Abstract
Background.
Recurrent coarctation (re-CoA) after stage I palliation in hypoplastic left heart syndrome (HLHS) is deleterious. We studied whether re-CoA had an effect on ventricular systolic function.
Methods.
Retrospectively reviewed were HLHS patients surviving stage I Norwood palliation (stage I) and cavopulmonary shunt (CPS) between January 2004 and February 2007. Echocardiographic right ventricular fractional area change (RV-FAC) was used to evaluate ventricular systolic function after stage I, before CPS, and before Fontan procedures. Cardiac catheterization and magnetic resonance imaging data before CPS were reviewed to assess re-CoA, using a coarctation index (CI_isthmusdiameter/descending aortic diameter).
Results.
Fifty-one patients were included, and 21 had a CI of less than 0.75 (mean, 0.82 ± 0.19; 21). Twelve patients required arch balloon dilation between CPS and Fontan. The change of RV-FAC for all patients between stage I and CPS was −2.2% ± 9.6%. Pearson correlation coefficient demonstrated a significant correlation between lower CI values and lower RV-FAC at the pre-CPS echocardiogram (r =.35, p = 0.03); and lower CI values and greater decrease in RV-FAC between stage I and pre-CPS evaluation (r = 0.40, p = 0.018). At follow-up pre-Fontan, RV-FAC for patients who underwent balloon dilation for re-CoA recovered to a level that was inferior but not significantly different from that of patients who did not need balloon dilation.
Conclusions.
Recurrent aortic arch obstruction after stage I for HLHS is associated with worse RV systolic function at the time of stage II operation. Timely intervention on the re-CoA results in recovery of RV function.
Introduction
The occurrence of a recurrent coarctation of the distal aortic arch (re-CoA) remains an important problem in patients with hypoplastic left heart syndrome (HLHS) who have undergone a successful stage I Norwood palliation [1, 2]. The reported incidence of re-CoA ranges from 5% to 37% [3–7], depending on the definition of re-CoA. Several studies have shown that the incidence of re-CoA can be influenced by surgical technique, patch materials, and baseline anatomy [1, 8–10]. After stage I, the reconstructed aortic arch exhibits increased wall stiffness and decreased distensibility. When added to a re-CoA, this decrease in aortic compliance results in increased afterload, and the energy cost of cardiac ejection augments further [11]. Furthermore, children with HLHS often have impaired systolic ventricular function, and even mild degrees of obstruction can be clinically significant [2]. As a consequence, re-CoA may be deleterious for right ventricular systolic function, influencing interstage survival and the suitability for a future cavopulmonary shunt (CPS) palliation. The aim of this study was to evaluate the consequences of re-CoA after stage I palliation for HLHS on ventricular systolic function.
Material and Methods
Study Patients and Design
This was a retrospective cohort study of 54 consecutive patients with HLHS who survived stage I and CPS operations from January 2004 to February 2007 at Children’s Hospital Boston. Variables collected for analysis included patient characteristics (age and weight at operation, noncardiac anomalies, anatomic subtype), procedural characteristics (cardiopulmonary bypass times, shunt type), and postoperative characteristics (reinterventions, length of stay, in-hospital mortality). Data were collected from the discharge and pre-CPS echocardiograms (as well as preintervention echo, for the re-CoA patients) to evaluate right ventricular systolic function. Catheterization and magnetic resonance imaging (MRI) studies were used to measure a coarctation index (CI) and for ventricular systolic function parameters. The study was approved by the Children’s Hospital Boston Institutional Review Board. Because of the retrospective nature of the study, the need for informed consent was waived.
Evaluation of Recoarctation
The CI was used to define recoarctation. It was calculated as the ratio of the distal neoaortic anastomosis to the descending aorta, as previously described [3, 12].
Evaluation of Right Ventricular Fractional Area Change
Right ventricular fractional area change (RV-FAC) was calculated from the end-diastolic area (EDA) and end-systolic areas (ESA) obtained from apical imaging planes, according to the formula: RV-FAC (EDA - ESA)/EDA [13, 14]. The RV-FAC was measured at discharge after stage I (stage I RV-FAC), at pre-CPS (pre-CPS RV-FAC), and at either before Fontan or the latest post-CPS echocardiographic study available. The differences (Δ) between the stage I and pre-CPS (ΔS1-S2 RV-FAC) and between pre-CPS and pre-Fontan (ΔS2-S3 RV-FAC) measurements were calculated.
Data Analysis
Data were summarized as number (percentage) for categoric variables and either mean ± standard deviation or median (range) for continuous variables. The relationships between CI and patient characteristics, procedural factors, and aortic arch diameter measurements were evaluated using linear regression analysis. Relationships between CI and measures of RV systolic function were assessed using the Pearson correlation coefficient. Measures of RV systolic function for 2 observers were compared for a random sample of half the echocardiograms; the mean difference was calculated along with a 95% confidence interval. Statistical significance was set at p < 0.05.
Results
We identified 54 patients, of whom 51 had pre-CPS aortic arch imaging available from either catheterization or MRI. The 3 remaining patients did not have aortic imaging because no gradient was identified at catheterization or MRI, and thus a CI could not be calculated; these patients are not included in subsequent analyses. At stage I, the median age was 5 days (range, 2 to 13 days), and the median weight was 3.1 kg (range, 1.4 to 4.5 kg; Table 1). The median length of stay was 18 days (range, 2 to 97). At CPS, the median age was 148 days (range, 74 to 232). Twelve patients required arch balloon dilation between stage I and CPS.
Table 1.
Patient and Procedure Characteristics
| Variable | No. or Median | % or Range |
|---|---|---|
|
| ||
| Patients | 51 | |
| Age at stage I, days | 5 | 2–13 |
| Weight, kg | 3.1 | 1.4–4.5 |
| Weight < 2500 g | 6 | 12 |
| Time from stage 1 to CPS, mon | 5 | 3–12 |
| Cardiopulmonary bypass, min | 146 | 100–251 |
| Cross-clamp, min | 63 | 0–110 |
| Circulatory arrest, min | 43 | 0–69 |
CPS = cavopulmonary shunt.
Ventricular Systolic Function Findings and CI
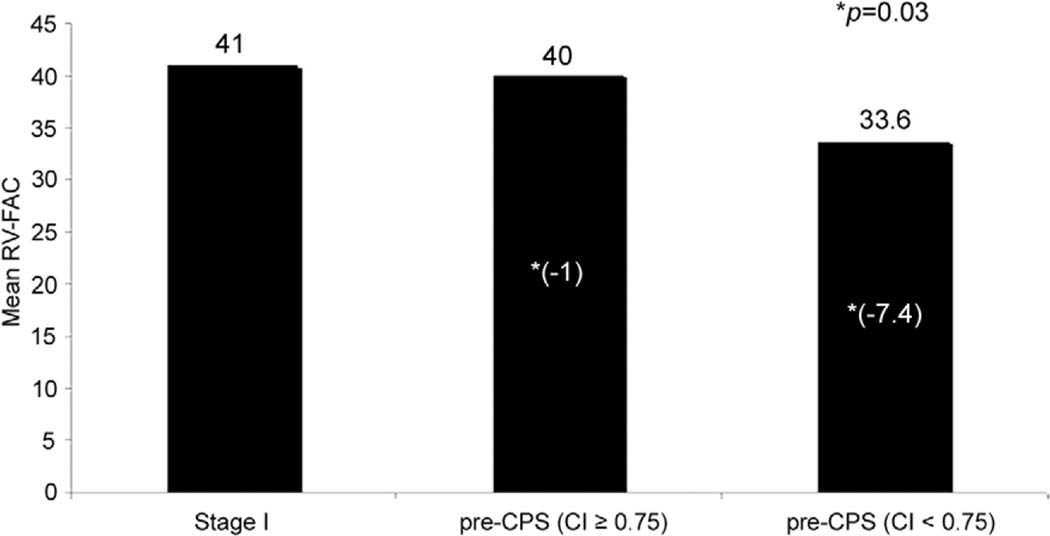
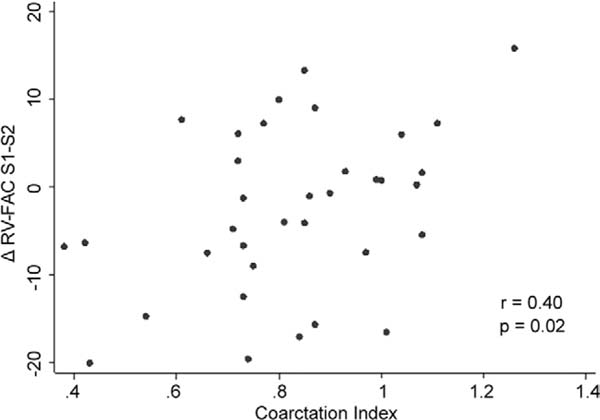
The CI was less than 0.75 in 21 patients. Table 2 presents the RV-FAC values for the stage I, pre-CPS, and pre- Fontan periods. Overall, the RV-FAC declined slightly between the stage I and pre-CPS periods (mean ΔS1-S2 RV-FAC: −2.2% ± 9.6%). As presented in Figure 1, patients with a lower CI of less than 0.75 had a significantly lower RV-FAC at pre-CPS than the patients with CI of 0.75 or more (p = 0.03, two-sample t test). Pearson correlation coefficient demonstrated a significant correlation between lower CI values and lower RV-FAC at the pre-CPS echocardiogram (r = 0.35, p = 0.03). A lower CI also correlated with a greater decrease in RV-FAC between stage I and pre-CPS evaluation (ΔS1-S2 RV-FAC; r = 0.40, p = 0.02; Fig 2 and Table 3).
Table 2.
Right Ventricular Fractional Area Change (%)
| Fractional Area Change (%) | ||
|---|---|---|
|
|
||
| Stage | Mean (SD) | Median (range) |
|
| ||
| After stage I | 40.6 (9.1) | 39.9 (20.5 to 58.2) |
| Before CPS | 37.7 (9.1) | 35.5 (17.6 to 54.3) |
| Before Fontan | 43.8 (9.6) | 45.3 (19.4 to 60.0) |
| Difference stage I to pre-CPS | −2.2 (9.6) | −1.2 (−20.1 to 15.8) |
| Difference pre-CPS to pre-Fontan | 6.8 (7.6) | 7.52 (−7.5 to 18.9) |
CPS = cavopulmonary shunt; SD = standard deviation.
Fig 1.
Mean change in right ventricular fractional area change (RV-FAC) for patients with recurrent coarctation was calculated using a coarctation index (CI) of 0.75 or higher as a cutoff value. Patients with a CI of less than 0.75 had a significantly lower RV-FAC at the pre-cavopulmonary shunt (CPS) evaluation than patients with a CI of 0.75 or higher. (Stage I = Norwood palliation.)
Fig 2.
The correlation is shown between coarctation index (CI) values and the difference in right ventricular function fractional area change (RV-FAC) between the post-stage I evaluation and the pre- cavopulmonary shunt (CPS) evaluation (ΔS1-S2 RV-FAC). A lower CI is associated with a greater decrease in RV function between stage I discharge and pre-CPS evaluation
Table 3.
Correlation of Coarctation Index vs Right Ventricular Fractional Area Change and Catheterization Values
| Coarctation Index vs | Pearson r | p Value |
|---|---|---|
|
| ||
| RV-FAC at stage I | −0.11 | 0.47 |
| RV-FAC pre-CPS | 0.35 | 0.03a |
| ΔS1-S2 RV-FAC | 0.40 | 0.02a |
| Cardiac index, L/min/m2 | 0.15 | 0.37 |
| Systemic venous oxygen saturation, % | 0.06 | 0.71 |
| Atrial pressure, mm Hg | 0.06 | 0.72 |
| Ventricular EDP, mm Hg | 0.25 | 0.10 |
Statistically significant (p < 0.05).
CPS = cavopulmonary shunt; EDP = end-diastolic pressure; RV- FAC = right ventricular fractional area change; ΔS1-S2 = difference between stage I and CPS.
Pre-Fontan Ventricular Systolic Function Findings
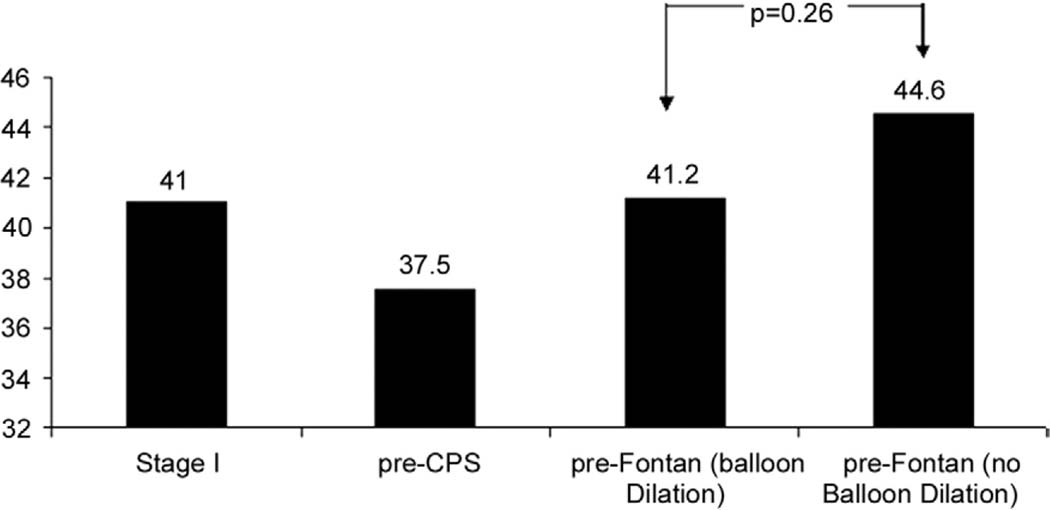
Four additional patients required arch balloon dilation between the CPS and the Fontan operations. The mean RV-FAC at the pre-Fontan evaluation was 43.8% ± 9.6%, showing a mean increase of 6.8% from the pre-CPS period (p < 0.001). For patients who underwent balloon dilation at any time before Fontan for recurrent coarctation, RV-FAC recovers to a level that is lower, but not statistically different, from that of patients who did not need balloon dilation for recurrent coarctation or did not have recurrent coarctation (Fig 3).
Fig 3.
For patients who underwent balloon dilation for recurrent coarctation, mean right ventricular-fractional area change recovers to a level at the pre-Fontan assessment that is not significantly different from that of patients who did not need balloon dilation for recurrent coarctation. (CPS = cavopulmonary shunt.)
Relationship Between the ΔS1-S2 RV-FAC and Other Patient Variables
A linear regression model demonstrated that patients with aortic atresia had a statistically greater drop in RV systolic function compared with patients without aortic atresia, with a mean difference of −7.5% (95% confidence interval, −13.6 to −1.4; p = 0.02). This is likely due to the colinear relationship between aortic atresia and the CI, where patients with aortic atresia are also more likely to have a lower CI. Because of the large amount of overlap, however, we cannot determine whether aortic atresia is an independent risk factor for lower systolic function. Other patient variables such as shunt type (modified Blalock-Taussig shunt vs right ventricle–pulmonary artery conduit), cardiopulmonary bypass, cross-clamp, and circulatory arrest times were not related to a decrease in RV-FAC.
Relationship Between CI and Other Patient Variables
A linear regression model with outcome CI did not demonstrate a relationship between a lower CI and patient demographic, anatomic or surgical characteristics.
Interobserver Variability
When we explored interobserver variability of the echocardiography measurements, RV-FAC was not different between the 2 observers (ST and DB). The mean difference in RV-FAC was 0.28% (95% confidence interval, −0.02% to 0.58%) at stage I and 0.08% (95% confidence interval, −0.19% to 0.34%) at the pre-CPS echocardiogram.
Comment
This study demonstrates that the occurrence of re-CoA during the interstage period after stage I palliation for HLHS results in a significant reduction in RV systolic function. The data also show that timely treatment of re-CoA results in an improvement of RV systolic function to a level that is lower, but not significantly different, from patients in whom re-CoA did not develop. One of the principal objectives of stage I palliation for HLHS is the reconstruction of an unobstructed aortic arch with potential for growth [5, 8, 9]. Several techniques of aortic arch reconstruction have been described, but no general agreement exists about which technique to use [3, 8, 10, 15, 16]. Interstage death, a persistent problem despite improved hospital survival, may also be causally related to the occurrence of re-CoA [7, 17]. Several studies have shown that mid- and long-term survival is affected by re-CoA, although no specific causality of this could be found [2, 5, 17]. This study links re-CoA with a decrease in the systolic RV function, with obvious consequences for short- and long-term survival [18]. It implies that surgeons should extend their utmost efforts to ensure that the distal aortic arch remains unobstructed not only at hospital discharge but also in the long-term.
Our experiences in a previous study [1] and this one have led us to recently modify our techniques of aortic arch reconstruction during stage I palliation by aggressively resecting the posterior shelf when present, minimizing the patch as much as possible, increasing the use of the interdigitating technique [3], and using autologous pericardium or a pulmonary homograft rather than an aortic homograft as patch material, when feasible [1].
This study also demonstrated that timely relief of re-CoA by means of catheter-based intervention results in some recovery of ventricular systolic function. At the time of the pre-Fontan evaluation, the median RV-FAC of the patients with treated re-CoA is lower, but not significantly different, from that of patients who did not have re-CoA. Although an increase in RV systolic function between pre-CPS and pre-Fontan should be expected given the volume unloading that occurs during CPS creation, this shows some ability by the systemic RV for reverse remodeling in response to a significant reduction in wall stress [2].
This study was not designed to study the effectiveness of balloon angioplasty for re-CoA, but it does complement the findings by Zeltser and colleagues [17], who demonstrated no difference in long-term survival between patients who did not have re-CoA and patients who had re-CoA treated with balloon angioplasty. Clearly, the long-term implications of even a short period of elevated afterload remain unclear, and this topic will require more detailed and long-term study. In particular, diastolic RV function may not recover as well as systolic function.
Besides its retrospective nature, the main limitation of this study is the use of a noninvasive method (echocardiography) to measure RV systolic function. Because of the unique shape of the RV, assessment of RV systolic function is complex and has commonly depended on a variety of indices and surrogate methods such as the evaluation of tricuspid regurgitation, RV myocardial performance indexes, and others [13, 14, 19, 20]. The method used in this study, 4-chamber fractional shortening, has been successfully used and validated as a reliable method to evaluate systolic ventricular function in HLHS patients [13]. Another possible limitation is that although the CI used in this study has been previously used and validated by others [3, 12], it only captures isthmic coarctations, and not coarctations at other levels of the aortic arch.
In conclusion, the occurrence of recurrent coarctation after stage I palliation for HLHS results in deterioration of RV systolic function. Close monitoring and prompt intervention is essential for these patients because intervention results in some recovery of ventricular systolic function.
Discussion
DR CHRISTOPHER A. CALDARONE (Toronto, Ontario, Canada): Marshall Jacobs gave a great discussion yesterday about arch reconstructions and talked about the various techniques available. Could you comment on the techniques that might have been used at the time of original stage I and how that may have related to the presence of coarctation.
DR LARRAZABAL: Yes, we looked for all the reconstruction techniques, because a couple of years ago there was another paper from our institution where Dr Bautista found that in Norwood stage I surgical cases where autologous pericardium was used, the rate of recoarctation was lower, and it was higher in the cases where homograft was used. So we tried to find if the patch material used was related to a decrease in ventricular function. We found a relationship between use of homograft and lower ventricular function measurements. This may just be a confounding factor telling us that when a homograft was used, there was a higher incidence of recoarctation, and therefore also lower measurements of right ventricular function. The relationship is not statistically significant in our series so that is why it was not shown here.
FRANK A. PIGULA (Boston, MA): Well, I think you were remarking about aortic homograft in the arch reconstruction rather than the pulmonary homograft, correct?
DR LARRAZABAL: Well, our calculation combined the aortic and pulmonary. They both have a higher rate of recoarctation and they both seem to have lower ventricular function measurements. I really don’t know the entire answer for this because I would need to know why they used the homograft instead of autologous pericardium, because maybe the anatomy was harder, maybe the arch was smaller and not suitable to use pericardium, and that is what had interfered.
DR PIGULA: Well, I think one of the other things that was instituted, again, after one of those reviews, was the resection of the coarctation. So one of the things that we have begun doing is resecting the area of coarctation entirely rather than just simply extending the aortotomy distal to that and patching it. So there are a lot of different changes that have happened. And over a certain period of time, it is very difficult to tell exactly which one is the operative factor, I think.
DR LARRAZABAL: I am sorry, I forgot to mention that, but when we looked in our series we found that the resection of the posterior shelf and the ductal tissue reduced the recoarctation, and we also found higher ventricular function measurements in those patients. So it was also correlated, but it was not presented here because our main focus was not on the reconstruction technique.
DR KOH TAKEUCHI (Tokyo, Japan): When you identified recoarctation, how can you repair? Do you repair by surgery or do the balloon dilatation? If you do the surgical repair, what kind of technique do you use, is it a patch augmentation or using the pericardium or homograft? What kind of technique do you use?
DR LARRAZABAL: You mean once the recoarctation occurs?
DR TAKEUCHI: Right.
DR LARRAZABAL: Yes, all of our patients had balloon aortic dilation through cardiac catheterization.
DR TAKEUCHI: No surgical intervention?
DR LARRAZABAL: Yes, all of them were interventional. And in all of them the intervention was successful. None of them needed surgical repair.
DR PIGULA: I’d like to say that one of the difficult issues with this sometimes is trying to determine who you are going to intervene on based on the gradient. The gradient can be very misleading with these patients, particularly if they have a BT [Blalock-Taussig] shunt. So we have become, I think, in general, more aggressive about dealing with the arch reconstructions even in the presence of a minimal gradient, and simply because of that. And some of these patients we will intervene on based on anatomic appearance of the arch as much as the physiologic or the gradient that we see. So I think we have become, in general, more aggressive about reintervening on the arches in these patients. And if it is unsuccessful in the cath lab, we would augment it at the time of surgery.
DR TAKEUCHI: So basically follow-up would be done by echocardiography, right, instead of the angio?
DR PIGULA: I’m sorry, I didn’t understand.
DR LARRAZABAL: No, they have an angio or MRI [magnetic resonance imaging] just before they have the cavopulmonary shunt, and after that that the basic follow-up is just echocardiography.
DR CALDARONE: We have kind of drifted off the message here just a little bit. And I just want to take the prerogative and ask one question, and that is, do you think that the presence of coarctation is what causes diminished ventricular function after a stage I procedure, or do you think it was some events happening at the time of the initial stage I procedure that precipitated the decreased ventricular function?
DR LARRAZABAL: No. We think that it is the presence of recoarctation. Because we explored other factors and none were significantly related to it. That is why in Table 1 you could see factors like the times for the cardiopulmonary bypass and everything. We looked for them; they were not related. We looked for complications; they were not related. Anatomical subtype; not related. It was only if the patient had a recoarctation.
And I think the finding is stronger because we correlated the size of the aortas measured as coarctation index and didn’t use just the occurrence of recoarctation as a yes or no variable. So even if the patient was not considered to have recoarctation, but there was a mismatch between the distal neoaortic anastomosis and the descending aorta, even if there was a small mismatch, which probably on the echo gradient would not prompt a cardiac intervention, we found that they still were likely to have had a lower right ventricular function measurement. So it appears we found a strong correlation that, as Dr Pigula was saying, may support a more aggressive approach in treating these patients.
DR MARSHALL L. JACOBS (Philadelphia, PA): I think the real important answer is when you intervene on these patients who have either hemodynamically important coarctation or some threshold index, dimensional difference between the distal arch and the descending thoracic aorta, do you, in fact, observe recovery of right ventricular function?
DR LARRAZABAL: Well, for some reason I suspected that Dr Marshall Jacobs was going to ask me that question, so we looked at the pre-Fontan echocardiography measurements, and we also explored that. And if I can get my backup slides. In this graph, we can see ventricular function at stage I and we can see how it decreased at the pre-cavopulmonary shunt stage. This column represents all the patients, and here of course, as we saw in Figure 1, the decrease was more significant for the patients with recoarctation. Now this is the function at pre-Fontan. And what is important here is to see that, first, the function improved after Glenn and pre-Fontan overall. This improvement was statistically significant. As you can see here, the mean increase in function from pre-Glenn to pre-Fontan was 6.8, and statistically significant. Also, the increase was no different between patients with recoarctation and the no-recoarctation patients. I am not sure why, but it may be because we are promptly treating patients with recoarctation through interventional catheterization, and perhaps doing so before the damage to the right ventricle is irreversible. So we found no difference in improvement of ventricular function for the two groups, which I think is encouraging.
DR CHRISTIAN PIZARRO (Wilmington, DE): This is great work, I wonder if you had the opportunity to look at echocardiographic indices of diastolic function, like tissue Doppler index. We presented a poster at this meeting on the follow-up of 55 patients after Fontan completion, who received either a RV-PA [right ventricle-pulmonary artery] or a Blalock-Taussig shunt, and found no difference in the overall outcome nor in ventricular function. Yet when we looked at other end points, like incidence of prolonged effusions, the only variable that came out positive in the multivariate analysis was the history of a previous coarctation that required intervention. And I wonder, having not seen a different systolic function, perhaps diastolic dysfunction is the problem and we are just not able to pick it up. Maybe you have that data.
DR LARRAZABAL: I don’t have the data. I wish I did. Because it is one of the arguments that we have been entertaining: what happens with these ventricles? And that is the reason we look for the pre-Fontan echo, and we found that function returns to normal. But then, what is the reason for that? Is it just that because the ventricle had to confront this obstruction it gets hypertrophied, and once the obstruction is released then the ventricle just seems to work fine? But on the diastolic part, we don’t really know if that has a long term consequence, so we think this needs further study and probably not just at the clinical but at the molecular level.
DR JAMES S. TWEDDELL (Milwaukee, WI): We looked at this subject in a series of patients from our institution, and we found an important difference, not in the incidence of recoarctation but in aortic dimension between the two techniques of stage I, a BT shunt vs RV-PA conduit. Not surprisingly, the patients with the BT shunt had slightly larger aortic dimensions all the way through the arch, descending aorta to the diaphragm. This almost certainly is the consequence of the increased volume and pressure load on the aorta with a BT shunt in comparison to the RV to PA conduit in which the pulmonary blood comes directly from the ventricle. I was wondering if you observed this difference in anatomy between the BT shunt and the RV to PA conduit?
DR LARRAZABAL: The only finding that seemed to be somehow related was the presence of aortic atresia, but other than that, shunt type did not seem to be related with aortic size or coarctation index.
References
- 1.Bautista-Hernandez V, Marx GR, Gauvreau K, et al. Coarctectomy reduces neoaortic arch obstruction in Hypoplastic left heart syndrome. J Thorac Cardiovasc Surg 2007;133: 1540–6. [DOI] [PubMed] [Google Scholar]
- 2.Tworetzky W, McElhinney DB, Burch GH, Teitel DF, Moore P. Balloon arterioplasty of recurrent coarctation after the modified Norwood procedure in infants. Catheter Cardiovasc Interv 2000;50:54–8. [DOI] [PubMed] [Google Scholar]
- 3.Burkhart HM, Ashburn DA, Konstantinov IE, et al. Interdigitating arch reconstruction eliminates recurrent coarctation after the Norwood procedure. J Thorac Cardiovasc Surg 2005;130:61–5. [DOI] [PubMed] [Google Scholar]
- 4.Chessa M, Dindar A, Vettukattil JJ, et al. Balloon angioplasty in infants with aortic obstruction after the modified stage I Norwood procedure. Am Heart J 2000;140:227–31. [DOI] [PubMed] [Google Scholar]
- 5.Fraisse A, Colan SD, Jonas RA, Gauvreau K, Geva T. Accuracy of echocardiography for detection of aortic arch obstruction after stage I Norwood procedure. Am Heart J 1998;135:230–6. [DOI] [PubMed] [Google Scholar]
- 6.Siewers RD, Ettedgui J, Pahl E, Tallman T, del Nido PJ. Coarctation and hypoplasia of the aortic arch: will the arch grow? Ann Thorac Surg 1991;52:608–13; discussion 13–4. [DOI] [PubMed] [Google Scholar]
- 7.Zellers TM. Balloon angioplasty for recurrent coarctation of the aorta in patients following staged palliation for Hypoplastic left heart syndrome. Am J Cardiol 1999;84:231–3, A9. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs ML. Aortic reconstruction in hypoplastic left heart syndrome-A reappraisal. J Thorac Cardiovasc Surg 2000;120: 872–4. [DOI] [PubMed] [Google Scholar]
- 9.Larrazabal LA, Pigula F, Del Nido PJ, et al. Measurement of technical performance in congenital heart surgery: the stage I Norwood procedure. J Thorac Cardiovasc Surg 2008; (in press) [DOI] [PubMed] [Google Scholar]
- 10.Poirier NC, Drummond-Webb JJ, Hisamochi K, Imamura M, Harrison AM, Mee RB. Modified Norwood procedure with a high-flow cardiopulmonary bypass strategy results in low mortality without late arch obstruction. J Thorac Cardiovasc Surg 2000;120:875–84. [DOI] [PubMed] [Google Scholar]
- 11.Cardis BM, Fyfe DA, Mahle WT. Elastic properties of the reconstructed aorta in hypoplastic left heart syndrome. Ann Thorac Surg 2006;81:988–91. [DOI] [PubMed] [Google Scholar]
- 12.Lemler MS, Zellers TM, Harris KA, Ramaciotti C. Coarctation index: identification of recurrent coarctation in infants with hypoplastic left heart syndrome after the Norwood procedure. Am J Cardiol 2000;86:697–9, A9. [DOI] [PubMed] [Google Scholar]
- 13.Anavekar NS, Gerson D, Skali H, Kwong RY, Yucel EK, Solomon SD. Two-dimensional assessment of right ventricular function: an echocardiographic-MRI correlative study. Echocardiography 2007;24:452–6. [DOI] [PubMed] [Google Scholar]
- 14.Jiang L, Levine RA, Weyman AE. Echocardiographic assessment of right ventricular volume and function. Echocardiography 1997;14:189–206. [DOI] [PubMed] [Google Scholar]
- 15.Gargiulo G, Napoleone CP, Solinas M, Frascaroli G, Pierangeli A. A new patch for the Norwood procedure. Ann Thorac Surg 1999;68:1873–4. [DOI] [PubMed] [Google Scholar]
- 16.Norwood WI Jr. Hypoplastic left heart syndrome. Ann Thorac Surg 1991;52:688–95. [DOI] [PubMed] [Google Scholar]
- 17.Zeltser I, Menteer J, Gaynor JW, Spray TL, Clark BJ, Kreutzer J, Rome JJ. Impact of re-coarctation following the Norwood operation on survival in the balloon angioplasty era. J Am Coll Cardiol 2005;45:1844–8. [DOI] [PubMed] [Google Scholar]
- 18.Hosein RB, Clarke AJ, McGuirk SP, et al. Factors influencing early and late outcome following the Fontan procedure in the current era. The ‘Two Commandments’? Eur J Cardiothorac Surg 2007;31:344–52; discussion 53. [DOI] [PubMed] [Google Scholar]
- 19.Helbing WA, Bosch HG, Maliepaard C, et al. Comparison of echocardiographic methods with magnetic resonance imaging for assessment of right ventricular function in children. Am J Cardiol 1995;76:589–94. [DOI] [PubMed] [Google Scholar]
- 20.Ishii M, Eto G, Tei C, et al. Quantitation of the global right ventricular function in children with normal heart and congenital heart disease: a right ventricular myocardial performance index. Pediatr Cardiol 2000;21:416–21. [DOI] [PubMed] [Google Scholar]