Abstract
PURPOSE
The anti-NECTIN4 antibody-drug conjugate enfortumab vedotin (EV) is approved for patients with metastatic urothelial cancer (mUC). However, durable benefit is only achieved in a small, yet uncharacterized patient subset. NECTIN4 is located on chromosome 1q23.3, and 1q23.3 gains represent frequent copy number variations (CNVs) in urothelial cancer. Here, we aimed to evaluate NECTIN4 amplifications as a genomic biomarker to predict EV response in patients with mUC.
MATERIALS AND METHODS
We established a NECTIN4-specific fluorescence in situ hybridization (FISH) assay to assess the predictive value of NECTIN4 CNVs in a multicenter EV-treated mUC patient cohort (mUC-EV, n = 108). CNVs were correlated with membranous NECTIN4 protein expression, EV treatment responses, and outcomes. We also assessed the prognostic value of NECTIN4 CNVs measured in metastatic biopsies of non–EV-treated mUC (mUC-non-EV, n = 103). Furthermore, we queried The Cancer Genome Atlas (TCGA) data sets (10,712 patients across 32 cancer types) for NECTIN4 CNVs.
RESULTS
NECTIN4 amplifications are frequent genomic events in muscle-invasive bladder cancer (TCGA bladder cancer data set: approximately 17%) and mUC (approximately 26% in our mUC cohorts). In mUC-EV, NECTIN4 amplification represents a stable genomic alteration during metastatic progression and associates with enhanced membranous NECTIN4 protein expression. Ninety-six percent (27 of 28) of patients with NECTIN4 amplifications demonstrated objective responses to EV compared with 32% (24 of 74) in the nonamplified subgroup (P < .001). In multivariable Cox analysis adjusted for age, sex, and Bellmunt risk factors, NECTIN4 amplifications led to a 92% risk reduction for death (hazard ratio, 0.08 [95% CI, 0.02 to 0.34]; P < .001). In the mUC-non-EV, NECTIN4 amplifications were not associated with outcomes. TCGA Pan-Cancer analysis demonstrated that NECTIN4 amplifications occur frequently in other cancers, for example, in 5%-10% of breast and lung cancers.
CONCLUSION
NECTIN4 amplifications are genomic predictors of EV responses and long-term survival in patients with mUC.
) NECTIN4 amplification predicts response to EV in mUC and it occurs frequently across solid tumors (app. 25% mUC)
INTRODUCTION
The anti-NECTIN4 antibody-drug conjugate (ADC) enfortumab vedotin (EV) has been approved for previously treated patients with metastatic urothelial cancer (mUC).1,2 The combination of EV plus pembrolizumab (EV/P) was recently approved in metastatic, treatment-naïve and cisplatin-ineligible patients with mUC. More recently, in EV-302, this combination proved to be superior to platinum plus gemcitabine and defined a new standard of care in the first-line setting.3-6
CONTEXT
Key Objective
Can NECTIN4 amplifications be used as a genomic biomarker to predict the response to the anti-NECTIN4 antibody-drug conjugate (ADC) enfortumab vedotin (EV) in patients with metastatic urothelial cancer (mUC)?
Knowledge Generated
NECTIN4 amplifications were found to be frequent genomic events in mUC, occurring in approximately 25% of cases. In the EV-treated mUC patient cohort, 96% of patients with NECTIN4 amplifications showed objective responses to EV compared with 32% in the nonamplified subgroup. The frequent occurrence of NECTIN4 amplifications in various cancer types, for example, lung and breast cancers, indicates that this biomarker holds promise for tumor-agnostic clinical development of NECTIN4-targeted ADC.
Relevance (M.A. Carducci)
-
This hypothesis generating study requires prospective evaluation as a predictive genomic biomarker for EV responses. Given the target of EV as an anti-NECTIN4 ADC, the results are highly plausible and may represent strong classifier for treatment response and improved clinical outcomes.*
*Relevance section written by JCO Associate Editor Michael A. Carducci, MD, FACP, FASCO.
EV is currently administered in an all-comer setting without rational biomarker-based patient selection although there is evidence that its target NECTIN4 is heterogeneously expressed in urothelial cancer (UC) molecular subtypes.7-9 In addition, we recently showed that membranous NECTIN4 expression frequently decreased during metastatic spread and correlates with EV response in patients with mUC.10 In light of other effective treatment alternatives such as trophoblast cell surface antigen 2 (TROP2)- or human epidermal growth factor receptor 2 (HER2)–directed ADC or fibroblast growth factor receptor inhibitors, a better understanding of the molecular basis for EV responses is urgently needed to improve the rational use of this effective drug for patients with mUC11-15 and to optimize its ongoing clinical development in earlier UC stages and other solid tumors.16-19
The relationship between copy number variation (CNV), mRNA, and protein expression has been known for decades. As a prime example, anti–HER2-targeted therapy conquered modern oncologic therapy of certain breast cancer subtypes and subsequently other entities in an unprecedented success story. The HER2-targeted ADC trastuzumab deruxtecan (T-DXd) proved to be effective in various HER2-expressing solid cancers, also mUC, with a close correlation with expression status.20,21 However, HER2-directed therapy is guided solely on the basis of biomarker testing that aims to identify HER2-overexpressing/ERBB2-amplified tumors. Unlike in this setting, anti–NECTIN4 EV, whose therapeutic efficacy has been shown to depend on the expression of its target,7,10 is applied without previous tumor biomarker testing. Similar to HER2, whose expression is strongly linked to CNV of ERBB2, previous reports linked NECTIN4 gene expression to gains/amplifications of 1q23.3—where the NECTIN4 gene is located—occurring in approximately 15%-20% of mUC22 with an enrichment of NECTIN4 amplifications in luminal molecular subtypes of mUC.23 Despite the frequency of NECTIN4 CNVs in mUC, to date, the link between NECTIN4 CNVs, membranous NECTIN4 protein expression, and especially the clinical potential of NECTIN4 CNVs to predict EV responses has not been assessed.
Thus, we here assessed NECTIN4 CNVs and their association with membranous NECTIN4 protein expression in a multicenter cohort of n = 108 EV-treated patients with mUC and correlated the results with EV responses and outcomes. Furthermore, we confirmed the correlation of NECTIN4 CNVs, mRNA, and protein expression in a The Cancer Genome Atlas (TCGA) pan-cancer analysis and explored the prevalence of NECTIN4 CNVs representing a potential tumor-agnostic genomic biomarker to predict EV response in multiple cancer entities.
MATERIALS AND METHODS
TCGA Data
CNV (Affymetrix single nucleotide polymorphism [SNP] 6.0 array data), transcriptome sequencing (RNA-Seq_v2, log2-transformed RNA-Seq by expectation maximization [RSEM] normalized values), and reverse phase protein arrays (RPPA, only for TCGA-BRCA) were downloaded via cBioPortal24 querying 10,712 samples/patients in a TCGA pan-cancer analysis including 32 studies. For the n = 408 bladder cancers from TCGA (TCGA-BLCA), clinical data (age, sex, outcomes) were downloaded from the University of California, Santa Cruz Xena browser.25 The TCGA Network calculated CNVs using GISTIC 2.0, and the following values were assigned: –2 = deep deletion; –1 = shallow deletion; 0 = diploid; 1 = gain; 2 = amplification.
Multicenter EV-Treated mUC Cohort
We retrospectively reviewed medical records of n = 108 EV-treated patients with mUC. All patients received EV as the standard of care. Treatment response was evaluated according to RECIST v.1.1 by site investigators.26 Progression-free survival (PFS) was defined as the time from EV initiation to radiologic or clinical progression or death from any cause. Representative formalin-fixed and paraffin-embedded (FFPE) tissue of the primary tumor (PRIM; transurethral resection of the bladder [TURB], cystectomy, or nephroureterectomy) and/or metastatic (MET) tissue was required for inclusion in our explorative biomarker study. When multiple tissue samples were available (matched PRIM + MET in n = 27), we considered the one closest to EV start for our outcome analyses. The study was approved by the ethical review board of the Friedrich-Alexander-University Erlangen-Nürnberg (approval numbers: 329_16B and 97_18Bc) and the Medical Faculty of the University of Bonn (approval number: 372/21). Our biomarker study conforms to REMARK guidelines.27
Non–EV-Treated mUC Cohort
Whole-genome sequencing (WGS) was previously conducted on fresh-frozen metastatic biopsy samples from 116 patients with mUC.23 These patients with mUC were enrolled in clinical trials (ClinicalTrials.gov identifiers: NCT01855477 and NCT02925234) for palliative systemic treatments, with none receiving EV (mUC-non-EV). This patient cohort was already described in detail by Nakauma-González et al.23 NECTIN4 CNVs were assessed using GISTIC 2.0.28 Sufficient clinical information on outcomes was available for n = 103 patients.
NECTIN4 Fluorescence In Situ Hybridization
The NECTIN4 fluorescence in situ hybridization (FISH) probe was purchased from Empire Genomics (Catalog No. NECTIN4-20-GR, Empire Genomics, Buffalo, NY). The probe is designed to specifically target and bind to the NECTIN4 gene (NCBI Gene ID: 81607). The probe consisted of a fluorescently labeled DNA probe that specifically binds to the NECTIN4 gene. All hybridizations were performed in an accredited specialized laboratory for clinical molecular pathology (accredited according to DIN EN ISO/IEC 17020) using a standard protocol.
The slides were analyzed using a fluorescence microscope equipped with appropriate filter sets to detect the fluorescence signal from NECTIN4 and CEN1 probes. Representative tumor areas for formal analysis were chosen by an experienced board-certified pathologist (ME; blinded to patient outcomes), and at least 50 nonoverlapping nuclei per sample were assessed. Green (NECTIN4) and red (CEN1) signals were manually quantified. The NECTIN4/CEN1 ratio was calculated, and a ratio of ≥2.0 qualified tumors as NECTIN4-amplified. Tumors with ratio values <2.0 were considered nonamplified. Furthermore, gene copy changes (≥4 NECTIN4 gene copies per nucleus) without qualifying for an amplification (NECTIN4/CEN1 ratio below <2.0) were considered as polysome tumors, and polysomy status was correlated with response to EV.
NECTIN4 Immunohistochemistry
Immunohistochemical staining of NECTIN4 was performed using a VENTANA BenchMark ULTRA autostainer (Ventana, Oro Valley, AZ), as previously described.10 The samples were categorized as negative (H-score, 0-14), weak (H-score, 15-99), moderate (H-score, 100-199), or strong (H-score, 200-300), as described previously.10,29
SNP Array
DNA from the cryopreserved tumor specimen was isolated using the AllPrep DNA/RNA Micro Kit (#80284, Qiagen, Hilden, Germany) following the manufacturer’s instructions. Infinium Global Screening Array-24 v3.0 Kit (Illumina, San Diego, CA) was used according to the manufacturer’s protocol for the detection of NECTIN4 CNVs. Data were analyzed using GenomeStudio version 2.0.5 (Illumina) with cnvPartition CNV Analysis Plugin version 3.2.0 to identify CNV regions and estimate CNV values. CNV values of higher than two were considered as amplification.
Statistical Analysis
Statistical analysis was performed using R (Version 4.3.0), R Studio (Version 2023.03.1 + 446), and GraphPad Prism (Version 9.4.0).
NECTIN4 CNV was correlated with NECTIN4 mRNA (log2-transformed RSEM-normalized values) and membranous protein expression (H-score). Nonparametric Mann-Whitney test was used to compare two groups. For comparisons involving multiple groups, the nonparametric Kruskal-Wallis test was used.
The predictive value of NECTIN4 amplification for response to EV was assessed by comparing best overall response (BOR), progression-free survival (PFS), and overall survival (OS) between NECTIN4-amplified and nonamplified tumors.
To evaluate the survival after the start of EV treatment, univariable Kaplan-Meier regressions were performed, and significance was determined using the log-rank test. Multivariate Cox regression analyses were conducted to compare the prognostic value of NECTIN4 CNV with baseline patient characteristics (age, sex) and the Bellmunt risk factors (Eastern Cooperative Oncology Group >0, hemoglobin level <10 g/dL, and the presence of liver metastasis) 30 in relation to PFS and OS after EV initiation.
All P values were calculated as two-sided, and a significance level of P < .05 was used to determine statistical significance.
RESULTS
NECTIN4 Amplifications Predict Responses and Favorable Outcomes to EV in mUC
We first established a NECTIN4 FISH assay to examine NECTIN4 CNVs. FISH images and corresponding IHC stainings for a NECTIN4 nonamplified UC lacking membranous NECTIN4 expression and a NECTIN4-amplified UC that demonstrates pronounced membranous NECTIN4 expression, respectively, are illustrated in Figures 1A and 1B. The NECTIN4 CNVs in these samples were validated using a SNP assay, which confirms the accuracy and specificity of our NECTIN4 FISH assay (Appendix Fig A1, online only). Next, we used FISH to determine NECTIN4 CNV in our multicenter EV-treated mUC cohort (mUC-EV, n = 108). Twenty-eight of 108 samples (26%) showed NECTIN4 amplifications (NECTIN4/CEN1 ratio ≥2.0), consistent with amplification frequencies observed in the non–EV-treated metastatic biopsy mUC cohort (mUC-non-EV, 26%, 27 of 103). Regarding baseline characteristics, 25 of 28 patients with NECTIN4 amplifications were male (P = .043) and tended to be older (P = .20; Appendix Table A1). In the mUC-non-EV cohort (ClinicalTrials.gov identifiers: NCT01855477 and NCT02925234), 27 of 27 patients with NECTIN4 amplification were male (P = .001), and again, there was a nonsignificant trend toward a higher frequency of NECTIN4 amplification in older patients with mUC (P = .069; Appendix Table A2). In TCGA-BLCA, there was a significant correlation between NECTIN4 amplification and older age (P = .013), and there was a nonsignificant trend toward higher amplification frequency in males (P = .15; Appendix Table A3). Next, we evaluated whether NECTIN4 CNVs correlated with membranous NECTIN4 protein expression, the prerequisite for EV binding, known to be correlated with EV response.10 NECTIN4-amplified tumors demonstrated significantly enhanced membranous NECTIN4 expression (median H-score: 295; IQR, 235-300) compared with NECTIN4 nonamplified tumors (median H-score, 90; IQR, 20-205; Fig 1C). In 27 matched primary (PRIM) and corresponding metastatic (MET) tumor tissues, NECTIN4 CNV was stable in 93% (25 of 27). Of eight NECTIN4-amplified PRIM with available matched MET, only one tumor lost NECTIN4 amplification during metastasis (Fig 1D). Membranous NECTIN4 expression of NECTIN4-amplified PRIM (median H-score, 290; range, 170-300) remained high in the corresponding MET (median H-score, 280; range, 20-300), except for the primary tumor, which lost its NECTIN4 amplification (Fig 1E). In only 1 of 27 matched PRIM and MET pairs, NECTIN4 amplification was exclusive in the metastatic sample (Fig 1D).
FIG 1.
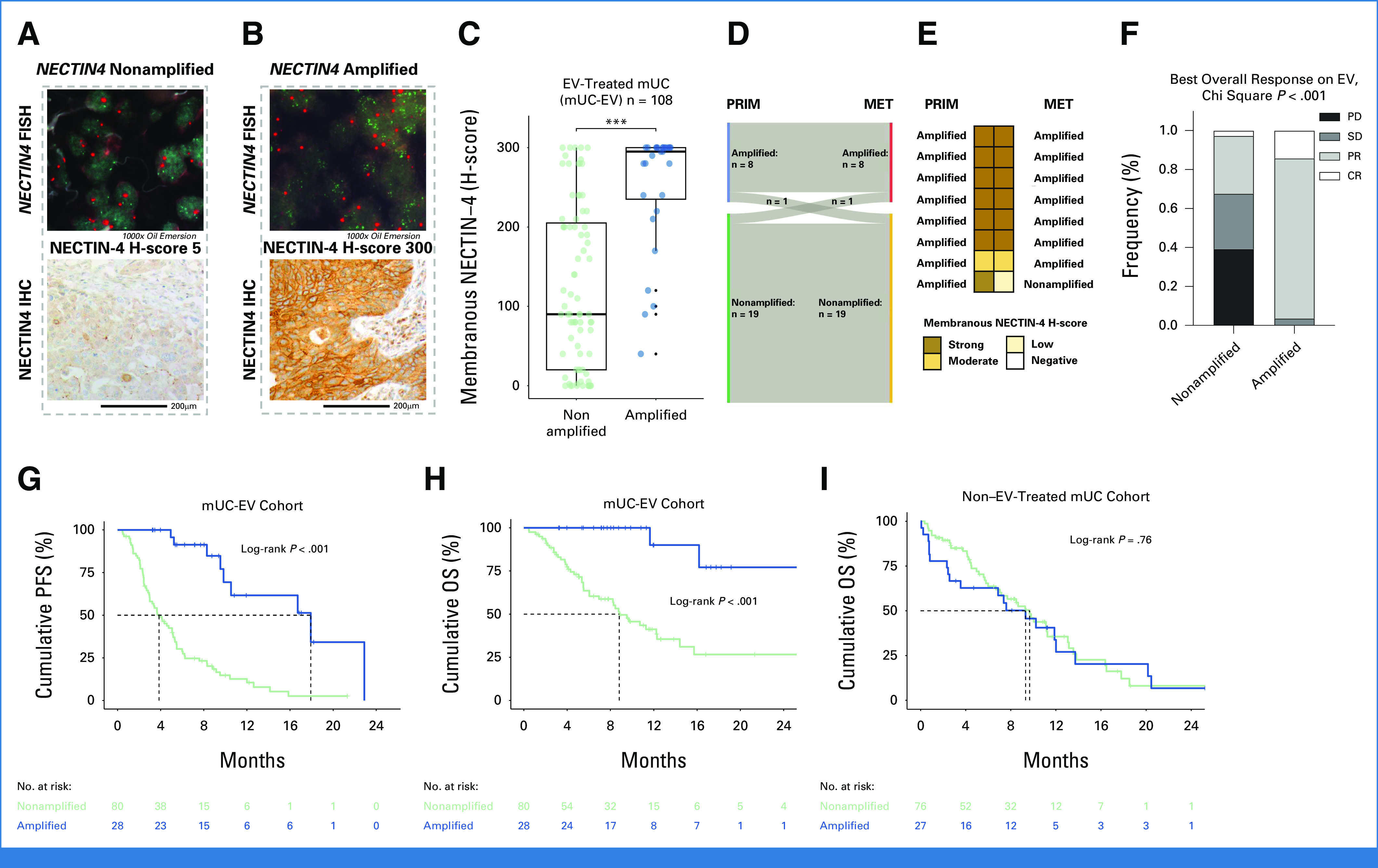
NECTIN4 amplification predicts EV response in mUC. (A and B) NECTIN4 FISH image (green signals = NECTIN4; red signals = centromere 1, 1,000× oil immersion) and (A) corresponding immunohistochemical NECTIN4 staining on NECTIN4 nonamplified and (B) NECTIN4-amplified urothelial cancers. The gray dashed box demonstrates the two patient cases. (C) Membranous NECTIN4 expression is significantly associated with FISH-detected NECTIN4 amplification in our EV-treated UC cohort (mUC-EV). Statistical significance (***P < .001) was determined using the Mann-Whitney U test. (D) Sankey plot of NECTIN4 amplification status in the 27 matched PRIM and MET samples. (E) Evolution of membranous NECTIN4 expression during metastatic spread in the eight NECTIN4-amplified PRIMs. (F) BOR on the mUC-EV cohort on the basis of NECTIN4 copy number status; BOR was available for n = 65 patients. NECTIN4 amplification status is associated with both prolonged (G) PFS and (H) OS since EV therapy start compared with nonamplified tumors. (I) NECTIN4 amplification is not associated with OS in non–EV-treated mUC. The log-rank P value is shown. The dashed lines demonstrate median PFS and OS when reached. BOR, best overall response; EV, enfortumab vedotin; FISH, fluorescence in situ hybridization; OS, overall survival; MET, metastatic; PFS, progression-free survival.
A total of 96% (27 of 28) patients with NECTIN4 amplification demonstrated an objective response (82%; partial response [PR] and 14% complete response [CR], one patient with stable disease [SD]) as BOR compared with 32% (including 3% with CR) of the NECTIN4 nonamplified tumors (Chi square P < .001; Fig 1F). NECTIN4 amplifications associated with prolonged PFS (Fig 1G) and OS (Fig 1H), with 90% 12-month survival rate and median OS not reached (95% CI, NR to NR) compared with 41% 12-month survival and a median OS of 8.8 months (95% CI, 6.1 to 14) for NECTIN4 nonamplified tumors. In multivariable Cox regression coadjusted for age, sex, and Bellmunt risk factors, NECTIN4 amplification status led to a 92% risk reduction for death compared with NECTIN4 nonamplified tumors (hazard ratio, 0.08 [95% CI, 0.02 to 0.34], P < .001; Table 1). In addition, NECTIN4 amplification was associated with prolonged PFS and OS compared with the patient subgroup of nonamplified tumors with strong membranous NECTIN4 expression (H-score ≥200; Appendix Fig A2). Furthermore, we explored whether polysome gene copy changes per nucleus (copy number ≥4.0) without qualifying for an amplification (NECTIN4/CEN1 ratio below <2.0) correlated with EV response and found that five of eight polysome tumors demonstrated an PR/CR or SD with disease control >6 months.
TABLE 1.
Multivariable Cox Regression Analyses in the Multicenter Enfortumab Vedotin Cohort
| Characteristic | PFS | OS | ||||||
|---|---|---|---|---|---|---|---|---|
| No. | HR | 95% CI | P | No. | HR | 95% CI | P | |
| NECTIN4 CNV status | <.001 | <.001 | ||||||
| Nonamplified | 80 | — | — | 80 | — | — | ||
| Amplified | 28 | 0.14 | 0.06 to 0.30 | 28 | 0.08 | 0.02 to 0.34 | ||
| Age, years | .25 | .71 | ||||||
| <75 | 86 | — | — | 86 | — | — | ||
| ≥75 | 22 | 0.70 | 0.38 to 1.30 | 22 | 1.16 | 0.54 to 2.47 | ||
| Sex | .28 | .58 | ||||||
| Male | 81 | — | — | 81 | — | — | ||
| Female | 27 | 1.36 | 0.79 to 2.34 | 27 | 0.83 | 0.41 to 1.65 | ||
| Liver metastasis | .31 | .023 | ||||||
| No | 76 | — | — | 76 | — | — | ||
| Yes | 32 | 0.77 | 0.46 to 1.29 | 32 | 2.06 | 1.11 to 3.82 | ||
| ECOG | .090 | <.001 | ||||||
| 0 | 36 | — | — | 36 | — | — | ||
| ≥1 | 72 | 1.53 | 0.92 to 2.55 | 72 | 4.84 | 2.01 to 11.7 | ||
| Hemoglobin, g/dL | .54 | .16 | ||||||
| ≥10 | 104 | — | — | 104 | — | — | ||
| <10 | 4 | 0.71 | 0.23 to 2.19 | 4 | 0.29 | 0.04 to 2.24 | ||
NOTE. Significant P values are highlighted in bold.
Abbreviations: CNV, copy number variation; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; OS, overall survival; PFS, progression-free survival.
To rule out a prognostic bias of NECTIN4 CNVs, we assessed their prognostic impact in non–EV-treated UC patient cohorts. In the mUC-non-EV cohort, NECTIN4 amplifications were assessed via whole-genome DNA sequencing in 103 metastatic biopsy samples obtained before palliative systemic treatment. In this cohort, NECTIN4 amplifications were found in 26% of tumors and were not associated with OS (Fig 1I). In the TCGA-BLCA cohort of muscle-invasive bladder cancer, NECTIN4 amplifications were also not associated with disease-specific survival and OS (Appendix Fig A3A and A3B).
NECTIN4 Amplification Occurs Frequently Across Entities
In the TCGA Pan-Cancer cohort, NECTIN4 amplifications were observed in 25 of 32 cancer types including various solid entities with NECTIN4 amplification frequency > 5% (Fig 2A). The highest prevalence of NECTIN4 amplifications was found in bladder cancer (BLCA, 17%), cholangiocarcinoma (CHOL, 14%), hepatocellular carcinoma (LIHC, 12%), breast cancer (BRCA, 9%), and lung adenocarcinoma (LUAD, 7%). NECTIN4-amplified samples or those with gains showed increased NECTIN4 mRNA levels compared with diploid samples (Fig 2B) on the pan-cancer level. In BLCA, BRCA, and LUAD—where EV is approved or in late-stage clinical development—NECTIN4 amplifications associated with increased NECTIN4 mRNA expression (Fig 2C; Appendix Figs A4A and A4C) and higher NECTIN4 protein levels in breast cancer (Appendix Fig A4B).
FIG 2.
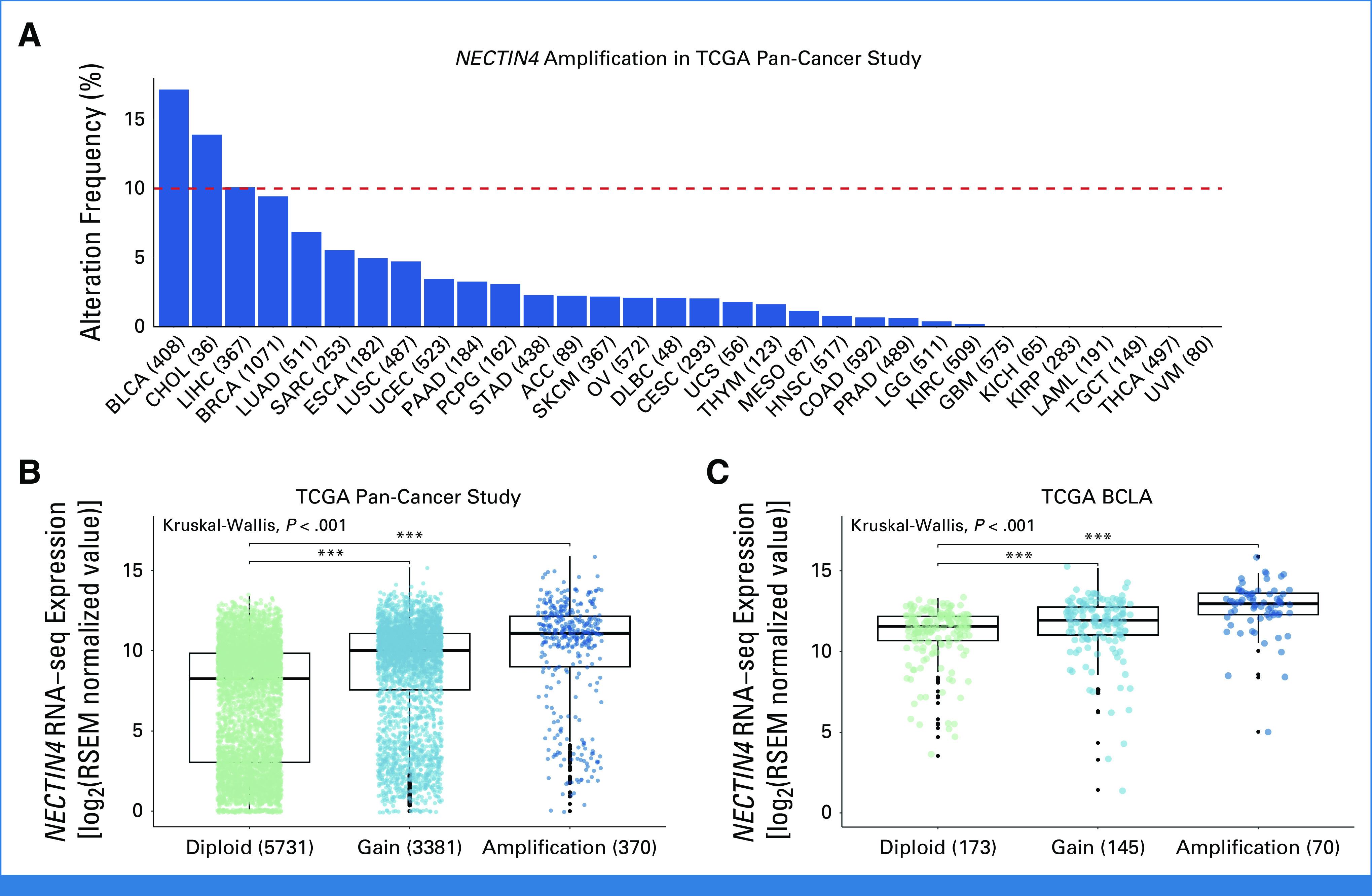
NECTIN4 amplifications occur frequently across solid tumors. (A) The frequency of NECTIN4 amplifications are depicted for 32 studies consisting of 10,712 samples/patients, with BLCA presenting the highest prevalence (17%). Positive correlation was observed between NECTIN4′ copy number variation and mRNA level in both (B) Pan-Cancer Study and (C) TCGA-BLCA. Standard TCGA study abbreviations were used.31 BLCA, Bladder Urothelial Carcinoma; TCGA, The Cancer Genome Atlas.
DISCUSSION
The identification of biomarkers to predict response to targeted therapies is crucial to improve the management of patients with cancer.32 Here, we provide data from a multicenter mUC patient cohort highlighting NECTIN4 amplifications as genomic biomarkers to predict EV responses and favorable outcomes. Importantly, in the non–EV-treated patients with mUC, NECTIN4 amplifications have no impact on OS,33 suggesting that NECTIN4 amplifications are neither indicating aggressive nor favorable tumor biology, strengthening its potential value as a pure predictive biomarker.34 NECTIN4 amplification was strongly associated with EV sensitivity (BOR, 96%). However, the response rate of 32% in the nonamplified subgroup is comparable with the expected outcomes (BOR app. 40%) observed in real-world settings and the pivotal phase III EV-301 study.1,35,36 With a median OS of 12 months (95% CI, 9.7 to NR) in our mUC-EV cohort, our data confirm the clinical activity of EV in previously treated patients with mUC (eg, EV-301, 12.9 months [95% CI, 10.6 to 15.2]). Therefore, EV again proves to be an effective drug in previously treated mUC also in the nonamplified context.
We recently showed that membranous NECTIN4 protein expression is volatile and often (>50%) decreases during metastatic progression of mUC.10 By contrast, 88% of PRIM with NECTIN4 amplifications retains their NECTIN4 amplification and subsequently a stable high membranous NECTIN4 protein expression during metastatic progression. This is in line with previous results from the study by Faltas et al37 demonstrating that early acquired genomic features including copy number alterations are rather stable during metastatic progression in comparison with parental primary tumors. Thus, treatment decisions for the metastatic stage could be based on NECTIN4 amplification status in primary tumor material, facilitating implementation into clinical trials. It is worth noting that this consideration does not apply to the assessment of membranous NECTIN4 protein expression, which decreases substantially during metastasis in UC without NECTIN4 amplifications.10 This difference could be explained by the inability of NECTIN4-amplified tumors to downregulate membranous expression of NECTIN4 at the transcriptional level. Because downregulation of the target is a known mechanism of resistance to ADCs,38,39 this could explain, at least in part, the exceptional and durable clinical efficacy of EV in NECTIN4-amplified tumors. Beside considerations of tissue choice for predictive biomarker testing, overcoming hurdles to implement biomarker tests into daily care is a major obstacle for biomarker-guided therapies. In the case of CNV assessment, a broad variety of cytogenetic and molecular techniques are available, including FISH/chromogenic in situ hybridization, SNP microarray, comparative genomic hybridization, multiplex ligation-dependent probe amplification, and sequencing methods like whole-exome or whole-genome sequencing.40 Among these options, FISH is the most frequently performed diagnostic assay to assess CNVs in clinical routine.41 Moreover, FISH as predictive biomarker assay has been proven to be a highly reproducible, easy-to-implement, fast, and cost-effective method in daily molecular pathology. Thus, we conclude that a NECTIN4 FISH assay could be quickly integrated into clinical trials and routine molecular pathology/daily patient care.
Other biomarkers were described to be associated with EV response and outcomes: Jindal et al42 conducted a comprehensive biomarker analysis within the UNITE study cohort, which comprised 303 patients receiving EV monotherapy with available next-generation sequencing data across 16 US sites. Among these patients, 207 had their tumor mutational burden (TMB) assessed and 146 had their PD-L1 status evaluated. Multivariate analysis revealed that alterations in ERBB2, KDM6A, and PIK3CA were associated with favorable treatment outcomes on EV. Conversely, patients with low TMB (<10 Mut/Mb) and high PD-L1 (CPS ≥10) exhibited less favorable outcomes on EV.42 It is known that alterations in ERBB2 and KDM6A are over-represented in luminal differentiated UC,43 which are known to be enriched for NECTIN4 amplification 23 and increased NECTIN4 mRNA and protein expression.7,44 Therefore, the prognostic value of these genomic alterations may depend on luminal differentiation and concomitant higher NECTIN4 expression. Consistent with this, the absence of squamous differentiation has been shown to correlate with response to EV.45 In addition, the occurrence of skin toxicity after initiation of EV treatment has been reported to be associated with favorable outcomes of EV treatment.46 In the context of ADC precision oncology, it is well established from several clinical trials that ADC response correlates with the respective target gene expression, for example, for HER214,20,21 and FOLR1-targeting ADC 47; we have demonstrated linear correlation also between membranous NECTIN-4 expression and EV response.10 Future biomarker analyses would therefore ideally need to integrate membranous NECTIN4 expression, NECTIN4 CNV, histomorphology, and further high throughput data to deepen our understanding of EV-responsive tumors.
Rational biomarker-guided therapy selection is urgently required to establish the optimal therapy sequence for patients with (m)UC.11,13,32,48 Consideration of NECTIN4 amplifications as predictive biomarkers could potentially rationalize EV drug development—also at earlier disease stages—by defining the patient subgroup with the highest chance of durable benefit. In this context, a strategic focus on biomarker-guided trials could greatly enhance our understanding of the potential of EV or other anti–NECTIN4-targeted therapies and open new avenues to optimize treatment and improve outcomes in patients with (m)UC.48,49
A wide range of surface targets, such as HER2 or TROP2, are present in different types of cancers, and there has been a growing interest to expand the use of ADC beyond specific cancer types in a tumor-agnostic fashion.16,17,50,51 Of note, in our TCGA Pan-Cancer analysis, NECTIN4 amplifications can be found in 5%-10% of breast cancer and non–small cell lung cancer, both tumor types with a high impact on all-cancer mortality, which are currently being evaluated for EV response in the multicohort phase II EV-202 trial (ClinicalTrials.gov identifier: NCT04225117).19 Thus, NECTIN4 CNV may be a valuable predictive biomarker to streamline clinical development of NECTIN4-targeted therapies in tumor entities beyond UC.52 The frequent occurrence of NECTIN4 amplifications across solid cancer types could thus pave the way for basket trial designs studying the efficacy of EV on the basis of NECTIN4 CNV status in a tumor-agnostic study framework,16,17 similar to the phase II DESTINY-PanTumor02 trial which assessed anti-HER2 ADC T-DXd in HER2-expressing solid tumors.20
Although our study certainly has important strengths, its main limitation is the use of a retrospectively assembled patient cohort, which consists of both archived primary (TURB, cystectomy or nephroureterectomy) and metastatic tumor specimens with varying ranges between tumor sampling and start of EV treatment. Therefore, our data are hypothesis-generating and prospective confirmation in larger, biomarker-driven trials is mandatory. As the combination of EV/P is the new standard of care in the first-line treatment of mUC, the predictive value of NECTIN4 amplification in this new treatment setting should be further investigated. In addition, our study does not include correlative data on NECTIN4 CNVs and responses to EV in other cancer entities, as mUC is the only approved standard-of-care setting for EV to date.
In conclusion, our study suggests that NECTIN4 amplification is a simple, valuable, and easy-to-implement predictive biomarker for EV in patients with mUC. The frequent occurrence of NECTIN4 amplifications in other cancer types suggests that this biomarker is a promising candidate with broader applicability for clinical development of NECTIN4-targeted ADCs in a tumor-agnostic context.
ACKNOWLEDGMENT
We thank the patients and their families who were patients of this study.
We thank the BioBank initiatives of the participating centers for their support of this study. In addition, we would like to thank the NGS Core Facility of the Medical Faculty at the University of Bonn and the TCGA Research Network.
APPENDIX
TABLE A1.
Baseline Characteristics of the mUC-EV Cohort
| Characteristic | Nonamplified (n = 80) | Amplified (n = 28) | P a |
|---|---|---|---|
| Sex, No. (%) | .043 | ||
| Male | 56 (70) | 25 (89) | |
| Female | 24 (30) | 3 (11) | |
| Age, years | .2 | ||
| Median (IQR) | 68, (58-73) | 69, (61-75) | |
| Range | 33-89 | 55-89 | |
| ECOG, No. (%) | .9 | ||
| 0 | 26 (33) | 10 (36) | |
| 1 | 43 (54) | 14 (50) | |
| ≥2 | 10 (13) | 4 (14) | |
| Hemoglobin, g/dL, No. (%) | .6 | ||
| ≥10 | 76 (95) | 28 (100) | |
| <10 | 4 (5.0) | 0 (0) | |
| Liver metastases, No. (%) | .011 | ||
| No | 51 (64) | 25 (89) | |
| Yes | 29 (36) | 3 (11) |
NOTE. Significant P values are highlighted in bold.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; mUC-EV, metastatic urothelial cancer-enfortumab vedotin.
Pearson's chi-squared test; Wilcoxon rank-sum test; Fisher's exact test.
TABLE A2.
Baseline Characteristics of mUC-Non-EV
| Characteristic | Nonamplified (n = 76) | Amplified (n = 27) | P a |
|---|---|---|---|
| Sex, No. (%) | .001 | ||
| Male | 53 (70) | 27 (100) | |
| Female | 23 (30) | 0 (0) | |
| Age, years | .069 | ||
| Median (IQR) | 67 (56-72) | 71 (62-75) | |
| Range | 39-82 | 25-85 |
NOTE. Significant P values are highlighted in bold.
Abbreviation: mUC-Non-EV, metastatic urothelial cancer-non enfortumab vedotin.
Pearson's chi-squared test; Wilcoxon rank-sum test.
TABLE A3.
Baseline Characteristics of TCGA-BCLA Cohort
| Characteristic | Nonamplified, n = 336 | Amplified, n = 72 | P a |
|---|---|---|---|
| Sex, No. (%) | .15 | ||
| Male | 243 (72) | 58 (81) | |
| Female | 93 (28) | 14 (19) | |
| Age, years | .013 | ||
| Median (IQR) | 68 (60-75) | 73 (66-78) | |
| Range | 34-90 | 44-88 |
NOTE. Significant P values are highlighted in bold.
Abbreviation: TCGA-BCLA, The Cancer Genome Atlas - Bladder Cancer.
Pearson's chi-squared test; Wilcoxon rank-sum test.
FIG A1.
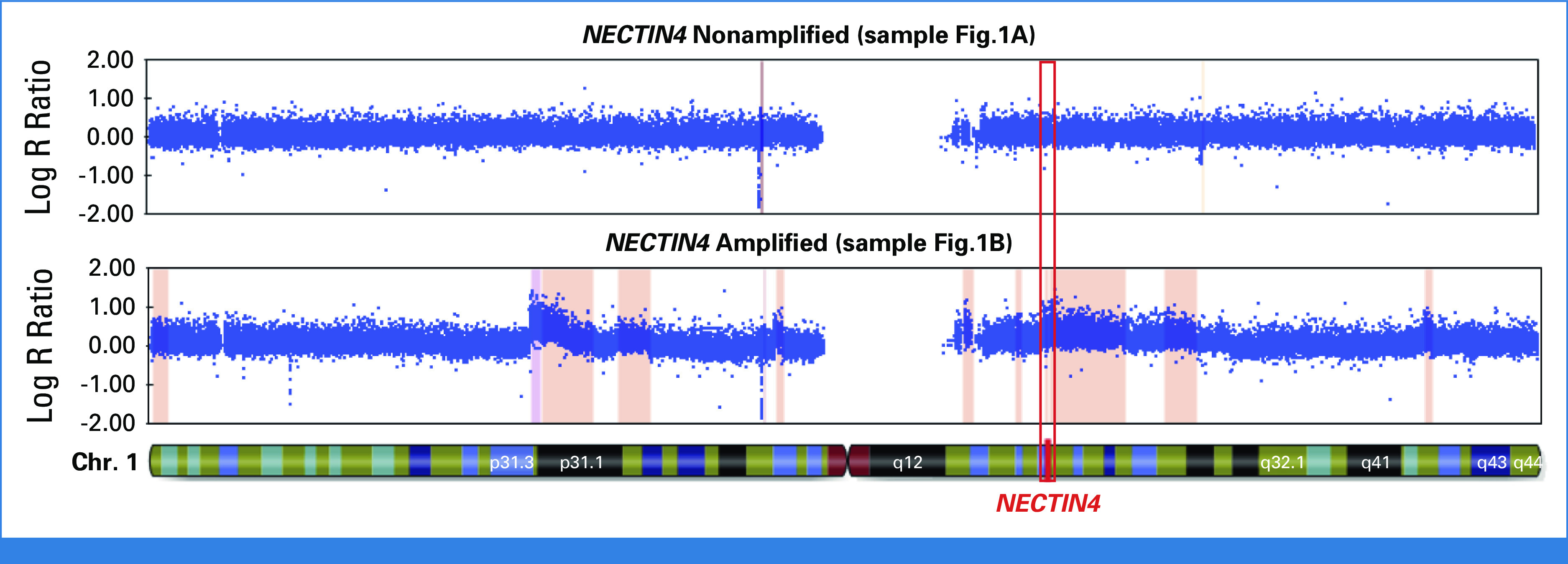
Illustration of copy number variation profiles derived by Illumina SNP arrays. Upper panel: NECTIN4 nonamplified tumor profile; lower panel: NECTIN4 amplified tumor profile. The NECTIN4 gene location on Chr. 1 shows higher copy numbers in the amplified tumors. Chr.1, chromosome 1; SNP, single nucleotide polymorphisms.
FIG A2.
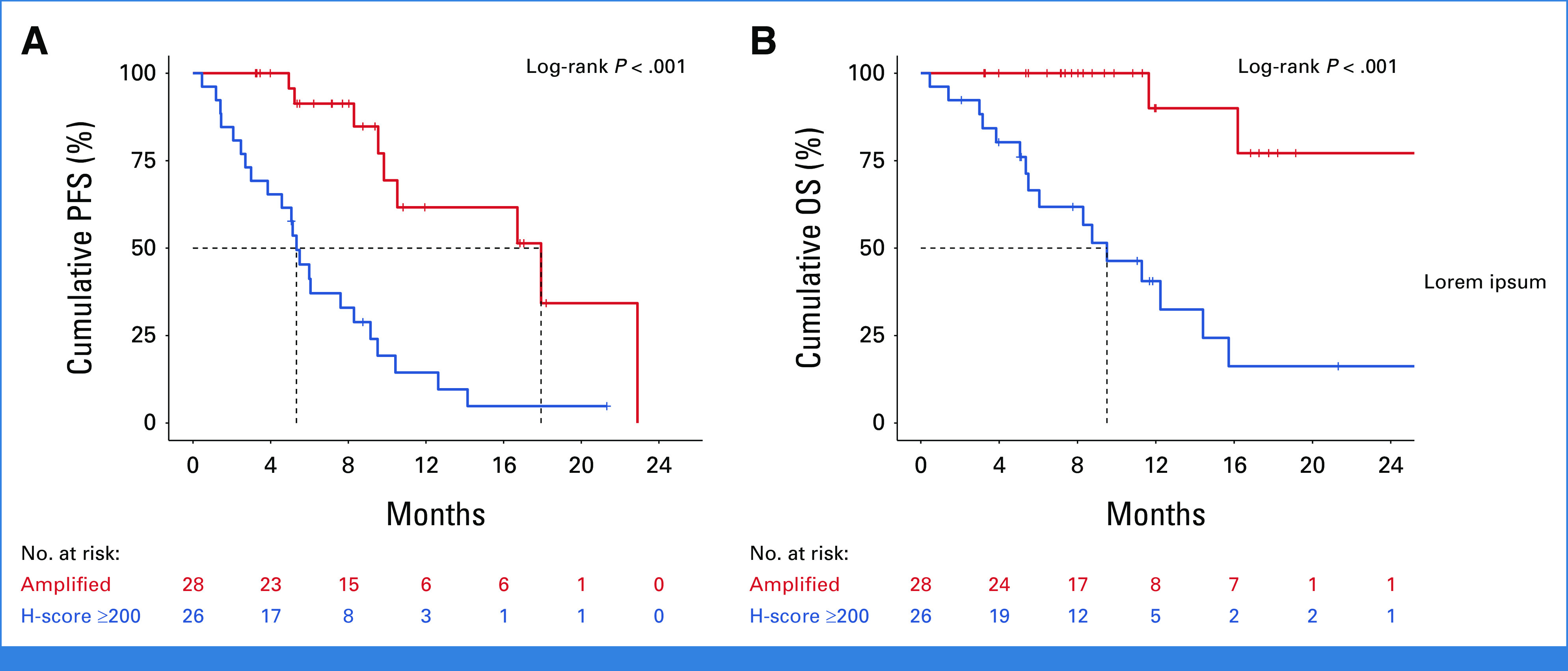
PFS (A) and OS (B) upon initiation of EV treatment stratified by presence of NECTIN4 gene amplification versus high membranous NECTIN4 protein expression without NECTIN4 gene amplification. EV, enfortumab vedotin; OS, overall survival; PFS, progression-free survival.
FIG A3.
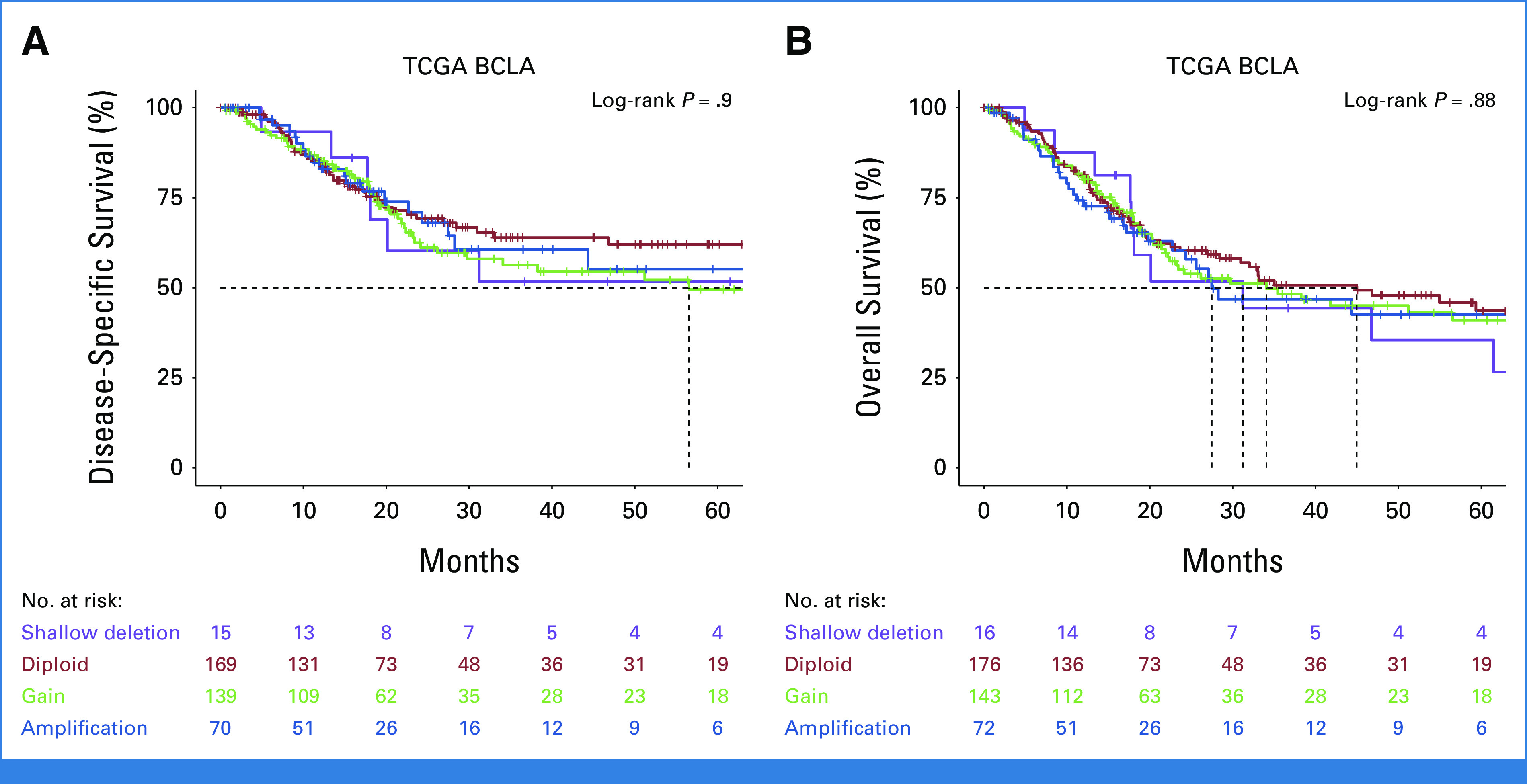
(A) Disease-specific survival of n = 393 patients of the TCGA-BLCA cohort stratified by copy number alterations of NECTIN4 (data were missing for 14 patients). (B) Overall survival of n = 407 patients of the TCGA-BLCA cohort stratified by copy number alterations of NECTIN4. TCGA-BLCA, The Cancer Genome Atlas-bladder cancer.
FIG A4.
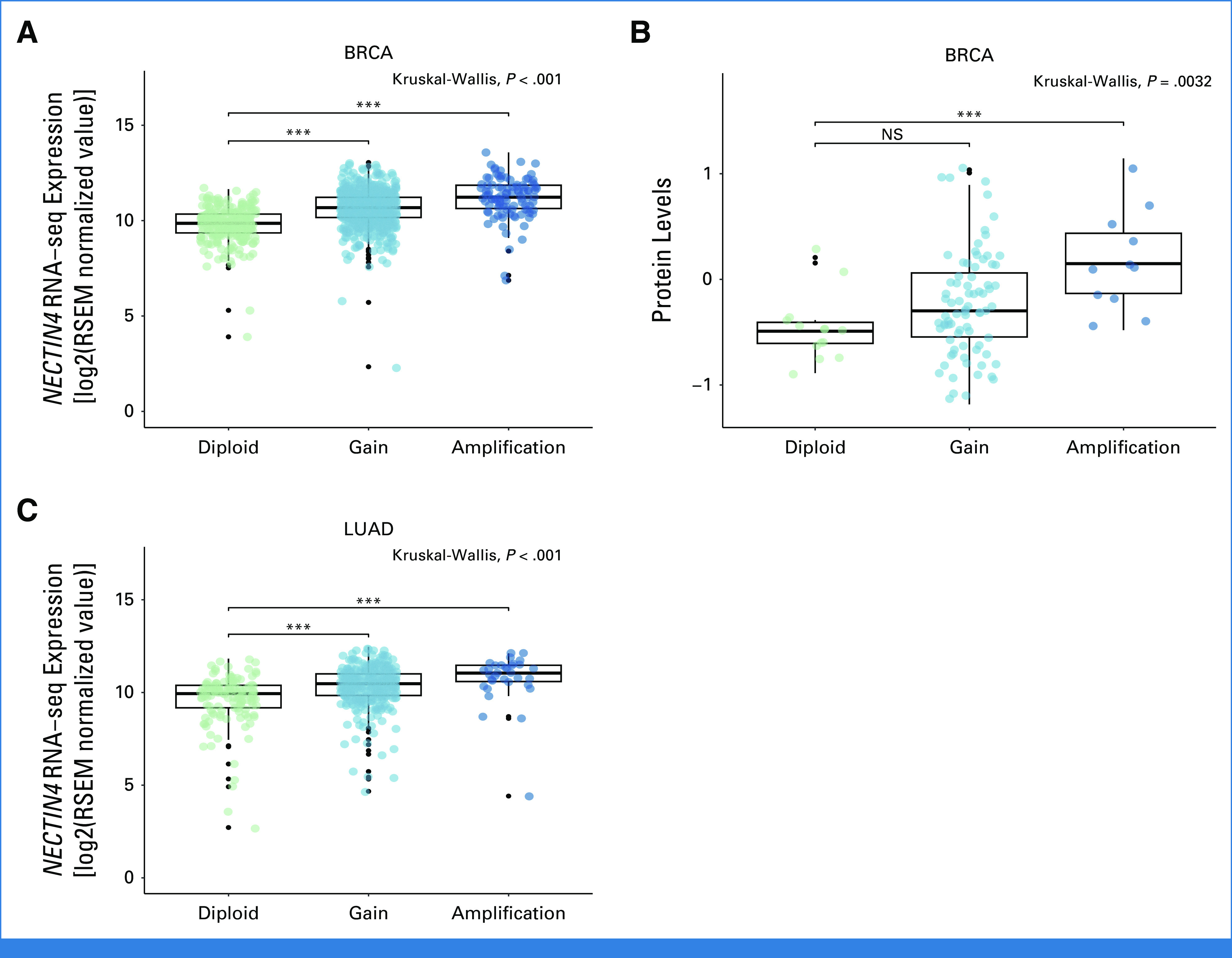
(A) NECTIN4 mRNA expression (log2 normalized RSEM values) in TCGA-BRCA cohort stratified by NECTIN4 copy number alterations. (B) NECTIN4 protein expression levels (Z-score scaled results from RPPA) in TCGA-BRCA cohort stratified by NECTIN4 copy number alterations. (C) NECTIN4 mRNA expression (log2 normalized RSEM values) in TCGA-LUAD cohort stratified by NECTIN4 copy number alterations. NS, not significant; RPPA, reverse-phase protein arrays; TCGA-BRCA, The Cancer Genome Atlas breast cancer; TCGA-LUAD; The Cancer Genome Atlas lung adenocarcinoma.
Niklas Klümper
Stock and Other Ownership Interests: Bicycle Therapeutics, Pfizer, Daiichi Sankyo/UCB Japan, immatics
Honoraria: Astellas Pharma, MSD Oncology
Consulting or Advisory Role: Astellas Pharma, MSD Oncology, Eisai
Travel, Accommodations, Expenses: Ipsen, Photocure, MSD Oncology
Stefanie Zschäbitz
Honoraria: Amgen, Eisai Germany, Merck Serono, Pfizer (Inst), MSD Oncology, BMS GmbH & Co KG, Janssen Oncology, Ipsen, Astellas Pharma, Novartis, Bayer
Consulting or Advisory Role: Pfizer (Inst), MSD Oncology, Merck, Sanofi/Aventis (Inst), EUSA Pharma, Roche, Bristol Myers Squibb (Inst), Eisai, Amgen, Astellas Pharma (Inst), Ipsen, Bayer (Inst), Gilead Sciences
Research Funding: Eisai (Inst)
Travel, Accommodations, Expenses: Merck, Pfizer, BMS GmbH & Co KG, Ipsen, Amgen, Astellas Pharma, Janssen Oncology
Oliver Hahn
Honoraria: Bayer, Bristol Myers Squibb, AstraZeneca, Eisai Germany, Medac, Merck
Consulting or Advisory Role: AstraZeneca, Bayer, Bristol Myers Squibb, Eisai
Travel, Accommodations, Expenses: Bayer
Thomas Büttner
Honoraria: Astellas Pharma
Travel, Accommodations, Expenses: Ipsen, MSD
Florian Roghmann
Consulting or Advisory Role: Janssen (Inst), BMS (Inst), Roche (Inst), Pfizer, Merck Serono (Inst), Merck (Inst), Astellas Pharma (Inst)
Travel, Accommodations, Expenses: Janssen (Inst), Merck Serono (Inst)
Christian Bolenz
Honoraria: Janssen-Cilag GmbH, AstraZeneca, Bayer, Bristol Myers Squibb
Consulting or Advisory Role: AstraZeneca, Bristol Myers Squibb
Research Funding: ERBE Elektromedizin (Inst), Thericon GmbH, AstraZeneca (Inst)
Travel, Accommodations, Expenses: Janssen Cilag
Uncompensated Relationships: Thericon GmbH
Friedemann Zengerling
Honoraria: Ipsen, Pfizer, Astellas Pharma, Janssen, Merck, Bayer/Vital, Amgen, Apogepha
Consulting or Advisory Role: Bristol Myers Squibb, IPSEN, Merck Sharp & Dohme, Bayer Health, Apogepha, Pfizer, Janssen, Roche, AstraZeneca, Merck, Astellas Pharma, Novartis
Travel, Accommodations, Expenses: Astellas Pharma, IPSEN, Janssen, Pfizer
Constantin Schwab
Stock and Other Ownership Interests: Illumina, Novo Nordisk
Honoraria: MSD, Bayer Germany
Marieta Toma
Honoraria: Gilead Sciences
Hendrik Heers
Honoraria: Janssen, Merck/Pfizer, BMS GmbH & Co KG, AstraZeneca
Consulting or Advisory Role: Novartis, Bayer
Research Funding: BMS GmbH & Co KG (Inst), Ipsen (Inst), Pfizer (Inst), Merck Serono (Inst)
Travel, Accommodations, Expenses: Merck/Pfizer, Janssen
Philipp Ivanyi
Stock and Other Ownership Interests: BB Biotech Ventures
Honoraria: Bayer/Vital, Bristol Myers Squibb, Ipsen, Eisai, AIMM Therapeutics, EUSA Pharma, Roche Pharma AG, MSD Oncology, Merck Serono, AstraZeneca
Consulting or Advisory Role: Bayer/Vital, Bristol Myers Squibb, Ipsen, Merck Serono, Pfizer, MSD, Eisai, ClinSol, Deciphera, EUSA Pharma
Travel, Accommodations, Expenses: Bristol Myers Squibb, Ipsen, Merck Serono
Other Relationship: Merck Serono, Pfizer
Uncompensated Relationships: German Cancer Society, AIO-Studien
Günter Niegisch
Honoraria: Roche Pharma AG, Medac, AstraZeneca, Astellas Pharma, Pfizer, BMS GmbH & Co KG, Eisai Germany, Janssen Oncology
Consulting or Advisory Role: Roche Pharma AG, Bristol Myers Squibb, Janssen Oncology, Pfizer, Merck/Pfizer, Astellas Pharma, AstraZeneca
Travel, Accommodations, Expenses: Janssen Oncology, Roche, AstraZeneca
Camilla Marisa Grunewald
Consulting or Advisory Role: Ferring
Christopher Darr
Honoraria: Janssen, Ipsen, Bayer
Consulting or Advisory Role: Janssen, Ipsen
Research Funding: Deutsche Forschungsgemeinschaft (Inst)
Travel, Accommodations, Expenses: Ipsen, Janssen, Bayer
Katrin Schlack
Honoraria: AAA HealthCare, Amgen (Inst), Astellas Pharma, AstraZeneca, Bayer Germany, Eisai Germany, Fosanis, Ipsen, Janssen, Merck Serono, MSD, Novartis, Pfizer
Consulting or Advisory Role: Apogepha, Bayer, BMS GmbH & Co KG, Ipsen, Janssen, Pfizer
Travel, Accommodations, Expenses: AstraZeneca, Bayer, Ipsen, Janssen, Pfizer
Can Aydogdu
Travel, Accommodations, Expenses: Ipsen, Eisai
Jozefina Casuscelli
Research Funding: AstraZeneca (Inst), MSD Oncology (Inst)
Expert Testimony: Janssen Cilag, MSD Oncology, Astellas Pharma
Travel, Accommodations, Expenses: Bayer, Janssen Oncology, Merck KGaA, Pfizer
Theresa Mokry
Honoraria: Bayer, Bracco Diagnostics, Deutsche Röntgengesellschaft, AKD Congress + Events
Research Funding: Philips Healthcare (Inst)
Dora Niedersüß-Beke
Consulting or Advisory Role: MSD Oncology, BMS GmbH & Co KG
Speakers' Bureau: BMS GmbH & Co KG, MSD Oncology, Astellas Pharma, Ipsen
Research Funding: Astellas Pharma
Steffen Rausch
Honoraria: Merck, Merck/Pfizer, Advanced Accelerator Applications, Janssen, Astellas Pharma, Bayer, Amgen, Ipsen, Bristol Myers Squibb/Sanofi
Consulting or Advisory Role: Ambu, Merck/Pfizer, Bayer, Bristol Myers Squibb/Sanofi, Eisai
Speakers' Bureau: Bayer
Research Funding: Ambu
Travel, Accommodations, Expenses: Janssen, ipsen, Bayer, Merck/Pfizer
Dimo Dietrich
Research Funding: Novartis (Inst)
Patents, Royalties, Other Intellectual Property: Patents (licensed to Qiagen)
Jonas Saal
Travel, Accommodations, Expenses: Janssen
Manuel Ritter
Honoraria: Medac, Medac, Janssen Oncology
Research Funding: Procept Biorobotics (Inst)
Travel, Accommodations, Expenses: Medac
Joshua Meeks
Honoraria: Janssen, Merck, AstraZeneca, incyte, Urogen pharma, Prokarium, Imvax, Astellas Pharma, Pfizer
Research Funding: Epizyme, Merck Sharp & Dohme
Patents, Royalties, Other Intellectual Property: NMIBC genetic classifier (Inst), TCGA Classifier (Inst)
Other Relationship: Olympus
Francisco E. Vera Badillo
Consulting or Advisory Role: Bayer, AstraZeneca, Janssen Oncology, Merck, Bayer, AstraZeneca, Janssen Oncology, Janssen Oncology, Merck, Merck Serono
Research Funding: AstraZeneca/MedImmune (Inst), Janssen Oncology (Inst)
Travel, Accommodations, Expenses: Janssen Oncology
Joost Boormans
Consulting or Advisory Role: Bristol Myers Squibb/Sanofi, Janssen, MSD, ISMAR Health Care, Merck KGaA
Research Funding: Merck KGaA, Vitroscan, Janssen
Kerstin Junker
Stock and Other Ownership Interests: Bayer
Honoraria: Ipsen
Arndt Hartmann
Honoraria: BMS, MSD, Roche, AstraZeneca, Boehringer Ingelheim, Abbvie, Janssen-Cilag, Ipsen
Consulting or Advisory Role: Bristol Myers Squibb, MSD, Roche, Cepheid, Qiagen, Janssen-Cilag, AstraZeneca, Ipsen, NanoString Technologies, Illumina, 3DHISTECH, Diaceutics
Research Funding: Cepheid, BioNTech, Roche, Janssen-Cilag, NanoString Technologies, AstraZeneca, Owkin (Inst), Phäon (Inst)
Expert Testimony: NanoString Technologies
Viktor Grünwald
Employment: University Hospital Essen
Stock and Other Ownership Interests: MSD, Bristol Myers Squibb, AstraZeneca, Genmab, Bicycle Therapeutics
Honoraria: Bristol Myers Squibb, Pfizer, Ipsen, Eisai, MSD Oncology, Merck Serono, AstraZeneca, Janssen-Cilag, Advanced Accelerator Applications/Novartis, Apogepha, Ono Pharmaceutical, Astellas Pharma, Amgen
Consulting or Advisory Role: Bristol Myers Squibb, Pfizer, Novartis, MSD Oncology, Ipsen, Janssen-Cilag, Eisai, Debiopharm Group, PCI Biotech, Gilead Sciences, Cureteq, Oncorena, Synthekine
Research Funding: Amgen (Inst), MSD Oncology (Inst), BMS (Inst), Seagen (Inst), Ipsen (Inst), Gilead Sciences (Inst), Bicycle Therapeutics (Inst)
Travel, Accommodations, Expenses: Pfizer, AstraZeneca, Janssen, Merck Serono, Ipsen
Michael Hölzel
Stock and Other Ownership Interests: Bicycle Therapeutics, Daiichi Sankyo/UCB Japan, immatics
Honoraria: Novartis, Bristol Myers Squibb/Roche, TME Pharma
Consulting or Advisory Role: TME Pharma, Novartis
Research Funding: TME Pharma
Patents, Royalties, Other Intellectual Property: EP 23 000 076.2 and EP 23 000 075.4 patent applications related to biomarkers for NOX-A12 therapy
Travel, Accommodations, Expenses: TME Pharma
Markus Eckstein
Employment: Diaceutics
Honoraria: AstraZeneca, Roche, Astellas Pharma, Genomic Health, Janssen-Cilag, Owkin, Diaceutics
Consulting or Advisory Role: AstraZeneca, Janssen-Cilag, Genomic Health, Diaceutics, Gilead Sciences, Owkin, MSD Oncology
Speakers' Bureau: Diaceutics, Roche, AstraZeneca, MSD, Astellas Pharma
Research Funding: STRATIFYER Molecular Pathology, Janssen, AstraZeneca/MedImmune, AstraZeneca/MedImmune, Owkin, Gilead Sciences
Travel, Accommodations, Expenses: AstraZeneca, Roche, MSD, Janssen-Cilag, Genomic Health, Diaceutics, Astellas Pharma
No other potential conflicts of interest were reported.
DISCLAIMER
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
SUPPORT
Supported by the BMBF-funded Advanced Clinician Scientist Program Bonn (ACCENT) of the Medical Faculty of the University of Bonn (grant ID: 01EO2107; N.K.) and the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) Young Investigator Academy UroAgeCare (grant ID: 511985506; N.K.); the DFG under Germany's Excellence Strategy (EXC2151–390873048; M.H.); and by the Else Kröner-Fresenius Foundation/EKFS (2020_EKEA.129; 2023_EKES.07; M.E.), the Clinician Scientist program of the IZKF of the FAU, the TOPeCS funding line of the IZKF (T04) of the FAU, an advanced research grant of the IZKF of the FAU Erlangen-Nürnberg (IZKF-FAU D41; M.E.), and a Young Clinical Scientist Fellowship of the Bavarian Center for Cancer Research (BZKF; YSF-TP01; M.E.).
N.K. and N.K.T. contributed equally to this work. M.H. and M.E. are joint senior authors.
DATA SHARING STATEMENT
The results are in part based upon publicly available data generated by the TCGA Research Network: https://www.cancer.gov/tcga. Further data that support the findings of this study are available from the corresponding author upon reasonable request.
AUTHOR CONTRIBUTIONS
Conception and design: Niklas Klümper, Ngoc Khanh Tran, Christian Bolenz, Steffen Rausch, Manuel Ritter, Arndt Hartmann, Michael Hölzel, Markus Eckstein
Financial support: Niklas Klümper, Arndt Hartmann, Michael Hölzel, Markus Eckstein
Administrative support: Niklas Klümper, Ngoc Khanh Tran, Florian Roghmann, Christian Bolenz, Manuel Ritter, Arndt Hartmann, Michael Hölzel, Markus Eckstein
Provision of study materials or patients: Niklas Klümper, Stefanie Zschäbitz, Oliver Hahn, Thomas Büttner, Florian Roghmann, Christian Bolenz, Friedemann Zengerling, Marieta Toma, Glen Kristiansen, Hendrik Heers, Philipp Ivanyi, Günter Niegisch, Christopher Darr, Arian Farid, Can Aydogdu, Dora Niedersüß-Beke, Steffen Rausch, Jonas Saal, Jörg Ellinger, Manuel Ritter, Markus Eckstein
Collection and assembly of data: Niklas Klümper, Ngoc Khanh Tran, Stefanie Zschäbitz, Oliver Hahn, Thomas Büttner, Florian Roghmann, Christian Bolenz, Friedemann Zengerling, Constantin Schwab, Dora Nagy, Marieta Toma, Hendrik Heers, Philipp Ivanyi, Günter Niegisch, Camilla Marisa Grunewald, Christopher Darr, Arian Farid, Katrin Schlack, Mahmoud Abbas, Can Aydogdu, Jozefina Casuscelli, Michael Mayr, Dora Niedersüß-Beke, Steffen Rausch, Jonas Saal, J. Alberto Nakauma-González, Arndt Hartmann, Viktor Grünwald, Michael Hölzel, Markus Eckstein
Data analysis and interpretation: Niklas Klümper, Ngoc Khanh Tran, Stefanie Zschäbitz, Oliver Hahn, Florian Roghmann, Christian Bolenz, Glen Kristiansen, Philipp Ivanyi, Theresa Mokry, Steffen Rausch, Dimo Dietrich, Jörg Ellinger, Manuel Ritter, Abdullah Alajati, Christoph Kuppe, Joshua Meeks, Francisco E. Vera Badillo, Joost Boormans, Kerstin Junker, Arndt Hartmann, Michael Hölzel, Markus Eckstein
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
NECTIN4 Amplification Is Frequent in Solid Tumors and Predicts Enfortumab Vedotin Response in Metastatic Urothelial Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Niklas Klümper
Stock and Other Ownership Interests: Bicycle Therapeutics, Pfizer, Daiichi Sankyo/UCB Japan, immatics
Honoraria: Astellas Pharma, MSD Oncology
Consulting or Advisory Role: Astellas Pharma, MSD Oncology, Eisai
Travel, Accommodations, Expenses: Ipsen, Photocure, MSD Oncology
Stefanie Zschäbitz
Honoraria: Amgen, Eisai Germany, Merck Serono, Pfizer (Inst), MSD Oncology, BMS GmbH & Co KG, Janssen Oncology, Ipsen, Astellas Pharma, Novartis, Bayer
Consulting or Advisory Role: Pfizer (Inst), MSD Oncology, Merck, Sanofi/Aventis (Inst), EUSA Pharma, Roche, Bristol Myers Squibb (Inst), Eisai, Amgen, Astellas Pharma (Inst), Ipsen, Bayer (Inst), Gilead Sciences
Research Funding: Eisai (Inst)
Travel, Accommodations, Expenses: Merck, Pfizer, BMS GmbH & Co KG, Ipsen, Amgen, Astellas Pharma, Janssen Oncology
Oliver Hahn
Honoraria: Bayer, Bristol Myers Squibb, AstraZeneca, Eisai Germany, Medac, Merck
Consulting or Advisory Role: AstraZeneca, Bayer, Bristol Myers Squibb, Eisai
Travel, Accommodations, Expenses: Bayer
Thomas Büttner
Honoraria: Astellas Pharma
Travel, Accommodations, Expenses: Ipsen, MSD
Florian Roghmann
Consulting or Advisory Role: Janssen (Inst), BMS (Inst), Roche (Inst), Pfizer, Merck Serono (Inst), Merck (Inst), Astellas Pharma (Inst)
Travel, Accommodations, Expenses: Janssen (Inst), Merck Serono (Inst)
Christian Bolenz
Honoraria: Janssen-Cilag GmbH, AstraZeneca, Bayer, Bristol Myers Squibb
Consulting or Advisory Role: AstraZeneca, Bristol Myers Squibb
Research Funding: ERBE Elektromedizin (Inst), Thericon GmbH, AstraZeneca (Inst)
Travel, Accommodations, Expenses: Janssen Cilag
Uncompensated Relationships: Thericon GmbH
Friedemann Zengerling
Honoraria: Ipsen, Pfizer, Astellas Pharma, Janssen, Merck, Bayer/Vital, Amgen, Apogepha
Consulting or Advisory Role: Bristol Myers Squibb, IPSEN, Merck Sharp & Dohme, Bayer Health, Apogepha, Pfizer, Janssen, Roche, AstraZeneca, Merck, Astellas Pharma, Novartis
Travel, Accommodations, Expenses: Astellas Pharma, IPSEN, Janssen, Pfizer
Constantin Schwab
Stock and Other Ownership Interests: Illumina, Novo Nordisk
Honoraria: MSD, Bayer Germany
Marieta Toma
Honoraria: Gilead Sciences
Hendrik Heers
Honoraria: Janssen, Merck/Pfizer, BMS GmbH & Co KG, AstraZeneca
Consulting or Advisory Role: Novartis, Bayer
Research Funding: BMS GmbH & Co KG (Inst), Ipsen (Inst), Pfizer (Inst), Merck Serono (Inst)
Travel, Accommodations, Expenses: Merck/Pfizer, Janssen
Philipp Ivanyi
Stock and Other Ownership Interests: BB Biotech Ventures
Honoraria: Bayer/Vital, Bristol Myers Squibb, Ipsen, Eisai, AIMM Therapeutics, EUSA Pharma, Roche Pharma AG, MSD Oncology, Merck Serono, AstraZeneca
Consulting or Advisory Role: Bayer/Vital, Bristol Myers Squibb, Ipsen, Merck Serono, Pfizer, MSD, Eisai, ClinSol, Deciphera, EUSA Pharma
Travel, Accommodations, Expenses: Bristol Myers Squibb, Ipsen, Merck Serono
Other Relationship: Merck Serono, Pfizer
Uncompensated Relationships: German Cancer Society, AIO-Studien
Günter Niegisch
Honoraria: Roche Pharma AG, Medac, AstraZeneca, Astellas Pharma, Pfizer, BMS GmbH & Co KG, Eisai Germany, Janssen Oncology
Consulting or Advisory Role: Roche Pharma AG, Bristol Myers Squibb, Janssen Oncology, Pfizer, Merck/Pfizer, Astellas Pharma, AstraZeneca
Travel, Accommodations, Expenses: Janssen Oncology, Roche, AstraZeneca
Camilla Marisa Grunewald
Consulting or Advisory Role: Ferring
Christopher Darr
Honoraria: Janssen, Ipsen, Bayer
Consulting or Advisory Role: Janssen, Ipsen
Research Funding: Deutsche Forschungsgemeinschaft (Inst)
Travel, Accommodations, Expenses: Ipsen, Janssen, Bayer
Katrin Schlack
Honoraria: AAA HealthCare, Amgen (Inst), Astellas Pharma, AstraZeneca, Bayer Germany, Eisai Germany, Fosanis, Ipsen, Janssen, Merck Serono, MSD, Novartis, Pfizer
Consulting or Advisory Role: Apogepha, Bayer, BMS GmbH & Co KG, Ipsen, Janssen, Pfizer
Travel, Accommodations, Expenses: AstraZeneca, Bayer, Ipsen, Janssen, Pfizer
Can Aydogdu
Travel, Accommodations, Expenses: Ipsen, Eisai
Jozefina Casuscelli
Research Funding: AstraZeneca (Inst), MSD Oncology (Inst)
Expert Testimony: Janssen Cilag, MSD Oncology, Astellas Pharma
Travel, Accommodations, Expenses: Bayer, Janssen Oncology, Merck KGaA, Pfizer
Theresa Mokry
Honoraria: Bayer, Bracco Diagnostics, Deutsche Röntgengesellschaft, AKD Congress + Events
Research Funding: Philips Healthcare (Inst)
Dora Niedersüß-Beke
Consulting or Advisory Role: MSD Oncology, BMS GmbH & Co KG
Speakers' Bureau: BMS GmbH & Co KG, MSD Oncology, Astellas Pharma, Ipsen
Research Funding: Astellas Pharma
Steffen Rausch
Honoraria: Merck, Merck/Pfizer, Advanced Accelerator Applications, Janssen, Astellas Pharma, Bayer, Amgen, Ipsen, Bristol Myers Squibb/Sanofi
Consulting or Advisory Role: Ambu, Merck/Pfizer, Bayer, Bristol Myers Squibb/Sanofi, Eisai
Speakers' Bureau: Bayer
Research Funding: Ambu
Travel, Accommodations, Expenses: Janssen, ipsen, Bayer, Merck/Pfizer
Dimo Dietrich
Research Funding: Novartis (Inst)
Patents, Royalties, Other Intellectual Property: Patents (licensed to Qiagen)
Jonas Saal
Travel, Accommodations, Expenses: Janssen
Manuel Ritter
Honoraria: Medac, Medac, Janssen Oncology
Research Funding: Procept Biorobotics (Inst)
Travel, Accommodations, Expenses: Medac
Joshua Meeks
Honoraria: Janssen, Merck, AstraZeneca, incyte, Urogen pharma, Prokarium, Imvax, Astellas Pharma, Pfizer
Research Funding: Epizyme, Merck Sharp & Dohme
Patents, Royalties, Other Intellectual Property: NMIBC genetic classifier (Inst), TCGA Classifier (Inst)
Other Relationship: Olympus
Francisco E. Vera Badillo
Consulting or Advisory Role: Bayer, AstraZeneca, Janssen Oncology, Merck, Bayer, AstraZeneca, Janssen Oncology, Janssen Oncology, Merck, Merck Serono
Research Funding: AstraZeneca/MedImmune (Inst), Janssen Oncology (Inst)
Travel, Accommodations, Expenses: Janssen Oncology
Joost Boormans
Consulting or Advisory Role: Bristol Myers Squibb/Sanofi, Janssen, MSD, ISMAR Health Care, Merck KGaA
Research Funding: Merck KGaA, Vitroscan, Janssen
Kerstin Junker
Stock and Other Ownership Interests: Bayer
Honoraria: Ipsen
Arndt Hartmann
Honoraria: BMS, MSD, Roche, AstraZeneca, Boehringer Ingelheim, Abbvie, Janssen-Cilag, Ipsen
Consulting or Advisory Role: Bristol Myers Squibb, MSD, Roche, Cepheid, Qiagen, Janssen-Cilag, AstraZeneca, Ipsen, NanoString Technologies, Illumina, 3DHISTECH, Diaceutics
Research Funding: Cepheid, BioNTech, Roche, Janssen-Cilag, NanoString Technologies, AstraZeneca, Owkin (Inst), Phäon (Inst)
Expert Testimony: NanoString Technologies
Viktor Grünwald
Employment: University Hospital Essen
Stock and Other Ownership Interests: MSD, Bristol Myers Squibb, AstraZeneca, Genmab, Bicycle Therapeutics
Honoraria: Bristol Myers Squibb, Pfizer, Ipsen, Eisai, MSD Oncology, Merck Serono, AstraZeneca, Janssen-Cilag, Advanced Accelerator Applications/Novartis, Apogepha, Ono Pharmaceutical, Astellas Pharma, Amgen
Consulting or Advisory Role: Bristol Myers Squibb, Pfizer, Novartis, MSD Oncology, Ipsen, Janssen-Cilag, Eisai, Debiopharm Group, PCI Biotech, Gilead Sciences, Cureteq, Oncorena, Synthekine
Research Funding: Amgen (Inst), MSD Oncology (Inst), BMS (Inst), Seagen (Inst), Ipsen (Inst), Gilead Sciences (Inst), Bicycle Therapeutics (Inst)
Travel, Accommodations, Expenses: Pfizer, AstraZeneca, Janssen, Merck Serono, Ipsen
Michael Hölzel
Stock and Other Ownership Interests: Bicycle Therapeutics, Daiichi Sankyo/UCB Japan, immatics
Honoraria: Novartis, Bristol Myers Squibb/Roche, TME Pharma
Consulting or Advisory Role: TME Pharma, Novartis
Research Funding: TME Pharma
Patents, Royalties, Other Intellectual Property: EP 23 000 076.2 and EP 23 000 075.4 patent applications related to biomarkers for NOX-A12 therapy
Travel, Accommodations, Expenses: TME Pharma
Markus Eckstein
Employment: Diaceutics
Honoraria: AstraZeneca, Roche, Astellas Pharma, Genomic Health, Janssen-Cilag, Owkin, Diaceutics
Consulting or Advisory Role: AstraZeneca, Janssen-Cilag, Genomic Health, Diaceutics, Gilead Sciences, Owkin, MSD Oncology
Speakers' Bureau: Diaceutics, Roche, AstraZeneca, MSD, Astellas Pharma
Research Funding: STRATIFYER Molecular Pathology, Janssen, AstraZeneca/MedImmune, AstraZeneca/MedImmune, Owkin, Gilead Sciences
Travel, Accommodations, Expenses: AstraZeneca, Roche, MSD, Janssen-Cilag, Genomic Health, Diaceutics, Astellas Pharma
No other potential conflicts of interest were reported.
REFERENCES
- 1.Powles T, Rosenberg JE, Sonpavde GP, et al. : Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med 384:1125-1135, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu EY, Petrylak DP, O’Donnell PH, et al. : Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV-201): A multicentre, single-arm, phase 2 trial. Lancet Oncol 22:872-882, 2021 [DOI] [PubMed] [Google Scholar]
- 3.Hoimes CJ, Flaig TW, Milowsky MI, et al. : Enfortumab vedotin plus pembrolizumab in previously untreated advanced urothelial cancer. J Clin Oncol 41:22-31, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Donnell PH, Milowsky MI, Petrylak DP, et al. : Enfortumab vedotin with or without pembrolizumab in cisplatin-ineligible patients with previously untreated locally advanced or metastatic urothelial cancer. J Clin Oncol 41:4107-4117, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powles TB, Perez Valderrama B, Gupta S, et al. : LBA6 EV-302/KEYNOTE-A39: Open-label, randomized phase III study of enfortumab vedotin in combination with pembrolizumab (EV+P) vs chemotherapy (Chemo) in previously untreated locally advanced metastatic urothelial carcinoma (la/mUC). Ann Oncol 34:S1340, 2023 [Google Scholar]
- 6.UC trials get standing ovation. Cancer Discov 13:2496, 2023 [DOI] [PubMed] [Google Scholar]
- 7.Chu CE, Sjöström M, Egusa EA, et al. : Heterogeneity in NECTIN4 expression across molecular subtypes of urothelial cancer mediates sensitivity to enfortumab vedotin. Clin Cancer Res 27:5123-5130, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffman-Censits J, Lombardo K, McConkey D, et al. : New and topics: Enfortumab vedotin mechanisms of response and resistance in urothelial cancer—What do we understand so far? Urol Oncol 39:619-622, 2021 [DOI] [PubMed] [Google Scholar]
- 9.Heath EI, Rosenberg JE: The biology and rationale of targeting nectin-4 in urothelial carcinoma. Nat Rev Urol 18:93-103, 2021 [DOI] [PubMed] [Google Scholar]
- 10.Klümper N, Ralser DJ, Ellinger J, et al. : Membranous NECTIN-4 expression frequently decreases during metastatic spread of urothelial carcinoma and is associated with enfortumab vedotin resistance. Clin Cancer Res 29:1496-1505, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tagawa ST, Balar AV, Petrylak DP, et al. : TROPHY-U-01: A phase II open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors. J Clin Oncol 39:2474-2485, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loriot Y, Necchi A, Park SH, et al. : Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med 381:338-348, 2019 [DOI] [PubMed] [Google Scholar]
- 13.Loriot Y, Matsubara N, Park SH, et al. : Phase 3 THOR study: Results of erdafitinib (erda) versus chemotherapy (chemo) in patients (pts) with advanced or metastatic urothelial cancer (mUC) with select fibroblast growth factor receptor alterations (FGFRalt). J Clin Oncol 41, 2023. (suppl 17; abstr LBA4619) [Google Scholar]
- 14.Sheng X, Wang L, He Z, et al. : Efficacy and safety of disitamab vedotin in patients with human epidermal growth factor receptor 2–positive locally advanced or metastatic urothelial carcinoma: A combined analysis of two phase II clinical trials. J Clin Oncol 42:1391-1402, 2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller EJ, Galsky MD: Precision medicine in urothelial carcinoma: Current markers to guide treatment and promising future directions. Curr Treat Options Oncol 24:1870-1888, 2023 [DOI] [PubMed] [Google Scholar]
- 16.Corti C, Antonarelli G, Valenza C, et al. : Histology-agnostic approvals for antibody–drug conjugates in solid tumours: Is the time ripe? Eur J Cancer 171:25-42, 2022 [DOI] [PubMed] [Google Scholar]
- 17.Tarantino P, Carmagnani Pestana R, Corti C, et al. : Antibody–drug conjugates: Smart chemotherapy delivery across tumor histologies. CA Cancer J Clin 72:165-182, 2022 [DOI] [PubMed] [Google Scholar]
- 18.Chatterjee S, Sinha S, Kundu CN: Nectin cell adhesion molecule-4 (NECTIN-4): A potential target for cancer therapy. Eur J Pharmacol 911:174516, 2021 [DOI] [PubMed] [Google Scholar]
- 19.Bruce JY, Pusztai L, Braiteh FS, et al. : EV-202: A phase II study of enfortumab vedotin in patients with select previously treated locally advanced or metastatic solid tumors. J Clin Oncol 38, 2020. (suppl 15; abstr TPS3647) [Google Scholar]
- 20.Meric-Bernstam F, Makker V, Oaknin A, et al. : Efficacy and safety of trastuzumab deruxtecan in patients with HER2-expressing solid tumors: Primary results from the DESTINY-PanTumor02 phase II trial. J Clin Oncol 42:47-58, 2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosele F, Deluche E, Lusque A, et al. : Trastuzumab deruxtecan in metastatic breast cancer with variable HER2 expression: The phase 2 DAISY trial. Nat Med 29:2110-2120, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riester M, Werner L, Bellmunt J, et al. : Integrative analysis of 1q23.3 copy-number gain in metastatic urothelial carcinoma. Clin Cancer Res 20:1873-1883, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakauma-González JA, Rijnders M, van Riet J, et al. : Comprehensive molecular characterization reveals genomic and transcriptomic subtypes of metastatic urothelial carcinoma. Eur Urol 81:331-336, 2022 [DOI] [PubMed] [Google Scholar]
- 24.Cerami E, Gao J, Dogrusoz U, et al. : The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2:401-404, 2012. [Erratum: Cancer Discov 2:960, 2012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldman MJ, Craft B, Hastie M, et al. : Visualizing and interpreting cancer genomics data via the Xena platform. Nat Biotechnol 38:675-678, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 27.McShane LM, Altman DG, Sauerbrei W, et al. : Reporting recommendations for tumor marker prognostic studies. J Clin Oncol 23:9067-9072, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Mermel CH, Schumacher SE, Hill B, et al. : GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome Biol 12:R41, 20112023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Challita-Eid PM, Satpayev D, Yang P, et al. : Enfortumab vedotin antibody-drug conjugate targeting nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer Res 76:3003-3013, 2016 [DOI] [PubMed] [Google Scholar]
- 30.Bellmunt J, Choueiri TK, Fougeray R, et al. : Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol 28:1850-1855, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Grossman RL, Heath AP, Ferretti V, et al. : Toward a shared vision for cancer genomic data. N Engl J Med 375:1109-1112, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wahida A, Buschhorn L, Fröhling S, et al. : The coming decade in precision oncology: Six riddles. Nat Rev Cancer 23:43-54, 2023 [DOI] [PubMed] [Google Scholar]
- 33.Liu J, Lichtenberg T, Hoadley KA, et al. : An integrated TCGA pan-cancer clinical data resource to drive high-quality survival outcome analytics. Cell 173:400-416.e11, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ballman KV: Biomarker: Predictive or prognostic? J Clin Oncol 33:3968-3971, 2015 [DOI] [PubMed] [Google Scholar]
- 35.Zschäbitz S, Biernath N, Hilser T, et al. : Enfortumab vedotin in metastatic urothelial carcinoma: Survival and safety in a European multicenter real-world patient cohort. Eur Urol Open Sci 53:31-37, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koshkin VS, Henderson N, James M, et al. : Efficacy of enfortumab vedotin in advanced urothelial cancer: Analysis from the Urothelial Cancer Network to Investigate Therapeutic Experiences (UNITE) study. Cancer 128:1194-1205, 2022 [DOI] [PubMed] [Google Scholar]
- 37.Faltas BM, Prandi D, Tagawa ST, et al. : Clonal evolution of chemotherapy-resistant urothelial carcinoma. Nat Genet 48:1490-1499, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coates JT, Sun S, Leshchiner I, et al. : Parallel genomic alterations of antigen and payload targets mediate polyclonal acquired clinical resistance to sacituzumab govitecan in triple-negative breast cancer. Cancer Discov 11:2436-2445, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loganzo F, Tan X, Sung M, et al. : Tumor cells chronically treated with a trastuzumab-maytansinoid antibody-drug conjugate develop varied resistance mechanisms but respond to alternate treatments. Mol Cancer Ther 14:952-963, 2015 [DOI] [PubMed] [Google Scholar]
- 40.Pös O, Radvanszky J, Buglyó G, et al. : DNA copy number variation: Main characteristics, evolutionary significance, and pathological aspects. Biomed J 44:548-559, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bayle A, Bonastre J, Chaltiel D, et al. : ESMO study on the availability and accessibility of biomolecular technologies in oncology in Europe. Ann Oncol 34:934-945, 2023 [DOI] [PubMed] [Google Scholar]
- 42.Jindal T, Zhang L, Jiang C, et al. : Independent biomarkers predictive of outcomes with enfortumab vedotin (EV) in patients (pts) with advanced urothelial carcinoma (aUC): Analysis of the UNITE study. J Clin Oncol 41, 2023. (suppl 16; abstr 4573) [Google Scholar]
- 43.Kamoun A, de Reyniès A, Allory Y, et al. : A consensus molecular classification of muscle-invasive bladder cancer. Eur Urol 77:420-433, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bahlinger V, Branz A, Strissel PL, et al. : Associations of TACSTD2/TROP2 and NECTIN‐4/NECTIN ‐4 with molecular subtypes, PD‐L1 expression, and FGFR3 mutational status in two advanced urothelial bladder cancer cohorts. Histopathology 84:863-876, 2024 [DOI] [PubMed] [Google Scholar]
- 45.Jindal T, Zhang L, Deshmukh P, et al. : Impact of squamous histology on clinical outcomes and molecular profiling in metastatic urothelial carcinoma PatientsTreated with immune checkpoint inhibitors or enfortumab vedotin. Clin Genitourin Cancer 21:e394-e404, 2023 [DOI] [PubMed] [Google Scholar]
- 46.Vlachou E, Matoso A, McConkey D, et al. : Enfortumab vedotin-related cutaneous toxicity and radiographic response in patients with urothelial cancer: A single-center experience and review of the literature. Eur Urol Open Sci 49:100-103, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moore KN, Oza AM, Colombo N, et al. : Phase III, randomized trial of mirvetuximab soravtansine versus chemotherapy in patients with platinum-resistant ovarian cancer: Primary analysis of FORWARD I. Ann Oncol 32:757-765, 2021 [DOI] [PubMed] [Google Scholar]
- 48.Hu C, Dignam JJ: Biomarker-driven oncology clinical trials: Key design elements, types, features, and practical considerations. JCO Precis Oncol 10.1200/PO.19.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reardon B, Moore ND, Moore NS, et al. : Integrating molecular profiles into clinical frameworks through the Molecular Oncology Almanac to prospectively guide precision oncology. Nat Cancer 2:1102-1112, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Criscitiello C, Morganti S, Curigliano G: Antibody–drug conjugates in solid tumors: A look into novel targets. J Hematol Oncol 14:20, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uzunparmak B, Haymaker C, Raso G, et al. : HER2-low expression in patients with advanced or metastatic solid tumors. Ann Oncol 34:1035-1046, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bouleftour W, Guillot A, Magné N: The anti-nectin 4: A promising tumor cells target. A systematic review. Mol Cancer Ther 21:493-501, 2022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The results are in part based upon publicly available data generated by the TCGA Research Network: https://www.cancer.gov/tcga. Further data that support the findings of this study are available from the corresponding author upon reasonable request.


