Abstract
Purpose
Laparoscopic pancreaticoduodenectomy (LPD) is a highly challenging procedure, which prevents its widespread adoption despite its advantages of being a minimally invasive procedure. This study analyzed the learning curve for LPD based on a single surgeon’s experience.
Methods
We retrospectively analyzed the medical records of 111 consecutive patients who underwent LPD by a single surgeon between March 2014 and October 2022. The learning curve was assessed using cumulative summation (CUSUM) and risk-adjusted CUSUM (RA-CUSUM) methods. Surgical failure was defined as conversion to an open procedure or the occurrence of severe complications (Clavien-Dindo grade ≥III). Based on the learning curve analysis, we divided the learning curve into the early and late phases and compared the operative outcomes in each phase.
Results
Based on the CUSUM analysis, the operation time decreased after the first 33 cases. Based on the RA-CUSUM analysis, the LPD technique stabilized after the 44th case. In the late phase, operation time, length of stay, and incidence of delayed gastric emptying, severe complications, and surgical failure were significantly lower than in the early phase.
Conclusion
Our results indicate that 44 cases are required for stabilization of the LPD technique and improvement of operative outcomes.
Keywords: Laparoscopy, Learning curve, Pancreaticoduodenectomy
INTRODUCTION
In 1994, Gagner and Pomp [1] performed the first laparoscopic pancreaticoduodenectomy (LPD) procedure, whereas Cuschieri et al. [2] reported performing the first laparoscopic distal pancreatectomy in 1996. LPD has not gained widespread adoption compared to laparoscopic distal pancreatectomy, and there has been less investigation into the learning curve of the procedure, primarily owing to its technical complexity and associated challenges. The complex anatomy surrounding the pancreatic head includes critical structures such as the portal vein (PV), hepatic artery (HA), and superior mesenteric artery (SMA). Even after a successful resection phase, laparoscopic reconstruction is also technically challenging, with laparoscopic pancreaticojejunostomy (PJ) being the most challenging procedure. Furthermore, the risk of critical complications such as postoperative pancreatic fistula (POPF) and postpancreatectomy hemorrhage (PPH) exists. Consequently, the learning curve for LPD is steep and varies based on the expertise and experience of the operator and the volume at the center. Several studies reported that 30–60 cases are required to overcome the learning curve for LPD [3,4,5,6,7,8]. This study aimed to analyze the learning curve of a single surgeon for LPD using cumulative summation (CUSUM) analysis and risk-adjusted CUSUM (RA-CUSUM) analysis methods.
METHODS
Ethics statement
This study was approved by the Institutional Review Board of Chonnam National University Hospital (No. CNUH-2022-141). This study was performed in accordance with the Declaration of Helsinki and written informed consent was waived due to its retrospective nature.
Study population and data collection
We retrospectively analyzed the medical records of the patients who underwent LPD at Chonnam National University Hospital by a single surgeon between March 2014 and October 2022. Demographic, clinical, pathologic, and operative data, including age, sex, American Society of Anesthesiologists (ASA) physical status, body mass index, frailty, pathological diagnosis, tumor size, diameter of the main pancreatic duct (MPD), the texture of pancreatic parenchyma, operation time, open conversion status, estimated blood loss, transfusion, hospital stay, margin status, harvested lymph node count, and postoperative complications were collected. Frailty was assessed using the 5-factor modified frailty index proposed by the American College of Surgeons [9]. All complications were recorded according to the Clavien-Dindo classification [10]. POPF, delayed gastric emptying (DGE), and PPH were graded using the International Study Group on Pancreatic Surgery (ISGPS) grading system [11,12,13].
The learning curve was analyzed using the CUSUM and RA-CUSUM methods. Operation time was used for CUSUM analysis. The CUSUM of operation time (CUSUM-OT) was defined as the summation of the difference of time in minutes between the operation time of each case and the mean operation time of all cases. The RA-CUSUM method overcomes the heterogeneity of each case by estimating the individual patient's risk of surgical failure using a logistic regression model [14,15]. Surgical failure was defined as conversion to open surgery or the occurrence of severe postoperative complications (Clavien-Dindo grade ≥III). The factors associated with the occurrence of complications and POPF including frailty, the MPD diameter (<5 mm or not), texture of the pancreatic parenchyma (soft pancreas or not), and pathology (pancreatic ductal adenocarcinoma or not) were used for risk adjustment [9,16].
Surgical procedure
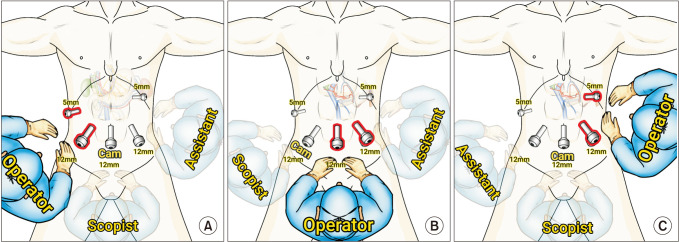
The patient was placed in the French position (supine split-leg position), with a 30° reverse Trendelenburg position placement. In the resection phase, the operator stood on the patient's right side, the first assistant stood on the patient's left side, and the scopist was positioned between the patient's legs (Fig. 1A). Five trocars were used for the procedure as depicted in Fig. 1A. First, the right gastric vessels were ligated and divided just above the pylorus. Then, the peritoneum overlying the porta hepatis was incised along the upper border of the duodenum, the common and proper HA (PHA), and the lower border of the liver. After locating the PHA, common bile duct (CBD), and gastroduodenal artery (GDA), the anterior surface of the PV was exposed between the PHA and CBD. Lymph node dissection was performed along the common HA (CHA) and PHA, and then the PHA was encircled. Subsequently, the GDA was doubly ligated with endoclips and divided. The PV was isolated and encircled, and the root of the right gastric vein, superior pancreaticoduodenal vein, and left gastric vein were identified and ligated. After gallbladder dissection from the gallbladder bed, the common hepatic duct was divided. At this step, the location of the right HA across the PV ought to be identified, and care should be taken to avoid injury to the right HA. All soft tissues around the PV, PHA, and CHA were dissected carefully. After ligation and division of right gastroepiploic vessels, the duodenum was transected 2–3 cm distal to the pylorus using an endoscopic linear stapler. The inferior border of the pancreatic neck was dissected carefully; subsequently, the anterior surface of the superior mesenteric vein (SMV) was exposed. The pancreatic neck was encircled with nylon tape. All branches of the gastrocolic trunk were ligated, and then the root of the gastrocolic trunk was ligated and divided. The hepatic flexure of the colon was mobilized sufficiently, and the Kocher maneuver was performed till the anterior surfaces of the aorta and left renal vein were exposed. Usually, the ligament of Treitz was opened during this step, and the proximal jejunum was pulled upward via the opened ligament of Treitz. The proximal jejunum was transected 10–15 cm distal to the pancreatic uncinate process with an endoscopic linear stapler. The pancreatic neck was transected using an endo-scissor that was connected to monopolar electrocautery. In the presumed area of the pancreatic duct, the pancreatic parenchyma was transected with an endo-scissor without electrocauterization. The SMV and PV were dissected completely from the surrounding tissues and pulled upward and to the patient's left side. Then, the SMA was identified. The uncinate process of the pancreas was dissected from the SMA to complete resection from the patient's caudal to cephalad aspects. The specimen was then retrieved using an endoscopic plastic bag through the extension of the umbilical port site. After the resection phase was completed, the surgeon and scopist switched places to perform a PJ (Fig. 1B). A double-layered duct-to-mucosa PJ was performed. An 8-cm internal stent was inserted during the PJ. After the PJ, the laparoscope was inserted via the umbilical port again, and the operator performed a hepaticojejunostomy using the 2 ports positioned on the left side of the patient's abdomen (Fig. 1C). After the placement of 2 drains around the PJ, the procedure was considered complete.
Fig. 1. Trocar placement and operator’s position. (A) In the resection phase, the operator stands on the patient’s right side. (B) In the pancreatico-jejunostomy (PJ) phase, the operator stands between the patient’s legs considering the direction of the instrument for PJ. (C) In the hepatico-jejunostomy phase, the operator stands on the patient’s left side.
Statistical analysis
The learning curve for LPD was analyzed using CUSUM and RA-CUSUM methods [3,4,5,7]. CUSUM-OT was defined as the summation of the difference of time in minutes between the operation time of each case and the mean operation time of all cases. The formula is as follows: where χi is the operation time of an individual case and µ is the overall mean operation time. Therefore, an upward slope in the CUSUM-OT graph implies a longer operation time than the mean operation time, and a downward slope implies a shorter operation time than the mean. RA-CUSUM was calculated as follows: where χi = 1 indicates surgical failure. In case of no surgical failure, χi = 0. The observed rate of surgical failure was represented by τ, and Pi is the expected rate of surgical failure calculated using a logistic regression model. An upward slope in the RA-CUSUM graph indicates an increasing trend of surgical failure, and a downward slope means a decreasing trend of surgical failure. The peak point in the RA-CUSUM graph was interpreted as the point of stabilization of the procedure. Normally distributed continuous variables are presented as means ± standard deviations, and non-normally distributed continuous variables are presented as medians (range). Continuous variables were compared using the independent Student t-test or Mann-Whitney U-test according to the result of the normality test. Categorical variables were compared using the chi-square test or Fisher exact test. A P-value of <0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics ver. 27.0 (IBM Corp.).
RESULTS
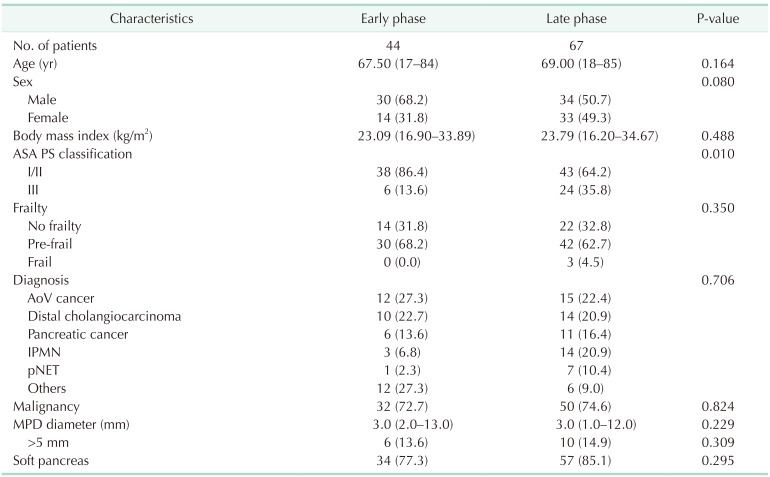
Baseline characteristics and overall perioperative outcomes
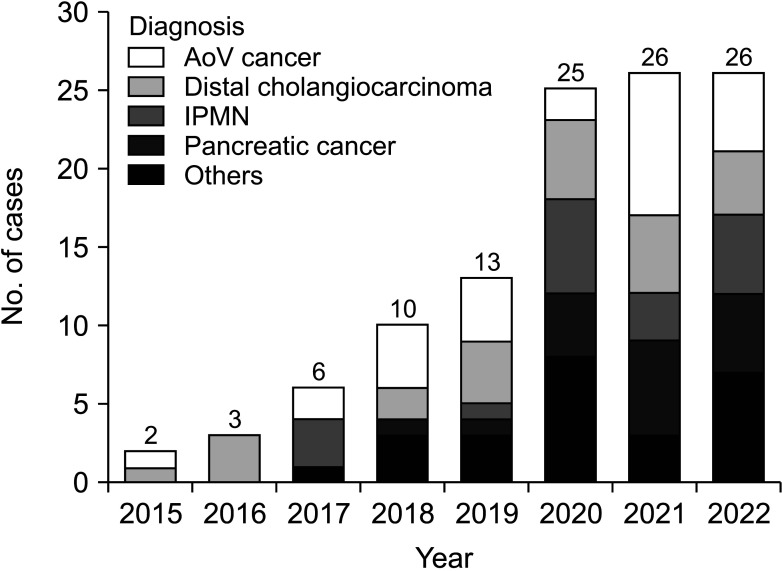
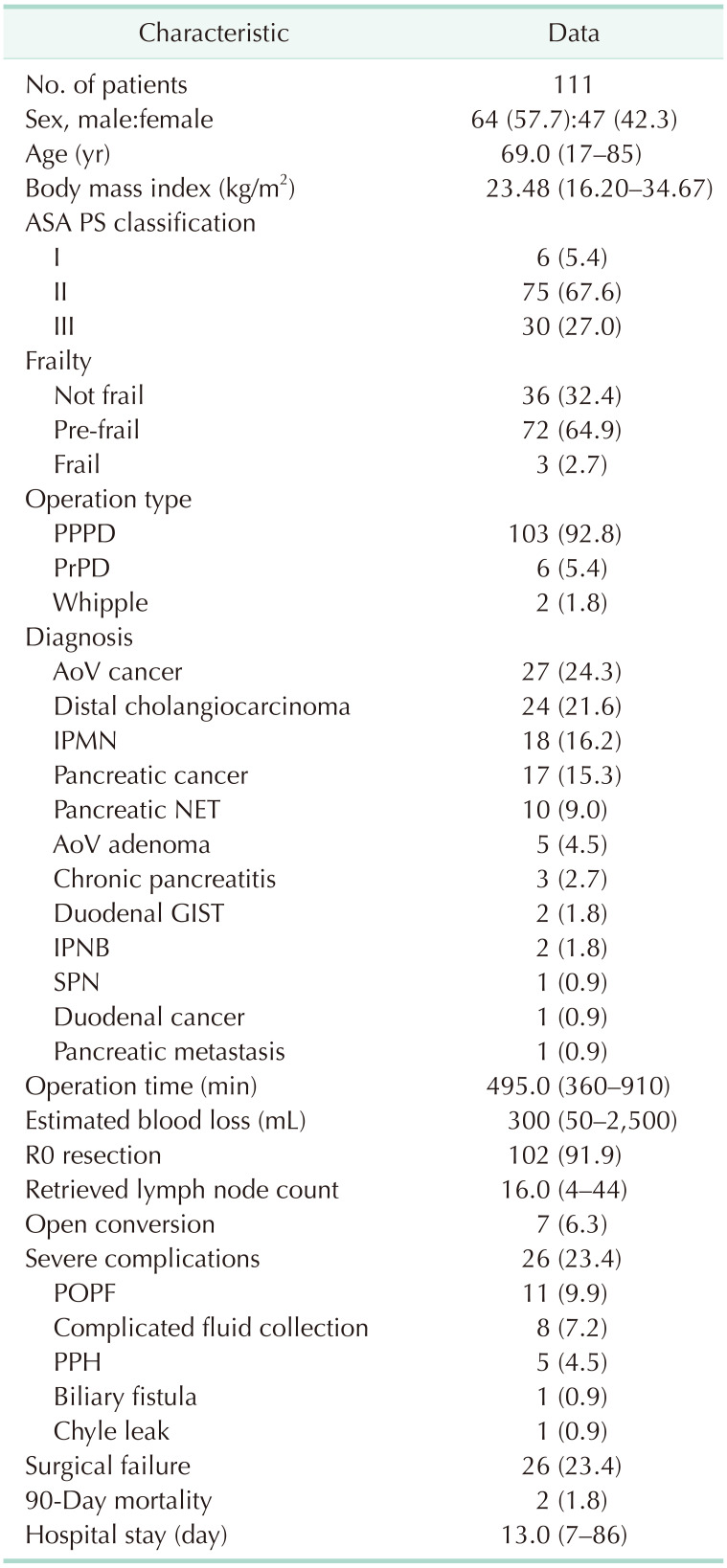
Between March 2014 and October 2022, 111 LPD procedures were performed at Chonnam National University Hospital by a single surgeon. In the early stages of LPD, patients with a history of major abdominal surgery or advanced cancer requiring combined organ resection or major vessel resection were excluded from candidates for LPD. The number of cases by year is demonstrated in Fig. 2. The baseline characteristics and overall perioperative outcomes of the 111 consecutive patients are described in Table 1. The median operation time and hospital stay were 495 minutes and 13 days, respectively. Conversion to open surgery was observed in 7 patients (6.3%). The most common cause of conversion to open surgery was bleeding (5 of 7), followed by severe adhesion around the pancreas. The overall surgical failure rate was 23.4% (26 of 111).
Fig. 2. Number of cases and indications by year. AoV, ampulla of Vater; IPMN, intraductal papillary mucinous neoplasm.
Table 1. Baseline characteristics and perioperative outcomes of the consecutive patients.
Values are presented as number only, number (%), or median (range).
ASA, American Society of Anesthesiologists; PS, physical status; PPPD, pylorus preserving pancreaticoduodenectomy; PrPD, pylorus resecting pancreaticoduodenectomy; AoV, ampulla of Vater; IPMN, intraductal papillary mucinous neoplasm; NET, neuroendocrine tumor; GIST, gastrointestinal stromal tumor; IPNB, intraductal papillary neoplasm of bile duct; SPN, solitary pseudopapillary neoplasm; POPF, postoperative pancreatic fistula; PPH, postpancreatectomy hemorrhage.
Learning curve analysis
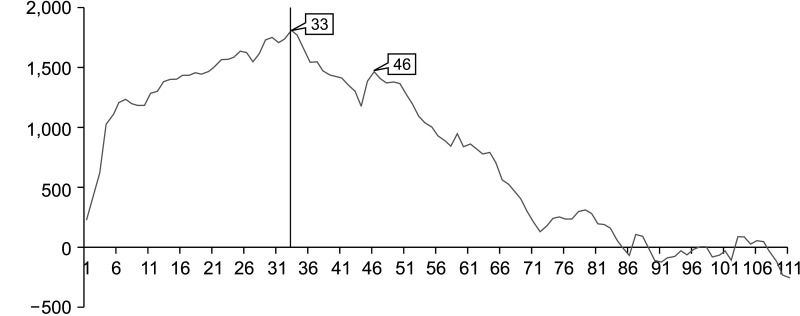
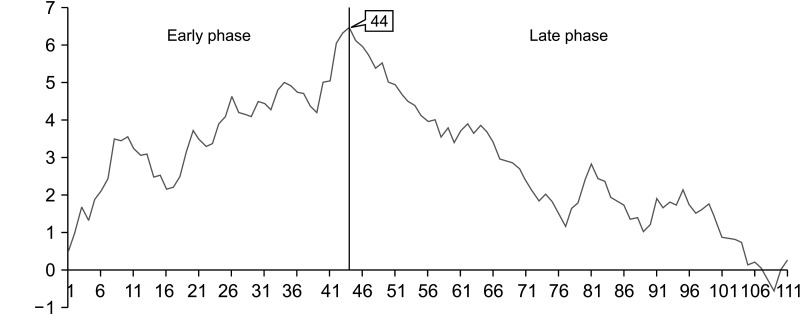
We observed the peak point on the CUSUM-OT graph at the 33rd case. A second peak point was observed at the 46th case. Subsequently, the slope of the CUSUM-OP graph trended downward (Fig. 3). On the RA-CUSUM graph, the surgical failure rate gradually increased till the 44th case, and the slope trended downward after the 44th case (Fig. 4). Therefore, the periods before and after the 44th case were classified as the early and late phases, respectively.
Fig. 3. Cumulative summation graph for the operation time. The peak point was observed at the 33rd case.
Fig. 4. Risk-adjusted cumulative summation graph for surgical failure in laparoscopic pancreaticoduodenectomy. The surgical failure rate gradually decreased after the 44th case. Therefore, the evaluation period was classified as early or late phase as before or after the 44th case, respectively.
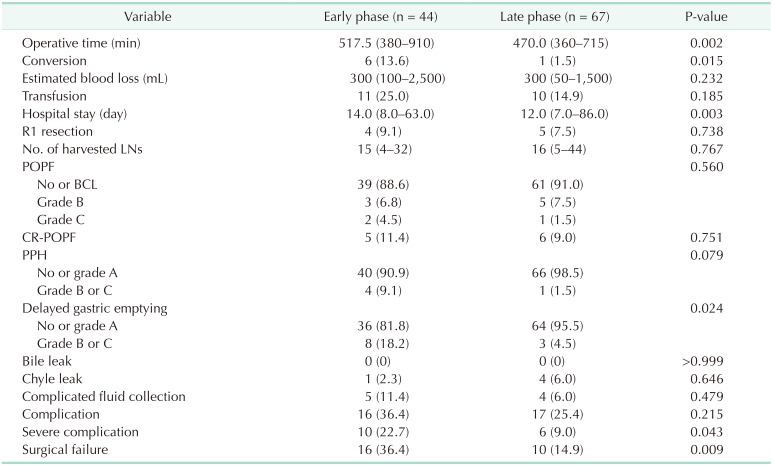
Comparison of surgical outcomes between the early and late phases
Table 2 compares the demographic and pathologic characteristics between the early and late phases. The late phase had a significantly greater number of patients with ASA class III physical status. The other parameters were comparable in both phases. Table 3 summarizes the perioperative outcomes in the early and late phases. The operation time, open conversion rate, hospital stay, rate of DGE, severe complication rate, and surgical failure rate were significantly lower in the late phase. Estimated blood loss and clinically relevant POPF rates were comparable in both phases. The PPH rate was lower in the late phase (9.1% vs. 1.5%) although not statistically significant (P = 0.079).
Table 2. Demographic and pathologic characteristics of the early and late phases defined by RA-CUSUM analysis.
Values are presented as number only, median (range), or number (%).
RA-CUSUM, risk-adjusted cumulative summation; ASA, American Society of Anesthesiologists; PS, physical status; AoV, ampulla of Vater; IPMN, intraductal papillary mucinous neoplasm; pNET, pancreatic neuroendocrine tumor; MPD, main pancreatic duct.
Table 3. Comparison of surgical outcomes between the early and late phases defined by RA-CUSUM analysis.
Values are presented as median (range) or number (%).
LN, lymph node; POPF, postoperative pancreatic fistula; BCL, biochemical leak; CR-POPF, clinically relevant POPF; PPH, postpancreatectomy hemorrhage.
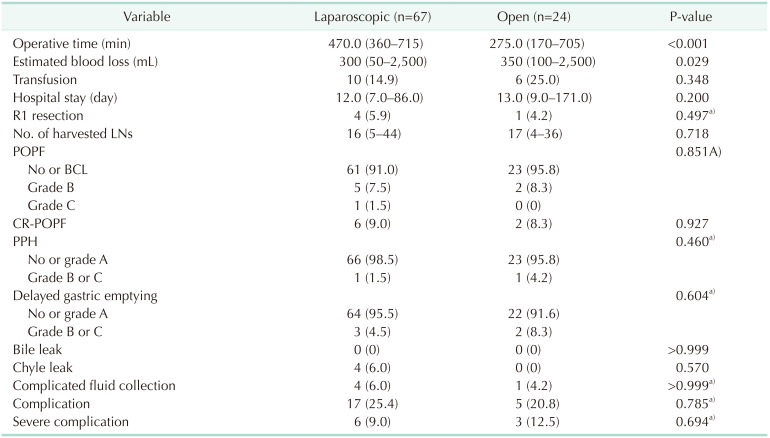
Comparison of surgical outcomes between laparoscopic and open pancreaticoduodenectomies in the late phase
Operation time was significantly shorter in the open group compared to the laparoscopic group (277.5 ± 105.0 minutes vs. 470 ± 95.0 minutes, P < 0.001). Estimated blood loss was significantly lower in the laparoscopic group. The other operative outcomes were comparable in both groups (Table 4).
Table 4. Comparison of surgical outcomes between laparoscopic and open pancreaticoduodenectomy in the late phase.
Values are presented as median (range) or number (%).
LN, lymph node; POPF, postoperative pancreatic fistula; BCL, biochemical leak; CR-POPF, clinically relevant POPF; PPH, postpancreatectomy hemorrhage.
a)Fisher exact test.
DISCUSSION
Previous studies have demonstrated the advantages of LPD over open pancreaticoduodenectomy (OPD) in terms of a shorter hospital stay, reduced blood loss, and faster recovery [17,18,19]. In addition, LPD is superior to OPD in terms of surgical education, given the surgeon and the assistant have the same view of the surgical field. However, LPD is a challenging procedure as it requires a thorough understanding of the complex anatomy around the pancreas head including major vessels such as the HA, SMA, and PV. Performing multiple complex anastomoses after the resection phase is another challenge in LPD. Specifically, the PJ is critical to LPD success. Consequently, LPD has a long and steep learning curve. The single surgeon of the present study (HJK) performed 62 cases of OPD as the primary surgeon before the first LPD and participated in approximately 150 OPD cases as the 1st assistant. Although the surgeon has no prior experience as a first assistant in LPD, the pancreatic neck tunneling technique was adopted from the experience with 56 cases of laparoscopic distal pancreatectomy before the first LPD, and the suture technique was acquired through repeated training using a laparoscopic training kit and experience with 32 cases of laparoscopic biloenteric anastomosis including choledochal cyst and biliary reconstruction for CBD stone. The Kocherization technique was learned from stomach surgeries. Recent studies that evaluated the learning curve for LPD with CUSUM and RA-CUSUM analysis reported that 30–60 cases are required to overcome the learning curve for LPD [3,4,5,6,7,8]. The present study demonstrated a similar result (44 cases) for the stabilization of LPD performance. In several studies, the RA-CUSUM graph showed 2 peak points before a continuous decreasing trend, and the researchers classified the learning curve into 3 phases [3,4,5]. However, the RA-CUSUM graph of our study showed a continuous downward slope for surgical failures after the 44th case. A study reported by Song et al. [8] demonstrated that the second-generation surgeon has a shorter and more stable learning curve. This result reflects that structured education could reduce the steep learning curve for LPD. The results of this study may have been affected by the ongoing efforts for LPD education by The Korean Study Group on Minimally Invasive Pancreatic Surgery (K-MIPS, http://kmips.or.kr). K-MIPS was founded in 2019 and has since strived to share minimally invasive pancreatic surgery experience and provide systematic education.
Operative outcomes after overcoming the learning curve in the present study were comparable with those of other studies. The median operation time after overcoming the learning curve was 408–469 minutes in other studies [3,4,5,8] compared to 470 minutes in our study. The incidences of clinically relevant POPF were 8.2%–28.3% in other studies compared to 9.0% in our study [3,4,5,6,8]. The incidences of PPH (0%–5.08% in other studies vs. 1.5% in our study), severe complications (2.73%–31.7% in other studies vs. 9.0% in our study), and open conversion (2.73%–12.9% in other studies vs. 1.5% in our study) were also similar [3,4,5,6,8]. The median postoperative hospital stay in our study was 12.0 ± 5.0 days compared to 10–21 days in other studies [3,4,5,6,8].
The limitation of this study is the relatively small number of cases compared to the duration of the study. It is anticipated that a shorter learning curve could be achieved than the results of this study if intensive experience is accumulated over a short period. However, this study is meaningful in that it presents the experience of pioneers who first attempted LPD.
In conclusion, the operation time reduced after the first 33 cases as per CUSUM-OT analysis, and the surgical failure rate decreased after the 44th case. After the 44th case, the operation time, conversion rate, postoperative hospital stays, DGE, and rate of severe complications significantly decreased. To overcome the long and steep learning curve for LPD, continued effort is required to improve the surgical outcomes for the next generation of surgeons.
Footnotes
Fund/Grant Support: None.
Conflict of Interest: Hee Joon Kim, serving as the associate editor of Annals of Surgical Treatment and Research, did not participate in the review process of this article. No other potential conflicts of interest pertinent to this article were reported.
- Conceptualization: HJK, CKC.
- Formal Analysis, Investigation, Project Administration: HJK.
- Methodology: CKC.
- Writing – Original Draft: HJK.
- Writing – Review & Editing: HJK, CKC.
References
- 1.Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc. 1994;8:408–410. doi: 10.1007/BF00642443. [DOI] [PubMed] [Google Scholar]
- 2.Cuschieri A, Jakimowicz JJ, van Spreeuwel J. Laparoscopic distal 70% pancreatectomy and splenectomy for chronic pancreatitis. Ann Surg. 1996;223:280–285. doi: 10.1097/00000658-199603000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim H, Choi HZ, Kang BM, Lee JW. Learning curve in laparoscopic pancreaticoduodenectomy: using risk-adjusted cumulative summation methods. J Laparoendosc Adv Surg Tech A. 2022;32:401–407. doi: 10.1089/lap.2021.0260. [DOI] [PubMed] [Google Scholar]
- 4.Kim S, Yoon YS, Han HS, Cho JY, Choi Y, Lee B. Evaluation of a single surgeon’s learning curve of laparoscopic pancreaticoduodenectomy: risk-adjusted cumulative summation analysis. Surg Endosc. 2021;35:2870–2878. doi: 10.1007/s00464-020-07724-z. [DOI] [PubMed] [Google Scholar]
- 5.Choi M, Hwang HK, Lee WJ, Kang CM. Total laparoscopic pancreaticoduodenectomy in patients with periampullary tumors: a learning curve analysis. Surg Endosc. 2021;35:2636–2644. doi: 10.1007/s00464-020-07684-4. [DOI] [PubMed] [Google Scholar]
- 6.Nagakawa Y, Nakamura Y, Honda G, Gotoh Y, Ohtsuka T, Ban D, et al. Learning curve and surgical factors influencing the surgical outcomes during the initial experience with laparoscopic pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci. 2018;25:498–507. doi: 10.1002/jhbp.586. [DOI] [PubMed] [Google Scholar]
- 7.Wang M, Meng L, Cai Y, Li Y, Wang X, Zhang Z, et al. Learning curve for laparoscopic pancreaticoduodenectomy: a CUSUM analysis. J Gastrointest Surg. 2016;20:924–935. doi: 10.1007/s11605-016-3105-3. [DOI] [PubMed] [Google Scholar]
- 8.Song KB, Kim SC, Lee W, Hwang DW, Lee JH, Kwon J, et al. Laparoscopic pancreaticoduodenectomy for periampullary tumors: lessons learned from 500 consecutive patients in a single center. Surg Endosc. 2020;34:1343–1352. doi: 10.1007/s00464-019-06913-9. [DOI] [PubMed] [Google Scholar]
- 9.Subramaniam S, Aalberg JJ, Soriano RP, Divino CM. New 5-factor modified frailty index using American College of Surgeons NSQIP data. J Am Coll Surg. 2018;226:173–181. doi: 10.1016/j.jamcollsurg.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017;161:584–591. doi: 10.1016/j.surg.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 12.Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2007;142:761–768. doi: 10.1016/j.surg.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142:20–25. doi: 10.1016/j.surg.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Steiner SH, Cook RJ, Farewell VT, Treasure T. Monitoring surgical performance using risk-adjusted cumulative sum charts. Biostatistics. 2000;1:441–452. doi: 10.1093/biostatistics/1.4.441. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Jiang J, Jiang X, Liu L. Risk-adjusted monitoring of surgical performance. PLoS One. 2018;13:e0200915. doi: 10.1371/journal.pone.0200915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM., Jr A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. 2013;216:1–14. doi: 10.1016/j.jamcollsurg.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Conrad C, Basso V, Passot G, Zorzi D, Li L, Chen HC, et al. Comparable long-term oncologic outcomes of laparoscopic versus open pancreaticoduodenectomy for adenocarcinoma: a propensity score weighting analysis. Surg Endosc. 2017;31:3970–3978. doi: 10.1007/s00464-017-5430-3. [DOI] [PubMed] [Google Scholar]
- 18.Croome KP, Farnell MB, Que FG, Reid-Lombardo KM, Truty MJ, Nagorney DM, et al. Total laparoscopic pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: oncologic advantages over open approaches? Ann Surg. 2014;260:633–640. doi: 10.1097/SLA.0000000000000937. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, Lan X, Peng B, Li B. Is total laparoscopic pancreaticoduodenectomy superior to open procedure?: a meta-analysis. World J Gastroenterol. 2019;25:5711–5731. doi: 10.3748/wjg.v25.i37.5711. [DOI] [PMC free article] [PubMed] [Google Scholar]