Abstract
Introduction
Over 265 000 women are living with HIV in the USA, but limited research has investigated the physical, mental and behavioural health outcomes among women living with HIV of reproductive age. Health status during the reproductive years before, during and after pregnancy affects pregnancy outcomes and long-term health. Understanding health outcomes among women living with HIV of reproductive age is of substantial public health importance, regardless of whether they experience pregnancy. The Health Outcomes around Pregnancy and Exposure to HIV/Antiretrovirals (HOPE) study is a prospective observational cohort study designed to investigate physical and mental health outcomes of young women living with HIV as they age, including HIV disease course, engagement in care, reproductive health and choices and cardiometabolic health. We describe the HOPE study design, and characteristics of the first 437 participants enrolled as of 1 January 2024.
Methods and analysis
The HOPE study seeks to enrol and follow 1630 women living with HIV of reproductive age, including those with perinatally-acquired HIV, at 12 clinical sites across 9 US states and Puerto Rico. HOPE studies multilevel dynamic determinants influencing physical, mental and social well-being and behaviours of women living with HIV across the reproductive life course (preconception, pregnancy, post partum, not or never-pregnant), informed by the socioecological model. Key research areas include the clinical course of HIV, relationship of HIV and antiretroviral medications to reproductive health, pregnancy outcomes and comorbidities and the influence of racism and social determinants of health. HOPE began enrolling in April 2022.
Ethics and dissemination
The HOPE study received approval from the Harvard Longwood Campus Institutional Review Board, the single institutional review board of record for all HOPE sites. Results will be disseminated through conference presentations, peer-reviewed journals and lay summaries.
Keywords: HIV & AIDS, Postpartum Women, Pregnant Women, Health Equity, Observational Study, MENTAL HEALTH
Strengths and limitations of this study.
The Health Outcomes around Pregnancy and Exposure to HIV/Antiretrovirals (HOPE) longitudinal study will enrol and follow a cohort of women of reproductive age living with HIV, including those with perinatally-acquired HIV, from across the USA and Puerto Rico to understand their health and well-being outcomes over time.
Most follow-up visits are conducted remotely, which encourages enrolment by offering flexibility for completing assessments and improves efficiency by reducing participant and staff burden compared with in-person visits.
A unique feature of the HOPE study design is recruitment of a subgroup of women living with perinatally-acquired HIV, making HOPE uniquely poised to assess distinct influences of lifelong HIV and antiretroviral exposure on health outcomes compared with individuals acquiring HIV later in life.
Because HOPE largely recruits participants from clinical sites, results may not be generalisable to women living with HIV who are not engaged in care.
HOPE does not enrol women who are HIV seronegative; thus, the absence of a comparison group of women without HIV limits the ability to evaluate the contribution of HIV status to risks of health conditions or behaviours of interest.
Introduction
Globally, there are 19.7 million women living with HIV, accounting for 54% of adults living with HIV.1 Each year, over 1 million women living with HIV experience pregnancy, with an increasing proportion receiving antiretroviral therapy in pregnancy, from 49% in 2010 to 82% in 2022.2 In the USA, over 265 000 women are living with HIV, comprising 22% of adults living with HIV.3 However, the physical, behavioural and mental health outcomes of young women living with HIV of reproductive age in the USA and individual and social determinants of these outcomes have not been well-studied.
Despite advances in HIV treatment and care, a suboptimal proportion of women living with HIV in the USA engage in HIV care, adhere to antiretrovirals and achieve viral suppression, which are all crucial to promoting health and reducing the risk of HIV transmission.4 In 2021 about 25% of all US adult women living with HIV were not receiving HIV care, and only 64% were virally suppressed at the time of their most recent clinical visit.5 Over 60% of postpartum women living with HIV are not virally suppressed 6–24 months after giving birth.6–10 Women with perinatally-acquired HIV (PHIV) have even lower rates of viral suppression, with 70–85% not virally suppressed at 12 months post partum.11 12
Women living with HIV are disproportionately black and/or Latina, reflecting the influence of structural racism in driving disparities in access to prevention and care, antiretroviral medication adherence, mental health, comorbidities and overall well-being.13 14 ‘Weathering’ induced by chronic exposure to racism and forms of social and economic disadvantage, has the potential to accelerate ageing and declines in health among minoritised women and is a possible mechanism linking structural racism to adverse health outcomes.15–17 Structural racism shapes racialised residential segregation, disproportionate exposures to environmental hazards, less access to quality healthcare, discrimination during medical encounters and increased negative experiences with the criminal legal system.18–21 Yet little is known about the specific effects of structural racism and other associated harmful social policies (eg, living in states with more laws criminalising HIV) on the health of women living with HIV.
Additionally, women experiencing HIV-related stigma may also experience higher levels of social isolation and depression, which could contribute to suboptimal adherence.22 The intersection of HIV-related stigma and racism, classism, sexism, disability and heterosexism, have been associated with suboptimal HIV care, posing additional threats to positive health outcomes.23 24 However, few studies to date have examined the impact of racism, and intersectional stigma and discrimination on the health of marginalised women living with HIV of childbearing age, despite the fact that HIV disproportionately impacts women who identify as black and Latina in the USA.
Although there has been extensive research examining the health of older women living with HIV in the USA,25–27 less is known about the health of women living with HIV currently of reproductive age in the USA. Investigating the complex milieu of biological, social, environmental and structural events influencing the health and well-being of women living with HIV across their reproductive life course, including those who are nulliparous, pregnant, post partum or parous and women who have never been or will not become pregnant is imperative for achieving health equity and informing interventions to optimise long-term health of women living with HIV.
There is an opioid crisis in the USA, with opioid use driven largely by use of prescription opioids.28 Additionally, cannabis legalisation in many US states for both medical and recreational use may affect substance use trends across the reproductive life course among women living with HIV.29 Substance use has been linked to food insecurity, suboptimal adherence and challenges in retention in HIV care among older women living with HIV in the USA,30 however, less is known about alcohol and other substance use and its treatment among younger women living with HIV.
Due to improvements in HIV treatment and medical advances which dramatically reduce the risk of perinatal HIV transmission, more women living with HIV are now choosing to have children.31–35 In the USA, approximately 3500 women living with HIV give birth annually.36 Pregnancy rates for women living with HIV are comparable to women without HIV.31 However, about 80% of pregnancies among women living with HIV in the USA are reported to be unintended, compared with 40–50% in the general population.12 37–39 This disparity highlights a need to better understand and support the reproductive intentions, concerns about HIV transmission and health of women living with HIV, as well as the environmental, psychosocial, economic and sociopolitical conditions in which they live. Women living with PHIV represent a unique subset of individuals, and there is a dearth of information on the potential impact of lifelong HIV and antiretrovirals on their long-term health and pregnancies.
Understanding individual, biological and social determinants of health of women living with HIV of reproductive age before, during and after pregnancy is of clinical and public health importance both to improve short-term pregnancy outcomes and to mitigate long-term chronic disease risk. Although pregnancy is a time of increased risk for physical and mental health complications, the contribution of pregnancy to long-term health of women living with HIV has not been sufficiently examined. For example, hypertensive disorders of pregnancy are potent signals for elevated risk of long-term cardiovascular (CVD) and metabolic disease40–43 and antiretroviral medications may influence CVD risk.44 Additionally, mental health conditions are prevalent among women living with HIV who are parenting,45 yet the influence of parenting on the physical and mental health of women living with HIV, and on their ability to engage in their own HIV care and maintain adherence to antiretrovirals, particularly if children have acute or chronic health needs, has not been well-studied. Finally, although neighbourhood deprivation has been linked to viral load, little is known about the relationship of other social and physical environmental factors (eg, air pollution and extreme temperature) to pregnancy and the overall well-being of women living with HIV.46–48
To address these scientific gaps, the Health Outcomes around Pregnancy and Exposure to HIV/Antiretrovirals (ARVs) (HOPE) longitudinal study, funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, will enrol and follow a large US-based cohort of women living with HIV of reproductive age, including those with PHIV, to understand their health and well-being outcomes over time. In this paper we present the HOPE study protocol, including the conceptual framework, aims, design, methods and characteristics of the first 437 participants enrolled as of 1 January 2024. Due to its focus on the reproductive life course, HOPE enrols only individuals assigned female sex at birth. For simplicity, the terms ‘woman’ and ‘women’ are used throughout this paper. However, the HOPE study population includes cisgender women, transgender men, non-binary individuals and gender-diverse people.
Methods and analysis
HOPE study objectives
The HOPE study has four primary aims: (1) Establish the HOPE cohort to evaluate the health and well-being of women living with HIV of reproductive age using innovative epidemiological study designs and cost-effective methods for enrolment, follow-up and data collection; (2) assess HIV-related outcomes in multiple domains (defined below) and overall health of women living with HIV over their reproductive life course, including reproductive health, coinfections, long-term non-communicable diseases, as well as potential inflammatory and epigenetic processes associated with these outcomes; and psychosocial determinants of health including mental health diagnoses, stigma, racism, inequity, disclosure of HIV and opioid and other substance use/misuse; (3) determine the association of HIV disease-related factors, including timing of acquisition, treatment, disease course and engagement in care, with the overall physical, mental and behavioural health of women living with HIV during their reproductive years; (4) assess the relationship of adverse infant or child health outcomes to the health of women living with HIV.
In addition to four overall study aims, the HOPE study is designed to address aims examining multilevel dynamic determinants of health within specific domains: Mental health, reproductive health, cardiometabolic health, coinfections, HIV outcomes, HIV care continuum, substance use and stigma, racism and social determinants of health. The domain-specific aims and selected hypotheses are summarised in online supplemental table 1. Key exposures include ARV regimens received over the life course, comorbidities and social and structural determinants of health, including HIV-related stigma, poverty, racism and discrimination. Many variables of interest in the HOPE study can be evaluated as exposures and as outcomes, depending on the research question.
bmjopen-2024-084835supp001.pdf (175.1KB, pdf)
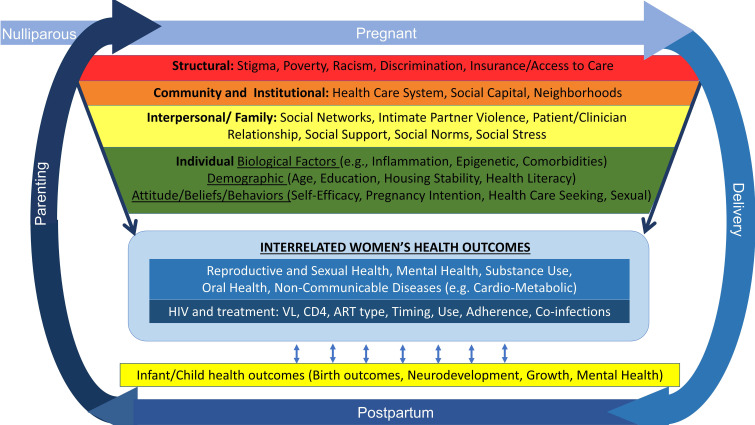
Conceptual framework
The HOPE study conceptual framework is informed by the socioecological model, which recognises that factors operating at multiple levels including individual, interpersonal and family, community and institutional and structural/societal, may affect individuals’ health and risk of adverse outcomes.49–51 The HOPE framework also incorporates a life course perspective and Developmental Origins of Health and Disease framework, which acknowledge that biological and social factors across generations, at early stages of development and across the reproductive life span, are critical determinants of reproductive health, which in turn, influence later chronic health conditions52–55 (figure 1). Changes in exposure to these factors, in HIV disease, as well as pregnancy and postpartum events, may affect health. Infections other than HIV, the mode of HIV acquisition and timing of HIV diagnosis, type and timing of ARV treatment and adherence can influence engagement in care, HIV outcomes and overall health of women living with HIV.56 57 The needs and health of children in turn may affect women’s health. This cyclical and dynamic relationship, occurring in the context of structural factors including HIV-related stigma, violence, racism, inequity and poverty as well as trauma and depression may negatively influence HIV outcomes through direct or indirect pathways, including toxic stress, potentiating health outcome disparities among women living with HIV.50 58–63 Conversely, resilience resources including individual, family and interpersonal resources, social support and availability and access to community resources may mitigate potential adverse effects on health and well-being and support positive outcomes.64–68
Figure 1.
Health Outcomes around Pregnancy and Exposure to HIV/Antiretrovirals conceptual model. ART, antiretroviral therapy; VL, viral load.
Leveraging the Pediatric HIV/AIDS Cohort Study infrastructure
The HOPE study is designed to address questions regarding the long-term health of women living with HIV of reproductive age during young adulthood, pregnancy and parenting (for those who have given birth to or are raising children as foster parents or through guardianship or adoption). HOPE is affiliated with the Pediatric HIV/AIDS Cohort Study (PHACS) network, a national multisite research network conducting longitudinal studies of long-term effects of HIV and ARV exposure on infants, children, adolescents and young adults with PHIV and those living with perinatal HIV exposure who are not living with HIV. The PHACS Surveillance Monitoring for ART Toxicities (SMARTT) study is an ongoing observational cohort study established in 2007 that follows children with perinatal HIV exposure who are not living with HIV from birth along with their biological mothers (women living with HIV) or other caregivers, to evaluate the safety of fetal exposure to ARVs.69 70 The PHACS Adolescent Master Protocols for Participants 18 Years of Age and Older (AMP Up Series) follow young adults living with PHIV and a comparison cohort of individuals with perinatal HIV exposure.71 Individuals in SMARTT and the AMP Up Series who meet HOPE eligibility criteria may co-enrol in HOPE, thus enriching the data collection for these individuals. The unique HOPE research platform fosters opportunities for multidisciplinary and cross-cutting research to inform public health policy for optimising the health of women living with HIV.
Patient and public involvement
The HOPE protocol, research focus and data collection instruments were designed in partnership with women living with HIV from the PHACS Community Advisory Board (CAB) and/or members of the PHACS Health Education and Community Core Community Task Force. A core principle of the HOPE study is to support research that reflects the perspectives and priorities of people living with HIV. Once all sites were open to accrual, we engaged HOPE participants to join a HOPE-specific CAB established to ensure representation of participants’ priorities in HOPE, drive the creation of health education materials and promote insights and opportunities to support participant retention. HOPE participants also serve on the PHACS Community Task Force, using the PHACS Health Education and Community Core infrastructure to work as paid consultants providing input and ensuring representation of HOPE participant priorities in all protocol activities. In collaboration with the HOPE protocol team, HOPE CAB members conduct reviews of research proposals using HOPE data, providing extensive written feedback. They also advise and assist with the development of resources supporting recruitment, retention and study conduct, coauthor publications and support the dissemination of study findings, aiming to use results to inform policy changes that promote health equity for women living with HIV. They have informed the creation of a video describing geocoding for participants and illustrated instructions to assist participants with vaginal and anal swab self-collection.
Study population and eligibility criteria
HOPE is a prospective observational cohort study enrolling women living with HIV who are 18–39 years of age at 12 clinical sites across 9 US states and Puerto Rico (figure 2). Individuals eligible to participate in HOPE are (1) woman, based on biological sex assignment at birth; (2) living with HIV as documented in their medical record; (3) 18 to <40 years of age if pregnant or parous and 18–30 years of age if nulliparous and non-pregnant; (4) at least 13 weeks of gestation at time of enrolment if pregnant; (5) willing to provide access to medical records and provide legal consent/assent and able to complete study assessments in English or Spanish. Individuals who are currently incarcerated and individuals concurrently enrolled in studies not approved by the HOPE protocol team are ineligible for HOPE.
Figure 2.
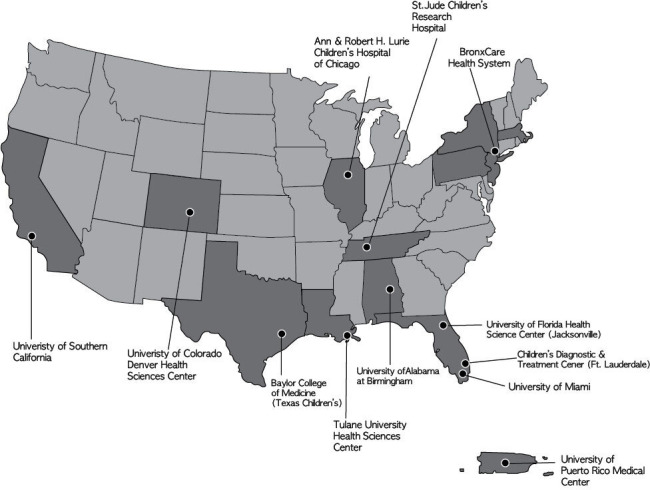
Geographical locations of HOPE study sites across the USA and Puerto Rico. States in darker shade are locations of Pediatric HIV/AIDS Cohort Study sites. HOPE, Health Outcomes around Pregnancy and Exposure to HIV/Antiretrovirals.
Recruitment and retention
The study began enrolling participants in April 2022 with a goal of enrolling 1630 participants over 4 years from the following four groups:
Nulliparous, non-pregnant participants 18–30 years of age (N~370).
Participants who are pregnant or recently gave birth (≤3 days) and are 18 to <40 years of age (N~430).
Postpartum (>3 days up to 12 months after delivery), non-pregnant participants 18 to <40 years of age (N~260).
Parous, non-pregnant (>12 months after delivery) participants 18 to <40 years of age (N~570).
The study aims to recruit a population that includes at least 15% women living with PHIV in all of the above categories. The HOPE team is in the process of modifying the HOPE protocol eligibility criteria to accelerate accrual. This includes increasing the maximum eligible age to 45 years of age, removing the minimum weeks of gestation in pregnancy and expanding eligibility to women whose preferred language for completing assessments is Haitian Creole.
In addition to co-enrolling eligible PHACS participants, each site enrols qualifying participants who are not part of the PHACS network through outreach to local CABs and to clinicians who provide care to women living with HIV. Recruitment strategies were informed by CAB members and site staff. Women living with HIV consenting to participate in the study will complete follow-up visits through 31 August 2025 (6 months prior to the funding period end date), or longer if the study receives renewed funding.
Multiple methods to retain participants in HOPE are informed by site staff, the HOPE CAB and HOPE members of the PHACS Community Task Force. Capitalising on existing patient-provider relationships, sites partner with clinical providers and local and national CABs throughout the course of this study to support participants for continued HOPE study retention. Study staff maintain contact with HOPE participants at least every 6 months to maintain rapport, inquire if they had become pregnant or experienced any other significant changes and to support study retention.
Schedule of evaluations
The HOPE study has two schedules of evaluations, one for participants who are pregnant or have recently given birth and another for participants who are not pregnant (online supplemental figure 1). Entry visits are in-person and annual follow-up visits are remote. The entry visit for participants who are pregnant can occur either during pregnancy or in the peripartum period. Participants who enrol while pregnant have follow-up visits at delivery, 6 weeks postpartum, 1-year postpartum and annual remote visits thereafter. Participants who are not pregnant have one in-person entry visit followed by annual remote visits. Annual visits are conducted remotely, and include completion of an online survey by the participant and medical chart abstraction by site staff. Participants who are not pregnant at enrolment but who subsequently experience pregnancy are invited to modify their frequency of evaluations from annually to the pregnancy schedule described above, followed by annual remote visits thereafter.
Data collection and measures
The data collection at each HOPE visit is summarised in table 1. Data collection at the entry visit consists of collection of residential addresses for geocoding, clinical assessments, an interviewer-administered medical and psychosocial history questionnaire, a self-completed online survey and collection of specimens for the HOPE Biorepository. Trained research staff complete medical record abstraction concurrently with each visit.
Table 1.
Health Outcomes around Pregnancy and Exposure to HIV/Antiretrovirals data collection
| Pregnant or recently delivered at enrolment | Not pregnant at enrolment | |||||
| Enrolment | Enrolment | Annual follow-up | ||||
| Pregnancy | Delivery | 6 weeks post partum | 1-year post partum and annually | |||
| In person | In person | In person | Remote | In person | Remote | |
| Residential address information/location for geocoding purposes | X | (X) | X | |||
| Contact and check-in between visits | X | X | X | X | X | X |
| Change in pregnancy status | X | X | X | X | ||
| Clinical assessments (collected in-person) | ||||||
| Height | X | (X) | X | X | ||
| Weight | X | X | X | X | X | X |
| Waist and hip circumference | X | X | X | |||
| Blood pressure | X | X | X | X | X | X |
| Interview | ||||||
| Family and personal medical history | X | (X) | X | |||
| Reproductive history | X | (X) | X | |||
| Depression* | X | (X) | X | X | ||
| Anxiety† | X | (X) | X | X | ||
| PTSD‡ | X | (X) | X | |||
| Health literacy§ | X | (X) | X | |||
| Social history | X | (X) | X | |||
| Online survey (detail provided in table 2) | X | X | X | X | X | X |
| Medical chart abstraction | ||||||
| Weight | X | X | X | X | X | X |
| Blood pressure | X | X | X | X | X | |
| HIV RNA level, lymphocytes and subsets | X | X | X | X | X | X |
| ART medications | X | X | X | X | X | X |
| Non-ART medications | X | X | X | X | X | X |
| HIV, primary care, OB, gynaecologic, mental healthcare engagement | X | X | X | X | X | X |
| Immunisations | X | X | X | X | X | X |
| Medical and mental health diagnoses and hospitalisation | X | X | X | X | X | X |
| Laboratory test results | X | X | X | X | X | X |
| Pregnancy and pregnancy outcomes | X | X | X | X | X | |
| Cervical and anal dysplasia screening | X | X | X | X | X | X |
| STI testing and results | X | X | X | X | X | |
| Sample collection/repository | ||||||
| Serum, plasma (EDTA and heparin) and non-viable PBMCs | X | X | X | |||
| Rectal swab for microbiome | X | (X) | X | |||
| Vaginal swab for microbiome | X | (X) | X | |||
| Vaginal swab for metabolomics | X | (X) | X | |||
| Vaginal swab for STI testing | X | X | X | |||
| Oral swab | X | X | X | |||
| Saliva | X | X | X | |||
| Hair | X | X | X | |||
Brackets () indicate that the assessment takes place at the delivery visit only if the delivery visit is an enrolment visit.
*Depressive symptoms assessed via interview using the Patient Health Questionnaire-9.75
†Anxiety symptoms assessed via interview using the Generalised Anxiety Disorder-7.76
‡Post-traumatic stress disorder assessed via interview using the Primary Care Post-Traumatic Stress Disorder Screen for DSM-5.77
§Health literacy assessed via interview using the Newest Vital Sign.73
ART, antiretroviral therapy; DSM-5, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; OB, obstetrical; PBMC, peripheral blood mononuclear cells; STI, sexually transmitted infection.
Geocoding
To examine structural racism and other area-based social, environmental and structural determinants of health (eg, historical redlining, air quality, green space and proximity to high-quality clinics/hospitals), HOPE employs geocoding. Participants who consent to provide their residential addresses for geocoding provide all addresses where they resided for the 2-year period prior to the entry visit and the address where they resided longest between ages 14–18 years. Site staff record the addresses and use ArcGIS Pro V.3.0 software72 to transform each address into a Federal Information Processing System code for the census tract corresponding to each location.
Clinical assessments
Height, weight, waist and hip circumference and blood pressure measurements are collected.
Interviewer-administered questionnaires
A questionnaire collects medical history including medications, diagnoses and family medical history. Participants complete assessments of pregnancy and reproductive history, health literacy measured using the Newest Vital Sign73 and social history (eg, food security via the Six-Item Short Form of the US Household Food Security Scale74 and housing security). In addition, interviewers screen participants for symptoms of depression via the Patient Health Questionnaire-9,75 anxiety symptoms via the Generalised Anxiety Disorder-7 Scale76 and Post-Traumatic Stress Disorder (PTSD) via the Primary Care PTSD screen for Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5).77
Specimen collection
Site staff collect bio-specimens from HOPE participants at the in-person entry visit. Specimens include serum, plasma, non-viable peripheral blood mononuclear cells, anal, oral and vaginal swabs, hair specimens and saliva. Participants who enrol while pregnant provide bio-specimens again at the delivery visit. All HOPE bio-specimens are stored in a bio-repository, allowing for future investigations of biological processes and conditions (eg, testing for sexually transmitted infections, inflammation, epigenetics, the microbiome and biological markers of stress).
Medical record abstraction
For participants who are nulliparous or enrolling more than 1 year after giving birth and not pregnant at enrolment, site staff abstract data from participant medical records for the period starting 12 months prior to the visit. For participants who are pregnant or recently gave birth or within the 1-year postpartum period, site staff abstract medical record data for the period beginning 6 months prior to conception of the most recent pregnancy. Health conditions abstracted from participants’ medical records include HIV history, general and obstetrical/gynaecological history, mental health history, medical diagnoses and care engagement details. HIV history includes ARV medications, CD4 count including nadir CD4 count and HIV viral load. General history includes non-ARV medications, immunisations, weight, blood pressure and results of selected laboratory tests (eg, lipids, blood urea nitrogen, white blood cell count). Obstetrical/gynaecological history includes gravidity, parity, pregnancy outcomes, results of STI testing, as well as normal and abnormal cervical cancer screening results and associated histology. Mental health diagnoses include depression, anxiety, PTSD, psychosis and substance use disorders. Medical diagnoses include, but are not limited to, diabetes (including gestational diabetes), hypertension (including hypertensive disorders of pregnancy), obesity, dyslipidaemia and anaemia. Care engagement details include HIV, primary care, obstetrics/gynaecologic and mental healthcare engagement.
Online survey
The online survey collects information on socio-demographic characteristics, physical and mental health, behaviours (eg, substance use) and social determinants of health, described in table 2,78–94 and assesses feasibility/acceptability of a wearable actigraphy device for collection of participant sleep and physical activity data. An online survey audio component reads the questions aloud to support the engagement of participants with limited literacy.
Table 2.
Health Outcomes around Pregnancy and Exposure to HIV/Antiretrovirals online survey data collection
| Survey domains | Not pregnant at enrolment | Pregnant or recently delivered at enrolment | ||||
| Entry | Annually | Pregnancy/ delivery (entry) | Delivery (follow-up) | 6 weeks postpartum | 1-year post partum and annually | |
| Background | ||||||
| Race and ethnicity | X | X | ||||
| Gender and sexual identity | X | X | X | X | ||
| Education, employment, income | (SH-I) | X | (SH-I) | X | X | X |
| Housing security/living situationˆˆˆ | (SH-I) | X | (SH-I) | X | X | X |
| Food Security* | (SH-I) | X | (SH-I) | X | ||
| Health and healthcare | ||||||
| HIV care engagement, access and medical insurance | X | X | X | X | X | X |
| Self-efficacy re: HIV care management | X | X | x | X | X | |
| Trust in healthcare system† and providers‡ | biannual (Y1, Y3, …) | biannual (Y1, Y3, …) | ||||
| Medication adherence§ | X | X | X | X | X | X |
| Life events | ||||||
| Self-rated health and pain | X | X | X | X | X | |
| Quality of life¶ | X | X | X | |||
| Physical activity | X | X | X | X | X | |
| Sleep quality** and shift work | X | X | X | X | X | |
| Perceived stress†† | X | X | X | X | X | |
| Life events checklist | X | X | X | X | X | X |
| Adverse childhood experiences | X | X | ||||
| Intimate partner violence (sexual, physical, emotional) | X | X | X | X | X | |
| Neighbourhood safety‡‡ | X | X | X | X | X | |
| Everyday discrimination, experiences of discrimination and reactions to race§§, ¶¶ | X | biannual (Y2, Y4, …) | X | X (healthcare setting version) | biannual (Y2, Y4, …) | |
| Internalised HIV stigma*** | X | biannual (Y2, Y4,) | X | biannual (Y2, Y4, …) | ||
| Disclosure | X | X | X | X | ||
| PLHIV Resilience Scale††† | X | biannual (Y2, Y4, …) | X | biannual (Y2, Y4,…) | ||
| Brief Resilience Scale‡‡‡ | X | X | ||||
| Social integration§§§ | X | X | ||||
| Social support¶¶¶ | X | X | X | X | ||
| Sexual behaviour | ||||||
| Number of sexual partners | X | X | X | X | X | |
| HIV risk reduction strategies (eg, condoms, PrEP, U=U) | X | X | X | X | X | |
| Perception of PrEP and U=U effectiveness | X | biannual (Y2, Y4 …) | X | biannual (Y2, Y4, …) | ||
| Female Sexual Function Index (three questions) | X | X | ||||
| Sexual Relationship Power Scale**** | X | X | ||||
| Reproductive Health | ||||||
| Pregnancy status and intention (current) | X | X | X | X | X | |
| Contraceptive use and discontinuation reasons | X | X | X | X | X | |
| STI testing | X | X | X | X | ||
| Pap smear | X | X | X | X | ||
| Influenza vaccination | X | X | X | X | X | |
| HPV vaccination | X | X | ||||
| Pregnancy history (intention, outcome) | X | X | X | X | X | |
| Postpartum care engagement | X | X | X | X | ||
| Breastfeeding intentions and practices | X | X | X | X | X | X |
| Children parented/parenting (relation, health conditions) | X | X | ||||
| Substance use and mental health | ||||||
| Substance use and abuse†††† | X | X | X | X | X | |
| Cannabis use | X | X | X | X | X | |
| SAMISS alcohol questions‡‡‡‡ | X | X | X | X | X | |
| Depression symptoms§§§§ | (ˆ) | X | (ˆ) | (ˆˆ) | X | |
| Anxiety symptoms¶¶¶¶ | (ˆ) | X | (ˆ) | (ˆˆ) | X | |
| Post-traumatic stress disorder symptoms***** | (ˆ) | (ˆ) | ||||
| Wearables for actigraphy | ||||||
| Feasibility and acceptability | X | X | ||||
ˆAsked separately at entry via interview.
ˆˆAsked separately at follow-up via interview.
ˆˆˆAccountable Health Communities Health-Related Social Needs Screening Tool.
*Six-Item Food Security Scale.74
†Health Care System Distrust Scale.78
‡Healthcare Relationship Trust Scale.79
§Three question adherence measure.80
¶Medical Outcomes Study SF-20.81
**Brief Pittsburgh Sleep Quality Index.82
††Perceived Stress Scale-4.83
‡‡Neighborhood safety.84
§§Everyday Discrimination Scale.85
¶¶Experiences of Discrimination.86
***Internalised HIV Stigma Scale.87
†††People Living with HIV Resilience Scale.88
‡‡‡Brief Resilience Scale.89
§§§Berkman Social Network Index.90
¶¶¶Medical Outcomes Study Social Support Survey.91
****Sexual Relationship Power Scale.92
††††Alcohol Smoking and Substance Involvement Screening Test.93
‡‡‡‡SAMISS alcohol questions.94
§§§§Patient Health Questionnaire-9.75
¶¶¶¶Generalised Anxiety Disorder-7.76
*****Primary Care Post-Traumatic Stress Disorder Screen for DSM-5.77
DSM-5, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; HPV, human papillomavirus; PLHIV, people living with HIV; PrEP, pre-exposure prophylaxis; SAMISS, Substance Use and Mental Illness Symptoms Screener; SF-20, 20-Item Short-Form Survey; SH-I, asked in social history interview at entry; STI, sexually transmitted infection; U=U, undetectable=untransmittable.
Data analysis and sample size considerations
Data analysis
The HOPE study is designed to evaluate the health of women living with HIV over their reproductive lifespan. Detailed statistical analysis plans will be developed for each specific HOPE study question. However, in general, for binary outcomes of interest, such as preterm birth, diagnoses of hypertension or depression, unsuppressed viral load, suboptimal retention in HIV care or substance use, the prevalence or the risk of such outcomes between different exposure groups will be compared using standard modelling techniques such as log-binomial regression, both unadjusted and adjusted for potential confounders. For continuous outcomes, such as body mass index or blood pressure, we will use general linear regression or generalised estimating equation (GEE) models, as appropriate, with and without adjustment for potential confounders. Examples of key ‘exposure’ groups include reproductive life stage and PHIV status.
Based on the longitudinal follow-up in the HOPE study, we will assess associations between exposures which may change over time and incidence rates of various conditions of interest, such as depression or hypertension, using Poisson regression models. We will evaluate key outcomes over time (eg, viral suppression, depression, hypertension, blood pressure, sleep quality), and changes in these outcomes. For binary conditions, we will use log-binomial models using GEEs for repeated measures and time-varying exposures or covariates. For continuous outcomes such as weight, log10 RNA or sleep duration, we will first visually inspect the trajectories by exposure group or risk factors using locally estimated scatterplot smoothing plots then use GEE or mixed effect models as appropriate to fit regression models as a function of age or elapsed follow-up time, spanning the reproductive life stages reflected by the HOPE protocol, adjusting for other risk factors or potential confounders. Finite mixture modelling techniques such as group-based trajectory modelling95 or growth mixture modelling96 will also be applied to identify subgroups with distinct patterns of trajectories.
To manage and account for data missingness, we will describe the reasons for missed study visits (eg, missed a visit due to hospitalisation, incarceration) and compare the characteristics of participants with missing versus non-missing data. Assumptions needed to obtain valid statistical inferences in the presence of missing data will be thoroughly investigated. When appropriate, for example, for accounting for missingness due to incomplete visit follow-up, factors associated with the propensity of missingness will be identified and included in analyses using missing data methods, such as multiple imputation and inverse probability weighting, to address potential selection bias.
Sample size
Some HOPE study aims and hypotheses will apply to all participants in the HOPE study, while others will be specific to a subgroup. For binary outcomes in cross-sectional comparisons, our target sample size of 1630 will provide 80% power at a 0.05 significance level to detect relative risks (RR) ranging from 1.17 to 2.5 depending on the underlying prevalence of the outcome of interest in the reference exposure group and the sample size distribution between the two exposure groups (online supplemental table 2). For continuous outcomes (eg, HIV stigma), the effect size is expressed as a difference in means between the exposure groups relative to a common SD. Online supplemental table 3 summarises the minimum detectable differences between two exposure groups based on a two-sample t-test at 80% power and alpha=0.05. For example, with a sample size of 1600 including 40% reporting food insecurity,97 the minimum detectable difference in mean stigma scores between food secure and food insecure participants is 0.14 SD. The power for longitudinal analyses will increase due to multiple measures for each participant and the corresponding minimal detectable RR or difference in mean will decrease.
Baseline characteristics of the first HOPE enrollees
Characteristics of the first 437 participants enrolled into HOPE as of 1 January 2024 are summarised in table 3.
Table 3.
Characteristics at entry of participants enrolled in the Health Outcomes around Pregnancy and Exposure to HIV/Antiretrovirals Cohort by enrolment group, 2022–2023
| Enrolment group | Total (N=437) | ||||
| Nulliparous (N=87) | Pregnant or recently delivered (N=59) | Post partum and non-pregnant (N=80) | Parous and non-pregnant (N=211) | ||
| Age (in years) | |||||
| ≤25 | 74 (85%) | 16 (27%) | 24 (30%) | 17 (8%) | 131 (30%) |
| 26 to 30 | 13 (15%) | 20 (34%) | 20 (25%) | 50 (24%) | 103 (24%) |
| 31 to 35 | 0 (0%) | 15 (25%) | 20 (25%) | 78 (37%) | 113 (26%) |
| 36 to <40 | 0 (0%) | 8 (14%) | 16 (20%) | 66 (31%) | 90 (21%) |
| Site region | |||||
| Northeast | 10 (11%) | 5 (8%) | 7 (9%) | 43 (20%) | 65 (15%) |
| Midwest | 7 (8%) | 10 (17%) | 7 (9%) | 22 (10%) | 46 (11%) |
| South | 54 (62%) | 22 (37%) | 45 (56%) | 85 (40%) | 206 (47%) |
| West | 15 (17%) | 18 (31%) | 20 (25%) | 47 (22%) | 100 (23%) |
| Puerto Rico | 1 (1%) | 4 (7%) | 1 (1%) | 14 (7%) | 20 (5%) |
| Race | |||||
| White | 17 (20%) | 13 (23%) | 18 (26%) | 53 (27%) | 101 (25%) |
| Black or African American | 67 (78%) | 41 (73%) | 50 (71%) | 139 (70%) | 297 (72%) |
| Asian | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0%) |
| American Indian | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1%) | 1 (0%) |
| More than one race | 1 (1%) | 2 (4%) | 2 (3%) | 5 (3%) | 10 (2%) |
| Unknown | 1 | 3 | 10 | 13 | 27 |
| Ethnicity | |||||
| Hispanic or Latina | 13 (15%) | 15 (25%) | 25 (32%) | 69 (33%) | 122 (28%) |
| Not Hispanic or Latina | 74 (85%) | 44 (75%) | 54 (68%) | 142 (67%) | 314 (72%) |
| Unknown | 0 | 0 | 1 | 0 | 1 |
| Gender identity | |||||
| Female | 67 (97%) | 50 (100%) | 61 (100%) | 162 (100%) | 340 (99%) |
| Male | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0%) |
| Non-binary | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0%) |
| Unknown* | 18 | 9 | 19 | 49 | 95 |
| Current living situation | |||||
| Steady place to live | 65 (90%) | 35 (73%) | 50 (85%) | 145 (86%) | 295 (85%) |
| Place to live today but worried about losing it | 4 (6%) | 6 (13%) | 6 (10%) | 15 (9%) | 31 (9%) |
| Do not have a steady place to live | 1 (1%) | 7 (15%) | 3 (5%) | 4 (2%) | 15 (4%) |
| Rather not answer | 2 (3%) | 0 (0%) | 0 (0%) | 4 (2%) | 6 (2%) |
| Unknown* | 15 | 11 | 21 | 43 | 90 |
| Food security status† | |||||
| High or marginal food security (0–1) | 53 (78%) | 38 (86%) | 37 (65%) | 104 (65%) | 232 (71%) |
| Low food security (2–4) | 7 (10%) | 3 (7%) | 11 (19%) | 29 (18%) | 50 (15%) |
| Very low food security (5–6) | 8 (12%) | 3 (7%) | 9 (16%) | 27 (17%) | 47 (14%) |
| Unknown* | 19 | 15 | 23 | 51 | 108 |
| Mode of HIV acquisition | |||||
| PHIV | 58 (76%) | 11 (21%) | 11 (19%) | 43 (25%) | 123 (34%) |
| Non-PHIV | 18 (24%) | 42 (79%) | 48 (81%) | 126 (75%) | 234 (66%) |
| Unknown* | 11 | 6 | 21 | 42 | 80 |
| Age first learning of HIV diagnosis, in years | |||||
| Median (IQR) | 12 (8–16) | 21.8 (17.1–25.6) | 22.0 (17.8–25.3) | 20.0 (15.8–23.9) | 19.20 (13.0–23.5) |
| HIV viral load (copies/mL) | |||||
| ≤50 | 37 (71%) | 15 (83%) | 45 (83%) | 71 (75%) | 168 (77%) |
| >50 to ≤400 | 5 (10%) | 2 (11%) | 4 (7%) | 6 (6%) | 17 (8%) |
| >400 to ≤1000 | 0 (0%) | 0 (0%) | 1 (2%) | 2 (2%) | 3 (1%) |
| >1000 | 10 (19%) | 1 (6%) | 4 (7%) | 16 (17%) | 31 (14%) |
| Unknown‡ | 35 | 41 | 26 | 116 | 218 |
| CD4 count (cells/mm³) | |||||
| 0 to 250 | 3 (6%) | 4 (15%) | 1 (2%) | 10 (11%) | 18 (8%) |
| 251 to 500 | 8 (15%) | 9 (35%) | 12 (25%) | 13 (14%) | 42 (19%) |
| 501 to 750 | 17 (32%) | 2 (8%) | 18 (38%) | 21 (23%) | 58 (26%) |
| 751 to 1000 | 12 (23%) | 6 (23%) | 8 (17%) | 29 (31%) | 55 (25%) |
| >1000 | 13 (25%) | 5 (19%) | 9 (19%) | 20 (22%) | 47 (21%) |
| Unknown‡ | 34 | 33 | 32 | 118 | 217 |
| Depressive symptoms§ | |||||
| Minimal (0–4) | 35 (48%) | 30 (56%) | 31 (51%) | 100 (60%) | 196 (55%) |
| Mild (5–9) | 25 (34%) | 16 (30%) | 18 (30%) | 42 (25%) | 101 (29%) |
| Moderate (10–14) | 10 (14%) | 5 (9%) | 5 (8%) | 18 (11%) | 38 (11%) |
| Moderately severe (15–19) | 3 (4%) | 3 (6%) | 4 (7%) | 3 (2%) | 13 (4%) |
| Severe (20–27) | 0 (0%) | 0 (0%) | 3 (5%) | 3 (2%) | 6 (2%) |
| Unknown* | 14 | 5 | 19 | 45 | 83 |
| Anxiety symptoms¶ | |||||
| Minimal (0–4) | 36 (49%) | 31 (57%) | 34 (55%) | 98 (59%) | 199 (56%) |
| Mild (5–9) | 24 (33%) | 15 (28%) | 17 (27%) | 42 (25%) | 98 (28%) |
| Moderate (10–14) | 10 (14%) | 6 (11%) | 6 (10%) | 19 (11%) | 41 (12%) |
| Severe (15–21) | 3 (4%) | 2 (4%) | 5 (8%) | 8 (5%) | 18 (5%) |
| Unknown* | 14 | 5 | 18 | 44 | 81 |
Data as of 1 January 2024.
*Unknown data reflect time lag between data collection and becoming available in the database (longer time lag for chart abstracted data), assessment not yet completed or no measurement at or prior to study entry visit.
†Six-Item Food Security Scale.62
‡Unknown viral load or CD4 data reflect time lag between data collection and becoming available in the database or no measurement at or prior to study entry visit. The viral load and CD4 data are from the most recent measurement at or prior to study entry visit.
§Depressive symptoms assessed via interview using the Patient Health Questionnaire-9 (Kroenke, 2001).
¶Anxiety symptoms assessed via interview using the Generalised Anxiety Disorder-7 (Spitzer, 2006).76
PHIV, perinatally-acquired HIV infection.
Ethics and dissemination
The Harvard Longwood Campus Institutional Review Board, the single Institutional Review Board of record for all sites in the HOPE study, reviewed and approved the HOPE protocol and all study-specific materials prior to initiating participant enrolment. All participants provided written informed consent. Study results will be presented at local, national and international conferences, published in peer-reviewed journals and disseminated through lay summaries. Results from HOPE aim to significantly advance our understanding of the multilevel determinants of the health of young women living with HIV, inform clinical guidelines and shape supportive interventions and policies that address the needs and priorities of women living with HIV and their families.
Acknowledgments
We thank the participants for their participation in HOPE, and the individuals and institutions involved in the conduct of HOPE. Data management services were provided by Frontier Science (Data Management Center Director: SS), and regulatory services and logistical support were provided by Westat, Inc (Project Director: TW). The team is grateful to George Seage III (deceased), one of the original Principal Investigators of HOPE and PHACS. The institutions, clinical site investigators and staff that participated in conducting HOPE in 2023 are listed under Collaborators section in alphabetical order.
Footnotes
Collaborators: The Health Outcomes around Pregnancy and Exposure to HIV/ARVs (HOPE) Study Team: Ann & Robert H. Lurie Children’s Hospital of Chicago: Jennifer Jao, Lela Lartey, Kathleen Malee; Baylor College of Medicine: Mary Paul, Alejandra Martinez, Lynnette Harris; BronxCare Health System: Murli Purswani, Martha Cavallo, Mahoobullah Mirza Baig, Alma Villegas-Schwalenberg; Children's Diagnostic & Treatment Center: Lisa-Gaye Robinson, Kierra Archer, Alan Bernegger, Patricia Garvie; St. Jude Children's Research Hospital: Katherine Knapp, Chloe Burkhead, Gheri Terry, Megan Wilkins; Tulane University School of Medicine: Margarita Silio, Dornese Jones, Medea Gabriel, Patricia Sirois; University of Alabama, Birmingham: Cecelia Hutto, Paige Hickman, Dan Marullo; University of Colorado, Denver: Elizabeth McFarland, Carrie Chambers, Robin McEvoy; University of Florida, Center for HIV/AIDS Research, Education and Service: Mobeen Rathore, Saniyyah Mahmoudi, Staci Routman; University of Miami: Gwendolyn Scott, Lorena Bracho, Anai Cuadra; University of Puerto Rico School of Medicine, Medical Science Campus: Zoe M. Rodriguez, Lizmarie Torres, Nydia Scalley; University of Southern California: Toniette Frederick, Mariam Davtyan, Cristina Hernandez, Guadalupe Morales Avendano
Contributors: DK wrote the manuscript, was integrally involved in the conception and design of the protocol, co-directed its implementation and oversaw the analysis. KMP and PLW were integrally involved in the conception and design of the protocol and data collection instruments, co-directed protocol implementation and provided critical input on the organisation and content of the manuscript. LMY and EGC were integrally involved in the conception and design of the protocol and data collection instruments, co-directed protocol implementation, were involved in the acquisition of data and provided critical input on the organisation and content of the manuscript. JJ and SS were integrally involved in the conception and design of the protocol and data collection instruments, were involved in the acquisition of data and provided critical input on the organisation and content of the manuscript. LBH, KMM and A-BM were integrally involved in the conception and design of the protocol, were involved in the development of the data collection instruments and provided important revisions to the manuscript. T-JY is the protocol statistician, was integrally involved in the conception and design of the protocol, the development of the data collection instruments and provided important revisions to the manuscript. JL was involved in the development of the protocol and data collection instruments, conducted the analysis for the manuscript and provided important revisions to the manuscript. MD was involved in the development of the protocol and data collection instruments, was involved in the acquisition of data and provided important revisions to the manuscript. L-GR was involved in the development of the data collection instruments, was involved in the acquisition of data and provided important revisions to the manuscript. CAB, KS and RAS were involved in the development of the protocol and data collection instruments, provided important revisions to the manuscript, led the formation of the HOPE CAB and facilitate partnership between the research team and the HOPE CAB and Task Force members. EAB, AD, AF, LH, DLJ, AK, TJ-T, KP and LS were involved in the development of the protocol and data collection instruments, and provided important revisions to the manuscript. JG and TW were integrally involved in the design and implementation of the protocol, and reviewed and provided important revisions to the manuscript.
Funding: The study was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD), through award R01HD101351 to the Harvard T.H. Chan School of Public Health (Principal Investigators: PLW, EGC, DK, KMP; Protocol Co-Chairs: DK, KMP, LMY; Senior Project Manager: Natalie Lewis-Vass). The study was also supported through the Pediatric HIV/AIDS Cohort Study (PHACS) 2020 award P01HD103133.
Disclaimer: The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health or US Department of Health and Human Services.
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographical or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
Collaborators: The Health Outcomes in Pregnancy and Exposure to HIV/Antiretrovirals (HOPE) Study, Jennifer Jao, Lela Lartey, Kathleen Malee, Mary Paul, Alejandra Martinez, Lynnette Harris, Murli Purswani, Martha Cavallo, Mahoobullah Mirza Baig, Alma Villegas-Schwalenberg, Lisa-Gaye Robinson, Kierra Archer, Alan Bernegger, Patricia Garvie, Katherine Knapp, Chloe Burkhead, Gheri Terry, Megan Wilkins, Margarita Silio, Dornese Jones, Medea Gabriel, Patricia Sirois, Cecelia Hutto, Paige Hickman, Dan Marullo, Elizabeth McFarland, Carrie Chambers, Robin McEvoy, Mobeen Rathore, Saniyyah Mahmoudi, Staci Routman, Gwendolyn Scott, Lorena Bracho, Anai Cuadra, Zoe M. Rodriguez, Lizmarie Torres, Nydia Scalley, Toniette Frederick, Mariam Davtyan, Cristina Hernandez, and Guadalupe Morales Avendano
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. World Health Organization . The Global Health Observatory: HIV/AIDS. 2022. Available: https://www.who.int/data/gho/data/themes/hiv-aids [Accessed 8 Apr 2023].
- 2. UNAIDS . Global HIV and AIDS Statistics – fact sheet. Available: https://www.unaids.org/en/resources/fact-sheet [Accessed 10 Aug 2023].
- 3. Centers for Disease Control and Prevention . Estimated HIV incidence and prevalence in the United States, 2017–2021. HIV Surveillance Supplemental Report; 2023. Available: https://www.cdc.gov/hiv/library/reports/hiv-surveillance/vol-28-no-3/index.html [Accessed 1 Jun 2023]. [Google Scholar]
- 4. O’Shea JG, Neblett Fanfair R, Dasgupta S, et al. Cisgender women with HIV in the United States: how have HIV care continuum outcomes changed over time? 2015-2020. AIDS 2023;37:347–53. 10.1097/QAD.0000000000003431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention . Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2021. HIV Surveillance Supplemental Report; 2023. Available: http://www.cdc.gov/hiv/library/reports/hiv-surveillance/vol-28-no-4/content/tables.html [Accessed 2 Jun 2023]. [Google Scholar]
- 6. Mellins CA, Chu C, Malee K, et al. Adherence to antiretroviral treatment among pregnant and postpartum HIV-infected women. AIDS Care 2008;20:958–68. 10.1080/09540120701767208 [DOI] [PubMed] [Google Scholar]
- 7. Chen JS, Pence BW, Rahangdale L, et al. Postpartum HIV care continuum outcomes in the southeastern US. AIDS 2019;33:637–44. 10.1097/QAD.0000000000002094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Patel M, Tedaldi E, Armon C, et al. HIV RNA suppression during and after pregnancy among women in the HIV outpatient study, 1996 to 2015. J Int Assoc Provid AIDS Care 2018;17. 10.1177/2325957417752259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Swain C-A, Smith LC, Nash D, et al. Postpartum loss to HIV care and HIV viral suppression among previously diagnosed HIV-infected women with a live birth in New York State. PLoS One 2016;11:e0160775. 10.1371/journal.pone.0160775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O’Brien BE, Williams PL, Huo Y, et al. Repeat pregnancies among US women living with HIV in the SMARTT study: temporal changes in HIV disease status and predictors of preterm birth. J Acquir Immune Defic Syndr 2020;85:346–54. 10.1097/QAI.0000000000002445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meade CM, Hussen SA, Momplaisir F, et al. Long term engagement in HIV care among postpartum women with perinatal HIV infection in the United States. AIDS Care 2018;30:488–92. 10.1080/09540121.2017.1417531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patel K, Karalius B, Powis K, et al. Trends in post-partum viral load among women living with perinatal HIV infection in the USA: a prospective cohort study. Lancet HIV 2020;7:e184–92. 10.1016/S2352-3018(19)30339-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Randolph SD, Golin C, Welgus H, et al. How perceived structural racism and discrimination and medical mistrust in the health system influences participation in HIV health services for black women living in the United States south: A qualitative descriptive study. J Assoc Nurses AIDS Care 2020;31:598–605. 10.1097/JNC.0000000000000189 [DOI] [PubMed] [Google Scholar]
- 14. Bowleg L, Malekzadeh AN, Mbaba M, et al. Ending the HIV epidemic for all not just some: structural racism as a fundamental but overlooked social-structural determinant of the US HIV epidemic. Curr Opin HIV AIDS 2022;17:40–5. 10.1097/COH.0000000000000724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Geronimus AT, Hicken M, Keene D, et al. Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health 2006;96:826–33. 10.2105/AJPH.2004.060749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Allen AM, Wang Y, Chae DH, et al. Racial discrimination, the superwoman schema, and allostatic load: exploring an integrative stress-coping model among African American women. Ann N Y Acad Sci 2019;1457:104–27. 10.1111/nyas.14188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thomas MD, Michaels EK, Reeves AN, et al. Differential associations between everyday versus institution-specific racial discrimination, self-reported health, and allostatic load among black women: implications for clinical assessment and epidemiologic studies. Ann Epidemiol 2019;35:20–8. 10.1016/j.annepidem.2019.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Relf MV, Pan W, Edmonds A, et al. Discrimination, medical distrust, stigma, depressive symptoms, antiretroviral medication adherence, engagement in care, and quality of life among women living with HIV in North Carolina: a mediated structural equation model. J Acquir Immune Defic Syndr 2019;81:328–35. 10.1097/QAI.0000000000002033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mendez DD, Hogan VK, Culhane JF. Institutional racism, neighborhood factors, stress, and preterm birth. Ethnicity & Health 2014;19:479–99. 10.1080/13557858.2013.846300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Williams DR, Lawrence JA, Davis BA. Racism and health: evidence and needed research. Annu Rev Public Health 2019;40:105–25. 10.1146/annurev-publhealth-040218-043750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hailu EM, Riddell CA, Bradshaw PT, et al. Structural racism, mass Incarceration, and racial and ethnic disparities in severe maternal morbidity. JAMA Netw Open 2024;7:e2353626. 10.1001/jamanetworkopen.2023.53626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Turan B, Smith W, Cohen MH, et al. Mechanisms for the negative effects of internalized HIV-related stigma on antiretroviral therapy adherence in women: the mediating roles of social isolation and depression. J Acquir Immune Defic Syndr 2016;72:198–205. 10.1097/QAI.0000000000000948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rice WS, Logie CH, Napoles TM, et al. Perceptions of intersectional stigma among diverse women living with HIV in the United States. Soc Sci Med 2018;208:9–17. 10.1016/j.socscimed.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wagner AC, Girard T, McShane KE, et al. HIV-related stigma and overlapping stigmas towards people living with HIV among health care trainees in Canada. AIDS Educ Prev 2017;29:364–76. 10.1521/aeap.2017.29.4.364 [DOI] [PubMed] [Google Scholar]
- 25. Adimora AA, Ramirez C, Benning L, et al. Cohort profile: the women’s interagency HIV study (WIHS). Int J Epidemiol 2018;47:393–394i. 10.1093/ije/dyy021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kassaye SG, Wang C, Ocampo JMF, et al. Viremia trajectories of HIV in HIV-positive women in the United States, 1994-2017. JAMA Netw Open 2019;2:e193822. 10.1001/jamanetworkopen.2019.3822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Collins LF, Sheth AN, Mehta CC, et al. The prevalence and burden of non-AIDS comorbidity among women living with or at risk for human immunodeficiency virus infection in the United States. Clin Infect Dis 2021;72:1301–11. 10.1093/cid/ciaa204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hodder SL, Feinberg J, Strathdee SA, et al. The opioid crisis and HIV in the USA: deadly synergies. The Lancet 2021;397:1139–50. 10.1016/S0140-6736(21)00391-3 [DOI] [PubMed] [Google Scholar]
- 29. Yee LM, Kacanek D, Brightwell C, et al. Marijuana, opioid and alcohol use among pregnant and postpartum individuals living with HIV in the US. JAMA Netw Open 2021;4:e2137162. 10.1001/jamanetworkopen.2021.37162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Whittle HJ, Sheira LA, Frongillo EA, et al. Longitudinal associations between food insecurity and substance use in a cohort of women with or at risk for HIV in the United States. Addiction 2019;114:127–36. 10.1111/add.14418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haddad LB, Wall KM, Mehta CC, et al. Trends of and factors associated with live-birth and abortion rates among HIV-positive and HIV-negative women. Am J Obstet Gynecol 2017;216:71. 10.1016/j.ajog.2016.09.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Blair JM, Hanson DL, Jones JL, et al. Trends in pregnancy rates among women with human immunodeficiency virus. Obstet Gynecol 2004;103:663–8. 10.1097/01.AOG.0000117083.33239.b5 [DOI] [PubMed] [Google Scholar]
- 33. Huntington SE, Thorne C, Bansi LK, et al. Predictors of pregnancy and changes in pregnancy incidence among HIV-positive women accessing HIV clinical care at 13 large UK clinics. AIDS 2013;27:95–103. 10.1097/QAD.0b013e3283565df1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sharma A, Feldman JG, Golub ET, et al. Live birth patterns among human immunodeficiency virus-infected women before and after the availability of highly active antiretroviral therapy. Am J Obstet Gynecol 2007;196:541. 10.1016/j.ajog.2007.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Massad LS, Springer G, Jacobson L, et al. Pregnancy rates and predictors of conception, miscarriage and abortion in US women with HIV. AIDS 2004;18:281–6. 10.1097/00002030-200401230-00018 [DOI] [PubMed] [Google Scholar]
- 36. Lampe MA, Nesheim SR, Oladapo KL, et al. Achieving elimination of perinatal HIV in the United States. Pediatrics 2023;151:e2022059604. 10.1542/peds.2022-059604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sutton MY, Zhou W, Frazier EL. Unplanned pregnancies and contraceptive use among HIV- positive women in care. PLOS ONE 2018;13:e0197216. 10.1371/journal.pone.0197216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med 2016;374:843–52. 10.1056/NEJMsa1506575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kost K, Zolna M, Murro R. Pregnancies in the United States by desire for pregnancy: estimates for 2009, 2011, 2013, and 2015. Demography 2023;60:837–63. 10.1215/00703370-10690005 [DOI] [PubMed] [Google Scholar]
- 40. Rich-Edwards JW, McElrath TF, Karumanchi SA, et al. Breathing life into the lifecourse approach: pregnancy history and cardiovascular disease in women. Hypertension 2010;56:331–4. 10.1161/HYPERTENSIONAHA.110.156810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wilcox AJ. Invited commentary: beyond Barker-mothers are the ones at risk. Am J Epidemiol 2023;192:878–81. 10.1093/aje/kwad056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Al Mamun A, Mannan M, O’Callaghan MJ, et al. Association between gestational weight gain and postpartum diabetes: evidence from a community based large cohort study. PLoS One 2013;8:e75679. 10.1371/journal.pone.0075679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu P, Haththotuwa R, Kwok CS, et al. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 2017;10:e003497. 10.1161/CIRCOUTCOMES.116.003497 [DOI] [PubMed] [Google Scholar]
- 44. Stone L, Looby SE, Zanni MV. Cardiovascular disease risk among women living with HIV in North America and Europe. Curr Opin HIV AIDS 2017;12:585–93. 10.1097/COH.0000000000000413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Malee KM, Mellins CA, Huo Y, et al. Prevalence, incidence and persistence of psychiatric and substance use disorders among mothers living with HIV. J Acquir Immune Defic Syndr 2014;65:526–34. 10.1097/QAI.0000000000000070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Burke-Miller JK, Weber K, Cohn SE, et al. Neighborhood community characteristics associated with HIV disease outcomes in a cohort of urban women living with HIV. AIDS Care 2016;28:1274–9. 10.1080/09540121.2016.1173642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Howe CJ, Siegel H, Dulin-Keita A. Neighborhood environments and sexual risk behaviors for HIV infection among US women: a systematic review. AIDS Behav 2017;21:3353–65. 10.1007/s10461-017-1771-0 [DOI] [PubMed] [Google Scholar]
- 48. Lieber M, Chin-Hong P, Whittle HJ, et al. The synergistic relationship between climate change and the HIV/AIDS epidemic: a conceptual framework. AIDS Behav 2021;25:2266–77. 10.1007/s10461-020-03155-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bronfenbrenner U. The Ecology of Human Development: Experiments by Nature and Design. Cambridge, MA: Harvard University Press, Available: https://www.degruyter.com/document/doi/10.4159/9780674028845/html [Google Scholar]
- 50. Bronfenbrenner U. Making Human Beings Human: Bioecological Perspectives on Human Development. Thousand Oaks, CA: Sage Publications, 2005. [Google Scholar]
- 51. Sallis J, Owen N, Fisher E. Ecological models of health behavior. In: Glanz K, Rimer B, Lewis F, eds. Health Behavior and Health Education: Theory, Research, and Practice. 4th edn. San Francisco, California, USA: John Wiley & Sons, 2008: 465–86. [Google Scholar]
- 52. Barker DJ. The fetal and infant origins of adult disease. BMJ 1990;301:1111. 10.1136/bmj.301.6761.1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mishra GD, Cooper R, Kuh D. A life course approach to reproductive health: theory and methods. Maturitas 2010;65:92–7. 10.1016/j.maturitas.2009.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lin B, Appleton AA. Developmental origins of pregnancy-related morbidity and mortality in black US women. Front Public Health 2022;10:853018. 10.3389/fpubh.2022.853018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Power C, Kuh D, Morton S. From developmental origins of adult disease to life course research on adult disease and aging: insights from birth cohort studies. Annu Rev Public Health 2013;34:7–28. 10.1146/annurev-publhealth-031912-114423 [DOI] [PubMed] [Google Scholar]
- 56. Katz IT, Leister E, Kacanek D, et al. Factors associated with lack of viral suppression at delivery among highly active antiretroviral therapy–naive women with HIV: a cohort study. Ann Intern Med 2015;162:90–9. 10.7326/M13-2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Momplaisir FM, Brady KA, Fekete T, et al. Time of HIV diagnosis and engagement in prenatal care impact virologic outcomes of pregnant women with HIV. PLoS One 2015;10:e0132262. 10.1371/journal.pone.0132262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Geter A, Herron AR, Sutton MY. HIV-related stigma by healthcare providers in the United States: a systematic review. AIDS Patient Care STDS 2018;32:418–24. 10.1089/apc.2018.0114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sweeney SM, Vanable PA. The association of HIV-related stigma to HIV medication adherence: a systematic review and synthesis of the literature. AIDS Behav 2016;20:29–50. 10.1007/s10461-015-1164-1 [DOI] [PubMed] [Google Scholar]
- 60. Earnshaw VA, Bogart LM, Dovidio JF, et al. Stigma and racial/ethnic HIV disparities: moving toward resilience. Am Psychol 2013;68:225–36. 10.1037/a0032705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Katz IT, Ryu AE, Onuegbu AG, et al. Impact of HIV-related stigma on treatment adherence: systematic review and meta-synthesis. J Int AIDS Soc 2013;16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Khazanchi R, Sayles H, Bares SH, et al. Neighborhood deprivation and racial/ ethnic disparities in HIV viral suppression: a single-center cross-sectional study in the U.S. Clinical Infectious Diseases 2021;72:e642–5. 10.1093/cid/ciaa1254 [DOI] [PubMed] [Google Scholar]
- 63. Shonkoff JP, Garner AS, Siegel BS, et al. The lifelong effects of early childhood adversity and toxic stress. Pediatrics 2012;129:e232–46. 10.1542/peds.2011-2663 [DOI] [PubMed] [Google Scholar]
- 64. Phiri S, Tweya H, van Lettow M, et al. Impact of facility- and community-based peer support models on maternal uptake and retention in Malawi’s option B+ HIV prevention of mother-to-child transmission program: a 3-arm cluster randomized controlled trial (PURE Malawi). J Acquir Immune Defic Syndr 2017;75:S140–8. 10.1097/QAI.0000000000001357 [DOI] [PubMed] [Google Scholar]
- 65. Momplaisir FM, Storm DS, Nkwihoreze H, et al. Improving postpartum retention in care for women living with HIV in the United States. AIDS 2018;32:133–42. 10.1097/QAD.0000000000001707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ungar M. Resilience, trauma, context, and culture. Trauma Violence Abuse 2013;14:255–66. 10.1177/1524838013487805 [DOI] [PubMed] [Google Scholar]
- 67. Dale SK, Safren SA. Resilience takes a village: black women utilize support from their community to foster resilience against multiple adversities. AIDS Care 2018;30:S18–26. 10.1080/09540121.2018.1503225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dulin AJ, Earnshaw VA, Dale SK, et al. A concept mapping study to understand multilevel resilience resources among African American/black adults living with HIV in the Southern United States. AIDS Behav 2021;25:773–86. 10.1007/s10461-020-03042-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Williams PL, Seage GR, Van Dyke RB, et al. A trigger-based design for evaluating the safety of in utero antiretroviral exposure in uninfected children of human immunodeficiency virus-infected mothers. Am J Epidemiol 2012;175:950–61. 10.1093/aje/kwr401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Van Dyke RB, Chadwick EG, Hazra R, et al. The PHACS SMARTT study: assessment of the safety of in utero exposure to antiretroviral drugs. Front Immunol 2016;7:199. 10.3389/fimmu.2016.00199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tassiopoulos K, Patel K, Alperen J, et al. Following young people with perinatal HIV infection from adolescence into adulthood: the protocol for PHACS AMP Up, a prospective cohort study. BMJ Open 2016;6:e011396. 10.1136/bmjopen-2016-011396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Environmental systems research Institute (ESRI). Redlands, CA: ArcGIS Pro 3.0.0; 2022. [Google Scholar]
- 73. Weiss BD, Mays MZ, Martz W, et al. Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med 2005;3:514–22. 10.1370/afm.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Blumberg SJ, Bialostosky K, Hamilton WL, et al. The effectiveness of a short form of the household food security scale. Am J Public Health 1999;89:1231–4. 10.2105/AJPH.89.8.1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092–7. 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 77. Prins A, Bovin MJ, Smolenski DJ, et al. The primary care PTSD screen for DSM-5 (PC-PTSD-5): development and evaluation within a veteran primary care sample. J GEN INTERN MED 2016;31:1206–11. 10.1007/s11606-016-3703-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Rose A, Peters N, Shea JA, et al. Development and testing of the health care system distrust scale. J Gen Intern Med 2004;19:57–63. 10.1111/j.1525-1497.2004.21146.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bova C, Fennie KP, Watrous E, et al. The health care relationship (HCR) trust scale: development and psychometric evaluation. Res Nurs Health 2006;29:477–88. 10.1002/nur.20158 [DOI] [PubMed] [Google Scholar]
- 80. Wilson IB, Lee Y, Michaud J, et al. Validation of a new self-report measure for medication adherence. AIDS Behav 2016;20:2700–8. 10.1007/s10461-016-1406-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Davies AR. Developing and testing the MOS 20-item short-form health survey: A general population application. In: Stewart AL, Ware JE, eds. Measuring functioning and well-being: The Medical Outcomes Study approach. Durham, NC: Duke University Press, 1992: 277–90. [Google Scholar]
- 82. Sancho-Domingo C, Carballo JL, Coloma-Carmona A, et al. Brief version of the Pittsburgh Sleep Quality Index (B-PSQI) and measurement invariance across gender and age in a population-based sample. Psychol Assess 2021;33:111–21. 10.1037/pas0000959 [DOI] [PubMed] [Google Scholar]
- 83. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385–96. 10.2307/2136404. [DOI] [PubMed] [Google Scholar]
- 84. Mujahid MS, Diez Roux AV, Morenoff JD, et al. Assessing the measurement properties of neighborhood scales: from psychometrics to ecometrics. Am J Epidemiol 2007;165:858–67. 10.1093/aje/kwm040 [DOI] [PubMed] [Google Scholar]
- 85. Williams DR, Jackson JS, et al. Racial differences in physical and mental health: socio-economic status, stress and discrimination. J Health Psychol 1997;2:335–51. 10.1177/135910539700200305 [DOI] [PubMed] [Google Scholar]
- 86. Krieger N, Smith K, Naishadham D, et al. Experiences of discrimination: validity and reliability of a self-report measure for population health research on racism and health. Soc Sci Med 2005;61:1576–96. 10.1016/j.socscimed.2005.03.006 [DOI] [PubMed] [Google Scholar]
- 87. Sayles JN, Wong MD, Kinsler JJ, et al. The association of stigma with self-reported access to medical care and antiretroviral therapy adherence in persons living with HIV/AIDS. J Gen Intern Med 2009;24:1101–8. 10.1007/s11606-009-1068-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gottert A, Friedland B, Geibel S, et al. The People Living with HIV (PLHIV) resilience scale: development and validation in three countries in the context of the PLHIV Stigma Index. AIDS Behav 2019;23:172–82. 10.1007/s10461-019-02594-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Smith BW, Dalen J, Wiggins K, et al. The brief resilience scale: assessing the ability to bounce back. Int J Behav Med 2008;15:194–200. 10.1080/10705500802222972 [DOI] [PubMed] [Google Scholar]
- 90. Wingard DL, Berkman LF. Mortality risk associated with sleeping patterns among adults. Sleep 1983;6:102–7. 10.1093/sleep/6.2.102 [DOI] [PubMed] [Google Scholar]
- 91. Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med 1991;32:705–14. 10.1016/0277-9536(91)90150-b [DOI] [PubMed] [Google Scholar]
- 92. Pulerwitz J, Gortmaker SL, DeJong W. Measuring sexual relationship power in HIV/STD research. Sex Roles 2000;42:637–60. 10.1023/A:1007051506972 [DOI] [Google Scholar]
- 93. Ali R, Elia A, Babor T. The alcohol smoking and substance involvement screening test (ASSIST): development, Reliability and feasibility. Addiction 2002;97:1183–94. 10.1046/j.1360-0443.2002.00185.x [DOI] [PubMed] [Google Scholar]
- 94. Pence BW, Gaynes BN, Whetten K, et al. Validation of a brief screening instrument for substance abuse and mental illness in HIV-positive patients. J Acquir Immune Defic Syndr 2005;40:434–44. 10.1097/01.qai.0000177512.30576.9c [DOI] [PubMed] [Google Scholar]
- 95. Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol 2010;6:109–38. 10.1146/annurev.clinpsy.121208.131413 [DOI] [PubMed] [Google Scholar]
- 96. Muthen B. Latent variable analysis: growth mixture modeling techniques for longitudinal data. In: Kaplan D, ed. The Sage handbook of quantitative methodology for the social sciences. Thousand Oaks, CA: Sage, 2004: 345–68. [Google Scholar]
- 97. Spinelli MA, Frongillo EA, Sheira LA, et al. Food insecurity is associated with poor HIV outcomes among women in the United States. AIDS Behav 2017;21:3473–7. 10.1007/s10461-017-1968-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2024-084835supp001.pdf (175.1KB, pdf)