Abstract
Endothelial cells (EC) are common targets of permissive infection by human cytomegalovirus (HCMV) in vivo during acute disease. However, studies of HCMV-EC interactions in vitro have generated discordant results. While lytic infection of cultured venous EC has been well established, Fish et al. (K. N. Fish, C. Soderberg Naucler, L. K. Mills, S. Stenglein, and J. A. Nelson, J. Virol. 72:5661–5668) have reported noncytopathic persistence of the virus in cultured aortic EC. We propose that interstrain differences in viral host cell tropism rather than the vascular bed of origin of infected EC might account for these discrepancies. In the present investigation we compared the responses of EC derived from human adult iliac artery, placental microvasculature, and umbilical vein to infection with various HCMV strains. Regardless of the vascular bed of origin, infection with EC-propagated HCMV strains induced 100% efficient cytopathic change progressing to complete lysis of inoculated monolayers. While fibroblast-propagated strains persisted at low titer in infected arterial EC cultures, they were also cytolytic for individual infected cells. The finding of cytopathic lytic infection of arterial EC by HCMV implicates a mechanism of vascular injury in the pathogenesis of HCMV infection.
Endothelial cells (EC) are major targets of human cytomegalovirus (HCMV) during acute infection of an immunocompromised host (18). In addition to contributing to hematogenous viral dissemination (7, 8, 13, 19, 23), infected EC may trigger direct vascular injury if viral induced cytopathogenicity occurs. In support of these in vivo data, susceptibility of cultured human venous EC (HUVEC) to productive lytic HCMV infection has been demonstrated (25). However, studies of interactions of HCMV with human arterial EC (HAEC) have generated conflicting results. Whereas reports about noncytopathic persistence of HCMV in cultured aortic EC (6) raised the possibility of different susceptibilities of EC depending on their vascular origin, Knight et al. (9) have described similar cytopathologies of HCMV in HUVEC, HAEC, and microvascular EC with regard to adhesion molecule induction. The respective usage of fibroblast-propagated and EC-propagated HCMV strains might provide an explanation for the discrepancies. While such interstrain differences in the cytopathic potential of HCMV in HUVEC are well established (11, 20, 22, 24), the lytic potentials of various HCMV strains in HAEC have not yet been determined.
In the present investigation we have tested the hypothesis that interstrain differences in viral host cell tropism, rather than properties inherent to EC of different vascular origins, determine the outcome of HCMV infection. To this end we have performed a detailed analysis of viral replication kinetics, long-term evolution of cytopathology, and lytic end points, comparing EC derived from human adult iliac artery, placental microvasculature, and umbilical vein following inoculation with various HCMV strains.
Efficient lytic infection of HAEC by EC-propagated HCMV strains.
In a first set of experiments, HCMV strains VHL/E (kindly provided by W. J. Waldman) (24) and TB40/E (22) were tested for cytopathic potential in HAEC. Both strains were propagated in HUVEC to preserve the natural endothelial cytopathogenicity of the original isolates. To obtain high-titer virus preparations, EC-propagated virus stocks were inoculated with fibroblasts for a single round of infection, harvested at 100% cytopathic effect (CPE), and made cell free by centrifugation. HAEC were isolated from adult human iliac arteries of bypass recipients by mechanically removing the endothelial layer as previously described (1, 2) and were cultured in EGM-2 medium (Clonetics, Walkersville, Md.) containing 2% fetal calf serum on culture flasks (Greiner, Frickenhausen, Germany) coated with 2% gelatin. For infection, cells were preincubated for 2 h in heparin-free medium, inoculated with cell-free virus at multiplicities of infection (MOI) of 0.1, 1, and 10, and incubated for 90 min before removal of the inoculum. The medium was replaced at 48-h intervals. The kinetics of viral antigen expression in HAEC cultures was analyzed by indirect immunoperoxidase detection of immediate-early antigen (UL122/123; monoclonal antibody [MAb] E13; Biosoft, Paris, France), early protein p52 (UL44; MAb BS510; Biotest, Dreieich, Germany), and late viral major capsid protein (UL86; MAb 28-4; kindly provided by W. Britt) in acetone-fixed cultures at various intervals postinoculation (p.i.), using diaminobenzidine as the chromogen. CPEs and lysis of infected cells were documented by sequential phase-contrast micrographs up to day 32 p.i.; i.e., identical frames of infected cultures were photographed daily with a Zeiss Axiovert 135 microscope. The accuracy of the method can be judged from Fig. 2D, where characteristic scratch structures were present on the surface of the culture plate, and from Fig. 4, where characteristic cell culture structures are indicated. All experiments described in this paper were repeated at least three times with identical results.
FIG. 2.
CPEs of HCMV strains TB40/E and TB40/F in HAEC cultures. CPEs were documented by sequential phase-contrast micrographs of HAEC cultures either mock infected (A and C), infected with HCMV strain TB40/E at an MOI of 10 (B), infected with HCMV strain TB40/E at an MOI of 0.1 (D), or infected with HCMV strain TB40/F at an MOI of 10 (E) at the indicated days p.i. (dpi).
FIG. 4.
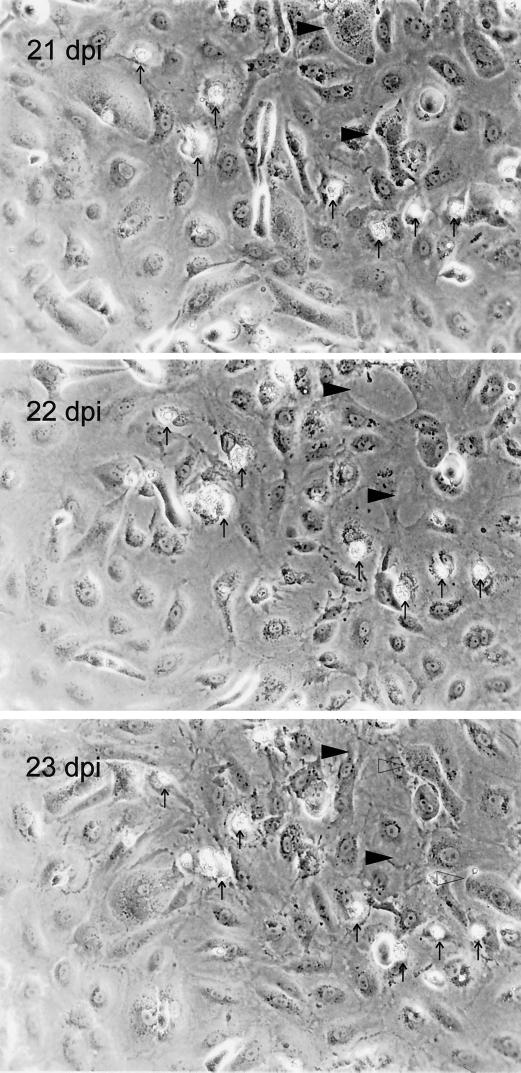
Sequential phase-contrast micrographs of HAEC cultures at 21, 22, and 23 days p.i. (dpi) with fibroblast-propagated strain TB40/F (MOI = 10). Identical frames of infected cultures were photographed daily with a Zeiss Axiovert 135 microscope. For better orientation, characteristic structures are indicated by arrows. Two late-stage infected cells (characterized by nuclear inclusions) at 21 dpi are indicated by filled arrowheads. At 22 dpi these cells are lysed and defects in the monolayer have occurred, which are filled by noncytopathic cells at 23 dpi. Adjacent to these sites two new late-stage infected cells appeared (open arrowheads) at 23 dpi.
Both strain TB40/E (Fig. 1 and 2B and D) and strain VHL/E (data not shown) established a fully permissive infection in HAEC in vitro. At a high MOI (MOI of 10), the majority of cells were infected by the initial inoculum, and within 48 h the late stage of viral replication was observed (Fig. 1). By day 12 p.i., lysis of infected cells occurred, and lysis was completed by day 17 p.i. (Fig. 2B). At a low MOI (0.1), only a minor fraction of cells were infected. However, both strains TB40/E (Fig. 2D) and VHL/E (data not shown) disseminated throughout HAEC monolayers, ultimately resulting in 100% CPE (by day 27 p.i.) as indicated by the appearance of characteristic nuclear inclusions in 100% of the cells. Subsequently, lysis of the infected cultures occurred (by day 32 p.i.) (Fig. 2D), while mock-infected cultures remained intact (Fig. 2A and C).
FIG. 1.
HCMV antigen expression in HAEC cells infected with HCMV strain TB40/E at an MOI of 10. (A to D) Detection of immediate-early antigen (MAb E13, UL122/123) 1 day after infection (A), of early antigen p52 (MAb BS510, UL44) 4 days after infection (B), of late antigen major capsid protein (MAb 28-4, UL86) 4 days after infection (C), and of the irrelevant antibody anticytokeratin (control for specificity of staining) 4 days after infection (D) in HAEC by the indirect immunoperoxidase technique. (E) Time course of appearance of viral antigens in infected HAEC.
Interstrain differences in HCMV infection of HAEC.
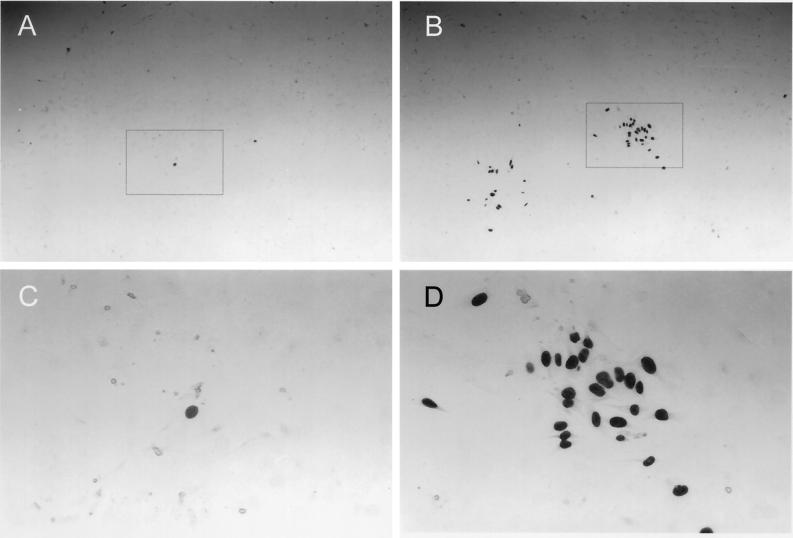
To test whether the efficiency of infection in HAEC distinguishes EC-propagated strains from fibroblast-propagated strains, quantitative analysis of cell-free infection and cell-associated infection of HAEC was performed, comparing fibroblast-propagated strains AD169, TB40/F (22), and VHL/F (kindly provided by W. J. Waldman) (24) with HUVEC-propagated strains TB40/E (22) and VHL/E (24). The efficiency of cell-free HCMV infection was determined by limiting-dilution analyses in human foreskin fibroblast (HFF) and HAEC cultures (Table 1). Cell-free supernatants of the various virus strains were harvested from HFF cultures displaying 100% late-stage CPE. Identical log step dilutions were incubated with HFFs and HAEC in 96-well plates for 24 h. Cultures were then fixed, and infected cells were detected by indirect immunoperoxidase staining of viral immediate-early antigen. The infectivity of a given virus preparation in HFFs or HAEC was calculated as the 50% tissue culture infective dose (TCID50) per milliliter by the method of Spearman and Kärber (described in reference 12). Cell-associated infectivity was determined by focus expansion (FE) assays (Table 2) as described previously (20). In FE assays, the potential of HCMV to spread from late-stage infected cells to adjacent uninfected HAEC or HFFs was quantified in cocultures. The number of infected cells per focus (FEHAEC or FEHFF) at day 5 after cocultivation was determined by immunostaining of immediate-early antigen. Although all strains established permissive infection in HAEC to some degree (including lysis of individual infected cells) (Fig. 2; see Fig. 4), quantitative analysis clearly distinguished endotheliotropic from nonendotheliotropic strains by the efficiency of infection and by the ability to disseminate in the infected HAEC cultures. All fibroblast-propagated strains clearly exhibited dramatically reduced cell-free efficiency of infection in HAEC compared to efficiency of infection in HFFs (Table 1). None of these strains was able to form infectious foci of more than three cells per focus (Fig. 3; Table 2) or to disseminate within HAEC cultures (Fig. 2E). In contrast, all HUVEC-propagated strains displayed higher relative efficiencies of infection (Table 1). More importantly, both HUVEC-propagated strains, TB40/E and VHL/E, were able to form infectious foci and disseminate in HAEC cultures, as indicated by focus expansion values of 39 and 51 cells/focus, respectively (Fig. 3; Table 2). Following cell-free infection of HAEC at an MOI of 10, endotheliotropic strains induced complete lysis of infected HAEC cultures within 17 days (Fig. 2B), whereas nonendotheliotropic strains persisted in the infected HAEC cultures for more than 32 days (data shown for TB40/F in Fig. 2E). The interstrain differences of virus dissemination and cytopathogenicity within HAEC cultures became particularly clear when the initial infectivity was equalized by a 100-fold-reduced MOI of the EC-propagated strain (Fig. 2D versus 2E). As documented by sequential phase-contrast micrographs of infected cultures, persistence of strain TB40/F in HAEC cultures seemed to reflect a state of equilibrium between lysis of the small fraction of infected cells and regeneration of uninfected cells (Fig. 4) rather than persistence of the virus in individual infected cells.
TABLE 1.
Comparison of supernatant-associated infection efficiency of various HCMV strains in HFFs and HAEC
| Virus preparation | Infectivity (TCID50/ml) in:
|
Ratio (TCID50 HFF/ TCID50 HAEC)a | |
|---|---|---|---|
| HFFs | HAEC | ||
| TB40/E | 105.5 | 103.8 | 50 |
| TB40/F | 106 | 101.4 | 40,000 |
| VHL/E | 105.3 | 103 | 200 |
| VHL/F | 103.5 | 0 | >3,200 |
| AD169 | 106 | 101.8 | 16,000 |
A high ratio indicates low efficiency in HAEC, whereas a low ratio indicates high efficiency in HAEC.
TABLE 2.
Comparison of cell-associated infection efficiency of various HCMV strains in HFFs, HAEC, HUVEC, and placental microvascular endothelial cells (HPEC-A1)
| Virus preparation | FE (infected cells/focus) in:
|
|||
|---|---|---|---|---|
| HFFs | HAEC | HUVEC | HPEC-A1 | |
| TB40/E | >300 | 39 | 70 | 53 |
| TB40/F | >300 | 3 | 0 | 4 |
| VHL/E | >300 | 51 | 40 | 63 |
| VHL/F | >300 | 3 | 3 | 8 |
| AD169 | >300 | 3 | 1 | 5 |
FIG. 3.
Interstrain differences in cell-associated infectivity of HCMV in HAEC as determined by FE assays. Detection of nuclear HCMV immediate-early antigen by the immunoperoxidase technique 5 days after cocultivation of few late-stage infected HFFs with abundand noninfected HAEC is shown. (A and C) Fibroblast-propagated HCMV strain TB40/F. (B and D) EC-propagated strain TB40/E. Panels C and D are magnifications of the frames indicated in panels A and B, respectively. Magnifications, ×40 (A and B) and ×170 (C and D).
Comparison of HCMV infection in various EC types.
It is well known that individual EC derived from different vascular beds exhibit distinguishing characteristics. To compare responses of EC of different vascular origins to HCMV infection, quantification of cell-associated infectivity was performed in HUVEC and in a placental microvascular cell line (HPEC-A1) (17), essentially as described above for HAEC and HFFs. FE values for the various virus strains in all four cell types were subsequently compared (Table 2), and lytic end points were documented by phase-contrast micrographs. The efficiency of infection in HAEC, HUVEC, and HPEC-A1 and the lytic potentials of various HCMV strains (data not shown) were essentially identical in each of these cell types. EC-propagated HCMV retained the potential for focal dissemination in all EC types, whereas their fibroblast-propagated counterparts were incapable of focal expansion in HAEC, HUVEC, and HPEC-A1 (Table 2).
In summary, the data generated from these experiments imply that the ultimate fate of HCMV-infected EC cultures (i.e., viral persistence versus lytic infection) is determined primarily by properties inherent in the virus itself, rather than by the vascular bed of origin of the EC. Specifically we have found that EC derived from adult artery, fetal vein, and placental microvasculature all respond to infection with each given strain of HCMV in an essentially identical manner with regard to efficiency of infection, cytopathic alterations, and cell lysis. Thus, although endothelia populating different vascular beds exhibit certain distinguishing characteristics, their responses to HCMV infection seem to be quite similar. This conclusion is further supported by studies of Knight et al. (9, 10), who demonstrated that patterns of HCMV-mediated modulation of endothelial HLA and adhesion molecule expression were essentially identical among EC derived from human umbilical vein and matched artery, human coronary artery, human aorta, and human dermal microvasculature. Although those studies did not monitor cytopathic change through to complete cell lysis, efficiency of infection and cytopathology were essentially identical among all endothelial isolates (9, 10; W. J. Waldman, personal communication).
Fish et al. (6) have reported that inoculation of human aortic EC with HCMV resulted in persistent, noncytopathic, and nonlytic infection. In contrast, our data clearly demonstrate that certain HCMV strains have the potential for extensive CPE (cytomegaly and appearance of inclusion bodies) and for complete lysis of the infected HAEC cultures. This apparent discrepancy might be mainly explained by the usage of EC-propagated strains that have been shown to retain the cell tropism of clinical isolates (22, 24). In contrast, isolate Po used by Fish et al. has been “passaged through HF [human fibroblasts] and frozen below passage 12 in liquid nitrogen” (5), and loss of natural EC cytopathogenicity might have occurred at that stage of fibroblast propagation. Interestingly, we also observed persistence of HCMV in HAEC cultures when fibroblast-propagated strains were used. However, even with these strains the occurrence of cytomegaly with nuclear inclusions indicated a CPE on a single-cell level (Fig. 2E and 4). Moreover, on a single-cell level, infection of HAEC by these strains also resulted in lytic infection (Fig. 4), suggesting that HCMV persistence in HAEC might possibly reflect a balance between lysis of infected cells and regeneration of uninfected cells rather than persistence on a single-cell level. It may be questioned whether this might also explain previous reports by Fish et al. (6) about persistence of HCMV in human aortic EC. In that work, the mitotic activity of infected cells had been assessed by judging their DNA content. However, this method seems to be inappropriate for permissively infected cells that produce numerous viral particles containing viral DNA (6). In addition, viral immediate-early antigen expression in mitotic cells had been shown to demonstrate that infected cells are capable of cell division. However, this pattern could as well reflect recent infection of a cell which was already in the state of mitosis at the time of infection. In contrast, early or late viral proteins, which should also be detectable when mitotic activity of productively infected cells is assumed, had not been demonstrated in dividing cells. Thus, based on the primary data obtained, these findings are not inconsistent with our finding of lytic infection in a fraction of cells when fibroblast-propagated isolates TB40/F and VHL/F were used. Clearly, it cannot be excluded that other cells in the population are not lytically infected, and differences in cells, culture conditions, and virus strains might further explain part of the discrepancies. Still, based on the evidence presented here, the assumption of a balance between lysis of infected cells and regeneration of uninfected cells would provide a simple explanation for persistence of fibroblast-adapted strains in EC cultures.
Our finding of cytopathic lytic infection of HAEC by HCMV has significant implications for considerations regarding the pathogenesis of HCMV infections. Release of infectious virus progeny from late-stage infected HAEC could promote the hematogenous spread of HCMV throughout the organism. More important for the development of organ disease, lysis of infected HAEC was demonstrated here for the first time on a single-cell level. If such a CPE also occurred in vivo, it would result in the development of lesions in the EC monolayer, followed by thrombocyte aggregations, attraction of leukocytes, and transmigration of immune cells into parenchymal tissue (4, 16). These events might then promote transport of HCMV into organ tissues by transmigrating leukocytes (3, 8, 14, 21, 23), vasculitic reactions at sites of vascular injury (15, 26), and the development of atherosclerotic lesions (4, 16).
Acknowledgments
The excellent technical assistance of Jutta Knapp and Heike Runge is greatly appreciated. We thank P. Friedl for providing cell line HPEC-A1.
This work was supported by the Bundesministerium für Bildung und Forschung (Projektnummer 01 KI 9602) and by the Stiftung zur Förderung und Erforschung von Ersatz- und Ergänzungsmethoden zur Einschränkung von Tierversuchen.
REFERENCES
- 1.Axel D I, Brehm B R, Wolburg Buchholz K, Betz E L, Koveker G, Karsch K R. Induction of cell-rich and lipid-rich plaques in a transfilter coculture system with human vascular cells. J Vasc Res. 1996;33:327–339. doi: 10.1159/000159160. [DOI] [PubMed] [Google Scholar]
- 2.Axel D I, Riessen R, Athanasiadis A, Runge H, Koveker G, Karsch K R. Growth factor expression of human arterial smooth muscle cells and endothelial cells in a transfilter coculture system. J Mol Cell Cardiol. 1997;29:2967–2978. doi: 10.1006/jmcc.1997.0541. [DOI] [PubMed] [Google Scholar]
- 3.Craigen J L, Yong K L, Jordan N J, MacCormac L P, Westwick J, Akbar A N, Grundy J E. Human cytomegalovirus infection up-regulates interleukin-8 gene expression and stimulates neutrophil transendothelial migration. Immunology. 1997;92:138–145. doi: 10.1046/j.1365-2567.1997.00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faggiotto A, Ross R. Studies of hypercholesterolemia in the nonhuman primate. II. Fatty streak conversion to fibrous plaque. Arteriosclerosis. 1984;4:341–356. doi: 10.1161/01.atv.4.4.341. [DOI] [PubMed] [Google Scholar]
- 5.Fish K N, Depto A S, Moses A V, Britt W, Nelson J A. Growth kinetics of human cytomegalovirus are altered in monocyte-derived macrophages. J Virol. 1995;69:3737–3743. doi: 10.1128/jvi.69.6.3737-3743.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fish K N, Soderberg Naucler C, Mills L K, Stenglein S, Nelson J A. Human cytomegalovirus persistently infects aortic endothelial cells. J Virol. 1998;72:5661–5668. doi: 10.1128/jvi.72.7.5661-5668.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grefte A, van der Giessen M, van Son W, The T H. Circulating cytomegalovirus (CMV)-infected endothelial cells in patients with an active CMV infection. J Infect Dis. 1993;167:270–277. doi: 10.1093/infdis/167.2.270. [DOI] [PubMed] [Google Scholar]
- 8.Grundy J E, Lawson K M, MacCormac L P, Fletcher J M, Yong K L. Cytomegalovirus-infected endothelial cells recruit neutrophils by the secretion of C-X-C chemokines and transmit virus by direct neutrophil-endothelial cell contact and during neutrophil transendothelial migration. J Infect Dis. 1998;177:1465–1474. doi: 10.1086/515300. [DOI] [PubMed] [Google Scholar]
- 9.Knight D A, Waldman W J, Sedmak D D. Cytomegalovirus-mediated modulation of adhesion molecule expression by human arterial and microvascular endothelial cells. Transplantation. 1999;68:1814–1818. doi: 10.1097/00007890-199912150-00030. [DOI] [PubMed] [Google Scholar]
- 10.Knight D A, Waldman W J, Sedmak D D. Human cytomegalovirus does not induce human leukocyte antigen class II expression on arterial endothelial cells. Transplantation. 1997;63:1366–1369. doi: 10.1097/00007890-199705150-00030. [DOI] [PubMed] [Google Scholar]
- 11.MacCormac L P, Grundy J E. Two clinical isolates and the Toledo strain of cytomegalovirus contain endothelial cell tropic variants that are not present in the AD169, Towne, or Davis strains. J Med Virol. 1999;57:298–307. doi: 10.1002/(sici)1096-9071(199903)57:3<298::aid-jmv14>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 12.Mahy B W J, Kangro H O. Virology methods manual. San Diego, Calif: Academic Press; 1996. [Google Scholar]
- 13.Percivalle E, Revello M G, Vago L, Morini F, Gerna G. Circulating endothelial giant cells permissive for human cytomegalovirus (HCMV) are detected in disseminated HCMV infections with organ involvement. J Clin Invest. 1993;92:663–670. doi: 10.1172/JCI116635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Revello M G, Percivalle E, Arbustini E, Pardi R, Sozzani S, Gerna G. In vitro generation of human cytomegalovirus pp65 antigenemia, viremia, and leukoDNAemia. J Clin Invest. 1998;101:2686–2692. doi: 10.1172/JCI1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts W H, Sneddon J M, Waldman J, Stephens R E. Cytomegalovirus infection of gastrointestinal endothelium demonstrated by simultaneous nucleic acid hybridization and immunohistochemistry. Arch Pathol Lab Med. 1989;113:461–464. [PubMed] [Google Scholar]
- 16.Ross R, Glomset J, Harker L. Response to injury and atherogenesis. Am J Pathol. 1977;86:675–684. [PMC free article] [PubMed] [Google Scholar]
- 17.Schutz M, Teifel M, Friedl P. Establishment of a human placental endothelial cell line with extended life span after transfection with SV 40 T-antigens. Eur J Cell Biol. 1997;74:315–320. [PubMed] [Google Scholar]
- 18.Sinzger C, Grefte A, Plachter B, Gouw A S, The T H, Jahn G. Fibroblasts, epithelial cells, endothelial cells and smooth muscle cells are major targets of human cytomegalovirus infection in lung and gastrointestinal tissues. J Gen Virol. 1995;76:741–750. doi: 10.1099/0022-1317-76-4-741. [DOI] [PubMed] [Google Scholar]
- 19.Sinzger C, Jahn G. Human cytomegalovirus cell tropism and pathogenesis. Intervirology. 1996;39:302–319. doi: 10.1159/000150502. [DOI] [PubMed] [Google Scholar]
- 20.Sinzger C, Knapp J, Plachter B, Schmidt K, Jahn G. Quantification of replication of clinical cytomegalovirus isolates in cultured endothelial cells and fibroblasts by a focus expansion assay. J Virol Methods. 1997;63:103–112. doi: 10.1016/s0166-0934(97)02082-x. [DOI] [PubMed] [Google Scholar]
- 21.Sinzger C, Plachter B, Grefte A, The T H, Jahn G. Tissue macrophages are infected by human cytomegalovirus in vivo. J Infect Dis. 1996;173:240–245. doi: 10.1093/infdis/173.1.240. [DOI] [PubMed] [Google Scholar]
- 22.Sinzger C, Schmidt K, Knapp J, Kahl M, Beck R, Waldman J, Hebart H, Einsele H, Jahn G. Modification of human cytomegalovirus tropism through propagation in vitro is associated with changes in the viral genome. J Gen Virol. 1999;80:2867–2877. doi: 10.1099/0022-1317-80-11-2867. [DOI] [PubMed] [Google Scholar]
- 23.Waldman W J, Knight D A, Huang E H, Sedmak D D. Bidirectional transmission of infectious cytomegalovirus between monocytes and vascular endothelial cells: an in vitro model. J Infect Dis. 1995;171:263–272. doi: 10.1093/infdis/171.2.263. [DOI] [PubMed] [Google Scholar]
- 24.Waldman W J, Roberts W H, Davis D H, Williams M V, Sedmak D D, Stephens R E. Preservation of natural endothelial cytopathogenicity of cytomegalovirus by propagation in endothelial cells. Arch Virol. 1991;117:143–164. doi: 10.1007/BF01310761. [DOI] [PubMed] [Google Scholar]
- 25.Waldman W J, Sneddon J M, Stephens R E, Roberts W H. Enhanced endothelial cytopathogenicity induced by a cytomegalovirus strain propagated in endothelial cells. J Med Virol. 1989;28:223–230. doi: 10.1002/jmv.1890280405. [DOI] [PubMed] [Google Scholar]
- 26.Wiley C A, Nelson J A. Role of human immunodeficiency virus and cytomegalovirus in AIDS encephalitis. Am J Pathol. 1988;133:73–81. [PMC free article] [PubMed] [Google Scholar]