Abstract
Introduction
Reviewing and updating research priorities is essential to assess progress and to ensure optimal allocation of financial and human resources in research. In 2001, WHO held a research priority setting workshop for herpes simplex virus type 2 (HSV-2) research in low-income and middle-income countries (LMICs). This study aimed to describe progress between 2000 and 2020 in three of the five key research priority areas outlined in the workshop: HSV-2/HIV interactions, HSV-2 control measures and HSV-2 mathematical modelling. The remaining priorities are addressed in a companion paper.
Method
A systematic literature search of MEDLINE, CINAHL, Global Health and Cochrane databases was carried out. Relevant primary research studies based in LMICs, written in English and published on 2000–2020 were included. Papers were screened by two independent reviewers, and suitable variables were selected for manual extraction from study texts. Data were organised into an Excel spreadsheet and analysed using IBM SPSS.
Results
In total, 3214 discrete papers were identified, of which 180 were eligible for inclusion (HSV-2/HIV interactions, 98; control measures, 58; mathematical modelling, 24). Most studies were conducted in East Africa. The majority of the 2001 WHO HSV-2 research priorities were addressed at least in part. Overall, despite several studies describing a strong relationship between HSV-2 and the acquisition and transmission of HIV, HSV-2 control repeatedly demonstrated little effect on HIV shedding or transmission. Further, although mathematical modelling predicted that vaccines could significantly impact HSV-2 indicators, HSV-2 vaccine studies were few. Studies of antiviral resistance were also few.
Conclusion
Since 2000, LMIC HSV-2 research addressing its control, HIV interactions and mathematical modelling has largely addressed the priorities set in the 2001 WHO HSV-2 workshop. However, key knowledge gaps remain in vaccine research, antiviral cost-effectiveness, antiviral resistance and specific geographical areas.
Keywords: Global Health, Public Health, HIV, Review, Mathematical modelling
WHAT IS ALREADY KNOWN ON THIS TOPIC
Herpes simplex virus type 2 (HSV-2) has a high disease burden in low-income and middle-income countries (LMICs) and is closely related to HIV. In 2001, WHO set some research priorities for HSV-2 in LMICs with the help of global experts. This study reviews the progress towards meeting these priorities.
WHAT THIS STUDY ADDS
Although many of the priorities were addressed, knowledge gaps were identified within vaccine research, antiviral cost-effectiveness, antiviral resistance and specific geographical areas.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study identified unaddressed HSV-2 research priorities that may be utilised to formulate updated research priorities for HSV-2 in LMICs.
Introduction
Herpes simplex virus type 2 (HSV-2) is a sexually transmitted infection which causes painful genital ulcers and affects over 400 million adults globally.1 Low-income and middle-income countries (LMICs) bear the greatest global burden of HSV-2 infection; for example, the population prevalence in Africa is over 30%, whereas in Europe, it is below 10%.1 Across all settings, women, men who have sex with men (MSM) and commercial sex workers are disproportionately affected.2 3 Cumulative evidence has implicated HSV-2 in HIV epidemics as a facilitator or HIV acquisition and transmission.4 5 Several studies have shown that people who are HSV-2 seropositive are up to five times more likely to acquire HIV, and people coinfected with HSV-2 and HIV are significantly more likely to transmit HIV to their sexual partners.6–9 Examination of this relationship and its potential as a means of HIV control have been major drivers of HSV-2 research in LMICs.
Against this background, in 2001, WHO held a 3-day workshop to identify priorities for research to address the high burden of HSV-2 in low-income and middle-income countries (LMICs).10 Delegates included 40 HSV-2 experts from 26 institutions in 12 countries further described in a companion paper.11 Research priorities were identified in five key areas: (1) the epidemiology of HSV-2, (2) the interaction between HSV-2 and HIV, (3) effective strategies for HSV-2 control, (4) mathematical modelling of HSV-2 and (5) HSV-2 diagnostics. Areas for further examination were explored in each area. These included evaluating and modelling the effectiveness of HSV-2 suppressive therapy and vaccination, the impact of antiherpetic therapy on HIV indicators and aciclovir resistance. Additionally, emphasis was put on the need to lobby for further vaccine development and affordable provision of aciclovir for low-income and middle-income countries.12
In order to achieve maximum benefit from priority setting activities, progress towards the defined priorities should be evaluated at appropriate time intervals.13 14 Further, since research priorities can influence the allocation of research funding, reviewing priorities allows optimal allocation of finite resources to maximise the likelihood of patient and population benefit.14 To our knowledge, there has been no published review of the progress towards the research priorities defined in 2001.
The current study therefore aimed to review the progress between 2000 and 2020 in addressing the 2001 WHO HSV-2 research priorities for LMICs in three out of the five key research priority areas identified by the workshop: HSV-2/HIV interactions (priority area 1), HSV-2 control measures (priority area 2) and HSV-2 mathematical modelling (priority area 3). Progress in the remaining priority areas is addressed in a companion paper.11
Methods
A systematic scoping of the literature was performed to assess the research progress made in the HSV-2 control measures, interactions with HIV and mathematical modelling between 2000 and 2020. Data were collected on numbers of studies, their objectives, main findings and geographical locations. Data were also collected on sample size, study design, use of randomisation and sources of bias to allow the assessment of quality of the evidence in the articles.
The databases MEDLINE Complete, CINAHL, Global Health and The Cochrane Library were systematically searched for relevant literature using keywords and subject headings. Search terms were refined through preliminary scoping. Independent searches were performed for each priority area evaluated in this study. Search terms were chosen for each priority area, for ‘low and middle-income countries’ and for ‘HSV-2’ and combined using Boolean operators ‘OR’ and ‘AND’. For example, to address priority area 1, the keyword search strategy was as follows:
(‘HSV-2’ OR ‘genital herpes’ OR ‘herpes genitalis’ OR ‘herpes simplex 2’ OR ‘human simplex virus 2’ OR ‘herpes virus 2’) AND
(‘developing countr*’ OR ‘LMIC’ OR ‘low-to-middle income’ OR ‘low to middle income’ OR ‘low income’ OR ‘middle income’ OR ‘least developed countr*’ OR ‘less developed countr*’ OR ‘underdeveloped countr*’ OR ‘under developed nation*’ OR ‘poor countr*’ OR ‘third world countr*’ OR ‘third world nation*’ OR ‘least developed nation*’ OR ‘less developed nation*’ OR ‘global South’ OR ‘sub-Saharan Africa’ OR ‘Asia’ OR ‘South America’) AND
(‘HIV’ OR ‘human immunodeficiency virus’ OR ‘AIDS’ OR ‘acquired immunodeficiency syndrome’)
See online supplemental appendix 1 for a comprehensive description of the search strategies.
bmjgh-2024-015167supp001.pdf (131.9KB, pdf)
Following the removal of duplicate records, the remaining titles and abstracts were screened independently by two reviewers for inclusion for full-text review. Disagreements were settled by a senior researcher. Articles were included if they reported research conducted in a LMIC between the years 2000 and 2020. LMICs were defined according to the United Nations (UN) Country Classification.15 The map in figure 1 illustrates the countries eligible for inclusion. Studies written in languages other than English, and studies describing aspects of HSV-2 other than interactions with HIV (priority area 1), control measures (priority area 2) or mathematical modelling (priority area 3), were excluded. Due to a relative shortage of primary research in priority areas 1 and 3, both primary and secondary researches were included, whereas there was a wealth of primary data for priority 2, and therefore, secondary data research was excluded. The systematic search was complemented by a supplementary manual scoping of reference lists of the selected studies for further articles that were not captured by the search terms. Additionally, the works of the eight key authors (Jérôme Legoff, Richard Hayes, Helen Weiss, Connie Celum, Esther Freeman, Nicolas Nagot, Katharine Looker and Jared Baeten) who had published the greatest number of individual articles from our included studies were systematically searched.
Figure 1.
This map displays in red the LMICs that were eligible for inclusion in this study based on the United Nations classification by income and development.15 LMICs, low-income and middle-income countries.
Using content analysis, information that addressed the research outcomes and the previously described quality indicators were manually extracted from the included studies and organised into a Microsoft Excel spreadsheet. The data were subsequently imported to IBM SPSS V.26 statistical software where they were cleaned and reorganised to undergo simple summary statistical analysis. Short summaries of the study outcomes were also made and assessed for similarities and differences between studies in each thematic area.
Patient and public Involvement
Patient and public involvement was not appropriate for this study as no new patient data were collected.
Results
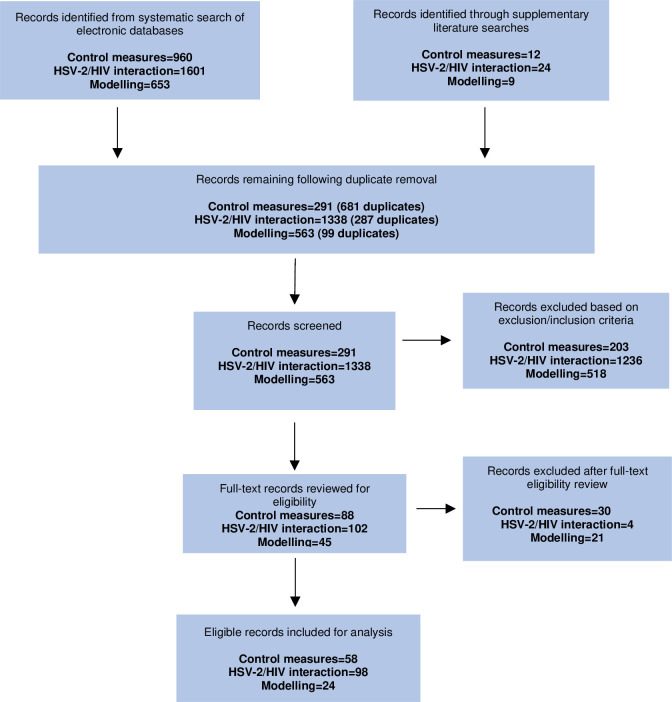
A combined total of 3214 literature records were identified from the 3 electronic database searches, and 45 additional records were retrieved through supplementary search strategies. The removal of duplicates excluded 1067 of these records, which left 2192 records to manually screen. Of these records, 235 were deemed eligible for full-text review against the criteria outlined in the methods. A final total of 180 eligible online records were included for content analysis. A full list of the included studies can be viewed in online supplemental appendix 2. There was high level of screening concordance (>99%) between reviewers for the three searches. A flow diagram of the literature selection for the three research areas is presented in figure 2.
Figure 2.
Flow chart showing study selection process for HSV-2 control measures, HSV-2/HIV interactions and HSV-2 mathematical modelling. See online supplemental appendix 3 for description of reasons for exclusion after full-text eligibility review for each research area.
bmjgh-2024-015167supp002.pdf (402.3KB, pdf)
bmjgh-2024-015167supp003.pdf (11.7KB, pdf)
HSV-2/HIV interactions
A total of 98 eligible studies examining the interaction between HSV-2 and HIV in LMICs between 2000 and 2020 were identified. The most commonly studied topic was the association between seroprevalences of HSV-2 and HIV (n=32, 33%), followed by the effect of HSV-2 control measures on HIV indicators (n=24, 24%), the effect of HSV-2 on HIV acquisition (n=14, 14%) and the effect of HSV-2 on HIV shedding and transmission (n=13, 13%). All other study topics (effect of HSV-2 on the clinical course of HIV, effect of HIV therapy on HSV-2 indicators, diagnostic techniques in HSV-2 and HIV-coinfected people, in vitro studies) included fewer than 10 studies. Geographically, the most common study locations were East Africa (n=44, 45%), Southern Africa (n=24, 24%) and West Africa (n=17, 17%). A total of nine studies were based in South America and South Asia, respectively (9%), six in Central Africa (6%), four in East Asia (4%) and no studies from North Africa, Central America or Western Asia. Specific study participants included people living with HIV (n=26, 27%), the general population (n=23, 23%), people living with HIV and HSV-2 (n=15, 15%), clinic attendees with presumed genital ulcer disease (GUD) (n=9, 9%), commercial sex workers (n=7, 7%), pregnant women (n=5, 5%), MSM (n=5, 5%), adolescents (n=2, 2%), HSV-2-seropositive (HIV-negative) persons (n=2, 2%) and market vendors (n=1, 1%). A total of 43% (n=42) of the studies included only female participants, 39% (n=38) included male and female participants and just 10% (n=10) included only male participants.
Association between HSV-2 and HIV prevalence
13 studies reported HSV-2 prevalence as being substantially higher in HIV-seropositive individuals compared with HIV seronegative.2 16–27 When reported as ORs, the odds of HSV-2 seropositivity in HIV-seropositive individuals was reported as varying between 4.0 and 7.9 times the odds in the HIV-seronegative individuals among different populations.28 29 Conversely, the odds of HIV seropositivity in HSV-2-infected people was also increased compared with the odds in HSV-2-seronegative people, although with a smaller effect size (OR=2.7).19 Only one study (based in Iran) reported no association between HSV-2 and HIV prevalence.30
Effect of HSV-2 on HIV indicators
Nine studies reported that HSV-2/HIV coinfection increased genital HIV shedding and/or transmission,8 9 31–37 while only one study found no association between coinfection and HIV shedding and/or transmission.38 More recently published studies recognised shedding and transmission as discrete outcomes.9 A 4-year prospective study of 174 HIV serodifferent couples in Uganda found that the rate of HIV transmission was greater in those with genital ulceration, compared with those without GUD (adjusted rate ratio 2.58 (95% CI, 1.03 to 5.69)). However, HIV transmission was not affected specifically by HSV-2 serological diagnosis.39
In terms of HIV acquisition, 10 studies agreed that HSV-2 seropositivity was associated with an increased risk of HIV acquisition.7 40–48 Six of these found the association to be stronger for incident HSV-2 compared with prevalent HSV-2.7 40 42 46–48 For example, Reynolds et al reported a HR of HIV acquisition of 1.67 and 3.81 for prevalent and incident HSV-2, respectively.40 One study in Mozambique found no association between HSV-2 and HIV acquisition.49 Freeman et al suggested that HSV-2 has a greater effect on HIV transmission than acquisition.41
Given the possible involvement of HSV-2 in enhancing HIV transmission/acquisition, researchers proceeded to investigate the impact of HSV-2 control measures on HIV indicators through clinical trials. Of these, eight studies found long-term HSV-2 suppressive therapy in coinfected individuals to be associated with reduced plasma and/or genital HIV levels,50–57 while five studies reported no significant reduction in HIV shedding with the use of suppressive therapy.50 58–61 Only one of these studies assessed both shedding and subsequent sexual transmission in HIV-discordant couples, and although it reported HSV-2 suppressive therapy to reduce HIV shedding, there was no evident effect on transmission.55 Conversely, two studies assessed the effect of HSV-2 antiviral therapy on the risk of HIV acquisition in HSV-2-seronegative individuals. Both studies found aciclovir to have no significant effect on HIV acquisition.62 63 The only study assessing the impact of a potential HSV-2 vaccine on HIV indicators estimated that a vaccine against HSV-2 could have a substantial effect on HIV incidence in LMIC.64
Population attributable fraction (PAF) of HSV-2 to HIV (the percentage of HIV cases attributable to HSV-2 infection) was reported in a total of six studies.41 42 47 65–67 They all estimated that a substantial proportion of HIV cases were attributable to HSV-2 with PAFs varying from 22% to 70% in different geographical populations.
Effect of HIV infection on HSV-2 indicators
A singe study of HIV serodiscordant couples with at least one HSV-2-seropositive partner reported HSV-2 incidence to be 2.5 times higher in partners living with HIV compared with HIV-negative people.68 Other studies found that tenofovir disoproxil and emtricitabine-based daily pre-exposure prophylaxis for HIV (PrEP) was associated with reduced HSV-2 incidence (as well as reduced HIV incidence),69 and antiretroviral therapy (ART) reduced HSV-2 shedding (as well as HIV shedding).70 Compared with HIV-seronegative people, people living with HIV were found to suffer more frequent and more severe HSV-2 recurrences.60 71
Table 1 displays the extent of the research progress according to the associated research priorities set in the 2001 WHO HSV-2 workshop.
Table 1.
Progress towards 2001 WHO research priorities: HSV-2 and HIV interactions
| 2001 WHO research priority | Has the priority been addressed? | Explanation | ||
| Yes | Partially | No | ||
| Effect of aciclovir antiviral on HIV viral load and genital shedding |

|
|
||
| Differences in HIV genital shedding during primary clinical HSV-2 episodes, subclinical recurrences and in-between recurrences |

|
|
||
| Differences in HIV viral shedding between genital lesions and from semen |

|
|
||
| Geographical differences in the effect of HSV-2 on HIV between Africa and other LMICs |

|
|
||
| Risk ratios/relative risk and PAF by age and sex, as well as the difference in these measures for incident and prevalent cases. More prospective studies would help achieve this |

|
|
||
| Studies involving HIV-discordant couples where at least one partner is HSV-2 positive to investigate the effect of HSV-1 on transmission and acquisition |

|
|
||
| More information on estimated PAFs of HSV-2 for HIV |

|
|
||
HSV-2, herpes simplex virus type 2; LMICs, low-income and middle-income countries; PAF, population attributable fraction.
HSV-2 control measures
A total of 58 studies were identified to address HSV-2 control measures, the most common study location being sub-Saharan Africa (n=24, 41%). The most frequently studied topic area was the use of antiviral therapy (n=27, 47%), followed by condom use (n=12, 21%), vaccine trials (n=10, 17%), microbicide use (n=6, 10%) and male circumcision (n=3, <1%). They encompassed a range of study designs including randomised control trials (n=26, 45%), literature reviews (n=13, 22%), laboratory studies (n=10, 17%), systematic reviews with meta-analyses (n=5, 9%) and cohort or cross-sectional studies (n=3, <1%).
Suppressive therapy
Six articles including a multiregion randomised placebo-controlled trial (RCT) among HSV-2-seropositive MSM and women in sub-Saharan Africa and South America found that suppressive therapy (400 mg twice daily aciclovir) reduced HSV-2 shedding and transmission rates.50 57 58 72–74 For example, Fuchs et al reported a 63% reduction in the frequency of genital ulcers with detectable HSV-2 and a 47% reduction in the rate of GUD recurrence overall.72 Analogous results were seen among male and female participants living with HIV.58 RCTs based in South and West Africa reported evidence to suggest that suppressive aciclovir may also be efficacious in reducing HIV shedding and disease progression.54 58 However, the results of further East African trials disputed this by showing no significant difference in genital and plasma HIV RNA between the control and intervention (suppressive aciclovir) arms61 and that suppressive aciclovir had no impact on the incidence/acquisition of HIV in HSV-2-seropositive women.62
Episodic therapy
A total of four studies investigated the effect of episodic aciclovir therapy on HSV-2 (and HIV in some instances).60 75–77 Episodic therapy was defined as a 5-day course of aciclovir, and the dose varied between studies from a total of 1.2 g to 1.6 g per day. Ulcer healing was the primary outcome for all four studies. Three of the four studies reported reduced ulcer duration with the use of episodic therapy, although a modest difference was reported by Baeten et al.60 75 77 On the contrary, Phiri et al reported no significant difference in ulcer healing with the use of aciclovir; however, this study used a relatively long duration of 14 days to assess for the presence of ulcers.76 HIV indicators were described as secondary outcomes in three of these studies.60 76 77 Reduced HIV shedding from ulcers was reported by all three studies on day 7; however, no study found a significant reduction in cervicovaginal or plasma HIV RNA.60 76 77
A limited number of studies (n=2) were found to evaluate the extent of HSV-2 resistance to antivirals in LMICs and the clinical benefit of early drug initiation following the onset of an ulcer lesion.78 79 Two studies evaluated the effect of ART in reducing HSV-2 shedding in dually infected people, with both reporting a reduction in HSV-2 shedding and acquisition, respectively.70 79
Vaccine research
Eight of the selected studies focused on the status of the HSV-2 vaccine trials and those currently under development.80–87 Three of these were literature reviews of the progress and challenges of developing HSV-2 vaccine,80 81 84 four were preclinical in vivo studies of specific candidate vaccines in mice or guinea pigs,83 85–87 and one was a mathematical modelling study of potential delivery methods of a theoretical vaccine.82 Of the vaccines tested in the preclinical studies, two were glycoprotein subunit vaccines,83 87 one was a live attenuated recombinant vaccine (AD472),86 and the other was an infected cell protein 0 (ICP0) vaccine.85 Both the glycoprotein subunit vaccines were found to be effective compared with the control group; however, the ICP0 vaccine showed between 10 and 100 times greater protection against HSV-2 compared with a glycoprotein subunit vaccine.85
Two strategies are being studied for an HSV-2 vaccine: a preventive vaccine that aims to offer protection against genital HSV-2 prior to exposure and a therapeutic vaccine that aims to reduce genital lesions and genital shedding in HSV-2-seropositive individuals. No effective vaccine is currently available for HSV-2 infection; however, six identified studies indicated that several candidate HSV-2 vaccines are in various phases of development in high-income countries (HICs). Neither of the two types of vaccines have been tested in LMICs. Despite this, there is much advocacy for the design of an HSV-2 vaccine that will be effective in LMICs.88 The most common products utilised for HSV-2 vaccines in clinical trials are glycoprotein subunit vaccines which work by stimulating mucosal immunity to prevent genital infection.
Other preventative strategies
A total of 12 articles (primary research, n=7; literature reviews, n=5) examined the impact of condom promotion or distribution in reducing HSV-2 acquisition.73 89–99 Of the interventional research studies, four studies (n=4, 44%) reported condom use as having no significant effect on HSV-2 acquisition,91 93 96 97 whereas three studies (n=3, 33%) reported a statistically significant reduction in HSV-2 acquisition.73 92 98
The potential effect of topical microbicides in controlling HSV-2 spread was the focus of five studies.100–104 Although no safe and effective microbicide has yet been approved for the prevention of HSV-2 transmission, numerous candidates showed encouraging results. All the microbicides investigated targeted broad categories of STIs, including HSV-2. The 2001 WHO workshop listed several potential topical microbicides for combatting HSV-2 infection, including Nonoxynol-9 and pH-modifying products (Buffergel).10 Following the result of the phase 2 clinical trials in Africa, Nonoxynol-9 was found to offer no protection against HIV and actually increased the risk of genital disease.102
Only three of the identified articles analysed the effect of male circumcision on HSV-2.42 105 106 For two of these studies, the primary outcome was the impact of male circumcision on HIV indicators, and HSV-2 was a secondary research outcome.42 106 Both Yuan et al and Tobian et al reported that male circumcision reduced the risk of HSV-2 seropositivity,105 106 while Sobngwi-Tambekou et al concluded that HSV-2 seropositivity did not have an impact on the protective effects of male circumcision on HIV acquisition.42
Table 2 displays the extent of the research progress according to the associated research priorities set in the 2001 WHO HSV-2 workshop.
Table 2.
Progress towards 2001 WHO research priorities: HSV-2 control measures
| 2001 WHO research priority: control measures | Has the priority been addressed? | Explanation | ||
| Yes | Partially | No | ||
| Development of African reference laboratories to monitor aciclovir resistance |

|
|
||
| Evaluation of the current syndromic management of GUD in areas of high HSV-2 prevalence (whether to add aciclovir) |

|
|
||
| Monitoring of the clinical/cost-effectiveness of aciclovir use in the syndromic management of GUD |

|
|
||
| Trials of episodic therapy, measuring the effect on HIV shedding and HSV-2 shedding |

|
|
||
| Trials of episodic therapy, measuring the effect on HIV acquisition or transmission |

|
|
||
| Trials of suppressive therapy in high-risk groups, measuring the effect on HIV acquisition among individuals at high risk of infection |

|
|
||
| Lobby for investment in prophylactic vaccine development |

|
|
||
| Develop locally administered vaccines |

|
|
||
| Evaluate counselling strategies for HSV-2-seropositive individuals |

|
|
||
| Include HSV-2 as an outcome in microbicide trials |

|
|
||
GUD, genital ulcer disease; HSV-2, herpes simplex virus type 2; STIs, sexually transmitted infections.
Mathematical modelling
A total of 24 eligible studies examining HSV-2 mathematical were identified. The most common study topics included the modelling of HSV-2 and HIV interactions (n=10, 42%) and the modelling of HSV-2 control measures (n=8, 33%). Other topic areas included modelling of HSV-2 transmission (n=5, 21%), modelling the burden of HSV-2 (n=2, 8%) and improving HSV-2 modelling techniques (n=2, 10%). The majority of the studies were based on data from East Africa (n=11, 45.8%); nine were from Southern Africa (42.9%) and four from both West Africa and South Asia (16.7%). The two most commonly used models in the studies were the STDSIM and Markov models (dynamic stochastic models).107 108 Both the studies that were aimed at further developing HSV-2 mathematical modelling capabilities concluded that existing models could be simplified without a significant loss of accuracy in order to aid their interpretation.108 109
Incorporating aciclovir into the syndromic management of HSV-2 was predicted to be a cost-effective strategy in reducing HSV-2 burden in LMIC areas with high prevalence.110 A South African study estimated that female-to-male sexual transmission of HSV-2 could be reduced by male circumcision,31 and schooling interventions were also predicted to reduce HSV-2 risk through its impact on future aspirations and likelihood of having sex.111 Tenofovir disoproxil vaginal microbicide was predicted to cause only a 4.9% reduction in HSV-2 incidence 15 years after its introduction,112 and the protective effects of tenofovir DF/emtricitabine HIV PrEP against HSV-2 were not sufficient as to enhance its overall cost-effectiveness.113 Studies modelling the impact of HSV-2 therapy (non-vaccine) on HIV indicators predicted modest results, with limitations including cost-effectiveness, and specific unrealistic assumptions underlying the conclusions.109 114 115 Studies modelling vaccine impact were few. It was predicted in two separate studies that an HSV-2 vaccine could positively impact both HSV-2 and HIV incidence in LMICs.64
Variations in HSV-2 sexual transmission rates were largely explained by sexual network characteristics such as marital status and number of partners.116 117 Among heterosexual married couples with HSV-2 infection, it was estimated that the virus was introduced by the male partner via sexual activity outside of marriage in 64.1% of cases.118 These studies were conducted to aid the targeting of interventions.
Table 3 displays the extent of the research progress according to the associated research priorities set in the 2001 WHO HSV-2 workshop.
Table 3.
Progress towards 2001 WHO research priorities: HSV-2 mathematical modelling
| 2001 WHO research priority: mathematical modelling | Has the priority been addressed? | Explanation | ||
| Yes | Partially | No | ||
| Further models of HSV-2 transmission, control measures and interaction with HIV |

|
|
||
| Modelling risk of antiviral resistance |

|
|
||
| Modelling the cost-effectiveness of specific control measures |

|
|
||
| Modelling the potential effect of a HSV-2 vaccine |

|
|
||
HSV-2, herpes simplex virus type 2.
Discussion
This review employed a content analysis methodology to describe the progress made in meeting the 2001 WHO research priorities for HSV-2/HIV interactions, HSV-2 control and mathematical modelling.12
Successfully addressed WHO HSV-2 priorities
The published research over the past 20 years in the areas of HSV-2/HIV interactions, HSV-2 control measures and mathematical modelling has certainly reflected the WHO research priorities set in 2001,12 with most of the identified studies addressing at least one of the documented priorities. The greatest number of the included studies were related to the interaction between HSV-2 and HIV; this was expected since the prospect of harnessing this relationship to reduce the burden of HIV has been a key driver of HSV-2 research over this period.119
All but one of the seven priorities for HSV-2 and HIV interactions were addressed in the literature. A considerable amount of research attention was given to the effect of HSV-2 therapeutics on HIV shedding and viral load. However, many of these studies extrapolated a positive link between HSV-2 therapeutics and reduced HIV shedding and/or viral load to inferring a link between HSV-2 therapeutics and reduced HIV transmission. Only two studies explored the effect of HSV-2 therapeutics on both HIV shedding and sexual transmission. The studies found that although aciclovir reduced HIV genital shedding in coinfected people, it did not result in a corresponding reduction in the rate of forward transmission.55 This highlights both the importance of avoiding this overinterpretation and the need for more studies to extend their outcome measures to include HIV sexual transmission.
A total of 52 studies on HSV-2 control measures were published within the selected time frame, and they addressed 6 of the 10 research priorities that were outlined at the WHO priority meeting. Most of the identified studies revealed that, currently, the two best-established and recommended prevention methods for HSV-2 infection are behavioural intervention and HSV-2 antiviral treatment.
A relatively small number (n=20) of mathematical modelling studies were published in the studied time frame; however, they did address three out of four of the 2001 research priorities. Although HSV-2 was found to have had a substantial bearing on the HIV epidemic and multiple authors emphasising the need for a vaccine, very few studies were based on a potential HSV-2 vaccine.
Unaddressed priorities and research gaps
The majority of unaddressed WHO priorities involved HSV-2 control measures (n=4). Unaddressed priorities from 2001 were identified across the three priority areas. These included the monitoring of the clinical/cost-effectiveness of aciclovir in GUD syndromic management, trials of episodic therapy measuring the effect on HIV acquisition or transmission, evaluation of counselling strategies and the development of locally administered vaccines. Although there were studies assessing the clinical effectiveness of aciclovir in the syndromic approach to GUD, none of these included cost-effectiveness as an outcome. This outcome measure would have been useful supporting evidence for developing GUD syndromic management in LMICs, where the use of aciclovir is much less frequent than in HICs. The impact of HSV-2 control measures on HIV indicators was frequently cited; however, this was always in the context of suppressive therapy, rather than episodic therapy. This may have been due to the more complex study design that would be required to investigate the effect of episodic therapy on HSV-2 shedding over multiple HSV-2 episodes.
The last two unaddressed priorities were the exploration of the differences in HIV genital shedding during primary clinical HSV-2 episodes, subclinical recurrences and in-between recurrences and the modelling of the risk of antiviral resistance. Antiviral resistance may not have been deemed a priority in LMIC during the studied time period because even by 2010 numerous African countries were yet to incorporate aciclovir into their GUD syndromic management algorithm, despite its patent expiring in the late 1990s.120 It is therefore unlikely that researchers could justify the study of aciclovir resistance when the drug was rarely used due to relative unavailability. Indeed, aciclovir resistance testing is not even available in many HICs.121 No studies were identified to address counselling strategies which may be owing to the high number of hours and skilled personnel required for the intervention.
HSV-2 vaccine
Despite consistent emphasis on the urgent need to focus on vaccine research and development, the progress has been poor in this respect. A possible reason for modest progress may be the relatively small size of the vaccine industry compared with the market for curative therapy which patients consume daily.122 There have been concerns for some time about disinvestment in research of vaccines because of their relative lack of profitability for pharmaceutical companies.123 Alternatively, this may be attributable to publication bias whereby studies obtaining negative results were not published. Nevertheless, the identified studies obtained encouraging results highlighting satisfactory cost-effectiveness and improvements in both HSV-2 and HIV indicators.64 117
In vitro vaccine studies conducted in HIC to develop vaccines for use in LMIC would not have been included, for example, Morello et al.124
Impact of the 2001 WHO workshop
Only one of the 180 included studies in this review cited the 2001 WHO HSV-2 workshop report in their work.125 However, many of the workshop attendees proceeded to publish research that addressed the priorities in the 2001 workshop report. The eight key authors listed in the methodology were responsible for 61% (n=111) of the included articles in this study, and many had collaborated in their research. Of these, 38% attended the workshop. This may therefore have influenced a notable proportion of the subsequent research.
Limitations
There are limitations to the analytical approach of this study that increase the risk of bias. First, although conducting the content analysis manually allowed researcher immersion in the data, this increased the risk of unconscious cognitive biases such as confirmation and attribution bias. This may have affected the interpretation of the included papers and hence how they were categorised and reported. Additionally, this study did not include an in-depth appraisal of the strengths and limitations of all the identified studies; therefore, the description of the studies’ main findings should be interpreted with caution. Further, although the research was conducted by a team of experienced clinical and research experts, a more holistic approach would have included a wider range of stakeholders, particularly from LMICs.
The search strategy also had its limitations. Due to the time and resource constraints, separate search terms for each developing country could not be used, although the search was conducted with the support of an experienced librarian. Also, EMBASE, a commonly used database, was not included in the search strategy because the host institutions did not have access to it. Finally, only English language studies were included. These limitations meant that relevant studies written in languages other than English will have been excluded. Additionally, some relevant literature within the EMBASE database and additional studies that could have been identified through including individual LMIC names in the search strategies will likely have been missed. This may particularly affect research published in French since it is the national language of many West and Central African countries. Unpublished research could also not be included which therefore risks publication bias. Finally, because a number of the vaccine studies were laboratory based and did not include any participants, studies that were conducted in HIC (exclusion criteria) with the ultimate aim of use in LMIC would not have been included, for example, Morello et al.124 Nevertheless, our systematic content analysis of so broad a range of HSV-2 research, over a 20-year period, provides information that may help inform future priority setting.
Future HSV-2 priority setting: issues for consideration in future HSV-2-related priority setting for each respective research area include the following.
HSV-2/HIV interactions
There was a relative lack of studies assessing the effect of HSV-2 on HIV shedding that included HIV transmission as an outcome measure. While in the past years, this paucity would have shaped future priority setting, addressing this research gap with the current knowledge of U=U (HIV undetectable = untransmittable) would be unethical.126 This exemplifies the importance of reviewing research priorities in dynamic research fields where new knowledge can significantly alter the landscape.
HSV-2 control measures
Further research on HSV-2 control measures involving young adults, particularly women, as an increased HSV-2 prevalence rate was noted in this age stratum.
Further assessment of microbicide candidates as many of them showed promising initial results. Further focus to be placed on evaluating the microbicide effect in terms of preventing HSV-2 infection in HSV-2 discordant couples.
Although few studies reported encouraging results for either HSV-2 vaccination type in laboratory-based studies in developed countries, no research of this nature was identified based in developing countries. Therefore, further HSV-2 vaccine research is needed in LMICs. The public health gains of a successful vaccine, given the high rates of acquisition in adolescence (e.g in Africa), could be substantial.
HSV-2 mathematical modelling
Further research modelling the impact of HSV-2 vaccination on HSV-2 and HIV indicators which better reflects disease burden
Further studies to model the cost-effectiveness of proposed interventions to better inform future programmes
Further research modelling the risk of antiviral resistance, since an increasing number of LMICs are incorporating aciclovir into the syndromic management algorithm for GUD
Conclusion
In summary, the research carried out between 2000 and 2020 in the areas of HSV-2 and HIV interactions, HSV-2 control measures and HSV-2 mathematical modelling has largely reflected the priorities set in the 2001 WHO HSV-2 workshop. Being the principal driver of HSV-2 research during this time, the most studied topic was the interaction between HSV-2 and HIV, and the most frequently studied geographical area was East Africa.
However, notable research gaps remain, in particular, in HSV-2 vaccine research, antiviral resistance and monitoring of the clinical/cost-effectiveness of aciclovir use in the syndromic management of GUD. Updated research priorities for HSV-2 may focus on increasing the number of vaccine and microbicide studies conducted in LMICs and prospective studies of HIV-discordant couples with HSV-2 and exploring neglected geographical areas such as North Africa and the Middle East.
Acknowledgments
We would like to thank Ms Alison Derbyshire (LSTM Librarian and Academic Liaison and Training Specialist) for her help in tailoring the literature search.
Footnotes
Handling editor: Seye Abimbola
@munajama17, @dremilyrclarke, @Obasi_TropMed
Contributors: All authors meet all four criteria for authorship in the ICMJE Recommendations. EC conceived the original idea; EC and AO supervised study design implementation and quality control. All authors contributed to instrument design and data acquisition. EMO and MJ led the analysis and produced the first draft of the manuscript. BN contributed to the second screening and identified articles. AO, EC, EMO and MJ contributed to the interpretation of results and drafting of manuscripts and their revisions and have agreed for the final version to be published. EMO and MJ are joint first authors; EC and AO are joint senior authors. AO is the guarantor of the study. A reflexivity statement is provided as a supporting document.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Map disclaimer: The depiction of boundaries on this map does not imply the expression of any opinion whatsoever on the part of BMJ (or any member of its group) concerning the legal status of any country, territory, jurisdiction or area, or its authorities. This map is provided without any warranty of any kind, either expressed or implied.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. Looker KJ, Magaret AS, Turner KME, et al. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS One 2015;10:e114989. 10.1371/journal.pone.0114989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lama JR, Lucchetti A, Suarez L, et al. Association of herpes simplex virus type 2 infection and syphilis with human immunodeficiency virus infection among men who have sex with men in Peru. J Infect Dis 2006;194:1459–66. 10.1086/508548 [DOI] [PubMed] [Google Scholar]
- 3. Baltzer H, Chege D, Rebbapragada A, et al. Relative HIV resistance in Kenyan sex workers is not due to an altered prevalence or mucosal immune impact of herpes simplex virus type 2 infection. Curr HIV Res 2009;7:504–7. 10.2174/157016209789346336 [DOI] [PubMed] [Google Scholar]
- 4. Freeman EE, Weiss HA, Glynn JR, et al. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. Aids 2006;20:73–83. 10.1097/01.aids.0000198081.09337.a7 [DOI] [PubMed] [Google Scholar]
- 5. Looker KJ, Elmes JAR, Gottlieb SL, et al. Effect of HSV-2 infection on subsequent HIV acquisition: an updated systematic review and meta-analysis. Lancet Infect Dis 2017;17:1303–16. 10.1016/S1473-3099(17)30405-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baeten JM, Benki S, Chohan V, et al. Hormonal contraceptive use, herpes simplex virus infection, and risk of HIV-1 acquisition among Kenyan women. AIDS 2007;21:1771–7. 10.1097/QAD.0b013e328270388a [DOI] [PubMed] [Google Scholar]
- 7. Kapiga SH, Sam NE, Bang H, et al. The role of herpes simplex virus type 2 and other genital infections in the acquisition of HIV-1 among high-risk women in Northern Tanzania. J Infect Dis 2007;195:1260–9. 10.1086/513566 [DOI] [PubMed] [Google Scholar]
- 8. Rebbapragada A, Wachihi C, Pettengell C, et al. Negative mucosal synergy between herpes simplex type 2 And HIV in the female genital tract. AIDS 2007;21:589–98. 10.1097/QAD.0b013e328012b896 [DOI] [PubMed] [Google Scholar]
- 9. Todd J, Riedner G, Maboko L, et al. Effect of genital herpes on cervicovaginal HIV shedding in women co-infected with HIV and HSV-2 in Tanzania. PLoS ONE 2013;8:e59037. 10.1371/journal.pone.0059037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization . Herpes simplex virus type 2: programmatic and research priorities in developing countries: report of a WHO/UNAIDS/LSHTM workshop, London, 14-16 February 2001. Geneva: World Health Organization, 2001. [Google Scholar]
- 11. Jama MO E, Obasi A, Clarke E. Twenty years of herpes smplex virus type 2 (HSV-2) research in low and middle-income countries: a systematic evaluation of progress made in addressing WHO priorities for research in HSV-2 epidemiology and HSV-2 diagnostics; 2023. [DOI] [PMC free article] [PubMed]
- 12. Herpes simplex virus type 2: programmatic and research priorities in developing countries. WHO/UNAIDS/LSHTM workshop, London, UK, 14-16 February 2001. Herpes simplex virus type 2: programmatic and research priorities in developing countries WHO/UNAIDS/LSHTM workshop, London, UK, 14-16 February 2001. Geneva; Switzerland: World Health Organization; 2001. [Google Scholar]
- 13. Council on Health Research for Development . Priority setting research for health. 2010. Available: http://www.cohred.org/wp-content/uploads/2012/05/Priority-Setting-brochure2.pdf
- 14. Montorzi G, De S, IJsselmuiden C. Priority setting for research for health: a management process for countries. Council on health research for development (COHRED); 2010.
- 15. UN . Country classification. Author New York. NY; 2014. [Google Scholar]
- 16. Ahmed HJ, Mbwana J, Gunnarsson E, et al. Etiology of genital ulcer disease and association with human immunodeficiency virus infection in two Tanzanian cities. Sex Transm Dis 2003;30:114–9. 10.1097/00007435-200302000-00004 [DOI] [PubMed] [Google Scholar]
- 17. Rama Rao Gr, Ramani T, Padmaja J, et al. Herpes simplex virus 2 infection: a risk factor for HIV infection in heterosexuals. Indian J Dermatol Venereol Leprol 2008;74:230. 10.4103/0378-6323.41367 [DOI] [PubMed] [Google Scholar]
- 18. Benjamin RJ, Busch MP, Fang CT, et al. Human immunodeficiency virus-1 infection correlates strongly with herpes simplex virus-2 (genital herpes) seropositivity in South African and United states blood donations. Transfusion 2008;48:295–303. 10.1111/j.1537-2995.2007.01523.x [DOI] [PubMed] [Google Scholar]
- 19. Ghebremichael M, Habtzgi D, Paintsil E. Deciphering the epidemic synergy of herpes simplex virus type 2 (HSV-2) on human immunodeficiency virus type 1 (HIV-1) infection among women in sub-Saharan Africa. BMC Res Notes 2012;5:451. 10.1186/1756-0500-5-451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Phiri S, Zadrozny S, Weiss HA, et al. Etiology of genital ulcer disease and association with HIV infection in Malawi. Sex Transm Dis 2013;40:923–8. 10.1097/OLQ.0000000000000051 [DOI] [PubMed] [Google Scholar]
- 21. Ankamma A, Nagaraja B, Radha P, et al. Study of Seroprevalence of HSV-2 among HIV Seropositive individuals at S.V.R.R.G.G.H Tirupati. International Journal of Pharmaceutical Research and Bio-Science 2014;3:510–5. [Google Scholar]
- 22. Kenyon C, Buyze J, Colebunders R. Classification of incidence and prevalence of certain sexually transmitted infections by world regions. Int J Infect Dis 2014;18:73–80. 10.1016/j.ijid.2013.09.014 [DOI] [PubMed] [Google Scholar]
- 23. Bharathi VC, Anaparty U. Sero evaluation of coinfection of HIV and HSV-2. J Pharm Sci Innov 2015;4:217–21. 10.7897/2277-4572.04449 [DOI] [Google Scholar]
- 24. Jacob S, Gopal T, Kanagasabai S, et al. Herpes simplex virus 2 infection in HIV-seropositive individuals in Tamil Nadu, India. Int J Med Sci Public Health 2015;4:404. 10.5455/ijmsph.2015.1411201483 [DOI] [Google Scholar]
- 25. Ao TTH, Sam NE, Masenga EJ, et al. Human immunodeficiency virus type 1 among bar and hotel workers in Northern Tanzania: the role of alcohol, sexual behavior, and herpes simplex virus type 2. Sex Transm Dis 2006;33:163–9. 10.1097/01.olq.0000187204.57006.b3 [DOI] [PubMed] [Google Scholar]
- 26. Lingappa JR, Kahle E, Mugo N, et al. Characteristics of HIV-1 discordant couples enrolled in a trial of HSV-2 suppression to reduce HIV-1 transmission: the partners study. PLoS ONE 2009;4:e5272. 10.1371/journal.pone.0005272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nakku-Joloba E, Kambugu F, Wasubire J, et al. Sero-prevalence of herpes simplex type 2 virus (HSV-2) and HIV infection in Kampala, Uganda. Afr Health Sci 2014;14:782–9. 10.4314/ahs.v14i4.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weiss HA, Buvé A, Robinson NJ, et al. The epidemiology of HSV-2 infection and its association with HIV infection in four urban African populations. AIDS 2001;15:S97–108. 10.1097/00002030-200108004-00011 [DOI] [PubMed] [Google Scholar]
- 29. Nakubulwa S, Mirembe FM, Kaye DK, et al. Association between HSV-2 and HIV serostatus in pregnant women of known HIV serostatus attending mulago hospital antenatal clinic, Kampala, Uganda. J Infect Dev Ctries 2009;3:803–6. 10.3855/jidc.230 [DOI] [PubMed] [Google Scholar]
- 30. Behling J, Chan AK, Zeh C, et al. Evaluating HIV prevention programs: herpes Simplex virus type 2 antibodies as biomarker for sexual risk behavior in young adults in resource-poor countries. PLoS One 2015;10:e0128370. 10.1371/journal.pone.0128370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mahiane S-G, Legeai C, Taljaard D, et al. Transmission probabilities of HIV and herpes simplex virus type 2, effect of male circumcision and interaction: a longitudinal study in a township of South Africa. AIDS 2009;23:377–83. 10.1097/qad.0b013e32831c5497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bollen LJ, Whitehead SJ, Mock PA, et al. Maternal herpes simplex virus type 2 coinfection increases the risk of perinatal HIV transmission: possibility to further decrease transmission? AIDS 2008;22:1169–76. 10.1097/QAD.0b013e3282fec42a [DOI] [PubMed] [Google Scholar]
- 33. McClelland RS, Wang CC, Overbaugh J, et al. Association between cervical shedding of herpes simplex virus and HIV-1. AIDS 2002;16:2425–30. 10.1097/00002030-200212060-00007 [DOI] [PubMed] [Google Scholar]
- 34. Mbopi-Kéou FX, Grésenguet G, Mayaud P, et al. Interactions between herpes simplex virus type 2 and human immunodeficiency virus type 1 infection in African women: opportunities for intervention. J Infect Dis 2000;182:1090–6. 10.1086/315836 [DOI] [PubMed] [Google Scholar]
- 35. Mbopi-Kéou F-X, Legoff J, Grésenguet G, et al. Genital shedding of herpes simplex virus-2 DNA and HIV-1 RNA and proviral DNA in HIV-1- and herpes simplex virus-2-coinfected African women. J Acquir Immune Defic Syndr 2003;33:121–4. 10.1097/00126334-200306010-00001 [DOI] [PubMed] [Google Scholar]
- 36. LeGoff J, Weiss HA, Gresenguet G, et al. Cervicovaginal HIV-1 and herpes simplex virus type 2 shedding during genital ulcer disease episodes. AIDS 2007;21:1569–78. 10.1097/QAD.0b013e32825a69bd [DOI] [PubMed] [Google Scholar]
- 37. Drake AL, John-Stewart GC, Wald A, et al. Herpes simplex virus type 2 and risk of Intrapartum human immunodeficiency virus transmission. Obstet Gynecol 2007;109:403–9. 10.1097/01.AOG.0000251511.27725.5c [DOI] [PubMed] [Google Scholar]
- 38. Cowan FF, Pascoe SJ, Barlow KL, et al. Association of genital shedding of herpes simplex virus type 2 And HIV-1 among sex workers in rural Zimbabwe. AIDS 2006;20:261–7. 10.1097/01.aids.0000198086.39831.4a [DOI] [PubMed] [Google Scholar]
- 39. Gray RH, Wawer MJ, Brookmeyer R, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet 2001;357:1149–53. 10.1016/S0140-6736(00)04331-2 [DOI] [PubMed] [Google Scholar]
- 40. Reynolds SJ, Risbud AR, Shepherd ME, et al. Recent herpes simplex virus type 2 infection and the risk of human immunodeficiency virus type 1 acquisition in India. J Infect Dis 2003;187:1513–21. 10.1086/368357 [DOI] [PubMed] [Google Scholar]
- 41. Freeman EE, Orroth KK, White RG, et al. Proportion of new HIV infections attributable to herpes simplex 2 increases over time: simulations of the changing role of sexually transmitted infections in sub-Saharan African HIV epidemics. Sex Transm Infect 2007;83 Suppl 1:i17–24. 10.1136/sti.2006.023549 [DOI] [PubMed] [Google Scholar]
- 42. Sobngwi-Tambekou J, Taljaard D, Lissouba P, et al. Effect of HSV-2 serostatus on acquisition of HIV by young men: results of a longitudinal study in orange farm, South Africa. J Infect Dis 2009;199:958–64. 10.1086/597208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Daniels B, Wand H, Ramjee G, et al. Prevalence of herpes simplex virus 2 (HSV-2) infection and associated risk factors in a cohort of HIV negative women in Durban, South Africa. BMC Res Notes 2016;9:510. 10.1186/s13104-016-2319-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bradley J, Floyd S, Piwowar-Manning E, et al. Sexually transmitted bedfellows: exquisite association between HIV and herpes simplex virus type 2 in 21 communities in Southern Africa in the HIV prevention trials network 071 (Popart) study. J Infect Dis 2018;218:443–52. 10.1093/infdis/jiy178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ramjee G, Williams B, Gouws E, et al. The impact of incident and prevalent herpes simplex Virus-2 infection on the incidence of HIV-1 infection among commercial sex workers in South Africa. J Acquir Immune Defic Syndr 2005;39:333–9. 10.1097/01.qai.0000144445.44518.ea [DOI] [PubMed] [Google Scholar]
- 46. Brown JM, Wald A, Hubbard A, et al. Incident and prevalent herpes simplex virus type 2 infection increases risk of HIV acquisition among women in Uganda and Zimbabwe. AIDS 2007;21:1515–23. 10.1097/QAD.0b013e3282004929 [DOI] [PubMed] [Google Scholar]
- 47. del Mar Pujades Rodríguez M, Obasi A, Mosha F, et al. Herpes simplex virus type 2 infection increases HIV incidence: a prospective study in rural Tanzania. AIDS 2002;16:451–62. 10.1097/00002030-200202150-00018 [DOI] [PubMed] [Google Scholar]
- 48. Tobian AAR, Ssempijja V, Kigozi G, et al. Incident HIV and herpes simplex virus type 2 infection among men in Rakai, Uganda. AIDS 2009;23:1589–94. 10.1097/QAD.0b013e32832d4042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Meque I, Dubé K, Feldblum PJ, et al. Prevalence, incidence and determinants of herpes Simplex virus type 2 infection among HIV-Seronegative women at high-risk of HIV infection: a prospective study in Beira, Mozambique. PLoS ONE 2014;9:e89705. 10.1371/journal.pone.0089705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Baeten JM, Strick LB, Lucchetti A, et al. Herpes simplex virus (HSV)-suppressive therapy decreases plasma and genital HIV-1 levels in HSV-2/HIV-1 coinfected women: a randomized, placebo-controlled, cross-over trial. J Infect Dis 2008;198:1804–8. 10.1086/593214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Roxby AC, Drake AL, Ongecha-Owuor F, et al. Effects of valacyclovir on markers of disease progression in postpartum women co-infected with HIV-1 and herpes simplex Virus-2. PLoS ONE 2012;7:e38622. 10.1371/journal.pone.0038622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zuckerman RA, Lucchetti A, Whittington WLH, et al. Herpes simplex virus (HSV) suppression with valacyclovir reduces Rectal and blood plasma HIV-1 levels in HIV-1/HSV-2-Seropositive men: a randomized, double-blind, placebo-controlled crossover trial. J Infect Dis 2007;196:1500–8. 10.1086/522523 [DOI] [PubMed] [Google Scholar]
- 53. Mugwanya K, Baeten JM, Mugo NR, et al. High-dose valacyclovir HSV-2 suppression results in greater reduction in plasma HIV-1 levels compared with standard dose acyclovir among HIV-1/HSV-2 coinfected persons: a randomized, crossover trial. J Infect Dis 2011;204:1912–7. 10.1093/infdis/jir649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nagot N, Ouédraogo A, Foulongne V, et al. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N Engl J Med 2007;356:790–9. 10.1056/NEJMoa062607 [DOI] [PubMed] [Google Scholar]
- 55. Celum C, Wald A, Lingappa JR, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med 2010;362:427–39. 10.1056/NEJMoa0904849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zuckerman RA, Lucchetti A, Whittington WL, et al. HSV suppression reduces seminal HIV-1 levels in HIV-1/HSV-2 co-infected men who have sex with men. AIDS 2009;23:479–83. 10.1097/QAD.0b013e328326ca62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dunne EF, Whitehead S, Sternberg M, et al. Suppressive acyclovir therapy reduces HIV cervicovaginal shedding in HIV- and HSV-2-infected women, Chiang Rai, Thailand. J Acquir Immune Defic Syndr 2008;49:77–83. 10.1097/QAI.0b013e3181831832 [DOI] [PubMed] [Google Scholar]
- 58. Cowan FM, Pascoe SJ, Barlow KL, et al. A randomised placebo-controlled trial to explore the effect of suppressive therapy with acyclovir on genital shedding of HIV-1 and herpes simplex virus type 2 among Zimbabwean sex workers. Sex Transm Infect 2008;84:548–53. 10.1136/sti.2008.031153 [DOI] [PubMed] [Google Scholar]
- 59. Baggaley RF, Griffin JT, Chapman R, et al. Estimating the public health impact of the effect of herpes simplex virus suppressive therapy on plasma HIV-1 viral load. AIDS 2009;23:1005–13. 10.1097/QAD.0b013e32832aadf2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mayaud P, Legoff J, Weiss HA, et al. Impact of acyclovir on genital and plasma HIV-1 RNA, genital herpes simplex virus type 2 DNA, and ulcer healing among HIV-1-infected African women with herpes ulcers: a randomized placebo-controlled trial. J Infect Dis 2009;200:216–26. 10.1086/599991 [DOI] [PubMed] [Google Scholar]
- 61. Tanton C, Weiss HA, Rusizoka M, et al. Long-term impact of Acyclovir suppressive therapy on genital and plasma HIV RNA in Tanzanian women: a randomized controlled trial. J Infect Dis 2010;201:1285–97. 10.1086/651696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Watson-Jones D, Weiss HA, Rusizoka M, et al. Effect of herpes Simplex suppression on incidence of HIV among women in Tanzania. N Engl J Med 2008;358:1560–71. 10.1056/NEJMoa0800260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Celum C, Wald A, Hughes J, et al. Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 Seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. Lancet 2008;371:2109–19. 10.1016/S0140-6736(08)60920-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Freeman EE, White RG, Bakker R, et al. Population-level effect of potential HSV2 prophylactic vaccines on HIV incidence in sub-Saharan Africa. Vaccine 2009;27:940–6. 10.1016/j.vaccine.2008.11.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Biraro S, Kamali A, White R, et al. Effect of HSV-2 on population-level trends in HIV incidence in Uganda between 1990 and 2007. Trop Med Int Health 2013;18:1257–66. 10.1111/tmi.12176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Abu-Raddad LJ, Magaret AS, Celum C, et al. Genital herpes has played a more important role than any other sexually transmitted infection in driving HIV prevalence in Africa. PLoS One 2008;3:e2230. 10.1371/journal.pone.0002230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Foss AM, Vickerman PT, Mayaud P, et al. Modelling the interactions between herpes simplex virus type 2 and HIV: implications for the HIV epidemic in Southern India. Sex Transm Infect 2011;87:22–7. 10.1136/sti.2009.041699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Muiru AN, Guthrie BL, Bosire R, et al. Incident HSV-2 infections are common among HIV-1-discordant couples. J Infect Dis 2013;208:1093–101. 10.1093/infdis/jit303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Celum C, Morrow RA, Donnell D, et al. Daily oral tenofovir and emtricitabine-tenofovir preexposure prophylaxis reduces herpes simplex virus type 2 acquisition among heterosexual HIV-1-uninfected men and women: a subgroup analysis of a randomized trial. Ann Intern Med 2014;161:11–9. 10.7326/M13-2471 [DOI] [PubMed] [Google Scholar]
- 70. Low AJ, Nagot N, Weiss HA, et al. Herpes simplex virus type-2 Cervicovaginal shedding among women living with HIV-1 and receiving antiretroviral therapy in Burkina Faso: an 8-year longitudinal study. J Infect Dis 2016;213:731–7. 10.1093/infdis/jiv495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nwadike VU, Anaedobe CG, Azeez RA, et al. Atypical presentation of genital herpes in a retroviral disease patient on highly active anti-retroviral therapy. Af J Clin Exp Micro 2018;19:121. 10.4314/ajcem.v19i2.7 [DOI] [Google Scholar]
- 72. Fuchs J, Celum C, Wang J, et al. Clinical and virologic efficacy of herpes simplex virus type 2 suppression by acyclovir in a multicontinent clinical trial. J Infect Dis 2010;201:1164–8. 10.1086/651381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jewkes R, Nduna M, Levin J, et al. Impact of stepping stones on incidence of HIV and HSV-2 and sexual behaviour in rural South Africa: cluster randomised controlled trial. BMJ 2008;337:a506. 10.1136/bmj.a506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mujugira A, Magaret AS, Celum C, et al. Daily acyclovir to decrease herpes simplex virus type 2 (HSV-2) transmission from HSV-2/HIV-1 coinfected persons: a randomized controlled trial. J Infect Dis 2013;208:1366–74. 10.1093/infdis/jit333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Baeten JM, Reid SE, Delany-Moretlwe S, et al. Clinical and virologic response to episodic acyclovir for genital ulcers among HIV-1 seronegative, herpes simplex virus type 2 Seropositive African women: a randomized, placebo-controlled trial. Sex Transm Dis 2012;39:21–4. 10.1097/OLQ.0b013e31823b50c6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Phiri S, Hoffman IF, Weiss HA, et al. Impact of aciclovir on ulcer healing, lesional, genital and plasma HIV-1 RNA among patients with genital ulcer disease in Malawi. Sex Transm Infect 2010;86:345–52. 10.1136/sti.2009.041814 [DOI] [PubMed] [Google Scholar]
- 77. Paz-Bailey G, Sternberg M, Puren AJ, et al. Improvement in healing and reduction in HIV shedding with episodic acyclovir therapy as part of syndromic management among men: a randomized, controlled trial. J Infect Dis 2009;200:1039–49. 10.1086/605647 [DOI] [PubMed] [Google Scholar]
- 78. Jiang Y-C, Feng H, Lin Y-C, et al. New strategies against drug resistance to herpes simplex virus. Int J Oral Sci 2016;8:1–6. 10.1038/ijos.2016.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Celum C, Baeten JM. Tenofovir-based pre-exposure prophylaxis for HIV prevention: evolving evidence. Curr Opin Infect Dis 2012;25:51–7. 10.1097/QCO.0b013e32834ef5ef [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Johnston C, Gottlieb SL, Wald A. Status of vaccine research and development of vaccines for herpes simplex virus. Vaccine 2016;34:2948–52. 10.1016/j.vaccine.2015.12.076 [DOI] [PubMed] [Google Scholar]
- 81. Johnston C, Koelle DM, Wald A. Current status and prospects for development of an HSV vaccine. Vaccine 2014;32:1553–60. 10.1016/j.vaccine.2013.08.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lou Y, Qesmi R, Wang Q, et al. Epidemiological impact of a genital herpes type 2 vaccine for young females. PLoS ONE 2012;7:e46027. 10.1371/journal.pone.0046027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Skoberne M, Cardin R, Lee A, et al. An adjuvanted herpes simplex virus 2 subunit vaccine elicits a T cell response in mice and is an effective therapeutic vaccine in guinea pigs. J Virol 2013;87:3930–42. 10.1128/JVI.02745-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Dropulic LK, Cohen JI. The challenge of developing a herpes simplex virus 2 vaccine. Expert Rev Vaccines 2012;11:1429–40. 10.1586/erv.12.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Halford WP, Püschel R, Gershburg E, et al. A live-attenuated HSV-2 Icp0− virus elicits 10 to 100 times greater protection against genital herpes than a glycoprotein D subunit vaccine. PLoS ONE 2011;6:e17748. 10.1371/journal.pone.0017748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Prichard MN, Kaiwar R, Jackman WT, et al. Evaluation of AD472, a live attenuated recombinant herpes simplex virus type 2 vaccine in guinea pigs. Vaccine 2005;23:5424–31. 10.1016/j.vaccine.2005.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Egan KP, Hook LM, Naughton A, et al. An HSV-2 nucleoside-modified mRNA genital herpes vaccine containing glycoproteins gC, gD, and gE protects mice against HSV-1 genital lesions and latent infection. PLOS Pathog 2020;16:e1008795. 10.1371/journal.ppat.1008795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gottlieb SL, Giersing BK, Hickling J, et al. Meeting report: initial World Health Organization consultation on herpes simplex virus (HSV) vaccine preferred product characteristics. Vaccine 2019;37:7408–18. 10.1016/j.vaccine.2017.10.084 [DOI] [PubMed] [Google Scholar]
- 89. Moreno R, Nababan HY, Ota E, et al. Structural and community‐level interventions for increasing condom use to prevent the transmission of HIV and other sexually transmitted infections. Cochrane Database Syst Rev 2014;2014:CD003363. 10.1002/14651858.CD003363.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Martin ET, Krantz E, Gottlieb SL, et al. A pooled analysis of the effect of condoms in preventing HSV-2 acquisition. Arch Intern Med 2009;169:1233–40. 10.1001/archinternmed.2009.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Cowan FM, Pascoe SJS, Langhaug LF, et al. The regai dzive shiri project: results of a randomized trial of an HIV prevention intervention for youth. AIDS 2010;24:2541–52. 10.1097/QAD.0b013e32833e77c9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kamali A, Quigley M, Nakiyingi J, et al. Syndromic management of sexually-transmitted infections and behaviour change interventions on transmission of HIV-1 in rural Uganda: a community randomised trial. Lancet 2003;361:645–52. 10.1016/S0140-6736(03)12598-6 [DOI] [PubMed] [Google Scholar]
- 93. Gregson S, Adamson S, Papaya S, et al. Impact and process evaluation of integrated community and clinic-based HIV-1 control: a cluster-randomised trial in Eastern Zimbabwe. PLoS Med 2007;4:e102. 10.1371/journal.pmed.0040102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Free C, Roberts IG, Abramsky T, et al. A systematic review of randomised controlled trials of interventions promoting effective condom use. J Epidemiol Community Health 2011;65:100–10. 10.1136/jech.2008.085456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hearst N, Chen S. Condom promotion for AIDS prevention in the developing world: is it working Stud Fam Plann 2004;35:39–47. 10.1111/j.1728-4465.2004.00004.x [DOI] [PubMed] [Google Scholar]
- 96. Feldblum PJ, Kuyoh MA, Bwayo JJ, et al. Female condom introduction and sexually transmitted infection prevalence: results of a community intervention trial in Kenya. AIDS 2001;15:1037–44. 10.1097/00002030-200105250-00012 [DOI] [PubMed] [Google Scholar]
- 97. Doyle AM, Ross DA, Maganja K, et al. Long-term biological and behavioural impact of an adolescent sexual health intervention in Tanzania: follow-up survey of the community-based MEMA Kwa Vijana trial. PLoS Med 2010;7:e1000287. 10.1371/journal.pmed.1000287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Magaret AS, Mujugira A, Hughes JP, et al. Effect of condom use on per-act HSV-2 transmission risk in HIV-1, HSV-2-discordant couples. Clin Infect Dis 2016;62:456–61. 10.1093/cid/civ908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. García PJ, Holmes KK, Cárcamo CP, et al. Prevention of sexually transmitted infections in urban communities (Peru PREVEN): a multicomponent community-randomised controlled trial. Lancet 2012;379:1120–8. 10.1016/S0140-6736(11)61846-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Farr Zuend C, Nomellini JF, Smit J, et al. Generation of a dual-target, safe, inexpensive Microbicide that protects against HIV-1 and HSV-2 disease. Sci Rep 2018;8:2786. 10.1038/s41598-018-21134-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Obiero J, Mwethera PG, Wiysonge CS. Topical microbicides for prevention of sexually transmitted infections. Cochrane Database Syst Rev 2012;2012:CD007961. 10.1002/14651858.CD007961.pub2 [DOI] [PubMed] [Google Scholar]
- 102. Shahzad N, Farooq R, Aslam B, et al. Microbicides for the prevention of HPV, HIV-1, and HSV-2: sexually transmitted viral infections. InTech; 2017. [Google Scholar]
- 103. Van Damme L, Ramjee G, Alary M, et al. Effectiveness of COL-1492, a Nonoxynol-9 vaginal GEL, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet 2002;360:971–7. 10.1016/s0140-6736(02)11079-8 [DOI] [PubMed] [Google Scholar]
- 104. Ceña-Diez R, Vacas-Córdoba E, García-Broncano P, et al. Prevention of vaginal and rectal herpes simplex virus type 2 transmission in mice: mechanism of antiviral action. Int J Nanomedicine 2016;11:2147–62. 10.2147/IJN.S95301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Tobian AAR, Serwadda D, Quinn TC, et al. Male circumcision for the prevention of HSV-2 and HPV infections and syphilis. N Engl J Med 2009;360:1298–309. 10.1056/NEJMoa0802556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Yuan T, Fitzpatrick T, Ko N-Y, et al. Circumcision to prevent HIV and other sexually transmitted infections in men who have sex with men: a systematic review and meta-analysis of global data. Lancet Global Health 2019;7:e436–47. 10.1016/S2214-109X(18)30567-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ndii MZ, Supriatna AK. Stochastic mathematical models in epidemiology. Information 2017;20:6185–96. [Google Scholar]
- 108. Siettos CI, Russo L. Mathematical modeling of infectious disease dynamics. Virulence 2013;4:295–306. 10.4161/viru.24041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Foss AM, Vickerman PT, Chalabi Z, et al. Dynamic modeling of herpes simplex virus type-2 (HSV-2) transmission: issues in structural uncertainty. Bull Math Biol 2009;71:720–49. 10.1007/s11538-008-9379-1 [DOI] [PubMed] [Google Scholar]
- 110. Vickerman P, Ndowa F, Mayaud P. Modelling the cost per ulcer treated of incorporating episodic treatment for HSV-2 into the syndromic algorithm for genital ulcer disease. Sex Transm Infect 2008;84:243–8. 10.1136/sti.2007.027136 [DOI] [PubMed] [Google Scholar]
- 111. Stoner MCD, Neilands TB, Kahn K, et al. Multilevel measures of education and pathways to incident herpes simplex virus type 2 in adolescent girls and young women in South Africa. J Adolesc Health 2019;65:723–9. 10.1016/j.jadohealth.2019.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Terris-Prestholt F, Foss AM, Cox AP, et al. Cost-effectiveness of tenofovir GEL in urban South Africa: model projections of HIV impact and threshold product prices. BMC Infect Dis 2014;14:14. 10.1186/1471-2334-14-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Jewell BL, Cremin I, Pickles M, et al. Estimating the cost-effectiveness of pre-exposure prophylaxis to reduce HIV-1 and HSV-2 incidence in HIV-serodiscordant couples in South Africa. PLoS ONE 2015;10:e0115511. 10.1371/journal.pone.0115511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Korenromp EL, Bakker R, de Vlas SJ, et al. HIV dynamics and behaviour change as determinants of the impact of sexually transmitted disease treatment on HIV transmission in the context of the Rakai trial. AIDS 2002;16:2209–18. 10.1097/00002030-200211080-00014 [DOI] [PubMed] [Google Scholar]
- 115. Vickerman P, Devine A, Foss AM, et al. The cost-effectiveness of herpes simplex virus-2 suppressive therapy with daily aciclovir for delaying HIV disease progression among HIV-1-infected women in South Africa. Sex Transm Dis 2011;38:401–9. 10.1097/OLQ.0b013e31820b8bc8 [DOI] [PubMed] [Google Scholar]
- 116. Omori R, Abu-Raddad LJ. Sexual network drivers of HIV and herpes simplex virus type 2 transmission. AIDS 2017;31:1721–32. 10.1097/QAD.0000000000001542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Alsallaq RA, Schiffer JT, Longini IM, et al. Population level impact of an imperfect prophylactic vaccine for herpes simplex Virus-2. Sex Transm Dis 2010;37:290–7. 10.1097/OLQ.0b013e3181d3d023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Arora P, Nagelkerke N, Sgaier SK, et al. HSV-2 and syphilis among married couples in India: patterns of discordance and concordance. Sex Transm Infect 2011;87:516–20. 10.1136/sextrans-2011-050203 [DOI] [PubMed] [Google Scholar]
- 119. Celum C, Levine R, Weaver M, et al. Genital herpes and human immunodeficiency virus: double trouble. Bull World Health Organ 2004;82:447–53. [PMC free article] [PubMed] [Google Scholar]
- 120. WHO . Sources and prices of selected medicines and diagnostics for people living with HIV/AIDS, Report no.: 924159179X. World Health Organization; 2004. [Google Scholar]
- 121. Centers For Disease Control and Prevention . 2021. Available: https://www.cdc.gov/std/treatment-guidelines/herpes.htm
- 122. Douglas RG, Samant VB. The vaccine industry. Plotkin’s Vaccines; 2018. 41. [Google Scholar]
- 123. Offit PA. Why are pharmaceutical companies gradually abandoning vaccines Health Affairs 2005;24:622–30. 10.1377/hlthaff.24.3.622 [DOI] [PubMed] [Google Scholar]
- 124. Morello CS, Kraynyak KA, Levinson MS, et al. Inactivated HSV-2 in MPL/alum adjuvant provides nearly complete protection against genital infection and shedding following long term challenge and rechallenge. Vaccine 2012;30:6541–50. 10.1016/j.vaccine.2012.08.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ndjoyi-Mbiguino A, Ozouaki F, Legoff J, et al. Comparison of washing and Swabbing procedures for collecting genital fluids to assess cervicovaginal shedding of herpes simplex virus type 2 DNA. J Clin Microbiol 2003;41:2662–4. 10.1128/JCM.41.6.2662-2664.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. U=U consensus statement: risk of sexual transmission of HIV from a person living with HIV who has an undetectable viral load. 2017. Available: https://preventionaccess.org/resource/consensus-statement-on-uu-in-criminal-law-reform/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2024-015167supp001.pdf (131.9KB, pdf)
bmjgh-2024-015167supp002.pdf (402.3KB, pdf)
bmjgh-2024-015167supp003.pdf (11.7KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.