Abstract
Background
There is no clear evidence on the comparative effectiveness of bone-marrow mononuclear cell (BMMNC) vs. mesenchymal stromal cell (MSC) stem cell therapy in patients with chronic heart failure (HF).
Methods
Using a systematic approach, eligible randomized controlled trials (RCTs) of stem cell therapy (BMMNCs or MSCs) in patients with HF were retrieved to perform a meta-analysis on clinical outcomes (major adverse cardiovascular events (MACE), hospitalization for HF, and mortality) and echocardiographic indices (including left ventricular ejection fraction (LVEF)) were performed using the random-effects model. A risk ratio (RR) or mean difference (MD) with corresponding 95% confidence interval (CI) were pooled based on the type of the outcome and subgroup analysis was performed to evaluate the potential differences between the types of cells.
Results
The analysis included a total of 36 RCTs (1549 HF patients receiving stem cells and 1252 patients in the control group). Transplantation of both types of cells in patients with HF resulted in a significant improvement in LVEF (BMMNCs: MD (95% CI) = 3.05 (1.11; 4.99) and MSCs: MD (95% CI) = 2.82 (1.19; 4.45), between-subgroup p = 0.86). Stem cell therapy did not lead to a significant change in the risk of MACE (MD (95% CI) = 0.83 (0.67; 1.06), BMMNCs: RR (95% CI) = 0.59 (0.31; 1.13) and MSCs: RR (95% CI) = 0.91 (0.70; 1.19), between-subgroup p = 0.12). There was a marginally decreased risk of all-cause death (MD (95% CI) = 0.82 (0.68; 0.99)) and rehospitalization (MD (95% CI) = 0.77 (0.61; 0.98)) with no difference among the cell types (p > 0.05).
Conclusion
Both types of stem cells are effective in improving LVEF in patients with heart failure without any noticeable difference between the cells. Transplantation of the stem cells could not decrease the risk of major adverse cardiovascular events compared with controls. Future trials should primarily focus on the impact of stem cell transplantation on clinical outcomes of HF patients to verify or refute the findings of this study.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13287-024-03829-7.
Keywords: Chronic heart failure, Stem cell therapy, Mesenchymal stem cells, Bone-marrow mononuclear cells
Background
Heart failure represents a significant global health burden, affecting a substantial proportion of the worldwide population [1]. Although significant progress has been made in the development of pharmacological interventions [2] and implantable cardiac devices [3, 4], the overall clinical outcomes for patients diagnosed with heart failure remain suboptimal, underscoring the need to investigate innovative therapeutic modalities. Stem cell-based therapies are novel approaches with the potential to improve the morbidity and mortality associated with heart failure [5]. It is believed that stem cell transplantation can result in higher regional blood flow, angiogenesis, and improved cardiac function, which is mediated by increased paracrine signaling pathways obtained from higher expression of interleukin-1β (IL-1β), tissue necrosis factor-α (TNF-α), and vascular endothelial growth factor (VEGF) [6–8]. Many types of stem cells have been frequently used in the past few years to improve the clinical outcomes of patients with heart failure (HF). Mesenchymal stromal cells (MSCs) [9] and bone marrow-derived mononuclear cells (BMMNCs) [10] transplantation have both emerged as possibly important therapies for HF patients due to their potential for cardiac repair. MSCs, multipotent stromal cells that can differentiate into a variety of cell types, including cardiomyocytes, have shown potential in preclinical and clinical studies [11]. Using the current criteria for isolation, MSCs produce heterogenous, non-clonal cultures, comprising stromal cells with varying multipotential capabilities, along with committed progenitors and differentiated cells. These cultures demonstrate a wide range of differentiation potentials resulting in a diverse array of cell types within the cultures [12, 13]. On the other hand, BMMNCs, a heterogeneous population of cells, including hematopoietic stem cells and endothelial progenitor cells, have also demonstrated beneficial effects on cardiac function and structure [14, 15]. Although a previous meta-analysis has compared BMMNCs with MSCs in patients with acute myocardial infarction, a clear comparison between the two types of therapies in chronic heart failure has not been established. This meta-analysis was conducted to determine and compare the cardiovascular outcomes and echocardiographic indices of MSCs and BMMNCs therapies in heart failure.
Methods
This systematic review was reported based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [16]. Prospective protocol registration was done with the registration ID of CRD42024504239.
Search sources and strategies
An extensive search of the literature was carried out in three different online databases including PubMed, Scopus, and Embase Library to find the eligible studies published from database inception up until February 20th, 2024. No time frame or restriction was placed on the search results. The search strategy of our study included the relevant keywords mentioned in Table S1.
Study selection and risk of bias
First, the duplicate records were removed and they were subsequently imported into the Rayyan web-based tool for managing systematic reviews [17]. Two reviewers (ND and SM) autonomously assessed the records based on their titles and abstracts. Subsequently, full texts were obtained for each study to undergo screening based on the eligibility criteria. Discrepancies were addressed through discussion with a third author (AH) until consensus was reached. Eligible studies were required to meet the following criteria: (a) randomized controlled trials (RCTs), (b) patients with chronic heart failure or cardiomyopathy (ischemic or non-ischemic), (c) administration of BMMNCs or MSCs in at least one trial arm, (d) presence of one or more control arms who were treated with standard therapy with or without placebo injection, and (e) reporting clinical outcomes or echocardiographic indices. Studies with potential overlapping population were identified and the study with the larger sample size was included.
For the present study, the primary outcome of interest was major adverse cardiovascular events (MACE), all-cause mortality, and hospitalization for heart failure at the longest available follow-up. The secondary outcomes were echocardiographic indices (left ventricular ejection fraction (LVEF), left ventricular end-diastolic volume (LVEDV), and left ventricular end-systolic volume (LVESV)), 6-min walk test (6MWT), and B-type natriuretic peptide (BNP).
The reviewers (ND, SM) extracted data into a predefined form within Microsoft Excel Spreadsheet Software. A third author (AH) cross-checked the data accuracy and resolved any extraction discrepancies through discussion. For each study, the following data were extracted: (a) study characteristics (first author, publication year, trial name, and country), (b) subject characteristics (sample size, type of heart failure, and baseline demographics of treatment and control group), (c) intervention specifics (dosage and type of stem cells administered), (d) clinical outcomes (MACE, all-cause mortality, and rehospitalization), (e) echocardiographic indices (baseline, final measurements, changes from baseline during follow-up period) including LVEF, LVEDV, and LVESV, and (f) the baseline and follow-up values of 6MWT and BNP. The clinical outcomes and echocardiographic indices were extracted at the longest available follow-up.
The quality assessment of the eligible studies was conducted using the Cochrane Collaboration's tool for evaluating bias in randomized trials [18]. The included RCTs each underwent quality assessment and were assigned to categories of high, low, or some concerns risk of bias within various domains. A total risk of bias was then assigned to each of the studies based on the risk of bias within each domains. Risk of Bias plots were generated using “Risk-of-bias visualization (robvis)” R package [19].
Data synthesis and ICEMAN tool assessment
The analyses performed for the present study was undertaken in R software version 4.3.2 with “meta” and “metaphor” packages being used. For binary outcomes, the number of events and total sample size were used to perform the analysis and generate a risk ratio (RR) and its corresponding 95% confidence interval (CI) with Mantel–Haenszel method being used. For continuous outcomes, based on the pre-post intervention data, a mean difference (MD) and standard error of the MD were calculated and inserted for the analysis with inverse variance method. We used both BNP and N-terminal proBNP (NT-proBNP) levels and since there is difference in measurement scales between the two parameters, we used standardized mean difference (SMD) to pool the results. For both types of outcomes, the random effects model was used. The outcomes were analyzed for both an overall effect and subgroup difference. The studies were stratified based on the type of the stem cell used (BMMNC vs. MSC) to test for any potential difference between the subgroups in all the analyses. A subgroup analysis was also conducted to investigate the difference between the routes of injection. For better evaluation of potential differences between the two types of stem cells, we used Instrument to assess the Credibility of Effect Modification Analyses (ICEMAN) in meta-analysis of randomized controlled trials. It is consisted of a total of eight questions to assess the credibility of the results and for each outcome, a total rating is given based on the answers to each questions [20]. The p-values were reported for the overall effect and subgroup differences. The statistical significance was met if the pooled estimate did not cross the null zone.
Results
Characteristics of the included studies
A total of 20 randomized controlled trials involving BMMNCs (622 participants receiving stem cell and 495 controls) [14, 21–39] and 17 trials [24, 40–55] with MSCs (927 receiving stem cells and 757 controls) were considered eligible for meta-analysis. The search of Scopus, PubMed, and Embase library yielded a total of 7316 citations. After adjusting for duplicates, 5393 records remained. Upon abstract review and screening, it was determined that 5147 of these studies did not fulfill the established criteria and were consequently excluded. The full-text of the remaining 246 citations underwent a detailed examination. It was determined that 211 studies did not meet the inclusion criteria as described and were subsequently discarded.
The final selection for the systematic review and meta-analysis comprised 36 studies (one study including data for both MSCs and BMMNCs [24]), all of which were randomized controlled trials (Fig. 1). In the included MSCs studies, the origin of the cells was diverse: Seven studies [24, 41–43, 45, 46, 53] utilized autologous bone marrow, while four studies [44, 47, 48, 54] employed allogenic bone marrow. MSCs derived from adipose tissue were used in three studies [49–51], and three studies used allogenic MSCs from umbilical cord [40, 52, 55]. The sample size ranged from 16 to 537 patients with HF. The lowest and highest LVEF among groups were 16.2 ± 6.0 and 54.0 ± 8.0, respectively. Table 1 provides an overview of the data pertaining to each individual study. This includes information such as the countries where the studies were conducted, the sample sizes used in each study, and the mean age range of the participants. Additionally, it also details the type of cells used in each study, among other relevant factors.
Fig. 1.
PRISMA flowchart demonstrating the search and screening process
Table 1.
General characteristics of the included trials
| Study | Country | Type of cell | Sample size | Mean age | % of Male | Baseline LVEF | Route of delivery | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inv | Ctrl | Inv | Ctrl | Inv | Ctrl | Inv | Ctrl | |||||
| Assmus [14] | Germany | BMMNCs | 62 | 39 | 61.0 ± 12.2 | 61.5 ± 9.9 | 82 | 85 | 34.4 ± 10.1 | 31.1 ± 12.2 | Intracoronary | |
| Choudhury [21] | United Kingdom | BMMNCs | 30 | 30 | 63.7 ± 9.5 | 61.6 ± 10.8 | 97 | 97 | 30.2 ± 8.9 | 30.3 ± 7.7 | Intracoronary or Intramyocardial | |
| Duan [22] | USA | BMMNCs | 24 | 18 | 57.9 ± 8.5 | 56.6 ± 9.1 | 96 | 94 | ND | ND | During CABG | |
| Hamshere [23] | United Kingdom | BMMNCs | 15 | 15 | 57.7 ± 12.3 | 54.9 ± 10.9 | 67 | 60 | 32.9 ± 16.5 | 41.7 ± 15.3 | Intracoronary | |
| Heldman [24] | USA | BMMNCs | 19 | 10 | 61.1 ± 8.4 | 61.3 ± 9.0 | 90 | 100 | 35.9 ± 8.2 | 36.2 ± 7.4 | Transendocardial | |
| Hu [25] | USA | BMMNCs | 31 | 29 | 56.6 ± 9.7 | 58.3 ± 8.9 | 93 | 23.3 ± 6.0 | 24.9 ± 6.6 | During CABG | ||
| Lehtinen [26] | Finland | BMMNCs | 20 | 19 | 69.3 ± 9.9 | 69.0 ± 11.2 | ND | ND | 38.5 ± 9.9 | 37.7 ± 14.8 | Intramyocardial | |
| Mann [27] | Netherlands | BMMNCs | 19 | 20 | 65.0 ± 7.0 | 65.0 ± 8.0 | 100 | 95 | 32.6 ± 12.3 | 29.0 ± 9.7 | Intramyocardial | |
| Martino [28] | Brazil | BMMNCs | 82 | 78 | 51.0 ± 11.1 | 49.6 ± 11.1 | 73 | 68 | 24 ± 10.8 | 24.3 ± 9.9 | Intracoronary | |
| Mozid [29] | United Kingdom | BMMNCs | 34 | 15 | 66.8 ± 9.8 | 66.0 ± 9.0 | 97 | 93 | 28.2 ± 7.0 | 28.0 ± 8.0 | Intracoronary and intramyocardial | |
| Pätilä [30] | Finland | BMMNCs | 20 | 19 | 65.0 ± 12.8 | 64.0 ± 9.6 | 95 | 95 | 36.9 ± 12.6 | 36.0 ± 13.1 | Intramyocardial | |
| Perin [31] | USA | BMMNCs | 20 | 10 | 60.5 ± 6.4 | 56.3 ± 8.6 | 80 | 50 | 37.3 ± 10.6 | 39.0 ± 9.1 | Transendocardial | |
| Perin [32] | USA | BMMNCs | 61 | 31 | 63.9 ± 10.9 | 62.3 ± 8.3 | 87 | 94 | 32.4 ± 9.2 | 30.2 ± 7.8 | Transendocardial | |
| Pokushalov [33] | Russia | BMMNCs | 55 | 54 | 61.0 ± 9.0 | 2.0 ± 5.0 | 87 | 85 | 27.8 ± 3.4 | 26.8 ± 3.8 | Intramyocardial | |
| Sant'Anna [34] | Brazil | BMMNCs | 20 | 10 | 48.3 ± 8.7 | 51.6 ± 7.8 | 65 | 50 | 21.8 ± 41.2 | 24.8 ± 4.6 | Intramyocardial | |
| Santoso [35] | Hong kong and indonesia | BMMNCs | 19 | 9 | 58.0 ± 9.9 | 60.0 ± 5.6 | 95 | 100 | 23.6 ± 8.4 | 26.8 ± 8.8 | Intramyocardial | |
| Seth [36] | India | BMMNCs | 41 | 40 | 45.0 ± 15.0 | 49.0 ± 9.0 | 81 | 88 | 22.5 ± 8.3 | 20.8 ± 9.3 | Intracoronary | |
| Trifunović [39] | Serbia | BMMNCs | 15 | 15 | 53.8 ± 10.1 | 60.0 ± 6.8 | 93 | 93 | 35.3 ± 3.9 | 36.5 ± 5.3 | Intramyocardial during CABG | |
| Wang [37] | China | BMMNCs | 17 | 16 | 65.6 ± 4.0 | 65.5 ± 5.6 | 45 | 45 | 34.0 ± 4.5 | 34.8 ± 2.9 | Intramyocardial | |
| Zhao [38] | China | BMMNCs | 18 | 18 | 60.3 ± 10.4 | 59.1 ± 15.7 | 83 | 83 | 35.8 ± 7.3 | 36.7 ± 9.2 | Intramyocardial | |
| Bartolucci [40] | Chile | MSCs | 15 | 15 | 57.3 ± 10.1 | 57.2 ± 11.6 | 80 | 93 | 33.5 ± 6.1 | 31.5 ± 4.9 | Intravenous | |
| Bartunek [41] | Belgium | MSCs | 21 | 15 | 55.7 ± 10.4 | 59.5 ± 8.0 | 95 | 92 | 27.5 ± 4.7 | 27.8 ± 4.0 | Intramyocardial | |
| Bartunek [42] | Belgium | MSCs | 120 | 151 | 61.6 ± 8.6 | 62.1 ± 8.7 | 89 | 90 | 27.3 ± 6.8 | 28.0 ± 6.0 | Intramyocardial | |
| Bolli [43] | USA | MSCs | 14 | 17 | 54.7 ± 12.8 | 58.2 ± 11.2 | 43 | 24 | 34.5 ± 2.9 | 33.3 ± 6.2 | Transendocardial | |
| Bolli [44] | USA | MSCs | 29 | 32 | 61.0 ± 11.1 | 63.1 ± 8.8 | 93 | 97 | 29.3 ± 5.9 | 29.7 ± 6.2 | Transendocardial | |
| Mathiasen [45] | Denmark | MSCs | 40 | 20 | 66.1 ± 7.7 | 64.2 ± 10.6 | 90 | 70 | 28.2 ± 9.3 | 25.1 ± 8.5 | Intramyocardial | |
| Mohyeddin [46] | Iran | MSCs | 8 | 8 | 49.0 ± 9.4 | 53.3 ± 6.7 | 88 | 75 | 38.8 ± 13.0 | 41.9 ± 8.4 | Intracoronary and Intramyocardial | |
| Heldman [24] | USA | MSCs | 19 | 11 | 57.1 ± 10.6 | 60.0 ± 12.0 | 95 | 91 | 35.7 ± 9.0 | 28.1 ± 9.8 | Transendocardial | |
| Perin [48] | USA | MSCs | 45 | 15 | 62.2 ± 10.3 | 62.7 ± 11.2 | 98 | 73 | 31.3 ± 8.6 | 34.6 ± 6.4 | Transendocardial | |
| Perin [47] | USA | MSCs | 265 | 272 | 62.7 ± 10.9 | 62.6 ± 10.4 | 78 | 78 | 28.6 ± 6.6 | 28.6 ± 7.0 | Transendocardial | |
| Qayyum [49] | Denmark | MSCs | 28 | 13 | 65.5 ± 9.7 | 65.3 ± 8.7 | 88 | 100 | 52.0 ± 8.0 | 54.0 ± 8.0 | Intramyocardial | |
| Qayyum [50] | Denmark | MSCs | 54 | 27 | 67.0 ± 9.0 | 66.6 ± 8.1 | 82 | 89 | 34.2 ± 7.9 | 31.4 ± 7.2 | Intramyocardial | |
| Qayyum [51] | Denmark | MSCs | 90 | 43 | 66.4 ± 8.1 | 64.0 ± 8.8 | 93 | 88 | 31.6 ± 7.2 | 32 ± 8.9 | Intramyocardial | |
| Ulus [52] | Turkey | MSCs | 26 | 16 | 61.8 ± 10.0 | 65.30 ± 6.8 | 100 | 100 | 34.8 ± 4.8 | 36.2 ± 5.6 | Intramyocardial | |
| Xiao [53] | China | MSCs | 17 | 20 | 51.6 ± 12.2 | 54.4 ± 11.6 | 71 | 70 | 34.1 ± 3.6 | 33.7 ± 4.0 | Intracoronary | |
| Yau [54] | Canada And USA | MSCs | 106 | 53 | 55.5 ± 12.3 | 56.9 ± 11.7 | 89 | 89 | 17.3 ± 5.8 | 16.2 ± 6.0 | Intramyocardial | |
| Zhao [55] | China | MSCs | 30 | 29 | 52.9 ± 16.3 | 53.2 ± 11.5 | 80 | 65 | 30.0 ± 4.5 | 28.0 ± 5.1 | Intracoronary | |
BMMNCs: bone-marrow mononuclear cells, MSCs: mesenchymal stem cells, Inv: intervention, Ctrl: control, LVEF: left ventricular ejection fraction, CABG: coronary artery bypass grafting
Risk of bias
Utilizing the Cochrane Collaboration’s tool for Risk of bias (ROB) assessment, a diverse range of bias risk across the evaluated studies was revealed. On average, nearly 44% of the studies were classified as having a low risk of bias. Approximately 28% of the studies fell into the category of “Some Concerns” risk of bias and 28% of the studies were identified as having a high risk of bias. A total of thirteen studies provided a comprehensive description of all domains as per the Cochrane Collaboration’s tool. Figure 2 and Figure S1 provide visual representations of ROB assessment of each study in every domain.
Fig. 2.
Risk of bias assessment
Clinical outcomes following stem cell therapy
A total of 33 studies (1402 patients in the stem cell group and 1166 patients in the control group) reported data regarding mortality. Pooled estimate showed a 18% decrease in the risk of all-cause mortality in the stem cell group compared with the controls (RR (95% CI) = 0.82 (0.68, 0.99), p = 0.04). Although the RR of mortality was marginally significant, BMMNC and MSC therapy could not decrease the risk of mortality in the longest follow-up period (BMMNC: RR (95% CI) = 0.84 (0.54, 1.32), MSC: RR (95% CI) = 0.83 (0.69, 1.01), between-subgroup p = 0.96). Stem cell therapy could not change the risk of long-term MACE compared to the controls (RR (95% CI) = 0.83 (0.64, 1.06), p = 0.13) and the subgroups were similar regarding risk reduction of MACE (BMMNC: RR (95% CI) = 0.59 (0.31, 1.13), MSC: RR (95% CI) = 0.91 (0.70, 1.19), between-subgroup p = 0.12). The transplantation of stem cells resulted in a marginally significant decrease in the risk of rehospitalization in long-term follow-up (RR (95% CI) = 0.77 (0.61, 0.98), p = 0.04) with no difference between subgroups (p = 0.68) (Fig. 3).
Fig. 3.
Forest plot demonstrating the comparison of stem cell transplantation compared with placebo stratified by the type of cell A: MACE, B: all-cause mortality, and C: rehospitalization for heart failure (MACE: major adverse cardiovascular events, RR: risk ratio, CI: confidence interval, BMMNC: bone-marrow mononuclear cell, MSC: mesenchymal stem cell)
Echocardiographic parameters
After inclusion of 17 studies performing BMMNC therapy and 16 studies with MSC transplantation, stem cell transplantation resulted in a significant increase in LVEF compared with the control group (MD (95% CI) = 2.94% (1.71, 4.17), p < 0.001). Both subgroups of BMMNCs and MSCs were effective in increasing LVEF although there was not a statistically significant difference between the subgroups (BMMNC: MD (95% CI) = 3.05% (1.11, 4.99), MSC: MD (95% CI) = 2.82% (1.19, 4.45), p = 0.86). There was no statistically significant difference in LVEDV change following stem cell therapy compared with the control group (MD (95% CI) = -4.11 (-10.35, 2.12), p = 0.20) with no difference across subgroups (p = 0.71). Regarding the change in LVESV, transplantation of stem cells was concomitant with a statistically significant decrease in LVESV (MD (95% CI) = -8.02 (-13.24, -2.80), p < 0.001) mainly driven from MSC therapy rather than BMMNC transplantation (BMMNC: MD (95% CI) = -9.16 (-18.98, 0.66), MSC: MD (95% CI) = -8.57 (-13.44, -3.71), p = 0.92) (Fig. 4).
Fig. 4.
Forest plot demonstrating the comparison of stem cell transplantation compared with placebo stratified by the type of cell A: LVEF, B: LVEDV, and C: LVESV (LVEF: left ventricular ejection fraction, LVEDV: left ventricular end-diastolic volume, LVESV: left ventricular end-systolic volume, MD: mean difference, CI: confidence interval, BMMNC: bone-marrow mononuclear cell, MSC: mesenchymal stem cell)
6-min walk test and brain natriuretic peptide
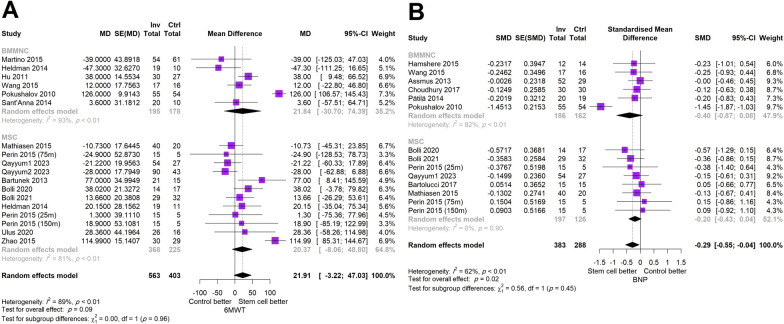
The results of the 6MWT from 18 studies were pooled and the estimate showed no statistically significant difference between stem cells and control group (MD (95% CI) = 21.91 (-3.22; 47.03), p = 0.09). No noticeable difference was also observed among the two types of stem cells (BMMNC: MD (95% CI) = 21.84 (-30.70, 74.39), MSC: MD (95% CI) = 20.37 (-8.06, 48.80), p = 0.96). After pooling the results of 14 studies reporting the changes in BNP, stem cell therapy led to a significant decrease in BNP levels compared with placebo (SMD (95% CI) = -0.29 (-0.55; -0.04), p = 0.02) although no difference was shown between BMMNCs (SMD (95% CI) = -0.40 (-0.87; 0.08)) and MSCs (SMD (95% CI) = -0.20 (-0.43; 0.04)) (Fig. 5).
Fig. 5.
Forest plot demonstrating the comparison of stem cell transplantation compared with placebo stratified by the type of cell A: 6MWT and B: BNP (6MWT: 6-min walk test, BNP: B-type natriuretic peptide, SMD: standardized mean difference, MD: mean difference, CI: confidence interval, BMMNC: bone-marrow mononuclear cell, MSC: mesenchymal stem cell)
Route of stem cell delivery
The routes of injection included intracoronary, intramyocardial, through graft vessel during coronary artery bypass grafting (CABG), transendocardial, and intravenous. Regarding LVEF, all of the injection routes including intracoronary, intramyocardial, and transendocardial could improve the ventricular function significantly. In terms of MACE, the transendocardial injection of stem cells was superior compared with other cell types. For other outcomes, there was no considerable difference among subgroups (Figure S2-S9).
ICEMAN credibility assessment
According to Table 2, all the included variables were assessed by the ICEMAN instrument. Except for one of the studied parameters (LVESV), the endpoints of interest were unlikely to have different magnitude of effect across the two subgroups (BMMNC and MSC) and therefore, they were rated as “likely no effect modification”. For LVESV, the MSC subgroup showed superior results compared with BMMNC as BMMNCs could not demonstrated a statistically significant change in LVESV compared with their controls.
Table 2.
ICEMAN criteria for meta-analysis of randomized controlled trials
| Variable | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Overall rating | Interpretation |
|---|---|---|---|---|---|---|---|---|---|---|
| MACE | Completely between | NA | Rather large | Unclear | Chance a very likely explanation (0.12) | Definitely yes | Definitely yes | NA | Maximum usually low | Likely no effect modification. Use overall effect for each subgroup but note remaining uncertainty |
| All-cause mortality | Completely between | NA | Large | Unclear | Chance a very likely explanation (0.96) | Definitely yes | Definitely yes | NA | Maximum usually low | Likely no effect modification. Use overall effect for each subgroup but note remaining uncertainty |
| Rehospitalization | Completely between | NA | Rather large | Unclear | Chance a very likely explanation (0.68) | Definitely yes | Definitely yes | NA | Maximum usually low | Likely no effect modification. Use overall effect for each subgroup but note remaining uncertainty |
| LVEF | Completely between | NA | Large | Unclear | Chance a very likely explanation (0.86) | Definitely yes | Definitely yes | NA | Maximum usually low | Likely no effect modification. Use overall effect for each subgroup but note remaining uncertainty |
| LVEDV | Completely between | NA | Large | Unclear | Chance a very likely explanation (0.71) | Definitely yes | Definitely yes | NA | Maximum usually low | Likely no effect modification. Use overall effect for each subgroup but note remaining uncertainty |
| LVESV | Completely between | NA | Large | Definitely yes | Chance a very likely explanation (0.92) | Definitely yes | Definitely yes | NA | Maximum usually moderate | Likely effect modification. Use separate effects for each subgroup but note remaining uncertainty |
| 6MWT | Completely between | NA | Rather large | Probably yes | Chance a very likely explanation (0.96) | Definitely yes | Definitely yes | NA | Maximum usually low | Likely no effect modification. Use overall effect for each subgroup but note remaining uncertainty |
| BNP | Completely between | NA | Rather large | Probably yes | Chance a very likely explanation (0.45) | Definitely yes | Definitely yes | NA | Maximum usually low | Likely no effect modification. Use overall effect for each subgroup but note remaining uncertainty |
Q1, Is the analysis of effect modification based on comparison within rather than between trials? Q2, for within-trial comparisons, is the effect modification similar from trial to trial? Q3, for between-trial comparisons, is the number of trials large? Q4, Was the direction of the effect modification correctly hypothesized priori? Q5, does a test for interaction suggest that chance is an unlikely explanation of the apparent effect modification? Q6, Did the authors test only a small number of effect modifiers? Q7, Did the authors use a random effects model? Q8, If the effect modifier is a continuous variable, were arbitrary cut points avoided?
Discussion
In this systematic review and meta-analysis, which included 35 clinical trials, effectiveness of stem cell transplantation therapy using mesenchymal stem cells (MSCs) or bone marrow-derived mononuclear cells (BMMNCs) in heart failure patients was assessed and compared using different clinical outcomes and echocardiographic indices. The primary findings of this meta-analysis are as follows: (1) The combined effect from these clinical trials supports the hypothesis that both MSC and BMMNC therapy are effective in increasing LVEF, with estimates slightly favoring BMMNCs (MD of 3.05% vs 2.82%), however there is no statistically significant difference between the effects of the interventions (p = 0.86). (2) The improvement in LVEF was not translated to superior results of stem cells compared with placebo regarding MACE although a marginally significant decrease in the risk of all-cause mortality and rehospitalization was noted compared with placebo. (3) Other secondary outcomes and indices extracted from the studies were also pooled to make a better comparison between the efficacy of MSCs and BMMNCs, however no statistical difference was observed between MSCs and BMMNCs groups in any of the indices (p-value of interaction ranging from 0.12 to 0.96). Based on our current understanding, although there have been meta-analyses which compared these cell types as a part of their subgroup analysis [56], analyses performed may have been underpowered, with few studies in each group. Subsequently, this meta-analysis represents a more inclusive investigation comparing the impact of these two distinct cell therapies in patients suffering from heart failure. Our results demonstrated that although the stem cells may result in a significant increase in LVEF, it may not cause a decrease in the rates of long-term MACE and future randomized trials powered to assess clinical outcomes are needed to evaluate the efficacy of stem cells compared with placebo in HF patients.
Prior studies have shown that MSC treatment exhibits remarkable efficacy in enhancing echocardiographic parameters among patients with acute myocardial infarction [57]. Similarly, BMMNC treatment has demonstrated comparable effects, showing promise as a viable therapeutic approach for patients with heart failure [58]. A meta-analysis done by Kalou et al. [59], was conducted to determine the efficacy of MSCs for heart failure treatment. Results from that study showed that treatment with MSCs resulted in a significant improvement in LVEF, with an increase of 4.43% when compared to the control groups [59]. Another systematic review and meta-analysis done by Fan et al. [60], made a pooled analysis of several indices to investigate efficacy of MSCs therapy in systolic heart failure. According to their study, MSCs therapy increased LVEF by 5.25% compared to the placebo group. The TAC-HFT trial was a randomized study including a direct comparison of MSCs with BMMNCs showing better function of MSCs in terms of reducing the infarct size and improving regional myocardial infarction compared with BMMNCs and placebo but no difference regarding LVEF change[24]. Regarding the subject of LVEF, the findings of these studies align with the results of our meta-analysis. An interesting finding in our analysis was that although both cells were proved to increase LVEF, there was no superior type of cell and this was further confirmed with assessing the credibility of the results using ICEMAN tool. It should be mentioned that a meta-analysis comparing MSCs and BMMNCs showed that MSCs may have better efficacy regarding LVEF in the setting of acute myocardial infarction [61] but according to this study, this was not the case for patients with chronic heart failure.
The findings from our meta-analysis suggest that treatment with MSCs and BM-MNCs is linked to a notable enhancement in LVEF and LVESV, as pooled mean differences of LVEF and LVESV both excluded the null zone and were statistically significant regardless of the type of cell therapy. However, overall mean difference of LVEDV was not statistically significant in any of the subgroups. A systematic review and meta-analysis carried out by Wang et al. [62], also found similar outcomes for these three echocardiographic parameters following stem cell transplantation. This consistency of results further substantiates the notion that, for reasons yet to be fully understood and further investigated, the transplantation of MSCs or BM-MNCs appears to improve ventricular function by increasing LVEF and reducing LVESV without having any considerable effect on LVEDV.
An earlier meta-analysis conducted by Fisher et al. concluded that cell therapy could decrease the risk of all-cause mortality with a medium grade efficacy [56]. However, our analysis does not support the previous finding. The combined risk ratio of all cell therapies (RR 0.82, 95% CI 0.68 to 0.99) for all-cause mortality was statistically significant and excluded the null zone but indicated a small grade efficacy that is borderline trivial. On the other hand, the combined effect size of BMMNCs (RR 0.84, 95% CI 0.54 to 1.32) and MSCs therapy (RR 0.83, 95% CI 0.69 to 1.01) suggests that the impact of this intervention on all-cause mortality is not even statistically significant. This rather considerable discrepancy between our study and Fisher et al., might be due to the larger number of participants in our study.
The pooled analysis on major adverse cardiac events (MACE) rates yielded no statistically significant difference between any of the cell therapy groups and the placebo, nor was there a significant difference among the cell therapy groups themselves. However, looking at the combined effect size from all studies, stem cell therapy can have a marginal effect in reducing the rate of rehospitalization (RR 0.77, 95% CI 0.61 to 0.98) in patients receiving it compared to placebo. Also, the pooled estimate showed a marginally significant risk reduction of all-cause death by 18% compared with placebo. This is the first meta-analysis comparing the clinical outcomes between MSCs and BMMNCs using randomized trials. Our results are consistent with a previous meta-analysis that showed MSCs could not reduce the risk of adverse clinical events[60]. The mentioned study showed better outcomes regarding readmission in comparison to the control group after inclusion of five trials. It is noteworthy that our analysis included 12 studies in the MSC subgroup which may have yielded more reliable results compared to previous ones and showed no difference efficacy compared with placebo. Furthermore, although the contemporary evidence has shown no superior efficacy of stem cells compared with placebo in HF patients, the majority of the trials were not powered to assess the clinical outcomes and this may have led to the non-significant results. Also, a previous meta-analysis has shown the better cardiovascular outcomes after BMMNC therapy in the setting of acute myocardial infarction[63]. Future well-designed and large-scale trials should primarily focus on clinical outcomes to assess if improvement in echocardiographic indices can be translated into favorable clinical outcomes or not.
Previous studies have presented 6MWT and BNP as potential prognostic factors in patients with heart failure. It has been demonstrated that each 100 pg/mL increase in BNP levels can increase the hazard of adverse outcomes by 14% [64, 65]. Our results showed that stem cell therapy can cause a more considerable decrease in serum BNP compared with no stem cell transplantation although no apparent difference was noted between BMMNCs and MSCs. Regarding the distance walked during 6MWT, stem cell group could increase the distance of 6MWT by 21.9 m but this difference was marginally non-significant. Also, there was no difference among the stem cell groups. It can be assumed that although stem cell transplantation may result in improvements in prognostic factors (as demonstrated by reduction in BNP levels and marginally non-significant improvement in 6MWT), there may be no superior type of stem cell in this regard and these results present opportunities for future investigations in randomized trials.
The primary limitation of this meta-analysis, similar to any review, lies in the fact that there is a lack of uniformity across studies in terms of the patient population, the implementation of the intervention, and the definitions of outcomes. Another limitation of our study was the fact that the quality of the studies varied. In the majority of the trials, the process of randomization was executed appropriately. However, there were three studies that did not clearly indicate that their data analysis was conducted in accordance with the intention-to-treat principle. This omission could potentially result in an overestimation of the treatment effect in these particular trials. Moreover, head-to-head comparison of cell types was not performed in any of the studies except one, hence, the only way to compare MSCs vs. BMMNCs was through subgroup analysis and the test to spot any difference between subgroups. Also, many of the included trials were focused on echocardiographic indices rather than long-term clinical outcomes and hence, they were not powered to compare clinical outcomes.
Conclusion
In conclusion, we showed that both MSCs and BMMNCs were effective in terms of improving LVEF and decreasing BNP compared with placebo in patients with chronic heart failure and no apparent difference was observed between the two types of cells. Transplantation of MSCs and BMMNCs was not translated into better MACE but a marginally significant improvement in rehospitalization and all-cause mortality at long-term. Future well-designed randomized trials are warranted to further investigate the possible benefits of stem cell therapy in patients with heart failure.
Supplementary Information
Acknowledgements
The authors declare that artificial intelligence is not used in this study.
Abbreviations
- IL-1β
Interleukin-1β
- TNF-α
Tissue necrosis factor-α
- VEGF
Vascular endothelial growth factor
- HF
Heart failure
- MSC
Mesenchymal stem cell
- BMMNC
Bone-marrow mononuclear cell
- PRISMA
Preferred reporting items for systematic reviews and meta-analyses
- RCT
Randomized controlled trial
- MACE
Major adverse cardiovascular events
- LVEF
Left ventricular ejection fraction
- LVEDV
Left ventricular end-diastolic volume
- LVESV
Left ventricular end-systolic volume
- RR
Risk ratio
- CI
Confidence interval
- MD
Mean difference
- ICEMAN
Instrument to assess the credibility of effect modification analyses
- CABG
Coronary artery bypass grafting
Author contributions
AA and AH contributed to conceptualization and design. Data collection was performed by ND, SAM, SK, and AH. Data analysis was performed by AH and AS. Primary draft was written by JK, AH, ND, and SAM. The primary draft was reviewed and edited by AA and AH. All the listed authors have contributed to the manuscript substantially and have agreed to the final submitted version.
Funding
This study received no funding.
Availability of data and materials
The data underlying this article will be shared on reasonable request from the corresponding author.
Declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Groenewegen A, Rutten F, Mosterd A, Hoes A. Epidemiology of heart failure. Eur J Heart Fail 2020. [DOI] [PMC free article] [PubMed]
- 2.Bozkurt B. Contemporary pharmacological treatment and management of heart failure. Nat Rev Cardiol (Print). 2024. [DOI] [PubMed]
- 3.Murphy C, Zafar H, Sharif F. An updated review of cardiac devices in heart failure. Ir J Med Sci 2017. [DOI] [PubMed]
- 4.Kolte D, Abbott J, Aronow HD. Interventional therapies for heart failure in older adults. Heart Fail Clin 2017. [DOI] [PubMed]
- 5.Lalu M, Mazzarello S, Zlepnig J, Dong Y, Montroy J, McIntyre L, et al. Safety and efficacy of adult stem cell therapy for acute myocardial infarction and ischemic heart failure (SafeCell Heart): a systematic review and meta-analysis. Stem Cells Transl Med 2018. [DOI] [PMC free article] [PubMed]
- 6.Tse HF, Siu CW, Zhu SG, Songyan L, Zhang QY, Lai WH, et al. Paracrine effects of direct intramyocardial implantation of bone marrow derived cells to enhance neovascularization in chronic ischaemic myocardium. Eur J Heart Fail. 2007;9(8):747–753. doi: 10.1016/j.ejheart.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104(9):1046–1052. doi: 10.1161/hc3501.093817. [DOI] [PubMed] [Google Scholar]
- 8.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410(6829):701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 9.Tieu A, Slobodian M, Fergusson D, Montroy J, Burger D, Stewart DJ, et al. Methods and efficacy of extracellular vesicles derived from mesenchymal stromal cells in animal models of disease: a preclinical systematic review protocol. Syst Rev 2019. [DOI] [PMC free article] [PubMed]
- 10.Hosseinpour A, Hosseinpour H, Attar A. Preventive effect of bone marrow mononuclear cell transplantation on acute myocardial infarction-induced heart failure: a meta-analysis of randomized controlled trials. 2022. [DOI] [PubMed]
- 11.Rehman A, Nigam A, Laino L, Russo D, Todisco C, Esposito G, et al. Mesenchymal stem cells in soft tissue regenerative medicine: a comprehensive review. Medicina (Kaunas). 2023;59(8). [DOI] [PMC free article] [PubMed]
- 12.Kulus M, Sibiak R, Stefańska K, Zdun M, Wieczorkiewicz M, Piotrowska-Kempisty H, et al. Mesenchymal stem/stromal cells derived from human and animal perinatal tissues-origins, characteristics, signaling pathways, and clinical trials. Cells. 2021;10(12). [DOI] [PMC free article] [PubMed]
- 13.Galderisi U, Peluso G, Di Bernardo G. Clinical Trials based on mesenchymal stromal cells are exponentially increasing: Where are we in recent years? Stem Cell Rev Rep. 2022;18(1):23–36. doi: 10.1007/s12015-021-10231-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Assmus B, Leistner DM, Schächinger V, Erbs S, Elsässer A, Haberbosch W, et al. Long-term clinical outcome after intracoronary application of bone marrow-derived mononuclear cells for acute myocardial infarction: migratory capacity of administered cells determines event-free survival. Eur Heart J. 2014;35(19):1275–1283. doi: 10.1093/eurheartj/ehu062. [DOI] [PubMed] [Google Scholar]
- 15.Silva GV, Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, et al. Catheter–based transendocardial delivery of autologous bone-marrow-derived mononuclear cells in patients listed for heart transplantation. Tex Heart Inst J. 2004;31(3):214. [PMC free article] [PubMed] [Google Scholar]
- 16.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 19.McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Research Synthesis Methods. 2020;n/a(n/a). [DOI] [PubMed]
- 20.Schandelmaier S, Briel M, Varadhan R, Schmid CH, Devasenapathy N, Hayward RA, et al. Development of the Instrument to assess the Credibility of Effect Modification Analyses (ICEMAN) in randomized controlled trials and meta-analyses. CMAJ. 2020;192(32):E901–E906. doi: 10.1503/cmaj.200077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choudhury T, Mozid A, Hamshere S, Yeo C, Pellaton C, Arnous S, et al. An exploratory randomized control study of combination cytokine and adult autologous bone marrow progenitor cell administration in patients with ischaemic cardiomyopathy: the REGENERATE-IHD clinical trial. Eur J Heart Fail. 2017;19(1):138–147. doi: 10.1002/ejhf.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duan F, Qi Z, Liu S, Lu X, Wang H, Gao Y, et al. Effectiveness of bone marrow mononuclear cells delivered through a graft vessel for patients with previous myocardial infarction and chronic heart failure: an echocardiographic study of left ventricular remodeling. Med Ultrason. 2015;17(2):160–166. doi: 10.11152/mu.2013.2066.172.effbm. [DOI] [PubMed] [Google Scholar]
- 23.Hamshere S, Arnous S, Choudhury T, Choudry F, Mozid A, Yeo C, et al. Randomized trial of combination cytokine and adult autologous bone marrow progenitor cell administration in patients with non-ischaemic dilated cardiomyopathy: the REGENERATE-DCM clinical trial. Eur Heart J. 2015;36(44):3061–3069. doi: 10.1093/eurheartj/ehv390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heldman AW, DiFede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014;311(1):62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu S, Liu S, Zheng Z, Yuan X, Li L, Lu M, et al. Isolated coronary artery bypass graft combined with bone marrow mononuclear cells delivered through a graft vessel for patients with previous myocardial infarction and chronic heart failure: a single-center, randomized, double-blind, placebo-controlled clinical trial. J Am Coll Cardiol. 2011;57(24):2409–2415. doi: 10.1016/j.jacc.2011.01.037. [DOI] [PubMed] [Google Scholar]
- 26.Lehtinen M, Pätilä T, Kankuri E, Lauerma K, Sinisalo J, Laine M, et al. Intramyocardial bone marrow mononuclear cell transplantation in ischemic heart failure: long-term follow-up. J Heart Lung Transplant. 2015;34(7):899–905. doi: 10.1016/j.healun.2015.01.989. [DOI] [PubMed] [Google Scholar]
- 27.Mann I, Tseng CCS, Rodrigo SF, Koudstaal S, van Ramshorst J, Beeres SL, et al. Intramyocardial bone marrow cell injection does not lead to functional improvement in patients with chronic ischaemic heart failure without considerable ischaemia. Neth Heart J. 2019;27(2):81–92. doi: 10.1007/s12471-018-1213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martino H, Brofman P, Greco O, Bueno R, Bodanese L, Clausell N, et al. Multicentre, randomized, double-blind trial of intracoronary autologous mononuclear bone marrow cell injection in non-ischaemic dilated cardiomyopathy (the dilated cardiomyopathy arm of the MiHeart study) Eur Heart J. 2015;36(42):2898–2904. doi: 10.1093/eurheartj/ehv477. [DOI] [PubMed] [Google Scholar]
- 29.Mozid A, Yeo C, Arnous S, Ako E, Saunders N, Locca D, et al. Safety and feasibility of intramyocardial versus intracoronary delivery of autologous cell therapy in advanced heart failure: the REGENERATE-IHD pilot study. Regen Med. 2014;9(3):269–278. doi: 10.2217/rme.14.3. [DOI] [PubMed] [Google Scholar]
- 30.Pätilä T, Lehtinen M, Vento A, Schildt J, Sinisalo J, Laine M, et al. Autologous bone marrow mononuclear cell transplantation in ischemic heart failure: a prospective, controlled, randomized, double-blind study of cell transplantation combined with coronary bypass. J Heart Lung Transplant. 2014;33(6):567–574. doi: 10.1016/j.healun.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Perin EC, Silva GV, Henry TD, Cabreira-Hansen MG, Moore WH, Coulter SA, et al. A randomized study of transendocardial injection of autologous bone marrow mononuclear cells and cell function analysis in ischemic heart failure (FOCUS-HF) Am Heart J. 2011;161(6):1078–87.e3. doi: 10.1016/j.ahj.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 32.Perin EC, Willerson JT, Pepine CJ, Henry TD, Ellis SG, Zhao DX, et al. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trial. JAMA. 2012;307(16):1717–1726. doi: 10.1001/jama.2012.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pokushalov E, Romanov A, Chernyavsky A, Larionov P, Terekhov I, Artyomenko S, et al. Efficiency of intramyocardial injections of autologous bone marrow mononuclear cells in patients with ischemic heart failure: a randomized study. J Cardiovasc Transl Res. 2010;3(2):160–168. doi: 10.1007/s12265-009-9123-8. [DOI] [PubMed] [Google Scholar]
- 34.Sant'Anna RT, Fracasso J, Valle FH, Castro I, Nardi NB, Sant'Anna JR, et al. Direct intramyocardial transthoracic transplantation of bone marrow mononuclear cells for non-ischemic dilated cardiomyopathy: INTRACELL, a prospective randomized controlled trial. Rev Bras Cir Cardiovasc. 2014;29(3):437–447. doi: 10.5935/1678-9741.20140091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santoso T, Siu CW, Irawan C, Chan WS, Alwi I, Yiu KH, et al. Endomyocardial implantation of autologous bone marrow mononuclear cells in advanced ischemic heart failure: a randomized placebo-controlled trial (END-HF) J Cardiovasc Transl Res. 2014;7(6):545–552. doi: 10.1007/s12265-014-9580-6. [DOI] [PubMed] [Google Scholar]
- 36.Seth S, Bhargava B, Narang R, Ray R, Mohanty S, Gulati G, et al. The ABCD (autologous bone marrow cells in dilated cardiomyopathy) trial a long-term follow-up study. J Am Coll Cardiol. 2010;55(15):1643–1644. doi: 10.1016/j.jacc.2009.11.070. [DOI] [PubMed] [Google Scholar]
- 37.Wang R, Zhang L, Wang Y, Gong Z, Xiao C, Wu Y, et al. Long-term outcome of intra-myocardial injection of autologous bone marrow mononuclear cells combined with isolated coronary artery bypass grafting for patients with chronic ischemic heart failure. Heart Surg Forum. 2016;19(3):E131–E138. doi: 10.1532/hsf.1505. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Q, Sun Y, Xia L, Chen A, Wang Z. Randomized study of mononuclear bone marrow cell transplantation in patients with coronary surgery. Ann Thorac Surg. 2008;86(6):1833–1840. doi: 10.1016/j.athoracsur.2008.08.068. [DOI] [PubMed] [Google Scholar]
- 39.Trifunović Z, Obradović S, Balint B, Ilić R, Vukić Z, Šišić M, et al. Functional recovery of patients with ischemic cardiomyopathy treated with coronary artery bypass surgery and concomitant intramyocardial bone marrow mononuclear cell implantation–a long-term follow-up study. Vojnosanit Pregl. 2015;72(3):225–232. doi: 10.2298/VSP140109071T. [DOI] [PubMed] [Google Scholar]
- 40.Bartolucci J, Verdugo FJ, González PL, Larrea RE, Abarzua E, Goset C, et al. Safety and efficacy of the intravenous infusion of umbilical cord mesenchymal stem cells in patients with heart failure: a phase 1/2 randomized controlled trial (RIMECARD trial [randomized clinical trial of intravenous infusion umbilical cord mesenchymal stem cells on cardiopathy]) Circ Res. 2017;121(10):1192–1204. doi: 10.1161/CIRCRESAHA.117.310712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bartunek J, Behfar A, Dolatabadi D, Vanderheyden M, Ostojic M, Dens J, et al. Cardiopoietic stem cell therapy in heart failure: the C-CURE (Cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. J Am Coll Cardiol. 2013;61(23):2329–2338. doi: 10.1016/j.jacc.2013.02.071. [DOI] [PubMed] [Google Scholar]
- 42.Bartunek J, Terzic A, Davison BA, Filippatos GS, Radovanovic S, Beleslin B, et al. Cardiopoietic cell therapy for advanced ischaemic heart failure: results at 39 weeks of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial. Eur Heart J. 2017;38(9):648–660. doi: 10.1093/eurheartj/ehw543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bolli R, Mitrani RD, Hare JM, Pepine CJ, Perin EC, Willerson JT, et al. A phase II study of autologous mesenchymal stromal cells and c-kit positive cardiac cells, alone or in combination, in patients with ischaemic heart failure: the CCTRN CONCERT-HF trial. Eur J Heart Fail. 2021;23(4):661–674. doi: 10.1002/ejhf.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bolli R, Perin EC, Willerson JT, Yang PC, Traverse JH, Henry TD, et al. Allogeneic mesenchymal cell therapy in anthracycline-induced cardiomyopathy heart failure patients: the CCTRN SENECA trial. JACC CardioOncol. 2020;2(4):581–595. doi: 10.1016/j.jaccao.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mathiasen AB, Qayyum AA, Jørgensen E, Helqvist S, Fischer-Nielsen A, Kofoed KF, et al. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: a randomized placebo-controlled trial (MSC-HF trial) Eur Heart J. 2015;36(27):1744–1753. doi: 10.1093/eurheartj/ehv136. [DOI] [PubMed] [Google Scholar]
- 46.Mohyeddin-Bonab M, Mohamad-Hassani MR, Alimoghaddam K, Sanatkar M, Gasemi M, Mirkhani H, et al. Autologous in vitro expanded mesenchymal stem cell therapy for human old myocardial infarction. Arch Iran Med. 2007;10(4):467–473. [PubMed] [Google Scholar]
- 47.Perin EC, Borow KM, Henry TD, Mendelsohn FO, Miller LW, Swiggum E, et al. Randomized trial of targeted transendocardial mesenchymal precursor cell therapy in patients with heart failure. J Am Coll Cardiol. 2023;81(9):849–863. doi: 10.1016/j.jacc.2022.11.061. [DOI] [PubMed] [Google Scholar]
- 48.Perin EC, Borow KM, Silva GV, DeMaria AN, Marroquin OC, Huang PP, et al. A phase II dose-escalation study of allogeneic mesenchymal precursor cells in patients with ischemic or nonischemic heart failure. Circ Res. 2015;117(6):576–584. doi: 10.1161/CIRCRESAHA.115.306332. [DOI] [PubMed] [Google Scholar]
- 49.Qayyum AA, Mathiasen AB, Helqvist S, Jørgensen E, Haack-Sørensen M, Ekblond A, et al. Autologous adipose-derived stromal cell treatment for patients with refractory angina (MyStromalCell Trial): 3-years follow-up results. J Transl Med. 2019;17(1):360. doi: 10.1186/s12967-019-2110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qayyum AA, Mouridsen M, Nilsson B, Gustafsson I, Schou M, Nielsen OW, et al. Danish phase II trial using adipose tissue derived mesenchymal stromal cells for patients with ischaemic heart failure. ESC Heart Fail. 2023;10(2):1170–1183. doi: 10.1002/ehf2.14281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qayyum AA, van Klarenbosch B, Frljak S, Cerar A, Poglajen G, Traxler-Weidenauer D, et al. Effect of allogeneic adipose tissue-derived mesenchymal stromal cell treatment in chronic ischaemic heart failure with reduced ejection fraction - the SCIENCE trial. Eur J Heart Fail. 2023;25(4):576–587. doi: 10.1002/ejhf.2772. [DOI] [PubMed] [Google Scholar]
- 52.Ulus AT, Mungan C, Kurtoglu M, Celikkan FT, Akyol M, Sucu M, et al. Intramyocardial transplantation of umbilical cord mesenchymal stromal cells in chronic ischemic cardiomyopathy: a controlled, randomized clinical trial (HUC-HEART Trial) Int J Stem Cells. 2020;13(3):364–376. doi: 10.15283/ijsc20075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiao W, Guo S, Gao C, Dai G, Gao Y, Li M, et al. A Randomized comparative study on the efficacy of intracoronary infusion of autologous bone marrow mononuclear cells and mesenchymal stem cells in patients with dilated cardiomyopathy. Int Heart J. 2017;58(2):238–244. doi: 10.1536/ihj.16-328. [DOI] [PubMed] [Google Scholar]
- 54.Yau TM, Pagani FD, Mancini DM, Chang HL, Lala A, Woo YJ, et al. Intramyocardial injection of mesenchymal precursor cells and successful temporary weaning from left ventricular assist device support in patients with advanced heart failure: a randomized clinical trial. JAMA. 2019;321(12):1176–1186. doi: 10.1001/jama.2019.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao XF, Xu Y, Zhu ZY, Gao CY, Shi YN. Clinical observation of umbilical cord mesenchymal stem cell treatment of severe systolic heart failure. Genet Mol Res. 2015;14(2):3010–3017. doi: 10.4238/2015.April.10.11. [DOI] [PubMed] [Google Scholar]
- 56.Fisher SA, Doree C, Mathur A, Taggart DP, Martin‐Rendon E. Stem cell therapy for chronic ischaemic heart disease and congestive heart failure. Cochrane Database of Systematic Reviews. 2016(12). [DOI] [PMC free article] [PubMed]
- 57.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54(24):2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beeres SL, Bax JJ, Dibbets P, Stokkel MP, Zeppenfeld K, Fibbe WE, et al. Effect of intramyocardial injection of autologous bone marrow-derived mononuclear cells on perfusion, function, and viability in patients with drug-refractory chronic ischemia. J Nucl Med. 2006;47(4):574–580. [PubMed] [Google Scholar]
- 59.Kalou Y, Al-Khani AM, Haider KH. Bone marrow mesenchymal stem cells for heart failure treatment: a systematic review and meta-analysis. Heart Lung Circ. 2023;32(7):870–880. doi: 10.1016/j.hlc.2023.01.012. [DOI] [PubMed] [Google Scholar]
- 60.Fan M, Huang Y, Chen Z, Xia Y, Chen A, Lu D, et al. Efficacy of mesenchymal stem cell therapy in systolic heart failure: a systematic review and meta-analysis. Stem Cell Res Ther. 2019;10(1):150. doi: 10.1186/s13287-019-1258-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hosseinpour A, Kheshti F, Kazemi A, Attar A. Comparing the effect of bone marrow mono-nuclear cells with mesenchymal stem cells after acute myocardial infarction on improvement of left ventricular function: a meta-analysis of clinical trials. Stem Cell Res Ther. 2022;13(1):203. doi: 10.1186/s13287-022-02883-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y, Xu F, Ma J, Shi J, Chen S, Liu Z, et al. Effect of stem cell transplantation on patients with ischemic heart failure: a systematic review and meta-analysis of randomized controlled trials. Stem Cell Res Ther. 2019;10(1):125. doi: 10.1186/s13287-019-1214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Attar A, Hosseinpour A, Hosseinpour H, Kazemi A. Major cardiovascular events after bone marrow mononuclear cell transplantation following acute myocardial infarction: an updated post-BAMI meta-analysis of randomized controlled trials. BMC Cardiovasc Disord. 2022;22(1):259. doi: 10.1186/s12872-022-02701-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scrutinio D, Guida P, Passantino A. Prognostic value of 6-minute walk test in advanced heart failure with reduced ejection fraction. Am J Cardiol. 2023;199:37–43. doi: 10.1016/j.amjcard.2023.04.041. [DOI] [PubMed] [Google Scholar]
- 65.Buchan TA, Ching C, Foroutan F, Malik A, Daza JF, Hing NNF, et al. Prognostic value of natriuretic peptides in heart failure: systematic review and meta-analysis. Heart Fail Rev. 2022;27(2):645–654. doi: 10.1007/s10741-021-10136-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request from the corresponding author.