Abstract
Introduction
A striking pattern in young children after severe TBI is when the entire cortical ribbon displays tissue damage: hemispheric hypodensity (HH). HH is often a result of abusive head trauma (AHT). We previously reported a model of HH in a gyrencephalic species where a combination of injuries consisting of (1) cortical impact, (2) midline shift, (3) subdural hematoma/subarachnoid hemorrhage, (4) traumatic seizures, and (5) brief apnea and hypoventilation resulted in extensive, hypoxic-ischemic-type injury. Importantly, this mechanism closely resembles that seen in children, with relative sparing of the contralateral cortex, thus ruling out a pure asphyxia mechanism. In this model, piglets of similar developmental stage to human toddlers (postnatal day 30, PND30) have extensive hypoxic-ischemic damage to the cortical ribbon with sparing of the contralateral hemisphere and deep gray matter areas. However, piglets of similar developmental stage to human infants (postnatal day 7, PND7) have less hypoxic-ischemic damage that is notably bilateral and patchy. We therefore sought to discover whether the extensive tissue damage observed in PND30 was due to a greater upregulation of matrix metalloproteinases (MMPs).
Materials and Methods
In PND7 or PND30 piglets receiving AHT injuries (cortical impact, midline shift, subdural hematoma/subarachnoid hemorrhage, traumatic seizures, and brief apnea and hypoventilation) or a sham injury, the pattern of albumin extravasation and MMP-9 upregulation throughout the brain was determined via immunohistochemistry, brain tissue adjacent to the cortical impact where the tissue damage spreads was collected for Western blots, and the gelatinase activity was determined over time in peripheral plasma. EEG was recorded, and piglets survived up to 24 h after injury administration.
Results
The pattern of albumin extravasation, indicating vasogenic edema, as well as increase in MMP-9, were both present at the same areas of hypoxic-ischemic tissue damage. Evidence from immunohistochemistry, Western blot, and zymogens demonstrate that MMP-2, -3, or -9 are constitutively expressed during immaturity and are not different between developmental stages; however, active forms are upregulated in PND30 but not PND7 after in response to AHT model injuries. Furthermore, peripheral active MMP-9 was downregulated after model injuries in PND7.
Conclusions
This differential response to AHT model injuries might confer protection to the PND7 brain. Additionally, we find that immature gyrencephalic species have a greater baseline and array of MMPs than previously demonstrated in rodent species. Treatment with an oral or intravenous broad-spectrum matrix metalloproteinase inhibitor might reduce the extensive spread of injury in PND30, but the exposure to metalloproteinase inhibitors must be acute as to not interfere with the homeostatic role of matrix metalloproteinases in normal postnatal brain development and plasticity as well as post-injury synaptogenesis and tissue repair.
Keywords: Animal model, Blood-brain barrier, Developing brain, Hypoxic-ischemic injury, Inflammation
Introduction
Brain injury due to abusive head trauma (AHT) is the leading cause of brain injury in infants [1]. In children aged 0–4, homicide is the third leading cause of death (3 per 100,000 deaths) – higher than the rate of all cancers combined [2]. In those who survive, AHT often results in long-term morbidity, ranging from learning and behavioral disorders to post-traumatic epilepsy, severe cognitive and motor impairments, and vegetative state [3]. Hemispheric hypodensity (HH) is a radiographic pattern of diffuse hemispheric edema followed by eventual atrophy and hypodensity on CT. It is primarily associated with subdural hematomas (SDH) following AHT in infants and toddlers, encompassing multiple vascular territories despite patent vessels [4–6]. If a unilateral SDH is observed, unilateral HH often follows [6]. However, little is understood about the underlying pathophysiology. Placement of SDH alone, for example, does not result in the extensive pattern of damage seen after AHT, including HH [7]. Clinically, HH has also been referred to in the literature as “Big Black Brain,” “Hemispheric Stroke,” and “Hypoxic-Ischemic Injury” when the hypoxic-ischemic damage spreads beyond the area of infarction that would occur due to a vessel stroke or focal contusion [5, 8, 9]. Though repetitive injuries likely play a part in AHT, the pattern of HH has been observed after a witnessed accidental bunk bed fall, and therefore, is a pattern that can result from a single event [6].
Until recently, a limitation in this area of research has been the lack of an animal model of severe traumatic brain injury (TBI) that replicates the injuries seen in children, in a species where development parallels that of humans [10]. We previously reported a model of HH in a gyrencephalic species where a sequence of injuries consisting of cortical impact, midline shift, SDH, subarachnoid hemorrhage, traumatic seizures, followed by apnea and hypoventilation, resulted in the extensive, hypoxic-ischemic-type injury seen in children, with relative sparing of the contralateral cortex ruling out a pure asphyxia mechanism [11, 12]. We combine these injuries and insults to cause the spreading hypoxic-ischemic injury that evolves in the hours after injury. Each individual injury or insult does not result in this pervasive pattern [13]. We have previously demonstrated that seizure appears to drive the hypoxic-ischemic injury in the location of the subarachnoid hemorrhage [11]. Spreading hypoxic-ischemic injury that evolves in the hours or days after severe TBI in children is not typically studied though a common pathoanatomic lesion [14, 15]. Understanding the pathophysiology of severe TBI, where the injuries and insults are synergistic, might lead to identification of therapeutic targets.
One mechanism that may contribute to the pathophysiology of extensive injury is the disruption of the blood-brain barrier and development of vasogenic edema, a process controlled by inflammatory mediators including matrix metalloproteinases (MMPs). MMPs are a family of 23 distinct Zn2+-dependent proteases classified by substrate specificity. Most MMPs are synthesized as latent enzymes, requiring conversion to the active form by either another MMP or plasminogen activators. MMPs are ubiquitously expressed in the central nervous system and are implicated in normal physiology, such as synapse remodeling, maintenance of the blood-brain barrier, and angiogenesis in the pathophysiology of TBI, epileptogenesis, stroke, multiple sclerosis, acting as master regulators of inflammation including ILβ, caspases, TNFα, NFκβ [16–21].
In this study, we direct our attention to MMP-2 (EC 3.4.24.24), MMP-3 (EC 3.4.24.17), and MMP-9 (EC 3.4.24.35), given their known targets in the blood-brain barrier. MMP-2, a gelatinase, targets major components of the basement membrane, including type IV collagen, laminin, and fibronectin. MMP-9, another gelatinase, has similar targets to MMP-2, although proteomic research suggests that possible targets of MMP-9 may reach in the hundreds [22]. MMP-9 can be activated by MMP-3, a stremolysin [23]. A variety of insults, including ischemic stroke, cortical impact, subarachnoid hemorrhage, hypoxia-ischemia, and seizures, stimulate MMP-9 production [24–26]. While a delayed and moderate increase in MMP-9 expression is likely beneficial for tissue repair, remodeling, and synaptogenesis immediately following injury, acute and expansive blood-brain barrier breakdown by MMPs might perpetuate secondary injury cascades [27, 28]. Strikingly, high concentrations of MMP-9 in plasma predict poor outcome in patients with severe TBI [29, 30]. Additionally, MMP-9 has been used in a biomarker panel to detect hemorrhage as a screen for AHT [31].
The pattern that develops in HH appears to be dependent on the developmental stage of immaturity. In children, HH is most often observed to involve both hemispheres in infants but is unilateral in toddlers [6]. Similarly, in our model of severe TBI, the injury results in unilateral HH in piglets developmentally similar to human toddlers (30 days old, PND30), and is less severe, patchy, and bilateral in piglets developmentally similar to human infants (7 days old, PND7) [11]. Here, we hypothesized that MMP-2, -3, and -9 may be developmentally regulated in response to severe TBI injuries, which might explain the age-dependent tissue damage pattern where PND7 piglets have less hypoxic-ischemic injury in response to AHT injuries scaled to the brain compared to PND30 piglets. If regulators of the matrisome are developmentally implicated in an age-dependent manner, future work may aim to reduce damage by inhibiting MMPs specific to developmental stage.
Materials and Methods
Surgery to Model HH
All procedures were approved by the MGH IACUC in accordance with the American Veterinary Medical Association and the National Institutes of Health Office of Laboratory Animal Welfare Guidelines (IACUC #2010N000198). Yorkshire, male piglets aged 7 days old (similar developmentally to infants, PND7) and 30 days old (developmentally similar to toddlers, PND30; Table 1) received model injuries scaled to brain size or a sham surgery [10].
Table 1.
Number of piglets used per outcome included on the experiment
| Age (developmental stage) and treatment | Number used per outcome | ||
|---|---|---|---|
| histology | zymography (performed on histology piglets) | Western blots | |
| 7-day old (“infant”) sham | 3 | 2 | 6 (4 at 1 h, 2 at 24 h) |
| 7-day old (“infant”) AHT model injuries | 8 | 8 | 5 (2 at 1 h, 3 at 24 h) |
| 1-month old (“toddler”) sham | 4 | 4 | 6 (4 at 1 h, 2 at 24 h) |
| 1-month old (“toddler”) AHT model injuries | 11 | 8 | 5 (2 at 1 h, 3 at 24 h) |
| Totals per outcome | 28 | 22 | 22 |
| Total piglets used | 50 (28 of which had damage patterns previously published) [11] | ||
Surgery and anesthesia protocols were employed as previously described, and injuries were scaled to the age of the piglets [11]. Briefly, general anesthesia was induced with isoflurane. Piglets were intubated and mechanically ventilated with room air. Prior to seizure induction with kainic acid, piglets were switched to a seizure-permissive anesthetic regimen to largely avoid the GABA receptor by utilizing a continuous infusion of dexmedetomidine, morphine, and boli of rocuronium [11]. A combination of injuries scaled to brain volume were induced as previously described including a cortical impact, creation of a mass effect, placement of a SDH with kainic acid mixed in the autologous blood (SDH often results in SAH), then 1 min of apnea followed by 10 min of hypoventilation/hypercarbia [11, 12]. Blood was collected prior to injury and at regular intervals after injury for blood gases, pH, lactate, glucose, and base excess of the extracellular compartment as described and reported previously [11]. Contact strip electrodes (4 per strip, AdTech) were placed epidurally in a bipolar array with 2 channels per hemisphere. A reference electrode was placed under the scalp and a ground electrode on the leg. Electrodes were attached to XL Tech amplifier and digitizer (Natus Neurology). Video electrocorticography (ECoG) was recorded until brain was collected or until the pig was recovered from anesthesia. In the sham group, piglets underwent placement of 2 burr holes, video ECoG was recorded, and were exposed to anesthetics for a similar duration. Arterial blood was collected pre-injury, 1–4 h after injury, and 24 h after injury or at comparable time point in shams.
Fresh tissue was collected for Western blots after 1 h of seizure or 24 h following initiation of injuries. Tissue from sham piglets was collected 1 or 24 h after placement of burr holes and exposure to anesthetics. The fresh tissue that was collected was the cortex rostral to the rostral gyrus. This area is distinct from the area of impact such that area of contusion was avoided and changes in protein expression would be expected from damage spread beyond the area of the contusion. Tissue was flash frozen on dry ice.
For immunohistochemistry (Fig. 1, 2), piglets were perfused transcardially 24 h after the end of injuries or after a sham surgery exposed to the equivalent duration of anesthetics as previously described. The damage analysis of pigs used for histology and zymography was previously published, while the piglets produced for Western blot are first presented here (Table 1) [11].
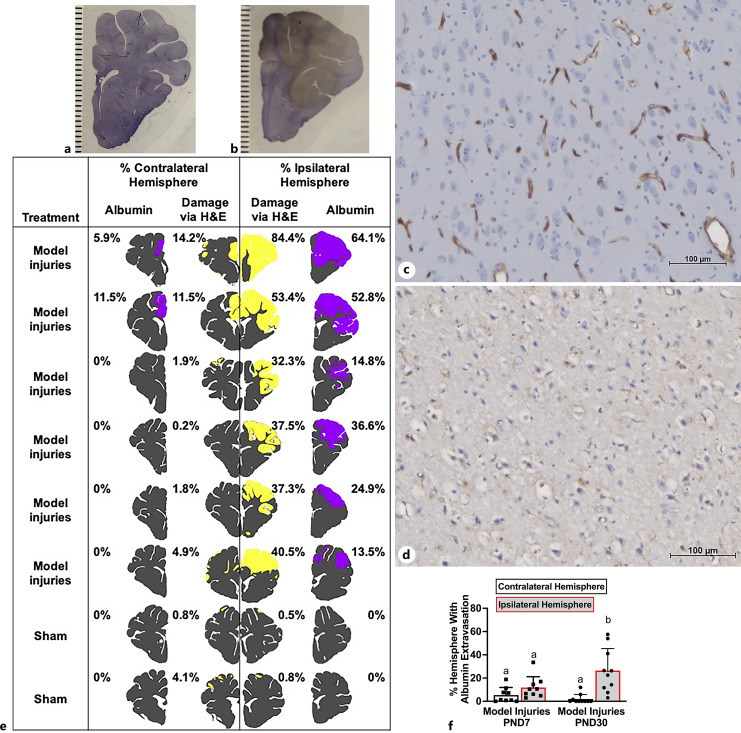
Fig. 1.
AHT model injuries caused widespread albumin extravasation in the ipsilateral hemisphere in PND30 pigs and minimal albumin extravasation in PND7 piglets. Albumin extravasation could be observed macroscopically in piglets with HH model injuries (albumin = brown, healthy tissue = purple; b) versus sham pigs (healthy tissue = purple; lines = 1 mm increments; a). Due to the potential for artifact, distribution was mapped by albumin extravasation microscopically. c Photomicrograph of healthy cortex where albumin is contained to the blood vessels (scale bar = 100 μM). d Photomicrograph of damaged cortex where albumin is widespread (scale bar = 100 μM). e A representative, single-section ipsilateral versus contralateral to focal injuries in representative piglets receiving model injuries or sham surgery demonstrating that the pattern of albumin extravasation (purple) is similar to the pattern of damage seen via H&E (yellow). f Percentage of the hemisphere with albumin extravasation ipsilateral or contralateral to focal injuries in PND7 and PND30. a,bMeans ± SD with different letters differ (p < 0.05).
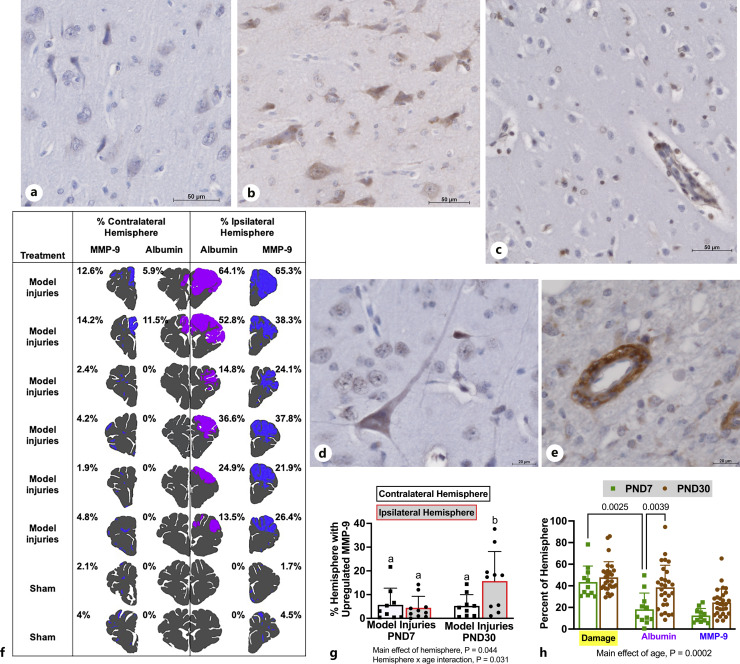
Fig. 2.
MMP-9 upregulation was increased in PND30 but not PND7 after AHT model injuries. a Minimal matrix metalloproteinase-9 (MMP-9) expression in healthy cortex (scale bar = 50 μM). MMP-9 was upregulated in neurons (scale bar = 50 μM, b; scale bar = 20 μM, d), glia (scale bar = 50 μM, c), and the basement membrane of blood vessels (scale bar = 20 μM, e) in damaged tissue. Though the interstitial matrix was often positive for MMP-9, areas were only mapped as positive if cells in that area were also positive. f Areas of albumin extravasation (purple) and increased MMP-9 expression (blue) were in a representative mapped section in injured and sham piglets. g The percentage of the hemisphere with MMP-9 upregulation was greater in the hemisphere ipsilateral to focal injuries compared to the contralateral hemisphere in PND30 but equivalent among hemispheres in PND7. h In sections with over 30% damage, damage areas via H&E (yellow) were equivalent, but PND30 had greater areas of vasogenic edema as detected via albumin extravasation compared to PND7. Albumin extravasation was less than the histologic tissue damage in PND7. Both these characteristics might indicate that the pathophysiology may be continuing to evolve in PND30 while spreading has ceased in PND7 piglets.
Seizure Analysis
Video ECoG was analyzed using NeuroScoreTM (Data Sciences International), and seizures were defined as paroxysmal spikes ≤100 ms in duration, deflecting at least 150% above baseline with a frequency of least 2 Hz lasting at least 10 s. One piglet only had 2 of 4 channels that functioned (1 channel per hemisphere) and was still included in the analysis.
Histology and Immunohistochemistry
Perfused brains were post-fixed in 10% neutral-buffered formalin for 3–5 days, cut in half, paraffin embedded, and cut into 5 mm slabs. Sections from each of the 5 mm slabs (18 total) were stained with H&E, and area of damage per section was determined as previously described [12]. Sections were deparaffinized and rehydrated in xylene and ethanol. For antigen retrieval, sections were placed in 0.1 M Tris 0.05 M EDTA in a pressure cooker reaching 120°C for 10 min and 90°C for 20 min. Sections were blocked using undiluted normal horse serum in Super Sensitive Wash Buffer (BioGenex) then incubated with primary antibodies overnight at 4°C. Primary antibodies used included anti-albumin (Abcam ab137885, 1:250) and anti-MMP-9 (full size, proenzyme; Abcam ab38898, 1:250). VECTASTAIN Universal Elite ABC Kit was then used (Vector Laboratories) followed by DAB Peroxidase Substrate Kit (Vector Laboratories). Sections were then counterstained with hematoxylin (Thermo Scientific) and a bluing reagent.
Specificity of antibodies for albumin IHC was determined by corresponding Western blotting and knockdown validation from the published literature (online suppl. Fig. 1; for all online suppl. material, see https://doi.org/10.1159/000536054). Each round of IHC included negative IgG controls to test for nonspecific binding of the secondary antibody. Additionally, specificity for albumin was confirmed microscopically via localization. In healthy tissue, albumin was confined to blood vessels with an intact blood-brain barrier (Fig. 1c). In injured tissue, denoted by shrunken and vacuolized neurons, albumin was observed outside of the blood vessels (Fig. 1d). These morphological features of albumin extravasation have been widely published [32].
MMP-9 is constitutively expressed in the brain; therefore, the antibody concentration was optimized such that the background was low and immunostaining of cells (neurons, glia) and basement membrane of blood vessels was specific. An internal positive control was neurons at a higher antibody concentration. The original IHC antibody (Abcam, ab38898), which is polyclonal anti-rat for the full length MMP-9 proenzyme yielded some smaller molecular weight binding via Western blot, perhaps due to antibodies recognizing various cleaved products for the full-length pro-MMMP-9 enzyme. Therefore, we used an MMP-9 antibody (10375-2-AP, Proteintech; KD/KO validated) that we validated specificity via Western blot (described below) in a subset of sections from blocks already characterized, and the pattern of MMP-9 upregulation was similar to the previous antibody, and the IHC quantification with ab38898 was considered valid.
The distribution of MMP-9 and albumin extravasation via IHC were quantified in the same manner as described previously for amyloid precursor protein and damage via H&E [11, 12]. Briefly, areas of MMP-9 positivity or areas of albumin extravasation were visualized microscopically with ×100 magnification on 6–15 sections and subsequently mapped to a photo of the slide. Areas of MMP-9 or albumin expression were quantified and are expressed as the percentage of the entire hemisphere that is positive for MMP-9 or albumin (%).
Western Blot
Tissue rostral or lateral to the site of cortical impact was mechanically homogenized in ×10 vol of N-PERTM (Thermo Scientific) supplemented with protease/phosphatase inhibitor cocktail (HALT, ×100, Thermo Fisher) and allowed to lyse with end-over-end rotation at 4°C. Following, samples were clarified at 14,000 g × 10 min. Sample concentrations were measured with Pierce BCA Assay from Thermo Scientific. Samples were diluted to equivalent concentration with nuclease-free water and reduced in ×5 Laemmli sample buffer with BME. Forty micrograms of total protein were loaded per well, and samples were run in duplicate on 4–15% TGX Gels (Bio-Rad) in Tris-Glycine Buffer. Membranes subjected to Ponceau S stain for total protein (total protein calculated by Image J AUC). Proteins were transferred to a 0.2 µM PVDF membrane in under semidry conditions (Bio-Rad Trans Blotter). Membranes were subsequently blocked with washes of 5% milk in TBST then incubated with primary antibodies at room temperature for 2 h. Primary antibodies used included anti-MMP-9 (10375-2-AP, 1:1,000, Proteintech; KD/KO validated), anti-MMP-3 (66338, 1:1,000, Proteintech; KD/KO validated), and anti-albumin (Abcam ab137885, 1:250). The antibodies Cell Signaling Technology 13667S (MMP-9) and Abcam ab52915 (MMP-3) were found not to be specific in swine. Westerns were attempted to quantify MMP-2 and IL-1β, but the antibodies tested did not yield a specific band (CST87809S, Cell Signaling Technologies; Abcam ab92536; CST12703S, Cell Signaling Technologies). Membranes were washed and incubated with an HRP-conjugated secondary antibody (Jackson ImmunoLabs) for 1 hr at room temperature and then washed extensively. ECL Select Western Blotting Substrate (Amersham) was used for signal detection, which was then imaged digitally with a LI-COR gel documentation system. Intensity of bands was analyzed in LI-COR software, averaged for each sample in duplicate, values were normalized to protein loaded detected via Ponceau stain and were expressed as arbitrary units (au). Westerns were stripped with restore (Thermo Fisher) and reincubated for beta-actin as a secondary loading control.
Zymography
Plasma collected pre-injury, 1–4 h post-injury, and 24 h post-injury was diluted 1:5 with Tris-Glycine SDS Sample Buffer (Life Technologies) then diluted an additional 1:10 with loading dye, resulting in a protein content of 20–25 ng/μL. 1.6 μL of the sample was loaded per lane. Samples were run in duplicate and separated by electrophoresis with tris-glycine gels with 0.1% gelatin (Invitrogen). Each series of each subject was run on the same gel, and the post-injury times expressed as a ratio to pre-injury. A positive control of MMP-9 protein was added in a subset of gels. After electrophoresis, gels were washed with Triton X-100 renaturing buffer (Life Technologies) for 1 h, then incubated at 37°C for overnight in Novex Zymogram Developing Buffer (Life Technologies). Gels were stained with a solution containing Coomassie Brilliant Blue R-250 for 1 h then destained. Density of bands was measured in Image J.
Statistics
Data were analyzed using GraphPad Prism® 9 and presented as means ± SD. Due to the heterogeneity of outbred swine and current consensus of “difference” by statisticians, p values <0.06 were considered significantly different [33]. The main effects of hemisphere and age and the interaction on the percent of the hemisphere displaying albumin extravasation and MMP-9 upregulation were tested with a two-way ANOVA followed by Tukey’s post hoc tests. The main effects of age, time, and the interaction on MMP protein abundance were tested via a two-way ANOVA followed by Tukey’s post hoc tests. The effect of age and time of collection and their interaction on the length of EEG recording was tested via a two-way ANOVA followed by Tukey’s post hoc tests. Potential correlations between seizure duration and MMP-9 and -3 were tested by determining Pearson correlation coefficients. The main effect of age and time and the interaction on plasma MMP activity were tested via a repeated measure two-way ANOVA followed by Tukey’s post hoc test.
Transparency, Rigor, and Reproducibility
Using our data from our cortical impact model, allowing detection of a 138% difference in lesion size in PND7 versus PND30 (1.8 vs. 10%; σ = 7.2%) yields n = 9/injured group with α = 0.05% and Power = 95% [11] with shams serving as benchmarks. This power analysis was used to determine the number of animals to determine the difference in area positive for MMP-9 and albumin extravasation via immunohistochemistry. This study was fully randomized. Analysis of tissue was performed by investigators blinded to age and treatment. The measurement of cortical MMP protein and MMP activity via zymography in peripheral plasma were pilot studies (Table 1). Animals were serially assigned with the analysis being performed with the investigator blinded to subject age. Western blots and zymogens were run in duplicate and any sample with over 20% coefficient of variation was reanalyzed. These pilot data are being used to plan replication studies. Of the 20 additional piglets assigned to the experiment, three piglets (PND30) were excluded due to early death prior to 24 h of injuries. One piglet died after a failed resuscitation attempts after an accidental extubation due to convulsions. The two other piglets had prolonged seizures, low blood pressure, and failed resuscitation attempts after cardiac events. No other animals nor data points were excluded.
Results
Distribution of MMP-9 Expression and Albumin Extravasation
In this model of severe TBI, which includes cortical impact, hemorrhage, seizure, and brief apnea and hypoventilation, we investigated the pattern of albumin extravasation as a marker of blood-brain barrier disruption, as well as the expression of MMP-9, a mediator of the breakdown of the blood-brain barrier, to determine if they matched the patterns of hypoxic-ischemic type injury (as observed via H&E), which is age-dependent [11].
In PND30 piglets receiving model injuries, albumin extravasation was widespread in the hemisphere ipsilateral to focal injuries and largely absent in the contralateral hemisphere but was distributed bilaterally in PND7 in a similar pattern to the hypoxic-ischemic damage (Fig. 1). The pattern of increased MMP-9 expression via IHC was similar to the distribution of albumin extravasation (Fig. 2f, g). MMP-9 expression was increased in the basement membrane of blood vessels, neurons, and inflammatory cells in damaged areas (Fig. 2a–f). The distribution of albumin extravasation was greater than MMP-9 in both PND7 and PND30 piglets (ipsilateral hemisphere: PND7: 11.8 ± 3.1% vs. 5.24 ± 0.016%, p = 0.01; PND30: 28.1 ± 6.4% vs. 16.8 ± 0.04, p < 0.001). Although there were no age differences observed in MMP-9 expression versus albumin extravasation averaged over the hemisphere, in sections from the ipsilateral hemisphere with over 30% damage, PND30 piglets had significantly more blood-brain barrier disruption than PND7 piglets (Fig. 2h). In PND7 piglets, albumin extravasation was less than the hypoxic-ischemic injury (Fig. 2h). In regions with extensive damage (>30%), there may be ongoing evolution of damage at 24 h post-injury in PND30 but not in PND7.
MMP Expression in the Cortex
MMP-9 protein was quantified in the cortex of PND7 and PND30 piglets at 1 h and 24 h post-injury compared to sham subjects in the region either rostral or lateral to the site of direct cortical impact. At 1 h post-injury, active MMP-9 was greater in PND30 piglets versus PND7 piglets (Fig. 3a, c).
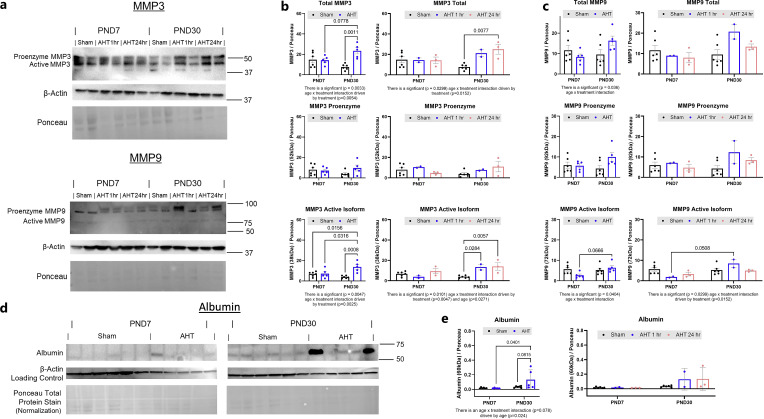
Fig. 3.
Active MMP-3 and MMP-9 is upregulated in PND30 but not PND7 piglets in response to AHT model injuries. a Western blot of matrix metalloproteinase-3 and -9 (MMP-3, -9), actin, and Ponceau total protein stain in the rostral gyrus rostral or lateral from the cortical impact or sham surgery of PND7 and PND30 piglets at injury 1 or 24 h after injury or sham injury (samples run in duplicate). b MMP-3 proenzyme did not increase in either age in response to injury, while active MMP-3 increased in PND30 piglets but not in PND7 piglets. c Pro-MMP-9 did not increase in either age, but active MMP-9 was greater at 1 h post-injury in PND30 versus PND7 piglets. Differences among groups were compared via two-way ANOVA, followed by Tukey’s post hoc tests, and p ≤ 0.06 was considered significant. d Western blot of albumin, actin, and Ponceau stain in the rostral gyrus rostral or lateral from the cortical impact or sham surgery of PND7 and PND30 piglets at injury 1 or 24 h after injury or sham injury (samples run in duplicate). e Albumin was increased in PND30 piglets after AHT model injuries.
As MMP-3 is a known activator of MMP-9, we measured cortical MMP-3 expression (Fig. 3) in the area rostral or lateral to the site of direct cortical impact. MMP-3 was upregulated in PND30 piglets but not PND7 piglets (Fig. 3a, b). AHT injuries did not increase either pro-MMP-3 or pro-MMP-9 in either developmental stage (Fig. 3b, c). A lack of upregulation in active MMP-3 in PND7 may impart resistance to AHT model injuries, including resistance to seizure and/or subarachnoid hemorrhage. Consistent with the pattern of albumin extravasation observed on tissue sections where it was higher in PND30 than PND7 piglets (Fig. 1g), we observed an increase in albumin protein via Western blot in PND30 piglets but not PND7 piglets (Fig. 3e). We were unable to identify an antibody that resulted in a specific band for porcine MMP-2 or IL-1β.
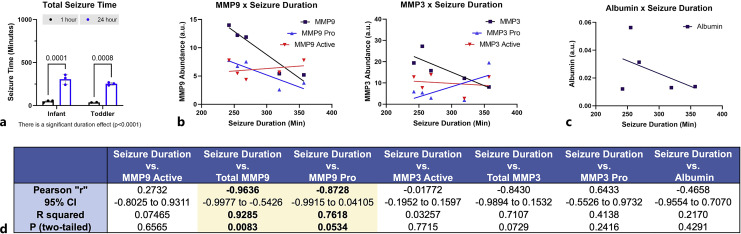
In our prior work, we demonstrated a positive correlation with the degree of tissue damage and seizure duration in PND30 piglets [11]. Here, seizure duration in piglets where tissue was collected at 1 or 24 h after injury did not differ between piglets aged PND7 and PND30 (Fig. 4a). Among ages, seizure duration was negatively correlated with pro-MMP-9 (Fig. 4b, d) and was not correlated to the amount of pro-MMP-3, active MMP-3, active MMP-9, nor albumin (Fig. 4b–d).
Fig. 4.
Interaction of inflammatory mediators and seizure. a Seizure duration was longer in piglets surviving 24 h versus 1 h, but there was no effect of age on seizure duration. Seizure duration was negatively correlated to total MMP-9 and pro-MMP-9 (b) but was not correlated with active MMP-9, pro-MMP-3, active MMP-3, nor albumin (c) (Pearson correlations). d Table of the correlation and p values for all mediators.
Plasma MMP Activity
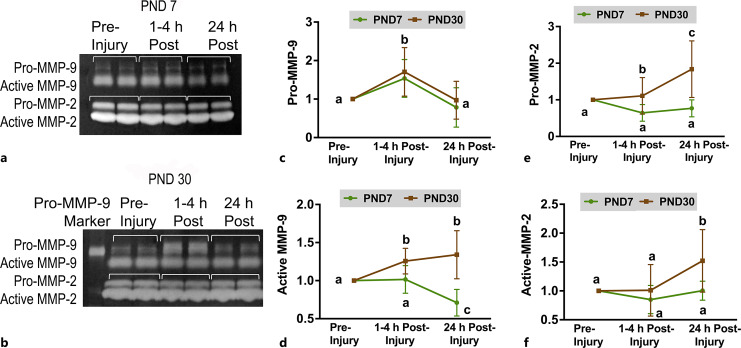
To assess if the peripheral activity of MMP-9 reflected what was observed in the cortex, gelatin zymography was performed with plasma collected pre-injury, 1–4 h post-injury, and 24 h post-injury. Pro-MMP-9 activity increased 1–4 h post-injury in piglets receiving model injuries and returned to pre-injury levels by 24 h post-injury in both ages (p = 0.01; Fig. 5), which mirrors findings in the cortex for PND30. Active MMP-9 activity increased in PND30 at 1–4 h post-injury and remained elevated at 24 h post-injury. In PND7, however, active MMP-9 decreased from 1 to 4 h post-injury to 24 h (Fig. 5). MMP-2 activity was also assessed as it is also a gelatinase. Both pro-MMP-2 and active MMP-2 were increased at 24 h post-injury compared to pre-injury in PND30 but not in PND7 piglets (Fig. 5).
Fig. 5.
The activity of MMP-2 and -9 in peripheral plasma in response to AHT model injuries is developmentally regulated. Gelatin zymography with matrix metalloproteinase-9 (MMP-9) standard and the pro- and active forms of MMP-2 and -9 in one PND7 (a) and two PND30 (b) piglets over time. c Pro-MMP-9 increased in both ages (MMP activity is expressed as a ratio to pre-injury). d Active MMP-9 only increased in PND30 (p = 0.01). Pro-MMP-2 (e) and active MMP-2 (f) only increased in PND30 piglets. Samples are displayed a ratio to pre-injury. a,b,cMeans ± SD with different letters differ p < 0.05.
Discussion
In young children, severe TBI is usually characterized by contusion, SDH, and subarachnoid hemorrhage, which are often accompanied by traumatic seizures and periods of apnea and hypoventilation, which result in pervasive hypoxic-ischemic injury. Diffuse axonal injury is rare [14, 15]. These areas of hypoxic-ischemic injury that are greater than can be explained by focal contusion or restriction of blood flow within a blood vessel territory and may even extend to an entire hemisphere or both hemispheres with sparing of the deep gray areas [4, 5, 8, 9, 34]. Few preclinical models study hypoxic-ischemic resulting from a combination of injuries, but instead, study the pathophysiology of one pathoanatomic lesion such as focal contusion or diffuse axonal injury. Clinically, rarely does any type of severe TBI present with a single pathoanatomic lesion. It should be noted that “hypoxic-ischemic injury” as observed histologically is termed “cytotoxic edema” by the common data elements of radiology based on the location of restricted movement of water: “cytotoxic edema” in the gray matter versus “vasogenic edema” in the white matter [35]. Here, we use the term “vasogenic edema” as extravasation of albumin and/or the appearance of “red neurons” with vacuolization of the blood vessels observed histologically no matter the location: white matter or gray matter or both. Certainly, cytotoxic edema may have preceded, and likely even caused, the observed vasogenic edema, but it occurs in a time span that is not observable with histological techniques and is an area of active inquiry. Furthermore, vasogenic edema may be the endpoint of cytotoxic edema. Both types of edema may be targets for reducing damage after severe TBI in children prone to swell.
Here, we use our large animal, gyrencephalic model of HH to further investigate the mechanisms by which these multi-injury insults scaled to brain size cause pervasive hypoxic-ischemic damage restricted to one hemisphere in piglets of similar developmental stage to human toddlers (PND30) but results in damage that is patchy, less severe, and bilateral in piglets of a developmental stage that is similar to human infants (PND7) [11]. We have previously demonstrated that seizure duration after brain trauma is positively correlated to the extent of damage and that the SDH results in a focal subarachnoid hemorrhage that directs damage to the cortex in PND30 piglets [11]. Here, we identify additional mechanisms associated with the differential age-related responses in PND7 and PND30 piglets to severe brain trauma. These processes include MMP activation and the development of vasogenic edema as demonstrated via albumin extravasation.
Vasogenic edema is a physiologic process controlled by inflammatory mediators and proteases. A variety of insults stimulate MMP-9, which breaks down the basement membrane of blood vessels, causing vasogenic edema. The extracellular matrix is comprised proteins and sulfated glycosaminoglycans and forms the basement membrane of blood vessels and the structure around neurons [36]. Though direct trauma causes breakdown of the blood-brain barrier, continuing blood-brain barrier breakdown is specifically controlled by cytokines, including IL-1β, which stimulates MMPs and is activated by MMP-9. Cortical impact, subarachnoid hemorrhage, hypoxia-ischemia, and seizures all increase MMP-9 production [25, 26, 37, 38]. Cortical impact would be expected to directly damage the blood vessels at the site of impact, but in our model, the hypoxic-ischemic damage spreads past the direct impact site of cortical impact indicating that the blood-brain barrier might be breached in these areas. A brief increase in blood-brain barrier permeability is likely helpful for immune cell invasion and softening of the extracellular matrix surrounding neurons requisite for tissue repair and remodeling [39–41]. However, sustained and expansive blood-brain barrier opening may be deleterious and perpetuate the secondary injury cascades. Indeed, inhibiting MMPs reduces vasogenic edema and tissue damage after brain injury [42–45]. Moreover, plasma concentrations of MMP-9 and CSF concentrations of IL-1β predict poor outcome in patients with severe TBI [29, 46]. HH is associated with several insults known to individually increase MMP-9 and result in vasogenic edema. Additionally, MMP-9 has been proposed as a viable biomarker for acute intracranial hemorrhage in pediatric patients MMP-9 served as a peripheral biomarker indicating the acute intracranial hemorrhage in pediatric patients – the same patients where the TBI was inflicted [31].
We have demonstrated that the pattern/location of blood-brain barrier breakdown as demonstrated by albumin extravasation is similar to the previously published pattern of hypoxic-ischemic-type injury [11]. The pattern of MMP-9 upregulation in tissue sections was a subset of the area demonstrating vasogenic edema. The only developmental difference noted was that in sections with over 30% damage (as quantified on sections stained with H&E), representing widespread damage, the areas positive for albumin extravasation were greater in PND30 versus PND7 piglets. At 24 h, the damage may still be evolving and expanding in these areas of widespread damage in PND30 acting in a feed-forward mechanism, while the opening of the blood-brain barrier has stopped expansion in PND7 piglets.
The immature brain may be particularly susceptible to vasogenic edema at specific stages. Swelling from a cortical impact is greater in piglets developmentally similar to toddlers versus piglets similar to infants [47]. In rodent models, the degree of vasogenic edema resulting from vessel occlusion is age-dependent; in developmental stages where less vasogenic edema results from this injury, there is a concurrent reduction of hypoxic-ischemic injury [48]. In human autopsy of diffuse brain swelling following TBI, adults primarily display vasogenic edema in small arteries and arterioles, but vasogenic edema occurs mostly in the capillaries in pediatric cases, potentially making the immature brain more susceptible to vasogenic edema [49]. Vasogenic edema may exacerbate damage, in part, through additional capillary collapse, which further exacerbates metabolic mismatch.
Immature piglets at either stage appear to constitutively express MMP-9 that is not different between developmental stages. In adult rodents, MMP-9 was not constitutively expressed but only increased after cortical impact [45]. Here, we demonstrate potential developmental regulation of MMPs in response to severe TBI injuries that may determine the degree of vasogenic edema. The cortex analyzed was collected rostral or lateral to the site of direct cortical impact, so that a response to cortical impact alone was not being analyzed. Active MMP-9 was greater at 1 h post-injury in PND30 compared to PND7 piglets. In peripheral plasma, we observed an increase in proenzyme MMP-9 in PND30 and its decline by 24 h, while active MMP-9 was high in both the cortex and plasma in PND30 piglets. In PND7 piglets, an increase in proenzyme MMP-9 at 1–4 h was only observed in the plasma and not the cortex. Active MMP-9 was increased in zymogens of peripheral plasma may be the best approach in quantifying MMPs as the tissue pattern is highly heterogenous, especially in PND7 where the hypoxic-ischemic damage, vasogenic edema, and MMP-9 expression are patchy and do not spread. An alternative approach may be measuring MMPs in a sample of a homogenized whole cortex or a mix of multiple locations, reducing the risk of sampling a small area of the cortex where damage did not spread. Additionally, extra-neuronal sources, such as the creation of the burr hole in the skull to create the injuries, may have contributed to the increase peripheral proenzyme MMP-9, similar to S-100B, which increases with bone fractures or fevers [50]. Zymogens allowed the detection of cleaved/active MMP-9, which was highly developmentally regulated increasing over time after injury in PND30 but decreasing over time in PND7. Cleaving of the proenzyme may constitute an additional level of regulation of the age-dependent response to severe TBI.
MMP-3 cleaves MMP-9 from the proenzyme to the active enzyme. MMP-3 is not a gelatinase, precluding evaluation via zymography, but an age by time after injury interaction was present such that MMP-3 protein in the cortex adjacent to the site of cortical impact increased after injury in PND30 but not in PND7 piglets. MMP-3 may impart development regulation, or alternatively, may have failed to have been upregulated due to lack of injury in the regions of cortex collected in PND7. MMP-3 protein upregulation by glia has been demonstrated via immunohistochemistry in adult rats after fluid percussion injury [27].
Both pro- and active MMP-2, a gelatinase, increased over time in PND30 but not in PND7 piglets. Unfortunately, we were not able to assess MMP-2 expression in the cortex via Western blotting given the lack of availability of a specific antibody. Both pro-MMP-2 and active MMP-2 were in greater abundance in zymogens of plasma from piglets than previously reported for rodents for both baseline and in response to injury [42]. In immature rodents, pro-MMP-2 and pro-MMP-9 are the predominant forms at baseline and are upregulated after cortical impact, resulting in an increase in active MMP-2 and active MMP-9. The difference in baseline MMPs in piglets versus rodents might be the anesthetics that piglets must be exposed to in order to collect the brain. GABA-acting anesthetics have been shown to open the blood-brain barrier such that albumin is extravasated focally around the blood vessel with the degree of extravasation dependent upon age and type of anesthetic agent [32]. The rodent brain can be collected immediately after rapid decapitation without exposure to GABA-acting anesthetics. We might have induced some slight albumin extravasation with our anesthetics used to collect the brain.
In this series, we demonstrate an inverse correlation of seizure duration and pro-MMP-9, while other mediators were not related. Seizure duration is correlated with the amount of hypoxic-ischemic damage after AHT in children and in our previous work in our animal model [11, 51]. This result is opposite to what we would have expected, but not all subjects had EEGs long enough to be included in the dataset due to our previous practice of recovering some PND30 piglets from anesthesia. We have since changed our protocols to be closer to the pediatric intensive care unit and keep all animals sedated, intubated, and mechanically ventilated overnight, which maximizes the collection of video ECoG data.
Future studies into other inflammatory mediators such as other MMPs, IL-1β, aquaporin-4, and targets of hypoxia-inducible factor 1-alpha will likely advance the understanding of the pathophysiology of HH and/or widespread hypoxic-ischemic injury. Another possible area of study would entail administering gelatinase-specific inhibitors, such as p-OH SB-3CT or doxycycline, which has been used in rodent models of trauma with variable success, at various time points post-injury to determine if tissue damage can be attenuated and whether there is a critical period for intervention to improve clinical outcome [42, 44]. Studies in which p-OH SB-3CT was administered to P21 mice within 48 h post-TBI did not improve long-term motor and cognitive outcomes. It also failed to reduce long-term neuronal loss. However, the rodent model of TBI entailed focal cortical impact only, which does not yield the extensive pattern of injury seen in HH in large animals. Additionally, perinatal and postnatal development in mice differs from that of humans, and there exists some disagreement about the matching of mice and human development; sometimes PND21 mice are considered similar to human toddlers, while other investigators equate them to young adults. Vaginal opening, the earliest external sign of puberty, initiates at PND25, which means that ovarian steroids were produced days prior to the external signs, which makes a comparison to human toddlers, who would not have gonadal steroids present, tenuous. Further studies with MMP-2 and MMP-9-specific inhibitors, and even perhaps broad inhibitors of MMP, are needed in large animal models of different age groups. It is possible that wide-spectrum MMP inhibitors are needed in more immature subjects as the switch to a predominately MMP-9-only regulated process during hemorrhage, stroke, and seizure may occur only during maturity.
Certainly, other age-dependent factors might also affect the expression of MMPs in response to AHT model injuries. Clinically, we have previous shown that PND7 piglets had persistently lower base excess of the extracellular fluid not resolved by 24 h after injury where it was resolved by 24 in PND30 piglets [14]. Neurologic scores were lower in PND7 piglets compared to PND30 piglets 8 h after injury but were resolved by 24 h post-injury [14]. Presumably, the difference in clinical signs is a result of a more severe brain injury. During the brief 1 min of apnea and 10 min of hypoventilation during the administration of injuries/insults, end tidal CO2 increased and oxygen saturation decreased as expected but were not different between ages [52]. Blood gases collected pre-injury and throughout the 30-h experiment revealed that pO2 and pCO2 were not different among ages nor treatment [52]. We have previously demonstrated a reduction of ventricular ejection fraction but did not have the number of subjects needed in order to determine an effect of developmental stage [12]. Human infants have a lower oxygen reserve than toddlers. Cardiac dysfunction, which might be developmentally influenced, might worsen brain injury and should be evaluated in future studies.
Limitations of this study include small group sizes and lack of randomization in the Western blot outcome experiment, limiting the examination of mediators to a few MMPs, lack of spatial resolution, and lack of knowledge if the collected areas were indeed injured. Another limitation of the study is only studying males in this pilot study. Future work will include females and consider exploring any potential effects of sex. The age-dependent effect of the response of the PND7 brain to AHT injuries might also be due to alterations in glutamate receptors, AMPA receptors, kainic acid receptor, NKCC1 versus KCC2, sodium channels, potassium channels, peptides, and glial cells, and the host other developmentally regulated proteins that influence neuronal excitability. Future work will leverage spatial proteomics or transcriptomics where we can achieve spatial resolution with markers that indicate acute tissue damage patterns, where the tissue damage will likely spread next, and tissue that is rarely injured in this model (deep gray matter) with a wide array of potential mediators.
In conclusion, we demonstrate widespread albumin extravasation in areas of hypoxic-ischemic injury that is greater in PND30 piglets than PND7 piglets that is similar to the pattern of damage in children where infants have less, patchy damage and toddlers have widespread, hemispheric damage mostly restricted to one hemisphere. Developmentally dependent upregulation of MMPs may be responsible for the widespread hypoxic-ischemic damage and vasogenic edema observed in PND30 piglets (comparable to human toddlers) after severe TBI. This work is consistent with previous work in rodent species where MMP-9 increased after TBI; however, we demonstrate here developmental and species differences where the immature gyrencephalic brain exhibits a greater baseline expression of MMPs and a greater array of MMPs upregulated in response to injury. The degree of MMP upregulation in response to severe TBI appears to be specific to stage of immaturity where we observed that PND7 piglets (similar to human infants) did not exhibit an upregulation of MMPs, and perhaps, even a downregulation. Further studies need to be performed to determine if specific or broad inhibition of MMPs reduces damage in this multi-injury model of severe pediatric TBI.
Acknowledgments
The authors thank Luis Martinez Ramirez, Jason Zhao, Andrew Ding, John Dempsey, Akhila Penumarthy, Tess Del Prado, and Caroline Kaplan with assistance with animal surgeries and overnight intensive care. We thank Dr. Declan McGuone for his guidance with optimization of the IHC for MMP-9 and review of the manuscript.
Statement of Ethics
All protocols and procedures were in accordance with the guidelines of the American Veterinary Association and the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee at Massachusetts General Hospital.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
Funding was provided by NICHD K01 HD083759, R01 HD099397, MGH Claflin Distinguished Scholar Award to B.C.B., and NINDS R01 NS91573 to J.L.
Author Contributions
Alexandra Hochstetler: investigation, formal analysis, visualization, and writing – review and editing; George Price: conceptualization, investigation, formal analysis, and writing – review and editing; Amy Baohan: conceptualization and writing – original draft; Melissa Li and Frances Rodriguez Lara: investigation and writing – review and editing; Josephine Lok: conceptualization, resources, and writing – review and editing; and Beth Costine-Bartell: conceptualization, formal analysis, investigation, writing – original draft, visualization, project administration, and funding acquisition.
Funding Statement
Funding was provided by NICHD K01 HD083759, R01 HD099397, MGH Claflin Distinguished Scholar Award to B.C.B., and NINDS R01 NS91573 to J.L.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Supplementary Material.
References
- 1. Keenan HT, Runyan DK, Marshall SW, Nocera MA, Merten DF, Sinal SH. A population-based study of inflicted traumatic brain injury in young children. JAMA. 2003;290(5):621–6. [DOI] [PubMed] [Google Scholar]
- 2. Bennett MD Jr., Hall J, Frazier L Jr, Patel N, Barker L, Shaw K. Homicide of children aged 0-4 years, 2003-04: results from the national violent death reporting system. Inj Prev. 2006;12(Suppl 2):ii39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Duhaime AC, Christian C, Moss E, Seidl T. Long-term outcome in infants with the shaking-impact syndrome. Pediatr Neurosurg. 1996;24(6):292–8. [DOI] [PubMed] [Google Scholar]
- 4. Foster KA, Recker MJ, Lee PS, Bell MJ, Tyler-Kabara EC, et al. Factors associated with hemispheric hypodensity after subdural hematoma following abusive head trauma in children. J Neurotrauma. 2014;31(19):1625–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khan NR, Fraser BD, Nguyen V, Moore K, Boop S, Vaughn BN, et al. Pediatric abusive head trauma and stroke. J Neurosurg Pediatr. 2017;20(2):183–90. [DOI] [PubMed] [Google Scholar]
- 6. Duhaime AC, Durham SR. Traumatic brain injury in infants: the phenomenon of subdural hemorrhage with hemispheric hypodensity (“Big Black Brain”). Prog Brain Res. 2007;161:293–302. [DOI] [PubMed] [Google Scholar]
- 7. Durham SR, Duhaime AC. Basic science; maturation-dependent response of the immature brain to experimental subdural hematoma. J Neurotrauma. 2007;24(1):5–14. [DOI] [PubMed] [Google Scholar]
- 8. Duhaime AC, Bilaniuk L, Zimmerman R. The “big black brain”: radiographic changes after severe inflicted head injury in infancy. J Neurotrauma. 1993;10(Suppl 1):S59. [Google Scholar]
- 9. Orru E, Huisman T, Izbudak I. Prevalence, patterns, and clinical relevance of hypoxic-ischemic injuries in children exposed to abusive head trauma. J Neuroimaging. 2018;28(6):608–14. [DOI] [PubMed] [Google Scholar]
- 10. Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3(1):79–83. [DOI] [PubMed] [Google Scholar]
- 11. Costine-Bartell B, Price G, Shen J, McGuone D, Staley K, Duhaime AC. A perfect storm: the distribution of tissue damage depends on seizure duration, hemorrhage, and developmental stage in a gyrencephalic, multi-factorial, severe traumatic brain injury model. Neurobiol Dis. 2021;154:105334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Costine-Bartell BA, McGuone D, Price G, Crawford E, Keeley KL, Munoz-Pareja J, et al. Development of a model of hemispheric hypodensity (“Big black brain”). J Neurotrauma. 2019;36(5):815–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Costine-Bartell BA, McGuone D, Price G, Crawford E, Keeley KL, Munoz-Pareja J, et al. Development of a model of hemispheric hypodensity (“big black brain”). J Neurotrauma. 2019;36(5):815–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Geddes JF, Hackshaw AK, Vowles GH, Nickols CD, Whitwell HL. Neuropathology of inflicted head injury in children. I. Patterns of brain damage. Brain. 2001;124(Pt 7):1290–8. [DOI] [PubMed] [Google Scholar]
- 15. Geddes JF, Vowles GH, Hackshaw AK, Nickols CD, Scott IS, Whitwell HL. Neuropathology of inflicted head injury in children. II. Microscopic brain injury in infants. Brain. 2001;124(Pt 7):1299–306. [DOI] [PubMed] [Google Scholar]
- 16. Lakhan S, Kirchgessner A, Tepper D, Leonard A. Matrix metalloproteinases and blood-brain barrier disruption in acute ischemic stroke. Front Neurol. 2013;4:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Meighan SE, Meighan PC, Choudhury P, Davis CJ, Olson ML, Zornes PA, et al. Effects of extracellular matrix-degrading proteases matrix metalloproteinases 3 and 9 on spatial learning and synaptic plasticity. J Neurochem. 2006;96(5):1227–41. [DOI] [PubMed] [Google Scholar]
- 18. Reinhard S, Razak K, Ethell I. A delicate balance: role of MMP-9 in brain development and pathophysiology of neurodevelopmental disorders. Front Cell Neurosci. 2015;9:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chandler S, Miller KM, Clements JM, Lury J, Corkill D, Anthony DC, et al. Matrix metalloproteinases, tumor necrosis factor and multiple sclerosis: an overview. J Neuroimmunol. 1997;72(2):155–61. [DOI] [PubMed] [Google Scholar]
- 20. Wilczynski GM, Konopacki FA, Wilczek E, Lasiecka Z, Gorlewicz A, Michaluk P, et al. Important role of matrix metalloproteinase 9 in epileptogenesis. J Cell Biol. 2008;180(5):1021–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abdul-Muneer PM, Pfister BJ, Haorah J, Chandra N. Role of matrix metalloproteinases in the pathogenesis of traumatic brain injury. Mol Neurobiol. 2016;53(9):6106–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bronisz E, Kurkowska-Jastrzebska I. Matrix metalloproteinase 9 in epilepsy: the role of neuroinflammation in seizure development. Mediators Inflamm. 2016;2016:7369020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gurney KJ, Estrada EY, Rosenberg GA. Blood brain barrier disruption by stromelysin-1 facilitates neutrophil infiltration in neuroinflammation. Neurobiol Dis. 2006;23(1):87–96. [DOI] [PubMed] [Google Scholar]
- 24. Turner RJ, Sharp FR. Implications of MMP9 for blood brain barrier disruption and hemorrhagic transformation following ischemic stroke. Front Cell Neurosci. 2016;10:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rosell A, Ortega-Aznar A, Alvarez-Sabín J, Fernández-Cadenas I, Ribó M, Molina CA, et al. Increased brain expression of matrix metalloproteinase-9 after ischemic and hemorrhagic human stroke. Stroke. 2006;37(6):1399–406. [DOI] [PubMed] [Google Scholar]
- 26. Cudna A, Jopowicz A, Mierzejewski P, Kurkowska-Jastrzębska I. Serum metalloproteinase 9 levels increase after generalized tonic-clonic seizures. Epilepsy Res. 2017;129:33–6. [DOI] [PubMed] [Google Scholar]
- 27. Kim HJ, Fillmore HL, Reeves TM, Phillips LL. Elevation of hippocampal MMP-3 expression and activity during trauma-induced synaptogenesis. Exp Neurol. 2005;192(1):60–72. [DOI] [PubMed] [Google Scholar]
- 28. Lee SR, Kim HY, Rogowska J, Zhao BQ, Bhide P, Parent JM, et al. Involvement of matrix metalloproteinase in neuroblast cell migration from the subventricular zone after stroke. J Neurosci. 2006;26(13):3491–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Simon D, Evaldt J, Nabinger DD, Fontana MF, Klein MG, do Amaral Gomes J, et al. Plasma matrix metalloproteinase-9 levels predict intensive care unit mortality early after severe traumatic brain injury. Brain Inj. 2017;31(3):390–5. [DOI] [PubMed] [Google Scholar]
- 30. Lima R, Simon D, Silva WDL, Nabinger DD, Regner A. Prognostic utility of early plasma matrix metalloproteinases -2 and -9 concentrations after severe traumatic brain injury. Rev Bras Ter Intensiva. 2020;32(3):418–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Berger RP, Pak BJ, Kolesnikova MD, Fromkin J, Saladino R, Herman BE, et al. Derivation and validation of a serum biomarker panel to identify infants with acute intracranial hemorrhage. JAMA Pediatr. 2017;171(6):e170429. [DOI] [PubMed] [Google Scholar]
- 32. Acharya NK, Goldwaser EL, Forsberg MM, Godsey GA, Johnson CA, Sarkar A, et al. Sevoflurane and Isoflurane induce structural changes in brain vascular endothelial cells and increase blood-brain barrier permeability: possible link to postoperative delirium and cognitive decline. Brain Res. 2015;1620:29–41. [DOI] [PubMed] [Google Scholar]
- 33. Wasserstein RL, Lazar NA. The ASA statement on p values: context, process, and purpose. Am Statistician. 2016;70(2):129–33. [Google Scholar]
- 34. Ajmera S, Motiwala M, Weeks M, Oravec CS, Hersh DS, Fraser BD, et al. What variables correlate with different clinical outcomes of abusive head injury? Neurosurgery. 2020;87(4):803–10. [DOI] [PubMed] [Google Scholar]
- 35. Duhaime AC, Holshouser B, Hunter JV, Tong K. Common data elements for neuroimaging of traumatic brain injury: pediatric considerations. J Neurotrauma. 2012;29(4):629–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Glykys J, Dzhala V, Egawa K, Kahle KT, Delpire E, Staley K. Chloride dysregulation, seizures, and cerebral edema: a relationship with therapeutic potential. Trends Neurosci. 2017;40(5):276–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Park KP, Rosell A, Foerch C, Xing C, Kim WJ, Lee S, et al. Plasma and brain matrix metalloproteinase-9 after acute focal cerebral ischemia in rats. Stroke. 2009;40(8):2836–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shi H, Wang HL, Pu HJ, Shi YJ, Zhang J, Zhang WT, et al. Ethyl pyruvate protects against blood-brain barrier damage and improves long-term neurological outcomes in a rat model of traumatic brain injury. CNS Neurosci Ther. 2015;21(4):374–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Taylor SR, Smith C, Harris BT, Costine BA, Duhaime AC. Maturation-dependent response of neurogenesis after traumatic brain injury in children. J Neurosurg Pediatr. 2013;12(6):545–54. [DOI] [PubMed] [Google Scholar]
- 40. Taylor SR, Smith CM, Keeley KL, McGuone D, Dodge CP, Duhaime AC, et al. Neuroblast distribution after cortical impact is influenced by white matter injury in the immature gyrencephalic brain. Front Neurosci. 2016;10:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Costine B, Missios S, Taylor SR, McGuone D, Smith CM, Dodge CP, et al. The subventricular zone in the immature piglet brain: anatomy and exodus of neuroblasts into white matter after traumatic brain injury. Dev Neurosci. 2015;37(2):115–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hadass O, Tomlinson BN, Gooyit M, Chen S, Purdy JJ, Walker JM, et al. Selective inhibition of matrix metalloproteinase-9 attenuates secondary damage resulting from severe traumatic brain injury. PLoS One. 2013;8(10):e76904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Svedin P, Hagberg H, Sävman K, Zhu C, Mallard C. Matrix metalloproteinase-9 gene knock-out protects the immature brain after cerebral hypoxia-ischemia. J Neurosci. 2007;27(7):1511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lee H, Park JW, Kim SP, Lo EH, Lee SR. Doxycycline inhibits matrix metalloproteinase-9 and laminin degradation after transient global cerebral ischemia. Neurobiol Dis. 2009;34(2):189–98. [DOI] [PubMed] [Google Scholar]
- 45. Wang X, Jung J, Asahi M, Chwang W, Russo L, Moskowitz MA, et al. Effects of matrix metalloproteinase-9 gene knock-out on morphological and motor outcomes after traumatic brain injury. J Neurosci. 2000;20(18):7037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nwachuku EL, Puccio AM, Adeboye A, Chang YF, Kim J, Okonkwo DO. Time course of cerebrospinal fluid inflammatory biomarkers and relationship to 6-month neurologic outcome in adult severe traumatic brain injury. Clin Neurol Neurosurg. 2016;149:1–5. [DOI] [PubMed] [Google Scholar]
- 47. Duhaime A-C, Hunter JV, Grate LL, Kim A, Golden J, Demidenko E, et al. Magnetic resonance imaging studies of age-dependent responses to scaled focal brain injury in the piglet. J Neurosurg. 2003;99(3):542–8. [DOI] [PubMed] [Google Scholar]
- 48. Muramatsu K, Fukuda A, Togari H, Wada Y, Nishino H. Vulnerability to cerebral hypoxic-ischemic insult in neonatal but not in adult rats is in parallel with disruption of the blood-brain barrier. Stroke. 1997;28(11):2281–9; discussion 2288-9. [DOI] [PubMed] [Google Scholar]
- 49. Fullerton J. Catastrophic disprution of the blood brain barrier in pediatric TBI: Annual National Neurotrauma Symposium; 2018. [Google Scholar]
- 50. Costine B, Quebeda-Clerkin PB, Dodge CP, Harris BT, Hillier SC, Duhaime AC. Neuron-specific enolase, but not S100B or myelin basic protein, increases in peripheral blood corresponding to lesion volume after cortical impact in piglets. J Neurotrauma. 2012;29(17):2689–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dingman AL, Stence NV, O’Neill BR, Sillau SH, Chapman KE. Seizure severity is correlated with severity of hypoxic-ischemic injury in abusive head trauma. Pediatr Neurol. 2018;82:29–35. [DOI] [PubMed] [Google Scholar]
- 52. Rodriguez FL. Brief apnea and hypoventilation reduces seizure duration and shifts seizure location for several hours in a model of severe traumatic brain injury: BioRxiv; 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.