Abstract
Functional neurological disorder (FND) is a common and disabling condition at the intersection of neurology and psychiatry. Despite remarkable progress over recent decades, the mechanisms of FND are still poorly understood and there are limited diagnostic tools and effective treatments. One potentially promising treatment modality for FND is virtual reality (VR), which has been increasingly applied to a broad range of conditions, including neuropsychiatric disorders. FND has unique features, many of which suggest the particular relevance for, and potential efficacy of, VR in both better understanding and managing the disorder. In this review, we describe how VR might be leveraged in the treatment and diagnosis of FND (with a primary focus on motor FND and persistent perceptual-postural dizziness given their prominence in the literature), as well as the elucidation of neurocognitive mechanisms and symptom phenomenology. First, we review what has been published to date on the applications of VR in FND and related neuropsychiatric disorders. We then discuss the hypothesised mechanism(s) underlying FND, focusing on the features that are most relevant to VR applications. Finally, we discuss the potential of VR in (1) advancing mechanistic understanding, focusing specifically on sense of agency, attention and suggestibility, (2) overcoming diagnostic challenges and (3) developing novel treatment modalities. This review aims to develop a theoretical foundation and research agenda for the use of VR in FND that might be applicable or adaptable to other related disorders.
Keywords: FUNCTIONAL NEUROLOGICAL DISORDER, MOVEMENT DISORDERS, NEUROPSYCHIATRY, ATTENTION, REHABILITATION
Introduction
Functional neurological disorder (FND) is frequently encountered by neurologists.1 FND encompasses a wide range of neurological symptoms and includes several subtypes, including functional movement disorder (FMD), which in turn is one of the most common movement disorders,2 and persistent perceptual-postural dizziness (PPPD).3 Motor symptoms in FND may include tremor, dystonia, myoclonus and/or paresis. Clinicians can distinguish these symptoms from other neurological disorders by using positive rule-in criteria, such as entrainment in tremor and Hoover’s sign in paresis, alongside more general characteristics such as distractibility and suggestibility.4 5
As FND falls into the gap between physical and mental health, historically, the disorder has remained relatively poorly understood and under-researched. However, over the last 10–15 years, there has been considerable progress in FND research across mechanisms, diagnosis and treatment.6 In particular, there has been a shift from purely psychological theories regarding conversion of stress to a broader biopsychosocial framework emphasising the importance of biological risk factors and mechanisms, and their compatibility and interaction with cognitive as well as psychological mechanisms as part of more nuanced multifactorial models.7 8 Despite these promising developments, there is still significant uncertainty in the field of FND, most evidently demonstrated by limited evidence-based treatments.7
There is increasing interest in and use of virtual reality (VR) technology across a wide range of neurological and psychiatric disorders.9 Despite there being several theoretical reasons why VR could be of utility in FND, there has been little research in this area. In this review, we provide a theoretical framework supporting the implementation of VR-based approaches in FND, with a primary focus on FMD (ie, motor FND) to illustrate and underpin the potential of VR which could be applied and adapted to most if not all FND subtypes.
Current applications of VR
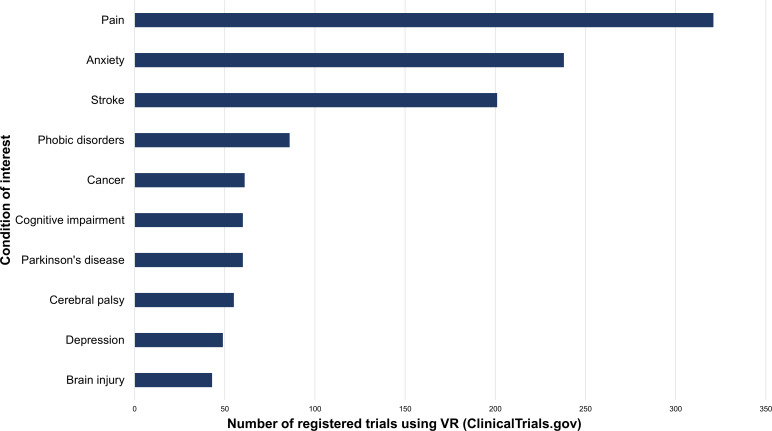
Over recent decades, VR has emerged as a safe and engaging tool leading to significant advances across medicine from direct therapeutic applications delivered in VR to using VR for training and educating healthcare professionals and investigating mechanisms of disorders.10 VR-based approaches hold particular appeal due to their ability to better maintain attention to tasks, replicate real-world rehabilitative interventions in a clinical setting and create novel scenarios not possible in the real world.11 In addition, studies of neurological and psychiatric conditions comprise a considerable proportion (70%) of clinical trials using VR, of which there are to date over 2000 registered on ClinicalTrials.gov12 (figure 1).
Figure 1.
Number of registered trials on ClinicalTrials.gov12 using VR shown for the 10 most commonly mentioned conditions of interest. VR, virtual reality.
Neurology
In neurology, most VR interventions are rehabilitative9 and largely target motor symptoms, replicating conventional rehabilitation programmes in a VR environment.13 In stroke, VR interventions have demonstrated effects on functional motor outcomes comparable with those of conventional rehabilitation, and when applied as adjunct have significantly augmented effects on upper limb function and manual dexterity.13 14 Furthermore, VR rehabilitation displayed comparable outcome effects on gait and balance in Parkinson’s disease.15
In addition to motor symptoms, VR rehabilitation has been applied in cognitive disorders, demonstrating moderate-to-large effects on global cognition, attention and memory in patients with mild cognitive impairment (MCI) or dementia.16 Besides therapeutic purposes, VR has been used in the diagnosis of MCI, showing substantial detection performance.17
Psychiatry
In psychiatry, VR has typically been used as a method of simulation and/or distraction.18 19 VR exposure therapy is the most studied VR intervention and uses simulation20 to allow controlled exposure to a wide range of situations, for example, for specific phobias, with preliminary evidence suggesting comparable efficacy with in vivo exposure.21 Just recently, the first VR treatment was added to the National Institute for Health and Care Excellence guidelines, as treatment for agoraphobia.22 Additionally, there are numerous VR training programmes that involve social interaction, such as social skills training in patients with psychosis or autism spectrum disorder.20 23 There are also several VR programmes that facilitate stress reduction through distraction.19 In addition, more experimental VR interventions are being developed24; for example, virtual body ownership illusions altering distorted body perceptions as a new treatment modality for eating disorders and obesity.25 26
For diagnosis, VR shows promise due to its capacity to simultaneously provoke and measure psychiatric symptoms.27 This is for instance done by measuring hyperactivity in attention-deficit/hyperactivity disorder (ADHD) by capturing bodily movements, and by assessing paranoia and social functioning in psychotic disorders.18 27
In both neurology and psychiatry, most VR interventions are treatment focused and based on conventional interventions. However, VR also allows for novel interventions that would be difficult or impossible in real life.
Pain
There is a significant potential role for VR in pain management, which is of particular relevance given the high prevalence of comorbid pain in FND.28 Distraction is the main mechanism, though other targets, such as improving mood or teaching psychological coping skills, exist.29
In acute pain, VR consistently demonstrated moderate effect sizes in pain reduction (varying depending on population, condition and timing).30 In chronic (musculoskeletal and/or neuropathic) pain, there is preliminary evidence suggesting significant effects on pain severity, with more research needed to support long-lasting effects.30 31
Several studies have explored the use of VR in fibromyalgia and have found positive impacts on quality of life and pain.32 33 One study that employed a progressive body invisibility illusion found strength of embodiment was positively associated with body perception disturbances and negatively with symptom intensity.34 In addition, VR mirror visual feedback therapy, which is used to create illusions normalising the distorted representation of the affected body part, has demonstrated potential effects in phantom pain35 and complex regional pain syndrome,36 in addition to its rehabilitative purposes in patients who had a stroke.37 However, these studies have not included placebo control conditions and multiple lines of evidence challenge the presumed mechanistic basis of these effects.38 39
Functional neurological disorder
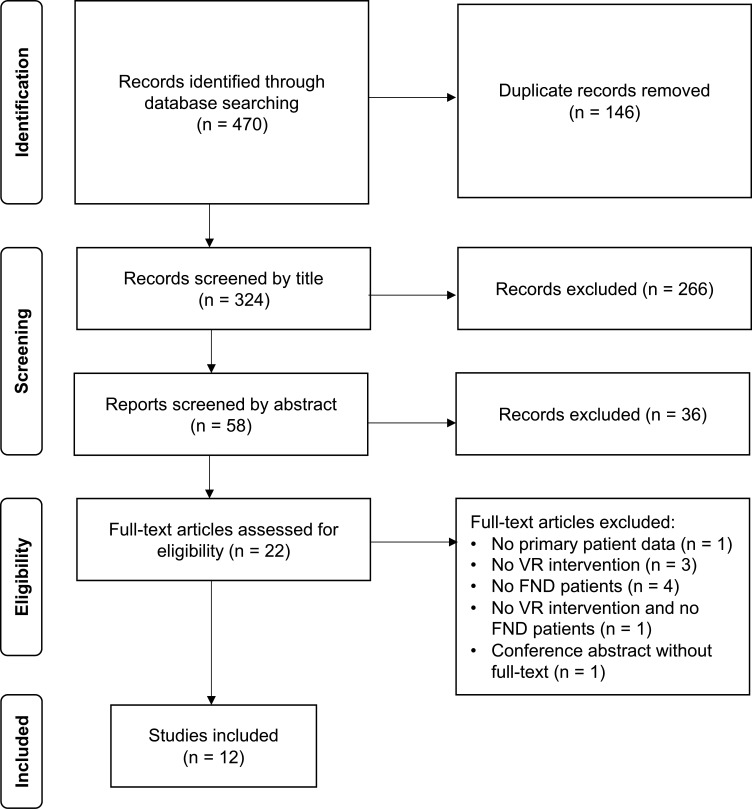
We systematically searched for both published studies and clinical trials currently being conducted on VR and FND (see online supplemental methods for search strategy). Our search identified 12 published studies on the application of VR in FND (figure 2), of which four focus on FMD specifically40–43 (table 1), seven focus on PPPD44–50 (table 2) and one on dissociative amnesia51 (table 1). Two trials currently in progress were also identified (table 1). In terms of study types, there were: four mechanistic functional MRI (fMRI) studies, three randomised controlled trials, three case–control pilot studies, one retrospective study and one case report.
Figure 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram for systematic search of literature on VR in FND. FND, functional neurological disorder; VR, virtual reality.
Table 1.
Summary of published and unpublished research on applications of VR in motor and cognitive FND
| Reference | Study type | Methodology | Findings | Relevance |
| Functional motor/movement disorder (FMD) | ||||
| Nahab et al 40 | Mechanistic fMRI study | Behavioural experiment using virtual hand illusion in individuals with FND (n=21) vs healthy controls (n=20), testing sense of agency over virtual hand that would or wouldn’t follow their own movements | Altered activity in regions associated with sense of agency on fMRI—right dorsolateral prefrontal cortex and pre-supplementary motor area did not respond differentially to loss of movement control. | Provides a paradigm for altering sense of agency in patients with FND using VR by altering synchronicity between real and virtual hand movements. |
| Bullock et al*41 | Single-blind randomised controlled pilot study (midpoint data) | Participants in the treatment arm (n=7) received 8 sessions of VR mirror visual feedback therapy. Starting from session 4, participants additionally received VR exposure therapy based on self-identified FND triggers. The primary outcome measure was symptom severity. | 86% completion rate (n=12/14). No side effects or adverse events. Symptom frequency and level of disability (Oxford Handicap Scale115) were reported but not statistically analysed. | Midpoint results support general feasibility in the FND patient population. |
| Nguyen et al 42 | Case report of VR physiotherapy | Use of three commercially available VR games to encourage either upper limb, lower limb or fine motor movements. Use of objective movement tracking application. | A 5-fold increase in total movement from physiotherapy rehabilitation VR sessions 1–2. With reduction in wheelchair requirement, improved balance and smoother gait. | Highlights utility of objective movement tracking in 3D space. |
| Gandolfi et al 43 | Case–control pilot study | Comparison of postural parameters in FMD (n=17) and healthy controls (n=19) under four attention-demanding conditions: simple fixation task (1) in a real room and (2) in 3D VR room-like condition; complex fixation task in a 3D VR city-like condition (3) avoiding distractors and (4) counting them. | Patients displayed reduced sway area and mediolateral centre of pressure displacement velocity dual task effect but only under most attention-demanding condition (4). | Preliminary evidence for potential utility of combined immersive VR environment with graded attention-demanding conditions in adapting postural control in FMD. |
| https://clinicaltrials.gov/study/NCT05086380 | Treatment trial (no further details) | Transcranial magnetic stimulation or mindfulness-based stress reduction therapy to modulate sense of agency and well-being in FND (symptom type unspecified) and other neurological disorders. | Currently recruiting | The study references use of VR to examine movement patterns and symptoms, but no further methodological details are available in the published protocol. |
| Dissociative amnesia | ||||
| Weniger et al 51 | Mechanistic fMRI study | 10 women with dissociative amnesia, and 4 with dissociative identity disorder, and 14 healthy controls underwent fMRI while navigating a virtual maze, controlling movements with a joystick. | Individuals with dissociative disorders where not impaired in learning the virtual maze compared with controls and showed similar though weaker patterns of activity change during egocentric spatial learning. | Though the study population is not entirely homogeneous, this serves as an example of the use of VR to study attentional and visuospatial memory function in functional cognitive disorder. |
*This study corresponds to the second trial found in the ClinicalTrials.gov database (https://clinicaltrials.gov/study/NCT02764476).
3D, three-dimensional; fMRI, functional MRI; FND, functional neurological disorder; VR, virtual reality.
Table 2.
Summary of published research on applications of VR in PPPD
| Reference | Study type | Methodology | Findings | Relevance |
| Riccelli et al 44 | Mechanistic fMRI study | VR rollercoaster with fMRI in patients with PPPD (n=15) and healthy controls (n=15). Comparisons between groups made for alteration in vestibular and visual cortical activity with vertical vs horizontal displacement. | Controls (but not patients with PPPD) displayed increased activity in anterior bank of central insular sulcus during vertical relative to horizontal motion. For the same comparison, dizziness handicap116 correlated positively with visual cortex activity in those with PPPD. | Provides some insight into PPPD functional alterations in brain processes that may affect balance control and reweighting of space–motion inputs to favour visual cues. |
| Passamonti et al 45 | Mechanistic fMRI study | Secondary analyses of Riccelli et al, exploring relationship between neuroticism and introversion (Revised NEO Personality Inventory117) | Neuroticism positively correlated with inferior frontal gyrus activity, and enhanced connectivity between inferior frontal gyrus and occipital regions in patients with PPPD relative to controls during vertical vs horizontal movement. | Raises question on role of personality traits in activity and connectivity of neural networks mediating attention to visual motion cues during vertical motion in PPPD. |
| Lubetzky et al 46 | Case–control pilot study | Changes in centre of pressure (COP) and head kinematics of people with PPPD (n=22) and healthy controls (n=20) were compared in response to different combinations of visual (static or moving stars) and cognitive perturbations (serial-3 subtraction) during a balance task. | Controls significantly increased all COP and head parameters with the cognitive task, whereas PPPD only increased COP mediolateral path and acceleration. | Some evidence for reduced movement with challenge in PPPD, particularly with regard to head position. |
| Aharoni et al 47 | Case–control pilot study | Individuals with PPPD (n=22) and controls (n=29) performed a square-shaped fast walking task in VR under three conditions (empty train platform, people moving, people and trains moving). | State anxiety and simulator sickness did not increase following testing. There were no significant between-group differences in head kinematics. In high visual load conditions, high trait anxiety and longer timed-up-and-go (TUG)118 duration were associated with reduced anteroposterior and mediolateral range of movement in those with PPPD. | Some associations between head kinematics and self-reported and functional outcomes in those with PPPD were noted. |
| Choi et al 48 | Randomised controlled trial | 30 individuals with PPPD experienced a VR-based vestibular exercise, of whom 15 also received optokinetic stimulation (consisting of stars rotating around the yaw, pitch and roll axes for 3 min in each axis). This was conducted once a week for 4 weeks. | From baseline to 4 weeks, significant improvements in dizziness handicap inventory,116 activities of daily living (ADLs), Visual Vertigo Analogue Scale119 and TUG118 were noted. Only ADL and TUG showed significant improvement with optokinetic stimulation. | Preliminary evidence suggesting potential benefit of VR-based vestibular exercises in PPPD. |
| Mempouo et al 49 | Retrospective study | Retrospective review of outcomes in 1000 patients with Situational Characteristic Questionnaire120 >0.9/4 indicating diagnosis of visual vertigo or PPPD who received VR-based therapy along with usual vestibular rehabilitation. Varied VR environments allowed identification of individual triggers and tailored desensitisation therapies. | Significant improvement was noted in Situational Characteristic Questionnaire (symptom severity),121 Nijmegen Questionnaire (hyperventilation),122 and Dizziness Handicap Inventory.116 | Provides an example of a clinical physiotherapy service already making use of VR-based rehabilitation to provide more individualised treatment. |
| Yamaguchi et al 50 | Randomised controlled trial | The VR group (n=12) included patients who underwent dual task, trunk balance training for PPPD for 100 tasks (10 min). This involved catching falling virtual objects and touching stationary virtual items in a video game-like training environment. The control group (n=14) received standard care. | The VR group displayed significant improvement in static and dynamic postural stability when comparing pre-single and post-single session outcomes. They also had significant improvement in Hospital Anxiety and Depression Scale123 and Niigata PPPD Questionnaire124 1 week post-session. | Evidence for potential impact of dual task-based VR balance training on postural control and mood-related outcomes. |
fMRI, functional MRI; PPPD, persistent perceptual-postural dizziness; VR, virtual reality.
bmjno-2023-000622supp001.pdf (79.6KB, pdf)
VR applications varied though largely focused on attention. For FMD, VR mirror visual feedback training and exposure therapy were explored in a randomised controlled trial with midpoint data showing general feasibility (though efficacy remains to be determined).41 One case report made use of commercially available VR games involving physical activity for rehabilitation.42 Graded attentional dual tasks were also used in FMD to explore impact on postural stability.43 Similarly, PPPD studies (which comprise the bulk of the literature) largely used VR for balance training and vestibular rehabilitation via visual directional motion and rotation cues (eg, virtual rollercoasters, moving stars, trains) with several also making use of graded levels of dual-task attentional demands.46 47 50 The single study of functional cognitive disorder used a virtual maze to explore attentional and visuospatial learning in dissociative amnesia.51 Of note, none of the papers identified made use of augmented reality-based approaches. Overall, the current evidence is limited and warrants further exploration in larger studies.
Mechanisms of FND
Many aspects of the mechanism(s) proposed to underlie FND indicate it is a disorder for which VR applications are potentially relevant. Current explanatory models recognise FND as a multifaceted disorder at the intersection of neurology and psychiatry, with intricate interplay between body and mind.52 One such explanation of FND symptoms is based on a Bayesian model of the brain,53 54 in which perception is described as a hierarchical process of inference, combining (top-down) prior expectations and (bottom-up) sensory input to minimise prediction errors. Functional symptoms then arise due to prediction errors that are not adequately updated, resulting in a multilevel cascade of dysfunction.3 Generally, it is understood that abnormal priors (prior expectations), which are given pathologically high precision (weight) through attentional processes, lie at the basis of these prediction errors.53 Overall, FND is then conceptualised as an abnormality in predictive processing.3
Some studies have generated empirical evidence supporting this framework; for example, Lin et al 55 provided evidence for the role of predictive processing abnormalities in patients with functional gait disorder, using the broken escalator phenomenon.56 Additionally, a recent study by Weissbach et al 57 further substantiated the role of altered perception–action integration processes in patients with FMD.
Our central premise is that VR, by creating a wide range of ways to study and modulate predictive coding abnormalities, could be used to directly manipulate and thus elucidate the hypothesised pathophysiological mechanisms underlying FND, thereby interrogating the Bayesian model of FND. For example, it can be used to conduct similar experiments to the broken escalator phenomenon, such as the VR plank falling experience, in which a decreased H-reflex is demonstrated during falling, implicating a direct link between the VR experience and motor response.58
The three most fundamental concepts in this Bayesian model, as components of predictive processes, are (1) attention, (2) sense of agency and (3) suggestibility.53 59 We explore each in turn as well as the potential interplay with VR.
Sense of agency
Sense of agency in FND
A distorted sense of agency is understood to be a fundamental element of FND.40 53 Sense of agency is a cognitive phenomenon accompanying voluntary movements, and is defined as the feeling of being in control of one’s (motor) actions.60 61 It is related to sense of ownership, which refers to the feeling that your body parts, feelings or thoughts are your own.62 Theoretically, sense of agency is generated through the comparison of efferent predictions and afferent sensory feedback, that is, through sensorimotor integration.63 It consequently increases when there is a predictive match and diminishes with prediction errors.3 60
Although sense of agency is considered to be a crucial aspect of FND in general, its role is most evident in FMD, where patients experience physiologically willed movements as involuntary and show deficits in explicit motor control leading to difficulty performing simple motor tasks.6 64 Several experiments have been conducted on different implicit markers of sense of agency to substantiate its role in FMD. One such marker is temporal (action–outcome) binding, the perceived contraction of temporal interval between a motor action and its sensory effect, which is reported to be greater in healthy controls, but low in patients with FMD.65 Similarly, sensory attenuation, the phenomenon of reduced intensity of sensory perceptions when a movement is perceived as self-generated, was found to be reduced in patients with FMD compared with healthy controls.66 Despite this, a study of explicit sense of agency in individuals with FMD found that self-reported agency over tapping movements (Rubber Hand Illusion Questionnaire67) did not differ from that in healthy controls,68 suggesting a potential discrepancy between explicit and implicit sense of agency in this population.
Sense of agency in VR
Turning to VR, there is evidence regarding its potential to alter sense of agency.61 69 On a theoretical level, one can imagine how manipulation of sensory feedback may alter sense of agency by increasing or decreasing sensorimotor congruence.
Immersion in VR often leads to individuals experiencing a sense of ownership and agency regarding their virtual body and movements.70 This phenomenon was originally exemplified by the VR variant of the classic rubber hand illusion (RHI), where participants perceive ownership of a rubber hand being stroked synchronously with their real hand.71 72 Building on this, virtual limb and even virtual body illusions have been developed, where participants experience a complete virtual body as their own.73 74
One illuminating study using VR to alter sense of agency was conducted by Aoyagi et al.61 Subjects were instructed to make repetitive hand movements, following a designated circular trajectory. Delaying visual feedback resulted in a weakened self-reported sense of agency, whereas positional correction of the virtual hand towards the designated trajectory enhanced sense of agency.61 These results support the hypothesis that sense of agency can be altered by creating spatiotemporal discrepancies between self-generated movement and visual feedback.
Sense of agency in VR and FND
The foregoing results suggest that VR represents a viable method for altering sense of agency in patients with FND, thereby allowing investigation of the precise aetiological role of sense of agency in functional symptoms. Mechanistic research of sense of agency in FND using VR has been limited beyond the landmark study by Nahab et al,40 in which one notable finding was the tendency of patients with FMD to overestimate control over the virtual hand in comparison with healthy subjects. Indeed, when control over the virtual hand was entirely lost, patients failed to recognise that loss of control. In addition, by conducting the experiment during fMRI, the authors observed that the right dorsolateral prefrontal cortex and pre-supplementary motor area (regions involved in the cerebral network associated with sense of agency) were less responsive during loss of movement control in FMD compared with controls.40 This study lends weight to the involvement of an impaired sense of agency in FMD, but emphasises the complexity and uncertainty surrounding its precise role.
Compared with other means of studying sense of agency, VR is better equipped to manipulate sensory feedback, up to the point of manipulating perception of movement itself (which is particularly relevant in PPPD), suggesting multiple fruitful paths for future mechanistic research. First, it would be valuable to conduct experiments similar to those by Nahab et al,40 including varying the amount of perceived control by manipulating sensory feedback. The methods of sensory feedback manipulation could also be developed further, for example, by creating different spatiotemporal alterations, thereby offering a broad scope for conducting diverse experiments. Specifically, it would be useful to include functional symptom severity as outcome measures, to explore how variations in sense of agency influence symptoms. Second, comparable experiments in a third-person perspective may show promise, due to the potential for full-body ownership illusions to influence sense of agency.24 As first-person VR interventions seem limited to targeting upper limbs, third-person paradigms or mirror-based VR experiments could be used to target the whole body, allowing exploration of a wider range of symptoms. Comparing third-person to first-person experiences could offer deeper insights into the role of sense of agency. Third, incorporating markers of sense of agency such as sensory attenuation into VR experiments may deepen understanding of its role and the reliability of these markers.74
Attention
Attention in FND
Alongside sense of agency, attention is widely theorised to play a fundamental role in FND.3 In Bayesian models of perception, attention determines and modulates the relative precision given to both prior expectations and sensory information and thereby in turn the relative weight (precision weighting) of these variables in modulating perceptual states.53 For example, in cases of priors with high precision combined with imprecise sensory information, posterior (perceptual) states will be relatively close to the prior beliefs. Therefore, from a Bayesian perspective, attention represents a crucial factor in both the development and persistence of functional symptoms by affording excessive precision to abnormal prior expectations.53 This is broadly supported by empirical evidence, for instance, of individuals with FND having a body-focused attentional bias, and consequently displaying increased attention towards symptoms.3 Moreover, studies have shown patients with FMD seem to have an abnormal allocation of attention, leading to a cognitive bias in favour of feedforward predictions and a deficit in processing new sensory information.75
In a clinical setting, the role of attention is readily demonstrated by worsening of functional symptoms through body-focused attention, whereas distraction generally leads to improvement.3 76
Attention in VR
Targeting attentional processes is a fundamental aspect of VR and is closely related to the concept of immersion. Immersion entails the subjective feeling of being ‘present’ in a VR environment.77 Since real-world visual input is substituted by virtual visual input, a significant degree of attention is diverted to the virtual world, though non-visual sensory input generally remains unchanged and immersion is never all-encompassing. It is however possible to additionally provide auditory, haptic and proprioceptive feedback to create a more immersive experience. Consequently, several VR interventions have been developed to modulate attentional processes, with particular success in pain management.29 In addition to distractive interventions, there is some evidence for the ability of VR to alter attentional biases. For example, in social anxiety disorders, where patients have an attentional bias towards external stimuli, a VR intervention resulted in significant reduction of this bias.78 Similarly, a reduction in bodily focused attentional bias through VR interventions has been demonstrated in healthy participants.79 Furthermore, VR training programmes have been demonstrated to improve attention in patients with ADHD.80
Attention in VR and FND
Returning to FND, our proposal is that VR can be an effective intervention modality to manipulate attentional processes in patients with FND. For one, these interventions engender distraction. Simple VR distraction methods, which are potentially more comprehensive and immersive than real-life counterparts, could be used to explore the effects of distraction and deepen understanding of the role of attention in symptom expression. A simple experiment would be to monitor severity of FND symptoms during various distractive VR interventions. This could be expanded by incorporating stepped levels of distraction, potentially using multisensory input to explore its effect on symptom severity. This has already been explored in several aforementioned studies such as Gandolfi et al’s study of postural stability in FMD during increasingly demanding virtual attentional dual tasks,43 as well as several VR studies of PPPD.46 47 50 Moreover, adaptive psychophysics could be used to identify the specific level of distraction that engenders symptom attenuation (ie, a symptom attention threshold) and this could be used as an outcome measure in therapy. Second, the use of VR to manipulate attentional biases78 79 may elucidate the precise role of attention in FND. By shifting attention towards external stimuli, specifically new sensory inputs provided by the VR system, distorted learning processes and the role of attentional mechanisms could be examined further. In addition, differences between explicit and implicit motor control observed in individuals with FMD could be further explored through the use of VR-based dual tasks to modulate attention.64
Suggestibility
Suggestibility in FND
A suggestion can be understood as a communication for a change in awareness or behaviour that is typically experienced as involuntary.81 82 Individuals vary considerably in their trait responsiveness to verbal suggestions (direct verbal suggestibility83). In a recent meta-analysis, Wieder et al 84 found evidence for elevated responsiveness to verbal suggestions in patients with FND compared with healthy controls. The analyses specifically highlighted how patients with FND are especially responsive to symptom-specific provocation methods (suggestive symptom induction), and to a lesser extent, responsive to general suggestions (standardised suggestibility scales). This corroborates the long-held hypothesis that elevated suggestibility plays an important role in FND, with additional implications for the incorporation of suggestion in the treatment of FND.85 86 This result also aligns with the Bayesian model of FND, where suggestibility is thought to reflect the tendency to form overly precise priors that are overweighted relative to sensory input.84 87 Nevertheless, the specific neurocognitive mechanisms underlying atypical suggestibility in FND remain poorly understood. In particular, it remains unclear whether elevated responsiveness to symptom suggestions exemplifies suggestibility as a risk factor for FND or the result of a kindling or conditioning process whereby repeated symptom experience engenders an attenuation of patients’ symptom thresholds.59 84
Within the context of FND, suggestion effects can be reflected in a variety of cues that could be used to provoke, reinforce or reduce symptoms.85 86 A previous meta-analysis found that inclusion of suggestion appears to improve treatment outcomes in FND.88 However, it should be noted that elevated suggestibility is not unique to patients with FND, nor are all patients with FND suggestible.84
Suggestibility in VR
Given that a primary feature of VR is to induce visual illusions and changes in experience, there are evident parallels with suggestion effects. In turn, this would imply that elevated suggestibility may translate to increased responsiveness to VR interventions. For instance, individual differences in the RHI are weakly associated with (hypnotic) suggestibility, such that highly suggestible participants experience more sense of ownership over the rubber hand.89 90 A recent study90 interpreted this association to reflect that the RHI is partly confounded by suggestions, expectations and demand characteristics. Debate continues on the specific role of suggestibility in the RHI and other embodiment experiments, whereby it is accepted that the effects of the RHI are at least partially influenced by suggestions.91–93 Hence, it seems reasonable that experimental and clinical manipulations of sense of agency or body ownership should consider the role of suggestibility.
The link between suggestibility and proneness to illusion similarly extends to VR. There is a general association between VR and suggestibility, such that direct verbal suggestibility is associated with the experience of presence in the VR environment.94 In addition, demand characteristics have been found to impact on experience of presence and embodiment in VR.95 Accordingly, it is plausible that highly suggestible individuals will be more easily immersed in a virtual world.
As for specific interventions, aside from the body ownership illusions, several studies discuss the combination of VR and hypnosis, proposing VR as an augmentation of therapies making use of hypnotic suggestions.96 VR hypnosis is an effective analgesic intervention, combining immersion in VR with hypnotic suggestions to modulate pain perception, and seems more effective than VR interventions or hypnosis individually.97 However, it remains unclear whether VR shapes therapeutic outcomes through suggestion/placebo effects, induction of dissociative states (eg, depersonalisation) or whether the analgesic effects of VR are simply due to its distractive capacities.97
Suggestibility in FND and VR
We propose several possibilities as to how VR may serve as a tool for harnessing suggestion in FND. VR can be used to explore the aforementioned relationship between sense of agency and suggestibility, and deepen our understanding of their interaction in FND, using VR-based illusory experiments such as body ownership illusions or VR mirror visual feedback illusions.37 Using VR to induce illusory experiences that are not predictable or expected may offer unique opportunities to dissociate demand characteristic effects from other contributing factors. Full body illusions may show promise, since they are impossible in real life and allow for a wide range of suggestive interventions, as opposed to the RHI. Subsequently, comparable experiments may be executed to measure different components of suggestibility in patients with FND, thereby providing more insight into the impact of different types of suggestibility in these patients, for instance, by exploring the role of visual suggestions as opposed to verbal suggestions.84 Equally, VR may help to clarify the differences between general and symptom-specific suggestibility.98
Diagnosis
In addition to potentially advancing mechanistic understanding, it is also possible that VR could help develop novel, more reliable ways of diagnosing FND. Existing diagnostic criteria predominantly revolve around the underlying concept of attention, and to a lesser extent, suggestibility.3 Manipulating these mechanisms in VR using the aforementioned methods will therefore likely have diagnostic utility.
Due to its potential to create a wide range of environments, scenarios and tasks to facilitate distraction and suggestion, VR could be used to facilitate diagnosis of FND subclasses. For instance, as functional dystonia remains difficult to diagnose, future research could explore the potential of VR to distinguish between functional and non-functional dystonia,6 with one possible approach being comparison of susceptibility to VR mirror visual feedback illusions. Another possible experiment with potential diagnostic utility would be that of measuring gait alteration in functional gait disorder when observing an avatar of oneself in VR. In addition, beyond altering audiovisual feedback, changes in haptic feedback through vibrations in VR handheld controllers may merit research on its potential capacity to entrain functional tremors.
Specifically, hypnotic suggestions, as well as visual and verbal suggestions (which can still be effective and administered outside of the context of hypnosis) provided through VR, could be used to provoke functional symptoms, thereby strengthening suggestive symptom induction methods and aiding diagnosis.99
In addition, VR could be a promising method for identifying biomarkers for FMD, as previous research has yet to produce a reliable biomarker.3 An asset of VR lies in its ability to capture and measure outcome measures, such as movement patterns. Should a VR paradigm capable of influencing functional motor symptoms be developed, specific changes in body movements could be tracked and measured within the virtual environment and used as a quantitative biomarker. Furthermore, VR could aid in further developing other potential biomarkers that have already been identified and hold potential for exploration within VR, such as impaired information processing and abnormal attention.75 100
Treatment
Current treatment options for FND broadly consist of physiotherapy, multidisciplinary rehabilitation and psychotherapy, as well as more novel methods currently under research such as transcranial magnetic stimulation.7 Future research should aim to provide additional evidence on current treatment options, deepening understanding of the mechanisms underlying therapeutic effects, and developing new and symptom-specific treatments. We propose several ways in which VR interventions may serve as a promising treatment modality for FND.
VR as a therapeutic approach for FND
VR could function as an adjunct to existing treatment modalities, such as physiotherapy and rehabilitation.6 VR-based rehabilitation has been effectively implemented in other neurological disorders, leading to practical benefits such as cost-effectiveness and accessibility.13 VR may in fact expand the domain of rehabilitative interventions, creating training situations that are impracticable in real life. Furthermore, VR may augment existing hypnotic interventions for FND, potentially increasing their efficacy and accessibility.87 96 Therefore, VR may contribute to evidence supporting hypnotic interventions in FND, as current evidence remains limited.86
The use of VR in combination with brain–computer interface (BCI) technology has demonstrated motor and other rehabilitation potential in stroke and spinal cord injury due to BCI’s capacity for motor imagery-linked functional electrical stimulation of an affected limb (to trigger real movement) as well as the use of motor imagery rehearsal to move an avatar in VR.101 102 Similar combined approaches have yet to be studied in FND and may merit exploration.
As outlined previously, VR-based interventions for FND may serve as a low-grade (though alterable) form of exposure given their capacity to provide a relatively stress-free environment, as demonstrated by the extensive study of VR exposure therapy in psychiatric conditions,20 thus countering avoidance which is a perpetuating factor in many patients. In addition, the use of VR to create a ‘normal’ movement state rather than that experienced by individuals with FMD could potentially be coupled with the use of certain actions or sensory inputs as a form of conditioning.
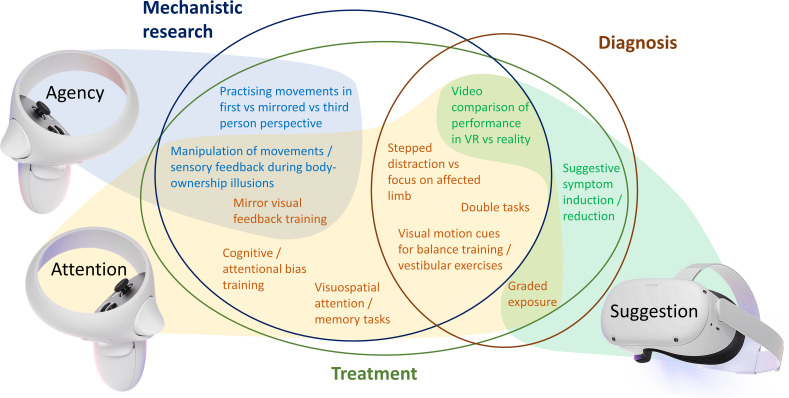
More importantly, we believe VR may lead to completely new treatment modalities that are not possible in the real world. In our view, these interventions would predominantly rely on simulation, intervening with the predictive coding abnormalities underlying FND, and targeting sense of agency, attention and suggestibility (figure 3). Conducting studies to explore the effect of different body ownership illusions combined with manipulation of sensory feedback (as per Bullock et al 41) could pave the path to new treatment modalities. More specifically, VR could aid in developing more symptom-specific interventions, for instance, through tailor-made programmes targeting specific motor symptoms such as tremor or dystonia, or virtual vestibular rehabilitation using visual motion cues and balance training for functional dizziness in PPPD48 50 which is already being used in some services to provide individualised virtual interventions based on the patient’s personal triggers,49 thereby individualising treatment.3
Figure 3.
Overview of proposed VR experiments and interventions, their suggested relation to underlying FND mechanisms (shaded) and potential relevance to FND mechanistic research, diagnosis and treatment (circled). Figure created by author HM using images from Oculus Quest 2 press kit under fair use (source: https://about.fb.com/news/2020/09/introducing-oculus-quest-2-the-next-generation-of-all-in-one-vr/). FND, functional neurological disorder; VR, virtual reality.
Furthermore, since delivery and acceptance of the FND diagnosis are the (often difficult) first steps of effective treatment, VR may facilitate diagnosis acceptance, contributing to psychoeducation.103 Should VR be able to alter FND symptoms through the mechanisms described earlier, video recordings of the patient’s performance could be used to reflect on the objective alteration of symptoms during the VR intervention which (similar to presenting a positive Hoover’s sign) may assist in psychoeducation and acceptance. In addition, a double-blind single-case trial by Ulubas et al 104 demonstrated how suggestions may be used to persuade the patient to acknowledge the diagnosis of FND. Given VR’s suggestive potential, similar experiments may be conducted in a VR environment using video recordings to reflect on the experience afterwards and facilitate acceptance of the diagnosis.
Though the majority of VR applications for neurological conditions have focused on rehabilitation-based motor symptom amelioration, approaches taken for VR-based cognitive rehabilitation of working memory in traumatic brain injury may serve as a promising framework for treatment of cognitive symptoms in FND.105 106 In addition, functional pain syndromes may potentially respond to VR-based pain distraction, as indicated by altered laser-evoked potential subjective pain rating in patients with migraine depending on the nature of the experienced virtual waiting area.107 VR-based behavioural activation may also merit study for FND-related fatigue given its ongoing exploration for use in depression.108
More generally, VR-based interventions may improve sustainability and access to treatment due to their potential to be held remotely109 or even be delivered through a self-help framework.108 This may particularly benefit patients facing difficulties in attending clinics (mobility related or otherwise) though research will be required to determine if either approach would be acceptable or effective in an FND population.
Overall, VR interventions may elucidate the as-of-yet unclear relationship between underlying pathophysiology and observed treatment effects.6
Limitations, pitfalls and concerns for future research
As the ideas we have proposed for future research are largely based on theoretical inferences, it is important to consider potential risks when translating these concepts into practice.
First, it is pertinent to note that not all aspects of FMD treatment can or should be provided through VR, as a broader treatment approach is required when considering psychiatric comorbidity, complex symptoms or extensive disturbance of quality of life. Future studies should not only evaluate VR-based interventions, but also compare them with existing interventions to determine if VR provides a benefit comparable with that conferred in vivo, either as sole treatment or in combination with existing therapeutic modalities.110
In addition, it should be noted that some individuals may experience adverse events from VR such as nausea111 (although there are methods to minimise this112), dissociation113 and other symptoms. Further research is required to explore whether augmented/mixed reality-based approaches (in which participants see the real environment around them through a camera but with virtual elements superimposed) could potentially be less disembodying than traditional VR when used for rehabilitation. It is theoretically possible that in some cases, VR-induced dissociative experiences may in fact exacerbate FND symptoms. Therefore, we emphasise that current implementations of VR should be conducted in the presence of a healthcare professional attentive to potential adverse effects, even though VR may eventually create opportunities for home-based treatment.
Third, we should provide a note of caution on the potential effects of altering the pathophysiological mechanisms underlying FMD. Huys et al 114 demonstrated that artificially manipulating visual feedback on a computer screen while participants were moving their finger on a mousepad did not improve functional tremor severity, even though one might expect altering sensory feedback would influence functional symptoms. The authors emphasise the potential risk of reinforcing pathological movement patterns when manipulating visual feedback. As we share that concern, we stress that VR interventions should not simply aim to correct visual ‘deficits’, and that manipulating the underlying mechanisms of FMD is complex and demands nuanced, thoughtful interventions.
Fourth, when conducting clinical research using wearable health technology, it is important to consider the ethical implications of data storage and ownership. Such concerns are of particular importance when collaborating with commercial partners to develop VR-based interventions, or when interventions involve movement tracking or collection of other individual data.
Finally, though this theoretical overview has largely focused on the potential applications of VR for motor symptoms and dizziness in FND, this is reflective of the existing literature which is sparse and predominantly composed of such approaches. Given the roles of agency, attention and suggestibility across FND more widely, and existing evidence of VR interventions in cognitive, pain and psychiatric disorders, we believe there is a need for research on VR applications across all FND subtypes, as well as prevalent comorbid symptoms.
Conclusion
In this review, we have provided a theoretical framework proposing how VR interventions could advance understanding of pathophysiological mechanisms, diagnosis and treatment in patients with FND. Given the lack of empirical evidence on this topic, our proposals are grounded in theoretical inferences, combining the existing literature on FND with research on VR. This review is therefore meant to lay out the groundwork for future clinical research. We believe VR could result in significant advancements in overcoming current challenges in FND research, and subsequently contribute to continuation of the momentum observed in recent decades.6
Footnotes
@Hammy_UK
DB and HM contributed equally.
JG and PS contributed equally.
Contributors: JG and PS provided project supervision. DB and HM conducted the literature review, wrote the first draft and revised the final draft. TRN, DBT, MJE, JG and PS reviewed the manuscript and contributed throughout.
Funding: HM (Academic Clinical Fellow, ACF-2023-17-008) is funded by the National Institute for Health and Care Research (NIHR) for this research project.
Disclaimer: The views expressed in this publication are those of the author(s) and not necessarily those of the NIHR, NHS or the UK Department of Health and Social Care.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. Stone J, Carson A, Duncan R, et al. Who is referred to neurology Clinics?--The diagnoses made in 3781 new patients. Clin Neurol Neurosurg 2010;112:747–51. 10.1016/j.clineuro.2010.05.011 [DOI] [PubMed] [Google Scholar]
- 2. Factor SA, Podskalny GD, Molho ES. Psychogenic movement disorders: frequency, clinical profile, and characteristics. J Neurol Neurosurg Psychiatry 1995;59:406–12. 10.1136/jnnp.59.4.406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hallett M, Aybek S, Dworetzky BA, et al. Functional neurological disorder: new subtypes and shared mechanisms. Lancet Neurol 2022;21:537–50. 10.1016/S1474-4422(21)00422-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 2013. Available: https://psychiatryonline.org/doi/book/10.1176/appi.books.9780890425596
- 5. World Health Organization . ICD-11: International classification of diseases. (11th revision). 2022.
- 6. Perez DL, Edwards MJ, Nielsen G, et al. Decade of progress in motor functional neurological disorder: continuing the momentum. J Neurol Neurosurg Psychiatry 2021. 10.1136/jnnp-2020-323953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aybek S, Perez DL. Diagnosis and management of functional neurological disorder. BMJ 2022;376:64. 10.1136/bmj.o64 [DOI] [PubMed] [Google Scholar]
- 8. Keynejad RC, Frodl T, Kanaan R, et al. Stress and functional neurological disorders: mechanistic insights. J Neurol Neurosurg Psychiatry 2019;90:813–21. 10.1136/jnnp-2018-318297 [DOI] [PubMed] [Google Scholar]
- 9. Scott H, Griffin C, Coggins W, et al. Virtual reality in the neurosciences: current practice and future directions. Front Surg 2021;8:807195. 10.3389/fsurg.2021.807195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dhar E, Upadhyay U, Huang Y, et al. A scoping review to assess the effects of virtual reality in medical education and clinical care. Digit Health 2023;9:20552076231158022. 10.1177/20552076231158022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li G, Anguera JA, Javed SV, et al. Enhanced attention using head-mounted virtual reality. J Cogn Neurosci 2020;32:1438–54. 10.1162/jocn_a_01560 [DOI] [PubMed] [Google Scholar]
- 12. National Library of Medicine . Clinicaltrials.Gov search for the terms “virtual reality" [ClinicalTrials.Gov]. 2023. Available: https://www.clinicaltrials.gov/search?intr=Virtual%20Reality [Accessed 27 Oct 2023].
- 13. Rutkowski S, Kiper P, Cacciante L, et al. Use of virtual reality-based training in different fields of rehabilitation: a systematic review and meta-analysis. J Rehabil Med 2020;52:jrm00121. 10.2340/16501977-2755 [DOI] [PubMed] [Google Scholar]
- 14. Fang Z, Wu T, Lv M, et al. Effect of traditional plus virtual reality rehabilitation on prognosis of stroke survivors: a systematic review and meta-analysis of randomized controlled trials. Am J Phys Med Rehabil 2022;101:217–28. 10.1097/PHM.0000000000001775 [DOI] [PubMed] [Google Scholar]
- 15. Dockx K, Bekkers EM, Van den Bergh V, et al. Virtual reality for rehabilitation in Parkinson’s disease. Cochrane Database Syst Rev 2016;2016:CD010760. 10.1002/14651858.CD010760.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Papaioannou T, Voinescu A, Petrini K, et al. Efficacy and moderators of virtual reality for cognitive training in people with dementia and mild cognitive impairment: a systematic review and meta-analysis. JAD 2022;88:1341–70. 10.3233/JAD-210672 [DOI] [PubMed] [Google Scholar]
- 17. Liu Q, Song H, Yan M, et al. Virtual reality technology in the detection of mild cognitive impairment: a systematic review and meta-analysis. Ageing Research Reviews 2023;87:101889. 10.1016/j.arr.2023.101889 [DOI] [PubMed] [Google Scholar]
- 18. Freeman D, Reeve S, Robinson A, et al. Virtual reality in the assessment, understanding, and treatment of mental health disorders. Psychol Med 2017;47:2393–400. 10.1017/S003329171700040X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park MJ, Kim DJ, Lee U, et al. A literature overview of virtual reality (VR) in treatment of psychiatric disorders: recent advances and limitations. Front Psychiatry 2019;10:505. 10.3389/fpsyt.2019.00505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wiebe A, Kannen K, Selaskowski B, et al. Virtual reality in the diagnostic and therapy for mental disorders: a systematic review. Clin Psychol Rev 2022;98:102213. 10.1016/j.cpr.2022.102213 [DOI] [PubMed] [Google Scholar]
- 21. Carl E, Stein AT, Levihn-Coon A, et al. Virtual reality exposure therapy for anxiety and related disorders: a meta-analysis of randomized controlled trials. J Anxiety Disord 2019;61:27–36. 10.1016/j.janxdis.2018.08.003 [DOI] [PubMed] [Google Scholar]
- 22. National Institute for Health and Care Excellence (NICE) . Virtual reality technologies for treating agoraphobia or agoraphobic avoidance: early value assessment. 2023. Available: https://www.nice.org.uk/guidance/HTE15/chapter/1-Recommendations
- 23. Rus-Calafell M, Garety P, Sason E, et al. Virtual reality in the assessment and treatment of psychosis: a systematic review of its utility, acceptability and effectiveness. Psychol Med 2018;48:362–91. 10.1017/S0033291717001945 [DOI] [PubMed] [Google Scholar]
- 24. Matamala-Gomez M, Maselli A, Malighetti C, et al. Virtual body ownership illusions for mental health: a narrative review. J Clin Med 2021;10:139. 10.3390/jcm10010139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Keizer A, van Elburg A, Helms R, et al. A virtual reality full body illusion improves body image disturbance in anorexia nervosa. PLoS One 2016;11:e0163921. 10.1371/journal.pone.0163921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scarpina F, Serino S, Keizer A, et al. The effect of a virtual-reality full-body illusion on body representation in obesity. J Clin Med 2019;8:1330. 10.3390/jcm8091330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Bennekom MJ, de Koning PP, Denys D. Virtual reality objectifies the diagnosis of psychiatric disorders: a literature review. Front Psychiatry 2017;8:163. 10.3389/fpsyt.2017.00163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maggio J, Alluri PR, Paredes-Echeverri S, et al. Briquet syndrome revisited: implications for functional neurological disorder. Brain Commun 2020;2:fcaa156. 10.1093/braincomms/fcaa156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Trost Z, France C, Anam M, et al. Virtual reality approaches to pain: toward a state of the science. Pain 2021;162:325–31. 10.1097/j.pain.0000000000002060 [DOI] [PubMed] [Google Scholar]
- 30. Mallari B, Spaeth EK, Goh H, et al. Virtual reality as an analgesic for acute and chronic pain in adults: a systematic review and meta-analysis. J Pain Res 2019;12:2053–85. 10.2147/JPR.S200498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goudman L, Jansen J, Billot M, et al. Virtual reality applications in chronic pain management: systematic review and meta-analysis. JMIR Serious Games 2022;10:e34402. 10.2196/34402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Polat M, Kahveci A, Muci B, et al. The effect of virtual reality exercises on pain, functionality, cardiopulmonary capacity, and quality of life in fibromyalgia syndrome: a randomized controlled study. Games Health J 2021;10:165–73. 10.1089/g4h.2020.0162 [DOI] [PubMed] [Google Scholar]
- 33. Christensen SWM, Almsborg H, Vain TS, et al. The effect of virtual reality on cold pain sensitivity in patients with fibromyalgia and pain-free individuals: a randomized crossover study. Games Health J 2023;12:295–301. 10.1089/g4h.2022.0138 [DOI] [PubMed] [Google Scholar]
- 34. Świdrak J, Arias A, de la Calle ER, et al. Virtual embodiment in fibromyalgia. Sci Rep 2023;13:10719. 10.1038/s41598-023-36861-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Osumi M, Ichinose A, Sumitani M, et al. Restoring movement representation and alleviating phantom limb pain through short-term neurorehabilitation with a virtual reality system. Eur J Pain 2017;21:140–7. 10.1002/ejp.910 [DOI] [PubMed] [Google Scholar]
- 36. Sato K, Fukumori S, Matsusaki T, et al. Nonimmersive virtual reality mirror visual feedback therapy and its application for the treatment of complex regional pain syndrome: an open-label pilot study. Pain Med 2010;11:622–9. 10.1111/j.1526-4637.2010.00819.x [DOI] [PubMed] [Google Scholar]
- 37. Ramachandran VS, Altschuler EL. The use of visual feedback, in particular mirror visual feedback, in restoring brain function. Brain 2009;132:1693–710. 10.1093/brain/awp135 [DOI] [PubMed] [Google Scholar]
- 38. Amoruso E, Terhune DB, Kromm M, et al. Reassessing referral of touch following peripheral deafferentation: the role of contextual bias. Cortex 2023;167:167–77. 10.1016/j.cortex.2023.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Makin TR. Phantom limb pain: thinking outside the (mirror) box. Brain 2021;144:1929–32. 10.1093/brain/awab139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nahab FB, Kundu P, Maurer C, et al. Impaired sense of agency in functional movement disorders: an fMRI study. PLoS One 2017;12:e0172502. 10.1371/journal.pone.0172502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bullock K, Won AS, Bailenson J, et al. Virtual reality-delivered mirror visual feedback and exposure therapy for FND: a midpoint report of a randomized controlled feasibility study. J Neuropsychiatry Clin Neurosci 2020;32:90–4. 10.1176/appi.neuropsych.19030071 [DOI] [PubMed] [Google Scholar]
- 42. Nguyen AT, Hemphill S, Donahue B, et al. Use of virtual reality for targeted physical rehabilitation: case report on managing functional motor disorder. J Pediatr Rehabil Med 2023;16:415–23. 10.3233/PRM-210009 [DOI] [PubMed] [Google Scholar]
- 43. Gandolfi M, Sandri A, Menaspà Z, et al. How does postural control in patients with functional motor disorders adapt to multitasking-based immersive virtual reality Movement Disord Clin Pract 2024;11:337–45. 10.1002/mdc3.13961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Riccelli R, Passamonti L, Toschi N, et al. Altered insular and occipital responses to simulated vertical self-motion in patients with persistent postural-perceptual dizziness. Front Neurol 2017;8:529. 10.3389/fneur.2017.00529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Passamonti L, Riccelli R, Lacquaniti F, et al. Brain responses to virtual reality visual motion stimulation are affected by neurotic personality traits in patients with persistent postural-perceptual dizziness. VES 2018;28:369–78. 10.3233/VES-190653 [DOI] [PubMed] [Google Scholar]
- 46. Lubetzky AV, Aharoni MMH, Arie L, et al. People with persistent postural-perceptual dizziness demonstrate altered postural strategies in complex visual and cognitive environments. VES 2021;31:505–17. 10.3233/VES-201552 [DOI] [PubMed] [Google Scholar]
- 47. Aharoni MMH, Lubetzky AV, Arie L, et al. Factors associated with dynamic balance in people with persistent postural perceptual dizziness (PPPD): a cross-sectional study using a virtual-reality four square step test. J Neuroeng Rehabil 2021;18:55. 10.1186/s12984-021-00852-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Choi SY, Choi JH, Oh EH, et al. Effect of vestibular exercise and Optokinetic stimulation using virtual reality in persistent postural-perceptual dizziness. Sci Rep 2021;11:14437. 10.1038/s41598-021-93940-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mempouo E, Lau K, Green F, et al. Customised vestibular rehabilitation with the addition of virtual reality based therapy in the management of persistent postural-perceptual dizziness. J Laryngol Otol 2021;135:887–91. 10.1017/S0022215121002127 [DOI] [PubMed] [Google Scholar]
- 50. Yamaguchi T, Miwa T, Tamura K, et al. Temporal virtual reality-guided, dual-task, trunk balance training in a sitting position improves persistent postural-perceptual dizziness: proof of concept. J Neuroeng Rehabil 2022;19:92. 10.1186/s12984-022-01068-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Weniger G, Siemerkus J, Barke A, et al. Egocentric virtual maze learning in adult survivors of childhood abuse with Dissociative disorders: evidence from functional magnetic resonance imaging. Psychiatr Res Neuroimaging 2013;212:116–24. 10.1016/j.pscychresns.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 52. Perez DL, Aybek S, Nicholson TR, et al. Functional neurological (conversion) disorder: a core neuropsychiatric disorder. J Neuropsychiatry Clin Neurosci 2020;32:1–3. 10.1176/appi.neuropsych.19090204 [DOI] [PubMed] [Google Scholar]
- 53. Edwards MJ, Adams RA, Brown H, et al. A Bayesian account of “hysteria". Brain 2012;135:3495–512. 10.1093/brain/aws129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Friston K. The free-energy principle: a unified brain theory? Nat Rev Neurosci 2010;11:127–38. 10.1038/nrn2787 [DOI] [PubMed] [Google Scholar]
- 55. Lin D, Castro P, Edwards A, et al. Dissociated motor learning and de-adaptation in patients with functional gait disorders. Brain 2020;143:2594–606. 10.1093/brain/awaa190 [DOI] [PubMed] [Google Scholar]
- 56. Perez DL. Persistence of the 'Broken Escalator' phenomenon in functional gait disorder: mechanistic insights. Brain 2020;143:2338–40. 10.1093/brain/awaa202 [DOI] [PubMed] [Google Scholar]
- 57. Weissbach A, Moyé J, Takacs A, et al. Perception-action integration is altered in functional movement disorders. Movement Disorders 2023;38:1399–409. 10.1002/mds.29458 [DOI] [PubMed] [Google Scholar]
- 58. Grosprêtre S, Eon P, Marcel-Millet P. Virtual reality does not fool the brain only: spinal excitability changes during virtually simulated falling. J Neurophysiol 2023;129:368–79. 10.1152/jn.00383.2022 [DOI] [PubMed] [Google Scholar]
- 59. Fiorio M, Braga M, Marotta A, et al. Functional neurological disorder and placebo and Nocebo effects: shared mechanisms. Nat Rev Neurol 2022;18:624–35. 10.1038/s41582-022-00711-z [DOI] [PubMed] [Google Scholar]
- 60. Moore JW. What is the sense of agency and why does it matter? Front Psychol 2016;7:1272. 10.3389/fpsyg.2016.01272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Aoyagi K, Wen W, An Q, et al. Modified sensory feedback enhances the sense of agency during continuous body movements in virtual reality. Sci Rep 2021;11:2553. 10.1038/s41598-021-82154-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Braun N, Debener S, Spychala N, et al. The senses of agency and ownership: a review. Front Psychol 2018;9:535. 10.3389/fpsyg.2018.00535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Haggard P. Sense of agency in the human brain. Nat Rev Neurosci 2017;18:196–207. 10.1038/nrn.2017.14 [DOI] [PubMed] [Google Scholar]
- 64. Pareés I, Kassavetis P, Saifee TA, et al. Failure of explicit movement control in patients with functional motor symptoms. Mov Disord 2013;28:517–23. 10.1002/mds.25287 [DOI] [PubMed] [Google Scholar]
- 65. Kranick SM, Moore JW, Yusuf N, et al. Action-effect binding is decreased in motor conversion disorder: implications for sense of agency. Mov Disord 2013;28:1110–6. 10.1002/mds.25408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pareés I, Brown H, Nuruki A, et al. Loss of sensory attenuation in patients with functional (psychogenic) movement disorders. Brain 2014;137:2916–21. 10.1093/brain/awu237 [DOI] [PubMed] [Google Scholar]
- 67. Kalckert A, Ehrsson HH. Moving a rubber hand that feels like your own: a dissociation of ownership and agency. Front Hum Neurosci 2012;6:40. 10.3389/fnhum.2012.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Marotta A, Bombieri F, Zampini M, et al. The moving rubber hand illusion reveals that explicit sense of agency for tapping movements is preserved in functional movement disorders. Front Hum Neurosci 2017;11:291. 10.3389/fnhum.2017.00291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wang L, Huang M, Qin C, et al. Movement augmentation in virtual reality: impact on sense of agency measured by subjective responses and electroencephalography. 2022 IEEE Conference on Virtual Reality and 3D User Interfaces Abstracts and Workshops (VRW); 2022:832–3. Available: https://ieeexplore.ieee.org/document/9757590 [Google Scholar]
- 70. Kilteni K, Groten R, Slater M. The sense of embodiment in virtual reality. Presence Teleoperators Virtual Environ 2012;21:373–87. 10.1162/PRES_a_00124 [DOI] [Google Scholar]
- 71. Botvinick M, Cohen J. Rubber hands 'feel' touch that eyes see. Nature 1998;391:756. 10.1038/35784 [DOI] [PubMed] [Google Scholar]
- 72. Slater M, Perez-Marcos D, Ehrsson HH, et al. Inducing illusory ownership of a virtual body. Front Neurosci 2009;3:214–20. 10.3389/neuro.01.029.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Maselli A, Slater M. The building blocks of the full body ownership illusion. Front Hum Neurosci 2013;7:83. 10.3389/fnhum.2013.00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fritz C, Flick M, Zimmermann E. Tactile motor attention induces sensory attenuation for sounds. Conscious Cogn 2022;104:103386. 10.1016/j.concog.2022.103386 [DOI] [PubMed] [Google Scholar]
- 75. Sadnicka A, Daum C, Meppelink AM, et al. Reduced drift rate: a biomarker of impaired information processing in functional movement disorders. Brain 2020;143:674–83. 10.1093/brain/awz387 [DOI] [PubMed] [Google Scholar]
- 76. Huys A, Haggard P, Bhatia KP, et al. Misdirected attentional focus in functional tremor. Brain 2021;144:3436–50. 10.1093/brain/awab230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Loetscher T, Barrett AM, Billinghurst M, et al. Immersive medical virtual reality: still a novelty or already a necessity? J Neurol Neurosurg Psychiatry 2023;94:499–501. 10.1136/jnnp-2022-330207 [DOI] [PubMed] [Google Scholar]
- 78. Urech A, Krieger T, Chesham A, et al. Virtual reality-based attention bias modification training for social anxiety: a feasibility and proof of concept study. Front Psychiatry 2015;6:154. 10.3389/fpsyt.2015.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Miquel-Nabau H, Briseño-Oloriz N, Porras-Garcia B, et al. Modification of body-related attentional bias through virtual reality and eye-tracking in healthy participants: implications for anorexia nervosa treatments. Brain Sci 2023;13:764. 10.3390/brainsci13050764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Corrigan N, Păsărelu CR, Voinescu A. Immersive virtual reality for improving cognitive deficits in children with ADHD: a systematic review and meta-analysis. Virtual Real 2023;1–20. 10.1007/s10055-023-00768-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Halligan PW, Oakley DA. Hypnosis and beyond: exploring the broader domain of suggestion. Psychol Conscious: Theory, Res, Pract 2014;1:105–22. 10.1037/cns0000019 [DOI] [Google Scholar]
- 82. Kirsch I. Clinical hypnosis as a nondeceptive placebo: empirically derived techniques. Am J Clin Hypn 1994;37:95–106. 10.1080/00029157.1994.10403122 [DOI] [PubMed] [Google Scholar]
- 83. Oakley DA, Walsh E, Mehta MA, et al. Direct verbal suggestibility: measurement and significance. Conscious Cogn 2021;89:103036. 10.1016/j.concog.2020.103036 [DOI] [PubMed] [Google Scholar]
- 84. Wieder L, Brown R, Thompson T, et al. Suggestibility in functional neurological disorder: a meta-analysis. J Neurol Neurosurg Psychiatry 2021;92:150–7. 10.1136/jnnp-2020-323706 [DOI] [PubMed] [Google Scholar]
- 85. Deeley Q. Hypnosis as a model of functional neurologic disorders. Handb Clin Neurol 2016;139:95–103. 10.1016/B978-0-12-801772-2.00009-6 [DOI] [PubMed] [Google Scholar]
- 86. Deeley Q. Hypnosis as therapy for functional neurologic disorders. Handb Clin Neurol 2016;139:585–95. 10.1016/B978-0-12-801772-2.00047-3 [DOI] [PubMed] [Google Scholar]
- 87. Bell V, Oakley DA, Halligan PW, et al. Dissociation in hysteria and hypnosis: evidence from cognitive neuroscience. J Neurol Neurosurg Psychiatry 2011;82:332–9. 10.1136/jnnp.2009.199158 [DOI] [PubMed] [Google Scholar]
- 88. Poole NA, Wuerz A, Agrawal N. Abreaction for conversion disorder: systematic review with meta-analysis. Br J Psychiatry 2010;197:91–5. 10.1192/bjp.bp.109.066894 [DOI] [PubMed] [Google Scholar]
- 89. Marotta A, Tinazzi M, Cavedini C, et al. Individual differences in the rubber hand illusion are related to sensory suggestibility. PLoS One 2016;11:e0168489. 10.1371/journal.pone.0168489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lush P, Botan V, Scott RB, et al. Trait phenomenological control predicts experience of mirror Synaesthesia and the rubber hand illusion. Nat Commun 2020;11:4853. 10.1038/s41467-020-18591-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lush P, Seth AK. Reply to: no specific relationship between hypnotic suggestibility and the rubber hand illusion. Nat Commun 2022;13:563. 10.1038/s41467-022-28178-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Slater M, Ehrsson HH. Multisensory integration dominates Hypnotisability and expectations in the rubber hand illusion. Front Hum Neurosci 2022;16:834492. 10.3389/fnhum.2022.834492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ehrsson HH, Fotopoulou A, Radziun D, et al. No specific relationship between hypnotic suggestibility and the rubber hand illusion. Nat Commun 2022;13:564. 10.1038/s41467-022-28177-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Jicol C, Clarke C, Tor E, et al. Imagine that! imaginative suggestibility affects presence in virtual reality. CHI 23 Proc 2023 CHI Conf Hum Factors Comput Syst; 2023. 10.1145/3544548.3581212 [DOI] [Google Scholar]
- 95. Forster PP, Karimpur H, Fiehler K. Demand characteristics challenge effects in embodiment and presence. Sci Rep 2022;12:14084. 10.1038/s41598-022-18160-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Askay SW, Patterson DR, Sharar SR. Virtual reality hypnosis. Contemporary Hypnosis 2009;26:40–7. 10.1002/ch.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Rousseaux F, Panda R, Toussaint C, et al. Virtual reality hypnosis in the management of pain: self-reported and neurophysiological measures in healthy subjects. Eur J Pain 2023;27:148–62. 10.1002/ejp.2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Huys A, Beck B, Haggard P, et al. No increased suggestibility to placebo in functional neurological disorder. Eur J Neurol 2021;28:2367–71. 10.1111/ene.14816 [DOI] [PubMed] [Google Scholar]
- 99. Espay AJ, Aybek S, Carson A, et al. Current concepts in diagnosis and treatment of functional neurological disorders. JAMA Neurol 2018;75:1132. 10.1001/jamaneurol.2018.1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Teodoro T, Koreki A, Meppelink AM, et al. Contingent negative variation: a biomarker of abnormal attention in functional movement disorders. Eur J Neurol 2020;27:985–94. 10.1111/ene.14189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Levett JJ, Elkaim LM, Niazi F, et al. Invasive brain computer interface for motor restoration in spinal cord injury: a systematic review. Neuromodulation 2024;27:597–603. 10.1016/j.neurom.2023.10.006 [DOI] [PubMed] [Google Scholar]
- 102. Mane R, Chouhan T, Guan C. BCI for stroke rehabilitation: motor and beyond. J Neural Eng 2020;17:041001. 10.1088/1741-2552/aba162 [DOI] [PubMed] [Google Scholar]
- 103. Stone J, Carson A, Hallett M. Explanation as treatment for functional neurologic disorders. Handb Clin Neurol 2016;139:543–53. 10.1016/B978-0-12-801772-2.00044-8 [DOI] [PubMed] [Google Scholar]
- 104. Ulubas M, Gelauff J, Beudel M. Treat by suggestion: the potential use of N = 1 trials in functional neurological disorders. Mov Disord Clin Pract 2022;9:843–5. 10.1002/mdc3.13496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ettenhofer ML, Guise B, Brandler B, et al. Neurocognitive driving rehabilitation in virtual environments (Neurodrive): a pilot clinical trial for chronic traumatic brain injury. NeuroRehabilitation 2019;44:531–44. 10.3233/NRE-192718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Gimbel SI, Ettenhofer ML, Cordero E, et al. Brain bases of recovery following cognitive rehabilitation for traumatic brain injury: a preliminary study. Brain Imaging Behav 2021;15:410–20. 10.1007/s11682-020-00269-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. de Tommaso M, Ricci K, Laneve L, et al. Virtual visual effect of hospital waiting room on pain modulation in healthy subjects and patients with chronic migraine. Pain Res Treat 2013;2013:515730. 10.1155/2013/515730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Paul M, Bullock K, Bailenson J. Virtual reality behavioral activation for adults with major depressive disorder: feasibility randomized controlled trial. JMIR Ment Health 2022;9:e35526. 10.2196/35526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Duan S, Valmaggia L, Fennema D, et al. Remote virtual reality assessment Elucidates self-blame-related action tendencies in depression. J Psychiatr Res 2023;161:77–83. 10.1016/j.jpsychires.2023.02.031 [DOI] [PubMed] [Google Scholar]
- 110. Kiper P, Szczudlik A, Agostini M, et al. Virtual reality for upper limb rehabilitation in subacute and chronic stroke: a randomized controlled trial. Arch Phys Med Rehabil 2018;99:834–42. 10.1016/j.apmr.2018.01.023 [DOI] [PubMed] [Google Scholar]
- 111. Munafo J, Diedrick M, Stoffregen TA. The virtual reality head-mounted display Oculus rift induces motion sickness and is Sexist in its effects. Exp Brain Res 2017;235:889–901. 10.1007/s00221-016-4846-7 [DOI] [PubMed] [Google Scholar]
- 112. Buttussi F, Chittaro L. Locomotion in place in virtual reality: a comparative evaluation of Joystick, Teleport, and leaning. IEEE Trans Vis Comput Graph 2021;27:125–36. 10.1109/TVCG.2019.2928304 [DOI] [PubMed] [Google Scholar]
- 113. van Heugten-van der Kloet D, Cosgrave J, van Rheede J, et al. Out-of-body experience in virtual reality induces acute dissociation. Psychol Conscious: Theory, Res, Pract 2018;5:346–57. 10.1037/cns0000172 [DOI] [Google Scholar]
- 114. Huys A, Haggard P, Bhatia KP, et al. A note of caution on distorted visual feedback as a treatment for functional movement disorders. Mov Disord Clin Pract 2022;9:275–7. 10.1002/mdc3.13386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604–7. 10.1161/01.str.19.5.604 [DOI] [PubMed] [Google Scholar]
- 116. Mutlu B, Serbetcioglu B. Discussion of the dizziness handicap inventory. J Vestib Res 2013;23:271–7. 10.3233/VES-130488 [DOI] [PubMed] [Google Scholar]
- 117. Costa PT, McCrae RR. The revised NEO personality inventory (NEO-PI-R). In: The SAGE handbook of personality theory and assessment: volume 2 — personality measurement and testing. 1 Oliver’s Yard, 55 City Road, London EC1Y 1SP, United Kingdom: SAGE Publications Ltd, 2008: 179–98. Available: https://sk.sagepub.com/reference/hdbk_personalitytheory2/n9.xml [Google Scholar]
- 118. Kear BM, Guck TP, McGaha AL. Timed up and go (TUG) test: normative reference values for ages 20 to 59 years and relationships with physical and mental health risk factors. J Prim Care Community Health 2017;8:9–13. 10.1177/2150131916659282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Dannenbaum E, Chilingaryan G, Fung J. Visual vertigo analogue scale: an assessment questionnaire for visual vertigo. J Vestib Res 2011;21:153–9. 10.3233/VES-2011-0412 [DOI] [PubMed] [Google Scholar]
- 120. Jacob RG, Lilienfeld SO, Furman JMR, et al. Panic disorder with vestibular dysfunction: further clinical observations and description of space and motion Phobic stimuli. J Anxiety Disord 1989;3:117–30. 10.1016/0887-6185(89)90006-6 [DOI] [Google Scholar]
- 121. Pavlou M, Davies RA, Bronstein AM. The assessment of increased sensitivity to visual stimuli in patients with chronic dizziness. VES 2007;16:223–31. 10.3233/VES-2006-164-509 [DOI] [PubMed] [Google Scholar]
- 122. van Dixhoorn J, Duivenvoorden HJ. Efficacy of Nijmegen questionnaire in recognition of the hyperventilation syndrome. J Psychosom Res 1985;29:199–206. 10.1016/0022-3999(85)90042-x [DOI] [PubMed] [Google Scholar]
- 123. Bjelland I, Dahl AA, Haug TT, et al. The validity of the hospital anxiety and depression scale. J Psychosom Res 2002;52:69–77. 10.1016/S0022-3999(01)00296-3 [DOI] [PubMed] [Google Scholar]
- 124. Yagi C, Morita Y, Kitazawa M, et al. A validated questionnaire to assess the severity of persistent postural-perceptual dizziness (PPPD): the Niigata PPPD questionnaire (NPQ). Otol Neurotol 2019;40:e747–52. 10.1097/MAO.0000000000002325 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjno-2023-000622supp001.pdf (79.6KB, pdf)