Abstract
Background
Local anesthetic transperineal prostate biopsy (LATP) is a widely used diagnostic procedure for prostate cancer. As a diagnostic procedure, it should carry minimal risk. However, morbidity resulting from prostate biopsy is frequent. Prostate biopsy, like any other intervention, carries a significant risk of various infections, ranging from urinary tract infections (UTIs) to potentially life-threatening conditions like sepsis.
Aim
This study examined the rate of infections following a prostate biopsy at a single center and sought to identify risk factors that could increase the likelihood of developing an infection.
Methods
A retrospective review was conducted on all 168 patients who underwent LATP biopsy between 01/04/2022 and 01/04/2023. Data were collected from the Clinical Record and Reporting System (CRRS). Patient characteristics, including age, prostate-specific antigen (PSA) levels, prostate volume, the main indication for the biopsy, number of cores taken, antibiotic prophylaxis, and comorbidities were analyzed. The inclusion criteria encompassed all patients receiving this procedure within the specified timeframe, without restrictions on age, underlying health conditions, or medical history. No exclusion criteria were applied, aiming to comprehensively analyze and capture the full spectrum of patient outcomes and characteristics associated with these biopsies during the study period.
Results
In terms of socio-demographics, all patients were male with an average age (mean) of 65.5 years, a mean PSA level of 13.9 ng/dL, and an average prostate volume of 66.1 mL. On average, 23.2 biopsy cores were taken. All patients received antibiotic prophylaxis, mainly ciprofloxacin. Despite this, 1.78% of patients (n=3) developed post-biopsy infections. Two of these patients had diabetes mellitus, and two had a large prostate volume of 95 mL.
Keywords: post-latp infection, transrectal prostate biopsy, risk factors of infection, antibiotic prophylaxis, prostate biopsy, transperineal prostate biopsy, latp
Introduction
Prostate cancer is one of the most common malignancies affecting men worldwide, ranking second in diagnosis frequency and fifth in global mortality. Timely detection and accurate diagnosis are imperative for optimizing treatment and enhancing patient outcomes [1].
Transrectal (TR) prostate biopsy remains the most commonly used diagnostic tool for prostate cancer in most regions worldwide [2]. It has an infection risk ranging from 5% to 7% [3].
Local anesthetic transperineal prostate biopsy (LATP) is a widely accepted and well-tolerated procedure for diagnosing prostate cancer. It offers several advantages over traditional TR biopsy, including reduced infection and sepsis risk, improved sampling of the apical and anterior prostate regions, and potentially higher cancer detection rates [4-6].
Among men under active surveillance for low-risk prostate cancer, LATP is more adept at identifying clinically significant cases, likely due to improved sampling of the anterior prostate region [7].
A systematic review found that using antibiotic prophylaxis for LATP did not significantly reduce the rates of infection, fever, sepsis, or hospital readmission compared to cases without antibiotic prophylaxis [8]. However, despite its benefits, LATP is an invasive procedure that inevitably carries some risk of post-biopsy infection which can range from uncomplicated urinary tract infections (UTIs) to severe sepsis, potentially leading to hospitalizations and increased healthcare costs [9].
Recognizing risk factors for post-biopsy infections is vital for prevention and enhancing patient care. This study evaluated the post-biopsy infection rate following LATP at a single center and sought to identify potential risk factors contributing to infection.
Materials and methods
This was a retrospective, single-center study conducted at the University Hospitals of Coventry and Warwickshire in the United Kingdom. Data were collected from the Clinical Record and Reporting System (CRRS) for all patients who underwent LATP biopsy from 01/04/2022 to 01/04/2023. A total of 168 patients were included in the study.
The collected data included patient characteristics like age, prostate-specific antigen (PSA) levels, prostate volume, main indication for the biopsy, number of biopsy cores, antibiotic prophylaxis use, and co-morbidities linked to immunosuppression or increased infection risk (e.g., diabetes mellitus, hematologic disorders, and self-catheterization). Microsoft Excel was used to calculate the average (mean), standard deviations (SD), and any other statistical analyses carried out.
Results
The study included 168 patients who underwent LATP biopsy. The average (mean) age of the patients was 65.5 years (SD 8.E+00). The mean PSA level was 13.9 ng/dL (SD 3.E+01), and the average prostate volume was 66.1 mL (3.E+01). The average number of core biopsies taken was 23.2 (4.E+00). All patients received antibiotic prophylaxis, with ciprofloxacin being the most used (167 patients), followed by gentamicin (one patient only).
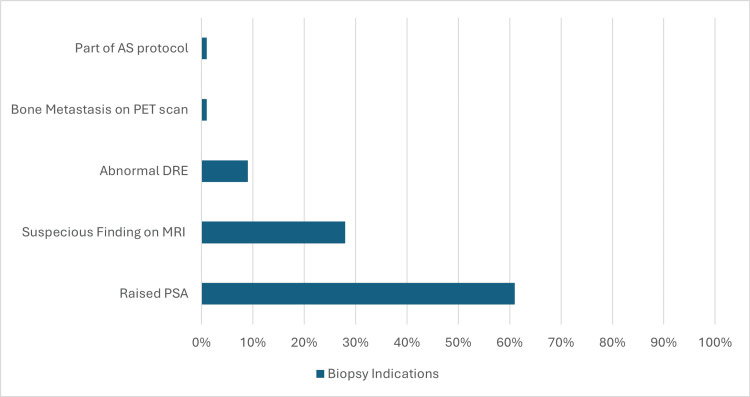
The main indications for biopsy were raised PSA (103 patients), suspicious findings on MRI (48 patients), abnormal digital rectal examination (DRE) (15 patients), and bone metastasis on positron emission tomography (PET) scan (one patient), as part of active surveillance protocol (one patient) (Figure 1).
Figure 1. Main indications for biopsy.
AS: active surveillance, PET: positron emission tomography, DRE: digital rectal examination, MRI: magnetic resonance imaging, PSA: prostatic specific antigen
A subset of patients had comorbidities linked to immunosuppression or increased infection risk: 11 patients had diabetes mellitus, two had hematological disorders, and one was on intermittent self-catheterization (ISC). None had documented UTI before the procedure. Out of 168 patients, three (1.78%) developed post-biopsy infections. Two of these patients had diabetes mellitus, and two had a large prostate volume of 95 mL. None required ICU admission or additional procedures. One patient required four days of hospitalization (Table 1).
Table 1. Details of the patients with post-biopsy infections.
| Case | Date of Biopsy | Date of Infection | Urine Culture | Blood Culture | Hospitalization | Treatment | Additional Procedure | Risk Factors | Antibiotic Prophylaxis | Number of Cores |
| 1 | 20/2/23 | 21/2/23 | Negative | Not taken | 4 days | 10 days of co-amoxiclav | No | Large prostate volume (95 mL) | Ciprofloxacin | 18 |
| 2 | 13/2/23 | 30/2/23 | Negative | Not taken | Not required | Ofloxacin for 14 days | No | Diabetes mellitus, large prostate volume (95 mL) | Ciprofloxacin | 18 |
| 3 | 4/4/23 | 14/4/23 | Not taken | Not taken | No required | Nitrofurantoin for 7 days | No | Diabetes mellitus, moderately enlarged prostate (50 mL) | Ciprofloxacin | 24 |
Discussion
Prostate biopsy is a crucial procedure for acquiring tissue samples for histopathological assessment. Historically, clinicians have relied on PSA levels and DRE findings to determine the necessity of performing a prostate biopsy [10,11].
The preferred method for obtaining a prostate tissue sample has traditionally been the TR approach under ultrasound guidance. Initially, the sextant technique was regarded as the gold standard [12]. This approach systematically but randomly sampled six cores from the prostate. However, the literature highlights a high false-negative rate for this procedure, reaching up to 30% [13]. The use of quinolone-class antibiotics, typically ciprofloxacin, was recommended as prophylaxis [14]. Quinolone-class antibiotics, such as ciprofloxacin, were recommended due to their strong bactericidal activity against gram-negative bacteria (e.g., Escherichia coli), which are a heightened risk with TR biopsy, and their excellent penetration into prostate tissue. Additionally, there was a weak recommendation for a self-administered cleansing enema on the morning of the procedure [15].
Efforts to address issues like false-negative biopsies, over-sampling, and under-sampling have led to the adoption of MRI-guided biopsies. However, the TR approach is inadequate for accessing the apical and anterior regions of the prostate. Consequently, the transperineal (TP) biopsy has emerged as an alternative to overcome the limitations of the TR biopsy [16].
Though numerous studies investigate infection risk factors in TR prostate biopsy, research on risk factors of infection in LATP is sparse, likely due to procedural similarities. However, further dedicated studies are warranted to comprehensively explore this specific area.
The first step for prevention is a preoperative assessment of risk factors which includes diabetes, significant comorbidities, immunosuppression, urinary tract infections, prostatitis, the higher cumulative number of biopsies, large prostate volume, recent antibiotic use, recent international travel to resistant-endemic areas, antibiotic use for traveler’s diarrhea prevention, healthcare worker status, and colonization with resistant bacteria like fluoroquinolone-resistant E. coli [3].
Proper infection control measures, including equipment sterilization, using sterile ultrasound gel, avoiding contamination of tissue samples, and correct ultrasound transducer processing, are essential to prevent infectious complications after prostate biopsy [3].
Men with lower urinary tract symptoms should have a urine culture done, any infections treated, and a repeat culture performed before undergoing a prostate biopsy [3].
Interestingly, two out of the three patients who developed post-biopsy infections had diabetes mellitus. Diabetes mellitus is known to be associated with an increased risk of infections due to impaired immune function and other factors [17].
This finding suggests that patients with diabetes may be at a higher risk for post-biopsy infections following LATP and may benefit from additional preventive measures and closer monitoring.
Another notable observation was the presence of a large prostate volume in two of the cases with post-biopsy infections. It is possible that larger prostate volumes may increase the risk of complications, including infection [18].
It is important to note that all three cases of post-biopsy infection were managed with appropriate antibiotic treatment, and none of the patients required additional procedures or intensive care unit (ICU) admission.
Potential limitations of our study include its single-center design, which may limit the generalizability of the findings to other healthcare settings or populations. Additionally, the small sample size of 168 patients may restrict the statistical power to detect significant associations or risk factors. The retrospective design, relying on data from medical records, is subject to potential errors or incomplete documentation. Furthermore, the study did not account for the duration of follow-up after the biopsy procedure, which may have led to missed or undetected infections that developed later.
Conclusions
At this center, the post-biopsy infection rate was 1.78%, with diabetes mellitus and large prostate volume identified as potential risk factors. Larger studies are necessary to confirm these findings, identify additional risk factors and high-risk patient groups undergoing LATP, and establish necessary precautionary measures.
Disclosures
Human subjects: Consent was obtained or waived by all participants in this study. University Hospitals of Coventry and Warwickshire issued approval 9579.
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Baidar Khalabazyane, Christine Mizzi, Lawrence Adesunloro, Priyadarshi Kumar, Rahel Rashid, Roza Salah, Israa Kadhmawi
Acquisition, analysis, or interpretation of data: Baidar Khalabazyane, Christine Mizzi, Lawrence Adesunloro, Priyadarshi Kumar, Rahel Rashid, Roza Salah, Israa Kadhmawi
Drafting of the manuscript: Baidar Khalabazyane, Christine Mizzi, Lawrence Adesunloro, Priyadarshi Kumar, Rahel Rashid, Roza Salah, Israa Kadhmawi
Critical review of the manuscript for important intellectual content: Baidar Khalabazyane, Christine Mizzi, Lawrence Adesunloro, Priyadarshi Kumar, Rahel Rashid, Roza Salah, Israa Kadhmawi
Supervision: Priyadarshi Kumar
References
- 1.Epidemiology of prostate cancer. Rawla P. World J Oncol. 2019;10:63–89. doi: 10.14740/wjon1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antibiotic resistance, hospitalizations, and mortality related to prostate biopsy: first report from the Norwegian Patient Registry. Johansen TE, Zahl PH, Baco E, et al. World J Urol. 2020;38:17–26. doi: 10.1007/s00345-019-02837-0. [DOI] [PubMed] [Google Scholar]
- 3.An update of the American Urological Association white paper on the prevention and treatment of the more common complications related to prostate biopsy. Liss MA, Ehdaie B, Loeb S, Meng MV, Raman JD, Spears V, Stroup SP. J Urol. 2017;198:329–334. doi: 10.1016/j.juro.2017.01.103. [DOI] [PubMed] [Google Scholar]
- 4.The value of transperineal apical prostate biopsy in predicting urethral/apical margin status after radical prostatectomy. Dai J, Zhang X, Zhao J, et al. Medicine (Baltimore) 2019;98:0. doi: 10.1097/MD.0000000000017633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MP24-16 local anaesthetic transperineal prostate (LATP) biopsy using the precision point access system: a multi-centre outcome analysis. Campbell A, Omer A, Stroman L, et al. Urol J. 2019;201:0. [Google Scholar]
- 6.Transperineal biopsy of the prostate—is this the future? Chang DT, Challacombe B, Lawrentschuk N. Nat Rev Urol. 2013;10:690–702. doi: 10.1038/nrurol.2013.195. [DOI] [PubMed] [Google Scholar]
- 7.Transperineal prostate biopsy improves the detection of clinically significant prostate cancer among men on active surveillance. Meyer AR, Mamawala M, Winoker JS, et al. J Urol. 2021;205:1069–1074. doi: 10.1097/JU.0000000000001523. [DOI] [PubMed] [Google Scholar]
- 8.Infection rate after transperineal prostate biopsy with and without prophylactic antibiotics: results from a systematic review and meta-analysis of Comparative Studies. Castellani D, Pirola GM, Law YX, et al. J Urol. 2022;207:25–34. doi: 10.1097/JU.0000000000002251. [DOI] [PubMed] [Google Scholar]
- 9.Local anaesthetic transperineal (LATP) prostate biopsy using a probe-mounted transperineal access system: a multicentre prospective outcome analysis. Lopez JF, Campbell A, Omer A, et al. BJU Int. 2021;128:311–318. doi: 10.1111/bju.15337. [DOI] [PubMed] [Google Scholar]
- 10.Prostate cancer detection in a clinical urological practice by ultrasonography, digital rectal examination and prostate specific antigen. Cooner WH, Mosley BR, Rutherford Jr CL, et al. J Urol. 1990;143:1146–1152. doi: 10.1016/s0022-5347(17)40211-4. [DOI] [PubMed] [Google Scholar]
- 11.Prostate biopsy: indications and technique. Matlaga BR, Eskew LA, McCullough DL. J Urol. 2003;169:12–19. doi: 10.1016/S0022-5347(05)64024-4. [DOI] [PubMed] [Google Scholar]
- 12.Random systematic versus directed ultrasound guided transrectal core biopsies of the prostate. Hodge KK, McNeal JE, Terris MK, Stamey TA. J Urol. 1989;142:71–74. doi: 10.1016/s0022-5347(17)38664-0. [DOI] [PubMed] [Google Scholar]
- 13.Does transrectal ultrasound probe configuration really matter? End fire versus side fire probe prostate cancer detection rates. Ching CB, Moussa AS, Li J, Lane BR, Zippe C, Jones JS. J Urol. 2009;181:2077–2083. doi: 10.1016/j.juro.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 14.Best practice policy statement on urologic surgery antimicrobial prophylaxis. Wolf JS Jr, Bennett CJ, Dmochowski RR, Hollenbeck BK, Pearle MS, Schaeffer AJ. J Urol. 2008;179:1379–1390. doi: 10.1016/j.juro.2008.01.068. [DOI] [PubMed] [Google Scholar]
- 15.Complications of transrectal ultrasound-guided prostate biopsy: impact of prebiopsy enema. Kam SC, Choi SM, Yoon S, et al. Korean J Urol. 2014;55:732–736. doi: 10.4111/kju.2014.55.11.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shifting to transperineal prostate biopsy: a narrative review. Chung Y, Hong SK. Prostate Int. 2024;12:10–14. doi: 10.1016/j.prnil.2023.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Risk of infection in type 1 and type 2 diabetes compared with the general population: a matched cohort study. Carey IM, Critchley JA, DeWilde S, Harris T, Hosking FJ, Cook DG. Diabetes Care. 2018;41:513–521. doi: 10.2337/dc17-2131. [DOI] [PubMed] [Google Scholar]
- 18.Larger prostate causes higher frequency of infectious complications in prostate biopsy. Shigemura K, Arakawa S, Nakano Y, Hara I, Tanaka K, Fujisawa M. Urol Int. 2006;76:321–326. doi: 10.1159/000092055. [DOI] [PubMed] [Google Scholar]