Abstract
Rationale
Nontuberculous mycobacteria (NTM) has been reported to be transmitted between people with cystic fibrosis (CF) attending CF centres. A suspected Mycobacterium abscessus outbreak was investigated at the University of Texas Southwestern (UTSW) Adult CF Program using a combination of pathogen genomic sequencing and epidemiologic methods. The objectives of the present study were to apply the Healthcare-Associated Links in Transmission of NTM (HALT NTM) study to investigate the occurrence of potential healthcare-associated transmission and/or acquisition of NTM among people with CF infected with genetically similar NTM isolates.
Methods
Whole-genome sequencing of respiratory M. abscessus isolates from 50 people with CF receiving care at UTSW was performed to identify genetically similar isolates. Epidemiologic investigation, comparison of respiratory and environmental isolates, and home residence watershed mapping were studied.
Measurements and main results
Whole-genome sequencing analysis demonstrated seven clusters of genetically similar M. abscessus (four ssp. abscessus and three ssp. massiliense). Epidemiologic investigation revealed potential opportunities for healthcare-associated transmission within three of these clusters. Healthcare environmental sampling did not recover M. abscessus, but did recover four human disease-causing species of NTM. No subjects having clustered infections lived in the same home residence watershed. Some subjects were infected with more than one M. abscessus genotype, both within and outside of the dominant circulating clones.
Conclusions
Healthcare-associated person-to-person transmission of M. abscessus appears to be rare at this centre. However, polyclonal infections of M. abscessus species and subspecies, not originating from the endemic hospital environment, suggest multiple shared modes of acquisition outside the healthcare setting.
Shareable abstract
Nontuberculous mycobacteria have been reported to be transmitted between people with cystic fibrosis. A single-centre Mycobacterium abscessus outbreak was investigated at an adult programme and revealed low potential for healthcare-associated transmission. https://bit.ly/4a4v1uh
Introduction
Nontuberculous mycobacteria (NTM) are opportunistic pathogens that can cause lung disease in people with cystic fibrosis (CF). NTM infections are challenging to eradicate and may require lengthy, multidrug therapy that is frequently complicated by side-effects and toxicity [1, 2]. Sources of exposure and modes of NTM transmission among people with CF include environmental acquisition and potential person-to-person transmission. Environmental acquisition sources include NTM-laden bioaerosols in soil and water [3–6] as well as biofilms in water systems from built environments including healthcare facilities [7–12] and CF centres [13, 14]. Among both people with CF and non-CF populations, home dust has also been linked to infection [15, 16]. NTM outbreaks have been documented in healthcare settings, including susceptible populations that were exposed to medical devices or aqueous environments contaminated by NTM [17]. There have been numerous reports of pulmonary Mycobacterium abscessus infections linked to CF centres [18]. Whole-genome sequencing (WGS) has become the gold standard to infer potential person-to-person transmission including early reports of M. abscessus ssp. massiliense among people with CF, where comprehensive environmental sampling of the healthcare setting failed to isolate a source of infection [19, 20]. Dominant circulating clones (DCC) were identified and have been associated with worse clinical outcomes and greater antibiotic resistance [21]. CF NTM outbreak investigations have produced contradictory conclusions, some supporting evidence of person-to-person transmission [13, 22, 23], while others found little evidence of person-to-person transmission [14, 24–27]. This discrepancy can be attributed to unique features of each healthcare centre, differing methods of isolate identification, and varying application of epidemiologic investigation. Therefore, improving our understanding of healthcare-associated NTM outbreaks remains a priority [28].
The evidence-based Healthcare-Associated Links in Transmission of NTM (HALT NTM; clinicaltrials.gov identifier NCT04024423) study has standardised epidemiologic investigation, supported by environmental sampling, watershed analysis and pangenomic analysis of isolates for NTM outbreak investigation [28, 29]. The HALT NTM toolkit has been applied in CF centre investigations, and can distinguish between healthcare-associated hospital-endemic environmental acquisition and person-to-person transmission [13, 14, 29].
The HALT NTM study utilises the largest collection of WGS data from respiratory CF NTM isolates in the United States, and is banked at the Colorado National Resource Centers (CO-NRC) biorepository [30–32]. WGS analysis of core genome similarities identified clusters of CF NTM isolates from people with CF in single United States CF Care Centers [30, 31] and raised concern for potential centre-specific healthcare-associated NTM outbreaks. Herein, we report an investigation of seven NTM clusters (four M. abscessus ssp. abscessus and three Mycobacterium abscessus ssp. massiliense) from people with CF who received care at the University of Texas Southwestern Medical Center (UTSW) Adult CF Program between January 2013 and December 2018.
Methods
Overview
This is a genomic epidemiologic outbreak investigation of potential healthcare-associated transmission and/or environmental acquisition of NTM among people with CF at the UTSW Adult CF Care Center having infections with genetically similar NTM isolates. The HALT NTM toolkit [29] was used to perform 1) WGS core genome analysis of respiratory NTM isolates to identify potential clustered infections; 2) epidemiologic investigation via electronic health record review of people with CF infected with genetically similar NTM isolates; 3) accessory genome analysis to ascertain pangenome relatedness; 4) healthcare environmental sampling of dust and water biofilms for NTM culture and WGS comparison to respiratory isolates; and 5) watershed mapping of people with CF home addresses to assess the possibility of common acquisition from the home environment.
Patients and respiratory sample collection
Sputum samples from the UTSW Adult CF Program were collected during routine clinical care of 294 people with CF (2013–2018). Among the 294 people with CF, 71 had one or more positive cultures for NTM. NTM isolates from 50 people with CF were submitted to the CO-NRC for research purposes.
Healthcare environmental sample collection
Video teleconferencing support was provided to identify a comprehensive list of healthcare environmental sampling locations, targeted at spaces where people with CF enter, exit and interact with the healthcare facility as well as all clinic, hospital and research spaces where people with CF received care from 2013 to 2018. Following an environmental sampling protocol, healthcare environmental samples of water biofilms and dust were collected by UTSW personnel from the clinic, hospital and research settings. In the clinic, hospital and research setting, all rooms where people with CF received care between 2013 and 2018 were sampled for all in-room water biofilms and dust from air vents was sampled when available. Additionally, water biofilms from common access areas including lobbies, waiting rooms, family rooms, restrooms and hallways were sampled. Water biofilm samples included (when available) sink faucets, water fountains, ice dispensers, water dispensers, coffee machines, soda machines, showerhead faces, shower hoses, endoscopy suite cleaning equipment and decorative water features. Dust samples were collected from ceiling vents. Biofilm samples were collected as published [33] using sterile synthetic flock dual-tipped swab applicators (HydraFlock sterile flocked collection devices #25–3306 2HBT; Puritan, Guilford, ME, USA). Standard microbiological approaches were used for the isolation of environmental NTM. In brief, swabs were immersed in 2 mL of autoclaved ultrapure water and vortexed on “high” for 1 min. Next, 450 µL of sample was transferred to a sterile tube with 50 µL of 1% cetylpyridinium chloride, vortexed for 1 min on “high”, and incubated at room temperature for 30 min. After incubation, the sample was vortexed and 100 µL was spread-plated on duplicate Middlebrook 7H10 agar plates with oleic acid/glycerol enrichment. One plate was incubated at 30°C and the other at 37°C for 3 weeks. Following incubation, colonies were picked and grown in supplemented Middlebrook 7H9 broth for the isolation of genomic DNA. 1 mL of culture was centrifuged at 1300×g for 1 min at room temperature after which the supernatant was discarded and the bacterial pellet stored at −80°C to be used for NTM identification. Intact genomic DNA was extracted from bacterial pellets as described [34] including the optional bead-beating step. Isolates were identified to the subspecies level using amplification and sequencing of the RNA polymerase-β subunit (rpoβ) gene [33, 35, 36]. Human pulmonary disease causing environmental NTM (M. avium complex (MAC), M. kansasii, M xenopi and M. abscessus) [37] isolates underwent WGS.
Whole-genome sequencing and phylogenetic analysis
DNA extraction, WGS and single nucleotide polymorphism (SNP) analyses were performed on respiratory and environmental NTM isolates [30, 34]. NTM respiratory isolates were deemed genetically related and falling into a cluster using a core genome SNP difference threshold of ≤30 for M. abscessus, similar to other studies [19, 21, 23, 24, 26, 30, 38, 39]. Phylogenetic trees were generated for environmental and respiratory isolates using the first respiratory isolate available for research purposes and may not represent the first respiratory NTM isolate recovered. NTM cluster network analysis was performed [30] and combined genetic plots were created (GraphPad Prism 8.0). WGS are available at the National Center for Biotechnology Information (BioProject identifier PRJNA319839).
Pangenome analysis
NTM isolate WGS were assembled using Unicycler [40] and annotated with Prokka [41]. Pangenome comparisons were analysed using Panaroo [42]. Pairwise accessory genome comparisons were completed [43].
Epidemiologic investigation
The HALT NTM toolkit [29] analysed healthcare setting dates, location, and procedure data to identify opportunities for transmission events between subjects having a clustered infection based phylogenetic analysis of WGS. The first respiratory NTM isolate available for research purposes and WGS may not represent the first time each subject cultured respiratory NTM. All respiratory NTM culture data were included in epidemiologic analyses. Patient demographics and microbiological data from the electronic health record were assembled and managed using REDCap electronic data capture tools hosted at National Jewish Health.
Watershed data collection
Patient addresses were extracted based on year of first positive NTM identification via WGS and not necessarily the first time NTM was cultured locally. Addresses were converted to latitude and longitude coordinates using the R package ggmap [44] with mapping visualisation performed using ArcGIS 10.2 (Esr, Boston, MA, USA) and using the USGS Hydrologic Unit Code level 8 watersheds [45] for watershed mapping.
Results
Cluster identification via genomic analysis
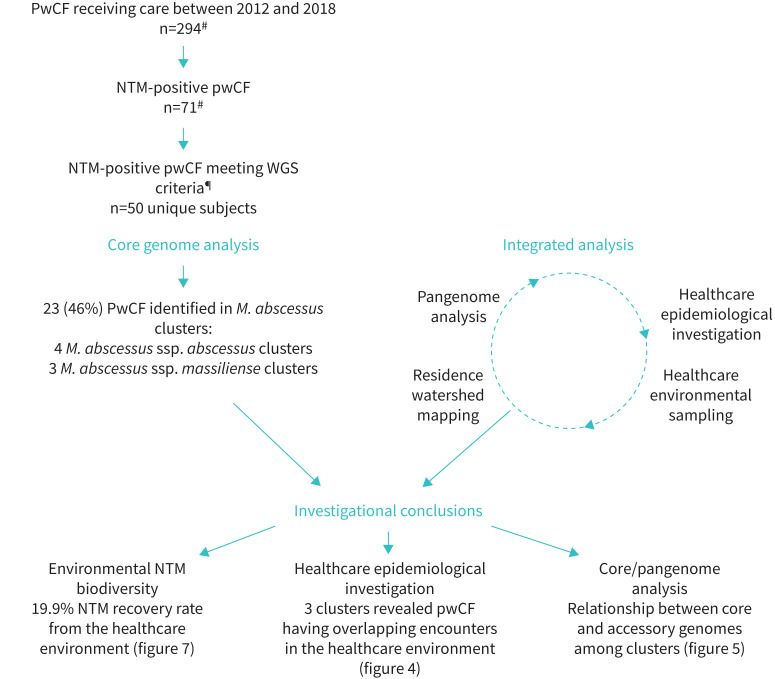
Between 1 January 2013 and 31 December 2018, respiratory NTM was cultured at least once from 71 (24.1%) patients out of 294 people with CF cared for at the UTSW Adult CF Program. MAC isolates were identified by the local clinical laboratory, but were not available for research analysis. 50 (70.4%) out of 71 subjects had at least one M. abscessus positive culture. From the 50 subjects, 36 subjects had more than one isolate available for WGS. 145 isolates underwent WGS (figure 1).
FIGURE 1.
Overview of the healthcare-associated transmission and/or environmental acquisition of nontuberculous mycobacteria (NTM) investigation. Investigational outcomes include healthcare environmental sample collection of the clinic and hospital settings demonstrating NTM diversity; the number of clusters with overlapping encounters in the healthcare environment; and pangenome analysis of paired respiratory isolates. Whole-genome sequencing (WGS) criteria were defined as more than one positive NTM culture within the abstraction timeframe. pwCF: people with cystic fibrosis; M. abscessus: Mycobacterium abscessus. #: largest number identified between 2013 and 2018; ¶: defined as having more than one NTM isolate and available for research purposes.
Seven clusters of respiratory NTM were identified by core genome analysis. 23 (46.0%) out of 50 subjects were infected with isolates comprising seven M. abscessus clusters. Three clusters were M. abscessus ssp. massiliense (clusters A, F, G) and four were M. abscessus ssp. abscessus (clusters B, C, D, E) (figure 2). Clusters B and D consist of isolates of ssp. abscessus DCC1 [30] (figure 2). Each cluster is indicated by a letter (A–G) and the subject by a number (1–7) (figure 3). Subject 1 was defined as the individual with the longest period with M. abscessus-positive culture based on clinical data as identified by the local laboratory and subject 2 was defined as the individual with the second longest M. abscessus-positive culture. Culture data for M. abscessus are shown on timeline analysis (figure 4). Notably, four subjects had clustered polyclonal M. abscessus infections.
FIGURE 2.
Phylogenetic analysis of Mycobacterium abscessus isolates among people with cystic fibrosis (CF) at the University of Texas Southwestern Medical Center (UTSW) Adult CF Program. Reference isolates, including type strains and previously published isolate genomes, are shown in grey circles with labels. Isolates from subjects not identified in a cluster are represented in unlabelled grey circles. The sets of clustered nontuberculous mycobacteria isolates are represented as coloured circles, with each letter representing a cluster. ATCC: American Type Culture Collection; T: type strain; DCC: dominant circulating clone; SNP: single nucleotide polymorphism.
FIGURE 3.
Cluster network analysis. Nontuberculous mycobacteria species are identified by colour, and the letter identifies the cluster. Nodes represent each subject within a cluster. The number in the node identifies the within-cluster subject order based on the first positive culture. Four subjects are identified in one or more Mycobacterium abscessus clusters (M. abscessus polyclonal clustered infections). 10 subjects identified with clustered M. abscessus infections also grew M. avium complex. Core genome single nucleotide polymorphism (SNP) distances are shown as solid lines connecting the nodes.
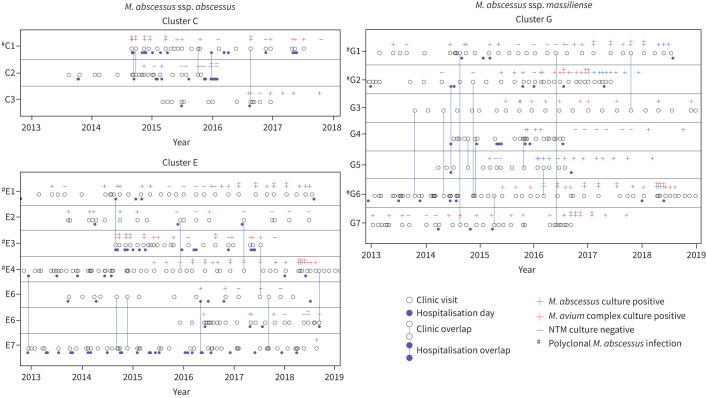
FIGURE 4.
Timeline overlap analysis of people with cystic fibrosis identified in Mycobacterium abscessus clusters demonstrating clinic visits, hospitalisation days, same-day clinic overlap and hospitalisation overlap. Clusters that did not have contact overlaps are not shown. NTM: nontuberculous mycobacteria. #: subjects with more than one isolate.
Subjects with more than one clustered infection (figure 3) include subject G2/B2 (one clustered isolate of ssp. massiliense and one clustered isolate of ssp. abscessus), subject G1/E1 (one clustered isolate of ssp. massiliense and one clustered isolate of ssp. abscessus), subject E3/C1 (two clustered isolates of ssp. abscessus) and subject G6/E4/F2 (two clustered isolates of ssp. massiliense and one clustered isolate of ssp. abscessus). Two other subjects were identified with polyclonal M. abscessus infections, including subject D1 (one clustered, one unclustered ssp. abscessus isolate) and another subject with two unclustered ssp. abscessus isolates (not shown).
Participant and NTM cluster characteristics
The demographic characteristics of the subjects identified with clustered infections (table 1) are representative of an adult CF population [46].
TABLE 1.
Subject characteristics within nontuberculous mycobacteria (NTM) clusters
| Total patients | 23 |
| Female | 11 (47.8) |
| Mean age years | 34.5 |
| Patients in more than one cluster | 4 |
| Race | |
| White | 21 (91.3) |
| Asian | 2 (8.6) |
| Ethnicity | |
| Non-Hispanic | 19 (82.6) |
| Hispanic | 4 (17.4) |
| Clinical descriptives | |
| CF centres visited by subjects | 1.5 (1–3) |
| Clinic visits per subject# | 1–70 |
| Admissions# | 0–27 |
| Subjects growing more than one subspecies of M. abscessus | 4 (17.4) |
| Subjects on NTM treatment | 13 (56.5) |
| Subjects with ATS NTM infection criteria¶ | 13 (56.5) |
| Number of patients in a cluster | 3.4 (2–7) |
| Overlaps | |
| Clinics | 9 |
| Admissions | 3 |
| Length of admission overlap days | 4.3 (1–10) |
Data are presented as n, n (%), mean (range) or range. CF: cystic fibrosis; M. abscessus: Mycobacterium abscessus; ATS: American Thoracic Society. #: during the extraction range period; ¶: based on clinical assessment by the primary CF physician.
Epidemiological investigation
Opportunities for healthcare-associated NTM transmission between subjects were investigated by plotting care episodes along with subject-specific respiratory NTM culture data. Overlaps are defined as a time within a same-day healthcare setting during which two subjects received care in the same general location based on electronic health record review. Timeline overlap analysis (figure 4) demonstrated healthcare-associated overlaps which serve as potential opportunities for transmission in two (50.0%) out of four of the ssp. abscessus clusters (clusters C and E) and one (33.3%) out of three ssp. massiliense clusters (cluster G). Clusters without healthcare-associated contact overlaps are not shown.
In ssp. abscessus cluster C, subjects C1 and C2 had four overlaps, two clinic visits and two hospitalisations. Of the four overlaps between C1 and C2, one overlapping hospitalisation (2 days) and one clinic visit occurred prior to subject C2 becoming ssp. abscessus culture-positive. Within 2 months while subject C1 was recurrently ssp. abscessus culture-positive and having a total of 3 days of observed healthcare overlaps (2 days hospitalisation, 1 day clinic), subject C2 converted to ssp. abscessus culture-positive. Notably, during the first C1 and C2 hospitalisation overlap, subject C1 was admitted from 28 August to 11 September, while subject C2 was admitted from 10 to 18 September of the same year, during which time C1 was cared for in a third-floor room then moved to the seventh floor; C2 was admitted 12 days later to the same third-floor hospital room as C1 for the duration of the hospitalisation. Subjects C1 and C3 also had one clinic overlap that occurred while C1 was ssp. abscessus culture-positive, but the overlap occurred after C3 converted to ssp. abscessus culture-positivity, thus excluding this overlap as a transmission event.
Timeline overlap in ssp. abscessus cluster E revealed overlapping healthcare encounters among multiple members of the cluster. In some cases, both subjects were culture-negative prior and during the observed overlap (E1 and E4, E4 and E7). In contrast, in some cases, both subjects were culture-positive prior to the observed overlap (E4 and E6, E3 and E5). Cases where the overlap served as a plausible opportunity for transmission are described: E1 and E3 (one hospital overlap during which E1 was intermittently culture-positive and E3 became first-time culture-positive on the day of overlap). E2 and E4 (one clinic overlap during which E2 was historically culture-positive, but culture-negative just before the overlap, at which time both performed spirometry). E5 and E7 (three clinic overlaps, 1-day hospital overlap). During the E5 and E7 overlaps, the first two clinic overlaps were prior to either subject being first-time culture-positive. The third overlap (hospital) was first-time culture-positive for E5 and never-positive for E7 until 2 years after the hospital overlap. During the fourth overlap (clinic), both subjects were seen and performed spirometry in the same room on the same day, one in the morning and the other in the afternoon.
In our investigation, ssp. massiliense cluster G revealed two pairs of subjects (G1 and G7, G3 and G6) having a stringent level of relatedness at the pangenome level, and were identified with opportunities for healthcare-associated transmission (figure 5). Within the first pair (G1 and G7), G1 was intermittently ssp. massiliense culture-positive from 2014 to 2018 and G7 was culture-positive on four occasions from 2016 to 2018. G1 and G7 had two overlapping clinic encounters with the first overlap in 2014 when G1 was culture-positive with ssp. massiliense, 2 years before G7 became culture-positive. During the first overlap in 2014, G1 and G7 shared an afternoon clinic visit where both subjects performed spirometry 2 h apart in the clinic room; however, room location was not searchable via the electronic health record. The second overlap was in 2016 when G1 was acid-fast bacillus culture-negative and 3 months later G7 became culture-positive. At that time, subject G7 had a research study visit without spirometry and subject G1 had a routine clinic visit with spirometry, and both visits occurred in the same CF clinic space with G7 arriving in the late morning and G1 arriving early afternoon. The high degree of relatedness at the pangenome level (figure 5) between G1 and G7 respiratory isolates increases the concern for a transmission event via droplet exposure, specifically in the setting where respiratory droplets may have been aerosolised via cough and/or spirometry. M. abscessus survives aerosolisation, providing supporting evidence that NTM transmission may occur person-to-person via droplets [47] and was suggested in a hospital-based M. abscessus molecular epidemiological investigation in people with CF [21]. The second clinic overlap is not suggestive of a transmission event. The second pair in ssp. massiliense cluster G (G3 and G6) met stringent criteria for genetic relatedness (figure 5) and had two overlapping clinic encounters. Both encounters occurred prior to either subject becoming culture-positive. However, it has been reported that culture-positivity lags infection and clinical decline precedes NTM positive culture [48, 49] and may suggest that NTM culture recovery from sputum is delayed compared to the initial patient exposure to the infection; thus, there is potential for a transmission event within a year prior to the first positive culture. Several overlaps occurred prior to either subject being culture positive (G2 and G5, G4 and G5, G2 and G6, G6 and G7) and one pair (G1 and G3) was culture-positive prior to the overlap. A clinical overlap between G5 and G6 was observed during which G5 was recurrently positive and G6 became positive on the day of overlap. The remaining people with CF identified as having clustered infections either did not have overlapping sources of care or did not meet the stringent criteria for genetic relatedness to support healthcare-associated transmission.
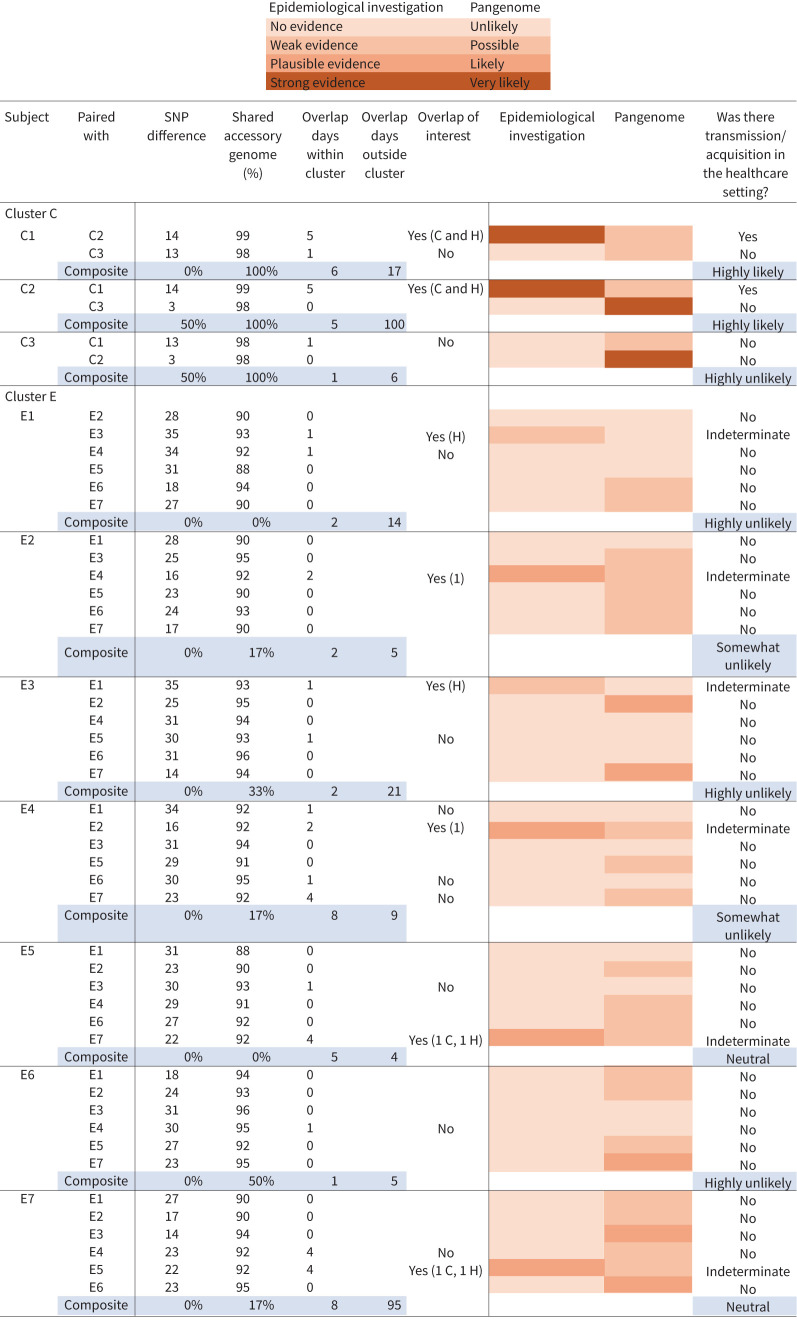
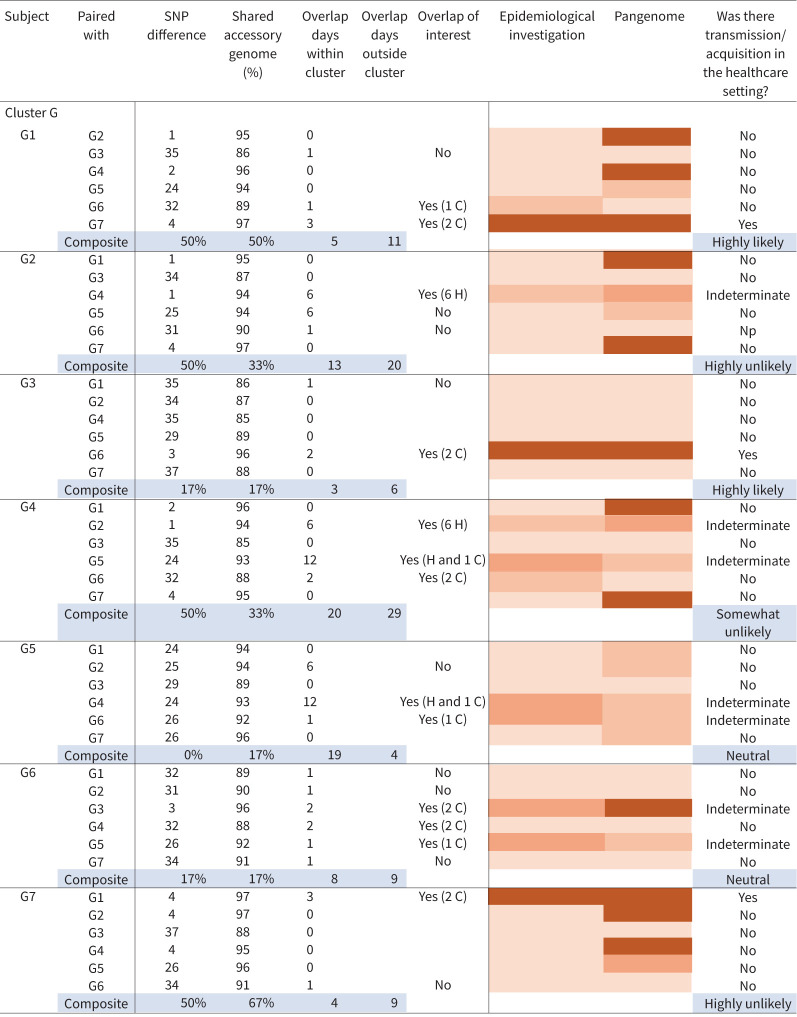
FIGURE 5.

Integrated pangenome analysis comparing single nucleotide polymorphism (SNP) differences between pairs of Mycobacterium abscessus isolates within clusters versus percentage shared accessory genome. Cluster G as well as M. abscessus ssp. abscessus clusters C and E demonstrated episodes of healthcare-associated opportunities for transmission. Cut-off points of ≤10 SNPs and ≥95% shared accessory genome are noted in the dashed-line box, representing the most stringent degree of clustered isolate similarity. Multiple clustered isolate pairs fall within the stringent genetic similarity cut-offs. Two paired isolates from M. abscessus ssp. massiliense cluster G with healthcare-associated opportunity for transmission from G1 to G7 and G3 to G6 fall within the stringent similarity, while the remaining isolate pairs that fall within the stringent similarity do not have observed healthcare overlaps. M. abscessus ssp. abscessus cluster D, consisting of two subjects with no evidence of healthcare-associated opportunity for transmission, also falls within the stringent similarity cut-offs. Cluster C, consisting of three subjects, has one pair without observed overlaps falling into the stringent similarity cut-offs, while two isolate pairs with healthcare overlaps fall just outside the SNP cut-off for stringent similarity, but meet the shared accessory genome cut-off.
Two (66.7%) ssp. massiliense clusters and two (50.0%) ssp. abscessus clusters revealed no healthcare-associated opportunities for transmission. Of interest, the two clusters (B and D) belonging to DCC1 did not show overlap events between subjects. There were no known social links (defined as siblings, spouses, housemates with CF or interactions between subjects outside the healthcare system, as determined by clinical staff and electronic health record review) observed between subjects.
Pangenome analysis
In addition to core genome comparisons, an accessory genome analysis was performed to assess pangenome relatedness of clustered isolates. Core genome SNP differences of paired isolates within clusters were plotted against the percentage shared genome of the same isolate pairs, creating a pangenome analysis (figure 5). Cut-off points of ≤10 SNPs and ≥95% shared accessory genome and have been described as representing the most stringent level of isolate similarity within the pangenome, and paired isolates falling within this area are highly suggestive of recent common source acquisition and/or transmission [13, 14]. Only one full cluster comparison (ssp. abscessus cluster D, DCC1) fell within the stringent cut-off points of isolate similarity and had no evidence of overlapping encounters in the healthcare setting. However, within ssp. massiliense cluster G, two sets of respiratory isolate pairs had overlapping healthcare encounters (G1 and G7, G3 and G6) and fell within the most stringent level of pangenome isolate similarity.
In order to assess the rate of M. abscessus infection among subjects, we applied two calculations that were both restricted to subjects identified with clustered infections. Among the seven NTM clusters identified, there were 28 M. abscessus infections observed in 23 subjects. Including all subjects identified with clustered infections, there were 290 unique person-day overlaps (supplementary material), defined as the total number of days that unique pairs of subjects with clustered infections were at the facility on the same day. Thus, the infection rate was (28×100)/290=9.7 per 100 unique person-day overlaps. When accounting for all the days these subjects were at the facility (regardless of whether there was another subject with a clustered infection at the facility), the infection rate was (28×100)/1322=2.1 per 100 person-days at the facility. The supplementary material includes more detailed definitions and examples. Ordinal levels of likelihood of person-to-person NTM transmission among subjects with clustered infections were created (figure 6).
FIGURE 6.
Ordinal levels of transmission. Ordinal levels for Mycobacterium abscessus transmission among subjects identified with a clustered infection were created in the following manner. Each subject was paired with each of the other subjects in the cluster. Single nucleotide polymorphism (SNP) distances and shared accessory genome values were used to create the pangenome heatmap. An “unlikely” event was defined as >30 SNPs difference or <90% shared genome. A “possible” event was defined as >15–30 SNPs difference and >90–95% shared genome. A “likely” event was defined as 0–15 SNPs or >95% shared genome. A “very likely” event was defined as ≤10 SNPs difference and ≥95% shared genome. Overlaps were described as the number of days two subjects were in the same healthcare space, the location they occurred, and if they were of interest. The determination of overlap of interest was assigned “yes” if there was biological plausibility for a potential transmission event, either acquisition or transmission from another subject based on epidemiologic review of the healthcare record. An overlap of interest was assigned “no” if both subjects were M. abscessus positive at the time the overlap was observed or the overlap occurred, but the locations where subjects received care was in two different healthcare spaces. The epidemiological investigation heatmap was estimated based on biological plausibility of transmission based on the strength of the epidemiological findings, and categories included no evidence, weak evidence, plausible evidence and strong evidence. Composite variables were determined in the following manner. The composite SNP variable for a subject is the percentage of pairs with <10 SNP difference among all possible pairs between the given subject and others in the same cluster. The composite shared accessory genome variable is defined similarly, but considering pairs with ≥95% shared data. H: hospital; C: clinic.
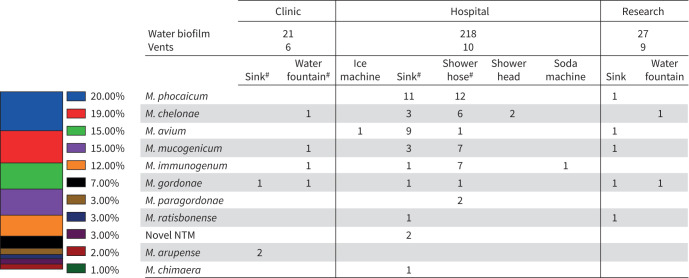
Healthcare environmental sampling
Water biofilms and vent dust from the healthcare setting were sampled (n=291) (figure 7). NTM was recovered in 19.9% of healthcare environmental samples, although none was recovered from vent dust (figure 7). Multiple healthcare environmental swabs recovered more than one NTM species. Disease-causing NTM isolates (M. chelonae, M. avium, M. gordonae, and M. chimaera) recovered from healthcare water biofilm samples underwent WGS. No respiratory isolates clustered with any of the healthcare environmental isolates.
FIGURE 7.
Environmental collection summary at University of Texas Southwestern Medical Center. M.: Mycobacterium; NTM: nontuberculous mycobacteria. #: locations with multiple NTM isolates recovered.
Watershed analysis
Watersheds have been used [50] to investigate NTM infection risk from water exposure in the absence of home environmental sampling [13, 28, 51]. We analysed home-of-residence addresses, when available, based on watershed distribution for people with CF having clustered M. abscessus infections [13]. There was no evidence of subjects from any cluster living in the same watershed, but not all subjects had addresses available for analysis (data not shown).
Discussion
We report a molecular epidemiological investigation of a healthcare-associated M. abscessus outbreak among people with CF receiving care at the UTSW Adult CF Program between 2013 and 2018. While WGS analysis of core genomes from available respiratory M. abscessus isolates identified seven clusters, only three clusters demonstrated opportunities for healthcare-associated transmission. Pangenome analysis supported stringent isolate similarity among two sets of ssp. massiliense isolate pairs (G1 and G7, G3 and G6) and one ssp. abscessus pair (C1 and C2) from people with CF who also had overlapping encounters in the healthcare system, consistent with healthcare-associated transmission. Although the watershed data was limited, there was no evidence of individuals from any cluster living in the same watershed, implying clustered NTM acquisition from the home environment was unlikely. Additionally, extensive sampling of the healthcare environment did not recover M. abscessus, making clustered healthcare-related acquisition unlikely. An unusual aspect of this investigation was the finding of co-infections with more than one M. abscessus strain among six subjects. Four subjects were identified with multiple clonal clustered M. abscessus infections B2 and G2 (ssp. massiliense and ssp. abscessus); G1 and E1 (ssp. massiliense and ssp. abscessus); E3 and C1 (two strains of ssp. abscessus); E4 and G6 and F2 (two strains of ssp. massiliense and one ssp. abscessus)); a fifth subject had one clustered M. abscessus infection and a second unclustered infection (D1); and a sixth subject had two unclustered M. abscessus infections (not shown). Furthermore, 43.5% of all people with CF having an M. abscessus clustered infection also had co-infection with MAC (identified to the species level at the local laboratory) (figure 3). In this study, the high frequency of co-infection with MAC and clustered M. abscessus as well as multiple clustered clones of M. abscessus from people with CF suggests multiple modes of infection acquisition in the majority of clustered infections.
Early CF centre outbreak investigations that utilised WGS described clusters of ssp. massiliense and ssp. abscessus localised only within DCC1 [19, 21]. Population surveys found that most people with CF were infected with isolates from DCC1, which were reported by some [21], but not all [30], to have greater antibiotic resistance and virulence and have been referred to as person-to-person “transmissible” strains [19, 52, 53]. However, among our subjects identified within DCC1 clusters (B and D), we did not observe any overlapping healthcare encounters, eliminating healthcare-associated person-to-person transmission as a source of infection. In this study, we also identified clusters within DCC2 (cluster C), DCC3 (cluster F) and DCC7 (cluster G) (figure 2). Pairs within both clusters C and G were identified as person-to-person transmission, but the remainder of subjects appear to be infected from environmental acquisition outside the healthcare environment. These findings suggest that DCCs may more likely be acquired from the environment than transmitted to another person.
As described previously [13, 23, 30], we observed that 50.0% of our ssp. abscessus clusters were in the DCC1 [21]. M. abscessus ssp. abscessus cluster C (DCC2) demonstrated that C1 and C2 had two discrete overlapping encounters including a 2-day hospitalisation and one clinic overlap with pangenome analysis demonstrating 14 SNP differences at the core genome and >98% relatedness of the accessory genome, falling just outside the stringent criteria for relatedness. During the 2-day hospitalisation overlap, C1 and C2 independently resided in the same hospital room 12 days apart, raising concern for indirect person-to-person transmission via fomites. Fomites are inanimate passive carriers of infections and have been proposed as a source of NTM transmission [21, 54]. One study identified transmission of M. abscessus ssp. massiliense (DCC1) via fomites from a hospital room contaminated from an index case and spread to three people with CF in three separate events, with persistent NTM recovered from sampling of sinks and surface rails after multiple routine cleaning procedures [21]. In the laboratory setting, dust particles can serve as a fomite enhancing survival of M. abscessus two weeks after desiccation, supporting the possibility of cross infection via exposure to dust-laden desiccated NTM droplets [54]. We did not recover M. abscessus from the endemic healthcare setting; however, our findings are similar to previous studies [21] and increase the plausibility that noncontemporaneously shared rooms can serve as a source of indirect patient-to-patient transmission of M. abscessus. This finding highlights the importance of reviewing current infection prevention and control guidelines [55] that may be inadequate to thoroughly decontaminate rooms in which people with CF infected with NTM reside. This finding seems to be based on the duration of room exposure to NTM droplets, since healthcare-associated fomite-driven indirect patient-to-patient transmission has been demonstrated previously within inpatient hospital rooms [21], and use of specific cleaning agents including Clinell wipes, concentrated perchlorate solution and hydrogen peroxide vapour all resulted in decontamination of M. abscessus from inpatient surfaces [21].
Limitations of this study include the lack of research access to all clinical NTM isolates and the single-centre nature of the study. Investigation of human-transmissible MAC was reported recently and concluded that healthcare-associated person-to-person transmission of M. avium occurred in a CF care centre [13, 56]. Unfortunately, in the current centre investigation MAC was only identified to the species level at the local laboratory and not banked for research, limiting analysis of MAC as well as other NTM of human significance in this study. Additionally, healthcare environmental sampling was limited to plumbing biofilms and surface dust from vents. Others have recovered NTM from free water [57], but hydrophobic NTM attach to surfaces and form biofilms, and it is the persistence of biofilms that serve as the source of infection transmission via NTM-enriched aerosols and droplets [58]. Water biofilms are our preferred sampling source over free water because the preference for surface adherence over suspension in water results in more concentrated NTM from biofilms (1000–15 000 CFU·cm−2) compared to free water (10–100 CFU·mL−1) [59]. Furthermore, the study lacks serial sampling of the healthcare environment as well as timed healthcare environmental sampling aligned with clinical isolate collection. There is evidence to support that NTM are stable in plumbing water biofilms over prolonged periods [58, 59], including one report of stable M. avium biofilm recovery from a recirculating hospital hot water system over a 41-month time frame [60]. The ongoing multicentre study, Prospective Healthcare-Associated Links in Transmission of Nontuberculous Mycobacteria (pHALT NTM; clinicaltrials.gov identifier NCT05686837) will address these limitations through the collection and analysis of prospective of all respiratory NTM from people with CF as well as serial environmental healthcare sampling over a 2-year period.
Remarkably, 19.9% of healthcare environmental samples recovered NTM, significantly higher than previous reports using the HALT NTM toolkit [13, 14]. Notably, M. abscessus was not recovered from the healthcare environment in this study (figure 6), but has been recovered in our previous outbreak investigation [14]. Similarly to our previous reports [13, 14], healthcare-associated environmental MAC was recovered, including M. avium and M. intracellulare ssp. chimaera. Future comparison of these healthcare environmental isolates to isolates recovered from people with CF is warranted. Additionally, WGS comparisons of all clinical respiratory NTM isolates to environmental isolates is necessary to determine whether the healthcare setting can be a source of acquisition of respiratory NTM among people with CF, but such extensive analysis exceeded the scope of this study.
We have previously used watersheds as a proxy for home water exposure in NTM outbreak investigations [13, 14]. However, the use of addresses based on the year the first NTM isolate was available for research and the inability to recover home addresses for all subjects limits the use of home watershed data in this study.
A unique finding of this study is the high frequency of polyclonal infection with MAC and M. abscessus as well as isolation of polyclonal M. abscessus among people with CF identified as having a clustered M. abscessus infection. MAC is the most frequently isolated respiratory NTM among people with CF [61]. A recent study demonstrated that 27% of people with CF with more than one MAC isolate had polyclonal infections, and the findings of polyclonal infections and high genetic similarity among MAC isolates is consistent with multiple modes of acquisition [31]. We are conducting a prospective multisite study that will include longitudinal analysis of all respiratory NTM collected from people with CF as well as serial sampling of the healthcare environment that will improve our understanding of polyclonal infections and help further identify sources of transmission and acquisition of NTM.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00165-2024.SUPPLEMENT (145.4KB, pdf)
Footnotes
Provenance: Submitted article, peer reviewed.
This study is registered at www.clinicaltrials.gov as HALT NTM (identifier number NCT04024423). Individual participant data that underlie the results reported in this article, after deidentification, will be shared upon request. The study protocol, statistical analysis plan and analytic code will be available immediately following publication with no end date, and available to investigators who provide a written request for data analysis. Requests should be directed to grossjane@njhealth.org.
Ethics statement: This study underwent expedited review with BRANY and was determined to meet criteria for waiver of informed consent (protocol number HS-3175).
Author contributions: Conceptualisation: J.E. Gross and J.A. Nick. Study design: J.E. Gross, C.L. Daley and J.A. Nick. Data collection: J.E. Gross, J.D. Finklea, S.M. Caceres, K.R. Poch, N.A. Hasan, L.E. Epperson, E.M. Lipner, C.K. Vang, V.C. Nogueira de Moura and J.R. Honda. Data analysis: J.E. Gross, S.M. Caceres, K.R. Poch, N.A. Hasan, F. Jia, L.E. Epperson, E.M. Lipner, C.K. Vang, J.R. Honda, M.J. Strand, M. Strong and J.A. Nick. Funding acquisition: J.E. Gross and J.A. Nick. All authors contributed to the manuscript and approved the final version submitted for publication.
Conflict of interest: J.E. Gross reports support for the present manuscript from the Cystic Fibrosis (CF) Foundation and grants or contracts from CF Foundation outside the submitted work; and is a board member of the Rocky Mountain Chapter of the Cystic Fibrosis Foundation and Chair of the American Board of Pediatrics Pulmonology Subboard, disclosures made outside the submitted work.
Conflict of interest: J.D. Finklea reports support for the present manuscript from a CF Foundation QI improvement grant, CF Foundation Success with Therapies Research Consortium.
Conflict of interest: C.K. Vang reports support for the present manuscript from the CF Foundation.
Conflict of interest: J.R. Honda reports support for the present manuscript from the Padosi Foundation and the University of Texas Health Science Center at Tyler; grants or contracts from the National Science Foundation, National Institutes of Health, CF Foundation and the John Chapman Endowed Professorship in Microbiology, outside the submitted work; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from the North American CF Foundation, Yale University, National Jewish Health, NTM Research Consortium and Albert Einstein College of Medicine, outside the submitted work; support for attending meetings and/or travel from the Padosi Foundation, National Science Foundation, National Jewish Health, NIH AGOLD-PRIDE, Albert Einstein College of Medicine, Sam Houston State University, American Thoracic Society, Texas Tech University, Colorado State University, NTM Research Consortium and North American CF Foundation, outside the submitted work; a leadership or fiduciary role for the American Thoracic Society, outside the submitted work; and receipt of equipment, materials, drugs, medical writing, gifts or other services from the University of Texas Health Science Center at Tyler, outside the submitted work.
Conflict of interest: M. Strand reports support for the present manuscript from Effort from CF Foundation grant “Prospective Healthcare-Associated Transmission of Nontuberculous Mycobacteria”.
Conflict of interest: C.L. Daley reports support for the present manuscript from CF Foundation; grants or contracts from AN2, Bugworks, Insmed, Juvabis and Paratek, outside the submitted work; consulting fees from Genentech and Pfizer, outside the submitted work; and participation on a data safety monitoring or advisory board for AN2, AstraZeneca, Hyfe, Insmed, MannKind, Matinas Biopharma, Nob Hill, Paratek, Spero and Zambon, outside the submitted work.
Conflict of interest: The remaining authors have nothing to disclose.
Support statement: This work was supported by the Departments of Pediatrics and Medicine, and the Center for Genes, Environment, and Health at National Jewish Health, as well as by the Cystic Fibrosis Foundation (GROSS19Q0 (J.E. Gross), NICK20Y2SVC (J.A. Nick), NICK20Y2OUT (J.A. Nick) and NICK15RO (J.A. Nick)). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Floto RA, Olivier KN, Saiman L, et al. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis. Thorax 2016; 71: Suppl. 1, i1–i22. doi: 10.1136/thoraxjnl-2015-207360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martiniano SL, Nick JA, Daley CL. Nontuberculous mycobacterial infections in cystic fibrosis. Clin Chest Med 2022; 43: 697–716. doi: 10.1016/j.ccm.2022.06.010 [DOI] [PubMed] [Google Scholar]
- 3.Falkinham JO 3rd. Nontuberculous mycobacteria from household plumbing of patients with nontuberculous mycobacteria disease. Emerg Infect Dis 2011; 17: 419–424. doi: 10.3201/eid1703.101510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Honda JR. Environmental sources and transmission of nontuberculous mycobacteria. Clin Chest Med 2023; 44: 661–674. doi: 10.1016/j.ccm.2023.07.001 [DOI] [PubMed] [Google Scholar]
- 5.Honda JR, Virdi R, Chan ED. Global environmental nontuberculous mycobacteria and their contemporaneous man-made and natural niches. Front Microbiol 2018; 9: 2029. doi: 10.3389/fmicb.2018.02029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Primm TP, Lucero CA, Falkinham JO 3rd. Health impacts of environmental mycobacteria. Clin Microbiol Rev 2004; 17: 98–106. doi: 10.1128/CMR.17.1.98-106.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker AW, Lewis SS, Alexander BD, et al. Two-phase hospital-associated outbreak of Mycobacterium abscessus: investigation and mitigation. Clin Infect Dis 2017; 64: 902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davidson RM, Nick SE, Kammlade SM, et al. Genomic analysis of a hospital-associated outbreak of Mycobacterium abscessus: implications on transmission. J Clin Microbiol 2022; 60: e0154721. doi: 10.1128/JCM.01547-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.du Moulin GC, Stottmeier KD, Pelletier PA, et al. Concentration of Mycobacterium avium by hospital hot water systems. JAMA 1988; 260: 1599–1601. doi: 10.1001/jama.1988.03410110107037 [DOI] [PubMed] [Google Scholar]
- 10.Kanamori H, Weber DJ, Rutala WA. Healthcare-associated Mycobacterium chimaera transmission and infection prevention challenges: role of heater-cooler units as a water source in cardiac surgery. Clin Infect Dis 2017; 64: 343–346. doi: 10.1093/cid/ciw755 [DOI] [PubMed] [Google Scholar]
- 11.Shin JH, Lee EJ, Lee HR, et al. Prevalence of non-tuberculous mycobacteria in a hospital environment. J Hosp Infect 2007; 65: 143–148. doi: 10.1016/j.jhin.2006.10.004 [DOI] [PubMed] [Google Scholar]
- 12.Williams MM, Armbruster CR, Arduino MJ. Plumbing of hospital premises is a reservoir for opportunistically pathogenic microorganisms: a review. Biofouling 2013; 29: 147–162. doi: 10.1080/08927014.2012.757308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gross JE, Caceres S, Poch K, et al. Investigating nontuberculous mycobacteria transmission at the Colorado Adult Cystic Fibrosis Program. Am J Respir Crit Care Med 2022; 205: 1064–1074. doi: 10.1164/rccm.202108-1911OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gross JE, Teneback CC, Sweet JG, et al. Molecular epidemiologic investigation of Mycobacterium intracellulare subspecies chimaera lung infections at an adult cystic fibrosis program. Ann Am Thorac Soc 2023; 20: 677–686. doi: 10.1513/AnnalsATS.202209-779OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaevska M, Slana I, Kralik P, et al. ‘Mycobacterium avium subsp. hominissuis’ in neck lymph nodes of children and their environment examined by culture and triplex quantitative real-time PCR. J Clin Microbiol 2011; 49: 167–172. doi: 10.1128/JCM.00802-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lahiri A, Kneisel J, Kloster I, et al. Abundance of Mycobacterium avium ssp. hominissuis in soil and dust in Germany – implications for the infection route. Lett Appl Microbiol 2014; 59: 65–70. doi: 10.1111/lam.12243 [DOI] [PubMed] [Google Scholar]
- 17.Desai AN, Hurtado RM. Infections and outbreaks of nontuberculous mycobacteria in hospital settings. Curr Treat Options Infect Dis 2018; 10: 169–181. doi: 10.1007/s40506-018-0165-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bolden N, Mell JC, Logan JB, et al. Phylogenomics of nontuberculous mycobacteria respiratory infections in people with cystic fibrosis. Paediatr Respir Rev 2023; 46: 63–70. doi: 10.1016/j.prrv.2023.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bryant JM, Grogono DM, Greaves D, et al. Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: a retrospective cohort study. Lancet 2013; 381: 1551–1560. doi: 10.1016/S0140-6736(13)60632-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aitken ML, Limaye A, Pottinger P, et al. Respiratory outbreak of Mycobacterium abscessus subspecies massiliense in a lung transplant and cystic fibrosis center. Am J Respir Crit Care Med 2012; 185: 231–232. doi: 10.1164/ajrccm.185.2.231 [DOI] [PubMed] [Google Scholar]
- 21.Bryant JM, Grogono DM, Rodriguez-Rincon D, et al. Emergence and spread of a human-transmissible multidrug-resistant nontuberculous mycobacterium. Science 2016; 354: 751–757. doi: 10.1126/science.aaf8156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnston DI, Chisty Z, Gross JE, et al. Investigation of Mycobacterium abscessus outbreak among cystic fibrosis patients, Hawaii 2012. J Hosp Infect 2016; 94: 198–200. doi: 10.1016/j.jhin.2016.04.015 [DOI] [PubMed] [Google Scholar]
- 23.Yan J, Kevat A, Martinez E, et al. Investigating transmission of Mycobacterium abscessus amongst children in an Australian cystic fibrosis centre. J Cyst Fibros 2020; 19: 219–224. doi: 10.1016/j.jcf.2019.02.011 [DOI] [PubMed] [Google Scholar]
- 24.Doyle RM, Rubio M, Dixon G, et al. Cross-transmission is not the source of new Mycobacterium abscessus infections in a multicenter cohort of cystic fibrosis patients. Clin Infect Dis 2020; 70: 1855–1864. doi: 10.1093/cid/ciz526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris KA, Underwood A, Kenna DT, et al. Whole-genome sequencing and epidemiological analysis do not provide evidence for cross-transmission of Mycobacterium abscessus in a cohort of pediatric cystic fibrosis patients. Clin Infect Dis 2015; 60: 1007–1016. doi: 10.1093/cid/ciu967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tortoli E, Kohl TA, Trovato A, et al. Mycobacterium abscessus in patients with cystic fibrosis: low impact of inter-human transmission in Italy. Eur Respir J 2017; 50: 1602525. doi: 10.1183/13993003.02525-2016 [DOI] [PubMed] [Google Scholar]
- 27.Wetzstein N, Kohl TA, Schultze TG, et al. Antimicrobial susceptibility and phylogenetic relations in a German cohort infected with Mycobacterium abscessus. J Clin Microbiol 2020; 58: e01813-20. doi: 10.1128/JCM.01813-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gross JE, Martiniano SL, Nick JA. Prevention of transmission of Mycobacterium abscessus among patients with cystic fibrosis. Curr Opin Pulm Med 2019; 25: 646–653. doi: 10.1097/MCP.0000000000000621 [DOI] [PubMed] [Google Scholar]
- 29.Gross JE, Caceres S, Poch K, et al. Healthcare-associated links in transmission of nontuberculous mycobacteria among people with cystic fibrosis (HALT NTM) study: rationale and study design. PLoS One 2021; 16: e0261628. doi: 10.1371/journal.pone.0261628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davidson RM, Hasan NA, Epperson LE, et al. Population genomics of Mycobacterium abscessus from U.S. cystic fibrosis care centers. Ann Am Thorac Soc 2021; 18: 1960–1969. doi: 10.1513/AnnalsATS.202009-1214OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hasan NA, Davidson RM, Epperson LE, et al. Population genomics and inference of Mycobacterium avium complex clusters in cystic fibrosis care centers, United States. Emerg Infect Dis 2021; 27: 2836–2846. doi: 10.3201/eid2711.210124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hasan NA, Davidson RM, Epperson LE, et al. Population genomics of nontuberculous mycobacteria recovered from United States cystic fibrosis patients. bioRxiv 2019; preprint [ 10.1101/663559]. doi: 10.1101/663559 [DOI] [Google Scholar]
- 33.Virdi R, Lowe ME, Norton GJ, et al. Lower recovery of nontuberculous mycobacteria from outdoor Hawai'i environmental water biofilms compared to indoor samples. Microorganisms 2021; 9: 224. doi: 10.3390/microorganisms9020224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Epperson LE, Strong M. A scalable, efficient, and safe method to prepare high quality DNA from mycobacteria and other challenging cells. J Clin Tuberc Other Mycobact Dis 2020; 19: 100150. doi: 10.1016/j.jctube.2020.100150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adékambi T, Colson P, Drancourt M. rpoB-based identification of nonpigmented and late-pigmenting rapidly growing mycobacteria. J Clin Microbiol 2003; 41: 5699–5708. doi: 10.1128/JCM.41.12.5699-5708.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Honda JR, Hasan NA, Davidson RM, et al. Environmental nontuberculous mycobacteria in the Hawaiian islands. PLoS Negl Trop Dis 2016; 10: e0005068. doi: 10.1371/journal.pntd.0005068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daley CL, Iaccarino JM, Lange C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J 2020; 56: 2000535. doi: 10.1183/13993003.00535-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaw LP, Doyle RM, Kavaliunaite E, et al. Children with cystic fibrosis are infected with multiple subpopulations of Mycobacterium abscessus with different antimicrobial resistance profiles. Clin Infect Dis 2019; 69: 1678–1686. doi: 10.1093/cid/ciz069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoon JK, Kim TS, Kim JI, et al. Whole genome sequencing of nontuberculous mycobacterium (NTM) isolates from sputum specimens of co-habiting patients with NTM pulmonary disease and NTM isolates from their environment. BMC Genomics 2020; 21: 322. doi: 10.1186/s12864-020-6738-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wick RR, Judd LM, Gorrie CL, et al. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol 2017; 13: e1005595. doi: 10.1371/journal.pcbi.1005595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics 2014; 30: 2068–2069. doi: 10.1093/bioinformatics/btu153 [DOI] [PubMed] [Google Scholar]
- 42.Tonkin-Hill G, MacAlasdair N, Ruis C, et al. Producing polished prokaryotic pangenomes with the Panaroo pipeline. Genome Biol 2020; 21: 180. doi: 10.1186/s13059-020-02090-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davidson RM, Benoit JB, Kammlade SM, et al. Genomic characterization of sporadic isolates of the dominant clone of Mycobacterium abscessus subspecies massiliense. Sci Rep 2021; 11: 15336. doi: 10.1038/s41598-021-94789-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kahle D, Wickham H. ggmap: spatial visualization with ggplot2. R J 2013; 5: 144–161. doi: 10.32614/RJ-2013-014 [DOI] [Google Scholar]
- 45.U.S. Department of Agriculture Natural Resources Conservation Service (USDA-NRCS) . The Watershed Boundary Dataset. https://usgs.gov/national-hydrography/watershed-boundary-dataset#:~:text=The%20Watershed%20Boundary. Date last accessed: 1 March 2022.
- 46.Cystic Fibrosis Foundation . Patient Registry 2021 Annual Data Report. Bethesda, Cystic Fibrosis Foundation, 2022. [Google Scholar]
- 47.Fletcher LA, Chen Y, Whitaker P, et al. Survival of Mycobacterium abscessus isolated from people with cystic fibrosis in artificially generated aerosols. Eur Respir J 2016; 48: 1789–1791. doi: 10.1183/13993003.00849-2016 [DOI] [PubMed] [Google Scholar]
- 48.Martiniano SL, Sontag MK, Daley CL, et al. Clinical significance of a first positive nontuberculous mycobacteria culture in cystic fibrosis. Ann Am Thorac Soc 2014; 11: 36–44. doi: 10.1513/AnnalsATS.201309-310OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nick JA, Dedrick RM, Gray AL, et al. Host and pathogen response to bacteriophage engineered against Mycobacterium abscessus lung infection. Cell 2022; 185: 1860–1874. doi: 10.1016/j.cell.2022.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kolok AS, Beseler CL, Chen XH, et al. The watershed as a conceptual framework for the study of environmental and human health. Environ Health Insights 2009; 3: 1–10. doi: 10.4137/EHI.S1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lipner EM, Knox D, French J, et al. A geospatial epidemiologic analysis of nontuberculous mycobacterial infection: an ecological study in Colorado. Ann Am Thorac Soc 2017; 14: 1523–1532. doi: 10.1513/AnnalsATS.201701-081OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davidson RM, Hasan NA, de Moura VC, et al. Phylogenomics of Brazilian epidemic isolates of Mycobacterium abscessus subsp. bolletii reveals relationships of global outbreak strains. Infect Genet Evol 2013; 20: 292–297. doi: 10.1016/j.meegid.2013.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tettelin H, Davidson RM, Agrawal S, et al. High-level relatedness among Mycobacterium abscessus subsp. massiliense strains from widely separated outbreaks. Emerg Infect Dis 2014; 20: 364–371. doi: 10.3201/eid2003.131106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malcolm KC, Caceres SM, Honda JR, et al. Mycobacterium abscessus displays fitness for fomite transmission. Appl Environ Microbiol 2017; 83: e00562-17. doi: 10.1128/AEM.00562-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saiman L, Siegel JD, LiPuma JJ, et al. Infection prevention and control guideline for cystic fibrosis: 2013 update. Infect Control Hosp Epidemiol 2014; 35: Suppl. 1, S1–S67. doi: 10.1086/676882 [DOI] [PubMed] [Google Scholar]
- 56.Tonder A, Ellis HC, Churchward C, et al. Mycobacterium avium complex (MAC) genomics and transmission in a London hospital. Eur Respir J 2023; 61: 2201237. doi: 10.1183/13993003.01237-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomson R, Tolson C, Carter R, et al. Isolation of nontuberculous mycobacteria (NTM) from household water and shower aerosols in patients with pulmonary disease caused by NTM. J Clin Microbiol 2013; 51: 3006–3011. doi: 10.1128/JCM.00899-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mullis SN, Falkinham JO 3rd. Adherence and biofilm formation of Mycobacterium avium, Mycobacterium intracellulare and Mycobacterium abscessus to household plumbing materials. J Appl Microbiol 2013; 115: 908–914. doi: 10.1111/jam.12272 [DOI] [PubMed] [Google Scholar]
- 59.Falkinham JO 3rd. Environmental sources of nontuberculous mycobacteria. Clin Chest Med 2015; 36: 35–41. 10.1016/j.ccm.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 60.von Reyn CF, Maslow JN, Barber TW. Persistent colonisation of potable water as a source of Mycobacterium avium infection in AIDS. Lancet 1994; 343: 1137–1141. doi: 10.1016/S0140-6736(94)90239-9 [DOI] [PubMed] [Google Scholar]
- 61.Adjemian J, Olivier KN, Seitz AE, et al. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med 2012; 185: 881–886. doi: 10.1164/rccm.201111-2016OC [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00165-2024.SUPPLEMENT (145.4KB, pdf)