Abstract
Background
Radial endobronchial ultrasound (rEBUS) guide sheath (GS) transbronchial lung biopsy (TBLB) improves the diagnostic yield of peripheral lung lesions (PLL). However, its diagnostic yield is approximately 60%. We aimed to evaluate the diagnostic utility of adding rEBUS GS transbronchial needle aspiration (TBNA) using PeriView FLEX needle (Olympus, Tokyo, Japan) to rEBUS GS TBLB.
Methods
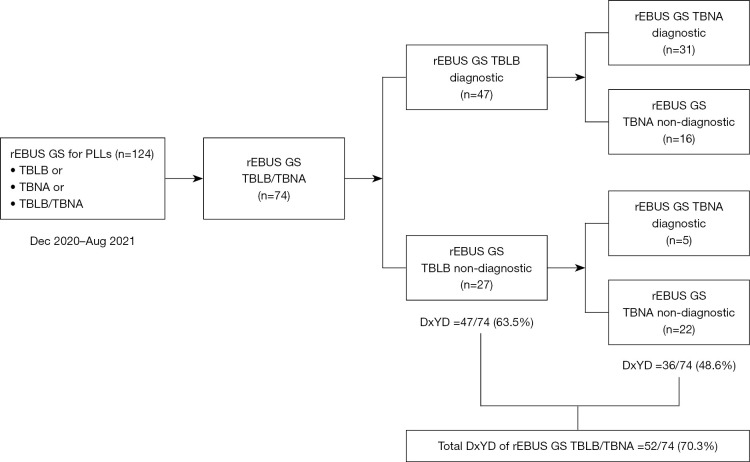
In this retrospective study, we initially screened 124 PLLs in 123 patients who underwent rEBUS GS procedures for PLLs from December 2020 to August 2021. The analysis was performed on 74 PLLs in 73 patients who underwent both rEBUS GS TBLB and TBNA.
Results
PLLs showed the following characteristics: lesion size [mean ± standard deviation (SD)], 24±12 mm; nature (solid vs. subsolid), 59 (79.7%) vs. 15 (20.3%); distance from the pleura (mean ± SD), 14±14 mm; rEBUS visualization type (probe within PLL vs. probe adjacent to PLL), 56 (75.7%) vs. 18 (24.3%). Among 74 PLLs, 47 (63.5%) were successfully diagnosed by rEBUS GS TBLB. In 27 PLLs not diagnosed by rEBUS GS TBLB, 5 (18.5%) were further diagnosed by rEBUS GS TBNA [overall diagnostic yield: 70.3% (52/74)]. EBUS visualization type of “probe adjacent to PLL” was a significant factor associated with the diagnostic yield of additional rEBUS GS TBNA.
Conclusions
In rEBUS GS procedures for PLLs, the diagnostic yield might be improved by implementing TBNA in addition to TBLB. In particular, additional TBNA is preferable if the probe is adjacent to the lesion rather than within the lesion on rEBUS.
Keywords: Solitary pulmonary nodule, image-guided biopsy, ultrasonography, bronchoscopy
Highlight box.
Key findings
• Adding transbronchial needle aspiration (TBNA) (using PeriView FLEX needle) could increase diagnostic yield in radial endobronchial ultrasound (rEBUS) guide sheath (GS) procedures for peripheral lung lesions (PLL).
What is known and what is new?
• rEBUS GS transbronchial lung biopsy (TBLB) is a very useful tool for the diagnosis of PLLs. However, its diagnostic yield is approximately 60%. Therefore, more efforts are needed to improve diagnostic yield.
• In this study, among 74 PLLs, 47 (63.5%) were successfully diagnosed by rEBUS GS TBLB. In 27 PLLs not diagnosed by rEBUS GS TBLB, 5 (18.5%) were further diagnosed by rEBUS GS TBNA [overall diagnostic yield: 70.3% (52/74)]. EBUS visualization type of “probe adjacent to PLL” was a significant factor associated with the diagnostic yield of additional rEBUS GS TBNA.
What is the implication, and what should change now?
• When performing a rEBUS GS procedure for tissue biopsy of PLLs, adding rEBUS GS TBNA using the PeriView FLEX TBNA needle in addition to rEBUS GS TBLB could increase diagnostic yield if the lesion is adjacent.
Introduction
The discovery of a lung lesion prompts a decision regarding observation, direct surgery, or tissue biopsy (1-3). If the identified lesion is located on the periphery and is small (i.e., a solid nodule <15 mm or a part-solid nodule with a solid component <6–8 mm), the patient can be placed under observation (3). However, if the lesion is peripheral but of considerable size (i.e., a solid nodule of 15–30 mm or a part-solid nodule with solid component ≥6–8 mm) or growing, direct surgery is desirable and most cost-effective unless a distant or nodal metastasis is suspected (3,4). Except for the above situations, such as in cases the nodule (tumor) is >30 mm, a central lesion, multiple lesions, distant metastasis, or visible lymphadenopathy on imaging studies, or the possibility of benign disease that can be treated medically, a tissue biopsy will eventually be required (3,5). The preferred initial site for the tissue biopsy should be easily and safely accessible, and the approach may vary depending upon experience and available facilities (5). For peripheral lung lesions (PLL), it can be selected from transthoracic needle biopsy (TTNB) or special bronchoscopic procedures [using electromagnetic navigation bronchoscopy, radial endobronchial ultrasound (rEBUS), or robotic bronchoscopy] (5,6).
Since 2016, we have performed rEBUS transbronchial lung biopsy (TBLB) with a guide sheath (GS) for PLL. In our previous study, the strict diagnostic yield (i.e., if only definite pathologies were included) of rEBUS GS TBLB was 63% (7). However, we believe that more efforts are needed to improve diagnostic yield. Conventional transbronchial biopsy with larger forceps following rEBUS GS TBLB (8,9), can increase diagnostic yield from 65% to 82% (8). Alternatively, transbronchial cryobiopsy using a 1.1-mm cryoprobe following rEBUS GS TBLB (10-12) can increase yield from 61% to 79% (10).
Recently, the PeriView FLEX transbronchial needle aspiration (TBNA) (Olympus, Tokyo, Japan) was introduced to increase the diagnostic yield of rEBUS GS TBLB. The maximal diameter of the aspiration needle is 1.5 mm, making it suitable for use within the GS and compatible with 4 mm outer diameter thin bronchoscopes with a 2 mm working channel. A study of the diagnostic effectiveness thereof revealed that specimens were satisfactory for evaluation and provided sufficient tissue for ancillary studies (13). However, to date, there have been no studies on the additional diagnostic yield when using the PeriView FLEX TBNA needle in rEBUS GS TBLB. We, therefore, aimed to determine the diagnostic utility of adding rEBUS GS TBNA (using the PeriView FLEX TBNA needle) to rEBUS GS TBLB in the biopsy of PLL and to identify situations in which this is particularly helpful. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1598/rc).
Methods
Subjects
We identified cases where rEBUS GS was performed for PLL from the Ulsan University Hospital registry between 10 December 2020 and 19 August 2021, when the PeriView FLEX TBNA needle was introduced. Patients in whom both rEBUS GS TBLB and rEBUS GS TBNA (using the PeriView FLEX TBNA needle) were performed were retrospectively reviewed and cross-sectionally analyzed. Subjects with missing data were excluded from the study. PLL was defined as lesions in the outer half of the lungs, surrounded by normal lung parenchyma, and connected by a small bronchus (<5 mm in diameter), which were unlikely to be accessed by conventional bronchoscopy (outer diameter, 5.0–6.0 mm) (7). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board of Ulsan University Hospital (No. UUH 2023-03-002) and individual consent for this retrospective analysis was waived.
rEBUS GS procedure
All patients underwent a high-quality CT scan (256-MDCT scanners: Somatom Definition AS+ and Somatom Definition Flash; Siemens Healthcare, Forchheim, Germany) before rEBUS to enable hand-drawing of a bronchial map for a target PLL. Two bronchoscopists (J.H.K. and T.L.) performed EBUS GS as previously described (7). First, a thin bronchoscope (outer diameter, 4.0–4.2 mm, BF-P260F or BF-P290, Olympus, Tokyo, Japan) was inserted as far as possible into the bronchus nearest to the PLL. Second, a rEBUS probe (UM-S20–17S; Olympus) was inserted with a GS [K-201 (outer diameter 1.95 mm); Olympus] through the working channel of the bronchoscope. Lastly, biopsies (TBLB and TBNA) were performed only after rEBUS imaging confirmed that the probe had reached the target lesion (within or adjacent to). If the image was within the lesion, rEBUS GS TBLB was performed first, followed by rEBUS GS TBNA; if the image was adjacent to the lesion, this was reversed. All processes were performed using a GS.
rEBUS GS TBLB was performed by alternating cycles of one brush and two biopsies through the GS with a GS-dedicated cytology brush (BC-204D-2010; Olympus) and biopsy forceps (FB-233D; Olympus); this process was repeated ≥3 times until at least four biopsy specimens were obtained. rEBUS GS TBNA was performed through the GS using a PeriView FLEX TBNA needle. At least two needle passes were performed until core tissue was obtained, with more than 50 to-and-fro needle agitations per pass under 20 mL of negative pressure. The core tissue was separated and fixed with formalin and send for the histopathology and molecular analysis (13). Rapid on-site evaluation of pathological specimens was not conducted. All procedures were performed with the help of fluoroscopy under conscious sedation. A routine chest radiograph was obtained within 2 hours of the end of the procedure.
Baseline data collection and final diagnosis
Baseline characteristics [age, sex, PLL size (the average of the long and short axes), PLL distance from the pleura, lobar location of the PLL, bronchus sign (presence of an open bronchus connected from a proximal airway in the PLL), and nature of the PLL on chest CT] of all enrolled patients were collected. Final diagnoses were established based on the pathological results. If not diagnosed using rEBUS, further examination (TTNB or surgical resection) was performed.
Primary outcome
The primary outcome was diagnostic yield, which is the proportion of PLL with a final diagnosis confirmed by rEBUS GS procedures.
Statistical analysis
Data were analyzed using SPSS version 24 (IBM Corporation, Armonk, NY). Continuous variables are expressed as mean ± standard deviation (SD), and categorical variables are presented as percentages. The independent t-test (for continuous variables) and the chi-square or Fisher’s exact test (for dichotomous variables) were used to identify any potential associations. Diagnostic yields were analyzed using the chi-square test. To identify factors that may affect the diagnostic yield of rEBUS GS TBLB, multivariate logistic regression analyses were performed by setting the following variables as covariates: age (years), sex (male), lesion size (≥20 mm), chest CT nature (solid), chest CT bronchus sign (presence), and rEBUS visualization type (within). A P value <0.05 was considered statistically significant in all analyses.
Results
During the study period, rEBUS GS was performed on 124 PLL from 123 patients. Of these, TBLB and TBNA were used for 74 lesions (73 patients) (Figure 1). The lesions showed the following characteristics: lesion size, 24±12 mm; nature (solid vs. subsolid), 59 (79.7%) vs. 15 (20.3%); distance from pleura, 14±14 mm; rEBUS visualization type (probe within PLL vs. probe adjacent to PLL), 56 (75.7%) vs. 18 (24.3%). In terms of complications, no significant incidents of bleeding or pneumothorax were observed in either group (Table 1). The final diagnoses of all PLL are shown in Table 2.
Figure 1.
Flowchart showing how the peripheral lung lesions were divided for analysis (n=74). DxYD, diagnostic yield; GS, guide sheath; rEBUS, radial endobronchial ultrasound; TBLB, transbronchial lung biopsy; TBNA, transbronchial needle aspiration; PLL, peripheral lung lesion.
Table 1. Baseline characteristics of 74 PLLs.
| Variables | Total | Diagnostic in rEBUS GS TBLB (n=47) |
Non-diagnostic in rEBUS GS TBLB (n=27) |
P value |
|---|---|---|---|---|
| Age, years | 68±11 | 68±12 | 69±8 | 0.98 |
| Gender | 0.75 | |||
| Male | 51 (68.9) | 33 (70.2) | 18 (66.7) | |
| Female | 23 (31.1) | 14 (29.8) | 9 (33.3) | |
| Lesion size, mm | 24±12 | 26±11 | 21±13 | 0.046 |
| Distribution by size | 0.02 | |||
| ≤20 mm | 31 (41.9) | 15 (31.9) | 16 (59.3) | |
| >20 mm | 43 (58.1) | 32 (68.1) | 11 (40.7) | |
| Distance from pleura, mm | 14±14 | 13±14 | 14±16 | 0.78 |
| Location of PLL | 0.39 | |||
| Right upper lobe | 27 (36.5) | 19 (40.4) | 8 (29.6) | |
| Right middle lobe | 4 (5.4) | 3 (6.4) | 1 (3.7) | |
| Right lower lobe | 13 (17.6) | 10 (21.3) | 3 (11.1) | |
| Left upper lobe | 24 (32.4) | 12 (25.5) | 12 (44.4) | |
| Left lower lobe | 6 (8.1) | 3 (6.4) | 3 (11.1) | |
| Opacity of PLL on chest CT | 0.36 | |||
| Subsolid opacity | 15 (20.3) | 8 (17.0) | 7 (25.9) | |
| Solid opacity | 59 (79.7) | 39 (83.0) | 20 (74.1) | |
| Cavity formation on chest CT | 0.41 | |||
| Present | 7 (9.5) | 6 (12.8) | 1 (3.7) | |
| Absent | 67 (90.5) | 41 (87.2) | 26 (96.3) | |
| Bronchus sign on chest CT | 0.16 | |||
| Present | 64 (86.5) | 43 (91.5) | 21 (77.8) | |
| Absent | 10 (13.5) | 4 (8.5) | 6 (22.2) | |
| rEBUS visualization | <0.001 | |||
| Within PLL | 56 (75.7) | 44 (93.6) | 12 (44.4) | |
| Adjacent to PLL | 18 (24.3) | 3 (6.4) | 15 (55.6) | |
| Complication after procedure | NA | |||
| Significant bleeding | 0 (0) | 0 (0) | 0 (0) | |
| Pneumothorax | 0 (0) | 0 (0) | 0 (0) | |
| Final diagnosis | <0.001 | |||
| Malignant | 47 (63.5) | 38 (80.9) | 9 (33.3) | |
| Benign | 19 (25.7) | 9 (19.1) | 10 (37) | |
| Undetermined | 8 (10.8) | 0 (0) | 8 (29.6) |
Data are presented as n (%) or mean ± SD. CT, computed tomography; GS, guide sheath; NA, non-applicable; PLL, peripheral lung lesion; rEBUS, radial endobronchial ultrasound; SD, standard deviation; TBLB, transbronchial lung biopsy.
Table 2. Final diagnosis of 74 PLLs who underwent rEBUS GS TBLB/TBNA.
| Variables | Values |
|---|---|
| Diagnosed with rEBUS GS TBLB/TBNA (n=52) | |
| Malignant disease, n (%) | 43 (82.7) |
| Adenocarcinoma | 28 (65.1) |
| Squamous cell carcinoma | 9 (20.9) |
| NSCLC NOS | 2 (4.7) |
| Small cell lung cancer | 3 (7.0) |
| Carcinoid | 1 (2.3) |
| Benign disease, n (%) | 9 (17.3) |
| Tuberculosis | 5 (55.6) |
| Aspergillosis | 2 (22.2) |
| Cryptococcosis | 1 (11.1) |
| Organizing pneumonia | 1 (11.1) |
| Undiagnosed with rEBUS GS TBLB/TBNA (n=22) | |
| Malignant disease, n (%) | 4 (18.2) |
| Adenocarcinoma | 1 (25.0) |
| Squamous cell carcinoma | 1 (25.0) |
| Large cell carcinoma | 1 (25.0) |
| Sarcomatoid carcinoma | 1 (25.0) |
| Benign disease, n (%) | 10 (45.5) |
| Tuberculosis | 2 (20.0) |
| NTM | 1 (10.0) |
| Aspergillosis | 1 (10.0) |
| Chronic inflammation | 6 (60.0) |
| Unknown, n (%) | 8 (36.4) |
GS, guide sheath; NSCLC NOS, non-small cell lung cancer not otherwise specified; NTM, non-tuberculous mycobacterium; PLL, peripheral lung lesion; rEBUS, radial endobronchial ultrasound; TBLB, transbronchial lung biopsy; TBNA, transbronchial needle aspiration.
Diagnostic yield
Among the 74 PLL, 47 (63.5%) were successfully diagnosed using rEBUS GS TBLB. Of the 27 PLL not diagnosed using rEBUS GS TBLB, 5 (18.5%) were further diagnosed using rEBUS GS TBNA: adenocarcinoma (n=2), non-small cell lung cancer not otherwise specified (n=2), small cell lung cancer (n=1). Additional TBNA increased the diagnostic yield by 6.8% (5/74), resulting in an overall diagnostic yield of 70.3% (52/74) (Figure 1 and Figure 2A). Diagnostic yields for each modality (including biopsy forceps, brushing, or washing, etc.) are shown in Table S1.
Figure 2.
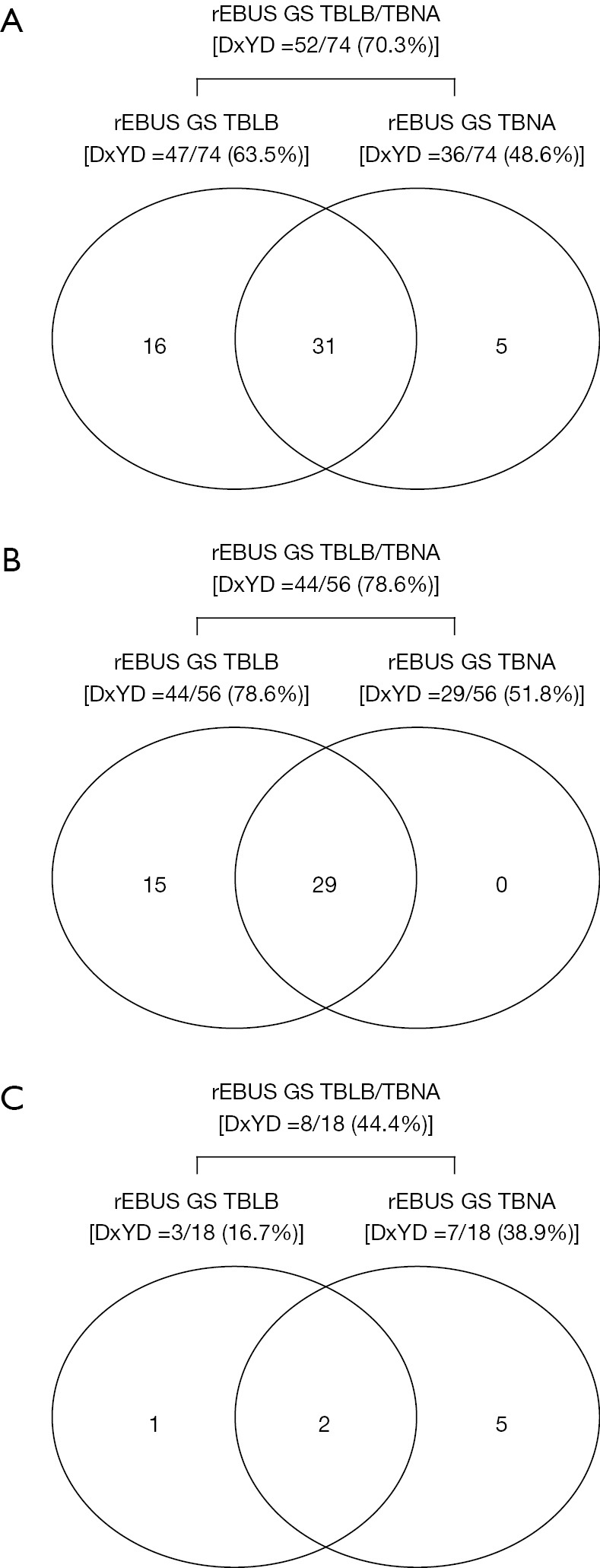
Diagnostic yields for rEBUS GS TBLB, rEBUS GS TBNA, and rEBUS GS TBLB/TBNA according to rEBUS visualization types. (A) All peripheral lung lesions (n=74); (B) peripheral lung lesions with within-type rEBUS visualization (n=56); (C) peripheral lung lesions with adjacent to-type rEBUS visualization (n=18). DxYD, diagnostic yield; GS, guide sheath; rEBUS, radial endobronchial ultrasound; TBLB, transbronchial lung biopsy; TBNA, transbronchial needle aspiration.
TBLB-diagnosed group vs. TBLB-undiagnosed group
The rEBUS GS TBLB-diagnosed group (n=47) and the rEBUS GS TBLB-undiagnosed group (n=27) were separated for further analysis (Figure 1). PLL size >20 mm [32 (68.1%) vs. 11 (40.7%), P=0.02] and within-type rEBUS visualization [44 (93.6%) vs. 12 (44.4%), P<0.001] were significantly higher in the rEBUS GS TBLB-diagnosed group (Table 1). Age, sex, distance from the pleura, location, nature of the PLL, and bronchus sign were not significantly different between the groups.
Factors affecting diagnostic yield of rEBUS GS TBLB
In the univariate analysis, lesions >20 mm and within-type rEBUS visualization were associated with rEBUS GS TBLB diagnosis; however, in the multivariate analysis, only within-type rEBUS visualization was independently associated with rEBUS GS TBLB diagnosis [adjusted OR (aOR) 19.609, 95% confidence interval (CI): 4.080–94.242, P<0.001] (Table 3).
Table 3. Factors affecting diagnostic yield of rEBUS GS TBLB.
| Variables | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | aOR | 95% CI | P value | ||
| Age, years | 0.999 | 0.956–1.044 | 0.98 | 0.989 | 0.932–1.049 | 0.71 | |
| Male sex | 0.751 | 0.427–1.179 | >0.99 | 1.523 | 0.391–5.934 | 0.55 | |
| Lesion size >20 mm | 3.103 | 1.162–8.289 | 0.02 | 1.598 | 0.432–5.912 | 0.48 | |
| Solid nature PLL on chest CT | 1.706 | 0.541–5.382 | 0.36 | 2.479 | 0.598–10.268 | 0.21 | |
| Presence of bronchus sign on chest CT | 3.071 | 0.782–12.069 | 0.11 | 1.056 | 0.138–8.077 | 0.95 | |
| Within-type rEBUS visualization | 18.333 | 4.547–73.921 | <0.001 | 19.609 | 4.080–94.242 | <0.001 | |
aOR, adjusted odds ratio; CI, confidence interval; CT, computed tomography; GS, guide sheath; OR, odds ratio; PLL, peripheral lung lesion; rEBUS, radial endobronchial ultrasound; TBLB, transbronchial lung biopsy.
Subgroup analysis for the rEBUS GS TBLB-undiagnosed group
For the 27 rEBUS GS TBLB-undiagnosed cases, the rEBUS GS TBNA-diagnosed subgroup (n=5) and the rEBUS GS TBNA-undiagnosed subgroup (n=22) were compared in a subgroup analysis. TBNA diagnosis was significantly associated with adjacent-to-type EBUS visualization [100.0% (5/5) vs. 45.5% (10/22), P=0.047] (Table 4).
Table 4. Subgroup analysis of 27 PLLs which were not diagnosed by rEBUS GS TBLB and underwent rEBUS GS TBNA.
| Variables | rEBUS GS TBNA | P value | |
|---|---|---|---|
| Diagnostic (n=5) | Non-diagnostic (n=22) | ||
| Age, years | 71±8 | 68±8 | 0.47 |
| Gender | >0.99 | ||
| Male | 3 (60.0) | 15 (68.2) | |
| Female | 2 (40.0) | 7 (31.8) | |
| Lesion size, mm | 20±4 | 21±14 | 0.83 |
| Distribution by size | >0.99 | ||
| ≤20 mm | 3 (60.0) | 13 (59.1) | |
| >20 mm | 2 (40.0) | 9 (40.9) | |
| Distance from pleura, mm | 21±21 | 13±14 | 0.32 |
| Location of PLL | 0.47 | ||
| Right upper lobe | 1 (20.0) | 7 (31.8) | |
| Right middle lobe | 0 | 1 (4.5) | |
| Right lower lobe | 0 | 3 (13.6) | |
| Left upper lobe | 4 (80.0) | 8 (36.4) | |
| Left lower lobe | 0 | 3 (13.6) | |
| Opacity of PLL on chest CT | 0.58 | ||
| Subsolid opacity | 2 (40.0) | 5 (22.7) | |
| Solid opacity | 3 (60.0) | 17 (77.3) | |
| Cavity formation on chest CT | >0.99 | ||
| Present | 0 | 1 (4.5) | |
| Absent | 5 (100.0) | 21 (95.5) | |
| Bronchus sign on chest CT | 0.30 | ||
| Present | 3 (60.0) | 18 (81.8) | |
| Absent | 2 (40.0) | 4 (18.2) | |
| rEBUS visualization | 0.047 | ||
| Within PLL | 0 | 12 (54.5) | |
| Adjacent to PLL | 5 (100.0) | 10 (45.5) | |
Data are presented as n (%) or mean ± SD. CT, computed tomography; GS, guide sheath; PLL, peripheral lung lesion; rEBUS, radial endobronchial ultrasound; SD, standard deviation; TBLB, transbronchial lung biopsy; TBNA, transbronchial needle aspiration.
Diagnostic yields according to rEBUS visualization types
Based on the subgroup analysis results, we analyzed the diagnostic yields according to the EBUS visualization type. For 56 within-type PLL, the overall diagnostic yield (through rEBUS GS TBLB/TBNA) was 78.6% (44/56), and no cases were exclusively diagnosed using rEBUS GS TBNA alone. Thus, additional rEBUS GS TBNA did not increase the diagnostic yield of within-type PLL (Figure 2B). In 18 adjacent-to-type PLL, the overall diagnostic yield (through rEBUS GS TBLB/TBNA) was 44.4% (8/18), and there were five cases exclusively diagnosed with rEBUS GS TBNA. Thus, the additional rEBUS GS-TBNA significantly increased the diagnostic yield of adjacent-to-type PLL from 16.7% (3/18) to 44.4% (8/18). Among the 18 adjacent-to-types PLL, only one was exclusively diagnosed with rEBUS GS TBLB (Figure 2C).
Discussion
When performing tissue biopsy via rEBUS GS-guided bronchoscopy on PLL, a PeriView FLEX TBNA needle can be used in addition to TBLB to improve the diagnostic yield. It is preferable to perform additional TBNA when the rEBUS probe is adjacent to the lesion rather than within the lesion.
Among the various techniques for biopsy of PLL, TTNB has the highest diagnostic yield (approximately 90%) (5,14) and the highest complication rate (pneumothorax, 15–20%; and hemoptysis, 1–5%) (15,16). Special bronchoscopic procedures such as electromagnetic navigation bronchoscopy, rEBUS, or robotic bronchoscopy have been developed for safer tissue biopsies. rEBUS is more difficult to learn than electromagnetic navigation bronchoscopy and requires a longer training time, but has the advantage of being more cost effective than electromagnetic navigation bronchoscopy despite a similar diagnostic yield (7). We used both rEBUS and electromagnetic navigation bronchoscopy, but recently started using only rEBUS because of cost issues. rEBUS is very safe (pneumothorax, 0–3%; hemoptysis, 0–1%), but its diagnostic yield is relatively low (60–70%) compared to that of TTNB (7,16). In terms of the robotic bronchoscopy, while the authors have yet to gain firsthand experience, research suggests a higher diagnostic yield (around 80%) compared to rEBUS or electromagnetic navigation bronchoscopy (6). However, its availability is limited due to the high setup costs (17).
To increase the diagnostic yield of rEBUS, the first attempted method was conventional transbronchial biopsy using larger forceps, following rEBUS GS TBLB (8,9). Additional conventional transbronchial biopsy increased the diagnostic yield by 14–17% (8,9). Kunimasa et al. found that additional conventional transbronchial biopsy was particularly helpful when the lesion was a ground-glass opacity nodule and the EBUS visualization type was adjacent to or eccentric within (which means the probe-to-lesion margin is <2.5 mm) (8). An alternative method to increase diagnostic yield was transbronchial cryobiopsy using a 1.1 mm Cryoprobe (ERBE, Germany) following rEBUS GS TBLB (10-12). Additional cryobiopsy increased the diagnostic yield by 15–18% (10,12). The significant factors associated with increased diagnostic yield through additional cryobiopsy were small (<22 mm in diameter) lesions (10) and adjacent-to-type EBUS visualization (12).
Although both conventional transbronchial biopsy and cryobiopsy are good adjunct modalities for rEBUS TBLB with GS, they have inevitable technical drawbacks. Regarding additional conventional transbronchial biopsy, the procedure should be performed after the removal of the GS. When using the GS, an accurate biopsy is possible within or directly in front of the lesion; however, without the GS, the forceps may not reach the lesion accurately. In cases of additional cryobiopsy, the procedure through GS is possible if a 1.1 mm Cryoprobe is used, but the scope must be removed at the same time unless a dedicated oversheath (outer diameter 2.6 mm) is used, which is impossible with a thin bronchoscope (outer diameter, 4.0–4.2 mm; working channel, 2 mm); therefore, repeat procedures may be difficult, and the risk of bleeding is relatively high. The cost of cryobiopsy is also considerable.
The PeriView FLEX TBNA needle has recently been developed as a novel diagnostic tool. Its maximal diameter is 1.5 mm, which is sufficient for the GS used in a 2 mm working channel bronchoscope. Using GS, needle aspiration biopsy can be performed immediately in front of or inside the lesion and repeat procedures can be performed easily. Another advantage is the flexibility of the needle. Even if the scope is bent, the needle can pass through the working channel without much difficulty, which is important to access lesions in the upper lobes.
Our results revealed that additional rEBUS GS TBNA using a PeriView FLEX TBNA needle increased the diagnostic yield when performed in combination with rEBUS GS TBLB. rEBUS GS TBNA was particularly helpful when adjacent to the lesion on EBUS. These results are consistent with those of Sumi et al. (18), who evaluated the diagnostic performance of the PeriView FLEX TBNA needle with ultrathin bronchoscopy (GS was not used) and found that rEBUS TBNA improved the diagnostic yield for small PLL when the lesions were adjacent to the rEBUS probe.
In comparison, the additional rEBUS GS TBNA using the PeriView FLEX TBNA needle was not helpful for within-type lesions on rEBUS. In our study, additional rEBUS GS TBNA did not at all increase the diagnostic yield of within-type PLL on rEBUS. It is though that, in the case of within-type PLL on rEBUS, performing additional rEBUS GS TBNA may yield tissue samples from the same location where rEBUS GS TBLB was performed. Therefore, in the case of within-type PLL on rEBUS, only rEBUS GS-TBLB was deemed sufficient. Our multivariate analysis (within-type rEBUS visualization was the only independently associated factor for rEBUS GS-TBLB diagnosis) supported these results. After confirming our results, rEBUS GS TBNA has not generally been performed in within-type PLL at our institution, except when the lesion is too difficult to open with the rEBUS GS TBLB forceps (on fluoroscopy).
The present study had some limitations. First, this was a retrospective, single-institution study with a small sample size, which may have had various sources of selection bias that were not identified or controlled. In particular, the number of patients diagnosed using rEBUS GS TBNA was small. Second, the time required for additional rEBUS GS-TBNA, which is a quality assessment factor, was not measured. In these regards, our results should be reaffirmed through a prospective study with a larger sample.
Conclusions
In conclusion, when performing the rEBUS GS procedure for tissue biopsy of PLL, if the lesion is adjacent, the diagnostic yield might be increased by adding rEBUS GS TBNA using the PeriView FLEX TBNA needle in addition to rEBUS GS TBLB.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: The present study was funded by the National Research Foundation of Korea (NRF-2023R1A2C2002510).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board of Ulsan University Hospital (No. UUH 2023-03-002) and individual consent for this retrospective analysis was waived.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1598/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1598/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1598/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1598/coif). The authors have no conflicts of interest to declare.
References
- 1.Chelala L, Hossain R, Kazerooni EA, et al. Lung-RADS Version 1.1: Challenges and a Look Ahead, From the AJR Special Series on Radiology Reporting and Data Systems. AJR Am J Roentgenol 2021;216:1411-22. 10.2214/AJR.20.24807 [DOI] [PubMed] [Google Scholar]
- 2.Dyer SC, Bartholmai BJ, Koo CW. Implications of the updated Lung CT Screening Reporting and Data System (Lung-RADS version 1.1) for lung cancer screening. J Thorac Dis 2020;12:6966-77. 10.21037/jtd-2019-cptn-02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin MD, Kanne JP, Broderick LS, et al. RadioGraphics Update: Lung-RADS 2022. Radiographics 2023;43:e230037. 10.1148/rg.230037 [DOI] [PubMed] [Google Scholar]
- 4.Na KJ, Park IK, Park S, et al. Efficacy and Cost-effectiveness of Surgical Biopsy for Histologic Diagnosis of Indeterminate Nodules Suspected for Early Stage Lung Cancer: Comparison with Percutaneous Needle Biopsy. J Korean Med Sci 2020;35:e261. 10.3346/jkms.2020.35.e261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e142S-65S. [DOI] [PubMed] [Google Scholar]
- 6.Ali MS, Ghori UK, Wayne MT, et al. Diagnostic Performance and Safety Profile of Robotic-assisted Bronchoscopy: A Systematic Review and Meta-Analysis. Ann Am Thorac Soc 2023;20:1801-12. 10.1513/AnnalsATS.202301-075OC [DOI] [PubMed] [Google Scholar]
- 7.Bae S, Lim S, Ahn JJ, et al. Diagnosing peripheral lung lesions using endobronchial ultrasonography with guide sheath: A prospective registry study to assess the effect of virtual bronchoscopic navigation using a computed tomography workstation. Medicine (Baltimore) 2020;99:e19870. 10.1097/MD.0000000000019870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kunimasa K, Tachihara M, Tamura D, et al. Diagnostic utility of additional conventional techniques after endobronchial ultrasonography guidance during transbronchial biopsy. Respirology 2016;21:1100-5. 10.1111/resp.12813 [DOI] [PubMed] [Google Scholar]
- 9.Park S, Yoon HY, Han Y, et al. Diagnostic yield of additional conventional transbronchial lung biopsy following radial endobronchial ultrasound lung biopsy for peripheral pulmonary lesions. Thorac Cancer 2020;11:1639-46. 10.1111/1759-7714.13446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SH, Mok J, Jo EJ, et al. The Additive Impact of Transbronchial Cryobiopsy Using a 1.1-mm Diameter Cryoprobe on Conventional Biopsy for Peripheral Lung Nodules. Cancer Res Treat 2023;55:506-12. 10.4143/crt.2022.1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen H, Yu X, Yu Y, et al. Diagnostic performance of cryobiopsy guided by radial-probe EBUS with a guide sheath for peripheral pulmonary lesions. J Bras Pneumol 2023;49:e20220200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakai T, Watanabe T, Kaimi Y, et al. Diagnostic Utility and Safety of Non-Intubated Cryobiopsy Technique Using a Novel Ultrathin Cryoprobe in Addition to Conventional Biopsy Techniques for Peripheral Pulmonary Lesions. Respiration 2023;102:503-14. 10.1159/000531010 [DOI] [PubMed] [Google Scholar]
- 13.Naso J, Bras J, Villamil C, et al. Cytologic features and diagnostic value of PeriView FLEX transbronchial needle aspiration targeting pulmonary nodules. Cancer Cytopathol 2020;128:333-40. 10.1002/cncy.22240 [DOI] [PubMed] [Google Scholar]
- 14.Asano F, Eberhardt R, Herth FJ. Virtual bronchoscopic navigation for peripheral pulmonary lesions. Respiration 2014;88:430-40. 10.1159/000367900 [DOI] [PubMed] [Google Scholar]
- 15.Wiener RS, Schwartz LM, Woloshin S, et al. Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records. Ann Intern Med 2011;155:137-44. 10.7326/0003-4819-155-3-201108020-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e93S-e120S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diddams MJ, Lee HJ. Robotic Bronchoscopy: Review of Three Systems. Life (Basel) 2023;13:354. 10.3390/life13020354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sumi T, Shijubou N, Sawai T, et al. Transbronchial needle aspiration with endobronchial ultrasonography and ultrathin bronchoscopy for peripheral pulmonary lesions. Respir Investig 2021;59:766-71. 10.1016/j.resinv.2021.04.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as