Key Clinical Message
We report on the successful use of chemotherapy for treatment of stage 2B testicular seminoma in a carrier of the Leber's hereditary optic neuropathy 11778 mitochondrial mutation. Neurotoxic chemotherapy may not prompt disease conversion.
Keywords: antineoplastic agents; optic atrophy, hereditary, Leber; seminoma
1. INTRODUCTION
Leber's hereditary optic neuropathy (LHON) is one of the most prevalent inherited mitochondrial disorders and the most common mitochondrial optic neuropathy. 1 The condition has been reported to be held as a carrier gene mutation in up to 1 in 9000 people and affect as many as 1 in 30,000 individuals of European ancestry. 1 It exhibits a heavy male predominance with 80%–90% of those affected being male. 2 The disorder typically presents within the second and third decade of life and is characterized by progressive optic neuropathy which can result in irreversible bilateral central vision loss. 1 Therapies for LHON are limited with only one treatment registered for use (idebenone) and ongoing clinical trials for other disease‐modifying treatments including gene therapy. 3 , 4
The disorder is most commonly caused by mutations on nucleotide positions 3460, 11778, and 14484 of mitochondrial DNA. 5 These mutations predominantly affect genes encoding the subunit proteins of NADH dehydrogenase or complex I in the mitochondrial respiratory chain. 4 This is thought to result in dysfunction of oxidative phosphorylation with reduced adenosine triphosphate production and an increase in reactive oxygen species. 6 , 7 Cybrid experimentation on cell lines transmutated with mitochondrial DNA (mtDNA) possessing these LHON‐associated mutations have demonstrated greater susceptibility to apoptotic cell death via the fatty acid synthesis (FAS) and apoptosis‐inducing factor pathways. 8 , 9 These changes are thought to subsequently result in retinal ganglion cell degeneration and consequent optic neuropathy. 10
Environmental exposures including toxic medications, smoking, and significant alcohol intake have been associated with activation of disease in carriers resulting in vision loss. 10 For this reason, LHON patients with malignancy have presented a therapeutic dilemma, balancing a fear of chemotherapy‐induced vision loss against the threat of the malignancy requiring treatment.
2. CASE HISTORY
A 31‐year‐old male had been diagnosed as a carrier of the LHON 11778 mutation after familial genetic testing prompted by a relative's diagnosis of LHON. His maternal uncle had become blind at age 28 following a course of antibiotic therapy, possibly compounded by other behavioral and environmental exposures including excess alcohol intake and passive smoking. Our patient had not experienced any visual loss.
Localized testicular cancer was diagnosed in our patient following investigations for infertility. In 2016, a left‐sided testicular mass was identified on scrotal ultrasound. Staging imaging showed no evidence of metastatic disease. A left orchidectomy was performed with histopathology demonstrating 45‐mm (pT1) pure seminoma without lymphovascular invasion and no rete testis involvement. Adjuvant chemotherapy was discussed but due to the low risk of recurrence and concern regarding chemotherapy‐induced conversion of LHON, the patient instead chose to undergo active surveillance.
3. PROGRESSION, INVESTIGATIONS AND TREATMENT
Surveillance imaging 13 months post‐orchidectomy in 2017 with computed tomography (CT) of the abdomen/pelvis (Figure 1) demonstrated disease recurrence and progression to metastatic testicular seminoma stage 2B, with evidence of a new 37‐mm para‐aortic lymph node. Positron emission tomography (PET) imaging confirmed an isolated glucose avid lesion consistent with a site of active disease. He was referred for radiotherapy due to concerns for LHON conversion secondary to cisplatin‐based chemotherapy (BEP). The patient was deemed not suitable for radiotherapy given unacceptable levels of left kidney irradiation required due to the extent of macroscopic disease and the tumor proximity to the renal hilum. The patient elected not to receive bleomycin given his uncle's reported conversion following exposure to glycopeptide antibiotic therapy and case reports of conversion in the literature following erythromycin, 11 instead receiving four cycles of cisplatin and etoposide. High‐dose intravenous vitamin C was administered during the last week of each chemotherapy cycle, with a previous study reporting a benefit of supplementation in LHON. 12 This was in addition to regular supplements idebenone 500 mg daily and coenzyme Q10 150 mg twice daily, taken in consultation with his neuro‐ophthalmologist during treatment cycles.
FIGURE 1.
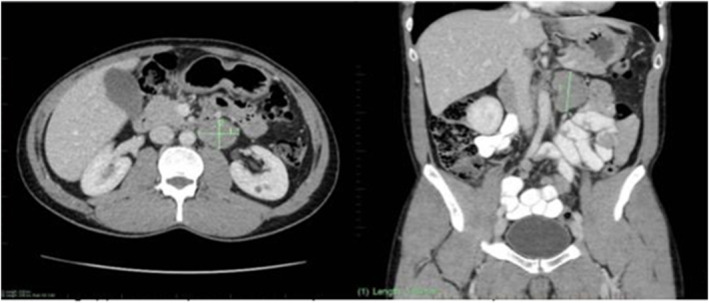
Surveillance imaging with CT abdomen/pelvis in 2017 demonstrating disease recurrence and progression to metastatic testicular seminoma stage 2B with evidence of a new 37‐mm para‐aortic lymph node.
4. OUTCOME AND FOLLOW‐UP
Following treatment, interval resolution was seen in the left para‐aortic lymph node on progress MRI in 2019. Our patient did develop transient Grade 1 sensory neuropathy after cycle 2 of chemotherapy. There was no deterioration of vision during the course of the treatment and he currently remains asymptomatic from a visual perspective 5 years post‐completion of chemotherapy. He had no evidence of cancer recurrence and he was discharged from regular oncology follow‐up.
5. DISCUSSION
Exposure to chemotherapy may conceivably represent an environmental trigger for LHON conversion, as the pathophysiology of LHON is postulated to involve cellular apoptotic sensitivity. 5 Neurotoxicity is a well‐known possible complication of chemotherapy such as platinum agents, taxanes and bortezimib, typically occurring in a dose‐dependent manner with pre‐existing neuropathies serving as important potential risk factors. 13 In this way, patients with congenital neuropathies presenting with malignancy pose a difficult challenge in selecting the appropriate chemotherapy regimen to both minimize toxicity and provide optimal efficacy.
In consideration of the cytotoxics used in our case, although cisplatin commonly causes large fiber sensory neuropathy it could also have been thought to mediate mitochondrial damage relevant to the pathogenesis of LHON. Cisplatin preferentially has uptake in the dorsal root ganglia resulting in a dose‐dependent large fiber sensory neuropathy. 14 The mechanism thought to underpin this involves DNA binding and interruption of synthesis which has been shown to cause inhibition of axonal growth, alterations to sensory ganglion cell body nucleoli, and neuronal atrophy. 14 These effects however may manifest at lower cumulative dose totals if risk factors including a background history of neuropathy are present. 14 Cisplatin has also been shown to mediate mitochondrial damage in tumor‐bearing mice studies via the oxidation of proteins and lipids via decreased antioxidant activity and increased free radicals. 15 Furthermore, data linkage studies have demonstrated an association between cisplatin‐induced ototoxicity and mitochondrial haplogroup J (also associated with LHON). 16 Cisplatin neuropathy could theoretically also be mediated via alternate mitochondrial pathways, affecting those with mitochondrial mutations who may be more vulnerable. It is interesting that despite these theoretical risks of exposure and the potential role for the mitochondrion in the pathogenesis of certain chemotherapy toxicity, this was not supported in our case. One explanation for this could be through viewing LHON as a disorder predominantly of "mitochondrial dysfunction" as opposed to being mediated via the same cytotoxic biomolecular pathways in which chemotherapy classically exert their neuropathic effects. Other drugs which are known to cause conversion of LHON are associated with mitochondrial effects, including erythromycin which was shown to cause blindness in one LHON patient as well as inhibition of cybrids containing their 11778 mitochondrial DNA. 11 On this basis, chemotherapeutic choice may then be analyzed based on their risk to the mitochondrion and ROS generation rather than purely the risk of neuropathy.
There are two other documented cases in the literature of successful administration of chemotherapy in LHON patients. The first case report detailed the successful treatment of a 36‐year‐old male with LHON (11778 mtDNA mutation) and stage 2A non‐Hodgkin's lymphoma with six cycles of dose reduced cyclophosphamide, vincristine, epirubicin, and prednisolone. 5 The patient had no observed acute or chronic side effects for 4 years following treatment at the time of publication. An in vitro assay performed on the patient's mononuclear peripheral blood cells did not demonstrate altered vulnerability to mafosfamide and hence the authors concluded that the 11778 mutation did not clinically change cellular response to cytotoxic therapy. The second case involved use of intrathecal methotrexate for treatment of acute lymphoblastic leukemia in a 3‐year‐old patient. 17 Marked leukoencephalopathic changes were observed on brain magnetic resonance imaging but measured language skills remained stable. No visual impairment or disease conversion as a consequence of therapy had been reported by the authors. It is interesting that methotrexate in this case did not cause conversion particularly given its folate inhibition has been shown to result in a severe optic neuropathy which is thought to be mediated by mitochondrial dysfunction, even resulting in similar visual field deficits as LHON in one case report. 18 To our knowledge, and as noted by other studies, there are no documented cases of chemotherapeutics resulting in LHON conversion and blindness. 19
The relative safety of chemotherapy in LHON may be bolstered through the use of adjunct antioxidant treatments. Our patient was treated with high‐dose vitamin C based on evidence of faster visual recovery for LHON patients noted in one study for those treated with idebenone, vitamins B2 and C. 12 Some studies have shown improved biochemical marker profiles, compound muscle action potential amplitudes and motor performance in rats with cisplatin‐induced neuropathy treated with vitamin C. 20 However, the role of chemoprotective agents more generally in mitigating the neurotoxicity of platin‐based chemotherapy agents remains controversial, with insufficient evidence to support routine use. There have been traditional concerns that treatment efficacy may be compromised by antioxidant activity, although this has not been supported in the literature as found by one systematic review. 21 However, there could perhaps be a greater role for antioxidants in patients with pre‐existing congenital neuropathies such as LHON who are undergoing cytotoxic therapy.
We would argue that LHON is not an absolute contraindication to use of chemotherapy. The incidence of neuropathy associated with a specific chemotherapy drug does not appear to increase the risk of LHON, and instead selection should be based on risk of mitochondrial effects. This case demonstrates a further example of a case of hereditary optic neuropathy without conversion following exposure to cytotoxic therapy. We would propose the need for further studies to assess the safety of chemotherapeutic regimens in these patients to prevent an entire cohort of individuals from being excluded from potentially efficacious lines of treatment which may not necessarily pose the risks previously thought.
AUTHOR CONTRIBUTIONS
Jean‐Luc Vrisakis: Writing – original draft; writing – review and editing. Clare L. Fraser: Writing – review and editing. Udit Nindra: Writing – review and editing. Adel Shahnam: Writing – review and editing. Peter Grimison: Conceptualization; supervision; writing – review and editing.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
ETHICS STATEMENT
None.
CONSENT
Written informed consent was obtained from the patient for publication of this case report in accordance with the journal's patient consent policy.
ACKNOWLEDGMENTS
None.
Vrisakis J‐L, Fraser CL, Shahnam A, Nindra U, Grimison P. A case for the use of chemotherapy in hereditary mitochondrial optic neuropathies: Successful administration of cisplatin/etoposide in a male patient with testicular seminoma and Leber's hereditary optic neuropathy. Clin Case Rep. 2024;12:e9045. doi: 10.1002/ccr3.9045
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Bahr T, Welburn K, Donnelly J, Bai Y. Emerging model systems and treatment approaches for Leber's hereditary optic neuropathy: challenges and opportunities. Biochim Biophys Acta Mol Basis Dis. 2020;1866(6):165743. doi: 10.1016/j.bbadis.2020.165743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Theodorou‐Kanakari A, Karampitianis S, Karageorgou V, et al. Current and emerging treatment modalities for Leber's hereditary optic neuropathy: a review of the literature. Adv Ther. 2018;35(10):1510‐1518. doi: 10.1007/s12325-018-0776-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Catarino CB, von Livonius B, Priglinger C, et al. Real‐world clinical experience with Idebenone in the treatment of Leber hereditary optic neuropathy. J Neuroophthalmol. 2020;40(4):558‐565. doi: 10.1097/WNO.0000000000001023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Newman NJ, Yu‐Wai‐Man P, Biousse V, Carelli V. Understanding the molecular basis and pathogenesis of hereditary optic neuropathies: towards improved diagnosis and management. Lancet Neurol. 2023;22(2):172‐188. doi: 10.1016/S1474-4422(22)00174-0 [DOI] [PubMed] [Google Scholar]
- 5. Zanssen S, Buse G. Successful chemotherapy in a male patient with malignant lymphoma and Leber's hereditary optic neuropathy (LHON). Am J Hematol. 2003;72(4):263‐266. doi: 10.1002/ajh.10301 [DOI] [PubMed] [Google Scholar]
- 6. Zhang J, Ji Y, Lu Y, et al. Leber's hereditary optic neuropathy (LHON)‐associated ND5 12338 T > C mutation altered the assembly and function of complex I, apoptosis and mitophagy. Hum Mol Genet. 2018;27(11):1999‐2011. doi: 10.1093/hmg/ddy107 [DOI] [PubMed] [Google Scholar]
- 7. Chi SC, Cheng HC, Wang AG. Leber hereditary optic neuropathy: molecular pathophysiology and updates on gene therapy. Biomedicine. 2022;10(8):1930. doi: 10.3390/biomedicines10081930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Danielson SR, Wong A, Carelli V, Martinuzzi A, Schapira AHV, Cortopassi GA. Cells bearing mutations causing Leber's hereditary optic neuropathy are sensitized to Fas‐induced apoptosis. J Biol Chem. 2002;277(8):5810‐5815. doi: 10.1074/jbc.M110119200 [DOI] [PubMed] [Google Scholar]
- 9. Zanna C, Ghelli A, Am P, Carelli V, Martinuzzi A, Rugolo M. Apoptotic cell death of cybrid cells bearing Leber's hereditary optic neuropathy mutations is caspase independent. Ann N Y Acad Sci. 2003;1010(1):213‐217. doi: 10.1196/annals.1299.037 [DOI] [PubMed] [Google Scholar]
- 10. Lopez Sanchez MIG, Kearns LS, Staffieri SE, et al. Establishing risk of vision loss in Leber hereditary optic neuropathy. Am J Hum Genet. 2021;108(11):2159‐2170. doi: 10.1016/j.ajhg.2021.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Luca CC, Lam BL, Moraes CT. Erythromycin as a potential precipitating agent in the onset of Leber's hereditary optic neuropathy. Mitochondrion. 2004;4(1):31‐36. doi: 10.1016/j.mito.2004.05.002 [DOI] [PubMed] [Google Scholar]
- 12. Mashima Y, Kigasawa K, Wakakura M, Oguchi Y. Do Idebenone and vitamin therapy shorten the time to achieve visual recovery in Leber hereditary optic neuropathy? J Neuroophthalmol. 2000;20(3):166‐170. doi: 10.1097/00041327-200020030-00006 [DOI] [PubMed] [Google Scholar]
- 13. Jordan B, Margulies A, Cardoso F, et al. Systemic anticancer therapy‐induced peripheral and central neurotoxicity: ESMO–EONS–EANO clinical practice guidelines for diagnosis, prevention, treatment and follow‐up. Ann Oncol. 2020;31(10):1306‐1319. doi: 10.1016/j.annonc.2020.07.003 [DOI] [PubMed] [Google Scholar]
- 14. Albers JW, Chaudhry V, Cavaletti G, Donehower RC. Interventions for preventing neuropathy caused by cisplatin and related compounds. Cochrane Database Syst Rev Published Online March 31. 2014;2014:CD005228. doi: 10.1002/14651858.CD005228.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Khynriam D, Prasad SB. Changes in glutathione‐related enzymes in tumor‐bearing mice after cisplatin treatment. Cell Biol Toxicol. 2002;18(6):349‐358. doi: 10.1023/A:1020899221192 [DOI] [PubMed] [Google Scholar]
- 16. Peters U, Preisler‐Adams S, Lanvers‐Kaminsky C, Jürgens H, Lamprecht‐Dinnesen A. Sequence variations of mitochondrial DNA and individual sensitivity to the ototoxic effect of cisplatin. Anticancer Res. 2003;23(2B):1249‐1255. [PubMed] [Google Scholar]
- 17. Lewis FM, Coman DJ, Murdoch BE. Language skills in a child with Leber hereditary optic neuropathy following intrathecal chemotherapy for acute lymphoblastic leukemia. Pediatr Hematol Oncol. 2010;27(8):626‐635. doi: 10.3109/08880018.2010.503340 [DOI] [PubMed] [Google Scholar]
- 18. Clare G, Colley S, Kennett R, Elston JS. Reversible optic neuropathy associated with low‐dose methotrexate therapy. J Neuroophthalmol. 2005;25(2):109‐112. doi: 10.1097/01.WNO.0000166061.73483.CE [DOI] [PubMed] [Google Scholar]
- 19. Kogachi K, Ter‐Zakarian A, Asanad S, Sadun A, Karanjia R. Toxic medications in Leber's hereditary optic neuropathy. Mitochondrion. 2019;46:270‐277. doi: 10.1016/j.mito.2018.07.007 [DOI] [PubMed] [Google Scholar]
- 20. Pala EE, Pala HG, Ekmekci S, Erbas O. Vitamin C (ascorbic acid) protects from neuropathy caused by cisplatin, through enhanced heat shock protein‐70 and reduced oxidant effect. Rev Assoc Med Bras. 2022;68(8):1017‐1022. doi: 10.1590/1806-9282.20220032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Block KI, Koch AC, Mead MN, Tothy PK, Newman RA, Gyllenhaal C. Impact of antioxidant supplementation on chemotherapeutic efficacy: a systematic review of the evidence from randomized controlled trials. Cancer Treat Rev. 2007;33(5):407‐418. doi: 10.1016/j.ctrv.2007.01.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


