Abstract
Chronicity after infection with the hepatitis B virus (HBV) can occur for a variety of reasons. However, once established, chronicity may be maintained by high levels of viral proteins circulating in the serum. To examine the characteristics of T cells capable of coexisting with the secreted hepatitis B e antigen (HBeAg), T-cell receptor (TCR) transgenic (Tg) mice were produced. To ensure that HBeAg-specific T cells would not be deleted in the presence of serum HBeAg, the TCR α- and β-chain genes used to produce the TCR-Tg mice were derived from T-cell hybridomas produced from immunizing HBeAg-Tg mice. A TCR-Tg lineage (11/4-12) was produced that possessed a high frequency (∼67%) of CD4+ T cells that expressed a Tg TCR specific for the HBeAg. As predicted, when 11/4-12 TCR-Tg mice were bred with HBeAg-Tg mice no deletion of the HBeAg-specific CD4+ T cells occurred in the thymus or the spleen. Functional analysis of the TCR-Tg T cells revealed that the HBeAg-specific CD4+ T cells escaped deletion in the thymus and periphery by virtue of low avidity. Regardless of their low avidity, HBeAg-specific TCR-Tg T cells could be activated by exogenous HBeAg, as measured by cytokine production in vitro and T-helper-cell function for anti-HBe antibody production in vitro and in vivo. Furthermore, activated TCR-Tg HBeAg-specific T cells polarized to the Th1 subset were able to elicit liver injury when transferred into HBeAg or HBcAg-Tg recipients. Therefore, HBeAg-specific CD4+ T cells that can survive deletion or anergy in the presence of circulating HBeAg nonetheless are capable of being activated and of mediating liver injury in vivo. The 11/4-12 TCR-Tg lineage may serve as a monoclonal model for the HBe/HBcAg-specific CD4+ T-cell repertoire present in chronically infected HBV patients.
The nucleoprotein of the hepatitis B virus (HBV) exists in two structural forms. The particulate nucleocapsid (hepatitis B c antigen [HBcAg]), which encapsulates the viral genome, and a monomeric secreted form (HBeAg). We have postulated that maternally derived HBeAg traverses the placenta and acts as a tolerogen in utero, thereby predisposing perinatally infected babies to chronic infection (14). Neonatal tolerance studies in mice demonstrated that tolerance to the HBeAg was major histocompatibility complex (MHC) dependent inasmuch as the CD4+ T cells of H-2s mice were very sensitive to tolerance induction by HBeAg, whereas some proportion of CD4+ T cells of H-2b mice were resistant to neonatal tolerance induction with HBeAg (15). These studies were extended by the production of HBeAg-expressing transgenic (Tg) mice. The CD4+ T cells of HBeAg-Tg mice on an H-2s background (B10.S) were highly tolerant, yet CD4+ T cells in HBeAg-Tg mice on an H-2b background (B10) were incompletely tolerant (16). Incomplete tolerance in H-2b HBeAg-Tg mice permitted functional studies of the HBeAg-specific CD4+ T-cell repertoire that escaped tolerance and coexisted with circulating HBeAg. These studies revealed that HBeAg-specific CD4+ T cells that evaded tolerance induction in HBeAg-Tg H-2b mice were of relatively low avidity, possessed a unique fine specificity pattern, and tended to belong to the Th2 subset (17).
In order to further examine HBeAg-specific CD4+ T cells that can coexist with circulating HBeAg and remain functional in vivo, we produced mice transgenic for T-cell receptors (TCR) specific for HBeAg. First, T-cell hybridomas were produced by immunizing B10 wild-type (+/+) mice or HBeAg-Tg (B10 e/e) mice with HBeAg. These immunizations yielded 100 T-cell hybridomas from the B10 +/+ mice and 13 T-cell hybridomas from the B10 e/e mice (17). The TCR-α/β genes derived from selected HBeAg-specific T-cell hybridomas were sequenced and inserted into T-cell expression shuttle vectors for use in the generation of TCR-α/β–Tg mice.
The TCR-α/β–Tg lineage 11/4-12, derived from HBeAg-Tg (B10 e/e) mice, is the subject of this report. A second TCR-α/β–Tg lineage, 8/3-11, derived from B10 +/+ mice, is included as a comparative control. As predicted from the fact that the 11/4-12 TCR was derived from immunized B10 HBeAg-Tg mice, CD4+ T cells bearing this Tg-TCR are not deleted in the thymus or periphery of TCR-Tg × HBeAg-Tg mice (i.e., “double Tg” mice). This provided the opportunity to examine the functional status of HBeAg-specific and/or self-reactive CD4+ T cells bearing the 11/4-12 TCR-α/β chains in single- and/or double-Tg mice, respectively.
MATERIALS AND METHODS
Production of HBeAg-specific T-cell hybridomas.
HBeAg-specific T-cell hybridomas were generated using a standard protocol. Briefly, draining LN cells from B10 HBeAg-Tg mice were harvested 15 days after injection in the hind footpads with 10 μg of HBeAg emulsified in complete Freund adjuvant. LN cells were pooled, and single-cell suspensions were stimulated in vitro with HBeAg (0.1 μg/ml) for 3 days. Thereafter, the cells were washed and recultured with interleukin-2 (IL-2; 20 U/ml) for an additional 2 days. The cells were then washed and hybridized with the hypoxanthine-aminopterin-thymidine-sensitive fusion partner BW5147, which does not contain functional mRNA for the α or β chains of the TCR. A number of HBeAg-specific hybridomas were cloned by limiting dilution.
Sequence determination of the V(D)J regions of the TCR derived from the hybridomas.
The Vα usage of each T-cell hybridoma was determined by reverse transcription-PCR (RT-PCR) using primers specific for each Vα gene family. Total RNA was isolated from 2 × 106 cells of each hybridoma using Trizol reagent (Gibco BRL, Grand Island, N.Y.). Following extraction, 1 μg of RNA was reverse transcribed to cDNA with Moloney murine leukemia virus reverse transcriptase (Gibco BRL) and pd(T)12–18 primer (Amersham Pharmacia Biotech, Piscataway, N.J.). Aliquots of cDNA were subjected to 30 cycles of PCR under stringent primer annealing conditions, with the reverse primer located at the constant region of the α chain and each of the 13 forward primers specific for each Vα gene family (24).
The Vβ usage was determined with a Vβ multiprobe RNase protection assay (6). Mixtures of radiolabeled riboprobes specific for each Vβ gene family were hybridized with 10 μg of extracted RNA, digested with RNase, and resolved on denaturing polyacrylamide gels. Protected RNA fragments were visualized on autoradiography and identified in comparison to the unprotected Vβ probes.
From the Vα and Vβ typing results, primers were designed to isolate the V(D)J and partial constant regions of the hybridomas. Following 30 thermal cycles, the PCR products were purified from agarose and cloned into the pSPORT plasmid vectors. Three individual colonies from each TCR α- and β-chain cDNA were sequenced using M13 forward and reverse primers.
Generation of TCR α-chain and β-chain transgenes and Tg mice.
After sequence analysis, the V(D)J regions of the TCR were PCR amplified with Pfu DNA polymerase (Stratagene, La Jolla, Calif.) using gene-specific primers. Restriction sites, intron sequence, and splice donor/acceptors were also introduced into the VDJ fragments. The modified α-chain VJ fragment was inserted into a XhoI-NotI-excised TCR α-chain shuttle vector which contains a rearranged TCR α-chain genomic DNA and the endogenous TCR α enhancer. The modified β-chain V(D)J fragment was inserted into a ClaI-NotI-excised TCR β-chain shuttle vector that contains a rearranged TCR β-chain genomic DNA and the endogenous β enhancer. The shuttle vectors (8) were kindly provided by M. M. Davis, Stanford University. To ensure that no mutation had been introduced, the V(D)J regions from each TCR construct were subcloned into pUC19 vector and resequenced. Prior to microinjection, the bacterial sequences were removed, and the 15.4-kb α-chain and the 19.8-kb β-chain TCR DNA fragments were comicroinjected into fertilized mouse (C57BL/10; B10) embryos. Progeny mice were screened for the presence of the transgenes in PBL by PCR analysis using primers located on the V and the CDR3 region for each TCR transgene. The expression of the TCR transgenes in peripheral blood lymphocytes (PBL) and lymphoid tissues was confirmed by immunofluorescence and RT-PCR by using monoclonal antibodies (MAbs) and oligonucleotide primers specific for the transgene TCRs. The Tg mice expressing the HBeAg (10 ng/ml) or the HBcAg (0.25 ng/mg of soluble liver protein) were produced at the Scripps Research Institute Transgenic Research Facility as previously described (14, 18).
rHBeAg and synthetic peptides.
An Escherichia coli-derived rHBeAg corresponding in sequence to serum-derived HBeAg encompassing the 10 precore amino acids remaining after cleavage of the precursor and residues 1 to 149 of hepatitis B core antigen (ayw subtype) was provided by Florian Schödel (EVAX, Munich, Germany). The presence of the 10 precore amino acids prevents particle formation, and the rHBeAg preparation is recognized efficiently by HBeAg-specific MAbs but displays little hepatitis B core antigenicity (23). Peptides were synthesized by the simultaneous multiple peptide synthesis method. The following HBcAg- and HBeAg-derived synthetic peptides representing Th-cell recognition sites were used and designated by amino acid position from the N terminus of HBcAg: 129-140 amino acids 129 to 140), PPAYRPPNAPIL; and 120-140 (amino acids 120 to 140), VSFGVWIRTPPAYRPPNAPIL.
Serology.
HBeAg was measured in diluted Tg mouse sera by a commercial enzyme-linked immunosorbent assay (ELISA; HBe enzyme immunoassay; Abbott Laboratories, Chicago, Ill.), and rHBeAg was used as a standard. Anti-HBc and anti-HBe immunoglobulin G (IgG) antibodies were measured in murine sera by an indirect solid-phase ELISA using rHBcAg or rHBeAg as the solid-phase ligands as described previously (19). The data are expressed as antibody titers representing the reciprocal of the highest dilution of sera required to yield an optical density at 492 nm (OD492) three times the equal dilution of preimmunization sera. IgG isotype-specific ELISAs were performed using IgG1-, IG2a, IgG2b, and IgG3-specific second antibodies (Southern Biotechnology, Birmingham, Ala.).
Cytokine analysis.
Spleen cells from either unprimed or primed TCR-Tg or wild-type mice were cultured (6 × 106/ml) with various concentrations of a series of antigens. Culture supernatants (SNs) were harvested at 24 h for IL-2 determination and at 48 h for IL-4 and gamma-interferon (IFN-γ) determinations. Cytokines were measured by two-site ELISA using pairs of cytokine-specific MAbs. One unlabeled MAb was absorbed to the microtiter plate well and used as a capture antibody, and the other labeled MAb served as the probe. Alternatively, a CELL-ELISA (1) was utilized. In this case the cytokine-specific capture MAb was bound to the solid phase of a cell culture well, and the last 24 h of the cell culture was conducted in the presence of the capture MAb. The CELL-ELISA is more sensitive than the SN-ELISA because the cytokines are directly bound by solid-phase MAb and are less likely to be absorbed by the cellular cytokine receptors (1).
Tg autoantibody model.
Because HBeAg-expressing transgenic mice on a B10(H-2b) background are not completely T-cell tolerant, injection of the synthetic Th-cell site 129-140 results in anti-HBe or autoantibody production (16). This Tg model is useful for screening immunomodulatory drugs or therapies. Groups of e/+ or TCR double-Tg mice were injected with the peptide Th-cell site 129-140 (50 μg in incomplete Freund adjuvant). The mice were bled before injection and at 2-week intervals for the determination of total IgG anti-HBe as well as isotype-specific anti-HBe antibody levels by ELISA.
Liver injury model.
TCR-Tg or wild-type mice served as donors of spleen cells for adoptive transfer into HBeAg-Tg or HBcAg-Tg recipients. Unprimed spleen cells from donor mice were cultured in two cycles (5 days/cycle) with HBeAg (5 μg/ml) in the presence of IL-12 (2.0 ng/ml) and anti-IL-4 (MAb 11B11) in order to polarize the activated T cells toward the Th1 subset. Virtually 100% of the T cells were CD4+ after the second culture cycle. Activated Th1 cells (20 × 106) were transferred into sublethally irradiated (400 R) HBeAg-Tg or HBcAg-Tg recipients. Hepatocellular injury was monitored biochemically by measuring serum alanine aminotransferase (ALT) activity. Mice were killed by cervical dislocation 6 months after adoptive transfer, and necropsy was performed. Liver tissue was fixed in 10% zinc-buffered formalin, embedded in parafin, sectioned (3 μm), and stained with hematoxylin and eosin (H&E).
Flow cytometry.
Single cell suspensions of thymus or spleen were prepared. Before staining, cells were incubated with an anti-Fc MAb (2.4G2) to block nonspecific Fc receptor uptake. For staining with directly labeled antibodies, 106 cells were incubated with antibodies at 4°C for 15 min. Cells were washed three times and analyzed with a FACScan (Becton Dickinson). Gates were set only on viable cells and usually >104 cells were analyzed using LYSIS II (Becton Dickinson). The murine antibodies used for two- and three-color staining were as follows: anti-TCR Vβ4 (KT4), anti-TCR Vβ11 (RR3-15), anti-TCR Vα11 (RR8-1), anti-CD4 (H129.19), and anti-CD8a (53-6.7) (PharMingen, Palo Alto, Calif.).
RESULTS
Characterization of HBeAg-specific T-cell hybridomas.
A number of HBeAg-specific T-cell hybridomas were derived after immunization of B10.S +/+ mice, and B10 +/+ mice, and B10 e/e Tg mice with HBeAg (17). Approximately 25 HBeAg-specific T-cell hybridomas were selected for analysis as candidate donors of TCR genes for the production of TCR-Tg mice. Interestingly, the HBeAg-specific T-cell hybridomas derived from B10 e/e Tg mice preferentially expressed Vβ4 (7 of 9), whereas those derived from B10 +/+ mice preferentially expressed Vβ11 (6 of 10). The TCR α- and β-chain genes from five T-cell hybridomas were cloned and sequenced, and the V gene usage was determined against known TCR sequences deposited in GenBank. The two T-cell hybridomas (2B2 and 1B9) derived from B10 e/e Tg mice both utilize Vβ4 and Vα11.1 in combination with Jβ1.4 and Jα14 (Table 1). In contrast, the T-cell hybridomas (4E4 and 7B7) derived from B10 +/+ mice utilize Vβ11 and Vα5. The 4E4 hybridoma utilizes Jβ2.6 and Jα33 and hybridoma 7B7 utilizes Jβ2.6 and Jα26. An insertion in the 3′ end of the Vα gene of the 7B7 hybridoma results in a stop codon at position 95.
TABLE 1.
Characterization of Vβ, Vα, Jβ, and Jα, usage and VDJ junctional region sequence of β chains and VJ junctional regions of α chains from five HBeAg-specific T-cell hybridomas
| T-cell hybridoma | Source | Epitope | V region | CDR3 region | J region | TCR-Tg lineage |
|---|---|---|---|---|---|---|
| 2B2 | B10 e/e | 129-140 | α11.1 | GAGCCGACTAACAGTGCAGGGAACAAGCTA | 14α | |
| E P T N S A G N K L | 11/4-12 | |||||
| β4 | AGCCCAGATAGGCAAAACGAAAGGTTA | 1.4β | ||||
| S Q D R Q N E R L | ||||||
| 1B9 | B10 e/e | 129-140 | α11.1 | GAGGCCTCTAACAGTGCAGGGAACAAGCTA | 14α | |
| E A S N S A G N K L | —a | |||||
| β4 | AGCAGGGACAGACAGAACGAAAGATTA | 1.4β | ||||
| S R D R Q N E R L | ||||||
| 4E4 | B10 +/+ | 129-140 | α5 | AGTTCAAATACAGGAAACTACAAATAC | 33α | |
| S S N T G N Y K Y | 8/3-11 | |||||
| β11 | AGCTTGGATGAACAG | 2.6β | ||||
| S L D E Q | ||||||
| 7B7 | B10 +/+ | 129-140 | α5 | AGTGGATAG | 26α | |
| S G | — | |||||
| β11 | AGCTTGGATGAACAG | 2.6β | ||||
| S L D E Q | ||||||
| 1E9 | B10.S +/+ | 120-131 | α4.2 | GGTGACCAGGGAGGCAGAGCTCTG | 12α | |
| G D Q G G R A L | — | |||||
| β4 | AGCCAACCGGACTGGGGGTATGAACAG | 2.6β | ||||
| S Q P D W G Y E Q |
—, none.
The CDR3 sequences of the VβDβJβ and VαJα junctional regions were determined and are shown in Table 1. The four T-cell hybridomas derived from B10 mice are specific for the 129-140 epitope within HBeAg, whereas the T-cell hybridoma derived from the B10.S mouse (IE9) is specific for residues 120 to 131 within HBeAg. Note that aspartic acid (D) and glutamine (Q) at β-chain positions 100 and 102 are conserved in all four hybridomas specific for the 129-140 epitope of HBeAg. Positions 100 and 102 within the TCR CDR3 region are known to be important peptide antigen contact residues. The IE9 T-cell hybridoma specific for residues 120 to 131 on HBeAg possesses different amino acids in the critical 100 and 102 positions of the β chain. With respect to the 129-140/IAb epitope, the TCR sequences indicate that multiple TCR can be used to recognize this epitope and suggest the possibility that Vβ-chain residues 100 and 102 must be conserved. This suggests that regions other than Vβ-chain residues 100 and 102 may contribute to the avidity of the TCR rather than the specificity and that hybridomas 2B2 and 1B9 represent T cells that survived negative selection in B10 e/e Tg mice due to their low avidity as discussed below.
Establishment of the TCR-Tg lineage 11/4-12.
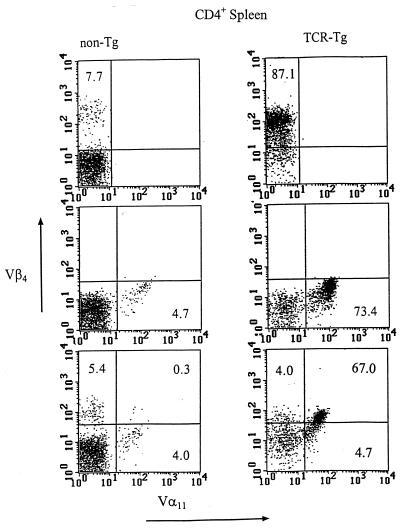
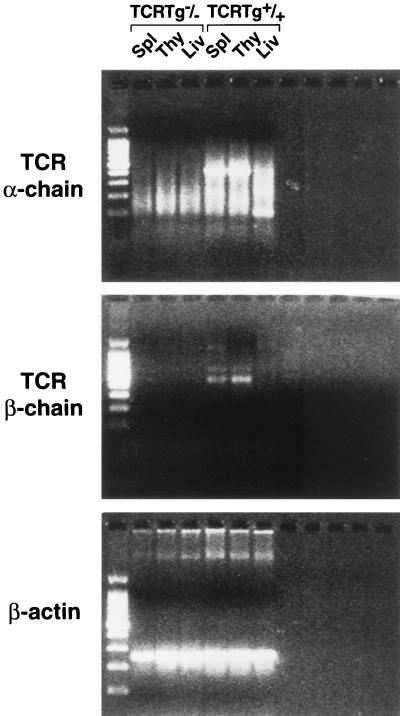
After microinjection of the 2B2 hybridoma-derived Vα- and Vβ-chain gene constructs, putative founder mice were screened for the presence of the transgenes by PCR analysis of DNA extracted from PBL. Mice positive for both TCR transgenes were examined for TCR expression by fluorescence-activated cell sorter (FACS) analysis (Fig. 1) and RT-PCR (Fig. 2). In TCR-Tg lineage 11/4-12, 87.1% of splenic CD4+ T cells express Vβ4 compared to 7.7% of control CD4+ spleen cells and Vα11 is expressed on 73.4% of CD4+ T cells in lineage 11/4-12 compared to 4.7% in control mice (Fig. 1). Splenic T cells expressing both Vβ4 and Vα11 represented 67% of the CD4+ population in the 11/4-12 TCR-Tg lineage compared to 0.3% in control mice. Analysis by RT-PCR confirmed the relatively high level of expression of the transgenic Vα and Vβ chains in the thymus and spleen but not the liver of the TCR-Tg 11/4-12 lineage (Fig. 2).
FIG. 1.
One- and two-color FACS analysis of non-Tg and 11/4-12 TCR-Tg splenic CD4+ T cells. Purified CD4+ splenic T cells were stained with anti-Vβ4 (top panels), anti-Vα11 (middle panels), or both anti-Vβ4 and anti-Vα11 (bottom panels).
FIG. 2.
RT-PCR analysis of mRNA expression in 11/4-12 TCR-Tg mice. Total RNA was isolated from spleen, thymus, and liver of 11/4-12 TCR-Tg mice, and 1 μg of isolated RNA was used for cDNA synthesis. The cDNA synthesis was initiated using either the oligo(dT)15 primer, the Cα primer (AGAGGGTGCTGTCCTGAGAC), or the Cβ primer (GCCGTCGACCTCAAACAAGGAGACCTTGGGT). For detection of the transgenic TCR α-chain mRNA, an aliquot of cDNA was subjected to 35 thermal cycles in a PCR reaction containing the Cα- and the Vα (CCAACAGAATTCCAGGGGCAGC) primers. For detection of the transgenic TCR β-chain mRNA, the Cβ and Vβ (GTCCAGTCGACCCGAAAATTA) primers were used. Spleen from a non-Tg littermate was used as a negative control. The φX174DNA-HaeIII digest was used as DNA size marker.
TCR-Tg mice were also derived from microinjection of Vα- and Vβ-chain genes derived from the 4E4 T cell hybridoma, which originated from B10 +/+ mice immunized with HBeAg (lineage 8/3-11) (Table 1). In the case of 8/3-11 TCR-Tg mice, only 11.5% of splenic CD4+ T cells express the transgenic Vβ11 chain compared to 4.5% in control mice (data not shown).
Functional analysis of HBeAg-specific CD4+ T cells in TCR-Tg lineages 11/4-12 and 8/3-11.
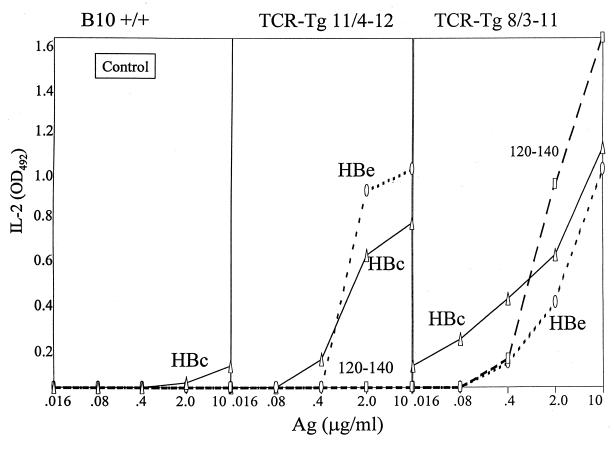
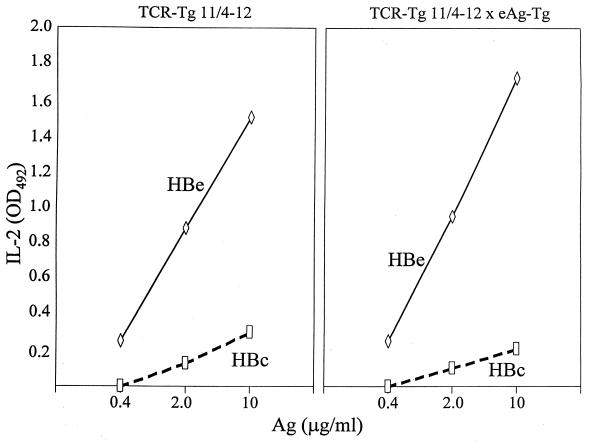
To ascertain the functional ability of the CD4+ T cells expressing the TCR transgenes to respond to antigen, unprimed spleen cells from 11/4-12 and 8/3-11 TCR-Tg mice were cultured for 2 days (11/4-12) or 3 days (8/3-11) either with HBcAg (particulate form), HBeAg (monomeric form), or peptide 120-140, and the antigen-specific IL-2 production was determined. The whole spleen was used because CD8+ cell-depleted spleen behaved equivalently to whole spleen (not shown). As shown in Fig. 3, naive spleen cells from non-TCR-Tg mice did not respond to the antigen panel, establishing that the endogenous HBeAg- and HBcAg-specific T-cell repertoire requires in vivo expansion by priming in order to be detected. In contrast, naive splenic T cells from both TCR-Tg lineages produced IL-2 in response to culture with the HBeAg. However, significant differences existed between the two TCR-Tg lineages. Splenic T cells from 11/4-12 TCR-Tg mice “recognized” HBeAg preferentially to the particulate HBcAg and were not activated by peptide 120-140. Splenic T cells from 8/3-11 TCR-Tg mice recognized HBcAg preferentially to HBeAg and were activated by peptide 120-140. Also note that 8/3-11 T cells produced higher levels of IL-2 and responded to lower doses of in vitro antigen than 11/4-12 T cells, even though the frequency of HBe/HBcAg-specific CD4+ T cells expressing a transgenic TCR is much lower in 8/3-11 mice (11.5%) than in 11/4-12 mice (67%).
FIG. 3.
Antigen-specific IL-2 production by naive splenic T cells from non-Tg (B10 +/+), TCR-Tg 11/4-12 and TCR-Tg 8/3-11 mice. Unprimed spleen cells were cultured with concentrations of the indicated antigens in vitro for 3 days (except for TCR-Tg 11/4-12 spleen cells, which were cultured for 2 days), and SNs were collected and assayed for IL-2 by ELISA. Comparative IL-2 levels are expressed in OD units. This is one of four assays and is representative.
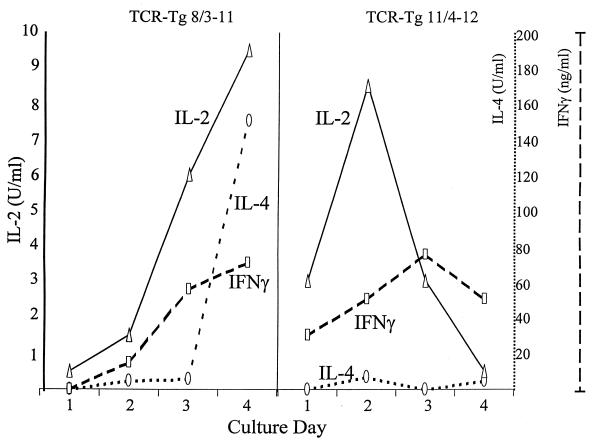
In addition to IL-2, production of IFN-γ and IL-4 by naive spleen cells from 11/4-12 and 8/3-11 TCR-Tg mice was monitored by CELL-ELISA over a 4-day culture period. As shown in Fig. 4, CD4+ T cells from 8/3-11 TCR-Tg mice produced increasing amounts of all three cytokines upon culture with the HBcAg beginning at day 1 for IL-2 and day 2 for IFN-γ and IL-4. Maximum cytokine levels were present in 4-day cultures. In contrast, CD4+ T cells from 11/4-12 TCR-Tg mice cultured with HBeAg produced maximum levels of IL-2 on day 2, produced IFN-γ on day 3, and produced very little IL-4 at all time points. Cytokine levels were decreasing by day 4 in the 11/4-12 TCR-Tg splenic cultures, indicating that in vitro T-cell activation was less sustainable in 11/4-12 as opposed to 8/3-11 TCR-Tg mice. The transient cytokine production was not due to T-cell death, since T-cell number and viability remained relatively stable throughout the 4-day culture (data not shown).
FIG. 4.
Kinetics of cytokine production by TCR-Tg 11/4-12 and TCR-Tg 8/3-11 naive, splenic T cells cultured with antigen. Unprimed TCR-Tg 8/3-11 spleen cells were cultured with HBcAg (1.0 μg/ml), and TCR-Tg 11/4-12 spleen cells were cultured with HBeAg (5.0 μg/ml) for from 1 to 4 days. Amounts of IL-2, IFN-γ, and IL-4 levels produced each day were determined by CELL-ELISA. Spleen cells were transferred to CELL-ELISA plates during the last 24 h of culture to measure contemporaneous rather than accumulated cytokine production. This experiment was performed on three separate occasions and is representative.
T cells expressing the 11/4-12 transgenic TCR are not deleted in the thymus or the periphery in TCR-Tg 11/4-12 × HBeAg-Tg double-Tg mice.
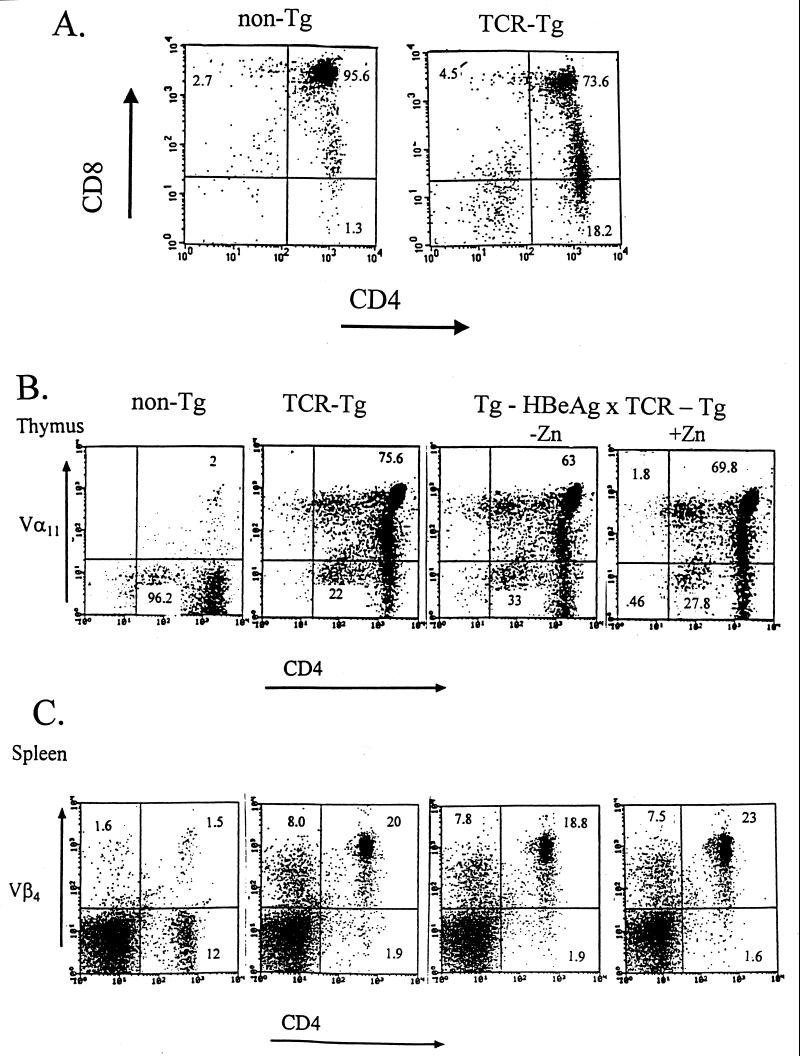
Since the TCR genes used to generate 11/4-12 TCR Tg mice originated from immunization of HBeAg-Tg mice, it was predicted that the transgenic TCR-bearing CD4+ cells would not be deleted in the presence of HBeAg, at least not at the HBeAg concentration occurring in the original HBeAg-Tg mouse (i.e., 10 ng/ml). To test this prediction, TCR-Tg 11/4-12 mice were bred with HBeAg-Tg mice, and the frequencies of the transgenic Vα11 chain or the transgenic Vβ4 chain among CD4+ T cells in the thymus or the spleen in non-Tg, single-TCR–Tg, or double-TCR–Tg × HBeAg-Tg mice were compared (Fig. 5). In the normal thymus, the vast majority (95.6%) of T cells are CD4+ CD8+ double-positive cells. Note that in the TCR-Tg 11/4-12 thymus 73.6% of T cells are double positive and 15.2% of thymic T cells are CD4+ single positive (Fig. 5A). The skewing toward the more mature CD4+ population in the thymus is typical in TCR-Tg mice expressing an MHC class II-restricted TCR. In Fig. 5B, the frequencies of CD4+ T cells expressing the TCR transgenic Vα11-chain in the thymus are shown in non-Tg, TCR-Tg (11/4-12), TCR-Tg × HBeAg-Tg, and double-Tg mice given Zn+ to induce the HBeAg-specific MT promoter, which increases the HBeAg concentration in the serum approximately 7-fold to ∼70 ng/ml (14). No significant decreases in the frequency of the transgenic Vα11-chain occurred in the thymus of 11/4-12 TCR-Tg mice also expressing the HBeAg in the serum at levels of 10 ng/ml (−Zn) or 70 ng/ml (+Zn) (Fig. 5B). Similarly, the presence of HBeAg in the serum did not deplete the CD4+ T cells in the spleen carrying the TCR transgenic Vβ4 chain (Fig. 5C). It is clear from this FACS analysis that the HBeAg-specific, CD4+ T cells bearing the 11/4-12 transgenic TCR are not negatively selected in the thymus or physically depleted in the periphery by exposure to circulating HBeAg.
FIG. 5.
Two-color FACS analysis of thymic and splenic T cells derived from non-Tg mice, TCR-Tg 11/4-12 mice, HBeAg-Tg × TCR-Tg 11/4-12 (double-Tg mice), and double-Tg mice given zinc sulfate (Zn, 25 mM) in the drinking water. (A) Thymic cells from non-Tg and TCR-Tg 11/4-12 mice were double stained with anti-CD8 and anti-CD4. (B) Thymic cells from non-Tg, TCR-Tg, and double-Tg mice without (−) and with (+) Zn treatment were double stained with anti-Vα11 and anti-CD4. (C) Splenic cells from non-Tg, TCR-Tg, and double-Tg mice without and with Zn treatment were double stained with anti-Vβ4 and anti-CD4.
T cells bearing the 11/4-12 transgenic TCR are not functionally altered by exposure to HBeAg in the serum of double-Tg mice.
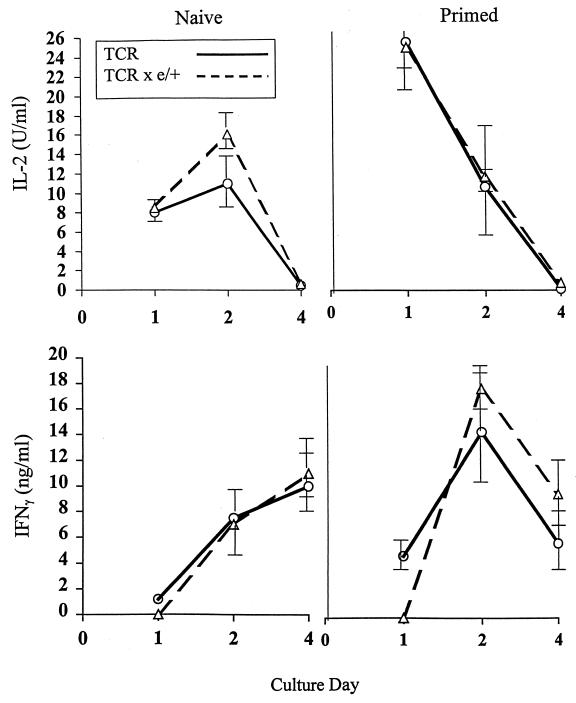
Because CD4+ T cells expressing the 11/4-12 transgenic TCR were not physically deleted by HBeAg in the thymus or the periphery, the next issue was to determine if exposure to circulating HBeAg altered the functional capacity of these T cells. For this purpose, naive splenic T cells from TCR-Tg 11/4-12 or double-Tg mice were cultured in vitro with HBeAg and HBcAg, and T-cell activation was monitored by IL-2 production after 2 days in culture (Fig. 6). Splenic T cells derived from naive single-TCR-Tg mice or TCR × HBeAg double-Tg mice produced equivalent amounts of IL-2 upon exposure to HBeAg and HBcAg in vitro. T cells from double-Tg mice required exposure to HBeAg in vitro to elicit IL-2 production, indicating that the T cells were not spontaneously activated by endogenous HBeAg in vivo. Similarly, FACS analysis revealed that the CD4+ T cells bearing the transgenic TCR derived from both single- and double-Tg mice possessed a resting phenotype as opposed to an activated or memory phenotype prior to in vitro culture (data not shown). Similarly, the kinetics of cytokine production in vitro were not significantly different between T cells derived from single-TCR-Tg or double-Tg mice regardless of whether the mice were unprimed or primed in vivo with soluble HBeAg (40 μg) (Fig. 7). HBeAg-specific IL-2 production peaked at day 2 of culture and declined to very low levels by day 4 in unprimed TCR-Tg and double-Tg splenic cultures. Priming of the mice with 40 μg of soluble HBeAg in vivo 3 days prior to culture had the same effect on in vitro T-cell IL-2 production in single-TCR-Tg and double-Tg mice. Priming resulted in a faster onset of IL-2 production in vitro, which was maximal during the first 24 h of culture, but did not affect the transient nature of IL-2 production (Fig. 7). Likewise, in vitro IFN-γ production in naive and HBeAg-primed single TCR-Tg or double-Tg mice was not significantly different. Priming with HBeAg increased maximum IFN-γ production at day 2 of culture in single-TCR-Tg and double-Tg mice. Splenic T cells from wild-type B10 +/+ mice either unprimed or primed with soluble HBeAg 3 days prior to culture did not produce IL-2 or IFN-γ upon culture with HBeAg (data not shown). Therefore, the results indicate that the T cells bearing the 11/4-12 transgenic TCR can be activated in vivo by priming with soluble HBeAg and that the presence of endogenous HBeAg in the serum of double-Tg mice neither suppresses nor enhances T-cell activation in vivo.
FIG. 6.
Comparative antigen-specific IL-2 production by naive splenic T cells derived from TCR-Tg 11/4-12 and double-Tg mice. Spleen cells from TCR-Tg 11/4-12 and double-Tg mice were cultured with various concentrations of HBeAg or HBcAg for 2 days, at which time the SNs were collected in order to measure IL-2 levels by ELISA. Comparative IL-2 levels are expressed in OD units. This experiment is representative of four separate assays.
FIG. 7.
Kinetics of cytokine production in TCR-Tg 11/4-12 and double-Tg mice either unprimed or primed with HBeAg in vivo. Spleen cells from TCR-Tg 11/4-12 and double-Tg mice either unprimed (naive) or primed in vivo with HBeAg (40 μg in saline) 3 days prior to culture were incubated with HBeAg (10 μg/ml) for 1 to 4 days. At culture days 1, 2, and 4, IL-2 and IFN-γ production were measured by CELL-ELISA. Each datum point represents the mean (± the standard deviation) cytokine measurement from three mice.
T cells bearing the transgenic 11/4-12 TCR mediate anti-HBe antibody production in vitro and in vivo.
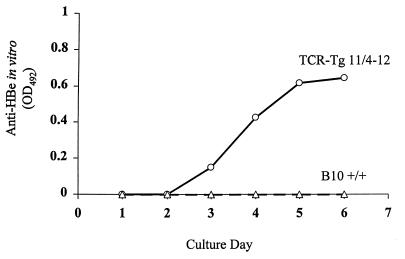
To determine if 11/4-12 TCR-Tg T cells could function as T helper cells for HBeAg-specific B cells and mediate antibody production, 7-day spleen cultures from B10 +/+ and 11/4-12 TCR-Tg mice in the presence or absence of HBeAg were monitored for the presence of anti-HBe antibodies in the SN (Fig. 8). Control cultures from both strains not containing HBeAg yielded no anti-HBe antibody production (data not shown). In the presence of HBeAg (0.2 to 5.0 μg/ml), only spleen cells from 11/4-12 TCR-Tg mice produced anti-HBe antibodies detectable in the SN. Anti-HBe antibodies were first detectable on day 3 of culture and continued to accumulate in the SN throughout the 7-day culture (Fig. 8). Apparently, the high frequency of HBeAg-specific CD4+ T cells present in 11/4-12 TCR-Tg spleen (67%) was sufficient to mediate a primary anti-HBe antibody response in vitro. It is notable that the anti-HBe response consisted exclusively of IgM antibodies; no IgG antibodies were detected.
FIG. 8.
In vitro anti-HBe antibody production in splenic cultures from naive TCR-Tg 11/4-12 and B10 wild-type (+/+) mice. Spleen cells were cultured for 7 days with from 0.2 to 5.0 μg/ml of HBeAg (only the data from the 5.0-μg/ml cultures are shown), and the SNs was harvested daily for the measurement of IgM anti-HBe antibodies by ELISA. Undiluted SN was used in the ELISA, and comparative anti-HBe levels are expressed as OD units. This assay is representative of three separate experiments.
Because 11/4-12 TCR-Tg T cells were functional as Th cells for antibody production in vitro, 11/4-12 TCR-Tg mice were immunized with 10 μg of HBeAg in saline and the in vivo anti-HBe antibody production was determined (Fig. 9). Although HBeAg-immunized 11/4-12 TCR-Tg mice were competent to produce IgG anti-HBe antibodies, the levels of anti-HBe were approximately 100-fold less than with HBeAg-immunized wild-type B10 +/+ mice. Therefore, production of high-titer IgG anti-HBe antibodies in vivo was dependent on factors other than HBeAg-specific Th-cell frequency. As shown in Fig. 9, the presence of endogenous HBeAg in double-TCR-Tg mice did not suppress in vivo anti-HBe antibody production and may have enhanced it by eliciting an “autoantibody” component as discussed in the following section.
FIG. 9.
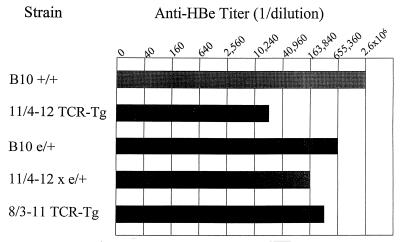
In vivo anti-HBe antibody production in TCR-Tg, HBeAg-Tg, double-Tg, and wild-type mice. Groups of four mice each were immunized in vivo with HBeAg (10 μg) in saline, and 4 weeks later sera were collected, pooled, and assayed for determination of IgG anti-HBe antibodies by ELISA. The anti-HBe endpoint titer is expressed as a reciprocal of the highest dilution of serum yielding an OD492 value three times that of preimmunization sera.
Variable anti-HBe autoantibody production in 11/4-12 TCR-double-Tg mice.
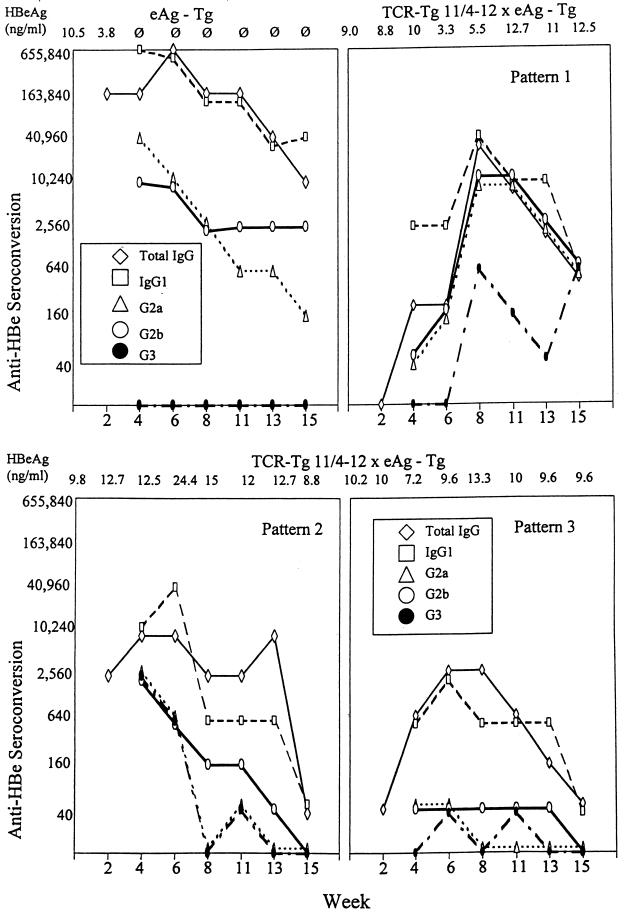
Because HBeAg-expressing Tg mice on an H-2b background are only partially tolerant, injection of the peptidic T-cell epitope 129-140 activates Th cells in vivo, which mediate anti-HBe seroconversion (16). Anti-HBe autoantibody is produced in excess of HBeAg, which becomes undetectable in the serum (see Fig. 10, first panel). Therefore, it was of interest to determine if the T cells bearing the transgenic 11/4-12 TCR in double-Tg mice could be activated by injection of the 129-140 peptidic T-cell epitope and to what extent this model autoimmune response might differ in HBeAg-Tg versus TCR-double-Tg mice. Injection of HBeAg-Tg mice with p129-140 results in anti-HBe autoantibody production dominated by the IgG1 subclass with little IgG2b and IgG2a anti-HBe antibodies and no IgG3 (Fig. 10, first panel). Injection of 11/4-12 TCR-double-Tg mice with p129-140 elicited variable responses which could be characterized by three distinct patterns approximately equally distributed. Approximately 30% of 11/4-12 TCR-double-Tg mice injected with p129-140 produced a slightly delayed (week 4) anti-HBe autoantibody response with a much broader IgG subclass distribution consisting of IgG1, IgG2a, IgG2b, and IgG3 compared to HBeAg-Tg mice (i.e., pattern 1, Fig. 10). The pattern 1 response is also characterized by a lower total IgG anti-HBe titer and delayed and less-efficient HBeAg serum clearance (i.e., reduced serum HBeAg levels only during weeks 6 through 8) compared to the response in HBeAg-Tg mice. Another 30% of 11/4-12 TCR-double-Tg mice injected with p129-140 demonstrated a response very similar to that of the HBeAg-Tg mice but of a lower magnitude and with no clearance of serum HBeAg (pattern 2). The third pattern is characterized by very inefficient anti-HBe autoantibody production (i.e., ≤1:2,560) and no clearance of serum HBeAg (Fig. 10). It is interesting that the autoantibody response can be more complex (pattern 1) with more mouse-to-mouse variation in TCR-double-Tg mice, which represents a limited TCR repertoire, compared to the consistently IgG1-dominated response exhibited by HBeAg-Tg mice, in which a polyclonal TCR repertoire exists. We have never observed any mouse-to-mouse variation in the anti-HBe seroconversion response of at least 50 HBeAg-Tg mice injected with p129-140. Perhaps the polyclonal response is more susceptible to crossregulation by a dominating Th2 cell phenotype as opposed to a monoclonal T-cell population, which may be influenced by environmental factors.
FIG. 10.
Seroconversion from HBeAg positivity to anti-HBe production in HBeAg-Tg and HBeAg-Tg × TCR-Tg 11/4-12 double-Tg mice elicited by priming with a synthetic T-cell peptide. Groups of 6 HBeAg-Tg and 12 double-Tg mice were injected with the peptidic T-cell epitope 129-140 (50 μg, incomplete Freund adjuvant) at time zero. Thereafter, sera were collected bimonthly and individually assayed for anti-HBe antibodies (total IgG and isotype specific) and HBeAg by ELISA assays. The HBeAg serum concentrations are shown across the top of each graph. Three patterns of anti-HBe seroconversion occurred in double-Tg mice, and representative data from single mice depict each pattern. Seroconversion in six HBeAg-Tg mice was nonvariable.
Adoptive transfer of 11/4-12 TCR-Tg T cells polarized to the Th1 subset can mediate liver injury in HBeAg- and HBcAg-expressing Tg recipients.
Because 11/4-12 TCR-Tg T cells were not deleted or functionally altered by exposure to endogenous HBeAg yet could be activated in vivo by exogenous HBeAg, it was of interest to determine the potential of these T cells to mediate liver injury upon adoptive transfer into HBeAg- or HBcAg-expressing Tg recipients. For this purpose, T cells derived from B10 +/+ mice or 11/4-12 TCR-Tg mice were cultured for two “cycles” with HBeAg in the presence of IL-12 and anti-IL-4 in order to activate and polarize the HBeAg-specific CD4+ T cells toward the Th1 subset. These polarized Th1 cells secrete low levels of IL-2 and high levels of IFN-γ and no IL-4 upon further culture with HBeAg (data not shown). Activated HBeAg-specific Th1 cells were then transferred into sublethally irradiated HBeAg or HBcAg-expressing Tg recipients. Serum samples were collected on a daily or weekly basis and serum ALT levels were determined as a measure of liver injury. Normal ALT levels in our mouse colony ranged between 20 and 60 U/liter, with occasional elevations at single time points; therefore, ALT values of ≥80 U/liter at multiple time points were considered significant elevations.
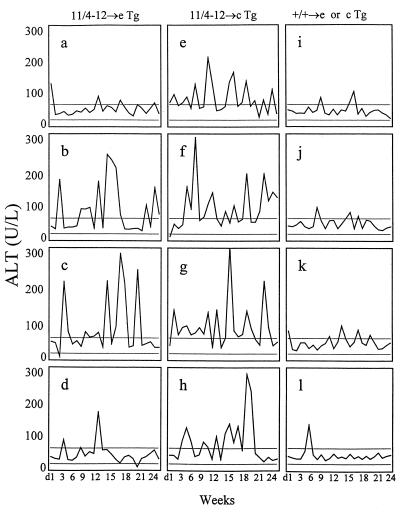
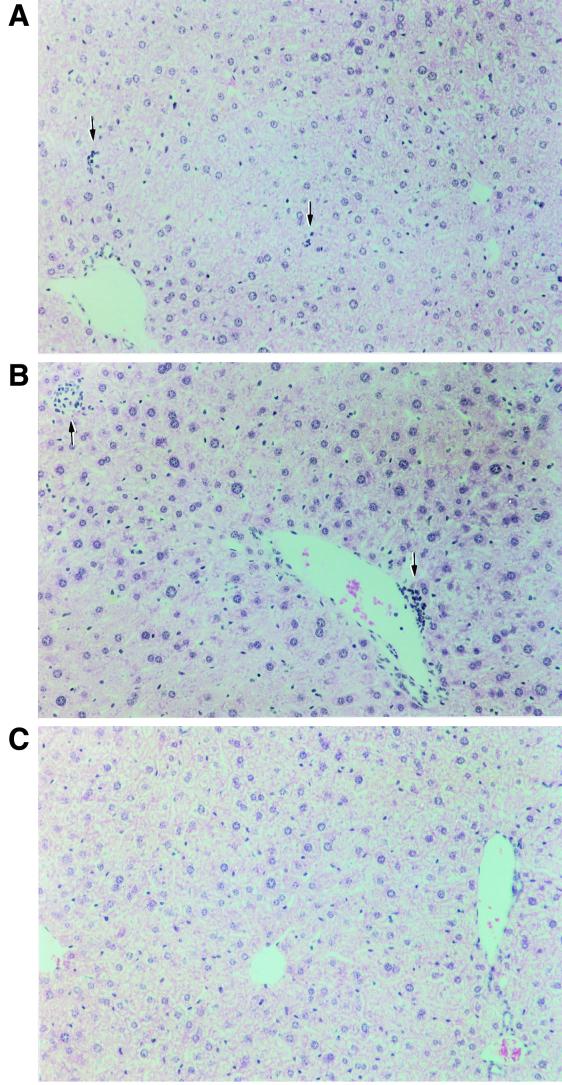
As shown in Fig. 11, adoptive transfer of in vitro-activated Th1 cells from 11/4-12 TCR-Tg mice into both HBeAg-Tg and HBcAg-Tg recipients resulted in relatively mild relapsing and remitting chronic liver injury, whereas adoptive transfer of Th1 cells cultured in vitro with HBeAg from non-Tg control mice elicited no liver injury. However, there were differences between HBeAg-Tg and HBcAg-Tg recipients of 11/4-12 TCR-Tg Th1 cells. Only 50% of HBeAg-Tg recipients demonstrated chronic liver injury compared to 100% of the HBcAg-Tg recipients, and the onset of liver injury appeared sooner in the HBcAg-Tg recipients (Fig. 11). The kinetics of liver injury in both groups was unique in each recipient and quite variable from mouse to mouse. At the termination of adoptive transfer experiments (6 months), liver sections of recipient mice were prepared for histological examination. The H&E-stained liver sections were read in a blinded fashion by a pathologist (S.N.T.). The pathology observed, which included portal inflammation, parenchymal necrosis, and pleiomorphic hepatocytes, was semiquantitated (Fig. 12 and Table 2). Portal inflammation (PI) was minimal in all livers: in HBeAg-Tg recipients one of seven livers demonstrated 1+ PI; in HBcAg-Tg recipients three of eight livers had evidence of PI of 1+ to 2+ severity; no inflammation was observed in the controls. Foci of parenchymal necrosis (FN) graded 2+ were observed in seven of eight HBcAg recipients and in one of seven HBeAg-Tg recipients; the recipients transferred with non-Tg Th1 cells showed 1+ FN. The presence of hepatocytes with large nuclei (pleiomorphic hepatocytes) graded as 2+ were observed in one of seven HBeAg-Tg recipients and in five of eight HBcAg-Tg recipients (Table 2). In summary, the HBcAg-Tg recipients demonstrated a greater degree of focal necrosis and pleiomorphic hepatocytes than the HBeAg-Tg recipients, and portal inflammation was minimal in both groups. This histology is consistent with chronic lobular hepatitis, which does not usually progress to cirrhosis.
FIG. 11.
Adoptive transfer of 11/4-12 TCR-Tg Th1 cells mediates liver injury in HBeAg- and HBcAg-expressing Tg recipients. Unprimed, donor T cells derived from either B10 +/+ mice or from 11/4-12 TCR-Tg mice were cultured with HBeAg (5 μg/ml) in the presence of IL-12 and anti-IL-4 for two cycles of 5 days each. In vitro-activated and Th1-polarized 11/4-12 TCR-Tg T cells (20 × 106) were then transferred into either HBeAg-Tg recipients (a to d) or HBcAg-Tg recipients (e to h). Donor cells from control (+/+) mice were transferred into HBeAg-Tg (i and j) or HBcAg-Tg (k and l) recipients. The recipients were sublethally irradiated (400 R) at the time of adoptive transfer. Hepatocellular injury was monitored by measuring serum ALT levels on a weekly basis. The normal range of serum ALT values in our mouse colony was 20 to 60 U/liter, as depicted by the horizontal bars. Each graph represents an individual mouse.
FIG. 12.
Liver histology in recipients of TCR-Tg 11/4-12 Th1 cells. (A) Rare and small foci of parenchymal necrosis and inflammation (arrows, +1) in HBeAg-Tg recipients of TCR-Tg 11/4-12 Th1 cells. (B) Rare and larger foci of parenchymal necrosis and inflammation (arrows, +2) in HBcAg-Tg recipients. Pleiomorphism of hepatocyte nuclei is also more pronounced in HBcAg-Tg recipients. (C) HBcAg-Tg recipients of T cells from control (+/+) donor mice show no necrosis or inflammation.
TABLE 2.
Histology of liver sections prepared 6 months after adoptive transfer of Th1 cells
| Donor | Recipient group (no. of animals) | Histology (no. positive/total no. [score]a
|
||
|---|---|---|---|---|
| PI | FN | PH | ||
| Tg-11/4-12 | HBeAg-Tg (7) | 1/7 (1+) | 1/7 (2+):6/7 (1+) | 1/7 (2+):6/7 (1+) |
| Tg-11/4-12 | HBcAg-Tg (8) | 1/8 (2+):2/8 (1+) | 7/8 (2+):1/8 (1+) | 5/8 (2+):3/8 (1+) |
| +/+ | HBeAg-Tg (1) | 0 | ± | 1+ |
| +/+ | HBcAg-Tg (1) | 0 | ± | 1+ |
PI, portal inflammation; FN, focal necrosis; PH, pleiomorphic hepatocytes.
DISCUSSION
The functional studies of 11/4-12 TCR-Tg mice suggest that T cells expressing the transgenic HBeAg-specific TCR survive in the presence of HBeAg in double-Tg mice by virtue of low avidity. The evidence for the low avidity of the 11/4-12 TCR-Tg CD4+ T cells is functionally defined and includes (i) a right-shifted dose-response curve after primary activation in vitro, (ii) the transient nature of T-cell activation in vitro, (iii) lack of clonal deletion in the thymus and the periphery in the context of HBeAg concentrations of 10 to 70 ng/ml in the serum, (iv) lack of anergy of peripheral CD4+ T cells, (v) altered fine specificity, and (vi) inefficient activation in vivo demonstrated by low levels of anti-HBe antibodies produced after immunization. Presumably, HBeAg-specific T cells demonstrating higher avidities than 11/4-12 TCR-Tg T cells would be either deleted in the thymus or anergized in the periphery upon exposure to serum HBeAg. For example, HBeAg-Tg mice on an H-2s background are totally tolerant at the level of CD4+ T cells in the context of a 10-ng/ml serum concentration of HBeAg (16, 18), and HBeAg-Tg mice on an H-2b background are partially tolerant (17).
Studies in HBV chronically infected patients suggest that HBeAg-specific CD4+ T cells are present even in the context of relatively high levels of circulating HBeAg (2, 9, 12, 27). We suggest that the transgenic HBeAg-specific CD4+ T-cell population existing in 11/4-12 TCR-Tg mice may represent a monoclonal model for the HBeAg-specific CD4+ T-cell repertoire present in long-term chronic carriers of HBV. Like TCR transgenic HBeAg-specific CD4+ T cells in double-Tg mice, HBeAg-specific CD4+ T cells in chronically infected patients must be able to coexist with serum HBeAg. In 11/4-12 TCR-double Tg mice, the transgenic T cells are quiescent in vivo and are neither spontaneously activated nor inactivated by deletion or anergy. However, exposure to exogenous HBeAg in vitro or in vivo is capable of activating 11/4-12 TCR-Tg CD4+ T cells derived from TCR single-Tg or double-Tg mice sufficiently to elicit cytokine production and T-helper-cell function for anti-HBe antibody production in vitro and in vivo. It is notable that the minimum HBeAg concentrations necessary to activate 11/4-12 TCR-Tg CD4+ T cells in vitro and in vivo are greater than the endogenous HBeAg concentrations (i.e., 10 to 70 ng/ml) examined. This suggests that the well-recognized fluctuations in HBeAg concentration during natural HBV infection may variably effect HBeAg-specific CD4+ T-cell activation. It has been previously proposed that increases of HBeAg concentration in the serum and accumulation of HBcAg intracellularly triggers HBeAg- and HBcAg-specific CD4+ T-cell activation and at least partially may account for the cyclic pattern of liver cell injury often observed in chronically infected patients (13). Similarly, the ability to detect Th-cell sensitization to the HBeAg and HBcAg in the PBL of chronic HBV patients coincides with periods of liver cell injury (27). These findings support the contention that the HBeAg- and HBcAg-specific CD4+ T-cell repertoire remaining in long-term chronically infected patients may be of low avidity. This would explain the limited ability to detect HBeAg- and HBcAg-specific T-cell proliferation or cytokine production in the PBL of chronic HBV patients (2, 9, 27). The presence of low-avidity, HBeAg- and HBcAg-specific CD4+ T cells may also explain the production of IgG-restricted anti-HBe antibodies, which are present in 50% of asymptomatic chronic carriers and IgG-unrestricted anti-HBe antibodies present in 100% of symptomatic chronic carriers, even though HBeAg remains in excess in the serum (12). If there were a total absence of HBeAg-specific CD4+ T cells in these chronic HBV patients, no anti-HBe antibody would be produced and the presence of high-avidity CD4+ T cells may result in HBeAg to anti-HBe seroconversion.
The preferential recognition of the monomeric HBeAg over the particulate HBcAg by 11/4-12 TCR-Tg T cells is an important observation. This finding establishes that CD4+ T cells with this unusual fine specificity do exist. CD4+ T cells that recognize the HBeAg preferentially to the HBcAg may represent at least one type of effector cell capable of selecting the HBeAg-negative mutant of the HBV (22). Generally, it has been observed that the HBeAg and HBcAg are fully cross-reactive at the level of CD4+ T-cell recognition due to the shared amino acid sequence between these two antigens, with the exception of the 10 residual precore amino acids present on serum HBeAg (3, 19). The 11/4-12 TCR-Tg T cells do not recognize the precore sequence (data not shown). The HBcAg is recognized preferentially to the HBeAg by HBeAg- and HBcAg-specific CD4+ T cells, presumably because the particulate structure confers an advantage in terms of antigen uptake by antigen-presenting cells (APC) (20). It appears that the epitope recognized by 11/4-12 TCR-Tg T cells is generated more efficiently by the presentation and processing of the HBeAg monomer than by the particulate HBcAg. Future studies will address questions regarding the fine specificity of the epitope, although the epitope does reside within residues 129 to 140, and whether this unique epitope is generated differentially depending on the APC type.
It was somewhat surprising that 11/4-12 TCR-Tg T cells, which are of sufficiently low avidity to escape deletion in the thymus and in the periphery, nevertheless can under the appropriate conditions mediate liver injury, as demonstrated by the adoptive-transfer experiments. First, it was not expected that low-avidity CD4+ T cells would be competent to mediate liver injury because hepatocytes express such limited amounts of MHC class II molecules. Secondly, it was of interest that the liver injury elicited was of a chronic relapsing and remitting nature, which is often observed in chronic HBV patients and in many autoimmune disorders as well. The low avidity of the 11/4-12 TCR-Tg CD4+ T cells may contribute to the relapsing and remitting course of liver injury. The partial or transient activation observed for these low-avidity T cells may permit their continued survival in the liver due to less-efficient downregulation via apoptosis or other regulatory mechanisms. An absence of sustained T-cell activation has been attributed to low TCR density (28). Similarly, low-avidity TCR binding resulting in fewer engagements of the TCR with peptide-MHC complexes may also deter sustained T-cell activation. We are currently investigating whether 11/4-12 TCR-Tg CD4+ T cells directly cause liver injury or perhaps mediate it through the activation of endogenous HBeAg- and HBcAg-specific CD8+ cytotoxic T lymphocytes. This seems unlikely because recipient mice received sublethal irradiation and because of the rather rapid onset of liver injury after adoptive transfer of the CD4+ T cells, especially in HBcAg-expressing Tg recipients. It is also possible that the CD4+ T cells mediate liver injury directly through a FAS-FASL mechanism since FAS+ hepatocytes are very sensitive to apoptosis (4, 21, 25, 26). Alternatively, proinflammatory cytokines produced by CD4+, Th1-like cells such as IFN-γ and tumor necrosis factor alpha, may mediate liver injury. The rather mild liver injury observed in this model most likely reflects limiting antigen concentrations in the Tg recipients rather than limited effector cell function. Lastly, it was notable that the HBcAg-Tg recipients experienced more liver injury than HBeAg-Tg recipients, even though 11/4-12 TCR-Tg T cells preferentially recognize the HBeAg, at least in the in vitro cultures.
Nondeletional TCR-Tg models have been described which have specificity for experimental antigens and autoantigens (5, 7, 10, 11). This study describes a nondeletional TCR-Tg model which has relevance to an infectious agent. The development of HBeAg- and HBcAg-specific TCR-Tg mice should enable us to obtain a greater understanding of the role of T-cell tolerance in the complex interactions between the immune response to the HBV nucleocapsid antigens, liver injury, and HBV clearance. We are currently examining additional HBeAg- and HBcAg-specific TCR-Tg lineages possessing an array of specificities and avidities in order to obtain a more complete view of the HBeAg- and HBcAg-specific CD4+ T-cell repertoire in the context of constant exposure to the HBe and HBc antigens much like what occurs in chronic HBV infection.
ACKNOWLEDGMENTS
We thank A. Theofilopoulos (The Scripps Research Institute) for performing the Vβ multiprobe RNase protection assays, M. M. Davis (Stanford) for providing the TCR shuttle vectors, and F. Schödel (EVAX) for providing recombinant HBeAg.
This research was supported by NIH grant AI 20720 and grants from the Swedish Medical Research Council (B95-16X-11219-01A) and the Swedish Work Environment Fund.
REFERENCES
- 1.Beech J T, Bainbridge T, Thompson S J. Incorporation of cells into an ELISA system enhances antigen-driven lymphokine detection. J Immunol Methods. 1997;205:163–168. doi: 10.1016/s0022-1759(97)00072-0. [DOI] [PubMed] [Google Scholar]
- 2.Ferrari C, Penna A, Bertoletti A, Valli A, Degli Antoni A M, Giuberti T, Cavalli A, Petit M A, Fiaccadori F. Cellular immune response to hepatitis B virus encoded antigens in acute and chronic hepatitis B virus infection. J Immunol. 1990;145:3442–3449. [PubMed] [Google Scholar]
- 3.Ferrari C, Bertoletti A, Penna A, Cavalli A, Missale G, Pilli M, Fowler P, Giuberti T, Chisari F V, Fiaccadori F. Identification of immunodominant T cell epitopes of the hepatitis B virus nucleocapsid antigen. J Clin Investig. 1991;88:214–222. doi: 10.1172/JCI115280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galle P R, Hofmann W J, Walczak H, Schaller H, Otto G, Stremmel W, Krammer P H, Runkel L. Involvement of the CD95 (APO-1/Fas)receptor and ligand in liver damage. J Exp Med. 1995;182:1223–1230. doi: 10.1084/jem.182.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Girgis L, Davis M M, de St. Groth B F. The avidity spectrum of T cell receptor interactions accounts for T cell anergy in a double transgenic model. J Exp Med. 1999;189:265–277. doi: 10.1084/jem.189.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez-Quintial R, Theophilopoulos A N. V beta gene repertoires in aging mice. J Immunol. 1992;149:230–236. [PubMed] [Google Scholar]
- 7.Goverman J, Woods A, Larson L, Weiner L P, Hood L, Zaller D M. Transgenic mice that express a myelin basic protein-specific T cell receptor develop spontaneous autoimmunity. Cell. 1993;72:551–560. doi: 10.1016/0092-8674(93)90074-z. [DOI] [PubMed] [Google Scholar]
- 8.Ho W Y, Cooke M P, Goodnow C C, Davis M M. Resting and anergic B cells are defective in CD28-dependent costimulation of naive CD4+ T cells. J Exp Med. 1994;179:1539–1549. doi: 10.1084/jem.179.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung M C, Diepolder H M, Spengler U, Wierenga E A, Zachoval R, Hoffmann R M, Eichenlaub D, Frosner G, Will H, Pape G R. Activation of a heterogeneous hepatitis B (HB) core and e antigen-specific CD4+ T cell population during seroconversion to anti-HBe and Anti-HBs in hepatitis B virus infection. J Virol. 1995;69:3358–3368. doi: 10.1128/jvi.69.6.3358-3368.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katz J D, Wang B, Haskins K, Benoist C, Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993;74:1089–1100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- 11.Lafaille J J, Nagashima K, Katsuki M, Tonegawa S. High incidence of spontaneous autoimmune encephalomyelitis in immunodeficient anti-myelin basic protein T cell receptor transgenic mice. Cell. 1994;78:399–408. doi: 10.1016/0092-8674(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 12.Maruyama T, McLachlan A, Iino S, Koike K, Kurokawa K, Milich D R. The serology of chronic hepatitis B infection revisited. J Clin Investig. 1993;91:2586–2595. doi: 10.1172/JCI116497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maruyama T, Iino S, Koike K, Yasuda K, Milich D R. Serology of acute exacerbation in chronic hepatitis B virus infection. Gastroenterology. 1993;105:1141–1151. doi: 10.1016/0016-5085(93)90960-k. [DOI] [PubMed] [Google Scholar]
- 14.Milich D R, Jones J E, Hughes J L, Price J, Raney A K, McLachlan A. Is a function of the secreted hepatitis B E antigen to induce immunologic tolerance in utero. Proc Natl Acad Sci USA. 1990;87:6599–6603. doi: 10.1073/pnas.87.17.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milich D R, Jones J E, McLachlan A, Houghten R, Thornton G B, Hughes J L. Distinction between immunogenicity and tolerogenicity among HBcAg T cell determinants: influence of peptide-MHC interaction. J Immunol. 1989;143:3148–3156. [PubMed] [Google Scholar]
- 16.Milich D R, McLachlan A, Raney A K, Houghten F, Thornton G B, Maruyama T, Hughes J L, Jones J E. Autoantibody production in HBeAg transgenic mice elicited with a self T cell peptide and inhibited with non-self peptides. Proc Natl Acad Sci USA. 1991;88:4348. doi: 10.1073/pnas.88.10.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milich D R, Schödel F, Peterson D L, Jones J E, Hughes J L. Characterization of self-reactive T cells that evade tolerance in HBeAg transgenic mice. Eur J Immunol. 1995;25:1663–1672. doi: 10.1002/eji.1830250628. [DOI] [PubMed] [Google Scholar]
- 18.Milich D R, Jones J E, Hughes J L, Maruyama T, Price J, Melhado I, Jirik F. Extrathymic expression of the intracellular hepatitis B core antigen results in T cell tolerance in transgenic mice. J Immunol. 1994;152:455–466. [PubMed] [Google Scholar]
- 19.Milich D R, McLachlan A, Stahl S, Wingfield P, Thornton G B, Hughes J L, Jones J. Comparative immunogenicity of hepatitis B virus core and E antigens. J Immunol. 1988;141:3617–3624. [PubMed] [Google Scholar]
- 20.Milich D R, Chen M, Schödel F, Peterson D L, Jones J E, Hughes J L. Role of B cells in antigen presentation of the hepatitis B core. Proc Natl Acad Sci USA. 1997;94:14648–14653. doi: 10.1073/pnas.94.26.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mita E, Hayashi N, Lio S, Takehara T, Hijioka T, Kasahar A, Fusamoto H, Kamada T. Role of Fas ligand in apoptosis induced by hepatitis C virus infection. Biochem Biophys Res Commun. 1994;204:468–474. doi: 10.1006/bbrc.1994.2483. [DOI] [PubMed] [Google Scholar]
- 22.Raimondo G, Schneider R, Stemler M, Smedile V, Rodino G, Will H. A new hepatitis B virus variant in a chronic carrier with multiple episodes of viral reactivation and acute hepatitis. Virology. 1990;179:64–68. doi: 10.1016/0042-6822(90)90274-u. [DOI] [PubMed] [Google Scholar]
- 23.Schödel F, Peterson D, Zheng J, Jones J E, Hughes J L, Milich D R. Structure of hepatitis B virus core and E-antigen: a single precore amino acid prevents nucleocapsid assembly. J Biol Chem. 1993;268:1332–1337. [PubMed] [Google Scholar]
- 24.Solheim J C, Alexander-Miller M A, Martinko J M, Connolly J M. Biased T cell receptor usage by Ld-restricted, turn-peptide-specific cytotoxic T lymphocyte clones. J Immunol. 1993;150:800–811. [PubMed] [Google Scholar]
- 25.Strand S, Hofmann W J, Grambihler A, Hug H, Volkmann M, Otto G, Wesch H, Mariani S M, Hach V, Stremmel W, Krammer P H, Galle P R. Hepatic failure and liver cell damage in acute Wilson's disease involve CD95 (APO-1/Fas) mediated apoptosis. Nat Med. 1998;4:588–593. doi: 10.1038/nm0598-588. [DOI] [PubMed] [Google Scholar]
- 26.Tagawa Y, Kakuta S, Iwakura Y. Involvement of Fas/Fas ligand system-mediated apoptosis in the development of concanavalin A-induced hepatitis. Eur J Immunol. 1998;28:4105–4113. doi: 10.1002/(SICI)1521-4141(199812)28:12<4105::AID-IMMU4105>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 27.Tsai S-L, Chen P J, Lai M Y, Yang P M, Sung J-L, Huang J-H, Hwang L H, Chang T-H, Chen D S. Acute exacerbations of chronic type B hepatitis are accompanied by increased T cell responses to hepatitis B core and e antigens. J Clin Investig. 1992;89:87–96. doi: 10.1172/JCI115590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viola A, Lanzavecchia A. T cell activation determined by T cell receptor number and tunable thresholds. Science. 1996;273:104–106. doi: 10.1126/science.273.5271.104. [DOI] [PubMed] [Google Scholar]