Abstract
PURPOSE
This multicenter, single-arm, open-label, phase Ib study was designed to determine the recommended phase II dose (RP2D) and to evaluate the safety and preliminary efficacy of unesbulin plus dacarbazine (DTIC) in patients with advanced leiomyosarcoma (LMS).
PATIENTS AND METHODS
Adult subjects with locally advanced, unresectable or metastatic, relapsed or refractory LMS were treated with escalating doses of unesbulin orally twice per week in combination with DTIC 1,000 mg/m2 intravenously (IV) once every 21 days. The time-to-event continual reassessment method was used to determine the RP2D on the basis of dose-limiting toxicities (DLTs) assessed during the first two 21-day treatment cycles. All explored doses of unesbulin (200 mg up to 400 mg) were in combination with DTIC. An expansion cohort was enrolled to evaluate the safety and efficacy of unesbulin at the RP2D.
RESULTS
Unesbulin 300 mg administered orally twice per week in combination with DTIC 1,000 mg/m2 IV once every 21 days was identified as the RP2D. On the basis of data from 27 subjects who were deemed DLT-evaluable, toxicity was higher in the unesbulin 400 mg group, with three of four subjects (75%) experiencing DLTs versus one of four subjects (25%) in the 200 mg group and three of 19 subjects (15.8%) in the 300 mg group. The most commonly reported DLTs and treatment-related grade 3 and 4 adverse events were thrombocytopenia and neutropenia. At the RP2D, seven subjects who were efficacy evaluable achieved partial response for an objective response rate of 24.1%.
CONCLUSION
Unesbulin 300 mg twice per week plus DTIC 1,000 mg/m2 once every 21 days was identified as the RP2D, demonstrating a favorable benefit-risk profile in a heavily pretreated population of adults with advanced LMS.
INTRODUCTION
Leiomyosarcoma (LMS) is an aggressive subtype of soft tissue sarcoma (STS), a rare and heterogeneous group of cancers of mesenchymal origin.1-3 LMS represents approximately 20%-25% of all STS.4-7 LMS is a cytogenetically complex sarcoma with numerous and often nonrecurrent chromosomal aberrations.8
CONTEXT
Key Objective
What dose of unesbulin in combination with dacarbazine (DTIC) provides the optimal benefit-risk profile in adult patients with locally recurrent, unresectable or metastatic, relapsed or refractory leiomyosarcoma (LMS)?
Knowledge Generated
A recommended oral dose of unesbulin 300 mg two times per week with intravenous DTIC 1,000 mg/m2 once every 21 days was identified on the basis of dose-limiting toxicities reported during the first two treatment cycles (6 weeks). At the recommended dose of 300 mg, the objective response rate and disease control rate were 24.1% and 55.2%, respectively.
Relevance (R.G. Maki)
This phase I trial demonstrates notable activity of the combination of DTIC with the microtubule active agent unesbulin in LMS patients, who lack therapy that provides durable benefit. Whether the activity of the combination is greater than DTIC alone is the topic of a phase III trial.*
*Relevance section written by JCO Associate Editor Robert G. Maki, MD, PhD, FACP, FASCO.
Surgical resection with or without radiation is the standard treatment for patients with localized LMS independent of the site of origin. For patients with locally advanced or metastatic disease, first-line treatment includes anthracycline-based regimens such as doxorubicin with or without ifosfamide or trabectedin, or gemcitabine plus docetaxel.9,10 Commonly used second-line or later-line treatments include trabectedin and pazopanib.9-11 However, these agents have shown limited improvement in median progression-free survival (<5 months) with no significant improvement in overall survival.12,13
Unesbulin is an orally bioavailable, small molecule that binds to a unique site within the colchicine-binding region of tubulin that results in destabilization of tubulin polymers and microtubules.14,15 Preclinically, unesbulin exhibits broad-spectrum anticancer activity. The antitumor efficacy of unesbulin as a single agent and in combination with other standard-of-care anticancer agents, including dacarbazine (DTIC), has been demonstrated in studies using cell-line xenograft models of LMS.14 The combination of unesbulin and DTIC demonstrated a synergistic antitumor effect and was more effective than either agent administered as monotherapy. These findings prompted this phase Ib study that evaluated the combination of unesbulin and DTIC in patients with advanced LMS.
The primary objectives of the present phase Ib study were to determine the maximum tolerated dose (MTD), recommended phase II dose (RP2D), and safety of unesbulin plus DTIC in patients with locally advanced, unresectable or metastatic, relapsed or refractory LMS. The secondary objectives were to evaluate the antitumor activity and the pharmacokinetics (PK) of unesbulin, DTIC, and 5-amino-imidazole-4-carboxamide, the inactive metabolite of DTIC. This report presents the safety and efficacy results from this study as of May 2023.
PATIENTS AND METHODS
Patient Selection
Eligible patients were age 18 years and older with locally advanced, unresectable or metastatic, relapsed or refractory LMS of any anatomic site or origin who previously received at least one previous systemic cytotoxic therapy (ClinicalTrials.gov identifier: NCT03761095). Patients were required to have adequate bone marrow, renal, pulmonary, and liver functions, and an Eastern Cooperative Oncology Group performance status score of ≤1. Previous treatment with DTIC was allowed. Patients with major comorbidities that might jeopardize patient safety, the study procedures, or interpretation of the data were excluded.
Ethics
The study was conducted in accordance with the Declaration of Helsinki, the International Council for Harmonisation Principles of Good Clinical Practice, and applicable regulatory requirements. The study was approved by the respective institutional review boards of each participating site. All patients provided written informed consent before any study-related procedures.
Study Design and Treatment
This multicenter, single-arm, open-label, phase Ib study was conducted at four sites in the United States. Subjects were treated with unesbulin orally twice per week in combination with DTIC administered intravenously (IV) once every 21 days. DTIC was administered on day 1 and unesbulin was administered on days 2, 5, 8, 12, 15, and 19 of each 21-day treatment cycle in a staggered manner to minimize unesbulin inhibition on DTIC metabolism.
This study used the time-to-event continual reassessment method (TITE-CRM) for dose finding.16 For each subject enrolled in the dose-escalation portion of the study, dose-limiting toxicities (DLTs) were assessed over the course of the first two treatment cycles (6 weeks). DLTs were defined as adverse events (AEs) that were assessed as being possibly, probably, or definitely related to study treatment administration and not due to the underlying malignancy. Protocol-defined DLTs included any grade 3 or higher nonhematologic toxicity (except fatigue, nausea, vomiting, and/or diarrhea, which must have persisted 5 or more days despite optimal supportive care to be considered dose limiting), any grade 4 or higher hematologic toxicity that persisted more than 7 days, grade 3 neutropenia with fever, grade 3 thrombocytopenia with clinically significant bleeding, abnormal liver function testing, any other laboratory abnormality that was associated with clinical sequelae or did not resolve to grade 1 or lower in fewer than 3 days, and any event leading to a dose reduction in unesbulin or permanent discontinuation of study treatment. The use of supportive treatments, including filgrastim, pegfilgrastim, and romiplostim, was permitted in the study.
The first subject accrued to the study was assigned unesbulin 200 mg orally twice per week plus DTIC 1,000 mg/m2 IV once every 21 days. For subsequent subjects, the assigned dose was selected on the basis of the TITE-CRM up to a maximum dose of unesbulin 400 mg twice per week plus DTIC 1,000 mg/m2 IV once every 21 days. Criteria for dose reductions and dose delays because of treatment-associated toxicities were standardized in the protocol.
An expansion cohort was added for a food effect assessment and to further evaluate the safety and antitumor activity of unesbulin at the RP2D. Twelve subjects in the expansion cohort were analyzed for both safety and efficacy but not for the determination of the RP2D. Results of the food effect assessment will be reported separately.
End Points
The primary end points include (1) MTD and RP2D of unesbulin in combination with DTIC as determined using the TITE-CRM for dose finding, and (2) type, frequency, severity, and timing of AEs and their relation to the study treatment(s). Secondary efficacy end points per RECIST version 1.1 include objective response rate (ORR; defined as a confirmed best overall response of complete response [CR] or partial response [PR]) and disease control (defined as attaining a best overall response of CR, PR, or at least 3 months of stable disease [SD]).
Assessments
Safety and tolerability were assessed by monitoring of AEs, clinical laboratory tests, including complete blood count and comprehensive metabolic panel, vital signs, physical examination, and electrocardiograms.
Tumor response was evaluated using RECIST version 1.1. Subjects were assessed by the same imaging procedure (magnetic resonance imaging or computed tomography) throughout the study, performed every 6 weeks until cycle 9 and then every 9 weeks.
Statistical Analysis
The MTD was defined as the dose associated with a target probability of DLT of 0.25. For each subject, DLTs were assessed over the course of the first two treatment cycles; partial toxicity information from patients who received 80% or more of the planned unesbulin doses was included for dose assignments for newly enrolled subjects and estimation of the MTD. At the end of the study, the final MTD for the combination was estimated using the TITE-CRM on the basis of the proportion of DLT at each dose level.16
The efficacy population included subjects who received at least 50% of two cycles of treatment with unesbulin (ie, six doses), received at least two doses of DTIC, and had a baseline and at least one postbaseline response assessment using RECIST version 1.1 criteria; or subjects who died before cycle 3 day 1 and received at least one dose of unesbulin. Best overall response per RECIST version 1.1 was evaluated locally per investigator assessment. ORR was defined as the proportion of subjects who achieved a confirmed best overall response of CR or PR per investigator assessment, and disease control rate (DCR) was defined as the proportion of subjects with best overall response of CR, PR, or at least 3 months of SD. CIs for ORR and DCR were based on the Clopper-Pearson method.
All subjects who received at least one dose of unesbulin or DTIC were analyzed for safety. The frequency and count of subjects experiencing a specific AE were summarized by grade. Results of PK analyses are reported separately.
RESULTS
Patients and Analysis Populations
Fifty-six subjects with advanced LMS were screened and 41 subjects were enrolled between April 4, 2019 (first subject, first visit), and October 19, 2022 (last subject, first visit). Analyses were performed using a data cutoff date of May 8, 2023.
Twenty-nine subjects were initially enrolled and treated in the dose-finding part of the study; 27 of these initial 29 subjects were considered DLT-evaluable per protocol. The DLT-evaluable cohort included four subjects treated with unesbulin 200 mg twice per week, 19 subjects treated with unesbulin 300 mg twice per week, and four subjects treated with unesbulin 400 mg, as per the TITE-CRM, administered in 21-day treatment cycles. All subjects received unesbulin in combination with DTIC.
The expansion cohort included the subsequent 12 enrolled subjects who were treated with the RP2D of unesbulin in combination with DTIC.
Demographics and Baseline Disease Characteristics
Demographic and baseline disease characteristics for the overall subject population are summarized in Table 1. The majority of patients were White (n = 29 [70.7%]) followed by Black or African American (n = 6 [14.6%]). The median age of the overall study population was 62 years, with a range from 32 to 73 years. The overall study population (n = 41) included a greater number of female subjects (n = 34 [82.9%]) than male subjects (n = 7 [17.1%]). Within the subpopulation of subjects with nonuterine LMS (n = 20), there was also a greater proportion of female than male subjects (13 [65%] v 7 [35%]).
TABLE 1.
Demographic and Baseline Disease Characteristics by Dose Level
| Characteristic | Unesbulin 200 mg + DTIC 1,000 mg/m2 (n = 4) | Unesbulin 300 mg + DTIC 1,000 mg/m2 (n = 33)a | Unesbulin 400 mg + DTIC 1,000 mg/m2 (n = 4) | All Subjects (N = 41) |
|---|---|---|---|---|
| Age, years | ||||
| Mean (SD) | 67.8 (4.50) | 58.1 (10.74) | 61.8 (7.80) | 59.4 (10.36) |
| Median | 67.0 | 61.0 | 62.0 | 62.0 |
| Min-max | 64-73 | 32-73 | 52-71 | 32-73 |
| Sex, No. (%) | ||||
| Male | 0 | 7 (21.2) | 0 | 7 (17.1) |
| Female | 4 (100.0) | 26 (78.8) | 4 (100.0) | 34 (82.9) |
| Race, No. (%)b | ||||
| White | 2 (50.0) | 23 (69.7) | 4 (100.0) | 29 (70.7) |
| Black or African American | 1 (25.0) | 5 (15.2) | 0 | 6 (14.6) |
| Asian | 0 | 1 (3.0) | 0 | 1 (2.4) |
| Multiple | 0 | 1 (3.0) | 0 | 1 (2.4) |
| Other | 0 | 2 (6.1) | 0 | 2 (4.9) |
| Not reported | 1 (25.0) | 1 (3.0) | 0 | 2 (4.9) |
| Weight at baseline, kg | ||||
| Mean (SD) | 72.85 (10.206) | 81.49 (20.384) | 93.65 (37.723) | 81.83 (21.658) |
| Min-max | 62.8-84.6 | 44.7-122.0 | 54.8-143.0 | 44.7-143.0 |
| BMI at baseline, kg/m2c | ||||
| Mean (SD) | 29.48 (3.672) | 29.42 (6.976) | 33.78 (15.406) | 29.85 (7.710) |
| Min-max | 25.8-34.5 | 17.5-46.0 | 21.2-55.2 | 17.5-55.2 |
| ECOGd | ||||
| 0 | 3 (75.0) | 21 (63.6) | 0 | 24 (58.5) |
| 1 | 1 (25.0) | 12 (36.4) | 4 (100.0) | 17 (41.5) |
| Type of LMS, No. (%) | ||||
| Uterine | 1 (25.0) | 19 (57.6) | 1 (25.0) | 21 (51.2) |
| Nonuterine | 3 (75.0) | 14 (42.4) | 3 (75.0) | 20 (48.8) |
| Male | 0 | 7 (50.0) | 0 | 7 (35.0) |
| Female | 3 (100) | 7 (50.0) | 3 (100) | 13 (65.0) |
| Previous lines of therapy, No. (%) | ||||
| 1 | 1 (25.0) | 3 (9.1) | 1 (25.0) | 5 (12.2) |
| 2 | 1 (25.0) | 5 (15.2) | 0 | 6 (14.6) |
| 3 | 0 | 9 (27.3) | 2 (50.0) | 11 (26.8) |
| 4 or more | 2 (50.0) | 16 (48.5) | 1 (25.0) | 19 (46.3) |
| Previous anticancer therapy, No. (%) | ||||
| Anthracycline | 4 (100) | 31 (93.9) | 4 (100) | 39 (95.1) |
| Gemcitabine | 3 (75.0) | 26 (78.8) | 3 (75.0) | 32 (78.0) |
| Taxane | 3 (75.0) | 25 (75.8) | 2 (50.0) | 30 (73.2) |
| Trabectedin | 2 (50.0) | 12 (36.4) | 0 | 14 (34.1) |
| DTIC | 0 | 4 (12.1) | 0 | 4 (9.8) |
| Pazopanib | 2 (50.0) | 10 (30.3) | 0 | 12 (29.3) |
Abbreviations: DTIC, dacarbazine; ECOG, Eastern Cooperative Oncology Group; LMS, leiomyosarcoma; max, maximum; min, minimum; SD, standard deviation.
Includes all subjects who were treated with unesbulin 300 mg, including subjects who participated in the dose-finding and expansion parts of the study.
More than one choice could be selected, so percentages may total >100%.
BMI was calculated as the individual’s body mass divided by the square of their height, with the value universally being given in units of kg/m2.
ECOG score: 0 = fully active, able to carry on all predisease performance without restriction; 1 = restricted in physically strenuous activity, but ambulatory and able to carry out work of a light or sedentary nature (eg, light housework, office work); 2 = ambulatory and capable of all self-care, but unable to carry out any work activities; up and about more than 50% of waking hours; 3 = capable of only limited self-care, confined to bed or chair more than 50% of waking hours; 4 = completely disabled; cannot carry on any self-care; totally confined to bed or chair; 5 = dead.
The study population was heavily pretreated (Table 1). All subjects had received previous systemic therapy for LMS. The median number of previous lines of systemic therapy was three, and 19 of 41 subjects (46.3%) had four or greater previous lines of treatment. The three most reported previous anticancer therapies included anthracycline (95.1%), gemcitabine (78.0%), and taxane (73.2%; Table 1). Four subjects (9.8%) had previous treatment with DTIC.
Time on Study Treatments
Of the 41 subjects enrolled and treated, 37 subjects (90.2%) had discontinued the study treatment. Disease progression was the most common reason for discontinuation (29 [70.7%]), followed by AE (2 [4.9%]), withdrawal of consent (2 [4.9%]), investigator decision (2 [4.9%]), and death (1 [2.4%]). Reason for study treatment discontinuation was not reported for one subject.
Twenty-eight subjects (68.3%) completed at least two treatment cycles, and 21 subjects (51.2%) completed more than four cycles of treatment.
Safety and Tolerability
The TITE-CRM determined a RP2D of unesbulin 300 mg twice per week on the basis of DLTs reported during the first two treatment cycles (6 weeks) of subjects in the DLT-evaluable cohort (Table 2). Unesbulin 400 mg was associated with DLTs in three of four subjects (75%) versus one of four subjects (25%) in the 200 mg group and three of 19 subjects (15.8%) in the 300 mg group. Except for one case each of sepsis and gastric perforation, all other DLTs were either grade 3 or 4 thrombocytopenia or neutropenia. All DLT events resolved within 7 days except in three cases: gastric perforation, thrombocytopenia, and sepsis.
TABLE 2.
Summary of DLTs by Dose Level—DLT-Evaluable Cohort
| Variable | Unesbulin Dose Level | ||
|---|---|---|---|
| 200 mg | 300 mg | 400 mg | |
| Subjects, No. | 4 | 19 | 4 |
| Subjects with DLTs, No. (%) | 1 (25) | 3 (15.8) | 3 (75) |
| DLT event | Thrombocytopenia (grade 3) | Thrombocytopenia (grade 3) and neutropenia (grade 4) | Neutropenia (grade 4) and thrombocytopenia (grade 3) |
| Neutropenic fever and sepsis (grade 3) | Neutropenia (grade 4) | ||
| Gastric perforation (grade 3) | Thrombocytopenia (grade 4) and clinically significant bleeding | ||
NOTE. A DLT event was assessed as per DLT definitions in version 5.0 of the protocol. The subject with GI perforation had a history of GI perforation and was receiving ibuprofen and diclofenac.
Abbreviation: DLT, dose-limiting toxicity.
All 41 treated subjects reported at least one TEAE in this study, and 35 subjects (85.4%) had at least one grade 3 or grade 4 TEAE (Table 3). The most commonly reported (>20%) grade 3/4 TEAEs included neutrophil count decreased (51.2%), platelet count decreased (46.3%), WBC count decreased (31.7%), anemia (24.4%), and lymphocyte count decreased (22.0%; Table 3). These commonly reported events, along with fatigue, diarrhea, nausea, vomiting, and decreased appetite, were often assessed as related to study treatment (unesbulin and/or DTIC). A similar pattern and incidence of TEAEs were reported at the RP2D.
TABLE 3.
Summary of TEAEs Reported by >10% of All Treated Subjects and Grade 3/4 Treatment-Emergent Laboratory Abnormalities Reported by >5% of All Treated Subjects
| Adverse Event Category and Term | Unesbulin 200 mg + DTIC 1,000 mg/m2 (n = 4), No. (%) | Unesbulin 300 mg + DTIC 1,000 mg/m2 (n = 33), No. (%) | Unesbulin 400 mg + DTIC 1,000 mg/m2 (n = 4), No. (%) | All Treated Subjects (N = 41), No. (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 3 | Grade 4 | All | Grade 3 | Grade 4 | All | Grade 3 | Grade 4 | All | Grade 3 | Grade 4 | All | |
| Subjects with any TEAE | 2 (50.0) | 1 (25.0) | 4 (100) | 18 (54.5) | 10 (30.3) | 33 (100) | 0 | 4 (100) | 4 (100) | 20 (48.8) | 15 (36.6) | 41 (100) |
| Blood and lymphatic system disorders | ||||||||||||
| Anemia | 1 (25.0) | 0 | 1 (25.0) | 7 (21.2) | 0 | 16 (48.5) | 2 (50.0) | 0 | 2 (50.0) | 10 (24.4) | 0 | 19 (46.3) |
| GI | ||||||||||||
| Diarrhea | 0 | 0 | 1 (25.0) | 2 (6.1) | 0 | 19 (57.6) | 0 | 0 | 2 (50.0) | 2 (4.9) | 0 | 22 (53.7) |
| Nausea | 0 | 0 | 1 (25.0) | 1 (3.0) | 0 | 18 (54.5) | 0 | 0 | 1 (25.0) | 1 (2.4) | 0 | 20 (48.8) |
| Vomiting | 0 | 0 | 0 | 1 (3.0) | 0 | 9 (27.3) | 0 | 0 | 0 | 1 (2.4) | 0 | 9 (22.0) |
| Constipation | 0 | 0 | 0 | 0 | 0 | 6 (18.2) | 0 | 0 | 1 (25.0) | 0 | 0 | 7 (17.1) |
| Abdominal distension | 0 | 0 | 1 (25.0) | 0 | 0 | 4 (12.1) | 0 | 0 | 1 (25.0) | 0 | 0 | 6 (14.6) |
| Abdominal pain | 0 | 0 | 0 | 0 | 0 | 4 (12.1) | 0 | 0 | 2 (50.0) | 0 | 0 | 6 (14.6) |
| General disorders and administration site conditions | ||||||||||||
| Fatigue | 0 | 0 | 1 (25.0) | 4 (12.1) | 0 | 20 (60.6) | 0 | 0 | 3 (75.0) | 4 (9.8) | 0 | 24 (58.5) |
| Investigations | ||||||||||||
| Platelet count decreased | 1 (25.0) | 1 (25.0) | 3 (75.0) | 9 (27.3) | 5 (15.2) | 19 (57.6) | 0 | 3 (75.0) | 4 (100) | 10 (24.4) | 9 (22.0) | 26 (63.4) |
| Neutrophil count decreased | 1 (25.0) | 1 (25.0) | 2 (50.0) | 10 (30.3) | 6 (18.2) | 19 (57.6) | 0 | 3 (75.0) | 4 (100) | 11 (26.8) | 10 (24.4) | 25 (61.0) |
| Lymphocyte count decreased | 1 (25.0) | 0 | 2 (50.0) | 8 (24.2) | 0 | 16 (48.5) | 0 | 0 | 0 | 9 (22.0) | 0 | 18 (43.9) |
| WBC count decreased | 2 (50.0) | 0 | 2 (50.0) | 6 (18.2) | 1 (3.0) | 12 (36.4) | 4 (100) | 0 | 4 (100) | 12 (29.3) | 1 (2.4) | 18 (43.9) |
| Blood creatinine increased | 0 | 0 | 1 (25.0) | 0 | 0 | 5 (15.2) | 0 | 0 | 0 | 0 | 0 | 6 (14.6) |
| Cardiac disorders | ||||||||||||
| Sinus tachycardia | 0 | 0 | 0 | 0 | 0 | 4 (12.1) | 0 | 0 | 3 (75.0) | 0 | 0 | 7 (17.1) |
| Metabolism and nutrition disorders | ||||||||||||
| Decreased appetite | 0 | 0 | 1 (25.0) | 0 | 0 | 5 (15.2) | 0 | 0 | 1 (25.0) | 0 | 0 | 7 (17.1) |
| Musculoskeletal and connective tissue disorders | ||||||||||||
| Bone pain | 0 | 0 | 0 | 0 | 0 | 6 (18.2) | 0 | 0 | 1 (25.0) | 0 | 0 | 7 (17.1) |
| Myalgia | 0 | 0 | 0 | 0 | 0 | 2 (6.1) | 0 | 0 | 3 (75.0) | 0 | 0 | 5 (12.2) |
| Pain in extremity | 0 | 0 | 0 | 1 (3.0) | 0 | 4 (12.1) | 0 | 0 | 1 (25.0) | 1 (2.4) | 0 | 5 (12.2) |
| Nervous system disorders | ||||||||||||
| Headache | 0 | 0 | 0 | 0 | 0 | 5 (15.2) | 0 | 0 | 1 (25.0) | 0 | 0 | 6 (14.6) |
| Respiratory, thoracic, and mediastinal disorders | ||||||||||||
| Dyspnea | 0 | 0 | 0 | 0 | 0 | 5 (15.2) | 0 | 0 | 0 | 0 | 0 | 5 (12.2) |
| Vascular disorders | ||||||||||||
| Hypertension | 0 | 0 | 0 | 0 | 0 | 3 (9.1) | 0 | 0 | 2 (50.0) | 0 | 0 | 5 (12.2) |
NOTE. The number of subjects for each column, and the denominator for all percentages, is the number of subjects in the safety population by group. TEAE is defined as an AE with an onset date or worsening date on or after the date on which unesbulin or DTIC was first administered and within 30 days after the last dose. If a subject experienced multiple AEs under the same SOC, the subject was counted only once for the SOC with the greatest severity. Similarly, if a subject experienced multiple AEs under the same PT and SOC, the subject was counted only once for the PT with the greatest severity. The AE toxicity was graded on the basis of NCI CTCAE version 5.0. If a subject experienced the same AE multiple times, the maximum grade that the subject experienced the event was summarized. A DLT was defined as one of the AEs (listed in the protocol) assessed as being possible or probably related to study treatment administration that was not because of the underlying malignancy and had no clear evidence of an alternative etiology in the opinion of the investigator.
Abbreviations: AE, adverse event; DLT, dose-limiting toxicity; DTIC, dacarbazine; NCI CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events; PT, preferred term; SOC, System Organ Class; TEAE, treatment-emergent adverse event.
There were no reported treatment-related deaths in the study. Treatment-emergent serious adverse events (SAEs) were reported for 14 (34.1%) subjects. Most SAEs were considered unrelated to study treatment except platelet count decreased and sepsis in two subjects each, and vomiting, hemoglobin decreased, and gastric perforation in one subject each.
Efficacy
There were 37 subjects evaluable for efficacy (Fig 1). At the time of the data cutoff, two subjects had been treated for more than 1 year, including one who had been in the study for more than 2 years. Eight subjects (21.6%) had achieved a PR, including seven subjects treated with unesbulin 300 mg and one subject treated with unesbulin 200 mg.
FIG 1.
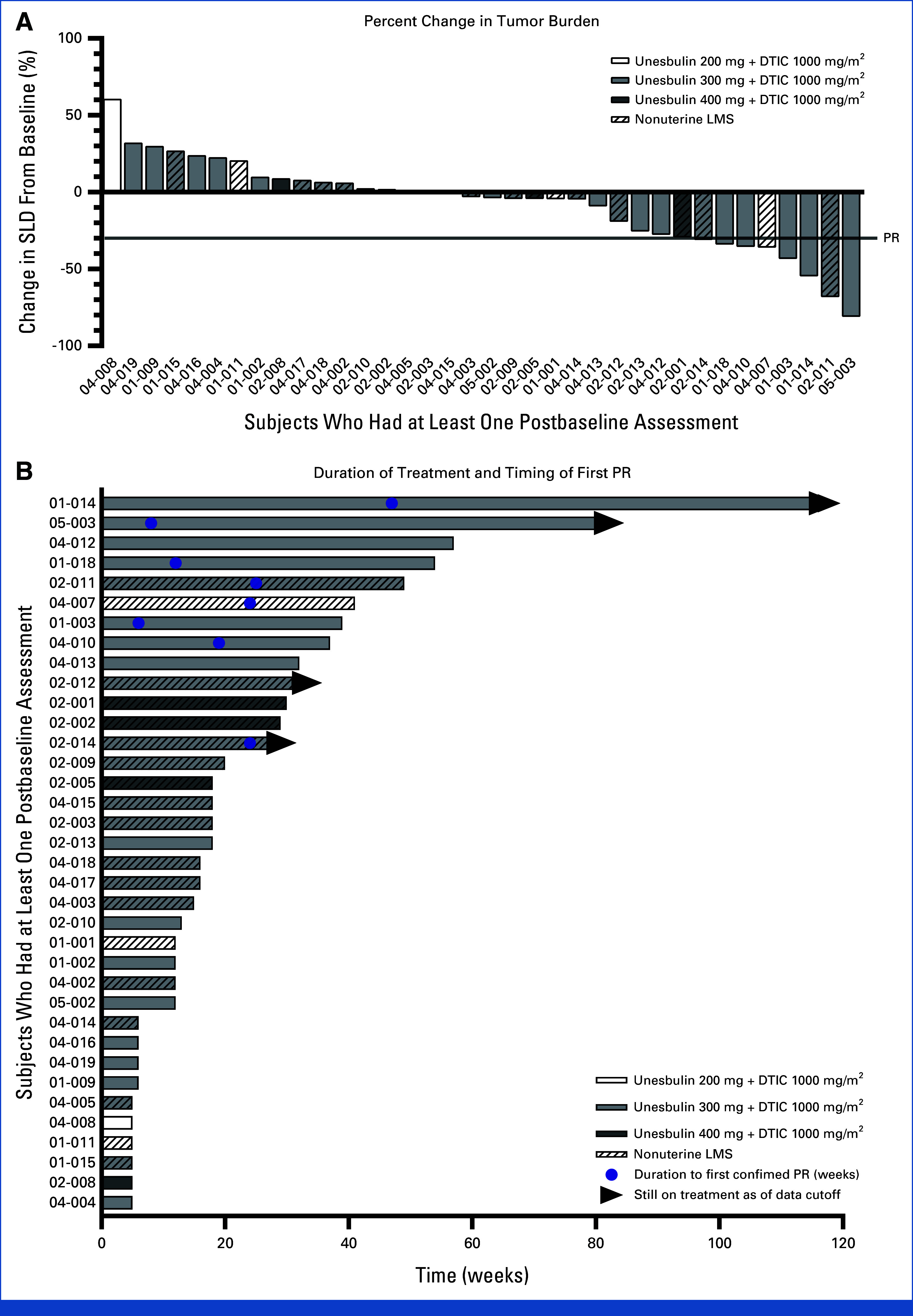
Best tumor reduction and time on treatment by unesbulin (PTC596) dose level—efficacy population. (A) The waterfall plot is best tumor reduction from baseline in sum of diameters of all target lesions in the efficacy population. (B) Swimmer chart depicts time on treatment for the efficacy population. Among the 37 treated subjects, the following subject did not have a percent change of diameter noted: 01-010. Subjects with nonuterine LMS are designated with diagonal lines within the bars. Time on treatment (weeks) was measured as the time in weeks from the first dose date to the last dose date calculated as (last dose date – first dose date + 1). DTIC, dacarbazine; LMS, leiomyosarcoma; PR, partial response; SLD, sum of longest diameters.
In the overall efficacy population, the ORR was 21.6% (8/37 subjects), and the DCR (defined as the percentage of subjects with CR, PR, and SD for ≥3 months) was 54.1% (20/37 subjects). At the RP2D of 300 mg (n = 29), the ORR and DCR were 24.1% and 55.2%, respectively. Previous anticancer treatment had no meaningful effect on ORR (anthracycline [22.9%; 95% CI, 10.4 to 40.1], gemcitabine [25.0%; 95% CI, 10.7 to 44.9], taxane [23.1%; 95% CI, 9 to 43.6], trabectedin [23.1%; 95% CI, 5.0 to 53.8], and pazopanib [16.7%; 95% CI, 2.1 to 48.4]). The number of subjects with previous exposure to DTIC was too limited to provide any meaningful interpretation.
Although no subjects in the study achieved CR, most subjects in the unesbulin 300 mg treatment group (22 of 29 [75.8%]) and all four subjects (100%) in the unesbulin 400 mg treatment group achieved a BOR of SD or PR compared with two of four subjects (50%) in the 200 mg group.
Antitumor activity was observed in subjects with uterine and nonuterine LMS. Of the 19 subjects who had a decrease in tumor lesion diameters from baseline, 10 had nonuterine LMS (Fig 1). The ORR was higher among subjects with uterine LMS than nonuterine LMS in the efficacy population (27.8% [5/18] v 15.8% [3/19]). However, the DCR was higher among subjects with nonuterine LMS than uterine LMS (57.9% [11/19] v 50% [9/18]).
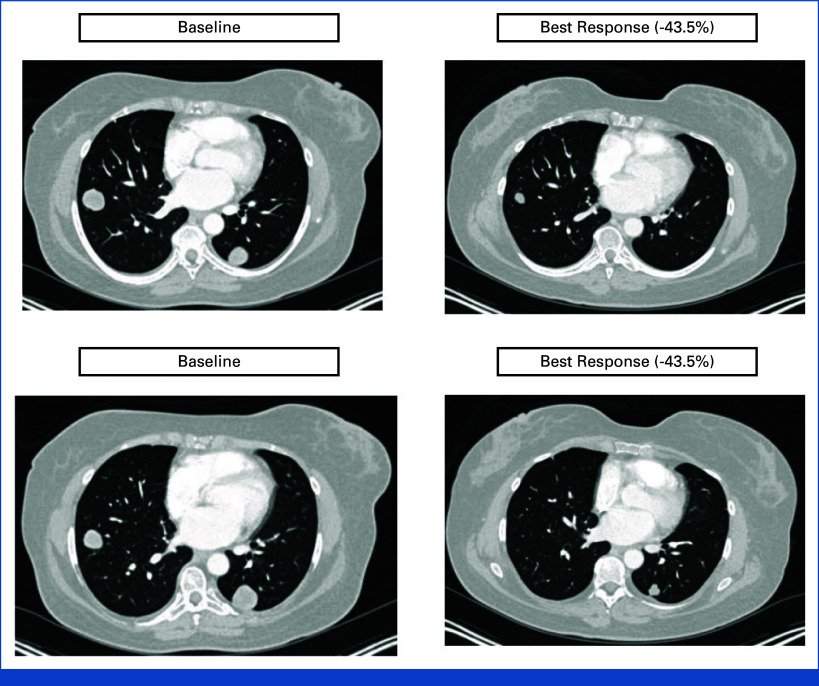
A best overall tumor reduction of 43.5%, including resolution of several sites of metastatic disease in the lung, was observed in one subject with high-grade uterine LMS who was treated with 12 cycles of unesbulin 300 mg and DTIC 1,000 mg/m2 (Fig 2). The subject completed 12 cycles of treatment with a clinically significant response and resolution of several sites of metastatic disease in the lung. After an interdisciplinary discussion, the subject was removed from the study to undergo surgery, rendering her radiographically disease-free. She remained off all other systemic therapies for more than 2 years after stopping unesbulin/DTIC, and when a relapse occurred, she received a different experimental therapy.
FIG 2.
Tumor CT scan at baseline and after 12 cycles of unesbulin in combination with dacarbazine. CT scan of tumor lesions (left lower lobe [top] and right lower lobe [bottom]) from a 61-year-old woman diagnosed with an unresectable 13.5-cm high-grade uterine leiomyosarcoma at baseline (left) and at best response (–43.5%) after approximately 24 weeks of treatment (right). CT, computed tomography.
DISCUSSION
This phase I study using TITE-CRM determined a RP2D of unesbulin 300 mg twice per week administered in combination with DTIC 1,000 mg/m2 once every 21 days. At the 400 mg dose, three of four subjects (75%) experienced DLTs versus one of four subjects (25%) in the 200 mg group and three of 19 subjects (15.8%) in the 300 mg group. Higher rates of AEs, including grade 4 thrombocytopenia and neutropenia, were observed in the 400 mg dose group compared with the 300 mg dose.
This open-label clinical study supports a favorable benefit-risk profile for unesbulin for the treatment of advanced LMS after failure of previous cytotoxic chemotherapy. Unesbulin in combination with DTIC was well tolerated. There were no unexpected safety findings. Although peripheral neurotoxicity is a well-known toxicity associated with chemotherapeutic agents, including tubulin inhibitors, only one case of grade 1 and 2 cases of grade 2 peripheral neuropathy were reported in this study.
Consistent with a heavily pretreated population with advanced disease, grade 3 to 4 toxicities were primarily observed in laboratory-based measures of myelotoxicity. Hematologic toxicities were mitigated and well managed through adjustments in DTIC and supportive care, as needed. Other commonly reported treatment-related AEs included fatigue, nausea, diarrhea, vomiting, and decreased appetite. The nature of the toxicities observed in this study is generally consistent with what has been previously reported for DTIC monotherapy in this patient population with advanced disease.13,17
Although the majority of subjects in the study had received three or more previous lines of systemic therapy, antitumor activity was seen at all unesbulin dose levels and in subjects with uterine and nonuterine LMS. In the overall efficacy population, the ORR was 21.6% and the DCR was 54.1%. At the 300 mg dose level, the ORR and DCR were 24.1% and 55.2%, respectively. The ORR and DCR were 27.8% and 50%, respectively, in subjects with uterine LMS and 15.8% and 57.9%, respectively, in subjects with nonuterine LMS. In comparison, other treatments (trabectedin, pazopanib, and DTIC) for unresectable or metastatic, relapsed or refractory LMS have demonstrated an ORR range of between 7% and 11%.12,13,18
Unesbulin demonstrates favorable pharmacologic properties and an attractive PK profile, including a half-life suitable for twice per week dosing and an effective biodistribution.15,19 In a phase I, dose-escalation study in subjects with advanced solid tumors, unesbulin was rapidly absorbed, reaching Tmax at 2-4 hours after dosing with no accumulation after multiple administrations up to 7.0 mg/kg.19 Another important pharmacologic feature of unesbulin is the lack of P-glycoprotein (P-gp) substrate activity.15 P-gp has been implicated in chemoresistance.20-22 Therefore, the antitumor activity of unesbulin is not expected to diminish with long-term use.
In conclusion, the results of this interim analysis indicate a favorable benefit-risk profile for unesbulin 300 mg twice per week in combination with DTIC once every 21 days in a heavily pretreated population of patients with advanced LMS. On the basis of the positive findings from this study, an international, multicenter, randomized, double-blind, placebo-controlled phase II/III study (SUNRISE-LMS, ClinicalTrials.gov identifier: NCT05269355) is ongoing to compare the safety and efficacy of unesbulin plus DTIC versus placebo plus DTIC in 345 adult subjects with unresectable or metastatic, relapsed or refractory LMS who have received at least one previous line of systemic therapy.
ACKNOWLEDGMENT
The authors thank the patients who volunteered to participate in this study and their families; the investigators, coordinators, and study teams at each of the clinical sites; and the sponsor staff involved in data collection and analyses. This manuscript is dedicated in memory of Dr Mona Wahba, who was an instrumental contributor to the study and passionate about the lives of the patients for whom this study was conducted.
Brian A. Van Tine
Leadership: Polaris
Honoraria: Iterion Therapeutics, Inc, Total Health Conference
Consulting or Advisory Role: Epizyme, Daiichi Sankyo, Bayer, Deciphera, ADRx, Ayala Pharmaceuticals, Intellisphere, PTC Therapeutics, Boehringer Ingelheim, EcoR1 Capital, Deciphera Pharmaceuticals, Advenchen Laboratories, Putnam Associates, Salarius Pharmaceuticals, Inc, Boxer Capital LLC, Acuta Capital Partners, LLC, Aadi, Race Oncology, Hinge Bio, Inc, Kronos Bio, Inc, Agenus, Regeneron, Curis
Research Funding: Pfizer, Merck, TRACON Pharma, GlaxoSmithKline, Polaris
Patents, Royalties, Other Intellectual Property: Patent on the use of ME1 as a biomarker, Patent on ALEXT3102, Accuronix Therapeutics-Licensing agreement. Sigma-2 Receptor Ligands and Therapeutic uses therefor (006766), Modular Platform for Targeted Therapeutic Delivery (006755), Sigma-2 Receptor Ligand Drug Conjugates as Antitumor Compounds, Methods of synthesis and Uses Thereof (014229)
Expert Testimony: Health Advances
Travel, Accommodations, Expenses: Adaptimmune, Advenchen Laboratories, Kronos Bio, Inc
Matthew A. Ingham
Employment: Regeneron
Stock and Other Ownership Interests: Regeneron
Consulting or Advisory Role: Daiichi Sankyo, Xencor, Apexigen, Epizyme, Caris Life Sciences
Research Funding: Apexigen (Inst), Mirati Therapeutics (Inst), PTC Therapeutics (Inst), Apices, Intensity (Inst), Boehringer Ingelheim (Inst), BioAtla (Inst), Merck (Inst), Astellas Pharma (Inst), AstraZeneca (Inst)
Steven Attia
Research Funding: TRACON Pharma (Inst), Bayer (Inst), Novartis (Inst), Lilly (Inst), Karyopharm Therapeutics (Inst), Epizyme (Inst), Blueprint Medicines (Inst), Genmab (Inst), CBA Pharma (Inst), Desmoid Tumor Research Foundation, Merck (Inst), Philogen (Inst), Gradalis (Inst), Deciphera (Inst), Takeda (Inst), Incyte (Inst), SpringWorks Therapeutics (Inst), Adaptimmune (Inst), Advenchen Laboratories (Inst), Bavarian Nordic (Inst), BTG (Inst), PTC Therapeutics (Inst), GlaxoSmithKline (Inst), FORMA Therapeutics (Inst), Ayala Pharmaceuticals (Inst), Trillium Therapeutics (Inst), Boehringer Ingelheim (Inst), Salarius Pharmaceuticals (Inst), Theseus Pharmaceuticals (Inst), Monopar Therapeutics (Inst), C4 Therapeutics (Inst), InhibRx (Inst), Noxopharm (Inst), Rain Therapeutics (Inst), Guardant Health, Cogent Biosciences (Inst), Jazz Pharmaceuticals (Inst), Shanghai Pharma (Inst), PharmaMar (Inst)
Christian F. Meyer
Consulting or Advisory Role: Deciphera, Intellisphere, Aadi
Speakers’ Bureau: Novartis
Other Relationship: UpToDate
Uncompensated Relationships: Desmoid Tumor Research Foundation, Chrondrosarcoma Foundation
John D. Baird
Employment: PTC Therapeutics
Stock and Other Ownership Interests: PTC Therapeutics
Patents, Royalties, Other Intellectual Property: Inventor listed on PTC Therapeutics patent for antimicrobial compounds
Travel, Accommodations, Expenses: PTC Therapeutics
Esther Brooks-Asplund
Employment: Catalent, inSeption Group
Stock and Other Ownership Interests: Catalent
Consulting or Advisory Role: PTC Therapeutics, Bristol Myers Squibb/Celgene
Travel, Accommodations, Expenses: PTC Therapeutics
Dhiren D’Silva
Employment: PTC Therapeutics
Leadership: PTC Therapeutics
Stock and Other Ownership Interests: PTC Therapeutics
Travel, Accommodations, Expenses: PTC Therapeutics
Ronald Kong
Employment: PTC Therapeutics
Stock and Other Ownership Interests: PTC Therapeutics
Research Funding: PTC Therapeutics
Anthony Mwatha
Employment: PTC Therapeutics, Zymeworks
Stock and Other Ownership Interests: PTC Therapeutics, Zymeworks
Kylie O’Keefe
Employment: PTC Therapeutics
Stock and Other Ownership Interests: PTC Therapeutics (Inst)
Marla Weetall
Employment: PTC Therapeutics
Stock and Other Ownership Interests: PTC Therapeutics
Research Funding: PTC Therapeutics
Travel, Accommodations, Expenses: PTC Therapeutics
Robert Spiegel
Employment: Spiegel Consulting LLC
Leadership: Ayala Pharmaceuticals, Geron, Cyclacel, Sun Pharma Advanced Research Company, Athenex
Stock and Other Ownership Interests: Merck, PTC Therapeutics
Consulting or Advisory Role: PTC Therapeutics
Travel, Accommodations, Expenses: PTC Therapeutics
Gary K. Schwartz
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Stock and Other Ownership Interests: GenCirq, Bionaut Labs, January Therapeutics
Consulting or Advisory Role: Bionaut Labs, Gencirq, OnCusp Therapeutics, Concarlo, Shanghai Pharma, Astex Pharmaceuticals, January Therapeutics, PureTech, Aadi, Kirilys Therapeutics, Agenus, Boehringer Ingelheim, Ipsen, Oncogenuity
Research Funding: Astex Pharmaceuticals, Oxford BioTherapeutics (Inst), SpringWorks Therapeutics (Inst), TRACON Pharma (Inst), PTC Therapeutics (Inst), Rain Therapeutics (Inst)
Patents, Royalties, Other Intellectual Property: Companion diagnostics for CD4 inhibitors (Inst), patent granted to develop a new technology called PNAs for cancer therapy
Travel, Accommodations, Expenses: Boehringer Ingelheim
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the 2022 European Society of Medical Oncology Annual Meeting, Paris, France, September 9-13, 2022 (abstr 1528TiP); the 2022 ASCO Annual Meeting, Chicago, IL, June 3-7, 2022 (abstr 11507); and the 2022 Connective tissue Oncology Society Annual Meeting, Vancouver, BC, Canada, November 16-19, 2022 (paper 17).
SUPPORT
Supported by PTC Therapeutics, Inc. This work was funded in part by a Seeding Drug Discovery grant from the Wellcome Trust.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Matthew A. Ingham, Steven Attia, John D. Baird, Ronald Kong, Kylie O'Keefe, Robert Spiegel, Gary K. Schwartz
Provision of study materials or patients: Brian A. Van Tine, Matthew A. Ingham, Christian F. Meyer, Gary K. Schwartz
Collection and assembly of data: Brian A. Van Tine, Matthew A. Ingham, Steven Attia, Ronald Kong, Anthony Mwatha, Gary K. Schwartz
Data analysis and interpretation: Brian A. Van Tine, Steven Attia, Christian F. Meyer, John D. Baird, Esther Brooks-Asplund, Dhiren D'Silva, Ronald Kong, Anthony Mwatha, Kylie O'Keefe, Marla Weetall, Robert Spiegel, Gary K. Schwartz
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase Ib Study of Unesbulin (PTC596) Plus Dacarbazine for the Treatment of Locally Recurrent, Unresectable or Metastatic, Relapsed or Refractory Leiomyosarcoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Brian A. Van Tine
Leadership: Polaris
Honoraria: Iterion Therapeutics, Inc, Total Health Conference
Consulting or Advisory Role: Epizyme, Daiichi Sankyo, Bayer, Deciphera, ADRx, Ayala Pharmaceuticals, Intellisphere, PTC Therapeutics, Boehringer Ingelheim, EcoR1 Capital, Deciphera Pharmaceuticals, Advenchen Laboratories, Putnam Associates, Salarius Pharmaceuticals, Inc, Boxer Capital LLC, Acuta Capital Partners, LLC, Aadi, Race Oncology, Hinge Bio, Inc, Kronos Bio, Inc, Agenus, Regeneron, Curis
Research Funding: Pfizer, Merck, TRACON Pharma, GlaxoSmithKline, Polaris
Patents, Royalties, Other Intellectual Property: Patent on the use of ME1 as a biomarker, Patent on ALEXT3102, Accuronix Therapeutics-Licensing agreement. Sigma-2 Receptor Ligands and Therapeutic uses therefor (006766), Modular Platform for Targeted Therapeutic Delivery (006755), Sigma-2 Receptor Ligand Drug Conjugates as Antitumor Compounds, Methods of synthesis and Uses Thereof (014229)
Expert Testimony: Health Advances
Travel, Accommodations, Expenses: Adaptimmune, Advenchen Laboratories, Kronos Bio, Inc
Matthew A. Ingham
Employment: Regeneron
Stock and Other Ownership Interests: Regeneron
Consulting or Advisory Role: Daiichi Sankyo, Xencor, Apexigen, Epizyme, Caris Life Sciences
Research Funding: Apexigen (Inst), Mirati Therapeutics (Inst), PTC Therapeutics (Inst), Apices, Intensity (Inst), Boehringer Ingelheim (Inst), BioAtla (Inst), Merck (Inst), Astellas Pharma (Inst), AstraZeneca (Inst)
Steven Attia
Research Funding: TRACON Pharma (Inst), Bayer (Inst), Novartis (Inst), Lilly (Inst), Karyopharm Therapeutics (Inst), Epizyme (Inst), Blueprint Medicines (Inst), Genmab (Inst), CBA Pharma (Inst), Desmoid Tumor Research Foundation, Merck (Inst), Philogen (Inst), Gradalis (Inst), Deciphera (Inst), Takeda (Inst), Incyte (Inst), SpringWorks Therapeutics (Inst), Adaptimmune (Inst), Advenchen Laboratories (Inst), Bavarian Nordic (Inst), BTG (Inst), PTC Therapeutics (Inst), GlaxoSmithKline (Inst), FORMA Therapeutics (Inst), Ayala Pharmaceuticals (Inst), Trillium Therapeutics (Inst), Boehringer Ingelheim (Inst), Salarius Pharmaceuticals (Inst), Theseus Pharmaceuticals (Inst), Monopar Therapeutics (Inst), C4 Therapeutics (Inst), InhibRx (Inst), Noxopharm (Inst), Rain Therapeutics (Inst), Guardant Health, Cogent Biosciences (Inst), Jazz Pharmaceuticals (Inst), Shanghai Pharma (Inst), PharmaMar (Inst)
Christian F. Meyer
Consulting or Advisory Role: Deciphera, Intellisphere, Aadi
Speakers’ Bureau: Novartis
Other Relationship: UpToDate
Uncompensated Relationships: Desmoid Tumor Research Foundation, Chrondrosarcoma Foundation
John D. Baird
Employment: PTC Therapeutics
Stock and Other Ownership Interests: PTC Therapeutics
Patents, Royalties, Other Intellectual Property: Inventor listed on PTC Therapeutics patent for antimicrobial compounds
Travel, Accommodations, Expenses: PTC Therapeutics
Esther Brooks-Asplund
Employment: Catalent, inSeption Group
Stock and Other Ownership Interests: Catalent
Consulting or Advisory Role: PTC Therapeutics, Bristol Myers Squibb/Celgene
Travel, Accommodations, Expenses: PTC Therapeutics
Dhiren D’Silva
Employment: PTC Therapeutics
Leadership: PTC Therapeutics
Stock and Other Ownership Interests: PTC Therapeutics
Travel, Accommodations, Expenses: PTC Therapeutics
Ronald Kong
Employment: PTC Therapeutics
Stock and Other Ownership Interests: PTC Therapeutics
Research Funding: PTC Therapeutics
Anthony Mwatha
Employment: PTC Therapeutics, Zymeworks
Stock and Other Ownership Interests: PTC Therapeutics, Zymeworks
Kylie O’Keefe
Employment: PTC Therapeutics
Stock and Other Ownership Interests: PTC Therapeutics (Inst)
Marla Weetall
Employment: PTC Therapeutics
Stock and Other Ownership Interests: PTC Therapeutics
Research Funding: PTC Therapeutics
Travel, Accommodations, Expenses: PTC Therapeutics
Robert Spiegel
Employment: Spiegel Consulting LLC
Leadership: Ayala Pharmaceuticals, Geron, Cyclacel, Sun Pharma Advanced Research Company, Athenex
Stock and Other Ownership Interests: Merck, PTC Therapeutics
Consulting or Advisory Role: PTC Therapeutics
Travel, Accommodations, Expenses: PTC Therapeutics
Gary K. Schwartz
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Stock and Other Ownership Interests: GenCirq, Bionaut Labs, January Therapeutics
Consulting or Advisory Role: Bionaut Labs, Gencirq, OnCusp Therapeutics, Concarlo, Shanghai Pharma, Astex Pharmaceuticals, January Therapeutics, PureTech, Aadi, Kirilys Therapeutics, Agenus, Boehringer Ingelheim, Ipsen, Oncogenuity
Research Funding: Astex Pharmaceuticals, Oxford BioTherapeutics (Inst), SpringWorks Therapeutics (Inst), TRACON Pharma (Inst), PTC Therapeutics (Inst), Rain Therapeutics (Inst)
Patents, Royalties, Other Intellectual Property: Companion diagnostics for CD4 inhibitors (Inst), patent granted to develop a new technology called PNAs for cancer therapy
Travel, Accommodations, Expenses: Boehringer Ingelheim
No other potential conflicts of interest were reported.
REFERENCES
- 1.George S, Serrano C, Hensley ML, et al. : Soft tissue and uterine leiomyosarcoma. J Clin Oncol 36:144-150, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Worhunsky DJ, Gupta M, Gholami S, et al. : Leiomyosarcoma: One disease or distinct biologic entities based on site of origin? J Surg Oncol 111:808-812, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Sbaraglia M, Bellan E, Dei Tos AP: The 2020 WHO classification of soft tissue tumours: News and perspectives. Pathologica 113:70-84, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bessen T, Caughey GE, Shakib S, et al. : A population-based study of soft tissue sarcoma incidence and survival in Australia: An analysis of 26,970 cases. Cancer Epidemiol 63:101590, 2019 [DOI] [PubMed] [Google Scholar]
- 5.Parikh RC, Lorenzo M, Hess LM, et al. : Treatment patterns and survival among older adults in the United States with advanced soft-tissue sarcomas. Clin Sarcoma Res 8:8, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toro JR, Travis LB, Wu HJ, et al. : Incidence patterns of soft tissue sarcomas, regardless of primary site, in the Surveillance, Epidemiology and End Results Program, 1978-2001: An analysis of 26,758 cases. Int J Cancer 119:2922-2930, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Saltus CW, Calingaert B, Candrilli S, et al. : Epidemiology of adult soft-tissue sarcomas in Germany. Sarcoma 2018:5671926, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agaram NP, Zhang L, LeLoarer F, et al. : Targeted exome sequencing profiles genetic alterations in leiomyosarcoma. Genes Chromosomes Cancer 55:124-130, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network: Clinical Practice Guidelines in Oncology : Soft tissue sarcoma. Version 2.2023. https://NCCN.org
- 10.Gronchi A, Miah AB, Dei Tos AP, et al. : Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 32:1348-1365, 2021 [DOI] [PubMed] [Google Scholar]
- 11.In GK, Hu JS, Tseng WW: Treatment of advanced, metastatic soft tissue sarcoma: Latest evidence and clinical considerations. Ther Adv Med Oncol 9:533-550, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Der Graaf WT, Blay J-Y, Chawla SP, et al. : Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 379:1879-1886, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Patel S, von Mehren M, Reed DR, et al. : Overall survival and histology-specific subgroup analyses from a phase 3, randomized controlled study of trabectedin or dacarbazine in patients with advanced liposarcoma or leiomyosarcoma. Cancer 125:2610-2620, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jernigan F, Branstrom A, Baird J, et al. : Preclinical and early clinical development of PTC596, a novel small-molecule tubulin-binding agent. Mol Cancer Ther 20:1846-1857, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eberle-Singh JA, Sagalovskiy I, Maurer HC, et al. : Effective delivery of a microtubule polymerization inhibitor synergizes with standard regimens in models of pancreatic ductal adenocarcinoma. Clin Cancer Res 25:5548-5560, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Cheung YK, Chappell R: Sequential designs for phase I clinical trials with late-onset toxicities. Biometrics 56:1177-1182, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Demetri GD, Von Mehren M, Jones RL, et al. : Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: Results of a phase III randomized multicenter clinical trial. J Clin Oncol 34:786-793, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hensley ML, Patel SR, von Mehren M, et al. : Efficacy and safety of trabectedin or dacarbazine in patients with advanced uterine leiomyosarcoma after failure of anthracycline-based chemotherapy: Subgroup analysis of a phase 3, randomized clinical trial. Gynecol Oncol 146:531-537, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shapiro GI, O’Mara E, Laskin OL, et al. : Pharmacokinetics and safety of PTC596, a novel tubulin-binding agent, in subjects with advanced solid tumors. Clin Pharmacol Drug Dev 10:940-949, 2021 [DOI] [PubMed] [Google Scholar]
- 20.Moiseeva NI, Laletina LA, Fetisov TI, et al. : Analysis of multiple drug resistance mechanism in different types of soft tissue sarcomas: Assessment of the expression of ABC-transporters, MVP, YB-1, and analysis of their correlation with chemosensitivity of cancer cells. J Mol Sci 23:3183, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oda Y, Saito T, Tateishi N, et al. : ATP-binding cassette superfamily transporter gene expression in human soft tissue sarcomas. Int J Cancer 114:854-862, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Levine EA, Holzmayer T, Bacus S, et al. : Evaluation of newer prognostic markers for adult soft tissue sarcomas. J Clin Oncol 15:3249-3257, 1997 [DOI] [PubMed] [Google Scholar]