Key Teaching Points.
-
•
BRASH syndrome stands for a cluster of symptoms defined as bradycardia, renal failure, atrioventricular blockade, shock, and hyperkalemia.
-
•
Prompt diagnosis and management of BRASH syndrome can help avoid the necessity of pacemaker implantation, consequently reducing the risk of pacemaker-related infections.
-
•
Identifying and addressing the underlying triggers of BRASH syndrome will prevent lethal complications of BRASH syndrome.
-
•
Although BRASH syndrome may manifest with diverse symptoms, its diagnosis can be aided by specific laboratory tests and electrocardiographic findings.
Introduction
BRASH syndrome is defined as a constellation of symptoms including bradycardia, renal failure, atrioventricular (AV) nodal blocker, shock, and hyperkalemia.1 It is known to be caused by the synergistic effects of AV nodal blockers and renal dysfunction escalating the effect of the AV nodal blockade. Patients presenting with this syndrome can develop a wide range of clinical symptoms from asymptomatic bradycardia to cardiogenic shock. Unfortunately, it has become an underdiagnosed reversible cause of symptomatic bradycardia, which has led to premature pacemaker implantation. While it is difficult to see the incidence of unwarranted implantation of pacemakers, there was a study done that evaluated 382 pacemaker implants at 30 hospitals in Philadelphia County. This study showed 168 implants were indicated, 137 possibly indicated, and 77 (20%) not indicated.2 This is worrisome, as about 1%–2% of pacemakers implanted will end up causing infections, which can lead to lethal complications.3 Given the annual placement of more than 1 million pacemakers, precise identification of reversible causes takes precedence over preemptive pacemaker insertion.4 We describe a rare case wherein a patient presented with symptomatic bradycardia and not only explained why we refrained from pacemaker insertion, but highlighted the important clinical signs that lead to the diagnosis and treatment of BRASH syndrome.
Case report
A 57-year-old female patient with history of coronary artery disease with multiple percutaneous stent placements, grade 2 diastolic dysfunction with preserved left ventricular ejection fraction, end-stage renal disease on hemodialysis, hypertension, and diabetes who presented to the emergency department with weakness, fatigue, and shortness of breath. She reported recently missing dialysis and had missed multiple sessions in the past. Her home medications included carvedilol 6.25 mg twice a day, losartan 100 mg twice a day, amlodipine 10 mg, aspirin 81 mg, and atorvastatin 80 mg. On admission, the patient was bradycardic, with a heart rate in the 30s. Blood pressure was 116/36 mm Hg. The physical examination was notable for generalized weakness, bradycardia, mild crackles on lung examination, and bilateral lower extremity edema. Labs were significant for a potassium of 6.0, creatinine 9.1, BUN 46, high-sensitivity troponin 57, NT-proBNP 50,333. Initial electrocardiogram (ECG) showed a junctional escape rhythm at 42 beats per minute (Figure 1). Echocardiogram showed an ejection fraction of 65%. The patient was given atropine 1 mg intravenously ×2, which only increased her heart rate to the 40s. She was eventually transferred to the intensive care unit and started on a dopamine drip owing to continuous bradycardia with associated hypotension. Simultaneously, the patient received albuterol, calcium gluconate, insulin, and sodium zirconium cyclosilicate, followed by emergent dialysis.
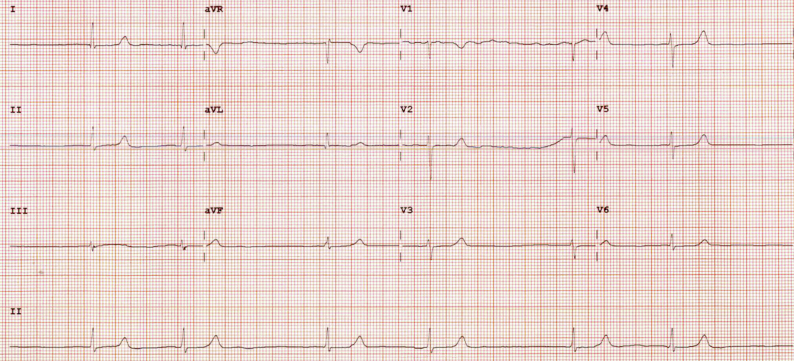
Figure 1.
Initial electrocardiogram showed sinus arrest with junctional escape with a heart rate of 42 beats/min.
The initiation of the dopamine drip elevated the heart rate to the 70s and she became normotensive shortly after. Subsequently, after the completion of the dialysis, the patient’s potassium level was normalized at 3.9. Following notable improvements in the patient’s vital signs and laboratory values, it was determined to gradually taper the dopamine dosage after 24 hours. However, during this process, the patient became increasingly dizzy and lightheaded, and her heart rate dropped to 40 beats per minute. Consequently, the patient was then reverted onto the dopamine drip and successfully tapered off it within an additional 24 hours, summing up to a total 48 hours on the dopamine drip.
Repeat ECG after dopamine was successfully discontinued showed normal sinus rhythm (Figure 2). She then was downgraded to the medicine team for further monitoring and was discharged with a new set of medications, including nifedipine and hydralazine, while her losartan and carvedilol were discontinued. Prior to discharge, proper education was given to avoid recurrence of BRASH syndrome and the patient was discharged with no further complications.
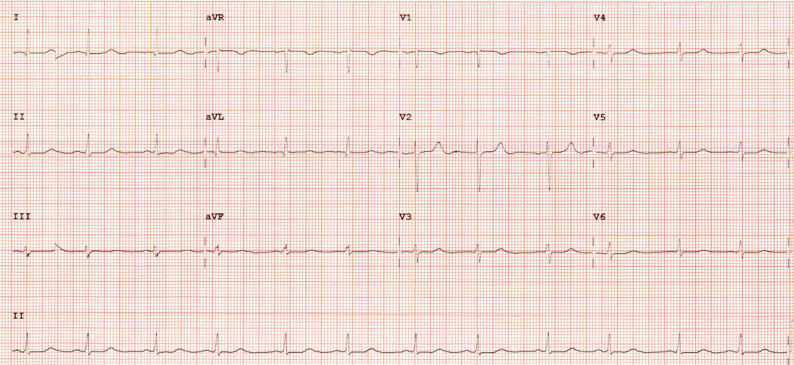
Figure 2.
After treatment, electrocardiogram showed normal sinus rhythm with heart rate in the 70s.
Discussion
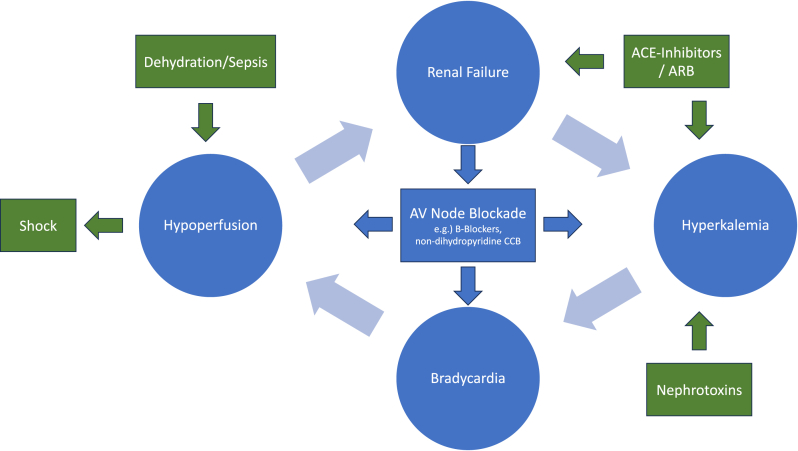
BRASH syndrome is typically due to the synergistic effects of AV nodal blocking medications and hyperkalemia in the setting of renal dysfunction. To fully understand how these synergistic effects could eventually lead to life-threatening complications it is important to understand how hyperkalemia itself can lead to bradycardia. Excess potassium will decrease membrane excitability and overall affect the cardiac action potentials by preventing repolarization. This will eventually decrease cardiac conduction that will then lead to isolated bradycardia.5 On the other hand, AV nodal blockers will act on the beta-1 receptors that are found on cardiomyocytes, which will suppress the conduction of electrical signals from the sinus atrial node, leading to bradycardia.6 BRASH syndrome is not simply excessive hyperkalemia or AV nodal blockade toxicity, but a vicious cycle that, when left unchecked, can progress to multiorgan failure (Figure 3).7 A big component of BRASH syndrome is the renal dysfunction. As AV nodal blockers and the hyperkalemia cause bradycardia, it will lead to decreased renal perfusion. This will lead to even worse renal dysfunction, which will eventually prolong the effects of AV nodal blockers and cause more hyperkalemia.8
Figure 3.
Pathophysiology/triggers of BRASH syndrome.
Patients with BRASH syndrome may have a wide variety of clinical symptoms ranging from asymptomatic bradycardia to cardiogenic shock. However, other than a patient’s clinical history, there are other objective indicators that can contribute to the diagnosis of BRASH syndrome, with an ECG being a pivotal diagnostic tool. Numerous studies show that the distinguishing factor on an ECG between BRASH syndrome and a patient solely with hyperkalemia is the lack of hyperkalemic ECG manifestations. However, the precise reason for this differentiation remains unknown. In the case of our patient, she had an elevated potassium level at 6.0, but there were no signs of peaked t waves (seen in potassium levels between 5.5 and 6.5), PR interval prolongation (seen in potassium levels 6.5–7.0), or QRS widening that is usually seen in isolated hyperkalemia (seen in potassium levels greater than 7.0).1
It is important to distinguish BRASH syndrome from symptomatic bradycardia, as the treatment differs from the advanced cardiovascular life support (ACLS) protocol. The ACLS algorithm involving atropine and cardiac pacing is not effective in these patients.8,9 Treatment of BRASH revolves around 3 approaches: treating the hyperkalemia; supporting the hemodynamics, which will involve bradycardia and hypotension; and identifying the trigger.8 In the management of hyperkalemia seen in BRASH syndrome, the first step is the administration of intravenous calcium, which is not seen in the ACLS protocol. Calcium is imperative in these patients for its cardioprotective measures by stabilizing the cardiac membrane.7 Other treatment options would be giving potassium-lowering agents, including intravenous insulin and dextrose, sodium zirconium cyclosilicate, and dialysis, which our patient required. It is important to additionally provide hemodynamic support. In our case, the patient’s hemodynamic instability stemmed mainly from bradycardia, leading to the necessity of a 48-hour dopamine drip. When the patient was initially weaned off dopamine, a discussion was had whether the patient would require a permanent pacemaker. However, given the notable rise of infections associated with permanent pacemakers, the decision was made to purse continued medical management.3 We suspected that the patient was still experiencing the lingering effects of the AV nodal blocking agents she received, particularly in the setting of renal failure. It was anticipated that hemodynamic support would only be necessary until these effects left her system, which would make permanent pacemaker placement inappropriate. A recent study, using the EMBASE, CINAHL, and Web of Science databases, conducted a systemic review of 595 reported BRASH syndrome cases until February 2022, revealing that none of the patients underwent permanent pacemaker implantation.10
Lastly, it is important to recognize the causative factor that causes BRASH syndrome and reverse it to avoid future episodes. Although BRASH syndrome can stem from various triggers, identifying its underlying cause requires a comprehensive understanding of the disease’s pathophysiology. As depicted in Figure 3, factors contributing to renal failure, hyperkalemia, bradycardia, or hypoperfusion can incite BRASH syndrome. Addressing or mitigating these triggers is key to minimizing the risk of developing this syndrome.
Conclusion
BRASH syndrome is a critically underdiagnosed syndrome that can have destructive complications if not recognized early. We presented a rare case of BRASH syndrome and emphasized the important symptoms, ranging from asymptomatic bradycardia to multiorgan failure. Additionally, we highlighted clinical signs such as bradycardia, renal failure, and hyperkalemia, notably lacking the typical hyperkalemic ECG manifestations. These findings played a pivotal role in our diagnostic process. The recognition of this syndrome allowed for reversal of the underlying cause, preventing unnecessary pacemaker implantation.
Disclosures:
No conflict of interest to report.
Acknowledgments
Funding Sources
None.
References
- 1.Lizyness K., Dewald O. StatPearls [Internet]. Treasure Island (FL) StatPearls Publishing; 2023. BRASH syndrome. 2023 Mar 27. Jan–. PMID: 34033405. [PubMed] [Google Scholar]
- 2.Greenspan A.M., Kay H.R., Berger B.C., Greenberg R.M., Greenspon A.J., Gaughan M.J. Incidence of unwarranted implantation of permanent cardiac pacemakers in a large medical population. N Engl J Med. 1988;318:158–163. doi: 10.1056/NEJM198801213180306. [DOI] [PubMed] [Google Scholar]
- 3.Klug D., Balde M., Pavin D., et al. PEOPLE Study Group Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation. 2007;116:1349–1355. doi: 10.1161/CIRCULATIONAHA.106.678664. [DOI] [PubMed] [Google Scholar]
- 4.Bhatia N., El-Chami M. Leadless pacemakers: a contemporary review. J Geriatr Cardiol. 2018;15:249–253. doi: 10.11909/j.issn.1671-5411.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parham W.A., Mehdirad A.A., Biermann K.M., Fredman C.S. Hyperkalemia revisited. Tex Heart Inst J. 2006;33:40–47. [PMC free article] [PubMed] [Google Scholar]
- 6.Ghumman G.M., Kumar A. BRASH syndrome leading to cardiac arrest and diffuse anoxic brain injury: an underdiagnosed entity. Cureus. 2021;13 doi: 10.7759/cureus.18628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farkas J.D., Long B., Koyfman A., Menson K. BRASH syndrome: bradycardia, renal failure, AV blockade, shock, and hyperkalemia. J Emerg Med. 2020;59:216–223. doi: 10.1016/j.jemermed.2020.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Wong C.K., Jaafar M.J. Bradycardia, renal failure, atrioventricular nodal blockade, shock, and hyperkalemia: an important syndrome to recognize. Turk J Emerg Med. 2021;21:86–89. doi: 10.4103/2452-2473.309138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neumar R.W., Shuster M., Callaway C.W., et al. Part 1: executive summary: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132:S315–S367. doi: 10.1161/CIR.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 10.Majeed H., Khan U., Khan A.M., et al. BRASH syndrome: a systematic review of reported cases. Curr Probl Cardiol. 2023;48 doi: 10.1016/j.cpcardiol.2023.101663. [DOI] [PubMed] [Google Scholar]