Abstract
Silicosis is the most common type of pneumoconiosis, having a high incidence in workers chronically exposed to crystalline silica (CS). No specific medication exists for this condition. GHK, a tripeptide naturally occurring in human blood and urine, has antioxidant effects. We aimed to investigate the therapeutic effect of GHK-Cu on silicosis and its potential underlying molecular mechanism. An experimental silicosis mouse model was established to observe the effects of GHK-Cu on lung inflammation and fibrosis. Moreover, the effects of GHK-Cu on the alveolar macrophages (AM) were examined using the RAW264.7 cell line. Its molecular target, peroxiredoxin 6 (PRDX6), has been identified, and GHK-Cu can bind to PRDX6, thus attenuating lung inflammation and fibrosis in silicosis mice without significant systemic toxicity. These effects were partly related to the inhibition of the CS-induced oxidative stress in AM induced by GHK-Cu. Thus, our results suggest that GHK-Cu acts as a potential drug by attenuating alveolar macrophage oxidative stress. This, in turn, attenuates the progression of pulmonary inflammation and fibrosis, which provides a reference for the treatment of silicosis.
Keywords: Silicosis, GHK-Cu, Oxidative stress, Macrophage, PRDX6
Highlights
-
•
Plasma levels of GHK are lower in patients with silicosis, and GHK plasma levels correlate with lung function.
-
•
Exogenous supplementation of GHK-Cu attenuates crystalline silica-induced lung injury.
-
•
GHK-Cu exerts anti-oxidative stress effects by targeting PRDX6 to macrophages exposed to crystalline silica.
1. Introduction
Silicosis is a systemic disease characterized by extensive nodular fibrosis of the lungs, which is caused by inhalation of large amounts of free silica dust [1]. Although silicosis occurs worldwide, it remains the most serious occupational disease among mining, construction, and manufacturing workers in many developing countries, and ideal therapeutic agents for this condition are yet to be developed [[2], [3], [4]]. Oxidative stress is an imbalance between the oxidative damage mediated by reactive oxygen species and the antioxidant defense system. Alveolar macrophages (AM) are the first line of defense in the distal lung [5], and if the AM-ingested silica particles are not cleared from the lungs, the phagocytosed particles induce reactive oxygen species (ROS) production and damage the macrophage lysosomes. This leads to an inflammatory cascade that further recruits inflammatory cells and perpetuates the pathology [[6], [7], [8], [9]]. The secreted inflammatory factors induce an effective pro-fibrotic response, leading to collagen deposition, fibrosis, and ultimately progressive massive fibrosis (PMF) [[10], [11], [12]]. AM oxidative stress and the inflammatory response, which are important causative factors of silicosis, may be potential targets for treating this condition.
Glycine-Histidine-Lysine (GHK) is a tripeptide with a glycyl-histidyl-lysine amino acid sequence, as the name indicates, and is a normal component of human plasma, urine, and saliva [13]. GHK has a wide range of biological actions that are beneficial to human health. The ability of GHK to improve tissue repair has been demonstrated in skin, lung, bone, liver, and stomach tissue [[14], [15], [16], [17]]. GHK can easily form a GHK-Cu complex with Cu, which can improve the GHK bioavailability, and the metal ion Cu2+ can be used as an endogenous antioxidant to activate superoxide dismutase (SOD), which makes the GHK-Cu complex have a stronger antioxidant effect than that of the original GHK [18]. GHK-Cu complexes can increase the SOD activity to reduce the production of ROS and decrease the release of the pro-inflammatory cytokine tumor necrosis factor-α (TNF-α) in a lipopolysaccharide (LPS)-induced acute lung injury mouse model [19,20]. Furthermore, in wound healing and skin repair, GHK-Cu can reduce the release of the transforming growth factor beta1 (TGF-β1) [21]. Based on the previous research done by our group, we found that GHK can be used to inhibit the insulin-like-1 (IGF-1) and TGF-β1/Smads signaling pathways, and thus inhibit bleomycin-induced pulmonary fibrosis in mice [22]. Therefore, this GHK-Cu property makes it a drug that can be used for the treatment of silicosis. We aimed to investigate the protective effect of GHK-Cu against silica-induced pulmonary fibrosis in mice and its possible underlying mechanism.
2. Materials and methods
2.1. Human subjects and measurement of the plasma GHK-Cu level
Patients with silicosis (n = 15) and age-matched healthy controls (n = 11) were recruited in the China-Japan Friendship Hospital between December 2021 and March 2022. The inclusion criterion was a silicosis diagnosis. The exclusion criteria were as follows: silicosis exacerbation within the last month; having severe cardiovascular disease or active lung disease; and having an inability to read or understand the informed consent documents. The plasma GHK-Cu level was detected as previously reported [23].
Details of animal model, cell culture, treatment and other experimental procedures are supplied in the supplementary materials.
3. Results and discussion
3.1. The plasma level and clinical relevance of GHK-Cu in patients with silicosis
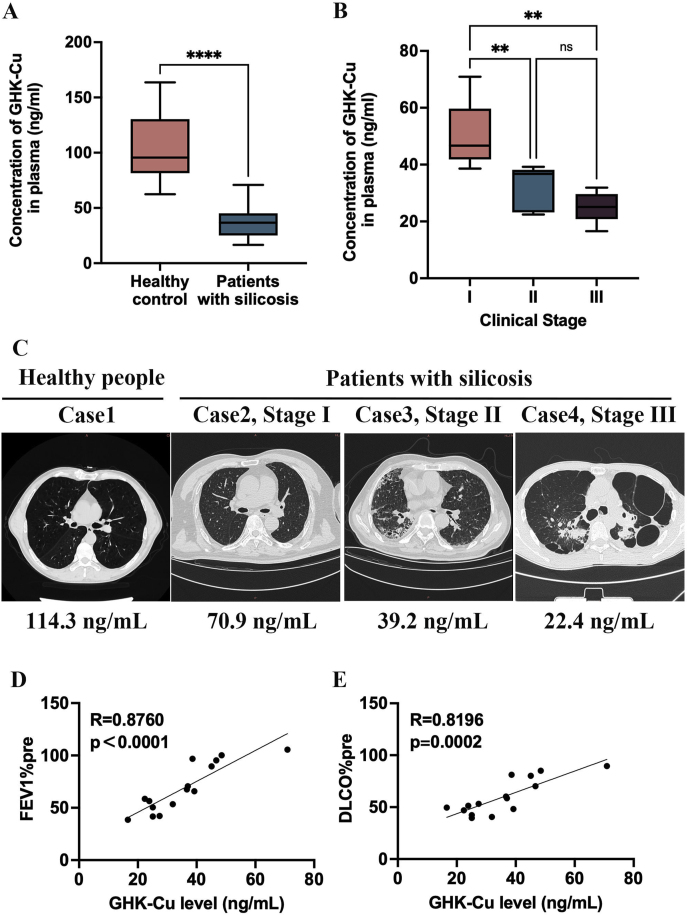
The baseline characteristics of the healthy subjects and patients with silicosis are listed in Supplemental Table 1. Pulmonary function (FEV1% predicted and DLCO% predicted) was significantly lower in patients with silicosis than in healthy controls. To analyze the expression level and clinical relevance of GHK in patients with silicosis and healthy subjects, the plasma levels of GHK, inflammatory factors, and antioxidative stress factors were determined. Our data show that the plasma GHK levels of patients with silicosis were lower (35.67 ± 13.69 ng/mL vs. 105.50 ± 31.94 ng/mL, p < 0.0001) (Fig. 1A) than those of healthy subjects. The plasma GHK level decreased as the clinical stage of patients with silicosis progressed (Fig. 1B and C). Next, the correlation between the plasma GHK level and lung function was assessed. As shown in Fig. 1D, the plasma GHK levels were positively correlated with the FEV1% and the DLCO% levels predicted (R = 0.8760, p < 0.0001 and R = 0.8196, p = 0.0002, respectively). Our previous studies have shown that the GHK levels are significantly lower in the plasma of patients with chronic obstructive pulmonary disease and asthma than in the healthy subjects and that these correlate with an altered lung function, which can be improved via exogenous supplementation with GHK-Cu [23,24]. Therefore, based on the fact that the GHK levels were significantly lower in patients with silicosis than in healthy subjects, we hypothesized that the exogenous supplementation with GHK-Cu could improve lung injury and function in patients with silicosis.
Fig. 1.
The plasma level and clinical relevance of GHK in patients with silicosis: A. Plasma GHK levels in healthy subjects and patients with silicosis. B. Plasma GHK levels in patients with silicosis in different clinical stages of the disease. C. Lung imaging manifestations and plasma GHK levels in a healthy control population and in patients in different clinical stages of silicosis. D. Correlation between the plasma GHK levels and FEV1%pre in patients with silicosis. E. Correlation between the plasma GHK levels and the DLCO%pre in patients with silicosis. ns: p ≥ 0.05, **p < 0.01, ****p < 0.0001.
3.2. GHK-Cu attenuates lung inflammation and fibrosis in silicosis mice
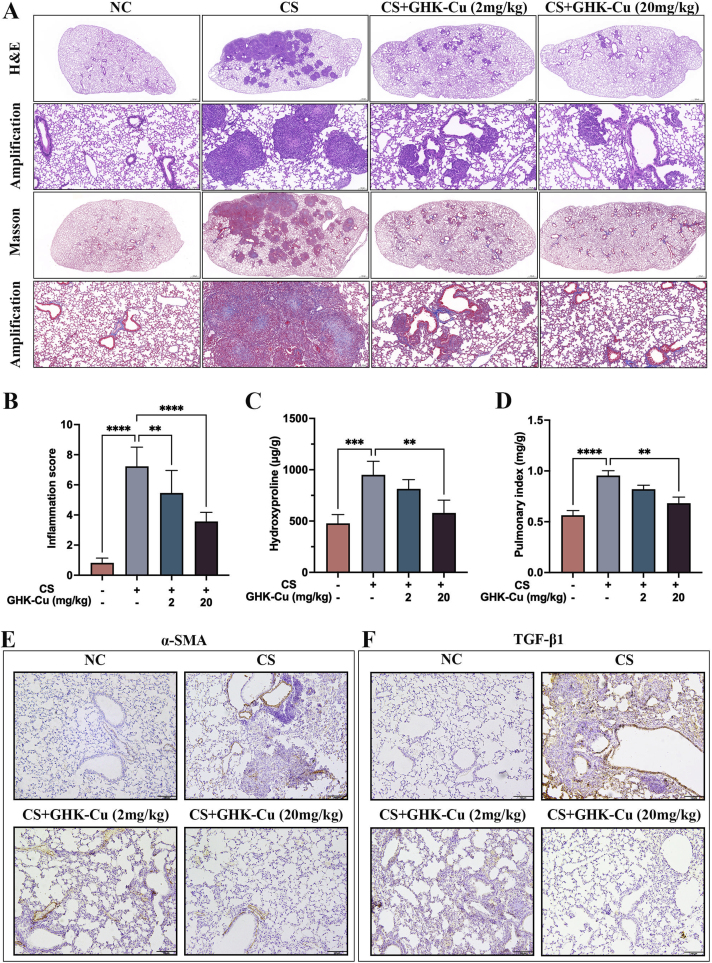
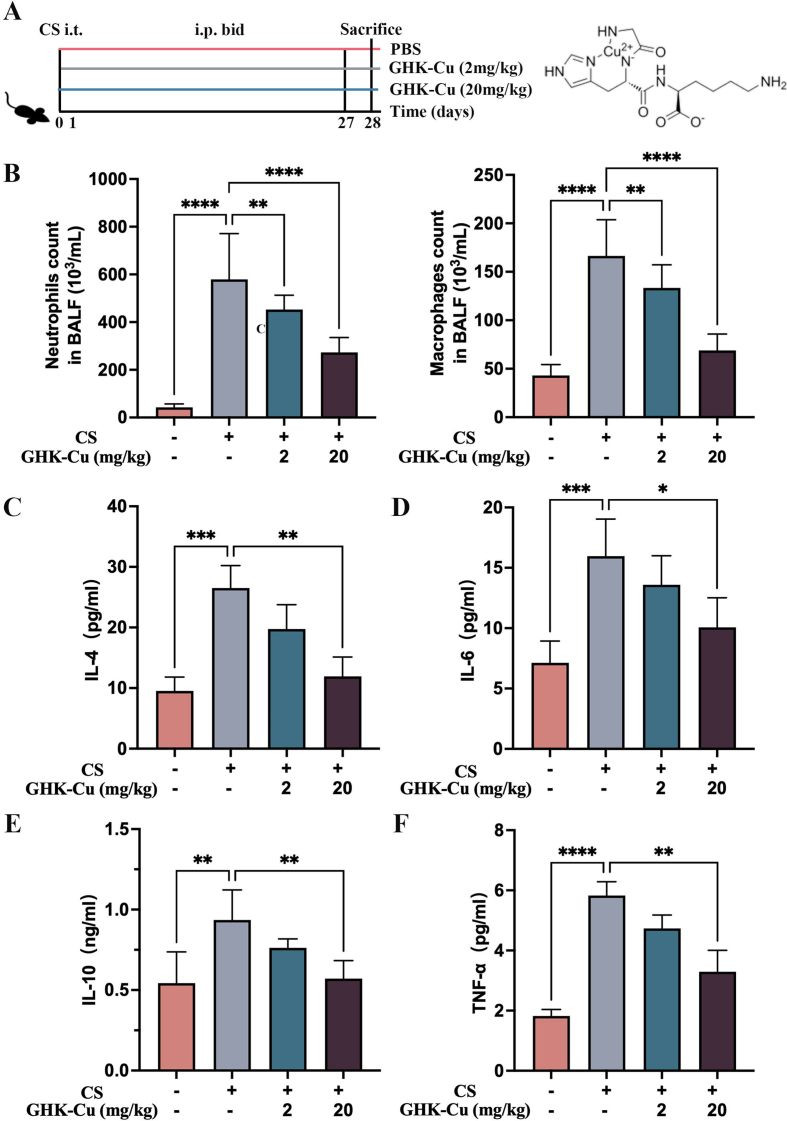
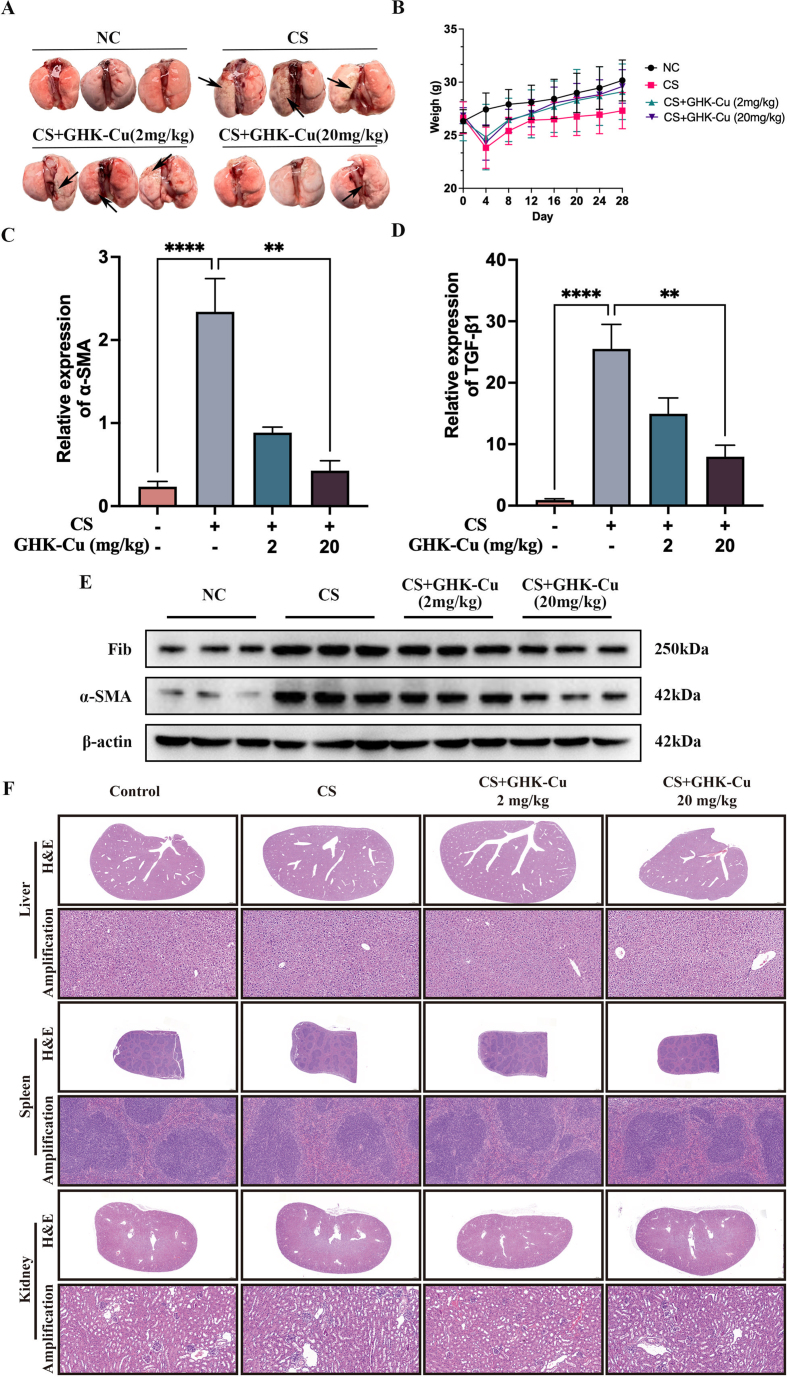
To investigate the role of GHK-Cu in the silicosis process, we examined a series of inflammatory indicators in the lungs of silicosis mouse models and the corresponding controls (Fig. S1). The morphological examinations revealed that GHK-Cu markedly alleviated the alveolar lesions, pulmonary injury, and fibrosis caused by CS stimulation (Fig. 2A and B). The levels of macrophages and other subtypes of infiltrating cells found in the BALF in the control group were notably lower than those found in the CS group (Fig. S1B). ELISA was used to detect the levels of the main inflammatory factors among cytokines and chemokines, such as TNF-α, IL-4, IL-6, and IL-10, which returned to normal levels due to the high-dose GHK-Cu (20 mg/kg) intervention (Figs. S1C–F). GHK-Cu can alleviate pulmonary fibrosis in silicosis mice (Figs. S2A–B). The detection of the hydroxyproline content also indicated that GHK-Cu inhibited the silica-induced collagen deposition in the lungs (Fig. 2C). GHK-Cu also significantly reduced the pulmonary index (Fig. 2D). Furthermore, the immunohistochemistry results clearly showed that the α-SMA and TGF-β1 expression was reversed by GHK-Cu (Fig. 2E–F and S2C-D). Previous studies have also shown that GHK-Cu ameliorates bleomycin-induced pulmonary fibrosis in mice via TGF-β pathway inhibition [22]. Taken together, the silicosis mice might have benefited from the GHK-Cu intervention done here.
Fig. 2.
GHK-Cu attenuates lung inflammation and fibrosis in silicosis mice: A. H&E and Masson staining of the left lung tissues of CS-exposed mice treated with intraperitoneal injections of different doses of GHK-Cu (2 and 20 mg/kg). n = 5. B. The lung inflammatory scores were calculated according to the sum of the cell infiltration and damage levels, as assessed based on the lung sections. n = 5. C. Quantification of the collagen amount present in the lung sections, represented by the hydroxyproline content. D. The pulmonary index was determined to show the alveolar swelling and interstitial fibrosis of the lung sections treated with GHK-Cu or not. E. Immunohistochemical staining of the lung sections for α-SMA was performed after 28 days of CS exposure. F. Immunohistochemical staining of the lung sections for TGF-β1 was performed after 28 days of CS exposure. **p < 0.01, ***p < 0.001, ****p < 0.0001.
3.3. GHK-Cu attenuates cellular injury and oxidative stress in CS-exposed macrophages
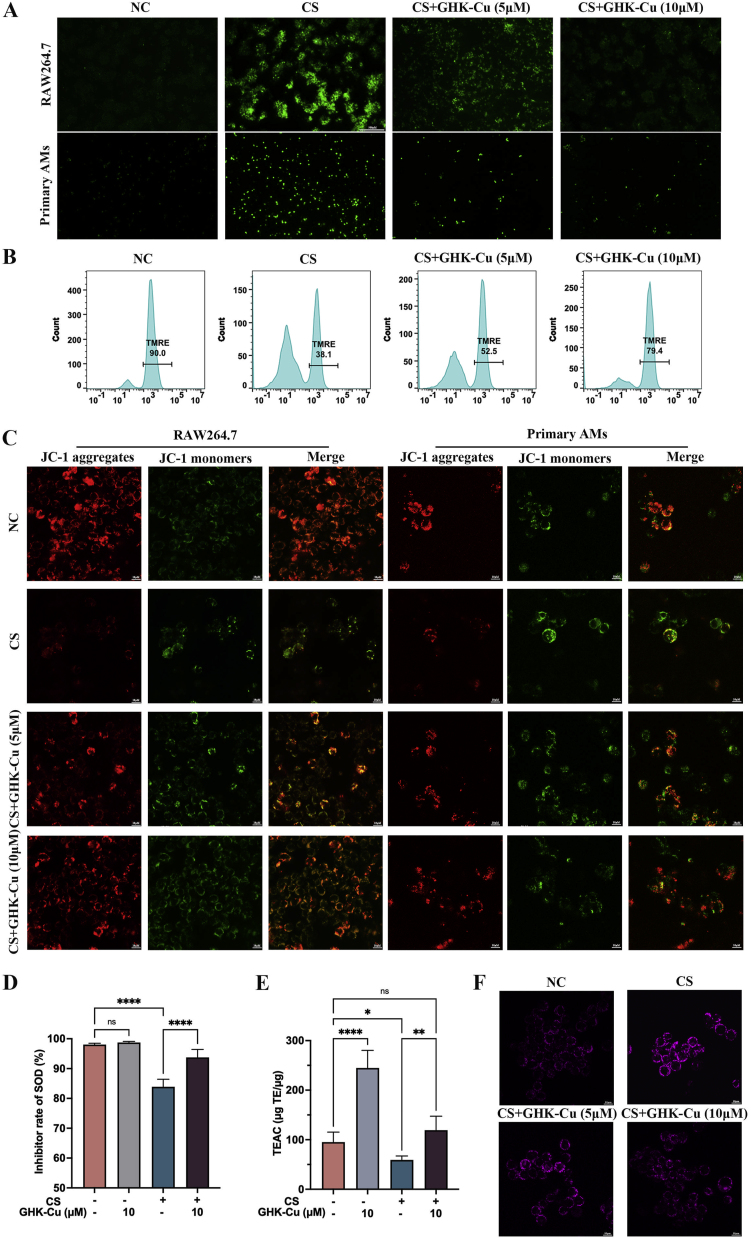
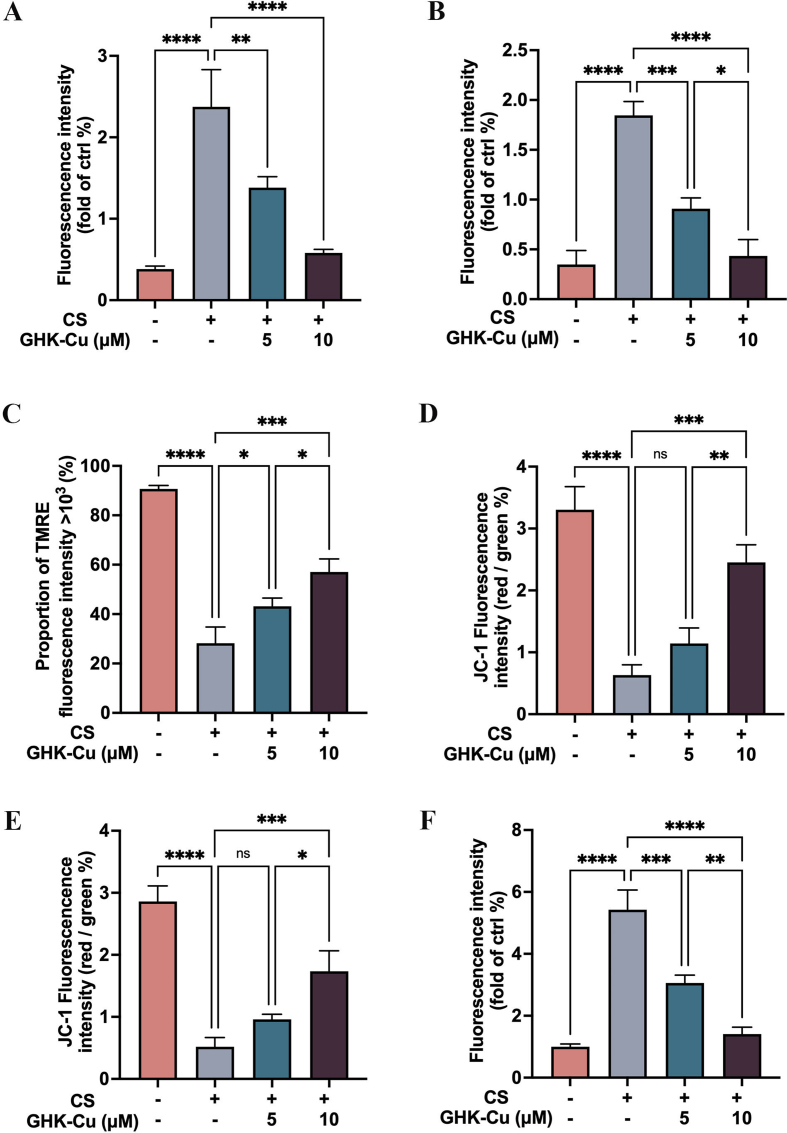
To facilitate the mechanistic investigation, we focused our attention on AM because these are the primary host defense cells that respond to CS phagocytosis and antigen presentation in silicosis. Thus, we established an in vitro CS culture system comprising RAW264.7 cells that could mimic the response of intrapulmonary macrophages to silicosis. After confirming the concentrations and times of administration of CS and GHK-Cu to cells, we observed that RAW264.7 cells and primary AMs produce more ROS due to an excessive phagocytosis of CS (50 μg/cm2), whereas GHK-Cu reversed this phenomenon (Fig. 3A and S3A-B). We detected the cell membrane puncta using flow cytometry and the TMRE assay showed that GHK-Cu reversed the impaired mitochondrial membrane potential (Fig. 3B and S3C). Consistent with the TERM assay results, the JC-1 staining results also showed that GHK-Cu could ameliorate the impaired mitochondrial membrane potential dose-dependently (Fig. 3C and S3D-E). We further examined the effect of GHK-Cu on the SOD and TEAC levels in RAW264.7 cells after CS exposure, and the results showed that GHK-Cu could rescue the SOD and TEAC levels (Fig. 3D and E). Moreover, we found that GHK-Cu could improve mitochondrial autophagy even further (Fig. 3F and S3F). Previous studies have also shown that the level of oxidative stress in AM influences the subsequent inflammatory cascade and promotes fibrosis development [6,25,26]. Based on our findings, we concluded that GHK-Cu exerted a direct regulatory effect on maintaining the homoeostasis of CS-injured AM through antioxidative stress pathways. RAW264.7 cell line is widely used in research related to silicosis and be considered as a reliable cell line to conduct macrophage-related experimental investigations. However, we have to be aware that using RAW264.7 cell line rather than primary alveolar macrophages is one of the limitations of this study because we did not validate all the experiments with primary AMs.
Fig. 3.
GHK-Cu attenuates the cellular injury and oxidative stress in CS-exposed macrophages: A. ROS levels in CS-exposed RAW264.7 cells and primary AMs, as well as after the treatment with different doses of GHK-Cu. B. Mitochondrial membrane potential in CS-exposed RAW264.7 cells, as well as after the treatment with different doses of GHK-Cu, was detected using flow cytometry via TRME staining. C. Mitochondrial membrane potential in CS-exposed RAW264.7 cells and primary AMs, as well as after the treatment with different doses of GHK-Cu, was detected using fluorescence confocal microscopy via JC-1 staining. D. SOD inhibition rate of RAW264.7 cells exposed to CS and treated with different doses of GHK-Cu. E. Antioxidant activity of RAW264.7 cells exposed to CS and treated with different doses of GHK-Cu. F. Detection of the level of mitophagy of RAW264.7 cells exposed to CS and treated with different doses of GHK-Cu according to the fluorescence results. ns: p ≥ 0.05, *p < 0.05, **p < 0.01, ****p < 0.0001.
3.4. GHK-Cu targets PRDX6 and protects against CS-exposed oxidative stress via PRDX6
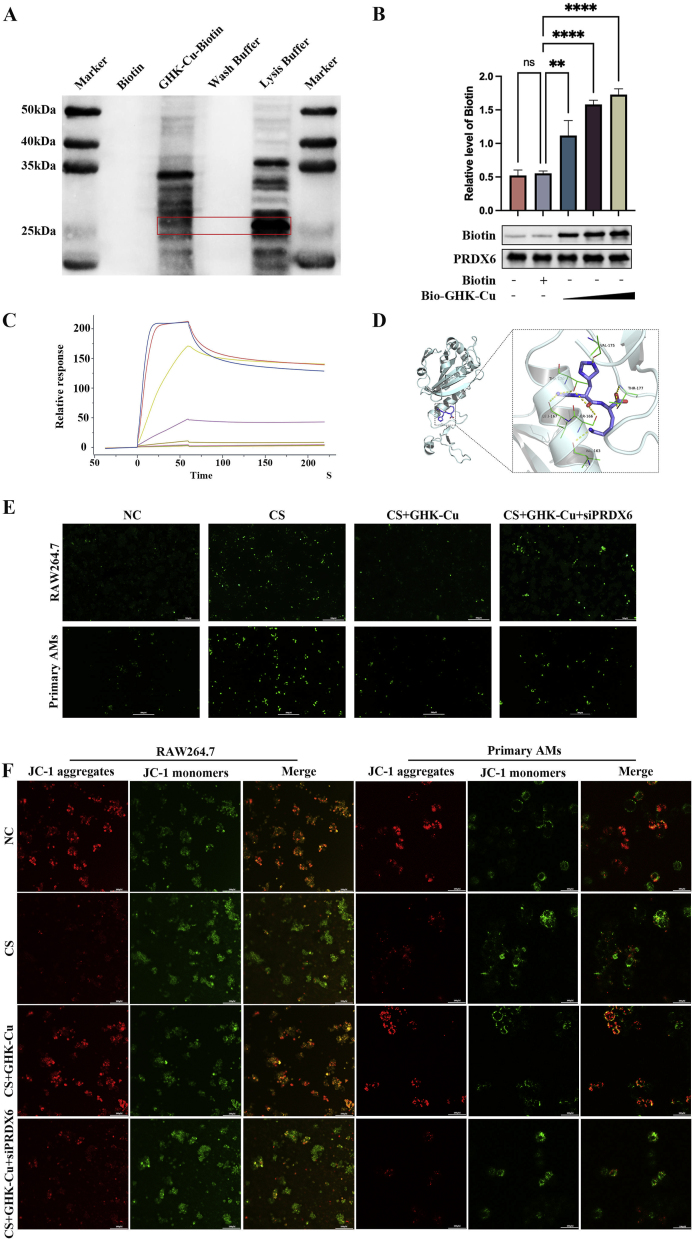
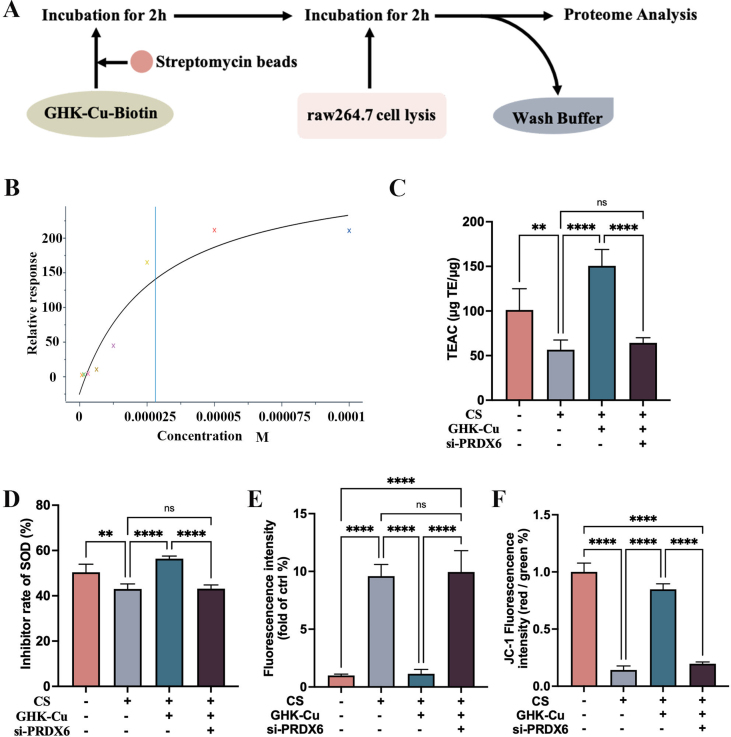
The promising outcomes of GHK-Cu in silicosis models indicate that it is worthy of further examination. To further reveal the molecular targets of GHK-Cu in modulating macrophage function, we used biotinylated GHK-Cu (bio-GHK-Cu) to unravel its targets in RAW264.7 cells. To provide direct evidence of the GHK-Cu target, we incubated the RAW264.7 cell lysate with bio-GHK-Cu and conducted proteome analysis by paying special attention to PRDX6, which is an important antioxidative stress molecule (Fig. S4A). Biotinylated immunoprecipitation, biotinylated pull-down and Western blot analyses demonstrated the binding between GHK-Cu and PRDX6 (Fig. 4A and B). We then used surface plasmon resonance (SPR) to measure the equilibrium dissociation constant between GHK-Cu and PRDX6 and found that the binding affinity (Kd) was 2.81 × 105 M (Fig. 4C and S4B). Spatial docking simulation was used to visualize the binding state between GHK-Cu and PRDX6 and showed that Thr177, which is important to the activity of c-PLA2, is one of the binding sites (Fig. 4D). Collectively, we inferred that GHK-Cu plays a covalent binding role in targeting the PRDX6 protein. To confirm that PRDX6 is a pathologic molecule for the direct GHK-Cu interaction, we silenced PRDX6 in RAW264.7 cells. This antagonized the effect of GHK-Cu in reducing the ROS levels produced by RAW264.7 cells and primary AMs after CS exposure (Fig. 4E and S4E). Then, we further showed that the protective effect of GHK-Cu on the mitochondrial membrane potential was also lost after silencing PRDX6, as per the JC-1 staining results (Fig. 4F and S4F). The results of the SOD inhibition rate and TEAC also show that the antioxidant capacity of GHK-Cu to rescue RAW264.7 cells was antagonized after silencing PRDX6 (Fig. S4C-D). Our results demonstrate that GHK-Cu indeed exerts antioxidant effects in macrophages by targeting PRDX6.
Fig. 4.
GHK-Cu targets PRDX6 and protection against CS-exposed oxidative stress via PRDX6: A. Pull-down and Western blot analysis of the interaction between biotin and biotin-GHK-Cu with the cell lysates of RAW264.7 cells treated with CS. B. Purified PRDX6 was incubated with different concentrations of Biotin–GHK-Cu for 2 h and then detected by SDS-PAGE. C. The SPR analysis showed a direct kinetic interaction between GHK-Cu and the purified PRDX6 protein. D. Ribbon representation of the binding of GHK-Cu to the dimer link position of PRDX6, with the inset image showing the structural interactions. E. ROS levels in CS-exposed RAW264.7 cells and primary AMs after the treatment with GHK-Cu and after silencing PRDX6. F. JC-1 staining of CS-exposed RAW264.7 cells and primary AMs after the treatment with GHK-Cu and after silencing PRDX6 was performed and detected using fluorescence confocal microscopy. ns: p ≥ 0.05, **p < 0.01, ****p < 0.0001.
Here, we demonstrated that GHK-Cu attenuates silica-induced pneumonitis and pulmonary fibrosis by targeting the binding to PRDX6. Thus, our results have rekindled our interest in the fact that the inhibition of oxidative stress-mediated signaling is essential for preventing silicosis. Alterations in the PRDX6 levels in rats with silica-induced silicosis have previously been reported, suggesting that PRDX6 may be involved in silicosis development [27]. Moreover, some studies have suggested that PRDX6 has an antioxidant role in certain lung diseases [[28], [29], [30], [31]]. However exactly how PRDX6 works in silicosis has not yet been reported in studies. Although some studies have shown that other immune cells such as lymphocytes, monocytes and centrocytes play a role in the fibrosis of silicosis [[32], [33], [34]], previous studies have been more explicit about the critical role of AMs in silicosis fibrosis. Based on accumulating evidence, the activation of AMs is the beginning step in a complicated inflammatory cascade and are central to fibrosis, both in terms of the histopathology of silicosis and in terms of pathogenesis [35]. Our results show that the binding of GHK-Cu to PRDX6 reduced macrophage ROS production and the oxidative stress levels, and might have prevented silicosis development. Therefore, GHK-Cu is a new effective drug candidate for silicosis treatment. Moreover, combination therapy using different classes of agents may be a promising therapeutic strategy to delay the progression of silicosis. In future, we will pay attention on exploring the functions of other immune cells in the process of fibrosis or silicosis.
4. Conclusion
Taken together, the present work reveals the novel pharmacological role of GHK-Cu of attenuating pneumonia and pulmonary fibrosis. We determined that the regulatory effect of GHK-Cu on PRDX6 is, at least, partially involved in the molecular mechanism underlying the treatment of silicosis. Our results, which show that GHK-Cu plays a protective role in preventing silicosis, suggest that a nebulized inhaler form of this compound could be developed and used for the prevention and adjunctive treatment of lung diseases in future clinical practice. Thus, our study demonstrates that GUK-Cu is a beneficial tripeptide for the adjunctive management of silicosis, providing a reference for the treatment of silicosis.
Funding
This research was supported by National High Level Hospital Clinical Research Funding (No. 2022-NHLHCRF-LX-01), National Natural Science Foundation of China (No. 82300053), China Postdoctoral Science Foundation (No. 2023M733987), the Non-profit Central Research Institute Fund of Chinese Academy of Medical Science (No. 2020-PT320-005).
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
CRediT authorship contribution statement
Yiding Bian: Writing – original draft, Visualization, Methodology, Data curation. Mingming Deng: Writing – original draft, Methodology, Data curation. Jia Liu: Methodology, Formal analysis, Data curation. Jiaye Li: Visualization, Methodology, Formal analysis. Qin Zhang: Methodology, Formal analysis. Zilin Wang: Methodology, Formal analysis, Data curation. Liwei Liao: Writing – review & editing. Jinrui Miao: Formal analysis, Data curation. Ruixia Li: Writing – review & editing. Xiaoming Zhou: Writing – review & editing, Resources, Project administration. Gang Hou: Writing – review & editing, Writing – original draft, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank our patients for their confidence in the research team.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2024.103237.
Contributor Information
Xiaoming Zhou, Email: zhouxmcmu@163.com.
Gang Hou, Email: hougangcmu@163.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
figs3.
figs4.
Data availability
Data will be made available on request.
References
- 1.Zhu Z., Li Q., Xu C., Zhao J., Li S., Wang Y., et al. Sodium tanshinone IIA sulfonate attenuates silica-induced pulmonary fibrosis in rats via activation of the Nrf2 and thioredoxin system. Environ. Toxicol. Pharmacol. 2020;80 doi: 10.1016/j.etap.2020.103461. [DOI] [PubMed] [Google Scholar]
- 2.Leung C.C., Yu I.T., Chen W. Silicosis. Lancet. 2012;379(9830):2008–2018. doi: 10.1016/S0140-6736(12)60235-9. [DOI] [PubMed] [Google Scholar]
- 3.Helal M.G., Said E. Carvedilol attenuates experimentally induced silicosis in rats via modulation of P-AKT/mTOR/TGFβ1 signaling. Int. Immunopharmacol. 2019;70:47–55. doi: 10.1016/j.intimp.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Wang M., Zhang Z., Liu J., Song M., Zhang T., Chen Y., et al. Gefitinib and fostamatinib target EGFR and SYK to attenuate silicosis: a multi-omics study with drug exploration. Signal Transduct. Target Ther. 2022;7(1):157. doi: 10.1038/s41392-022-00959-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox L.A., Jr. An exposure-response threshold for lung diseases and lung cancer caused by crystalline silica. Risk Anal. 2011;31(10):1543–1560. doi: 10.1111/j.1539-6924.2011.01610.x. [DOI] [PubMed] [Google Scholar]
- 6.Vanhée D., Gosset P., Boitelle A., Wallaert B., Tonnel A.B. Cytokines and cytokine network in silicosis and coal workers' pneumoconiosis. Eur. Respir. J. 1995;8(5):834–842. [PubMed] [Google Scholar]
- 7.Wei Y., You Y., Zhang J., Ban J., Min H., Li C., et al. Crystalline silica-induced macrophage pyroptosis interacting with mitophagy contributes to pulmonary fibrosis via modulating mitochondria homeostasis. J. Hazard Mater. 2023;454 doi: 10.1016/j.jhazmat.2023.131562. [DOI] [PubMed] [Google Scholar]
- 8.Tian Y., Shi H., Zhang D., Wang C., Zhao F., Li L., et al. Nebulized inhalation of LPAE-HDAC10 inhibits acetylation-mediated ROS/NF-κB pathway for silicosis treatment. J. Control Rel. 2023;364:618–631. doi: 10.1016/j.jconrel.2023.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Dostert C., Pétrilli V., Van Bruggen R., Steele C., Mossman B.T., Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320(5876):674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marrocco A., Ortiz L.A. Role of metabolic reprogramming in pro-inflammatory cytokine secretion from LPS or silica-activated macrophages. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.936167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weissman D.N. Progressive massive fibrosis: an overview of the recent literature. Pharmacol. Ther. 2022;240 doi: 10.1016/j.pharmthera.2022.108232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar A., Abdelmalak B., Inoue Y., Culver D.A. Pulmonary alveolar proteinosis in adults: pathophysiology and clinical approach. Lancet Respir. Med. 2018;6(7):554–565. doi: 10.1016/S2213-2600(18)30043-2. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H., Wang Y., He Z. Glycine-histidine-lysine (GHK) alleviates neuronal apoptosis due to intracerebral hemorrhage via the miR-339-5p/VEGFA pathway. Front. Neurosci. 2018;12:644. doi: 10.3389/fnins.2018.00644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pickart L., Margolina A. Regenerative and protective actions of the GHK-Cu peptide in the light of the new gene data. Int. J. Mol. Sci. 2018;19(7) doi: 10.3390/ijms19071987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klontzas M.E., Reakasame S., Silva R., Morais J.C.F., Vernardis S., MacFarlane R.J., et al. Oxidized alginate hydrogels with the GHK peptide enhance cord blood mesenchymal stem cell osteogenesis: a paradigm for metabolomics-based evaluation of biomaterial design. Acta Biomater. 2019;88:224–240. doi: 10.1016/j.actbio.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 16.Pickart L., Vasquez-Soltero J.M., Margolina A. GHK peptide as a natural modulator of multiple cellular pathways in skin regeneration. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/648108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pickart L., Vasquez-Soltero J.M., Margolina A. The effect of the human peptide GHK on gene expression relevant to nervous system function and cognitive decline. Brain Sci. 2017;7(2) doi: 10.3390/brainsci7020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pickart L. The human tri-peptide GHK and tissue remodeling. J. Biomater. Sci. Polym. Ed. 2008;19(8):969–988. doi: 10.1163/156856208784909435. [DOI] [PubMed] [Google Scholar]
- 19.Park J.R., Lee H., Kim S.I., Yang S.R. The tri-peptide GHK-Cu complex ameliorates lipopolysaccharide-induced acute lung injury in mice. Oncotarget. 2016;7(36):58405–58417. doi: 10.18632/oncotarget.11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X., Liu B., Xu Q., Sun H., Shi M., Wang D., et al. GHK-Cu-liposomes accelerate scald wound healing in mice by promoting cell proliferation and angiogenesis. Wound Repair Regen. 2017;25(2):270–278. doi: 10.1111/wrr.12520. [DOI] [PubMed] [Google Scholar]
- 21.Gruchlik A., Chodurek E., Dzierzewicz Z. Effect of GLY-HIS-LYS and its copper complex on TGF-β secretion in normal human dermal fibroblasts. Acta Pol. Pharm. 2014;71(6):954–958. [PubMed] [Google Scholar]
- 22.Ma W.H., Li M., Ma H.F., Li W., Liu L., Yin Y., et al. Protective effects of GHK-Cu in bleomycin-induced pulmonary fibrosis via anti-oxidative stress and anti-inflammation pathways. Life Sci. 2020;241 doi: 10.1016/j.lfs.2019.117139. [DOI] [PubMed] [Google Scholar]
- 23.Deng M., Zhang Q., Yan L., Bian Y., Li R., Gao J., et al. Glycyl-l-histidyl-l-lysine-Cu(2+) rescues cigarette smoking-induced skeletal muscle dysfunction via a sirtuin 1-dependent pathway. J. Cachexia SarcopeN. Musc. 2023;14(3):1365–1380. doi: 10.1002/jcsm.13213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Q., Liu J., Deng M.M., Tong R., Hou G. Relief of ovalbumin-induced airway remodeling by the glycyl-l-histidyl-l-lysine-Cu(2+) tripeptide complex via activation of SIRT1 in airway epithelial cells. Biomed. Pharmacother. 2023;164 doi: 10.1016/j.biopha.2023.114936. [DOI] [PubMed] [Google Scholar]
- 25.Ou L., Zhang P., Huang Z., Cheng Y., Miao Q., Niu R., et al. Targeting STING-mediated pro-inflammatory and pro-fibrotic effects of alveolar macrophages and fibroblasts blunts silicosis caused by silica particles. J. Hazard Mater. 2023;458 doi: 10.1016/j.jhazmat.2023.131907. [DOI] [PubMed] [Google Scholar]
- 26.Benmerzoug S., Rose S., Bounab B., Gosset D., Duneau L., Chenuet P., et al. STING-dependent sensing of self-DNA drives silica-induced lung inflammation. Nat. Commun. 2018;9(1):5226. doi: 10.1038/s41467-018-07425-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu N., Xue L., Guan Y., Li Q.Z., Cao F.Y., Pang S.L., et al. Expression of peroxiredoxins and pulmonary surfactant protein A induced by silica in rat lung tissue. Biomed. Environ. Sci. 2016;29(8):584–588. doi: 10.3967/bes2016.077. [DOI] [PubMed] [Google Scholar]
- 28.Park M.H., Yun H.M., Hwang C.J., Park S.I., Han S.B., Hwang D.Y., et al. Presenilin mutation suppresses lung tumorigenesis via inhibition of peroxiredoxin 6 activity and expression. Theranostics. 2017;7(15):3624–3637. doi: 10.7150/thno.21408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benipal B., Feinstein S.I., Chatterjee S., Dodia C., Fisher A.B. Inhibition of the phospholipase A2 activity of peroxiredoxin 6 prevents lung damage with exposure to hyperoxia. Redox Biol. 2015;4:321–327. doi: 10.1016/j.redox.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang D., Song Y., Wang X., Sun J., Ben Y., An X., et al. Deletion of peroxiredoxin 6 potentiates lipopolysaccharide-induced acute lung injury in mice. Crit. Care Med. 2011;39(4):756–764. doi: 10.1097/CCM.0b013e318206befd. [DOI] [PubMed] [Google Scholar]
- 31.Cheng C., Liu K., Shen F., Zhang J., Xie Y., Li S., et al. Astragaloside IV targets PRDX6, inhibits the activation of RAC subunit in NADPH oxidase 2 for oxidative damage. Phytomedicine. 2023;114 doi: 10.1016/j.phymed.2023.154795. [DOI] [PubMed] [Google Scholar]
- 32.Zhao Y., Hao C., Li M., Qu Y., Guo Y., Deng X., et al. PD-1/PD-L1 inhibitor ameliorates silica-induced pulmonary fibrosis by maintaining systemic immune homeostasis. Biomed. Pharmacother. 2022;148 doi: 10.1016/j.biopha.2022.112768. [DOI] [PubMed] [Google Scholar]
- 33.Li C., He Y.Y., Zhang Y.T., You Y.C., Yuan H.Y., Wei Y.G., et al. Tauroursodeoxycholic acid (TUDCA) disparate pharmacological effects to lung tissue-resident memory T cells contribute to alleviated silicosis. Biomed. Pharmacother. 2022;151 doi: 10.1016/j.biopha.2022.113173. [DOI] [PubMed] [Google Scholar]
- 34.Cheng D., Lian W., Wang T., Xi S., Jia X., Li Z., et al. The interplay of Cxcl10(+)/Mmp14(+) monocytes and Ccl3(+) neutrophils proactively mediates silica-induced pulmonary fibrosis. J. Hazard Mater. 2024;467 doi: 10.1016/j.jhazmat.2024.133713. [DOI] [PubMed] [Google Scholar]
- 35.Hoy R.F., Chambers D.C. Silica-related diseases in the modern world. Allergy. 2020;75(11):2805–2817. doi: 10.1111/all.14202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.