Abstract
The herpes simplex virus type 1 (HSV-1) immediate-early protein ICP27 is an RNA-binding protein that performs multiple functions required for the expression of HSV-1 genes during a productive infection. One essential function involves shuttling between the nucleus and the cytoplasm. Some of the domains identified in ICP27 include a leucine-rich nuclear export sequence (NES), a nuclear localization signal, three KH-like RNA-binding domains, and an RGG-box type RNA-binding motif. To study the contribution of two of the essential domains in ICP27 to HSV gene expression, we generated recombinant herpesviruses carrying deleterious mutations in the NES and KH domains of ICP27. To accomplish this, we fused the green fluorescent protein (GFP) to ICP27 and utilized fluorescence as a marker to isolate recombinant herpesviruses. Fusion of GFP to wild-type ICP27 did not disturb its localization or function or significantly reduce virus yield. Analysis of HSV gene expression in cells infected with a recombinant virus carrying a point mutation in the first KH-like RNA-binding domain revealed that nuclear export of ICP27 was not blocked, and the expression of only a subset of ICP27-dependent late genes was affected. These findings suggest that individual KH-like RNA-binding motifs in ICP27 may be involved in binding distinct RNAs. Analysis of recombinant viruses carrying a lethal mutation in the NES of ICP27 was not accomplished because this mutation results in a strong dominant-negative phenotype. Finally, we demonstrate that shuttling by ICP27 is regulated by an export control sequence adjacent to its NES that functions like the inhibitory sequence element found adjacent to the NES of NS1 from influenza virus.
During a productive infection, herpes simplex virus type 1 (HSV-1) gene expression proceeds in a tightly regulated cascade (15, 16). Based on their temporal expression during a productive infection, the genes of HSV-1 are classified into three kinetic classes, immediate early (α), early (β), and late (γ). The immediate-early gene products ICP4, ICP0, ICP27, and ICP22 cooperatively regulate the expression of all kinetic classes of virus genes (5, 6, 9, 10, 22, 28, 29, 35–37). ICP4, ICP0, and ICP22 activate transcription of early and late genes. In contrast, ICP27 is not a transcriptional activator per se; however, it is required for expression of early and late genes at both the transcriptional and posttranscriptional levels (22, 31, 37, 38, 48). Two functions of ICP27 that have been characterized are (i) shuttling to mediate the nucleocytoplasmic export of some late HSV-1 intronless RNAs and (ii) the inhibition of pre-mRNA splicing to mediate the shutoff of host cell protein synthesis (11, 13, 24, 25, 32, 33, 39, 40, 43, 50, 51).
ICP27 is an RNA-binding and export protein that contains two types of RNA-binding domains, an RGG-box and three KH-like RNA-binding domains (1, 18, 23, 25, 39, 51). KH motifs were first identified in the human heterogeneous nuclear ribonucleoprotein (hnRNP) K protein as a triple repeat (46). Subsequently, a number of proteins such as FMR-1, Nova-1, and ribosomal S3 proteins were also found to contain KH motifs. It is now clear that KH domains bind single-stranded RNA either singly or collectively and often nonspecifically (2).
A leucine-rich nuclear export sequence (NES) was first identified in Rev from human immunodeficiency virus (HIV) and has subsequently been found in a number of proteins, including ICP27 (19, 26, 34, 39, 50, 54). The NES, together with a nuclear localization signal (NLS), allows ICP27 and Rev to shuttle between the nucleus and cytoplasm. The cellular protein CRM1 belongs to the karyopherin family of proteins and binds this type of NES to mediate nuclear export via complex formation with the small GTPase Ran (34). Leptomycin B (LMB) inhibits this function of CRM1 and thus has been utilized to investigate the role of proteins that require CRM1 to exit the nucleus (7, 8, 30). Previously, we identified the cargo RNAs that are exported by ICP27 using LMB to inhibit nuclear export of ICP27 (51). In that study, we found that a subset of intronless HSV RNAs do not accumulate in the cytoplasm of infected cells in the presence of LMB.
The localization signal sequences and RNA-binding domains are considered central to the function of ICP27 in RNA export of HSV late RNAs. Previously, we presented evidence that RNA export is an essential function of ICP27 (50, 51). We identified the KH1, KH3, and NES domains in ICP27 to be essential. To determine the role of the first KH domain in ICP27, we have now introduced a mutation equivalent to the deleterious mutation in the KH1 domain of FMR-1 into the α27 gene (45). To determine the role of the NES domain, A's were substituted for L11 and L13 in the NES of ICP27. This approach was based on similar loss-of-function mutations identified in other NESs (54). To isolate HSV-1 recombinant viruses carrying point mutations in these domains of ICP27, we used a green fluorescent protein (GFP) tag as a marker to identify recombinant viruses. The analysis of HSV gene expression from these viruses suggests how some of these domains may contribute to the function of ICP27.
NES and NLS elements are thought to allow a protein to continuously shuttle between the nucleus and cytoplasm (34). However, we suspect that nuclear export of ICP27 is regulated because RNA binding appears to be a prerequisite to its nuclear export (50, 51). This hypothesis is based on two observations. First, a temperature-sensitive (ts) mutation in the KH-like domain that disrupts RNA-binding in vivo blocks nuclear export of ICP27. Second, ICP27 only exits the nucleus in the presence of late HSV RNAs. In infected cells, ICP27 does not shuttle at early times or when late gene transcription is reduced because of a block in DNA replication. In transfected cells, ICP27 required the coexpression of a late RNA to accumulate in the cytoplasm, although we and others have shown that it can shuttle constitutively when overexpressed in transient assays (24, 32, 39, 50). These findings led us to investigate whether ICP27 contains any elements that may inhibit nuclear export. Two distinct elements that inhibit nuclear export have been identified. One such element was found in hnRNP C, it overrides NES elements and retains any protein fused to it in the nucleus (27). In the NS1 protein from influenza virus, an inhibitory element adjacent to the NES of NS1 was identified that negatively regulates nuclear export of that protein (21). Therefore, we investigated whether ICP27 contained similar intrinsic signals that could regulate nuclear export of ICP27. An export control sequence (ECS) with properties similar to the element found in NS1 was identified in ICP27.
MATERIALS AND METHODS
Cells and viruses.
Vero cells were grown and maintained in Dulbecco modified Eagle medium (DMEM; Gibco BRL, Grand Island, N.Y.) containing 5% bovine calf serum (HyClone Laboratories, Inc., Logan, Utah) and supplemented with 100 U of penicillin and 100 μg of streptomycin (Gibco BRL) per ml. The ICP27 complementing cell line 2-2 is a derivative of Vero cells that express ICP27 under its own promoter (44). These cells were maintained in DMEM supplemented with 500 μg of G418 (Geneticin; Gibco BRL) per ml.
The strain of wild-type HSV-1 used in this study was KOS 1.1A. The transplacement vector, vBSΔ27, contains a lacZ gene fragment in place of the sequences encoding ICP27 and was described previously (50). vBSLG4 the ts HSV-1 mutant, (R480H), was described previously (12, 42, 50).
The viruses vBSGFP27, vBSECS, and vBStsECS were generated from vBSΔ27 by marker rescue for growth on Vero cells using a BamHI-linearized fragment containing the α27 allele from plasmids pBS7GFP27, pBSDD32AA, or pBSDD/LG4, as previously described (50). The HSV-1 mutant virus vBSGFPKH1 carrying a mutation in the KH1 domain was also generated by marker rescue using a BamHI-linearized fragment containing the α27 allele from plasmid pBSGFP303 but was isolated on 2-2 cells using the fluorescence of GFP as a marker for replicating virus. Unsuccessful attempts to isolate HSV-1 mutant viruses carrying a lethal mutation in the NES, vBSGFPNES, were performed as for vBSGFPKH1 using the linearized plasmid pBSGFPnes. All recombinant viruses were plaque purified three times.
Plasmid construction.
To fuse the DNA sequence encoding an enhanced GFP for use in eukaryotic cells (EGFP; Clontech, Palo Alto, Calif.) to ICP27, a BamHI/EcoRI fragment containing the ICP27 open reading frame and 3′ untranslated region from an ICP27 expression vector was introduced into the BglII/EcoRI sites of pEGFP-C1 (Clontech). The BspEI site was cut, filled with Klenow fragment, and religated to create an in-frame fusion with ICP27 as confirmed by DNA sequence analysis. The AgeI/EcoRI fragment containing the coding sequences of the GFP-ICP27 fusion and the 3′ untranslated region of ICP27 was ligated to the AgeI/EcoRI site in pBS727 to generate a recombination vector, pBS7GFP27, containing the ICP27 promoter driving the expression of a GFP-ICP27 fusion protein. Plasmid pBS727 contains the α27 gene from BamHI to SacI in a derivative of pUC19, pXW7, that had the DrdI sites removed by filling and religating. Plasmid pBSGFP303 was generated by removing the BssHII to SacI fragment containing the C-terminal coding region of ICP27 from pBS7GFP27 and ligating a similar BssHII to SacI fragment from pBSF303N that carries a point mutation (F303N) in ICP27.
Site-directed mutagenesis was performed to introduce mutations into the ICP27 alleles using the QuikChange Site-Directed Mutagenesis Kit as described by the manufacturer (Stratagene, La Jolla, Calif.). To verify that these mutations were introduced, plasmid DNAs were sequenced in the Columbia University Cancer Center DNA Sequencing Facility using oligonucleotide primers specific for the ICP27 open reading frame. The template used to generate some of these mutations is pBS27; it contains a wild-type copy of the α27 gene (51).
Plasmids pBS7GFP27nes and pBS27nes were generated using oligonucleotides (oligos) 27NES5′ and 27NES3′ on pBS7GFP27 and pBS27, respectively. Plasmid pBSF303N was generated using oligos 27 F303N 5′ and 27 F303N 3′ on pBS27. Plasmid pBSDD32 was generated using oligos 27 DD32 5′ and 27 DD32 3′ on pBS27.
Oligos 27NES5′ (5′-GCTAATTGACGCCGGCGCGGACCTCTCCG-3′) and 27NES3′ (5′-CGGAGAGGTCCGCGCCGGCGTCAATTAGC-3′) were designed to substitute A's for the critical L residues at amino acids 11 and 13 in the NES of ICP27.
Oligos 27F303N5′ (5′-GGAGGGCCCAATGACGCCGAG) and 27F303N3′ (5′-CTCGGCGTCATTGGGCCCTCC) were designed to substitute N for F at position 303.
Oligos 27 DD32 5′ (GAGCCGCCGCGCCGCCCTGGAATC) and 27 DD32 3′ (GATTCCAGGGCGGCGCGGCGGCTC) were designed to substitute A's for D's at residues 32 and 33.
pBSDD/LG4 was generated by replacing the BssHII to SacI fragment from pBSDD32 with the BssHII to SacI fragment from pBSLG4, a plasmid that contains the α27 gene from the LG4 virus as described previously (50).
Southern blot analysis.
Viral DNA was isolated from cytoplasmic extracts of infected Vero cells. Briefly, a 10-cm dish containing 2 × 107 Vero cells was infected with 2 × 108 PFU of HSV-1 for 24 h. Cells were washed, scraped into phosphate-buffered saline, and pelleted at 2,500 rpm in a Beckman centrifuge at 4°C. The pellet was resuspended in 1 ml of cold nuclear buffer (20 mM Tris, pH 7.5; 140 mM NaCl; 1 mM EDTA; 2 mM CaCl2; 3 mM MgCl2), and Triton X-100 was added to 0.5%. After 10 min on ice, nuclei were removed by centrifugation at 2,000 rpm in a Beckman centrifuge for 10 min. To the cytoplasmic fraction, proteinase K was added to 100 μg/ml, followed by incubation at 56°C for 2 h. The resulting cytoplasmic fraction was extracted with a mixture of phenol and chloroform and then precipitated with 2 volumes of ice-cold ethanol. The pellet was resuspend in 50 μl of Tris-EDTA with 0.5% sodium dodecyl sulfate (SDS), 10 mM EDTA and 10 μg of RNase A per ml. After incubation at 37°C for 30 min, the resulting nucleic acid solution was extracted with phenol-chloroform and precipitated with ethanol.
Viral DNAs from cells infected with HSV-1, vBSΔ27, vBSGFP27, and vBSGFPNES were digested with PstI and SalI, electrophoresed on a 1% agarose gel with 32P-end-labeled λ HindIII markers, and transferred to a Nytran membrane (Schleicher & Schuell, Keene, N.H.). The membrane was probed with a 32P-labeled DNA fragment derived from a BamHI-to-SacI digestion of the α27 gene from pBS27 (see Fig. 7) (50).
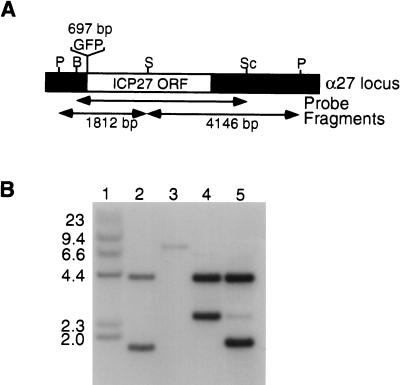
FIG. 7.
Southern blot analysis of recombinant GFP herpesviruses. (A) Schematic representation of the α27 locus from HSV-1. Shown are the locations of restriction sites for PstI (P), BamHI (B), SalI (S), and SacI (Sc). The ICP27 open reading frame is indicated by the white box and the GFP open reading frame is indicated by the shaded box above the diagram. The probe used is also indicated by the arrow. The predicted sizes for the restriction fragments are indicated below the arrows for the respective fragments and above the GFP box. (B) Autoradiogram of a Southern blot probed with an α27-specific probe. Lane 1, end-labeled λ HindIII size markers with the adjacent size (in kilodaltons) indicated. Lanes 2 to 5, viral DNA digested with PstI and SalI. The lane order is as follows: 2, HSV-1; 3, vBSΔ27; 4, vBSGFP27; and 5, vBSGFPNES. The expected sizes for the fragments from HSV-1 are 4,146 and 1,812 bp. The expected sizes for the fragment from vBSΔ27 is 8,023 bp; those for vBSGFP27 and vBSGFPNES are 4,146 and 2,509 bp, respectively, due to the addition of 697 bp encoding for GFP to the smaller fragment expected for HSV-1.
GFP27 localization and immunofluorescence.
Vero cells seeded onto coverslips were infected with vBSGFP27 or vBSGFPKH1 at a multiplicity of infection (MOI) of 10 and, following a 1-h treatment with cycloheximide at 8 h postinfection, were visualized “live.”
Vero cells were infected with 10 PFU of HSV-1 or pBSDD32 per cell for 6 h and then treated with 100 μg of cycloheximide per ml for 2 h and harvested. Where indicated, cells were treated with 300 μg of phosphonoacetic acid (PAA) and 25 ng of LMB (provided by Minoru Yoshida, University of Tokyo) per ml. Cells were fixed with 3.7% formaldehyde, permeabilized with acetone, and incubated with the ICP27-specific polyclonal antibody, Clu38. ICP27 was visualized by addition of fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit immunoglobulin G (Kirkegaard and Perry, Gaithersburg, Md.).
Preparations were viewed using a Leitz Dialux microscope with optical systems for the visualization of fluorescein and GFP. Representative fields of cells were photographed using Kodak Ektachrome P1600 film (Eastman Kodak, Rochester, N.Y.) and a Nikon UFX-DXII photographic system. Images of the developed slides were secured using a Polaroid ES plus scanner and Adobe Photoshop software. Digitized images were assembled using Canvas 7.1 on a Macintosh G3 computer.
Virus growth and complementation assays.
Vero cells (106) were seeded in 35-mm dishes and infected the next day at an MOI of 0.1. The infections were allowed to proceed at the temperatures and times indicated. Infections were halted by freezing the cultures at −80°C. Infected cells were subjected to five cycles of freezing and thawing, and virus yields were determined by titration on Vero cells. Growth curves represent the average of three independent infections, each titrated in duplicate.
To determine the plating efficiency on Vero cell lines expressing different ICP27 alleles, viruses were titrated in duplicate on cell monolayers in six-well plates.
For complementation assays, Vero cells (4 × 105) were seeded in 60-mm dishes. The next day cells were cotransfected with nucleocapsids from HSV-1 (47) and 15 μg of a plasmid expressing different alleles of ICP27 under its natural promoter or, as a control, the empty vector (pIBI31). At 48 h posttransfection, viruses were harvested by four sequential freeze-thaw cycles and then titrated on Vero cells to determine virus yield.
Immunoblots.
Cells were infected at an MOI of 10 and harvested at the indicated times. Proteins were separated by electrophoresis on SDS–7.5% polyacrylamide gels (SDS-polyacrylamide gel electrophoresis [PAGE]) and transferred to nitrocellulose. The primary antibodies used were as follows: ICP4, mouse monoclonal 58S; ICP0, Clu7; ICP27, Clu38; UL9, R-anti-UL9 (provided by M. Challberg, National Institutes of Health); VP5, NC-1 (provided by G. Cohen, Department of Microbiology, University of Pennsylvania); VP16 (Clontech); gB, R69 (provided by G. Cohen); and gC, R47 (provided by G. Cohen). Secondary antibodies were horseradish peroxidase-conjugated goat anti-mouse and anti-rabbit antibodies (Kirkegaard and Perry). Detection utilized the chemiluminescent substrate LumiGLO (Kirkegaard and Perry), followed by exposure to Biomar Blue film (March Biochemical, Rochester, N.Y.).
RESULTS
Construction of a recombinant HSV that expresses a GFP-ICP27 fusion protein.
ICP27 is an essential protein required for multiple functions during an infection with HSV-1 (11, 13, 31, 35, 38, 39, 48, 50). Examination of the contributions of putative functional domains in ICP27 required the construction of recombinant herpesviruses expressing mutant alleles of ICP27. Isolation of recombinant viruses carrying mutations in essential domains of ICP27 is difficult because of the inability to select on the complementing cell line. To overcome this obstacle, we sought to determine whether GFP could be fused to ICP27 and utilized both as a marker for recombination and as a tool to study the trafficking of ICP27. Therefore, a recombinant herpesvirus that expressed a wild-type GFP-ICP27 fusion protein was generated as described in Materials and Methods. To determine if the recombinant viruses expressed a biologically active form of ICP27, virus pools were tested for the ability to form plaques on Vero cells. All of the recombinant viruses successfully formed plaques on Vero cells, and these proved to be fluorescent when viewed by fluorescent microscopy. Therefore, the GFP-ICP27 fusion protein complements virus growth. One recombinant virus was isolated and designated vBSGFP27.
To determine if the fusion of GFP encumbered the function of ICP27, we analyzed the kinetics of virus growth. The growth of vBSGFP27 was compared to growth of the wild-type HSV-1 (Kos1.1A) on Vero cells. The results demonstrate a slightly slower growth rate for vBSGFP27 compared to HSV-1; however, by 48 h postinfection the yields are similar. This indicates that GFP does not significantly hinder ICP27 in its functions required for virus replication in tissue culture (Fig. 1).
FIG. 1.
Analysis of GFP virus growth kinetics. Vero cells were infected at an MOI of 0.1 with HSV-1 (○) or vBSGFP27 (■) and incubated at 37°C. The infections were stopped at the indicated times, and the virus yields were determined by titration on Vero cells. Datum points represent the average of three independent infections, each titrated in duplicate.
Based on our finding that the fusion of GFP does not significantly affect virus growth, we assumed that function of ICP27 was unimpaired. Therefore, we chose to utilize the fluorescence of GFP as a marker to simplify the isolation of recombinant herpesviruses carrying lethal mutations in ICP27. Our goal was to generate recombinant herpesviruses expressing alleles of ICP27 carrying point mutations in domains that might be required for the RNA export function of ICP27. The domains targeted were the NES and KH1 domains of ICP27. Figure 2 shows the position of amino acid changes in the KH domains of ICP27 that were previously determined to be lethal (51). The genotype and phenotype of the viruses constructed for this study and the plasmids used to generate them are described in Table 1.
FIG. 2.
Schematic diagram of domains in ICP27. The characterized domains include an NES, an NLS, and an RGG-box type RNA-binding motif, three KH motifs (KH1, KH2, and KH3), and an SM motif. Also shown is the position of mutations described here. These are the ts mutation R480H, the Fmr-like mutation in KH1, F303N, and the mutation DD32AA in the proposed ECS domain resembling the alterations that disrupt the inhibitory sequence in NS1.
TABLE 1.
Viruses and plasmids
| Virus or plasmida | Genotype | Phenotype | Source or reference(s) |
|---|---|---|---|
| vBSΔ27 | α27 gene deleted | Requires complementing cell line for growth | 50 |
| vBSGFP27 | GFP fused to wild-type ICP27 | Growth on Vero cells | This study |
| vBSGFPKH1 | GFP fused to ICP27 with KH1 mutation (F303N) | Requires complementing cell line for growth | This study |
| vBSGFPNES | GFP fused to ICP27 with NES mutations (L11,13A) | Unable to maintain genomic stability on complementing cell line | This study |
| vBSECS | ICP27 with ECS mutations (D32,33A) | Growth on Vero cells | This study |
| vBSLG4 | ICP27 with KH3 ts mutation (R480H) | Growth on Vero cells only at 32°C | 12, 42, 50 |
| vBStsECS | ICP27 with ECS mutations (D32,33A) and KH3 ts mutation (R480H) | Growth on Vero cells only at 32°C | This study |
| pBS27 | Wild-type ICP27 | Complements vBSΔ27 | 50 |
| pBS27nes | ICP27 with NES mutations (L11,13A) | Unable to complement vBSΔ27 | 51 |
| pBSF303N | ICP27 with KH1 mutation (F303N) | Unable to complement vBSΔ27 | 51 |
A “v” identifies recombinant virus constructs, whereas a “p” identifies plasmid constructs.
Plasmid constructs were generated that carry these alleles of ICP27 fused in frame to GFP. These alleles were then recombined into the vBSΔ27 genome as described earlier (51). After cotransfection, the lysates were plated on the complementing cell line, 2-2. Fluorescent plaques indicative of recombinant virus were observed. Fluorescent plaques were purified at least three times on 2-2 cells until all plaques that formed fluoresced. The viruses were designated vBSGFPNES and vBSGFPKH1 for the NES and KH1 mutant alleles, respectively. These viruses are unable to form plaques on the noncomplementing cell line (Vero), a finding consistent with our previous suggestion that these mutations are lethal (51).
Analysis of virus protein accumulation in cells infected with vBSGFP27 and vBSGFPKH1.
We wanted to analyze the effect a lethal mutation in the KH1 RNA-binding domain of ICP27 would have on HSV gene expression. Accordingly, the kinetics of virus-specified protein accumulation was assessed by Western blots during a time course of infection in cells infected with vBSGFP27 and vBSGFPKH1. Vero cells were infected and extracts were harvested at the times indicated (Fig. 3). Proteins were separated by SDS-PAGE and transferred to nitrocellulose. To determine the integrity of the GFP-ICP27 fusion product, Western blot analysis was performed using an ICP27-specific polyclonal antibody, Clu38. The lane corresponding to cells infected with HSV-1 has a prominent band representing wild-type ICP27 at its reported Mr of 63 kDa (Fig. 3A). In the control lane corresponding to mock-infected cells no bands are evident, thus demonstrating the specificity of the antiserum. In the lanes corresponding to cells infected with vBSGFP27 and vBSGFPKH1, a prominent band of approximately 96 kDa is evident. This is the size expected if a 27-kDa GFP polypeptide was fused to ICP27, confirming the fusion of GFP to ICP27. Further analysis of the lanes representing GFP-ICP27 fusion proteins revealed additional bands that migrate with mobilities that are similar to that of native ICP27. To determine if these additional bands represented degradation products or alternative translational products, we analyzed the migration of newly synthesized and mature ICP27 polypeptides by incorporating [35S]methionine-[35S]cysteine. Infected cells were pulsed labeled for 15 min at 5 h postinfection and harvested (pulse) or replaced with medium and harvested 2 h later (chase). Lysates were then immunoprecipitated with an ICP27-specific antibody, and the extracts were subjected to SDS-PAGE analysis. The resulting autoradiogram (Fig. 4) revealed that the full-length ICP27 and GFP-ICP27 fusion products were synthesized and endured for 2 h. However, the half-life of GFP-ICP27 fusion products is reduced compared to ICP27 alone. Synthesis of the wild-type ICP27 polypeptide also produces a faster-migrating species, observed after the pulse that is not found in the chase. This species may represent an unstable polypeptide that was either prematurely truncated or alternatively initiated. The GFP-ICP27 fusion proteins also appear to have at least two smaller products that comigrate with ICP27 and the unstable faster-migrating immunoreactive species. The KH1 mutation appears to increase the abundance of the most rapidly migrating species (46 kDa). These results correspond with and may explain the appearance of polypeptides detected by Western blotting (Fig. 3) that correspond in size to ICP27 from cells infected with viruses expressing fusion proteins.
FIG. 3.
Analysis of virus-specified protein accumulation by a KH1 mutant. Vero cells were either mock infected (M) or infected with vBSGPF27, vBSGPKH1, or HSV-1 at an MOI of 10 at 37°C. At the times indicated, protein extracts from infected cells were harvested, subjected to SDS-PAGE analysis, and transferred to nitrocellulose. Shown are representative Western blots using a series of antibodies specific for HSV-1 proteins ICP27, ICP4, ICP0, UL9, VP16, VP5, gB, and gC. (A) Expected mobility of the GFP-ICP27 fusion protein (GFP-27) and ICP27. (B) Relative protein accumulation of HSV-1 specified proteins at various times comparing cells infected with vBSGFP27 to vBSGFPKH1.
FIG. 4.
Pulse-chase analysis of ICP27 proteins synthesized in HSV-infected cells. Vero cells were either mock infected (M) or infected with HSV-1, vBSGFP27, or vBSGFPKH1 at an MOI of 10 and then incubated at 37°C. The cells were then pulse-labeled with Tran35S-label for 15 min and either harvested (P) or chased by replacing the medium, incubated for 2 h, and then harvested (C). Whole-cell extracts were immunoprecipitated with an ICP27-specific polyclonal antibody, Clu38, and then subjected to SDS-PAGE analysis. Labeled proteins were visualized by autoradiography. Full-length ICP27 or GFP-ICP27 proteins are labeled as such. Molecular mass standards are indicated in kilodaltons.
We next examined the accumulation of HSV proteins belonging to each of the three major temporally regulated classes using antiserum specific for the immediate-early proteins ICP4 and ICP0, the early protein UL9, and the late proteins VP5, VP16, gB, and gC. The results (Fig. 3B) show that only the expression of gC was significantly reduced in cells infected with vBSGFPKH1 compared to its wild-type counterpart, vBSGFP27. This defect in HSV gene expression differs from when nuclear export of ICP27 was blocked using LMB or when the KH3 domain of ICP27 carries a ts mutation (51). In those experiments, a defect was found in the expression of several HSV-1 late genes, including VP16, VP5, gB, and gC. Therefore, although the mutation in the KH1 domain is lethal, only a subset of HSV late genes, whose expression is regulated by ICP27, was affected.
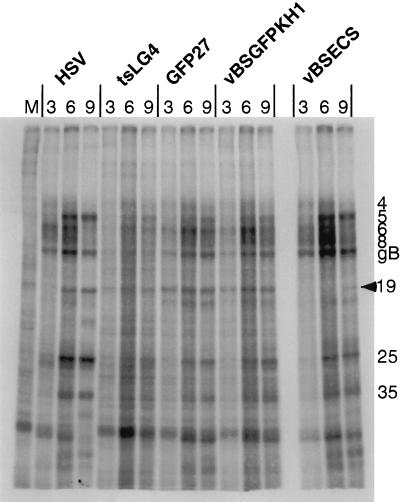
To confirm these results, virus protein synthesis was compared in cells infected with HSV mutants carrying lethal mutations in the KH1 domain, i.e., vBSGFPKH1, and the KH3 domain, i.e., vBSLG4. We found that virus protein synthesis differed significantly (Fig. 5) as fewer virus-specified proteins were synthesized in cells infected vBSLG4 than were synthesized in cells infected with vBSGFPKH1. This finding confirmed that lethal mutations in the KH1 and KH3 domains do not result in the same defects in HSV gene expression.
FIG. 5.
Analysis of protein synthesis in HSV-infected cells. Cells were either mock infected (M) or infected with the viruses HSV-1, vBSLG4, vBSGFP27, vBSGFPKH1, or vBSECS as indicated at an MOI of 10 and incubated at 39.5°C. At the times as indicated, cells were pulsed-labeled for 30 min with Tran35S-label. Extracts were subjected to SDS-PAGE analysis on a 7.5% acrylamide gel. An autoradiogram is shown. The arrowhead indicates the protein discussed in the text.
Would the mutation in the KH1 domain block nuclear export of ICP27 as was demonstrated for the tsR480H mutation in the KH3 domain? To address this question, we observed the intracellular localization of the GFP-ICP27 fusion proteins in “live” cells at 8 h postinfection. The wild-type GFP fusion protein was observed in several distinct patterns that reflect previously observed patterns seen by staining for ICP27 using immunofluorescence. However, visualization of ICP27 localization patterns using GFP was significantly clearer than when standard immunofluorescent techniques were used. In Fig. 6A, the protein appears in the nucleus in two distinct size clusters. The small clusters resemble what is observed when ICP27 reorganizes and colocalizes with snRNPs (40, 41). The large cluster resembles HSV DNA replication compartments or could represent ICP27 accumulating in the nucleolus, both of which are sites where ICP27 is known to localize (4, 23). In Fig. 6B, the protein appears diffusely in the nucleus and in the cytoplasm, consistent with observations that the protein can shuttle between the two compartments. In cells infected with vBSGFPKH1, the fusion protein is detected in subnuclear structures similar to the wild-type GFP-ICP27 fusion as well as in the cytoplasm of some cells (Fig. 6C). This demonstrates that the mutation in the KH1 motif does not affect the localization or trafficking of ICP27. This finding again differs from results obtained with the ts mutant vBSLG4, where nuclear export is blocked at the restrictive temperature (50, 51).
FIG. 6.
GFP-ICP27 localization in infected cells. Vero cells were infected at an MOI of 10 with vBSGFP27 (A and B) or vBSGFPKH1 (C). Infected cells were visualized for fluorescence “live” at 8 h postinfection following a 2-h treatment with cycloheximide. The selected fields photographed represent the different localization patterns observed.
The lethal NES mutation in ICP27 also confers a dominant-negative phenotype.
Mutant viruses carrying an inactivating mutation in the NES of ICP27 were examined to determine the role of this domain on HSV gene expression. Examination of the genetic organization of the recombinant viruses described here by Southern blot hybridization revealed the expected makeup, except for vBSGFPNES (Fig. 7). DNA extracted from vBSGFP27 hybridized to two bands of 4,146 and 2,509 bp corresponding to the correct integration of the targeting construct. However, in the lane corresponding to viral DNA isolated from the stock of vBSGFPNES, an abundant smaller fragment of approximately 1,850 bp was present, in addition to the expected two fragments. This smaller 1,850-bp fragment appears to be represented to the same extent as the larger expected fragment of 4,146 bp. In addition, the expected 2,509-bp fragment is present but is significantly less abundant than either of the other two bands. This shows that a deletion event took place in the 2,509-bp fragment resulting in most of the genomes being deleted of approximately 700 bp. Because these viruses maintain the GFP coding region, as judged by the fact that all plaques fluoresce, it is likely that the deletion maps to the amino-terminal half of the ICP27 open reading frame.
A simple interpretation of this result is that this virus (vBSGFPNES) has undergone a spontaneous deletion event. Alternatively, we considered that virus growth might have been inhibited by the expression of ICP27 containing the NES mutation, even when the virus is grown in a complementing cell line that expresses wild-type ICP27. If this interpretation is correct, then the NES mutation conveys a dominant-negative phenotype. The deletion that was observed in the recombinant virus might have been required to relieve this phenotype and allow virus growth. To distinguish between these possibilities, additional GFPNES isolates from independent transfections were examined by Southern blot analysis. The results obtained reveal that the genomes isolated from fluorescent plaques all contained deletions of various sizes in the same DNA fragment containing the coding region for GFP and the amino-terminal half of ICP27 (data not shown). The screening of these additional recombinant viruses was complicated because the fluorescence was often lost after a few rounds of purification for some isolates. This suggested that further deletion events, which include the GFP and the ICP27 coding regions, were occurring in these cases. We also observed a lower frequency of recombinant viruses for the mutant NES allele compared to the frequency observed with the mutant KH1 allele of ICP27. Implicit in these findings is the suggestion that the presence of an allele carrying the NES mutation is deleterious for virus growth even in the presence of wild-type ICP27. Furthermore, all virus isolates that were studied had undergone deletions of this region, presumably to relieve the dominant-negative phenotype.
To confirm that the ICP27 NES mutation was sufficient to confer a dominant-negative phenotype in the absence of GFP, a mutant ICP27 was expressed in trans and virus growth was quantified. Wild-type HSV-1 nucleocapsids were cotransfected with plasmids expressing either a wild-type allele of ICP27 or one carrying the lethal NES or KH1 mutations. Virus yields from the transfections were then quantified by plating the extracts on Vero cells and counting plaques. The HSV yields with the cotransfected plasmids were as follows: vector, 2 × 108; pBS27, 1 × 109; pBS27nes, 2 × 106; and pBSF303N, 4 × 108. The virus yield was thus reduced 2 logs in the presence of the transiently expressed ICP27 NES mutant. This finding supports our hypothesis that the NES mutation confers a dominant-negative phenotype.
Analysis of cis-acting shuttling control elements in ICP27.
During our analysis, we searched for possible cis-acting elements that could regulate export of ICP27. Examination of the ICP27 open reading frame revealed a stretch of amino acids adjacent to the amino-terminal NES that were similar to a sequence that inhibits the nuclear export of NS1 from influenza virus (21) (Fig. 8A). When the R and E residues in NS1 were mutated to A's, the ability to retain NS1 in the nucleus was lost. The amino acids adjacent to the NES in ICP27, which resembled the residues in NS1, were mutated to determine if they affected the export of ICP27. The D repeat at positions 32 and 33 in ICP27, resembling the dipeptide repeat of glutamic acid residues in NS1, was altered to A's. A recombinant HSV carrying this mutant allele of ICP27, vBSECS, was isolated on Vero cells as described above.
FIG. 8.
Identification of an ECS in ICP27. (A) Sequence alignment of the NES and inhibitory sequence of NS1 from influenza virus with the corresponding amino acids in ICP27 from HSV-1. The NES residues for both NS1 and ICP27 are underlined. The residues in NS1 that are critical for inhibiting the NES function and the corresponding amino acids in ICP27 that were altered to alanine in vBSECS are shown in boldface. (B) Vero cells were infected at an MOI of 10 with HSV-1 or vBSECS as indicated. After 1 h of adsorption, HSV-infected cells were either left untreated or were treated with PAA or LMB as indicated. Infected cells were processed for immunofluorescence at 8 h postinfection following a 2-h treatment with cycloheximide. The intracellular distribution of ICP27 was examined with an antibody specific for ICP27 and an FITC-labeled secondary antibody.
Previously, we defined conditions where wild-type ICP27 was unable to shuttle when PAA or a conditional lethal DNA− phenotype was used to limit the transcription of HSV late genes through the inhibition of HSV DNA replication (51). To determine if mutation of the putative ECS in ICP27 abrogated control on exiting the nucleus under conditions where DNA synthesis was inhibited, cells were infected with HSV or vBSECS and the intracellular localization of ICP27 was examined by use of immunofluorescence. To confirm that the accumulation of ICP27 in the cytoplasm is the result of shuttling, we utilized LMB as a control. ICP27 requires CRM1 to exit the nucleus (51). In the presence of LMB, CRM1 is inhibited, resulting in nuclear retention of ICP27. The results show (Fig. 8B) that ICP27 accumulates in the cytoplasm of Vero cells infected with either HSV or vBSECS, but not in the presence of LMB. This demonstrates that the accumulation of ICP27 in the cytoplasm, in the absence of LMB, is the result of nuclear export. However, ICP27 from wild-type virus does not accumulate in the cytoplasm in cells infected in the presence of PAA. In contrast, ICP27 in cells infected with vBSECS continues to accumulate in the cytoplasm in the presence of PAA. Parenthetically, we note that vBSECS has the same reduced plaquing efficiency as wild-type virus in the presence of PAA and thus retains its sensitivity to inhibition by the drug. These data suggest that this allele of ICP27 is no longer regulated for shuttling. We propose that ICP27 contains a cis-acting sequence element that regulates its nuclear export. This ECS not only appears adjacent to the NES in ICP27 as in NS1 but also resembles and functions like the element found in the NS1 protein. Thus, these proteins may use a common mechanism to regulate their nuclear export.
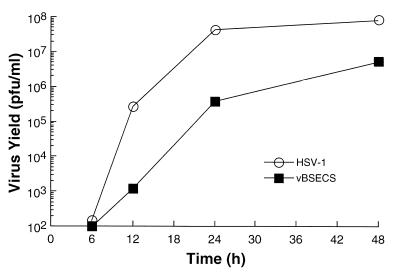
To determine if the mutations in the ECS of ICP27 that permit constitutive shuttling of the protein interfere with its function, we analyzed the kinetics of growth of the mutant virus. Figure 9 shows that the kinetics of growth is slower and that virus yield is reduced for the mutant. This suggests that continuous shuttling by ICP27 may restrict its ability to perform some biological functions. To determine how the ECS mutation affects the function of ICP27, we analyzed virus protein synthesis in cells infected with vBSECS compared to wild-type HSV-1 at 3, 6, and 9 h postinfection. Protein synthesis in the infected cells does not appear to be significantly different, with the exception of ICP19, an HSV late gene (Fig. 5). Thus, further analysis will be required to provide an explanation of why the mutation in the ECS of ICP27 results in diminished virus yield.
FIG. 9.
Analysis of the growth kinetics of an ECS mutant virus. Vero cells were infected at an MOI of 0.1 with HSV-1 (○) or vBSECS (■) and incubated at 37°C. The infections were stopped at the indicated times, and the virus yields were determined by titration on Vero cells. Datum points represent the average of three independent infections each titrated in duplicate.
We next asked if the mutation in the ECS could rescue shuttling of ICP27 with the KH3 ts mutation. This mutation disrupts the ability of ICP27 to be exported from the nucleus at the nonpermissive temperature (50, 51). If the ECS is required to regulate nuclear export of ICP27, then the mutation in the ECS should allow the KH3 mutant to shuttle constitutively. Accordingly, the double mutant virus vBStsECS was constructed. This virus maintains the ts phenotype as it only forms plaques at the permissive temperature.
Vero cells infected with HSV-1, vBStsECS, or vBSLG4 were incubated at the restrictive temperature for 7 h, treated with cycloheximide for 1 h, and processed for analysis at 8 h postinfection. ICP27 accumulates in both the nuclear and cytoplasmic compartments of 50 to 80% of the cells infected with wild-type virus (Fig. 10). In contrast, ICP27 is localized exclusively in the nucleus of cells infected with vBSLG4 (Fig. 10). In cells infected with the double mutant vBStsECS, ICP27 appears in the cytoplasm of 30 to 60% of the cells as well as in the nucleus (Fig. 10). Thus, the ECS mutation partially rescues the ts phenotype of LG4, allowing tsICP27 to accumulate in the cytoplasm of infected cells at the restrictive temperature. These results confirm that the ECS in ICP27 regulates nuclear export.
FIG. 10.
The ECS in ICP27 is required for nuclear retention of tsICP27. Vero cells were infected at an MOI of 10 with HSV-1, vBSLG4, or vBStsECS as indicated and incubated at 39.5°C. Infected cells were processed for immunofluorescence at 8 h postinfection following a 2-h treatment with cycloheximide. The intracellular distribution of ICP27 was examined with an antibody specific for ICP27 and an FITC-labeled secondary antibody.
DISCUSSION
ICP27 is an RNA-binding protein that shuttles between the nucleus and cytoplasm (1, 23–25, 32, 39, 50, 51): it is essential for HSV replication and one function appears to involve the export of some HSV late RNAs (50, 51). Study of the essential KH1 and NES domains of ICP27 required the development of a simple strategy for the rapid isolation of recombinant viruses carrying the mutant alleles of ICP27. To accomplish this, we first constructed an HSV-1 recombinant virus carrying a functional GFP-ICP27 fusion protein. This virus expresses a full-length fluorescent fusion protein. Using GFP to follow the movement of ICP27, we found localization patterns similar to those previously reported for ICP27 (3, 4, 14, 23, 24, 32, 39, 41, 50). However, visualization of GFP-ICP27 fusion proteins allows for detection in live cells and provides a clearer visualization of the localization of ICP27 in subnuclear compartments when compared to immunofluorescent staining. The use of GFP also permitted a simple screen to identify recombinant viruses carrying lethal point mutations in specific domains of interest in ICP27. The fusion of GFP to heterologous proteins of interest may provide a general method to identify recombinant viruses. This is particularly useful in our case, as fusion of GFP did not appear to significantly affect the function of ICP27.
ICP27 appears to be required to export some HSV late RNAs (50, 51). Previously, we demonstrated that only some HSV RNAs were exported by ICP27 and that when ICP27 was retained in the nucleus some virus-specified RNAs were still exported (50, 51). That analysis revealed that RNA export by ICP27 is required for normal accumulation of at least the late genes encoding VP5, VP16, gB, and gC, but not for immediate-early and early genes encoding ICP4, ICP27, ICP0, or UL9. RNA binding is a central component of RNA export and ICP27 contains an RGG-box and three KH-like RNA-binding domains (1, 18, 25, 39, 51). One question is: how do these RNA-binding domains contribute to the RNA export function of ICP27? In a limited mutational analysis of these RNA-binding domains, only the first and third KH domains were found to be essential for virus growth (51). The tsR480H mutation in the KH3 domain results in loss of expression of HSV late gene products that are coded for by ICP27-dependent RNAs and nuclear export of ICP27 is blocked (50, 51). This suggests that the KH3 domain is required for the export of all ICP27-dependent RNAs.
Here, we analyzed gene expression in cells infected with a virus carrying a lethal mutation (F303N) in the KH1 RNA-binding domain of ICP27. This analysis revealed a distinct defect in virus late gene expression, such that only a subset of genes that was dependent on the RNA export function of ICP27 were affected. While we were only able to demonstrate a difference in abundance of gC in the KH1 mutant, gC is not an essential gene. Since the KH1 mutation is lethal, the expression or function of at least one other essential HSV gene must also be affected. We also studied the overall pattern of protein synthesis by this mutant and were unable to find any appreciable differences compared to the wild-type control (vBSGFP27). However, this approach only allows for comparison of the major viral proteins. We also found that this KH1 mutation does not block ICP27 from exiting the nucleus. In another report, a lethal mutation that maps to what we now define as the KH1 domain was found to shuttle (24). In addition, a lethal mutation mapping to the KH3 domain was found to almost completely inhibit nuclear export of ICP27 (50). Those observations correlate well with our findings. They suggest that ICP27 carrying mutations in the KH1 domain can still bind to and export some of its cargo RNAs. In contrast, mutations in the KH3 domain can block the nuclear export of ICP27 and cause a complete loss of ICP27-dependent late gene expression (50, 51). Thus, one possibility is that individual KH-like RNA-binding motifs may have a role in recognition or interaction with distinct RNA cargoes. When the KH1 domain is mutated, the loss of expression of a subset of HSV late genes may therefore be the result of a loss of interaction with the RNAs that encode those genes. This could in turn result in these RNAs not being exported to the cytoplasm. Alternatively, these RNAs may not be appropriately processed in other ways when unable to be bound by ICP27.
In constructing a recombinant HSV carrying a lethal point mutation in the NES of ICP27, we found that this allele confers a strong dominant-negative phenotype. This result is not surprising considering that mutations in the NES of Rev, another viral RNA export protein from HIV, also result in a dominant-negative phenotype (17, 52). Previously, other mutations in the carboxy-terminal domain of ICP27 were also found to confer a dominant-negative phenotype (49). Our analysis of the carboxy-terminal mutant F303N in the KH1 domain of ICP27 was found to only modestly diminish virus yield in trans. However, vBSGFPKH1, the virus carrying this allele of ICP27 grows more slowly on 2-2 cells than an ICP27 deletion virus. One explanation for these results is that the NES mutation confers a more severe dominant-negative phenotype than do the carboxy-terminal mutations.
In the transient assay for the dominant-negative phenotype a reduction in virus yield was observed (as shown above). However, we feel that the extent of virus growth inhibition was better represented by the observation that viruses carrying this allele of ICP27 could not be isolated. The viruses we were able to isolate all had deleted portions of the α27 locus. This suggests that the dominant-negative phenotype was extremely detrimental to virus growth. Currently, vectors expressing dominant-negative alleles of Rev are being used in clinical trials to inhibit the growth of HIV. Here we have identified a potent dominant-negative allele of ICP27 that severely inhibits growth of HSV-1.
A dominant-negative mutant would require that the mutant protein dimerize with the wild-type protein to inhibit its function. In two separate studies it was demonstrated that ICP27 can homodimerize (53, 55). Because the dominant-negative ICP27 carries a mutation in the NES we postulate that the mechanism of interference may result from blocking the export of the wild-type ICP27 after heterodimer formation. This hypothesis led us to speculate that ICP27 could be actively retained in the nucleus by another protein in the absence of a functional NES. The active retention of the NES mutant ICP27 molecule in the nucleus could override the export of the ICP27 molecule with an active NES. Support for this model comes from the finding that ICP27 contains an ECS.
Nuclear export of ICP27 appears to be regulated. In this report we identify a sequence adjacent to the NES of ICP27 that is required to regulate nuclear export of ICP27. This sequence was identified based on its similarity to an element in the influenza virus NS1 protein that also regulates the export of that protein (21). The element in NS1 is described as masking the NES element in uninfected cells such that the protein remains nuclear. In infected cells, NS1 is able to accumulate in the cytoplasm, suggesting that the NES can be unmasked. These observations correspond well with the observation that ICP27 also remains nuclear in uninfected cells and can accumulate in the cytoplasm of infected cells, but only at late times postinfection (50). The NES and ECS elements found in both ICP27 and NS1 appear to function similarly. We propose that these proteins encode a novel sequence element that we term an ECS. Because this element is found in two proteins expressed by distinct viruses, it is likely that a cellular protein recognizes this sequence to regulate nuclear export of these proteins.
While we have demonstrated the presence of a functional ECS element in ICP27, we have not defined the borders of this element. The inhibitory element found in the NS1 protein is located close to the NES element, and spacing between these elements was found to be critical. In contrast, at least part of the ECS element found in ICP27 is spaced further from the NES than it is in the NS1 protein from influenza virus (21). Thus, further research is required to determine the boundaries of the ECS in ICP27 and whether spacing is also critical.
We proposed that RNA binding by ICP27 is a prerequisite to its nuclear export (50, 51). Our model predicts that ICP27 in the nucleus binds RNA and that this interaction induces a conformational change that exposes the NES and allows export of the RNA-protein complex. Once in the cytoplasm, the RNA is released for translation and ICP27 returns to the nucleus for further rounds of export. In light of the findings presented here, the correlation between the regulation of nuclear export and RNA binding by ICP27 leads us to propose that RNA binding may be a mechanism ICP27 utilizes to unmask its NES and release it from regulation by the adjacent ECS.
Previously, we identified intragenic suppressors of the tsR480H mutation in the KH3 domain of ICP27 that restore shuttling and virus growth at the restrictive temperature (50). Here, we engineered a mutation into ICP27 that restores shuttling of ICP27 carrying the ts KH3 mutation without restoring virus growth. The difference between these mutations is that the intragenic suppressor mutations map to the putative RNA-binding domains and likely restore RNA-binding that is required for shuttling. In contrast, the ECS mutation only restores nuclear export. This result provides further evidence that ICP27 exports HSV RNAs. Parenthetically, we found that the residues corresponding to the ts and suppressor mutations are likely to be critical to the interaction of a KH domain with its cognate RNA. The recent crystal structure of the KH3 domain from Nova-1 bound to RNA reveals a platform in KH domains that recognizes RNA (20). In examining the location of the ts and suppressor mutations in a computer-generated structural model of the KH3 domain of ICP27 (51), we found that the residues correspond to critical residues in the crystal structure that form the RNA-binding platform and contact the RNA itself. Therefore, these residues may be important for determining the sequence specificity of the RNA interaction.
ACKNOWLEDGMENTS
We thank Minoru Yoshida for generously providing LMB, David Knipe for suggesting an important control, and Kiran Musunuru for helpful discussions regarding the structure of KH domains.
This study was supported by Public Health Service grant AI-33952 to Saul J. Silverstein.
REFERENCES
- 1.Brown C R, Nakamura M S, Mosca J D, Hayward G S, Straus S E, Perera L P. Herpes simplex virus trans-regulatory protein ICP27 stabilizes and binds to 3′ ends of labile mRNA. J Virol. 1995;69:7187–7195. doi: 10.1128/jvi.69.11.7187-7195.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burd C G, Dreyfuss G. Conserved structures and diversity of functions of RNA-binding proteins. Science. 1994;265:615–621. doi: 10.1126/science.8036511. [DOI] [PubMed] [Google Scholar]
- 3.Curtin K D, Knipe D M. Altered properties of the herpes simplex virus ICP8 DNA-binding protein in cells infected with ICP27 mutant viruses. Virology. 1993;196:1–14. doi: 10.1006/viro.1993.1449. [DOI] [PubMed] [Google Scholar]
- 4.de Bruyn Kops A, Uprichard S L, Chen M, Knipe D M. Comparison of the intranuclear distributions of herpes simplex virus proteins involved in various viral functions. Virology. 1998;252:162–178. doi: 10.1006/viro.1998.9450. [DOI] [PubMed] [Google Scholar]
- 5.Everett R D. The products of herpes simplex virus type 1 (HSV-1) immediate early genes 1, 2, and 3 can activate HSV-1 gene expression in trans. J Gen Virol. 1986;68:2507–2513. doi: 10.1099/0022-1317-67-11-2507. [DOI] [PubMed] [Google Scholar]
- 6.Everett R D. Trans-activation of transcription by herpes virus products: requirement for two HSV-1 immediate-early polypeptides for maximum activity. EMBO J. 1984;3:3135–3141. doi: 10.1002/j.1460-2075.1984.tb02270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fornerod M, Ohno M, Yoshida M, Mattaj I W. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 8.Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M, Yanagida M, Nishida E. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature. 1997;390:308–311. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- 9.Gelman I H, Silverstein S. Coordinate regulation of herpes simplex virus gene expression is mediated by the functional interaction of two immediate early gene products. J Mol Biol. 1986;191:395–409. doi: 10.1016/0022-2836(86)90135-x. [DOI] [PubMed] [Google Scholar]
- 10.Gelman I H, Silverstein S. Identification of immediate early genes from herpes simplex virus that transactivate the virus thymidine kinase gene. Proc Natl Acad Sci USA. 1985;82:5265–5269. doi: 10.1073/pnas.82.16.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardwicke M A, Sandri-Goldin R M. The herpes simplex virus regulatory protein ICP27 contributes to the decrease in cellular mRNA levels during infection. J Virol. 1994;68:4797–4810. doi: 10.1128/jvi.68.8.4797-4810.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardwicke M A, Vaughan P J, Sekulovich R E, O'Conner R, Sandri-Goldin R M. The regions important for the activator and repressor functions of herpes simplex virus type 1 α protein ICP27 map to the C-terminal half of the molecule. J Virol. 1989;63:4590–4602. doi: 10.1128/jvi.63.11.4590-4602.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardy W R, Sandri-Goldin R M. Herpes simplex virus inhibits host cell splicing, and regulatory protein ICP27 is required for this effect. J Virol. 1994;68:7790–7799. doi: 10.1128/jvi.68.12.7790-7799.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hibbard M K, Sandri-Goldin R M. Arginine-rich regions succeeding the nuclear localization region of the herpes simplex virus type 1 regulatory protein ICP27 are required for efficient nuclear localization and late gene expression. J Virol. 1995;69:4656–4667. doi: 10.1128/jvi.69.8.4656-4667.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Honess R W, Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974;14:8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Honess R W, Roizman B. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc Natl Acad Sci USA. 1975;72:1276–1280. doi: 10.1073/pnas.72.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hope T J, Klein N P, Elder M E, Parslow T G. trans-dominant inhibition of human immunodeficiency virus type 1 Rev occurs through formation of inactive protein complexes. J Virol. 1992;66:1849–1855. doi: 10.1128/jvi.66.4.1849-1855.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ingram A, Phelan A, Dunlop J, Clements J B. Immediate early protein IE63 of herpes simplex virus type 1 binds RNA directly. J Gen Virol. 1996;77:1847–1851. doi: 10.1099/0022-1317-77-8-1847. [DOI] [PubMed] [Google Scholar]
- 19.Kalland K, Szilvay A M, Brokstad K A, Saetrevik W, Haukenes G. The human immunodeficiency virus type 1 Rev protein shuttles between the nucleus and the cytoplasm. Mol Cell Biol. 1994;14:7436–7444. doi: 10.1128/mcb.14.11.7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis H A, Musunuru K, Jensen K B, Edo C, Chen H, Darnell R B, Burley S K. Sequence-specific RNA binding by a nova KH domain: Implications for paraneoplastic disease and the fragile X syndrome. Cell. 2000;100:323–332. doi: 10.1016/s0092-8674(00)80668-6. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Yamakita Y, Krug R M. Regulation of a nuclear export signal by an adjacent inhibitory sequence: the effector domain of the influenza virus NS1 protein. Proc Natl Acad Sci USA. 1998;95:4864–4869. doi: 10.1073/pnas.95.9.4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCarthy A M, McMahan L, Schaffer P A. Herpes simplex virus type 1 ICP27 deletion mutants exhibit altered patterns of transcription and are DNA deficient. J Virol. 1989;63:18–27. doi: 10.1128/jvi.63.1.18-27.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mears W E, Lam V, Rice S A. Identification of nuclear and nucleolar localization signals in the herpes simplex virus regulatory protein ICP27. J Virol. 1995;69:935–947. doi: 10.1128/jvi.69.2.935-947.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mears W E, Rice S A. The herpes simplex virus immediate-early protein ICP27 shuttles between nucleus and cytoplasm. Virology. 1998;242:128–137. doi: 10.1006/viro.1997.9006. [DOI] [PubMed] [Google Scholar]
- 25.Mears W E, Rice S A. The RGG box motif of the herpes simplex virus ICP27 protein mediates an RNA-binding activity and determines in vivo methylation. J Virol. 1996;70:7445–7453. doi: 10.1128/jvi.70.11.7445-7453.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer B E, Malim M H. The HIV-1 rev trans-activator shuttles between the nucleus and the cytoplasm. Genes Dev. 1994;8:1538–1547. doi: 10.1101/gad.8.13.1538. [DOI] [PubMed] [Google Scholar]
- 27.Nakielny S, Dreyfuss G. The hnRNP C proteins contain a nuclear retention sequence that can override nuclear export signals. J Cell Biol. 1996;134:1365–1373. doi: 10.1083/jcb.134.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Hare P, Hayward G S. Evidence for a direct role for both the 175,000 and 110,000 molecular-weight immediate early proteins of herpes simplex virus in the transactivation of delayed-early promoters. J Virol. 1985;53:751–760. doi: 10.1128/jvi.53.3.751-760.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Hare P, Hayward G S. Three trans-acting regulatory proteins of herpes simplex virus modulate immediate-early gene expression in a pathway involving positive and negative feedback regulation. J Virol. 1985;56:723–733. doi: 10.1128/jvi.56.3.723-733.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ossareh-Nazari B, Bachelerie F, Dargemont C. Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science. 1997;278:141–144. doi: 10.1126/science.278.5335.141. [DOI] [PubMed] [Google Scholar]
- 31.Panagiotidis C A, Lium E K, Silverstein S J. Physical and functional interactions between herpes simplex virus immediate-early proteins ICP4 and ICP27. J Virol. 1997;71:1547–1557. doi: 10.1128/jvi.71.2.1547-1557.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phelan A, Clements J B. Herpes simplex virus type 1 immediate early protein IE63 shuttles between nuclear compartments and the cytoplasm. J Gen Virol. 1997;78:3327–3331. doi: 10.1099/0022-1317-78-12-3327. [DOI] [PubMed] [Google Scholar]
- 33.Phelan A, Dunlop J, Clements J B. Herpes simplex virus type 1 protein IE63 affects the nuclear export of virus intron-containing transcripts. J Virol. 1996;70:5255–5265. doi: 10.1128/jvi.70.8.5255-5265.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pollard V W, Malim M H. The HIV-1 Rev protein. Annu Rev Microbiol. 1998;52:491–532. doi: 10.1146/annurev.micro.52.1.491. [DOI] [PubMed] [Google Scholar]
- 35.Rice S A, Lam V, Knipe D M. The acidic amino-terminal region of herpes simplex virus type 1 alpha protein ICP27 is required for an essential lytic function. J Virol. 1993;67:1778–1787. doi: 10.1128/jvi.67.4.1778-1787.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roizman B, Sears A. The human herpes viruses: biology, pathogenesis, and treatment. New York, N.Y: Raven Press; 1993. [Google Scholar]
- 37.Sacks W R, Greene C C, Aschman D P, Schaffer P A. Herpes simplex virus type 1 ICP27 is an essential regulatory protein. J Virol. 1985;55:796–805. doi: 10.1128/jvi.55.3.796-805.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samaniego L A, Webb A L, DeLuca N A. Functional interactions between herpes simplex virus immediate-early proteins during infection: Gene expression as a consequence of ICP27 and different domains of ICP4. J Virol. 1995;69:5705–5715. doi: 10.1128/jvi.69.9.5705-5715.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sandri-Goldin R M. ICP27 mediates HSV RNA export by shuttling through a leucine-rich nuclear export signal and binding viral intronless RNAs through an RGG motif. Genes Dev. 1998;12:868–879. doi: 10.1101/gad.12.6.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandri-Goldin R M, Hibbard M K. The herpes simplex virus type 1 regulatory protein ICP27 coimmunoprecipitates with anti-sm antiserum, and the C terminus appears to be required for this interaction. J Virol. 1996;70:108–118. doi: 10.1128/jvi.70.1.108-118.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sandri-Goldin R M, Hibbard M K, Hardwicke M A. The C-terminal repressor region of herpes simplex virus type 1 ICP27 is required for the redistribution of small nuclear ribonucleoprotein particles and splicing factor SC35; however, these alterations are not sufficient to inhibit host cell splicing. J Virol. 1995;69:6063–6076. doi: 10.1128/jvi.69.10.6063-6076.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sandri-Goldin R M, Levine M, Glorioso J C. Method for induction of mutations in physically defined regions of the herpes simplex virus genome. J Virol. 1981;38:41–49. doi: 10.1128/jvi.38.1.41-49.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sandri-Goldin R M, Mendoza G E. A herpesvirus regulatory protein appears to act post-transcriptionally by affecting mRNA processing. Genes Dev. 1992;6:848–863. doi: 10.1101/gad.6.5.848. [DOI] [PubMed] [Google Scholar]
- 44.Sekulovich R E, Leary K, Sandri-Goldin R M. The herpes simplex virus type 1 α protein ICP27 can act as a trans-repressor or a trans-activator in combination with ICP4 and ICP0. J Virol. 1988;62:4510–4522. doi: 10.1128/jvi.62.12.4510-4522.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siomi H, Choi M, Siomi M C, Nussbaum R L, Dreyfuss G. Essential role for KH domains in RNA binding: impaired RNA binding by a mutation in the KH domain of FMR1 that causes fragile X syndrome. Cell. 1994;77:33–39. doi: 10.1016/0092-8674(94)90232-1. [DOI] [PubMed] [Google Scholar]
- 46.Siomi H, Matunis M J, Michael W M, Dreyfuss G. The pre-mRNA binding K protein contains a novel evolutionarily conserved motif. Nucleic Acids Res. 1993;21:1193–1198. doi: 10.1093/nar/21.5.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smiley J. Construction in vitro and rescue of a thymidine kinase deficient deletion mutant of herpes simplex virus. Nature. 1980;285:333–335. doi: 10.1038/285333a0. [DOI] [PubMed] [Google Scholar]
- 48.Smith I L, Hardwicke M A, Sandri-Goldin R M. Evidence that the herpes simplex virus immediate early protein ICP27 acts post-transcriptionally during infection to regulate gene expression. Virology. 1992;186:74–86. doi: 10.1016/0042-6822(92)90062-t. [DOI] [PubMed] [Google Scholar]
- 49.Smith I L, Sekulovich R E, Hardwicke M A, Sandri-Goldin R M. Mutations in the activation region of herpes simplex virus regulatory protein ICP27 can be trans dominant. J Virol. 1991;65:3656–3666. doi: 10.1128/jvi.65.7.3656-3666.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soliman T M, Sandri-Goldin R M, Silverstein S J. Shuttling of the herpes simplex virus type 1 regulatory protein ICP27 between the nucleus and cytoplasm mediates the expression of late proteins. J Virol. 1997;71:9188–9197. doi: 10.1128/jvi.71.12.9188-9197.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soliman, T. M., and S. J. Silverstein. 2000. Herpesvirus mRNAs are sorted for export via Crm1-dependent and independent pathways. J. Virol., in press. [DOI] [PMC free article] [PubMed]
- 52.Venkatesh L K, Chinnadurai G. Mutants in a conserved region near the carboxy-terminus of HIV-1 Rev identify functionally important residues and exhibit a dominant negative phenotype. Virology. 1990;178:327–330. doi: 10.1016/0042-6822(90)90414-m. [DOI] [PubMed] [Google Scholar]
- 53.Wadd S, Bryant H, Filhol O, Scott J E, Hsieh T Y, Everett R D, Clements J B. The multifunctional herpes simplex virus IE63 protein interacts with heterogeneous ribonucleoprotein K and with casein kinase 2. J Biol Chem. 1999;274:28991–28998. doi: 10.1074/jbc.274.41.28991. [DOI] [PubMed] [Google Scholar]
- 54.Wen W, Meinkoth J L, Tsien R Y, Taylor S S. Identification of signal for rapid nuclear export of proteins from the nucleus. Cell. 1995;82:463–473. doi: 10.1016/0092-8674(95)90435-2. [DOI] [PubMed] [Google Scholar]
- 55.Zhi Y, Sciabica K S, Sandri-Goldin R M. Self-interaction of the herpes simplex virus type 1 regulatory protein ICP27. Virology. 1999;257:341–351. doi: 10.1006/viro.1999.9698. [DOI] [PubMed] [Google Scholar]