‐ | Between‐patient design | 1 | 0 | 0 | 0 | 1 |
| ‐ | Within‐patient design | 0 | 0 | 0 | 0 | 0 |
| ‐ | Treatment duration | 52 wks | ‐ | ‐ | ‐ | 52 wks |
For acronyms, see Table 1.
One trial was included in this comparison; Kragballe 2006 was a between‐patient trial that compared three 52‐week regimens with all treatments used once daily:
first, combination treatment with calcipotriol and betamethasone dipropionate;
second, alternating treatment with combination therapy for 4 weeks, then calcipotriol for 4 weeks; and
third, combination therapy for 4 weeks, then calcipotriol for 48 weeks.
In total, 297 participants contributed IGA data to the analysis. Data were unsuitable for pooling because the same participants contributed to more than one analysis.
According to the investigators' assessment, there was no significant difference between the three long‐term regimens. One year's combination therapy was not significantly better than either the alternating regimen (SMD ‐0.09; 95% CI ‐0.36 to 0.18) or the regimen of treatment with 4 weeks of combination therapy followed by 48 weeks of calcipotriol (SMD ‐0.18; 95% CI ‐0.47 to 0.10). In both these comparisons, combination therapy achieved a larger absolute benefit, but the difference was not statistically significant. When alternating therapy was compared with the regimen of 4 weeks' combination therapy followed by 48 weeks of calcipotriol, the SMD for the IAGI also indicated the two regimens were not statistically significantly different: ‐0.09 (95% CI ‐0.37 to 0.19).
Analysis 15: Vitamin D analogues versus other treatment
This comparison incorporated all other vitamin D head‐to‐head comparisons of treatments for psoriasis of the body (excluding inverse psoriasis) that had not already been included (see Analysis 15.1 and Table 18). We included 12 intervention‐comparator contrasts, with IAGI data available for 6 of these contrasts. Eight between‐patient trials and 2 within‐patient trials provided data for 1386 participants. Trial duration ranged between 4 and 12 weeks. In light of the pharmacological diversity amongst the comparators, we only pooled data within subgroups.
15.1. Analysis.
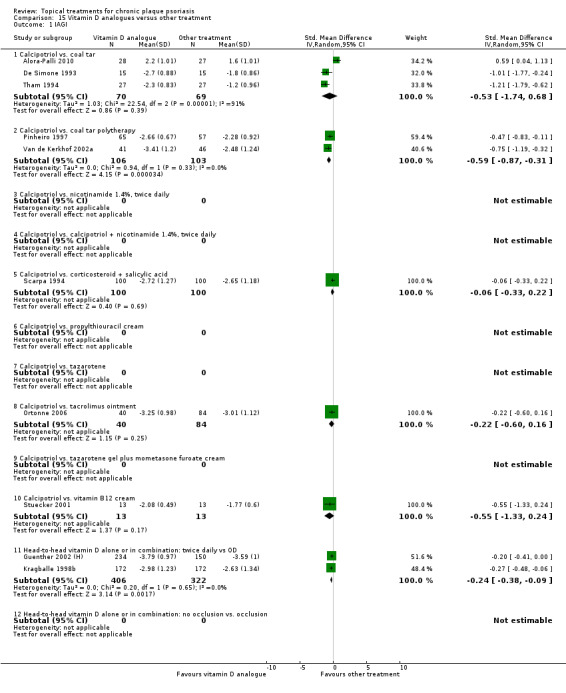
Comparison 15 Vitamin D analogues versus other treatment, Outcome 1 IAGI.
18. Analysis 15: Trial characteristics and outcomes: vitamin D/other treatment.
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Calcipotriol vs. coal tar | Effect size [CI] | (SMD ‐0.53; 95% CI ‐1.74 to 0.68) | ‐ | (SMD ‐0.10; 95% CI ‐1.54 to 1.35) | (SMD ‐0.10; 95% CI ‐1.54 to 1.35) | (SMD ‐0.53; 95% CI ‐1.74 to 0.68) |
| 02 Calcipotriol vs. coal tar polytherapy | Effect size [CI] | (SMD ‐0.59; 95% CI ‐0.87 to ‐0.31) | (SMD ‐0.51; 95% CI ‐0.86 to ‐0.16) | (SMD ‐0.63; 95% CI ‐1.06 to ‐0.20) | (SMD ‐0.63; 95% CI ‐1.06 to ‐0.20) | (SMD ‐0.59; 95% CI ‐0.87 to ‐0.31) |
| 03 Calcipotriol vs. nicotinamide 1.4%, BD | Effect size [CI] | ‐ | (SMD ‐0.09; 95% CI ‐0.49 to 0.31) | ‐ | ‐ | (SMD ‐0.09; 95% CI ‐0.49 to 0.31) |
| 04 Calcipotriol vs. calcipotriol + nicotinamide 1.4%, BD | Effect size [CI] | ‐ | (SMD 0.19; 95% CI ‐0.14 to 0.52) | ‐ | ‐ | (SMD 0.19; 95% CI ‐0.14 to 0.52) |
| 05 Calcipotriol vs. corticosteroid + salicylic acid | Effect size [CI] | (SMD ‐0.06; 95% CI ‐0.33 to 0.22) | ‐ | (SMD ‐0.05; 95% CI ‐0.36 to 0.26) | (SMD ‐0.49; 95% CI ‐0.79 to ‐0.20) | (SMD ‐0.05; 95% CI ‐0.26 to 0.15) |
| 06 Calcipotriol vs. propylthiouracil cream | Effect size [CI] | ‐ | ‐ | (SMD ‐2.24; 95% CI ‐3.23 to ‐1.25) | ‐ | (SMD ‐2.24; 95% CI ‐3.23 to ‐1.25) |
| 07 Calcipotriol vs. tacrolimus ointment | Effect size [CI] | ‐ | (SMD ‐0.35; 95% CI ‐1.51 to 0.81) | (SMD ‐0.13; 95% CI ‐0.51 to 0.24) | (SMD ‐0.55; 95% CI ‐1.28 to 0.17) | |
| 08 Calcipotriol vs. tazarotene | Effect size [CI] | (SMD ‐0.22; 95% CI ‐0.60 to 0.16) | (SMD ‐0.05; 95% CI ‐0.33 to 0.23) | ‐ | (SMD ‐0.35; 95% CI ‐0.99 to 0.29) | (SMD ‐0.10; 95% CI ‐0.35 to 0.16) |
| 09 Calcipotriol vs. tazarotene gel plus mometasone furoate cream | Effect size [CI] | ‐ | ‐ | ‐ | ‐ | ‐ |
| 10 Calcipotriol vs. vitamin B12 cream | Effect size [CI] | (SMD ‐0.55; 95% CI ‐1.33 to 0.24) | ‐ | (SMD ‐0.01; 95% CI ‐0.78 to 0.75) | (SMD ‐0.55; 95% CI ‐1.33 to 0.24) | (SMD ‐0.55; 95% CI ‐1.33 to 0.24) |
| 11 Head‐to‐head vitamin D alone or in combination: dosing | Effect size [CI] | (SMD ‐0.24; 95% CI ‐0.38 to ‐0.09) | ‐ | (SMD ‐0.12; 95% CI ‐0.25 to 0.00) | ‐ | (SMD ‐0.20; 95% CI ‐0.32 to ‐0.07) |
| 12 Head‐to‐head vitamin D alone or in combination: occlusion | Effect size [CI] | ‐ | (SMD ‐0.18; 95% CI ‐2.04 to 1.68) | ‐ | ‐ | (SMD ‐0.18; 95% CI ‐2.04 to 1.68) |
| All treatments | (not pooled) | ‐ | ‐ | ‐ | ‐ | ‐ |
| ‐ | No. participants | 1386 | 898 | 1228 | 456 | 2364 |
| ‐ | Between‐patient design | 8 | 5 | 6 | 3 | 13 |
| ‐ | Within‐patient design | 2 | 2 | 3 | 3 | 6 |
| ‐ | Treatment duration | 4 wks to 12 wks | 6 wks to 12 wks | 4 wks to 12 wks | 4 wks to 12 wks | 4 wks to 12 wks |
For acronyms, see Table 1.
According to the investigator's global assessment, twice‐daily calcipotriol was significantly more effective than coal tar polytherapy (SMD ‐0.59; 95% CI ‐0.87 to ‐0.31; I² statistic = 0%). Once‐daily vitamin D was significantly less effective than a twice‐daily application (SMD ‐0.24; 95% CI ‐0.38 to ‐0.09; I² statistic = 0%). This contrast included a comparison of once‐ versus twice‐daily dosing of calcipotriol (SMD ‐0.27; 95% CI ‐0.48 to ‐0.06) and a dosing comparison combination treatment with calcipotriol and betamethasone dipropionate (SMD ‐0.20; 95% CI ‐0.41 to 0.00).
In the remaining four intervention‐comparator contrasts, no significant difference was found between twice‐daily calcipotriol and the comparators. This finding held for the comparison against coal tar monotherapy (SMD ‐0.53; 95% CI ‐1.74 to 0.68; I² statistic = 91.1%), against betamethasone dipropionate ointment and salicylic acid (SMD ‐0.06; 95% CI ‐0.33 to 0.22; I² statistic = NA), against tacrolimus ointment (SMD ‐0.22; 95% CI ‐0.60 to 0.16; I² statistic = NA), and against vitamin B12 cream (SMD ‐0.55; 95% CI ‐1.33 to 0.24; I² statistic = NA).
Two of the three trials comparing calcipotriol with coal tar monotherapy found a significant difference in favour of calcipotriol; the other trial found a significant difference in favour of coal tar (Alora‐Palli 2010). This apparent contradiction may reflect different formulations of coal tar, treatment durations, or different baseline disease severity.
Analysis 16: Flexural/facial psoriasis: placebo‐controlled trials
This comparison included placebo‐controlled trials of topical treatments for inverse or facial psoriasis (see Analysis 16.1 and Table 19). Evidence on four treatments was found in this comparison: the potent steroid betamethasone valerate; the vitamin D analogue calcipotriol; and two topical calcineurin inhibitors, pimecrolimus and tacrolimus. We only found one trial that evaluated tacrolimus ointment (Lebwohl 2004), but the study did not report any effectiveness data suitable for this review. However, the study did contribute data on adverse events and withdrawal rates.
16.1. Analysis.
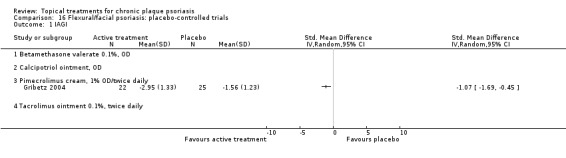
Comparison 16 Flexural/facial psoriasis: placebo‐controlled trials, Outcome 1 IAGI.
19. Analysis 16: Trial characteristics and outcomes: flexural/facial psoriasis: placebo trials.
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Betamethasone valerate 0.1%, OD | Effect size [CI] | ‐ | ‐ | (SMD ‐2.83; 95% CI ‐3.79 to ‐1.88) | ‐ | (SMD ‐2.83; 95% CI ‐3.79 to ‐1.88) |
| 02 Calcipotriol ointment, OD | Effect size [CI] | ‐ | ‐ | (SMD ‐1.08; 95% CI ‐1.77 to ‐0.40) | ‐ | (SMD ‐1.08; 95% CI ‐1.77 to ‐0.40) |
| 03 Pimecrolimus cream, 1% OD/BD | Effect size [CI] | (SMD ‐1.07; 95% CI ‐1.69 to ‐0.45) | (SMD ‐1.37; 95% CI ‐1.95 to ‐0.79) | (SMD ‐0.62; 95% CI ‐1.27 to 0.02) | (SMD ‐0.65; 95% CI ‐1.24 to ‐0.06) | (SMD ‐0.86; 95% CI ‐1.30 to ‐0.41) |
| 04 Tacrolimus ointment 0.1%, BD | Effect size [CI] | ‐ | ‐ | ‐ | ‐ | ‐ |
| All treatments | (no pooling) | ‐ | ‐ | ‐ | ‐ | ‐ |
| ‐ | No. participants | 47 | 57 | 75 | 47 | 122 |
| ‐ | Between‐patient design | 1 | 1 | 1 | 1 | 2 |
| ‐ | Within‐patient design | 0 | 0 | 0 | 0 | 0 |
| ‐ | Treatment duration | 8 wks | 8 wks | 4 wks | 8 wks | 4 wks to 8 wks |
For acronyms, see Table 1.
IAGI outcome data were available for one of the four topical treatments for inverse psoriasis. One 8‐week between‐patient study with 47 participants (Gribetz 2004) reported placebo‐controlled data on pimecrolimus 1% cream, which demonstrated a statistically significant difference in favour of twice‐daily pimecrolimus (SMD ‐1.07; 95% CI ‐1.69 to ‐0.45).
Analysis 17: Flexural/facial psoriasis: vitamin D alone or in combination versus other treatment
This comparison included head‐to‐head trials of treatments for inverse psoriasis, which compared vitamin D with an active control (see Analysis 17.1 and Table 20). We identified five intervention‐comparator contrasts. Four treatments were compared with calcipotriol: once‐daily betamethasone valerate, combined treatment with calcipotriol and hydrocortisone, calcitriol, and pimecrolimus. Calcitriol was compared with tacrolimus.
20. Analysis 17: Trial characteristics and outcomes: flexural/facial psoriasis: vitamin D vs. other treatment.
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Calcipotriol vs. BMV | Effect size [CI] | ‐ | ‐ | (SMD 2.02; 95% CI 1.20 to 2.84) | ‐ | (SMD 2.02; 95% CI 1.20 to 2.84) |
| 02 Calcipotriol vs. calcipotriol + hydrocortisone | Effect size [CI] | (SMD 0.30; 95% CI 0.11 to 0.50) | ‐ | (SMD 0.32; 95% CI 0.12 to 0.51) | ‐ | (SMD 0.30; 95% CI 0.11 to 0.50) |
| 03 Calcipotriol vs. calcitriol | Effect size [CI] | ‐ | (SMD 0.61; 95% CI 0.28 to 0.94) | ‐ | ‐ | (SMD 0.61; 95% CI 0.28 to 0.94) |
| 04 Calcipotriol vs. pimecrolimus | Effect size [CI] | ‐ | ‐ | (SMD ‐0.53; 95% CI ‐1.17 to 0.11) | (SMD ‐0.53; 95% CI ‐1.17 to 0.11) | |
| 05 Calcitriol vs. tacrolimus | Effect size [CI] | (SMD 0.42; 95% CI ‐0.15 to 0.98) | (SMD 0.29; 95% CI ‐0.27 to 0.85) | ‐ | ‐ | (SMD 0.42; 95% CI ‐0.15 to 0.98) |
| All treatments | (no pooling) | ‐ | ‐ | ‐ | ‐ | ‐ |
| ‐ | No. participants | 457 | 124 | 464 | 0 | 588 |
| ‐ | Between‐patient design | 2 | 1 | 2 | 0 | 3 |
| ‐ | Within‐patient design | 0 | 1 | 0 | 0 | 1 |
| ‐ | Treatment duration | 6 wks to 8 wks | 6 wks | 4 wks to 8 wks | 0 | 4 wks to 8 wks |
For acronyms, see Table 1.
Two between‐patient studies contributed IAGI data from 457 study participants on 2 of the 5 intervention‐comparator contrasts. Trial duration ranged from six to eight weeks. When applied once daily to inverse psoriasis, calcipotriol was significantly less effective than combined treatment with calcipotriol and hydrocortisone (SMD 0.30; 95% CI 0.11 to 0.50) (Ortonne 2010). However, there was no significant difference in effect between twice daily treatment with calcitriol and tacrolimus (SMD 0.42; 95% CI ‐0.15 to 0.98) (Liao 2007).
Analysis 18: Scalp psoriasis: placebo‐controlled trials
This comparison included placebo‐controlled trials of treatments for scalp psoriasis (see Analysis 18.1 and Table 21). We included evidence on 11 treatments in this comparison, with IAGI data available for 9 treatments. Ten trials, 9 of which were between‐patient studies, contributed data from 2472 participants. One study was a within‐patient trial (Lepaw 1978). Trial duration ranged between two and eight weeks. IAGI data were not available for betamethasone valerate or ciclopirox olamine shampoo.
21. Analysis 18: Trial characteristics and outcomes: scalp psoriasis: placebo‐controlled trials.
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Vitamin D: calcipotriol | Effect size [CI] | (SMD ‐0.72; 95% CI ‐1.28 to ‐0.16) | (SMD ‐0.44; 95% CI ‐0.64 to ‐0.25) | ‐ | (SMD ‐0.66; 95% CI ‐1.28 to ‐0.05) | (SMD ‐0.72; 95% CI ‐1.28 to ‐0.16) |
| 02 Potent steroid: betamethasone dipropionate | Effect size [CI] | (SMD ‐1.09; 95% CI ‐1.29 to ‐0.90) | (SMD ‐1.00; 95% CI ‐1.19 to ‐0.81) | ‐ | (SMD ‐1.23; 95% CI ‐1.43 to ‐1.03) | (SMD ‐1.09; 95% CI ‐1.29 to ‐0.90) |
| 03 Potent steroid: betamethasone valerate | Effect size [CI] | ‐ | (SMD ‐1.40; 95% CI ‐1.75 to ‐1.05) | ‐ | ‐ | (SMD ‐1.40; 95% CI ‐1.75 to ‐1.05) |
| 04 Very potent steroid: amcinonide | Effect size [CI] | (SMD ‐1.42; 95% CI ‐1.80 to ‐1.04) | (SMD ‐1.58; 95% CI ‐1.98 to ‐1.18) | ‐ | (SMD ‐0.97; 95% CI ‐1.33 to ‐0.61) | (SMD ‐1.42; 95% CI ‐1.80 to ‐1.04) |
| 05 Very potent steroid: clobetasol propionate | Effect size [CI] | (SMD ‐1.73; 95% CI ‐1.99 to ‐1.48) | (SMD ‐1.53; 95% CI ‐1.77 to ‐1.28) | ‐ | ‐ | (SMD ‐1.57; 95% CI ‐1.81 to ‐1.34) |
| 06 Very potent steroid: halcinonide | Effect size [CI] | (SMD ‐1.11; 95% CI ‐1.69 to ‐0.53) | ‐ | ‐ | ‐ | (SMD ‐1.11; 95% CI ‐1.69 to ‐0.53) |
| 07 Vitamin D in combination: calcipotriol + BMD | Effect size [CI] | (SMD ‐0.97; 95% CI ‐1.61 to ‐0.32) | (SMD ‐0.92; 95% CI ‐1.42 to ‐0.43) | ‐ | (SMD ‐1.00; 95% CI ‐1.79 to ‐0.22) | (SMD ‐0.97; 95% CI ‐1.61 to ‐0.32) |
| 08 Other treatment: betamethasone‐17,21‐dipropionate plus salicylic acid | Effect size [CI] | (SMD ‐1.48; 95% CI ‐2.50 to ‐0.47) | (SMD ‐1.15; 95% CI ‐2.11 to ‐0.19) | ‐ | ‐ | (SMD ‐1.48; 95% CI ‐2.50 to ‐0.47) |
| 09 Other treatment: ciclopirox olamine shampoo | Effect size [CI] | ‐ | (SMD ‐0.07; 95% CI ‐0.82 to 0.68) | ‐ | (SMD ‐0.11; 95% CI ‐0.86 to 0.64) | (SMD ‐0.07; 95% CI ‐0.82 to 0.68) |
| 10 Other treatment: fluocinolone acetonide, plus occlusion | Effect size [CI] | (SMD ‐1.22; 95% CI ‐1.69 to ‐0.76) | (SMD ‐0.89; 95% CI ‐1.34 to ‐0.44) | ‐ | ‐ | (SMD ‐1.22; 95% CI ‐1.69 to ‐0.76) |
| 11 Other treatment: salicylic acid | Effect size [CI] | (SMD ‐0.86; 95% CI ‐1.79 to 0.06) | (SMD ‐0.57; 95% CI ‐1.47 to 0.32) | ‐ | ‐ | (SMD ‐0.86; 95% CI ‐1.79 to 0.06) |
| All treatments | No. participants | 2472 | 2897 | 0 | 1875 | 3011 |
| ‐ | Between‐patient design | 9 | 12 | 0 | 5 | 13 |
| ‐ | Within‐patient design | 1 | 0 | 0 | 0 | 1 |
| ‐ | Treatment duration | 2 wks to 8 wks | 2 wks to 8 wks | ‐ | 3 wks to 8 wks | 2 wks to 8 wks |
| Sensitivity analysis: potent corticosteroids | Effect size [CI]; I² statistic | ‐ | ‐ | ‐ | ‐ | (SMD ‐1.18; 95% CI ‐1.40 to ‐0.96); I² statistic: 19.9% |
| Sensitivity analysis: very potent corticosteroids | Effect size [CI]; I² statistic | ‐ | ‐ | ‐ | ‐ | (SMD ‐1.51; 95% CI ‐1.70 to ‐1.31); I² statistic: 37.5% |
For acronyms, see Table 1.
Eight treatments for scalp psoriasis that were assessed using the IAGI scale were significantly more effective than placebo. The least effective treatment was calcipotriol (SMD ‐0.72; 95% CI ‐1.28 to ‐0.16; I² statistic = 69.2%), and the most effective treatment was clobetasol propionate (SMD ‐1.73; 95% CI ‐1.99 to ‐1.48; I² statistic = 14.3%). Combination treatment with calcipotriol and betamethasone dipropionate was also effective (SMD ‐0.97; 95% CI ‐1.61 to ‐0.32; I² statistic = 90.2%). Data from the three very potent corticosteroids were pooled (amcinonide, clobetasol propionate, halcinonide). The SMD across these treatments was ‐1.57 (95% CI ‐1.85 to ‐1.28; I² statistic = 49.7%). Only salicylic acid was not significantly more effective than placebo (SMD ‐0.86; 95% CI ‐1.79 to 0.06).
Analysis 19: Scalp psoriasis: vitamin D alone or in combination versus other treatment
This comparison included head‐to‐head trials of treatments for scalp psoriasis in which one of the interventions was a vitamin D product (used either as monotherapy or in combination with another product) (see Analysis 19.1 and Table 22). We identified six intervention‐comparator contrasts, and IAGI data were available for five of these contrasts. All studies were parallel‐group in design (between‐patient). Ten studies contributed IAGI data from 5175 participants, and trial duration ranged from 4 to 52 weeks.
19.1. Analysis.
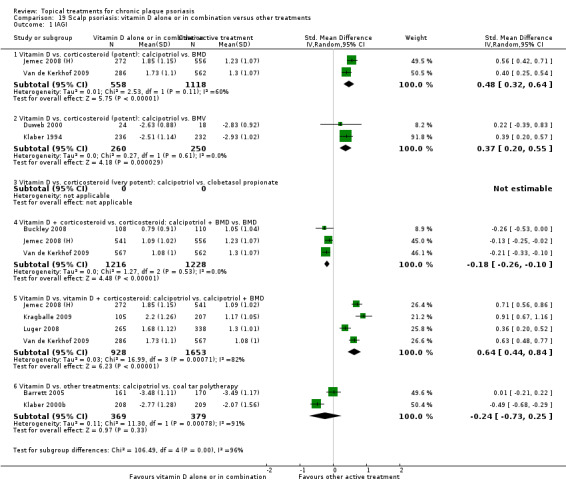
Comparison 19 Scalp psoriasis: vitamin D alone or in combination versus other treatments, Outcome 1 IAGI.
22. Analysis 19: Trial characteristics and outcomes: scalp psoriasis: vitamin D vs. other treatment.
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMD | Effect size [CI] | (SMD 0.48; 95% CI 0.32 to 0.64) | (SMD 0.45; 95% CI 0.28 to 0.63) | ‐ | (SMD 0.56; 95% CI 0.31 to 0.81) | (SMD 0.48; 95% CI 0.32 to 0.64) |
| 02 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMV | Effect size [CI] | (SMD 0.37; 95% CI 0.20 to 0.55) | (SMD 0.09; 95% CI ‐0.09 to 0.27) | ‐ | (SMD 0.41; 95% CI 0.22 to 0.59) | (SMD 0.37; 95% CI 0.20 to 0.55) |
| 03 Vitamin D vs. corticosteroid (very potent): calcipotriol vs. clobetasol propionate | Effect size [CI] | ‐ | (SMD 0.37; 95% CI 0.05 to 0.69) | ‐ | ‐ | (SMD 0.37; 95% CI 0.05 to 0.69) |
| 04 Vitamin D + corticosteroid vs. corticosteroid: calcipotriol + BMD vs. BMD | Effect size [CI] | (SMD ‐0.18; 95% CI ‐0.26 to ‐0.10) | (SMD ‐0.19; 95% CI ‐0.27 to ‐0.11) | ‐ | (SMD ‐0.17; 95% CI ‐0.25 to ‐0.09) | (SMD ‐0.18; 95% CI ‐0.26 to ‐0.10) |
| 05 Vitamin D vs. vitamin D + corticosteroid: calcipotriol vs. calcipotriol + BMD | Effect size [CI] | (SMD 0.64; 95% CI 0.44 to 0.84) | (SMD 0.70; 95% CI 0.56 to 0.84) | ‐ | (SMD 0.84; 95% CI 0.61 to 1.08) | (SMD 0.64; 95% CI 0.44 to 0.84) |
| 06 Vitamin D vs. other treatments: calcipotriol vs. coal tar polytherapy | Effect size [CI] | (SMD ‐0.24; 95% CI ‐0.73 to 0.25) | (SMD ‐0.30; 95% CI ‐0.84 to 0.24) | ‐ | ‐ | (SMD ‐0.45; 95% CI ‐0.92 to 0.02) |
| All treatments | No. participants | 5175 | 4877 | 0 | 3742 | 5413 |
| ‐ | Between‐patient design | 10 | 11 | 0 | 6 | 12 |
| ‐ | Within‐patient design | 0 | 0 | 0 | 0 | 0 |
| ‐ | Treatment duration | 4 wks to 52 wks | 4 wks to 8 wks | 0 | 4 wks to 8 wks | 4 wks to 52 wks |
For acronyms, see Table 1.
Vitamin D for scalp psoriasis was significantly less effective than potent steroids, either alone or in combination with vitamin D. Specifically, calcipotriol was less effective than three comparator treatments: betamethasone dipropionate (SMD 0.48; 95% CI 0.32 to 0.64; I² statistic = 60.4%), betamethasone valerate (SMD 0.37; 95% CI 0.20 to 0.55; I² statistic = 0%), and combination treatment with calcipotriol and betamethasone dipropionate (SMD 0.64; 95% CI 0.44 to 0.84; I² statistic = 82.3%). Combination treatment (calcipotriol/betamethasone dipropionate) was significantly more effective than betamethasone dipropionate alone (SMD ‐0.18; 95% CI ‐0.26 to ‐0.10; I² statistic = 0%). The efficacy of calcipotriol and coal tar polytherapy was similar: SMD ‐0.24 (95% CI ‐0.73 to 0.25; I² statistic = 91.1%).
(b) Total Severity Scores (TSS)
Analysis 1: Vitamin D analogues versus placebo
Our review included eight vitamin D analogues for body psoriasis in this comparison (see Analysis 1.2 and Table 5). Nineteen studies reported TSS data with 2647 participants contributing data. Nine trials were between‐patient design, and 10 were within‐patient studies. Treatment duration ranged from 4 weeks to 12 weeks. The average effect size across all 8 treatments for TSS was ‐1.04; (95% CI ‐1.33 to ‐0.74; I² statistic = 93.0%), but two of these treatments were not significantly more effective than placebo. The high level of heterogeneity means that the estimated average benefit should be treated with caution; there was considerable variation between effective treatments, with the TSS ranging from ‐0.46 (becocalcidiol twice daily) to ‐2.15 (paricalcitol once daily). Therefore, we removed pooling across subgroups (Higgins 2011).
1.2. Analysis.
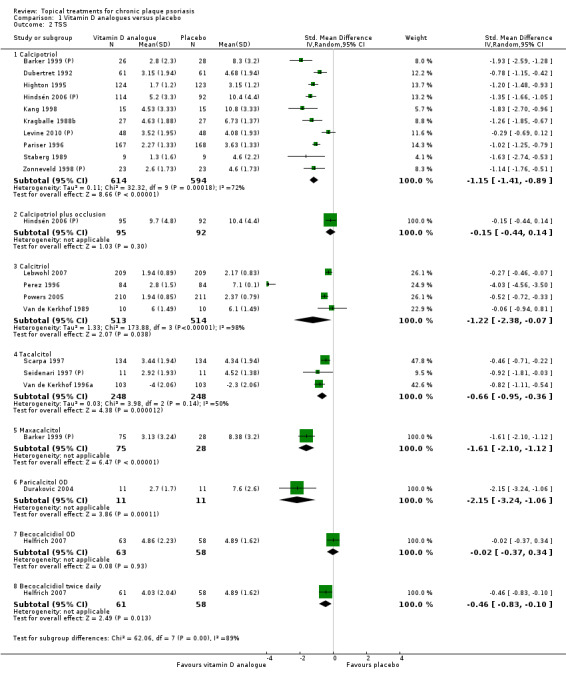
Comparison 1 Vitamin D analogues versus placebo, Outcome 2 TSS.
Four studies contributed data for the SMD for the TSS of calcitriol (SMD ‐1.22; 95% CI ‐2.38 to ‐0.07), but there was considerable heterogeneity within the subgroup (I² statistic = 98.3%). One of the studies (Van de Kerkhof 1989) found no statistically significant difference between calcitriol and placebo (SMD ‐0.06; 95% CI ‐0.94 to 0.81), whereas the study by Perez 1996 found a large and statistically significant difference (‐4.03; 95% CI ‐4.56 to ‐3.50). We considered this finding in more detail in the 'combined end point' section (e) below.
Analysis 2: Corticosteroid (potent) versus placebo
Our review included 10 potent corticosteroids for body psoriasis in this comparison (see Analysis 2.2 and Table 6). Seven studies reported TSS data contributed by 553 participants on 8 of these 10 treatments. Six trials were between‐patient studies, and there was one within‐patient design (Ormerod 1997). Treatment duration ranged from 2 to 12 weeks. The average effect size across all 8 treatments for TSS was ‐0.77 (95% CI ‐1.01 to ‐0.52; I² statistic = 46.7%). We found all treatments, except for diflorasone diacetate, to be statistically significantly superior to placebo: SMD ‐0.32; 95% CI ‐0.73 to 0.09).
2.2. Analysis.
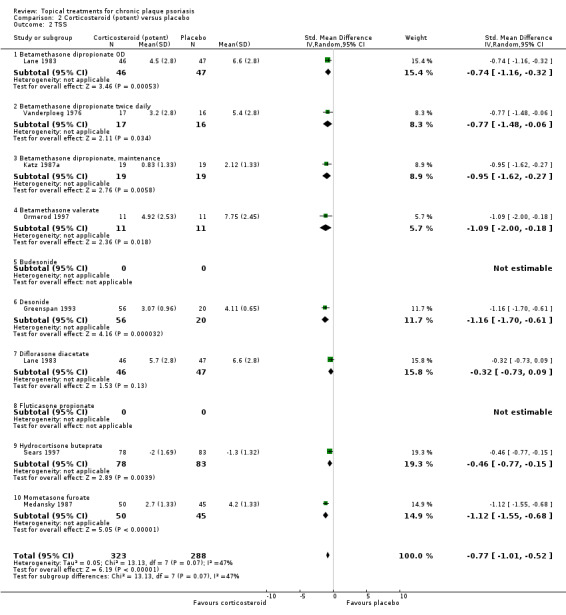
Comparison 2 Corticosteroid (potent) versus placebo, Outcome 2 TSS.
Analysis 3: Corticosteroid (very potent) versus placebo
Of the three very potent corticosteroids for body psoriasis included in this comparison, TSS data were available only for clobetasol propionate (see Analysis 3.2 and Table 7). Three studies, all of which were between‐patient trials, reported TSS data for 545 participants. Treatment duration ranged from two to four weeks. The SMD for the TSS was ‐1.35 (95% CI ‐1.80 to ‐0.89; I² statistic = 75.3%). In Analysis 18.2, we reported TSS data on the use of very potent steroids on the scalp.
3.2. Analysis.
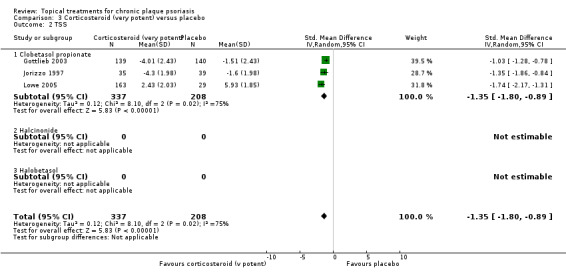
Comparison 3 Corticosteroid (very potent) versus placebo, Outcome 2 TSS.
18.2. Analysis.
Comparison 18 Scalp psoriasis: placebo‐controlled trials, Outcome 2 TSS.
Analysis 4: Dithranol versus placebo
This comparison considered dithranol against placebo (see Analysis 4.2 and Table 23). Three within‐patient trials reported TSS data for 47 participants. Treatment duration ranged from three to eight weeks. The SMD for the TSS was ‐1.06 (95% CI ‐1.66 to ‐0.46; I² statistic = 37.4%).
4.2. Analysis.
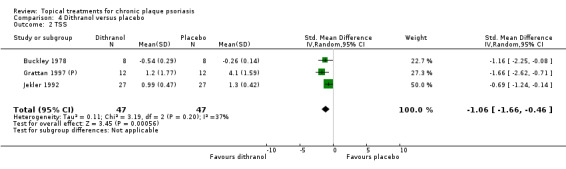
Comparison 4 Dithranol versus placebo, Outcome 2 TSS.
23. Analysis 04: Trial characteristics and outcomes: dithranol vs. placebo.
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Dithranol | Effect size [CI], N, I² statistic | ‐ | (SMD ‐1.06; 95% CI ‐1.66 to ‐0.46); I² statistic: 37.4% | ‐ | ‐ | (SMD ‐1.06; 95% CI ‐1.66 to ‐0.46); I² statistic: 37.4% |
| ‐ | No. participants | 0 | 47 | 0 | 0 | 47 |
| ‐ | Between‐patient design | 0 | 0 | 0 | 0 | 0 |
| ‐ | Within‐patient design | 0 | 3 | 0 | 0 | 3 |
| ‐ | Treatment duration | 3 wks to 8 wks | ‐ | ‐ | 3 wks to 8 wks | |
| ‐ | correlation coefficient (rho) = 0 All trials |
‐ | ‐ | ‐ | (SMD ‐0.98; 95% CI ‐1.56 to ‐0.41) I² statistic: 13.9% |
|
| ‐ | rho = 0 Btw‐patient trials rho = 0.25 Within‐patient trials |
‐ | ‐ | ‐ | ‐ | (SMD ‐1.05; 95% CI ‐1.67 to ‐0.44) I² statistic: 35.4% |
| ‐ | rho = 0 Btw‐patient trials rho = 0.50 Within‐patient trials |
‐ | ‐ | ‐ | ‐ | (SMD ‐1.12; 95% CI ‐1.75 to ‐0.48) I² statistic: 56.9% |
| ‐ | rho = 0 Btw‐patient trials rho = 0.75 Within‐patient trials |
‐ | ‐ | ‐ | ‐ | (SMD ‐1.17; 95% CI ‐1.81 to ‐0.52) I² statistic: 78.5% |
For acronyms, see Table 1.
Analysis 5: Vitamin D combination products versus placebo
Our review did not identify any trial reporting TSS data for this comparison.
Analysis 6: Other treatment versus placebo
This comparison comprised all other treatments for body psoriasis that were not included in the first five comparisons. None of the studies assessed the same treatment, which means that findings should be interpreted with caution.
In total, we included 26 treatments in this analysis (see Analysis 6.2 and Table 9). Seventeen studies with 907 participants reported TSS data on 17 of these 26 treatments. Five trials were between‐patient design, and 12 were within‐patient studies. Treatment duration ranged from 3 to 12 weeks.
6.2. Analysis.
Comparison 6 Other treatment versus placebo, Outcome 2 TSS.
Ten treatments performed significantly better than placebo: anti‐IL‐8 monoclonal antibody cream, calcipotriene 0.005% ointment + nicotinamide, fish oil plus occlusion, hexafluoro‐1,25‐dihydroxyvitamin D3, indigo naturalise 1.4% ointment, methotrexate gel, mycophenolic acid ointment, oleum horwathiensis, PTH (1‐34) in Novasome cream®, and tazarotene. The effect size for the TSS ranged from ‐0.48 (Levine 2010 (P); calcipotriene 0.005% ointment + nicotinamide) to ‐2.31 (Holick 2003; PTH (1‐34) in Novasome cream®).
In seven treatments, the difference relative to placebo was not statistically significant: kukui nut oil, NG‐monomethyl‐L‐arginine (L‐NMMA) cream, nicotinamide 1.4%, polymyxin B cream, topical sirolimus, topical tacrolimus, and tar.
Analysis 7: Vitamin D analogues versus corticosteroid (potent)
There were eight vitamin D analogue‐potent corticosteroid comparisons for body psoriasis (see Analysis 7.2 and Table 10). Six studies with 891 participants reported TSS data for 5 of the 8 intervention‐comparator contrasts. Two trials were between‐patient design, and four were within‐patient studies. Treatment duration ranged from three to six weeks. The SMD across all 5 treatments for TSS indicated that there was no significant difference between the vitamin D derivatives and potent corticosteroid: SMD 0.11 (95% CI ‐0.22 to 0.44; I² statistic = 86.7%). However, there was considerable variation between the individual contrasts underlying the pooled effect. In light of this heterogeneity, we removed pooling (Higgins 2011).
7.2. Analysis.
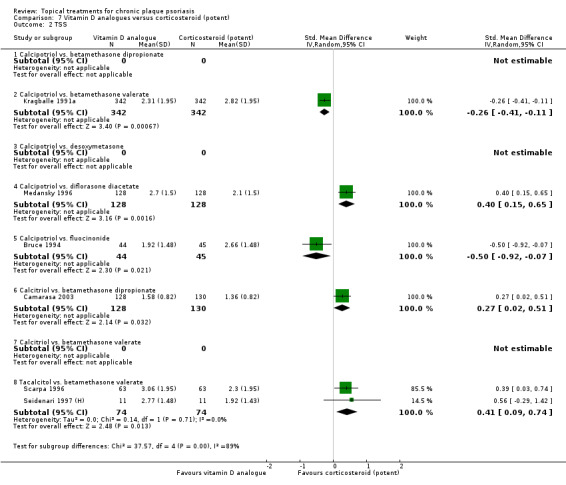
Comparison 7 Vitamin D analogues versus corticosteroid (potent), Outcome 2 TSS.
In two of the five vitamin D‐potent corticosteroid comparisons, the vitamin D analogue performed statistically significantly better than the potent corticosteroid: The SMD for calcipotriol against fluocinonide 0.05% ointment was ‐0.50 (95% CI ‐0.92 to ‐0.07; I² statistic = NA) (Bruce 1994). Similarly, the comparison of calcipotriol against betamethasone valerate showed a significant difference in favour of calcipotriol (SMD ‐0.26; 95% CI ‐0.41 to ‐0.11), a finding also based on a single study (Kragballe 1991a).
In three comparisons, the vitamin D analogue was statistically significantly less effective than the potent corticosteroid: calcipotriol versus diflorasone diacetate (SMD 0.40; 95% CI 0.15 to 0.65), calcitriol versus betamethasone dipropionate (SMD 0.27; 95% CI 0.02 to 0.51), and tacalcitol versus betamethasone valerate (SMD 0.41; 95% CI 0.09 to 0.74).
Analysis 8: Vitamin D analogues versus corticosteroid (very potent)
Our review did not identify any study of psoriasis of the body that compared vitamin D analogues against very potent corticosteroids and that reported TSS data.
Analysis 9: Vitamin D combined with corticosteroid versus corticosteroid
This comparison considered vitamin D analogues‐steroid combination against potent or very potent corticosteroid for body psoriasis (see Analysis 9.2 and Table 12). One four‐week between‐patient trial reported TSS data for 122 participants with moderate to severe psoriasis (Menter 2009). The combined treatment with calcipotriol and betamethasone dipropionate was found to be significantly less effective than clobetasol propionate spray alone (0.45; 95% CI 0.09 to 0.81).
9.2. Analysis.
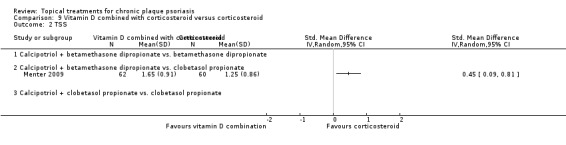
Comparison 9 Vitamin D combined with corticosteroid versus corticosteroid, Outcome 2 TSS.
Analysis 10: Vitamin D alone or in combination versus dithranol
This comparison considered vitamin D analogues against dithranol (see Analysis 10.2 and Table 13). We identified three intervention‐comparator contrasts: calcipotriol versus dithranol, calcitriol versus dithranol, and tacalcitol versus dithranol. Three between‐patient trials and 1 within‐patient trial (Grattan 1997 (H)) reported TSS data for 386 participants. Treatment duration ranged from four weeks to eight weeks. There was some variation in the dithranol regimens employed by trials and in the baseline severity of trial participants. These factors may help explain the substantial heterogeneity found in the pooled results (Higgins 2011).
10.2. Analysis.
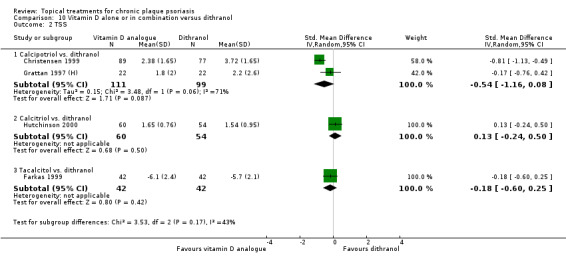
Comparison 10 Vitamin D alone or in combination versus dithranol, Outcome 2 TSS.
The SMD for the TSS was ‐0.27 (95% CI ‐0.73 to 0.20; I² statistic = 80.6%). Data from two trials contributed to the SMD for the calcipotriol versus dithranol: ‐0.54 (95% CI ‐1.16 to 0.08; I² statistic = 71.2%) (Christensen 1999; Grattan 1997 (H)). Data from one trial contributed to the SMD for the calcitriol versus dithranol: 0.13 (95% CI ‐0.24 to 0.50; I² statistic = NA) (Hutchinson 2000). Data from one trial contributed to the SMD for the tacalcitol versus dithranol: ‐0.18 (95% CI ‐0.60 to 0.25; I² statistic = NA) (Farkas 1999). Therefore, neither the summary statistic for the TSS nor the pooled data for individual intervention‐comparator contrasts provided evidence of a statistically significant advantage of a vitamin D analogue over dithranol or vice versa.
Analysis 11: Vitamin D alone or in combination versus other vitamin D analogue
Our review identified three intervention‐comparator contrasts for body psoriasis in this comparison: calcipotriol versus calcitriol, calcipotriol versus tacalcitol, and calcipotriol versus maxacalcitol (see Analysis 11.2 and Table 14). Three trials involving 563 participants contributed TSS data for all 3 of these intervention‐comparator contrasts. Two trials were between‐patient, and one was within‐patient in design (Barker 1999 (H)). Treatment duration ranged from 8 to 12 weeks. The SMD for the TSS indicated that there was a statistically significant difference in favour of calcipotriol: SMD ‐0.31; 95% CI ‐0.55 to ‐0.06; I² statistic = 46.9%. When assessed by the TSS, calcipotriol was statistically significantly more effective than calcitriol (SMD ‐0.32; 95% CI ‐0.57 to ‐0.07). This finding contrasts with the IAGI assessment from the same study, which found no significant difference (see Ji 2008;Analysis 11.1). Calcipotriol was also significantly more effective than tacalcitol (SMD ‐0.45; 95% CI ‐0.68 to ‐0.22) and similar in efficacy relative to maxacalcitol (SMD 0.13; 95% CI ‐0.41 to 0.68).
11.2. Analysis.
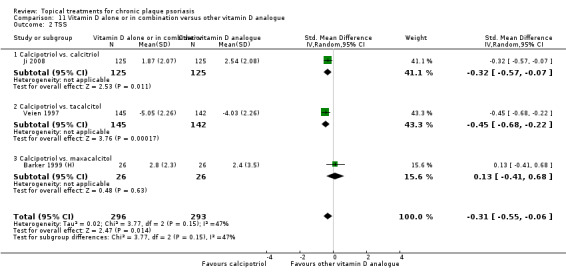
Comparison 11 Vitamin D alone or in combination versus other vitamin D analogue, Outcome 2 TSS.
Analysis 12: Vitamin D alone or in combination versus vitamin D + corticosteroid
Our review identified 12 intervention‐comparator contrasts for body psoriasis, involving 3 vitamin D analogues, 2 combination products, and 7 different corticosteroids (see Analysis 12.1 and Table 15). One 4‐week parallel‐group trial contributed TSS data from 301 participants for 1 of these 12 intervention‐comparator contrasts (Huang 2009). Twice‐daily calcipotriol was found to be statistically significantly less effective than once‐daily treatment with a combined product containing calcipotriol and betamethasone dipropionate (SMD 0.25; 95% CI 0.03 to 0.48).
Analysis 13: Vitamin D alone or in combination versus other treatments: complex regimens
This comparison summarises findings on complex regimens for body psoriasis, defined here as treatment sequences that do not consist of a simple head‐to‐head comparison between two active treatments (see Analysis 13.2 and Table 16). We identified 12 intervention‐comparator contrasts and TSS data were available for one of these. Data from 1 6‐week within‐patient trial with 46 participants (Austad 1998) contributed to the TSS analysis. Austad 1998 compared six weeks of twice‐daily calcipotriol with a regimen of two weeks' treatment with clobetasol propionate followed by four weeks with calcipotriol. The complex regimen was significantly more effective than monotherapy with calcipotriol (SMD 0.63; 95% CI 0.21 to 1.05).
13.2. Analysis.

Comparison 13 Vitamin D alone or in combination versus other treatments: complex regimens, Outcome 2 TSS.
Analysis 14: Vitamin D alone or in combination versus other treatment: long‐term studies (> 24 weeks)
We did not identify any relevant study that provided TSS data for this comparison.
Analysis 15: Vitamin D analogues versus other treatment
This comparison incorporated all other vitamin D head‐to‐head comparisons of treatments for psoriasis of the body (excluding inverse psoriasis) that had not already been included (see Analysis 15.2 and Table 18). We included 12 intervention‐comparator contrasts, with TSS data available for 6 of these contrasts. Five between‐patient trials and 2 within‐patient trials provided data from 898 participants. Trial duration ranged between 6 and 12 weeks. In light of the pharmacological diversity of the comparators, we only pooled data within subgroups.
15.2. Analysis.
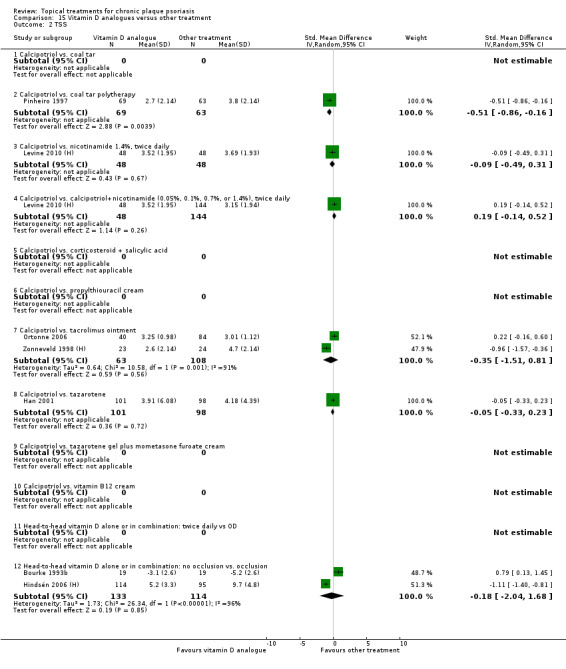
Comparison 15 Vitamin D analogues versus other treatment, Outcome 2 TSS.
According to the TSS assessment, twice‐daily calcipotriol was significantly more effective than coal tar polytherapy (SMD ‐0.51; 95% CI ‐0.86 to ‐0.16; I² statistic = NA). In the remaining five intervention‐comparator contrasts, we found no significant difference between twice‐daily calcipotriol and the comparators.
Analysis 16: Flexural/facial psoriasis: placebo‐controlled trials
This comparison included placebo‐controlled trials of topical treatments for inverse or facial psoriasis (see Analysis 16.2 and Table 19). Evidence on four treatments was found in this comparison: the potent steroid betamethasone valerate; the vitamin D analogue calcipotriol; and two topical calcineurin inhibitors, pimecrolimus and tacrolimus. We found only one placebo‐controlled trial that evaluated tacrolimus ointment (Lebwohl 2004), but the study did not report any effectiveness data suitable for this review. However, the study did contribute data on adverse events and withdrawal rates.
16.2. Analysis.
Comparison 16 Flexural/facial psoriasis: placebo‐controlled trials, Outcome 2 TSS.
TSS data were available for one of the four topical treatments for inverse psoriasis. One 8‐week between‐patient study contributed data on pimecrolimus 1% cream from 57 participants (Gribetz 2004), 10 participants more than those with an investigator's assessment (see Analysis 16.1). Findings from the TSS were consistent with those of the IAGI: the SMD for the TSS also found a statistically significant difference in favour of twice‐daily pimecrolimus (SMD ‐1.37; 95% CI ‐1.95 to ‐0.79).
Analysis 17: Flexural/facial psoriasis: vitamin D alone or in combination versus other treatment
This comparison included head‐to‐head trials of treatments for inverse psoriasis, which compared vitamin D with an active control (see Analysis 17.2 and Table 20). We identified five intervention‐comparator contrasts. Four treatments were compared with calcipotriol: once‐daily betamethasone valerate, combined treatment with calcipotriol and hydrocortisone, calcitriol, and pimecrolimus. Calcitriol was compared with tacrolimus.
17.2. Analysis.
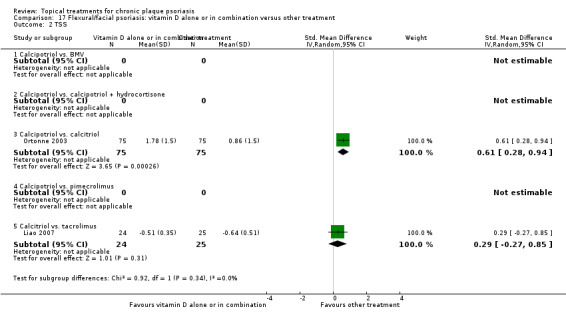
Comparison 17 Flexural/facial psoriasis: vitamin D alone or in combination versus other treatment, Outcome 2 TSS.
Two 6‐week studies contributed TSS data from 124 study participants on 2 of the 5 intervention‐comparator contrasts. Calcipotriol was significantly less effective than calcitriol (SMD 0.61; 95% CI 0.28 to 0.94) (Ortonne 2003). In this within‐patient study, participants applied treatments twice daily to 'sensitive areas', including the face, hairline, retro‐auricular, and flexural areas. When they applied treatments to the facial and genitofemoral areas, there was no significant difference in effect between twice‐daily treatment with calcitriol and tacrolimus (SMD 0.29; 95% CI ‐0.27 to 0.85) (Liao 2007). In this between‐patient study, participants applied both treatments twice daily.
Analysis 18: Scalp psoriasis: placebo‐controlled trials
This comparison included placebo‐controlled trials of treatments for scalp psoriasis (see Analysis 18.2 and Table 21). We included evidence on 11 treatments in this comparison, with TSS data available for 10 treatments. Twelve between‐patient trials contributed data from 2897 participants. Trial duration ranged between two and eight weeks. TSS data were not available for halcinonide (classed as a very potent corticosteroid).
Eight of the 10 treatments for scalp psoriasis that were assessed using the TSS scale were significantly more effective than placebo. The least effective treatment was calcipotriol (SMD ‐0.44; 95% CI ‐0.64 to ‐0.25; I² statistic = 0%), and the most effective were the 2 very potent steroids, amcinonide (SMD ‐1.58; 95% CI ‐1.98 to ‐1.18) and clobetasol propionate (SMD ‐1.53; 95% CI ‐1.77 to ‐1.28; I² statistic = 46.3%). Combination treatment with calcipotriol and betamethasone dipropionate was also effective (SMD ‐0.92; 95% CI ‐1.42 to ‐0.43; I² statistic = 83.2%).
We pooled data from the two potent corticosteroids, betamethasone valerate and betamethasone dipropionate (SMD ‐1.13; 95% CI ‐1.44 to ‐0.81; I² statistic = 52.2%). We also pooled data from the two very potent corticosteroids, amcinonide and clobetasol propionate (SMD ‐1.55; 95% CI ‐1.73 to ‐1.37; I² statistic = 19.6%).
Two treatments were not significantly different to placebo: ciclopirox olamine shampoo (SMD ‐0.07; 95% CI ‐0.82 to 0.68) and salicylic acid (SMD ‐0.57; 95% CI ‐1.47 to 0.32).
Analysis 19: Scalp psoriasis: vitamin D alone or in combination versus other treatment
This comparison included head‐to‐head trials of treatments for scalp psoriasis in which one of the interventions was a vitamin D product (used either as monotherapy or in combination with another product) (see Analysis 19.2 and Table 22).
19.2. Analysis.
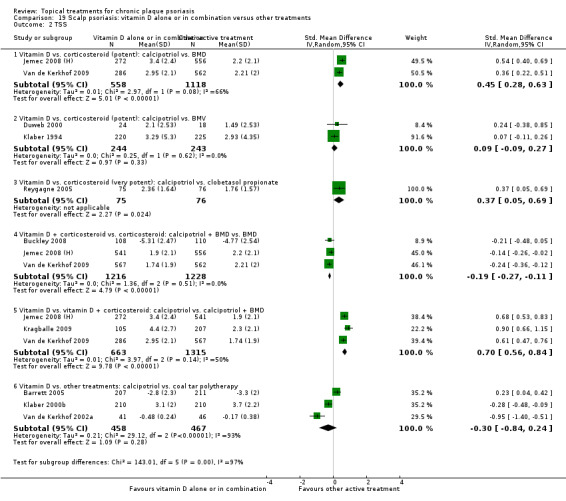
Comparison 19 Scalp psoriasis: vitamin D alone or in combination versus other treatments, Outcome 2 TSS.
We identified six intervention‐comparator contrasts, and TSS data were available for all six of these contrasts. All studies were parallel‐group in design (between‐patient). Eleven studies contributed TSS data from 4877 participants, and trial duration ranged from 4 to 8 weeks.
Based on Total Severity Scores, calcipotriol was significantly less effective than betamethasone dipropionate (SMD 0.45; 95% CI 0.28 to 0.63; I² statistic = 66.3%), clobetasol propionate (SMD 0.37; 95% CI 0.05 to 0.69; I² statistic = NA), and combination treatment with calcipotriol and betamethasone dipropionate (SMD 0.70; 95% CI 0.56 to 0.84; I² statistic = 49.6%). There was no statistically significant difference between calcipotriol and betamethasone valerate (SMD 0.09; 95% CI ‐0.09 to 0.27; I² statistic = 0%). Combination treatment (calcipotriol/betamethasone dipropionate) was significantly more effective than betamethasone dipropionate alone (SMD ‐0.19; 95% CI ‐0.27 to ‐0.11; I² statistic = 0%). The efficacy of calcipotriol and coal tar polytherapy was not significantly different (SMD ‐0.30; 95% CI ‐0.84 to 0.24; I² statistic = 93.1%).
(c) Psoriasis Area and Severity Index (PASI)
Analysis 1: Vitamin D analogues versus placebo
Our review included eight vitamin D analogues for body psoriasis in this comparison (see Analysis 1.3 and Table 5). Nine studies reported PASI data, with 2357 participants contributing data on 2 (calcipotriol and tacalcitol) of these 8 treatments. Eight trials were between‐patient design, and one was a within‐patient study (Dubertret 1992). Treatment duration ranged from three weeks to eight weeks. The average effect size across the 2 treatments for PASI was SMD ‐0.58 (95% CI ‐0.71 to ‐0.45; I² statistic = 42.3%).
1.3. Analysis.
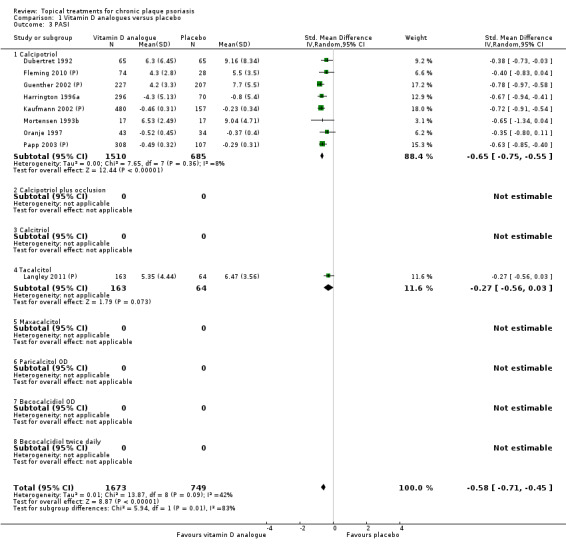
Comparison 1 Vitamin D analogues versus placebo, Outcome 3 PASI.
Analysis 2: Corticosteroid (potent) versus placebo
Our review included 10 potent corticosteroids for body psoriasis in this comparison (see Analysis 2.3 and Table 6). Three between‐patient studies reported PASI data from 1158 participants on once‐daily or twice‐daily doses of betamethasone dipropionate (2 of the 10 potent steroid regimens considered in this group). Trial duration ranged between four and eight weeks. The average PASI effect size across both application frequencies was ‐0.97 (95% CI ‐1.31 to ‐0.62; I² statistic = 79.6%).
2.3. Analysis.
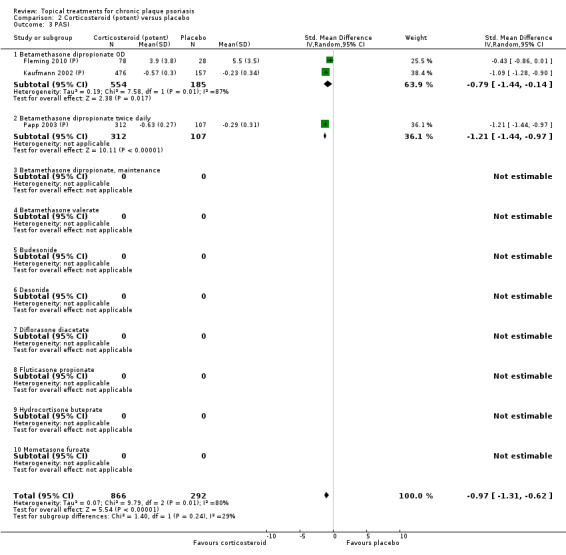
Comparison 2 Corticosteroid (potent) versus placebo, Outcome 3 PASI.
Analysis 3: Corticosteroid (very potent) versus placebo
Our review did not identify any study that compared very potent corticosteroids against placebo and also reported PASI data. This may be because whole‐body application of very potent corticosteroids is not recommended, so a whole‐body assessment measure like the PASI is inappropriate.
Analysis 4: Dithranol versus placebo
Our review did not identify any study that compared dithranol against placebo and also reported PASI data.
Analysis 5: Vitamin D combination products versus placebo
This comparison included treatment with combined calcipotriol and betamethasone dipropionate used either once or twice daily on the body (see Analysis 5.3 and Table 8). Five parallel‐group studies with 2056 participants contributed PASI data on both dosing options. Treatment duration ranged from four to eight weeks. The PASI SMD across treatments was ‐1.24 (95% CI ‐1.53 to ‐0.95; I² statistic = 87.6%). Although twice‐daily combination treatment (SMD ‐1.41; 95% CI ‐1.86 to ‐0.97) achieved a larger effect than once‐daily treatment (SMD ‐1.14; 95% CI ‐1.57 to ‐0.70), the difference was not statistically significant at the 5% level. This finding contrasts with the assessment of the relative benefit of once‐ versus twice‐daily dosing using the IAGI metric (see Analysis 5.1).
Analysis 6: Other treatment versus placebo
This comparison comprised all other treatments for body psoriasis that were not included in the first five comparisons; therefore, we removed pooling. None of the studies assessed the same treatment, which means that findings should be interpreted with caution.
In total, we included 26 treatments in this analysis (see Analysis 6.3 and Table 9). Nine studies with 529 participants reported PASI data on 9 of these 26 treatments. Eight trials were between‐patient studies, and one was within‐patient in design (Vali 2005). Treatment duration ranged from 2 to 12 weeks.
6.3. Analysis.
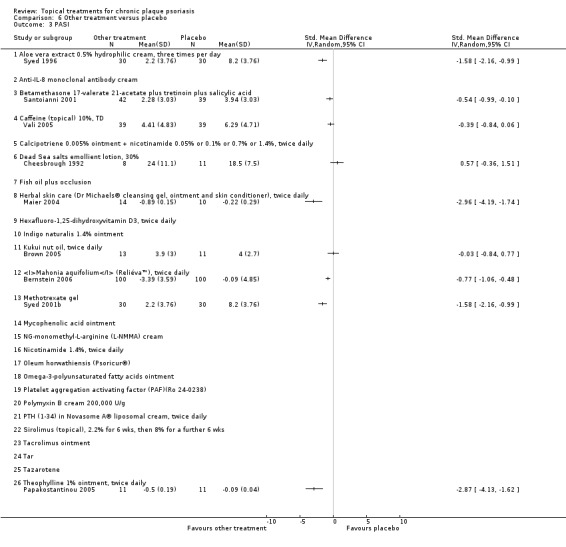
Comparison 6 Other treatment versus placebo, Outcome 3 PASI.
Six treatments performed statistically significantly better than placebo: aloe vera extract, betamethasone 17‐valerate 21‐acetate plus tretinoin plus salicylic acid, a herbal skin care product, Mahonia aquifolium, methotrexate gel, and theophylline ointment. Effects sizes ranged from ‐0.54 (95% CI ‐0.99 to ‐0.10) for the betamethasone 17‐valerate 21 product (Santoianni 2001) to ‐2.96 (95% CI ‐4.19 to ‐1.74) for the herbal skin care treatment (Maier 2004).
In three treatments, we found no statistically significant difference relative to placebo in the PASI assessments. These comprised topical caffeine (Vali 2005), dead sea salts emollient lotion (Cheesbrough 1992), and kukui nut oil (Brown 2005).
Analysis 7: Vitamin D analogues versus corticosteroid (potent)
Our review identified eight vitamin D analogue‐potent corticosteroid comparisons for body psoriasis (see Analysis 7.3 and Table 10). Nine studies with 3185 participants reported PASI data for 4 of the 8 intervention‐comparator contrasts. Seven trials were between‐patient design, and two were within‐patient studies. Treatment duration ranged from four to eight weeks. The PASI SMD across all four intervention‐comparator contrasts indicated that there was no statistically significant difference between the vitamin D derivatives and potent corticosteroid (SMD 0.12; 95% CI ‐0.07 to 0.32; I² statistic = 86.2%).
7.3. Analysis.
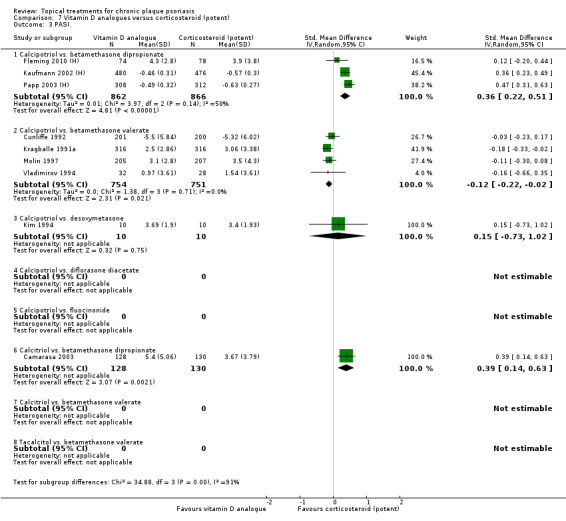
Comparison 7 Vitamin D analogues versus corticosteroid (potent), Outcome 3 PASI.
In one vitamin D analogue versus potent corticosteroid comparison, the vitamin D analogue performed statistically significantly better than the potent corticosteroid: four studies contributed data to analysis of calcipotriol versus betamethasone valerate (SMD ‐0.12; 95% CI ‐0.22 to ‐0.02; I² statistic = 0%).
In two intervention‐comparator contrasts, the vitamin D analogue was statistically significantly less effective than the potent corticosteroid: Betamethasone dipropionate was more effective than both calcipotriol (SMD 0.36; 95% CI 0.22 to 0.51; I² statistic = 49.6%) and calcitriol (SMD 0.39; 95% CI 0.14 to 0.63; I² statistic = NA).
We found no statistically significant difference between calcipotriol and desoxymetasone.
Analysis 8: Vitamin D analogues versus corticosteroid (very potent)
This comparison considered vitamin D analogues against very potent corticosteroids for treating psoriasis of the body (see Analysis 8.3 and Table 11). We found data on one intervention‐comparator contrast: calcipotriol versus clobetasol propionate. One between‐patient trial reported PASI data for 40 participants. This trial had a treatment duration of six weeks (Landi 1993). The SMD for the PASI indicated that there was no statistically significant difference between the very potent corticosteroid and the vitamin D analogue: SMD ‐0.32 (95% CI ‐0.95 to 0.30; I² statistic = NA).
8.3. Analysis.

Comparison 8 Vitamin D analogues versus corticosteroid (very potent), Outcome 3 PASI.
Analysis 9: Vitamin D combined with corticosteroid versus corticosteroid
This comparison considered vitamin D analogue‐steroid combination against potent or very potent corticosteroid for body psoriasis (Table 12). Three between‐patient trials reported PASI data for 1876 participants (see Analysis 9.3). All 3 trials compared combination treatment with calcipotriol and betamethasone dipropionate with betamethasone dipropionate as monotherapy, but varied in their treatment duration (4 to 8 weeks). The SMD for the PASI was ‐0.44 (95% CI ‐0.55 to ‐0.33; I² statistic = 22.4%), indicating a significantly greater effect for combination treatment.
9.3. Analysis.
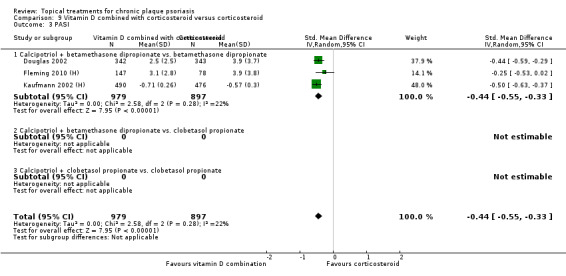
Comparison 9 Vitamin D combined with corticosteroid versus corticosteroid, Outcome 3 PASI.
Analysis 10: Vitamin D alone or in combination versus dithranol
This comparison considered vitamin D analogues against dithranol (see Analysis 10.3 and Table 13). We identified three intervention‐comparator contrasts: calcipotriol versus dithranol, calcitriol versus dithranol, and tacalcitol versus dithranol. Five between‐patient trials reported PASI data for 796 participants. Treatment duration ranged from 8 to 12 weeks. There was some variation in the dithranol regimens employed by trials and in the baseline severity of trial participants. These factors may explain the considerable level of heterogeneity found in the pooled results (Higgins 2011).
10.3. Analysis.
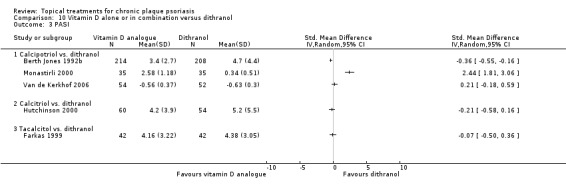
Comparison 10 Vitamin D alone or in combination versus dithranol, Outcome 3 PASI.
The SMD for the PASI was 0.36 (95% CI ‐0.33 to 1.04; I² statistic = 94.5%). Data from three trials contributed to the SMD for the calcipotriol versus dithranol: 0.73 (95% CI ‐0.55 to 2.00; I² statistic = 97.2%) (Berth Jones 1992b; Monastirli 2000; Van de Kerkhof 2006). One of these three trials found a large and statistically significant difference in favour of calcipotriol (Berth Jones 1992b); Monastirli 2000 found a significant difference in favour of dithranol, and the trial by Van de Kerkhof 2006 found no difference between the two treatments. In the light of this heterogeneity, we removed all pooling from this comparison.
Analysis 11: Vitamin D alone or in combination versus other vitamin D analogue
Our review identified three intervention‐comparator contrasts for body psoriasis in this comparison: calcipotriol versus calcitriol, calcipotriol versus tacalcitol, and calcipotriol versus maxacalcitol (see Analysis 11.3 and Table 14). One between‐patient trial involving 15 participants contributed PASI data for the comparison of calcipotriol and calcitriol (Bourke 1997). Treatment duration was eight weeks. Although there was a trend towards a greater effect for calcipotriol, the SMD for the PASI was not statistically significant: ‐1.11 (95% CI ‐2.22 to 0.01; I² statistic = NA).
11.3. Analysis.
Comparison 11 Vitamin D alone or in combination versus other vitamin D analogue, Outcome 3 PASI.
Analysis 12: Vitamin D alone or in combination versus vitamin D + corticosteroid
Our review identified 12 intervention‐comparator contrasts, involving 3 vitamin D analogues, 2 combination products, and 7 different corticosteroids (see Analysis 12.3 and Table 15). Sixteen trials involving 5703 participants contributed PASI data for 11 of these 12 intervention‐comparator contrasts. Fifteen trials were between‐patient, and 1 was within‐patient in design (Salmhofer 2000). Treatment duration ranged from 2 to 12 weeks. We did not identify any PASI data for the comparison of calcipotriol against clobetasol propionate then calcipotriol. Overall, vitamin D plus corticosteroid appeared to be more effective than vitamin D alone: The SMD for the PASI was 0.47 (95% CI 0.34 to 0.59; I² statistic = 82.3%). However, there was considerable variation between the findings of the intervention‐comparator contrasts. In light of the observed heterogeneity, we only pooled subtotals (Higgins 2011).
12.3. Analysis.
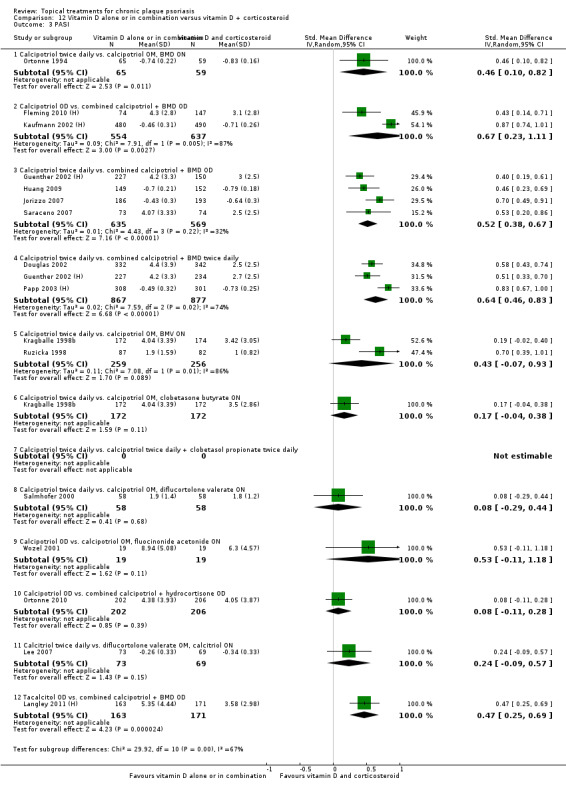
Comparison 12 Vitamin D alone or in combination versus vitamin D + corticosteroid, Outcome 3 PASI.
In six of the intervention‐comparator contrasts, the analysis of the PASI measure found no significant difference between the treatments. This was the case for twice‐daily calcipotriol compared with the following:
a regimen with calcipotriol applied in the morning and the night‐time application of betamethasone valerate (Kragballe 1998b; Ruzicka 1998);
clobetasone butyrate (Kragballe 1998b); or
diflucortolone valerate (Salmhofer 2000).
Once‐daily calcipotriol was no more effective than combined treatment with fluocinonide acetonide (Wozel 2001) or combined with hydrocortisone (Ortonne 2010). Lastly, no significant difference was found between twice‐daily calcitriol and combined treatment with diflucortolone valerate (mornings) and calcitriol (night time) (Lee 2007).
In contrast, combined treatment with calcipotriol and betamethasone dipropionate was significantly more effective than twice‐daily calcipotriol alone. This finding held for regimens involving night‐time applications of betamethasone dipropionate (SMD 0.46; 95% CI 0.10 to 0.82; I² statistic = NA) and for treatment with a combined product, whether applied once daily (SMD 0.52; 95% CI 0.38 to 0.67; I² statistic = 32.3%) or twice daily (SMD 0.64; 95% CI 0.46 to 0.83; I² statistic = 73.6%). Once‐daily treatment with the combined calcipotriol/betamethasone dipropionate product was also significantly more effective than once‐daily calcipotriol (SMD 0.67; 95% CI 0.23 to 1.11; I² statistic = 87.4%) as well as once‐daily tacalcitol (SMD 0.47; 95% CI 0.25 to 0.69; I² statistic = NA).
Analysis 13: Vitamin D alone or in combination versus other treatments: complex regimens
This comparison summarises findings on complex regimens for body psoriasis, defined here as treatment sequences that do not consist of a simple head‐to‐head comparison between two active treatments (see Analysis 13.3 and Table 16). PASI data were available for 11 of the 12 intervention‐comparator contrasts identified. Data from 2991 participants contributed to the PASI analysis, based on findings from 8 between‐patient trials. Trial duration varied between 2 and 12 weeks. Because the interventions and comparators were highly variable and two trials each contributed three pair‐wise contrasts, we did not pool the data.
13.3. Analysis.
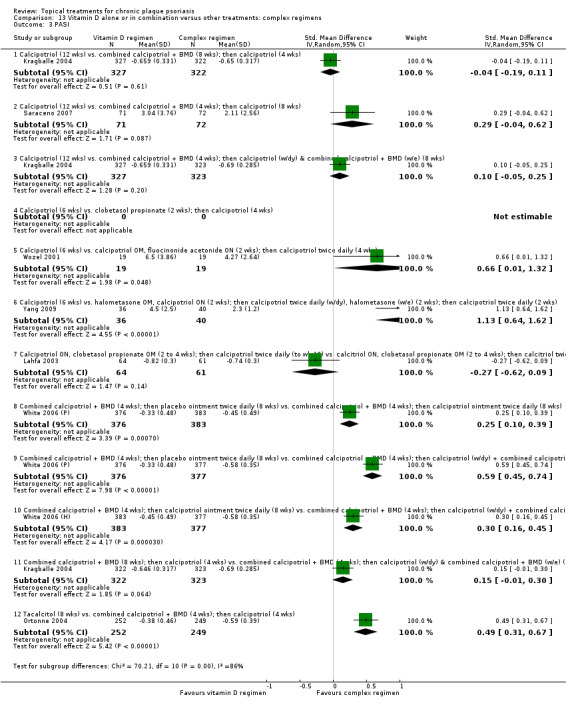
Comparison 13 Vitamin D alone or in combination versus other treatments: complex regimens, Outcome 3 PASI.
Six intervention‐comparator contrasts for which PASI data were available found a significant difference between regimens. A six‐week course of calcipotriol was compared with two different complex regimens. Monotherapy with calcipotriol was significantly less effective than two weeks of treatment with calcipotriol and fluocinonide acetonide followed by four weeks of calcipotriol (SMD 0.66; 95% CI 0.01 to 1.32) (Wozel 2001). Calcipotriol monotherapy was also less effective than treatment with calcipotriol to which halometasone was added (SMD 1.13; 95% CI 0.64 to 1.62) (Yang 2009). Monotherapy with tacalcitol (8 weeks) was less effective than sequential treatment with a combined calcipotriol and betamethasone dipropionate product (4 weeks) followed by calcipotriol monotherapy for a further 4 weeks (SMD 0.49; 95% CI 0.31 to 0.67) (Ortonne 2004). White 2006 (P) compared three regimens, all of which included an initial phase in which participants applied combination treatment with calcipotriol and betamethasone dipropionate once a day for four weeks. The subsequent eight‐week maintenance phase using placebo ointment was significantly less effective than maintenance with either twice‐daily calcipotriol (SMD 0.25; 95% CI 0.10 to 0.39), or maintenance with calcipotriol on weekdays and combination treatment at weekends (SMD 0.59; 95% CI 0.45 to 0.74). When the two active maintenance regimens were compared directly, the alternating (weekday/weekend) regimen was significantly more efficacious (SMD 0.30; 95% CI 0.16 to 0.45) (White 2006 (H)).
In the remaining four intervention‐comparator contrasts, we did not detect any significant difference in the PASI assessments (see Analysis 13.3). PASI assessments in the study by Kragballe 2004 found the difference between two complex regimens not to be significant (SMD 0.15; 95% CI ‐0.01 to 0.30). This result was on the borderline of significance and contrasted with the IAGI assessment in the same study, which was statistically significant in favour of the complex regimen (see Analysis 13.1).
Analysis 14: Vitamin D alone or in combination versus other treatment: long‐term studies (> 24 weeks)
We did not identify any relevant study that provided PASI data for this analysis.
Analysis 15: Vitamin D analogues versus other treatment
This comparison incorporated all other vitamin D head‐to‐head comparisons of treatments for psoriasis of the body (excluding inverse psoriasis) that had not already been included (see Analysis 15.3 and Table 18). We included 12 intervention‐comparator contrasts, with PASI data available for 6 of these contrasts. There were six between‐patient trials and three within‐patient trials, with data for 1228 participants. Trial duration ranged between 4 and 12 weeks. In light of the pharmacological diversity of the comparators, we only pooled data within subgroups (Higgins 2011).
15.3. Analysis.
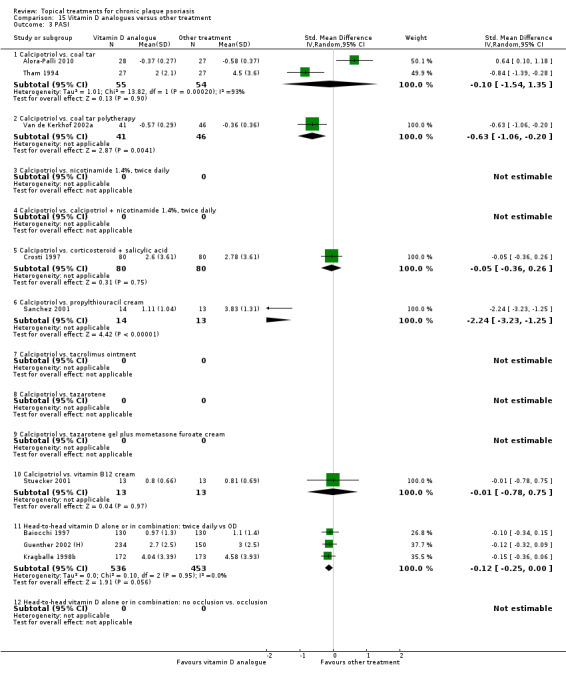
Comparison 15 Vitamin D analogues versus other treatment, Outcome 3 PASI.
According to the PASI assessment, twice‐daily calcipotriol was significantly more effective than coal tar polytherapy (SMD ‐0.63; 95% CI ‐1.06 to ‐0.20; I² statistic = NA) as well as propylthiouracil cream (SMD ‐2.24; 95% CI ‐3.23 to ‐1.25; I² statistic = NA). Twice‐daily calcipotriol was not significantly more effective than coal tar (SMD ‐0.10; 95% CI ‐1.54 to 1.35; I² statistic = 92.8%), betamethasone dipropionate and salicylic acid (SMD ‐0.05; 95% CI ‐0.36 to 0.26; I² statistic = NA), or vitamin B12 cream (SMD ‐0.01; 95% CI ‐0.78 to 0.75; I² statistic = NA). The high level of heterogeneity observed in the pooled analysis of calcipotriol versus coal tar reflects contradictory findings from two studies, one finding in favour of calcipotriol (Tham 1994) and the other in favour of coal tar (Alora‐Palli 2010).
When compared with once‐daily dosing, twice‐daily vitamin D was borderline in demonstrating a significantly better effect (SMD ‐0.12; 95% CI ‐0.25 to 0.00; I² statistic = 0%). The subgroups contributing to this result were dosing comparisons of calcipotriol (SMD ‐0.12; 95% CI ‐0.28 to 0.03; I² statistic = 0%) and combination treatment with calcipotriol and betamethasone dipropionate (SMD ‐0.12; 95% CI ‐0.32 to 0.09; I² statistic = NA).
Analysis 16: Flexural/facial psoriasis: placebo‐controlled trials
This comparison included placebo‐controlled trials of topical treatments for inverse or facial psoriasis (see Analysis 16.3 and Table 19). We found evidence on four treatments in this comparison: the potent steroid betamethasone valerate; the vitamin D analogue calcipotriol; and two topical calcineurin inhibitors, pimecrolimus and tacrolimus. We only identified one placebo‐controlled trial evaluating tacrolimus ointment (Lebwohl 2004), but the study did not report any effectiveness data suitable for this review. However, the study did contribute data on adverse events and withdrawal rates.
16.3. Analysis.
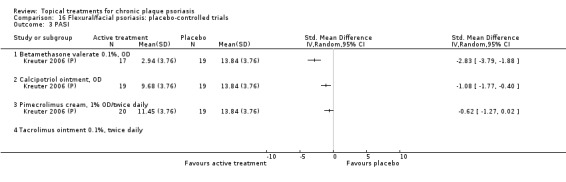
Comparison 16 Flexural/facial psoriasis: placebo‐controlled trials, Outcome 3 PASI.
PASI data were available for 3 of the 4 topical treatments for inverse psoriasis, all reported by 1 4‐week between‐patient study with 75 participants (Kreuter 2006 (P)). In this study, participants applied all treatments once daily. The SMD for the PASI found a statistically significant difference in favour of betamethasone valerate 0.1% (SMD ‐2.83; 95% CI ‐3.79 to ‐1.88) as well as calcipotriol ointment (SMD ‐1.08; 95% CI ‐1.77 to ‐0.40). However, PASI data on once‐daily pimecrolimus cream indicated the difference relative to vehicle was not statistically significant (SMD ‐0.62; 95% CI ‐1.27 to 0.02).
Analysis 17: Flexural/facial psoriasis: vitamin D alone or in combination versus other treatment
This comparison included head‐to‐head trials of treatments for inverse psoriasis, where vitamin D was compared with an active control (see Analysis 17.3 and Table 20). We identified five intervention‐comparator contrasts. We compared four treatments with calcipotriol: once‐daily betamethasone valerate, combined treatment with calcipotriol and hydrocortisone, calcitriol, and pimecrolimus. Calcitriol was compared with tacrolimus.
17.3. Analysis.
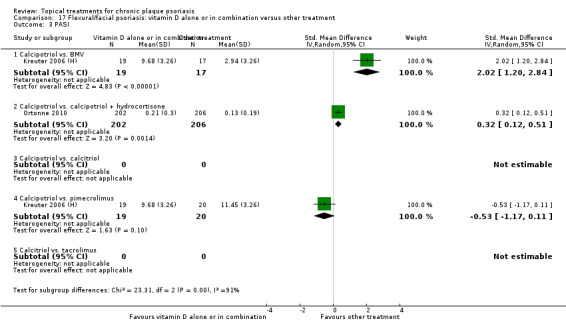
Comparison 17 Flexural/facial psoriasis: vitamin D alone or in combination versus other treatment, Outcome 3 PASI.
Two between‐patient studies contributed PASI data from 464 study participants on 3 of the 5 intervention‐comparator contrasts. Kreuter 2006 (H) found that calcipotriol was significantly less effective than betamethasone valerate (SMD 2.02; 95% CI 1.20 to 2.84), but not significantly different in effect to pimecrolimus (SMD ‐0.53; 95% CI ‐1.17 to 0.11). Participants in the Kreuter 2006 (H) trial applied all treatments once daily. When assessed using the PASI, Ortonne 2010 found that calcipotriol was significantly less effective on inverse psoriasis than combined treatment with calcipotriol and hydrocortisone (SMD 0.32; 95% CI 0.12 to 0.51). This finding was consistent with the IAGI assessment in the same trial (see Analysis 17.1).
Analysis 18: Scalp psoriasis: placebo‐controlled trials
No PASI data were available for this comparison.
Analysis 19: Scalp psoriasis: vitamin D alone or in combination versus other treatment
No PASI data were available for this comparison.
(d) Patient Assessment of overall Global Improvement (PAGI)/Patient Global Assessment of Disease Severity (PGA)
Analysis 1: Vitamin D analogues versus placebo
Our review included eight vitamin D analogues for body psoriasis in this comparison, with PAGI data available for three treatments (see Analysis 1.4 and Table 5). Five studies reported PAGI data, all of which were between‐patient design, and 1467 participants contributed data. In all six studies, treatment duration was eight weeks. The pooled effect across the 3 studies reporting PAGI data was SMD ‐0.54; 95% CI ‐0.72 to ‐0.36; I² statistic = 55.5%.
1.4. Analysis.
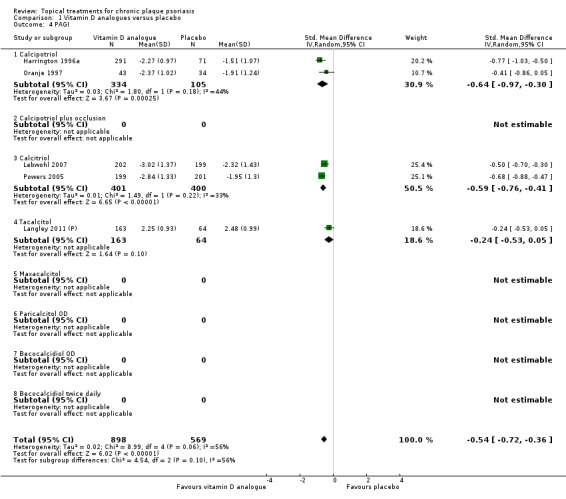
Comparison 1 Vitamin D analogues versus placebo, Outcome 4 PAGI.
Analysis 2: Corticosteroid (potent) versus placebo
Our review did not identify any study that compared potent corticosteroids against placebo and reported PAGI data.
Analysis 3: Corticosteroid (very potent) versus placebo
We included three very potent corticosteroids for body psoriasis in this comparison for this outcome (see Analysis 3.4 and Table 7). Three studies reported PAGI data for 283 participants on 2 of these 3 treatments. There was one between‐patient trial (Lebwohl 2002) and two within‐patient studies. In all three studies, treatment duration was two weeks. The pooled effect across both treatments for PAGI was (SMD ‐1.22; 95% CI ‐1.42 to ‐1.02; I² statistic = 0%). Both treatments performed statistically significantly better than placebo. PAGI data on the use of very potent steroids on the scalp are reported in Analysis 18.4.
3.4. Analysis.
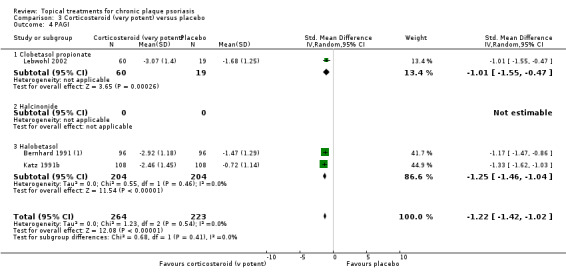
Comparison 3 Corticosteroid (very potent) versus placebo, Outcome 4 PAGI.
18.4. Analysis.
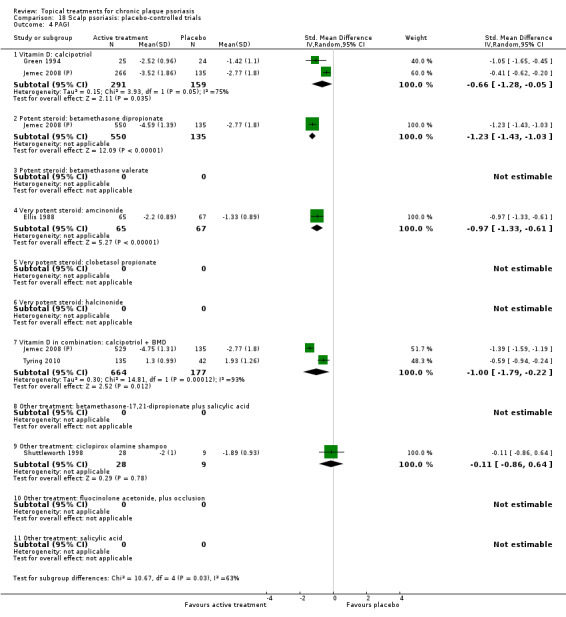
Comparison 18 Scalp psoriasis: placebo‐controlled trials, Outcome 4 PAGI.
Analysis 4: Dithranol versus placebo
Our review did not identify any study comparing dithranol against placebo and that reported PAGI data.
Analysis 5: Vitamin D combination products versus placebo
This comparison included treatment with combined calcipotriol and betamethasone dipropionate used either once or twice daily on the body (see Analysis 5.4 and Table 8). None of the studies of twice‐daily treatment reported PAGI data, but one parallel‐group study with 235 participants contributed data on once‐daily treatment (Langley 2011 (P)). Treatment duration was eight weeks. The PAGI SMD was ‐0.69 (95% CI ‐0.98 to ‐0.40; I² statistic = NA). Placebo‐controlled trials of combination vitamin D/steroid treatments for scalp psoriasis are reported in Analysis 18.4.
5.4. Analysis.
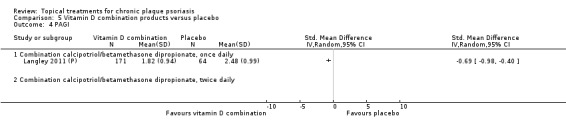
Comparison 5 Vitamin D combination products versus placebo, Outcome 4 PAGI.
Analysis 6: Other treatment versus placebo
This comparison comprised all other treatments for body psoriasis that we did not include in the first five comparisons; therefore, we removed pooling. None of the studies assessed the same treatment, which means that findings should be interpreted with caution.
In total, we included 26 treatments in this analysis (see Analysis 6.4 and Table 9). Two studies with 105 participants reported PAGI data on 2 of these 26 treatments. Both trials were between‐patient studies. Treatment duration ranged from 3 to 12 weeks. According to participants' assessments (PAGI), betamethasone 17‐valerate 21 acetate plus tretinoin plus salicylic acid performed significantly better than placebo (SMD ‐0.80; 95% CI ‐1.26 to ‐0.35), whilst kukui nut oil was no more effective than placebo (SMD 0.00; 95% CI ‐0.80 to 0.80). These findings were very similar to the investigators' assessments (see Analysis 6.1).
6.4. Analysis.
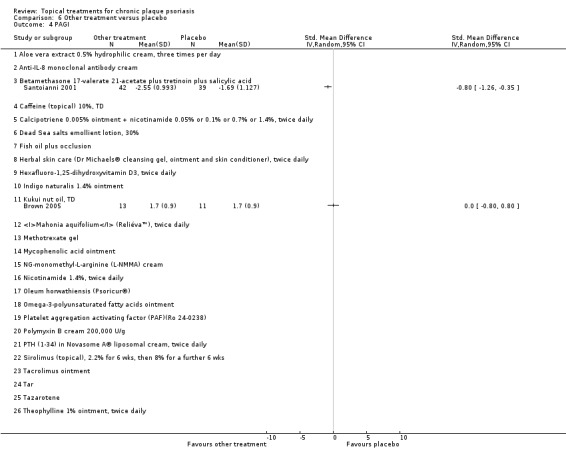
Comparison 6 Other treatment versus placebo, Outcome 4 PAGI.
Analysis 7: Vitamin D analogues versus corticosteroid (potent)
Our review identified eight vitamin D analogue‐potent corticosteroid comparisons for body psoriasis (see Analysis 7.4 and Table 10). Two studies with 738 participants reported PAGI data for 1 of these 8 comparisons. One trial was a parallel‐group study (Cunliffe 1992), and one was a within‐patient study (Kragballe 1991a), both with a treatment duration of six weeks. The PAGI SMD indicated that the vitamin D analogue calcipotriol was significantly more effective than betamethasone valerate (SMD ‐0.26; 95% CI ‐0.38 to ‐0.14; I² statistic = 0%).
7.4. Analysis.
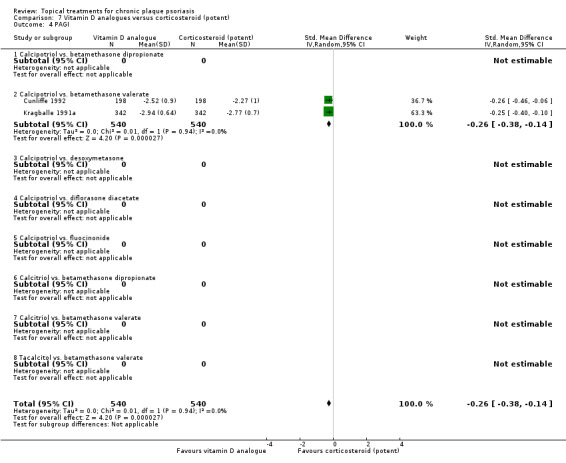
Comparison 7 Vitamin D analogues versus corticosteroid (potent), Outcome 4 PAGI.
Analysis 8: Vitamin D analogues versus corticosteroid (very potent)
This comparison included one vitamin D analogue and a very potent corticosteroid contrast, calcipotriol ointment versus clobetasol propionate foam for body psoriasis (see Table 11 and Analysis 8.4). One study with 42 participants reported PAGI data. Koo 2006 was a between‐patient study with a treatment duration of two weeks. Findings for the participant‐reported assessment (SMD 0.42; 95% CI ‐0.20 to 1.03) were similar, the investigators' assessment from the same study (SMD 0.19; 95% CI ‐0.42 to 0.80; see Analysis 8.1).
8.4. Analysis.

Comparison 8 Vitamin D analogues versus corticosteroid (very potent), Outcome 4 PAGI.
Analysis 9: Vitamin D combined with corticosteroid versus corticosteroid
We found one study comparing a vitamin D analogue‐steroid combination against corticosteroids for body psoriasis that reported PAGI/PGA data (see Table 12 and Analysis 9.4). This two‐week between‐patient study reported PAGI data for 65 participants (Koo 2006), comparing combined treatment with calcipotriol and clobetasol propionate against the very potent corticosteroid alone. According to the participants' assessment (PAGI), there was no statistically significant difference between the treatment options (SMD ‐0.28; 95% CI ‐0.80 to 0.24). This finding contrasts with the investigators' assessment (IAGI), which found in favour of combination treatment (SMD ‐0.69; 95% CI ‐1.22 to ‐0.15) (see Analysis 9.1).
9.4. Analysis.
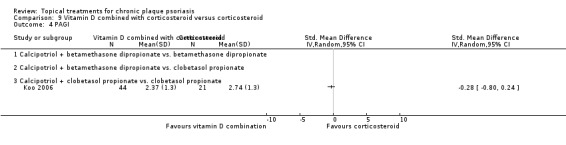
Comparison 9 Vitamin D combined with corticosteroid versus corticosteroid, Outcome 4 PAGI.
Analysis 10: Vitamin D alone or in combination versus dithranol
This comparison considered vitamin D analogues against dithranol (see Analysis 10.4 and Table 13). We identified three intervention‐comparator contrasts: calcipotriol versus dithranol, calcitriol versus dithranol, and tacalcitol versus dithranol. PAGI data were available only for the intervention‐comparator contrast of calcipotriol versus dithranol. Two between‐patient trials reported PAGI data for 544 participants (Berth Jones 1992b; Van de Kerkhof 2006). Treatment duration ranged from 8 to 12 weeks. The SMD for the PAGI was ‐0.05 (95% CI ‐0.90 to 0.80; I² statistic = 92.5%), indicating no statistically significant advantage for calcipotriol or dithranol according to the participants' assessment.
10.4. Analysis.
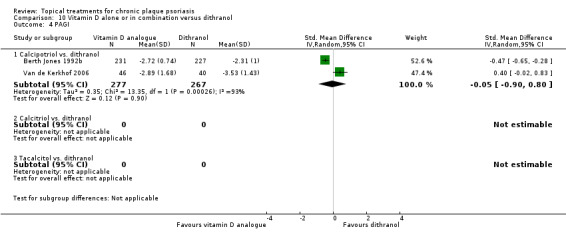
Comparison 10 Vitamin D alone or in combination versus dithranol, Outcome 4 PAGI.
Analysis 11: Vitamin D alone or in combination versus other vitamin D analogue
Our review identified three intervention‐comparator contrasts for body psoriasis in this comparison: calcipotriol versus calcitriol, calcipotriol versus tacalcitol, and calcipotriol versus maxacalcitol (see Analysis 11.4 and Table 14). One between‐patient trial involving 250 participants contributed PAGI data for the comparison of calcipotriol and calcitriol (Ji 2008). Treatment duration was 12 weeks. The SMD for the PAGI indicated that there was no statistically significant difference between calcipotriol and calcitriol (SMD 0.04; 95% CI ‐0.21 to 0.29; I² statistic = NA). This study used three measures to assess the effects of calcipotriol and calcitriol. The investigators' assessment supported the participant assessment (no difference) (see Analysis 11.1), but the TSS found a significant difference in favour of calcipotriol (Analysis 11.2).
11.4. Analysis.
Comparison 11 Vitamin D alone or in combination versus other vitamin D analogue, Outcome 4 PAGI.
Analysis 12: Vitamin D alone or in combination versus vitamin D + corticosteroid
Our review identified 12 intervention‐comparator contrasts for body psoriasis, involving 3 vitamin D analogues, 2 combination products, and 7 different corticosteroids (see Analysis 12.4 and Table 15). Two parallel‐group trials contributed PAGI data from 399 participants for 2 of these 12 intervention‐comparator contrasts. Treatment duration ranged between two and eight weeks. According to the participant assessment, twice‐daily calcipotriol was significantly less effective than twice‐daily treatment with calcipotriol and clobetasol propionate (SMD 0.70; 95% CI 0.16 to 1.23). Treatment with tacalcitol was also less effective than treatment with a combined product containing calcipotriol and betamethasone dipropionate, when both treatments were applied once daily (SMD 0.46; 95% CI 0.24 to 0.68). The pooled SMD for these two trials (0.49; 95% CI 0.29 to 0.69; I² statistic = 0%) gave greater weight to the comparison with tacalcitol, since this was the larger trial (334 participants; Langley 2011 (H)).
12.4. Analysis.

Comparison 12 Vitamin D alone or in combination versus vitamin D + corticosteroid, Outcome 4 PAGI.
Analysis 13: Vitamin D alone or in combination versus other treatments: complex regimens
This comparison summarises findings on complex regimens for body psoriasis, defined here as treatment sequences that do not consist of a simple head‐to‐head comparison between two active treatments (see Analysis 13.4 and Table 16). We identified 12 intervention‐comparator contrasts and data from the Patient Assessment of Global Improvement (PAGI) or Patient Global Assessment of Disease Severity (PGA) were available for 7 of these. Data from 2508 participants contributed to the PAGI analysis, based on findings from 4 between‐patient trials. Trial duration varied between 8 and 12 weeks. Because the interventions and comparators were highly variable and two trials each contributed three pair‐wise contrasts, we did not pool the data.
13.4. Analysis.
Comparison 13 Vitamin D alone or in combination versus other treatments: complex regimens, Outcome 4 PAGI.
Four intervention‐comparator contrasts for which PAGI data were available found a significant difference between regimens. Ortonne 2004 found that four weeks of treatment with a combination product of calcipotriol and betamethasone dipropionate, followed by monotherapy with calcipotriol for four weeks, was significantly more effective than monotherapy with tacalcitol (SMD 0.54; 95% CI 0.36 to 0.72). Findings from the patient‐reported outcome were identical to the investigators' assessment (Analysis 13.1). The trial by White 2006 (H)/White 2006 (P) compared three regimens. All included an initial phase in which participants applied once‐daily combination treatment with calcipotriol and betamethasone dipropionate for four weeks, but differed in their subsequent eight‐week maintenance phase. According to the participants' assessment, maintenance therapy with twice‐daily placebo ointment was significantly less effective than maintenance with either calcipotriol ointment (SMD 0.28; 95% CI 0.13 to 0.42) or maintenance with calcipotriol on weekdays and combination treatment at weekends (SMD 0.71; 95% CI 0.56 to 0.85). We compared the 2 regimens with active maintenance options directly; the alternating (weekday/weekend) approach was significantly more effective (SMD 0.44; 95% CI 0.29 to 0.58). These findings, based on the participants' assessment of severity, were aligned with those of both the investigators' assessment (Analysis 13.1) and the PASI assessment (Analysis 13.3).
A head‐to‐head comparison of two complex regimens found a significant difference (Kragballe 2004). Once‐daily combination treatment (calcipotriol/betamethasone dipropionate) for 8 weeks followed by once‐daily calcipotriol for 4 weeks was significantly less effective than a regimen consisting of combined treatment for 4 weeks, followed by 8 weeks of once‐daily therapy with calcipotriol on weekdays and combined therapy at weekends (SMD 0.23; 95% CI 0.07 to 0.39).
In three of the seven intervention‐comparator contrasts, no significant difference was found. Kragballe 2004 also compared 12 weeks of calcipotriol monotherapy with the 2 complex regimens described in the paragraph above. Compared with monotherapy, participants who applied once‐daily combination treatment (calcipotriol/betamethasone dipropionate) for 8 weeks followed by once‐daily calcipotriol for 4 weeks did not experience a significantly greater improvement in their psoriasis (SMD ‐0.14; 95% CI ‐0.30 to 0.02). Similarly, the psoriasis of participants on monotherapy with twice‐daily calcipotriol did not improve significantly differently compared to those who applied once‐daily combination treatment (calcipotriol/betamethasone dipropionate) for 4 weeks, followed by 8 weeks using calcipotriol once daily (weekdays) and combination therapy (weekends) (SMD 0.10; 95% CI ‐0.06 to 0.26).
Analysis 14: Vitamin D alone or in combination versus other treatment: long‐term studies (> 24 weeks)
We did not identify any relevant study that provided PAGI data for this analysis.
Analysis 15: Vitamin D analogues versus other treatment
This comparison incorporated all other vitamin D head‐to‐head comparisons of treatments for psoriasis of the body (excluding inverse psoriasis) that we had not already included (see Analysis 15.4 and Table 18). We included 12 intervention‐comparator contrasts, with PAGI data available for 6 of these contrasts: 3 between‐patient trials and 3 within‐patient trials data for 456 participants. Trial duration ranged between 4 and 12 weeks. In light of the pharmacological diversity of the comparators, we only pooled data within subgroups.
15.4. Analysis.
Comparison 15 Vitamin D analogues versus other treatment, Outcome 4 PAGI.
According to the participants' assessment, twice‐daily calcipotriol was significantly more effective than three comparators, with evidence on each based on a single trial. Calcipotriol was more effective than coal tar both as monotherapy (SMD ‐1.51; 95% CI ‐2.12 to ‐0.90; Tham 1994) and as polytherapy (SMD ‐0.56; 95% CI ‐0.99 to ‐0.13; Van de Kerkhof 2002a), and more effective than betamethasone dipropionate and salicylic acid (SMD ‐0.49; 95% CI ‐0.79 to ‐0.20; Scarpa 1994). No significant difference was evident when calcipotriol was compared to tacrolimus ointment (Ortonne 2006), tazarotene (Tzung 2005), or vitamin B12 cream (Stuecker 2001).
Analysis 16: Flexural/facial psoriasis: placebo‐controlled trials
This comparison included placebo‐controlled trials of topical treatments for inverse or facial psoriasis (see Analysis 16.4 and Table 19). We found evidence on four treatments in this comparison: the potent steroid betamethasone valerate; the vitamin D analogue calcipotriol; and two topical calcineurin inhibitors, pimecrolimus and tacrolimus. We identified only one placebo‐controlled trial evaluating tacrolimus ointment (Lebwohl 2004), but the study did not report any effectiveness data suitable for this review. However, the study did contribute data on adverse events and withdrawal rates.
16.4. Analysis.
Comparison 16 Flexural/facial psoriasis: placebo‐controlled trials, Outcome 4 PAGI.
PAGI (patient‐assessment) data were available for one of the four topical treatments for inverse psoriasis. One 8‐week between‐patient study (Gribetz 2004) reported data from 47 participants. Relative to placebo, the SMD for the PAGI found a statistically significant difference in favour of twice‐daily pimecrolimus cream (SMD ‐0.65; 95% CI ‐1.24 to ‐0.06).
Analysis 17: Flexural/facial psoriasis: vitamin D alone or in combination versus other treatment
We did not identify any relevant study that provided PAGI data for this analysis.
Analysis 18: Scalp psoriasis: placebo‐controlled trials
This comparison included placebo‐controlled trials of treatments for scalp psoriasis (see Analysis 18.4 and Table 21). We included evidence on 11 treatments in this comparison, with PAGI data available for 5 treatments. Five between‐patient trials contributed data from 1875 participants. Trial duration ranged between three and eight weeks.
Four of the five treatments for scalp psoriasis that were evaluated using the Patient Global Assessment Scale were significantly more effective than placebo. The least effective treatment was calcipotriol (SMD ‐0.66; 95% CI ‐1.28 to ‐0.05; I² statistic = 74.5%), and the most effective was betamethasone dipropionate (SMD ‐1.23; 95% CI ‐1.43 to ‐1.03; I² statistic = NA). Effects for the very potent steroid amcinonide (SMD ‐0.97; 95% CI ‐1.33 to ‐0.61 I² statistic = NA) and combination treatment with calcipotriol and betamethasone dipropionate (SMD ‐1.00; 95% CI ‐1.79 to ‐0.22; I² statistic = 93.2%) fell between these two extremes. There were no data suitable for pooling across subcategories.
Only for ciclopirox olamine shampoo was the difference relative to placebo found to be non‐significant: SMD ‐0.11 (95% CI ‐0.86 to 0.64; I² statistic = NA).
Analysis 19: Scalp psoriasis: vitamin D alone or in combination versus other treatment
This comparison included head‐to‐head trials of treatments for scalp psoriasis in which one of the interventions was a vitamin D product (used either as monotherapy or in combination with another product) (see Analysis 19.4 and Table 22).
19.4. Analysis.
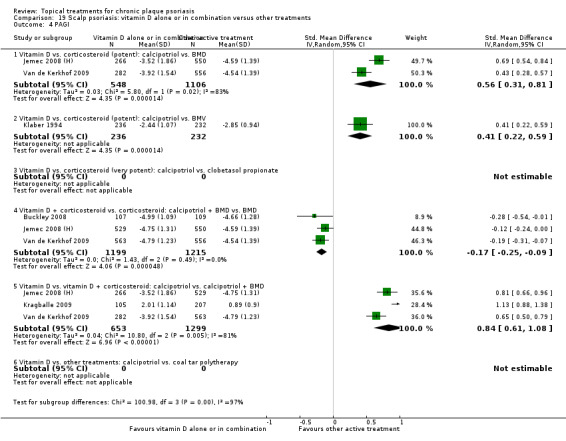
Comparison 19 Scalp psoriasis: vitamin D alone or in combination versus other treatments, Outcome 4 PAGI.
We identified six intervention‐comparator contrasts, and PAGI data were available for four of these contrasts (there were no participant‐assessed outcomes data for the comparison of calcipotriol against either clobetasol propionate or against coal tar polytherapy). All studies were parallel‐group in design (between‐patient). Six studies contributed PAGI data from 3742 participants, and trial duration ranged from 4 to 8 weeks.
Based on the participant assessment scores, calcipotriol was significantly less effective than betamethasone dipropionate (SMD 0.56; 95% CI 0.31 to 0.81; I² statistic = 82.8%), betamethasone valerate (SMD 0.41; 95% CI 0.22 to 0.59; I² statistic = NA), and combination treatment with calcipotriol and betamethasone dipropionate (SMD 0.84; 95% CI 0.61 to 1.08; I² statistic = 81.5%). Combination treatment (calcipotriol/betamethasone dipropionate) was significantly more effective than betamethasone dipropionate alone (SMD ‐0.17; 95% CI ‐0.25 to ‐0.09; I² statistic = 0%).
(e) Combined end point (IAGI/TSS/PASI/PAGI)
Analysis 1: Vitamin D analogues versus placebo
Our review included eight vitamin D analogues for body psoriasis in this comparison (see Analysis 1.5 and Table 5). Thirty studies, involving 4986 participants, contributed data. Eighteen trials were between‐patient design, and 12 were within‐patient studies. Treatment duration ranged from 3 to 12 weeks. Six studies had adequately concealed treatment allocation. The pooled effect across all 30 studies was ‐0.90 (95% CI ‐1.07 to ‐0.72; I² statistic = 87.5%), which equates to 1.03 on a 6‐point IAGI scale. However, the summary statistic conceals considerable variation between treatments. In two treatments, the effect was not statistically significantly different to placebo (twice‐daily calcipotriol plus occlusion for 2 weeks followed by 4 weeks with no treatment, and once‐daily becocalcidiol). In treatments that were significantly more effective than placebo, the effect ranged from ‐0.67 (twice‐daily becocalcidiol) to ‐1.66 (once‐daily paricalcitol); on a 6‐point IAGI scale, these effects translate into 0.80 and 1.91 points, respectively. However, given the high level of heterogeneity found in the meta‐analysis, it may be more informative to look at individual products within the class, rather than treating this class as a single group. We removed the pooling across the class, so that we only pooled subtotals.
1.5. Analysis.
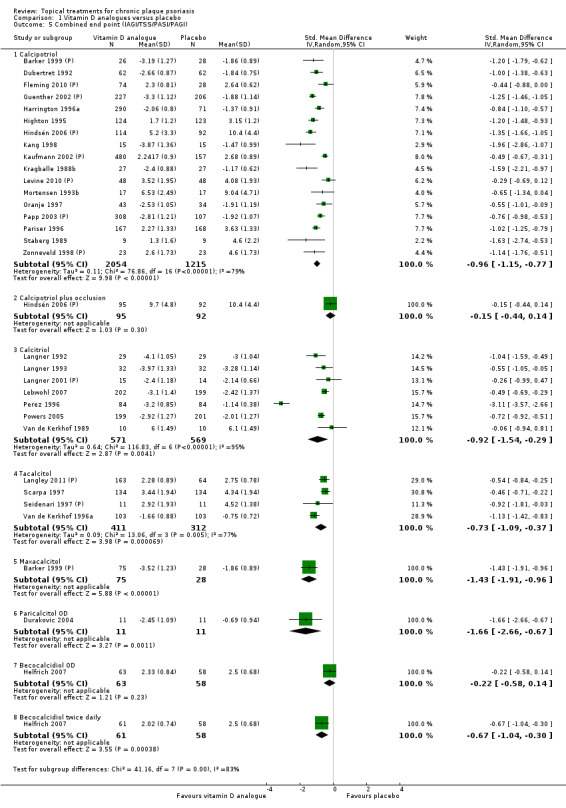
Comparison 1 Vitamin D analogues versus placebo, Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).
We reported placebo‐controlled trials of vitamin D products for scalp psoriasis (Analysis 18.5) and inverse psoriasis (Analysis 16.5) elsewhere.
18.5. Analysis.
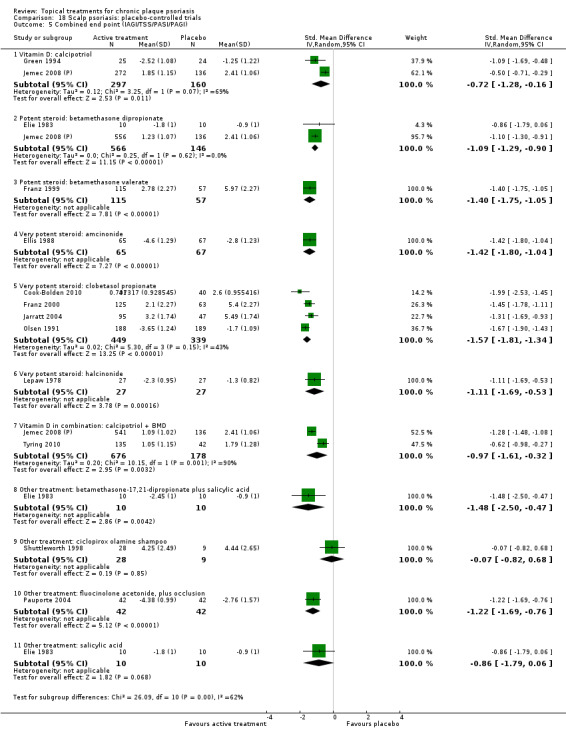
Comparison 18 Scalp psoriasis: placebo‐controlled trials, Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).
16.5. Analysis.
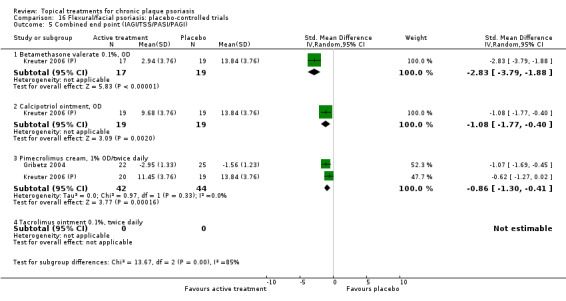
Comparison 16 Flexural/facial psoriasis: placebo‐controlled trials, Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).
Sensitivity analyses
We explored differences in within‐patient and between‐patient designs using one‐way sensitivity analysis (Table 5). The SMD for the 12 within‐patient studies (600 participants) was ‐1.11 (95% CI ‐1.58 to ‐0.64; I² statistic = 91.5%). This translates into a change of 1.27 on a 6‐point IAGI scale, slightly larger than the effect size for all studies. The SMD for the 18 between‐patient studies (4386 participants) was ‐0.80 (95% CI ‐0.96 to ‐0.63; I² statistic = 83.2%). This translates into a change of 0.91 on a 6‐point IAGI scale, slightly smaller than the effect size for the within‐patient studies.
We also used sensitivity analysis to explore the impact of varying the correlation coefficient (rho) for the 12 within‐patient studies. As no trial in the review reported this statistic, we tested values of 0, 0.25, 0.50, and 0.75. Varying the value of rho for the within‐patient studies had no significant effect on the findings: The pooled SMD ranged from ‐0.85 (95% CI ‐1.00 to ‐0.71; I² statistic = 87.8%; 30 studies), when we assumed the correlation to be zero, to ‐0.91 (95% CI ‐1.07 to ‐0.75; I² statistic = 93.2%; 30 studies), when we assumed the correlation to be 0.75.
The study by Perez 1996 comparing calcitriol and placebo reported large and statistically significant differences for both TSS and IAGI outcomes. In both outcomes, the magnitude of the effect was the largest across all comparisons and treatments. Perez 1996 was a 10‐week within‐patient study involving 84 participants. The study included people with severe disease (mean TSS at baseline: 7.6 on a 10‐point scale, with at least 10% of body surface area affected) who had previously had an unsatisfactory response to at least 1 previous treatment including topical steroids, UVB, PUVA, and methotrexate. The dramatic improvement observed in the intervention group is difficult to interpret in the context of findings from other trials, and we explored the impact of removing this study in a sensitivity analysis. When we removed Perez 1996 from the pooled analysis for calcitriol, the effect size was smaller but still statistically significantly different from placebo: SMD with Perez 1996 was ‐0.92 (95% CI ‐1.54 to ‐0.29; I² statistic = 94.9%; 7 studies), and SMD without Perez 1996 was ‐0.60 (95% CI ‐0.78 to ‐0.41; I² statistic = 30%; 6 studies). On a 6‐point IAGI scale, these effect sizes equate to 1.05 and 0.68, respectively (Table 5).
The trials of calcipotriol varied by dose, treatment duration, and dosing frequency; where trials reported more than one dose (Kragballe 1988b) or type of vehicle (Harrington 1996a), we estimated the weighted mean and standard deviation across the trial. The effect size for twice‐daily regimens was ‐1.02 (95% CI ‐1.23 to ‐0.82 I² statistic = 73.5%; 13 studies); this equates to 1.18 on a 6‐point IAGI scale. For once‐daily calcipotriol, the corresponding figures were ‐0.76 (95% CI ‐1.13 to ‐0.40; I² statistic = 82.3%; 4 studies) and 0.87 on a 6‐point IAGI scale. Therefore, both dosing frequencies were more effective than placebo, but once‐daily applications had a smaller effect (Table 5).
Analysis 2: Corticosteroid (potent) versus placebo
Our review identified 10 potent corticosteroids for body psoriasis in this comparison (see Analysis 2.5 and Table 6). The treatments included three betamethasone dipropionate regimens, but we identified no effectiveness evidence for budesonide and no study reporting PAGI data. Therefore, 13 studies involving 2216 participants contributed data on 9 of the 10 treatments. Eleven trials were between‐patient design, and two were within‐patient studies. Treatment allocation was adequately concealed in four studies. Treatment duration ranged from 2 to 12 weeks. Participant numbers for individual studies ranged from 9 (Wortzel 1975 (2)) to 633 (Kaufmann 2002 (P)). The pooled effect across all 13 studies was ‐0.89 (95% CI ‐1.06 to ‐0.72; I² statistic = 65.1%). This equates to 1.02 on a 6‐point IAGI scale. With the exception of diflorasone diacetate (Lane 1983), all potent corticosteroids were significantly more effective than placebo at the 5% level of significance. We reported elsewhere placebo‐controlled trials of potent corticosteroid products for scalp psoriasis (Analysis 18.5) and inverse psoriasis (Analysis 16.5).
2.5. Analysis.
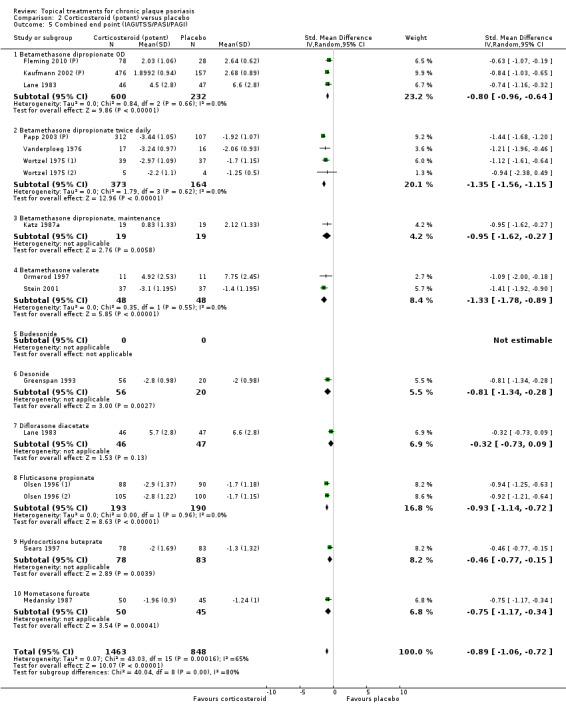
Comparison 2 Corticosteroid (potent) versus placebo, Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).
Sensitivity analyses
We explored differences in within‐patient and between‐patient designs using one‐way sensitivity analysis (Table 6). The SMD for the 2 within‐patient studies was ‐1.33 (95% CI ‐1.78 to ‐0.89; I² statistic = 0%). This translates into a change of 1.53 on a 6‐point IAGI scale, which is larger than the effect for all studies. However, as just 48 participants contributed data to this analysis, robust inferences cannot be drawn. The SMD for the 11 between‐patient studies (2168 participants) was ‐0.85 (95% CI ‐1.03 to ‐0.67; I² statistic = 66.7%). This translates into a change of 0.98 on a 6‐point IAGI scale, slightly smaller than the effect for all studies.
We also used sensitivity analysis to explore the impact of varying the correlation coefficient (rho) for the 2 within‐patient studies. As no trial in the review reported this statistic, we tested values of 0, 0.25, 0.50, and 0.75. Varying the value of rho for the within‐patient studies had no significant effect on the findings: the pooled SMD ranged from ‐0.89 (95% CI ‐1.06 to ‐0.72; I² statistic = 77.7%; 14 studies), when we assumed the correlation was to be zero, to ‐0.91 (95% CI ‐1.08 to ‐0.74; I² statistic = 80.2%; 13 studies), when we assumed the correlation to be 0.75.
Analysis 3: Corticosteroid (very potent) versus placebo
Our review identified three very potent corticosteroids for body psoriasis in this comparison (see Analysis 3.5 and Table 7), but effectiveness data were not available for one of these treatments (halcinonide). Ten studies reported data for 1264 participants. There were seven between‐patient trials and three within‐patient studies. Treatment duration ranged from two to four weeks. In no study was treatment allocation adequately concealed. The SMD across the 2 treatments was ‐1.56 (95% CI ‐1.87 to ‐1.26; I² statistic = 81.7%; 10 studies). This equates to 1.80 points on a 6‐point IAGI scale. Both clobetasol propionate (SMD ‐1.65; 95% CI ‐2.10 to ‐1.20; I² statistic = 86.3%; 7 studies) and halobetasol (SMD ‐1.36; 95% CI ‐1.65 to ‐1.07 I² statistic = 47.1%; 3 studies) performed significantly better than placebo.
3.5. Analysis.
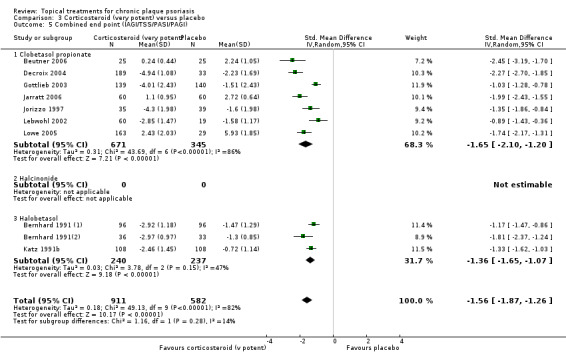
Comparison 3 Corticosteroid (very potent) versus placebo, Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).
We reported elsewhere placebo‐controlled trials of very potent corticosteroid products for scalp psoriasis (Analysis 18.5).
Sensitivity analyses
We explored differences in within‐patient and between‐patient designs using one‐way sensitivity analysis (Table 7). The SMD for the 3 within‐patient studies (229 participants) was ‐1.52 (95% CI ‐2.02 to ‐1.02; I² statistic = 79.3%). This translates into a change of 1.74 on a 6‐point IAGI scale. The SMD for the 7 between‐patient studies (1035 participants) was ‐1.58 (95% CI ‐1.99 to ‐1.17; I² statistic = 84.4%). This translates into a change of 1.81 on a 6‐point IAGI scale.
We also used sensitivity analysis to explore the impact of varying the correlation coefficient (rho) for the 3 within‐patient studies. As no trial in the review reported this statistic, we tested values of 0, 0.25, 0.50, and 0.75. Varying the value of rho for the within‐patient studies had no significant effect on the findings: The pooled SMD ranged from ‐1.52 (95% CI ‐1.80 to ‐1.24; I² statistic = 81.6%; 10 studies), when we assumed the correlation to be zero, to ‐1.55 (95% CI ‐1.80 to ‐1.29; I² statistic = 85.9%; 10 studies), when we assumed the correlation to be 0.75.
Analysis 4: Dithranol versus placebo
This comparison considered dithranol against placebo (see Analysis 4.5 and Table 23). Three within‐patient trials reported data for 47 participants. The only outcome reported by these trials was the TSS, so results for the combined end point are identical to the TSS results section. The types of treatment comprised the following: dithranol 0.1% in a carbamide (17% urea) base twice daily (Buckley 1978), dithranol in aqueous gel (dose titration 0.1% to 2.0%) twice daily (Grattan 1997 (P)), and dithranol 2% ointment 'one minute therapy' once daily (Jekler 1992).
4.5. Analysis.
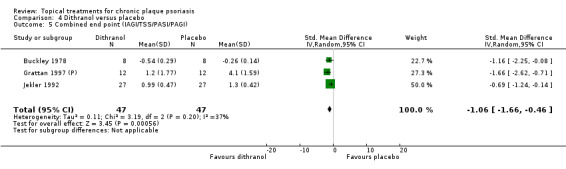
Comparison 4 Dithranol versus placebo, Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).
Treatment duration ranged from three to eight weeks. In all three studies, the adequacy of the concealment of treatment allocation was unclear. The SMD for the combined end point was ‐1.06 (95% CI ‐1.66 to ‐0.46; I² statistic = 37.4%; 3 studies), which equates to 1.22 on a 6‐point IAGI scale. All three trials found a statistically significant effect in favour of dithranol.
Sensitivity analysis
We used sensitivity analysis to explore the impact of varying the correlation coefficient (rho) for the 3 within‐patient studies. As no trial in the review reported this statistic, we tested values of 0, 0.25, 0.50, and 0.75. Varying the value of rho for the within‐patient studies had no significant effect on the findings: The pooled SMD ranged from ‐0.98 (95% CI ‐1.56 to ‐0.41; I² statistic = 13.9%; 3 studies), when we assumed the correlation to be zero, to ‐1.17 (95% CI ‐1.81 to ‐0.52; I² statistic = 78.5%), when we assumed the correlation to be 0.75.
Analysis 5: Vitamin D combination products versus placebo
This comparison included treatment with combined calcipotriol and betamethasone dipropionate used either once or twice daily on the body (see Analysis 5.5 and Table 8). As all five studies reported IAGI data, results for the combined end point are identical to those in Analysis 5.1. Five parallel‐group studies with 2058 participants contributed data. Treatment duration ranged from four to eight weeks. Three trials adequately concealed treatment allocation. The SMD for the combined end point across treatments was ‐1.44 (95% CI ‐1.76 to ‐1.12; I² statistic = 89.4%; 5 studies), with twice‐daily combination treatment (SMD ‐1.90; 95% CI ‐2.09 to ‐1.71; 2 studies) achieving a significantly larger effect than once‐daily treatment (SMD ‐1.21; 95% CI ‐1.50 to ‐0.91; 3 studies). On a 6‐point IAGI scale, these equate to improvements of 2.18 and 1.39 points, respectively.
5.5. Analysis.
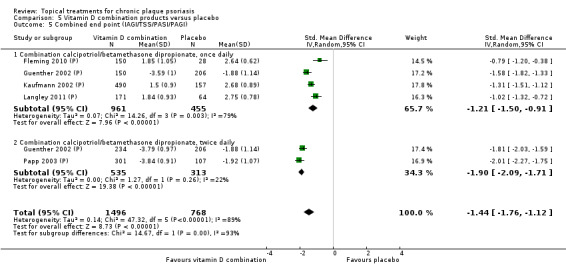
Comparison 5 Vitamin D combination products versus placebo, Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).
However, the PASI assessments differed from the IAGI: although twice‐daily combination treatment achieved a larger effect than once‐daily treatment (SMD ‐1.41 and ‐1.14, respectively), the difference was not statistically significant (see Analysis 5.3).
In Analysis 18.5, we reported placebo‐controlled trials of combination vitamin D/steroid treatments for scalp psoriasis.
Analysis 6: Other treatment versus placebo
This comparison comprised all other treatments for psoriasis of the body that we did not include in the first five comparisons; therefore, we removed pooling. None of the studies assessed the same treatment, which means that findings should be interpreted with caution.
In total, we included 26 treatments in this analysis (see Analysis 6.5 and Table 9). Twenty‐six studies, with 1450 participants, reported data on 25 of these 26 treatments. The study of omega‐3‐polyunsaturated fatty acids ointment (Henneicke‐v. Z. 1993) did not report usable effectiveness data, but did contribute data to the analysis of withdrawals. Twelve trials were between‐patient design, and 14 were within‐patient studies. Treatment duration ranged from 2 to 12 weeks. Two studies adequately concealed treatment allocation (Levine 2010 (P); Stutz 1996).
6.5. Analysis.
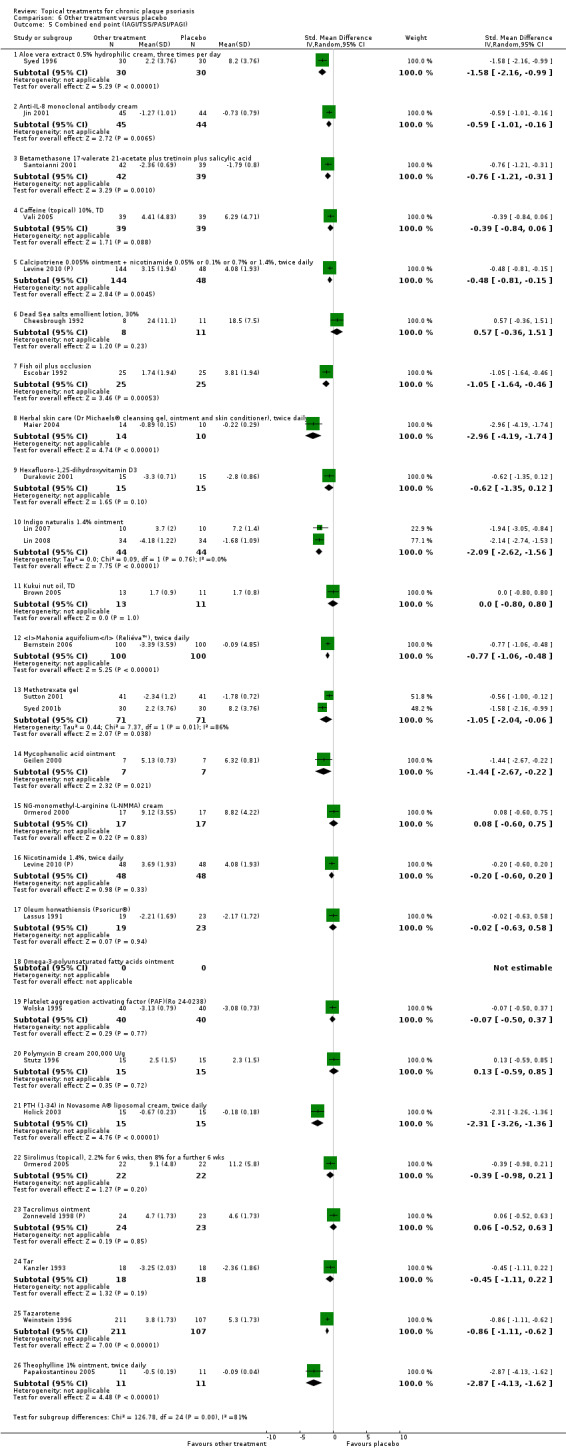
Comparison 6 Other treatment versus placebo, Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).
As assessed by the combined end point, just over half the treatments (13/25) performed statistically significantly better than placebo: aloe vera cream, anti‐IL‐8 monoclonal antibody cream, betamethasone 17‐valerate 21‐acetate plus tretinoin plus salicylic acid, combined treatment with calcipotriene and nicotinamide, fish oil plus occlusion, herbal skin care products, indigo naturalise 1.4% ointment, Mahonia aquifolium, methotrexate gel, mycophenolic acid ointment, PTH (1‐34) in Novasome cream®, tazarotene, and theophylline 1% ointment. The effect size (SMD) for the combined end point ranged from ‐0.48 (Levine 2010 (P); calcipotriene combined with nicotinamide) to ‐2.96 (Maier 2004; herbal skin care products), or 0.55 to 3.40, respectively, on a 6‐point IAGI scale.
The trial of herbal skin care products enrolled just 34 participants, of which 24 contributed data (Maier 2004), so this study is classed as having a high risk of attrition bias (Figure 3; see Risk of bias in included studies). The results of this study were published as an abstract, and our searches failed to locate a full publication. Therefore, findings should be treated with caution.
In the remaining 12 treatments, the difference relative to placebo using the combined end point was not statistically significant. These included topical caffeine, Dead Sea salts emollient lotion, hexafluoro‐1,25‐dihydroxyvitamin D3, kukui nut oil, NG‐monomethyl‐L‐arginine (L‐NMMA) cream, nicotinamide 1.4%, oleum horwathiensis, platelet aggregation activating factor (PAF), polymyxin B cream, topical sirolimus, topical tacrolimus, and tar.
In two treatments, findings by different outcomes were inconsistent. When assessed by the TSS, hexafluoro‐1,25‐dihydroxyvitamin D3 was significantly more effective than placebo (‐1.13; 95% CI ‐1.91 to ‐0.35) (Analysis 6.2), whereas the difference assessed by the IAGI was non‐significant (‐0.62; 95% CI ‐1.35 to 0.12) (Analysis 6.1). We based these findings on a single trial (Durakovic 2001). Similarly, the difference for oleum horwathiensis was statistically significant using the TSS (‐0.77; 95% CI ‐1.40 to ‐0.14) (Analysis 6.2) whereas the difference assessed by the IAGI was not (‐0.02; 95% CI ‐0.63 to 0.58) (Analysis 6.1). We also based findings for this treatment on a single trial (Lassus 1991).
We reported elsewhere placebo‐controlled trials of other treatments for scalp psoriasis (Analysis 18.5) and inverse psoriasis (Analysis 16.5).
Analysis 7: Vitamin D analogues versus corticosteroid (potent)
Our review identified eight vitamin D analogue‐potent corticosteroid comparisons for body psoriasis for this comparison (see Analysis 7.5 and Table 10). Fourteen studies with 3542 participants reported data for these 8 intervention‐comparator contrasts. Nine trials were between‐patient design, and five were within‐patient studies. Treatment duration ranged from three to eight weeks. One study adequately concealed treatment allocation (Papp 2003 (H)).
7.5. Analysis.
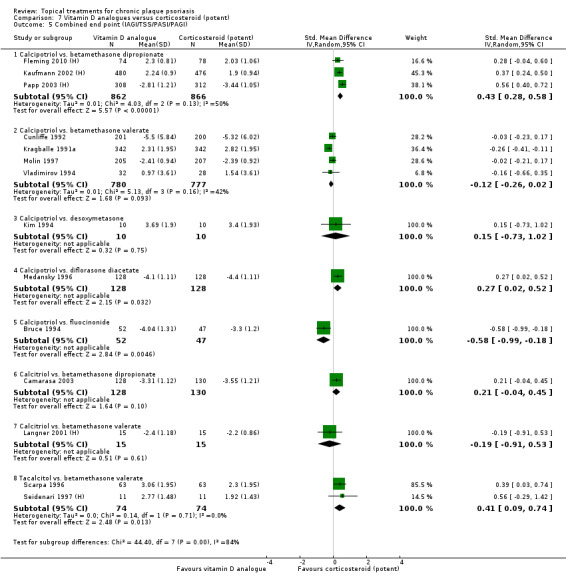
Comparison 7 Vitamin D analogues versus corticosteroid (potent), Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).
The combined end point SMD indicated that, overall, there was no statistically significant difference between vitamin D and potent corticosteroids: SMD 0.11 (95% CI ‐0.07 to 0.30; I² statistic = 85.6%; 14 studies). There was however substantial heterogeneity and variation in effect underlying this summary statistic. Therefore, we only pooled subtotals.
The difference between the vitamin D analogue and the potent corticosteroid was not statistically significant for four intervention‐comparator contrasts (calcipotriol versus betamethasone valerate, calcipotriol versus desoxymetasone, calcitriol versus betamethasone dipropionate, calcitriol versus betamethasone valerate). In the comparison of calcipotriol and betamethasone valerate, the non‐significant result was borderline (SMD ‐0.12; 95% CI ‐0.26 to 0.02; I² statistic = 41.6%; 4 studies). When assessed using the TSS (one study), PASI (four studies), and PAGI (two studies), calcipotriol was significantly more effective than betamethasone valerate (see Analysis 7.2, Analysis 7.3, and Analysis 7.4). There were similar inconsistencies between the outcome assessments for the comparison of calcitriol with betamethasone dipropionate, all of which we based on a single study (Camarasa 2003). According to the TSS and PASI, calcitriol was significantly less effective than betamethasone dipropionate, but the IAGI indicated that the difference was not significant (see Analysis 7.1).
In one intervention‐comparator contrast (calcipotriol versus fluocinonide), the vitamin D analogue was significantly more effective than potent corticosteroid: SMD ‐0.58 (95% CI: ‐0.99 to ‐0.18; I² statistic = NA). This effect is equivalent to a gain of approximately two‐thirds of a point (0.64) on a 6‐point IAGI scale. In three intervention‐comparator contrasts, the potent corticosteroid was significantly more effective than the vitamin D analogue (calcipotriol versus betamethasone dipropionate, calcipotriol versus diflorasone diacetate, tacalcitol versus betamethasone valerate). The SMD effect sizes for these were 0.43, 0.27, and 0.41, respectively, equivalent to improvements of 0.47, 0.30, and 0.45 on a 6‐point IAGI scale.
We reported elsewhere trials comparing vitamin D and potent corticosteroids for scalp psoriasis (Analysis 19.5) and inverse psoriasis (Analysis 17.5).
19.5. Analysis.
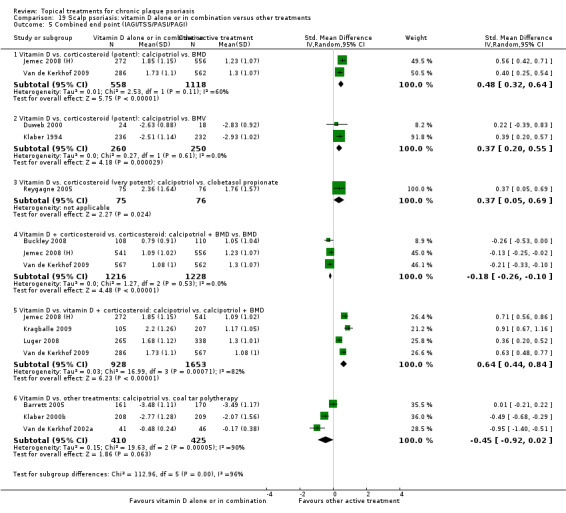
Comparison 19 Scalp psoriasis: vitamin D alone or in combination versus other treatments, Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).
17.5. Analysis.
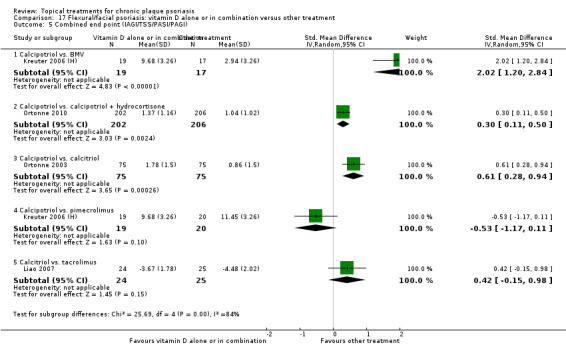
Comparison 17 Flexural/facial psoriasis: vitamin D alone or in combination versus other treatment, Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).
Sensitivity analyses
We explored differences in within‐patient and between‐patient designs using one‐way sensitivity analysis (Table 10). In both types of design, the difference between vitamin D and potent corticosteroid was not statistically significant, but we associated the pooled results with substantial levels of heterogeneity (Higgins 2011). The SMD for the 5 within‐patient studies (554 participants) was 0.17 (95% CI ‐0.20 to 0.54; I² statistic = 81.9%). The SMD for the 9 between‐patient studies (2988 participants) was 0.10 (95% CI ‐0.11 to 0.31; I² statistic = 84.9%).
We also used sensitivity analysis to explore the impact of varying the correlation coefficient (rho) for the 5 within‐patient studies. As no trial in the review reported this statistic, we tested values of 0, 0.25, 0.50, and 0.75. Varying the value of rho for the within‐patient studies had no significant effect on the findings: The pooled SMD ranged from 0.10 (95% CI ‐0.08 to 0.28; I² statistic = 90.5%; 14 studies), when we assumed the correlation to be zero, to 0.12 (95% CI ‐0.06 to 0.30; I² statistic = 94.3%), when we assumed the correlation to be 0.75.
Analysis 8: Vitamin D analogues versus corticosteroid (very potent)
This comparison considered vitamin D analogues against very potent corticosteroids for body psoriasis (see Analysis 8.5 and Table 11). We found data on one intervention‐comparator contrast: calcipotriol versus clobetasol propionate. Two between‐patient trials reported data for 82 participants. Treatment duration ranged between two and six weeks. The adequacy of treatment allocation was unclear in both studies. The SMD for the combined end point indicated that there was no statistically significant difference between the vitamin D analogue and the very potent corticosteroid: ‐0.06 (95% CI ‐0.57 to 0.44; I² statistic = 25.7%; 2 studies).
8.5. Analysis.
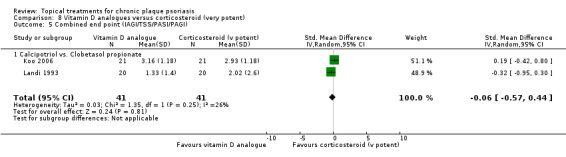
Comparison 8 Vitamin D analogues versus corticosteroid (very potent), Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).
We reported elsewhere trials comparing vitamin D and very potent corticosteroids for scalp psoriasis (Analysis 19.5).
Analysis 9: Vitamin D combined with corticosteroid versus corticosteroid
This comparison considered combined treatment with vitamin D analogues and corticosteroids against potent or very potent corticosteroid for body psoriasis (see Analysis 9.5 and Table 12). Five parallel‐group studies, ranging in treatment duration from 2 to 8 weeks, provided data on 2113 participants. The adequacy of the concealment of treatment allocation was unclear in all five trials.
9.5. Analysis.
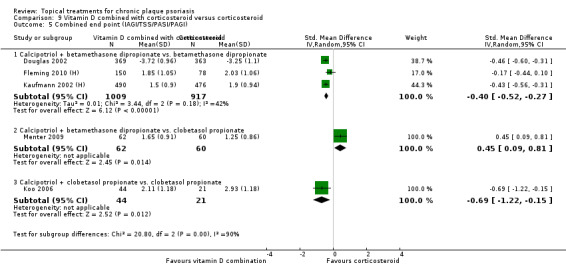
Comparison 9 Vitamin D combined with corticosteroid versus corticosteroid, Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).
Effectiveness data were available for three contrasts:
calcipotriol plus betamethasone dipropionate versus betamethasone dipropionate;
calcipotriol plus betamethasone dipropionate versus clobetasol propionate; and
calcipotriol plus clobetasol propionate versus clobetasol propionate.
Overall, combination treatment was more effective than monotherapy with corticosteroids: SMD ‐0.26 (95% CI ‐0.52 to ‐0.00; I² statistic = 84.4%; 5 studies). This effect is equivalent to a change of 0.29 on a 6‐point IAGI scale. However, the pooled finding masks important diversity between the three intervention‐comparator contrasts. When we compared combination treatment with calcipotriol and betamethasone dipropionate with a potent steroid (betamethasone dipropionate alone), combination treatment was more effective (SMD ‐0.40; 95% CI ‐0.52 to ‐0.27; I² statistic = 41.8%; 3 studies). However, the same combination treatment was significantly less effective than monotherapy with a very potent steroid (clobetasol propionate) (SMD 0.45; 95% CI 0.09 to 0.81; I² statistic = NA). Calcipotriol in combination with clobetasol propionate however was more effective than the very potent steroid used alone (SMD ‐0.69; 95% CI ‐1.22 to ‐0.15; I² statistic = NA). Therefore, we removed pooling for this analysis, but the findings demonstrate that combination treatment with vitamin D analogue and a corticosteroid is more effective than the same steroid used as a monotherapy.
We reported elsewhere trials comparing combination therapy (with vitamin D and potent corticosteroids) against potent steroids for scalp psoriasis (Analysis 19.5).
Analysis 10: Vitamin D alone or in combination versus dithranol
This comparison considered vitamin D analogues against dithranol for body psoriasis (see Analysis 10.5 and Table 13). We identified three intervention‐comparator contrasts: calcipotriol versus dithranol, calcitriol versus dithranol, and tacalcitol versus dithranol. Seven between‐patient trials and one within‐patient trial (Grattan 1997 (H)) reported data for 1284 participants. We considered concealment of treatment allocation to be adequate in one trial (Van de Kerkhof 2006). Treatment duration ranged from 4 weeks to 12 weeks. In addition, there was some variation in the dithranol regimens employed by trials and in the baseline severity of trial participants. These factors may explain the high level of heterogeneity found in the pooled results.
10.5. Analysis.
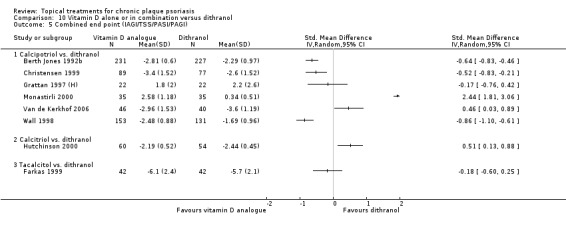
Comparison 10 Vitamin D alone or in combination versus dithranol, Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).
The SMD for the combined end point was 0.09 (95% CI ‐0.44 to 0.63; I² statistic = 94.9%; 8 studies), indicating that there was no evidence of a statistically significant difference in effect between vitamin D analogues and dithranol. The pooled findings for calcipotriol against dithranol (SMD 0.07; 95% CI ‐0.57 to 0.71; I² statistic = 95.7%; 6 studies) and tacalcitol versus dithranol (SMD ‐0.18; 95% CI ‐0.60 to 0.25; I² statistic = NA) were aligned with, and drove, this result. However, the high levels of heterogeneity mean that findings merit closer scrutiny. Six studies contributed data from 1086 participants to the comparison of calcipotriol and dithranol. In three of these studies, calcipotriol performed significantly better than dithranol; two trials found significant differences in favour of dithranol; and one study found no significant difference between the treatments (see Analysis 10.5). A single study of 114 participants compared calcitriol against dithranol (Hutchinson 2000) and found a statistically significant difference in favour of dithranol (SMD 0.51; 95% CI 0.14 to 0.88; I² statistic = NA). This equates to just over half a point (0.56 of a point) improvement on a 6‐point IAGI scale. However, the same study found no significant difference between the treatments in the assessment using the TSS (Analysis 10.2) or the PASI (Analysis 10.3).
In light of the considerable level of observed variation within and between studies (Higgins 2011), we removed pooling from this comparison.
Analysis 11: Vitamin D alone or in combination versus other vitamin D analogue
Our review identified three intervention‐comparator contrasts for body psoriasis in this comparison: calcipotriol versus calcitriol, calcipotriol versus tacalcitol, and calcipotriol versus maxacalcitol (see Analysis 11.5 and Table 14). Four trials involving 513 participants contributed data. One trial was within‐patient (Barker 1999 (H)); three were between‐patient in design; and no trials demonstrated the concealment of treatment allocation to be adequate. Treatment duration ranged from 8 to 12 weeks.
11.5. Analysis.
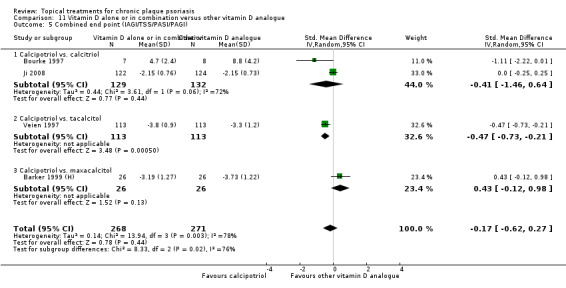
Comparison 11 Vitamin D alone or in combination versus other vitamin D analogue, Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).
The SMD for the combined end point indicated that there was no statistically significant difference between the treatments: ‐0.17 (95% CI ‐0.62 to 0.27; I² statistic = 78.5%; 4 studies). When we considered individual intervention‐comparator contrasts using the combined end point, calcipotriol was more effective than tacalcitol (SMD ‐0.47; 95% CI ‐0.73 to ‐0.21; I² statistic = NA), which equates to an improvement of 0.52 on a 6‐point IAGI scale. However, there was no statistically significant difference between calcipotriol and calcitriol (SMD ‐0.41; 95% CI ‐1.46 to 0.64; I² statistic = 72.3%; 2 studies) or between calcipotriol and maxacalcitol (SMD 0.43; 95% CI ‐0.12 to 0.98; I² statistic = NA). Participants in all trials applied treatments twice daily, with the exception of tacalcitol, which was applied once daily (Veien 1997).
Sensitivity analysis
The trial by Ji 2008 used three outcome measures to assess the effects of calcipotriol and calcitriol. Both the investigators' assessment (Analysis 11.1) and the participants' assessment found no difference between the two vitamin D products (Analysis 11.4), but the TSS found a statistically significant difference in favour of calcipotriol (Analysis 11.2). As findings for Ji 2008 varied depending on the outcome measure used, we tested the impact of using TSS data for the combined end point instead of IAGI data. The sensitivity analysis demonstrated that findings were robust, both for the SMD for the combined end point for all treatments (SMD ‐0.28; 95% CI ‐0.66 to 0.10; I² statistic = 70.6%; 4 studies) and for the comparison of calcipotriol and calcitriol (SMD ‐0.52; 95% CI ‐1.19 to 0.15; I² statistic = 44.9%; 2 studies).
Analysis 12: Vitamin D alone or in combination versus vitamin D + corticosteroid
Our review identified 12 intervention‐comparator contrasts for body psoriasis, involving 3 vitamin D analogues, 2 combination products, and 7 different corticosteroids (see Analysis 12.5 and Table 15). Seventeen trials involving 5856 participants contributed data. Sixteen trials were between‐patient, and 1 was within‐patient in design (Salmhofer 2000). Treatment duration ranged from 2 to 12 weeks. Four trials adequately concealed treatment allocation, but concealment was inadequate in one trial (in the other trials, insufficient data were reported to allow an assessment of adequacy).
12.5. Analysis.
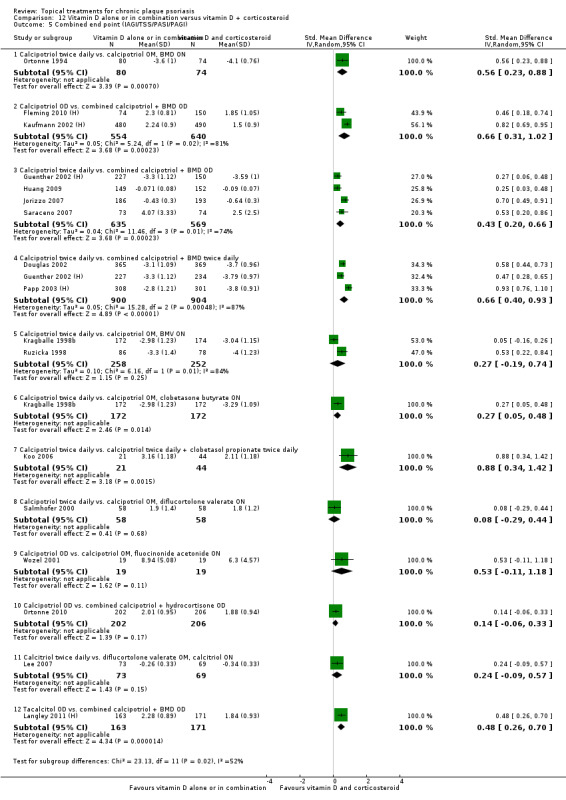
Comparison 12 Vitamin D alone or in combination versus vitamin D + corticosteroid, Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).
Overall, vitamin D plus corticosteroid was more effective than vitamin D alone: The SMD for the combined end point was 0.46 (95% CI 0.33 to 0.59; I² statistic = 83.3%; 17 studies), which translates into half of 1 point (0.50) on a 6‐point IAGI scale. The substantial level of heterogeneity reflects not only different dosing schedules of the intervention and comparator products, but also the different potency of the corticosteroids and the different vitamin D analogues evaluated; we therefore removed pooling (Higgins 2011).
Twice‐daily calcipotriol was significantly less effective than combination treatment with betamethasone dipropionate. This finding held whether participants applied the corticosteroid separately at night time (SMD 0.56; equivalent to 0.61 on a 6‐point IAGI scale) or as a combined product used once daily or twice daily (SMD 0.43 and 0.66, respectively, translating into improvements of 0.48 and 0.73 points on a 6‐point IAGI scale). Twice‐daily calcipotriol was also significantly less effective than combination treatment with clobetasone butyrate (SMD 0.27; 95% CI 0.05 to 0.48; I² statistic = NA; 0.29 of a point on a 6‐point IAGI) or clobetasol propionate (SMD 0.88; 95% CI 0.34 to 1.42; I² statistic = NA; 0.97 of a point on a 6‐point IAGI scale). Once‐daily combination treatment with calcipotriol and betamethasone dipropionate was significantly more effective than once‐daily treatment with either calcipotriol (SMD 0.66; 95% CI 0.31 to 1.02; I² statistic = 80.9%; 2 studies) or tacalcitol (SMD 0.48; 95% CI 0.26 to 0.70; I² statistic = NA).
In none of the 12 intervention‐comparator contrasts was the vitamin D analogue significantly more effective than combined vitamin D plus corticosteroid. However, in five instances, there was no significant difference between vitamin D and the comparator (combination treatment). These included twice‐daily calcipotriol against combination treatment with betamethasone valerate or diflucortolone valerate, once‐daily calcipotriol against combination treatment with fluocinonide acetonide or hydrocortisone, and twice‐daily calcitriol against combination treatment with diflucortolone valerate.
We reported elsewhere trials comparing vitamin D and combination therapy with vitamin D/potent corticosteroids for scalp psoriasis (Analysis 19.5).
Analysis 13: Vitamin D alone or in combination versus other treatments: complex regimens
This comparison summarises complex regimens for body psoriasis, defined here as treatment sequences that do not consist of a simple head‐to‐head comparison between two active treatments (see Analysis 13.5 and Table 16). Using the combined end point, data were available for all 12 intervention‐comparator contrasts (Figure 4). Data were available from 2936 participants from 8 between‐patient trials and 1 within‐patient trial (Austad 1998). Trial duration varied between 2 and 12 weeks. One trial adequately concealed treatment allocation. As the interventions and comparators were highly variable and because two trials each contributed three pair‐wise contrasts, we did not pool the data.
13.5. Analysis.
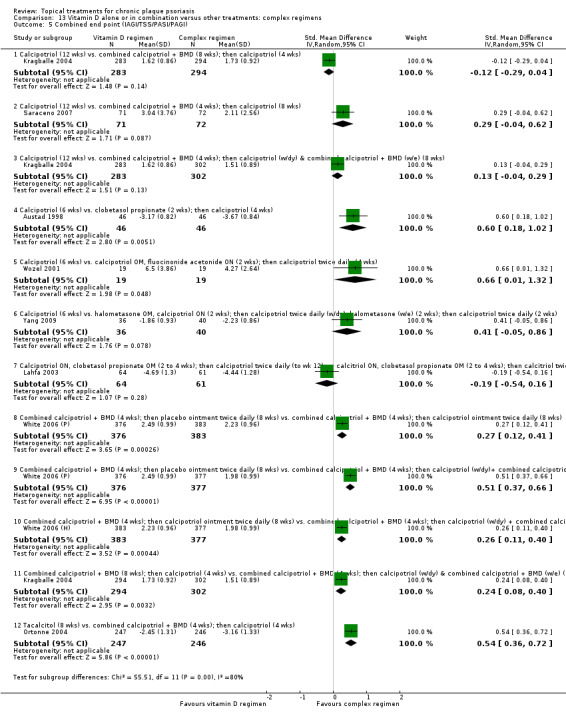
Comparison 13 Vitamin D alone or in combination versus other treatments: complex regimens, Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).
4.
Forest plot of comparison: 13 Vitamin D alone or in combination vs. other treatments: complex regimens, outcome: 13.5 Combined end point (IAGI/TSS/PASI/PAGI).
Seven intervention‐comparator contrasts found a significant difference between regimens. Six weeks' monotherapy with twice‐daily calcipotriol was less effective than clobetasol propionate (2 weeks) followed by 4 weeks' monotherapy with calcipotriol (SMD 0.60; 95% CI 0.18 to 1.02) (Austad 1998). A similar effect was evident when calcipotriol monotherapy (once daily for two weeks, then twice daily for four weeks) was compared with two weeks of treatment with calcipotriol (mornings) and fluocinonide acetonide (evenings), followed by 4 weeks' monotherapy with calcipotriol (SMD 0.66; 95% CI 0.01 to 1.32) (Wozel 2001). These benefits translated into improvements of 0.66 and 0.72 (i.e. around two‐thirds of a point) on a 6‐point IAGI scale.
The 12‐week trial by White 2006 (H)/White 2006 (P) compared 3 regimens; all included an initial phase in which participants applied once‐daily combination treatment with calcipotriol and betamethasone dipropionate for 4 weeks, but differed in their subsequent 8‐week maintenance phase. Maintenance therapy with twice‐daily placebo ointment was significantly less effective than maintenance with either calcipotriol ointment (SMD 0.27; 95% CI 0.12 to 0.41) or maintenance with calcipotriol on weekdays and combination treatment at weekends (SMD 0.51; 95% CI 0.37 to 0.66). When we directly compared the two regimens with active maintenance options, the alternating (weekday/weekend) approach was significantly more effective (SMD 0.26; 95% CI 0.11 to 0.40). The benefits of these 3 regimens equate to an improvement of 0.29, 0.56, and 0.28 of a point, respectively, on a 6‐point IAGI scale. These findings, based on the investigators' assessment of severity, were aligned with those of both the PASI assessment (Analysis 13.3) and the participants' assessment (PAGI) (Analysis 13.4).
Ortonne 2004 demonstrated that four weeks of treatment with a combination product of calcipotriol and betamethasone dipropionate followed by monotherapy with calcipotriol for four weeks was significantly more effective than monotherapy with tacalcitol (SMD 0.54; 95% CI 0.36 to 0.72) (0.59 on a 6‐point IAGI scale). Findings from the investigators' assessment (Analysis 13.1) were aligned with the PASI assessment (Analysis 13.3) and the participants' assessment (Analysis 13.4).
Kragballe 2004 compared 12 weeks of calcipotriol monotherapy with 2 complex regimens, both of which involved different sequences of combination therapy (calcipotriol/betamethasone dipropionate) and calcipotriol. When we directly compared these two complex regimens, 8 weeks of combination therapy followed by 4 weeks of once‐daily calcipotriol was significantly less effective than a routine involving 4 weeks of combination therapy followed by 8 weeks of once‐daily calcipotriol on weekdays and combination therapy at the weekend (SMD 0.24; 95% CI 0.08 to 0.40, equivalent to 0.27 (¼ of 1 point) on a 6‐point IAGI scale). This finding was based on the investigators' assessment (IAGI), which was very similar to the participants' (PAGI) assessment (Analysis 13.4). However, the PASI found a non‐significant trend in favour of the weekday/weekend regimen (Analysis 13.3).
In five intervention‐comparator contrasts, we found no significant difference. A single study reported two of the non‐significant intervention‐comparator contrasts: Kragballe 2004 compared 12 weeks of calcipotriol monotherapy with each of the 2 complex regimens described in the paragraph above. Compared with monotherapy, participants who applied once‐daily combination treatment (calcipotriol/betamethasone dipropionate) for 8 weeks followed by once‐daily calcipotriol for 4 weeks did not experience a significantly greater improvement (SMD ‐0.12; 95% CI ‐0.29 to 0.04). Similarly, the psoriasis of participants on monotherapy with twice‐daily calcipotriol did not improve significantly more or less than the disease in participants who applied once‐daily combination treatment (calcipotriol/betamethasone dipropionate) for 4 weeks followed by 8 weeks using once‐daily calcipotriol (weekdays) and once‐daily combination therapy (weekends) (SMD 0.13; 95% CI ‐0.04 to 0.29). These findings were based on the investigators' assessment (IAGI), which were consistent with those of the participants (Analysis 13.4) and with the PASI assessment (Analysis 13.3).
Similarly to Kragballe 2004, Saraceno 2007 compared 12 weeks of calcipotriol monotherapy with 4 weeks of combined therapy followed by 8 weeks of twice‐daily calcipotriol. The SMD for the combined end point (0.29; 95% CI ‐0.04 to 0.62) showed a non‐significant trend in favour of the complex regimen. Yang 2009 also explored how monotherapy with calcipotriol compared with combined vitamin D/corticosteroid treatment. A 6‐week course of monotherapy with calcipotriol was not significantly different to a sequential regimen involving halometasone (SMD 0.41; 95% CI ‐0.05 to 0.86). Lahfa 2003 found that the effect of clobetasol propionate combined with calcipotriol was similar to the effect of a regimen where the same corticosteroid was used in conjunction with calcitriol: SMD ‐0.19 (95% CI ‐0.54 to 0.16).
Analysis 14: Vitamin D alone or in combination versus other treatment: long‐term studies (> 24 weeks)
This comparison included active‐controlled studies of psoriasis of the body that were at least 24 weeks in duration (see Analysis 14.5 and Table 17). One longer‐term placebo‐controlled trial of the body (Katz 1991a) and two long‐term scalp trials (Luger 2008; Poulin 2010) did not meet the inclusion criteria for this comparison, so we evaluated them elsewhere (Analyses 2, 19, and 18, respectively).
14.5. Analysis.
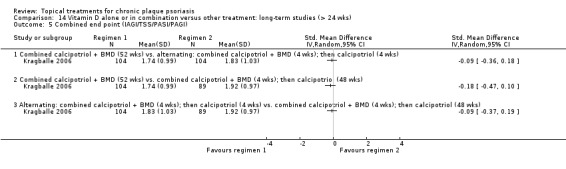
Comparison 14 Vitamin D alone or in combination versus other treatment: long‐term studies (> 24 wks), Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).
This comparison included one trial, Kragballe 2006, which was a between‐patient trial that compared 3 52‐week regimens with all treatments used once daily:
first, combination treatment with calcipotriol and betamethasone dipropionate;
second, alternating treatment with combination therapy for 4 weeks, then calcipotriol for 4 weeks; and
third, combination therapy for 4 weeks, then calcipotriol for 48 weeks.
In total, 297 participants contributed data to the analysis. The only outcome measure from the trial that could be included in our review was the Investigator's Global Assessment of Disease Severity (scored from 0, absent, to 5, severe); therefore, results from this section are identical to those in Analysis 14.1. Data were unsuitable for pooling because the same participants contributed to more than one analysis. The adequacy of the concealment of treatment allocation was unclear in this trial.
Data from the combined end point showed there was no significant difference between the three long‐term regimens (Figure 5). One year's combination therapy was not significantly better than either the alternating regimen (SMD ‐0.09; 95% CI ‐0.36 to 0.18) or the regimen of treatment with 4 weeks of combination therapy followed by 48 weeks of calcipotriol (SMD ‐0.18; 95% CI ‐0.47 to 0.10). In both these comparisons, combination therapy achieved a larger absolute benefit, but the difference was not statistically significant. When we compared the alternating therapy with the regimen of 4 weeks' combination therapy followed by 48 weeks of calcipotriol, the SMD for the IAGI also indicated the 2 regimens were not statistically significantly different: ‐0.09 (95% CI ‐0.37 to 0.19).
5.

Forest plot of comparison: 14 Vitamin D alone or in combination vs. other treatment: long term studies (>24wks), outcome: 14.5 Combined end point (IAGI/TSS/PASI/PAGI).
Findings were based on unpublished data supplied by the trial sponsor. Although these data were described as 'intention‐to treat', just 47% of the enrolled participants contributed data to the 52‐week assessment. The reasons why participants withdrew are summarised in Analysis 14.6, Analysis 14.7, and Analysis 14.8.
14.6. Analysis.
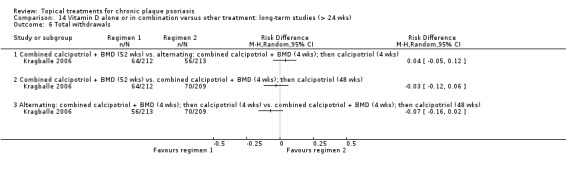
Comparison 14 Vitamin D alone or in combination versus other treatment: long‐term studies (> 24 wks), Outcome 6 Total withdrawals.
14.7. Analysis.
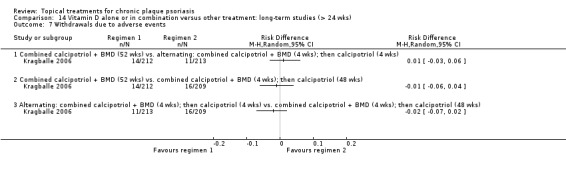
Comparison 14 Vitamin D alone or in combination versus other treatment: long‐term studies (> 24 wks), Outcome 7 Withdrawals due to adverse events.
14.8. Analysis.
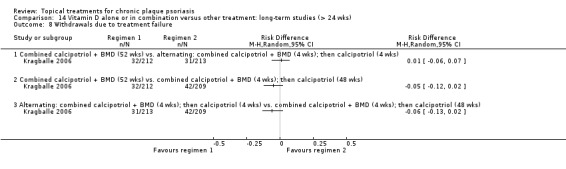
Comparison 14 Vitamin D alone or in combination versus other treatment: long‐term studies (> 24 wks), Outcome 8 Withdrawals due to treatment failure.
Analysis 15: Vitamin D analogues versus other treatment
This comparison incorporated all other vitamin D head‐to‐head comparisons of treatments for psoriasis of the body (excluding inverse psoriasis) that we had not already included (see Analysis 15.5 and Table 18). We included 12 intervention‐comparator contrasts, with data using the combined end point available for 11 of these contrasts. No effectiveness data were available for the comparison of calcipotriol and combined treatment with tazarotene gel plus mometasone furoate cream (Guenther 2000), but the trial did report data on withdrawal and adverse events. Thirteen between‐patient trials and 6 within‐patient trials provided data for the combined end point from 2364 participants. Trial duration ranged between 4 and 12 weeks. Three studies adequately concealed treatment allocation. In light of the pharmacological diversity of the comparators, we only pooled data within subgroups.
15.5. Analysis.
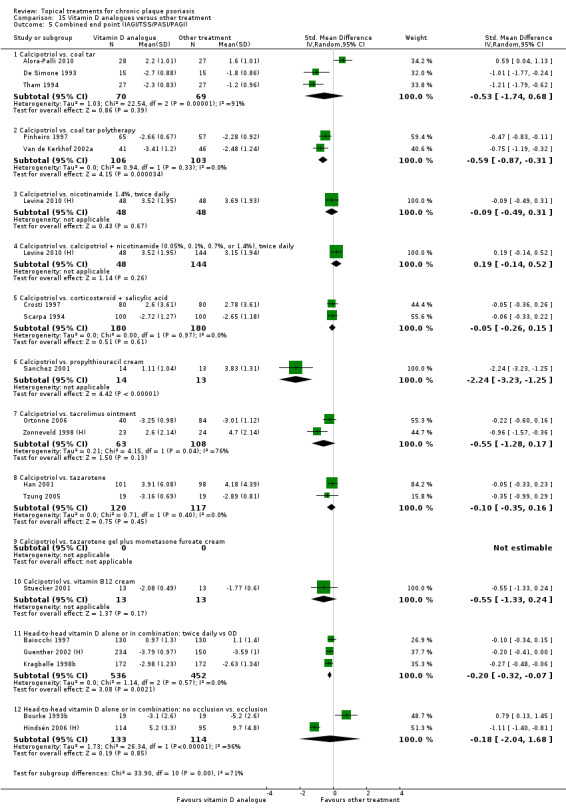
Comparison 15 Vitamin D analogues versus other treatment, Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).
In three intervention‐comparator contrasts, vitamin D was significantly more effective than the comparator. Specifically, twice‐daily calcipotriol was more effective than coal tar polytherapy (SMD ‐0.59; 95% CI ‐0.87 to ‐0.31; I² statistic = 0%; 2 studies) and propylthiouracil cream (SMD ‐2.24; 95% CI ‐3.23 to ‐1.25; I² statistic = NA). On a 6‐point IAGI scale, these equate to improvements of 0.65 of a point and 2.46 points, respectively. Twice‐daily vitamin D was significantly more effective than once‐daily treatment (SMD ‐0.20; 95% CI ‐0.32 to ‐0.07; I² statistic = 0%; 3 studies), achieving an additional improvement of around 0.22 of a point on a 6‐point IAGI scale. This finding was based on dosing assessments of 2 vitamin D products: calcipotriol (SMD ‐0.19; 95% CI ‐0.37 to ‐0.02; I² statistic = 12%; 2 studies) and combination treatment with calcipotriol and betamethasone dipropionate (SMD ‐0.20; 95% CI ‐0.41 to 0.00; I² statistic = NA).
In the remaining eight intervention‐comparator contrasts, we found no significant difference between twice‐daily calcipotriol and the comparators. This finding held for the comparison against the following products: coal tar monotherapy (SMD ‐0.53; 95% CI ‐1.74 to 0.68; I² statistic = 91.1%; 3 studies); nicotinamide, either alone (SMD ‐0.09; 95% CI ‐0.49 to 0.31; I² statistic: NA) or in combination with calcipotriol (SMD 0.19; 95% CI ‐0.14 to 0.52; I² statistic = NA); betamethasone dipropionate ointment and salicylic acid (SMD ‐0.05; 95% CI ‐0.26 to 0.15; I² statistic = NA); tacrolimus ointment (SMD ‐0.55; 95% CI ‐1.28 to 0.17; I² statistic = 75.9%; 2 studies); tazarotene (SMD ‐0.10; 95% CI ‐0.35 to 0.16; I² statistic = 0%; 2 studies); and vitamin B12 cream (SMD ‐0.55; 95% CI ‐1.33 to 0.24; I² statistic = NA). The addition of occlusion to calcipotriol did not significantly improve the drug's effectiveness (SMD ‐0.18; 95% CI ‐2.04 to 1.68; I² statistic = 96.2%; 2 studies).
Two of the three trials comparing calcipotriol with coal tar monotherapy found a significant difference in favour of calcipotriol (De Simone 1993; Tham 1994); the other trial found a significant difference in favour of coal tar (Alora‐Palli 2010). This apparent contradiction may reflect different formulations of coal tar, treatment durations, or different baseline disease severity. Alora‐Palli 2010 also assessed effectiveness and quality of life over the six‐week post‐treatment period, finding that the coal tar group maintained their improvement significantly better than those treated with calcipotriol (see section (c) Quality of life measures).
The two occlusion trials used very different regimens and may be better considered separately. Hindsén 2006 (H) compared calcipotriol with occlusion (applied once weekly for 2 weeks) followed by 4 weeks off treatment against 6 weeks of calcipotriol monotherapy. This trial does not provide a simple assessment of occlusion, since, additionally, treatment is withdrawn. This parallel‐group trial contributed data from 209 participants with moderately severe disease and found monotherapy was significantly more effective at the 6‐week end point (SMD ‐1.11; 95% CI ‐1.40 to ‐0.81). In the other trial, 19 participants were treated for 8 weeks with twice‐daily calcipotriol on both sides of the body, and one side was randomised to receive overnight occlusion with polythene. Bourke 1993b found a significant difference in favour of occlusion (SMD 0.79; 95% CI 0.13 to 1.45); the trial did not explicitly state the severity of participants.
We reported elsewhere trials comparing vitamin D and other treatments for scalp psoriasis (Analysis 19.5) and inverse psoriasis (Analysis 17.5).
Analysis 16: Flexural/facial psoriasis: placebo‐controlled trials
This comparison included placebo‐controlled trials of topical treatments for inverse or facial psoriasis (see Analysis 16.5 and Table 19). We found evidence on four treatments in this comparison: the potent steroid betamethasone valerate; the vitamin D analogue calcipotriol; and two topical calcineurin inhibitors, pimecrolimus and tacrolimus. We identified only one placebo‐controlled trial evaluating tacrolimus ointment (Lebwohl 2004), but the study did not report any effectiveness data suitable for this review. However, the study did contribute data on adverse events and withdrawal rates. We pooled only subtotals in this comparison.
Using the combined end point, data were available for three of the four topical treatments for inverse psoriasis. Two between‐patient trials contributed data from 122 participants. Gribetz 2004 was a eight‐week study that evaluated twice‐daily pimecrolimus cream. The four‐week trial by Kreuter 2006 (P) compared betamethasone valerate, calcipotriol, and pimecrolimus against placebo; all treatments were applied once daily. Treatment allocation was adequately concealed in one trial (Gribetz 2004). Therefore, we based the findings for each treatment on a single study.
The SMD for the combined end point found a statistically significant difference in favour of betamethasone valerate (SMD ‐2.83; 95% CI ‐3.79 to ‐1.88) and calcipotriol ointment (SMD ‐1.08; 95% CI ‐1.77 to ‐0.40). These equate to improvements of 3.25 points and 1.24 points on a 6‐point IAGI scale. Pimecrolimus cream was also significantly more effective than vehicle (SMD ‐0.86; 95% CI ‐1.30 to ‐0.41; I² statistic = 0%), equivalent to almost 1 point on a 6‐point IAGI. This result pooled data on twice‐daily applications for 8 weeks (SMD ‐1.07; 95% CI ‐1.69 to ‐0.45) and once‐daily pimecrolimus cream for 4 weeks (SMD ‐0.62; 95% CI ‐1.27 to 0.02). These findings suggest that different dosing schedules may influence efficacy.
Analysis 17: Flexural/facial psoriasis: vitamin D alone or in combination versus other treatment
This comparison included head‐to‐head trials of treatments for inverse psoriasis, where we compared vitamin D with an active control (see Analysis 17.5 and Table 20). We identified five intervention‐comparator contrasts. Four treatments were compared with calcipotriol: once‐daily betamethasone valerate, combined treatment with calcipotriol and hydrocortisone, calcitriol, and pimecrolimus. Calcitriol was compared with tacrolimus.
Using the combined end point, data were available for all five intervention‐comparator contrasts. Four trials, varying in duration from 4 to 8 weeks, contributed data from 588 participants. Three studies were between‐patient trials, and one was a within‐patient study (Ortonne 2003). The adequacy of concealment of treatment allocation was unclear in all four trials.
When applied to sensitive areas of the body, calcipotriol was significantly less effective than three of the comparators. These included betamethasone valerate (SMD 2.02; 95% CI 1.20 to 2.84) (Kreuter 2006 (H)), combination treatment with calcipotriol and hydrocortisone (SMD 0.30; 95% CI 0.11 to 0.50) (Ortonne 2010), and calcitriol (SMD 0.61; 95% CI 0.28 to 0.94) (Ortonne 2003). On a 6‐point IAGI scale, the additional benefit equates to 2.22 points for betamethasone valerate, ⅓ of a point for combination treatment with hydrocortisone, and ⅔ of a point for calcitriol.
There was no significant difference between vitamin D and the topical calcineurin inhibitors (calcipotriol versus pimecrolimus: SMD ‐0.53 (95% CI ‐1.17 to 0.11; I² statistic = NA); calcitriol versus tacrolimus: SMD 0.42 (95% CI ‐0.15 to 0.98; I² statistic = NA)).
Analysis 18: Scalp psoriasis: placebo‐controlled trials
This comparison included placebo‐controlled trials of treatments for scalp psoriasis (see Analysis 18.5, Figure 6, and Table 21). We included evidence on 11 treatments in this comparison, with data from the combined end point available for all 11 treatments. Thirteen between‐patient trials and 1 within‐patient trial (Lepaw 1978) contributed data from 3011 participants. Trial duration ranged between two and eight weeks. Concealment of treatment allocation was adequate in one trial.
6.
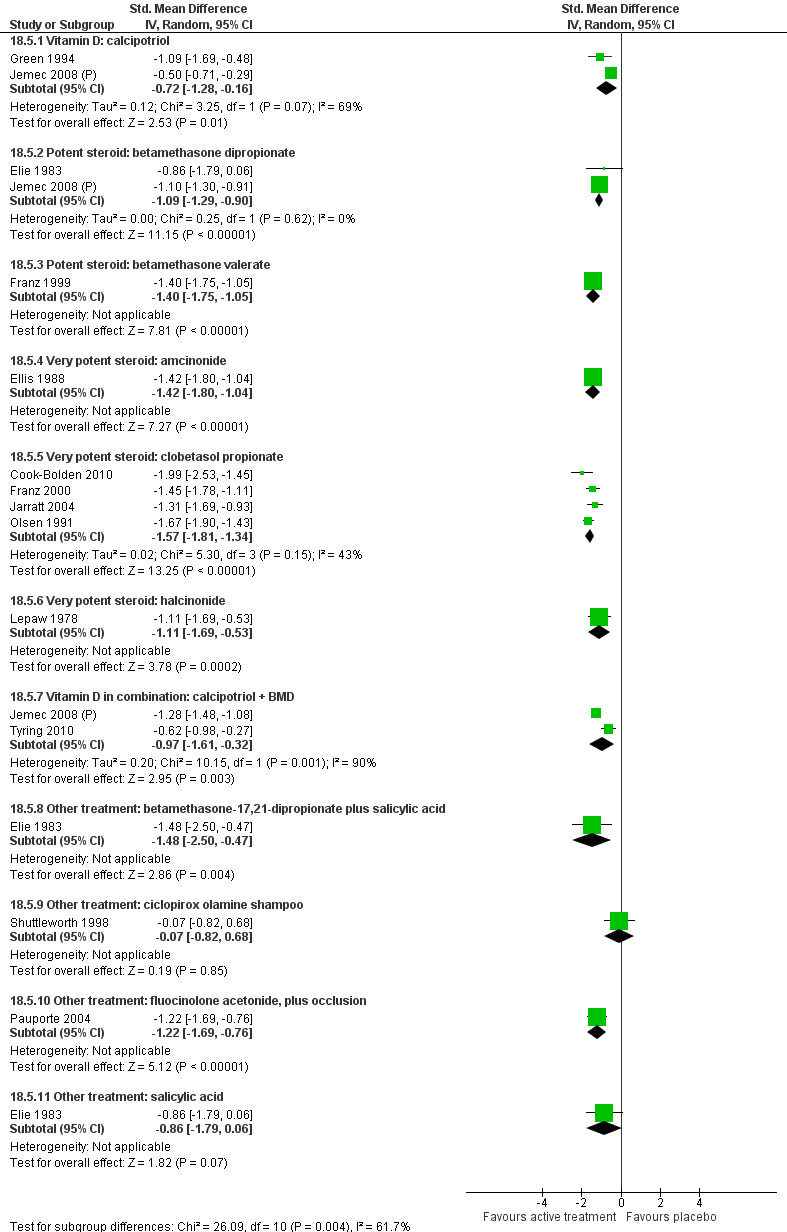
Forest plot of comparison: 18 Scalp psoriasis: placebo‐controlled trials, outcome: 18.5 Combined end point (IAGI/TSS/PASI/PAGI).
Two treatments were not significantly more effective than placebo. These were ciclopirox olamine shampoo (SMD ‐0.07; 95% CI ‐0.82 to 0.68; I² statistic = NA) and salicylic acid (SMD ‐0.86; 95% CI ‐1.79 to 0.06; I² statistic = NA). Of the nine treatments demonstrated to be significantly more effective than placebo, corticosteroids achieved the largest effects. Two very potent corticosteroids, amcinonide (SMD ‐1.42; 95% CI ‐1.80 to ‐1.04; I² statistic = NA) and clobetasol propionate (SMD ‐1.57; 95% CI ‐1.81 to ‐1.34; I² statistic = 43.3%; 4 studies), delivered benefits equating to almost 2 points (1.70 and 1.88 points, respectively) on a 6‐point IAGI (Investigator's Assessment of Global Improvement) scale. Betamethasone dipropionate combined with salicylic acid achieved a similar effect (SMD ‐1.48; 95% CI ‐2.50 to ‐0.47; I² statistic = NA), translating into 1.77 point improvement on the 6‐point IAGI over and above placebo.
When used as monotherapy, the 2 potent corticosteroids evaluated were both more effective than placebo: Betamethasone dipropionate (SMD ‐1.09; 95% CI ‐1.29 to ‐0.90; I² statistic = 0%; 2 studies) had a smaller effect than betamethasone valerate (SMD ‐1.40; 95% CI ‐1.75 to ‐1.05; I² statistic = NA), although the difference was not statistically significant. Calcipotriol had the smallest observed benefit (SMD ‐0.72; 95% CI ‐1.28 to ‐0.16; I² statistic = 69.2%; 2 studies). When calcipotriol was combined with betamethasone dipropionate, the pooled effect was larger than for calcipotriol used alone (SMD ‐0.97; 95% CI ‐1.61 to ‐0.32; I² statistic = 90.2%; 2 studies), or 1.16 points on a 6‐point improvement scale. Figure 6 shows that both trials found combination therapy to be significantly more effective, but Jemec 2008 (P) found a significantly larger effect (SMD ‐1.28; 95% CI ‐1.48 to ‐1.08) than Tyring 2010 (SMD ‐0.62; 95% CI ‐0.98 to ‐0.27). Participants in the two trials contributing data to the analysis of combination therapy differed in their ethnicity: Tyring 2010 recruited 177 people of Hispanic, Latino, Black, and African American ethnicity; in the trial by Jemec 2008 (P), over 96% of the 1505 enrollees were white. In addition, the trial by Jemec 2008 (P) included a larger proportion of people with severe or very severe disease (37% versus 20%). These factors may help explain the considerable level of heterogeneity (Higgins 2011) observed in the pooled effect for combination therapy.
Sensitivity analysis
We pooled data for the two potent corticosteroids, betamethasone dipropionate and betamethasone valerate. The SMD for the combined end point was ‐1.18 (95% CI ‐1.40 to ‐0.96; I² statistic = 19.9%; 3 studies), equating to 1.41 points on a 6‐point IAGI scale. For the 3 very potent corticosteroids, the SMD was ‐1.51 (95% CI ‐1.70 to ‐1.31; I² statistic = 37.5%; 6 studies), translating as a 1.80 point improvement over placebo on the IAGI.
Analysis 19: Scalp psoriasis: vitamin D alone or in combination versus other treatment
This comparison included head‐to‐head trials of treatments for scalp psoriasis in which one of the interventions was a vitamin D product (used either as monotherapy or in combination with another product) (see Analysis 19.5 and Table 22).
We identified six intervention‐comparator contrasts, and data on the combined end point were available for all these contrasts. All studies were parallel‐group in design (between‐patient). Twelve studies contributed data from 5413 participants. Trial duration ranged from 4 to 8 weeks in 11 studies, but duration was 52 weeks in one trial (Luger 2008). The adequacy of the concealment of treatment allocation was unclear in all 12 trials.
Based on the combined end point scores, monotherapy with vitamin D was consistently less effective than monotherapy with potent or very potent corticosteroids, and it was also significantly less effective than combination therapy with vitamin D and a potent steroid. Specifically, calcipotriol was significantly less effective than betamethasone dipropionate (SMD 0.48; 95% CI 0.32 to 0.64; I² statistic = 60.4%; 2 studies), betamethasone valerate (SMD 0.37; 95% CI 0.20 to 0.55; I² statistic = 0%; 2 studies), and clobetasol propionate (SMD 0.37; 95% CI 0.05 to 0.69; I² statistic = NA). On a 6‐point investigators' global assessment of improvement (IAGI) scale, these SMDs translate into 0.62, 0.48, and 0.48 points, respectively.
Combination treatment achieved a significantly greater benefit than either calcipotriol alone or betamethasone dipropionate alone. Calcipotriol was significantly less effective than combination treatment (SMD 0.64; 95% CI 0.44 to 0.84; I² statistic = 82.3%; 4 studies), with combined therapy delivering a benefit equivalent to 0.83 of a point on a 6‐point IAGI. Combination treatment was significantly more effective than monotherapy with betamethasone dipropionate (SMD ‐0.18; 95% CI ‐0.26 to ‐0.10; I² statistic = 0%; 3 studies), translating into a net benefit of 0.24 of a point on a 6‐point IAGI scale.
Compared with coal tar polytherapy, calcipotriol achieved a larger benefit, though this was not statistically significant at the 5% level (SMD ‐0.45; 95% CI ‐0.92 to 0.02; I² statistic = 89.8%; 3 studies).
(2) Secondary outcome measures
(a) Withdrawal rates (total rate; withdrawal due to adverse events; withdrawal due to treatment failure)
Analysis 1: Vitamin D analogues versus placebo
We pooled withdrawal data from seven of the eight vitamin D analogues using a random‐effects risk difference (RD) metric (see Analysis 1.6, Analysis 1.7, and Analysis 1.8). There were no withdrawal data for the comparison of calcipotriol plus occlusion versus placebo.
1.6. Analysis.
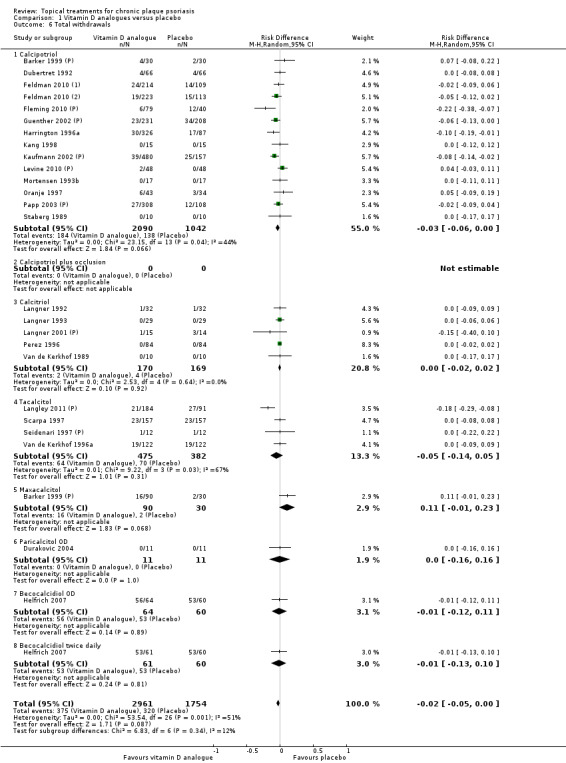
Comparison 1 Vitamin D analogues versus placebo, Outcome 6 Total withdrawals.
1.7. Analysis.
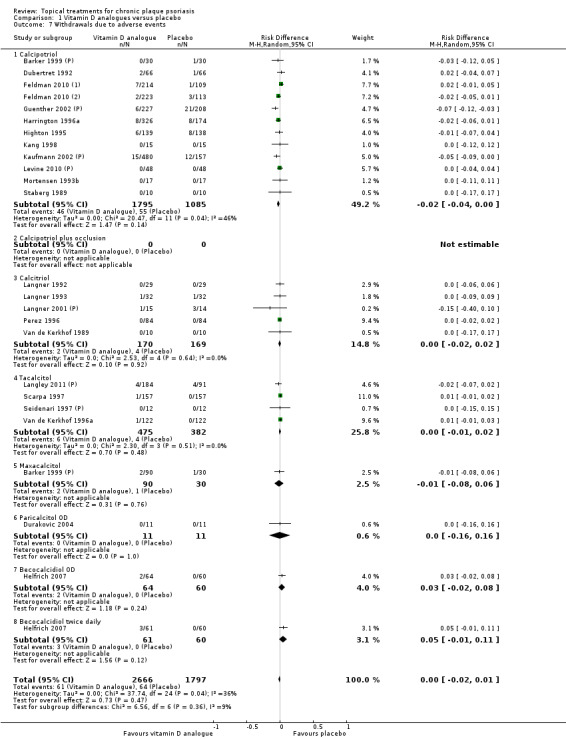
Comparison 1 Vitamin D analogues versus placebo, Outcome 7 Withdrawals due to adverse events.
1.8. Analysis.
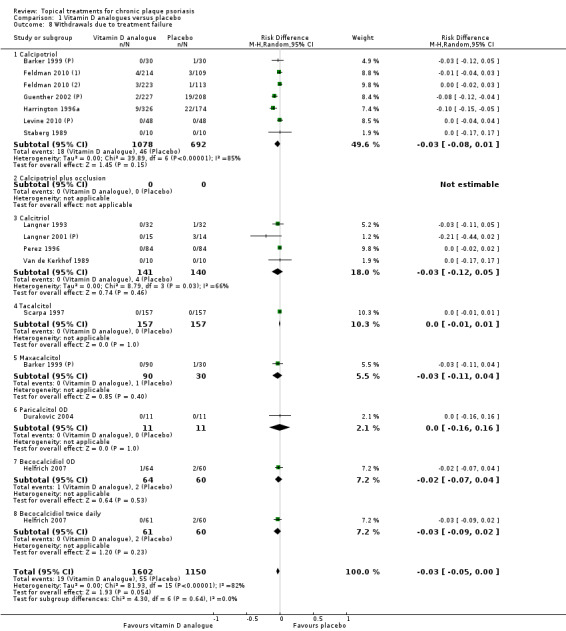
Comparison 1 Vitamin D analogues versus placebo, Outcome 8 Withdrawals due to treatment failure.
There was no significant difference in the pooled rate of withdrawals from the trials for any reason (total withdrawals) (RD: ‐0.02; (95% CI ‐0.05 to 0.00; I² statistic = 51.4%). Rates of withdrawals due to adverse events were not statistically significantly different (RD ‐0.00; 95% CI ‐0.02 to 0.01; I² statistic = 36.4%), and neither were withdrawals due to treatment failure (RD ‐0.03; 95% CI ‐0.05 to 0.00; I² statistic = 81.7%). Individually, none of the eight vitamin D analogues were significantly different to placebo in any of the three withdrawal rates.
Analysis 2: Corticosteroid (potent) versus placebo
This comparison included 10 potent corticosteroids. No withdrawal data were available for diflorasone diacetate, and some withdrawal data were missing for fluticasone propionate, mometasone furoate, and betamethasone dipropionate. Where available, we pooled data using a random‐effects risk difference metric (see Analysis 2.6, Analysis 2.7, and Analysis 2.8). There was a small but statistically significant difference in favour of potent corticosteroids for withdrawals from the trials for any reason (total withdrawals): RD ‐0.14 (‐0.22 to ‐0.05; I² statistic = 81.5%). This finding was driven by two large trials of once‐daily betamethasone dipropionate (Fleming 2010 (P); Kaufmann 2002 (P)) and two small trials of betamethasone dipropionate used as maintenance (weekend pulse therapy) (Katz 1987a; Katz 1991a). In the other corticosteroids, the RD showed no statistically significant difference.
2.6. Analysis.
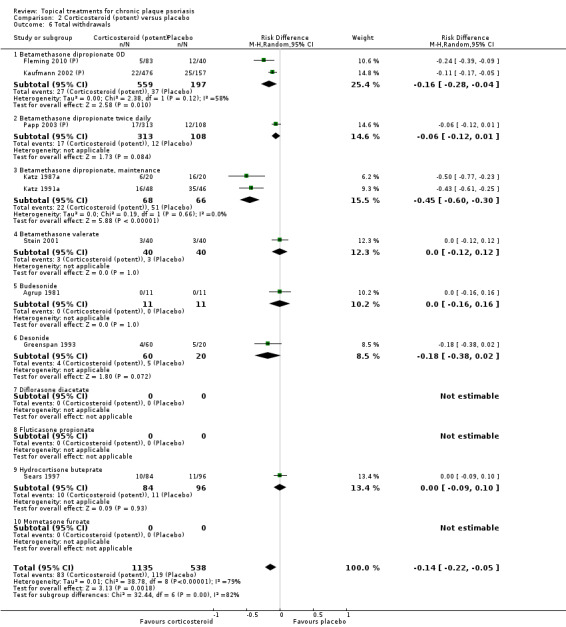
Comparison 2 Corticosteroid (potent) versus placebo, Outcome 6 Total withdrawals.
2.7. Analysis.
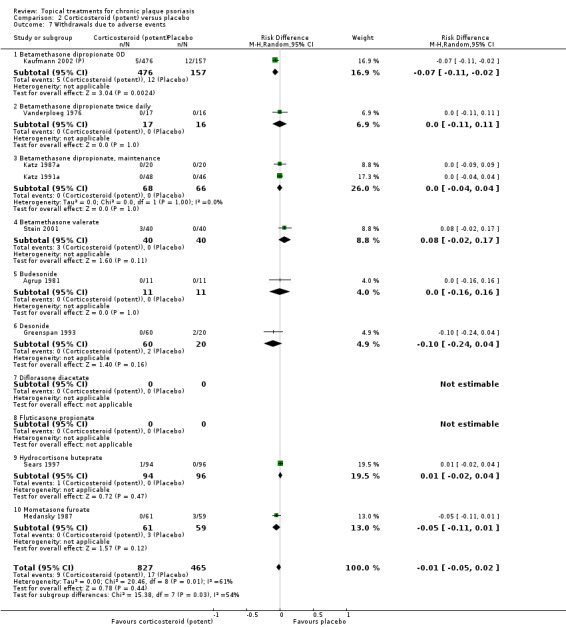
Comparison 2 Corticosteroid (potent) versus placebo, Outcome 7 Withdrawals due to adverse events.
2.8. Analysis.
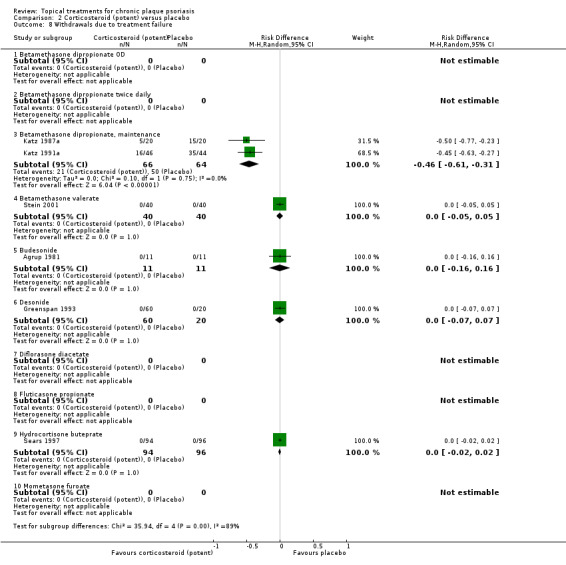
Comparison 2 Corticosteroid (potent) versus placebo, Outcome 8 Withdrawals due to treatment failure.
There was no significant difference in the pooled rate of withdrawals due to adverse events (RD ‐0.01; 95% CI ‐0.05 to 0.02; I² statistic = 60.9%). However, betamethasone dipropionate once daily was associated with statistically significantly lower rates of withdrawal due to adverse events than placebo (RD ‐0.07; 95% CI ‐0.11 to ‐0.02; I² statistic = NA).
For the rate of withdrawals due to treatment failure (Analysis 2.8), findings differed by treatment duration; therefore, we only pooled subgroup data. For short‐term treatments, there was no difference relative to placebo (RD 0.00; 95% CI ‐0.02 to 0.02; I² statistic = 0.0%). This is the pooled effect for the placebo comparisons with betamethasone valerate, budesonide, desonide, and hydrocortisone buteprate (data on withdrawals due to treatment failure for once‐daily and twice‐daily betamethasone dipropionate were not available). For maintenance treatment with betamethasone dipropionate, there was a statistically significant difference in favour of maintenance treatment when compared with placebo: RD ‐0.46; 95% CI ‐0.61 to ‐0.31; I² statistic = 0.0% (Katz 1987a; Katz 1991a).
Analysis 3: Corticosteroid (very potent) versus placebo
We identified withdrawal data for two of the three very potent corticosteroids in this comparison and pooled data using a random‐effects risk difference metric (see Analysis 3.6, Analysis 3.7, and Analysis 3.8). There were no withdrawal data available for halcinonide. There were no statistically significant differences between very potent corticosteroids and placebo for any type of withdrawal or for any individual treatment. For total withdrawals, the risk difference was ‐0.05 (95% CI ‐0.10 to 0.01; I² statistic = 83.7%). Differences in withdrawals due to adverse events (RD ‐0.00; 95% CI ‐0.01 to 0.01; I² statistic = 0.0%) and withdrawals due to treatment failure (RD ‐0.00; 95% CI ‐0.02 to 0.01); I² statistic = 13.5%) were also small and non‐significant.
3.6. Analysis.
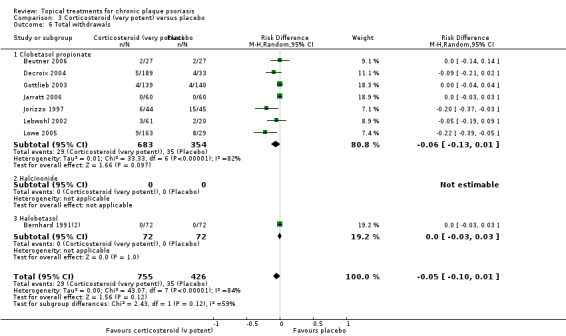
Comparison 3 Corticosteroid (very potent) versus placebo, Outcome 6 Total withdrawals.
3.7. Analysis.
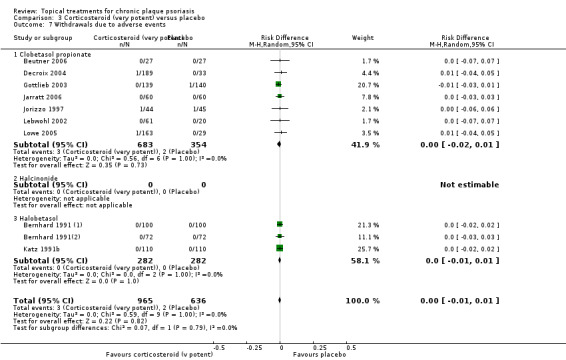
Comparison 3 Corticosteroid (very potent) versus placebo, Outcome 7 Withdrawals due to adverse events.
3.8. Analysis.
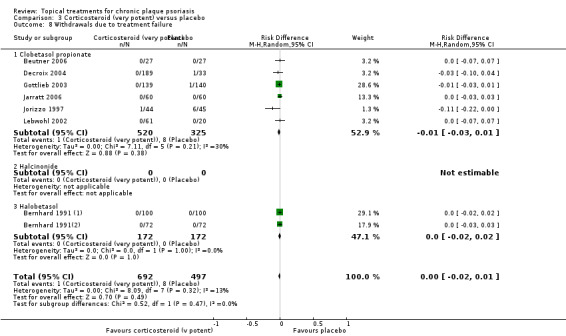
Comparison 3 Corticosteroid (very potent) versus placebo, Outcome 8 Withdrawals due to treatment failure.
Analysis 4: Dithranol versus placebo
We pooled withdrawal data on dithranol versus placebo using a random‐effects risk difference metric (see Analysis 4.6, Analysis 4.7, and Analysis 4.8). There were no statistically significant differences between dithranol and placebo for any type of withdrawal. For total withdrawals, the risk difference was 0.00 (95% CI ‐0.09 to 0.09; I² statistic = 0%). Differences in withdrawals due to adverse events (RD 0.00; 95% CI ‐0.05 to 0.05; I² statistic = 0%) and withdrawals due to treatment failure (RD 0.00; 95% CI ‐0.11 to 0.11; I² statistic = 0%) were also small and non‐significant.
4.6. Analysis.
Comparison 4 Dithranol versus placebo, Outcome 6 Total withdrawals.
4.7. Analysis.
Comparison 4 Dithranol versus placebo, Outcome 7 Withdrawals due to adverse events.
4.8. Analysis.
Comparison 4 Dithranol versus placebo, Outcome 8 Withdrawals due to treatment failure.
Analysis 5: Vitamin D combination products versus placebo
We pooled data from trials of vitamin D combination products using a random‐effects risk difference (RD) metric (see Analysis 5.6, Analysis 5.7, and Analysis 5.8). In all three withdrawal measures, combined treatment ‐ whether used once or twice daily ‐ was significantly less likely to lead to withdrawal from the trial. This finding held for total withdrawals (RD ‐0.12; 95% CI ‐0.17 to ‐0.07; I² statistic = 59.3%), withdrawals due to adverse events (RD ‐0.07; 95% CI ‐0.11 to ‐0.04; I² statistic = 55.0%), and withdrawals due to treatment failure (RD ‐0.09; 95% CI ‐0.12 to ‐0.06; I² statistic = 0%).
5.6. Analysis.
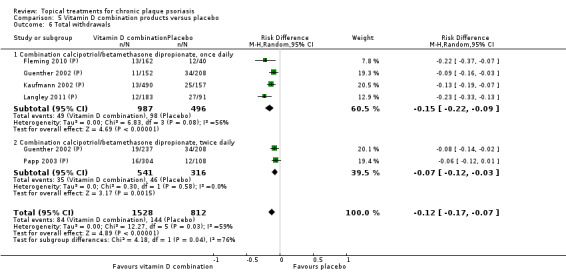
Comparison 5 Vitamin D combination products versus placebo, Outcome 6 Total withdrawals.
5.7. Analysis.
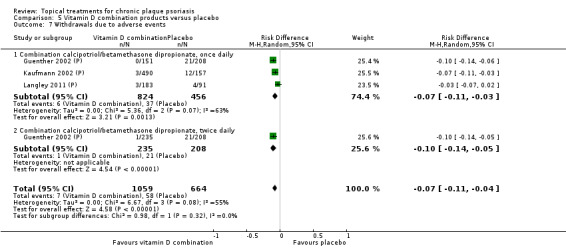
Comparison 5 Vitamin D combination products versus placebo, Outcome 7 Withdrawals due to adverse events.
5.8. Analysis.
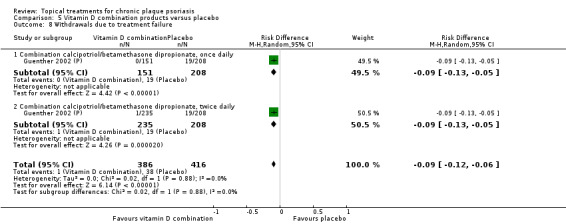
Comparison 5 Vitamin D combination products versus placebo, Outcome 8 Withdrawals due to treatment failure.
Analysis 6: Other treatment versus placebo
We report findings separately for 22 of the 26 treatments considered; no withdrawal data were available for four placebo comparisons: those involving a herbal skin care product, topical sirolimus, topical tacrolimus, or coal tar. Data on total withdrawals were available for 22 treatments; for withdrawals due to adverse events or treatment failure, data were available for 18 treatments (see Analysis 6.6, Analysis 6.7, and Analysis 6.8).
6.6. Analysis.
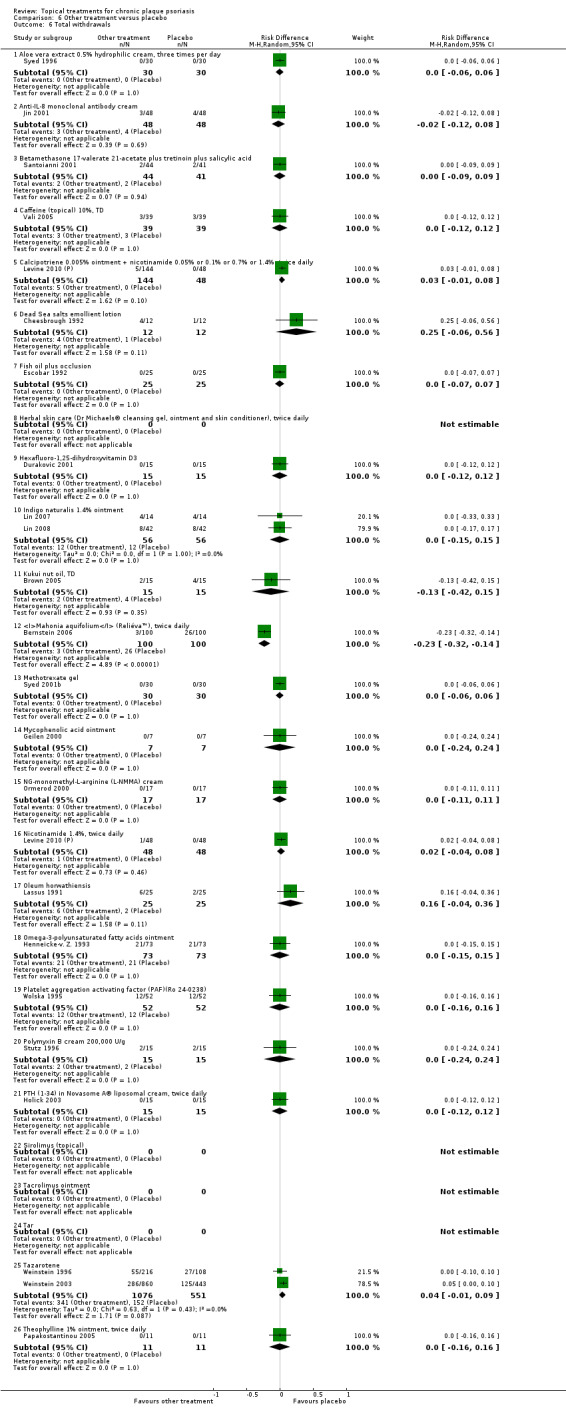
Comparison 6 Other treatment versus placebo, Outcome 6 Total withdrawals.
6.7. Analysis.
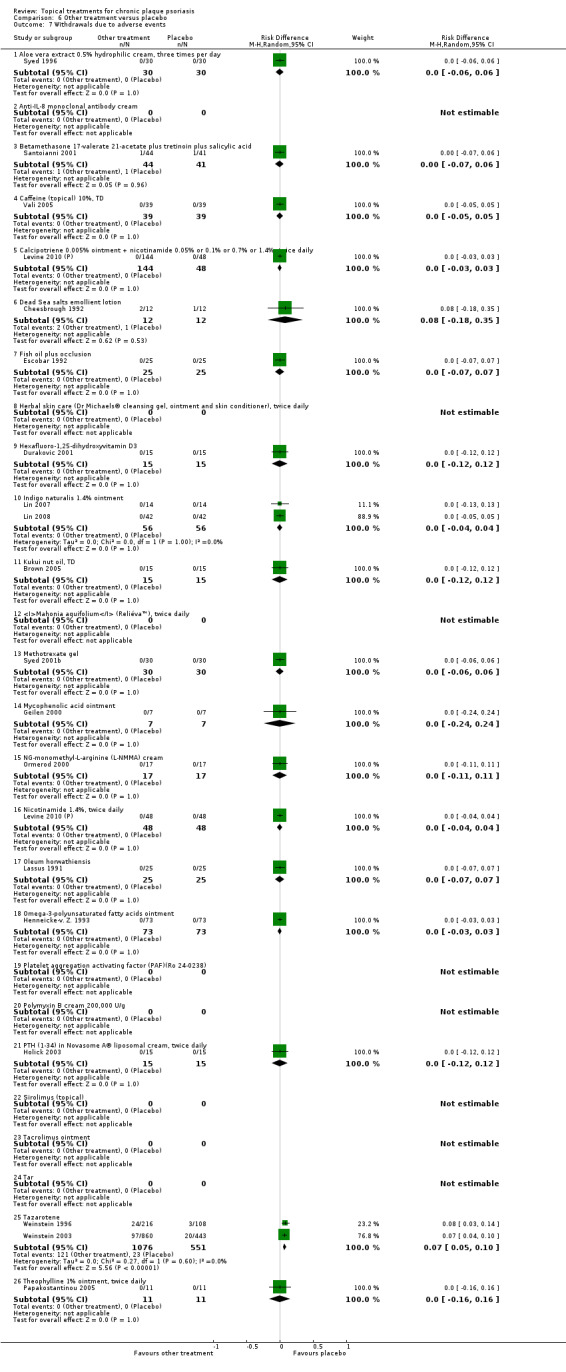
Comparison 6 Other treatment versus placebo, Outcome 7 Withdrawals due to adverse events.
6.8. Analysis.
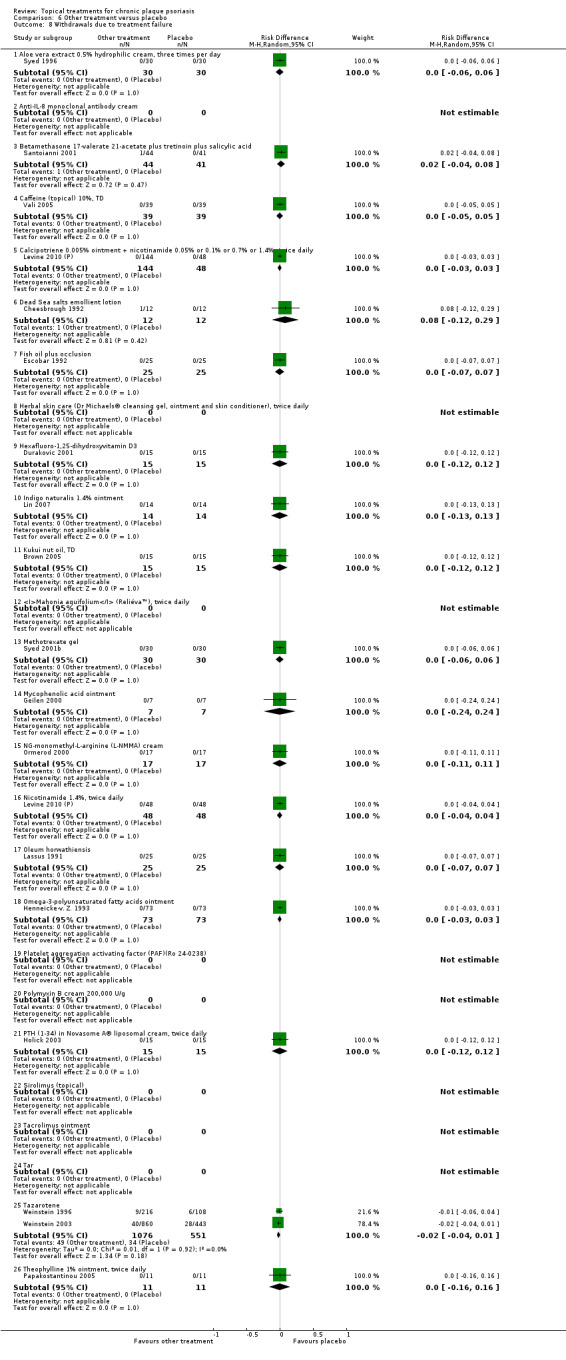
Comparison 6 Other treatment versus placebo, Outcome 8 Withdrawals due to treatment failure.
We assessed withdrawal data using a random‐effects risk difference metric. Relative to placebo, tazarotene was associated with a significantly higher rate of withdrawal due to adverse events (RD 0.07; 95% CI 0.05 to 0.10) and Mahonia aquifolium was associated with a significantly lower rate of total withdrawal (i.e. withdrawal from the trial for any reason) (RD ‐0.23; 95% CI ‐0.32 to ‐0.14). No other treatment was significantly different from placebo on any of the three withdrawal measures assessed.
Aloe vera extract 0.5% hydrophilic cream, three times per day
Sixty participants contributed data (Syed 1996). The comparison with placebo found no statistically significant difference for aloe vera extract, for total withdrawals, withdrawals due to adverse events, or withdrawals due to treatment failure.
Anti‐IL‐8 monoclonal antibody cream
Ninety‐six participants contributed data (Jin 2001). For total withdrawals, the comparison with placebo found no statistically significant difference for anti‐IL‐8 monoclonal antibody cream. The study did not report data on withdrawals due to adverse events or treatment failure.
Betamethasone 17‐valerate 21‐acetate plus tretinoin plus salicylic acid
Eighty‐five participants contributed data (Santoianni 2001). The comparison with placebo found no statistically significant difference for betamethasone 17‐valerate 21‐acetate plus tretinoin plus salicylic acid for total withdrawals, withdrawals due to adverse events, or withdrawals due to treatment failure.
Caffeine (topical) 10%, three times per day
Thirty‐nine participants contributed data (Vali 2005). The comparison with placebo found no statistically significant difference for topical caffeine for total withdrawals, withdrawals due to adverse events, or withdrawals due to treatment failure.
Calcipotriene 0.005% ointment + nicotinamide 0.05% or 0.1% or 0.7% or 1.4%, twice daily
One hundred and sixty participants contributed data (Levine 2010 (P)). The comparison with placebo found no statistically significant difference for combination therapy with calcipotriol and nicotinamide for total withdrawals, withdrawals due to adverse events, or withdrawals due to treatment failure.
Dead Sea salts emollient lotion
Twenty‐four participants contributed data (Cheesbrough 1992). The comparison with placebo found no statistically significant difference for Dead Sea salts emollient lotion for total withdrawals, withdrawals due to adverse events, or withdrawals due to treatment failure.
Fish oil plus occlusion
Twenty‐five participants contributed data (Escobar 1992). The comparison with placebo found no statistically significant difference for fish oil plus occlusion for total withdrawals, withdrawals due to adverse events, or withdrawals due to treatment failure.
Herbal skin care (Dr Michaels® cleansing gel, ointment, and skin conditioner), twice daily
No withdrawal data were available for this comparison.
Hexafluoro‐1,25‐dihydroxyvitamin D3
Fifteen participants contributed data (Durakovic 2001). The comparison with placebo found no statistically significant difference for hexafluoro‐1,25‐dihydroxyvitamin D3 for total withdrawals, withdrawals due to adverse events, or withdrawals due to treatment failure.
Indigo naturalise 1.4% ointment
Fifty‐six participants contributed data from two within‐patient trials (Lin 2007; Lin 2008). The comparison with placebo found no statistically significant difference for indigo naturalise ointment for total withdrawals, withdrawals due to adverse events, or withdrawals due to treatment failure.
Kukui nut oil, three times per day
Thirty participants contributed data (Brown 2005). The comparison with placebo found no statistically significant difference for kukui nut oil for total withdrawals, withdrawals due to adverse events, or withdrawals due to treatment failure.
Mahonia aquifolium (Reliéva™), twice daily
Two hundred participants contributed data (Bernstein 2006). For total withdrawals, the comparison with placebo found a statistically significant difference for Mahonia aquifolium (RD ‐0.23; 95% CI ‐0.32 to ‐0.14). The trial did not report data on withdrawals due to adverse events or treatment failure.
Methotrexate gel
Sixty participants contributed data (Syed 2001b). The study by Sutton 2001 on methotrexate gel did not report withdrawal data. The comparison with placebo found no statistically significant difference for methotrexate gel for total withdrawals, withdrawals due to adverse events, or withdrawals due to treatment failure.
Mycophenolic acid ointment
Seven participants contributed data (Geilen 2000). The comparison with placebo found no statistically significant difference for mycophenolic acid ointment for total withdrawals, withdrawals due to adverse events, or withdrawals due to treatment failure.
NG‐monomethyl‐L‐arginine (L‐NMMA) cream
Seventeen participants contributed data (Ormerod 2000). The comparison with placebo found no statistically significant difference for NG‐monomethyl‐L‐arginine (L‐NMMA) cream for total withdrawals, withdrawals due to adverse events, or withdrawals due to treatment failure.
Nicotinamide 1.4%, twice daily
Eighty‐eight participants contributed data (Levine 2010 (P)). The comparison with placebo found no statistically significant difference for nicotinamide for total withdrawals, withdrawals due to adverse events, or withdrawals due to treatment failure.
Oleum horwathiensis
Fifty participants contributed data (Lassus 1991). The comparison with placebo found no statistically significant difference for oleum horwathiensis for total withdrawals, withdrawals due to adverse events, or withdrawals due to treatment failure.
Omega‐3 polyunsaturated fatty acids ointment
Seventy‐three participants contributed data (Henneicke‐v. Z. 1993). The comparison with placebo found no statistically significant difference for omega‐3 polyunsaturated fatty acids ointment for total withdrawals, withdrawals due to adverse events, or withdrawals due to treatment failure.
Platelet aggregation activating factor (PAF) (Ro 24‐0238)
Fifty‐two participants contributed data (Wolska 1995). The comparison with placebo found no statistically significant difference for platelet aggregation activating factor for total withdrawals. The trial did not report data on withdrawals due to adverse events or treatment failure.
Polymyxin B cream, 200,000 U/g
Fifteen participants contributed data (Stutz 1996). The comparison with placebo found no statistically significant difference for polymyxin B cream for total withdrawals. The trial did not report data on withdrawals due to adverse events or treatment failure.
PTH (1‐34) in Novasome A® liposomal cream, twice daily
Fifteen participants contributed data (Holick 2003). The comparison with placebo found no statistically significant difference for PTH (1‐34) in Novasome A® liposomal cream for total withdrawals, withdrawals due to adverse events, or withdrawals due to treatment failure.
Sirolimus (topical)
No withdrawal data were available for this comparison.
Tacrolimus ointment
No withdrawal data were available for this comparison.
Tar
No withdrawal data were available for this comparison.
Tazarotene
Two studies, with 1627 participants, contributed data (Weinstein 1996; Weinstein 2003). The comparison with placebo found no statistically significant difference for tazarotene for total withdrawals or withdrawals due to treatment failure. However, tazarotene was significantly more likely to lead to withdrawal from the trial due to adverse events (RD 0.07; 95% CI 0.05 to 0.10; I² statistic = 0%).
Theophylline 1% ointment, twice daily
Twenty‐two participants contributed data (Papakostantinou 2005). The comparison with placebo found no statistically significant difference for theophylline ointment for total withdrawals, withdrawals due to adverse events, or withdrawals due to treatment failure.
Analysis 7: Vitamin D analogues versus corticosteroid (potent)
Seven of the eight vitamin D analogues that were compared with potent corticosteroids reported withdrawal data (withdrawal data on calcipotriol versus desoxymetasone were not available) (see Analysis 7.6, Analysis 7.7, and Analysis 7.8). We pooled withdrawal data on these seven treatment comparison pairs using a random‐effects risk difference (RD) metric.
7.6. Analysis.
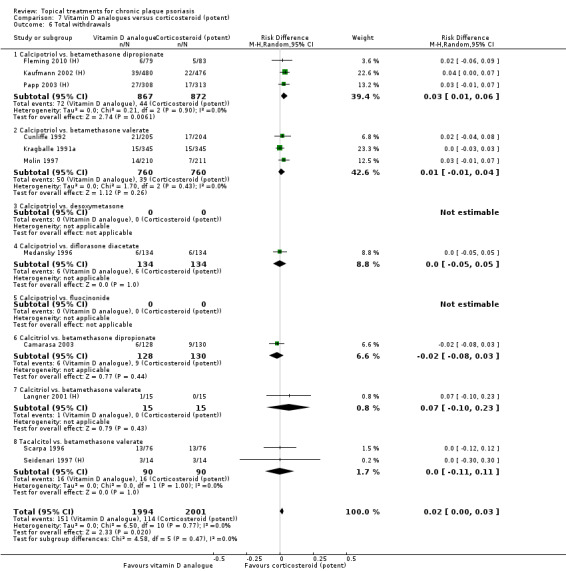
Comparison 7 Vitamin D analogues versus corticosteroid (potent), Outcome 6 Total withdrawals.
7.7. Analysis.
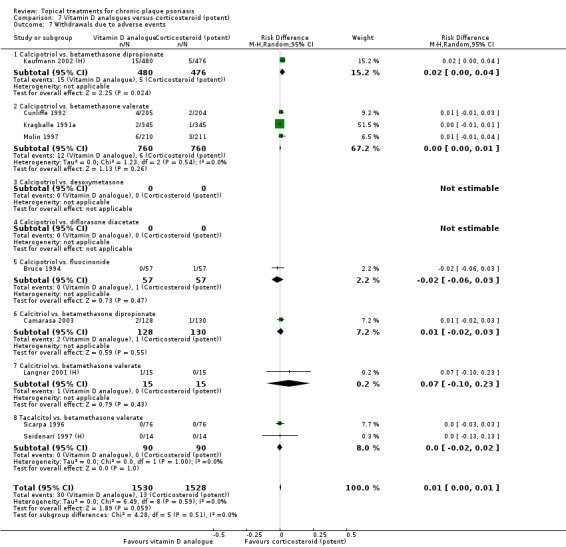
Comparison 7 Vitamin D analogues versus corticosteroid (potent), Outcome 7 Withdrawals due to adverse events.
7.8. Analysis.
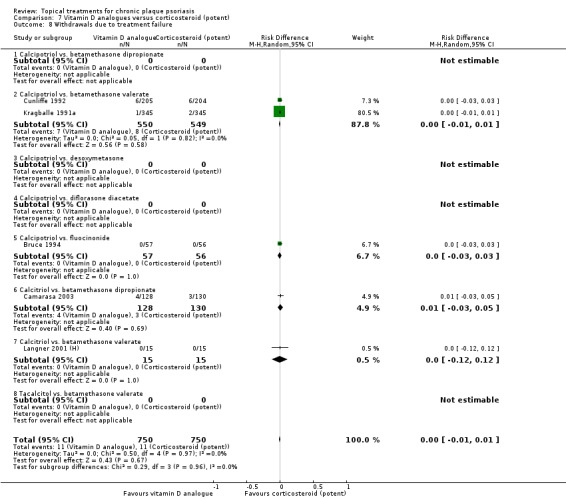
Comparison 7 Vitamin D analogues versus corticosteroid (potent), Outcome 8 Withdrawals due to treatment failure.
Relative to potent corticosteroid, there was a statistically significant difference in total withdrawals in favour of corticosteroids: RD 0.02 (95% CI 0.00 to 0.03; I² statistic = 0%). Pooled rates of withdrawals due to adverse events or treatment failure were not statistically significantly different. Regarding individual vitamin D analogues, the only statistically significant differences in withdrawals relative to corticosteroid were for the comparison of calcipotriol against betamethasone dipropionate (total withdrawals: RD 0.03 (95% CI 0.01 to 0.06; I² statistic = 0%); withdrawals due to adverse events: RD 0.02 (95% CI 0.00 to 0.04; I² statistic = NA)).
Analysis 8: Vitamin D analogues versus corticosteroid (very potent)
We pooled withdrawal data on calcipotriol against clobetasol propionate using a random‐effects risk difference (RD) metric (see Analysis 8.6, Analysis 8.7, and Analysis 8.8). Relative to the very potent corticosteroid, we found no statistically significant difference for calcipotriol on any of the withdrawal assessments: total withdrawals, withdrawals due to adverse events, or withdrawals due to treatment failure.
8.6. Analysis.
Comparison 8 Vitamin D analogues versus corticosteroid (very potent), Outcome 6 Total withdrawals.
8.7. Analysis.
Comparison 8 Vitamin D analogues versus corticosteroid (very potent), Outcome 7 Withdrawals due to adverse events.
8.8. Analysis.
Comparison 8 Vitamin D analogues versus corticosteroid (very potent), Outcome 8 Withdrawals due to treatment failure.
Analysis 9: Vitamin D combined with corticosteroid versus corticosteroid
We pooled withdrawal data on combination treatment (vitamin D and a corticosteroid) against monotherapy with a corticosteroid using a random‐effects risk difference (RD) metric (see Analysis 9.6, Analysis 9.7, and Analysis 9.8).
9.6. Analysis.
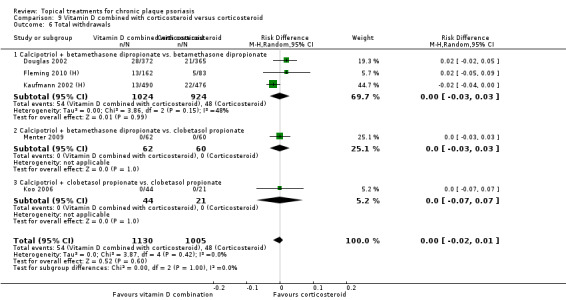
Comparison 9 Vitamin D combined with corticosteroid versus corticosteroid, Outcome 6 Total withdrawals.
9.7. Analysis.
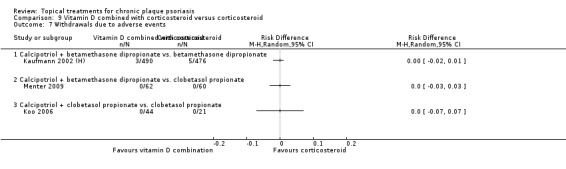
Comparison 9 Vitamin D combined with corticosteroid versus corticosteroid, Outcome 7 Withdrawals due to adverse events.
9.8. Analysis.
Comparison 9 Vitamin D combined with corticosteroid versus corticosteroid, Outcome 8 Withdrawals due to treatment failure.
Relative to the corticosteroid, we found no statistically significant difference for combination treatment on total withdrawals, withdrawals due to adverse events, or withdrawals due to treatment failure. There were no data on the rate of withdrawals due to treatment failure for the comparison of combined treatment with calcipotriol and betamethasone dipropionate versus betamethasone dipropionate monotherapy.
Analysis 10: vitamin D alone or in combination versus dithranol
Withdrawal data were available for three intervention‐comparator pairs: calcipotriol versus dithranol, calcitriol versus dithranol, and tacalcitol versus dithranol. We pooled these data using a random‐effects risk difference (RD) metric (see Analysis 10.6, Analysis 10.7, and Analysis 10.8). For the comparison of tacalcitol versus dithranol, there were no data available on withdrawals due to adverse events or treatment failure.
10.6. Analysis.
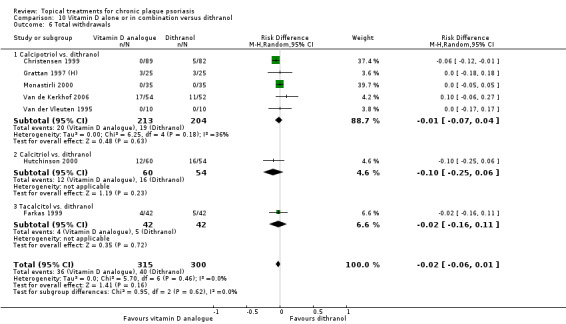
Comparison 10 Vitamin D alone or in combination versus dithranol, Outcome 6 Total withdrawals.
10.7. Analysis.
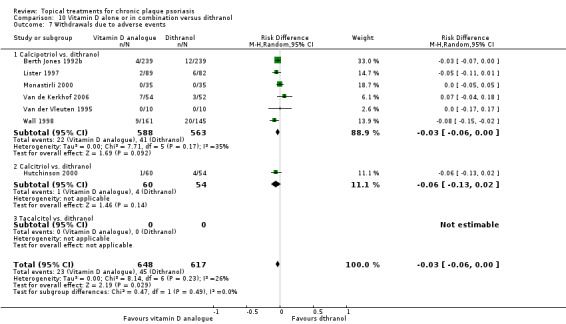
Comparison 10 Vitamin D alone or in combination versus dithranol, Outcome 7 Withdrawals due to adverse events.
10.8. Analysis.
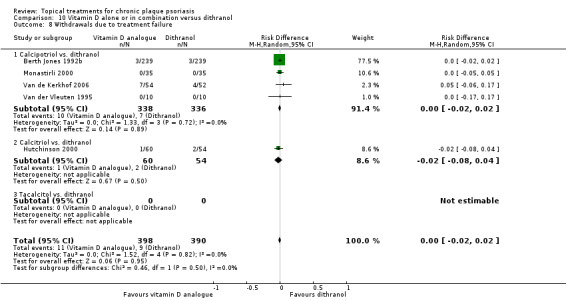
Comparison 10 Vitamin D alone or in combination versus dithranol, Outcome 8 Withdrawals due to treatment failure.
There was no statistically significant difference for total withdrawals, either for individual intervention‐comparator pairs or for the pooled effect (RD ‐0.02; 95% CI ‐0.06 to 0.01; I² statistic = 0%). The pooled risk difference showed a significant difference in favour of vitamin D for withdrawals due to adverse events (RD ‐0.03; 95% CI ‐0.06 to ‐0.00; I² statistic = 26.3%), but no significant difference in the rate of withdrawals due to treatment failure (RD ‐0.00; 95% CI ‐0.02 to 0.02; I² statistic = 0%).
Analysis 11: Vitamin D alone or in combination versus other vitamin D analogue
Two of the three vitamin D analogues head‐to‐head comparisons reported withdrawal data (withdrawal data on calcipotriol versus tacalcitol were not available). We pooled withdrawal data on calcipotriol versus calcitriol, and calcipotriol versus maxacalcitol, using a random‐effects risk difference (RD) metric (see Analysis 11.6, Analysis 11.7, and Analysis 11.8). We found no statistically significant difference on any of the withdrawal assessments: total withdrawals, withdrawals due to adverse events, or withdrawals due to treatment failure. There was also no significant difference in the withdrawal rates for the individual contrasts, calcipotriol versus calcitriol, and calcipotriol versus maxacalcitol.
11.6. Analysis.
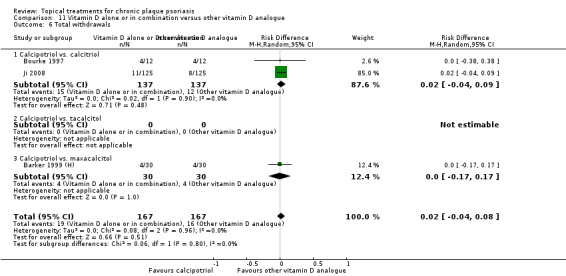
Comparison 11 Vitamin D alone or in combination versus other vitamin D analogue, Outcome 6 Total withdrawals.
11.7. Analysis.
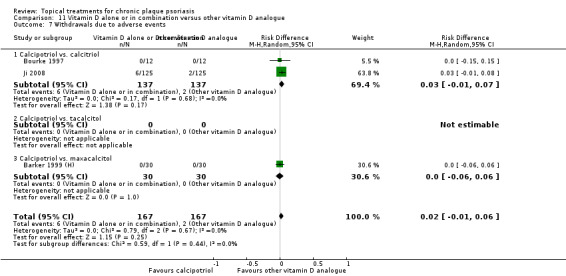
Comparison 11 Vitamin D alone or in combination versus other vitamin D analogue, Outcome 7 Withdrawals due to adverse events.
11.8. Analysis.
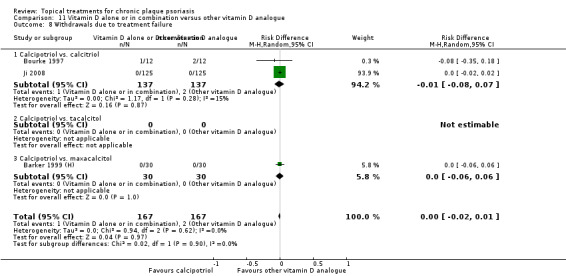
Comparison 11 Vitamin D alone or in combination versus other vitamin D analogue, Outcome 8 Withdrawals due to treatment failure.
Analysis 12: Vitamin D alone or in combination versus vitamin D + corticosteroid
To simplify the analysis, we summarised withdrawal data by grouping the first 10 intervention‐comparator pairs in a single category: calcipotriol versus calcipotriol and corticosteroid. The remaining comparisons were calcitriol versus calcitriol and corticosteroid, and tacalcitol versus calcipotriol and corticosteroid. We pooled withdrawal data using a random‐effects risk difference (RD) metric (see Analysis 12.6, Analysis 12.7, and Analysis 12.8). There were no data on withdrawals due to treatment failure for the comparisons of combined therapy versus calcitriol or combined therapy versus tacalcitol.
12.6. Analysis.
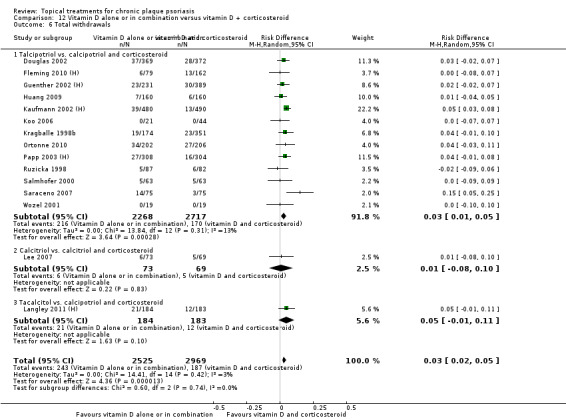
Comparison 12 Vitamin D alone or in combination versus vitamin D + corticosteroid, Outcome 6 Total withdrawals.
12.7. Analysis.
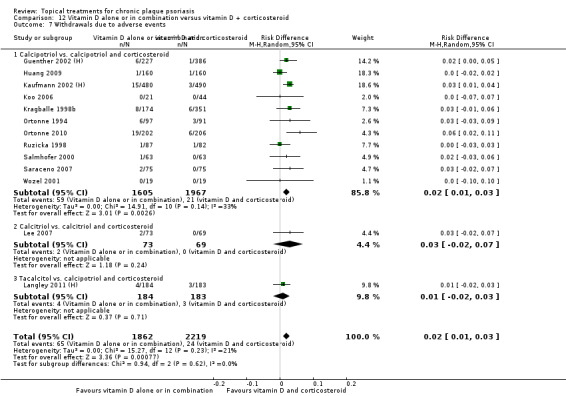
Comparison 12 Vitamin D alone or in combination versus vitamin D + corticosteroid, Outcome 7 Withdrawals due to adverse events.
12.8. Analysis.
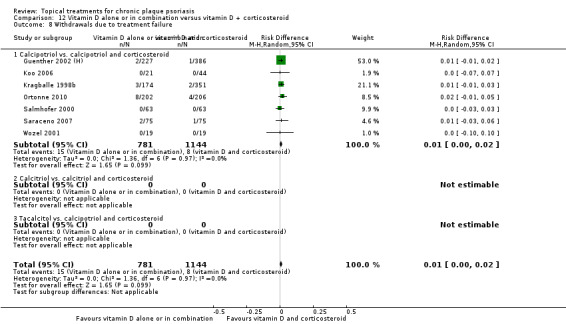
Comparison 12 Vitamin D alone or in combination versus vitamin D + corticosteroid, Outcome 8 Withdrawals due to treatment failure.
In the comparison of calcipotriol against calcipotriol plus corticosteroid, total withdrawals were statistically significantly different in favour of polytherapy: RD 0.03 (95% CI 0.01 to 0.05; I² statistic = 13.3%). We based this on findings from 4922 participants in 13 trials. Similarly, a significant difference in favour of polytherapy was evident from the data on withdrawals due to adverse events: RD 0.02 (95% CI 0.01 to 0.03; I² statistic = 32.9%). However, we found no statistically significant difference for withdrawals due to treatment failure (RD 0.01; 95% CI ‐0.00 to 0.02; I² statistic = 0%). When either calcitriol or tacalcitol was compared with combination therapy, differences in total withdrawals and in withdrawals due to adverse events were not statistically significant.
Analysis 13: Vitamin D alone or in combination versus other treatments: complex regimens
This comparison covers complex regimens, defined here as treatment sequences that do not consist of a simple head‐to‐head comparison between two active treatments. We summarised withdrawals rates on twelve intervention‐comparator contrasts (see Analysis 13.6, Analysis 13.7, and Analysis 13.8). There were data on total withdrawal rates for all 12 contrasts, but data on withdrawals due to adverse events were missing for 3 contrasts, and there were no data on withdrawals due to treatment failure for 6 contrasts.
13.6. Analysis.
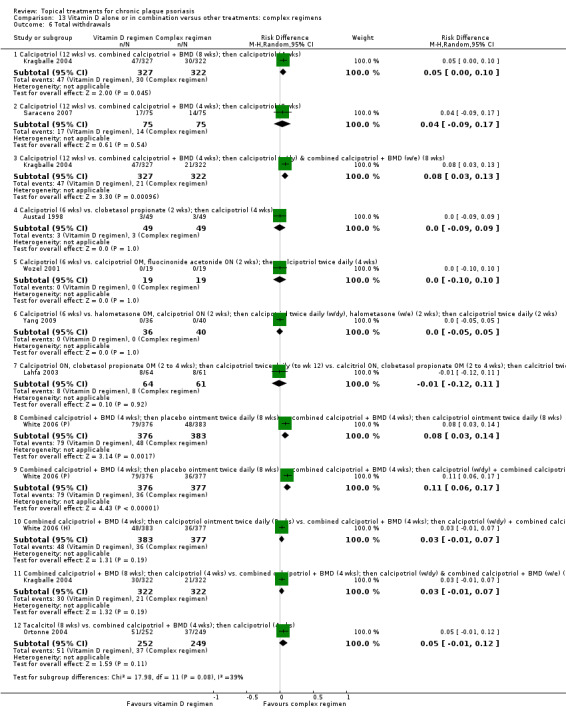
Comparison 13 Vitamin D alone or in combination versus other treatments: complex regimens, Outcome 6 Total withdrawals.
13.7. Analysis.
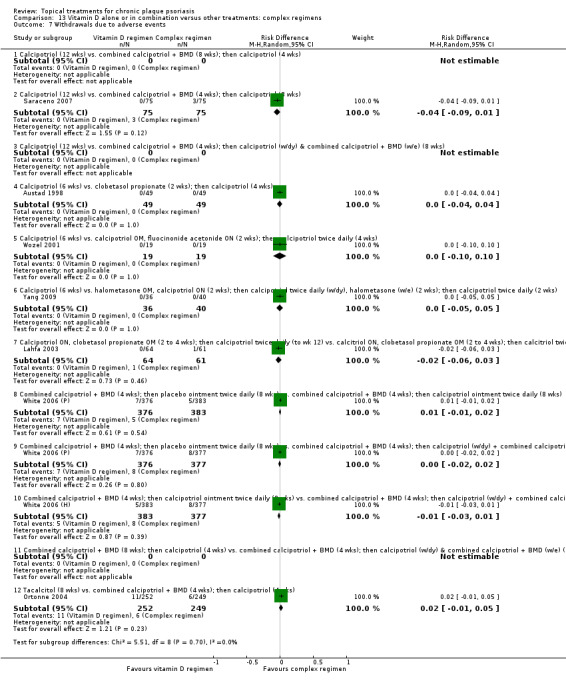
Comparison 13 Vitamin D alone or in combination versus other treatments: complex regimens, Outcome 7 Withdrawals due to adverse events.
13.8. Analysis.
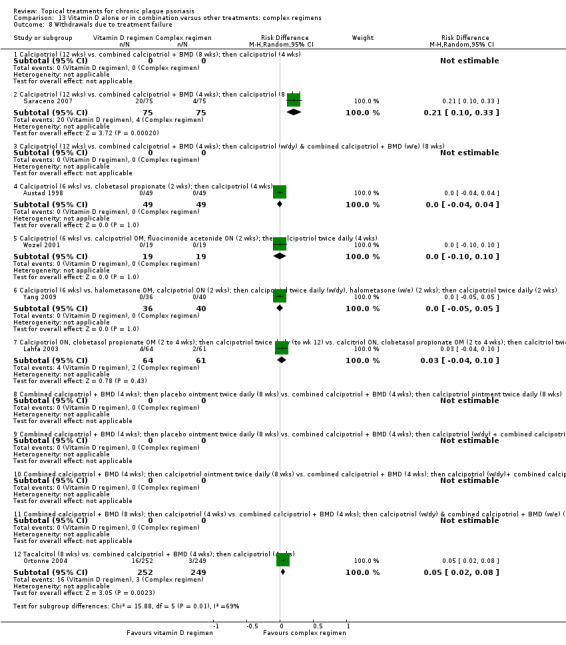
Comparison 13 Vitamin D alone or in combination versus other treatments: complex regimens, Outcome 8 Withdrawals due to treatment failure.
In the analysis of total withdrawals (Analysis 13.6), there was a significant difference in favour of the complex regimen for four contrasts:
Participants were significantly more likely to withdraw from the trial after 12 weeks of calcipotriol than participants who received combination therapy (8 weeks) followed by calcipotriol (4 weeks) (RD 0.05; 95% CI 0.00 to 0.10).
Trial withdrawal rates were also significantly higher in participants receiving 12 weeks of monotherapy with calcipotriol than those treated with combination therapy (4 weeks) followed by alternating calcipotriol on weekdays and combination therapy at the weekend (8 weeks) (RD 0.08; 95% CI 0.03 to 0.13).
After an initial 4‐week phase with combination ointment, participants who received 8 weeks' maintenance with calcipotriol (RD 0.08; 95% CI 0.03 to 0.14) or 8 weeks' maintenance with an alternating calcipotriol/combined therapy routine (RD 0.11; 95% CI 0.06 to 0.17) were significantly less likely to withdraw than participants who used vehicle ointment for maintenance.
In the analysis of withdrawals due to adverse events (Analysis 13.7), there were no significant differences between vitamin D and any of the complex regimens.
In the analysis of withdrawals due to treatment failure (Analysis 13.8), there were significant differences in two of the six contrasts for which we had reported data. After 12 weeks of calcipotriol, participants were significantly more likely to withdraw from the trial because of treatment failure than those who received combination therapy followed by calcipotriol (RD 0.21; 95% CI 0.10 to 0.33). Withdrawals due to treatment failure were more likely in participants who received 8 weeks' monotherapy with tacalcitol than those who received combination therapy followed by calcipotriol (RD 0.05; 95% CI 0.02 to 0.08).
Analysis 14: Vitamin D alone or in combination versus other treatment: long‐term studies (> 24 weeks)
This comparison included active‐controlled trials of psoriasis of the body that were at least 24 weeks in duration (see Analysis 14.6, Analysis 14.7, and Analysis 14.8). There were three intervention‐comparator contrasts, all 52 weeks in duration and all reported by a single study (Kragballe 2006).
Participants received one of three long‐term treatments:
once‐daily combined calcipotriol and betamethasone dipropionate;
combination therapy (4 weeks) followed by calcipotriol (for 4 weeks), rotated over 52 weeks; or
combination therapy for 4 weeks followed by calcipotriol for 48 weeks.
There were no significant differences between these three regimens in any of the withdrawal assessments: total withdrawals, withdrawals due to adverse events, or withdrawals due to treatment failure.
Analysis 15: Vitamin D analogues versus other treatment
This comparison compared vitamin D analogue with 12 other treatments. We assessed the results from the analyses of withdrawal data using a random‐effects risk difference (RD) metric, and we report these separately (see Analysis 15.6, Analysis 15.7, and Analysis 15.8). Withdrawal data were missing only in the head‐to‐head comparison of vitamin D (alone or in combination) versus calcipotriol with occlusion.
15.6. Analysis.
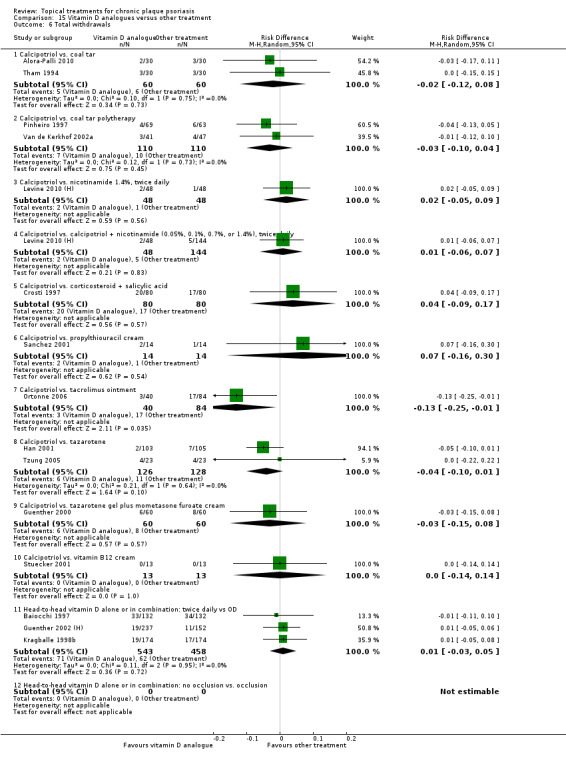
Comparison 15 Vitamin D analogues versus other treatment, Outcome 6 Total withdrawals.
15.7. Analysis.
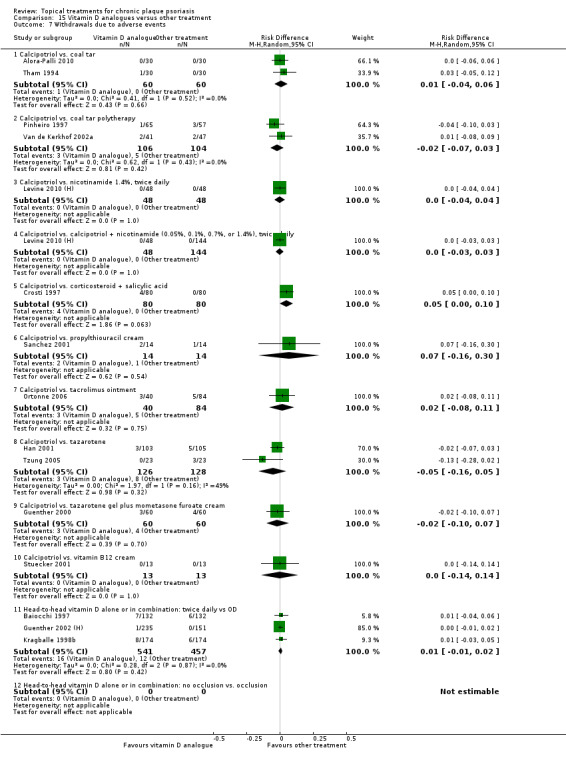
Comparison 15 Vitamin D analogues versus other treatment, Outcome 7 Withdrawals due to adverse events.
15.8. Analysis.
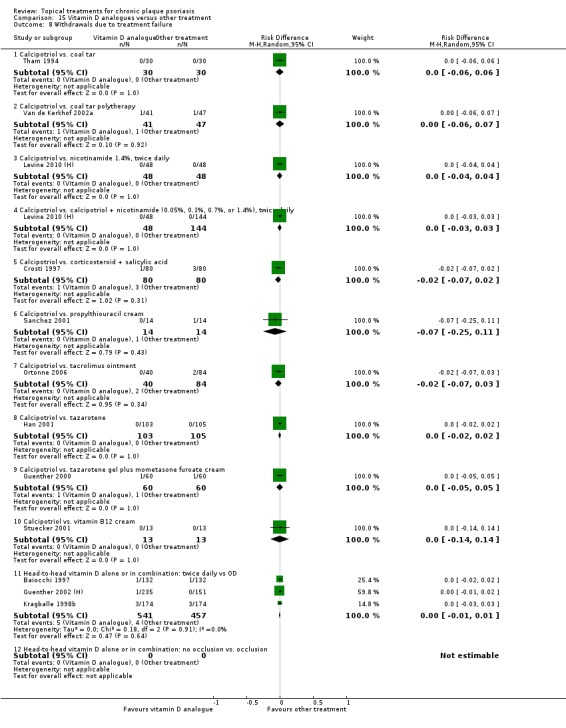
Comparison 15 Vitamin D analogues versus other treatment, Outcome 8 Withdrawals due to treatment failure.
Of the remaining 11 intervention‐comparator contrasts, we found only 1 statistically significant difference in withdrawal rates: The rate of total withdrawals was significantly lower for calcipotriol than for tacrolimus (RD ‐0.13; 95% CI ‐0.25 to ‐0.01).
Calcipotriol versus coal tar
In the comparison of calcipotriol and coal tar, we found no statistically significant difference on any of the withdrawal assessments: total withdrawals, withdrawals due to adverse events, or withdrawals due to treatment failure.
Calcipotriol versus coal tar polytherapy
In the comparison of calcipotriol and coal tar polytherapy, we found no statistically significant difference on any of the withdrawal assessments: total withdrawals, withdrawals due to adverse events, or withdrawals due to treatment failure.
Calcipotriol versus nicotinamide 1.4%, twice daily
We found no statistically significant difference on any of the withdrawal assessments: total withdrawals, withdrawals due to adverse events, or withdrawals due to treatment failure.
Calcipotriol versus calcipotriol + nicotinamide (0.05%, 0.1%, 0.7%, or 1.4%), twice daily
We found no statistically significant difference on any of the withdrawal assessments: total withdrawals, withdrawals due to adverse events, or withdrawals due to treatment failure.
Calcipotriol versus corticosteroid + salicylic acid
We found no statistically significant difference on any of the withdrawal assessments: total withdrawals, withdrawals due to adverse events, or withdrawals due to treatment failure. However, the risk difference for the withdrawal rate for adverse events was almost statistically significant in favour of combined therapy (RD 0.05; 95% CI ‐0.00 to 0.10).
Calcipotriol versus propylthiouracil cream
We found no statistically significant difference on any of the withdrawal assessments: total withdrawals, withdrawals due to adverse events, or withdrawals due to treatment failure.
Calcipotriol versus tacrolimus ointment
We found no statistically significant difference in withdrawals due to adverse events or withdrawals due to treatment failure. However, the rate of total withdrawals was significantly lower for calcipotriol than for tacrolimus (RD ‐0.13; 95% CI ‐0.25 to ‐0.01) (Ortonne 2006).
Calcipotriol versus tazarotene
We found no statistically significant difference on any of the withdrawal assessments: total withdrawals, withdrawals due to adverse events, or withdrawals due to treatment failure.
Calcipotriol versus tazarotene gel plus mometasone furoate cream
We found no statistically significant difference on any of the withdrawal assessments: total withdrawals, withdrawals due to adverse events, or withdrawals due to treatment failure.
Calcipotriol versus vitamin B12 cream
We found no statistically significant difference on any of the withdrawal assessments: total withdrawals, withdrawals due to adverse events, or withdrawals due to treatment failure.
Head‐to‐head vitamin D alone or in combination: dosing
We found no statistically significant difference on any of the withdrawal assessments: total withdrawals, withdrawals due to adverse events, or withdrawals due to treatment failure.
Head‐to‐head vitamin D alone or in combination: occlusion
We found no usable withdrawal data reported for this comparison.
Analysis 16: Flexural/facial psoriasis: placebo‐controlled trials
This comparison included placebo‐controlled trials of four topical treatments for inverse or facial psoriasis. Data on three withdrawal rates were available for all four treatments: the potent steroid betamethasone valerate; the vitamin D analogue calcipotriol; and two topical calcineurin inhibitors, pimecrolimus and tacrolimus (see Analysis 16.6, Analysis 16.7, and Analysis 16.8).
16.6. Analysis.
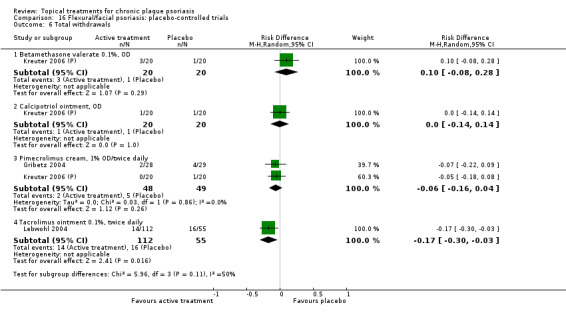
Comparison 16 Flexural/facial psoriasis: placebo‐controlled trials, Outcome 6 Total withdrawals.
16.7. Analysis.
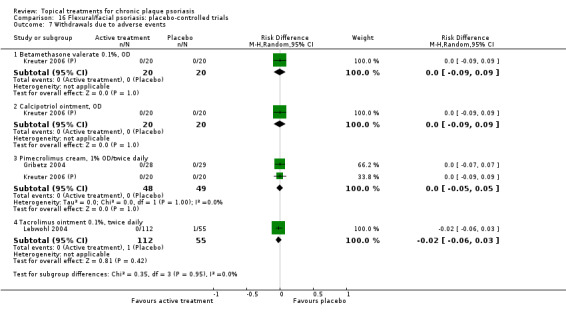
Comparison 16 Flexural/facial psoriasis: placebo‐controlled trials, Outcome 7 Withdrawals due to adverse events.
16.8. Analysis.
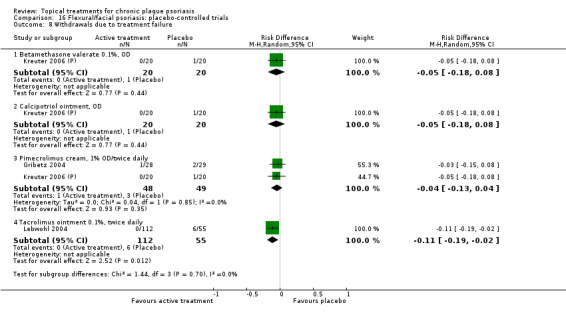
Comparison 16 Flexural/facial psoriasis: placebo‐controlled trials, Outcome 8 Withdrawals due to treatment failure.
Relative to placebo, participants receiving topical tacrolimus were significantly less likely to withdraw from treatment for any reason (RD ‐0.17; 95% CI ‐0.30 to ‐0.03) or because of treatment failure (RD ‐0.11; 95% CI ‐0.19 to ‐0.02). No other treatment was significantly different from placebo on any of the three withdrawal measures assessed.
Analysis 17: Flexural/facial psoriasis: vitamin D alone or in combination versus other treatment
This comparison included head‐to‐head trials of treatments for flexural or facial psoriasis, where we compared vitamin D with an active control (see Analysis 17.6, Analysis 17.7, and Analysis 17.8). We identified five intervention‐comparator contrasts, and no withdrawal data were missing. Four treatments were compared with calcipotriol: once‐daily betamethasone valerate, combined treatment with calcipotriol and hydrocortisone, calcitriol, and pimecrolimus. Calcitriol was compared with tacrolimus.
17.6. Analysis.
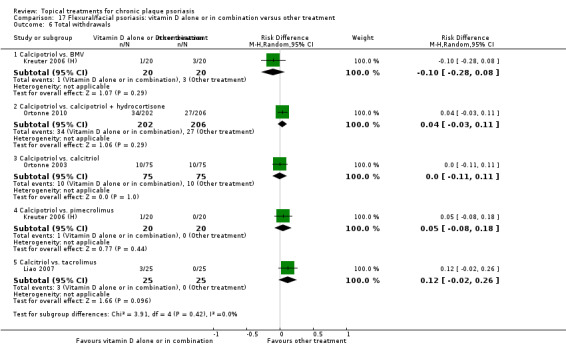
Comparison 17 Flexural/facial psoriasis: vitamin D alone or in combination versus other treatment, Outcome 6 Total withdrawals.
17.7. Analysis.
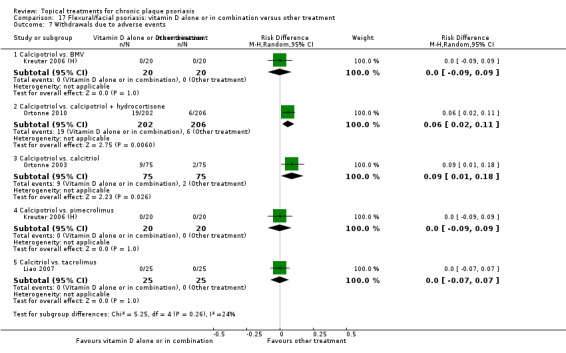
Comparison 17 Flexural/facial psoriasis: vitamin D alone or in combination versus other treatment, Outcome 7 Withdrawals due to adverse events.
17.8. Analysis.
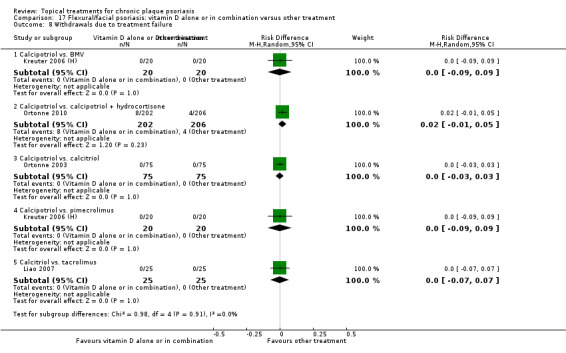
Comparison 17 Flexural/facial psoriasis: vitamin D alone or in combination versus other treatment, Outcome 8 Withdrawals due to treatment failure.
The rate of withdrawals due to adverse events was significantly higher in people treated with calcipotriol compared to those receiving combination therapy with calcipotriol and hydrocortisone for facial psoriasis (RD 0.06; 95% CI 0.02 to 0.11) and compared to those receiving calcitriol (RD 0.09; 95% CI 0.01 to 0.18). We found no other significant differences in withdrawal rates.
Analysis 18: Scalp psoriasis: placebo‐controlled trial
This comparison included placebo‐controlled trials of 11 topical treatments for scalp psoriasis (see Analysis 18.6, Analysis 18.7, and Analysis 18.8). All treatments had data on at least one withdrawal measure, which we assessed using a risk difference (RD) metric.
18.6. Analysis.
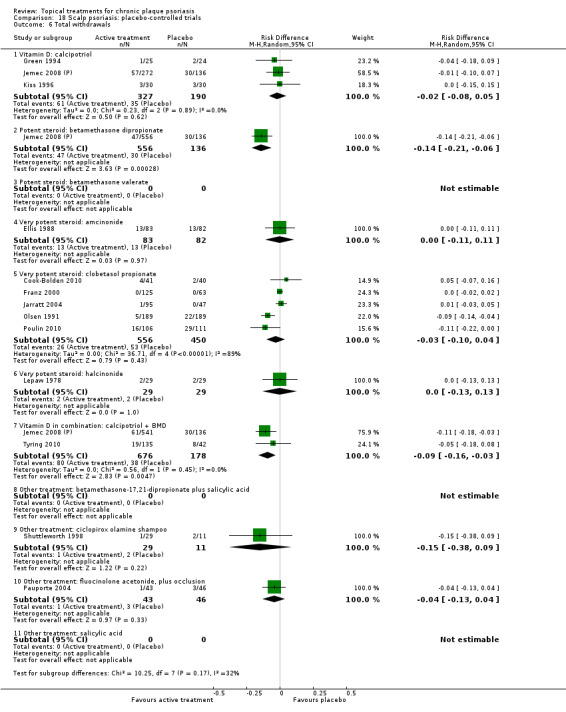
Comparison 18 Scalp psoriasis: placebo‐controlled trials, Outcome 6 Total withdrawals.
18.7. Analysis.
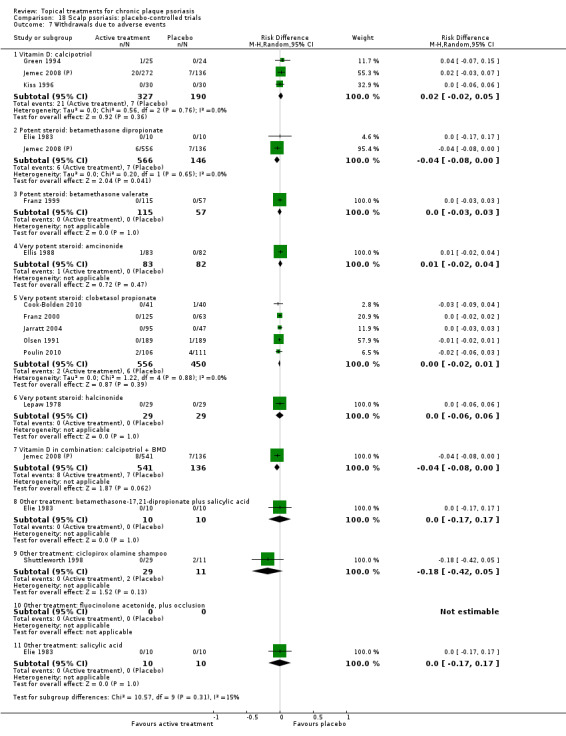
Comparison 18 Scalp psoriasis: placebo‐controlled trials, Outcome 7 Withdrawals due to adverse events.
18.8. Analysis.
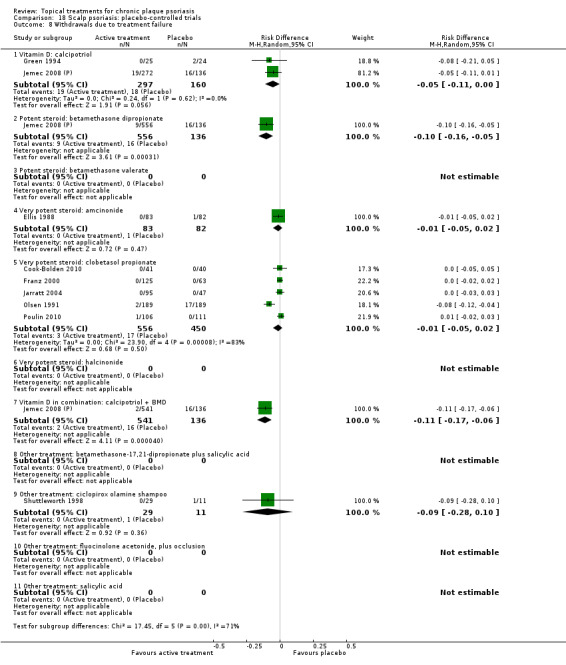
Comparison 18 Scalp psoriasis: placebo‐controlled trials, Outcome 8 Withdrawals due to treatment failure.
Relative to placebo, participants treated with betamethasone dipropionate were significantly less likely to withdraw from the trial for any reason (total withdrawals) (RD ‐0.14; 95% CI ‐0.21 to ‐0.06), because of adverse events (RD ‐0.04; 95% CI ‐0.08 to ‐0.00), or because of treatment failure (RD ‐0.10; 95% CI ‐0.16 to ‐0.05). Participants receiving combination therapy with calcipotriol and betamethasone dipropionate were significantly less likely to withdraw from the trial for any reason (total withdrawals) (RD ‐0.09; 95% CI ‐0.16 to ‐0.03) or because of treatment failure (RD ‐0.11; 95% CI ‐0.17 to ‐0.06). We found no other significant differences in withdrawal rates.
Analysis 19: Scalp psoriasis: vitamin D alone or in combination versus other treatment
This comparison included head‐to‐head trials of treatments for scalp psoriasis in which one of the interventions was a vitamin D product (used either as monotherapy or in combination with another product) (see Analysis 19.6, Analysis 19.7, and Analysis 19.8). We identified six intervention‐comparator contrasts. There were no data for withdrawal due to treatment failure for one of these contrasts (calcipotriol versus coal tar polytherapy). No other withdrawal data were missing.
19.6. Analysis.
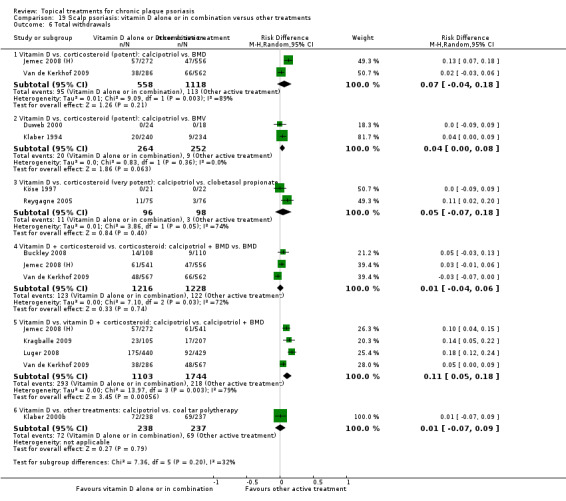
Comparison 19 Scalp psoriasis: vitamin D alone or in combination versus other treatments, Outcome 6 Total withdrawals.
19.7. Analysis.
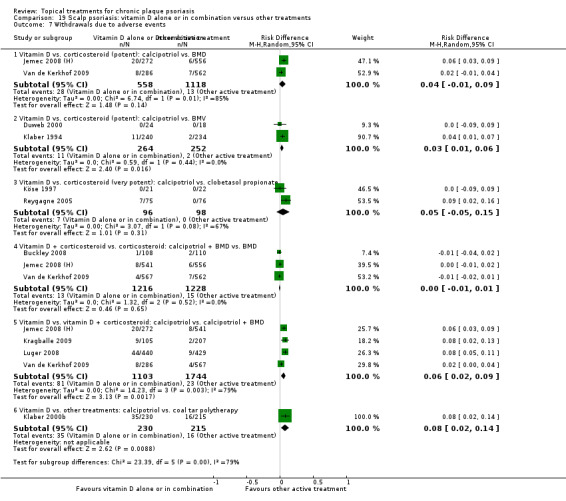
Comparison 19 Scalp psoriasis: vitamin D alone or in combination versus other treatments, Outcome 7 Withdrawals due to adverse events.
19.8. Analysis.
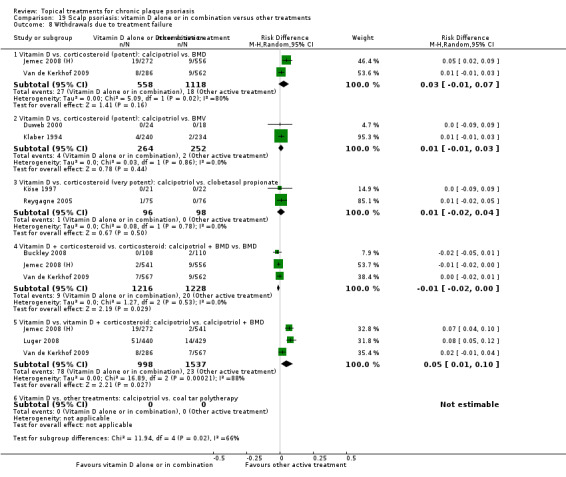
Comparison 19 Scalp psoriasis: vitamin D alone or in combination versus other treatments, Outcome 8 Withdrawals due to treatment failure.
Relative to combination treatment with calcipotriol and betamethasone dipropionate, calcipotriol monotherapy was associated with a significantly higher rate of total withdrawals (RD 0.11; 95% CI 0.05 to 0.18; I² statistic = 78.5%), withdrawals due to adverse events (RD 0.06; 95% CI 0.02 to 0.09; I² statistic = 78.9%) and withdrawals due to treatment failure (RD 0.05; 95% CI 0.01 to 0.10; I² statistic = 88.2%). Compared with betamethasone dipropionate, combination therapy was also significantly less likely to result in participant withdrawal due to treatment failure (RD ‐0.01; 95% CI ‐0.02 to ‐0.00; I² statistic = 0%).
Study participants treated with calcipotriol were significantly more likely to withdraw because of adverse events than participants receiving betamethasone valerate (RD 0.03; 95% CI 0.01 to 0.06; I² statistic = 0%) or coal tar polytherapy (RD 0.08; 95% CI 0.02 to 0.14; I² statistic = NA).
(b) Adverse events (local (cutaneous) and systemic)
(i) Findings from the main review
Analysis 1: Vitamin D analogues versus placebo
We pooled data on adverse events using a random‐effects risk difference metric (see Analysis 1.9 and Analysis 1.10). There were no adverse events data for the comparison of calcipotriol plus occlusion versus placebo. Therefore, data were available for seven of the eight vitamin D analogues evaluated against placebo
1.9. Analysis.
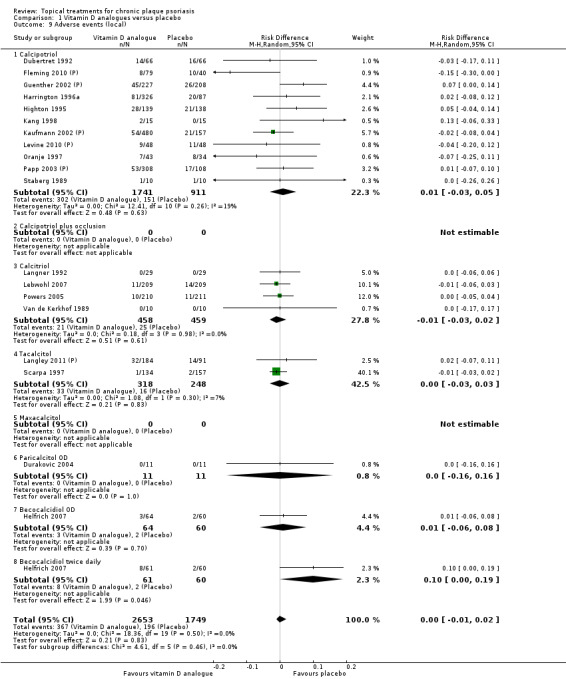
Comparison 1 Vitamin D analogues versus placebo, Outcome 9 Adverse events (local).
1.10. Analysis.
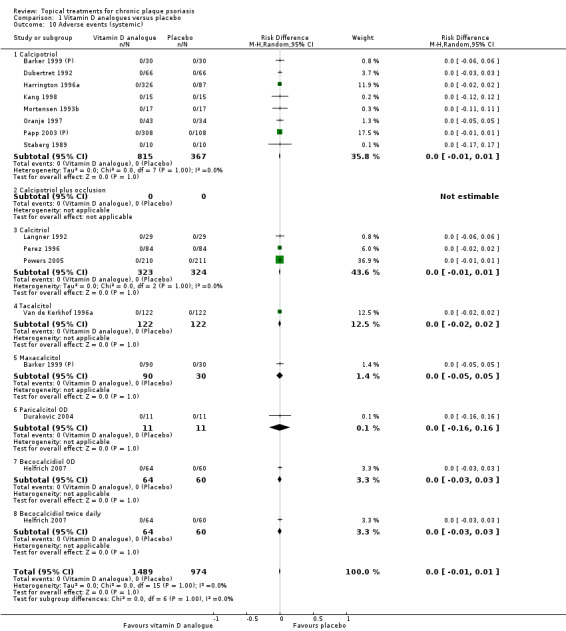
Comparison 1 Vitamin D analogues versus placebo, Outcome 10 Adverse events (systemic).
Of the seven vitamin D analogues evaluated against placebo, we found only one statistically significantly difference. Twice‐daily becocalcidiol was significantly more likely to cause local adverse events than placebo (RD 0.10; 95% CI 0.00 to 0.19). There were no significant differences for the pooled risk difference for either local adverse events (RD 0.00; 95% CI ‐0.01 to 0.02; I² statistic = 0%) or systemic adverse events (RD 0.00; 95% CI ‐0.01 to 0.01; I² statistic = 0%).
Analysis 2: Corticosteroid (potent) versus placebo
We pooled data on adverse events using a random‐effects risk difference metric (see Analysis 2.9 and Analysis 2.10). Data on local adverse events were available for 7 of the 10 corticosteroids; data on systemic adverse events were available only for betamethasone dipropionate twice daily, betamethasone dipropionate (maintenance), and mometasone furoate.
2.9. Analysis.
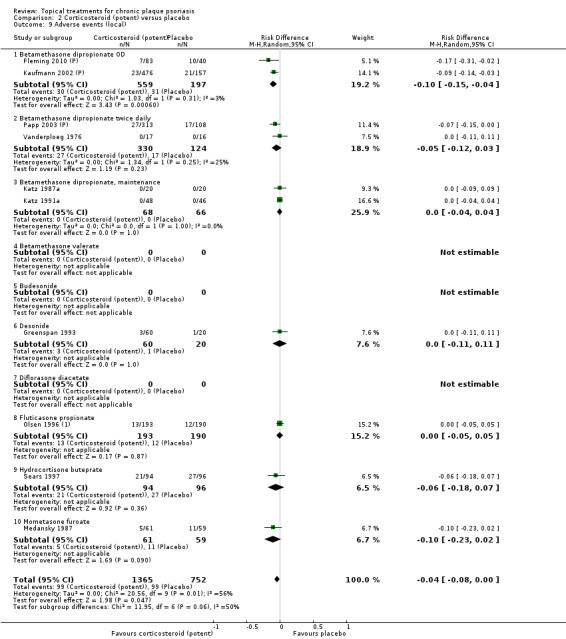
Comparison 2 Corticosteroid (potent) versus placebo, Outcome 9 Adverse events (local).
2.10. Analysis.
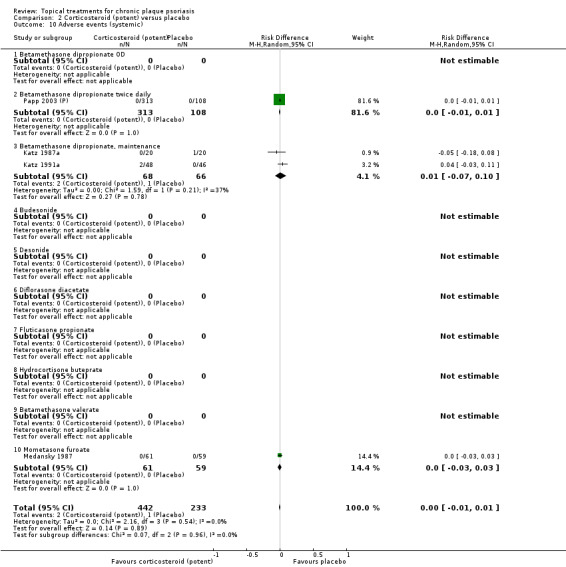
Comparison 2 Corticosteroid (potent) versus placebo, Outcome 10 Adverse events (systemic).
The pooled analysis of local adverse events found a significant difference in favour of corticosteroids (RD ‐0.04; 95% CI ‐0.08 to ‐0.00; I² statistic = 56.2%). Among the individual potent corticosteroids evaluated against placebo, we found only one statistically significantly difference. Once‐daily betamethasone dipropionate was less likely than placebo to be associated with local adverse events: RD ‐0.10 (95% CI ‐0.15 to ‐0.04; I² statistic = 2.5%). We found no significant difference in the pooled analyses of systemic adverse events data.
Analysis 3: Corticosteroid (very potent) versus placebo
We pooled data on adverse events using a random‐effects risk difference metric (see Analysis 3.9 and Analysis 3.10). The comparison with placebo found no statistically significant difference for any of the three very potent corticosteroids considered for either local adverse events or systemic adverse events.
3.9. Analysis.
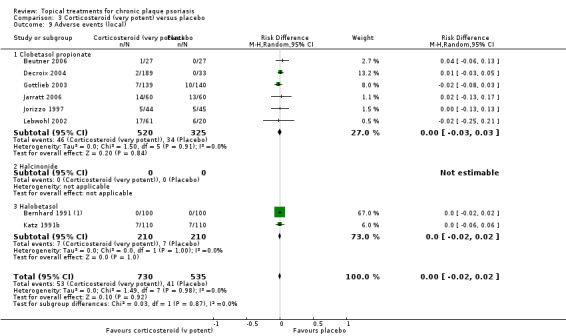
Comparison 3 Corticosteroid (very potent) versus placebo, Outcome 9 Adverse events (local).
3.10. Analysis.
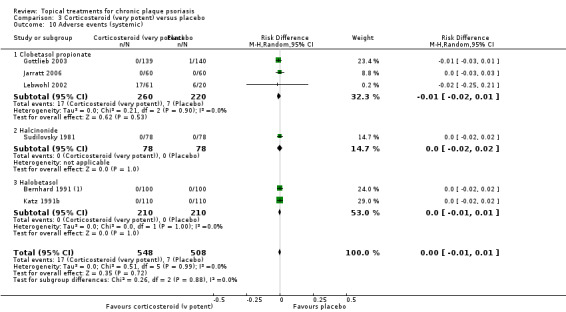
Comparison 3 Corticosteroid (very potent) versus placebo, Outcome 10 Adverse events (systemic).
Analysis 4: Dithranol versus placebo
We pooled data on adverse events using a random‐effects risk difference metric (see Analysis 4.9 and Analysis 4.10). The comparison with placebo found no statistically significant difference for pooled findings on local adverse events or systemic adverse events. In one study, a significant difference in favour of placebo was evident: RD 0.40 (95% CI 0.08 to 0.72; I² statistic = NA) (Volden 1992).
4.9. Analysis.
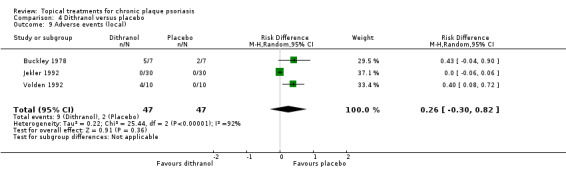
Comparison 4 Dithranol versus placebo, Outcome 9 Adverse events (local).
4.10. Analysis.
Comparison 4 Dithranol versus placebo, Outcome 10 Adverse events (systemic).
Analysis 5: Vitamin D combination products versus placebo
We pooled data on adverse events using a random‐effects risk difference metric (see Analysis 5.9 and Analysis 5.10). The rate of local adverse events was significantly lower for combination therapy compared with placebo (RD ‐0.05; 95% CI ‐0.08 to ‐0.02; I² statistic = 10.0%). There was no significant difference in the rate of systemic adverse events.
5.9. Analysis.
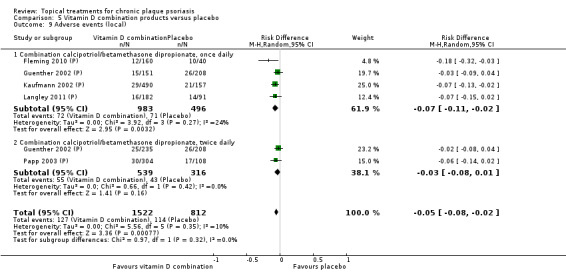
Comparison 5 Vitamin D combination products versus placebo, Outcome 9 Adverse events (local).
5.10. Analysis.
Comparison 5 Vitamin D combination products versus placebo, Outcome 10 Adverse events (systemic).
Analysis 6: Other treatment versus placebo
Our review reports findings separately for 22 of the 26 comparisons considered (see Analysis 6.9 and Analysis 6.10). No data on adverse events were available for placebo comparisons involving polymyxin B cream, topical sirolimus, topical tacrolimus, coal tar, or tazarotene. Data on local adverse events were available for 22 comparisons, but data on systemic adverse events were reported for just 9 of the 26 comparisons. We assessed data on adverse events using a random‐effects risk difference metric. The comparison with placebo found no statistically significant difference in the rate of either local or systemic adverse events for any treatment.
6.9. Analysis.
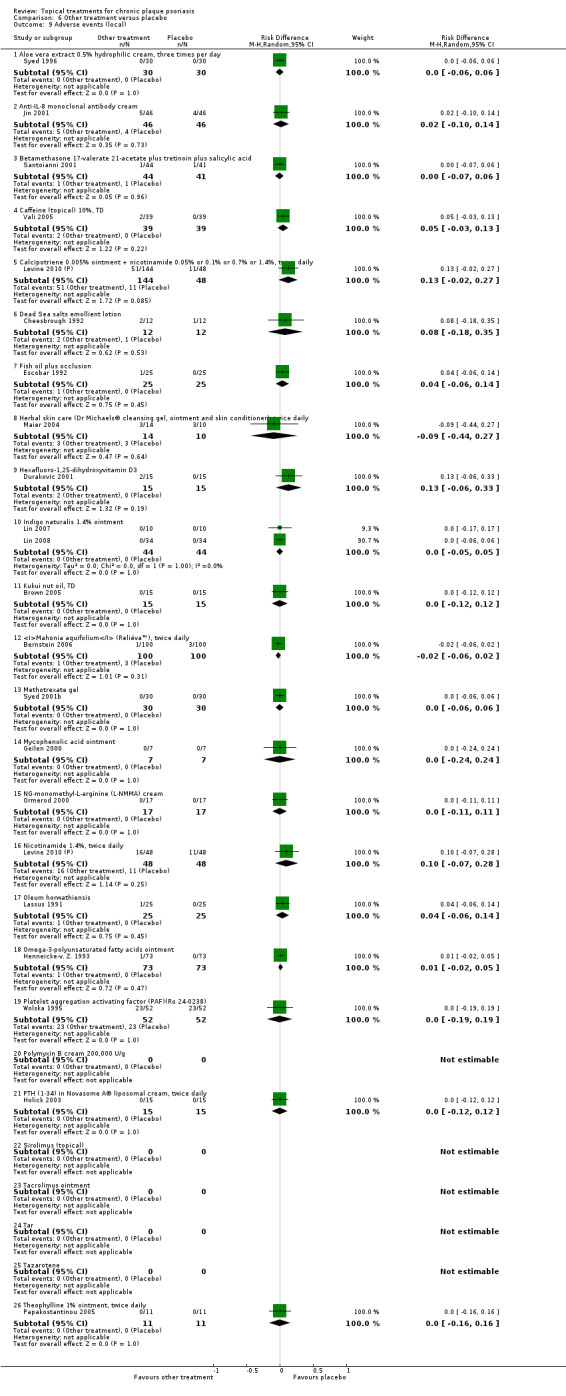
Comparison 6 Other treatment versus placebo, Outcome 9 Adverse events (local).
6.10. Analysis.
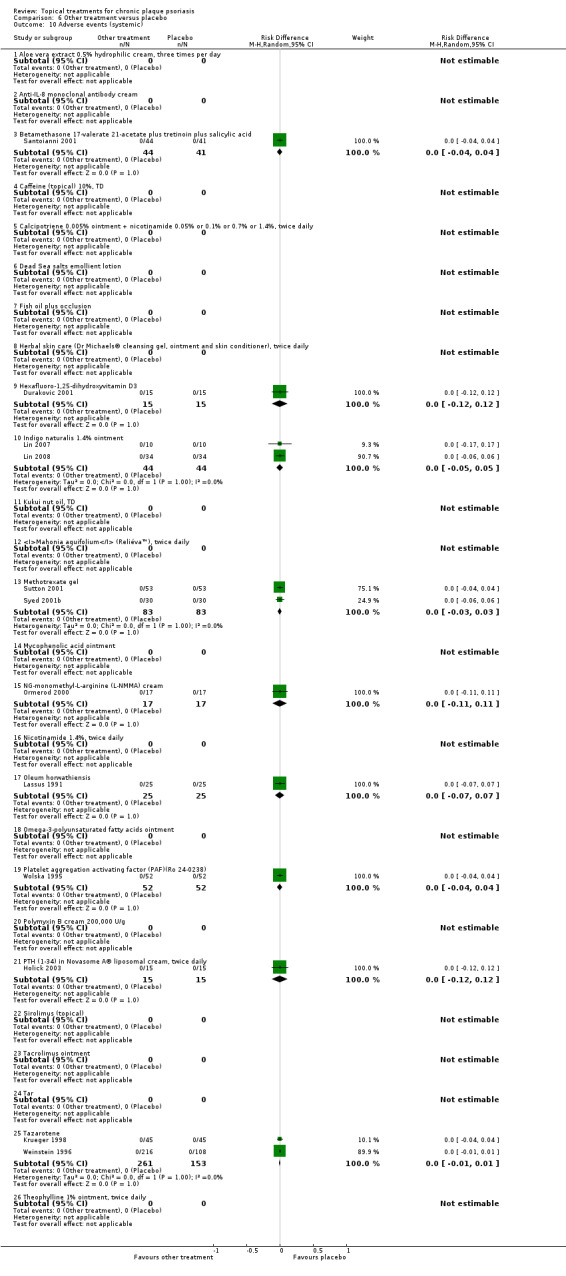
Comparison 6 Other treatment versus placebo, Outcome 10 Adverse events (systemic).
Analysis 7: Vitamin D analogues versus corticosteroid (potent)
Of the eight vitamin D analogues that were compared with potent corticosteroids, data on local adverse events were available for five comparisons, and data on systemic events were available for four comparisons (see Analysis 7.9 and Analysis 7.10). There were no data on adverse events for calcipotriol versus desoxymetasone, calcipotriol versus diflorasone diacetate, and calcitriol versus betamethasone valerate. In addition, systemic adverse events data were unavailable for calcipotriol versus fluocinonide. We pooled data on adverse effects using a random‐effects risk difference metric.
7.9. Analysis.
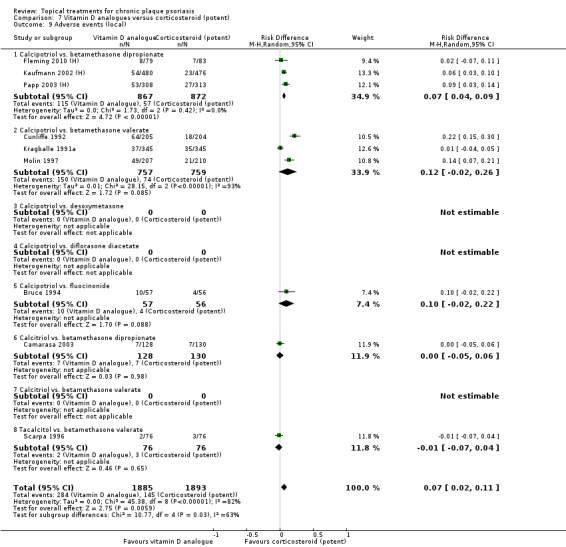
Comparison 7 Vitamin D analogues versus corticosteroid (potent), Outcome 9 Adverse events (local).
7.10. Analysis.
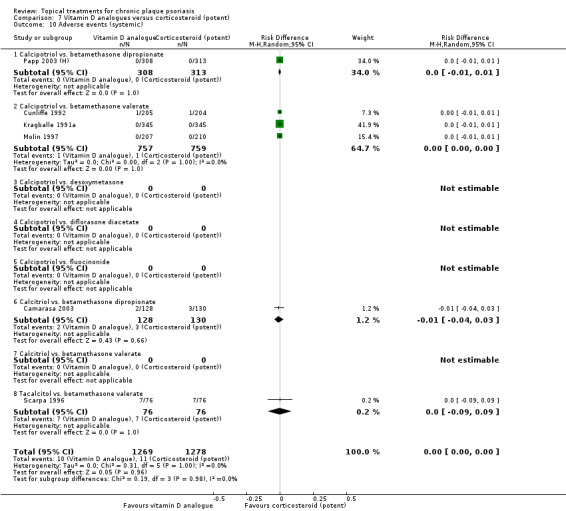
Comparison 7 Vitamin D analogues versus corticosteroid (potent), Outcome 10 Adverse events (systemic).
In the comparison of individual vitamin D analogues and potent corticosteroids, our review found one statistically significant difference in favour of potent corticosteroids. Pooled data from 3 trials with 1739 participants indicated that the rate of local adverse events was significantly higher in the calcipotriol group than in the group receiving betamethasone dipropionate (RD 0.07; 95% CI 0.04 to 0.09; I² statistic = 0%). This finding drove the pooled (class) result for local adverse events (RD 0.07; 95% CI 0.02 to 0.11; I² statistic = 82.4%). Our review found no other statistically significant differences for local or systemic adverse events in this comparison.
Analysis 8: Vitamin D analogues versus corticosteroid (very potent)
We pooled withdrawal data on adverse effects using a random‐effects risk difference metric (see Analysis 8.9 and Analysis 8.10). There was no statistically significantly difference between calcipotriol and clobetasol propionate, either for local adverse events (RD ‐0.05; 95% CI ‐0.18 to 0.08) or systemic events (RD ‐0.05; 95% CI ‐0.18 to 0.08).
8.9. Analysis.
Comparison 8 Vitamin D analogues versus corticosteroid (very potent), Outcome 9 Adverse events (local).
8.10. Analysis.
Comparison 8 Vitamin D analogues versus corticosteroid (very potent), Outcome 10 Adverse events (systemic).
Analysis 9: Vitamin D combined with corticosteroid versus corticosteroid
We pooled withdrawal data on combination treatment (vitamin D and a corticosteroid) against monotherapy with a corticosteroid using a random‐effects risk difference metric (see Analysis 9.9 and Analysis 9.10). No adverse events data were available for the comparison of combination (calcipotriol/clobetasol propionate) therapy and clobetasol propionate.
9.9. Analysis.
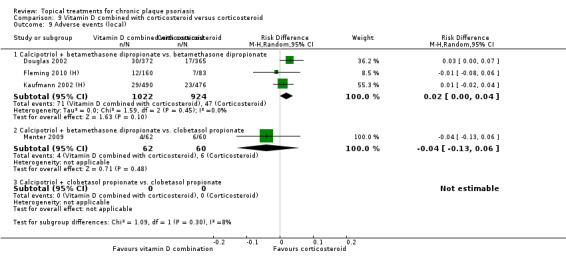
Comparison 9 Vitamin D combined with corticosteroid versus corticosteroid, Outcome 9 Adverse events (local).
9.10. Analysis.
Comparison 9 Vitamin D combined with corticosteroid versus corticosteroid, Outcome 10 Adverse events (systemic).
The pooled analysis found no statistically significant difference in adverse event rates between combination (calcipotriol/betamethasone dipropionate) therapy and betamethasone dipropionate. There was no significant difference in local adverse events for combination (calcipotriol/betamethasone) therapy and clobetasol propionate, and there were no data on systemic adverse events for this comparison.
Analysis 10: Vitamin D alone or in combination versus dithranol
Adverse events data were available for three intervention‐comparator pairs: calcipotriol versus dithranol, calcitriol versus dithranol, and tacalcitol versus dithranol. We pooled these data using a random‐effects risk difference metric (see Analysis 10.9 and Analysis 10.10).
10.9. Analysis.
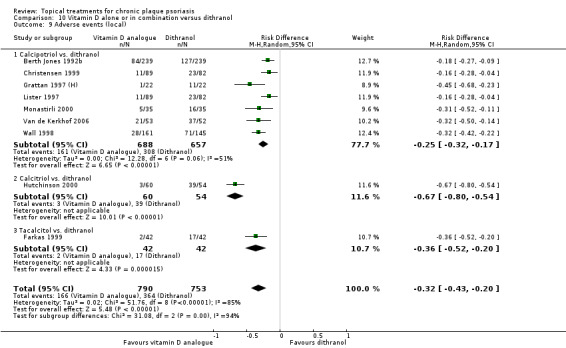
Comparison 10 Vitamin D alone or in combination versus dithranol, Outcome 9 Adverse events (local).
10.10. Analysis.
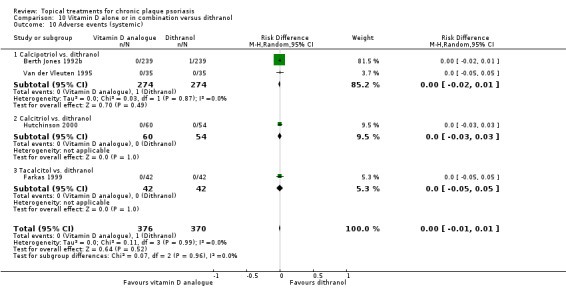
Comparison 10 Vitamin D alone or in combination versus dithranol, Outcome 10 Adverse events (systemic).
Vitamin D was statistically significantly less likely to cause local adverse events (RD ‐0.32; 95% CI ‐0.43 to ‐0.20; I² statistic = 84.5%), although substantial heterogeneity was evident in the pooled statistic (Higgins 2011). This finding also held for each of the three intervention‐comparator pairs, with the effect size ranging from ‐0.25 (calcipotriol versus dithranol) to ‐0.67 (calcitriol versus dithranol). However, the analysis of systemic adverse events found no statistically significant differences.
Analysis 11: Vitamin D alone or in combination versus other vitamin D analogue
We reported local adverse events data on two of the vitamin D analogues head‐to‐head comparisons (see Analysis 11.9 and Analysis 11.10). Systemic adverse events data were not available for any of the three comparisons. We pooled data on adverse effects using a random‐effects risk difference metric. Overall, our review found no statistically significant difference on either local or systemic adverse events when comparing pooled vitamin D analogues head‐to‐head. However, in the comparison of calcipotriol against calcitriol, the rate of local adverse events was significantly lower for calcitriol: RD 0.07 (95% CI 0.01 to 0.14; I² statistic = NA).
11.9. Analysis.
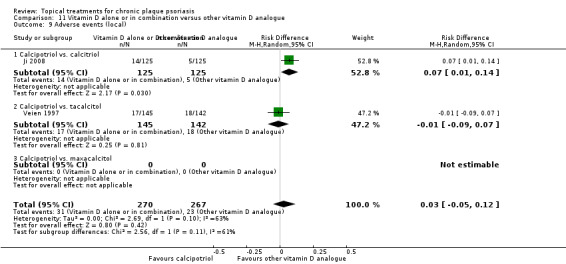
Comparison 11 Vitamin D alone or in combination versus other vitamin D analogue, Outcome 9 Adverse events (local).
11.10. Analysis.
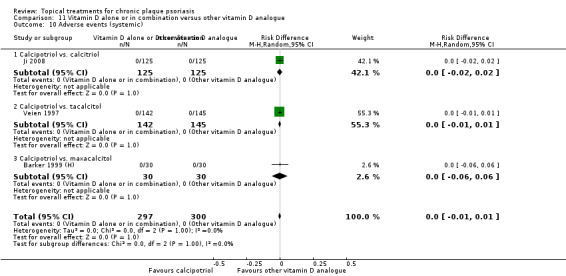
Comparison 11 Vitamin D alone or in combination versus other vitamin D analogue, Outcome 10 Adverse events (systemic).
Analysis 12: Vitamin D alone or in combination versus vitamin D + corticosteroid
To simplify the comparison, we summarised adverse events data by grouping the first 10 intervention‐comparator pairs in a single category: calcipotriol versus calcipotriol and corticosteroid. The remaining comparisons were 1) calcitriol versus calcitriol and 2) corticosteroid and tacalcitol versus calcipotriol and corticosteroid. There were no data on systemic adverse events for the comparison of tacalcitol against calcipotriol and corticosteroid.
We pooled data on adverse effects using a random‐effects risk difference (RD) metric (see Analysis 12.9 and Analysis 12.10). In the local adverse events comparison of vitamin D against vitamin D plus corticosteroid, our review found a statistically significantly difference in favour of combination therapy: RD 0.06 (95% CI 0.05 to 0.08; I² statistic = 4.1%). This finding also held for each individual intervention‐comparator pair. Our analysis found no statistically significantly difference in the rates of systemic adverse events.
12.9. Analysis.
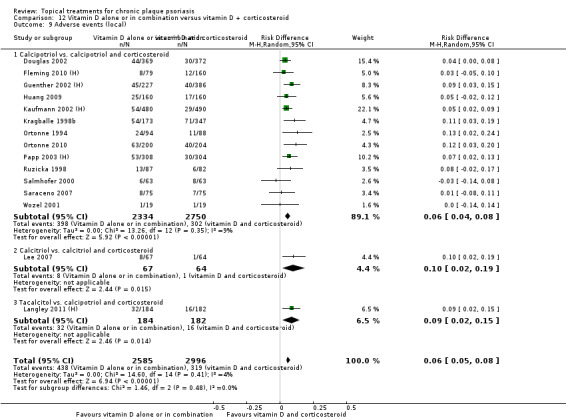
Comparison 12 Vitamin D alone or in combination versus vitamin D + corticosteroid, Outcome 9 Adverse events (local).
12.10. Analysis.
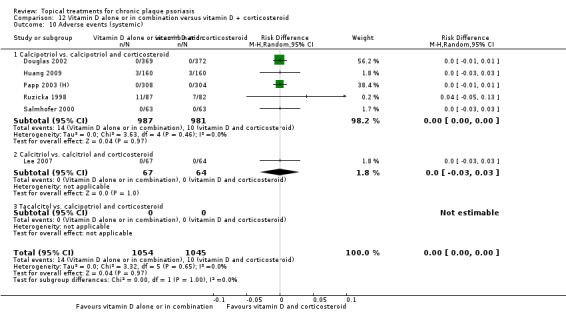
Comparison 12 Vitamin D alone or in combination versus vitamin D + corticosteroid, Outcome 10 Adverse events (systemic).
Analysis 13: Vitamin D alone or in combination versus other treatments: complex regimens
This comparison included 12 intervention‐comparator contrasts (see Analysis 13.9 and Analysis 13.10). Data on local adverse events were available for 11 contrasts, but there were no data on the comparison of calcipotriol (12 weeks) versus combination therapy (4 weeks) followed by calcipotriol (8 weeks). No trial in this comparison reported data on systemic adverse events.
13.9. Analysis.
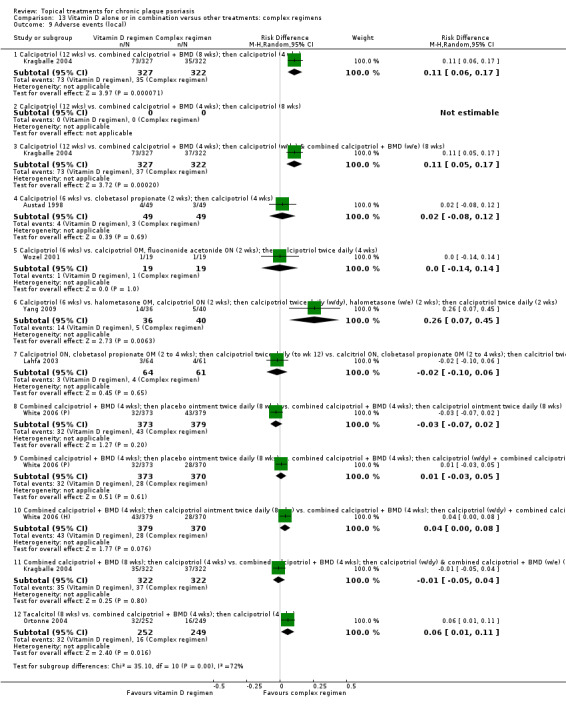
Comparison 13 Vitamin D alone or in combination versus other treatments: complex regimens, Outcome 9 Adverse events (local).
In 4 of the 11 contrasts for which data were available, the complex regimen was associated with a significantly lower rate of local adverse events than when the vitamin D analogue was used alone. Twelve weeks of calcipotriol monotherapy was associated with significantly higher rates of local adverse events than two complex regimen comparators. Specifically, the rate of local adverse events was higher for calcipotriol monotherapy than for a regimen consisting of 8 weeks of combination therapy followed by 4 weeks of once‐daily calcipotriol (RD 0.11; 95% CI 0.06 to 0.17). Calcipotriol monotherapy was also more likely to be associated with local adverse events than 4 weeks of combination therapy followed by 8 weeks of once‐daily calcipotriol on weekdays and combination therapy at the weekend (RD 0.11; 95% CI 0.05 to 0.17) (Kragballe 2004). When we compared the two complex regimens directly, the rates of cutaneous adverse events were not significantly different.
Relative to calcipotriol, combination therapy with halometasone and calcipotriol had a significantly lower rate of adverse events of the skin (RD 0.26; 95% CI 0.07 to 0.45). An 8‐week course of tacalcitol had significantly higher rates of adverse events than a routine of 4 weeks' combined (calcipotriol/betamethasone dipropionate) therapy followed by 4 weeks' monotherapy with calcipotriol (RD 0.06; 95% CI 0.01 to 0.11).
There was no significant difference in the rate of local adverse events in any of the remaining seven intervention‐comparator contrasts.
Analysis 14: Vitamin D alone or in combination versus other treatment: long‐term studies (> 24 weeks)
This comparison included active‐controlled studies of psoriasis of the body that were at least 24 weeks in duration (see Analysis 14.9 and Analysis 14.10). No data on systemic adverse events were reported. We included one trial in this comparison: Kragballe 2006 compared 3 52‐week regimens with all treatments used once daily.
14.9. Analysis.
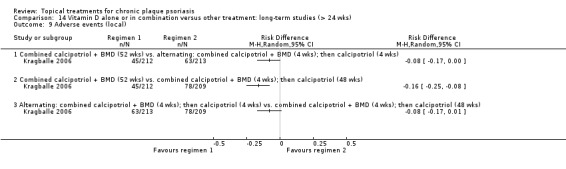
Comparison 14 Vitamin D alone or in combination versus other treatment: long‐term studies (> 24 wks), Outcome 9 Adverse events (local).
Combination therapy with calcipotriol and betamethasone dipropionate was associated with significantly lower rates of adverse events than either of the two 'complex' long‐term regimens:
alternating treatment with combination therapy for 4 weeks, then calcipotriol for 4 weeks (RD ‐0.08; 95% CI ‐0.17 to ‐0.00); and
combination therapy for 4 weeks, then calcipotriol for 48 weeks (RD ‐0.16; 95% CI ‐0.25 to ‐0.08).
The difference between the two 'complex' regimens was not statistically significant at the 5% level (RD ‐0.08; 95% CI ‐0.17 to 0.01), although there was a trend in favour of alternating therapy.
Analysis 15: vitamin D analogues versus other treatment
A vitamin D analogue was compared with 12 other treatments (see Analysis 15.9 and Analysis 15.10). Data on local adverse events were available for 10 intervention‐comparator contrasts, and systemic adverse events data were available for 8 intervention‐comparator contrasts.
15.9. Analysis.

Comparison 15 Vitamin D analogues versus other treatment, Outcome 9 Adverse events (local).
15.10. Analysis.
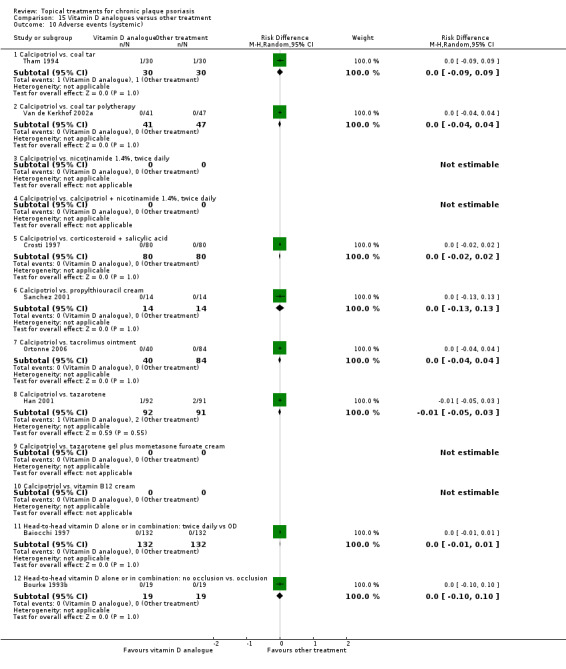
Comparison 15 Vitamin D analogues versus other treatment, Outcome 10 Adverse events (systemic).
We assessed results from the analyses of adverse events data using a random‐effects risk difference metric and report these separately. For local adverse events, three intervention‐comparator contrasts were significantly different. These were the comparison of calcipotriol against calcipotriol + nicotinamide (RD ‐0.17; 95% CI ‐0.30 to ‐0.03), calcipotriol versus corticosteroid + salicylic acid (RD 0.09; 95% CI 0.02 to 0.15), and calcipotriol versus tacrolimus ointment (RD ‐0.19; 95% CI ‐0.37 to ‐0.01). No trial that reported the rate of systemic adverse events found a significant difference in this outcome.
Calcipotriol versus coal tar
Our review found no statistically significant difference for local or systemic adverse events.
Calcipotriol versus coal tar polytherapy
Our review found no statistically significant difference for local or systemic adverse events.
Calcipotriol versus nicotinamide 1.4%, twice daily
Systemic adverse events data were not reported for this analysis. There was no significant difference between the local adverse event rates, although there was a trend in favour of calcipotriol (RD ‐0.15; 95% CI ‐0.32 to 0.03; I² statistic = NA).
Calcipotriol versus calcipotriol + nicotinamide (0.05%, 0.1%, 0.7%, or 1.4%), twice daily
Systemic adverse events data were not reported for this analysis. Monotherapy with calcipotriol was significantly less likely to cause local adverse events than combined therapy with nicotinamide (RD ‐0.17; 95% CI ‐0.30 to ‐0.03; I² statistic = NA).
Calcipotriol versus corticosteroid + salicylic acid
Relative to calcipotriol alone, combination therapy with betamethasone dipropionate and salicylic acid was significantly less likely to be associated with local adverse events (RD 0.09; 95% CI 0.02 to 0.15). There was no significant difference in the rate of systemic adverse events.
Calcipotriol versus propylthiouracil cream
Our review found no statistically significant difference for local or systemic adverse events.
Calcipotriol versus tacrolimus ointment
Relative to calcipotriol, tacrolimus was associated with a significantly higher rate of local adverse events (RD ‐0.19; 95% CI ‐0.37 to ‐0.01). There was no significant difference in the rate of systemic adverse events.
Calcipotriol versus tazarotene
Our review found no statistically significant difference for local or systemic adverse events.
Calcipotriol versus tazarotene gel plus mometasone furoate cream
Adverse events data were not reported for this analysis.
Calcipotriol versus vitamin B12 cream
Our review found no statistically significant difference in the rates of local adverse events. Data on systemic adverse events were not reported.
Head‐to‐head vitamin D alone or in combination: dosing
Our review found no statistically significant difference for local or systemic adverse events.
Head‐to‐head vitamin D alone or in combination: occlusion
Local adverse events data were not reported for this analysis. Our review found no statistically significant difference in the rate of systemic adverse events.
Analysis 16: Flexural/facial psoriasis: placebo‐controlled trials
This comparison included placebo‐controlled trials of four topical treatments for inverse or facial psoriasis. Data on local adverse events were available for all four treatments, but data on systemic events were reported only for one treatment, i.e. the comparison of tacrolimus against placebo (see Analysis 16.9 and Analysis 16.10).
16.9. Analysis.
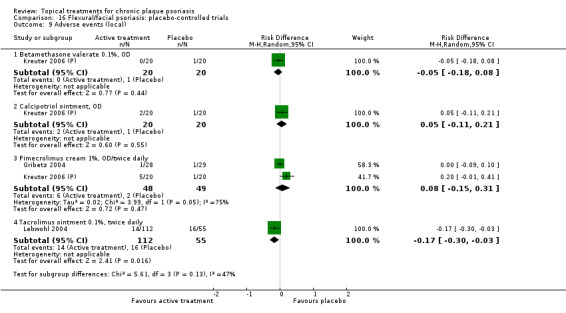
Comparison 16 Flexural/facial psoriasis: placebo‐controlled trials, Outcome 9 Adverse events (local).
16.10. Analysis.
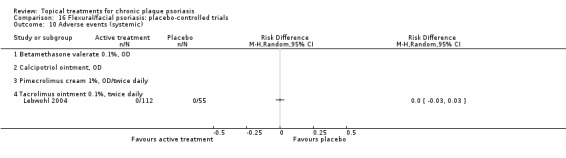
Comparison 16 Flexural/facial psoriasis: placebo‐controlled trials, Outcome 10 Adverse events (systemic).
Relative to placebo, participants receiving topical tacrolimus were significantly less likely to report local adverse events (RD ‐0.17; 95% CI ‐0.30 to ‐0.03), but there was no significant difference in the rate of systemic events. No other treatment was significantly different from placebo.
Analysis 17: Flexural/facial psoriasis: vitamin D alone or in combination versus other treatment
This comparison included head‐to‐head trials of treatments for flexural or facial psoriasis, where vitamin D was compared with an active control. We identified five intervention‐comparator contrasts (see Analysis 17.9 and Analysis 17.10). Local adverse events data were missing for the comparison of calcitriol and tacrolimus. There were no data on systemic events for any of the five contrasts.
17.9. Analysis.
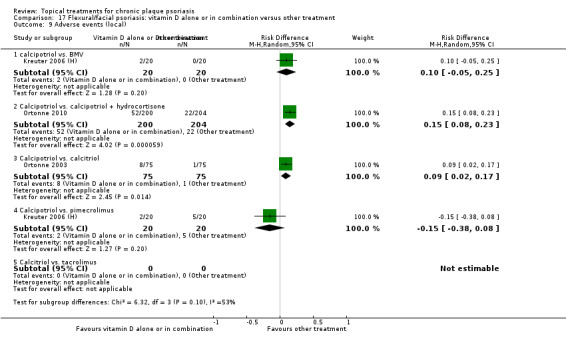
Comparison 17 Flexural/facial psoriasis: vitamin D alone or in combination versus other treatment, Outcome 9 Adverse events (local).
In people treated with calcipotriol, the rate of local adverse events was significantly higher compared to those receiving combination therapy with calcipotriol and hydrocortisone for facial psoriasis (RD 0.15; 95% CI 0.08 to 0.23) and also compared to those receiving calcitriol (RD 0.09; 95% CI 0.02 to 0.17). These findings aligned with the withdrawal rates for adverse events (Analysis 17.7). We found no other significant differences in adverse events rates.
Analysis 18: Scalp psoriasis: placebo‐controlled trials
This comparison included placebo‐controlled trials of 11 topical treatments for scalp psoriasis (see Analysis 18.9 and Analysis 18.10). There were no adverse events data for two treatments, betamethasone valerate and amcinonide. Data on systemic events were available for four treatments.
18.9. Analysis.
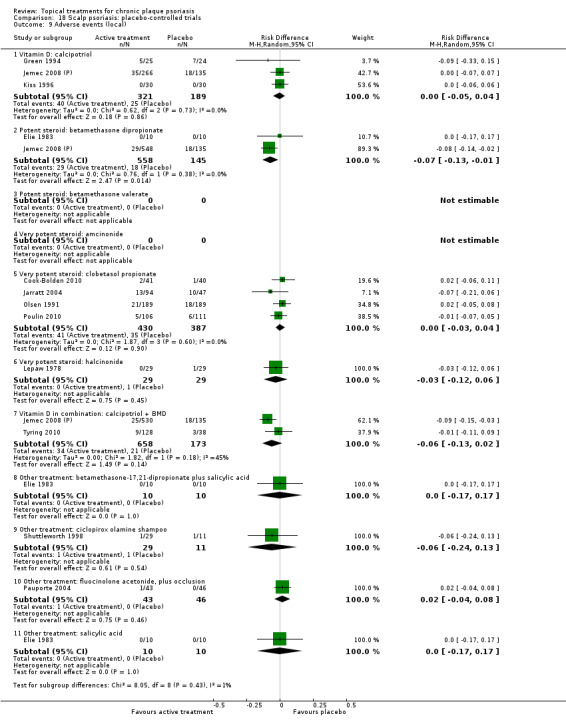
Comparison 18 Scalp psoriasis: placebo‐controlled trials, Outcome 9 Adverse events (local).
18.10. Analysis.
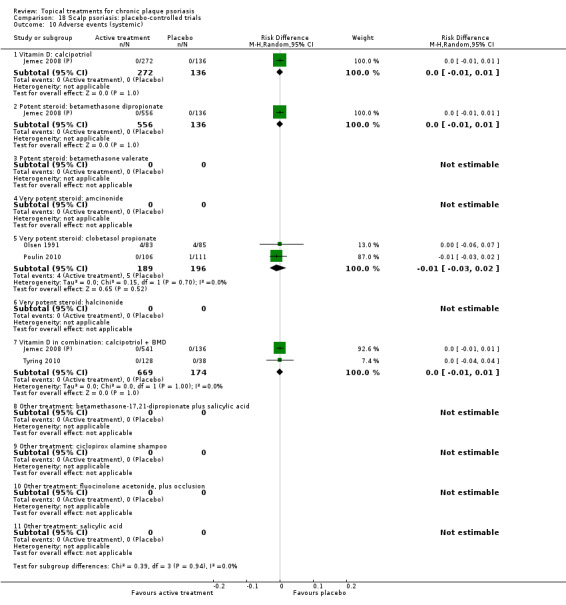
Comparison 18 Scalp psoriasis: placebo‐controlled trials, Outcome 10 Adverse events (systemic).
Relative to placebo, participants treated with betamethasone dipropionate were significantly less likely to suffer local adverse events (RD ‐0.07; 95% CI ‐0.13 to ‐0.01; I² statistic = 0%). These findings aligned with the withdrawal rates for adverse events (Analysis 18.7). We found no other significant differences in adverse events rates.
Analysis 19: Scalp psoriasis: vitamin D alone or in combination versus other treatment
This comparison included head‐to‐head trials of treatments for scalp psoriasis in which one of the interventions was a vitamin D product (used either as monotherapy or in combination with another product) (see Analysis 19.9 and Analysis 19.10). We identified six intervention‐comparator contrasts, and there were no adverse events data.
19.9. Analysis.
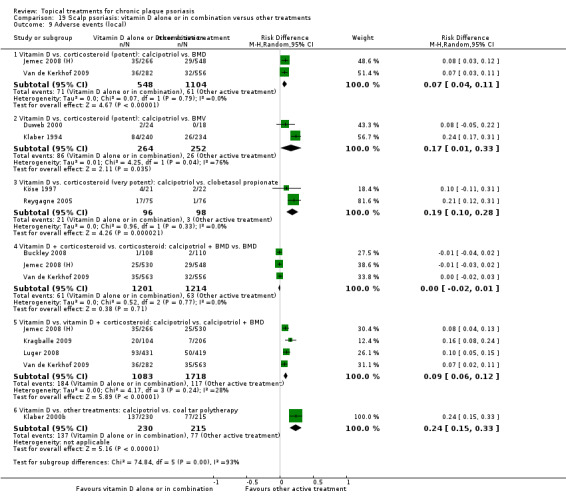
Comparison 19 Scalp psoriasis: vitamin D alone or in combination versus other treatments, Outcome 9 Adverse events (local).
19.10. Analysis.
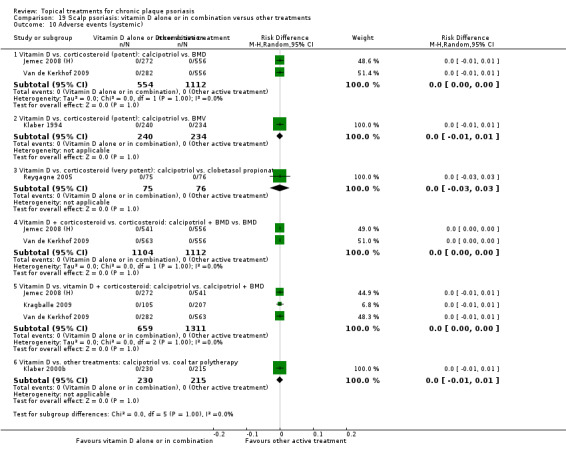
Comparison 19 Scalp psoriasis: vitamin D alone or in combination versus other treatments, Outcome 10 Adverse events (systemic).
Calcipotriol monotherapy was associated with a significantly higher rate of local adverse events than five of the comparator treatments; these included betamethasone dipropionate (RD 0.07; 95% CI 0.04 to 0.11; I² statistic = 0%), betamethasone valerate (RD 0.17; 95% CI 0.01 to 0.33; I² statistic = 76.5%), and clobetasol propionate (RD 0.19; 95% CI 0.10 to 0.28; I² statistic = 0%). Relative to calcipotriol, combined therapy with calcipotriol and betamethasone dipropionate (RD 0.09; 95% CI 0.06 to 0.12; I² statistic = 28.1%), and coal tar polytherapy (RD 0.24; 95% CI 0.15 to 0.33; I² statistic = NA), were also associated with significantly lower rates of adverse events. Compared with betamethasone dipropionate monotherapy, the rate of adverse events for combined therapy with calcipotriol and betamethasone dipropionate was not significantly different (RD ‐0.00; 95% CI ‐0.02 to 0.01; I² statistic = NA). There were no significant differences in the rates of systemic adverse events.
(ii) Findings from the separate search for additional studies of adverse events
In addition to findings on adverse events from the main review, we undertook a separate search for additional safety and tolerability studies. The update searches in 2011 identified 3 new literature reviews and 12 potentially relevant records. Eight studies (reported in seven references) met the inclusion criteria, and we excluded five studies from the review (Breneman 2007; Carboni 2005; Feldman 2007a; Hong 2010; and Hong 2011; Jacobi 2008) in addition to the 13 trials excluded in the previous edition of this review (Aste 2004; Bos 2002; Floden 1975; Franssen 1999; Kang 1998; Lebwohl 1996; Park 2002; Senter 1983; Singh 2000; Stevanovic 1977; Traulsen 2003; Uhoda 2003; Vissers 2004). Table 24 details the characteristics and key findings of the included studies, and Table 25 lists the excluded studies. Langner 1996 and van de Kerkhof 1996c also reported the study by Gerritsen 2001. Two papers reported two studies (Berth‐Jones 1993; Kimball 2008), which we analysed separately. Kimball 2008 was a review reporting findings from phase II and phase III trials of clobetasol foam (we did not locate any other reports of these studies). The study by van de Kerkhof 2002c reported a two‐phase open study of tacalcitol, where 'responders' to part 1 were eligible for part 2. Full results for part 2 were not reported separately (e.g. incidence of local adverse events was reported only for all trial participants). The study by Lambert 2002 appeared very similar to the second phase of the study reported by van de Kerkhof 2002c, but as there was no explicit evidence of an association, we analysed it as a separate trial.
24. Included studies of adverse events.
| Study | Methods | Participants | Intervention(s) | Outcomes (AEs) | Summary findings | Notes | Allocation concealment |
| Andres 2006 | DESIGN: between‐patient patient delivery ALLOCATION: random Method of randomisation: computer‐generated list Concealment: unclear BLINDING: single‐blind (investigator) WITHDRAWAL/DROPOUT: described |
N: 26 TD: 4 wks; FU: 4 wks LF: 0 (0%) BC: characteristics reported, but not demonstrated to be comparable (shampoo group had more severe disease and higher proportion of males) Age: 34.3 (9.5SD) Gender (per cent men): 58% Severity: DSS: 5.3 (1.3SD) % scalp affected: 63.8% INCLUSION CRITERIA: people with scalp psoriasis affecting > = 25% scalp; DSS > = 3 EXCLUSION CRITERIA: pregnancy or risk thereof; lactation; ophthalmological disorder; contact lens wearer | Clobetasol propionate 0.05% shampoo, OD. Applied to dry scalp, rinsed off after 15 minutes Clobetasol propionate 0.05% gel, OD. Applied to dry scalp and left in. |
Serum cortisol atrophogenicity (ultrasound measurement of skin thickness (epidermis + dermis) (mm), averaged over 3 sites of the scalp) ocular safety (intraocular pressure) DSS (10‐pt; sum of erythema, adherent desquamation, and plaque thickening; 0 (none) to 3 (severe) with half‐point ratings permitted) Patient‐reported ocular stinging (0 to 3) Compliance |
Neither formulation had an impact on ocular safety, no report of ocular stinging. LAE: CS: 1/14; CG: 2/14 HPA suppression: CS: 0/14; CG: 2/14 Atrophy: CS: 0/14; CG: 0/14 Decrease in skin thickness from baseline: mean difference: CS: 0.04 mm CG: ‐0.24 mm (difference: P < = 0.025) Efficacy results of the 2 formulations were similar. Compliance with protocol was good in both groups. |
Exploratory safety study Sponsored by Galderma Laboratories |
Unclear |
| Barnes 2000 | DESIGN: within‐patient
patient delivery ALLOCATION: non‐random Method of randomisation: NA Concealment: NA BLINDING: open WITHDRAWAL/DROPOUT: described |
N: 202 TD: 52 wks; FU: 52 wks LF: 64 (32%) BC: NA Age: 46 (14.5SD) Gender (per cent men): 60% Severity: Scalp: TSS (0 to 12): 5.9; Overall assessment (investigator): mild (31%); moderate (58%); severe (11%) Body: PASI (modified): 6.8 Overall assessment (investigator): mild (41.5%); moderate (55%); severe (3.5%) INCLUSION CRITERIA: chronic plaque psoriasis on scalp and body; adult (≥18); outpatient EXCLUSION CRITERIA: pregnancy or risk thereof; severe (i.e. requiring additional therapy) or unstable psoriasis; hypercalcaemia; history of hypo‐ or hyperparathyroidism, renal/hepatic disease; systemic or phototherapies within previous 6 wks; other medication that could affect course of disease | Calcipotriol scalp solution 50 mcg/ml BD plus calcipotriol cream 50 mcg/g BD (up to 70 g/wk) No control | Local AEs:
number of AEs/participant
% severe AEs
withdrawals due to adverse events (WA)
Systemic AEs: serum calcium serum PTH urinary calcium/creatinine ratio |
Local AEs: the most common local AE was facial irritation (60/202 participants at wk 2), though the incidence declined rapidly over time (1/141 at wk 46). 20% of local AEs considered by investigator to be 'severe'. 14% of participants withdrew because of adverse events Systemic AEs: no significant changes observed | Sponsored by Leo Pharmaceuticals | Not applicable |
| Berth‐Jones 1993; Berth‐Jones 1992c | DESIGN: uncontrolled study
patient delivery
ALLOCATION: non‐random
Method of randomisation: NA Concealment: NA BLINDING: open WITHDRAWAL/DROPOUT: NA |
STUDY A: N: 20 TD: 52 wks; FU: 52 wks LF: 0 (0%) BC: NA Age: 43 Gender (per cent men): 65% Severity: mean PASI: 7.6 (3.5SD) STUDY B: N: intervention: 10 {32 controls} TD: 4 wks; FU: 4 wks LF: 0 (0%) BC: not demonstrated Age: 48 {42} Gender (per cent men): 50% {44%} Severity: mean PASI: 18.0 (13.9SD) {NR} INCLUSION CRITERIA: people with chronic plaque psoriasis; aged ≥18; under long‐term care of investigators. Study A: compliant patients, responsive to calcipotriol. Study B: more extensive disease, failing to respond to low doses of topical agents. Controls received no calcipotriol. EXCLUSION CRITERIA: pregnancy | Study A: calcipotriol ointment 50 mcg/g BD up to 100 g/wk No control Study B: calcipotriol ointment 50 mcg/g BD, using 100 g/wk for 4 wks Control: people using alternative therapies | Local AEs: not assessed Systemic AEs: urine calcium and phosphate excretion; serum total calcium, phosphate and alkaline phosphatase | Study A: no significant trend in urine calcium excretion Study B: significant increase in urine calcium excretion (relative to controls and to baseline) | Sponsorship not reported For study B, baseline comparability of intervention and control groups not demonstrated. Berth Jones 1992 reports findings for study A at 10 mths | Not applicable |
| Bleiker 1998 | DESIGN: uncontrolled study
Delivery: unclear ALLOCATION: non‐random Method of randomisation: NA Concealment: NA BLINDING: open WITHDRAWAL/DROPOUT: not described |
N: 28 TD: 2 wks; FU: 26 wks LF: unclear BC: NA Age: 47 (range: 18 to 83) Gender (per cent men): 50% Severity: PASI: 21.4 (range: 8.2 to 53.7) INCLUSION CRITERIA: inpatients with severe chronic plaque psoriasis (> 15% BSA) EXCLUSION CRITERIA: renal impairment, pregnancy, lactation, systemic treatment, diuretics | STUDY A: 200 g calcipotriol ointment 50 mcg/g (wk 1) plus 300 g 50 mcg/g calcipotriol (wk 2) STUDY B: Calcipotriol 50 mcg/g PRN < = 360 g/wk | Local AEs: not assessed Systemic AEs: serum total adjusted calcium urinary calcium | 5 participants developed hypercalcaemia during treatment, all had received a dose > 5 g/kg 9 participants became hypercalciuric during treatment; this was uncorrelated with dose | Sponsorship not reported | Not applicable |
| Brodell 2011b | DESIGN: uncontrolled study patient delivery ALLOCATION: non‐randomised BLINDING: open WITHDRAWAL/DROPOUT: not described | N: 305
TD: 12 wks; FU: 12
LF: unclear
BC: NA
Age: 50.3 (13.7SD); range: 22 to 84
Gender (per cent men): 61.8%
Severity:
ODS: all patients scored as moderate/severe/very severe
% BSA: 7.1% % white: 91.8% INCLUSION CRITERIA: people with moderate to severe plaque psoriasis; affected; aged 18 to 80 EXCLUSION CRITERIA: not stated |
Clobetasol propionate 0.05% spray BD (2 to 4 wks); treatment responders (ODS < = 3) then treated with calcitriol 3 mg/g ointment (8 wks) | Pruritis, telangiectasias, burning/stinging (0 to 3), skin atrophy, folliculitis Overall disease severity (ODS) (5‐pt: 0 = clear to 4 = severe/very severe) based on erythema, scaling, and plaque elevation. Treatment success (change from baseline ODS > = 1 at wk 12) |
At 4 wks: skin atrophy 7/285 telangiectasias 2/285 stinging/Burning 39/285 folliculitis 11/285 At 12 wks: skin atrophy 2/235 telangiectasias 5/235 stinging/Burning 35/235 folliculitis 3/235 Any adverse event: 100/305 |
Sponsored by Galderma laboratories | Not applicable |
| Corbett 1976 | DESIGN: within‐patient patient delivery ALLOCATION: random Method of randomisation: NR Concealment: unclear BLINDING: double‐blind (participant/investigator) WITHDRAWAL/DROPOUT: not described | N: 14
TD: 26 wks; FU: 26 wks
LF: 2 (14.3%)
BC (clinical): NR Age: 44 (18.4SD) Gender (per cent men): 64%
Severity: NR INCLUSION CRITERIA: bilateral psoriasis involving < = 15% BSA; willing to participate for 6 months EXCLUSION CRITERIA: NR |
Clobetasol propionate 0.05% ointment, BD Betamethasone valerate 0.1% ointment, BD | Local AEs: NR Systemic AEs: synacthen test for function of pituitary‐adrenal axis at 0, 3, and 6 months | Quantities used by study participants were small (mean: 7 g/wk) No pituitary‐adrenal suppression observed | Sponsorship not reported | Unclear |
| Gerritsen 2001; Langner 1996; van de Kerkhof 1996c | DESIGN: uncontrolled study patient delivery ALLOCATION: NA Method of randomisation: NA Concealment: NA BLINDING: open WITHDRAWAL/DROPOUT: described |
N: 257 TD: < = 78 wks; FU: <= 78 wks LF: 4 (1.6%) BC: NA Age: 42 (13SD) Gender (per cent men): 61.3% Severity: BSA: 14.0% (14.2%SD); PASI: 9.7; 47% had severe disease INCLUSION CRITERIA: chronic plaque psoriasis; aged ≥18 EXCLUSION CRITERIA: non‐compliant; pregnancy; use of systemic/phototherapy within previous 2 mths; use of topical therapy within previous 1 wk; concomitant disease; clinically relevant abnormality in laboratory assessments; known hypersensitivity to vitamin D/vehicle | Calcitriol 3 mcg/g BD No control | Local AEs: serious AEs reported; withdrawals due to adverse events (WA) Systemic AEs: laboratory levels for: protein albumin; calcium, phosphorus, sodium, potassium, plasma calcitriol Urinary calcium, creatinine, phosphorus; creatinine clearance; urinary calcium/creatinine ratio | Local AEs: WA: 7/253; AEs: 37/353; no serious local adverse events Systemic AEs: WA: 1/253. 4 additional participants experienced hypercalcaemia. All mean values for all parameters remained within normal levels. Mean use: 6 g/day (range: 1 to 24 g/day) | Sponsored by Solvay‐Duphar BV Excludes scalp | Not applicable |
| Guzzo 1996 | DESIGN: between‐patient
patient delivery ALLOCATION: random Method of randomisation: NR Concealment: unclear BLINDING: double‐blind (participant/investigator) WITHDRAWAL/DROPOUT: described |
N: 78
TD: 8 wks; FU: 8 wks
LF: 2 (2.6%)
BC: no (1 statistically significant difference (% BSA higher in intervention group)
Age: 48
Gender (per cent men): 67%
Severity: mean BSA: 9%
INCLUSION CRITERIA: aged ≥18; stable plaque psoriasis; BSA: 5% to 20% EXCLUSION CRITERIA: hypercalcaemia, bone, thyroid or parathyroid disease; topical therapy within previous 2 wks; systemic/phototherapy within previous 8 wks |
Calcipotriol 50 mcg/g ointment BD, up to 120 g/wk Placebo | Local AEs: not assessed Systemic AEs: blood and urine chemistry analysis: parathyroid hormone, serum calcium, bone‐specific alkaline phosphatase, urinary hyroxyproline, 24‐hr urinary calcium excretion. Bone densitometry | No adverse effects on bone metabolism or calcium | Sponsored by Bristol‐Myers Squibb | Unclear |
| Heng 1990 | DESIGN: between‐patient (retrospective study)
patient delivery ALLOCATION: non‐random Method of randomisation: NA Concealment: NA BLINDING: NA WITHDRAWAL/DROPOUT: NA |
N: 28
TD: 6 mths to 12 ys; FU: 6 mths to 12 years
LF: NA
BC: demographic: yes; clinical: not demonstrated
Age: 49 (13SD)
Gender (per cent men): 82%
Severity: NR INCLUSION CRITERIA: psoriasis (any severity; types include plaque (16), generalised, seborrhoeic, guttate, erythrodermic); previous prolonged treatment with topical fluorinated steroids (range: 6 mths to 12 years). Control group matched for age and gender EXCLUSION CRITERIA: NR |
Previous prolonged treatment with topical fluorinated steroids Control: 'steroid‐negative' group ‐ previous tar/UVB/sunlight or no treatment | Local AEs: light/electron microscopy for examination of basal keratinocyte herniation (BKH); layers of basement membrane Systemic AEs: NR | Local AEs: light microscopy revealed no between‐group differences. Electron microscopy revealed multi‐layered, fragmented and disorganised basal laminae in the steroid group, which appeared to be correlated with duration of treatment. Fragmentation was not observed in the control group | Sponsorship not reported Non‐psoriatic control group also considered 16/28 participants had plaque psoriasis | Not applicable |
| Katz 1987b | DESIGN: between‐patient
patient delivery ALLOCATION: random Method of randomisation: NS Concealment: unclear BLINDING: double‐blind (participant/investigator) WITHDRAWAL/DROPOUT: described |
N: 40 TD: 3 wks; FU: 3 wks LF: NA BC: demographic: yes; clinical: yes (gender imbalance) Age: 44 (range: 18 to 66) Gender (per cent men): 53% Severity: 55% moderate disease; 45% severe disease INCLUSION CRITERIA: aged ≥ 18; stable or worsening, moderate or severe chronic plaque psoriasis; baseline laboratory values within normal range (5 to 25 mcg%) EXCLUSION CRITERIA: pregnancy or risk thereof; lactation; hypersensitivity to study medications; concurrent medication that could affect study outcomes; use of systemic therapies within previous 4 wks; use of topical therapies within previous 2 wks | Betamethasone dipropionate (0.05%) in optimised vehicle BD; Clobetasol 17 propionate (0.05%) ointment BD | Local AEs: not assessed Systemic AEs: morning plasma cortisol levels; 24‐hr urine steroid levels; FBC, blood chemistry, urinalysis |
Temporary reversible suppression of the hypothalamic‐pituitary‐adrenal axis in 8/40 participants | Sponsorship not reported; 1 author from Schering Corporation | Unclear |
| Katz 1989 | DESIGN: within‐patient
patient delivery ALLOCATION: random Method of randomisation: unclear Concealment: unclear BLINDING: double‐blind (participant/investigator) WITHDRAWAL/DROPOUT: described |
N: 30 TD: 2 wks; FU: 4 wks LF: 0 (0%) BC: yes Age: 55 (range: 36 to 69) Gender (per cent men): 53% Severity: NR INCLUSION CRITERIA: bilateral symmetric chronic plaque psoriasis EXCLUSION CRITERIA: pregnancy or risk thereof; people with overt signs of atrophy | Betamethasone dipropionate (0.05%) in optimised vehicle BD (BMD) Clobetasol propionate (0.05%) ointment BD (CP) Uninvolved (non‐psoriatic) area used as test area for each participant | Local AEs: skin surface microscopic examination with photographic documentation; oil and magnifying (8 x) lens to detect 'preatrophy' (visibility of subpapillary vascular plexus caused by thinning of epidermis and papillary dermis) Systemic AEs: NR | Local AEs: no serious adverse events observed with either treatment. Preatrophy identified in 20% of involved plaques (BMD: 11/59; CP: 12/59) and was more likely in females. In the test area (non‐psoriatic skin), 5% of plaques showed preatrophy (BMD: 2/30; CP: 1/30). Preatrophy appeared to be usually reversible following treatment cessation. | Sponsored by Schering Corporation | Unclear |
|
Kimball 2008 Phase II |
DESIGN: uncontrolled
patient delivery
ALLOCATION: unclear BLINDING: open WITHDRAWAL/DROPOUT: described |
N: 32 TD: 2 wks; FU: NS LF: 1 (3.1%) BC: NA Age: 24 to 72 Gender (per cent men): NS Severity: NS % white: 94% INCLUSION CRITERIA people with mild to moderate plaque psoriasis; aged > = 12 EXCLUSION CRITERIA: NS | Clobetasol 0.05% foam BD Clobetasol 0.05% ointment BD (= 7 g/day, up to 50 g/wk) |
Maximal plasma concentration of clobetasol propionate |
Higher but non‐significant levels of clobetasol in ointment group | Supported by Stiefel Laboratories, Inc. Review of phase II studies on AD and psoriasis (clobetasol foam) |
Not applicable |
|
Kimball 2008 Phase III |
DESIGN: between‐patient patient delivery ALLOCATION: random Method of randomisation: not stated Concealment: unclear BLINDING: double‐blind (participant/investigator) WITHDRAWAL/DROPOUT: described | N: 497 TD: 2 wks; FU: NS LF: 16 (3%) BC: NS Age: NS Gender (per cent men): NS Severity: most had baseline ISGA of 3 INCLUSION CRITERIA: people with mild to moderate plaque psoriasis; aged > = 12 EXCLUSION CRITERIA: NS | Clobetasol 0.05% foam BD Clobetasol 0.05% ointment BD Placebo foam BD Face, scalp, and intertriginous areas excluded from treatment |
Treatment‐related adverse events:
ISGA (investigator's static global assessment score)(scale NR) |
Atrophy: C foam: 2% (N = 253) C ointment: NS (N = 121) Placebo foam: 1% (N = 123) Burning: C foam: 2% (N = 253) C ointment: NS (N = 121) Placebo foam: 2% (N = 123) All treatment‐related adverse events: C foam: 8% (N = 253) C ointment: 2% (N = 121) Placebo foam: 7% (N = 123) |
Supported by Stiefel Laboratories Inc. Review of phase III studies on AD and psoriasis (clobetasol foam). |
Unclear |
| Kragballe 1991b | DESIGN: uncontrolled study patient delivery ALLOCATION: non‐random Method of randomisation: NA Concealment: NA BLINDING: open WITHDRAWAL/DROPOUT: described |
N: 15
TD: 26 wks; FU: 26 wks
LF: 1(6.7%)
BC: NA
Age: 42 (range: 21 to 71)
Gender (per cent men): 53%
Severity: % BSA: 14% (range: 5% to 30%)
Most 'moderate' severity
INCLUSION CRITERIA: participants previously responding to calcipotriol during 8‐wk clinical trial, but who had since relapsed EXCLUSION CRITERIA: hypercalcaemia, impaired renal/hepatic function, daily receiving > 400 i.u. vitamin D |
Calcipotriol ointment 50 mcg/g BD (max: 100 g/wk) No control | Local AEs: patient report of adverse events Investigator report of adverse events (skin examination). Skin biopsies to determine histologic signs of epidermal and dermal atrophy. Systemic AEs: laboratory tests: FBC, serum alkaline phosphatase, aspartate aminotransferase, bilirubin, creatinine, total calcium, total phosphate | Local AEs: AE(L): 3/15 (reported to be transient & mild). Cases of mild to moderate atrophy found in 4/8 participants Systemic AEs: no consistent changes in laboratory analyses, with no clinically important changes in serum calcium | Sponsorship not reported. Leo Pharmaceuticals supplied study medication Face and scalp treated with emollient or hydrocortisone cream 1% (not calcipotriol) | Not applicable |
| Lambert 2002 | DESIGN: uncontrolled study patient delivery ALLOCATION: non‐random Method of randomisation: NA Concealment: NA BLINDING: open WITHDRAWAL/DROPOUT: described |
N: 157
TD: 26 wks; FU: 26 wks
LF: 8 (5.1%)
BC: NA
Age: 44.4 (14.0SD)
Gender (per cent men): 57%
Severity: mean BSA: 13%; mean PASI: 9.4 (5.4SD) INCLUSION CRITERIA: chronic plaque psoriasis; aged 18 to 70; BSA 7% to 20%; laboratory parameters normal at baseline EXCLUSION CRITERIA: pregnancy or risk thereof; topical antipsoriatic therapy within previous 2 wks; systemic antipsoriatic therapy within previous 6 wks; retinoids within previous 52 wks.; history of hyperparathyroidism; concomitant use of drugs affecting calcium metabolism |
Tacalcitol ointment, 4 mcg/g OD. No control | Local AEs: participants asked about adverse events. Tolerability assessed by investigator (4‐pt: 1 = excellent to 4 = poor). Systemic AEs: Routine haematology and biochemistry: FBC, haemoglobin, bilirubin, creatinine, alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, gamma glutamyltranspeptidase, calcium, phosphate, sodium, potassium, glucose, urea, albumin. Urinalysis: calcium, creatinine, phosphate. | Local AEs: WA: 5/157 (3.4%) (treatment‐related). AE(L): 26/157 (transient skin irritation) Tolerability at least moderate in 95% of participants Systemic AEs: no serious adverse events and no hypercalcaemia | Sponsored by Hermal/BHI, Germany Scalp excluded Similar study to van de Kerkhof 2002, but unclear whether Lambert 2002 is a report of a subgroup or a distinct study | Not applicable |
| Lebwohl 1998b | DESIGN: between‐patient
patient delivery ALLOCATION: random. Method of randomisation: unclear Concealment: unclear BLINDING: double‐blind (participant/investigator) WITHDRAWAL/DROPOUT: described |
N: 40
TD: 26 wks; FU: 26 wks
LF: 4 (10%)
BC: no (Group A had less severe disease at baseline)
Age: NR
Gender (per cent men): NR
Severity: mild to moderate psoriasis
INCLUSION CRITERIA: people with at least moderate improvement in response to initial 2‐wk therapy regimen; aged ≥18; stable disease; BSA < = 20% (excluding face/scalp); plaque elevation at least moderate; willing to comply with study protocol EXCLUSION CRITERIA: history of sensitivity to study ingredients; topical antipsoriatics within previous 2 wks; UVB/PUVA within previous 8 wks; history of hypercalcaemia, recurrent illness |
Initial regimen: all participants received 2 wks of calcipotriol (OM), halobetasol ointment (ON) Group A: Calcipotriol ointment 50 mcg/g (weekdays) plus halobetasol 0.05% ointment BD (weekends) Group B: Placebo ointment (weekdays) plus halobetasol 0.05% ointment BD (weekends) | Local AEs: treatment‐related adverse events Systemic AEs: not assessed | Local AEs: AE(L) (treatment‐related; all irritant contact dermatitis): Group A: 4/17 Group B: 1/20 No cutaneous atrophy observed | Sponsored by Westwood Squibb Pharmaceuticals | Unclear |
| Lebwohl 2001 | DESIGN: between‐patient patient delivery ALLOCATION: random Method of randomisation: NR Concealment: unclear BLINDING: double‐blind (participant/investigator) WITHDRAWAL/DROPOUT: not described |
N: 50 TD: 26 wks; FU: 26 wks LF: NR BC: NR Age: 55 Gender (per cent men): NR Severity: NR INCLUSION CRITERIA: moderate to severe plaque psoriasis; BSA < = 15%. All participants participated in an open‐label treatment phase for 6 wks (tazarotene gel 0.1% OM, clobetasol propionate ointment 0.05% ON) EXCLUSION CRITERIA: topical antipsoriatic treatment within previous 2 wks; UV treatment within previous 4 wks; systemic antipsoriatic treatment within previous 8 wks | Open‐label phase: tazarotene 0.1% gel plus clobetasol propionate 0.05% ointment for 6 wks. Once daily initially, then 'tapered'. Maintenance phase (20 wks): tazarotene gel, 0.1%, OM (3/7 days), plus clobetasol propionate 0.05% ointment ON (2/7days) (TC) Tazarotene gel, 0.1%, OM (3/7 days), placebo ointment OM (2/7 days), placebo ointment ON (2/7 days) (TP) Placebo gel OM (3/7 days), placebo ointment ON (2/7 days) (P) | Local AEs: no. steroid‐specific side‐effects Withdrawals due to adverse events (WA) Drug‐related adverse events Systemic AEs: not assessed | Local AEs: no steroid‐specific side‐effects WA: 0/50 AE(L) (treatment‐related): TC: 24% TP: 29% P: 0% | Sponsorship: not reported No adequate effectiveness data reported. Numbers of participants in each group NR | Unclear |
| Menter 2007; Feldman 2007a | DESIGN: uncontrolled
patient delivery
ALLOCATION: NA BLINDING: open WITHDRAWAL/DROPOUT: described |
N: 1423 TD: 4 wks; FU: 4 wks LF: 2 (0.1%) BC: NA Age: 49.7 (14.7SD) Gender (per cent men): NS Severity: Duration (yrs): 12.5 (13SD) BSA: 10.6% (5.75% SD) INCLUSION CRITERIA: people with moderate to severe plaque psoriasis; 3% to 20% BSA involvement EXCLUSION CRITERIA: current systemic therapy, phototherapy or topical therapy | Clobetasol 0.05% foam BD, up to 50 g/wk either as monotherapy or adjunctive to existing therapy |
Erythema, peeling/scaling, dryness, stinging/burning (0 none to 3 severe). Telangiectasia, skin atrophy, pruritus, folliculitis (absent/present) Also assessed efficacy and QoL |
Erythema: 23.7%
peeling/scaling: 21.0%
dryness: 28.3%
stinging/burning: 15.1% Telangiectasia, skin atrophy, folliculitis: present in less than 1% of participants (findings not reported separately) Pruritus: 5.7% |
Clobex Spray Community‐Based Research Assessment (COBRA) Sponsorship not reported |
Not applicable |
| Miyachi 2002 | DESIGN: uncontrolled study patient delivery ALLOCATION: non‐random Method of randomisation: NA Concealment: NA BLINDING: open WITHDRAWAL/DROPOUT: described |
N: 160 TD: 54 wks; FU: 54 wks LF: 6 (3.8%) BC: NA Age: 48.2 (16.1SD) Gender (per cent men): 82% Severity: mean PASI: 22.49 (10.2SD) INCLUSION CRITERIA: inpatients and outpatients with BSA ≥10% EXCLUSION CRITERIA: pregnancy; lactation; severe liver disease, heart disease, impaired renal function, hypercalcaemia; treatment with topical, UV or systemic antipsoriatics within previous 2 wks | Tacalcitol ointment 20 mcg/g OD (max: 10 g/day) No control | Local AEs: treatment‐related adverse events Systemic events: haematological tests (FBC), blood biochemical tests (calcium, inorganic phosphorus, albumin, protein, bilirubin, urea nitrogen, creatinine, GP/AST, GPT/ALT, alkaline phosphatase, LDH, intact PTH), urinalysis (glucose, protein); serum tacalcitol and vitamin D₃ levels. | Local AEs: AE(L): 16/154 (29 events, all mild to moderate) Systemic AEs: AE(S): 85/154 (155 events, of which 6 were considered treatment‐related). Serum levels of intact PTH and tacalcitol decreased, suggesting percutaneous absorption of tacalcitol. However, mean levels of serum calcium remained within the standard level. Data on individual responses not reported. High‐dose tacalcitol affected serum calcium in participants with reduced renal function | Sponsorship: not reported Scalp treated in 74/154 participants Usual dosing regimen for tacalcitol is 4 mcg/g OD | Not applicable |
| Poyner 1993 | DESIGN: uncontrolled study patient delivery ALLOCATION: non‐random Method of randomisation: NA Concealment: NA BLINDING: open WITHDRAWAL/DROPOUT: described |
N: 203 TD: 48 wks; FU: 48 wks LF: 59 (29.1%) BC: NA Age: 43.8 (range: 17 to 80) Gender (per cent men): 52.7% Severity (assessment methods NR): mild (8%); moderate (63%); severe (30%) INCLUSION CRITERIA: aged ≥18; chronic plaque psoriasis ≥ 100 cm². EXCLUSION CRITERIA: PUVA within previous 8 wks; elevated serum calcium, unstable disease, impaired hepatic/renal function' pregnancy; concomitant oral calcium/vitamin D. topical antipsoriatics, lithium, systemic steroids | Calcipotriol 50 mcg/g ointment No control | Loca AEs: self report of adverse events. Withdrawals due to adverse events (WA) Systemic AEs: biochemical and haematological tests Compliance: self‐reported usage at each visit; weighing of ointment tubes | Local AEs: WA: 8/203 AE(L): 83/203 142 events reported by 83 (41%) participants with 20.2% being lesional/perilesional irritation Systemic AEs: no significant changes in haematological values. Mean serum calcium did not change significantly over study period. Significant fall in serum urate in those treated ≥ 36 wks Compliance: median weekly use (wks 0 to 5): 16.5g; (wks 43 to 48): 11.6g | Sponsored by Leo Pharmaceuticals Face/scalp excluded | Not applicable |
| Ramsay 1994 | DESIGN: uncontrolled open study
patient delivery ALLOCATION: non‐random Method of randomisation: NA Concealment: NA BLINDING: open WITHDRAWAL/DROPOUT: described |
N: 167 TD: 52 wks; FU: 52 wks LF: 39 (23.4%) BC: NA Age: 49 (range: 20 to 85) Gender (per cent men): 60% Severity: PASI (modified): 8.1 (6.7SD) INCLUSION CRITERIA: chronic plaque psoriasis; previous response to calcipotriol; managed by specialists EXCLUSION CRITERIA: pregnancy or risk thereof; abnormal serum calcium or phosphate; impaired hepatic/renal function; concomitant oral calcium/vitamin D; systemic therapy within previous 8 wks; topical therapy within previous 4 wks |
Calcipotriol 50 mcg/g ointment. Max dose: 100 g/wk; 2500 g/pa Face/scalp/neck excluded No control | Local AEs: self report of adverse events: mild, moderate, severe; unlikely, possibly, or probably treatment‐related Systemic AEs: haematology (erythrocyte, haemoglobin, leukocyte, platelet counts) and biochemistry (bilirubin, AST/ALT, alkaline phosphatase, albumin, urate, creatinine, phosphate, total calcium) tests Compliance: self report of number tubes used and number daily doses | AE(L): 52/161 60 (46 considered to be treatment‐related) events reported by 52 of 161 participants. 1 participant developed a significant rise in serum calcium. No other abnormalities in haematology or biochemistry tests. 118/161 participants reported continuous medication use and 80% to 90% used it twice daily. Mean use: 35.1 g/wk ('initially') to 23.4 g/wk during last 6 mths | Sponsored by Leo Pharmaceuticals | Not applicable |
| Roelofzen 2010 | DESIGN: observational study using retrospective data (medical records, cancer registry) and prospective (survey) data (treatments/lifestyle). Multivariate proportional hazards regression. ALLOCATION: NA BLINDING: NA WITHDRAWAL/DROPOUT: described | N: 4315 TD: pix lithantracis (med): 4 mths (1 to 300 mths) liquor carbonis detergens (med): 6 mths (1 to 500 mths) FU: (med) 21 yrs LF: 329 (7.6%) BC: NA Age (at diagnosis): 31 (0 to 95.7) Gender (per cent men): 52% Severity: % BSA < 1%: 9% % BSA 2% to 9%: 30% % BSA 10% to 30%: 39% % BSA > 30%: 22% INCLUSION CRITERIA: people with psoriasis or eczema, diagnosed 1960 to 1990 and treated in 1 of 3 Dutch hospitals; EXCLUSION CRITERIA: people who could not be traced | All topical, phototherapy, and systemic therapies. Focus of study is on coal tar:
|
Any cancer Skin cancer Internal malignancies Specific tumour groups:
No statistically significant increased risk of any cancer. Hazard ratios: Any cancer: LCD: 0.85 (95% CI 0.60 to 1.19) PL: 0.64 (95% CI 0.40 to 1.03) Skin cancer: LCD: 1.35 (95% CI 0.53 to 3.44) PL: 0.33 (95% CI 0.07 to 1.69) |
Analysis adjusted for smoking status, other treatments, skin type, alcohol consumption. However, data on duration of tar therapy, smoking status and alcohol consumption were missing for most participants | Not applicable‐ | |
| van de Kerkhof 1997b | DESIGN: uncontrolled study patient delivery ALLOCATION: non‐random Method of randomisation: NA Concealment: NA BLINDING: open WITHDRAWAL/DROPOUT: described |
N: 58 TD: < = 60 wks; FU: < = 60 wks LF: 16 (27.6%) BC: NA Age: 45 (range: 19 to 78) Gender (per cent men): 69.0% Severity: BSA: 8.6% (3.9SD); TSS (0 to 12):7.9 (2.1SD) INCLUSION CRITERIA: people with chronic plaque psoriasis participating in previous double‐blind study (Van de Kerkhof 1996b); aged 25 to 80; normal serum calcium/phosphate. EXCLUSION CRITERIA: pregnancy or risk thereof; topical therapy within previous 4 wks; systemic therapy within previous 8 wks; serious disease; known allergy to study medication; concomitant medication that could interfere with study drug or systemic calcium metabolism | Part 1: double‐blind study (8 wks): tacalcitol 4 mcg/g ointment OD Placebo Part 2: open follow‐up study (4 wk wash‐out period): tacalcitol 4 mcg/g ointment OD, < = 20 mg/day and < 2000 g per participant over study period. Participants could discontinue treatment after 12 wks No control | Local AEs: occurrence of adverse events (duration, severity, and whether treatment‐related) Participant and investigator assessments of tolerability (4‐pt: v. good (3) to insufficient (0)) Systemic AEs: haematology (erythrocytes, platelets, haemoglobin, haematocrit); blood chemistry (serum calcium, inorganic phosphate, creatinine, ASAT, alkaline phosphatase, LDH) | Local AEs:
WA: 0/58
AE(L): 10/58 (19 events) AE(L)(treatment‐related): 8/58
Tolerability: investigator assessment: 2.60 (0.53SD, N = 58); participant assessment: 2.53 (0.63SD, N = 58)
Systemic AEs:
AE(S): 0/58 No case of hypercalcaemia |
Sponsorship not reported Follow‐up study to Van de Kerkhof 1996b ‐ 3 of 15 centres participated Scalp excluded | Not applicable |
| van de Kerkhof 2002c (see also Lambert 2002) | DESIGN: uncontrolled study patient delivery ALLOCATION: non‐random Method of randomisation: NA Concealment: NA BLINDING: open WITHDRAWAL/DROPOUT: described |
Part 1: N: 304 TD: 13 wks; FU: 13 wks LF: 47 (15.5%) BC: NA Age: 44 (range: 15 to 76) Gender (per cent men): 57% Severity: median PASI (modified to exclude head): 9.5 (range: 2.2 to 24.4); TSS (0 to 12): 6.0 Part 2: n: 197 TD: 65 wks; FU: 65 wks LF: 83 (42.1%) BC: NA Age: NR Gender (per cent men): NR Severity: NR INCLUSION CRITERIA: chronic plaque psoriasis; BSA 7% to 20% (excluding scalp); aged 18 to 70; normal baseline laboratory values Part 2 of study: responders to part 1 (≥ 30% reduction in sum score (TSS) from baseline) EXCLUSION CRITERIA: topical steroids in previous 2 wks; systemic antipsoriatics within previous 6 wks; retinoids within previous 52 wks; known hypersensitivity to vitamin D₃ analogues; serious concomitant disease; disease that might interfere with study assessments; concomitant use of oral calcium/vitamin D; pregnancy or risk thereof | Tacalcitol 4 mcg/g OD. Treatment discontinued during remission and restarted if relapse No control | Local AEs: number treatment‐related adverse events; withdrawals due to adverse events (WA); investigator assessment of tolerability; participant assessment of tolerability Systemic AEs: Haematology: serum calcium, parathyroid hormone (PTH), calcitonin, calcitriol Urine: calcium, creatinine, calcium/creatinine ratio. Compliance with medication | Local AEs: WA: 18/304 AE(L): 65/304 Tolerability excellent/good in 76% (patient assessment) to 92% (investigator assessment) of participants at final assessment. Systemic AEs: No clinically significant changes in routine haematology, urinalysis or serum chemistry. Compliance with treatment regimen varied between 82% and 92%. However, 54% of those with BSA 10% to 20% exceeded recommended daily dose of 5 g (up to 13 g daily), but there was no effect on calcium homeostasis. Duration of excess dosing not reported | Sponsored by Hermal/BHI, Germany Scalp excluded | Not applicable |
| Vazquez‐Lopez 2004 | DESIGN: uncontrolled study patient delivery ALLOCATION: non‐random Method of randomisation: NA Concealment: NA BLINDING: open WITHDRAWAL/DROPOUT: described |
N: 20
TD: 26 wks; FU: 34 wks
LF: 0 (0%)
BC: NA
Age: 28.2 (range: 20 to 55)
Gender (per cent men): 40%
Severity: NR INCLUSION CRITERIA: absence of visible or dermascopic red lines (linear telangiectasias) EXCLUSION CRITERIA: use of topical steroids in previous 2 mths |
Clobetasol propionate 0.05% cream, OD (weekends) plus calcipotriol 50 mcg/g ointment BD (weekdays) No control | Local AEs: clinical (naked eye) examination of psoriatic plaque and surrounding area Dermoscopic examination of psoriatic plaque and surrounding area Systemic AEs: NR Compliance: quantity and frequency of study drug use (tubes weighed) | Overuse of topical steroids resulted in appearance of clinically unapparent but dermoscopically apparent linear telangiectasias. 7/20 participants failed to adhere to recommended steroid dosing schedules. Dermoscopic red lines not apparent in 15/20 participants. Dermoscopic red lines apparent in 5/20 participants, of whom 4 had overused topical steroid cream. Steroid discontinued in participants with red lines and there was complete resolution within 2 mths | Links compliance with adverse events Study received no funding | Not applicable |
| Veraldi 2006 | DESIGN: uncontrolled study patient delivery ALLOCATION: sequential recruitment BLINDING: open WITHDRAWAL/DROPOUT: described | N: 48 TD: 45 dys; FU: 45 dys LF: 5 (10.4%) BC: NA Age: 48.9 (range: 21 to 71) Gender (per cent men): 62.5% Severity: NR INCLUSION CRITERIA: people with chronic stable plaque psoriasis; % BSA < = 20% EXCLUSION CRITERIA: use of antipsoriatic therapy within previous 2 wks; concurrent use of other topical, photo or systemic therapies | 0.1% tazarotene gel, short contact therapy (applied OD for 20 minutes then rinsed off with water) | Pruritis (4‐pt: 0 = absent to 3 = severe) Burning (4‐pt: 0 = absent to 3 = severe) |
At day 45: pruritis (0 to 3): 0.17 (0.38SD) 14/43 had mild pruritis Burning (0 to 3) 0.17 (0.38SD) 14/43 14/43 had mild burning No participant withdrew because of irritation on treated lesion |
Sponsorship not reported | Not applicable |
| Wishart 1994 | DESIGN: uncontrolled study patient delivery ALLOCATION: groups determined according to BSA affected. BLINDING: open WITHDRAWAL/DROPOUT: described |
N: 30 TD: 6 wks; FU: 6 wks LF: 1 (3%) BC: NA Age: 42.5 (13.2SD) Gender (per cent men): 47% Severity: Duration (mths): 202 (176SD) INCLUSION CRITERIA: people aged >18 with severe chronic plaque psoriasis; lesion severity > = 3 (GSS 0 to 4) EXCLUSION CRITERIA: pregnancy, other type of psoriasis, concurrent use of medicines containing calcium or vitamin D, antacids or digitalis | Calcitriol 15 mcg/g ointment OD Quantity of study drug varied by group: Group 1 (N =12): 4% to 8% BSA treated (300 to 600 cm^2) Group 2 (N = 10): 8% to 15% BSA treated (600 to 1200 cm^2) Group 3 (N = 8): 15% to 30% BSA treated (1200 to 2400 cm^2) |
IAGI (6‐pt) ECG Haematology, biochemistry, urine protein and glucose. Serum calcium, phosphorus, plasma PTH, serum 25‐hydroxyvitaim D, 1‐alpha,25dihydroxy‐vitaim D, 24 hr urine tests for calcium, creatinine and phosphorus. Compliance also assessed (medication weight) |
Mean daily usage: 74.0 to 306.1 mcg. No systemic adverse events, no skin irritation. No clinically relevant changes in vital signs, haematology, biochemistry, urine or ECGs. IAGI (0 to 5): ‐3.57 (1.01SD, N = 30) |
Usual dose is 3 mcg/g BD, max. 30 g daily Sponsored by Solvay Duphar |
Not applicable |
per cent men: per cent male; AE(L): number local adverse events/number participants; AE(S): number systemic adverse events/number participants; AE: adverse events; BC: baseline comparability; BD: twice daily; BSA: body surface area; FU: follow up (includes TD); N: number enrolled; NA: not applicable; NR: not reported; OD: once daily; PASI: Psoriasis Area and Severity Index; PRN: as required; TD: treatment duration; TSS: Total Severity Score; WA: withdrawal due to adverse events
25. Excluded studies of adverse events.
| Study | Reason for exclusion |
| Aste 2004 | Follow‐up under 12 wks and not focused on adverse events |
| Bos 2002 | Not psoriasis, short review (letter) |
| Breneman 2007 | Not a product included in our review (bexarotene gel 1%) |
| Carboni 2005 | Not focused on adverse events |
| Feldman 2007a | Evaluated add‐on clobetasol for participants treated concurrently with topical or systemic therapy |
| Floden 1975 | Inadequate reporting of adverse events |
| Franssen 1999 | Small (N = 54) retrospective study using participant questionnaires ‐ aimed to identify teratogenetic effects of tar, but many women unable to recall whether tar used in pregnancy |
| Hong 2010; Hong 2011 | Not chronic plaque psoriasis: paediatric dermatology participants had eczema or "eczema–psoriasis overlap (atopic dermatitis with associated features of psoriasis)" |
| Jacobi 2008 | Small uncontrolled short‐term and already reflected in results from main review |
| Kang 1998 | Short‐term and already reflected in results from main review |
| Lebwohl 1996 | Follow‐up under 12 wks and not focused on adverse events |
| Park 2002 | Case study |
| Senter 1983 | Adverse events not reported |
| Singh 2000 | Short‐term (4 weeks) and brief mention of adverse events |
| Stevanovic 1977 | Short‐term, unclear if psoriasis, small numbers (N = 6) |
| Traulsen 2003 | Participants were healthy volunteers |
| Uhoda 2003 | Not about adverse events |
| Vissers 2004 | Not about adverse events |
Of the 29 included adverse events studies, 20 were uncontrolled (Barnes 2000; Berth‐Jones 1992c; Berth‐Jones 1993; Bleiker 1998; Brodell 2011b; Cullen 1996; Gerritsen 2001 and Langner 1996; Kimball 2008 (phase II trial); Kragballe 1991b; Lambert 2002; Menter 2007; Miyachi 2002; Poyner 1993; Ramsay 1994; Roelofzen 2010; van de Kerkhof 1997b; van de Kerkhof 2002c; Vazquez‐Lopez 2004; Veraldi 2006; Wishart 1994), 8 were randomised trials (Andres 2006; Corbett 1976; Guzzo 1996; Katz 1987b; Katz 1989; Kimball 2008 (phase III trial); Lebwohl 1998b; Lebwohl 2001), 1 was a retrospective controlled study (Heng 1990), and 1 reported a control group of people using treatment other than calcipotriol (Berth‐Jones 1993).
We did not include any of the eight RCTs in the main review. Six RCTs did not meet the inclusion criteria for the main review, because they did not address comparisons of interest (Andres 2006; Corbett 1976; Katz 1987b; Katz 1989; Lebwohl 1998b) or effectiveness (Guzzo 1996). We excluded one RCT from the main review because it did not report the numbers of participants in each arm of the trial (Lebwohl 2001). In all eight RCTs, the adequacy of concealment of treatment allocation was unclear, the method of randomisation was unclear, and all were double‐blind (participant/investigator) trials. Five of the eight trials reported baseline comparability, with two studies demonstrating between‐group comparability in both clinical and demographic characteristics (Katz 1987b; Katz 1989), two reporting differences in baseline severity (Guzzo 1996; Lebwohl 1998b), and one with some significant differences in demographics and clinical severity (Andres 2006). Four of the eight RCTs were placebo‐controlled and six included active controls.
Of the nine controlled studies, two were within‐patient designs (Corbett 1976; Katz 1989), and the remainder were between‐patient (parallel‐group) designs.
Trials ranged in size from 10 to over 4000 participants. Treatment duration ranged from 2 weeks to 18 months. Loss to follow up averaged 9.5% (range = 0% to 63%). The mean age of participants was 48 (range = 15 to 85), and participants were more likely to be men (mean: 55%; range = 40 to 82%). In 12 studies, the baseline severity of participants was unclear. Remaining studies recruited participants with mild to moderate disease (N = 4), mild to severe disease (N = 4), moderate to severe disease (N = 6), and severe disease (N = 3).
Study treatments included vitamin D products (18/29), topical corticosteroids (12/29), coal tar (1/29), and tazarotene (2/29). Some comprised of combination regimens, such as vitamin D and corticosteroid (Lebwohl 1996; Lebwohl 1998b; Vazquez‐Lopez 2004) or tazarotene and corticosteroid (Lebwohl 2001). No study of dithranol met the inclusion criteria for the review. Twenty‐one of the studies assessed local adverse events, 18 assessed systemic effects, and 11 studies assessed both types. Although six RCTs reported some data on cutaneous adverse events (Andres 2006; Corbett 1976; Katz 1987b; Katz 1989; Lebwohl 1998b), these were neither suitable nor adequate for pooling.
Vitamin D products (N = 18)
Eleven studies evaluated local (N = 7) or systemic (N = 8) adverse effects associated with calcipotriol, or both. The rate of withdrawals due to local adverse events ranged from 4% to 14% and the rate of adverse events ranged between 20% and 41%; larger trials reported higher rates (weighted mean: 36%). In the 52‐week trial by Barnes 2000, facial irritation affected 30% of participants in the early stages of the trial, but the incidence declined over time. The incidence of systemic effects was less common: 5/8 studies found no significant effects. Bleiker 1998's study of inpatients with severe disease found 5/28 developed hypercalcaemia after receiving a dose greater than 5 g/kg. The study by Berth‐Jones 1993, in which 10 trial participants received a weekly dose of 100 g of calcipotriol for 4 weeks, found that urinary calcium increased significantly from baseline levels.
Four studies evaluated both local and systemic adverse effects associated with tacalcitol. The rate of withdrawals due to local adverse events ranged from 0% to 6%, and the rate of adverse events ranged between 10% and 21%. Three studies found no systemic effects. The study by van de Kerkhof 2002c found that over half of study participants with psoriasis affecting 10% to 20% of their body surface area exceeded the recommended daily dose of 5 g/day (up to 13 g daily), but there was no effect on calcium homeostasis. Systemic effects were identified in over half enrolled participants in the trial by Miyachi 2002, but only 6/155 events were considered to be treatment‐related in this uncontrolled study.
Three studies looked at adverse events associated with calcitriol (Brodell 2011b; Gerritsen 2001; Wishart 1994). One study examined the tolerability and systemic effects of calcitriol used as monotherapy (3 mcg/g ointment applied twice daily, as per licence) (Gerritsen 2001). Three per cent of participants withdrew due to adverse events, and 15% reported local adverse events. The withdrawal rate due to systemic effects was low (0.4%), but 4 cases of hypercalcaemia were reported (N = 253). Mean daily use of calcitriol in this trial was 6 g (range = 1 to 24 g). In Brodell 2011b, participants who had responded to treatment with clobetasol spray then received 8 weeks of maintenance treatment with calcitriol 3 mcg/g ointment twice‐daily. Around 15% (35/235) of participants reported burning or stinging at the end of treatment. Wishart 1994 tested the effects of high‐dose calcitriol (15 mcg/g once‐daily) on 3 groups of participants, with the quantity used proportional to the area affected The trial recruited participants with psoriasis affecting up to 30% of the body surface area. Mean daily drug use ranged from 74 to 306 mcg. The study did not observe any systemic adverse events, skin irritation, or 'clinically relevant' changes in vital signs, haematology, biochemistry, urine, or electrocardiograms.
Corticosteroids (N = 12)
The 12 studies of adverse events associated with steroids adopted a range of different study designs. Four studies had no comparator group (Brodell 2011b; Kimball 2008 (Phase II); Menter 2007; Vazquez‐Lopez 2004). Seven studies were randomised trials (Andres 2006; Corbett 1976; Katz 1987b; Katz 1989; Kimball 2008 (Phase III); Lebwohl 1998b; Lebwohl 2001), of which six used active treatment controls and three were placebo‐controlled. The remaining study retrospectively compared 13 participants who had used topical corticosteroids for between 6 months and 12 years with a 'no steroid' group (N = 15).
Eight of the 12 corticosteroid studies assessed atrophy or preatrophy in people with psoriasis who were treated with topical steroids. Three studies explicitly described the methods used to assess atrophy, and some studies did not clearly state the numbers of participants affected by atrophy or its extent.
The retrospective study by Heng 1990 compared 13 participants who had used topical corticosteroids for between 6 months and 12 years with a 'no steroid' group: These 15 individuals had previously used tar, UVB, or were untreated. Light microscopy revealed no between‐group differences. However, electron microscopy revealed multi‐layered, fragmented, and disorganised basal laminae (the lining of the outer surface of the cell membrane) in the steroid group; this appeared to be correlated with duration of treatment. Fragmentation was not observed in the control group.
The 4‐week study by Katz 1989 identified preatrophy in 20% of involved plaques, using a hand‐held magnifying lens. Two long‐term (26‐week) studies of combination maintenance therapy with steroids (Lebwohl 1998b; Lebwohl 2001) did not observe cutaneous atrophy, but neither study reported the assessment method. A 4‐week trial of clobetasol propionate for scalp psoriasis assessed atrophy using an ultrasound probe (20 MHz) (Andres 2006). No case of cutaneous atrophy was detected in either the gel (which was left in) or shampoo formulations (which was rinsed out after 15 minutes). However, there was a significantly greater reduction in skin thickness in participants treated with the gel, compared to the shampoo group (Andres 2006).
An uncontrolled study of clobetasol spray (Brodell 2011b) detected atrophy in 7/285 participants after 4 weeks of treatment, though the study did not describe the assessment method. A randomised trial compared 2 weeks of treatment with clobetasol propionate foam (N = 253), clobetasol propionate ointment (N = 121), or placebo foam (N = 123) (Kimball 2008, Phase III). The paper did not describe the assessment method. The trial found five cases of skin atrophy in the clobetasol foam group and one case in the placebo group, but the paper did not report the number of atrophy cases in the clobetasol ointment group. COBRA (Clobex Spray Community‐Based Research Assessment) was an open‐label 4‐week study where 1421 people with psoriasis used clobetasol spray 0.05% twice daily as monotherapy (Menter 2007). Cases of telangiectasia, skin atrophy, or folliculitis each occurred in less than 1% of participants (the study did not report the numbers affected or the assessment method).
Four studies examined systemic effects associated with corticosteroids. The study by Corbett 1976 compared betamethasone valerate with clobetasol propionate. Quantities used by study participants were small (mean: 7 g/wk), and the study observed no pituitary‐adrenal suppression. Katz 1987b compared two 'superpotent' corticosteroids and identified temporary and reversible adrenal suppression in 20% (8/40) of study participants. In the study by Andres 2006, none of the 14 participants assigned to clobetasol propionate shampoo experienced hypothalamic‐pituitary‐adrenal (HPA) axis suppression. Conversely, 2 of the 12 participants in the gel group experienced HPA axis suppression. Neither formulation had an impact on ocular safety. In a phase II study (Kimball 2008), higher levels of clobetasol were found in the plasma tests in ointment group than in the shampoo group, though the difference was not statistically significant.
Tazarotene (N = 2)
The study by Lebwohl 2001 compared three types of maintenance therapy: tazarotene plus steroid, tazarotene plus placebo, and placebo alone. In this 6‐month trial, there were no withdrawals due to adverse events, but 24% of participants in the tazarotene/steroid group and 29% of participants in the tazarotene/placebo group experienced adverse events. There were no adverse events in the placebo group, and the study did not assess systemic effects. Veraldi 2006 evaluated short‐contact treatment with tazarotene gel in 43 participants. The study drug was applied once daily, left for 20 minutes, and then rinsed off with water. The number of participants reporting pruritis and burning decreased over the 45‐day study period, with 14/43 participants reporting mild pruritis at the end of treatment, and the same number reporting mild burning. The study did not assess systemic effects.
(c) Quality of life measures
Eight of the 177 studies included in the main review formally assessed quality of life (QoL) (Alora‐Palli 2010; Bernstein 2006; Cook‐Bolden 2010; Guenther 2002 (H) and Guenther 2002 (P); Hutchinson 2000; Saraceno 2007; Van de Kerkhof 2006; Wall 1998).
The trials used different QoL measures. Alora‐Palli 2010 used the Dermatology Quality of Life Index (DLQI), and Bernstein 2006 used the Quality of Life Index. In both measures, higher scores indicate poorer QoL. The trial by Guenther (2002) measured quality of life using the Psoriasis Disability Index (PDI) and the EuroQOL (EQ‐5D and EQ‐VAS), reporting it in a separate publication (van de Kerkhof 2004). Hutchinson 2000 and Wall 1998 also assessed quality of life using the Psoriasis Disability Index; Wall 1998 also used the Sickness Impact Profile (SIP). Although four studies used the PDI to measure quality of life, they did not report data in sufficient detail to allow pooling, so we reported findings narratively. Four trials included participants with mild to moderate disease; the other three trials included participants with at least moderately severe disease. The number of participants ranged from 60 (Alora‐Palli 2010) to 828 (Guenther 2002 (P)), although PDI scores were obtained for only 51% of participants in this trial (van de Kerkhof 2004). Saraceno 2007 and Van de Kerkhof 2006 used the Skindex‐29, with Van de Kerkhof 2006 also employing the SF‐36. One trial used a QoL instrument designed specifically for the scalp, the Scalpdex (Cook‐Bolden 2010).
Dermatology Quality of Life Index (DLQI) (N = 1)
The Dermatology Quality of Life Index (DLQI) is scored from 0 to 30, where higher scores indicate poorer QoL. Alora‐Palli 2010 used the DLQI to compare calcipotriol and liquid carbonis distillate (LCD) 15% solution. Both treatments improved QoL relative to baseline, but there was no significant difference between the groups at the end of the 12‐week treatment period. Participants were followed up for 6 weeks post‐treatment; in the LCD group, quality of life continued to improve, but it deteriorated in the calcipotriol group. There was a significant between‐group difference in the DLQI scores at the 18‐week follow‐up assessment. This QoL effect reflected the PASI and physician global assessments, which demonstrated significantly lower recurrence rates in the LCD group at 18 weeks.
EuroQOL (EQ‐5D and EQ‐VAS) (N = 1)
The EQ5D is a generic quality of life measure. van de Kerkhof 2004 reported quality of life data from the trial by Guenther 2002. The study presented the EQ5D scores in a non‐standard method, making their interpretation problematic. EQ‐VAS (scored from 1 to 100) supported findings from the PDI assessments, with all groups improving quality of life scores relative to baseline. However, the EQ‐VAS score for the once‐daily combined calcipotriol/betamethasone dipropionate (plus placebo) group was higher (with a better quality of life) than the corresponding score in the twice‐daily group. Relative to baseline, improvements in mean quality of life scores were statistically significant in all treatment groups, but the significance of between‐group differences was not reported.
Psoriasis Disability Index (PDI) (N = 4)
In all four trials reporting this measure, there was an improvement in mean quality of life scores for participants in every group. The trial by Guenther 2002 found the greatest improvement (reduction in PDI from baseline, i.e. improvement in QoL) for twice‐daily combination treatment with calcipotriol and betamethasone (50%). Corresponding figures were as follows: once‐daily combination treatment with calcipotriol and betamethasone (plus placebo): 41%, calcipotriol twice daily: 31%, and placebo twice daily: 9%. In terms of absolute scores, the group using twice‐daily combination treatment with calcipotriol and betamethasone had the best (lowest) quality of life score, followed by the once‐daily combined treatment group, calcipotriol group, and placebo group. Relative to baseline, improvements in mean quality of life scores were statistically significant in all treatment groups, but the statistical significance of between‐group differences was not reported. In the study by Hutchinson 2000, quality of life improved from baseline significantly more in the group treated with calcitriol relative to the dithranol group. The comparison of dithranol and calcipotriol by Wall 1998 found that the magnitude of the difference was greater in the calcipotriol group, but the difference was not statistically significant at the 5% level.
Quality of Life Index (N = 1)
Bernstein 2006 used the Quality of Life Index to compare Mahonia aquifolium with placebo in participants with mild to moderate psoriasis. The index ranges from 0 to 120, with higher scores indicating poorer quality of life. Mahonia aquifolium was significantly more effective than placebo, and this was also reflected in the QoL index.
Scalpdex (N = 1)
Cook‐Bolden 2010 used the Scalpdex, a scalp dermatitis‐specific quality of life instrument comprising 23 questions, each scored 0 (never) to 100 (all the time). Relative to baseline scores, the study found a significant improvement in QoL for participants in the clobetasol spray arm of the trial and no significant improvement in the placebo spray arm.
SF‐36 (N = 1)
The SF‐36 is a generic short‐form health survey with 36 questions covering physical and mental health. The study by Van de Kerkhof 2006 compared calcipotriol and dithranol administered in a day‐care setting. The study found no significant difference in quality of life, either for the Skindex‐29 or for the SF‐36.
Sickness Impact Profile (SIP) (N = 1)
The SIP is a generic quality of life measure. Wall 1998 assessed participants using the SIP, which has a maximum score of 136. As with the PDI, the SIP found a statistically significant improvement from baseline in both groups, but the between‐group difference was not statistically significant.
Skindex‐29 (N = 2)
The Skindex‐29 is a dermatology‐specific QoL index that includes three domains: emotions, symptoms, and functioning. Two trials used the Skindex‐29, both enrolling participants with mild to moderate disease. Saraceno 2007 compared a 12‐week course of calcipotriol against 4 weeks of combination therapy (calcipotriol/betamethasone dipropionate) followed by maintenance with calcipotriol for 8 weeks. Quality of life improved in both groups relative to baseline. Van de Kerkhof 2006's study in a day‐care treatment setting found no significant difference between calcipotriol and dithranol, either for the Skindex‐29 or for the SF‐36.
(d) Economic outcomes
(THIS SECTION HAS NOT BEEN UPDATED)
A number of studies have looked at economic aspects of topical treatment for psoriasis. These include cost‐of‐illness studies (Feldman 1997; Jenner 2002; Poyner 1999), quality‐of‐life studies (Leu 1985; Lundberg 1999; Ortonne 2000; Schiffner 2003; Zachariae 2002; Zug 1995), methodological issues (Lambert 1996; Lambert 1999), willingness to pay analyses (Lundberg 1999; Poyner 2000; Schiffner 2003), cost analysis (Feldman 2000), and cost‐effectiveness analyses (Ashcroft 2000; de Tiedra 1997; Harrington 1995; Köse 1997; Marchetti 1998; Oh 1997; Owen 1993; Parodi 1991; Schwicker 1992; Sorensen 2002; Stern 1988). These analyses involve a range of modelling approaches and assumptions and have not been formally reviewed here, although they were part of our original review (Mason 2002a). Our updated review revealed no substantial variations in tolerability or effectiveness for most treatment comparisons, and no trials provided robust resource data on the consequences of managing treatment failure. Consequently, any economic models extrapolating beyond the duration of the trials may be largely speculative and uninformative. In the light of available data, a 'cost and consequences' approach, in which costs and outcomes are reported separately, may be most informative to clinical decision‐makers. The relative short‐term clinical performance of topical antipsoriatic treatments can be set against their reimbursed costs. While it is accepted that long‐term sequelae in participants not responding to treatment may be very important when considering overall costs and benefits, there are no good comparative data on these costs with which to distinguish between treatments (Mason 2002a).
(e) Concordance or adherence with treatment
(THIS SECTION HAS NOT BEEN UPDATED)
The separate search for studies of concordance/adherence identified 246 potentially relevant studies for screening. Of these, we retrieved 18 papers, and we included in the review 12 papers reporting on 11 studies (see Table 26) (Balkrishnan 2003; Carroll 2004a; Carroll 2004b; Feldman 2007; Ferrandiz 1998; Fouere 2005; Gokdemir 2008; Richards 1999; van de Kerkhof 1998c; van de Kerkhof 2000; van de Kerkhof 2001; Zaghloul 2004). Some studies were pilots (e.g. Balkrishnan 2003; van de Kerkhof 1998c) for other studies (i.e. Carroll 2004a; van de Kerkhof 2000). We listed studies that did not meet the inclusion criteria in Table 27 (Atkinson 2004; Chu 2000; Gupta 2007; Lee 2006; Osborne 2002; Richards 2006; Szeimies 2004). We identified three reviews and bibliographies were checked for further potentially relevant studies (Gupta 2007; Lee 2006; Richards 2006). Of the 11 studies included in the review, 2 were randomised controlled trials (Carroll 2004a; Ferrandiz 1998) and 4 were questionnaire surveys Fouere 2005; Richards 1999; van de Kerkhof 1998; van de Kerkhof 2000). The remaining five studies were prospective experiments, of which one included a control group (van de Kerkhof 2001). The number of participants included in the adherence studies ranged from 10 to 1281, and the study duration ranged from 1 to 16 weeks. In total, 11 studies enrolled 5541 participants, of which 39% were men and the mean age was 47.0 (range = 4 to 91). Study size ranged from 10 to 1281 participants, with the questionnaire surveys reporting the largest sample sizes. Studies covered four main issues. First, some reported methodological issues on the measurement of adherence (Balkrishnan 2003; Carroll 2004a; Fouere 2005; Zaghloul 2004). Second, some studies reported adherence rates (Balkrishnan 2003; Carroll 2004a; Fouere 2005; Richards 1999; van de Kerkhof 2000; van de Kerkhof 2001; Zaghloul 2004). Third, seven studies considered reasons for non‐adherence (Carroll 2004a; Gokdemir 2008; Fouere 2005; Richards 1999; van de Kerkhof 1998c; van de Kerkhof 2000; Zaghloul 2004). Fourth, three studies assessed interventions to improve adherence (Feldman 2007; Ferrandiz 1998; van de Kerkhof 2001).
26. Included studies of compliance.
| Study | Methods | Participants | Interventions | Outcomes (compliance | Summary findings | Notes | Allocation concealment |
| Balkrishnan 2003 | DESIGN: uncontrolled study patient delivery ALLOCATION: non‐random Method of randomisation: NA Concealment: NA BLINDING: single‐blind (participants unaware of electronic compliance assessment) WITHDRAWAL/DROPOUT: described | N: 10 TD: 1 wk; FU: 1 wk LF: 0 (0%) BC: NA Age: NR Gender (per cent men): NR Severity: NR INCLUSION CRITERIA: participants with psoriasis who already enrolling in a study with salicylic acid and topical tacrolimus ointment (Protopic) combination therapy. EXCLUSION CRITERIA: NR | Topical salicylic acid 6% No control | Medication adherence: (1) MEMS cap: medication bottle cap with microprocessor to record time/date of every opening of the bottle. (2) Patient log (self report) of compliance Mean adherence rate: method 1: 67% (32% SD); method 2: 92% (7% SD) | Medication adherence measured by method 1 (electronic) much lower than by method 2 (patient log) | Sponsorship not reported | D |
| Carroll 2004a; Carroll 2004b; Carroll 2005 | DESIGN: within‐patient patient delivery ALLOCATION: random Method of randomisation: NR Concealment: unclear BLINDING: Single‐blind (participants unaware of electronic compliance assessment) WITHDRAWAL/DROPOUT: described | N: 30 TD: 8 wks; FU: 12 wks LF: 6 (20%) BC: Yes Age: 43.6 (range 18 to 70) Gender (per cent men): 50% Severity: TSS (0 to 8): 5.3 INCLUSION CRITERIA: participants aged ≥18; symmetrical plaque‐type psoriasis; BSA < = 10%; symmetrical target plaque 1cm² with each with a score of at least 1 for erythema, thickness, and scale EXCLUSION CRITERIA: pregnancy or risk thereof; topical treatment within previous 2 wks; phototherapy or systemic therapy within previous 4 wks | Topical salicylic acid 6% plus 0.1% tacrolimus ointment BD Topical salicylic acid 6% plus placebo BD | Medication adherence: (1) MEMS cap: medication bottle cap with microprocessor to record time/date of every opening of the bottle. (2) Patient log (self report) of compliance (3) medication weights | Adherence decreased over time. On the intervention side, a decrease in adherence rate of 10% was associated with a 1‐point increase in severity (P < 0.05). For the placebo‐treated side, adherence was not significantly correlated with changes in severity. Poor compliance appears to have an impact on treatment outcomes in psoriasis Mean adherence (method 1): % (doses taken/doses expected): 55%; % (days with twice‐daily dose/total days): 39.1% Higher adherence rate for women and older participants | Sponsored by Fujisawa Healthcare, Inc. and by Wake Forest University School of Medicine. Excluded from effectiveness review (comparator is not placebo) | B |
| Feldman 2007 | DESIGN: uncontrolled study patient delivery ALLOCATION: non‐random Method of randomisation: NA Concealment: NA BLINDING: single‐blind (participants unaware of electronic compliance assessment) WITHDRAWAL/DROPOUT: described | N: 29 TD: 8 wks; FU: 8 wks LF: NR BC: NA Age: 43.5 Gender (per cent men): NR Severity: NR INCLUSION CRITERIA: NR EXCLUSION CRITERIA: NR | 6% salicylic acid gel BD No control | Impact of office visits on participants' adherence to topical treatment. Adherence assessed using MEMS cap: medication bottle cap | Adherence statistically significantly higher at time of office visit. Mean adherence over the study duration was 55%. Mean applications/day: 1.1 (range: 0.72 to 1.4) | Sponsored in part by Astellas Pharma US, Inc. The Center for Dermatology Research is funded by a grant from GaldermaLaboratories, LP. (see also Balkrishnan 2003; Carroll 2004a, 2004b, 2005) | D |
| Ferrandiz 1998 | DESIGN: between‐patient patient delivery (therapy) Clinician delivery (programme) ALLOCATION: random Method of randomisation: NR Concealment: unclear BLINDING: open WITHDRAWAL/DROPOUT: described | N: 881 TD: 16 wks; FU: 16 wks LF: 127 (12.6%) BC: Yes Age: 43.3 (16.9SD) Gender (per cent men): NR Severity: mean PASI: 7.0 INCLUSION CRITERIA: moderately severe chronic plaque psoriasis; BSA < = 30%; aged 18 to 70; under specialist supervision EXCLUSION CRITERIA: pregnancy or lactation; history of intolerance to calcipotriol/excipients; concurrent vitamin D (> 400 units/day) or calcium tablets; psoriasis mainly on face or hirsute areas | Calcipotriol plus reinforcement programme Calcipotriol without reinforcement programme | Reinforcement therapeutic programme to enhance adherence: dermatologist provided participant education with explanation of disease characteristics and treatment efficacy and application, plus written information card | The reinforcement programme had no effect on treatment efficacy | Sponsorship not reported | B |
| Fouere 2005 | DESIGN: questionnaire survey (observational cross‐sectional study) ALLOCATION: non‐random Method of randomisation: NA Concealment: NA BLINDING: open WITHDRAWAL/DROPOUT: response rate not reported | N: 1281 TD: NA; FU: NA LF: NA BC: NA Age: 51.9 (SD 14.8) Gender (per cent men): 48% Severity: 74% considered their psoriasis as at least moderately severe INCLUSION CRITERIA: members of the national psoriasis patient associations in France, UK, Belgium, Germany, and the Netherlands. EXCLUSION CRITERIA: not stated | Any antipsoriatic therapy | Compliance measured against PMAQ‐3w scale (patient medication adherence questionnaire): strict adherence to prescribed regimen over previous 3 days and last weekend Reasons for non‐compliance Perceived necessary measures to increase compliance | 73% reported non‐compliance with current treatment Main reasons for non‐compliance: lack of efficacy, messiness, and time constraints To improve compliance, patients suggested improved efficacy, less greasy, sticky and smelly treatment, and fewer side‐effects. | Sponsorship not reported. 70% of responders used topical therapy | D |
| Gokdemir 2008 | DESIGN: open uncontrolled study patient delivery ALLOCATION: non‐random Method of randomisation: NA Concealment: NA BLINDING: open WITHDRAWAL/DROPOUT: described | N: 109 TD: 8 wks; FU: 8 wks LF: 6 (6%) BC: NA Age: 40 (range: 16 to 70) Gender (per cent men): 43% Severity: PASI: 9.1 (range: 1.2 to 35) INCLUSION CRITERIA: chronic plaque psoriasis; received prescribed antipsoriatic therapy; aged ≥16; attending outpatient clinic in Istanbul. EXCLUSION CRITERIA: other types of psoriasis; hospitalised; pregnancy | Any prescribed antipsoriatic therapy | Medication adherence: number prescribed doses taken/number prescribed doses prescribed (see Zaghloul 2004). | Mean adherence for topical therapy: 72% (31%SD) Adherence rate was correlated with being unmarried, more highly educated, and being satisfied with treatment Main reasons for non‐adherence were busyness and 'being fed up' | Findings relate to any treatment for psoriasis (not just topical therapy) Sponsorship not reported | D |
| Richards 1999 | DESIGN: questionnaire survey (cross‐sectional uncontrolled study) patient delivery ALLOCATION: non‐random Method of randomisation: NA Concealment: NA BLINDING: open WITHDRAWAL/DROPOUT: Response rate not reported | N: 120 TD: NA; FU: NA LF: NA BC: NA Age: 49 (18 to 84) Gender (per cent men): 54% Severity: Duration: range: 1 to 63 yrs INCLUSION CRITERIA: consecutive participants attending tertiary psoriasis specialty clinic; psoriasis. EXCLUSION CRITERIA: not stated | Any antipsoriatic therapy | Per cent complying with treatment (self report): scale not reported | 39 per cent reported non‐compliance (sometimes/never complying) with prescribed treatment. The non‐compliant group had a higher self‐rated disease severity, were younger, and had a younger age at onset. The non‐compliant group reported that psoriasis had a greater impact on daily life Factors affecting compliance included the doctor‐participant relationship; optimism with the treatment prescribed; and a limited 'nuisance' value of treatment in terms of side‐effects and hassle of use | Sponsorship not reported 55% of participants were using topical therapies | D |
| van de Kerkhof 1998 | DESIGN: questionnaire survey (uncontrolled study)
patient delivery
ALLOCATION: non‐random
Method of randomisation: NA
Concealment: NA BLINDING: NA WITHDRAWAL/DROPOUT: Response rate reported |
N: 972 TD: NA; FU: NA Response rate: 13% BC: NA Age: 45.8 (range: 5 to 87) Gender (per cent men): 43% Severity: duration of psoriasis > 10 yrs in 67% of responders INCLUSION CRITERIA: subscribers to 'Psoriasis', the journal of the Dutch Psoriasis Patient Organisation EXCLUSION CRITERIA: none stated | Any topical antipsoriatic therapy | Per cent complying with frequency of application of prescribed topical therapies Reason for non‐compliance | 29% of responders reported that the prescriber did not specify dosage frequency. Where dosage frequency was specified, 33% (39%) complied with twice (once) daily regimens Main reasons for non‐adherence were preference for less frequent dosage; greasiness; lack of efficacy; and higher‐than expected efficacy | Sponsorship not reported. 14‐item questionnaire mailed in 1996 to 6100 subscribers of Psoriasis, the Journal of the Dutch Psoriasis Patient Organisation Responders asked to report on compliance over past 6 mths | D |
| van de Kerkhof 2000 | DESIGN: questionnaire survey (uncontrolled study) ALLOCATION: non‐random Method of randomisation: NA Concealment: NA BLINDING: single‐blind WITHDRAWAL/DROPOUT: response rate reported | N: 839 TD: NA; FU: NA LF: NA Response rate: 14% BC: NA Age: 48.5 (range: 4 to 91) Gender (per cent men): 46% Severity: duration of psoriasis ≥ 11 years in 62% of responders INCLUSION CRITERIA: subscribers to 'Psoriasis', the Journal of the Dutch Psoriasis Patient Organisation EXCLUSION CRITERIA: none stated | Any antipsoriatic therapy including topical treatments, photo(chemo)therapy and systemic therapy | Per cent complying with duration of prescribed treatment (topical therapies) Per cent complying with frequency of application of prescribed treatment (topical therapies) Reason for non‐compliance | Per cent complying with duration of prescribed treatment (topical therapies): 71% Per cent complying with frequency of application of prescribed treatment (topical therapies): 51% Main reasons for non‐adherence were preference for minimum dosage; time constraints; and lack of confidence in efficacy | Sponsorship not reported 41‐item questionnaire mailed to 6100 subscribers of Psoriasis, the Journal of the Dutch Psoriasis Patient Organisation Responders asked to report on compliance over past 6 mths | D |
| van de Kerkhof 2001 | DESIGN: within‐patient (see Notes) patient delivery ALLOCATION: non‐random Method of randomisation: NA concealment: NA BLINDING: open WITHDRAWAL/DROPOUT: described | N: 976 TD: 8 wks; FU: 8 wks LF: 93 (9.5%) BC: NR Age: 45.6 (range: 7.4 to 88.4) Gender (per cent men): 52% Severity: BSA ≥ 10% in 51% of participants INCLUSION CRITERIA: psoriasis (type NR); eligible for treatment with calcipotriol EXCLUSION CRITERIA: concomitant topical or systemic antipsoriatic therapy; co‐existing skin disorder other than psoriasis | Calcipotriol cream OM plus calcipotriol ointment ON Calcipotriol ointment BD | Compliance: self‐reported number of days cream/ointment regimen applied | At wk 3, 72% of participants applied the regimen on most days. By wk 8, this statistic had fallen to 61% 51% of the 309 participants with previous experience of calcipotriol ointment monotherapy reported that their compliance with the cream/ointment regimen was higher | Sponsorship not reported Control group comprised retrospective self‐reported experience of calcipotriol ointment monotherapy by 35% of participants in the intervention group | D |
| Zaghloul 2004 | DESIGN: uncontrolled study patient delivery ALLOCATION: non‐random Method of randomisation: NA Concealment: NA BLINDING: single‐blind (participants unaware that study focused on compliance) WITHDRAWAL/DROPOUT: described | N: 294 TD: 12 wks; FU: 12 wks LF: 93 (31.6%) BC: NA Age: 45.1 (range: 20 to 65) Gender (per cent men): 44.3% Severity: NR INCLUSION CRITERIA: psoriasis (unclear if chronic plaque only); aged 18 to 65; prescribed oral, topical or combined treatment EXCLUSION CRITERIA: pregnancy, lactation, concomitant disease | Topical, oral, or combined antipsoriatic medication No control | Medication adherence: (1) number prescribed doses taken/number prescribed doses prescribed (2) patient self‐report Quality of Life (DLQI) (0 to 30; higher score implies lower quality of life) | Medication adherence measured by method 1 (objective) much lower than by method 2 (patient self report). Mean rate: 60.6% (33.0%SD); (range: 0% to169%) Direct correlation observed between medication adherence and quality of life Adherence rate higher for participants who were women, married, employed, or not paying for prescriptions Adherence greater for topical (vs. systemic) therapy, once daily, or first‐time use | Authors report no relevant financial interests | D |
per cent men: per cent male; AE(L): number local adverse events/number participants; AE(S): number systemic adverse events/number participants; AE: adverse events; BC: baseline comparability; BD: twice daily; BSA: body surface area; FU: follow up (includes TD); N: number enrolled; NA: not applicable; NR: not reported; OD: once daily; PASI: Psoriasis Area and Severity Index; PRN: as required; TD: treatment duration; TSS: Total Severity Score; WA: withdrawal due to adverse events
27. Excluded studies of compliance.
| Study | Reason for exclusion |
| Atkinson 2004 | Adherence not assessed |
| Chu 2000 | Treatment guideline (not primary study) |
| Gupta 2007 | Review/think piece |
| Lee 2006 | Review |
| Osborne 2002 | Study focused on non‐responsive participants rather than those that are specifically non‐compliant |
| Richards 2006 | Review |
| Szeimies 2004 | Think piece (not primary study) |
Methodological issues relating to adherence measurement and adherence rates
Some studies used self‐reported adherence rates (Gokdemir 2008; Richards 1999; van de Kerkhof 2000; van de Kerkhof 2001; Zaghloul 2004). In these studies, which included short‐term prospective trials and cross‐sectional questionnaire surveys, rates varied between 61% and 72%. However, other studies adopted more objective assessment methods. The study by Fouere used a semistructured participant questionnaire: The PMAQ‐3w scale (Patient Medication Adherence Questionnaire) asked about strict adherence to prescribed regimen over the previous three days and previous weekend. Using this method, the study (Fouere 2005) deemed just 27% of participants as 'compliant'. Some studies adopted a single‐blind approach to assess electronic bottle caps (i.e. participants were unaware that bottles were fitted with electronic measuring devices) (Balkrishnan 2003; Carroll 2004a; Feldman 2007). Known as a 'MEMS cap' (Medication Event Monitoring System), the medication bottle cap was fitted with a microprocessor to record the time/date of every opening of the bottle. When compared with participant logs, electronic methods suggested that adherence was considerably lower than rates reported by participants. For example, Balkrishnan's 1‐week pilot study of 10 participants found that the adherence rate was 67% by electronic assessment compared with a self‐reported rate of 92%. Carroll and colleagues took this pilot study forward using an 8‐week trial of 30 participants (Carroll 2004a). Adherence rates fell over time and averaged 55% over the study period when assessed using the MEMS cap. Twice‐daily dosing was achieved on 39% of the treatment days.
Reasons for non‐adherence
Reasons for non‐adherence comprised therapy characteristics (real or perceived), participants' clinical characteristics, and participants' demographic characteristics. Regarding therapy characteristics, the included studies identified the following as influences on adherence: efficacy (Fouere 2005; van de Kerkhof 1998c; van de Kerkhof 2000), side‐effects (Fouere 2005; Richards 1999; Zaghloul 2004), time for application (Fouere 2005; Gokdemir 2008; van de Kerkhof 2000), and 'messiness' (Fouere 2005; Richards 1999; van de Kerkhof 1998c). Adherence was higher for topical therapy compared with systemic treatment (Zaghloul 2004), but adherence rates also varied by type of topical treatment (Fouere 2005). Disease severity was inversely related to adherence (Carroll 2004a; Richards 1999), although the direction of causality is unclear. Higher adherence rates were variously associated with female gender (Carroll 2004a; Zaghloul 2004), older age (Carroll 2004a; Richards 1999), higher educational achievement (Gokdemir 2008), and older age of disease onset (Richards 1999), but findings on marital status were mixed (Gokdemir 2008; Zaghloul 2004).
Methods to improve adherence
Office visits to clinicians were found to temporarily increase adherence rates (Carroll 2004a; Feldman 2007). An educational programme designed to improve adherence had no impact on the effectiveness of treatment (Ferrandiz 1998). Van de Kerkhof's study comparing ointment/cream regimen with ointment‐only treatment found no clear impact, with 51% of participants reporting better adherence with the cream/ointment option (van de Kerkhof 2001).
Discussion
Summary of main results
Effectiveness
This review analysed evidence from 177 studies that included 34,808 participants. We reported results separately for treatments of the body, inverse psoriasis, and psoriasis of the scalp.
Treatments for psoriasis of the body
We analysed six placebo‐controlled comparisons of treatments for psoriasis of the body. Most treatments were significantly more effective than placebo, with the equivalent pooled effect on a 6‐point improvement (IAGI) scale ranging from 1.02 (potent corticosteroid) to 1.80 (very potent corticosteroid). Pooled effects indicated similar improvements for vitamin D analogues (1.03 points), dithranol (1.22 points), and combination therapy with vitamin D and potent corticosteroid (1.65) on a 6‐point scale.
Amongst less frequently researched treatments, 13 were significantly more effective than placebo. For these treatments, the equivalent effect on a 6‐point IAGI scale ranged from 0.55 (calcipotriol plus nicotinamide) to 3.40 (herbal skin care products). Tazarotene achieved an equivalent improvement of 0.99 on the 6‐point IAGI scale. One small within‐patient study found that tar was no more effective than placebo (Kanzler 1993). Findings from single studies should be treated with caution and regarded as requiring further evidence before guiding treatment.
We evaluated nine head‐to‐head comparisons. Findings from the comparisons of vitamin D against potent or very potent corticosteroids were mixed, and they varied depending on which vitamin D and which corticosteroid were analysed. Combination treatment with vitamin D/corticosteroid was usually more effective than monotherapy with the same vitamin D, and it was always more effective than the same corticosteroid used alone.
Twelve complex regimens (i.e. treatment sequences that do not consist of a simple head‐to‐head comparison between two active treatments) were compared with vitamin D. Seven of the complex regimens were more effective than vitamin D, achieving an additional benefit of between 0.27 points and 0.72 points on an equivalent 6‐point IAGI. A comparison of three long‐term regimens found no significant difference in effect, but some were better tolerated (see below). These studies were interpreted independently, reflecting the differing management regimens. None of the studies presented have been replicated precisely. Therefore, while supportive evidence for findings can be drawn from similar studies, trial findings for complex regimens should be interpreted with caution when informing clinical decisions.
Treatments for inverse psoriasis
The review included four placebo‐controlled comparisons of treatments for psoriasis of the sensitive areas of the body. As there were no effectiveness data for tacrolimus, the review evaluated benefits for three treatments. All three were significantly more effective than placebo, with the pooled effect on a 6‐point IAGI scale ranging from 0.98 (pimecrolimus) to 3.25 (betamethasone valerate). Five treatments were compared head‐to‐head against vitamin D. Betamethasone valerate, combined treatment with calcipotriol and hydrocortisone, and calcitriol were all significantly more effective than calcipotriol alone. On an equivalent 6‐point IAGI, these treatments achieved an additional benefit of 2.22, 0.33, and 0.67 points, respectively.
Treatments for psoriasis of the scalp
We compared 11 treatments for scalp psoriasis with placebo. All but two were significantly more effective than vehicle alone; the most effective was the very potent corticosteroid, clobetasol propionate, which delivered a benefit of almost 2 points (1.88) on an equivalent 6‐point IAGI scale. When compared head‐to‐head, vitamin D was significantly less effective than both potent and very potent corticosteroids. On an equivalent 6‐point IAGI scale, the additional benefit was between 0.48 and 0.67 points. Combination therapy with calcipotriol and betamethasone dipropionate was significantly more effective than either product used alone.
Adverse effects
Randomised evidence found that vitamin D was significantly more likely than potent corticosteroids to cause local adverse effects (15% versus 8%, P = 0.006), and participants were (borderline) more likely to withdraw for this reason (2% versus 1%, P = 0.059). These findings were supported by indirect comparisons of placebo‐controlled trials. Participants tolerated combined treatment with vitamin D/corticosteroid on either the body or the scalp as well as potent corticosteroids and significantly better than vitamin D alone. No comparison of topical agents found a significant difference in systemic adverse effects.
Studies of longer‐term adverse events found that up to 40% of those using vitamin D analogues experienced cutaneous effects and that up to 14% of participants stopped using vitamin D analogues for this reason. There was some evidence to suggest that using vitamin D analogues at high doses could cause systemic adverse effects.
Two of the major cutaneous adverse effects of topical corticosteroids are dermal and epidermal atrophy (Hengge 2006; Kragballe 2006). Only 25 of the 78 RCTs of corticosteroids included in the review explicitly assessed cutaneous atrophy, and in these the duration of the trials, skin sites assessed, and methods employed reduced the chance of detection. The studies either did not support the methods used to assess atrophy (14/25 trials), physicians undertook them (10/25), study participants self‐reported them (5/25), or a combination of the aforementioned. Where physician assessments were conducted, the methods used were not explicit (for example, whether microscopy or ultrasound was used). Two of the 25 trials featured an independent 'adjudication committee', which assessed whether atrophy was likely to be treatment‐related (Kragballe 2006; Poulin 2010). Trial duration of the 25 trials was typically between 4 and 8 weeks, but there were 2 6‐month studies (Katz 1991a; Poulin 2010) and 2 52‐week studies (Kragballe 2006; Luger 2008).
Two trials that assessed atrophy did not report whether or not any cases of atrophy occurred (Huang 2009; Koo 2006). Ten RCTs reported cases of atrophy, and these were trials of either very potent corticosteroids (Cook‐Bolden 2010; Decroix 2004; Lowe 2005; Poulin 2010; Reygagne 2005) or combination treatment with potent corticosteroids (Guenther 2002 (H); Kragballe 2004; Kragballe 2006; Papp 2003 (H) and Papp 2003 (P); Yang 2009). Three of the 10 were trials of scalp psoriasis (Cook‐Bolden 2010; Poulin 2010; Reygagne 2005), all of which investigated clobetasol propionate.
Some studies reviewed as part of our search for additional studies of adverse events of longer‐term use of topical corticosteroids revealed atrophy and damage to basal laminae, the extent of which appeared to be correlated with dosage and duration of use. Research of very potent steroids on the skin of normal volunteers (Kao 2003). Longer‐term use with topical retinoids caused cutaneous effects in around 30% of participants, but systemic effects were not assessed.
Concordance/adherence
Thirty‐four of the 177 RCTs assessed adherence ('compliance'), and 26 trials reported findings. Trials used various methods such as self‐report by participants, medication weight, and treatment completion. Most of the 26 studies that reported findings on adherence found it to be suboptimal. However, Gupta has argued that the relevant notion is concordance rather than compliance or adherence (Gupta 2007). Whereas adherence implies that people comply with clinicians' prescribed treatment, concordance involves a negotiated doctor‐patient agreement about the treatment regimen. Van de Kerkhof's survey of over 800 European people with psoriasis found that some chose not to comply, preferring to apply the 'minimum' dose needed to achieve the effect they wanted (van de Kerkhof 2000). If prescribed regimens reflected the wishes of the person with psoriasis, 'adherence' rates might be expected to rise. Nonetheless, poor adherence to treatment regimens is likely to impair the effectiveness of treatment.
The review identified one study that used an educational programme to improve adherence rates in people with psoriasis (Ferrandiz 1998). This study found no statistically significant difference in outcomes, a finding which contrasts with evidence concerning an educational programme for atopic dermatitis (Cork 2003). However, whereas the programme in the Ferrandiz 1998 study provided education about the disease, the study by Cork involved specialist nurses teaching methods for applying topical treatments. Therefore, the findings from Ferrandiz 1998 may not generalise to reflect the value of other educational programmes.
Sensitivity analysis
In four comparisons, we used sensitivity analysis to explore whether within‐patient versus between‐patient study designs were associated with different effect sizes. When comparing within‐patient and between‐patient study designs, our review found no statistically significant difference in treatment effect size. If within‐patient studies featured some correlation, due to systemic effects or cross contamination, then one would expect systematically smaller effect sizes from within‐patient studies. If there is no correlation, then one would expect the variance to be smaller (as other sources of between‐patient variation have been removed) but the effect size to be similar. We found no evidence to support a correlation for within‐patient studies, and variances were similar for within and between‐patient studies (Table 3), supporting the pooling of these, although it is accepted that within‐patient designs are 'underweighted' in meta‐analyses.
In the placebo‐controlled studies of vitamin D products, there was little difference when comparing within‐patient and between‐patient study findings. On average, within‐patient studies identified a slightly greater effect than between‐patient studies (mean difference: 0.36 points on a 6‐point IAGI scale). For placebo‐controlled potent corticosteroids, there was a more pronounced difference in favour of within‐patient studies (mean difference: 0.55 points on a 6‐point IAGI scale). In the case of very potent corticosteroids, the mean effect relative to placebo was slightly larger in the between‐patient studies (mean difference: 0.07 points on a 6‐point IAGI scale), and the analysis of vitamin D against potent corticosteroid was similar in this respect (a small positive difference in favour of between‐patient studies). These non‐statistically significant differences may be chance findings.
Quality of the evidence
All included trials were randomised, but only 47 studies (27%) clearly reported the method used to randomise participants (Figure 3). Just 15 trials (9%) adequately concealed treatment allocation, but most (74%) masked participants to treatment. Of the 177 studies assessed, 151 reported loss to follow up, and 143 demonstrated that groups were comparable at baseline. Based on the 100 studies that reported assessable data on baseline severity, it was apparent that a wide range of severity was included within and between trials. If the PASI is unreliable for mild‐to‐moderate disease (PASI scores less than 10) (Brownell 2007), it is possible that some trials may have contributed unreliable PASI data to this review.
Trials varied in their treatment duration and in their outcome measures (see Included studies section for details). One reason for the variation in trial treatment duration is that the time taken for an intervention to be effective differs between treatments. The analyses and forest plots do not provide information on treatment duration, but this is an important consideration for clinical decisions. Trials also used different outcome measures; for example, only 113 studies reported data on the IAGI or IGA, and 65 studies reported PASI data. In order to maximise the number of studies contributing data for a particular treatment comparison, we used a combined end point that incorporated different outcome measures. This approach is not ideal; although all outcome measures essentially assess redness, thickness, and scaling (and sometimes area of psoriatic involvement), they differ in their construct. Therefore, the composite end points should be seen as indicative rather than definitive.
Medical text books commonly document the risk of skin atrophy and tachyphylaxis (diminution of the effects of a drug with continual use) as problems associated with topical corticosteroids (Bos 2008). Randomised evidence reported in this review suggests that maintenance regimens using intermittent dosing are safe and effective, and no RCT included in the review detected a statistically significant difference in systemic effects. However, the RCTs did not clearly report the methods employed (e.g. whether the investigator used ultrasound) and may have reduced the chance of detection. We undertook a separate search for longer‐term studies of adverse events, which identified 12 relevant studies of corticosteroids. Studies were heterogeneous in terms of design, assessment methods, comparators considered, and doses used, so we did not pool data (Higgins 2011). Eight studies assessed atrophy, and six found some evidence of basal damage and atrophy. We identified no studies reporting adequate data on tachyphylaxis, despite including this term in our searches.
Potential biases in the review process
There are a number of limitations to this review. First, one author extracted data and a second checked it. Ideally, two authors should extract data independently (NHSCRD 2001). However, this approach was not feasible for this review because of funding constraints. Second, requests for unpublished data from trialists and sponsors were of variable success; requests were more likely to be successful when made for more recently published studies, products still on patent, or both.
This review included 19 comparisons with 10 potential outcomes, so multiple analyses may be expected to generate occasional chance findings. Thus, greater importance is attached where supportive findings are found from placebo‐controlled and head‐to‐head studies as well as linked therapeutic modalities.
Agreements and disagreements with other studies or reviews
A systematic review by Ashcroft and colleagues focused on head‐to‐head trials of one vitamin D analogue, calcipotriol (Ashcroft 2000a). Based on data from 37 studies with over 6000 participants, the review found that calcipotriol was at least as effective as potent topical corticosteroids and more effective than calcitriol, tacalcitol, coal tar, and short contact dithranol. Our review generally supported these findings. However, we found no significant difference for the comparison of calcipotriol and calcitriol (SMD ‐0.41; 95% CI ‐1.46 to 0.64), a finding based on 2 head‐to‐head trials with 261 participants. Our review also found that calcitriol is more efficacious than calcipotriol when used for inverse psoriasis, and it is also better tolerated. Our findings on the effectiveness of short‐contact dithranol compared with calcipotriol were mixed, but physicians and people with psoriasis may be interested to know that inpatient treatment with dithranol appears more effective than calcipotriol (Monastirli 2000). Our review also supported findings from the review by Ashcroft 2000a that although calcipotriol caused significantly more skin irritation than potent topical corticosteroids, skin irritation rarely led to withdrawal of calcipotriol treatment.
The review by Afifi 2005 concluded that combined treatment with steroids and vitamin D analogues or topical retinoids appeared the most promising current treatment on account of its superior efficacy and favourable side‐effects profile. However, longer‐term adverse effects were not addressed by this review. In contrast, the review by Bruner 2003a focused on adverse effects. This review supported our findings that there is little robust evidence on longer‐term adverse effects; therefore, concluding that since clearance is not a realistic expectation, "reasonable goals" are needed to avoid increasing the risk of cutaneous and systemic side‐effects through over use.
Authors' conclusions
Implications for practice.
Evidence from large numbers of trials indicates that most of the topical treatments tested in the trials reviewed here alleviate the symptoms of psoriasis. However, it was not possible to assess the performance of treatments at different levels of severity of psoriasis.
The evidence suggests that vitamin D products are more effective than emollient alone. Potent and very potent corticosteroids are also effective, and very potent corticosteroids are more effective than either potent corticosteroids or vitamin D products. The effectiveness of dithranol and tazarotene appears to be similar to that of vitamin D products. Although vitamin D and corticosteroids are equally effective for treating psoriasis of the body, corticosteroids appear to be more effective than vitamin D for treating psoriasis of the scalp. Combined treatment of vitamin D with corticosteroid is more effective than either vitamin D alone or corticosteroid alone. Vitamin D is more effective than coal tar, but findings on the relative effectiveness of vitamin D and dithranol were mixed. Occlusion enhances the effectiveness of vitamin D, as does twice‐daily rather than once‐daily application.
Compared with vitamin D alone, combined therapy that uses two products separately (vitamin D in the morning and corticosteroid at night) can achieve similar effects and be as well tolerated as using a combined product. However, some corticosteroids seem to perform better than others when used separately (see Analysis 12.5 and Analysis 12.9), and disease severity may also affect treatment performance, though poor reporting of baseline severity in trials means that we cannot confirm this. Use of a combined product may also enhance concordance, and there is evidence to suggest that adherence is higher when application time is shorter.
Potent corticosteroids are less likely than vitamin D to cause local adverse events, and treatment with corticosteroids is less likely to result in discontinued use because of these adverse events. Tazarotene is more likely than emollient to cause local adverse events. Our review found no difference between placebo and any other topical treatment in the assessment of systemic adverse events. However, this may reflect an absence of evidence (trials failing to appropriately assess these events over adequate time periods) rather than being evidence of absence.
Although current evidence demonstrates that topical steroids are as effective as and at least as well tolerated as vitamin D analogues, concern remains about the potential safety problems associated with corticosteroids (Bos 2008). Concerns include the risk of rebound (a worsening of disease following treatment discontinuation), skin atrophy (skin thinning), and tachyphylaxis (decreasing response to the drug) after long‐term use (Hengge 2006). Methods to assess rebound have been developed and should be used in future research (Carey 2006). Regarding skin thinning, one problem with psoriasis is that the skin is very thick and a goal of therapy is to reduce the thickness of lesional (epidermal) skin. Damage to the surrounding normal skin may occur, and for that reason, people should use topical corticosteroids for limited periods or sparingly in delicate areas, such as the face or folds of the skin.
Although topical corticosteroids have been in use for about 50 years, there is a surprising lack of relevant evidence addressing steroid‐associated skin dermal atrophy in people with chronic plaque psoriasis requiring long‐term treatment. It is improbable that short‐term (less than three weeks) courses of topical corticosteroids cause dermal skin atrophy, except in delicate areas such as the face and flexures (groin, axillae, inframammary). However, treatment may be long‐term and continuous for people with more severe chronic psoriasis. Current assessments of cutaneous dermal atrophy within trials have largely been limited to clinical observation or symptom and sign reporting by trial participants (which will only detect severe atrophy of the dermis). More sensitive and reliable methods, such as high frequency ultrasound, are routinely available (Cossmann 2006). Dermal atrophy might be monitored in part if trials routinely adopted more robust methods, such as high frequency ultrasound, to assess dermal skin atrophy skin‐thinning, providing useful information for patients and clinicians.
Excessive use of topical corticosteroids can also cause a substantial thinning of the epidermis (Kao 2003). However, thickening of the epidermis is part of the problem in psoriasis and so thinning of the epidermis (not dermis) that has been caused by topical corticosteroids may be of benefit in psoriasis. Evidence drawn from healthy volunteers or severe long‐term cases cannot be generalised. Specifically, evidence is required concerning the frequency and spectrum of atrophy in people with chronic psoriasis requiring long‐term treatment.
Topical vitamin D analogues (calcipotriol, tacalcitol) and topical calcineurin inhibitors (tacrolimus and pimecrolimus) do not cause cutaneous atrophy. The issue may become a historical one if newer safer steroids, currently being developed and evaluated for atopic dermatitis, subsequently demonstrate efficacy and safety in chronic plaque psoriasis.
We found no evidence on tachyphylaxis, but if treatment response were to decline, this could lead to over‐use, increasing the risk of percutaneous absorption. As the evidence base on longer‐term adverse effects in psoriasis is inadequate, the preferences of people with psoriasis and their attitudes to these perceived risks should inform treatment choice. Further research is required to inform approaches to long‐term maintenance.
Implications for research.
Evidence showing that treatments improve the symptoms of psoriasis has focused mainly on treatments with relatively short duration. Although improving, there is still relatively limited randomised evidence to tell us about the long‐term effect of using these treatments; good quality head‐to‐head evidence is therefore needed to quantify and compare long‐term adverse events and to explore the feasibility of long‐term treatment. There are important sources of heterogeneity in currently available trial findings, which it is not possible to explore in anything other than a qualitative sense. For example, the properties of the vehicle preparation are known to deliver wide variation in response to treatment. This is important when interpreting the findings of this review. The value of the active ingredient may be worth one point on a six‐point (IAGI) scale, but possibly one to two points are also being contributed by the vehicle. However, an analysis of vehicle performance was outside the scope of the review. Trial publications included in this review span 45 years. Reporting standards within these trials are generally suboptimal by today's standards, and it would be useful to ensure that current trials adhere to CONSORT (CONsolidated Standards of Reporting Trials) standards to help future reviews to interpret findings appropriately. For example, where trials enrol participants with a wide range of baseline severity, stratifying the randomisation by baseline severity would be a useful design feature. We are not aware of studies that have adopted this approach. Trialists might usefully consider including more homogeneous participant groups in terms of severity, so that the clinical implications of findings are clearer.
Given the importance of safety to patients and to resolve clinical uncertainties, it would be valuable to obtain reliable data on the safety of corticosteroids used long‐term at recommended doses, including step‐down or intermittent management. Historically, these uncertainties have been driven by unrepresentative case series of non‐standard use of steroids. Given the variety of methods used to assess atrophy and other sequelae, it would be valuable to reach clinical consensus about reliable assessment methods (Cossmann 2006).
What's new
| Date | Event | Description |
|---|---|---|
| 9 December 2015 | Amended | Author information (affiliation) updated |
History
Protocol first published: Issue 4, 2004 Review first published: Issue 2, 2009
| Date | Event | Description |
|---|---|---|
| 2 April 2013 | Amended | 'Declarations of interest' section updated. |
| 25 February 2013 | New citation required but conclusions have not changed | This review was updated, but there was no change to the conclusions. |
| 25 February 2013 | New search has been performed | 48 new RCTs added. Comparisons revised to reflect new evidence. Scalp trials moved to separate comparison groups ready for removal at next update (#18; #19). Inverse psoriasis trials also moved to separate comparison groups (#16; #17). Nail trials removed, as now part of separate Cochrane review. |
| 15 December 2008 | Amended | Review amended to reflect peer review comments |
| 25 June 2008 | Amended | Converted to new review format. |
| 29 September 2003 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The Cochrane Skin Group editorial base wishes to thank Luigi Naldi who was the Key Editor for this review; Jo Leonardi‐Bee and Philippa Middleton who were the Statistical and Methods Editors, respectively; the clinical referees, Phyllis Spuls and Steven Chow; and the consumer referee, Carolyn Hughes.
The review team are indebted to Jane Harrison (formerly of the Centre for Reviews and Dissemination, University of York) for devising the original search strategies in 1999, Julie Glanville (formerly of the Centre for Reviews and Dissemination, University of York) for running the effectiveness and adverse events search strategies in 2002 and 2005, Kate Light and Kath Wright (Centre for Reviews and Dissemination, University of York) for updating the searches in 2008 and 2011, and Finola Delamere and Liz Doney of the Cochrane Skin Group for searching the Cochrane Skin Group's Specialist Skin Trials Register. We also acknowledge the valuable contribution made by Gladys Edwards, who co‐authored a previous version of this review.
Last but not least, we would like to thank the authors and sponsors who provided unpublished data, which has greatly enriched this review.
Appendices
Appendix 1. Specialised Register search strategy (RCTs)
Searched 04/12/04 Search updated on 17/11/2008: from January 2005 to date Search updated on 3/02/2011: from November 2008 to date. One additional term added to 1st part: 'taclonex'. Search updated 29/08/12: from February 2011 to date.
1st part
(*psorias* or *psoriat*) AND (*tar* or *gel* or alphosyl or carbodome or exorex or balneum or cocois or capasal or ceanel or ionil or meted or pentrax or anthralin or dithr* or micanol or psorin or psoriderm or salicylic or (vitamin and D) or calcipo* or dovo* or *calcit* or curatoderm or tazarotene or zorac or silkis or acitretin or neotigason or ciclosporin or cyclosporin or methotrexate or tacrolimus or pimecrolimus or protopic or elidel or retinoid* or macrolactam* or immunosuppressant* or taclonex) AND topical*
2nd part
(*psorias* or *psoriat*) AND ((adrenal and cortex and hormone*) or *steroid* or hydrocort* or cobadex or efcortelan or *derm or *dermal or *movate or mildison or calmurid or locoid or alclometasone or modrasone or beclo* or betametha* or betacap or betnovate or bettamousse or dipro* or clobetaso* or desox* or stiedex or diflucortolone or nerisone or fluocino* or synalar or metosyn or fluocortolone or ultralanum or flurandrenolone or fludroxycortide or haelan or fluticasone or cutivate or halci* or mometasone or elocon or triamcinolone or *cort or *cortyl)
Appendix 2. CENTRAL (Cochrane Library CD‐ROM) and National Research Register (NRR) (CD‐ROM interface) search strategies (RCTs)
CENTRAL (Cochrane Library CD‐ROM 2005 issue 1): publication years 2001 to 2005‐02‐24 Searched on 17/11/08: CENTRAL (The Cochrane Library: http://www.thecochranelibrary.com/) Searched on 02/02/11: CENTRAL (The Cochrane Library: http://www.thecochranelibrary.com/) Searched on 23/08/12: CENTRAL (The Cochrane Library: http://www.thecochranelibrary.com/)
National Research Register (CD‐ROM interface, issue 2004/4): all projects with a start date of 2001 to 2005 Website browsed on 20/11/08: UK Clinical Research Network Study Portfolio (http://public.ukcrn.org.uk/search/) Website browsed on 22/02/11: UK Clinical Research Network Study Portfolio (http://public.ukcrn.org.uk/search/) Website browsed on 10/09/12: UK Clinical Research Network Study Portfolio (http://public.ukcrn.org.uk/search/)
#1 MeSH descriptor Psoriasis explode all trees #2 (Psorias* or psoriat* or antipsoria*) #3 MeSH descriptor Coal Tar, this term only #4 "coal tar" #5 (alphosyl) #6 "carbo dome" #7 (clinitar or exorex or gelcosal or gelcotar) #8 (pragmatar or psorigel or balneum or polytar) #9 (psoriderm or tarcortin or cocois) #10 "T gel" #11 (capasal or ceanel or clinitar or ionil) #12 (meted or pentrax) #13 MeSH descriptor Anthralin, this term only #14 (Dithranol or dithrocream or micanol or psorin) #15 (salicylic next acid*) #16 (vitamin next d next analogue*) #17 (vitamin next d next derivative*) #18 (calcipotriol or calcipotriene or dovonex or dovobet) #19 (tacalcitol or curatoderm or tazarotene or zorac) #20 MeSH descriptor Calcitriol, this term only #21 (silkis or maxacalcitol) #22 (#3 OR #4 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21) #23 MeSH descriptor Acitretin, this term only #24 MeSH descriptor Cyclosporins explode all trees #25 MeSH descriptor Methotrexate, this term only #26 MeSH descriptor Tacrolimus, this term only #27 (methotrexate or tacrolimus) #28 (pimecrolimus or elidel or protopic) #29 (acitretin or neotigason or cyclosporin or ciclosporin) #30 (#23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29) #31 (topical) #32 (#30 AND #31) #33 (topical next retinoid*) #34 (topical next macrolactam*) #35 (topical next immunosuppressant*) #36 MeSH descriptor Dermatologic Agents, this term only #37 MeSH descriptor Adrenal Cortex Hormones explode all trees #38 (corticosteroid* or (cortico next steroid*)) #39 MeSH descriptor Hydrocortisone explode all trees #40 (hydrocortisone or cobadex or dioderm or efcortelan or hydrocortisyl mildison or alphaderm or calmurid) #41 "hydrocortisone butyrate" #42 "alclometasone dipropionate" #43 (modrasone or locoid or propaderm) #44 MeSH descriptor Beclomethasone, this term only #45 "beclomethasone dipropionate" #46 MeSH descriptor Betamethasone explode all trees #47 "betamethasone esters" #48 (betamethasone or betacap or betnovate or diprosone) #49 (diprosalic or bettamousse) #50 "clobetasol propionate" #51 "clobetasone butyrate" #52 (eumovate or trimovate or dermovate) #53 MeSH descriptor Desoximetasone, this term only #54 (desoxymethasone or desoximetasone or stiedex) #55 MeSH descriptor Diflucortolone, this term only #56 "diflucortolone valerate" #57 MeSH descriptor Fluocinolone Acetonide explode all trees #58 "fluocinolone acetonide" #59 (synalar or fluocinonide or metosyn or nerisone or elocon) #60 MeSH descriptor Fluocortolone explode all trees #61 (fluocortolone or ultralanum or flurandrenolone or haelan) #62 MeSH descriptor Flurandrenolone, this term only #63 (fludroxycortide) #64 (fluticasone next propionate) #65 (cutivate or halcinonide or halciderm) #66 "mometasone furoate" #67 MeSH descriptor Triamcinolone explode all trees #68 "triamcinolone acetonide" #69 (adcortyl or aureocort or nystadermal or triadcortyl) #70 MeSH descriptor Steroids explode all trees #71 (steroid*) #72 (#33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40) #73 (#41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48) #74 (#49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56) #75 (#57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64) #76 (#65 OR #66 OR #67 OR #68 OR #69 OR #70 OR #71) #77 (#22 OR #32 OR #72 OR #73 OR #74 OR #75 OR #76) #78 (( #1 OR #2 ) AND #77), from 2008 to 2011
Appendix 3. MEDLINE (OVID) search strategy (RCTs)
MEDLINE (OvidSP Online http://www.ovid.com/): database updates 2002/07 to 2005/02 week 2 Search updated on 17/11/2008: 1950 to November Week 1 2008 Search updated on 22/02/2011: 1948 to January Week 3 2011 Search updated on 23/08/2012: 1946 to Present
1 randomized controlled trial.pt. 2 randomized controlled trial/ 3 Random Allocation/ 4 Double‐Blind Method/ 5 single‐blind method/ 6 clinical trial.pt. 7 exp clinical trial/ 8 ((clinical$ or intervention$) adj5 (trial$ or study or studies)).tw. 9 ((singl$ or doubl$ or trebl$ or tripl$) adj5 (blind$ or mask$)).tw. 10 Placebos/ 11 (placebo$ or random$).tw. 12 Research Design/ 13 Comparative Study/ 14 exp evaluation studies as topic/ 15 Follow‐Up Studies/ 16 Prospective Studies/ 17 (control$ or prospectiv$ or volunteer$).tw. 18 Animals/ 19 Humans/ 20 or/1‐17 21 18 not (18 and 19) 22 20 not 21 23 exp Psoriasis/ 24 psorias$.tw. 25 psoriat$.tw. 26 or/23‐25 27 coal tar/ 28 coal tar.tw. 29 alphosyl.tw. 30 carbo dome.tw. 31 clinitar.tw. 32 exorex.tw. 33 gelcosal.tw. 34 gelcotar.tw. 35 pragmatar.tw. 36 psorigel.tw. 37 balneum.tw. 38 polytar.tw. 39 psoriderm.tw. 40 tarcortin.tw. 41 cocois.tw. 42 (T adj gel).tw. 43 capasal.tw. 44 ceanel.tw. 45 clinitar.tw. 46 ionil.tw. 47 meted.tw. 48 pentrax.tw. 49 Anthralin/ 50 dithranol.tw. 51 dithrocream.tw. 52 micanol.tw. 53 psorin.tw. 54 salicylic acid$.tw. 55 (vitamin adj d adj2 analogue$).tw. 56 (vitamin adj d adj2 derivative$).tw. 57 calcipotriol.tw. 58 calcipotriene.tw. 59 dovonex.tw. 60 dovobet.tw. 61 tacalcitol.tw. 62 curatoderm.tw. 63 tazarotene.tw. 64 zorac.tw. 65 Calcitriol/ 66 silkis.tw. 67 maxacalcitol.tw. 68 or/27‐67 69 Acitretin/ 70 acitretin.tw. 71 neotigason.tw. 72 exp Cyclosporins/ 73 cyclosporin.tw. 74 ciclosporin.tw. 75 Methotrexate/ 76 methotrexate.tw. 77 Tacrolimus/ 78 tacrolimus.tw. 79 protopic.tw. 80 pimecrolimus.tw. 81 elidel.tw. 82 or/69‐81 83 topical.tw. 84 82 and 83 85 topical retinoid$.tw. 86 topical macrolactam$.tw. 87 topical immunosuppressant$.tw. 88 or/84‐87 89 68 or 88 90 antipsoriat$.tw. 91 antipsorias$.tw. 92 26 or 90 or 91 93 exp Psoriasis/th, pc, dt [Therapy, Prevention & Control, Drug Therapy] 94 Dermatologic Agents/ 95 92 and 22 96 93 and 22 97 94 and 92 and 22 98 or/95‐97 99 exp Adrenal Cortex Hormones/ 100 corticosteroid$.tw. 101 cortico steroid$.tw. 102 exp Hydrocortisone/ 103 hydrocortisone.tw. 104 cobadex.tw. 105 dioderm.tw. 106 efcortelan.tw. 107 hydrocortisyl.tw. 108 mildison.tw. 109 alphaderm.tw. 110 calmurid.tw. 111 hydrocortisone butyrate.tw. 112 locoid.tw. 113 alclometasone dipropionate.tw. 114 modrasone.tw. 115 Beclomethasone/ 116 beclomet$asone dipropionate.tw. 117 propaderm.tw. 118 exp Betamethasone/ 119 betamethasone esters.tw. 120 betamethasone.tw. 121 betacap.tw. 122 betnovate.tw. 123 diprosone.tw. 124 diprosalic.tw. 125 bettamousse.tw. 126 clobetasol propionate.tw. 127 dermovate.tw. 128 clobetasone butyrate.tw. 129 eumovate.tw. 130 trimovate.tw. 131 Desoximetasone/ 132 desoxymethasone.tw. 133 desoximetasone.tw. 134 stiedex.tw. 135 Diflucortolone/ 136 diflucortolone valerate.tw. 137 nerisone.tw. 138 exp Fluocinolone Acetonide/ 139 fluocinolone acetonide.tw. 140 synalar.tw. 141 fluocinonide.tw. 142 metosyn.tw. 143 exp Fluocortolone/ 144 fluocortolone.tw. 145 ultralanum.tw. 146 Flurandrenolone/ 147 flurandrenolone.tw. 148 fludroxycortid$.tw. 149 haelan.tw. 150 fluticasone propionate.tw. 151 cutivate.tw. 152 halcinonide.tw. 153 halciderm.tw. 154 mometasone furoate.tw. 155 elocon.tw. 156 exp Triamcinolone/ 157 triamcinolone acetonide.tw. 158 adcortyl.tw. 159 aureocort.tw. 160 nystadermal.tw. 161 tri‐adcortyl.tw. 162 exp Steroids/ 163 steroid$.tw. 164 or/99‐163 165 22 and 92 and 164 166 22 and 92 and 89 167 98 or 165 or 166 168 (200811$ or 200812$).ed. 169 (2009$ or 2010$ or 2011$).ed. 170 168 or 169 171 167 and 170
Appendix 4. EMBASE (OVID) search strategy (RCTs)
EMBASE (OvidSP Online http://www.ovid.com/): database updates 2002/08 to 2005/08 Search updated on 17/11/2008: 1980 to 2008 Week 46 Search updated on 31/01/2011: 1980 to 2011 Week 04 Search updated on 23/08/2012: 1996 to 2012 Week 33
1 Coal Tar/ 2 dithranol/ or tazarotene/ or 22 Oxacalcitriol/ 3 (coal tar or alphosyl or carbo dome or clinitar or exorex or cocois or T gel or capasal or ceanel or ionil or meted or pentrax).ti,ab. 4 (gelcosal or gelcotar or pragmatar or psoriderm or psorigel or balneum or polytar or tarcortin or dithranol or dithrocream).ti,ab. 5 (anthralin or micanol or psorin or salicylic acid$ or vitamin d analogue$ or vitamin d derivative$).ti,ab. 6 (calcipotriol or calcipotriene or dovonex or dovobet or tacalcitol or curatoderm or tazarotene or zorac or silkis or maxacalcitol).ti,ab. 7 vitamin d derivative/ or calcipotriol/ or calcitriol/ or tacalcitol/ or Salicylic Acid/ 8 or/1‐7 9 Etretin/ or Immunosuppressive Agent/ or Tacrolimus/ 10 Cyclosporin/ or Retinoid/ or Pimecrolimus/ 11 Methotrexate/ 12 ((immunosuppressant$ or acitretin or neotigason or cyclosporin$ or ciclosporin$ or methotrexate or retinoid$ or macrolactam) adj5 topical$).ti,ab. 13 ((tacrolimus or protopic or pimecrolimus or elidel) adj5 topical$).ti,ab. 14 or/9‐11 15 topical.ti,ab. or topical treatment/ or topical drug administration/ 16 (14 and 15) or 12 or 13 17 exp Corticosteroid/ 18 Hydrocortisone/ 19 (corticosteroid$ or cortico steroid$ or hydrocortisone or cobadex or dioderm or efcortelan).ti,ab. 20 (hydrocortisyl or mildison or alphaderm or calmurid).ti,ab. 21 (locoid or modrasone or beclomethasone dipropionate).ti,ab. 22 (alclometasone diproprionate or propaderm or betamethasone or betacap or betnovate or diprosone).ti,ab. 23 (diprosalic or bettamousse or clobetasol propionate or dermovate or clobetasone butyrate).ti,ab. 24 (eumovate or trimovate or desoxymethasone or desoxymetasone or desoximethasone or desoximetasone or stiedex).ti,ab. 25 (diflucortolone valerate or nerisone or fluocinolone acetonide or synalar or fluocinonide or metosyn).ti,ab. 26 (ultralanum or flurandrenolone or haelan or fluticasone propionate or cutivate or halcinonide or halciderm).ti,ab. 27 (mometasone furoate or elocon or adcortyl or aureocort or nystadermal or tri adcortyl or steroid$).ti,ab. 28 beclometasone/ or psoralon/ or psoraderm/ or psoradexan/ or psorin/ 29 Beclometasone Dipropionate/ or Urea/ or hydrocortisone butyrate/ or hydrocortisone plus urea/ 30 alclometasone dipropionate/ or betamethasone dipropionate/ or betamethasone valerate/ or diflucortolone/ 31 clobetasol propionate/ or clobetasone butyrate/ or desoximetasone/ or diflucortolone valerate/ or fluocinonide/ or Fluticasone Propionate/ 32 fluocinolone/ or halcinonide/ or mometasone furoate.mp. or triamcinolone acetonide/ 33 Fluocortolone/ 34 Fludroxycortide/ 35 Triamcinolone/ 36 exp Steroid/ 37 exp Steroid Hormone/ 38 or/17‐37 39 exp Antipsoriasis Agent/ 40 8 or 16 or 38 or 39 41 exp Psoriasis/ 42 (psorias$ or psoriat$ or antipsorias$ or antipsoriat$).ti,ab. 43 or/41‐42 44 40 and 43 45 randomization/ 46 Single Blind Procedure/ 47 Double Blind Procedure/ 48 exp clinical trial/ 49 Placebo/ 50 Methodology/ 51 comparative study/ 52 exp drug comparison/ 53 evaluation/ 54 Follow Up/ 55 Prospective Study/ 56 Crossover Procedure/ 57 (clinical$ adj3 (trial$ or study or studies)).ti,ab. 58 (intervention$ adj3 (trial$ or study or studies)).ti,ab. 59 ((single$ or doubl$ or trebl$ or tripl$) adj3 blind$).ti,ab. 60 ((single$ or doubl$ or trebl$ or tripl$) adj3 mask$).ti,ab. 61 (placebo or placebos or random$ or control$ or prospectiv$ or volunteer$).ti,ab. 62 or/45‐61 63 44 and 62 64 (2008$ or 2009$ or 2010$ or 2011$).em. 65 63 and 64 66 from 65 keep 1‐2041
Appendix 5. Science Citation Index (ISI web of Knowledge interface) and Conference Proceedings Citation Index ‐ Science (ISI web of Knowledge interface) search strategies (RCTs)
Science Citation Index (SCI Web Of Knowledge http://wos.mimas.ac.uk/): publication years 2000 to 2005 Search updated on 17/11/2008: All lines linted as follows: DocType=All document types; Language=All languages; Database=SCI EXPANDED; Timespan=2005‐2008 Search updated on 02/02/2011: All lines linted as follows: Databases=SCI‐EXPANDED Timespan=2008‐2011 Search updated on 23/08/12: (limited to 2011‐01‐01 ‐ 2012‐08‐23)
Conference Proceedings Citation Index ‐ Science (SCI Web Of Knowledge http://wos.mimas.ac.uk/) Search updated on 02/02/2011: All lines linted as follows: Databases=CPCI‐S Timespan=2008‐2011 Search updated on 23/08/12: (limited to 2011‐01‐01 ‐ 2012‐08‐23)
# 1 Topic=((psoria* OR antipsoria*) AND (trial* OR random* OR control OR controls OR double blind OR doubleblind or single blind OR singleblind OR placebo* or evaluation*)) # 2 Topic=(coaltar or coal tar or alphosyl or carbo dome or clinitar or exorex or gelcosal or gelcotar or pragmatar) # 3 Topic=(psorigel or balneum or polytar or psoriderm or tarcortin or cocois or t gel or tgel or capasal or ceanel or clinitar or ionil or meted or pentrax) # 4 Topic=(eumovate or trimovate or desoxime$asone or desoxyme$asone or stiedex or diflucortolone or nerisone or fluocinolone or synalar) # 5 Topic=(anthralin OR dithranol or dithrocream or micanol or psorin or salicylic acid or salicylic acids or vitamin d or calcipotriol or calcipotriene or dovonex or dovobet or tacalcitol or curatoderm or tazarotene or zorac) # 6 Topic=(fluocinonide or metosyn or fluocortolone or ultralanum or flurandrenolone or fludroxycortide or haelan or fluticasone propionate) # 7 Topic=(calcitriol or silkis or maxacalcitol) # 8 Topic=(cutivate or halcinonide or halciderm or mometasone furoate or elocon or triamcinolone or adcortyl or aureocort or nystadermal or triadcortyl or steroid or steroids or steroidal) # 9 Topic=((acitretin or neotigason or cyclosporin* or ciclosporin* or methotrexate or tacrolimus or protopic or pimecrolimus or elidel or retinoid* or macrolactam* or immunosuppressant*) same topical) # 10 Topic=(adrenal cortex hormone* or corticosteroid* or cortico steroid* or hydrocortisone or cobadex or dioderm or efcortelan or hydrocortisyl or mildison) # 11 Topic=(alphaderm or calmurid or locoid or alclometasone dipropionate or modrasone or beclomet$asone or propaderm) # 12 Topic=(betamethasone or betacap or betnovate or diprosone or diprosalic or bettamousse or clobetasol propionate or dermovate or clobetasone butyrate) # 13 #12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 # 14 #13 AND #1
Appendix 6. BIOSIS (EDINAinterface) search strategy (RCTs)
BIOSIS (EDINAinterface): publication years 2001 to 2005
((((((((((((((al: betamethasone or betacap or betnovate or diprosone or diprosalic or bettamousse or "clobetasol propionate" or dermovate or "clobetasone butyrate") and (sy: 2001‐2005)) or ((al: alphaderm or calmurid or locoid or "alclometasone dipropionate" or modrasone or beclomethasone beclometasone or propaderm) and (sy: 2001‐2005))) or ((al: "adrenal cortex hormone*" or corticosteroid* or "cortico steroid*" or hydrocortisone or cobadex or dioderm or efcortelan or hydrocortisyl or mildison) and (sy: 2001‐2005))) or ((al: (acitretin or neotigason or cyclosporin* or ciclosporin* or methotrexate or tacrolimus or protopic or pimecrolimus or elidel or retinoid* or macrolactam* or immunosuppressant*)) and (sy: 2001‐2005))) or ((al: cutivate or halcinonide or halciderm or "mometasone furoate" or elocon or triamcinolone or adcortyl or aureocort or nystadermal or triadcortyl or steroid or steroids or steroidal) and (sy: 2001‐2005))) or ((al: (calcitriol or silkis or maxacalcitol)) and (sy: 2001‐2005))) or ((al: fluocinonide or metosyn or fluocortolone or ultralanum or flurandrenolone or fludroxycortide or haelan or "fluticasone propionate") and (sy: 2001‐2005))) or ((al: anthralin OR dithranol or dithrocream or micanol or psorin or "salicylic acid" or "salicylic acids" or "vitamin d" or calcipotriol or calcipotriene or dovonex or dovobet or tacalcitol or curatoderm or tazarotene or zorac) and (sy: 2001‐2005))) or ((al: (eumovate or trimovate or desoxime$asone or desoxyme$asone or stiedex or diflucortolone or nerisone or fluocinolone or synalar)) and (sy: 2001‐2005))) or (al: (psorigel or balneum or polytar or psoriderm or tarcortin or cocois or t gel or tgel or capasal or ceanel or clinitar or ionil or meted or pentrax))) or (al: (coaltar or coal tar or alphosyl or carbo dome or clinitar or exorex or gelcosal or gelcotar or pragmatar)))) and ((((al: trial* or random* or "double blind" or doubleblind or "single blind" or singleblind or evaluation* or placebo* or control or controls) and (sy: 2001‐2005)) and ((al: (psoria* or antipsoria*)) and (sy: 2001‐2005)))))
Appendix 7. Dissertation Abstracts (Dialog Classic interface) and Inside Conferences (Dialog Classic interface) search strategies (RCTs)
Dissertation Abstracts (Dialog Classic interface): all publication years Inside Conferences (Dialog Classic interface): all publication years Biosis (Dialog Classic interface): all publication years
Search updated 17/11/2008: 1993‐2008/Nov W2 Search updated 01/02/2011: 1993‐2011/Jan W4 Search updated on 24/08/2012: 1993‐2012/Aug W3
1 s (coal()tar or coaltar or alphosyl or carbo()dome or clinitar or exorex or cocois)/ti,ab,de 2 s (t()gel or tgel or capasal or ceanel or clinitar or ionil or meted or pentrax)/ti,ab,de 3 s (dithranol or gelcosal or gelcotar or pragmatar or psoriderm or psorigel or balneum or 4. polytar or tarcortin or dithrocream)/ti,ab,de 4 s (micanol or psorin or salicylic()acid? ? or vitamin()d()analogue? or vitamin()d()derivative?)/ti,ab,de 5 s (calcipotriol or calcipotriene or dovonex or dovobet or tacalcitol or curatoderm or tazarotene or zorac)/ti,ab,de 6 s (calcitriol or silkis or maxacalcitol or adrenal()cortex()hormone? ?)/ti,ab,de 7 s ((cyclosporin or ciclosporin or methotrexate or acitretin or neotigason)(4w)topical?)/ti,ab,de 8 s ((retinoid? ? or immunosuppressant? ? or tacrolimus or protopic or pimecrolimus or macorlactam? ?)(4w)topical?)/ti,ab,de 9 s (corticosteroid? ? or cortico()steroid? ? or hydrocortisone or cobadex or dioderm or efcortelan)/ti,ab,de 10 s (hydrocortisyl or mildison or alphaderm or calmurid)/ti,ab,de 11 s (locoid or alclometasone()dipropionate or modrasone or beclomethasone()dipropionate)/ti,ab,de 12 s (propaderm or betamethasone or betacap or betnovate or diprosone)/ti,ab,de 13 s (diprosalic or bettamousse or clobetasol()propionate or dermovate or clobetasone()butyrate)/ti,ab,de 14 s (eumovate or trimovate or desoxymethasone or desoxymetasone or desoximetasone or desoximethasone or stiedex)/ti,ab,de 15 s (diflucortolone()valerate or nerisone or fluocinolone()acetonide or synalar or fluocinonide or metosyn)/ti,ab,de 16 s (fluocortolone or ultralanum or haelan or fluticasone()propionate or cutivate or halcinonide or halciderm or flurandrenolone or triamcinolone)/ti,ab,de 17 s (mometasone()furoate or elocon or adcortyl or aureocort or nystadermal or triadcortyl or steroid? ? or steroidal)/ti,ab,de 18 s (beclometasone()dipropionate or fludroxycortide or triamcinolone)/ti,ab,de 19 s s1:s18 20 s (psoria? or antipsoria?)/ti,ab,de 21 s (random? or single()blind or singleblind or double()blind or doubleblind)/ti,ab,de 22 s (placebo or comparative()study or evaluation or prospective()study or crossover or trial or trials or triallist? ?)/ti,ab,de 23 s (control or controls? or prospectiv?)/ti,ab,de 24 s s21:s23 25 s s19 and s20 and s24 26 s UD=200812:201102 27 s s25 and s26 28 s rd s27
Appendix 8. SIGLE (WebSPIRS interface) search strategy (RCTs)
SIGLE (WebSPIRS interface): publication years 2001 to 2005 (database issue 2004/12) SIGLE has not been updated since 2005, so searches were not rerun.
#1 psoriat* or psorias*(83 records) #2 (2002 in PY) or (2003 in PY) or (2004 in PY) or (2005 in PY)(49881 records) #3 ((2002 in PY) or (2003 in PY) or (2004 in PY) or (2005 in PY)) and (psoriat* or psorias*)(5 records)
Appendix 9. MEDLINE (OVID) search strategy (adverse events)
MEDLINE (OvidSP Online http://www.ovid.com/): database updates 1990 to 2005/02 week 2 Search updated on 17/11/08: 1950 to November Week 1 2008 Search updated on 02/02/11: 1948 to January Week 3 2011 Search updated on 23/08/12: 1946 to present
1 (coal adj tar).tw. 2 alphosyl.tw. 3 (carbo adj dome).tw. 4 clinitar.tw. 5 exorex.tw. 6 gelcosal.tw. 7 gelcotar.tw. 8 pragmatar.tw. 9 psorigel.tw. 10 balneum.tw. 11 polytar.tw. 12 psoriderm.tw. 13 tarcortin.tw. 14 cocois.tw. 15 (T adj gel).tw. 16 capasal.tw. 17 ceanel.tw. 18 ionil.tw. 19 meted.tw. 20 pentrax.tw. 21 dithranol.tw. 22 dithrocream.tw. 23 micanol.tw. 24 psorin.tw. 25 (salicylic adj acid$).tw. 26 (vitamin adj d adj2 analogue$).tw. 27 (vitamin adj d adj2 derivative$).tw. 28 calcipotriol.tw. 29 calcipotriene.tw. 30 dovonex.tw. 31 dovobet.tw. 32 tacalcitol.tw. 33 curatoderm.tw. 34 tazarotene.tw. 35 zorac.tw. 36 silkis.tw. 37 maxacalcitol.tw. 38 antipsoriat$.tw. 39 antipsorias$.tw. 40 corticosteroid$.tw. 41 (cortico adj steroid$).tw. 42 hydrocortisone.tw. 43 cobadex.tw. 44 dioderm.tw. 45 efcortelan.tw. 46 hydrocortisyl.tw. 47 mildison.tw. 48 alphaderm.tw. 49 calmurid.tw. 50 (hydrocortisone adj butyrate).tw. 51 locoid.tw. 52 (alclometasone adj dipropionate).tw. 53 modrasone.tw. 54 (beclomet$asone adj dipropionate).tw. 55 propaderm.tw. 56 (betamethasone adj esters).tw. 57 betamethasone.tw. 58 betacap.tw. 59 betnovate.tw. 60 diprosone.tw. 61 diprosalic.tw. 62 bettamousse.tw. 63 (clobetasol adj propionate).tw. 64 dermovate.tw. 65 (clobetasone adj butyrate).tw. 66 eumovate.tw. 67 trimovate.tw. 68 desoxymethasone.tw. 69 desoximetasone.tw. 70 stiedex.tw. 71 (diflucortolone adj valerate).tw. 72 nerisone.tw. 73 (fluocinolone adj acetonide).tw. 74 synalar.tw. 75 fluocinonide.tw. 76 metosyn.tw. 77 fluocortolone.tw. 78 ultralanum.tw. 79 flurandrenolone.tw. 80 haelan.tw. 81 (fluticasone adj propionate).tw. 82 cutivate.tw. 83 halcinonide.tw. 84 halciderm.tw. 85 (mometasone adj furoate).tw. 86 elocon.tw. 87 (triamcinolone adj acetonide).tw. 88 adcortyl.tw. 89 aureocort.tw. 90 nystadermal.tw. 91 tri‐adcortyl.tw. 92 steroid$.tw. 93 Coal Tar/ 94 Anthralin/ 95 Calcitriol/ 96 exp Cyclosporins/ 97 Tacrolimus/ 98 Dermatologic Agents/ 99 exp Adrenal Cortex Hormones/ 100 exp Hydrocortisone/ 101 Beclomethasone/ 102 exp Betamethasone/ 103 Desoximetasone/ 104 Diflucortolone/ 105 exp Fluocinolone Acetonide/ 106 Fluocortolone/ 107 Flurandrenolone/ 108 Flurandrenolone/ 109 exp Triamcinolone/ 110 exp Steroids/ 111 or/1‐110 112 Acitretin/ 113 Immunosuppressive Agents/ 114 Cyclosporine/ 115 Retinoids/ 116 Methotrexate/ 117 or/112‐116 118 topical.ti,ab. or topical treatment/ or topical drug administration/ 119 117 and 118 120 ((tacrolimus or protopic or pimecrolimus or elidel or immunosuppressant$ or acitretin or neotigason or cyclosporin$ or ciclosporin$ or methotrexate or retinoid$ or macrolactam) adj5 topical$).tw. 121 119 or 120 or 111 122 (safe or safety).tw. 123 side effect$.tw. 124 treatment emergent.tw. 125 undesirable effect$.tw. 126 tolerability.tw. 127 toxicity.tw. 128 adrs.tw. 129 (adverse adj3 (effect or effects or reaction or reactions or event or events or outcome or outcomes)).tw. 130 Adverse Drug Reaction Reporting Systems/ 131 drug hypersensitivity/ 132 hypersensit$.tw. 133 harm$.tw. 134 exp Substance Withdrawal Syndrome/ci [Chemically Induced] 135 rebound.tw. 136 Hypercalcemia/ci [Chemically Induced] 137 exp Urinary Calculi/ci [Chemically Induced] 138 Tachyphylaxis/ci, de [Chemically Induced, Drug Effects] 139 exp Substance Withdrawal Syndrome/ci [Chemically Induced] 140 exp Atrophy/ci [Chemically Induced] 141 exp Telangiectasis/ci [Chemically Induced] 142 cutaneous atrophy.tw. 143 striae.tw. 144 skin atrophy.tw. 145 exp Abnormalities, Drug‐Induced/ 146 exp Drug Toxicity/ 147 or/122‐146 148 Coal Tar/ae [Adverse Effects] 149 Anthralin/ae [Adverse Effects] 150 Calcitriol/ae [Adverse Effects] 151 Acitretin/ae [Adverse Effects] 152 exp Cyclosporins/de, ae [Drug Effects, Adverse Effects] 153 Methotrexate/ae [Adverse Effects] 154 Tacrolimus/ae [Adverse Effects] 155 Dermatologic Agents/ae [Adverse Effects] 156 exp Adrenal Cortex Hormones/ae, de [Adverse Effects, Drug Effects] 157 exp Hydrocortisone/ae [Adverse Effects] 158 Beclomethasone/ae [Adverse Effects] 159 exp Betamethasone/ae [Adverse Effects] 160 Desoximetasone/ae [Adverse Effects] 161 Diflucortolone/ae [Adverse Effects] 162 exp Fluocinolone Acetonide/ae [Adverse Effects] 163 Fluocortolone/ae [Adverse Effects] 164 Flurandrenolone/ae [Adverse Effects] 165 exp Triamcinolone/ae [Adverse Effects] 166 exp Steroids/ae [Adverse Effects] 167 or/148‐166 168 147 or 167 169 (psorias$ or psoriat$).tw. 170 exp psoriasis/ 171 or/169‐170 172 121 and 168 and 171 173 exp animals/ not (exp animals/ and humans/) 174 172 not 173 175 (comment or editorial).pt. 176 174 not 175 177 (2008$ or 2009$ or 2010$ or 2011$).ed. 178 176 and 177
Appendix 10. EMBASE (OVID) search strategy (adverse events)
EMBASE (OvidSP Online http://www.ovid.com/): 1990 to 2005/08
Search updated on 11/12/08: 1980 to 2008 Week 49 Search updated on 02/02/11: 1980 to 2011 week 4 Search updated on 23/08/12: 1996 to 2012 Week 34
Note: A pragmatic approach was taken to reduce irrelevant records and to negate the over indexing of records in EMBASE; EMTREE terms were focused in this strategy
1 (coal adj tar).ti,ab. 2 alphosyl.ti,ab. 3 (carbo adj dome).ti,ab. 4 clinitar.ti,ab. 5 exorex.ti,ab. 6 gelcosal.ti,ab. 7 gelcotar.ti,ab. 8 pragmatar.ti,ab. 9 psorigel.ti,ab. 10 balneum.ti,ab. 11 polytar.ti,ab. 12 psoriderm.ti,ab. 13 tarcortin.ti,ab. 14 cocois.ti,ab. 15 (T adj gel).ti,ab. 16 capasal.ti,ab. 17 ceanel.ti,ab. 18 ionil.ti,ab. 19 meted.ti,ab. 20 pentrax.ti,ab. 21 dithranol.ti,ab. 22 dithrocream.ti,ab. 23 micanol.ti,ab. 24 psorin.ti,ab. 25 (salicylic adj acid$).ti,ab. 26 (vitamin adj d adj2 analogue$).ti,ab. 27 (vitamin adj d adj2 derivative$).ti,ab. 28 calcipotriol.ti,ab. 29 calcipotriene.ti,ab. 30 dovonex.ti,ab. 31 dovobet.ti,ab. 32 tacalcitol.ti,ab. 33 curatoderm.ti,ab. 34 tazarotene.ti,ab. 35 zorac.ti,ab. 36 silkis.ti,ab. 37 maxacalcitol.ti,ab. 38 antipsoriat$.ti,ab. 39 antipsorias$.ti,ab. 40 corticosteroid$.ti,ab. 41 (cortico adj steroid$).ti,ab. 42 hydrocortisone.ti,ab. 43 cobadex.ti,ab. 44 dioderm.ti,ab. 45 efcortelan.ti,ab. 46 hydrocortisyl.ti,ab. 47 mildison.ti,ab. 48 alphaderm.ti,ab. 49 calmurid.ti,ab. 50 (hydrocortisone adj butyrate).ti,ab. 51 locoid.ti,ab. 52 (alclometasone adj dipropionate).ti,ab. 53 modrasone.ti,ab. 54 (beclomet$asone adj dipropionate).ti,ab. 55 propaderm.ti,ab. 56 (betamethasone adj esters).ti,ab. 57 betamethasone.ti,ab. 58 betacap.ti,ab. 59 betnovate.ti,ab. 60 diprosone.ti,ab. 61 diprosalic.ti,ab. 62 bettamousse.ti,ab. 63 (clobetasol adj propionate).ti,ab. 64 dermovate.ti,ab. 65 (clobetasone adj butyrate).ti,ab. 66 eumovate.ti,ab. 67 trimovate.ti,ab. 68 desoxymethasone.ti,ab. 69 desoximetasone.ti,ab. 70 stiedex.ti,ab. 71 (diflucortolone adj valerate).ti,ab. 72 nerisone.ti,ab. 73 (fluocinolone adj acetonide).ti,ab. 74 synalar.ti,ab. 75 fluocinonide.ti,ab. 76 metosyn.ti,ab. 77 fluocortolone.ti,ab. 78 ultralanum.ti,ab. 79 flurandrenolone.ti,ab. 80 haelan.ti,ab. 81 (fluticasone adj propionate).ti,ab. 82 cutivate.ti,ab. 83 halcinonide.ti,ab. 84 halciderm.ti,ab. 85 (mometasone adj furoate).ti,ab. 86 elocon.ti,ab. 87 (triamcinolone adj acetonide).ti,ab. 88 adcortyl.ti,ab. 89 aureocort.ti,ab. 90 nystadermal.ti,ab. 91 tri‐adcortyl.ti,ab. 92 steroid$.ti,ab. 93 *Coal Tar/ 94 *alphosyl/ 95 *carbo dome/ 96 *Salicylic Acid/ 97 *capasal/ 98 *meted/ 99 *Dithranol/ 100 *Psorin/ 101 *Vitamin d Derivative/ 102 *Calcipotriol/ 103 *Betamethasone Dipropionate Plus Calcipotriol/ 104 *Tacalcitol/ 105 *Tazarotene/ 106 *Calcitriol/ 107 *22 Oxacalcitriol/ 108 *Corticosteroid/ 109 *Hydrocortisone/ 110 *Urea/ 111 *Hydrocortisone Butyrate/ 112 *Alclometasone Dipropionate/ 113 *Beclometasone Dipropionate/ 114 *Betamethasone/ 115 *Betamethasone Valerate/ 116 *Betamethasone Dipropionate/ 117 *Clobetasol Propionate/ 118 *Clobetasone Butyrate/ 119 *trimovate/ 120 *Desoximetasone/ 121 *Diflucortolone Valerate/ 122 *Fluocinolone Acetonide/ 123 *Fluocinonide/ 124 *Fluocortolone/ 125 *Fludroxycortide/ 126 *Fluticasone Propionate/ 127 *Halcinonide/ 128 *Mometasone Furoate/ 129 *Triamcinolone Acetonide/ 130 *Triamcinolone/ 131 *Mycolog/ 132 *exp Steroid/ 133 *Cyclosporin Derivative/ or *Cyclosporin/ 134 *Tacrolimus/ 135 *Dermatological Agent/ 136 or/1‐135 137 *Tacrolimus/ 138 *Pimecrolimus/ 139 *Immunosuppressive Agent/ 140 *Etretin/ 141 *Cyclosporin/ 142 *Cyclosporin A/ 143 *Methotrexate/ 144 *Retinoid/ 145 or/137‐144 146 topical.ti,ab. or topical treatment/ or topical drug administration/ 147 145 and 146 148 ((tacrolimus or protopic or pimecrolimus or elidel or immunosuppressant$ or acitretin or neotigason or cyclosporin$ or ciclosporin$ or methotrexate or retinoid$ or macrolactam) adj5 topical$).ti,ab. 149 136 or 147 or 148 150 (safe or safety).ti,ab. 151 side effect$.ti,ab. 152 treatment emergent.ti,ab. 153 undesirable effect$.ti,ab. 154 tolerability.ti,ab. 155 toxicity.ti,ab. 156 adrs.ti,ab. 157 (adverse adj3 (effect or effects or reaction or reactions or event or events or outcome or outcomes)).ti,ab. 158 *Safety/ or *Drug Safety/ 159 *Side Effect/ 160 *Adverse Drug Reaction/ 161 *Drug Tolerability/ 162 *Toxicity/ or *Drug Toxicity/ 163 *Drug Surveillance Program/ 164 *Adverse Outcome/ 165 hypersensit$.ti,ab. 166 harm$.ti,ab. 167 rebound.ti,ab. 168 *Drug Hypersensitivity/ 169 *Rebound/ 170 *Withdrawal Syndrome/ 171 *Hypercalcemia/ 172 *Urolithiasis/ 173 *Tachyphylaxis/ 174 *Drug Withdrawal/ 175 *Atrophy/ 176 *Telangiectasia/ 177 cutaneous atrophy.ti,ab. 178 striae.ti,ab. 179 skin atrophy.ti,ab. 180 *Skin Atrophy/ 181 *Stria/ 182 or/150‐181 183 *Coal Tar/ae, to [Adverse Drug Reaction, Drug Toxicity] 184 *alphosyl/ae [Adverse Drug Reaction] 185 *Salicylic Acid/ae, to [Adverse Drug Reaction, Drug Toxicity] 186 *Dithranol/ae, to [Adverse Drug Reaction, Drug Toxicity] 187 *Psorin/ae [Adverse Drug Reaction] 188 *Vitamin d Derivative/ae, to [Adverse Drug Reaction, Drug Toxicity] 189 *Calcipotriol/ae, to [Adverse Drug Reaction, Drug Toxicity] 190 *Betamethasone Dipropionate Plus Calcipotriol/to, ae [Drug Toxicity, Adverse Drug Reaction] 191 *Tacalcitol/ae, to [Adverse Drug Reaction, Drug Toxicity] 192 *Tazarotene/ae, to [Adverse Drug Reaction, Drug Toxicity] 193 *Calcitriol/ae, to [Adverse Drug Reaction, Drug Toxicity] 194 *22 Oxacalcitriol/ae, to [Adverse Drug Reaction, Drug Toxicity] 195 *Corticosteroid/ae, to [Adverse Drug Reaction, Drug Toxicity] 196 *Hydrocortisone/ae, to [Adverse Drug Reaction, Drug Toxicity] 197 *Urea/ae, to [Adverse Drug Reaction, Drug Toxicity] 198 *Hydrocortisone Butyrate/ae, to [Adverse Drug Reaction, Drug Toxicity] 199 *Alclometasone Dipropionate/ae, to [Adverse Drug Reaction, Drug Toxicity] 200 *Beclometasone Dipropionate/ae, to [Adverse Drug Reaction, Drug Toxicity] 201 *Betamethasone/ae, to [Adverse Drug Reaction, Drug Toxicity] 202 *Betamethasone Valerate/ae, to [Adverse Drug Reaction, Drug Toxicity] 203 *Betamethasone Dipropionate/ae, to [Adverse Drug Reaction, Drug Toxicity] 204 *Clobetasol Propionate/ae, to [Adverse Drug Reaction, Drug Toxicity] 205 *Clobetasone Butyrate/ae [Adverse Drug Reaction] 206 *trimovate/ae [Adverse Drug Reaction] 207 *Desoximetasone/ae, to [Adverse Drug Reaction, Drug Toxicity] 208 *Diflucortolone Valerate/to, ae [Drug Toxicity, Adverse Drug Reaction] 209 *Fluocinolone Acetonide/ae, to [Adverse Drug Reaction, Drug Toxicity] 210 *Fluocinonide/ae, to [Adverse Drug Reaction, Drug Toxicity] 211 *Fluocortolone/ae, to [Adverse Drug Reaction, Drug Toxicity] 212 *Fludroxycortide/ae [Adverse Drug Reaction] 213 *Fluticasone Propionate/ae, to [Adverse Drug Reaction, Drug Toxicity] 214 *Halcinonide/ae [Adverse Drug Reaction] 215 *Mometasone Furoate/ae, to [Adverse Drug Reaction, Drug Toxicity] 216 *Triamcinolone Acetonide/ae, to [Adverse Drug Reaction, Drug Toxicity] 217 *Triamcinolone/ae, to [Adverse Drug Reaction, Drug Toxicity] 218 *Mycolog/ae [Adverse Drug Reaction] 219 *exp Steroid/ae, to [Adverse Drug Reaction, Drug Toxicity] 220 *Cyclosporin Derivative/ae, to [Adverse Drug Reaction, Drug Toxicity] 221 *Cyclosporin/ae, to [Adverse Drug Reaction, Drug Toxicity] 222 *Tacrolimus/ae, to [Adverse Drug Reaction, Drug Toxicity] 223 *Dermatological Agent/ae, to [Adverse Drug Reaction, Drug Toxicity] 224 *Pimecrolimus/ae, to [Adverse Drug Reaction, Drug Toxicity] 225 *Immunosuppressive Agent/ae, to [Adverse Drug Reaction, Drug Toxicity] 226 *Etretin/ae, to [Adverse Drug Reaction, Drug Toxicity] 227 *Cyclosporin A/ae, to [Adverse Drug Reaction, Drug Toxicity] 228 *Methotrexate/ae, to [Adverse Drug Reaction, Drug Toxicity] 229 *Retinoid/ae, to [Adverse Drug Reaction, Drug Toxicity] 230 or/183‐229 231 182 or 230 232 (psorias$ or psoriat$).ti,ab. 233 *exp Psoriasis/ 234 232 or 233 235 149 and 231 and 234 236 animal/ not (animal/ and human/) 237 235 not 236 238 ("200800" or "200900").em. 239 (2010$ or 2011$).em. 240 238 or 239 241 237 and 240
Appendix 11. EMBASE (OVID) search strategy (compliance)
EMBASE (OvidSP Online http://www.ovid.com/): 1980 to 2007 Week 49 Not updated in 2011
1 compliance$.ti,ab. (46694) 2 complied.ti,ab. (1455) 3 compliance/ (1674) 4 comply.ti,ab. (3613) 5 (medicat$ adj4 adher$).ti,ab. (1732) 6 (drug$ adj4 adher$).ti,ab. (777) 7 (medicine$ adj4 adher$).ti,ab. (62) 8 (treatment adj4 adher$).ti,ab. (3072) 9 concordance$.ti,ab. (12825) 10 or/1‐9 (68155) 11 psor$.ti,ab. (20549) 12 10 and 11 (171) 13 from 12 keep 1‐171 (171)
Appendix 12. MEDLINE (OVID) search strategy (compliance)
MEDLINE(OvidSP Online http://www.ovid.com/): 1950 to November Week 2 2007 Not updated in 2011
1. compliance$.ti,ab. (52891) 2. complied.ti,ab. (1748) 3. compliance/ (2982) 4. comply.ti,ab. (4307) 5. (medicat$ adj4 adher$).ti,ab. (2047) 6. (drug$ adj4 adher$).ti,ab. (878) 7. (medicine$ adj4 adher$).ti,ab. (76) 8. (treatment adj4 adher$).ti,ab. (3442) 9. concordance$.ti,ab. (14973) 10. or/1‐9 (78297) 11. psor$.ti,ab. (25210) 12. 10 and 11 (162)
Data and analyses
Comparison 1. Vitamin D analogues versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 IAGI | 20 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Calcipotriol | 10 | 2287 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐1.17, ‐0.68] |
| 1.2 Calcipotriol plus occlusion | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Calcitriol | 6 | 1120 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐1.71, ‐0.36] |
| 1.4 Tacalcitol | 2 | 433 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.41, ‐0.26] |
| 1.5 Maxacalcitol | 1 | 103 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.43 [‐1.91, ‐0.96] |
| 1.6 Paricalcitol OD | 1 | 22 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.66 [‐2.66, ‐0.67] |
| 1.7 Becocalcidiol OD | 1 | 121 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.58, 0.14] |
| 1.8 Becocalcidiol twice daily | 1 | 119 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.04, ‐0.30] |
| 2 TSS | 19 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Calcipotriol | 10 | 1208 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.15 [‐1.41, ‐0.89] |
| 2.2 Calcipotriol plus occlusion | 1 | 187 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.44, 0.14] |
| 2.3 Calcitriol | 4 | 1027 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐2.38, ‐0.07] |
| 2.4 Tacalcitol | 3 | 496 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐0.95, ‐0.36] |
| 2.5 Maxacalcitol | 1 | 103 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.61 [‐2.10, ‐1.12] |
| 2.6 Paricalcitol OD | 1 | 22 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.15 [‐3.24, ‐1.06] |
| 2.7 Becocalcidiol OD | 1 | 121 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.37, 0.34] |
| 2.8 Becocalcidiol twice daily | 1 | 119 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.83, ‐0.10] |
| 3 PASI | 9 | 2422 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐0.71, ‐0.45] |
| 3.1 Calcipotriol | 8 | 2195 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐0.75, ‐0.55] |
| 3.2 Calcipotriol plus occlusion | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Calcitriol | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Tacalcitol | 1 | 227 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.56, 0.03] |
| 3.5 Maxacalcitol | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.6 Paricalcitol OD | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.7 Becocalcidiol OD | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.8 Becocalcidiol twice daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 PAGI | 5 | 1467 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.72, ‐0.36] |
| 4.1 Calcipotriol | 2 | 439 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.97, ‐0.30] |
| 4.2 Calcipotriol plus occlusion | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Calcitriol | 2 | 801 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐0.76, ‐0.41] |
| 4.4 Tacalcitol | 1 | 227 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.53, 0.05] |
| 4.5 Maxacalcitol | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 Paricalcitol OD | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.7 Becocalcidiol OD | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.8 Becocalcidiol twice daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) | 30 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Calcipotriol | 17 | 3269 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐1.15, ‐0.77] |
| 5.2 Calcipotriol plus occlusion | 1 | 187 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.44, 0.14] |
| 5.3 Calcitriol | 7 | 1140 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐1.54, ‐0.29] |
| 5.4 Tacalcitol | 4 | 723 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.73 [‐1.09, ‐0.37] |
| 5.5 Maxacalcitol | 1 | 103 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.43 [‐1.91, ‐0.96] |
| 5.6 Paricalcitol OD | 1 | 22 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.66 [‐2.66, ‐0.67] |
| 5.7 Becocalcidiol OD | 1 | 121 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.58, 0.14] |
| 5.8 Becocalcidiol twice daily | 1 | 119 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.04, ‐0.30] |
| 6 Total withdrawals | 25 | 4715 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.05, 0.00] |
| 6.1 Calcipotriol | 14 | 3132 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.06, 0.00] |
| 6.2 Calcipotriol plus occlusion | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Calcitriol | 5 | 339 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.02, 0.02] |
| 6.4 Tacalcitol | 4 | 857 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.14, 0.05] |
| 6.5 Maxacalcitol | 1 | 120 | Risk Difference (M‐H, Random, 95% CI) | 0.11 [‐0.01, 0.23] |
| 6.6 Paricalcitol OD | 1 | 22 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.16, 0.16] |
| 6.7 Becocalcidiol OD | 1 | 124 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.12, 0.11] |
| 6.8 Becocalcidiol twice daily | 1 | 121 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.13, 0.10] |
| 7 Withdrawals due to adverse events | 23 | 4463 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 7.1 Calcipotriol | 12 | 2880 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.04, 0.00] |
| 7.2 Calcipotriol plus occlusion | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Calcitriol | 5 | 339 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.02, 0.02] |
| 7.4 Tacalcitol | 4 | 857 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.02] |
| 7.5 Maxacalcitol | 1 | 120 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.08, 0.06] |
| 7.6 Paricalcitol OD | 1 | 22 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.16, 0.16] |
| 7.7 Becocalcidiol OD | 1 | 124 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.02, 0.08] |
| 7.8 Becocalcidiol twice daily | 1 | 121 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [‐0.01, 0.11] |
| 8 Withdrawals due to treatment failure | 14 | 2752 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.05, 0.00] |
| 8.1 Calcipotriol | 7 | 1770 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.08, 0.01] |
| 8.2 Calcipotriol plus occlusion | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Calcitriol | 4 | 281 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.12, 0.05] |
| 8.4 Tacalcitol | 1 | 314 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 8.5 Maxacalcitol | 1 | 120 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.11, 0.04] |
| 8.6 Paricalcitol OD | 1 | 22 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.16, 0.16] |
| 8.7 Becocalcidiol OD | 1 | 124 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.07, 0.04] |
| 8.8 Becocalcidiol twice daily | 1 | 121 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.09, 0.02] |
| 9 Adverse events (local) | 19 | 4402 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.02] |
| 9.1 Calcipotriol | 11 | 2652 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.03, 0.05] |
| 9.2 Calcipotriol plus occlusion | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Calcitriol | 4 | 917 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.03, 0.02] |
| 9.4 Tacalcitol | 2 | 566 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.03, 0.03] |
| 9.5 Maxacalcitol | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.6 Paricalcitol OD | 1 | 22 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.16, 0.16] |
| 9.7 Becocalcidiol OD | 1 | 124 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.06, 0.08] |
| 9.8 Becocalcidiol twice daily | 1 | 121 | Risk Difference (M‐H, Random, 95% CI) | 0.10 [0.00, 0.19] |
| 10 Adverse events (systemic) | 14 | 2463 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 10.1 Calcipotriol | 8 | 1182 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 10.2 Calcipotriol plus occlusion | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Calcitriol | 3 | 647 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 10.4 Tacalcitol | 1 | 244 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 10.5 Maxacalcitol | 1 | 120 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
| 10.6 Paricalcitol OD | 1 | 22 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.16, 0.16] |
| 10.7 Becocalcidiol OD | 1 | 124 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 10.8 Becocalcidiol twice daily | 1 | 124 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
Comparison 2. Corticosteroid (potent) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 IAGI | 11 | 1904 | Std. Mean Difference (IV, Random, 95% CI) | 1.00 [‐1.18, ‐0.82] |
| 1.1 Betamethasone dipropionate OD | 2 | 739 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐0.98, ‐0.64] |
| 1.2 Betamethasone dipropionate twice daily | 4 | 537 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.35 [‐1.56, ‐1.15] |
| 1.3 Betamethasone dipropionate, maintenance | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Betamethasone valerate | 1 | 74 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.41 [‐1.92, ‐0.90] |
| 1.5 Budesonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.6 Desonide | 1 | 76 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.34, ‐0.28] |
| 1.7 Diflorasone diacetate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.8 Fluticasone propionate | 2 | 383 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐1.14, ‐0.72] |
| 1.9 Hydrocortisone buteprate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.10 Mometasone furoate | 1 | 95 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.17, ‐0.34] |
| 2 TSS | 7 | 611 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐1.01, ‐0.52] |
| 2.1 Betamethasone dipropionate OD | 1 | 93 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐1.16, ‐0.32] |
| 2.2 Betamethasone dipropionate twice daily | 1 | 33 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐1.48, ‐0.06] |
| 2.3 Betamethasone dipropionate, maintenance | 1 | 38 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐1.62, ‐0.27] |
| 2.4 Betamethasone valerate | 1 | 22 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐2.00, ‐0.18] |
| 2.5 Budesonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 Desonide | 1 | 76 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.16 [‐1.70, ‐0.61] |
| 2.7 Diflorasone diacetate | 1 | 93 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.73, 0.09] |
| 2.8 Fluticasone propionate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.9 Hydrocortisone buteprate | 1 | 161 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.77, ‐0.15] |
| 2.10 Mometasone furoate | 1 | 95 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.12 [‐1.55, ‐0.68] |
| 3 PASI | 3 | 1158 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐1.31, ‐0.62] |
| 3.1 Betamethasone dipropionate OD | 2 | 739 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.44, ‐0.14] |
| 3.2 Betamethasone dipropionate twice daily | 1 | 419 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.21 [‐1.44, ‐0.97] |
| 3.3 Betamethasone dipropionate, maintenance | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Betamethasone valerate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.5 Budesonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.6 Desonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.7 Diflorasone diacetate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.8 Fluticasone propionate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.9 Hydrocortisone buteprate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.10 Mometasone furoate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 PAGI | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Betamethasone dipropionate OD | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Betamethasone dipropionate twice daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Betamethasone dipropionate, maintenance | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Betamethasone valerate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 Budesonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 Desonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.7 Diflorasone diacetate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.8 Fluticasone propionate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.9 Hydrocortisone buteprate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.10 Mometasone furoate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) | 15 | 2311 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.06, ‐0.72] |
| 5.1 Betamethasone dipropionate OD | 3 | 832 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐0.96, ‐0.64] |
| 5.2 Betamethasone dipropionate twice daily | 4 | 537 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.35 [‐1.56, ‐1.15] |
| 5.3 Betamethasone dipropionate, maintenance | 1 | 38 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐1.62, ‐0.27] |
| 5.4 Betamethasone valerate | 2 | 96 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.33 [‐1.78, ‐0.89] |
| 5.5 Budesonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.6 Desonide | 1 | 76 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.34, ‐0.28] |
| 5.7 Diflorasone diacetate | 1 | 93 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.73, 0.09] |
| 5.8 Fluticasone propionate | 2 | 383 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐1.14, ‐0.72] |
| 5.9 Hydrocortisone buteprate | 1 | 161 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.77, ‐0.15] |
| 5.10 Mometasone furoate | 1 | 95 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.17, ‐0.34] |
| 6 Total withdrawals | 9 | 1673 | Risk Difference (M‐H, Random, 95% CI) | ‐0.14 [‐0.22, ‐0.05] |
| 6.1 Betamethasone dipropionate OD | 2 | 756 | Risk Difference (M‐H, Random, 95% CI) | ‐0.16 [‐0.28, ‐0.04] |
| 6.2 Betamethasone dipropionate twice daily | 1 | 421 | Risk Difference (M‐H, Random, 95% CI) | ‐0.06 [‐0.12, 0.01] |
| 6.3 Betamethasone dipropionate, maintenance | 2 | 134 | Risk Difference (M‐H, Random, 95% CI) | ‐0.45 [‐0.60, ‐0.30] |
| 6.4 Betamethasone valerate | 1 | 80 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.12, 0.12] |
| 6.5 Budesonide | 1 | 22 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.16, 0.16] |
| 6.6 Desonide | 1 | 80 | Risk Difference (M‐H, Random, 95% CI) | ‐0.18 [‐0.38, 0.02] |
| 6.7 Diflorasone diacetate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.8 Fluticasone propionate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.9 Hydrocortisone buteprate | 1 | 180 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.09, 0.10] |
| 6.10 Mometasone furoate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Withdrawals due to adverse events | 9 | 1292 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.05, 0.02] |
| 7.1 Betamethasone dipropionate OD | 1 | 633 | Risk Difference (M‐H, Random, 95% CI) | ‐0.07 [‐0.11, ‐0.02] |
| 7.2 Betamethasone dipropionate twice daily | 1 | 33 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.11, 0.11] |
| 7.3 Betamethasone dipropionate, maintenance | 2 | 134 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 7.4 Betamethasone valerate | 1 | 80 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [‐0.02, 0.17] |
| 7.5 Budesonide | 1 | 22 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.16, 0.16] |
| 7.6 Desonide | 1 | 80 | Risk Difference (M‐H, Random, 95% CI) | ‐0.1 [‐0.24, 0.04] |
| 7.7 Diflorasone diacetate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.8 Fluticasone propionate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.9 Hydrocortisone buteprate | 1 | 190 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.02, 0.04] |
| 7.10 Mometasone furoate | 1 | 120 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.11, 0.01] |
| 8 Withdrawals due to treatment failure | 6 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Betamethasone dipropionate OD | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Betamethasone dipropionate twice daily | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Betamethasone dipropionate, maintenance | 2 | 130 | Risk Difference (M‐H, Random, 95% CI) | ‐0.46 [‐0.61, ‐0.31] |
| 8.4 Betamethasone valerate | 1 | 80 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
| 8.5 Budesonide | 1 | 22 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.16, 0.16] |
| 8.6 Desonide | 1 | 80 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.07, 0.07] |
| 8.7 Diflorasone diacetate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.8 Fluticasone propionate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.9 Hydrocortisone buteprate | 1 | 190 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 8.10 Mometasone furoate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Adverse events (local) | 10 | 2117 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.08, ‐0.00] |
| 9.1 Betamethasone dipropionate OD | 2 | 756 | Risk Difference (M‐H, Random, 95% CI) | ‐0.10 [‐0.15, ‐0.04] |
| 9.2 Betamethasone dipropionate twice daily | 2 | 454 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.12, 0.03] |
| 9.3 Betamethasone dipropionate, maintenance | 2 | 134 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 9.4 Betamethasone valerate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.5 Budesonide | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.6 Desonide | 1 | 80 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.11, 0.11] |
| 9.7 Diflorasone diacetate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.8 Fluticasone propionate | 1 | 383 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.05, 0.05] |
| 9.9 Hydrocortisone buteprate | 1 | 190 | Risk Difference (M‐H, Random, 95% CI) | ‐0.06 [‐0.18, 0.07] |
| 9.10 Mometasone furoate | 1 | 120 | Risk Difference (M‐H, Random, 95% CI) | ‐0.10 [‐0.23, 0.02] |
| 10 Adverse events (systemic) | 4 | 675 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.01] |
| 10.1 Betamethasone dipropionate OD | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Betamethasone dipropionate twice daily | 1 | 421 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 10.3 Betamethasone dipropionate, maintenance | 2 | 134 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.07, 0.10] |
| 10.4 Budesonide | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.5 Desonide | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.6 Diflorasone diacetate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.7 Fluticasone propionate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.8 Hydrocortisone buteprate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.9 Betamethasone valerate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.10 Mometasone furoate | 1 | 120 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
Comparison 3. Corticosteroid (very potent) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 IAGI | 5 | 540 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.87 [‐2.38, ‐1.36] |
| 1.1 Clobetasol propionate | 4 | 471 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.89 [‐2.53, ‐1.24] |
| 1.2 Halcinonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Halobetasol | 1 | 69 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.81 [‐2.37, ‐1.24] |
| 2 TSS | 3 | 545 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.35 [‐1.80, ‐0.89] |
| 2.1 Clobetasol propionate | 3 | 545 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.35 [‐1.80, ‐0.89] |
| 2.2 Halcinonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Halobetasol | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 PASI | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Clobetasol propionate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Halcinonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Halobetasol | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 PAGI | 3 | 487 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐1.42, ‐1.02] |
| 4.1 Clobetasol propionate | 1 | 79 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐1.55, ‐0.47] |
| 4.2 Halcinonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Halobetasol | 2 | 408 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.25 [‐1.46, ‐1.04] |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) | 10 | 1493 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.56 [‐1.87, ‐1.26] |
| 5.1 Clobetasol propionate | 7 | 1016 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.65 [‐2.10, ‐1.20] |
| 5.2 Halcinonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Halobetasol | 3 | 477 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.36 [‐1.65, ‐1.07] |
| 6 Total withdrawals | 8 | 1181 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.10, 0.01] |
| 6.1 Clobetasol propionate | 7 | 1037 | Risk Difference (M‐H, Random, 95% CI) | ‐0.06 [‐0.13, 0.01] |
| 6.2 Halcinonide | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Halobetasol | 1 | 144 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 7 Withdrawals due to adverse events | 10 | 1601 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.01, 0.01] |
| 7.1 Clobetasol propionate | 7 | 1037 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 7.2 Halcinonide | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Halobetasol | 3 | 564 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 8 Withdrawals due to treatment failure | 8 | 1189 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 8.1 Clobetasol propionate | 6 | 845 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| 8.2 Halcinonide | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Halobetasol | 2 | 344 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 9 Adverse events (local) | 8 | 1265 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.02, 0.02] |
| 9.1 Clobetasol propionate | 6 | 845 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.03, 0.03] |
| 9.2 Halcinonide | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Halobetasol | 2 | 420 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 10 Adverse events (systemic) | 6 | 1056 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.01, 0.01] |
| 10.1 Clobetasol propionate | 3 | 480 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.02, 0.01] |
| 10.2 Halcinonide | 1 | 156 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 10.3 Halobetasol | 2 | 420 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
Comparison 4. Dithranol versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 IAGI | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 TSS | 3 | 94 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐1.66, ‐0.46] |
| 3 PASI | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 PAGI | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) | 3 | 94 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐1.66, ‐0.46] |
| 6 Total withdrawals | 4 | 124 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.09, 0.09] |
| 7 Withdrawals due to adverse events | 3 | 104 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
| 8 Withdrawals due to treatment failure | 2 | 44 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.11, 0.11] |
| 9 Adverse events (local) | 3 | 94 | Risk Difference (M‐H, Random, 95% CI) | 0.26 [‐0.30, 0.82] |
| 10 Adverse events (systemic) | 1 | 20 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.35, 0.35] |
Comparison 5. Vitamin D combination products versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 IAGI | 5 | 2264 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.44 [‐1.76, ‐1.12] |
| 1.1 Combination calcipotriol/betamethasone dipropionate, once daily | 4 | 1416 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.21 [‐1.50, ‐0.91] |
| 1.2 Combination calcipotriol/betamethasone dipropionate, twice daily | 2 | 848 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐2.09, ‐1.71] |
| 2 TSS | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Combination calcipotriol/betamethasone dipropionate, once daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Combination calcipotriol/betamethasone dipropionate, twice daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 PASI | 5 | 2263 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.24 [‐1.53, ‐0.95] |
| 3.1 Combination calcipotriol/betamethasone dipropionate, once daily | 4 | 1414 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.14 [‐1.57, ‐0.70] |
| 3.2 Combination calcipotriol/betamethasone dipropionate, twice daily | 2 | 849 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.41 [‐1.86, ‐0.97] |
| 4 PAGI | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Combination calcipotriol/betamethasone dipropionate, once daily | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Combination calcipotriol/betamethasone dipropionate, twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) | 5 | 2264 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.44 [‐1.76, ‐1.12] |
| 5.1 Combination calcipotriol/betamethasone dipropionate, once daily | 4 | 1416 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.21 [‐1.50, ‐0.91] |
| 5.2 Combination calcipotriol/betamethasone dipropionate, twice daily | 2 | 848 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐2.09, ‐1.71] |
| 6 Total withdrawals | 5 | 2340 | Risk Difference (M‐H, Random, 95% CI) | ‐0.12 [‐0.17, ‐0.07] |
| 6.1 Combination calcipotriol/betamethasone dipropionate, once daily | 4 | 1483 | Risk Difference (M‐H, Random, 95% CI) | ‐0.15 [‐0.22, ‐0.09] |
| 6.2 Combination calcipotriol/betamethasone dipropionate, twice daily | 2 | 857 | Risk Difference (M‐H, Random, 95% CI) | ‐0.07 [‐0.12, ‐0.03] |
| 7 Withdrawals due to adverse events | 3 | 1723 | Risk Difference (M‐H, Random, 95% CI) | ‐0.07 [‐0.11, ‐0.04] |
| 7.1 Combination calcipotriol/betamethasone dipropionate, once daily | 3 | 1280 | Risk Difference (M‐H, Random, 95% CI) | ‐0.07 [‐0.11, ‐0.03] |
| 7.2 Combination calcipotriol/betamethasone dipropionate, twice daily | 1 | 443 | Risk Difference (M‐H, Random, 95% CI) | ‐0.10 [‐0.14, ‐0.05] |
| 8 Withdrawals due to treatment failure | 1 | 802 | Risk Difference (M‐H, Random, 95% CI) | ‐0.09 [‐0.12, ‐0.06] |
| 8.1 Combination calcipotriol/betamethasone dipropionate, once daily | 1 | 359 | Risk Difference (M‐H, Random, 95% CI) | ‐0.09 [‐0.13, ‐0.05] |
| 8.2 Combination calcipotriol/betamethasone dipropionate, twice daily | 1 | 443 | Risk Difference (M‐H, Random, 95% CI) | ‐0.09 [‐0.13, ‐0.05] |
| 9 Adverse events (local) | 5 | 2334 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.08, ‐0.02] |
| 9.1 Combination calcipotriol/betamethasone dipropionate, once daily | 4 | 1479 | Risk Difference (M‐H, Random, 95% CI) | ‐0.07 [‐0.11, ‐0.02] |
| 9.2 Combination calcipotriol/betamethasone dipropionate, twice daily | 2 | 855 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.08, 0.01] |
| 10 Adverse events (systemic) | 1 | 412 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 10.1 Combination calcipotriol/betamethasone dipropionate, once daily | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Combination calcipotriol/betamethasone dipropionate, twice daily | 1 | 412 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
Comparison 6. Other treatment versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 IAGI | 8 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Aloe vera extract 0.5% hydrophilic cream, three times per day | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Anti‐IL‐8 monoclonal antibody cream | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Betamethasone 17‐valerate 21‐acetate plus tretinoin plus salicylic acid | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Caffeine (topical) 10%, TD | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Calcipotriene 0.005% ointment + nicotinamide 0.05% or 0.1% or 0.7% or 1.4%, twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Dead Sea salts emollient lotion | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.7 Fish oil plus occlusion | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.8 Herbal skin care (Dr Michaels® cleansing gel, ointment and skin conditioner), twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.9 Hexafluoro‐1,25‐dihydroxyvitamin D3 | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.10 Indigo naturalis 1.4% ointment | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.11 Kukui nut oil, TD | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.12 Mahonia aquifolium (Reliéva™), twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.13 Methotrexate gel | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.14 Mycophenolic acid ointment | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.15 NG‐monomethyl‐L‐arginine (L‐NMMA) cream | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.16 Nicotinamide 1.4%, twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.17 Oleum horwathiensis (Psoricur®) | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.18 Omega‐3‐polyunsaturated fatty acids ointment | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.19 Platelet aggregation activating factor (PAF)(Ro 24‐0238) | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.20 Polymyxin B cream 200,000 U/g | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.21 PTH (1‐34) in Novasome A® liposomal cream, twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.22 Sirolimus (topical), 2.2% for 6 wks, then 8% for a further 6 wks | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.23 Tacrolimus ointment | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.24 Tar | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.25 Tazarotene | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.26 Theophylline 1% ointment, twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 TSS | 17 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Aloe vera extract 0.5% hydrophilic cream, three times per day | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Anti‐IL‐8 monoclonal antibody cream | 1 | 89 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐1.13, ‐0.27] |
| 2.3 Betamethasone 17‐valerate 21‐acetate plus tretinoin plus salicylic acid | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Caffeine (topical) 10%, TD | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 Calcipotriene 0.005% ointment + nicotinamide 0.05% or 0.1% or 0.7% or 1.4%, twice daily | 1 | 192 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.81, ‐0.15] |
| 2.6 Dead Sea salts emollient lotion | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.7 Fish oil plus occlusion | 1 | 50 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐1.64, ‐0.46] |
| 2.8 Herbal skin care (Dr Michaels® cleansing gel, ointment and skin conditioner), twice daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.9 Hexafluoro‐1,25‐dihydroxyvitamin D3, twice daily | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.13 [‐1.91, ‐0.35] |
| 2.10 Indigo naturalis 1.4% ointment | 2 | 88 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.64 [‐2.13, ‐1.15] |
| 2.11 Kukui nut oil, TD | 1 | 24 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [‐0.48, 1.14] |
| 2.12 Mahonia aquifolium (Reliéva™), twice daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.13 Methotrexate gel | 1 | 82 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.92, ‐0.04] |
| 2.14 Mycophenolic acid ointment | 1 | 14 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.44 [‐2.67, ‐0.22] |
| 2.15 NG‐monomethyl‐L‐arginine (L‐NMMA) cream | 1 | 34 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.60, 0.75] |
| 2.16 Nicotinamide 1.4%, twice daily | 1 | 96 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.60, 0.20] |
| 2.17 Oleum horwathiensis (Psoricur®) | 1 | 42 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐1.40, ‐0.14] |
| 2.18 Omega‐3‐polyunsaturated fatty acids ointment | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.19 Platelet aggregation activating factor (PAF)(Ro 24‐0238) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.20 Polymyxin B cream 200,000 U/g | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.59, 0.85] |
| 2.21 PTH (1‐34) in Novasome A® liposomal cream, twice daily | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.31 [‐3.26, ‐1.36] |
| 2.22 Sirolimus (topical), 2.2% for 6 wks, then 8% for a further 6 wks | 1 | 44 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.98, 0.21] |
| 2.23 Tacrolimus ointment | 1 | 47 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.52, 0.63] |
| 2.24 Tar | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐1.11, 0.22] |
| 2.25 Tazarotene | 1 | 318 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.11, ‐0.62] |
| 2.26 Theophylline 1% ointment, twice daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 PASI | 9 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Aloe vera extract 0.5% hydrophilic cream, three times per day | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Anti‐IL‐8 monoclonal antibody cream | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Betamethasone 17‐valerate 21‐acetate plus tretinoin plus salicylic acid | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Caffeine (topical) 10%, TD | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Calcipotriene 0.005% ointment + nicotinamide 0.05% or 0.1% or 0.7% or 1.4%, twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Dead Sea salts emollient lotion, 30% | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 Fish oil plus occlusion | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.8 Herbal skin care (Dr Michaels® cleansing gel, ointment and skin conditioner), twice daily | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.9 Hexafluoro‐1,25‐dihydroxyvitamin D3, twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.10 Indigo naturalis 1.4% ointment | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.11 Kukui nut oil, twice daily | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.12 Mahonia aquifolium (Reliéva™), twice daily | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.13 Methotrexate gel | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.14 Mycophenolic acid ointment | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.15 NG‐monomethyl‐L‐arginine (L‐NMMA) cream | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.16 Nicotinamide 1.4%, twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.17 Oleum horwathiensis (Psoricur®) | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.18 Omega‐3‐polyunsaturated fatty acids ointment | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.19 Platelet aggregation activating factor (PAF)(Ro 24‐0238) | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.20 Polymyxin B cream 200,000 U/g | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.21 PTH (1‐34) in Novasome A® liposomal cream, twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.22 Sirolimus (topical), 2.2% for 6 wks, then 8% for a further 6 wks | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.23 Tacrolimus ointment | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.24 Tar | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.25 Tazarotene | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.26 Theophylline 1% ointment, twice daily | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 PAGI | 2 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Aloe vera extract 0.5% hydrophilic cream, three times per day | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Anti‐IL‐8 monoclonal antibody cream | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Betamethasone 17‐valerate 21‐acetate plus tretinoin plus salicylic acid | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Caffeine (topical) 10%, TD | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Calcipotriene 0.005% ointment + nicotinamide 0.05% or 0.1% or 0.7% or 1.4%, twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Dead Sea salts emollient lotion, 30% | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 Fish oil plus occlusion | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.8 Herbal skin care (Dr Michaels® cleansing gel, ointment and skin conditioner), twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.9 Hexafluoro‐1,25‐dihydroxyvitamin D3, twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.10 Indigo naturalis 1.4% ointment | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.11 Kukui nut oil, TD | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.12 Mahonia aquifolium (Reliéva™), twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.13 Methotrexate gel | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.14 Mycophenolic acid ointment | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.15 NG‐monomethyl‐L‐arginine (L‐NMMA) cream | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.16 Nicotinamide 1.4%, twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.17 Oleum horwathiensis (Psoricur®) | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.18 Omega‐3‐polyunsaturated fatty acids ointment | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.19 Platelet aggregation activating factor (PAF)(Ro 24‐0238) | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.20 Polymyxin B cream 200,000 U/g | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.21 PTH (1‐34) in Novasome A® liposomal cream, twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.22 Sirolimus (topical), 2.2% for 6 wks, then 8% for a further 6 wks | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.23 Tacrolimus ointment | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.24 Tar | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.25 Tazarotene | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.26 Theophylline 1% ointment, twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) | 26 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Aloe vera extract 0.5% hydrophilic cream, three times per day | 1 | 60 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.58 [‐2.16, ‐0.99] |
| 5.2 Anti‐IL‐8 monoclonal antibody cream | 1 | 89 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐1.01, ‐0.16] |
| 5.3 Betamethasone 17‐valerate 21‐acetate plus tretinoin plus salicylic acid | 1 | 81 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.21, ‐0.31] |
| 5.4 Caffeine (topical) 10%, TD | 1 | 78 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.84, 0.06] |
| 5.5 Calcipotriene 0.005% ointment + nicotinamide 0.05% or 0.1% or 0.7% or 1.4%, twice daily | 1 | 192 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.81, ‐0.15] |
| 5.6 Dead Sea salts emollient lotion, 30% | 1 | 19 | Std. Mean Difference (IV, Random, 95% CI) | 0.57 [‐0.36, 1.51] |
| 5.7 Fish oil plus occlusion | 1 | 50 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐1.64, ‐0.46] |
| 5.8 Herbal skin care (Dr Michaels® cleansing gel, ointment and skin conditioner), twice daily | 1 | 24 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.96 [‐4.19, ‐1.74] |
| 5.9 Hexafluoro‐1,25‐dihydroxyvitamin D3 | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐1.35, 0.12] |
| 5.10 Indigo naturalis 1.4% ointment | 2 | 88 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.09 [‐2.62, ‐1.56] |
| 5.11 Kukui nut oil, TD | 1 | 24 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.80, 0.80] |
| 5.12 Mahonia aquifolium (Reliéva™), twice daily | 1 | 200 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐1.06, ‐0.48] |
| 5.13 Methotrexate gel | 2 | 142 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐2.04, ‐0.06] |
| 5.14 Mycophenolic acid ointment | 1 | 14 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.44 [‐2.67, ‐0.22] |
| 5.15 NG‐monomethyl‐L‐arginine (L‐NMMA) cream | 1 | 34 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.60, 0.75] |
| 5.16 Nicotinamide 1.4%, twice daily | 1 | 96 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.60, 0.20] |
| 5.17 Oleum horwathiensis (Psoricur®) | 1 | 42 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.63, 0.58] |
| 5.18 Omega‐3‐polyunsaturated fatty acids ointment | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.19 Platelet aggregation activating factor (PAF)(Ro 24‐0238) | 1 | 80 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.50, 0.37] |
| 5.20 Polymyxin B cream 200,000 U/g | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.59, 0.85] |
| 5.21 PTH (1‐34) in Novasome A® liposomal cream, twice daily | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.31 [‐3.26, ‐1.36] |
| 5.22 Sirolimus (topical), 2.2% for 6 wks, then 8% for a further 6 wks | 1 | 44 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.98, 0.21] |
| 5.23 Tacrolimus ointment | 1 | 47 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.52, 0.63] |
| 5.24 Tar | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐1.11, 0.22] |
| 5.25 Tazarotene | 1 | 318 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.11, ‐0.62] |
| 5.26 Theophylline 1% ointment, twice daily | 1 | 22 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.87 [‐4.13, ‐1.62] |
| 6 Total withdrawals | 23 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Aloe vera extract 0.5% hydrophilic cream, three times per day | 1 | 60 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.06, 0.06] |
| 6.2 Anti‐IL‐8 monoclonal antibody cream | 1 | 96 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.12, 0.08] |
| 6.3 Betamethasone 17‐valerate 21‐acetate plus tretinoin plus salicylic acid | 1 | 85 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.09, 0.09] |
| 6.4 Caffeine (topical) 10%, TD | 1 | 78 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.12, 0.12] |
| 6.5 Calcipotriene 0.005% ointment + nicotinamide 0.05% or 0.1% or 0.7% or 1.4%, twice daily | 1 | 192 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.01, 0.08] |
| 6.6 Dead Sea salts emollient lotion | 1 | 24 | Risk Difference (M‐H, Random, 95% CI) | 0.25 [‐0.06, 0.56] |
| 6.7 Fish oil plus occlusion | 1 | 50 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.07, 0.07] |
| 6.8 Herbal skin care (Dr Michaels® cleansing gel, ointment and skin conditioner), twice daily | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.9 Hexafluoro‐1,25‐dihydroxyvitamin D3 | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.12, 0.12] |
| 6.10 Indigo naturalis 1.4% ointment | 2 | 112 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.15, 0.15] |
| 6.11 Kukui nut oil, TD | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | ‐0.13 [‐0.42, 0.15] |
| 6.12 Mahonia aquifolium (Reliéva™), twice daily | 1 | 200 | Risk Difference (M‐H, Random, 95% CI) | ‐0.23 [‐0.32, ‐0.14] |
| 6.13 Methotrexate gel | 1 | 60 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.06, 0.06] |
| 6.14 Mycophenolic acid ointment | 1 | 14 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.24, 0.24] |
| 6.15 NG‐monomethyl‐L‐arginine (L‐NMMA) cream | 1 | 34 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.11, 0.11] |
| 6.16 Nicotinamide 1.4%, twice daily | 1 | 96 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.04, 0.08] |
| 6.17 Oleum horwathiensis | 1 | 50 | Risk Difference (M‐H, Random, 95% CI) | 0.16 [‐0.04, 0.36] |
| 6.18 Omega‐3‐polyunsaturated fatty acids ointment | 1 | 146 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.15, 0.15] |
| 6.19 Platelet aggregation activating factor (PAF)(Ro 24‐0238) | 1 | 104 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.16, 0.16] |
| 6.20 Polymyxin B cream 200,000 U/g | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.24, 0.24] |
| 6.21 PTH (1‐34) in Novasome A® liposomal cream, twice daily | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.12, 0.12] |
| 6.22 Sirolimus (topical) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.23 Tacrolimus ointment | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.24 Tar | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.25 Tazarotene | 2 | 1627 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.01, 0.09] |
| 6.26 Theophylline 1% ointment, twice daily | 1 | 22 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.16, 0.16] |
| 7 Withdrawals due to adverse events | 19 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Aloe vera extract 0.5% hydrophilic cream, three times per day | 1 | 60 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.06, 0.06] |
| 7.2 Anti‐IL‐8 monoclonal antibody cream | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Betamethasone 17‐valerate 21‐acetate plus tretinoin plus salicylic acid | 1 | 85 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.07, 0.06] |
| 7.4 Caffeine (topical) 10%, TD | 1 | 78 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
| 7.5 Calcipotriene 0.005% ointment + nicotinamide 0.05% or 0.1% or 0.7% or 1.4%, twice daily | 1 | 192 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 7.6 Dead Sea salts emollient lotion | 1 | 24 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [‐0.18, 0.35] |
| 7.7 Fish oil plus occlusion | 1 | 50 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.07, 0.07] |
| 7.8 Herbal skin care (Dr Michaels® cleansing gel, ointment and skin conditioner), twice daily | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.9 Hexafluoro‐1,25‐dihydroxyvitamin D3 | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.12, 0.12] |
| 7.10 Indigo naturalis 1.4% ointment | 2 | 112 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 7.11 Kukui nut oil, TD | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.12, 0.12] |
| 7.12 Mahonia aquifolium (Reliéva™), twice daily | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.13 Methotrexate gel | 1 | 60 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.06, 0.06] |
| 7.14 Mycophenolic acid ointment | 1 | 14 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.24, 0.24] |
| 7.15 NG‐monomethyl‐L‐arginine (L‐NMMA) cream | 1 | 34 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.11, 0.11] |
| 7.16 Nicotinamide 1.4%, twice daily | 1 | 96 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 7.17 Oleum horwathiensis | 1 | 50 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.07, 0.07] |
| 7.18 Omega‐3‐polyunsaturated fatty acids ointment | 1 | 146 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 7.19 Platelet aggregation activating factor (PAF)(Ro 24‐0238) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.20 Polymyxin B cream 200,000 U/g | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.21 PTH (1‐34) in Novasome A® liposomal cream, twice daily | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.12, 0.12] |
| 7.22 Sirolimus (topical) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.23 Tacrolimus ointment | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.24 Tar | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.25 Tazarotene | 2 | 1627 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [0.05, 0.10] |
| 7.26 Theophylline 1% ointment, twice daily | 1 | 22 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.16, 0.16] |
| 8 Withdrawals due to treatment failure | 18 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Aloe vera extract 0.5% hydrophilic cream, three times per day | 1 | 60 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.06, 0.06] |
| 8.2 Anti‐IL‐8 monoclonal antibody cream | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Betamethasone 17‐valerate 21‐acetate plus tretinoin plus salicylic acid | 1 | 85 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.04, 0.08] |
| 8.4 Caffeine (topical) 10%, TD | 1 | 78 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
| 8.5 Calcipotriene 0.005% ointment + nicotinamide 0.05% or 0.1% or 0.7% or 1.4%, twice daily | 1 | 192 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 8.6 Dead Sea salts emollient lotion | 1 | 24 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [‐0.12, 0.29] |
| 8.7 Fish oil plus occlusion | 1 | 50 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.07, 0.07] |
| 8.8 Herbal skin care (Dr Michaels® cleansing gel, ointment and skin conditioner), twice daily | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.9 Hexafluoro‐1,25‐dihydroxyvitamin D3 | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.12, 0.12] |
| 8.10 Indigo naturalis 1.4% ointment | 1 | 28 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.13, 0.13] |
| 8.11 Kukui nut oil, TD | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.12, 0.12] |
| 8.12 Mahonia aquifolium (Reliéva™), twice daily | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.13 Methotrexate gel | 1 | 60 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.06, 0.06] |
| 8.14 Mycophenolic acid ointment | 1 | 14 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.24, 0.24] |
| 8.15 NG‐monomethyl‐L‐arginine (L‐NMMA) cream | 1 | 34 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.11, 0.11] |
| 8.16 Nicotinamide 1.4%, twice daily | 1 | 96 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 8.17 Oleum horwathiensis | 1 | 50 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.07, 0.07] |
| 8.18 Omega‐3‐polyunsaturated fatty acids ointment | 1 | 146 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 8.19 Platelet aggregation activating factor (PAF)(Ro 24‐0238) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.20 Polymyxin B cream 200,000 U/g | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.21 PTH (1‐34) in Novasome A® liposomal cream, twice daily | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.12, 0.12] |
| 8.22 Sirolimus (topical) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.23 Tacrolimus ointment | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.24 Tar | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.25 Tazarotene | 2 | 1627 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.04, 0.01] |
| 8.26 Theophylline 1% ointment, twice daily | 1 | 22 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.16, 0.16] |
| 9 Adverse events (local) | 21 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Aloe vera extract 0.5% hydrophilic cream, three times per day | 1 | 60 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.06, 0.06] |
| 9.2 Anti‐IL‐8 monoclonal antibody cream | 1 | 92 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.10, 0.14] |
| 9.3 Betamethasone 17‐valerate 21‐acetate plus tretinoin plus salicylic acid | 1 | 85 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.07, 0.06] |
| 9.4 Caffeine (topical) 10%, TD | 1 | 78 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [‐0.03, 0.13] |
| 9.5 Calcipotriene 0.005% ointment + nicotinamide 0.05% or 0.1% or 0.7% or 1.4%, twice daily | 1 | 192 | Risk Difference (M‐H, Random, 95% CI) | 0.13 [‐0.02, 0.27] |
| 9.6 Dead Sea salts emollient lotion | 1 | 24 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [‐0.18, 0.35] |
| 9.7 Fish oil plus occlusion | 1 | 50 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.06, 0.14] |
| 9.8 Herbal skin care (Dr Michaels® cleansing gel, ointment and skin conditioner), twice daily | 1 | 24 | Risk Difference (M‐H, Random, 95% CI) | ‐0.09 [‐0.44, 0.27] |
| 9.9 Hexafluoro‐1,25‐dihydroxyvitamin D3 | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | 0.13 [‐0.06, 0.33] |
| 9.10 Indigo naturalis 1.4% ointment | 2 | 88 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
| 9.11 Kukui nut oil, TD | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.12, 0.12] |
| 9.12 Mahonia aquifolium (Reliéva™), twice daily | 1 | 200 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.06, 0.02] |
| 9.13 Methotrexate gel | 1 | 60 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.06, 0.06] |
| 9.14 Mycophenolic acid ointment | 1 | 14 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.24, 0.24] |
| 9.15 NG‐monomethyl‐L‐arginine (L‐NMMA) cream | 1 | 34 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.11, 0.11] |
| 9.16 Nicotinamide 1.4%, twice daily | 1 | 96 | Risk Difference (M‐H, Random, 95% CI) | 0.10 [‐0.07, 0.28] |
| 9.17 Oleum horwathiensis | 1 | 50 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.06, 0.14] |
| 9.18 Omega‐3‐polyunsaturated fatty acids ointment | 1 | 146 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.02, 0.05] |
| 9.19 Platelet aggregation activating factor (PAF)(Ro 24‐0238) | 1 | 104 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.19, 0.19] |
| 9.20 Polymyxin B cream 200,000 U/g | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.21 PTH (1‐34) in Novasome A® liposomal cream, twice daily | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.12, 0.12] |
| 9.22 Sirolimus (topical) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.23 Tacrolimus ointment | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.24 Tar | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.25 Tazarotene | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.26 Theophylline 1% ointment, twice daily | 1 | 22 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.16, 0.16] |
| 10 Adverse events (systemic) | 12 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Aloe vera extract 0.5% hydrophilic cream, three times per day | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Anti‐IL‐8 monoclonal antibody cream | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Betamethasone 17‐valerate 21‐acetate plus tretinoin plus salicylic acid | 1 | 85 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 10.4 Caffeine (topical) 10%, TD | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.5 Calcipotriene 0.005% ointment + nicotinamide 0.05% or 0.1% or 0.7% or 1.4%, twice daily | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.6 Dead Sea salts emollient lotion | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.7 Fish oil plus occlusion | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.8 Herbal skin care (Dr Michaels® cleansing gel, ointment and skin conditioner), twice daily | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.9 Hexafluoro‐1,25‐dihydroxyvitamin D3 | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.12, 0.12] |
| 10.10 Indigo naturalis 1.4% ointment | 2 | 88 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
| 10.11 Kukui nut oil, TD | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.12 Mahonia aquifolium (Reliéva™), twice daily | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.13 Methotrexate gel | 2 | 166 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 10.14 Mycophenolic acid ointment | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.15 NG‐monomethyl‐L‐arginine (L‐NMMA) cream | 1 | 34 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.11, 0.11] |
| 10.16 Nicotinamide 1.4%, twice daily | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.17 Oleum horwathiensis | 1 | 50 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.07, 0.07] |
| 10.18 Omega‐3‐polyunsaturated fatty acids ointment | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.19 Platelet aggregation activating factor (PAF)(Ro 24‐0238) | 1 | 104 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 10.20 Polymyxin B cream 200,000 U/g | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.21 PTH (1‐34) in Novasome A® liposomal cream, twice daily | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.12, 0.12] |
| 10.22 Sirolimus (topical) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.23 Tacrolimus ointment | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.24 Tar | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.25 Tazarotene | 2 | 414 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 10.26 Theophylline 1% ointment, twice daily | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 7. Vitamin D analogues versus corticosteroid (potent).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 IAGI | 8 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Calcipotriol vs. betamethasone dipropionate | 3 | 1728 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [0.28, 0.58] |
| 1.2 Calcipotriol vs. betamethasone valerate | 1 | 412 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.21, 0.17] |
| 1.3 Calcipotriol vs. desoxymetasone | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Calcipotriol vs. diflorasone diacetate | 1 | 256 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [0.02, 0.52] |
| 1.5 Calcipotriol vs. fluocinonide | 1 | 99 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐0.99, ‐0.18] |
| 1.6 Calcitriol vs. betamethasone dipropionate | 1 | 258 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.04, 0.45] |
| 1.7 Calcitriol vs. betamethasone valerate | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.91, 0.53] |
| 1.8 Tacalcitol vs. betamethasone valerate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 TSS | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Calcipotriol vs. betamethasone dipropionate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Calcipotriol vs. betamethasone valerate | 1 | 684 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.41, ‐0.11] |
| 2.3 Calcipotriol vs. desoxymetasone | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Calcipotriol vs. diflorasone diacetate | 1 | 256 | Std. Mean Difference (IV, Random, 95% CI) | 0.40 [0.15, 0.65] |
| 2.5 Calcipotriol vs. fluocinonide | 1 | 89 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.92, ‐0.07] |
| 2.6 Calcitriol vs. betamethasone dipropionate | 1 | 258 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [0.02, 0.51] |
| 2.7 Calcitriol vs. betamethasone valerate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.8 Tacalcitol vs. betamethasone valerate | 2 | 148 | Std. Mean Difference (IV, Random, 95% CI) | 0.41 [0.09, 0.74] |
| 3 PASI | 9 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Calcipotriol vs. betamethasone dipropionate | 3 | 1728 | Std. Mean Difference (IV, Random, 95% CI) | 0.36 [0.22, 0.51] |
| 3.2 Calcipotriol vs. betamethasone valerate | 4 | 1505 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.22, ‐0.02] |
| 3.3 Calcipotriol vs. desoxymetasone | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.73, 1.02] |
| 3.4 Calcipotriol vs. diflorasone diacetate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.5 Calcipotriol vs. fluocinonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.6 Calcitriol vs. betamethasone dipropionate | 1 | 258 | Std. Mean Difference (IV, Random, 95% CI) | 0.39 [0.14, 0.63] |
| 3.7 Calcitriol vs. betamethasone valerate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.8 Tacalcitol vs. betamethasone valerate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 PAGI | 2 | 1080 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.38, ‐0.14] |
| 4.1 Calcipotriol vs. betamethasone dipropionate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Calcipotriol vs. betamethasone valerate | 2 | 1080 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.38, ‐0.14] |
| 4.3 Calcipotriol vs. desoxymetasone | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Calcipotriol vs. diflorasone diacetate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 Calcipotriol vs. fluocinonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 Calcitriol vs. betamethasone dipropionate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.7 Calcitriol vs. betamethasone valerate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.8 Tacalcitol vs. betamethasone valerate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) | 14 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Calcipotriol vs. betamethasone dipropionate | 3 | 1728 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [0.28, 0.58] |
| 5.2 Calcipotriol vs. betamethasone valerate | 4 | 1557 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.26, 0.02] |
| 5.3 Calcipotriol vs. desoxymetasone | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.73, 1.02] |
| 5.4 Calcipotriol vs. diflorasone diacetate | 1 | 256 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [0.02, 0.52] |
| 5.5 Calcipotriol vs. fluocinonide | 1 | 99 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐0.99, ‐0.18] |
| 5.6 Calcitriol vs. betamethasone dipropionate | 1 | 258 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.04, 0.45] |
| 5.7 Calcitriol vs. betamethasone valerate | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.91, 0.53] |
| 5.8 Tacalcitol vs. betamethasone valerate | 2 | 148 | Std. Mean Difference (IV, Random, 95% CI) | 0.41 [0.09, 0.74] |
| 6 Total withdrawals | 11 | 3995 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [0.00, 0.03] |
| 6.1 Calcipotriol vs. betamethasone dipropionate | 3 | 1739 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [0.01, 0.06] |
| 6.2 Calcipotriol vs. betamethasone valerate | 3 | 1520 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.04] |
| 6.3 Calcipotriol vs. desoxymetasone | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 Calcipotriol vs. diflorasone diacetate | 1 | 268 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
| 6.5 Calcipotriol vs. fluocinonide | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.6 Calcitriol vs. betamethasone dipropionate | 1 | 258 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.08, 0.03] |
| 6.7 Calcitriol vs. betamethasone valerate | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [‐0.10, 0.23] |
| 6.8 Tacalcitol vs. betamethasone valerate | 2 | 180 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.11, 0.11] |
| 7 Withdrawals due to adverse events | 9 | 3058 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.00, 0.01] |
| 7.1 Calcipotriol vs. betamethasone dipropionate | 1 | 956 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [0.00, 0.04] |
| 7.2 Calcipotriol vs. betamethasone valerate | 3 | 1520 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.00, 0.01] |
| 7.3 Calcipotriol vs. desoxymetasone | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 Calcipotriol vs. diflorasone diacetate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.5 Calcipotriol vs. fluocinonide | 1 | 114 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.06, 0.03] |
| 7.6 Calcitriol vs. betamethasone dipropionate | 1 | 258 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.02, 0.03] |
| 7.7 Calcitriol vs. betamethasone valerate | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [‐0.10, 0.23] |
| 7.8 Tacalcitol vs. betamethasone valerate | 2 | 180 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 8 Withdrawals due to treatment failure | 5 | 1500 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.01, 0.01] |
| 8.1 Calcipotriol vs. betamethasone dipropionate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Calcipotriol vs. betamethasone valerate | 2 | 1099 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.01, 0.01] |
| 8.3 Calcipotriol vs. desoxymetasone | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.4 Calcipotriol vs. diflorasone diacetate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.5 Calcipotriol vs. fluocinonide | 1 | 113 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 8.6 Calcitriol vs. betamethasone dipropionate | 1 | 258 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.03, 0.05] |
| 8.7 Calcitriol vs. betamethasone valerate | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.12, 0.12] |
| 8.8 Tacalcitol vs. betamethasone valerate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Adverse events (local) | 9 | 3778 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [0.02, 0.11] |
| 9.1 Calcipotriol vs. betamethasone dipropionate | 3 | 1739 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [0.04, 0.09] |
| 9.2 Calcipotriol vs. betamethasone valerate | 3 | 1516 | Risk Difference (M‐H, Random, 95% CI) | 0.12 [‐0.02, 0.26] |
| 9.3 Calcipotriol vs. desoxymetasone | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.4 Calcipotriol vs. diflorasone diacetate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.5 Calcipotriol vs. fluocinonide | 1 | 113 | Risk Difference (M‐H, Random, 95% CI) | 0.10 [‐0.02, 0.22] |
| 9.6 Calcitriol vs. betamethasone dipropionate | 1 | 258 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.05, 0.06] |
| 9.7 Calcitriol vs. betamethasone valerate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.8 Tacalcitol vs. betamethasone valerate | 1 | 152 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.07, 0.04] |
| 10 Adverse events (systemic) | 6 | 2547 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.00, 0.00] |
| 10.1 Calcipotriol vs. betamethasone dipropionate | 1 | 621 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 10.2 Calcipotriol vs. betamethasone valerate | 3 | 1516 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.00, 0.00] |
| 10.3 Calcipotriol vs. desoxymetasone | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.4 Calcipotriol vs. diflorasone diacetate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.5 Calcipotriol vs. fluocinonide | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.6 Calcitriol vs. betamethasone dipropionate | 1 | 258 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.04, 0.03] |
| 10.7 Calcitriol vs. betamethasone valerate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.8 Tacalcitol vs. betamethasone valerate | 1 | 152 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.09, 0.09] |
Comparison 8. Vitamin D analogues versus corticosteroid (very potent).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 IAGI | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Calcipotriol vs. Clobetasol propionate | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 TSS | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Calcipotriol vs. Clobetasol propionate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 PASI | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Calcipotriol vs. Clobetasol propionate | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 PAGI | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Calcipotriol vs. Clobetasol propionate | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) | 2 | 82 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.57, 0.44] |
| 5.1 Calcipotriol vs. Clobetasol propionate | 2 | 82 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.57, 0.44] |
| 6 Total withdrawals | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 Calcipotriol vs. Clobetasol propionate | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Withdrawals due to adverse events | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1 Calcipotriol vs. Clobetasol propionate | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Withdrawals due to treatment failure | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 8.1 Calcipotriol vs. Clobetasol propionate | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Adverse events (local) | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 9.1 Calcipotriol vs. Clobetasol propionate | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Adverse events (systemic) | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 10.1 Calcipotriol vs. Clobetasol propionate | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 9. Vitamin D combined with corticosteroid versus corticosteroid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 IAGI | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Calcipotriol + betamethasone dipropionate vs. betamethasone dipropionate | 3 | 1926 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.52, ‐0.27] |
| 1.2 Calcipotriol + betamethasone dipropionate vs. clobetasol propionate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Calcipotriol + clobetasol propionate vs. clobetasol propionate | 1 | 65 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐1.22, ‐0.15] |
| 2 TSS | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Calcipotriol + betamethasone dipropionate vs. betamethasone dipropionate | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Calcipotriol + betamethasone dipropionate vs. clobetasol propionate | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Calcipotriol + clobetasol propionate vs. clobetasol propionate | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 PASI | 3 | 1876 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.55, ‐0.33] |
| 3.1 Calcipotriol + betamethasone dipropionate vs. betamethasone dipropionate | 3 | 1876 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.55, ‐0.33] |
| 3.2 Calcipotriol + betamethasone dipropionate vs. clobetasol propionate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Calcipotriol + clobetasol propionate vs. clobetasol propionate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 PAGI | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Calcipotriol + betamethasone dipropionate vs. betamethasone dipropionate | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Calcipotriol + betamethasone dipropionate vs. clobetasol propionate | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Calcipotriol + clobetasol propionate vs. clobetasol propionate | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Calcipotriol + betamethasone dipropionate vs. betamethasone dipropionate | 3 | 1926 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.52, ‐0.27] |
| 5.2 Calcipotriol + betamethasone dipropionate vs. clobetasol propionate | 1 | 122 | Std. Mean Difference (IV, Random, 95% CI) | 0.45 [0.09, 0.81] |
| 5.3 Calcipotriol + clobetasol propionate vs. clobetasol propionate | 1 | 65 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐1.22, ‐0.15] |
| 6 Total withdrawals | 5 | 2135 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 6.1 Calcipotriol + betamethasone dipropionate vs. betamethasone dipropionate | 3 | 1948 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.03, 0.03] |
| 6.2 Calcipotriol + betamethasone dipropionate vs. clobetasol propionate | 1 | 122 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 6.3 Calcipotriol + clobetasol propionate vs. clobetasol propionate | 1 | 65 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.07, 0.07] |
| 7 Withdrawals due to adverse events | 3 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1 Calcipotriol + betamethasone dipropionate vs. betamethasone dipropionate | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Calcipotriol + betamethasone dipropionate vs. clobetasol propionate | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Calcipotriol + clobetasol propionate vs. clobetasol propionate | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Withdrawals due to treatment failure | 2 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 8.1 Calcipotriol + betamethasone dipropionate vs. betamethasone dipropionate | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Calcipotriol + betamethasone dipropionate vs. clobetasol propionate | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Calcipotriol + clobetasol propionate vs. clobetasol propionate | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Adverse events (local) | 4 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Calcipotriol + betamethasone dipropionate vs. betamethasone dipropionate | 3 | 1946 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.00, 0.04] |
| 9.2 Calcipotriol + betamethasone dipropionate vs. clobetasol propionate | 1 | 122 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.13, 0.06] |
| 9.3 Calcipotriol + clobetasol propionate vs. clobetasol propionate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Adverse events (systemic) | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 10.1 Calcipotriol + betamethasone dipropionate vs. betamethasone dipropionate | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Calcipotriol + betamethasone dipropionate vs. clobetasol propionate | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 Calcipotriol + clobetasol propionate vs. clobetasol propionate | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 10. Vitamin D alone or in combination versus dithranol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 IAGI | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Calcipotriol vs. dithranol | 4 | 994 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.85, ‐0.01] |
| 1.2 Calcitriol vs. dithranol | 1 | 114 | Std. Mean Difference (IV, Random, 95% CI) | 0.51 [0.13, 0.88] |
| 1.3 Tacalcitol vs. dithranol | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 TSS | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Calcipotriol vs. dithranol | 2 | 210 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐1.16, 0.08] |
| 2.2 Calcitriol vs. dithranol | 1 | 114 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.24, 0.50] |
| 2.3 Tacalcitol vs. dithranol | 1 | 84 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.60, 0.25] |
| 3 PASI | 5 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Calcipotriol vs. dithranol | 3 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Calcitriol vs. dithranol | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Tacalcitol vs. dithranol | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 PAGI | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Calcipotriol vs. dithranol | 2 | 544 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.90, 0.80] |
| 4.2 Calcitriol vs. dithranol | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Tacalcitol vs. dithranol | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) | 8 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 Calcipotriol vs. dithranol | 6 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Calcitriol vs. dithranol | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Tacalcitol vs. dithranol | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Total withdrawals | 7 | 615 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.06, 0.01] |
| 6.1 Calcipotriol vs. dithranol | 5 | 417 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.07, 0.04] |
| 6.2 Calcitriol vs. dithranol | 1 | 114 | Risk Difference (M‐H, Random, 95% CI) | ‐0.10 [‐0.25, 0.06] |
| 6.3 Tacalcitol vs. dithranol | 1 | 84 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.16, 0.11] |
| 7 Withdrawals due to adverse events | 7 | 1265 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.06, ‐0.00] |
| 7.1 Calcipotriol vs. dithranol | 6 | 1151 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.06, 0.00] |
| 7.2 Calcitriol vs. dithranol | 1 | 114 | Risk Difference (M‐H, Random, 95% CI) | ‐0.06 [‐0.13, 0.02] |
| 7.3 Tacalcitol vs. dithranol | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Withdrawals due to treatment failure | 5 | 788 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.02, 0.02] |
| 8.1 Calcipotriol vs. dithranol | 4 | 674 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.02, 0.02] |
| 8.2 Calcitriol vs. dithranol | 1 | 114 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.08, 0.04] |
| 8.3 Tacalcitol vs. dithranol | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Adverse events (local) | 9 | 1543 | Risk Difference (M‐H, Random, 95% CI) | ‐0.32 [‐0.43, ‐0.20] |
| 9.1 Calcipotriol vs. dithranol | 7 | 1345 | Risk Difference (M‐H, Random, 95% CI) | ‐0.25 [‐0.32, ‐0.17] |
| 9.2 Calcitriol vs. dithranol | 1 | 114 | Risk Difference (M‐H, Random, 95% CI) | ‐0.67 [‐0.80, ‐0.54] |
| 9.3 Tacalcitol vs. dithranol | 1 | 84 | Risk Difference (M‐H, Random, 95% CI) | ‐0.36 [‐0.52, ‐0.20] |
| 10 Adverse events (systemic) | 4 | 746 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.01, 0.01] |
| 10.1 Calcipotriol vs. dithranol | 2 | 548 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 10.2 Calcitriol vs. dithranol | 1 | 114 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 10.3 Tacalcitol vs. dithranol | 1 | 84 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
Comparison 11. Vitamin D alone or in combination versus other vitamin D analogue.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 IAGI | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Calcipotriol vs. calcitriol | 1 | 246 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.25, 0.25] |
| 1.2 Calcipotriol vs. tacalcitol | 1 | 226 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.73, ‐0.21] |
| 1.3 Calcipotriol vs. maxacalcitol | 1 | 52 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.12, 0.98] |
| 2 TSS | 3 | 589 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.55, ‐0.06] |
| 2.1 Calcipotriol vs. calcitriol | 1 | 250 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.57, ‐0.07] |
| 2.2 Calcipotriol vs. tacalcitol | 1 | 287 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.68, ‐0.22] |
| 2.3 Calcipotriol vs. maxacalcitol | 1 | 52 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.41, 0.68] |
| 3 PASI | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Calcipotriol vs. calcitriol | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Calcipotriol vs. tacalcitol | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Calcipotriol vs. maxacalcitol | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 PAGI | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Calcipotriol vs. calcitriol | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Calcipotriol vs. tacalcitol | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Calcipotriol vs. maxacalcitol | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) | 4 | 539 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.62, 0.27] |
| 5.1 Calcipotriol vs. calcitriol | 2 | 261 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐1.46, 0.64] |
| 5.2 Calcipotriol vs. tacalcitol | 1 | 226 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.73, ‐0.21] |
| 5.3 Calcipotriol vs. maxacalcitol | 1 | 52 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.12, 0.98] |
| 6 Total withdrawals | 3 | 334 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.04, 0.08] |
| 6.1 Calcipotriol vs. calcitriol | 2 | 274 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.04, 0.09] |
| 6.2 Calcipotriol vs. tacalcitol | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Calcipotriol vs. maxacalcitol | 1 | 60 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.17, 0.17] |
| 7 Withdrawals due to adverse events | 3 | 334 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.01, 0.06] |
| 7.1 Calcipotriol vs. calcitriol | 2 | 274 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.01, 0.07] |
| 7.2 Calcipotriol vs. tacalcitol | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Calcipotriol vs. maxacalcitol | 1 | 60 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.06, 0.06] |
| 8 Withdrawals due to treatment failure | 3 | 334 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 8.1 Calcipotriol vs. calcitriol | 2 | 274 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.08, 0.07] |
| 8.2 Calcipotriol vs. tacalcitol | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Calcipotriol vs. maxacalcitol | 1 | 60 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.06, 0.06] |
| 9 Adverse events (local) | 2 | 537 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.05, 0.12] |
| 9.1 Calcipotriol vs. calcitriol | 1 | 250 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [0.01, 0.14] |
| 9.2 Calcipotriol vs. tacalcitol | 1 | 287 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.09, 0.07] |
| 9.3 Calcipotriol vs. maxacalcitol | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Adverse events (systemic) | 3 | 597 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 10.1 Calcipotriol vs. calcitriol | 1 | 250 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 10.2 Calcipotriol vs. tacalcitol | 1 | 287 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 10.3 Calcipotriol vs. maxacalcitol | 1 | 60 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.06, 0.06] |
Comparison 12. Vitamin D alone or in combination versus vitamin D + corticosteroid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 IAGI | 11 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Calcipotriol twice daily vs. calcipotriol OM, BMD ON | 1 | 154 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [0.23, 0.88] |
| 1.2 Calcipotriol OD vs. combined calcipotriol + BMD OD | 2 | 1194 | Std. Mean Difference (IV, Random, 95% CI) | 0.66 [0.31, 1.02] |
| 1.3 Calcipotriol twice daily vs. combined calcipotriol + BMD OD | 1 | 377 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [0.06, 0.48] |
| 1.4 Calcipotriol twice daily vs. combined calcipotriol + BMD twice daily | 3 | 1804 | Std. Mean Difference (IV, Random, 95% CI) | 0.66 [0.40, 0.93] |
| 1.5 Calcipotriol twice daily vs. calcipotriol OM, BMV ON | 2 | 510 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.19, 0.74] |
| 1.6 Calcipotriol twice daily vs. calcipotriol OM, clobetasone butyrate ON | 1 | 344 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [0.05, 0.48] |
| 1.7 Calcipotriol twice daily vs. calcipotriol twice daily + clobetasol propionate twice daily | 1 | 65 | Std. Mean Difference (IV, Random, 95% CI) | 0.88 [0.34, 1.42] |
| 1.8 Calcipotriol twice daily vs. calcipotriol OM, diflucortolone valerate ON | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.9 Calcipotriol OD vs. calcipotriol OM, fluocinonide acetonide ON | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.10 Calcipotriol OD vs. combined calcipotriol + hydrocortisone OD | 1 | 408 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.06, 0.33] |
| 1.11 Calcitriol twice daily vs. diflucortolone valerate OM, calcitriol ON | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.12 Tacalcitol OD vs. combined calcipotriol + BMD OD | 1 | 334 | Std. Mean Difference (IV, Random, 95% CI) | 0.48 [0.26, 0.70] |
| 2 TSS | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Calcipotriol twice daily vs. calcipotriol OM, BMD ON | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Calcipotriol OD vs. combined calcipotriol + BMD OD | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Calcipotriol twice daily vs. combined calcipotriol + BMD OD | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Calcipotriol twice daily vs. combined calcipotriol + BMD twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Calcipotriol twice daily vs. calcipotriol OM, BMV ON | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Calcipotriol twice daily vs. calcipotriol OM, clobetasone butyrate ON | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Calcipotriol twice daily vs. calcipotriol twice daily + clobetasol propionate twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 Calcipotriol twice daily vs. calcipotriol OM, diflucortolone valerate ON | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Calcipotriol OD vs. calcipotriol OM, fluocinonide acetonide ON | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.10 Calcipotriol OD vs. combined calcipotriol + hydrocortisone OD | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.11 Calcitriol twice daily vs. diflucortolone valerate OM, calcitriol ON | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.12 Tacalcitol OD vs. combined calcipotriol + BMD OD | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 PASI | 16 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Calcipotriol twice daily vs. calcipotriol OM, BMD ON | 1 | 124 | Std. Mean Difference (IV, Random, 95% CI) | 0.46 [0.10, 0.82] |
| 3.2 Calcipotriol OD vs. combined calcipotriol + BMD OD | 2 | 1191 | Std. Mean Difference (IV, Random, 95% CI) | 0.67 [0.23, 1.11] |
| 3.3 Calcipotriol twice daily vs. combined calcipotriol + BMD OD | 4 | 1204 | Std. Mean Difference (IV, Random, 95% CI) | 0.52 [0.38, 0.67] |
| 3.4 Calcipotriol twice daily vs. combined calcipotriol + BMD twice daily | 3 | 1744 | Std. Mean Difference (IV, Random, 95% CI) | 0.64 [0.46, 0.83] |
| 3.5 Calcipotriol twice daily vs. calcipotriol OM, BMV ON | 2 | 515 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.07, 0.93] |
| 3.6 Calcipotriol twice daily vs. calcipotriol OM, clobetasone butyrate ON | 1 | 344 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.04, 0.38] |
| 3.7 Calcipotriol twice daily vs. calcipotriol twice daily + clobetasol propionate twice daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.8 Calcipotriol twice daily vs. calcipotriol OM, diflucortolone valerate ON | 1 | 116 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.29, 0.44] |
| 3.9 Calcipotriol OD vs. calcipotriol OM, fluocinonide acetonide ON | 1 | 38 | Std. Mean Difference (IV, Random, 95% CI) | 0.53 [‐0.11, 1.18] |
| 3.10 Calcipotriol OD vs. combined calcipotriol + hydrocortisone OD | 1 | 408 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.11, 0.28] |
| 3.11 Calcitriol twice daily vs. diflucortolone valerate OM, calcitriol ON | 1 | 142 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.09, 0.57] |
| 3.12 Tacalcitol OD vs. combined calcipotriol + BMD OD | 1 | 334 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [0.25, 0.69] |
| 4 PAGI | 2 | 399 | Std. Mean Difference (IV, Random, 95% CI) | 0.49 [0.29, 0.69] |
| 4.1 Calcipotriol twice daily vs. calcipotriol OM, BMD ON | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Calcipotriol OD vs. combined calcipotriol + BMD OD | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Calcipotriol twice daily vs. combined calcipotriol + BMD OD | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Calcipotriol twice daily vs. combined calcipotriol + BMD twice daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 Calcipotriol twice daily vs. calcipotriol OM, BMV ON | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 Calcipotriol twice daily vs. calcipotriol OM, clobetasone butyrate ON | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.7 Calcipotriol twice daily vs. calcipotriol twice daily + clobetasol propionate twice daily | 1 | 65 | Std. Mean Difference (IV, Random, 95% CI) | 0.70 [0.16, 1.23] |
| 4.8 Calcipotriol twice daily vs. calcipotriol OM, diflucortolone valerate ON | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.9 Calcipotriol OD vs. calcipotriol OM, fluocinonide acetonide ON | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.10 Calcipotriol OD vs. combined calcipotriol + hydrocortisone OD | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.11 Calcitriol twice daily vs. diflucortolone valerate OM, calcitriol ON | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.12 Tacalcitol OD vs. combined calcipotriol + BMD OD | 1 | 334 | Std. Mean Difference (IV, Random, 95% CI) | 0.46 [0.24, 0.68] |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) | 17 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Calcipotriol twice daily vs. calcipotriol OM, BMD ON | 1 | 154 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [0.23, 0.88] |
| 5.2 Calcipotriol OD vs. combined calcipotriol + BMD OD | 2 | 1194 | Std. Mean Difference (IV, Random, 95% CI) | 0.66 [0.31, 1.02] |
| 5.3 Calcipotriol twice daily vs. combined calcipotriol + BMD OD | 4 | 1204 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [0.20, 0.66] |
| 5.4 Calcipotriol twice daily vs. combined calcipotriol + BMD twice daily | 3 | 1804 | Std. Mean Difference (IV, Random, 95% CI) | 0.66 [0.40, 0.93] |
| 5.5 Calcipotriol twice daily vs. calcipotriol OM, BMV ON | 2 | 510 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.19, 0.74] |
| 5.6 Calcipotriol twice daily vs. calcipotriol OM, clobetasone butyrate ON | 1 | 344 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [0.05, 0.48] |
| 5.7 Calcipotriol twice daily vs. calcipotriol twice daily + clobetasol propionate twice daily | 1 | 65 | Std. Mean Difference (IV, Random, 95% CI) | 0.88 [0.34, 1.42] |
| 5.8 Calcipotriol twice daily vs. calcipotriol OM, diflucortolone valerate ON | 1 | 116 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.29, 0.44] |
| 5.9 Calcipotriol OD vs. calcipotriol OM, fluocinonide acetonide ON | 1 | 38 | Std. Mean Difference (IV, Random, 95% CI) | 0.53 [‐0.11, 1.18] |
| 5.10 Calcipotriol OD vs. combined calcipotriol + hydrocortisone OD | 1 | 408 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.06, 0.33] |
| 5.11 Calcitriol twice daily vs. diflucortolone valerate OM, calcitriol ON | 1 | 142 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.09, 0.57] |
| 5.12 Tacalcitol OD vs. combined calcipotriol + BMD OD | 1 | 334 | Std. Mean Difference (IV, Random, 95% CI) | 0.48 [0.26, 0.70] |
| 6 Total withdrawals | 15 | 5494 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [0.02, 0.05] |
| 6.1 Talcipotriol vs. calcipotriol and corticosteroid | 13 | 4985 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [0.01, 0.05] |
| 6.2 Calcitriol vs. calcitriol and corticosteroid | 1 | 142 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.08, 0.10] |
| 6.3 Tacalcitol vs. calcipotriol and corticosteroid | 1 | 367 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [‐0.01, 0.11] |
| 7 Withdrawals due to adverse events | 13 | 4081 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [0.01, 0.03] |
| 7.1 Calcipotriol vs. calcipotriol and corticosteroid | 11 | 3572 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [0.01, 0.03] |
| 7.2 Calcitriol vs. calcitriol and corticosteroid | 1 | 142 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.02, 0.07] |
| 7.3 Tacalcitol vs. calcipotriol and corticosteroid | 1 | 367 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.02, 0.03] |
| 8 Withdrawals due to treatment failure | 7 | 1925 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.00, 0.02] |
| 8.1 Calcipotriol vs. calcipotriol and corticosteroid | 7 | 1925 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.00, 0.02] |
| 8.2 Calcitriol vs. calcitriol and corticosteroid | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Tacalcitol vs. calcipotriol and corticosteroid | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Adverse events (local) | 15 | 5581 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [0.05, 0.08] |
| 9.1 Calcipotriol vs. calcipotriol and corticosteroid | 13 | 5084 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [0.04, 0.08] |
| 9.2 Calcitriol vs. calcitriol and corticosteroid | 1 | 131 | Risk Difference (M‐H, Random, 95% CI) | 0.10 [0.02, 0.19] |
| 9.3 Tacalcitol vs. calcipotriol and corticosteroid | 1 | 366 | Risk Difference (M‐H, Random, 95% CI) | 0.09 [0.02, 0.15] |
| 10 Adverse events (systemic) | 6 | 2099 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.00, 0.00] |
| 10.1 Calcipotriol vs. calcipotriol and corticosteroid | 5 | 1968 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.00, 0.00] |
| 10.2 Calcitriol vs. calcitriol and corticosteroid | 1 | 131 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 10.3 Tacalcitol vs. calcipotriol and corticosteroid | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
12.2. Analysis.
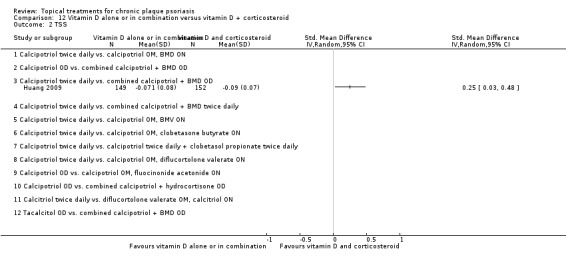
Comparison 12 Vitamin D alone or in combination versus vitamin D + corticosteroid, Outcome 2 TSS.
Comparison 13. Vitamin D alone or in combination versus other treatments: complex regimens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 IAGI | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) | 1 | 577 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.29, 0.04] |
| 1.2 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (8 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 1 | 585 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.04, 0.29] |
| 1.4 Calcipotriol (6 wks) vs. clobetasol propionate (2 wks); then calcipotriol (4 wks) | 1 | 92 | Std. Mean Difference (IV, Random, 95% CI) | 0.60 [0.18, 1.02] |
| 1.5 Calcipotriol (6 wks) vs. calcipotriol OM, fluocinonide acetonide ON (2 wks); then calcipotriol twice daily (4 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.6 Calcipotriol (6 wks) vs. halometasone OM, calcipotriol ON (2 wks); then calcipotriol twice daily (w/dy), halometasone (w/e) (2 wks); then calcipotriol twice daily (2wks) | 1 | 76 | Std. Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.05, 0.86] |
| 1.7 Calcipotriol ON, clobetasol propionate OM (2 to 4 wks); then calcipotriol twice daily (to wk 12) vs. calcitriol ON, clobetasol propionate OM (2 to 4 wks); then calcitriol twice daily (to wk 12) | 1 | 125 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.54, 0.16] |
| 1.8 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) | 1 | 759 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [0.12, 0.41] |
| 1.9 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy)+ combined calcipotriol + BMD (w/e) (8 wks) | 1 | 753 | Std. Mean Difference (IV, Random, 95% CI) | 0.51 [0.37, 0.66] |
| 1.10 Combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy)+ combined calcipotriol + BMD (w/e) (8 wks) | 1 | 760 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [0.11, 0.40] |
| 1.11 Combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 1 | 596 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [0.08, 0.40] |
| 1.12 Tacalcitol (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 1 | 493 | Std. Mean Difference (IV, Random, 95% CI) | 0.54 [0.36, 0.72] |
| 2 TSS | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (8 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Calcipotriol (6 wks) vs. clobetasol propionate (2wks); then calcipotriol (4 wks) | 1 | 92 | Std. Mean Difference (IV, Random, 95% CI) | 0.63 [0.21, 1.05] |
| 2.5 Calcipotriol (6 wks) vs. calcipotriol OM, fluocinonide acetonide ON (2 wks); then calcipotriol twice daily (4 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 Calcipotriol (6 wks) vs. halometasone OM, calcipotriol ON (2 wks); then calcipotriol twice daily (w/dy), halometasone (w/e) (2 wks); then calcipotriol twice daily (2 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.7 Calcipotriol ON, clobetasol propionate OM (2 to 4 wks); then calcipotriol twice daily (to wk 12) vs. calcitriol ON, clobetasol propionate OM (2 to 4 wks); then calcitriol twice daily (to wk 12) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.8 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.9 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) + combined calcipotriol + BMD (w/e) (8 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.10 Combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) + combined calcipotriol + BMD (w/e) (8 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.11 Combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.12 Tacalcitol (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 PASI | 8 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) | 1 | 649 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.19, 0.11] |
| 3.2 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (8 wks) | 1 | 143 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.04, 0.62] |
| 3.3 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 1 | 650 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.05, 0.25] |
| 3.4 Calcipotriol (6 wks) vs. clobetasol propionate (2 wks); then calcipotriol (4 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.5 Calcipotriol (6 wks) vs. calcipotriol OM, fluocinonide acetonide ON (2 wks); then calcipotriol twice daily (4 wks) | 1 | 38 | Std. Mean Difference (IV, Random, 95% CI) | 0.66 [0.01, 1.32] |
| 3.6 Calcipotriol (6 wks) vs. halometasone OM, calcipotriol ON (2 wks); then calcipotriol twice daily (w/dy), halometasone (w/e) (2 wks); then calcipotriol twice daily (2 wks) | 1 | 76 | Std. Mean Difference (IV, Random, 95% CI) | 1.13 [0.64, 1.62] |
| 3.7 Calcipotriol ON, clobetasol propionate OM (2 to 4 wks); then calcipotriol twice daily (to wk 12) vs. calcitriol ON, clobetasol propionate OM (2 to 4 wks); then calcitriol twice daily (to wk 12) | 1 | 125 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.62, 0.09] |
| 3.8 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) | 1 | 759 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [0.10, 0.39] |
| 3.9 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) + combined calcipotriol + BMD (w/e) (8 wks) | 1 | 753 | Std. Mean Difference (IV, Random, 95% CI) | 0.59 [0.45, 0.74] |
| 3.10 Combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) + combined calcipotriol + BMD (w/e) (8 wks) | 1 | 760 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [0.16, 0.45] |
| 3.11 Combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 1 | 645 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.01, 0.30] |
| 3.12 Tacalcitol (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 1 | 501 | Std. Mean Difference (IV, Random, 95% CI) | 0.49 [0.31, 0.67] |
| 4 PAGI | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) | 1 | 577 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.30, 0.02] |
| 4.2 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (8 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 1 | 585 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.06, 0.26] |
| 4.4 Calcipotriol (6 wks) vs. clobetasol propionate (2 wks); then calcipotriol (4 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 Calcipotriol (6 wks) vs. calcipotriol OM, fluocinonide acetonide ON (2 wks); then calcipotriol twice daily (4 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 Calcipotriol (6 wks) vs. halometasone OM, calcipotriol ON (2 wks); then calcipotriol twice daily (w/dy), halometasone (w/e) (2 wks); then calcipotriol twice daily (2 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.7 Calcipotriol ON, clobetasol propionate OM (2 to 4 wks); then calcipotriol twice daily (to wk 12) vs. calcitriol ON, clobetasol propionate OM (2 to 4 wks); then calcitriol twice daily (to wk 12) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.8 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) | 1 | 759 | Std. Mean Difference (IV, Random, 95% CI) | 0.28 [0.13, 0.42] |
| 4.9 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) + combined calcipotriol + BMD (w/e) (8 wks) | 1 | 753 | Std. Mean Difference (IV, Random, 95% CI) | 0.71 [0.56, 0.85] |
| 4.10 Combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) + combined calcipotriol + BMD (w/e) (8 wks) | 1 | 760 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [0.29, 0.58] |
| 4.11 Combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 1 | 596 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [0.07, 0.39] |
| 4.12 Tacalcitol (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 1 | 493 | Std. Mean Difference (IV, Random, 95% CI) | 0.54 [0.36, 0.72] |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) | 9 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) | 1 | 577 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.29, 0.04] |
| 5.2 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (8 wks) | 1 | 143 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.04, 0.62] |
| 5.3 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 1 | 585 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.04, 0.29] |
| 5.4 Calcipotriol (6 wks) vs. clobetasol propionate (2 wks); then calcipotriol (4 wks) | 1 | 92 | Std. Mean Difference (IV, Random, 95% CI) | 0.60 [0.18, 1.02] |
| 5.5 Calcipotriol (6 wks) vs. calcipotriol OM, fluocinonide acetonide ON (2 wks); then calcipotriol twice daily (4 wks) | 1 | 38 | Std. Mean Difference (IV, Random, 95% CI) | 0.66 [0.01, 1.32] |
| 5.6 Calcipotriol (6 wks) vs. halometasone OM, calcipotriol ON (2 wks); then calcipotriol twice daily (w/dy), halometasone (w/e) (2 wks); then calcipotriol twice daily (2 wks) | 1 | 76 | Std. Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.05, 0.86] |
| 5.7 Calcipotriol ON, clobetasol propionate OM (2 to 4 wks); then calcipotriol twice daily (to wk 12) vs. calcitriol ON, clobetasol propionate OM (2 to 4 wks); then calcitriol twice daily (to wk 12) | 1 | 125 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.54, 0.16] |
| 5.8 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) | 1 | 759 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [0.12, 0.41] |
| 5.9 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy)+ combined calcipotriol + BMD (w/e) (8 wks) | 1 | 753 | Std. Mean Difference (IV, Random, 95% CI) | 0.51 [0.37, 0.66] |
| 5.10 Combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) + combined calcipotriol + BMD (w/e) (8 wks) | 1 | 760 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [0.11, 0.40] |
| 5.11 Combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 1 | 596 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [0.08, 0.40] |
| 5.12 Tacalcitol (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 1 | 493 | Std. Mean Difference (IV, Random, 95% CI) | 0.54 [0.36, 0.72] |
| 6 Total withdrawals | 9 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) | 1 | 649 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [0.00, 0.10] |
| 6.2 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (8 wks) | 1 | 150 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.09, 0.17] |
| 6.3 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 1 | 649 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [0.03, 0.13] |
| 6.4 Calcipotriol (6 wks) vs. clobetasol propionate (2 wks); then calcipotriol (4 wks) | 1 | 98 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.09, 0.09] |
| 6.5 Calcipotriol (6 wks) vs. calcipotriol OM, fluocinonide acetonide ON (2 wks); then calcipotriol twice daily (4 wks) | 1 | 38 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.10, 0.10] |
| 6.6 Calcipotriol (6 wks) vs. halometasone OM, calcipotriol ON (2 wks); then calcipotriol twice daily (w/dy), halometasone (w/e) (2 wks); then calcipotriol twice daily (2 wks) | 1 | 76 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
| 6.7 Calcipotriol ON, clobetasol propionate OM (2 to 4 wks); then calcipotriol twice daily (to wk 12) vs. calcitriol ON, clobetasol propionate OM (2 to 4 wks); then calcitriol twice daily (to wk 12) | 1 | 125 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.12, 0.11] |
| 6.8 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) | 1 | 759 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [0.03, 0.14] |
| 6.9 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) + combined calcipotriol + BMD (w/e) (8 wks) | 1 | 753 | Risk Difference (M‐H, Random, 95% CI) | 0.11 [0.06, 0.17] |
| 6.10 Combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) + combined calcipotriol + BMD (w/e) (8 wks) | 1 | 760 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.01, 0.07] |
| 6.11 Combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 1 | 644 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.01, 0.07] |
| 6.12 Tacalcitol (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 1 | 501 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [‐0.01, 0.12] |
| 7 Withdrawals due to adverse events | 8 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (8 wks) | 1 | 150 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.09, 0.01] |
| 7.3 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 Calcipotriol (6 wks) vs. clobetasol propionate (2 wks); then calcipotriol (4 wks) | 1 | 98 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 7.5 Calcipotriol (6 wks) vs. calcipotriol OM, fluocinonide acetonide ON (2 wks); then calcipotriol twice daily (4 wks) | 1 | 38 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.10, 0.10] |
| 7.6 Calcipotriol (6 wks) vs. halometasone OM, calcipotriol ON (2 wks); then calcipotriol twice daily (w/dy), halometasone (w/e) (2 wks); then calcipotriol twice daily (2 wks) | 1 | 76 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
| 7.7 Calcipotriol ON, clobetasol propionate OM (2 to 4 wks); then calcipotriol twice daily (to wk 12) vs. calcitriol ON, clobetasol propionate OM (2 to 4 wks); then calcitriol twice daily (to wk 12) | 1 | 125 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.06, 0.03] |
| 7.8 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) | 1 | 759 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.02] |
| 7.9 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) + combined calcipotriol + BMD (w/e) (8 wks) | 1 | 753 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.02, 0.02] |
| 7.10 Combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) + combined calcipotriol + BMD (w/e) (8 wks) | 1 | 760 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| 7.11 Combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.12 Tacalcitol (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 1 | 501 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.01, 0.05] |
| 8 Withdrawals due to treatment failure | 6 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (8 wks) | 1 | 150 | Risk Difference (M‐H, Random, 95% CI) | 0.21 [0.10, 0.33] |
| 8.3 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.4 Calcipotriol (6 wks) vs. clobetasol propionate (2 wks); then calcipotriol (4 wks) | 1 | 98 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 8.5 Calcipotriol (6 wks) vs. calcipotriol OM, fluocinonide acetonide ON (2 wks); then calcipotriol twice daily (4 wks) | 1 | 38 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.10, 0.10] |
| 8.6 Calcipotriol (6 wks) vs. halometasone OM, calcipotriol ON (2 wks); then calcipotriol twice daily (w/dy), halometasone (w/e) (2 wks); then calcipotriol twice daily (2 wks) | 1 | 76 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
| 8.7 Calcipotriol ON, clobetasol propionate OM (2 to 4 wks); then calcipotriol twice daily (to wk 12) vs. calcitriol ON, clobetasol propionate OM (2 to 4 wks); then calcitriol twice daily (to wk 12) | 1 | 125 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.04, 0.10] |
| 8.8 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.9 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) + combined calcipotriol + BMD (w/e) (8 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.10 Combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy)+ combined calcipotriol + BMD (w/e) (8 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.11 Combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.12 Tacalcitol (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 1 | 501 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [0.02, 0.08] |
| 9 Adverse events (local) | 8 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) | 1 | 649 | Risk Difference (M‐H, Random, 95% CI) | 0.11 [0.06, 0.17] |
| 9.2 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (8 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 1 | 649 | Risk Difference (M‐H, Random, 95% CI) | 0.11 [0.05, 0.17] |
| 9.4 Calcipotriol (6 wks) vs. clobetasol propionate (2 wks); then calcipotriol (4 wks) | 1 | 98 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.08, 0.12] |
| 9.5 Calcipotriol (6 wks) vs. calcipotriol OM, fluocinonide acetonide ON (2 wks); then calcipotriol twice daily (4 wks) | 1 | 38 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.14, 0.14] |
| 9.6 Calcipotriol (6 wks) vs. halometasone OM, calcipotriol ON (2 wks); then calcipotriol twice daily (w/dy), halometasone (w/e) (2 wks); then calcipotriol twice daily (2 wks) | 1 | 76 | Risk Difference (M‐H, Random, 95% CI) | 0.26 [0.07, 0.45] |
| 9.7 Calcipotriol ON, clobetasol propionate OM (2 to 4 wks); then calcipotriol twice daily (to wk 12) vs. calcitriol ON, clobetasol propionate OM (2 to 4 wks); then calcitriol twice daily (to wk 12) | 1 | 125 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.10, 0.06] |
| 9.8 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) | 1 | 752 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.07, 0.02] |
| 9.9 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) + combined calcipotriol + BMD (w/e) (8 wks) | 1 | 743 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.03, 0.05] |
| 9.10 Combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) + combined calcipotriol + BMD (w/e) (8 wks) | 1 | 749 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.00, 0.08] |
| 9.11 Combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 1 | 644 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.05, 0.04] |
| 9.12 Tacalcitol (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 1 | 501 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [0.01, 0.11] |
| 10 Adverse events (systemic) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (8 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.4 Calcipotriol (6 wks) vs. clobetasol propionate (2 wks); then calcipotriol (4 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.5 Calcipotriol (6 wks) vs. calcipotriol OM, fluocinonide acetonide ON (2 wks); then calcipotriol twice daily (4 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.6 Calcipotriol (6 wks) vs. halometasone OM, calcipotriol ON (2 wks); then calcipotriol twice daily (w/dy), halometasone (w/e) (2 wks); then calcipotriol twice daily (2 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.7 Calcipotriol ON, clobetasol propionate OM (2 to 4 wks); then calcipotriol twice daily (to wk 12) vs. calcitriol ON, clobetasol propionate OM (2 to 4 wks); then calcitriol twice daily (to wk 12) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.8 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.9 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) + combined calcipotriol + BMD (w/e) (8 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.10 Combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) + combined calcipotriol + BMD (w/e) (8 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.11 Combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.12 Tacalcitol (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 14. Vitamin D alone or in combination versus other treatment: long‐term studies (> 24 wks).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 IAGI | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Combined calcipotriol + BMD (52 wks) vs. alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Combined calcipotriol + BMD (52 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 TSS | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Combined calcipotriol + BMD (52 wks) vs. alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Combined calcipotriol + BMD (52 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 PASI | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Combined calcipotriol + BMD (52 wks) vs. alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Combined calcipotriol + BMD (52 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 PAGI | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Combined calcipotriol + BMD (52 wks) vs. alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Combined calcipotriol + BMD (52 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 Combined calcipotriol + BMD (52 wks) vs. alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Combined calcipotriol + BMD (52 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Total withdrawals | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 Combined calcipotriol + BMD (52 wks) vs. alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Combined calcipotriol + BMD (52 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Withdrawals due to adverse events | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1 Combined calcipotriol + BMD (52 wks) vs. alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Combined calcipotriol + BMD (52 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Withdrawals due to treatment failure | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 8.1 Combined calcipotriol + BMD (52 wks) vs. alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Combined calcipotriol + BMD (52 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Adverse events (local) | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 9.1 Combined calcipotriol + BMD (52 wks) vs. alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Combined calcipotriol + BMD (52 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Adverse events (systemic) | 0 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Combined calcipotriol + BMD (52 wks) vs. alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Combined calcipotriol + BMD (52 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 15. Vitamin D analogues versus other treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 IAGI | 10 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Calcipotriol vs. coal tar | 3 | 139 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐1.74, 0.68] |
| 1.2 Calcipotriol vs. coal tar polytherapy | 2 | 209 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐0.87, ‐0.31] |
| 1.3 Calcipotriol vs. nicotinamide 1.4%, twice daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Calcipotriol vs. calcipotriol + nicotinamide 1.4%, twice daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 Calcipotriol vs. corticosteroid + salicylic acid | 1 | 200 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.33, 0.22] |
| 1.6 Calcipotriol vs. propylthiouracil cream | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.7 Calcipotriol vs. tazarotene | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.8 Calcipotriol vs. tacrolimus ointment | 1 | 124 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.60, 0.16] |
| 1.9 Calcipotriol vs. tazarotene gel plus mometasone furoate cream | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.10 Calcipotriol vs. vitamin B12 cream | 1 | 26 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐1.33, 0.24] |
| 1.11 Head‐to‐head vitamin D alone or in combination: twice daily vs OD | 2 | 728 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.38, ‐0.09] |
| 1.12 Head‐to‐head vitamin D alone or in combination: no occlusion vs. occlusion | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 TSS | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Calcipotriol vs. coal tar | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Calcipotriol vs. coal tar polytherapy | 1 | 132 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.86, ‐0.16] |
| 2.3 Calcipotriol vs. nicotinamide 1.4%, twice daily | 1 | 96 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.49, 0.31] |
| 2.4 Calcipotriol vs. calcipotriol+nicotinamide (0.05%, 0.1%, 0.7%, or 1.4%), twice daily | 1 | 192 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.14, 0.52] |
| 2.5 Calcipotriol vs. corticosteroid + salicylic acid | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 Calcipotriol vs. propylthiouracil cream | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.7 Calcipotriol vs. tacrolimus ointment | 2 | 171 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐1.51, 0.81] |
| 2.8 Calcipotriol vs. tazarotene | 1 | 199 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.33, 0.23] |
| 2.9 Calcipotriol vs. tazarotene gel plus mometasone furoate cream | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.10 Calcipotriol vs. vitamin B12 cream | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.11 Head‐to‐head vitamin D alone or in combination: twice daily vs OD | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.12 Head‐to‐head vitamin D alone or in combination: no occlusion vs. occlusion | 2 | 247 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐2.04, 1.68] |
| 3 PASI | 9 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Calcipotriol vs. coal tar | 2 | 109 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐1.54, 1.35] |
| 3.2 Calcipotriol vs. coal tar polytherapy | 1 | 87 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐1.06, ‐0.20] |
| 3.3 Calcipotriol vs. nicotinamide 1.4%, twice daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Calcipotriol vs. calcipotriol + nicotinamide 1.4%, twice daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.5 Calcipotriol vs. corticosteroid + salicylic acid | 1 | 160 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.36, 0.26] |
| 3.6 Calcipotriol vs. propylthiouracil cream | 1 | 27 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.24 [‐3.23, ‐1.25] |
| 3.7 Calcipotriol vs. tacrolimus ointment | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.8 Calcipotriol vs. tazarotene | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.9 Calcipotriol vs. tazarotene gel plus mometasone furoate cream | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.10 Calcipotriol vs. vitamin B12 cream | 1 | 26 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.78, 0.75] |
| 3.11 Head‐to‐head vitamin D alone or in combination: twice daily vs OD | 3 | 989 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.25, 0.00] |
| 3.12 Head‐to‐head vitamin D alone or in combination: no occlusion vs. occlusion | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 PAGI | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Calcipotriol vs. coal tar | 1 | 54 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.51 [‐2.12, ‐0.90] |
| 4.2 Calcipotriol vs. coal tar polytherapy | 1 | 87 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐0.99, ‐0.13] |
| 4.3 Calcipotriol vs. nicotinamide 1.4%, twice daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Calcipotriol vs. calcipotriol + nicotinamide 1.4%, twice daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 Calcipotriol vs. corticosteroid + salicylic acid | 1 | 186 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.79, ‐0.20] |
| 4.6 Calcipotriol vs. propylthiouracil cream | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.7 Calcipotriol vs. tacrolimus ointment | 1 | 124 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.51, 0.24] |
| 4.8 Calcipotriol vs. tazarotene | 1 | 38 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.99, 0.29] |
| 4.9 Calcipotriol vs. tazarotene gel plus mometasone furoate cream | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.10 Calcipotriol vs. vitamin B12 cream | 1 | 26 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐1.33, 0.24] |
| 4.11 Head‐to‐head vitamin D alone or in combination: twice daily vs OD | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.12 Head‐to‐head vitamin D alone or in combination: no occlusion vs. occlusion | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) | 19 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Calcipotriol vs. coal tar | 3 | 139 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐1.74, 0.68] |
| 5.2 Calcipotriol vs. coal tar polytherapy | 2 | 209 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐0.87, ‐0.31] |
| 5.3 Calcipotriol vs. nicotinamide 1.4%, twice daily | 1 | 96 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.49, 0.31] |
| 5.4 Calcipotriol vs. calcipotriol + nicotinamide (0.05%, 0.1%, 0.7%, or 1.4%), twice daily | 1 | 192 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.14, 0.52] |
| 5.5 Calcipotriol vs. corticosteroid + salicylic acid | 2 | 360 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.26, 0.15] |
| 5.6 Calcipotriol vs. propylthiouracil cream | 1 | 27 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.24 [‐3.23, ‐1.25] |
| 5.7 Calcipotriol vs. tacrolimus ointment | 2 | 171 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐1.28, 0.17] |
| 5.8 Calcipotriol vs. tazarotene | 2 | 237 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.35, 0.16] |
| 5.9 Calcipotriol vs. tazarotene gel plus mometasone furoate cream | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.10 Calcipotriol vs. vitamin B12 cream | 1 | 26 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐1.33, 0.24] |
| 5.11 Head‐to‐head vitamin D alone or in combination: twice daily vs OD | 3 | 988 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.32, ‐0.07] |
| 5.12 Head‐to‐head vitamin D alone or in combination: no occlusion vs. occlusion | 2 | 247 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐2.04, 1.68] |
| 6 Total withdrawals | 15 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Calcipotriol vs. coal tar | 2 | 120 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.12, 0.08] |
| 6.2 Calcipotriol vs. coal tar polytherapy | 2 | 220 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.10, 0.04] |
| 6.3 Calcipotriol vs. nicotinamide 1.4%, twice daily | 1 | 96 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.05, 0.09] |
| 6.4 Calcipotriol vs. calcipotriol + nicotinamide (0.05%, 0.1%, 0.7%, or 1.4%), twice daily | 1 | 192 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.06, 0.07] |
| 6.5 Calcipotriol vs. corticosteroid + salicylic acid | 1 | 160 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.09, 0.17] |
| 6.6 Calcipotriol vs. propylthiouracil cream | 1 | 28 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [‐0.16, 0.30] |
| 6.7 Calcipotriol vs. tacrolimus ointment | 1 | 124 | Risk Difference (M‐H, Random, 95% CI) | ‐0.13 [‐0.25, ‐0.01] |
| 6.8 Calcipotriol vs. tazarotene | 2 | 254 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.10, 0.01] |
| 6.9 Calcipotriol vs. tazarotene gel plus mometasone furoate cream | 1 | 120 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.15, 0.08] |
| 6.10 Calcipotriol vs. vitamin B12 cream | 1 | 26 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.14, 0.14] |
| 6.11 Head‐to‐head vitamin D alone or in combination: twice daily vs OD | 3 | 1001 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.03, 0.05] |
| 6.12 Head‐to‐head vitamin D alone or in combination: no occlusion vs. occlusion | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Withdrawals due to adverse events | 15 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Calcipotriol vs. coal tar | 2 | 120 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.04, 0.06] |
| 7.2 Calcipotriol vs. coal tar polytherapy | 2 | 210 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.07, 0.03] |
| 7.3 Calcipotriol vs. nicotinamide 1.4%, twice daily | 1 | 96 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 7.4 Calcipotriol vs. calcipotriol + nicotinamide (0.05%, 0.1%, 0.7%, or 1.4%), twice daily | 1 | 192 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 7.5 Calcipotriol vs. corticosteroid + salicylic acid | 1 | 160 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [‐0.00, 0.10] |
| 7.6 Calcipotriol vs. propylthiouracil cream | 1 | 28 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [‐0.16, 0.30] |
| 7.7 Calcipotriol vs. tacrolimus ointment | 1 | 124 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.08, 0.11] |
| 7.8 Calcipotriol vs. tazarotene | 2 | 254 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.16, 0.05] |
| 7.9 Calcipotriol vs. tazarotene gel plus mometasone furoate cream | 1 | 120 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.10, 0.07] |
| 7.10 Calcipotriol vs. vitamin B12 cream | 1 | 26 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.14, 0.14] |
| 7.11 Head‐to‐head vitamin D alone or in combination: twice daily vs OD | 3 | 998 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.02] |
| 7.12 Head‐to‐head vitamin D alone or in combination: no occlusion vs. occlusion | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Withdrawals due to treatment failure | 12 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Calcipotriol vs. coal tar | 1 | 60 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.06, 0.06] |
| 8.2 Calcipotriol vs. coal tar polytherapy | 1 | 88 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.06, 0.07] |
| 8.3 Calcipotriol vs. nicotinamide 1.4%, twice daily | 1 | 96 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 8.4 Calcipotriol vs. calcipotriol + nicotinamide (0.05%, 0.1%, 0.7%, or 1.4%), twice daily | 1 | 192 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 8.5 Calcipotriol vs. corticosteroid + salicylic acid | 1 | 160 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.07, 0.02] |
| 8.6 Calcipotriol vs. propylthiouracil cream | 1 | 28 | Risk Difference (M‐H, Random, 95% CI) | ‐0.07 [‐0.25, 0.11] |
| 8.7 Calcipotriol vs. tacrolimus ointment | 1 | 124 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.07, 0.03] |
| 8.8 Calcipotriol vs. tazarotene | 1 | 208 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 8.9 Calcipotriol vs. tazarotene gel plus mometasone furoate cream | 1 | 120 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
| 8.10 Calcipotriol vs. vitamin B12 cream | 1 | 26 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.14, 0.14] |
| 8.11 Head‐to‐head vitamin D alone or in combination: twice daily vs OD | 3 | 998 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.01] |
| 8.12 Head‐to‐head vitamin D alone or in combination: no occlusion vs. occlusion | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Adverse events (local) | 11 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Calcipotriol vs. coal tar | 2 | 120 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.06, 0.10] |
| 9.2 Calcipotriol vs. coal tar polytherapy | 1 | 122 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [‐0.09, 0.20] |
| 9.3 Calcipotriol vs. nicotinamide 1.4%, twice daily | 1 | 96 | Risk Difference (M‐H, Random, 95% CI) | ‐0.15 [‐0.32, 0.03] |
| 9.4 Calcipotriol vs. calcipotriol + nicotinamide (0.05%, 0.1%, 0.7%, or 1.4%), twice daily | 1 | 192 | Risk Difference (M‐H, Random, 95% CI) | ‐0.17 [‐0.30, ‐0.03] |
| 9.5 Calcipotriol vs. corticosteroid + salicylic acid | 1 | 160 | Risk Difference (M‐H, Random, 95% CI) | 0.09 [0.02, 0.15] |
| 9.6 Calcipotriol vs. propylthiouracil cream | 1 | 28 | Risk Difference (M‐H, Random, 95% CI) | ‐0.07 [‐0.25, 0.11] |
| 9.7 Calcipotriol vs. tacrolimus ointment | 1 | 124 | Risk Difference (M‐H, Random, 95% CI) | ‐0.19 [‐0.37, ‐0.01] |
| 9.8 Calcipotriol vs. tazarotene | 1 | 204 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.11, 0.06] |
| 9.9 Calcipotriol vs. tazarotene gel plus mometasone furoate cream | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.10 Calcipotriol vs. vitamin B12 cream | 1 | 26 | Risk Difference (M‐H, Random, 95% CI) | 0.23 [‐0.06, 0.52] |
| 9.11 Head‐to‐head vitamin D alone or in combination: twice daily vs OD | 2 | 731 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.06, 0.05] |
| 9.12 Head‐to‐head vitamin D alone or in combination: no occlusion vs. occlusion | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Adverse events (systemic) | 8 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Calcipotriol vs. coal tar | 1 | 60 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.09, 0.09] |
| 10.2 Calcipotriol vs. coal tar polytherapy | 1 | 88 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 10.3 Calcipotriol vs. nicotinamide 1.4%, twice daily | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.4 Calcipotriol vs. calcipotriol + nicotinamide 1.4%, twice daily | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.5 Calcipotriol vs. corticosteroid + salicylic acid | 1 | 160 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 10.6 Calcipotriol vs. propylthiouracil cream | 1 | 28 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.13, 0.13] |
| 10.7 Calcipotriol vs. tacrolimus ointment | 1 | 124 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 10.8 Calcipotriol vs. tazarotene | 1 | 183 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.05, 0.03] |
| 10.9 Calcipotriol vs. tazarotene gel plus mometasone furoate cream | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.10 Calcipotriol vs. vitamin B12 cream | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.11 Head‐to‐head vitamin D alone or in combination: twice daily vs OD | 1 | 264 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 10.12 Head‐to‐head vitamin D alone or in combination: no occlusion vs. occlusion | 1 | 38 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.10, 0.10] |
Comparison 16. Flexural/facial psoriasis: placebo‐controlled trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 IAGI | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Betamethasone valerate 0.1%, OD | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Calcipotriol ointment, OD | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Pimecrolimus cream, 1% OD/twice daily | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Tacrolimus ointment 0.1%, twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 TSS | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Betamethasone valerate 0.1%, OD | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Calcipotriol ointment, OD | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Pimecrolimus cream, 1% OD/twice daily | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Tacrolimus ointment 0.1%, twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 PASI | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Betamethasone valerate 0.1%, OD | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Calcipotriol ointment, OD | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Pimecrolimus cream, 1% OD/twice daily | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Tacrolimus ointment 0.1%, twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 PAGI | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Betamethasone valerate 0.1%, OD | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Calcipotriol ointment, OD | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Pimecrolimus cream, 1% OD/twice daily | 1 | 47 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.24, ‐0.06] |
| 4.4 Tacrolimus ointment 0.1%, twice daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Betamethasone valerate 0.1%, OD | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.83 [‐3.79, ‐1.88] |
| 5.2 Calcipotriol ointment, OD | 1 | 38 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.08 [‐1.77, ‐0.40] |
| 5.3 Pimecrolimus cream, 1% OD/twice daily | 2 | 86 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.30, ‐0.41] |
| 5.4 Tacrolimus ointment 0.1%, twice daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Total withdrawals | 3 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Betamethasone valerate 0.1%, OD | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | 0.10 [‐0.08, 0.28] |
| 6.2 Calcipotriol ointment, OD | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.14, 0.14] |
| 6.3 Pimecrolimus cream, 1% OD/twice daily | 2 | 97 | Risk Difference (M‐H, Random, 95% CI) | ‐0.06 [‐0.16, 0.04] |
| 6.4 Tacrolimus ointment 0.1%, twice daily | 1 | 167 | Risk Difference (M‐H, Random, 95% CI) | ‐0.17 [‐0.30, ‐0.03] |
| 7 Withdrawals due to adverse events | 3 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Betamethasone valerate 0.1%, OD | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.09, 0.09] |
| 7.2 Calcipotriol ointment, OD | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.09, 0.09] |
| 7.3 Pimecrolimus cream, 1% OD/twice daily | 2 | 97 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
| 7.4 Tacrolimus ointment 0.1%, twice daily | 1 | 167 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.06, 0.03] |
| 8 Withdrawals due to treatment failure | 3 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Betamethasone valerate 0.1%, OD | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.18, 0.08] |
| 8.2 Calcipotriol ointment, OD | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.18, 0.08] |
| 8.3 Pimecrolimus cream, 1% OD/twice daily | 2 | 97 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.13, 0.04] |
| 8.4 Tacrolimus ointment 0.1%, twice daily | 1 | 167 | Risk Difference (M‐H, Random, 95% CI) | ‐0.11 [‐0.19, ‐0.02] |
| 9 Adverse events (local) | 3 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Betamethasone valerate 0.1%, OD | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.18, 0.08] |
| 9.2 Calcipotriol ointment, OD | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [‐0.11, 0.21] |
| 9.3 Pimecrolimus cream 1%, OD/twice daily | 2 | 97 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [‐0.15, 0.31] |
| 9.4 Tacrolimus ointment 0.1%, twice daily | 1 | 167 | Risk Difference (M‐H, Random, 95% CI) | ‐0.17 [‐0.30, ‐0.03] |
| 10 Adverse events (systemic) | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 10.1 Betamethasone valerate 0.1%, OD | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Calcipotriol ointment, OD | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 Pimecrolimus cream 1%, OD/twice daily | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.4 Tacrolimus ointment 0.1%, twice daily | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 17. Flexural/facial psoriasis: vitamin D alone or in combination versus other treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 IAGI | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Calcipotriol vs. BMV | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Calcipotriol vs. calcipotriol + hydrocortisone | 1 | 408 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [0.11, 0.50] |
| 1.3 Calcipotriol vs. calcitriol | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Calcipotriol vs. pimecrolimus | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 Calcitriol vs. tacrolimus | 1 | 49 | Std. Mean Difference (IV, Random, 95% CI) | 0.42 [‐0.15, 0.98] |
| 2 TSS | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Calcipotriol vs. BMV | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Calcipotriol vs. calcipotriol + hydrocortisone | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Calcipotriol vs. calcitriol | 1 | 150 | Std. Mean Difference (IV, Random, 95% CI) | 0.61 [0.28, 0.94] |
| 2.4 Calcipotriol vs. pimecrolimus | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 Calcitriol vs. tacrolimus | 1 | 49 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.27, 0.85] |
| 3 PASI | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Calcipotriol vs. BMV | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | 2.02 [1.20, 2.84] |
| 3.2 Calcipotriol vs. calcipotriol + hydrocortisone | 1 | 408 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [0.12, 0.51] |
| 3.3 Calcipotriol vs. calcitriol | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Calcipotriol vs. pimecrolimus | 1 | 39 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐1.17, 0.11] |
| 3.5 Calcitriol vs. tacrolimus | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 PAGI | 0 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Calcipotriol vs. BMV | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Calcipotriol vs. calcipotriol + hydrocortisone | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Calcipotriol vs. calcitriol | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Calcipotriol vs. pimecrolimus | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 Calcitriol vs. tacrolimus | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Calcipotriol vs. BMV | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | 2.02 [1.20, 2.84] |
| 5.2 Calcipotriol vs. calcipotriol + hydrocortisone | 1 | 408 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [0.11, 0.50] |
| 5.3 Calcipotriol vs. calcitriol | 1 | 150 | Std. Mean Difference (IV, Random, 95% CI) | 0.61 [0.28, 0.94] |
| 5.4 Calcipotriol vs. pimecrolimus | 1 | 39 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐1.17, 0.11] |
| 5.5 Calcitriol vs. tacrolimus | 1 | 49 | Std. Mean Difference (IV, Random, 95% CI) | 0.42 [‐0.15, 0.98] |
| 6 Total withdrawals | 4 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Calcipotriol vs. BMV | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | ‐0.10 [‐0.28, 0.08] |
| 6.2 Calcipotriol vs. calcipotriol + hydrocortisone | 1 | 408 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.03, 0.11] |
| 6.3 Calcipotriol vs. calcitriol | 1 | 150 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.11, 0.11] |
| 6.4 Calcipotriol vs. pimecrolimus | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [‐0.08, 0.18] |
| 6.5 Calcitriol vs. tacrolimus | 1 | 50 | Risk Difference (M‐H, Random, 95% CI) | 0.12 [‐0.02, 0.26] |
| 7 Withdrawals due to adverse events | 4 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Calcipotriol vs. BMV | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.09, 0.09] |
| 7.2 Calcipotriol vs. calcipotriol + hydrocortisone | 1 | 408 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [0.02, 0.11] |
| 7.3 Calcipotriol vs. calcitriol | 1 | 150 | Risk Difference (M‐H, Random, 95% CI) | 0.09 [0.01, 0.18] |
| 7.4 Calcipotriol vs. pimecrolimus | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.09, 0.09] |
| 7.5 Calcitriol vs. tacrolimus | 1 | 50 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.07, 0.07] |
| 8 Withdrawals due to treatment failure | 4 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Calcipotriol vs. BMV | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.09, 0.09] |
| 8.2 Calcipotriol vs. calcipotriol + hydrocortisone | 1 | 408 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.01, 0.05] |
| 8.3 Calcipotriol vs. calcitriol | 1 | 150 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 8.4 Calcipotriol vs. pimecrolimus | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.09, 0.09] |
| 8.5 Calcitriol vs. tacrolimus | 1 | 50 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.07, 0.07] |
| 9 Adverse events (local) | 3 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 calcipotriol vs. BMV | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | 0.10 [‐0.05, 0.25] |
| 9.2 Calcipotriol vs. calcipotriol + hydrocortisone | 1 | 404 | Risk Difference (M‐H, Random, 95% CI) | 0.15 [0.08, 0.23] |
| 9.3 Calcipotriol vs. calcitriol | 1 | 150 | Risk Difference (M‐H, Random, 95% CI) | 0.09 [0.02, 0.17] |
| 9.4 Calcipotriol vs. pimecrolimus | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | ‐0.15 [‐0.38, 0.08] |
| 9.5 Calcitriol vs. tacrolimus | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Adverse events (systemic) | 0 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Calcipotriol vs. BMV | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Calcipotriol vs. calcipotriol + hydrocortisone | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Calcipotriol vs. calcitriol | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.4 Calcipotriol vs. pimecrolimus | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.5 Calcitriol vs. tacrolimus | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 18. Scalp psoriasis: placebo‐controlled trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 IAGI | 9 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Vitamin D: calcipotriol | 2 | 457 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.28, ‐0.16] |
| 1.2 Potent steroid: betamethasone dipropionate | 2 | 712 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐1.29, ‐0.90] |
| 1.3 Potent steroid: betamethasone valerate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Very potent steroid: amcinonide | 1 | 132 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.42 [‐1.80, ‐1.04] |
| 1.5 Very potent steroid: clobetasol propionate | 2 | 458 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.73 [‐1.99, ‐1.48] |
| 1.6 Very potent steroid: halcinonide | 1 | 54 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.11 [‐1.69, ‐0.53] |
| 1.7 Vitamin D in combination: calcipotriol + BMD | 2 | 854 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐1.61, ‐0.32] |
| 1.8 Other treatment: betamethasone‐17,21‐dipropionate plus salicylic acid | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.48 [‐2.50, ‐0.47] |
| 1.9 Other treatment: ciclopirox olamine shampoo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.10 Other treatment: fluocinolone acetonide, plus occlusion | 1 | 84 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐1.69, ‐0.76] |
| 1.11 Other treatment: salicylic acid | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.79, 0.06] |
| 2 TSS | 11 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Vitamin D: calcipotriol | 2 | 457 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.64, ‐0.25] |
| 2.2 Potent steroid: betamethasone dipropionate | 2 | 712 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐1.19, ‐0.81] |
| 2.3 Potent steroid: betamethasone valerate | 1 | 172 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐1.75, ‐1.05] |
| 2.4 Very potent steroid: amcinonide | 1 | 126 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.58 [‐1.98, ‐1.18] |
| 2.5 Very potent steroid: clobetasol propionate | 3 | 707 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.53 [‐1.77, ‐1.28] |
| 2.6 Very potent steroid: halcinonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.7 Vitamin D in combination: calcipotriol + BMD | 2 | 854 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐1.42, ‐0.43] |
| 2.8 Other treatment: betamethasone‐17,21‐dipropionate plus salicylic acid | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.15 [‐2.11, ‐0.19] |
| 2.9 Other treatment: ciclopirox olamine shampoo | 1 | 37 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.82, 0.68] |
| 2.10 Other treatment: fluocinolone acetonide, plus occlusion | 1 | 84 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.34, ‐0.44] |
| 2.11 Other treatment: salicylic acid | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐1.47, 0.32] |
| 3 PASI | 0 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Vitamin D: calcipotriol | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Potent steroid: betamethasone dipropionate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Potent steroid: betamethasone valerate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Very potent steroid: amcinonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.5 Very potent steroid: clobetasol propionate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.6 Very potent steroid: halcinonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.7 Vitamin D in combination: calcipotriol + BMD | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.8 Other treatment: betamethasone‐17,21‐dipropionate plus salicylic acid | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.9 Other treatment: ciclopirox olamine shampoo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.10 Other treatment: fluocinolone acetonide, plus occlusion | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.11 Other treatment: salicylic acid | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 PAGI | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Vitamin D: calcipotriol | 2 | 450 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.28, ‐0.05] |
| 4.2 Potent steroid: betamethasone dipropionate | 1 | 685 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.23 [‐1.43, ‐1.03] |
| 4.3 Potent steroid: betamethasone valerate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Very potent steroid: amcinonide | 1 | 132 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐1.33, ‐0.61] |
| 4.5 Very potent steroid: clobetasol propionate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 Very potent steroid: halcinonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.7 Vitamin D in combination: calcipotriol + BMD | 2 | 841 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐1.79, ‐0.22] |
| 4.8 Other treatment: betamethasone‐17,21‐dipropionate plus salicylic acid | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.9 Other treatment: ciclopirox olamine shampoo | 1 | 37 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.86, 0.64] |
| 4.10 Other treatment: fluocinolone acetonide, plus occlusion | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.11 Other treatment: salicylic acid | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) | 13 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Vitamin D: calcipotriol | 2 | 457 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.28, ‐0.16] |
| 5.2 Potent steroid: betamethasone dipropionate | 2 | 712 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐1.29, ‐0.90] |
| 5.3 Potent steroid: betamethasone valerate | 1 | 172 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐1.75, ‐1.05] |
| 5.4 Very potent steroid: amcinonide | 1 | 132 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.42 [‐1.80, ‐1.04] |
| 5.5 Very potent steroid: clobetasol propionate | 4 | 788 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.57 [‐1.81, ‐1.34] |
| 5.6 Very potent steroid: halcinonide | 1 | 54 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.11 [‐1.69, ‐0.53] |
| 5.7 Vitamin D in combination: calcipotriol + BMD | 2 | 854 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐1.61, ‐0.32] |
| 5.8 Other treatment: betamethasone‐17,21‐dipropionate plus salicylic acid | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.48 [‐2.50, ‐0.47] |
| 5.9 Other treatment: ciclopirox olamine shampoo | 1 | 37 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.82, 0.68] |
| 5.10 Other treatment: fluocinolone acetonide, plus occlusion | 1 | 84 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐1.69, ‐0.76] |
| 5.11 Other treatment: salicylic acid | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.79, 0.06] |
| 6 Total withdrawals | 13 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Vitamin D: calcipotriol | 3 | 517 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.08, 0.05] |
| 6.2 Potent steroid: betamethasone dipropionate | 1 | 692 | Risk Difference (M‐H, Random, 95% CI) | ‐0.14 [‐0.21, ‐0.06] |
| 6.3 Potent steroid: betamethasone valerate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 Very potent steroid: amcinonide | 1 | 165 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.11, 0.11] |
| 6.5 Very potent steroid: clobetasol propionate | 5 | 1006 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.10, 0.04] |
| 6.6 Very potent steroid: halcinonide | 1 | 58 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.13, 0.13] |
| 6.7 Vitamin D in combination: calcipotriol + BMD | 2 | 854 | Risk Difference (M‐H, Random, 95% CI) | ‐0.09 [‐0.16, ‐0.03] |
| 6.8 Other treatment: betamethasone‐17,21‐dipropionate plus salicylic acid | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.9 Other treatment: ciclopirox olamine shampoo | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | ‐0.15 [‐0.38, 0.09] |
| 6.10 Other treatment: fluocinolone acetonide, plus occlusion | 1 | 89 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.13, 0.04] |
| 6.11 Other treatment: salicylic acid | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Withdrawals due to adverse events | 13 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Vitamin D: calcipotriol | 3 | 517 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.02, 0.05] |
| 7.2 Potent steroid: betamethasone dipropionate | 2 | 712 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.08, ‐0.00] |
| 7.3 Potent steroid: betamethasone valerate | 1 | 172 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 7.4 Very potent steroid: amcinonide | 1 | 165 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.02, 0.04] |
| 7.5 Very potent steroid: clobetasol propionate | 5 | 1006 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 7.6 Very potent steroid: halcinonide | 1 | 58 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.06, 0.06] |
| 7.7 Vitamin D in combination: calcipotriol + BMD | 1 | 677 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.08, 0.00] |
| 7.8 Other treatment: betamethasone‐17,21‐dipropionate plus salicylic acid | 1 | 20 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.17, 0.17] |
| 7.9 Other treatment: ciclopirox olamine shampoo | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | ‐0.18 [‐0.42, 0.05] |
| 7.10 Other treatment: fluocinolone acetonide, plus occlusion | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.11 Other treatment: salicylic acid | 1 | 20 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.17, 0.17] |
| 8 Withdrawals due to treatment failure | 9 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Vitamin D: calcipotriol | 2 | 457 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.11, 0.00] |
| 8.2 Potent steroid: betamethasone dipropionate | 1 | 692 | Risk Difference (M‐H, Random, 95% CI) | ‐0.10 [‐0.16, ‐0.05] |
| 8.3 Potent steroid: betamethasone valerate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.4 Very potent steroid: amcinonide | 1 | 165 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.05, 0.02] |
| 8.5 Very potent steroid: clobetasol propionate | 5 | 1006 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.05, 0.02] |
| 8.6 Very potent steroid: halcinonide | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.7 Vitamin D in combination: calcipotriol + BMD | 1 | 677 | Risk Difference (M‐H, Random, 95% CI) | ‐0.11 [‐0.17, ‐0.06] |
| 8.8 Other treatment: betamethasone‐17,21‐dipropionate plus salicylic acid | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.9 Other treatment: ciclopirox olamine shampoo | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | ‐0.09 [‐0.28, 0.10] |
| 8.10 Other treatment: fluocinolone acetonide, plus occlusion | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.11 Other treatment: salicylic acid | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Adverse events (local) | 12 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Vitamin D: calcipotriol | 3 | 510 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.05, 0.04] |
| 9.2 Potent steroid: betamethasone dipropionate | 2 | 703 | Risk Difference (M‐H, Random, 95% CI) | ‐0.07 [‐0.13, ‐0.01] |
| 9.3 Potent steroid: betamethasone valerate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.4 Very potent steroid: amcinonide | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.5 Very potent steroid: clobetasol propionate | 4 | 817 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.03, 0.04] |
| 9.6 Very potent steroid: halcinonide | 1 | 58 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.12, 0.06] |
| 9.7 Vitamin D in combination: calcipotriol + BMD | 2 | 831 | Risk Difference (M‐H, Random, 95% CI) | ‐0.06 [‐0.13, 0.02] |
| 9.8 Other treatment: betamethasone‐17,21‐dipropionate plus salicylic acid | 1 | 20 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.17, 0.17] |
| 9.9 Other treatment: ciclopirox olamine shampoo | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | ‐0.06 [‐0.24, 0.13] |
| 9.10 Other treatment: fluocinolone acetonide, plus occlusion | 1 | 89 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.04, 0.08] |
| 9.11 Other treatment: salicylic acid | 1 | 20 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.17, 0.17] |
| 10 Adverse events (systemic) | 4 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Vitamin D: calcipotriol | 1 | 408 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 10.2 Potent steroid: betamethasone dipropionate | 1 | 692 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 10.3 Potent steroid: betamethasone valerate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.4 Very potent steroid: amcinonide | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.5 Very potent steroid: clobetasol propionate | 2 | 385 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.03, 0.02] |
| 10.6 Very potent steroid: halcinonide | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.7 Vitamin D in combination: calcipotriol + BMD | 2 | 843 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 10.8 Other treatment: betamethasone‐17,21‐dipropionate plus salicylic acid | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.9 Other treatment: ciclopirox olamine shampoo | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.10 Other treatment: fluocinolone acetonide, plus occlusion | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.11 Other treatment: salicylic acid | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 19. Scalp psoriasis: vitamin D alone or in combination versus other treatments.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 IAGI | 9 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMD | 2 | 1676 | Std. Mean Difference (IV, Random, 95% CI) | 0.48 [0.32, 0.64] |
| 1.2 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMV | 2 | 510 | Std. Mean Difference (IV, Random, 95% CI) | 0.37 [0.20, 0.55] |
| 1.3 Vitamin D vs. corticosteroid (very potent): calcipotriol vs. clobetasol propionate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Vitamin D + corticosteroid vs. corticosteroid: calcipotriol + BMD vs. BMD | 3 | 2444 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.26, ‐0.10] |
| 1.5 Vitamin D vs. vitamin D + corticosteroid: calcipotriol vs. calcipotriol + BMD | 4 | 2581 | Std. Mean Difference (IV, Random, 95% CI) | 0.64 [0.44, 0.84] |
| 1.6 Vitamin D vs. other treatments: calcipotriol vs. coal tar polytherapy | 2 | 748 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.73, 0.25] |
| 2 TSS | 10 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMD | 2 | 1676 | Std. Mean Difference (IV, Random, 95% CI) | 0.45 [0.28, 0.63] |
| 2.2 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMV | 2 | 487 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.09, 0.27] |
| 2.3 Vitamin D vs. corticosteroid (very potent): calcipotriol vs. clobetasol propionate | 1 | 151 | Std. Mean Difference (IV, Random, 95% CI) | 0.37 [0.05, 0.69] |
| 2.4 Vitamin D + corticosteroid vs. corticosteroid: calcipotriol + BMD vs. BMD | 3 | 2444 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.27, ‐0.11] |
| 2.5 Vitamin D vs. vitamin D + corticosteroid: calcipotriol vs. calcipotriol + BMD | 3 | 1978 | Std. Mean Difference (IV, Random, 95% CI) | 0.70 [0.56, 0.84] |
| 2.6 Vitamin D vs. other treatments: calcipotriol vs. coal tar polytherapy | 3 | 925 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.84, 0.24] |
| 3 PASI | 0 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMD | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMV | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Vitamin D vs. corticosteroid (very potent): calcipotriol vs. clobetasol propionate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Vitamin D + corticosteroid vs. corticosteroid: calcipotriol + BMD vs. BMD | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.5 Vitamin D vs. vitamin D + corticosteroid: calcipotriol vs. calcipotriol + BMD | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.6 Vitamin D vs. other treatments: calcipotriol vs. coal tar polytherapy | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 PAGI | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMD | 2 | 1654 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [0.31, 0.81] |
| 4.2 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMV | 1 | 468 | Std. Mean Difference (IV, Random, 95% CI) | 0.41 [0.22, 0.59] |
| 4.3 Vitamin D vs. corticosteroid (very potent): calcipotriol vs. clobetasol propionate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Vitamin D + corticosteroid vs. corticosteroid: calcipotriol + BMD vs. BMD | 3 | 2414 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.25, ‐0.09] |
| 4.5 Vitamin D vs. vitamin D + corticosteroid: calcipotriol vs. calcipotriol + BMD | 3 | 1952 | Std. Mean Difference (IV, Random, 95% CI) | 0.84 [0.61, 1.08] |
| 4.6 Vitamin D vs. other treatments: calcipotriol vs. coal tar polytherapy | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) | 11 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMD | 2 | 1676 | Std. Mean Difference (IV, Random, 95% CI) | 0.48 [0.32, 0.64] |
| 5.2 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMV | 2 | 510 | Std. Mean Difference (IV, Random, 95% CI) | 0.37 [0.20, 0.55] |
| 5.3 Vitamin D vs. corticosteroid (very potent): calcipotriol vs. clobetasol propionate | 1 | 151 | Std. Mean Difference (IV, Random, 95% CI) | 0.37 [0.05, 0.69] |
| 5.4 Vitamin D + corticosteroid vs. corticosteroid: calcipotriol + BMD vs. BMD | 3 | 2444 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.26, ‐0.10] |
| 5.5 Vitamin D vs. vitamin D + corticosteroid: calcipotriol vs. calcipotriol + BMD | 4 | 2581 | Std. Mean Difference (IV, Random, 95% CI) | 0.64 [0.44, 0.84] |
| 5.6 Vitamin D vs. other treatments: calcipotriol vs. coal tar polytherapy | 3 | 835 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.92, 0.02] |
| 6 Total withdrawals | 10 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMD | 2 | 1676 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [‐0.04, 0.18] |
| 6.2 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMV | 2 | 516 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.00, 0.08] |
| 6.3 Vitamin D vs. corticosteroid (very potent): calcipotriol vs. clobetasol propionate | 2 | 194 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [‐0.07, 0.18] |
| 6.4 Vitamin D + corticosteroid vs. corticosteroid: calcipotriol + BMD vs. BMD | 3 | 2444 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.04, 0.06] |
| 6.5 Vitamin D vs. vitamin D + corticosteroid: calcipotriol vs. calcipotriol + BMD | 4 | 2847 | Risk Difference (M‐H, Random, 95% CI) | 0.11 [0.05, 0.18] |
| 6.6 Vitamin D vs. other treatments: calcipotriol vs. coal tar polytherapy | 1 | 475 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.07, 0.09] |
| 7 Withdrawals due to adverse events | 10 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMD | 2 | 1676 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.01, 0.09] |
| 7.2 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMV | 2 | 516 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [0.01, 0.06] |
| 7.3 Vitamin D vs. corticosteroid (very potent): calcipotriol vs. clobetasol propionate | 2 | 194 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [‐0.05, 0.15] |
| 7.4 Vitamin D + corticosteroid vs. corticosteroid: calcipotriol + BMD vs. BMD | 3 | 2444 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.01, 0.01] |
| 7.5 Vitamin D vs. vitamin D + corticosteroid: calcipotriol vs. calcipotriol + BMD | 4 | 2847 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [0.02, 0.09] |
| 7.6 Vitamin D vs. other treatments: calcipotriol vs. coal tar polytherapy | 1 | 445 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [0.02, 0.14] |
| 8 Withdrawals due to treatment failure | 8 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMD | 2 | 1676 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.01, 0.07] |
| 8.2 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMV | 2 | 516 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.03] |
| 8.3 Vitamin D vs. corticosteroid (very potent): calcipotriol vs. clobetasol propionate | 2 | 194 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.02, 0.04] |
| 8.4 Vitamin D + corticosteroid vs. corticosteroid: calcipotriol + BMD vs. BMD | 3 | 2444 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.02, ‐0.00] |
| 8.5 Vitamin D vs. vitamin D + corticosteroid: calcipotriol vs. calcipotriol + BMD | 3 | 2535 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [0.01, 0.10] |
| 8.6 Vitamin D vs. other treatments: calcipotriol vs. coal tar polytherapy | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Adverse events (local) | 10 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMD | 2 | 1652 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [0.04, 0.11] |
| 9.2 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMV | 2 | 516 | Risk Difference (M‐H, Random, 95% CI) | 0.17 [0.01, 0.33] |
| 9.3 Vitamin D vs. corticosteroid (very potent): calcipotriol vs. clobetasol propionate | 2 | 194 | Risk Difference (M‐H, Random, 95% CI) | 0.19 [0.10, 0.28] |
| 9.4 Vitamin D + corticosteroid vs. corticosteroid: calcipotriol + BMD vs. BMD | 3 | 2415 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 9.5 Vitamin D vs. vitamin D + corticosteroid: calcipotriol vs. calcipotriol + BMD | 4 | 2801 | Risk Difference (M‐H, Random, 95% CI) | 0.09 [0.06, 0.12] |
| 9.6 Vitamin D vs. other treatments: calcipotriol vs. coal tar polytherapy | 1 | 445 | Risk Difference (M‐H, Random, 95% CI) | 0.24 [0.15, 0.33] |
| 10 Adverse events (systemic) | 6 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMD | 2 | 1666 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.00, 0.00] |
| 10.2 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMV | 1 | 474 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 10.3 Vitamin D vs. corticosteroid (very potent): calcipotriol vs. clobetasol propionate | 1 | 151 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 10.4 Vitamin D + corticosteroid vs. corticosteroid: calcipotriol + BMD vs. BMD | 2 | 2216 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.00, 0.00] |
| 10.5 Vitamin D vs. vitamin D + corticosteroid: calcipotriol vs. calcipotriol + BMD | 3 | 1970 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.00, 0.00] |
| 10.6 Vitamin D vs. other treatments: calcipotriol vs. coal tar polytherapy | 1 | 445 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agrup 1981.
| Methods |
DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: unclear Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described |
|
| Participants | N: 11 Treatment duration: 3 wks; FU: 3 wks LF: 0 (0%) BC: not reported Age: not reported Gender (per cent men): not reported Severity: not reported INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Unclear risk | The trial did not report this. |
Alora‐Palli 2010.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: computer‐generated list Concealment: unclear BLINDING Single‐blind (investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 60 Treatment duration: 12 wks; FU: 18 wks LF: 5 (8.3%) BC: Yes Age: 48.5 (15.4SD); range = 19 to 77 Gender (per cent men): 56.7% Severity: mPASI = 7.09 (3.14SD); PGA = 3.05 Duration (yrs): 16.5 (13.0SD); range = 1 to 62 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | NeoStrata company, Inc. sponsored the trial. There was significant improvement in DLQI scores relative to baseline in both groups. Compliance: 96% of participants in both groups reported they applied the study medication twice daily on most days. Recurrence rates (loss of PASI50 response): C: 7/9; T: 4/16 Recurrence rates (PGA): C: 14/20; T: 5/22 The trial author supplied unpublished data. There was SD imputation (TSS). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The study was single‐blind (investigator). |
| Randomisation method reported | Low risk | A computer‐generated list was used for randomisation. |
| Loss to follow up | Low risk | 8.3% |
| Baseline assessments | Low risk | The trial reported demographic and clinical characteristics. |
| Baseline comparability demonstrated | Low risk | This was demonstrated. |
Austad 1998.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 49 Treatment duration: 6 wks; FU: 10 wks LF: 3 (6.1%) BC: yes Age: 42.4 (13.9SD; range = 18 to 68) Gender (per cent men): 63% Severity: TSS (0 to 9) = 6.4 (0.5SD; range = 6.0 to 8.0) Duration: 15.6 (12.3SD; range = 1 to 57) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Glaxo Wellcome Research and Development, Norway, sponsored the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | High risk | The trial did not report this. |
| Loss to follow up | Low risk | 6.1% |
| Baseline assessments | Low risk | The trial did not report these. |
| Baseline comparability demonstrated | Low risk | ‐ |
Baiocchi 1997.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: block randomisation (4 participants) Concealment: unclear BLINDING Open WITHDRAWAL/DROPOUT Described | |
| Participants | N: 132 Treatment duration: 8 wks; FU: 8 wks LF: 2 (1.5%) BC: yes Age: 46.8 (15.2SD; range = 18 to 89) Gender (per cent men): 67.4% Severity: PASI = 4.4 (2.1SD) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report on sponsorship. All participants had a bath with salicylic acid 3 to 4 days before starting study treatments. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was open. |
| Randomisation method reported | Low risk | Block randomisation was used. |
| Loss to follow up | Low risk | 1.5% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | ‐ |
Barker 1999 (H).
| Methods | DESIGN Within‐patient Participant delivery Method of randomisation: unclear Concealment: unclear BLINDING Double‐blind (participant/assessor) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 30 Treatment duration: 8 wks; FU: 8 wks LF: 4 (13.3%) BC: demographics similar; clinical characteristics not reported Age: 47.2 (14.5SD, N = 144) (range = 20 to 75) Gender (per cent men): 59.7% (86/144) Severity: not reported INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions | Dose‐ranging study including placebo; calcipotriol 50 mcg/g; maxacalcitol 6, 12.5, 25, and 50 mcg/g OD. Contrast included the following:
|
|
| Outcomes |
|
|
| Notes | Non‐target plaques received emollient or coal tar throughout. Chugai Pharma Europe sponsored the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/assessor). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 13.3% |
| Baseline assessments | Low risk | These were partially reported (demographics only). |
| Baseline comparability demonstrated | Unclear risk | This was partially demonstrated. |
Barker 1999 (P).
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: unclear Concealment: unclear BLINDING Double‐blind (unclear) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 60 Treatment duration: 8 wks; FU: 8 wks LF: 6 (10.0%) BC: demographics similar; clinical characteristics not reported Age: 47.2 (14.5SD, N = 144) (range = 20 to 75) Gender (per cent men): 59.7% (86/144) Severity: not reported INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions | Dose‐ranging study including placebo; calcipotriol 50 mcg/g; maxacalcitol ointment 6, 12.5, 25, and 50 mcg/g OD. Contrast included the following:
|
|
| Outcomes |
|
|
| Notes | Non‐target plaques received emollient or coal tar throughout the study. Chugai Pharma Europe sponsored the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/assessor). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 10.0% |
| Baseline assessments | Low risk | These were partially reported (demographics only). |
| Baseline comparability demonstrated | Unclear risk | These were partially done. |
Barrett 2005.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Open WITHDRAWAL/DROPOUT Not described | |
| Participants | N: 420 Treatment duration: 8 wks; FU: 8 wks LF: unclear BC: not reported Age: not reported Gender (per cent men): not reported Severity: not reported INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
At visit 1, all participants were treated with calcipotriol scalp solution twice daily. Participants were randomly assigned to treatment with either twice‐weekly Polytar® liquid or a non‐medicated shampoo twice weekly. |
|
| Outcomes |
|
|
| Notes | Leo Pharmaceuticals sponsored the trial. The sponsor supplied unpublished outcomes data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial did not report this. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was open (impossible to blind participants when tar‐based products are used). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Unclear risk | The trial did not report this. |
| Baseline assessments | Unclear risk | The trial did not report these. |
| Baseline comparability demonstrated | Unclear risk | The trial did not report this. |
Bernhard 1991 (1).
| Methods | DESIGN Within‐patient Delivery unclear ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 100 Treatment duration: 2 wks; FU: 2 wks LF: 4 (4%) BC: yes Age: 49 (range = 20 to 77) Gender (per cent men): 61.5% Severity: at least 2 signs or symptoms ≥ 2 on a 4‐pt scale Duration (yrs): 18.2 (range = 1 to 53) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Westwood‐Squibb Pharmaceuticals (BMS) sponsored the trial with an educational grant. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 4.0% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | ‐ |
Bernhard 1991(2).
| Methods | DESIGN Between‐patient Delivery unclear ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 72 Treatment duration: 2 wks; FU: 2 wks LF: 0 (0%) BC: yes (demographics); clinical comparability unclear Age: 53 (range = 23 to 86) Gender (per cent men): 52.8% Severity: signs > = 4 on a 7‐pt scale; BSA = 1% to 20% Duration: 22.7 (range = 1 to 62) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Westwood‐Squibb Pharmaceuticals (BMS) sponsored the trial with an educational grant. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Unclear risk | This was partially demonstrated. |
Bernstein 2006.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 200 Treatment duration: 12 wks; FU: 12 wks LF: 29 (14.5%) BC: yes Age: 48.3 (13.9SD) Gender (per cent men): 46.5% Severity: PASI (0 to 12) = 6.89 (2.8SD); QLI (0 to 120) = 58.74 (31.5SD) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Apollo Pharmaceuticals sponsored the trial. 'PASI' is similar to TSS (range = 0 to 12) as it examines small BSA. However, the PASI score was also adjusted by area. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 14.5% |
| Baseline assessments | Low risk | The trial reported this. |
| Baseline comparability demonstrated | Low risk | ‐ |
Berth Jones 1992b.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: balanced blocks of 4 using computer‐generated random numbers Concealment: unclear BLINDING Open WITHDRAWAL/DROPOUT Described | |
| Participants | N: 478 Treatment duration: 8 wks; FU: 8 wks LF: for PASI: 56 (11.7%); for Response: 20 (4.2%) BC: yes Age: 44 (range = 18 to 85) Gender (per cent men): 55% Severity: PASI = 9.3 Duration (yrs): 18 (12SD) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Leo Pharmaceutical Products, Denmark, sponsored the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was open. |
| Randomisation method reported | Low risk | A computer‐generated block list was used for randomisation. |
| Loss to follow up | Low risk | 11.7% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | ‐ |
Beutner 2006.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 27 Treatment duration: 4 wks; FU: 4 wks LF: 2 (7.4%) BC: yes Age: 51.6 (12.8SD); range = 21 to 75 Gender (per cent men): 67% Ethnicity (% white): 85% Severity: overall target severity score (0 to 4) = 2.67 (0.58SD) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Galderma Laboratories, L.P. sponsored the study. There was SD imputation (TSS). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 7.4% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | ‐ |
Bourke 1993b.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: participants were randomised into groups A and B, then randomised to left/right application with sealed envelopes Concealment: unclear BLINDING Single‐blind (investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 19 (evaluable) Treatment duration: 8 wks; FU: 8 wks LF: NR BC: yes (clinical only) Age: not reported Gender (per cent men): not reported Severity: TSS = 7.9 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Participants were randomised into groups A (calcipotriol BD) and B (occlusion ON), then each participant was randomised to left/right application: group A (occlusion ON/no occlusion); group B (calcipotriol BD or placebo BD). The study reported findings for group A. The trial did not report sponsorship. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was single‐blind (investigator). |
| Randomisation method reported | Low risk | Envelopes were used. |
| Loss to follow up | Unclear risk | The trial did not report this. |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Unclear risk | This was partially demonstrated. |
Bourke 1997.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/assessor) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 24 Treatment duration: 8 wks LF: 4 (16.7%) BC: yes (clinical only reported) Age: not reported Gender (per cent men): 41.7% Severity: PASI mean = 14.0 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Solvay‐Duphar Ltd sponsored the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/assessor). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 16.7% |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Unclear risk | This was partially demonstrated. |
Brown 2005.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 30 Treatment duration: 12 wks; FU: 12 wks LF: 6 (20%) BC: unclear Age: 55 (12.6SD); range = 20 to 75 Gender (per cent men): 50% Ethnicity (% white): 46.7% Severity: TSS (0 to 16) mean = 6.97 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Hawaii Community Foundation sponsored the study. The trial author supplied unpublished data. There was SD imputation (TSS). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Unclear risk | 20% |
| Baseline assessments | Unclear risk | The trial did not report these. |
| Baseline comparability demonstrated | Unclear risk | The trial did not report this. |
Bruce 1994.
| Methods |
DESIGN
Between‐patient
Participant delivery
ALLOCATION
Random
Method of randomisation: not reported
Concealment: unclear
BLINDING
Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described |
|
| Participants | N: 114 Treatment duration: 6 wks; FU: 6 wks LF: 15 (13.2%) BC: yes Age: 44.1 (14.6SD; range = 20 to 77) Gender (per cent men): 60.2% Severity: mean duration of current episode (days) = 142 (range = 0 to 601) Overall severity score (mean): 4.5 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Westwood Squibb Pharmaceuticals Inc. sponsored the trial. There was SD imputation (TSS). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 13.2% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Buckley 1978.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 10 Treatment duration: 3 wks; FU: 3 wks LF: 2 (20%) BC: not reported Age: 21.4 (range = 9 to 41) Gender (per cent men): 50% Severity: not reported INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 20.0% |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Unclear risk | The trial did not report this. |
Buckley 2008.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 218 Treatment duration: 8 wks; FU: 10 wks LF: 5 (2.3%) BC: yes Age: 48.4 (15.48SD) Gender (per cent men): 45.0% Ethnicity: 97.7% Severity: TSS (0 to 12) = 6.80 (1.58SD) Duration (yrs): 14.6 (13.87SD); range = 0 to 65 Extent of scalp psoriasis: 3.39 (1.36SD) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
Participants achieving absence of disease (IGA = 5) at weeks 2 to 5 could withdraw from the study. |
|
| Outcomes |
|
|
| Notes | Leo Pharma A/S, Ballerup, Denmark, sponsored the study. Treatment duration (wks): C‐B: 6.1 (2.4 SD), N = 108; B: 6.8 (2.2 SD), N = 110 Compliance (missed < = 20% applications): C‐B: 93/108; B: 103/110 The sponsor supplied unpublished data., |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 2.3% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | ‐ |
Camarasa 2003.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Conducted in 20 centres; stratification not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 258 Treatment duration: 6 wks; FU: 14 wks LF: 15 (5.8%) BC: yes Age: 43.5 (14.3SD: range = 15 to 83) Gender (per cent men): 64.3% Severity: per cent BSA = 25.5 (22.9SD: range = 1 to 95); PASI = 15.4 (10.6SD) Duration of psoriasis (mths) mean: 199.2 (157.5SD: range = 1 to 745) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | There was a 1‐wk run‐in period without treatment, except tar shampoo and emollients. Follow up was for responders only (defined as achieving clearance or considerable improvement). The scalp was excluded. Galderma Laboratories sponsored the trial. There was SD imputation (TSS). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 5.8% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Cheesbrough 1992.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 24 Treatment duration: 12 wks; FU: 12 wks LF: 5 (20.8 %) BC: yes Age (mean): 47 Gender (per cent men): 54.2% Severity: PASI mean = 26 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Finders Dead Sea Salt Co and Dead Sea Salt works supplied the study treatments. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Unclear risk | 20.8% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | ‐ |
Christensen 1999.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Single‐blind at inclusion only (investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 171 Treatment duration: 8 wks; FU: 16 wks (N = 95) LF: 5 (2.9%) BC: yes Age: 47.4 (range = 17 to 88) Gender (per cent men): 62.6% Severity: mean TSS (0 to 9) = 6.24; mean duration of psoriasis = 18.5 (range = 1 to 58) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The study did not report sponsorship. The study also included 16‐week follow‐up data for consenting participants who achieved at least 50% improvement from baseline (Investigator scale). There was SD imputation (TSS). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was single‐blind (investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 2.9% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Cook‐Bolden 2010.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: unclear Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 81
Treatment duration: 4 wks; FU: 4 wks
LF: 3 (4%)
BC: yes
Age: 43.7 (14.69SD)
Ethnicity (% white): 78%
Gender (per cent men): 39.5%
Duration (yrs): 11.1 (10.5SD) Severity: GSS = 3 (moderate) (67.9%); GSS = 4 (severe) (32.1%) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
Max usage/wk: 50 g Participants achieving GSS = 0 at 2 wks completed ("withdrew" from) the study. |
|
| Outcomes |
|
|
| Notes | Galderma Laboratories, LP, sponsored the trial. Galderma supplied safety data for this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 4% |
| Baseline assessments | Low risk | These were made. |
| Baseline comparability demonstrated | Low risk | The trial demonstrated demographic and clinical comparability. |
Crosti 1997.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Unclear WITHDRAWAL/DROPOUT Described | |
| Participants | N: 160 Treatment duration: 6 wks; FU: 10 wks LF: 8 (5%) BC: yes Age: 49.9 (14.2SD) Gender (per cent men): 68.1% Severity: mean PASI = 7.6 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. There was SD imputation (PASI). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial did not report this. |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 5.0% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Cunliffe 1992.
| Methods | DESIGN Between‐patient Delivery unclear ALLOCATION Random Method of randomisation: balanced blocks of 10 according to a computer‐generated random numbers table Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 409 Treatment duration: 6 wks; FU: 6 wks LF: 8 (2.0%) BC: yes Age: 44.9 (range = 17 to 83) Gender (per cent men): 55.7% Severity: mean PASI = 9.0 (range = 0.6 to 41.2); mean duration psoriasis = 16.2 (range = 0.2 to 57) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Leo Pharmaceuticals sponsored the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Low risk | A computer‐generated block list was used for randomisation. |
| Loss to follow up | Low risk | 2.0% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
De Simone 1993.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Blinding unclear WITHDRAWAL/DROPOUT Not described | |
| Participants | N: 30 Treatment duration: 6 wks; FU: 10 wks LF: 0 (0%) BC: not reported Age: range = 18 to 84 Gender (per cent men): 70.0% Severity: PASI range = 2.7 to 24.3 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial did not report this. |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Unclear risk | The trial did not report this. |
Decroix 2004.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Single‐blind (investigator); as cream and lotion were compared, not possible to blind participants WITHDRAWAL/DROPOUT Described | |
| Participants | N: 222 Treatment duration: 4 wks; FU: 4 wks LF: 0 (0%) BC: yes Age: 48.4 (15.0SD) Gender (per cent men): 55.9% Ethnicity (% white): 100% Severity: DSS (0 to 12) = 8.44 (1.45SD); atrophy (0 to 3) = 1.01; telangiectasia (0 to 3) = 0.01 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
Participants were randomised to clobetasol propionate cream or lotion; results were aggregated for review purposes. |
|
| Outcomes |
|
|
| Notes | Galderma R&D, Sophia Antipolis, France, sponsored the trial. There was SD imputation (TSS). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study was single‐blind (investigator); as the cream and lotion were compared, it was not possible to blind participants. |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | This was demonstrated. |
Douglas 2002.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: computer‐generated randomisation schedule Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 1106 Treatment duration: 4 wks; FU: 4 wks LF: 86 (7.8%) BC: yes Age: mean = 47.1 (range = 18 to 89) Gender (per cent men): 59.8% Severity: PASI = 10.7 (range = 2.1 to 39.6) Duration: mean = 18.4 (range = 0 to 65) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
All groups then received 4 weeks of maintenance therapy with calcipotriol BD. |
|
| Outcomes |
|
|
| Notes | Leo Pharmaceuticals sponsored the trial. 4‐week follow‐up study also reported (open design: all participants received calcipotriol). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Low risk | Randomisation was computer‐generated. |
| Loss to follow up | Low risk | 7.8% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Dubertret 1992.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: unclear Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 66 Treatment duration: 4 wks; FU: 8 wks LF: 6 (9.1%) BC: yes Age: 43 (range = 21 to 84) Gender (per cent men): 69.7% Severity: PASI mean = 14.15 Duration: 13.3 (range = 0.3 to 40.0) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Leo Pharmaceuticals sponsored the trial.
We contacted Leo for patient outcome data. There was the SD imputation (TSS). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 9.1% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Durakovic 2001.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 15 Treatment duration: 12 wks; FU: 20 wks LF: 0 (0%) BC: yes Age: 49 (range = 27 to 76) Gender (per cent men): 80% Severity: TSS (0 to 24) = 13.7 (14.7SD) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | National Institutes of Health and by Penederm Inc. sponsored the trial. 8‐week follow up (open design: all participants received study drug) study also reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Durakovic 2004.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 11
Treatment duration: 12 wks; FU: 12 wks
LF: 0 (0%)
BC: yes
Age: 46 (range = 29 to 65)
Gender (per cent men): 72.7%
Severity: TSS (0 to 12) = 9.30
INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial was sponsored in part by grant from the National Institutes for Health. US Abbott Laboratories supplied the study drug. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Duweb 2000.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Unclear WITHDRAWAL/DROPOUT Described | |
| Participants | N: 42 Treatment duration: 6 wks; FU: unclear LF: 0 (0%) BC: clinical only Age: 33.5 (range = 6 to 61) Gender (per cent men): 69% Severity: TSS (0 to 12) = 5.2 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Leo Pharmaceuticals sponsored the trial. There was SD imputation (TSS). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial did not report this. |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | This was partially demonstrated. |
Elie 1983.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: unclear Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 40 (55% psoriasis) Treatment duration: 3 wks; FU: 3 wks LF: not reported BC: yes Age: 36.5 (range = 20 to 63) Gender (per cent men): 40% Severity: not reported INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Schering Canada Inc. sponsored the trial. This was a scalp trial. There was SD imputation (TSS/IAGI). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Unclear risk | The trial did not report this. |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Low risk | ‐ |
Ellis 1988.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: computer‐generated randomisation list (1:1) Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 165 Treatment duration: 3 wks; FU: 3 wks LF: 33 (20%) BC: yes Age: 49.1 (range = 19 to 82) Gender (per cent men): 51.6% Severity: not reported INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. Compliance was checked by counting returned bottles. This was a scalp trial. There was SD imputation (TSS). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Low risk | Randomisation was computer‐generated. |
| Loss to follow up | Low risk | 20.0% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Escobar 1992.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: 'randomised code' Concealment: unclear BLINDING Single‐blind (investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 25 Treatment duration: 4 wks; FU: 8 wks LF: 0 (0%) BC: yes Age: 40.3 (14.1SD); range = 18 to 66 Gender (per cent men): 56.0% Severity: mean TSS (0 to 12) = 7.83 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. There was SD imputation (TSS). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was single‐blind (investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Farkas 1999.
| Methods | DESIGN Between‐patient Delivery unclear ALLOCATION Random Method of randomisation: computer programme randomised participants in blocks of 10 Concealment: unclear BLINDING Open WITHDRAWAL/DROPOUT Described | |
| Participants | N: 84 Treatment duration: 8 wks; FU: 12 wks LF: 0 (0%) BC: yes Age: 45.1 (range = 18 to 69) Gender (per cent men): 60.7% Severity: mean PASI = 13.2%; BSA (mean) = 16.5% INCLUSION CRITERIA
EXCLUSION CRITERIA
Serious co‐morbidity |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Hermal sponsored the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was open. |
| Randomisation method reported | Low risk | A computer‐generated block list was used for randomisation. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Feldman 2010 (1).
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 323
Treatment duration: 8 wks; FU: 8 wks
LF: 7 (2.2%)
BC: yes
Age: 47.8 (15.2SD)
Gender (per cent men): 48.3%
Per cent white: 90.7%
Severity: ISGA = mild disease: 26.9%; ISGA = moderate disease: 73.1%; SGA = 3.69 (0.89SD); % BSA = 6.4% (4.9SD) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | STUDY 1: nCT00689481 Stiefel Laboratories, a GSK Company, sponsored the trial. Atrophy was not reported. We sought data, but they were not received. No usable effectiveness data were reported or available from sponsor. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 2.2% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | This was demonstrated. |
Feldman 2010 (2).
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 336
Treatment duration: 8 wks; FU: 8 wks
LF: 6 (1.8%)
BC: yes
Age: 48.7 (14.4SD)
Gender (per cent men): 60.4%
% white: 86.0%
Severity: ISGA = mild disease: 31.8%; ISGA = moderate disease: 68.2%; SGA = 3.69 (0.83SD); % BSA = 6.2% (4.8SD) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | STUDY 2: nCT00688519 Stiefel Laboratories, a GSK Company, sponsored the trial. Atrophy was not reported. We sought data, but they were not received. No usable effectiveness data were reported or available from sponsor. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 1.8% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | This was demonstrated. |
Fleming 2010 (H).
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: computer‐generated schedule Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 364 Treatment duration: 8 wks; FU: 10 wks LF: 2 (0.5%) BC: yes Age: 51.1 (14.7SD) Gender (per cent men): 58.8% Per cent white: 98.1% Severity: PASI (0 to 64.8) = 7.8 (4.4SD), range = 1 to 25; IGA = 2.97 (0.66SD), range = mild (2) to very severe (5) Duration (yrs): 18.9 (13.8SD) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
Concomitant use of corticosteroids (WHO group I or II), dithranol, tar, and retinoids on face, scalp, and flexures was permitted. |
|
| Outcomes |
|
|
| Notes | Leo Pharma, Ballerup, Denmark, sponsored the trial. Compliance: mean weekly us =: C‐B: 22.7 g; C: 22.4g; B: 25.9 g Most participants (73.5% to 80%) were fully compliant and those non‐compliant missed < 10% applications. Safety data were available for 362/364 participants. Leo Pharmaceuticals supplied unpublished data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Low risk | A computer‐generated randomisation schedule was used. |
| Loss to follow up | Low risk | 0.5% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | This was demonstrated. |
Fleming 2010 (P).
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: computer‐generated schedule Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 364
Treatment duration: 8 wks; FU: 10 wks
LF: 2 (0.5%)
BC: yes
Age: 51.1 (14.7SD)
Gender (per cent men): 58.8%
Per cent white: 98.1%
Severity: PASI (0 to 64.8) = 7.8 (4.4SD), range = 1 to 25; IGA = 2.97 (0.66SD), range = mild (2) to very severe (5) Duration (yrs): 18.9 (13.8SD) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
Concomitant use of corticosteroids (WHO group I or II), dithranol, tar, and retinoids on face, scalp, and flexures were permitted. |
|
| Outcomes |
|
|
| Notes | Leo Pharma, Ballerup, Denmark, sponsored the trial. Compliance: mean weekly us =: C‐B: 22.7 g; C: 22.4g; B: 25.9 g; P: 26.1 g Most participants (73.5% to 80%) were fully compliant and those non‐compliant missed < 10% applications. Safety data were available for 362/364 participants. Leo Pharmaceuticals supplied unpublished data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Low risk | A computer‐generated randomisation schedule was used. |
| Loss to follow up | Low risk | 0.5% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | This was demonstrated. |
Franz 1999.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: unclear Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 190 Treatment duration: 2 wks; FU: 4 wks LF: 18 (9.5%) BC: yes Age: 49.6 Gender (per cent men): 49.3% Severity: mean TSS (0 to 12) = 7.92 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
Findings reported for foam and lotion combined:
|
|
| Outcomes |
|
|
| Notes | Connectics Corporation sponsored by the trial. This was a scalp trial. There was SD imputation (TSS). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 9.5% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Franz 2000.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 188 Treatment duration: 2 wks; FU: 4 wks LF: 0 (0%) BC: unclear Age: not reported Gender (per cent men): 49.5% Severity: mean TSS (0 to 12) = 7.25 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
Findings reported for foam and lotion combined:
|
|
| Outcomes |
|
|
| Notes | Connectics Corporation sponsored by the trial. This was a scalp trial. There was SD imputation (TSS). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Unclear risk | The trial did not report this. |
Geilen 2000.
| Methods | DESIGN Within‐patient Nurse delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 7 Treatment duration: 3 wks; FU: 3 wks LF: 0 (0%) BC: yes Age: 36 to 67 Gender (per cent men): 100% Severity: TSS (0 to 8) = 6.72 (0.76SD) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Low risk | ‐ |
Gottlieb 2003.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Conducted in 17 centres; stratification not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 279 Treatment duration: 2 wks; FU: 4 wks LF: 8 (2.9%) BC: yes (clinical only) Age: 47 (range = 19 to 82) Gender (per cent men): 57% Severity: BSA = 6.7% INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
Limit of 50 g of medication/wk to non‐scalp sites only |
|
| Outcomes |
|
|
| Notes | Connetics Corporation sponsored the trial. There was SD imputation (TSS). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | ‐ |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | This was partially demonstrated. |
Grattan 1997 (H).
| Methods | DESIGN Within‐patient Delivery unclear ALLOCATION Random Method of randomisation: pre‐determined randomisation schedule Concealment: unclear BLINDING Open WITHDRAWAL/DROPOUT Described | |
| Participants | N: 25 Treatment duration: 4 wks; FU: 16 weeks LF: not reported BC: yes Age: 44.0 (range = 20 to 72) Gender (per cent men): 52.0%) Severity: BSA = 16.1% (range = 4.1% to 47.8%); TSS (target sites) = 6.3 (range = NR) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | There was inpatient treatment to ensure high level of compliance. Dermal Laboratories sponsored the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was open. |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Unclear risk | The trial did not report this. |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | This was demonstrated. |
Grattan 1997 (P).
| Methods | DESIGN Within‐patient Delivery unclear ALLOCATION Random Method of randomisation: pre‐determined randomisation schedule Concealment: unclear BLINDING Open WITHDRAWAL/DROPOUT Described | |
| Participants | N: 12 Treatment duration: 4 wks: FU: 16 wks LF: 0 (0%) BC: yes Age: 50.3 (range = 33 to 75) Gender (per cent men): 33% (4/12) Severity: BSA 17.1% (range = 4.7% to 45.7%); TSS = 6.3 (range = 5 to 7) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | There was inpatient treatment to ensure high level of compliance. Dermal Laboratories sponsored the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was open. |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Green 1994.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 49 Treatment duration: 4 wks; FU: 4 wks LF: 3 (6.1%) BC: unclear Age: not reported Gender (per cent men): not reported Severity: mean TSS (0 to 12) = 6.7 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Leo Pharmaceutical Products sponsored the trial. Compliance was assessed by unused medication returned at each visit. The compliance rate for participants in each group was > 90%. This was a scalp trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 6.1% |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Unclear risk | The trial did not report this. |
Greenspan 1993.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 80 Treatment duration: 3 wks; FU: 3 wks LF: 9 (11.3%) BC: unclear Age: 51.5 (range = 20 to 77) Gender (per cent men): 43.8% Severity: not reported INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship, but 3 of the authors were employed by Owen/Galderma laboratories. There was SD imputation (IAGI). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 11.3% |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Unclear risk | The trial did not report this. |
Gribetz 2004.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: 'validated system that automates the random assignment of treatment codes' Concealment: adequate BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 57 Treatment duration: 8 wks; FU: 8 wks LF: 6 (10.5%) BC: yes Age: 47.8 (range = 21 to 88) Gender (per cent men): 50.9% Severity: PGA, per cent moderate = 72%; PGA, per cent severe = 29.8%; TSS = 5.34 (range = 3.0 to 9.0) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Novartis Pharmaceuticals Group sponsored the trial. No instances of skin atrophy were reported. This was an inverse psoriasis trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Novartis supplied identical tubes identified only by randomisation number (participants and investigators blinded to tube contents). |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Low risk | Randomisation was by a validated system that automated the random assignment of treatment codes. |
| Loss to follow up | Low risk | 10.5% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | ‐ |
Guenther 2000.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Single‐blind (investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 120 Treatment duration: 8 wks; FU: 20 wks LF: 14 (11.7%) BC: yes Age: 48.5 Gender (per cent men): 60.8% Severity: not reported INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
12 weeks' maintenance for both groups with emollient only. |
|
| Outcomes |
|
|
| Notes | Allergan Inc. sponsored the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial was single‐blind (investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 11.7% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Guenther 2002 (H).
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: computer‐generated random numbers table Concealment: adequate BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 828 Treatment duration: 4 wks; FU: 4 wks LF: 10 (1.2%) BC: yes Age: 48.5 (14.3SD) Gender (per cent men): 64.0% Severity: mean PASI = 10.5; mean duration psoriasis = 18.3 yrs INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Leo Pharmaceuticals sponsored the trial. The trial was conducted across 57 centres in 8 countries. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | All study personnel and participants were blinded to treatment assignment for the duration of the study. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Low risk | Randomisation was computer‐generated. |
| Loss to follow up | Low risk | 1.2% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Guenther 2002 (P).
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: computer‐generated random numbers table Concealment: adequate BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 828
Treatment duration: 4 wks; FU: 4 wks
LF: 10 (1.2%)
BC: yes
Age: 48.5 (14.3SD)
Gender (per cent men): 64.0%
Severity: mean PASI = 10.5; mean duration psoriasis = 18.3 yrs INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Leo Pharmaceuticals sponsored the trial. The trial was conducted across 57 centres in 8 countries. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | All study personnel and participants were blinded to treatment assignment for the duration of the study. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Low risk | Randomisation was computer‐generated. |
| Loss to follow up | Low risk | 1.2% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | ‐ |
Han 2001.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Open WITHDRAWAL/DROPOUT Described | |
| Participants | N: 208 Treatment duration: 12 wks; FU: 12 wks LF: 9 (4.3%) BC: yes Age: 40.4 (8.9SD) Gender (per cent men): 59.8% Severity: TSS (0 to 20) = 16.1 (11.0SD) Duration (yrs): 9.4 (8.9SD) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. Translation support was received for data extraction. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was open. |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 4.3% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | ‐ |
Harrington 1996a.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 413 Treatment duration: 8 wks; FU: 8 wks LF: 47 (11.4%) BC: yes, except average age in placebo group higher than for A and B (P = 0.02) Age: 44.6 Gender (per cent men): 52.8% Severity: PASI (modified) = 8.3 (range = 0.6 to 59.4) Duration (yrs): 17.7 (range = 0.04 to 70) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Leo Pharmaceuticals sponsored the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 11.4% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Unclear risk | This was partially demonstrated. |
Helfrich 2007.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: unclear (method inadequately described) Concealment: unclear (inadequately described) BLINDING Double‐blind (participant/investigator/assessor) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 185 Treatment duration: 8 wks; FU: 8 wks LF: 3 (1.6%) BC: yes Age: 49.0 (range = 19 to 83) Gender (per cent men): 56.8% Severity: PGA = 3.2 (range = 3 to 4); PSS = 8.1 (range = 4.7 to 12) Ethnicity (per cent white): NR Duration: 17.3 (range = 0.5 to 65) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
Max: 8 g ointment/day Participants were permitted concurrent oral vitamin D (= < 400 IU/day), oral calcium (< = 1200 mg/day), or both; or tar shampoo for scalp psoriasis. |
|
| Outcomes |
|
|
| Notes | QuatRx Pharmaceuticals (product now owned by Deltanoid Pharmaceuticals) sponsored the trial. The sponsor supplied unpublished data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant and investigator/assessor). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 1.6% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | This was demonstrated (clinical and demographic). |
Henneicke‐v. Z. 1993.
| Methods | DESIGN Within‐patient (placebo) Between‐patient (active) Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 73 Treatment duration: 8 wks; FU: 8 wks LF: 21 (28.8%) BC: yes Age: 40.5 (median); range = 18 to 71 (N = 52) Gender (per cent men): 65.8% (N = 73) Severity: TSS (0 to 12) = 7.4 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | High risk | 28.8% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | ‐ |
Highton 1995.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 277 Treatment duration: 8 wks LF: 30 (10.8%) BC: clinical severity comparable, demographics unclear Age: not reported Gender (per cent men): not reported Severity: TSS (0 to 8) = 3.90; BSA = 9.1% INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Bristol Myers Squibb sponsored the trial. There was SD imputation (TSS). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 10.8% |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Unclear risk | This was partially demonstrated. |
Hindsén 2006 (H).
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Not described | |
| Participants | N: 360 Treatment duration: 2 or 6 wks; FU: 6 or 10 wks LF: NR BC: not demonstrated Age: NR Gender (per cent men): NR Severity: NR INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
[Placebo ointment plus occlusion, once/week for 2 wks (P)] |
|
| Outcomes |
|
|
| Notes | Leo Pharma, Ballerup, sponsored the trial. It was unclear how participants were blinded to treatment options where 2/3 involved occlusion. The sponsor supplied unpublished data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial was reported to be double‐blind (participant/investigator), but it was unclear how they blinded participants to treatment options where 2/3 involved occlusion. |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Unclear risk | The trial did not report this. |
| Baseline assessments | Unclear risk | The trial did not report these. |
| Baseline comparability demonstrated | Unclear risk | This was not demonstrated. |
Hindsén 2006 (P).
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Not described | |
| Participants | N: 360
Treatment duration: 2 or 6 wks; FU: 6 or 10 wks
LF: NR
BC: not demonstrated
Age: NR
Gender (per cent men): NR
Severity: NR
INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Leo Pharma, Ballerup, sponsored the trial. It was unclear how participants were blinded to treatment options where 2/3 involved occlusion. The sponsor supplied unpublished data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial was reported to be double‐blind (participant/investigator), but it was unclear how they blinded participants to treatment options where 2/3 involved occlusion. |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Unclear risk | The trial did not report this. |
| Baseline assessments | Unclear risk | The trial did not report these. |
| Baseline comparability demonstrated | Unclear risk | This was not demonstrated. |
Holick 2003.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 15 Treatment duration: 8 wks; FU: 8 wks LF: 0 (0%) BC: not reported Age: 56 (range = 25 to 74) Gender (per cent men): 67 % Severity: not reported INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The National Institutes for Health (Department of Health and Human Services) and the Department of Defense Small Business Innovation Research (SBIR) Program sponsored the trial through grants. The trial also reported a uncontrolled open, large area, study for N = 10 (PTH applied OD for up to 11 months). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Unclear risk | The trial did not report this. |
Huang 2009.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: unclear Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 320 Treatment duration: 4 wks; FU: 4 wks LF: 0 (0%) BC: stated no significant difference in clinical or demographic characteristics (data not shown) Age: NR Gender (per cent men): NR Severity: NR INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
Participant gave written consent to use < = 100 g/wk. |
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. We received translation support from native speaker. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0% |
| Baseline assessments | Unclear risk | The trial authors state these were undertaken. |
| Baseline comparability demonstrated | Unclear risk | The trial authors state that groups were comparable at baseline (not demonstrated). |
Hutchinson 2000.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Open WITHDRAWAL/DROPOUT Described | |
| Participants | N: 114 Treatment duration: 8 wks; FU: 8 wks LF: 28 (24.6%) BC: yes Age: 42.3 Gender (per cent men): 74.4% Severity: PASI (mean) = 11.8 Duration of psoriasis (mths) (mean): 185.1 (range = 1 to 85) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was open. |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Unclear risk | 24.6% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | This was demonstrated. |
Jarratt 2004.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: computer‐generated list Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Unclear | |
| Participants | N: 142 Treatment duration: 4 wks; FU: 6 wks LF: 1 (0.7%) BC: yes Age: 45.1 (15.37SD) Gender (per cent men): 42.3% Severity: TSS (0 to 9) = 6.6; GSS (6‐pt (rescaled: 0 = very severe to 5 = clear) = 1.65 (0.61SD), N = 142 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Galderma R&D Inc sponsored the trial. Missing data imputed using last observation carried forward. No cases of skin atrophy, teleangiectasia, or acne were reported. This was a scalp trial. There was SD imputation (TSS). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Low risk | ‐ |
| Loss to follow up | Low risk | 0.7% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Jarratt 2006.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 120 Treatment duration: 4 wks; FU: 8 wks LF: 0 (0%) BC: yes Age: 48.0 (12.9SD) Gender (per cent men): 60.0% Severity: % BSA = 7.69% (6.17%SD); ODS (moderate) = 90.8%; ODS (severe/very severe) = 9.2% Ethnicity (per cent white): 94.2% Duration: NR INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
Then 4‐week treatment‐free follow‐up period. There was 8 hrs between applications, and the treated area was unwashed for 4 hours after application. |
|
| Outcomes |
|
|
| Notes | Galderma Pharmaceuticals sponsored the trial. The trial also reported CIC data on a safety RCT (2 or 4 wks with clobetasol propionate 0.05% spray, < = 50 g/wk): 8/36 had HPA suppression; all returned to normal within 2 wks of end of treatment (EOT). The trialist supplied unpublished data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0% |
| Baseline assessments | Low risk | The trial reported these. |
| Baseline comparability demonstrated | Low risk | This was demonstrated. |
Jekler 1992.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 30 Treatment duration: 8 wks LF: 3 (10%) BC: not reported Age: 45.2 (14.0SD; N = 27) Gender (per cent men): 77.8%; N = 27 Severity: severity (mean score, 0 to 3) = 1.9; N = 27 Duration disease (years): 20.5 (14.8SD; N = 27) Duration exacerbation (mths): 5.1 (6.4SD; N = 27) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | E Merck AB sponsored the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 10.0% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Unclear risk | The trial did not report this. |
Jemec 2008 (H).
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: pre‐planned computer‐generated randomisation code list (2:4:4:1) Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 1505 Treatment duration: 8 wks; FU: 8 wks LF: 32 (2.1%) BC: yes Age: 49.0 (15.8SD); range = 17 to 97 Gender (per cent men): 44.8% Ethnicity (per cent white): 96.3% Severity: IGA mild disease = 6.5%; IGA moderate disease = 56.2%; IGA severe disease = 31.6%; IGA very severe disease = 5.7%; TSS (0 to 12) = 6.82 (1.85SD) Duration (yrs): 16.5 (13.6SD) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Leo Pharma A/S, Ballerup, Denmark, sponsored the trial. The sponsor supplied unpublished data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Low risk | A preplanned computer‐generated randomisation code list was used. |
| Loss to follow up | Low risk | 2.1% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | This was demonstrated. |
Jemec 2008 (P).
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: pre‐planned computer‐generated randomisation code list (2:4:4:1) Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 1505
Treatment duration: 8 wks; FU: 8 wks
LF: 32 (2.1%)
BC: yes
Age: 49.0 (15.8SD); range = 17 to 97
Gender (per cent men): 44.8%
Ethnicity (% white): 96.3%
Severity: IGA mild disease = 6.5%; IGA moderate disease = 56.2%; IGA severe disease = 31.6%; IGA very severe disease = 5.7%; TSS (0 to 12) = 6.82 (1.85SD) Duration (yrs): 16.5 (13.6SD) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Leo Pharma A/S, Ballerup, Denmark, sponsored the trial. The sponsor supplied unpublished data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Low risk | A preplanned computer‐generated randomisation code list was used. |
| Loss to follow up | Low risk | 2.1% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | This was demonstrated. |
Ji 2008.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Single‐blind (investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 250
Treatment duration: 12 wks; FU: 12 wks
LF: 7 (2.8%)
BC: yes
Age: 41.7 (12.2SD)
Gender (per cent men): 65.6%
Ethnicity (per cent Asian): 100%
Severity: per cent BSA = 18%, range = 1% to 35%; DSS (0 to 12) = 8.12 (1.97SD), range = 3 to 12 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
Usage restrictions:
|
|
| Outcomes |
|
|
| Notes | Galderma Laboratories LP sponsored the trial. The trial was set in China. We received translation support for data extraction. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The study was single‐blind (investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 2.8% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | This was demonstrated. |
Jin 2001.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: Not stated Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 96 Treatment duration: 6 wks; FU: 6 wks LF: 7 (7.3%) BC: yes Age: 35.8 (range = 18 to 65) Gender (per cent men): 57.3% Severity: TSS (0 to 20) = 9.4 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Biological Technical Medical Trade Limited sponsored the trial. We received translation support for data extraction. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 7.3% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | ‐ |
Jorizzo 1997.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 89 Treatment duration: 4 wks; FU: 6 wks LF: Unclear BC: yes Age: 49.7 (range = 21 to 84) Gender (per cent men): 65% Severity: per cent BSA affected = 8.1% Duration of psoriasis (range, years): 1 to 57 Duration of exacerbation (range, wks): 3 to 2080 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Glaxo Wellcome Inc. sponsored the trial. There was SD imputation (TSS). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Unclear risk | The trial did not report this. |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | ‐ |
Jorizzo 2007.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Not described | |
| Participants | N: 379 Treatment duration: 6 wks; FU: 6 wks LF: NR BC: yes (only clinical comparability demonstrated) Age: NR Gender (per cent men): NR Severity: PASI = 7.9 (5.3SD); IGA moderate = 76.2%; IGA severe = 22.2% INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
< = 100 g/wk ‐ the daily dose was not specified. |
|
| Outcomes |
|
|
| Notes | Warner‐Chilcott sponsored the trial. This was a conference abstract only. We sought data but received no response. There was SD imputation (PASI). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Unclear risk | The trial did not report this. |
| Baseline assessments | Unclear risk | The trial only reported clinical assessments (demographic characteristics not reported). |
| Baseline comparability demonstrated | Unclear risk | This was partially demonstrated (demographic characteristics not assessed). |
Kang 1998.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: identical tubes with computer‐generated codes Concealment: adequate BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 30 Treatment duration: 6 wks LF: 0 (0%) BC: psoriasis comparable, demographics unclear Age: 41 (range = 18 to 66) Gender (per cent men): 66.7% Severity: TSS (0 to 24) = 12.27 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Bristol‐Myers Squibb Corporation and from Babcock Dermatologic Endowment (University of Michigan) sponsored the trial through grants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Participants were assigned by computer‐generated code. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Low risk | Randomisation was computer‐generated. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Unclear risk | This was partially demonstrated. |
Kanzler 1993.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: identical containers labelled right and left; labelling method not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Not described | |
| Participants | N: 18 Treatment duration: 4 wks; FU: 4 wks LF: 0 (0%) BC: not reported Age: 45.4 (range = 21 to 66) Gender (per cent men): 55.6% Severity: not reported INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. Compliance was assessed by unused medication returned at each visit. The compliance rate for participants in each group was > 90%. This was a scalp trial. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Unclear risk | The trial did not report this. |
Katz 1987a.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 40 Treatment duration: 12 wks; FU: 12 wks LF: 2 (5%) BC: yes Age: 46.7 Gender (per cent men): 60% Severity: per cent participants with BSA affected < 10% = 68% Duration (years): 20.8 INCLUSION CRITERIA
Note: 38/59 (64%) achieved remission during the acute phase EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial was supported in part by a grant from Schering Plough Corporation. There was SD imputation (TSS). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 5.0% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Katz 1991a.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: computer‐generated code Concealment unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 94 Treatment duration: 24 wks; FU: 24 wks LF: 4 (4.3%) BC: yes Age: 46.0 (range = 21 to 86) Gender (per cent men): 67.8% Severity: overall score not reported INCLUSION CRITERIA
Note: 94/123 (76%) achieved remission during acute phase EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship, but the Schering Corporation employed the corresponding author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Low risk | Randomisation was computer‐generated. |
| Loss to follow up | Low risk | 4.3% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | ‐ |
Katz 1991b.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: identical tubes labelled by computer‐generated code Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 110 Treatment duration: 2 wks LF: 2 (1.8%) BC: inadequately reported Age: 51.7 (range = 19 to 84) Gender (per cent men): 80.6% Severity: Total Severity Score (0 to 12) (median) = 8 Duration of disease (years): 21 (range 1 to 57) Duration of exacerbation (years): 15.4 (range < 1 to 57) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial was supported by an educational grant from Westwood‐Squibb Pharmaceuticals, a Bristol‐Myers Squibb company. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Low risk | Randomisation was computer‐generated. |
| Loss to follow up | Low risk | 1.8% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Unclear risk | The trial did not report this. |
Kaufmann 2002 (H).
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: computer‐generated randomisation schedule Concealment: unclear BLINDING Double‐blind (participant/assessor) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 1603 Treatment duration: 4 wks; FU: 4 wks LF: 0 (0%) BC: yes Age: 48.4 (range = 17 to 90) Gender (per cent men): 60.5% Severity: PASI mean = 10.0 (range = 1.2 to 49.5) Duration: 19.2 (range = 0 to 75) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Leo Pharmaceuticals sponsored the trial. Compliance rates were reported for each regimen. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/assessor). |
| Randomisation method reported | Low risk | Randomisation was computer‐generated. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | ‐ |
Kaufmann 2002 (P).
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: computer‐generated randomisation schedule Concealment: unclear BLINDING Double‐blind (participant/assessor) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 1603
Treatment duration: 4 wks; FU: 4 wks
LF: 0 (0%)
BC: yes
Age: 48.4 (range = 17 to 90)
Gender (per cent men): 60.5%
Severity: PASI mean = 10.0 (range = 1.2 to 49.5)
Duration: 19.2 (range = 0 to 75) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Leo Pharmaceuticals sponsored the trial. Compliance were rates reported for each regimen. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/assessor). |
| Randomisation method reported | Low risk | Randomisation was computer‐generated. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | ‐ |
Kim 1994.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: unclear Concealment: unclear BLINDING Double‐blind (unclear who was blinded) WITHDRAWAL/DROPOUT Not described | |
| Participants | N: 10 Treatment duration: 8 wks; FU: 8 wks LF: 0 (0%) BC: yes Age: 32.1 (range 20 to 52) Gender (per cent men): 60% Severity: PASI = 10.49 (1.60SD) Duration: 6.7 years (range 0.2 to 15) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (unclear who was blinded). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | ‐ |
Kiss 1996.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 239 Treatment duration: 8 wks LF: 29 (12.1%) BC: not reported Age: not reported Gender (per cent men): not reported Severity: not reported INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Bristol Myers Squibb Pharmaceuticals sponsored the trial. Carder 1996 reports finding for subgroup (N = 29). This was a scalp trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 12.1% |
| Baseline assessments | Unclear risk | The trial did not report these. |
| Baseline comparability demonstrated | Unclear risk | The trial did not report this. |
Klaber 1994.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: Concealment: unclear BLINDING Double‐blind (participant/assessor) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 474 Treatment duration: 4 wks LF: assessment = 6 (1.3%) TSS: 29 (6.1%) BC: yes Age: 44.1 (range = 18 to 90) Gender (per cent men): 51.5% Severity: TSS (0 to 12) = 6.5 (range = 2 to 12) Duration of scalp psoriasis (yrs): 13.1 (range = 0.1 to 67.0) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Leo Pharmaceutical Products sponsored the trial. This was a scalp trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/assessor). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 1.3% |
| Baseline assessments | Low risk | reported |
| Baseline comparability demonstrated | Low risk | ‐ |
Klaber 2000b.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Open WITHDRAWAL/DROPOUT Described | |
| Participants | N: 475 Treatment duration: 8 wks; FU: 24 wks (N = 166) LF: 52 (10.9%) BC: yes Age: 45.3 Gender (per cent men): 52.0% Severity: Total Severity Score (0 to 12) = 5.1 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Leo Pharmaceuticals sponsored the trial. All participants who achieved at least a slight improvement in scalp psoriasis then received 16 weeks of treatment with calcipotriol BD. This was a scalp trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was open. |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 10.9% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | ‐ |
Koo 2006.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated (1:1:2) Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 86 Treatment duration: 2 wks; FU: 2 wks LF: 0 (0%) BC: not demonstrated Age: NR Gender (per cent men): NR Severity: NR INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
Part 1
Part 2
|
|
| Outcomes |
|
|
| Notes | Connetics Corporation, Palo Alto, California, US, sponsored the trial. We sought data, but we received no response. There was SD imputation (IGA/PGA). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0% |
| Baseline assessments | Unclear risk | The trial did not report these. |
| Baseline comparability demonstrated | Unclear risk | This was not demonstrated. |
Kragballe 1988b.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Not described | |
| Participants | N: 30 Treatment duration: 6 wks LF: 3 (10%) BC: yes Age: 39 (range = 18 to 65) Gender (per cent men): 30.0% Severity: TSS (0 to 9) = 6.9; per cent BSA = 18.7% (range = 12% to 50%) Duration (yrs): 15.3 (range = 1 to 35) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 10.0% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Kragballe 1991a.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Double‐blind (participant/assessor) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 345 Treatment duration: 6 wks LF: 3 (0.9%) BC: yes Age: 45.2 (range = 18 to 90) Gender (per cent men): 58.8% Severity: PASI = 8.35 (range = 0.60 to 48.5) Duration (yrs): 19.5 (range = 0.5 to 76) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Leo Pharmaceuticals sponsored the trial Trial participants were inpatients and outpatients. There was SD imputation (TSS). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/assessor). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0.9% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Kragballe 1998b.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: not stated BLINDING Double‐blind (participant/assessor) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 699 Treatment duration: 8 wks; FU: 8 wks LF: 8 (1.1%) BC: psoriasis comparable, demographics unclear Age: not stated Gender (per cent men): not stated Severity: not stated INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Leo Pharmaceuticals sponsored the trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The study was double‐blind (participant/assessor). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 1.1% |
| Baseline assessments | Unclear risk | The trial did not report these. |
| Baseline comparability demonstrated | Unclear risk | This was partially demonstrated. |
Kragballe 2004.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: computer‐generated randomisation schedule, using centralised telephone voice response system Concealment: adequate BLINDING Double‐blind (participant/investigator) (Groups A and B) Single‐blind (investigator) (Group C) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 972 Treatment duration: 8 wk; FU: 12 wks LF: 99 (10.2%) BC: yes Age: 47.7 (range = 18 to 97) Gender (per cent men): 63.8% Severity: PASI = 10.5 (range = 2 to 49); per cent with moderate disease = 64.3% Duration (yrs): 18.5 (range = 0 to 70) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Leo Pharmaceuticals sponsored the trial. Request data: none supplied Reversible skin atrophy: A: 1/322; B: 0/322; C: 0/327 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A centralised telephone voice response system was used for the participant assignment, and this removed the opportunity for investigator bias during randomisation. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Groups A and B were double‐blind (participant/investigator); group C was single‐blind (investigator). |
| Randomisation method reported | Low risk | Randomisation was computer‐generated. |
| Loss to follow up | Low risk | 10.2% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Kragballe 2006.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: computer‐generated randomisation schedule (1:1:1) Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 634
Treatment duration: 52 wks; FU: 52 wks
LF: 8 (1.3%)
BC: yes
Age: 48.7 (14.2SD)
Gender (per cent men): 61.0%
Ethnicity (per cent white): 97.3%
Severity: per cent IGA moderate = 69.1%; per cent IGA severe = 27.9%; per cent IGA very severe = 3.0%
Duration (yrs): mean = 19.7; median = 17.0; range = 1 to 65
Use of topical corticosteroids during last decade (mths): 33.8 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
< = 100 g/wk |
|
| Outcomes |
|
|
| Notes | Leo Pharma A/S sponsored the trial. The sponsor supplied unpublished data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Low risk | A computer‐generated randomisation schedule was used (1:1:1). |
| Loss to follow up | Low risk | 1.3% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Unclear risk | The trial only reported mean values (no measure of spread). They described groups as "well balanced". |
Kragballe 2009.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: computer‐generated schedule (1:2) Concealment: unclear BLINDING Single‐blind (investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 312 Treatment duration: 8 wks; FU: 16 wks LF: 2 (0.6%) BC: yes Age: 51.0 (15.4SD); range = 18 to 91 Gender (per cent men): 43.0% Ethnicity (per cent white): 99.0% Severity: IGA moderate = 56.7%; IGA severe = 35.9%; IGA very severe = 7.4%; TSS (0 to 12) = 7.3 (1.7SD) Duration (yrs): 18.7 (14.6SD); range = 0 to 70 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
There was no concomitant topical treatment, emollient, or medicated shampoo or conditioner. |
|
| Outcomes |
|
|
| Notes | Leo Pharma A/S, Ballerup, Denmark, sponsored the trial. The sponsor supplied unpublished data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Low risk | A computer‐generated randomisation schedule was used (1:2). |
| Loss to follow up | Low risk | 0.6% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | This was demonstrated. |
Kreuter 2006 (H).
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: computer‐generated randomisation lists Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 80 Treatment duration: 4 wks; FU: 10 wks LF: 2 (2.5%) BC: yes Age: 52.4 (14.3SD); range = 28 to 87 Gender (per cent men): 61.3% Ethnicity: NR Severity: mPASI = 21.2 (13.4SD); range = 3 to 54; VAS (0 to 10) = 4.8 (2.7SD); range = 1 to 10 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
No concomitant therapies were permitted (including emollients). |
|
| Outcomes |
|
|
| Notes | Novartis Pharma GmbH sponsored the trial. Atrophy was not reported. We sought unpublished data, but it was reported to be unavailable. There was SD imputation (PASI). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Low risk | Computer‐generated randomisation lists were used. |
| Loss to follow up | Low risk | 2.5% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | This was demonstrated. |
Kreuter 2006 (P).
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: computer‐generated randomisation lists Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 80
Treatment duration: 4 wks; FU: 10 wks
LF: 2 (2.5%)
BC: yes
Age: 52.4 (14.3SD); range = 28 to 87
Gender (per cent men): 61.3%
Ethnicity: NR
Severity: mPASI = 21.2 (13.4SD), range = 3 to 54; VAS (0 to 10) = 4.8 (2.7SD), range = 1 to 10 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
No concomitant therapies were permitted (including emollients). |
|
| Outcomes |
|
|
| Notes | Novartis Pharma GmbH sponsored the trial. Atrophy was not reported. We sought unpublished data, but it was reported to be unavailable. There was SD imputation (PASI). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Low risk | Computer‐generated randomisation lists were used. |
| Loss to follow up | Low risk | 2.5% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | This was demonstrated. |
Krueger 1998.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 45 Treatment duration: 6 wks LF: 0 (0%) BC: reported to be similar Age: 50 (range = 23 to 83) Gender (per cent men): 84% Severity: not reported INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Allergan, Inc, California, sponsored the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Unclear risk | These were partially done. |
| Baseline comparability demonstrated | Unclear risk | These were stated to be similar; comparability was not demonstrated. |
Köse 1997.
| Methods | DESIGN Between‐patient Delivery unclear ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Unclear WITHDRAWAL/DROPOUT Described | |
| Participants | N: 43 Treatment duration: 10 days FU: 20 days LF: 0 (0%) BC: yes Age: not stated Gender (per cent men): not stated Severity: not stated INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. This was a scalp trial. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial did not report this. |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Unclear risk | The trial did not report these. |
| Baseline comparability demonstrated | Unclear risk | The trial reported baseline comparability (demographic/clinical), but it was not demonstrated. |
Lahfa 2003.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Single‐blind (investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 125 Treatment duration: 12 wks; FU: 12 wks LF: 5 (4.0%) BC: yes Age: 49.5 (15.1SD) Gender (per cent men): 55.2% Ethnicity (per cent white/Caucasoid): 97.6% Severity: PASI = 6.8; per cent BSA = 13.2 (7.4SD) Duration (yrs): 16.7 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
In the initial phase, the participant applied dual therapy until achieving clearance or marked improvement or until 4 wks elapsed, then switched to monotherapy maintenance phase. |
|
| Outcomes |
|
|
| Notes | Galderma Laboratories sponsored the trial. We sought unpublished data, but it was reported to be unavailable. There was SD imputation (PASI). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study was single‐blind (investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 4.0% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | This was demonstrated. |
Landi 1993.
| Methods | DESIGN Between‐patient Delivery unclear ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Unclear WITHDRAWAL/DROPOUT Described | |
| Participants | N: 40 Treatment duration: 6 wks; FU: 10 wks LF: 0 (0%) BC: psoriasis comparable, demographics unclear Age: range = 17 to 84 Gender (per cent men): not stated Severity: PASI (mean) = 11.6 (range = 3.0 to 35.1) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Leo Pharmaceuticals sponsored the trial. Landi 1993 reported the findings of a single centre, 1 of 3 centres reported in Landi 1993 (N = 120). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial did not report this. |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Unclear risk | These were partially done. |
| Baseline comparability demonstrated | Unclear risk | This was partially demonstrated. |
Lane 1983.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Not described | |
| Participants | N: 157 Treatment duration: 3 wks; FU: 3 wks LF: 18 (11.5%) BC: yes Age: 39.6 Gender (per cent men): 52.5% Severity: TSS (0 to 20) = 10.6 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. There was SD imputation (TSS). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 11.5% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Langley 2011 (H).
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated (2:2:1) Concealment: adequate: treatments dispensed by non‐study staff BLINDING Single‐blind (investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 458 Treatment duration: 8 wks; FU: 16 wks LF: 0 (0%) BC: yes Age: 51.6 (14SD) Gender (per cent men): 62.2% Ethnicity (per cent white): 93.9% Severity: mPASI = 9.39, range = 2.4 to 59.4; IGA moderate = 68.3%; IGA severe = 29.5%; IGA very severe = 2.2%; per cent BSA = 9.3 (8.2SD) Duration (yrs): 19.8 (13.3SD) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
Participants achieving clear IGA stopped treatment and restarted if psoriasis reappeared. There was follow up of responders only (IGA clear or almost clear). |
|
| Outcomes |
|
|
| Notes | Leo Pharma A/S, Ballerup, Denmark, sponsored the trial. Participants could not be completely blinded to treatment allocation because of differences in formulation and packaging (bottles for gel vs. tubes for ointments). Atrophy was not assessed. The sponsor supplied unpublished data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A designated third person performed allocation of the investigational products at all 18 centres. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study was single‐blind (investigator); it was not possible to blind participants to treatments because of differences in vehicle formulations. |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | This was demonstrated. |
Langley 2011 (P).
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated (2:2:1) Concealment: adequate: treatments dispensed by non‐study staff. BLINDING Single‐blind (investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 458
Treatment duration: 8 wks; FU: 16 wks
LF: 0 (0%)
BC: yes
Age: 51.6 (14SD)
Gender (per cent men): 62.2%
Ethnicity (per cent white): 93.9% Severity: mPASI = 9.39, range = 2.4 to 59.4; IGA moderate = 68.3%; IGA severe = 29.5%; IGA very severe = 2.2%; per cent BSA = 9.3 (8.2SD) Duration (yrs): 19.8 (13.3SD) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
Participants achieving clear IGA stopped treatment and restarted if psoriasis reappeared. There was follow up of responders only (IGA clear or almost clear). |
|
| Outcomes |
|
|
| Notes | Leo Pharma A/S, Ballerup, Denmark, sponsored the trial. Participants could not be completely blinded to treatment allocation because of differences in formulation and packaging (bottles for gel vs. tubes for ointments). Atrophy was not assessed. The sponsor supplied unpublished data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A designated third person performed allocation of the investigational products at all 18 centres. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study was single‐blind (investigator); it was not possible to blind participants to treatments because of differences in vehicle formulations. |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | This was demonstrated. |
Langner 1992.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: unclear Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 29 Treatment duration: 6 wks; FU: 6 wks LF: 0 (0%) BC: yes Age: mean: 40.5 (range = 16‐77) Gender (per cent men): 69.0% Severity: not reported INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. All participants received 2 weeks' pre‐treatment with vehicle BD. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Low risk | This was demonstrated. |
Langner 1993.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: unclear Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 32 Treatment duration: 6 wks; FU: 6 wks LF: 2 (6.3%) BC: yes Age: mean: 42.4 (range = 16 to 77) Gender (per cent men): 62.5% Severity: global severity score (0 to 4) = 3.5 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. All participants received 2 weeks' pre‐treatment with vehicle BD. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 6.3% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Langner 2001 (H).
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: unclear Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 44 Treatment duration: 6 wks; FU: 6 wks (14 wks for responders) LF: 4 (9.1%) BC: not reported Age: not reported Gender (per cent men): 54.5% Severity: not reported INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. All participants received 2 weeks' pre‐treatment with vehicle BD. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 9.1% |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Unclear risk | The trial did not report this. |
Langner 2001 (P).
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: unclear Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 44 Treatment duration: 6 wks; FU: 6 wks (14 wks for responders) LF: 4 (9.1%) BC: not reported Age: not reported Gender (per cent men): 54.5% Severity: not reported INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. All participants received 2 weeks' pre‐treatment with vehicle BD. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 9.1% |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Unclear risk | The trial did not report this. |
Lassus 1991.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: block randomisation Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 50 Treatment duration: 12 wks; FU: 12 wks LF: 8 (16%) BC: yes Age: 42.8 (range = 22 to 50; N = 42) Gender (per cent men): 45.2% (N = 42) Severity: TSS (0 to 12) = 7.72 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. There was SD imputation (TSS). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Low risk | Block randomisation was used. |
| Loss to follow up | Low risk | 16.0% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Lebwohl 2002.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported (but ratio 3:1 used) Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 81 Treatment duration: 2 wks; FU: 4 wks LF: 5 (6.2%) BC: yes Age: not reported Gender (per cent men): not reported Severity: pruritis (0 to 4) = 2.11 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Connetics Corporation sponsored the trial. Only non‐scalp sites were treated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 6.2% |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Low risk | ‐ |
Lebwohl 2004.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 167 Treatment duration: 8 wks; FU: 8 wks LF: 30 (18%) BC: yes Age: 48.0 Gender (per cent men): 58.7% Severity: Static Severity Score (SSS) (0 to 6) (median) = 3 (range = 1.5 to 5.0); per cent with concurrent plaque‐type lesions = 85% INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Fujisawa Healthcare Inc sponsored the trial. Loss to follow up was reported as 11/167. Non‐study body sites received usual topical treatment. No adequate effectiveness data were reported or available from the sponsor. This was an inverse psoriasis trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 18.0% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Lebwohl 2007.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Not described | |
| Participants | N: 418 Treatment duration: 8 wks; FU: 8 wks LF: 0 (0%) BC: NR separately (see notes) Age: NR Gender (per cent men): NR Severity: GSS (0 to 5) = 2.81 (0.40SD); DSS (0 to 12) (bony areas) = 6.4 (1.24SD); (non‐bony areas) = 6.4 (1.33SD) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Galderma R&D Inc, Princeton, New Jersey, sponsored the trial. The main publication reports only pooled results and baseline statistics from 2 studies (Lebwohl 2007; Powers 2005). The sponsor supplied unpublished data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0% |
| Baseline assessments | Unclear risk | The trial did not report these. |
| Baseline comparability demonstrated | Unclear risk | This was not demonstrated. |
Lee 2007.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Single‐blind (investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 142 Treatment duration: 6 wks; FU: 14 wks LF: 11 (7.7%) BC: yes (clinical only) Age: NR Gender (per cent men): NR Severity: PASI = 3.75 (2.90SD) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Sponsor: NR The trial was set in Korea. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study was single‐blind (investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 7.7% |
| Baseline assessments | Unclear risk | Clinical assessments were reported; demographics were unclear. |
| Baseline comparability demonstrated | Unclear risk | Only clinical comparability was demonstrated. |
Lepaw 1978.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: predetermined schedule using identical containers coded with participant number Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 29 Treatment duration: 2 wks; FU: 2 wks LF: 2 (6.9%) BC: inadequately reported Age: 14 to 75 Gender (per cent men): 55.2% Severity: not reported INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. This was a scalp trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 6.9% |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Unclear risk | The trial did not report this. |
Levine 2010 (H).
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: computer‐generated randomisation table Concealment: adequate BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 168 Treatment duration: 12 wks; FU: 12 wks LF: 4 (2.4%) BC: stated, not demonstrated Age: not reported Gender (per cent men): not reported Severity: TLPSS = 6.69 (1.38 SD) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions | Participants were randomised to 2 of 7 treatments:
|
|
| Outcomes |
|
|
| Notes | Dermipsor Ltd sponsored the trial.
Compliance rates (medicine usage) were reported for each regimen. The trial author supplied unpublished data. As participants were randomised to 2 of 7 treatments, some comparisons were within‐patient and others between‐patient. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | The participants, investigators, study site personnel, sponsor, and laboratories were unaware of the treatment assignments; throughout the study, the study investigator retained a set of code envelopes that were to be opened by the investigator only if deemed necessary after a serious adverse event. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Low risk | A computer‐generated randomisation table was used. |
| Loss to follow up | Low risk | 2.4% |
| Baseline assessments | Unclear risk | The trial did not report these. |
| Baseline comparability demonstrated | Unclear risk | Clinical comparability was stated (not demonstrated). |
Levine 2010 (P).
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: computer‐generated randomisation table Concealment: adequate BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 168
Treatment duration: 12 wks; FU: 12 wks
LF: 4 (2.4%)
BC: stated, not demonstrated
Age: not reported
Gender (per cent men): not reported
Severity: TLPSS = 6.69 (1.38SD) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions | Participants were randomised to 2 of 7 treatments:
|
|
| Outcomes |
|
|
| Notes | Dermipsor Ltd sponsored the trial.
Compliance rates (medicine usage) were reported for each regimen. The trial author supplied unpublished data. As participants were randomised to 2 of 7 treatments, some comparisons were within‐patient and others between‐patient. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | The participants, investigators, study site personnel, sponsor, and laboratories were unaware of the treatment assignments; throughout the study, the study investigator retained a set of code envelopes that were to be opened by the investigator only if deemed necessary after a serious adverse event. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Low risk | A computer‐generated randomisation table was used. |
| Loss to follow up | Low risk | 2.4% |
| Baseline assessments | Unclear risk | The trial did not report these. |
| Baseline comparability demonstrated | Unclear risk | Clinical comparability was stated (not demonstrated). |
Liao 2007.
| Methods |
DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described |
|
| Participants | N: 50 Treatment duration: 6 wks; FU: 6 wks LF: 1 (2.0%) BC: yes Age: 39.6 (12.8SD); range 21 to 69 Gender (per cent men): 73.5% Target lesion: face (89.8%); genitofemoral (10.2%) Severity: TAS (0 to 12) = 6.2 (3.32SD) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
No concomitant topical therapies or emollients were permitted. Dosing frequency could be reduced or medication discontinued depending on level of irritancy. |
|
| Outcomes |
|
|
| Notes | Galderma, Taiwan, sponsored the trial. Individual safety measures were reported, but the total number of participants experiencing any adverse event were not reported. The trial author supplied unpublished data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 2.0% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | This was demonstrated. |
Lin 2007.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Single‐blind (investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 14 Treatment duration: 8 wks; FU: 8 wks LF: 4 (28.6%) BC: yes Age: 35.8 (10.4SD), range = 21 to 54 Gender (per cent men): 78.6% Severity: PSI (0 to 12) = 9.9 (1.2SD); PASI = 11.7 (4.8SD), range = 5.5 to 19.2; per cent BSA = 13.6 (6.1SD), range = 3% to 20% Duration (yrs): 110.8 (9.4SD); range = 2 to 30 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The sponsor was not reported. Assessments were conducted by 2 blinded investigators. A 3‐month uncontrolled follow‐study also conducted. The trial author supplied unpublished data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study was single‐blind (investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | High risk | 28.6% |
| Baseline assessments | Unclear risk | These were reported. |
| Baseline comparability demonstrated | Unclear risk | This was demonstrated. |
Lin 2008.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Single‐blind (investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 42 Treatment duration: 12 wks; FU: 12 wks LF: 0 (0%) BC: Yes Age: 34.6 (11.SD); range = 18 to 58 Gender (per cent men): 76.2% Severity: PASI (median) = 14.7; per cent BSA (median) = 18%; TSS (0 to 24) = 18.8 (3.03SD) Duration (yrs): 10 (median); range = 2 to 41 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
Participants achieving clearance before study end point could stop treatment. |
|
| Outcomes |
|
|
| Notes | The trial was supported by a grant: CMRPG33024 from Chang Gung Memorial Hospital. Participants were instructed to wash skin thoroughly before visits; assessments were based on photographs. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study was single‐blind (investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Unclear risk | These were reported. |
| Baseline comparability demonstrated | Unclear risk | This was demonstrated. |
Lister 1997.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Single‐blind (investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 171 Treatment duration: 8 wks; FU: 16 wks LF: not reported BC: psoriasis comparable, demographics unclear Age: not stated Gender (per cent men): not stated Severity: TSS (scale NR) = 6.24 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Bioglan Laboratories sponsored the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient detail. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was single‐blind (investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Unclear risk | The trial did not report this. |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Unclear risk | This was partially demonstrated. |
Lowe 2005.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: consecutive balanced blocks of 7 (3:3:1) Concealment: unclear BLINDING Single‐blind (investigator); as cream and lotion were compared, not possible to blind patients WITHDRAWAL/DROPOUT Described | |
| Participants | N: 192 Treatment duration: 4 wks; FU: 8 wks LF: 0 (0%) BC: yes Age: 48.65; range = 19 to 79 Gender (per cent men): 65.6% Ethnicity (per cent white): 82.8% Severity: DSS (0 to 12) = 7.60 (1.55SD) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
Participants were randomised to clobetasol propionate cream or lotion; results were aggregated for review purposes. Other affected areas could also be treated. There was a 4‐wk treatment‐ free follow‐up period. |
|
| Outcomes |
|
|
| Notes | Galderma R&D, Sophia Antipolis, France, sponsored the trial. We sought unpublished data, but it was reported to be unavailable. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study was single‐blind (investigator); as the cream and lotion were compared, it was not possible to blind participants. |
| Randomisation method reported | Low risk | Participants allocated in consecutive balanced blocks of 7 using the following ratios: 3:3:1. |
| Loss to follow up | Low risk | 0% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | This was demonstrated. |
Luger 2008.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 869 Treatment duration: 52 wks; FU: 52 wks LF: 55 (6.3%) BC: yes Age: 48.7 (15.0SD), range = 18 to 86 Gender (per cent men): 44.0% Ethnicity (per cent white): 96.7% Severity: IGA moderate = 55.5%; IGA severe = 37.8%; IGA very severe = 6.7% Duration (yrs): 17.6 (14.2SD) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
Participants achieving clearance could stop treatment, but remained in the trial, and restart as needed. The regimen was intended to reflect usual clinical practice. |
|
| Outcomes |
|
|
| Notes | Leo Pharma, Ballerup, Denmark,sponsored the trial. Adverse events were recorded by investigators and reviewed by an adjudication committee (Data Safety Monitoring Board) comprising of 3 dermatologists blinded to treatment assignment. The sponsor supplied unpublished data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | This was not stated. |
| Loss to follow up | Low risk | 6.3% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | This was demonstrated. |
Maier 2004.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Not described | |
| Participants | N: 34 Treatment duration: 8 wks; FU: 8 wks LF: 10 (29.4%) BC: yes (clinical only) Age: NR Gender (per cent men): 55.9% Severity: mPASI = 6.3 (2.2SD) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not state the sponsor. This was an abstract only. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | High risk | 29.4% |
| Baseline assessments | Unclear risk | Clinical assessments were reported. |
| Baseline comparability demonstrated | Unclear risk | Clinical comparability was demonstrated. |
Medansky 1987.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 121 Treatment duration: 3 wks; FU: 3 wks LF: 6 (5.0%) BC: yes, except duration of disease (P = 0.04) Age: 53.8 (range = 16 to 80) Gender (per cent men): 67.8% Severity: TSS (0 to 9) = 6.6 Duration (yrs): 17.9 (range = 1 to 52) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial was supported in part by a grant from Schering Corporation. There was SD imputation (TSS). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 67.8% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | Baseline comparability was demonstrated (only significant difference: duration of disease). |
Medansky 1996.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: "schedule" Concealment: unclear BLINDING Double‐blind (participant/assessor) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 134 Treatment duration: 3 wks; FU: 3 wks LF: 6 (4.5%) BC: unclear Age: 47 (range = 20 to 81) Gender (per cent men): not stated Severity: TSS (0 to 9) = 6.5; per cent unstable psoriasis = 28% INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Dermik Laboratories Inc. sponsored the trial. Adverse events: itching and burning There was SD imputation (TSS/IAGI). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/assessor). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 4.5% |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Unclear risk | The trial did not report this. |
Menter 2009.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Open (blinding NS but product vehicles differ) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 122
Treatment duration: 4 wks; FU: 8 wks
LF: 0 (0%)
BC: yes
Age: 46.5 (14.2SD)
Gender (per cent men): 61.1%
Duration (yrs): 16.4 (11.50SD)
Severity: ODS = 3.06 (0.43SD)
(participant demographics based on per‐protocol sample). INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Galderma Laboratories, LP, sponsored the trial. Atrophy was not assessed. Telangiectasia was not assessed. 29 participants were 'non‐compliant' with treatment, but intention‐to‐treat (ITT) analysis included all enrolled participants. The sponsor supplied unpublished data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was open (blinding not reported, but product vehicles differ). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | This was demonstrated. |
Molin 1997.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Double‐blind (participant/assessor) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 421 Treatment duration: 8 wks; FU: 8 wks LF: 4 (1%) BC: Psoriasis comparable, demographics not reported Age: not stated Gender (per cent men): not stated Severity: no summary measure reported INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Leo Pharmaceutical Products sponsored the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/assessor). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 1.0% |
| Baseline assessments | Unclear risk | The trial did not report these. |
| Baseline comparability demonstrated | Unclear risk | This was partially demonstrated. |
Monastirli 2000.
| Methods | DESIGN Between‐patient Delivery unclear ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Open WITHDRAWAL/DROPOUT Described | |
| Participants | N: 70 Treatment duration: 10 wks; FU: 10 wks LF: not reported BC: yes Age: 45.7 Gender (per cent men): 57.1% Severity: PASI =
Duration (yrs): 17 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. Treatment was delivered in an inpatient setting. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was open. |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Unclear risk | The trial did not report this. |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Mortensen 1993b.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 34 Treatment duration: 3 wks; FU: 4 wks LF: 0 (0%) BC: psoriasis comparable, demographics inadequately reported. Age: 43 (range = 26 to 75) Gender (per cent men): 58.8% Severity: PASI = 12.2 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Leo Pharmaceuticals, Denmark Duration (yrs) sponsored the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Unclear risk | This was partially demonstrated. |
Olsen 1991.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 378 Treatment duration: 2 wks; FU: 3 wks LF: 1 (0.3%) BC: yes Age: 46 (range = 18 to 88) Gender (per cent men): 45% Severity: 80% moderate (6 ≤ TSS < 7.5), 20% severe (TSS > 7.5) Duration (yr): 12.0 (9.7SD, N = 377); range = 0.4 to 55.0 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | In part, Glaxo Inc. sponsored the trial. This was a scalp trial. There was SD imputation (TSS). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0.3% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Olsen 1996 (1).
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described (for both trials together) | |
| Participants | N: 181 Treatment duration: 4 wks; FU: 4 wks LF: 3 (1.7%) BC: yes Age: 49 (range = 15 to 76) Gender (per cent men): 68.0% Severity: per cent BSA affected = 12.0% (range = 1% to 80%); per cent BSA treated = 11% (range = 1% to 80%) Duration (yrs): 19 (range = 1 to 60) | |
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 1.7% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Olsen 1996 (2).
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described (for both trials together) | |
| Participants | N: 207 Treatment duration: 4 wks; FU: 4 wks LF: 2 (1.0%) BC: yes Age: 45 (range = 12 to 87) Gender (per cent men): 52.7% Severity: per cent BSA affected = 12.5% (range = 1% to 80%); per cent BSA treated = 12% (range = 1% to 80%) Duration (yrs): 16 (range = 0.8 to 52) | |
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 1.0% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Oranje 1997.
| Methods | DESIGN Between‐patient Patient/parent delivery ALLOCATION Random Method of randomisation: computer‐generated random number table used by 7/14 centres to randomly select ≤ 3 participants Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 77 Treatment duration: 8 wks; FU: 8 wks LF: 0 (0%) BC: yes Age: 10 (range = 2 to 14) Gender (per cent men): 46.8% Severity: not reported INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Leo Pharmaceutical Products, Denmark, sponsored the trial. The trial participants were children. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Low risk | Randomisation was computer‐generated. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Low risk | ‐ |
Ormerod 1997.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Not described | |
| Participants | N: 12 Treatment duration: 2 wks; FU: 2 wks LF: unclear BC: unclear Age: not reported Gender (per cent men): not reported Severity: TSS (0 to 24) =12.2 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Wyeth‐Ayerst Research and Glaxo Dermatology sponsored the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Unclear risk | The trial did not report this. |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Unclear risk | The trial did not report this. |
Ormerod 2000.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 17 Treatment duration: 4 wks; FU: 4 wks LF: 0 (0%) BC: not reported Age: not reported Gender (per cent men): not reported Severity: TSS (0 to 24) = 15.5 (SD: 3.7; N = 17) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Unclear risk | The trial did not report this. |
Ormerod 2005.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: randomised in blocks of 4 by the pharmacy department using 'Minitab' Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Unclear | |
| Participants | N: 24 Treatment duration: 12 wks; FU: 12 wks LF: 2 (8.3%) BC: yes Age: 47.5 (range = 28 to 78) Gender (per cent men): 75.0% Severity: TSS (0 to 24) = 17.0 (3.3SD, N = 22) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
Dosing frequency was not reported. |
|
| Outcomes |
|
|
| Notes | Wyeth Research, Philadelphia, sponsored the trial. No systemic adverse event was considered clinically significant. The daily dosing frequency was unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Low risk | Block randomisation was used. |
| Loss to follow up | Low risk | 9.3% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Ortonne 1994.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: participants allocated sequentially upon inclusion Concealment: unclear BLINDING Double‐blind (participant/assessor) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 188 Treatment duration: 6 wks; FU: 6 wks LF: 32 (17.0%) BC: yes Age: 46.0 (range = 20 to 85) Gender (per cent men) 67.3% Severity: per cent BSA = 18.9%; PASI = 11.67; per cent unstable psoriasis = 40% Duration (yrs): 14.9 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Leo Pharmaceuticals sponsored the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/assessor). |
| Randomisation method reported | Low risk | Randomisation was sequential. |
| Loss to follow up | Low risk | 17.0% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Ortonne 2003.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: computer‐generated randomisation by blocks of 4 using RANUNI routine of the SAS system Concealment: unclear BLINDING Single‐blind (investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 75 Treatment duration: 6 wks; FU: 6 wks LF: 10 (13.3%) BC: yes, within‐patient design but comparability of lesions at baseline not reported Age: 44.5 (14.5SD: range = 18.8 to 70.7) Gender (per cent men): 53% Severity: not reported INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship, but 2 authors worked for Galderma, France This was an inverse psoriasis trial. There was SD imputation (TSS). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was single‐blind (investigator). |
| Randomisation method reported | Low risk | A computer‐generated block list was used for randomisation. |
| Loss to follow up | Low risk | 13.3% |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Unclear risk | This was partially demonstrated. |
Ortonne 2004.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: computer‐generated randomisation schedule Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 501 Treatment duration: 8 wks; FU: 8 wks LF: 21 (4.2%) BC: yes Age: 51.2 (15.0SD, N = 501) Gender (per cent men): 54.9% Severity: PASI = 9.8 (6.1SD, N = 501) Duration (yrs): 19.4 (14.6SD, N = 501) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Leo Pharmaceutical Products sponsored the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Low risk | Randomisation was computer‐generated. |
| Loss to follow up | Low risk | 4.2% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Ortonne 2006.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: generated by sponsor; stratified by centre (N = 16); Concealment: unclear BLINDING Single‐blind (investigator); as cream and gel were compared, it was not possible to blind participants WITHDRAWAL/DROPOUT Described | |
| Participants | N: 124 Treatment duration: 12 wks; FU: 14 wks LF: 0 (0%) BC: yes Age: 48.2 (14.8SD); range = 18 to 82 Gender (per cent men): 68.5% Ethnicity (per cent white): 96.0% Severity: per cent BSA = 6.3 ((2.2SD), range = 1% to 10%; LPSI (0 to 12) = 7.2 (1.7SD), range = 4 to 12; PASI (range NS) = 7.1 (3.7SD), range = 1.4 to 21.8 Duration (yrs): 18.9 (13.9SD); range = 0.7 to 69.3 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
There were10 to 14 hours between applications; lesions were treated for 7 days following clearance. Participants were randomised to tacrolimus cream or gel; results were aggregated for review purposes. |
|
| Outcomes |
|
|
| Notes | Fujisawa GmbH, Munich, Germany, sponsored the trial. Vissers 2008 (secondary reference under Ortonne 2006) reports immunohistochemistry findings for subgroup (N = 18). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study was single‐blind (investigator); as the cream and gel were compared, it was not possible to blind participants. |
| Randomisation method reported | Low risk | Randomisation was generated by the sponsor and stratified by centre. |
| Loss to follow up | Low risk | 0% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | This was demonstrated. |
Ortonne 2010.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: pre‐planned computer‐generated schedule Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 408 Treatment duration: 8 wks; FU: 8 wks LF: 9 (2.2%) BC: yes Age: 45.6; range = 18 to 86 Gender (per cent men): 54.7% Ethnicity (per cent white): 97.8% Severity: PASI = 9.6, range = 0.9 to 32.7; IGA moderate = 68.6% Duration (yrs): 17.7; range = 0 to 70 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes | Outcomes were assessed for the face and body overall and for the face only:
|
|
| Notes | Leo Pharma A/S sponsored the trial. The sponsor supplied unpublished data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Low risk | A preplanned computer‐generated schedule was used. |
| Loss to follow up | Low risk | 2.2% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | This was demonstrated. |
Papakostantinou 2005.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 22 Treatment duration: 2 wks; FU: 2 wks LF: 0 (0%) BC: no Age: 41.3 (3.94SD) Gender (per cent men): 45.5% Severity: NS INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The sponsor was not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details (abstract only). |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0% |
| Baseline assessments | Unclear risk | The trial did not report this (but this was a within‐patient study, so demographics will be comparable by definition). |
| Baseline comparability demonstrated | Unclear risk | This was not demonstrated (see comment above). |
Papp 2003 (H).
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: computer‐generated random code Concealment: adequate BLINDING Double‐blind (participant/assessor) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 1043 Treatment duration: 4 wks; FU: 4 wks LF: 15 (1.4%) BC: yes Age: 47.1 Gender (per cent men): 58.4% Severity: mean PASI = 10.8 (range = 1 to 36) Duration: 18.7 years INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Leo Pharmaceuticals sponsored the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Treatments were assigned by computer‐generated code and assigned chronologically at each centre. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). Treatments were identifiable only by a code number. |
| Randomisation method reported | Low risk | Randomisation was computer‐generated. (3:3:3:1) |
| Loss to follow up | Low risk | 1.4% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Papp 2003 (P).
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: computer‐generated random code Concealment: adequate BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 1043
Treatment duration: 4 wks; FU: 4 wks
LF: 15 (1.4%)
BC: yes
Age: 47.1
Gender (per cent men): 58.4%
Severity: mean PASI = 10.8 (range = 1 to 36)
Duration: 18.7 years INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Leo Pharmaceuticals sponsored the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Treatments were assigned by computer‐generated code and assigned chronologically at each centre. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator), and treatments were identifiable only by a code number. |
| Randomisation method reported | Low risk | Randomisation was computer‐generated. (3:3:3:1) |
| Loss to follow up | Low risk | 1.4% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Pariser 1996.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/assessor) WITHDRAWAL/DROPOUT Not described | |
| Participants | N: 235 Treatment duration: 8 wks; FU: 8 wks LF: Unclear BC: psoriasis comparable, demographics not reported. Age: 45.1 (range = 18 to 86) Gender (per cent men): not reported Severity: TSS (0 to 9) = 4.75 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Bristol‐Myers Squibb Pharmaceutical Research Institute sponsored the trial. There was SD imputation (TSS). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/assessor). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Unclear risk | The trial did not report this. |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Unclear risk | This was partially demonstrated. |
Pauporte 2004.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 89 Treatment duration: 3 wks; FU: 4 wks LF: 4 (4.5%) BC: yes Age: 46 (15SD) Gender (per cent men): 44.8% Severity: TSS (0 to 9) = 7.15 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
Participants washed their hair with a non‐medicated shampoo after treatment. |
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship, but the corresponding author worked for Hill Dermaceuticals Inc, US. To be eligible for inclusion in the efficacy analysis, participants were permitted to deviate from the treatment plan < = 2 consecutive days and < = 4/10 days. No case of skin atrophy or telangiectasia were reported. This was a scalp trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 4.5% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Perez 1996.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 84 Treatment duration: 10 wks: FU: 52 wks LF: 0 (0%) BC: yes Age: 46 (range = 19 to 76) Gender (per cent men): 65.5% Severity: TSS (0 to 9) = 7.6 (range = 5 to 9) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The NIH General Clinical Research Center supported the trial. There was also an uncontrolled follow up study (N = 22) involving large‐area administration of calcitriol; 12‐month results were based on results from 6 participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Pinheiro 1997.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Open WITHDRAWAL/DROPOUT Described | |
| Participants | N: 132 Treatment duration: 8 wks; FU: 8 wks LF: 10 (7.6%) BC: yes Age: 48.2 (range = 17 to 90) Gender (per cent men): 59.1% Severity: per cent severe = 13.6% Duration (yrs): 16.9 (range = 0.5 to 60) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Leo Pharmaceuticals sponsored the trial. There was SD imputation (TSS). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was open. |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 7.6% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Poulin 2010.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 217
Treatment duration: 6 mths; FU: 6 mths
LF: 45 (20.7%)
BC: yes
Age: 50.4; range = 18 to 82
Gender (per cent men): 44.7%
Ethnicity (per cent white): 91.2%
Severity: GSS = 0 (clear): 10.1%; GSS = 0 (very mild): 33.7%; GSS = 0 (mild): 56.2%; < 20% scalp affected = 76.5%; mPASI = 4.2 (4.2SD) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Galderma Laboratories, LP, sponsored the trial. The trial (JDT) reported findings for a subset with moderate scalp psoriasis. The sponsor supplied unpublished safety data. No useable efficacy data were available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Unclear risk | 20.7% |
| Baseline assessments | Low risk | These were reported (clinical and demographic). |
| Baseline comparability demonstrated | Low risk | This was demonstrated. |
Powers 2005.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: unclear Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Not described | |
| Participants | N: 421
Treatment duration: 8 wks; FU: 8 wks
LF: 0 (0%)
BC: yes (clinically), demographics NR (abstract)
Age: NR
Gender (per cent men): NR
Per cent white: NR
Severity: GSS (0 to 5) = 2.71 (0.45SD) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
Max 30 g/day |
|
| Outcomes |
|
|
| Notes | Galderma Laboratories, LP, sponsored the trial. The sponsor supplied unpublished data. This was an abstract only. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0% |
| Baseline assessments | Unclear risk | Clinical assessments were reported (demographic characteristics was unclear). |
| Baseline comparability demonstrated | Unclear risk | Clinical comparability was demonstrated (demographic comparability was unclear). |
Reygagne 2005.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: computer‐generated randomisation list Concealment: unclear BLINDING Single‐blind (investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 151
Treatment duration: 4 wks; FU: 4 wks
LF: 0 (0%)
BC: yes
Age: 45.3 (17.1SD)(range = 10 to 89)
Gender (per cent men): 47.0%
Per cent white: 98.7%
Severity: TSS (0 to 9) = 4.90 (1.74SD); GSS (0 to 5) = 1.50 (0.60SD)
Per cent scalp affected: 45% (SD28%) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Galderma Laboratories, LP, sponsored the trial. The sponsor supplied unpublished data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study was single‐blind (investigator). |
| Randomisation method reported | Low risk | A computer‐generated randomisation list was used. |
| Loss to follow up | Low risk | 0% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | This was demonstrated. |
Ruzicka 1998.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Double‐blind (participant/assessor) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 178 Treatment duration: 2 + 4 wks; FU: 14 wks LF: 7 (3.9%) BC: psoriasis comparable, demographics not reported Age: 42 (range = 18 to 80) Gender (per cent men): 55.6% Severity: PASI = 6.0 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not state sponsorship. Schering AG employed 1 author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 3.9% |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Unclear risk | This was partially demonstrated. |
Salmhofer 2000.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: NR BLINDING Double‐blind (participant/assessor) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 63 Treatment duration: 4 wks; FU: 8 wks LF: 5 (7.9%) BC: yes Age: 47 (15.4SD, range = 19 to 83) Gender (per cent men): 54.0% Severity: PASI = 5.5 (2.65SD) Duration (months): 141 (124SD) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Schering Wien GmbH sponsored the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/assessor). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 7.9% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Sanchez 2001.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Unclear WITHDRAWAL/DROPOUT Described | |
| Participants | N: 28 Treatment duration: 8 wks; FU: 8 wks LF: 3 (10.7%) BC: yes Age: 49.5 (range = 22 to 73) Gender (per cent men): 50% Severity: PASI = 7.7 (range = 4 to 10) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial did not report this. |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 10.7% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Santoianni 2001.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: computer‐generated list using block randomisation; pharmacy‐dispensed treatments in identical tubes Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 85 Treatment duration: 3 wks; FU: 3 wks LF: 4 (4.7%) BC: clinical only Age: 51.9 (range = 18.8 to 88.5) Gender (per cent men): 55.3% Severity: PASI = 6.2 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | IDI Farmaceuticia SpA sponsored the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Low risk | Randomisation was computer‐generated. |
| Loss to follow up | Low risk | 4.7% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Unclear risk | This was partially demonstrated. |
Saraceno 2007.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: computer‐generated randomisation schedule; boxes dispensed sequentially Concealment: inadequate BLINDING Open WITHDRAWAL/DROPOUT Described | |
| Participants | N: 150 Treatment duration: 12 wks; FU: 12 wks LF: 3 (2.0%) BC: yes Age: 47.7 (16.5SD); range = 18 to 83 Gender (per cent men): 66.0% Severity: per cent BSA = 18.6 (7.6SD), range 2 to 30; PASI = 9.2 (4.8SD), range = 1.6 to 33.0 Duration (yrs): 13.8 (13.4SD); range = 0 to 60.1 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Prodotti Formenti Srl, Milano, Italy, sponsored the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Consecutive coded treatment boxes were assigned to participants in a 1:1 ratio. It was unclear if boxes were of identical appearance. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was open. |
| Randomisation method reported | Low risk | A computer‐generated randomisation schedule was used. |
| Loss to follow up | Low risk | 2.0% |
| Baseline assessments | Low risk | These were reported (clinical and demographic). |
| Baseline comparability demonstrated | Low risk | This was demonstrated (clinical and demographic). |
Scarpa 1994.
| Methods | DESIGN Between‐patient Delivery unclear ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Blinding unclear WITHDRAWAL/DROPOUT Not described | |
| Participants | N: 160 Treatment duration: 6 wks; FU: 10 wks LF: not reported BC: demographics comparable, severity not reported Age: 50 Gender (per cent men): 68.1% Severity: not reported INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial did not report this. |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Unclear risk | The trial did not report this. |
| Baseline assessments | Unclear risk | These were partially done. |
| Baseline comparability demonstrated | Unclear risk | This was partially demonstrated. |
Scarpa 1996.
| Methods | DESIGN Within‐patient Delivery unclear ALLOCATION Random Method of randomisation: block randomisation (6 participants) Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 76 Treatment duration: 6 wks; FU: 8 wks LF: 13 (17.1%) BC: yes Age: not reported Gender (per cent men): not reported Severity: TSS (0 to 12) = 7.92 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. There was SD imputation (TSS). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Low risk | Block randomisation was used. |
| Loss to follow up | Low risk | 17.1% |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Low risk | ‐ |
Scarpa 1997.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported; tubes labelled left or right and with participant ID number and tube ID number Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 157 Treatment duration: 6 wks; FU: 7 wks LF: 23 (14.6%) BC: yes Age: 49 (15SD; N = 134) Gender (per cent men): 65.6% (N = 157) Severity: TSS (0 to 12) = 7.7 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship, but Istituto Gentili SpA provided medications and appeared to have undertaken the randomisation. There was SD imputation (TSS). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 14.6% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Sears 1997.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 190 participants Treatment duration: 3 wks; FU: 3 wks LF: 21 (11%) BC: yes Age: 44 (range = 19 to 73) Gender (per cent men): 47.9% Severity: TSS (0 to 9) = 6.0 Duration (yrs): 17 (range = 1 to 56) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 11% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | ‐ |
Seidenari 1997 (H).
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/assessor) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 14 Treatment duration: 6 wks; FU: 8 wks LF: 3 (21.4%) BC: yes Age: 46 (range 23 to 69, N = 26) Gender (per cent men): 46.2% (N = 26) Severity: TSS = 6.31 (1.25SD, N = 11) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Demographic characteristics were summarised over both studies, placebo and active controls (N = 26). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/assessor). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Unclear risk | 21.4% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | ‐ |
Seidenari 1997 (P).
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/assessor) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 12
Treatment duration: 6 wks; FU: 8 wks
LF: 1 (8%)
BC: yes
Age: 46 (range 23 to 69, N = 26)
Gender (per cent men): 46.2% (N = 26)
Severity: TSS = 6.50 (0.76SD, N = 11) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Demographic characteristics were summarised over both studies, placebo and active controls (N = 26). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/assessor). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 8.0% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | ‐ |
Shuttleworth 1998.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: predetermined randomisation schedule in blocks of 10 Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 40 Treatment duration: 4 wks; FU: 4 wks LF: 3 (7.5%) BC: yes Age: 41.4 (12.0SD) Gender (per cent men):60.0% Severity: investigator's overall assessment (0 to 9) = 4.85; patient's overall assessment (0 to 4) = 2.43 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Stiefel Laboratories sponsored the trial. The study was underpowered to detect a statistically significant difference due to recruitment difficulties. This was a scalp trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Low risk | Block randomisation was used. |
| Loss to follow up | Low risk | 7.5% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Staberg 1989.
| Methods | DESIGN Within‐patient Delivery unclear ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 10 Treatment duration: 6 wks; FU: 6 wks LF: 1 (10%) BC: yes Age: 50 (26 to 76) Gender (per cent men): not reported Severity: TSS (0 to 9) = 7.3 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Leo Pharmaceutical Products supplied the drugs and undertook the statistical analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 10.0% |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Low risk | ‐ |
Stein 2001.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: investigator undertook randomisation Concealment: inadequate BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 40 Treatment duration: 12 wks; FU: 12 wks LF: 3 (7.5%) BC: unclear Age: range = 20 to 70 + Gender (per cent men): not reported Severity: TSS (elbows) (0 to 12) = 7.0 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The Connetics Corporation sponsored the trial. There was SD imputation (IAGI). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Allocation concealment was inadequate: The investigator undertook randomisation. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 7.5% |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Unclear risk | The trial did not report this. |
Stuecker 2001.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Blinding unclear WITHDRAWAL/DROPOUT Described | |
| Participants | N: 13 Treatment duration: 12 wks; FU: 12 wks LF: 2 (15.4%) BC: yes Age: 52.9 (12.2SD, range = 38 to 67) Gender (per cent men): 76.9% Severity: PASI = 9.11 (4.88SD, range = 2.20 to 18.70) Duration: 20.8 (12.7SD) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Regeneratio Pharma AG sponsored the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial did not report this. |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 15.4% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Stutz 1996.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: computer‐generated randomisation code to allocate sides Concealment: adequate BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Not described | |
| Participants | N: 15 Treatment duration: 3 wks; FU: 3 wks LF: 2 (13.3%) BC: not reported Age: range = 21 to 68 Gender (per cent men): The trial did not report this. Severity: TSS (scale not reported) = 2.8 (0.3SD) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Babcock Dermatologic Endowment and the alumni of the Department of Dermatology, University of Michigan Medical Center, MI, sponsored the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Body sites were selected then assigned a computer‐generated randomisation code that was concealed from both investigators and participants. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Low risk | Randomisation was computer‐generated. |
| Loss to follow up | Low risk | 13.3% |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Unclear risk | The trial did not report this. |
Sudilovsky 1981.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: identical tubes allocated by random numbers table Concealment: adequate BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 78 (57% psoriasis) Treatment duration: 3 wks; FU: 3 wks LF: 0% BC: Inadequately reported Age: not reported Gender (per cent men): not reported Severity: not reported INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The Squibb Institute for Medical Research sponsored the trial. Part of a larger study involving participants with atopic dermatitis and trialling other dosages; aggregated demographics were reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | The part of the study (I or II) and side of the body chosen for treatment were concealed from investigators and determined by random numbers table. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Unclear risk | The trial did not report these. |
| Baseline comparability demonstrated | Unclear risk | The trial did not report this. |
Sutton 2001.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 53 participants Treatment duration: 8 wks; FU: 12 wks LF: 5 (9.4%) BC: yes Age: range = 26 to 67 Gender (per cent men): 58.5% Severity: duration (yrs) = 2 to 50 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Cato Research Ltd and Durham Pharmaceuticals LLC, NC, sponsored the trial. They treated lesions < = 20% BSA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 9.4% |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Low risk | ‐ |
Syed 1996.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 60 Treatment duration: 4 wks; FU: 52 wks LF: 0 (0%) BC: yes Age: 25.6 (range = 18 to 50) Gender (per cent men): 60.0% Severity: PASI = 9.3 (range = 4.8 to 16.7) Duration (yrs): 8.5 (range = 1 to 21) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. Concomitant water soluble emollients were permitted. SDs were imputed (PASI). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Syed 2001b.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 60 participants Treatment duration: 4 wks; FU: 8 wks LF: 0 (0%) BC: yes Age: 29.3 (range = 18 to 70) Gender (per cent men): 61.7% Severity: PASI = 9.8 (range = 5.3 to 17.5) Duration (yrs): 9.6 (range = 1 to 24) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. SDs were imputation (PASI). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Tham 1994.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: computer‐generated random numbers Concealment: unclear BLINDING Single‐blind (investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 30 Treatment duration: 6 wks; FU: 6 wks LF: 3 (10%) BC: yes Age: 40 (range = 20 to 74) Gender (per cent men): 56.7% Ethnicity: Chinese (70.0%), Indian (16.7%), Malay (10.0%), and Sikh (3.3%) Severity: PASI = 6.65 Duration (years): 9.7 (range = 2 to 20) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Leo Pharmaceutical Products sponsored the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was single‐blind (investigator). |
| Randomisation method reported | Low risk | Randomisation was computer‐generated. |
| Loss to follow up | Low risk | 10.0% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Tyring 2010.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: computer‐generated schedule (3:1), stratified by ethnicity; Concealment: adequate BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 177 Treatment duration: 8 wks; FU: 52 wks LF: 0 (0%) (at 8 wks) BC: yes Age: 44.7 (range = 18 to 76) Gender (per cent men): 63.3% Ethnicity: per cent white = 0%; per cent Hispanic/Latino: 56%; per cent black/African American = 44% Severity: scalp IGA (moderate) = 80.2%; scalp IGA (severe/very severe) = 19.8%; TSS (0 to 12) = 6.3, range = 4 to 11 Duration (yrs): 10.8, range = 1 to 50 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
Maximum of 40 g/wk. Treatment was stopped if scalp psoriasis cleared. Concurrent (scalp) antipsoriatics and 'chemical treatments of the hair' were not permitted. All participants received C‐B ointment for trunk/limbs. |
|
| Outcomes |
|
|
| Notes | Leo Pharma A/S sponsored the trial This was part of a 52‐wk study: Following 8 wks of double‐blind treatment, all participants received C‐B scalp formulation for 44 wks. Data were reported only for the 8‐wk end point. The sponsor supplied unpublished data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Participants were assigned the next consecutive randomisation number available at the site for his/her ethnic category. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | Randomisation was preplanned according to a computer‐generated randomisation schedule in a ratio of 3:1, stratified by ethnicity. |
| Loss to follow up | Unclear risk | 0% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Unclear risk | This was partially demonstrated (reported means, but not standard, deviations; proportions reported). |
Tzung 2005.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Single‐blind (investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 23 Treatment duration: 12 wks; FU: 16 wks LF: 4 (17.4%) BC: yes Age: 60.2; range = 12 to 80 Gender (per cent men): 91.3% Severity: Signs = mean baseline scores ranged from 2.1 to 2.3 (SD range = 0.71 to 0.80) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not state the sponsor. The number of adverse events was described, but the number of participants experiencing ADRs was not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study was single‐blind (investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Unclear risk | 17.4% |
| Baseline assessments | Low risk | Clinical assessments were reported (within‐patient study, so demographics comparable). |
| Baseline comparability demonstrated | Low risk | Clinical comparability was demonstrated. |
Vali 2005.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: flip of coin Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 42 Treatment duration: 8 wks; FU: 8 wks LF: 3 (7.1%) BC: yes Age: 32 (12.22SD) Gender (per cent men): 66.7% Severity: PASI = 10.86 (5.15SD) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not state the sponsor. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Low risk | Randomisation was by the flip of a coin. |
| Loss to follow up | Low risk | 7.1% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | This was demonstrated. |
Van de Kerkhof 1989.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: adequate BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 10 Treatment duration: 4 wks; FU: 4 wks LF: 0 (0%) BC: yes Age: range = 28 to 72 Gender (per cent men): 70% Severity: TSS (0 to 9) = 7.2 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship, but Hoffmann‐La Roche, Switzerland , employed 1 of the authors. The authors stated that allocation was concealed to the investigator, but provided no justification. Treatment was given at subtherapeutic dose. There was SD imputation (TSS). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A randomisation code was generated centrally and concealed from the investigator until after trial completion. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Low risk | ‐ |
Van de Kerkhof 1996a.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 122 Treatment duration: 8 wks; FU: 12 wks LF: 19 (15.6%) BC: inadequately reported Age: 44.8 (13.69SD) Gender (per cent men): 62.3% Severity: BSA = 5.6% Duration (mths): 233.5 (175.9SD) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Hermal Kurt Herrmann sponsored the trial. Maximum treatment area: 10% BSA There was SD imputation (TSS). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 15.6% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Unclear risk | The trial did not report this. |
Van de Kerkhof 2002a.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Open WITHDRAWAL/DROPOUT Described | |
| Participants | N: 88 participants Treatment duration: 4 wks; FU: 5 wks LF: 7 (8.0%) BC: yes Age: not reported Gender (per cent men): not reported Severity: PASI = 16.9 (range = 4.3 to 48.0) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Leo Pharmaceutical Products sponsored the trial. The trial included inpatients. The trial reported medication quantities used: "patients complied well". 'High dose' calcipotriol was given (above recommended dosage). IAGI/PAGI: combined scalp/body TSS: scalp only PASI: body only |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was open. |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 8.0% |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Low risk | ‐ |
Van de Kerkhof 2006.
| Methods | DESIGN Between‐patient Nurse and participant delivery ALLOCATION Random Method of randomisation: computer‐generated system using telephone response Concealment: adequate BLINDING Open WITHDRAWAL/DROPOUT Described | |
| Participants | N: 106 Treatment duration: 12 wks; FU: 12 wks LF: 0 (0%) BC: yes Age (mean): 51.2; range = 25 to 83 Gender (per cent men): 59/100 (reported by de Korte 2008 ‐ this is a secondary reference under Van de Kerkhof 2006); 6 participants missing) Ethnicity: NR Severity: mPASI (mean) = 9.9, range = 2.7 to 27.0 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
Participants intolerant of 0.1% dithranol used 0.05% dose. Cases of extensive hyperkeratosis, scales of participants in both treatment groups could be removed with salicylic acid (5%) in petrolatum before wk 0. Participants achieving clearance exited the study. |
|
| Outcomes |
PRIMARY OUTCOMES
SECONDARY OUTCOMES
|
|
| Notes | Leo Pharma sponsored the trial. Secondary response criteria did not demonstrate a statistically significant difference between treatments. The sponsor supplied unpublished data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | The treatment was assignment using a telephone voice response system, to ensure that the investigators' decision to randomise the participant preceded knowledge of the randomised treatment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was open. |
| Randomisation method reported | Low risk | Randomisation was through a computer‐generated system using telephone response. |
| Loss to follow up | Low risk | 0% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Unclear risk | This was partially demonstrated (de Korte 2008). There were significantly more men in the dithranol group (72% versus 46%). However, de Korte does not provide data on 6 participants. If all these participants are assumed to be of the gender that minimises between group differences, the difference is almost statistically significant at the 5% level (= 0.0502; Fisher's exact test). This may indicate a flawed randomisation process, or it may be chance. The primary reference does not report variance around mean PASI baseline estimates, so comparability was unclear. |
Van de Kerkhof 2009.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: computer‐generated schedule (1:2:2) Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 1417 Treatment duration: 8 wks; FU: 8 wks LF: 2 (0.1%) BC: yes Age: 48.3 (16.4SD); range = 18 to 92 Gender (per cent men): 44.8% Ethnicity (per cent white): 97.2% Severity: IGA (0 to 5) = 3.32 (0.71SD), range = 2 to 5; TSS (0 to 12) = 6.8 (1.8SD) Duration (yrs): 15.9 (13.4SD) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
Participants achieving IGA = 0 could stop medication during the study. |
|
| Outcomes |
|
|
| Notes | Leo Pharma A/S, Ballerup, Denmark, sponsored the trial. The sponsor supplied unpublished data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Low risk | A computer‐generated randomisation schedule was used (1:2:2). |
| Loss to follow up | Low risk | 0.1% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | This was demonstrated. |
Van der Vleuten 1995.
| Methods | DESIGN Within‐patient Delivery unclear ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Open WITHDRAWAL/DROPOUT Described | |
| Participants | N: 10 Treatment duration: 2 wks; FU: 2 wks LF: 0 (0%) BC: inadequately reported Age: range = 20 to 72 Gender (per cent men): 40% Severity: PASI (modified) = 17.1 (2.1SEM) Duration (yrs): 3 to 53 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. Treatment was delivered in an inpatient setting. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was open. |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Unclear risk | The trial did not report this. |
Vanderploeg 1976.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: identical tubes allocated by sequential admission number, corresponding to a standard randomisation schedule using a double‐blind code Concealment: adequate BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 36 Treatment duration: 3 wks; FU: 3 wks LF: 3 of 36 (8.3%) BC: yes Age: 45.7 (range = 10 to 66; N = 33) Gender (per cent men): 48.5% (N = 33) Severity: TSS (0 to 20) = 9.9 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. This was part of a larger trial that included participants with atopic dermatitis (50% psoriasis). There was SD imputation (TSS). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | The sequential trial admission number corresponded to the randomisation schedule; the randomisation code was 'double blind'. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Low risk | Randomisation was sequential. |
| Loss to follow up | Low risk | 8.3% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Veien 1997.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: block Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 287 Treatment duration: 8 wks; FU: 12 wks LF: 0 (0%) BC: psoriasis comparable, demographics inadequately reported Age: 45.0 Gender (per cent men): 53.7% Severity: BSA = 7.9% (range = 1% to 75%); TSS (0 to 12) = 7.59 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Nycomed Pharma sponsored the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Low risk | Block randomisation was used. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Unclear risk | The trial did not report this. |
Vladimirov 1994.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Not described | |
| Participants | N: 60 Treatment duration: 6 wks; FU: 6 wks LF: 0 (0%) BC: inadequately reported Age: range = 18 to 70 Gender (per cent men): not reported Severity: PASI = 2.92 Duration (yrs): range = 0.2 to 30 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Leo Pharmaceuticals sponsored the trial. There was SD imputation (PASI). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Unclear risk | The trial did not report this. |
Volden 1992.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 10 Treatment duration: 4 wks; FU: 4 wks LF: 1 (10%) BC: yes Age: not reported Gender (per cent men): not reported Severity: BSA = 5% to 15% Duration (mean years): 20 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship, but Hydro Pharma, Swedenone, employed 1 of the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 10.0% |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Low risk | ‐ |
Wall 1998.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Open WITHDRAWAL/DROPOUT Not described | |
| Participants | N: 306 Treatment duration: 3 mths LF: 28 (7.2%) BC: yes Age: 46.7 Gender (per cent men): 47.1% Severity: duration (yrs) = 18.7 Signs and extent reported, but not by summary measure INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Leo Pharmaceuticals sponsored the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was open. |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 7.2% |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Low risk | ‐ |
Weinstein 1996.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 324 Treatment duration: 12 wks; FU: 24 wks LF: 6 (1.9%) BC: yes Age: 46.8 (range = 12 to 83) Gender (per cent men): 67% Severity: per cent BSA = 6.9 (5.2SD); TSS (0 to 12) = 7.3 Duration (yrs): 17.5 (12.7SD) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Allergan Inc. sponsored the trial. The authors stated that concealment of treatment allocation was achieved for participants and clinicians, as tubes were identical. There was SD imputation (TSS). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 1.9% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Weinstein 2003.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: randomised in blocks of 6 Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 1303 Treatment duration: 12 wks; FU: 24 wks LF: 411 (31.5%) BC: yes Age: 48.2 (range = 18 to 84) Gender (per cent men): 62.6% Severity: OLA (0 to 5) (mean) = 3.6; BSA affected (mean) = 10.5% Duration (mean yrs): 18.4 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Allergan Inc. sponsored the trial. The paper reported 2 trials; only study A reported follow‐up data after 12 weeks (N = 108). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Low risk | Block randomisation was used. |
| Loss to follow up | High risk | 31.5% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
White 2006 (H).
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: computer‐generated schedule Concealment: unclear BLINDING Open (acute phase and maintenance phase for CB‐W); double‐blind (participant/investigator) (maintenance phase for CB‐C and CB‐P) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 1136 Treatment duration: 12 wks; FU: 12 wks LF: 0 (0%) BC: yes Age: 50.7; range = 18 to 89 Gender (per cent men): 60.7% Ethnicity (per cent white): 96.9% Severity: per cent BSA = 12.1%; mPASI = 8.9 (3.8SD), range = 2.4 to 30.9; IGA moderate = 76%; IGA severe = 24% Duration (yrs): 0 to 75 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
Use < = 100 g/wk |
|
| Outcomes |
|
|
| Notes | Leo Pharma A/S sponsored the trial. Atrophy was not assessed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was open in the acute phase and maintenance phase for CB‐W and double‐blind (participant/investigator) in the maintenance phase for CB‐C and CB‐P. It was not possible to blind the alternating group (CB‐W), as the vehicles were different. |
| Randomisation method reported | Low risk | A preplanned computer‐generated randomisation schedule was used (1:1:1). |
| Loss to follow up | Low risk | 0% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Unclear risk | This was partially demonstrated (reported means, but not standard, deviations; proportions reported). |
White 2006 (P).
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: computer‐generated schedule Concealment: unclear BLINDING Open (acute phase and maintenance phase for CB‐W); double‐blind (participant/investigator) (maintenance phase for CB‐C and CB‐P) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 1136 Treatment duration: 12 wks; FU: 12 wks LF: 0 (0%) BC: yes Age: 50.7; range = 18 to 89 Gender (per cent men): 60.7% Ethnicity (per cent white): 96.9% Severity: per cent BSA = 12.1%; mPASI = 8.9 (3.8SD), range = 2.4 to 30.9; IGA moderate = 76%; IGA severe = 24% Duration (yrs): 0 to 75 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
Use < = 100 g/wk |
|
| Outcomes |
|
|
| Notes | Leo Pharma A/S sponsored the trial. Atrophy was not assessed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was open in the acute phase and maintenance phase for CB‐W and double‐blind (participant/investigator) in the maintenance phase for CB‐C and CB‐P. It was not possible to blind the alternating group (CB‐W), as the vehicles were different. |
| Randomisation method reported | Low risk | A preplanned computer‐generated randomisation schedule was used (1:1:1). |
| Loss to follow up | Low risk | 0% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Unclear risk | This was partially demonstrated (reported means, but not standard, deviations; proportions reported). |
Wolska 1995.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 52 Treatment duration: 4 wks; FU: 4 wks LF: 12 (23.1%) BC: yes Age: 44.7 (range = 18 to 77; N = 40) Gender (per cent men): 70%; N = 40 Severity: TSS (0 to 9) (median) = 7.1 (range = 6.0 to 9.0) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. Inpatients PLS CAN YOU PUT THIS IN A SENTENCE?] | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/investigator). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Unclear risk | 23.1% |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Low risk | ‐ |
Wortzel 1975 (1).
| Methods | DESIGN Between‐patient Delivery unclear ALLOCATION Random Method of randomisation: sequential admission number Concealment: adequate BLINDING Double‐blind (participant/physician) WITHDRAWAL/DROPOUT Not described | |
| Participants | N: 76 Treatment duration: 3 wks; FU: 3 wks LF: 0 (0%) BC: not reported Age: not reported Gender (per cent men): not reported Severity: not reported INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Leo Pharmaceuticals sponsored the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | The sequential trial admission number corresponded to a treatment unit on a randomisation schedule. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/physician). |
| Randomisation method reported | Low risk | Randomisation was sequential. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Unclear risk | The trial did not report these. |
| Baseline comparability demonstrated | Unclear risk | The trial did not report this. |
Wortzel 1975 (2).
| Methods | DESIGN Between‐patient Delivery unclear ALLOCATION Random Method of randomisation: sequential admission number Concealment: adequate BLINDING Double‐blind (participant/physician) WITHDRAWAL/DROPOUT Not described | |
| Participants | N: 9 Treatment duration: 3 wks; FU: 3 wks LF: 0 (0%) BC: The trial did not report this. Age: not reported Gender (per cent men): not reported Severity: not reported INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. Concomitant water soluble emollients were permitted. There was SD imputation (PASI). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | The sequential trial admission number corresponded to a treatment unit on a randomisation schedule. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (participant/physician). |
| Randomisation method reported | Low risk | Randomisation was sequential. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Unclear risk | The trial did not report these. |
| Baseline comparability demonstrated | Unclear risk | The trial did not report this. |
Wozel 2001.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (unclear) WITHDRAWAL/DROPOUT Described | |
| Participants | N: 38 Treatment duration: 2 wks; FU: 6 wks LF: 0 (0%) BC: yes (statistical significance not reported) Age: not reported Gender (per cent men): not reported Severity: not reported INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The trial did not report sponsorship. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient detail. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (it was unclear who was blinded). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0.0% |
| Baseline assessments | Unclear risk | The trial did not report these. |
| Baseline comparability demonstrated | Low risk | ‐ |
Yang 2009.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Not stated WITHDRAWAL/DROPOUT Described | |
| Participants | N: 76 Treatment duration: 6 wks; FU: 6 wks LF: 0 (0%) BC: yes Age: range = 18 to 65 Gender (per cent men): 68.4% Severity: PASI = 14.3 (5.5SD), range = 4.2 to 19.6 Duration (yrs): range = 0.5 to 34 INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
Treatments were stopped when 90% improvement achieved. |
|
| Outcomes |
|
|
| Notes | The trial did not state the sponsor. There was 1 case of skin atrophy in the monotherapy group. We received translation support for data extraction. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial did not report this. |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Low risk | 0% |
| Baseline assessments | Low risk | These were reported. |
| Baseline comparability demonstrated | Low risk | This was demonstrated. |
Zonneveld 1998 (H).
| Methods | DESIGN Between‐patient Delivery unclear ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (but unmatched regimens) (participant/assessor) WITHDRAWAL/DROPOUT Not described | |
| Participants | N: 70 Treatment duration: 6 wks; FU: 6 wks LF: not reported BC: Yes (clinical); demographics not reported Age: not reported Gender (per cent men): not reported Severity: median LPSI ranged from 7.0 (C;T) to 8.0 (P) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The study was double‐blind (but unmatched regimens). Fujisawa GmbH sponsored the trial. There was SD imputation (TSS). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (but unmatched regimens) (participant/assessor). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Unclear risk | The trial did not report this. |
| Baseline assessments | Low risk | These were partially done. |
| Baseline comparability demonstrated | Unclear risk | This was partially demonstrated. |
Zonneveld 1998 (P).
| Methods | DESIGN Between‐patient Delivery unclear ALLOCATION Random Method of randomisation: not reported Concealment: unclear BLINDING Double‐blind (but unmatched regimens) (participant/assessor) WITHDRAWAL/DROPOUT Not described | |
| Participants | N: 70 Treatment duration: 6 wks; FU: 6 wks LF: not reported BC: yes (clinical), demographics not reported Age: not reported Gender (per cent men): not reported Severity: median LPSI ranged from 7.0 (C;T) to 8.0 (P) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The study was double‐blind (but unmatched regimens). Fujisawa GmbH sponsored the trial. There was SD imputation (TSS). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | The trial reported insufficient details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was double‐blind (but unmatched regimens) (participant/assessor). |
| Randomisation method reported | Unclear risk | The trial did not report this. |
| Loss to follow up | Unclear risk | The trial did not report this. |
| Baseline assessments | Low risk | ‐ |
| Baseline comparability demonstrated | Unclear risk | This was partially demonstrated. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agrawal 2010 | The study did not provide a comparison of interest (compared acitretin gel in 2 formulations). |
| Akerman 2009 | The trialists did not report or make available adequate data. |
| Ambroziak 2002 | The study compared steroid against no treatment (rather than against placebo). |
| Baadsgaard 1995 | The study was within‐patient, but did not adopt clear left‐right design and assessed multiple plaques for each participant. |
| Baran 1999 | The trialists did not report or make available adequate data. |
| Bianchi 2003 | The study compared steroid against no treatment (rather than against placebo). |
| Brodell 2011a | The sponsor did not report or make available adequate data. |
| Buder 2010 | The study assessed multiple plaques for each participant (7 per participant). |
| Callen 1996 | The trialists or sponsor did not report or make available adequate data. |
| Cannavo 2003 | This was a nail psoriasis trial, which was removed in the 2011 update. |
| Carroll 2005 | The comparator was not strictly placebo (salicylic acid in vehicle). |
| De Jong 1999 | The comparator was not strictly placebo (urea in vehicle). |
| Elias 1994 | It was unclear if it was a valid randomised controlled trial. |
| Esposito 2009 | The study did not provide a comparison of interest. |
| Friedrich 2004 | The sponsors did not report or make available adequate data. |
| Hsia 2010 | The study did not assess patient outcomes/tolerability |
| Insa 2009 | The study did not assess patient outcomes/tolerability. |
| Iraji 2010 | The trialists did not report or make available adequate data. |
| Ito 2005 | The study was not randomised. |
| Jansen 1986 | No adequate data were reported. |
| Kaur 2004 | The study was not randomised. |
| Kleyn 2005 | The study did not provide a comparison of interest. |
| Kragballe 1989 | Participants were randomised to the 2 substudies, but within the substudies, treatments were applied without randomisation. |
| Kragballe 1994 | This was a dose‐ranging study of an unlicensed product not subsequently marketed. |
| Lebwohl 1998b | The study did not provide a simple comparison against a vitamin D₃ derivative treatment. |
| Lebwohl 2001 | The trialists or sponsor did not report or make available adequate data. |
| Levin 2003 | The trialists or sponsor did not report or make available adequate data. |
| Meyrat 1996 | The study did not provide a comparison of interest. |
| Palazon 2005 | The study did not provide a comparison of interest. |
| Reygagne 2002a | The trialists or sponsor did not report or make available adequate data. |
| Rhemus 2006 | The study did not provide a comparison of interest (product unlicensed). |
| Ruzicka 2004 | The trialists or sponsor did not report or make available adequate data. |
| Sander 1998 | The study did not provide a simple comparison against a vitamin D₃ derivative treatment. |
| Saraswat 2007 | The study did not provide a simple comparison against a vitamin D₃ derivative treatment. |
| Scher 2001 | This was a nail psoriasis trial, which was removed in the 2011 update. |
| Sefton 1984 | The trialists or sponsor did not report or make available adequate data. |
| Sharma 2003 | The study used concomitant UV light. |
| Syed 2001a | The trialists did not report or make available adequate data. |
| Tokura 2004 | No translation was available. |
| Tosti 1998 | This was a nail psoriasis trial, which was removed in the 2011 update. |
| Tzaneva 2003 | The study used concomitant UV light. |
| van de Kerkhof 1996b | The trialists did not report or make available adequate data. |
| Vena 2005 | The study was not a randomised controlled trial. |
Characteristics of studies awaiting assessment [ordered by study ID]
Bissonnette 2011.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: computer‐generated code Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described |
| Participants | N: 61 Treatment duration: 12 wks; FU: 12 wks LF: 0 (0%) BC: yes Age: 50.6 (22 to 65) Gender (per cent men): 83.6% Severity: PGA = 3.3 (0.6SD); PASI = 6.0 (2.5SD); BSA = 3.2% (2.0SD) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
| Interventions |
|
| Outcomes |
|
| Notes | Welichem Biotech Inc. sponsored the trial. |
Callis Duffin 2010.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Not described |
| Participants | N: 200
Treatment duration: 12 wks; FU: 12 wks
LF: NS
BC: NS
Age: NS
Gender (per cent men): NS
Severity: NS INCLUSION CRITERIA
EXCLUSION CRITERIA
|
| Interventions |
|
| Outcomes |
|
| Notes | Incyte Corporation sponsored the trial. |
Calzavara‐Pinton 2011.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Single‐blind (investigator/outcome assessor) WITHDRAWAL/DROPOUT Described |
| Participants | N: 20 Treatment duration: 8 wks; FU: 16 wks LF: 2 (10%) BC: yes Age: 40.2 (range = 19 to 68) Gender (per cent men): 40% Severity: PSI = median 8 (IQR = 7 to 9.25) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
| Interventions |
|
| Outcomes |
|
| Notes | The sponsor was not stated. |
Henry 2011.
| Methods |
DESIGN
Within‐patient
Participant delivery
ALLOCATION
Random
Method of randomisation: not stated
Concealment: unclear
BLINDING
Single‐blind (investigator)
WITHDRAWAL Described |
| Participants | N: 13 Treatment duration: 4 wks; FU: 4 wks LF: 3 (23.0%) BC: NS Age: NS Gender (per cent men): NS Severity: NS INCLUSION CRITERIA
EXCLUSION CRITERIA
|
| Interventions |
|
| Outcomes |
|
| Notes | Galderma sponsored the trial. This was an abstract only. |
Katoh 2003.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Not stated WITHDRAWAL/DROPOUT Described |
| Participants | N: 61 Treatment duration: 12 wks; FU: 12 wks LF: 2 (3%) BC: yes Age: 49.2 (14.3SD) Gender (per cent men): 34% Severity: PASI = 12.9 (5.3SD) Ethnicity: Japanese INCLUSION CRITERIA
EXCLUSION CRITERIA
|
| Interventions |
Treatments were applied after bathing. Participants achieving 50% reduction in baseline PASI then used calcipotriol once daily for a further 12 weeks. |
| Outcomes |
|
| Notes | The Japanese Ministry of Education, Science, Sports, and Culture sponsored the trial. |
Matheson 2011.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Open WITHDRAWAL/DROPOUT Not described |
| Participants | N: 32 Treatment duration: 2 wks; FU: 2 wks LF: NS BC: no Age: NS Gender (per cent men): NS Severity: NS INCLUSION CRITERIA
EXCLUSION CRITERIA
|
| Interventions |
|
| Outcomes |
|
| Notes | Stiefel, a GSK company, sponsored the trial. |
Paulsen 2005.
| Methods |
DESIGN
Within‐patient
Participant delivery
ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described |
| Participants | N: 41 Treatment duration: 4 wks; FU: 12 wks LF: 1 (2%) BC: yes Age (median): 44 (23 to 77) Gender (per cent men): 65% Severity: duration (yrs; median) = 15 (range = 2 to 60) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
| Interventions |
|
| Outcomes |
|
| Notes | Psoriasisfonden sponsored the trial. |
Sadeghian 2011.
| Methods | DESIGN Between‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described |
| Participants | N: 60 Treatment duration: 12 wks; FU: 12 wks LF: 0 (0%) BC: yes Age: 32.5 (15.6SD) Gender (per cent men): 51.7% Severity: PASI = 3.9 (1.9SD) INCLUSION CRITERIA
EXCLUSION CRITERIA
|
| Interventions |
|
| Outcomes |
|
| Notes | The sponsor was not stated. |
Vahlquist 2004.
| Methods | DESIGN Within‐patient Participant delivery ALLOCATION Random Method of randomisation: not stated Concealment: unclear BLINDING Double‐blind (participant/investigator) WITHDRAWAL/DROPOUT Described |
| Participants | N: 12 Treatment duration: 8 wks; FU: x wks LF: 0 (0%) BC: no Age: 43.8 (range = 21 to 58) Gender (per cent men): 83.3% Severity: NS INCLUSION CRITERIA
EXCLUSION CRITERIA
|
| Interventions |
|
| Outcomes |
|
| Notes | ‐ |
Yang 1999.
| Methods | This will need to be translated; details to follow at next update. |
| Participants | N: 60 Treatment duration: 6 wks; FU: 6 wks LF: unclear BC: unclear Age: range = 14 to 65 Gender (per cent men): unclear Severity: unclear INCLUSION CRITERIA
EXCLUSION CRITERIA
|
| Interventions |
|
| Outcomes | This will need to be translated; details to follow at next update. |
| Notes | This will need to be translated; details to follow at next update. |
Characteristics of ongoing studies [ordered by study ID]
NCT00824980.
| Trial name or title | Combined Inhibition of Dipeptidyl Peptidase IV (DPIV/CD26) and Aminopeptidase N (APN/CD13) in the Treatment of Psoriasis ‐ Phase II Single Center Study |
| Methods | Allocation: randomised End point classification: safety/efficacy study Intervention model: parallel assignment Masking: double‐blind (participant, caregiver, investigator, outcomes assessor) Primary purpose: treatment |
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | January 2009 |
| Contact information | Alexander Narvarini, MD Telephone: +41 44 255 1111 alexander.navarini@usz.ch |
| Notes | Immune Technologies & Medicine GmbH http://clinicaltrials.gov/show/NCT00824980 |
NCT01018134.
| Trial name or title | A Double‐Blind, Vehicle‐Controlled, Randomized, Dose Ranging, Multiple‐Site Clinical Study to Evaluate the Efficacy and Safety of Desoximetasone Topical Sprays (0.05%, 0.25%) in Patients With Moderate to Severe Plaque Psoriasis |
| Methods | Allocation: randomised End point classification: safety/efficacy study Intervention model: parallel assignment Masking: double‐blind (participant, caregiver, investigator, outcomes assessor) Primary purpose: treatment |
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | November 2009 |
| Contact information | Darin B Brimhall, D.O., FACP Study Director Novum Pharmaceutical Research Services |
| Notes | Taro Pharmaceuticals USA http://clinicaltrials.gov/show/NCT01018134 |
NCT01206387.
| Trial name or title | A Double‐Blind, Vehicle‐Controlled, Randomized, Parallel Design, Multiple‐Site Clinical Study to Evaluate the Efficacy and Safety of Desoximetasone 0.25% Topical Spray in Patients With Moderate to Severe Plaque Psoriasis |
| Methods | Allocation: randomised End point classification: safety/efficacy study Intervention model: parallel assignment Masking: double‐blind (participant, investigator, outcomes assessor) Primary purpose: treatment |
| Participants | Genders eligible for study: both Accepts healthy volunteers: no
|
| Interventions |
|
| Outcomes |
SECONDARY OUTCOMES
|
| Starting date | August 2010 |
| Contact information | Gail Gongas Telephone: 412 363 3300 ext: 522 GDGongas@novumprs.com |
| Notes | Taro Pharmaceuticals USA Study ID number: DSXS‐0808 http://clinicaltrials.gov/show/NCT01206387 |
NCT01206660.
| Trial name or title | A Double‐Blind, Vehicle‐Controlled, Randomized, Parallel Design, Multiple‐Site Clinical Study to Evaluate the Efficacy and Safety of Desoximetasone 0.25% Topical Spray in Patients With Moderate to Severe Plaque Psoriasis |
| Methods | Study type: Interventional Study design: Allocation: randomised End point classification: safety/efficacy study Intervention model: parallel assignment Masking: double‐blind (participant, investigator, outcomes assessor) Primary purpose: treatment |
| Participants | Accepts healthy volunteers: no
|
| Interventions |
|
| Outcomes |
PRIMARY OUTCOMES
SECONDARY OUTCOMES
|
| Starting date | August 2010 |
| Contact information | Gail Gongas Telephone: 412 363 3300 ext: 522 GDGongas@novumprs.com |
| Notes | Taro Pharmaceuticals USA Study ID number: DSXS‐0914 http://clinicaltrials.gov/show/NCT01206660 |
NCT01246583.
| Trial name or title | A Phase 2a, Multi Site, Randomized, Double Blind, Placebo Controlled, Parallel Group Study Of The Pilot Efficacy, Safety, And Pharmacokinetics Of 2 Ointment Formulations Of CP‐690,550 In Subjects With Mild To Moderate Chronic Plaque Psoriasis |
| Methods | Study type: interventional Study design: allocation: randomised End point classification: safety/efficacy study Intervention model: parallel assignment Masking: double‐blind (participant, investigator) Primary purpose: treatment |
| Participants | Ages eligible for study: 18 years and older Genders eligible for study: both Accepts healthy volunteers: no INCLUSION CRITERIA
EXCLUSION CRITERIA
|
| Interventions |
|
| Outcomes |
PRIMARY OUTCOMES
SECONDARY OUTCOMES
|
| Starting date | November 22 2010 |
| Contact information | Pfizer CT.gov Call Center |
| Notes | Pfizer |
NCT01247818.
| Trial name or title | A Phase 2 Dose‐Randomized, Vehicle‐Controlled Study of PH‐10‐Aqueous Hydrogel for the Treatment of Plaque Psoriasis |
| Methods | Study type: interventional Study design: allocation: randomised End point classification: safety/efficacy study Intervention model: parallel assignment Masking: single‐blind (outcomes assessor) Primary purpose: treatment |
| Participants | Accepts healthy volunteers: no
|
| Interventions |
|
| Outcomes |
PRIMARY OUTCOMES
SECONDARY OUTCOMES
|
| Starting date | December 2010 |
| Contact information | Eric Wachter, PhD Provectus Pharmaceuticals |
| Notes | Provectus Pharmaceuticals http://clinicaltrials.gov/show/NCT01247818 |
NCT01422434.
| Trial name or title | Efficacy and Safety of LEO 90105 Ointment (Calcipotriol Hydrate Plus Betamethasone Dipropionate) in Japanese Subjects With Psoriasis Vulgaris |
| Methods | Study type: interventional Study design: allocation: randomised End point classification: efficacy study Intervention model: parallel assignment Masking: double‐blind (participant, caregiver, investigator, outcomes assessor) Primary purpose: treatment |
| Participants | Accepts healthy volunteers: No Participants having understood and signed a written informed consent form prior to any study related procedures being carried out.
|
| Interventions |
|
| Outcomes |
PRIMARY OUTCOMES
SECONDARY OUTCOMES
|
| Starting date | July 2011 |
| Contact information | Akira Ozawa, MD, Professor, Principal Investigator Tokai University School of Medicine |
| Notes | LEO 90105 ointment contains both calcipotriol and betamethasone dipropionate. It has been approved for the treatment of psoriasis in more than 60 centres, including most European countries, the US, China, Korea, and Taiwan. This trial will investigate its safety and efficacy in the treatment of Japanese participants with psoriasis. LEO Pharma http://clinicaltrials.gov/show/NCT01422434 |
NCT01465282.
| Trial name or title | A Randomized, Placebo‐controlled, Phase IIb Study to Evaluate the Efficacy, Safety and Tolerability of 0.05%, 0.1% and 0.5% w/w Topical CT327 When Applied Twice Daily in Subjects With Psoriasis Vulgaris |
| Methods | Study type: interventional Study design: allocation: randomised End point classification: safety/efficacy study Intervention model: single group assignment Masking: double‐blind (participant, caregiver, investigator, outcomes assessor) Primary purpose: treatment |
| Participants | Accepts healthy volunteers: no INCLUSION CRITERIA
EXCLUSION CRITERIA
|
| Interventions |
|
| Outcomes |
PRIMARY OUTCOMES
SECONDARY OUTCOMES
|
| Starting date | November 1, 2011 |
| Contact information | No contacts or locations provided |
| Notes | Creabilis SA |
NCT01536886.
| Trial name or title | LEO 90100 Compared With Calcipotriol Plus Betamethasone Dipropionate Ointment, LEO 90100 Vehicle and Ointment Vehicle in Subjects With Psoriasis Vulgaris |
| Methods | Allocation: randomised End point classification: efficacy study Intervention model: parallel assignment Masking: double‐blind (caregiver, investigator, outcomes assessor) Primary purpose: treatment |
| Participants | Accepts healthy volunteers: no INCLUSION CRITERIA
EXCLUSION CRITERIA
|
| Interventions |
|
| Outcomes |
|
| Starting date | May 2012 |
| Contact information | Anders Ninn Hansen, MSc Pharm Telephone: +45 72 26 28 18 ext: 2818 anders.n‐hansen@leo‐pharma.com |
| Notes | ‐ |
NCT01536938.
| Trial name or title | LEO 90100 in the Treatment of Psoriasis Vulgaris |
| Methods | Study type: interventional Study design: allocation: randomised End point classification: efficacy study Intervention model: parallel assignment Masking: double‐blind (participant, caregiver, investigator, outcomes assessor) Primary purpose: treatment |
| Participants | Accepts healthy volunteers: no INCLUSION CRITERIA
EXCLUSION CRITERIA
|
| Interventions |
|
| Outcomes |
(Designated as safety issue: no) |
| Starting date | May 2012 |
| Contact information | Anders Ninn Hansen, MSc Pharm Telephone: +45 72 26 28 18 ext: 2818 anders.n‐hansen@leo‐pharma.com |
| Notes | ‐ |
Differences between protocol and review
There are some differences between the protocol and the review.
1. In our protocol, we stated our intention to adjust for the precision of findings from within‐patient studies for within‐patient correlation. However, we were unsuccessful in our attempts to identify estimates of this correlation from published or unpublished sources. We therefore undertook sensitivity analysis to investigate differences between within‐patient and between‐patient studies.
2. In our protocol, we listed three primary outcome measures for data extraction. In the review, we also included the Patient Assessment of Global Improvement.
3. In our protocol, we stated that there would be no language restrictions when searching for publications. However, the search for longer‐term studies of adverse events included a restriction to publications in English.
4. In our protocol, we stated our intention that studies meeting only some of the inclusion criteria stated above would be listed as excluded studies. However, as large numbers of studies would need to be listed, this was not feasible. Therefore, we listed as excluded studies only those studies that we deemed potentially eligible for inclusion and for which we retrieved full papers, but which we subsequently found to fail to meet the inclusion criteria.
5. Throughout the text, we replaced all references to vitamin D3 with 'vitamin D analogues'.
6. Under Types of studies, we relaxed the condition that studies were of at least two weeks duration. In the same section, we added the following sentence: "If no useful effectiveness, withdrawal or adverse events data were available, either from the published paper or from sponsors or trialists, we excluded the study."
7. Under Types of interventions, we added the sentence "The potency of topical corticosteroids was based on classifications from a previous review (Mason 2002b)".
8. Under Methods, Selection of studies, and our explanations of the studies excluded, we added the searches for studies exploring adverse events and compliance studies.
9. Under Methods/Data extraction and management, we added the phrase 'between‐patient design'.
10. Under Methods/Assessment of risk of bias in included studies, we removed the phrase 'in each arm'.
11. Under Methods/Unit of analysis issues/'Summarising primary outcomes with standardised mean differences', we revised the text in this section to include the PAGI outcome and to provide a fuller explanation of our analytic approach.
12. Under Methods/Unit of analysis issues/Secondary outcomes, we gave an explanation regarding the different method of analysis used.
13 Under Methods/Data collection and analysis/Sensitivity analysis, we added text to describe the different types of sensitivity analysis undertaken.
14 We replaced 'standardised weighted mean difference' with 'standardised mean difference' throughout the text to reflect Cochrane terminology.
15. Under Methods/Data and analyses/Subgroup analysis and investigation of heterogeneity, we inserted two paragraphs to explain our approach to statistical heterogeneity.
Contributions of authors
The following contributions were made by the authors stated. Link with editorial base and co‐ordinate contributions from co‐authors (AM). Draft protocol (AM with contributions from MC, GD, GE, and JM). Run searches (adherence) (AM). Identify relevant titles and abstracts from searches, i.e. broad screen (AM and JM). Obtain copies of trials (AM). Select which trials to include (AM, JM, and MC as arbitrator when necessary). Extract data from trials (AM, JM, and HH). Enter data into RevMan (AM). Carry out analysis (AM and JM) Interpret analysis (AM, JM, and HH). Draft final review (AM with contribution from MC, GD, HH, and JM). Update review (AM, JM, HH, and MC).
Sources of support
Internal sources
Funding from Centre for Reviews and Dissemination to update review (2002) for UK products only, UK.
Award from University of York Fund for Staff on Fixed‐Term Contracts, UK.
External sources
Grant from Crookes Healthcare Ltd. to do original systematic review (1999), UK.
Grant from the Psoriasis Association to update review (2011), Other.
Declarations of interest
Mike Cork: "I gave a lecture for Leo Pharmaceuticals about psoriasis and atopic eczema in 2012."
Anne R Mason: none declared
Gordon Dooley: none declared
James Mason: none declared
Helen Hancock: none declared
The clinical referee for this review, Dr Phyllis Spuls, stated the following potential conflict of interest on her comments form: "I have participated in an advisory board of LEO Pharma to give guidance to general practitioners regarding what to do if patients used calcipotriol, which has been removed from the market. I am involved as a Principal Investigator in many clinical trials with systemic agents for psoriasis and now in one for the improvement of adherence to Dovobet."
Edited (no change to conclusions)
References
References to studies included in this review
Agrup 1981 {published data only}
- Agrup G, Bjornberg A, Elmros T, Groth O, Hannuksela M, Lassus A, et al. Clinical Trial of a Potent Non‐Halogenated Topical Steroid, Budesonide. Acta Dermato Venereologica 1981;61(2):180‐2. [PUBMED: 6165205] [PubMed] [Google Scholar]
Alora‐Palli 2010 {published and unpublished data}
- Alora‐Palli MB, Kimball AB, Cott A. A new topically applied liquor carbonis distillate (coal tar) solution helps reduce regression of plaque psoriasis after 12 weeks of treatment: A 6‐week comparison to calcipotriol cream. Journal of the American Academy of Dermatology 2009;60(3 Suppl 1):AB174. [Google Scholar]
- Alora‐Palli Maria B, Perkins Alexis C, Cott Alicia, Kimball Alexandra B. Efficacy and tolerability of a cosmetically acceptable coal tar solution in the treatment of moderate plaque psoriasis: a controlled comparison with calcipotriene (calcipotriol) cream. American Journal of Clinical Dermatology 2010;11(4):275‐83. [PUBMED: 20513160] [DOI] [PubMed] [Google Scholar]
- Brouda I, Edison B, Cott A, Green BA. Tolerability and cosmetic acceptability of liquor carbonis distillate (coal tar) solution 15% as topical therapy for plaque psoriasis. Cutis 2010;85(4):214‐20. [PubMed] [Google Scholar]
Austad 1998 {published and unpublished data}
- Austad J, Bjerke JR, Gjertsen BT, Helland S, Livden JK, Morken T, et al. Clobetasol Propionate Followed by Calcipotriol Is Superior to Calcipotriol Alone in Topical Treatment of Psoriasis. Journal of the European Academy of Dermatology & Venereology 1998;11(1):19‐24. [PUBMED: 9731961] [PubMed] [Google Scholar]
Baiocchi 1997 {published data only}
- Baiocchi R, Bertani E, Biggio P, Calandra P, Marchi R, Franchi A, et al. Controlled Trial of the Efficacy and Safety of Calcipotriol Ointment Applied Once or Twice a Day in Psoriasis Vulgaris Studio [Controllato Per Emiparti Sull'efficacia E La Tollerabilita Di Calcipotriolo Unguento 1 Applicazione/Die Versus 2 Applicazioni/Die, Nella Psoriasi Volgare]. Giornale Italiano di Dermatologia e Venereologia 1997;132(2):139‐47. [Google Scholar]
Barker 1999 (H) {published and unpublished data}
- Barker N, Ashton RE, Marks R, Harris RI, Berth‐Jones J. Topical maxacalcitol for the treatment of psoriasis vulgaris: a placebo‐controlled, double‐blind, dose‐finding study with active comparator. British Journal Of Dermatology 1999;141(2):274‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Barker 1999 (P) {published and unpublished data}
- Barker N, Ashton RE, Marks R, Harris RI, Berth‐Jones J. Topical maxacalcitol for the treatment of psoriasis vulgaris: a placebo‐controlled, double‐blind, dose‐finding study with active comparator. British Journal Of Dermatology 1999;141(2):274‐8. [PUBMED: 10468799] [DOI] [PubMed] [Google Scholar]
- Berth‐Jones J. Maxacalcitol ‐ a new, potent and safe vitamin D analogue for treatment of psoriasis. British Journal Of Dermatology 1999;141:992. [DOI] [PubMed] [Google Scholar]
Barrett 2005 {published and unpublished data}
- Barrett C, Lowson D, Blades K J, investigators Mcs Int. Limited benefit of combined use of tar‐based shampoo with 50 microg/ml calcipotriol solution in scalp psoriasis. Journal of Dermatological Treatment 2005;16(3):175. [PUBMED: 16211775] [DOI] [PubMed] [Google Scholar]
Bernhard 1991 (1) {published data only}
- Bernhard J, Whitmore C, Guzzo C, Kantor I, Kalb RE, Ellis C, et al. Evaluation of Halobetasol Propionate Ointment in the Treatment of Plaque Psoriasis: Report on Two Double‐Blind, Vehicle‐Controlled Studies. Journal of the American Academy of Dermatology 1991;25(6 Pt 2):1170‐4. [PUBMED: 1757612] [DOI] [PubMed] [Google Scholar]
Bernhard 1991(2) {published data only}
- Bernhard J, Whitmore C, Guzzo C, Kantor I, Kalb RE, Ellis C, et al. Evaluation of Halobetasol Propionate Ointment in the Treatment of Plaque Psoriasis: Report on Two Double‐Blind, Vehicle‐Controlled Studies. Journal of the American Academy of Dermatology 1991;25(6 Pt 2):1170‐4. [PUBMED: 1757612] [DOI] [PubMed] [Google Scholar]
Bernstein 2006 {published data only}
Berth Jones 1992b {published data only}
- Berth Jones J, Chu AC, Dodd WA, Ganpule M, Griffiths WA, Haydey RP, et al. A Multicentre, Parallel‐Group Comparison of Calcipotriol Ointment and Short‐Contact Dithranol Therapy in Chronic Plaque Psoriasis. British Journal Of Dermatology 1992;127(3):266‐71. [PUBMED: 1390171] [DOI] [PubMed] [Google Scholar]
Beutner 2006 {published data only}
- Beutner K, Chakrabarty A. An intra‐individual safety and efficacy comparison of clobetasol propionate 0.05% spray and its vehicle in the treatment of plaque psoriasis. [Abstract P2858.] American Academy of Dermatology 64th Annual Meeting 3‐7 March 2006. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB212. [PubMed] [Google Scholar]
- Beutner K, Chakrabarty A, Lemke S, Yu K. An intra‐individual randomized safety and efficacy comparison of clobetasol propionate 0.05% spray and its vehicle in the treatment of plaque psoriasis. Journal of Drugs in Dermatology 2006;5(4):357‐60. [PUBMED: 16673804] [PubMed] [Google Scholar]
Bourke 1993b {published data only}
- Bourke JF, Berth‐Jones J, Hutchinson PE. Occlusion enhances the efficacy of topical calcipotriol in the treatment of psoriasis vulgaris. Clinical & Experimental Dermatology 1993;18(6):504‐6. [PUBMED: 8252786] [DOI] [PubMed] [Google Scholar]
Bourke 1997 {published and unpublished data}
- Bourke JF, Featherstone S, Iqbal SJ, Hutchinson PE. A Double‐Blind Comparison of Topical Calcitriol (3 mcg/g) and Calcipotriol (50 mcg/g) in the Treatment of Chronic Plaque Psoriasis Vulgaris. British Journal Of Dermatology 1995;133(Suppl 45):17. [Google Scholar]
- Bourke JF, Iqbal SJ, Hutchinson PE. A Randomized Double‐Blind Comparison of the Effects on Systemic Calcium Homeostasis of Topical Calcipotriol (3 Mu G/G) and Calcipotriol (50 U M/G) in the Treatment of Chronic Plaque Psoriasis Vulgaris. Acta Dermato Venereologica 1997;77(3):228‐30. [PUBMED: 9188878] [DOI] [PubMed] [Google Scholar]
Brown 2005 {published and unpublished data}
Bruce 1994 {published and unpublished data}
- Bruce S, Epinette WW, Funicella T, Ison A, Jones EL, Loss RJ, et al. Comparative Study of Calcipotriene (Mc 903) Ointment and Fluocinonide Ointment in the Treatment of Psoriasis. Journal of the American Academy of Dermatology 1994;31(5 Pt 1):755‐9. [PUBMED: 7929921] [DOI] [PubMed] [Google Scholar]
- Siskin SB. Efficacy and Safety of Calcipotriol Ointment Compared to Fluocinonide. 2nd International Symposium on Calcipotriol. Monaco, 1993.
Buckley 1978 {published data only}
- Buckley DB. A Double‐Blind Comparison of 0.1% Dithranol in a 17% Urea Base ("Psoradrate") and Base Alone in the Treatment of Active Chronic Psoriasis. Current Medical Research & Opinion 1978;5(6):489‐94. [PUBMED: 350501 ] [DOI] [PubMed] [Google Scholar]
Buckley 2008 {published and unpublished data}
- Buckley C, Hoffmann V. Calcipotriol plus betamethasone dipropionate gel is effective and safe in the treatment of scalp psoriasis (a phase II study). [Abstract P06.57.] 14th Congress of the European Academy of Dermatology and Venereology, 12‐15th October 2005, London UK. Journal of the European Academy of Dermatology & Venereology 2005;19(Suppl 2):175. [Google Scholar]
- Buckley C, Hoffmann V, Shapiro J, Saari S, Cambazard F, Milsgaard M. Calcipotriol plus betamethasone dipropionate scalp formulation is effective and well tolerated in the treatment of scalp psoriasis: a phase II study. Dermatology 2008; Vol. 217, issue 2:107‐13. [PUBMED: 18463448] [DOI] [PubMed]
Camarasa 2003 {published data only}
- Camarasa JM, Ortonne JP, Dubertret L. Calcitriol shows greater persistence of treatment effect than betamethasone dipropionate in topical psoriasis therapy. Journal of Dermatological Treatment 2003;14(1):8‐13. [PUBMED: 12745849] [DOI] [PubMed] [Google Scholar]
Cheesbrough 1992 {published data only}
- Cheesbrough MJ. Treatment of psoriasis with 30% Dead Sea salt lotion. Journal Of Dermatological Treatment 1992;3(4):201‐203. [EMBASE: 1993020259] [Google Scholar]
Christensen 1999 {published data only}
- Christensen OB, Mork NJ, Ashton R, Daniel F, Anehus S. Comparison of a treatment phase and a follow‐up phase of short‐contact dithranol and calcipotriol in outpatients with chronic plaque psoriasis. Journal Of Dermatological Treatment 1999;10(4):261‐5. [EMBASE: 2000044219] [Google Scholar]
Cook‐Bolden 2010 {published and unpublished data}
- Cook‐Bolden FE, Goffe BS, Hudson CP, Sofen HL. Efficacy and safety results from a randomized, double‐blind, vehicle‐controlled study of clobetasol propionate spray for the treatment of moderate to severe plaque psoriasis of the scalp [Abstract]. Conference: 68th Annual Meeting of the American Academy of Dermatology, AAD Miami, FL United States. Conference Start: 20100305 Conference End: 20100309. Journal of the American Academy of Dermatology 2010;62(3 Suppl 1):AB140. [EMBASE: 70142994; NCT00881868] [Google Scholar]
- Cook‐Bolden FE, Goffe BS, Hudson CP, Sofen HL. Patient satisfaction with clobetasol propionate spray, 0.05% for the management of moderate to severe plaque psoriasis of the scalp [Abstract]. Conference: 70th Annual Meeting of the American Academy of Dermatology San Diego, CA United States. Conference Start: 20120316 Conference End: 20120320. Journal of the American Academy of Dermatology 2012;66(4 Suppl 1):AB197. [EMBASE: 70704639] [Google Scholar]
- Cook‐Bolden FE, Goffe BS, Sofen HL, Colon LE, Gottschalk RW. Successful treatment of moderate to severe plaque psoriasis of the scalp with clobetasol propionate spray, 0.05% [Abstract]. Conference: 69th Annual Meeting of the American Academy of Dermatology New Orleans, LA United States. Conference Start: 20110204 Conference End: 20110208. Journal of the American Academy of Dermatology 2011;64(2 Suppl 1):AB157. [EMBASE: 70331905] [Google Scholar]
- Cook‐Bolden FE, Goffe BS, Sofen HL, Hudson CP, Colon LE, Gottschalk RW, et al. Patient satisfaction with clobetasol propionate spray, 0.05% for the management of moderate to severe plaque psoriasis of the scalp [Abstract]. Conference: 36th Annual Hawaii Dermatology Seminar of the Skin Disease Education Foundation Waikoloa, HI United States. Conference Start: 20120219 Conference End: 20120224.. Seminars in Cutaneous Medicine & Surgery 2012;31(1):A9‐A10. [EMBASE: 70706927] [Google Scholar]
- Jarratt M, Sofen HL, Caveney S. The effects of clobetasol propionate spray, 0.05% vs. vehicle spray on the signs of plaque psoriasis on the body and scalp [Abstract]. Conference: 36th Annual Hawaii Dermatology Seminar of the Skin Disease Education Foundation Waikoloa, HI United States. Conference Start: 20120219 Conference End: 20120224. Seminars in Cutaneous Medicine & Surgery 2012;31(1):A11. [EMBASE: 70706930] [Google Scholar]
- Jarratt M, Sofen HL, Caveney SW. The effects of clobetasol propionate spray, 0.05% versus vehicle spray on the signs of plaque psoriasis on the body and scalp [Abstract]. Conference: 70th Annual Meeting of the American Academy of Dermatology San Diego, CA United States. Conference Start: 20120316 Conference End: 20120320. Journal of the American Academy of Dermatology 2012;66(4 Suppl 1):AB204. [EMBASE: 70704666] [Google Scholar]
- NCT00881868. Clobetasol Propionate Spray Versus Vehicle Spray for the Management of Moderate to Severe Plaque Psoriasis of the Scalp. clinicaltrials.gov/ct2/show/NCT00881868 (accessed 2011).
- Sofen H, Hudson CP, Cook‐Bolden FE, Preston N, Colon LE, Caveney SW, et al. Clobetasol propionate 0.05% spray for the management of moderate‐to‐severe plaque psoriasis of the scalp: results from a randomized controlled trial. Journal of Drugs in Dermatology 2011;10(8):885‐92. [MEDLINE: ] [PubMed] [Google Scholar]
Crosti 1997 {published data only}
- Crosti C, Finzi AF, Mian E, Scarpa C. Calcipotriol in psoriasis vulgaris: a controlled trial comparing betamethasone dipropionate + salicylic acid. International Journal Of Dermatology 1997;36(7):537‐9. [PUBMED: 9268756] [DOI] [PubMed] [Google Scholar]
Cunliffe 1992 {published data only}
- Cunliffe WJ, Berth Jones J, Claudy A, Fairiss GM, Goldin D, Gratton D, et al. Comparative study of calcipotriol (MC 903) ointment and betamethasone 17‐valerate ointment in patients with psoriasis vulgaris. Journal of the American Academy of Dermatology 1992;26(5 Pt 1):736‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Decroix 2004 {published data only}
- Decroix J, Pres H, Tsankov N, Poncet M, Arsonnaud S. Clobetasol propionate lotion in the treatment of moderate to severe plaque‐type psoriasis. Cutis 2004;74(3):201‐6. [PUBMED: 15499763] [PubMed] [Google Scholar]
De Simone 1993 {published data only}
- Simone C, Guerriero C, Morini P, Venier A. Treatment of Psoriasis with Topical Calcipotriol [Il Calcipotriol Nella Terapia Locale Della Psoriasi]. Giornale Italiano di Dermatologia e Venereologia 1993;128(10):Ic‐Ciii. [Google Scholar]
Douglas 2002 {published and unpublished data}
- Douglas WS, Poulin Y, Decroix J, Ortonne JP, Mrowietz U, Gulliver W, et al. A new calcipotriol/betamethasone formulation with rapid onset of action was superior to monotherapy with betamethasone dipropionate or calcipotriol in psoriasis vulgaris. Acta Dermato Venereologica 2002;82(2):131‐5. [PUBMED: 12125943 ] [DOI] [PubMed] [Google Scholar]
Dubertret 1992 {published and unpublished data}
- Dubertret L, Wallach D, Souteyrand P, Perussel M, Kalis B, Meynadier J, et al. Efficacy and Safety of Calcipotriol (Mc 903) Ointment in Psoriasis Vulgaris. A Randomized, Double‐Blind, Right/Left Comparative, Vehicle‐Controlled Study. Journal of the American Academy of Dermatology 1992;27(6 Pt 1):983‐8. [PUBMED: 1479106] [DOI] [PubMed] [Google Scholar]
Durakovic 2001 {published data only}
- Durakovic C, Malabanan A, Holick MF. Rationale for use and clinical responsiveness of hexafluoro‐1,25‐dihydroxyvitamin D3 for the treatment of plaque psoriasis: a pilot study. British Journal Of Dermatology 2001;144(3):500‐6. [PUBMED: 11260006] [DOI] [PubMed] [Google Scholar]
Durakovic 2004 {published data only}
- Durakovic C, Ray S, Holick MF. Topical paricalcitol (19‐nor‐1 alpha,25‐dihydroxyvitamin D2) is a novel, safe and effective treatment for plaque psoriasis: a pilot study. British Journal of Dermatology 2004;151(1):190‐5. [PUBMED: 15270890] [DOI] [PubMed] [Google Scholar]
Duweb 2000 {published data only}
- Duweb GA, Abuzariba O, Rahim M, al‐Taweel M, Abdulla SA. Scalp psoriasis: topical calcipotriol 50 micrograms/g/ml solution vs. betamethasone valerate 1% lotion. International Journal of Clinical Pharmacology Research 2000;20(3‐4):65‐8. [PUBMED: 11314240 ] [PubMed] [Google Scholar]
Elie 1983 {published data only}
- Elie R, Durocher LP, Kavalec EC. Effect of Salicylic Acid on the Activity of Betamethasone‐17,21‐Dipropionate in the Treatment of Erythematous Squamous Dermatoses. Journal of International Medical Research 1983;11(2):108‐12. [PUBMED: 6852357 ] [DOI] [PubMed] [Google Scholar]
Ellis 1988 {published data only}
- Ellis CN, Horwitz SN, Menter A. Amcinonide Lotion 0.1% in the Treatment of Patients with Psoriasis of the Scalp. Current Therapeutic Research ‐ Clinical & Experimental 1988;44(2):315‐24. [EMBASE: 1989002095] [Google Scholar]
Escobar 1992 {published data only}
- Escobar SO, Achenbach R, Iannantuono R, Torem V. Topical fish oil in psoriasis‐‐a controlled and blind study. Clinical & Experimental Dermatology 1992;17(3):159‐62. [PUBMED: 1451289 ] [DOI] [PubMed] [Google Scholar]
Farkas 1999 {published and unpublished data}
- Farkas B, Dobozy A. Comparison of Tacalcitol with Dithranol. 3rd European Hermal Symposium. Hamburg, 1995.
- Farkas B, Dobozy A, Horvath A, Hunyadi J, Schneider I. Comparison of tacalcitol ointment with short‐contact dithranol therapy in the treatment of psoriasis vulgaris: a randomized multicentre, open prospective study on efficacy and safety. Journal of Dermatological Treatment 1999;10(2):93‐9. [EMBASE: 1999259658] [Google Scholar]
Feldman 2010 (1) {published data only (unpublished sought but not used)}
- Feldman S, Wyres M, Markowitz O, Matheson R, Bruce S. Calcipotriene foam for the treatment of plaque‐type psoriasis: Results of two phase III studies. Journal of the American Academy of Dermatology 2010;62(3 Suppl 1):AB138. [NCT00689481] [Google Scholar]
- Feldman SR, Matheson R, Bruce S, Grande K, Markowitz O, Kempers S, et al. Efficacy and safety of calcipotriene 0.005% foam for the treatment of plaque‐type psoriasis: Results of two multicenter, randomized, double‐blind, vehicle‐controlled, phase III clinical trials. American Journal of Clinical Dermatology 2012;13(4):261‐71. [EMBASE: 2012323159] [DOI] [PubMed] [Google Scholar]
- NCT00689481. Study to Compare U0267 Against Vehicle in Subjects With Plaque‐type Psoriasis Two of Two Phase 3 Studies. clinicaltrials.gov/ct2/results?term=NCT00689481 (accessed 2010).
Feldman 2010 (2) {published data only (unpublished sought but not used)}
- Feldman S R, Matheson R, Bruce S, Grande K, Markowitz O, Kempers S, et al. Efficacy and safety of calcipotriene 0.005% foam for the treatment of plaque‐type psoriasis: Results of two multicenter, randomized, double‐blind, vehicle‐controlled, phase III clinical trials. AMERICAN JOURNAL OF CLINICAL DERMATOLOGY 2012;13(4):261‐71. [DOI] [PubMed] [Google Scholar]
- Feldman S, Wyres M, Markowitz O, Matheson R, Bruce S. Calcipotriene foam for the treatment of plaque‐type psoriasis: Results of two phase III studies. Journal of the American Academy of Dermatology 2010;62(3 Suppl 1):AB138. [NCT00689481] [Google Scholar]
- NCT00688519. Study to Compare U0267 Against Vehicle in Subjects With Plaque‐type Psoriasis One of Two Phase 3 Studies. clinicaltrials.gov/ct2/results?term=NCT00688519 (accessed 2010).
Fleming 2010 (H) {published and unpublished data}
- Fleming C, Ganslandt C, Guenther L, Johannesson A, Buckley C, Simon JC, et al. Calcipotriol plus betamethasone dipropionate gel compared with its active components in the same vehicle and the vehicle alone in the treatment of psoriasis vulgaris: a randomised, parallel group, double‐blind, exploratory study. European Journal of Dermatology 2010;20(4):465‐71. [PUBMED: 20413372] [DOI] [PubMed] [Google Scholar]
- Fleming C, Ganslandt C, Leese GP. Short‐ and long‐term safety assessment of a two‐compound ointment containing calcipotriene/betamethasone dipropionate (Taclonex/Daivobet/Dovobet ointment): hypothalamic‐pituitary‐adrenal axis function in patients with psoriasis vulgaris. Journal of Drugs in Dermatology 2010;9(8):969‐74. [PUBMED: 20684147] [PubMed] [Google Scholar]
Fleming 2010 (P) {published and unpublished data}
- Fleming C, Ganslandt C, Guenther L, Johannesson A, Buckley C, Simon JC, et al. Calcipotriol plus betamethasone dipropionate gel compared with its active components in the same vehicle and the vehicle alone in the treatment of psoriasis vulgaris: a randomised, parallel group, double‐blind, exploratory study. European Journal of Dermatology 2010;20(4):465‐71. [PUBMED: 20413372] [DOI] [PubMed] [Google Scholar]
- Fleming C, Ganslandt C, Leese GP. Short‐ and long‐term safety assessment of a two‐compound ointment containing calcipotriene/betamethasone dipropionate (Taclonex/Daivobet/Dovobet ointment): hypothalamic‐pituitary‐adrenal axis function in patients with psoriasis vulgaris. Journal of Drugs in Dermatology 2010;9(8):969‐74. [PUBMED: 20684147] [PubMed] [Google Scholar]
Franz 1999 {published data only}
- Franz TJ, Parsell DA, Halualani RM, Hannigan JF, Kalbach JP, Harkonen WS. Betamethasone valerate foam 0.12%: a novel vehicle with enhanced delivery and efficacy. International Journal Of Dermatology 1999;38(8):628‐32. [PUBMED: 10487457 ] [DOI] [PubMed] [Google Scholar]
Franz 2000 {published data only}
- Franz TJ, Parsell DA, Myers JA, Hannigan JF. Clobetasol propionate foam 0.05%: a novel vehicle with enhanced delivery. International Journal Of Dermatology 2000;39(7):535‐8. [PUBMED: 10940121 ] [DOI] [PubMed] [Google Scholar]
Geilen 2000 {published data only}
- Geilen CC, Mrowietz U. Lack of efficacy of topical mycophenolic acid in psoriasis vulgaris. Journal of the American Academy of Dermatology 2000;42(5 Pt 1):837‐40. [PUBMED: 10775867 ] [DOI] [PubMed] [Google Scholar]
Gottlieb 2003 {published data only}
- Gottlieb AB, Ford RO, Spellman MC. The efficacy and tolerability of clobetasol propionate foam 0.05% in the treatment of mild to moderate plaque‐type psoriasis of nonscalp regions. Journal of Cutaneous Medicine & Surgery 2003;7(3):185‐92. [PUBMED: 12704534 ] [DOI] [PubMed] [Google Scholar]
Grattan 1997 (H) {published data only}
- Grattan CEH, Hallam F, Whitefield M. A New Aqueous Dithranol Gel for Psoriasis: Comparison with Placebo and Calcipotriol Ointment. Journal Of Dermatological Treatment 1997;8(1):11‐5. [EMBASE: 1997109958] [Google Scholar]
Grattan 1997 (P) {published data only}
- Grattan CEH, Hallam F, Whitefield M. A New Aqueous Dithranol Gel for Psoriasis: Comparison with Placebo and Calcipotriol Ointment. Journal Of Dermatological Treatment 1997;8(1):11‐5. [EMBASE: 1997109958] [Google Scholar]
Green 1994 {published and unpublished data}
- Green C, Ganpule M, Harris D, Kavanagh G, Kennedy C, Mallett R, et al. Comparative Effects of Calcipotriol (Mc903) Solution and Placebo (Vehicle of Mc903) in the Treatment of Psoriasis of the Scalp. British Journal Of Dermatology 1994;130(4):483‐7. [PUBMED: 8186114 ] [DOI] [PubMed] [Google Scholar]
Greenspan 1993 {published and unpublished data}
- Greenspan A, Herndon JJ, Baker MD, Cheney T. Controlled Evaluation of 0.05% Desonide Lotion and Desonide Cream in Psoriasis. Current Therapeutic Research ‐ Clinical & Experimental 1993;53(6):614‐20. [EMBASE: 1993261649] [Google Scholar]
Gribetz 2004 {published and unpublished data}
- Gribetz C, Ling M, Lebwohl M, Pariser D, Draelos Z, Gottlieb AB, et al. Pimecrolimus cream 1% in the treatment of intertriginous psoriasis: A double‐blind, randomized study. Journal of the American Academy of Dermatology 2004;51(5):731‐8. [PUBMED: 15523351] [DOI] [PubMed] [Google Scholar]
Guenther 2000 {published data only}
- Guenther LC, Poulin YP, Pariser DM. A comparison of tazarotene 0.1% gel once daily plus mometasone furoate 0.1% cream once daily versus calcipotriene 0.005% ointment twice daily in the treatment of plaque psoriasis. Clinical Therapeutics 2000;22(10):1225‐38. [PUBMED: 11110233] [DOI] [PubMed] [Google Scholar]
Guenther 2002 (H) {published and unpublished data}
- Guenther L, Kerkhof PCM, Kragballe K, Chu AC, Tegner E, Garcia‐Diez A, et al. Efficacy and safety of a new combination of calcipotriol and betamethasone dipropionate (once or twice daily) compared to calcipotriol (twice daily) in the treatment of psoriasis vulgaris: a randomized, double‐blind, vehicle‐controlled clinical trial. British Journal Of Dermatology 2002;147(2):316‐23. [PUBMED: 12174105] [DOI] [PubMed] [Google Scholar]
- Kerkhof PCM. Once daily vs. twice daily applications of topical treatments in psoriasis [15]. British Journal of Dermatology 2005; Vol. 153, issue 6:1245. [DOI] [PubMed]
- Kerkhof PC. The impact of a two‐compound product containing calcipotriol and betamethasone dipropionate (Daivobet/ Dovobet) on the quality of life in patients with psoriasis vulgaris: a randomized controlled trial. British Journal of Dermatology 2004;151(3):663‐8. [PUBMED: 15377355] [DOI] [PubMed] [Google Scholar]
- Kerkhof PCM. A fixed combination of calcipotriol/betamethasone dipropionate improves quality of life in patients with psoriasis vulgaris. 11th Congress of the European Academy of Dermatology and Venereology; 2002 Oct 2‐6; Prague. 2002:27‐2.
Guenther 2002 (P) {published and unpublished data}
- Guenther L, Kerkhof PCM, Kragballe K, Chu AC, Tegner E, Garcia‐Diez A, et al. Efficacy and safety of a new combination of calcipotriol and betamethasone dipropionate (once or twice daily) compared to calcipotriol (twice daily) in the treatment of psoriasis vulgaris: a randomized, double‐blind, vehicle‐controlled clinical trial. British Journal Of Dermatology 2002;147(2):316‐23. [PUBMED: 12174105] [DOI] [PubMed] [Google Scholar]
- Kerkhof PCM. Once daily vs. twice daily applications of topical treatments in psoriasis [15]. British Journal of Dermatology 2005; Vol. 153, issue 6:1245. [DOI] [PubMed]
- Kerkhof PC. The impact of a two‐compound product containing calcipotriol and betamethasone dipropionate (Daivobet/ Dovobet) on the quality of life in patients with psoriasis vulgaris: a randomized controlled trial. British Journal of Dermatology 2004;151(3):663‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kerkhof PCM. A fixed combination of calcipotriol/betamethasone dipropionate improves quality of life in patients with psoriasis vulgaris. 11th Congress of the European Academy of Dermatology and Venereology; 2002 Oct 2‐6; Prague. 2002:P27‐2.
Han 2001 {published data only}
- Han GW, Yu BT, Li H, Zhu XJ, Wang BX, Li GM, et al. A randomized controlled multicenter clinical trial on tazarotene gel versus calcipotriol ointment in the treatment of plaque psoriasis vulgaris. Chinese Journal of Clinical Pharmacology 2001;17(6):419‐22. [Google Scholar]
Harrington 1996a {published data only}
- Harrington CI, Goldin D, Lovell CR, Kerkhof P, Nieboer C, Austad J, et al. Comparative Effects of Two Different Calcipotriol (Mc 903) Cream Formulations Versus Placebo in Psoriasis Vulgaris. A Randomised, Double‐Blind, Placebo‐Controlled, Parallel Group Multi‐Centre Study 1. Journal of the European Academy of Dermatology & Venereology 1996;6(2):152‐8. [EMBASE: 1996096735] [Google Scholar]
Helfrich 2007 {published and unpublished data}
Henneicke‐v. Z. 1993 {published data only}
- Henneicke‐von Zepelin HH, Mrowietz U, Farber L, Bruck‐Borchers K, Schober C, Huber J, et al. Highly purified omega‐3‐polyunsaturated fatty acids for topical treatment of psoriasis. Results of a double‐blind, placebo‐controlled multicentre study. British Journal Of Dermatology 1993;129(6):713‐7. [PUBMED: 8286257] [DOI] [PubMed] [Google Scholar]
Highton 1995 {published data only}
- Highton A, Quell J, Breneman D, Cullen S, Goffe B, Griffiths C, et al. Calcipotriene ointment 0.005% for psoriasis: A safety and efficacy study. Journal of the American Academy of Dermatology 1995;32(1):67‐72. [PUBMED: 7822519] [DOI] [PubMed] [Google Scholar]
Hindsén 2006 (H) {published and unpublished data}
- Hindsén M, Traulsen J. Calcipotriol ointment under occlusion gives a fast onset of action. Journal of the European Academy of Dermatology & Venereology 2006;20(6):764‐5. [PUBMED: 16836527] [DOI] [PubMed] [Google Scholar]
Hindsén 2006 (P) {published and unpublished data}
- Hindsén M, Traulsen J. Calcipotriol ointment under occlusion gives a fast onset of action. Journal of the European Academy of Dermatology & Venereology 2006;20(6):764‐5. [PUBMED: 16836527] [DOI] [PubMed] [Google Scholar]
Holick 2003 {published data only}
- Holick MF, Chimeh FN, Ray S. Topical PTH (1‐34) is a novel, safe and effective treatment for psoriasis: a randomized self‐controlled trial and an open trial. British Journal of Dermatology 2003;149(2):370‐6. [PUBMED: 12932245] [DOI] [PubMed] [Google Scholar]
Huang 2009 {published data only}
- Huang L, Ma L, Huang Q, Yang QP, Zheng ZZ, Zhu XJ, et al. Calcipotriol betamethasone ointment in the treatment of psoriasis vulgaris: a randomized, double‐blind, active‐controlled, parallel group study. Zhonghua Pifuke Zazhi (Chinese Journal of Dermatology) 2009;42(10):691‐4. [NCT00248456] [Google Scholar]
Hutchinson 2000 {published data only}
- Hutchinson PE, Marks R, White J. The efficacy, safety and tolerance of calcitriol 3 microg/g ointment in the treatment of plaque psoriasis: a comparison with short‐contact dithranol. Dermatology 2000;201(2):139‐45. [PUBMED: 11053917] [DOI] [PubMed] [Google Scholar]
Jarratt 2004 {published data only}
- Jarratt M, Breneman D, Gottlieb AB, Poulin Y, Liu Y, Foley V. Clobetasol propionate shampoo 0.05%: a new option to treat patients with moderate to severe scalp psoriasis. Journal of Drugs in Dermatology 2004;3(4):367‐73. [PUBMED: 15303780] [PubMed] [Google Scholar]
Jarratt 2006 {published and unpublished data}
- Jarratt M, Sofen HL, Caveney S. The effects of clobetasol propionate spray, 0.05% vs. vehicle spray on the signs of plaque psoriasis on the body and scalp [Abstract]. Conference: 36th Annual Hawaii Dermatology Seminar of the Skin Disease Education Foundation Waikoloa, HI United States. Conference Start: 20120219 Conference End: 20120224. Seminars in Cutaneous Medicine & Surgery 2012;31(1):A11. [EMBASE: 70706930] [Google Scholar]
- Jarratt MT, Clark SD, Savin RC, Swinyer LJ, Safley CF, Brodell RT, et al. Evaluation of the efficacy and safety of clobetasol propionate spray in the treatment of plaque‐type psoriasis. Cutis 2006; Vol. 78, issue 5:348‐54. [PUBMED: 17186795] [PubMed]
Jekler 1992 {published data only}
- Jekler J, Swanbeck G. One‐Minute Dithranol Therapy in Psoriasis: A Placebo‐Controlled Paired Comparative Study. Acta Dermato Venereologica 1992;72(6):449‐50. [PUBMED: 1362841] [PubMed] [Google Scholar]
Jemec 2008 (H) {published and unpublished data}
- Combemale P. A new treatment in scalp psoriasis: Xamiol (R) formulation and results at 8 weeks. Annales de Dermatologie et de Venereologie 2009;136(Suppl 3):S42‐5. [Google Scholar]
- Jemec GBE, Burden D, Poulin Y, Ortonne JP. A new scalp formulation of calcipotriene plus betamethasone in the treatment of scalp psoriasis compared to its active ingredients and the vehicle [Abstract P2733.] American Academy of Dermatology 65th Annual Meeting 2‐6 February 2007. Journal of the American Academy of Dermatology 2007; Vol. 56, issue 2:AB182. [DOI] [PubMed]
- Jemec GBE, Ganslandt C, Ortonne J‐P, Poulin Y, Burden AD, Unamuno P, et al. A new scalp formulation of calcipotriene plus betamethasone compared with its active ingredients and the vehicle in the treatment of scalp psoriasis: a randomized, double‐blind, controlled trial. Journal of the American Academy of Dermatology 2008; Vol. 59, issue 3:455‐63. [PUBMED: 18694678] [DOI] [PubMed]
Jemec 2008 (P) {published and unpublished data}
- Combemale P. A new treatment in scalp psoriasis: Xamiol (R) formulation and results at 8 weeks. Annales de Dermatologie et de Venereologie 2009;136(Suppl 3):S42‐5. [Google Scholar]
- Jemec GBE, Burden D, Poulin Y, Ortonne JP. A new scalp formulation of calcipotriene plus betamethasone in the treatment of scalp psoriasis compared to its active ingredients and the vehicle [Abstract P2733.] American Academy of Dermatology 65th Annual Meeting 2‐6 February 2007. Journal of the American Academy of Dermatology 2007; Vol. 56, issue 2:AB182. [DOI] [PubMed]
- Jemec GBE, Ganslandt C, Ortonne J‐P, Poulin Y, Burden AD, Unamuno P, et al. A new scalp formulation of calcipotriene plus betamethasone compared with its active ingredients and the vehicle in the treatment of scalp psoriasis: a randomized, double‐blind, controlled trial. Journal of the American Academy of Dermatology 2008; Vol. 59, issue 3:455‐63. [PUBMED: 18694678] [DOI] [PubMed]
Ji 2008 {published data only}
- Gottschalk RW, Johnson LA. Calcitriol 3 micrograms/g versus calcipotriol 50 micrograms/g for plaque psoriasis: treatment, maintenance and cost [Abstract P2720.] American Academy of Dermatology 65th Annual Meeting 2‐6 February 2007. Journal of the American Academy of Dermatology 2007; Vol. 56, issue 2:AB179.
- Ji S, Chen X, Wang B, Jin H, Zhao G, Wang Y, et al. Calcitriol ointment versus calcipotriol ointment in the treatment of psoriasis: a single‐blind, randomized, multicenter trial. Zhonghua Pifuke Zazhi (Chinese Journal of Dermatology) 2008; Vol. 41, issue 3:153‐6.
- Xuejun Z, Baoxi W, Guang Z, Jun G, Zhiqiang C, Briantais P, et al. A comparison of the efficacy and safety of twice daily applications of calcitriol 3 lg/g ointment versus calcipotriol 50 mcg/g ointment in subjects with mild to moderate chronic plaque‐type psoriasis. [Abstract P0647.] 14th Congress of the European Academy of Dermatology and Venereology, 12‐15th October 2005, London, UK. Journal of the European Academy of Dermatology & Venereology 2005;19(Suppl 2):172. [DOI] [PubMed] [Google Scholar]
- Zhu X, Wang B, Zhao G, Gu J, Chen Z, Briantais P, et al. An investigator‐masked comparison of the efficacy and safety of twice daily applications of calcitriol 3 microg/g ointment vs. calcipotriol 50 microg/g ointment in subjects with mild to moderate chronic plaque‐type psoriasis. Journal of the European Academy of Dermatology & Venereology 2007; Vol. 21, issue 4:466‐72. [PUBMED: 17373972] [DOI] [PubMed]
Jin 2001 {published data only}
- Jin HZ, Wang JB, He ZX, Xie Y. A Randomized, Placebo Controlled, Double Blind Trial Study on IL‐8 Monoclonal Antibody Cream in the Treatment of Psoriasis Vulgaris. Chinese Journal of Clinical Pharmacology 2001;17(1):36‐40. [Google Scholar]
Jorizzo 1997 {published data only}
- Jorizzo Jl, Magee K, Stewart DM, Lebwohl MG, Rajagopalan R, Brown JJ. Clobetasol propionate emollient 0.05 percent: hypothalamic‐pituitary‐adrenal‐axis safety and four‐week clinical efficacy results in plaque‐type psoriasis. Cutis 1997;60(1):55‐60. [PUBMED: 9252738] [PubMed] [Google Scholar]
Jorizzo 2007 {published data only (unpublished sought but not used)}
- Jorizzo J. Efficacy with calcipotriene and betamethasone dipropionate ointment in patients with moderate and severe psoriasis [Abstract P2780.] American Academy of Dermatology 65th Annual Meeting 2‐6 February, 2007. Journal of the American Academy of Dermatology 2007; Vol. 56, issue 2:AB194.
Kang 1998 {published and unpublished data}
- Kang S, Yi S, Griffiths CEM, Fancher L, Hamilton TA, Choi JH. Calcipotriene‐Induced Improvement in Psoriasis Is Associated with Reduced Interleukin‐8 and Increased Interleukin‐10 Levels within Lesions. British Journal Of Dermatology 1998;138(1):77‐83. [PUBMED: 9536226] [DOI] [PubMed] [Google Scholar]
Kanzler 1993 {published and unpublished data}
- Kanzler MH, Gorsulowsky DC. Efficacy of topical 5% liquor carbonis detergens vs. its emollient base in the treatment of psoriasis. British Journal Of Dermatology 1993;129(3):310‐4. [PUBMED: 8286230] [DOI] [PubMed] [Google Scholar]
Katz 1987a {published data only}
- Katz HI, Hien NT, Prawer S, Scot JC, Grivna EM. Betamethasone Dipropionate in Optimized Vehicle. Intermittent Pulse Dosing for Extended Maintenance Treatment of Psoriasis. Archives of Dermatology 1987;123(10):1308‐11. [PUBMED: 3662562] [DOI] [PubMed] [Google Scholar]
Katz 1991a {published data only}
- Katz HI, Prawer SE, Medansky RS, Krueger GG, Mooney JJ, Jones ML, et al. Intermittent corticosteroid maintenance treatment of psoriasis: a double‐blind multicenter trial of augmented betamethasone dipropionate ointment in a pulse dose treatment regimen. Dermatologica 1991;183(4):269‐74. [PUBMED: 1809589] [DOI] [PubMed] [Google Scholar]
Katz 1991b {published data only}
- Katz HI, Gross E, Buxman M, Prawer SE, Schwartzel EH, Gibson JR. A double‐blind, vehicle‐controlled paired comparison of halobetasol propionate cream on patients with plaque psoriasis. Journal of the American Academy of Dermatology 1991;25(6 Pt 2):1175‐8. [PUBMED: 1757613] [DOI] [PubMed] [Google Scholar]
Kaufmann 2002 (H) {published and unpublished data}
- Kaufmann R, Bibby AJ, Bissonnette R, Cambazard F, Chu AC, Decroix J, et al. A New Calcipotriol/Betamethasone Dipropionate Formulation (Daivobet(TM)) Is an Effective Once‐Daily Treatment for Psoriasis vulgaris. Dermatology 2002;205(4):389‐93. [PUBMED: 12444337] [DOI] [PubMed] [Google Scholar]
Kaufmann 2002 (P) {published and unpublished data}
- Kaufmann R, Bibby AJ, Bissonnette R, Cambazard F, Chu AC, Decroix J, et al. A New Calcipotriol/Betamethasone Dipropionate Formulation (Daivobet(TM)) Is an Effective Once‐Daily Treatment for Psoriasis vulgaris. Dermatology 2002;205(4):389‐93. [PUBMED: 12444337] [DOI] [PubMed] [Google Scholar]
Kim 1994 {published data only}
- Kim JY, You YH, Kim TY, Kim CW. Comparative Study of Calcipotriol and Desoxymethasone Ointments in the Treatment of Psoriasis Vulgaris. The Clinical Effect and Immunohistochemical Change. Korean Journal of Dermatology 1994;32(6):1054‐63. [EMBASE: 1995058111] [Google Scholar]
Kiss 1996 {published data only}
- Carder KR. A randomized double blind parallel group dose‐ranging comparison of the efficacy and safety of calcipotriene solution in the treatment of scalp psoriasis [Abstract 844]. Journal of Investigative Dermatology 1996;106(4):946. [Google Scholar]
- Kiss I, McCreary HL, Siskin SB, Epinette WW. A Randomized, Double‐ Blind, Parallel Group, Dose‐Ranging Comparison of the Efficacy and Safety of Calcipotriene Solution in the Treatment of Scalp Psoriasis. 54th Annual Meeting of the American Academy of Dermatology, 10‐15 February 1996. Journal of the American Academy of Dermatology. 1996:P301.
Klaber 1994 {published data only}
- Klaber MR, Hutchinson PE, Pedvis‐Leftick A, Kragballe K, Reunala TL, Kerkhof PC, et al. Comparative effects of calcipotriol solution (50 micrograms/ml) and betamethasone 17‐valerate solution (1 mg/ml) in the treatment of scalp psoriasis. British Journal Of Dermatology 1994;131(5):678‐83. [PUBMED: 7999600] [DOI] [PubMed] [Google Scholar]
Klaber 2000b {published and unpublished data}
- Klaber MR, McKinnon C. Calcipotriol (DovonexR) scalp solution in the treatment of scalp psoriasis: Comparative efficacy with 1% coal tar/1% coconut oil/0.5% salicylic acid (CapasalR) shampoo, and long‐term experience. Journal Of Dermatological Treatment 2000;11(1):21‐8. [EMBASE: 2000114933] [Google Scholar]
Koo 2006 {published data only (unpublished sought but not used)}
- Koo J, Blum RR, Lebwohl M. A randomized, multicenter study of calcipotriene ointment and clobetasol propionate foam in the sequential treatment of localized plaque‐type psoriasis: short‐ and long‐term outcomes. Journal of the American Academy of Dermatology 2006; Vol. 55, issue 4:637‐41. [PUBMED: 17010744] [DOI] [PubMed]
Köse 1997 {published data only}
- Köse O. Calcipotriol ointment vs clobetasol solution in scalp psoriasis. Journal Of Dermatological Treatment 1997;8:287. [EMBASE: 1998022654] [Google Scholar]
Kragballe 1988b {published and unpublished data}
- Kragballe K, Beck HI, Søgaard H. Improvement of Psoriasis by a Topical Vitamin D3 Analogue (Mc 903) in a Double‐Blind Study. British Journal Of Dermatology 1988;119(2):223‐30. [EMBASE: 1988203615] [DOI] [PubMed] [Google Scholar]
Kragballe 1991a {published and unpublished data}
- Kragballe K, Gjertsen BT, Hoop D, Karlsmark T, Kerkhof PC, Larko O, et al. Double‐blind, right/left comparison of calcipotriol and betamethasone valerate in treatment of psoriasis vulgaris. Lancet 1991;337(8735):193‐6. [PUBMED: 1670840] [DOI] [PubMed] [Google Scholar]
Kragballe 1998b {published and unpublished data}
- Glade CP, Erp PE, Kerkhof PC. Epidermal cell DNA content and intermediate filaments keratin 10 and vimentin after treatment of psoriasis with calcipotriol cream once daily, twice daily and in combination with clobetasone 17‐butyrate cream or betamethasone 17‐valerate cream: a comparative flow cytometric study. British Journal Of Dermatology 1996;135(3):379‐84. [PUBMED: 8949429] [PubMed] [Google Scholar]
- Kragballe K, Barnes L, Hamberg KJ, Hutchinson P, Murphy F, Moller S, et al. Calcipotriol Cream with or without Concurrent Topical Corticosteroid in Psoriasis: Tolerability and Efficacy. British Journal Of Dermatology 1998;139(4):649‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kerkhof PC. Calcipotriol cream and concurrent corticosteroids in psoriasis. Journal of the European Academy of Dermatology & Venereology 1995; Vol. 5, issue Suppl 1:S184.
Kragballe 2004 {published and unpublished data}
- Kragballe K, Noerrelund KL, Lui H, Ortonne JP, Wozel G, Uurasmaa T, et al. Efficacy of once‐daily treatment regimens with calcipotriol/betamethasone dipropionate ointment and calcipotriol ointment in psoriasis vulgaris. British Journal of Dermatology 2004;150(6):1167‐73. [PUBMED: 15214905] [DOI] [PubMed] [Google Scholar]
Kragballe 2006 {published and unpublished data}
- Gold LS. Correlation between amount of drug used versus efficacy of calcipotriene/betamethasone in severe psoriasis during a 52‐week study [Abstract P2873.] American Academy of Dermatology 64th Annual Meeting 3‐7 March, 2006. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB216. [Google Scholar]
- Kragballe K, Austad J, Barnes L, Bibby A, Brassinne M, Cambazard F, et al. A 52‐week randomized safety study of a calcipotriol/betamethasone dipropionate two‐compound product (Dovobet/Daivobet/Taclonex) in the treatment of psoriasis vulgaris. British Journal of Dermatology 2006; Vol. 154, issue 6:1155‐60. [PUBMED: 16704648] [DOI] [PubMed]
- Kragballe K, Austad J, Barnes L, Bibby A, Brassinne M, Cambazard F, et al. Efficacy results of a 52‐week, randomised, double‐blind, safety study of a calcipotriol/betamethasone dipropionate two‐compound product (Daivobet/Dovobet/Taclonex) in the treatment of psoriasis vulgaris. Dermatology 2006; Vol. 213, issue 4:319‐26. [PUBMED: 17135738] [DOI] [PubMed]
- Kragballe K, Bibby A. Long‐term efficacy of a calcipotriene/betamethasone dipropionate two compound product in Psoriasis vulgaris [Abstract P2891] American Academy of Dermatology 64th Annual Meeting 3‐7 March, 2006. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB220. [Google Scholar]
Kragballe 2009 {published and unpublished data}
- Kragballe K, Ganslandt C, Ortonne J P. A calcipotriol plus betamethasone dipropionate scalp formulation significantly reduces itching in scalp psoriasis. Journal of the American Academy of Dermatology 2009;60(3 Suppl 1):AB172. [Google Scholar]
- Kragballe K, Hoffmann V, Ortonne J P, Tan J, Nordin P, Segaert S. Efficacy and safety of calcipotriol plus betamethasone dipropionate scalp formulation compared with calcipotriol scalp solution in the treatment of scalp psoriasis: a randomized controlled trial. British Journal of Dermatology 2009;161(1):159‐66. [PUBMED: 19416259] [DOI] [PubMed] [Google Scholar]
- Ortonne JP, Ganslandt C, Tan J, Nordin P, Kragballe K, Segaert S. Quality of life in patients with scalp psoriasis treated with calcipotriol/betamethasone dipropionate scalp formulation: a randomized controlled trial. Journal of the European Academy of Dermatology & Venereology 2009;23(8):919‐26. [DOI] [PubMed] [Google Scholar]
Kreuter 2006 (H) {published data only (unpublished sought but not used)}
- Kreuter A, Sommer A, Hyun J, Brautigam M, Brockmeyer NH, Altmeyer P, et al. 1% pimecrolimus, 0.005% calcipotriol, and 0.1% betamethasone in the treatment of intertriginous psoriasis: a double‐blind, randomized controlled study. Archives of Dermatology 2006; Vol. 142, issue 9:1138‐43. [PUBMED: 16983001] [DOI] [PubMed]
Kreuter 2006 (P) {published data only (unpublished sought but not used)}
- Kreuter A, Sommer A, Hyun J, Brautigam M, Brockmeyer NH, Altmeyer P, et al. 1% pimecrolimus, 0.005% calcipotriol, and 0.1% betamethasone in the treatment of intertriginous psoriasis: a double‐blind, randomized controlled study. Archives of Dermatology 2006; Vol. 142, issue 9:1138‐43. [PUBMED: 16983001] [DOI] [PubMed]
Krueger 1998 {published data only}
- Krueger GG, Drake LA, Elias PM, Lowe NJ, Guzzo C, Weinstein GD, et al. The safety and efficacy of tazarotene gel, a topical acetylenic retinoid, in the treatment of psoriasis. Archives of Dermatology 1998;134(1):57‐60. [PUBMED: 9449910] [DOI] [PubMed] [Google Scholar]
Lahfa 2003 {published data only (unpublished sought but not used)}
- Lahfa M, Mrowietz U, Koenig M, Simon JC. Calcitriol ointment and clobetasol propionate cream: a new regimen for the treatment of plaque psoriasis. European Journal of Dermatology 2003; Vol. 13, issue 3:261‐5. [PubMed]
Landi 1993 {published data only}
- Landi G. Efficacy and safety of calcipotriol ointment compared to clobetasol ointment in psoriasis vulgaris. 3rd Congress of the European Academy of Dermatology & Venereology. Copenhagen, Denmark, 1993:199.
- Landi G, Pierleoni M, Polverelli M, Fioravanti F. Calcipotriol, a New Topical Product in the Therapy of Psoriasis: Controlled Study Versus Clobetasol [Il Calcipotriol, Nuovo Topico Nella Terapia Della Psoriasi: Studio Controllato Versus Clobetasol]. Giornale Italiano di Dermatologia e Venereologia 1993;128(9):89‐93. [EMBASE: 1993360641] [Google Scholar]
Lane 1983 {published data only}
- Lane AT, Wachs GN, Weston WL. Once‐Daily Treatment of Psoriasis with Topical Glucocorticosteroid Ointments. Journal of the American Academy of Dermatology 1983;8(4):523‐5. [PUBMED: 6853785] [DOI] [PubMed] [Google Scholar]
Langley 2011 (H) {published and unpublished data}
- Langley R, Gupta A, Papp K, Wexler D, Østerdal M, Ćurčić D. Calcipotriol plus Betamethasone Dipropionate Gel Compared with Tacalcitol Ointment and the Gel Vehicle Alone in Patients with Psoriasis Vulgaris: A Randomized, Controlled Clinical Trial. Dermatology 2011; Vol. 222, issue 2:148‐56. [PUBMED: 21293107] [DOI] [PubMed]
- Langley R, Gupta A, Wexler D, Curcic D, Papp K. Efficacy and safety of calcipotriene plus betamethasone dipropionate gel compared to tacalcitol ointment and gel vehicle alone in patients with psoriasis vulgaris: A randomized controlled trial. Journal of the American Academy of Dermatology 2010;62(3 Suppl 1):AB134. [DOI] [PubMed] [Google Scholar]
- NCT00670241. Efficacy and Safety of Calcipotriol Plus Betamethasone Dipropionate Gel Compared With Tacalcitol Ointment and the Gel Vehicle Alone in Patients With Psoriasis Vulgaris. clinicaltrials.gov/ct2/results?term=NCT00670241 (accessed 2010).
Langley 2011 (P) {published and unpublished data}
- Langley R, Gupta A, Papp K, Wexler D, Østerdal M, Ćurčić D. Calcipotriol plus Betamethasone Dipropionate Gel Compared with Tacalcitol Ointment and the Gel Vehicle Alone in Patients with Psoriasis Vulgaris: A Randomized, Controlled Clinical Trial. Dermatology 2011; Vol. 222, issue 2:148‐56. [PUBMED: 21293107] [DOI] [PubMed]
- Langley R, Gupta A, Wexler D, Curcic D, Papp K. Efficacy and safety of calcipotriene plus betamethasone dipropionate gel compared to tacalcitol ointment and gel vehicle alone in patients with psoriasis vulgaris: A randomized controlled trial. Journal of the American Academy of Dermatology 2010;62(3 Suppl 1):AB134. [DOI] [PubMed] [Google Scholar]
- NCT00670241. Efficacy and Safety of Calcipotriol Plus Betamethasone Dipropionate Gel Compared With Tacalcitol Ointment and the Gel Vehicle Alone in Patients With Psoriasis Vulgaris. clinicaltrials.gov/ct2/results?term=NCT00670241 (accessed 2010).
Langner 1992 {published data only}
- Langner A, Verjans H, Stapor V, Mol M, Fraczykowska M. 1 Alpha 25‐Dihydroxyvitamin D‐3 (Calcitriol) Ointment in Psoriasis. Journal Of Dermatological Treatment 1992;3(4):177‐80. [EMBASE: 1993020253] [Google Scholar]
Langner 1993 {published data only}
- Langner A, Verjans H, Stapor V, Mol M, Fraczykowska M. Topical Calcitriol in the Treatment of Chronic Plaque Psoriasis: A Double‐Blind Study. British Journal Of Dermatology 1993;128(5):566‐71. [PUBMED: 8504051] [DOI] [PubMed] [Google Scholar]
Langner 2001 (H) {published data only}
- Langner A, Stapor W, Ambroziak M. Efficacy and tolerance of topical calcitriol 3 microg g(‐1) in psoriasis treatment: a review of our experience in Poland. British Journal Of Dermatology 2001;58:11‐16. [PUBMED: 11501507] [DOI] [PubMed] [Google Scholar]
Langner 2001 (P) {published data only}
- Langner A, Stapor W, Ambroziak M. Efficacy and tolerance of topical calcitriol 3 microg g(‐1) in psoriasis treatment: a review of our experience in Poland. British Journal Of Dermatology 2001;58:11‐16. [PUBMED: 11501507] [DOI] [PubMed] [Google Scholar]
Lassus 1991 {published data only}
- Lassus A, Forsstrom S. A double‐blind study comparing oleum horwathiensis with placebo in the treatment of psoriasis. Journal of International Medical Research 1991;19(2):137‐46. [PUBMED: 1864450] [DOI] [PubMed] [Google Scholar]
Lebwohl 2002 {published data only}
- Lebwohl M, Scher RK, Washenik K, Krueger G, Menter A, Koo J, et al. A randomized, double‐blind placebo‐controlled study of the safety and efficacy of clobetasol propionate 0.05% foam in the treatment of non‐scalp psoriasis. Skin Pharmacology & Applied Skin Physiology 2001;14:168. [Google Scholar]
- Lebwohl M, Sherer D, Washenik K, Krueger GG, Menter A, Koo J, Feldman SR. A randomized, double‐blind, placebo‐controlled study of clobetasol propionate 0.05% foam in the treatment of nonscalp psoriasis. International Journal Of Dermatology 2002;41(5):269‐74. [PUBMED: 12100701] [DOI] [PubMed] [Google Scholar]
Lebwohl 2004 {published data only (unpublished sought but not used)}
- Lebwohl M, Freeman AK, Chapman MS, Feldman SR, Hartle JE, Henning A. Tacrolimus ointment is effective for facial and intertriginous psoriasis. Journal of the American Academy of Dermatology 2004;51(5):723‐30. [PUBMED: 15523350] [DOI] [PubMed] [Google Scholar]
Lebwohl 2007 {published and unpublished data}
- Lebwohl M, Menter A, Weiss J, Clark SD, Flores J, Powers J, et al. Calcitriol 3 microg/g ointment in the management of mild to moderate plaque type psoriasis: results from 2 placebo‐controlled, multicenter, randomized double‐blind, clinical studies. Journal of Drugs in Dermatology 2007; Vol. 6, issue 4:428‐35. [PUBMED: 17668541] [PubMed]
Lee 2007 {published data only}
- Lee J, Youn J, Kim N, Kim K, Kim T, Choi J, et al. A randomized investigator‐blinded comparative study of calcitriol twice a day vs. diflucortolone valerate morning plus calcitriol evening application in the treatment of mild to moderate psoriasis. Journal of the European Academy of Dermatology & Venereology 2007; Vol. 21, issue Suppl 1:19.
Lepaw 1978 {published data only}
- Lepaw MI. Double‐Blind Comparison of Halcinonide Solution and Placebo Control in Treatment of Psoriasis of the Scalp. Cutis 1978;21(4):571‐3. [PUBMED: 346315] [PubMed] [Google Scholar]
Levine 2010 (H) {published and unpublished data}
- Levine D, Even‐Chen Z, Lipets I, Pritulo OA, Svyatenko TV, Andrashko Y, et al. Pilot, multicenter, double‐blind, randomized placebo‐controlled bilateral comparative study of a combination of calcipotriene and nicotinamide for the treatment of psoriasis. Journal of the American Academy of Dermatology 2010;63(5):775‐81. [PUBMED: 20599292] [DOI] [PubMed] [Google Scholar]
Levine 2010 (P) {published and unpublished data}
- Levine D, Even‐Chen Z, Lipets I, Pritulo OA, Svyatenko TV, Andrashko Y, et al. Pilot, multicenter, double‐blind, randomized placebo‐controlled bilateral comparative study of a combination of calcipotriene and nicotinamide for the treatment of psoriasis. Journal of the American Academy of Dermatology 2010;63(5):775‐81. [PUBMED: 20599292] [DOI] [PubMed] [Google Scholar]
Liao 2007 {published and unpublished data}
- Liao YH, Chiu HC, Tseng YS, Tsai TF. Comparison of cutaneous tolerance and efficacy of calcitriol 3 microg g(‐1) ointment and tacrolimus 0.3 mg g(‐1) ointment in chronic plaque psoriasis involving facial or genitofemoral areas: a double‐blind, randomized controlled trial. British Journal of Dermatology 2007; Vol. 157, issue 5:1005‐12. [PUBMED: 17935517] [DOI] [PubMed]
Lin 2007 {published and unpublished data}
- Lin YK, Wong WR, Chang YC, Chang CJ, Tsay PK, Chang SC, et al. The efficacy and safety of topically applied indigo naturalis ointment in patients with plaque‐type psoriasis. Dermatology 2007;214(2):155‐61. [PUBMED: 17341866] [DOI] [PubMed] [Google Scholar]
Lin 2008 {published data only}
- Lin YK, Chang CJ, Chang YC, Wong WR, Chang SC, Pang JHS. Clinical assessment of patients with recalcitrant psoriasis in a randomized, observer‐blind, vehicle‐controlled trial using indigo naturalis. Archives of Dermatology 2008;144(11):1457‐64. [PUBMED: 19015420] [DOI] [PubMed] [Google Scholar]
Lister 1997 {published data only}
- Lister RK, Woodrow Sl, Hughes JH, Cerio R, Norris PG, Griffiths CEM, et al. Can dithranol have a lasting effect and be more acceptable to patients? Micanol Cream ‐ a trial of 171 patients with psoriasis. British Journal of Dermatology 1997;137(Suppl 50):17. [Google Scholar]
Lowe 2005 {published data only}
- Lowe N, Feldman SR, Sherer D, Weiss J, Shavin JS, Lin YL, et al. Clobetasol propionate lotion, an efficient and safe alternative to clobetasol propionate emollient cream in subjects with moderate to severe plaque‐type psoriasis. Journal of Dermatological Treatment 2005; Vol. 16, issue 3:158‐64. [PUBMED: 16096182] [DOI] [PubMed]
Luger 2008 {published and unpublished data}
- Cambazard F. Xamiol, a new treatment in scalp psoriasis: short‐ and long‐term results [Un nouveau traitement dans le psoriasis du cuir chevelu : Xamiol : Resultats court terme et a long terme]. Annales de Dermatologie et de Venereologie 2009;136(Suppl 3):546‐50. [Google Scholar]
- Luger T, Kidson P, Cambazard F, Larsen FG. A 1‐year, randomized, double‐blind safety study of long‐term treatment of a new gel formulation containing calcipotriene plus betamethasone dipropionate in scalp psoriasis. Journal of the American Academy of Dermatology 2008; Vol. 58, issue 2:AB134.
- Luger TA, Cambazard F, Larsen FG, Bourcier M, Gupta G, Clonier F, et al. A Study of the Safety and Efficacy of Calcipotriol and Betamethasone Dipropionate Scalp Formulation in the Long‐Term Management of Scalp Psoriasis. Dermatology 2008; Vol. 217, issue 4:321‐8. [PUBMED: 18787325] [DOI] [PMC free article] [PubMed]
Maier 2004 {published data only}
- Maier H. Prospective, randomized controlled double‐blind study on the efficacy and safety of a series of herbal skin‐care products for stable chronic plaque psoriasis [Abstract P061] European Congress on Psoriasis 2004. Journal of the European Academy of Dermatology & Venereology 2004;18(6):794. [Google Scholar]
Medansky 1987 {published data only}
- Medansky RS, Brody NI, Kanof NB, Russo GJ, Peets EA. Clinical Investigations of Mometasone Furoate ‐ a Novel Nonfluorinated, Topical Corticosteroid. Seminars In Dermatology 1987;6(2):94‐100. [EMBASE: 1988051740] [Google Scholar]
Medansky 1996 {published data only}
- Medansky RS, Greenspan A, Kraus SJ, Todd Plott R. The Comparative Efficacy of Diflorasone Diacetate Ointment 0.05% (Psorcon®) vs Calcipotriene Ointment (Dovonex®) in the Treatment of Psoriasis. Journal of Geriatric Dermatology 1996;4(1):20‐24. [Google Scholar]
Menter 2009 {published and unpublished data}
- Menter A, Abramovits W, Colon LE, Johnson LA, Gottschalk RW. Comparing clobetasol propionate 0.05% spray to calcipotriene 0.005% betamethasone dipropionate 0.064% ointment for the treatment of moderate to severe plaque psoriasis. Journal of Drugs in Dermatology 2009;8(1):52‐7. [PUBMED: 19180896] [PubMed] [Google Scholar]
- Menter A, Colon L, Johnson L, Gottschalk R. Results from a randomized study comparing clobetasol propionate 0.05% spray to calcipotriene 0.005%, betamethasone dipropionate 0.064% ointment for the treatment of plaque psoriasis. Journal of the American Academy of Dermatology 2008; Vol. 58, issue 2:AB124.
Molin 1997 {published and unpublished data}
- Molin L, Cutler TP, Helander I, Nyfors B. Calcipotriol cream and betamethasone valerate cream of equal efficacy in psoriasis. Journal of the European Academy of Dermatology & Venereology 1995;5(Suppl 1):S92. [Google Scholar]
- Molin L, Cutler TP, Helander I, Nyfors B. Comparative Efficacy of Calcipotriol Cream and Betamethasone Valerate Cream in the Treatment of Psoriasis. Journal of Investigative Dermatology. Symposium Proceedings 1996;1(1):110. [PubMed] [Google Scholar]
- Molin L, Cutler TP, Helander I, Nyfors B, Downes N. Comparative Efficacy of Calcipotriol (Mc903) Cream and Betamethasone 17‐Valerate Cream in the Treatment of Chronic Plaque Psoriasis. A Randomized, Double‐Blind, Parallel Group Multicentre Study. Calcipotriol Study Group. British Journal Of Dermatology 1997;136(1):89‐93. [PUBMED: 9039301] [PubMed] [Google Scholar]
Monastirli 2000 {published data only}
- Monastirli A, Zografakis CH, Braun H, Pasmatzi E, Georgiou S, Sakkis TH, et al. Calcipotriol vs. Anthralin in the treatment of chronic plaque psoriasis. H+G Zeitschrift Fur Hautkrankheiten. 2000;75(11):626‐9. [EMBASE: 2000428558] [Google Scholar]
Mortensen 1993b {published and unpublished data}
- Mortensen L, Kragballe K, Wegmann E, Schifter S, Risteli J, Charles P. Treatment of Psoriasis Vulgaris with Topical Calcipotriol Has No Short‐Term Effect on Calcium or Bone Metabolism. A Randomized, Double‐Blind, Placebo‐Controlled Study. Acta Dermato Venereologica 1993;73(4):300‐4. [PUBMED: 7904106] [DOI] [PubMed] [Google Scholar]
Olsen 1991 {published data only}
- Olsen EA, Cram DL, Ellis CN, Hickman JG, Jacobson C, Jenkins EE, et al. A double‐blind, vehicle‐controlled study of clobetasol propionate 0.05% (Temovate) scalp application in the treatment of moderate to severe scalp psoriasis. Journal of the American Academy of Dermatology 1991;24(3):443‐7. [PUBMED: 2061442] [DOI] [PubMed] [Google Scholar]
Olsen 1996 (1) {published data only}
- Olsen EA. Efficacy and Safety of Fluticasone Propionate 0.005% Ointment in the Treatment of Psoriasis. Cutis 1996;57(2 Suppl):57‐61. [PUBMED: 8646872] [PubMed] [Google Scholar]
Olsen 1996 (2) {published data only}
- Olsen EA. Efficacy and Safety of Fluticasone Propionate 0.005% Ointment in the Treatment of Psoriasis. Cutis 1996;2 Suppl:57‐61. [PUBMED: 8646872] [PubMed] [Google Scholar]
Oranje 1997 {published data only}
- Oranje AP, Marcoux DSA, Prendiville J, Krafchik B, Toole J, Rosenthal D, et al. Topical calcipotriol in childhood psoriasis. Journal of the American Academy of Dermatology 1997;36(2 Pt 1):203‐8. [PUBMED: 9039169] [DOI] [PubMed] [Google Scholar]
Ormerod 1997 {published and unpublished data}
- Ormerod AD, Dwyer CM, Weller R, Cox DH, Price R. A comparison of subjective and objective measures of reduction of psoriasis with the use of ultrasound, reflectance colorimetry, computerized video image analysis, and nitric oxide production. Journal of the American Academy of Dermatology 1997;37(1):51‐7. [PUBMED: 9216523 ] [DOI] [PubMed] [Google Scholar]
Ormerod 2000 {published data only}
- Ormerod AD, Copeland P, Shah SA. Treatment of psoriasis with topical NG‐monomethyl‐L‐arginine, an inhibitor of nitric oxide synthesis. British Journal Of Dermatology 2000;142(5):985‐90. [PUBMED: 10809860] [DOI] [PubMed] [Google Scholar]
Ormerod 2005 {published data only}
- Ormerod AD, Shah SA, Copeland P, Omar G, Winfield A. Treatment of psoriasis with topical sirolimus: preclinical development and a randomized, double‐blind trial. British Journal of Dermatology 2005;152(4):758‐64. [PUBMED: 15840110 ] [DOI] [PubMed] [Google Scholar]
Ortonne 1994 {published data only}
- Ortonne JP, Bazex J, Binet O, Bombart M, Brun P, Carreau O, et al. Psoriasis: new Therapeutic Modality by Calcipotriol and Betamethasone Dipropionate. Nouvelles Dermatologiques 1994;13(10):746‐51. [EMBASE: 1995010294] [Google Scholar]
Ortonne 2003 {published data only}
- Nicolas JF. Calcitriol vs calcipotriol in psoriasis of sensitive areas [Abstract]. 20th World Congress of Dermatology 1‐5 July 2002, Paris. Paris, 2002:SA1048.
- Ortonne JP, Humbert P, Nicolas JF, Tsankov N, Tonev SD, Janin A, et al. Intra‐individual comparison of the cutaneous safety and efficacy of calcitriol 3 microg g(‐1) ointment and calcipotriol 50 microg g(‐1) ointment on chronic plaque psoriasis localized in facial, hairline, retroauricular or flexural areas. British Journal of Dermatology 2003;148(2):326‐33. [PUBMED: 12588387] [DOI] [PubMed] [Google Scholar]
Ortonne 2004 {published and unpublished data}
- Ortonne JP, Kaufmann R, Lecha M, Goodfield M. Efficacy of treatment with calcipotriol/betamethasone dipropionate followed by calcipotriol alone compared with tacalcitol for the treatment of psoriasis vulgaris: A randomised, double‐blind trial. Dermatology 2004;209(4):308‐13. [PUBMED: 15539894] [DOI] [PubMed] [Google Scholar]
- Ortonne JP, Traulsen J, Kaufmann R, Lecha M, Goodfield M. Calcipotriol/betamethasone dipropionate ointment once daily versus tacalcitol once daily in psoriasis vulgaris [Abstract P27‐49.] 12th Congress of the European Academy of Dermatology and Venereology. 15‐18 October 2003, Barcelona, Spain. Journal of the European Academy of Dermatology & Venereology 2003;17(Suppl 3):374. [Google Scholar]
Ortonne 2006 {published data only}
- Ortonne JP, Kerkhof PCM, Prinz JC, Bieber T, Lahfa M, Rubins A, et al. 0.3% Tacrolimus gel and 0.5% Tacrolimus cream show efficacy in mild to moderate plaque psoriasis: Results of a randomized, open‐label, observer‐blinded study. Acta Dermato‐Venereologica 2006; Vol. 86, issue 1:29‐33. [PUBMED: 16585986] [DOI] [PubMed]
- Vissers W H, Vlijmen I, Erp P E, Jong E M, Kerkhof P C. Topical treatment of mild to moderate plaque psoriasis with 0.3% tacrolimus gel and 0.5% tacrolimus cream: the effect on SUM score, epidermal proliferation, keratinization, T‐cell subsets and HLA‐DR expression. British Journal of Dermatology 2008;158(4):705‐12. [DOI] [PubMed] [Google Scholar]
Ortonne 2010 {published and unpublished data}
- Ortonne JP, Noerrelund KL, Papp K, Herpe L, Sebastian M, Herrera E, et al. Comparison of two different dose combinations of calcipotriol/hydrocortisone ointment used once daily for the treatment of psoriasis vulgaris on the face and body. European Journal of Dermatology 2010;20(5):585‐9. [PUBMED: 20732849] [DOI] [PubMed] [Google Scholar]
Papakostantinou 2005 {published data only}
- Papakostantinou E, Xenos K, Markantonis S L, Druska S, Stratigos A, Katsambas A. Efficacy of 2 weeks' application of theophylline ointment in psoriasis vulgaris. Journal of Dermatological Treatment 2005;16(3):169‐70. [PUBMED: 16096184] [DOI] [PubMed] [Google Scholar]
Papp 2003 (H) {published and unpublished data}
- Papp KA, Guenther L, Boyden B, Larsen FG, Harvima RJ, Guilhou JJ, et al. Early onset of action and efficacy of a combination of calcipotriene and betamethasone dipropionate in the treatment of psoriasis. Journal of the American Academy of Dermatology 2003;48(1):48‐54. [PUBMED: 12522370] [DOI] [PubMed] [Google Scholar]
Papp 2003 (P) {published and unpublished data}
- Papp KA, Guenther L, Boyden B, Larsen FG, Harvima RJ, Guilhou JJ, et al. Early onset of action and efficacy of a combination of calcipotriene and betamethasone dipropionate in the treatment of psoriasis. Journal of the American Academy of Dermatology 2003;48(1):48‐54. [PUBMED: 12522370] [DOI] [PubMed] [Google Scholar]
Pariser 1996 {published data only}
- Pariser DM, Pariser RJ, Breneman D, Lebwohl M, Kalb R, Moore J, et al. Calcipotriene ointment applied once a day for psoriasis: A double‐ blind, multicenter, placebo‐controlled study. Archives of Dermatology 1996;132(12):1527. [PUBMED: 8961897] [PubMed] [Google Scholar]
Pauporte 2004 {published and unpublished data}
- Pauporte M, Maibach H, Lowe N, Pugliese M, Friedman DJ, Mendelsohn H, et al. Fluocinolone acetonide topical oil for scalp psoriasis. Journal of Dermatological Treatment 2004;15(6):360‐4. [PUBMED: 15764047] [DOI] [PubMed] [Google Scholar]
Perez 1996 {published and unpublished data}
- Perez A, Chen TC, Turner A, Raab R, Bhawan J, Poche P, et al. Efficacy and Safety of Topical Calcitriol (1,25‐Dihydroxyvitamin D3) for the Treatment of Psoriasis. British Journal Of Dermatology 1996;134(2):238‐46. [PUBMED: 8746336] [PubMed] [Google Scholar]
Pinheiro 1997 {published and unpublished data}
- Pinheiro N. Comparative Effects of Calcipotriol Ointment (50 Micrograms/G) and 5% Coal Tar/2% Allantoin/0.5% Hydrocortisone Cream in Treating Plaque Psoriasis. British Journal of Clinical Practice 1997;51(1):16‐9. [PUBMED: 9158266] [PubMed] [Google Scholar]
Poulin 2010 {published and unpublished data}
- Poulin Y, Papp K, Bissonnette R, Barber K, Kerrouche N, Villemagne H, et al. Clobetasol propionate shampoo 0.05% is efficacious and safe for long‐term control of moderate scalp psoriasis. Journal of Dermatological Treatment 2010;21(3):185‐92. [PUBMED: 20131980] [DOI] [PubMed] [Google Scholar]
- Poulin Y, Papp K, Bissonnette R, Guenther L, Tan J, Lynde C, et al. Clobetasol propionate shampoo 0.05% is efficacious and safe for long‐term control of scalp psoriasis. Cutis 2010;85(1):43‐50. [PUBMED: 20184211] [PubMed] [Google Scholar]
- Poulin Y, Papp K, Guenther L, Bissonnette R. Twice‐weekly use of clobetasol propionate 0.05% shampoo is effective and safe for the long‐term control of scalp psoriasis. Journal of the American Academy of Dermatology 2010; Vol. 62, issue 3 Suppl 1:AB132. [0190‐9622]
Powers 2005 {published and unpublished data}
- Powers J. Assessment of the Efficacy and Safety of Calcitriol 3mcg/g Ointment in the Treatment of Chronic Plaque Psoriasis. 7th Asian Congress of Dermatology incorporating the 5th Regional Conference of Paediatric Dermatology Kuala Lumpur, Malaysia 28 September ‐1 October, 2005 2005:367.
- Powers J, Balin AK, Kempers S, Glinert RJ, Fleming T, Graeber M. Assessment of the efficacy and safety of calcitriol 3ugg‐1 ointment in the treatment of chronic plaque psoriasis [Abstract P‐40.] 85th BAD Annual Meeting 5‐8 July 2005, Glasgow, UK. British Journal of Dermatology 2005; Vol. 153, issue Suppl 1:32.
Reygagne 2005 {published and unpublished data}
- Reygagne P, Mrowietz U, Decroix J, Waard‐Van Der Spek FB, Acebes LO, Figueiredo A, et al. Clobetasol propionate shampoo 0.05% and calcipotriol solution 0.005%: A randomized comparison of efficacy and safety in subjects with scalp psoriasis. Journal of Dermatological Treatment 2005; Vol. 16, issue 1:31‐6. [PUBMED: 15897165] [DOI] [PubMed]
- Reygagne P, Mrowietz U, Decroix J, Spek W, Olmos Acebes L, Figueiredo A, et al. Four‐week efficacy and safety comparison of a new clobetasol shampoo and calcipotriol solution 0.005% in subjects with scalp psoriasis. 11th Congress of the European Academy of Dermatology & Venereology, 2‐6 October, 2002, Prague. 2002:27‐36.
Ruzicka 1998 {published data only}
- Ruzicka T, Kallinschnigg G, Lorenz B, Schroder G. Clinical Efficacy of a Monotherapy with Calcipotriol‐Ointment Compared to Combination Therapy with Calcipotriol Ointment and Betamethasone Valerate Ointment in Psoriasis Vulgaris. Journal of Investigative Dermatology 1996;1(1):107. [Google Scholar]
- Ruzicka T, Lorenz B. Comparison of Calcipotriol Monotherapy and a Combination of Calcipotriol and Betamethasone Valerate after 2 Weeks' Treatment with Calcipotriol in the Topical Therapy of Psoriasis Vulgaris: A Multicentre, Double‐Blind, Randomized Study. British Journal Of Dermatology 1998;138(2):254‐8. [PUBMED: 9602870] [DOI] [PubMed] [Google Scholar]
Salmhofer 2000 {published data only}
- Salmhofer W, Maier H, Soyer HP, Honigsmann H, Hodl S. Double‐blind, placebo‐controlled, randomized, right‐left study comparing calcipotriol monotherapy with a combined treatment of calcipotriol and diflucortolone valerate in chronic plaque psoriasis. Acta Dermato Venereologica 2000;Suppl 211:5‐8. [PUBMED: 11234559] [DOI] [PubMed] [Google Scholar]
Sanchez 2001 {published and unpublished data}
- Sanchez RM, Iglesias SM, Umbert MP. Treatment of plague‐type psoriasis with topical propylthiouracil. Actas Dermo Sifiliograficas 2001;92(4):174‐6. [EMBASE: 2001206374] [Google Scholar]
Santoianni 2001 {published data only}
- Santoianni P, Iorio S, Giannetti A, Manfredi G, Rebora A, Landi G, et al. Short‐term clinical efficacy of the association betamethasone 17‐valerate 21‐acetate + tretinoine + salicylic acid in outpatients affected with disseminated keratotic plaque psoriasis: A double blinded placebo‐controlled multicentric randomized clinical trial. Giornale Italiano di Dermatologia e Venereologia 2001;136(1):77‐83. [EMBASE: 2001160666] [Google Scholar]
Saraceno 2007 {published data only (unpublished sought but not used)}
- Saraceno R, Andreassi L, Ayala F, Bongiorno MR, Giannetti A, Lisi P, et al. Efficacy, safety and quality of life of calcipotriol/betamethasone dipropionate (Dovobet) versus calcipotriol (Daivonex) in the treatment of psoriasis vulgaris: a randomized, multicentre, clinical trial. Journal of Dermatological Treatment 2007; Vol. 18, issue 6:361‐5. [PUBMED: 17934937] [DOI] [PubMed]
Scarpa 1994 {published data only}
- Scarpa C. Calcipotriol: Clinical Trial Versus Betamethasone Dipropionate + Salicylic Acid. Acta Dermato Venereologica 1994;186(Suppl):47. [PUBMED: 8073835] [PubMed] [Google Scholar]
Scarpa 1996 {published data only}
- Scarpa C. Tacalcitol Ointment Is an Efficacious and Well Tolerated Treatment for Psoriasis. Journal of the European Academy of Dermatology & Venereology 1996;6(2):142‐6. [EMBASE: 1996096733] [Google Scholar]
- Scarpa C, Kokelj F, Plozzer C, Lavaroni G. Efficacy and Tolerability of Tacalcitol Ointment on Psoriatic Skin: Study in 63 Patients. Journal of Investigative Dermatology. Symposium Proceedings 1996;1(1):110. [Google Scholar]
Scarpa 1997 {published data only}
- Scarpa C, Kokelj F, Plozzer C, Lavaroni G, Torsello P. Efficacy and Tolerability of Tacalcitol Administered Once Daily in the Treatment of Psoriasis Vulgaris (Double‐Blind, Randomized, Placebo Controlled Italian Multicenter Study). Giornale Italiano di Dermatologia e Venereologia 1997;132(5):335‐8. [EMBASE: 1997363332] [Google Scholar]
Sears 1997 {published data only}
- Sears HW, Bailer JW, Yeadon A. A Double‐Blind, Randomized, Placebo‐Controlled Evaluation of the Efficacy and Safety of Hydrocortisone Buteprate 0.1% Cream in the Treatment of Psoriasis. Advances In Therapy 1997;14(3):140‐9. [EMBASE: 1997223749] [Google Scholar]
Seidenari 1997 (H) {published data only}
- Seidenari S, Magni R, Giannetti A. Assessment of the Activity of Tacalcitol on Psoriatic Plaques by Means of Colorimetry and High‐Frequency Ultrasound: A Double‐Blind Intrasubject Half‐Side Right‐Left Comparison with Betamethasone Valerate and Placebo. Skin Pharmacology 1997;10(1):40‐7. [EMBASE: 1997139265] [Google Scholar]
Seidenari 1997 (P) {published data only}
- Seidenari S, Magni R, Giannetti A. Assessment of the Activity of Tacalcitol on Psoriatic Plaques by Means of Colorimetry and High‐Frequency Ultrasound: A Double‐Blind Intrasubject Half‐Side Right‐Left Comparison with Betamethasone Valerate and Placebo. Skin Pharmacology 1997;10(1):40‐7. [EMBASE: 1997139265] [Google Scholar]
Shuttleworth 1998 {published data only}
- Shuttleworth D, Galloway DB, Boorman GC, Donald AE. A double‐blind, placebo‐controlled study of the clinical efficacy of ciclopirox olamine (1.5%) shampoo for the control of scalp psoriasis. Journal Of Dermatological Treatment 1998;9(3):163‐7. [EMBASE: 1998346163] [Google Scholar]
Staberg 1989 {published data only}
- Staberg B, Roed‐Petersen J, Menne T. Efficacy of Topical Treatment in Psoriasis with Mc903, a New Vitamin D Analogue. Acta Dermato Venereologica 1989;69(2):147‐50. [PUBMED: 2564233] [PubMed] [Google Scholar]
Stein 2001 {published data only}
- Stein LF, Sherr A, Solodkina G, Gottlieb AB, Chaudhari U. Betamethasone valerate foam for treatment of nonscalp psoriasis. Journal of Cutaneous Medicine & Surgery 2001;5(4):303‐7. [PUBMED: 11907840] [DOI] [PubMed] [Google Scholar]
Stuecker 2001 {published and unpublished data}
- Hoffmann M, Memmel U, Altmeyer P, Stuecker M. Topical treatment of chronic‐stationary plaque psoriasis with vitamin B12. Zeitschrift Fur Hautkrankheiten 2001;Suppl 1(76):S12. [Google Scholar]
- Stuecker M, Memmel U, Hoffmann M, Hartung J, Altmeyer P. Vitamin B(12) cream containing avocado oil in the therapy of plaque psoriasis. Dermatology 2001;203(2):141‐7. [PUBMED: 11586013] [DOI] [PubMed] [Google Scholar]
Stutz 1996 {published data only}
- Stutz JA, Ellis CN, Kang S. Failure of topical polymyxin B to improve mild plaque psoriasis [letter]. Archives of Dermatology 1996;132(2):231. [PUBMED: 8629837] [DOI] [PubMed] [Google Scholar]
Sudilovsky 1981 {published data only}
- Sudilovsky A, Muir JG, Bocobo FC. A Comparison of Single and Multiple Applications of Halcinonide Cream. International Journal Of Dermatology 1981;20(9):609‐13. [PUBMED: 7030988] [DOI] [PubMed] [Google Scholar]
Sutton 2001 {published data only}
- Sutton L, Swinehart JM, Cato A, Kaplan AS. A clinical study to determine the efficacy and safety of 1% methotrexate/Azone (MAZ) gel applied topically once daily in patients with psoriasis vulgaris. International Journal Of Dermatology 2001;40(7):464‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Syed 1996 {published data only}
- Syed TA, Ahmad SA, Holt AH, Ahmad SA, Ahmad SH, Afzal M. Management of psoriasis with Aloe vera extract in a hydrophilic cream: a placebo‐controlled, double‐blind study. Tropical Medicine & International Health 1996;1(4):505‐9. [PUBMED: 8765459] [DOI] [PubMed] [Google Scholar]
Syed 2001b {published data only}
- Syed TA, Hadi SM, Qureshi ZA, Nordstrom CG, Ali SM. Management of psoriasis vulgaris with methotrexate 0.25% in a hydrophilic gel: a placebo‐controlled, double‐blind study. Journal of Cutaneous Medicine & Surgery 2001;5(4):299‐302. [PUBMED: 11907839] [DOI] [PubMed] [Google Scholar]
Tham 1994 {published data only}
- Tham SN, Lun KC, Cheong WK. A comparative study of calcipotriol ointment and tar in chronic plaque psoriasis. British Journal Of Dermatology 1994;131(5):673‐7. [PUBMED: 7999599] [DOI] [PubMed] [Google Scholar]
Tyring 2010 {published and unpublished data}
- Tyring S, Mendoza N, Appell M, Bibby A, Foster R, Hamilton T, et al. A calcipotriene/betamethasone dipropionate two‐compound scalp formulation in the treatment of scalp psoriasis in Hispanic/Latino and Black/African American patients: Results of the randomized, 8‐week, double‐blind phase of a clinical trial. International Journal of Dermatology 2010;49(11):1328‐33. [PUBMED: 20964660] [DOI] [PubMed] [Google Scholar]
Tzung 2005 {published data only}
Vali 2005 {published data only}
- Vali A, Asilian A, Khalesi E, Khoddami L, Shahtalebi M, Mohammady M. Evaluation of the efficacy of topical caffeine in the treatment of psoriasis vulgaris. Journal of Dermatological Treatment 2005; Vol. 16, issue 4:234‐7. [PUBMED: 216249145] [DOI] [PubMed]
- Vali A, Asilian A, Khalesi E, Khoddami L, Shahtalebi M, Mohammady M. Evaluation of the efficacy of topical caffeine in the treatment of psoriasis vulgaris: A randomized, double‐blind clinical trial [Abstract]. Iranian Journal of Dermatology 2006; Vol. 8, issue 6:87. [DOI] [PubMed]
Van de Kerkhof 1989 {published data only}
- Kerkhof PCM, Bokhoven M, Zultak M, Czarnetzki BM. A Double‐Blind Study of Topical 1 Alpha,25‐Dihydroxyvitamin D3 in Psoriasis. British Journal Of Dermatology 1989;120(5):661‐4. [PUBMED: 2667612] [DOI] [PubMed] [Google Scholar]
Van de Kerkhof 1996a {published data only}
- Kerkhof PCM, Werfel T, Haustein UF, Luger T, Czarnetzki BM, Niemann R, et al. Tacalcitol Ointment in the Treatment of Psoriasis Vulgaris: A Multicentre, Placebo‐ Controlled, Double‐ Blind Study on Efficacy and Safety. British Journal Of Dermatology 1996;135(5):758‐65. [PUBMED: 8977677] [PubMed] [Google Scholar]
Van de Kerkhof 2002a {published data only}
- Kerkhof PCM, Green C, Hamberg KJ, Hutchinson PE, Jensen JK, Kidson P, et al. Safety and efficacy of combined high‐dose treatment with calcipotriol ointment and solution in patients with psoriasis. Dermatology 2002;204(3):214‐21. [PUBMED: 12037450] [DOI] [PubMed] [Google Scholar]
Van de Kerkhof 2006 {published and unpublished data}
- Korte J, Valk PG, Sprangers MA, Damstra RJ, Kunkeler AC, Lijnen RL, et al. A comparison of twice‐daily calcipotriol ointment with once‐daily short‐contact dithranol cream therapy: quality‐of‐life outcomes of a randomized controlled trial of supervised treatment of psoriasis in a day‐care setting. British Journal of Dermatology 2008; Vol. 158, issue 2:375‐81. [DOI] [PubMed]
- Kerkhof PC, Valk PG, Swinkels OQ, Kucharekova M, Rie MA, Vries HJ, et al. A comparison of twice‐daily calcipotriol ointment with once‐daily short‐contact dithranol cream therapy: a randomized controlled trial of supervised treatment of psoriasis vulgaris in a day‐care setting. British Journal of Dermatology 2006; Vol. 155, issue 4:800‐7. [PUBMED: 16965431] [DOI] [PubMed]
Van de Kerkhof 2009 {published and unpublished data}
- Kerkhof P, Anstey A, Barnes L, Bolduc C. A new scalp formulation of calcipotriene plus betamethasone in the treatment of scalp psoriasis compared to its active ingredients in the same vehicle [Abstract P2734.] American Academy of Dermatology 65th Annual Meeting 2‐6 February, 2007. Journal of the American Academy of Dermatology 2007;56(2):AB182. [Google Scholar]
- Kerkhof PCM, Hoffmann V, Anstey A, Barnes L, Bolduc C, Reich K, et al. A new scalp formulation of calcipotriol plus betamethasone dipropionate compared with each of its active ingredients in the same vehicle for the treatment of scalp psoriasis: a randomized, double‐blind, controlled trial. British Journal of Dermatology 2009; Vol. 160, issue 1:170‐6. [PUBMED: 18694678] [DOI] [PubMed]
Vanderploeg 1976 {published data only}
- Vanderploeg DE. Betamethasone Dipropionate Ointment in the Treatment of Psoriasis and Atopic Dermatitis: A Double‐Blind Study. Southern Medical Journal 1976;69(7):862‐3. [PUBMED: 781848] [DOI] [PubMed] [Google Scholar]
Van der Vleuten 1995 {published data only}
- Vleuten CJM, Jong EMGJ, Rulo EHFC, Gerritsen MJP, Kerkhof PCM. In‐Patient Treatment with Calcipotriol Versus Dithranol in Refractory Psoriasis. European Journal of Dermatology 1995;5(8):676‐9. [EMBASE: 1996010645] [Google Scholar]
Veien 1997 {published and unpublished data}
- Veien NK, Bjerke JR, Rossmann‐Ringdahl I, Jakobsen HB. Once daily treatment of psoriasis with tacalcitol compared with twice daily treatment with calcipotriol. A double‐blind trial. British Journal Of Dermatology 1997;137(4):581‐6. [PUBMED: 9390335] [DOI] [PubMed] [Google Scholar]
Vladimirov 1994 {published data only}
- Vladimirov VV, Tcherjomukchina IJ, Kurjanova ON, Menshikova LV, Mazina NM. Efficacy of calcipotriol ointment compared to betamethasone 17‐valerate ointment in the treatment of psoriasis [Abstract]. Skin Therapy Update '94. Crete, Greece, 1994:219.
Volden 1992 {published data only}
- Volden G, Bjornberg A, Tegner E, Pedersen NB, Arles UB, Agren S, et al. Short‐Contact Treatment at Home with Micanol. Acta Dermato Venereologica 1992;172(Suppl):20‐2. [PUBMED: 1585757] [PubMed] [Google Scholar]
Wall 1998 {published and unpublished data}
- Wall AR, Poyner TF. Psoriasis; the Burden of Disease Before, During and After Treatment with Dovonex Ointment or Dithrocream. British Journal Of Dermatology 1997;137(Suppl 50):55. [Google Scholar]
- Wall ARJ, Poyner TF, Menday AP. A comparison of treatment with dithranol and calcipotriol on the clinical severity and quality of life in patients with psoriasis. British Journal Of Dermatology 1998;139(6):1005‐11. [PUBMED: 9990363] [DOI] [PubMed] [Google Scholar]
Weinstein 1996 {published data only}
- Weinstein GD. Safety, Efficacy and Duration of Therapeutic Effect of Tazarotene Used in the Treatment of Plaque Psoriasis. British Journal Of Dermatology 1996;135(Suppl 49):32‐6. [PUBMED: 9035703] [DOI] [PubMed] [Google Scholar]
- Weinstein GD, Krueger GG, Lowe NJ, Duvic M, Friedman DJ, Jegasothy BV, et al. Tazarotene gel, a new retinoid, for topical therapy of psoriasis: vehicle‐controlled study of safety, efficacy, and duration of therapeutic effect. Journal of the American Academy of Dermatology 1997;37(1):85‐92. [PUBMED: 9216528] [DOI] [PubMed] [Google Scholar]
Weinstein 2003 {published data only}
- Weinstein GD. Tazarotene cream in the treatment of plaque psoriasis [Abstract]. 20th World Congress of Dermatology, 1‐5 July 2002, Paris. 2002:P2043.
- Weinstein GD, Koo JY, Krueger GG, Lebwohl MG, Lowe NJ, Menter MA, et al. Tazarotene cream in the treatment of psoriasis: Two multicenter, double‐blind, randomized, vehicle‐controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. Journal of the American Academy of Dermatology 2003;48(5):760‐7. [PUBMED: 12734506] [DOI] [PubMed] [Google Scholar]
White 2006 (H) {published data only}
- White S, Vender R, Thaci D, Haverkamp C, Naeyaert J‐M, Foster R, et al. Use of calcipotriene cream (Dovonex cream) following acute treatment of psoriasis vulgaris with the calcipotriene/betamethasone dipropionate two‐compound product (Taclonex): a randomized, parallel‐group clinical trial. American Journal of Clinical Dermatology 2006; Vol. 7, issue 3:177‐84. [PUBMED: 16734505] [DOI] [PubMed]
White 2006 (P) {published data only}
- White S, Vender R, Thaci D, Haverkamp C, Naeyaert J‐M, Foster R, et al. Use of calcipotriene cream (Dovonex cream) following acute treatment of psoriasis vulgaris with the calcipotriene/betamethasone dipropionate two‐compound product (Taclonex): a randomized, parallel‐group clinical trial. American Journal of Clinical Dermatology 2006; Vol. 7, issue 3:177‐84. [PUBMED: 16734505] [DOI] [PubMed]
Wolska 1995 {published data only}
- Wolska H, Laws S, Schulz‐Kiesow M, Grossman R, Jablonska S, Stadlers R, et al. Clinical evaluation of a PAF‐antagonist in psoriasis vulgaris. Journal Of Dermatological Treatment 1995;6(1):43‐5. [EMBASE: 1995125049] [Google Scholar]
Wortzel 1975 (1) {published data only}
- Wortzel MH. A new corticosteroid for moderate/severe dermatoses. Clinical Medicine 1975;82(3):23‐6. [Google Scholar]
Wortzel 1975 (2) {published data only}
- Wortzel WH. A new corticosteroid for moderate/severe dermatoses. Clinical Medicine 1975;82(3):23‐6. [Google Scholar]
Wozel 2001 {published and unpublished data}
- Wozel G. Treatment of chronic‐stationary psoriasis with fluocinolone acetonide and calcipotriol. Zeitschrift Fur Hautkrankheiten 2001;76(7‐8):482. [Google Scholar]
Yang 2009 {published data only}
- Yang DQ, Zhang LX, Bai YP. Clinical observation of sequential therapy of calcipotriol cream with halometasone cream in plaque type psoriasis. Journal of Clinical Dermatology 2009;38(6):401‐3. [EMBASE: 2010312999] [Google Scholar]
Zonneveld 1998 (H) {published data only}
- Zonneveld IM, Rubins A, Jablonska S, Dobozy A, Ruzicka T, Kind P, et al. Topical tacrolimus is not effective in chronic plaque psoriasis. A pilot study. Archives of Dermatology 1998;134(9):1101‐2. [PUBMED: 9762021] [DOI] [PubMed] [Google Scholar]
Zonneveld 1998 (P) {published data only}
- Zonneveld IM, Rubins A, Jablonska S, Dobozy A, Ruzicka T, Kind P, et al. Topical tacrolimus is not effective in chronic plaque psoriasis. A pilot study. Archives of Dermatology 1998;134(9):1101‐2. [PUBMED: 9762021] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Agrawal 2010 {published data only}
Akerman 2009 {published data only (unpublished sought but not used)}
- Akerman L, Sredni B, Albeck M, David M. Topical Ammonium‐trichlorotellurate (AS101) in the treatment of mild plaque‐type psoriasis; double‐blind placebo controlled study. Journal of Investigative Dermatology 2009;129(Suppl. 1):S50. [Google Scholar]
Ambroziak 2002 {published data only}
- Ambroziak M, Stapor W, Langner A, Kwiek B. Clinical evaluation of the scalp psoriasis treatment with clobetasol propionate 0.05% lotion [Ocena skutecznosci I bezpieczenstwa stosowania plynu zawierajacego 0,05% propionianu klobetazolu w leczeniu przewleklej luszczycy plackowatej skory owlosionej glowy]. Przeglad Dermatologiczny 2002;89(3):237‐40. [Google Scholar]
Baadsgaard 1995 {published data only}
- Baadsgaard O, Traulsen J, Roed Petersen J, Jakobsen HB. Optimal concentration of tacalcitol in once‐daily treatment of psoriasis. Journal Of Dermatological Treatment 1995;6(3):145‐50. [Google Scholar]
Baran 1999 {published data only}
- Baran R, Tosti A. Topical treatment of nail psoriasis with a new corticoid‐containing nail lacquer formulation. Journal Of Dermatological Treatment 1999;10:201‐4. [Google Scholar]
Bianchi 2003 {published data only}
- Bianchi L, Soda R, Diluvio L, Chimenti S. Tazarotene 0.1% gel for psoriasis of the fingernails and toenails: an open, prospective study. British Journal of Dermatology 2003;149(1):207‐9. [DOI] [PubMed] [Google Scholar]
Brodell 2011a {published data only (unpublished sought but not used)}
- Brodell R, Goffe BS, Weiss JS, Bruce S. Safety and efficacy of two regimens involving clobetasol spray, 0.05% and calcitriol ointment, 3 mug/g for moderate plaque psoriasis. Journal of the American Academy of Dermatology 2011; Vol. 64, issue 2 Suppl 1:AB149.
Buder 2010 {published data only}
- Buder K, Knuschke P, Wozel G. Evaluation of methylprednisolone aceponate, tacrolimus and combination thereof in the psoriasis plaque test using sum score, 20‐MHz‐ultrasonography and optical coherence tomography. International Journal of Clinical Pharmacology & Therapeutics 2010;48(12):814‐20. [DOI] [PubMed] [Google Scholar]
Callen 1996 {published data only}
- Callen J. Comparison of Safety and Efficacy of Fluticasone Propionate Cream, 0.05%, and Betamethasone Valerate Cream, 0.1%, in the Treatment of Moderate‐to‐Severe Psoriasis. Cutis 1996;57(2 Suppl):45‐50. [PubMed] [Google Scholar]
Cannavo 2003 {published and unpublished data}
- Cannavo SP, Guarneri F, Vaccaro M, Borgia F, Guarneri B. Treatment of psoriatic nails with topical cyclosporin: a prospective, randomized placebo‐controlled study. Dermatology 2003; Vol. 206, issue 2:153‐6. [MEDLINE: ] [DOI] [PubMed]
- Cannavo SP, Guarneri F, Vaccaro M, Borgia FAD, Institute of Dermatology University of Messina Italy. Treatment of psoriatic nails with topical cyclosporin. 11th Congress of the European Academy of Dermatology and Venereology. 2002:P27‐89.
Carroll 2005 {published data only}
- Carroll CL, Clarke J, Camacho F, Balkrishnan R, Feldman SR. Topical tacrolimus ointment combined with 6% salicylic acid gel for plaque psoriasis treatment. Archives of Dermatology 2005;141(1):43‐6. [DOI] [PubMed] [Google Scholar]
De Jong 1999 {published and unpublished data}
- Jong EM, Menke HE, Praag MC, Kerkhof PC. Dystrophic psoriatic fingernails treated with 1% 5‐fluorouracil in a nail penetration‐enhancing vehicle: a double‐blind study. Dermatology 1999;199(4):313‐8. [DOI] [PubMed] [Google Scholar]
Elias 1994 {published data only}
- Elias AN, Dangaran K, Barr RJ, Rohan MK, Goodman MM. A controlled trial of topical propylthiouracil in the treatment of patients with psoriasis. Journal of the American Academy of Dermatology 1994;31(3 Pt 1):455‐8. [DOI] [PubMed] [Google Scholar]
Esposito 2009 {published data only}
- Esposito M. The therapeutic advantages on BMV plaster in chronic plaque psoriasis. Results from a new European multicentric study [Les avantages therapeutiques d'un topique bio‐adhesif a base de VBM dans le traitement du psoriasis en plaques. Presentation des resultats d'une nouvelle etude europeenne, multicentrique]. Annales de Dermatologie et de Venereologie 2009; Vol. 136, issue Suppl 8:S476‐80. [0151‐9638]
Friedrich 2004 {published data only}
- Friedrich M, Vollhardt K, Zahlten R, Sterry W, Wolff G. Demonstration of antipsoriatic efficacy of a new topical formulation of the small molecule selectin antagonist bimosiamose [Abstract P016]. European Congress on Psoriasis 2004. Journal of the European Academy of Dermatology & Venereology 2004; Vol. 18, issue 6:779.
Hsia 2010 {published data only}
- Hsia E, Hofland H, Therrien J‐P, Chern W. In vitro activity and safety assessment of a novel calcipotriene foam formulation. 68th Annual Meeting of the American Academy of Dermatology, Miami, FL United States. Journal of the American Academy of Dermatology 2010;62(3 Suppl 1):AB138. [Google Scholar]
Insa 2009 {published data only}
- Insa R, Cruz G, Gassmueller J, Leon M. Topical cyclosporin proves efficacy in psoriatic patients in a phase I/II clinical trial. 67th Annual Meeting of the American Academy of Dermatology, San Francisco, CA United States. Journal of the American Academy of Dermatology 2009; Vol. 60, issue 3 Suppl 1:AB180. [0190‐9622]
Iraji 2010 {published data only}
- Iraji F, Faghihi G, Siadat AH, Enshaieh S, Shahmoradi Z, Joia A, et al. Efficacy of 15% azelaic acid in psoriasis vulgaris: a randomized, controlled clinical trial. Journal of Drugs in Dermatology 2010;9(8):964‐8. [PubMed] [Google Scholar]
Ito 2005 {published data only}
- Ito Y, Nakagawa H. A comparative study of maxacalcitol monotherapy, clobetasol propionate monotherapy and the combination of each agent as a premixed ointment for the treatment of psoriasis. [Japanese]. Nishinihon Journal of Dermatology 2005;67(5):522‐6. [Google Scholar]
Jansen 1986 {published data only}
- Jansen C, Lammintausta K, Bullingham RES, Forsstrom S. A Clinical Trial of Lonapalene Fluocinolone Acetonide and Vehicle in Psoriasis [Abstract]. Journal of Investigative Dermatology 1986;86(4):483. [Google Scholar]
Kaur 2004 {published data only}
- Kaur I, Jain R, Kumar B. Comparative study of calcipotriol (0.005%) vs tazarotene (0.05%, 0.1%) in stable plaque psoriasis [Abstract P015]. European Congress on Psoriasis 2004. Journal of the European Academy of Dermatology & Venereology 2004; Vol. 18, issue 6:779.
Kleyn 2005 {published data only}
- Kleyn CE, Woodcock D, Sharpe GR. The efficacy of 0.1% tacrolimus ointment compared with clobetasone butyrate 0.05% ointment in patients with facial, flexural or genital psoriasis [Abstract P‐41]. The 85th BAD Annual Meeting 5‐8th July 2005, Glasgow, UK. British Journal of Dermatology 2005;153(Suppl 1):33. [Google Scholar]
Kragballe 1989 {published data only}
- Kragballe K. Treatment of psoriasis by the topical application of the novel cholecalciferol analogue calcipotriol (MC 903). Archives of Dermatology 1989;125(12):1647‐52. [PubMed] [Google Scholar]
Kragballe 1994 {published data only}
- Kragballe K, Dam TN, Hansen ER, Baadsgaard O, Gronhoj Larsen F, Sondergaard J, et al. Efficacy and Safety of the 20‐Epi‐Vitamin D3 Analogue Kh 1060 in the Topical Therapy of Psoriasis: Results of a Dose‐Ranging Study. Acta Dermato‐Venereologica 1994;74(5):398‐402. [DOI] [PubMed] [Google Scholar]
Lebwohl 1998b {published data only}
- Lebwohl M, Yoles A, Lombardi K, Lou W. Calcipotriene Ointment and Halobetasol Ointment in the Long‐Term Treatment of Psoriasis: Effects on the Duration of Improvement. Journal of the American Academy of Dermatology 1998;39(3):447‐50. [DOI] [PubMed] [Google Scholar]
Lebwohl 2001 {published data only}
- Lebwohl M, Lombardi K, Tan MH. Duration of improvement in psoriasis after treatment with tazarotene 0.1% gel plus clobetasol propionate 0.05% ointment: comparison of maintenance treatments. International Journal Of Dermatology 2001;40(1):64‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Levin 2003 {published data only}
- Levin C, Fiorentino DF, Vosganian G, Chon S, Kimball AB. The safety of topical cyclosporin A conjugate (CGC1072) in the treatment of mild to moderate psoriasis [Abstract 1201] International Investigative Dermatology. The 4th Joint Meeting of the ESDR, Japanese SID & SID, 30th April‐4th May 2003, Florida, USA. Journal of Investigative Dermatology 2003;121(1):201. [Google Scholar]
Meyrat 1996 {published and unpublished data}
- Meyrat R, Muller I. Daivonex registered trade mark ointment twice a day versus Daivonex registered trade mark cream in the morning and Daivonex registered trade mark ointment in the evening [Daivonex Salbe zwei mal taglich versus Daivonex Creme morgens und Daivonex Salbe abends]. Ars Medici 1996;86(20):1218‐20. [Google Scholar]
Palazon 2005 {published data only}
- Palazon DAB, Sanchez‐Regana M, Mateos FL, Ezquerra GM, Acosta EH, Vidal I, et al. A double blind aleatory and prospective study of topical vitamin b12 in psoriasis [Abstract P06.21]. The 14th Congress of the European Academy of Dermatology and Venereology, London, UK. 12‐15th October 2005. Journal of the European Academy of Dermatology & Venereology 2005;19(Suppl 2):165. [Google Scholar]
Reygagne 2002a {published data only}
- Reygagne P, Diaconu J, Près H, Ernst TM, Meyer KG, S.A.D.A. Efficacy and safety comparison of clobetasol propionate shampoo, gel and vehicle in scalp psoriasis. 11th Congress of the European Academy of Dermatology and Venereology. 2002:P27‐9.
Rhemus 2006 {published data only}
- Rhemus W, Kim J, Kern D, Murase J. A double‐blind, placebo‐controlled study of a natural topical anti‐inflammatory for treatment of atopic dermatitis and psoriasis [Abstract P2849]. American Academy of Dermatology 64th Annual Meeting March 3‐7, 2006. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB210. [Google Scholar]
Ruzicka 2004 {published data only}
- Ruzicka T, Trompke C. Treatment of scalp psoriasis. An effective and safe tacalcitol emulsion [Behandlung der kopfhaut‐psoriasis. Gute wirksamkeit und sicherheit durch Tacalcitol‐emulsion]. Hautarzt 2004;55(2):165‐70. [DOI] [PubMed] [Google Scholar]
Sander 1998 {published data only}
- Sander P, Stucker M, Hermes N, Hoffmann K, Altmeyer P. Mometasone and calcipotriol optimise the initial therapeutic effect of dithranol on chronic plaque psoriasis [Mometason Und Calcipotriol Optimieren Den Therapeutischen Initialeffekt Von Dithranol Auf Die Chronisch Stationare Psoriasis (CSP)]. Hautarzt 1998;49(4):291‐4. [DOI] [PubMed] [Google Scholar]
Saraswat 2007 {published data only}
Scher 2001 {published data only}
- Scher R. Tazarotene 0.1% gel in the treatment of nail psoriasis [Abstract]. 20th World Congress of Dermatology, 1st to 5th July 2002. 2002:P0791.
- Scher RK, Stiller M, Zhu YI. Tazarotene 0.1% gel in the treatment of fingernail psoriasis: a double‐blind, randomized, vehicle‐controlled study. Cutis 2001;68(5):355‐8. [PUBMED: 11766122 ] [PubMed] [Google Scholar]
- Scher RK, Stiller M, Zhu YI. Treating fingernail psoriasis with tazarotene 0.1% gel: A vehicle‐controlled study [Abstract]. 2nd Joint Meeting International Psoriasis Symposium and European Congress on Psoriasis. Skin Pharmacology & Applied Skin Physiology 2001;14(3):170. [Google Scholar]
Sefton 1984 {published data only}
- Sefton J, Loder JS, Kyriakopoulos AA. Clinical Evaluation of Hydrocortisone Valerate 0.2% Ointment. Clinical Therapeutics 1984;6(3):282‐93. [PubMed] [Google Scholar]
Sharma 2003 {published data only}
- Sharma V, Kaur I, Kumar B. Calcipotriol versus coal tar: a prospective randomized study in stable plaque psoriasis. International Journal of Dermatology 2003;42(10):834‐8. [DOI] [PubMed] [Google Scholar]
Syed 2001a {published data only}
- Syed TA, Qureshi ZA. Management of psoriasis of the scalp with methotrexate (0.25%) in an aerosolized spray gel. A placebo‐controlled, double‐blind study [Abstract]. 2nd Joint Meeting International Psoriasis Symposium and European Congress on Psoriasis. Skin Pharmacology & Applied Skin Physiology 2001;14(3):167. [Google Scholar]
Tokura 2004 {published data only}
- Tokura Y. Effectiveness of Combination between Diflucortolone Valerate and Vitamin D3 Analogue in the Treatment of Psoriasis Vulgaris. [Japanese]. Nishinihon Journal of Dermatology 2004;66(2):188‐191. [Google Scholar]
Tosti 1998 {published data only}
- Tosti A, Piraccini BM, Cameli N, Kokely F, Plozzer C, Cannata GE, et al. Calcipotriol Ointment in Nail Psoriasis: A Controlled Double‐Blind Comparison with Betamethasone Dipropionate and Salicylic Acid. British Journal Of Dermatology 1998;139(4):655‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tzaneva 2003 {published data only}
- Tzaneva S, Honigsmann H, Tanew A. Observer‐blind, randomized, intrapatient comparison of a novel 1% coal tar preparation (Exorex) and calcipotriol cream in the treatment of plaque type psoriasis. British Journal of Dermatology 2003;149(2):350‐3. [EMBASE: 2003371589] [DOI] [PubMed] [Google Scholar]
van de Kerkhof 1996b {published data only}
- Kerkhof PCM, Kuypers A. A randomised, double blind, left right study to compare hydrocortisone 17‐butyrate 0.1% emulsion with vehicle in the treatment of patients suffering from psoriasis vulgaris [Poster P16]. 6th International Skin Symposium. Brussels, 1996.
Vena 2005 {published data only}
- Vena GA, Cassano N, Agnusdei CP, Bellini M, Calabretta S, Centofanti S, et al. Treatment of psoriasis vulgaris with calcipotriol betamethasone dipropionate combination followed by calcipotriol and assessment of the adjuvant basic use of urea‐based emollients. European Journal of Inflammation 2005; Vol. 3, issue 1:37‐41.
References to studies awaiting assessment
Bissonnette 2011 {published data only}
- Bissonnette R, Bolduc C, Maari C, Nigen S, Webster JM, Tang L, et al. Efficacy and safety of topical WBI‐1001 in patients with mild to moderate psoriasis: results from a randomized double‐blind placebo‐controlled, phase II trial. Journal of the European Academy of Dermatology & Venereology 2012;26(12):1516‐21. [DOI: 10.1111/j.1468-3083.2011.04332.x; NCT01098721; PUBMED: 22077962] [DOI] [PubMed] [Google Scholar]
- NCT01098721. A Safety/Efficacy Study of a Non‐steroid, Topical Cream Treatment of Psoriasis Over 12‐weeks (134993). clinicaltrials.gov/ct2/show/NCT01098721 (accessed January 2013).
Callis Duffin 2010 {published data only}
- Callis Duffin K, Luchi M, Fidelus‐Gort R, Newton R, Fridman J, Burn T, et al. Novel mechanism for topical treatment of plaque psoriasis – results of a randomized, double blind, concentration ranging, vehicle controlled 12 week study with JAK 1/2 inhibitor INCB018424 cream (Abstract 261). 2010 Annual Meeting of the Society for Investigative Dermatology, Hilton Atlanta, Atlanta, Georgia, May 5‐8. Journal of Investigative Dermatology 2010;130(Suppl 1):S44. [Google Scholar]
- NCT00778700. A Dose Ranging Study of the Effect of INCB018424 Phosphate Cream When Applied to Patients With Plaque Psoriasis. clinicaltrials.gov/show/NCT00778700 (accessed January 2013).
Calzavara‐Pinton 2011 {published data only}
- Calzavara‐Pinton P, Rossi M T, Sala R, Venturini M. The separate daily application of tacalcitol 4 microg/g ointment and budesonide 0.25 mg/g cream is more effective than the single daily application of a two compound ointment containing calcipotriol 50 microg/g and betamethasone dipropionate 0.5 mg/g. Giornale Italiano di Dermatologia e Venereologia 2011;146(4):295‐9. [PubMed] [Google Scholar]
Henry 2011 {published data only}
- Henry M, Frankel A, Emer J, Lebwohl M. Bilateral comparison study on the order of application of combination clobetasol proprionate spray and calcitriol ointment in the treatment of plaque psoriasis. (Abstract P3343). Conference: 69th Annual Meeting of the American Academy of Dermatology New Orleans, LA United States. Conference Start: 20110204 Conference End: 20110208. Conference Publication. Journal of the American Academy of Dermatology 2011;64(2 Suppl 1):AB156. [Google Scholar]
Katoh 2003 {published data only}
- Katoh N, Kishimoto S. Combination of calcipotriol and clobetasol propionate as a premixed ointment for the treatment of psoriasis. European Journal of Dermatology 2003;13(4):382‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Matheson 2011 {published data only}
- Matheson R, Hsia E, Ge X. Safety and bioavailability of two calcipotriene formulations in subjects with mild to moderate plaque‐type psoriasis. (Abstract P3348). Conference: 69th Annual Meeting of the American Academy of Dermatology New Orleans, LA United States. Conference Start: 20110204 Conference End: 20110208. Journal of the American Academy of Dermatology 2011;64(2 Suppl 1):AB157. [Google Scholar]
Paulsen 2005 {published data only}
- Paulsen E, Korsholm L, Brandrup F. A double‐blind, placebo‐controlled study of a commercial Aloe vera gel in the treatment of slight to moderate psoriasis vulgaris. Journal Of The European Academy Of Dermatology & Venereology 2005;19(3):326‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sadeghian 2011 {published data only}
- Sadeghian G, Ziaei H, Nilforoushzadeh M A. Treatment of localized psoriasis with a topical formulation of zinc pyrithione. Acta Dermatovenerologica Alpina, Panonica et Adriatica 2011;20(4):187‐90. [PubMed] [Google Scholar]
Vahlquist 2004 {published data only}
- Vahlquist A, Torma H, Carlsson B. Inefficacy of topical thyroid hormone analogue TriAc in plaque psoriasis: results of a double‐blind placebo‐controlled trial. British Journal of Dermatology 2004;151(2):489‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yang 1999 {published data only}
- Yang XY, Lin L, Cui PG, Jia H, Jin PY, Liu XQ. Comparison of the efficacy of tacalcitol ointment and 17 a hydrocortisone cream in the treatment of psoriasis (Chinese). Chinese Journal of Dermatology 1999;32(6):425. [Google Scholar]
References to ongoing studies
NCT00824980 {unpublished data only}
- NCT00824980. Combined Inhibition of Dipeptidyl Peptidase IV (DPIV/CD26) and Aminopeptidase N (APN/CD13) in the Treatment of Psoriasis. clinicaltrials.gov/ct2/show/NCT00824980 (accessed December 2011).
NCT01018134 {unpublished data only}
- NCT01018134. Desoximetasone Spray 0.05%, 0.25%; Dose Ranging Study. clinicaltrials.gov/ct2/show/NCT01018134 (accessed December 2011).
NCT01206387 {unpublished data only}
- NCT01206387 . Effects of Desoximetasone Spray 0.25% on Moderate to Severe Plaque Psoriasis. clinicaltrials.gov/ct2/show/NCT01206387 (accessed December 2011).
NCT01206660 {unpublished data only}
- NCT01206660. Effects of Desoximetasone Spray 0.25% in Patients With Moderate to Severe Plaque Psoriasis. clinicaltrials.gov/ct2/show/NCT01206660 (accessed December 2011).
NCT01246583 {unpublished data only}
- NCT01246583. CP‐690‐550 Ointment For Chronic Plaque Psoriasis. clinicaltrials.gov/ct2/show/NCT01246583 (accessed December 2011).
NCT01247818 {unpublished data only}
- NCT01247818 . Randomized Study of PH‐10 for Psoriasis. clinicaltrials.gov/ct2/show/NCT01247818 (accessed December 2011).
NCT01422434 {unpublished data only}
- NCT01422434. LEO 90105 Ointment in Japanese Subjects With Psoriasis. clinicaltrials.gov/ct2/show/results/NCT01422434 (accessed December 2011).
NCT01465282 {unpublished data only}
- NCT01465282. Evaluation of the Efficacy, Safety and Toleration of CT327 Ointment in Patients With Psoriasis Vulgaris. clinicaltrials.gov/ct2/show/NCT01465282 (accessed December 2011).
NCT01536886 {unpublished data only}
- NCT01536886. LEO 90100 Compared With Calcipotriol Plus Betamethasone Dipropionate Ointment, LEO 90100 Vehicle and Ointment Vehicle in Subjects With Psoriasis Vulgaris. clinicaltrials.gov/show/NCT01536886 (accessed August 2012).
NCT01536938 {unpublished data only}
- NCT01536938. LEO 90100 in the Treatment of Psoriasis Vulgaris. clinicaltrials.gov/show/NCT01536938 (accessed August 2012).
Additional references
Afifi 2005
- Afifi T, Gannes G, Huang C, Zhou Y. Topical therapies for psoriasis: evidence‐based review. Canadian Family Physician 2005; Vol. 51:519‐25. [MEDLINE: ] [PMC free article] [PubMed]
Andres 2006
- Andres P, Poncet M, Farzaneh S, Soto P. Short‐term safety assessment of clobetasol propionate 0.05% shampoo: hypothalamic‐pituitary‐adrenal axis suppression, atrophogenicity, and ocular safety in subjects with scalp psoriasis. Journal of Drugs in Dermatology 2006; Vol. 5, issue 4:328‐32. [MEDLINE: ] [PubMed]
Ashcroft 2000
- Ashcroft DM, Li Wan Po A, Williams HC, Griffiths CE. Cost‐effectiveness analysis of topical calcipotriol versus short‐contact dithranol in the treatment of mild to moderate plaque psoriasis. Pharmacoeconomics 2000;18(5):469‐76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ashcroft 2000a
- Ashcroft DM, Po AL, Williams HC, Griffiths CE. Systematic review of comparative efficacy and tolerability of calcipotriol in treating chronic plaque psoriasis. BMJ 2000; Vol. 320, issue 7240:963‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed]
Aste 2004
- Aste N. Tacalcitol ointment in the treatment of psoriasis [Tacalcitolo unguento nel trattamento della psoriasi]. Giornale Italiano di Dermatologia e Venereologia 2004;139(1):81‐4. [EMBASE: 2004234976] [Google Scholar]
Atkinson 2004
- Atkinson MJ, Sinha A, Hass SL, Colman SS, Kumar RN, Brod M, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health & Quality of Life Outcomes 2004;2(1):12. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Balkrishnan 2003
- Balkrishnan R, Carroll CL, Camacho FT, Feldman SR. Electronic monitoring of medication adherence in skin disease: Results of a pilot study. Journal of the American Academy of Dermatology 2003;49(4):651‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Barisic‐Drusko 1994
- Barisic‐Drusko V, Dobric I, Pasic A, Paljan D, Jukic Z, Basta‐Juzbasic A, et al. Frequency of psoriatic arthritis in general population and among the psoriatics in department of dermatology. Acta Dermato‐Venereologica. Supplementum. 1994;186:107‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Barker 1991
- Barker JN. The pathophysiology of psoriasis. Lancet 1991;338(8761):227‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Barnes 2000
- Barnes L, Altmeyer P, Forstrom L, Stenstrom MH. Long‐term treatment of psoriasis with calcipotriol scalp solution and cream. European Journal of Dermatology 2000;10(3):199‐204. [MEDLINE: ] [PubMed] [Google Scholar]
Berth‐Jones 1992c
- Berth‐Jones J. Immediate and long term effects of topical calcipotriol on calcium homeostasis during treatment of psoriasis [Abstract]. British Journal of Dermatology 1992;127(Suppl 40):17. [Google Scholar]
Berth‐Jones 1993
- Berth‐Jones J, Bourke JF, Iqbal SJ, Hutchinson PE. Urine Calcium Excretion During Treatment of Psoriasis with Topical Calcipotriol. British Journal Of Dermatology 1993;129(4):411‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bhalerao 1998
- Bhalerao J, Bowcock AM. The genetics of psoriasis: a complex disorder of the skin and immune system. Human Molecular Genetics 1998;7(10):1537‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bleiker 1998
- Bleiker TO, Bourke JF, Mumford R, Hutchinson PE. Long‐Term Outcome of Severe Chronic Plaque Psoriasis Following Treatment with High‐Dose Topical Calcipotriol. British Journal Of Dermatology 1998;139(2):285‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
BMA 2012
- Joint Formulary Committee. British National Formulary (BNF) 63. London: Pharmaceutical Press, 2012. [Google Scholar]
Bonifati 1998
- Bonifati C, Carducci M, Mussi A, D'Auria L, Ameglio F. Recognition and treatment of psoriasis: special considerations in elderly patients. Drugs & Aging 1998;12(3):177‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bos 2002
- Bos JD. Topical tacrolimus and pimecrolimus are not associated with skin atrophy. British Journal of Dermatology 2002;146(2):342. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bos 2008
Bourke 1993a
- Bourke JF, Berth‐Jones J, Iqbal SJ, Hutchinson PE. High‐dose topical calcipotriol in the treatment of extensive psoriasis vulgaris. British Journal of Dermatology 1993;129(1):74‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bourke 1994
- Bourke JF, Berth‐Jones J, Mumford R, Iqbal SJ, Hutchinson PE. High dose topical calcipotriol consistently reduces serum parathyroid hormone levels. Clinical Endocrinology 1994;41(3):295‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brandrup 1978
- Brandrup F, Hauge M, Henningsen K, Eriksen B. Psoriasis in an unselected series of twins. Archives of Dermatology 1978;114(6):874‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Breneman 2007
- Breneman D, Sheth P, Berger V, Naini V, Stevens V. Phase II clinical trial of bexarotene gel 1% in psoriasis. Journal of Drugs in Dermatology 2007; Vol. 6, issue 5:501‐6. [MEDLINE: ; 1545‐9616] [PubMed]
Brodell 2011b
- Brodell RT, Bruce S, Hudson CP, Weiss JS, Colon LE, Johnson LA, et al. A multi‐center, open‐label study to evaluate the safety and efficacy of a sequential treatment regimen of clobetasol propionate 0.05% spray followed by Calcitriol 3 mg/g ointment in the management of plaque psoriasis. Journal of Drugs in Dermatology 2011; Vol. 10, issue 2:158‐64. [MEDLINE: ; 1545‐9616] [PubMed]
Brownell 2007
Bruner 2003a
- Bruner CR, Feldman SR, Ventrapragada M, Fleischer AB Jr. A systematic review of adverse effects associated with topical treatments for psoriasis. Dermatology Online Journal 2003; Vol. 9, issue 1:2. [MEDLINE: ] [PubMed]
Camp 1992
- Camp RDR. Psoriasis. In: Champion RH, Burton JL, Ebling FJG editor(s). Textbook of Dermatology. Oxford: Blackwell Science, 1992:1391‐458. [Google Scholar]
Carboni 2005
- Carboni I, Felice C, Bergamin A, Chimenti S. Topical use of calcitriol 3 microg/g ointment in the treatment of mild‐to‐moderate psoriasis: results from an open‐label study. Journal of the European Academy of Dermatology & Venereology 2005; Vol. 19, issue Suppl 3:11‐3. [MEDLINE: ; 0926‐9959] [DOI] [PubMed]
Carey 2006
Carroll 2004a
- Carroll CL, Feldman SR, Camacho FT, Balkrishnan R. Better medication adherence results in greater improvement in severity of psoriasis. British Journal of Dermatology 2004;151(4):895‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Carroll 2004b
- Carroll CL, Feldman SR, Camacho FT, Manuel JC, Balkrishnan R. Adherence to topical therapy decreases during the course of an 8‐week psoriasis clinical trial: commonly used methods of measuring adherence to topical therapy overestimate actual use. Journal of the American Academy of Dermatology 2004;51(2):212‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chalmers 2000
- Chalmers RJ, O'Sullivan T, Owen CM, Griffiths CE. Interventions for guttate psoriasis. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD001213] [DOI] [PubMed] [Google Scholar]
Chalmers 2006
- Chalmers R, Hollis S, Leonardi‐Bee J, Griffiths CEM, Marsland A. Interventions for chronic palmoplantar pustulosis. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2011
- Chen X, Cheng Y, Yang M, Liu GJ, Zhang M. Narrow‐band ultraviolet B phototherapy versus broad‐band ultraviolet B or psoralen‐ultraviolet A photochemotherapy for psoriasis. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD009481] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chu 2000
- Chu T. Better patient compliance in psoriasis. Practitioner 2000;244(1608):238‐42. [MEDLINE: ] [PubMed] [Google Scholar]
Corbett 1976
- Corbett MF. The response of psoriasis to betamethasone valerate and clobetasol propionate: a 6‐month controlled study. British Journal Of Dermatology 1976;94(Suppl 12):89‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cork 2003
- Cork MJ, Britton J, Butler L, Young S, Murphy R, Keohane SG. Comparison of parent knowledge, therapy utilization and severity of atopic eczema before and after explanation and demonstration of topical therapies by a specialist dermatology nurse. British Journal of Dermatology 2003;149(3):582‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cossmann 2006
- Cossmann M, Welzel J. Evaluation of the atrophogenic potential of different glucocorticoids using optical coherence tomography, 20‐MHz ultrasound and profilometry; a double‐blind, placebo‐controlled trial. British Journal of Dermatology 2006; Vol. 155, issue 4:700‐6. [PUBMED: 16965418] [DOI] [PubMed]
Cullen 1996
- Cullen SI. Long‐Term Effectiveness and Safety of Topical Calcipotriene for Psoriasis. Calcipotriene Study Group. Southern Medical Journal 1996;89(11):1053‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
de Jong 1997
- Jong EM. The course of psoriasis. Clinics in Dermatology 1997;15(5):687‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
de Tiedra 1997
- Tiedra A, Mercadal J, Lozano R. Prednicarbate versus fluocortin for inflammatory dermatoses. A cost‐effectiveness study. Pharmacoeconomics 1997;12(2 Pt 1):193‐208. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
de Vries 2013
- Vries ACQ, Bogaards NA, Hooft L, Velema M, Pasch M, Lebwohl M, et al. Interventions for nail psoriasis. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD007633.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
du Vivier 1975
- du Vivier A, Stoughton RB. Tachyphylaxis to the action of topically applied corticosteroids. Archives of Dermatology 1975;111(5):581‐3. [MEDLINE: ] [PubMed] [Google Scholar]
Farber 1974
- Farber EM, Nall ML, Watson W. Natural history of psoriasis in 61 twin pairs. Archives of Dermatology 1974;109(2):207‐11. [MEDLINE: ] [PubMed] [Google Scholar]
Feldman 1997
- Feldman SR, Fleischer AB, Reboussin DM, Rapp SR, Bradham DD, Exum ML, et al. The economic impact of psoriasis increases with psoriasis severity. Journal of the American Academy of Dermatology 1997;37(4):564‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Feldman 2000
- Feldman SR, Sahu S, Fleischer AB, Dezii CM. Per‐gram cost of medication is by itself a poor indicator for comparing costs of different psoriasis treatments: a retrospective cohort study of the cost of psoriasis treatment with topical corticosteroids versus topical calcipotriene. Journal of Cutaneous Medicine & Surgery 2000;4(3):121‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Feldman 2007
- Feldman SR, Camacho FT, Krejci‐Manwaring J, Carroll CL, Balkrishnan R. Adherence to topical therapy increases around the time of office visits. Journal of the American Academy of Dermatology 2007;57(1):81‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Feldman 2007a
- Feldman SR. Effectiveness of clobetasol propionate spray 0.05% added to other stable treatments: add‐on therapy in the COBRA trial. Cutis 2007; Vol. 80, issue 5 Suppl:20‐8. [MEDLINE: ; 0011‐4162] [PubMed]
Ferrandiz 1998
- Ferrandiz C, Bielsa I, Ribera M, Fuente MJ, Carrascosa JM. Reinforcement Therapy in Patients with Psoriasis Treated with Calcipotriol Cream Terapia De Refuerzo En Pacientes Con Psoriasis Tratada Con Calcipotriol [Terapia de refuerzo en pacientes con psoriasis tratada con calcipotriol]. Actas Dermo Sifiliograficas 1998;89(11):626‐30. [EMBASE: 1998399783] [Google Scholar]
Finlay 1994
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) ‐ a simple practical measure for routine clinical use. Clinical & Experimental Dermatology 1994;19(3):210‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Finlay 1995a
- Finlay AY, Coles EC. The effect of severe psoriasis on the quality of life of 369 patients. British Journal of Dermatology 1995;132(2):236‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Finlay 1995b
- Finlay AY. Coping with psoriasis. BMJ 1995;310(6995):1673. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Finlay 2001
- Finlay AY. Psoriasis from the patient's point of view. Archives of Dermatology 2001;137(3):352‐3. [MEDLINE: ] [PubMed] [Google Scholar]
Finlay 2005
- Finlay AY. Current severe psoriasis and the rule of tens. British Journal of Dermatology 2005;152(5):861‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Floden 1975
- Floden CH, Woodbridge P, Samman P, Kurwa AR. Comparison of the Response of Psoriasis, over a 6‐Month Period, to Clobetasol Propionate and Fluocinolone Acetonide Ointments. Current Medical Research & Opinion 1975;3(6):375‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fluhr 2008
Fortune 2003
- Fortune DG, Richards HL, Kirby B, McElhone K, Markham T, Rogers S, et al. Psychological distress impairs clearance of psoriasis in patients treated with photochemotherapy. Archives of Dermatology 2003;139(6):752‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fouere 2005
- Fouere S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. Journal of the European Academy of Dermatology & Venereology 2005;19(Suppl 3):2‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Franssen 1999
- Franssen ME, Wilt GJ, Jong PC, Bos RP, Arnold WP. A retrospective study of the teratogenicity of dermatological coal tar products. Acta Dermato‐Venereologica 1999;79(5):390‐1. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fredriksson 1980
- Fredriksson T, Lassus A, Bleeker J. Treatment of psoriasis and atopic dermatitis with halcinonide cream applied once and three times daily. British Journal of Dermatology 1980;102(5):575‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Genetic Analysis of Psoriasis Consortium 2010
- Genetic Analysis of Psoriasis Consortium, the Wellcome Trust Case Control Consortium, Strange A, Capon F, Spencer CC, Knight J, Weale ME, et al. A genome‐wide association study identifies new psoriasis susceptibility loci and an interaction between HLA‐C and ERAP1. Nature Genetics 2010; Vol. 42, issue 11:985‐90. [PUBMED: 20953190] [DOI] [PMC free article] [PubMed]
Gerritsen 2001
- Gerritsen MJ, Kerkhof PC, Langner A. Long‐term safety of topical calcitriol 3 microg g(‐1) ointment. British Journal Of Dermatology 2001;144(Suppl 58):17‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ghoreschi 2007
Goeckerman 1931
- Goeckerman WH. Treatment of psoriasis. AMA Archives of Dermatology & Syphilology 1931;24:446‐50. [Google Scholar]
Gokdemir 2008
- Gokdemir G, Ari S, Köslü A. Adherence to treatment in patients with psoriasis vulgaris: Turkish experience. Journal of the European Academy of Dermatology & Venereology 2008;22(3):330‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Griffiths 2003
- Griffiths CE. The immunological basis of psoriasis. Journal of the European Academy of Dermatology & Venereology 2003;17(Suppl 2):1‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Griffiths 2004
Griffiths 2007
Griffiths 2007a
Gupta 1998
- Gupta MA, Gupta AK, Watteel GN. Perceived deprivation of social touch in psoriasis is associated with greater psychologic morbidity: an index of the stigma experience in dermatologic disorders. Cutis 1998;61(6):339‐42. [MEDLINE: ] [PubMed] [Google Scholar]
Gupta 2007
- Gupta G, Bhattacharya S. Psoriasis: maximizing topical treatment concordance. British Journal of Hospital Medicine 2007;68(5):263‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Guzzo 1996
- Guzzo C, Lazarus G, Goffe BS, Katz HI, Lowe NJ, Pincus SH. Topical Calcipotriene Has No Short‐Term Effect on Calcium and Bone Metabolism of Patients with Psoriasis. Journal of the American Academy of Dermatology 1996;34(3):429‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Harrington 1995
- Harrington CI. Cost‐Effectiveness Analysis of Calcipotriol Ointment and 'Short‐Contact' Dithranol in Treating Mild‐to‐Moderate Psoriasis. British Journal Of Medical Economics 1995;8(1):27‐32. [EMBASE: 1995157081] [Google Scholar]
Hellgren 1967
- Hellgren L. Psoriasis. The prevalence in sex, age and occupational groups in total populations in Sweden: morphology, inheritance and association with other skin and rheumatic diseases. Stockholm: Almqvist & Wiksell, 1967. [Google Scholar]
Heng 1990
- Heng MC, Heng Hl, Allen SG. Basement Membrane Changes in Psoriatic Patients on Long‐Term Topical Corticosteroid Therapy. Clinical & Experimental Dermatology 1990;15(2):83‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hengge 2006
- Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. Journal of the American Academy of Dermatology 2006;54(1):1‐15; quiz 16‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Henseler 1992
- Henseler T, Koch F, Westphal E, Nair RP, Vorhees JJ, Elder JT, et al. Presence of HLA‐DR7 in type‐I‐psoriasis. Clinical Research 1992;40:A457. [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holick 1996
- Holick MF, Chen ML, Kong XF, Sanan DK. Clinical uses for calciotropic hormones 1, 25‐dihydroxyvitamin D3 and parathyroid hormone‐related peptide in dermatology: a new perspective. Journal of Investigative Dermatology. Symposium Proceedings 1996;1(1):1‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Hong 2010
- Hong E, Smith SD, Fischer G. Evaluation of the atrophogenic potential of topical corticosteroid in dermatology paediatric patients [Abstract]. 43rd Annual Scientific Meeting of the Australasian College of Dermatologists Darwin, NT Australia, 16‐19 May 2010. Australasian Journal of Dermatology 2010; Vol. 51:A17. [EMBASE: 70162819; 0004‐8380]
Hong 2011
Ingram 1953
- Ingram JT. The approach to psoriasis. British Medical Journal 1953;2:591‐4. [doi: 10.1136/bmj.2.4836.591] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacobi 2008
Jales 2012
- Jales RD, Nast A, Saconato H, Atallah ÁN, Hirata SH. Topical treatments for scalp psoriasis. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD009687] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jenner 2002
- Jenner N, Campbell J, Plunkett A, Marks R. Cost of psoriasis: a study on the morbidity and financial effects of having psoriasis in Australia. Australasian Journal of Dermatology 2002;43(4):255‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Juni 2001
- Juni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kao 2003
- Kao JS, Fluhr JW, Man MQ, Fowler AJ, Hachem JP, Crumrine D, et al. Short‐term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: inhibition of epidermal lipid synthesis accounts for functional abnormalities. Journal of Investigative Dermatology 2003; Vol. 120, issue 3:456‐64. [MEDLINE: ] [DOI] [PubMed]
Katz 1987b
- Katz HI, Hien NT, Prawer SE, Mastbaum LI, Mooney JJ, Samson CR. Superpotent Topical Steroid Treatment of Psoriasis Vulgaris‐‐Clinical Efficacy and Adrenal Function. Journal of the American Academy of Dermatology 1987;16(4):804‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Katz 1989
- Katz HI, Prawer SE, Mooney JJ, Samson CR. Preatrophy: Covert Sign of Thinned Skin. Journal of the American Academy of Dermatology 1989;20(5 Pt 1):731‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kimball 2008
- Kimball AB, Gold MH, Zib B, Davis MW, Clobetasol Propionate Emulsion Formulation Foam Phase IIICSG. Clobetasol propionate emulsion formulation foam 0.05%: review of phase II open‐label and phase III randomized controlled trials in steroid‐responsive dermatoses in adults and adolescents. Journal of the American Academy of Dermatology 2008; Vol. 59, issue 3:448‐54. [MEDLINE: ; 1097‐6787] [DOI] [PubMed]
Kragballe 1988
- Kragballe K, Beck HI, Søgaard H. Improvement of psoriasis by a topical vitamin D3 analogue (Mc 903) in a double‐blind study. British Journal of Dermatology 1988;119(2):223‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kragballe 1991b
- Kragballe K, Fogh K, Sogaard H. Long‐Term Efficacy and Tolerability of Topical Calcipotriol in Psoriasis. Results of an Open Study. Acta Dermato‐Venereologica 1991;71(6):475‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Krueger 1984
- Krueger GG, Bergstresser PR, Lowe NJ, Voorhees JJ, Weinstein GD. Psoriasis. Journal of the American Academy of Dermatology 1984;11(5 Pt 2):937‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Krueger 2000
- Krueger GG, Feldman SR, Camisa C, Duvic M, Elder JT, Gottlieb AB, et al. Two considerations for patients with psoriasis and their clinicians: what defines mild, moderate, and severe psoriasis? What constitutes a clinically significant improvement when treating psoriasis?. Journal of the American Academy of Dermatology 2000;43(2 Pt 1):281‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lambert 1996
- Lambert JRMG. Economic Analyses of the Treatment of Psoriasis. European Journal of Dermatology 1996;6(8):543‐7. [EMBASE: 1997003685] [Google Scholar]
Lambert 1999
- Lambert JRMG. Cost‐Effectiveness of Treatments in Psoriasis. Journal Of Dermatological Treatment 1999;10(Suppl 1):S9‐S13. [EMBASE: 1999115355] [Google Scholar]
Lambert 2002
- Lambert J, Trompke C. Tacalcitol ointment for long‐term control of chronic plaque psoriasis in dermatological practice. Dermatology 2002;204(4):321‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Langner 1996
- Langner A, Ashton P, Kerkhof PC, Verjans H. A long‐term multicentre assessment of the safety and tolerability of calcitriol ointment in the treatment of chronic plaque psoriasis. British Journal of Dermatology 1996;135(3):385‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Lebwohl 1996
- Lebwohl M, Siskin SB, Epinette W, Breneman D, Funicella T, Kalb R, et al. A Multicenter Trial of Calcipotriene Ointment and Halobetasol Ointment Compared with Either Agent Alone for the Treatment of Psoriasis. Journal of the American Academy of Dermatology 1996;35(2 Pt 1):268‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lee 1990
- Lee FI, Bellary SV, Francis C. Increased occurrence of psoriasis in patients with Crohn's disease and their relatives [comment]. American Journal of Gastroenterology 1990;85(8):962‐3. [MEDLINE: ] [PubMed] [Google Scholar]
Lee 1998
- Lee JY, Maibach HI. Corticosteroid skin atrophogenicity: assessment methods. Skin Research & Technology 1998;4(4):161‐6. [EMBASE: 1998377637] [DOI] [PubMed] [Google Scholar]
Lee 2006
- Lee IA, Maibach HI. Pharmionics in dermatology: a review of topical medication adherence. American Journal of Clinical Dermatology 2006;7(4):231‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Leu 1985
- Leu RE. Economic Evaluation of New Drug Therapies in Terms of Improved Life Quality. Social Science & Medicine 1985;21(10):1153‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lomholt 1963
- Lomholt G. Psoriasis, prevalence, spontaneous course and genetics. Published Ph.D Thesis. Copenhagen: G.E.C. Gad, 1963:31‐3. [Google Scholar]
Lundberg 1999
- Lundberg L, Johannesson M, Silverdahl M, Hermansson C, Lindberg M. Quality of life, health‐state utilities and willingness to pay in patients with psoriasis and atopic eczema. British Journal of Dermatology 1999;141(6):1067‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marchetti 1998
- Marchetti A, Pensee K, An P. A Pharmacoeconomic Analysis of Topical Therapies for Patients with Mild‐to‐Moderate Stable Plaque Psoriasis: A US Study. Clinical Therapeutics 1998;20(4):851‐69. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Matthews 1996
- Matthews D, Fry L, Powles A, Weber J, McCarthy M, Fisher E, et al. Evidence that a locus for familial psoriasis maps to chromosome 4q. Nature Genetics 1996;14(2):231‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McKenna 2003
- McKenna SP, Cook SA, Whalley D, Doward LC, Richards HL, Griffiths CE, et al. Development of the PSORIQoL, a psoriasis‐specific measure of quality of life designed for use in clinical practice and trials. British Journal of Dermatology 2003;149(2):323‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mee 1998
- Mee JB, Cork MJ. Vitamin D receptor polymorphism and calcipotriol response in patients with psoriasis. Journal of Investigative Dermatology 1998;110(3):301‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Menter 2007
- Menter A. Topical monotherapy with clobetasol propionate spray 0.05% in the COBRA trial. Cutis 2007;80(5 Suppl):12‐9. [MEDLINE: ; 0011‐4162] [PubMed] [Google Scholar]
Miyachi 2002
- Miyachi Y, Ohkawara A, Ohkido M, Harada S, Tamaki K, Nakagawa H, et al. Long‐term safety and efficacy of high‐concentration (20 microg/g) tacalcitol ointment in psoriasis vulgaris. European Journal of Dermatology 2002;12(5):463‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Mortensen 1993
- Mortensen L, Kragballe K, Wegmann E, Schifter S, Risteli J, Charles P. Treatment of psoriasis vulgaris with topical calcipotriol has no short‐term effect on calcium or bone metabolism. A randomized, double‐blind, placebo‐controlled study. Acta Dermato‐Venereologica 1993;73(4):300‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nair 1997
- Nair RP, Henseler T, Jenisch S, Stuart P, Bichakjian CK, Lenk W, et al. Evidence for two psoriasis susceptibility loci (HLA and 17q) and two novel candidate regions (16q and 20p) by genome‐wide scan. Human Molecular Genetics 1997;6(8):1349‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NHSCRD 2001
- NHS Centre for Reviews & Dissemination. Undertaking Systematic Reviews of Research on Effectiveness: CRD guidelines for those Carrying Out or Commissioning Reviews. 2nd Edition. Vol. CRD Report Number 4 (2nd edition), York: CRD, 2001. [Google Scholar]
Oh 1997
- Oh PI, Gupta AK, Einarson TR, Maerov P, Shear NH. Calcipotriol in the Treatment of Psoriasis of Limited Severity: Pharmacoeconomic Evaluation. Journal of Cutaneous Medicine & Surgery 1997;2(1):7‐15. [EMBASE: 1998192329] [Google Scholar]
Ortonne 2000
- Ortonne JP. Psoriasis: evaluation of quality of life. Annales de Dermatologie et de Venereologie 2000;127(Suppl 2):2s19‐22. [MEDLINE: ] [PubMed] [Google Scholar]
Osborne 2002
- Osborne JE, Hutchinson PE. The importance of accurate dosage of topical agents: a method of estimating involved area and application to calcipotriol treatment failures. Journal of the European Academy of Dermatology & Venereology 2002;16(4):367‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Owen 1993
- Owen OG. Healthy returns come from costly drug ‐ prescribing an expensive drug may be the most cost‐effective way to treat patients. Healthcare Management 1993;January:52‐3. [Google Scholar]
Owen 2000
- Owen CM, Chalmers RJ, O'Sullivan T, Griffiths CEM. Antistreptococcal interventions for guttate and chronic plaque psoriasis. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD001976] [DOI] [PubMed] [Google Scholar]
Park 2002
- Park YK, Lee JH, Chung WG. Allergic contact dermatitis from calcipotriol. Acta Dermato‐Venereologica 2002;82(1):71‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Parodi 1991
- Parodi A, Guarrera M, Volonte MV. Medicated Tape Versus Cream Formulation ‐ a Major Saving of Fluocinolone Acetonide. Journal Of Dermatological Treatment 1991;1(6):305‐6. [EMBASE: 1991180520] [Google Scholar]
Poyner 1993
- Poyner T, Hughes IW, Dass BK, Adnitt PI. Long‐Term Treatment of Chronic Plaque Psoriasis with Calcipotriol. Journal Of Dermatological Treatment 1993;4(4):173‐7. [EMBASE: 1994040748] [Google Scholar]
Poyner 1999
- Poyner TF, Wall A, Adnitt PI, Menday AP. Economic Impact of Psoriasis Treatment on the Patient and on the National Health Service. Journal Of Dermatological Treatment 1999;10(1):25‐9. [EMBASE: 1999115428] [Google Scholar]
Poyner 2000
- Poyner TF, Menday AP, Williams ZV. Patient attitudes to topical antipsoriatic treatment with calcipotriol and dithranol. Journal of the European Academy of Dermatology & Venereology 2000;14(3):153‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ramsay 1994
- Ramsay CA, Berth‐Jones J, Brundin G, Cunliffe WJ, Dubertret L, Kerkhof PC, et al. Long‐Term Use of Topical Calcipotriol in Chronic Plaque Psoriasis. Dermatology 1994;189(3):260‐4. [PUBMED: 7949479] [DOI] [PubMed] [Google Scholar]
Richards 1999
- Richards HL, Fortune DG, O'Sullivan TM, Main CJ, Griffiths CE. Patients with psoriasis and their compliance with medication. Journal of the American Academy of Dermatology 1999;41(4):581‐3. [MEDLINE: ] [PubMed] [Google Scholar]
Richards 2003
- Richards HL, Fortune DG, Main CJ, Griffiths CE. Stigmatization and psoriasis. British Journal of Dermatology 2003;149(1):209‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Richards 2006
- Richards HL, Fortune DG, Griffiths CE. Adherence to treatment in patients with psoriasis. Journal of the European Academy of Dermatology & Venereology 2006;20(4):370‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Roberts 2011
- Roberts C, Angus JE, Williams HC, Villanueva E, Saeterdal I, Jobling R. Ustekinumab for plaque psoriasis. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD008947] [DOI] [Google Scholar]
Roelofzen 2010
Russell 1972
- Russell TJ, Schultes LM, Kuban DJ. Histocompatibility (HL‐A) antigens associated with psoriasis. New England Journal of Medicine 1972;287(15):738‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Salvarani 1995
- Salvarani C, Lo Scocco G, Macchioni P, Cremonesi T, Rossi F, Mantovani W, et al. Prevalence of psoriatic arthritis in Italian psoriatic patients. Journal of Rheumatology 1995;22(8):1499‐503. [MEDLINE: ] [PubMed] [Google Scholar]
Schiffner 2003
- Schiffner R, Schiffner‐Rohe J, Gerstenhauer M, Hofstadter F, Landthaler M, Stolz W. Willingness to pay and time trade‐off: sensitive to changes of quality of life in psoriasis patients?. British Journal of Dermatology 2003;148(6):1153‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schwicker 1992
- Schwicker D, Dinkel R, Antunes HC. A Cost‐Comparison Study: Ulobetasol Versus Clobetasol in Severe Localized Psoriasis. Journal Of Dermatological Treatment 1992;2(4):127‐31. [EMBASE: 1992107343] [DOI] [PubMed] [Google Scholar]
Senter 1983
- Senter TP. Topical fluocinonide and tachyphylaxis letter. Archives of Dermatology 1983;119(5):363‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Simons 1949
- Simons RD. Additional studies on psoriasis in the tropics and in starvation camps. Journal of Investigative Dermatology 1949;12(5):285‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Singh 2000
- Singh S, Reddy DC, Pandey SS. Topical therapy for psoriasis with the use of augmented betamethasone and calcipotriene on alternate weeks. Journal of the American Academy of Dermatology 2000;43(1 Pt 1):61‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sorensen 2002
- Sorensen M, Norregaard J. Pharmacoeconomic evaluation of a new two compound ointment (Daivobet (R)) and calcipotriol (Daivonex (R)) in the treatment of psoriasis vulgaris in Sweden. Value Health 2002;5(6):554‐5. [Google Scholar]
Stern 1988
- Stern RS. The Benefits, Costs and Risks of Topical Tar Preparations in the Treatment of Psoriasis: Considerations of Cost Effectiveness. Annals Of The Academy Of Medicine, Singapore 1988;17(4):473‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Stern 1995
- Stern RS. Epidemiology of psoriasis. Dermatologic Clinics 1995;13(4):717‐22. [MEDLINE: ] [PubMed] [Google Scholar]
Stern 1997
- Stern RS. Psoriasis. Lancet 1997;350(9074):349‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stevanovic 1977
- Stevanovic DV, Wilson L, Sparkes CG. A separation of clinical from epidermal thinning effect in the topical glucocorticoid clobetasone butyrate. British Journal of Dermatology 1977;96(1):67‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Svejgaard 1974
- Svejgaard A, Nielsen LS, Svejgaard E, Nielsen FK, Hjortshoj A, Zachariae H. HL‐A in psoriasis vulgaris and in pustular psoriasis ‐ population and family studies. British Journal of Dermatology 1974;91(2):145‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Szeimies 2004
- Szeimies RM. Psoriasis: Patient‐oriented treatment improves compliance [Psoriasis: Patientenorientierte behandlung verbessert compliance]. Haut 2004;15(5):221‐3. [EMBASE: 2004422749] [Google Scholar]
Tagami 1997
- Tagami H. Triggering factors. Clinics in Dermatology 1997;15(5):677‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Taylor 2006
Tazi‐Ahnini 1999a
- Tazi Ahnini R, Camp NJ, Cork MJ, Mee JB, Keohane SG, Duff GW, et al. Novel genetic association between the corneodesmosin (MHC S) gene and susceptibility to psoriasis. Human Molecular Genetics 1999;8(6):1135‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tazi‐Ahnini 1999b
- Tazi‐Ahnini R, Giovine FS, Cox A, Keohane SG, Cork MJ. Corneodesmosin (MHC S) gene in guttate psoriasis. Lancet 1999;354(9178):597. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tomfohrde 1994
- Tomfohrde J, Silverman A, Barnes R, Fernandez‐Vina MA, Young M, Lory D, et al. Gene for familial psoriasis susceptibility mapped to the distal end of human chromosome 17q. Science 1994;264(5162):1141‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Traulsen 2003
- Traulsen J, Hughes‐Formella BJ. The atrophogenic potential and dermal tolerance of calcipotriol/betamethasone dipropionate ointment compared with betamethasone dipropionate ointment. Dermatology 2003;207(2):166‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Trembath 1997
- Trembath RC, Clough RL, Rosbotham JL, Jones AB, Camp RD, Frodsham A, et al. Identification of a major susceptibility locus on chromosome 6p and evidence for further disease loci revealed by a two stage genome‐wide search in psoriasis. Human Molecular Genetics 1997;6(5):813‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tsoi 2012
- Tsoi LC, Spain SL, Knight J, Ellinghaus E, Stuart PE, Capon F, et al. Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nature Genetics 2012;44(12):1341‐8. [PUBMED: 3510312] [DOI] [PMC free article] [PubMed] [Google Scholar]
Uhoda 2003
- Uhoda I, Quatresooz P, Hermanns‐Le T, Pierard‐Franchimont C, Arrese JE, Pierard GE. Histometric assessment of psoriatic plaques treated by vitamin D3 derivatives. Dermatology 2003;206(4):366‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Unna 1916
- Unna PG. Cignolin als Heilmittel der Psoriasis. Dermatologische Wochenschrift 1916;62:116‐37. [Google Scholar]
van de Kerkhof 1996c
- Kerkhof PCM, Harten J, Verjans H. A Long‐Term Assessment of the Safety and Tolerability of Calcitriol Ointment in the Treatment of Chronic Plaque Psoriasis. Journal Of Dermatological Treatment 1996;7(Suppl 1):S11‐14. [EMBASE: 1996266413] [Google Scholar]
van de Kerkhof 1997a
- Kerkhof PC. Combinations and comparisons. Clinics in Dermatology 1997;15(5):831‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
van de Kerkhof 1997b
- Kerkhof P, Vleuten C, Gerritsen M, Glade C, Luger T, Werfel T, et al. Long‐Term Efficacy and Safety of Once Daily Treatment of Chronic Plaque Psoriasis with Tacalcitol Ointment. European Journal of Dermatology 1997;7(6):421‐5. [EMBASE: 1997293421] [Google Scholar]
van de Kerkhof 1998
- Kerkhof PC. An update on vitamin D3 analogues in the treatment of psoriasis. Skin Pharmacology & Applied Skin Physiology 1998;11(1):2‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
van de Kerkhof 1998c
- Kerkhof PCM, Steegers Theunissen RP, Kuipers MV. Evaluation of topical drug treatment in psoriasis. Dermatology 1998;197(1):31‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
van de Kerkhof 2000
- Kerkhof PC, Hoop D, Korte J, Cobelens SA, Kuipers MV. Patient compliance and disease management in the treatment of psoriasis in the Netherlands. Dermatology 2000;200(4):292‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
van de Kerkhof 2001
- Kerkhof PC, Franssen M, Brassine M, Kuipers M. Calcipotriol cream in the morning and ointment in the evening: a novel regimen to improve compliance. Journal of Dermatological Treatment 2001;12(2):75‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
van de Kerkhof 2002c
- Kerkhof PC, Berth‐Jones J, Griffiths CE, Harrison PV, Honigsmann H, Marks R, et al. Long‐term efficacy and safety of tacalcitol ointment in patients with chronic plaque psoriasis. British Journal of Dermatology 2002;146(3):414‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
van de Kerkhof 2004
- Kerkhof PC. The impact of a two‐compound product containing calcipotriol and betamethasone dipropionate (Daivobet/ Dovobet) on the quality of life in patients with psoriasis vulgaris: a randomized controlled trial. British Journal of Dermatology 2004;151(3):663‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Van de Kerkhof 2008
Vazquez‐Lopez 2004
- Vazquez‐Lopez F, Marghoob AA. Dermoscopic assessment of long‐term topical therapies with potent steroids in chronic psoriasis. Journal of the American Academy of Dermatology 2004;51(5):811‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Velema 2009
- Velema M, Hooft L, Lebwohl M, Spuls PI. Interventions for nail psoriasis. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD007633] [DOI] [PMC free article] [PubMed] [Google Scholar]
Veraldi 2006
Vissers 2004
- Vissers WH, Berends M, Muys L, Erp PE, Jong EM, Kerkhof PC. The effect of the combination of calcipotriol and betamethasone dipropionate versus both monotherapies on epidermal proliferation, keratinization and T‐cell subsets in chronic plaque psoriasis. Experimental Dermatology 2004;13(2):106‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Watts 1998
- Watts J. Helping people to remain in control of their psoriasis. Community Nurse 1998;4(7):19‐21. [MEDLINE: ] [PubMed] [Google Scholar]
Willan 1808
- Willan R. On cutaneous diseases. London: Johnson, 1808. [Google Scholar]
Wishart 1994
Yip 1984
- Yip SY. The prevalence of psoriasis in the Mongoloid race. Journal of the American Academy of Dermatology 1984;10(6):965‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zachariae 2002
- Zachariae R, Zachariae H, Blomqvist K, Davidsson S, Molin L, Mork C, et al. Quality of life in 6497 Nordic patients with psoriasis. British Journal of Dermatology 2002;146(6):1006‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zaghloul 2004
- Zaghloul SS, Goodfield MJ. Objective assessment of compliance with psoriasis treatment. Archives of Dermatology 2004;140(4):408‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zanolli 1992
- Zanolli MD, Wikle JS. Joint complaints in psoriasis patients. International Journal of Dermatology 1992;31(7):488‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zug 1995
- Zug KA, Littenberg B, Baughman RD, Kneeland T, Nease RF, Sumner W, et al. Assessing the Preferences of Patients with Psoriasis: A Quantitative, Utility Approach. Archives of Dermatology 1995;131(5):561‐8. [MEDLINE: ] [PubMed] [Google Scholar]
References to other published versions of this review
Mason 2002a
- Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: a systematic review. British Journal Of Dermatology 2002;146(3):351‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mason 2002b
- Mason J, Mason A, Cork M. Topical preparations for the treatment of psoriasis in primary care: a systematic review. University of York (OP41). York: University of York, 2002. [DOI] [PubMed] [Google Scholar]
Mason 2009
- Mason AR, Mason J, Cork M, Dooley G, Edwards G. Topical treatments for chronic plaque psoriasis. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD005028.pub2] [DOI] [PubMed] [Google Scholar]