Abstract
Background
In patients with esophageal squamous cell carcinoma (ESCC), accurately predicting a pathologic complete response (pCR) to preoperative chemoradiotherapy (PCRT) has the potential to enable an active surveillance strategy without esophagectomy. We aimed to establish a reliable multiparameter nomogram model that combines tumor characteristics, imaging modalities, and hematologic markers to predict pCR in patients with ESCC who underwent PCRT and esophagectomy.
Methods
We retrospectively reviewed the medical records of 457 patients with ESCC who received PCRT followed by esophagectomy between January 2005 and October 2020. The nomogram model was developed using logistic regression analysis with a training cohort and externally validated with a validation cohort.
Results
In the training and validation cohorts, 44.2% (126/285) and 48.3% (83/172) of patients, respectively, achieved pCR after PCRT. The 5-year rates of overall survival, progression-free survival, and freedom from local progression in the training cohort were 51.6%, 48.5%, and 77.6%, respectively. The parameters included in the nomogram were histologic grade, clinical N stage, maximum standardized uptake value on positron emission tomography, and post-PCRT biopsy. Hematologic markers were significantly associated with survival outcomes but not with pCR. The area under the receiver operating characteristic curve of the nomogram was 0.717, 0.704, and 0.707 for the training cohort, internal validation cohort, and external validation cohort, respectively.
Conclusion
Our nomogram model based on four parameters obtained from standard clinical practice demonstrated good performance in both the training and validation cohorts and could be useful to aid clinical decision-making to determine whether surgery or active surveillance strategy should be pursued.
Keywords: esophageal squamous cell carcinoma, nomograms, treatment outcome, chemoradiotherapy, esophagectomy
Introduction
Esophageal malignancy ranks sixth in terms of cancer-related deaths worldwide, and treatment outcomes remain poor due to frequent early-stage metastasis and significant treatment-related morbidities. Despite recent advances in treatment modalities, the 5-year overall survival (OS) rate for patients with esophageal cancer is approximately 20% [1]. While esophagectomy is the primary curative treatment for esophageal cancer, preoperative chemoradiotherapy (PCRT) followed by surgery is currently the most preferred strategy for patients with locally advanced stages disease. This approach has demonstrated tumor downstaging, increased rates of complete resection, and improved oncologic outcomes compared with esophagectomy alone [2, 3]. In particular, a substantial number of patients can achieve a pathologic complete response (pCR) after PCRT, suggesting that an active surveillance strategy can be considered as an alternative to avoid complications associated with esophagectomy. In these cases, patients can experience both adequate disease control and improved quality of life, and surgical resection can be postponed until salvage treatment is necessary.
There are two distinct histologic subtypes of esophageal cancer: esophageal squamous cell carcinoma (ESCC), which is predominant in Asia, and esophageal adenocarcinoma (EAC), which is more prevalent in Western countries [4]. These subtypes have different susceptibilities to PCRT; ESCC shows a higher response rate than EAC. In many previous studies, patients with ESCC achieved pCR rates of 40% to 50%, while those with EAC achieved pCR rate of only about 20% [2, 5]. Since patients with ESCC show higher rates of pCR, they are more likely to benefit from an active surveillance strategy than those with EAC. Therefore, although current treatment strategies are similar for both histologic subtypes, the choice between surgery and active surveillance after PCRT should be made depending on the histologic subtype.
Conversely, an active surveillance strategy is significantly limited in that it is difficult to preoperatively distinguish patients with pCR from others. In previous research, diagnostic modalities such as computed tomography (CT), positron emission tomography-CT (PET-CT), endoscopic ultrasonography (EUS), esophagogastroduodenoscopy (EGD), and biopsy after PCRT have shown unsatisfactory accuracy in predicting pCR. However, a multiparameter predictive model that combines clinicopathologic factors and findings from various diagnostic modalities could potentially increase the accuracy of response prediction. Although several previous studies have developed models to predict pCR in patients with esophageal cancer, these studies have primarily focused on patients with EAC or have used non-standard diagnostic and treatment protocols that may not be applicable to other institutions [6–8]. Therefore, we conducted this study to establish a reliable predictive model that combines tumor characteristics, imaging modalities, and hematologic markers to predict pCR in patients with ESCC who have undergone PCRT and esophagectomy.
Methods
Study population
We conducted a retrospective review of the medical records of patients who underwent PCRT and curative esophagectomy for locally advanced ESCC between January 2005 and October 2020. The eligibility criteria were as follows: (a) clinical stage T3/4 or N+, (b) Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2, (c) no distant metastasis except the supraclavicular lymph node at initial evaluation, and (d) evaluation of a PET-CT scan with maximum standardized uptake values (mSUVs) at diagnosis and after PCRT. Patients who received radiotherapy doses of less than 38 Gy or did not undergo esophagectomy immediately after PCRT and later underwent salvage esophagectomy were excluded. This study was approved by the Institutional Review Board of Asan Medical Center, and informed consent was waived due to the retrospective nature of the study (IRB no. 2020–1781).
Treatments and follow-up
The initial patient evaluation included a medical history, physical examination, complete blood count, standard blood chemistry panel, EGD, EUS, chest CT, and PET-CT. Hematologic markers derived from laboratory tests were measured at three timepoints: before treatment, at the end of PCRT, and one month after PCRT. The neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) were calculated by dividing the neutrophil or platelet count by the lymphocyte count. The prognostic nutrition index (PNI) was calculated based on the albumin concentration and lymphocyte count [9].
All patients received PCRT, and the decision to administer induction chemotherapy before PCRT was made by the treating physicians. The most common chemotherapy regimens were capecitabine plus cisplatin (XP) and oxaliplatin plus TS-1 (SOX). For radiotherapy, the most common schemes included three-dimensional conformal radiotherapy or intensity-modulated radiotherapy techniques of 50 Gy in 25 fractions or 50.4 Gy in 28 fractions. The primary tumor and metastatic lymph nodes were identified as the gross tumor volume (GTV) in simulation CT images. Diagnostic CT, PET-CT, and EGD findings were utilized to aid in the delineation of the GTV. The clinical target volume (CTV) included a longitudinal expansion of at least 3 cm, a radial expansion of 1 cm around the primary tumor, and a 1-cm expansion in all directions for regional lymph nodes unless it would compromise adjacent critical normal organs, such as the lungs and heart. Further elective nodal areas were included in the CTV based on the extent and location of the disease as well as the patient’s medical condition. The planning target volume was determined by expanding the CTV by 7 mm radially and 10 mm longitudinally.
One month after the completion of PCRT, chest CT, PET-CT, EGD, and biopsy were performed to assess the response to treatment. Either Ivor–Lewis or McKeown esophagectomy was performed six to eight weeks after the completion of PCRT, and pCR was defined as the absence of tumor cells in the surgical specimens of the esophagus and lymph nodes.
After treatment, regular follow-up examinations were performed every three months for the first two years and every six months thereafter. Chest CT and EGD were performed every six months for five years and annually thereafter. Additional imaging studies were conducted whenever clinically indicated.
Statistical analysis
We classified the patients into a training cohort (before December 2015) and a validation cohort (from January 2016) based on the year of diagnosis. The patient characteristics between the two cohorts were compared using Student's t-test for continuous variables and the χ2 test or Fisher's exact test for categorical variables. The Kaplan–Meier method was used to estimate the rates of freedom from local progression (FFLP), progression-free survival (PFS), and OS. FFLP was calculated from the date of diagnosis to the date of local progression as determined through radiologic or pathologic examination. OS and PFS were calculated from the date of diagnosis to the date of death from any cause and to the date of death from any cause or disease progression, respectively. To develop the prediction model, univariate and multivariable analyses were performed using logistic regression models. Variables that showed statistical significance in univariate analyses were included in the multivariate model. Missing data were considered at random and handled using imputation with the iterative Markov chain Monte Carlo method. A backward elimination procedure was repeated for each of the 1,000 bootstrap resamplings for the model. A 40% relative frequency of selection of bootstrap resampling was the criterion for the inclusion of predictors in the final model, and the points associated with each category of each risk factor were computed using regression coefficients. Finally, we estimated the pCR rate for each point total and formulated the logistic regression model. The established model was internally validated using the bootstrap self-sampling method and externally validated using the validation cohort. The performance of the point system was assessed using the receiver operating characteristic (ROC) curve with the area under the curve (AUC) and calibration curve, which reflect the agreement between predicted and actual outcomes in both the training and validation cohorts. A P value of <0.05 was considered statistically significant. All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA) and R version 4.1.2 program (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics and treatment
Patient characteristics are summarized in Table 1. Out of the 457 patients, 285 were included in the training cohort, and 172 were included in the validation cohort. The median age was 63 years (range, 39–80 years), and 425 (93.0%) patients were male. Clinically positive lymph nodes were suspected in 356 (77.9%) patients at the initial evaluation, and 375 (82.1%) patients received at least one cycle of induction chemotherapy. The median radiotherapy dose was 46.0 Gy (range, 38.0–50.4 Gy); except for five patients who received 38 Gy or 40 Gy, all other patients received 42 Gy or higher. The concurrent chemotherapy regimens were XP in 372 (81.4%) patients, SOX in 59 (12.9%), paclitaxel plus carboplatin in 22 (4.8%), and 5-fluorouracil plus cisplatin in 4 (0.9%). Post-PCRT EGD and biopsy were performed in 455 (99.6%) and 394 (86.2%) patients, respectively. The endoscopic findings were used instead of the biopsy results for 61 patients. Ivor–Lewis and McKeown esophagectomy were performed in 299 and 158 patients, respectively, at a median of 8 weeks (range, 4–16 weeks) after PCRT. Patients in the validation cohort had more advanced T- and N-stage tumors, higher initial mSUVs, and received higher radiation doses than those in the training cohort. In the training cohort, 126 patients (44.2%) achieved pCR following surgery, while in the validation cohort, 83 patients (48.3%) achieved pCR.
Table 1.
Patient characteristics of the training and validation cohorts
| Variable | Total (n = 457) | Training (n = 285) | Validation (n = 172) | P Value |
|---|---|---|---|---|
| Age (years) | 63 (39–80) | 61 (391–76) | 64 (44–80) | <0.001 |
| Sex | 0.582 | |||
| Male | 425 (93.0%) | 267 (93.7%) | 158 (91.9%) | – |
| Female | 32 (7.0%) | 18 (6.3%) | 14 (8.1%) | – |
| ECOG PS | 0.059 | |||
| 0 | 97 (21.2%) | 69 (24.2%) | 28 (16.3%) | – |
| 1 | 360 (78.8%) | 216 (75.8%) | 144 (83.7%) | – |
| Charlson–Deyo score | 0.277 | |||
| 0 | 352 (77.0%) | 211 (74.0%) | 141 (82.0%) | – |
| 1 | 84 (18.4%) | 59 (20.7%) | 25 (14.5%) | – |
| 2 | 17 (3.7%) | 12 (4.2%) | 5 (2.9%) | – |
| 3 | 4 (0.9%) | 3 (1.1%) | 1 (0.6%) | – |
| Alcohol consumption | 0.953 | |||
| No | 55 (12.0%) | 35 (12.3%) | 20 (11.6%) | – |
| Yes | 402 (88.0%) | 250 (87.7%) | 152 (88.4%) | – |
| Smoking history | 0.879 | |||
| No | 88 (19.3%) | 56 (19.6%) | 32 (18.6%) | – |
| Yes | 369 (80.7%) | 229 (80.4%) | 140 (81.4%) | – |
| Histologic grade | 0.576 | |||
| Well-differentiated | 57 (12.9%) | 35 (12.8%) | 22 (13.2%) | |
| Moderately differentiated | 337 (76.4%) | 213 (77.7%) | 124 (74.3%) | |
| Poorly differentiated | 47 (10.7%) | 26 (9.5%) | 21 (12.6%) | |
| Clinical T stage | 0.103 | |||
| 1–2 | 180 (39.4%) | 77 (27.0%) | 24 (14.0%) | – |
| 3–4 | 277 (60.6%) | 208 (73.0%) | 148 (86.0%) | – |
| Clinical N stage | <0.001 | |||
| N0 | 176 (38.5%) | 137 (48.1%) | 39 (22.7%) | – |
| N+ | 281 (61.5%) | 148 (51.9%) | 133 (77.3%) | – |
| Initial mSUV | 12.5 (1.3–38.0) | 11.0 (1.3–34.9) | 14.3 (1.3–38.0) | <0.001 |
| Induction chemotherapy | <0.001 | |||
| None | 82 (17.9%) | 34 (11.9%) | 48 (27.9%) | – |
| Yes | 375 (82.1%) | 251 (88.1%) | 124 (72.1%) | – |
| Chemotherapy regimen | 0.044 | |||
| XP/FP | 376 (82.3%) | 226 (79.3%) | 150 (87.2%) | |
| Others | 81 (17.7%) | 59 (20.7%) | 22 (12.8%) | |
| RT dose (Gy) | 46.0 (38.0–50.4) | 46.0 (38.0–50.4) | 50.0 (40.0–50.4) | <0.001 |
| Post-PCRT endoscopy | 0.262 | |||
| CR | 138 (30.3%) | 80 (28.3%) | 58 (33.7%) | – |
| Non-CR | 317 (69.7%) | 203 (71.7%) | 114 (66.3%) | – |
| Post-PCRT biopsy | 0.969 | |||
| CR | 380 (83.5%) | 237 (83.7%) | 143 (83.1%) | – |
| Non-CR | 75 (16.5%) | 46 (16.3%) | 29 (16.9%) | – |
| Post-CCRT mSUV | 3.4 (1.2–22.0) | 3.1 (1.3–22.0) | 4.3 (1.2–18.2) | <0.001 |
| ΔmSUV (%) | 68.2 (−607–94.4) | 68.8 (−607–94.4) | 67.5 (−453–93.8) | 0.719 |
| Pathologic response | 0.457 | |||
| CR | 209 (45.7%) | 126 (44.2%) | 83 (48.3%) | – |
| Non-CR | 248 (54.3%) | 159 (55.8%) | 89 (51.7%) | – |
ECOG PS = Eastern Cooperative Oncology Group performance status, mSUV = maximum standardized uptake value, XP = capecitabine plus cisplatin, FP = 5-fluorouracil plus cisplatin, RT = radiotherapy, PCRT = preoperative chemoradiotherapy, CR = complete response.
Survival and recurrence
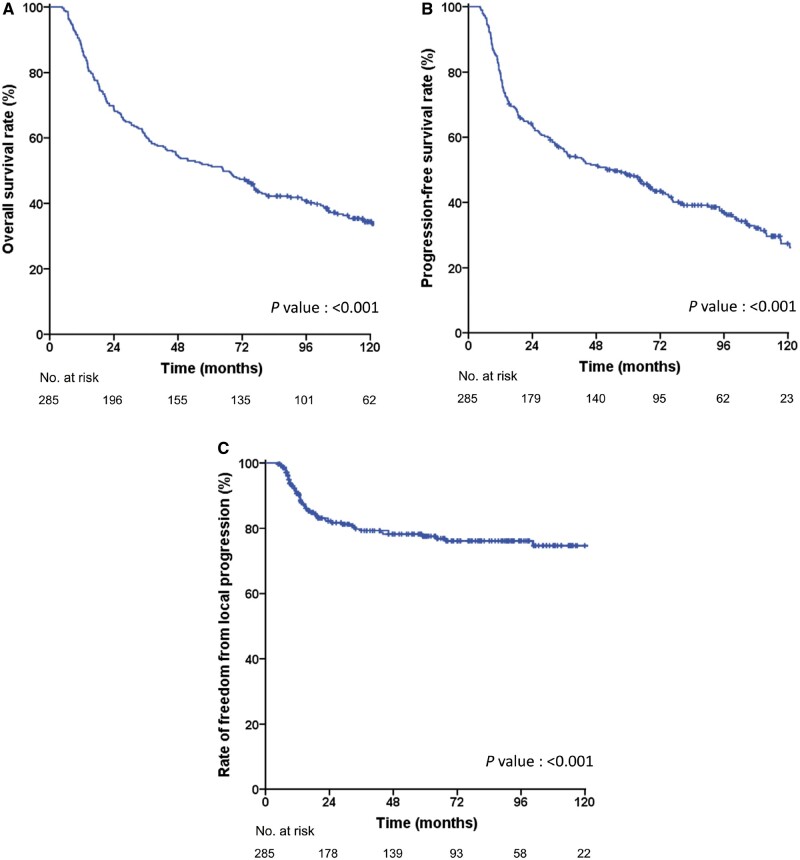
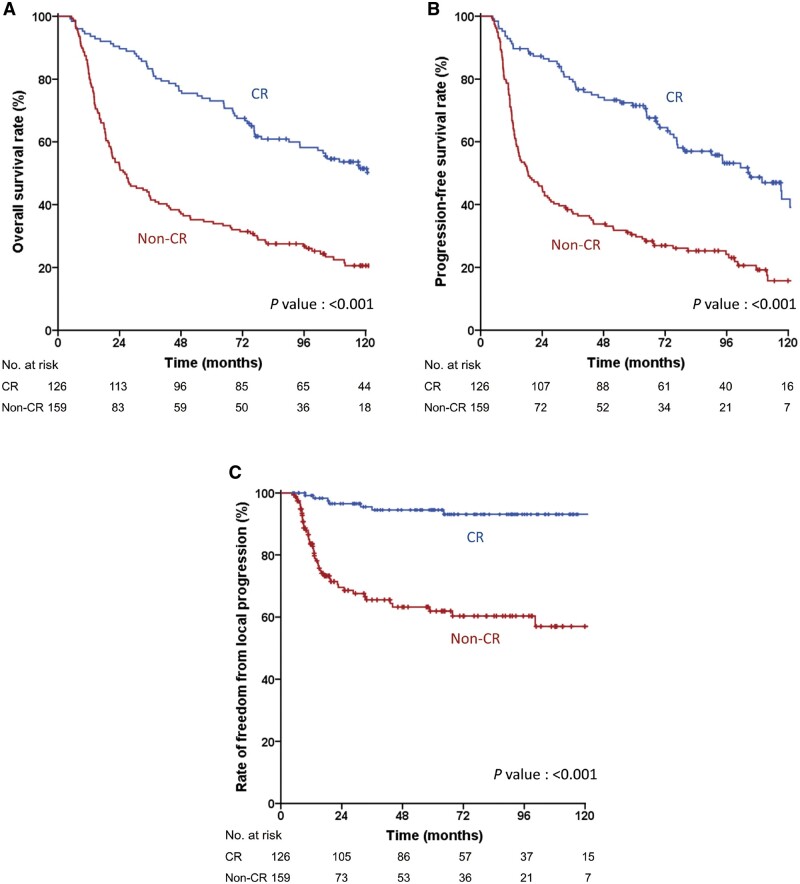
Survival outcomes were only evaluated in the training cohort (n = 285) because the follow-up duration of the validation cohort was insufficient. During a median follow-up duration of 45.6 months (range, 4.7–164.0 months), 84 (29.5%) patients experienced recurrences, including 29 in locoregional sites, 26 in distant sites, and 29 in both locoregional and distant sites simultaneously. The 5-year OS, PFS, and FFLP rates in the training cohort were 51.6%, 48.5%, and 77.6%, respectively (Figure 1). Patients who achieved pCR showed significantly higher 5-year rates of OS (73.0% vs 34.6%, P < 0.001), PFS (71.5% vs 30.5%, P < 0.001), and FFLP (94.5% vs 61.9%, P < 0.001) than those who did not achieve pCR (Figure 2). In the univariate analysis, age, ECOG performance status, clinical T stage, clinical N stage, initial mSUV, post-PCRT biopsy, post-PCRT mSUV, and pCR were found to be significantly associated with both OS and PFS (Tables 2 and 3). In the multivariate analysis, all variables except the clinical T stage and initial mSUV remained in the final model for OS, while all variables except the clinical T stage remained for PFS.
Figure 1.
Recurrence and survival outcomes in the training cohort. (A) Overall survival (OS); (B) Progression-free survival (PFS); (C) Freedom from local progression (FFLP).
Figure 2.
Recurrence and survival outcomes in the training cohort according to pathologic complete response (pCR). (A) Overall survival (OS); (B) Progression-free survival (PFS); (C) Freedom from local progression (FFLP). CR, clinical response.
Table 2.
Univariate and multivariate analysis for overall survival (OS) in the training cohort
| Variable | Univariate |
Multivariate |
||
|---|---|---|---|---|
| P Value | HR (95% CI) | P Value | HR (95% CI) | |
| Age (years) | 0.027 |
|
<0.001 |
|
| Sex (Males vs Females) | 0.618 |
|
– | – |
| ECOG PS (0 vs 1–2) | <0.001 |
|
<0.001 |
|
|
0.334 |
|
– | – |
| Alcohol consumption (No vs Yes) | 0.264 |
|
– | – |
| Smoking history (No vs Yes) | 0.306 |
|
– | – |
|
0.566 |
|
– | – |
|
0.001 |
|
– | – |
|
0.004 |
|
0.030 |
|
| Initial mSUV | <0.001 |
|
– | – |
|
0.726 |
|
– | – |
|
0.964 |
|
– | – |
|
0.153 |
|
– | – |
|
<0.001 |
|
0.005 |
|
| Post-PCRT mSUV | <0.001 |
|
<0.001 |
|
| Pathologic CR | <0.001 |
|
<0.001 |
|
ECOG PS = Eastern Cooperative Oncology Group performance status, mSUV = maximum standardized uptake value, XP = capecitabine plus cisplatin, FP = 5-fluorouracil plus cisplatin, RT = radiotherapy, PCRT = preoperative chemoradiotherapy, CR = complete response, HR = hazard ratio, CI = confidence interval.
Table 3.
Univariate and multivariate analysis for progression-free survival (PFS) in the training cohort
| Variable | Univariate |
Multivariate |
||
|---|---|---|---|---|
| P Value | HR (95% CI) | P Value | HR (95% CI) | |
| Age (years) | 0.011 |
|
<0.001 |
|
| Sex (Males vs Females) | 0.447 |
|
– | – |
| ECOG PS (0 vs 1–2) | 0.001 |
|
0.035 |
|
|
0.258 |
|
– | – |
| Alcohol consumption (No vs Yes) | 0.167 |
|
– | – |
| Smoking history (No vs Yes) | 0.653 |
|
– | – |
|
0.253 |
|
– | – |
|
0.001 |
|
||
|
0.001 |
|
0.015 |
|
| Initial mSUV | <0.001 |
|
0.026 |
|
|
0.424 |
|
– | – |
|
0.964 |
|
– | – |
|
0.078 |
|
– | – |
|
<0.001 |
|
0.015 |
|
| Post-PCRT mSUV | <0.001 |
|
<0.001 |
|
| Pathologic CR | <0.001 |
|
<0.001 |
|
ECOG PS = Eastern Cooperative Oncology Group performance status, mSUV = maximum standardized uptake value, XP = capecitabine plus cisplatin, FP = 5-fluorouracil plus cisplatin, RT = radiotherapy, PCRT = preoperative chemoradiotherapy, CR = complete response, HR = hazard ratio, CI = confidence interval.
Hematologic markers
To evaluate the predictive value of hematologic markers, we analyzed the NLR, PLR, PNI, and serum albumin levels at three timepoints as well as the changes in these values between timepoints. In the univariate analysis for pCR, none of these variables showed statistical significance (Supplementary Table S1). In the univariate analysis for OS and PFS, NLR (pretreatment), PNI (pretreatment), PNI (end of PCRT), serum albumin level (end of PCRT), PNI (one month post-PCRT), and serum albumin level (one month post-PCRT) were significant predictors (Supplementary Table S1). The serum albumin level (end of PCRT) and PNI (one month post-PCRT) remained statistically significant in the multivariate analysis, which included both clinical and hematologic variables for OS and PFS (Supplementary Table S2).
Development of a prediction model for pCR
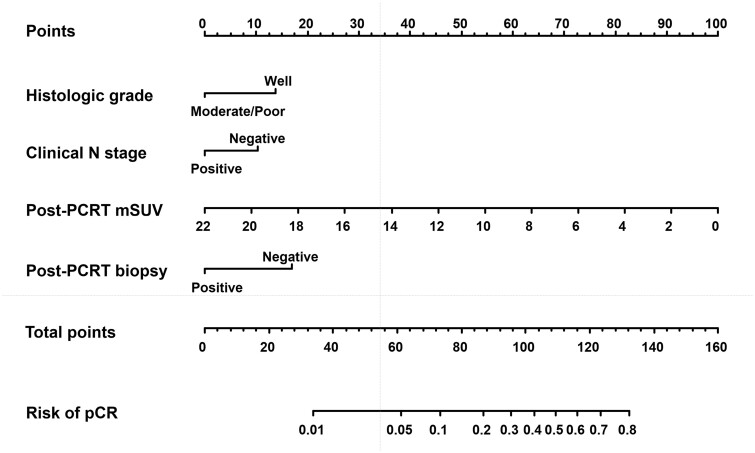
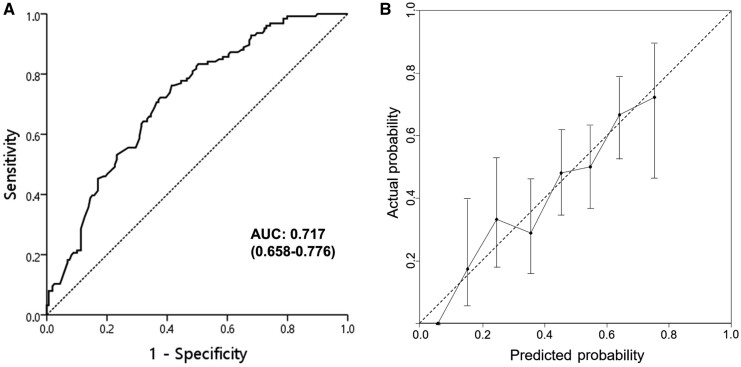
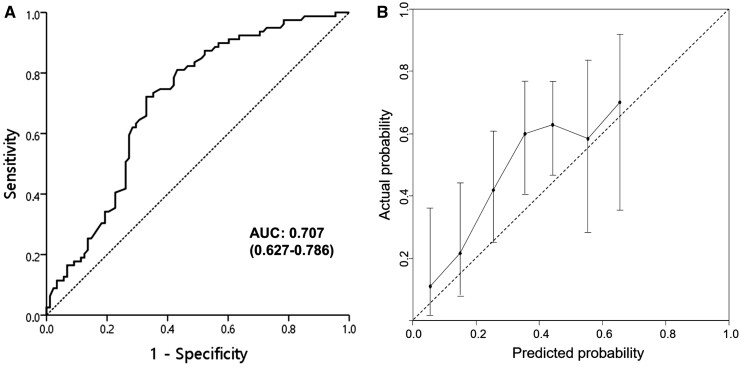
Univariate analysis showed that pCR was significantly associated with ECOG performance status, alcohol consumption, smoking status, clinical N stage, histologic grade, initial and post-PCRT mSUVs, post-PCRT EGD, and biopsy (Table 4). In the multivariate analysis, histologic grade, clinical N stage, post-PCRT mSUV, and biopsy were significantly associated with pCR. These four variables remained in the repeated bootstrap sampling and were included in the final model (Figure 3). In the final model, the effects of each factor were converted into points to calculate the total points that represent the probability of achieving pCR. For example, a patient with a node-negative or well-differentiated tumor showed an approximately 80% probability of pCR when a negative biopsy and no residual hypermetabolic lesion were observed after PCRT. The calibration curve for the probability of pCR showed good agreement between the nomogram-based prediction and the observed outcomes, and the AUC was 0.717 (95% confidence interval [CI], 0.658–0.776) (Figure 4). The bias-corrected C-statistic was 0.704 in the internal validation using the bootstrap sampling method. In the validation cohort, where the pCR rate was higher than that in the training cohort, the model tended to underestimate the actual probability. However, the calibration curve still demonstrated good agreement with a slope close to 1, and the AUC was 0.707 (95% CI, 0.627–0.786) (Figure 5).
Table 4.
Univariate and multivariate analysis for pathologic complete response (pCR) in the training cohort
| Variable | Univariate |
Multivariate |
||
|---|---|---|---|---|
| P Value | OR (95% CI) | P Value | OR (95% CI) | |
| Age (years) | 0.065 |
|
– | – |
| Sex (Males vs Females) | 0.321 |
|
– | – |
| ECOG PS (0 vs 1–2) | 0.038 |
|
– | – |
|
0.641 |
|
– | – |
| Alcohol consumption (No vs Yes) | 0.048 |
|
– | – |
| Smoking history (No vs Yes) | 0.031 |
|
– | – |
|
0.020 |
|
0.034 |
|
|
0.071 |
|
||
|
0.007 |
|
0.016 |
|
| Initial mSUV | <0.001 |
|
||
|
0.470 |
|
– | – |
|
0.575 |
|
– | – |
|
<0.001 |
|
– | – |
|
<0.001 |
|
0.019 |
|
| Post-CCRT mSUV | <0.001 |
|
<0.001 |
|
| ΔmSUV (%) | 0.842 |
|
||
ECOG PS = Eastern Cooperative Oncology Group performance status, mSUV = maximum standardized uptake value, XP = capecitabine plus cisplatin, FP = 5-fluorouracil plus cisplatin, RT = radiotherapy, PCRT = preoperative chemoradiotherapy, OR = odds ratio, CI = confidence interval.
Figure 3.
Nomogram for predicting pathologic complete response (pCR). Points for each factor can be read using the top point scale bar. The total points from four factors were used to estimate the probability of pCR on the bottom scale bar. PCRT, preoperative chemoradiotherapy; mSUV, maximum standardized uptake value.
Figure 4.
Receiver operating characteristic (ROC) curve (A) and calibration plot (B) of the nomogram in the training cohort. AUC, area under the curve.
Figure 5.
Receiver operating characteristic (ROC) curve (A) and calibration plot (B) of the nomogram in the validation cohort. AUC, area under the curve.
Discussion
Preserving patient quality of life is as important as controlling tumors in cancer treatment. Although esophagectomy is the standard treatment for patients with ESCC, it is associated with significant morbidity and decreased quality of life. As an alternative, if applied to appropriate candidates who have achieved a complete response after PCRT, an active surveillance strategy can help patients avoid the deterioration in quality of life that may occur. To enhance the accuracy of pCR prediction, we analyzed a large cohort of patients with ESCC and developed a multiparameter nomogram model. Our patients were treated with a current standard PCRT scheme similar to that used in two previous randomized trials [2, 3] and showed comparable pCR rates (45.7%) and 5-year OS rates (51.6%), which supports the reliability of our model. Our model utilized four parameters (histologic grade, clinical N stage, post-PCRT mSUV, and post-PCRT biopsy findings) and demonstrated good performance in both the training and validation cohorts. These four parameters are typically obtained through standard workups for locally advanced ESCC; therefore, our nomogram appears to be easily applicable to other institutions and may serve as a useful tool to aid treatment-related decision-making.
Several studies have previously reported the development of nomogram models for predicting pCR in esophageal cancer, and two of them specifically evaluated patients with predominantly EAC. A study from MD Anderson Cancer Center developed a nomogram model in 302 patients with EAC and 20 patients with ESCC [6]. The parameters included in their nomogram were similar to ours: sex, T stage, post-PCRT mSUV, post-PCRT biopsy results, and histologic grade. The post-PCRT mSUV had the greatest influence on the probability of achieving pCR in the study. Toxopeus et al. [7] established a model involving 292 patients with EAC and 89 patients with ESCC. Histologic subtype and T stage had the greatest impact on the model. Gender and histologic grade were also included; however, PET-CT parameters were not included due to their limited usage.
In the two studies that exclusively included patients with ESCC [8, 10], Chao et al. [8] constructed a nomogram using a total of 392 patients (274 in the training cohort and 118 in the validation cohort). Likely due to the lower-than-standard radiation dose of 30 Gy, their pCR rate was 25.8%. However, the institution’s unique classification of post-PCRT endoscopic findings was the strongest factor in the model, and other factors included age, tumor length, post-PCRT albumin level, and a prior history of metachronous head and neck cancer [8]. Wu et al. [10] developed a nomogram involving 306 patients with ESCC, and the pCR rate was 40.5%. The model consisted of six variables: sex, chemotherapy regimen, EGD findings, pre-PCRT NLR, post-PCRT PLR, and absolute leukocyte nadir during PCRT. In both ESCC model studies, PET parameters were not analyzed due to limited utilization of this modality. Except for the EGD results, the factors in our model do not overlap with those in the previous two ESCC studies likely because we exclusively used PET parameters and found no significant association between pCR and hematologic markers in our study.
However, the parameters in our model are consistent with other research studies on predictors of pCR. The post-PCRT mSUV had the greatest impact on the probability of achieving pCR in our model. Although the prediction accuracy of PET-CT is limited in tumors with low metabolism or in patients with extensive treatment-related esophagitis, PET-CT is currently considered one of the most accurate imaging modalities for predicting pCR. Parameters such as the post-PCRT mSUV, change in mSUV, total lesion glycolysis, and metabolic complete response have been reported to be closely associated with pCR [11–15], and a recent meta-analysis found that the sensitivities and specificities of PET parameters were as high as 65% to 75% [12].
The inclusion of the clinical N stage and histologic grade in our model indicates that advanced or aggressive tumors are less sensitive to PCRT and are less likely to achieve pCR. These tumors more frequently evade or resist the treatment mechanism, which is consistent with previous research indicating that they are less responsive to chemoradiation [6, 16, 17].
Hematologic indicators obtained from routinely performed laboratory tests in clinical practice have been identified as convenient and useful predictors of clinical outcomes in previous studies on esophageal cancer [18]. These variables are believed to reflect the nutritional status, immune function, and tumor microenvironment of the patient. As two previous studies that developed ESCC pCR prediction models reported hematologic indicators as significant predictors of pCR [8, 10], we evaluated them at three different timepoints. However, these markers were significant predictors of survival but not of pCR in our study. Only the serum albumin level and PNI after treatment were found to be significantly associated with OS and PFS in the multivariate model, suggesting that patients who recovered well after PCRT have a more favorable prognosis. Due to the contradictory results of previous studies, further research is necessary to determine the relationship between hematologic markers and pCR as well as the most appropriate timing for evaluating these markers.
Several emerging modalities for response prediction were not considered in our study, and their inclusion may help to improve the accuracy of the model in the future. Although not widely used as a standard evaluation modality, the role of functional magnetic resonance imaging (MRI) in response evaluation is gaining attention. Two recent studies reported that PET-CT and diffusion-weighted MRI parameters were equally effective in response prediction and that combining the two modalities could improve prediction accuracy [11, 19]. According to these studies, MRI appears to be capable of predicting the PCRT response, even when performed in the middle of the treatment, allowing for quicker decision-making regarding the necessity of surgical resection.
A new biopsy method has been suggested to improve prediction accuracy. In our study, the post-PCRT biopsy result was found to be a significant predictor of pCR, and it demonstrated a high positive predictive value in confirming the presence of residual tumor. However, this test has a limited sensitivity of approximately 25% [20]. A Dutch study group suggested the “bite on bite” biopsy procedure, in which a second deep biopsy is performed in the same location, and showed an increased sensitivity and specificity of 70% to 80% [21]. Although their ongoing “SANO” trial, which investigates an active surveillance strategy, is based on this result [22], this procedure is not commonly performed elsewhere likely due to the complexity of the procedure and concerns about procedure-related complications. Finally, ongoing studies are examining the relationship between the expression of various genes in individual tumors and treatment response. Studies on the p53 gene have shown that the wild-type p53 gene is associated with a higher pCR rate in ESCC, and there is increasing evidence that various molecular markers, combinations of markers, and molecular mutational burden may be useful predictors of treatment response [23].
Investigations into active surveillance strategies have continued despite the ongoing lack of high-level evidence. Although two older randomized trials comparing PCRT followed by surgery with definitive chemoradiation found no significant difference in OS between the two groups among responders to PCRT [24, 25], these studies are outdated when compared with current practices in terms of surgical mortality and response evaluation methods. Two recent systematic reviews and meta-analyses also demonstrated favorable OS with PCRT and active surveillance compared with PCRT followed by surgery in patients who achieved clinical CR [26, 27]. We have also reported prospective and retrospective studies comparing PCRT followed by surgery and active surveillance. In patients who achieved a clinical complete response, the addition of surgery was associated with improved local control but not with OS [28, 29]. Further investigations including the two ongoing randomized trials are still needed [30].
Our study has several limitations. First, due to its retrospective nature, our study could have potential selection biases. As patients who did not undergo surgery after PCRT were excluded, caution should be exercised in interpreting the results; furthermore, the decision to pursue active surveillance may be influenced by factors such as a patient’s medical condition, clinical tumor response, and allocation to active surveillance in a prospective study. Second, because our nomogram was only validated in a single institution, further verification by other institutions is still necessary. Although the parameters of our nomogram are usually obtained through current standard evaluations for patients with ESCC, the values of these parameters could vary based on the institutional equipment, operating physician, and schedule of the procedure. Third, while our study involved four different chemotherapy regimens, which might have influenced pCR rates, comparing the superiority of these regimens was beyond the scope. However, we believe the impact of the chemotherapy regimen is limited in our study as it was not significantly associated with pCR or survival. In addition, as immunotherapy, which was not used in our cohort, is emerging as a potential neoadjuvant treatment option, modifications to our model or inclusion of factors such as PD-L1 expression may be required if immunotherapy becomes a standard treatment [31]. Finally, our model may not provide sufficiently high pCR probabilities for some patients, and high pCR probabilities do not necessarily guarantee favorable outcomes in terms of recurrence or prognosis. Therefore, our model alone may not be sufficient to determine active surveillance strategies. Nonetheless, we believe improving pCR prediction accuracy can aid clinical decision-making and contribute to enhancing the efficacy of such strategies.
In conclusion, our predictive model for pCR, which was developed using a large cohort of patients with ESCC who underwent examination and treatment according to the current standard protocol including PET-CT, exhibited good performance. It can serve as a useful tool to aid clinical decision-making regarding whether to pursue surgery or an active surveillance strategy after PCRT; thus, it has the potential to help maintain patient quality of life without compromising disease control. As our model was built upon routinely available clinical parameters, it can be readily applied in other institutions. However, further validation across multiple institutions and incorporation of additional factors such as MRI findings, second deep biopsy results, and novel biomarkers could enhance the effectiveness and reliability of the model.
Statement of Ethics
This study protocol was approved by the Institutional Review Board of Asan Medical Center, and the need for informed consent was waived due to its retrospective nature.
Supplementary Data
Supplementary data is available at Gastroenterology Report online.
Authors’ Contributions
J.H.K. conceived and designed the analysis. Y.S.S., J.Y.J., Y.J.Y., J.Y., K.H.S., and Y.Y.J. collected the data. S.-B.K., S.R.P., H.J.S., Y.-H.K., H.R.K., and J.H.K. contributed data or analysis tools. Y.S.S. performed the analysis. Y.S.S. and J.H.K. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
We have no conflicts of interest to declare.
Supplementary Material
Contributor Information
Young Seob Shin, Department of Radiation Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Jeong Yun Jang, Department of Radiation Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Ye Jin Yoo, Department of Radiation Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Jesang Yu, Department of Radiation Oncology, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea.
Kye Jin Song, Department of Radiation Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Yoon Young Jo, Department of Radiation Oncology, Yeungnam University Medical Centre, University of Yeungnam College of Medicine, Daegu, Korea.
Sung-Bae Kim, Department of Medical Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Sook Ryun Park, Department of Medical Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Ho June Song, Department of Gastroenterology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Yong-Hee Kim, Department of Thoracic and Cardiovascular Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Hyeong Ryul Kim, Department of Thoracic and Cardiovascular Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Jong Hoon Kim, Department of Radiation Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Data Availability
The datasets used and/or analyzed during this study are available from the corresponding author upon reasonable request.
References
- 1. Then EO, Lopez M, Saleem S. et al. Esophageal cancer: an updated surveillance epidemiology and end results database analysis. World J Oncol 2020;11:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shapiro J, van Lanschot JJB, Hulshof M. et al. ; CROSS Study Group. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090–8. [DOI] [PubMed] [Google Scholar]
- 3. Yang H, Liu H, Chen Y. et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized, open-label clinical trial. J Clin Oncol 2018;36:2796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sung H, Ferlay J, Siegel RL. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- 5. Oh D, Kim JH.. The current evidence on neoadjuvant therapy for locally advanced esophageal squamous cell carcinoma. Korean J Thorac Cardiovasc Surg 2020;53:160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ajani JA, Correa AM, Hofstetter WL. et al. Clinical parameters model for predicting pathologic complete response following preoperative chemoradiation in patients with esophageal cancer. Ann Oncol 2012;23:2638–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Toxopeus EL, Nieboer D, Shapiro J. et al. Nomogram for predicting pathologically complete response after neoadjuvant chemoradiotherapy for oesophageal cancer. Radiother Oncol 2015;115:392–8. [DOI] [PubMed] [Google Scholar]
- 8. Chao Y-K, Ku H-Y, Chen C-Y. et al. Development of a nomogram for the prediction of pathological complete response after neoadjuvant chemoradiotherapy in patients with esophageal squamous cell carcinoma. Dis Esophagus 2017;30:1–7. [DOI] [PubMed] [Google Scholar]
- 9. Onodera T, Goseki N, Kosaki G.. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 1984;85:1001–5. [PubMed] [Google Scholar]
- 10. Wu Y, Chen J, Zhao L. et al. Prediction of pathologic response to neoadjuvant chemoradiotherapy in patients with esophageal squamous cell carcinoma incorporating hematological biomarkers. Cancer Res Treat 2021;53:172–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Borggreve AS, Goense L, van Rossum PSN. et al. Preoperative prediction of pathologic response to neoadjuvant chemoradiotherapy in patients with esophageal cancer using 18F-FDG PET/CT and DW-MRI: a prospective multicenter study. Int J Radiat Oncol Biol Phys 2020;106:998–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eyck BM, Onstenk BD, Noordman BJ. et al. Accuracy of detecting residual disease after neoadjuvant chemoradiotherapy for esophageal cancer: a systematic review and meta-analysis. Ann Surg 2020;271:245–56. [DOI] [PubMed] [Google Scholar]
- 13. Kim MK, Ryu JS, Kim SB. et al. Value of complete metabolic response by 18F-fluorodeoxyglucose-positron emission tomography in oesophageal cancer for prediction of pathologic response and survival after preoperative chemoradiotherapy. Eur J Cancer 2007;43:1385–91. [DOI] [PubMed] [Google Scholar]
- 14. Im HS, Kang J, Kim YH. et al. The role of comprehensive evaluation for clinical complete response in predicting pathologic complete response in patients treated with neoadjuvant chemoradiation for esophageal squamous cell carcinoma (ESCC). Ann Oncol 2018;29:VIII213. [Google Scholar]
- 15. Song SY, Kim JH, Ryu JS. et al. FDG-PET in the prediction of pathologic response after neoadjuvant chemoradiotherapy in locally advanced, resectable esophageal cancer. Int J Radiat Oncol Biol Phys 2005;63:1053–9. [DOI] [PubMed] [Google Scholar]
- 16. Wang C, Zhao K, Hu S. et al. A predictive model for treatment response in patients with locally advanced esophageal squamous cell carcinoma after concurrent chemoradiotherapy: based on SUVmean and NLR. BMC Cancer 2020;20:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nam SY, Jeon SW, Lee SJ. et al. Clinical factors to predict the response to concurrent chemoradiotherapy and survival in esophageal cancer patients. Gut Liver 2020;14:450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qiu J, Yang J, Yu Y. et al. Prognostic value of pre-therapeutic nutritional risk factors in elderly patients with locally advanced esophageal squamous cell carcinoma receiving definitive chemoradiotherapy or radiotherapy. BMC Cancer 2023;23:597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu X, Sun ZY, Wu HW. et al. Diffusion-weighted MRI and 18F-FDG PET/CT in assessing the response to neoadjuvant chemoradiotherapy in locally advanced esophageal squamous cell carcinoma. Radiat Oncol 2021;16:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang Q, Cleary KR, Yao JC. et al. Significance of post-chemoradiation biopsy in predicting residual esophageal carcinoma in the surgical specimen. Dis Esophagus 2004;17:38–43. [DOI] [PubMed] [Google Scholar]
- 21. Noordman BJ, Spaander MCW, Valkema R. et al. Detection of residual disease after neoadjuvant chemoradiotherapy for oesophageal cancer (preSANO): a prospective multicentre, diagnostic cohort study. Lancet Oncol 2018;19:965–74. [DOI] [PubMed] [Google Scholar]
- 22. Noordman BJ, Wijnhoven BPL, Lagarde SM. et al. ; SANO-Study Group. Neoadjuvant chemoradiotherapy plus surgery versus active surveillance for oesophageal cancer: a stepped-wedge cluster randomised trial. BMC Cancer 2018;18:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li Y, Liu J, Cai XW. et al. Biomarkers for the prediction of esophageal cancer neoadjuvant chemoradiotherapy response: a systemic review. Crit Rev Oncol Hematol 2021;167:103466. [DOI] [PubMed] [Google Scholar]
- 24. Stahl M, Stuschke M, Lehmann N. et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol 2005;23:2310–7. [DOI] [PubMed] [Google Scholar]
- 25. Bedenne L, Michel P, Bouché O. et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007;25:1160–8. [DOI] [PubMed] [Google Scholar]
- 26. Park J, Yea JW, Oh SA. et al. Omitting surgery in esophageal cancer patients with complete response after neoadjuvant chemoradiotherapy: a systematic review and meta-analysis. Radiat Oncol 2021;16:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hipp J, Nagavci B, Schmoor C. et al. Post-neoadjuvant surveillance and surgery as needed compared with post-neoadjuvant surgery on principle in multimodal treatment for esophageal cancer: a scoping review. Cancers (Basel) 2021;13:429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Park SR, Yoon DH, Kim JH. et al. A randomized phase III trial on the role of esophagectomy in complete responders to preoperative chemoradiotherapy for esophageal squamous cell carcinoma (ESOPRESSO). Anticancer Res 2019;39:5123–33. [DOI] [PubMed] [Google Scholar]
- 29. Yu J, Kim JH, Kim SB. et al. Role of esophagectomy after chemoradiation therapy in patients with locally advanced squamous cell carcinoma: a comparative analysis stratified by clinical response to chemoradiation therapy. Cancer Res Treat 2022;54:1148–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eyck BM, van der Wilk BJ, Noordman BJ. et al. ; SANO-Study Group. Updated protocol of the SANO trial: a stepped-wedge cluster randomised trial comparing surgery with active surveillance after neoadjuvant chemoradiotherapy for oesophageal cancer. Trials 2021;22:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu Y. Perioperative immunotherapy for esophageal squamous cell carcinoma: now and future. World J Gastroenterol 2023;29:5020–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during this study are available from the corresponding author upon reasonable request.