Abstract
Background
Endoscopic staplers are common surgical devices used for the ligation and division of vasculature in thoracic procedures. When a stapler ligates and divides pulmonary vasculature, potentially catastrophic intraoperative bleeding at the staple-line may occur. The aim of this study was to confirm the safety and discuss the utility of a two-row stapler reload, by assessing the incidence of clinically necessary intraoperative hemostatic intervention when applied to pulmonary vasculature in real-world applications.
Methods
This study was designed as a prospective non-comparative registry study conducted in seven centers across the United States, to confirm the safety and performance of Signia™ Small Diameter Reloads (SDR) when used for indicated thoracic surgical procedures. The primary endpoint was the incidence of hemostatic intervention related to the ligation and division of pulmonary arteries and veins. A five-point Likert scale scored hemostasis of each SDR staple-line. Secondary endpoints included the incidence of device-related only adverse events (AEs), device deficiencies, and procedure-related hospital readmission up to and including 30 days post operation.
Results
SDR was fired 302 times across pulmonary vasculature in 120 subjects. Three firings required clinically necessary hemostatic intervention for an intervention rate of 0.99% (3 of 302 firings). Moreover, 97.5% (117 of 120 subjects) had intact SDR staple-lines regardless of surgical access or stapler handle preference. Only 4 (3.3%) thoracoscopic and robotic procedures converted to open, but none were due to SDR staple-lines. There was no statistically significant difference between the Likert score of transected arteries compared to veins (P=0.61). There were no device deficiencies or device-only related AEs reported.
Conclusions
In this study, the two-row stapler reloads demonstrated favorable safety and efficacy profiles when fired across hilar vessels in the thoracic space with a 99% hemostatic rate, independent of surgical access and stapler handle preference.
Keywords: Surgical stapler, hemostasis, pulmonary vasculature
Highlight box.
Key findings
• Signia™ Small Diameter Reload (SDR) staple-lines had a clinically necessary intraoperative hemostatic intervention rate of only 0.99%.
• There was no statistical difference between SDR’s performance, in relation to staple-line bleeding, regardless of vasculature, surgical access or stapler handle preference.
• There were no reported device-related injuries to organs or surrounding tissue.
What is known and what is new?
• Three-row surgical staplers are reliable devices that ligate and divide pulmonary vasculature; however, the need to optimize a surgical solution for difficult to reach structures is clear. SDR addresses this unmet need without introducing new risks or harms to subjects.
What is the implication, and what should change now?
• This study demonstrates that SDR is an adequate device to ligate and divide pulmonary vasculature with added benefits of access due to its narrow profile.
Introduction
Background
Since the inception of the first mechanical stapling device in 1906, stapling technology has advanced dramatically. What began with wire staples and staggered rows over a century ago transformed mechanical linear stapling to what is now a typical instrument applied to pulmonary vasculature in thoracic procedures (1). Although the stapler has evolved significantly since the early 1900s, especially in physical design, the fundamental principles of surgical stapling have remained the same. The use of B-formed staples for strong tissue approximation and the formation of staggered staple-lines to preserve blood supply are foundational characteristics that ensure adequate performance of a surgical stapler (2). Stapling technology has advanced dramatically, offering a range of features and functionalities to enable minimally invasive techniques to assist physicians in performing consistent, effective, and reliable internal repairs (3-6). Minimally invasive surgical staplers have typically employed three staggered rows of titanium staples on either side of the cutline with a blade dividing tissue longitudinally. The necessary width for application of six total rows has proven limiting as access has become more minimally invasive. Regardless of the impressive advances in the technology of surgical stapling over the last century, there remains a need for improved hemostasis to reduce detrimental staple-line failures which can cause significant complications, including fatal hemorrhage (7).
Rationale and knowledge gap
Staple-line bleeding can be indicative of a failed staple-line due to a malfunction of the stapler itself or caused by other factors such as tissue fragility, stapler motion during stapling, stapler-tissue thickness mismatch, or technical failure (8,9). For this reason, the true cause of intraoperative hemostatic failure is difficult to pinpoint. A review of literature demonstrates the rate of intraoperative pulmonary vasculature bleeding ranges between 0.3% and 8.3% (9-11). Major adverse outcomes can include a longer time under general anesthesia, uncontrolled bleeding requiring a blood transfusion, injuries to vasculature, airway, and other structures, need for more extensive lung resection, and conversion to an open procedure (12-14). Moreover, conversions to open procedures may increase operating time, recovery time, chance of adjacent tissue injury due to more lung manipulation, and respiratory complications (6,15,16).
Objective
Though the benefit of standard surgical staplers is clear, the need for smaller reloads has grown over the years; surgeons prefer an optimized solution for procedures where structures are difficult to reach due to size, space, and location, especially when firing in the thoracic cavity. This real-world study evaluated the safety and utilization of a smaller two-row stapler, with four total rows of staples applied, for indicated thoracic procedures. To confirm the safety and performance of Signia™ Small Diameter Reload (SDR) in thoracic procedures, this study specifically focused on its application on pulmonary vasculature. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-179/rc).
Methods
Study design
This study was designed as a post-market prospective, observational study focusing on the safety and performance of a commercially available two-row stapler reload in indicated surgical procedures to support a regulatory body submission. The thoracic cohort data described here is part of a larger study with adult abdominal and pediatric abdominal cohorts as well. Thoracic enrollment was conducted in seven United States hospitals between August 2021 through May 2023 using Medtronic’s Post Market Safety Registry Platform (PSR) to characterize the safety and performance of the reload in a real-world setting. The seven hospitals included: Duke University, Cooper University Health Care, Cedars-Sinai, Rush University, University of Pittsburgh Medical Center, Mary Washington Hospital, and Virginia Cancer Specialists at Inova Fairfax Hospital. Although the enrollment period spanned 22 months, not all seven sites were activated at the start of the study; site activation was staggered, and Principal Investigators began enrolling subjects once activation was complete. Because this study was conducted on a platform intended to capture real-world device use, surgeons used a compatible stapler handle of their preference to fire the reload and chose which reload type to use for transection of pulmonary vasculature, as all three commercially available SDR reload configurations have vascular indication. Reload type and vessel diameter were not collected due to the observational nature of the study.
The study was conducted in accordance with Good Clinical Practice and the Declaration of Helsinki (as revised in 2013), as well as all other applicable local, state, and federal regulatory requirements, and is registered with ClinicalTrials.gov (NCT05095935). All seven sites received IRB approval prior to site activation. The reviewing entities included: Duke Health IRB (Pro0010867-KSP-7.0), Western Institutional Review Board-Copernicus Group for three of the sites (IRB for Virginia Cancer Specialists: 1342927, Cooper: 1301179 and UPMC: 1324230), Cedars Sinai Office of Research Compliance and Quality Improvement (IRB 00002913), Rush University IRB (ORA Number: 15033005-IRB01-AM09) and Mary Washington Healthcare IRB (IRB 2022-02). Six of the seven sites’ IRBs approved the waiver of written informed consent. Rush University IRB required written informed consent. There were no protocol deviations during this study.
Hemostatic intervention
The primary outcome measure of this study was the incidence of intraoperative hemostatic intervention related to ligation and division of pulmonary arteries and veins. After each firing of SDR, surgeons were asked to assess each staple-line for bleeding and to describe any additional intervention applied to the staple-line. Hemostatic intervention was defined as staple-line bleeding detected and controlled intraoperatively by applying additional stapler reloads, over-sewing with suture, placing clips, applying compression greater than what is considered typical, use of hemostatic agents and/or buttress, and/or use of energy or addressing bleeding that occurs intraoperatively requiring blood or blood product transfusion or an additional surgical procedure (i.e., conversion to open). Surgeons characterized the type of intervention as standard of care (typical surgeon practice) or clinically necessary intervention (to preclude injury to the subject); only clinically necessary staple-line interventions were counted towards the primary endpoint. As this was a registry study, no deviation of the surgeon’s typical practice was requested.
Safety and efficacy
Additional data points were collected for safety and efficacy analyses as secondary measures. All adverse events (AEs) related to the device only or both procedure and device and device deficiencies affecting the intended performance of the device up to and including 30 days following each procedure were captured. Additionally, the incidence of repeat hospital admission for primary procedure-related complications and intraoperative and postoperative staple-line assessments were analyzed. All safety events were reported based on surgeon assessment and reviewed internally with Medtronic’s Patient Safety team.
Due to the observational nature of the study, exploratory comparative statistical analyses using Fisher exact tests were conducted to determine correlations in staple-line integrity with other variables such as pulmonary veins versus arteries, stapler handle preference, conversions to open procedures and types of procedures. Moreover, a surgeon satisfaction survey collected the surgeons’ opinions on the usability of the stapler as well as their preferences of SDR in comparison to other commercially available staplers for ligating and dividing pulmonary vasculature.
Study population
Subjects undergoing thoracic procedures during which SDR was used for ligating and dividing pulmonary vasculature were enrolled in this study if they were surgical candidates per the IFU and consented within the enrollment window of the therapy received. Subjects who were, or were expected to be, inaccessible for follow-up, and subjects also enrolled in, or planned to enroll in, any concurrent drug/device study that may have confounded the results within this study were excluded from the study. Because the registry was utilized as the mechanism for data collection, inclusion and exclusion criteria was intentionally broad to reduce bias and ensure a wide subject population was captured.
Device
The study device used for every firing on pulmonary vasculature was the Signia™ Small Diameter Reload (SDR) (Covidien, Mansfield, MA, USA). SDR was developed to optimize a solution for procedures where structures are difficult to reach due to size, space, or location. SDR exemplifies an incremental change to an existing technology. Figure 1 is an example of one available configuration. SDR can be used with compatible manual and powered handles.
Figure 1.

SigniaTM Small Diameter Reloads (Medtronic) have a narrow shaft allowing to pass through 8 mm or larger ports (©2023 Medtronic. All rights reserved. Used with the permission of Medtronic).
Compared to existing 12 mm three-row staplers, SDR’s narrow profile allows for a smaller trocar (8 mm) to be leveraged for thoracoscopic or robotic surgeries and offers an alternative device to dissect vascular structures and/or thin tissue (compared to larger staplers or bipolar energy devices). The curved tip on the distal end of the reloads can aid in positioning the reload around target tissues or vessels for firing and placement of staples. The reloads place two staggered rows of titanium staples on either side of the cutline and subsequently divide the tissue. The height of the staples deployed is determined by the selection of the single use reload.
Statistical analysis
The primary safety objective was to determine if the upper limit of the one-sided 97.5% confidence interval (CI) of intervention incidence was below the pre-specified acceptance threshold of 7.88%. To achieve the overall primary objective analysis of the study, approximately 299 firings across 100 subjects was needed for the thoracic cohort.
Descriptive statistics were used to analyze the primary and secondary outcomes using SAS 9.4 (SAS, Cary, NC, USA). Where appropriate, the mean, standard deviations, counts and 95% CIs were used to describe the study outcomes. Comparative analyses were conducted when appropriate using Fisher exact tests to assess the impact of differences in surgical approaches and stapler handle. Fisher exact tests were leveraged with a significance level of 5%.
Results
A total of 120 subjects ranging from 23.7 to 86.3 (mean 65.8±11.9) years of age underwent thoracic procedures where pulmonary vasculature was ligated and divided. All subjects were followed for 30 days postoperatively, and all subjects completed their 30-day follow-up visit. Table 1 displays the characteristics of the subject population included in this study.
Table 1. Baseline characteristics.
| Attribute | Subjects treated with SDR (N=120) |
|---|---|
| Sex | |
| Male | 56 (46.7) |
| Female | 64 (53.3) |
| Age (years) | |
| Mean ± SD | 65.8±11.9 |
| Median (min, max) | 67.4 (23.7, 86.3) |
| Ethnicity | |
| Hispanic or Latino | 5 (4.2) |
| Not Hispanic or Latino | 107 (89.2) |
| Not reported | 6 (5.0) |
| Unknown | 2 (1.7) |
| Race | |
| American Indian or Alaska Native | 1 (0.8) |
| Asian | 3 (2.5) |
| Black or African American | 6 (5.0) |
| White | 104 (86.7) |
| Other | 6 (5.0) |
| ASA physical status | |
| ASA I | 1 (0.8) |
| ASA II | 13 (10.8) |
| ASA III | 62 (51.7) |
| ASA IV | 5 (4.2) |
| Not available | 39 (32.5) |
Data are presented as n (%). SD, standard deviation; ASA, American Society of Anesthesiologists physical status classification; SDR, Signia™ Small Diameter Reloads.
Because the intent of utilizing the PSR as a mechanism of data acquisition was to collect data representative of real-world use of the device, a wide array of thoracic procedures was performed on enrolled subjects. Table 2 describes the types of procedures, access, and any conversions to open. The majority of the subjects underwent lobectomies (72 of 120, 60.0%), followed by lung transplants (35 of 120, 29.2%).
Table 2. Surgical characteristics.
| Operative data | Subjects treated with SDR (N=120) |
|---|---|
| Procedure type | |
| Esophagectomy† | 2 (1.7) |
| Lobectomy | 72 (60.0) |
| Lung transplant | 35 (29.2) |
| Segmentectomy | 11 (9.2) |
| Surgical access | |
| Open thoracotomy‡ | 60 (50.0) |
| Thoracoscopic | 57 (47.5) |
| Robotic assisted | 3 (2.5) |
| Estimated blood loss (mL) | |
| Mean ± SD | 238.7±310.0 |
| Median (min, max) | 100 (0, 1,200) |
| Operative time (hr:min) | |
| Mean ± SD | 4:22±2:46 |
| Median (min, max) | 3:15 (0:37, 14:42) |
Data are presented as n (%). †, for these cases, esophagectomy was listed as the primary procedure but concomitant lung resection was performed as a secondary procedure; staple fires were on the pulmonary vasculature; ‡, 4 procedures (3 thoracoscopic and 1 robotic assisted) were converted to open thoracotomy. SD, standard deviation; SDR, Signia™ Small Diameter Reloads.
Based on the procedures performed, inclusive of conversions, the number of subjects undergoing thoracoscopic or open procedures were evenly distributed (60 of 120, 50% for open, 57 of 120, 47.5% for thoracoscopic).
Intraoperative staple-line performance
A total of 302 reloads transected pulmonary vasculature in 120 subjects. Surgeons applied SDR on hilar pulmonary arteries and veins evenly (48% of firings on arteries, 52% on veins). Reinforcement was applied to 14 firings (4.63%) of the 302 SDR firings on pulmonary vasculature. Three (0.99%) were categorized as needing clinically necessary intervention by the operating surgeon with the remaining 11 SDR firings having reinforcement applied as per the surgeons’ typical standard practice (Table 3).
Table 3. Incidence of intraoperative hemostatic intervention.
| Hemostatic intervention | Total firings (n=302) |
|---|---|
| Firing location (n=302 firings) | |
| Pulmonary arteries | 145 (48.0) |
| Pulmonary veins | 157 (52.0) |
| Number of transactions requiring clinically necessary intervention | 3 (0.99) |
| 95% confidence interval min and max | 0.21%, 2.88% |
| Types of intervention (n=14 firings) | |
| SOC intervention | 11 (78.6) |
| Clinically necessary intervention | 3 (21.4) |
| Specific types of clinically necessary intervention (n=3 firings) | |
| Additional stapler loads, pressure and hemostatic agent | 1 (33.3) |
| Additional stapler loads | 1 (33.3) |
| Endoscopic clip | 1 (33.3) |
Data are presented as n (%). SOC, standard of care.
The three firings resulting in clinically necessary hemostatic intervention occurred in separate subjects, all during lobectomies. None of these procedures required conversion to an open procedure, a blood transfusion, suture placement, or the need for more extensive pulmonary parenchymal resection.
Likert scale
SDR firings were assessed using a 5-point Likert scale (17) as described in Table 4. Acceptable staple-lines were described as firings requiring no intervention (scores 1 to 3) and unacceptable staple-lines were described as firings requiring mild or extensive intervention (scores of 4 or 5).
Table 4. Hemostatic Likert score assessment of SDR firings on pulmonary arteries and pulmonary veins.
| Assessment and hemostatic score | SDR firings on arteries (n=145) | SDR firings on veins (n=157) | Total SDR firings (n=302) |
|---|---|---|---|
| Acceptable | 143 (98.6) | 156 (99.4) | 299 (99.0) |
| 1. No bleeding at tissue site after initial blotting of staple-line | 127 (88.8) | 148 (94.9) | 275 (92.0) |
| 2. Blood oozing at tissue site; stops prior to 15 seconds; no intervention needed | 4 (2.8) | 0 (0.0) | 4 (1.3) |
| 3. Blood oozing, still progressive after 15 sec., no intervention needed | 4 (2.8) | 0 (0.0) | 4 (1.3) |
| With Likert scores (1–3) | 135 (94.4) | 148 (94.9) | 283 (94.6) |
| Likert scores not recorded† | 8 (5.6) | 8 (5.1) | 16 (5.4) |
| Not acceptable | 2 (1.4) | 1 (0.6) | 3 (1.0) |
| 4. Blood oozing at tissue site, mild intervention (i.e., cautery) | 2 (100.0) | 1 (100.0) | 3 (100.0) |
| 5. Significant bleeding requiring intervention such as extensive coagulation or ligation with clips | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| With Likert scores [4, 5] | 2 (100.0) | 1 (100.0) | 3 (100.0) |
| Likert scores not recorded† | 0 | 0 | 0 |
Data are presented as n (%). †, there were sixteen firings that did not require hemostatic intervention based on surgeon review of their operative notes, however a Likert scale score was not assigned to these firings by the surgeons. For this reason, all sixteen firings are grouped with scores 1–3 and considered acceptable staple-lines not requiring intervention. SDR, Signia™ Small Diameter Reloads.
A Likert score three or lower was considered acceptable, hemostatic staple-lines with no clinically necessary interventions required. A Likert score of four or five was for staple-lines requiring intervention to control bleeding. Approximately 99% of total firings with SDR were integrous (Table 4), with no need for clinical intervention. Of the 299 firings characterized as acceptable, 283 firings (94.6%) received a score of 1 through 3. The remaining 16 firings, eight on pulmonary arteries and eight on pulmonary veins, were assessed for hemostatic intervention, but Likert scores (the numeric value) were not reported by the operating surgeons. Although a score was not provided for these 11 subjects, these firings were included in this analysis because the surgeons confirmed that the SDR staple-lines were acceptable based on their operative notes. There were no firings ranked as a five on the Likert scale. Moreover, there was no significant difference in acceptable staple-line integrity for arteries or veins (P=0.61) as described in Table 5.
Table 5. Stratification of acceptable SDR fired on pulmonary arteries and pulmonary veins.
| Location of SDR firings | Acceptable SDR firings (n=299) |
P value |
|---|---|---|
| Arteries | 143 of 145 (98.6%) | 0.61 |
| Veins | 156 of 157 (99.4%) |
SDR, Signia™ Small Diameter Reloads.
Figure 2 describes the breakout of individual available Likert scores based on the type of pulmonary vasculature. The 16 firings reported acceptable by surgeons but not assigned a Likert score are not characterized in Figure 2.
Figure 2.
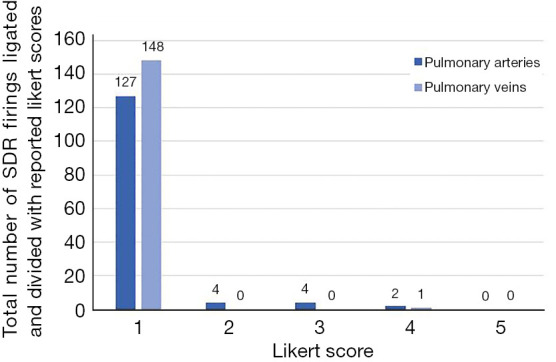
Comparison of reported Likert scores between pulmonary arteries and veins ligated and divided using SigniaTM Small Diameter Reloads. 16 firings without Likert scores were not included in this figure, despite an ‘acceptable’ in this study refers to a score of 1, 2, or 3. SDR, Signia™ Small Diameter Reloads.
As seen in Figure 2, most firings on pulmonary arteries and veins (96.2%) had a Likert score of 1. Although other firings on arteries were ranked a score of 2 or 3, these staple-lines were still considered acceptable with no clinically necessary intervention needed.
Additional outcomes
Surgeon’s stapler handle preference did not impact the subject outcomes as seen by the low incidence of bleeding events, reported in a subset analysis characterized in Table 6. Of note, 60% of the firings were performed with a manual handle and 40% with a powered handle. All three patients requiring clinically necessary intervention were in the manual handle subgroup accounting for 4.2% of these firings, however this difference did not meet statistical significance (P=0.27).
Table 6. Firing mechanism used to deploy SDR.
| Stapler handle | Subjects treated with SDR (N=120) | P value |
|---|---|---|
| Powered | 48 (40.0) | 0.27 |
| Subjects requiring clinically necessary hemostatic intervention | 0 (0.00) | |
| Manual | 72 (60.0) | |
| Subjects requiring clinically necessary hemostatic intervention | 3 (4.2) | |
| Overall | 120 (100.0) | – |
| Subjects with staple-line integrity | 117 (97.5) | |
| Subjects requiring clinically necessary hemostatic intervention | 3 (2.5) |
Data are presented as n (%). SDR, Signia™ Small Diameter Reloads.
There were no device-related injuries to organs or surrounding tissue experienced by the utilization of the two-row stapler, regardless of handle preference or surgical access. All 4 conversions (3.3%) to open procedures were unrelated to the study device. Three subjects required conversions to open thoracotomies from thoracoscopic procedures; in all three cases, the reason for conversion was access. There was one subject who required conversion to open thoracotomy from a robotic assisted procedure. The reason for conversion was inability to identify nodule.
This supports the utility of SDR to effectively be leveraged in minimally invasive procedure without impediment.
Safety and utility
No subjects experienced AEs directly related to the study device alone (i.e., there were no reported malfunctions of staple-line formation and division). The device-related AEs and postoperative safety data are described in Table 7 below. Three out of 44 subjects (6.8%) experienced AEs that were possibly related to or caused by both the study device and the procedure. We have classified it this way to better evaluate the efficacy of the two-row stapler. One subject experienced a small tear and bleeding adjacent to the SDR staple-line that was quickly resolved with an endoscopic clip. Similarly, a second subject experienced bleeding when the stapler was applied very distally on a 3-mm vessel due to adhesions, which was resolved using another SDR reload and a hemostatic agent. The third subject experienced slight staple-line bleeding that was quickly controlled with a second reload, gentle pressure, and a hemostatic agent. All events were resolved intraoperatively without further subject sequelae. Bleeding in all three scenarios were less than 50 cc and were controlled with reapplication of the stapler and/or gentle pressure, hemostatic agents, or clips without the need for any complex repair or open conversion. There were no device deficiencies reported and no postoperative interventions needed to treat staple-line failure. There was one incidence of a repeat hospitalization for a primary procedure-related complication. The subject experienced a wound infection at day 22 postoperatively, which was unrelated to the study device.
Table 7. Postoperative and safety outcomes.
| Safety measure | Subjects treated with SDR (N=120) |
|---|---|
| Device deficiencies | 0 (0.0) |
| Adverse events | 44 (36.7) |
| Procedure-related only | 41 (93.2) |
| Device-related only | 0 (0.0) |
| Device-related and procedure-related | 3 (6.8) |
| Repeat hospitalization for primary procedure-related complications | 1 (0.8) |
| Post-operative assessment for additional intervention to treat staple-line firings | 0 (0.0) |
Data are presented as n (%). SDR, Signia™ Small Diameter Reloads.
Surgeon satisfaction survey
After the last subject completed the 30-day follow-up visit, all investigators completed a surgeon satisfaction questionnaire characterizing their experiences with SDR. All surveyed surgeons agreed that they could effortlessly maneuver SDR in tight spaces in comparison to a 12-mm reload in the same space and that the reload offers appropriate flexibility to be used across a variety of cases. Most surgeons (88%) felt their firings were more precise due to the smaller profile of the device and preferred use of SDR over similar devices on the market. However, only 63% responders preferred SDR over 12 mm reloads on the market suggesting that further investigations with a control group are necessary.
Discussion
Key findings
In this real-world study, the two-row stapler reload was fired over 300 times across 120 subjects. There are a number of ligation strategies which include suture ligation, clips, energy devices, and stapling devices. The decision of which ligation strategy to employ is a combination of surgeon preference and familiarity, but it is well recognized that both arterial and venous vasculature in the pulmonary circuit exists at low pressures. Given the typically low pressures in the chest, considerations to moving to a two-row stapler from a three-row stapler may be advantageous given its smaller profile. In addition, we were pleased to find that even in the pulmonary transplant population, these staple-lines were sufficiently hemostatic despite the known increased pressures in those operations. Of note, the pulmonary and venous system was decompressed at the time of firing the stapler in all the lung transplant cases.
As an observational study, variability in procedures, surgical access and handle preference organically occurred. Regardless of this variability, the staple-line integrity among the cohort remained consistent with only three hemostatic interventions required (0.99%) and no reported device-related surrounding tissue or organ injuries. The staple-lines requiring hemostatic intervention did not result in the need for further suturing, open conversion, blood transfusion, or further parenchymal pulmonary resection. These findings suggest that SDR may be a reliable option with acceptable hemostasis to address structures that are difficult to access due to size, space, or location.
Safety and utility
With the rise of adoption of robotic and uniportal thoracoscopic surgery in recent years, the need for finer but equally durable instrumentation paved the way for devices such as SDR. Difficulties in stapling in a narrow workspace and narrow, limited vision may impact outcomes and lead to pulmonary complications (18). While the minimally invasive surgical approaches have appealing outcomes, they have not eliminated the need for intraoperative hemostatic interventions. Intraoperative bleeding remains one of the most common and potentially fatal reasons for conversions to open thoracotomy (19). The low conversion rate (3.3%) coupled with the exceptional hemostatic rate (99%) observed in this study supports that a two-row stapler may be a sound alternative to larger staplers or bipolar energy devices to dissect vascular structures; especially those which are fragile and/or difficult to expose.
The smaller profile of the stapler allows the user to leverage the various access sites in thoracoscopic procedures without the need to upsize from an 8-mm port. The narrow shaft of the reload provides an opportunity to couple the stapler with robotic technology and provide even more surgeon flexibility with hybrid bedside stapling techniques. Given the smaller profile of the two-row stapler, its intended use is for branch segmental vessels in difficult to access locations. This was not stipulated and there was acceptable hemostasis in a variety of vessel diameters and clinical scenarios. SDR delivers technological and performance characteristics substantively equivalent to three-row staplers, which provide a compelling argument to the non-inferior nature of the dual line SDR stapler compared to standard 12 mm shaft three-row staplers, despite a 33.3% decrease in delivered staples (20)a.
Strengths and limitations
This study provided a first real-world prospective look at the use of the two-row stapler in thoracic procedures which resulted in a 99% hemostatic rate. The sample size was powered at the firing level to ensure that intraoperative performance of the staple-line was appropriately assessed. The subject population did not exclude complex subjects, which allowed for a more realistic snapshot of device use and outcomes.
A limitation of this study is the lack of a baseline control group due to the nature of the study design. The authors recommend a prospective, randomized study to address limitations of the observational data using energy or three-row stapling devices for the control group. All conclusions drawn around the safety and efficacy profile of the SDR device were compared to rates and results described in literature.
Comparison with similar research
In addition to surgical stapling, the use of energy devices to divide pulmonary vessels is an alternative therapy that is well established in literature. Liberman et al. describes a 1.3% intraoperative bleed rate with ultrasonic vessel-sealing devices compared to 2.2% with endostaplers in 150 subjects; 239 pulmonary artery branches were divided with the ultrasonic vessel-sealing device and 181 with endostaplers (21). Similarly, in a smaller retrospective study of 16 subjects conducted by Tomoyasu et al., 1.4% experienced intraoperative bleeding with either Ligasure™ or EndoGIA™ reloads (22).
Molins et al. describes a randomized control trial conducted in seven centers within the United States, comparing intraoperative hemostatic rates of three-row staplers to Ethicon’s Echelon™ Powered Vascular Stapler (EPVS), a two-row stapler. In this study, 8.3% of EPVS firings on pulmonary vasculature required intraoperative hemostatic intervention compared to 5.3% in the three-row stapler arm; the results were not statistically significant (P=0.14) (10). The definition of incidence of hemostatic intervention in this study aligns with that which Molins et al. noted. Compared to the 8.3% intervention rate reported with EPVS, SDR had a 0.99% intervention rate. However, because this study was solely an observational study, future studies involving control groups of manual or coagulative closure devices would be necessary to further confirm study findings.
Current literature suggests improvements in intraoperative bleeding for thoracic procedures when powered staplers are utilized instead of manual staplers (23-25). This real-world study revealed a stratification in powered (40%) and manual stapler handles (60%) with no difference in hemostatic outcomes (P=0.27) or Likert assessments. These findings suggest that the reload chosen may have a greater impact on the bleeding outcomes than the stapler handle alone.
Explanations of findings
This preliminary study of a two-row small diameter stapler reload showed no differences in bleeding events regardless of observed splits in procedures, access and stapler handles. These findings suggest that the size and maneuverability of the reload may favorably impact the surgical outcomes. The smaller 8 mm size may help improve outcomes for thoracoscopic or robotic bedside use by reducing the need to increase the port size or convert to an open procedure as noted by the low 3.3% conversion rate due to access seen in this study.
Implications and actions needed
Future investigations involving control groups would confirm the findings of this study. Future studies comparing a two-row stapler to other mechanical and coagulative closure devices would further define the benefits of the smaller stapler in thoracic procedures. Vessel diameters and reload type were not collected as part of the methodology for the study; further work in this domain could help clarify optimal utility of the devices available. A separate analysis of the lung transplant subset is needed to better characterize the benefits of using SDR for whole organ transplants.
Conclusions
The two-row stapler reloads were successfully utilized in a variety of thoracic procedures, including lung transplants, while supporting surgeon preference of stapler handle and surgical access with a 0.99% clinically necessary intervention rate. The real-world advantage of using a smaller two-row stapler, such as SDR, is the ability to easily fit through 8 mm ports with improved maneuverability, optimizing control and access, and minimizing the risk of device-related injury to pulmonary vasculature while delivering integrous staple lines.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors would like to thank the study investigators (Dr. Harmik Soukiasian. Dr. Michael Liptay, Dr. Clark Fuller, and Dr. J. Timothy Sherwood), research coordinators and support staff for the enrollment of subjects included in this dataset.
Funding: The study was sponsored and funded by Medtronic, which contributed to the study design, data collection analysis, and manuscript writing in accordance with the ICMJE criteria.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with Good Clinical Practice and the Declaration of Helsinki (as revised in 2013), as well as all other applicable local, state, and federal regulatory requirements, and is registered with ClinicalTrials.gov (NCT05095935). All seven sites received IRB approval prior to site activation. The reviewing entities included: Duke Health IRB (Pro0010867-KSP-7.0), Western Institutional Review Board-Copernicus Group for three of the sites (IRB for Virginia Cancer Specialists: 1342927, Cooper: 1301179 and UPMC: 1324230), Cedars Sinai Office of Research Compliance and Quality Improvement (IRB 00002913), Rush University IRB (ORA Number: 15033005-IRB01-AM09) and Mary Washington Healthcare IRB (IRB 2022-02). Six of the seven sites’ IRBs approved the waiver of written informed consent. Rush University IRB required written informed consent.
Footnotes
Reporting Checklist: The authors have completed the STROBE checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-179/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-179/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-179/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-179/coif). All the authors report that the study was sponsored and funded by Medtronic, which contributed to the study design, data collection analysis, and manuscript writing. S.J.K., D.D.S., C.W.S. and P.G.S. are investigators in the Medtronic-sponsored Signia™ Small Diameter Reload Registry study and their institutions have received funds for participation. S.J.K. is a consultant of Medtronic. D.D.S. is an educational consultant of Covidien. N.P.D. and S.A. are current full-time employees of Medtronic and have stock options through Employee Stock Purchase Plan (ESPP). The authors have no other conflicts of interest to declare.
Percent decrease calculated based on the number of staples in SDR reloads with cartridge lengths of 30 and 45 mm compared to the number of staples in Tri-Staple™ reloads with cartridge lengths of 30 and 45 mm.
References
- 1.Akopov A, Artioukh DY, Molnar TF. Surgical Staplers: The History of Conception and Adoption. Ann Thorac Surg 2021;112:1716-21. 10.1016/j.athoracsur.2021.03.107 [DOI] [PubMed] [Google Scholar]
- 2.Baker RS, Foote J, Kemmeter P, et al. The science of stapling and leaks. Obes Surg 2004;14:1290-8. 10.1381/0960892042583888 [DOI] [PubMed] [Google Scholar]
- 3.Asamura H, Suzuki K, Kondo H, et al. Mechanical vascular division in lung resection. Eur J Cardiothorac Surg 2002;21:879-82. 10.1016/S1010-7940(02)00101-X [DOI] [PubMed] [Google Scholar]
- 4.Szwerc MF, Landreneau RJ, Santos RS, et al. Minithoracotomy combined with mechanically stapled bronchial and vascular ligation for anatomical lung resection. Ann Thorac Surg 2004;77:1904-9; discussion 1909-10. 10.1016/j.athoracsur.2003.12.003 [DOI] [PubMed] [Google Scholar]
- 5.Gossot D, Merlusca G, Tudor A, et al. Pitfalls related to the use of endostaplers during video-assisted thoracic surgery. Surg Endosc 2009;23:189-92. 10.1007/s00464-008-9765-7 [DOI] [PubMed] [Google Scholar]
- 6.Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. 10.1016/j.jtcvs.2009.08.026 [DOI] [PubMed] [Google Scholar]
- 7.Shimizu N, Tanaka Y, Okamoto T, et al. How to prevent adverse events of vascular stapling in thoracic surgery: recommendations based on a clinical and experimental study. J Thorac Dis 2018;10:6466-71. 10.21037/jtd.2018.11.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Subotic D, Hojski A, Wiese M, et al. Use of staplers and adverse events in thoracic surgery. J Thorac Dis 2019;11:S1216-21. 10.21037/jtd.2019.03.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yano M, Takao M, Fujinaga T, et al. Adverse events of pulmonary vascular stapling in thoracic surgery. Interact Cardiovasc Thorac Surg 2013;17:280-4. 10.1093/icvts/ivt130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molins L, Lanuti M, Force S, et al. Evaluation of a Powered Vascular Stapler in Video-Assisted Thoracic Surgery Lobectomy. J Surg Res 2020;253:26-33. 10.1016/j.jss.2020.03.023 [DOI] [PubMed] [Google Scholar]
- 11.Mei J, Pu Q, Liao H, et al. A novel method for troubleshooting vascular injury during anatomic thoracoscopic pulmonary resection without conversion to thoracotomy. Surg Endosc 2013;27:530-7. 10.1007/s00464-012-2475-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Decaluwe H, Petersen RH, Hansen H, et al. Major intraoperative complications during video-assisted thoracoscopic anatomical lung resections: an intention-to-treat analysis. Eur J Cardiothorac Surg 2015;48:588-98; discussion 599. 10.1093/ejcts/ezv287 [DOI] [PubMed] [Google Scholar]
- 13.Cerfolio RJ, Bess KM, Wei B, et al. Incidence, Results, and Our Current Intraoperative Technique to Control Major Vascular Injuries During Minimally Invasive Robotic Thoracic Surgery. Ann Thorac Surg 2016;102:394-9. 10.1016/j.athoracsur.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 14.Tane S, Tanaka Y, Nishikubo M, et al. Console and bedside surgeon fused robot-assisted thoracic surgery. Gen Thorac Cardiovasc Surg 2023;71:730-2. 10.1007/s11748-023-01964-1 [DOI] [PubMed] [Google Scholar]
- 15.Byun CS, Lee S, Kim DJ, et al. Analysis of Unexpected Conversion to Thoracotomy During Thoracoscopic Lobectomy in Lung Cancer. Ann Thorac Surg 2015;100:968-73. 10.1016/j.athoracsur.2015.04.032 [DOI] [PubMed] [Google Scholar]
- 16.Safdie FM, Sanchez MV, Sarkaria IS. Prevention and management of intraoperative crisis in VATS and open chest surgery: how to avoid emergency conversion. J Vis Surg 2017;3:87. 10.21037/jovs.2017.05.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siegel J, Cummings J, Clymer J. Reproducible, Repeatable and Clinically-relevant Hemostasis Scoring. J Adv Méd Pharm Sci 2014;1:30-9. 10.9734/JAMPS/2014/11653 [DOI] [Google Scholar]
- 18.Osoegawa A, Abe M, Miyawaki M, et al. Challenges in Robotic Lung Lobectomy through the Anterior Approach. Ann Thorac Cardiovasc Surg 2024;30:23-00146. 10.5761/atcs.oa.23-00146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu L, Mei J, He J, et al. International expert consensus on the management of bleeding during VATS lung surgery. Ann Transl Med 2019;7:712. 10.21037/atm.2019.11.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.U.S. Food & Drug Administration 10903 New Hampshire Avenue Doc ID # 04 017.04. 06 Silver Spring, MD 20993 www.fda.gov.
- 21.Liberman M, Goudie E, Morse C, et al. Prospective, multicenter, international phase 2 trial evaluating ultrasonic energy for pulmonary artery branch sealing in video-assisted thoracoscopic surgery lobectomy. J Thorac Cardiovasc Surg 2020;159:301-11. 10.1016/j.jtcvs.2019.09.061 [DOI] [PubMed] [Google Scholar]
- 22.Tomoyasu M, Deguchi H, Kudo S, et al. Evaluation of pulmonary artery bleeding during thoracoscopic pulmonary resection for lung cancer. Thorac Cancer 2022;13:3001-6. 10.1111/1759-7714.14649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roy S, Yoo A, Yadalam S, et al. Comparison of economic and clinical outcomes between patients undergoing laparoscopic bariatric surgery with powered versus manual endoscopic surgical staplers. J Med Econ 2017;20:423-33. 10.1080/13696998.2017.1296453 [DOI] [PubMed] [Google Scholar]
- 24.Miller DL, Roy S, Kassis ES, et al. Impact of Powered and Tissue-Specific Endoscopic Stapling Technology on Clinical and Economic Outcomes of Video-Assisted Thoracic Surgery Lobectomy Procedures: A Retrospective, Observational Study. Adv Ther 2018;35:707-23. 10.1007/s12325-018-0679-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsunezuka Y, Tanaka N, Fujimori H. The Impact of Endoscopic Stapler Selection on Bleeding at the Vascular Stump in Pulmonary Artery Transection. Med Devices (Auckl) 2020;13:41-7. 10.2147/MDER.S240343 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as


