Abstract
Human herpesviruses are characterized by distinct states of infection. Typically in permissive herpesvirus infection, abundant virus production results in cell lysis. In latent transforming Epstein-Barr virus (EBV) infection, viral proteins that induce cell growth are expressed. The immunodeficiency-associated hairy leukoplakia (HLP) lesion is the only pathologic manifestation of permissive EBV infection; however, within HLP, viral proteins characteristic of latent infection have also been detected. In this study, we further analyzed expression of EBV latent genes and investigated their contribution to the unique histologic phenotype of HLP. Coexpression of lytic and transforming viral proteins was detected simultaneously within individual HLP keratinocytes. LMP1 has now been shown to be uniformly expressed in the affected tissue, and it is associated and colocalizes with tumor necrosis factor receptor-associated factor (TRAF) signaling molecules. Effects induced by activated TRAF signaling that were detected in HLP included activation of NF-κB and c-Jun terminal kinase 1 (JNK1) and upregulated expression of epidermal growth factor receptor (EGFR), CD40, A20, and TRAFs. This study identifies a novel state of EBV infection with concurrent expression of replicative and transforming proteins. It is probable that both replicative and latent proteins contribute to HLP development and induce many of the histologic features of HLP, such as acanthosis and hyperproliferation. In contrast to other permissive herpesvirus infections, expression of EBV transforming proteins within the permissively infected HLP tissue enables epithelial cell survival and may enhance viral replication.
Normal oral mucosa is comprised of stratified squamous epithelium that is divided into four distinct differentiation states: a mitotically active basal layer, a spinous layer containing differentiation-associated keratins, a granular layer where a cornified scaffold is deposited beneath the plasma membrane, and a stratum corneum with metabolically inert cells (12). Basal cells expressing keratins K14 and K5, Bcl-2, and the epidermal growth factor receptor (EGFR) maintain proliferative capacity (12, 24). The EGFR is located primarily on the surface of basal cells and when bound to ligand influences mitogenesis and cell migration (24). As basal cells differentiate, the EGFR is no longer detected, and differentiation-specific cornifying keratins K1 and K10 are expressed suprabasally (12). Expression of the antiapoptotic molecule Bcl-2 in the basal cell layer decreases upon stratification (24). Epithelial cell differentiation involves anoikus, a form of apoptosis induced by loss of contact with the extracellular matrix (23). The granular layer of epithelium contains apoptotic cells, and the stratum corneum is marked by enucleated cells densely packed with keratin fibrils that form a protective barrier against extracellular insults.
Epstein-Barr virus (EBV) is a ubiquitous oral pathogen that infects lymphoid and epithelial cells. Multiple EBV-associated malignancies, including Burkitt's lymphoma and nasopharyngeal carcinoma, are characterized by latent EBV infection and cellular proliferation. In contrast, oral hairy leukoplakia (HLP) is a permissive EBV infection with abundant viral replication within the squamous epithelial cells of the lateral tongue border (15). HLP often develops in patients infected with the human immunodeficiency virus (HIV) and in persons with other significant immunodeficiencies. HLP is a hyperproliferative lesion characterized histologically by intracellular edema, epithelial acanthosis (thickening), lack of inflammatory infiltrate, and hyperkeratosis. These cellular characteristics are also found in the histologically identical pseudohairy leukoplakia lesion (PHLP); however, EBV DNA is not detected (14).
Expression of EBV LMP1, an integral membrane protein, has been detected in HLP and in EBV-associated malignancies (34, 43). LMP1 modulates cellular growth and differentiation in a variety of cell types. LMP1 expression is transforming in rodent fibroblasts, resulting in loss of contact inhibition and induction of tumorigenicity in nude mice (41). LMP1 induces expression of multiple cell surface markers, cell activation antigens, and cell adhesion molecules (33, 42). The carboxy-terminal region of LMP1 is essential for signal transduction and activates NF-κB-mediated transcription from two effector domains, carboxy-terminal activating region 1 (CTAR1) and CTAR2 (18). CTAR1, in addition to NF-κB activation, induces EGFR expression through interaction with tumor necrosis factor (TNF)-associated factors (TRAFs) (29). CTAR2 does not induce EGFR expression but activates NF-κB and the c-jun N-terminal kinase (JNK) signaling pathway, leading to activation of AP-1-dependent transcription (19). Epithelial cells expressing LMP1 also express CD40 and ICAM1, and organotypic cultures of these cells are much thicker and less organized, with poor intercellular contacts (5).
In this study, expression of LMP1, the viral transcriptional regulators of LMP1, Epstein-Barr nuclear antigen 1 (EBNA1), EBNA2, and EBNA-LP, and activation of the LMP1 signaling cascade were evaluated in HLP. EGFR expression in HLP was examined, as were other transcriptional targets of LMP1 signaling. The data suggest that expression of EBNA2 and LMP1 significantly influences the unique pathologic phenotype of HLP.
MATERIALS AND METHODS
Patients and tissues.
Biopsy specimens of oral HLP were obtained from HIV-seropositive patients identified at the University of North Carolina (UNC) Hospitals. Specimens were immediately frozen and stored at −80°C. A portion of each biopsy was fixed in formalin and sent to UNC Hospitals Department of Pathology for histopathologic diagnosis. The presence of EBV within the HLP biopsies was determined by identification of the terminal restriction enzyme fragments on Southern blots (13).
Cell lines.
Epithelial cell lines C33A, HT29, and H1299 were grown at 37°C in DMEM-H supplemented with 10% fetal calf serum (Irvine Scientific) and antibiotics. LMP1-, TRAF1-, and TRAF3-expressing derivative cell lines were produced and maintained as previously described (26). The lymphoid cell lines B95-8, X50-7, and cord blood cells transformed with B95-8 virus (CB B95-8) were maintained in suspension at 37°C in RPMI 1640 supplemented with 10% fetal calf serum and antibiotics.
RNA isolation, cDNA synthesis, and reverse transcription-PCR (RT-PCR).
DNA and RNA were extracted by pulverizing frozen tissue and ultracentrifugation on a 4 M guanidine isothiocyanate-cesium chloride step gradient as previously described (13). Removal of residual DNA in the RNA was accomplished by digestion with DNase I (Boehringer Mannheim). Subsequent to priming with oligo(dTTP), cDNA was synthesized from 10 μg of HLP RNA with Superscript reverse transcriptase (Gibco BRL) in the presence of 100 μM dATP, dCTP, dGTP, and dTTP.
Single-stranded DNA oligonucleotides were synthesized for PCR primers. Primer design allowed amplification across introns to distinguish processed mRNA from genomic DNA or unprocessed RNA. PCR amplifications were performed with 150 to 200 ng of cDNA as previously described, with amplification proceeding for a total of 38 cycles. Gels were stained with ethidium, and then cDNA was transferred to a membrane and hybridized with an end-labeled probe that was internal to the initial primer set (5).
Immunoblotting.
HLP biopsy specimens and HIV-negative tongue tissues were processed as previously described (10). Following sodium dodecyl sulfate-polyacrylamide gel electrophoresis, protein samples were electrophoretically transferred to nitrocellulose, blocked, and probed with either EBNA-LP monoclonal antibody JF186, LMP1 monoclonal antibody OT22C, pooled EBNA1 polyclonal antibodies (K12, K67, and K17), or TRAF1 polyclonal (Santa Cruz), TRAF2 polyclonal, TRAF3 polyclonal, and phospho-ERK1/2 or phospho-JNK monoclonal (Promega) antibodies as previously described (27).
Immunocytochemistry.
Biopsy tissues were placed in neutral buffered formalin and paraffin embedded. Specimens were serially sectioned at 4-μm thickness. Tissue sections were adhered to poly-l-lysine-coated slides, deparaffinized, and washed with phosphate-buffered saline. Slides were then incubated in 3% hydrogen peroxide and blocked with blocking agent (DAKO Corporation). Primary antibodies for BZLF1 (Argene), BHRF1 (Chemicon), LMP1 (mouse monoclonal OT22C or rabbit polyclonal LYDMA), EGFR (rabbit polyclonal EGFR 22-013/ECRT or Clone1 monoclonal), EBNA2 (mouse monoclonal PE2), CD40 (rabbit polyclonal C20; Santa Cruz), TRAF1 (rabbit polyclonal S19; Santa Cruz), TRAF2 (rabbit polyclonal C20; Santa Cruz), TRAF3 (rabbit polyclonal N19; Santa Cruz), and anti-NF-κB, p65 subunit (mouse monoclonal; Boehringer Mannheim) were incubated, and a DAKO LSAB+ peroxidase kit was used according to the manufacturer's specifications. Subsequent to application of 3,3′-diaminobenzidine chromagen solution, slides were counterstained with methyl green or hematoxylin (Sigma) and then mounted with coverslips using Permount (Fisher Scientific).
Immunofluorescence.
Frozen tissue sections were cut to 5-μm thickness and placed on poly-l-lysine-coated slides. Tissue sections and cell lines were fixed in a chilled 1:1 mixture of methanol and acetone, blocked with 20% normal goat serum, stained with primary antibody, washed, and then stained with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin G or lissamine rhodamine goat anti-rabbit immunoglobulin G. Slides were mounted with coverslips using Vectashield (Vector Laboratories) and then subjected to confocal microscopy. Confocal images were overlaid; using the image processing tool kit, only pixels that stained positive for both fluorescein and rhodamine colocalized and were visualized as yellow (36).
Coimmunoprecipitations.
Pulverized tissues or cell lines were dissolved in NP-40 lysis buffer. The supernatant was clarified by centrifugation and precleared by incubation with normal mouse or rabbit serum, and LMP1 and associated proteins were immunopurified with Gammabind Plus beads and TRAF1-, TRAF3-, or LMP1-specific antibodies overnight at 4°C. Bound LMP1 and TRAF proteins were detected by immunoblotting as described elsewhere (26).
RESULTS
HLP is a predominantly permissive infection whose molecular pathogenesis is thought to reflect pure EBV lytic replication. Identification of terminal EBV restriction enzyme fragments in HLP specimens revealed ladder arrays of restriction enzyme fragments characteristic of productive EBV infection (data not shown). Analysis of the EBV terminal repeat structure indicated that these specimens contained linear virion DNA. Within this permissive lesion, EBER expression was detected only in trace amounts on Northern blot analysis and after two sets, 38 cycles each, of RT-PCR (data not shown) (13).
BZLF1 is the initial immediate-early protein expressed during induction of EBV productive infection, and BZLF1 and BRLF1 are the major immediate-early transcriptional regulators of replicative viral gene expression (38). In HLP, BZLF1 and BRLF1 were detected in the nuclei of HLP cells in the mid to upper strata (Table 1; Fig. 1A1 and A3). Staining for BRLF1 detected its expression in condensed nuclei in the koilocyte cells. The early gene product, BHRF1, was expressed in the same region of the strata but was detected in the cytoplasm (Table 1; Fig. 1B2 and B4). BHRF1 is a Bcl-2 homolog with antiapoptotic activity (17). BHRF1 expression was also detected in the mid to upper strata within the stratum spinosum and stratum granulosum layer. This region is where apoptosis occurs during normal stratified squamous differentiation, a process potentially affected by BHRF1.
TABLE 1.
Immunohistochemical detection of viral and cellular proteins in HLPa
| Sample | Reaction after staining:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| For LMP1-associated and -inducible gene products
|
With monoclonal antibody specific to:
|
PBS | ||||||||||
| CD40 | TRAF 1 | TRAF 2 | TRAF 3 | NFκB p65 | EGFR | EBNA2 | EBNA1 | BHRF1 | BZLF1 | LMP1 | ||
| HIV-A | ∗ | − | ND | ND | − | ∗ | − | − | − | − | − | − |
| HIV-B | ∗ | ND | + | ± | ND | ∗ | − | ND | ± | − | − | − |
| HLP1 | + | + | ND | ND | + | + | + | + | + | + | + | − |
| HLP2 | + | ND | − | ± | + | + | + | + | ND | ND | + | − |
| HLP3 | + | − | + | ± | − | + | + | + | + | + | + | − |
| HLP4 | ND | − | + | + | ND | ND | ND | ND | ND | ND | + | − |
| HLP5 | ND | + | + | + | + | ND | + | ND | ND | ND | + | − |
Peroxidase-based immunocytochemical analysis was performed on paraffin-embedded sections of tongue biopsy specimens from two HIV-negative individuals with hyperkeratosis in the region of biopsy and five HIV-positive individuals with HLP. Sections were stained as indicated; all sections were analyzed using phosphate-buffered saline (PBS) and secondary antibody alone as negative controls. Scoring: +, positive suprabasal staining; ∗, staining of the basal layer; −, no staining; +/−, weak signal. ND, not determined.
FIG. 1.
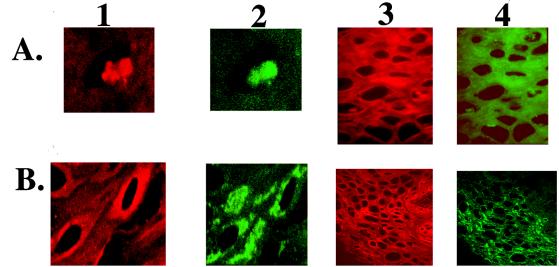
(A) Nuclear expression of EBV-encoded proteins associated with permissive and transforming infection in HLP. Frozen sections of HLP were stained with a BRLF1 polyclonal antiserum and EBNA2 monoclonal antiserum. Rhodamine-conjugated anti-rabbit secondary antibody detected BRLF1 (red fluorescence) in the nuclei of cells within HLP (A1 and A3). FITC-conjugated anti-mouse secondary antibody detected EBNA2 (green fluorescence) in the nuclei and cytoplasm of keratinocytes within HLP (A2 and A4). (B) Cytoplasmic expression of EBV-encoded proteins associated with permissive and transforming infection in HLP. Frozen sections of HLP were stained with a BHRF1 monoclonal antibody and an LMP1 rabbit polyclonal antibody. Rhodamine-conjugated anti-rabbit secondary antibody detected LMP1 (red fluorescence) in the cytoplasm of cells within HLP (B1 and B3). Likewise, FITC-conjugated anti-mouse secondary antibody detected BHRF1 (green fluorescence) in the cytoplasm of keratinocytes within HLP (B2 and B4).
In addition to these lytic cycle antigens, proteins essential for transforming infection, LMP1, EBNA1, EBNA2, and EBNA-LP, are also expressed in HLP (Table 1; Fig. 1 and 2). EBV transforming proteins expressed in HLP were detected together with lytic gene products within the same keratinocytes of the mid to upper stratum spinosum. Confocal microscopy detected expression of BRLF1 within condensed nuclei of the same cells of HLP that express EBNA2 (Fig. 1A1 and A2). Similarly, within tissue sections, LMP1 and BHRF1 were found in the cytoplasm of HLP cells (Fig. 1B). Although the distribution of the proteins within the tissue differed, 50 to 60% of infected cells expressed both antigens simultaneously (Fig. 1A3, A4, B3, and B4). This coexpression implies that there are not distinct lytic and latent cell populations within HLP. The variability in staining reflects cells expressing viral antigens at different points in the lytic cycle. Staining was not detected in HLP stained with FITC-conjugated antibodies or rhodamine-conjugated secondary antibodies alone or in normal tongue stained with LMP1, BHRF1, BRLF1, or EBNA2 and their secondary antibodies (data not shown).
FIG. 2.
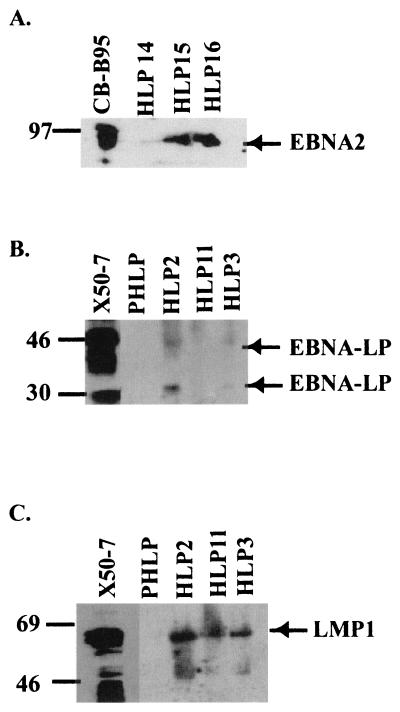
Immunoblot detection of EBV proteins from tongue biopsy specimens. (A) EBNA2 was detected in the three HLP samples (HLP14, -15, and -16) and control lymphoblastoid cell line CB-B95-8. (B) EBNA-LP was detected at 44 kDa in the EBV-positive lymphoblastoid line X50-7, and 33- and 42-kDa proteins were detected in the HLP2 and -13 samples. (C) LMP1 was detected in HLP2, -11, and -13 samples and control lymphoblastoid cell line X50-7 but was not detected in PHLP. Locations of the viral proteins and the molecular mass markers (in kilodaltons) are indicated.
In lymphocytes, LMP1 expression is transcriptionally regulated by viral proteins EBNA2 and EBNA-LP through interactions with the DNA binding proteins RBP-Jκ and PU.1. EBNA-LP has recently been shown to greatly enhance EBNA2-mediated transactivation in lymphocytes (16, 30). EBNA2 was detected by immunohistochemistry, and the 85-kDa protein was detected by immunoblotting in three of three HLP samples (Fig. 1A2 and A4; Fig. 2A). An immunoblot probed with a monoclonal antibody specific for EBNA-LP detected proteins migrating at 42 and 35 kDa in HLP. EBNA-LP was detected in two of three HLPs but not in PHLP (Fig. 2B). EBNA-LP varies in size due to differing numbers of IR1 repeats; thus, the size of the protein may range from 28 to 90 kDa (8). The 63-kDa LMP1 protein was also detected on immunoblots in six of six HLP samples (Fig. 2C and 6). The differences between EBV protein levels in the tissue samples and cell lines likely reflect differences in the proportion of uninfected cells within the tissue sample.
FIG. 6.
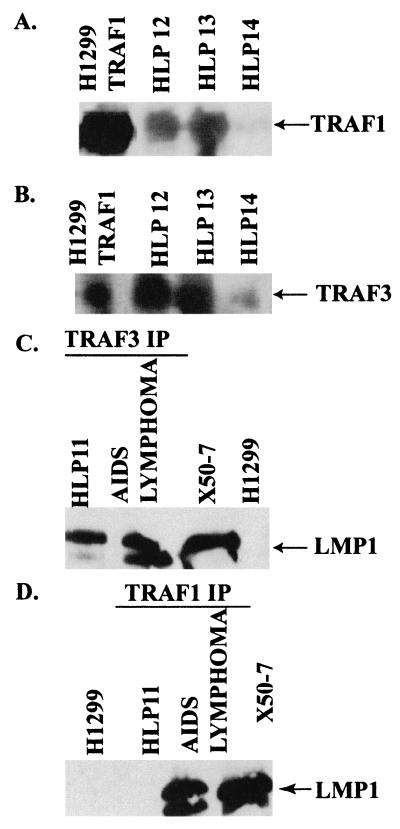
Expression of TRAF1 and TRAF3 and detection of LMP1-TRAF complexes in an HLP biopsy specimen. Protein lysates from HLP and AIDS lymphoma specimens were prepared and either immunoblotted for TRAF1 (A) or TRAF3 (B) or immunoprecipitated (IP) with antibodies for TRAF3 (C) or TRAF1 (D) and analyzed for LMP1 by immunoblotting. TRAF1 and TRAF3 were detected in three of three HLP specimens (HLP12, -13, and -14) and in H1299 cells transfected with TRAF1 and TRAF3 by immunoblotting (A and B). LMP1 was detected in complexes immunoprecipitated for TRAF3 in the HLP (C) but not in complexes that contained TRAF1 (D). Direct loads of protein lysates from the LMP1-positive cell line X50-7 and the EBV-negative epithelial cell line H1299 were included as controls for LMP1 expression.
The mRNA encoding LMP1, an EBV signaling molecule and oncogene, has been consistently detected in HLP (43). Previous studies identified occasional foci of LMP1-positive cells in the middle to upper epithelial layers of the HLP, using monoclonal antibodies S12 and CS1-4, although the mRNA was distributed throughout all layers as detected by in situ hybridization (37, 39). In this study, immunohistochemistry with OT22C, an LMP1-specific monoclonal antibody with increased affinity and specificity, detected LMP1-positive cells with characteristic membrane patching throughout HLP, predominately in the mid to upper strata (Fig. 3B).
FIG. 3.
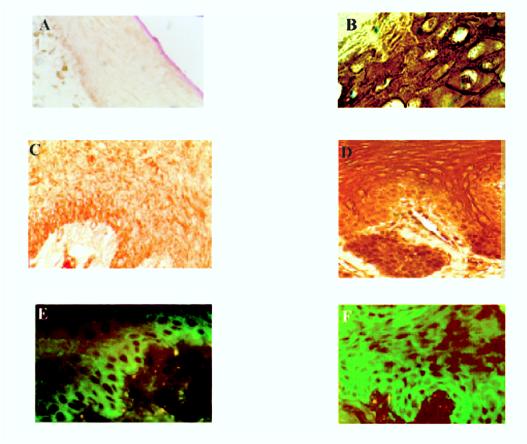
LMP1 and EGFR expression in HLP. Images show immunofluorescence and immunoperoxidase-based staining of frozen and paraffin-embedded HIV-negative tongue sections (A, C, and E) or HLP (B, D, and F). Membrane and cytoplasmic staining of LMP1 with monoclonal antibody OT22C (1:20) was detected in suprabasal cells of HLP (staining of the upper stratum spinosum shown in panel B) and not in tongue specimens from HIV-negative individuals at a magnification of ×200 (A). Both immunoperoxidase-based staining and immunofluorescence detect significant suprabasal EGFR staining in HLP (staining of the lower to middle stratum spinosum shown in panels D and F), while staining of the control tissue was associated only with the basal layer of epithelial cells (C and E) (magnification, ×400).
LMP1 alters cell function by inducing expression of cellular genes and in epithelial cells induces expression of the EGFR (27). EGFR expression within HLP was determined by immunohistochemistry and immunoblotting (Fig. 3 and data not shown). In normal stratified epithelia, EGFR expression was localized to the basal layer (Fig. 3C and E), while in HLP high levels of EGFR expression were detected in suprabasal cells within the stratum spinosum (Fig. 3D and F).
In normal tissues, basal cells lose their proliferative ability as they migrate upward to become spinous cells (11). EGFR activates the mitogen-activated protein kinase (MAPK) pathway, providing a stimulus for cellular growth. Use of phosphospecific antibodies for ERK1/2, members of the MAPK pathway, detected activated forms of both proteins in three of three HLP specimens, while trace amounts of ERK1 were detected in the HIV-control tongue specimen (Fig. 4). The suprabasal detection of EGFR and activation of downstream effectors of EGFR signaling in HLP suggests that the spinous cells retain proliferative ability, resulting in the marked thickening of the spinous cell layer that is characteristic of HLP.
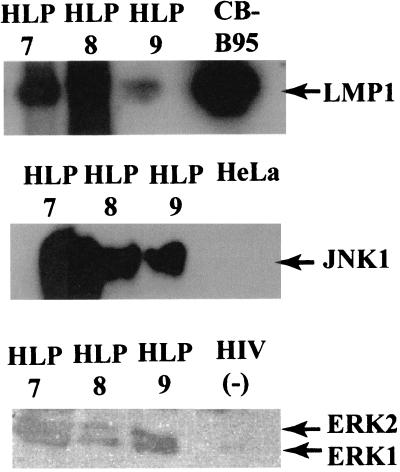
FIG. 4.
Expression of LMP1 and activated JNK1 and ERK1/2 in HLP. Protein lysates of HLP7, -9, and -10, of HIV-negative tongue specimens (CB-B95), and of EBV-negative cell lines (HeLa and H1299) were subjected to Western blot analysis. (Top) LMP1. (Middle and bottom) Blots probed with polyclonal antibodies specific for phosphorylated forms of MAPK and JNK detected active JNK1 and ERK1/2 in HLP and ERK1 in the HIV-negative control.
LMP1 has also been shown to activate JNK1 (19). JNK1 activation was assayed by immunoblotting of HLP lysates probed with an antibody specific for the activated, phosphorylated forms of JNK. Activation of JNK1 but not JNK2 was detected in three of three HLP specimens tested but was not detected in the epithelial cell line HeLa and was detected at very low levels in an HIV-negative tongue specimen (Fig. 4 and data not shown).
The expression of other cellular genes induced by LMP1 was analyzed by RT-PCR (Table 2). LMP1 induces expression of the antiapoptotic molecule A20 by activating NF-κB in both epithelial cells and lymphocytes (21). Interestingly, in two of three HLPs examined A20 was expressed, while low levels were detected in the PHLP and in HIV-negative tongue. CD40, a lymphoid cell activation marker and signaling molecule recently detected in the basal layer of normal epidermis (32), was also detected in HLP at higher levels than in PHLP (Table 1). CD40 upregulation by LMP1 has been reported for nasopharyngeal carcinoma (1). CD40 transcription was detected in three of three HLPs assayed and also in the PHLP but not in the HIV-negative tongue specimens. EGFR mRNA was also detected in three of three HLP specimens but was barely detected in the HIV-negative tongue specimens and PHL (Table 2). Low-level transcription of these genes was detected in RNA from nondiseased tissues. In RNA from HLP, the transcription of these genes was significantly increased.
TABLE 2.
Detection of EGFR, A20, and CD40 mRNAs in HLPa
| Molecule | Signal
|
|||||
|---|---|---|---|---|---|---|
| HLP6 | HLP7 | HLP8 | PHL | HIV-A | HIV-C | |
| A20 | + | + | − | ± | − | ± |
| EGFR | + | + | + | ± | − | ± |
| CD40 | + | + | + | ± | ± | − |
| BZLF1 | + | + | + | − | − | − |
| Actin | + | + | + | + | + | + |
Analysis of the expression of molecules known to be upregulated by LMP1 by RT-PCR allowed assessment of LMP1 function within HLP at the level of mRNA. mRNA samples from three patients with HLP (HLP6, HLP7, and HLP8), a patient with PHL, and two HIV-negative subjects (HIV-A and HIV-C) were subjected to RT-PCR with the relevant primer-probe combinations. BZLF1 expression was analyzed to confirm, along with Southern blot analysis, that the HLPs contained replicating EBV and that actin was amplified to control for amplification efficiency. As expected, BZLF1 was not detected in the PHL or HIV-negative tongue specimens. Scoring: +, positive signal detectable at the level of ethidium; −, no signal; ±, weak signal detectable only after hybridization.
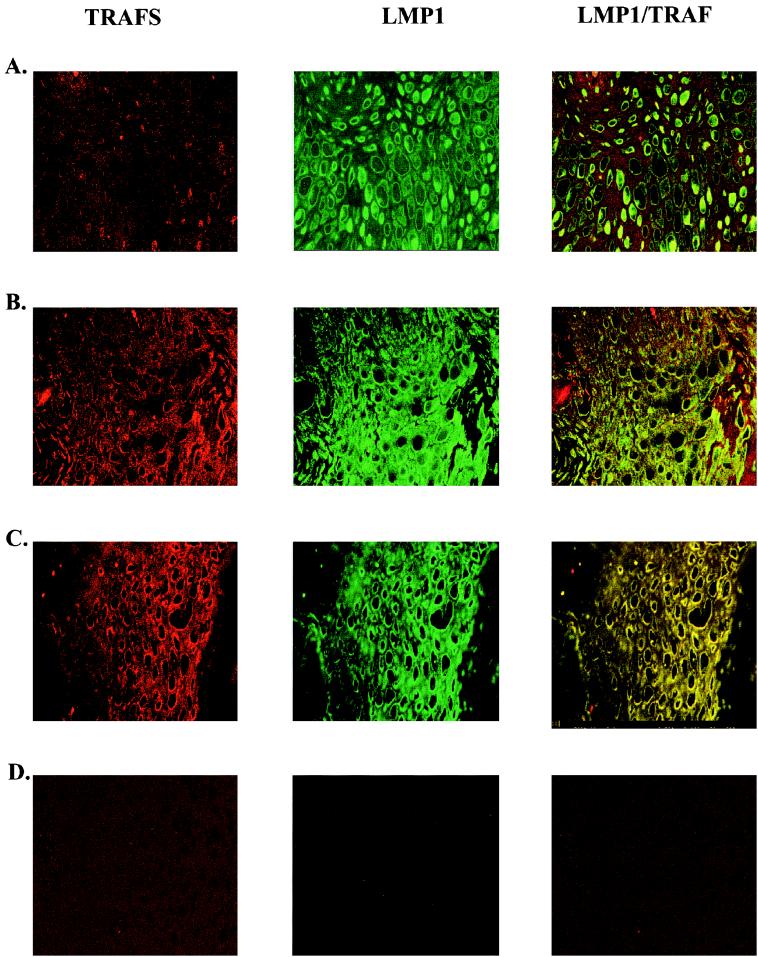
LMP1 interacts with TRAF molecules and is thought to be a constitutively active member of the TNF receptor (TNFR) family (28). In LMP1, amino acids 204 to 208, the PQQAT TRAF binding motif, binds TRAF1, TRAF2, and TRAF3 (6, 28). Expression of all three of these molecules was detected in HLP, with TRAF1, TRAF2, and TRAF3 expressed at higher levels in HLP than in normal tissue, as indicated by immunohistochemistry (Fig. 5; Table 1). Confocal microscopy detected an association of LMP1 with TRAF1 throughout the stratum spinosum (Fig. 5A). The characteristic membrane patching of LMP1 and colocalization with both TRAF2 and TRAF3 was readily visualized throughout the stratum spinosum (Fig. 5B and C).
FIG. 5.
LMP1 and TRAF molecules colocalize by confocal microscopy in HLP. Frozen sections of HLP and HIV-negative tongue specimens were stained with an LMP1 monoclonal antibody and rabbit polyclonal antibodies for TRAF1, TRAF2, and TRAF3. Rhodamine-conjugated secondary antibody detects TRAFs (red fluorescence), and FITC-conjugated secondary antibody detects LMP1 (green fluorescence) throughout the stratum spinosum of HLP. In panels A to C, colocalization of molecules in HLP is detected by yellow staining (magnification, ×400). (A) TRAF1-LMP1 colocalization; (B) colocalization of TRAF2 and LMP1; (C) colocalization of TRAF3 and LMP1. Staining was not detected in HLP stained with FITC or rhodamine alone (data not shown) or in normal tongue stained with LMP1, TRAF1, TRAF2, and TRAF3 and their secondary antibodies (D).
Expression of TRAF1 and TRAF3 was readily detected on immunoblots of HLP and in epithelial cells transfected with expression constructs (Fig. 6A and B). In complexes that were immunoprecipitated with antibodies specific for TRAF3 from HLP11, LMP1 was also detected at levels equivalent to that detected in an EBV-associated lymphoma that developed in a patient with AIDS (Fig. 6C). In contrast, although LMP1 could be detected in the TRAF1 immune complexes from the AIDS lymphoma, it was not detected in the TRAF1 complex from HLP11 (Fig. 6D). This may be due to lower levels of TRAF1 in epithelial cells or differences in the relative TRAF1 abundance in the LMP1 complexes in HLP. Although colocalization of TRAF1, TRAF3, and LMP1 was detected in all HLP tissue sections by immunohistochemistry, coimmunoprecipitation was achieved in only one sample. The variation in detection of immunoprecipitated TRAF-LMP1 complexes may reflect disruption of molecular complexes during tissue processing of frozen biopsy specimens (Fig. 6C and D).
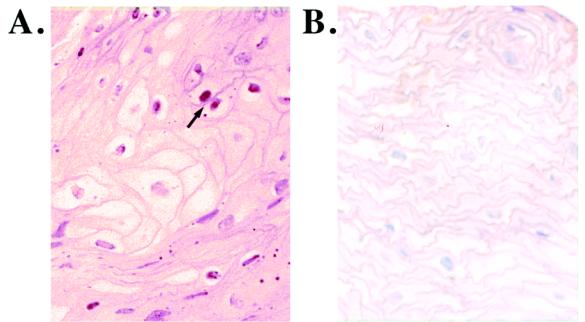
The LMP1-TRAF association is known to activate the transcription factor NF-κB; in epithelial cells, three distinct NF-κB complexes have been detected, with the major complex containing p52-p65 heterodimers (7, 22, 25, 26, 31). To determine if NF-κB is activated in HLP, antibodies specific for the p65 nuclear localization signal were used to detect the activated form of the transcription factor by immunohistochemistry. Activated p65 was detected in the nuclei of multiple cells within HLP but was not detected in normal tissue, which was stained only by the counterstain (Fig. 7; Table 1).
FIG. 7.
Activated NF-κB is detected in HLP. A monoclonal antibody specific for the nuclear localization signal of the NF-κB subunit p65 was used to detect the activated form of the transcription factor in nuclei of several cells within HLP. The arrow in panel A indicates NF-κB-positive brown-staining nuclei in HLP tissue. This signal was not detected in the control specimens, in the HIV-negative control subject shown in panel B, or with secondary antibody alone (data not shown).
These data indicate that in HLP, LMP1 activates the same signaling pathways, including TRAF, NF-κB, and JNK1, as it does during the transformation of lymphocytes. The resulting effects on expression of EGFR, A20, and CD40 are likely to be important to HLP pathogenesis.
DISCUSSION
This study reveals a novel state of EBV infection within HLP, where multiple EBV gene products characteristic of permissive and transforming infection are consistently expressed and appear to contribute to pathogenesis. Cells within the mid to upper stratum spinosum of HLP simultaneously express latent and lytic EBV proteins. Not only are these transforming proteins expressed in HLP, it is likely that their function within the lesion together with lytic cycle proteins is critical to development of this lesion. Unlike other herpesviruses, such as herpes simplex virus, where fulminant replication results in cell lysis and ulcer formation, EBV in HLP instead causes acanthosis. It appears that by inducing cell proliferation and enhancing cell survival within the lesion, the transforming proteins create an optimal environment for viral replication, subsequent infection of neighboring cells, and possible superinfection of infected cells, which results in the intertypic recombinants characteristic of HLP (40). Interestingly, the expression of these proteins is dependent on viral replication within HLP, as administration of the antiviral agent acyclovir, which targets the viral DNA polymerase, terminates expression of all viral proteins within HLP, transforming and replicative alike (35).
As EBV infection distinguishes HLP from the HIV-negative control specimens, differences reflected in cellular properties are presumably a direct result of viral gene expression. In HLP, LMP1 and other viral proteins may play a role in the abnormal cell growth of keratinocytes in the absence of malignancy. This is the first report of consistent, uniform expression of full-length LMP1 and its viral regulators, EBNA1, EBNA2, and EBNA-LP, within HLP tissues. The data presented in this study suggest that in HLP, as in malignancy, LMP1 is active and functions as a signaling molecule. LMP1 colocalization with cellular TRAFs and activation of known pathways by LMP1 in HLP are indicative of LMP1 signaling. It is likely that activation of these key signaling intermediates modulates cell proliferation. LMP1-TRAF interactions have recently been found in EBV-associated posttransplant lymphoproliferative disease and in this study were detected in EBV-associated AIDS lymphomas (22). In another hyperproliferative acanthotic epidermal lesion, psoriasis, the type 1 TNFR is abundantly expressed (20). This may indicate that the type 1 TNFR, which also interacts with TRAF molecules, may affect epithelial cell proliferation similarly to LMP1 in HLP. LMP1 is a known transforming protein whose expression in lymphoid cells significantly enhances B-cell proliferation. While epithelial proliferation rates in HLP are similar to those for control tongue specimens, the virus modulates the cell phenotype within HLP, and suprabasal cells are not postmitotic but maintain proliferative ability (39). This is evident by the suprabasal expression of basal cell markers, K5 and K14, and by upregulation of proliferation-associated proteins CD40 and EGFR in HLP (44). LMP1 transgenic mice have acanthotic hyperplastic skin lesions which are similar to HLP in the distribution of keratin markers (45, 39). It is likely that LMP1-enhanced suprabasal expression of EGFR and CD40 may stimulate growth of virus-infected tissue. The activation of MAPK within HLP suggests that the EGFR is also active and contributes to proliferation, providing further evidence that EBV infection within these tissues provides signals for cell growth.
Additionally, cells within HLP defer commitment to cell death. The minimal expression of hyperproliferation-associated keratins indicates that the cell turnover is reduced in HLP (44). Interestingly, levels of the apoptosis-associated proteins Bcl-2, Bcl-x, and Bax in HLP are minimally altered (2). The EBV BHRF1 gene product, a homologue of Bcl-2, is expressed at high levels within HLP and may inhibit the normal program of cell death, delaying differentiation and enhancing cell survival (3, 4). A20 expression provides a second antiapoptotic mechanism. LMP1-induced expression of A20 has been shown to protect epithelial cells from p53-mediated apoptosis (9, 21). BHRF1 expression together with A20 may extend cell survival in HLP, perhaps contributing to the acanthosis characteristic of HLP.
These studies suggest that in HLP, by expressing genes characteristic of transforming and permissive infection, EBV interrupts normal epithelial growth patterns. EBV delays anoikus by antiapoptotic mechanisms and provides an additional growth advantage to its host cells through activation of NFκB, MAPK, and JNK and by upregulation of such molecules as EGFR and CD40. These events are likely mediated by LMP1 signaling.
ACKNOWLEDGMENTS
We thank members of the UNC Hospitals divisions of Oral Medicine and Infectious Disease for aid in patient identification, members of the Raab-Traub lab and M. H. McNary for critical reviews and helpful discussions, and all patients involved in the study. We thank H. Shelton Earp for the anti-ECRT rabbit antiserum, Elliot Kieff, Matthew Davenport, Val Zacny, Wanla Kulwichit, and Shannon Kenney for various viral antibodies, histologists Cynthia Suggs and Theresa Bone-Turrentine for tissue section preparation, and Robert Bagnell and Vickie Madden of the UNC microscopy facility.
This work was supported by National Institutes of Health grants T32A10 7151-21 and P30HD37260 (to J.W.-C.) and DE11644 and CA19014 (to N.R.-T.)
REFERENCES
- 1.Busson P, Zhang Q, Guillon J M, Gregory C D, Young L S, Clausse B, Lipinski M, Rickinson A B, Tursz T. Elevated expression of ICAM1 (CD54) and minimal expression of LFA3 (CD58) in Epstein-Barr-virus-positive nasopharyngeal carcinoma cells. Int J Cancer. 1992;50:863–867. doi: 10.1002/ijc.2910500605. [DOI] [PubMed] [Google Scholar]
- 2.Chrysomali E, Greenspan J S, Dekker N, Greenspan D, Regezi J A. Apoptosis-associated proteins in oral hairy leukoplakia. Oral Dis. 1996;2:279–284. doi: 10.1111/j.1601-0825.1996.tb00238.x. [DOI] [PubMed] [Google Scholar]
- 3.Dawson C W, Dawson J, Jones R, Ward K, Young L S. Functional differences between BHRF1, the Epstein-Barr virus-encoded Bcl-2 homologue, and Bcl-2 in human epithelial cells. J Virol. 1998;72:9016–9024. doi: 10.1128/jvi.72.11.9016-9024.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dawson C W, Eliopoulos A G, Dawson J, Young L S. BHRF1, a viral homologue of the Bcl-2 oncogene, disturbs epithelial cell differentiation. Oncogene. 1995;10:69–77. [PubMed] [Google Scholar]
- 5.Dawson C W, Rickinson A B, Young L S. Epstein-Barr virus latent membrane protein inhibits human epithelial cell differentiation. Nature. 1990;344:777–780. doi: 10.1038/344777a0. [DOI] [PubMed] [Google Scholar]
- 6.Devergne O, Hatzivassiliou E, Izumi K M, Kaye K M, Kleijnen M F, Kieff E, Mosialos G. Association of TRAF1, TRAF2, and TRAF3 with an Epstein-Barr virus LMP1 domain important for B-lymphocyte transformation: role in NF-κB activation. Mol Cell Biol. 1996;16:7098–7108. doi: 10.1128/mcb.16.12.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eliopoulos A G, Stack M, Dawson C W, Kaye K M, Hodgkin L, Sihota S, Rowe M, Young L S. Epstein-Barr virus-encoded LMP1 and CD40 mediate IL-6 production in epithelial cells via an NF-κB pathway involving TNF receptor-associated factors. Oncogene. 1997;14:2899–2916. doi: 10.1038/sj.onc.1201258. [DOI] [PubMed] [Google Scholar]
- 8.Finke J, Rowe M, Kallin B, Ernberg I, Rosen A, Dillner J, Klein G. Monoclonal and polyclonal antibodies against Epstein-Barr virus nuclear antigen 5 (EBNA-5) detect multiple protein species in Burkitt's lymphoma and lymphoblastoid cell lines. J Virol. 1987;61:3870–3878. doi: 10.1128/jvi.61.12.3870-3878.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fries K L, Miller W E, Raab-Traub N. Epstein-Barr virus latent membrane protein 1 blocks p53-mediated apoptosis through the induction of the A20 gene. J Virol. 1996;70:8653–8659. doi: 10.1128/jvi.70.12.8653-8659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fries K L, Sculley T B, Webster-Cyriaque J, Rajadurai P, Sadler R H, Raab-Traub N. Identification of a novel protein encoded by the BamHI A region of the Epstein-Barr virus. J Virol. 1997;71:2765–2771. doi: 10.1128/jvi.71.4.2765-2771.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuchs E. Epidermal differentiation. Curr Opin Cell Biol. 1990;2:1028–1035. doi: 10.1016/0955-0674(90)90152-5. [DOI] [PubMed] [Google Scholar]
- 12.Fuchs E, Byrne C. The epidermis: rising to the surface. Curr Opin Genet Dev. 1994;4:725–736. doi: 10.1016/0959-437x(94)90140-x. [DOI] [PubMed] [Google Scholar]
- 13.Gilligan K, Rajadurai P, Resnick L, Raab-Traub N. Epstein-Barr virus small nuclear RNAs are not expressed in permissively infected cells in AIDS-associated leukoplakia. Proc Natl Acad Sci USA. 1990;87:8790–8794. doi: 10.1073/pnas.87.22.8790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green T L, Greenspan J S, Greenspan D, De Souza Y G. Oral lesions mimicking hairy leukoplakia: a diagnostic dilemma. Oral Surg Oral Med Oral Pathol. 1989;67:422–426. doi: 10.1016/0030-4220(89)90385-x. [DOI] [PubMed] [Google Scholar]
- 15.Greenspan J S, Greenspan D, Lennette E T, Abrams D I, Conant M A, Petersen V, Freese U K. Replication of Epstein-Barr virus within the epithelial cells of oral “hairy” leukoplakia, an AIDS-associated lesion. N Engl J Med. 1985;313:1564–1571. doi: 10.1056/NEJM198512193132502. [DOI] [PubMed] [Google Scholar]
- 16.Harada S, Kieff E. Epstein-Barr virus nuclear protein LP stimulates EBNA-2 acidic domain-mediated transcriptional activation. J Virol. 1997;71:6611–6618. doi: 10.1128/jvi.71.9.6611-6618.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henderson S, Huen D, Rowe M, Dawson C, Johnson G, Rickinson A. Epstein-Barr virus-coded BHRF1 protein, a viral homologue of Bcl-2, protects human B cells from programmed cell death. Proc Natl Acad Sci USA. 1993;90:8479–8483. doi: 10.1073/pnas.90.18.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huen D S, Henderson S A, Croom-Carter D, Rowe M. The Epstein-Barr virus latent membrane protein-1 (LMP1) mediates activation of NF-kappa B and cell surface phenotype via two effector regions in its carboxy-terminal cytoplasmic domain. Oncogene. 1995;10:549–560. [PubMed] [Google Scholar]
- 19.Kieser A, Kilger E, Gires O, Ueffing M, Kolch W, Hammerschmidt W. Epstein-Barr virus latent membrane protein-1 triggers AP-1 activity via the c-Jun N-terminal kinase cascade. EMBO J. 1997;16:6478–6485. doi: 10.1093/emboj/16.21.6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kristensen M, Chu C Q, Eedy D J, Feldmann M, Brennan F M, Breathnach S M. Localization of tumour necrosis factor-alpha (TNF-alpha) and its receptors in normal and psoriatic skin: epidermal cells express the 55-kD but not the 75-kD TNF receptor. Clin Exp Immunol. 1993;94:354–362. doi: 10.1111/j.1365-2249.1993.tb03457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laherty C D, Hu H M, Opipari A W, Wang F, Dixit V M. The Epstein-Barr virus LMP1 gene product induces A20 zinc finger protein expression by activating nuclear factor kappa B. J Biol Chem. 1992;267:24157–24160. [PubMed] [Google Scholar]
- 22.Liebowitz D. Epstein-Barr virus and a cellular signaling pathway in lymphomas from immunosuppressed patients. N Engl J Med. 1998;338:1413–1421. doi: 10.1056/NEJM199805143382003. [DOI] [PubMed] [Google Scholar]
- 23.Lovas J G. Apoptosis in human epidermis: a postmortem study by light and electron microscopy. Australas J Dermatol. 1986;27:1–5. doi: 10.1111/j.1440-0960.1986.tb00274.x. [DOI] [PubMed] [Google Scholar]
- 24.Marthinuss J, Lawrence L, Seiberg M. Apoptosis in Pam212, an epidermal keratinocyte cell line: a possible role for bcl-2 in epidermal differentiation. Cell Growth Differ. 1995;6:239–250. [PubMed] [Google Scholar]
- 25.Miller W E, Cheshire J L, Baldwin A S, Jr, Raab-Traub N. The NPC derived C15 LMP1 protein confers enhanced activation of NF-kappa B and induction of the EGFR in epithelial cells. Oncogene. 1998;16:1869–1877. doi: 10.1038/sj.onc.1201696. [DOI] [PubMed] [Google Scholar]
- 26.Miller W E, Cheshire J L, Raab-Traub N. Interaction of tumor necrosis factor receptor-associated factor signaling proteins with the latent membrane protein 1 PXQXT motif is essential for induction of epidermal growth factor receptor expression. Mol Cell Biol. 1998;18:2835–2844. doi: 10.1128/mcb.18.5.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller W E, Earp H S, Raab-Traub N. The Epstein-Barr virus latent membrane protein 1 induces expression of the epidermal growth factor receptor. J Virol. 1995;69:4390–4398. doi: 10.1128/jvi.69.7.4390-4398.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller W E, Mosialos G, Kieff E, Raab-Traub N. Epstein-Barr virus LMP1 induction of the epidermal growth factor receptor is mediated through a TRAF signaling pathway distinct from NF-κB activation. J Virol. 1997;71:586–594. doi: 10.1128/jvi.71.1.586-594.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mosialos G, Birkenbach M, Yalamanchili R, VanArsdale T, Ware C, Kieff E. The Epstein-Barr virus transforming protein LMP1 engages signaling proteins for the tumor necrosis factor receptor family. Cell. 1995;80:389–399. doi: 10.1016/0092-8674(95)90489-1. [DOI] [PubMed] [Google Scholar]
- 30.Nitsche F, Bell A, Rickinson A. Epstein-Barr virus leader protein enhances EBNA-2-mediated transactivation of latent membrane protein 1 expression: a role for the W1W2 repeat domain. J Virol. 1997;71:6619–6628. doi: 10.1128/jvi.71.9.6619-6628.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paine E, Scheinman R I, Baldwin A S, Jr, Raab-Traub N. Expression of LMP1 in epithelial cells leads to the activation of a select subset of NF-κB/Rel family proteins. J Virol. 1995;69:4572–4576. doi: 10.1128/jvi.69.7.4572-4576.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peguet-Navarro J, Dalbiez-Gauthier C, Moulon C, Berthier O, Reano A, Gaucherand M, Banchereau J, Rousset F, Schmitt D. CD40 ligation of human keratinocytes inhibits their proliferation and induces their differentiation. J Immunol. 1997;158:144–152. [PubMed] [Google Scholar]
- 33.Peng M, Lundgren E. Transient expression of the Epstein-Barr virus LMP1 gene in human primary B cells induces cellular activation and DNA synthesis. Oncogene. 1992;7:1775–1782. [PubMed] [Google Scholar]
- 34.Raab-Traub N. Pathogenesis of Epstein-Barr virus and its associated malignancies. Semin Virol. 1996;7:315–323. [Google Scholar]
- 35.Resnick L, Herbst J S, Raab-Traub N. Oral hairy leukoplakia. J Am Acad Dermatol. 1990;22:1278–1282. doi: 10.1016/0190-9622(90)70174-g. [DOI] [PubMed] [Google Scholar]
- 36.Russ J, editor. The image processing handbook. 2nd ed. Boca Raton, Fla: CRC Press; 1995. [Google Scholar]
- 37.Sandvej K, Krenacs L, Hamilton-Dutoit S J, Rindum J L, Pindborg J J, Pallesen G. Epstein-Barr virus latent and replicative gene expression in oral hairy leukoplakia. Histopathology. 1992;20:387–395. doi: 10.1111/j.1365-2559.1992.tb01008.x. [DOI] [PubMed] [Google Scholar]
- 38.Takada K, Ono Y. Synchronous and sequential activation of latently infected Epstein-Barr virus genomes. J Virol. 1989;63:445–449. doi: 10.1128/jvi.63.1.445-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas J A, Felix D H, Wray D, Southam J C, Cubie H A, Crawford D H. Epstein-Barr virus gene expression and epithelial cell differentiation in oral hairy leukoplakia. Am J Pathol. 1991;139:1369–1380. [PMC free article] [PubMed] [Google Scholar]
- 40.Walling D M, Raab-Traub N. Epstein-Barr virus intrastrain recombination in oral hairy leukoplakia. J Virol. 1994;68:7909–7917. doi: 10.1128/jvi.68.12.7909-7917.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang D, Liebowitz D, Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985;43:831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- 42.Wang F, Gregory C, Sample C, Rowe M, Liebowitz D, Murray R, Rickinson A, Kieff E. Epstein-Barr virus latent membrane protein (LMP1) and nuclear proteins 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA-2 and LMP1 cooperatively induce CD23. J Virol. 1990;64:2309–2318. doi: 10.1128/jvi.64.5.2309-2318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Webster-Cyriaque J, Raab-Traub N. Transcription of Epstein-Barr virus latent cycle genes in oral hairy leukoplakia. Virology. 1998;248:53–65. doi: 10.1006/viro.1998.9268. [DOI] [PubMed] [Google Scholar]
- 44.Williams D M, Leigh I M, Greenspan D, Greenspan J S. Altered patterns of keratin expression in oral hairy leukoplakia: prognostic implications. J Oral Pathol Med. 1991;20:167–171. doi: 10.1111/j.1600-0714.1991.tb00914.x. [DOI] [PubMed] [Google Scholar]
- 45.Wilson J B, Weinberg W, Johnson R, Yuspa S, Levine A J. Expression of the BNLF-1 oncogene of Epstein-Barr virus in the skin of transgenic mice induces hyperplasia and aberrant expression of keratin 6. Cell. 1990;61:1315–1327. doi: 10.1016/0092-8674(90)90695-b. [DOI] [PubMed] [Google Scholar]