Abstract
Expression of the measles virus (MV) F/H complex on the surface of viral particles, infected cells, or cells transfected to express these proteins (presenter cells [PC]) is necessary and sufficient to induce proliferative arrest in both human and rodent lymphoid cells (responder cells [RC]). This inhibition was found to occur independent of apoptosis and soluble mediators excluded by a pore size filter of 200 nm released from either PC or RC. We now show that reactive oxygen intermediates which might be released by RC or PC also do not contribute to MV-induced immunosuppression in vitro. Using an inhibitor of Golgi-resident mannosidases (deoxymannojirimycin), we found that complex glycosylation of the F and H proteins is not required for the induction of proliferative arrest of RC. As revealed by our previous studies, proteolytic cleavage of the MV F protein precursor into its F1 and F2 subunits, but not of F/H-mediated cellular fusion, was found to be required, since fusion-inhibitory peptides such as Z-d-Phe-l-Phe-Gly (Z-fFG) did not interfere with the induction of proliferative inhibition. We now show that Z-fFG inhibits cellular fusion at the stage of hemifusion by preventing lipid mixing of the outer membrane layer. These results provide strong evidence for a receptor-mediated signal elicited by the MV F/H complex which can be uncoupled from its fusogenic activity is required for the induction of proliferative arrest of human lymphocytes.
In the course of acute measles, an efficient virus-specific immune response is generated which leads to viral clearance from the peripheral blood and the establishment of lifelong immunity to reinfection. Paradoxically, measles virus (MV) also causes a marked suppression of the host's immune responses that accounts for high susceptibility to opportunistic infections; that is the major reason for the high rates of measles-related morbidity and mortality worldwide (7). It is a key finding in MV-induced immunosuppression that peripheral blood lymphocytes (PBL) isolated during and for weeks after acute measles largely fail to proliferate in response to mitogenic, allogenic, and recall antigen stimulation (5, 33). Although MV infects cells of the lymphoid/monocytic lineage and induces cell cycle arrest in these cells (21–23, 40), the frequency of infected peripheral blood mononuclear cells (PBMC) is usually low at any stage of the disease. This indicates that the general failure of lymphocytes to response to mitogenic stimulation is not likely to result from directly infection-dependent cell loss or cell cycle arrest. Thus, independent mechanisms such as the release of inhibitory soluble mediators from infected PBMC (12, 36) or surface contact-mediated negative signaling between MV glycoproteins and cellular receptor molecules have been suggested. These include MV H-protein-mediated downregulation of CD46 from the surface of uninfected cells or downregulation of interleukin-12 release from uninfected monocytes following CD46 cross-linking by MV or CD46 ligation by specific antibodies (15, 32) and induction of apoptosis in thymocytes as shown in SCID-hu mice (2).
Using an in vitro system to study MV-induced immunosuppression, we found that expression of the MV glycoproteins on the surface of UV-inactivated viral particles, or UV-inactivated cells infected with either MV vaccine or wild-type strains or transfected to express the MV glycoproteins, is necessary and sufficient to induce a state of unresponsiveness to mitogenic stimulation in uninfected human or rodent lymphocytes (24, 34). The very same effector structure, the MV glycoprotein complex, was also able to induce immunosuppression in cotton rats (24). As revealed by transwell assays, soluble inhibitory factors were not released from presenter cells (PC) or PC-cocultivated responder cells (RC), and as with primary lymphocytes, proliferation of cell lines of lymphocytic/monocytic origin was also prevented in the presence of UV-inactivated viral particles or UV-inactivated cells expressing the F/H complex (31). The well-known fusogenic activity of this complex was not required for the induction of immunosuppression in vitro since (i) fusion with PC but not proliferative inhibition was observed when human cells of nonhematopoietic origin were used as RC, (ii) proliferative inhibition but not fusion was seen after cocultivation of rodent RC with human PC (25, 31), and (iii) the presence of fusion-inhibitory peptides did not interfere with the induction of immunosuppression by PC (38). Although F/H-mediated cellular fusion was obviously not involved, proteolytic cleavage of the MV F0 precursor by a cellular subtilisin-like protease, furin, was found to be essential for the immunosuppressive activity of the complex (38).
In this study, we show that the generation of reactive oxygen intermediates and nitric oxide during PC-RC coculture is not likely to be involved in the induction of RC unresponsiveness. We further show that complex glycosylation of the F/H complex is not required for its immunosuppressive activity. Lipid mixing of the outer membrane bilayers of the membranes, also referred to as hemifusion, is essential for cellular fusion. We now show that Z-d-Phe-l-Phe-Gly (Z-fFG) inhibits hemifusion but not proliferative inhibition by MV-infected PC, indicating that the immunosuppressive activity of the F/H complex is a receptor-mediated signaling via a surface receptor and can be uncoupled from even early events in viral fusion.
MATERIALS AND METHODS
Cells, viruses, antibodies, and detection kits.
Lymphoid and monocytic cell lines (BJAB [human lymphoblastoid B cells], BJAB-EDp [BJAB cells persistently infected with MV vaccine strain Edmonston-B {MV-ED}], Jurkat cell clone J16 [34], U937 [human monocytic cells], and B95a [adherent subclone of Epstein-Barr virus-transformed marmoset B cells]) were maintained in RPMI 1640 medium containing 10% fetal calf serum (FCS), Vero (African green monkey kidney) cells were grown in minimal essential medium containing 5% FCS, and LoVo (human colon adenocarcinoma) cells were grown in 50% Ham's F-12 medium–50% Dulbecco modified Eagle medium supplemented with 10% FCS. PBMC were isolated by Ficoll-Paque (Amersham Pharmacia Biotech, Freiburg, Germany) density gradient centrifugation of heparinized blood obtained from healthy adult donors and were depleted of monocytes by plastic adherence. PBL were cultured in RPMI 1640 medium containing 10% FCS. MV-ED was grown and propagated on Vero cells. Cell surface staining was performed with monoclonal antibodies directed against MV F (A5047) or H (K83) protein (generated in our laboratory) or with immunoglobulin G isotype controls (Becton Dickinson); for immunoprecipitation, an MV hyperimmune serum or a monospecific serum against the cytoplasmic domain of the MV-ED H protein was used. The nitrate-nitrite colorimetric assay kit was obtained from Alexis (Grünberg, Germany).
Immunoprecipitation.
Cells treated or untreated with 1-deoxymannojirimycin (DMJ; Calbiochem-Novabiochem, Bad Soden, Germany) at the concentrations indicated were treated with sulfo-NHS-LC-biotin (0.5 mg/ml) (Pierce, Rockford, Ill.) twice for 30 min each time at 4°C, extensively washed with medium containing 10% FCS, and finally washed with phosphate-buffered saline prior to lysis in radioimmunoprecipitation assay detergent (150 mM NaCl, 10 mM Tris-HCl [pH 7.4], 1% sodium deoxycholate, 1% Triton X-100, 0.1% sodium dodecyl sulfate [SDS], 1 mM phenylmethylsulfonyl fluoride). Protein lysates were immunoprecipitated with an H-specific serum or an MV hyperimmune serum; the precipitates were resuspended in 0.02% SDS–100 mM β-mercaptoethanol, boiled for 10 min, and recentrifuged. Supernatants were adjusted to a final pH of 5.5 by addition of sodium acetate and, when indicated, digested with 50 mU of endoglycosidase H (endo H; Boehringer, Mannheim, Germany) for 16 h at 37°C. Products were separated by standard SDS-polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membranes, and detected using peroxidase-conjugated streptavidin (Amersham, Braunschweig, Germany).
In vitro proliferation assay.
PC (uninfected BJAB cells or BJAB cells infected with MV-ED at the multiplicity of infection [MOI] indicated in the presence or absence of DMJ or BJAB cells persistently infected with MV-ED [BJAB-EDp]) were inactivated by UV irradiation in a biolinker (0.25 J/cm2). Alternatively, UV-inactivated (1.5 J/cm2 in a biolinker) MV (UV-MV) was used. Then 105 RC (Jurkat cell clone J16 or PBL in the presence of 2.5 μg/ml of phytohemagglutinin were seeded into a 96-well plate in a volume of 100 μl. The PC were added at the concentrations indicated in a volume of 100 μl per well and were incubated for 48 h. When indicated, l-ascorbic acid, N-acetyl-l-cysteine, or catalase (all from Calbiochem-Novabiochem) was added. Proliferation rates were determined following a 16-h labeling period with [3H]thymidine (0.5 μCi/200 μl). Assays were routinely performed in triplicate, cells were harvested, and the rates of incorporation of [3H]thymidine were determined using a β-plate reader. Proliferative inhibition of the RC was determined as a percentage of the proliferation rate seen in cocultures with control cells.
Lipid mixing and fusion assay.
After washing in RPMI 1640, 106 BJAB or BJAB-EDp cells were labeled in RPMI 1640 containing octadecylrhodamine (R18; final concentration, 4 μM; Molecular Probes, Eugene, Oreg.) for 15 min at 37°C in the dark. Unbound R18 was subsequently removed by three washing steps in RPMI 1640 containing 10% FCS. Labeled cells (104 in a total volume of 100 μl of RPMI 1640 containing 10% FCS) were laid onto a monolayer of B95a cells seeded onto a coverslip. When indicated Z-Gly-l-Phe-l-Ala (Z-GFA) or Z-fFG (both from Bachem, Heidelberg, Germany) was added at a final concentration of 0.2 mM. The coverslips were incubated at 4°C for 30 min and for 60 min at 37°C, washed once with ice-cold phosphate-buffered saline, mounted, and analyzed by fluorescence microscopy using a rhodamine filter set. For the fusion assay, 105 BJAB-EDp cells were laid onto a monolayer of Vero, LoVo, or B95a cells in a six-well plate in the presence or absence of 0.2 mM Z-fFG for 20 h and analyzed for syncytium formation by light microscopy.
RESULTS
Generation of reactive oxygen intermediates is not involved in MV-induced immunosuppression in vitro.
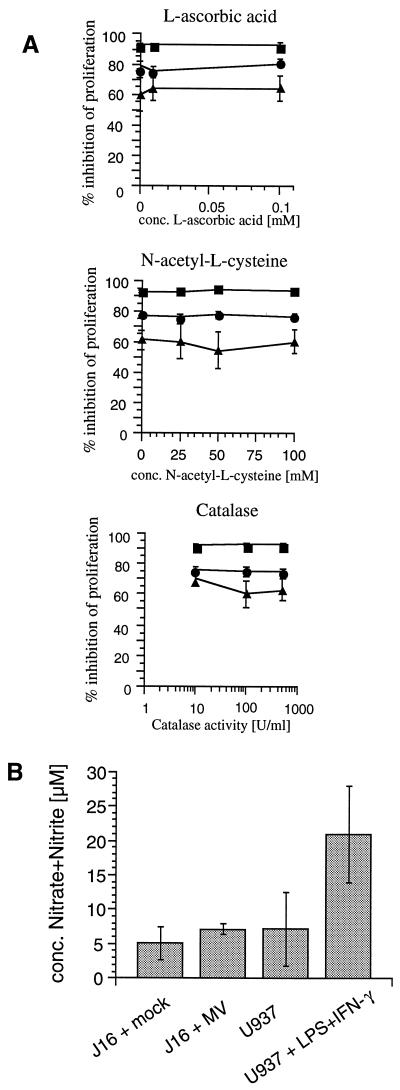
Using a transwell system (exclusion size of 200 nm), we have shown that neither MV-infected, UV-irradiated cells (PC) nor mitogen-stimulated uninfected PBL or lymphocytic cell lines (RC) after cocultivation with infected PC released soluble mediators that block mitogen-dependent proliferation of a second or third population of RC, respectively (31). We could, however, not exclude that factors released into the microenvironment by either PC or RC cocultivated with PC, such as reactive oxygen intermediates, could contribute to proliferative arrest of the RC. Thus, RC (Jurkat clone J16 cells, which are sensitive to immunosuppression in vitro [34]) were cocultivated with PC (UV-irradiated BJAB-EDp cells or, for a control, uninfected BJAB cells) at the PC/RC ratios indicated in the absence or presence of increasing concentrations of l-ascorbic acid, N-acetyl-l-cysteine, or catalase, all of which are known to interfere with the generation or release of reactive oxygen intermediates. The presence of the compounds added at the concentrations tested did not interfere with the viability of proliferative activity of J16 cells in the absence of PC (not shown). Levels of proliferative inhibition of the RC by BJAB-EDp cells were more than 90% (PC/RC ratio of 1/10), 75 to 80% (PC/RC ratio of 1/50), and 60 to 65% (PC/RC ratio of 1/100) both in the absence and in the presence of increasing concentrations of the inhibitors (Fig. 1A). These data indicate that generation of reactive oxygen intermediates either by PC or by PC-contacted RC is not involved in the induction of immunosuppression in vitro. Similarly, generation of nitric oxide apparently does not contribute to MV-induced proliferative inhibition in this system. This is because inducible nitric oxide synthase is barely detectable on the protein level in mock-treated Jurkat cells by Western blotting and fluorescence-activated cell sorting analysis and is not induced in these cells following treatment with UV-MV (not shown). Moreover, only trace amounts of nitrate and nitrite as a marker of NO production could be measured in supernatants of J16 cells treated with UV-MV although the proliferative inhibition was 55% in this experiment (Fig. 1B).
FIG. 1.
MV-induced immunosuppression in vitro is unaffected in the presence of antioxidants and does not involve generation of nitrite or nitrate. (A) Jurkat clone J16 cells (RC) were cocultivated with UV-inactivated BJAB-EDp cells (or, for a control, with UV-inactivated BJAB cells) in the presence of increasing concentrations of l-ascorbic acid, N-acetyl-l-cysteine, or catalase, or left untreated, at a PC/RC ratio of 1/10 (■), 1/50 (●), or 1/100 (▴) for 48 h followed by a 16-h labeling period. (B) Nitrate and nitrite concentrations were determined in supernatants of J16 cells (105) 48 h following treatment with UV-MV (MOI of 1) or mock supernatant or, for a control, in supernatants of U937 cells treated with phorbol myristate acetate (5 ng/ml) for 48 h in the presence or absence of lipopolysaccharide (LPS; 100 ng/ml) and gamma interferon (IFN-γ; 100 U/ml). Proliferative inhibition of J16 cells was determined compared to J16 cells cocultured with uninfected BJAB cells cultured using identical conditions with respect to added compounds (A) or mock supernatant (B).
Complex glycosylation of MV F/H is not required for immunosuppression in vitro.
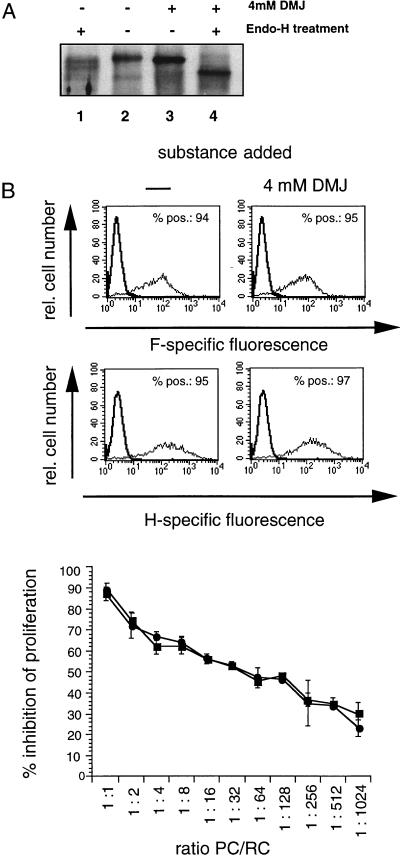
MV F/H complexes expressed on the surface of UV-irradiated MV-infected cells, UV-inactivated viral particles, and cells transfected to express these proteins are necessary and sufficient to induce immunosuppression in vitro and in vivo (25, 33), and proteolytic processing of the MV F protein is a prerequisite for both the fusogenic and immunosuppressive activities of the complex (38). To assess the role of complex glycosylation of the MV F/H complex for both activities, we used an inhibitor of Golgi-resident mannosidases, DMJ, for treatment of BJAB cells immediately after infection with MV-ED (MOI of 0.5). The inhibitor did not affect the viability of mock- or MV-infected BJAB cells at any concentration applied (not shown). As revealed by endo H sensitivity, DMJ efficiently prevented formation of complex carbohydrate chains of the MV F and H surface proteins (Fig. 2A and not shown). The overall levels of F and H proteins detected on the cell surface after DMJ treatment were identical to those seen in the absence of the inhibitor, indicating that the inhibitors did not affect the transport of viral glycoproteins and their cell surface accumulation (Fig. 2B). UV-irradiated MV-infected BJAB cells cultured in the presence or absence of DMJ (shown in Fig. 2B) revealed indistinguishable inhibitory activities when used as PC in a cocultivation assay with mitogen-stimulated human PBL as RC over a wide range of PC/RC ratios, indicating that complex glycosylation is not required for immunosuppression.
FIG. 2.
Impact of DMJ treatment on MV F and H surface expression and inhibitory activity of MV-infected BJAB cells. (A) BJAB cells were treated after a 1-h infection (MOI of 0.5) with DMJ (4 mM; Fig. 2A, lanes 3 and 4) or left untreated (Fig. 2A, lanes 1 and 2). At 24 h postinfection, cell surface proteins were biotinylated, cell extracts were harvested for immunoprecipitation using an H-specific serum, and precipitates were subjected to endo H digestion (lanes 1 and 4). H-specific bands were detected using peroxidase-conjugated streptavidin. (B) MV-infected BJAB cells (MOI of 0.5) treated with DMJ (4 mM; ●) or left untreated (■) were harvested 24 h postinfection. Aliquots of the cells were stained for expression of the MV H or F protein using monoclonal antibodies or were used as PC in a coculture assay with human mitogen-stimulated PBL as RC in a standard assay for 48 h followed by a 16-h labeling period. Proliferative inhibition was determined compared to RC cocultured with uninfected BJAB cells cultured using identical conditions with respect to added compounds. POS., positive.
Z-fFG prevents cellular fusion at the level of hemifusion which is not required for the induction of immunosuppression in vitro.
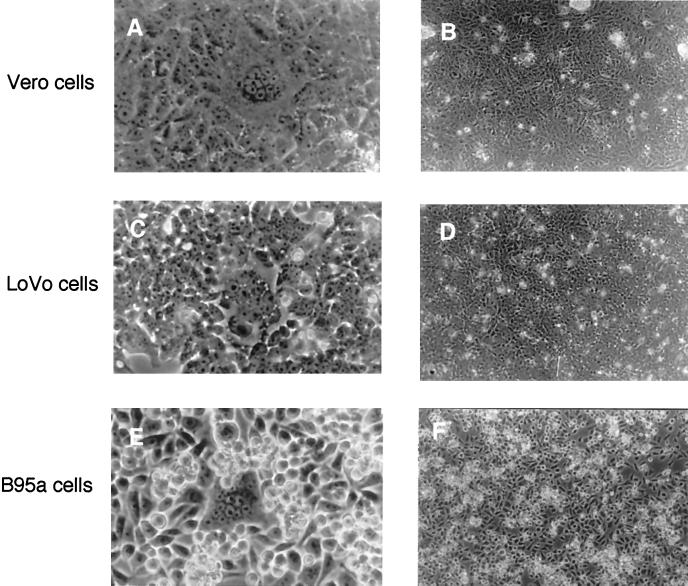
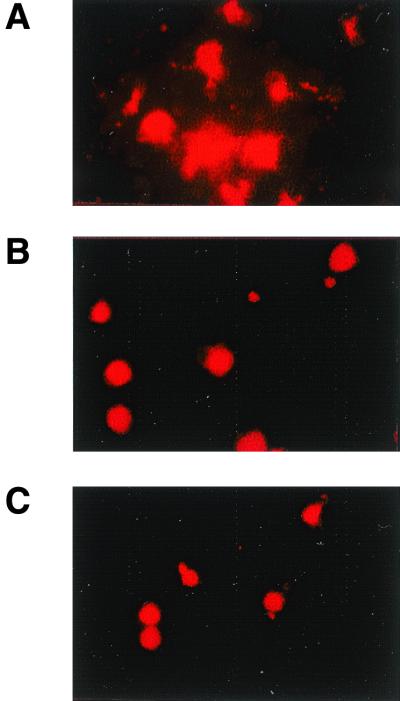
Peptide inhibitors such as Z-fFG and the HRB peptide (corresponding to the leucine zipper domain juxtaposed to the transmembrane region of the MV F protein [6, 39]) are known to prevent MV-induced membrane fusion (27, 28, 39) but not immunosuppression in vitro (38), indicating that the two activities can be uncoupled. We aimed to identify the step at which Z-fFG interfered with MV F/H-induced membrane fusion to pinpoint further the requirements of the membrane interaction of this complex for immunosuppression. BJAB-EDp, cells which do not undergo cellular fusion, efficiently induced syncytium formation when overlaid onto Vero or B95a cells for 20 h (Fig. 3A and E). Formation of syncytia containing up to 10 to 15 nuclei was also observed after 20 h when BJAB-EDp cells were laid onto LoVo cell cultures (Fig. 3C), which are unable to produce infectious MV since they are defective for furin. This indicates that syncytium formation does not result from viral spread but is induced by MV F/H complexes on the BJAB-EDp cells. The presence of Z-fFG during the coculture with BJAB-EDp cells completely abolished syncytium formation in Vero, B95a, and LoVo cell monolayers (Fig. 3B, D, and F). Since we have previously shown that the proliferation of B95a cells is sensitive to PC-induced inhibition, and this inhibition is not affected in the presence of Z-fFG, we aimed to define the step during membrane fusion which is sensitive to Z-fFG-induced inhibition in a coculture of B95a and BJAB-EDp cells. For this purpose, BJAB-EDp cells or, for a control, uninfected BJAB cells were loaded with the lipid dye R18 and overlaid onto a monolayer of B95a cells. After a 1-h incubation, the lipid dye was still retained in the membrane of uninfected BJAB cells (Fig. 4B), whereas spread of the dye to the B95a cells overlaid with BJAB-EDp cells was observed in the presence of a control peptide, Z-GFA (Fig. 4A), indicating that hemifusion by lipid mixing had occurred. Addition of Z-fFG during the coculture had no effect on the R18 distribution in BJAB-B95a cocultures (not shown) but completely abolished lipid mixing between BJAB-EDp and B95a cells (Fig. 4C), indicating that Z-fFG interferes with the mixing of the outer membrane leaflets during hemifusion. This finding strongly supports the concept that immunosuppression induced by the MV F/H complex is based on a receptor-mediated signal and does not involve any step of membrane fusion.
FIG. 3.
Formation of syncytia by BJAB-EDp cells in Vero, LoVo, and B95a cell monolayers is abolished in the presence of Z-fFG. BJAB-EDp cells (105) were layered onto Vero (A and B), LoVo (C and D), or B95a (E and F) cell monolayers in the absence (A, C, and E) or presence (B, D, and F) of 0.2 mM Z-fFG for 20 h and analyzed for syncytium formation (magnifications: A, C, and E, ×580; B, C, and F, ×180).
FIG. 4.
Z-fFG prevents lipid mixing of the outer membrane leaflets. BJAB-EDp cells (A and C) or BJAB cells (B) were labeled with R18 and overlaid (104 cells per 100 μl) onto a monolayer of B95a cells in the presence of 0.2 mM Z-GFA (A) or 0.2 mM Z-fFG (C). Redistribution of R18 was analyzed 1 h later by microscopy (magnification, ×400).
DISCUSSION
Indirect mechanisms such as the release of inhibitory mediators from infected cells or surface contact-mediated signaling leading to growth arrest or apoptosis in uninfected cells appear particularly attractive to explain the pronounced suppression of immune functions during acute measles. We have previously shown that the MV F and H proteins can act as an effector structure which, after contact with the surface of an excess amount of uninfected cells, elicits proliferative unresponsiveness to mitogenic stimulation in primary human and rodent lymphocytes, or cell lines of lymphocytic/monocytic origin in vitro (9, 31, 35). Moreover, the very same effector molecules efficiently induced immunosuppression following transfer of cells transfected to express these proteins in cotton rats (24, 25).
Soluble factors inhibiting antigen-specific proliferation of T-cell lines have been found in supernatants of MV-infected B and T cells but have not been identified (12, 36). In our system, the involvement of soluble inhibitory factors in the induction of proliferative inhibition did not appear very likely since (i) these factors would have to act species nonspecifically, as rodent lymphocytes were sensitive to inhibition by human PC, (ii) the inhibition was also induced by UV-inactivated MV but not by mock supernatants or inactivated vesicular stomatitis virus UV-VSV, and (iii) separation of PC or a PC-RC coculture from a second population of RC by a filter with a pore size of 200 nm completely abolished the induction of proliferative unresponsiveness (31). Based on the latter finding, we could not, however, rule out that factors such as reactive oxygen intermediates released from the RC after PC cocultivation into the microenvironment could act on the RC in an autocrine manner. N-Acetyl-l-cysteine, l-ascorbic acid, and catalase have been previously found to prevent growth arrest and apoptosis in cell lines, primary ovary follicle cells and primary fetal hepatocytes, by reducing reactive oxygen intermediates (1, 14, 29, 37). Since neither of these compounds had any effect in our system (Fig. 1A), it is unlikely that reactive oxygen intermediates are generated following PC-RC interaction and contribute to the induction of proliferative unresponsiveness. This interpretation is further supported by our finding that lymphocytes isolated from cotton rats transferred with F/H-expressing 293 cells reveal an impaired proliferative activity ex vivo (24), and unresponsiveness to mitogenic stimulation is observed in vitro when PC are removed from the RC population up to 96 h prior to mitogenic stimulation (31). Similarly, production of nitric oxide from RC is not likely to contribute to proliferative inhibition in our system since we failed to detect induction of iNOS on the protein level in Jurkat cells treated with mock supernatant and UV-MV (not shown), and nitrate and nitrite were essentially not produced (Fig. 1B), although proliferative inhibition did occur.
Since we have previously shown that proteolytic cleavage of the MV F protein is an essential requirement for the inhibitory activity of the F/H complex, we aimed to evaluate the role of other posttranslational modifications of these proteins such as glycosylation for immunosuppression. Since complete inhibition of MV glycoprotein glycosylation by tunicamycin blocks the transport of these proteins to the cell surface (26, 30), we have chosen to use an inhibitor of Golgi-resident mannosidases essential for complex glycosylation, DMJ (8, 20). Treatment with this compound did not affect transport of MV F/H proteins to the cell surface and their surface expression levels (Fig. 2B); however, DMJ prevented complex glycosylation of the MV F and H proteins since these proteins were sensitive to endo H digestion after DMJ treatment (Fig. 2A and data not shown). The partial endo H sensitivity of MV H protein in the absence of the trimming inhibitor was observed previously (4). In contrast, MV H and F proteins were completely endo H sensitive (Fig. 2A and data not shown), indicating that at most, only trace amounts of complex glycosylated proteins were present. Since DMJ treatment did not affect their immunosuppressive activity, MV glycoproteins containing carbohydrate chains with terminal mannose residues still retain their inhibitory phenotype. Although not detectable in our analysis (Fig. 2A), trace amounts of MV glycoproteins carrying complex oligosaccharide chains after DMJ treatment would not induce immunosuppression in vitro as efficiently as MV F/H proteins synthesized in the absence of the inhibitor over a wide range of PC/RC ratios (Fig. 2B). This is because the induction of immunosuppression in vitro is strongly dependent on the surface expression level of MV F/H complexes with inhibitory activity (38).
As indicated by previous findings, F/H-mediated immunosuppression is independent of cellular fusion (25, 38). This has clearly been demonstrated using peptides with known fusion inhibitory activity (Z-fFG [27, 28] and HRB [19]), the presence of which did not interfere with the induction of proliferative unresponsiveness by the F/H complex (38). Although formally not shown for MV F, it is likely that in analogy to simian virus 5, the HRB peptide inhibits formation of an F protein conformation necessary for fusion by interacting with an α-helical domain within the fusion domain which is thought to interact with the leucine zipper domain juxtaposed to the transmembrane region (3). Since this peptide does not interfere with the immunosuppressive activity of the MV F/H complex, conformational requirements for this activity and fusion are apparently different.
The precise mechanism underlying the inhibition of virus-induced membrane fusion (27, 28) and fusion of vesicles (16, 17) by Z-fFG is not understood. It has been suggested that Z-fFG binds to and stabilizes both membrane leaflets, thereby altering the lateral mobility of membrane components (10). Hemifusion is an intermediate step during membrane fusion which involves the lipid mixing of the outer leaflets of the membranes and has been extensively studied for influenza A virus hemagglutinin HA-mediated fusion of erythrocytes (11, 18). Using R18-labeled BJAB-EDp cells, we found that Z-fFG inhibits membrane fusion already at the step of hemifusion (Fig. 4). It is unlikely that redistribution of R18 in the absence of Z-fFG (or the presence of the control peptide Z-GFA) was due to fusion of BJAB-EDp cell membranes because it was not observed in R18-labeled BJAB-EDp cell cultures alone, most likely due to downregulation of CD46 (13, 32). Syncytium formation and redistribution of R18 were induced by BJAB-EDp cells only in the presence of Vero, LoVo, or B95a cells and was completely abolished in the presence of Z-fFG (Fig. 3 and 4). Our findings thus indicate that Z-fFG most likely intercalates into the membrane of B95a cells, thereby preventing lipid mixing and hemifusion induced by BJAB-EDp cells but not the induction of proliferative inhibition (38). Thus, immunosuppression is induced in vitro as a consequence of a mere contact of MV F/H with the surface of the RC followed by intracellular signaling and does not involve any step of membrane fusion.
ACKNOWLEDGMENTS
We thank Bert Rima, Jürgen Schneider-Schaulies, and Wolfgang Garten for helpful discussion; we also thank Anselm Ebert and Marion Seufert for excellent technical assistance.
We thank the Deutsche Forschungsgemeinschaft, the Bundesministerium for Bildung and Forschung, and the WHO for financial support.
REFERENCES
- 1.Arakaki N, Kajihara T, Arakaki R, Ohnishi T, Kazi A J, Nakashima H, Daikuhara Y. Involvement of oxidative stress in tumor cytotoxic activity of hepatocyte growth factor/scatter factor. J Biochem. 1999;274:13541–13546. doi: 10.1074/jbc.274.19.13541. [DOI] [PubMed] [Google Scholar]
- 2.Auwaerter P G, Kaneshima H, McCune J M, Wiegand G, Griffin D E. Measles virus infection of thymic epithelium in the SCID-hu mouse leads to thymocyte apoptosis. J Virol. 1996;70:3734–3740. doi: 10.1128/jvi.70.6.3734-3740.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker K A, Dutch R E, Lamb R A, Jardeztky T S. Structural basis for paramyxovirus-mediated membrane fusion. Mol Cell. 1999;3:309–319. doi: 10.1016/s1097-2765(00)80458-x. [DOI] [PubMed] [Google Scholar]
- 4.Bolt G, Pedersen I, Blixenkrone-Moller M. Processing of N-linked oligosaccharides on the measles virus glycoproteins: importance for antigenicity and for production of infectious virus particles. Virus Res. 1999;61:43–51. doi: 10.1016/s0168-1702(99)00025-8. [DOI] [PubMed] [Google Scholar]
- 5.Borrow P, Oldstone M B A. Measles virus-mononuclear cell interactions. In: Billeter M, ter Meulen V, editors. Measles virus. Berlin, Germany: Springer-Verlag KG; 1995. pp. 51–64. [DOI] [PubMed] [Google Scholar]
- 6.Buckland R, Malvoisin E, Beauverger P, Wild F. A leucine zipper structure present in the measles virus fusion protein is not required for its tetramerization but is essential for fusion. J Virol. 1992;73:1703–1707. doi: 10.1099/0022-1317-73-7-1703. [DOI] [PubMed] [Google Scholar]
- 7.Clements C J, Cutts F T. The epidemiology of measles: thirty years of vaccination. Curr Top Microbiol Immunol. 1995;191:13–34. doi: 10.1007/978-3-642-78621-1_2. [DOI] [PubMed] [Google Scholar]
- 8.Elbein A D. Inhibitors of the biosynthesis and processing of N-linked oligosaccharide chains. Annu Rev Biochem. 1987;56:439–497. doi: 10.1146/annurev.bi.56.070187.002433. [DOI] [PubMed] [Google Scholar]
- 9.Engelking O, Fedorov L M, Lilischkis R, ter Meulen V, Schneider-Schaulies S. Measles virus-induced immunosuppression in vitro is associated with deregulation of G1 cell cycle control proteins. J Gen Virol. 1999;80:1599–1608. doi: 10.1099/0022-1317-80-7-1599. [DOI] [PubMed] [Google Scholar]
- 10.Epand R M. Virus replication inhibitory peptide inhibits the conversion of phospholipid bilayers to the hexagonal phase. Biosci Rep. 1986;6:647–653. doi: 10.1007/BF01114759. [DOI] [PubMed] [Google Scholar]
- 11.Fischer C, Schroth-Diez B, Herrmann A, Garten W, Klenk H D. Acylation of the influenza hemagglutinin modulates fusion activity. Virology. 1998;248:284–294. doi: 10.1006/viro.1998.9286. [DOI] [PubMed] [Google Scholar]
- 12.Fujinami R S, Sun X, Howell J M, Jenkin J C, Burns J B. Modulation of immune system function by measles virus infection: role of soluble factor and direct infection. J Virol. 1998;72:9421–9427. doi: 10.1128/jvi.72.12.9421-9427.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirano A, Yant S, Iwata K, Korte-Sarfaty J, Seya T, Nagawasa S, Wong T C. Human cell receptor CD46 is down regulated through recognition of a membrane-proximal region of the cytoplasmic domain in persistent measles virus infection. J Virol. 1996;70:6929–6936. doi: 10.1128/jvi.70.10.6929-6936.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hockenbery D M, Oltvai Z N, Yin X M, Milliman C L, Korsmeyer S J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 15.Karp C L, Wysocka M, Wahl L M, Ahearn J M, Cuomo P J, Sherry B, Trinchieri G, Griffin D E. Mechanism of suppression of cell-mediated immunity by measles virus. Science. 1996;273:228–231. doi: 10.1126/science.273.5272.228. [DOI] [PubMed] [Google Scholar]
- 16.Kelsey D R, Flanagan T D, Young J, Yeagle L P. Peptide inhibitors of enveloped virus infection inhibit phospholipid vesicle fusion and Sendai virus fusion with phospholipid vesicles. J Biol Chem. 1990;265:12178–12183. [PubMed] [Google Scholar]
- 17.Kelsey D R, Flanagan T D, Young J E, Yeagle L P. Inhibition of Sendai virus fusion with phospholipid vesicles and human erythrocyte membranes by hydrophobic peptides. Virology. 1991;182:690–702. doi: 10.1016/0042-6822(91)90610-n. [DOI] [PubMed] [Google Scholar]
- 18.Kemble G W, Danieli T, White J M. Lipid-anchored influenza hemagglutinin promotes hemifusion, not complete fusion. Cell. 1994;76:383–391. doi: 10.1016/0092-8674(94)90344-1. [DOI] [PubMed] [Google Scholar]
- 19.Lambert D M, Barney S, Lambert A L, Guthrie K, Medinas R, Davis D E, Bucy T, Ericson J, Merutka G, Petteway S R. Peptides from conserved regions of paramyxovirus fusion (F) proteins are potent inhibitors of viral fusion. Proc Natl Acad Sci USA. 1996;93:2186–2191. doi: 10.1073/pnas.93.5.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Legler G, Julich E. Synthesis of 5-amino-5-deoxy-d-mannopyranose and 1,5-dideoxy-1,5-imino-d-mannitol, and of alpha- and beta-d-mannosidases. Carbohydr Res. 1984;15:61–72. doi: 10.1016/0008-6215(84)85084-3. [DOI] [PubMed] [Google Scholar]
- 21.McChesney M B, Kehrl J H, Valsamakis A, Fauci A S, Oldstone M B A. Measles virus infection of B lymphocytes permits cellular activation but blocks progression through the cell cycle. J Virol. 1987;61:3441–3447. doi: 10.1128/jvi.61.11.3441-3447.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McChesney M B, Altman A, Oldstone M B A. Suppression of T lymphocyte function by measles virus is due to cell cycle arrest in G1. J Immunol. 1988;140:1269–1273. [PubMed] [Google Scholar]
- 23.Naniche D, Reed S I, Oldstone M B A. Cell cycle arrest during measles virus infection: a G0-like block leads to suppression of retinoblastoma protein expression. J Virol. 1999;73:1894–1901. doi: 10.1128/jvi.73.3.1894-1901.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niewiesk S, Eisenhut I, Fooks A, Clegg J C S, Schnorr J J, Schneider-Schaulies S, Meulen V. Measles virus-induced suppression in the cotton rat (Sigmodon hispidus) model depends on viral glycoproteins. J Virol. 1997;71:7214–7219. doi: 10.1128/jvi.71.10.7214-7219.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niewiesk S, Ohnimus H, Schnorr J J, Götzelmann M, Schneider-Schaulies S, Jassoy C, ter Meulen V. Measles virus-induced immunosuppression in cotton rats is associated with a cell cycle retardation in uninfected lymphocytes. J Gen Virol. 1999;80:2023–2029. doi: 10.1099/0022-1317-80-8-2023. [DOI] [PubMed] [Google Scholar]
- 26.Ogura H, Sato H, Kamiya S, Nakamura S. Glycosylation of measles virus haemagglutinin protein in infected cells. J Gen Virol. 1991;72:2679–2684. doi: 10.1099/0022-1317-72-11-2679. [DOI] [PubMed] [Google Scholar]
- 27.Richardson C D, Scheid A, Choppin P W. Specific inhibition of paramyxovirus and myxovirus replication by oligopeptides with amino acid sequences similar to those at the N-termini of the F1 or HA2 viral polypeptides. Virology. 1980;105:205–222. doi: 10.1016/0042-6822(80)90168-3. [DOI] [PubMed] [Google Scholar]
- 28.Richardson C D, Choppin P W. Oligopeptides that specifically inhibit membrane fusion by paramyxoviruses: studies on the site of action. Virology. 1983;131:518–532. doi: 10.1016/0042-6822(83)90517-2. [DOI] [PubMed] [Google Scholar]
- 29.Sánchez A, Álvarez A, Benito M, Fabregat I. Apoptosis induced by transforming growth factor-β in fetal hepatocyte primary cultures. J Biol Chem. 1996;271:7416–7422. doi: 10.1074/jbc.271.13.7416. [DOI] [PubMed] [Google Scholar]
- 30.Sato T A, Kohama T, Sugiura A. Intracellular processing of measles virus fusion protein. Arch Virol. 1988;98:39–50. doi: 10.1007/BF01321004. [DOI] [PubMed] [Google Scholar]
- 31.Schlender J, Schnorr J J, Spielhofer P, Cathomen T, Cattaneo R, Billeter M A, ter Meulen V, Schneider-Schaulies S. Interaction of measles virus glycoproteins with the surface of uninfected peripheral blood lymphocytes induces immunosuppression in vitro. Proc Natl Acad Sci USA. 1996;93:13194–13199. doi: 10.1073/pnas.93.23.13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider-Schaulies J, Schnorr J J, Schlender J, Dunster L M, Schneider-Schaulies S, ter Meulen V. Receptor (CD46) modulation and complement-mediated lysis of uninfected cells after contact with measles virus-infected cells. J Virol. 1996;70:255–263. doi: 10.1128/jvi.70.1.255-263.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider-Schaulies S, ter Meulen V. Measles virus induced immunosuppression. Nova Acta Leopold. 1999;307:185–197. [Google Scholar]
- 34.Schnorr J-J, Seufert M, Schlender J, Borst J, Johnston I C, ter Meulen V, Schneider-Schaulies S. Cell cycle arrest rather than apoptosis is associated with measles virus contact-mediated immunosuppression in vitro. J Gen Virol. 1997;78:3217–3226. doi: 10.1099/0022-1317-78-12-3217. [DOI] [PubMed] [Google Scholar]
- 35.Schnorr J-J, Xanthakos S, Keikavoussi P, Kämpgen E, ter Meulen V, Schneider-Schaulies S. Induction of maturation of human blood dendritic cell precursors by measles virus is associated with immunosuppression. Proc Natl Acad Sci USA. 1997;94:5326–5331. doi: 10.1073/pnas.94.10.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun X, Burns J B, Howell J M, Fujinami R S. Suppression of antigen-specific T cell proliferation by measles virus infection: role of a soluble factor in suppression. Virology. 1998;246:24–33. doi: 10.1006/viro.1998.9186. [DOI] [PubMed] [Google Scholar]
- 37.Tilly J L, Tilly K I. Inhibitors of oxidative stress mimic the ability of follicle-stimulating hormone to suppress apoptosis in cultured rat ovarian follicles. Endocrinology. 1995;136:242–252. doi: 10.1210/endo.136.1.7828537. [DOI] [PubMed] [Google Scholar]
- 38.Weidmann A, Maisner A, Garten W, Seufert M, ter Meulen V, Schneider-Schaulies S. Proteolytic cleavage of the fusion protein but not membrane fusion is required for measles virus-induced immunosuppression in vitro. J Virol. 2000;74:1985–1993. doi: 10.1128/jvi.74.4.1985-1993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wild F T, Buckland R. Inhibition of measles virus infection and fusion with peptides corresponding to the leucine zipper region of the fusion protein. J Virol. 1997;78:107–111. doi: 10.1099/0022-1317-78-1-107. [DOI] [PubMed] [Google Scholar]
- 40.Yanagi Y, Cubitt B A, Oldstone M B A. Measles virus inhibits mitogen-induced T cell proliferation but does not directly perturb the T cell activation process inside the cell. Virology. 1992;187:280–289. doi: 10.1016/0042-6822(92)90316-h. [DOI] [PubMed] [Google Scholar]