Abstract
The structure of cucumber mosaic virus (CMV; strain Fny) has been determined to a 3.2-Å resolution using X-ray crystallography. Despite the fact that CMV has only 19% capsid protein sequence identity (34% similarity) to cowpea chlorotic mottle virus (CCMV), the core structures of these two members of the Bromoviridae family are highly homologous. As suggested by a previous low-resolution structural study, the 305-Å diameter (maximum) of CMV is ∼12 Å larger than that of CCMV. In CCMV, the structures of the A, B, and C subunits are nearly identical except in their N termini. In contrast, the structures of two loops in subunit A of CMV differ from those in B and C. These loops are 6 and 7 residues longer than the analogous regions in CCMV. Unlike that of CCMV, the capsid of CMV does not undergo swelling at pH 7.0 and is stable at pH 9.0. This may be partly due to the fact that the N termini of the B and C subunits form a unique bundle of six amphipathic helices oriented down into the virion core at the threefold axes. In addition, while CCMV has a cluster of aspartic acid residues at the quasi-threefold axis that are proposed to bind metal in a pH-dependent manner, this cluster is replaced by complementing acids and bases in CMV. Finally, this structure clearly demonstrates that the residues important for aphid transmission lie at the outermost portion of the βH-βI loop and yields details of the portions of the virus that are hypothesized to mediate binding to aphid mouthparts.
Cucumber mosaic virus (CMV) is the type member of the genus Cucumovirus, family Bromoviridae, which infects over 800 plant species and causes economically important diseases of many crops worldwide (18). CMV isolates are divided into two main subgroups based on their serological and nucleic acids properties (18). Serologically, the two subgroups are closely related, as has been shown by cross-reactivity to polyclonal antibodies (18, 29). Some monoclonal antibodies produced against the coat proteins of subgroups I and II can differentiate the two, indicating the presence of unique epitopes for each (22, 29).
Recently, the molecular structure of CMV was determined to ∼8-Å resolution using cryo-transmission electron microscopy (cryo-TEM) and X-ray crystallography (30). A remarkable similarity was demonstrated between the structure of CMV and that of cowpea chlorotic mottle virus (CCMV), another member of the Bromoviridae. In CCMV, the N termini of the B and C subunits form an unusual β-hexamer at the icosahedral threefold axes (27). This structure is believed to also exist in CMV (30) and is a variant of the β-annulus observed in many plant virus capsids. The domain connecting the N-terminal basic R domain to the β-barrel domain has been observed in the electron densities of the C subunits in several T=3 plant viruses, including southern bean mosaic virus (1) and tomato busy stunt virus (6). The N-terminal arms of the three C subunits extend along an inner edge of the protein shell and loop around the threefold axes, interdigitating in sets of three to form the β-annulus motif.
While the overall architecture of CCMV has been shown to be homologous to that of CMV (30), it is clear that there are a number of properties not shared by these two viruses. CCMV is stable and RNase resistant at pH 5.0 and has a sedimentation coefficient of 88S. At pH 7.0, when the ionic strength is kept at less than 0.1, the particle swells by ∼10% and the sedimentation coefficient drops to 78S (3). This swelling is reversed by dropping the pH back to 5.0 or by increasing the calcium or magnesium concentrations to 50 mM. However, if the pH is raised to 7.5 and the ionic strength is greater than 0.4, the CCMV capsid disassociates into ∼40S, dimeric, ribonuclear-protein species. These particles can be subsequently reassembled into T=1, T=3, and T=7 singly and multishelled spherical capsids as well as sheets, tubes, and rosettes. In contrast, CMV has a sedimentation coefficient of 99S and does not disassemble under these conditions but is RNase sensitive over a wide range of in vitro conditions. It should be noted, however, that the correlation between in vitro and in vivo RNase sensitivities is unclear. Therefore, a structural comparison of CCMV and CMV will elucidate the reasons why these homologous viruses have such marked differences in assembly and stability.
It is also of interest to examine the structure of CMV to understand the process of insect transmission. CMV is transmitted by aphids in a nonpersistent manner, i.e., they do not circulate or replicate in the aphid (5, 21). The virus can be both acquired from and transmitted to a host within seconds to minutes after feeding. To accomplish this, the virus interacts with the anterior portion of the alimentary tract (food canal to foregut), from which it can be subsequently inoculated by egestion. Unlike some of the other nonpersistently transmitted plant viruses, CMV does not require helper proteins for transmission, and therefore the aphid recognition motifs must reside on the capsid itself. The CMV capsid protein is also essential for normal cell-to-cell and systemic movement within the host plant (9). Viral movement does not require the formation of virions; it appears that an alternative ribonucleoprotein complex is transported.
Here is presented the crystal structure of CMV determined to an ∼3.2-Å resolution. While the overall fold of CMV is very similar to that of CCMV, the β-hexamer of CCMV has been replaced by a bundle of six helices that is unique among the structures of any virus determined to date. This and other details in the CMV structure may explain differences in capsid stability between it and CCMV. Finally, this structure facilitates analysis of the functional significance of residues crucial for aphid transmission and viral movement in the host.
MATERIALS AND METHODS
CMV was propagated in Nicotiana tabacum and purified by differential centrifugation followed by sucrose gradient fractionation (14). The sucrose-fractionated virus was dialyzed against 10 mM Tris, pH 7.0, and concentrated to 2 mg/ml. Crystals were grown using vapor diffusion and the sitting-drop method. The reservoir contained 2 M sodium formate, 0.1 M sodium acetate buffer (pH 4.6), and 0.05 to 0.125% polyethylene glycol (PEG) 8000. To the sitting drop, 10 μl of this solution was added to 8 μl of the virus solution and 2 μl of a 24 mM (10 times the critical micelle concentration) solution of CYMAL-5 (cyclohexyl-pentyl-β-d-maltoside) was then added. The detergent improved crystal size by decreasing the number of nucleation sites. It did not improve diffraction resolution other than by virtue of increasing the crystal volume. Crystals with a rhombic dodecahedron habit grew to dimensions of ∼3.0 mm within 2 weeks and diffracted X rays to ∼3.0-Å resolution. To prepare the crystals for freezing, drops that did not have usable crystals were pooled and centrifuged to remove precipitate. This solution was then used to make 10, 20, and 30% solutions of PEG 400. The crystals were transferred to the increasing PEG solutions, with 0.5-h incubations at each step. The crystals were then frozen in a liquid nitrogen stream that was at 110 K. Data was collected using a Rigaku rotating anode X-ray source and an R-axis IV image plate system.
Intensities were integrated with the program DENZO (16) and scaled together with the program SCALEPACK (17). The crystals were found to belong to the P23 space group with a unit cell dimension of 336.0 Å (see Table 1 for data statistics). This finding was initially of some concern since no other virus crystal had been shown to exhibit this symmetry. However, self-rotation function calculations verified this space-group assignment. The self-rotation function clearly showed that there were two particles in the unit cell that differed in orientation by exactly 90°. With the P23 symmetry, this places the two particles at 0,0,0 and 1/2,1/2,1/2 and yields 10 copies of the viral proteins A, B, and C in the crystallographic asymmetric unit. A previous study had shown that CMV appeared to have remarkable structural similarity to CCMV but that it had a 7-Å-larger radius (30). Therefore, the coordinates for CCMV were swollen radially by 3% and two of these swollen particles were placed into the P23 unit cell per the self-rotation function results. This model was then used to calculate structure factors to 10 Å. The CCP4 suite (2, 4), the mask-generating program MAMA (11) using a 5-Å probe radius, and the real-space-averaging program RAVE (10) were used to average the 10 asymmetric units and extend these phases to a 5-Å resolution. At this resolution, it was possible to move major secondary segments of the CCMV model to better fit the density. In addition, the long α-helices at the N termini of the B and C subunits were quite apparent and were also modeled into the density. This updated model was then used to recalculate the phases and the mask, and phases were extended to 3.2 Å. The final map had an overall averaging R factor of 27.5% and correlation coefficient of 83.7%. This map was of sufficient quality to unambiguously assign residues 29 to 218 to B and C subunits and residues 62 to 218 to the A subunit. The model was built using the program O (8). All but Fig. 1, 3, and 7A were created using the program MolView (26; http://bilbo.bio.purdue.edu/∼tom). For refinement, the calculated structure factors from the averaged electron density map were used rather than the raw data. This not only improved the R factor but also improved the geometry of the model. This final model has an R factor of 24.7% when data between resolutions of 8 and 3.2 Å, with an RMS deviation in bond length of 0.018 Å and a root mean square deviation in bond angles of 2.4°, are used. All other geometrical parameters are better than the average of those of other structures determined to this resolution (13).
TABLE 1.
CMV data statistics
| Resolution (Å) | Rsym (%)a | Completeness (%) |
|---|---|---|
| 30.0–6.4 | 2.7 | 80.2 |
| 6.4–5.1 | 4.2 | 74.3 |
| 5.1–4.5 | 4.2 | 73.1 |
| 4.5–4.1 | 4.9 | 70.7 |
| 4.1–3.8 | 6.4 | 66.0 |
| 3.8–3.6 | 7.7 | 61.9 |
| 3.6–3.4 | 9.2 | 56.3 |
| 3.4–3.2 | 10.2 | 39.9 |
| 3.2–3.1 | 12.0 | 27.5 |
| 3.1–3.0 | 13.1 | 18.6 |
| Overall | 4.6 | 57.0 |
Rsym, Σ|Ii − (Ii)|/Σ|Ii|.
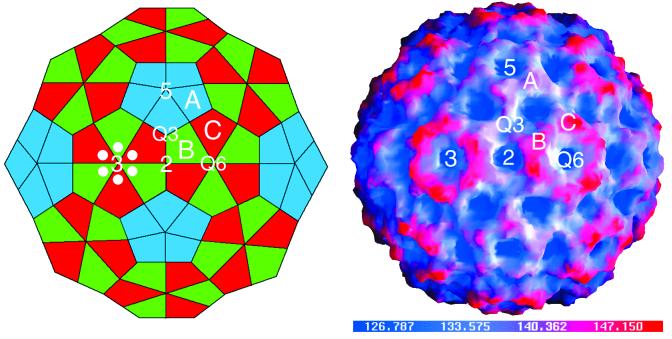
FIG. 1.
Schematic representation of the T=3, truncated icosahedron (left) and a surface representation of the CMV capsid colored according to radial distance (right). In the schematic, the labeled A, B, and C subunits are those that are in the general orientation used for the following diagrams. The subunits used to represent the icosahedral asymmetric unit were chosen to demonstrate the quasi-sixfold axis and are not related by a quasi-threefold axis. In both images the icosahedral threefold (quasi-sixfold [Q6]), fivefold, twofold, and the quasi-threefold (Q3) axes are labeled. In the schematic, six white circles are positioned around one of the quasi-sixfold axes to approximate the location of the hexameric bundle of N-terminal helices described in the following figures.
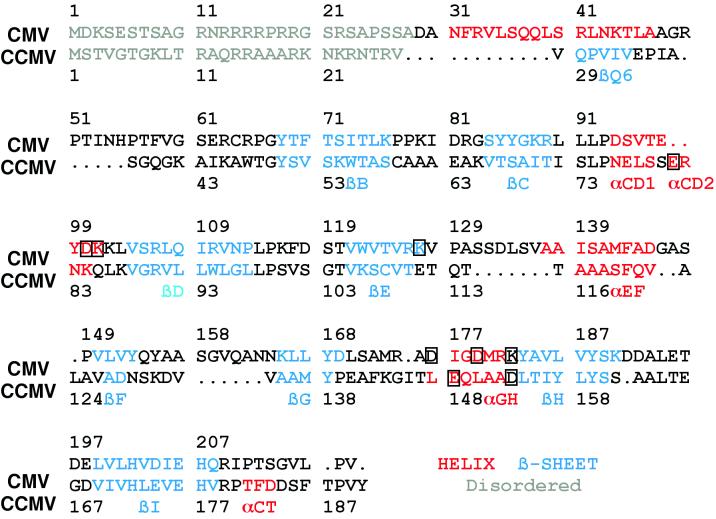
FIG. 3.
Sequence homology of CMV and CCMV based on structural alignments. The gray regions represent disordered regions, the red regions are helices, and the blue regions are β-strands. The nomenclature used for secondary elements is the same as that used for CCMV. The boxed amino acids are those involved in the subunit contacts about the quasi-threefold axes.
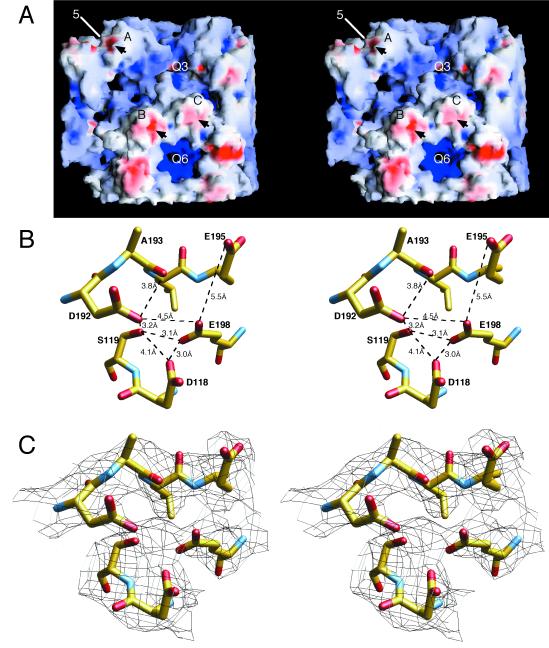
FIG. 7.
Structure of an external loop involved in aphid transmission. (A) van der Waals surface of a portion of the CMV capsid, with the negatively and positively charged electrostatic fields being shown in red and blue, respectively, created with the program GRASP (15). Arrows denote the locations of the loops described below. Note that the only negatively charged patch on the entire capsid surface is about the βH-βI loop. (B) Distances between the residues involved in this negatively charged patch. The atoms are colored according to atom type as defined in the legend to Fig. 5. The side chains in the area are very close to each other and may be indicative of a counterbalancing cation. (C) Same region and view as those shown in panel B, with the electron density contoured at 1 ς, represented by black lines. Note the patch of density between D118, S119, E198, and D192, which may represent a bound, divalent cation. The distances between the center of this patch of density and the oxygen atoms are between 2.3 and 3.0 Å. Upon deprotonation at neutral pH, these distances may decrease to those of typical oxygen-calcium contacts (∼2.3 Å). Q3 and Q6, quasi-three- and quasi-sixfold axes, respectively.
The coordinates of our models have been deposited in the Protein Data Bank (identification code 1F15) and can also be found at the Virus Particle Explorer (VIPER) website (http://mmtsb.scripps.edu/viper/viper.html).
RESULTS
The overall architecture of the CMV viral capsid is the same as that of CCMV. This T=3 virus is best represented as a truncated icosahedron (Fig. 1). There are three copies of capsid protein in each icosahedral asymmetric unit. Lying between the three subunits on each icosahedral face are quasi-equivalent threefold axes. At the icosahedral threefold axes, the B and C subunits are arranged with nearly perfect quasi-sixfold symmetry and form a hexameric, torus-like protrusion (Fig. 1). About the fivefold axes, the A subunits form pentameric capsomers that do not protrude as far above the surface as the hexameric structures. Similar to what occurs in CCMV, 62 of the N-terminal residues of the A subunit are mostly disordered whereas only 29 residues in the B and C subunits are disordered. This break down in quasi-equivalence is due to a hexameric bundle of helices that can form about the quasi-sixfold but not the fivefold axes. As also observed in CCMV, the carboxyl termini of the capsid proteins form extensive interactions between the pentameric and hexameric structures (27).
The core structure of CMV is remarkably homologous to that of CCMV even though their capsid proteins share only 19% sequence identity. The structures of CMV and CCMV were aligned (Fig. 2) using the program MolView (26). Using a distance cutoff of 1.5 Å during the alignment process, 91 residues yielded an RMS deviation of 1.3 Å between the two structures. The first 60 residues could not be used for alignment because they were either disordered or had disparate structures. From this structural alignment, the sequence alignment was updated (Fig. 3) from that of the previously published report (30).
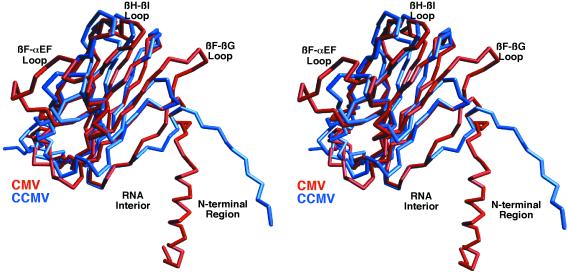
FIG. 2.
Comparison of C subunits of CMV and CCMV. The C-α backbone of CMV is shown in red, and that of CCMV is shown in blue. The program MolView was used for this alignment, and 91 residues yielded a root-mean-square deviation of 1.3 Å. Some of the key areas of differences are labeled.
The most obvious difference between the two viruses is that CMV has ∼12-Å larger diameter (305-Å maximum diameter) than that of CCMV. This is in agreement with the hypothesis that CMV is in a somewhat permanent “swollen” state compared to CCMV. However, as previously determined by cryo-TEM and X-ray crystallography, CMV does not have the same structure as the swollen form of CCMV (27, 30). CMV has only an ∼4%-larger diameter than that of CCMV, whereas the expansion in CCMV is ∼10%.
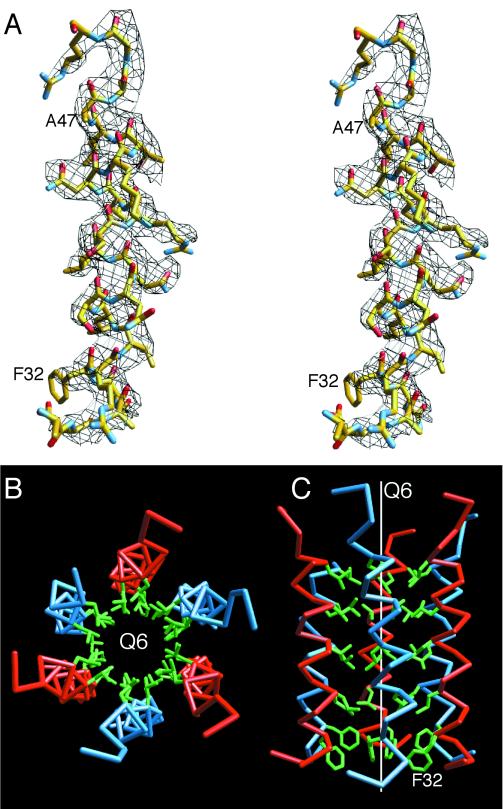
The second most obvious difference is at the N termini of the B and C subunits. In CCMV, residues 29 to 33 form the β-hexameric annulus at the quasi-sixfold (icosahedral threefold) axes. In contrast, residues 31 to 47 in these subunits of CMV form a hexameric bundle of helices unique among the known structures of plant viruses (Fig. 4). These helices start at ∼85 Å from the center of the virion and run parallel to the quasi-sixfold axis up to the capsid-RNA interface. The inner core of this bundle is entirely composed of leucine residues with a phenylalanine plug at the N terminus. Compared to those of CCMV, these helices give the appearance that the capsid of CMV is suspended on stilts over the RNA core.
FIG. 4.
Amphipathic hexameric helical bundles found at the quasi-sixfold axes. (A) A stereo view of the electron density at 1 ς and the fitted model at the N termini of the C subunits are shown. The model is colored according to atom type. (B and C) Six helices about the quasi-sixfold axis (Q6). The C-α backbones for the B and C subunits are shown in blue and red, respectively. The side chains of the inner, hydrophobic residues are shown in green. All the residues shown in green are leucine except for the most N-terminal residue, which is a phenylalanine. The view from the capsid toward the RNA interior in panel B is parallel to the icosahedral axis, whereas the view in panel C is perpendicular to the quasi-sixfold axis.
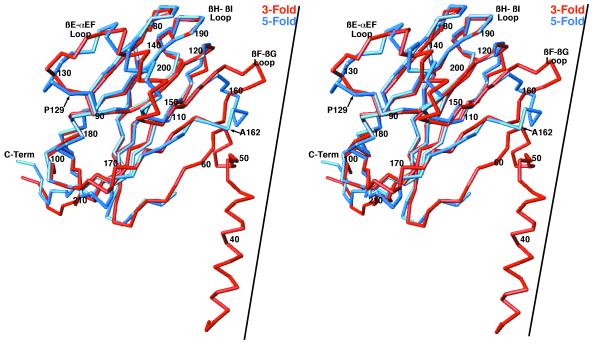
There are several areas of smaller structural differences between CCMV and CMV that may also pertain to the differences in capsid radii and stability (Fig. 2). When the C subunits are compared, the N-terminal coil between residues 38 and 49 in CCMV diverge further from the main β-barrel than does the homologous region in CMV. This is likely due to the differences in the quaternary structures at the quasi-sixfold axes. In CCMV, αCD1 and αCD2 are linked by a short coil and together form a loop structure (residues 72 to 87), whereas in CMV, this loop is shorter by 2 residues and forms a single helix.
The core structures of all three subunits in CCMV are nearly identical to each other, whereas the B and C subunits differ significantly from subunit A in CMV. Compared to CCMV, the βF and βG strands and the βF-βG loop are all longer in CMV than in CCMV. In the B and C subunits, this loop has an extended structure and points toward the threefold axes. In contrast, this loop in the A subunit points toward the fivefold axis and is bent away from the surface (Fig. 5). Another example of nonequivalence in CMV is that the αEF helix region makes contact among the A subunits about the fivefold axes and forms the contact surface between B and C subunits about the icosahedral threefold axes. This βE-αEF loop is 7 residues longer than that in CCMV and has nonidentical structures at the fivefold versus the threefold axes. All of these insertions relative to the sequence of CCMV may fill in the gaps between the subunits in this expanded capsid or may be used to accommodate quasi-equivalent differences. However, there is still a great deal of space between the A, B, and C subunits (see Fig. 7A) that may account for the RNase sensitivity.
FIG. 5.
Comparison of the CMV A- and C-subunit structures. The C-α backbones of the A and C subunits are shown in blue and red, respectively. The RNA interior is toward the bottom of the diagram. The approximate locations of the threefold axis (for the C subunit) and the fivefold axis (for the A subunit) are represented by the black lines. C-Term, C terminus.
A major difference between CMV and CCMV is that extensive RNA-capsid interactions are observed (27) in CCMV but that only several small pieces of isolated density are observed at the capsid-RNA interface in CMV. This is not due to the mask used for averaging since a large probe radius was initially used to generate the mask and the original virus model had a radius smaller than that of the final CMV virion. The reason for the lack of observable RNA density is unclear, especially since the interior of CMV is highly basic. Clearly, RNA interactions must occur with the capsid, but these interactions apparently are not specific to icosahedral symmetry.
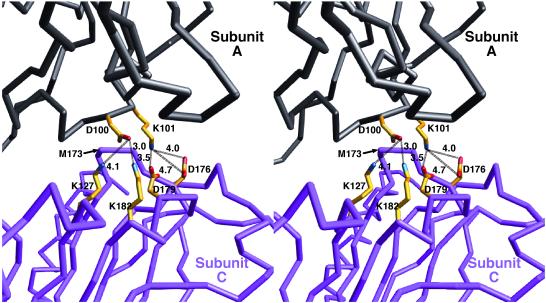
The other difference between CMV and CCMV is in the proposed metal binding regions. In CCMV, the quasi threefold interactions are mediated by three carboxyl side chains: two from one subunit and one from the other (27). While the low pH and the presence of EDTA during crystallization presumably removed the metals from this site, the orientations and distances between these side chains make it likely that this is a metal-chelating site. These interactions help explain the role of metal and pH in CCMV capsid swelling and disassociation (27). An analogous cluster of acidic residues is not found in CMV. Instead, the equivalent interactions in CMV are mostly acid-base interactions (Fig. 6). These direct interactions between the quasi-equivalent subunits offer one explanation for the insensitivity of the capsid to changes in pH and metal concentrations.
FIG. 6.
Subunit interactions about the quasi-threefold axes. For clarity, only the interactions between two of three subunits are shown. The C-α backbone of an A subunit is shown in black, and that of a C subunit is shown in purple. The side chains of the residues at this interface are colored according to atom type: nitrogen atoms are blue, carbon atoms are yellow, and oxygen atoms are red. In CCMV, this same interface is entirely composed of acidic residues that are proposed to interact via a divalent cation.
There is, however, one location of the capsid that may be involved in metal chelation. As shown in Fig. 7A, the entire interior and exterior surfaces of the capsid are mostly basic, with the exception of one small acidic patch on the top of each subunit. Unlike CCMV, this patch is not at a subunit interface but instead may help define the structure of an outermost loop. As shown in Fig. 7B, the side chains of D192, E198, D118, and S119 are in close proximity with each other, with E195 lying on the perimeter of these interactions. Above the main cluster of acidic side chains is a portion of electron density not assigned to the protein structure that may represent a bound metal cation (Fig. 7C). It should be noted that CMV was crystallized at relatively low pH (4.6) and that the virus was treated with EDTA during preparation; therefore, some of the metal ions may have been removed. In the presence of a metal cation and at higher pH, E195 may interact to a greater degree with this cluster. Perhaps the most interesting aspect of this region is that amino acids in the βH-βI loop were shown to be remarkably conserved among strains of CMV and other cucumoviruses (K. Perry, unpublished results). Seven of the 9 amino acids in this loop (positions 190 to 198) are invariant among cucumoviruses, and of these 7, 6 have charged side chains. The βH-βI loop is highly antigenic (7), and it plays an essential role in aphid transmission (K. Perry, unpublished results).
DISCUSSION
The previous finding that CCMV and CMV had homologous structures (30) was essential for this structure determination. It is therefore interesting to note where sequence alignment procedures succeeded and failed. As in CCMV, the first 28 residues are disordered in subunits B and C. In CCMV, residues 28 to 67 form mostly a random coil, with the residues comprising the β-annulus structure located at residues 29 to 33. In CMV, this region (residues 29 to 67) has a disparate sequence and structure. As with CCMV, a torus-shaped density was observed in the low-resolution structure of CMV at the quasi-sixfold axis (30). While it was proposed that this represented a β-annulus similar to that of CCMV, it was in fact a hexameric bundle of helices in CMV. In hindsight, the density of this region in CMV (Fig. 1 of reference 30) is thicker and has a larger radius than the β-annulus in CCMV. In addition, the program NNpredict (12) predicted that residues 30 to 45 formed a helix. However, a prediction of this structure would have been surprising, given that this hexameric helical structure has never before been observed in a virus structure. It is not surprising that, with such low sequence homology, the sequence alignment algorithms failed in this particular region.
The main core region, residues 61 to 129, in CMV was correctly aligned with residues 43 to 113 in CCMV in spite of relatively low sequence homology. In the subsequent section, the alignment was confounded by weak homology and relatively large insertions in CMV. It was proposed that there was a large insertion between βG and aGH in CMV relative to the sequence of CCMV. However, this insertion was actually distributed at two different places: the βE-αEF and the βF-βG loops. The major consequence of this is that the βF, βG, and αGh strands and part of the βH strand are incorrectly aligned. This is likely due to the relatively low sequence identity in this region and the fact that the βF and βG strands are longer in CMV than in CCMV. Interestingly, the alignment is in sync again toward the end of the βH strand and then proceeds without any insertions to the C terminus. The last significant difference is that the αCT helix does not exist in CMV, presumably because of a disrupting proline at position 211. In spite of these problems, the previous model for CMV was fairly accurate and proves the importance of combining crystallography, cryo-TEM, and secondary-structure prediction to model virus structures.
The availability of a CMV structure facilitates a review and reinterpretation of biological and genetic data on the roles of specific amino acids in the CMV capsid protein. While the following discussion attempts to interpret many of these results in the context of the assembled virion, it is also possible that some of these mutations affect the function of the individual subunit during host infection. Of particular interest is a conserved proline residue at position 129. CMV variants altered at this position have been identified because they induce a striking chlorosis in infected plants, presumably due to a profound disruption of chloroplast function (24, 25, 28). The proline residue at position 129 is also required for transmission by two species of aphid vectors (19, 20) and for efficient cell-to-cell and systemic movement in squash (31). The structural reasons for the importance of proline at this position are unclear. P129 lies in the loop between βE and αEF and is not directly involved in any interface interactions. While this residue does not lie on the outermost portion of the capsid, it is exposed to solvent and may be accessible to host or vector interactions. However, the βE and αEF loop that immediately follows P129 is more elaborate than its CCMV counterpart and protrudes toward the quasi-threefold axis. The importance of this proline, therefore, may be to position αEF in the proper orientation for subunit interactions. It may also affect the acid-base interactions discussed above that are mediated by the proximal lysine at position 127.
Mutation of alanine at position 162 has also been shown to profoundly affect aphid transmission. Two independently isolated A162T mutants in different wild-type backgrounds have been shown to have a significant effect on virion stability as measured in vitro (J. Ng and K. Perry, unpublished results). The effects of this mutation on aphid transmission may be due to a loss in capsid stability. A162 points toward the loop connecting the unusual N-terminal helix and βB. Perhaps more importantly, A162 lies within the loop that has a different structure at the icosahedral threefold and fivefold axes (Fig. 5 and 8). Therefore, mutations in this loop may affect the plasticity of the capsid protein and its ability to fulfill the needs of quasi-equivalence.
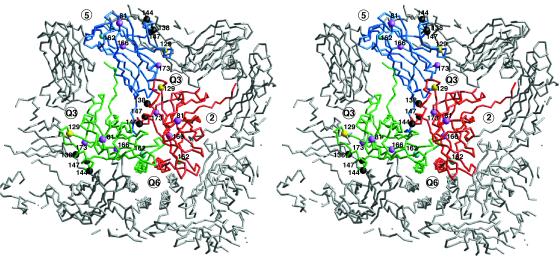
FIG. 8.
Locations of some of the mutations that affect aphid transmission and movement within the plant. The C-α backbones of one of the A, B, and C subunits are shown in blue, green, and red, respectively. The surrounding icosahedrally related subunits are shown in gray. The nearest five- and threefold (quasi-sixfold [Q6]) axes are labeled. The positions of the various mutation sites are represented by colored spheres. P129, represented by yellow balls, appears to be involved in aphid transmission and host symptomalogy. S129F can be compensated for by mutations that lie on αEF (residues 138, 144, and 147), represented by black balls. Mutations at A162 (cyan balls) affect aphid transmission, perhaps by decreasing capsid stability. The destabilizing effects of deleting residues 15 to 40 can be partially circumvented by the mutations at residues 81, 166, and 173, denoted by mauve balls.
Changes in amino acid positions 129 and 162 are paradoxical in that they appear to offer a selective advantage in populations of the virus passaged mechanically in the absence of an aphid vector. One can infer that these changes enhance the competitive ability of the variants, either in replication or some aspect of cell-to-cell or systemic movement (Ng and Perry, unpublished results). Both of these mutations lie in major insertions relative to the sequence of CCMV, and both regions of insertion differ structurally in the A versus the B and C subunits. These mutations, therefore, may affect capsid assembly and/or stability.
A number of mutations have been made in the N-terminal region that yield some insight as to the roles of the basic region in the first 30 residues and of the unusual helix at residues 31 to 47. Deleting the first 12 amino acids does not disrupt virion formation (23). This was also true for mutants with a deletion of the four consecutive Arg residues at positions 14 to 17 or with the replacement of the two Arg residues at positions 19 and 20 with Ala. These results suggest that a redundancy of basic residues in this disordered region is required but that the exact locations of these residues are not conserved. These conclusions are consistent with the proposal that these residues serve to neutralize the RNA charge via nonspecific interactions.
The virus does not, however, tolerate the deletion of amino acid positions 2 to 20, 14 to 20, or 15 to 40 (6, 23). The deletion of amino acids 2 to 20 removes most of the basic residues in the disordered N terminus. This finding suggests that there are limits to the number of basic residues that can be removed in this region. The deletions of amino acids 14 to 20 and 15 to 40 not only remove most of the basic residues but also more than half of the N-terminal helix that extends into the viral core. The deletion of amino acids 26 to 40 leaves all of the N-terminal basic residues intact but removes more than half of the N-terminal helix. When residues 26 to 40 were deleted, virions could still be recovered, but when residues 15 to 40 were deleted, the accumulation and stability of virions were affected (9). These results suggest that the unusual N-terminal helix, in addition to the basic residues at the N terminus, has a stabilizing role in the capsid.
Remarkably, a suppressor of the mutant with positions 15 to 40 deleted was recovered in which two sizes of virions with altered stabilities and levels of accumulation were observed (9). The boundaries of the original deletion were the same, but three additional amino acid changes had been selected: D81E, L166V, and M173R (9). It seems likely that this deletion destabilizes the virion by removing more than half of the N-terminal α-helix and that the compensatory mutations act by stabilizing the capsid. M173 is immediately adjacent to E98 on the quasi-threefold related subunit. The M173R mutation might be expected to stabilize the capsid by increasing the coulombic interactions shown in Fig. 6. Similarly, L166 lies at the interface between subunits about the quasi-sixfold axis. This environment is hydrophobic, with P56 and I53 lying at the adjacent subunit. While it is not clear why mutating L166 to a valine might stabilize the capsid, it is interesting that this residue makes significant subunit contact only about the icosahedral threefold axes (above the quasi-sixfold helices) and not the fivefold axes. The final compensatory mutation, D81E, is harder to explain. It lies near the βH-βI external loop that is involved in aphid transmission (Fig. 8) and is not involved in any subunit interactions. It may be that this mutation enhances the metal binding environment, since it seems coincidental that this is a mutation to an acidic residue at the only acidic patch on the virion surface.
Compensatory mutants were also recovered when position 129 was mutated. A S129F mutation eliminated systemic movement in tobacco, but three compensatory mutants could be recovered, those with an A138D, A144E, or A147S mutation (28). Again, while the exact reasons why these mutations abrogate the effect of the mutation at position 129 are unclear, it is interesting to note the locations of these mutations. As discussed above, position 129 lies on the loop preceding the αEF helix, and the position of this helix in the A subunit differs from its positions in the quasi-equivalent B and C subunits. In each case, mutations in position 129 are compensated for by increasing the hydrophilicity of αEF. It is not clear how this could compensate for the loss in systemic movement caused by the mutations at position 129. However, it is again coincidental that these mutations cluster about one of the three sites (the αEF helix, the βH-βI loop, and the βF-βG loop) that apparently play an important role in a wide variety of viral processes.
The availability of this structure will now allow us to further examine how specific regions of the capsid protein affect virus-host interactions and vector transmission. For example, it will be interesting to swap the N-terminal domains of CCMV and CMV to see the roles of these regions in viral stability, transmission, and particle size. One could also interchange the CCMV and CMV subunit interactions at the quasi-threefold axis to try to either add or subtract the metal dependency. Finally, armed with the structural details of the βH-βI loop, we can start to probe the fine details of the interactions between virions and the aphid vector.
ACKNOWLEDGMENTS
We thank V. Reddy and J. Johnson for their advice and support and V. Reddy for preparing the rendered capsid shown in Fig. 1.
This work was supported by National Institutes of Health grants GM10704 and AI45976 to T.J.S. and by USDA NRICGP grant 199902511 to K.L.P.
REFERENCES
- 1.Abad-Zapatero C, Abdel-Meguid S S, Johnson J E, Leslie A G W, Rayment I, Rossmann M G, Suck D, Tsukihara T. Structure of southern bean mosaic virus at 2.8Å resolution. Nature (London) 1980;286:33–39. doi: 10.1038/286033a0. [DOI] [PubMed] [Google Scholar]
- 2.Bailey S. The CCP4 suite: programs for protein crystallography. Acta Crystallogr Sect D. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 3.Bancroft J B, Hills G J, Markham R. A study of the self-assembly process in a small spherical virus. Formation of organized structures from protein subunits in vitro. Virology. 1967;31:354–379. doi: 10.1016/0042-6822(67)90180-8. [DOI] [PubMed] [Google Scholar]
- 4.Collaborative Computational Project n. The CCP4 suite: programs for protein crystallography. Acta Crystallogr Sect D. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 5.Gray S M. Plant virus proteins involved in natural vector transmission. Trends Microbiol. 1996;4:259–264. doi: 10.1016/0966-842X(96)10040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrison S C, Olson A J, Schutt C E, Winkler F K, Bricogne G. Tomato bushy stunt virus at 2.9Å resolution. Nature (London) 1978;276:368–373. doi: 10.1038/276368a0. [DOI] [PubMed] [Google Scholar]
- 7.He X, Liu S, Perry K L. Identification of epitopes in cucumber mosaic virus using a phage-displayed random peptide library. J Gen Virol. 1998;79:3145–3153. doi: 10.1099/0022-1317-79-12-3145. [DOI] [PubMed] [Google Scholar]
- 8.Jones T A, Zou J-Y, Cowan S W. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr Sect A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan I B, Zhang L, Palukaitis P. Characterization of cucumber mosaic virus, V: cell-to-cell movement requires capsid protein, but not virions. Virology. 1998;246:221–231. doi: 10.1006/viro.1998.9192. [DOI] [PubMed] [Google Scholar]
- 10.Kleywegt G J, Jones T A. Halloween…masks and bones. In: Bailey S, Hubbard R, Waller D, editors. From first map to final model. Daresbury, United Kingdom: SERC Daresbury Laboratory; 1994. pp. 59–66. [Google Scholar]
- 11.Kleywegt G J, Jones T A. Software for handling macromolecular envelopes. Acta Crystallogr Sect D. 1999;55:941–944. doi: 10.1107/s0907444999001031. [DOI] [PubMed] [Google Scholar]
- 12.Kneller D G, Cohen F E, Langridge R. Improvements in protein secondary structure prediction by an enhanced neural network. J Mol Biol. 1990;214:171–182. doi: 10.1016/0022-2836(90)90154-E. [DOI] [PubMed] [Google Scholar]
- 13.Laskowski R A, MacArthur M W, Moss D S, Thornton J M. PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr. 1993;26:283–291. [Google Scholar]
- 14.Lot H, Marrou J, Quiot J B, Esvan C. Contribution à l'étude de virus de la mosaïque du concombre (CMV). II. Méthode de purification rapide du virus. Ann Phytopathol. 1972;4:25–38. [Google Scholar]
- 15.Nicholls A. GRASP: graphical representation and analysis of surface properties. New York, N.Y: Columbia University; 1993. [Google Scholar]
- 16.Otwinowski Z. DENZO. In: Sawyer L, Isaacs N, Bailey S, editors. Data collection and processing. Warrington, United Kingdom: SERC Daresbury Laboratory; 1993. pp. 56–62. [Google Scholar]
- 17.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 18.Palukaitis P, Roossinck M J, Dietzgen R G, Francki R I B. Cucumber mosaic virus. Adv Virus Res. 1992;41:281–348. doi: 10.1016/s0065-3527(08)60039-1. [DOI] [PubMed] [Google Scholar]
- 19.Perry K L, Zhang L, Palukaitis P. Amino acid changes in the coat protein of cucumber mosaic virus differentially affect transmission by the aphids Myzus persicae and Aphis gossypii. Virology. 1998;242:204–210. doi: 10.1006/viro.1998.8991. [DOI] [PubMed] [Google Scholar]
- 20.Perry K L, Zhang L, Shintaku M H, Palukaitis P. Mapping determinants in cucumber mosaic virus for transmission by Aphis gossypii. Virology. 1994;205:591–595. doi: 10.1006/viro.1994.1686. [DOI] [PubMed] [Google Scholar]
- 21.Pirone T P. Viral genes and gene products that determine insect transmissibility. Semin Virol. 1991;2:81–87. [Google Scholar]
- 22.Porta C, Devergne J C, Cardin L, Briand J P, Van Regenmortel M H V. Serotype specificity of monoclonal antibodies to cucumber mosaic virus. Arch Virol. 1989;104:271–285. doi: 10.1007/BF01315549. [DOI] [PubMed] [Google Scholar]
- 23.Schmitz I, Rao A L N. Deletions in the conserved amino-terminal basic arm of cucumber mosaic virus coat protein disrupt virion assembly but do not abolish infectivity and cell-to-cell movement. Virology. 1998;248:323–331. doi: 10.1006/viro.1998.9257. [DOI] [PubMed] [Google Scholar]
- 24.Shintaku M. Coat protein gene sequences of two cucumber mosaic virus strains reveal a single amino acid change correlating with chlorosis induction. J Gen Virol. 1991;72:2587–2589. doi: 10.1099/0022-1317-72-10-2587. [DOI] [PubMed] [Google Scholar]
- 25.Shintaku M H, Zhang L, Palukaitis P. A single amino acid substitution in the coat protein of cucumber mosaic virus induces chlorosis in tobacco. Plant Cell. 1992;4:751–757. doi: 10.1105/tpc.4.7.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith T J. MolView: a program to analyze and display atomic structures on the Macintosh personal computer. J Mol Graphics. 1995;13:122–125. doi: 10.1016/0263-7855(94)00019-o. [DOI] [PubMed] [Google Scholar]
- 27.Speir J A, Munshi S, Wang G, Baker T S, Johnson J E. Structures of the native and swollen forms of cowpea chlorotic mottle virus determined by X-ray crystallography and cryo-electron microscopy. Structure. 1995;3:63–78. doi: 10.1016/s0969-2126(01)00135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki M, Kuwata S, Masuta C, Takanami Y. Point mutations in the coat protein of cucumber mosaic virus affect symptom expression and virion accumulation. J Gen Virol. 1995;76:1791–1799. doi: 10.1099/0022-1317-76-7-1791. [DOI] [PubMed] [Google Scholar]
- 29.Wahyuni W S, Dietzgen R G, Hanada K, Francki R I B. Serological and biological variation between and within subgroup I and II strains of cucumber mosaic virus. Plant Pathol. 1991;41:282–297. [Google Scholar]
- 30.Wikoff W R, Tsai C J, Wang G, Baker T S, Johnson J E. The structure of cucumber mosaic virus—cryoelectron microscopy, X-ray crystallography and sequence analysis. Virology. 1997;232:91–97. doi: 10.1006/viro.1997.8543. [DOI] [PubMed] [Google Scholar]
- 31.Wong S, Thio S S, Shintaku M H, Palukaitis P. The rate of cell-to-cell movement in squash of cucumber mosaic virus is affected by sequences of the capsid protein. Mol Plant-Microbe Interact. 1999;12:628–632. [Google Scholar]