Abstract
Background
Calcium channel blockers are a heterogeneous class of drugs, including dihydropyridine and non‐dihydropyridine subgroups, commonly used in the treatment of hypertension. A systematic review of the 24‐hour time course of the blood pressure‐lowering effect has not been published.
Objectives
To assess how much variation there is in hourly systolic and diastolic blood pressure lowering by dihydropyridine calcium channel blockers over a 24‐hour period in people with hypertension aged 18 years or over, with baseline systolic blood pressure of at least 140 mmHg or diastolic blood pressure of at least 90 mmHg, or both.
Search methods
We performed electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 1, 2014), MEDLINE (1946 to February 2014), EMBASE (1974 to February 2014), and ClinicalTrials.gov (to February 2014). We also screened references of published studies and reviews to identify additional trials.
Selection criteria
We included all randomized, placebo‐controlled trials assessing the hourly effects of dihydropyridine calcium channel blockers by ambulatory blood pressure monitoring in adults with hypertension with a follow‐up of at least three weeks.
Data collection and analysis
Two authors independently selected the included trials, evaluated the risk of bias, and analyzed the data.
Main results
We included 16 randomized controlled trials of dihydropyridine calcium channel blockers in this systematic review, with 2768 randomized participants. Drugs studied included amlodipine, lercanidipine, mandipine, nifedipine, and felodipine (all administered once daily) and nicardipine (administered twice daily). We analyzed and presented data by hour post dose. The blood pressure‐lowering effect was stable over time; there were no clinically important differences in blood pressure‐lowering effect of calcium channel blockers between each hour for either systolic blood pressure (estimated mean hourly differences ranged between 9.45 mmHg and 13.2 mmHg) or diastolic blood pressure (estimated mean hourly differences ranged between 5.85 mmHg and 8.5 mmHg). However, there was a moderate risk of bias for this finding. Once‐daily dihydropyridine calcium channel blockers appeared to lower blood pressure by a relatively constant amount throughout the 24‐hour dosing interval.
Authors' conclusions
Six dihydropyridine calcium channel blockers studied in this review lowered blood pressure by a relatively similar amount each hour over the course of 24 hours. The benefits and harms of this pattern of blood pressure lowering are unknown. Further trials are needed with accurate recording of time of drug intake and with reporting of standard deviation of blood pressure at each hour. We did not attempt to assess adverse effects in this review due to the lack of reporting and the short duration of follow‐up.
Keywords: Adult, Female, Humans, Male, Antihypertensive Agents, Antihypertensive Agents/administration & dosage, Antihypertensive Agents/therapeutic use, Blood Pressure, Blood Pressure/drug effects, Blood Pressure/physiology, Calcium Channel Blockers, Calcium Channel Blockers/therapeutic use, Circadian Rhythm, Dihydropyridines, Dihydropyridines/administration & dosage, Dihydropyridines/therapeutic use, Drug Administration Schedule, Hypertension, Hypertension/drug therapy, Randomized Controlled Trials as Topic, Time Factors
Plain language summary
Is the blood pressure lowering effect of dihydropyridine calcium channel blockers consistent or variable throughout 24 hours?
Background
High blood pressure, also known as hypertension, is a risk factor for adverse cardiovascular events such as stroke and heart attack. Blood pressure varies widely in an individual but certain patterns in its rise and fall have been identified in the general population; blood pressure increases in the early morning hours and decreases during the night. There is a variety of treatment options available for treating high blood pressure. Dihydropyridine calcium channel blockers are a group of drugs used to lower blood pressure.
Study characteristics
This review explores whether the blood pressure lowering effect of dihydropyridine calcium channel blockers in adults (aged 18 years or over) with high blood pressure (systolic blood pressure (the upper blood pressure reading) of at least 140 mmHg or diastolic blood pressure (the lower blood pressure reading) of at least 90 mmHg, or both of these) is consistent or variable over a 24‐hour period. We performed a review of studies that compared the 24‐hour blood pressure lowering effects of six of these drugs versus a control treatment for at least three weeks. Blood pressure needed to be measured by an ambulatory blood pressure monitor, which is a device that automatically measures blood pressure at regular intervals. We performed searches for clinical trials up to February 2014.
Key results
We found 16 trials involving 2768 participants that studied five drugs given once a day (amlodipine, lercanidipine, mandipine, nifedipine, and felodipine) and one drug given twice a day (nicardipine). The amount of blood pressure lowering by dihydropyridine calcium channel blockers stayed relatively the same at every hour throughout a 24‐hour day. The average hourly differences in blood pressure were between 9.45 mmHg and 13.2 mmHg for systolic blood pressure and between 5.85 mmHg and 8.5 mmHg for diastolic blood pressure. At the present time, the benefits and harms of this pattern of blood pressure lowering are unknown.
Quality of the evidence
We judged the overall quality of the evidence to be moderate. Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Summary of findings
Summary of findings for the main comparison. Dihydropyridine calcium channel blockers compared with placebo for hypertension.
| Dihydropyridine calcium channel blockers compared with placebo for hypertension | |||
|
Patient or population: adults with primary hypertension Settings: outpatient Intervention: dihydropyridine calcium channel blockers (CCB) at maximum doses Comparison: placebo | |||
| Outcomes | No of participants (studies) | Quality of the evidence (GRADE) | Comments |
|
Variation in the decrease in 24‐hour ambulatory hourly systolic blood pressure at 3‐12 weeks |
2768 (16) | ⊕⊕⊕⊝ moderate2 | A relatively constant blood pressure‐lowering effect at each hour. No subgroup differences demonstrated1 |
|
Variation in the decrease in 24‐hour ambulatory hourly diastolic blood pressure at 3‐12 weeks |
2768 (16) | ⊕⊕⊕⊝ moderate2 | A relatively constant blood pressure‐lowering effect at each hour. No subgroup differences demonstrated1 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
1. ANOVA F tests done on each mixed model analysis rarely failed to reject the null hypothesis in tests for heterogeneity.
2. High risk of bias for finding of no difference between hours as Industry‐funded studies were likely designed to show no difference.
Background
Description of the condition
Cardiovascular diseases are widespread and represent the leading cause of death globally (Turnbull 2003). The positive association between increased blood pressure (BP) and the risk of major cardiovascular disease is well established, as are the effects of BP‐lowering drugs to lower these risks in people with moderate to severe elevations in BP (Psaty 2003; Wright 2009).
In the general population, some distinct circadian patterns in BP have been identified. BP declines during sleep, and rises in the early morning hours (Elliott 1999). While the morning spike of BP is associated with an increase in some cardiovascular events (Elliott 1999), disturbances in night‐time patterns (such as blunted drops in BP (non‐dippers) and marked decreases in BP (extreme dippers)) are also associated with increased cardiovascular risks (Kario 2004).
Each of the various classes of drugs used to lower BP act through different modes of action and have been shown to vary in their ability to reduce the risk of various cardiovascular events. For example, thiazide‐type diuretics are more efficacious than calcium channel blockers (CCB) and angiotensin‐converting enzyme inhibitors in preventing heart failure, but are not different in their ability to reduce total cardiovascular events (ALLHAT 2002; Chen 2010).
Potential variability in outcomes not only arises from the class of antihypertensive drugs prescribed but also how BP is measured following treatment. Twenty‐four‐hour monitoring of BP provides more information than clinic measurements as it allows observation of how the BP‐lowering effect of a drug changes over time.
Description of the intervention
CCBs are a heterogeneous class of drugs including dihydropyridines (DHPs), phenylalkylamines, benzothiazepines, and nonselective CCBs. They are used to treat a variety of cardiovascular diseases including hypertension and angina. First‐line treatment with CCBs has been shown to reduce risks of total major cardiovascular events and stroke when compared with a placebo (Turnbull 2003). This review is limited to studying the DHP CCBs. These compounds are more potent vasodilators than drugs in the phenylalkylamine and benzothiazepine subclass (Basile 2004; Sica 2006).
The earliest CCBs were nifedipine (a DHP), diltiazem (a benzothiazepine), and verapamil (a phenylalkylamine). They displayed variability in dose response, had short durations of action, and were associated with numerous adverse effects (Toyo‐Oka 1996). Later, CCBs were developed to decrease negative adverse effects, and increase the duration of action plus decrease the frequency of dosing of the drugs.
How the intervention might work
DHP CCBs prevent the entry of calcium through L‐type calcium channels in the myocardium and vasculature. This reduces contractility of the cardiac muscle, conduction velocities of the sinoatrial and atrioventricular nodes, and causes vasodilation of the vascular smooth muscle (Elliott 2011). DHPs preferentially bind the L‐type calcium channels in the vasculature rather than those of the cardiac muscle (Basile 2004). This general mechanism of action is shared between all DHP CCBs; however, there are pharmacokinetic differences within this subclass. For example, half‐lives of CCBs vary from relatively short (0.2 to 1 hour for nifedipine) to long (44 hours or greater for amlodipine) (Elliott 2011). This suggests possible differences in the time course of effects depending on the drug used.
Why it is important to do this review
A systematic review of the time course of DHP CCBs has not been done. The information from this review will tell us whether there are differences in the time course of BP‐lowering among different drugs within this class. It will also provide valuable information about this class of drugs that can be compared with similar reviews of other classes of drugs (Sekhon 2008). It is possible that different mortality and morbidity effects of BP‐lowering drugs can be explained by differences in the time course of BP lowering.
Objectives
To assess how much variation there is in hourly systolic and diastolic BP lowering by DHP CCBs over a 24‐hour period in people with hypertension aged 18 years or over, with baseline systolic blood pressure (SBP) of at least 140 mmHg or diastolic blood pressure (DBP) of at least 90 mmHg, or both.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) with random allocation to a standard dose* of a DHP CCB and to a parallel placebo group.
In addition, they had to meet the following criteria:
duration of follow‐up of at least three weeks;
BP measured using 24‐hour ambulatory blood pressure monitoring (ABPM) at one or more time points after week three.
*Standard doses defined as any dose within the dose range recommended by the manufacturer for the treatment of hypertension.
Types of participants
People with primary hypertension who were aged over 18 years. Participants had to have a baseline SBP of at least 140 mmHg or DBP of at least 90 mmHg, or both.
We assumed that age does not impact the temporal BP‐lowering effect of this class of drugs.
Types of interventions
Intervention: CCBs of the DHP type including: amlodipine, aranidipine, azelnidipine, barnidipine, benidipine, cilnidipine, clevidipine, darodipine, efonidipine, elgodipine, felodipine, isradipine, lacidipine, lercanidipine, manidipine, nicardipine, nifedipine, niguldipine, nilvadipine, nimodipine, nisoldipine, and nitrendipine. When more than one dose was studied in a single RCT, we used the highest dose within the recommended dose range to increase the chance of finding a difference in effect at different times.
Control: placebo.
Types of outcome measures
Primary outcomes
Endpoint hourly BP using a 24‐hour ABPM.
Search methods for identification of studies
We searched the Database of Abstracts of Reviews of Effectiveness (DARE) and The Cochrane Database of Systematic Reviews for related reviews.
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 1, 2014), Ovid MEDLINE (1946 to February 2014), Ovid EMBASE (1974 to February 2014), and ClinicalTrials.gov (ClinicalTrials.gov) (to February 2014) for RCTs.
We used the Cochrane Highly Sensitive Search Strategy for identifying RCTs in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision) with selected MeSH terms and free‐text terms relating to CCBs and hypertension. We applied no language restrictions. We adapted the MEDLINE search strategy (Appendix 1) into strategies for CENTRAL (Appendix 2), EMBASE (Appendix 3), and ClincialTrials.gov (Appendix 4) using the appropriate controlled vocabulary as applicable.
Searching other resources
We handsearched reference lists of all papers and relevant reviews identified and ISI Web of Science for papers that cite studies included in the review. We contacted authors of relevant papers regarding any further published or unpublished work and authors of trials reporting incomplete information to request the missing information.
Data collection and analysis
Selection of studies
We selected studies primarily based on abstracts and titles, and rejected studies that did not meet the inclusion criteria or that fulfilled the exclusion criteria. For those studies selected, we reviewed the full texts for their overall applicability based on the inclusion criteria. We also examined the reference list of the full‐text papers for their relevance. Two review authors independently assessed the selected studies for inclusion.
Data extraction and management
We entered data into a data extraction form and two review authors independently cross‐checked entries. A second review author double checked all interpolations and calculations. We contacted the investigators of the specific trials to request any missing data.
Assessment of risk of bias in included studies
We assessed the risk of bias following the methodology described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), under the subheadings: sequence generation, allocation sequence concealment, blinding of participants, incomplete outcome data, selective outcome reporting, and other biases.
Measures of treatment effect
The treatment effect was the mean change in systolic and DBP in mmHg (a continuous variable) for each hour over a 24‐hour period. For example, if a trial used 24‐hour ABPM at different points in time between week three and 12, we used the mean of all the measurements.
Unit of analysis issues
We developed the approach to assessing statistical heterogeneity in order to avoid unit of analysis errors.
Dealing with missing data
We attempted to contact the authors of selected articles via email or telephone to request missing data and noted any replies.
Standard deviation data at endpoints are often not included in published reports or are of an unrealistic magnitude. In the event that this was the case and the information could not be obtained from the authors, we imputed standard deviations according to the following hierarchy.
Standard deviation of the change in endpoint BP obtained from the same trial.
Weighted mean standard deviation of BP at endpoint calculated from at least three other trials using the same drug and dose regimen.
Weighted mean standard deviation of BP at endpoint calculated from other trials using the same drug.
Weighted mean standard deviation of BP at endpoint calculated from all other trials (any drug and dose).
Assessment of heterogeneity
We could not use the Review Manager software's built‐in test for heterogeneity of treatment effect to test for differences in BP‐lowering effect at different hours because of correlated errors introduced by repeated observations on the same participants. Instead, we analyzed the 307 observations in the SBP analysis and the 356 observations in the DBP analysis using linear regression models that compensated for the correlated observations. We performed the linear regressions 1000 times each for the SBP and DBP data. We needed an iterative process because estimating only one SBP or DBP linear model with the reported mean difference (MD) for each hour and study combination would have ignored the variation around each observation (i.e. the variation around the MD for study i in hour j). A single iteration of the process involved generating 307 SBP values (356 for the DBP dataset) randomly selected from normal distributions defined by the reported MD and respective 95% confidence interval (CI). The generated values were then inputted into a linear regression to obtain an estimated total MD across all studies and hours. We repeated this process 1000 times to obtain a distribution of total MDs. We used the Kernel density estimation to identify a normal density function for the 1000 values, and then extracted the mean, upper 95% CI, and lower 95% CI from the density. These analyses were completed using PROC MIXED and PROC KDE in SAS versions 9.4 (SAS Institute Inc., Cary, NC). To account for correlated observations, we assumed variance‐covariance matrices in each linear regression to be heterogeneous compound symmetric. We used the generated values from each iteration to conduct analyses of variance (ANOVA). We computed F‐tests for each iteration. We assumed that observations across hours were likely to be homogeneous if the F‐tests rarely exceeded the critical F values of 1.564 (SBP) or 1.559 (DBP).
Assessment of reporting biases
We assessed publication bias using funnel plots, as outlined in Cochrane Handbook for Systematic Reviews of Interventions (Sterne 2011).
Data synthesis
We entered the mean change from control or baseline plus the standard deviation for each trial and for each hour.
Subgroup analysis and investigation of heterogeneity
We performed a subgroup analysis of individual DHP CCBs or of once‐daily or twice‐daily dosing if possible.
Sensitivity analysis
We planned sensitivity analyses according to participant characteristics, gender, or baseline BP.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
We identified 20 potentially eligible studies, from which we excluded four after screening the full texts. Reasons for exclusion included: smoothed data points causing inaccurate hourly data extraction (Carr 1992; Zachariah 1990), lack of placebo data provided (Viskoper 1991), and too long a time range between data points to accurately assess hourly effects (Honorato 1989).
We reviewed 16 RCTs with 2768 randomized participants for inclusion in the review (Asmar 1992; Bellet 1987; Chrysant 2003; Fagan 1993; Fogari 1996; Fogari 1999; Grimm 2002; Kuschnir 1996; Lacourciere 1998; Mroczek 1988; Omboni 1998; Pandita‐Gunawardena 1999; Toal 1997; van Ree 1996; White 2010; Zanchetti 1993). This total value varied at some hours as some studies provided bi‐hourly data (Asmar 1992), had missing data points for certain hours (Fagan 1993), provided less than 24 hours of data (Bellet 1987), or only provided diastolic data (Kuschnir 1996; Pandita‐Gunawardena 1999).
All study participants had hypertension. Each trial began with a two‐ to four‐week washout of previous antihypertensive medication or placebo run‐in. The criteria for entry differed between the trials and are documented in the Characteristics of included studies table.
All but one of the 16 RCTs explicitly stated both male and female participants were recruited (Zanchetti 1993). However, no RCT reported hourly BP results separately in men and women. Requirements for age varied among the studies and are documented in the Characteristics of included studies table.
This review includes investigations of six different DHPs. Seven of the RCTs studied amlodipine (Chrysant 2003; Grimm 2002; Kuschnir 1996; Lacourciere 1998; Mroczek 1988; Pandita‐Gunawardena 1999; White 2010). Two RCTs studied nicardipine in a twice‐daily regimen, but in different formulations: slow release (SR) (Fagan 1993) and long‐acting (LA) (Bellet 1987). The remaining study drugs were: lercanidipine (Omboni 1998), manidipine (Fogari 1996; Fogari 1999), nifedipine gastrointestinal therapeutic system (GITS) (Toal 1997; Zanchetti 1993), felodipine extended release (ER) (van Ree 1996), and nitrendipine (Asmar 1992). We only included trials that did not allow the use of supplemental antihypertensive agents other than study drugs.
Titrated doses were used in four RCTs (Grimm 2002; Lacourciere 1998; Pandita‐Gunawardena 1999; White 2010). Multiple doses were used in five RCTs (Fagan 1993; Fogari 1996; Omboni 1998; van Ree 1996; White 2010), and, in these RCTs, we used the data points from the highest dose. Five studies did not provide the time of drug administration (Fogari 1996; Fogari 1999; Mroczek 1988; Pandita‐Gunawardena 1999; Zanchetti 1993); in these studies, we chose 8 a.m. as the most likely time of drug administration. Five trials provided standard deviation or standard error (Asmar 1992; Fogari 1996; Toal 1997; van Ree 1996; Zanchetti 1993); however, we deemed the values provided in two of these studies to be too low to be realistic values (Asmar 1992; van Ree 1996). We used imputed standard deviations in these studies, and the remaining stud from Perez 2009. We imputed the standard deviations as 17 mmHg for SBP and 13 mmHg for DBP. These values were the mean standard deviations that were calculated from hourly individual participant data.
The mean duration of follow‐up of the included trials was about seven weeks, and ranged from three weeks (Bellet 1987) to 20 weeks (Grimm 2002). Due to the short duration of these trials, we did not attempt to quantify adverse effects of the study drugs in this review.
Results of the search
Figure 1 summarizes the PRISMA flow diagram for the screening process.
1.
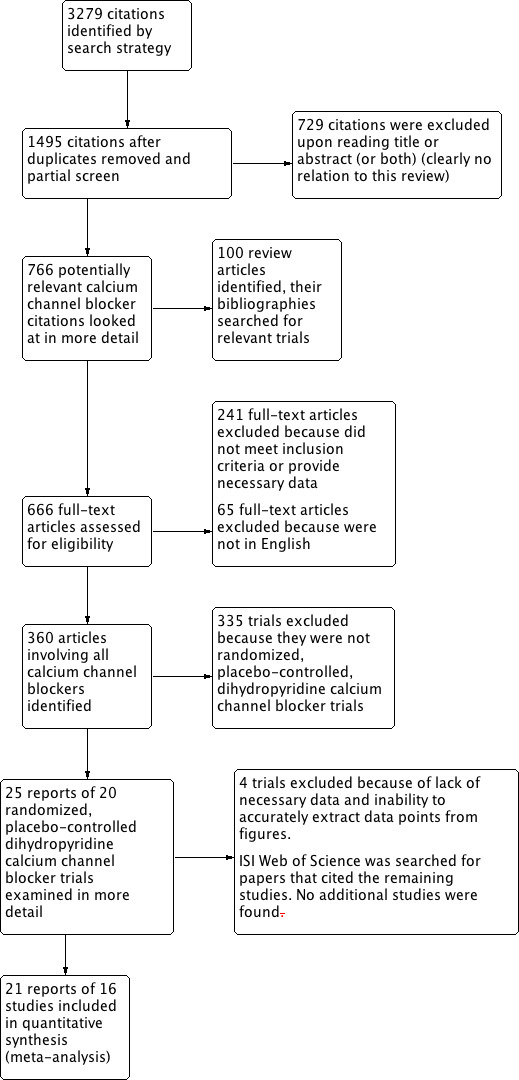
Study flow diagram.
Included studies
See Included studies; Characteristics of included studies table.
Excluded studies
See Excluded studies; Characteristics of excluded studies table.
Risk of bias in included studies
The risk of bias judgments and reasons can be found in Risk of bias in included studies.
Allocation
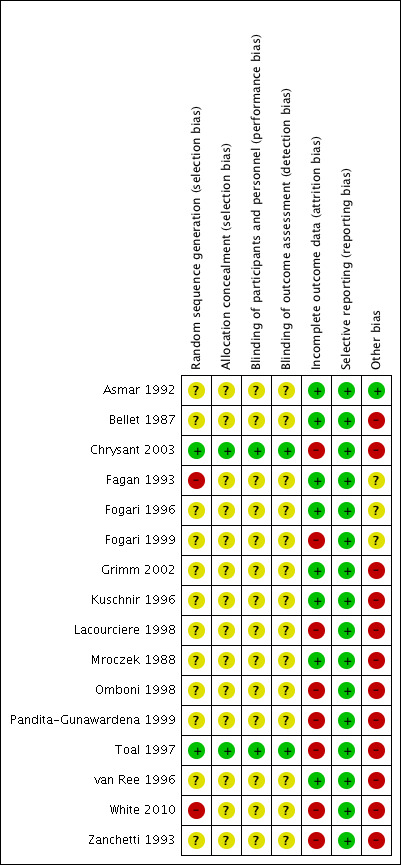
All of the included trials stated that they were randomized; however, only two of the trials, in which we were able to contact the lead author and received a response, provided information on how randomization took place (Chrysant 2003; Toal 1997). We deemed these two trials to have a low risk of random sequence generation bias. We judged two trials to have high risk of random sequence generation bias (Fagan 1993; White 2010). In these studies, subgroups of the originally randomized participant population were used for ABPM substudies, with no description of how the subgroup populations were selected. The remaining trials did not address how randomization took place and we assessed them as having an unclear risk of selection bias for randomization (Asmar 1992; Bellet 1987; Chrysant 2003; Fogari 1996; Fogari 1999; Grimm 2002; Kuschnir 1996; Lacourciere 1998; Mroczek 1988; Omboni 1998; Pandita‐Gunawardena 1999; van Ree 1996; Zanchetti 1993).
Only two trials provided information on allocation concealment (Chrysant 2003; Toal 1997). We judged these as having a low risk of bias for this field. The remaining trials did not describe methods of allocation concealment and we deemed them to have an unclear risk of bias.
Blinding
All of the 16 trials declared that their studies were double blinded. Only two studies provided information on methods of double blinding, and we deemed them to be at low risk for both performance and detection bias (Chrysant 2003; Toal 1997). Two trials described methods of blinding participants to treatment, but not blinding of personnel or outcome assessment (Bellet 1987; Fogari 1996). We assessed these as having unclear risk of performance and detection bias, as with the remaining studies.
Incomplete outcome data
We assessed eight of the trials as high risk of attrition bias. In six of these trials, we included only ABPM data that were deemed valid by that trial in the analysis (Chrysant 2003; Fogari 1999; Omboni 1998; Toal 1997; White 2010; Zanchetti 1993). The two remaining high‐risk trials did not have balanced numbers or reasons for withdrawals between groups (Lacourciere 1998; Pandita‐Gunawardena 1999).
Selective reporting
We judged all trials to have a low risk of selective reporting bias.
Other potential sources of bias
Twelve of the 16 trials were funded by or involved a pharmaceutical company and we deemed this as a high risk of other potential bias (Bellet 1987; Chrysant 2003; Grimm 2002; Kuschnir 1996; Lacourciere 1998; Mroczek 1988; Omboni 1998; Pandita‐Gunawardena 1999; Toal 1997; van Ree 1996; White 2010).
Figure 2 and Figure 3 provide a summary of the overall risk of bias and, since there is a paucity of low risk of bias, we judged the review to have a moderate to high risk of bias. That is certainly the case for the magnitude of BP lowering shown here and possibly also for the main finding of a no clinically important variation in BP lowering over the 24‐hour period.
2.
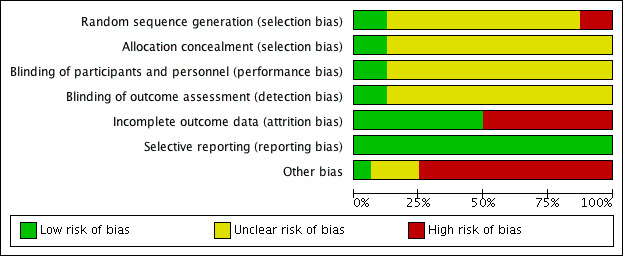
3.
Effects of interventions
See: Table 1
At each hour throughout the 24‐hour dosing interval, DHP CCBs significantly lowered BP more than placebo (P value < 0.00001 for both SBP and DBP). Estimated mean hourly differences ranged between 9.45 mmHg and 13.2 mmHg for SBP (Analysis 1.1) and ranged between 5.85 mmHg and 8.5 mmHg for DBP (Analysis 1.2). For both the hourly SBP and hourly DBP, the mean BP‐lowering effect remained relatively constant over time with no evidence of any pattern. In order to test whether there were any differences between BP‐lowering effects at the different hours, we estimated the total meta‐analytic effect of BP change across 24 hours from linear regression models (repeated 1000 times each for SBP and DBP). We performed ANOVA F tests on each mixed model analysis, which rarely failed to reject the null hypothesis in tests for heterogeneity (F < Critical F = 1.564 on all but seven of 1000 SBP iterations; F < Critical F = 1.559 on all but one of 1000 DBP iterations).
1.1. Analysis.
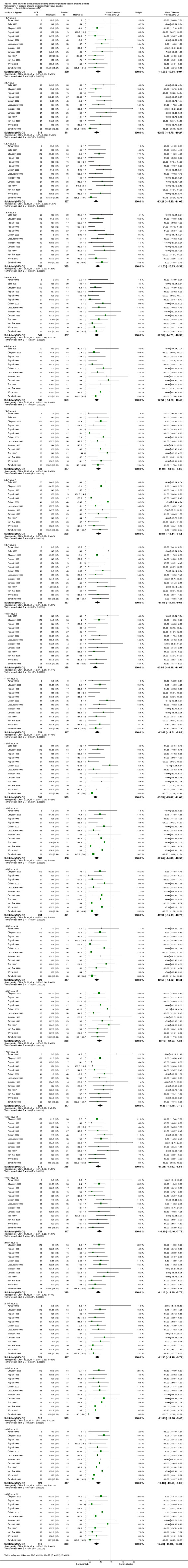
Comparison 1 Calcium channel blockeres (CCB) versus placebo, Outcome 1 Systolic blood pressure (BP).
1.2. Analysis.
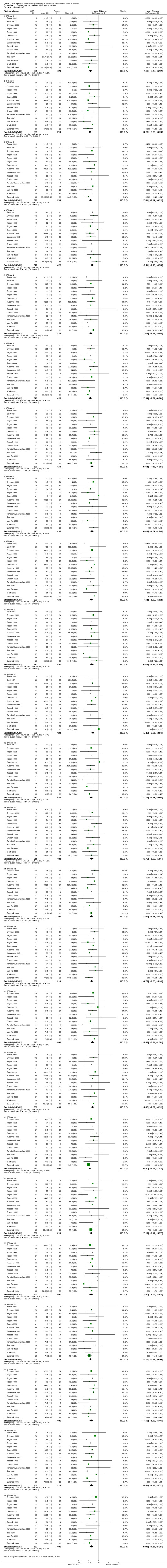
Comparison 1 Calcium channel blockeres (CCB) versus placebo, Outcome 2 Diastolic BP.
For most hours, there was no significant heterogeneity. The only exceptions were hours one, two, three, and 11 from the SBP data (where I2 ≥ 50%). We judged these infrequent occurrences to be most likely due to chance, as it was unlikely that there were sources of clinical or methodologic heterogeneity in this review. Because heterogeneity was found in only 16.6% of subgroups in SBP data, we deemed the fixed‐effect model to be most appropriate for analysis of both sets of data.
Adverse effects were inconsistently reported in these trials and, since the trials were short and this was not one of the objectives of this review, we did not attempt to quantify them.
Discussion
Summary of main results
We included 16 RCTs in this systematic review, with 2768 randomized participants. We analyzed data by hourly subgroups and found no significant differences in the BP‐lowering effects of DHP CCBs between each hour, over the course of 24 hours. This result was found for both SBP and DBP. This suggests that the DHP CCBs studied in this review lowered BP by a consistent magnitude throughout the 24‐hour dosing interval. This finding was the same if the seven RCTs studying amlodipine were analyzed alone and was the same when the other once‐daily RCTs analyzing nifedipine, manidipine, felodipine, and lercanidine were analyzed together. We have not calculated the overall BP‐lowering effect, as we were only interested in the variation of BP‐lowering over the 24‐hour period. The magnitude of BP lowering is not meaningful in this review as the included studies used different doses and approaches, for example dose titration. In addition, the BP‐lowering magnitude observed represents an exaggeration of the mean effect, as we specifically selected the highest dose in trials where several doses were studied and there is a high risk of bias for industry‐funded trials such as these.
It is not known at the present time whether the pattern of BP lowering (consistent over the 24‐hour period) is desirable or not. BP normally is reduced significantly during sleep as compared with during the day. It is not known whether further lowering of BP during sleep is desirable or not. It will be important to compare drug and drug class effectiveness in reducing mortality and morbidity with the pattern of BP lowering. Therefore, it is important to do systematic reviews studying the BP‐lowering profile of all drugs and classes of drugs that have been studied in long‐term mortality and morbidity outcome trials (Wright 2009).
Fourteen of the 16 trials used a once‐daily regimen. The remaining two trials studied twice‐daily nicardipine (Bellet 1987; Fagan 1993). When these two nicardipine trials were removed from the analysis, the conclusions of the review were unchanged. One trial of nitrendipine showed a loss of BP‐lowering effect during the second 12 hours after a once‐daily dose (Asmar 1992). When this trial was removed from the analysis it also had no effect on overall BP‐lowering profile or the on the conclusions of the review.
Overall completeness and applicability of evidence
The review authors originally planned to assess the time‐course profile of all CCBs, and include not only RCTs, but cross‐over, and baseline‐controlled trials as well. The first set of searches reflected these goals. However, due to the large amount of relevant trials that were found from the searches, it was deemed that the objectives could be obtained by limiting criteria to the most rigorous trial design, that is, randomized, placebo‐controlled double‐blind trials. In addition, we decided to focus the review on the largest subclass of CCBs, that is, DHPs. One limitation of the review is that we only included studies published in English.
Standard deviations were only reported accurately in three of the 16 included trials. BP variability (standard deviation) is constant in human populations and effect size is relatively insensitive to standard deviation. Therefore, we used imputed standard deviations in the 13 remaining studies using data from Perez 2009. The values provided in this study are from individual participant data, which we believe to be more accurate than pooled values, and are relatively more conservative than any of the values provided in the included studies. This represents a limitation but is unlikely to introduce a potential bias.
Most of the DHP CCBs included in this review were developed to have an antihypertensive effect over a 24‐hour period (Toyo‐Oka 1996). The results of this systematic review demonstrate that the five DHP CCBs (amlodipine, lercanidipine, mandipine, nifedipine, and felodipine) control BP by a relatively constant amount throughout a 24‐hour dosing interval. The evidence is strongest for amlodipine with seven RCTs, intermediate for nifedipine and manidipine with two RCTs each, and weakest for felodipine and lercanidipine with one RCT each.
Quality of the evidence
All included studies stated that they were randomized trials; however, most studies did not address how treatment randomization occurred or how allocation of treatment was concealed, and, therefore, had an unclear risk of selection bias. All included studies also stated that they were double‐blinded trials, but again, most did not describe how double blinding was ensured throughout the trial. Since BP was measured by a computer‐generated program, the chance of loss of blinding having an effect on the BP values was reduced. We assess most of the studies as having an unclear risk of performance and detection bias. We found high risk of attrition bias in eight of the 16 trials, mainly because of the inclusion of study‐defined "valid" ABPM data and exclusion of the remainder. All of the studies had a low risk for reporting bias. Other biases, in the form of pharmaceutical company funding or sponsoring, were found in 12 of the 16 included studies. It is possible that these studies were deliberately designed to show a constant BP‐lowering effect over a 24‐hour period so for this category we judged there to be a high risk of bias. We judged the overall risk of bias to be moderate to high. We have judged it to be high for the magnitude of BP lowering so this review should not be used to estimate the BP‐lowering effect of DHP CCBs. We judged the risk of bias for the main conclusion, no clinically important variation between the 24 different hourly measurements, to be moderate.
Potential biases in the review process
A potential limitation of this review is that due to time constrictions of the review authors, we included only studies written in English. Another limitation is that the time of drug administration was not reported or provided following attempted communication in four of the 16 trials. As a result, time of dosing for 'hour 0' in these trials was estimated as 8 a.m. Funnel plots of the SBP and DBP data did not suggest asymmetry, but there were not enough trials for the funnel plot to provide a good measure of the likelihood of publication bias.
Agreements and disagreements with other studies or reviews
We believe this is the first review of its kind.
Authors' conclusions
Implications for practice.
The dihydropyridine CCBs amlodipine, nifedipine, manidipine, felodipine, and lercanidipine taken once daily consistently lower blood pressure by a similar amount over the course of 24 hours. However, the clinical benefits or harms of equal blood pressure lowering throughout the night and day are unknown.
Implications for research.
In order to improve the validity of this type of review, trials investigating blood pressure‐lowering effects of drugs over 24‐hours should accurately record the time of drug intake and report the blood pressure data with zero hour being the time of drug intake. These trials also should be required to report standard deviations for each hourly measurement. More, high‐quality trials are needed for dihydropyridine CCBs where the evidence is weak (e.g. felodipine and lercanidipine) and for all the dihydropyridine CCBs being used where such randomized controlled trials have not been carried out.
Acknowledgements
The review authors would like to acknowledge the help provided by the Cochrane Hypertension Group.
Appendices
Appendix 1. MEDLINE search strategy
Database: Ovid MEDLINE(R) 1946 to Present with Daily Update Search date: 28 February 2014 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp calcium channel blockers/ 2 (amlodipine or amrinone or aranidipine or barnidipine or bencyclane or benidipine or bepridil or cilnidipine or cinnarizine or clentiazem or darodipine or diltiazem or efonidipine or elgodipine or etafenone or fantofarone or felodipine or fendiline or flunarizine or gallopamil or isradipine or lacidipine or lercanidipine or lidoflazine or lomerizine or manidipine or mibefradil or nicardipine or nifedipine or niguldipine or nilvadipine or nimodipine or nisoldipine or nitrendipine or perhexiline or prenylamine or semotiadil or terodiline or tiapamil or verapamil).tw. 3 (calcium adj2 (antagonist? or block$ or inhibit$)).tw. 4 or/1‐3 5 blood pressure monitoring, ambulatory/ 6 ((blood pressure or bp or dbp or sbp) adj10 (ambulatory or monitor$)).tw. 7 ((24 hour? or 24h or 24hr or 24 hr or 24‐h or hourly) adj10 (ambulatory or blood pressure or bp or dbp or monitor$ or sbp)).tw. 8 (abp or abpm).tw. 9 *time factors/ 10 time course?.tw. 11 circadian.mp. 12 or/5‐11 13 hypertension/ 14 (anti‐hypertens$ or antihypertens$ or hypertens$).tw. 15 exp blood pressure/ 16 (blood pressure or bloodpressure).tw. 17 or/13‐16 18 randomized controlled trial.pt. 19 controlled clinical trial.pt. 20 randomi?ed.ab. 21 placebo.ab. 22 clinical trials as topic/ 23 randomly.ab. 24 trial.ti. 25 or/18‐24 26 animals/ not (humans/ and animals/) 27 25 not 26 28 4 and 12 and 17 and 27
Appendix 2. CENTRAL search strategy
Database: Wiley ‐ Cochrane Central Register of Controlled Trials <2014 Issue 1> Search date: 28 February 2014 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ ID Search #1 (amlodipine or aranidipine or azelnidipine or barnidipine or benidipine or cilnidipine or clevidipine or darodipine or efonidipine or elgodipine or felodipine or isradipine or lacidipine or lercanidipine or manidipine or nicardipine or nifedipine or niguldipine or nilvadipine or nimodipine or nisoldipine or nitrendipine):ti,ab,kw #2 calcium near/2 (inhibit* or antagonist* or block*):ti,ab #3 #1 or #2 #4 MeSH descriptor: [Blood Pressure Monitoring, Ambulatory] explode all trees #5 ("blood pressure" or bp) near/5 (ambulatory or monitor*):ti,ab #6 (24 next hour* or 24h or 24hr or 24 next hr or 24‐h or hourly) near/5 (ambulatory or monitor*):ti,ab #7 (abp or abpm):ti,ab #8 #4 or #5 or #6 or #7 #9 MeSH descriptor: [Hypertension] this term only #10 (anti‐hypertens* or antihypertens* or hypertens*):ti,ab #11 MeSH descriptor: [Blood Pressure] explode all trees #12 ("blood pressure" or bloodpressure):ti,ab #13 #9 or #10 or #11 or #12 #14 #3 and #8 and #13
Appendix 3. EMBASE search strategy
Database: EMBASE <1974 to 2014 week 08> Search date: 28 February 2014 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 calcium channel blocking agent/ 2 (amlodipine or amrinone or aranidipine or barnidipine or bencyclane or benidipine or bepridil or cilnidipine or cinnarizine or clentiazem or darodipine or diltiazem or efonidipine or elgodipine or etafenone or fantofarone or felodipine or fendiline or flunarizine or gallopamil or isradipine or lacidipine or lercanidipine or lidoflazine or lomerizine or manidipine or mibefradil or nicardipine or nifedipine or niguldipine or nilvadipine or nimodipine or nisoldipine or nitrendipine or perhexiline or prenylamine or semotiadil or terodiline or tiapamil or verapamil).tw. 3 (calcium adj2 (antagonist? or block$ or inhibit$)).tw. 4 or/1‐3 5 blood pressure monitoring/ 6 ((blood pressure or bp or dbp or sbp) adj10 (ambulatory or monitor$)).tw. 7 ((24 hour? or 24h or 24hr or 24 hr or 24‐h or hourly) adj10 (ambulatory or blood pressure or bp or dbp or monitor$ or sbp)).tw. 8 (abp or abpm).tw. 9 time/ 10 time course?.tw. 11 circadian.mp. 12 or/5‐11 13 exp hypertension/ 14 (anti‐hypertens$ or antihypertens$ or hypertens$).tw. 15 exp blood pressure/ 16 (blood pressure or bloodpressure).tw. 17 or/13‐16 18 randomized controlled trial/ 19 crossover procedure/ 20 double‐blind procedure/ 21 (randomi?ed or randomly).tw. 22 (crossover$ or cross‐over$).tw. 23 placebo.ab. 24 doubl$ blind$.tw. 25 assign$.ab. 26 allocat$.ab. 27 or/18‐26 28 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 29 27 not 28 30 4 and 12 and 17 and 29
Appendix 4. ClinicalTrials.gov (via Cochrane Register of Studies)
Database: ClinicalTrials.gov Search date: 28 February 2014 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Search Terms: Randomized AND (24 hour* OR 24h OR 24hr OR “24 hr” OR “24‐h” OR ABPM OR chronotherap* OR circadian OR hourly OR “time course” OR "time factors" OR “time response”) Study type: Interventional Studies Conditions: hypertension Interventions: "calcium channel blockers" OR amlodipine OR benidipine OR cilnidipine OR clevidipine OR felodipine OR isradipine OR lacidipine OR lercanidipine OR manidipine OR nicardipine OR nifedipine OR nilvadipine OR nimodipine OR nisoldipine OR nitrendipine Outcome Measures: "blood pressure"
Data and analyses
Comparison 1. Calcium channel blockeres (CCB) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Systolic blood pressure (BP) | 14 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 BP hour 0 | 13 | 872 | Mean Difference (IV, Fixed, 95% CI) | ‐11.35 [‐13.64, ‐9.07] |
| 1.2 BP hour 1 | 12 | 855 | Mean Difference (IV, Fixed, 95% CI) | ‐12.53 [‐14.79, ‐10.27] |
| 1.3 BP hour 2 | 14 | 908 | Mean Difference (IV, Fixed, 95% CI) | ‐13.24 [‐15.40, ‐11.09] |
| 1.4 BP hour 3 | 13 | 891 | Mean Difference (IV, Fixed, 95% CI) | ‐11.53 [‐13.73, ‐9.32] |
| 1.5 BP hour 4 | 14 | 908 | Mean Difference (IV, Fixed, 95% CI) | ‐12.59 [‐14.78, ‐10.39] |
| 1.6 BP hour 5 | 13 | 891 | Mean Difference (IV, Fixed, 95% CI) | ‐12.58 [‐14.70, ‐10.46] |
| 1.7 BP hour 6 | 14 | 908 | Mean Difference (IV, Fixed, 95% CI) | ‐11.02 [‐13.19, ‐8.85] |
| 1.8 BP hour 7 | 13 | 891 | Mean Difference (IV, Fixed, 95% CI) | ‐10.84 [‐13.14, ‐8.54] |
| 1.9 BP hour 8 | 14 | 908 | Mean Difference (IV, Fixed, 95% CI) | ‐11.88 [‐14.15, ‐9.61] |
| 1.10 BP hour 9 | 13 | 891 | Mean Difference (IV, Fixed, 95% CI) | ‐13.89 [‐16.16, ‐11.63] |
| 1.11 BP hour 10 | 13 | 868 | Mean Difference (IV, Fixed, 95% CI) | ‐12.07 [‐14.31, ‐9.82] |
| 1.12 BP hour 11 | 13 | 891 | Mean Difference (IV, Fixed, 95% CI) | ‐13.76 [‐15.87, ‐11.66] |
| 1.13 BP hour 12 | 12 | 832 | Mean Difference (IV, Fixed, 95% CI) | ‐12.65 [‐14.80, ‐10.50] |
| 1.14 BP hour 13 | 11 | 815 | Mean Difference (IV, Fixed, 95% CI) | ‐12.91 [‐15.13, ‐10.70] |
| 1.15 BP hour 14 | 13 | 868 | Mean Difference (IV, Fixed, 95% CI) | ‐12.53 [‐14.62, ‐10.44] |
| 1.16 BP hour 15 | 12 | 851 | Mean Difference (IV, Fixed, 95% CI) | ‐10.19 [‐12.50, ‐7.88] |
| 1.17 BP hour 16 | 13 | 868 | Mean Difference (IV, Fixed, 95% CI) | ‐9.45 [‐11.78, ‐7.12] |
| 1.18 BP hour 17 | 12 | 851 | Mean Difference (IV, Fixed, 95% CI) | ‐11.24 [‐13.62, ‐8.86] |
| 1.19 BP hour 18 | 13 | 868 | Mean Difference (IV, Fixed, 95% CI) | ‐10.10 [‐12.46, ‐7.75] |
| 1.20 BP hour 19 | 12 | 851 | Mean Difference (IV, Fixed, 95% CI) | ‐11.13 [‐13.49, ‐8.76] |
| 1.21 BP hour 20 | 13 | 868 | Mean Difference (IV, Fixed, 95% CI) | ‐11.95 [‐14.18, ‐9.71] |
| 1.22 BP hour 21 | 12 | 851 | Mean Difference (IV, Fixed, 95% CI) | ‐11.83 [‐14.20, ‐9.47] |
| 1.23 BP hour 22 | 13 | 868 | Mean Difference (IV, Fixed, 95% CI) | ‐11.18 [‐13.48, ‐8.89] |
| 1.24 BP hour 23 | 12 | 851 | Mean Difference (IV, Fixed, 95% CI) | ‐12.73 [‐15.08, ‐10.38] |
| 2 Diastolic BP | 16 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 BP hour 0 | 15 | 1022 | Mean Difference (IV, Fixed, 95% CI) | ‐7.79 [‐9.45, ‐6.12] |
| 2.2 BP hour 1 | 14 | 1011 | Mean Difference (IV, Fixed, 95% CI) | ‐8.36 [‐9.99, ‐6.73] |
| 2.3 BP hour 2 | 16 | 1058 | Mean Difference (IV, Fixed, 95% CI) | ‐7.81 [‐9.41, ‐6.22] |
| 2.4 BP hour 3 | 15 | 1041 | Mean Difference (IV, Fixed, 95% CI) | ‐6.14 [‐7.76, ‐4.53] |
| 2.5 BP hour 4 | 16 | 1058 | Mean Difference (IV, Fixed, 95% CI) | ‐7.46 [‐8.98, ‐5.95] |
| 2.6 BP hour 5 | 15 | 1041 | Mean Difference (IV, Fixed, 95% CI) | ‐7.91 [‐9.53, ‐6.28] |
| 2.7 BP hour 6 | 16 | 1058 | Mean Difference (IV, Fixed, 95% CI) | ‐6.44 [‐7.89, ‐4.98] |
| 2.8 BP hour 7 | 15 | 1041 | Mean Difference (IV, Fixed, 95% CI) | ‐6.45 [‐8.04, ‐4.86] |
| 2.9 BP hour 8 | 16 | 1058 | Mean Difference (IV, Fixed, 95% CI) | ‐7.11 [‐8.70, ‐5.52] |
| 2.10 BP hour 9 | 15 | 1041 | Mean Difference (IV, Fixed, 95% CI) | ‐6.53 [‐8.17, ‐4.89] |
| 2.11 BP hour 10 | 16 | 1058 | Mean Difference (IV, Fixed, 95% CI) | ‐5.46 [‐6.98, ‐3.94] |
| 2.12 BP hour 11 | 15 | 1041 | Mean Difference (IV, Fixed, 95% CI) | ‐7.17 [‐8.71, ‐5.64] |
| 2.13 BP hour 12 | 14 | 982 | Mean Difference (IV, Fixed, 95% CI) | ‐6.70 [‐8.39, ‐5.01] |
| 2.14 BP hour 13 | 13 | 965 | Mean Difference (IV, Fixed, 95% CI) | ‐7.02 [‐8.61, ‐5.43] |
| 2.15 BP hour 14 | 15 | 1018 | Mean Difference (IV, Fixed, 95% CI) | ‐6.72 [‐8.30, ‐5.14] |
| 2.16 BP hour 15 | 14 | 1001 | Mean Difference (IV, Fixed, 95% CI) | ‐5.94 [‐7.61, ‐4.28] |
| 2.17 BP hour 16 | 15 | 1091 | Mean Difference (IV, Fixed, 95% CI) | ‐5.85 [‐7.39, ‐4.32] |
| 2.18 BP hour 17 | 14 | 1001 | Mean Difference (IV, Fixed, 95% CI) | ‐8.50 [‐9.58, ‐7.42] |
| 2.19 BP hour 18 | 15 | 1018 | Mean Difference (IV, Fixed, 95% CI) | ‐7.12 [‐8.47, ‐5.77] |
| 2.20 BP hour 19 | 14 | 1001 | Mean Difference (IV, Fixed, 95% CI) | ‐7.29 [‐8.93, ‐5.64] |
| 2.21 BP hour 20 | 15 | 1018 | Mean Difference (IV, Fixed, 95% CI) | ‐7.90 [‐9.24, ‐6.56] |
| 2.22 BP hour 21 | 14 | 1001 | Mean Difference (IV, Fixed, 95% CI) | ‐7.13 [‐8.79, ‐5.46] |
| 2.23 BP hour 22 | 15 | 1018 | Mean Difference (IV, Fixed, 95% CI) | ‐6.95 [‐8.63, ‐5.27] |
| 2.24 BP hour 23 | 14 | 1001 | Mean Difference (IV, Fixed, 95% CI) | ‐6.90 [‐8.51, ‐5.28] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Asmar 1992.
| Methods | Single‐blind 15‐day placebo‐wash‐out period followed by a 4‐week placebo‐controlled, double‐blind, active treatment period. ABPM was performed at the end of the single‐blind and double‐blind period | |
| Participants | Participants, aged 36‐64 years (mean age ± SD 50 ± 8 years) with essential, moderate, and uncomplicated hypertension with DBP ≥ 95 mmHg at the end of the single‐blind period were eligible for the randomized double‐blind period (17 participants) | |
| Interventions | Nitrendipine 20 mg (8 participants) or placebo (9 participants), once daily between 8 a.m. and 10 a.m. for 4 weeks | |
| Outcomes | Circadian rhythm of arterial pressure and heart rate using 24‐hour ABPM Effects on arterial distensibility using measurements of pulse wave velocity |
|
| Notes | Time of dose was listed as 8 a.m. to 10 a.m. For analysis in this review, we used 9 a.m. as the time of dosing; 'hour 0' Emailed lead author asking about unclear risks in bias assessment with no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of the process of randomization |
| Allocation concealment (selection bias) | Unclear risk | No description of process |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description of process |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description of process |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data for randomized participants |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods were reported |
| Other bias | Low risk | The study was funded by a non‐industry source; Institut National de la Santé et de la Recherche Médicale (INSERM) |
Bellet 1987.
| Methods | Single‐blind 2‐week placebo period followed by randomized allocation to active treatment or its matched placebo for about 3 weeks (mean 23 days, range 18‐30 days). ABPM was performed at the beginning and end of the double‐blind period | |
| Participants | Participants, aged 27‐72 years (mean age (± SD) 53 ± 10 years) with no cardiovascular complications and chronic disease, and supine DBP 95‐120 mmHg following single‐blind period were randomized (40 participants) | |
| Interventions | Nicardipine log acting (LA), 50 mg twice daily (20 participants) or placebo (20 participants) at 9 a.m. and 9 p.m. daily | |
| Outcomes | Antihypertensive effect of chronic oral nicardipine LA treatment | |
| Notes | Lead author contact email not found to ask about unclear risks in bias assessment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of the process of randomization |
| Allocation concealment (selection bias) | Unclear risk | Randomization schedule was kept in the pharmacy; however, does not describe who had access to it |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Low risk of blinding participants (matched placebo, both sets of tablets unmarked), but no mention of how personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description of process |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data for randomized participants |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods were reported |
| Other bias | High risk | Funded by Sandoz France |
Chrysant 2003.
| Methods | Single‐blind, 4‐week, placebo run‐in period followed by an 8‐week randomized, double‐blind, placebo‐controlled trial conducted at 43 study centers. Analysis by intention‐to‐treat population, defined as participants who were randomized to treatment and received at least 1 dose of their assigned treatment, and had at least 1 post‐baseline ABPM measurement. ABPM was performed at baseline and at the end of the 8‐week treatment period. Only hours with at least 1 BP measurement were considered valid, and data from the entire period were rejected if there were ≥ 2 consecutive hours or ≥ 6 nonconsecutive hours with no ABPM readings | |
| Participants | Participants (mean age 51.5 years) with mild‐to‐moderate hypertension, defined as mean seated DBP of 100‐115 mmHg during weeks 3 and 4 of placebo run‐in (with a difference of ≤ 10 mm Hg between the 2 visit means) and a mean daytime DBP of 90‐119 mmHg measured with ABPM were randomized in a 3 : 3 : 1 ratio (440 participants). 397 participants were included in the intention‐to‐treat population | |
| Interventions | Following the placebo run‐in, olmesartan medoxomil 20 mg (188 participants), amlodipine besylate 5 mg (186 participants), or placebo (66 participants), once‐daily orally as close to 8 a.m. as possible (± 1.5 hours). Only the amlodipine 5 mg arm data and the placebo arm data were used in this review | |
| Outcomes | Primary endpoint: change from baseline in mean 24‐hour DBP by ABPM at week 8 of treatment Secondary endpoints: change from baseline in mean 24‐hour ABPM SBP at week 8 and change in mean cuff seated DBP and cuff seated SBP at week 8 |
|
| Notes | The review authors would like to thank Dr. Chrysant for providing answers to questions regarding the risk of bias assessment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Dr. Chrysant stated that the study used interactive voice response system for randomization |
| Allocation concealment (selection bias) | Low risk | Central allocation with the use of interactive voice response system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Dr. Chrysant stated this was a double‐blind study that all personnel involved with the study were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Dr. Chrysant stated that the data gathered from the ABPM device were assessed by an independent company, and that all personnel involved with the study were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All randomized participants were not followed to the end of the study; intention‐to‐treat population did not include all randomized participants. Only participants with study defined "valid" BP measurements included in analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods were reported |
| Other bias | High risk | Sponsored by Sankyo Pharma Inc. |
Fagan 1993.
| Methods | Single‐blind 14‐day placebo run‐in period followed by 12‐week double‐blind active treatment period conducted at 12 sites. ABPM was performed at the end of the single‐blind period and repeated during the fourth and eighth weeks of active treatment on a subset of participants from 5 centers | |
| Participants | Participants, aged 22‐75 years, with supine DBP of 95‐114 mmHg on 2 consecutive visits during the single‐blind period with a difference no greater than 10 mmHg between the 2 values were randomized (230 participants). A subset of participants were included in the ABPM portion of the study (71 participants) | |
| Interventions | Nicardipine sustained release (SR) 30 mg (57 participants), 45 mg (55 participants), or 60 mg(60 participants), or placebo (58 participants), twice‐daily dosing at 12‐hour intervals (9 a.m. and 9 p.m.). For the ABPM substudy, 30 mg had18 participants, 45 mg had 19 participants, and 60 mg had 17 participants. Only the 60 mg arm and the placebo arm data were used in this review | |
| Outcomes | Safety and efficacy of nicardipine SR | |
| Notes | Only the nicardipine SR 60 mg and placebo were analyzed in this review Lead author contact email not found to ask about unclear risks in bias assessment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No evidence of randomization of ABPM substudy |
| Allocation concealment (selection bias) | Unclear risk | No description of the process |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description of the process |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description of the process |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data for randomized participant s |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods were reported |
| Other bias | Unclear risk | Funding not specified |
Fogari 1996.
| Methods | Single‐blind 2‐week placebo run‐in period, followed by a 4‐week randomized, double‐blind, placebo‐controlled period. The ABPM device recorded measurements for 24‐hours at the end of the single‐blind period and at the end of the double‐blind period | |
| Participants | Participants, aged 40‐63 years, with mild‐to‐moderate essential hypertension defined as supine DBP ≥ 95 mmHg and ≤ 115 mmHg and SBP < 210 mmHg at the end of the single‐blind period were randomized (52 participants) | |
| Interventions | Manidipine hydrochloride 10, 20, or 40 mg or placebo, 1 capsule daily after breakfast (13 participants for each group) | |
| Outcomes | Antihypertensive efficacy | |
| Notes | Only the manidipine hydrochloride 40 mg and placebo were analyzed No information about time of dosing provided. Emailed first author with no response. Assumed 8 a.m. dosing; 'hour 0' |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of the process of randomization |
| Allocation concealment (selection bias) | Unclear risk | No description of the process |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Low risk of loss of blinding participants (test treatments were identical in appearance, taste, and smell and were identically labelled), but no mention of how personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description of the process |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data for randomized participants |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods were reported |
| Other bias | Unclear risk | Funding not specified |
Fogari 1999.
| Methods | After a 4‐week placebo washout period, eligible participants were included in the randomized, double‐blind period. ABPM was performed before randomization and after 8 weeks of treatment for 24 hours. Recordings were only included in analysis if number of readings was > 75% | |
| Participants | Participants, aged 76‐89 years (mean age (± SD) 81.8 ± 4.4 years), with mild‐to‐moderate essential hypertension, defined as a sitting DBP > 90 mmHg, and < 110 mmHg and SBP > 160 mmHg were randomized (54 participants) | |
| Interventions | Manidipine 10 mg (27 participants) or placebo (27 participants) at a dosage of 1 capsule, once daily after breakfast | |
| Outcomes | Antihypertensive efficacy | |
| Notes | No information about time of dosing provided. Emailed first author with no response. Assumed 8 a.m. dosing; 'hour 0' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of the process of randomization |
| Allocation concealment (selection bias) | Unclear risk | No description of the process |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description of the process |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description of the process |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only participants with study defined "valid" BP measurements included in analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods were reported |
| Other bias | Unclear risk | Funding not specified |
Grimm 2002.
| Methods | Multicenter trial. Following a 4‐week placebo run‐in phase, participants were randomized in a double‐blind, parallel‐group method for 20 weeks (median duration of therapy for all participants). ABPM was performed at the end of the placebo run‐in and at the end of the treatment period | |
| Participants | Participants, aged ≥ 50 years with stage 1 isolated systolic hypertension defined as mean of 2 sitting SBP measurements of 140‐159 mmHg on 2 consecutive visits during the placebo run‐in phase were randomized (150 participants) | |
| Interventions | Amlodipine 5 mg (48 participants, 41 completed), chlorthalidone 15 mg (50 participants, 45 completed), or placebo (52 participants, 48 completed), once daily. During the first 8 weeks of treatment (titration phase), the dosage of each drug could be doubled after 4 weeks of treatment if the SBP goal was not reached. After the titration phase, the dose was maintained for an additional 12 weeks | |
| Outcomes | Primary outcome: mean sitting SBP Secondary outcomes: number of participants reaching sitting SBP goal, pulse pressure, standing SBP, sitting and standing DBP, and 24‐hour ABPM |
|
| Notes | No information was provided about time of dosing. Email was sent to author with no reply; we assumed 8 a.m. dosing; 'hour 0' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of the process of randomization |
| Allocation concealment (selection bias) | Unclear risk | No description of the process |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description of the process |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description of the process |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing participants relatively balanced across groups |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods were reported |
| Other bias | High risk | Funded by Pfizer Inc. |
Kuschnir 1996.
| Methods | Single‐blind placebo run‐in period of 2‐4 weeks followed by randomization into double‐blind treatment period of 8 weeks. ABPM was performed at randomization and at the end of the treatment period | |
| Participants | Participants with uncomplicated primary hypertension, with a mean sitting DBP ≥ 100 mmHg and ≤ 120 mmHg, and mean sitting SBP that did not differ by more than 10 mmHg at screening and randomization visit, were randomized (308 participants) and evaluated for tolerability and safety. 307 participants were included in the intention‐to‐treat analysis of efficacy (1 participant discontinued before any post‐randomization efficacy data gathered). Trial completed by 285 participants | |
| Interventions | Amlodipine 5 mg/benazepril 20 mg (administered as separate components), amlodipine 5 mg, benazepril 20 mg, or placebo once daily around 8 a.m. (77 participants per group at randomization) | |
| Outcomes | Efficacy, tolerability, and safety of dual therapy with a calcium antagonist and angiotensin‐converting enzyme inhibitor | |
| Notes | Only DBP provided. Contact information for first author could not be found | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of the process of randomization |
| Allocation concealment (selection bias) | Unclear risk | No description of the process |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description of the process |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description of the process |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing participants relatively balanced across groups |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods were reported |
| Other bias | High risk | Funded by Ciba‐Geigy Corporation |
Lacourciere 1998.
| Methods | Multicenter study starting with 3‐ to 14‐day pre‐qualification washout period, followed by a 4‐week single‐blind, placebo run‐in period. Eligible participants then entered a 12‐week, randomized double‐blind treatment period. ABPM was performed at the end of the placebo run‐in and at the end of the double‐blind period. Performed intention‐to‐treat analysis | |
| Participants | Adults, aged 28‐78 years (mean age 54.3 years) with a trough supine clinic DBP of 95‐114 mmHg were eligible for the single‐blind period. Participants with mean trough supine DBP of 95‐114 mmHg that had not changed by more than 7 mmHg at weeks 2 and 4 of the single‐blind period were randomized for treatment. 232 participants entered the double‐blind period and were included in the intention‐to‐treat and safety analyses | |
| Interventions | Telmisartan 40 mg (73 participants), amlodipine 5 mg (78 participants), or placebo (81 participants) once daily between 6 a.m. and 9 a.m. Dosage of amlodipine could be increased to 10 mg after 8 weeks of therapy if supine DBP remained > 90 mmHg | |
| Outcomes | BP‐lowering efficacy over 24‐hour period | |
| Notes | Lead author contact email not found to ask about unclear risks in bias assessment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of the process of randomization |
| Allocation concealment (selection bias) | Unclear risk | No description of the process |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description of the process |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description of the process |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Number of participants reported in ABPM graphs did not match the number of randomized participants following participant withdrawals. Reasons for withdrawals not balanced across groups |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods were reported |
| Other bias | High risk | Funded by Boehringer Ingelheim (Canada) Ltd. |
Mroczek 1988.
| Methods | Single‐blind, 4‐week, placebo run‐in period followed by a 4‐week double‐blind treatment period randomized in a 2 : 1 ratio. ABPM was performed at the end of the placebo run‐in and double‐blind periods (mean duration 27.5 days for amlodipine and 32 days for placebo) | |
| Participants | Participants with untreated DBP of 95‐114 mmHg in both supine and standing positions, 24 hours after placebo administration (16 participants). 1 participant was withdrawn before commencing the double‐blind period due to uncontrolled hypertension in the single‐blind period. 15 participants were included in the efficacy analysis | |
| Interventions | Amlodipine 5 mg (10 participants) or placebo (5 participants), once daily | |
| Outcomes | Antihypertensive efficacy and antihypertensive efficacy on circadian pattern | |
| Notes | No information was provided about time of dosing. Corresponding author not available; we assumed 8 a.m. dosing; 'hour 0' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of the process of randomization |
| Allocation concealment (selection bias) | Unclear risk | No description of the process |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description of the process |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description of the process |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data for randomized participants |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods were reported |
| Other bias | High risk | Funded by Pfizer Inc. |
Omboni 1998.
| Methods | Multicenter study beginning with 3‐week placebo run‐in, followed by double‐blind, randomized, placebo‐controlled treatment period of 4 weeks. ABPM was performed at the end of the placebo run‐in and at the end of the 4‐week treatment period. Only recordings with at least 24 hours of data, 75% of valid readings over 24 hours and a starting hour between 8 and 10 am were included in the final analysis. | |
| Participants | Adults, mean age (SD) 51 ± 8 years, with mild‐to‐moderate essential hypertension, defined as supine DBP 90‐109 mmHg during and at the end of the placebo run‐in period were randomized to double‐blind treatment (243 participants). 105 participants had valid ABPM readings | |
| Interventions | Lercanidipine 2.5 mg (28 participants), 5 mg (27 participants), 10 mg (27 participants) or placebo (23 participants), once daily | |
| Outcomes | Antihypertensive efficacy | |
| Notes | Time of dose was listed as 8 a.m. to 10 a.m., for analysis in this review, we used 9 a.m. as the time of dosing; 'hour 0' We analyzed only lercanidipine 10 mg and placebo Lead author contact email not found to ask about unclear risks in bias assessment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of the process of randomization |
| Allocation concealment (selection bias) | Unclear risk | No description of the process |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description of the process |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description of the process |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only participants with study defined "valid" BP measurements included in analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods were reported |
| Other bias | High risk | Funded by Recordati S.p.A., Pharmaceutical R&D Division |
Pandita‐Gunawardena 1999.
| Methods | 4‐week placebo run‐in followed by an 8‐week, double‐blind, placebo‐controlled treatment period. ABPM was performed after the run‐in and after the treatment period | |
| Participants | Adults, aged ≥ 60 years, with supine DBP ≥ 95 mmHg with upper limits of 105 mmHg in participants aged 60‐74 years, 110 mmHg in participants aged 75‐84 years and 115 mmHg in participants aged ≥ 85 years were randomized (26 participants). | |
| Interventions | Amlodipine 5 mg (13 participants) or placebo (13 participants), once daily. If supine DBP remained > 90 mmHg after 4 weeks, dose was doubled to 10 mg for the remaining 4 weeks | |
| Outcomes | Antihypertensive efficacy and regional cerebral blood flow | |
| Notes | Only provided DBP data. First author was emailed for SBP data with no response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of the process of randomization |
| Allocation concealment (selection bias) | Unclear risk | No description of the process |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description of the process |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description of the process |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reasons for missing outcome data not balanced in number or reason across groups |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods were reported |
| Other bias | High risk | Funded by Pfizer Inc. |
Toal 1997.
| Methods | Multicenter study with 2‐week placebo run‐in period followed by 4‐week double‐blind, randomized, placebo‐controlled treatment phase. ABPM was recorded for 26 hours in 5 of the 15 centers. During the day, 5/8 readings/hour were required for recording to be considered valid and included in analysis | |
| Participants | Participants with mild‐to‐moderate essential hypertension defined as sitting DBP 95‐114 mmHg were randomized (187 participants). 66 participants at 5 sites completed at least 1 ABPM recording. 47 had valid recordings at both baseline and at the end of treatment | |
| Interventions | Nifedipine gastrointestinal therapeutic system (GITS) 20 mg or placebo, once daily. Of those in the ABPM portion of study, 33 participants were randomized each to nifedipine GITS and placebo | |
| Outcomes | Antihypertensive efficacy in clinic and over 24‐hour period, and incidence and severity of spontaneously reported adverse events | |
| Notes | The review authors would like to thank Dr. Toal for providing answers to questions regarding the risk of bias assessment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Dr. Toal stated that they used randomization program to allocate participants to groups, using randomized blocks to try to ensure equal distribution of participants to each regimen, considering the different sites (ABPM was only performed at certain sites because many sites did not have the devices or had no experience with the devices. So, only sites that had identical devices and experience were chosen for the study) |
| Allocation concealment (selection bias) | Low risk | Dr. Toal stated participant codes were concealed in numbered and sealed envelopes. No envelopes were opened |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Dr. Toal stated that all placebo and active drugs were identical in shape, size, and color. Investigators, study coordinators (usually nurses), monitors, and statisticians were all blind for medication allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Dr. Toal stated that the data collected by the ABPM devices was assessed by an internal statistician at Bayer Inc. who completed all analyses before breaking the code (participants just identified as group A or B) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only participants with study defined "valid" BP measurements included in analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods were reported |
| Other bias | High risk | Funded by Bayer Inc. |
van Ree 1996.
| Methods | 4‐week placebo run‐in period followed by 6‐week double‐blind, randomized, placebo‐controlled treatment period. ABPM was performed at the start and end of the double‐blind period for 24 hours | |
| Participants | Participants with primary hypertension and a casual sitting DBP of 100‐115 mmHg and a SBP of 140‐200 mmHg at the start of the study were randomized (88 participants). Before starting the all‐participants‐treated analysis, it appeared 2 participants had a sitting DBP at randomization < 100 mm Hg and 1 had a DBP > 115, these participants were excluded and 85 participants were included in the analysis | |
| Interventions | Felodipine extended release (ER) 2.5 mg (29 participants) or 5 mg (27 participants), or placebo (29 participants). | |
| Outcomes | Antihypertensive efficacy and tolerability | |
| Notes | Only the felodipine 5 mg dose and placebo were analyzed. Time of dose was listed at 8 a.m. to 10 a.m. For analysis in this review, we used 9 a.m. as the time of dosing; 'hour 0' Lead author contact email not found to ask about unclear risks in bias assessment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of the process of randomization |
| Allocation concealment (selection bias) | Unclear risk | No description of the process |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description of the process |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description of the process |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data for randomized participants |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods were reported |
| Other bias | High risk | Funded by Astra Pharmaceutica BV, Rijswijk, The Netherlands |
White 2010.
| Methods | Multicenter study involving 3‐ to 4‐week single‐blind, placebo run‐in period, followed by an 8‐week double‐blind, double‐dummy, placebo‐controlled treatment period. Approximately 50% of the randomized participants in the main study were included in the ABPM substudy. ABPM was performed at baseline and after the end of the treatment period. AMBP data were considered valid if: they had a minimum of 18 hourly means available within 24 hours after monitor hookup, and no more than 3 consecutive hours of missing data. If these criteria were not met, participant was asked to repeat the procedure within 3 days. If repeat was unsuccessful, AMBP data not included in analysis | |
| Participants | Participants with hypertension defined as clinic DBP ≥ 95 mmHg and ≤ 119 mmHg were randomized to participate in the main study (1451 participants). 562 of these patients were included in the ABPM substudy with valid data | |
| Interventions | Telmisartan (20, 40, or 80 mg) alone, amlodipine (2.5, 5, or 10 mg) alone, each of the 9 combination therapies of telmisartan plus amlodipine, and placebo. We used the arms of the 58 participants who received amlodipine 10 mg and 16 participants who received placebo | |
| Outcomes | Antihypertensive efficacy | |
| Notes | Only the 10 mg dose of amlodipine and placebo was analyzed. Lead author was contacted to ask about unclear risks in bias assessment but was not able to respond by deadline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Did not describe how ABPM substudy was selected or randomized |
| Allocation concealment (selection bias) | Unclear risk | No description of the process |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Double‐blind, double dummy", no further description |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description of the process |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only participants with study defined "valid" BP measurements included in analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods were reported |
| Other bias | High risk | Funded by Boehringer‐Ingelheim GMBH, Ingelheim, Germany |
Zanchetti 1993.
| Methods | Multicenter study involving 2‐week placebo run‐in period, followed by double‐blind, placebo‐controlled treatment period of 4 weeks. ABPM was performed at the end of the placebo run‐in period and on the last day of treatment for a period of 24‐36 hours. Recordings were considered valid if had at least 1 valid BP measurement per hour and at least 24 hours of continuous BP recordings after removal of outlying values by an automatic procedure | |
| Participants | Participants with mild‐to‐moderate essential hypertension defined as a sitting clinic DBP 95‐114 mmHg were randomized (126 participants). 81 had valid ABPM data and were included in the analysis | |
| Interventions | Nifedipine GITS 30 mg (25 participants), nifedipine GITS 60 mg (28 participants), or placebo (28 participants), once daily for 4 weeks. Nifedipine 60 mg and placebo were used for this analysis | |
| Outcomes | Antihypertensive efficacy | |
| Notes | No information about time of dosing provided. Emailed first author with no response. Assumed 8 a.m. dosing; 'hour 0' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of the process of randomization |
| Allocation concealment (selection bias) | Unclear risk | No description of the process |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description of the process |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description of the process |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only participants with study defined "valid" BP measurements included in analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods were reported |
| Other bias | High risk | Bayer SpA, Milan involved in study group |
ABPM: ambulatory blood pressure monitoring; DBP: diastolic blood pressure; SBP: systolic blood pressure; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Carr 1992 | ABPM data were smoothed over, and deemed not possible to extract hourly data points accurately. Email contact information could not be found |
| Honorato 1989 | Provided data points for large ranges of hours rather than hourly data points. Email contact information could not be found |
| Viskoper 1991 | ABPM data not provided for placebo. Correspondence with author was attempted with no response |
| Zachariah 1990 | ABPM data were smoothed over, and deemed not possible to extract hourly data points accurately. Correspondence with second author was made, informed that the first author has retired and does not know if the data were retained. Could not find contact information of first author |
ABPM: ambulatory blood pressure monitoring.
Differences between protocol and review
Colin Dormuth joined the team of authors after the publication of the protocol.
We limited this review to randomized, placebo‐controlled trials published in English.
We developed the approach to assessing statistical heterogeneity after the publication of the protocol and designed the assessment in order to avoid unit of analysis errors.
Contributions of authors
Niousha Ghamami developed the basis for, and contributed to, the protocol; and took the lead role in: searching, identifying, and assessing studies; data extraction and analyses; and writing up the review.
James Wright formulated the idea for the review; developed the basis for, and contributed to, the protocol; independently screened for trials to be included; and independently conducted data extraction.
Sandy Chiang developed the basis for, and contributed to, the protocol; independently screened for trials to be included; and independently conducted the data extraction.
Colin Dormuth developed the approach to assessing statistical heterogeneity in order to avoid unit of analysis errors and performed the statistical analysis.
Sources of support
Internal sources
McMaster University, Biology and Pharmacology Coop Program, Canada.
University of British Columbia, Department of Anesthesiology, Pharmacology & Therapeutics, Canada.
External sources
Canadian Institutes of Health Research, Canada.
Declarations of interest
Ghamami N: nothing to declare.
Wright JM: nothing to declare.
Chiang SHY: nothing to declare.
Dormuth C: nothing to declare.
New
References
References to studies included in this review
Asmar 1992 {published data only}
- Asmar R, Benetos A, Brahimi M, Chaouche K, Safar M. Arterial and antihypertensive effects of nitrendipine: a double‐blind comparison versus placebo. Journal of Cardiovascular Pharmacology 1992;20(6):858‐63. [PUBMED: 1282585] [DOI] [PubMed] [Google Scholar]
Bellet 1987 {published data only}
- Bellet M, Pagny JY, Chatellier G, Corvol P, Menard J. Evaluation of slow release nicardipine in essential hypertension by casual and ambulatory blood pressure measurements. Effects of acute versus chronic administration. Journal of Hypertension 1987;5(5):599‐604. [PUBMED: 3429863] [DOI] [PubMed] [Google Scholar]
Chrysant 2003 {published data only}
- Chrysant SG, Marbury TC, Robinson TD. Antihypertensive efficacy and safety of olmesartan medoxomil compared with amlodipine for mild‐to‐moderate hypertension. Journal of Human Hypertension 2003;17(6):425‐32. [PUBMED: 12764406] [DOI] [PubMed] [Google Scholar]
- Chrysant SG, Marbury TC, Silfani TN. Use of 24‐h ambulatory blood pressure monitoring to assess blood pressure control: a comparison of olmesartan medoxomil and amlodipine besylate. Blood Press Monitoring 2006;11(3):135‐41. [PUBMED: 16702822] [DOI] [PubMed] [Google Scholar]
Fagan 1993 {published data only}
- Fagan TC, Tyler ED, Reitman MA, Kenley S, Weber MA. Sustained‐release nicardipine in mild‐to‐moderate hypertension. Chest 1993;104(2):427‐33. [PUBMED: 8339631] [DOI] [PubMed] [Google Scholar]
Fogari 1996 {published data only}
- Fogari R, Zoppi A, Lusardi P, Preti P, Poletti L, Mugellini A. Evaluation by 24‐hour ambulatory blood pressure monitoring of efficacy of manidipine hydrochloride 10, 20 or 40 mg once daily as compared to placebo in treating mild to moderate essential hypertension: a double‐blind, randomized, parallel group, placebo‐controlled study. Blood Pressure. Supplement 1996;5:16‐23. [PUBMED: 8973788] [PubMed] [Google Scholar]
Fogari 1999 {published data only}
- Fogari R, Zoppi A, Mugellini A, Preti P, Corradi L, Lusardi P. Effect of low‐dose manidipine on ambulatory blood pressure in very elderly hypertensives. Cardiovascular Drugs and Therapy 1999;13(3):243‐8. [PUBMED: 10439887] [DOI] [PubMed] [Google Scholar]
Grimm 2002 {published data only}
- Grimm RH Jr, Black H, Rowen R, Lewin A, Shi H, Ghadanfar M. Amlodipine versus chlorthalidone versus placebo in the treatment of stage I isolated systolic hypertension. American Journal of Hypertension 2002;15(1 Pt 1):31‐6. [PUBMED: 11824857] [DOI] [PubMed] [Google Scholar]
Kuschnir 1996 {published data only}
- Kuschnir E, Acuna E, Sevilla D, Vasquez J, Bendersky M, Resk J, et al. Treatment of patients with essential hypertension: amlodipine 5 mg/benazepril 20 mg compared with amlodipine 5 mg, benazepril 20 mg, and placebo. Clinical Therapeutics 1996;18(6):1213‐24. [PUBMED: 9001838] [DOI] [PubMed] [Google Scholar]
Lacourciere 1998 {published data only}
- Lacourciere Y, Lenis J, Orchard R, Lewanczuk R, Houde M, Pesant Y, et al. A comparison of the efficacies and duration of action of the angiotensin II receptor blockers telmisartan and amlodipine. Blood Pressure Monitoring 1998;3(5):295‐302. [PUBMED: 10212369] [PubMed] [Google Scholar]
Mroczek 1988 {published data only}
- Burris JF, Allenby KS, Mroczek WJ. The effect of amlodipine on ambulatory blood pressure in hypertensive patients. American Journal of Cardiology 1994;73(3):39A‐43A. [PUBMED: 8310975] [DOI] [PubMed] [Google Scholar]
- Mroczek WJ, Burris JF, Allenby KS. A double‐blind evaluation of the effect of amlodipine on ambulatory blood pressure in hypertensive patients. Journal of Cardiovascular Pharmacology 1988;12 Suppl 7:S79‐84. [PUBMED: 2467136] [DOI] [PubMed] [Google Scholar]
- Mroczek WJ, Burris JF, Klein J. A double‐blind evaluation of the effect of amlodipine on ambulatory blood pressure. Postgraduate Medical Journal 1991;67 Suppl 5:S24‐7. [PUBMED: 1839434] [PubMed] [Google Scholar]
Omboni 1998 {published data only}
- Omboni S, Zanchetti A. Antihypertensive efficacy of lercanidipine at 2.5, 5 and 10 mg in mild to moderate essential hypertensives assessed by clinic and ambulatory blood pressure measurements. Multicenter Study Investigators. Journal of Hypertension 1998;16(12 Pt 1):1831‐8. [PUBMED: 9869018] [DOI] [PubMed] [Google Scholar]
Pandita‐Gunawardena 1999 {published data only}
- Pandita‐Gunawardena ND, Clarke SE. Amlodipine lowers blood pressure without affecting cerebral blood flow as measured by single photon emission computed tomography in elderly hypertensive subjects. Age and Ageing 1999;28(5):451‐7. [PUBMED: 10529039] [DOI] [PubMed] [Google Scholar]
Toal 1997 {published data only}
- Toal CB. Efficacy of a low dose nifedipine GITS (20 mg) in patients with mild to moderate hypertension. Canadian Journal of Cardiology 1997;13(10):921‐7. [PUBMED: 9374948] [PubMed] [Google Scholar]
van Ree 1996 {published data only}
- Ree JW, Pol GA. Low dosages of felodipine ER once daily as monotherapy in elderly hypertensive patients: effect on ambulatory blood pressure and quality of life. Journal of Human Hypertension 1996;10(9):613‐8. [PUBMED: 8953207] [PubMed] [Google Scholar]
White 2010 {published data only}
- White WB, Littlejohn TW, Majul CR, Oigman W, Olvera R, Seeber M, et al. Effects of telmisartan and amlodipine in combination on ambulatory blood pressure in stages 1‐2 hypertension. Blood Pressure Monitoring 2010;15(4):205‐12. [PUBMED: 20613496] [DOI] [PubMed] [Google Scholar]
Zanchetti 1993 {published data only}
- Zanchetti A. The 24‐hour efficacy of a new once‐daily formulation of nifedipine. Italian Nifedipine GITS Study Group. Drugs 1994;48 Suppl 1:23‐30; discussion 30‐1. [PUBMED: 7533703] [DOI] [PubMed] [Google Scholar]
- Zanchetti A. Trough and peak effects of a single daily dose of nifedipine gastrointestinal therapeutic system (GITS) as assessed by ambulatory blood pressure monitoring. Italian Nifedipine GITS Study Group. Journal of Hypertension Supplement 1994;12(5):S23‐7. [PUBMED: 7965282] [PubMed] [Google Scholar]
- Zanchetti A, Bianchi L, Amigoni S, Omboni S, Villani A, Ravogli A. Antihypertensive effects of nifedipine gastrointestinal therapeutic system on clinic and ambulatory blood pressure in essential hypertensives. Italian GITS Study Group. Journal of Hypertension Supplement 1993;11(5):S334‐5. [PUBMED: 8158410] [PubMed] [Google Scholar]
References to studies excluded from this review
Carr 1992 {published data only}
- Carr AA, Bottini PB, Feig P, Prisant LM, Mulligan S, Devane JG, et al. Effectiveness of once‐daily monotherapy with a new nifedipine sustained release calcium antagonist. American Journal of Cardiology 1992;69(13):28E‐32E. [PUBMED: 1575174] [DOI] [PubMed] [Google Scholar]
Honorato 1989 {published data only}
- Honorato J, Azanza J, Guindo N, Suarez JR. Double‐blind placebo‐controlled study of the antihypertensive efficacy of nitrendipine in patients submitted to ambulatory blood pressure monitoring. Current Therapeutic Research, Clinical and Experimental 1989;46(5):932‐9. [Google Scholar]
Viskoper 1991 {published data only}
- Viskoper JR, Laszt A, Farragi D. The antihypertensive action of isradipine in mild essential hypertension. Journal of Cardiovascular Pharmacology 1991;18 Suppl 3:S9‐11. [PUBMED: 1720488] [PubMed] [Google Scholar]
Zachariah 1990 {published data only}
- Zachariah PK, Schwartz GL, Sheps SG, Schirger A, Carlson CA, Moore AG. Antihypertensive effects of a new sustained‐release formulation of nifedipine. Journal of Clinical Pharmacology 1990;30(11):1012‐9. [PUBMED: 2243148] [DOI] [PubMed] [Google Scholar]
Additional references
ALLHAT 2002
- The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002;288:2981‐97. [DOI] [PubMed] [Google Scholar]
Basile 2004
- Basile J. The role of existing and newer calcium channel blockers in the treatment of hypertension. Journal of Clinical Hypertension (Greenwich) 2004;6(11):621‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2010
- Chen N, Zhou M, Yang M, Guo J, Zhu C, Yang J, et al. Calcium channel blockers versus other classes of drugs for hypertension. Cochrane Database of Systematic Reviews 2010, Issue 8. [DOI: 10.1002/14651858.CD003654] [DOI] [PubMed] [Google Scholar]
Elliott 1999
- Elliott WJ. Circadian variation in blood pressure: implications for the elderly patient. American Journal of Hypertension 1999;12(2 Pt 2):43S‐9S. [PUBMED: 10090294] [DOI] [PubMed] [Google Scholar]
Elliott 2011
- Elliott WJ, Ram CV. Calcium channel blockers. Journal of Clinical Hypertension (Greenwich) 2011;13(9):687‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kario 2004
- Kario K, Shimada K. Risers and extreme‐dippers of nocturnal blood pressure in hypertension: antihypertensive strategy for nocturnal blood pressure. Clinical and Experimental Hypertension 2004;26(2):177‐89. [PUBMED: 15038628] [DOI] [PubMed] [Google Scholar]
Perez 2009
- Perez MI, Linden W, Perry T Jr, Puil LJ, Wright JM. Failure of psychological interventions to lower blood pressure: a randomized controlled trial. Open Medicine 2009;3(2):e92‐e100. [PUBMED: 19946397] [PMC free article] [PubMed] [Google Scholar]
Psaty 2003
- Psaty BM, Lumley T, Furberg CD, Schellenbaum G, Pahor M, Alderman MH, et al. Health outcomes associated with various antihypertensive therapies used as first‐line agents: a network meta‐analysis. JAMA 2003;289:2534‐44. [DOI] [PubMed] [Google Scholar]
Sekhon 2008
- Sekhon R, Perez MI, Wright JM. Time course for blood pressure lowering of thiazides. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD007436] [DOI] [Google Scholar]
Sica 2006
- Sica DA. Pharmacotherapy review: calcium channel blockers. Journal of Clinical Hypertension (Greenwich) 2006;8(1):53‐6. [PUBMED: 16407690] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Toyo‐Oka 1996
- Toyo‐Oka T, Nayler WG. Third generation calcium entry blockers. Blood Pressure 1996;5(4):206‐8. [PUBMED: 8809370] [DOI] [PubMed] [Google Scholar]
Turnbull 2003
- Turnbull F, Blood Pressure Lowering Treatment Trialists' Collaboration. Effects of different blood‐pressure‐lowering regimens on major cardiovascular events: results of prospectively‐designed overviews of randomised trials. Lancet 2003;362(9359):1527‐35. [DOI] [PubMed] [Google Scholar]
Wright 2009
- Wright JM, Musini VM. First‐line drugs for hypertension. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD001841] [DOI] [PubMed] [Google Scholar]


