Abstract
Background
The capsule formulation of CDK4/6 inhibitor palbociclib has reduced solubility at gastric pH > 4.5 and may have decreased activity when used with proton-pump inhibitors (PPI). Herein, we report the effect of PPI on palbociclib capsule activity and safety in the PARSIFAL study.
Methods
First-line endocrine-sensitive, hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2-) advanced breast cancer (ABC) patients received palbociclib capsules plus fulvestrant or letrozole. The primary endpoint was progression-free survival (PFS). This post-hoc analysis compared PPI use. Patients were PPI-naïve (N-PPI) if not on PPI during the study, and either early (E-PPI) or long-term PPI (LT-PPI) if on PPI at study entry or for at least ≥⅔ of treatment, respectively. PPI groups were not mutually exclusive.
Results
Among 486 patients, 66.9 % were N-PPI, 13.2 % E-PPI, 18.7 % LT-PPI, and 11.5 % of the PPI users were defined as neither. Median PFS (mPFS) was 29.6 months in the study population, 28.7 months in N-PPI, 23.0 months in E-PPI (Hazard Ratio [HR] 1.5; 95%Confidence Interval [CI] 1.1–2.2; p = 0.024), and 23.0 months in LT-PPI (HR 1.4; 95%CI 1.0–1.9; p = 0.035). By landmark analysis, PPI use was associated with poorer mPFS at 3 and 12 months. Grade ≥3 hematological adverse events occurred in 71.7 % of N-PPI, 57.8 % of E-PPI (p = 0.021), and 54.9 % of LT-PPI (p = 0.003). Dose reductions and dosing delays due to hematological toxicity occurred in 70.8 % of N-PPI, 56.3 % of E-PPI (p = 0.018), and 52.7 % of LT-PPI (p = 0.002).
Conclusions
PPI use may reduce palbociclib capsule toxicity, dose modifications, and clinical activity in HR+/HER2- ABC.
Keywords: Palbociclib, Endocrine therapy, Advanced breast cancer, Proton pump inhibitors, Pharmacokinetic interaction, Absorption
Highlights
-
•
Concomitant proton pump inhibitor and palbociclib was retrospectively analyzed.
-
•
Median progression-free survival was shorter with proton pump inhibitor use.
-
•
Adverse events and dose reductions were lower with proton pump inhibitors.
-
•
These results reveal proton pump inhibitors may interact with palbociclib capsules.
Trial registration
ClinicalTrials.gov Identifier: NCT02491983; https://clinicaltrials.gov/ct2/show/NCT02491983.
Meeting presentation
Part of the data was presented in the following meeting:
•American Society of Clinical Oncology (ASCO) Virtual Meeting ©2020. May 29-June 02, 2020. “1007P - PARSIFAL: A randomized, multicenter, open-label, phase II trial to evaluate palbociclib in combination with fulvestrant or letrozole in endocrine-sensitive patients with estrogen receptor (ER)[+]/HER2[-] metastatic breast cancer”: Abstract Oral Session ID 312547.
•San Antonio Breast Cancer Symposium (SABCS®) 2022. December 6–10, 2022. “Impact of Proton Pump Inhibitors (PPI) on Palbociclib (PAL) Outcomes in Hormone Receptor-Positive, HER2-Negative Advanced Breast Cancer (HR+/HER2- ABC): Exploratory Analysis of the PARSIFAL Trial”: Abstract Poster Session ID 1699.
1. Introduction
Proton pump inhibitors (PPI) are the most widely prescribed drugs for acid-related diseases [1,2] due to superior efficacy compared to other anti-acids, low cost, and over-the-counter availability in some countries [3,4]. More than one-quarter of patients on anticancer treatment use PPI [5], mainly as prophylaxis for non-steroidal anti-inflammatory and steroid drug-associated upper gastrointestinal complications.
However, long-term suppression of gastric acids may reduce the absorption of weakly basic substances (including many anti-tumor agents) which effects efficacy, and ultimately clinical outcomes for cancer patients [5,6].
The oral cyclin-dependent kinase 4/6 inhibitors (CDK4/6i) palbociclib, ribociclib, and abemaciclib, block the transition of cell cycle phases [7]. In hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2-) advanced breast cancer (ABC) the combination of CDK4/6i with endocrine therapy proved to be superior to endocrine therapy alone in prolonging survival and has become the new standard of care [8]. The absorption of CDK4/6i has been extensively evaluated [[9], [10], [11], [12]]. Much of these data stem from early clinical development, wherein gastric pH elevations failed to impair CDK4/6i ability to dissolve and reach systemic circulation [[9], [10], [11]]. Only the solubility of the capsule formulation of palbociclib appeared to be reduced at pH values above 4.5 but this was mitigated by food intake [12].
Retrospective observational studies have reported that concomitant use of PPIs and CDK4/6i leads to decreased efficacy [13,14]. Although PPI use is highly prevalent among ABC patients, all CDK4/6i pivotal studies excluded or limited the use of PPI, therefore there is little evidence of the clinical effects of the potential interaction between both agents. In this analysis, we aimed to investigate the effects of PPI on palbociclib capsule efficacy and safety in HR+/HER2- ABC patients treated within the PARSIFAL study, where the use of PPI was allowed [15].
2. Materials and methods
2.1. Study design and patients
PARSIFAL (NCT02491983) was an international, multicenter, open-label, randomized, phase II clinical trial designed to compare the efficacy and safety of palbociclib combined with fulvestrant or letrozole in women of any menopausal status with HR+/HER2- ABC and no previous systemic therapy for advanced disease. Palbociclib capsules were used in this study and were recommended under fed conditions and PPI use was permitted. An institutional review board/independent ethics committee approved the protocol; the study was conducted according to current ethical principles as set out by the Declaration of Helsinki and Good Clinical Practice. All patients were provided written informed consent before any study procedures. Additional patient eligibility criteria and study design details have been previously described [15].
2.2. Data source for post hoc analysis
All patients in the intention-to-treat set of the PARSIFAL study were included in this exploratory analysis. Records of concomitant medication were reviewed to identify patients who had a PPI prescription of omeprazole, pantoprazole, lansoprazole, rabeprazole, or esomeprazole. They included the drug, dose, and dates of treatment. Patients who received at least one dose of PPI during the palbociclib-based regimen were defined as PPI or PPI naïve (N-PPI) if no PPI was administered over the study treatment period. Early PPI users (E-PPI) were patients already on PPI at palbociclib initiation. Long-term PPI users (LT-PPI) were those who received PPI for at least two-thirds of their treatment with palbociclib.
2.3. Outcomes
PPI use was evaluated with respect to patient demographics and baseline disease characteristics. Investigator-assessed progression-free survival (PFS) was defined as the time from the date of randomization to the date of first documentation of objective progression of disease or death from any cause in the absence of documented progression of disease, whichever occurred first. Overall survival (OS) was defined as the time from randomization to death from any cause. Safety and tolerability of palbociclib plus endocrine therapy was assessed by using Common Terminology Criteria for Adverse Events (CTCAE) version 4.0, including incidence of hematological adverse events (AEs), or number of patients requiring palbociclib dose reductions or schedule delay due to hematological toxicity.
2.4. Statistical analysis
Efficacy and safety were assessed in three analysis sets. First, the early set for comparing N-PPI versus E-PPI groups. This set excluded patients that started PPI treatment after palbociclib initiation because only survivors without progressive disease could start a concomitant treatment after palbociclib initiation (immortal time bias). Second, the long-term set for comparing N-PPI versus LT-PPI patients. This set excluded patients treated with low exposure to the PPI treatment. Thirdly, the intent-to-treat set, which included all patients who had undergone randomization in the PARSIFAL trial. This analysis was planned to avoid selection bias. Moreover, we adjusted for multiplicity declaring a positive achievement only when all three analyses simultaneously yielded statistically significant results.
Investigator-assessed PFS based on concomitant use of PPI was assessed by Cox regression proportional-hazards models adjusted for patient characteristics to reduce bias from confounding factors such as age, Eastern Cooperative Oncology Group (ECOG) performance status, body mass index (BMI), type of disease, disease site, and number of disease sites. The survival estimates were calculated using the Kaplan-Meier method and 95% confidence intervals (CIs). The p-values and 95% CIs for Hazard Ratio [HR] were calculated using the Wald test. The Breslow method for tie handling in survival analysis was used. The interaction terms between patient characteristics and concomitant use of PPI were evaluated in a model-based likelihood ratio test. Immortal time bias and misclassification for the intention-to-treat population were addressed by analyzing PFS with landmark analysis. We compared Kaplan–Meier estimates for the percentage of patients who were alive without disease progression at 12 months (1-year PFS) after different landmark times post randomization (3, 6, 12, 18, 24, and 30 months). Patients whose treatment duration was shorter than the landmark time were excluded. Patients who started PPI treatment before the landmark were assigned to the group of PPI users, while those who did not receive PPI before the landmark were assigned to the group of N-PPI. A similar approach was used for OS analysis. We analyzed Kaplan–Meier estimates for the percentage of patients who were alive at 3 years after the planned landmark times (3-year OS rate). The safety analysis was conducted with logistic regressions models adjusted for patient characteristics. The interaction between endocrine therapy and co-administration of PPI was evaluated in a model-based likelihood ratio test. For all endpoints two-sided p-values with an alpha ≤0.05 level of significance and 95% CI were used. The analysis of interaction terms was set at 0.1 alpha level. As the primary objective of the PARSIFAL trial was not met, CIs and p-values in these post hoc analyses are intended to be descriptive. Analyses were done using the R software version 4.0.2. released on June 22, 2020.
3. Results
3.1. Study patients
Of 486 patients randomized in the PARSIFAL study, 325 (66.9 %) were N-PPI. Among 161 (33.1 %) PPI users, 64 (13.2 %) were E-PPI, 91 (18.7 %) were LT-PPI, and 56 (11.5 %) were other PPI users. Omeprazole was the most prescribed PPI (80.7 %). Median PPI use was 15.9 months (interquartile range [IQR] 0.9–52.2) for E-PPI, and 19.4 months (IQR 0.9–52.2) for LT-PPI. Compared with N-PPI, E-PPI and LT-PPI were older (median age, 60.5 versus [vs] 66.5 vs 67.0 years, respectively; p < 0.001), had a worse functional status (ECOG Performance Status of 0, 60.0 % vs 34.0 % vs 43.0 %, respectively; p = 0.002), and had greater BMI (median BMI (Kg/m2) of 26.2 vs 26.9 vs 27.7; respectively; p = 0.009). Demographic and baseline disease characteristics in the overall PARSIFAL population and according to PPI use are reported in Table 1. Additional baseline characteristics are in Supplementary Table 1, with a summary of baseline characteristics for all patients combined and the group of ‘other PPI users’ in Supplementary Table 2.
Table 1.
Patient characteristics at baseline.
| Characteristic | N-PPI (n = 325) | PPI users (n = 161) | E-PPI (n = 64) | LT-PPI (n = 91) |
|---|---|---|---|---|
| Age, years, median (range) | 60 (25–90) | 66 (34–88) | 67.5 (34–88) | 68 (34–88) |
| P value | ref. | <0.001 | <0.001 | <0.001 |
| Race | ||||
| Asian | 1 (0.3) | 2 (1.2) | 0 | 0 |
| Black | 3 (0.9) | 1 (0.6) | 0 | 0 |
| White | 306 (94.2) | 155 (96.3) | 64 (100) | 91 (100) |
| Unknown | 15 (4.6) | 3 (1.9) | 0 | 0 |
| P-value | ref. | 0.273 | 0.269 | 0.134 |
| ECOG performance status | ||||
| 0 | 195 (60) | 80 (49.7) | 22 (34.4) | 39 (42.9) |
| 1 | 120 (36.9) | 67 (41.6) | 36 (56.2) | 42 (46.2) |
| 2 | 10 (3.1) | 14 (8.7) | 6 (9.4) | 10 (11) |
| P-value | ref. | 0.009 | <0.001 | <0.001 |
| BMI kg/m2, median (range) | 26.2 (15.1–44.6) | 27.6 (16.2–47.6) | 26.9 (16.2–43.8) | 27.7 (17.4–42.4) |
| ref. | 0.009 | 0.19 | 0.006 | |
| ≥30 | 80 (24.6) | 49 (30.4) | 18 (28.1) | 26 (28.6) |
| <30 | 245 (75.4) | 112 (69.6) | 46 (71.9) | 65 (71.4) |
| P value | ref. | 0.208 | 0.665 | 0.529 |
| Menopausal status | ||||
| Premenopausal | 29 (8.9) | 8 (5) | 3 (4.7) | 3 (3.3) |
| Postmenopausal | 296 (91.1) | 153 (95) | 61 (95.3) | 88 (96.7) |
| P-value | ref. | 0.172 | 0.38 | 0.119 |
| Duration of palbociclib treatment, months, median (IQR) | 25.5 (12.2–34.6) | 24.6 (11.8–33.8) | 20.4 (9–28.6) | 20 (9.5–31.2) |
| P-value | ref. | 0.654 | 0.015 | 0.014 |
| HT administered in combination with palbociclib | ||||
| Fulvestrant | 153 (47.1) | 90 (55.9) | 35 (54.7) | 53 (58.2) |
| Letrozole | 172 (52.9) | 71 (44.1) | 29 (45.3) | 38 (41.8) |
| P-value | ref. | 0.083 | 0.329 | 0.078 |
| Type of disease | ||||
| De novo | 130 (40) | 68 (42.2) | 25 (39.1) | 36 (39.6) |
| Recurrent | 195 (60) | 93 (57.8) | 39 (60.9) | 55 (60.4) |
| P-value | ref. | 0.708 | 1 | 1 |
| Disease site | ||||
| Visceral | 161 (49.5) | 72 (44.7) | 30 (46.9) | 47 (51.6) |
| Nonvisceral | 164 (50.5) | 89 (55.3) | 34 (53.1) | 44 (52.7) |
| P-value | ref. | 0.366 | 0.8 | 0.813 |
| Number of disease sites | ||||
| <3 | 189 (58.2) | 85 (52,8) | 30 (46.9) | 43 (47.3) |
| ≥3 | 136 (41.8) | 76 (47.2) | 34 (53.1) | 48 (52.7) |
| P-value | ref. | 0.306 | 0.127 | 0.083 |
| Measurable disease | ||||
| Yes | 255 (78.5) | 121 (75.2) | 45 (70.3) | 69 (75.8) |
| No | 70 (21.5) | 40 (24.8) | 19 (29.7) | 22 (24.2) |
| P-value | ref. | 0.481 | 0.209 | 0.694 |
| Previous treatment with PPI | ||||
| No | 320 (98.5) | 87 (54.0) | 5 (7.8) | 33(36.3) |
| Yes | 5 (1.5) | < 74 (46.0) | 59 (92.2) | 58 (63.7) |
| P-value | ref. | <0.001 | <0.001 | <0.001 |
| Duration of previous PPI treatment, n | ||||
| Months, median (IQR) | 2.1 (1.1-2.4) | 8.8 (1.6-49.7) | 9.1(1.6-53.9) | 9.2 (1.4-58.2) |
| P-value | ref. | 0.255 | 0.271 | 0.258 |
| Type of concomitant PPI | ||||
| Omeprazole | 0 | 130 (80.7) | 54 (84.4) | 74 (81.3) |
| Pantoprazole | 0 | 33 (20.5) | 6 (9.4) | 19 (20.9) |
| Esomeprazole | 0 | 24 (14.9) | 4 (6.2) | 14 (15.4) |
| Lansoprazole | 0 | 11 (6.8) | 3 (4.7) | 6 (6.6) |
| Rabeprazole | 0 | 3 (1.9) | 2 (3.1) | 3 (3.3) |
| Treatment duration with concomitant PPI, n | ||||
| Months, median (IQR) | 0 | 11 (0.9–52.2) | 15.4 (2.4–52.2) | 18.1 (9.1–52.2) |
| Time to PPI treatment start since randomization, n | ||||
| Months, median (IQR) | 0 | 1 (0–13.5) | 0 | 0 (0–2.7) |
NOTE. Data presented as No. (%) unless otherwise noted.
Abbreviations: E-PPI, early PPI users; ECOG, Eastern Cooperative Oncology Group; HT, Hormone therapy; IQR, Interquartile range, defined as percentile 25 and percentile 75; LT-PPI, Long-term PPI users; N-PPI, PPI naïve; PPI, Proton-pump-inhibitors; Range, minimum and maximum values; Ref, reference category.
3.2. PPI use and palbociclib efficacy
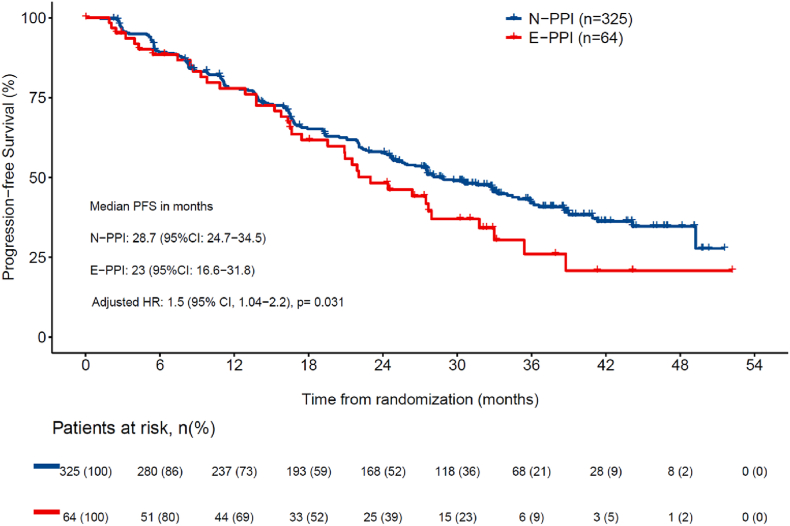
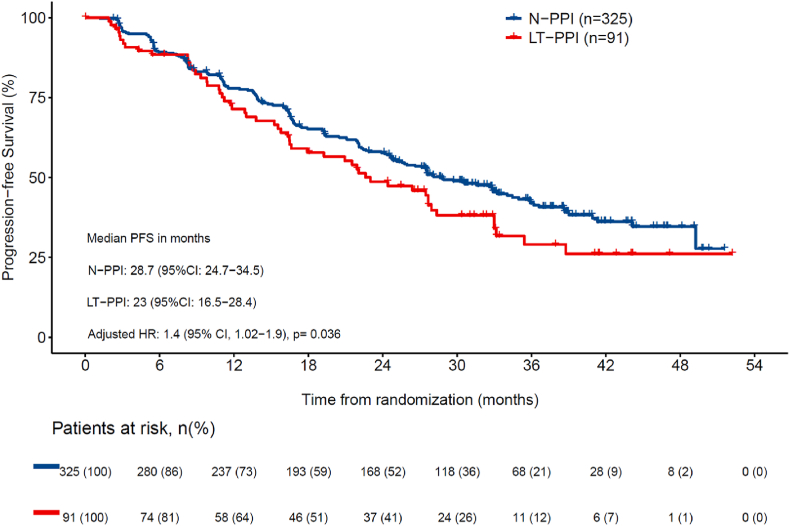
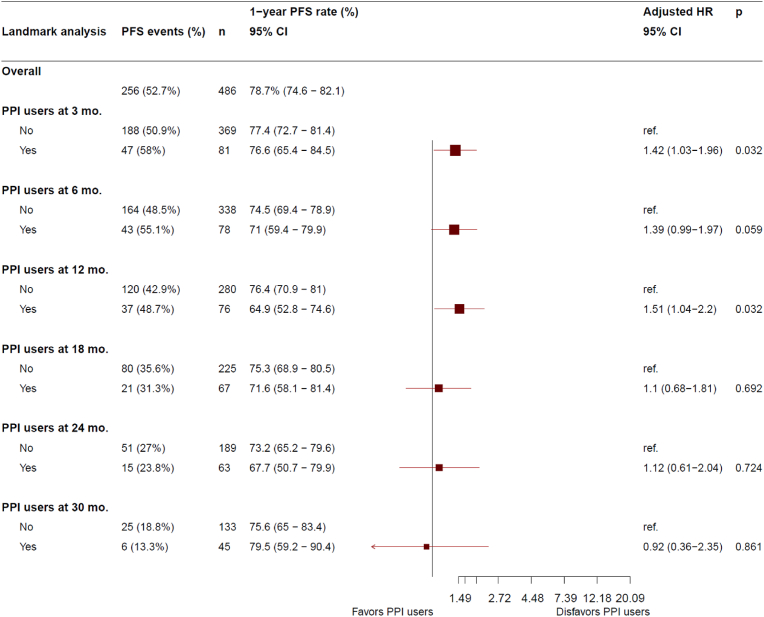
Data cutoff was January 31, 2020, when the target number of PFS events (n = 256) was met. There were 175 (53.8 %) events for N-PPI and 81 (50.3 %) for PPI users (38 [59.4 %] in E-PPI and 54 [59.3 %] in LT-PPI]). Median follow-up for the whole study population was 32.0 months. Median PFS was statistically significantly longer for the N-PPI, with a PFS of 28.7 months (95%CI 24.7–34.5), compared to 23.0 months (95%CI 16.5–28.4) in E-PPI (HR 1.5; 95%CI 1.0–2.2; p = 0.031) (Fig. 1a), and 23.0 months (95%CI 16.5–28.4) in LT-PPI (HR 1.4; 95%CI 1.02–1.9; p = 0.036) (Fig. 1b). In the landmark comparison for patients who had at least a 3-month follow-up, 1-year PFS rate was also statistically significantly higher (HR 1.4; 1.0–2.0; p = 0.032), with a rate of 77.4 % (95%CI 72.7–81.4) for N-PPI, compared to 76.6 % (95%CI 65.4–84.5) in PPI users. Landmark comparison for patients with at least 12 months of follow-up also showed a statistically significant difference (HR 1.5; 95%CI 1.0–2.2; p = 0.032), with a 1-year PFS rate of 76.4 % (95%CI 70.9–81) in N-PPI compared to 64.9 % (95%CI 52.8–74.6) in PPI users (Fig. 2).
Fig. 1a.
Progression-free survival between N-PPI and E-PPI users 95 % CI: 95 % confidence interval; HR: Hazard ratio; E-PPI, Early PPI user; N-PPI, PPI naïve; PFS: Progression-free survival; PPI: Proton pump inhibitors.
Fig. 1b.
Progression-free survival between N-PPI and LT-PPI users. Abbreviations: 95 % CI, 95 % confidence interval; HR, Hazard ratio; LT-PPI, Long term PPI user; N-PPI, PPI naïve; PFS, progression-free survival; PPI, proton pump inhibitors.
Fig. 2.
Landmark analysis of progression-free survival at 3, 6, 12, 18, 24, and 30 months in N-PPI and PPI users. Abbreviations: 95 % CI, 95 % confidence interval; HR, Hazard ratio; Mo, months; N-PPI, PPI naïve; Nr, number; PFS, progression-free survival; PPI, proton pump inhibitors; Ref: reference category.
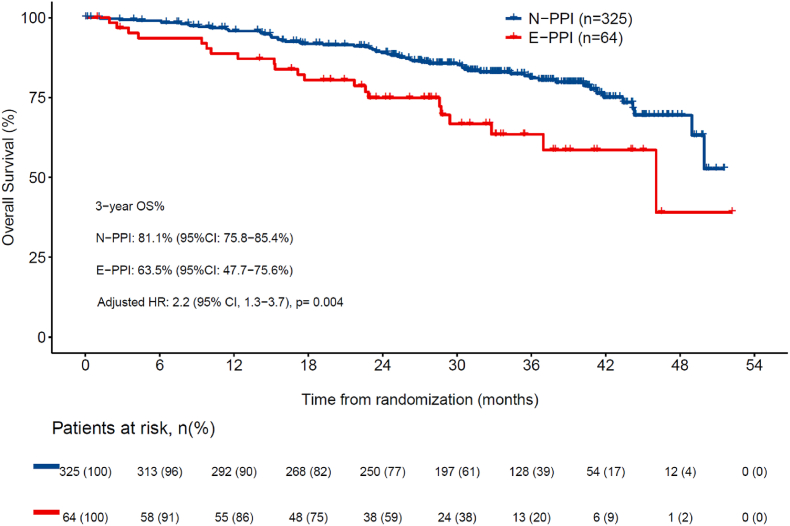
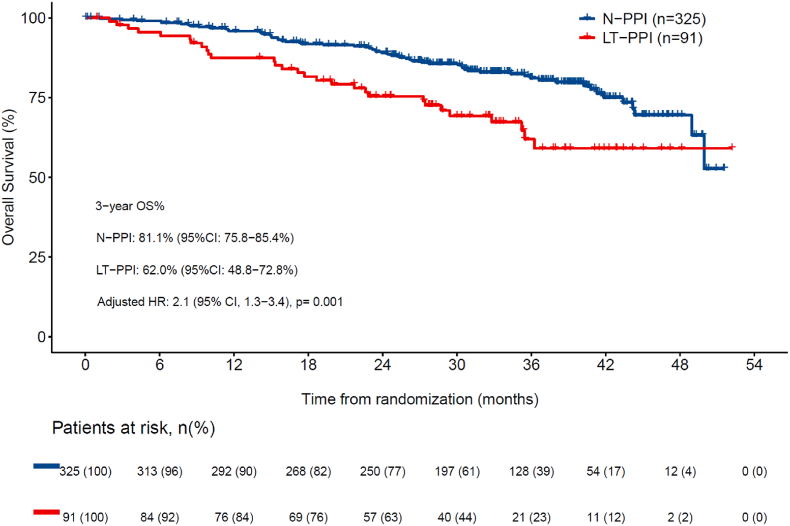
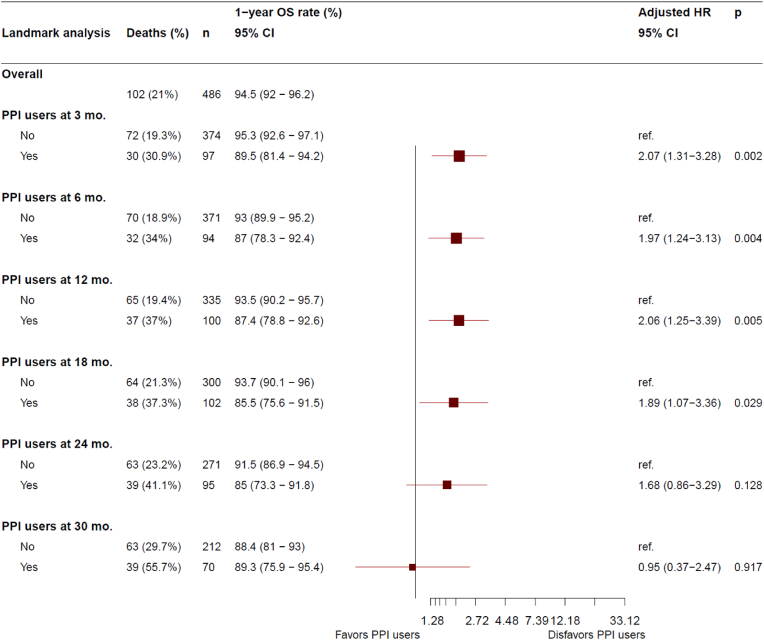
The number of deaths at data cutoff was 102 (21.0 %), with 62 (19.1 %) in N-PPI, and 40 (24.8 %) in PPI (21 [32.8 %] in E-PPI, and 29 [31.9 %] in LT-PPI). Three-year OS rate was statistically higher in N-PPI, with a rate of 81.1 % (95%CI 75.8–85.4) compared to 63.5 % (95%CI 47.7–75.6) in E-PPI (HR 2.2; 95%CI 1.3–3.7; p = 0.004) (Fig. 3a) and 62.0 % (95%CI 48.8–72.8) in LT-PPI (HR 2.1; 95%CI 1.3–3.4; p = 0.001) (Fig. 3b). In the landmark comparison for patients who had at least a 3-month follow-up, 1-year OS rate was also statistically higher for N-PPI, with a rate of 95.3 % (95%CI 92.6–97.1) compared to 89.5 % (95%CI, 81.4%–94.2 %) in PPI users (HR 2.1; 95%CI 1.3–3.3; p = 0.002). Landmark comparisons for patients who had at least a 6-, 12-, and 18-months follow-up also showed 1-year OS rate that statistically favored N-PPI, with a rate of 93.0 %, 93.5 %, and 93.7 %, respectively, in N-PPI compared with 87.0 % (p = 0.004), 87.4 % (p = 0.005), and 85.5 % (p = 0.03), respectively, for PPI users (Fig. 4).
Fig. 3a.
Overall survival between N-PPI and E-PPI users. 95 % CI: 95 % confidence interval; HR: Hazard ratio; E-PPI, Early PPI user; N-PPI, PPI naïve; OS: overall survival; PPI: Proton pump inhibitors.
Fig. 3b.
Overall survival between N-PPI and LT-PPI users. 95 % CI: 95 % confidence interval; HR: Hazard ratio; LT-PPI, Long term PPI user; N-PPI, PPI naïve; OS, overall survival; PPI: Proton pump inhibitors.
Fig. 4.
Landmark analysis of overall survival at 3, 6, 12, 18, 24, and 30 months in N-PPI and PPI users. 95 % CI: 95 % confidence interval; HR: Hazard ratio; Mo: months; N-PPI, PPI naïve; Nr. Number; PFS: Progression-free survival; PPI: Proton pump inhibitors; ref: reference category.
3.3. PPI use and palbociclib safety
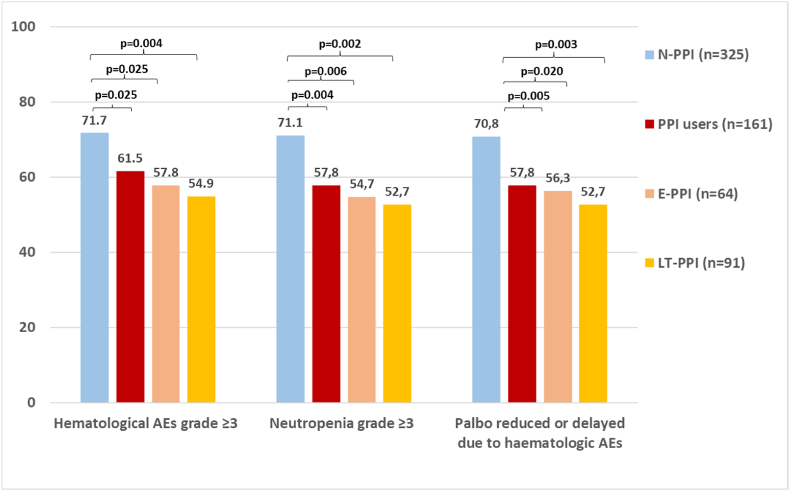
PPI use and safety data is reported in Fig. 5. The incidence of grade 3 or 4 hematological AEs was statistically significantly higher (p = 0.025) for N-PPI, with a rate of 71.7 % (95%CI 66.5–66.5) in N-PPI compared to 61.5 % (95%CI 53.5–69.0; p = 0.025) in all PPI users (E-PPI: 57.8 %, p = 0.025; LT-PPI: 54.9 %; p = 0.003). Grade 3 or 4 neutropenia occurred statistically more frequently (p = 0.003) for N-PPI (71.1 %; 95%CI 65.8–75.9) compared to PPI users (57.8 %; 95%CI, 49.7–65.5). Additionally, dose reductions and dosing delays due to hematological toxicity were also statistically more common (p = 0.005) for N-PPI (70.8 %; 95%CI 65.5–75.7) than for PPI users (57.8 %; 95%CI 49.7–65.5).
Fig. 5.
Hematological adverse events in N-PPI and PPI users. Abbreviations: AEs, Adverse events; E-PPI, early PPI users; LT-PPI, Long-term PPI users; N-PPI, PPI naïve users; PPI: Proton pump inhibitors.
3.4. Subgroup analyses
Subgroup analysis on progression-free survival was performed on N-PPI compared to both E-PPI (Supplementary Fig. 1) and to LT-PPI (Supplementary Fig. 2). For N-PPI vs. E-PPI, a statistically significant interaction (p = 0.05) was noted between relative dose intensity of palbociclib (≥95 % vs. <95 %) and PPI coadministration for PFS. Patients with a relative dose intensity of palbociclib ≥95 % had a median PFS of 29.7 months (95%CI 23.9–NA) for N-PPI compared to 21.5 months (95%CI 9.3–33.0) for E-PPI (HR 2.5; 95%CI 1.4–4.5; p = 0.003). This effect was not seen in N-PPI vs. LT-PPI use (Supplementary Fig. 2). PPI use did not have an observed effect for patients with relative dose intensity of palbociclib <95 % for either type PPI user. Another statistically significant interaction emerged between type of disease (de novo vs. recurrent) and PPI coadministration for PFS (N-PPI vs. E-PPI; p = 0.012; Supplementary Fig. 1). For patients with de novo disease, median PFS was longer for N-PPI, with a PFS of 31.6 months (95%CI 24.6–40.9) compared to only 20.9 months (95%CI 15.2–24.4) in E-PPI (HR 2.6; 95%CI 1.5–4.3; p < 0.001). No difference was noted for patient with recurrent disease. Similar interactions with type of disease were observed for N-PPI vs. LT-PPI (p = 0.024). In patients with de novo disease, median PFS was 31.6 months (95%CI 24.6–40.9) for N-PPI compared to only 16.6 months (95%CI 10.8–24.4) in LT-PPI (HR 2.2; 95%CI 1.4–3.5; p = 0.001) (Supplementary Fig. 2). No other significant interactions were noted in the rest of the baseline characteristics (Supplementary Figs. 1 and 2).
4. Discussion
This exploratory analysis suggests that PPI use could be associated with reduced palbociclib capsule efficacy, hematological toxicities, and dose modifications in HR+/HER2- ABC patients, which were evident in early and/or sustained PPIs users.
Preclinical models and studies with healthy volunteers demonstrate that the reduction in absorption of palbociclib capsule at pH values above 4.5 is abrogated by food intake [12]. Therefore, PPI use is not contraindicated for palbociclib capsules when taken with food. However, two retrospective studies reported a detrimental effect of PPI on the efficacy of CDK4/6i regimens in ABC patients, particularly palbociclib [13,14]. Our exploratory analysis on the PARSIFAL study seems to show that patients using PPI experienced less toxicities and dose reductions but also a shorter PFS with palbociclib capsules. While we recognize that patients on PPI were elderly and had a worse performance status which could account for the differences in PFS, it could not explain the improved safety profile. However, we lack the pharmacokinetic data to further explore this observation.
The strengths of this study include the sample size, prospective cohort design, the adjustment for main confounders, and an advanced analytical model. We used PARSIFAL longitudinally collected data from 486 patients on concomitant medications, including PPI- type, duration, and timing of use. The number of patients enrolled allowed us to mitigate challenges posed by drug-drug interaction studies [16]. Specifically, the potential immortal time bias and misclassification were handled using landmark analyses. If we had considered PPI users as being exposed from the date of entry into the PARSIFAL study, we would have introduced an immortal time bias because progression and/or death cannot occur before receiving the first PPI dispensation after enrollment [17]. Additionally, it is important to account for a latency period of the drug effect and reduce the potential protopathic bias between symptoms of gastroesophageal reflux disease and PPI prescription [18].
The PARSIFAL study did not achieve its primary endpoint, finding comparable efficacy between letrozole and fulvestrant [15]. The current analysis does not compromise these results, as no trends were noted between PPI use and either drug. Both endocrine therapies pointed in the same direction, further confirming the already known drug-drug interaction between concomitant use of PPI and the use of palbociclib capsule formulation.
Real-world studies have explored palbociclib and PPI interactions. Del Re and colleagues analyzed 112 endocrine sensitive or resistant patients with 50.0 % of them being on long PPI and saw a strong deleterious impact on PFS (HR 2.77) [13]. Eser and colleagues explored the effect of PPI on palbociclib or ribociclib regimens in 217 patients and noted stronger differences (HR 7.85 for palbociclib, HR 2.90 for ribociclib) [14]. In our study, differences related to PPI use are less significant (HR 1.5), potentially due to the previous studies being retrospective and less patients. In PARSIFAL, omeprazole was the most prevalent PPI agent whereas more potent PPIs as lansoprazole or pantoprazole were commonly used in the retrospective studies [19]. Finally, unlike the PALOMA-2 and 3 studies, which had an early amendment prohibiting the use of PPIs, the PARSIFAL study permitted the concomitant use of palbociclib capsules and PPI but patients were instructed to take palbociclib capsules under fed conditions [20,21], although patients could not have followed the recommendations, and may be a limiting factor of the study.
We performed two different analyses on PPI exposure to provide conclusive evidence of a relationship between time exposure and outcome. The first analysis was on patients already on a PPI at the time of inclusion, defined as E-PPI, and the second on patients that were on a PPI at least two thirds of the time that they were taking palbociclib (LT-PPI). In both analyses, after considering other prognostic factors in multivariable analysis, the effects of PPI on PFS outcomes were consistent; with an HR of 1.5 and 1.4, respectively. Interestingly, among 56 PPI users defined as neither E-PPI nor LT-PPI, the median time on PPI was only 1.4 months, and the median PFS was 31 months. This PFS was superior to any other patient group, including those PPI naïve, suggesting a possible immortal time bias effect.
Although our analysis identified a potential impact of PPI use on OS, differences in patient characteristics, particularly age and performance status, may have played a role. Additionally, the number of events at the time of the final analysis was still small. Of note, palbociclib has not shown a significant OS gain in any of the two registration studies. The reasons behind this lack of benefit may be the broad patient criteria in PALOMA-3 and the lower power for statistical analysis of OS in PALOMA-2 [21]. However, large real-world studies report a clear benefit in survival for palbociclib in the general population [22].
It is intriguing that this analysis found an association between PPIs treatment with reduced palbociclib capsule efficacy is stronger in patients with de novo advanced breast cancer, however, it is based on a post-hoc subgroup analysis and should be considered with caution given the small sample size.
Other oral anticancer agents with pH-dependent absorption have also reported possible negative effect of PPI coadministration and recommend avoiding PPI use concomitantly with anticancer treatments [[23], [24], [25], [26]]. It's important to note that the PARSIFAL study utilized palbociclib capsules but a new tablet formulation replacing capsules has been released and is associated with a lower interaction with PPI. In healthy subjects, a single 125 mg palbociclib capsule with multiple doses of rabeprazole under fasted conditions decreased palbociclib absorption but administration of a single 125 mg palbociclib tablet under the same conditions had no effect [12]. Additionally, a recent retrospective found that PPI use with palbociclib tablets did not significantly reduce PFS in patients with HR+/HER2 ABC [27].
Since the solubility and absorption of CDK4/6 inhibitors depends on different pH, it is important to consider drug-drug interactions. PPI cause gastric pH changes and therefore may alter the oral bioavailability of agents that have pH dependent solubility. However, the interactions appear to be different among the three main CDK4/6 inhibitors specifically when palbociclib is in the capsule formulation. It has been noted that ribociclib exhibits different dissolution properties than palbociclib capsules, and therefore concomitant PPI use has been found to have less or no effect on ribociclib efficacy [14,28,29]. Similarly, no effect has been noted with abemaciclib [30]. It should be noted that the pharmaceutical formulation (capsules vs. tablets) impacts absorption and consequent efficacy. For palbociclib, concurrent PPI use with palbociclib capsules has been associated with reduced PFS, an effect not observed with concurrent PPI use and palbociclib tablets [27].
Potential interactions from PPIs were not suspected when the study was designed, therefore, indications for PPI use were not collected and may be a confounding factor. Given the retrospective nature of this exploratory study, inaccuracies regarding concomitant medication may have occurred and the use of over-the-counter PPIs could have not been captured during the study's implementation. The lack of pharmacokinetic data prevented us from fully corroborating an overall neutral effect and a possible detrimental effect due to reduced absorption among elderly or functionally impaired patients independently of PPI.
In conclusion, this exploratory analysis of PARSIFAL suggests a detrimental effect of PPI on the anti-tumor efficacy of palbociclib capsule in unadjusted, adjusted, and timing-dependent covariate survival analyses. Studies on oral therapies should consider concomitant therapies, especially for frailer populations who are higher consumers of drugs but also seem particularly susceptible to drug interactions.
Data availability
Data are available from the authors upon reasonable request.
Funding
The PARSIFAL study was supported by Medica Scientia Innovation Research (MEDSIR) who sponsored the study; and funded by Pfizer. Fulvestrant was provided by AstraZeneca.
Ethical approval and consent to participate
An institutional review board/independent ethics committee approved the protocol; the study was conducted according to current ethical principles as set out by the Declaration of Helsinki and Good Clinical Practice. All patients were provided written informed consent before any study procedures.
CRediT authorship contribution statement
Serena Di Cosimo: Writing – original draft, Investigation. José Manuel Pérez-García: Writing – review & editing, Investigation, Conceptualization. Meritxell Bellet: Writing – review & editing, Investigation. Florence Dalenc: Writing – review & editing, Investigation. Miguel J. Gil Gil: Writing – review & editing, Supervision, Investigation. Manuel Ruiz-Borrego: Writing – review & editing, Supervision, Investigation. Joaquín Gavilá: Writing – review & editing, Investigation. Elena Aguirre: Writing – review & editing, Supervision, Conceptualization. Peter Schmid: Writing – review & editing, Supervision. Frederik Marmé: Writing – review & editing, Investigation. Joseph Gligorov: Writing – review & editing, Investigation. Andreas Schneeweiss: Writing – review & editing, Project administration, Investigation, Conceptualization. Joan Albanell: Writing – review & editing, Investigation. Pilar Zamora: Writing – review & editing, Supervision, Investigation, Conceptualization. Duncan Wheatley: Writing – review & editing, Supervision, Project administration, Investigation. Eduardo Martínez de Dueñas: Writing – review & editing, Investigation. Kepa Amillano: Writing – review & editing, Supervision, Investigation, Conceptualization. Eileen Shimizu: Writing – review & editing. Miguel Sampayo-Cordero: Writing – review & editing, Writing – original draft, Visualization, Methodology, Formal analysis. Javier Cortés: Writing – review & editing, Validation, Supervision, Methodology, Investigation, Conceptualization. Antonio Llombart-Cussac: Writing – review & editing, Supervision, Methodology, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
SDC reports consulting for MEDSIR and IQVIA and honoraria from Pierre-Fabre, Novartis, and AstraZeneca. JMPG reports consulting for Roche, Eiasi, Daichii Sankyo, AstraZeneca, Gilead, MSD and Seagen and travel expenses from Roche. MB reports consulting fees from Pfizer, Novartis, Lilly, and Steamline-Menarini; honoraria from Pfizer, Novartis, and Lilly; and trave support from Pfizer. FD reports travel grants for Daichii, Novartis, and Pfizer, and participation in monitor/advisory boards for Daiichi, Seagen, Novartis, Gilead, Lilly, and MSD. MGG reports honoraria from Pfizer, Novartis, and AstraZeneca, travel grants from Lilly, Daiichi-Sankyo, and Pfizer, and participation in monitoring/advisory boards for AstraZeneca, Seagen, and Gilead. MRB reports honoraria from Pfizer, Novartis, AstraZenca, and Daiichi Sankyo. JG reports consulting fees from Novartis, Pfizer, AstraZeneca; honoraria from Novartis; and travel support from Roche and AstraZeneca. EAO reports consulting for AstraZeneca, Daichii, Gilead, MSD, and Seagen; honoraria from AstraZeneca, Daichii, Lilly, MSD, and Seagen; travel support from Daichii, Lilly, Gilead, and AstraZeneca; and participation in monitor/advisory boards for Gilead, AstraZeneca, Seagen, and Dachii. PS reports honoraria from Pfizer, AstraZeneca, Novartis, Roche, Merck, and Boehringer Ingelheim; a consulting role for Pfizer, AstraZeneca, Novartis, Roche, Merck, Boehringer Ingelheim, Bayer, Eisai, Celgene, and Puma; and institutional grants from Roche, Genentech, Oncogenex, and Novartis. FM reports grants from Roche, Novartis, AstraZeneca, GSK, MSD, Clovis, Vaccibody, Gilead Sciences, and Esai; consulting fees from AstraZeneca, GSK, and Roche; honoraria from AstraZeneca, MSD, Lilly, Pfizer, Novartis, GSK, Clovis, Myriad, Daiich Sankyo, Seagen, Pierre-Fabre, and Agendia; travel support from AstraZeneca, GSK, Pfizer, and Roche; and participation in advisory boards for Palleos and Amgen. JG reports grant/research support from Roche, Eiasi, and Exact Science; honoraria or consultation fees from AstraZeneca, Daiichi, Eisai, Exact Science, Evapharm, GE Healthcare, Gilead, Lilly, MSD, Novartis, Pfizer, Pierre Fabre, Roche, Seagen, Sothema and participation in company sponsored speaker's bureaus for Exact Science, Lilly, and Novartis. AS reports grants from Celgene, Roche, Abbvie; travel grants from Celgene, Roche, Pfizer, AstraZeneca, and honoraria from Roche, Celgene, Pfizer, AstraZeneca, Novartis, MSD, Tesaro, Lilly, Seagen, Gilead, GSK, Bayer, Amgen, Pierre Fabre. JA reports trial funding from MEDSIR and consulting for Pfizer, Lilly, and Novartis. PZ reports honoraria from Pfizer, Novartis, Roche, and Pierre Fabre; and travel support from Pfizer, Novartis, Roche, and Lilly. DW reports honoraria from Pfizer, Novartis, AstraZeneca and travel grants from Roche, and Novartis. JC reports consulting for Roche, Celgene, Cellestia, AstraZeneca, Seattle Genetics, Daiichi Sankyo, Erytech, Athenex, Polyphor, Lilly, Merck Sharp&Dohme, GSK, Leuko, Bioasis, Clovis Oncology, Boehringer Ingelheim, Ellipses, Hibercell, BioInvent, Gemoab, Gilead, Menarini, Zymeworks, Reveal Genomics, Expres2ion Biotechnologies; honoraria from Roche, Novartis, Celgene, Eisai, Pfizer, Samsung Bioepis, Lilly, Merck Sharp&Dohme, Daiichi Sankyo.; research funding to the Institution from Roche, Ariad pharmaceuticals, AstraZeneca, Baxalta GMBH/Servier Affaires, Bayer healthcare, Eisai, F. Hoffman-La Roche, Guardanth health, Merck Sharp&Dohme, Pfizer, Piqur Therapeutics, Puma C, Queen Mary University of London; stock shares for MEDSIR, Nektar Pharmaceuticals, Leuko (relative); Travel, accommodation, expenses from Roche, Novartis, Eisai, Pfizer, Daiichi Sankyo, Astrazeneca, Gilead; and Patents: Pharmaceutical Combinations of A Pi3k Inhibitor And A Microtubule Destabilizing Agent. Javier Cortés Castán, Alejandro Piris Giménez, Violeta Serra Elizalde. WO 2014/199294 A. ISSUED Her2 as a predictor of response to dual HER2 blockade in the absence of cytotoxic therapy. Aleix Prat, Antonio Llombart, Javier Cortés. US 2019/0338368 A1. LICENSED. ALC reports research support from Roche, Agendia, Lilly, Pfizer, Novartis, Merck Sharp & Dohme, Gilead, and Daichii-Sanyo; consulting for Lilly, Roche, Pfizer, and Novartis; participation in speakers' bureaus for Lilly, AstraZeneca, Merck Sharp & Dohme; travel support from Roche, Pfizer, AstraZeneca; and stock or other ownership in MEDSIR and Initia-Research. The remaining authors declare no conflicts of interest.
Acknowledgements
We wish to thank the patients who kindly participated in our study and their families. We acknowledge the Investigators and their teams from the recruiting centers who enrolled patients in the PARSIFAL study. We would also like to thank the PARSIFAL steering committee, the trial unit staff at MEDSIR, Pfizer Inc, and AstraZeneca, who generously participated in this trial.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2024.103761.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Rui O., P T. National Ambulatory Medical Care Survey: 2015 State and National Summary Tables. 2015 [cdc.gov/nchs/ahcd/ahcd_ products.htm] [Google Scholar]
- 2.C. for M. and M. Services. 2., “Medicare Provider Utilization and Payment Data: pt D Prescriber CY 2015.” Accessed: December. 22, 2022. [Online]. Available: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Provider-Charge-Data/PartD2015.html.
- 3.Heidelbaugh J.J., Kim A.H., Chang R., Walker P.C. Overutilization of proton-pump inhibitors: what the clinician needs to know. Ther. Adv. Gastroenterol. Jul. 2012;5(4):219–232. doi: 10.1177/1756283X12437358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Food and Drug Administration Proton pump inhibitors BPCA drug use review and duration of use analysis 2010. 2010. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/UCM214657.pdf [Online]. Available:
- 5.Raoul J.-L., Guérin-Charbonnel C., Edeline J., Simmet V., Gilabert M., Frenel J.-S. Prevalence of proton pump inhibitor Use among patients with cancer. JAMA Netw Open. Jun. 2021;4(6) doi: 10.1001/jamanetworkopen.2021.13739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Savarino V., Marabotto E., Furnari M., Zingone F., Zentilin P., Savarino E. Latest insights into the hot question of proton pump inhibitor safety – a narrative review. Dig Liver Dis. Aug. 2020;52(8):842–852. doi: 10.1016/j.dld.2020.04.020. [DOI] [PubMed] [Google Scholar]
- 7.Asghar U., Witkiewicz A.K., Turner N.C., Knudsen E.S. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov. Feb. 2015;14(2):130–146. doi: 10.1038/nrd4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Groot A.F., Kuijpers C.J., Kroep J.R. CDK4/6 inhibition in early and metastatic breast cancer: a review. Cancer Treat Rev. Nov. 2017;60:130–138. doi: 10.1016/j.ctrv.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Clark A.S., et al. Palbociclib (PD0332991)—a selective and potent cyclin-dependent kinase inhibitor: a review of pharmacodynamics and clinical development. JAMA Oncol. Feb. 2016;2(2):253. doi: 10.1001/jamaoncol.2015.4701. [DOI] [PubMed] [Google Scholar]
- 10.Robert M., et al. Pharmacokinetic drug evaluation of abemaciclib for advanced breast cancer. Expet Opin Drug Metabol Toxicol. Feb. 2019;15(2):85–91. doi: 10.1080/17425255.2019.1559816. [DOI] [PubMed] [Google Scholar]
- 11.Curigliano G., Criscitiello C., Esposito A., Intra M., Minucci S. Pharmacokinetic drug evaluation of ribociclib for the treatment of metastatic, hormone-positive breast cancer. Expet Opin Drug Metabol Toxicol. May 2017;13(5):575–581. doi: 10.1080/17425255.2017.1318848. [DOI] [PubMed] [Google Scholar]
- 12.Sun W., et al. Impact of acid-reducing agents on the pharmacokinetics of palbociclib, a weak base with pH-dependent solubility, with different food intake conditions. Clin. Pharmacol. Drug Dev. Nov. 2017;6(6):614–626. doi: 10.1002/cpdd.356. [DOI] [PubMed] [Google Scholar]
- 13.Del Re M., et al. Drug-drug interactions between palbociclib and proton pump inhibitors may significantly affect clinical outcome of metastatic breast cancer patients. ESMO Open. Oct. 2021;6(5) doi: 10.1016/j.esmoop.2021.100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eser K., et al. Proton pump inhibitors may reduce the efficacy of ribociclib and palbociclib in metastatic breast cancer patients based on an observational study. BMC Cancer. Dec. 2022;22(1):516. doi: 10.1186/s12885-022-09624-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Llombart-Cussac A., et al. Fulvestrant-palbociclib vs letrozole-palbociclib as initial therapy for endocrine-sensitive, hormone receptor–positive, ERBB2 -negative advanced breast cancer: a randomized clinical trial. JAMA Oncol. Dec. 2021;7(12):1791. doi: 10.1001/jamaoncol.2021.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suissa S. Immortal time bias in pharmacoepidemiology. Am J Epidemiol. Jan. 2008;167(4):492–499. doi: 10.1093/aje/kwm324. [DOI] [PubMed] [Google Scholar]
- 17.Levesque L.E., Hanley J.A., Kezouh A., Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ. Mar. 2010;340(mar12 1) doi: 10.1136/bmj.b5087. b5087–b5087. [DOI] [PubMed] [Google Scholar]
- 18.Horwitz R.I., Feinstein A.R. The problem of ‘protopathic bias’ in case-control studies. Am J Med. Feb. 1980;68(2):255–258. doi: 10.1016/0002-9343(80)90363-0. [DOI] [PubMed] [Google Scholar]
- 19.Janczewska I., Sagar M., Sjösted S. Comparison of the effect of lansoprazole and omeprazole on intragastric acidity and gastroesophageal reflux in patients with gastroesophageal reflux disease. Scand J Gastroenterol. Jan. 1998;33(12):1239–1243. doi: 10.1080/00365529850172304. [DOI] [PubMed] [Google Scholar]
- 20.Finn R.S., et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925–1936. doi: 10.1056/NEJMoa1607303. 17. [DOI] [PubMed] [Google Scholar]
- 21.Turner N.C., et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. Nov. 2018;379(20):1926–1936. doi: 10.1056/NEJMoa1810527. [DOI] [PubMed] [Google Scholar]
- 22.DeMichele A., et al. Comparative effectiveness of first-line palbociclib plus letrozole versus letrozole alone for HR+/HER2− metastatic breast cancer in US real-world clinical practice. Breast Cancer Res. Dec. 2021;23(1):37. doi: 10.1186/s13058-021-01409-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee C.-H., et al. Proton pump inhibitors reduce the survival of advanced lung cancer patients with therapy of gefitinib or erlotinib. Sci Rep. Apr. 2022;12(1):7002. doi: 10.1038/s41598-022-10938-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlack K., et al. Effect of antacid intake on the therapeutic efficacy of sunitinib (SUN) in metastatic renal cell carcinoma (mRCC) patients (pts): a sub-analysis of the STAR-TOR registry. Ann Oncol. Oct. 2019;30:v386. doi: 10.1093/annonc/mdz249.051. [DOI] [Google Scholar]
- 25.Chu M.P., et al. Association of proton pump inhibitors and capecitabine efficacy in advanced gastroesophageal cancer: secondary analysis of the TRIO-013/LOGiC randomized clinical trial. JAMA Oncol. Jun. 2017;3(6):767. doi: 10.1001/jamaoncol.2016.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitazume Y., et al. Proton pump inhibitors affect capecitabine efficacy in patients with stage II–III colorectal cancer: a multicenter retrospective study. Sci Rep. Apr. 2022;12(1):6561. doi: 10.1038/s41598-022-10008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schieber T., et al. Effect of concurrent proton pump inhibitors with palbociclib tablets for metastatic breast cancer. Clin Breast Cancer. May 2023 doi: 10.1016/j.clbc.2023.05.009. S1526820923001313. [DOI] [PubMed] [Google Scholar]
- 28.Del Re M., et al. Concomitant administration of proton pump inhibitors does not significantly affect clinical outcomes in metastatic breast cancer patients treated with ribociclib. Breast. Dec. 2022;66:157–161. doi: 10.1016/j.breast.2022.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Çağlayan D., et al. The effect of concomitant use of proton pump inhibitors with CDK 4/6 inhibitors on survival in metastatic breast cancer. Eur J Clin Pharmacol. Feb. 2023;79(2):243–248. doi: 10.1007/s00228-022-03435-7. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi K., et al. Proton pump inhibitors and cyclin-dependent kinase 4/6 inhibitors in patients with breast cancer. Oncol. Feb. 2024:oyae015. doi: 10.1093/oncolo/oyae015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the authors upon reasonable request.