Abstract
A problem associated with the use of vaccinia virus recombinants as vaccines is the existence of a large human population with preexisting immunity to the vector. Here we showed that after a booster with attenuated recombinant modified vaccinia virus Ankara (rMVA), higher humoral and cellular immune responses to foreign antigens (human immunodeficiency virus type 1 Env and β-galactosidase) were found in mice preimmunized with rMVA than in mice primed with the virulent Western Reserve strain and boosted with rMVA. This enhancement correlated with higher levels of expression of foreign antigens after the booster.
Live-virus vector-based vaccines have been proposed as excellent candidates for the design of novel vaccines able to elicit long-term and protective humoral and cell-mediated immune (CMI) responses, similar to the case during natural infection with the corresponding pathogen. Certain properties of poxvirus-derived vectors make them suitable for the development of vaccines. Among properties of interest are the feasibility of expressing in the same vector diverse and nonrelated antigens, the potent and long-lasting immunity generated after immunization, and the well-established protection raised upon vaccination with recombinant vaccinia viruses (rVVs) in animal models and in field trials (15). Low-cost production, temperature stability, and easy administration are essential features when considering development of vaccines for use worldwide, and poxvirus-derived vectors meet those requirements (4). More recently, the beneficial immunological effects when rVVs were administered during the booster in bimodal immunization schemes have encouraged the use of poxvirus vectors for human use against a variety of pathogens and malignancies (22). However, safety concerns are present with the use of live-virus-based vaccines, and particularly with rVV, as nondesired side effects were demonstrated during the World Health Organization-directed smallpox eradication program. Thus, the development of strains of poxviruses that are highly safe for human use are required for clinical trials. Modified vaccinia virus Ankara (MVA) does not grow productively in cells of human origin, and in contrast to the case for avirulent avipoxviruses, its beneficial potential was evaluated in humans and showed no undesired effects, even in immunocompromised individuals (14). Moreover, the levels of expression of recombinant products from MVA in cultured cells are higher than those from a wild-type strain, and vaccinated animals developed protective immune responses to pathogens (16).
We have recently characterized in the mouse model the main immunological features following infection with MVA by a systemic route (19). We found that in comparison with a virulent laboratory strain (Western Reserve [WR]), the immune response elicited against the viral antigens was highly reduced, as neutralization antibodies were not significantly raised against MVA unless high viral doses were employed. In addition, the specific cellular immune response against MVA antigens was nearly five times lower than that to WR, irrespective of the viral dose utilized. Significantly, the immune response elicited against a foreign MVA-encoded antigen was at least as high, or even higher at certain viral doses, than that with the virulent WR strain (19). These data are of interest since in most systems during repeated immunizations, a strong immune response against viral vector proteins is associated with a lower effective immune response against vector-expressed foreign antigens. In the case of VV this is critical, as a large human population was vaccinated against smallpox and still maintains a perdurable immunity against VV (2, 7). Moreover, if recombinant poxvirus vectors are applied to humans, preimmunized persons could not be recipients of further vaccines based on the same vectors. This is an important issue considering that several fatal diseases are endemic in third world and developing countries, and for safety reasons, the immune status of the potential vaccinees should thus be taken into account in the design of live-virus vector-based vaccines.
The effects of preexisting immunity against VV upon secondary inoculations with either the same vector encoding different exogenous antigens or an rVV with distinct virulence have long been studied in the context of the humoral immune response (6, 9, 18). Briefly, it was demonstrated that immunity to virulent VV strains influences negatively both the titer and duration of the antibody response induced by a second distinct rVV (12) and that prior immunization with an rVV reduces the protective immunity against the pathogen achieved when the humoral immune response was raised after a single or multiple inoculations of the rVV expressing the desired antigen (21). However, a similar detailed analysis focusing on the implications of the immunity elicited against the vector antigens after priming, i.e., on the specific CMI response induced against a new foreign antigen delivered by the vector following a secondary immunization, has not been carried out. Our previous MVA studies prompted us to analyze the implications of the fact that the humoral and cellular immune responses elicited after priming against MVA were low, while the CMI response against a foreign product expressed from rMVA was similar to or even higher than that triggered by rWR (19). Here, we compared the immune responses elicited when either the virulent WR strain or MVA was employed in the first immunization and the booster was given with rMVA.
The extent of preexisting immunity to VV antigens determines the specific immune response against foreign antigens expressed upon secondary immunization with rMVA.
The rVVs employed have the inserted foreign genes in the thymidine kinase locus and have been previously described (11, 20), except for MVAenv. They all express β-galactosidase (β-Gal) under control of the p11 late promoter. WRluc and MVAluc express luciferase under control of the p7.5 early-late promoter, WRenv expresses human immunodeficiency virus type 1 (HIV-1) IIIB gp160 under control of the p7.5 early-late promoter, as does MVAenv, for which the levels of expression in baby hamster kidney (BHK21) cells, as evaluated by Western blotting, were comparable to those obtained with WRenv (data not shown). MVA derivatives were grown in BHK21 cells and plaque purified six times. WR derivatives were grown in human HeLa cells. Sucrose cushion-purified viral stocks of rMVA and rWR were titrated in BHK21 or African green monkey kidney BSC-40 cell monolayers by immunostaining of fixed infected cultures with polyclonal serum reactive against VV proteins (19) or by plaque assays, respectively.
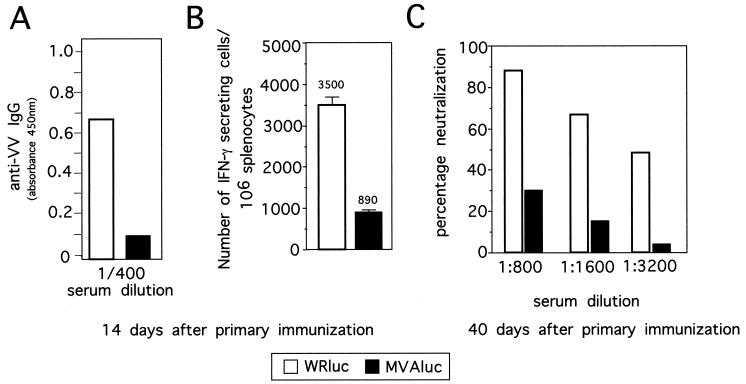
Groups of four 6 -to -8 week-old BALB/c mice were primed by intraperitoneal (i.p.) immunization with 107 PFU of rWR or rMVA expressing the β-Gal gene and the luciferase gene (WRluc and MVAluc, respectively). We first compared the humoral and cellular immune responses after priming of mice with rMVA or rWR. Inoculated animals were bled from the retro-orbital plexus, and immunoglobulin G (IgG) antibodies to VV antigens at 14 days postinoculation were determined by enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well flat-bottom plates were coated with 100 μl of extracted proteins from WR virions (1 μg/ml) (10) in carbonate buffer at 4°C overnight. Antibodies were screened by plating serial dilutions of sera, incubated (37°C, 1 h), and washed with phosphate-buffered saline (PBS) plus 0.05% Tween 20 (PBS-T). After incubation (37°C, 1 h) with peroxidase-conjugated goat anti-mouse IgG specific antibodies (Southern Biotechnology, Birmingham, Ala.), the color was developed with tetramethylbenzidine reagent (Sigma, St. Louis, Mo.) for 5 to 10 min, and after the reaction was stopped with 2 N SO4H2, absorbance was measured at 450 nm on a Multiskan Plus plate reader (Labsystems, Chicago, Ill.). The levels of antibodies to VV antigens are shown in Fig. 1A. As expected (19), at 14 days after the first immunization, MVAluc-inoculated mice had an IgG response against VV that was sixfold lower than that of mice given WRluc. Comparable results were obtained when ELISA plates were covered with proteins extracted from purified MVA virions and reacted with sera from the same group of animals (data not shown).
FIG. 1.
Humoral and CMI responses elicited against VV antigens in mice primed with rMVA or rWR. Mice (four per group) were i.p. inoculated with 107 PFU of MVAluc or WRluc and at 14 days postinoculation pooled sera were tested for specific IgG antibodies against VV by ELISA. (A) Averages of triplicate measurements of absorbance at 450 nm of a 1/400 dilution of sera. (B) The number of IFN-γ-secreting CD8+ T cells specific for VV antigens in spleens was determined by ELISPOT assay; bars represent averages for three pooled samples (± standard deviations) from triplicate cultures. (C) In an independent experiment, groups of four mice were inoculated as described above, and neutralizing antibodies 40 days after the virus inoculation were determined by measuring the inhibition of virus plaque formation on BSC-40 cells using the wild-type WR strain. The percentage of neutralization was calculated as (number of PFU with immune serum/number of PFU with naive serum) × 100. Averages of two independent measurements are shown.
The specific anti-VV CMI response elicited 14 days after the first inoculation was measured by an enzyme-linked immunospot (ELISPOT) assay (Fig. 1B), allowing quantification of the number of VV-specific CD8+ gamma interferon (IFN-γ)-secreting T cells (10). Briefly, 96-well nitrocellulose plates (Millipore Corporation, Bedford, Mass.) were coated with 8 μg of anti-mouse IFN-γ monoclonal antibody R4-6A2 (PharMingen, San Diego, Calif.) per ml in 75 μl of PBS. After overnight incubation at room temperature, wells were washed with RPMI complete medium and blocked with RPMI plus 10% fetal calf serum during 1 h at 37°C. Afterwards, triplicates of erythrocyte-depleted spleen cells were plated in twofold dilutions starting with 5 × 105 cells/well. P815 cells (a mastocytoma cell line that expresses only major histocompatibility complex class I molecules) were used as antigen-presenting cells (105 cells/well), after infection with 5 PFU of WRluc per cell and treatment with mitomycin C (30 μg/ml) (Sigma) at 4.5 h postinfection. The control was mock-infected P815 cells. Plates were incubated in a humidified atmosphere (37°C, 24 h), washed extensively with PBS-T, and incubated (room temperature, 2 h) with a solution in PBS-T of biotinylated anti-mouse IFN-γ monoclonal antibody XMG1.2 (2 μg/ml) (PharMingen). The plates were washed with PBS-T, and 100 μl of peroxidase-labeled avidin (1/800 dilution in PBS-T) (Sigma) was added to each well and incubated at room temperature for 1 h. After washing, spots were developed by adding the substrate 3,3′-diaminobenzidine tetrahydrochloride (1 μg/ml) (Sigma) in 50 mM Tris-HCl (pH 7.5) containing 0.015% hydrogen peroxide and counted using an MZ122 APO and Imaging System QWIN software (Leica, Cambridge, United Kingdom). As depicted in Fig. 1B, 14 days after primary inoculation, the number of anti-VV CD8+ IFN-γ-secreting T cells was approximately four times lower in mice inoculated with MVAluc than in mice inoculated with WRluc. The results in Fig. 1A and B show that the humoral as well as the cellular immune response induced against VV antigens was quantitatively different in animals primed with rMVA versus rWR.
Neutralizing antibody titers in heat-inactivated (56°C, 30 min) sera from immunized and control mice were evaluated. Serial dilutions of sera in PBS plus 2% fetal calf serum were incubated with 200 PFU of wild-type WR (37°C, 1 h); confluent BSC-40 cell monolayers were infected in triplicate, and plaques were visualized at 48 h postinfection by crystal violet staining and counted. As a negative control, sera from mock-infected mice were used. The number of plaques obtained with each serum was referred to the control value and used to calculate the neutralization titers (Fig. 1C). Clearly, the neutralization titers were considerably lower in sera from mice primed with MVA than in those from mice primed with WR.
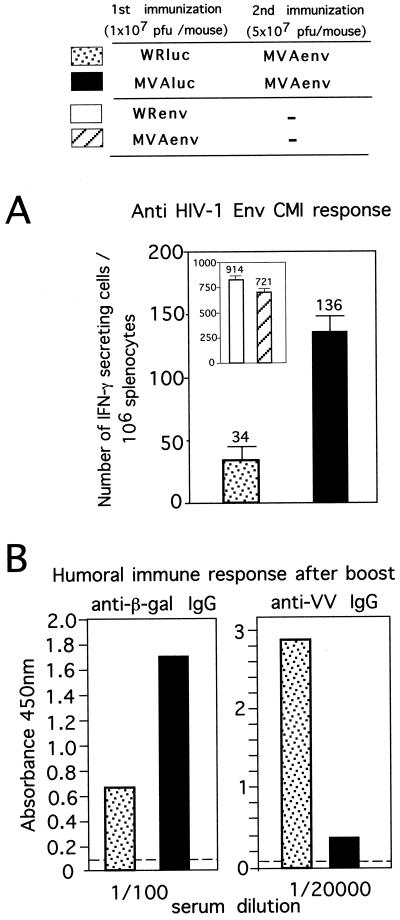
Next, we compared the immune responses with a prime-boost combination of vectors. Mice primed as for Fig. 1 were boosted 40 days later with 5 × 107 PFU of MVAenv expressing the HIV-1 Env gene of clade B (IIIB). Ten days after the second immunization with MVAenv, the specific cellular immune response against an epitope on the V3 loop was evaluated by ELISPOT assay as described for Fig. 1B, except that P815 cells were pulsed with a 10−6 M concentration of the 10-amino-acid specific peptide RGPGRAFVTI (24). Control mice received only one inoculation with either MVAenv or the similar WR-based virus (WRenv). A single immunization with MVAenv or WRenv induces a specific anti-Env CD8+-T-cell response of similar magnitudes for both vectors (Fig. 2A, insert). When mice were preimmunized with either MVAluc or WRluc, the total amount of anti-Env CD8+ IFN-γ-secreting T cells diminished after the booster (Fig. 2A). However, nearly fourfold more specific CD8+ T cells were present after immunization with MVAenv in mice preimmunized with MVA compared to mice preimmunized with WR. Thus, it appears that the lower immune response raised against the MVA antigens compared to WR antigens after a primary inoculation allowed a more potent response to an MVA-delivered antigen to which mice were naive (the HIV-1 Env). The low titer of anti-gp160 antibodies generated limited our ability to measure the antibody response in primed mice to a newly presented antigen delivered by immunization with a second recombinant virus (data not shown). The limitation of preexisting immunity to VV antigens in mounting of a humoral immune response to a foreign antigen delivered from a second recombinant VV vector has been extensively studied by other authors (12, 20).
FIG. 2.
Effect of preimmunization with either MVAluc or WRluc on cell-mediated and humoral immune responses elicited against foreign antigens after booster with MVAenv. (A) Number of IFN-γ-secreting CD8+ T cells specific for the HIV-1 Env product raised in mice preimmunized with either WRluc (stippled bar) or MVAluc (filled bar) and boosted with MVAenv. BALB/C mice were i.p. inoculated with the corresponding virus at priming and boosted 40 days later with MVAenv. After 10 days, spleen cells from mice were used as responder cells in the ELISPOT assay. The inset shows data from mice inoculated once with either WRenv (open bar) or MVAenv (hatched bar). Bars represent averages for triplicate cultures (± standard deviations) from pooled samples of four mice. (B) Humoral immune response against the β-Gal product (left panel) and against VV envelope antigens (right panel) 10 days postboost. Levels of antibodies were measured by ELISA from pooled serum samples for the same animals as in panel A. Bars represent mean absorbance values at 450 nm for triplicates at the indicated dilutions; the dashed lines indicate background levels obtained with naive serum.
The ability to boost an antibody response against the β-Gal antigen (a protein that must be expressed after viral infection to be exposed to the immune system) delivered by rMVA and rWR was also analyzed. The levels of IgG antibodies against the β-Gal antigen were evaluated by ELISA as described above, except that plates were coated with 5 μg of a solution of commercial β-Gal (Sigma) per ml. Anti-β-Gal antibodies in pooled serum were measured 14 days after priming with either WRluc or MVAluc and also 14 days after the booster with MVAenv. While after priming, low antibody titers to β-Gal are produced (data not shown) (19), after the booster, specific IgG levels were nearly three times higher in animals preimmunized with MVAluc than in those animals preimmunized with WRluc (Fig. 2B, left panel). As expected, the anamnestic response against VV envelope antigens (which are exposed on the virus particles and do not need to be expressed to affect the immune system) was different. While anti-WR antibody titers were high after a booster with MVAenv, these levels were significantly lower in mice preimmunized with MVAluc and boosted with MVAenv (Fig. 2B, right panel), in correlation with the primary anti-VV antibody response observed in WR-primed mice with respect to those preimmunized with MVAluc (Fig. 1A).
The outcome of the immune response in animals primed with VV is determined by the levels of expression of the recombinant antigen during the booster.
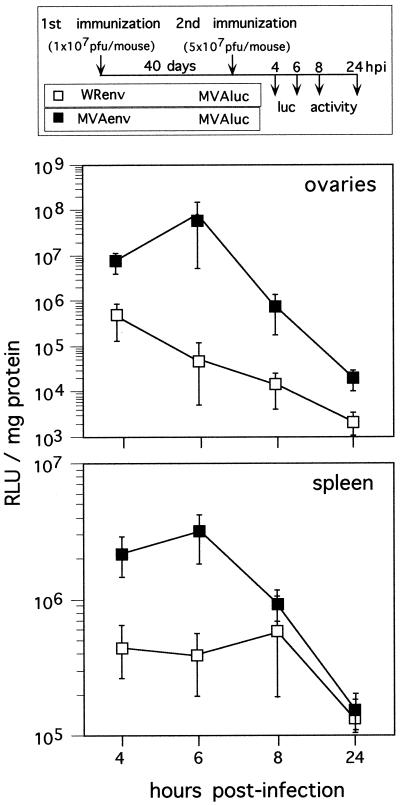
We next investigated whether differences in the anti-Env and anti-β-Gal immune responses in mice primed with WRluc or MVAluc and boosted with MVAenv could correlate with a different expression of the antigen product upon secondary MVA inoculation. To circumvent difficulties associated with the quantitation of expressed Env protein in animals, we primed mice with 1 × 107 PFU of WRenv or MVAenv and then carried out a secondary inoculation with 5 × 107 PFU of MVAluc (Fig. 3). Since MVAluc expresses the luciferase gene under control of the same early-late promoter as the rVV expressing the HIV-1 Env gene, luciferase levels at short times postinoculation indicate the extent of virus gene expression in target tissues. Luciferase activity in spleens and ovaries of infected mice was measured at various times postinoculation from cleared tissue homogenates in extraction buffer (300 μl/spleen and 100 μl/ovary) (Promega Corp., Madison, Wis.) in the presence of luciferin and ATP using a Lumat LB 9501 Berthold luminometer (Berthold, Nashua, N.H.) and was expressed as relative luciferase units per milligram of protein. Protein content in tissue extracts was measured with the bicinchoninic acid protein assay reagent kit (Pierce Co., Rockford, Ill.). As shown in Fig. 3, after the booster with MVAluc there is a clear difference in levels of luciferase expression between WRenv-primed mice (with a strong anti-VV immune response) and animals primed with MVAenv (with a low anti-VV immune response). At 6 h postbooster, when MVA reaches maximum values of gene expression in vivo (19), the differences between groups are 2.2 log units in ovaries and about 1 log unit in spleen. These findings highlight the differences in gene expression when animals are primed with WR or MVA and then boosted with rMVA, which are related to the virulence of the virus strain used for priming.
FIG. 3.
Gene expression from rMVA inoculated at booster in mice primed with rMVA or rWR virus. Mice were immunized with either WRenv (open squares) or MVAenv (closed squares) and boosted with an rMVA expressing the luciferase gene (MVAluc) according to the scheme shown in the upper part of the figure. At each time point postbooster, four mice were sacrificed and luciferase activity present in the ovaries and spleens was measured. Results represent mean relative luciferase units (RLU) (± standard deviations) per milligram of protein measured in samples from four animals. hpi, hours postinfection.
Early studies with rVV demonstrated the efficacy of poxvirus-derived live-virus-based vaccines in the development of protective immune responses in animal models (5, 17, 18). The problem of preexisting immunity to VV in future vaccine applications with the homologous virus (2, 3) has been addressed by examining the efficacy of single or double immunization with rVV expressing a foreign antigen (9), the importance of antibodies raised against VV to prevent a boosting effect of further immunizations with a different rVVs (12), and the effect of VV virulence on immunogenicity of single and double immunization with rVV expressing different antigens (13). To circumvent this problem, the use of different combinations of vectors and/or routes of immunization has been implemented (1, 8).
In the present study, using the mouse model, we have defined the immune responses elicited after booster in animals with preexisting immunity against the vector, MVA or WR. The immune response induced against a recombinant antigen expressed after a booster with MVA was then compared with that in animals primed with the virulent strain WR. Our findings showed that in an rMVA prime-rMVA boost combination, there is a stronger CMI response against an antigen expressed only in the second immunization (HIV-1 Env) and a higher anamnestic humoral response to an antigen expressed in both inoculations (β-Gal) than in an rWR prime-rMVA boost combination. Although here we have not characterized the boosting capacity of MVA against HIV-1 Env, it has been demonstrated (23) that anti-simian immunodeficiency virus Gag/Pol cytotoxic-T-lymphocyte responses can be boosted with the same MVA recombinant upon intramuscular injection of rhesus monkeys. By contrast, Belyakov et al. (1) showed in mice that there was no increase in the CMI elicited upon repeated subcutaneous injections with MVA expressing the HIV-89.6 Env protein (rMVA89.6) in comparison with a single vector injection; moreover, in mice preimmunized with WR-based recombinant virus, there was no response to HIV-89.6 Env upon booster with rMVA89.6 (1). In this investigation we showed that although prior immunity to the MVA vector causes a significant inhibitory effect on the CMI response against the HIV-1 Env, this immune response is measurable and is nearly fourfold higher than that observed in mice preimmunized with the WR vector (Fig. 2). Differences due to the inoculation routes (i.p. versus subcutaneous) and viral doses used in the two studies might explain these discrepancies, as the virus input has a relevant effect on the extent of the specific humoral and CMI responses against MVA antigens (19). Moreover, Belyakov et al. evaluated the specific cytotoxic-T-lymphocyte response after a longer period of time following the booster than we did in our study, and also they used an assay less sensitive than the ELISPOT assay.
The stronger response to foreign antigens elicited by MVA after a booster in mice primed with MVA versus mice primed with WR is, in part, due to levels of expression of the antigen. Indeed, higher levels of luciferase are produced in mice with preexisting immunity against MVA antigens than in those with immunity against WR (Fig. 3). This is probably because virus-neutralizing antibodies are lower in MVA-primed mice than in WR-primed mice (Fig. 1C). We found that those differences were linked to the ability of MVA administered in the second dose to infect target cells, which has also implications for the anamnestic antibody responses against an antigen expressed from rVV. Indeed, those reasons were pointed out by several authors (6, 9, 21) but were not demonstrated. The preexisting immunity to VV proteins, revealed by both neutralizing antibody titers and anti-VV CD8+ T cells present in mice at the time of the boost (Fig. 1), will limit the virus input that can reach and infect target tissues during booster and, hence, decrease the expression of virus-encoded genes.
Our findings establish benefits, besides safety concerns, of the use of MVA as a live-virus-based vaccine, due to the ability to elicit specific secondary immune responses upon repeated inoculations with MVA. This in conjunction with the proven efficient boosting capacity of MVA in bimodal immunization protocols (22) makes MVA a strain with potential use as a VV-based vaccine vector. Obviously, the intrinsic properties of MVA as an immunogen can be improved further by varying the time in the immunization scheme, increasing the strength of the VV promoter used to express the antigens, or coexpressing immunomodulatory molecules.
Acknowledgments
Juan C. Ramírez and M. Magdalena Gherardi contributed equally to this work.
The excellent technical assistance of M. Victoria Jiménez is acknowledged.
This work was supported by grants 08.6/0020/97 from Comunidad Autónoma de Madrid (CAM), SAF98-0056 from Comision Interministerial de Ciencia y Tecnología (CICYT), Spain, and BIO4-CT98-0456 from the European Union. J.C.R. is a recipient of postdoctoral fellowship from the CAM, Spain. M.M.G. is a researcher from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina.
REFERENCES
- 1.Belyakov I M, Moss B, Strober W, Berzofsky J A. Mucosal vaccination overcomes the barrier to recombinant vaccinia immunization caused by preexisting poxvirus immunity. Proc Natl Acad Sci USA. 1999;96:4512–4517. doi: 10.1073/pnas.96.8.4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooney E L, Collier A C, Greenberg P D, Coombs R W, Zarling J, Arditti D E, Hoffman M C, Hu S L, Corey L. Safety of and immunological response to a recombinant vaccinia virus vaccine expresing HIV envelope glycoprotein. Lancet. 1991;337:567–572. doi: 10.1016/0140-6736(91)91636-9. [DOI] [PubMed] [Google Scholar]
- 3.Cooney E L, McElrath M J, Corey L, Hu S, Collier A C, Arditti D, Hoffman M, Coombs R W, Smith G E, Greenberg P D. Enhanced immunity to HIV envelope elicited by a combined vaccine regimen consisting of priming with a vaccinia recombinant expressing HIV envelope and boosting with gp160 protein. Proc Natl Acad Sci USA. 1993;90:1882–1886. doi: 10.1073/pnas.90.5.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox W I, Tartaglia J, Paoletti E. Poxvirus recombinants as live vaccines. In: Binns M M, Smith G L, editors. Recombinant poxviruses. Boca Raton, Fla: CRC Press; 1992. pp. 123–162. [Google Scholar]
- 5.Cremer K, Mackett M, Wohlenberg C, Notkins A L, Moss B. Vaccinia virus recombinants expressing herpes simplex type 1 glycoprotein D prevents latent herpes in mice. Science. 1985;228:737–740. doi: 10.1126/science.2986288. [DOI] [PubMed] [Google Scholar]
- 6.Dallo S, Maa J-S, Rodriguez J R, Rodriguez D, Esteban M. Humoral immune response elicited by highly attenuated variants of vaccinia virus and by attenuated recombinant expressing HIV-1 envelope protein. Virology. 1989;173:323–329. doi: 10.1016/0042-6822(89)90250-x. [DOI] [PubMed] [Google Scholar]
- 7.Erickson A L, Walker C M. Class I major histocompatibility complex-restricted cytotoxic T cell responses to vaccinia virus in humans. J Gen Virol. 1993;74:751–754. doi: 10.1099/0022-1317-74-4-751. [DOI] [PubMed] [Google Scholar]
- 8.Etlinger H, Altenburger W. Overcoming inhibition of antibody responses to a malaria recombinant vaccinia virus caused by prior exposure to wild type virus. Vaccine. 1991;9:470–472. doi: 10.1016/0264-410x(91)90030-a. [DOI] [PubMed] [Google Scholar]
- 9.Flexner C, Murphy B R, Rooney J F, Wohlenberg C, Yuferov V, Notkins A L, Moss B. Succesful vaccination with a polyvalent live vector despite existing immunity to an expressed antigen. Nature. 1988;335:259–262. doi: 10.1038/335259a0. [DOI] [PubMed] [Google Scholar]
- 10.Gherardi M M, Esteban M. Mucosal and systemic immune responses induced after oral delivery of vaccinia virus recombinants. Vaccine. 1999;17:1074–1083. doi: 10.1016/s0264-410x(98)00324-7. [DOI] [PubMed] [Google Scholar]
- 11.Gherardi M M, Ramirez J C, Rodriguez D, Rodriguez J R, Sano G, Zavala F, Esteban M. IL-12 delivery from recombinant vaccinia virus attenuates the vector and enhances the cellular immune response against HIV-1 env in a dose-dependent manner. J Immunol. 1999;162:6724–6733. [PubMed] [Google Scholar]
- 12.Kündig T M, Kalberer C P, Hengartner H, Zinkernagel R M. Vaccination with two different vaccinia recombinants viruses: long-term inhibition of secondary vaccination. Vaccine. 1993;11:1154–1158. doi: 10.1016/0264-410x(93)90079-d. [DOI] [PubMed] [Google Scholar]
- 13.Kutinovä L, Ludvíkova V, Maresová L, Broucek J, Hainz P, Vonka V. Effect of virulence on immunogenicity of single and double vaccinia virus recombinants expressing differently immunogenic antigens: antibody-response inhibition induced by immunization with a mixture of recombinants differing in virulence. J Gen Virol. 1999;80:2901–2908. doi: 10.1099/0022-1317-80-11-2901. [DOI] [PubMed] [Google Scholar]
- 14.Mayr A, Stickl H, Muller H K, Denner K, Singer H. The smallpox vaccination strain MVA: marker, genetic structure, experience gained with the parenteral vaccination and behaviour in organisms with a debilitated defence mechanism. Zentbl Backteriol Hyg B. 1978;167:375–390. [PubMed] [Google Scholar]
- 15.Moss B. Genetically engineered poxviruses for recombinant gene expression, vaccination and safety. Proc Natl Acad Sci USA. 1996;93:11341–11348. doi: 10.1073/pnas.93.21.11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moss B, Carroll M W, Wyatt L S, Bennink J R, Hirsch V, Golstein S, Elkins W R, Fuerst T R, Lifson J D, Piatak M, Restifo N P, Overwijk W, Chamberlain R, Rosenberg S A, Sutter G. Host range restricted non-replicating vaccinia virus vectors as vaccine candidates. Adv Exp Med Biol. 1996;397:7–13. doi: 10.1007/978-1-4899-1382-1_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paoletti E, Lipinskas B R, Samsonoff C, Mercer S, Panicalli D. Construction of live vaccines using genetically engineered poxviruses: biological activity of vaccinia virus recombinants expressing the hepatitis B virus surface antigen and the herpes simplex virus glycoprotein D. Proc Natl Acad Sci USA. 1984;81:193–197. doi: 10.1073/pnas.81.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perkus M E, Piccini A, Lipinskas B R, Paoletti E. Recombinant vaccinia virus: immunization against multiple pathogens. Science. 1985;229:981–984. doi: 10.1126/science.2992092. [DOI] [PubMed] [Google Scholar]
- 19.Ramírez J C, Gherardi M M, Esteban M. The biology of attenuated modified vaccinia virus Ankara recombinant vector in mice: fate and activation of B- and T-cell immune responses in comparison with the Western Reserve strain and advantages as a vaccine. J Virol. 2000;74:923–933. doi: 10.1128/jvi.74.2.923-933.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodríguez J F, Rodríguez D, Rodríguez J R, McGowan E B, Esteban M. Expression of the firefly luciferase gene in vaccinia virus: a highly sensitive gene marker to follow virus dissemination in tissues of infected animals. Proc Natl Acad Sci USA. 1988;85:1667–1671. doi: 10.1073/pnas.85.5.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rooney J F, Wohlenberg C, Cremer K J, Moss B, Notkins A L. Immunization with a vaccinia virus recombinant expressing herpes simplex virustype 1 glycoprotein D: long-term protection and effect of revaccination. J Virol. 1988;62:1530–1534. doi: 10.1128/jvi.62.5.1530-1534.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider J, Gilbert S C, Hannan C M, Degano P, prieur E, Sheu E G, Plebanski M, Hill A V S. Induction of CD8+ T cells using heterologous prime-boost immunisation strategies. Immunol Rev. 1999;170:29–38. doi: 10.1111/j.1600-065x.1999.tb01326.x. [DOI] [PubMed] [Google Scholar]
- 23.Seth A, Ourmanov I, Kuroda M J, Schmitz J E, Carroll M W, Wyatt L S, Moss B, Forman M A, Hirsch V M, Letvin N L. Recombinant modified vaccinia virus Ankara-simian immunodeficiency virus gag pol elicits cytotoxic T lymphocytes in rhesus monkeys detected by a major histocompatibility complex class I/peptide tetramer. Proc Natl Acad Sci USA. 1998;95:10112–10116. doi: 10.1073/pnas.95.17.10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeshita T, Takahashi H, Kozlowski S, Ahlers J D, Pendleton c D, Moore R L, Nakagawa Y, Yokomuro K, Fox B S, Margulies D H, Berzofsky J A. Molecular analysis of the same HIV peptide functionally binding to both a class I and a class II MHC molecule. J Immunol. 1995;154:1973–1986. [PubMed] [Google Scholar]