Abstract
Background:
Pembrolizumab has a manageable safety profile as described in its label, which was primarily based on 2799 patients who participated in clinical trials for melanoma or non-small cell lung cancer. Here, we evaluated the safety of pembrolizumab in a broader population of patients from 31 advanced cancer clinical trials across 19 cancer types.
Methods:
Safety was analyzed in patients who received at least one dose of pembrolizumab (200 mg every 3 weeks [Q3W], 10 mg/kg Q2W or Q3W, or 2 mg/kg Q3W). Adverse events (AEs) and immune-mediated AEs and infusion reactions were evaluated.
Results:
Safety data from 8937 patients in 31 trials of pembrolizumab monotherapy were pooled (median, seven administrations; range, 1–59). Median duration on treatment was 4.1 months (range, 0.03–40.1). AEs occurred in 96.6% of patients. Grade 3–5 AEs occurred in 50.6% of patients. AEs led to pembrolizumab discontinuation in 12.7% of patients and death in 5.9%. Immune-mediated AEs and infusion reactions occurred in 23.7% of patients (4.6% experienced multiple immune-mediated AEs/infusion reactions) and led to pembrolizumab discontinuation in 3.6% and death in 0.2%. Grade 3–5 immune-mediated AEs occurred in 6.3% of patients. Serious immune-mediated AEs and infusion reactions occurred in 6.0% of patients. Median time to immune-mediated AE onset was 85 days (range, 13–163). Of 2657 immune-mediated AEs, 22.3% were initially treated with prednisone ≥ 40 mg/day or equivalent, and 8.3% were initially treated with lower steroid doses.
Conclusions:
This pooled analysis of 31 clinical trials showed that pembrolizumab has a consistent safety profile across indications.
Keywords: Safety, Pembrolizumab, Programmed cell death 1 receptor, Neoplasms
Trial registry information.
| KEYNOTE-001 | NCT01295827 |
| KEYNOTE-002 | NCT01704287 |
| KEYNOTE-006 | NCT01866319 |
| KEYNOTE-010 | NCT01905657 |
| KEYNOTE-012 | NCT01848834 |
| KEYNOTE-013 | NCT01953692 |
| CITN-09/KEYNOTE-017 | NCT02267603 |
| KEYNOTE-024 | NCT02142738 |
| KEYNOTE-028 | NCT02054806 |
| KEYNOTE-040 | NCT02252042 |
| KEYNOTE-042 | NCT02220894 |
| KEYNOTE-045 | NCT02256436 |
| KEYNOTE-048 | NCT02358031 |
| KEYNOTE-052 | NCT02335424 |
| KEYNOTE-055 | NCT02255097 |
| KEYNOTE-057 | NCT02625961 |
| KEYNOTE-059 | NCT02335411 |
| KEYNOTE-061 | NCT02370498 |
| KEYNOTE-062 | NCT02494583 |
| KEYNOTE-087 | NCT02453594 |
| KEYNOTE-158 | NCT02628067 |
| KEYNOTE-164 | NCT02460198 |
| KEYNOTE-170 | NCT02576990 |
| KEYNOTE-177 | NCT02563002 |
| KEYNOTE-180 | NCT02559687 |
| KEYNOTE-181 | NCT02564263 |
| KEYNOTE-204 | NCT02684292 |
| KEYNOTE-224 | NCT02702414 |
| KEYNOTE-361 | NCT02853305 |
| KEYNOTE-427 | NCT02853344 |
| KEYNOTE-629 | NCT03284424 |
1. Introduction
Since the approval of programmed cell death protein 1/ligand 1 (PD-1/L1) inhibitors in 2014, the survival of patients with many tumor types has substantially improved. The PD-1 inhibitor pembrolizumab is approved for use as monotherapy and in combination with other agents for the treatment of solid tumors and hematologic malignancies in more than 95 countries. Pembrolizumab has a manageable and consistent safety profile across indications that has been established using continuous signal detection and evaluation of safety data [1,2]. Due to its mechanism of action, pembrolizumab can be associated with the development of immune-mediated adverse events (AEs), which can occur in any organ system or tissue at any time after initiating treatment or after discontinuation. The most common immune-mediated AEs occurring with pembrolizumab are endocrinopathies, pneumonitis, colitis, dermatologic adverse reactions, hepatitis and nephritis [1,2]. Although these are generally low grade and manageable and resolve with treatment, they can rarely become severe or fatal [1,2]. Immune-mediated AEs may also last longer than 6 months (e.g. endocrinopathies) and have a late onset. [3] Management of immune-mediated AEs depends on severity and can include withholding or permanently discontinuing pembrolizumab and/or administering systemic corticosteroids, other immunosuppressants or other supportive therapies [1,2]. As with any intravenous antibody-based therapies, the administration of pembrolizumab can also result in infusion reactions, although these are less common. Since the initial filings for pembrolizumab monotherapy, a pooled safety dataset containing information on the incidence, nature, severity and clinical management of immune-mediated AEs and infusion reactions has been maintained to provide a consistent data source for submissions. This consists of a locked dataset of 2799 patients with advanced melanoma or non-small cell lung cancer (NSCLC) from three randomized, open-label, active--controlled clinical trials (KEYNOTE-002, KEYNOTE-006 and KEYNOTE-010) and one single-arm trial (KEYNOTE-001), referred to here as the label safety dataset [1,2]. We sought to assess the safety profile in a broader population of patients. This pooled analysis of data from 31 clinical trials further evaluates the safety profile of pembrolizumab monotherapy across additional tumor types, with particular emphasis on the incidence, characterization and management of immune-mediated AEs and infusion reactions.
2. Materials and methods
Patients included in the current analysis had advanced cancer and received at least one dose of pembrolizumab monotherapy in one of 31 phase 1–3 clinical trials (Appendix Table A.1). Of the 8937 patients, 65.4% received pembrolizumab 200 mg every 3 weeks, 25.8% received pembrolizumab 10 mg/kg every 2 or 3 weeks and 8.9% received pembrolizumab 2 mg/kg every 3 weeks. In most trials, patients received pembrolizumab for ≤ 2 years (35 cycles) or until disease progression, unacceptable toxicity or patient or investigator decision to withdraw. The studies were conducted in accordance with the principles of Good Clinical Practice and were approved by the appropriate institutional review boards and regulatory agencies. All patients provided written informed consent. AEs reflected in this evaluation were coded using the Medical Dictionary for Regulatory Activities, version 25.1, and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. Safety evaluations included any-cause AEs, immune-mediated AEs and infusion reactions and other events of clinical interest. Any-cause AEs were defined as any untoward medical occurrence that was temporally associated with pembrolizumab, whether or not it was considered related to pembrolizumab treatment. Immune-mediated AEs and infusion reactions were based on a list of preferred terms intended to capture known risks of pembrolizumab and were considered regardless of attribution to study treatment by the investigator. These events are pneumonitis, colitis, hepatitis, nephritis, adrenal insufficiency, hypophysitis, hyperthyroidism, hypothyroidism, thyroiditis, type 1 diabetes mellitus, severe skin reactions, uveitis, pancreatitis, myositis, Guillain-Barré syndrome, myocarditis, encephalitis, sarcoidosis, infusion reactions, myasthenic syndrome, myelitis, vasculitis, cholangitis sclerosing, hypoparathyroidism, arthritis, hemophagocytic lymphohistiocytosis, optic neuritis and infusion reaction. Other events of particular interest to PD-1 inhibitor treatment that are not in the list of terms for an immune-mediated AEs were graft-versus-host disease and pericarditis. Patients treated with pembrolizumab who have received allogenic hematopoietic stem cell transplant are at risk of graft-versus-host disease. Pericarditis remains under routine monitoring as there is not sufficient evidence for causality. Safety data were pooled from the 31 trials listed in Appendix Table A.1. The incidence, nature and severity of immune-mediated AEs and infusion reactions were also summarized for the four trials that are the basis for the label safety dataset (KEYNOTE-001, KEYNOTE-002, KEYNOTE-006 and KEYNOTE-010). The conditional incidence rates for immune-mediated AEs and infusion reactions in the 31-trial pooled safety dataset were calculated based on the number of patients experiencing the first occurrence of the event in each time period (6 weeks) divided by the effective number of patients still on study at risk for the event (and naive to the specific event) in each respective period, expressed as a percentage.
3. Results
3.1. Patients
A total of 8937 patients were included in the 31-trial pooled safety dataset. The median age was 63 years (range, 15–95), 66.1% of patients were male, 77.6% were White, 16% were Asian, 7.5% were of Hispanic or Latino ethnicity, 53.4% had an Eastern Cooperative Oncology Group performance status of 1, and 70.0% were enrolled outside the United States (Table 1). Patients in the 31-trial pooled safety dataset received a median of seven administrations (range, 1–59) of pembrolizumab, and the median duration on treatment was 4.1 months (range, 1 day to 40.1 months). Baseline characteristics were generally comparable between the 31-trial pooled safety dataset and the label safety dataset (Table 1). Similarly, in the label safety dataset, patients received a median of seven pembrolizumab administrations (range, 1–59), and the median duration of treatment was 4.2 months (range, 1 day to 30.4 months).
Table 1.
Baseline characteristics.
| 31-trial pooled safety dataset | Label safety dataset | |
|---|---|---|
| N = 8937 | N = 2799 | |
| Age, median (range), years | 63 (15–95) | 62 (15–94) |
| Age category | ||
| < 65 years | 4935 (55.2) | 1587 (56.7) |
| ≥ 65 years | 4002 (44.8) | 1212 (43.3) |
| Sex | ||
| Male | 5908 (66.1) | 1659 (59.3) |
| Female | 3029 (33.9) | 1140 (40.7) |
| Race | ||
| American Indian or Alaska Native | 69 (0.8) | 7 (0.3) |
| Asian | 1432 (16.0) | 233 (8.3) |
| Black or African American | 171 (1.9) | 48 (1.7) |
| Multiracial | 97 (1.1) | 11 (0.4) |
| Native Hawaiian or Other Pacific Islander |
10 (0.1) | 4 (0.1) |
| White | 6931 (77.6) | 2474 (88.4) |
| Unknown/missing | 227 (2.5) | 22 (0.8) |
| Ethnicity | ||
| Hispanic or Latino | 669 (7.5) | 128 (4.6) |
| Not Hispanic or Latino | 7660 (85.7) | 2582 (92.2) |
| Not reported | 406 (4.5) | 47 (1.7) |
| Unknown/missing | 202 (2.3) | 42 (1.5) |
| ECOG performance status | ||
| 0 | 3848 (43.1) | 1446 (51.7) |
| 1 | 4773 (53.4) | 1347 (48.1) |
| Other/missing | 316 (3.5) | 6 (0.2) |
| Geographic region | ||
| United States | 2678 (30.0) | 1250 (44.7) |
| Rest of world | 6259 (70.0) | 1549 (55.3) |
| Primary cancer type | ||
| Bladder cancer | 1198 (13.4) | 0 |
| Cervical cancer | 106 (1.2) | 0 |
| Cervical cancer, TMB-H | 16 (0.2) | 0 |
| Classical Hodgkin lymphoma | 389 (4.4) | 0 |
| Colorectal cancer | 277 (3.1) | 0 |
| Cutaneous squamous cell carcinoma | 159 (1.8) | 0 |
| Esophageal cancer | 458 (5.1) | 0 |
| Gastric cancer | 877 (9.9) | 0 |
| Head and neck squamous cell carcinoma | 909 (10.2) | 0 |
| Hepatocellular carcinoma | 104 (1.2) | 0 |
| Melanoma | 1567 (17.6) | 1567 (56.0) |
| Merkel cell carcinoma | 50 (0.6) | 0 |
| Microsatellite instability–high cancer | 351 (4.0) | 0 |
| Non-small cell lung cancer | 2106 (23.6) | 1232 (44.0) |
| Primary mediastinal large B-cell lymphoma | 74 (0.8) | 0 |
| Renal cell carcinoma | 110 (1.2) | 0 |
| Small cell lung cancer | 97 (1.1) | 0 |
| Small cell lung cancer, TMB-H | 34 (0.4) | 0 |
| TMB-H cancer | 55 (0.6) | 0 |
Data are n (%) unless otherwise specified.
ECOG, Eastern Cooperative Oncology Group; TMB-H, tumor mutational burden–high.
3.2. AEs
In the 31-trial pooled safety dataset, almost all patients (96.6%) who received pembrolizumab experienced at least one AE of any cause (Table 2). The most frequently reported any-cause AEs (≥20%) were fatigue (29.7%), nausea (20.4%) and decreased appetite (20.3%). Any-cause grade 3–5 AEs occurred in 50.6% of patients and were most commonly (≥ 2%) anemia (5.4%), pneumonia (4.0%), hyponatremia (2.7%), fatigue (2.6%) and dyspnea (2.1%) (Appendix Table A.2). Any-cause serious AEs were reported by 39.3% of patients, with pneumonia being the only serious AE occurring in ≥ 2% of patients (Appendix Table A.3). AEs that led to discontinuation of pembrolizumab occurred in 12.7% of patients, and the most common (≥ 1%) was pneumonitis (1.4%) (Appendix Table A.4). A total of 5.9% of patients died because of an any-cause AE (Appendix Table A.5). The AE profile summary in the 31-trial pooled safety dataset was comparable to that observed in the label safety dataset (Table 2).
Table 2.
Summary of all-cause AEs.
| 31-trial pooled safety dataset | Label safety dataset | |
|---|---|---|
| N = 8937 | N = 2799 | |
| AEs of any cause | 8630 (96.6) | 2727 (97.4) |
| Grade 3–5 AEs | 4525 (50.6) | 1273 (45.5) |
| Serious AEs | 3513 (39.3) | 1042 (37.2) |
| Led to discontinuation | 1135 (12.7) | 334 (11.9) |
| Led to death | 527 (5.9) | 110 (3.9) |
| Any AE in ≥ 10% of patients in either dataset | ||
| Fatigue | 2654 (29.7) | 1044 (37.3) |
| Nausea | 1827 (20.4) | 685 (24.5) |
| Decreased appetite | 1812 (20.3) | 630 (22.5) |
| Diarrhea | 1773 (19.8) | 625 (22.3) |
| Constipation | 1585 (17.7) | 498 (17.8) |
| Cough | 1570 (17.6) | 615 (22.0) |
| Pruritus | 1522 (17.0) | 580 (20.7) |
| Arthralgia | 1480 (16.6) | 636 (22.7) |
| Anemia | 1458 (16.3) | 347 (12.4) |
| Dyspnea | 1332 (14.9) | 534 (19.1) |
| Rash | 1212 (13.6) | 508 (18.1) |
| Vomiting | 1198 (13.4) | 387 (13.8) |
| Pyrexia | 1182 (13.2) | 357 (12.8) |
| Asthenia | 1059 (11.8) | 362 (12.9) |
| Back pain | 1019 (11.4) | 344 (12.3) |
| Hypothyroidism | 935 (10.5) | 236 (8.4) |
| Abdominal pain | 923 (10.3) | 274 (9.8) |
| Headache | 885 (9.9) | 400 (14.3) |
Data are n (%).
AE, adverse event.
3.3. Immune-mediated AEs and infusion reactions
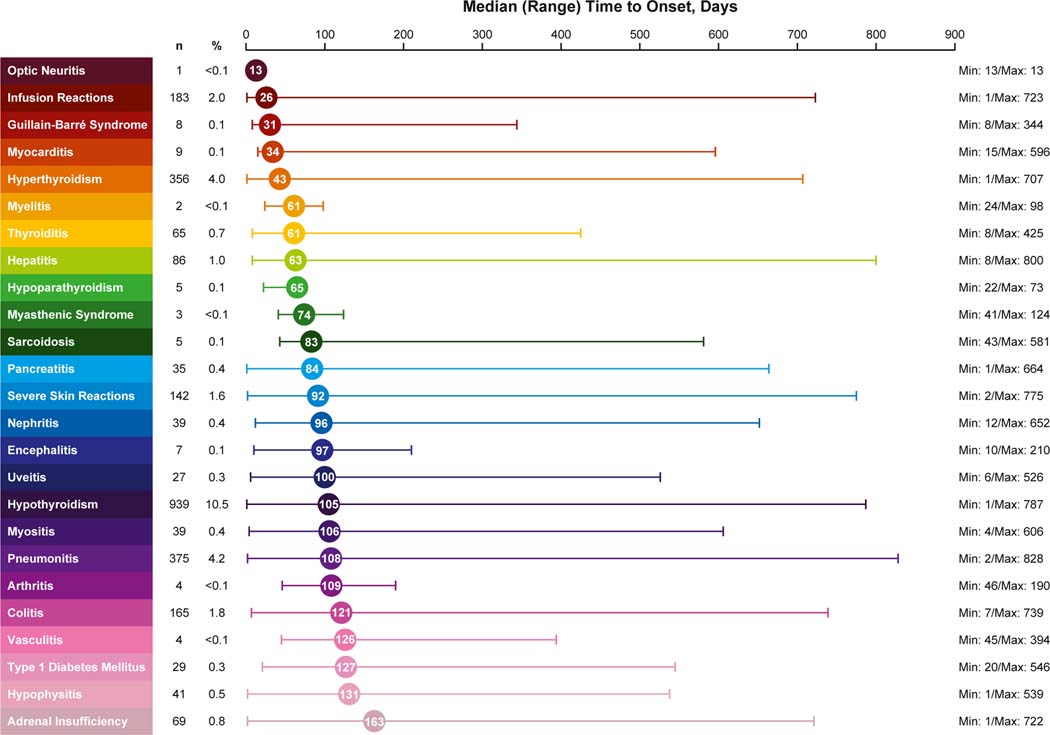
In the 31-trial pooled safety dataset, any-grade immune-mediated AEs and infusion reactions were reported by 23.7% of patients, and the most common (≥ 3%) were hypothyroidism (10.5%), pneumonitis (4.2%) and hyperthyroidism (4.0%) (Table 3; Fig 1). Grade 3–5 immune-mediated AEs and infusion reactions occurred in 6.3% of patients, and the majority were grade 3 (5.3%) (Appendix Table A.6). The most common grade 3–5 events were pneumonitis (1.4%), severe skin reactions (1.2%) and colitis (1.1%). Serious immune-mediated AEs and infusion reactions occurred in 6.0% of patients, and 3.6% of patients discontinued pembrolizumab because of an immune-mediated AE or infusion reaction (Table 3). Of the 8937 patients, 22 (0.2%) died of an immune-mediated AE (pneumonitis, n = 15 [0.2%]; colitis, n = 2 [<0.01%]; myocarditis, n = 2 [< 0.01%]; Guillain-Barré syndrome, n = 1 [< 0.01%]; myositis, n = 1 [< 0.01%] and severe skin reaction, n = 1 [< 0.01%]) (Appendix Table A.6). The immune-mediated AE and infusion reaction profile was comparable to that reported for the label safety dataset (Table 3). The time to onset of any immune-mediated AE or infusion reaction after initiating pembrolizumab ranged from 13 to 163 days (median, 85) (Fig 1). The risk of some immune-mediated events, such as hypothyroidism, hyperthyroidism, thyroiditis and infusion reactions, was higher earlier during the treatment period. This is reflected by a higher conditional incidence rate for these events, which peaked in the first 3–4 months of treatment and leveled off throughout subsequent treatment periods (see hypothyroidism example in Appendix Figure A.1). For the remaining immune-mediated events, the conditional incidence was generally consistent over the treatment course (see pneumonitis example in Appendix Figure A.2). Overall, 2657 immune-mediated AE episodes (excluding infusion reactions) occurred in 1984 of the 8937 patients (22.2%) in the 31-trial pooled safety dataset. Of those 2657 episodes, 592 (22.3%) required initial treatment with prednisone ≥ 40 mg/day or equivalent; the median starting dose for these episodes was 75 mg/day (range, 40–1250), and the median duration of initial treatment at doses of ≥ 40 mg/day was 7 days, after which the dose was tapered to < 40 mg/day (Table 4). Of all immune-mediated AE episodes (excluding infusion reactions), 1842 (69.3%) were not treated with corticosteroids. Many immune-mediated AEs were endocrinopathies, which may require long-term end-organ hormone replacement therapy rather than anti-inflammatory corticosteroid medication. Hormone replacement therapy was required for 81.4% of patients with an immune-mediated endocrinopathy, most commonly hypothyroidism (757 of 939 patients [80.6%]), adrenal insufficiency (65 of 69 patients [94.2%]), thyroiditis (45 of 65 patients [69.2%]), hypophysitis (40 of 41 patients [97.6%]) and type 1 diabetes mellitus (23 of 29 patients [79.3%]). At the time of AE reporting, most of the immune-mediated AEs were reported as resolved or resolving. The more common events that were usually unresolved at the time of AE reporting were pneumonitis and, due to the need for long-term hormone replacement, endocrinopathies. Some immune-mediated AEs were more likely to be observed in certain indications. Hypothyroidism occurred more frequently in patients with head and neck carcinoma (16.2% vs 9.8% with other types of cancer), pneumonitis occurred more frequently in patients with lung cancer (4.9% vs 3.4% with other types of cancer), and vitiligo occurred more frequently in patients with melanoma (10.8% vs 0.2% with other types of cancer). Among the 8937 patients, 407 (4.6%) experienced more than one type of immune-mediated AE involving different organ systems: 350 (3.9%) had two immune-mediated AEs, 50 (0.6%) had three immune-mediated AEs and seven (< 0.1%) had four immune-mediated AEs. No specific pattern of multiple immune-mediated AEs was observed. Immune-mediated AEs affecting more than one body system can occur simultaneously, but this was not a pattern for all patients. Other events of clinical interest with pembrolizumab included graft-versus-host disease and pericarditis. In this analysis, graft-versus-host disease occurred in six patients; all six had hematopoietic malignancies and received a bone marrow transplant within 20 days to 2 months after discontinuing pembrolizumab. The median time to onset for graft-versus-host disease was 213 days (range, 142–407), and four episodes required treatment with corticosteroids. Two episodes were grade 2 in severity, two were grade 3, one was grade 4, and one was grade 5. Four episodes were reported as resolved. Pericarditis occurred in 13 patients (0.1%), with a median time to onset of 46 days (range, 3–566). Ten of 16 episodes required treatment with corticosteroids, with eight requiring prednisone ≥ 40 mg/day or equivalent (median dose, 68 mg/day prednisone equivalent). Eleven patients were reported as having pericarditis resolved or resolving; pericarditis was resolved for two patients. There were no deaths due to pericarditis.
Table 3.
Summary of immune-mediated AEs and infusion reactions.
| 31-trial pooled safety dataset | Label safety dataset [1] | |||
|---|---|---|---|---|
| N = 8937 | N = 2799 | |||
|
| ||||
| Immune-mediated AEs or infusion reactions | 2121 (23.7) | 601 (21.5) | ||
| Grade 3–5 AEs | 563 (6.3) | 155 (5.5) | ||
| Serious AEs | 538 (6.0) | 162 (5.8) | ||
| Led to discontinuation | 326 (3.6) | 86 (3.1) | ||
| Led to death | 22 (0.2)a | 4 (0.1)b | ||
| Any immune-mediated AE or infusion reaction | Any grade | Grade 3–5 | Any grade | Grade 3–5 |
| Hypothyroidism | 939 (10.5) | 11 (0.1) | 237 (8.5) | 3 (0.1) |
| Pneumonitis | 375 (4.2) | 125 (1.4) | 94 (3.4) | 36 (1.3) |
| Hyperthyroidism | 356 (4.0) | 8 (0.1) | 96 (3.4) | 4 (0.1) |
| Infusion reactions | 183 (2.0) | 16 (0.2) | 70 (2.5) | 6 (0.2) |
| Colitis | 165 (1.8) | 95 (1.1) | 48 (1.7) | 32 (1.1) |
| Severe skin reactions | 142 (1.6) | 109 (1.2) | 39 (1.4) | 30 (1.1) |
| Hepatitis | 86 (1.0) | 68 (0.8) | 18 (0.6) | 13 (0.5) |
| Adrenal insufficiency | 69 (0.8) | 36 (0.4) | 22 (0.8) | 10 (0.4) |
| Thyroiditis | 65 (0.7) | 4 (< 0.1) | 16 (0.6) | 0 |
| Hypophysitis | 41 (0.5) | 23 (0.3) | 17 (0.6) | 9 (0.3) |
| Myositis | 39 (0.4) | 11 (0.1) | 11 (0.4) | 1 (< 0.1) |
| Nephritis | 39 (0.4) | 20 (0.2) | 9 (0.3) | 5 (0.2) |
| Pancreatitis | 35 (0.4) | 22 (0.2) | 9 (0.3) | 6 (0.2) |
| Type 1 diabetes mellitus | 29 (0.3) | 26 (0.3) | 6 (0.2) | 5 (0.2) |
| Uveitis | 27 (0.3) | 3 (< 0.1) | 14 (0.5) | 1 (< 0.1) |
| Myocarditis | 9 (0.1) | 8 (0.1) | 0 | 0 |
| Guillain-Barré syndrome | 8 (0.1) | 6 (0.1) | 2 (0.1) | 1 (< 0.1) |
| Encephalitis | 7 (0.1) | 6 (0.1) | 1 (< 0.1) | 1 (< 0.1) |
| Hypoparathyroidism | 5 (0.1) | 0 | 1 (< 0.1) | 0 |
| Sarcoidosis | 5 (0.1) | 0 | 2 (0.1) | 0 |
| Arthritis | 4 (< 0.1) | 4 (< 0.1) | 0 | 0 |
| Vasculitis | 4 (< 0.1) | 3 (< 0.1) | 2 (0.1) | 1 (< 0.1) |
| Myasthenic syndrome | 3 (< 0.1) | 2 (< 0.1) | 2 (0.1) | 1 (< 0.1) |
| Myelitis | 2 (< 0.1) | 2 (< 0.1) | 1 (< 0.1) | 1 (< 0.1) |
| Optic neuritis | 1 (< 0.1) | 0 | 1 (< 0.1) | 0 |
AE, adverse event.
Data are n (%).
A total of 22 patients (0.2%) in the 31-trial pooled safety dataset died due to an immune-mediated AE (pneumonitis, n = 15; colitis, n = 2; myocarditis, n = 2; Guillain-Barré syndrome, n = 1; myositis, n = 1; severe skin reaction, n = 1).
Four patients (0.1%) in the label safety dataset died due to an immune-mediated AE (pneumonitis, n = 4).
Fig. 1.
Time to onset of first occurrence of immune-mediated adverse events or infusion reactions.
Table 4.
Summary of concomitant corticosteroid use for immune-mediated AEs.
| No corticosteroid | Low-dose corticosteroid (40 mg/day prednisone equivalent) | High-dose corticosteroid (≥ 40 mg/day prednisone equivalent) | |||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Episodes, n/N (%) | Episodes, n/N (%) | Starting dose, median (range), mg/day | Duration, median (range), days | Episodes, n/N (%) | Starting dose, median (range), mg/day | Duration, median (range), days | |
| Any immune-mediated AE | 1842/2657 (69.3) | 220/2657 (8.3) | 20 (1–38) | 8 (1–1239) | 592/2657 (22.3) | 75 (40–1250) | 7 (1–1607) |
| Adrenal insufficiencya | 12/72 (16.7) | 44/72 (61.1) | 8 (1–38) | 16 (1–504) | 15/72 (20.8) | 67 (40–126) | 2 (1–164) |
| Arthritis | 1/5 (20.0) | 1/5 (20.0) | 5 (5–5) | 120 (120–120) | 3/5 (60.0) | 53 (40–60) | 3 (2–5) |
| Colitisa | 59/184 (32.1) | 14/184 (7.6) | 28 (1–33) | 7 (2–31) | 110/184 (59.8) | 64 (40–625) | 7 (1–369) |
| Encephalitis | 2/7 (28.6) | 2/7 (28.6) | 2 (1–3) | 5 (4–5) | 3/7 (42.9) | 267 (80–1250) | 2 (1–3) |
| Guillain-Barré syndrome | 5/8 (62.5) | 0/8 (0) | – | – | 3/8 (37.5) | 90 (63–107) | 7 (1–421) |
| Hepatitis | 27/89 (30.3) | 6/89 (6.7) | 23 (10–27) | 8 (1–715) | 56/89 (62.9) | 80 (40–625) | 5 (1–66) |
| Hyperthyroidism | 354/370 (95.7) | 4/370 (1.1) | 18 (10–30) | 31 (13–44) | 12/370 (3.2) | 73 (40–100) | 6 (1–50) |
| Hypoparathyroidism | 5/5 (100) | 0/5 (0) | – | – | 0/5 (0) | – | – |
| Hypophysitis | 4/42 (9.5) | 25/42 (59.5) | 10 (3–25) | 60 (1–1239) | 13/42 (31.0) | 63 (40 –160) | 4 (1–29) |
| Hypothyroidism | 1001/1018 (98.3) | 9/1018 (0.9) | 10 (3–25) | 57 (1–296) | 8/1018 (0.8) | 60 (40–625) | 6 (2–22) |
| Myasthenic syndrome | 1/3 (33.3) | 0/3 (0) | – | – | 2/3 (66.7) | 675 (100–1250) | 5 (3–7) |
| Myelitis | 0/2 (0) | 1/2 (50.0) | 1 (1–1) | 3 (3–3) | 1/2 (50.0) | 142 (142–142) | 1 (1–1) |
| Myocarditis | 1/9 (11.1) | 2/9 (22.2) | 19 (4–33) | 4 (3–5) | 6/9 (66.7) | 103 (65–725) | 5 (1–9) |
| Myositis | 24/44 (54.5) | 7/44 (15.9) | 20 (5–27) | 15 (4–22) | 13/44 (29.5) | 80 (40–1000) | 9 (1–71) |
| Nephritis | 5/39 (12.8) | 2/39 (5.1) | 25 (20–30) | 29 (12–46) | 32/39 (82.1) | 73 (40–625) | 9 (1–121) |
| Optic neuritis | 0/1 (0) | 0/1 (0) | – | – | 1/1 (100) | 80 (80–80) | 10 (10–10) |
| Pancreatitis | 21/38 (55.3) | 3/38 (7.9) | 13 (10–20) | 7 (2–318) | 14/38 (36.8) | 78 (40–213) | 6 (1–1110) |
| Pneumonitisa | 120/423 (28.4) | 58/423 (13.7) | 25 (1–38) | 8 (1–168) | 244/423 (57.7) | 75 (40–1250) | 7 (1–1607) |
| Sarcoidosis | 4/5 (80.0) | 0/5 (0) | – | – | 1/5 (20.0) | 60 (60–60) | 36 (36–36) |
| Severe skin reactions | 74/155 (47.7) | 37/155 (23.9) | 20 (1–38) | 8 (1–198) | 44/155 (28.4) | 75 (40–267) | 7 (1–153) |
| Thyroiditis | 61/68 (89.7) | 3/68 (4.4) | 8 (5–8) | 5 (3–22) | 4/68 (5.9) | 74 (60–725) | 4 (1–22) |
| Type 1 diabetes mellitus | 36/38 (94.7) | 0/38 (0) | – | – | 2/38 (5.3) | 64 (60–69) | 4 (3–4) |
| Uveitis | 24/28 (85.7) | 2/28 (7.1) | 30 (30–30) | 6 (1–11) | 2/28 (7.1) | 50 (40–60) | 12 (7–17) |
| Vasculitis | 1/4 (25.0) | 0/4 (0) | – | – | 3/4 (75.0) | 60 (60–75) | 7 (6–29) |
AE, adverse event.
Starting dose missing for one episode.
4. Discussion
This pooled analysis of 8937 patients from 31 clinical trials represents the largest analysis to date of the safety of pembrolizumab monotherapy in patients with advanced cancer. The safety profile was consistent with that of the labeled profile of pembrolizumab, despite a larger dataset and more diverse cancer population [1]. No new safety signals were observed, and the immune-mediated AE and infusion reaction profile for pembrolizumab remained unchanged from that observed in the label safety dataset [1,4,5]. The most frequent any-cause AEs in the 31-trial pooled safety dataset and the label safety dataset were similar, although only fatigue, nausea and decreased appetite were reported in ≥ 20% of patients in the 31-trial pooled safety dataset. The incidence of immune-mediated AEs and infusion reactions in the 31-trial pooled safety dataset was 23.7%, and were also generally consistent with the label safety dataset [1]. The most commonly reported immune-mediated AEs (> 1%) in both datasets were hypothyroidism, pneumonitis, hyperthyroidism, colitis and severe skin reactions, consistent with the label safety dataset [1]. Type 1 diabetes mellitus and arthritis were relatively rare in both datasets (≤ 0.3% of patients). A higher rate of hypothyroidism was observed in patients with head and neck carcinoma than other types of cancer, which may be because of prior radiation exposure. Infusion reactions occurred in 2.0% of patients in the 31-trial pooled safety dataset (grade 3–5, 0.2%) compared with 2.5% (grade 3–5, 0.2%) in the label safety dataset. Twenty-two patients (0.2%) died of immune-mediated AEs in the 31-trial pooled safety dataset; the majority of these deaths were due to pneumonitis (n = 15). Few patients (4.6%) experienced multiple immune-mediated AEs. For each type of immune-mediated AE in the 31-trial pooled safety dataset, there was a wide range of time to onset. The risks of experiencing immune-mediated AEs affecting the thyroid (i.e. hypothyroidism, hyperthyroidism and thyroiditis) and infusion reactions were higher earlier in treatment. This was reflected by conditional incidence rates for these events that peaked earlier in treatment before leveling off throughout subsequent time periods. In contrast, other events, such as pneumonitis, had consistent conditional incidence rates across time periods. The strategies recommended to manage AEs and immune-mediated AEs that occur with pembrolizumab include: treatment interruption or permanent discontinuation; use of immunosuppressants, most commonly systemic glucocorticoids; hormone replacement therapy; and other supportive strategies [1]. Only a small proportion of patients discontinued treatment due to toxicity in this analysis (12.7% because of all-cause AEs and 3.6% because of immune-mediated AEs or infusion reactions). Corticosteroid doses of ≥ 40 mg/day prednisone or equivalent were used for 22.3% of immune-mediated AE episodes, with a median duration of 7 days. Initial treatment with < 40 mg/day of prednisone or equivalent was required for 8.3% of episodes, and 69.3% of immune-mediated AE episodes were not treated with corticosteroids. Notably, investigators chose to administer the highest starting doses of corticosteroids for neurologic events, including myasthenic syndrome (median, 675 mg/day), encephalitis (median, 267 mg/day) and myelitis (median, 142 mg/day). The most frequent immune-mediated AEs and infusion reactions were thyroid endocrinopathies, which are managed with hormone replacement rather than anti-inflammatory steroid use. National cancer organizations and other published treatment guidelines provide recommendations for the use of additional immunomodulators beyond steroids [6,7]. Although the larger clinical trial dataset allows for more precision in defining the AE profile relative to the label safety dataset and demonstrates the consistency of the AE profile with the label safety dataset, there are some limitations to this study. Very rare immune-mediated events may have not been observed in the nearly 9000 patients included in the 31-trial pooled safety dataset. There was also a lack of data regarding time to immune-mediated AE resolution as AEs were only captured up to 30 days after discontinuing pembrolizumab treatment. The safety profile of pembrolizumab continues to be monitored using postmarketing and ongoing trial data, and safety information is updated as necessary.
5. Conclusions
The results of this large, pooled analysis of data from 31 clinical trials show that pembrolizumab had a consistent safety profile across tumor types.
Supplementary Material
Acknowledgments
The authors thank the participants and their families for participating in the study and all investigators and site personnel. Medical writing and/or editorial assistance was provided by Jemimah Walker, PhD, and Robert Steger, PhD, of ApotheCom (Yardley, PA, USA). This assistance was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Funding
Funding for this study was provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD). The funder participated in study design, data analysis and interpretation, and manuscript writing. This work was also supported by a grant from the National Cancer Institute, USA Kidney SPORE (5P50CA101942).
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships that may be considered as potential competing interests:
1. Julie R. Brahmer reports grants or contracts from AstraZeneca and Bristol Myers Squibb; consulting fees from AstraZeneca, BMS, Incyte, Genentech/Roche, Regeneron, MSD, Johnson & Johnson, Janssen, and Sanofi; participation on a Data Safety Monitoring Board or Advisory Board for Johnson & Johnson/Janssen and GlaxoSmithKline; a leadership or fiduciary role at the Society for Immunotherapy of Cancer; and receipt of drugs from Bristol Myers Squibb and Syndax.
2. Georgina V. Long reports consulting fees from Agenus Inc, Amgen Inc, Array Biopharma, Boehringer Ingelheim International GmbH, Bristol Myers Squibb, Evaxion Biotech A/S, Hexal AG (Sandoz Company), Highlight Therapeutics S.L., Innovent Biologics USA Inc., MSD, Novartis Pharma AG, OncoSec Medical Australia, Pierre Fabre, Provectus Australia, QBiotics Group Limited, and Regeneron Pharmaceuticals; payment or honoraria for lectures from Bristol Myers Squibb and Pierre Fabre; and participation on a Data Safety Monitoring or Advisory Board for Agenus Inc, Amgen Inc, Array Biopharma, Boehringer Ingelheim International GmbH, Bristol Myers Squibb, Evaxion Biotech A/S, Hexal AG (Sandoz Company), Highlight Therapeutics S.L., Innovent Biologics USA Inc., Merck Sharp & Dohme (Australia) Pty Limited, Novartis Pharma AG, OncoSec Medical Australia, Pierre Fabre, Provectus Australia, QBiotics Group Limited, and Regeneron Pharmaceuticals.
3. Omid Hamid reports support for the present manuscript from Merck; consulting fees from Alkermes, Amgen, Bactonix, BeiGene, BioAtla, Bristol-Myers Squibb, Eisai, Roche Genentech, Georgiamune, GigaGen, Grit Bio, GlaxoSmithKline, Idera, Immunocore, Incyte, Instil Bio, Iovance, Janssen, KSQ, Merck, Moderna, Novartis, Obsidian, Pfizer, Regeneron, Sanofi, Seattle Genetics, Tempus, Vial Health, and Zelluna; payment or honoraria for speakers bureaus from Bristol-Myers Squibb, Immunocore, Novartis, Pfizer, and Regeneron; support for attending meetings and/or travel from Alkermes, Amgen, Bactonix, BeiGene, BioAtla, Bristol-Myers Squibb, Eisai, Roche Genentech, Georgiamune, GigaGen, Grit Bio, GlaxoSmithKline, Idera, Immunocore, Incyte, Instil Bio, Iovance, Janssen, KSQ, Merck, Moderna, Novartis, Obsidian, Pfizer, Regeneron, Sanofi, Seattle Genetics, Tempus, Vial Health, and Zelluna; participation on an Advisory Board for Alkermes, Amgen, Bactonix, BeiGene, BioAtla, Bristol-Myers Squibb, Eisai, Roche Genentech, Georgiamune, GigaGen, Grit Bio, GlaxoSmithKline, Idera, Immunocore, Incyte, Instil Bio, Iovance, Janssen, KSQ, Merck, Moderna, Novartis, Obsidian, Pfizer, Regeneron, Sanofi, Seattle Genetics, Tempus, Vial Health, and Zelluna; stock or stock options in Bactonix; and institutional research funding from Arcus, Aduro, Akeso, Amgen, BioAtla, Bristol-Myers Squibb, CytomX Therapeutics, Exelixis, Roche Genentech, GlaxoSmithKline, Immunocore, Idera, Incyte, Iovance, Merck, Merck Serono, Moderna, NextCure, Novartis, Pfizer, Regeneron, Seattle Genetics, Torque, and Zelluna.
4. Edward B. Garon reports advisory and/or consultancy roles for AbbVie, ABL-Bio, Arcus, AstraZeneca, Atreca, Boehringer-Ingelheim, BridgeBio, Bristol Myers Squibb, EMD Serono, Eisai, Eli Lilly, Gilead, GlaxoSmithKline, Merck, Merus, Novartis, Nuvalent, Personalis, Regeneron, Sanofi, Seagan, Sensei, Sumitomo, Summit, Synthekine, Xilio, and Zymeworks; reports grants/research support from ABL-Bio, ArriVent, AstraZeneca, Bristol Myers Squibb, Daiichi Sanko, Dynavax Technologies, Eli Lilly, EMD Serono, Genentech, Gilead, Iovance Biotherapeutics, Merck, Mirati Therapeutics, Neon, Novartis, and Synthekine; sponsored Independent Medical Education for Daiichi-Sankyo and Ipsen; and support for travel from A2 Bio and Novartis.
5. Roy S. Herbst reports support for the present manuscript from Merck; consulting fees from Immunocore, AstraZeneca, Bristol Myers Squibb, DynamiCure Biotechnology, eFFECTOR Therapeutics Inc., Eli Lilly and Company, Genentech, Gilead, HiberCell Inc., Janssen, Johnson & Johnson, Loxo Oncology, Mirati Therapeutics, NextCure, Oncocyte Corp, Oncternal Therapeutics, Pfizer, Regeneron Pharmaceuticals, Roche, Sanofi, and Seattle Genetics; support for attending meetings and/or travel from the American Association for Cancer Research, the International Association for the Study of Lung Cancer, the Society for Immunotherapy of Cancer, and the Southwest Oncology Group; participation on a Data Safety Monitoring Board or Advisory Board for Bolt Biotherapeutics, Candel Therapeutics Inc., Checkpoint Therapeutics, Cybrexa Therapeutics, EMD Serono, I-Mab Biopharma, Immune-Onc Therapeutics Inc., Immunocore, Normunity, Novartis, Ocean Biomedical Inc., Revelar Biotherapeutics Inc., Ribbon Therapeutics, and Xencor; leadership or fiduciary roles with Immunocore and Junshi Pharmaceuticals; stock or stock options in Immunocore, Bolt Biotherapeutics, Checkpoint Therapeutics, and Normunity; and research support from AstraZeneca, Eli Lilly and Company, Genentech/Roche, and Merck and Company.
6. Thierry Andre reports consulting fees from AbbVie, Aptitude health, Bristol Myers Squibb, Gritstone Oncology, GamaMabs Pharma SA, Gilead, Nordic, GlaxoSmithKline, Merck & Co. Inc., Seagen, Servier, and Takeda; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Bristol Myers Squibb, Gritstone Oncology, GlaxoSmithKline, Merck & Co. Inc., Merck Serono, Roche/Ventana, Seagen, and Servier; support for attending meetings and/or travel from Bristol Myers Squibb and Merck & Co. Inc.; participation on a Data Safety Monitoring Board for Inspirna: Phase 2 randomized controlled trial of ompenaclid in 2nd line RAS mutant metastatic colorectal cancer; and a leadership or fiduciary role with the ARCAD foundation.
7. Philippe Armand reports consulting roles for Merck, BMS, Pfizer, Affimed, Adaptive, Infinity, ADC Therapeutics, Celgene, MorphoSys, Daiichi Sankyo, Miltenyi, Tessa, Genmab, C4, Enterome, Regeneron, Epizyme, Astra Zeneca, Genentech/Roche, Xencor, Foresight, and ATB Therapeutics; research funding from Kite, Merck, BMS, Affimed, Adaptive, Tensha, Otsuka, Sigma Tau, Genentech/Roche, IGM, and Astra Zeneca; and honoraria from Merck and BMS.
8. Dean Bajorin reports grants or contracts from Merck Sharp & Dohme and Bristol Myers Squibb; consulting fees from Merck Sharp & Dohme and Bristol Myers Squibb; and participation on a Data Safety Monitoring Board or Advisory Board for Merck Sharp & Dohme.
9. Joaquim Bellmunt reports royalties or licenses from UpToDate; consulting fees from Merck, MSD-Serono, Pfizer, AstraZeneca, BMS, and Genentech; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Pfizer, MSD Serono, Genentech, and Ipsen; support for attending meetings and/or travel from Pfizer; and participation on a Data Safety Monitoring Board or Advisory Board for Merck, Pfizer, MSD Serono, Genentech, and AstraZeneca.
10. Barbara Burtness reports support for the current manuscript from Merck; grants or contracts from Merck, Merck KgA, Vaccinex, and GSK; royalties or licenses from UpToDate; and consulting fees from AbbVie, AstraZeneca, Cue BioPharma, GSK, Seagen, Nektar, Kura, IO Biotech, Vaccinex, Merck, Merck KgA, Debiopharm, Mirati, and Genentech.
11. Toni K. Choueiri reports support from Alkermes, AstraZeneca, Aravive, Aveo, Bayer, Bristol Myers Squibb, Circle Pharma, Eisai, EMD Serono, Exelixis, GlaxoSmithKline, IQVA, Infiniti, Ipsen, Kanaph, Lilly, Merck, Nikang, Novartis, Nuscan, Pfizer, Roche, Sanofi/Aventis, Surface Oncology, Takeda, Tempest, Up-To-Date, and for CME events (Peerview, PER, MJH Life Sciences, Research to Practice, France Foundation, Springer, WebMed, ASiM Ce, Caribou Publishing, and others); grants or contracts from AstraZeneca, Aveo, Arcus, Bayer, Bristol Myers Squibb, Eisai, EMD Serono, Exelixis, GlaxoSmithKline, Lilly, Merck, Nikang, Novartis, Pfizer, Roche, Sanofi/Aventis, and Takeda; consulting fees from AstraZeneca, Aravive, Aveo, Bayer, Bristol Myers Squibb, Circle Pharma, Eisai, EMD Serono, Exelixis, GlaxoSmithKline, IQVA, Infiniti, Ipsen, Kanaph, Lilly, Merck, Nikang, Novartis, Nuscan, Pfizer, Roche, Sanofi/Aventis, Surface Oncology, Takeda, Tempest, Up-To-Date, and for CME events (Peerview, PER, MJH life sciences, Research to Practice, France Foundation, Springer, WebMed, ASiM Ce, Caribou Publishing); payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AstraZeneca, Aravive, Aveo, Bayer, Bristol Myers Squibb, Eisai, EMD Serono, Exelixis, GlaxoSmithKline, IQVA, Infiniti, Ipsen, Kanaph, Lilly, Merck, Nikang, Novartis, Pfizer, Roche, Sanofi/Aventis, Takeda, Tempest, Up-To-Date, and for CME events (Peerview, PER, MJH Life Sciences, Research to Practice, France Foundation, Springer, WebMed, ASiM Ce, Caribou Publishing); participation on a Data Safety Monitoring Board or Advisory Board for Aravive; leadership or fiduciary roles for KidneyCAN, ASCO, ESMO, NCCN, and the GU Steering Committee of the NCI; stock or stock options in Pionyr, Tempest, Precede Bio, Osel, Curesponse, Immdura, and Primium; and other support from the Dana-Farber/Harvard Cancer Center Kidney SPORE (2P50CA101942–16) and Program 5P30CA006516–56, the Kohlberg Chair at Harvard Medical School and the Trust Family, Michael Brigham, Pan-Mass Challenge, Hinda and Arthur Marcus Fund and Loker Pinard Funds for Kidney Cancer Research at DFCI.
12. Ezra E. W. Cohen reports honoraria for advisory boards from MSD.
13. Luis A. Diaz Jr is a member of the board of directors of Quest Diagnostics and Epitope. He is a compensated consultant to PetDx, Innovatus CP, Se’er, Delfi, Blackstone and Neophore. LD is an inventor of multiple licensed patents related to technology for circulating tumor DNA analyses and mismatch repair deficiency for diagnosis and therapy. Some of these licenses and relationships are associated with equity or royalty payments to the inventors. He holds equity in Quest Diagnostics, Epitope, PetDx, Se’er, Delfi and Neophore. He divested his equity in Personal Genome Diagnostics to LabCorp in February 2022 and divested his equity in Thrive Earlier Detection to Exact Biosciences in January 2021. His spouse holds equity in Amgen. The terms of all these arrangements are being managed by Memorial Sloan Kettering in accordance with their conflict-of-interest policy.
14. Kohei Shitara reports grants or contracts from Astellas Pharma, Ono Pharmaceutical, Daiichi Sankyo, Taiho Pharmaceutical, Chugai Pharma, MSD, Amgen, Eisai, PRA Health Sciences, and Syneos Health; consulting fees from Bristol-Myers Squibb, Takeda Pharmaceuticals, Ono Pharmaceutical, Novartis, Daiichi Sankyo, Amgen, Boehringer Ingelheim, MSD, Astellas, Guardant Health Japan, Janssen, AstraZeneca, Zymeworks Biopharmaceuticals Inc, ALX Oncology Inc., and Bayer; and payments or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Bristol-Myers Squibb, Ono Pharmaceutical, Janssen, Eli Lilly, Astellas, and AstraZeneca.
15. Girish Kulkarni reports grants or contracts from Janssen; consulting fees from Tolmar, Janssen, Merck, Pfizer, Astellas, Knight Therapeutics, Astra Zeneca, EMD Serono, BMS, Novartis, Pfizer, and Ferring; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from BMS, Photocure, EMD Serono, Theralase, Novartis, Pfizer, and Verity; and a leadership or fiduciary role with Bladder Cancer Canada.
16. David McDermott reports honoraria from BMS, Pfizer, Merck, Eisai Inc., Xilio, Aveo, Genentech, Cullinan, and Exelixis and research support from BMS, Merck, Genentech, Pfizer, Exelixis, X4 Pharma, and Alkermes Inc.
17. Manish Shah reports grants or contracts from Merck.
18. Josep Tabernero reports consulting fees from Array Biopharma, AstraZeneca, Bayer, Boehringer Ingelheim, Cardiff Oncology, Chugai, Daiichi Sankyo, F. Hoffmann-La Roche Ltd, Genentech Inc, HalioDX SAS, Hutchison Medi Pharma International, Ikena Oncology, Inspirna Inc, IQVIA, Lilly, Menarini, Merck Serono, Merus, MSD, Mirati, NeoPhore, Novartis, Ona Therapeutics, Orion Biotechnology, Peptomyc, Pfizer, Pierre Fabre, Samsung Bioepis, Sanofi, Scandion Oncology, Scorpion Therapeutics, Seattle Genetics, Servier, SOTIO Biotech, Taiho, Tessa Therapeutics, TheraMyc, and Tolremo Therapeutics; payments or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Imedex/HMP, Medscape Education, MJH Life Sciences, and PeerView Institute for Medical Education and Physicians Education Resource (PER); and stock or stock options in ONIRIA Therapeutics.
19. Arndt Vogel reports consulting fees from AstraZeneca, Amgen, BeiGene, Boehringer Mannheim, BMS, BTG, Daichi Sankyo, EISAI, Incyte, Ipsen, MSD, Pierre Fabre, Roche, Servier, Sirtex, Tahio, and Terumo; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AstraZeneca, Amgen, BeiGene, Boehringer Mannheim, BMS, BTG, Daichi Sankyo, EISAI, GSK, Imaging Equipment Ltd (AAA), Incyte, Ipsen, Jiangsu Hengrui Medicines MSD, Pierre Fabre, Roche, Servier, Sirtex, Tahio, and Terumo; participation on a Data Safety Monitoring Board or Advisory Board for AstraZeneca, Amgen, BeiGene, Boehringer Mannheim, BMS, BTG, Daichi Sankyo, EISAI, Incyte, Ipsen, MSD, Pierre Fabre, Roche, Servier, Sirtex, Tahio, and Terumo.
20. Pier Luigi Zinzani reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Takeda, BMS, MSD, Kyowa Kirin, Roche, Janssen, AstraZeneca, and Sobi.
21. Niusha Jafari reports employment at Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
22. Steven Bird, Ellen Snyder, Christine Gause, Oswaldo L. Bracco, M. Catherine Pietanza, and Todd Gruber report employment at Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and stock ownership in Merck & Co., Inc., Rahway, NJ, USA.
23. Antoni Ribas reports support for the present manuscript from Merck; participation on a Data Safety Monitoring Board or Advisory Board for Amgen, Bristol Myers Squibb, Merck, and Novartis; and stock or stock options in Appia, Apricity, Arcus, Compugen, Highlight, ImaginAb, ImmPACT, Inspirna, Larkspur, Lutris, MapKure, Merus, Pluto, Synthekine, Tango, and Gilead.
Footnotes
CRediT authorship contribution statement
Julie R. Brahmer: Conceptualization, Writing – Review and Editing. Georgina V. Long: Data curation, Investigation, Methodology, Resources, Writing – Review and Editing. Omid Hamid: Conceptualization, Methodology, Investigation, Formal Analysis, Writing – Original Draft, Writing – Review and Editing. Edward B. Garon: Investigation, Writing – Review and Editing. Roy S. Herbst: Conceptualization, Data Curation, Formal Analysis, Investigation, Methodology, Supervision, Validation, Writing – Original Draft, Writing – Review and Editing. Thierry Andre: Writing – Review and Editing. Philippe Armand: Investigation, Writing – Review and Editing. Dean Bajorin: Writing – Review and Editing. Joaquim Bellmunt: Investigation, Methodology, Resources, Visualization, Writing – Review and Editing. Barbara Burtness: Data Curation, Writing – Review and Editing. Toni K. Choueiri: Writing – Review and Editing. Ezra E. W. Cohen: Investigation, Writing – Review and Editing. Luis A. Diaz Jr: Conceptualization, Formal Analysis, Investigation, Supervision, Writing – Original Draft, Writing – Review and Editing. Kohei Shitara: Investigation, Writing – Review and Editing. Girish Kulkarni: Writing – Review and Editing. David McDermott: Conceptualization, Investigation, Supervision, Writing – Review and Editing. Manish Shah: Writing – Review and Editing. Josep Tabernero: Conceptualization, Supervision, Validation, Writing – Review and Editing. Arndt Vogel: Data Curation, Investigation, Writing – Original Draft, Writing – Review and Editing. Pier Luigi Zinzani: Data Curation, Writing – Review and Editing. Niusha Jafari: Data Curation, Formal Analysis, Validation. Steven Bird: Writing – Review and Editing. Ellen Snyder: Conceptualization, Formal Analysis, Investigation, Methodology, Supervision, Visualization, Writing – Review and Editing. Christine Gause: Conceptualization, Data Curation, Formal Analysis, Methodology, Resources, Supervision, Writing – Review and Editing. Oswaldo L. Bracco: Conceptualization, Data Curation, Formal Analysis, Methodology, Supervision, Writing – Original Draft, Writing – Review and Editing. M. Catherine Pietanza: Formal Analysis, Investigation, Writing – Original Draft, Writing – Review and Editing. Todd Gruber: Conceptualization, Data Curation. Antoni Ribas: Conceptualization, Formal Analysis, Investigation, Supervision, Writing – Original Draft, Writing – Review and Editing.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.ejca.2024.113530.
Data Sharing Statement
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD) is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the US and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
References
- [1].KEYTRUDA® (pembrolizumab) injection, for intravenous use. 04/2023. Merck & Co., Inc., Rahway, NJ, USA. [Google Scholar]
- [2].KEYTRUDA 25 mg/mL concentrate for solution for infusio (summary of product characteristics). 2023, Merck Sharp & Dohme B.V., Haarlem, Netherlands. [Google Scholar]
- [3].Ghisoni E, et al. Late-onset and long-lasting immune-related adverse events from immune checkpoint-inhibitors: an overlooked aspect in immunotherapy. Eur J Cancer 2021;149:153–64. 10.1016/j.ejca.2021.03.010. [DOI] [PubMed] [Google Scholar]
- [4].Wang PF, et al. Immune-related adverse events associated with anti-PD-1/PD-L1 treatment for malignancies: a meta-analysis. Front Pharm 2017;8:730. 10.3389/fphar.2017.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Robert C, et al. Long-term safety of pembrolizumab monotherapy and relationship with clinical outcome: a landmark analysis in patients with advanced melanoma. Eur J Cancer 2021;144:182–91. 10.1016/j.ejca.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Haanen J, et al. Management of toxicities from immunotherapy: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol 2022;33(12): 1217–38. 10.1016/j.annonc.2022.10.001. [DOI] [PubMed] [Google Scholar]
- [7].Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol 2021;39(36):4073–126. 10.1200/JCO.21.01440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare the following financial interests/personal relationships that may be considered as potential competing interests:
1. Julie R. Brahmer reports grants or contracts from AstraZeneca and Bristol Myers Squibb; consulting fees from AstraZeneca, BMS, Incyte, Genentech/Roche, Regeneron, MSD, Johnson & Johnson, Janssen, and Sanofi; participation on a Data Safety Monitoring Board or Advisory Board for Johnson & Johnson/Janssen and GlaxoSmithKline; a leadership or fiduciary role at the Society for Immunotherapy of Cancer; and receipt of drugs from Bristol Myers Squibb and Syndax.
2. Georgina V. Long reports consulting fees from Agenus Inc, Amgen Inc, Array Biopharma, Boehringer Ingelheim International GmbH, Bristol Myers Squibb, Evaxion Biotech A/S, Hexal AG (Sandoz Company), Highlight Therapeutics S.L., Innovent Biologics USA Inc., MSD, Novartis Pharma AG, OncoSec Medical Australia, Pierre Fabre, Provectus Australia, QBiotics Group Limited, and Regeneron Pharmaceuticals; payment or honoraria for lectures from Bristol Myers Squibb and Pierre Fabre; and participation on a Data Safety Monitoring or Advisory Board for Agenus Inc, Amgen Inc, Array Biopharma, Boehringer Ingelheim International GmbH, Bristol Myers Squibb, Evaxion Biotech A/S, Hexal AG (Sandoz Company), Highlight Therapeutics S.L., Innovent Biologics USA Inc., Merck Sharp & Dohme (Australia) Pty Limited, Novartis Pharma AG, OncoSec Medical Australia, Pierre Fabre, Provectus Australia, QBiotics Group Limited, and Regeneron Pharmaceuticals.
3. Omid Hamid reports support for the present manuscript from Merck; consulting fees from Alkermes, Amgen, Bactonix, BeiGene, BioAtla, Bristol-Myers Squibb, Eisai, Roche Genentech, Georgiamune, GigaGen, Grit Bio, GlaxoSmithKline, Idera, Immunocore, Incyte, Instil Bio, Iovance, Janssen, KSQ, Merck, Moderna, Novartis, Obsidian, Pfizer, Regeneron, Sanofi, Seattle Genetics, Tempus, Vial Health, and Zelluna; payment or honoraria for speakers bureaus from Bristol-Myers Squibb, Immunocore, Novartis, Pfizer, and Regeneron; support for attending meetings and/or travel from Alkermes, Amgen, Bactonix, BeiGene, BioAtla, Bristol-Myers Squibb, Eisai, Roche Genentech, Georgiamune, GigaGen, Grit Bio, GlaxoSmithKline, Idera, Immunocore, Incyte, Instil Bio, Iovance, Janssen, KSQ, Merck, Moderna, Novartis, Obsidian, Pfizer, Regeneron, Sanofi, Seattle Genetics, Tempus, Vial Health, and Zelluna; participation on an Advisory Board for Alkermes, Amgen, Bactonix, BeiGene, BioAtla, Bristol-Myers Squibb, Eisai, Roche Genentech, Georgiamune, GigaGen, Grit Bio, GlaxoSmithKline, Idera, Immunocore, Incyte, Instil Bio, Iovance, Janssen, KSQ, Merck, Moderna, Novartis, Obsidian, Pfizer, Regeneron, Sanofi, Seattle Genetics, Tempus, Vial Health, and Zelluna; stock or stock options in Bactonix; and institutional research funding from Arcus, Aduro, Akeso, Amgen, BioAtla, Bristol-Myers Squibb, CytomX Therapeutics, Exelixis, Roche Genentech, GlaxoSmithKline, Immunocore, Idera, Incyte, Iovance, Merck, Merck Serono, Moderna, NextCure, Novartis, Pfizer, Regeneron, Seattle Genetics, Torque, and Zelluna.
4. Edward B. Garon reports advisory and/or consultancy roles for AbbVie, ABL-Bio, Arcus, AstraZeneca, Atreca, Boehringer-Ingelheim, BridgeBio, Bristol Myers Squibb, EMD Serono, Eisai, Eli Lilly, Gilead, GlaxoSmithKline, Merck, Merus, Novartis, Nuvalent, Personalis, Regeneron, Sanofi, Seagan, Sensei, Sumitomo, Summit, Synthekine, Xilio, and Zymeworks; reports grants/research support from ABL-Bio, ArriVent, AstraZeneca, Bristol Myers Squibb, Daiichi Sanko, Dynavax Technologies, Eli Lilly, EMD Serono, Genentech, Gilead, Iovance Biotherapeutics, Merck, Mirati Therapeutics, Neon, Novartis, and Synthekine; sponsored Independent Medical Education for Daiichi-Sankyo and Ipsen; and support for travel from A2 Bio and Novartis.
5. Roy S. Herbst reports support for the present manuscript from Merck; consulting fees from Immunocore, AstraZeneca, Bristol Myers Squibb, DynamiCure Biotechnology, eFFECTOR Therapeutics Inc., Eli Lilly and Company, Genentech, Gilead, HiberCell Inc., Janssen, Johnson & Johnson, Loxo Oncology, Mirati Therapeutics, NextCure, Oncocyte Corp, Oncternal Therapeutics, Pfizer, Regeneron Pharmaceuticals, Roche, Sanofi, and Seattle Genetics; support for attending meetings and/or travel from the American Association for Cancer Research, the International Association for the Study of Lung Cancer, the Society for Immunotherapy of Cancer, and the Southwest Oncology Group; participation on a Data Safety Monitoring Board or Advisory Board for Bolt Biotherapeutics, Candel Therapeutics Inc., Checkpoint Therapeutics, Cybrexa Therapeutics, EMD Serono, I-Mab Biopharma, Immune-Onc Therapeutics Inc., Immunocore, Normunity, Novartis, Ocean Biomedical Inc., Revelar Biotherapeutics Inc., Ribbon Therapeutics, and Xencor; leadership or fiduciary roles with Immunocore and Junshi Pharmaceuticals; stock or stock options in Immunocore, Bolt Biotherapeutics, Checkpoint Therapeutics, and Normunity; and research support from AstraZeneca, Eli Lilly and Company, Genentech/Roche, and Merck and Company.
6. Thierry Andre reports consulting fees from AbbVie, Aptitude health, Bristol Myers Squibb, Gritstone Oncology, GamaMabs Pharma SA, Gilead, Nordic, GlaxoSmithKline, Merck & Co. Inc., Seagen, Servier, and Takeda; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Bristol Myers Squibb, Gritstone Oncology, GlaxoSmithKline, Merck & Co. Inc., Merck Serono, Roche/Ventana, Seagen, and Servier; support for attending meetings and/or travel from Bristol Myers Squibb and Merck & Co. Inc.; participation on a Data Safety Monitoring Board for Inspirna: Phase 2 randomized controlled trial of ompenaclid in 2nd line RAS mutant metastatic colorectal cancer; and a leadership or fiduciary role with the ARCAD foundation.
7. Philippe Armand reports consulting roles for Merck, BMS, Pfizer, Affimed, Adaptive, Infinity, ADC Therapeutics, Celgene, MorphoSys, Daiichi Sankyo, Miltenyi, Tessa, Genmab, C4, Enterome, Regeneron, Epizyme, Astra Zeneca, Genentech/Roche, Xencor, Foresight, and ATB Therapeutics; research funding from Kite, Merck, BMS, Affimed, Adaptive, Tensha, Otsuka, Sigma Tau, Genentech/Roche, IGM, and Astra Zeneca; and honoraria from Merck and BMS.
8. Dean Bajorin reports grants or contracts from Merck Sharp & Dohme and Bristol Myers Squibb; consulting fees from Merck Sharp & Dohme and Bristol Myers Squibb; and participation on a Data Safety Monitoring Board or Advisory Board for Merck Sharp & Dohme.
9. Joaquim Bellmunt reports royalties or licenses from UpToDate; consulting fees from Merck, MSD-Serono, Pfizer, AstraZeneca, BMS, and Genentech; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Pfizer, MSD Serono, Genentech, and Ipsen; support for attending meetings and/or travel from Pfizer; and participation on a Data Safety Monitoring Board or Advisory Board for Merck, Pfizer, MSD Serono, Genentech, and AstraZeneca.
10. Barbara Burtness reports support for the current manuscript from Merck; grants or contracts from Merck, Merck KgA, Vaccinex, and GSK; royalties or licenses from UpToDate; and consulting fees from AbbVie, AstraZeneca, Cue BioPharma, GSK, Seagen, Nektar, Kura, IO Biotech, Vaccinex, Merck, Merck KgA, Debiopharm, Mirati, and Genentech.
11. Toni K. Choueiri reports support from Alkermes, AstraZeneca, Aravive, Aveo, Bayer, Bristol Myers Squibb, Circle Pharma, Eisai, EMD Serono, Exelixis, GlaxoSmithKline, IQVA, Infiniti, Ipsen, Kanaph, Lilly, Merck, Nikang, Novartis, Nuscan, Pfizer, Roche, Sanofi/Aventis, Surface Oncology, Takeda, Tempest, Up-To-Date, and for CME events (Peerview, PER, MJH Life Sciences, Research to Practice, France Foundation, Springer, WebMed, ASiM Ce, Caribou Publishing, and others); grants or contracts from AstraZeneca, Aveo, Arcus, Bayer, Bristol Myers Squibb, Eisai, EMD Serono, Exelixis, GlaxoSmithKline, Lilly, Merck, Nikang, Novartis, Pfizer, Roche, Sanofi/Aventis, and Takeda; consulting fees from AstraZeneca, Aravive, Aveo, Bayer, Bristol Myers Squibb, Circle Pharma, Eisai, EMD Serono, Exelixis, GlaxoSmithKline, IQVA, Infiniti, Ipsen, Kanaph, Lilly, Merck, Nikang, Novartis, Nuscan, Pfizer, Roche, Sanofi/Aventis, Surface Oncology, Takeda, Tempest, Up-To-Date, and for CME events (Peerview, PER, MJH life sciences, Research to Practice, France Foundation, Springer, WebMed, ASiM Ce, Caribou Publishing); payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AstraZeneca, Aravive, Aveo, Bayer, Bristol Myers Squibb, Eisai, EMD Serono, Exelixis, GlaxoSmithKline, IQVA, Infiniti, Ipsen, Kanaph, Lilly, Merck, Nikang, Novartis, Pfizer, Roche, Sanofi/Aventis, Takeda, Tempest, Up-To-Date, and for CME events (Peerview, PER, MJH Life Sciences, Research to Practice, France Foundation, Springer, WebMed, ASiM Ce, Caribou Publishing); participation on a Data Safety Monitoring Board or Advisory Board for Aravive; leadership or fiduciary roles for KidneyCAN, ASCO, ESMO, NCCN, and the GU Steering Committee of the NCI; stock or stock options in Pionyr, Tempest, Precede Bio, Osel, Curesponse, Immdura, and Primium; and other support from the Dana-Farber/Harvard Cancer Center Kidney SPORE (2P50CA101942–16) and Program 5P30CA006516–56, the Kohlberg Chair at Harvard Medical School and the Trust Family, Michael Brigham, Pan-Mass Challenge, Hinda and Arthur Marcus Fund and Loker Pinard Funds for Kidney Cancer Research at DFCI.
12. Ezra E. W. Cohen reports honoraria for advisory boards from MSD.
13. Luis A. Diaz Jr is a member of the board of directors of Quest Diagnostics and Epitope. He is a compensated consultant to PetDx, Innovatus CP, Se’er, Delfi, Blackstone and Neophore. LD is an inventor of multiple licensed patents related to technology for circulating tumor DNA analyses and mismatch repair deficiency for diagnosis and therapy. Some of these licenses and relationships are associated with equity or royalty payments to the inventors. He holds equity in Quest Diagnostics, Epitope, PetDx, Se’er, Delfi and Neophore. He divested his equity in Personal Genome Diagnostics to LabCorp in February 2022 and divested his equity in Thrive Earlier Detection to Exact Biosciences in January 2021. His spouse holds equity in Amgen. The terms of all these arrangements are being managed by Memorial Sloan Kettering in accordance with their conflict-of-interest policy.
14. Kohei Shitara reports grants or contracts from Astellas Pharma, Ono Pharmaceutical, Daiichi Sankyo, Taiho Pharmaceutical, Chugai Pharma, MSD, Amgen, Eisai, PRA Health Sciences, and Syneos Health; consulting fees from Bristol-Myers Squibb, Takeda Pharmaceuticals, Ono Pharmaceutical, Novartis, Daiichi Sankyo, Amgen, Boehringer Ingelheim, MSD, Astellas, Guardant Health Japan, Janssen, AstraZeneca, Zymeworks Biopharmaceuticals Inc, ALX Oncology Inc., and Bayer; and payments or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Bristol-Myers Squibb, Ono Pharmaceutical, Janssen, Eli Lilly, Astellas, and AstraZeneca.
15. Girish Kulkarni reports grants or contracts from Janssen; consulting fees from Tolmar, Janssen, Merck, Pfizer, Astellas, Knight Therapeutics, Astra Zeneca, EMD Serono, BMS, Novartis, Pfizer, and Ferring; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from BMS, Photocure, EMD Serono, Theralase, Novartis, Pfizer, and Verity; and a leadership or fiduciary role with Bladder Cancer Canada.
16. David McDermott reports honoraria from BMS, Pfizer, Merck, Eisai Inc., Xilio, Aveo, Genentech, Cullinan, and Exelixis and research support from BMS, Merck, Genentech, Pfizer, Exelixis, X4 Pharma, and Alkermes Inc.
17. Manish Shah reports grants or contracts from Merck.
18. Josep Tabernero reports consulting fees from Array Biopharma, AstraZeneca, Bayer, Boehringer Ingelheim, Cardiff Oncology, Chugai, Daiichi Sankyo, F. Hoffmann-La Roche Ltd, Genentech Inc, HalioDX SAS, Hutchison Medi Pharma International, Ikena Oncology, Inspirna Inc, IQVIA, Lilly, Menarini, Merck Serono, Merus, MSD, Mirati, NeoPhore, Novartis, Ona Therapeutics, Orion Biotechnology, Peptomyc, Pfizer, Pierre Fabre, Samsung Bioepis, Sanofi, Scandion Oncology, Scorpion Therapeutics, Seattle Genetics, Servier, SOTIO Biotech, Taiho, Tessa Therapeutics, TheraMyc, and Tolremo Therapeutics; payments or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Imedex/HMP, Medscape Education, MJH Life Sciences, and PeerView Institute for Medical Education and Physicians Education Resource (PER); and stock or stock options in ONIRIA Therapeutics.
19. Arndt Vogel reports consulting fees from AstraZeneca, Amgen, BeiGene, Boehringer Mannheim, BMS, BTG, Daichi Sankyo, EISAI, Incyte, Ipsen, MSD, Pierre Fabre, Roche, Servier, Sirtex, Tahio, and Terumo; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AstraZeneca, Amgen, BeiGene, Boehringer Mannheim, BMS, BTG, Daichi Sankyo, EISAI, GSK, Imaging Equipment Ltd (AAA), Incyte, Ipsen, Jiangsu Hengrui Medicines MSD, Pierre Fabre, Roche, Servier, Sirtex, Tahio, and Terumo; participation on a Data Safety Monitoring Board or Advisory Board for AstraZeneca, Amgen, BeiGene, Boehringer Mannheim, BMS, BTG, Daichi Sankyo, EISAI, Incyte, Ipsen, MSD, Pierre Fabre, Roche, Servier, Sirtex, Tahio, and Terumo.
20. Pier Luigi Zinzani reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Takeda, BMS, MSD, Kyowa Kirin, Roche, Janssen, AstraZeneca, and Sobi.
21. Niusha Jafari reports employment at Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
22. Steven Bird, Ellen Snyder, Christine Gause, Oswaldo L. Bracco, M. Catherine Pietanza, and Todd Gruber report employment at Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and stock ownership in Merck & Co., Inc., Rahway, NJ, USA.
23. Antoni Ribas reports support for the present manuscript from Merck; participation on a Data Safety Monitoring Board or Advisory Board for Amgen, Bristol Myers Squibb, Merck, and Novartis; and stock or stock options in Appia, Apricity, Arcus, Compugen, Highlight, ImaginAb, ImmPACT, Inspirna, Larkspur, Lutris, MapKure, Merus, Pluto, Synthekine, Tango, and Gilead.
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD) is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the US and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.