Abstract
Canine distemper virus (CDV) causes a life-threatening disease in several carnivores including domestic dogs. Recently, we identified a molecule, CD9, a member of the tetraspan transmembrane protein family, which facilitates, and antibodies to which inhibit, the infection of tissue culture cells with CDV (strain Onderstepoort). Here we describe that an anti-CD9 monoclonal antibody (MAb K41) did not interfere with binding of CDV to cells and uptake of virus. In addition, in single-step growth experiments, MAb K41 did not induce differences in the levels of viral mRNA and proteins. However, the virus release of syncytium-forming strains of CDV, the virus-induced cell-cell fusion in lytically infected cultures, and the cell-cell fusion of uninfected with persistently CDV-infected HeLa cells were strongly inhibited by MAb K41. These data indicate that anti-CD9 antibodies selectively block virus-induced cell-cell fusion, whereas virus-cell fusion is not affected.
Canine distemper virus (CDV) causes in carnivores (canines, felids, ferrets, raccoons, and seals) a highly contagious disease with many similarities to human measles but also with a significant difference, as it is much more neurotropic and causes acute encephalitis in about half of the infected animals (2, 22). The disease is characterized by fever, coryza, conjunctivitis, gastroenteritis, and pneumonitis. The mortality rates following CDV infection vary with the host species, ranging from 0% in domestic cats to approximately 50% in domestic dogs and 100% in ferrets. Encephalomyelitis is the most common cause of death of CDV-infected animals (2, 40, 43). In dogs, CDV infection results in a progressive demyelinating encephalomyelitis, probably due to a bystander mechanism in which macrophages play an important role (46). The onset of encephalitis appears to be influenced by humoral immune responses to CDV (33). Canine distemper is also associated with transient immunosuppression that may result in significant morbidity and mortality through opportunistic infections (6, 19).
The cellular receptor for CDV is not known. It has been shown by complementation analysis with the help of recombinant envelope proteins of CDV and measles virus MV that the CDV H protein is responsible for the selective tropism of CDV in cell culture (39). Human-mouse somatic cell hybrids were used to demonstrate that human chromosome 19 encodes a CDV receptor on human cells (39). Recently, we obtained a monoclonal antibody (MAb K41) which was able to inhibit CDV infection and found that it recognizes the tetraspan transmembrane (TM4) protein CD9 (21), the gene of which is localized on human chromosome 12 (4, 5). However, direct binding of CDV to CD9 could not be demonstrated, suggesting that CD9 is not a receptor for CDV.
CD9 has also been discussed as a possible cellular receptor for feline immunodeficiency virus (FIV) (44), and a different member of the TM4 superfamily, C33 (CD82), was found to be involved in syncytium formation by human T-cell leukemia virus type 1 (HTLV-1) (14). Similar to our findings, direct binding of neither FIV to feline CD9 nor HTLV-1 to CD82 was demonstrated, suggesting again indirect functions of these two members of the TM4 family in virus replication and spread. Recently it was found that infection of cells with FIV is inhibited by antibodies to CD9 in a step occurring after virus uptake (9, 45). The authors suggested that FIV release is affected by anti-CD9 antibodies. In the present study, we investigated which step of CDV infection is impaired by anti-CD9 antibodies and found that virus release and virus-induced cell-cell fusion by syncytium-inducing strains is selectively inhibited, whereas virus-cell fusion is not affected.
MATERIALS AND METHODS
Propagation of cells and canine distemper virus strains.
The cell lines HeLa (human cervix carcinoma; ATCC CCL 2) and Vero (African green monkey; ATCC CRL 6318) were cultured in minimal essential medium containing 10% fetal calf serum, penicillin, and streptomycin. Primary dog brain cell cultures (DBCC) were grown on poly-l-lysine-coated glass coverslips as described elsewhere (49). These cultures contain predominantly astrocytes and can be maintained for several months.
CDV strains Onderstepoort (large- and small-plaque variants OND-LP and OND-SP) and Rockborn (RB), a dog isolate from Belfast (Dog/NI) (22), and strains A75/17-V (wild-type A75/17 adapted to growth in Vero cells), BUS (Bussel), and HAN2544/95 (a gift from L. Haas and V. von Messling, Tierärztliche Hochschule, Hannover, Germany) were propagated using Vero cells. Briefly, Vero cells in minimal essential medium containing 5% fetal calf serum were infected at a multiplicity of infection (MOI) of 0.01 at 37°C and incubated at 37°C for 3 to 5 days, depending on when the optimal titer of infectious CDV was produced. CDV was harvested by one cycle of freezing-thawing and centrifugation at 200 × g for 10 min to remove cell debris and then stored at −70°C. The CDV wild-type isolate A75/17 (a gift from M. Appel, Cornell University, Ithaca, N.Y.) was propagated in specific-pathogen-free dogs (Federal Institute of Virus Diseases and Immune Prophylaxis, Mittelhäusern, Switzerland) as described elsewhere (49). Lymphoid tissue from these dogs containing large quantities of virus was homogenized and frozen in aliquots at −70°C until used.
Antibodies, fluorescent dyes, and FIP.
Mouse anti-CD9 MAb K41 was raised against cell surface epitopes by inoculating BALB/c mice intraperitoneally with Vero cells (21). MAb 8D1, against the CDV H protein, was produced in our laboratory. Hybridoma cells were grown in RPMI 1640 medium, and MAbs were purified by protein G affinity chromatography. The dog polyclonal anti-CDV hyperimmune serum was a gift from M. Appel. Secondary antibodies (goat anti-mouse and anti-dog, both conjugated to fluorescein isothiocyanate [FITC]) were obtained from Dako and Immunotech. The fluorescent dyes rhodamine R18 (for staining of the membrane) and calcein (for cytoplasmic staining) were purchased from Molecular Probes; the dye Hoechst H33258, for staining DNA, was purchased from Sigma. The fusion-inhibiting peptide (FIP) Z-d-Phe-l-Phe-Gly-OH (9, 25) was purchased from Bachem (Bubendorf, Switzerland).
Virus-cell binding assay and flow cytometry.
Similar MOIs or amounts of proteins of virus preparations of various strains of CDV were used in the virus-cell binding assays. The MOIs were determined according to titration on Vero cells. Cells (2 × 104 in 100 μl of phosphate-buffered saline [PBS]) were incubated at 4°C for 2 h with virus at a given MOI or amount of viral protein, washed with FACS (fluorescence activated cell sorting) buffer (PBS containing 0.4% bovine serum albumin and 0.02% sodium azide), and incubated with polyclonal dog anti-CDV serum and FITC-conjugated goat anti-dog antibodies. The amount of bound virus was determined by flow cytometry.
Flow cytometric analyses were performed as described elsewhere (35). Briefly, 105 cells were incubated for 30 min on ice with 1 μg of MAb in 100 μl of FACS buffer. Cells were washed twice in FACS buffer and incubated with 200 μl of a 1:100 dilution of FITC-conjugated goat anti-mouse immunoglobulin (Dako) on ice for further 30 min. After three washes with FACS buffer, flow cytometric analysis was performed on a FACScan (Becton Dickinson).
Virus uptake assay by reverse transcription-PCR (RT-PCR).
Cells were infected with various MOIs of CDV for 1 h in the absence or presence of anti-CD9 antibodies. After infection, cells were washed once with an acidic buffer (0.14 M NaCl, 1 mg of bovine serum albumin/ml, 8 mM glycine [pH 2.5]) to destroy viral particles attached to the outside of the cells and twice with PBS. RNA was prepared (Qiagen RNA preparation kit), and 3 μg each used for reverse transcription and subsequent PCR. Primers were chosen to be specific for the viral genome spanning a region of 1,027 nucleotides (nt) between the fusion (F) and hemagglutinin (H) genes (forward primer, 5′ AGG TAC AAA CTT AGG GAA CGC 3′; reverse primer, 5′ AAA CTT TGC CTA CTG AAG TAG 3′). The universal morbillivirus primers spanning a region of 429 nt in the phosphoprotein (3) were also used.
RNA isolation and Northern blotting.
Vero cells (5 × 106) were lysed in situ by 4 M guanidinium isothiocyanate buffer (5 ml), and total cellular RNA was purified using an RNA purification kit (Qiagen). Total RNA was separated on 1.5% agarose gels containing 6.3% formaldehyde, blotted on Hybond-N filters (Amersham), and cross-linked with UV light (0.6 J/cm2). The hybridization probe for CDV N (nucleocapsid) was an 800-bp fragment of CDV N cloned in pBR322. For control of intact RNA, the 1.4-kb PstI fragment of rat glyceraldehyde 3-phosphate dehydrogenase (GAPDH) cDNA was used. The hybridization probes were radioactively labeled with [32P]dCTP, using a random prime labeling kit (Boehringer Mannheim). Blots were exposed to a screen for qualitative and quantitative evaluation with a PhosphorImager (Molecular Dynamics).
[35S]methionine-[35S]cysteine labeling and immunoprecipitation.
For immunoprecipitation of viral proteins, Vero cells (8 × 105/well) were infected with CDV at an MOI of 3. They were starved for 30 min for cysteine and methionine and labeled with 40 μCi of [35S]methionine and [35S]cysteine (Amersham) for 5 h before harvesting at certain time points. For radioimmunoprecipitation (RIPA), the cells were dissolved in 1× RIPA-det (150 mM NaCl, 10 mM Tris, 0.1% sodium dodecyl sulfate [SDS], 1% sodium deoxycholate, 1% Triton X-100). CDV-specific proteins were precipitated with polyclonal dog hyperimmune serum (3 μl/well) and precipitated with protein A-Sepharose (Pharmacia) as described elsewhere (33). Proteins were then separated by SDS–10% polyacrylamide gel electrophoresis. The gels were dryed and exposed to a PhosphorImager screen, and the signals were detected with a PhosphorImager (Molecular Dynamics).
RESULTS
Binding to and uptake of CDV by target cells is not altered by anti-CD9 antibodies.
The influence of CD9 antibodies on the binding of CDV to Vero cells was measured by flow cytometry. As found earlier, pretreatment of cells with anti-CD9 MAb K41 at a concentration of 15 μg/ml inhibits plaque formation by 80 to 99%. To determine the effect of MAb K41 on virus binding, we incubated Vero cells with a saturating concentration of K41 (50 μg/ml) for 1 h prior to incubation with CDV (MOIs of 5 to 10) for 1 h at 0°C. Bound CDV (strain OND-LP) was then detected using a polyclonal serum against CDV (Fig. 1A), and the mean fluorescence intensities of the signals in the absence and presence of K41 were compared (Fig. 1B). MAb K41 had no influence on the binding of viral particles to the cells. Similar results were obtained with CDV strains OND-SP, RB, Dog/NI, and A75/17-V (not shown). Thus, the attachment of CDV particles to the target cells is not inhibited by antibodies to CD9.
FIG. 1.
Anti-CD9 antibodies do not inhibit the binding to and the uptake of CDV by cells. CDV strain OND-SP (MOI = 10) was bound to Vero cells at 4°C, and the bound virus was detected by flow cytometry with a polyclonal dog hyperimmune serum (A). Bound virus shifted the signal from background (Vero) to high values of mean fluorescence intensity (Vero + CDV). Preincubation with MAb K41 (50 μg/ml) did not block the binding of virus and gives similar signals as virus alone (Vero + K41 + CDV). The mean values of the median fluorescence intensities of three binding experiments in the presence and absence of MAb K41 are shown in panel B. To measure the uptake of CDV by RT-PCR (C), Vero cells were infected with decreasing MOIs of CDV (1, 0.5, 0.1, 0.05, and 0.01) in the absence and presence of MAb K41 (lanes 1 to 5 and 6 to 10, respectively). The negative controls (lane 11 and 17) were RNA from uninfected cells; the positive control (lane 12) was RNA from 48-h CDV-infected Vero cells (MOI = 0.1). As a control, Vero cells were incubated with CDV at MOIs 3 and 1 at 4°C (lanes 13 and 14) and at 37°C (lanes 15 and 16) for 2 h and washed with the acidic buffer and PBS. Reverse transcription was primed with the primer specific for the CDV genome (forward primer). PCR with the F/H primers amplifies a 1,027-bp fragment spanning parts of the F and H genes of CDV. The RNA used for the RT-PCRs is shown in panel D.
The uptake of CDV by Vero cells was measured by RT-PCR with primers amplifying a 1,027-nt region spanning the region between and parts of the F and H genes. The orientation of the primers was chosen to detect genomic viral RNAs. To achieve semiquantitative results, Vero cells were infected with decreasing MOIs of CDV (1, 0.5, 0.1, 0.05, and 0.01) in the absence or presence of MAb K41. After 2 h, cells were washed once with an acidic buffer destroying residual virus and twice with PBS. Total cellular RNA was isolated (Fig. 1D), and RT-PCR was performed with the F/H primers. Signals were similar in the absence (Fig. 1C, lanes 1 to 5) and presence (lanes 6 to 10) of MAb K41. In a control experiment, cells were incubated with CDV at MOIs of 3 and 1 at 4°C (lanes 13 and 14) and at 37°C (lanes 15 and 16) for 2 h and washed with the acidic buffer and PBS. This control shows that only at 37°C a substantial amount of virus is taken up by the cells and is detected by RT-PCR. Similar results were obtained with primers (3) for the viral phosphoprotein (not shown).
Anti-CD9 antibodies inhibit the CDV-production in a step after the uptake of virus.
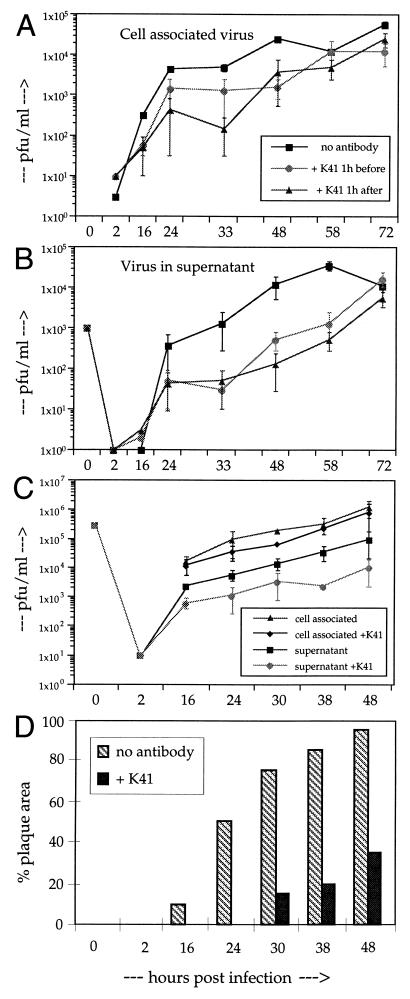
Since the uptake of virus is not blocked, we investigated whether MAb K41 can exert its inhibitory effect only before or also after infection of cells. We treated Vero cells with MAb K41 at 1 h before and 1 h after the infection with CDV strain OND-SP (MOI of 0.01). The duration of the treatment was 1 h in each case. Cell-associated and cell-free virus was then isolated and titrated up to 72 h postinfection (hpi). Regardless of when MAb K41 was added to the cells (even at 16 hpi [not shown]) there was always a considerable reduction in syncytium formation and virus production. Cell-associated virus was reduced approximately 10-fold between 16 and 48 h pi, with a smaller reduction at later time points (Fig. 2A). Virus in the supernatant was reduced approximately 30-fold up to 60 hpi (Fig. 2B). At 72 hpi, the titer in mock-treated cells had already dropped due to the exhaustive formation of syncytia, whereas titers of K41-treated cultures continued to increase. These results indicate that (i) a short (1-h) transient treatment of the cells with MAb K41 is sufficient to exert the inhibitory effect and (ii) anti-CD9 antibodies are effective in a step after the uptake of virus.
FIG. 2.
Titration of cell-associated and released virus from CDV-infected cells. (A and B) Vero cells were mock treated or treated with MAb K41 for 1 h before or 1 h after infection with OND-SP (as indicated; MOI = 0.01). After treatment with K41 (12 μg/ml), the cells were washed to remove unbound antibodies and incubated for the indicated times. Cell-associated virus released by one cycle of freezing-thawing (A) and supernatant (B) was titrated on Vero cells (n = 3). (C and D) Vero cells were infected at an MOI of 3.0 in the presence or absence of MAb K41, and cell-associated and cell-free virus was titrated (C). The percentage of the culture area in plaques was calculated using an image assistant program by computer, considering the area of syncytia containing three or more nuclei (D).
We titrated the yield of cell-associated and cell-free virus also under conditions of a single-step growth kinetic (infection of cells at an MOI of 3 [Fig. 2C and D]). In this case, the amount of cell-associated virus was not significantly reduced in the presence of MAb K41, whereas the virus titer in the supernatant was reduced by a factor of 5 to 10. Under these single-step growth conditions, we found again a strong effect of MAb K41 on syncytium formation in the cultures (Fig. 2D). In the presence of MAb K41, syncytium formation started later and the plaque area in the cultures was reduced by 75 to 90% after 30 to 48 hpi. These data indicate that the anti-CD9 antibody simultaneously induces a reduction in plaque area (cell-cell fusion) and of virus release.
Viral mRNA and protein levels in single-step growth kinetics are not affected by anti-CD9 antibodies.
To investigate a possible direct effect of MAb K41 on viral mRNA synthesis, we isolated viral mRNAs 6 and 12 h after infection of cells with CDV strain OND-SP (MOI of 0.5) in the presence and absence of K41. Northern blots of the RNA samples were hybridized with probes for CDV N and GAPDH as control for the amount of intact RNA (Fig. 3A and B). The signals were quantified with a PhosphorImager and expressed as the CDV N/GAPDH signal ratio (Fig. 3C). There was no detectable direct influence of anti-CD9 antibodies on viral mRNA synthesis.
FIG. 3.
No effect of MAb K41 on CDV N-specific mRNA levels. Vero cells were infected with OND-SP (MOI = 0.5), the inoculum was removed, and MAb K41 (12 μg/ml) was added to the cultures. RNA was harvested after 0, 6, and 12 hpi and blotted on Hybond-N filters. The filters were hybridized with 32P-labeled probes specific for CDV N (A) and GAPDH (B). Two experiments are shown (lanes 1 to 4 and lanes 5 to 8). Mean values of the N/GAPDH signal ratio are given in panel C.
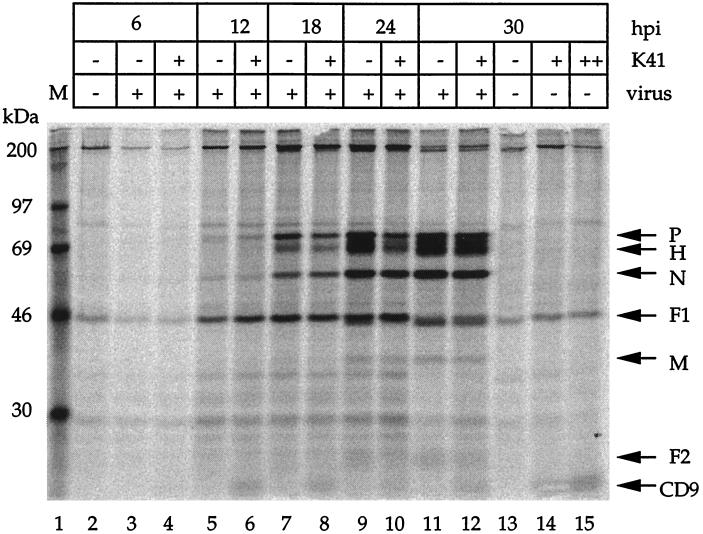
To assess a possible effect of MAb K41 on viral protein synthesis in a single-step growth kinetic, we infected cells with OND-SP at an MOI of 3 for 6, 12, 18, 24, and 30 h in the presence or absence of K41. Under these conditions, a high percentage of cells is initially infected and viral gene expression is not influenced by virus spread from cell to cell. Viral protein synthesis was measured by labeling cells during the last 5 h with [35S]methionine and [35S]cysteine for each time point, followed by immunoprecipitation with a polyclonal anti-CDV antiserum (Fig. 4). The viral proteins were synthesized at all time points without any significant effect of the CD9 antibody up to 30 hpi. In addition, processing of the viral proteins, including the cleavage of F0 to F1 and F2, appeared to be fully functional in the presence of K41.
FIG. 4.
MAb K41 does not inhibit viral protein synthesis. Vero cells were infected with OND-SP (MOI = 3) for 6, 12, 18, 24, and 30 h in the presence and absence of K41 (12 μg/ml), as indicated. Cells were labeled for 5 h with [35S]methionine and [35S]cysteine and subsequently immunoprecipitated with a polyclonal anti-CDV antiserum (lanes 1 to 14) or MAb K41 (lane 15) and protein A-Sepharose beads. In lanes 4, 6, 8, 10, and 12, MAb K41 was added after the infection (at 1 hpi) and also during the incubation with [35S]methionine and [35S]cysteine. The viral proteins P, H, N, F1 (lower band below actin), M, and F2 were detected as published elsewhere (33). Superimposed on the F1 band, a background signal is present in all lanes. MAb K41 present in the cultures led to a visible CD9 band (lanes 4, 6, 8, 10, 12, and 14), similar to the result obtained when it was used for precipitation (lane 15).
Inhibition of CDV-induced cell-cell fusion by anti-CD9 antibodies.
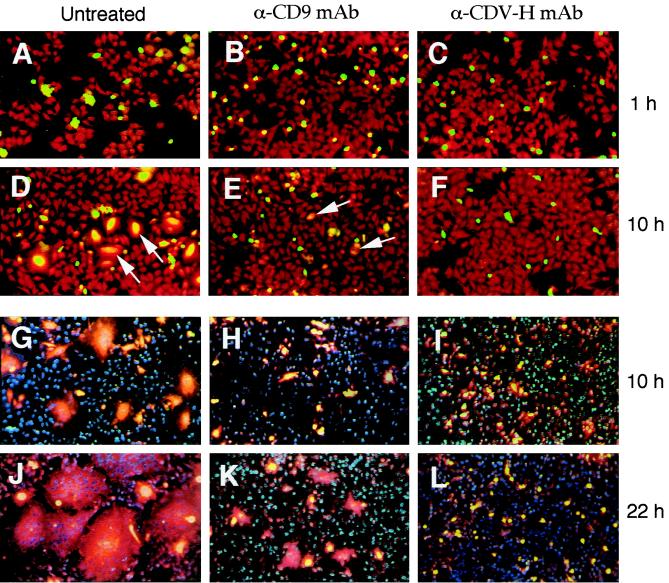
We described earlier that MAb K41 strongly inhibits the formation of large syncytia in CDV-infected cell cultures (21). Here we mixed uninfected with persistently CDV-infected HeLa cells in the absence or presence of MAb K41 in order to specifically measure the effect of K41 on the virally induced cell-cell fusion. A neutralizing anti-CDV H MAb was used as a control. For early time points, uninfected cells were labeled with rhodamine R18 and infected cells were labeled with calcein, and the extent of membrane mixing (hemifusion) and complete fusion was observed in a fluorescence microscope (Fig. 5A to F). Hemifusion was always followed by complete cell-cell fusion. In case of cell-cell fusion, the color of syncytia turns to orange (Fig. 5D). In the presence of K41, only a few small syncytia were observed after 10 h (Fig. 5E). In the presence of the neutralizing antibody against CDV H, cell-cell fusion was completely inhibited after 10 h (Fig. 5F, I, and L). Since the dyes calcein and R18 cannot be distinguished later than 16 hpi, we used other dyes for later time points. The nuclei of uninfected cells were then labeled with Hoechst H33258, and infected cells were labeled with rhodamine R18 plus calcein (Fig. 5G to L). In the absence of antibodies, large syncytia were formed and stained red (Fig. 5J). The size of syncytia between uninfected and persistently CDV-infected HeLa cells was strongly reduced by MAb K41 (Fig. 5K). The effect of K41 is clearly different from the effect of anti-CDV H, which completely blocked fusion (Fig. 5L). Since small syncytia are formed in the presence of K41, these findings indicate that the main effect of the anti-CD9 antibody is not to inhibit syncytium formation completely but to reduce the speed of the syncytium formation.
FIG. 5.
Determination of the effect of MAb K41 on the extent of cell-cell fusion. Uninfected and persistently CDV-infected HeLa cells were mixed in the absence of antibodies (A, D, G, and J) or in the presence of MAb K41 (B, E, H, and K) or anti-CDV-H MAb (C, F, I, and L). Coverslips were processed for immunofluorescence after 1, 10, and 22 h. (A to F) For early time points, uninfected cells were labeled with rhodamine R18 (red), and infected cells were labeled with calcein (green). In the case of cell-cell fusion, the color of growing syncytia changes from green to orange (D, arrows). In the presence of K41, only a few small syncytia are observed after 10 h (E, arrows). In the presence of anti-CDV H, cell-cell fusion is completely inhibited (F). (G to L) To visualize larger syncytia at later time points, the nuclei of uninfected cells were labeled with Hoechst H33258 (blue), and infected cells were labeled with rhodamine R18 plus calcein (yellow). Large syncytia are formed in the absence of antibodies and turn red (J). In the presence of K41, small syncytia develop (K), whereas in the presence of anti-CDV H, only single persistently infected cells are present and cell-cell fusion is completely blocked (L).
Inhibition of the CDV-induced cell-cell fusion indirectly reduces viral mRNA levels at later times after infection.
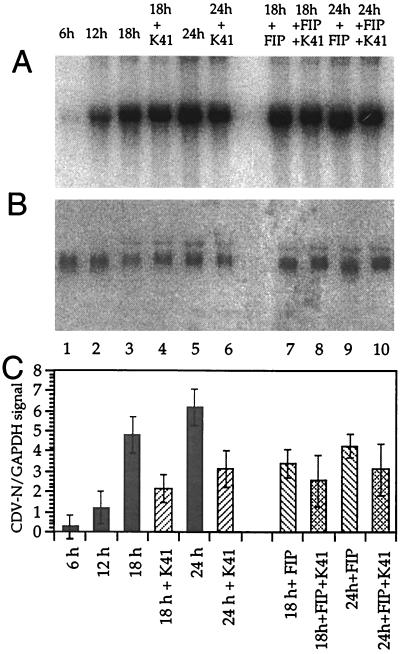
When the cell-cell fusion is reduced by anti-CD9 antibodies, a similar effect should be observed when fusion is inhibited by other means such as the FIP Z-d-Phe-l-Phe-Gly-OH). To analyze how the inhibition of the cell-cell fusion influences viral mRNA levels in the culture, cells were infected with CDV strain OND-SP at a low MOI of 0.1, and viral RNA levels were compared at times after infection when syncytia are formed. In contrast to the conditions presented in Fig. 4 (high MOI), the virus growth in culture now depended on cell-to-cell spread. MAb K41 alone, FIP alone, or a combination of K41 and FIP was added to the cultures at 12 hpi, to allow similar growth of virus in the cultures up to this time point. Total RNA was isolated 6, 12, 18, and 24 hpi. CDV N and GAPDH signals were quantified with a PhosphorImager (Fig. 6). Under these conditions, the viral N mRNA levels were reduced in the presence of K41 by 55 and 50% at 18 and 24 hpi, respectively, and in the presence of 200 μM FIP alone by approximately 30% (Fig. 6, lanes 7 and 9). In the presence of both FIP and K41, the N mRNA levels were reduced by approximately 50% (Fig. 6, lanes 8 and 10). Since there is no additive effect of FIP and K41 on the reduction of viral mRNA synthesis, we conclude that the observed reduction of mRNA levels at later time points after infection is an indirect effect of the inhibition of cell-cell fusion and virus spread by syncytium formation in the tissue culture.
FIG. 6.
Effect of MAb K41 and FIP on CDV N mRNA levels under conditions allowing virus spread by cell-cell fusion. Vero cells were infected with CDV strain OND-SP at a low MOI of 0.1. MAb K41 (12 μg/ml) alone, FIP (200 μg/ml) alone, or combinations of the two were added to the cultures after 12 h as indicated. RNA was harvested at 0, 6, 12, 18, and 24 hpi, blotted on Hybond-N filters, and hybridized to 32P-labeled probes for CDV N (A) and GAPDH (B). Mean values of the N/GAPDH signal ratio are given in panel C.
The inhibitory effect of anti-CD9 antibodies is CDV strain dependent.
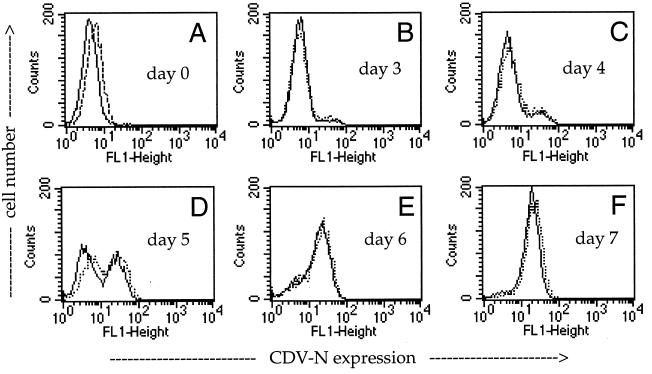
Using Vero cells, CDV strains OND-SP, OND-LP, and BUS and the Vero cell-adapted wild-type strain A75/17-V form readily visible syncytia after approximately 20 hpi. In contrast, CDV strains RB, Dog/NI, and HAN2544/95 do not form syncytia. At late times of infection, RB can induce the formation of small plaques. Analyzing the infection of Vero cells with the various CDV strains in the presence and absence of MAb K41, we found that the spread of all syncytium-inducing CDV strains was drastically inhibited by MAb K41, whereas spread in the culture and the virus yield of non-syncytium-inducing CDV strains were not affected. Nucleocapsid expression in cells infected with CDV strain RB in the absence or presence of MAb K41 is shown as an example (Fig. 7). We obtained similar results using the dog brain-propagated wild-type isolate A75/17, the envelope proteins of which are considerably different from those of OND (7). The initial spread of A75/17 in DBCC is not associated with a cytopathic effect. Although MAb K41 recognized its antigen on DBCC as efficiently as on Vero cells, the spread of CDV strain A75/17 in these primary DBCC was not inhibited by K41 (not shown).
FIG. 7.
No effect of MAb K41 on infection of Vero cells with CDV strain RB. Vero cells were infected with the non-syncytium-forming CDV strain RB (MOI = 0.01) for 1 h prior to addition of anti-CD9 MAb K41 (12 μg/ml) and analyzed by flow cytometry after 0, 3, 4, 5, 6, and 7 dpi. The CDV N-specific signal in the absence (full line) and presence (dashed line) of K41 is shown.
DISCUSSION
Cell-to-cell spread of virus in infected tissues as well as in tissue culture depends on the capacity of the virus to induce membrane fusion mechanisms overcoming the natural barriers between cells. Nonviral proteins in the host cell membrane are essential for successful fusion events. Recently, we described that infection of Vero cell cultures with CDV strain OND is drastically inhibited by antibodies to CD9 (21). Here we determined the step at which virus spread and production are inhibited. We found that neither virus uptake nor viral mRNA or protein levels are directly affected by anti-CD9 antibodies. Also, the processing of viral proteins including cleavage of the F protein and surface expression of viral proteins appeared to be normal. However, what is drastically affected by MAb K41 is syncytium formation in infected cultures and virus release. In an assay of fusion of uninfected with persistently infected HeLa cells, we found that MAb K41 directly impaired CDV-induced cell-cell fusion. The reductions of virus yield and of viral mRNA levels observed late after infection in the presence of MAb K41 were similar to those observed in the presence of a FIP and therefore most likely are a secondary effect of the inhibition of syncytium formation. From these data, we conclude that antibodies against CD9 specifically inhibit CDV-induced cell-cell fusion but not virus-cell fusion.
Cell-to-cell spread of MV, most likely involving localized fusion events at cell contact points, was demonstrated in vivo and in tissue culture (1, 10, 12, 24, 25, 42) and recently in real time in human astrocytoma cells, using a recombinant virus expressing the enhanced green fluorescent protein (11). Johnston et al. showed that cell tropism and the type of cytopathic effect of MV strains in tissue culture is governed by both the H and F proteins of MV, on the level of receptor usage (18). These experiments also indicated that cell-to-cell spread and infection of cells with extracellular virus are differentially regulated steps dependent on certain combinations of viral envelope proteins and cell surface molecules.
Interesting cell surface proteins which play a role in cell-cell fusion induced by viruses have been identified as fusion regulation protein 1 (FRP-1) and FRP-2. These proteins were initially found with MAbs stimulating Newcastle disease virus-induced cell-cell fusion and recognizing 80- and 135-kDa proteins, respectively (15, 16, 27). Interestingly, antibodies to FRP-1 stimulate Newcastle disease virus-induced and inhibit parainfluenza virus type 2-induced cell fusion (29). Recent data indicate that human immunodeficiency virus (HIV)-induced cell fusion is also regulated by FRP-1, integrins, and the activation of tyrosine kinases (28, 41) and that HIV DNA may also spread from cell to cell in a CD4-independent way via apoptotic bodies (38). In parallel, syncytium formation of HTLV-1 is regulated in a cell-type-specific manner by ICAM-1, ICAM-3, and VCAM-1 and can be inhibited by antibodies to integrin β2 or β7 (8). Thus, cell-to-cell spread of viruses is an important mechanism observed in a variety of viral diseases, contributing considerably to pathogenesis especially in infections affecting the central nervous system, such as poliovirus, herpesviruses, HIV, HTLV, and MV. Since the involved cell surface molecules are usually excluded from the viral membranes during budding, they are not involved in virus-cell fusion, which clearly distinguishes virus-cell fusion mechanistically from virus-induced cell fusion.
Among the known functions of CD9 is the activation of platelet aggregation (13, 20). Several molecules are involved in this process: CD9, the FcγRII receptor, and the platelet integrins αIIbβ3 (GPIIbIIIa or CD41 [17, 37]). CD9 is also known to participate in adhesive and migratory events via integrins of the β1 family, especially the α4β1 and α5β1 integrins (34). So far there is no experimental evidence that one of these integrins is involved in CDV-induced cell-cell fusion. It has been reported that several signal transduction pathways are affected by antibodies against CD9 (30, 31, 47, 48), that small G proteins are associated with CD9 (36), and that the binding activity of integrins is regulated by CD9 (23). Therefore, it is possible that antibodies to CD9 might regulate other proteins not identical with the directly associated integrins which may bind CDV or are involved in membrane fusion. The identification of such proteins and mechanisms of cell-cell fusion in contrast to virus-cell fusion requires further investigations.
ACKNOWLEDGMENTS
We thank S. Löffler and F. Dimpfel for technical assistance.
We thank the Deutsche Forschungsgemeinschaft for financial support.
REFERENCES
- 1.Allen I V, McQuaid S, McMahon J, Kirk J, McConnel R. The significance of measles virus antigen and genome distribution in the CNS in SSPE for mechanisms of viral spread and demyelination. J Neuropathol Exp Neurol. 1996;55:471–480. doi: 10.1097/00005072-199604000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Appel M J G, Gillespie J H. Canine distemper virus. Virol Monogr. 1972;11:1–96. [Google Scholar]
- 3.Barrett T, Visser I K G, Mamaev L, Goatley L, van Bressem M-F, Osterhaus A D M E. Dolphin and porpoise morbilliviruses are genetically distinct from phocine distemper virus. Virology. 1993;193:1010–1012. doi: 10.1006/viro.1993.1217. [DOI] [PubMed] [Google Scholar]
- 4.Benoit P, Gross M S, Frachet P, Frezal J, Uzan G, Boucheix C, Nguyen V C. Assignment of the human CD9 gene to chromosome 12 (region P13) by use of human specific DNA probes. Hum Genet. 1991;86:268–272. doi: 10.1007/BF00202407. [DOI] [PubMed] [Google Scholar]
- 5.Boucheix C, Benoit P, Frachet P, Billard M, Worthington R E, Gagnon J, Uzan G. Molecular cloning of the CD9 antigen. A new family of cell surface proteins. J Biol Chem. 1991;266:117–122. [PubMed] [Google Scholar]
- 6.Ceruti-Sola S, Kristensen F, Vandevelde M, Bichse P, Kihm U. Lymphocyte responsiveness to lectin and myelin antigens in canine distemper infection in relation to the development of demyelinating lesions. J Neuroimmunol. 1983;4:77–90. doi: 10.1016/0165-5728(83)90013-9. [DOI] [PubMed] [Google Scholar]
- 7.Cherpillod P, Beck K, Zurbriggen A, Wittek R. Sequence analysis and expression of the attachment and fusion proteins of canine distemper virus wild-type strain A75/17. J Virol. 1999;73:2263–2269. doi: 10.1128/jvi.73.3.2263-2269.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daenke S, McCracken S A, Booth S. Human T-cell leukaemia/lymphoma virus type 1 syncytium formation is regulated in a cell-specific manner by ICAM-1, ICAM-3 and VCAM-1 and can be inhibited by antibodies to integrin β2 or β7. J Gen Virol. 1999;80:1429–1436. doi: 10.1099/0022-1317-80-6-1429. [DOI] [PubMed] [Google Scholar]
- 9.de Parseval A, Lerner D L, Borrow P, Willett B J, Elder J H. Blocking of feline immunodeficiency virus infection by a monoclonal antibody to CD9 is via inhibition of virus release rather than interference with receptor binding. J Virol. 1997;71:5742–5749. doi: 10.1128/jvi.71.8.5742-5749.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duprex W P, Duffy I, McQuaid S, Hamill L, Cosby S L, Billeter M A, Schneider-Schaulies J, ter Meulen V, Rima B K. The H gene of rodent brain-adapted measles virus confers neurovirulence to the Edmonston vaccine strain. J Virol. 1999;73:6916–6922. doi: 10.1128/jvi.73.8.6916-6922.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duprex W P, McQuaid S, Hangartner L, Billeter M A, Rima B K. Observation of measles virus cell-to-cell spread in astrocytoma cells by using a green fluorescent protein-expressing recombinant virus. J Virol. 1999;73:9568–9575. doi: 10.1128/jvi.73.11.9568-9575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Firsching R, Buchholz C J, Schneider U, Cattaneo R, ter Meulen V, Schneider-Schaulies J. Measles virus spread by cell-cell contacts: uncoupling of contact-mediated receptor (CD46) downregulation from virus uptake. J Virol. 1999;73:5265–5273. doi: 10.1128/jvi.73.7.5265-5273.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horejsi V, Vlcek P. Novel structurally distinct family of leukocyte surface glycoproteins including CD9, CD37, CD53 and CD63. FEBS Lett. 1991;288:1–8. doi: 10.1016/0014-5793(91)80988-f. [DOI] [PubMed] [Google Scholar]
- 14.Imai T, Fukudome K, Takagi S, Nagira M, Furuse M, Fukuhara N, Nishimura M, Himuma Y, Yoshie O. C33 antigen recognized by monoclonal antibodies inhibitory to human T cell leukemia virus type 1-induced syncytium formation is a member of a new family of transmembrane proteins including CD9, CD37, CD53, and CD63. J Immunol. 1992;149:2879–2886. [PubMed] [Google Scholar]
- 15.Ito Y, Komada H, Kusagawa S, Tsurudome M, Matsumara H, Kawano M, Ohta H, Nishio M. Fusion regulations proteins on the cell surface: isolation and characterization of monoclonal antibodies which enhance giant polykarocyte formation in Newcastle disease virus-infected cell lines of human origin. J Virol. 1992;66:5999–6007. doi: 10.1128/jvi.66.10.5999-6007.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito Y, Tsurudome M, Hishiyama M. Induction of cell fusion in Newcastle disease virus-infected L929 cells by anti-L929 cell antisera. J Gen Virol. 1987;68:1261–1266. doi: 10.1099/0022-1317-68-5-1261. [DOI] [PubMed] [Google Scholar]
- 17.Jennings L K, Fox C F, Kouns W C, McKay C P, Ballou L R, Schultz H E. The activation of human platelets mediated by anti-human platelet p24/CD9 monoclonal antibodies. J Biol Chem. 1990;265:3815–3822. [PubMed] [Google Scholar]
- 18.Johnston I C D, ter Meulen V, Schneider-Schaulies J, Schneider-Schaulies S. A recombinant measles vaccine virus expressing wild-type glycoproteins: consequences for viral spread and cell tropism. J Virol. 1999;73:6903–6915. doi: 10.1128/jvi.73.8.6903-6915.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krakowka S, Higgins R J, Koestner A. Canine distemper virus: review of structural and functional modulations in lymphoid tissues. Am J Vet Res. 1980;41:284–292. [PubMed] [Google Scholar]
- 20.Lanza F, Wolf D, Fox C F, Kieffer N, Seyer J M, Fried V A, Coughlin S R, Phillips D R, Jennings L K. CDNA cloning and expression of platelet p24/CD9. Evidence for a new family of multiple membrane-spanning proteins. J Biol Chem. 1991;266:10638–10645. [PubMed] [Google Scholar]
- 21.Löffler S, Lottspeich F, Lanza F, Azorsa D O, ter Meulen V, Schneider-Schaulies J. CD9, a tetraspan transmembrane protein, renders cells susceptible to canine distemper virus. J Virol. 1997;71:42–49. doi: 10.1128/jvi.71.1.42-49.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mamaev L V, Visser I K, Belikov S I, Denikina N N, Harder T, Goatley L, Rima B, Edgington B, Osterhaus A D M E, Barrett T. Canine distemper virus in lake Baikal seals (Phoca siberica) Vet Rec. 1996;138:437–439. doi: 10.1136/vr.138.18.437. [DOI] [PubMed] [Google Scholar]
- 23.Masellis-Smith A, Shaw A R E. CD9-regulated adhesion. J Immunol. 1994;152:2768–2777. [PubMed] [Google Scholar]
- 24.McQuaid S, Campbell S, Wallace I J C, Kirk J, Cosby S L. Measles virus infection and replication in undifferentiated and differentiated human neuronal cells in culture. J Virol. 1998;72:5245–5250. doi: 10.1128/jvi.72.6.5245-5250.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meissner N, Koschel K. Downregulation of endothelin receptor mRNA synthesis in C6 rat astrocytoma cells by persistent measles virus and canine distemper virus infections. J Virol. 1995;69:5191–5194. doi: 10.1128/jvi.69.8.5191-5194.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norrby E. The effect of a carboxy tripeptide on the biological activities of measles virus. Virology. 1971;44:599–608. doi: 10.1016/0042-6822(71)90374-6. [DOI] [PubMed] [Google Scholar]
- 27.Ohgimoto S, Tabata N, Suga S, Nishio M, Ohta H, Tsurudome M, Komada H, Kawano M, Watanabe N, Ito Y. Molecular characterization of fusion regulatory protein-1 (FRP-1) that induces multinucleated giant cell formation of monocytes and HIV gp160-mediated cell fusion: FRP-1 and 4F2/CD98 are identical molecules. J Immunol. 1995;135:3585–3592. [PubMed] [Google Scholar]
- 28.Ohta H, Tsurudome M, Matsumura H, Koga Y, Morikawa S, Kawano M, Kusugawa S, Komada H, Nishio M, Ito Y. Molecular and biological characterization of fusion regulatory proteins (FRPs): anti-FRP mAb induced HIV-mediated cell fusion via an integrin system. EMBO J. 1994;13:2044–2055. doi: 10.1002/j.1460-2075.1994.tb06479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okamoto K, Tsurudome M, Ohgimoto S, Kawano M, Nishio M, Komada H, Ito M, Sakakura Y, Ito Y. An anti-fusion regulatory protein-1 monoclonal antibody suppresses human parainflueza virus type 2-induced cell fusion. J Gen Virol. 1997;78:83–89. doi: 10.1099/0022-1317-78-1-83. [DOI] [PubMed] [Google Scholar]
- 30.Ozaki Y, Matsumoto Y, Yatomi Y, Higashihara M, Kume S. Two-step mobilization of arachidonic acid in platelet activation induced by low concentrations of TP82, a monoclonal antibody against CD9 antigen. Eur J Biochem. 1990;199:347–354. doi: 10.1111/j.1432-1033.1991.tb16130.x. [DOI] [PubMed] [Google Scholar]
- 31.Ozaki Y, Satoh K, Kuroda K, Qi R, Yatomi Y, Yanagi S, Sada K, Yamamura H, Yanabu M, Nomura S, Kume S. Anti-CD9 monoclonal antibody activates p72syk in human platelets. J Biol Chem. 1995;270:15119–15124. doi: 10.1074/jbc.270.25.15119. [DOI] [PubMed] [Google Scholar]
- 32.Richardson C D, Choppin P W. Oligopeptides that specifically inhibit membrane fusion by paramyxoviruses: studies on the site of action. Virology. 1983;131:518–532. doi: 10.1016/0042-6822(83)90517-2. [DOI] [PubMed] [Google Scholar]
- 33.Rima B K, Baczko K, Imagawa D T, ter Meulen V. Humoral immune response in dogs with old dog encephalitis and chronic distemper meningo-encephalitis. J Gen Virol. 1987;68:1723–1735. doi: 10.1099/0022-1317-68-6-1723. [DOI] [PubMed] [Google Scholar]
- 34.Rubinstein E, Le Naour F, Billard M, Prenant M, Boucheix C. CD9 antigen is an accessory subunit of the VLA integrin complexes. Eur J Immunol. 1994;24:3005–3013. doi: 10.1002/eji.1830241213. [DOI] [PubMed] [Google Scholar]
- 35.Schneider-Schaulies J, Schnorr J-J, Brinckmann U, Dunster L M, Baczko K, Liebert U G, Schneider-Schaulies S, ter Meulen V. Receptor usage and differential downregulation of CD46 by measles virus wild type and vaccine strains. Proc Natl Acad Sci USA. 1995;92:3943–3947. doi: 10.1073/pnas.92.9.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seehafer J G, Shaw A R. Evidence that the signal-initiating membrane protein CD9 is associated with small GTP-binding proteins. Biochem Biophys Res Commun. 1991;179:401–406. doi: 10.1016/0006-291x(91)91384-o. [DOI] [PubMed] [Google Scholar]
- 37.Slupsky J R, Seehafer J G, Tang S C, Masellis-Smith A, Shaw A R E. Evidence that monoclonal antibodies against CD9 antigen induce specific association between CD9 and the glycoprotein IIb-IIIa complex. Biol Chem. 1989;264:12289–12293. [PubMed] [Google Scholar]
- 38.Spetz A L, Patterson B K, Lore K, Andersson J, Holmgren L. Functional gene transfer of HIV DNA by an HIV receptor-independent mechanism. J Immunol. 1999;163:736–742. [PubMed] [Google Scholar]
- 39.Stern L B-L, Greenberg M, Gershoni J M, Rozenblatt S. The hemagglutinin envelope protein of canine distemper virus (CDV) confers cell tropism as illustrated by CDV and measles virus complementation analysis. J Virol. 1995;69:1661–1668. doi: 10.1128/jvi.69.3.1661-1668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Summers B A, Appel M J. Aspects of canine distemper virus and measles virus encephalomyelitis. Neuropathol Appl Neurobiol. 1994;20:525–534. doi: 10.1111/j.1365-2990.1994.tb01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tabata N, Ido M, Suga S, Ohgimoto S, Tsurudome M, Kawano M, Nishio M, Watanabe N, Okamoto K, Komada H, Sakurai M, Ito Y. Protein tyrosine kinase activation provides an early and obligatory signal in anti-FRP-1/CD98/4F2 monoclonal antibody induced cell fusion mediated by HIV gp160. Med Microbiol Immunol Berl. 1998;186:115–123. doi: 10.1007/s004300050053. [DOI] [PubMed] [Google Scholar]
- 42.Urbanska E M, Chambers B J, Ljunggren H G, Norrby E, Kristensson K. Spread of measles virus through axonal pathways into limbic structures in the brain of TAP −/− mice. J Med Virol. 1997;52:362–369. doi: 10.1002/(sici)1096-9071(199708)52:4<362::aid-jmv3>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 43.Vandevelde M, Zurbriggen A. The neurobiology of canine distemper virus infection. Vet Microbiol. 1995;44:271–280. doi: 10.1016/0378-1135(95)00021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Willett B J, Hosie M J, Jarrett O, Neil J C. Identification of a putative cellular receptor for feline immunodeficiency virus as the feline homologue of CD9. Immunology. 1994;81:228–233. [PMC free article] [PubMed] [Google Scholar]
- 45.Willett B J, Hosie M J, Shaw A, Neil J C. Inhibition of feline immunodeficiency virus infection by CD9 antibody operates after virus entry and is independent of virus tropism. J Gen Virol. 1997;78:611–618. doi: 10.1099/0022-1317-78-3-611. [DOI] [PubMed] [Google Scholar]
- 46.Wisniewski H, Raine C S, Kay W J. Observations on viral demyelinating encephalomyelitis. Canine distemper. Lab Investig. 1972;26:589–599. [PubMed] [Google Scholar]
- 47.Yatomi Y, Higashihara M, Ozaki Y, Kume S, Kurokawa K. Intracellular ionized calcium mobilization of CD9 monoclonal antibody-activated human platelets. Biochem Biophys Res Commun. 1990;171:109–115. doi: 10.1016/0006-291x(90)91363-w. [DOI] [PubMed] [Google Scholar]
- 48.Yatomi Y, Ozaki Y, Satoh K, Kume S. Anti-CD9 monoclonal antibody elicits staurosporin inhibitable phosphatidyl 4,5-bisphosphate hydrolysis, phosphatidylinositol 3,4-bisphosphate synthesis, and protein-tyrosine phosphorylation in human platelets. FEBS Lett. 1993;322:285–290. doi: 10.1016/0014-5793(93)81587-p. [DOI] [PubMed] [Google Scholar]
- 49.Zurbriggen A, Graber H U, Wagner A, Vandevelde M. Canine distemper virus persistence in the nervous system is associated with noncytolytic selective virus spread. J Virol. 1995;69:1678–1686. doi: 10.1128/jvi.69.3.1678-1686.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]