Abstract
The first chromosome-scale reference genome of the rare narrow-endemic African moss Physcomitrellopsis africana (P. africana) is presented here. Assembled from 73 × Oxford Nanopore Technologies (ONT) long reads and 163 × Beijing Genomics Institute (BGI)-seq short reads, the 414 Mb reference comprises 26 chromosomes and 22,925 protein-coding genes [Benchmarking Universal Single-Copy Ortholog (BUSCO) scores: C:94.8% (D:13.9%)]. This genome holds 2 genes that withstood rigorous filtration of microbial contaminants, have no homolog in other land plants, and are thus interpreted as resulting from 2 unique horizontal gene transfers (HGTs) from microbes. Further, P. africana shares 176 of the 273 published HGT candidates identified in Physcomitrium patens (P. patens), but lacks 98 of these, highlighting that perhaps as many as 91 genes were acquired in P. patens in the last 40 million years following its divergence from its common ancestor with P. africana. These observations suggest rather continuous gene gains via HGT followed by potential losses during the diversification of the Funariaceae. Our findings showcase both dynamic flux in plant HGTs over evolutionarily “short” timescales, alongside enduring impacts of successful integrations, like those still functionally maintained in extant P. africana. Furthermore, this study describes the informatic processes employed to distinguish contaminants from candidate HGT events.
Keywords: Funariaceae, reference genome, Physcomitrium, bryophyta, HGT, contamination
Introduction
Horizontal gene transfer (HGT), also referred to as lateral gene transfer (Zhaxybayeva and Doolittle 2011), is the lateral movement of genetic material between distant branches of the tree of life. This process is ubiquitous among bacteria, facilitating rapid adaptation through exchange of ecologically important genes (Aminov 2011). While HGT is less common in eukaryotes than in prokaryotes, it plays a role in shaping eukaryotic evolution with about 0.04–6.49% of eukaryotic genes originating from HGT from microbes (Van Etten and Bhattacharya 2020). During the evolution of land plants, increasing interactions with rhizosphere microbes, particularly bacteria and mycorrhizal fungi, have enabled occasional horizontal transfer of functional genes between these distantly related lineages (Martin et al. 2017). For example, the gene for Killer Protein 4 (KP4) was likely acquired by mosses through HGT from ascomycete fungi (Guan et al. 2023). Similarly, the domain of the heterokaryon incompatibility proteins (HET), common in fungal heterokaryon incompatibility genes and involved in self/non-self-recognition, was identified as a truncated form in P. patens and may play a role in defenses against microbial pathogens (Sun et al. 2020). Van Etten and Bhattacharya (2020) suggested that rather than being merely anecdotal, HGT has been an important evolutionary force driving the adaptations of land plants to new habitats and stressors throughout their evolution, as previously already hypothesized by Yue et al. (2012).
Accurately detecting the taxonomic origin of genes and confirming HGT events is challenging. Many reported cases of HGT in published genomes have been proven to be artifacts resulting from contamination that had gone undetected. For example, the initial report that 17% of the genes in the genome of the tardigrade Hypsibius dujardini originated from HGT (Boothby et al. 2015) has subsequently been revised to only 0.2% (Koutsovoulos et al. 2016). Similarly, claims of HGT in mammals also turned out to be erroneous due to bacterial contamination (Douvlataniotis et al. 2020), highlighting the need for careful analyses and experimental validation. Systematic screening of 43 arthropod genomes by Francois et al. (2020) revealed extensive bacterial contaminants, often outnumbering true HGT events. For example, in the bumblebee Bombus impatiens, most contaminating genes were concentrated on just 30 scaffolds. Based on the size and number of contaminant sequences, it was concluded that the genome of the symbiont Candidatus Schmidhempelia bombi was co-assembled with that of its host. Strategies to confidently identify HGT, including taxonomic assignment of candidates via BLASTp/DIAMOND, phylogenetic analysis with candidate and donor proteins, synteny analysis of flanking genes, and quantitative PCR validation have been presented to address these issues (Francois et al. 2020).
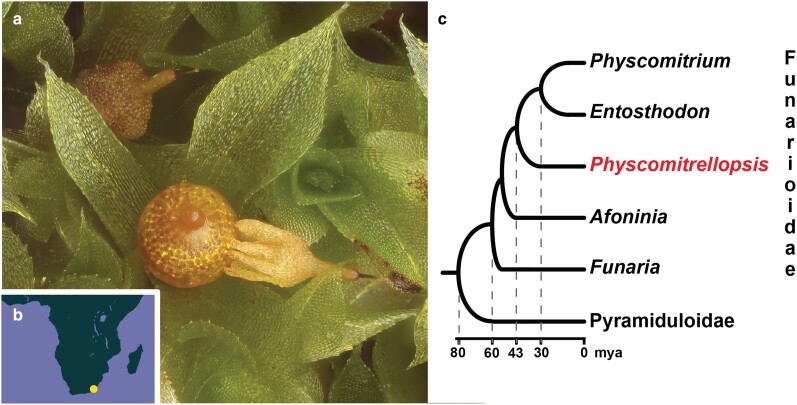
Physcomitrellopsis africana (P. africana) inhabits transitional zones between grassland and forests in coastal habitats in South Africa. It is currently the sole species of the genus, which likely should also include several species of the paraphyletic genus Entosthodon (Wilding 2015). Its genome of 414 Mb, the first genome of an African bryophyte, is presented here. This resource complements those available for Funaria hygrometrica and P. patens (Rensing et al. 2008; Kirbis et al. 2022) and thereby constitutes a fundamental resource to further the reconstruction of the evolution of the genome of the model P. patens (Rensing et al. 2020). Through rigorous contamination screening and verification, 2 genes emerge as candidates resulting from unique HGT events. Informatically validating HGT events in P. africana yields evolutionary insights while underscoring the need for stringent standards to support HGT conclusions from genomics data.
Materials and methods
Sample collection and culturing
A population of P. africana was sampled in October 2010 from a coastal forest in the Eastern Cape Province of South Africa (Dwesa National Park, along trail to chalets behind campground, coordinates 32 °18.222′ S 28 °49.666′, at ± sea level). The voucher specimens collected by Goffinet (collection numbers 10,326 and 10,329, with TAH and NW) are deposited in the George Safford Torrey Herbarium (CONN) under accession numbers CONN00235389 and CONN00235388, respectively. Specimen 10,326 (culture and long read library DNA #5074) provided DNA for long-read sequencing, whereas 10,329 [RNA and Beijing Genomics Institute (BGI)-Seq DNA #5075] provided RNA and DNA for short-read sequencing. Sterile cultures were first established on Knop medium using spores from a single operculate capsule. The gametophytes were harvested, ground, and spread on a rich sandy loam soil in PlantCon tissue culture containers (MP Biomedicals, Solon, OH, USA), and maintained in a growth chamber under 16 h of daylight at about 24°C.
Genomic DNA and RNA extraction
Gametophytic tissue consisting of stems and leaves of P. africana was harvested from fresh soil cultures under a dissecting microscope and ground in liquid nitrogen. DNA was extracted by following a modified protocol by Young (2022). The quality of the DNA sample 5,074 was assessed by quantitative PCR prior to sequencing, yielding a DNA integrity number (DIN) score of 7.0 and concentration of 40.9 ng/μL.
DNA for short-read sequencing was extracted using the NucleoSpin Plant midi DNA extraction kit, following the manufacturer's protocol (Macherey-Nagel, Düren, Germany). DNA quality was evaluated using a Qubit 3.0 Fluorometer (Thermo Fisher Scientific, USA). Total RNA was extracted from approximately 1 g of fresh gametophytic tissue using the RNeasy Plant Mini Kit (Qiagen, Valencia, CA, USA).
Genome and transcriptome library preparation and sequencing
DNA was prepared for long-read PromethION sequencing through a DNA repair step, generating blunt ends, and ligating sequencing adapters followed by priming of the flow cell, as described in the Oxford Nanopore Technologies Amplicons by Ligation (SQK-LSK109) protocol. The HMW DNA library was sequenced on an Oxford Nanopore PromethION (Center for Genome Innovation, UConn) using a FLO-PRO0002 flow cell. The 2 short-read DNA libraries were prepared following the methods used by Yu et al (2020), and were sequenced [150 bp Paired-end (PE)] on 2 lanes of a BGISEQ-500 (BGI-Shenzhen, China).
Approximately 1 μg of RNA was used to generate a paired-end library with an insert fragment size of 200–300 bp of the corresponding cDNA. RNA purification, reverse transcription, library construction, and sequencing were performed at WuXi NextCode (Shanghai, China). The captured coding regions of the transcriptome from total RNA were prepared using the TruSeq RNA Exome Library Preparation Kit. The 2 RNA libraries were sequenced on one lane of an Illumina HiSeq 2,000 (100 bp PE) at the WuXi NextCode (Shanghai, China).
Quality control of genomic and transcriptomic reads
The genomic short reads were first assessed with FASTQC v.0.11.7 (Andrews 2010). In preparation for assembly with Haslr and Wengan, Sickle v.1.33 with a minimum quality score threshold of 30 (-q) and a minimum length of 50 bp (-l) was employed for read trimming. Nanoplot v.1.21.0 was used to quantify and assess the quality of the nanopore reads. To detect potential contamination, the long reads were aligned against the preindexed bacterial, human, and viral databases with a metagenomic classifier, Centrifuge v.1.0.4-beta (p + h + v; min-hit length was increased to 50 bp) (Kim et al. 2016). Reads aligning to the database were removed. The quality of the transcriptomic short reads was assessed with FASTQC v.0.11.7 (Andrews 2010). The reads were trimmed with Sickle v1.33 (Joshi and Fass 2011) with a minimum quality score threshold of 30 (-q) and a minimum length of 40 bp (-l).
Genome size estimation
A single lane of short-read genomic data from accession 5,075 was employed to estimate the genome size. The k-mer distribution was calculated using Jellyfish v2.2.6 (Marçais and Kingsford 2011) and size estimates were processed with GenomeScope v2.0 (Ranallo-Benavidez et al. 2020; Supplementary Fig. 1).
Transcriptome assembly
The transcriptome was independently assembled to provide protein-level evidence for the structural annotation of the genome, using Trinity v2.6.6 (Grabherr et al. 2011), with a minimum contig length of 300 bp. Contigs with minimal read support, post assembly, were removed (fragments per kilobase of transcript per million mapped reads or FPKM > 0.5) with RSEM v1.3.0 (Li and Dewey 2011). Transdecoder v3.0.1 (Haas and Papanicolaou 2012) was used to translate the remaining contigs into open reading frames (ORFs) and remove sequences without a viable frame. To aid Transdecoder in the identification of ORFs, searches against the Pfam database were performed with HMMER v3.1b2 (Zhang and Wood 2003). The Transdecoder annotated the putative transcripts as complete, partial, and internal. Those without a defined start and stop codon (defined as internals) were removed (split_frames.py). The final set of peptide sequences were functionally annotated with EnTAP v0.8.0 (Hart et al. 2020) against NCBI's nr protein database and the UniProt/Swiss-prot reference databases. EnTAP was run with contaminant filters that included bacteria, archaea, fungi, and insecta. Transcripts with high confidence alignments to these organisms were removed (contam_removal.py).
Genome assembly
Hybrid genome assembly, integrating both long- and short-read data, was conducted with MaSuRCA v.4.0.3 (Zimin et al. 2013), Wengan v.0.2 (Di Genova et al. 2021), and Haslr v.0.8a1 (Haghshenas et al. 2020). Additionally, a separate assembly using only long reads as input was conducted with Flye v.2.5 (with three polishing iterations) (Kolmogorov et al. 2019).
The Flye, Wengan, and Haslr assemblies were polished following long-read alignment with Medaka v1.3.2 (github.com/nanoporetech/medaka). Post assembly with Wengan, the assembly was filtered to remove scaffolds less than 3 kb. To further improve the accuracy of the assemblies, the hybrid assembly generated by MaSuRCA was polished with the short reads using Pilon v.1.24 (Walker et al. 2014). Subsequently, the selected MaSuRCA assembly was processed with Purge Haplotigs v.1.0 (Roach et al. 2018).
The Purge Haplotigs pipeline categorized the assembled scaffolds into four coverage levels based on the distribution of mapped reads. This categorization enabled the identification and removal of redundant sequences exhibiting low coverage, presumed to represent erroneous duplicates given the haploid genome. Specific cutoff values of 0, 7, and 65 for the coverage levels were selected to delineate scaffolds to be retained or discarded, based on read depths (Supplementary Fig. 1). The term “allele” is used to describe these redundant sequences for convenience, although it is technically inaccurate for a haploid genome. The coverage analysis and purging allowed isolation of the primary genome sequence from duplications and artifacts generated during assembly.
To evaluate the quality of the assemblies, QUAST v5.2.0 (Gurevich et al. 2013) and Benchmarking Universal Single-Copy Ortholog (BUSCO) v4.1.2 (viridiplantae_odb10:425 single copy orthologues) (Manni et al. 2021) were employed. Each assembly was also evaluated with Merqury v1.3 (Rhie et al. 2020).
Genome annotation
Repeat library construction and masking
The repeat library for the final MaSuRCA assembly was generated using RepeatModeler v.2.01 (Flynn et al. 2020) with the long terminal repeat (LTR) discovery pipeline enabled. The genome was then soft-masked with RepeatMasker v.4.0.9-p2 using the consensus repeat library from RepeatModeler (Smit et al. 2013).
Structural and functional genome annotation
The RNA reads were aligned to the soft-masked MaSuRCA assembly with HISAT2 v.2.1.0 (Kim et al. 2019) to provide evidence for protein-coding gene prediction. Two gene prediction analyses were run on the soft-masked assembly using BRAKER v.2.1.5 (Brůna et al. 2021), one with RNA-Seq alignment evidence and 1 with protein evidence originating from de novo assembled transcriptome. Gene predictions from both BRAKER runs were integrated with TSEBRA v.1.0.3 (Gabriel et al. 2021). From this point, separate assessments were conducted on the RNA-Seq evidence gene predictions (BRAKER) and the final TSEBRA gene predictions to select the best approach. Putative genes were removed from both sets if they did not contain a complete protein domain. This filter was applied with Interproscan v.5.35–74.0 (Jones et al. 2014) using the Pfam database v32.0 (Finn et al. 2014). It is worth noting that mono-exonic genes can be the result of fragmented annotations and the target metric of 0.2 (mono:multi-exon gene ratio) is often achieved through protein domain filters (Vuruputoor et al. 2022). Metrics for the gene predictions were generated with Another Gtf/Gff Analysis Tool (AGAT; Dainat 2024) and BUSCO. After assessment, the filtered BRAKER gene predictions were selected for functional annotation with EnTAP v.0.10.8 (Hart et al. 2020). Functional annotation reports from EnTAP (both sequence similarity search and EggNog taxonomy scope classifications) allowed for the identification of nontarget species scaffolds in the assembly (Huerta-Cepas et al. 2019).
Assembly-level contaminant filtering
Using the functional annotation results from EnTAP, contaminated scaffolds were removed. Scaffolds with a length of 10 kb or less, and with 40% or more of their total genes classified as archaea, bacteria, or fungi, were removed. Additionally, scaffolds greater than or equal to 10 kb with 55% or more genes classified as archaea, bacteria, or fungi were also excluded. The final annotation was then assessed for the annotation rate using EnTAP, the mono:multi ratio using AGAT, and BUSCO completeness.
HGT candidate identification
To identify candidate HGTs in P. africana, protein sequence similarity searches were conducted with Diamond v2.1.8 (Buchfink et al. 2021). The protein sequences of P. africana were aligned against “donor” databases, which included sequences from bacteria, fungi, archaea, and metazoa from NCBI's nr database. Additionally, the same proteins were aligned to “recipient” databases containing sequences from Streptophyta, Tracheophyta, Embryophyta, Viridiplantae, and Spermatophyta (Ma et al. 2022). Although these categories are not fully exclusive, each database was utilized separately to systematically assess presence across plants at different evolutionary divergence points.
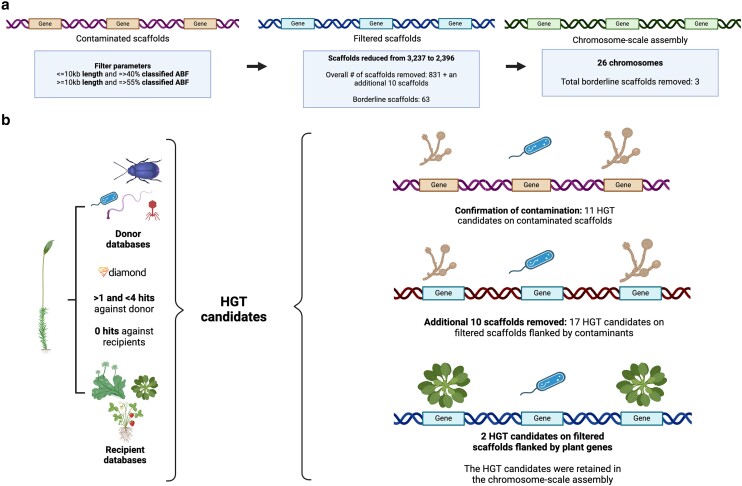
To identify candidate genes representing putative HGT events unique to P. africana, the following criteria were utilized: Genes were required to have between one and four significant sequence alignments (E-value <1e–5) to microbial donor databases, while exhibiting no significant sequence similarity to plant recipient databases. This range of one to four microbial alignments was selected to capture potential HGTs while avoiding ubiquitous domains shared across many microbes. The lack of hits to plant databases was intended to enrich for P. africana-specific sequences, rather than those conserved across plants through vertical inheritance. At this stage, any scaffolds containing only bacterial or fungal genes, without any plant-related genes, were removed from the assembly.
To assess the validity of the 2 proposed HGT candidates, the target proteins were independently aligned to the reference genome with Miniprot v0.7. To examine independent transcriptomic support, the RNA reads were aligned to the genome with HISAT2 v2.1.0 and assembled via StringTie2 v2.2.1 (Kovaka et al. 2019).
Analyzing HGT candidates from P. patens
The 273 putative HGTs previously identified in P. patens (Yue et al. 2012; Ma et al. 2022) were independently searched against the P. africana and F. hygrometrica protein sets using DIAMOND v2.1.8 (Buchfink et al. 2021). DIAMOND searches were conducted with an E-value cutoff of 1e-5 and max target sequences set to 1. Hits against P. africana and F. hygrometrica were collected and merged to generate a summary table with P. patens HGTs and the respective top hits in each species.
Scaffolding the genome to chromosome scale
A single HiC library was prepared for P. africana using Proximo Hi-C kit (Phase Genomics) and sequenced as paired-end reads on an Illumina NovaSeq platform (Center for Genome Innovation, UConn). The reads were aligned to the MaSuRCA genome assembly using Burrows-Wheeler Aligner (BWA) v0.7.17 but did not provide sufficient coverage due to contamination. Instead, the P. patens T2T reference genome assembly (Zhao 2023; Bi et al. 2024) was used to scaffold the P. africana assembly to chromosome-scale with RagTag v2.1.0 under default parameters, except a minimum fragment size threshold of 1 kb was specified.
Comparative genome analyses
A comparative analysis of the protein-coding gene space was conducted with OrthoFinder v.2.5.1 (Emms and Kelly 2019) with F. hygrometrica (Kirbis et al. 2022) and P. patens v3 (Lang et al. 2018). To provide a preliminary estimate of gene family size dynamics, gene counts from each species in the assembled orthogroups were categorized as neutral, expanded, or contracted. The first and third quartiles were calculated for the distinct gene counts within a gene family for each species. If the number of genes from a species was lower than the first quartile or higher than the third quartile, then the gene family was categorized as “contracted” or “expanded”, respectively. If the number of genes did not fit with either of these 2 criteria, then the gene family was considered “neutral”. The longest gene for each orthogroup was used to assign functional attributes to all genes in the group from the original EnTAP annotation. If the longest gene did not originate from P. africana, then the functional annotation was derived from either P. patens or F. hygrometrica.
GOSeq (Young et al. 2010) enrichment analysis was performed in R v4.2.0. Gene ontology (GO) terms were extracted for each gene from the EnTAP run. Enrichment analysis was investigated separately for the Biological Process and Molecular Function GO categories. Paralogs of Light-Harvesting Complex (LHC), Serine/Threonine-protein kinase (STN) 7, and STN8 were identified with Diamond v2.8.1 (E-value <1e–5).
Whole genome duplication analysis
Chromosome-scale genomes of F. hygrometrica and P. patens were assessed with the reference genome generated for P. africana, with wgd (v.2.0.19) to characterize whole genome duplication (WGD) events (Chen et al. 2024). Each species was compared against itself. Nucleotide coding sequences (CDS) were used as input to Blast & Markov clustering (MCL) (Altschul et al. 1997; van Dongen 2000). A Ks distribution was constructed using the “ksd” subcommand (Yang 2007; Katoh and Toh 2008; Price et al. 2010). Next, a collinearity analysis was performed with “syn” using the structural genome annotations (Proost et al. 2011). Model fit was evaluated with the Bayesian and Akaike information criterion (BIC/AIC).
Results and discussion
Sequencing and quality control of genomic reads
The single Oxford Nanopore Technologies (ONT) run generated 16 million reads (N50: 7,518 bp; 127× coverage: 404 Mb; 116× coverage: 440 Mb; Supplementary Table 1). Centrifuge filtering reduced this set to approximately 14 M reads (N50: 4,561 bp; 80× coverage: 404 Mb; 73× coverage: 440 Mb). The primary contaminants included bacteria from Xanthomonadaceae (10.68%) followed by Bradyrhizobiaceae (0.7%). The short-read genomic libraries (100 bp PE) generated 529 M reads. Following trimming with Sickle, 360 M reads remained (178× coverage: 404 Mb; 163× coverage: 440 Mb; Supplementary Table 2). Coverage estimates are provided for both the original estimate (404 Mb) and the final assembled genome size (440 Mb).
Transcriptome assembly
A total of 36.58 M RNA short reads were generated. Following quality filtering using Trimmomatic, the dataset was reduced to 31.84 M reads. The de novo assembly process with Trinity yielded 143,150 contigs. After expression filtering via RSEM, the number of contigs was reduced to 123,373. Identifying ORFs in the contigs resulted in 110,852 successfully translated transcripts. The average sequence length of these translated ORFs was 803 bp, with an N50 value of 1,038 bp. To enhance the quality of the transcriptome assembly, internal sequences and putative contaminants were removed, resulting in 74,997 total transcripts. After removing internal sequences and contamination, the total unique sequences with a sequence similarity search alignment are 51,982 (69.3%). An assessment of contamination revealed that 8,720 transcripts, 16.78% of the transcriptome, originated from various sources such as amoeba (0.06%), bacteria (0.64%), fungi (15.93%), or insecta (0.12%), as identified by EnTAP. A further 15,500 (18.5%) sequences remained unannotated. The final BUSCO score of the remaining transcripts was C:82%[D:35.7%]. A total of 30,657 (36.6%) transcripts aligned to P. patens proteins.
Genome assembly
Initial assembly
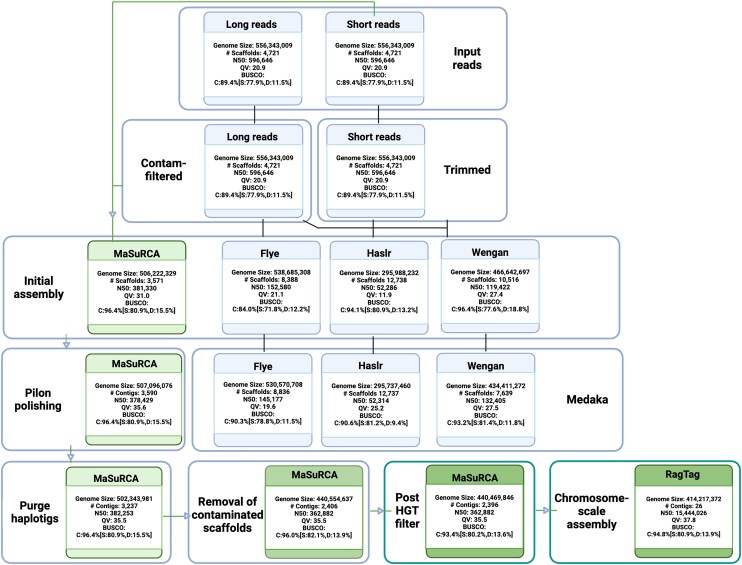
Multiple genome assembly approaches were employed to generate comprehensive draft assemblies of the P. africana genome (Fig. 1). The long-read approach, Flye, assembled 538.68 Mb in 8,388 scaffolds, with an N50 of 152.58 Kb. The BUSCO completeness score was C:84.0% [D:12.2%] and the Merqury Quality Value (QV) score was 21.1. Among the hybrid approaches, Haslr produced a 295.98 Mb reference distributed across 12,738 scaffolds, with an N50 of 52.29 Kb. The BUSCO score was C:94.1% [D:13.2%], and the QV score was 11.9. Wengan assembled a total of 466.64 Mb across 10,516 scaffolds, with an N50 of 119.42 Kb, a BUSCO score of C:96.4% [D:18.8%], and a QV score of 27.4. Finally, MaSuRCA assembled 506.22 Mb across 3,571 scaffolds, with an N50 of 381.33 Kb, (BUSCO: C:96.4% [D:15.5%], and a QV score of 31.0).
Fig. 1.
Workflow and statistics for read QC, genome assembly, polishing, and haplotype phasing. Short reads generated from BGI-seq were subject to trimming and the long reads (ONT) were filtered for contaminants with Centrifuge. Filtered and trimmed reads were utilized as input for four different genome assembly software tools: Flye (long-read only), Haslr, Wengan, and MaSuRCA. The MaSuRCA assembly was selected for further analysis. After Pilon polishing, the MaSuRCA assembly was used as input for Purge haplotigs. The final assembly was phased with Purge haplotigs. A total of 831 scaffolds were removed as a result of contaminant filtering using EnTAP following structural genome annotation. Ten additional scaffolds were removed after HGT candidate assessment. The assembly was then scaffolded against the P. patens V4 genome using RagTag, capturing 94% of the total assembly across 26 chromosomes. Summary statistics derived from Quast (N50—scaffold/contig length at which 50% of the genome is contained in scaffolds/contigs of this size or greater), BUSCO, and Merqury (QV—Quality Value, an assessment of assembly accuracy) are displayed for each process.
Polishing and improving genome assembly
The Medaka polished Flye assembly had a genome size of 530.57 Mb in 8,386 scaffolds, and the N50 was decreased to 145.17 Kb. The BUSCO dropped to C: 90:3% [D:11.5%]. The QV score decreased to 19.6. Polishing of the Haslr assembly resulted in a genome size of 295.73 Mb, across 12,737 scaffolds. The N50 remained almost unchanged at 52.31 Kb, and the BUSCO score reduced to C:90.6% [D:9.4%]. The QV score more than doubled to 25.2. Filtering for scaffolds <3 Kb, followed by Medaka, resulted in a smaller genome size for the Wengan assembly at 434.41 Mb across 7,639 scaffolds. The N50 increased to 132.40 Kb. The BUSCO score was reduced to C:93.2% [D:11.8%], and the QV increased slightly to 27.5.
Polishing with Pilon had very minimal influence on the completeness, as expected, and substantial impact on the accuracy of the MaSuRCA assembly. The polished MaSuRCA hybrid assembly size increased slightly to 507.10 Mb across 3,590 scaffolds, with a slightly decreased N50 of 378.43 Kb. The BUSCO completeness remained the same at C:96.4% [D:15.5%], and the QV score increased to 35.6.
Refinement of genome assemblies with purge haplotigs
The MaSuRCA assembly was selected for further refinement. This decision was based on the overall quality assessed by Merqury, BUSCO completeness, and overall contiguity. The MaSuRCA assembly was refined with purge haplotigs, which reduced the assembly length to 502.34 Mb across 3,237 scaffolds with an N50 value of 382.25 Kb. The BUSCO completeness score remained the same (e.g. 96.4%) and the QV score was minimally reduced to 35.5. At 502.34 Mb, the assembled genome is ∼100 Mb longer than the k-mer based estimate (440 Mb, Supplementary Fig. 2).
Genome annotation
Repeat identification
RepeatModeler produced a library containing 580 unique repeats that were used to softmask 50.22% of the final assembly (Supplementary Table 3). Of the repeats, 36.53% were composed of Ty3/Gypsy and 1.46% of Ty1/Copia. This is similar to the pattern in P. patens, wherein approximately 57% of the genome is composed of repeat elements, with LTRs, particularly the Gypsy family, accounting for 48% of the masked genome (Lang et al. 2018). These findings align with observations by Kirbis et al. (2022), suggesting a common pattern regarding the activation of Gypsy elements in P. africana and P. patens. By contrast, F. hygrometrica, which diverged from P. patens 60–80 MYA (Medina et al. 2018; Bechteler et al. 2023), exhibits a lower overall repeat estimate of 35%. Here, Gypsy elements contribute less to the LTR content, i.e. roughly 10%, whereas Copia elements contribute 17%.
Protein-coding gene identification
The P. africana RNA-Seq library had an overall alignment rate of 78.43%, likely due to contaminant content in the source extraction. Regardless, a substantial set of 37 M reads were retained, exceeding the minimum needed for prediction. The first BRAKER2 predictions, using RNA-Seq evidence alone, generated 60,917 protein-coding genes with a BUSCO completeness score of C:95.8% [D:15.3]. The mono:multi exonic ratio was 0.52. With only protein evidence, BRAKER2 generated 37,752 gene predictions with a BUSCO score of C:46.1% [D:16.0]. Merging these predictions, as recommended by TSEBRA, resulted in a set of 45,737 genes with a BUSCO score of C:91.3% [D:15.1], and a mono:multi exonic ratio of 1.06. The gene prediction set generated with RNA-Seq alignment evidence exclusively was selected for further refinement due to its higher BUSCO score and lower mono:multi ratio compared with the merged TSEBRA transcripts. Through protein domain filtering (InterProScan filter), the number of mono-exonic genes was further reduced from 20,081 to 12,696, producing a total of 23,561 genes (BUSCO: C:88.5% [D:13.8%]; mono:multi ratio: 0.08).
Based on the functional annotations of the gene space, we removed 831 scaffolds and 1,250 genes related to contaminants. This also reduced the assembly length to 440 Mb in 2,406 scaffolds with an N50 of 363 Kb, and an assembly BUSCO score of C:96% [D:13.9%]. This substantial reduction led to an assembly within ∼40 Mb of the original k-mer-based estimate (e.g. 404 Mb).
The annotated protein-coding gene space in the contaminant-filtered assembly included 1,708 mono-exonic and 21,853 multiexonic genes. A BUSCO score of C:93.8% [D:13.6%] and mono:multi ratio of 0.08 was reported. Reciprocal BLAST conducted by EnTAP produced an annotation rate of 78%, of which 93.6% aligned to P. patens (Table 1; Supplementary File 1). This contrasts with the annotation rate of 50% in F. hygrometrica (Kirbis et al. 2022), attributed to significant divergence time from P. patens, and the lack of closely related species in public genomic databases (Kirbis et al. 2020; Rahmatpour et al. 2021). The higher annotation rate in this study suggests that many genes found in P. patens, but not F. hygrometrica, may have been acquired in the ancestor of the Physcomitrellopsis–Entosthodon–Physcomitrium clade (Medina et al. 2019).
Table 1.
Genome annotation statistics for P. africana.
| Annotation method | Total genes | BUSCO (viridiplantae) | Mono: Multi ratio | Annotation rate |
|---|---|---|---|---|
| BRAKER (RNA) | 60,917 | C:95.8% [D:15.3] | 0.52 | 65% |
| BRAKER (protein) | 37,752 | C:46.1% [D:16.0] | 0.95 | 73% |
| TSEBRA (BRAKER RNA + BRAKER protein) | 45,737 | C:91.3% [D:15.1] | 1.02 | 74% |
| BRAKER (RNA) + InterProScan filter + scaffold contam filter) | 23,561 | C:93.8% [D:13.6%] | 0.08 | 78% |
| BRAKER (RNA) + InterProScan filter + scaffold contam filter + HGT filter) | 23,535 | C:88.5% [D:11.3%] | 0.07 | 83% |
| Scaffolded to 26 chromosomes with RagTag | 22,925 | C:86.4% [D:11.1%] | 0.07 | 75% |
Scaffolding the genome assembly to chromosome-scale
The T2T assembly of P. patens (V4; Zhao 2023; Bi et al. 2024) was used to scaffold the P. africana genome assembly. The final assembly contained 440.65 Mb in 583 scaffolds with an N50 length of 15.04 Mb. The 26 largest pseudomolecules, corresponding to the P. patens V4 haploid chromosome number, covered 414.21 Mb or 94% of the 440mMb assembly. From here on, we will refer to these pseudomolecules as chromosomes, numbered according to their lengths. The final genome assembly had a BUSCO completeness of C:94.8% [S:80.9%, D:13.9%], F:1.2%, M:4.0%, n:425, and a total number of 22,925 protein coding genes. While most of the annotated gene space was retained, the BUSCO duplication score remained at 13%, compared with 11–15% for the F. hygrometrica and P. patens genomes (Kirbis et al. 2022). Of the 557 contigs/scaffolds that did not contribute to the 26 chromosomes, 185 (33.34%) contained at least 1 protein coding gene annotation from the first annotation. Among those, 3 (1.6%) contained at least 1 gene that was annotated as a contaminant but did not meet the original thresholds that removed full scaffolds.
Comparative genome analysis
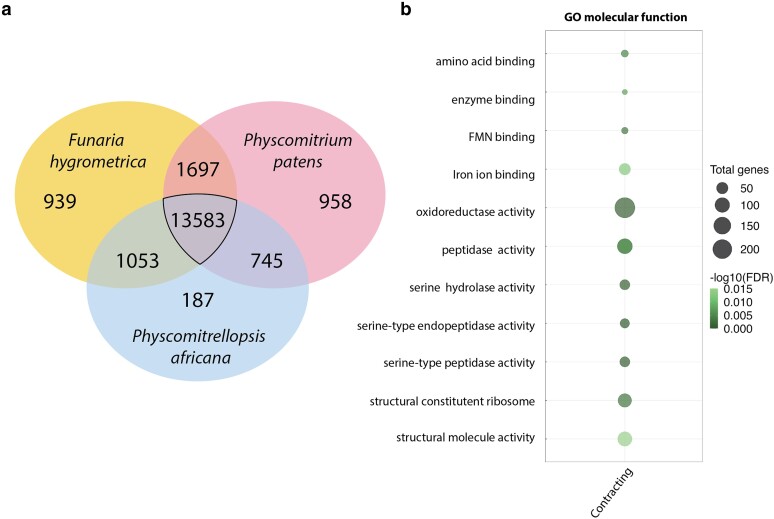
The comparison of the genomes of P. africana, F. hygrometrica, and P. patens reveals that they share 13.5 K orthogroups, but also hold 187, 939, and 958 unique orthogroups, respectively. F. hygrometrica and P. africana exclusively share more (1,053) orthogroups than P. africana and P. patens (745) (Fig. 2a), despite the latter sharing a more recent unique common ancestor (Fig. 3). Thus, whereas Funariaceae share a rather conserved architecture of their vegetative body even after at least 60 MY of divergence, their gene space varies considerably (Kirbis et al. 2022). Such differentiation was previously noted based on highly diverging transcriptomes (Rahmatpour et al. 2021), and interpreted as reflective of strong ecophysiological adaptations, which may be the driving force in bryophyte evolution (Glime 1990). Signatures of 2 ancient WGD events were found in both P. patens and F. hygrometrica. Previous studies have shown that genes related to regulation were preferentially retained after the first WGD in P. patens (Lang et al. 2018). Our analysis of P. africana also retrieved 2 peaks, in agreement with the 2 WGD events in F. hygrometrica and P. patens. The Ks peaks were at 0.56 and 0.92, and the AIC and BIC values accorded with 2 WGD events. To assess the gene expansion and contraction analysis, of the putative gene families from the P. africana annotation, 809 and 1,694 were, respectively, categorized as expanded and contracted (Supplementary File 2). Enrichment analysis examined through GO's Biological Process category revealed 167 expanded terms and 6 contracted terms. By contrast, within the Molecular Function GO category, 62 were contracted, and no terms were significantly expanded.
Fig. 2.
a) The total shared and unique orthogroups among F. hygrometrica, P. africana, and P. patens. b) Enriched Molecular function GO terms for gene families in a comparative analysis between P. africana, P. patens, and F. hygrometrica, showed contraction in the GO terms related to oxidoreductase activity, as well as serine peptidase activity and FMN binding for P. africana. The size of each bubble represents the number of gene families, and the gradient indicates the significance level of enrichment—darker shades denote more significant enrichment.
Fig. 3.
a) P. africana exhibits a reduced architectural complexity of the sporophyte similar to that observed in P. patens, namely, a sessile, aperistomate, and cleistocarpous sporangial capsule. b) The known geographic distribution of P. africana, a rare narrow species endemic to the Eastern Cape Region in South Africa. c) Phylogenetic relationships and chronology of the evolution of Physcomitrellopsis based on Medina et al. (2018).
Although expanded Biological Process GO terms were excessively broad, orthogroup analysis revealed a pattern of contraction in GO terms pertaining to oxidoreductase activity, flavin mono nucleotide (FMN) binding, and serine protease activity (Fig. 2b). Whether this unique suite of downregulated categories diagnoses photosynthetic properties of P. africana only or of the expanded genus sensu Wilding (2015) remains to be tested. The observed changes in GO term enrichment related to oxidoreductase activity, FMN binding, and serine protease activity suggest potential differences in photosynthetic processes between P. africana and other moss species.
The complement of LHC genes is expanded in P. patens compared with algae and vascular plants (Alboresi et al. 2008; Iwai et al. 2018). LHC proteins bind chlorophylls and carotenoids to facilitate light absorption and energy transfer to the reaction centers of Photosystems (PS) I and II. The LHC genes are classified into 2 groups: Lhca encodes antenna proteins for PSI (LHCI) whereas Lhcb encodes antenna proteins for PSII (LHCII). Although ancestral land plants contain several LHC homologs, further expansion occurred in P. patens after WGD events (Alboresi et al. 2008; Rensing et al. 2008; Zimmer et al. 2013; Sun et al. 2023). This led to a larger repertoire of LHC genes compared with the alga Chlamydomonas reinhardtii and the vascular plant Arabidopsis thaliana. Specific Lhca and Lhcb paralogs are present in multiple copies in the P. patens genome compared with 1 to 2 copies in C. reinhardtii and A. thaliana. The major antenna proteins encoded by Lhcbm also show greater redundancy and diversity in P. patens (Iwai et al. 2018; Sun et al. 2023).
Comparing the genomes of P. africana, F. hygrometrica, and P. patens reveals both conservation and divergence of LHC genes. For example, P. africana has 8 distinct Lhca genes, whereas P. patens has 12, and F. hygrometrica has 7. Similarly, whereas P. africana has seven distinct Lhcb genes, P. patens has 12, and F. hygrometrica has nine. Finally, P. africana has 2 distinct Lhcbm genes, P. patens has 14, and F. hygrometrica has 8. However, echoing Kirbis et al. (2022), more LHC gene duplicates were retained in P. patens than in F. hygrometrica and P. africana (Supplementary Fig. 3, Kirbis et al. 2022). Whereas P. patens retained multiple LHC paralogs of possible WGD origin or gene duplications, F. hygrometrica and P. africana may have lost some of this gene redundancy. This pattern of differential retention is further supported by assessing the number of paralogs and orthologues for each LHC gene family across the three genomes (Supplementary Table 4). Although some copies of LHC genes were lost in F. hygrometrica and P. africana, key STN7 and STN8 kinases involved in photosynthetic acclimation are conserved and retained in all 3 genomes, suggesting retention of core light signaling components.
Contaminant filtering and the identification of HGT events
In plants, horizontal transfer of genes can occur at all phylogenetic depths, via introgression following hybridization between plant species (Pfennig 2021) to acquisition of genetic material from other domains (i.e. bacteria) or phyla of eukaryotes (e.g. fungi) (Soucy et al. 2015; Husnik and McCutcheon 2018). Identifying such transfers, and hence the donors, is challenged by the occurrence of the plant's microbiome (Koutsovoulos et al. 2016; Francois et al. 2020) and hence requires critical screening of sequencing outputs. Filtering before and after the assembly of P. africana was employed, illustrating that iterative approaches at all stages enhanced the final quality of the reference. Here, metagenomic tools (Centrifuge; Kim et al. 2016), optimized for long reads, identified contaminants before assembly. Since this initial filter did not include the short reads or assess fungal contributions in either sequence set, further filtering was conducted after assembly and annotation. An estimated 22% of the genes were of fungal origin and 31% were of bacterial origin. Scaffolds that contained numerous genes originating from algae, bacteria, or fungi were removed. Separately, the annotated gene space was aligned to a set of “donor” and “recipient” databases. This identified 31 potential HGT events. Following the set of best practices outlined by Francois et al. (2020), an additional set of 10 scaffolds was found to be contaminated as the flanking genes of the candidates were not of plant origin. This resulted in their removal (HGT filter). Two unique HGT candidates survived these filters, and the final scaffolding (Fig. 4).
Fig. 4.
a) Contamination vs HGT in the P. africana genome. Parameters used for removing a set of contaminated scaffolds from the draft genome based on functional characterization of the annotated gene space. Scaffolds with a length of 10 kb or less, and those with 40% or more of their total genes classified as archaea, bacteria, or fungi, were removed. Additionally, scaffolds with a length greater than or equal to 10 kb and having 55% or more genes classified as archaea, bacteria, or fungi were also excluded. In total, 831 scaffolds were removed because of this filtering process. Altogether 63 scaffolds contained at least one gene annotated as a contaminant but did not meet the original thresholds for removing full scaffolds. When the chromosome-scale assembly was generated with RagTag scaffolding, 3 of these scaffolds were not placed in the 26 chromosomes. b) Identification of HGT candidates via sequence similarity comparisons of P. africana proteins against both “donor” databases (archaea, bacteria, fungal, and metazoan) and “recipient” databases (Streptophyta, Tracheophyta, and Spermatophyta). Proteins with >1 and <4 hits against all donor databases, and no hit against recipient databases, were labeled as HGT candidates. This analysis was conducted on the contaminated scaffolds removed in A, confirming their contamination status. On the post-filtered scaffolds, there were some putative HGTs that were flanked by contaminants. These scaffolds were also removed from the assembly (additional 10), resulting in the retention of 2 HGT candidates in the final analysis.
The first HGT candidate, Pa1_19801.1, aligns to C-type lectin (CTL) mannose-binding isoform-like XP_032077963.1. C-type lectins are the most frequent binding sites across all plants and animals. In vertebrates, CTLs have a function of pathogen recognition. HGTs in the context of plant defense and immunity are not new and have been identified before (Yue et al. 2012; Ma et al. 2022). In fact, Ma et al. (2022) identified a lectin that was acquired from bacteria. The second candidate, Pa1_12964.1, aligns to a hypothetical protein found in Endogone sp. FLAS-F59071 (RUS16308.1). While there is no information on the function of this gene, transfers from fungi are not surprising. As mentioned previously, fungal killer proteins KP4 have been acquired by mosses and play an important role in protonemal development (Guan et al. 2023).
These 2 candidates were manually validated by comparing alignments of de novo assembled transcriptomes. Both candidates are directly flanked by well-annotated plant (moss) genes. Additionally, aligning the full set of P. patens and Ceratodon purpureus proteins to the P. africana scaffolds produced no alignments in proximity to the candidates, further suggesting that these HGT candidates are specific to P. africana (Fig. 5).
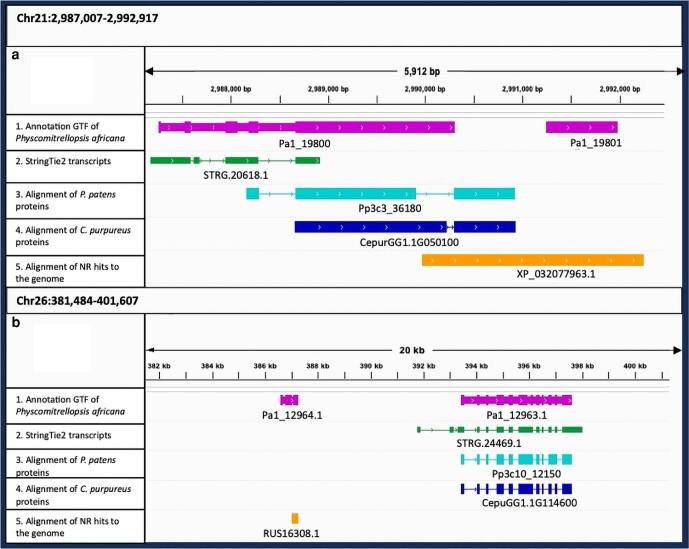
Fig. 5.
Integrated Genome Viewer (IGV) screens depicting 5 tracks of the Physcomitrellopsis africana genome. Track 1 shows the protein-coding structural annotation in context to the genome. Track 2 displays genome-guided transcript assemblies via StringTie2. Tracks 3 and 4 show the alignments of P. patens and C. purpureus proteins onto Chromosomes 2 and 26. Track 5 illustrates the alignment of the HGT candidate (nr database) to the genome. a) and b) show the HGT candidates Pa1_19801.1 and Pa1_12964.1 where the HGT candidate alignment validates the presence of a HGT protein. In both cases, independent RNA assemblies, via StringTie2, were not able to generate a supporting model. None of the other moss proteins from P. patens and C. purpureus aligned to the region hosting the HGT candidates in P. africana.
Analyzing HGT candidates from P. patens
The genomes of P. africana and F. hygrometrica were screened for the 273 putative HGTs previously identified in P. patens (Yue et al. 2012; Ma et al. 2022). Approximately 91 of these genes (33%) were not found in the other 2 species, whereas 15 (5%) were shared only by P. africana and P. patens, and seven (3%), only by P. patens and F. hygrometrica (Supplementary Fig. 4). The greater number of shared HGTs between P. patens and P. africana likely reflects their more recent divergence (at least 40 MYA) compared with that of P. patens and F. hygrometrica (at least 60 MYA) (Medina et al. 2018; Bechteler et al. 2023). These findings suggest that HGT may have played a role in the evolution of mosses, potentially facilitating their adaptation to diverse environments over their long evolutionary history dating back 500 million years (Bechteler et al. 2023). The life cycle of mosses, with stages where cells are exposed to microbes, such as the egg or zygote within the open archegonium or motile sperm cells, could provide opportunities for the uptake of foreign genetic material from bacteria or fungi (Yue et al. 2012; Huang 2013). These vulnerable stages raise the hypothesis that putative microbial symbionts or associates of mosses may have been sources of HTGs, potentially aiding mosses in adapting to harsh environmental conditions encountered as some of the earliest terrestrial plants.
Supplementary Material
Acknowledgments
The authors would like to thank the Institute for Systems Genomics (ISG) at the University of Connecticut, including the Center for Genome Innovation for sequencing support and the Computational Biology Core for software support and access to high-performance computing.
Contributor Information
Vidya S Vuruputoor, Department of Ecology and Evolutionary Biology, University of Connecticut, Storrs, CT 06269, USA.
Andrew Starovoitov, Department of Ecology and Evolutionary Biology, University of Connecticut, Storrs, CT 06269, USA.
Yuqing Cai, State Key Laboratory of Agricultural Genomics, BGI-Shenzhen, Shenzhen 518083, China; Key Laboratory of Southern Subtropical Plant Diversity, Fairy Lake 518004, China.
Yang Liu, State Key Laboratory of Agricultural Genomics, BGI-Shenzhen, Shenzhen 518083, China; Key Laboratory of Southern Subtropical Plant Diversity, Fairy Lake 518004, China.
Nasim Rahmatpour, Department of Ecology and Evolutionary Biology, University of Connecticut, Storrs, CT 06269, USA.
Terry A Hedderson, Department of Biological Sciences, Bolus Herbarium, University of Cape Town, Private Bag, 7701 Rondebosch, South Africa.
Nicholas Wilding, UMR PVBMT, BP 7151, Université de La Réunion, chemin de l’IRAT, 97410 Saint-Pierre, La Réunion, France; Missouri Botanical Garden, P.O. Box 299, St. Louis, MO 63166-0299, USA.
Jill L Wegrzyn, Department of Ecology and Evolutionary Biology, University of Connecticut, Storrs, CT 06269, USA; Institute for Systems Genomics, University of Connecticut, Storrs, CT 06269, USA.
Bernard Goffinet, Department of Ecology and Evolutionary Biology, University of Connecticut, Storrs, CT 06269, USA.
Data availability
All scripts and data are described in https://doi.org/10.5281/zenodo.11094703. NCBI BioProject ID PRJNA1020579 contains the genomic short reads and nanopore long reads (SRR26596311, SRR26596310, and SRR26666216) and the RNA reads (SRR26586950), de novo transcriptome assembly (GKQB00000000.1), as well as the whole genome assembly (GCA_036785485.1). The annotation files are available at https://doi.org/10.6084/m9.figshare.25724079.
Supplemental material is available at G3 online.
Funding
This study was made possible through the US National Science Foundation grants DEB-0919284 (fieldwork), DEB-1753811 awarded to B.G., and DBI-1943371 awarded to J.L.W.
Author contributions
J.L.W. and B.G. designed the study. B.G., N.W., and T.A.H. conducted fieldwork to sample the wild population. N.R., Y.C., and Y.L. generated genomic and transcriptomic data. A.S. and V.S.V. conducted all analyses. V.S.V., J.L.W., and B.G. wrote the paper. All authors approved of the final version.
Literature cited
- Alboresi A, Caffarri S, Nogue F, Bassi R, Morosinotto T. 2008. In silico and biochemical analysis of Physcomitrella patens photosynthetic antenna: identification of subunits which evolved upon land adaptation. PLoS One. 3(4):e2033. doi: 10.1371/journal.pone.0002033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25(17):3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminov RI. 2011. Horizontal gene exchange in environmental microbiota. Front Microbiol. 2:158. doi: 10.3389/fmicb.2011.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews S. 2010. FastQC: A Quality Control Tool for High Throughput Sequence Data. Available from http://www.bioinformatics.babraham.ac.uk/projects/fastqc.
- Bechteler J, Peñaloza-Bojacá G, Bell D, Burleigh JG, McDaniel SF, Davis EC, Sessa EB, Bippus A, Cargill DC, Chantanoarrapint S, et al. 2023. Comprehensive phylogenomic time tree of bryophytes reveals deep relationships and uncovers gene incongruences in the last 500 million years of diversification. Am J Bot. 110(11):e16249. doi: 10.1002/ajb2.16249. [DOI] [PubMed] [Google Scholar]
- Bi G, Zhao S, Yao J, Wang H, Zhao M, Sun Y, Hou X, Hass FB, Varshney D, Prigge M, et al. 2024. Near telomere-to-telomere genome of the model plant Physcomitrium patens. Nat Plants. 10(2):327–343. doi: 10.1038/s41477-023-01614-7. [DOI] [PubMed] [Google Scholar]
- Boothby TC, Tenlen JR, Smith FW, Wang JR, Patanella KA, Nishimura EO, Tintori SC, Li Q, Jones CD, Yandell M, et al. 2015. Evidence for extensive horizontal gene transfer from the draft genome of a tardigrade. Proc Natl Acad Sci U S A. 112(52):15976–15981. doi: 10.1073/pnas.1510461112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brůna T, Hoff KJ, Lomsadze A, Stanke M, Borodovsky M. 2021. BRAKER2: automatic eukaryotic genome annotation with GeneMark-EP+ and AUGUSTUS supported by a protein database. NAR Genom Bioinform. 3(1):lqaa108. doi: 10.1093/nargab/lqaa108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchfink B, Reuter K, Drost H-G. 2021. Sensitive protein alignments at tree-of-life scale using DIAMOND. Nat Methods. 18(4):366–368. doi: 10.1038/s41592-021-01101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zwaenepoel A, Van de Peer Y. 2024. wgd v2: a suite of tools to uncover and date ancient polyploidy and whole-genome duplication. Bioinformatics. 40(5):btae272. doi: 10.1093/bioinformatics/btae272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dainat J. 2024. AGAT: Another Gff Analysis Toolkit to Handle Annotations in Any GTF/GFF Format (Version v0.8.0). Zenodo. doi: 10.5281/zenodo.3552717. [DOI]
- Di Genova A, Buena-Atienza E, Ossowski S, Sagot M-F. 2021. Efficient hybrid de novo assembly of human genomes with WENGAN. Nat Biotechnol. 39(4):422–430. doi: 10.1038/s41587-020-00747-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douvlataniotis K, Bensberg M, Lentini A, Gylemo B, Nestor CE. 2020. No evidence for DNA N 6-methyladenine in mammals. Sci Adv. 6(12):eaay3335. doi: 10.1126/sciadv.aay3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emms DM, Kelly S. 2019. OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biol. 20(1):238. doi: 10.1186/s13059-019-1832-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RD, Bateman A, Clements J, Coggill P, Eberhardt RY, Eddy SR, Heger A, Hetherington K, Holm L, Mistry J, et al. 2014. Pfam: the protein families database. Nucleic Acids Res. 42(D1):D222–D230. doi: 10.1093/nar/gkt1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JM, Hubley R, Goubert C, Rosen J, Clark AG, Feschotte C, Smit AF. 2020. RepeatModeler2 for automated genomic discovery of transposable element families. Proc Natl Acad Sci U S A. 117(17):9451–9457. doi: 10.1073/pnas.1921046117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois CM, Durand F, Figuet E, Galtier N. 2020. Prevalence and implications of contamination in public genomic resources: a case study of 43 reference arthropod assemblies. G3 (Bethesda). 10(2):721–730. doi: 10.1534/g3.119.400758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel L, Hoff KJ, Brůna T, Borodovsky M, Stanke M. 2021. TSEBRA: transcript selector for BRAKER. BMC Bioinformatics. 22(1):566. doi: 10.1186/s12859-021-04482-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glime JM. 1990. The ecology column: introduction. Bryological Times. 55:5–7. [Google Scholar]
- Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q, et al. 2011. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 29(7):644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Ma L, Wang Q, Zhao J, Wang S, Wu J, Liu Y, Sun H, Huang J. 2023. Horizontally acquired fungal killer protein genes affect cell development in mosses. Plant J. 113(4):665–676. doi: 10.1111/tpj.16060. [DOI] [PubMed] [Google Scholar]
- Gurevich A, Saveliev V, Vyahhi N, Tesler G. 2013. QUAST: quality assessment tool for genome assemblies. Bioinformatics. 29(8):1072–1075. doi: 10.1093/bioinformatics/btt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas B, Papanicolaou A. 2012. TransDecoder (Find Coding Regions within Transcripts) [WWW Document]. Available from https://transdecoder.github.io/.
- Haghshenas E, Asghari H, Stoye J, Chauve C, Hach F. 2020. HASLR: fast hybrid assembly of long reads. iScience. 23(8):101389. doi: 10.1016/j.isci.2020.101389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AJ, Ginzburg S, Xu MS, Fisher CR, Rahmatpour N, Mitton JB, Paul R, Wegrzyn JL. 2020. EnTAP: bringing faster and smarter functional annotation to non-model eukaryotic transcriptomes. Mol Ecol Resour. 20(2):591–604. doi: 10.1111/1755-0998.13106. [DOI] [PubMed] [Google Scholar]
- Huang J. 2013. Horizontal gene transfer in eukaryotes: the weak-link model. Bioessays. 35(10):868–875. doi: 10.1002/bies.201300007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta-Cepas J, Szklarczyk D, Heller D, Hernández-Plaza A, Forslund SK, Cook H, Mende DR, Letunic I, Rattei T, Jensen LJ, et al. 2019. eggNOG 5.0: a hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 47(D1):D309–D314. doi: 10.1093/nar/gky1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husnik F, McCutcheon JP. 2018. Functional horizontal gene transfer from bacteria to eukaryotes. Nat Rev Microbiol. 16(2):67–79. doi: 10.1038/nrmicro.2017.137. [DOI] [PubMed] [Google Scholar]
- Iwai M, Grob P, Iavarone AT, Nogales E, Niyogi KK. 2018. A unique supramolecular organization of photosystem I in the moss Physcomitrella patens. Nat Plants. 4(11):904–909. doi: 10.1038/s41477-018-0271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P, Binns D, Chang H-Y, Fraser M, Li W, McAnulla C, McWilliam H, Maslen J, Mitchell A, Nuka G, et al. 2014. InterProScan 5: genome-scale protein function classification. Bioinformatics. 30(9):1236–1240. doi: 10.1093/bioinformatics/btu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi NA, Fass JN. 2011. Sickle: A Sliding-Window, Adaptive, Quality-based Trimming Tool for FastQ Files (Version 1.33) [Software]. Available from https://github.com/najoshi/sickle.
- Katoh K, Toh H. 2008. Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinform. 9(4):286–298. doi: 10.1093/bib/bbn013. [DOI] [PubMed] [Google Scholar]
- Kim D, Paggi JM, Park C, Bennett C, Salzberg SL. 2019. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol. 37(8):907–915. doi: 10.1038/s41587-019-0201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Song L, Breitwieser FP, Salzberg SL. 2016. Centrifuge: rapid and sensitive classification of metagenomic sequences. Genome Res. 26(12):1721–1729. doi: 10.1101/gr.210641.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirbis A, Rahmatpour N, Dong S, Yu J, van Gessel N, Waller M, Reski R, Lang D, Rensing SA, Temsch EM, et al. Genome dynamics in mosses: Extensive synteny coexists with a highly dynamic gene space. bioRxiv 492078, doi: 10.1101/2022.05.17.492078, 18 May 2022, preprint: not peer reviewed. [DOI]
- Kirbis A, Waller M, Ricca M, Bont Z, Neubauer A, Goffinet B, Szövényi P. 2020. Transcriptional landscapes of divergent sporophyte development in two mosses, Physcomitrium (Physcomitrella) patens and Funaria hygrometrica. Front Plant Sci. 11:747. doi: 10.3389/fpls.2020.00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolmogorov M, Yuan J, Lin Y, Pevzner PA. 2019. Assembly of long, error-prone reads using repeat graphs. Nat Biotechnol. 37(5):540–546. doi: 10.1038/s41587-019-0072-8. [DOI] [PubMed] [Google Scholar]
- Koutsovoulos G, Kumar S, Laetsch DR, Stevens L, Daub J, Conlon C, Maroon H, Thomas F, Aboobaker AA, Blaxter M. 2016. No evidence for extensive horizontal gene transfer in the genome of the tardigrade Hypsibius dujardini. Proc Natl Acad Sci U S A. 113(18):5053–5058. doi: 10.1073/pnas.1600338113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovaka S, Zimin AV, Pertea GM, Razaghi R, Salzberg SL, Pertea M. 2019. Transcriptome assembly from long-read RNA-seq alignments with StringTie2. Genome Biol. 20(1):278. doi: 10.1186/s13059-019-1910-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D, Ullrich KK, Murat F, Fuchs J, Jenkins J, Haas FB, Piednoel M, Gundlach H, Van Bel M, Meyberg R, et al. 2018. The Physcomitrella patens chromosome-scale assembly reveals moss genome structure and evolution. Plant J. 93(3):515–533. doi: 10.1111/tpj.13801. [DOI] [PubMed] [Google Scholar]
- Li B, Dewey CN. 2011. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 12(1):323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Wang S, Zhu X, Sun G, Chang G, Li L, Hu X, Zhang S, Zhou Y, Song C-P, et al. 2022. Major episodes of horizontal gene transfer drove the evolution of land plants. Mol Plant. 15(5):857–871. doi: 10.1016/j.molp.2022.02.001. [DOI] [PubMed] [Google Scholar]
- Manni M, Berkeley MR, Seppey M, Zdobnov EM. 2021. BUSCO: assessing genomic data quality and beyond. Curr Protoc. 1(12):e323. doi: 10.1002/cpz1.323. [DOI] [PubMed] [Google Scholar]
- Marçais G, Kingsford C. 2011. A fast, lock-free approach for efficient parallel counting of occurrences of k-mers. Bioinformatics. 27(6):764–770. doi: 10.1093/bioinformatics/btr011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin FM, Uroz S, Barker DG. 2017. Ancestral alliances: plant mutualistic symbioses with fungi and bacteria. Science. 356(6340):ea.ad4501. doi: 10.1126/science.aad4501. [DOI] [PubMed] [Google Scholar]
- Medina R, Johnson MG, Liu Y, Wickett NJ, Shaw AJ, Goffinet B. 2019. Phylogenomic delineation of Physcomitrium (Bryophyta: Funariaceae) based on nuclear targeted exons and their flanking regions rejects the retention of Physcomitrella, Physcomitridium and Aphanorrhegma. J Syst Evol. 57(4):404–417. doi: 10.1111/jse.12516. [DOI] [Google Scholar]
- Medina R, Johnson MG, Liu Y, Wilding N, Hedderson TA, Wickett N, Goffinet B. 2018. Evolutionary dynamism in bryophytes: phylogenomic inferences confirm rapid radiation in the moss family Funariaceae. Mol Phylogenet Evol. 120:240–247. doi: 10.1016/j.ympev.2017.12.002. [DOI] [PubMed] [Google Scholar]
- Pfennig KS. 2021. Biased hybridization and its impact on adaptive introgression. Trends Ecol Evol. 36(6):488–497. doi: 10.1016/j.tree.2021.02.010. [DOI] [PubMed] [Google Scholar]
- Price MN, Dehal PS, Arkin AP. 2010. FastTree 2—approximately maximum-likelihood trees for large alignments. PLoS One. 5(3):e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proost S, Fostier J, De Witte D, Dhoedt B, Demeester P, Van de Peer Y, Vandepoele K. 2011. i-ADHoRe 3.0—fast and sensitive detection of genomic homology in extremely large data sets. Nucleic Acids Res. 40(2):e11–e11. doi: 10.1093/nar/gkr955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmatpour N, Perera NV, Singh V, Wegrzyn JL, Goffinet B. 2021. High gene space divergence contrasts with frozen vegetative architecture in the moss family Funariaceae. Mol Phylogenet Evol. 154:106965. doi: 10.1016/j.ympev.2020.106965. [DOI] [PubMed] [Google Scholar]
- Ranallo-Benavidez TR, Jaron KS, Schatz MC. 2020. GenomeScope 2.0 and Smudgeplot for reference-free profiling of polyploid genomes. Nat Comm. 11(1):1432. doi: 10.1038/s41467-020-14998-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensing SA, Goffinet B, Meyberg R, Wu S-Z, Bezanilla M. 2020. The moss Physcomitrium (Physcomitrella) patens: a model organism for non-seed plants. Plant Cell. 32(5):1361–1376. doi: 10.1105/tpc.19.00828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensing SA, Lang D, Zimmer AD, Terry A, Salamov A, Shapiro H, Nishiyama T, Perroud P-F, Lindquist EA, Kamisugi Y, et al. 2008. The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science. 319(5859):64–69. doi: 10.1126/science.1150646. [DOI] [PubMed] [Google Scholar]
- Rhie A, Walenz BP, Koren S, Phillippy AM. 2020. Merqury: reference-free quality, completeness, and phasing assessment for genome assemblies. Genome Biol. 21(1):245. doi: 10.1186/s13059-020-02134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach MJ, Schmidt SA, Borneman AR. 2018. Purge Haplotigs: allelic contig reassignment for third-gen diploid genome assemblies. BMC Bioinformatics. 19(1):460. doi: 10.1186/s12859-018-2485-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit AFA, Hubley R, Green P. 2013. RepeatMasker Open-4.0; [cited 2022 Sep 7]. Available from: http://www.repeatmasker.org.
- Soucy SM, Huang J, Gogarten JP. 2015. Horizontal gene transfer: building the web of life. Nat Rev Genet. 16(8):472–482. doi: 10.1038/nrg3962. [DOI] [PubMed] [Google Scholar]
- Sun G, Bai S, Guan Y, Wang S, Wang Q, Liu Y, Liu H, Goffinet B, Zhou Y, Paoletti M, et al. 2020. Are fungi-derived genomic regions related to antagonism towards fungi in mosses? New Phytol. 228(4):1169–1175. doi: 10.1111/nph.16776. [DOI] [PubMed] [Google Scholar]
- Sun H, Shang H, Pan X, Li M. 2023. Structural insights into the assembly and energy transfer of the Lhcb9-dependent photosystem I from moss Physcomitrium patens. Nat Plants. 9(8):1347–1358. doi: 10.1038/s41477-023-01463-4. [DOI] [PubMed] [Google Scholar]
- van Dongen SM. 2000. Graph Clustering by Flow Simulation [Doctoral Dissertation]. Utrecht, The Netherlands: Utrecht University. [Google Scholar]
- Van Etten J, Bhattacharya D. 2020. Horizontal gene transfer in eukaryotes: not if, but how much? Trends Genet. 36(12):915–925. doi: 10.1016/j.tig.2020.08.006. [DOI] [PubMed] [Google Scholar]
- Vuruputoor VS, Monyak D, Fetter KC, Webster C, Bhattarai A, Shrestha B, Zaman S, Bennett J, McEvoy SL, Caballero M, et al. 2022. Welcome to the big leaves: best practices for improving genome annotation in non-model plant genomes. App Plant Sci. 11(4):e11533. doi: 10.1002/aps3.11533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BJ, Abeel T, Shea T, Priest M, Abouelliel A, Sakthikumar S, Cuomo CA, Zeng Q, Wortman J, Young SK, et al. 2014. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One. 9(11):e112963. doi: 10.1371/journal.pone.0112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding N. 2015. Systematics, Biogeography and Morphological Evolution in Entosthodon Schwägr. (Bryopsida, Funariaceae) with a Revision of the Genus in Africa [Doctoral Dissertation]. South Africa: University of Cape Town. [Google Scholar]
- Yang Z. 2007. PAML 4: phylogenetic analysis by Maximum likelihood. Mol Biol Evol. 24(8):1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- Young LA. 2022. Relationships among AA-Genome Chenopodium Diploids and a Whole Genome Assembly of the North American species, C. watsonii [Doctoral Dissertation]. Utah, USA: Brigham Young University. [Google Scholar]
- Young MD, Wakefield MJ, Smyth GK, Oshlack A. 2010. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol. 11(2):R14. doi: 10.1186/gb-2010-11-2-r14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Li L, Wang S, Dong S, Chen Z, Patel N, Goffinet B, Chen H, Liu H, Liu Y. 2020. Draft genome of the aquatic moss Fontinalis antipyretica (Fontinalaceae, Bryophyta). GigaByte. 2020:gigabyte8. doi: 10.46471/gigabyte.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue J, Hu X, Sun H, Yang Y, Huang J. 2012. Widespread impact of horizontal gene transfer on plant colonization of land. Nat Commun. 3(1):1152. doi: 10.1038/ncomms2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Wood WI. 2003. A profile hidden Markov model for signal peptides generated by HMMER. Bioinformatics. 19(2):307–308. doi: 10.1093/bioinformatics/19.2.307. [DOI] [PubMed] [Google Scholar]
- Zhao S. 2023. Telomere-to-telomere (T2T) genome of the model plant Physcomitrium patens. Figshare. Online resource. 10.6084/m9.figshare.22975925.v2. [DOI] [PubMed]
- Zhaxybayeva O, Doolittle WF. 2011. Lateral gene transfer. Curr Biol. 21(7):R242–R246. doi: 10.1016/j.cub.2011.01.045. [DOI] [PubMed] [Google Scholar]
- Zimin AV, Marçais G, Puiu D, Roberts M, Salzberg SL, Yorke JA. 2013. The MaSuRCA genome assembler. Bioinformatics. 29(21):2669–2677. doi: 10.1093/bioinformatics/btt476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer AD, Lang D, Buchta K, Rombauts S, Nishiyama T, Hasebe M, Van de Peer Y, Rensing SA, Reski R. 2013. Reannotation and extended community resources for the genome of the non-seed plant Physcomitrella patens provide insights into the evolution of plant gene structures and functions. BMC Genomics. 14(1):498. doi: 10.1186/1471-2164-14-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Dainat J. 2024. AGAT: Another Gff Analysis Toolkit to Handle Annotations in Any GTF/GFF Format (Version v0.8.0). Zenodo. doi: 10.5281/zenodo.3552717. [DOI]
- Zhao S. 2023. Telomere-to-telomere (T2T) genome of the model plant Physcomitrium patens. Figshare. Online resource. 10.6084/m9.figshare.22975925.v2. [DOI] [PubMed]
Supplementary Materials
Data Availability Statement
All scripts and data are described in https://doi.org/10.5281/zenodo.11094703. NCBI BioProject ID PRJNA1020579 contains the genomic short reads and nanopore long reads (SRR26596311, SRR26596310, and SRR26666216) and the RNA reads (SRR26586950), de novo transcriptome assembly (GKQB00000000.1), as well as the whole genome assembly (GCA_036785485.1). The annotation files are available at https://doi.org/10.6084/m9.figshare.25724079.
Supplemental material is available at G3 online.