Abstract
Background
Given the continued rise in cesarean birth rate and the increased risk of surgical site infections after cesarean birth compared with vaginal birth, effective interventions must be established for prevention of surgical site infections. Prophylactic intravenous (IV) antibiotic administration 60 minutes prior to skin incision is recommended for abdominal gynecologic surgery; however, administration of prophylactic antibiotics has traditionally been withheld until after neonatal umbilical cord clamping during cesarean delivery due to the concern for potential transfer of antibiotics to the neonate.
Objectives
To compare the effects of cesarean antibiotic prophylaxis administered preoperatively versus after neonatal cord clamp on postoperative infectious complications for both the mother and the neonate.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (1 March 2014) and reference lists of retrieved papers.
Selection criteria
Randomized controlled trials (RCTs) comparing maternal and neonatal outcomes following prophylactic antibiotics administered prior to skin incision versus after neonatal cord clamping during cesarean delivery. Cluster‐RCTs were eligible for inclusion but none were identified. Quasi‐RCT and trials using a cross‐over design were not eligible for inclusion in this review. Studies published in abstract form only were eligible for inclusion if sufficient information was available in the report.
Data collection and analysis
At least two review authors independently assessed the studies for inclusion, assessed risk of bias, abstracted data and checked entries for accuracy. We assessed the quality of evidence using the GRADE approach.
Main results
We included 10 studies (12 trial reports) from which 5041 women contributed data for the primary outcome. The overall risk of bias was low.
When comparing prophylactic intravenous (IV) antibiotic administration in women undergoing cesarean delivery, there was a reduction in composite maternal infectious morbidity (risk ratio (RR) 0.57, 95% confidence interval (CI) 0.45 to 0.72, high quality evidence), which was specifically due to the reduction in endometritis (RR 0.54, 95% CI 0.36 to 0.79, high quality evidence) and wound infection (RR 0.59, 95% CI 0.44 to 0.81, high quality evidence) in those that received antibiotics preoperatively as compared to those who received antibiotics after neonatal cord clamping. There were no clear differences in neonatal sepsis (RR 0.76, 95% CI 0.51 to 1.13, moderate quality evidence).
There were no clear differences for other maternal outcomes such as urinary tract infection (UTI), cystitis and pyelonephritis (moderate quality evidence), respiratory infection (low quality evidence), or any neonatal outcomes. Maternal side effects were not reported in the included studies.
The quality of the evidence using GRADE was high for composite morbidity, endomyometritis, wound infection and neonatal intensive care unit admission, moderate for UTI/cystitis/pyelonephritis and neonatal sepsis, and low for maternal respiratory infection.
Authors' conclusions
Based on high quality evidence from studies whose overall risk of bias is low, intravenous prophylactic antibiotics for cesarean administered preoperatively significantly decreases the incidence of composite maternal postpartum infectious morbidity as compared with administration after cord clamp. There were no clear differences in adverse neonatal outcomes reported. Women undergoing cesarean delivery should receive antibiotic prophylaxis preoperatively to reduce maternal infectious morbidities. Further research may be needed to elucidate short‐ and long‐term adverse effects for neonates.
Keywords: Female; Humans; Pregnancy; Anti‐Bacterial Agents; Anti‐Bacterial Agents/administration & dosage; Antibiotic Prophylaxis; Antibiotic Prophylaxis/standards; Cesarean Section; Cesarean Section/adverse effects; Drug Administration Schedule; Endometritis; Endometritis/prevention & control; Injections, Intravenous; Randomized Controlled Trials as Topic; Surgical Wound Infection; Surgical Wound Infection/prevention & control; Urinary Tract Infections; Urinary Tract Infections/prevention & control
Plain language summary
When should antibiotics be given to prevent infectious complications after cesarean birth?
People who undergo surgery are at risk of developing infections, which complicate their recovery. In order to prevent these infections and reduce complications, antibiotics are sometimes given as a preventative (or prophylactic) treatment. The antibiotics are generally given approximately 60 minutes before the operation so that adequate tissue concentrations are reached before the skin is cut. For cesarean deliveries however, the effect of the antibiotic on the baby has to be considered, and for this reason antibiotics have been administered to women after the baby’s umbilical cord is clamped. This may not allow for adequate tissue penetration in the mother for the prevention of surgery‐related infections; additionally deferring antibiotics may not benefit the newborn.
This review of randomized controlled studies looked at the different timing options for administration of prophylactic antibiotics to prevent infectious complications in women undergoing cesarean delivery. We compared preoperative administration to administration after the cord had been clamped.
The review includes 10 studies (with data from 5041 women). The studies were at a low risk of bias. Antibiotics given to women before cesarean delivery nearly halved the risks of combined infections (43%), endometritis (46%), and wound infection (41%) compared to giving the antibiotics after clamping of the baby’s umbilical. Other maternal infections such as urinary or lung infections were no different between the two groups of women, nor were adverse effects in newborns. High quality evidence shows that preoperative intravenous antibiotic administration decreases postpartum infections and is, therefore, beneficial for the mother. Maternal side effects were not consistently reported. Numbers were limited with respect to information on newborns and any adverse outcomes. Further research may be needed to determine adverse effects on the babies.
Summary of findings
Summary of findings for the main comparison. Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping.
| Prophylactic antibiotics for preventing postpartum infectious morbidity in women and infants after cesarean delivery | ||||||
| Population: women undergoing cesarean delivery. Settings: eight trials were conducted in developed countries: seven from US, one from Austria. Five trials were conducted in developing countries: two trials from India, one trial each from Egypt, South Africa and Turkey. Intervention: prophylactic intravenous antibiotic administration for cesarean birth administered prior to skin incision versus after neonatal umbilical cord clamping. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Maternal and neonatal postpartum infectious morbidity | |||||
| Maternal composite morbidity | Study population | RR 0.67 (0.54 to 0.82) | 5041 (10 studies) | ⊕⊕⊕⊕ high | ||
| 85 per 1000 | 57 per 1000 (46 to 70) | |||||
| Moderate | ||||||
| 97 per 1000 | 65 per 1000 (52 to 80) | |||||
| Maternal endomyometritis | Study population | RR 0.54 (0.36 to 0.79) | 5041 (10 studies) | ⊕⊕⊕⊕ high | ||
| 28 per 1000 | 15 per 1000 (10 to 22) | |||||
| Moderate | ||||||
| 26 per 1000 | 14 per 1000 (9 to 21) | |||||
| Maternal wound Infection | Study population | RR 0.59 (0.44 to 0.81) | 5041 (10 studies) | ⊕⊕⊕⊕ high | ||
| 41 per 1000 | 24 per 1000 (17 to 33) | |||||
| Moderate | ||||||
| 51 per 1000 | 30 per 1000 (22 to 41) | |||||
| Maternal UTI/cystitis/pyelonephritis | Study population | RR 1.02 (0.65 to 1.59) | 4001 (8 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 18 per 1000 | 18 per 1000 (12 to 29) | |||||
| Moderate | ||||||
| 13 per 1000 | 13 per 1000 (8 to 21) | |||||
| Maternal respiratory infection (pneumonia) | Study population | RR 2.3 (0.34 to 15.45) | 1158 (2 studies) | ⊕⊕⊝⊝ low2 | ||
| 2 per 1000 | 4 per 1000 (1 to 27) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Neonatal sepsis | Study population | RR 0.76 (0.51 to 1.13) | 2907 (5 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 37 per 1000 | 28 per 1000 (19 to 42) | |||||
| Moderate | ||||||
| 40 per 1000 | 30 per 1000 (20 to 45) | |||||
| Neonatal ICU admission | Study population | RR 0.91 (0.74 to 1.13) | 3708 (6 studies) | ⊕⊕⊕⊕ high | ||
| 86 per 1000 | 78 per 1000 (64 to 97) | |||||
| Moderate | ||||||
| 71 per 1000 | 65 per 1000 (53 to 80) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1 Wide confidence interval crossing the line of no effect. 2 Wide confidence interval crossing the line of no effect, few events and small sample size.
Background
Description of the condition
Cesarean birth is one of the most common surgical procedures performed worldwide. Internationally, 46 countries are noted to have cesarean delivery rates greater than 20%. Of these 46, China and Brazil account for almost 50% of total cesarean births (Gibbons 2012). The World Health Organization (WHO) Multicountry Survey on Maternal and Newborn Health survey conducted in 29 countries showed the overall rate of cesarean delivery was 28.6%, and the coverage of prophylactic antibiotics for cesarean birth was 87.3%, globally (Souza 2013). Worldwide, infectious morbidity consisting primarily of endomyometritis and wound infection occurs in approximately 5% to 10% of cesarean births (Henderson 1995; Olsen 2008; Opoien 2007; Yokoe 2001). As the cesarean birth rate continues to rise in most developed countries, postpartum infectious morbidity will become an even more significant problem. Therefore, measures aimed at decreasing postpartum infectious morbidity are an important area of focus.
Surgical site infections are the most common nosocomial infections among surgical patients, accounting for about 23% of all such infections from the data reported to the National Healthcare Safety Network (NHSN) in the United States during 2009 to 2010 (Sievert 2013). A prospective multicenter cohort study conducted in England reported that 9.6% of women in the study developed a postsurgical infection after cesarean delivery (Wloch 2012). Infectious complications after cesarean birth include, but are not limited to, wound infection, endomyometritis, urinary tract infection, pelvic abscess, septic shock, septic pelvic thrombophlebitis, necrotizing fasciitis, and pneumonia.
The risk of postpartum infection after cesarean birth is nearly five‐fold that of vaginal birth (Leth 2009). Many interventions have been studied in an attempt to decrease the incidence of surgical site infections after cesarean birth including prophylactic antibiotics, surgical hand antisepsis, skin preparation methods, surgical techniques, closure of subcutaneous fat, subcutaneous drain placement, and postoperative surveillance (Barwolff 2006; Dahlke 2013; Edi‐Osagie 1998; Hellums 2007; Lorenz 1988; Magann 1993; Starr 2005; Ventolini 2004).
Description of the intervention
In 2008, the WHO established a 'Safe Surgery Saves Lives' campaign. An intraoperative surgical checklist was developed to reduce post‐surgical infection as well as other associated morbidities. This checklist acknowledged the evidence‐based value of prophylactic antibiotic administration 60 minutes prior to skin incision (Soar 2009).
The obstetric population poses a unique challenge for antibiotic prophylaxis as there is known transplacental delivery of antibiotics to the fetus. Traditionally, the administration of prophylactic antibiotics has been withheld until after neonatal umbilical cord clamping during cesarean birth in order to avoid transfer of antibiotics to the fetus. The theoretical concerns of neonatal antibiotic exposure include the masking of neonatal infection, interference with sepsis workup and the selection of antibiotic‐resistant bacterial strains in both neonatal colonization and infection (Cunningham 1983). Due to these theoretical risks, the Centers for Disease Control and Prevention's 'Guideline for Prevention of Surgical Site Infection, 1999' stated with high level evidence, that for high‐risk cesarean birth, prophylactic antimicrobial agents should be given immediately after umbilical cord clamping, rather than preoperatively (Mangram 1999).
How the intervention might work
The general surgery tenet of antibiotic prophylaxis was born out of animal studies that demonstrated maximum suppression of infection when adequate tissue antibiotic levels were present at the time of microbial contamination (Burke 1961). These findings were confirmed in clinical practice: less surgical‐wound infections were noted when antibiotics were administered within two hours before skin incision as compared to when antibiotics were administered postoperatively (Classen 1992). One of the most commonly used antibiotics for cesarean birth is cefazolin. Pharmacokinetic studies of cefazolin demonstrate that mean inhibiting concentration levels for group B streptococcus are attained in maternal, fetal and amniotic fluid samples within 30 minutes of administration (Fiore 2001). It has also been demonstrated that bactericidal levels against group B streptococcus are achieved in maternal, fetal and amniotic fluid samples within five minutes of ampicillin administration (Bloom 1996).
Why it is important to do this review
The benefit of using prophylactic antibiotics in order to prevent surgical site and other infections has been demonstrated in the obstetric literature. A 2010 Cochrane review concluded that antibiotic prophylaxis, as compared to no prophylaxis, was associated with a reduction in the incidence of febrile morbidity, wound infection, endomyometritis and other serious maternal infectious complications (Smaill 2010). This prior review stated that data were insufficient to compare timing of antibiotic administration.
Several recent studies have evaluated timing of the administration of prophylactic antibiotics, specifically administration prior to skin incision versus after neonatal umbilical cord clamping. A meta‐analysis comparing the administration of prophylactic antibiotics prior to skin incision versus after clamping of the umbilical cord concluded that pre‐incision antibiotic prophylaxis for cesarean birth not only decreased the incidence of postpartum endomyometritis and total infectious morbidity, but also did not adversely affect neonatal outcomes (Costantine 2008). Several institutions have evaluated the impact of protocol changes in the timing of perioperative antibiotic administration on postoperative infectious complications, and have found decreased infectious complications with antibiotics given prior to skin incision compared to after cord clamping (Kaimal 2008; Owens 2009).
In response to the recent attention on the timing of prophylactic antibiotics in cesarean birth, the American College of Obstetricians and Gynecologists (ACOG) recommended antimicrobial prophylaxis for all cesarean births, and further stated that prophylaxis should be administered within 60 minutes of the start of the cesarean delivery (ACOG 2010). These recommendations are consistent with recommendations from the National Surgical Infection Prevention Project (Bratzler 2005).
Given the continued rise in cesarean birth rate and the relatively high risk of surgical site infections after cesarean birth as compared to other surgical procedures (NNIS System 2004), measures must be taken to prevent surgical site infections. Prior Cochrane reviews have looked at the maternal morbidity associated in relation to administration of prophylactic antibiotics at time of cesarean delivery (Smaill 2010) and various antibiotic regimens for cesarean delivery (Hopkins 1999). This review will focus on studies comparing antibiotic prophylaxis administered prior to skin incision compared with administration after umbilical cord clamping for cesarean birth. We will compare infectious morbidity and address the concern for neonatal harm.
Objectives
To assess the differences in infectious morbidity for mother and neonate when prophylactic cesarean antibiotics are administered preoperatively versus after neonatal cord clamping.
Methods
Criteria for considering studies for this review
Types of studies
Only randomized controlled trials were included. We excluded quasi‐randomized studies.
Abstracts were included if they provided sufficient information to make an assessment of methodologic quality, i.e., information allows for completion of at least some of the domains within the 'Risk of bias' tables. We attempted to contact authors of such manuscripts before deciding to exclude a study on these grounds.
Types of participants
Pregnant women who have undergone cesarean delivery and received prophylactic antibiotics.
Types of interventions
Prophylactic intravenous (IV) antibiotic administration for cesarean birth 0 to 30 and 30 to 60 minutes prior to skin incision versus prophylactic antibiotic administration for cesarean birth after neonatal umbilical cord clamping. We planned to exclude studies of women who received antibiotics after skin incision but before cord clamping.
Types of outcome measures
Primary outcomes
Composite maternal postpartum infectious morbidity (including serious infectious complications such as sepsis (including septic shock), endomyometritis, wound infection, or death attributed to infection). Though not pre‐specified in the protocol, we stratified composite morbidity by whether 1 g or 2 g of cephalosporin were administered.
Secondary outcomes
Maternal
1. Maternal mortality.
2. Maternal postpartum infection (the following are as defined by the individual trials):
endomyometritis (though not pre‐specified in the protocol, we stratified endomyometritis by whether 1 g or 2 g of cephalosporin were administered);
wound infection (though not pre‐specified in the protocol, we stratified wound infection by whether 1 g or 2 g of cephalosporin were administered);
urinary tract infection (UTI), cystitis and pyelonephritis;
sepsis, including septic shock;
pelvic abscess;
septic pelvic thrombophlebitis;
respiratory infection (e.g. pneumonia);
length of hospital stay (days);
Intensive care unit (ICU) admission;
cost of antibiotics;
antibiotic‐related adverse events (e.g. anaphylaxis);
febrile illness.
3. Placental transfer of antibiotics
4. Breastfeeding
Neonatal outcomes
1. Neonatal mortality.
2. Neonatal morbidity (the following are as defined by the individual trials):
sepsis;
neonatal sepsis workup;
infection with resistant organism;
infection (other);
admission to ICU;
length of ICU stay (days);
neonatal antibiotic treatment;
febrile illness.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We contacted the Trials Search Co‐ordinator to search the Cochrane Pregnancy and Childbirth Group’s Trials Register (1 March 2014).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of Embase;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We searched reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
At least two review authors independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion with the whole group.
Data extraction and management
We designed a form to abstract data. For eligible studies, two review authors independently abstracted the data using the agreed form. We resolved discrepancies through group discussion. We entered data into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for the studies using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We have assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomization);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. However, as overall methodologic quality was considered low risk, sensitivity analyses were not undertaken. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ see Sensitivity analysis.
The quality of the evidence was assessed using the GRADE approach (Schunemann 2009) in order to assess the quality of the body of evidence relating to the following key outcomes for the main comparison.
Composite morbidity
Endomyometritis
Wound infection
Urinary tract infection (UTI)/cystitis/pyelonephritis
Respiratory infections (e.g. pneumonia)
Neonatal sepsis
Neonatal ICU admission
GRADEprofiler (GRADE 2008) was used to import data from Review Manager 5.3 (RevMan 2014) in order to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of quality for each of the above outcomes were produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes are measured in the same way between trials. If necessary, we planned to use the standardized mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
We did not identify any cluster‐randomized trials, but we would include cluster‐randomized trials along with individually‐randomized trials in the analysis in future updates, if identified. We will adjust their sample size using the methods described in the Handbook using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify cluster‐randomized trials, we plan to synthesize the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomization unit is considered to be unlikely. We will also acknowledge heterogeneity in the randomization unit and perform a sensitivity analysis to investigate the effects of the randomization unit.
We did not include cross‐over trials.
Dealing with missing data
For included studies, we noted levels of attrition. In a subsequent update of this review, we will include a sensitivity analysis excluding poor quality studies with high levels of attrition.
For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomized to each group in the analyses. The denominator for each outcome in each trial was the number randomized minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either a Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
As there were 10 studies in the meta‐analysis for the primary outcome, we investigated reporting biases (such as publication bias) using a funnel plot. We assessed funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment in the future update, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it is reasonable to assume that studies are estimating the same underlying treatment effect: i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and the clinical implications of treatment effects differing between trials were discussed. If the average treatment effect was not clinically meaningful, we did not combine trials. Where we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
Substantial heterogeneity was not present, therefore we did not perform subgroup analyses in relation to heterogeneity. In future updates, if we identify substantial heterogeneity, we will perform subgroup analyses for composite maternal infectious morbidity based on:
cesarean as compared to labor: laboring versus non‐laboring (i.e. prelabor);
cesarean performed in presence of chorioamnionitis with treatment versus without treatment;
cesarean performed on women who have received antibiotic prophylaxis for other indications (e.g. GroupBeta Streptococcus) versus those without antibiotic treatment for any other indication;
timing of antibiotics prior to skin incision (e.g. less than 30 minutes to skin incision versus greater than 30 minutes prior to skin incision).
We considered whether an overall summary was meaningful and used random‐effects analysis to produce it. We assessed subgroup differences based on dose of cephalosporin (1 g versus 2 g) by interaction tests available within RevMan (RevMan 2014). Interaction test results are reported, along with the Chi² statistic, P value, and I² value.
Sensitivity analysis
We planned to carry out sensitivity analysis for the primary outcomes by restricting our analysis to trials assessed as having a low risk of bias for the domain attrition bias; if > 30% of participants were lost to follow‐up, these studies would have been excluded from sensitivity analyses. This was not necessary because none of the studies were assessed as having a high risk of bias. Planned sensitivity analysis will be conducted in future updates of this review, if appropriate.
Results
Description of studies
Results of the search
The search of the Cochrane Pregnancy and Childbirth Group's Register retrieved 25 reports (see: Figure 1). Ten studies (12 trial reports) were included with 5041 women contributing data to the primary outcome. Eleven trials were excluded. One study is awaiting classification (Pevzner 2009). One study is ongoing (Zhang 2012) (see Characteristics of ongoing studies).
1.
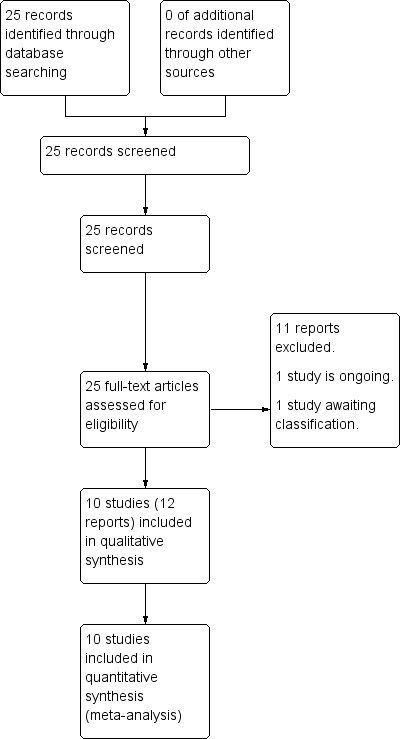
Study flow diagram.
Included studies
Ten trials met our inclusion criteria and contributed data on the 5041 women they enrolled for the primary outcome of composite infectious morbidity: 2531 received antibiotics preoperatively and 2510 received antibiotics after cord clamp.
For detailed information on all studies, see Characteristics of included studies tables.
Participants
Most studies described characteristics of the women who were included and excluded in detail. Most studies included women with non‐emergent or elective cesarean delivery at term (some studies included from 24 weeks estimated gestational age, others included from 34 or 36 weeks of gestation). Several studies excluded women with obstetric complications, while other studies only excluded women with infection at enrolment or allergy to antibiotics.
Settings
Eight trials were conducted in developed countries, seven from the United States of America and one from Austria. Five trials were performed in developing countries, with two trials from India and one trial each from Egypt, South Africa and Turkey.
Interventions
Antibiotics for prophylaxis were administered intravenously either before the incision versus after clamping of the neonatal umbilical cord. Studies administered antibiotics before incision at various time frames with the vast majority ranging from 15 to 60 minutes.
The antimicrobial agents used included a first generation cephalosporin (cefazolin 1 g or 2 g) in seven trials (Kandil 2014 (2 g); Macones 2012 (1 g); Sullivan 2007 (1 g); Thigpen 2005 (2 g); Wax 1997 (1 g); Witt 2011 (2 g); Yildirim 2009 (1 g)). The remaining three trials used a third generation cephalosporin (ceftriaxone 1 g or 2 g) (Bhattacharjee 2013 (2 g); Francis 2013 (2 g); Kalaranjini 2013 (1 g)). Clindamycin was typically the agent of choice for women who had known allergy to cephalosporins.
Outcomes
The primary outcome was a composite of maternal postpartum infectious morbidities including septic shock, endometritis, wound infection, or death attributed to infection. However, these were defined individually by the trialists.
The clinical criteria listed to define endometritis and urinary tract infection were remarkably consistent across trials. Wound infection was usually a clinical diagnosis and generally included induration, erythema, cellulitis or various degrees of drainage. Duration of maternal hospital stay and neonatal ICU length of stay were included when reported.
Two studies reported on septic pelvic thrombophlebitis, though there were no occurrences (Kalaranjini 2013; Yildirim 2009). One study (Kalaranjini 2013) reported on septic shock and maternal death, though there were no occurrences. None of the included studies reported on ICU admission, cost of antibiotics, antibiotic‐related adverse events, placental transfer of antibiotics, breastfeeding or neonatal mortality.
Excluded studies
Seven trials were excluded from analysis as antibiotic administrations were not limited to preoperative versus post‐cord clamp: women continued to receive antibiotics postoperatively in five (De Palma 1980; Gordon 1979; Gul 1999; Nokiani 2009; Tassi 1987) and single dose was compared to multiple day regimens in two (van Beekhuizen 2008; Van Velzen 2009). One study was excluded as antibiotics were given preoperatively and postoperatively, but were not given at neonatal cord clamp (Xu 1997). One study was excluded because it was not a randomized controlled trial (Cunningham 1983). Three studies published in abstract form did not provide sufficient information: two were excluded (Rodriguez 1990; Seton 1996) and one (Pevzner 2009) is awaiting classification pending publication of the final manuscript.
(Please refer to Characteristics of excluded studies for further details).
Risk of bias in included studies
The overall methodological quality of the trials 10 trials that contributed data towards the analysis was considered low risk of bias (see Figure 2; Figure 3).
2.

'Risk of bias. graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
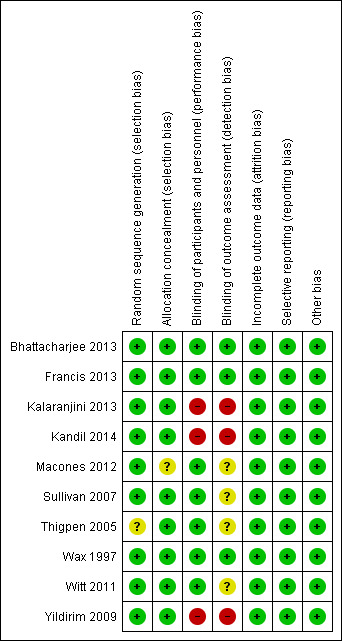
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
There was no serious publication bias in the primary outcome due to the large number of women in the symmetrical part of the funnel plot (Figure 4).
4.
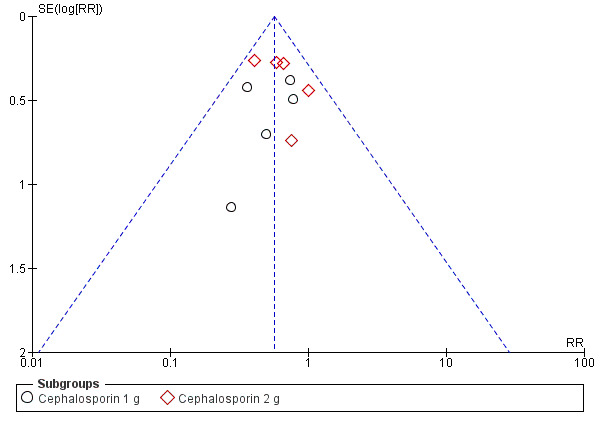
Funnel plot of comparison: 1 Maternal postpartum infectious morbidity, outcome: 1.1 Composite morbidity (as defined by trials).
Allocation
The studies used various methods of randomization, e.g. computer‐generated (Bhattacharjee 2013; Wax 1997), cards shuffled (Kandil 2014), and random number tables (Sullivan 2007), all of which were judged as low risk, except for Thigpen 2005 which was judged as unclear risk as it was only mentioned that randomization was performed by pharmacy personnel.
Four studies used sealed envelopes for allocation concealment (Bhattacharjee 2013; Kalaranjini 2013; Kandil 2014; Yildirim 2009) and five had pharmacy or nursing staff provide the antibiotics (Francis 2013; Sullivan 2007; Thigpen 2005; Wax 1997; Witt 2011). Macones 2012 does not mention method of allocation concealment.
Blinding
Seven trials were adequately blinded in regards to participants as well as providers, and therefore, deemed to have a low risk of performance bias. Three studies did not report performance bias but we suspect (from the study methods) that no blinding occurred and therefore categorized them as high risk of bias (Kalaranjini 2013; Kandil 2014; Yildirim 2009) for both blinding domains.
Four trials did not clearly report detection bias and we were unable to infer the potential for bias from the methods, so it was judged to be unclear risk (Macones 2012; Sullivan 2007; Thigpen 2005; Witt 2011). Three trials that described blinding of outcome assessment and were categorized as low risk of bias (Bhattacharjee 2013; Francis 2013; Wax 1997).Kalaranjini 2013; Yildirim 2009; and Kandil 2014 were again deemed high risk of bias as no mention was made in the associated text about detection bias and we suspect that no blinding occurred.
Incomplete outcome data
All 10 studies reported that all women who were initially randomized were included in the analysis; loss to follow‐up was low and typically reasons were provided.
Selective reporting
We found the reporting bias to be low risk as the primary outcomes were stated in the study methods of all 10 studies.
Other potential sources of bias
No other potential sources of bias were identified in the 10 trials.
Effects of interventions
See: Table 1
The overall risk of bias was low. The quality of the evidence using GRADE was high for composite morbidity, endomyometritis, wound infection and neonatal intensive care unit admission, moderate for UTI/cystitis/pyelonephritis and neonatal sepsis, and low for maternal respiratory infection.
1. Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes) ‐ Analyses 1.1 to 1.10
Primary outcomes
Composite morbidity (as defined by trials)
There were significant reductions in composite morbidity (as defined by trials) for women who received antibiotics preoperatively as compared to those who received antibiotics after cord clamp (risk ratio (RR) 0.57; 95% confidence interval (CI) 0.45 to 0.72, 10 trials, 5041 women, high quality evidence) (Analysis 1.1). An interaction test for subgroup differences (1 g versus 2 g of cephalosporin) found no clear difference between studies using 1g of cephalosporin and those using 2 g of cephalosporin (Chi² = 0.02, df = 1 (P = 0.88); I² = 0%).
1.1. Analysis.
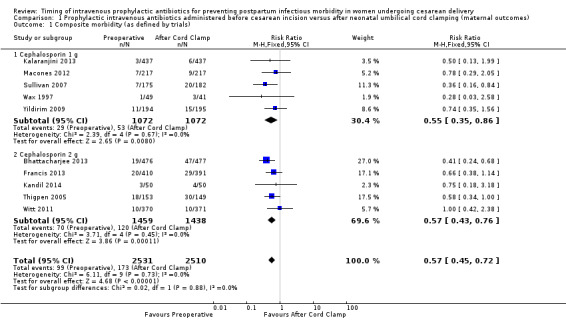
Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 1 Composite morbidity (as defined by trials).
Secondary outcomes (maternal)
There were significant reductions in endomyometritis (RR 0.54; 95% CI 0.36 to 0.79, 10 trials, 5041 women, high quality evidence (Analysis 1.2); wound infection (RR 0.59; 95% CI 0.44 to 0.81, 10 trials, 5041 women, high quality evidence (Analysis 1.3)) and length of hospital stay (mean difference (MD) ‐0.17; 95% CI ‐0.30 to ‐0.04, two trials, 1342 women (Analysis 1.7)) in women who received antibiotics preoperatively as compared to those who received antibiotics after cord clamp.
1.2. Analysis.
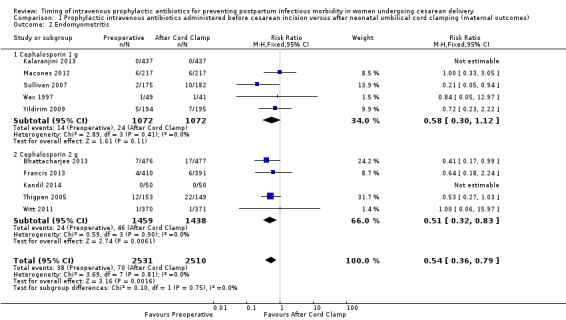
Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 2 Endomyometritis.
1.3. Analysis.
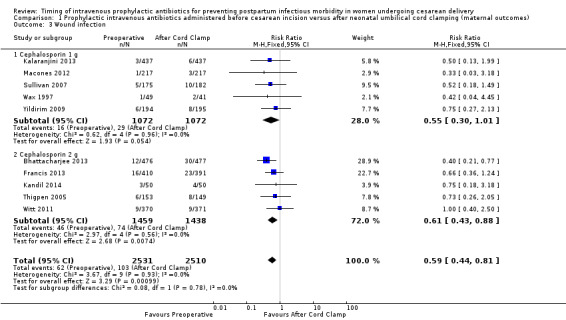
Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 3 Wound infection.
1.7. Analysis.
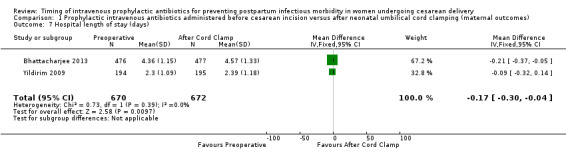
Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 7 Hospital length of stay (days).
An interaction test for subgroup differences (1 g versus 2 g of cephalosporin) found no clear difference between studies for endometritis (Chi² = 0.10, (P = 0.75); I² = 0%) or wound infections (Chi² = 0.08, (P = 0.78); I² = 0%).
There were no clear differences in occurrence of UTI/cystitis/pyelonephritis (RR 1.02; 95% CI 0.65 to 1.59, eight trials, 4001 women, moderate quality evidence (Analysis 1.4)); pelvic abscesses (RR 1.00; 95% CI 0.06 to 15.97, one trial, 741 women (Analysis 1.5)); respiratory infections (e.g. pneumonia) (RR 2.30; 95% CI 0.34 to 15.45, four trials, 1849 women, low quality evidence (Analysis 1.6)); or febrile illness (RR 0.93; 95% CI 0.63 to 1.35, four trials, 2650 women (Analysis 1.8)) between the groups.
1.4. Analysis.
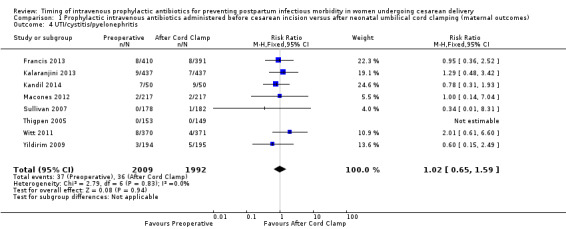
Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 4 UTI/cystitis/pyelonephritis.
1.5. Analysis.
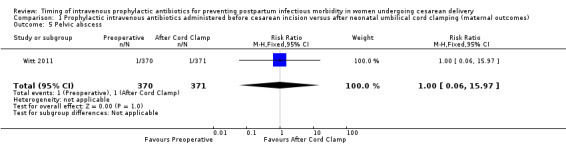
Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 5 Pelvic abscess.
1.6. Analysis.
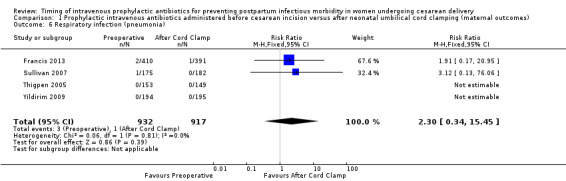
Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 6 Respiratory infection (pneumonia).
1.8. Analysis.
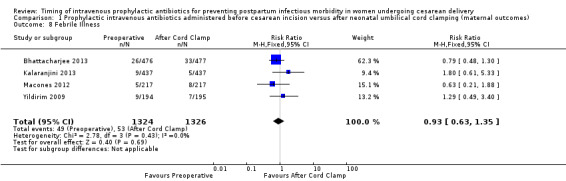
Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 8 Febrile Illness.
Two studies reported on septic pelvic thrombophlebitis, though there were no occurrences (Kalaranjini 2013; Yildirim 2009); so RR could not be calculated. One study (Kalaranjini 2013) reported on septic shock and maternal death, though there were no occurrences; so RR could not be calculated. None of the included studies reported on ICU admission, cost of antibiotics, antibiotic‐related adverse events, placental transfer of antibiotics, breastfeeding or neonatal mortality.
2. Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes) ‐ Analyses 2.1 to 2.8
Secondary outcomes
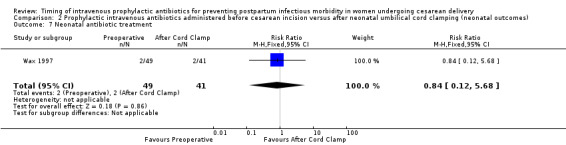
There were no clear differences for any of the neonatal outcomes when comparing preoperative administration of antibiotics to administration after cord clamp: neonatal sepsis (RR 0.76; 95% CI 0.51 to 1.13, five trials, 2907 neonates, moderate quality evidence (Analysis 2.1)); neonatal sepsis work up (RR 0.92; 95% CI 0.69 to 1.23, four trials, 1170 neonates) (Analysis 2.2)); infection with a resistant organism (RR 0.70; 95% CI 0.12 to 4.14, one trial, 379 neonates (Analysis 2.3)); infection (other) (RR 0.93; 95% CI 0.52 to 1.64, one trial, 302 neonates (Analysis 2.4)); ICU admission (RR 0.91; 95% CI 0.74 to 1.13, six trials, 3708 neonates (Analysis 2.5));ICU length of stay (days) (MD ‐0.07; 95% CI ‐2.60 to 2.46, three trials, 1731 neonates, random‐effects, Tau² = 4.07; I² = 97% (Analysis 2.6)); neonatal antibiotic treatment (RR 0.84; 95% CI 0.12 to 5.68, one trial, 90 neonates (Analysis 2.7)); and febrile illness (RR 0.67; 95% CI 0.28 to 1.62, one trial, 953 neonates (Analysis 2.8)).
2.1. Analysis.
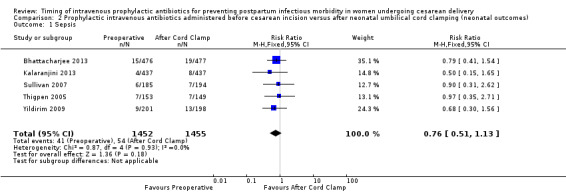
Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 1 Sepsis.
2.2. Analysis.
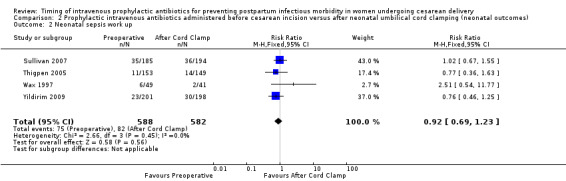
Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 2 Neonatal sepsis work up.
2.3. Analysis.
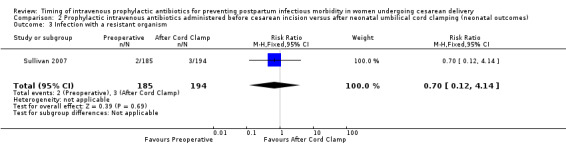
Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 3 Infection with a resistant organism.
2.4. Analysis.
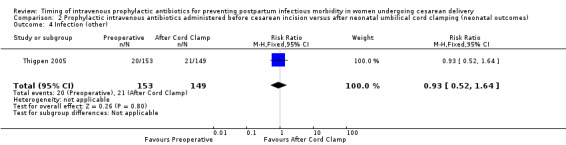
Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 4 Infection (other).
2.5. Analysis.
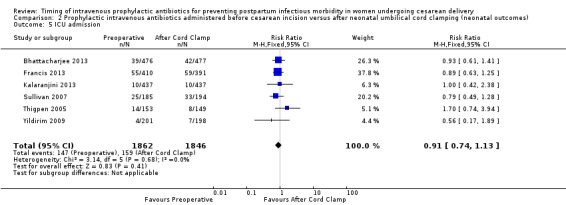
Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 5 ICU admission.
2.6. Analysis.
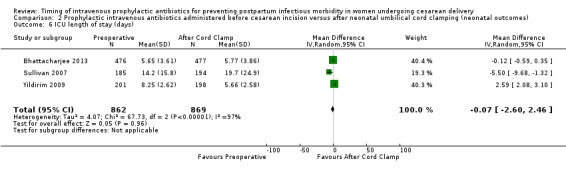
Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 6 ICU length of stay (days).
2.7. Analysis.
Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 7 Neonatal antibiotic treatment.
2.8. Analysis.
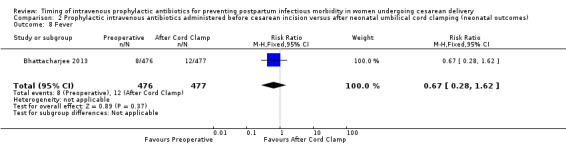
Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 8 Fever.
Among the included studies, no data were available for the neonatal outcome of mortality.
Discussion
Summary of main results
This review included 10 trials of 5041 women who received prophylactic antibiotic administration for cesarean birth prior to skin incision versus after neonatal umbilical cord clamping to assess which is superior in preventing postpartum infectious morbidity.
Those who received antibiotics preoperatively were 43% less likely to have infectious morbidity as compared to those who received antibiotics after neonatal cord clamping. Those who received antibiotics preoperatively were 46% and 41% less likely to have endomyometritis and wound infection, respectively, as compared to those who received antibiotics after neonatal cord clamping.
Although a significant difference was noted in the hospital length of stay favoring preoperative antibiotics, the difference is unlikely to be of clinical significance. For other maternal infections such as UTI, pelvic abscess, respiratory infection (pneumonia), febrile illness, and fever showed no differences. There were no clear differences in neonatal outcomes regardless of when antibiotics were administered (Table 1).
Overall completeness and applicability of evidence
The included trials enrolled 5041 women from six (developing and developed) countries from 1990 to 2013. Overall, the trials in this review were of reasonably sound methodology and the results were generally consistent across the trials.
Prophylactic administration of antibiotics for cesarean delivery given prior to skin incision was more effective in preventing infectious morbidity (specifically, endomyometritis and wound infection) than when antibiotics were given after neonatal cord clamp.
Some secondary outcomes were not (or rarely) reported: septic pelvic thrombophlebitis, septic shock, maternal death, pelvic abscess, hospital length of stay, ICU admission, cost of antibiotics, antibiotic‐related adverse events, placental transfer of antibiotics, breastfeeding. Maternal side effects were not reported (or might not have been collected consistently across trials) in the included studies; however, all the studies consistently reported on wound infection and endomyometritis,which are included in the composite primary outcome. Neonatal outcomes were not consistently reported from all trials, although no evidence of adverse effects were found regardless of timing of antibiotic administration. No study reported on neonatal mortality.
The lack of consistent reporting of neonatal outcomes is certainly a limitation of this review. Although the primary outcome of composite maternal morbidity is well represented, the lack of neonatal data within several of the included studies creates an artificial separation of the effects of antibiotics on the maternal/fetal diad. Additionally, no maternal deaths or ICU admissions were noted in any of the studies.
Quality of the evidence
The quality of the evidence using GRADE was high for composite morbidity, endomyometritis, wound infection and neonatal ICU admission. UTI/cystitis/pyelonephritis and neonatal sepsis were moderate for quality of the evidence, downgraded one due to wide confidence interval crossing the line of no effect. Maternal respiratory infection (pneumonia) was considered to be of low quality of evidence due to wide confidence intervals crossing the line of no effect, few events and small sample size (Table 1).
Potential biases in the review process
We followed to the Cochrane Pregnancy and Childbirth Group search strategies and review process. Two review authors conducted the study selection, data collection independently to avoid potential biases, and we are not aware of any potential bias in the review process.
Agreements and disagreements with other studies or reviews
There are two reviews that examined maternal and neonatal infectious morbidity in women undergoing cesarean delivery receiving preoperative prophylaxis compared with those receiving intraoperative administration (Baaqeel 2013; Costantine 2008). Costantine 2008 included three trials. Baaqeel 2013 included six trials. Our review includes 10 trials. Overall, the results of our review are in agreement with the previous reviews. All three reviews found that preoperative administration of antibiotics lead to a significant decrease in endomyometritis and total maternal infectious morbidity. Additionally, all three reviews found no significant differences in neonatal sepsis or neonatal ICU admission. In contrast, this review was able to demonstrate a significant decrease in wound infection when women received preoperative antibiotics, whereas the previous two reviews were only able to demonstrate a non‐significant trend towards decrease in wound infection. Other outcomes of the two previous reviews (including maternal febrile morbidity and neonatal morbidity) were found to have non‐significant reductions which was consistent with this review.
Authors' conclusions
Implications for practice.
Cesarean antibiotic prophylaxis administered preoperatively significantly reduced the incidence of maternal infection especially endomyometritis and wound infection compared with administration of antibiotics after neonatal umbilical cord clamping (based on high quality evidence with an overall low risk of bias). There were no adverse neonatal outcomes detected.
Implications for research.
The current evidence supports preoperative prophylactic antibiotic administration to decrease infectious morbidity after cesarean section, especially endometritis and wound infection. There is not enough information regarding genitourinary infections, pelvic abscess occurrence, respiratory infections or febrile illness to show whether administration preoperatively is superior to administration of antibiotics after cord clamp. Additional research may be able to provide further insight into adverse effects for neonates. We were not able to determine whether there is a specific time frame preoperatively that is preferred, for example, 30 to 60 minutes versus within 30 minutes.
Acknowledgements
Erika Ota's work was financially supported by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization. The named authors alone are responsible for the views expressed in this publication.
The authors would like to thank Tanya Ohly and Samantha Weed for helping develop the protocol (Baxter 2011).
As part of the pre‐publication editorial process, this review has been commented on by two peers (an editor and referee who is external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Data and analyses
Comparison 1. Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Composite morbidity (as defined by trials) | 10 | 5041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.45, 0.72] |
| 1.1 Cephalosporin 1 g | 5 | 2144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.35, 0.86] |
| 1.2 Cephalosporin 2 g | 5 | 2897 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.43, 0.76] |
| 2 Endomyometritis | 10 | 5041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.36, 0.79] |
| 2.1 Cephalosporin 1 g | 5 | 2144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.30, 1.12] |
| 2.2 Cephalosporin 2 g | 5 | 2897 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.32, 0.83] |
| 3 Wound infection | 10 | 5041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.44, 0.81] |
| 3.1 Cephalosporin 1 g | 5 | 2144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.30, 1.01] |
| 3.2 Cephalosporin 2 g | 5 | 2897 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.43, 0.88] |
| 4 UTI/cystitis/pyelonephritis | 8 | 4001 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.65, 1.59] |
| 5 Pelvic abscess | 1 | 741 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 15.97] |
| 6 Respiratory infection (pneumonia) | 4 | 1849 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [0.34, 15.45] |
| 7 Hospital length of stay (days) | 2 | 1342 | Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.30, ‐0.04] |
| 8 Febrile Illness | 4 | 2650 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.63, 1.35] |
Comparison 2. Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sepsis | 5 | 2907 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.51, 1.13] |
| 2 Neonatal sepsis work up | 4 | 1170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.69, 1.23] |
| 3 Infection with a resistant organism | 1 | 379 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.12, 4.14] |
| 4 Infection (other) | 1 | 302 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.52, 1.64] |
| 5 ICU admission | 6 | 3708 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.74, 1.13] |
| 6 ICU length of stay (days) | 3 | 1731 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐2.60, 2.46] |
| 7 Neonatal antibiotic treatment | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.12, 5.68] |
| 8 Fever | 1 | 953 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.28, 1.62] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bhattacharjee 2013.
| Methods | Randomized controlled trial. | |
| Participants | 953 pregnant women, more than 34 weeks of gestation, requiring cesarean deliveries. Excluded:obstetric complications (pre‐eclampsia, antepartum hemorrhage, etc), renal disease, heart disease, diabetes mellitus, febrile during or prior to screening, ruptured membranes with or without antibiotic prophylaxis, any exposure to antibiotic during past 1 week, obstetrical indication for emergency cesarean delivery during labor, penicillin or cephalosporin allergy. |
|
| Interventions | Group A received prophylactic single‐dose intravenous antibiotic (2 g ceftriaxone mixed with 10 mL) prior to incision and intravenous placebo (10 mL water) after cord clamp (n = 476). Group B received intravenous placebo (10 mL water) prior to incision and intravenous ceftriaxone 2 g in 10 mL after cord clamp (n = 477). |
|
| Outcomes | Primary outcome: postoperative maternal infectious morbidity. Secondary outcomes: neonatal complications, postoperative hospital stay of mother and stay of neonates at NICU (days). |
|
| Notes | July 2010 to December 2011, at 2 teaching hospitals in West Bengal India. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer‐generated randomisation sequence" was used. |
| Allocation concealment (selection bias) | Low risk | "The allocation was concealed in sealed, sequentially numbered, brown envelopes (opaque), which had been prepared by the statistician of each centre and handed over to the sister‐in‐charge of the operation theatre" was used. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The drugs were supplied in small sealed bags", "Both vials were identical", conducted double‐blinded manner. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Postoperative follow‐up was done by resident doctors who were blinded to the patients' and babies' identity." Outcome assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All the women randomized included in the analysis (intention‐to‐treat analysis), however, the number of women who completed the intervention group was n = 458 (96.2%), and in the control group was n = 456 (95.6%). |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes stated. |
| Other bias | Low risk | No other bias evident. |
Francis 2013.
| Methods | Randomized controlled trial. | |
| Participants | 896 women undergoing non‐emergent cesarean delivery including women with ruptured membranes and experiencing labor, and scheduled cesarean deliveries. Excluded:fever > 38°C, age < 18 years old, diagnosed as chorioamnionitis before delivery, allergy to cefazolin and clindamycin, any exposure to antibiotic within 1 week before delivery. 95 women lost to follow‐ up: 39 from Group 1; 56 from Group 2 (cord clamp). |
|
| Interventions | Prophylactic single‐dose antibiotic (2 g ceftriaxone or clindamycin 900 mg if women allergic to penicillin) within 30 to 60 minutes of expected skin incision (n = 410) and placebo after umbilical cord clamping or placebo within 30 to 60 minutes of expected incision and the antibiotic (2 g ceftriaxone or clindamycin 900 mg if women allergic to penicillin) after umbilical cord clamping (n = 391). | |
| Outcomes | Primary outcomes: maternal infectious morbidity including wound infection, UTI, endometritis and pneumonia. Secondary outcomes: neonatal antibiotic administration, admission to the NICU, and hospital readmission rates. |
|
| Notes | Single site: St Vincent Hospital in Indianapolis, Indiana, USA, September 2006 to January 2011. Underpowered as noted in article. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The randomisation sequence was generated by the hospital statistician.” “The randomisation list was e‐mailed to the research pharmacist.” Comments: unclear technique used to generate randomization list, but we suspect that method was appropriate as it was done by a statistician. |
| Allocation concealment (selection bias) | Low risk | “The randomisation list was e‐mailed to the research pharmacist, who was the only person with access to the randomisation information for the duration of the study.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “Treatment assignment was performed in the pharmacy and all physicians and patients were blinded to which bag contained the antibiotic and which one had saline.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Only pharmacy was aware of timing of antibiotic. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The 95 women lost to follow‐ up (10.6%), 39 (4.4%) were in the skin incision group and 56 (6.2%) were in the cord clamp group. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes stated. |
| Other bias | Low risk | No other bias evident. |
Kalaranjini 2013.
| Methods | Randomized controlled trial. | |
| Participants | 874 women undergoing elective cesarean delivery. 437 women in each group. Excluded: history of diabetes mellitus, severe anemia, obese women (BMI >= 25) ruptured membranes, retro positive women, immune‐suppressant drugs, history of allergy to ceftriaxone No women lost to follow‐up. |
|
| Interventions | Single dose of ceftriaxone 1 g intravenously 15‐45 minutes before skin incision (n = 437) versus the same medication after cord clamping (n = 437). | |
| Outcomes | Primary outcome: maternal postoperative infectious morbidities such as surgical site wound infection, febrile morbidity, endometritis, UTIs and neonatal sepsis. | |
| Notes | Single site: conducted from October 2010 to July 2012. 1 hospital in Puducherry, India. About 27 women (17 in before skin incision and 10 in cord clamping) continued receiving antibiotics after surgery due to various infections. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The patients were randomly categorized into two groups using serially numbered opaque sealed envelope (SNOPE) technique.” |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelope was used. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was not specified, but we suspect that no blinding occurred. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | This was not specified, but we suspect that no blinding occurred. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women accounted for, no loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were stated. |
| Other bias | Low risk | No other bias evident. |
Kandil 2014.
| Methods | Randomized controlled trial. | |
| Participants | 100 primigravid women with singleton pregnancy at term undergoing elective cesarean. Excluded: age < 20 or > 30 years; BMI < 19 or >= 25; exposure to antibiotics within 1 week of delivery; premature rupture of membranes; indication for emergency cesarean; hypersensitivity to cephalosporins; temperature > 37.8 degrees celsius. |
|
| Interventions | 2 g cefazolin administered 30 minutes preoperatively (n = 50) versus after cord clamp (n = 50). | |
| Outcomes | Endometritis, wound infection, UTI. | |
| Notes | 1 hospital in Egypt. June 2011‐December 2012. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 50 cards were prepared for each intervention. The cards were placed into opaque envelopes and "shuffled to produce a form of random assignment". |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was not specified, but we suspect that no blinding occurred. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | This was not specified, but we suspect that no blinding occurred. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None reported. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes stated. |
| Other bias | Low risk | No other bias evident. |
Macones 2012.
| Methods | Randomized controlled trial. | |
| Participants | 434 women undergoing non‐emergency cesarean deliveries >= 36 weeks excluding women with known fetal anomaly, antibiotics within 7 days of admission, overt intrapartum infection, or ruptured membranes > 18 hours. | |
| Interventions | 1 g cefazolin administered < 30 minutes preoperatively (n = 217) versus after cord clamp (n = 217). They did not specify intravenous treatment, but we included this trial as it was assumed that cefazolin was administered intravenously. | |
| Outcomes | Primary: postoperative fever, wound infection, endomyometritis, UTI. Secondary: NICU admission, proven or suspected neonatal sepsis (with resistant bacteria), neonatal length of stay (days). |
|
| Notes | 2 centers in USA. Study period not defined. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted blocks. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Adminstered by anesthesiologist to maintain blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | This was not specified. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not specifically mentioned, but it appears that outcomes are available for all enrolled women. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes stated. |
| Other bias | Low risk | No other bias evident. |
Sullivan 2007.
| Methods | Randomized controlled trial. | |
| Participants | 367 women >= 24 weeks who were undergoing cesarean delivery excluding women with a cephalosporin allergy, age < 18 years, exposure to antibiotics within 7 days, need for emergency surgery. | |
| Interventions | 1 g cefazolin administered at 15‐60 minutes pre‐incision and normal saline after cord clamp (n = 175) versus normal saline at 15‐60 minutes pre‐incision and 1 g cefazolin after cord clamp (n = 182). They did not specify intravenous treatment, but we included this trial as it was assumed that cefazolin was administered intravenously. | |
| Outcomes | Primary: total infectious morbidity. | |
| Notes | Abstract and paper; study commenced January 2003. 1 hospital in the United States of America. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomization using random number tables. |
| Allocation concealment (selection bias) | Low risk | Investigational pharmacy staff delivered both antibiotics and placebo. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Intraoperative labeled bag given by anesthesia. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind trial but does not describe blinding method. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The preoperative group lost 3 women and the cord clamp group lost 5 women to attrition. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes stated. |
| Other bias | Low risk | No other bias evident. |
Thigpen 2005.
| Methods | Randomized controlled trial. | |
| Participants | 346 women in active labor excluding women with chorioamnionitis, cephalosporin allergy, antibiotics within 2 weeks of labor. | |
| Interventions | 2 g cefazolin administered pre‐incision and normal saline after cord clamp (n = 153) versus normal saline pre‐incision and 2 g cefazolin after cord clamp (n = 149). | |
| Outcomes | Postoperative infection, neonatal infections and sepsis. | |
| Notes | 1 center in the United States of America from November 2000 until April 2003. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study only states that randomization was performed by pharmacy personnel. |
| Allocation concealment (selection bias) | Low risk | Allocated by pharmacy personnel. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Care providers were unaware of which bag contained antibiotic versus placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not specifically mentioned, but it appears that outcomes are available for all enrolled women who were not excluded following randomization. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes stated. |
| Other bias | Low risk | No other bias evident. |
Wax 1997.
| Methods | Randomized controlled trial. | |
| Participants | 90 women undergoing cesarean in labor with a single fetus ≥ 37 weeks' gestation excluding women with an allergy to penicillin or cephalosporin, antibiotic use within 2 weeks of delivery, temperature ≥ 37.8°C in labor, administration of group B streptococcal or subacute bacterial endocarditis prophylaxis during labor, insulin‐dependent diabetes mellitus, human immunodeficiency virus infection, chronic glucocorticoid use, or multiple gestation. | |
| Interventions | 1 g cefazolin administered over approximately 5 minutes upon decision for cesarean delivery and normal saline after cord clamp, each intervention in identical 50 mL infusions (n = 49) versus normal saline over approximately 5 minutes upon decision for cesarean delivery and 1 g cefazolin after cord clamp (n = 41). | |
| Outcomes | Primary maternal outcomes: endometritis and wound infection. Secondary maternal outcomes: intra‐abdominal abscess, septic pelvic thrombophlebitis, or symptomatic UTI. Neonatal outcomes: sepsis screen, sepsis, pneumonia, and meningitis. |
|
| Notes | 1 center in the United States of America over a 12‐month period (exact dates not provided). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization. |
| Allocation concealment (selection bias) | Low risk | Randomization code was produced by, and known only by pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Each participants received 2 bags, effectively blinding to timing of antibiotic. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Only pharmacy was aware of timing of antibiotic. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 participants were lost to 2 week follow‐up. 14 participants were lost to 6 week follow‐up. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes. |
| Other bias | Low risk | No other bias evident. |
Witt 2011.
| Methods | Randomized controlled trial. | |
| Participants | 1112 women with a fetus ≥ 37 weeks' gestation undergoing elective cesarean delivery of a fetus with reassuring fetal heart rate tracing. | |
| Interventions | Group A: 2 g cefazolin in 100 mL saline 20‐30 minutes before incision (n = 370). Group B: 2 g cefazolin in 100 mL saline immediately after cord clamp (n = 371). Group C: 100 mL saline 20‐30 minutes before incision (n = 371). |
|
| Outcomes | Total postoperative infectious morbidity (endometritis, wound infection, UTI). | |
| Notes | 1 hospital in Vienna, Austria. 3/1/04 ‐ 1/31/10. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated permuted blocks of 5. |
| Allocation concealment (selection bias) | Low risk | Only the study nurse was not blinded and handed appropriate infusion bag to anesthesiologist. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Women and surgeons masked to administration schedule. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Infectious morbidity was evaluated by 2 residents who were masked to group assignments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Protocol violations in Groups 1, 2, and 3 respectively: 12, 7, and 13. 32 women lost to follow‐ up/protocol violations/withdrawal. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes. |
| Other bias | Low risk | No other bias evident. |
Yildirim 2009.
| Methods | Randomized controlled trial. | |
| Participants | 400 women undergoing elective cesarean delivery prior to labor initially included with 11 excluded due to blood transfusion leaving 389 participants. Additional exclusion criteria included the use of antibiotics in the last 24 hours, pathology that should be treated with antibiotics, pre‐existing maternal disease (such as diabetes, collagen vascular disease, immune system problems), chorioamnionitis, fever on admission, need of transfusion before or during cesarean delivery, ruptured membranes, emergency cesarean delivery and preterm cesarean delivery. | |
| Interventions | 1 g of cefazolin ≤ 45 minutes pre‐incision (n = 194) versus 1 g cefazolin after cord clamp (n = 195). They did not specify intravenous treatment, but we included this trial as it was assumed that cefazolin was administered intravenously. | |
| Outcomes | Rates of postoperative infectious morbidity (endometritis, wound infection, febrile morbidity, UTI), estimated blood loss and operative time. Neonatal outcomes: NICU admission, Apgar score less than 7 at 5 minutes, neonatal sepsis and sepsis workup. |
|
| Notes | Conducted June 2007‐December 2007 at 1 hospital inTurkey. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 2 parts, blocked randomization. |
| Allocation concealment (selection bias) | Low risk | Sealed, sequentially distributed envelopes indicating group A (pre‐incision) or group B (following cord camp) chosen by the participants, opened by the investigator. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was not specified, but we suspect that no blinding occurred. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | This was not specified, but we suspect that no blinding occurred. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None lost to follow‐ up. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes. |
| Other bias | Low risk | No other bias evident. |
BMI: body mass index NICU: neonatal intensive care unit UTI: urinary tract infection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cunningham 1983 | Not a randomized controlled trial. |
| De Palma 1980 | Antibiotic regimens were not limited to preoperative versus post‐cord clamp. Women continued to receive antibiotics postoperatively. |
| Gordon 1979 | Antibiotic regimens were not limited to preoperative versus post‐cord clamp. Women continued to receive antibiotics postoperatively. |
| Gul 1999 | Antibiotic regimens were not limited to preoperative versus post‐cord clamp. Women continued to receive antibiotics postoperatively. |
| Nokiani 2009 | Antibiotic regimens were not limited to preoperative versus post‐cord clamp. Women continued to receive antibiotics postoperatively. |
| Rodriguez 1990 | There is insufficient information provided in the abstract. There was no information on randomization (sequence generation and allocation concealment were not described). There was no blinding. The group sizes were very different 55 versus 84. It was not clear whether this was due to chance. There was no information on attrition. There were no usable data (most results were not reported according to randomization group but according to risk), the results that were reported by randomization group did not present the data (P values only)). |
| Seton 1996 | There is insufficient information provided in the abstract. This was described as a small pilot study. There was no information on sequence generation or allocation concealment although it was described as a double blind‐trial. It was not clear whether the placebo was identical. There was no information on attrition or missing data (it was not stated how many were randomized to each group although percentages in the results suggest 20 women were randomized to each arm). There were no SDs reported for continuous data. The intervention was not clear. The women were randomized to receive antibiotics before or after cord clamping; but the interval was not described (it was not clear whether the antibiotic was administered immediately before cord clamping, after skin incision or immediately after or a long period after). |
| Tassi 1987 | Antibiotic regimens were not limited to preoperative versus post‐cord clamp. Women continued to receive antibiotics postoperatively. |
| van Beekhuizen 2008 | Antibiotic regimens were not limited to preoperative versus post‐cord clamp. Comparison was made between single dose and multiple day regimens. |
| Van Velzen 2009 | Antibiotic regimens were not limited to preoperative versus post‐cord clamp. Comparison was made between single dose and multiple day regimens. |
| Xu 1997 | Antibiotic regimens were limited to preoperative versus postoperative but were not given at cord clamp. |
Characteristics of studies awaiting assessment [ordered by study ID]
Pevzner 2009.
| Methods | Randomized controlled trial. |
| Participants | 50 women undergoing scheduled cesarean delivery. |
| Interventions | 2 g cefazolin preoperatively versus after cord clamp (number randomized to each group not specified). |
| Outcomes | Primary: endometritis and wound infection. Secondary: neonatal sepsis work‐up, sepsis, pneumonia, and meningitis. Followed 6 weeks postoperatively for late complications. |
| Notes | Abstract pilot study, in USA. Does not currently provide enough information to make an assessment of methodologic quality. |
Characteristics of ongoing studies [ordered by study ID]
Zhang 2012.
| Trial name or title | Timing of perioperative antibiotics for cesarean: a multicenter randomized controlled study. |
| Methods | Randomized control trial. |
| Participants | Women were eligible for the trial if they were > 37 weeks' gestation and undergoing cesarean delivery. Exclusion criteria: allergy to cefathiamidine, exposure to any antibiotic agent within 1 week of delivery, > 37.5°C before cesarean or age < 18 years or > 40 years. |
| Interventions | Cefethiamidine administered 30 minutes prior to skin incision but no more than 120 minutes before versus after cord clamping. |
| Outcomes | Endomyometritis, wound infection, urinary tract infection, neonatal sepsis, neonatal sepsis workup, neonatal admission, neonatal dysbacteriosis, temperature after cesarean, number of white blood count and neutrophile granulocyte after cesarean, ratio of neonatal stool coccus and bacillus. |
| Starting date | December 2011‐June 2012. |
| Contact information | Zhang Chuan, Department of Pharmacy West China Second University Hospital, Sichuan University (email: zhang_chuan@yahoo.cn) |
| Notes | Settings ‐ 3 facilities (West China Second University Hospital Sichuan University, Sichuan Women and Children's Health, Nanchong Central Hospital). |
Differences between protocol and review
We have assessed the quality of the body of evidence using the GRADE approach (Schunemann 2009) ‐ this was not pre‐specified in our published protocol (Baxter 2011).
We added a subgroup analysis based on dose of cephalosporin (1 g versus 2 g).
We changed the definition of our primary outcome of composite morbidity to include sepsis (including septic shock), endomyometritis, wound infection, or death attributed to infection. We removed necrotizing fasciitis and bacteremia as these would have been included in infection and sepsis.
In addition, some sections of the methods have been updated in accordance with the Cochrane Pregnancy and Childbirth Group's standard methods text:
assessment of reporting biases;
subgroup analysis and investigation of heterogeneity.
We have also made minor edits to the list of planned outcomes:
Secondary maternal outcomes
'Urinary tract infection' has been edited to 'Urinary tract infection, cystitis and pyelonephritis'
'Upper respiratory infection (pneumonia) has been edited to 'Respiratory infection (e.g. pneumonia)'
'Fever' has been changed to 'Febrile illness'
'Sepsis' has been changed to 'Sepsis, including septic shock'
Clarified that the unit for hospital length of stay is in days
Secondary infant outcomes
'Neonatal workup for infection' has been edited to 'Neonatal sepsis workup'
Length of ICU stay has been clarified as 'Length of ICU stay (days)'
'Antibiotic treatment' has been edited to 'Neonatal antibiotic treatment'
Febrile illness has been added as a new secondary outcome
Contributions of authors
A. Dhanya Mackeen, Roger Packard and Erika Ota abstracted data from the studies and entered data into RevMan. A. Dhanya Mackeen, Roger Packard, Vincenzo Berghella, and Erika Ota prepared the first draft. Erika Ota prepared the 'Summary of findings' tables. All authors approved the final version of the update for publication. A. Dhanya Mackeen is guarantor for the review.
Sources of support
Internal sources
-
UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland.
Erika Ota's work was financially supported by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization.
External sources
No sources of support supplied
Declarations of interest
None known.
New
References
References to studies included in this review
Bhattacharjee 2013 {published data only}
- Bhattacharjee N, Saha SP, Patra KK, Mitra U, Ghoshroy SC. Optimal timing of prophylactic antibiotic for cesarean delivery: a randomized comparative study. Journal of Obstetrics and Gynaecology Research 2013;39(12):1560‐8. [DOI] [PubMed] [Google Scholar]
Francis 2013 {published data only}
- Francis C, Mumford M, Strand ML, Moore ES, Strand EA. Timing of prophylactic antibiotic at cesarean section: A double‐blinded, randomized trial. Journal of Perinatology 2013;33(10):759‐62. [DOI] [PubMed] [Google Scholar]
Kalaranjini 2013 {published data only}
- Kalaranjini S, Veena P, Rani R. Comparison of administration of single dose ceftriaxone for elective caesarean section before skin incision and after cord clamping in preventing post‐operative infectious morbidity. Archives of Gynecology and Obstetrics 2013;288(6):1263‐8. [DOI] [PubMed] [Google Scholar]
Kandil 2014 {published data only}
- Kandil M, Sanad Z, Gaber W. Antibiotic prophylaxis at elective cesarean section: a randomized controlled trial in a low resource setting. Journal of Maternal‐Fetal & Neonatal Medicine 2014;27(6):588‐91. [DOI] [PubMed] [Google Scholar]
Macones 2012 {published data only}
- Macones G, Cleary K, Odibo A, Gross G, Parry S, Rampersad R, et al. Timing of antibiotic prophylaxis at cesarean‐ results of an RCT. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S33. [Google Scholar]
- Macones GA, Cleary KL, Parry S, Stamilio DM, Cahill AG, Odibo AO, et al. The timing of antibiotics at cesarean: A randomized controlled trial. American Journal of Perinatology 2012;29(4):273‐6. [DOI] [PubMed] [Google Scholar]
Sullivan 2007 {published data only}
- Sullivan S, Smith T, Chang E, Hulsey T, Dorsten J, Soper D. Administration of cefazolin prior to skin incision is superior to cefazolin at cord clamping in preventing postcesarean infectious morbidity. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S17. [DOI] [PubMed] [Google Scholar]
- Sullivan SA, Smith T, Chang E, Hulsey T, Vandorsten JP, Soper D. Administration of cefazolin prior to skin incision is superior to cefazolin at cord clamping in preventing postcesarean infectious morbidity: a randomized, controlled trial. American Journal of Obstetrics and Gynecology 2007;196(5):455.e1‐455.e5. [DOI] [PubMed] [Google Scholar]
Thigpen 2005 {published data only}
- Thigpen BD, Hood WA, Chauhan S, Bufkin L, Bofill J, Magann E, et al. Timing of prophylactic antibiotic administration in the uninfected laboring gravida: a randomized clinical trial. American Journal of Obstetrics and Gynecology 2005;192:1864‐71. [DOI] [PubMed] [Google Scholar]
Wax 1997 {published data only}
- Wax JR, Hersey K, Philput C, Wright MS, Nichols KV, Eggleston MK, et al. Single dose cefazolin prophylaxis for postcesarean infections: before vs after cord clamping. Journal of Maternal Fetal Medicine 1997;6(1):61‐5. [DOI] [PubMed] [Google Scholar]
Witt 2011 {published data only}
- Witt A, Doner M, Petricevic L, Berger A, Germann P, Heinze G, et al. Antibiotic prophylaxis before surgery vs after cord clamping in elective cesarean delivery: a double‐blind, prospective, randomized, placebo‐controlled trial. Archives of Surgery 2011;146(12):1404‐9. [DOI] [PubMed] [Google Scholar]
Yildirim 2009 {published data only}
- Yildirim G, Gungorduk K, Guven HZ, Aslan H, Celikkol O, Sudolmus S, et al. When should we perform prophylactic antibiotics in elective cesarean cases?. Archives of Gynecology and Obstetrics 2009;280(1):13‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Cunningham 1983 {published data only}
- Cunningham FG, Leveno KJ, Palma RT, Roark ML, Rosenfeld CR. Perioperative antimicrobials for cesarean delivery: before or after cord clamping?. Obstetrics & Gynecology 1983;62:151‐4. [PubMed] [Google Scholar]
De Palma 1980 {published data only}
- Palma RT, Leveno KJ, Cunningham FG, Pope T, Kappus SS, Roark ML, et al. Identification and management of women at high risk for pelvic infection following cesarean section. Obstetrics & Gynecology 1980;55:185S‐192S. [DOI] [PubMed] [Google Scholar]
Gordon 1979 {published data only}
- Gordon HR, Phelps D, Blanchard K. Prophylactic cesarean section antibiotics: maternal and neonatal morbidity before or after cord clamping. Obstetrics & Gynecology 1979;53:151‐6. [PubMed] [Google Scholar]
Gul 1999 {published data only}
- Gul A, Zeteroilu I, Surucu R. The comparison of piperacillin use for prophylaxis of post cesarean section infection before and after clamping umbilical cord [abstract]. Infectious Diseases in Obstetrics & Gynecology 1999;7(6):306. [Google Scholar]
Nokiani 2009 {published data only}
- Nokiani FA, Akbari H, Rezaei M. Timing of prophylactic antibiotic administration in term cesarean section: a randomized clinical trial. Iranian Journal of Clinical Infectious Diseases 2009;4(2):71‐6. [Google Scholar]
Rodriguez 1990 {published data only}
- Rodriguez GC, Gall SA, Parsons MT. Timing of prophylactic antibiotic administration at cesarean section. Proceedings of 10th Annual Meeting of Society of Perinatal Obstetricians; 1990 Jan 23‐27; Houston, Texas, USA. 1990:311.
Seton 1996 {published data only (unpublished sought but not used)}
- Seton DN, Vellema DMM, Schoon MG, Grobler JM. Prophylactic antibiotics and caesarean section: comparing ceftriaxone administration pre‐ and post‐ umbilical cord clamping in terms of maternal and neonatal morbidity. 15th Conference on Priorities in Perinatal Care in Southern Africa; 1996 March 5‐8; Goudini Spa, South Africa. 1996.
Tassi 1987 {published data only}
- Tassi PG, Tarantini M, Cadenelli GP, Gastaldi A, Benedetti M. Ceftazidime in antibiotic prophylaxis for emergency cesarean section: a randomized prospective study. International Journal of Clinical Pharmacology, Therapeutics and Toxicology 1987;25:582‐8. [PubMed] [Google Scholar]
van Beekhuizen 2008 {published data only}
- Beekhuizen H. Prevention of post‐caesarean infections in low resource countries: is a single dose as adequate as a multiple dose antibiotic regiment? A randomised controlled trial. Current Controlled Trials (www.controlled‐trials.com/) [accessed 20 February 2008] 2008.
Van Velzen 2009 {published data only}
- Velzen C, Kolk P, Westen E, Mmuni N, Hamisi A, Nakua R, et al. Comparison of a single prophylactic dose of ampicillin and metronidazole before caesarean section with a multiple day regimen of these antibiotics in prevention of postpartum maternal infection in a low resource setting: a randomized controlled trial. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S369. [Google Scholar]
Xu 1997 {published data only}
- Xu X, Zhang W, He X. Use of cefotaxime to control post‐operative infection in patients who underwent C‐section. Acta Academiae Medicinae Hubei 1997;18:74‐6. [Google Scholar]
References to studies awaiting assessment
Pevzner 2009 {published data only (unpublished sought but not used)}
- Pevzner L, Chan K. Optimal timing for antibiotic prophylaxis during elective cesarean: before or after cord clamping. American Journal of Obstetrics and Gynecology 2009;201(6 Suppl 1):S225‐S226. [Google Scholar]
References to ongoing studies
Zhang 2012 {published data only}
- Zhang L. Timing of perioperative antibiotics for cesarean: a multicenter randomized controlled study. Chinese Clinical Trial Register [accessed 31 May 2013] 2012.
Additional references
ACOG 2010
- American College of Obstetricians and Gynecologists. Antimicrobial prophylaxis for cesarean delivery: timing of administration. Committee Opinion No. 465. Obstetrics & Gynecology 2010;116:791‐2. [DOI] [PubMed] [Google Scholar]
Baaqeel 2013
- Baaqeel H, Baaqeel R. Timing of administration of prophylactic antibiotics for caesarean section: a systematic review and meta‐analysis. BJOG: an international journal of obstetrics and gynaecology 2013;120(6):661‐9. [DOI: 10.1111/1471-0528.12036] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barwolff 2006
- Barwolff S, Sohr D, Feffers C, Brandt C, Vonberg RP, Halle H, et al. Reduction of surgical site infections after Cesarean delivery using surveillance. Journal of Hospital Infections 2006;64(2):156‐61. [DOI] [PubMed] [Google Scholar]
Baxter 2011
- Baxter JK, Berghella V, Mackeen AD, Ohly NT, Weed S. Timing of prophylactic antibiotics for preventing postpartum infectious morbidity in women undergoing cesarean delivery. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD009516] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bloom 1996
- Bloom SL, Cox SM, Bawdon RE, Gilstrap LC. Ampicillin for neonatal group B streptococcal prophylaxis: how rapidly can bactericidal concentrations be achieved?. American Journal of Obstetrics and Gynecology 1996;175(4 Pt 1):974‐6. [DOI] [PubMed] [Google Scholar]
Bratzler 2005
- Bratzler DW, Houck PM. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. American Journal of Surgery 2005;189(4):395‐404. [DOI] [PubMed] [Google Scholar]
Burke 1961
- Burke JF. The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery 1961;50:161‐8. [PubMed] [Google Scholar]
Classen 1992
- Classen DC, Evans RS, Pestotnik SL, Horn SD, Menlove RL, Burke JP. The timing of prophylactic administration of antibiotics and the risk of surgical‐wound infection. New England Journal of Medicine 1992;326(5):281‐6. [DOI] [PubMed] [Google Scholar]
Costantine 2008
- Costantine MM, Rahman M, Ghulmiyah L, Byers BD, Longo M, Wen T, et al. Timing of perioperative antibiotics for cesarean delivery: a metaanalysis. American Journal of Obstetrics and Gynecology 2008;199(3):301.e1‐301.e6. [DOI] [PubMed] [Google Scholar]
Dahlke 2013
- Dahlke J, Mendez‐Figueroa H, Rouse D, Berghella V, Baxter J, Chauhan, S. Evidence‐based surgery for cesarean delivery: an updated systematic review. American Journal of Obstetrics and Gynecology 2013;209(4):294‐306. [DOI] [PubMed] [Google Scholar]
Edi‐Osagie 1998
- Edi‐Osagie EC, Hopkins RE, Ogbo V, Lockhat‐Cleff F, Ayeko M, Akpala WO, et al. Uterine exteriorization at cesarean section: influence on maternal morbidity. British Journal of Obstetrics and Gynaecology 1998;105(10):1070‐8. [DOI] [PubMed] [Google Scholar]
Fiore 2001
- Fiore Mitchell T, Pearlman MD, Chapman RL, Bhatt‐Mehta V, Faiz RG. Maternal and transplacental pharmacokinetics of cefazolin. Obstetrics & Gynecology 2001;98(6):1075‐9. [DOI] [PubMed] [Google Scholar]
Gibbons 2012
- Gibbons L, Belizan JM, Lauer JA, Betran AP, Merialdi M, Althabe F. Inequities in the use of cesarean section deliveries in the world. American Journal of Obstetrics and Gynecology 2012;206(4):311‐e1. [DOI] [PubMed] [Google Scholar]
GRADE 2008 [Computer program]
- Jan Brozek, Andrew Oxman, Holger Schünemann. GRADEpro. Version 3.6 for Windows., 2008.
Hellums 2007
- Hellums EK, Lin MG, Ramsey PS. Prophylactic subcutaneous drainage for prevention of wound complications after cesarean delivery‐‐a metaanalysis. American Journal of Obstetrics and Gynecology 2007;197(3):229‐35. [DOI] [PubMed] [Google Scholar]
Henderson 1995
- Henderson E, Love EJ. Incidence of hospital‐acquired infections associated with cesarean section. Journal of Hospital Infection 1995;29:245‐55. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated September 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hopkins 1999
- Hopkins L, Smaill F. Antibiotic prophylaxis regimens and drugs for cesarean section. Cochrane Database of Systematic Reviews 1999, Issue 2. [DOI: 10.1002/14651858.CD001136] [DOI] [PubMed] [Google Scholar]
Kaimal 2008
- Kaimal AJ, Zlatnik MG, Cheng YW, Thiet MP, Connatty E, Creedy P, et al. Effect of a change in policy regarding the timing of prophylactic antibiotics on the rate of postcesarean delivery surgical‐site infections. American Journal of Obstetrics and Gynecology 2008;199(3):310.e1‐310.e5. [DOI] [PubMed] [Google Scholar]
Leth 2009
Lorenz 1988
- Lorenz RP, Botti JJ, Appelbaum PC, Bennett N. Skin preparation methods before cesarean section. A comparative study. Journal of Reproductive Medicine 1988;33(2):202‐4. [PubMed] [Google Scholar]
Magann 1993
- Magann EF, Dodson MK, Ray MA, Harris RL, Martin JN Jr, Morrison JC. Preoperative skin preparation and intraoperative pelvic irrigation: impact on post‐cesarean endometritis and wound infection. Obstetrics & Gynecology 1993;81(6):922‐5. [PubMed] [Google Scholar]
Mangram 1999
- Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999: Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. American Journal of Infection Control 1999;27:97‐132. [PubMed] [Google Scholar]
NNIS System 2004
- National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. American Journal of Infection Control 2004;32(8):470‐85. [DOI] [PubMed] [Google Scholar]
Olsen 2008
- Olsen MA, Butler AM, Willers DM, Devkota P, Gross GA, Fraser VJ. Risk factors for surgical site infection after low transverse cesarean section. Infection Control and Hospital Epidemiology 2008;29(6):477‐84. [DOI] [PubMed] [Google Scholar]
Opoien 2007
- Opoien HK, Valbo A, Grinde‐Andersen A, Walberg M. Post‐cesarean surgical site infections according to CDC standards: rates and risk factors. A prospective cohort study. Acta Obstetricia et Gynecologica Scandinavica 2007;86(9):1097‐102. [DOI] [PubMed] [Google Scholar]
Owens 2009
- Owens SM, Brozanski BS, Mevn LA, Wiesenfeld HC. Antimicrobial prophylaxis for cesarean delivery before skin incision. Obstetrics & Gynecology 2009;114(3):573‐9. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schunemann 2009
- Schunemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391‐400. [PUBMED: 19839216] [DOI] [PubMed] [Google Scholar]
Sievert 2013
- Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, et al. Antimicrobial‐resistant pathogens associated with healthcare‐associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009‐2010. Infection Control and Hospital Epidemiology : the official journal of the Society of Hospital Epidemiologists of America 2013;34(1):1‐14. [PUBMED: 23221186] [DOI] [PubMed] [Google Scholar]
Smaill 2010
- Smaill FM, Gyte GM. Antibiotic prophylaxis versus no prophylaxis for preventing infection after cesarean section. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD007482.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soar 2009
- Soar J, Peyton J, Leonard M, Pullyblank AM. Surgical safety checklists. BMJ 2009;338:b220. [DOI] [PubMed] [Google Scholar]
Souza 2013
- Souza JP, Gülmezoglu AM, Vogel J, Carroli G, Lumbiganon P, Qureshi Z, et al. Moving beyond essential interventions for reduction of maternal mortality (the WHO Multicountry Survey on Maternal and Newborn Health): a cross‐sectional study. Lancet 2013;381(9879):1747‐55. [DOI] [PubMed] [Google Scholar]
Starr 2005
- Starr RB, Zurawski J, Ismail M. Preoperative vaginal preparation with povidone‐iodine and the risk of postcesarean endometritis. Obstetrics & Gynecology 2005;105(5 Pt 1):1024‐6. [DOI] [PubMed] [Google Scholar]
Ventolini 2004
- Ventolini G, Neiger R, McKenna D. Decreasing infectious morbidity in cesarean delivery by changing gloves. Journal of Reproductive Medicine 2004;49(1):13‐6. [PubMed] [Google Scholar]
Wloch 2012
- Wloch C, Wilson J, Lamagni T, Harrington P, Charlett A, Sheridan E. Risk factors for surgical site infection following caesarean section in England: results from a multicentre cohort study. BJOG: An International Journal of Obstetrics & Gynaecology 2012;119(11):1324‐33. [DOI: 10.1111/j.1471-0528.2012.03452.x] [DOI] [PubMed] [Google Scholar]
Yokoe 2001
- Yokoe DS, Christiansen CL, Johnson R, Sands KE, Livingston J, Shtatland ES, et al. Epidemiology of and surveillance for postpartum infections. Emerging Infectious Diseases 2001;7:837‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]


