Abstract
Purpose
Peri-implant mucositis (PIM) and peri-implantitis (PI) are multicausal conditions with several risk factors contributing to their pathogenesis. In this study, we retrospectively investigated risk variables potentially associated with these peri-implant diseases (PIDs) over a follow-up period of 1 to 18 years.
Methods
The study sample consisted of 379 implants placed in 155 patients. Single-visit clinical and radiographic evaluations were employed to determine the presence or absence of PIDs. Parameters related to the patient, site, surgery, implant, and prosthetic restoration were documented. The relationships between risk variables and the occurrence of PIDs were individually examined and adjusted for confounders using multivariate binary logistic regression models.
Results
The prevalence rates of PIM and PI were 28.4% and 36.8% at the patient level and 33.5% and 24.5% at the implant level, respectively. Poor oral hygiene, active gingivitis/periodontitis, preoperative alveolar ridge deficiency, early or delayed implant placement, implant length of 11.0 mm or less, and poor restoration quality were strong and independent risk indicators for both PIDs. Furthermore, a follow-up period of more than 5 years and a loading time of more than 4 years were important indicators for PI. Simultaneously, age and smoking status acted as modifiers of the effect of mesiodistal (MD) and buccolingual (BL) widths of restoration on PI.
Conclusions
In this study population, oral hygiene, periodontal status, preoperative alveolar ridge status, implant placement protocol, implant length, and the quality of coronal restoration appear to be robust risk indicators for both PIM and PI. Additionally, the length of follow-up and functional loading time are robust indicators of PI. Furthermore, the potential modifying relationships of age and smoking status with the MD and BL widths of restoration may be crucial for the development of PI.
Keywords: Dental implants, Follow-up studies, Mucositis, Peri-implantitis, Prevalence, Risk assessment
Graphical Abstract
INTRODUCTION
Biological complications associated with dental implants, such as peri-implant mucositis (PIM) and peri-implantitis (PI), are inflammatory conditions affecting the soft tissues and/or bone surrounding these restorative devices, caused by the presence of bacteria and their byproducts [1]. PIM is characterized as an inflammatory condition limited to the peri-implant soft tissues without evidence of supporting bone breakdown following initial bone remodeling during the healing process, while PI involves both soft tissue inflammation and progressive supporting bone loss beyond normal biological osseous remodeling [2]. Although peri-implant diseases (PIDs) arise from an imbalance between bacterial populations and the host’s immunological response in the subgingival environment [3], they can be influenced by various risk factors, including demographic, behavioral, and environmental as well as local and systemic factors [2,4,5].
Epidemiological studies have reported a broad range of prevalence rates for PIDs in various populations. Global patient-level frequency estimates range from 19.0% to 82.1% for PIM [6,7,8,9,10] and from 1.0% to 47.1% for PI [7,8,10,11]. At the implant level, prevalence rates have been estimated to be between 29.4% and 85.3% for PIM and between 9.2% and 22.7% for PI [2,8,9,10,11,12,13]. The wide variation in rates across studies may reflect not only inconsistent definitions of diagnostic criteria [12], but also methodological issues regarding factors such as study design, number of cases, study populations, implant types, follow-up periods, and statistical concerns.
Many studies investigating the etiology of PID have been inconclusive, as they have only considered the influence of individual variables without examining their combined effects. To enhance the understanding of this subject and improve clinical and therapeutic approaches, numerous studies have assessed multiple variables potentially associated with PID [2,8,10,11,14,15,16]; however, the results have varied. Recognizing that a comprehensive evaluation of various risk indicators is necessary for understanding the onset of PID, this study was conducted to retrospectively identify patient-, site-, surgery-, implant-, and prosthetic restoration-related variables that may be strongly associated with PID over a follow-up period of 1 to 18 years.
MATERIALS AND METHODS
Ethical statement and study design
This retrospective follow-up study adhered to the ethical standards of the Helsinki Declaration. Ethical approval was obtained from the Research Ethics Committees of the Faculty of Dentistry (approval No. 34-2019) and the University Health Care Provider (approval No. 143-2020) at the University of Antioquia. The manuscript was prepared in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology statement guidelines for cohort, case-control, and cross-sectional studies (https://www.strobe-statement.org).
Sample selection
Based on a reported prevalence of 21% for PI at the implant level [11], a sample size calculation using an online calculator (https://www.calculator.net/sample-size-calculator.html) indicated that a minimum of 255 implants would be necessary to determine significant differences between the results with 95% confidence and an alpha error of 0.05. However, a larger number of cases were recruited to maintain an optimal level of precision. Eligible patients were those aged 18 years or older who had implants placed at crestal or subcrestal levels, with implant-supported restorations in function for at least 1 year. Exclusion criteria included pregnancy; implant-supported removable partial dentures or overdentures, supracrestal implant placement, or unrestored implants; a history of radiation therapy in the head/neck region; and incomplete clinical records.
Patient-related data collection
Patients who met the inclusion criteria were contacted and invited to participate in the study. Upon understanding the research objectives, all volunteers agreed to sign the consent form and were enrolled for a single-visit clinical and radiographic follow-up examination. The collected patient data included sex, age, smoking status, and alcohol consumption (current consumers/short-term abstainers [individuals currently consuming and those who had quit less than 5 years prior to the follow-up examination] vs. non-consumers/long-term abstainers [patients who had never smoked or consumed alcohol and those who had stopped more than 5 years before the examination]) [17,18], systemic condition (healthy patients vs. those with systemic involvement), history of periodontitis (yes vs. no), and compliance with supportive maintenance (regular compliance, with at least 2 regular visits per year vs. erratic/non-compliance, with 1 or no visits per year) [14].
Preoperative, intraoperative, and postoperative data acquisition
Preoperative site-related variables, gathered from clinical records, included implant location (anterior vs. posterior), jaw type (maxillary vs. mandibular), presence or absence of adjacent teeth, preoperative alveolar ridge status (adequate [thick tissue biotype with normal horizontal and vertical dimensions] vs. deficient [with localized horizontal, vertical, or combined defects]) [19], and minimum keratinized tissue width. Surgery-related variables encompassed information about prior or concomitant soft/hard tissue augmentation procedures (membrane barriers, bone grafts, or combined techniques vs. no augmentation procedures), intraoperative complications (yes [cortical bone perforation, implant instability, and/or bone dehiscence] vs. no), and implant placement protocol (immediate [type 1] vs. early/delayed [types 2, 3, and 4]) [20]. Implant-related variables consisted of implant type (conical vs. cylindrical), implant surface (treated vs. non-treated), collar surface (smooth vs. rough), loading time point (immediate [within 3 days after implant placement] vs. late [at least 2 months after secondary stability was confirmed by applying manual force to the implant]), as well as implant length and diameter. Variables related to prosthetic restoration involved the type of coronal restoration (definitive vs. temporary restoration), restoration method (bridge-fixed prosthesis vs. individual crown), connection type (internal vs. external), and crown retention mechanism (screw-retained vs. cement- or screw-cement-retained).
Clinical and radiographic follow-up examination
The follow-up examination was independently conducted by 2 experienced periodontists (A.M. O-E. and C. G-G), who were calibrated for clinical measurements by a single “gold-standard” clinical researcher (M.C. C-G.) prior to the study until they achieved intraclass correlation coefficient and kappa values of ≥0.9. The clinical records included the length of the follow-up period, functional loading time, and occlusal contact relationship, as well as an evaluation of the quality and the greater mesiodistal (MD) and buccolingual (BL) widths of the coronal restoration. The occlusal contact relationship was determined using 0.025 mm-thick articulating paper (Accu-film; Parkell, Farmingdale, NJ, USA) and categorized as adequate (absence of premature contacts in centric occlusion), supra-occlusion (when the highest position of the restoration exceeded the occlusal level), or infra-occlusion (when the restoration had not reached occlusion). Satisfactory restoration conditions included an adequate emergence profile with respect to over-contour and overhanging [21], no marginal discrepancy, proper proximal contacts, and no history of crown decementation. The MD and BL widths of the restorations were measured using a Boley gauge caliper (Salvin Dental, Charlotte, NC, USA) with a precision of 0.1 mm.
The oral hygiene status was evaluated and classified as either poor or good/acceptable, considering the presence of soft/hard plaque and food debris around the teeth, dental implants, and soft tissues. Following this, a comprehensive periodontal assessment was performed using a conventional periodontal probe (PCP UNC 15; Hu-Friedy, Chicago, IL, USA) to determine the current periodontal status, which was categorized as either healthy or gingivitis/periodontitis. Healthy individuals exhibited: 1) fewer than 10% of sites with bleeding on probing (BOP) at a probing depth (PD) of 3 mm or less, and 2) absence of gingival redness/edema, even with reduced clinical attachment and bone levels. Gingivitis was identified by the presence of 10% or more BOP sites with PD of 3 mm or less [22]. Periodontitis was defined by the presence of: 1) interdental clinical attachment loss (CAL) detectable in at least 2 non-adjacent teeth, and 2) buccal or lingual CAL of 3 mm or more with PD greater than 3 mm detectable in at least 2 teeth [23].
The peri-implant clinical parameters assessed included the presence or absence of redness, swelling, and/or suppuration. BOP was also documented at 6 sites (distobuccal, mid-buccal, mesiobuccal, distolingual, mid-lingual, and mesiolingual), with the number of sites exhibiting bleeding recorded as either absent/1 site of bleeding or ≥2 points of bleeding [14]. Peri-implant PD measurements, defined as the distance from the base of the peri-implant sulcus or pocket to the mucosal margin, were obtained at the same 6 sites per implant using a calibrated implant probe (PCVUNC12PT; Hu-Friedy). These measurements were rounded to the nearest millimeter, and only the highest value was considered for analysis.
Each implant was further examined using digital periapical radiographs, which were obtained with an X-ray unit (Elity 70; Fiad International, Envigado, Colombia) and a long-cone paralleling technique (Rinn; XCP Instruments, Elgin, IL, USA) fitted to a digital intraoral sensor (EZ Sensor P EX 1.5; Vatech, Fort Lee, NJ, USA). The interproximal radiographic bone loss (IRBL) was assessed at the mesial and distal aspects of the implants [15] and was defined as the distance between the implant platform and the most coronal point of bone-implant contact [5]. These measurements were then used to calculate the maximum percentage of IRBL in relation to implant length, as well as the IRBL/age ratio [24].
Case definitions
Peri-implant diagnosis was made based on clinical findings in conjunction with the IRBL [16]. The cases were categorized into 3 groups according to previously published definitions [1,14,25]: 1) healthy implants (Figure 1), which included peri-implant sites with no bleeding or BOP at only 1 site, no suppuration, PD ≤5 mm, and IRBL <2.0 mm; 2) PIM (Figure 2), which comprised peri-implant sites with overt bleeding at a minimum of 2 sites or tissue edema, redness, or shininess of the soft tissue surface with either minimal isolated or no suppuration, as well as PD ≤5 mm and/or IRBL ≤2 mm; and 3) PI (Figure 3), which consisted of peri-implant sites with BOP, redness, swelling, suppuration, PD ≥6 mm and/or IRBL ≥3 mm.
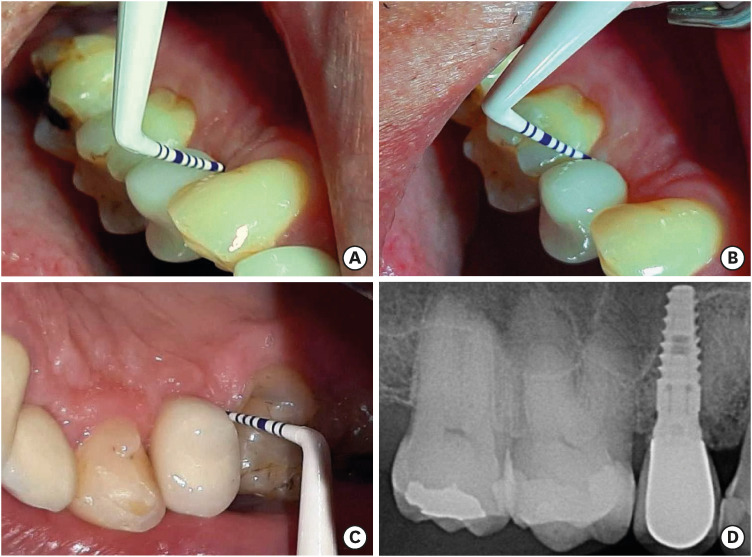
Figure 1. Representative photographs of a healthy implant placed in the region of a missing maxillary right first premolar. After 5 years of follow-up, a well-adapted, cement-retained, porcelain-fused-to-metal crown with no signs of peri-implant inflammation, no bleeding on probing, and probing depths of less than 5 mm in in both the buccal (A, B) and palatal surrounding mucosa (C) could be observed. A digital periapical radiograph of the implant site (D) shows the presence of a conical-type implant with an internal abutment connection and no signs of peri-implant bone loss.
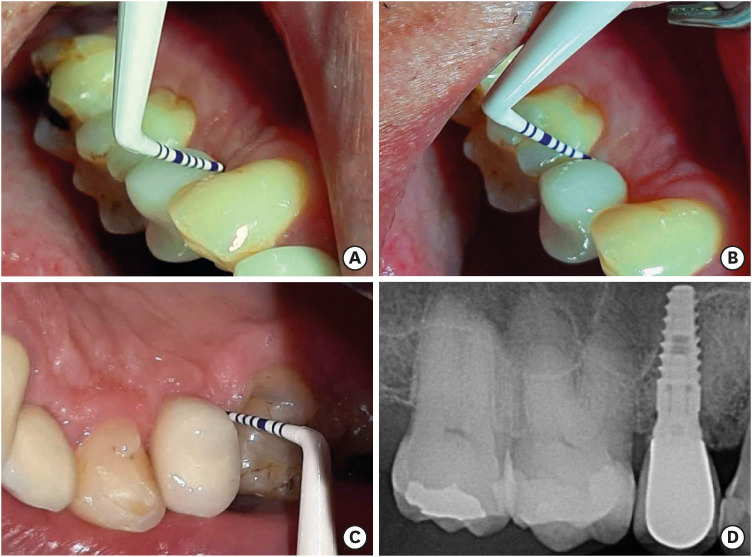
Figure 2. Representative photographs of a single-unit implant-supported maxillary right lateral incisor restoration exhibiting peri-implant mucositis. (A) Presentation of the restoration and its surrounding peri-implant mucosa during clinical exploration. After 4 years of follow-up, no suppuration was observed, and probing depths of 3 to 5 mm persisted on all surfaces. However, overt bleeding at 2 sites and mild tissue edema were apparent. (B) A periapical radiograph shows a conical-type implant with an internal abutment connection and no evidence of alveolar bone loss.
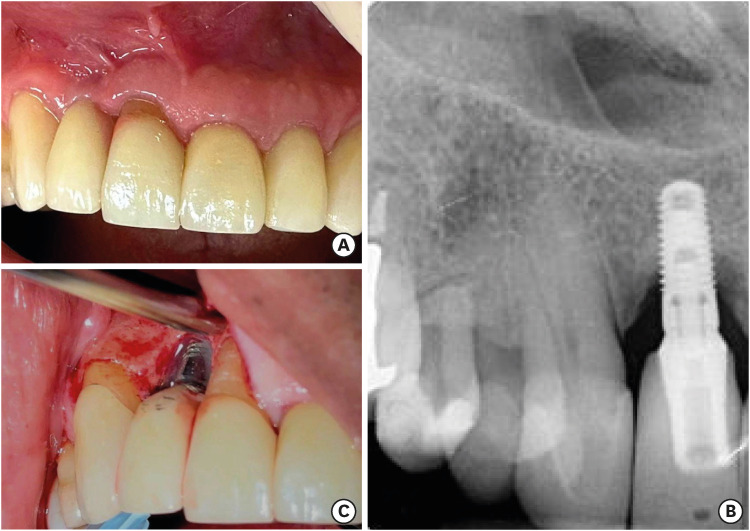
Figure 3. Representative photographs of peri-implantitis around a dental implant placed in the maxillary right lateral incisor position. (A) Clinical photograph displaying horizontal ridge deficiency and violaceous peri-implant tissues. (B) Digital periapical radiograph revealing a crater-shaped bone defect surrounding a conical-type implant with an internal abutment connection. (C) During surgical exploration to treat the affected implant, the untreated implant and collar surfaces supporting a single-unit restoration were exposed, showing an over-contoured emergence profile and marginal discrepancies.
Statistical methods
Data were analyzed using standard statistical software (SPSS v.27.0; IBM Corp., Armonk, NY, USA). The prevalence rates for PIM and PI at both patient and implant levels were estimated based on the percentage of cases detected at various time intervals (1 to ≤5 years, >5 years, and total). If a patient had both healthy and diseased peri-implant conditions simultaneously, the implant with the poorest diagnosis was used for the patient-level analyses. Bivariate analyses were conducted to identify differences related to explanatory variables and to detect potential indicators associated with disease statuses. The distribution mode of quantitative variables was analyzed using the Kolmogorov-Smirnov test. Since the data were normally distributed, they were analyzed using parametric 1-way analysis of variance (ANOVA) along with Games-Howell post hoc multiple comparison tests. The Pearson χ2 and Fisher exact tests were applied for categorical variables when appropriate. Univariate and multivariate binary logistic regression analyses were performed to confirm the association of potential indicators with each disease category while adjusting for confounding variables with P values ≤0.20 identified in the bivariate comparisons. For this purpose, all continuous data included in the models were dichotomized according to the optimal cut-off points obtained from receiver operating characteristic curve analysis. Positive associations were considered present when the odds ratio (OR) was greater than 1 and the 95% confidence interval (CI) was ≥1.0 for any of the constructs. The significance level was set at P<0.05.
RESULTS
Epidemiological and clinical profile of the study sample
A total of 155 patients received 379 implants between August 2003 and September 2021, meeting the inclusion criteria and undergoing clinical and radiographic follow-up assessments. Table 1 presents the prevalence rates observed at various follow-up periods for implant health, PIM, and PI at patient and implant levels. Overall, 54 patients (34.8%) and 159 implants (42.0%) were categorized as healthy within a period from 1 to 18 years (mean, 5.16±3.09 years); 44 patients (28.4%) and 127 implants (33.5%) were considered to have PIM within a follow-up period from 1 to 17 years (mean, 5.35±3.89 years); and 57 patients (36.8%) and 93 implants (24.5%) were classified as having PI within a follow-up period from 1.5 to 18 years (mean, 7.82±4.62).
Table 1. Prevalence of implant health, peri-implant mucositis, and peri-implantitis at the patient and implant levels as determined by combined clinical and radiographic findings, according to the follow-up period.
| Follow-up period | Patient levela) | Implant levelb) | ||||
|---|---|---|---|---|---|---|
| Implant health | Peri-implant mucositis | Peri-implantitis | Implant health | Peri-implant mucositis | Peri-implantitis | |
| 1 to ≤5 years | 31 (36.0) | 29 (33.7) | 26 (30.2) | 88 (46.3) | 70 (36.8) | 32 (16.8) |
| >5 years | 23 (33.3) | 15 (21.7) | 31 (44.9) | 71 (37.6) | 57 (30.2) | 61 (32.3) |
| Total | 54 (34.8) | 44 (28.4) | 57 (36.8) | 159 (42.0) | 127 (33.5) | 93 (24.5) |
Values are presented as number (%).
a)Data based on the number of patients within each follow-up period by diagnostic category. b)Data based on the number of implants within each follow-up period by diagnostic category.
In accordance with the case definitions, all examined clinical and radiographic parameters demonstrated significant between-group differences (Table 2; P<0.05, χ2 or 1-way ANOVA tests). Healthy peri-implant sites exhibited no swelling or redness, while PIM and PI sites displayed a significantly higher proportion of points with suppuration and BOP, which were significantly more frequent in PI cases. The mean values of PD, IRBL, percentage of IRBL, and IRBL/age ratio were significantly greater for PI sites compared to the other 2 groups (P<0.01, 1-way ANOVA/Games-Howell post hoc multiple-comparison tests). In contrast, no significant differences in these peri-implant health/disease status measurements were observed between healthy and PIM sites (P>0.05).
Table 2. Summary of clinical and radiographic findings from the study sample at the implant level by diagnostic category.
| Peri-implant parameters | Diagnostic category | Significancea) | |||||
|---|---|---|---|---|---|---|---|
| Healthy implant sites (n=159) | Peri-implant mucositis sites (n=127) | Peri-implantitis sites (n=93) | χ2 value | F test value | P value | ||
| Gingival swellingb) | 177.529 | - | <0.001c) | ||||
| Absent | 159 (100.0) | 42 (33.1) | 29 (31.2) | ||||
| Present | - | 85 (66.9)e) | 64 (68.8)e) | ||||
| Rednessb) | 206.979 | - | <0.001c) | ||||
| Absent | 159 (100.0) | 31 (24.4) | 26 (28.0) | ||||
| Present | - | 96 (75.6)e) | 67 (72.0)e) | ||||
| Suppurationb) | 39.994 | - | <0.001c) | ||||
| Absent | 159 (100.0) | 119 (93.7) | 73 (78.5) | ||||
| Present | - | 8 (6.3)e) | 20 (21.5)e)f) | ||||
| Bleeding on probingb) | 179.715 | - | <0.001c) | ||||
| Absent/1 site | 159 (100.0) | 51 (40.2) | 22 (23.7) | ||||
| ≥2 sites | - | 76 (59.8)e) | 71 (76.3)e)f) | ||||
| Maximum PD (mm) | 3.28±0.83 | 3.50±0.93 | 5.86±1.61e)f) | - | 179.531 | <0.001d) | |
| Maximum IRBL (mm) | 1.35±0.70 | 1.37±0.73 | 3.53±1.61e)f) | - | 160.651 | <0.001d) | |
| Maximum percentage of IRBL | 11.95±6.98 | 13.57±7.93 | 35.20±19.40e)f) | - | 133.747 | <0.001d) | |
| IRBL/age ratio | 0.21±0.14 | 0.23±0.15 | 0.57±0.33e)f) | - | 105.633 | <0.001d) | |
Values are presented as mean ± standard deviation or number (%).
PD: probing depth, IRBL: interproximal radiographic bone loss, df: degrees of freedom.
a)All df values = 2. b)Data based on the number of implants within each parameter by diagnostic category. c)Two-sided Pearson χ2 test. d)One-way analysis of variance. e)Statistically significant difference (P<0.05; χ2, Fisher exact, or Games-Howell post hoc comparison tests) compared with the healthy implant group. f)Statistically significant difference (P<0.05, χ2 or Games-Howell post hoc comparison tests) compared with the peri-implant mucositis group.
Bivariate comparisons between variables assessed at the patient level with reference to diagnostic category are summarized in Table 3. This table shows that patients diagnosed with PIM and PI had a significantly higher frequency of poor oral hygiene and gingivitis/periodontitis (P<0.05, χ2 post hoc pairwise-comparison tests) than healthy patients. In contrast, no significant differences (P>0.05, χ2 and 1-way ANOVA tests) were found between diagnostic categories regarding sex, age, smoking status, alcohol consumption, systemic condition, history of periodontitis, or supportive maintenance. However, both age and smoking status had a confounding influence on the results (P<0.20, χ2 and 1-way ANOVA tests). Regarding systemic condition, although no significant differences were observed between the study groups, it was notable that healthy controls had higher proportions of diabetes mellitus (2 of 22 cases, 9.1%), allergic and autoimmune diseases (2 of 22 cases, 9.1%), and chemotherapy for cancer (2 of 22 cases, 9.1%). In contrast, the presence of multiple systemic conditions, including the simultaneous occurrence of cardiovascular disease, thyroid diseases, diabetes, rheumatoid arthritis, and/or bone metabolism diseases, was more frequently detected in patients with PIM (5 of 23 cases, 21.7%). Similarly, patients with PI had a higher proportion of cardiovascular diseases (15 of 31 cases, 48.4%), thyroid diseases (5 of 31 cases, 16.1%), and bone metabolism diseases (3 of 31 cases, 9.7%).
Table 3. Comparison of patient-related characteristics by diagnostic category.
| Patient-related parameters | Diagnostic category | Significancea) | |||||
|---|---|---|---|---|---|---|---|
| Healthy patients (n=54) | Peri-implant mucositis patients (n=44) | Peri-implantitis patients (n=57) | χ2 value | F test value | P value | ||
| Sexb) | 2.957 | - | 0.228d) | ||||
| Male | 20 (37.0) | 18 (40.9) | 30 (52.6) | ||||
| Female | 34 (63.0) | 26 (59.1) | 27 (47.4) | ||||
| Age (yr) | 58.31±13.28 | 63.27±7.91 | 60.67±11.57 | - | 2.322 | 0.102e) | |
| Smoking statusb) | 4.216 | - | 0.122d) | ||||
| Current consumer/short-term abstainer | 8 (14.8) | 13 (29.5) | 17 (29.8) | ||||
| Non-consumer/long-term abstainer | 46 (85.2) | 31 (70.5) | 40 (70.2) | ||||
| Alcohol consumptionb) | 2.028 | - | 0.363d) | ||||
| Current consumer/short-term abstainer | 10 (18.5) | 12 (27.3) | 17 (29.8) | ||||
| Non-consumer/long-term abstainer | 44 (81.5) | 32 (72.7) | 40 (70.2) | ||||
| Systemic conditionb) | 2.324 | - | 0.313d) | ||||
| Healthy | 32 (59.3) | 21 (47.7) | 26 (45.6) | ||||
| Systemic involvementc) | 22 (40.7) | 23 (52.3) | 31 (54.4) | ||||
| History of periodontitisb) | 0.088 | - | 0.957d) | ||||
| Yes | 27 (50.0) | 23 (52.3) | 30 (52.6) | ||||
| No | 27 (50.0) | 21 (47.7) | 27 (47.4) | ||||
| Supportive maintenanceb) | 0.058 | - | 0.972d) | ||||
| Regular complier | 21 (38.9) | 17 (38.6) | 21 (36.8) | ||||
| Erratic/non-complier | 33 (61.1) | 27 (61.4) | 33 (66.2) | ||||
| Oral hygiene statusb) | 13.932 | - | 0.001d) | ||||
| Good/acceptable | 35 (64.8) | 14 (31.8) | 20 (35.1) | ||||
| Poor | 19 (35.2) | 30 (68.2)f) | 37 (64.9)f) | ||||
| Current periodontal statusb) | 18.294 | - | <0.001d) | ||||
| Periodontal health | 28 (51.9) | 6 (13.6) | 14 (24.6) | ||||
| Gingivitis/periodontitis | 26 (48.1) | 38 (86.4)f) | 43 (75.4)f) | ||||
Values are presented as mean ± standard deviation or number (%).
df: degrees of freedom.
a)All df values = 2. b)Data based on the number of patients within each parameter by diagnostic category. c)Including patients with diagnosis of bone metabolism disease (n=7), diabetes (n=5), allergic and autoimmune disease (n=5), cardiovascular disease (n=33), thyroid disease (n=11), cancer chemotherapy (n=3), and multiple systemic conditions (n=12). d)Two-sided Pearson χ2 test. e)One-way analysis of variance. f)Statistically significant difference (P<0.05, χ2 post hoc comparison tests) compared with the healthy implant group.
Results of implant-level analyses
Between-group comparisons regarding site-, surgery-, implant-, and prosthetic restoration-related variables in relation to diagnostic categories at the implant level are presented in Tables 4 and 5. Table 4 clearly shows that implant sites diagnosed with PIM and PI had a significantly higher frequency (P<0.05, χ2 test) of horizontal, vertical, or combined alveolar ridge defects, as well as a higher number of cases that underwent an early/delayed implant placement protocol relative to healthy implant sites. In terms of implant- and prosthetic restoration-related variables (Table 5), the length of follow-up, functional loading time, and MD/BL widths of coronal restoration were significantly associated with the proportion of PI sites (P<0.05, 1-way ANOVA/Games-Howell post hoc multiple-comparison tests). Additionally, implant length and the quality of coronal restoration had significant relationships (P<0.05, 1-way ANOVA/Games-Howell post hoc multiple-comparison and χ2 tests) with both PIM and PI. However, no significant differences (P>0.05, χ2 and 1-way ANOVA tests) were observed between the study groups regarding implant type, collar surface, time point of loading, implant diameter, type of coronal restoration, method of coronal restoration, connection type, mechanism of crown retention, or occlusal contact relationship.
Table 4. Bivariate comparisons of site- and surgery-related variables by diagnostic category.
| Site- and surgical-related parametersa) | Diagnostic category | Significancea) | ||||||
|---|---|---|---|---|---|---|---|---|
| Healthy implant sites (n=159) | Peri-implant mucositis sites (n=127) | Peri-implantitis sites (n=93) | χ2 value | F test value | P value | |||
| Site-related variables | ||||||||
| Location of implantb) | 0.279 | - | 0.870g) | |||||
| Anterior | 56 (35.2) | 41 (32.3) | 32 (34.4) | |||||
| Posterior | 103 (54.8) | 86 (67.7) | 61 (65.5) | |||||
| Type of jawb) | 2.214 | - | 0.331g) | |||||
| Maxillary | 93 (58.5) | 83 (65.4) | 62 (66.7) | |||||
| Mandibular | 66 (41.5) | 44 (34.6) | 31 (33.3) | |||||
| Adjacent teethb) | 1.963 | - | 0.375g) | |||||
| Present | 119 (74.8) | 90 (70.9) | 62 (66.7) | |||||
| Absent | 40 (25.2) | 37 (29.1) | 31 (33.3) | |||||
| Preoperative alveolar ridge statusb) | 22.769 | - | <0.001g) | |||||
| Adequate (with thick tissue biotype) | 91 (57.2) | 43 (33.9) | 29 (31.2) | |||||
| Deficient (with horizontal, vertical, or combined defects)c) | 68 (42.2) | 84 (66.1)h) | 64 (68.8)h) | |||||
| Minimum keratinized tissue width (mm) | 2.90±1.34 | 2.69±1.49 | 2.59±1.66 | - | 1.493 | 0.226i) | ||
| Surgery-related variables | ||||||||
| Tissue augmentation proceduresb) | 1.752 | - | 0.416g) | |||||
| Membrane barriers, bone grafts, or combined techniquesd) | 84 (52.8) | 60 (47.2) | 52 (55.9) | |||||
| No augmentation procedures | 75 (47.2) | 67 (52.8) | 41 (44.1) | |||||
| Intraoperative complicationsb) | 2.983 | - | 0.225g) | |||||
| Yese) | 10 (6.3) | 5 (3.9) | 9 (9.7) | |||||
| No | 149 (93.7) | 122 (96.1) | 84 (90.3) | |||||
| Implant placement protocolb) | 18.130 | - | <0.001g) | |||||
| Immediate | 53 (33.3) | 17 (13.4) | 16 (17.2) | |||||
| Early/delayedf) | 106 (66.7) | 110 (86.6)h) | 77 (82.8)h) | |||||
Values are presented as mean ± standard deviation or number (%).
df: degrees of freedom.
a)All df values = 2. b)Data based on the number of implants within each parameter by diagnostic category. c)Including horizontal ridge defects (n=96), vertical ridge defects (n=26), and combined ridge defects (n=94). d)Including membrane barriers (n=6), bone grafts (n=10), and combination of membrane barriers/grafting (n=180). e)Including cortical bone perforation (n=9), implant instability (n=8), and bone dehiscence (n=7). f)Including type 2 (after 4-8 weeks of soft tissue healing, n=30), type 3 (after 12-16 weeks of partial bone healing, n=29), and type 4 (after complete bone healing of at least 6 months, n=234). g)Two-sided Pearson χ2 test. h)Statistically significant difference (P<0.05, χ2 post hoc comparison tests) compared with the healthy implant group. i)One-way analysis of variance.
Table 5. Bivariate comparisons of implant- and prosthetic restoration-related variables by diagnostic category.
| Implant- and prosthetic restoration-related parameters | Diagnostic category | Significancea) | ||||||
|---|---|---|---|---|---|---|---|---|
| Healthy implant sites (n=159) | Peri-implant mucositis sites (n=127) | Peri-implantitis sites (n=93) | χ2 value | F test value | P value | |||
| Implant-related variables | ||||||||
| Implant typeb) | 0.035 | - | 0.983c) | |||||
| Conical | 141 (88.7) | 113 (89.0) | 82 (88.2) | |||||
| Cylindrical | 18 (11.3) | 14 (11.0) | 11 (11.8) | |||||
| Collar surfaceb) | 2.364 | - | 0.307c) | |||||
| Rough | 130 (81.8) | 112 (88.2) | 77 (82.8) | |||||
| Smooth | 29 (18.2) | 15 (11.8) | 16 (17.2) | |||||
| Time point of loadingb) | 1.133 | - | 0.568c) | |||||
| Immediate | 7 (4.4) | 8 (6.3) | 7 (7.5) | |||||
| Late | 152 (95.6) | 119 (93.7) | 86 (92.5) | |||||
| Implant length (mm) | 11.14±1.35 | 10.59±1.42e) | 10.34±1.79e) | - | 9.720 | <0.001d) | ||
| Implant diameter (mm) | 3.97±0.44 | 4.01±0.46 | 4.06±0.48 | - | 0.947 | 0.389d) | ||
| Length of follow-up (years) | 5.16±3.09 | 5.35±3.89 | 7.82±4.62e)f) | - | 16.438 | 0.002d) | ||
| Functional loading time (years) | 4.48±3.12 | 4.67±3.86 | 7.14±4.71e)f) | - | 16.217 | <0.001d) | ||
| Prosthetic restoration-related variables | ||||||||
| Type of coronal restorationb) | 3.020 | - | 0.221c) | |||||
| Definitive | 137 (86.2) | 103 (81.1) | 83 (89.2) | |||||
| Temporary | 22 (13.8) | 24 (18.9) | 10 (10.8) | |||||
| Method of coronal restorationb) | 2.354 | - | 0.308c) | |||||
| Bridge-fixed prosthesis | 50 (31.4) | 50 (39.4) | 36 (38.7) | |||||
| Individual crown | 109 (68.6) | 77 (60.6) | 57 (61.3) | |||||
| Connection typeb) | 1.901 | - | 0.386c) | |||||
| Internal | 150 (94.3) | 120 (94.5) | 84 (90.3) | |||||
| External | 9 (5.7) | 7 (5.5) | 9 (9.7) | |||||
| Mechanism of crown retentionb) | 2.584 | - | 0.275c) | |||||
| Screw-retained | 38 (23.9) | 28 (22.0) | 29 (31.2) | |||||
| Cement- or screw/cement-retained | 121 (76.1) | 99 (78.0) | 64 (68.8) | |||||
| Occlusal contact relationshipb) | 1.969 | - | 0.374c) | |||||
| Adequate | 133 (83.6) | 98 (77.2) | 76 (81.7) | |||||
| Infra-occlusion | 26 (16.4) | 29 (22.8) | 17 (18.3) | |||||
| Quality of coronal restorationb) | 12.286 | - | 0.002c) | |||||
| Satisfactory | 142 (89.3) | 95 (74.8) | 70 (75.3) | |||||
| Poor | 17 (10.7) | 32 (25.2)e) | 23 (24.7)e) | |||||
| MD width of coronal restoration (mm) | 7.93±1.63 | 7.90±1.50 | 8.39±1.37e)f) | - | 3.367 | 0.036d) | ||
| BL width of coronal restoration (mm) | 7.52±1.31 | 7.46±1.59 | 7.94±1.39e)f) | - | 3.555 | 0.030d) | ||
Values are presented as mean ± standard deviation or number (%).
MD: mesiodistal, BL: buccolingual, df: degrees of freedom.
a)All df values = 2. b)Data based on the number of implants within each parameter by diagnostic category. c)Two-sided Pearson χ2 test. d)One-way analysis of variance. e)Statistically significant difference (P<0.05, χ2 or Games-Howell post hoc comparison tests) compared with the healthy implant group. f)Statistically significant difference (P<0.05, Games-Howell post hoc comparison test) compared with the peri-implant mucositis group.
Results of univariate and multivariate logistic regression models
Data derived from regression models for estimating the risk of PIM and PI before and after adjusting for age stratum and smoking status are provided in Tables 6 and 7. For all comparisons, the optimal age cut-off point was estimated at 61.5 years. As seen in Table 6, the OR of PIM was significantly increased (P<0.01, Wald test) in cases with poor oral hygiene, active gingivitis/periodontitis, alveolar ridge deficiency, early/delayed implant placement, implant length ≤11.0 mm, and poor quality of coronal restoration. After adjusting for confounders, all of these candidate risk variables remained strongly associated with PIM status (P<0.01). It was also striking that although the OR of PI was significantly increased for the first 6 covariates (Table 7), a follow-up period >5.0 years, functional loading time >4.0 years, MD width of coronal restoration >8.0 mm, and BL width of coronal restoration >7.0 mm were also positively associated with an increased risk of the disease (P<0.05). However, after adjustment, a confounding effect could be identified for the MD/BL widths of coronal restoration, as the associations did not reach a statistically significant level (P>0.05). In contrast, we observed no confounding of the associations of poor oral hygiene, active gingivitis/periodontitis, alveolar ridge deficiency, early/delayed implant placement protocol, implant length ≤11.0 mm, follow-up period >5.0 years, functional loading time >4.0 years, or the poor quality of coronal restoration, as all of these covariates remained strongly and independently associated (P<0.05) with PI when adjusted for confounders.
Table 6. Univariate and multivariate binary logistic regression analyses examining the association of significant risk indicators with peri-implant mucositis after adjusting for age and smoking status.
| Clinical groups/parameters | Casesa) | Univariate analysis | Multivariate binary logistic regression analysis | |||
|---|---|---|---|---|---|---|
| Unadjusted OR (95% CI) | P valueb) | Adjusted OR (95% CI) | P valueb) | |||
| Oral hygiene status | <0.001 | 0.001 | ||||
| Good/acceptable | 39 (30.7) | Referent | ||||
| Poor | 88 (69.3) | 2.3 (1.4–3.7) | 2.3 (1.4–3.8) | |||
| Current periodontal status | <0.001 | <0.001 | ||||
| Periodontal health | 21 (16.5) | Referent | ||||
| Gingivitis/periodontitis | 106 (83.5) | 3.3 (1.9–5.8) | 3.4 (1.9–6.0) | |||
| Preoperative alveolar ridge status | <0.001 | <0.001 | ||||
| Adequate (thick tissue biotype) | 43 (33.9) | Referent | ||||
| Deficient (horizontal, vertical, or combined defects) | 84 (66.1) | 2.6 (1.6–4.2) | 2.7 (1.6–4.4) | |||
| Implant placement protocol | <0.001 | <0.001 | ||||
| Immediate | 17 (13.4) | Referent | ||||
| Early/delayed | 110 (86.6) | 3.2 (1.8–5.9) | 3.5 (1.8–6.5) | |||
| Implant length | <0.001 | <0.001 | ||||
| >11.0 mm | 45 (35.4) | Referent | ||||
| ≤11.0 mm | 82 (64.6) | 2.9 (1.8–4.6) | 2.9 (1.8–4.6) | |||
| Quality of coronal restoration | 0.003 | 0.003 | ||||
| Satisfactory | 95 (74.8) | Referent | ||||
| Poor | 32 (25.2) | 2.6 (1.4–4.9) | 2.7 (1.4–5.0) | |||
OR: odds ratio, CI: confidence interval.
a)Data based on the number of implants within each parameter. b)Wald test.
Table 7. Univariate and multivariate binary logistic regression analyses examining the association of significant risk indicators with peri-implantitis after adjusting for age and smoking status.
| Clinical groups/parameters | Casesa) | Univariate analysis | Multivariate binary logistic regression analysis | |||
|---|---|---|---|---|---|---|
| Unadjusted OR (95% CI) | P valueb) | Adjusted OR (95% CI) | P valueb) | |||
| Oral hygiene status | 0.003 | 0.002 | ||||
| Good/acceptable | 28 (30.1) | Referent | ||||
| Poor | 65 (69.9) | 2.3 (1.3–3.9) | 2.5 (1.4–4.3) | |||
| Current periodontal status | 0.015 | 0.008 | ||||
| Periodontal health | 22 (23.7) | Referent | ||||
| Gingivitis/periodontitis | 71 (76.3) | 2.1 (1.2–3.6) | 2.2 (1.2–4.0) | |||
| Preoperative alveolar ridge status | <0.001 | <0.001 | ||||
| Adequate | 29 (31.2) | Referent | ||||
| Deficient | 64 (68.8) | 2.9 (1.7–4.9) | 2.9 (1.7–5.0) | |||
| Implant placement protocol | 0.009 | 0.004 | ||||
| Immediate | 16 (17.2) | Referent | ||||
| Early/delayed | 77 (82.2) | 2.3 (1.2–4.4) | 2.6 (1.4–5.1) | |||
| Implant length | <0.001 | <0.001 | ||||
| >11.0 mm | 32 (34.4) | Referent | ||||
| ≤11.0 mm | 61 (65.6) | 2.9 (1.7–4.9) | 2.8 (1.6–4.9) | |||
| Length of follow-up | 0.001 | 0.004 | ||||
| ≤5.0 years | 32 (34.4) | Referent | ||||
| >5.0 years | 61 (65.6) | 2.4 (1.4–4.0) | 2.3 (1.3–4.0) | |||
| Functional loading time | 0.005 | 0.012 | ||||
| ≤4.0 years | 33 (35.5) | Referent | ||||
| >4.0 years | 60 (64.5) | 2.1 (1.3–3.6) | 2.1 (1.2–3.6) | |||
| Quality of coronal restoration | 0.005 | 0.013 | ||||
| Satisfactory | 70 (75.3) | Referent | ||||
| Poor | 23 (24.7) | 2.7 (1.4–5.4) | 2.5 (1.2–5.1) | |||
| MD width of coronal restoration | 0.034 | 0.052 | ||||
| ≤8 mm | 41 (44.1) | Referent | ||||
| >8 mm | 52 (55.9) | 1.7 (1.0–2.9) | 1.7 (0.9–2.9) | |||
| BL width of coronal restoration | 0.038 | 0.059 | ||||
| ≤7 mm | 23 (24.7) | Referent | ||||
| >7 mm | 70 (75.3) | 1.8 (1.0–3.2) | 1.7 (0.9–3.1) | |||
MD: mesiodistal, BL: buccolingual, OR: odds ratio, CI: confidence interval.
a)Data based on the number of implants within each parameter. b)Wald test.
DISCUSSION
Given the importance of understanding the prevalence of PIDs and potential risk indicators for their prevention [2], this study was conducted to evaluate and compare various risk variables associated with PIM and PI using updated case definitions [1,25]. The findings revealed that after a follow-up period of 1 to 18 years, 34.8% of patients exhibited peri-implant health, while 28.4% and 36.8% were diagnosed with PIM and PI, respectively. At the implant level, peri-implant health was observed in 42.0% of cases, PIM in 33.5%, and PI in 24.5%. Although the prevalence rates of PIDs vary across countries, other authors have reported similar rates, with 34% of patients and 21% of implants affected by PI [11] and 23.9% of patients and 27.4% of implants affected by PIM [9]. The differences in criteria used to define PIDs may greatly impact these estimates and partly explain the observed variability [1,2]. This study utilized the case definitions outlined in the 2017 World Workshop [1,25], but it is important to note that factors such as host susceptibility, oral hygiene habits, and prosthetic or restorative factors may also contribute to the differences in disease rates [2,10]. Additionally, minor changes in diagnostic criteria can lead to great differences in prevalence rates across studies [11]. It is crucial to emphasize that, in this study, all clinical and radiographic features associated with PIDs demonstrated statistically significant between-group differences regarding swelling, redness, suppuration, BOP, PD, IRBL, percentage of IRBL, and IRBL/age ratio scores according to the agreed-upon case definitions for PIM and PI [1,25].
Among the patient-related variables, only poor oral hygiene and the presence of active gingivitis/periodontitis were significantly associated with the proportion of cases of PIM and PI in the univariate analyses, and these factors remained robust indicators of disease status after adjustment for covariates. In agreement with the current results, it has been recognized that impaired access to oral hygiene procedures or poor self-performed oral hygiene measures can lead to biofilm accumulation on implants and their restorations, increasing the risk of PIDs [3,4,26]. Regarding the effect of current periodontal status, convincing evidence indicates that in susceptible individuals, the persistence of gingivitis and/or periodontitis, rather than the history of periodontal disease per se [7], may lead to the development of PIM and/or PI [6,11]. This may be attributable to the fact that both groups of diseases share common host factors, pathogenesis, and microbiota [8,27], although some differences have been identified in the biofilms associated with these entities [28].
Regarding the effects of site- and surgery-related variables on PIM and PI, we found that both preoperative alveolar ridge deficiency and early/delayed placement protocol were positively associated with the proportion of PIM and PI cases in the univariate analysis, and these associations remained robust after adjustment for covariates. The increased risk observed with deficient alveolar ridge dimensions is consistent with previous studies demonstrating that a thin tissue biotype [29] or severe ridge resorption (≤3 mm) [30] may pose potential risks for PIDs. Although the preoperative alveolar ridge status has been underestimated in the majority of studies related to implant treatment outcomes, it is generally accepted that a relationship may exist between alveolar ridge deficiency and the vulnerability of the peri-implant area to plaque-induced tissue breakdown [31]. Additionally, the strong and independent association of the early/delayed placement protocol with PIM and PI is roughly in line with previous results indicating lower frequencies of both PIM and PI associated with the immediate implant placement protocol [32]. Conversely, while some authors have reported a higher frequency of immediate implants affected by PIM [13] and PI [33], others have demonstrated statistically similar outcomes with delayed and immediate implants when assessing the prevalence of PIM [33,34] or PI [34]. Given that the early/delayed implant placement protocol is typically used when substantial soft and/or hard tissue deficiencies are present, it can be concluded that a residual bone defect may reduce the resistance of peri-implant tissues to inflammation [13].
Regarding implant-related variables, implant lengths of ≤11.0 mm were found to be positively associated with the proportions of PIM and PI cases. This finding contrasts with previous studies that found either no association with disease statuses [4,11] or a significant association between longer implants and greater marginal bone loss [35]. However, it has been also indicated that short implant length is associated with an increased failure rate [36]. In line with the data presented here, resonance frequency analysis has shown that reduced surface area of short implants at the bone-implant level leads to a decrease in the area of osseointegration and an increase in stress at the crestal bone [37]. If this stress concentration in the peri-implant bone exceeds physiological limits, the process of bone resorption will increase, yielding a higher risk of bone loss [38]. In addition to the above, other implant-related variables in this study, such as a follow-up period of >5.0 years and a functional loading time of >4.0 years, were also significantly related to the proportion of PI cases in the univariate analysis and remained robust indicators after adjustment for confounders. Several studies have emphasized that longer follow-up periods are associated with a greater prevalence of PI [10,13]. Moreover, it has been hypothesized that due to the inflammatory nature of PID, while the onset of the condition may occur early during follow-up [26], it requires time to develop and be detected [7]. Notably, however, in many studies, the observation periods generally begin with the loading of the implants. In this context, other reports [2,6] agree with the findings of this study, indicating that a functional loading time of >4.0 years is linked to the onset of PI. This may be related to stress concentration [2] or fatigue micro-damage [39] caused by a longer duration of occlusal loading, which is absorbed by the crestal bone-implant interface, resulting in bone resorption that may progress to PI.
The influence of variables related to prosthetic restorations on PIDs remains a relatively understudied subject. In this study, poor quality of coronal restorations was consistently associated with a higher proportion of cases of PIM and PI across various analyses. In line with this finding, some existing studies [10,21] suggest that this indicator may be particularly important insofar as it facilitates or prevents microbial biofilm accumulation beneath crown restoration margins. In contrast, although both the MD and BL widths of coronal restorations significantly contributed to the prevalence of PI in bivariate and univariate analyses, a confounding effect became apparent after adjusting for selected confounders in the final logistic regression model, as these parameters did not demonstrate a significant association. These results partially align with those of other study, which indicate that increased MD and BL widths of crowns [40] can act as stress multipliers, amplifying the total force exerted on the implant due to lever action or increased rotational load on the implant and implant-abutment connection during mastication, with detrimental consequences for the integrity of peri-implant tissues over time. Nonetheless, since confounding occurs when shared causes exist between the exposure and outcome, it is reasonable to infer that the heterogeneous effects on the immune system, vascularity, wound healing, or bone remodeling processes related to age and smoking status may be important modifiers for the impact of excessive occlusal load in their association with PI.
Several limitations were identified in the current study. First, the retrospective and cross-sectional design may have led to some selection biases and missing data, making it impossible to determine any correlation between the history of periodontitis and PIM and/or PI or the progression of PIM to PI. However, to enhance the information regarding the sample population, patients were recalled and underwent single-visit clinical and radiographic examinations. Second, the heterogeneity in case definitions for peri-implant conditions could limit the generalizability of the results. Third, the low frequency of cases associated with intraoperative complications, cylindrical implants, smooth collar surfaces, immediate loading, and external connections within the study groups prevented an adequate assessment of the possible role of these covariates in the pathogenesis of PIM and/or PI. Consequently, greater variability in the sample composition may yield different results.
In conclusion, the findings of this study show that oral hygiene, periodontal status, preoperative alveolar ridge status, implant placement protocol, implant length, and the quality of coronal restoration may be strong risk indicators for both PIM and PI. Additionally, the length of follow-up and functional loading time also served as robust indicators for PI in this study population. Furthermore, the potential modifying relationships of age and smoking status with the MD and BL widths of restoration could be crucial for the development of PI.
Footnotes
Funding: The study received full funding from the Technical Research Council of the Faculty of Dentistry of the University of Antioquia (reference No. 2019-25776) and the University Health Care Provider (reference No. IN30-2019).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: Ana María Ortiz-Echeverri, Carolina Gallego-González, María Catalina Castaño-Granada, Sergio Iván Tobón-Arroyave.
- Formal analysis: Sergio Iván Tobón-Arroyave.
- Investigation: Ana María Ortiz-Echeverri, Carolina Gallego-González, María Catalina Castaño-Granada.
- Methodology: Sergio Iván Tobón-Arroyave.
- Project administration: Sergio Iván Tobón-Arroyave.
- Writing - original draft: Ana María Ortiz-Echeverri, Carolina Gallego-González, María Catalina Castaño-Granada.
- Writing - review & editing: Sergio Iván Tobón-Arroyave.
References
- 1.Renvert S, Persson GR, Pirih FQ, Camargo PM. Peri-implant health, peri-implant mucositis, and peri-implantitis: case definitions and diagnostic considerations. J Periodontol. 2018;89(Suppl 1):S304–S312. doi: 10.1002/JPER.17-0588. [DOI] [PubMed] [Google Scholar]
- 2.Pimentel SP, Shiota R, Cirano FR, Casarin RCV, Pecorari VGA, Casati MZ, et al. Occurrence of peri-implant diseases and risk indicators at the patient and implant levels: a multilevel cross-sectional study. J Periodontol. 2018;89:1091–1100. doi: 10.1002/JPER.17-0599. [DOI] [PubMed] [Google Scholar]
- 3.Heitz-Mayfield LJ. Peri-implant diseases: diagnosis and risk indicators. J Clin Periodontol. 2008;35:292–304. doi: 10.1111/j.1600-051X.2008.01275.x. [DOI] [PubMed] [Google Scholar]
- 4.Dalago HR, Schuldt Filho G, Rodrigues MA, Renvert S, Bianchini MA. Risk indicators for peri-implantitis. a cross-sectional study with 916 implants. Clin Oral Implants Res. 2017;28:144–150. doi: 10.1111/clr.12772. [DOI] [PubMed] [Google Scholar]
- 5.Kumar PS, Dabdoub SM, Hegde R, Ranganathan N, Mariotti A. Site-level risk predictors of peri-implantitis: a retrospective analysis. J Clin Periodontol. 2018;45:597–604. doi: 10.1111/jcpe.12892. [DOI] [PubMed] [Google Scholar]
- 6.Máximo MB, de Mendonça AC, Alves JF, Cortelli SC, Peruzzo DC, Duarte PM. Peri-implant diseases may be associated with increased time loading and generalized periodontal bone loss: preliminary results. J Oral Implantol. 2008;34:268–273. doi: 10.1563/1548-1336(2008)34[269:PDMBAW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Atieh MA, Alsabeeha NH, Faggion CM, Jr, Duncan WJ. The frequency of peri-implant diseases: a systematic review and meta-analysis. J Periodontol. 2013;84:1586–1598. doi: 10.1902/jop.2012.120592. [DOI] [PubMed] [Google Scholar]
- 8.Daubert DM, Weinstein BF, Bordin S, Leroux BG, Flemming TF. Prevalence and predictive factors for peri-implant disease and implant failure: a cross-sectional analysis. J Periodontol. 2015;86:337–347. doi: 10.1902/jop.2014.140438. [DOI] [PubMed] [Google Scholar]
- 9.Wada M, Mameno T, Onodera Y, Matsuda H, Daimon K, Ikebe K. Prevalence of peri-implant disease and risk indicators in a Japanese population with at least 3 years in function - a multicentre retrospective study. Clin Oral Implants Res. 2019;30:111–120. doi: 10.1111/clr.13397. [DOI] [PubMed] [Google Scholar]
- 10.Kissa J, El Kholti W, Chemlali S, Kawtari H, Laalou Y, Albandar JM. Prevalence and risk indicators of peri-implant diseases in a group of Moroccan patients. J Periodontol. 2021;92:1096–1106. doi: 10.1002/JPER.20-0549. [DOI] [PubMed] [Google Scholar]
- 11.Kordbacheh Changi K, Finkelstein J, Papapanou PN. Peri-implantitis prevalence, incidence rate, and risk factors: a study of electronic health records at a U.S. dental school. Clin Oral Implants Res. 2019;30:306–314. doi: 10.1111/clr.13416. [DOI] [PubMed] [Google Scholar]
- 12.Derks J, Tomasi C. Peri-implant health and disease. A systematic review of current epidemiology. J Clin Periodontol. 2015;42(Suppl 16):S158–S171. doi: 10.1111/jcpe.12334. [DOI] [PubMed] [Google Scholar]
- 13.Lee CT, Huang YW, Zhu L, Weltman R. Prevalences of peri-implantitis and peri-implant mucositis: systematic review and meta-analysis. J Dent. 2017;62:1–12. doi: 10.1016/j.jdent.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Monje A, Wang HL, Nart J. Association of preventive maintenance therapy compliance and peri-implant diseases: a cross-sectional study. J Periodontol. 2017;88:1030–1041. doi: 10.1902/jop.2017.170135. [DOI] [PubMed] [Google Scholar]
- 15.Berglundh T, Wennström JL, Lindhe J. Long-term outcome of surgical treatment of peri-implantitis. A 2-11-year retrospective study. Clin Oral Implants Res. 2018;29:404–410. doi: 10.1111/clr.13138. [DOI] [PubMed] [Google Scholar]
- 16.Göthberg C, Gröndahl K, Omar O, Thomsen P, Slotte C. Bone and soft tissue outcomes, risk factors, and complications of implant-supported prostheses: 5-years RCT with different abutment types and loading protocols. Clin Implant Dent Relat Res. 2018;20:313–321. doi: 10.1111/cid.12587. [DOI] [PubMed] [Google Scholar]
- 17.Engebretson SP, Grbic JT, Singer R, Lamster IB. GCF IL-1beta profiles in periodontal disease. J Clin Periodontol. 2002;29:48–53. doi: 10.1034/j.1600-051x.2002.290108.x. [DOI] [PubMed] [Google Scholar]
- 18.Ahlner F, Falk Erhag H, Johansson L, Mellqvist Fässberg M, Rydberg Sterner T, Samuelsson J, et al. Patterns of alcohol consumption and associated factors in a population-based sample of 70-year-olds: data from the Gothenburg H70 Birth Cohort Study 2014-16. Int J Environ Res Public Health. 2022;19:8248. doi: 10.3390/ijerph19148248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang HL, Al-Shammari K. HVC ridge deficiency classification: a therapeutically oriented classification. Int J Periodontics Restorative Dent. 2002;22:335–343. [PubMed] [Google Scholar]
- 20.Gallucci GO, Hamilton A, Zhou W, Buser D, Chen S. Implant placement and loading protocols in partially edentulous patients: a systematic review. Clin Oral Implants Res. 2018;29(Suppl 16):106–134. doi: 10.1111/clr.13276. [DOI] [PubMed] [Google Scholar]
- 21.Katafuchi M, Weinstein BF, Leroux BG, Chen YW, Daubert DM. Restoration contour is a risk indicator for peri-implantitis: a cross-sectional radiographic analysis. J Clin Periodontol. 2018;45:225–232. doi: 10.1111/jcpe.12829. [DOI] [PubMed] [Google Scholar]
- 22.Chapple IL, Mealey BL, Van Dyke TE, Bartold PM, Dommisch H, Eickholz P, et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol. 2018;89(Suppl 1):S74–S84. doi: 10.1002/JPER.17-0719. [DOI] [PubMed] [Google Scholar]
- 23.Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, et al. Periodontitis: consensus report of workgroup 2 of the 2017 World Workshop on the classification of periodontal and peri-Implant diseases and conditions. J Periodontol. 2018;89(Suppl 1):S173–S182. doi: 10.1002/JPER.17-0721. [DOI] [PubMed] [Google Scholar]
- 24.Heitz-Mayfield LJ, Heitz F, Lang NP. Implant Disease Risk Assessment IDRA-a tool for preventing peri-implant disease. Clin Oral Implants Res. 2020;31:397–403. doi: 10.1111/clr.13585. [DOI] [PubMed] [Google Scholar]
- 25.Berglundh T, Armitage G, Araujo MG, Avila-Ortiz G, Blanco J, Camargo PM, et al. Peri-implant diseases and conditions: consensus report of workgroup 4 of the 2017 World Workshop on the classification of periodontal and peri-implant diseases and conditions. J Periodontol. 2018;89(Suppl 1):S313–S318. doi: 10.1002/JPER.17-0739. [DOI] [PubMed] [Google Scholar]
- 26.Schwarz F, Alcoforado G, Guerrero A, Jönsson D, Klinge B, Lang N, et al. Peri-implantitis: summary and consensus statements of group 3. The 6th EAO Consensus Conference 2021. Clin Oral Implants Res. 2021;32(Suppl 21):245–253. doi: 10.1111/clr.13827. [DOI] [PubMed] [Google Scholar]
- 27.Lang NP, Bosshardt DD, Lulic M. Do mucositis lesions around implants differ from gingivitis lesions around teeth? J Clin Periodontol. 2011;38(Suppl 11):182–187. doi: 10.1111/j.1600-051X.2010.01667.x. [DOI] [PubMed] [Google Scholar]
- 28.Dabdoub SM, Tsigarida AA, Kumar PS. Patient-specific analysis of periodontal and peri-implant microbiomes. J Dent Res. 2013;92:168S–75S. doi: 10.1177/0022034513504950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monje A, Galindo-Moreno P, Tözüm TF, Suárez-López del Amo F, Wang HL. Into the paradigm of local factors as contributors for peri-implant disease: short communication. Int J Oral Maxillofac Implants. 2016;31:288–292. doi: 10.11607/jomi.4265. [DOI] [PubMed] [Google Scholar]
- 30.Krennmair S, Hunger S, Forstner T, Malek M, Krennmair G, Stimmelmayr M. Implant health and factors affecting peri-implant marginal bone alteration for implants placed in staged maxillary sinus augmentation: a 5-year prospective study. Clin Implant Dent Relat Res. 2019;21:32–41. doi: 10.1111/cid.12684. [DOI] [PubMed] [Google Scholar]
- 31.Bassetti M, Kaufmann R, Salvi GE, Sculean A, Bassetti R. Soft tissue grafting to improve the attached mucosa at dental implants: a review of the literature and proposal of a decision tree. Quintessence Int. 2015;46:499–510. doi: 10.3290/j.qi.a33688. [DOI] [PubMed] [Google Scholar]
- 32.Parvini P, Obreja K, Becker K, Galarraga ME, Schwarz F, Ramanauskaite A. The prevalence of peri-implant disease following immediate implant placement and loading: a cross-sectional analysis after 2 to 10 years. Int J Implant Dent. 2020;6:63. doi: 10.1186/s40729-020-00259-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bianchi AE, Sanfilippo F. Single-tooth replacement by immediate implant and connective tissue graft: a 1-9-year clinical evaluation. Clin Oral Implants Res. 2004;15:269–277. doi: 10.1111/j.1600-0501.2004.01020.x. [DOI] [PubMed] [Google Scholar]
- 34.Schropp L, Wenzel A, Stavropoulos A. Early, delayed, or late single implant placement: 10-year results from a randomized controlled clinical trial. Clin Oral Implants Res. 2014;25:1359–1365. doi: 10.1111/clr.12273. [DOI] [PubMed] [Google Scholar]
- 35.Kim YM, Lee JB, Um HS, Chang BS, Lee JK. Long-term effect of implant-abutment connection type on marginal bone loss and survival of dental implants. J Periodontal Implant Sci. 2022;52:496–508. doi: 10.5051/jpis.2200960048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manzano G, Montero J, Martín-Vallejo J, Del Fabbro M, Bravo M, Testori T. Risk factors in early implant failure: a meta-analysis. Implant Dent. 2016;25:272–280. doi: 10.1097/ID.0000000000000386. [DOI] [PubMed] [Google Scholar]
- 37.Bataineh AB, Al-Dakes AM. The influence of length of implant on primary stability: an in vitro study using resonance frequency analysis. J Clin Exp Dent. 2017;9:e1–e6. doi: 10.4317/jced.53302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robau-Porrua A, Pérez-Rodríguez Y, Soris-Rodríguez LM, Pérez-Acosta O, González JE. The effect of diameter, length and elastic modulus of a dental implant on stress and strain levels in peri-implant bone: a 3D finite element analysis. Biomed Mater Eng. 2020;30:541–558. doi: 10.3233/BME-191073. [DOI] [PubMed] [Google Scholar]
- 39.Rokaya D, Srimaneepong V, Wisitrasameewon W, Humagain M, Thunyakitpisal P. Peri-implantitis update: risk indicators, diagnosis, and treatment. Eur J Dent. 2020;14:672–682. doi: 10.1055/s-0040-1715779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Urdaneta RA, Rodriguez S, McNeil DC, Weed M, Chuang SK. The effect of increased crown-to-implant ratio on single-tooth locking-taper implants. Int J Oral Maxillofac Implants. 2010;25:729–743. [PubMed] [Google Scholar]