Abstract
Intestinal epithelial cells secrete a protective luminal mucus barrier inhibiting viral gene transfer. Quiescent, polarized monolayers of primary epithelial cells from dog gallbladder and human colon are efficiently transduced through the apical mucus side by lentivirus vectors, suggesting their application to intestinal gene therapy.
Cystic fibrosis (CF) is associated with the inherited absence of CF transmembrane conductance regulator (CFTR) and is characterized by thick, tenacious mucus that leads to respiratory failure (5, 17). In the intestine the absence of CFTR causes a multitude of abnormalities and can lead to complete obstruction of the gastrointestinal tract (5). As treatments for the lung dysfunction of CF patients have improved, patients are living longer and there is an increasing need for CFTR gene transfer to the intestine and pancreas (17). Unfortunately, gene transfer into epithelial cells has proven difficult. Transduction of epithelia from the luminal (apical) side is desirable because this side is the most accessible. However, many epithelial cells secrete mucin from the apical side to act as a protective barrier, and this mucus layer can inhibit viral transduction (1, 2, 14). As model tissues to study gene transfer into intestinal epithelia, we used human CaCo-2 cells (16) and primary dog gallbladder epithelial (DGBE) cells (13). Both types of cells form well-differentiated, highly polarized, leak-tight epithelia, and DGBE cells retain many of the functions of gallbladder epithelial cells, such as abundant mucin production and response to second messengers (6, 8, 10).
We hypothesized that lentivirus vectors based on human immunodeficiency virus type 1 (HIV) (7, 11, 12) may transduce intestinal epithelial cells through the apical membrane. We investigated the efficiency of gene transfer into quiescent polarized intestinal epithelial cells by first-generation (3) and third-generation (4, 18) lentivirus vectors. We compared lentivirus transduction efficiency with that of a Moloney murine leukemia retroviral vector using viruses pseudotyped with vesicular stomatitis virus G protein (VSV-G).
Viral preparations.
Viral preparations were generated by calcium phosphate cotransfections in 293T cells and were all pseudotyped with VSV-G. Moloney murine leukemia retroviral vector LNCeGFP had enhanced green fluorescent protein (GFP) expression under control of the cytomegalovirus (CMV) promoter (15). First-generation lentivirus pRtatpeGFP was generated by cotransfections of a Gag/Pol, Tat, Rev, Vpu, Vif packaging construct (3), and enhanced GFP expression was under control of the HIV long terminal repeat (LTR). Third-generation lentivirus vector pRRLcmveGFPsin was generated by cotransfection of a HIV Gag/Pol packaging construct and Rev expression vector (18), and enhanced GFP expression was under control of the CMV promoter. Titers of viral preparations were determined by using 105 HeLa cells and determining the fraction of GFP-positive cells 3 days after infection by fluorescence microscopy. Titers of 106 infectious units/ml were routinely obtained for all viruses.
Cell culture and viral infections.
DGBE cells and human CaCo-2 cells were cultured in Dulbecco's modified Eagle's medium (9). To determine transduction of dividing cells, 2 × 105 DGBE cells or CaCo-2 cells were infected at a multiplicity of infection of 2 for 4 hrs in the presence of 10μg/ml DEAE dextran. Cells were harvested by trypsinization 4 days later, fixed with 10% formalin in phosphate-buffered saline, and analyzed by flow cytometry. Cells were seeded on transwell membrane inserts with a 3-μm pore size and 24-mm diameter (Corning Costar, Cambridge, Mass.) and grown to confluence in 3 to 4 days. Viral transductions were performed by adding virus in 2 ml of culture medium with 10 μg of DEAE dextran per ml to the apical or basolateral compartment for 4 h. In all cases, 106 viral particles (multiplicity of infection, 0.3 to 0.5) were used per well and the cells were not washed so the mucus layer would not be disturbed. Control experiments were performed by adding a 40 μM concentration of the reverse transcriptase inhibitor zidovudine (AZT) (Glaxo-Wellcome, Research Triangle Park, N.C.) during and after infection into both compartments. Three days after infection, the cells were fixed in situ for 30 min with 4% paraformaldehyde in phosphate-buffered saline and mounted with buffer containing the nuclear stain 4,6-diamino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, Calif.). Cells were labeled with 80 μM bromodeoxyuridine (BrdU) (Sigma, St. Louis, Mo.) in the apical and basolateral compartments for 24 h to determine the fraction of dividing cells. BrdU labeling was initiated concurrently with viral infections, and incorporation was determined immunologically using tetramethyl rhodamine isothiocyanate-labeled secondary antibody (Dako, Carpinteria, Calif.).
Transduction of proliferating DGBE and CaCo-2 cells.
Dividing primary DGBE and CaCo-2 cells cultured on plastic were infected by the murine retroviral vector LNCeGFP and two different lentivirus vectors, pRtatpeGFP and pRRLcmveGFPsin, and GFP expression was analyzed by fluorescence-activated cell sorting. All viruses were very effective in transducing dividing DGBE and CaCo-2 cells (Table 1). The mean fluorescence intensity shows that in DGBE cells, GFP expression driven by the CMV promoter in LNCeGFP and pRRLcmveGFPsin and GFP expression driven by the HIV LTR in pRtatpeGFP are equivalent. In contrast, in the human CaCo-2 cell line, the CMV promoter seems to be weaker than the HIV LTR (Table 1).
TABLE 1.
Transduction of dividing CaCo-2 and DGBE cellsa
| Cells and vector | % GFP-positive cells | Mean fluorescence intensity |
|---|---|---|
| CaCo-2 | ||
| Untransfected | 2.5 | 23.1 |
| pLNCeGFP | 44 | 140.6 |
| pRtatpeGFP | 99 | 2,781 |
| pRRLcmveGFPsin | 76 | 287.6 |
| DGBE | ||
| Untransfected | 1 | 22.6 |
| pLNCeGFP | 89 | 2,229 |
| pRtatpeGFP | 87 | 2,498 |
| pRRLcmveGFPsin | 96 | 1,609 |
Subconfluent CaCo-2 and DGBE cells were infected with murine retrovirus LNCeGFP, first-generation lentivirus pRtatpeGFP, and third-generation lentivirus pRRLcmveGFPsin. Cells were analyzed by flow cytometry. All viral vectors transduced target cells efficiently. Mean fluorescence levels are similar in DGBE cells, and pRtatpeGFP mediates higher expression levels in CaCo-2 cells.
Characterization of polarized epithelial cells.
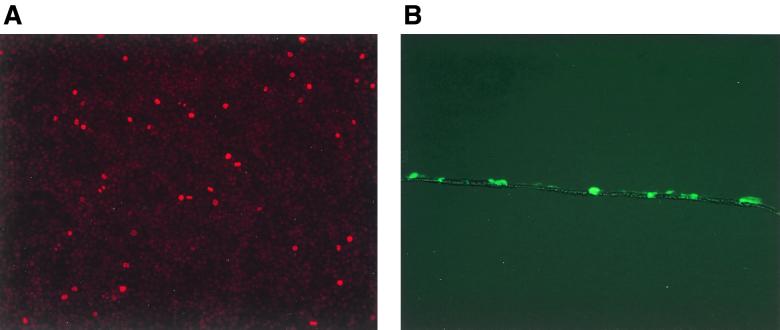
Cells grown on transwell membranes form highly polarized monolayers, allowing us to study infection through the basolateral and apical sides separately. To determine the fraction of polarized cells undergoing mitosis, confluent leak-tight monolayers of DGBE and CaCo-2 cells were labeled with BrdU for 24 h. BrdU labeling was started at the same time as the transduction and continued for 20 h afterwards. Only cells that divide in this 24-h period will incorporate BrdU (Fig. 1A). The average fraction of cells undergoing mitosis during and 20 h after transduction was 2.4% ± 0.7% (n = 3) for the DGBE cells and 2.2% ± 0.3% (n = 2) for the CaCo-2 cells. These data show low levels of proliferation in confluent polarized epithelial cells. A cross section of transduced DGBE cells on a transwell membrane shows a monolayer characteristic of differentiated epithelium (Fig. 1B). Mucin secretion by DGBE cells was confirmed by gel filtration of conditioned medium from DGBE cells that had been labeled with tritiated N-acetyl-d-glucosamine (9). Only conditioned medium from the apical side contained mucin, demonstrating unidirectional polarized secretion of mucin (not shown).
FIG. 1.
(A) BrdU labeling of DGBE cells. Confluent DGBE cells were labeled for 24 h with BrdU, and incorporation was detected using a mouse monoclonal anti-BrdU antibody and tetramethyl rhodamine isothiocyanate-conjugated anti-mouse immunoglobulins. Nuclei that have incorporated BrdU can be identified by their red fluorescence. A representative field is shown. Magnification, ×10. (B) Frozen cross sections of transwell membranes with transduced DGBE cells. Transwell membranes with DGBE cells transduced by pRtatpeGFP were fixed in 4% paraformaldehyde, embedded in Tissue-Tek O.C.T. compound (Sakura Finetek, Torrance, Calif.), frozen for 1 h at −80°C, and sectioned in a cryostat. A monolayer of cells covering the transwell membrane can be observed in a representative field with simultaneous fluorescent and bright-field illumination. Magnification, ×10.
Transduction of polarized intestinal epithelial cells with the murine retroviral vector LNCeGFP.
Transduction rates of CaCo-2 and DGBE cells with LNCeGFP were close to the fraction of dividing cells, i.e., 2.6% ± 2% and 2.9% ± 1%, respectively (Table 2). DGBE cells could be transduced by LNCeGFP with equal efficiency through the basolateral and apical sides, i.e., 2.4% ± 1% and 2.9% ± 1%, respectively, whereas transduction of CaCo-2 cells by the murine retroviral vector occurred only through the apical domain (Table 2). These experiments indicate that VSV-G-pseudotyped murine retroviral vectors can cross a mucus barrier and transduce dividing intestinal epithelial cells. Since DGBE cells were infected with equal efficiency through the apical and basolateral domains, these experiments also indicate that the presence of a transwell membrane at the basolateral surface does not act as a barrier to transduction.
TABLE 2.
Transduction of DGBE and CaCo-2 cells with lentivirus and murine retroviral vectorsa
| Cells and vector | % GFP-positive cellsb
|
|
|---|---|---|
| Apical infection | Basolateral infection | |
| CaCo-2 | ||
| pLNCeGFP | 2.6 ± 2 | <0.5 |
| pRtatpeGFP | 43 ± 17 | 4.7 ± 1 |
| pRRLcmveGFPsin | 24 ± 16 | <0.5 |
| DGBE | ||
| pLNCeGFP | 2.9 ± 1 | 2.4 ± 1 |
| pRtatpeGFP | 33 ± 25 | <0.5 |
| pRRLcmveGFPsin | 3.8 ± 0.5 | <0.5 |
Confluent monolayers of DGBE and CaCo-2 cells were transduced with first- and third-generation lentivirus vectors and a murine retroviral vector. Experiments were performed using at least two different viral preparations per virus type.
Results are averages and standard deviations from at least three different independent experiments.
Transduction of polarized intestinal epithelial cells with lentivirus vectors.
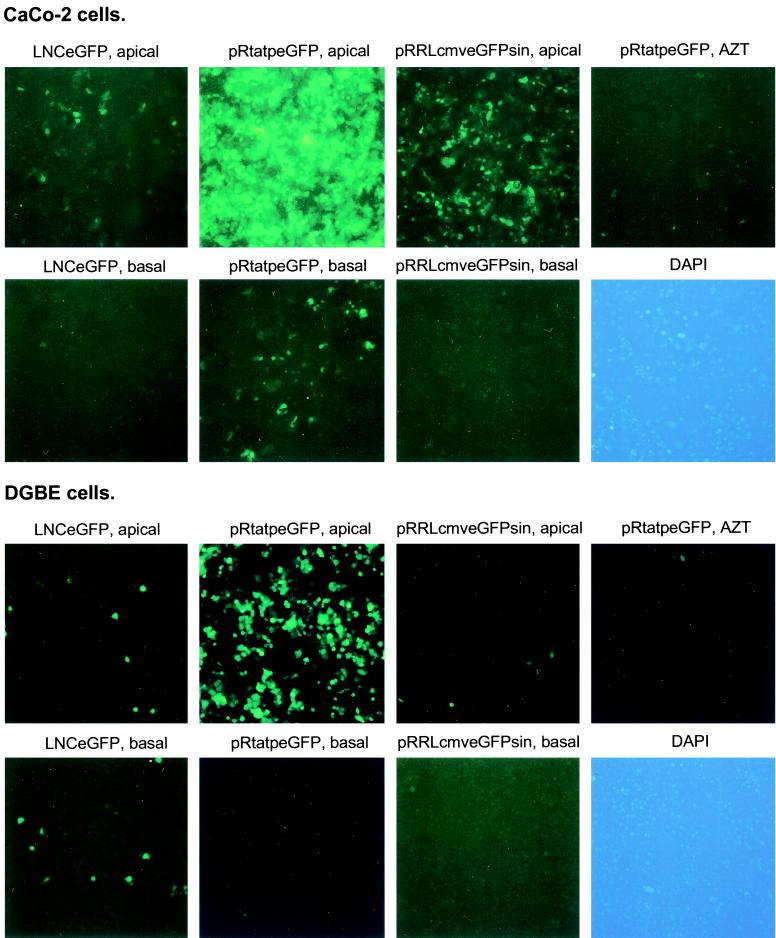
When CaCo-2 and DGBE cells were transduced through the apical compartment with the first-generation lentivirus vector pRtatpeGFP, 30 to 40% of the target cells became GFP positive, indicating that pRtatpeGFP is able to transduce quiescent epithelial cells (Table 2; Fig. 2). The HIV reverse transcriptase inhibitor AZT inhibited more than 99% of transduction (Fig. 2), indicating that viral transduction and not protein transfer or plasmid transfection had occurred. The third-generation lentivirus vector pRRLcmveGFPsin transduced quiescent CaCo-2 cells but not DGBE cells with greater efficiency than a murine retroviral vector, suggesting that the different lentivirus backbones and packaging systems might have different efficiencies in transducing nonhuman cells (Table 2; Fig. 2). Infection with lentivirus vectors was most efficient through the apical compartment, although 4.7% ± 1% of CaCo-2 cells were transduced by pRtatpeGFP through the basolateral side (Table 2; Fig. 2). DGBE cells were not significantly transduced through the basolateral side (Table 2; Fig. 2). Infections of subconfluent layers of DGBE cells on transwell membranes with pRtatpeGFP showed transduction efficiencies through the basolateral and apical sides of 31% ± 9% and 42% ± 6%, respectively. Thus the presence of a transwell membrane did not prevent viral particles from reaching the cells from the basolateral compartment.
FIG. 2.
Transduction of DGBE and CaCo-2 cells with lentivirus and murine retroviral vectors. DGBE and CaCo-2 cells were transduced and processed for microscopy. Exposure times were 10 s for all fields except DGBE cells transduced with pRtatpeGFP, which were exposed for 5s. The top two rows represent CaCo-2 cells, and the bottom two rows represent DGBE cells. The virus used and the polarity of infection are indicated above the panels. AZT, transduction with first-generation lentivirus from the apical compartment and simultaneous treatment with reverse transcriptase inhibitor AZT. DAPI, representative field of DAPI staining showing nuclei of all cells present. Magnification, ×10.
In summary, we showed that VSV-G-pseudotyped lentivirus was able to cross a mucus barrier and preferentially transduce DGBE and CaCo-2 cells through the apical side, which might be an important property for in vivo intestinal gene transfer.
Acknowledgments
We thank Chris Savard for many helpful suggestions and Janet Douglas for the original preparation of pRtatpeGFP. We also thank Didier Trono and Romain Zufferey, University of Geneva, Switzerland, for providing the third-generation lentivirus vectors.
The research described here was supported by NIH grants DK50686, DK43727, DK47754, DK50246, and AI39416 and Nederlandse Organisatie voor Wetenschappelijk Onderzoek grant 902-23-248.
REFERENCES
- 1.Arcasoy S M, Latoche J, Gondor M, Watkins S C, Henderson R A, Hughey R, Finn O J, Pilewski J M. MUC1 and other sialoglycoconjugates inhibit adenovirus-mediated gene transfer to epithelial cells. Am J Respir Cell Mol Biol. 1997;17:422–435. doi: 10.1165/ajrcmb.17.4.2714. [DOI] [PubMed] [Google Scholar]
- 2.Bals R, Xiao W, Sang N, Weiner D J, Meegalla R L, Wilson J M. Transduction of well-differentiated airway epithelium by recombinant adeno-associated virus is limited by vector entry. J Virol. 1999;73:6085–6088. doi: 10.1128/jvi.73.7.6085-6088.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Douglas J, Kelly P, Evans J T, Garcia J V. Efficient transduction of human lymphocytes and CD34+ cells via human immunodeficiency virus-based gene transfer vectors. Hum Gene Ther. 1999;10:935–945. doi: 10.1089/10430349950018337. [DOI] [PubMed] [Google Scholar]
- 4.Dull T, Zufferey R, Kelly M, Mandel R J, Nguyen M, Trono D, Naldini L. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eggermont E. Gastrointestinal manifestations in cystic fibrosis. Gastroenterol Hepatol. 1996;8:731–738. [PubMed] [Google Scholar]
- 6.Igimi H, Yamamoto F, Lee S P. Gallbladder mucosal function: studies in absorption and secretion in humans and in dog gallbladder epithelium. Am J Physiol. 1992;263:G69–G74. doi: 10.1152/ajpgi.1992.263.1.G69. [DOI] [PubMed] [Google Scholar]
- 7.Kafri T, Blomer U, Peterson D A, Gage F H, Verma I M. Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors. Nat Genet. 1997;17:314–317. doi: 10.1038/ng1197-314. [DOI] [PubMed] [Google Scholar]
- 8.Klinkspoor J H, Kuver R, Savard C E, Oda D, Azzouz H, Tytgat G N, Groen A K, Lee S P. Model bile and bile salts accelerate mucin secretion by cultured dog gallbladder epithelial cells. Gastroenterology. 1995;109:264–274. doi: 10.1016/0016-5085(95)90293-7. [DOI] [PubMed] [Google Scholar]
- 9.Kuver R, Ramesh N, Lau S, Savard C, Lee S P, Osborne W R A. Constitutive mucin secretion linked to CFTR expression. Biochem Biophys Res Commun. 1994;203:1457–1462. doi: 10.1006/bbrc.1994.2348. [DOI] [PubMed] [Google Scholar]
- 10.Kuver R, Savard C, Oda D, Lee S. PGE generates intracellular cAMP and accelerates mucin secretion by cultured dog gallbladder epithelial cells. Am J Physiol. 1994;267:G998–G1003. doi: 10.1152/ajpgi.1994.267.6.G998. [DOI] [PubMed] [Google Scholar]
- 11.Miyoshi H, Takahashi M, Gage F H, Verma I M. Stable and efficient gene transfer into the retina using an HIV-based lentiviral vector. Proc Natl Acad Sci USA. 1997;94:10319–10323. doi: 10.1073/pnas.94.19.10319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naldini L, Blomer U, Gage F H, Trono D, Verma I M. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oda D, Lee S P, Hayashi A. Long-term culture and partial characterization of dog gallbladder epithelial cells. Lab Invest. 1991;64:682–692. [PubMed] [Google Scholar]
- 14.Pickles R J, McCarty D, Matsui H, Hart P J, Randell S H, Boucher R C. Limited entry of adenovirus vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer. J Virol. 1998;72:6014–6023. doi: 10.1128/jvi.72.7.6014-6023.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soneoka Y, Cannon P M, Ramsdale E E, Griffiths J C, Romano G, Kingsmann S M, Kingsmann A J. A transient three-plasmid expression system for the production of high-titer retroviral vectors. Nucleic Acids Res. 1995;23:628. doi: 10.1093/nar/23.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tucker S P, Compans R W. Virus infection of polarized epithelial cells. Adv Virus Res. 1993;42:187–247. doi: 10.1016/S0065-3527(08)60086-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welsh M, Tsui L-C, Boat T, Beaudet A. Cystic fibrosis. In: Scriver C, Beaudet A, Sly W, Valle D, editors. The metabolic basis of inherited disease. 7th ed. II. New York, N.Y: McGraw-Hill; 1995. pp. 3799–3876. [Google Scholar]
- 18.Zufferey R, Dull T, Mendal R J, Bukovsky A, Quiroz D, Naldidni L, Trono D. Self-inactivating lentivirus vector for safe efficient in vivo gene delivery. J Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]