Abstract
Introduction
Non-ventilator-associated hospital-acquired pneumonia (nv-HAP) is the most common healthcare-associated infection (HCAI), is associated with high mortality and morbidity and places a major burden on healthcare systems. Diagnosis currently relies on chest x-rays to confirm pneumonia and sputum cultures to determine the microbiological cause. This approach leads to over-diagnosis of pneumonia, rarely identifies a causative pathogen and perpetuates unnecessary and imprecise antibiotic use. The HAP-FAST study aims to evaluate the feasibility of a randomised trial to evaluate the clinical impact of low-dose, non-contrast-enhanced thoracic CT scans and rapid molecular sputum analysis using the BIOFIRE® FILMARRAY® pneumonia plus panel (FAPP) for patients suspected with nv-HAP.
Methods and analysis
The HAP-FAST feasibility study consists of a pilot randomised trial, a qualitative study, a costing analysis and exploratory analyses of clinical samples to investigate the immune-pathophysiology of HAP. Participants are identified and recruited from four acute hospitals in the Northwest of the UK. Using a Research Without Prior Consent model, the pilot trial will recruit 220 adult participants, with or without mental capacity, and with suspected HAP. HAP-FAST is a non-blinded, sequential, multiple assignment, randomised trial with two possible stages of randomisation: first, chest x-ray (CXR) or CT; second, if treated as nv-HAP, FAPP or standard microbiological processing alone (no FAPP). Pathogen-specific antibiotic guidance will be provided for FAPP results. Randomisation uses a web-based platform and followed up for 90 days. The feasibility of a future trial will be determined by assessing trial processes, outcome measures and patient and staff experiences.
Ethics and dissemination
This study has undergone combined review by the UK NHS Research Ethics Committee and Health Research Authority. Results will be disseminated via peer-reviewed journals, via the funders’ website and through a range of media to engage the public.
Trial registration number
Keywords: Respiratory infections, RESPIRATORY MEDICINE (see Thoracic Medicine), Diagnostic microbiology, Diagnostic radiology
Strengths and limitations of this study.
Decentralised, clinician-led randomisation facilitates continual recruitment on all wards of participating hospitals, improving the representativeness of the study population and providing insights into recruitment patterns for future trial.
Low rates of self-expectorated sputum sample submission may mean the study will provide limited assessment of the use of the BIOFIRE® FILMARRAY® pneumonia plus panel (FAPP).
Qualitative sub-studies into the participant, carer and healthcare worked experiences of the trial will inform a future trial fully powered for clinical endpoints.
Introduction
Non-ventilator-associated hospital-acquired pneumonia (nv-HAP) is the most common healthcare-associated infection (HCAI).1 The UK in-patient mortality following nv-HAP is 24% and extends the length of hospital stay by, on average, 9 days.2 3 Among those who survive to discharge, compared with other HCAIs, nv-HAP has the highest level of disability-adjusted life years (DALYs).1 Nv-HAP therefore represents a major risk for patients and places a huge burden on healthcare systems.
Diagnostic uncertainty in nv-HAP
Pneumonia is a syndrome that is diagnosed based on a case definition with three components: signs and symptoms of a lower respiratory tract infection, evidence of a systemic inflammatory response and radiological change compatible with infection on chest imaging.4 Defining the specific aetiological cause requires microbiological tests. Traditional diagnostic methods, relying on chest x-rays for syndromic diagnosis of nv-HAP and sputum cultures for the microbiological diagnosis of cause, often lead to over-diagnosis, delayed treatment decisions and inappropriate antibiotic use.5 6 Collectively, these diagnostic inadequacies contribute to poor clinical outcomes, and the UK National Institute for Health and Care Excellence (NICE) have called for a research focus on diagnostics.7
Addressing this evidence gap, the HAP-FAST study aims to evaluate the use of low-dose, non-contrast-enhanced CT scans as an alternative to chest x-rays, and the BIOFIRE® FILMARRAY® pneumonia plus panel (FAPP) as an alternative to standard microbiological testing, both individually and in combination in patients suspected with nv-HAP.
Rationale for chosen diagnostics in this study
CT scans in nv-HAP
Chest x-rays (CXR) have limitations when diagnosing pneumonia.8–13 Using a CT scan as the gold standard, CXR had a positive predictive value of 27% in 3423 US patients with possible community-acquired pneumonia (CAP).10 Claessens et al demonstrated that performing a CT after a CXR in suspected CAP might avoid antibiotics in 14%.11 The diagnostic inaccuracy of CXR is further exacerbated in bedridden patients, as is often the case in nv-HAP, with CT scan reports changing the management plans based on CXR diagnosis in nearly half of patients.13 Prendki et al found that using a CT scan instead of a CXR avoided antibiotic use in 8.5% of elderly Swiss patients with suspected pneumonia.9 These non-randomised, observational studies are prone to bias, and we need a randomised controlled trial (RCT) to demonstrate the impact of CT scans on clinical outcomes following nv-HAP.
Rapid microbiological testing in nv-HAP
Empirical antibiotic treatment of nv-HAP is imprecise and hampered by conflicting evidence about the potential pathogens. A Spanish study demonstrated 60% of bacterial detections were Gram-positive, and a retrospective Scottish study found 71% were Gram-negative.14 15 Neither study tested for viruses but subsequent studies have detected viruses in up to 22% of patients with HAP.16 17 Clinical guidelines often extrapolate recommendations from literature about ventilator-associated pneumonia (VAP), but a comparative study suggests this comparison is invalid.18 Most recently, the INHALE research group compared two rapid molecular diagnostic tests to conventional microbiological testing of respiratory samples from patients with pneumonia on critical care. They reported superior sensitivity for pathogen detection for new rapid tests when compared with conventional methods and viruses were implicated in a significant proportion of cases.19
The BIOFIRE® FILMARRAY® pneumonia plus panel (FAPP) is a CE marked, US Food and Drug Administration (FDA) approved point-of-care test that can simultaneously detect 18 bacteria, nine viruses and seven antimicrobial resistance genes from a respiratory sample in 75 min.19 Compared with the traditional culture-based methods, the speed, sensitivity and specificity of this diagnostic test has the potential to dramatically change the way nv-HAP is managed. However, before it is widely implemented, questions relating to the interpretation of results and cost-effectiveness within the NHS setting need to be addressed.20 There are also key questions relating to the implementation of decentralised microbiology results within the clinical work flow, the feasibility of maximising time gains using the FAPP, the safety and effectiveness of antibiotic rationalisation based on results and the willingness of clinicians to deviate from traditional paradigms of empirical management.
Risks and benefits
During standard care, thoracic CT scans of various types are performed at some point during the care pathway for approximately 12% of patients managed for nv-HAP. Here we will trial the systematic use of low-dose, non-contrast, thoracic CT scans as the first test in those suspected of nv-HAP because there is evidence this may lead to improved patient outcomes.11 The CT scan used in HAP-FAST carries a radiation exposure of, on average, 1.5 mSv, which is greater than a CXR (0.05 mSv) but lower than annual UK background radiation exposure of 2.7 mSv.13 A recognised consequence of performing a thoracic CT scan at any point in a patient’s acute care is the detection of unexpected abnormalities such as anatomical variants, alternative diagnoses for the presenting symptoms and incidental findings such as pulmonary nodules. Given the frequency of detection of pulmonary nodules in routine care, there are well-established pathways for their investigation and follow-up supported by national guidelines.21 22
Patients who can self-expectorate sputum will be randomised to either a standard microbiological diagnostic pathway (no FAPP) with initial empirical antibiotic selection as per their local policy—or to analyse sputum using the FAPP. Clinicians are provided with an antibiotic guide with pathogen-targeted treatment options for those randomised to use the FAPP. It is possible that based on either empirical antibiotic prescribing or FAPP-guided treatment, a participant may receive antibiotics that are not effective against an undetected pathogen. This risk is always present due to the imperfect nature of microbiological tests, and so, it is the standard clinical practice for patients to be closely monitored for the response to treatment during the early stages of pneumonia, and this study protocol allows the clinicians treating the participant to escalate or change their therapy as clinically indicated.
Aims and objectives
This study aimed to determine the feasibility of a full-scale RCT comparing different diagnostic pathways in adult patients suspected with nv-HAP.
The following HAP-FAST objectives will assess feasibility parameters:
For each intervention, the effect size and dispersion are estimated for a range of possible outcomes to inform the sample size of a definitive study.
Evaluate the practicality and fidelity of a range of possible outcome measures using completion rates, missing data, effect size and dispersion.
Estimate eligibility, recruitment and consent rates.
Estimate rates of successful follow-up.
Assess the web-based randomisation process and incorporate clinical and researcher feedback.
Perform a costing analysis of nv-HAP to inform the cost-effectiveness analysis for any definitive study.
Assess human factors involved in the delivery of the study and how the different diagnostic tests influence the clinical decision-making by conducting qualitative interviews and focus groups with healthcare workers and researchers.
Evaluate the willingness of clinicians to recruit the study participants.
Evaluate the willingness of potential participants or their consultees to be recruited.
Evaluate adherence to antibiotic guidelines as outlined in the study protocol.
Assess the study participant and carer experience of participating in the study via qualitative interviews.
Methods and analysis
Study setting
Participants are identified and recruited from four acute hospitals in the Northwest of the UK: Aintree University Hospital, Royal Liverpool University Hospital, Royal Preston Hospital and Wythenshawe Hospital. Sites were selected to capture ethnic and socioeconomic diversity. Preliminary data from a longitudinal HAP improvement programme demonstrated a sufficiently large caseload potential participants in these settings within the study’s timeframe.23
Study design
HAP-FAST is a feasibility study consisting of a pilot study, two qualitative studies and a costing analysis. The study participants will also provide clinical samples to support exploratory analyses of the immune-pathophysiology of nv-HAP. The start date of the trial (first site) was on 07/06/2023, and the final date of follow-up will be 10/06/2024.
Pilot study
Participants and sample size
Since the aim is to assess feasibility, a sample size justification is given rather than a calculation. We aim to recruit 220 adult participants based on prospective audits of HAP in the UK Northwest revealing between 600 and 1000 cases per year at each of our recruiting sites and assuming 30% of cases are eligible of whom 40% are recruited to the trial. Recruitment targets will likely be affected by the seasonality of HAP, with a greater burden in winter and seasonal variation in pathogens, and thus, we aim to recruit across the majority of a calendar year.
HAP is potentially severe as evidenced by the in-patient mortality of 24%. NICE-recommend treatment is commenced within 4 hours. Clinicians therefore face a narrow timeframe during which patients must be clinically assessed and diagnostic tests must be ordered, completed, reported, interpreted and acted on. Patients with nv-HAP frequently have impaired mental capacity due to underlying cognitive impairment or acute delirium. Therefore, due to the emergency nature of HAP, in common with research in other emergency settings such as trauma and intensive care, HAP-FAST uses a Research Without Prior Consent (RWPC) model.24–26 The use of RWPC for nv-HAP trials has been studied previously and deemed acceptable by patients and the public.26
At the point of suspecting nv-HAP, treating clinicians at the recruiting sites can randomise, carry out the interventions and obtain the initial sample set. Randomisation leads to an automatic email alerting the site research team who then obtain written informed consent from the patient or for those lacking capacity from a personal or professional proxy before discharge. Every effort will be made to obtain written informed consent after discharge if a patient is discharged before consent is obtained. Patients who decline to provide consent or no longer wish to continue in the study will be withdrawn. Data collected up to the point of withdrawal will be included in the analysis, and permission will be sought to collect data from routine assessments to complete some outcome data.
Pilot study eligibility criteria
Eligibility criteria for stage 1 randomisation to CXR versus CT and stage 2 randomisation to FAPP or no FAPP can be seen in table 1. Patients who are ineligible for randomisation to stage 2 will still be able to participate in the trial.
Table 1.
Inclusion and exclusion criteria for stage 1 and 2 randomisation
| Stage 1 CXR versus CT | Stage 2 FAPP versus no FAPP (standard laboratory sputum analysis) | |
| Inclusion criteria | Age ≥18 years | The clinician intends to treat the patient for HAP or a hospital-acquired respiratory tract infection (RTI) |
| Suspected HAP (For the purposes of this study, HAP is defined as per the BTS and FDA definitions as pneumonia which develops 48 hours after an admission to the hospital for an alternative diagnosis; or a new presentation to hospital with pneumonia in a patient who has been discharged from an overnight stay in hospital within the last 10 days) |
A sputum sample has been obtained before the second dose of antibiotics | |
| Exclusion Criteria | Already received a CXR to confirm suspected HAP diagnosis | Following the CXR or CT, the clinician decides not to treat with antibiotics for either HAP or a hospital-acquired RTI |
| Diagnosis or suspected diagnosis of ventilator-associated pneumonia | ||
| Intention to palliate rather than cure | ||
| Interventions cannot be completed before administration of the second antibiotic dose* | ||
| Cannot be randomised to low-dose, non-contrast CT scan on clinical grounds, for example, strong suspicion of PE (a non-contrast, low-dose thoracic CT scan is an inappropriate test for a PE, and if it is high in the differential diagnosis, then tick yes here) |
||
| Pregnancy (A urine pregnancy test is required as part of the routine care before a CXR or CT scan. If the test reveals the patient is pregnant, they will not be eligible for the study) |
||
| Previous study participation (patients with second of third episodes of HAP will not be re-recruited) |
*In the circumstance where a patient is diagnosed with HAP while receiving antibiotics for a non-respiratory infection (eg, UTI) if the HAP diagnosis leads to a change in the antibiotic prescription to cover the HAP, then that patient will be eligible for recruitment. However, if the diagnosis of HAP would not result in a change in antibiotic, then the patient is not eligible.
Interventions and treatments
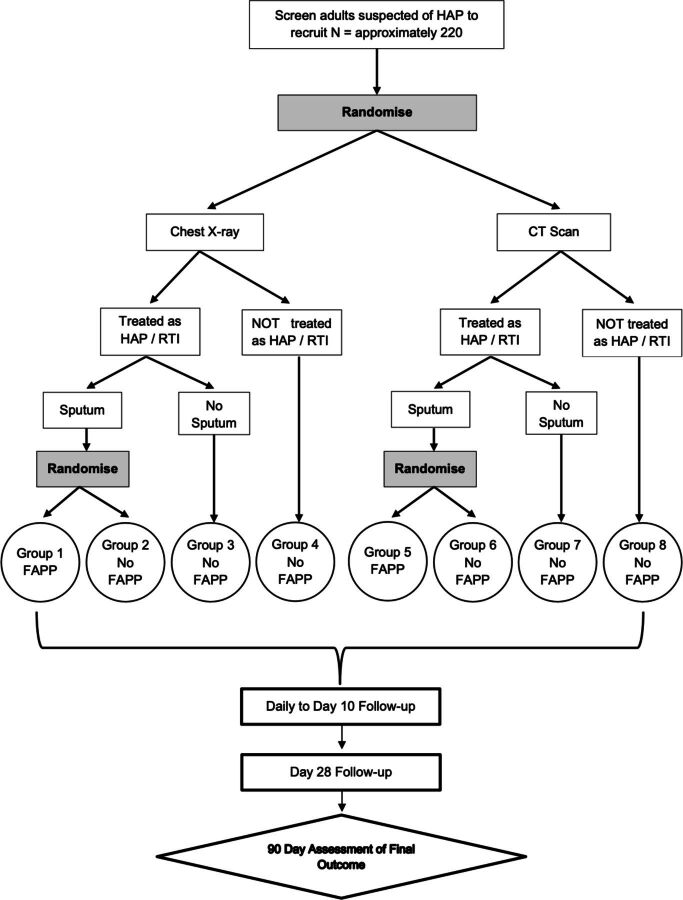
Participants are initially randomised between a standard-care CXR and low-dose, non-contrast, thoracic CT scan. If the clinician decides to give antibiotics to treat nv-HAP and the participant can produce a sputum sample before the administration of the second dose of antibiotics, they are further randomised between sputum testing by FAPP alongside local, standard of care microbiological processing or standard processing alone—no FAPP. A study-specific antibiotic guideline has been produced and approved by all recruiting sites for use with the results of the FAPP. It is anticipated that patients randomised to standard microbiological testing will receive an empirical antibiotic prescription supported by usual microbiological tests. Additional advice regarding the antibiotic treatment is available from microbiology specialists in line with local policies. Participants who cannot provide sputum and who are not randomised at stage 2 will be managed as per usual care. These interventions are summarised in table 2 and figure 1.
Table 2.
Treatment pathways in pilot study
| Result of stage 1 randomisation | Result of imaging | Sputum available? | Result of stage 2 randomisation | Treatment | Group |
| CXR | Clinician decides to treat for HAP/hospital-acquired RTI | YES | FAPP |
|
1 |
| Yes | No FAPP |
|
2 | ||
| No | N/A |
|
3 | ||
| Clinical diagnosis is not HAP/RTI | N/A | N/A |
|
4 | |
| CT Scan* | Clinician decides to treat for HAP/hospital acquired RTI | Yes | FAPP |
|
5 |
| Yes | No FAPP |
|
6 | ||
| No | N/A |
|
7 | ||
| Clinical diagnosis is not HAP/RTI | N/A | N/A |
|
8 |
Figure 1.
Pilot sequential multiple assignment randomised trial design.
Outcome measures
A key objective of HAP-FAST is to gather data to inform the choice of outcome measure for a fully powered RCT. We searched the COMET database for core outcome sets in HAP trials.27 Some groups advocate all-cause mortality assessed on a non-inferiority basis.28 However, others argue that discerning the mortality attributable to HAP, as opposed to underlying comorbidity, is difficult without unfeasibly large trials.29 One group proposed a hierarchical, composite, primary outcome of survival at day 28 and ‘clinical cure’ between days 7–10 but unfortunately did not provide a pragmatic definition of clinical cure.30 A group convened by the FDA suggested using mortality plus resolution of symptoms.31 HAP-FAST will therefore evaluate a range of outcomes including mortality, antibiotic usage and clinical cure incorporating a pneumonia-specific patient-reported outcome measure called the CAP-SYM score.
Pilot study randomisation
The pilot study has been designed as a sequential, multiple assignment, randomised trial (SMART) with a 1:1 allocation ratio, with the purpose to address study objectives 1–5.32 The randomisation list has been created by an independent statistician, and participant allocations are generated by completion of the web-based randomisation platform. The SMART study design is presented schematically in figure 1.
Pilot study blinding
The study is open-labelled, and treating clinicians, researchers and participants will know which intervention is being administered via the web-based randomisation process.
Pilot study outcome measures and participant timeline
Baseline and outcome data are collected at distinct time points according to the schedule in table 3 and in the supplementary table 4. Participants will be assessed by the study team daily until day 10 to track symptomatic recovery, changes in the quality of life (QOL) and determine time to clinical cure. Participants will have symptoms and QOL assessed on day 28 as an in- or out-patient. Follow-up will be conducted as a phone call 90 days (±14 days) following entry into the study to assess symptoms and QOL and to remind them to return a survey booklet on health and social care use up to day 90.
Table 3.
Schedule for recording of data outcomes
| Objective | ||
| Primary objective | ||
| The primary objective is to determine the feasibility of a full-scale randomised controlled trial (RCT) comparing different diagnostic dynamic treatment regimens (DTRs) in adult patients suspected of HAP. | ||
| Secondary Objective | ||
| Objective | Outcome | Time point |
| Inform the sample size of a definitive study | Time to clinical cure* | Day 90 |
| Antibiotic usage for the HAP episode | Day 90 | |
| EQ-5D-5L | Baseline, day 10, 28 and 90 | |
| Length of hospital stay post HAP diagnosis | Day 90 | |
| Mortality | Day 14, 28 and 90 | |
| To measure key outcome measures (completion rates, missing data, estimates and dispersion) | Estimate rates of completion of questionnaires - EQ5D5L, CAP-sym, economic evaluation Summary statistics and proportion of missing data for time to clinical care, antibiotic usage for HAP diagnosis, EQ-5D-5L, length of hospital stay post HAP diagnosis, mortality |
Screening Randomisation Follow-up End of Treatment End of Study |
| To estimate eligibility, recruitment and consent rates | Rate of recruitment Proportion screened that meet eligibility criteria** Proportion eligible that consents and where they present** Proportion consented and randomised that complete study pathway as per protocol Proportion consented and randomised that withdraw from study intervention or follow-up** |
Screening Randomisation Follow-up End of Treatment End of Study |
| Estimate rates of successful follow-up | Proportion consented and randomised that complete the study pathway as per protocol Proportion consented and randomised that withdraw from study intervention or follow-up** |
End of Study |
| Assess the web-based randomisation process and incorporate clinical and researcher feedback | Qualitative conclusions based on staff focus groups | Qualitative analysis |
| Perform a costing analysis of HAP to inform the cost-effectiveness analysis for any definitive study | Summary statistics for numbers and types of costs with comparison between DTRs | End of Study |
| Assess human factors involved in delivery of the study and how the different diagnostic tests influence clinical decision making by conducting qualitative interviews and focus groups with healthcare workers and researchers | Qualitative conclusions based on staff focus groups | Qualitative analysis |
| Evaluate the willingness of clinicians to recruit to the study | Qualitative conclusions based on staff focus groups | Qualitative analysis |
| Evaluate the willingness of potential participants or their consultees to be recruited | Qualitative conclusions based on participant and carer interviews | Qualitative analysis |
| Evaluate adherence to antibiotic guidelines and study protocol | Summary statistics relating to antibiotic use in the pilot study with a comparison between the DTRs | End of Study |
| Assess the study participant and carer experience of participating in the study | Qualitative interviews | Qualitative analysis |
*defined as the number of days from baseline when there is a combination of resolution of the signs and symptoms present at enrollment and improvement or lack of progression of radiological signs
**reasons why and stage will be collected to inform future trial design
Pilot study data analysis
All analyses will be carried out on an intention-to-treat basis, retaining all participants in their initially randomised groups, irrespective of any protocol deviations. The focus of analysis will be to assess the feasibility and recruitment for each participating site and overall pilot study as well as assessments of efficacy for each outcome for treatment arm comparisons of CXR versus CT (figure 1, group 1–4 vs group 5–8) and FAPP versus no FAPP (figure 1- group 1+5 vs groups 2 and 6). No inference will be drawn—all results will be treated as hypothesis generation.
Continuous data will be presented using median (IQR) and mean (SD) as appropriate, with boxplots summarising measurements at each time point by the treatment group. Categorical data will be presented as frequencies and percentages. Time-to-event data will be presented with Kaplan–Meier curves and summarised by median (95% CI) if possible.
As much information as possible will be collected about the reasons for missing outcome data; this will be used to inform any imputation approaches employed in the analysis. Such methods will be fully described in the full statistical analysis plan, which will be written before conducting any comparative analysis of the treatment arms, including methods employed for missing data.
Qualitative sub-studies
Clinicians
This qualitative sub-study will address objectives 5, 7, 8 and 10 to evaluate human factors involved in the delivery of the study, clinician willingness to recruit participants and adherence to antibiotic guidelines as per the study protocol (table 3).26 33 A range of clinical, allied health professional and research staff will be invited to participate in focus groups of approximately eight participants. Focus groups will be topic guided, yet conversational and exploratory and conducted in a comfortable private environment.
Patients and carers
This qualitative sub-study will address objectives 9 and 11 to evaluate patient willingness to participate in the study and their experience from recruitment to study follow-up (table 3).34 Approximately 15 participants (five from each of the three recruiting trusts) will be purposively recruited for in-depth semi-structured interviews based on age, gender and underlying comorbidity class (medical admission, surgical admission, acute admission). Relatives and carers of some study participants will also be interviewed.
Exploratory sub-study
Clinical samples are taken at enrolment to the pilot RCT, on day 3 and at day 28 and comprise venous blood, sputum and a nose swab and participants will be asked for additional consent for this sub-study. These samples will be used to explore the role of immune cells, and inflammatory mediators play in the pathophysiology of nv-HAP and how these vary with the pathogen. Samples from the HAP-FAST pilot study cohort (patients suspected of HAP) will be compared with equivalent samples from patients who chronically produce sputum, are not exacerbating and are being managed as out-patients in respiratory clinics. Specific consent questions will ask about retention of samples for future studies relating to pneumonia and for sharing samples with other non-commercial labs.
Health economic evaluation
This costing analysis will address objective 6 by capturing the direct costs in hospital associated with HAP as well as the post-discharge indirect costs with a bespoke questionnaire (up to 90 days following diagnosis). We will evaluate the performance of this questionnaire, which we have developed with reference to a range of similar studies.35–38 We will capture item completion rates and discuss participant and carer’s views of the questionnaire to refine it for the future full-scale RCT.
Data collection and management
Data management
For the HAP-FAST study, the responsibilities for data management, audit and monitoring are delegated to the Liverpool Clinical Trial Centre (LCTC). Data collection will be directly entered on to a secure, auditable and database as the source document, and this includes validation features to alert the user of inconsistent or missing data. Data of written informed consent processes and participation in the clinical trial will be added to the patient’s medical record chronologically.
Baseline assessment data will be obtained from patient medical notes, followed by the use of the CAP-SYM questionnaire,39 EQ-5D-5L questionnaire, research sample collection (for exploratory sub-study), monitoring of blood test results and a post-discharge indirect cost survey as shown in online supplemental table 4. Separate Data Management and Trial Monitoring Plans will detail the internal processes that will be conducted at the LCTC throughout the study in line with regulatory, ethical and legal obligations.
bmjopen-2024-088490supp002.pdf (102.2KB, pdf)
Confidentiality
This study will collect personal data (eg, participant names), including special category personal data (ie, participant medical information), and this will be handled in accordance with all applicable data protection legislation. Data (including special category) will only be collected, used and stored if necessary for the study (eg, evidencing provision of consent, for data management and central monitoring, statistical analysis, regulatory reporting). At all times, this data will be handled confidentially and securely.
Monitoring
Trial monitoring
Given this study is designed to evaluate feasibility rather that safety or efficacy, there is no on-site monitoring planned. LCTC will, however, be monitoring case report form completion, making consent checks and monitoring adherence. The Trial Management Group (TMG), including investigators, Patient and Public Involvement (PPI) representatives and LCTC members, will meet regularly to discuss the day-to-day conduct, management and progression of the study and troubleshoot issues such as adherence. The Trial Steering Committee (TSC) consists of an independent lay chairperson, two independent experts in the field, an independent biostatistician, the chief investigator and a second PPI representative to provide overall supervision of the study.
PPI
Patient and public representatives will be consulted throughout the duration of the study by acting as members of the TMG and TSC.
Ethics and dissemination
Research ethics approval
The study will be conducted in accordance with Good Clinical Practice (GCP) and will abide by the principles of the World Medical Association Declaration of Helsinki. The protocol, patient information sheet and all proposed public-facing materials were prepared along with our PPI team members and have undergone combined review by the UK NHS Research Ethics Committee (REC) and Health Research Authority (22/WA/0315). The committee was specifically configured to assess studies recruiting patients who lack capacity and reviewed Medical Physics Expert and Clinical Radiation Expert reports conducted in compliance with Ionising Radiation (Medical Exposure) Regulations (IRMER) legislation.
Protocol amendments
This publication has been based on version 3.0 of the protocol (online supplemental file). Version 1.0 was submitted to the REC, resulting in amendments and use of version 2.0 from the start of the trial. Further amendments, to improve clarity, were approved in October 2023 to the eligibility criteria (clarifying ‘the development of pneumonia within 10 days of discharge’ as a component of the definition of HAP and removing a fixed time-period requirement for stage 2 randomisation) patient information sheets (including format and hypostatical changes, additional consent statements using clinical samples, provision of a letter to deceased participant’s next of kin), consent processes (allowing verbal consent for the qualitative study, allowing postal consent for patients discharged before written informed consent obtained), study processes (removal of requirement for the statistical team to be blinded to participant allocation, adding a 7 day window for day 28 follow-up and reducing the frequency of collecting concomitant medication in the schedule of activities).
bmjopen-2024-088490supp001.pdf (1.7MB, pdf)
Protocol deviations
Deviations from, breaches or violations of or non-compliance to either the protocol, the conditions, or principles of GCP and REC requirements are handled based on their nature and severity by LCTC and reported to the trial oversight committees with serious breaches being reported to Sponsor and REC within 7 days.
Dissemination
The findings of HAP-FAST will be published and disseminated within scientific and lay communities regardless of the magnitude or direction of effect.
Supplementary Material
Footnotes
@stephen_aston
Contributors: DW wrote the grant and obtained the funding and is the Chief Investigator of the HAP-FAST trial and associated sub-studies. SA, LT, SJA, BY, FS, AA, SW, AJ and DW wrote and amended this trial protocol. AH, SA, NS and DW wrote the trial antibiotic guideline. LT, SA and DW led the writing of the mechanistic sub-study components. BY, FS and DW led the writing of the qualitative sub-study components. AA contributed a patient and public involvement perspective throughout the protocol drafting and approval process and chairs the HAP-FAST steering committee. SW and APJ are, respectively, trial manager and lead statistician for Liverpool Clinical Trial Centre and contributed to the protocol development and ongoing trial processes. SJA is the site Principal Investigator at one of the recruiting sites. NS is NIHR Associate Principal Investigator at one of the sites and drafted this protocol submission, and all other authors reviewed and edited this manuscript.
Funding: This work was supported by the National Institute of Health Research (NIHR300669).
Disclaimer: Sponsor is the University of Liverpool UoL001676.
Competing interests: The BIOFIRE® machines for each study site were loaned, free of charge by bioMérieux. FAPP test kits for running on those machines were also provided by bioMérieux. bioMérieux had no role in the content of the funding application, protocol, ethics application for this work nor will they have a role in handling or interpretation of the data or its dissemination. LT has received consulting fees from MHRA and from AstraZeneca and Synairgen, paid to the University of Liverpool; speakers’ fees from Eisai Ltd, and support for conference attendance from AstraZeneca. AH has received personal consulting fees from Pfizer and funding from Pfizer paid to the University of Liverpool for a public/practitioner engagement project.
Patient and public involvement: Patients and/or the public were involved in the design, conduct, reporting or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data sharing not applicable as no datasets generated and/or analysed for this study. Not Applicable— no datasets were analysed for this publication.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involved human participants and was approved by NIHR Ethics Committee: NIHR300669. Participants gave informed consent to participate in the study before taking part.
References
- 1. Suetens C, Kärki T, Plachouras D. Po2int prevalence survey of healthcare-associated infections and antimicrobial use in European acute care hospitals 2016-2017. European Centre for Disease Prevention and Control (ECDC); 2023. Available: https://www.ecdc.europa.eu/sites/default/files/documents/healthcare-associated--infections-antimicrobial-use-point-prevalence-survey-2016-2017.pdf [Google Scholar]
- 2. Wootton D, Higgins J, Jenks T. Hospital acquired pneumonia policy project. Advancing Quality Alliance (Aqua), NHS; 2023. Available: https://aqua.nhs.uk/wp-content/uploads/2023/04/HAP-policy-project-report-1-1.pdf [Google Scholar]
- 3. Wootton D, Cawthorne KR, Higgins J, et al. The incidence risk of hospital acquired pneumonia and associated mortality in England between 2018 and 2023. Advancing Quality Alliance (Aqua), NHS; 2023. Available: https://aqua.nhs.uk/wp-content/uploads/2023/09/HAP-policy-project-report-phase-2-July-2023.pdf [Google Scholar]
- 4. Lim WS, Baudouin SV, George RC, et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax 2009;64 Suppl 3:iii1–55. 10.1136/thx.2009.121434 [DOI] [PubMed] [Google Scholar]
- 5. Burton LA, Price R, Barr KE, et al. Hospital-acquired pneumonia incidence and diagnosis in older patients. Age Ageing 2016;45:171–4. 10.1093/ageing/afv168 [DOI] [PubMed] [Google Scholar]
- 6. Felton T, Wootton D. Antimicrobial prescribing for hospital-acquired pneumonia. Br J Hosp Med (Lond) 2020;81:1–3. 10.12968/hmed.2019.0325 [DOI] [PubMed] [Google Scholar]
- 7. Woodhead M. Pneumonia: diagnosis and management of community and hospital-acquired pneumonia in adults. 2014. [PubMed]
- 8. Singh B, Curtis J, Gordon SB, et al. Junior doctors' interpretation of CXRs is more consistent than consultants in the context of possible pneumonia. Thorax 2011;66:A169–70. 10.1136/thoraxjnl-2011-201054c.251 [DOI] [Google Scholar]
- 9. Wootton D, Feldman C. The diagnosis of pneumonia requires a chest radiograph (X-ray)–yes, no or sometimes? Pneumonia (Nathan) 2014;5:1–7. 10.15172/pneu.2014.5/464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Self WH, Courtney DM, McNaughton CD, et al. High discordance of chest X-ray and computed tomography for detection of pulmonary opacities in ED patients: implications for diagnosing pneumonia. Am J Emerg Med 2013;31:401–5. 10.1016/j.ajem.2012.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Claessens Y-E, Debray M-P, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med 2015;192:974–82. 10.1164/rccm.201501-0017OC [DOI] [PubMed] [Google Scholar]
- 12. Esayag Y, Nikitin I, Bar-Ziv J, et al. Diagnostic value of chest radiographs in bedridden patients suspected of having pneumonia. Am J Med 2010;123:88. 10.1016/j.amjmed.2009.09.012 [DOI] [PubMed] [Google Scholar]
- 13. Prendki V, Scheffler M, Huttner B, et al. Low-dose computed tomography for the diagnosis of pneumonia in elderly patients: a prospective, interventional cohort study. Eur Respir J 2018;51:02375–2017. 10.1183/13993003.02375-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Russell CD, Koch O, Laurenson IF, et al. Diagnosis and features of hospital-acquired pneumonia: a retrospective cohort study. J Hosp Infect 2016;92:273–9. 10.1016/j.jhin.2015.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sopena N, Sabrià M. Multicenter study of hospital-acquired pneumonia in non-ICU patients. Chest 2005;127:213–9. 10.1378/chest.127.1.213 [DOI] [PubMed] [Google Scholar]
- 16. Loubet P, Voiriot G, Houhou-Fidouh N, et al. Impact of respiratory viruses in hospital-acquired pneumonia in the intensive care unit: a single-center retrospective study. J Clin Virol 2017;91:52–7. 10.1016/j.jcv.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hong H-L, Hong S-B, Ko G-B, et al. Viral infection is not uncommon in adult patients with severe hospital-acquired pneumonia. PLoS One 2014;9:e95865. 10.1371/journal.pone.0095865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weber DJ, Rutala WA, Sickbert-Bennett EE, et al. Microbiology of ventilator-associated pneumonia compared with that of hospital-acquired pneumonia. Infect Control Hosp Epidemiol 2007;28:825–31. 10.1086/518460 [DOI] [PubMed] [Google Scholar]
- 19. Enne VI, Aydin A, Baldan R, et al. Multicentre evaluation of two multiplex PCR platforms for the rapid microbiological investigation of nosocomial pneumonia in UK ICUS: the INHALE WP1 study. Thorax 2022;77:1220–8. 10.1136/thoraxjnl-2021-216990 [DOI] [PubMed] [Google Scholar]
- 20. Wagner AP, Enne VI, Livermore DM, et al. Review of health economic models exploring and evaluating treatment and management of hospital-acquired pneumonia and ventilator- associated pneumonia. J Hosp Infect 2020;106:745–56. 10.1016/j.jhin.2020.09.012 [DOI] [PubMed] [Google Scholar]
- 21. Baldwin D, Callister M, Akram A, et al. British thoracic society quality standards for the investigation and management of pulmonary nodules. BMJ Open Respir Res 2018;5:e000273. 10.1136/bmjresp-2017-000273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. National Institute of Health and Social Care Excellence . Pneumonia (hospital-acquired): antimicrobial prescribing.
- 23. Advancing Quality Alliance (AQUA) . Hospital acquired pneumonia (HAP). n.d. Available: https://aqua.nhs.uk/solutions/aq-programme/hospital-acquired-pneumonia-hap/
- 24. Woolfall K, Frith L, Dawson A, et al. Fifteen-minute consultation: an evidence-based approach to research without prior consent (deferred consent) in neonatal and paediatric critical care trials. Arch Dis Child Educ Pract Ed 2016;101:49–53. 10.1136/archdischild-2015-309245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Woolfall K, Frith L, Gamble C, et al. How parents and practitioners experience research without prior consent (deferred consent) for emergency research involving children with life threatening conditions: a mixed method study. BMJ Open 2015;5:e008522. 10.1136/bmjopen-2015-008522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Corneli A, Perry B, Collyar D, et al. Assessment of the perceived acceptability of an early enrolment strategy using advance consent in health care-associated pneumonia. JAMA Netw Open 2018;1:e185816. 10.1001/jamanetworkopen.2018.5816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Powers JH. Recommendations for improving the design, conduct, and analysis of clinical trials in hospital-acquired pneumonia and ventilator-associated pneumonia. Clin Infect Dis 2010;51:S18–28. 10.1086/653036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Muscedere JG, Day A, Heyland DK. Mortality, attributable mortality, and clinical events as end points for clinical trials of ventilator-associated pneumonia and hospital-acquired pneumonia. Clin Infect Dis 2010;51 Suppl 1:S120–5. 10.1086/653060 [DOI] [PubMed] [Google Scholar]
- 29. Weiss E, Zahar J-R, Alder J, et al. Elaboration of consensus clinical endpoints to evaluate antimicrobial treatment efficacy in future hospital-acquired/ventilator-associated bacterial pneumonia clinical trials. Clin Infect Dis 2019;69:1912–8. 10.1093/cid/ciz093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Talbot GH, Das A, Cush S, et al. Foundation for the national Institutes of health biomarkers consortium HABP/VABP project team. evidence-based study design for hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. J Infect Dis 2019;219:1536–44. 10.1093/infdis/jiy578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vock DM, Almirall D. Wiley StatsRef: statistics reference online. Sequential Multiple Assignment Randomized Trial (SMART), n.d.:1–11. [Google Scholar]
- 32. Corneli A, Calvert SB, Powers JH, et al. Consensus on language for advance informed consent in health care–associated pneumonia clinical trials using a Delphi process. JAMA Netw Open 2020;3:e205435. 10.1001/jamanetworkopen.2020.5435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Woolfall K, Shilling V, Hickey H, et al. Parents’ agendas in paediatric clinical trial recruitment are different from researchers’ and often remain unvoiced: a qualitative study. PLoS ONE 2013;8:e67352. 10.1371/journal.pone.0067352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Manoukian S, Stewart S, Graves N, et al. Evaluating the post-discharge cost of healthcare-associated infection in NHS Scotland. J Hosp Infect 2021;114:51–8. 10.1016/j.jhin.2020.12.026 [DOI] [PubMed] [Google Scholar]
- 35. Pinto D, Robertson MC, Abbott JH, et al. Manual therapy, exercise therapy, or both, in addition to usual care, for osteoarthritis of the hip or knee. 2: economic evaluation alongside a randomized controlled trial. Osteoarthr Cartil 2013;21:1504–13. 10.1016/j.joca.2013.06.014 [DOI] [PubMed] [Google Scholar]
- 36. Peek G, Elbourne D, Mugford M, et al. Randomised controlled trial and parallel economic evaluation of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR). Health Technol Assess 2010;14. 10.3310/hta14350 [DOI] [PubMed] [Google Scholar]
- 37. Cross J, Elender F, Barton G, et al. A randomised controlled equivalence trial to determine the effectiveness and cost-utility of manual chest physiotherapy techniques in the management of exacerbations of chronic obstructive pulmonary disease (MATREX). Health Technol Assess 2010;14:1–147. 10.3310/hta14230 [DOI] [PubMed] [Google Scholar]
- 38. Wootton DG, Dickinson L, Pertinez H, et al. A longitudinal modelling study estimates acute symptoms of community acquired pneumonia recover to baseline by 10 days. Eur Respir J 2017;49:1602170. 10.1183/13993003.02170-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lamping DL, Schroter S, Marquis P, et al. The community-acquired pneumonia symptom questionnaire: a new, patient-based outcome measure to evaluate symptoms in patients with community-acquired pneumonia. Chest 2002;122:920–9. 10.1378/chest.122.3.920 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2024-088490supp002.pdf (102.2KB, pdf)
bmjopen-2024-088490supp001.pdf (1.7MB, pdf)
Data Availability Statement
Data sharing not applicable as no datasets generated and/or analysed for this study. Not Applicable— no datasets were analysed for this publication.