Editor—O'Beirne and Cairns reported a case of cholestatic hepatitis in association with celecoxib.1 This case does not, however, fulfil the accepted international criteria for cholestatic hepatitis as it was not confirmed histologically.2 Biochemical criteria of cholestatic hepatitis require the presence of alkaline phosphatase activity over twice the upper normal limit or a ratio of alkaline phosphatase to alanine aminotransferase activity >2, or both.2 Neither was fulfilled, even when using aspartate aminotransferase as a surrogate of alanine aminotransferase, which was not provided. Moreover, the patient did not experience an actual positive rechallenge as, during the first exposure to the drug, she experienced only pruritus, which is not specific for hepatitis.
The incidence of cholestatic hepatitis in the general population is unknown. But the incidence of acute liver injury in the general population is 4-5 per 100 000 person years.3 García-Rodriguez reviewed eight studies of drug induced acute liver injury and found that 50 of 102 cases (49%) were cholestatic hepatitis.4
Assuming that 49% of cases of acute liver injury in the general population are cholestatic hepatitis, its incidence can be estimated to be 2-2.5/100 000 person years. As of 30 June 2001, the worldwide exposure to celecoxib is estimated to be 10.6 million patient years (21.5 million patients with an average of six months' treatment). Therefore, the number of expected cases of cholestatic hepatitis should range between 208 and 260. As of 30 June 2001 we had received 14 cases of adverse events reported as cholestatic hepatitis in patients treated with celecoxib worldwide. The receipt of these is influenced by an unknown degree of under-reporting, inherent to the spontaneous reporting system. Regardless, the 14 reported cases fall within the limits of what could be expected, given the widespread use of celecoxib.
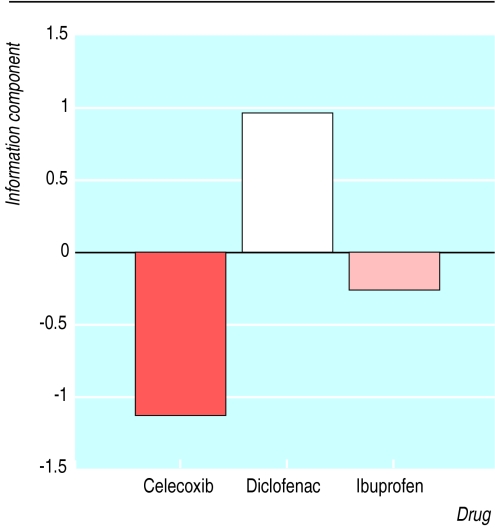
We conducted an analysis of the international drug monitoring database of the World Health Organization, which contains spontaneous reports from 57 countries.5 The figure shows that the information component (the measure of the association between adverse reaction and drug that is used by the WHO at the Uppsala Monitoring Centre to monitor safety signals) of cholestatic hepatitis (–1.15) for celecoxib is not significantly different from background expectation or ibuprofen (–0.30). The information component of cholestatic hepatitis for diclofenac (0.92) was significantly higher than background (P<0.05).
Causality can rarely be ruled out or attributed with certainty on the basis of single case reports. We continue to monitor the safety profile of celecoxib and welcome the reports of possible safety signals. The current existing evidence does not, however, support a causal association between celecoxib and cholestatic hepatitis.
Figure.
Information components for cholestatic hepatitis from WHO safety database
References
- 1.O'Beirne JP, Cairns SR. Cholestatic hepatitis in association with celecoxib. BMJ. 2001;7:23. doi: 10.1136/bmj.323.7303.23. . (7 July.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Council for International Organizations of Medical Sciences (CIOMS) Reporting adverse drug reactions. Geneva: CIOMS; 1999. [Google Scholar]
- 3.Rodriguez LAG, Gutthann SP, Walker AM, Lueck L. The role of non-steroidal anti-inflammatory drugs in acute liver injury. BMJ. 1992;305:865–868. doi: 10.1136/bmj.305.6858.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez LAG, Ruigomez A, Jick H. A review of epidemiologic research on drug-induced acute liver injury using the general practice research database in the United Kingdom. Pharmacotherapy. 1997;17:721–728. [PubMed] [Google Scholar]
- 5.Bate A, Lindquist M, Edwards IR, Olsson S, Orre R, Lansner A, et al. A bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol. 1998;54:315–311. doi: 10.1007/s002280050466. [DOI] [PubMed] [Google Scholar]