Abstract
Background
Subjective cognitive decline (SCD), i.e. self/other-reported concerns on one’s cognitive functioning without objective evidence of significant decline, is an indicator of dementia risk. There is little consensus on reliability and validity of the available SCD measures. Therefore, introducing a novel and psychometrically sound measure of SCD is timely.
Objective
The psychometric properties of a new SCD measure, the McCusker Subjective Cognitive Impairment Inventory–Self-Report (McSCI-S), are reported.
Methods
Through review of previously published measures as well as our clinical and research data on people with SCD, we developed a 46-item self-report questionnaire to assess concerns on six cognitive domains, namely, memory, language, orientation, attention and concentration, visuoconstruction abilities and executive function. The McSCI-S was examined in a cohort of 526 participants using factor analysis, item response theory analysis and receiver operating characteristic (ROC) curve.
Results
A unidimensional model provided acceptable fit (CFI = 0.94, TLI = 0.94, RMSEA [90% CI] = 0.052 [.049, 0.055], WRMR = 1.45). The McSCI-S internal consistency was excellent (.96). A cut-off score of ≥24 is proposed to identify participants with SCDs. Higher McSCI-S scores were associated with poorer general cognition, episodic verbal memory, executive function and greater memory complaints and depressive scores (P < .001), controlling for age, sex and education.
Conclusions
Excellent reliability and construct validity suggest the McSCI-S estimates SCDs with acceptable accuracy while capturing self-reported concerns for various cognitive domains. The psychometric analysis indicated that this measure can be used in cohort studies as well as on individual, clinical settings to assess SCDs.
Keywords: subjective cognitive decline, dementia, The McCusker Subjective Cognitive Impairment Inventory, cognition, McSCI-S, perceived cognitive decline, older people
Key Points
We address the urgent need for psychometrically sound measures of subjective cognitive decline (SCD) by proposing a novel measure.
The McCusker Subjective Cognitive Impairment Inventory–Self-Report (McSCI-S) measures cognitive concerns with excellent reliability and validity.
The McSCI-S can identify individuals with above average levels of SCD at 99.9% accuracy.
This SCD measure can reliably be used in research and clinical settings for clinical decision-making at individual and group levels.
Introduction
Subjective cognitive decline (SCD) refers to self or informant-reported cognitive decline relative to previous abilities that were perceived as normal [1]. Subjective memory complaint (SMC), a more specific component of SCD, includes self or others’ reported concerns about one’s memory abilities. SCD/SMC, in the absence of concurrent cognitive impairment, has been linked to future cognitive decline [1–3] and Alzheimer’s disease (AD) [4].
Available measures of SCD/SMC have major limitations, including small sample sizes used in the test validation phase, less than optimal psychometric properties, lack of a research-informed cut-off score(s) and most importantly, limited content validity as represented by the number of cognitive domains sampled. The existing SCD measures also have low sensitivity in detecting subtle subjective changes at the individual, compared to the cohort level [5], thus limiting their application in clinical settings. For instance, in individual-level decision-making about a patient, a test should have a reliability of greater than 0.95 [6]; a condition that many of the existing SCD measures do not meet [7–10]. Further, a review of 34 SCD measures used in 19 studies, noted that 61.8% (n = 21) of these measures had items that were not conceptually related to specific cognitive functions (e.g. items assessing emotional and psychological conditions or physical and motor functioning) [11].
The McCusker Subjective Cognitive Impairment Inventory (McSCI; pronounced: Mak·see) is developed with the goal of providing a clinically informed and psychometrically sound measure of SCD. Here, we report on the psychometric properties of the McSCI–Self-Report (McSCI-S), including its factor structure, reliability and construct validity.
Methods
Participants were recruited from several ongoing studies in Western Australia (WA) and New South Wales (NSW), Australia. These studies included (i) the Western Australia Memory Study (WAMS), a longitudinal study of the neuropsychological and biological markers of ageing among community-dwelling individuals aged 30+ without a history of psychiatric or neurological conditions [12, 13]; (ii) The Kerr Anglican Retirement Village Initiative in Ageing Health (KARVIAH) Study (NSW), a completed clinical trial that examined the efficacy of curcumin to prevent future risk of dementia in retirement village residents aged 65–90 (see [14]); (iii) The Australian Imaging, Biomarkers and Lifestyle (AIBL) Study of Ageing, an ongoing longitudinal observational study recruiting participants aged 60+ with preclinical, prodromal and clinical stages of AD [15]; (iv) The 56-week, Double-Blind, Randomised Study to Evaluate the Efficacy of Testosterone, With and Without DHA Supplementation on Cerebral Amyloid Load in Known Brain Amyloid-PET Positive Men with Subjective Memory Complaints [TotAL Study; (ACTRN12618000761268)]; and (v) The Dominantly Inherited Alzheimer Network (DIAN), an observational study of individuals aged 18 years and over from a family with a known mutation for AD [16].
Interested participants were provided with a unified, study-specific information and consent pack to sign prior to enrolling into the McSCI Study. This study was approved by the Ramsay Health Care WA/SA (Western Australia and South Australia) Human Research Ethics Committee.
Across all studies, participants were eligible for inclusion into the McSCI Study if they were living independently; were able to read and write fluent English and provide consent; had no major medical, psychiatric or neurological condition (e.g. dementia) affecting their cognitive abilities; had normal hearing and vision with or without correction, as per the parent studies’ inclusion/exclusion criteria; and were 30 years old or over at the time of recruitment.
Measures
The measures used in this study included the following.
The McCusker Subjective Cognitive Impairment Inventory–Self-Report1
In January 2013, we conducted a general PubMed search with key words including ‘subjective cognitive complaints,’ ‘subjective cognitive impairment,’ ‘subjective memory complaints,’ ‘self-reported cognitive problems,’ ‘self-reported memory problems,’ and ‘dementia,’ ‘mild cognitive impairment’ or ‘MCI’ and ‘Alzheimer’s disease’ or ‘AD’. We identified 205 papers on SMC/SCD, with ~30 that have used published questionnaires (our unpublished data). We also examined literature reporting cognitive decline in preclinical AD, MCI and dementia to determine cognitive domains/functions affected that should be included in the McSCI [17].
An initial bank of 96 items was developed. Two of the authors, with the assistance of their PhD students and research assistants as well as three independent psychologists (see the acknowledgement), rated these items for assessing specific cognitive domains, item clarity, appropriateness and face validity. Forty-six items were selected representing six cognitive domains, namely, language skills (LS, 6 items), orientation (O, 6 items), attention and concentration (AC, 6 items), visuoconstruction abilities (VC, 6 items), executive function (EF, 9 items) and memory (MA, 13 items) (see Table 1 for the McSCI-S Questionnaire, and Appendices for cognitive domains, items allocated to each cognitive domain and descriptive details) [18]. These 46 items were then given to participants to comment on wording, and where appropriate, we have revised the items to improve their comprehensibility. The McSCI-S items are scored on a 5-point Likert scale (0–4) with responses ranging from ‘Almost always true’ (scored: 4) to ‘Almost never true’ (scored: 0). The possible total McSCI score ranges from 0 to 184, with higher scores representing more concerns.
Table 1.
The McCusker subjective cognitive impairment inventory-self report (McSCI-S)
| This questionnaire asks about gradual changes in memory, language, concentration, and other mental abilities that you might have experienced or noticed during the last two years, as compared to five years ago. Please read each statement carefully and choose the answer (by a tick ✔or cross ✗) that describes your current mental abilities, irrespective of your physical health (e.g. arthritis, hearing or eyesight problems). For those phrases that you cannot choose an answer, please make your best guess of how good you will perform in such a given condition. | ||||||
|---|---|---|---|---|---|---|
| In the last two years | Almost Always True | Usually True | Occasionally True | Usually Not True | Almost Never True | |
| 1 | I have noticed more difficulties with my language and speech abilities. | |||||
| 2 | I have significant problems with my memory abilities. | |||||
| 3 | I have more difficulty concentrating on different tasks. | |||||
| 4 | I have more difficulty solving everyday problems that come up around the house. | |||||
| 5 | I cannot remember where I have put things. | |||||
| 6 | I lose track of the date, more often. | |||||
| 7 | I may forget the name of the person I am talking to even when I know them. | |||||
| 8 | It is difficult for me to repeat back something that I have just heard (e.g. phone number, address etc.). | |||||
| 9 | I forget details of an event that happened a couple of weeks ago. | |||||
| 10 | I am not as organised as I used to be. | |||||
| 11 | I get distracted quickly and cannot follow a conversation or a movie plot. | |||||
| 12 | I am slower at writing or typing. | |||||
| 13 | I forget things that I intend to do in the near future. | |||||
| 14 | I do things earlier or later than the time, they are expected to be done. | |||||
| 15 | I keep forgetting where I have parked my car in a carpark. | |||||
| 16 | I have trouble planning things ahead and carrying out these plans on time. | |||||
| 17 | I often forget basic things that I learned at school when I was young. | |||||
| 18 | Sometimes, I don’t understand what others say, regardless of my hearing ability. | |||||
| 19 | I am more likely to bump into objects or people. | |||||
| 20 | I have more trouble making decisions on everyday matters (e.g. which clothes to wear; which item to buy). | |||||
| 21 | Others have told me that I repeat myself (e.g. telling them the same story or asking the same questions). | |||||
| 22 | When driving/walking back to my home, I may pass my house without realising it. | |||||
| 23 | I fail to recognise well-known individuals (e.g. actor, singer, TV character) that I knew. | |||||
| 24 | It is difficult for me to manage a household emergency (e.g. a leaking tap; a spider or a small non-poisonous lizard in the bedroom etc.). | |||||
| 25 | I forget the name of everyday items (e.g. kitchen utensils; familiar household objects). | |||||
| 26 | I have to try harder to remember things that I have heard, read, or seen. | |||||
| 27 | I cannot keep my mind focused on a task even if I enjoyed it. | |||||
| 28 | I cannot do two things at once (e.g. washing dishes and talking to someone; driving and listening to the radio). | |||||
| 29 | More often, I forget what day of the week it is. | |||||
| 30 | I often forget how to use a gadget/tool that I have recently learned to use (e.g. computer; mobile/smart phones). | |||||
| 31 | I cannot find a friend’s/relatives’ address on the map. | |||||
| 32 | I can no longer play well in a game of skill (e.g. Bridge, Chess, Cards and Golf) or doing something I was good at. | |||||
| 33 | When speaking, I have difficulty finding the right words. | |||||
| 34 | My handwriting has dramatically changed. | |||||
| 35 | When I look at old photos, I cannot recognise some of the people I used to recognise. | |||||
| 36 | Nowadays, I have to read something a few times to understand it. | |||||
| 37 | I fail to pay attention to what is going on around me. | |||||
| 38 | I may not be able to accurately copy a drawing (e.g. a tree; a house, etc.). | |||||
| 39 | I can no longer do simple arithmetic calculations ‘in my head’. | |||||
| 40 | Sometimes, I get confused about how to get to a specific room (e.g. bathroom, bedroom, or lounge) within my home. | |||||
| 41 | If interrupted, I cannot pick up the conversation from where I stopped. | |||||
| 42 | When I see famous places or buildings, I may not recognise them. | |||||
| 43 | I have had difficulty giving directions to someone because I get the routes confused. | |||||
| 44 | It is difficult for me to stop something that I have just started doing. | |||||
| 45 | I forget my close relatives’ names or dates of birth (e.g. grandchildren, nieces and nephews). | |||||
| 46 | I have difficulty using things that I have previously used with confidence (e.g. electronic toothbrush; microwave; calculator). | |||||
Other measures
To examine the validity and relationship between the McSCI-S and established cognitive measures, we analysed the WAMS data collected at the same study visit. Global cognitive function was assessed using the Montreal Cognitive Assessment (MoCA) [19]. Verbal episodic memory was examined using the California Verbal Learning Test-II (CVLT-II) including total learning trials, short and long delay free recalls and recognition hits [20]. The Trail Making Test B (time: seconds) [21] was used to assess attention/processing speed and executive function [22]. The SMC was assessed using the Memory Assessment Clinic- Questionnaire (MAC-Q), a measure of perceived decline in memory that has six items with total scores ranging from 7 to 35. A cut-off score of ≥25 represents SMCs [8]. The Depression Anxiety Stress Scale-21 (DASS-21) is a 21-item self-report, and each of its item is rated on a 0–3 scale and the total score is multiplied by 2 to create a score range of 0–126 [23].
Data analysis
The psychometric properties of the McSCI-S were evaluated using item response theory (IRT) methods. This allows both the item difficulty parameters and the individual trait-level estimates to be placed on the same scale. Due to the polytomous nature of the McSCI-S items, the graded response model (GRM) introduced by Samejima [24] was used to fit the model and estimate item parameters.
Factor structure
Because the McSCI-S asks questions about concerns of decline across six different cognitive domains, we sought to determine whether the scores were better explained by a unidimensional or multidimensional factor structure. Using Mplus version 8 [25], we specified two competing models: a single-factor confirmatory factor analysis (CFA) model and a six-factor CFA model. Model fit was judged using standard fit criteria, including the comparative fit index (CFI), Tucker–Lewis index (TLI), root mean square error of approximation (RMSEA) and weighted root mean square residual (WRMR). The superiority of the multidimensional model would be indicated by an absence of estimation problems, superior model fit statistics, sufficiently dissociable factors (i.e. factor intercorrelations of < 0.9) and standardised factor loadings of sufficient magnitude (i.e. > 0.40) and sign (i.e. positive). The best model was then further evaluated using McDonald’s omega, calculated using the psych package (version 1.8.12) for R version 3.5.2 [26].
Graded response model
The unidimensional graded response IRT model was analysed using the mirt package (version 1.29) for R version 3.5.2. We estimated item discrimination (a) parameters and up to four threshold (b) parameters for each item. These parameter estimates were used to evaluate the McSCI-S scale-level psychometric properties, including total test information, standard error and reliability across the range of the underlying trait (θ).
Cut-off score calculation
We used Youden’s index [27] to identify a relatively sound cut-off point. Youden’s index has a score range of 0 to 1 and the cut-off points with higher scores are considered to perform better for screening or diagnostic purposes. Youden’s index was calculated using the following formula: Youden’s J = (sensitivity + specificity) − 1 [28, 29].
Results
The CFA results and associations between the McSCI-S and other measures are provided here. Where necessary, further information or results are provided in the Appendices, under Appendices.
Demographics
Table 2 provides demographic data for the CFA cohort (n = 526). Participants’ age ranged between 39 and 97 years (M = 71.47; SD = 7.28), and 35.4% were female. Years of education ranged from 6 to 24 (M = 13.06; SD = 3.08). In the IRT sample (n = 385), 269 participants (69.8%) and in the larger sample with full data (n = 503), 347 participants (68.9%) were memory complainers using MAC-Q cut-off ≥25.
Table 2.
Demographic data for the CFA cohort (n = 526).
| Variable | Overall | Range |
|---|---|---|
| Age, M (SD) | 71.47 (7.28) | 39–97 |
| Female sex, N (%) | 186 (35.4) | – |
| Education years, M (SD) | 13.06 (3.08) | 6–24 |
| McSCI-S Total, M (SD) | 36.96 (22.53) | 0–122 |
McSCI-S, The McCusker Subjective Cognitive Impairment Inventory- self report
Factor analysis, validity and reliability
McCusker Subjective Cognitive Impairment Inventory–Self-Report factor structure
The first CFA model tested the hypothesis that the McSCI-S item scores could be explained by a six-factor model, with each correlated factor corresponding to a unique cognitive domain (i.e. language, memory, attention and concentration, executive functioning, orientation and visuoconstruction). Although this appeared to fit well (CFI = 0.94, TLI = 0.94, RMSEA [90% CI] = 0.051 [.048, 0.054], WRMR = 1.41), there was insufficient discrimination between the factors. The estimated correlations between all the pairwise factor correlations exceeded 0.87, and 13 of the 15 factor correlations were g > 0.90, (95% CI [.99, 1.04]). These results strongly suggest redundancy among the factors and a more parsimonious model was warranted.
A unidimensional model also fit the data well, CFI = 0.94, TLI = 0.94, RMSEA [90% CI] = 0.052 [.049, 0.055], WRMR = 1.45, with almost no decrement in model fit relative to the six-factor model. In addition, McDonald’s omega for the unidimensional model was 0.96, which strongly supports a single-factor model.
Graded response model
After verifying that the unidimensionality assumption could be satisfied, Samejima’s GRM was used to estimate item and scale parameters. Discrimination and threshold estimates are shown in Table 3. All discrimination parameters performed well, ranging from a minimum of 1.04 to a maximum of 2.68. Threshold parameters for the first (lowest) threshold ranged from a low of −1.97 (Item 5) to a high of 2.00 (Item 40), suggesting that very low trait levels (q < −2) of SCD may not be reliably measured by the McSCI-S items. In contrast, the threshold parameters for the last (highest) threshold ranged from a low of 1.92 (Item 25) to a high of 4.77 (Item 44), suggesting that high and very high trait levels (q > 2) of SCD can be estimated precisely by the McSCI-S. Figure 1 shows the relationship between the underlying trait level of SCD and the expected score on the McSCI-S. The scale information and standard errors are shown in Appendices Figure-Supp File-A. As illustrated in Figure 1, the McSCI-S has high information (3 10) across a wide range of the SCD trait, roughly 2 SD below the mean to 4 SD above the mean. Consequently, the standard errors of estimated SCD within this interval are low (<0.31), meaning that the McSCI-S is capable of precisely estimating SCD for most levels of the trait. In addition, a consequence of the high information is the resulting high reliability across most of the latent ability continuum, as shown in Figure Supp-File (Appendices). In particular, reliability estimates of 0.90 or greater, indicative of excellent reliability, were found for ability levels ranging from −1.73 to 4.54. Reliability estimates of 0.95 or greater, a highly desirable measurement property, are possible within the range of ability levels from −0.89 to 3.72. In other words, for 99.99% of the population of individuals with above-average (i.e. q > 0) SCD, the precision of the McSCI-S is excellent (i.e. SEs < 0.23) and its reliability is in the most desirable range for individual- level SCD screening decision-making [6]. In contrast, the psychometric properties of the McSCI-S in the lower half of the SCD trait continuum was less desirable, but not inadequate, meaning that SCD estimates are less precise and reliable when individuals are very low on this trait (i.e. not reporting high levels of cognitive decline). Further details at the item level of the McSCI-S can be found in Table Supp-2 (providing the latent trait z-scores corresponding to observed scores). Figure Supp-2 shows item response category characteristic curves for each of the 46 McSCI items, providing a visual depiction of how each of the items’ response options operate as a function of the underlying trait being measured.
Table 3.
Parameter estimates for the graded response model
| Item | a | b1 | b2 | b3 | b4 |
|---|---|---|---|---|---|
| 1 | 1.27 | −0.59 | 0.53 | 2.44 | 3.92 |
| 2 | 1.28 | −1.96 | −0.37 | 1.68 | 3.14 |
| 3 | 1.91 | −0.77 | 0.50 | 1.70 | 3.02 |
| 4 | 1.96 | −0.20 | 1.24 | 2.46 | – |
| 5 | 1.24 | −1.97 | −0.45 | 2.11 | 3.91 |
| 6 | 1.46 | −0.89 | 0.58 | 2.09 | 3.49 |
| 7 | 1.22 | −0.91 | 0.34 | 2.65 | 3.94 |
| 8 | 1.58 | −1.45 | 0.08 | 1.63 | 2.94 |
| 9 | 1.51 | −1.20 | 0.36 | 2.09 | 3.70 |
| 10 | 1.85 | −0.46 | 1.01 | 2.22 | 3.17 |
| 11 | 1.99 | −0.45 | 0.92 | 2.25 | – |
| 12 | 1.83 | −0.30 | 0.95 | 1.86 | 3.06 |
| 13 | 1.72 | −0.88 | 0.76 | 2.37 | – |
| 14 | 2.02 | −0.03 | 1.51 | 2.78 | – |
| 15 | 1.48 | 0.07 | 1.83 | 3.77 | – |
| 16 | 1.90 | 0.29 | 1.73 | 3.05 | – |
| 17 | 1.54 | −0.37 | 1.02 | 2.37 | – |
| 18 | 2.13 | −0.16 | 1.04 | 2.50 | – |
| 19 | 1.40 | 0.48 | 1.73 | 3.61 | – |
| 20 | 1.93 | 0.27 | 1.46 | 2.66 | – |
| 21 | 1.15 | −0.36 | 1.06 | 3.52 | – |
| 22 | 1.92 | 1.69 | 3.12 | – | – |
| 23 | 1.04 | −0.35 | 1.39 | 3.96 | – |
| 24 | 1.41 | 1.24 | 2.67 | 3.26 | – |
| 25 | 1.31 | 0.60 | 1.92 | – | – |
| 26 | 1.92 | −0.69 | 0.56 | 1.96 | 3.06 |
| 27 | 2.68 | 0.23 | 1.25 | 2.19 | – |
| 28 | 1.41 | 0.45 | 1.57 | 2.75 | 3.67 |
| 29 | 1.42 | 0.41 | 1.92 | 3.20 | – |
| 30 | 1.48 | −0.59 | 0.52 | 1.98 | 3.26 |
| 31 | 2.00 | 0.95 | 2.22 | – | – |
| 32 | 1.56 | 0.19 | 1.61 | 2.81 | – |
| 33 | 1.51 | −0.94 | 0.52 | 2.59 | – |
| 34 | 1.30 | 0.08 | 1.35 | 2.37 | 3.65 |
| 35 | 1.68 | 0.48 | 1.76 | 3.25 | – |
| 36 | 1.80 | −0.75 | 0.50 | 1.97 | 3.36 |
| 37 | 1.74 | −0.13 | 1.20 | 2.82 | – |
| 38 | 1.19 | 0.79 | 2.50 | 3.35 | – |
| 39 | 1.50 | 0.55 | 1.64 | 2.88 | – |
| 40 | 2.00 | 2.00 | – | – | – |
| 41 | 1.30 | −0.43 | 1.17 | 3.21 | – |
| 42 | 1.57 | 0.50 | 2.04 | – | – |
| 43 | 1.97 | 0.36 | 1.61 | 2.67 | – |
| 44 | 1.09 | −0.16 | 1.39 | 2.85 | 4.77 |
| 45 | 1.26 | 0.06 | 1.23 | 2.47 | 3.88 |
| 46 | 1.33 | 1.28 | 2.80 | – | – |
Note. a = discrimination parameter; b = threshold parameter.
Figure 1.
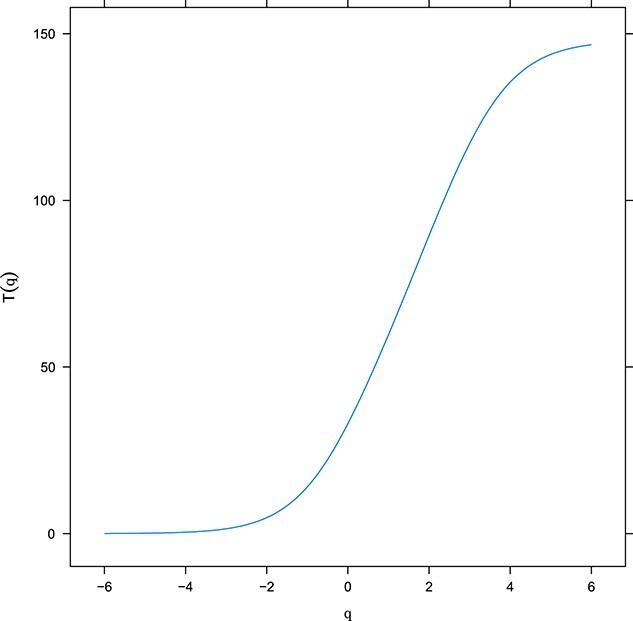
The relationship between SCD at trait level and expected score on the McSCI-S. Expected Total McSCI-S Score as a Function of Theta (Latent SCD). The x-axis represents the level of the latent trait (SCCs), with higher scores representing more complaints. The y-axis shows the expected total McSCI-S sum score. The s-shaped curve demonstrates the relationship between the latent trait and the sum score on the McSCI-S that would be expected on the basis of the underlying trait.
Association with other measures
A preliminary study was conducted with a cohort of 383 individuals aged 39–97 years old from our larger longitudinal study of ageing, the WAMS [18]. The demographic data for this sub-cohort are presented in Table Supp-3. Age was not significantly associated with the McSCI-S total or its IRT Factor scores (P > .05). The McSCI-S total score was negatively associated with global and specific cognitive functions including the MoCA, the CVLT-II learning trials 1–5 total score and CVLT-II short and long delay free recalls (r = −.14, −.21, −.13, −.13, respectively; P < .05). The McSCI-S total score was also associated with TMT-B and DASS depression score (P < .05; Table 4).
Table 4.
Associations between McSCI and other measures (n = 382)
| Age | Edu. | MoCA | MAC-Q | DASS-D (n = 69) | CVLT-L1-5 | CVLT- SD-FR | CVLT- LD-FR | CVLT-Recog. | TMT-B | |
|---|---|---|---|---|---|---|---|---|---|---|
| McSCI-S Total | .033 | −.14a | −.18b | . 53b | .45b | −.21b | −.13a | −.13a | −.041 | .24b |
| McSCI-S IRT Factor Score | 0.06 | −.14a | −.10 | .55b | .25b | −.15a | −.10 | −.09 | −.01 | .19a |
Pearson correlation coefficient (two-tailed) was used; CVLT, California Verbal Learning Test-II; CVLT-L1–5, CVLT-II learning trials 1–5 total score; CVLT-SD-FR, CVLT-II short delay free recall score; CVLT-II LD-FR, CVLT-II long delay free recall; CVLT-Recog., CVLT-II recognition Hits; DASS-total, Depression Anxiety Stress Scale; Edu, education years; M-Q, The Memory Complaint Questionnaire; McSCI, McCusker Subjective Cognitive Impairment Inventory (McSCI-S); McSCI-T, McSCI-S Total Score; MoCA, The Montreal Cognitive Assessment; TMT-B, Trail making Test-B.
a P < .05,
b P < .01.
The MAC-Q cut-off ≥25 was used to group participants into SMCs and noncomplainers. The two groups performed significantly differently on the McSCI-S total score, [t (df = 376) = −7.29, P < .001, (Figure Supp-3)]. Using the McSCI-S Factor scores, this significant difference between the MAC-Q-derived SMCs and noncomplainers was confirmed [t (df = 197.6) = 6.11, P < .01]. The association between the MAC-Q and McSCI-S total score or Factor score was significant (r = .53 and .55, respectively; P < .001); however, as 73% of the variance was not shared, the MAC-Q and McSCI-S appear to capture different aspects of SCD.
Cut-off score
To identify an optimal cut-off score for the McSCI-S, ROC curves analysis was conducted. The participants (n = 503) were classified into two groups based on the absence or presence of SMC using the MAC-Q cut-off score. This model resulted in an area under the curve (AUC) = 0.70; 95% CI = 0.65–0.75. Using the Youden index, a cut-off score of 24 and above resulted in sensitivity = 0.79 and specificity = 0.50 (data on ROC graphs, sensitivity, specificity and Youden’s J are presented in the Appendices: Figure Supp-4 and Table Supp-4). This cut-off was chosen for its higher sensitivity.
Discussion
The results clearly indicated that the McSCI-S is a reliable measure of SCD. In addition, the McSCI-S total scores (both total score and the IRT-based factor score) were significantly related to measures of cognitive function and memory complaints providing evidence of good concurrent validity.
The McSCI-S items were developed to capture concerns on most cognitive domains that have been previously identified as important by the SCD Initiative (SCD-I) Working Group including memory, attention and working memory, language, executive function, orientation and visuospatial skills [11]. However, the CFA findings on the McSCI-S showed that a single factor and not a six-factor model was the best fit for the data. While this finding indicates that a single total score may best represent the SCDs for a given respondent, it does not imply that domain specific scores of the McSCI-S should not be used in future research when individuals with higher risk or at confirmed (e.g. via biomarkers) prodromal and clinical stages of dementia or other neurological/psychiatric conditions are concerned. That is, in clinical cohorts, the patterns of responding may more clearly align with a multifactor model. Of note, recent research has provided increasingly convincing evidence that age-related cognitive decline progresses in a relatively uniform fashion across most cognitive domains, rather than in a domain-specific fashion [30]. Such findings, indirectly support the unidimensional factor structure of the McSCI-S.
Compared to previously published measures of SCD (e.g. [7–10]), the McSCI-S has shown significantly higher reliability and better validity statistics. For example, the McSCi’s omega coefficient was much higher than similar measures and we have previously reported strong reliability findings for the McSCI, using Cronbach’s alpha (.96; n = 367) in a smaller sample [18]. Furthermore, the IRT analyses added nuance to the reliability estimates because they do not assume that a single reliability value can describe the entire test. The IRT results for the McSCI-S showed that reliability was highest for average and above levels of SCD, but less reliable at lower levels of SCD. This is an important finding because it indicates that the McSCI-S can estimate SCD when it is high, which is the case in preclinical stage of AD, as compared to when it is low, as in the clinical stages of dementia [31], potentially due to decreased insight. Therefore, McSCI-S can serve better in detecting those at risk rather than those with dementia. These psychometric properties imply that the McSCI, as compared to other measures, performs better at detecting SCDs related to neurodegenerative processes, although this has not been assessed by us and requires further research to be supported.
Content validity was supported by the finding that the McSCI-S was significantly associated with another measure of SCD (MAC-Q) and there were significant differences between those with and without such complaints. Evidence for concurrent validity came from the finding that higher McSCI-S scores were associated with poorer performance on objective measures of cognition (e.g. the MoCA and CVLT-II), as it was expected. Previous studies on the relationship between SCD and objective measures of cognition have mostly been unsuccessful in stablishing convincing associations between the two or providing evidence of sufficient validity for SCD measures [32–35].
The cut-off score ≥24 is proposed for the McSCI-S, following the ROC analysis. This cut-off was chosen for better sensitivity at the cost of specificity, which was not the primary objective of this study. Of note, Youden’s index is based on the results of the ROC, which relies on the accuracy of the gold standard in identifying true-positive from true-negative cases. Here, we used the MAC-Q as it was the primary measure of SMC available across several cohort studies that we recruited participants from. Because of the less-than-ideal reliability data available for the MAC-Q (Cronbach’s α = .57) [36], future research with more robust SCD measures may result in slightly different cut-off scores for the McSCI-S.
Higher McSCI-S scores were also associated with higher depression scores. There is a wealth of evidence on the relationship between depression severity and SCD [37, 38]. In addition, depressive symptoms have been proposed as a risk factor for cognitive decline as well as dementia and can indicate those at higher risk of dementia [12]. However, a strong relationship between depression and SCD may also result from overlapping or unclear items. For example, depression measures often contain items that ask about memory and other cognitive concerns [39] or items that are confusing to complete. With this in mind, during the development phase of the McSCI, we excluded mood items and sought feedback from our participants in finalising the wording of the items to minimise such sources of bias [39]. Future research can determine how well the McSCI-S predicts the rate of cognitive decline and conversion to MCI and dementia after controlling for the effects of depression.
Conclusions
Research evidence suggests that SCDs are related to a wide range of conditions and represent various underlying aetiologies. Therefore, it is important to accurately capture these self-reported concerns about cognition to identify the patterns of SCD that are predictive of dementia as opposed to those patterns that are indicative of depression, personality or other factors. The McSCI-S, as compared to other measures, has shown powerful psychometric properties including very high reliability and validity and is, therefore, an appropriate measure to assess SCDs at both individual and group levels.
Supplementary Material
Acknowledgements:
Authors would like to thank Dr Chloe Marshall-Cary, Neuropsychologist, Dr Toni Musiello, Clinical Psychologist, and Dr Keira James, Neuropsychologist for providing input regarding the face validity of the items selected for the McSCI-S. The authors acknowledge the support of the followings who were our collaborators, project partners, PhD students, or research assistants at early stages of the McSCI-S development and data collection: Caleb Bishop, Sabine Bird, Samantha Gardener, Kevin Taddei, Mark Rodrigues, Shane Fernandez, Manja Laws, Sherilyn Tan, Shaun Markovic, Kathryn Goozee, Bethany Ball and Candice Man Yan. This study was not preregistered.
Footnotes
Any use of the McSCI-S follows Copyrights and intellectual property agreements. The McSCI-S can be used as it is, without any change in format, instructions and wording and items numbers, by researchers and university lecturers for academic purposes or by clinicians for single case patient assessments. However, such applications should give full referencing to this publication. Any application of this measure by pharmaceutical or other non-academic research entities (e.g., single or mass assessments, in any format including paper and pencil, electronic or online) should seek permission from the corresponding authors. In addition, the McSCI-S has not been translated into any other language. While such efforts are welcomed, they should be in consultation with the corresponding authors and after appropriate permissions are granted. Other forms of translation or application (e.g., computerised, web-based apps and online, paper and pencil, or audio) require permission from the corresponding authors to maintain the consistency and psychometric properties of the measure.
Contributor Information
Hamid R Sohrabi, Centre for Healthy Ageing, Health Futures Institute, Murdoch University, 90 South Street, Murdoch, WA 6150, Australia; School of Psychology, Murdoch University, Building 440, 90 South Street, Murdoch, WA 6150, Australia; School of Medical and Health Sciences, Edith Cowan University, 270 Joondalup Drive, Joondalup WA 6027, Australia; Department of Biomedical Sciences, Faculty of Medicine and Health Sciences, Macquarie University, 75 Talavera Road, Macquarie Park, NSW 2109, Australia.
Brandon E Gavett, School of Psychological Science, University of Western Australia, 35 Stirling Highway, Perth, Crawley WA 6009, Australia; Department of Neurology, University of California, Davis, Sacramento, CA 95817, USA.
Michael Weinborn, School of Medical and Health Sciences, Edith Cowan University, 270 Joondalup Drive, Joondalup WA 6027, Australia; School of Psychological Science, University of Western Australia, 35 Stirling Highway, Perth, Crawley WA 6009, Australia.
Craig P Speelman, Experimental Psychology Unit, School of Arts and Humanities, Edith Cowan University, 270 Joondalup Drive, Joondalup WA 6027, Australia.
Romola S Bucks, School of Psychological Science, University of Western Australia, 35 Stirling Highway, Perth, Crawley WA 6009, Australia.
Ralph N Martins, Centre for Healthy Ageing, Health Futures Institute, Murdoch University, 90 South Street, Murdoch, WA 6150, Australia; School of Medical and Health Sciences, Edith Cowan University, 270 Joondalup Drive, Joondalup WA 6027, Australia; Department of Biomedical Sciences, Faculty of Medicine and Health Sciences, Macquarie University, 75 Talavera Road, Macquarie Park, NSW 2109, Australia.
Data Availability:
The data presented in this study are available from corresponding authors upon written, formal request. The data are held in a password protected, secure cloud environment, based at our universities.
Declaration of Conflicts of Interest:
B.G., M.W., C.S. and R.B. report no conflict of interest directly or indirectly associated to this manuscript. H.R.S. and R.N.M. report being Directors of SMarT Minds WA, Australia. H.R.S. and R.N.M. have had or are receiving personal reimbursements or research support from the Pharmaceutical and Nutraceutical companies including Alector, Alnylam Pharmaceuticals, CWEK PTY LTD, WA, Australia, and Biogen pharmaceuticals.
Declaration of Sources of Funding:
The Western Australia Memory Study (WAMS), the primary study for this manuscript, has been supported by a grant from the National Health and Medical Research Council-Australia (Grant Number: 324100 awarded to RNM), an Early Career Researcher Grant (HRS; G1001512-2014) from Edith Cowan University, the Dementia Australia, the Cecilia Margaret Hudson Dementia Research Grant to H.R.S. (2014; Dementia Australia Research Foundation), the McCusker Charitable Foundation grant, the Alzheimer’s Research Australia (previously known as the Australian Alzheimer’s Research Foundation Inc.), the Hollywood Private Hospital and the Charlies Foundation Western Australia, Australia. The sponsors of this study had no contribution to the design, data collection, statistical analysis, interpretation of the results or the preparation and final submission of the manuscript.
References
- 1. Jessen F, Amariglio RE, Buckley RFet al. The characterisation of subjective cognitive decline. Lancet Neurol 2020;19:271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jessen F, Kleineidam L, Wolfsgruber Set al. Prediction of dementia of Alzheimer type by different types of subjective cognitive decline. Alzheimers Dement 2020;16:1745–9. [DOI] [PubMed] [Google Scholar]
- 3. Sohrabi HR, Weinborn M. Cognitive Impairments in Alzheimer's Disease and Other Neurodegenerative Diseases. Neurodegeneration and Alzheimer's Disease: The Role of Diabetes, Genetics, Hormones, and Lifestyle. West Sussex, UK: John Wiley & Sons Ltd., 2019; 267–90. [Google Scholar]
- 4. Zhao Z, Wang J, Wang Yet al. 18F-AV45 PET and MRI reveal the influencing factors of Alzheimer’s disease biomarkers in subjective cognitive decline population. J Alzheimers Dis 2023;93:585–94. [DOI] [PubMed] [Google Scholar]
- 5. Kielb S, Rogalski E, Weintraub Set al. Objective features of subjective cognitive decline in a United States national database. Alzheimers Dement 2017;13:1337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nunnally J, Bernstein IH. Psychometric Theory. 3rd edition. New York: McGraw-Hill, 1994. [Google Scholar]
- 7. Crawford J, Smith G, Maylor EAet al. The prospective and retrospective memory questionnaire (PRMQ): normative data and latent structure in a large non-clinical sample. Memory 2003;11:261–75. [DOI] [PubMed] [Google Scholar]
- 8. Crook TH, Feher EP, Larrabee GJ. Assessment of memory complaint in age-associated memory impairment: the MAC-Q. Int Psychogeriatr 1992;4:165–76. [DOI] [PubMed] [Google Scholar]
- 9. Gilewski MJ, Zelinski EM, Schaie KW. The memory functioning questionnaire for assessment of memory complaints in adulthood and old age. Psychol Aging 1990;5:482–90. [DOI] [PubMed] [Google Scholar]
- 10. Rami L, Mollica MA, García-Sanchez Cet al. The subjective cognitive decline questionnaire (SCD-Q): a validation study. J Alzheimers Dis 2014;41:453–66. [DOI] [PubMed] [Google Scholar]
- 11. Rabin LA, Smart CM, Crane PKet al. Subjective cognitive decline in older adults: an overview of self-report measures used across 19 international research studies. J Alzheimers Dis 2015;48:S63–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnson LA, Sohrabi HR, Hall JRet al. A depressive endophenotype of poorer cognition among cognitively healthy community-dwelling adults: results from the Western Australia memory study. Int J Geriatr Psychiatry 2015;30:881–6. [DOI] [PubMed] [Google Scholar]
- 13. Sohrabi HR, Bates KA, Rodrigues Met al. The relationship between memory complaints, perceived quality of life and mental health in apolipoprotein E epsilon4 carriers and non-carriers. J Alzheimers Dis 2009;17:69–79. [DOI] [PubMed] [Google Scholar]
- 14. Goozee K, Chatterjee P, James Iet al. Elevated plasma ferritin in elderly individuals with high neocortical amyloid-β load. Mol Psychiatry 2018;23:1807–12. [DOI] [PubMed] [Google Scholar]
- 15. Fowler C, Rainey-Smith SR, Bird Set al. Fifteen years of the Australian imaging, biomarkers and lifestyle (AIBL) study: Progress and observations from 2,359 older adults spanning the Spectrum from cognitive normality to Alzheimer’s disease. J Alzheimer's Dis Rep 2021;5:443–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bateman RJ, Aisen PS, De Strooper Bet al. Autosomal-dominant Alzheimer's disease: a review and proposal for the prevention of Alzheimer's disease. Alzheimer's Res Ther 2011;3:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sohrabi HR, Weinborn M, Martins RN. The McCusker Subjective Cognitive Impairment Inventory (McSCI): A New Self-Report Measure of Cognitive Concerns. In: 12TH International Conference on Alzheimer's & Parkinson's Diseases:AD/PDTM 2015. Nice, France, 2015.
- 18. Sohrabi HR, Weinborn M, Shen K, Martins RN.. McCusker Subjective Cognitive Decline Inventory- Development of a New Measure. In: 6th International Conference on Memory (ICOM6). Budapest, Hungary, 2016.
- 19. Nasreddine ZS, Phillips NA, Bédirian Vet al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–9. [DOI] [PubMed] [Google Scholar]
- 20. Delis DC, Kramer JH, Kaplan Eet al. Manual for the California Verbal Learning Test, (CVLT-II). San Antonio, TX: The Psychological Corporation, 2000. [Google Scholar]
- 21. Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills 1958;8:271–6. [Google Scholar]
- 22. Arbuthnott K, Frank J. Trail making test, part B as a measure of executive control: validation using a set-switching paradigm. J Clin Exp Neuropsychol 2000;22:518–28. [DOI] [PubMed] [Google Scholar]
- 23. Lovibond SH, Lovibond PF. Manual for the Depression Anxiety Stress Scales. Sydney, Australia: Psychology Foundation of Australia, 1995. [Google Scholar]
- 24. Samejima F. Graded response models. In: Handbook of Item Response Theory, Volume One. New York, USA: Chapman and Hall/CRC, 2016; 123–36. [Google Scholar]
- 25. Muthén B, Muthén L. Mplus. In: Handbook of Item Response Theory, Volume Three. New York, USA: Chapman and Hall/CRC, 2017;201–212. [Google Scholar]
- 26. Team RC. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2019. [Google Scholar]
- 27. Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32–5. [DOI] [PubMed] [Google Scholar]
- 28. Topcu DI, Çubukçu HC. Optimization of patient-based real-time quality control based on the Youden index. Clin Chim Acta 2022;534:50–6. [DOI] [PubMed] [Google Scholar]
- 29. Schisterman EF, Perkins NJ, Liu Aet al. Optimal cut-point and its corresponding Youden index to discriminate individuals using pooled blood samples. Epidemiology 2005;16:73–81. [DOI] [PubMed] [Google Scholar]
- 30. Tucker-Drob EM, Brandmaier AM, Lindenberger U. Coupled cognitive changes in adulthood: a meta-analysis. Psychol Bull 2019;145:273–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kuhn E, Perrotin A, Tomadesso Cet al. Subjective cognitive decline: opposite links to neurodegeneration across the Alzheimer’s continuum. Brain Commun 2021;3:fcab199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Slavin MJ, Sachdev PS, Kochan NAet al. Predicting cognitive, functional, and diagnostic change over 4 years using baseline subjective cognitive complaints in the Sydney memory and ageing study. Am J Geriatr Psychiatry 2015;23:906–14. [DOI] [PubMed] [Google Scholar]
- 33. Zlatar ZZ, Muniz MC, Espinoza SGet al. Subjective cognitive decline, objective cognition, and depression in older Hispanics screened for memory impairment. J Alzheimers Dis 2018;63:949–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sohrabi HR, Weinborn M, Laske Cet al. Subjective memory complaints predict baseline but not future cognitive function over three years: results from the Western Australia memory study. Int Psychogeriatr 2019;31:513–25. [DOI] [PubMed] [Google Scholar]
- 35. Reid M, Parkinson L, Gibson Ret al. Memory complaint questionnaire performed poorly as screening tool: validation against psychometric tests and affective measures. J Clin Epidemiol 2012;65:199–205. [DOI] [PubMed] [Google Scholar]
- 36. Ibnidris A, Robinson JN, Stubbs Met al. Evaluating measurement properties of subjective cognitive decline self-reported outcome measures: a systematic review. Syst Rev 2022;11:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zlatar ZZ, Muniz M, Galasko Det al. Subjective cognitive decline correlates with depression symptoms and not with concurrent objective cognition in a clinic-based sample of older adults. J Gerontol B 2018;73:1198–202. 10.1093/geronb/gbw207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Seo EH, Kim H, Choi KYet al. Association of subjective memory complaint and depressive symptoms with objective cognitive functions in prodromal Alzheimer's disease including pre-mild cognitive impairment. J Affect Disord 2017;217:24–8. [DOI] [PubMed] [Google Scholar]
- 39. Hill NL, Mogle J, Whitaker EBet al. Sources of response bias in cognitive self-report items: “which memory are you talking about?”. Gerontologist 2019;59:912–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available from corresponding authors upon written, formal request. The data are held in a password protected, secure cloud environment, based at our universities.


