Abstract
To compare preventive medications against graft failures in coronary artery bypass graft surgery (CABG) patients after a 1-year follow-up. Systematic review with Bayesian network meta-analysis and meta-regression analysis. We searched PubMed, Scopus, and Web of Science databases in February 2023 for randomized controlled trials, comparing preventive medications against graft failure in CABG patients. We included studies that reported outcomes at 1 year after surgery. Our primary outcome was graft failure After screening 11,898 studies, a total of 18 randomized trials were included. Acetylsalicylic acid (ASA) [odds ratios (OR) 0.51, 95% credibility interval (CrI) 0.28–0.95, meta-regression OR 0.54, 95% CrI 0.26–1.00], Clopidogrel + ASA (OR 0.27, 95% CrI 0.09–0.76, meta-regression OR 0.28, 95% CrI 0.09–0.85), dipyridamole + ASA (OR 0.50, 95% CrI 0.30–0.83, meta-regression OR 0.49, 95% CrI 0.26–0.90), ticagrelor (OR 0.40, 95% CrI 0.16–1.00, meta-regression OR 0.43, 95% CrI 0.15–1.2), and ticagrelor + ASA (OR 0.26, 95% CrI 0.10–0.62, meta-regression OR 0.28, 95% CrI 0.10–0.68) were superior to placebo in preventing graft failure. Rank probabilities suggested the highest likelihood to be the most efficacious for ticagrelor + ASA [surface under the cumulative ranking (SUCRA) 0.859] and clopidogrel + ASA (SUCRA 0.819). The 95% CrIs of ORs for mortality, bleeding, and major adverse cardio- and cerebrovascular events (MACE) were wide. A trend towards increased bleeding risk and decreased MACE risk was observed when any of the medication regimens were used when compared to placebo. Sensitivity analysis excluding studies with a high risk of bias yielded equivalent results. Of the reviewed medication regimens, dual antiplatelet therapy combining ASA with ticagrelor or clopidogrel was found to result in the lowest rate of graft failures.
Keywords: Graft failure, Coronary artery bypass grafting, Antiplatelet, Dual antiplatelet therapy
Introduction
Despite the increasing number of percutaneous coronary interventions performed worldwide, coronary artery bypass grafting (CABG) still remains a recommended treatment for patients with moderate to complex coronary artery disease.1,2 The bypassing may be performed using grafts such as internal thoracic arteries, radial artery, gastroepiploic artery or saphenous vein. Along with anastomosing the left internal thoracic artery to the left anterior descending coronary artery, saphenous vein is still the most common graft for secondary anastomoses.3–7 However, anastomosing saphenous vein to coronary arteries encompasses an increased risk for graft stenosis and failure when compared to arterial grafts, and up to 30–40% of saphenous vein grafts (SVG) have been reported to fail within a year from surgery.8,9 To improve saphenous graft patency, the use of antiplatelet medication, most commonly acetylsalicylic acid (ASA), is an established practice after CABG.1,2,10,11 However, as a cost of improved SVG patency, these medications possess an increased risk for bleeding complications.12–14
There are several agents available to improve SVG patency, and the use of double agents has gained increasing attention. During the previous decades, the body of knowledge has been increasing along with several randomized controlled trials, meta-analyses and, during the past few years, network meta-analyses.15–17 Still, uncertainty remains on which medication regimen would be the most beneficial, and the previous evidence-composing reviews have raised some methodological concerns affecting the applicability of the results.
First, there has been a high variability in follow-up times in studies in which results have been pooled in the previous network meta-analyses.15–17 In these works, the minimum follow-up period has either not been defined or has been limited to more than 3 months. However, with regards to the pathophysiology of SVG failure, early failures are predominantly related to operative and technical factors of CABG surgery and competitive flow rather than sporadic thrombosis, intimal hyperplasia and atherosclerosis, of which risk may be modified by appropriate preventive medication.3 Further, due to the slow pace of the pathophysiological process leading to SVG occlusion, the consequences of disease progression become visible after several months.3 Therefore, the efficacy of preventive should be assessed no earlier than 1 year from surgery and, subsequently, it is clear, that 3-month follow-up is too short.
Second, there have been discrepancies in the outcome measure definitions. The SVG failure rate may be reported per patient, per graft or per distal anastomosis. The previous network meta-analyses have either inappropriately pooled per patient and per graft data leading to guaranteed inaccuracy in the effect estimates or studied outcomes per graft only without accounting per patient data.15–17
Third, since the early course of SVG failure prevention trials several decades ago, there have been massive advances in operative techniques, equipment, perioperative care, and guidelines. Additionally, there may be differences in patient-related and institutional factors related to SVG failure rates between trials. These factors may have influenced the effect estimates in individual studies due to which it would be beneficial to conduct a meta-regression analysis with appropriate covariates.
With an appreciation of these aspects of previous literature, we conducted a Bayesian network meta-analysis and meta-regression to compare different medication regimens to prevent SVG failures that are not related to technical factors or competitive flow with a follow-up of 1 year.
Methods
Search and screening process
Search for this systematic review was performed on 19 February 2023. PubMed, Scopus, and Web of Science databases were searched from inception. The search strategy is described in Supplementary material online, Appendix S1. All authors contributed to the abstract screening and each abstract was screened by two individual authors. Abstracts accepted by both authors were included in full-text screening. Cases of discrepancy between the screening authors were solved by third author. Full report screening was carried out in a similar manner. Covidence software was used in the screening process. We did not search grey literature. The reference lists of the included studies were screened manually to find missed relevant studies for inclusion. Previous meta-analyses were also searched for relevant studies.
Inclusion and exclusion criteria
We included randomized controlled trials comparing medical treatment regimens to prevent vein graft failure after CABG surgery and reporting outcomes at 12 months from the surgery. All studies that reported observational data or did not report original data were excluded.
Patients
Patients were required to undergo CABG surgery due to coronary artery disease. Both chronic and acute-phase surgery patients were included. CABG surgery was defined as cardiac surgery procedure in which the blood flow of an occluded coronary artery has been restored by bypassing the occluded segment of the artery using a vein graft. Percutaneous coronary interventions were excluded.
Intervention
We included all medical treatment regimens that are targeted against vein graft failure including agents affecting blood coagulation and thrombus formation. The minimum number of patients per treatment regimen in all included studies was set to 100 and those regimens with less than 100 patients were excluded.
Comparator
Placebo was used as a comparator for all studied medication regimens in our network analysis.
Outcomes
Our main outcome was the graft failure which was defined as graft occlusion or thrombosis. Stenosis of anastomosis without occlusion was not considered as graft failure. The follow-up time was set to 12 months. Secondary outcomes were mortality, bleeding complications requiring intervention and major adverse cardio- or cerebrovascular events (MACE) during the 12 months of follow-up.
Data extraction
The following information was extracted from each study: authors, funding, competing interests, inclusion and exclusion criteria, study period, country, intervention definition, control definition, outcome definitions, number of included patients, number of events, and main outcome measures.
Evidence certainty
Evidence certainty was assessed by CINeMA (Confidence in Network Meta-Analysis) frameworks.18,19 Evidence certainty was ranked from very low to high.
Within-study bias was assessed by two authors independently according to Cochranes Risk of bias 2.0 tool.20 Risk of bias plots were generated using Robvis shinyapp.21 In the risk of bias assessment, the lack of blinding was not judged as an issue as the outcome assessment was considered not to be influenced by the knowledge of the intervention. Thus, we have utilized the same risk of bias assessment in all outcomes, as per the recommendation suggests considering the risk of bias for each outcome.
Reporting bias was considered generally low in all studies as no evidence of selective reporting or unpublished reports was noticed. Due to strict inclusion criteria with clearly defined outcome variables, we considered that indirectness was not an issue. Imprecision, heterogeneity and incoherence were assessed according to CINeMA framework.19 OR values 0.90–1.10 set as a range of equivalence.19
Statistical methods
We conducted a Bayesian network meta-analysis with four Markov chains. Due to the assumed between-study heterogeneity, we selected the random-effects model as our approach. The posterior distributions were estimated using Monte Carlo simulations. Simulation was performed in two phases. First, 5000 burn-in simulation iterations were performed to adapt the algorithm after which the results of these iterations were discarded. Second, 100 000 inference simulation iterations were performed to estimate the posterior distributions. Convergence of the algorithm was assessed by inspecting trace plots and calculating potential scale reduction factor (PSRF) values. PSFR values below 1.05 were considered to represent sufficient convergence. Inconsistency was assessed by conducting a node-splitting analysis. Non-significant differences (P > 0.05) between the direct and indirect effect estimates were interpreted as representing sufficient consistency of the network model.
After compiling the model, the crude effect estimates for each medication regimen were calculated as odds ratios (OR) along with 95% credibility intervals (95% CrI) with placebo set as a control treatment. The rank probabilities indicating the probability for each treatment to be the most efficacious were calculated. Further, the surface under the cumulative ranking (SUCRA) scores were calculated. Higher SUCRA score indicate higher likelihood of the medication regimen to be the most efficacious. A meta-regression analysis was performed to adjust for the between-study differences. Covariates included the mean age of patients, the proportion of female patients, the proportion of patients with acute coronary syndrome at the time of surgery, the proportion of patients operated using cardiopulmonary bypass, mean number of grafts used, number of years from study publication and overall failure rate in each study. The ‘failures per patient’ and ‘failures per graft’ analyses were performed separately. Sensitivity analysis was performed excluding high risk of bias studies. Since placebo was not studied in the low and moderate risk of bias studies, ASA was set to a control treatment in the sensitivity analysis.
Statistical analysis was performed using R statistical software [version 4.3.1, R Core Team (2023), R Foundation for Statistical Computing, Vienna, Austria].
This study has been conducted according to the guidelines in Cochrane handbook and reported according to the Preferred reporting items in systematic reviews and meta-analysis (PRISMA) guideline.22,23
Protocol registration
Protocol was registered to International prospective register of systematic reviews (PROSPERO; ID: CRD42023482354; available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023482354).
Results
Search results
The initial search provided a total of 11 898 studies after removing duplicates. After the screening, 18 studies were included in the synthesis (Figure 1).
Figure 1.
PRISMA flowchart of the study selection process.
Study and patient characteristics
Study characteristics are presented in Table 1. The mean age of patients varied between 50 and 68 and the proportion of female varied between 0% and 39% across the studies. The mean number of bypassed coronary arteries varied from 1.9 to 3.8 between studies. Of the included studies, the overall risk of bias was low in seven, had some concerns in three, and was high in eight studies (Figure 2). Most issues were due to bias in the selection of the reported results and bias arising from the randomization process and from selection of the reported results. Confidence ratings according to CINeMA framework for the effects of each medication regimen in relation to placebo are presented in Supplementary material online, Appendix S2 and S3 for per patient and per graft analyses, respectively.
Table 1.
Study characteristics
| Study | Country | Study period | Funding | COI | Blinding | Follow-up (months) | Overall mean age | Overall women (%) | Patients with acute coronary syndrome at the time of surgery (%) |
Patients operated using cardiopulmonary bypass(%) | Intervention | Mean number of bypassed coronary arteries per patient | Number of patients | Number of patients with graft failure (%) | Number of grafts | Number of failed grafts (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Agnew et al.24 | New Zealand | 1986–1988 | Reported | Not reported | Double | 12 months | 56 | 5 | 0 | 100 | Dipyridamole 300 mg × 1 + ASA 100 mg × 1 | 3.0 | 30 | 96 | 10 (10) | |
| ASA 100 mg × 1 | 3.0 | 31 | 96 | 10 (10) | ||||||||||||
| Brooks et al.25 | UK | 1978–1982 | Reported | Not reported | Double | 12 months | 54 | 12 | 0 | 100 | Dipyridamole 75 mg × 3 + ASA 330 mg × 3 | 3.5 | 133 | 33 (25) | 360 | 39 (11) |
| Placebo | 3.2 | 133 | 42 (32) | 352 | 46 (13) | |||||||||||
| Brown et al.26 | USA | 1976–1980 | Not reported | Not reported | Double | 12 months | Not reported (range 34–70) | 0 | 0 | 100 | Dipyridamole 75 mg × 3 + ASA 325 mg × 3 | 3.1 | 45 | 15 (33) | ||
| ASA 325 mg × 3 | 3.1 | 38 | 10 (26) | |||||||||||||
| Placebo | 3.3 | 44 | 14 (32) | |||||||||||||
| Chesebro et al.27 | USA | 1977–1981 | Reported | Not reported | Double | 12 months | 56 | 10 | 0 | 100 | Dipyridamole 75 mg × 1 + ASA 325 mg × 1 | 2.8 | 171 | 37 (22) | 478 | 53 (11) |
| Placebo | 2.8 | 172 | 81 (47) | 486 | 121 (25) | |||||||||||
| Ekeström et al.28 | Sweden | 1983–1987 | Reported | Not reported | Double | 12 months | 58 | 12 | 0 | 100 | Dipyridamole 100 mg × 1 | 3.2 | 126 | 146 | 35 (24) | |
| Placebo | 3.4 | 129 | 147 | 43 (29) | ||||||||||||
| Gao et al.29 | China | 2005–2007 | Not reported | Not reported | Single | 12 months | 62 | 17 | 0 | 63 | Clopidrogel 75 mg × 1 + ASA 100 mg × 1 | 2.7 | 95 | 10 (11) | ||
| Clopidogrel 75 mg × 1 | 2.5 | 102 | 13 (13) | |||||||||||||
| Gavaghan et al.30 | Australia | 1984–1987 | Not reported | Not reported | Double | 12 months | 56 | 26 | 0 | 100 | ASA 324 mg × 1 | 3.4 | 119 | 7 (5.9) | ||
| Placebo | 3.6 | 100 | 12 (12) | |||||||||||||
| Kim et al.31 | Republic of Korea | 2014–2020 | Reported | None declared | Open-label | 12 months | 68 | 22 | 0 | 0 | Ticagrelor 90 mg × 2 + ASA 100 mg × 1 | 3.1 | 102 | 4 (3.9) | ||
| Clopidogrel 75 mg × 1 + ASA 100 mg × 1 | 3.2 | 102 | 6 (5.9) | |||||||||||||
| Kulik et al.32 | Canada | 2006–2009 | Reported | None declared | Double | 12 months | 67 | 11 | 19 | 96 | Clopidogrel 75 mg × 1 + ASA 162 mg × 1 | 3.6 | 46 | 6 (13) | ||
| ASA 162 mg × 1 | 3.4 | 45 | 6 (13) | |||||||||||||
| Kulik et al.33 | Canada | 2014–2019 | Reported | None declared | Double | 12 months | 68 | 16 | 61 | 88 | Ticagrelor 90 mg × 2 | 2.9 | 100 | 17 (17) | 289 | 30 (10) |
| ASA 81 mg × 2 | 3.0 | 102 | 24 (24) | 299 | 34 (11) | |||||||||||
| Lamy et al.7 | Canada | 2015–2017 | Reported | None declared | Double | 12 months | 66 | 19 | 22 | 76 | Rivaroxaban 2,5 mg × 2 + ASA 100 mg × 1 | 3.1 | 396 | 86 (22) | 1242 | 113 (9.1) |
| ASA 100 mg × 1 | 3.1 | 362 | 75 (21) | 1154 | 92 (8.0) | |||||||||||
| Rivaroxaban 2,5 mg × 2 | 3.1 | 381 | 68 (18) | 1166 | 91 (7.8) | |||||||||||
| Mayer et al.34 | USA | 1973–1975 | Not reported | Not reported | Double | 12 months | 54 | 18 | 0 | 100 | Dipyridamole 50 mg × 2 + ASA 650 mg × 2 | 2.0 | 47 | 6 (13) | 93 | 6 (6.5) |
| Placebo | 1.8 | 66 | 20 (30) | 120 | 22 (18) | |||||||||||
| McEnany et al.35 | USA | 1979–1981 | Reported | Not reported | Double | 12 months | 50 | 9 | 13 | 100 | ASA 300 mg × 2 | 2.1 | 40 | 15 (37) | 81 | 16 (20) |
| Placebo | 2.0 | 37 | 16 (43) | 75 | 20 (27) | |||||||||||
| Mulder et al.36 | Netherlands | 1987–1990 | Not reported | Not reported | Double | 12 months | 58 | 15 | 0 | 100 | Dipyridamole 200 mg × 1 + ASA 50 mg × 1 | 3.6 | 30 | 107 | 24 (22) | |
| ASA 50 mg × 1 | 4.2 | 31 | 129 | 31 (24) | ||||||||||||
| Acenocoumarol or phenprocoumon (individual dosing) | 5.0 | 31 | 154 | 41 (27) | ||||||||||||
| Rajah et al.37 | UK | 1986–1989 | Not reported | Not reported | Double | 12 months | 55 | 14 | 0 | 100 | Indobufen 200 mg × 2 | 3.2 | 278 | 123 (44) | 883 | 163 (18) |
| Dipyradimole 75 mg × 3 + ASA 300 mg × 3 | 3.2 | 274 | 112 (41) | 870 | 144 (17) | |||||||||||
| Tang et al.38 | China | 2017–2018 | Reported | None declared | Open-label | 12 months | 64 | 39 | 0 | 69 | Ticagrelol 90 mg × 2 + ASA 100 mg × 1 | 3.2 | 70 | 224 | 15 (6.7) | |
| Clopidogrel 75 mg × 1 + ASA 100mg | 3.3 | 77 | 253 | 19 (8.7) | ||||||||||||
| Une et al.39 | Canada | 2006–2009 | Reported | None declared | Double | 12 months | 66 | 12 | 0 | 90 | Clopidrogel 75 mg × 1 + ASA 162 mg × 1 | Not reported | 46 | 4 (8.7) | (4.8) | |
| ASA 162mg | Not reported | 46 | 14 (30) | (4.5) | ||||||||||||
| van der Meer et al.40 | Netherlands/Switzerland/Germany | 1987–1990 | Reported | Not reported | Double | 12 months | 58 | 5 | 0 | 100 | ASA 50 mg × 1 | 3.6 | 270 | 73 (27) | 440 | 88 (20) |
| Dipyridamole 200 mg × 2 + ASA 50 mg × 1 | 3.9 | 249 | 65 (26) | 461 | 69 (15) | |||||||||||
| Acenocoumarol 4 mg × 1 or phenprocoumon 6 mg × 1 | 3.8 | 257 | 69 (27) | 448 | 85 (19) | |||||||||||
| Willemsen et al.41 | Netherlands | 2015–2019 | Reported | Reported | Double | 12 months | 68 | 13 | 31 | 95 | Ticagrelol 90 mg × 2 + ASA 80–100 mg × 1 | 3.7 | 219 | 26 (12) | 457 | 44 (9.6) |
| ASA 80–100 mg × 1 | 3.8 | 224 | 32 (14) | 497 | 50 (10) | |||||||||||
| Zhao et al.6 | China | 2014–2015 | Reported | Reported | Open-label | 12 months | 64 | 20 | 4 | 24 | Ticagrelor 90 mg × 2 + ASA 100 mg × 1 | 3.8 | 168 | 30 (18) | 458 | 29 (6.3) |
| ASA 100 mg × 1 | 3.8 | 166 | 58 (35) | 436 | 73 (17) | |||||||||||
| Ticagrelor 90 mg × 2 | 3.8 | 166 | 49 (30) | 445 | 55 (12) |
Figure 2.
Within-study risk of bias of the included studies.
Model diagnostics
Trace plot and PSRF values indicated acceptable convergence of the model with the selected burn-in and inference simulation iterations as all PSRF values estimated at 1.000 (upper bound of PSRF 95% confidence interval range 1.00–1.01). The node-splitting analysis showed no prominent differences (P > 0.05) between the direct and indirect effect estimates for any of the medication regimens indicating sufficient consistency of the algorithm.
Primary outcomes
Failures per patient
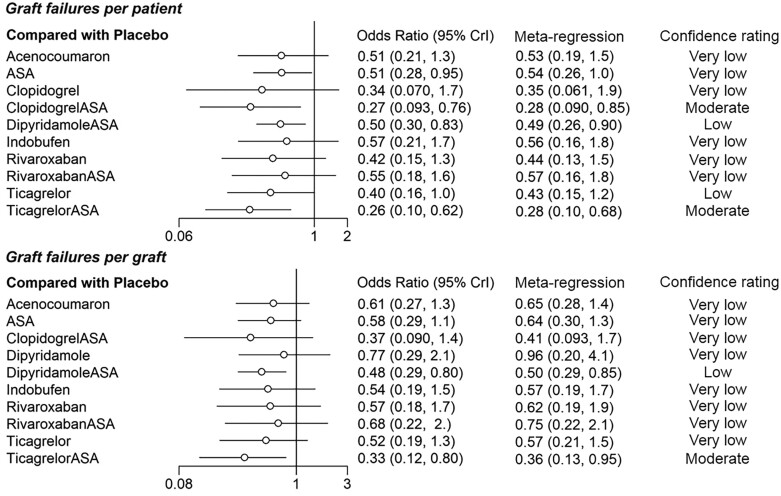
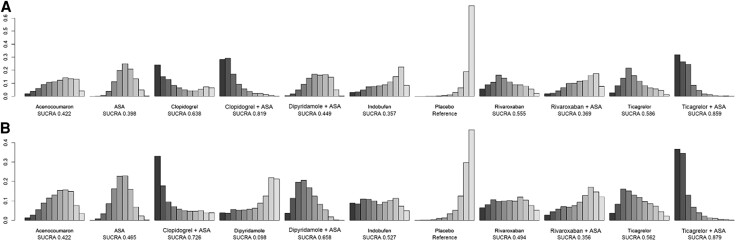
The per-patient data involved overall 5341 patients from 16 studies. A total of 1278 (24%) patients had graft failure. Eleven medication regimens were included in the analysis (Figure 3). The ORs of ASA, clopidogrel + ASA, dipyridamole + ASA, ticagrelor and ticagrelor + ASA showed these medication regimens to be superior to placebo by the means of the 95% credibility level (Figure 4). Rank probabilities suggested the superiority of ticagrelor + ASA (SUCRA 0.859) and clopidogrel + ASA (SUCRA 0.819) over other medication regimens in preventing graft failures per patient (Figure 5). Of single agents clopidogrel (SUCRA 0.638), ticagrelor (SUCRA 0.586), and rivaroxaban (SUCRA 0.555) were found the most effective. The results of the meta-regression analysis showed similar findings. Confidence ratings of the medication regimens’ observed effects with placebo set as a reference were mainly low to very low while the effect of ticagrelor + ASA and clopidogrel + ASA rated as moderate confidence. Sensitivity analysis with high risk of bias studies excluded showed, according to the rank probabilities, tendency towards superiority of ticagrelor + ASA and clopidogrel + ASA over the other medications in line with the main analysis (Figure 6). However, the evidence on differences between the medication regimens was inadequate, given the wide CrIs of ORs.
Figure 3.
The network plots between the medication regimens. (A) The network of graft failures per patient. (B) The network of graft failures per graft. The edge width corresponds with the number of studies on the given comparison.
Figure 4.
Crude and adjusted odds ratios for graft failure per patient and per graft of each medication regimen with placebo set as a control treatment. 95% Crl, 95% credibility interval.
Figure 5.
Rank probability distributions for each medication regimen. (A) Graft failures per patient. (B) Graft failures per graft. The leftmost bar signifies the first rank, i.e. the relative probability be the most efficacious medication regimen whereas the rightmost bar signifies the last rank, i.e. the relative probability to be the least efficacious regimen. The height of a bar shows the probability of the given rank. The surface under the cumulative ranking (SUCRA) score indicates the likelihood of a medication regimen to be the most efficacious with higher value indicating higher likelihood.
Figure 6.
Sensitivity analysis of low risk of bias studies only for per-patient data.
Failures per graft
Analysis of per-graft data involved a total of 12 942 grafts and 1711 observed graft failures in 14 studies. Ten medication regimens were analysed (Figure 3). The ORs of dipyridamole + ASA and ticagrelor + ASA appeared to be superior to placebo by means of the 95% credibility level (Figure 4). Rank probabilities suggested the superiority of ticagrelor + ASA (SUCRA 0.879) and clopidogrel + ASA (SUCRA 0.726) over the other medication regimens (Figure 5). Of single agents, ticagrelor (SUCRA 0.562) was found superior to other agents. The meta-regression analysis resulted in similar findings. Confidence ratings of the medication regimens’ observed effects with placebo set as a reference were generally low to very low while only the effect of ticagrelor + ASA rated as moderate confidence. Confidence ratings of the medication regimens’ observed effects with placebo set as a reference were generally very low except in ticagrelor + ASA and dipyridamole + ASA, which were rated as moderate and low confidence, respectively. In line with the main analysis, sensitivity analysis with a high risk of bias studies excluded showed, according to the rank probabilities, tendency towards superiority of ticagrelor + ASA over the other medications (Figure 7). In a light of wide CrIs of ORs, however, the evidence on differences between the medication regimens was insufficient.
Figure 7.
Sensitivity analysis of low risk of bias studies only for per-graft data.
Secondary outcomes
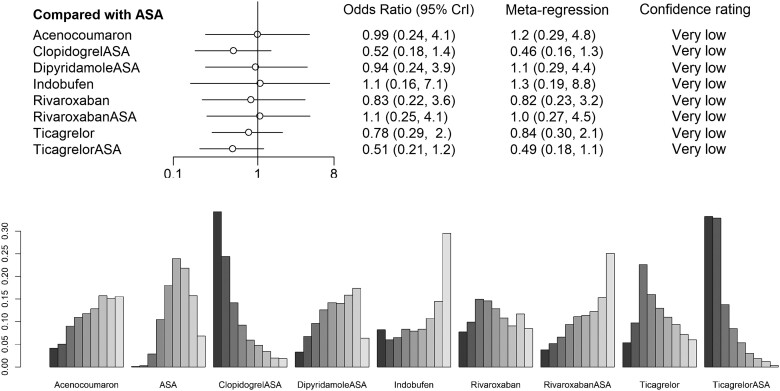
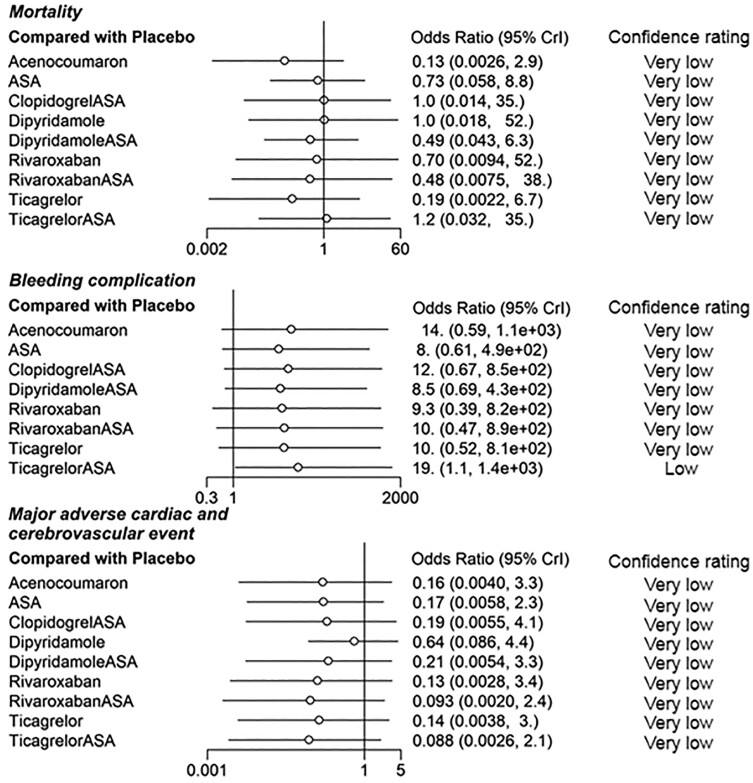
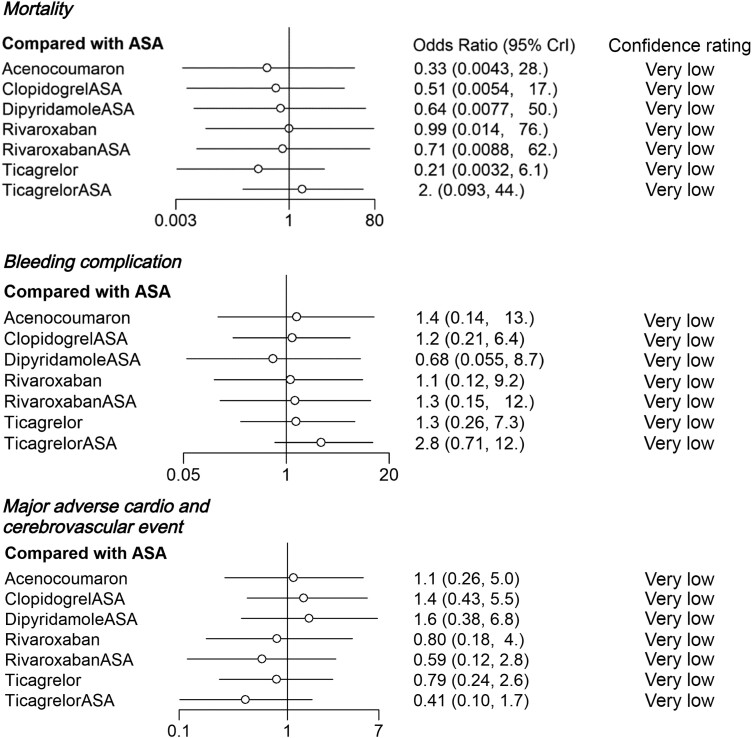
The 95% CrIs of ORs for mortality, bleeding and MACE were extremely wide (Figure 8). Although all the point estimates of ORs for bleeding complications showed increased risk and those for MACE showed decreased risk when compared to placebo, eligible evidence of increased complication risk was observed only in patients using ticagrelor + ASA (confidence rated as low), among which the OR indicated increased risk for bleeding complications. In the sensitivity analyses with high risk of bias studies excluded and ASA set as a reference, no eligible evidence of differences between the medication regimens was observed (Figure 9).
Figure 8.
Odds ratios for complications of each medication regimen with placebo set as a control treatment. 95% Crl, 95% credibility interval.
Figure 9.
Sensitivity analysis of complications with high risk of bias studies excluded. ASA is set as a control treatment. 95% Crl, 95% credibility interval.
Discussion
According to the results of this network meta-analysis and meta-regression, double antiplatelet treatment regimens ticagrelor + ASA and clopidogrel + ASA are most likely the most effective medication regimens to prevent SVG failure at 1 year from surgery, independent of whether the analysis was performed per patient or per graft. Of single agents, clopidogrel, ticagrelor, and rivaroxaban were found most likely the most effective although wide CrIs suggested high uncertainty of relative effectiveness between the studied agents. With regards to complications, uncertainty was high as observed by wide CrIs. However, evidence on increased bleeding risk related to ticagrelor + ASA combination was observed. These findings may be used in the assessment of risk for SVG failure and in planning of optimal medication regimen based on clinical and patient-related risk factors.
In the concurrent guidelines, ASA as a monotherapy is recommended as SVG failure preventing medication in CABG patients with stable coronary artery disease whereas in patients undergoing CABG due to acute coronary syndrome dual antiplatelet therapy should be used at least 1 year from surgery.1,2,10 However, evidence on benefits of dual antiplatelet therapy in CABG patients with stable coronary artery disease is scarce although there are some data suggesting that dual antiplatelet therapy may decrease SVG failure when compared to ASA monotherapy.2,11 With the existing knowledge gap, recommendations on antithrombotic medication after CABG in the current guidelines are cautious.1,2,10
While ASA monotherapy is a widely established practice recommended by the guidelines, there are some evidence suggesting benefit of dual antiplatelet therapy in certain patient groups. Beneficial effects seem more pronounced in patients undergoing off-pump CABG and when vein grafts are used.42–44 This in addition to the data suggesting higher graft failure rates in off-pump CABG especially when SVGs have been used, advocates towards favouring of dual treatment in these patients.42–44 With regards to the lacking guidelines, these findings underline the need of risk-benefit-assessment accounting individual patient characteristics when planning the medication strategy against graft failure after CABG. Moreover, some authors have even raised a concern on underutilization of dual treatment in coronary artery disease patients, especially after CABG surgery.45–47
The findings of this network meta-analysis provide leverage in this assessment with expectable reciprocal efficacy against graft failure and risk profile. In line with the guidelines, ASA was found related to improved graft patency when compared to placebo although the confidence level for the beneficial effect was very low. As expected, the dual therapy regimens with ASA combined with ticagrelor or clopidogrel, appeared to result in even higher graft patency than other regimens especially in per-patient analysis with moderate confidence level. Ticagrelor and clopidogrel did well also as monotherapies, although there was still rather high level of uncertainty due to which more research is needed before strong recommendations on the use of ticagrelor or clopidogrel as a monotherapy against graft failure.
The graft failure rate in patients with rivaroxaban was promising alluding that rivaroxaban may perform adequately in preventing graft failure in post-CABG patients with a simultaneous need for persistent anticoagulation therapy. Interestingly, combining rivaroxaban with ASA did not seem to improve graft patency compared to rivaroxaban monotherapy advocating rivaroxaban to be used only as a monotherapy.
With regards to complications, there was a trend of increased bleeding complications and decreased MACE with each medication regimen in relation to placebo although the evidence on complication rates were mainly inconclusive. However, the observed 1-year mortality was predominantly not related to medication regimens.
Strengths and limitations
There were several strengths in the current work. First, with regards to the previous network meta-analyses on medication regimens against graft failure in patients after CABG, this is the only work in which follow-up has been predefined to 1 year to meet the characteristics of the pathophysiological process of graft stenosis. Further, adjusting the effect estimates with potential confounders of graft patency risk enabled calculating of even more accurate effect estimates and reciprocal comparison of medication regimens. Acknowledging these strengths, the observed effect estimates were still in line with the previous network meta-analyses. The credibility of our results is affected by some limitations. First, as revealed by the risk of bias analysis, the overall risk of bias was moderate and over a half of the studies were affected by at least some concerns regarding risk of bias. In addition, with regards to some agents, the number of studies or patients was low increasing uncertainty on the effect estimate observed by wide credibility interval. There was inconsistency in the definitions and criteria for graft failure between studies. Lastly, despite the urge to control for the confounders of the association between medication regimens and graft failure, there may still be uncontrolled confounding which may have an influence on the effect estimates.
Conclusion
As a summary, of the reviewed medication regimens, dual antiplatelet therapy combining ASA with ticagrelor or clopidogrel was found to result in the lowest rate of graft failures. These findings suggest that especially the patients with increased risk for graft failure after CABG surgery, such as in patients with acute coronary syndrome, off-pump CABG or poor graft quality, may benefit from dual antiplatelet therapy combining ASA with ticagrelor or clopidogrel.
Supplementary Material
Contributor Information
Mikko Uimonen, Tampere University Hospital, Heart Hospital, Elämänaukio 1, 33520 Tampere, Finland; Faculty of Medicine and Health Technology, Tampere University, Arvo Ylpön katu 34, 33520 Tampere, Finland.
Rasmus Liukkonen, Faculty of Medicine and Health Technology, Tampere University, Arvo Ylpön katu 34, 33520 Tampere, Finland.
Ville Ponkilainen, Department of Surgery, Central Finland Hospital Nova, Jyväskylä, Finland.
Matias Vaajala, Faculty of Medicine and Health Technology, Tampere University, Arvo Ylpön katu 34, 33520 Tampere, Finland.
Jeremias Tarkiainen, Faculty of Medicine and Health Technology, Tampere University, Arvo Ylpön katu 34, 33520 Tampere, Finland.
Oskari Pakarinen, Department of Surgery, Päijät-Häme Central Hospital, Lahti, Finland.
Marjut Haapanen, Institute of Clinical Medicine, University of Eastern Finland, Kuopio, Finland.
Ilari Kuitunen, Institute of Clinical Medicine, University of Eastern Finland, Kuopio, Finland; Department of Pediatrics, Kuopio University Hospital, Kuopio, Finland.
Lead author biography

Dr Mikko Uimonen is a cardiothoracic surgery resident at Tampere Heart Hospital. Additionally, Dr Uimonen holds the position of Associate Professor at Tampere University. With a passion for advancing the management of coronary artery disease, Dr Uimonen is dedicated to achieving clinical excellence and conducting impactful academic research in the field of cardiothoracic surgery. His research focuses particularly on treatment selection, decision-making processes, and secondary prevention strategies, aiming to enhance patient care and outcomes through innovative approaches in cardiac surgery.
Data availability
The data supporting the findings of this analysis are presented in Table 1 of this manuscript.
Supplementary material
Supplementary material is available at European Heart Journal Open online.
Authors’ contributions
M.U.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, software, visualization, writing—original draft; R.L.: data curation, writing—review and editing; V.P.: supervision, methodology, validation, writing—review and editing; M.V.: data curation, writing—review and editing; J.T.: data curation, writing—review and editing; O.P.: data curation, writing—review and editing; M.H.: data curation, writing—review and editing; and I.K.: project administration, methodology, validation, writing—review and editing.
Funding
None.
References
- 1. Virani SS, Newby LK, Arnold SV, Bittner V, Brewer LC, Demeter SH, Dixon DL, Fearon WF, Hess B, Johnson HM, Kazi DS, Kolte D, Kumbhani DJ, LoFaso J, Mahtta D, Mark DB, Minissian M, Navar AM, Patel AR, Piano MR, Rodriguez F, Talbot AW, Taqueti VR, Thomas RJ, van Diepen S, Wiggins B, Williams MS. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA guideline for the management of patients with chronic coronary disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2023;148:e9–e119. [DOI] [PubMed] [Google Scholar]
- 2. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes: the task force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J 2020;41:407–477. [DOI] [PubMed] [Google Scholar]
- 3. Xenogiannis I, Zenati M, Bhatt DL, Rao SV, Rodés-Cabau J, Goldman S, Shunk KA, Mavromatis K, Banerjee S, Alaswad K, Nikolakopoulos I, Vemmou E, Karacsonyi J, Alexopoulos D, Burke MN, Bapat VN, Brilakis ES. Saphenous vein graft failure: from pathophysiology to prevention and treatment strategies. Circulation 2021;144:728–745. [DOI] [PubMed] [Google Scholar]
- 4. Gao G, Zheng Z, Pi Y, Lu B, Lu J, Hu S. Aspirin plus clopidogrel therapy increases early venous graft patency after coronary artery bypass surgery: a single-center, randomized, controlled trial. J Am Coll Cardiol 2010;56:1639–1643. [DOI] [PubMed] [Google Scholar]
- 5. Sun JC, Teoh KH, Lamy A, Sheth T, Ellins ML, Jung H, Yusuf S, Anand S, Connolly S, Whitlock RP, Eikelboom JW. Randomized trial of aspirin and clopidogrel versus aspirin alone for the prevention of coronary artery bypass graft occlusion: the preoperative aspirin and postoperative antiplatelets in coronary artery bypass grafting study. Am Heart J 2010;160:1178–1184. [DOI] [PubMed] [Google Scholar]
- 6. Zhao Q, Zhu Y, Xu Z, Xu Z, Cheng Z, Mei J, Chen X, Wang X. Effect of ticagrelor plus aspirin, ticagrelor alone, or aspirin alone on saphenous vein graft patency 1 year after coronary artery bypass grafting: a randomized clinical trial. JAMA 2018;319:1677–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lamy A, Eikelboom J, Sheth T, Connolly S, Bosch J, Fox KAA, Zhu J, Lonn E, Dagenais G, Widimsky P, Branch KRH, Bhatt DL, Zheng Z, Straka Z, Dagenais F, Kong Y, Marsden T, Lee SF, Copland I, Yusuf S. Rivaroxaban, aspirin, or both to prevent early coronary bypass graft occlusion: the COMPASS-CABG study. J Am Coll Cardiol 2019;73:121–130. [DOI] [PubMed] [Google Scholar]
- 8. Cooper G, Underwood M, Deverall P. Arterial and venous conduits for coronary artery bypass. A current review. Eur J Cardio-thorac Surg 1996;10:129–140. [DOI] [PubMed] [Google Scholar]
- 9. Alexander JH. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA 2005;294:2446–2454. [DOI] [PubMed] [Google Scholar]
- 10. Byrne RA, Rossello X, Coughlan J, Barbato E, Berry C, Chieffo A, Claeys MJ, Dan G-A, Dweck MR, Galbraith M, Gilard M, Hinterbuchner L, Jankowska EA, Jüni P, Kimura T, Kunadian V, Leosdottir M, Lorusso R, Pedretti RFE, Rigopoulos AG, Rubini Gimenez M, Thiele H, Vranckx P, Wassmann S, Wenger NK, Ibanez B; ESC Scientific Document Group . 2023 ESC guidelines for the management of acute coronary syndromes: developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J 2023;44:3720–3826. [DOI] [PubMed] [Google Scholar]
- 11. Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, Jüni P, Kastrati A, Kolh P, Mauri L, Montalescot G. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018;39:213–260. [DOI] [PubMed] [Google Scholar]
- 12. Solo K, Lavi S, Choudhury T, Martin J, Nevis IF, Kwok CS, Kotronias RA, Nishina N, Sponga S, Ayan D, Tzemos N, Mamas MA, Bagur R. Pre-operative use of aspirin in patients undergoing coronary artery bypass grafting: a systematic review and updated meta-analysis. J Thorac Dis 2018;10:3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hastings S, Myles P, McIlroy D. Aspirin and coronary artery surgery: a systematic review and meta-analysis. Br J Anaesth 2015;115:376–385. [DOI] [PubMed] [Google Scholar]
- 14. Hastings S, Myles P, McIlroy D. Aspirin and coronary artery surgery: an updated meta-analysis. Br J Anaesth 2016;116:716–717. [DOI] [PubMed] [Google Scholar]
- 15. Chakos A, Jbara D, Singh K, Yan TD, Tian DH. Network meta-analysis of antiplatelet therapy following coronary artery bypass grafting (CABG): none versus one versus two antiplatelet agents. Ann Cardiothorac Surg 2018;7:577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gupta S, Belley-Cote EP, Panchal P, Panchal P, Pandey A, Basha A, Pallo L, Rochwerg B, Mehta S, Schwalm J-D, Whitlock RP. Antiplatelet therapy and coronary artery bypass grafting: a systematic review and network meta-analysis. Interact Cardiovasc Thorac Surg 2020;31:354–363. [DOI] [PubMed] [Google Scholar]
- 17. Solo K, Lavi S, Kabali C, Levine GN, Kulik A, John-Baptiste AA, Fremes SE, Martin J, Eikelboom JW, Ruel M, Huitema AA, Choudhury T, Bhatt DL, Tzemos N, Mamas MA, Bagur R. Antithrombotic treatment after coronary artery bypass graft surgery: systematic review and network meta-analysis. BMJ 2019;367:l5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, deBeer H. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383–394. [DOI] [PubMed] [Google Scholar]
- 19. Nikolakopoulou A, Higgins JP, Papakonstantinou T, Del Giovane C, Egger M, Salanti G. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med 2020;17:e1003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng H-Y, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. Rob 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 21. McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and shiny web app for visualizing risk-of-bias assessments. Res Synth Methods 2021;12:55–61. [DOI] [PubMed] [Google Scholar]
- 22. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 2021;88:105906. [DOI] [PubMed] [Google Scholar]
- 23. Cumpston M, Li T, Page MJ, Welch VA, Higgins JP, Thomas J. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev 2019;2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Agnew T, Brandt P, French J, Kerr AR, Neutze JM, Webber BJ, Whitlock RML, Rutherford JD. The role of dipyridamole in addition to low dose aspirin in the prevention of occlusion of coronary artery bypass grafts. Aust N Z J Med 1992;22:665–670. [DOI] [PubMed] [Google Scholar]
- 25. Brooks N, Wright J, Sturridge M, Pepper J, Magee P, Walesby R, Layton C, Honey M, Balcon R. Randomised placebo controlled trial of aspirin and dipyridamole in the prevention of coronary vein graft occlusion. Heart 1985;53:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brown BG, Cukingnan R, DeRouen T, Goede LV, Wong M, Fee HJ, Roth JA, Carey JS. Improved graft patency in patients treated with platelet-inhibiting therapy after coronary bypass surgery. Circulation 1985;72:138–146. [DOI] [PubMed] [Google Scholar]
- 27. Chesebro JH, Fuster V, Elveback LR, Clements IP, Smith HC, Holmes DR, Bardsley WT, Pluth JR, Wallace RB, Puga FJ, Orszulak TA, Piehler JM, Danielson GK, Schaff HV, Frye RL. Effect of dipyridamole and aspirin on late vein-graft patency after coronary bypass operations. N Engl J Med 1984;310:209–214. [DOI] [PubMed] [Google Scholar]
- 28. Ekeström SA, Gunnes S, Brodin UB. Effect of dipyridamole (Persantin®) on blood flow and spatency of aortocoronary vein bypass grafts. Scand J Thorac Cardiovasc Surg 1990;24:191–196. [DOI] [PubMed] [Google Scholar]
- 29. Gao C, Ren C, Li D, Li L. Clopidogrel and aspirin versus clopidogrel alone on graft patency after coronary artery bypass grafting. Ann Thorac Surg 2009;88:59–62. [DOI] [PubMed] [Google Scholar]
- 30. Gavaghan TP, Gebski V, Baron D. Immediate postoperative aspirin improves vein graft patency early and late after coronary artery bypass graft surgery. A placebo-controlled, randomized study. Circulation 1991;83:1526–1533. [DOI] [PubMed] [Google Scholar]
- 31. Kim H-H, Yoo K-J, Youn Y-N. A randomized trial of clopidogrel vs ticagrelor after off-pump coronary bypass. Ann Thorac Surg 2023;115:1127–1134. [DOI] [PubMed] [Google Scholar]
- 32. Kulik A, Le May MR, Voisine P, Tardif J-C, DeLarochelliere R, Naidoo S, Wells GA, Mesana TG, Ruel M. Aspirin plus clopidogrel versus aspirin alone after coronary artery bypass grafting: the clopidogrel after surgery for coronary artery disease (CASCADE) trial. Circulation 2010;122:2680–2687. [DOI] [PubMed] [Google Scholar]
- 33. Kulik A, Abreu AM, Boronat V, Kouchoukos NT, Ruel M. Ticagrelor versus aspirin and vein graft patency after coronary bypass: a randomized trial. J Card Surg 2022;37:563–570. [DOI] [PubMed] [Google Scholar]
- 34. Mayer J Jr, Lindsay W, Castaneda W, Nicoloff D. Influence of aspirin and dipyridamole on patency of coronary artery bypass grafts. Ann Thorac Surg 1981;31:204–210. [DOI] [PubMed] [Google Scholar]
- 35. McEnany MT, Salzman EW, Mundth ED, DeSanctis RW, Warren Harthorne J, Weintraub RM, Gates S, Gerald Austen W. The effect of antithrombotic therapy on patency rates of saphenous vein coronary artery bypass grafts. J Thorac Cardiovasc Surg 1982;83:81–89. [PubMed] [Google Scholar]
- 36. Mulder B, Van der Doef R, Van der Wall E, Tijssen JGP, Piek JJ, Van Der Meer J, Dunning AJ. Effect of various antithrombotic regimens (aspirin, aspirin plus dipyridamole, anticoagulants) on the functional status of patients and grafts one year after coronary artery bypass grafting. Eur Heart J 1994;15:1129–1134. [DOI] [PubMed] [Google Scholar]
- 37. Rajah S, Nair U, Rees M, Saunders N, Walker D, Williams G, Critchley A, Beton D, Campbell C, Lawson RA, Rahman A, Nair KK, Dyet J, Kushwaha S, Rees A, Powell JD, Drake J. Effects of antiplatelet therapy with indobufen or aspirin-dipyridamole on graft patency one year after coronary artery bypass grafting. J Thorac Cardiovasc Surg 1994;107:1146–1153. [PubMed] [Google Scholar]
- 38. Tang Y, Fan X, Zhang B, Zhang J, Xue Q, Xu Z, Han L. Aspirin plus ticagrelor or clopidogrel on graft patency one year after coronary bypass grafting: a single-center, randomized, controlled trial. J Thorac Dis 2021;13:1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Une D, Al-Atassi T, Kulik A, Voisine P, Le May M, Ruel M. Impact of clopidogrel plus aspirin versus aspirin alone on the progression of native coronary artery disease after bypass surgery: analysis from the Clopidogrel After Surgery for Coronary Artery DiseasE (CASCADE) randomized trial. Circulation 2014;130:S12–S18. [DOI] [PubMed] [Google Scholar]
- 40. van der Meer J, Hillege HL, van Gilst W, Lie KI, Kootstra GJ, Ascoop CAPL, Pfisterer M. Prevention of one-year vein-graft occlusion after aortocoronary-bypass surgery: a comparison of low-dose aspirin, low-dose aspirin plus dipyridamole, and oral anticoagulants. Lancet 1993;342:257–264. [DOI] [PubMed] [Google Scholar]
- 41. Willemsen LM, Janssen PW, Peper J, Soliman-Hamad MA, van Straten AHM, Klein P, Hackeng CM, Sonker U, Bekker MWA, von Birgelen C, Brouwer MA, van der Harst P, Vlot EA, Deneer VHM, Chan Pin Yin DRPP, Gimbel ME, Beukema KF, Daeter EJ, Kelder JC, Tijssen JGP, Rensing BJWM, van Es HW, Swaans MJ, ten Berg JM. Effect of adding ticagrelor to standard aspirin on saphenous vein graft patency in patients undergoing coronary artery bypass grafting (POPular CABG): a randomized, double-blind, placebo-controlled trial. Circulation 2020;142:1799–1807. [DOI] [PubMed] [Google Scholar]
- 42. Zhou Z, Fu G, Feng K, Huang S, Chen G, Liang M, Wu Z. Randomized evidence on graft patency after off-pump versus on-pump coronary artery bypass grafting: an updated meta-analysis. Int J Surg 2022;98:106212. [DOI] [PubMed] [Google Scholar]
- 43. Deo SV, Dunlay SM, Shah IK, Altarabsheh SE, Erwin PJ, Boilson BA, Park SJ, Joyce LD. Dual anti-platelet therapy after coronary artery bypass grafting: is there any benefit? A systematic review and meta-analysis. J Card Surg 2013;28:109–116. [DOI] [PubMed] [Google Scholar]
- 44. Nocerino AG, Achenbach S, Taylor AJ. Meta-analysis of effect of single versus dual antiplatelet therapy on early patency of bypass conduits after coronary artery bypass grafting. Am J Cardiol 2013;112:1576–1579. [DOI] [PubMed] [Google Scholar]
- 45. Bomb R, Oliphant CS, Khouzam RN. Dual antiplatelet therapy after coronary artery bypass grafting in the setting of acute coronary syndrome. Am J Cardiol 2015;116:148–154. [DOI] [PubMed] [Google Scholar]
- 46. Benedetto U, Altman DG, Gerry S, Gray A, Lees B, Flather M, Taggart DP. Impact of dual antiplatelet therapy after coronary artery bypass surgery on 1-year outcomes in the arterial revascularization trial. Eur J Cardiothorac Surg 2017;52:456–461. [DOI] [PubMed] [Google Scholar]
- 47. Anastasius M, Lau JK, Hyun K, D'Souza M, Patel A, Rankin J, Walters D, Juergens C, Aliprandi-Costa B, Yan AT, Goodman SG, Chew D, Brieger D. The underutilisation of dual antiplatelet therapy in acute coronary syndrome. Int J Cardiol 2017;240:30–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this analysis are presented in Table 1 of this manuscript.