Abstract
Background:
Weight loss response after bariatric surgery is highly variable, and several demographic factors are associated with differential responses to surgery. Preclinical studies demonstrate numerous sex-specific responses to bariatric surgery, but whether these responses are also operation dependent is unknown.
Objective:
To examine sex-specific weight loss outcomes up to 5 years after laparoscopic Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG).
Setting:
Single center, university, United States.
Methods:
Retrospective, observational cohort study including RYGB (n = 5057) and vertical SG (n = 2041) patients from a single, academic health center. Percentage total weight loss (TWL) over time was examined with generalized linear mixed models to determine the main and interaction effects of surgery type on weight loss by sex.
Results:
TWL demonstrated a strong sex-by-procedure interaction, with women having a significant advantage with RYGB compared with SG (adjusted difference at 5 yr: 8.0% [95% CI: 7.5–8.5]; P < .001). Men also experienced greater TWL over time with RYGB or SG, but the difference was less and clinically insignificant (adjusted difference at 5 yr: 2.9% [2.0–3.8]; P < .001; P interaction between sex and procedure type = .0001). Overall, women had greater TWL than men, and RYGB patients had greater TWL than SG patients (adjusted difference at 5 yr: 3.1% [2.4–3.2] and 6.9% [6.5–7.3], respectively; both P < .0001). Patients with diabetes lost less weight compared with those without (adjusted difference at 5 yr: 3.0% [2.7–3.2]; P < .0001).
Conclusions:
Weight loss after bariatric surgery is sex- and procedure-dependent. There is an association suggesting a clinically insignificant difference in weight loss between RYGB and SG among male patients at both the 2- and 5-year postsurgery time points. (Surg Obes Relat Dis 2024;20:687–694.)
Keywords: Sexual dimorphism, Weight loss outcomes, Bariatric surgery
As the surgical and medical options for obesity treatment increase, understanding how patient factors such as sex, race, age, and underlying co-morbidities are predictive of outcomes is paramount to provide guidance in treatment selection. Current therapy options for surgical weight loss include laparoscopic sleeve gastrectomy (SG) and Roux-en-Y gastric bypass (RYGB), with other surgical options becoming more common place, including one-anastomosis gastric bypass and single-anastomosis duodenoileal bypass [1,2]. However, no guidelines currently exist to assist in the selection of a particular bariatric surgical therapy in a given patient population.
Numerous demographic and clinical factors have been associated with the degree of weight loss achieved after bariatric surgery, including age, race, baseline weight, diabetes status, duration of diabetes, and environmental and psychosocial factors [3–9]. Extensive research has explored the influence of sex on the extent of weight loss following bariatric surgery, yet there is ongoing debate about the specific effect of gender on weight loss directionality. Masrur et al. and Kitamura et al. both found in large retrospective case-control analyses that female patients achieved greater weight loss than male patients [6,10]; however, Shah et al. in a matched propensity score analysis failed to identify any sex-specific difference [11]. A meta-analysis by Risi et al., incorporating 27 studies, found that while women experienced more in terms of excess weight, men lost more in terms of total body mass index (BMI) [12]. Studies vary in how SG and RYGB compare in the amount of weight loss achieved in the short (1 yr), moderate (3 yr) [13], and long term (5 yr) [14], and little is known as to how the sex-specific differences interact with differences in weight loss achieved with different weight loss surgeries.
We thus aimed to evaluate the interaction of sex and operations undertaken on weight loss outcomes following bariatric surgery in our large single-institution cohort. Our primary hypothesis is that female patients achieve greater weight loss than male patients, which may be modified by procedure type.
Methods
Study population and data extraction
This study was approved by the local institutional review board. The data utilized for this study derived from the local Quality, Efficacy, and Safety (QES) Database, a prospective data registry that includes operative records (>8000) of metabolic and bariatric operations. The QES Database is unique to Vanderbilt University Medical Center and is solely for internal research and quality improvement purposes. Data are procured and securely stored using Research Electronic Data Capture (REDCap), a secure, web-based software platform designed to support data capture, providing: (1) an intuitive interface for validated data capture; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for data integration and interoperability with external sources [12,13].
For the current study, data extraction included cases and follow-up from January 1999 through June 2022. Variables included age, surgery date, sex (female/male), race (American Indian/Alaska Native, Asian, Native Hawaiian or Other Pacific Islander, Black or African American, White/Caucasian, Unknown/Not Reported, Declined), ethnicity (Hispanic or Latino, NOT Hispanic or Latino, Unknown/Not Reported, Declined), operation (RYGB, SG), preoperative body weight (kg), and preoperative height (cm). Preoperative weight was extracted as the highest weight within 6 weeks before surgery. Only laparoscopic RYGB and laparoscopic SG were included, and we limited our analysis to only Black/African American and White/Caucasian race for modeling purposes, as these racial groups accounted for ~98% of all records. Patients were classified as having type 2 diabetes (T2D) if they had an International Statistical Classification of Disease-9/10 code for diabetes. Records with duplicate identifiers were excluded.
Body weight and follow-up determination
A frequent limitation of bariatric surgical studies is poor follow-up and to circumvent this limitation, the QES includes all weight measurements within the hospital system network, even if patients were lost to bariatric follow-up. Using the date of surgery as a reference point, all weight measurements were extracted at 3-month intervals. In the case of multiple measurements within an interval, the measurement closest to the 3-month time interval was automatically included. For follow-up calculation, patients were considered to have follow-up to the year of interest if they had at least 1 measurement within a given year.
Statistical analysis and data visualization
Data analysis, manipulation, and visualization were conducted with R version 3.5.0 or higher (R Foundation for Statistical Computing, Vienna, Austria). Data were described using mean and standard deviation for continuous variables, while categorical variables were described using counts and percentages. Generalized linear mixed models (SAS) were used to estimate the effects of age, sex (female or male), race (Black/African American or White/Caucasian), operation (RYGB or vertical sleeve gastrectomy [VSG]), preoperative body weight, and preoperative insulin resistance/diabetes (present/absent) on total weight loss percentage with a spline function of time with prespecified knots at 0, 12, 18, 36, and 60 months. Interactions were included between spline function of time and the aforementioned variables given their effects may change over time.
Results
Cohort characteristics and follow-up
Cohort characteristics are shown in Table 1. For the entire cohort, the median age was 45.3, with 80% female, 81% White, and 38% having T2D at baseline. The majority of patients underwent RYGB (n = 5057) compared with SG (n = 2041). The median baseline weight was 135.3 kg, with a median baseline BMI of 47.9 kg/m2. Follow-up rates (at least 1 visit within 6 mo of time before or after) at 1 year, 2 years, and 5 years postsurgery were 83.7%, 54.2%, and 23.6%, respectively. Follow-up rates by male and female cohorts differed by <1% at year 1, 2, and 5.
Table 1.
Cohort baseline characteristics
| Characteristic | Total (n = 7098) | Female (n = 5669) | Male (n = 1429) | ||||
|---|---|---|---|---|---|---|---|
| RYGB (n = 4064) | SG (n = 1605) | P value | RYGB (n = 993) | SG (n = 436) | P value | ||
| Age (yr)* | 45.3 ± 10.9 | 44.9 ± 10.7 | 44.4 ± 11.1 | .10 | 47.8 ± 10.6 | 46.7 ± 11.2 | .09 |
| Race, n (%) | <.0001 | <.0001 | |||||
| White | 5747 (81.0) | 3393 (83.5) | 1138 (70.9) | 872 (87.8) | 344 (78.9) | ||
| Black | 1172 (16.5) | 592 (14.6) | 418 (26.0) | 89 (9.0) | 73 (16.7) | ||
| Others | 179 (2.5) | 79 (1.9) | 49 (3.1) | 32 (3.2) | 19 (4.4) | ||
| Presurgery weight (kg)* | 135.3 ± 25.9 | 131.6 ± 23.5 | 125.4 ± 20.6 | <.0001 | 158.0 ± 26.2 | 154.4 ± 25.3 | .02 |
| Presurgery BMI (kg/m2)* | 47.9 ± 7.7 | 48.3 ± 7.9 | 46.2 ± 6.8 | <.0001 | 48.8 ± 7.8 | 48.0 ± 7.4 | .06 |
| Diabetes status, n (%)† | <.0001 | <.0001 | |||||
| No | 4391 (61.9) | 2474 (60.9) | 1191 (74.2) | 468 (47.1) | 258 (59.2) | ||
| Yes | 2707 (38.1) | 1590 (39.1) | 414 (25.8) | 525 (52.9) | 178 (40.8) | ||
RYGB = Roux-en-Y gastric bypass; SG = sleeve gastrectomy; BMI = body mass index.
Mean ± SD.
Diabetes status was defined by International Statistical Classification of Disease (ICD) code (ICD-9: 250; ICD-10: E10 and E11).
Weight loss outcomes
Weight loss across the cohort was in line with prior studies, with 31.8% and 32.2% at 1 and 2 years postsurgery, respectively, but weight loss declined over time, with mean weight loss of 26% at 5 years (Table 2). Patients who underwent RYGB experienced greater weight loss than those who underwent SG at all time points (Fig. 1), with a covariate-adjusted difference of 5%, 6.6%, and 6.9% at years 1, 2, and 5, respectively (Table 2).
Table 2.
Effects of age, sex, race, preoperative body weight and diabetes status, and procedure choice on percentage total weight loss at postoperative years 1, 2, and 5*
| Mean weight loss % (95% CI) | Adjusted difference in weight loss % (95% CI) | P value for difference | |
|---|---|---|---|
| Year 1 | |||
| All patients | 31.8 (31.7, 31.9) | ||
| Age, per 5-yr increment | −.24 (−.30, −.18) | <.0001 | |
| Weight, per 25-kg increment | .32 (.19, .45) | <.0001 | |
| Female | 32.1 (32.0, 32.3) | Ref. | |
| Male | 30.3 (30.1, 30.6) | −2.18 (−2.55, −1.81) | <.0001 |
| White | 32.6 (32.5, 32.7) | Ref. | |
| Black | 28.4 (28.2, 28.7) | −3.91 (−4.27, −3.55) | <.0001 |
| No diabetes | 33.2 (33.0, 33.3) | Ref. | |
| Diabetes | 30.1 (29.9, 30.3) | −2.96 (−3.24, −2.68) | <.0001 |
| RYGB | 33.1 (33.0, 33.2) | Ref. | |
| VSG | 28.4 (28.2, 28.7) | −4.97 (−5.27, −4.67) | <.0001 |
| Year 2 | |||
| All patients | 32.2 (32.0, 32.3) | ||
| Age, per 5-yr increment | −.17 (−.23, −.11) | <.0001 | |
| Weight, per 25-kg increment | .72 (.58, .85) | <.0001 | |
| Female | 32.5 (32.3, 32.7) | Ref. | |
| Male | 30.8 (30.4, 31.2) | −2.81 (−3.20, −2.42) | <.0001 |
| White | 33.1 (32.9, 33.3) | Ref. | |
| Black | 28.6 (28.2, 29.0) | −4.13 (−4.50, −3.75) | <.0001 |
| No diabetes | 34.0 (33.8, 34.2) | Ref. | |
| Diabetes | 30.1 (29.9, 30.4) | −3.67 (−3.97, −3.38) | <.0001 |
| RYGB | 33.9 (33.7, 34.1) | Ref. | |
| VSG | 27.5 (27.1, 27.8) | −6.64 (−6.95, −6.32) | <.0001 |
| Year 5 | |||
| All patients | 26.8 (26.5, 27.1) | ||
| Age, per 5-yr increment | .04 (−.03, .11) | .25 | |
| Weight, per 25-kg increment | 1.90 (1.74, 2.05) | <.0001 | |
| Female | 27.4 (27.0, 27.7) | Ref. | |
| Male | 24.5 (23.8, 25.2) | −3.09 (−3.56, −2.62) | <.0001 |
| White | 27.6 (27.3, 28.0) | Ref. | |
| Black | 23.1 (22.5, 23.7) | −3.67 (−4.12, −3.22) | <.0001 |
| No diabetes | 29.0 (28.6, 29.5) | Ref. | |
| Diabetes | 24.9 (24.5, 25.2) | −2.83 (−3.18, −2.47) | <.0001 |
| RYGB | 28.0 (27.7, 28.3) | Ref. | |
| VSG | 21.9 (21.2, 22.6) | −6.92 (−7.33, −6.51) | <.0001 |
Ref. = reference; RYGB = Roux-en-Y gastric bypass; VSG = vertical sleeve gastrectomy.
Generalized linear mixed models adjusted for age (years), preoperative body weight (kilograms), sex (male versus female), race (Black versus White), diabetes status (diabetes versus no diabetes), procedure (VSG versus RYGB), time (months, modeled using a spline function with knots at 0, 12, 24, 36, and 63 mo), and interactions between time spline and the aforementioned variables given that their effects may change over time. The interaction P values are <.0001 for age * time, weight * time, sex * time, race * time, diabetes * time, and operation * time.
Fig. 1.
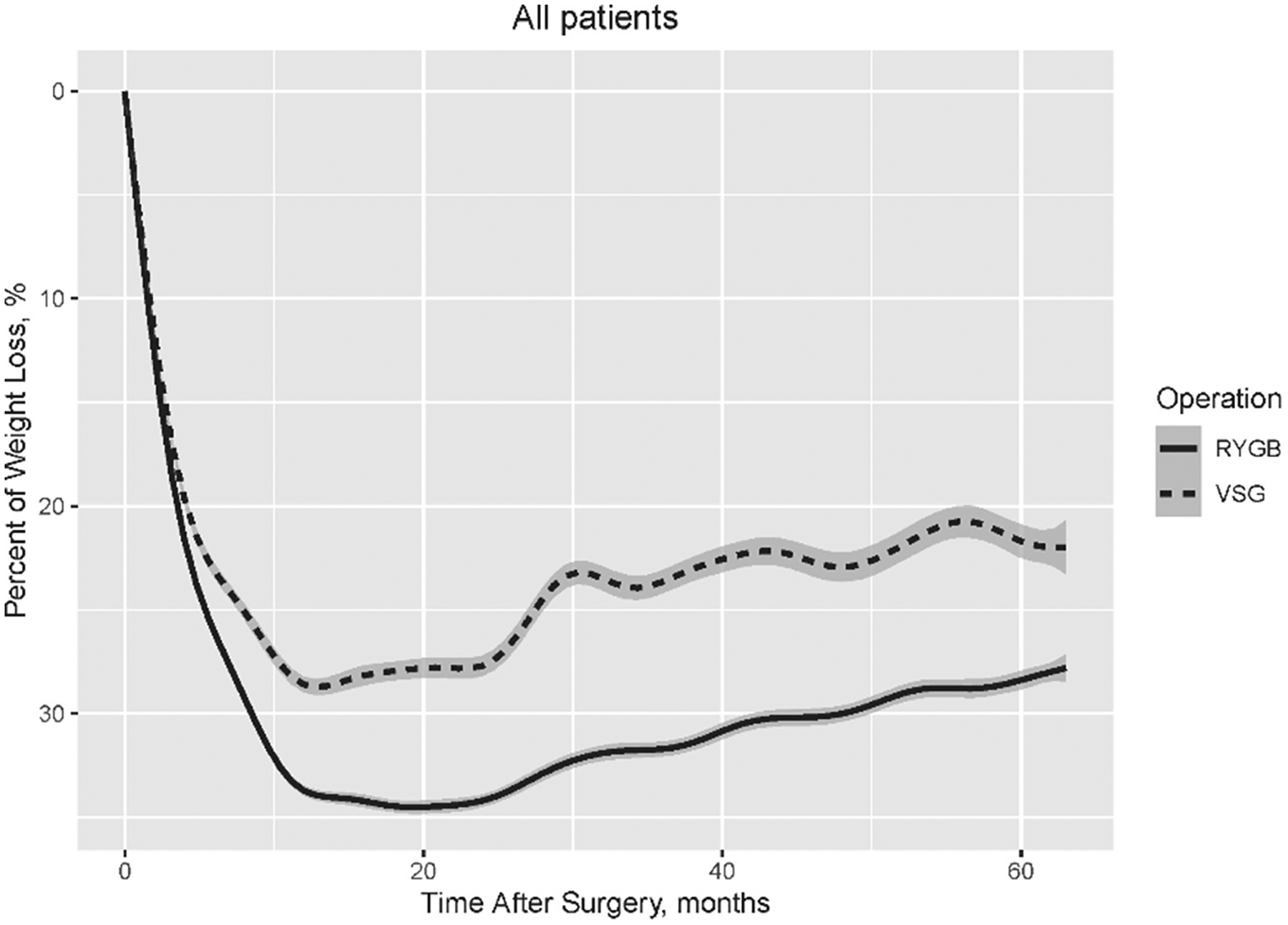
Percentage of weight loss by operation within 5 years after metabolic surgery among all patients.
Effect of sex and procedure type on weight loss
In comparing weight loss by sex, at 1, 2, and 5 years female patients experienced 2.2%, 2.8%, and 3.1% greater weight loss, respectively, when compared with male patients (< .0001) of either operation. In the first 2 years postsurgery, female patients had more weight loss after RYGB (female: 34.4% versus male: 31.7%) and less weight loss after SG (female: 27.3% versus male: 28.3%) than male patients, resulting in greater difference between RYGB and SG in female patients than male patients (adjusted difference: −7.43% versus −3.40%). For 2 to 5 years after surgery, female patients showed similar weight regain after RYGB and SG (both 5.7%), while male patients had more weight regain after RYGB than after SG (6.7% versus 5.4%), resulting in a smaller discrepancy between RYGB and SG in male patients at 5 years and further increasing the difference between RYGB and SG in female patients than male patients (adjusted difference: −8.00% versus −2.88%; Table 3, Fig. 2).
Table 3.
Effects of sex, diabetes status, and operation choice on percentage total weight loss at postoperative years 1, 2, and 5, stratified by sex*
| Female patients | Male patients | |||||
|---|---|---|---|---|---|---|
| Mean weight loss % (95% CI) | Adjusted difference % (95% CI) | P value for difference | Mean weight loss % (95% CI) | Adjusted difference % (95% CI) | P value for difference | |
| Year 1 | ||||||
| No diabetes | 33.3 (33.1, 33.4) | Ref. | 32.7 (32.4, 33.1) | Ref. | ||
| Diabetes | 30.6 (30.4, 30.8) | −2.83 (−3.14, −2.51) | <.0001 | 28.5 (28.2, 28.8) | −3.47 (−4.10, −2.83) | <.0001 |
| RYGB | 33.5 (33.4, 33.7) | Ref. | 31.3 (31.0, 31.6) | Ref. | ||
| VSG | 28.6 (28.3, 28.8) | −5.14 (−5.47, −4.80) | <.0001 | 27.9 (27.4, 28.5) | −4.31 (−4.99, −3.64) | <.0001 |
| Year 2 | ||||||
| No diabetes | 34.1 (33.9, 34.4) | Ref. | 33.2 (32.6, 33.8) | Ref. | ||
| Diabetes | 30.4 (30.1, 30.7) | −3.75 (−4.08, −3.43) | <.0001 | 29.3 (28.8, 29.9) | −3.39 (−4.07, −2.72) | <.0001 |
| RYGB | 34.4 (34.2, 34.6) | Ref. | 31.7 (31.3, 32.1) | Ref. | ||
| VSG | 27.3 (26.9, 27.7) | −7.43 (−7.79, −7.08) | <.0001 | 28.3 (27.4, 29.1) | −3.40 (−4.11, −2.68) | <.0001 |
| Year 5 | ||||||
| No diabetes | 28.9 (28.4, 29.3) | Ref. | 30.2 (28.9, 31.5) | Ref. | ||
| Diabetes | 25.8 (25.3, 26.2) | −2.91 (−3.30, −2.51) | <.0001 | 22.3 (21.5, 23.1) | −2.43 (−3.28, −1.59) | <.0001 |
| RYGB | 28.7 (28.4, 29.1) | Ref. | 25.0 (24.3, 25.7) | Ref. | ||
| VSG | 21.6 (20.9, 22.3) | −8.00 (−8.46, −7.54) | <.0001 | 22.9 (21.0, 24.8) | −2.88 (−3.79, −1.98) | <.0001 |
Ref. = reference; RYGB = Roux-en-Y gastric bypass; VSG = vertical sleeve gastrectomy.
Generalized linear mixed models adjusted for age (years), preoperative body weight (kilograms), race (Black versus White), diabetes status (diabetes versus no diabetes), procedure (VSG versus RYGB), time (months, modeled using a spline function with knots at 0, 12, 24, 36, and 63 mo), and interactions between time spline and the aforementioned variables.
Fig. 2.
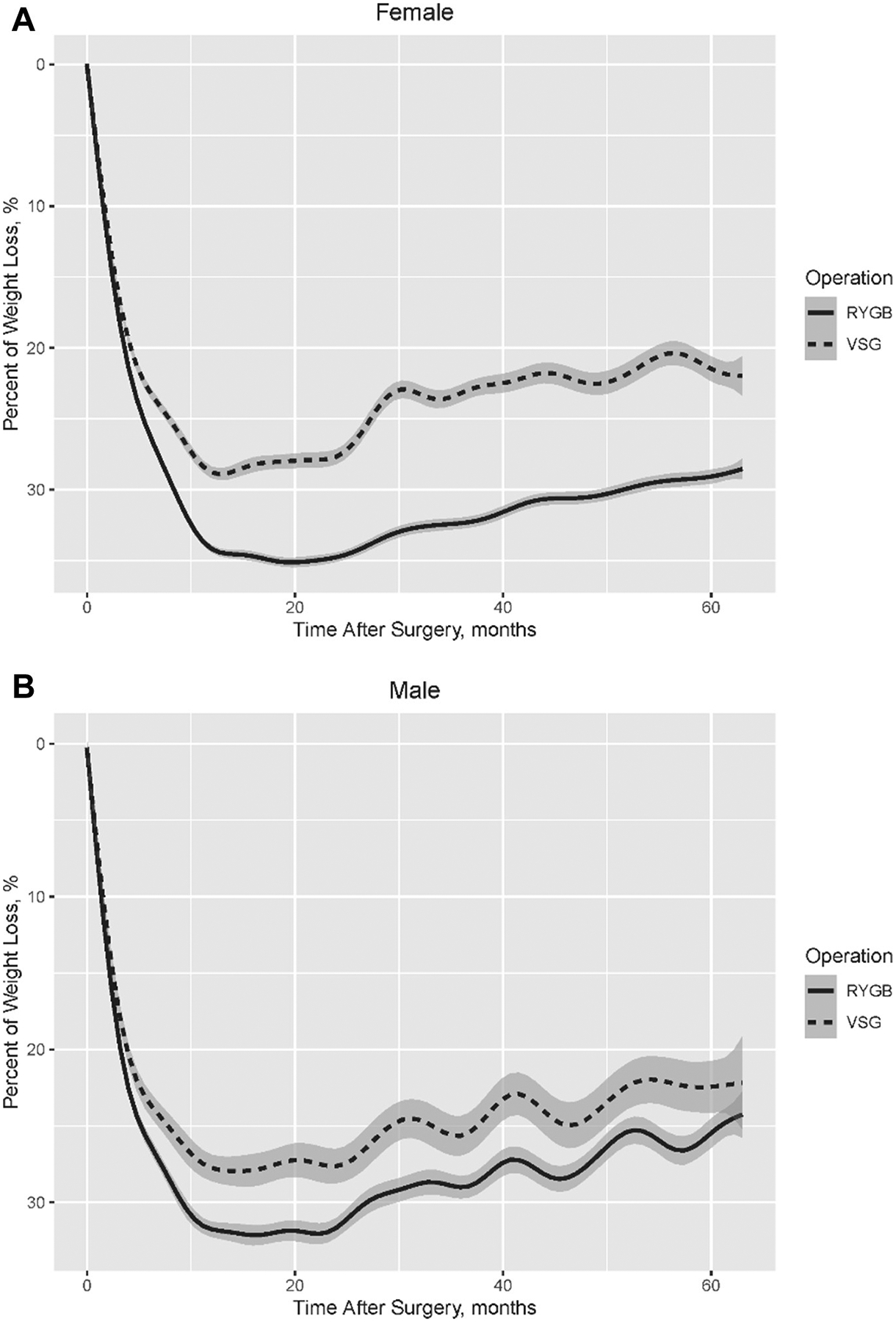
Percentage of weight loss by operation within 5 years after metabolic surgery among (A) female and (B) male patients.
As female patients represented most of the total cohort (80%), female weight loss over time mirrored the total cohort, with RYGB having a greater weight loss at all time points relative to SG (Fig. 2A). In contrast, weight loss trends by men undergoing RYGB converged with men undergoing SG (Fig. 2B). While men at 1 year undergoing RYGB experienced 4.3% more weight loss than those undergoing SG, that difference decreased at 2 and 5 years, with only a 3.4% and 2.9% greater weight loss after RYGB compared with SG, respectively (Table 3). This contrasts with female patients undergoing RYGB who experienced a further increase in weight loss over time compared with female patients undergoing SG, with female patients after RYGB experiencing 5.1%, 7.4%, and 8.0% more weight loss at 1, 2, and 5 years, respectively.
Effect of age, initial body weight, and race
Age weakly and inconsistently impacted weight loss over time. At 1 year, covariate-adjusted difference was .2% greater weight loss per 5-year increment (P <.001), but by 5 years, no difference with increasing age was found (P = .25; Table 2). Increasing body weight (per 25-kg increment) was similarly associated with greater weight loss at all time points, with the difference increasing over time, equating to a 1.9% difference at 5 years (P <.001; Table 2). Compared to White patients, Black patients lost less weight at all time points, with 3.9%, 4.1%, and 3.6% less weight loss at 1, 2, and 5 years, respectively (all P <.001).
Effect of diabetes history
T2D was associated with decreased weight loss at all time points with a covariate-adjusted difference of 3.0%, 3.7%, and 2.8% less weight loss at 1, 2, and 5 years, respectively (all P < .001). The impact of T2D was consistent across male and female patients (2.4 versus 2.9% at year 5, respectively; Table 3).
Discussion
In this study, we identified several factors impacting the degree of weight loss experienced both in short (1-yr) and long-term (5-yr) follow-up. First, female patients experienced greater weight loss than men at all follow-up time points. Patients with T2D experienced less weight loss than those without, although the difference was small in the long term. Similarly, although RYGB was associated with greater weight loss than SG in women, in men, the difference at 5 years was not clinically significant. These findings of minimal differences in weight loss between SG and RYGB in men and in patients with T2D raises the prospect of surgical decision making focusing less on degree of weight loss, and more on patient-focused variables such as need for medications and quality of life.
Several prior studies have evaluated sex as a biological variable impacting weight loss after bariatric surgery with studies disagreeing on the directionality of a sex-dependent difference. Anderson et al. contradictorily found that women achieved less weight loss after sleeve gastrectomy than men [15]. However, this single-center study included only 160 patients in total, compared with our 7000. Quadri et al., in a similarly small cohort over a 2-year follow-up period, did not identify a difference in weight loss between men and women after sleeve gastrectomy [16]. When evaluating sex as a biological variable in patients undergoing RYGB, Stroh et al. also found no difference in weight loss with 3-year follow-up between men and women [17]. Although our study found that women on average experienced greater weight loss than men, the difference was small with only 3% less weight loss in men over 5-year follow-up.
Intriguingly, we identified a clinically insignificant difference in weight loss achieved by men undergoing SG versus RYGB. While female patients in our cohort experienced a dramatic 8% greater weight loss after RYGB compared with SG, male patients experienced only a 2.9% difference. The 8% difference in women in our study is similar to that noted in the SLEEVEPASS trial, which followed both men and women for 10 years [18]. In contrast, the SM-BOSS trial failed to identify a difference between SG and RYGB after 5 years of follow-up [19]. Neither study evaluated sex as a separate biological variable or the procedure-by-sex interaction. The differences in these findings raise the prospect that small, but perceptible differences in surgical technique impact weight loss across studies. These differences include sizing of the sleeve or decision on an appropriate Roux limb length. Given the higher risk profile for RYGB and the potential absence of a clinically meaningful difference in long-term weight outcome in men, this could direct some male patients to select SG over RYGB in the absence of any operation-specific contraindications. The caveat to this scenario is that even though weight loss might not differ long-term, other metabolic benefits of RYGB independent of weight loss could persist [20].
While studies disagree on the impact of sex on weight loss following SG and RYGB, diabetes has consistently been linked to less weight loss after either bariatric surgery. Rebelos et al. found a significant association with T2D and diminished weight loss after either SG or RYGB up to 5 years after surgery, with over 3 kg/m2 less decrease in BMI after surgery [21]. Similarly, in patients undergoing SG, Diedisheim et al. found preexisting diabetes status was associated with an increased risk of losing 10% or less of initial body weight [22]. The mechanism in which diabetes leads to diminished weight loss after bariatric surgery is, as of yet, undefined. As duration of diabetes and preoperative use of insulin signaling have been associated with lower rates of diabetes remission and weight loss, derangements in insulin signaling and other endocrine signaling pathways likely play a critical role in impacting weight loss after bariatric surgery [23,24].
Our study faces a common challenge with long-term, real-world, retrospective data analyses with limited follow-up [25]. At 2 years postoperatively, our follow-up was 54% and by 5 years had decreased to only 24%. This increases the probability of unmeasured confounding as those who follow-up may systematically differ from those who do not. Reasons for lack of follow-up beyond 1 year are multifactorial including insurance coverage issues, patient satisfaction with weight loss or the converse, or patient relocation, to name a few [26,27]. The best method to prevent such missing data is to conduct prospective, well-controlled trials; however, these incur significant costs and face challenges with generalizability of findings [28]. Ultimately, despite the limited follow-up rates of our data, these data clearly support funding for such long-term prospective trials that ensure a diverse cohort with adequate follow-up rates.
This study has several limitations beyond the retrospective, observational design. First, the burden of comorbidities besides insulin resistance/diabetes could be unevenly distributed among sex or surgical type and could skew the results. However, such associated co-morbid disease states have not been strongly associated with differences in weight loss like with T2D. Second, another important factor affecting long-term weight loss that we are unable to assess was preoperative duration of diabetes or severity of insulin resistance/diabetes, both of which would impede weight loss. Third, the 5-year follow-up rate (24%) in our cohort was limited and may represent a selection bias, as patients who failed to follow-up as time progressed may systematically differ from those who we were able to collect data. Fourth, as we are unable to determine rates of remission of obesity-related disease states such as T2D and hyperlipidemia, we are unable to determine the clinical significance of the sex- and operation-specific differences in weight loss. This is a result of the inconsistency of data for HbA1C data collected and the QES data set not collecting medication data.
Conclusion
Overall, this study adds to the growing body of literature identifying patient subtypes and their impact on weight loss following bariatric surgery. Namely, our study found a novel sex-operation interaction with weight loss over time, with women and men having different weight loss responses to bariatric surgery. The underlying mechanism that could explain this sex-dependent difference remains undefined. Future studies should focus on identifying the clinical relevance of these sex-specific differences as they pertain to rates of disease remission (e.g., T2D, hypertension, hyperlipidemia).
Acknowledgments
This work has been supported by the Vanderbilt Institute for Clinical and Translational Research, which is funded by the National Center for Advancing Translational Sciences Clinical Translational Science Award Program (5UL1TR002243-03). Additionally, this work has been supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK126721, F32DK103474).
Footnotes
Disclosures
The authors have no commercial associations that might be a conflict of interest in relation to this article.
References
- [1].Nguyen NT, Varela JE. Bariatric surgery for obesity and metabolic disorders: state of the art. Nat Rev Gastroenterol Hepatol 2017;14(3): 160–9. [DOI] [PubMed] [Google Scholar]
- [2].Cottam D, Cottam S, Surve A. Single-anastomosis duodenal ileostomy with sleeve gastrectomy “continued innovation of the duodenal switch”. Surg Clin North Am 2021;101(2):189–98. [DOI] [PubMed] [Google Scholar]
- [3].Wood MH, Carlin AM, Ghaferi AA, et al. Association of race with bariatric surgery outcomes. JAMA Surg 2019;154(5):e190029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Courcoulas AP, King WC, Belle SH, et al. Seven-year weight trajectories and health outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) study. JAMA Surg 2018;153(5):427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nielsen MS, Christensen BJ, Schmidt JB, et al. Predictors of weight loss after bariatric surgery-a cross-disciplinary approach combining physiological, social, and psychological measures. Int J Obes (Lond) 2020;44(11):2291–302. [DOI] [PubMed] [Google Scholar]
- [6].Masrur M, Bustos R, Sanchez-Johnsen L, et al. Factors associated with weight loss after metabolic surgery in a multiethnic sample of 1012 patients. Obes Surg 2020;30(3):975–81. [DOI] [PubMed] [Google Scholar]
- [7].Coleman KJ, Brookey J. Gender and racial/ethnic background predict weight loss after Roux-en-Y gastric bypass independent of health and lifestyle behaviors. Obes Surg 2014;24(10):1729–36. [DOI] [PubMed] [Google Scholar]
- [8].Apovian CM, Istfan NW. Ethnic and racial disparities in the behavioral, pharmacologic, and surgical treatment of obesity. Endocr Pract 2016;22(11):1347–9. [DOI] [PubMed] [Google Scholar]
- [9].Ng J, Seip R, Stone A, et al. Ethnic variation in weight loss, but not comorbidity remission, after laparoscopic gastric banding and Roux-en-Y gastric bypass. Surg Obes Relat Dis 2015;11(1):94–100. [DOI] [PubMed] [Google Scholar]
- [10].Kitamura R, Chen R, Trickey A, Eisenberg D. Positive and negative independent predictive factors of weight loss after bariatric surgery in a veteran population. Obes Surg 2020;30(6):2124–30. [DOI] [PubMed] [Google Scholar]
- [11].Shah N, Greenberg JA, Leverson G, et al. Weight loss after bariatric surgery: a propensity score analysis. J Surg Res 2016;202(2):449–54. [DOI] [PubMed] [Google Scholar]
- [12].Risi R, Rossini G, Tozzi R, et al. Sex difference in the safety and efficacy of bariatric procedures: a systematic review and meta-analysis. Surg Obes Relat Dis 2022;18(7):983–96. [DOI] [PubMed] [Google Scholar]
- [13].Coleman KJ, Huang YC, Hendee F, et al. Three-year weight outcomes from a bariatric surgery registry in a large integrated healthcare system. Surg Obes Relat Dis 2014;10(3):396–403. [DOI] [PubMed] [Google Scholar]
- [14].Han Y, Jia Y, Wang H, Cao L, Zhao Y. Comparative analysis of weight loss and resolution of comorbidities between laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass: a systematic review and meta-analysis based on 18 studies. Int J Surg 2020;76:101–10. [DOI] [PubMed] [Google Scholar]
- [15].Anderson B, Zhan T, Swaszek L, et al. Increased incidence of marginal ulceration following conversion of sleeve gastrectomy to Roux-en-Y gastric bypass: a multi-institutional experience. Surg Endosc 2023;37(5):3974–81. [DOI] [PubMed] [Google Scholar]
- [16].Quadri P, Sanchez-Johnsen L, Gonzalez-Heredia R, et al. Sleeve gastrectomy among males and females who are super-super obese (body mass index ≥60 kg/m2). Bariatr Surg Pract Patient Care 2017;12(4):178–83. [Google Scholar]
- [17].Stroh C, Weiner R, Wolff S, Knoll C, Manger T. Influences of gender on complication rate and outcome after Roux-en-Y gastric bypass: data analysis of more than 10,000 operations from the German Bariatric Surgery Registry. Obes Surg 2014;24(10):1625–33. [DOI] [PubMed] [Google Scholar]
- [18].Salminen P, Grönroos S, Helmiö M, et al. Effect of laparoscopic sleeve gastrectomy vs Roux-en-Y gastric bypass on weight loss, comorbidities, and reflux at 10 years in adult patients with obesity: the SLEEVEPASS randomized clinical trial. JAMA Surg 2022; 157(8):656–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Peterli R, Wölnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA 2018;319(3):255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Aminian A, Jamal M, Augustin T, et al. Failed surgical weight loss does not necessarily mean failed metabolic effects. Diabetes Technol Ther 2015;17(10):682–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rebelos E, Moriconi D, Honka MJ, Anselmino M, Nannipieri M. Decreased weight loss following bariatric surgery in patients with type 2 diabetes. Obes Surg 2023;33(1):179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Diedisheim M, Poitou C, Genser L, et al. Weight loss after sleeve gastrectomy: does type 2 diabetes status impact weight and body composition trajectories? Obes Surg 2021;31(3):1046–54. [DOI] [PubMed] [Google Scholar]
- [23].Koliaki C, Liatis S, le Roux CW, Kokkinos A. The role of bariatric surgery to treat diabetes: current challenges and perspectives. BMC Endocr Disord 2017;17(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sandoval DA, Patti ME. Glucose metabolism after bariatric surgery: implications for T2DM remission and hypoglycaemia. Nat Rev Endocrinol 2023;19(3):164–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Schlottmann F, Baz C, Pirzada A, Masrur MA. Postoperative follow-up compliance: the Achilles’ heel of bariatric surgery. Obes Surg 2023;33(9):2945–8. [DOI] [PubMed] [Google Scholar]
- [26].Monfared S, Martin A, Selzer D, Butler A. Travel distance reduces follow-up compliance but has no effect on long-term weight loss success in bariatric patients. Surg Endosc 2021;35(4):1579–83. [DOI] [PubMed] [Google Scholar]
- [27].Vidal P, Ramón JM, Goday A, et al. Lack of adherence to follow-up visits after bariatric surgery: reasons and outcome. Obes Surg 2014;24(2):179–83. [DOI] [PubMed] [Google Scholar]
- [28].Liu F, Panagiotakos D. Real-world data: a brief review of the methods, applications, challenges and opportunities. BMC Med Res Methodol 2022;22(1):287. [DOI] [PMC free article] [PubMed] [Google Scholar]


