Highlights
-
•
Yeo’s index has good performance for identifying severity of rheumatic MS.
-
•
Yeo’s Index can be used in rheumatic MS with or without mixed valve disease.
-
•
Atrial fibrillation does not affect the performance of Yeo’s Index in assessing severity of rheumatic MS.
Keywords: Yeo’s Index, Mitral stenosis, Rheumatic heart disease, Mitral leaflet separation index
Abstract
Introduction
Yeo’s Index, product of the mitral leaflet separation index and dimensionless index, is a novel measure of the severity of rheumatic mitral stenosis (MS). We assess Yeo’s index in patients with rheumatic MS with or without mixed valve disease.
Methods
In a retrospective cohort study, Yeo’s index was measured in 237 cases of rheumatic MS − 124 in a transthoracic echocardiography validation cohort using mitral valve area (MVA) by pressure half-time and planimetry as comparator and 113 in a transesophageal echocardiography (TEE) validation cohort using TEE three-dimensional MVA as comparator. Patients were considered to have mixed valve disease if they had MS and concomitant mitral regurgitation or aortic valve disease.
Results
There were 113 patients with isolated MS and 124 patients with mixed valve disease. Overall, Yeo’s index ≤ 0.26 cm showed 93.0 % sensitivity and 87.5 % specificity for identifying severe MS (MVA ≤ 1.5 cm2). In isolated MS, Yeo’s index ≤ 0.26 cm showed sensitivity of 94.6 % and specificity of 90.0 % for identifying severe MS, while in mixed valve disease sensitivity was 90.6 % and specificity 86.7 %. Overall, Yeo’s index ≤ 0.15 cm showed 83.6 % sensitivity and 94.3 % specificity for very severe MS (MVA ≤ 1.0 cm2). In isolated MS, the threshold of ≤0.15 cm showed sensitivity of 84.4 % and specificity of 92.6 % for very severe MS, while in mixed valve disease sensitivity was 81.3 % and specificity 95.3 %. The presence of atrial fibrillation did not influence the performance of Yeo’s index.
Conclusion
Yeo’s Index accurately differentiates severity of rheumatic MS with or without mixed valve disease.
1. Introduction
Rheumatic heart disease is still the predominant etiology of mitral stenosis (MS) globally [1], [2]. Transthoracic echocardiography (TTE) is central to the diagnosis and assessment of severity of MS through identification of the characteristic anatomical changes of MS and measurement of mitral valve area (MVA), with measurement of the transmitral mean pressure gradient (MPG) providing supportive evidence of the severity of MS [3], [4]. MVA can be measured using direct two-dimensional (2D) planimetry of the mitral valve (MV) orifice, pressure half-time (PHT), continuity equation (CE) and the proximal isovelocity surface area (PISA) methods [3], [4], [5], [6]. In clinical practice, it is well-recognized that these different methods have their own respective limitations which can introduce sources of error [7]. For instance, while direct planimetry has been regarded the most accurate measurement for MVA on transthoracic echocardiography (TTE), it relies on visualization of the narrowest flow-limiting MV orifice in the parasternal short-axis view which is technically challenging especially in patients with suboptimal images [8], [9]. Similarly, the accuracy of the PHT method is adversely affected by atrioventricular compliance. In patients with abnormal left ventricular (LV) compliance or raised LV diastolic pressure from other causes such as aortic regurgitation (AR), PHT may overestimate MVA resulting in underestimation of the severity of MS [10]. The CE method is inaccurate in the presence of significant mitral or aortic regurgitation [5], [11]. Lastly, the use of the PISA method is limited in clinical practice by the need for an angle correction factor ⍺; this corrects for the conical-shaped isovelocity shells formed by the funnel-shaped mitral valve orifice [11], [12]. The angle ⍺ is challenging to measure and may require manual measurement by hand which limits the practicality of this method [12].
Our group recently proposed a novel index, termed Yeo’s index, as an additional tool for the assessment of rheumatic MS [13]. This index utilises the mitral leaflet separation index (MLSI), which was previously proposed by Seow, Koh and Yeo in 2006, measuring the maximal anatomical diastolic separation of the MV leaflets [14]. Yeo’s index was calculated by the product of the MLSI and the MV dimensionless index (DI). DI was calculated by the pulsed wave (PW) Doppler time velocity integral (TVI) of the left ventricular outflow tract (LVOT) divided by the continuous-wave (CW) Doppler TVI of the MV. In our previous study in patients with isolated rheumatic MS, we demonstrated that Yeo’s index was able to accurately identify severity of MS using MVA assessed by 2D planimetry, PHT, and CE methods as comparators [13].
However, the performance of Yeo’s index in patients with rheumatic MS and concomitant mitral regurgitation or aortic valve disease has not been studied. In the previous study, Yeo’s index was also not validated against transesophageal echocardiography (TEE) three dimensional (3D) MVA, the current gold standard measure of MVA. The objectives of our study are:
to validate the performance of Yeo’s index in patients with rheumatic MS with or without mixed valve disease.
to validate Yeo’s index against TEE 3D MVA.
2. Methods
We performed a retrospective cohort study on 237 cases with rheumatic MS, including both patients with severe and those with non-severe MS, with or without mixed valve disease who underwent echocardiography at our academic medical center. Patients were considered to have mixed valve disease if they have MS and concomitant mitral regurgitation or aortic valve disease of greater than mild severity.
The patients were divided into 2 cohorts:
a cohort of 124 cases with rheumatic MS with or without mixed valve disease who underwent TTE (the ‘TTE validation cohort’)
a cohort of 113 cases with rheumatic MS with or without mixed valve disease who underwent both TTE and transesophageal echocardiography (TEE) (the ‘TEE validation cohort’). We included only patients with TTE done within 1 year of the TEE.
The research protocol was approved by our center’s Institutional Review Board and conforms to the ethical principles of the 1975 Declaration of Helsinki.
Echocardiographic recordings and data as well as relevant clinical information were obtained from the electronic medical records and databases. The MLSI was determined using the method that was used in our group’s previous work [14]. We measured the MLSI as the mean of the maximal diastolic separation of the MV leaflet tips in the parasternal long-axis view and the apical four-chamber view. When patients were in sinus rhythm, a mean of 3 measurements were taken; when patients were in atrial fibrillation (AF), a mean of 5 measurements were taken. The MV DI was calculated by dividing the LVOT PW Doppler TVI by the MV CW Doppler TVI. Yeo’s index was calculated by multiplying the MLSI by the MV DI.
Severe MS was defined as MVA ≤ 1.5 cm2 and very-severe MS as MVA ≤ 1.0 cm2 [3]. For the TTE validation cohort, we considered patients to have severe MS and very severe MS if MVA by both PHT and planimetry were ≤1.5 cm2 and ≤1.0 cm2 respectively. We did not use MVA by CE to determine severity of MS as a significant number of patients had concomitant mitral regurgitation, rendering MVA by CE inaccurate. For the TEE validation cohort, the comparator used was TEE 3D MVA. TEE 3D MVA was measured by multiplanar reconstruction or direct planimetry of the valve orifice in accordance with our institution’s standard protocol for TEE studies [15]. We showed previously in the derivation cohort that Yeo’s index ≤ 0.26 cm and Yeo’s index ≤ 0.147 cm accurately identified severe and very severe MS respectively [13]. We seek to validate the performance of Yeo’s index in the 2 validation cohorts using these cut off values. For reasons of practicality, we used Yeo’s index ≤ 0.15 cm for very severe MS instead.
Continuous variables are presented as mean ± standard deviation and categorical variables as frequency and percentages. P-values less than 0.05 were deemed statistically significant. Statistical analysis was performed with IBM SPSS Statistics Version 23 (IBM Corp., Armonk, NY, USA) as well as MedCalc (MedCalc Software Ltd, Belgium). Intra- and inter-observer variability of Yeo’s index was assessed by intra-class correlation coefficients (ICC) with 95 % confidence intervals (CI) using SPSS reliability analyses [16].
3. Results
3.1. Clinical and echocardiographic characteristics
Baseline demographics and clinical information for the 2 cohorts are presented in Table 1. The patients were relatively older than historical cohort studies of rheumatic MS, with mean age of 60.6 (±13.4), and predominantly female, with 75.9 % of the overall cohort of female sex [17], [18]. The majority of patients in our study cohorts, 56.1 % in total, had pre-existing atrial fibrillation.
Table 1.
Baseline clinical characteristics for the study cohorts.
| TTE Validation Cohort (n = 124) | TEE Validation Cohort (n = 113) | Overall (n = 237) | ||
|---|---|---|---|---|
| Clinical variable | Number (percentage), or mean value (±1 standard deviation) | |||
| Age (years) | 58.8 (±13.9) | 62.6 (±12.6) | 60.6 (±13.4) | |
| Female sex | 96 (77.4 %) | 84 (74.3 %) | 180 (75.9 %) | |
| Ethnicity |
Chinese | 73 (58.9 %) | 71 (62.8 %) | 144 (60.8 %) |
| Malay | 27 (21.8 %) | 16 (14.2 %) | 43 (18.1 %) | |
| Indian | 8 (6.5 %) | 17 (15.0 %) | 25 (10.5 %) | |
| Other ethnicities | 16 (12.9 %) | 9 (8.0 %) | 25 (10.5 %) | |
| Height (cm) | 157.1 (±7.7) | 157.4 (±7.7) | 157.3 (±7.7) | |
| Weight (kg) | 60.3 (±12.9) | 62.3 (±16.2) | 61.3 (±14.5) | |
| BMI (kg/m2) | 24.4 (±4.9) | 25.0 (±5.9) | 24.7 (±5.4) | |
| BSA (m2) | 1.62 (±0.19) | 1.64 (±0.23) | 1.63 (±0.21) | |
| Blood pressure (mmHg) during TTE study | 128.3 (±24.6) / 69.2 (±11.7) | 128.5 (±21.5) / 70.5 (±11.4) | 128.4 (±23.1) / 69.9 (±11.5) | |
| Blood pressure (mmHg) during TEE study | NA | 132.0 (±22.6) / 72.5 (±13.5) | NA | |
| Heart rate (beats per minute) during TTE study | 81.4 (±18.0) | 79.1 (±21.8) | 80.3 (±19.9) | |
| Heart rate during TEE study (beats per minute) | NA | 76.7 (±18.1) | NA | |
| Hypertension | 54 (43.5 %) | 44 (38.9 %) | 98 (41.4 %) | |
| Hyperlipidemia | 55 (44.4 %) | 38 (33.6 %) | 93 (23.2 %) | |
| Diabetes mellitus | 37 (29.8 %) | 18 (15.9 %) | 55 (23.2 %) | |
| Ischaemic heart disease | 15 (12.1 %) | 17 (15.0 %) | 32 (13.5 %) | |
| Stroke or transient ischaemic attack | 21 (16.9 %) | 16 (14.2 %) | 37 (15.6 %) | |
| Atrial fibrillation | 72 (58.1 %) | 60 (54.0 %) | 133 (56.1 %) | |
| Heart failure | 27 (21.8 %) | 25 (22.1 %) | 52 (21.9 %) | |
| Chronic kidney disease | 16 (12.9 %) | 6 (5.3 %) | 22 (9.3 %) | |
| Peripheral vascular disease | 2 (1.6 %) | 0 (0.0 %) | 2 (0.8 %) | |
| Asthma or COPD | 13 (10.5 %) | 4 (3.5 %) | 17 (7.2 %) | |
| Antiplatelet | 33 (26.6 %) | 33 (29.2 %) | 66 (27.8 %) | |
| Anticoagulation | 62 (50.0 %) | 57 (50.4 %) | 119 (50.2 %) | |
| Beta-blocker | 69 (55.6 %) | 63 (55.8 %) | 132 (55.7 %) | |
| Calcium-channel-blocker | 16 (12.9 %) | 11 (9.7 %) | 27 (11.4 %) | |
| Diuretic | 39 (31.5 %) | 28 (24.8 %) | 67 (28.3 %) | |
| ACE-I/ARB | 30 (24.2 %) | 15 (13.3 %) | 45 (19.0 %) | |
| MRA | 3 (2.4 %) | 6 (5.3 %) | 9 (3.8 %) | |
Abbreviations: ACE-I; angiotensin converting enzyme-inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BSA, body surface area; COPD, chronic obstructive pulmonary disease; MRA, mineralocorticoid receptor antagonist.
The baseline echocardiographic data for the 2 cohorts are shown in Table 2. For the TEE validation cohort, the median time between the TTE study and the TEE study was 28 (interquartile range 7–162) days. As expected, the mean MVA by PHT, CE and planimetry in the TEE validation cohort was smaller than the TTE validation cohort as the indication for TEE in a significant number of these patients was planned MV intervention for significant MS. In the TTE cohort, the mean pressure gradient was 7.7 (±4.0) mmHg while in the TEE cohort the mean pressure gradient was 8.0 (±3.8) mmHg. There were 143 patients with severe MS (MVA ≤ 1.5 cm2). Of these, 97 (67.8 %) patients had low gradient severe MS and 46 (32.2 %) patients had high gradient severe MS. Yeo’s index tended to be lower in patients with high-gradient severe MS compared to those with low-gradient severe MS. (low-gradient severe MS, 0.20 ± 0.07 cm; high-gradient severe MS 0.15 ± 0.07 cm; p < 0.001). In patients with low gradient severe MS, 90.7 % had Yeo’s index ≤ 0.26 cm (indicating severe MS) whereas 95.7 % of patients with high gradient severe MS had Yeo’s index ≤ 0.26 cm. There was no statistically significant difference in the proportion of patients with severe MS whose Yeo’s index is also ≤0.26 cm in the low gradient compared to the high gradient group (Fisher exact test, p = NS).
Table 2.
Baseline echocardiographic parameters for the study cohorts.
| TTE Validation Cohort (n = 124) | TEE Validation Cohort (n = 113) | Overall (n = 237) | |
|---|---|---|---|
| Echocardiographic Parameters | Mean value (±1 standard deviation) | ||
| Left atrial diameter (mm) | 52.6 (±7.7) | 52.0 (±7.94) | 52.3 (±7.82) |
| Left atrial volume (ml) | 114.9 (±52.1) | 110.6 (±45.5) | 112.9 (±49.1) |
| Left atrial volume index (ml/m2) | 72.4 (±35.6) | 69.5 (±32.6) | 71.1 (±34.1) |
| Left ventricle mass index (g/m2) | 97.4 (±30.8) | 90.0 (±34.1) | 94.1 (±32.5) |
| Left ventricle end diastolic volume (ml) | 105.4 (±38.8) | 93.2 (±36.3) | 99.9 (±38.1) |
| Left ventricle end systolic volume (ml) | 41.4 (±23.0) | 35.5 (±21.4) | 38.7 (±22.4) |
| Left ventricle stroke volume (ml) | 64.0 (±25.0) | 57.7 (±20.2) | 61.2 (±23.1) |
| Left ventricle ejection fraction (%) | 58.0 (±10.3) | 57.7 (±7.6) | 57.9 (±9.1) |
| Left ventricle outflow tract diameter (mm) | 19.6 (±1.8) | 19.8 (±2.1) | 19.7 (±1.9) |
| Left ventricle outflow tract pulsed-wave TVI (cm) | 17.3 (±3.8) | 19.0 (±7.4) | 18.1 (±5.8) |
| Heart rate during echocardiographic study (bpm) | 80.8 (±18.9) | 72.8 (±16.8) | 77.2 (±18.4) |
| Estimated cardiac output (L/min) | 4.38 (±1.42) | 4.13 (±1.62) | 4.27 (±1.52) |
| Estimated cardiac index (L/min/m2) | 2.72 (±0.92) | 2.54 (±0.90) | 2.64 (±0.92) |
| Mitral valve continuous-wave TVI (cm) | 54.2 (±16.2) | 61.6 (±17.5) | 57.4 (±17.2) |
| Pulmonary artery systolic pressure (mmHg) | 49.5 (±16.4) | 47.8 (±15.9) | 48.7 (±16.2) |
| MVA by two-dimensional planimetry (cm2) | 1.55 (±0.58) | 1.17 (±0.38) | 1.37 (±0.53) |
| MVA by pressure half-time (cm2) | 1.59 (±0.58) | 1.37 (±0.46) | 1.46 (±0.54) |
| MVA by continuity (cm2) | N.A. | 0.97 (±0.44) | N.A. |
| MVA by three-dimensional planimetry (cm2) | N.A | 1.11 (±0.40) | N.A. |
| Transmitral mean pressure gradient (mmHg) | 7.7 (±4.0) | 8.0 (±3.8) | 7.8 (±3.9) |
| Transmitral maximum pressure gradient (mmHg) | 17.6 (±6.6) | 18.1 (±7.0) | 17.8 (±6.7) |
| Mitral valve DI | 0.345 (±0.113) | 0.328 (±0.133) | 0.320 (±0.104) |
| Mitral leaflet separation index | 0.89 (±0.29) | 0.62 (±0.20) | 0.76 (±0.28) |
| Yeo’s Index | 0.34 (±0.19) | 0.19 (±0.10) | 0.26 (±0.18) |
| Mixed valvular pathology, of which (n, %): |
96 (77.4 %) | 28 (24.8 %) | 124 (52.3 %) |
Abbreviations: DI, dimensionless index; MVA, mitral valve area; TVI, time-velocity integral.
There were 96 (77.4 %) and 28 (24.8 %) patients with mixed valve disease in the TTE and TEE validation cohort respectively. In the TTE validation cohort, the majority of those with mixed valve disease had concomitant MR (68 patients, 54.8 %) while a further 23 (18.5 %) had concomitant AS, and 5 (4.0 %) had both MR and AS. In the TEE validation cohort, 14 patients (11.4 %) had concomitant MR, while 8 (7.1 %) had AS, and 4 had AR (3.5 %). There was 1 patient (0.9 %) who had both MR and AS, and another patient (0.9 %) who had both AS and AR. In total, of 88 patients with concomitant MR, 58 had moderate MR while 30 had severe MR; of 38 patients with concomitant AS, 20 had moderate AS while 18 had severe AS. Regarding AR, 2 of 5 patients with concomitant AR had moderate AR while the remaining 3 had severe AR.
The intra-observer and inter-observer variabilities for Yeo’s index as assessed by the intraclass correlation coefficients (r) were 0.98 (95 % CI 0.90–0.99) and 0.96 (95 % CI 0.86–0.98), respectively.
3.2. Validation of Yeo’s index
Sensitivity and specificity of Yeo’s Index ≤ 0.26 cm and Yeo’s Index ≤ 0.15 cm in identifying severe and very severe MS in the TTE and TEE validation cohorts were shown in Table 3. Overall, when the TTE and TEE validation cohorts were combined, Yeo’s Index ≤ 0.26 cm showed 93.0 % sensitivity and 87.5 % specificity for severe MS (MVA ≤ 1.5 cm2), while Yeo’s Index ≤ 0.15 cm showed 83.6 % sensitivity and 94.3 % specificity for very severe MS (MVA ≤ 1.0 cm2). Both threshold values of Yeo’s index showed high sensitivity and specificity of identifying severity of MS in patients with isolated MS as well as patients with mixed valve disease.
Table 3.
Sensitivity and specificity of Yeo’s index for severe and very-severe mitral stenosis in the validation cohorts.
| Threshold Values | Sensitivity | Specificity | ||
|---|---|---|---|---|
| TEE Validation | Yeo’s Index ≤ 0.15 (very-severe MS, MVA ≤ 1.0 cm2) | 82.6 % | 89.6 % | |
| Yeo’s Index ≤ 0.26 (severe MS, MVA ≤ 1.5 cm2) | 95.2 % | 100.0 % | ||
| TTE Validation | Yeo’s Index ≤ 0.15 (very-severe MS, MVA ≤ 1.0 cm2) | 86.7 % | 97.2 % | |
| Yeo’s Index ≤ 0.26 (severe MS, MVA ≤ 1.5 cm2) | 88.7 % | 85.9 % | ||
| Combined Validation Cohorts |
Yeo’s Index ≤ 0.15 (very-severe MS, MVA ≤ 1.0 cm2) |
Isolated MS | 84.4 % | 92.6 % |
| Mixed Valve Disease | 81.3 % | 95.3 % | ||
| Combined | 83.6 % | 94.3 % | ||
| Yeo’s Index ≤ 0.26 (severe MS, MVA ≤ 1.5 cm2) | Isolated MS | 94.6 % | 90.0 % | |
| Mixed Valve Disease | 90.6 % | 86.7 % | ||
| Combined | 93.0 % | 87.5 % | ||
Abbreviations: MS, mitral stenosis; MVA, mitral valve area; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography.
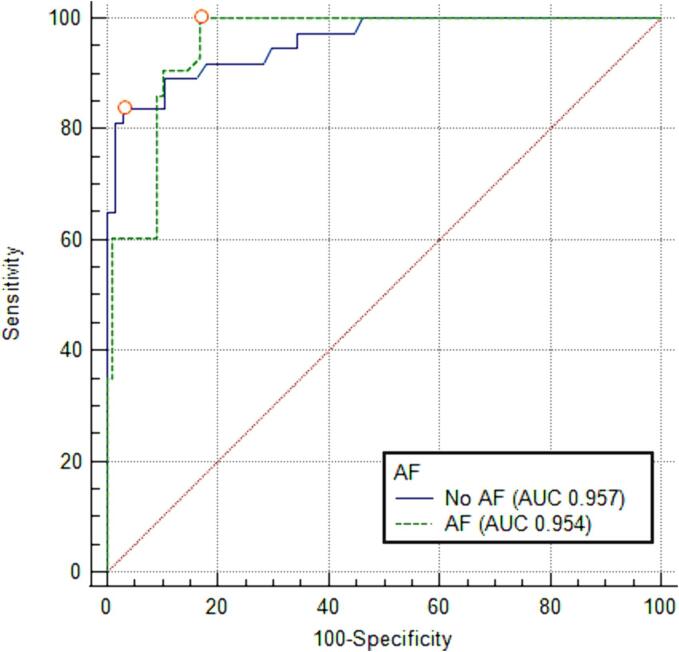
The presence or absence of atrial fibrillation (AF) did not significantly affect the performance of Yeo’s index (AF absent, n = 104, area under the curve (AUC) = 0.957 (95 % CI 0.919–0.994); AF present, n = 133, AUC 0.954 (95 % CI 0.923–0.985); p-value nonsignificant [NS]). The receiver operating characteristic (ROC) curves comparing the performance of Yeo’s index in patients with and without AF are presented in Fig. 1.
Fig. 1.
Receiver operating characteristic curves comparing the performance of Yeo’s index in patients with and without AF for severe MS, MVA ≤ 1.5 cm2. Abbreviations: AF, atrial fibrillation; AUC, area-under-the-curve.
As there was a time interval between the TTE and TEE studies for the TEE validation cohort with a median time interval of 28 days, we divided the TEE cohort into two subgroups with time intervals ≤28 days and >28 days between the studies respectively to perform subgroup analysis. We undertook ROC analysis to evaluate Yeo’s index against TEE 3D MVA. For the classification of severe MS, the AUC values were 0.951 (95 % CI 0.893–1.000) for the subgroup ≤28 days compared to 0.988 (95 % CI 0.959–1.000) for the subgroup > 28 days, p = NS. In comparison, for very severe MS, the AUC values were 0.917 (95 % CI 0.822–1.000) for the subgroup ≤28 days and 0.888 (95 % CI 0.801–0.976) for the subgroup > 28 days, p = NS.
As our patients were relatively older than historical cohort studies of rheumatic MS, with mean age of 60.6 (±13.4) years, we subdivided the patients into 2 groups (≤60 years old and > 60 years old) to evaluate the effect of age on the performance of Yeo’s index. We found that the AUC values for Yeo’s index in classifying severe MS were not significantly different between the 2 groups (AUC 0.948 (95 % CI 0.896–––1.000) versus AUC 0.957 (95 % CI 0.928–0.987) respectively, p = NS). Therefore, Yeo’s index had similar performance in younger and older patients.
4. Discussion
Our study showed that Yeo’s Index had high sensitivity and specificity for the identification of severe and very severe MS in patients with rheumatic MS with or without mixed valve disease when compared with MVA by PHT and planimetry in the TTE validation cohort and TEE 3D MVA in the TEE validation cohort.
Yeo’s Index was first proposed as a potentially useful novel adjunct to conventional measures of MS severity on the basis of its performance compared to MVA by PHT, planimetry and CE as comparators [13]. Yeo’s index was conceived as conceptually advantageous as it combined an anatomical measure of MS severity (the MLSI) with a functional or haemodynamic measure of severity of MS (the DI), unlike existing forms of assessment in MS which assess the severity of the valve lesion by either one, but not both, of these parameters. It may also avoid some limitations of existing methods to assess MVA such as technical difficulties in planimetering the narrowest mitral valve orifice. In contrast, the narrowest part of the mitral valve orifice can be easily identified in the parasternal long axis and apical 4 chamber views which are used to determine the MLSI. Unlike the transmitral gradient which is flow dependent, DI is relatively flow independent because it incorporates both the mitral TVI and LVOT TVI. This helps to avoid flow-dependency of the index as changes in flow would have similar directional effect on the mitral TVI and LVOT TVI. Importantly, the measurements required to determine Yeo’s index can be obtained from standard echocardiographic study without the need for acquisition of additional images. Therefore, the diagnostic performance of Yeo’s index does not come at the expense of longer scan time.
In our earlier work, a Yeo’s Index of ≤ 0.26 cm was posited to accurately identify patients with severe MS (≤1.5 cm2), and Yeo’s index ≤ 0.15 cm accurately identified very severe MS (≤1.0 cm2). However, these cut-off values were derived in patients with isolated rheumatic MS, which limited its real-world clinical applicability as mixed rheumatic valve disease is not uncommon. The EuroHeart study demonstrated 20.2 % prevalence of unspecified multiple valve pathologies in patients with valvular heart disease [2]. More recently, in an epidemiological study by Andell et al., 28.3 % of patients with MS had concomitant AS, while a further 17.9 % had concomitant MR [19]. A further limitation to the use of Yeo’s Index was that there was no validation of Yeo’s index against TEE 3D MVA which has been shown to yield highly accurate measurements of MVA [15], [20], [21].
Our definition of severe MS and very severe MS in the TTE validation cohort requires that MVA by both 2D planimetry and PHT must be ≤1.5 cm2 and ≤1.0 cm2 respectively. This rigorous definition ensured that the identified patients truly had severe and very severe MS respectively. MVA by continuity equation was not used because the study patients included patients with mitral regurgitation which made MVA assessment by continuity equation invalid. Recently, TEE 3D MVA has emerged as the gold standard measure of MS severity [15]. The validation against TEE 3D MVA is important as it validated Yeo’s index against the current gold standard measure of MVA. We believe that this is a strength of our study.
We found that Yeo’s index was able to identify severe and very severe MS in patients with isolated MS as well as those with mixed valve disease with high sensitivity and specificity. Hence, the result of this study extended the applicability of Yeo’s Index to the frequently-encountered setting of patients with rheumatic MS and concomitant valve lesions such as mitral regurgitation and aortic valve disease.
The performance of Yeo’s index in correctly identifying severity of MS is independent of the presence of AF. This is important as AF is commonly associated with MS. Although there is a time interval between the TTE and TEE study, it is not expected to affect the validity of the study as the progression of mitral stenosis is slow [22], [23]. Furthermore, there was no significant difference in the AUC values in patients with a short time interval between the 2 studies (≤28 days) compared with those with a longer time interval (>28 days).
4.1. Limitations
We did not study patients with non-rheumatic MS, a group consisting of patients with degenerative or calcific MS. With an aging population, the prevalence of degenerative MS is expected to increase in the developed world. Even so, the majority of patients with MS are still of rheumatic etiology even in European populations. We did not correlate with findings on cardiac catheterization. In contemporary practice, cardiac catheterization is rarely performed for assessment of MS and TEE 3D MVA is widely considered as the new gold standard method of assessing MVA. Another key limitation is the median time interval of 28 days between the TTE and comparator TEE study which may have introduced bias due to variability in haemodynamic conditions between the two studies. However, in a subgroup analysis within the TEE cohort to evaluate this concern, there was no significant difference in AUC values for Yeo’s index based on stratification into two subgroups ≤28 days and >28 days between the TTE and TEE study. Our study population, with a mean age of 60.6 years, is relatively older compared to other cohorts with MS and further validation should be sought in younger populations, who may have relatively fewer comorbidities and relatively less diastolic dysfunction which is associated with advanced age.
5. Conclusions
Our study validated the high sensitivity and specificity of Yeo’s Index for the identification of severe and very severe MS in patients with rheumatic MS with or without mixed valve disease. Yeo’s index may be a useful addition to current measures of MS severity, and can be particularly useful in situations where there are discrepancies in MVA derived by existing methods.
Sources of funding
CHS was supported by the National University of Singapore’s Junior Academic Fellowship Scheme as well as the Singapore Ministry of Health National Medical Research Council’s Transition Award (MOH-001368-00).
CRediT authorship contribution statement
Ryan Leow: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Tony Yi-Wei Li: Writing – review & editing, Validation, Software, Resources, Investigation, Formal analysis, Data curation. William K.F. Kong: Writing – review & editing, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Conceptualization. Kian-Keong Poh: Writing – review & editing, Validation, Supervision, Resources, Project administration, Investigation, Funding acquisition, Conceptualization. Ivandito Kuntjoro: Writing – review & editing, Visualization, Supervision, Investigation, Data curation. Ching-Hui Sia: . Tiong-Cheng Yeo: Writing – review & editing, Writing – original draft, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2024.101447.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
A patient with known rheumatic heart disease was evaluated for exertional dyspnoea. Transthoracic echocardiography demonstrated mitral stenosis with discrepant mitral valve area with two-dimensional planimetered mitral valve area of 0.90cm2 compared to a pressure half-time derived mitral valve area of 1.58cm2. The mitral leaflet separation index was measured as shown with a value of 0.50cm and the mitral valve dimensionless index was 0.32. Yeo’s Index was calculated at 0.16cm which was suggestive of severe mitral stenosis. Transoesophageal echocardiography confirmed severe mitral stenosis with three-dimensional planimetered mitral valve area of 1.11cm2 as shown with representative images. Percutaneous transmitral commissurotomy was successfully undertaken for relief of symptomatic mitral stenosis with substantial improvement in symptoms.
References
- 1.Chandrashekhar Y., Westaby S., Narula J. Mitral stenosis. Lancet. 2009;374(9697):1271–1283. doi: 10.1016/S0140-6736(09)60994-6. Epub 2009 Sep 9 PMID: 19747723. [DOI] [PubMed] [Google Scholar]
- 2.Iung B., Baron G., Butchart E.G., et al. A prospective survey of patients with valvular heart disease in Europe: the EuroHeart Survey on valvular heart disease. Eur. Heart J. 2003;24:1231–1243. doi: 10.1016/s0195-668x(03)00201-x. [DOI] [PubMed] [Google Scholar]
- 3.C.M. Otto, R.A. Nishimura, R.O. Bonow, et al., ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021 Feb 2;143(5):e35-e71. doi: 10.1161/CIR.0000000000000932. Epub 2020 Dec 17. Erratum in: Circulation. 2021 Feb 2;143(5):e228. Erratum in: Circulation. 2021 Mar 9;143(10):e784. PMID: 33332149. [DOI] [PubMed]
- 4.Baumgartner H., Hung J., Bermejo J., et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J. Am. Soc. Echocardiogr. 2009;22:1–23. doi: 10.1016/j.echo.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 5.Nakatani S., Masuyama T., Kodama K., et al. Value and limitations of Doppler echocardiography in the quantification of stenotic mitral valve area: comparison of the pressure half-time and the continuity equation methods. Circulation. 1988;77(1):78–85. doi: 10.1161/01.cir.77.1.78. PMID: 3335074. [DOI] [PubMed] [Google Scholar]
- 6.Rifkin R.D., Harper K., Tighe D. Comparison of proximal isovelocity surface area method with pressure half-time and planimetry in evaluation of mitral stenosis. J. Am. Coll. Cardiol. 1995;26(2):458–465. doi: 10.1016/0735-1097(95)80023-a. PMID: 7608451. [DOI] [PubMed] [Google Scholar]
- 7.Wunderlich N.C., Beigel R., Siegel R.J. Management of mitral stenosis using 2D and 3D echo-Doppler imaging. J. Am. Coll. Cardiol. Img. 2013 Nov;6(11):1191–1205. doi: 10.1016/j.jcmg.2013.07.008. PMID: 24229772. [DOI] [PubMed] [Google Scholar]
- 8.Silbiger J.J. Advances in rheumatic mitral stenosis: echocardiographic, pathophysiologic, and hemodynamic considerations. J. Am. Soc. Echocardiogr. 2021 Jul;34(7):709–722.e1. doi: 10.1016/j.echo.2021.02.015. Epub 2021 Feb 27 PMID: 33652082. [DOI] [PubMed] [Google Scholar]
- 9.Faletra F., Pezzano A., Jr, Fusco R., et al. Measurement of mitral valve area in mitral stenosis: four echocardiographic methods compared with direct measurement of anatomic orifices. J. Am. Coll. Cardiol. 1996;28(5):1190–1197. doi: 10.1016/S0735-1097(96)00326-9. PMID: 8890815. [DOI] [PubMed] [Google Scholar]
- 10.Thomas J.D., Wilkins G.T., Choong C.Y., et al. Inaccuracy of mitral pressure half-time immediately after percutaneous mitral valvotomy. Dependence on transmitral gradient and left atrial and ventricular compliance. Circulation. 1988;78(4):980–993. doi: 10.1161/01.cir.78.4.980. PMID: 3168200. [DOI] [PubMed] [Google Scholar]
- 11.Robinson S., Ring L., Augustine D.X., et al. The assessment of mitral valve disease: a guideline from the British Society of Echocardiography. Echo Res. Pract. 2021;8(1):G87–G136. doi: 10.1530/ERP-20-0034. PMID: 34061768; PMCID: PMC8495880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Messika-Zeitoun D., Cachier A., Brochet E., Cormier B., Iung B., Vahanian A. Evaluation of mitral valve area by the proximal isovelocity surface area method in mitral stenosis: could it be simplified? Eur. J. Echocardiogr. 2007;8(2):116–121. doi: 10.1016/j.euje.2006.02.007. Epub 2006 Apr 17 PMID: 16616646. [DOI] [PubMed] [Google Scholar]
- 13.Leow R., Kong W.K.F., Li T.Y., Poh K.K., Sia C.H., Yeo T.C. Yeo's index: a novel index that combines anatomic and haemodynamic assessment of the severity of mitral stenosis. Int. J. Cardiol. 2023 Dec;1(392) doi: 10.1016/j.ijcard.2023.131350. Epub 2023 Sep 7. PMID: 37689399. [DOI] [PubMed] [Google Scholar]
- 14.Seow S.C., Koh L.P., Yeo T.C. Hemodynamic significance of mitral stenosis: use of a simple, novel index by 2-dimensional echocardiography. J. Am. Soc. Echocardiogr. 2006;19(1):102–106. doi: 10.1016/j.echo.2005.07.012. PMID: 16423677. [DOI] [PubMed] [Google Scholar]
- 15.Schlosshan D., Aggarwal G., Mathur G., et al. Real-time 3D transesophageal echocardiography for the evaluation of rheumatic mitral stenosis. J. Am. Coll. Cardiol. Img. 2011;4(6):580–588. doi: 10.1016/j.jcmg.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Shrout P.E., Fleiss J.L. Intraclass correlations: uses in assessing rater reliability. Psychol. Bull. 1979;86(2):420–428. doi: 10.1037//0033-2909.86.2.420. PMID: 18839484. [DOI] [PubMed] [Google Scholar]
- 17.Olesen K.H. The natural history of 271 patients with mitral stenosis under medical treatment. Br. Heart J. 1962;24(3):349–357. doi: 10.1136/hrt.24.3.349. PMID: 14481743; PMCID: PMC1017892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rowe J.C., Bland E.F., Sprague H.B., et al. The course of mitral stenosis without surgery: ten- and twenty-year perspectives. Ann. Intern. Med. 1960;52:741–749. doi: 10.7326/0003-4819-52-4-741. PMID: 14439687. [DOI] [PubMed] [Google Scholar]
- 19.Andell P., Li X., Martinsson A., et al. Epidemiology of valvular heart disease in a Swedish nationwide hospital-based register study. Heart. 2017;103(21):1696–1703. doi: 10.1136/heartjnl-2016-310894. Epub 2017 Apr 21. PMID: 28432156; PMCID: PMC5749343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Min S., Song J., Kim Y., et al. Discrepancy between mitral valve areas measured by two-dimensional planimetry and three-dimensional transoesophageal echocardiography in patients with mitral stenosis. Heart. 2013;99:253–258. doi: 10.1136/heartjnl-2012-302742. [DOI] [PubMed] [Google Scholar]
- 21.Xie M.X., Wang X.F., Cheng T.O., Wang J., Lu Q. Comparison of accuracy of mitral valve area in mitral stenosis by real-time, three-dimensional echocardiography versus two-dimensional echocardiography versus Doppler pressure half-time. Am. J. Cardiol. 2005;95(12):1496–1499. doi: 10.1016/j.amjcard.2005.02.023. PMID: 15950582. [DOI] [PubMed] [Google Scholar]
- 22.Sagie A., Freitas N., Padial L.R., et al. Doppler echocardiographic assessment of long-term progression of mitral stenosis in 103 patients: valve area and right heart disease. J. Am. Coll. Cardiol. 1996;28:472–479. doi: 10.1016/0735-1097(96)00153-2. [DOI] [PubMed] [Google Scholar]
- 23.Zühlke L., Engel M.E., Karthikeyan G., Rangarajan S., Mackie P., Cupido B., et al. Characteristics, complications, and gaps in evidence-based interventions in rheumatic heart disease: the Global Rheumatic Heart Disease Registry (the REMEDY study) Eur. Heart J. 2014;36(18):1115–1122. doi: 10.1093/eurheartj/ehu449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A patient with known rheumatic heart disease was evaluated for exertional dyspnoea. Transthoracic echocardiography demonstrated mitral stenosis with discrepant mitral valve area with two-dimensional planimetered mitral valve area of 0.90cm2 compared to a pressure half-time derived mitral valve area of 1.58cm2. The mitral leaflet separation index was measured as shown with a value of 0.50cm and the mitral valve dimensionless index was 0.32. Yeo’s Index was calculated at 0.16cm which was suggestive of severe mitral stenosis. Transoesophageal echocardiography confirmed severe mitral stenosis with three-dimensional planimetered mitral valve area of 1.11cm2 as shown with representative images. Percutaneous transmitral commissurotomy was successfully undertaken for relief of symptomatic mitral stenosis with substantial improvement in symptoms.