Abstract
Background
Nintedanib is a small molecule tyrosine kinase inhibitor (TKI) targeting vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor (PDGFR), and fibroblast growth factor receptor (FGFR). The purpose of the study was to evaluate the response rate for patients with advanced non-small cell lung cancer (NSCLC) with mutations in TP53, VEGFR1–3, PDGFR-A, PDGFR-B, and FGFR1–3 treated with nintedanib as part of an open-label, single-arm pilot study.
Methods
Patients with advanced NSCLC previously treated with platinum-doublet chemotherapy with the above mutations were enrolled. Exclusion criteria included necrotic tumors with invasion of blood vessels, history of recent thromboembolic events, increased risk of bleeding or thrombosis, myocardial infarction, and weight loss >10% within past 6 months. Nintedanib was administered at a dose of 200 mg orally twice daily until disease progression or unacceptable toxicity. The primary endpoint was objective response rate (ORR) by Response Evaluation Criteria in Solid Tumors (RECIST) 1.1. Secondary endpoints included progression-free survival (PFS) and correlating outcomes with specific mutations. This study was registered with ClinicalTrials.gov, number NCT02299141.
Results
Between 2015 and 2019, 20 patients were enrolled with a median age was 66 years, 15 (75%) were females, 15 (75%) had adenocarcinoma, and 17 patients had a TP53 mutation (85%). Seventeen (85%) had received prior immunotherapy and 11 (55%) had received at least three prior lines of systemic therapy. The ORR was 15% with three partial responses (PR), while 12 patients had stable disease (SD), with disease control rate (DCR) consisting of a PR and SD greater than or equal to 16 weeks of 65% (n=13). Median PFS was 4.3 months [95% confidence interval (CI): 1.8–7.9] and median overall survival (OS) was 11.3 months (95% CI: 3.5–44.2). Three patients experienced prolonged clinical benefit from nintedanib, remaining on treatment for over 1 year and all three had a TP53 mutation and received prior immunotherapy. The most common adverse events of any grade included nausea (80%), fatigue (70%), diarrhea (60%), and anorexia (60%).
Conclusions
In this pilot study in heavily pretreated and molecularly selected patients with metastatic NSCLC, nintedanib showed modest activity.
Keywords: Nintedanib, non-small cell lung cancer (NSCLC), adenocarcinoma, multikinase inhibitor, vascular endothelial growth factor tyrosine kinase inhibitor (VEGF TKI)
Highlight box.
Key findings
• Nintedanib monotherapy has promising results in molecularly selected patients with advanced non-small cell lung cancer (NSCLC).
What is known and what is new?
• Nintedanib is a small molecule tyrosine kinase inhibitor targeting vascular endothelial growth factor (VEGF) receptor, platelet-derived growth factor receptor, and fibroblast growth factor receptor. Patients with NSCLC and mutated TP53 may benefit from agents targeting VEGF.
• In this pilot study, nintedanib monotherapy in molecularly selected patients had similar outcomes to nintedanib and chemotherapy combination in an unselected population.
What is the implication, and what should change now?
• Further prospective randomized controlled studies are needed to explore the effect of nintedanib in molecularly-selected patients. Ongoing studies are evaluating nintedanib in combination with immunotherapy, and there may be benefit of examining these combinations in a molecularly-selected population.
Introduction
Angiogenesis plays a fundamental role in the tumor development, proliferation, and metastasis (1,2). The molecular pathways intrinsic to angiogenesis provide several targets for effective therapies in non-small cell lung cancer (NSCLC) (1). Such targets include vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), and their corresponding receptors (2). VEGF is a well-known key regulator of angiogenesis and signals mainly through the VEGF receptor 2 (VEGFR2) promoting the development of vascular structures, regulating vascular permeability, and inducing vascular leakage (3). Tyrosine kinase inhibitors (TKIs) inhibit multiple receptors in this pathway by competing with adenosine triphosphate (ATP) for the active site of the kinase domain (4). The PDGF pathway plays a role in the development of vascular structures and stabilization of blood vessels through its effects on pericytes and vascular smooth muscle cells (5). FGF receptor (FGFR) kinases activate signaling pathways resulting in endothelial cell activation, recruitment of pericytes and vascular smooth muscle cells, and affecting vascular integrity (2). Activation of PDGF and FGF pathways has been implicated in VEGF resistance, and all three pathways produce a synergistic effect on tumor angiogenesis (1,2,5). Inhibiting multiple tyrosine kinase receptors may prove more effective in preventing angiogenesis and the development of resistance.
Pre-clinical studies have demonstrated a relationship between TP53 and angiogenesis. Loss of p53 results in increased levels of hypoxia-inducible factor 1ɑ in tumor cells and subsequently increased VEGF expression in hypoxia (6,7). Specifically in NSCLC patients, the presence of a TP53 mutation has been associated with increased levels of VEGF (8,9). In a multiple regression analysis of transcriptomic data, TP53 mutations were associated with higher VEGF-A expression in NSCLC patients (10). This was suggested as the possible mechanism for a significantly longer progression-free survival (PFS) with bevacizumab-containing regimens compared to non-bevacizumab containing regimens in patients with mutated TP53 (median 11.0 vs. 4.0 months, P<0.001) (11). NSCLC patients with mutated TP53 may benefit from angiogenesis inhibitors and therapeutic agents targeting VEGF.
Nintedanib is an oral TKI that targets VEGFR1, VEGFR2, VEGFR3, PDGF receptor (PDGFR)-alpha, PDGFR-beta, FGFR1, FGFR2, and FGFR3 (12). In phase I studies, nintedanib was well tolerated with dose limiting toxicities of elevations of serum liver enzymes (13,14). Other toxicities included mild to moderate nausea, diarrhea, vomiting, abdominal pain, and fatigue (13,14). A phase II trial in locally advanced or metastatic NSCLC found that patients treated with nintedanib as monotherapy had a median overall survival (OS) of 21.9 weeks and a median PFS of 11.6 weeks with tumor stabilization obtained in 46% of patients (15). Phase III trials have combined nintedanib with chemotherapy. In LUME-Lung 1, nintedanib was combined with docetaxel in patients with stage IIIB or IV NSCLC progressing after first-line chemotherapy compared to docetaxel alone. PFS was significantly improved in the nintedanib and docetaxel group compared to docetaxel alone [median 3.4 vs. 2.7 months; hazard ratio (HR), 0.79; P=0.002]. OS was also significantly improved for patients with adenocarcinoma histology in the nintedanib and docetaxel group (median 12.6 vs. 10.3 months; HR, 0.83; P=0.036) (16). LUME-Lung 2 compared nintedanib and pemetrexed to placebo and pemetrexed in advanced non-squamous NSCLC (17). Study enrollment ended prematurely due to a pre-planned futility analysis using investigator-assessed PFS suggesting that the study was unlikely to reach predefined efficacy criteria. PFS by central independent review demonstrated significant prolongation in the nintedanib and pemetrexed arm (median 4.4 vs. 3.6 months; HR, 0.83; P=0.044) (17).
Thus far, nintedanib has been examined in molecularly unselected locally advanced and metastatic NSCLC. Nevertheless, the presence of VEGF, PDGFR, FGFR, and TP53 mutations may predict for clinically meaningful benefit from nintedanib. Therefore, we hypothesized that use of nintedanib as monotherapy in patients with molecular alterations may result in an improved objective response rate (ORR) and PFS compared to unselected patients. We present this article in accordance with the TREND reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1717/rc).
Methods
Study design and participants
This was an open-label, single-arm pilot study designed to obtain preliminary information on the efficacy of nintedanib monotherapy. All patients were recruited from Washington University in St. Louis outpatient clinics and had advanced metastatic or unresectable NSCLC with molecular alterations in VEGFR1–3, TP53, PDGFR-A, PDGFR-B, or FGFR1–3 confirmed by Clinical Laboratory Improvement Amendments (CLIA) certified-lab testing. Next-generation sequencing was performed on tumor tissue and consisted of targeted sequencing of clinically significant cancer genes and cell-free DNA testing in a CLIA-certified lab and was performed on peripheral blood, either was permitted for the purpose of this study. Programmed death-ligand 1 (PD-L1) testing was performed in a CLIA-certified lab as appropriate based on guidelines at the time of enrollment and displayed as a tumor proportion score. Patients must have progressed after platinum-doublet chemotherapy prior to enrollment and were required to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1 and at least one measurable lesion by Response Evaluation Criteria in Solid Tumors (RECIST) criteria version 1.1 (18). Patients were required to have adequate renal function with a creatinine within normal institutional limits or creatinine clearance >45 mL/min and normal hepatic function with total bilirubin and aspartate transaminase/alanine transaminase ≤1.5 the institutional upper limit of normal for patients without liver metastases and ≤2.5 the institutional upper limit of normal for patients with liver metastases. Patients were required to have adequate bone marrow function with a leukocyte count ≥3,000/mcL, absolute neutrophil count ≥1,500/mcL, platelets ≥100,000/mcL, and hemoglobin ≥9.0 g/dL.
Exclusion criteria included prior treatment with VEGFR TKIs; radiotherapy to a target lesion in the 3 months preceding treatment initiation; symptomatic brain metastases; leptomeningeal disease; cavitary or necrotic tumors; centrally located tumors with local invasion of major blood vessels; symptomatic heart failure; active coronary artery disease; serious cardiac arrhythmias; uncontrolled hypertension; uncontrolled seizure disorder; major injuries or surgery with incomplete wound healing in the 4 weeks preceding treatment initiation; clinically significant hemorrhagic or thromboembolic event in 6 months preceding treatment or predisposition to such events; active or chronic hepatitis B or C infection or human immunodeficiency virus (HIV) positivity. Patients with brain metastases were eligible if asymptomatic and previously treated. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board (IRB) of Washington University in St. Louis (No. IRB00009237) and informed consent was obtained from all individual participants.
Treatment and evaluation
Patients were treated with 200 mg of nintedanib orally twice daily on a 28-day cycle received from the outpatient investigational drug service pharmacy. The dose of 200 mg twice daily was chosen based on the overall safety profile in phase I studies (14). Treatment continued until disease progression or unacceptable toxicity. A medication diary was provided to record adherence to nintedanib. Dose reductions in 100 mg increments per day were permitted (or 50 mg per dosing) if deemed necessary by the investigator based on individual safety and tolerability. Nintedanib was discontinued if treatment was not tolerated at the 100 mg twice daily dose. There were no restrictions on supportive care or concomitant medication administration, and strong P-gp inhibitors and inducers were monitored closely and carefully considered.
Radiographic evaluation and tumor assessment was performed at screening, every 8 weeks and confirmatory scans were completed 4 weeks following documentation of an objective, complete response (CR) or partial response (PR). The objective response was assessed using the revised RECIST criteria (version 1.1) (18). Treatment-related adverse events included any unfavorable medical occurrence including any abnormal sign symptom or disease beginning after the first dose of nintedanib until 30 days after the end of treatment and were graded using the revised National Cancer Institution Common Terminology for Adverse Events (CTCAE) version 4.0.
Statistical analysis
The primary endpoint was ORR for patients with advanced NSCLC with mutations in the target genes for nintedanib. Secondary endpoints were PFS and correlating outcomes with specific mutations. PFS was defined as the duration of time from the start of treatment to time of progression or death, whichever took place first. Disease control rate (DCR) was defined as a CR, PR, or stable disease (SD) for 16 weeks or greater. The intention-to-treat population included all patients who received at least one dose of medication was used to analyze efficacy parameters.
As a pilot study to assess preliminary efficacy, the sample size (n=20 patients) was determined mainly based on clinical feasibility rather than statistical power. Based on extensive simulation studies, however, Piantadosi recommended that a sample size of 10–20 patients will provide a reasonable precision in estimating preliminary information (19). Descriptive statistics describe the demographic and clinical characteristics of the population as well as response and toxicity. A Kaplan-Meier product limit estimator was used to describe the distribution of the PFS and OS with a 95% confidence interval (CI). PFS and OS were compared between patients with different molecular profiles and between patients receiving treatment with immunotherapy using the log-rank test. All the statistical analyses were performed using SAS 9.4 (SAS Institutes, Cary, NC, USA).
Results
Patients
Twenty patients were enrolled from May 2015 to November 2019. Most patients had adenocarcinoma (75%), were female (75%), and 30% of patients had brain metastases (Table 1). The median age was 66 years. The majority of patients had tumors with somatic TP53 alterations (85%). There were no VEGF1–3 or PDGFR-B alterations, 15% of patients had tumors with an alteration in FGFR1–3, and 35% had a PDGFR-A mutation. In total, 25% of tumors contained both a TP53 and PDGFR-A mutation (Table 2), 55% received three or more prior lines of treatment, and 85% of patients received immunotherapy prior to starting nintedanib for a median of 25.3 months (range, 6.3–61.7 months).
Table 1. Patient characteristics.
| Characteristics | Values |
|---|---|
| Age (years), median [range] | 66 [48–78] |
| Histology, n [%] | |
| Adenocarcinoma | 15 [75] |
| Squamous | 3 [15] |
| Poorly differentiated | 2 [10] |
| Gender, n [%] | |
| Female | 15 [75] |
| Male | 5 [25] |
| Mutations, n [%] | |
| TP53 mutant | 17 [85] |
| PDGFR-A mutant | 7 [35] |
| TP53 and PDGFR mutant | 5 [25] |
| FGFR1–3 | 3 [15] |
| Number of previous lines of treatment, n [%] | |
| 1 | 3 [15] |
| 2 | 6 [30] |
| 3 | 5 [25] |
| 4 | 6 [30] |
| Number of previous lines of immunotherapy (n=17), n [%] | |
| 1 | 12 [71] |
| 2 | 5 [29] |
| Duration of immunotherapy (weeks), median [range] |
25.3 [6.3–61.7] |
| History of brain metastases, n [%] | 6 [30] |
PDGFR, platelet-derived growth factor receptor; FGFR, fibroblast growth factor receptor.
Table 2. Molecular alterations in NSCLC patients.
| Subject | Specimen | TP53 | PDGFR-A | FGFR1–3 | Other mutations | PD-L1 |
|---|---|---|---|---|---|---|
| 1 | Tumor | A159D, A27D, P72R | S478P | – | KRAS p.G13C, ASXL p.D741V, ASXL p.L815P, EGFR p.R521K, EGFR p.D393N, RB1 p.A525G, ALK p.D1529E, ALK p.I1461V, ALK p.K1491R, ATM p.N1983S, ATM p.D814E, FGFR4 p.P136L, FLT3 p.D7G, FGFR4 p.G388R FLT3 p.V5571, RB1 p.A525G, RET p.E867D, TET2 p.L1721W, APC p.V1822D, NOTCH1 p.A2331T | NA |
| 3 | Tumor | P72R, P72 | – | – | TET2 p.V1718L, ASXL1 p.M1249V, ASXL1 p.L815P, ABL1 p.K628_K628del, ABL1 p.K609_K609del, ALK p.I1461V, ALK p.K1491R, ALK p.D1529E, APC p.V1822D, ATM p.N1983S, ERBB2 p.P1140A, ERBB2 p.P1170A, FGFR4 p.P136L, FGFR4 p.V10OI, FLT3 p.T227M, EGFR p.R521K, RET p.135A | NA |
| 4 | Tumor | S183†, S51†, P72R |
G79D | FGFR1: E84†, E117†, E76† | PIK3CA p.H1047Y, IDH1 p.R100Q, BRCA1 p.E1380K, BRCA1 p.E1333K, BRCA1 p.E277K, NF1 p.P1840T, NF1 p.P1819T, STK11 p.598-1G>T, ROS1 p.Y1239C, ROS1 p.D2213N, ROS1 p.K2228Q, ROS1 p.S2229C, ROS1 p.R167Q, ALK p.A586G, ALK p.I1461V, JAK2 p.D378H, JAK2 p.H538Y, JAK2 p.E1028K, MTOR p.Q1931H, NOTCH1 p.E848K, NTRK1 p.G607V, NTRK1 p.G613V, NTRK1 p.G557V, NTRK1 p.H598Y, NTRK1 p.H604Y, NTRK1 p.H568Y, BRCA2 p.N372H, BRCA2 p.V2466A, ATM p.N1983S, ERBB2 p.I625V, ERBB2 p.I655V, ERBB2 p.P1140A, ERBB2 p.P1170A, KIT p.M537L, KIT p.M541L, MLH1 p.I219V, MLH1 p.I121V | NA |
| 5 | Tumor | – | E74K | – | KRAS p.G12V | NA |
| 6 | Tumor | – | H920N | – | KRAS p.G12C‡, BRCA1 p.Q1299L, BRCA1 p.1E1725Q, BRCA1 p.E1678Q, BRCA1 p.E621Q, BRCA1 p.E1746Q, BRCA1 p.Q1299L, BRCA1 p.Q1252L, BRCA1 p.P871L, BRCA1 p.P824L, BRCA1 p.S509G, BRCA1 p.S1566G, BRCA1 p.S1613G, BRCA1 p.S1634G, BRCA1 p.K1183R, BRCA1 p.E1038G, BRCA1 p.E991G, NOTCH2 p.E38K, NOTCH2 p.C19W, TSC1 p.M271T, TSC1 p.M322T, KDR p.V297I, KDR p.Q472H, FLT4 p.H890Q, ALK p.D1529E, ALK p.K1491R, ALK p.I1461V, ATM p.D1853N, ATM p.N1983S, BRCA2 p.V2466A, BRCA2 p.N372H, BRIP1 p.S919P, EGFR p.R521K, ERBB2 p.P1170A, ERBB2 p.I625V, ERBB2 p.I655V, FANCA p.G501S, FANCA p.V6D, FGFR4 p.P136L, FLT3 p.T227M, FLT3 p.D7G, FLT4 p.R1324L, FLT4 p.N149D, FLT4 p.R1146H, FLT4 p.T494A, KIT p.M537L, KIT p.M541L, NTRK1, RAD54B p.D880G, ROS1 p.D2213N: ROS1 p.S2229C, ROS1 p.K2228Q, RB1 | NA |
| 8 | cfDNA | R158L | – | – | CDKN2A p.Q57H, STK11 p.E57†, MET amplification | NA |
| 9 | cfDNA | V157F | A827T | – | KRAS p.G12C‡, MET p.E34†, CDK6 p.S321L | NA |
| 14 | cfDNA | D281E | G166E | – | EGFR exon 20 insertion (p.N771dup)‡, EGFR p.G724S, ARAF p.R209C | NA |
| 16 | cfDNA | R249S, V143M, I195T | – | – | EGFR E746_A750 exon 19 deletion‡, EGFR I821S exon 3 deletion, EGFR p.P644P | NA |
| 19 | Tumor | exon7 C242F | – | FGFR3 exon7 p.S249C | RRM1, TOP2A, TUBB3, CSF1R p.E745K | NA |
| 21 | Tumor | A159V, A27V, P72R | – | – | KRAS p.G12C‡, BRCA1 c.5075-2A>T, DDR2 p.D661Y, ATM p.E1664K, MET p.Q318K, NF1 p.E725†, RAD54B p.D747H, PALB2 p.Q559R, FLT4 p.H890Q, FANCA p.G809D, ALK p.D1529E, ALK p.K1491R, ALK p.I1461V, ATM p.N1983S, BRCA2 p.V2466A, BRIP1 p.S919P, CREBBP p.L5131, CREBBP p.L551I, CSF1R p.H362R, EGFR p.R521K, ERBB2 p.P1140A, ERBB2 p.P1170A, FANCA p.G501S, FANCA p.T266A, FANCA p.V6D, FGFR4 p.P136L, FGFR4 p.G338R, FLT4 p.R1324L, MLH1 p.I219V, MLH1 p.I121V, MYC p.N26S, NTRK1 | 50% |
| 23 | cfDNA | R342P, D207fs | – | – | CDKN2A P81fs, NOTCH1 p.T2076T, MAPK3 p.V63V | <1% |
| 25 | cfDNA | E2BGfs†59, Y234H | – | – | Not detected | <1% |
| 26 | cfDNA | M160_A161del | – | – | EML4-ALK fusion‡, ALK p.F1174L, ALK p.G1269, PIK3CA amplification, CDK6 p.D110V, MET p.L903L, MAP2K2 p.E66K, CCDN2 p.T280A | 60% |
| 29 | cfDNA | V216M | – | – | Not detected | NA |
| 30 | cfDNA | T125R | W549fs (exon 11 deletion) | – | KRAS p.G12C‡, MYC amplification, CCNE1 p.P207T, CCNE1 amplification, EGFR p.P243R, JAK2 p.V617F | 70% |
| 31 | Tumor | Y234 | – | – | Not detected | Negative |
| 33 | cfDNA | – | – | FGFR1 amplification | EGFR amplification, PIK3CA amplification, MYC amplification, CCNE1 amplification, NF1 p.C187F | <1% |
| 34 | Tumor | R116Q, R248Q, P72R | – | – | ERBB2 E740_A771insAYVM, ERBB2 p.I625V, ERBB2 p.I655V, ERBB2 p.P1140A, ERBB2 p.P1170A, ALK p.G1345E, ALK p.D1529E, ALK p.K1491R, ALK p.I1461V, ELT3 p.V194M, FANCA p.R1186G, FANCA p.G809D, FANCA p.266A, NTRK1 p.R557Q, NTRK1 p.R587Q, NTRK1 p.R593Q, PALB2 p.Q559R, PALB2 p.E672Q, PALB2 p.G998E, FLT4 p.H890Q, BRCA1 p.S1613G, BRCA1 p.S1566G, BRCA1 p.S509G, BRCA1 p.S1634G, BRCA1 p.K1183R, BRCA1 p.K1136R, BRCA1 p.E1038G, BRCA1 p.E991G, BRCA1 p.P871L, BRCA1 p.P824L, BRCA1 p.D693N, BRCA1 p.D646N, BRCA1 p.Q356R, BRCA1 p.Q309R, BRCA1 p.N372H, BRCA1 p.V2466A, BRIP1 p.S919P, FGFR4 p.G388R, FLT3 p.G388R, FLT3 p.D7G, GNAS p.R314W, GNAS p.P376L, KDR p.Q472H, ROS1 p.R167Q, KRAS, MET, HER-2 amplification | 90% |
| 35 | Tumor | R273L | – | – | KRAS p.G12C‡ | Negative |
†, indicates the presence of a stop codon; ‡, actionable alterations at the time of data collection. NSCLC, non-small cell lung cancer; PDGFR, platelet-derived growth factor receptor; FGFR, fibroblast growth factor receptor; PD-L1, programmed death ligand 1; NA, not available; cfDNA, cell-free DNA.
Response rate and survival
Nineteen patients had evaluable results with a median follow-up of 11.3 months (range, 1.3–87.0 months). ORR was 15% after 2 months of treatment with three PRs and no CRs. SD was observed in 12 (60%) of patients (Figure 1). Disease control consisting of PR and SD for greater than or equal to 16 weeks was observed at a rate of 65%. At the time of analysis, 16 (80%) patients had expired. Median PFS was 4.3 months (95% CI: 1.8–7.9) (Figure 2A) and median OS was 11.3 months (95% CI: 3.5–44.2) (Figure 2B). No significant difference was observed in PFS (P=0.61) or OS (P=0.43) between patients who received immunotherapy prior to nintedanib and those who did not (n=3).
Figure 1.
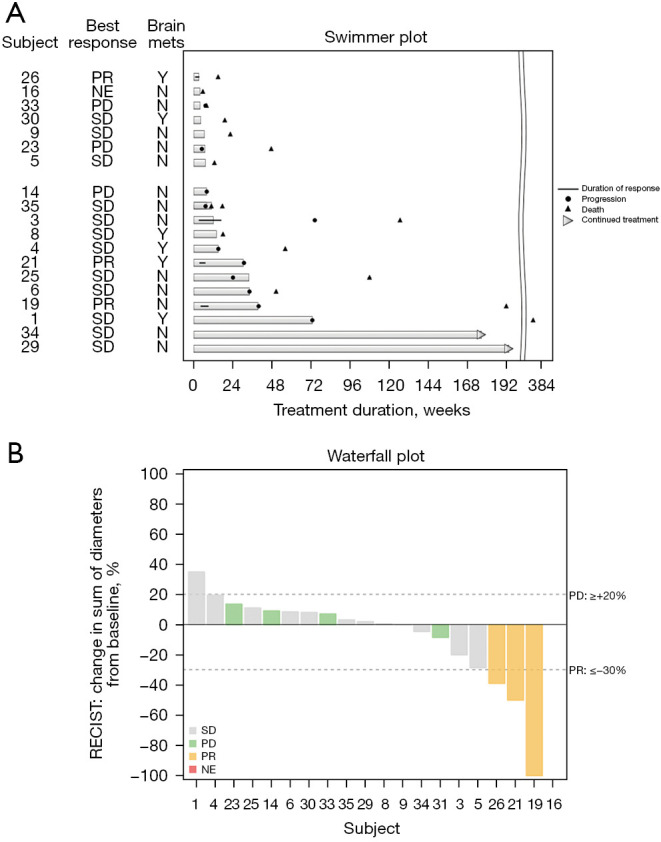
Efficacy of nintedanib monotherapy. (A) Swimmer plot of duration of response and patient status at time of data analysis. (B) Waterfall plot of best percent change in measurable disease. The sum of the longest diameters of all target lesions was measured at baseline and at time of best response. PR, partial response; Y, yes; NE, not evaluable; N, no; PD, progressive disease; SD, stable disease; RECIST, Response Evaluation Criteria in Solid Tumors.
Figure 2.
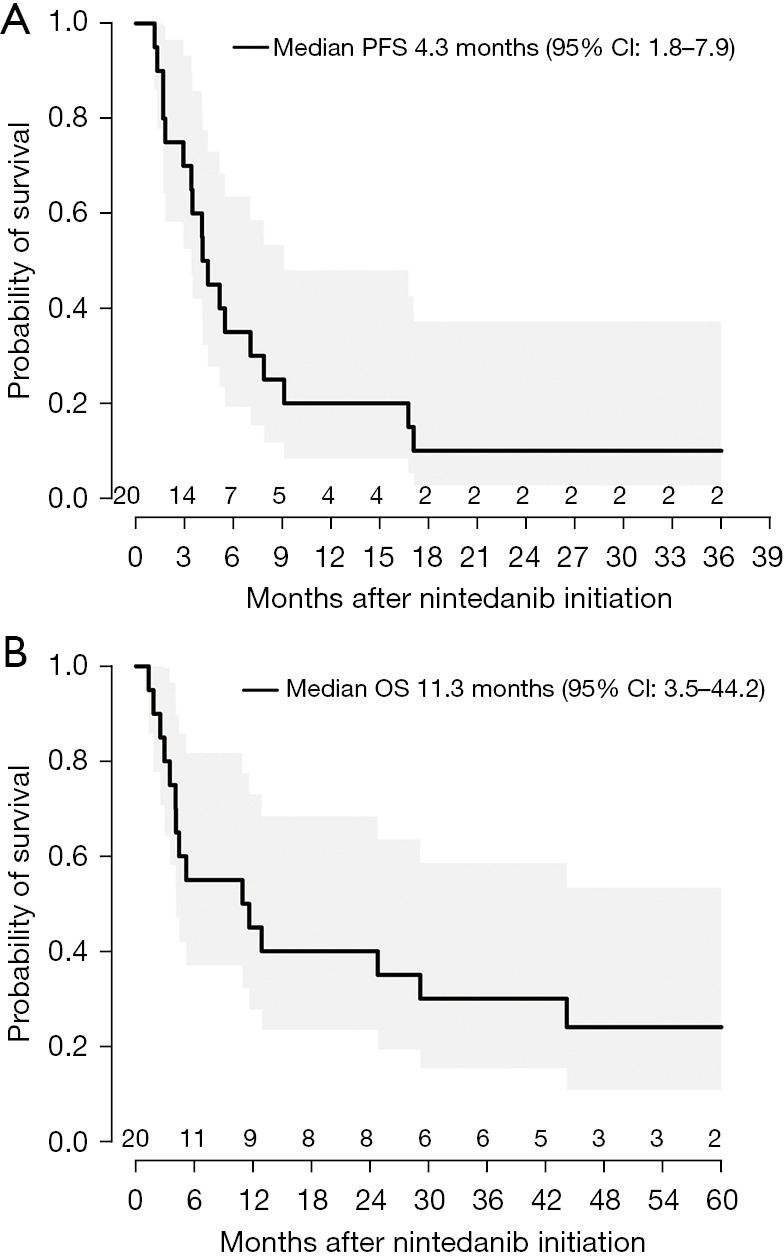
Kaplan-Meier curves of clinical outcomes of nintedanib monotherapy. (A) PFS. (B) OS. PFS, progression-free survival; CI, confidence interval; OS, overall survival.
Subgroup analyses exploring the association between specific mutations and outcomes including OS and PFS did not demonstrate any statistically significant associations. All three patients who experienced a PR had tumors with TP53 mutations, poorly differentiated or squamous histology and two of the three patients were on treatment for over 6 months. An additional three patients had prolonged clinical benefit and remained on nintedanib for over 1 year with one patient still on treatment. All three of these patients had TP53 mutations and adenocarcinoma histology.
Toxicity
Treatment-related adverse events were observed in 16 of 20 patients (Table 3). The most common adverse event was nausea with 12 (60%) of patients with grade one nausea, 3 (15%) with grade 2, and 1 (5%) patient with grade 3 nausea. Other common adverse events included fatigue (70%), diarrhea (60%), and anorexia (60%). There were five patients with grade 3 events and two patients with grade 4 events. Grade 4 events included elevated liver function tests (10%) and cerebral edema (5%). Grade 3 events included elevated liver function tests (25%), anorexia (5%), fatigue (5%), hypertension (5%), and nausea (5%). No fatal adverse events related to nintedanib occurred. No major bleeding events occurred.
Table 3. Treatment-related adverse events.
| Adverse events | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Nausea | 12 [60] | 3 [15] | 1 [5] | 0 |
| Fatigue | 10 [50] | 3 [15] | 1 [5] | 0 |
| Diarrhea | 10 [50] | 2 [10] | 0 | 0 |
| Vomiting | 8 [40] | 2 [10] | 0 | 0 |
| Anorexia | 7 [35] | 4 [20] | 1 [5] | 0 |
| Dyspepsia | 6 [30] | 2 [10] | 0 | 0 |
| Oral mucositis | 5 [25] | 0 | 0 | 0 |
| Myalgia | 3 [15] | 1 [5] | 0 | 0 |
| Hypertension | 2 [10] | 3 [15] | 1 [5] | 0 |
| Arthralgia | 2 [10] | 0 | 0 | 0 |
| Dizziness | 2 [10] | 0 | 0 | 0 |
| Edema | 2 [10] | 0 | 0 | 0 |
| Headache | 2 [10] | 0 | 0 | 0 |
| Rash | 2 [10] | 0 | 0 | 0 |
| Sweating | 2 [10] | 0 | 0 | 0 |
| Tremor | 2 [10] | 0 | 0 | 0 |
| Fever | 1 [5] | 1 [5] | 0 | 0 |
| Alopecia | 1 [5] | 0 | 0 | 0 |
| Constipation | 1 [5] | 0 | 0 | 0 |
| Cough | 1 [5] | 0 | 0 | 0 |
| Dry mouth | 1 [5] | 0 | 0 | 0 |
| Dry skin | 1 [5] | 0 | 0 | 0 |
| Dysgeusia | 1 [5] | 0 | 0 | 0 |
| Dysphagia | 1 [5] | 0 | 0 | 0 |
| Feet cramps | 1 [5] | 0 | 0 | 0 |
| Hot flashes | 1 [5] | 0 | 0 | 0 |
| Hyperpigmentation | 1 [5] | 0 | 0 | 0 |
| Insomnia | 1 [5] | 0 | 0 | 0 |
| Lightheadedness | 1 [5] | 0 | 0 | 0 |
| Non-cardiac chest pain | 1 [5] | 0 | 0 | 0 |
| Bilateral leg pain | 1 [5] | 0 | 0 | 0 |
| Infection | 0 | 3 [15] | 0 | 0 |
| Peptic ulcer disease | 0 | 1 [5] | 0 | 0 |
| Seizure | 0 | 1 [5] | 0 | 0 |
| Weight loss | 0 | 1 [5] | 0 | 0 |
| Alanine aminotransferase increased | 0 | 0 | 2 [10] | 1 [5] |
| Aspartate aminotransferase increased | 0 | 0 | 2 [10] | 1 [5] |
| Bilirubin increased | 0 | 0 | 1 [5] | 0 |
| Cerebral edema | 0 | 0 | 0 | 1 [5] |
Data are presented as n [%].
Discussion
This pilot study of nintedanib as monotherapy in molecularly selected patients demonstrated that nintedanib 200 mg twice daily was well tolerated and results in disease control. Most patients in our study were heavily pre-treated with over half receiving three or more lines of treatment. Our results with median PFS of 3.4 months, median OS of 11.3 months, and DCR of 65% were numerically higher than nintedanib monotherapy and similar to combination nintedanib and chemotherapy in a molecularly unselected population (15-17). These studies examining combination nintedanib and chemotherapy were conducted prior to the advent of immunochemotherapy as first-line treatment in advanced stage NSCLC. The optimal sequencing of angiogenesis inhibitors following progression on platinum-based chemotherapy and immunotherapy remains undefined. In the molecularly selected population of our study, there was no significant difference in PFS or OS in those who received prior immunotherapy compared to those who did not. Given the small sample size of this study, it is possible there was not enough signal to detect a significant difference or alternatively, there may be a role for nintedanib monotherapy in a molecularly selected population irrespective of use of prior immunotherapy.
It is theorized that VEGF and its role in angiogenesis may contribute to resistance to immunotherapy, and concurrent use of VEGF inhibitors may increase the efficacy of immunotherapy by normalizing tumor vasculature (20,21). Combination VEGF TKIs and immunotherapy have approval in hepatocellular carcinoma and renal cell carcinoma (22,23). Similarly in NSCLC, the combination of ramucirumab and pembrolizumab demonstrated a statistically improved median OS in a phase II study and is being further explored with an ongoing phase III trial, Pragmatica-Lung (NCT05633602) (24). Combination lenvatinib and pembrolizumab demonstrated improvement in PFS and ORR compared to pembrolizumab alone and this combination is undergoing further investigation with a phase III trial (NCT03976375) (25). Ongoing clinical trials are in progress examining combinations with nintedanib and immunotherapy in NSCLC, including nintedanib with nivolumab (NCT04046614) and nintedanib, nivolumab, and ipilimumab (NCT03377023).
Most patients in the study had tumors carrying mutations in TP53, which is known to portend a poor prognosis in NSCLC (26). Our results suggest that this patient population may derive benefit from VEGF-targeting agents, which corresponds to previous investigation with bevacizumab-containing therapy in malignancies with mutated TP53 (11). Preclinical studies have shown that lenvatinib and cabozantinib were particularly effective in hepatocellular carcinoma cell lines with mutated or deleted TP53 (27). Nintedanib has been shown to restore sensitivity to paclitaxel in endometrial cancer cells with alterations in p53 and induce cell death (28).
Another use of nintedanib is first-line treatment for patients with chronic fibrosing interstitial lung disease. In patients with idiopathic pulmonary fibrosis, nintedanib monotherapy has been shown to reduce the decline in forced vital capacity possibly slowing disease progression (29). Patients with NSCLC and interstitial lung disease are a particularly challenging patient population to treat with fewer options available to them. Nintedanib may be an option for this patient population and could be expanded to include patients who have experienced radiation pneumonitis or checkpoint-inhibitor pneumonitis. One patient in our pilot study had stage IV NSCLC and previously developed grade 3 pneumonitis after treatment with nivolumab in combination with an oral indoleamine 2,3-dioxygenase inhibitor. Both agents were stopped, he required multiple courses of steroids and mycophenolate mofetil, and he was initiated on nintedanib 15 months after his diagnosis of pneumonitis. His scans after 4 months of treatment showed improvement spirometry and ground glass opacities on imaging. At the time of data collection, he was still on treatment with nintedanib and had SD. A phase III trial of patients with NSCLC and idiopathic pulmonary fibrosis treated with carboplatin, nab-paclitaxel with or without nintedanib, though powered to evaluate exacerbation-free survival, demonstrated improvement in median PFS (6.2 vs. 5.5 months; HR, 0.68; 95% CI: 0.50–0.92) and improvement in OS for patients with nonsquamous histology (HR, 0.61; 95% CI: 0.38–0.98) (30). Further studies are needed to determine if nintedanib monotherapy or combination therapy may have clinical benefit in patients with NSCLC and complications related to immunotherapy, radiation, or idiopathic pulmonary fibrosis.
Twice daily treatment with nintedanib was overall well tolerated in this population. Most treatment-related adverse effects were mild to moderate. There were no new safety signals or unexpected toxicities. The most common adverse events were nausea, fatigue, diarrhea, and anorexia and were most often grade 1 adverse events. There were only three grade 4 events which were cerebral edema and elevated liver function tests. These results are similar to those described in other studies, and no bleeding events were observed (16,31). When used in combination with chemotherapy or immunotherapy nintedanib continues to be well tolerated and requires dose reductions 19–34% of patients (16,17,32).
This pilot study has some limitations, most notable being the molecular selection criteria. Testing for molecular alterations was performed on tumor or peripheral blood using cell-free DNA with 50% of testing performed on the latter. It is possible that some of the molecular alterations discovered on cell-free DNA testing were clonal hematopoiesis of indetermined potential (CHIP). CHIP has been known to increase in frequency with age and smoking. Mutations in DNA damage response genes, such as TP53, are more common in solid tumor malignancies than hematologic malignancies (33). It is thought some of these clones selectively expand after use of cytotoxic therapies (34). Furthermore, 80% of TP53 mutations in all cancer subtypes are missense mutations in the DNA-binding domain of the protein and have been posited to exert dominant negative effects that in turn provide a selective advantage to cells after DNA damage (35). The functional consequences of molecular alterations VEGFR1–3, PDGFR-A, PDGFR-B, and FGFR1–3 in the patients in our study are unknown. The small sample size of this pilot study of molecularly selected patients is another limitation and may limit our ability to detect differences particularly in patients who did not receive immunotherapy prior to treatment with nintedanib.
Conclusions
In conclusion, nintedanib monotherapy is a safe and has potential in molecularly selected patients with NSCLC. Ongoing studies are evaluating the combination of nintedanib with immunotherapy to evaluate for a possible synergistic response. Based on those outcomes, further prospective randomized controlled studies could explore multi-targeted angiokinase inhibitors in combination with immunotherapy to determine if outcomes are improved in a molecularly selected population.
Supplementary
The article’s supplementary files as
Acknowledgments
This study was submitted to the American Society of Clinical Oncology annual conference meeting in 2020 and selected for electronic publication (https://doi.org/10.1200/JCO.2020.38.15_suppl.e21694).
Funding: This study was funded by the National Comprehensive Cancer Network (NCCN) Oncology Research Program as an independent, investigator-initiated study supported through a grant from Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI). BIPI had no role in the design, analysis, or interpretation of the results in this study; BIPI was given the opportunity to review the manuscript for medical and scientific accuracy as it relates to BIPI substances, as well as intellectual property considerations.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board (IRB) of Washington University in St. Louis (No. IRB00009237) and informed consent was obtained from all individual participants.
Footnotes
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1717/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1717/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1717/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1717/coif). D.M. serves as an unpaid editorial board member of Journal of Thoracic Disease from October 2022 to September 2024. All authors report that the study was funded by the National Comprehensive Cancer Network (NCCN) Oncology Research program as an independent, investigator-initiated study supported through a grant from Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI) with funding supplied to the institution. S.D. reports an advisory role for AstraZeneca, Genentech, Merus and Jazz pharmaceuticals and royalties from Springer publications. D.M. reports research funding from Heat Biologics, Merck, Celgene, AstraZeneca, Baxter, Incyte, AbbVie, Bristol Myers Squibb, EpicentRx, Pfizer, Roche, Lilly, Altum Pharmaceuticals, Array BioPharma, Surface Oncology, Arcus Biosciences, Boehringer Ingelheim, Y-mAbs Therapeutics and serves in an advisory role for AbbVie, G1 Therapeutics, Lilly Medical, Miratic Therapeutics, Arcus Biosciences. S.N.W. reports research funding from SWOG-Clinical Trials Partnership, American Society of Hematology, AbbVie, Gilead, Immunomedics Inc., Daiichi Sankyo, Cullinan Pearl, Verastem Inc, Janssen Research and Development LLC, Elevation Oncology, Genentech, Verastem, Advenchen, Ribon, Loxo Oncology, Takeda, Hoffman-LaRoche, honoraria from ASCO, MJH Life Sciences, travel support from Gilead, and advisory roles for Janssen, Gilead, AstraZeneca, and Hoosier Cancer Research Network. The authors have no other conflicts of interest to declare.
References
- 1.Crinò L, Metro G. Therapeutic options targeting angiogenesis in nonsmall cell lung cancer. Eur Respir Rev 2014;23:79-91. 10.1183/09059180.00008913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballas MS, Chachoua A. Rationale for targeting VEGF, FGF, and PDGF for the treatment of NSCLC. Onco Targets Ther 2011;4:43-58. 10.2147/OTT.S18155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subramanian J, Morgensztern D, Govindan R. Vascular endothelial growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. Clin Lung Cancer 2010;11:311-9. 10.3816/CLC.2010.n.039 [DOI] [PubMed] [Google Scholar]
- 4.Morgensztern D, Herbst RS. Multitargeted tyrosine kinase inhibitors in unselected patients with advanced non-small-cell lung cancer (NSCLC): impressions from MONET (the motesanib NSCLC efficacy and tolerability study). J Clin Oncol 2012;30:2805-8. 10.1200/JCO.2012.42.7260 [DOI] [PubMed] [Google Scholar]
- 5.Rogosin S, Sandler AB. Beyond bevacizumab: antiangiogenic agents. Clin Lung Cancer 2012;13:326-33. 10.1016/j.cllc.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 6.Ravi R, Mookerjee B, Bhujwalla ZM, et al. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev 2000;14:34-44. 10.1101/gad.14.1.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamat CD, Green DE, Warnke L, et al. Mutant p53 facilitates pro-angiogenic, hyperproliferative phenotype in response to chronic relative hypoxia. Cancer Lett 2007;249:209-19. 10.1016/j.canlet.2006.08.017 [DOI] [PubMed] [Google Scholar]
- 8.Yuan A, Yu CJ, Luh KT, et al. Aberrant p53 expression correlates with expression of vascular endothelial growth factor mRNA and interleukin-8 mRNA and neoangiogenesis in non-small-cell lung cancer. J Clin Oncol 2002;20:900-10. [DOI] [PubMed] [Google Scholar]
- 9.Niklińska W, Burzykowski T, Chyczewski L, et al. Expression of vascular endothelial growth factor (VEGF) in non-small cell lung cancer (NSCLC): association with p53 gene mutation and prognosis. Lung Cancer 2001;34 Suppl 2:S59-64. 10.1016/S0169-5002(01)00346-4 [DOI] [PubMed] [Google Scholar]
- 10.Schwaederlé M, Lazar V, Validire P, et al. VEGF-A Expression Correlates with TP53 Mutations in Non-Small Cell Lung Cancer: Implications for Antiangiogenesis Therapy. Cancer Res 2015;75:1187-90. 10.1158/0008-5472.CAN-14-2305 [DOI] [PubMed] [Google Scholar]
- 11.Said R, Hong DS, Warneke CL, et al. P53 mutations in advanced cancers: clinical characteristics, outcomes, and correlation between progression-free survival and bevacizumab-containing therapy. Oncotarget 2013;4:705-14. 10.18632/oncotarget.974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hilberg F, Roth GJ, Krssak M, et al. BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res 2008;68:4774-82. 10.1158/0008-5472.CAN-07-6307 [DOI] [PubMed] [Google Scholar]
- 13.Mross K, Stefanic M, Gmehling D, et al. Phase I study of the angiogenesis inhibitor BIBF 1120 in patients with advanced solid tumors. Clin Cancer Res 2010;16:311-9. 10.1158/1078-0432.CCR-09-0694 [DOI] [PubMed] [Google Scholar]
- 14.Okamoto I, Kaneda H, Satoh T, et al. Phase I safety, pharmacokinetic, and biomarker study of BIBF 1120, an oral triple tyrosine kinase inhibitor in patients with advanced solid tumors. Mol Cancer Ther 2010;9:2825-33. 10.1158/1535-7163.MCT-10-0379 [DOI] [PubMed] [Google Scholar]
- 15.Reck M, Kaiser R, Eschbach C, et al. A phase II double-blind study to investigate efficacy and safety of two doses of the triple angiokinase inhibitor BIBF 1120 in patients with relapsed advanced non-small-cell lung cancer. Ann Oncol 2011;22:1374-81. 10.1093/annonc/mdq618 [DOI] [PubMed] [Google Scholar]
- 16.Reck M, Kaiser R, Mellemgaard A, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol 2014;15:143-55. 10.1016/S1470-2045(13)70586-2 [DOI] [PubMed] [Google Scholar]
- 17.Hanna NH, Kaiser R, Sullivan RN, et al. Nintedanib plus pemetrexed versus placebo plus pemetrexed in patients with relapsed or refractory, advanced non-small cell lung cancer (LUME-Lung 2): A randomized, double-blind, phase III trial. Lung Cancer 2016;102:65-73. 10.1016/j.lungcan.2016.10.011 [DOI] [PubMed] [Google Scholar]
- 18.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 19.Piantadosi S. Translational clinical trials: an entropy-based approach to sample size. Clin Trials 2005;2:182-92. 10.1191/1740774505cn078oa [DOI] [PubMed] [Google Scholar]
- 20.Fukumura D, Kloepper J, Amoozgar Z, et al. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol 2018;15:325-40. 10.1038/nrclinonc.2018.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Popat S, Grohé C, Corral J, et al. Anti-angiogenic agents in the age of resistance to immune checkpoint inhibitors: Do they have a role in non-oncogene-addicted non-small cell lung cancer? Lung Cancer 2020;144:76-84. 10.1016/j.lungcan.2020.04.009 [DOI] [PubMed] [Google Scholar]
- 22.Amin A, Plimack ER, Ernstoff MS, et al. Safety and efficacy of nivolumab in combination with sunitinib or pazopanib in advanced or metastatic renal cell carcinoma: the CheckMate 016 study. J Immunother Cancer 2018;6:109. Erratum in: J Immunother Cancer 2019;7:73. 10.1186/s40425-018-0420-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor MH, Lee CH, Makker V, et al. Phase IB/II Trial of Lenvatinib Plus Pembrolizumab in Patients With Advanced Renal Cell Carcinoma, Endometrial Cancer, and Other Selected Advanced Solid Tumors. J Clin Oncol 2020;38:1154-63. 10.1200/JCO.19.01598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reckamp KL, Redman MW, Dragnev KH, et al. Phase II Randomized Study of Ramucirumab and Pembrolizumab Versus Standard of Care in Advanced Non-Small-Cell Lung Cancer Previously Treated With Immunotherapy-Lung-MAP S1800A. J Clin Oncol 2022;40:2295-306. 10.1200/JCO.22.00912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang JCH, Luft A, Jiménez EDLM, et al. 120O Pembrolizumab (Pembro) with or without lenvatinib (Lenva) in first-line metastatic NSCLC with PD-L1 TPS≥ 1%(LEAP-007): A phase III, randomized, double-blind study. Ann Oncol 2021;32:S1429-30. 10.1016/j.annonc.2021.10.139 [DOI] [Google Scholar]
- 26.Steels E, Paesmans M, Berghmans T, et al. Role of p53 as a prognostic factor for survival in lung cancer: a systematic review of the literature with a meta-analysis. Eur Respir J 2001;18:705-19. 10.1183/09031936.01.00062201 [DOI] [PubMed] [Google Scholar]
- 27.Rodríguez-Hernández MA, Chapresto-Garzón R, Cadenas M, et al. Differential effectiveness of tyrosine kinase inhibitors in 2D/3D culture according to cell differentiation, p53 status and mitochondrial respiration in liver cancer cells. Cell Death Dis 2020;11:339. 10.1038/s41419-020-2558-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meng X, Dizon DS, Yang S, et al. Strategies for Molecularly Enhanced Chemotherapy to Achieve Synthetic Lethality in Endometrial Tumors with Mutant p53. Obstet Gynecol Int 2013;2013:828165. 10.1155/2013/828165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2071-82. 10.1056/NEJMoa1402584 [DOI] [PubMed] [Google Scholar]
- 30.Otsubo K, Kishimoto J, Ando M, et al. Nintedanib plus chemotherapy for nonsmall cell lung cancer with idiopathic pulmonary fibrosis: a randomised phase 3 trial. Eur Respir J 2022;60:2200380. 10.1183/13993003.00380-2022 [DOI] [PubMed] [Google Scholar]
- 31.Ellis PM, Kaiser R, Zhao Y, et al. Phase I open-label study of continuous treatment with BIBF 1120, a triple angiokinase inhibitor, and pemetrexed in pretreated non-small cell lung cancer patients. Clin Cancer Res 2010;16:2881-9. 10.1158/1078-0432.CCR-09-2944 [DOI] [PubMed] [Google Scholar]
- 32.Grohé C, Wehler T, Dechow T, et al. Nintedanib plus docetaxel after progression on first-line immunochemotherapy in patients with lung adenocarcinoma: Cohort C of the non-interventional study, VARGADO. Transl Lung Cancer Res 2022;11:2010-21. 10.21037/tlcr-21-1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaiswal S, Ebert BL. Clonal hematopoiesis in human aging and disease. Science 2019;366:eaan4673. 10.1126/science.aan4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong TN, Ramsingh G, Young AL, et al. Role of TP53 mutations in the origin and evolution of therapy-related acute myeloid leukaemia. Nature 2015;518:552-5. 10.1038/nature13968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boettcher S, Miller PG, Sharma R, et al. A dominant-negative effect drives selection of TP53 missense mutations in myeloid malignancies. Science 2019;365:599-604. 10.1126/science.aax3649 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as


