Abstract
Although durvalumab plus tremelimumab (Dur/Tre) has been approved as first-line therapy for patients with unresectable hepatocellular carcinoma (u-HCC), its outcomes in real-world clinical practice are unclear. The present study aimed to evaluate the efficacy and safety of Dur/Tre treatment. This multicenter study was conducted between March 2023 and January 2024, and included 120 patients with u-HCC treated with Dur/Tre. Among the patients, 44 had no history of systemic treatment. Progression-free survival (PFS), therapeutic response and adverse events (AEs) were assessed. The objective response rate (ORR) and disease control rates (DCR) were 15.8 and 53.3%, respectively. The median PFS was 3.9 months. The incidence rates of AEs of any grade and those grade 3 or higher were 83.3 and 36.7%, respectively. Liver injury was the most frequent AE of any grade and grade 3 or higher. Although there was no significant difference in ORR and PFS between the first and later line groups (ORR 15.8 vs. 15.7%, P=0.986; PFS 4.5 vs. 3.6 months, P=0.213), there was a significant difference in DCR between the two groups (65.8 vs. 45.9%, P=0.034). No significant differences were noted between the first- and later-line treatment groups regarding the incidence rate of AEs. Decision tree analysis revealed that poor liver function and advanced age were significant variables for discontinuation owing to AEs. In conclusion, Dur/Tre as first-line therapy had better disease control responses compared with later-line therapy; however, this regimen should be carefully administered to patients with deteriorating hepatic function or advanced age.
Keywords: Dur/Tre, HCC, STRIDE, durvalumab, tremelimumab, combination therapy
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related deaths and has become a global health issue, with an increasing incidence worldwide (1,2), as HCC is often diagnosed at an advanced stage (3). Although various systemic therapies such as molecular-targeted agents have been developed for patients with unresectable HCC (u-HCC), few patients achieve durable benefits, and long-term survival rates remain unsatisfactory (4). Recently, immune checkpoint inhibitors (ICIs) have revolutionized the treatment strategy for u-HCC, and remarkable changes have occurred in systemic treatment settings (5,6). After the combination of atezolizumab and bevacizumab (Atez/Bev) was established for u-HCC patients, durvalumab plus tremelimumab (Dur/Tre) was recently approved as a first-line therapy for dual ICIs (7).
The Single Tremelimumab Regular Interval Durvalumab (STRIDE) regimen is a combination immunotherapy with tremelimumab, an anti-cytotoxic T-lymphocyte-associated antigen 4 inhibitor, and durvalumab, an anti-programmed cell death ligand-1 inhibitor (5). Based on the findings of the HIMALAYA phase 3 clinical trial, this first immunotherapy combination showed non-inferior progression-free survival (PFS) but improved overall survival (OS) compared to sorafenib (7) for patients with u-HCC. Following the results of this clinical trial, the STRIDE regimen has become the preferred first-line treatment along with Atez/Bev for u-HCC (8). Compared with other clinical trials for u-HCC, the STRIDE regimen is characterized by a longer observation period, where favorable results have been reported, with a 3-year survival rate of 30.7%. Furthermore, When compared to patients treated with sorafenib, patients treated with the STRIDE regimen had a lower relative risk of reduced quality of life (9). Several phase 3 trials have also reported the efficacy and safety of the STRIDE regimen in other cancers (10,11). However, ICIs largely affect all internal organs, and their use can lead to immune-related adverse events (irAEs) that require treatment with immunosuppressive medications in moderate to severe cases (12,13). In fact, the rate of cases with irAE requiring high-dose steroids (≥ prednisolone 40 mg/day) was 20.1% in the HIMALAYA phase 3 clinical trial. However, the efficacy and safety of Dur/Tre for u-HCC in real-world clinical practice remains unknown.
This study aimed to evaluate the overall therapeutic outcomes and safety with the initial use of Dur/Tre for u-HCC treatment.
Patients and methods
Study design and patients
The present retrospective study evaluated 139 patients who were treated with Dur/Tre between March 2023 and January 2024 at 16 Japanese institutions: Kurume University Hospital (Kurume, Japan), Yamaguchi University Hospital (Yamaguchi, Japan), Tokushima University Hospital (Tokushima, Japan), Nagoya University Hospital (Nagoya, Japan), Kagawa University Hospital (Kagawa, Japan), Okayama University Hospital (Okayama, Japan), Japanese Red Cross Nagoya Daiichi Hospital (Nagoya, Japan), Toyohashi Municipal Hospital (Aichi, Japan), Anjo Kosei Hospital (Anjo, Japan), National Hospital Organization Takasaki General Medical Center (Gunma, Japan), Gunma Saiseikai Maebashi Hospital (Gunma, Japan), Kawasaki Medical School (Okayama, Japan), Fukuyama City Hospital (Okayama, Japan), Fukuyama Medical Center (Okayama, Japan), Iwamoto Internal Medical Clinic (Kitakyushu, Japan), and Kurume Central Hospital (Kurume, Japan). The following eligibility criteria were used: i) age >18 years, ii) Eastern Cooperative Oncology Group performance status (PS) <3, and iii) available clinical data and followed-up until study cessation (January 2024) or death. The exclusion criteria were: i) Child-Pugh class C and ii) a history of autoimmune disease, and iii) Barcelona Clinic Liver Cancer stage 0 or A. Therefore, 19 patients were excluded from the study. In total, 120 patients were enrolled in this study (Fig. S1). This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Kurume University School of Medicine (approval code: 23153), and an implementation permit was obtained from each of the 15 other institutions. An opt-out approach was used to obtain informed consent from patients. The cutoff date for this analysis was January 2024.
Durvalumab plus tremelimumab treatment and safety evaluation
The patients were administered tremelimumab for one dose of 300 mg intravenously and durvalumab at a dose of 1,500 mg once every 4 weeks. The treatment was continued until unacceptable AEs or progressive disease occurred. AEs were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0).
Assessment of hepatic reserve function
Liver function was evaluated using Child-Pugh class (14) and albumin-bilirubin (ALBI) scores (15). ALBI score was calculated based on serum albumin and total bilirubin levels: ALBI score=[log10 bilirubin (µmol/l) ×0.66] + [albumin (g/l) × −0.085], and was graded as follows: ≤-2.60=ALBI grade 1; >-2.60 to ≤-1.39=ALBI grade 2; >-1.39=ALBI grade 3. Based on the ALBI score, we also assessed liver function using the modified ALBI grade (mALBI grade) (16). We evaluated the change in the ALBI score from baseline at 4, 8, and 12 weeks.
Assessment of therapeutic response
The therapeutic response of HCC was assessed at the time of best response using dynamic computed tomography or magnetic resonance imaging every 4–8 weeks based on the Response Evaluation Criteria In Solid Tumors version 1.1 (RECIST v1.1) (17).
Statistical analysis
All categorical variables are presented as numbers or medians (ranges). Progression-free survival (PFS) was calculated using the Kaplan-Meier method and analyzed using the log-rank test. Continuous variables were compared using a one-way analysis of variance with Scheffe's post-hoc test, and categorical variables were compared between groups using χ2 test or Fisher's exact analysis. We also performed a decision tree analysis to identify factors associated with discontinuation owing to AEs. Statistical significance was defined as a two-tailed P-value of <0.05. All statistical analyses were performed using JMP Pro® version 15 (SAS Institute Inc.).
Results
Clinical characteristics
Patient characteristics are presented in Table I. The median age was 73 years, 96 patients (80.0%) were men, and 100 patients (83.3%) had a performance status of 0. Child-Pugh classes A and B were observed in 110 (91.7%) and 10 (8.4%) patients, respectively. The median ALBI score was −2.27, and m-ALBI grade 1 was observed in 29 patients (24.1%). Barcelona Clinic Liver Cancer stage B was observed in 67 patients (55.8%). Macrovascular invasion and extrahepatic spread were observed in 22 (18.3%) and 36 (30.0 %) patients, respectively. Dur/Tre treatment was introduced as the 1st-line, 2nd, 3rd, 4th, 5th, 6th, and 7th treatment in 44 (36.6%), 36 (30.0%), 18 (15.0%), 11 (9.2%), 5 (4.2%), 5 (4.2%), and 1 (0.8 %) patients, respectively. The median follow-up was 5.6 (1.0–9.0) months.
Table I.
Patient characteristics.
| Characteristic | Value |
|---|---|
| N | 120 |
| Median age, years (range) | 73 (42–92) |
| Sex, female/male | 24/96 |
| PS, 0/1/2 | 100/18/2 |
| Median body mass index, kg/m2 (range) | 23.3 (15.9–38.8) |
| Cause of HCC, HBV/HCV/Non-B,C | 23/46/51 |
| Child-Pugh class/score | |
| A (5/6) | 110 (54/56) |
| B (7/8) | 10 (7/3) |
| Median AST, U/l (range) | 34 (10–274) |
| Median ALT, U/l (range) | 24 (4–208) |
| Median serum albumin, g/dl (range) | 3.6 (2.4–4.6) |
| Median total bilirubin, mg/dl (range) | 0.8 (0.3–2.0) |
| Median ALBI score (range) | −2.27 |
| (−3.19 to −1.04) | |
| mALBI grade, 1/2a/2b/3 | 29/33/57/1 |
| BCLC stage, B/C | 67/53 |
| Macrovascular invasion, yes/no | 22/98 |
| Extrahepatic spread, yes/no | 36/84 |
| Median AFP, ng/ml (range) | 56.5 |
| (1.6–2580,000.0) | |
| Treatment line | |
| First-line | 44 |
| Later-line (2nd/3rd/4th/5th/6th/7th) | 76 |
| (36/18/11/5/5/1) | |
| Pre-ICI treatment, yes/no | 67/53 |
| HIMALAYA trial exclusion criteria, yes/no | 82/38 |
| Later-line | 65 |
| Later-line + Vp 4 | 3 |
| Later-line + Child-Pugh class B | 6 |
| Later-line + Increased AST or ALT | 2 |
| Child-Pugh class B | 4 |
| Increased AST or ALT | 2 |
| Median follow-up duration, months (range) | 5.6 (1.0–9.0) |
PS, performance status; HBV, hepatitis B virus; HCV, hepatitis C virus; AST, aspartate aminotransferase; ALT, alanine aminotransferase; mALBI, modified albumin-bilirubin; BCLC, Barcelona Clinic Liver Cancer; AFP, α-fetoprotein; ICI, immune checkpoint inhibitor.
Clinical therapeutic outcomes of durvalumab plus tremelimumab
The therapeutic responses are presented in Fig. 1A. Complete response (CR) was observed in three patients (2.5%), and partial response (PR) was observed in 16 patients (13.3%). The objective response rate (ORR) was 15.8%. Stable disease (SD) was observed in 45 patients (37.5%), and progressive disease (PD) was observed in 56 patients (46.7%). The overall disease control rate (DCR) was 53.3%. The median overall PFS was 3.9 months (Fig. 2A). There were no cases of pseudoprogression.
Figure 1.
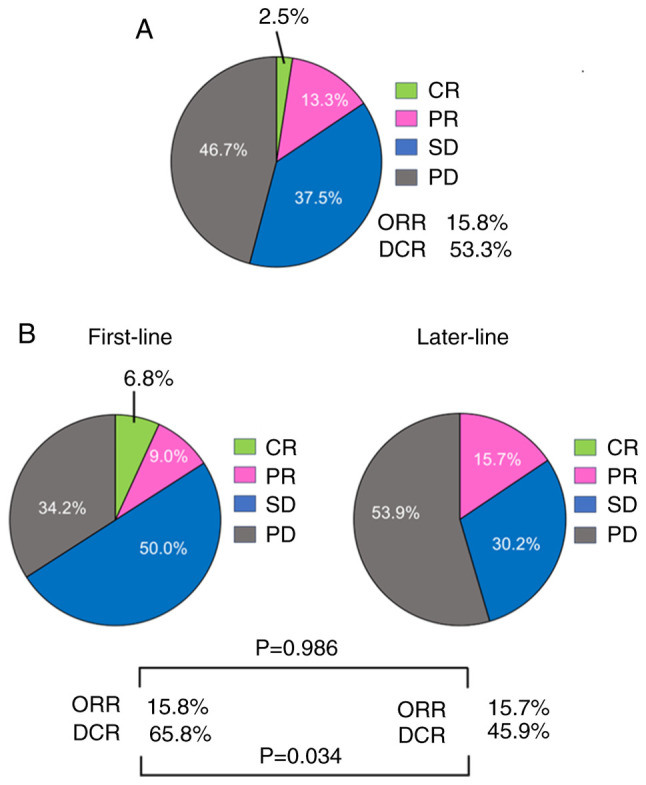
Therapeutic response to Dur/Tre using RECIST criteria. (A) Therapeutic response to Dur/Tre. (B) Therapeutic response to Dur/Tre in the first-line and later-line groups. Dur/Tre, durvalumab plus tremelimumab; RECIST, Response Evaluation Criteria In Solid Tumors; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; DCR, disease control rate.
Figure 2.
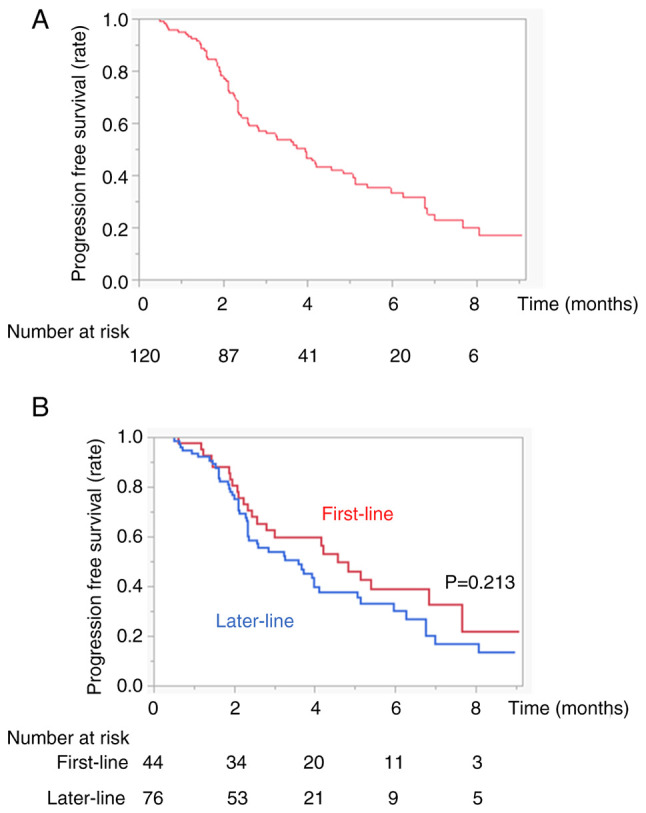
PFS in patients with HCC treated with Dur/Tre. (A) Kaplan-Meier curves for PFS of all patients. (B) Kaplan-Meier curves for PFS of the first-line and later-line groups. The red and blue lines indicate the first-line and later-line groups, respectively. PFS, progression-free survival; HCC, hepatocellular carcinoma Dur/Tre, durvalumab plus tremelimumab.
Clinical safety
The AEs that occurred during Dur/Tre treatment are shown in Table II. The overall incidence rates of any grade and grade 3 or higher were 83.3 and 36.7%, respectively. Among them, 61 patients (50.8%) had increased aspartate aminotransferase (AST) levels, 45 (37.5%) had increased alanine aminotransferase (ALT) levels, 45 patients (37.5%) experienced rash, 27 (22.5%) experienced fever, 22 (18.3%) had diarrhea, 6 (5.0%) had pituitary or adrenal insufficiency and drug-induced pneumonia, 26 (21.6%) had fatigue, and 29 (24.1%) experienced decreased appetite. Grade 3 or higher AEs included elevated AST levels (13.3%), elevated ALT levels (10.8%), diarrhea (10.0%), and rash (8.3%). High-dose steroids were administered to 27 patients (22.5%).
Table II.
Adverse events associated with Dur/Tre (n=120).
| Adverse event | Any, n (%) | Grade 3 or higher, n (%) |
|---|---|---|
| Total adverse events | 100 (83.3) | 44 (36.7) |
| AST evaluation | 61 (50.8) | 16 (13.3) |
| ALT evaluation | 45 (37.5) | 13 (10.8) |
| Rash | 45 (37.5) | 10 (8.3) |
| Fever | 27 (22.5) | 1 (0.8) |
| Diarrhea | 22 (18.3) | 12 (10.0) |
| Abdominal pain | 8 (6.7) | 2 (1.6) |
| Pituitary or adrenal insufficiency | 6 (5.0) | 6 (5.0) |
| Drug-induced pneumonia | 6 (5.0) | 4 (3.3) |
| Pancreatitis | 3 (2.4) | 2 (1.6) |
| Infusion reaction | 4 (3.3) | 1 (0.8) |
| Other irAEs | 8 (6.7) | 4 (3.3) |
| Fatigue | 26 (21.6) | 5 (4.1) |
| Decreased appetite | 29 (24.1) | 1 (0.8) |
| Factors | ||
| Requiring high-dose steroid | 27 (22.5) |
Dur/Tre, durvalumab plus tremelimumab; AST, aspartate aminotransferase; ALT, alanine aminotransferase; irAEs, immune-related adverse events.
Comparison of therapeutic outcomes and safety according to first or later lines of durvalumab plus tremelimumab
The therapeutic responses to the first or later lines are shown in Fig. 1B. Although there was no significant difference in the ORR between the first- or later-line treatment groups (ORR, 15.8 vs. 15.7%, P=0.986), there was a significant difference in the DCR between the two groups (65.8 vs. 45.9%, P=0.034). There was no significant difference in PFS between the two groups (PFS 4.5 vs. 3.6 months, P=0.213) (Fig. 2B). Differences in treatment-related AEs between the first- or later-line treatments are shown in Table III. There was no significant difference in the frequency of AEs between the first- and later-line treatment groups at any grade or grade 3 or higher (Table III).
Table III.
The difference in adverse events associated with first-line and later-line groups.
| Characteristic | First-line group | Later-line group | P-value |
|---|---|---|---|
| n | 44 | 76 | |
| Total | |||
| Any grade | 37 (84.1%) | 63 (82.8%) | 0.865 |
| Grade ≥3 | 15 (34.0%) | 29 (38.1%) | 0.655 |
| AST evaluation | |||
| Any grade | 22 (50.0%) | 39 (51.3%) | 0.889 |
| Grade ≥3 | 5 (11.3%) | 11 (14.4%) | 0.625 |
| ALT evaluation | |||
| Any grade | 14 (31.8%) | 31 (40.7%) | 0.325 |
| Grade ≥3 | 4 (9.1%) | 9 (11.8%) | 0.635 |
| Rash | |||
| Any grade | 17 (38.6%) | 28 (36.8%) | 0.619 |
| Grade ≥3 | 5 (11.3%) | 5 (6.5%) | 0.368 |
| Fever | |||
| Any grade | 11 (25.0%) | 16 (21.0%) | 0.521 |
| Grade ≥3 | 0 (0.0%) | 1 (1.3%) | 0.337 |
| Diarrhea | |||
| Any grade | 12 (27.2%) | 10 (13.1%) | 0.058 |
| Grade ≥3 | 7 (15.9%) | 5 (6.6%) | 0.107 |
| Abdominal pain | |||
| Any grade | 2 (4.5%) | 6 (7.8%) | 0.466 |
| Grade ≥3 | 1 (2.2%) | 1 (1.3%) | 0.698 |
| Pituitary or adrenal insufficiency | |||
| Any grade | 3 (6.8%) | 3 (3.9%) | 0.486 |
| Grade ≥3 | 3 (6.8%) | 3 (3.9%) | 0.486 |
| Drug-induced pneumonia | |||
| Any grade | 2 (4.5%) | 4 (5.2%) | 0.862 |
| Grade ≥3 | 1 (2.2%) | 3 (3.9%) | 0.612 |
| Pancreatitis | |||
| Any grade | 2 (4.5%) | 1 (1.3%) | 0.274 |
| Grade ≥3 | 1 (2.2%) | 1 (1.3%) | 0.693 |
| Infusion reaction | |||
| Any grade | 2 (4.5%) | 2 (2.6%) | 0.580 |
| Grade ≥3 | 1 (2.2%) | 0 (0.0%) | 0.186 |
| Other irAEs | |||
| Any grade | 4 (4.5%) | 4 (2.6%) | 0.623 |
| Grade ≥3 | 2 (4.5%) | 2 (2.6%) | 0.580 |
| Fatigue | |||
| Any grade | 9 (20.4%) | 17 (22.3%) | 0.805 |
| Grade ≥3 | 1 (2.2%) | 4 (5.2%) | 0.402 |
| Decreased appetite | |||
| Any grade | 10 (22.7%) | 19 (25.0%) | 0.779 |
| Grade ≥3 | 0 (0.0%) | 1 (1.3%) | 0.429 |
| High-dose steroid | 12 (27.2%) | 15 (19.8%) | 0.340 |
AST, aspartate aminotransferase; ALT, alanine aminotransferase; irAEs, immune-related adverse events.
Analyses of changes in ALBI score using durvalumab plus tremelimumab
Fig. S2 shows the changes in ALBI scores at 4, 8, and 12 weeks from baseline after Dur/Tre treatment. The median ALBI scores at baseline, 4, 8, and 12 weeks after introducing Dur/Tre were −2.28, −2.10, −2.14, and −2.11, respectively. Although the deterioration of ALBI score was significant at 4 weeks compared to baseline (−2.28 vs. −2.10, P= 0.012), there were no significant differences at 8 weeks compared to baseline (−2.28 vs. −2.14, P=0.056). No deterioration in the ALBI score was observed during subsequent treatment.
Decision-tree analysis for discontinuation of durvalumab plus tremelimumab due to AEs
In this study, discontinuation due to AEs in all subjects was 31.8% at the time of study discontinuation (Fig. 3). The Child-Pugh score was identified as the first splitting variable for the rate of discontinuation owing to AEs. Although the discontinuation rate due to AEs was only 27.9% in patients in Child-Pugh class A, it was 63.2% in patients in Child-Pugh class B. The second splitting variable was age in Child-Pugh class A patients. In patients with Child-Pugh class A aged <70 years, the discontinuation rate due to AEs was only 16.5%.
Figure 3.
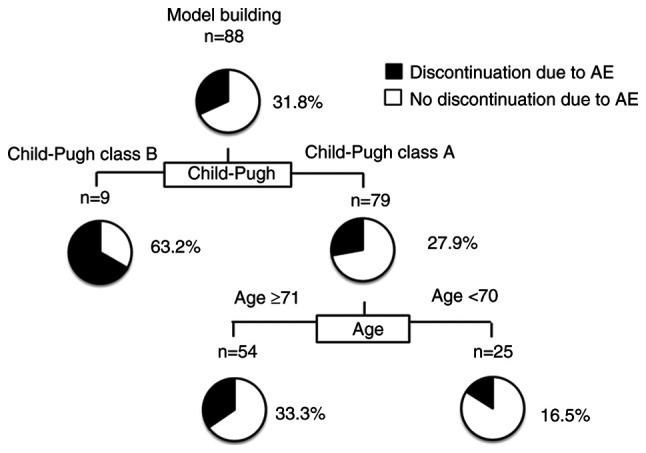
Profiles associated with discontinuation due to AEs in patients with HCC treated with Dur/Tre. Decision-tree algorithm for discontinuation due to AEs. The pie graphs indicate the percentage of no discontinuation due to AEs (white)/ discontinuation due to AEs (black) in each group. HCC, hepatocellular carcinoma; Dur/Tre, durvalumab plus tremelimumab.
Comparison of safety according to Age or Child-Pugh classification
Differences in treatment-related AEs between the age <70 and age ≥70 groups are shown in Table IV. There was no significant difference in each frequency of AEs between age <70 and age ≥70 patients. Also, there was no significant difference in each frequency of AEs between Child-Pugh A and Child-Pugh B (Table V).
Table IV.
Differences in AEs associated with age.
| AE | Age <70 | Age ≥70 | P-value |
|---|---|---|---|
| n | 38 | 82 | |
| Total | |||
| Any grade | 32 (84.2%) | 68 (82.9%) | 0.860 |
| Grade ≥3 | 17 (44.7%) | 27 (32.9%) | 0.211 |
| AST evaluation | |||
| Any grade | 20 (52.6%) | 41 (50.0%) | 0.788 |
| Grade ≥3 | 7 (18.4%) | 9 (10.9%) | 0.264 |
| ALT evaluation | |||
| Any grade | 16 (42.1%) | 29 (35.3%) | 0.478 |
| Grade ≥3 | 5 (13.1%) | 8 (9.7%) | 0.577 |
| Rash | |||
| Any grade | 14 (36.8%) | 31 (37.8%) | 0.919 |
| Grade ≥3 | 4 (10.5%) | 6 (7.3%) | 0.554 |
| Fever | |||
| Any grade | 10 (26.3%) | 17 (20.7%) | 0.495 |
| Grade ≥3 | 1 (2.6%) | 0 (0.0%) | 0.127 |
| Diarrhea | |||
| Any grade | 8 (21.1%) | 14 (17.0%) | 0.600 |
| Grade ≥3 | 7 (18.4%) | 5 (6.1%) | 0.059 |
| Abdominal pain | |||
| Any grade | 3 (7.8%) | 5 (6.1%) | 0.717 |
| Grade ≥3 | 1 (2.6%) | 1 (1.2%) | 0.574 |
| Pituitary or adrenal insufficiency | |||
| Any grade | 3 (7.8%) | 3 (3.6%) | 0.321 |
| Grade ≥3 | 3 (7.8%) | 3 (3.6%) | 0.321 |
| Drug-induced pneumonia | |||
| Any grade | 0 (0.0%) | 6 (7.3%) | 0.087 |
| Grade ≥3 | 0 (0.0%) | 4 (4.8%) | 0.161 |
| Pancreatitis | |||
| Any grade | 2 (5.2%) | 1 (1.2%) | 0.186 |
| Grade ≥3 | 1 (2.6%) | 1 (1.2%) | 0.574 |
| Infusion reaction | |||
| Any grade | 3 (7.8%) | 1 (1.2%) | 0.070 |
| Grade ≥3 | 1 (2.6%) | 0 (0.0%) | 0.140 |
| Other irAEs | |||
| Any grade | 3 (7.8%) | 5 (6.1%) | 0.717 |
| Grade ≥3 | 0 (0.0%) | 4 (4.8%) | 0.161 |
| Fatigue | |||
| Any grade | 9 (23.6%) | 17 (20.7%) | 0.715 |
| Grade ≥3 | 2 (5.2%) | 3 (3.6%) | 0.682 |
| Decreased appetite | |||
| Any grade | 10 (26.3%) | 19 (22.3%) | 0.709 |
| Grade ≥3 | 1 (2.6%) | 0 (0.0%) | 0.140 |
| High-dose steroid | 12 (31.5%) | 15 (18.2%) | 0.117 |
AE, adverse event; AST, aspartate aminotransferase; ALT, alanine aminotransferase; irAEs, immune-related adverse events.
Table V.
Differences in AEs associated with Child-Pugh classification.
| AE | Child-Pugh A (n=110) | Child-Pugh B (n=10) | P-value |
|---|---|---|---|
| Total | |||
| Any grade | 92 (83.6%) | 8 (80.0%) | 0.767 |
| Grade ≥3 | 39 (35.4%) | 5 (50.0%) | 0.360 |
| High-dose steroid | 23 (20.9%) | 4 (40.0%) | 0.166 |
AE, adverse event.
Discussion
This multicenter study showed that Dur/Tre therapy was effective and safe for patients with u-HCC under real-world conditions. Patients who received durvalumab plus tremelimumab as 1st-line therapy had better disease control responses than those who received durvalumab plus tremelimumab as later lines. However, the STRIDE regimen should be carefully administered to patients with deteriorating hepatic function or advanced age.
The ORR, DCR, and PFS in the HIMALAYA study were 20.1%, 60.1%, and 3.78 months, respectively. Under real-world conditions in this study, the ORR, DCR, and PFS were 15.0%, 53.3%, and 3.7 months, respectively, similar to those observed in the HIMALAYA study (7). In this study, although 38.4% of patients received Dur/Tre treatment as first-line therapy, Dur/Tre treatment presented promising results. However, Dur/Tre treatment as first-line therapy had a better DCR than later-line therapy. A previous study reported that patients who received immunotherapy as first-line therapy had better clinical outcomes than those who received immunotherapy as a later-line therapy (18). Also, all patients in this study who received late-line therapy received anti-VEGF therapy including Atez/Bev during previous systemic treatment. Yang et al previously reported that discontinuation of anti-VEGF therapy promoted metastasis through a liver revascularization mechanism (19). Therefore, we cannot deny the possibility that these factors influenced the efficacy of patients with later-line therapy. However, it has been reported that patients who achieved DCR during Dur/Tre treatment had longer median survival (20). Thus, achieving DCR during immunotherapy is important for longer survival (21). Although there was no significant difference in PFS between the first-line and later-line therapies in this study, this result may change if the observation period is extended. Therefore, in the future, it will be necessary to establish biomarkers related to treatment responses in patients receiving Dur/Tre treatment.
Several phase 3 trials have also reported the efficacy and safety of the STRIDE regimen in other cancers (10,11). Currently, although patients with higher levels of tumor PD-L1 expression received a clinical benefit from anti-PD-L1 therapy, it has become considered that patients with a low PD-L1 expression or PD-L1 negative are resistant to anti-PD-L1 therapy (22). Thus, new therapeutic strategies with immunotherapy combinations are needed for these patients. Tremelimumab enhances the binding of CD80 and CD86 to CD28, and it causes diversified T-cell responses and leads to increased tumor infiltration (23,24). Given their mechanisms, the addition of tremelimumab to a durvalumab-based regimen is expected to overcome resistance to PD-L1 therapy. In this study, although even with the high prevalence of later-line, Dur/Tre therapy was effective and safe for patients with u-HCC, we should compare to durvalumab alone therapy to build a more robust of efficacy of Dur/Tre therapy in real-world practice.
In this study, liver injury was the most common AE, and irAEs caused by Dur/Tre treatment were more common than in the clinical trial results. The higher rate of liver function deterioration and later-line therapy included in this study compared with clinical trials might have increased the frequency of liver injury and other AEs. Deterioration of liver function has been reportedly associated with a higher prevalence of AEs (25). Moreover, patients with prior irAEs due to ICIs are considered at risk of irAEs, even with a different ICI (26,27). In fact, 88% (67/76) of patients who received late-line therapy received Atez/Bev therapy during previous systemic treatment. In immunotherapy, liver injury is thought to be caused by the infiltration of activated T cells (28,29). Most HCCs also develop due to chronic liver disease or cirrhosis (30), making them susceptible to liver injury. These factors may explain the high rate of AEs, including liver damage. However, regarding treatment safety with Dur/Tre, there were no significant differences in AEs, even for the later-line cases, compared with first-line therapy. Thus, a detailed analysis of the AEs that occur with Dur/Tre treatment requires data accumulation and a longer observation period.
Currently, sequential therapy involving switching across systemic therapies is the primary evidence-based treatment strategy for u-HCC patients (31). Preserved hepatic function is a significant independent factor for sequential therapy in HCC (32,33). Furthermore, discontinuation due to AEs should be avoided during sequential therapy (34). Discontinuation due to AEs hampers the uptake of subsequent lines of systemic treatment and is associated with poorer prognosis (35). In this study, we found that Child-Pugh Class was the initial splitting variable for discontinuation due to AEs in patients with HCC treated with Dur/Tre, followed by age in the decision tree analysis. Additionally, there was no significant difference in the frequency of AEs between Child-Pugh A and Child-Pugh B or age <70 and age ≥70 patients, it results show that Child-Pugh and age are important factors related to discontinuation due to AEs. Particularly, it has been reported that advanced age is an independent factor that causes the discontinuation of AEs in systemic therapy (36). One reason for this may be that older patients are more vulnerable to the toxicity of anticancer drugs (37). Therefore, advanced age may be an important factor associated with treatment discontinuation due to AEs. However, discontinuation due to AEs in Dur/Tre treatment remains unclear. Therefore, Clinicians should be vigilant in monitoring AEs during treatment with Dur/Tre. Further studies are needed to establish a grading system, to predict discontinuation due to AEs.
This study had several limitations. First, this was a retrospective study. Second, although this was a multicenter study, the treatment observation period was relatively short and could not evaluate OS. Despite its limitations, to our knowledge, this study is the first to evaluate the efficacy and safety of Dur/Tre for the treatment of unresectable HCC in real-world clinical practice. Additional studies with larger sample sizes and longer observation periods are needed to further evaluate the efficacy and safety of Dur/Tre.
In conclusion, we demonstrated that patients who received durvalumab plus tremelimumab as first-line therapy had a better tumor response than those who received durvalumab plus tremelimumab in later lines. Although there were no significant differences in AEs between the first- and later-line groups, this regimen should be carefully administered to patients with deteriorating hepatic function or advanced age to prevent discontinuation due to AEs.
Supplementary Material
Acknowledgements
Not applicable.
Glossary
Abbreviations
- ALBI
albumin-bilirubin
- DCR
disease control rate
- Dur/Tre
durvalumab plus tremelimumab
- HCC
hepatocellular carcinoma
- ICIs
immune checkpoint inhibitors
- irAEs
immune-related adverse events
- ORR
objective response rate
- PFS
progression-free survival
- STRIDE
Single Tremelimumab Regular Interval Durvalumab
Funding Statement
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
SS participated in study conception and design, data acquisition, data interpretation and manuscript drafting. SS and IS confirm the authenticity of all the raw data. IS, TeTo, TI, JT, YT, NY, TN, MT, SK, TH, KS, TeY, MS, HI, SI, TS, NT, TaY and AN participated in data acquisition. SN, MO, HK, TeTa, TaTa and TK participated in data analysis, data interpretation, and manuscript drafting. All authors participated in the critical revision of the manuscript, and read and approved the final version of the manuscript.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Kurume University School of Medicine (approval code: 23153), and an implementation permit was obtained from each of the 15 other institutions. An opt-out approach was used to obtain informed consent from the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, Laversanne M, McGlynn KA, Soerjomataram I. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77:1598–1606. doi: 10.1016/j.jhep.2022.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400:1345–1362. doi: 10.1016/S0140-6736(22)01200-4. [DOI] [PubMed] [Google Scholar]
- 3.Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380:1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 4.Kudo M. Recent advances in systemic therapy for hepatocellular carcinoma in an aging society: 2020 update. Liver Cancer. 2020;9:640–662. doi: 10.1159/000511001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rimassa L, Finn RS, Sangro B. Combination immunotherapy for hepatocellular carcinoma. J Hepatol. 2023;79:506–515. doi: 10.1016/j.jhep.2023.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Singal AG, Llovet JM, Yarchoan M, Mehta N, Heimbach JK, Dawson LA, Jou JH, Kulik LM, Agopian VG, Marrero JA, et al. AASLD practice guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. 2023;78:1922–1965. doi: 10.1097/HEP.0000000000000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, Sukeepaisarnjaroen W, Kang YK, Van Dao T, De Toni EN, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022;1:EVIDoa2100070. doi: 10.1056/EVIDoa2100070. [DOI] [PubMed] [Google Scholar]
- 8.Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, Sukeepaisarnjaroen W, Kang YK, Dao TV, De Toni EN, et al. Plain language summary of the HIMALAYA study: Tremelimumab and durvalumab for unresectable hepatocellular carcinoma (liver cancer) Future Oncol. 2023;19:2505–2516. doi: 10.2217/fon-2023-0486. [DOI] [PubMed] [Google Scholar]
- 10.Johnson ML, Cho BC, Luft A, Alatorre-Alexander J, Geater SL, Laktionov K, Kim SW, Ursol G, Hussein M, Lim FL, et al. Durvalumab with or without tremelimumab in combination with chemotherapy as first-line therapy for metastatic non-small-cell lung cancer: The phase III POSEIDON study. J Clin Oncol. 2023;41:1213–1227. doi: 10.1200/JCO.22.00975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Psyrri A, Fayette J, Harrington K, Gillison M, Ahn MJ, Takahashi S, Weiss J, Machiels JP, Baxi S, Vasilyev A, et al. Durvalumab with or without tremelimumab versus the EXTREME regimen as first-line treatment for recurrent or metastatic squamous cell carcinoma of the head and neck: KESTREL, a randomized, open-label, phase III study. Ann Oncol. 2023;34:262–274. doi: 10.1016/j.annonc.2022.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Horvat TZ, Adel NG, Dang TO, Momtaz P, Postow MA, Callahan MK, Carvajal RD, Dickson MA, D'Angelo SP, Woo KM, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at memorial sloan kettering cancer center. J Clin Oncol. 2015;33:3193–3198. doi: 10.1200/JCO.2015.60.8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 14.Cholongitas E, Papatheodoridis GV, Vangeli M, Terreni N, Patch D, Burroughs AK. Systematic review: The model for end-stage liver disease-should it replace Child-Pugh's classification for assessing prognosis in cirrhosis? Aliment Pharmacol Ther. 2005;22:1079–1089. doi: 10.1111/j.1365-2036.2005.02691.x. [DOI] [PubMed] [Google Scholar]
- 15.Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A, Palmer D, et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550–558. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatanaka T, Kakizaki S, Hiraoka A, Tada T, Hirooka M, Kariyama K, Tani J, Atsukawa M, Takaguchi K, Itobayashi E, et al. Comparing the impact of atezolizumab plus bevacizumab and lenvatinib on the liver function in hepatocellular carcinoma patients: A mixed-effects regression model approach. Cancer Med. 2023;12:21680–21693. doi: 10.1002/cam4.6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Hayakawa Y, Tsuchiya K, Kurosaki M, Yasui Y, Kaneko S, Tanaka Y, Ishido S, Inada K, Kirino S, Yamashita K, et al. Early experience of atezolizumab plus bevacizumab therapy in Japanese patients with unresectable hepatocellular carcinoma in real-world practice. Invest New Drugs. 2022;40:392–402. doi: 10.1007/s10637-021-01185-4. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y, Zhang Y, Iwamoto H, Hosaka K, Seki T, Andersson P, Lim S, Fischer C, Nakamura M, Abe M, et al. Discontinuation of anti-VEGF cancer therapy promotes metastasis through a liver revascularization mechanism. Nat Commun. 2016;7:12680. doi: 10.1038/ncomms12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sangro B, Chan SL, Kelly RK, Lau G, Kudo M, Sukeepaisarnjaroen W, Yarchoan M, De Toni EN, Furuse J, Kang YK, et al. Four-year overall survival update from the phase 3 HIMALAYA study of tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. Ann Oncol. 2024;35:448–457. doi: 10.1016/j.annonc.2024.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Tanabe N, Saeki I, Aibe Y, Matsuda T, Hanazono T, Nishi M, Hidaka I, Kuwashiro S, Shiratsuki S, Matsuura K, et al. Early prediction of response focused on tumor markers in atezolizumab plus bevacizumab therapy for hepatocellular carcinoma. Cancers (Basel) 2023;15:2927. doi: 10.3390/cancers15112927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waterhouse D, Lam J, Betts KA, Yin L, Gao S, Yuan Y, Hartman J, Rao S, Lubinga S, Stenehjem D. Real-world outcomes of immunotherapy-based regimens in first-line advanced non-small cell lung cancer. Lung Cancer. 2021;156:41–49. doi: 10.1016/j.lungcan.2021.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: Similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016;39:98–106. doi: 10.1097/COC.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robert L, Tsoi J, Wang X, Emerson R, Homet B, Chodon T, Mok S, Huang RR, Cochran AJ, Comin-Anduix B, et al. CTLA4 blockade broadens the peripheral T-cell receptor repertoire. Clin Cancer Res. 2014;20:2424–2432. doi: 10.1158/1078-0432.CCR-13-2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimose S, Hiraoka A, Tanaka M, Iwamoto H, Tanaka T, Noguchi K, Aino H, Yamaguchi T, Itano S, Suga H, et al. Deterioration of liver function and aging disturb sequential systemic therapy for unresectable hepatocellular carcinoma. Sci Rep. 2022;12:17018. doi: 10.1038/s41598-022-21528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delire B, De Martin E, Meunier L, Larrey D, Horsmans Y. Immunotherapy and gene therapy: New challenges in the diagnosis and management of drug-induced liver injury. Front Pharmacol. 2021;12:786174. doi: 10.3389/fphar.2021.786174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ito T, Ishigami M, Yamamoto T, Mizuno K, Yamamoto K, Imai N, Ishizu Y, Honda T, Kawashima H, Yasuda S, et al. Clinical course of liver injury induced by immune checkpoint inhibitors in patients with advanced malignancies. Hepatol Int. 2021;15:1278–1287. doi: 10.1007/s12072-021-10238-y. [DOI] [PubMed] [Google Scholar]
- 28.Dudek M, Pfister D, Donakonda S, Filpe P, Schneider A, Laschinger M, Hartmann D, Hüser N, Meiser P, Bayerl F, et al. Auto-aggressive CXCR6+ CD8 T cells cause liver immune pathology in NASH. Nature. 2021;592:444–449. doi: 10.1038/s41586-021-03233-8. [DOI] [PubMed] [Google Scholar]
- 29.Pfister D, Núñez NG, Pinyol R, Govaere O, Pinter M, Szydlowska M, Gupta R, Qiu M, Deczkowska A, Weiner A, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592:450–456. doi: 10.1038/s41586-021-03362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tani J, Senoh T, Moriya A, Ogawa C, Deguchi A, Sakamoto T, Takuma K, Nakahara M, Oura K, Tadokoro T, et al. Long-term outcomes and evaluation of hepatocellular carcinoma recurrence after hepatitis C virus eradication by direct-acting antiviral treatment: All kagawa liver disease group (AKLDG) study. Cancers (Basel) 2021;13:2257. doi: 10.3390/cancers13092257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Persano M, Rimini M, Tada T, Suda G, Shimose S, Kudo M, Cheon J, Finkelmeier F, Lim HY, Presa J, et al. Sequential therapies after atezolizumab plus bevacizumab or lenvatinib first-line treatments in hepatocellular carcinoma patients. Eur J Cancer. 2023;189:112933. doi: 10.1016/j.ejca.2023.05.021. [DOI] [PubMed] [Google Scholar]
- 32.Takeuchi Y, Nouso K, Fujioka SI, Kariyama K, Kobashi H, Uematsu S, Moriya A, Hagihara H, Takabatake H, Nakamura S, et al. The prediction of early progressive disease in patients with hepatocellular carcinoma receiving atezolizumab plus bevacizumab. Cancer Med. 2023;12:17559–17568. doi: 10.1002/cam4.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomonari T, Tani J, Sato Y, Tanaka H, Tanaka T, Taniguchi T, Asahiro M, Okamoto K, Sogabe M, Miyamoto H, et al. Initial therapeutic results of atezolizumab plus bevacizumab for unresectable advanced hepatocellular carcinoma and the importance of hepatic functional reserve. Cancer Med. 2023;12:2646–2657. doi: 10.1002/cam4.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hatanaka T, Kakizaki S, Hiraoka A, Tada T, Hirooka M, Kariyama K, Tani J, Atsukawa M, Takaguchi K, Itobayashi E, et al. Comparative analysis of the therapeutic outcomes of atezolizumab plus bevacizumab and lenvatinib for hepatocellular carcinoma patients aged 80 years and older: Multicenter study. Hepatol Res. 2023;54:382–391. doi: 10.1111/hepr.13991. [DOI] [PubMed] [Google Scholar]
- 35.Iavarone M, Cabibbo G, Biolato M, Corte CD, Maida M, Barbara M, Basso M, Vavassori S, Craxì A, Grieco A, et al. Predictors of survival in patients with advanced hepatocellular carcinoma who permanently discontinued sorafenib. Hepatology. 2015;62:784–791. doi: 10.1002/hep.27729. [DOI] [PubMed] [Google Scholar]
- 36.Shimose S, Iwamoto H, Niizeki T, Shirono T, Noda Y, Kamachi N, Okamura S, Nakano M, Suga H, Kuromatsu R, et al. Clinical significance of adverse events for patients with unresectable hepatocellular carcinoma treated with lenvatinib: A multicenter retrospective study. Cancers (Basel) 2020;12:1867. doi: 10.3390/cancers12071867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dale W, Mohile SG, Eldadah BA, Trimble EL, Schilsky RL, Cohen HJ, Muss HB, Schmader KE, Ferrell B, Extermann M, et al. Biological, clinical, and psychosocial correlates at the interface of cancer and aging research. J Natl Cancer Inst. 2012;104:581–589. doi: 10.1093/jnci/djs145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in the present study may be requested from the corresponding author.


