Abstract
Introduction
Despite many technological advances, the diagnostic yield of bronchoscopic peripheral lung nodule analysis remains limited due to frequent mispositioning. Needle-based confocal laser endomicroscopy (nCLE) enables real-time microscopic feedback on needle positioning, potentially improving the sampling location and diagnostic yield. Previous studies have defined and validated nCLE criteria for malignancy, airway and lung parenchyma. Larger studies demonstrating the effect of nCLE on diagnostic yield are lacking. We aim to investigate if nCLE-imaging integrated with conventional bronchoscopy results in a higher diagnostic yield compared with conventional bronchoscopy without nCLE.
Methods and analysis
This is a parallel-group randomised controlled trial. Recruitment is performed at pulmonology outpatient clinics in universities and general hospitals in six different European countries and one hospital in the USA. Consecutive patients with a for malignancy suspected peripheral lung nodule (10–30 mm) with an indication for diagnostic bronchoscopy will be screened, and 208 patients will be included. Web-based randomisation (1:1) between the two procedures will be performed. The primary outcome is diagnostic yield. Secondary outcomes include diagnostic sensitivity for malignancy, needle repositionings, procedure and fluoroscopy duration, and complications. Pathologists will be blinded to procedure type; patients and endoscopists will not.
Ethics and dissemination
Primary approval by the Ethics Committee of the Amsterdam University Medical Center. Dissemination involves publication in a peer-reviewed journal.
Support
Financial and material support from Mauna Kea Technologies.
Trial registration number
Keywords: Biopsy, Clinical Trial, Diagnostic Imaging, Respiratory tract tumours, Cytopathology
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This is an (international multicentre) randomised controlled trial evaluating a novel sampling technique needle-based confocal laser endomicoscopy (nCLE) with the current standard for bronchoscopic diagnosis of peripheral lung nodules.
The definition of diagnostic yield is under debate. In this study, the diagnostic yield will be reported based on two different definitions for better comparison with existing and future studies.
Each participating centre uses their own methods for conventional bronchoscopic diagnosis of peripheral lung nodules and will, therefore, not be completely uniform across all centres. Each centre will keep conventional methods uniform in both the control and intervention group to ensure differences can be attributed to the nCLE technique.
In this study, only peripheral pulmonary nodules between 1 and 3 cm are included.
Introduction
Lung cancer remains the leading cause of cancer-related deaths, with 2.09 million new diagnoses and 1.76 million deaths worldwide per year.1 2 The increased use of chest CT and the future implementation of low-dose CT lung cancer screening result in an increased detection of lung nodules.3 4 Consequently, more early-stage lung cancer is detected, which is most often located in the periphery of the lung.5 6 Depending on lesion characteristics and associated risk factors, tissue sampling is needed to establish a definitive diagnosis and determine the appropriate treatment.
Bronchoscopic analysis of peripheral lung nodules remains challenging despite many technological innovations. The procedure comprises three essential pillars needed for a diagnostic success: navigation to the lesion, confirmation of tool location within the lesion (ie, tool-in-lesion confirmation) and successful tissue sampling. In the past years, fluoroscopy, radial probe endobronchial ultrasound (r-EBUS), electromagnetic navigation (EMN), virtual bronchoscopy (VB) or cone beam CT (CBCT) combined with augmented fluoroscopy have improved navigation with or without tool-in-lesion confirmation.7 Additionally, rapid on-site evaluation (ROSE) is sometimes used for direct feedback on the representativeness of the sample and forming a preliminary diagnosis. Nevertheless, diagnostic yield rarely exceeds 71%,8 as it depends highly on factors such as nodule size, bronchus sign on preprocedural CT, eccentric versus concentric r-EBUS pattern, pretest probability of malignancy and sampling tools used.9–12 The arrival of robotic bronchoscopy platforms combined with existing techniques has shown promising results with high navigation success rates. However, diagnostic yield remains behind due to substantial mispositioning rates, retaining a large gap between navigation success and diagnostic yield.13–15 The persistently low diagnostic yield calls for complementary techniques providing real-time information for fine-tuning the needle position.
Confocal laser endomicroscopy (CLE) is a high-resolution microscopic technique that visualises individual cells in real time. It has proven useful in the gastroenterology field, where it has been demonstrated that CLE could be used for rapid diagnosis, targeting of biopsies and prediction of neoplasms.16 CLE has been recently introduced in the respiratory tract, including for the peripheral lung nodule analysis.17–19 CLE probes are thin enough to fit through 18G biopsy needles to provide microscopic feedback at the tip of the needle (needle-based CLE (nCLE)). Fluorescein dye is used as a contrast agent and binds to the extracellular matrix, resulting in a highly fluorescent background in which individual cells can be seen. Previous studies have identified three nCLE image characteristics for malignancy in the lung,19 and criteria for airway and lung parenchyma.18 The identification of malignancy and distinction from airway and lung parenchyma were accurate based on these criteria.18 19
A recent study demonstrated a high needle mispositioning rate, as nCLE-imaging resulted in a repositioning of the biopsy needle in 9 out of 20 patients.20 nCLE could, therefore, potentially bridge the gap between navigation success and diagnostic yield.
To date, larger studies investigating the effect of the addition of nCLE to bronchoscopic peripheral lung nodule analysis are lacking. The improved diagnostic yield could reduce the necessity of further or more invasive diagnostic interventions such as CT-guided transthoracic biopsies or diagnostic surgery. In this multicentre randomised controlled trial, we aim to investigate if nCLE-imaging integrated with conventional bronchoscopy results in a higher diagnostic yield compared with conventional bronchoscopy without nCLE in diagnosing peripheral lung nodules.
Methods and analysis
Study design
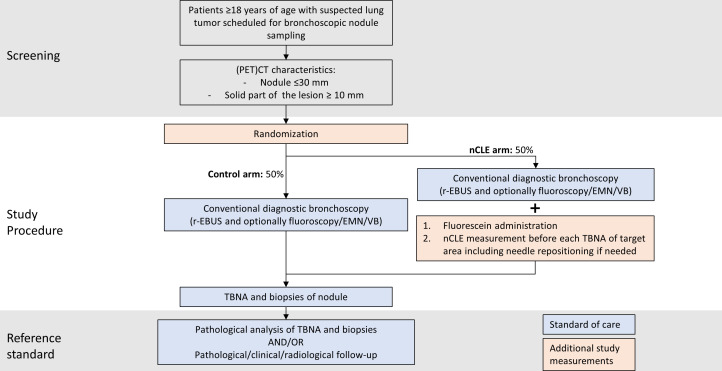
This study is an investigator-initiated, international, multicentre, parallel-group randomised controlled trial comparing two bronchoscopy procedures (with or without nCLE) for the diagnosis of suspected peripheral lung nodules. The study flow chart is shown in figure 1.
Figure 1.
Study flow chart. EMN, electromagnetic navigation; nCLE, neelde-based confocal laser endomicroscopy; PET, positron emission tomography; r-EBUS, radial endobronchial ultrasound; TBNA, transbronchial needle aspiration; VB, virtual bronchoscopy.
Participating centres
The study was executed in university or general hospitals in six countries in Europe and one hospital in the USA. Study inclusion started on 18 October 2023. Other centres will start including in 2024 and the estimated duration of the study is 24 months including follow-up.
Randomisation
After the participant has given written informed consent, patient data are entered into a digital database (CASTOR Electronic Data Capture (EDC) electronic case report form (eCRF)). We will use a web-based block-randomisation module in Castor to randomise participants into the control and intervention group (1:1). Randomisation will be stratified by participating centre to ensure that the nCLE and non-nCLE group is of the same size in each centre. As nodule size has a significant impact on diagnostic yield,8 we will stratify for nodule size (≤20 mm and >20 mm) to ensure that size is evenly distributed across study arms.
Patients and endoscopists will not be blinded since the physician needs to know if nCLE images must be acquired during bronchoscopy. Pathologists will be blinded to procedure type and raters who will analyse the nCLE videos after the procedure will be blinded to the patient history and histopathological outcome of the tissue samples.
Study population
Consecutive patients will be recruited by their treating physician at pulmonology outpatient clinics of participating centres. Patients are eligible for inclusion if they meet the following inclusion criteria:
≥18 years of age.
Suspected malignant peripheral lung lesion with an indication for a bronchoscopic diagnostic workup as determined by the attending physician or tumour board. Peripheral pulmonary lesions are defined as lesions located beyond the visible segmental bronchi, not detectable by regular flexible bronchoscopy.
Bronchus sign on preprocedural CT or estimated confidence for successful navigation to the nodule resulting in a r-EBUS signal.
Solid part of the lesion must be ≧10 mm.
The largest dimension of lesion size on CT 30 mm (long axis).
Ability to understand and willingness to sign a written informed consent.
A potential subject who meets any of the following criteria will be excluded from participation in this study:
Inability or non-willingness to provide informed consent.
Endobronchial visible malignancy on bronchoscopic inspection.
Target lesion within reach of the linear EBUS scope.
Failure to comply with the study protocol.
Known allergy or risk factors for an allergic reaction to fluorescein.
Pregnancy or breast feeding.
Haemodynamic instability.
Refractory hypoxaemia.
Therapeutic anticoagulant use that cannot be withheld for an appropriate interval before the procedure.
Unable to tolerate general anaesthesia according to the anaesthesiologist.
Undergoing chemotherapy as several chemotherapies have fluorescent properties at the same wavelength (eg, doxorubicin).
Primary outcome measure
Diagnostic yield (defined as the proportion of patients in whom the bronchoscopic procedure results in a definitive diagnosis (either malignant, specific benign or non-specific benign confirmed as benign in follow-up), relative to the total number of patients that underwent the diagnostic bronchoscopic procedure). If patients with multiple lesions are included, the diagnostic yield will be computed per nodule.
Secondary outcome measures
Diagnostic sensitivity for malignancy (defined as the proportion of patients in whom the bronchoscopic procedure diagnoses malignancy relative to the total number of patients with a final diagnosis of malignancy as determined by the reference standard).
Diagnostic yield according to the strict definition by Vachani et al 21 (defined as the proportion of patients in whom the bronchoscopic procedure results in a definitive diagnosis (either malignant or specific benign diagnosis), relative to the total number of patients that underwent the diagnostic bronchoscopic procedure).
Procedure duration (from bronchoscope insertion until removal).
Percentage of patients in which the needle was fine-tuned (defined as moving the needle within the same distal airway) or repositioned (defined as the selection of a different distal airway for tissue sampling) based on nCLE feedback (defined as the number of patients the needle was fine-tuned/repositioned divided by the total number of patients in which nCLE imaging was used).
Fluoroscopy radiation time and dose.
Diagnostic yield of ROSE (defined as the proportion of patients in whom ROSE resulted in a classifying diagnosis (malignant or specific benign diagnosis), relative to the total number of patients).
The proportion of patients in which ROSE provided tool-in-lesion confirmation, meaning that the acquired tissue shows signs of a malignant or non-malignant diagnosis and was not related to airway/lung parenchyma sampling such as bronchus epithelium/blood contamination, and tissue not suitable for a specific diagnosis such as atypical cells.
Complication rate (defined as any complication or complication categories occurring during or directly after the bronchoscopic procedure or any procedure-related complication within 1 week after the procedure).
Requirement of additional diagnostic procedures (CT-guided transthoracic biopsies, surgical diagnostics and/or additional bronchoscopy) during the 6-month follow-up period.
Exploratory endpoints
As an exploratory endpoint, we aim to identify potential new nCLE image characteristics for malignant and benign pathologies. We will also create an algorithm for automated nCLE criteria recognition using machine-learning or deep-learning methods.
Outcome parameters
Box 1 shows the baseline patient characteristics and corresponding procedural information that will be collected at the time of study inclusion, during the procedure and 6-month follow-up period.
Box 1. Data to be collected.
Patient characteristics
Age.
Sex.
BMI.
Smoking history.
Patient cancer history.
Family history of lung cancer.
Preprocedural (PET) CT scan lesion characteristics
CT scan quality (slice thickness).
Size (largest diameter).
Localisation (segmental level).
Lesion appearance/nodule type (solid, non-solid/ground glass, partially solid).
Bronchus sign (present(concentric/eccentric)/absent/insufficient CT scan quality).
Spiculation sign (present/absent).
Emphysema (present/absent).
PET uptake (not performed/no uptake/faint (SUV<1)/moderate (SUV 1–2.5) /intense (SUV>2.5)).
Intraprocedural information
r-EBUS sign (eccentric, concentric, absent).
Location of tissue sampling (lung segment).
nCLE image observations (for every needle pass).
Needle fine-tuning and repositioning done (for every needle pass).
Sampling techniques used (TBNA, (cryo)biopsy, brush).
ROSE results of tissue sample (if available).
Bronchoscopy start and end time.
Fluoroscopy duration.
Additional procedures performed (eg, EBUS/EUS-B/etc).
(Serious) complications.
Postprocedural information
(Serious) complications (up to 1 week week after the procedure).
Final pathological diagnosis (cytology and/or histology).
(Additional) Diagnostic follow-up procedures needed (eg, transthoracic needle biopsies, surgery, additional bronchoscopy, follow-up imaging) including (altered) diagnosis and/or results of follow-up CT-scans of the lesion(s).
BMI, body mass index; nCLE, needle-based confocal laser endomicroscopy; PET, positron emission tomography; r-EBUS, radial endobronchial ultrasound; ROSE, rapid on-site evaluation; SUV, standard uptake value; TBNA, transbronchial needle aspiration.
Investigational product
The Cellvizio CLE system with the corresponding AQ-Flex 19 miniprobe (Mauna Kea technologies (MKT), Paris, France) is the investigational medical device of this study. The probe has a compatible operating diameter of 0.91 mm, a resolution of 3,5 µm, a penetration depth of 40–50 µm and a maximum field of view of 325 µm. The device and corresponding probes are CE-marked and will be used for the intended purpose.22
The technique uses a laser beam (488 nm) focused by an objective lens to illuminate the tissue, with the illumination focus at a predefined depth. The light strikes the tissue resulting in fluorescent light emission back from autofluorescent structures such as elastin in the airways or an exogenous fluorescent dye such as fluorescein, a contrast dye used for nCLE imaging in the lung. Light originating from the focal layer is focused by the objective lens at the opening of a pinhole and detected while light from out-of-focus layers is rejected by the pinhole. This results in high-resolution imaging of individual cells and structures at a specific point with limited influence of (scattered) light from out-of-focus areas.22
Study procedures
Conventional diagnostic bronchoscopy (control group & intervention group)
The following procedure will be performed routinely (regardless of study participation): Bronchoscopic procedures will be performed by experienced pulmonologists specifically trained in navigation bronchoscopy and nCLE-imaging. All procedures are performed according to institutional practice, usually on an outpatient basis. Patient preparation and sedation will be done according to institutional practice and might include propofol or midazolam sedation and the use of topical anaesthesia. Vital parameters will be monitored during and after the procedure.
Systematic bronchoscopic inspection of the airways will be performed, followed by r-EBUS imaging (guide sheath optional) to select the distal airway with the highest probability of reaching the lesion. The use of fluoroscopy, EMN, VB or ultrathin bronchoscope is optional if regularly used at that institution. CBCT navigation with or without augmented fluoroscopy and robotic bronchoscopy will not be used in patients included in this trial. Bronchoscopist may use these techniques after following all actions related to this protocol while ensuring tissue samples are processed separately. Transbronchial needle aspirations (TBNA) using the 18G FleXNeedle (Broncus Medical, San Jose, SA) and (cryo)biopsies will be performed to acquire tissue for pathological evaluation (a recommended minimum of three TBNA and three biopsies). During the bronchoscopic workup, some of the cytological aspirations will be evaluated on site (ROSE) and the representativeness of the samples will be reported to the bronchoscopist. ROSE will always be performed for the first TBNA pass. For the following passes, the bronchoscopist decides if it is indicated.
Addition of nCLE imaging (intervention group)
The same procedure will be performed as described above for the patients randomised to the intervention arm, except for the addition of fluorescein administration and nCLE imaging before TBNA. Prior to the procedure, an 18G needle is preloaded with the CLE probe (AQ-Flex 19 Miniprobe, MKT, Paris, France). The CLE probe is advanced through the needle until the probe is positioned approximately 4 mm past the needle tip and secured using a locking device to maintain the probe position relative to the needle tip.
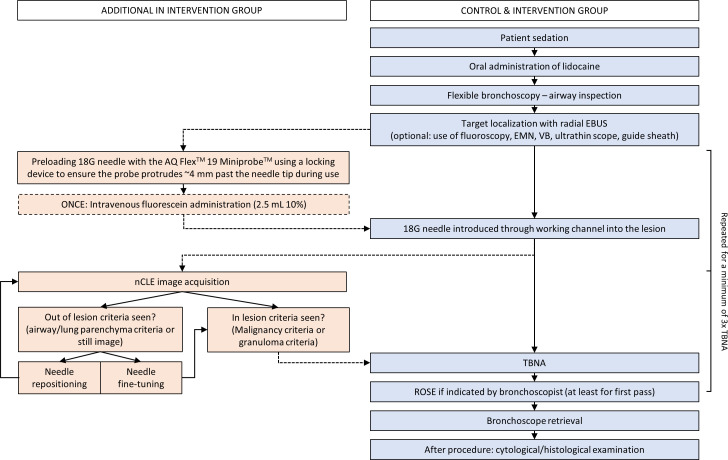
After determining the sample location based on r-EBUS and/or fluoroscopy, fluorescein (2.5 mL of 10% fluoresceindinatrium solution) is administered intravenously. Then, the preloaded 18G needle punctures the target area, followed by the insertion of the CLE probe through the biopsy needle for real-time microscopic feedback. In case nCLE visualises airway or lung parenchyma, indicating a near-miss, the biopsy needle is fine-tuned (ie, the needle is moved within the same distal airway) or repositioned (ie, a different distal airway is chosen). If nCLE demonstrates that the biopsy needle is placed within the lesion, the CLE probe is removed from the biopsy needle while holding the needle in position, followed by tissue sampling at the same location (repeated for at least three TBNAs). A flow chart of the procedure steps for both the conventional bronchoscopy and the nCLE-guided bronchoscopy is shown in figure 2.
Figure 2.
Procedure flow chart for control and intervention group (without and with nCLE). Fluorescein administration is only done once before the first puncture. EBUS, radial endobronchial ultrasound; EMN, electromagnetic navigation; nCLE, neelde-based confocal laser endomicroscopy; PET, positron emission tomography; ROSE, rapid on-site evaluation; TBNA, transbronchial needle aspiration; VB, virtual bronchoscopy.
nCLE image interpretation
The airway and lung parenchyma nCLE criteria as described by Kramer et al 18 will be considered as ‘out-of-lesion’ criteria indicating mispositioning of the needle. Currently known criteria for ‘tool-in-lesion’ are malignancy criteria and granuloma criteria.18 19 23 nCLE images will be interpreted during the procedure by the performing bronchoscopist and their team. Additionally, all videos are rated postprocedure by blinded raters of the initiating centre to establish a ground truth interpretation of the images.
Pathological examination
The cytological and histological examination will be done according to standard hospital procedure. In case the bronchoscopic procedure is considered non-diagnostic, additional procedures (transthoracic needle aspiration, surgical procedure, etc) could follow to obtain a definite diagnosis. Results of the nCLE imaging do not influence the indication for additional diagnostic procedures. If a surgical procedure is indicated, the histological images will be collected to compare this with the nCLE imaging.
In this study, the final pathological diagnosis will be subdivided into four categories as described by Vachani et al,21 namely (1) malignant, (2) non-malignant, which is divided into specific benign (including granulomatous, infectious and lymphocyte-predominant patterns) or non-specific benign (eg, inflammation) and (3) non-diagnostic (ie, insufficient material for classifying diagnosis or in case atypical cells could not be classified further).
Reference standard
For the primary outcome, diagnostic yield will be calculated using the intermediate method described by Vachani et al.21 The above-mentioned final pathological diagnosis categories will be used regardless of the results of the reference standard, except for initial non-specific benign diagnoses. In these cases, results from the reference standard will be considered. If the initial benign diagnosis is confirmed benign in follow-up, the bronchoscopic procedure will be considered diagnostic.
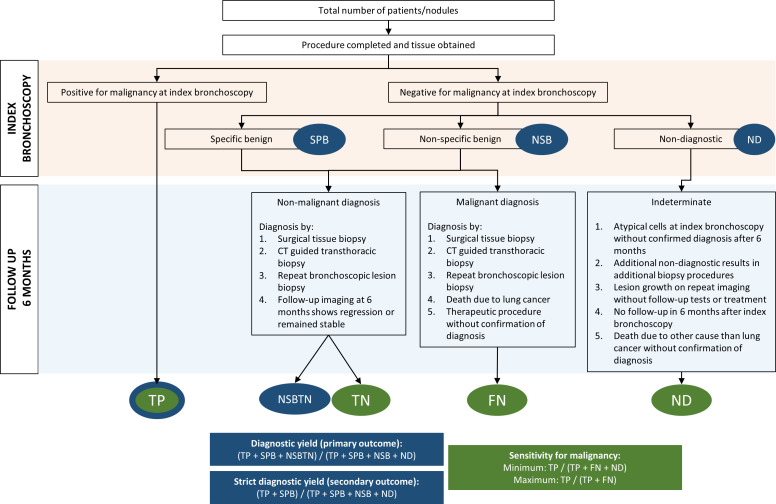
For the calculation of diagnostic sensitivity, malignant cases identified by the procedures under investigation will be considered as true positive since false positive results (almost) never occur. Benign (either specific or non-specific) and non-diagnostic samples will undergo a reference standard, which can be a subsequent sampling method such as transthoracic needle biopsy or surgery. Alternatively, if no subsequent sampling method is performed, clinical and radiological follow-up at 6 months is considered the reference standard. If follow-up CT imaging shows regression or resolution of the nodule or in case a nodule remains stable, it will be considered as a confirmation of non-malignant diagnosis (ie, true negative). Cases that are benign (either specific or non-specific) or non-diagnostic at the index bronchoscopy will be considered false negative if a malignancy diagnosis is established by the reference standard or if therapeutic procedures are done without confirmation of diagnosis. Figure 3 gives a schematic overview of the calculation methods of diagnostic yield and sensitivity for malignancy.
Figure 3.
Flow chart explaining calculation methods of diagnostic yield and sensitivity of malignancy. FN, false negative; ND, non-diagnostic; NSB, non-specific benign; SPB, specific benign; TN, true negative; TP, true positive.
Informed consent procedure
Patients will be recruited by their treating physician. If the patient is willing to receive more information about study participation, information will be provided by the local investigator. The eligible participants will have sufficient time to consider their consent. Written informed consent must be provided before any study-related procedures take place. The English template of the informed consent is provided as online supplemental file. After informed consent, patients will be randomised using Castor EDC software and assigned to the control or intervention group. The bronchoscopy will then be performed according to the study protocol. In case patients decline participation in the study, they will be treated to the usual local clinical practices and guidelines.
bmjopen-2023-081148supp001.pdf (175.4KB, pdf)
Quality assurance
Only experienced pulmonologists will perform the procedures to ensure high-quality bronchoscopic procedures. Additionally, all participating centres will be trained in the use of the CLE Cellvizio device and to maintain homogeneous quality of the nCLE image acquisition and interpretation over all centres. Training entails theoretic and practical training by the initiating centre with extensive nCLE experience and MKT representatives.
Sample size justification
Based on previous studies and meta-analyses, we expect the diagnostic yield in patients with a lesion <30 mm in the conventional bronchoscopy arm to be 62%.24 25 We hypothesise that additional nCLE guidance in the intervention arm will result in a diagnostic yield of 80%. In total, 198 patients are needed to show that nCLE guidance results in a diagnostic yield that is 18% point higher than the conventional bronchoscopy arm (alpha=0.05 and power=0.80). Taking into account a 5% study drop-out, a total of 208 patients will be included. We believe an increase in the diagnostic yield (from 62% to 80%) demonstrates a clinically relevant improvement in lung cancer diagnosis.
Data analysis
Results for continuous variables will be expressed as means and SDs or medians with IQRs. Categorical variables will be expressed as frequencies and percentages. The χ2 test will be used to compare diagnostic yield (or other comparisons between categorical variables) between the two randomisation groups. Continuous variables will be compared using Student’s t-test or Mann-Whitney U tests. A two-tailed p<0.05 will be considered statistically significant. All analyses are done based on an intention-to-treat approach, meaning that patients are analysed as part of the intervention group they were assigned to, even if nCLE imaging was not executed in a patient in the intervention arm due to unforeseen circumstances. These specific cases will be reported in the manuscript. Patients not undergoing the planned bronchoscopy procedure after randomisation are excluded from the analysis. Patients with missing outcome data will be excluded from analysis. Patients with incomplete essential follow-up information will also be excluded from the calculation of diagnostic sensitivity. However, we will also calculate diagnostic sensitivity based on a ‘worst-case scenario’, in which these patients are considered false negatives. For the primary outcome, subgroup analysis will be performed for several lesions and procedural characteristics (lesion size (≤20 mm vs >20 mm), radial EBUS image (eccentric vs concentric vs absent), location (upper lobe (without lingual) vs middle lobe/lingual vs lower lobe), pretest probability that the nodule is cancerous (<10%, 10%–35%, 36%–70% and >70%) based on the Brock score.26
Protocol amendments
Substantive protocol amendments will be assessed by the METC Amsterdam UMC. A substantial amendment is already incorporated in this publication. In the course of subject screening, it was observed that certain patients, integral to the population that could potentially benefit from nCLE, were excluded. Initially, the presence of a positive bronchus sign was obligatory. After inclusion of 12 patients, we also include patients if the bronchoscopist has estimated confidence for successful navigation to the nodule resulting in a r-EBUS signal without a clear bronchus sign on chest CT. As only 5% of patients were included at a single centre at the moment of the change, effects on the outcomes are negligible. In the event of other substantial amendments, all changes with a rationale will be reported in future publications arising from this protocol.
Patient and public involvement
There was no patients or public involvement in the design of this study. An original research manuscript will be prepared to present the study results.
Ethics and dissemination
The CLEVER study will be conducted in accordance with the Declaration of Helsinki (64th WMA General Assembly, Fortaleza, Brazil, October 2013) and the Medical Research Involving Human Subjects Act (WMO, The Netherlands) principles. To date, the Medical Ethical Committee of the Amsterdam UMC (NL83257.018.22), Athens Chest Hospital (21583/25-08-23) and General University Hospital in Prague (č.j. 143/23 S) have approved the study. All participating sites will obtain local ethical approval prior to starting inclusions. Written informed consent will be obtained prior to randomisation and any study-related procedures. In case of major changes to the protocol, the ethical review board will be notified, and it will be communicated with all participating centres and registered on clinicaltrials.gov.
Data management and safety
After informed consent, the patient will be given a code. This code will be used on all (pseudonymised) data, including CLE images and eCRF data. Castor EDC ecosystem (International Organization of Standardization 27001 and 9001 certified) will be used to collect key patient information described in outcome parameters. The key to the code is safeguarded by the local principal investigator and access to all records is limited to directly involved researchers. The coordinating investigator will centralise patients’ data, and principal investigators will have direct access to their own site’s data sets and to other sites’ data on reasonable request. All principal investigators will maintain records, including signed patient informed consent forms and information on adverse events (AEs).
Data management of all data (collection, storage and analysis) will be done according to the local data management plan. All records will be stored for a period of 15 years following the completion or termination of the study. Monitoring will be done according to a monitoring plan with specific attention paid to informed consent, completion of the eCRF, and storage of CLE video data.
Patient safety and AEs
The study was deemed a negligible risk study (according to the Nederlandse Federatie van Universitaire Medisch Centra (NFU) descriptions) by the ethical committee of the Amsterdam UMC. Previous study publications showed that nCLE-imaging and intravenous fluorescein administration are safe.27 Fluorescein adverse reactions are rare and mostly mild in nature. No study-related AEs occurred in the prior bronchoscopic nCLE studies in the Amsterdam UMC.18 19 Estimated prolonged endoscopy time due to study participation is approximately 10 min. Patients will not be aware of this as they will already be sedated for the bronchoscopic procedure.
In case any (serious) AE ((S)AE) occur during the procedure or up to 1 week after the procedure, the sponsor will register SAEs through the web portal Toetsingonline to the accredited METC that has approved the protocol. AEs are defined as any undesirable experience occurring to a subject during the study, whether or not considered related to the trial procedure. The severity and possible relatedness to the investigational product or the procedure will be documented. Investigators of the participating centres will report all SAEs to the coordinating and principal investigator of the initiating site. Reporting of SAEs that result in death or are life-threatening will be done within 7 days after initial identification, followed by a period of a maximum of 8 days to complete the preliminary report. All other SAEs will be reported within 15 days after first knowledge of the SAE.
Annual progress report
The sponsor will ensure that a progress report is submitted to the medical ethics committee once a year. Information on the start date of inclusion, number of subjects included to date, number of subjects that have completed participation, SAEs and amendments.
Dissemination
We aim to publish the study results in a peer-reviewed journal. Reporting will be in line with Consolidated Standards of Reporting Trials and Standard for Reporting Diagnostic Accuracy (STARD) 2015 reporting guidelines.28 29
Discussion
In this multicentre, investigator-initiated, randomised controlled trial, we aim to determine if the addition of nCLE-imaging to bronchoscopic peripheral lung lesion analysis results in an improved diagnostic yield.
Since there is still is a gap between the success rate of navigating the tissue sampling instrument towards the target lesion and the final diagnostic yield, there is a need for real-time tool-in-lesion confirmation. The addition of high-resolution microscopic nCLE imaging at the tip of the needle could potentially lead to a decrease in mispositioning rates and an improved diagnostic yield. As a result, fewer patients would need additional diagnostic procedures such as transthoracic needle biopsy or surgery, which are more invasive and have higher incidences of complications such as pneumothorax and haemorrhage.30 Previous smaller studies have already shown that nCLE is safe, and raters can distinguish different image characteristics with high accuracy. On top of that, it has also been demonstrated that fine-tuning the needle based on these image characteristics is often done, even when navigation to the lesion is successful.18–20 However, nCLE image interpretation remains subjective and challenging, especially when interpreting images live in the bronchoscopy suite. As described by Tian et al,31 the role of artificial intelligence might be important to make the technique routinely implementable in clinical practice. An exploratory endpoint of this study is to develop a deep-learning network for automated image interpretation. This is the first step towards easier, quicker and reproducible image interpretation.
Current literature on nCLE imaging for this purpose remains limited to smaller patient groups and the clinical benefit remains to be demonstrated. The results of the CLEVER study provide a formal comparison between conventional image-guided diagnostic bronchoscopy and conventional bronchoscopy with the addition of nCLE in a large randomised patient group. The results of this trial will clarify the added benefit of nCLE for bronchoscopic diagnosis of peripheral lung nodules and identify which patients could benefit from the use of this technique.
Supplementary Material
Footnotes
@SadoughiAli
Contributors: SvH, TK, DAK, DdB, PIB and JTA were involved in conception and trial design. SvH, TK, DAK, PIB and JTA were involved in drafting of the study protocol. DAK provided statistical expertise. All authors were involved in editing and final approval of the protocol. SvH, DG, CB, JH, KJ, VP, CR, AS, GS, KB, EK, NA, JV, ZS, MAH, JD, PIB and JTA will be involved in the conduct of the study and data acquisition. SvH, DAK, DdB, PIB and JTA will be involved in the data analysis and interpretation. SvH, DAK, PIB and JTA will be involved in drafting of the final manuscript for dissemination. All authors will provide editing and approval of the final manuscript for publication.
Funding: This is an investigator-initiated study with financial and material support from Mauna Kea Technologies (9 Rue d'Enghien, 75010 Paris, France).
Disclaimer: The funder has no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: JTA declares material and financial support to the sponsor’s institution from Mauna Kea Technologies for this study. All Cellvizio equipment needed for the conduct of the study is provided in-kind to participating centers. All other authors declare no other conflicts of interest.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Bade BC, Dela Cruz CS. Lung cancer 2020: epidemiology, etiology, and prevention. Clin Chest Med 2020;41:1–24. 10.1016/j.ccm.2019.10.001 [DOI] [PubMed] [Google Scholar]
- 2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 3. Hoffman RM, Atallah RP, Struble RD, et al. Lung cancer screening with low-dose CT: a meta-analysis. J Gen Intern Med 2020;35:3015–25. 10.1007/s11606-020-05951-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hyldgaard C, Trolle C, Harders SMW, et al. Increased use of diagnostic CT imaging increases the detection of stage IA lung cancer: pathways and patient characteristics. BMC Cancer 2022;22:464. 10.1186/s12885-022-09585-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang EW, Shepard J-AO, Kuo A, et al. Characteristics and outcomes of lung cancers detected on low-dose lung cancer screening CT. Cancer Epidemiol Biomarkers Prev 2021;30:1472–9. 10.1158/1055-9965.EPI-20-1847 [DOI] [PubMed] [Google Scholar]
- 6. Horeweg N, van der Aalst CM, Thunnissen E, et al. Characteristics of lung cancers detected by computer tomography screening in the randomized NELSON trial. Am J Respir Crit Care Med 2013;187:848–54. 10.1164/rccm.201209-1651OC [DOI] [PubMed] [Google Scholar]
- 7. Kramer T, Annema JT. Advanced bronchoscopic techniques for the diagnosis and treatment of peripheral lung cancer. Lung Cancer 2021;161:152–62. 10.1016/j.lungcan.2021.09.015 [DOI] [PubMed] [Google Scholar]
- 8. Kops SEP, Heus P, Korevaar DA, et al. Diagnostic yield and safety of navigation bronchoscopy: a systematic review and meta-analysis. Lung Cancer 2023;180:107196. 10.1016/j.lungcan.2023.107196 [DOI] [PubMed] [Google Scholar]
- 9. Nadig TR, Thomas N, Nietert PJ, et al. Guided bronchoscopy for the evaluation of pulmonary lesions: an updated meta-analysis. Chest 2023;163:1589–98. 10.1016/j.chest.2022.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen A, Chenna P, Loiselle A, et al. Radial probe endobronchial ultrasound for peripheral pulmonary lesions. A 5-year institutional experience. Ann Am Thorac Soc 2014;11:578–82. 10.1513/AnnalsATS.201311-384OC [DOI] [PubMed] [Google Scholar]
- 11. Mondoni M, Rinaldo RF, Carlucci P, et al. Bronchoscopic sampling techniques in the era of technological bronchoscopy. Pulmonology 2022;28:461–71. 10.1016/j.pulmoe.2020.06.007 [DOI] [PubMed] [Google Scholar]
- 12. Tang Y, Tian S, Chen H, et al. Transbronchial lung cryobiopsy for peripheral pulmonary lesions. a narrative review. Pulmonology 2023. 10.1016/j.pulmoe.2023.08.010 [DOI] [PubMed] [Google Scholar]
- 13. Chen AC, Pastis NJ, Mahajan AK, et al. Robotic Bronchoscopy for peripheral pulmonary lesions: A multicenter pilot and feasibility study (BENEFIT). Chest 2021;159:845–52. 10.1016/j.chest.2020.08.2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chaddha U, Kovacs SP, Manley C, et al. Robot-assisted bronchoscopy for pulmonary lesion diagnosis: results from the initial multicenter experience. BMC Pulm Med 2019;19:243. 10.1186/s12890-019-1010-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gex G, Pralong JA, Combescure C, et al. Diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules: a systematic review and meta-analysis. Respiration 2014;87:165–76. 10.1159/000355710 [DOI] [PubMed] [Google Scholar]
- 16. Fugazza A, Gaiani F, Carra MC, et al. Confocal laser endomicroscopy in gastrointestinal and pancreatobiliary diseases: a systematic review and meta-analysis. Biomed Res Int 2016;2016:4638683. 10.1155/2016/4638683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goorsenberg A, Kalverda KA, Annema J, et al. Advances in optical coherence tomography and confocal laser endomicroscopy in pulmonary diseases. Respiration 2020;99:190–205. 10.1159/000503261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kramer T, Wijmans L, de Bruin M, et al. Bronchoscopic needle-based confocal laser endomicroscopy (nCLE) as a real-time detection tool for peripheral lung cancer. Thorax 2022;77:370–7. 10.1136/thoraxjnl-2021-216885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wijmans L, Yared J, de Bruin DM, et al. Needle-based confocal laser endomicroscopy for real-time diagnosing and staging of lung cancer. Eur Respir J 2019;53:1801520. 10.1183/13993003.01520-2018 [DOI] [PubMed] [Google Scholar]
- 20. Manley CJ, Kramer T, Kumar R, et al. Robotic bronchoscopic needle-based confocal laser endomicroscopy to diagnose peripheral lung nodules. Respirology 2023;28:475–83. 10.1111/resp.14438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vachani A, Maldonado F, Laxmanan B, et al. The impact of alternative approaches to diagnostic yield calculation in studies of bronchoscopy. Chest 2022;161:1426–8. 10.1016/j.chest.2021.08.074 [DOI] [PubMed] [Google Scholar]
- 22. Chauhan SS, Abu Dayyeh BK, Bhat YM, et al. Confocal laser endomicroscopy. Gastrointestinal Endoscopy 2014;80:928–38. 10.1016/j.gie.2014.06.021 [DOI] [PubMed] [Google Scholar]
- 23. Kramer T, Wijmans L, van Heumen S, et al. Needle-based confocal laser endomicroscopy for real-time granuloma detection. Respirology 2023;28:934–41. 10.1111/resp.14542 [DOI] [PubMed] [Google Scholar]
- 24. Ali MS, Trick W, Mba BI, et al. Radial endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions: a systematic review and meta-analysis. Respirology 2017;22:443–53. 10.1111/resp.12980 [DOI] [PubMed] [Google Scholar]
- 25. Lee J, Song JU. Diagnostic yield of radial probe endobronchial ultrasonography-guided transbronchial biopsy without fluoroscopy in peripheral pulmonary lesions: a systematic review and meta-analysis. Thorac Cancer 2023;14:195–205. 10.1111/1759-7714.14733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med 2013;369:910–9. 10.1056/NEJMoa1214726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wallace MB, Meining A, Canto MI, et al. The safety of intravenous fluorescein for confocal laser endomicroscopy in the gastrointestinal tract. Aliment Pharmacol Ther 2010;31:548–52. 10.1111/j.1365-2036.2009.04207.x [DOI] [PubMed] [Google Scholar]
- 28. Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015;351:h5527. 10.1136/bmj.h5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schulz KF, Altman DG, Moher D, et al. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. 10.1136/bmj.c332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ho ATN, Gorthi R, Lee R, et al. Solitary lung Nodule: CT-guided transthoracic biopsy vs transbronchial biopsy with endobronchial ultrasound and flexible bronchoscope, a meta-analysis of randomized controlled trials. Lung 2023;201:85–93. 10.1007/s00408-023-00596-9 [DOI] [PubMed] [Google Scholar]
- 31. Tian S, Huang H, Zhang Y, et al. The role of confocal laser endomicroscopy in pulmonary medicine. Eur Respir Rev 2023;32:220185. 10.1183/16000617.0185-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-081148supp001.pdf (175.4KB, pdf)