Abstract
We have investigated whether hypoviruses, viral agents responsible for virulence attenuation (hypovirulence) of the chestnut blight fungus Cryphonectria parasitica, could serve as gene expression vectors. The infectious cDNA clone of the prototypic hypovirus CHV1-EP713 was modified to generate 20 different vector candidates. Although transient expression was achieved for a subset of vectors that contained the green fluorescent protein gene from Aequorea victoria, long-term expression (past day 8) was not observed for any vector construct. Analysis of viral RNAs recovered from transfected fungal colonies revealed that the foreign genes were readily deleted from the replicating virus, although small portions of foreign sequences were retained by some vectors after months of replication. However, the results of vector viability and progeny characterization provided unexpected new insights into essential and dispensable elements of hypovirus replication. The N-terminal portion (codons 1 to 24) of the 5′-proximal open reading frame (ORF), ORF A, was found to be required for virus replication, while the remaining 598 codons of this ORF were completely dispensable. Substantial alterations were tolerated in the pentanucleotide UAAUG that contains the ORF A termination codon and the overlapping putative initiation codon of the second of the two hypovirus ORFs, ORF B. Replication competence was maintained following either a frameshift mutation that caused a two-codon extension of ORF A or a modification that produced a single-ORF genomic organization. These results are discussed in terms of determinants of hypovirus replication, the potential utility of hypoviruses as gene expression vectors, and possible mechanisms by which hypoviruses recognize and delete foreign sequences.
Fungi representing all major taxons have been reported to harbor viruses and related virus-like double-stranded (ds) RNA genetic elements (1, 17, 29). Similar to viruses of animals and plants, mycoviruses have potential utility for elucidating host functions and manipulating host phenotype (see reference 41). Evidence of progress in developing this potential is provided by recent studies with members of the family Hypoviridae that cause an attenuation of virulence (hypovirulence) and alter dependent developmental process, e.g., asexual and sexual sporulation, of their host, the chestnut blight fungus Cryphonectria parasitica. For example, efforts to understand the molecular basis for hypovirulence revealed a crucial role for G-protein signal transduction in a wide range of vital fungal physiological processes that included pathogenesis (4, 9, 15, 16, 23, 24, 40). In related studies, Chen and Nuss (5) used full-length infectious cDNA clones of genomic RNAs derived from severe and mild hypovirus strains to differentially influence the fungal phenotype and the interaction between C. parasitica and its plant host.
The availability of a reverse genetics system for hypoviruses (3, 7) also provides opportunities to examine the consequences of specific mutations of the viral genome and explore the potential for development of hypoviruses as fungal gene expression vectors. In this regard, deletion of 88% of the coding region for the papain-like protease p29 from the infectious cDNA clone of the prototypic hypovirus CHV1-EP713 demonstrated that this viral protein was dispensable for viral replication and revealed the extent of its contribution to virus-mediated reduction in fungal pigmentation and asexual sporulation (10). This observation also implied that one could replace the deleted portion of the viral genome with heterologous sequences, thus generating a hypovirus RNA cytoplasmically replicating gene expression vector system. Additionally, since CHV1-EP713 RNA is not encapsidated, constraints on the size of heterologous inserts, as are often encountered with many other virus vector systems, were expected to be minimal.
Several potential applications can be envisioned for a cytoplasmically transmissible RNA-based hypovirus gene expression vector. The incorporation of a visual reporter gene could provide a convenient means for monitoring hypovirus movement from cell to cell during anastomosis or long distance through a fungal colony or even a fungal population. It might be possible to enhance ecological fitness of hypovirulent C. parasitica strains by expressing nuclear genes that are normally downregulated by hypovirus infection, e.g., viral expression of the mating pheromone gene Mat-2 (43) might relieve virus-mediated female infertility. Hypovirus host range might be extended to a number of pathogenic fungi by incorporating C. parasitica host range determinants, once identified, within recombinant viral constructs. It is also conceivable that the phenotypic changes caused by hypovirus infection could be further modified by the incorporation of foreign genes that might alter specific host metabolic or signaling pathways. Hypoviruses may even be exploited to deliver secretable gene products from infected fungal cells to difficult to transform chestnut plant cells, e.g., to enhance plant defense responses.
This study describes extensive efforts to modify the infectious CHV1-EP713 cDNA clone to express foreign genes. Although stable expression constructs were not obtained, transient expression of introduced foreign genes was confirmed. Moreover, significant new insights into essential and dispensable virus-encoded elements of replication were revealed by analysis of vector constructs and resulting progeny viruses.
MATERIALS AND METHODS
Source of heterologous gene inserts.
Four genes of nonhypovirus origin were selected for insertion into the full-length infectious cDNA clone of prototypic hypovirus CHV1-EP713. These included two reporter genes, the enhanced green fluorescent protein gene (EGFP) from Aequorea victoria (30) and the Escherichia coli hygromycin B phosphotransferase gene (HYG) (11). The coding regions of these reporters were amplified from plasmids pEGFP (Clontech Laboratories, Inc., Palo Alto, Calif.) and pCPXHY1 (10), respectively, by PCR using primers that also introduced specific terminal restriction sites and cloned into pPCR-Script SK(+) (Stratagene, La Jolla, Calif.). Two nonreporter genes that included the foot-and-mouth disease virus 2A protease gene (2A) (32) and the Mat-2 mating type pheromone gene (PH) of C. parasitica (43) were generated as synthetic oligonucleotides. The codon usage for the 2A gene, which encodes the 17-residue peptide NFDLLKLAGDVESNPGP, was modified to match that of C. parasitica nuclear genes (AAA-TTT-GAC-TTG-CTC-AAG-TTG-GCC-GGT-GAC-GTC-GAG-TCC-AAC-CCG-GGT-CCC).
Construction of virus expression vectors.
Modification of and gene insertion into the full-length hypovirus cDNA was facilitated by the development of a mutation/modification cassette system. The foundation plasmid pTRN was generated by PCR-mediated replacement (21) of the original multiple cloning site of the commercially available plasmid pTZ19R (U.S. Biochemicals, Cleveland, Ohio) with oligonucleotides specifying a multiple cloning site (NotI, SacII, AscI, XbaI, NheI, KpnI, RsrII, and SpeI) that contained combinations of the unique restriction sites found in the plasmid harboring the full-length CHV1-EP713 viral cDNA, pLDST. Construction of the pTRN-based cassette used in this study, pTNR4, involved insertion of the 5′-terminal 3.7-kb XbaI-NheI CHV1-EP713 cDNA fragment derived from pLDST (7), followed by modifications of the multiple cloning site for applications not described in this study that included destruction of the XbaI site and introduction of an additional NotI site following the SpeI site. Following appropriate alterations of, or insertion of foreign gene sequences into, the viral cDNA fragment in pTRN4, modified full-length CHV1-EP713 viral cDNAs were reconstituted by insertion of the 3′-terminal 8.9-kb NheI-SpeI CHV1-EP713 cDNA fragment, also derived from pLDST. The resulting plasmids were linearized at the SpeI site and used as a template for in vitro synthesis of viral vector transcripts from the T7 polymerase promoter present in the pLDST-derived XbaI-NheI fragment. All synthetic viral-vector transcripts contained the wild-type CHV1-EP713 5′- and 3′-noncoding terminal sequences with the exception of the group II vectors, which contained a two-nucleotide change immediately upstream of the open reading frame (ORF) A initiator codon from taATGg to ccATGg. Transfection of C. parasitica spheroplasts and recovery of infected fungal colonies was performed as described by Suzuki et al. (38). The integrity of each vector construct was verified by sequence analysis at insert junctions and modification sites. Detailed descriptions of cloning steps are available from the authors upon request.
GFP visualization.
Sterile glass coverslips (22 by 22 mm) were placed on cellophane overlaying potato dextrose agar (PDA) and hyphae of transfected fungal colonies were allowed to grow as a thin layer between the cellophane and coverslip from a small agar inoculation plug placed at the edge of the coverslip. Coverslips with attached hyphae were transferred to a glass slide, mounted with distilled water, and observed with a Leica fluorescence stereomicroscope model MZ FLIII (Leica Microscopy Systems, Heerbrugg, Switzerland). Emission of green fluorescence was observed with a GFP plant fluorescent filter set (GFP3; Leica Microscopy Systems) and photographed with a Spot 1.3.0 charge-coupled device digital camera (Diagnostic Instruments, Inc., Sterling Heights, Mich.). A C. parasitica transformant (strain EGFP-CP) in which EGFP gene expression was driven by the C. parasitica glyceraldehyde-3-phosphate dehydrogenase (GPD) gene promoter (Pgpd) was used as a positive control.
dsRNA isolation and ClampR analysis.
dsRNA was isolated from fungal colonies transfected with transcripts of each of the vector constructs and subjected to agarose gel electrophoretic analysis as described by Suzuki et al. (38). A single-tube, reverse transcriptase PCR protocol, ClampR (25), was performed on isolated dsRNA preparations as described previously (38). The 25-μl reaction mixture contained 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, appropriate primer sets (10 pmol each) annealed to dsRNA (100 ng) after a previous denaturation and annealing step, 1 U of avian myeloblastosis virus reverse transcriptase (Life Technologies, Gaithersburg, Md.), and 1 U of AmpliTaq DNA polymerase (Perkin-Elmer, Branchburg, N.J.). The reaction mixtures were incubated at 42°C for 30 min, followed by 30 PCR cycles of 94°C for 1 min, 45°C for 1.5 min, and 72°C for 2 min. Amplified PCR products were subcloned into pPCRScript and subjected to sequence analysis.
RESULTS
Strategies for design of hypovirus expression vectors.
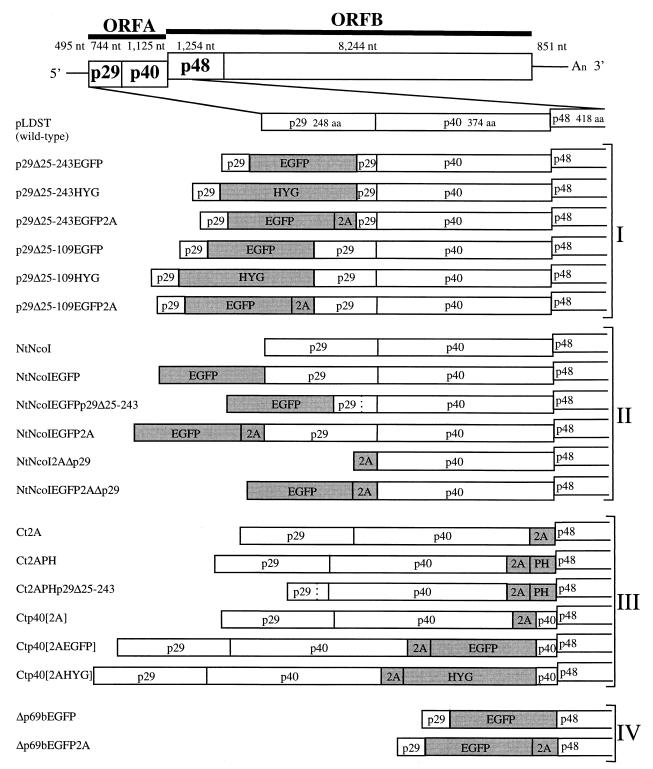
As indicated in Fig. 1, hypovirus CHV1-EP713 contains two large ORFs designated ORF A and ORF B (34). ORF A encodes two polypeptides, p29 and p40, that are released from polyprotein p69 by an autocatalytic event mediated by the papain-like protease domain within p29 (6). Expression of ORF B also involves an autoproteolytic event in which p48, also a papain-like protease, is released from the N-terminal portion of the encoded polyprotein. The junction between ORFs A and B consists of the sequence 5′-UAAUG-3′, where the UAA portion serves as the termination codon of ORF A and the AUG portion is the 5′-proximal translation initiation codon of ORF B.
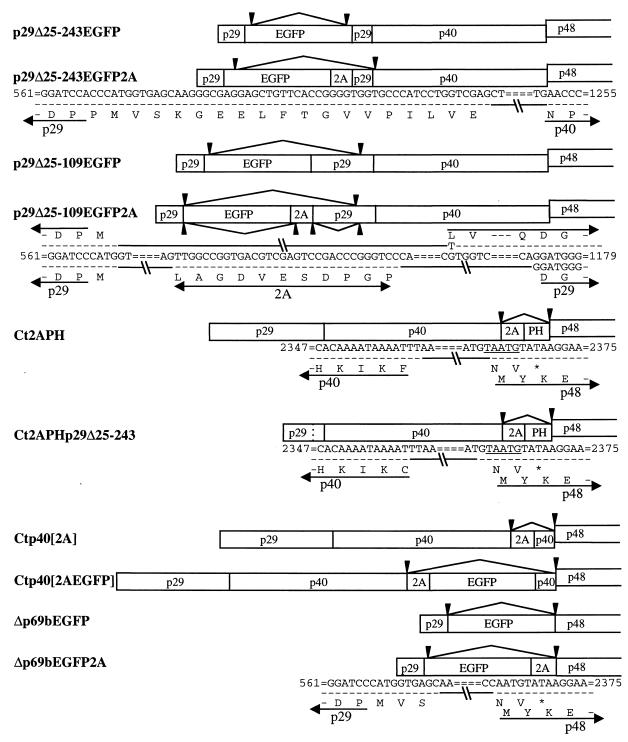
FIG. 1.
Schematic representation of vector constructs. The genetic organization of the infectious cDNA clone of wild-type hypovirus CHV1-EP713 in plasmid pLDST (7) is shown at the top. The coding strand is 12,712 nucleotides in length, excluding the poly(A) tract, and contains two ORFs, A and B (34). ORF A encodes two polypeptides, p29 and p40, that are released from polyprotein p69 by an autocatalytic event mediated by the papain-like protease domain within p29 (6). Expression of ORF B also involves an autoproteolytic event in which p48, also a papain-like protease, is released from the N-terminal portion of the encoded polyprotein. The junction between ORFs A and B consists of the sequence 5′-UAAUG-3′ (map positions 2362 to 2366) where the UAA portion serves as the termination codon of ORF A and the AUG portion is the 5′-proximal translation initiation codon of ORF B. Diagrams of 20 CHV1-EP713-based gene expression vector candidates, organized within four groups (I to IV) on the basis of four design strategies, are shown below the wild-type CHV1-EP713 cDNA. Four foreign genes (indicated by shaded boxes) were incorporated into the vectors: the enhanced A. victoria green fluorescent protein gene (EGFP), the E. coli hygromycin B phosphotransferase gene (HYG), the foot-and-mouth disease virus 2A protease gene (2A), and the Mat-2 mating type pheromone gene (PH) of C. parasitica. The dashed lines shown in vector constructs NtNcoIEGFPp29Δ25-243 and Ct2APHp29Δ25-243 indicate the deletion of the BamHI fragment (bases 562 to 1218) of the p29 coding domain leading to the fusion of p29 proline residue 24 with p29 leucine residue 244. The cloning operations used to construct individual expression vector candidates are described in detail in Methods and Materials and in Results.
Four basic strategies were explored to construct a total of 20 hypovirus expression vector candidates. The observation that 88% of the p29 papain-like protease coding domain of ORF A was dispensable for viral replication (10) provided the basis for the first group of vectors, targeting the p29 coding region as a candidate for insertion of foreign gene sequences. Two foreign genes previously demonstrated to express efficiently in transformed C. parasitica were utilized for these initial studies. The E. coli hygromycin phosphotransferase gene (HYG) is the basic selection marker used in a large number of C. parasitica transformation vectors (2, 8, 10, 16). The enhanced mutant form of the GFP (EGFP) gene from Aequorea victoria (30) is expressed efficiently under the control of the C. parasitica GPD promoter after ectopic chromosomal integration (see Fig. 3).
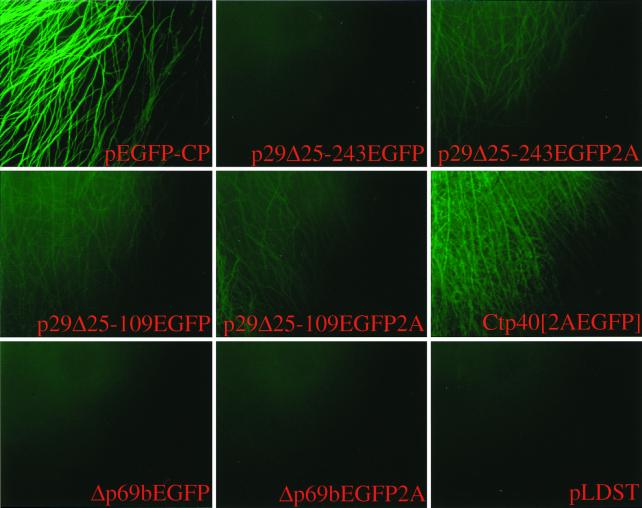
FIG. 3.
Micrographs of EGFP-expressing C. parasitica strains. Agar plugs containing transfected fungal mycelia were transferred from regeneration plates to cellophane-overlaid PDA plates on day 4 posttransfection and placed at the edge of a glass coverslip. The mycelia were allowed to grow between the coverslip and the cellophane for 2 days and then transferred to a glass slide for observation (see Materials and Methods). Mycelia attached to the coverglass were observed under a fluorescent microscope at ×100 magnification. Vector constructs used for transfection are indicated at the bottom of each panel and include p29Δ25-243EGFP, p29Δ25-243EGFP2A, p29Δ25-109EGFP, p29Δ25-109EGFP2A, Ctp40[2AEGFP], Δp69bEGFP, and Δp69bEGFP2A. A colony transfected with synthetic transcripts derived from wild-type virus cDNA, pLDST, served as a negative control, while a transformant in which the EGFP gene (pEGFP-CP) is expressed from the C. parasitica GPD gene promoter (Pgpd) was used as a positive control.
For group I vectors, the reporter genes were inserted in frame into previously characterized p29 deletion mutant infectious cDNAs. The first of these mutants, originally described by Craven et al. (10) and designated Δp29 (referred to here as p29Δ25-243), contained a deletion of the BamHI fragment spanning map positions 562 to 1218, thereby fusing codon Pro24 with codon Leu244 (see reference 34 for the CHV1-EP713 sequence map coordinates). The second mutant, designated p29Δ25-109, contained a smaller deletion extending from map position 562 to 822, fusing Pro24 with Glu110 (38). Since the active p29 protease domain (amino acid [aa] residues 135 to 248) and cleavage site (Gly248-Gly249) remained intact in the latter mutant (6), the p29-reporter fusion protein encoded in vectors p29Δ25-109EGFP and p29Δ25-109HYG was predicted to be cleaved from the polyprotein at the normal p29 cleavage site. In contrast, the p29-reporter fusion protein produced by the Δp29-based constructs p29Δ25-243EGFP and p29Δ25-243HYG, which lacked the p29 protease domain, was predicted to be further fused to p40. Two additional group I vectors (p29Δ25-109EGFP2A and p29Δ25-243EGFP2A) were engineered to incorporate the foot-and-mouth disease virus 2A protease (2A) (32) with the intention of liberating an N-terminal p29-reporter fusion protein.
Vectors in group II contained modifications or gene insertions at the precise N terminus (designated Nt in vector constructs) of ORF A. This was accomplished by first using PCR mutagenesis to modify the ORF A translation initiation codon from TAATGG (map positions 494 to 499) to CCATGG, thus creating an NcoI restriction site in the foundation vector construct NtNcoI. Other constructs in this group were then generated by insertions of reporter genes into the NcoI site. Vector NtNcoIEGFP was predicted to produce EGFP fused to p29, while deletion of the p29 BamHI fragment (map positions 562 to 1218) in vector NtNcoIEGFPp29Δ25-243 was predicted to produce EGFP flanked by p29 aa residues 1 to 24 at the N terminus and by p29 aa residues 244 to 248 at the C terminus all fused to p40. The 2A protease domain was introduced into the 3′-flanking region of the EGFP in vector NtNcoIEGFP2AΔp29 with the intention of liberating the EGFP-2A fusion protein from p29. An NcoI-modified translation initiation codon was also engineered at the N terminus of a vector in which the entire p29 coding domain was deleted and a 2A gene was fused to the p40 coding domain. The NcoI site in this vector, NtNcoI2AΔp29, was then used to introduce the EGFP sequence to form vector NtNcoIEGFP2AΔp29.
Vectors in group III contained gene inserts in the 3′-terminal portion of the p40 coding domain near or adjacent to the pentanucleotide UAAUG that separates ORF A from ORF B, i.e., in the C terminus (designated Ct in vector constructs) of the ORF A-encoded polyprotein. Vectors Ct2A and Ct2APH contained, respectively, the 2A domain and the 2A domain fused to the C. parasitica Mat-2 pheromone gene (PH) inserted immediately upstream of the pentanucleotide. The latter construct was predicted to produce an unfused pheromone protein. Vector Ct2APHp29Δ25-243 was similar to Ct2APH but was in the background of the original Δp29Δ25-243 recombinant virus. In vector Ctp40[2A], the 3′-terminal sequence of the p40 coding domain (map positions 2,318 to 2,361) was duplicated to form an intermediate construct that contained an SbfI site for subsequent insertion of the EGFP and HYG genes to produce vectors Ctp40[2AEGFP] and Ctp40[2AHYG], respectively. These last two vectors were predicted to produce reporter proteins fused at the C terminus with p40 amino acid residues 361 to 374.
The final group of vectors, group IV, consisted of two constructs in which the EGFP gene was inserted in place of most of ORF A (the p69 polyprotein coding domain). Vectors Δp69bEGFP and Δp69bEGFP2A contained the EGFP and EGFP–C-terminal 2A fusion genes inserted between the BamHI site at position 562 and a new BamHI site engineered precisely at the pentanucleotide in such a way that the stop codon was destroyed (UAAUG to CCAUG) while retaining the AUG initiator codon in frame with the upstream region. That is, these vectors have a single large ORF that is predicted to produce the EGFP fused at the N terminus with p29 aa 1 to 24 and at the C terminus with p48 of ORF B or unfused at the C terminus due to 2A protease activity, respectively.
Replication competence of expression vector constructs.
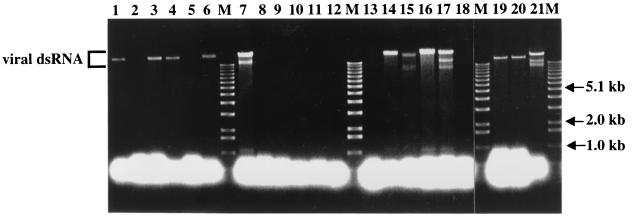
The replication competence of each vector construct was examined by transfecting spheroplasts prepared from virus-free C. parasitica EP155 and scoring for the recovery of viral dsRNA from regenerated mycelial cultures (results are summarized in Table 1). Although the HYG gene is efficiently expressed in C. parasitica from integrated plasmid vectors (7, 8), neither of the group I vectors that contained HYG as the foreign gene (p29Δ25-243HYG and p29Δ25-109HYG) were found to be replication competent (Fig. 2, lanes 2 and 5). In contrast, transcripts derived from all EGFP-containing vectors were able to readily establish productive infections (Fig. 2, lanes 1, 3, 4, and 6).
TABLE 1.
Properties of vector constructs
| Group and vector | Replication competencya | Green fluorescenceb | Stabilityc |
|---|---|---|---|
| I | |||
| p29Δ25-243EGFP | + | − | − |
| p29Δ25-243HYG | − | NAd | NA |
| p29Δ25-243EGFP2A | + | + | − |
| p29Δ25-109EGFP | + | + | − |
| p29Δ25-109HYG | − | NA | NA |
| p29Δ25-109EGFP2A | + | + | − |
| II | |||
| NtNcoI | + | NA | ++ |
| NtNcoIEGFP | − | NA | NA |
| NtNcoIEGFPp29Δ25-243 | − | NA | NA |
| NtNcoIEGFP2A | − | NA | NA |
| NtNcoI2AΔp29 | − | NA | NA |
| NtNcoIEGFP2AΔp29 | − | NA | NA |
| III | |||
| Ct2A | − | NA | NA |
| Ct2APH | + | NA | + |
| Ct2APHp29Δ25-243 | + | NA | + |
| Ctp40[2A] | + | NA | + |
| Ctp40[2AEGFP] | + | +++ | − |
| Ctp40[2AHYG] | − | NA | NA |
| IV | |||
| Δp69bEGFP | + | − | + |
| Δp69bEGFP2A | + | − | + |
Vector viability was examined based on virus-specific dsRNA recovery as shown in Fig. 2.
The ability of a vector virus to fluoresce is indicated as +++ (moderately highly fluorescent), + (faintly fluorescent), and − (negative).
The stability of vectors was analyzed by ClampR: ++, stable as long as 9 weeks posttransfection; +, stable as long as 3 weeks posttransfection; −, underwent deletion before 3 weeks posttransfection.
NA, not applicable.
FIG. 2.
Agarose gel electrophoretic analysis of viral dsRNAs recovered from transfected C. parasitica colonies 3 weeks posttransfection. Transfected fungal colonies were transferred from regeneration media to PDA plates 7 days posttransfection, cultured for 1 week, transferred to liquid growth media for an additional week of culturing, harvested, and processed to recover dsRNA according to the protocol of Hillman et al. (19). Partially purified viral dsRNA preparations were treated with S1 nuclease (5 U) to digest single-stranded RNA (3) and examined by agarose (0.7%) gel electrophoresis. Full-length viral dsRNAs are the slowest-migrating bands in each lane. The faster-migrating species observed in lanes 7, 15, 17, and 21 correspond to internally deleted defective viral dsRNAs previously identified in hypovirus-infected isolates (2, 35). Lanes marked with an M contain 200 ng of 1-kb DNA ladder (Life Technologies) as relative size markers, with arrows indicating the positions of the 5.1-, 2.0-, and 1.0-kb bands. Lane 1, p29Δ25-243EGFP; lane 2, p29Δ25-243HYG; lane 3, p29Δ25-243EGFP2A; lane 4, p29Δ25-109EGFP; lane 5, p29Δ25-109HYG; lane 6, p29Δ25-109EGFP2A; lane 7, NtNcoI; lane 8, NtNcoIEGFP; lane 9, NtNcoIEGFPp29Δ25-243; lane 10, NtNcoIEGFP2A; lane 11, NtNcoI2AΔp29; lane 12, NtNcoIEGFP2AΔp29; lane 13, Ct2A; lane 14, Ct2APH; lane 15, Ct2APHp29Δ25-243; lane 16, Ctp40[2A]; lane 17, Ctp40[2AEGFP]; lane 18, Ctp40[2AHYG]; lane 19, Δp69bEGFP; lane 20, Δp69bEGFP2A; lane 21, wild-type virus cDNA pLDST.
The group II foundation vector, NtNcoI, readily established an infection upon transfection (Fig. 2, lane 7) and stably retained the altered sequence (TAATGG to CCATGG) adjacent to the ORF A initiation codon (data not shown), indicating that the mutation required to introduce the NcoI site had no gross negative effect on virus replication. Interestingly, the introduction of foreign gene sequences into this NcoI site resulted in loss of replication competency (Fig. 2, lanes 8 to 12). Moreover, transcripts derived from vectors NtNcoI2AΔp29 and NtNcoIEGFP2AΔp29, both of which lacked any of the p29 coding domain, also failed to initiate productive infections. The potential role for the 5′ proximal portion of the p29 coding domain in CHV1-EP713 replication will be addressed in a subsequent section.
Insertion of foreign sequences adjacent to the UAAUG pentanucleotide separating ORFs A and B in the group III vectors had mixed consequences for virus replication. Vector Ct2A, in which the 2A protease gene was inserted immediately upstream of the UAAUG pentanucleotide, was replication incompetent (Fig. 2, lane 13). However, insertion of the C. parasitica Mat-2 pheromone gene (43) preceded by the 2A gene into the same position, either in the context of the full-length CHV1-EP713 sequence (Ct2APH) or the Δp29 background (Ct2APHp29Δ25-243), was tolerated and yielded infectious transcripts (Fig. 2, lanes 14 and 15). Similarly, transcripts derived from vectors Ctp40[2A] and Ctp40[2AEGFP], in which the 3′-terminal sequence of the p40 coding domain (nucleotides 2,318 to 2,361) was duplicated to form an SbfI site for insertion of foreign genes, were infectious (Fig. 2, lanes 16 and 17). Finally, as was observed for group I vectors containing the HYG gene, vector Ctp40[2AHYG] was not replication competent.
Group IV expression vectors were constructed with the anticipation that they might be replication incompetent. Although it was clearly established that 88% of the p29 coding domain could be deleted without abolishing CHV1-EP713 replication (10), there was no evidence to suggest that the p40 coding domain would also be dispensable. Additionally, the pentanucleotide separating ORFs A and B was mutated to remove the ORF A translation stop codon, creating an in-frame single ORF genome organization. Surprisingly, transcripts derived from both vectors, Δp69bEGFP and Δp69bEGFP2A, readily initiated infections (Fig. 2, lanes 19 and 20). Thus, p40, like most of p29, is dispensable for CHV1-EP713 replication. The role of the UAAUG pentanucleotide will be considered in more detail in a later section.
EGFP expression in fungal cells infected with expression vector constructs.
Replication-competent EGFP constructs p29Δ25-243EGFP, p29Δ25-243EGFP2A, p29Δ25-109EGFP, p29Δ25-109EGFP2A, Ctp40[2AEGFP], Δp69bEGFP, and Δp69bEGFP2A were examined for green fluorescence on day 6 posttransfection (results are summarized in Table 1). The highest level of fluorescence was observed for mycelia infected with vector Ctp40[2AEGFP] (Fig. 3). However, the level of fluorescence was considerably lower than that exhibited by mycelia stably transformed with the EGFP gene under the control of the C. parasitica GPD promoter, pEGFP-CP (Fig. 3). Fluorescence was also detected at very low levels in mycelia transfected with vectors p29Δ25-243EGFP2A, p29Δ25-109EGFP, and p29Δ25-109EGFP2A (Fig. 3). Importantly, green fluorescence was lost for each of the EGFP-positive transfectants within several weeks of culturing following transfer of a portion of the mature colony to new PDA plates. No fluorescence was observed at any time posttransfection for mycelia infected with transcripts derived from vectors p29Δ25-243EGFP, Δp69bEGFP, and Δp69bEGFP2A or for colonies transfected with wild-type CHV1-713 transcripts (pLDST) (Fig. 3).
Stability of expression vectors after transfection.
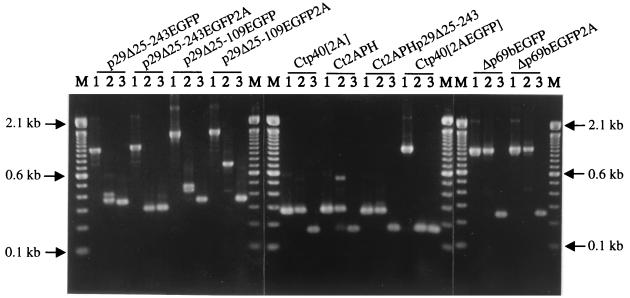
The transient nature of GFP fluorescence expression suggested either a significant reduction in vector replication or in the stability of the foreign gene within the replicating vector RNA. Since dsRNA was found to be present in infected mycelia even months after transfection (data not shown), a single-tube reverse transcription PCR analysis, ClampR (25), was performed on dsRNAs recovered from infected mycelium at 3 and 9 weeks posttransfection in an effort to examine foreign gene stability. As indicated in Fig. 4, the foreign gene sequences were partially or completely deleted from all group I vector RNAs by 3 weeks posttransfection. In contrast, foreign genes were retained in three of the four infectious group III vectors, Ct2A40, Ct2APH, and Ct2APHp29Δ25-243, at week 3. Similar results were observed for the two infectious group IV vectors. However, foreign genes appeared to be deleted from replicating vector RNAs in all groups by week 9.
FIG. 4.
ClampR analysis of dsRNA recovered from fungal colonies transfected with candidate expression vectors. For each replication-competent vector (shown at the top) ClampR was performed on the full-length cDNA clone (lane 1 in each set) and on dsRNA isolated 3 weeks (lane 2 of each set) and 9 weeks (lane 3 of each set) posttransfection. Transfected hyphae were transferred from regeneration plates to PDA plates at 7 days posttransfection. After incubation for 7 days, the cultures were transferred to liquid medium for an additional week of culturing and then harvested for recovery of dsRNA (3-week samples) or transferred weekly to new PDA plates for 6 weeks (9-week samples) before transfer to liquid medium. Primer sets were chosen to amplify fragments spanning the foreign gene insert as follows: NS7 and BR54 (nucleotide map positions 476 to 493 and 1386 to 1402, respectively) for vectors p29Δ25-243EGFP, p29Δ25-243EGFP2A, p29Δ25-109EGFP, and p29Δ25-109EGFP2A; NS21 and NS22 (nucleotide map positions 2240 to 2259 and 2382 to 2401, respectively) for Ctp40[2A], Ct2APH, Ct2APHp29Δ25-243, and Ctp40[2AEGFP]; and BR16 and NS22 (nucleotide map positions 364 to 382 and 2382 to 2401, respectively) for Δp69bEGFP and Δp69bEGFP2A. Amplified fragments were electrophoresed in a 2.0% agarose gel in 1× Tris-borate-EDTA. M refers to the 100-bp DNA ladder size markers (Life Technologies). Arrows indicate the migration positions of the 2.1-, 0.6-, and 0.1-kbp size standards.
To gain further insight into the deletion process, ClampR fragments generated from 9-week-old colonies (Fig. 4) were cloned and sequenced. With the exception of p29Δ25-109EGFP2A, a single deletion breakpoint was observed for five individual subcloned ClampR fragments generated from the progeny RNA of each vector, suggesting that a population consisting of a major deletion species was stabilized by 9 weeks posttransfection. As indicated in Fig. 5, progeny of all replication-competent vector constructs in group I retained the 5′-proximal p29 coding region upstream of the BamHI site at position 562 used for foreign gene insertion and a short sequence encoding from 1 (p29Δ25-109EGFP2A) to 19 (p29Δ25-243EGFP2A) aa residues of the foreign EGFP sequence. The position of the 3′-terminal deletion breakpoints varied considerably for the different vectors. For example, the deletion progeny generated from vector p29Δ25-243EGFP retained 10 codons from the C terminus of the EGFP protein, while the 3′-deletion breakpoints for progeny derived from vectors p29Δ25-243EGFP2A, p29Δ25-109EGFP, and p29Δ25-109EGFP2A extended to different regions of the p29 or p40 coding domains. In the case of progeny of vector p29Δ25-109EGFP2A, three of the five ClampR clones retained a portion of the FMDV 2A protease sequence, whereas the other two did not. Thus, although entire foreign genes were unstable, portions of foreign sequences were retained in some hypovirus vector progeny for long periods of culturing.
FIG. 5.
Illustration of deletion breakpoints in progeny RNA recovered from mycelia transfected with hypovirus vector constructs. DNA fragments amplified by ClampR for genomic dsRNA templates recovered from infected mycelia at 9 weeks posttransfection (Fig. 4) were cloned into the SrfI site of pPCRScript (Stratagene Systems), and five independent clones were subsequently sequenced. The breakpoints for each deletion progeny are indicated by arrowheads. Nucleotide and amino acid sequences are shown below the schematic representations for some of the more informative deletion breakpoints. Map positions are indicated at each end of the sequence information. Arrows show the amino acid sequence for viral proteins p29, p40, and p48. The symbols =, −, and -//- are used to indicate sequences not shown due to space limitations, the same sequence as found in the original parental vector shown in the top row, and deleted sequences, respectively.
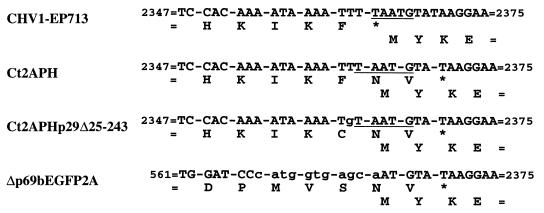
Foreign gene sequences were completely deleted from each of the group III replication-competent vector genomes, as diagrammed in Fig. 5. Vectors Ctp40[2A] and Ctp40[2AEGFP] reverted precisely to their parental virus, CHV1-EP713, suggesting a homologous recombination mediated by the duplicated p40 coding sequence of the vector constructs. The deletion events that led to removal of foreign sequences from vector Ct2APH included the additional deletion of a viral U residue immediately 5′ of the ORF A translation stop codon, causing a frameshift and a resulting two-codon extension of ORF A relative to that present in CHV1-EP713 (Fig. 5 and 6) (AUUUUAAUGUAUAA to AUUUAAUGUAUAA, the pentanucleotide within bases 2358 to 2371 is shown in boldface). A similar frameshifted deletion product was generated for Ct2APHp29Δ25-243. However, the events leading to the formation of this progeny must have involved deletion of two viral U residues since the G residue present at the third position of the C-terminal Met codon of the PH gene was retained (Fig. 5 and 6).
FIG. 6.
Sequences in the pentanucleotide region of vector construct progeny. ClampR fragments were obtained with primer set NS21 and NS22 (map positions 2240 to 2259 and 2382 to 2401, respectively) for progeny dsRNAs of vectors Ct2APH, Ct2APHp29Δ25-243, and Δp69bEGFP2A 9 weeks after transfection and sequenced. Nucleotide sequences and deduced amino acid sequences for the ORF A-ORF B junction of CHV1-EP713 are shown for reference. Asterisks indicate termination codons of ORF A, and the underlined sequence refers to the pentanucleotide. Nonviral nucleotides are shown in lowercase letters.
Different deletion patterns were observed for the two group IV vectors that were distinguished by the presence of a single ORF rather than the prototypical two-ORF organization. Vector Δp69bEGFP underwent complete deletion of the EGFP gene to revert to its progenitor, Δp69b, indicating that the progeny of this vector were able to replicate with the UAAUG pentanucleotide modified so as to fuse p29 codons 1 to 24 with the N terminus of ORF B in a single-ORF configuration. In contrast, the single-ORF vector Δp69bEGFP2A was converted to a deletion product containing two ORFs with a junction similar to that seen for the deletion products of group II vectors Ct2APH and Ct2APHp29Δ25-243, i.e., the equivalent of deleting a U residue just prior to the ORF A stop codon causing a two-codon extension into the p48 coding region and reconstruction of the ORF B coding domain.
The recovery of multiple independent viable deletion progeny that contained a two-codon extension of the 5′-proximal ORF was surprising (Fig. 6), given the prediction (34) that the UAAUG pentanucleotide junction separating ORFs A and B is likely to regulate the termination of ORF A translation and reinitiation of ribosomes for subsequent translation of ORF B. To reduce the possibility that the viability of these deletion mutants is dependent on compensatory mutations, a mutated virus cDNA, designated FS1, was constructed in which the first residue of the pentanucleotide, U 2362, was deleted within the context of the infectious virus cDNA. FS1 transcript derived from the reconstituted virus cDNA was able to replicate and to induce the same set of phenotypic changes caused by wild-type CHV1-EP713 (data not shown). The sequence integrity of the mutated region was confirmed by ClampR and sequence analyses, indicating that the mutant virus is viable and stable. We conclude that the single frameshift deletion of U 2362 and the resulting two codon extension of ORF A is tolerated without observable changes in virus replication or virus-mediated alterations of fungal phenotype.
Deletion mutation of the 5′ portion of the p29 coding domain abolishes replication competency.
With the exception of the foundation vector NtNcoI, all group II vectors were replication incompetent. Comparison of these vectors with those that could initiate an infection revealed one obvious distinguishing feature: all replication-competent vectors contained at least 66 nucleotides (map positions 496 to 561, Met1 through Pro24) of the 5′ portion of the p29 coding domain. The possibility that the 5′-proximal portion of the p29 coding domain plays a role in virus replication was investigated by further deletion from the Pro24 codon toward the N terminus of the p29 coding region within the context of the replication-competent, foreign-gene-free p29Δ25-243 and Δp69b mutants. In one set of deletion mutants, p29 codon Val12 was fused with Asp242 of p29 (Δp29-based mutant NS36a) or with Met1 of p48 (Δp69b-based mutant NS36b). In the second set of mutants, the deletion was extended to p29 translation initiation codon Met1, completely eliminating the p29 N-terminal coding domain. Both sets of deletions resulted in loss of replication competency when tested repeatedly under transfection conditions in which transcripts from the original p29Δ25-243 and Δp69b vectors consistently initiated productive infections.
DISCUSSION
Animal viruses have been modified for delivery of foreign genes of potential therapeutic value, while plant viruses are being harnessed for large scale in planta production of protein products. The availability of infectious full-length hypovirus cDNA clones (3, 5, 7), the only viral reverse genetics system currently available for the kingdom Fungi, provides the platform for manipulating a fungal virus for analogous applications. A potential for engineering hypoviruses as gene expression vectors was suggested by the observation that 88% of the p29 coding domain was dispensable for virus replication (10) and by the absence of a virus-encoded coat protein (34) that might place packaging constraints on insert size. Moreover, transcripts generated from chimeric cDNA clones derived from a severe and a mild strain were recently shown to be fully infectious and genetically stable (5). However, the performance of the 20 independent hypovirus vector candidates described in this report has failed to meet initial expectations.
It was possible to demonstrate that the CHV1-EP713 infectious transcript could maintain replication competence while accommodating the insertion of foreign genes at several different sites within ORF A. Moreover, transient expression of the inserted foreign gene was achieved for several of the vector candidates. However, long-term expression was compromised by the ability of the viral replication machinery to recognize and delete all or most of the foreign sequences within a few weeks posttransfection. In this regard, it is noteworthy that some vector progeny did retain as many as 60 nucleotides of foreign sequence for an extended time period. Efforts to find a correlation between sequence instability and differences in codon usage between viral ORFs and foreign gene sequences were not successful. The ill-defined and generally unpredictable nature of foreign gene instability in RNA virus vectors has been reviewed in detail by Scholthof et al. (33). As noted by these authors, several RNA virus vectors have found considerable utility in spite of the problem of long-term foreign gene instability.
In related studies, functionally conserved coding domains derived from hypovirus CHV1-Euro7 that differed as much as 13% at the nucleotide level from that of the corresponding CHV1-EP713 domain were stably maintained in CHV1-EP713/CHV1-Euro7 chimeric viruses (reference 5 and data not shown). Now that hypoviruses belonging to three distinct species have been sequenced in full (5, 20, 34, 36), it will be of considerable interest to determine whether genes derived from more distantly related hypoviruses will be stably retained in CHV1-EP713 chimeric viruses or recognized as foreign and deleted.
Although only transient expression of foreign genes was achieved in this study, the possibility that hypoviruses can be developed as vectors for stable gene expression is not excluded. Insertions were restricted in this study to ORF A. It is quite conceivable that regions within ORF B are more tolerant of gene insertion. An alternative vector strategy was also suggested by the observation that the 5′-terminal portion of the p29 coding domain was required for vector RNA replication. Shapira et al. (35) previously characterized internally deleted dsRNAs recovered from CHV1-EP713 infected colonies that retained only the terminal noncoding regions: ∼150 bp from the terminus corresponding to the 5′ end of the coding strand and ∼450 bp from the other terminus. Although these small dsRNAs replicated in the presence of the full-length viral RNA, efforts to use these terminal sequences to construct a helper virus-dependent replicon as a gene expression vector were unsuccessful (B. Chen and D. L. Nuss, unpublished results). The absence of a N-terminal p29 coding domain in those constructs may have been responsible for the failure to observe expression of inserted genes, a possibility currently under investigation.
The fact that defective RNAs lacking the N-terminal portion of p29 can replicate in the presence of the full-length hypovirus RNA suggests that this element does not play a direct role in the replication process. Could the N-terminal portion of p29 play a role in facilitating translation of the CHV1-EP713 coding domains? In this context, it is intriguing to consider that the long CHV1-EP713 5′-noncoding region contains seven mini-ORFs and has been shown to inhibit in vitro translation (31), properties often associated with internal ribosome entry sites (IRES) (28, 42). This raises the possibility that the 5′-terminal noncoding region and the N-terminal portion of p29 might function coordinately to facilitate an IRES-guided translation mechanism. Certainly, precedents exist for the involvement of coding domain elements in facilitating both viral RNA amplification (e.g., references 18 and 27) and cap-dependent or cap-independent translation (14, 26, 39). The role of the N-terminal portion of p29 in hypovirus replication and gene expression clearly warrants further detailed investigation.
Analyses of viable vectors and recovered progeny also provided unexpected revelations concerning the UAAUG pentanucleotide that separates ORFs A and B. As indicated by Hillman et al. (20), this pentanucleotide is conserved in the prototype members of two of the three recognized species within the genus Hypovirus, CHV1-EP713 and CHV2-NB58, that share only 60% overall nucleotide sequence identity. These authors further reported the presence of an A/U string immediately preceding the pentanucleotide for both hypovirus species: the sequences 5′-AAAAUAAAAUUUUAAUG-3′ for CHV1-EP713 (pentanucleotide in boldface) and 5′-AAAAUUUUAAUUAAUUAAUG-3′ for CHV2-NB58. Interestingly, insertion of the 2A domain immediately preceding the pentanucleotide resulted in a replication-incompetent vector, Ct2A, even though this domain was tolerated at several other sites within ORF A. In contrast, insertion of the C. parasitica pheromone gene at this site resulted in viable vectors, Ct2APH and Ct2APHp29Δ25-243. The potential secondary structure for the two sequences 5′-UCCAACCCGGGUUAAUG-3′ for the 2A gene and 5′-UGCGUUGUCAUGUAAUG-3′ for the pheromone gene, may provide a clue for the observed differences; the 2A sequence has the potential for forming a 10-residue hairpin structure that involves the first U of the pentanucleotide, U 2362.
Nucleotides immediately upstream of position 2362 are also notable in a second interesting observation regarding the pentanucleotide. Viral uracil residues were deleted in the progeny RNA recovered from mycelia transfected with two independent vector constructs (vectors Ct2APH and Ct2APHp29Δ25-243), resulting in a two-codon extension of ORF A. Additionally, a similar dicistronic genome structure was generated in the progeny of virus Δp69bEGFP2A that was engineered to have a monocistronic genome. Based on evidence from mutational analysis of an identical pentanucleotide in the dicistronic influenza virus RNA7 (22), it was suggested that the hypovirus pentanucleotide facilitated a ribosome termination and reinitiation mechanism that might, in turn, regulate the relative translation of the two ORFs (34). This would be functionally analogous to the −1 ribosomal frameshift in the yeast killer virus that regulates the relative production of the Gag and Gag-Pol fusion proteins (12) or the −1 termination-reinitiation proposed for the production of coat protein and RDRP protein by the Helminthsporium victoriae 190S totivirus (37). Even a twofold change in the efficiency of frameshifting from the normal 1.9% can have a significant effect on killer virus RNA replication (13). Thus, it was surprising that specific alteration of the pentanucleotide in mutant FS1 that extended ORF A by two codons did not cause any detectable change in viral RNA accumulation or infected host symptom expression.
An even more surprising result regarding the UAAUG pentanucleotide was the stable replication of the progeny derived from expression vector Δp69bEGFP2A, in which the two-ORF genetic configuration was abolished by a modification of the ORF A termination codon that fused Pro24 of p29 directly in frame with Met1 of p48. This result suggests that the UAAUG pentanucleotide is altogether dispensable for virus replication. In this regard, Smart et al. (36) recently reported a single-ORF configuration for hypovirus GH2, a proposed member of a third hypovirus species very distantly related to CHV1-EP713.
An additional unexpected revelation from this study was that p40 is not required for CHV1-EP713 replication. Although no function has previously been assigned to p40, computer-assisted analysis of the deduced p40 amino acid sequence indicated a very high pKa value, suggesting a possible role as a basic RNA binding protein. However, the ability to retain replication competence in the absence of any portion of the p40 coding domain (vectors Δp69bEGFP and Δp69bEGFP2A) eliminates this virus-encoded protein as playing an essential role in either viral transcription or replication.
The apparent dispensability of the pentanucleotide, most of p29 and all of p40 for virus replication, raises the question of why they are retained in the wild-type virus. The most obvious explanation is that these elements in some way contribute to viral fitness under natural field conditions. p29 has been shown to contribute to virus-mediated reductions in host pigment production, asexual sporulation, and laccase production (8, 10), and the symptom determinant has been mapped to a region extending from Phe25 through Gln73 (38). However, it is unclear just how these traits benefit virus reproduction. In this regard, preliminary studies suggest that portions of ORF A do influence the efficiency of virus transmission to asexual progeny (N. Suzuki et al., unpublished results). Fortunately, the C. parasitica-hypovirus experimental system lends itself to detailed studies of molecular host-pathogen interactions. The vector progeny recovered in this study provide the basis for future mutagenesis studies to determine the contribution of p40 to virus-mediated alteration of fungal phenotype, including hypovirulence, and the role of the pentanucleotide in regulating the relative expression of viral gene products and the role of the N terminus of p29 in translation initiation. It is anticipated that continued efforts to develop hypovirus expression vectors by gene insertions in ORF B or the use of defective RNA platforms will lead to additional revelations about hypovirus molecular biology.
ACKNOWLEDGMENTS
This work was supported in part by National Institutes of Health grant GM55981 to D.L.N.
We are grateful to Todd Parsley, Angus Dawe, and Gert Segers for helpful discussions.
REFERENCES
- 1.Buck K W. Fungal virology—an overview. In: Buck K W, editor. Fungal virology. Boca Raton, Fla: CRC Press; 1986. pp. 2–84. [Google Scholar]
- 2.Chen B, Choi G H, Nuss D L. Mitotic stability and nuclear inheritance of integrated viral cDNA in engineered hypovirulent strains of the chestnut blight fungus. EMBO J. 1993;12:2991–2998. doi: 10.1002/j.1460-2075.1993.tb05967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen B, Choi G H, Nuss D L. Attenuation of fungal virulence by synthetic infectious hypovirus transcripts. Science. 1994;264:1762–1764. doi: 10.1126/science.8209256. [DOI] [PubMed] [Google Scholar]
- 4.Chen B, Gao S, Choi G H, Nuss D L. Extensive alteration of fungal gene transcript accumulation and elevation of G-protein-regulated cAMP levels by a virulence-attenuating hypovirus. Proc Natl Acad Sci USA. 1996;93:7996–8000. doi: 10.1073/pnas.93.15.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen B, Nuss D L. Infectious cDNA clone of hypovirus CHV1-Euro7: a comparative virology approach to investigate virus-mediated hypovirulence of the chestnut blight fungus Cryphonectria parasitica. J Virol. 1999;73:985–992. doi: 10.1128/jvi.73.2.985-992.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi G H, Shapira R, Nuss D L. Cotranslational autoproteolysis involved in gene expression from a double-stranded RNA genetic element associated with hypovirulence of the chestnut blight fungus. Proc Natl Acad Sci USA. 1991;88:1167–1171. doi: 10.1073/pnas.88.4.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi G H, Nuss D L. Hypovirulence of chestnut blight fungus conferred by an infectious viral cDNA. Science. 1992;257:800–803. doi: 10.1126/science.1496400. [DOI] [PubMed] [Google Scholar]
- 8.Choi G H, Nuss D L. A viral gene confers hypovirulence-associated traits to the chestnut blight fungus. EMBO J. 1992;11:473–477. doi: 10.1002/j.1460-2075.1992.tb05077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi G H, Chen B, Nuss D L. Virus-mediated or transgenic suppression of a G protein α subunit and attenuation of fungal virulence. Proc Natl Acad Sci USA. 1995;92:305–309. doi: 10.1073/pnas.92.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craven M G, Pawlyk D M, Choi G H, Nuss D L. Papain-like protease p29 as a symptom determinant encoded by a hypovirulence-associated virus of the chestnut blight fungus. J Virol. 1993;67:6513–6521. doi: 10.1128/jvi.67.11.6513-6521.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cullen D, Leong S A, Wilson L J, Henner D J. Transformation of Aspergillus nidulans with the hygromycin resistance gene, hph. Gene. 1987;57:21–26. doi: 10.1016/0378-1119(87)90172-7. [DOI] [PubMed] [Google Scholar]
- 12.Dinman J D, Icho T, Wickner R B. A −1 ribosomal frameshift in a double-stranded RNA virus of yeast forms a gag-pol fusion protein. Proc Natl Acad Sci USA. 1991;88:174–178. doi: 10.1073/pnas.88.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinman J D, Wickner R B. Ribosomal frameshifting efficiency and Gag/Gag-Pol ratio are critical for yeast M1 double stranded RNA virus propagation. J Virol. 1992;66:3669–3676. doi: 10.1128/jvi.66.6.3669-3676.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frolov I, Schlesinger S. Translation of Sindbis virus mRNA: analysis of sequences downstream of the initiating AUG codon that enhance translation. J Virol. 1996;70:1182–1190. doi: 10.1128/jvi.70.2.1182-1190.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao S, Nuss D L. Distinct roles for two G-protein α subunits in fungal virulence, morphology and reproduction revealed by targeted gene disruption. Proc Natl Acad Sci USA. 1996;93:14122–14127. doi: 10.1073/pnas.93.24.14122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao S, Nuss D L. Mutagenesis of putative acylation sites alters function, localization and accumulation of a Giα subunit of the chestnut blight fungus Cryphonectria parasitica. Mol Plant-Microbe Interact. 1998;11:1130–1135. doi: 10.1094/MPMI.1998.11.11.1130. [DOI] [PubMed] [Google Scholar]
- 17.Ghabrial S A. Origin, adaption and evolutionary pathways of fungal viruses. Virus Genes. 1998;16:119–131. doi: 10.1023/A:1007966229595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodfellow I, Chaudhry Y, Richardson A, Meredith J, Almond J W, Barclay W, Evans D J. Identification of a cis-acting replication element within the poliovirus coding region. J Virol. 2000;74:4590–4600. doi: 10.1128/jvi.74.10.4590-4600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hillman B I, Shapira R, Nuss D L. Hypovirulence-associated suppression of host functions in Cryphonectria parasitica can be partially relieved by high light intensity. Phytopathology. 1990;80:950–956. [Google Scholar]
- 20.Hillman B I, Halpern B T, Brown M P. A viral dsRNA element of the chestnut blight fungus with a distinct genetic organization. Virology. 1994;201:241–250. doi: 10.1006/viro.1994.1289. [DOI] [PubMed] [Google Scholar]
- 21.Horton R M, Cai Z, Ho S, Pease L R. Gene splicing by overlap extension: tailor made genes using the polymerase chain reaction. BioTechniques. 1990;8:528–535. [PubMed] [Google Scholar]
- 22.Horvath C M, Williams M A, Lamb R A. Eukaryotic coupled translation of tandem cistrons: identification of the influenza B virus BM2 polypeptide. EMBO J. 1990;9:2639–2647. doi: 10.1002/j.1460-2075.1990.tb07446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasahara S, Nuss D L. Targeted disruption of a fungal G-protein β subunit gene results in increased growth but reduced virulence. Mol Plant-Microbe Interact. 1997;10:984–993. doi: 10.1094/MPMI.1997.10.8.984. [DOI] [PubMed] [Google Scholar]
- 24.Kasahara S, Nuss D L. Identification of bdm-1, a gene involved in G protein β-subunit function and α-subunit accumulation. Proc Natl Acad Sci USA. 2000;97:412–417. doi: 10.1073/pnas.97.1.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kowalik T F, Yang Y-Y, Li J K-K. Molecular cloning and comparative sequence analysis of bluetongue virus S1 by selective synthesis of specific full-length DNA copies of dsRNA genes. Virology. 1990;177:820–823. doi: 10.1016/0042-6822(90)90557-8. [DOI] [PubMed] [Google Scholar]
- 26.Lu H-H, Wimmer E. Poliovirus chimeras replicating under the translationals control of genetic elements of hepatitis C virus reveal unusual properties of the internal ribosome entry site of hepatitis C virus. Proc Natl Acad Sci USA. 1996;93:1412–1417. doi: 10.1073/pnas.93.4.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahajan S, Dolja V V, Carrington J C. Roles of the sequence encoding tobacco etch virus capsid protein in genome amplification: requirements for the translation process and a cis-active element. J Virol. 1996;70:4370–4379. doi: 10.1128/jvi.70.7.4370-4379.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McBratney S, Chen C Y, Sarnow P. Internal initiation of translation. Curr Opin Cell Biol. 1993;5:961–965. doi: 10.1016/0955-0674(93)90077-4. [DOI] [PubMed] [Google Scholar]
- 29.Nuss D L, Koltin Y. Significance of dsRNA genetic elements in plant pathogenic fungi. Annu Rev Phytopathol. 1990;28:37–58. doi: 10.1146/annurev.py.28.090190.000345. [DOI] [PubMed] [Google Scholar]
- 30.Prasher D C, Eckenrode V K, Ward W W, Prendergast F G, Cormier M J. Primary structure of the Aequorea victoria green-fluorescent protein. Gene. 1992;15:229–233. doi: 10.1016/0378-1119(92)90691-h. [DOI] [PubMed] [Google Scholar]
- 31.Rae B P, Hillman B I, Tartaglia J, Nuss D L. Characterization of double-stranded RNA genetic elements associated with biological control of chestnut blight: organization of terminal domains and identification of gene products. EMBO J. 1989;8:657–663. doi: 10.1002/j.1460-2075.1989.tb03423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryan M D, King A M, Thomas G P. Cleavage of foot-and-mouth disease virus polyprotein is mediated by residues located within a 19 amino acid sequence. J Gen Virol. 1991;72:2727–2732. doi: 10.1099/0022-1317-72-11-2727. [DOI] [PubMed] [Google Scholar]
- 33.Scholthof H B, Scholthof K-B G, Jackson A O. Plant virus gene vectors for transient expression of foreign proteins in plants. Annu Rev Phytopathol. 1996;34:299–323. doi: 10.1146/annurev.phyto.34.1.299. [DOI] [PubMed] [Google Scholar]
- 34.Shapira R, Choi G H, Nuss D L. Virus-like genetic organization and expression strategy for a double-stranded RNA genetic element associated with biological control of chestnut blight. EMBO J. 1991;10:731–739. doi: 10.1002/j.1460-2075.1991.tb08004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shapira R, Choi G H, Hillman B I, Nuss D L. The contribution of defective RNAs to complexity of viral-encoded double-stranded RNA populations present in hypovirulent strains of the chestnut blight fungus, Cryphonectria parasitica. EMBO J. 1991;10:741–746. doi: 10.1002/j.1460-2075.1991.tb08005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smart C D, Yuan W, Foglia R, Nuss D L, Fulbright D W, Hillman B I. Cryphonectria hypovirus 3, a virus species in the family Hypoviridae with a single open reading frame. Virology. 2000;265:66–73. doi: 10.1006/viro.1999.0039. [DOI] [PubMed] [Google Scholar]
- 37.Soldevila A, Ghabrial S. Expression in S. pombe of the HV 190S mycovirus RNA-dependent RNA polymerase gene from dicistronic constructs. J Virol. 2000;74:997–1003. doi: 10.1128/jvi.74.2.997-1003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suzuki N, Chen B, Nuss D L. Mapping of a hypovirus p29 protease symptom determinant domain with sequence similarity to potyvirus HC-Pro protease. J Virol. 1999;73:9478–9484. doi: 10.1128/jvi.73.11.9478-9484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verver J, Le Gall O, van Kammen A, Wellink J. The sequence between nucleotides 161 and 512 of cowpea mosaic virus M RNA is able to support internal initiation of translation in vitro. J Gen Virol. 1991;72:2339–2345. doi: 10.1099/0022-1317-72-10-2339. [DOI] [PubMed] [Google Scholar]
- 40.Wang P, Nuss D L. Induction of cellobiohydrolase I gene from C. parasitica is suppressed by hypovirus infection and regulated by a G-protein mediated signaling pathway involved in fungal pathogenesis. Proc Natl Acad Sci USA. 1995;92:11529–11533. doi: 10.1073/pnas.92.25.11529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wickner R B. Viruses of yeast, fungi and parasitic microorganisms. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 557–585. [Google Scholar]
- 42.Wimmer E, Hellen C U T, Cao X M. Genetics of poliovirus. Annu Rev Genet. 1993;27:353–436. doi: 10.1146/annurev.ge.27.120193.002033. [DOI] [PubMed] [Google Scholar]
- 43.Zhang L, Baasiri R A, Van Alfen N K. Viral repression of fungal pheromone precursor gene expression. Mol Cell Biol. 1998;18:953–959. doi: 10.1128/mcb.18.2.953. [DOI] [PMC free article] [PubMed] [Google Scholar]